WO2014109407A1 - Hard coat film, curable resin composition for hard coat layers, and method for producing hard coat film - Google Patents
Hard coat film, curable resin composition for hard coat layers, and method for producing hard coat film Download PDFInfo
- Publication number
- WO2014109407A1 WO2014109407A1 PCT/JP2014/050379 JP2014050379W WO2014109407A1 WO 2014109407 A1 WO2014109407 A1 WO 2014109407A1 JP 2014050379 W JP2014050379 W JP 2014050379W WO 2014109407 A1 WO2014109407 A1 WO 2014109407A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hard coat
- coat layer
- fine particles
- silica fine
- curable resin
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D4/00—Coating compositions, e.g. paints, varnishes or lacquers, based on organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond ; Coating compositions, based on monomers of macromolecular compounds of groups C09D183/00 - C09D183/16
- C09D4/06—Organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond in combination with a macromolecular compound other than an unsaturated polymer of groups C09D159/00 - C09D187/00
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/30—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers
-
- G02B1/105—
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/10—Optical coatings produced by application to, or surface treatment of, optical elements
- G02B1/14—Protective coatings, e.g. hard coatings
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1335—Structural association of cells with optical devices, e.g. polarisers or reflectors
- G02F1/133502—Antiglare, refractive index matching layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/10—Coating on the layer surface on synthetic resin layer or on natural or synthetic rubber layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/26—Polymeric coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/28—Multiple coating on one surface
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2264/00—Composition or properties of particles which form a particulate layer or are present as additives
- B32B2264/10—Inorganic particles
- B32B2264/102—Oxide or hydroxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/30—Properties of the layers or laminate having particular thermal properties
- B32B2307/306—Resistant to heat
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/40—Properties of the layers or laminate having particular optical properties
- B32B2307/412—Transparent
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/536—Hardness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/584—Scratch resistance
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2457/00—Electrical equipment
- B32B2457/20—Displays, e.g. liquid crystal displays, plasma displays
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2571/00—Protective equipment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/08—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/30—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers
- B32B27/308—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers comprising acrylic (co)polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/12—Interconnection of layers using interposed adhesives or interposed materials with bonding properties
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/02—Diffusing elements; Afocal elements
- G02B5/0205—Diffusing elements; Afocal elements characterised by the diffusing properties
- G02B5/0236—Diffusing elements; Afocal elements characterised by the diffusing properties the diffusion taking place within the volume of the element
- G02B5/0242—Diffusing elements; Afocal elements characterised by the diffusing properties the diffusion taking place within the volume of the element by means of dispersed particles
Definitions
- the present invention relates to a hard coat film used for the purpose of protecting the surface of a display or the like.
- Image display surfaces in image display devices such as liquid crystal displays, CRT displays, projection displays, plasma displays, and electroluminescence displays are required to be provided with scratch resistance so that they are not damaged during handling.
- the performance required for hard coat films has been increasing in recent years, and there is a demand for further improved hardness and scratch resistance.
- Patent Document 1 As means for improving the hardness of the hard coat film, for example, in Patent Document 1, a substrate having a specific configuration is used, and the total thickness of the hard coat film, the thickness of the acrylic resin layer constituting the substrate, and the thickness of the hard coat layer are set. A method for making a specific range is disclosed. Patent Document 2 discloses a method of forming a hard coat layer using a curable composition to which inorganic oxide particles are added. Further, Patent Documents 3 to 7 disclose methods for forming a hard coat layer using a curable composition to which reactive irregularly shaped silica fine particles are added.
- Patent Document 5 discloses that good flexibility can be obtained by adding reactive irregular-shaped silica fine particles to a curable resin composition for a hard coat layer and further adding a polymer. However, there is a concern that the hardness is lowered and the scratch resistance is deteriorated simply by adding a polymer, and it is difficult to achieve both high hardness and workability.
- Patent Document 7 proposes that a reactive polymer having a specific structure and a weight average molecular weight is contained in a curable resin composition for a hard coat layer in order to improve hardness and reduce curl. However, the workability has not been studied.
- the present invention has been made in view of the above problems, and has as its main object to provide a hard coat film that is excellent in hardness, scratch resistance and workability.
- the present inventors form a hard coat layer using a curable resin composition containing reactive irregularly shaped silica fine particles, a monomer, and a specific polymer. As a result, it was found that a hard coat film having both high hardness and workability was obtained, and the present invention was completed.
- the present invention is a hard coat film in which a hard coat layer is formed on a substrate, and the hard coat layer has irregular-shaped silica fine particles and a weight average molecular weight in the range of 30,000 to 110,000.
- a hard coat film comprising an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200 and a matrix resin is provided.
- the hard coat layer contains irregular shaped silica fine particles, whereby the hardness and scratch resistance can be improved. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to improve workability while maintaining high hardness.
- the hard coat layer may contain a polymerization initiator.
- the hard coat layer may contain a blue color material. This is because when the hard coat film of the present invention is used in an image display device, yellowness can be suppressed and visibility and color reproducibility can be improved.
- an antiglare layer may be formed on the hard coat layer. Even in the case where the antiglare layer is formed on the hard coat layer, the hard coat layer is excellent in hardness, so that high hardness can be achieved.
- the present invention is a hard coat film in which a hard coat layer is formed on a substrate, the hard coat layer is a curable resin composition containing reactive irregular silica fine particles, the acrylic polymer, and a monomer.
- a hard coat film comprising a cured product is provided.
- hardness and scratch resistance can be improved by using reactive irregularly shaped silica fine particles in the hard coat layer. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to improve workability while maintaining high hardness.
- the present invention also provides reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200, and a monomer.
- a curable resin composition for a hard coat layer is provided.
- the curable resin composition for a hard coat layer contains reactive irregularly shaped silica fine particles, a hard coat layer having excellent hardness and scratch resistance can be obtained. Moreover, it is possible to achieve both high hardness and workability by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range to the curable resin composition for a hard coat layer.
- the curable resin composition for a hard coat layer of the present invention may further contain a polymerization initiator.
- the curable resin composition for a hard coat layer of the present invention may further contain a blue color material. This is because when used in an image display device, a hard coat film capable of suppressing yellowness and improving visibility and color reproducibility can be obtained.
- the present invention also provides a reactive irregularly shaped silica fine particle on a substrate, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200.
- a method for producing a hard coat film comprising a hard coat layer forming step of applying a curable resin composition for a hard coat layer containing a monomer and curing to form a hard coat layer.
- the present invention also relates to a hard coat film having a hard coat layer formed on a substrate, wherein the hard coat layer has reactive irregularly shaped silica fine particles and a weight average molecular weight in the range of 30,000 to 110,000.
- a hard coat film comprising a cured product of a curable resin composition containing an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200, a monomer, and a polymerization initiator To do.
- the present invention also includes reactive irregularly shaped silica particles, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200, a monomer, A curable resin composition for a hard coat layer comprising a polymerization initiator is provided.
- the hardness and scratch resistance can be improved by including the reactive deformed silica fine particles in the hard coat layer. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to improve workability while maintaining high hardness.
- the present invention has an effect that it is possible to improve any of hardness, scratch resistance and workability.
- the hard coat film of the present invention is a hard coat film in which a hard coat layer is formed on a substrate, and the hard coat layer has irregular-shaped silica fine particles and a weight average molecular weight of 30,000 to 110,000. And an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200 and a matrix resin.
- the “hard coat film” is a concept including a member that can also be called a sheet or a plate.
- the hard coat layer contains irregular-shaped silica fine particles
- a hard coat layer having excellent hardness and scratch resistance can be obtained.
- an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer it is possible to achieve both high hardness and workability.
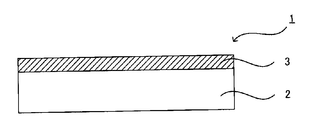
- FIG. 1 is a schematic sectional view showing an example of the hard coat film of the present invention.
- the hard coat film 1 includes a substrate 2 and a hard coat layer 3 formed on the substrate 2.
- the hard coat layer 3 contains irregular-shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within predetermined ranges, and a matrix resin. That is, the hard coat layer 3 is composed of a cured product of a curable resin composition containing reactive irregular shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Yes.
- each structure in the hard coat film of this invention is demonstrated.
- Hard coat layer The hard coat layer in this invention is formed on a board
- the hard coat layer of the first embodiment contains irregular-shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within predetermined ranges, and a matrix resin.
- the hard coat layer of the second aspect is a cured product of a curable resin composition containing reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Is included.
- the configuration of the hard coat layer will be described.
- Curable resin composition used in the present invention contains reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Is. Hereinafter, each component in the curable resin composition will be described.
- the reactive deformed silica fine particle is a component that contributes to improving the hardness of the hard coat layer.
- the reactive irregular shaped silica fine particles usually have a reactive functional group.
- a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group.
- Specific examples include ethylenically unsaturated bonds such as (meth) acryloyl groups, vinyl groups, allyl groups, and epoxy groups.
- (meth) acryloyl means at least one of acryloyl and methacryloyl
- (meth) acrylate means at least one of acrylate and methacrylate
- (meth) acryl is at least one of acrylic and methacrylic. Means.
- Examples of reactive irregularly shaped silica fine particles include reactive irregularly shaped silica fine particles in which a plurality of fine silica particles are bonded by an inorganic chemical bond.
- the reactive irregularly shaped silica fine particles are reactive irregularly shaped silica fine particles having 3 to 20 silica fine particles having an average primary particle diameter of 1 nm to 100 nm bonded by an inorganic chemical bond and having a reactive functional group on the surface.
- the reactive irregularly shaped silica fine particles have a reactive functional group, so that they can undergo a curing reaction that crosslinks with the reactive irregularly shaped silica fine particles and the monomer, and can impart scratch resistance and hardness to the hard coat layer. .
- the average primary particle diameter of the silica fine particles constituting the reactive irregularly shaped silica fine particles is preferably in the range of 1 nm to 100 nm, and more preferably in the range of 5 nm to 80 nm. If the average primary particle size of the silica fine particles is small, only reactive irregularly shaped silica fine particles having a small average secondary particle size can be obtained, and sufficient hardness may not be imparted to the hard coat layer.
- the average primary particle size of the silica fine particles is large, the average secondary particle size of the reactive irregularly shaped silica fine particles tends to be large, and if the average secondary particle size is large, the transparency of the hard coat layer is lowered and the transmittance Deterioration and haze increase may occur.
- the average secondary particle size of the reactive irregularly shaped silica fine particles is preferably in the range of 5 nm to 300 nm, and more preferably in the range of 10 nm to 200 nm. When the average two particle diameters of the reactive irregularly shaped silica fine particles are within the above range, it is easy to impart hardness to the hard coat layer and maintain the transparency of the hard coat layer.
- the average primary particle size of the silica fine particles is determined by measuring the silica fine particles in the curable resin composition by a dynamic light scattering method, and the 50% particle size (d50 median) when the particle size distribution is expressed as a cumulative distribution. Diameter).
- the average primary particle size can be measured using a Microtrac particle size analyzer or a Nanotrac particle size analyzer manufactured by Nikkiso Co., Ltd.
- the average secondary particle size of the reactive irregularly shaped silica fine particles can be determined by the same method as the average primary particle size in the curable resin composition.
- the average secondary particle size of the reactive irregularly shaped silica fine particles is such that, in the hard coat layer, the cross section of the hard coat layer is observed using an SEM photograph or a TEM photograph, and the observed cured reactive irregularly shaped silica fine particles are 100. It can be obtained as an average value of individual selection.
- Silica fine particles do not exclude the use of particles having pores or porous structures inside the particles, such as hollow particles, but solid particles having no pores or porous structures inside the particles should be used. It is more preferable from the viewpoint of improving the hardness.
- the reactive irregularly shaped silica fine particles are formed by bonding the above-mentioned silica fine particles, preferably 3 to 20, more preferably 3 to 10, by inorganic chemical bonds.
- the number of silica fine particles bonded by inorganic chemical bonds is small, it is substantially the same as monodisperse particles, and it is difficult to obtain a hard coat layer excellent in adhesion to the substrate, scratch resistance, and pencil hardness.
- bonded by the inorganic chemical bond the transparency of a hard-coat layer will fall, and the deterioration of the transmittance
- inorganic chemical bonds include ionic bonds, metal bonds, coordination bonds, and covalent bonds.
- a bond in which the bonded silica fine particles are not dispersed even when the reactive deformed silica fine particles are added to the polar solvent specifically, a metal bond, a coordination bond, and a covalent bond is preferable, and a covalent bond is particularly preferable.
- the polar solvent include water and lower alcohols such as methanol, ethanol and isopropyl alcohol.
- silica fine particles As the particle state of the reactive irregularly shaped silica fine particles, 3 to 20 silica fine particles are bonded by an inorganic chemical bond and aggregated particles (aggregated particles), and 3 to 20 silica fine particles are inorganic. Examples thereof include chain particles bonded by chemical bonds and bonded in a chain. Among these, from the viewpoint of increasing the hardness of the hard coat layer, chain particles are preferable as the particle state of the reactive irregularly shaped silica fine particles. Further, it is preferable that the chain particles are contained in at least a part of the reactive irregular shaped silica fine particles.
- the average number of bonds of the silica fine particles is determined by observing the cross section of the hard coat layer using an SEM photograph or a TEM photograph and observing the cured reactive irregularly shaped silica fine particles. 100 are selected, the silica fine particles contained in each reactive deformed silica fine particle are counted, and the average value can be obtained.
- the method for producing reactive irregularly shaped silica fine particles is not particularly limited as long as the above-mentioned silica fine particles are bonded by an inorganic bond, and a conventionally known method can be appropriately selected and used.
- a conventionally known method can be appropriately selected and used.
- it can be obtained by adjusting the concentration or pH of the monodispersed silica fine particle dispersion and performing hydrothermal treatment at a high temperature of 100 ° C. or higher.
- a binder component can be added to promote the binding of the silica fine particles.
- ions may be removed by passing the silica fine particle dispersion used through an ion exchange resin. Such ion exchange treatment can promote the binding of silica fine particles. After the hydrothermal treatment, the ion exchange treatment may be performed again.
- the reactive irregular shaped silica fine particles have at least a part of the surface coated with an organic component, and have a reactive functional group introduced by the organic component on the surface.
- the organic component is a component containing carbon.
- a compound containing an organic component such as a silane coupling agent reacts with a hydroxyl group present on the surface of the silica fine particles to cause a part of the surface
- silica Examples include a mode in which an organic component is attached to a hydroxyl group present on the surface of the fine particle by an interaction such as hydrogen bonding, a mode in which silica fine particles are contained in the polymer particle, and the
- At least a part of the surface is coated with an organic component, and a method for preparing reactive deformed silica fine particles having a reactive functional group introduced by the organic component on the surface is a reactive functional group to be introduced into the reactive deformed silica fine particles.
- a conventionally known method can be appropriately selected and used.
- any one of the following reactive deformed silica fine particles (i) and (ii) may be appropriately selected and used. preferable.
- Reactive deformed silica fine particles having a reactive functional group on the surface obtained by (Ii) a compound containing a reactive functional group to be introduced into the deformed silica fine particle before coating, a group represented by the following chemical formula (1), and a silanol group or a group that generates a silanol group by hydrolysis, and metal oxide fine particles: Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by bonding.
- Reactive deformed silica fine particles having a reactive functional group on the surface obtained by When the reactive irregularly shaped silica fine particles (i) are used, there is an advantage that the strength of the hard coat layer can be improved even if the organic component content is small.
- the surface-modifying compound used for the reactive deformed silica fine particles (i) is a carboxyl group, acid anhydride group, acid chloride group, acid amide group, ester group, imino group, nitrile group, isonitrile group, hydroxyl group, thiol.
- the chemical bond here preferably includes a covalent bond, an ionic bond or a coordination bond, but also includes a hydrogen bond.
- the coordination bond is considered to be complex formation.
- an acid-base reaction, complex formation or esterification according to Bronsted or Lewis occurs between the functional group of the surface modifying compound and the group on the surface of the deformed silica fine particle.
- the surface modification compound used for the reactive irregular shaped silica fine particles (i) can be used alone or in combination of two or more.
- the surface-modifying compound is usually added to at least one functional group capable of participating in chemical bonding with the surface group of the irregular-shaped silica fine particle, and after binding to the surface-modifying compound via this functional group, the irregular-shaped silica fine particle is newly added. It has molecular residues that give unique properties.
- at least one functional group that can participate in chemical bonding with a group on the surface of the irregular shaped silica fine particle is referred to as a first functional group.
- the molecular residue or a part thereof is hydrophobic or hydrophilic and, for example, stabilizes, integrates, or activates irregular shaped silica fine particles.
- the hydrophobic molecular residue includes an alkyl, aryl, alkaryl, aralkyl, or fluorine-containing alkyl group that causes inactivation or repulsion.
- the hydrophilic group include a hydroxy group, an alkoxy group, and a polyester group.
- the reactive functional group introduced to the surface so that the reactive irregular shaped silica fine particles can react with the monomer described later is appropriately selected according to the monomer.
- a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group.
- Specific examples thereof include ethylenically unsaturated bonds such as (meth) acryloyl group, vinyl group and allyl group, and epoxy group.
- the first functional group contained in the surface modifying compound is reacted with the surface of the deformed silica fine particle.
- a surface modification compound further having a polymerizable unsaturated group is preferable.
- a second reactive functional group is contained, and the second reactive functional group is used as a foothold for the reactive deformed silica fine particles of (i).
- a reactive functional group capable of reacting with the monomer may be introduced on the surface.
- a hydrogen bond-forming group capable of hydrogen bonding such as a hydroxyl group and an oxy group is introduced as the second reactive functional group, and another surface modification compound is added to the hydrogen bond-forming group introduced on the surface of the fine particles. It is preferable to introduce a reactive functional group capable of reacting with the monomer by the reaction of the hydrogen bond forming group.
- a compound having a hydrogen bond forming group and a compound having a reactive functional group capable of reacting with a monomer such as a polymerizable unsaturated group and a compound having a hydrogen bond forming group in combination As mentioned.
- Specific examples of the hydrogen bond-forming group indicate a functional group such as a hydroxyl group, a carboxyl group, an epoxy group, a glycidyl group, an amide group, or an amide bond.
- the term “amide bond” means that the bond unit contains —NHC (O) or> NC (O) —.
- a carboxyl group, a hydroxyl group, and an amide group are particularly preferable.
- the surface-modifying compound used in the reactive irregularly shaped silica fine particles (i) above preferably has a molecular weight of 500 or less, particularly a molecular weight not exceeding 400, and particularly a molecular weight not exceeding 200. Since it has such a low molecular weight, it is presumed that the surface of the silica fine particles can be rapidly occupied and aggregation of the reactive irregular shaped silica fine particles can be prevented.
- the surface-modifying compound used in the reactive deformed silica fine particles (i) is preferably a liquid under the reaction conditions for surface modification, and is preferably soluble or at least emulsifiable in a dispersion medium.
- the polymer is dissolved in the dispersion medium and uniformly distributed as discrete molecules or molecular ions in the dispersion medium.
- Saturated or unsaturated carboxylic acids have 1 to 24 carbon atoms, such as formic acid, acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, acrylic acid, methacrylic acid, crotonic acid, citric acid, Examples include adipic acid, succinic acid, glutaric acid, oxalic acid, maleic acid, fumaric acid, itaconic acid and stearic acid, and the corresponding acid anhydrides, chlorides, esters and amides such as caprolactam. Further, when an unsaturated carboxylic acid is used, a polymerizable unsaturated group can be introduced.
- n 0, 1 or 2.
- Residue Q is independently alkyl having 1 to 12, especially 1 to 6, particularly preferably 1 to 4, carbon atoms such as methyl, ethyl, n-propyl, i-propyl and butyl, and 6 to 24 Represents aryl, alkaryl or aralkyl having 5 carbon atoms such as phenyl, naphthyl, tolyl and benzyl.
- Examples of preferred amines include polyalkyleneamines, and specific examples are methylamine, dimethylamine, trimethylamine, ethylamine, aniline, N-methylaniline, diphenylamine, triphenylamine, toluidine, ethylenediamine, and diethylenetriamine. .
- Preferred ⁇ -dicarbonyl compounds are those having 4 to 12, particularly 5 to 8 carbon atoms, such as diketones such as acetylacetone, 2,3-hexanedione, 3,5-heptanedione, acetoacetate, acetoacetate Acetoacetic acid-C 1 -C 4 -alkyl esters such as ethyl esters, diacetyl and acetonyl acetone.
- Examples of amino acids include ⁇ -alanine, glycine, valine, aminocaproic acid, leucine and isoleucine.
- Preferred silanes are hydrolyzable organosilanes having at least one hydrolyzable group or hydroxy group and at least one non-hydrolyzable residue.
- examples of the hydrolyzable group include a halogen, an alkoxy group, and an acyloxy group.
- the non-hydrolyzable residue a non-hydrolyzable residue having a reactive functional group or not having a reactive functional group is used.
- Silanes having at least partially organic residues substituted with fluorine may also be used.
- the silane coupling agent is not particularly limited, and may include known ones such as KBM-502, KBM-503, KBE-502, KBE-503 manufactured by Shin-Etsu Chemical Co., Ltd. be able to.
- Examples of the metal compound having a functional group include a metal compound of metal M from at least one of the first group III to V and the second group II to IV of the periodic table. Specific examples include zirconium and titanium alkoxides represented by the following chemical formula. M (OR) 4 In the above formula, M is Ti or Zr. A part of the OR group is substituted with a complexing agent such as a ⁇ -dicarbonyl compound or a monocarboxylic acid. When a compound having a polymerizable unsaturated group such as methacrylic acid is used as a complexing agent, a polymerizable unsaturated group can be introduced.
- the dispersion medium at least one of water and an organic solvent is preferably used.
- a particularly preferred dispersion medium is distilled pure water.
- organic solvent polar, nonpolar and aprotic solvents are preferred. Examples thereof include alcohols such as C 1-6 aliphatic alcohols such as methanol, ethanol, n- and i-propanol and butanol, ketones such as methyl ethyl ketone, diethyl ketone, methyl isobutyl ketone, acetone and butanone, acetic acid Esters such as ethyl; ethers such as diethyl ether, tetrahydrofuran and tetrahydropyran; amides such as dimethylacetamide and dimethylformamide; sulfoxides and sulfones such as sulfolane and dimethyl sulfoxide; and optionally, such as pentane, hexane and cyclohexane Aliphatic hydro
- the dispersion medium preferably has a boiling point that can be easily removed by distillation, optionally under reduced pressure, and a solvent having a boiling point of 200 ° C. or lower, particularly 150 ° C. or lower is preferable.
- the concentration of the dispersion medium is usually within the range of 40% by mass to 90% by mass, preferably within the range of 50% by mass to 80% by mass, and particularly 55% by mass to 75%. It is in the range of mass%.
- the rest of the dispersion is composed of untreated silica fine particles and the surface modifying compound.
- the weight ratio of silica fine particles: surface modification compound is preferably 100: 1 to 4: 1, more preferably 50: 1 to 8: 1, and particularly preferably 25: 1 to 10: 1.
- Preparation of the reactive deformed silica fine particles (i) is preferably carried out in a temperature range from room temperature of about 20 ° C. to the boiling point of the dispersion medium.
- the dispersion temperature is 50 ° C. to 100 ° C.
- the dispersion time depends in particular on the type of material used, but is generally from a few minutes to a few hours, for example 1 to 24 hours.
- the compound may be referred to as a reactive functional group-modified hydrolyzable silane.
- the reactive functional group-modified hydrolyzable silane the reactive functional group to be introduced into the silica fine particles is not particularly limited as long as it is appropriately selected so as to be capable of reacting with a monomer described later. Suitable for introducing polymerizable unsaturated groups as described above.
- These groups can be used alone or in combination of two or more.
- At least one of a [—O—C ( ⁇ O) —] group, a [—O—C ( ⁇ S) —] group, and a [—S—C ( ⁇ O) —] group It is preferable to use one type in combination.
- Examples of the group that generates a silanol group by hydrolysis include groups having an alkoxy group, an aryloxy group, an acetoxy group, an amino group, a halogen atom, etc. on the silicon atom.
- An oxysilyl group is preferred.
- a silanol group or a group that generates a silanol group by hydrolysis can be bonded to the metal oxide fine particles by a condensation reaction or a condensation reaction that occurs following hydrolysis.
- the reactive functional group-modified hydrolyzable silane include, for example, compounds represented by the following chemical formulas (2) and (3), and the compound represented by the following chemical formula (3) is from the point of hardness. More preferably used.
- R a and R b may be the same or different, but are a hydrogen atom or a C 1 to C 8 alkyl group or aryl group, for example, methyl, ethyl, propyl , Butyl, octyl, phenyl, xylyl group and the like.
- m is 1, 2 or 3.
- Examples of the group represented by [(R a O) m R b 3-m Si—] include a trimethoxysilyl group, a triethoxysilyl group, a triphenoxysilyl group, a methyldimethoxysilyl group, a dimethylmethoxysilyl group, and the like. Can be mentioned. Of these groups, a trimethoxysilyl group or a triethoxysilyl group is preferable.
- R c is a divalent organic group having a C 1 to C 12 aliphatic or aromatic structure, and may contain a chain, branched or cyclic structure.
- examples of such an organic group include methylene, ethylene, propylene, butylene, hexamethylene, cyclohexylene, phenylene, xylylene, and dodecamethylene.
- preferred examples are methylene, propylene, cyclohexylene, phenylene and the like.
- R d is a divalent organic group and is usually selected from divalent organic groups having a molecular weight of 14 to 10,000, preferably a molecular weight of 76 to 500.
- chain polyalkylene groups such as hexamethylene, octamethylene, dodecamethylene; alicyclic or polycyclic divalent organic groups such as cyclohexylene and norbornylene; divalent groups such as phenylene, naphthylene, biphenylene and polyphenylene An aromatic group; and these alkyl group-substituted and aryl group-substituted products.
- these divalent organic groups may contain an atomic group containing an element other than carbon and hydrogen atoms, and include a polyether bond, a polyester bond, a polyamide bond, a polycarbonate bond, and a group represented by the above formula (1). Can also be included.
- R e is an (n + 1) valent organic group, preferably selected from a chain, branched or cyclic saturated hydrocarbon group and unsaturated hydrocarbon group.
- Y ′ represents a monovalent organic group having a reactive functional group.
- the reactive functional group itself as described above may be used.
- the reactive functional group is selected from a polymerizable unsaturated group, (meth) acryloyl (oxy) group, vinyl (oxy) group, propenyl (oxy) group, butadienyl (oxy) group, styryl (oxy) group, Examples include ethynyl (oxy) group, cinnamoyl (oxy) group, maleate group, (meth) acrylamide group and the like.
- N is preferably a positive integer of 1 to 20, more preferably 1 to 10, and particularly preferably 1 to 5.
- the method described in JP-A-9-100111 can be used. That is, for example, when it is desired to introduce a polymerizable unsaturated group, (A) an addition reaction between a mercaptoalkoxysilane, a polyisocyanate compound, and an active hydrogen group-containing polymerizable unsaturated compound capable of reacting with an isocyanate group is performed. it can. Moreover, it can carry out by (B) direct reaction with the compound which has an alkoxy silyl group and an isocyanate group in a molecule
- the reactive deformed silica fine particles of (ii) after separately hydrolyzing the reactive functional group-modified hydrolyzable silane, this is mixed with the deformed silica fine particles, followed by heating and stirring operation, Alternatively, a method of hydrolyzing a reactive functional group-modified hydrolyzable silane in the presence of deformed silica fine particles, and other components such as polyunsaturated organic compounds, monounsaturated organic compounds, radiation polymerization initiators, etc.
- the method of performing the surface treatment of the deformed silica fine particles in the presence of can be selected, a method of hydrolyzing the reactive functional group-modified hydrolyzable silane in the presence of the deformed silica fine particles is preferable.
- the temperature is usually 20 ° C. or higher and 150 ° C. or lower, and the treatment time is in the range of 5 minutes to 24 hours.
- an acid, salt or base may be added as a catalyst.
- the acid include organic acids and unsaturated organic acids
- examples of the base include tertiary amines or quaternary ammonium hydroxides.
- the addition amount of these acid or base catalysts is within the range of 0.001% by mass to 1.0% by mass, preferably 0.01% by mass to 0.1% by mass with respect to the reactive functional group-modified hydrolyzable silane. Within range.
- powdery fine particles not containing a dispersion medium may be used.
- the hard coat layer may include not only those in which the reactive functional group of the reactive irregular shaped silica fine particles has reacted but also those in which the reactive functional group of the reactive irregular shaped silica fine particles has not reacted.
- the content of the reactive irregularly shaped silica fine particles is preferably in the range of 40% by mass to 70% by mass, and in the range of 50% by mass to 60% by mass with respect to the total solid content of the curable resin composition. It is more preferable.
- the content is small, there is a possibility that sufficient hardness cannot be imparted to the hard coat layer.
- the content is large, the filling rate is excessively increased, the adhesion between the reactive irregularly shaped silica fine particles and the monomer is deteriorated, and the hardness of the hard coat layer may be lowered.
- solid content means things other than a solvent among the components contained in curable resin composition.
- the acrylic polymer used in the present invention has a weight average molecular weight and an acrylic equivalent within predetermined ranges, and is a component that contributes to improving the workability of the hard coat film.
- the weight average molecular weight of the acrylic polymer is in the range of 30,000 to 110,000, particularly in the range of 50,000 to 110,000, from the viewpoint of imparting flexibility to the hard coat layer and preventing cracks during processing. It is preferably within the range of 60,000 to 80,000.
- the weight average molecular weight refers to a weight average molecular weight which is a polystyrene conversion value measured by gel permeation chromatography.
- the acrylic polymer has an acrylic equivalent in the range of 200 to 1,200, and preferably in the range of 200 to 1,000.
- the acrylic equivalent indicates a value obtained by dividing the weight average molecular weight of the acrylic polymer by the number of (meth) acrylic groups in one molecule.
- the acrylic polymer is not particularly limited as long as it satisfies the above weight average molecular weight and acrylic equivalent, but it is a polymer of glycerol (meth) acrylate or a compound of a compound obtained by addition polymerization of (meth) acrylic acid to glycidyl methacrylate. It is preferably a coalescence. Specifically, a polymer of an acrylic monomer represented by the following chemical formula (4) or (5) is preferably used.
- R 1 to R 3 are each independently an acrylate group, a methacrylate group or a hydrogen atom, and one or more of R 1 to R 3 are an acrylate group or a methacrylate group. That is, the glycerol (meth) acrylate represented by the above formula (4) may be monofunctional, bifunctional, or trifunctional.
- R is an acrylic acid group or a methacrylic acid group.
- acrylic polymer is BL-2002 manufactured by Seiko PMC Co., Ltd.
- acrylic polymer you may use individually by 1 type, and may mix and use 2 or more types suitably.
- the content of the acrylic polymer is preferably in the range of 3% by mass to 20% by mass, more preferably in the range of 5% by mass to 10% by mass, based on the total solid content of the curable resin composition. More preferably, it is in the range of 6% by mass to 8% by mass. If the content of the acrylic polymer is within the above range, the workability of the hard coat film can be improved while maintaining the hardness of the hard coat layer.
- the content of the acrylic polymer can be set in the range of 5 to 80 parts by weight with respect to 100 parts by weight of the monomer described later, and is in the range of 20 to 40 parts by weight. Preferably, it is in the range of 10 to 30 parts by weight.
- a monomer is a component used as the matrix resin of a hard-coat layer.
- the monomer usually has a reactive functional group.
- Monomers crosslink between monomers when cured.
- the reactive functional group of the monomer has cross-linking reactivity with the reactive functional group of the reactive irregularly shaped silica fine particles, the monomer is crosslinked with the reactive irregularly shaped silica fine particles, forming a network structure, and the hardness of the hard coat layer And further improve the scratch resistance.
- a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group.
- the reactive functional group of the monomer may be the same as or different from the reactive functional group of the reactive deformed silica fine particle.
- a curable organic resin is preferable, and a translucent material that transmits light when it is used as a coating film is preferable.
- An ionizing radiation curable resin that is a resin that is cured by ionizing radiation represented by ultraviolet rays or electron beams, Other known curable resins and the like may be appropriately employed according to required performance.
- the ionizing radiation curable resin include acrylate-based, oxetane-based, and silicone-based resins.
- the monomer one type or two or more types of monomers can be used.
- the monomer preferably has three or more reactive functional groups from the viewpoint of increasing the crosslinking density.
- the polyfunctional monomer having three or more reactive functional groups include pentaerythritol tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexa (meth) acrylate, and dipentaerythritol penta (meth) acrylate. , Trimethylolpropane tri (meth) acrylate, trimethylolpropane hexa (meth) acrylate, and modified products thereof.
- pentaerythritol triacrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexaacrylate, pentaerythritol tetraacrylate, and dipentaerythritol pentaacrylate are preferably used, and dipentaerythritol hexaacrylate, penta Erythritol tetraacrylate and dipentaerythritol pentaacrylate are particularly preferably used.
- the hard coat layer may contain not only a monomer that is crosslinked but also a monomer that is not crosslinked.
- the monomer content is preferably in the range of 25% by mass to 44% by mass and more preferably in the range of 30% by mass to 40% by mass with respect to the total solid content of the curable resin composition. .
- the content is small, there is a possibility that sufficient hardness cannot be imparted to the hard coat layer.
- the content is large, the hardness of the hard coat layer is excessively increased, and the content of the acrylic polymer is relatively decreased, which may deteriorate the workability of the hard coat film.
- the curable resin composition may contain a polymerization initiator.
- a polymerization initiator is used when the curable resin composition is cured by ultraviolet irradiation or heating, but when it is cured by electron beam irradiation, a polymerization initiator is unnecessary.
- the polymerization initiator is decomposed by at least one of light and heat to generate radicals or cations to advance radical polymerization and cationic polymerization.
- radical polymerization initiators, cationic polymerization initiators, radicals and cationic polymerization initiators can be appropriately selected and used.
- the radical polymerization initiator only needs to be capable of releasing a substance that initiates radical polymerization by at least one of light and heat.
- photo radical polymerization initiators include imidazole derivatives, bisimidazole derivatives, N-aryl glycine derivatives, organic azide compounds, titanocenes, aluminate complexes, organic peroxides, N-alkoxypyridinium salts, thioxanthone derivatives, and the like. It is done. Specific examples include those described in JP 2010-102123 A and JP 2010-120182 A.
- the cationic polymerization initiator should just be able to discharge
- the cationic polymerization initiator include sulfonic acid ester, imide sulfonate, dialkyl-4-hydroxysulfonium salt, arylsulfonic acid-p-nitrobenzyl ester, silanol-aluminum complex, ( ⁇ 6 -benzene) ( ⁇ 5 -cyclopentadidiene).
- radical polymerization initiators and cationic polymerization initiators examples include aromatic iodonium salts, aromatic sulfonium salts, aromatic diazonium salts, aromatic phosphonium salts, triazine compounds, and iron arene complexes. Specific examples include those described in JP 2010-102123 A and JP 2010-120182 A.
- the polymerization initiator preferably has a relatively low absorption rate in the visible light region. This is because if the absorption rate in the visible light region is high, the light transmittance of the hard coat film may be lowered.
- the content of the polymerization initiator is preferably in the range of 2% by mass to 5% by mass with respect to the total solid content of the curable resin composition, and is preferably in the range of 2% by mass to 2.5% by mass. It is more preferable. If the content is small, the polymerization reaction of monomers or the like does not proceed sufficiently, and there is a possibility that sufficient hardness cannot be imparted to the hard coat layer. Moreover, when there is much content, polymerization reaction, such as a monomer, advances rapidly, and there exists a possibility that workability
- the curable resin composition used in the present invention may further contain a surfactant.
- the surfactant is a component that imparts coating stability, slipperiness, antifouling properties, and scratch resistance.
- examples of the surfactant include a fluorine-based surfactant, a silicon-based surfactant, and a fluorine-silicon-based surfactant. Of these, a fluorosilicone surfactant is preferably used because of its good slipperiness.
- surfactant a commercially available one can be used, and for example, leveling agents described in JP 2010-102123 A and JP 2010-120182 A can be used.
- fluorosilicone surfactant examples include a compound having a perfluoroalkyl group and a siloxane bond. Specifically, a compound having a perfluoroalkyl group and a copolymer of siloxane and polyether. Is mentioned.
- the perfluoroalkyl group has, for example, 4 to 10 carbon atoms.
- the perfluoroalkyl group may be linear or branched.
- the polyether group include a polyethylene oxide chain, a polypropylene oxide chain, and a copolymer thereof.
- fluorine silicon surfactants include compounds represented by the following chemical formula (6).
- R is a perfluoroalkyl group having 4 to 10 carbon atoms
- Q is a polyethylene oxide chain or a polypropylene oxide chain
- k and m are each 0 or 1
- n is 1, 2 or 3 It is.
- the fluorosilicone surfactant may have a reactive functional group.
- a reactive functional group for example, a polymerizable unsaturated group is used, specifically a photocurable unsaturated group, and more specifically an ionizing radiation curable unsaturated group.
- Specific examples of the reactive functional group include a (meth) acryloyl group.
- fluorosilicone surfactant examples include X-71-1203M, X-70-090, X-70-091, X-70-092, X-70-093, manufactured by Shin-Etsu Chemical Co., Ltd., DIC Corporation. Examples thereof include Megafac R-08, XRB-4, and the like.
- the hard coat layer has good surface wettability so that the antiglare layer can be formed on the hard coat layer. It is preferable to use a surfactant capable of obtaining Such a surfactant can be appropriately selected from the above and used.
- the content of the surfactant is preferably 0.2% by mass or less, and preferably in the range of 0.08% by mass to 0.1% by mass with respect to the total solid content of the curable resin composition. More preferred.
- the curable resin composition used in the present invention may further contain a blue color material. This is because when the hard coat film of the present invention is used in an image display device, yellowness can be suppressed and visibility and color reproducibility can be improved.
- a blue color material general pigments and dyes can be used. Specific examples include phthalocyanine pigments and indanthrene blue pigments.
- the content of the blue color material may be an amount such that the transmittance of the hard coat layer described later is 85% or more, and preferably 90% or more, and is appropriately adjusted. For example, the content of the blue color material is preferably 0.05% by mass or less with respect to the total solid content of the curable resin composition.
- the curable resin composition used in the present invention may further contain urethane acrylate. This is because by adding urethane acrylate, flexibility can be imparted to the hard coat layer and the occurrence of warpage can be suppressed.
- urethane acrylate flexibility can be imparted to the hard coat layer and the occurrence of warpage can be suppressed.
- a curable resin composition to which urethane acrylate is added is used. By forming the hard coat layer 3 and the second hard coat layer 4, it is possible to further suppress the occurrence of warping and improve the impact resistance.
- urethane acrylate for example, a general urethane acrylate used for a hard coat layer can be used. Specific examples include those described in JP 2011-31527 A, JP 2009-84328 A, and International Publication No. 2012/8444.
- the content of urethane acrylate may be an amount such that the pencil hardness of the hard coat layer, which will be described later, falls within a predetermined range, and is appropriately adjusted.
- the content of urethane acrylate can be set within the range of 4 to 100 parts by weight with respect to 100 parts by weight of the monomer.
- the curable resin composition used in the present invention usually contains a solvent.
- the solvent is not particularly limited, but a non-permeable solvent is preferable from the viewpoint of increasing the hardness of the hard coat film.
- permeation refers to dissolving or swelling the substrate.
- Specific examples of the non-permeable solvent include methyl isobutyl ketone, propylene glycol monomethyl ether, normal propanol, isopropanol, normal butanol, sec-butanol, isobutanol, and tert-butanol.
- the curable resin composition used for this invention may contain the antistatic agent, the glare-proof agent, various sensitizers, etc. as needed.
- Curable resin composition The curable resin composition is prepared by mixing a dispersion of reactive irregularly shaped silica fine particles, an acrylic polymer, a monomer, a polymerization initiator and the like in a solvent according to a general preparation method, and dispersing the mixture. Can do. For mixing and dispersing, a paint shaker or a bead mill can be used.
- the deformed silica fine particles contained in the hard coat layer in the present invention are obtained by crosslinking the reactive deformed silica fine particles contained in the curable resin composition, or the reactive deformed silica fine particles It is formed by crosslinking with monomers. That is, the hard coat layer contains cross-linked irregular-shaped silica fine particles or irregular-shaped silica fine particles crosslinked with the matrix resin.
- the hard coat layer includes not only the reactive functional group of the reactive irregular shaped silica fine particles reacted but also the reactive functional group of the reactive irregular shaped silica fine particles not reacting as the irregular shaped silica fine particles. It may be.
- the content of the irregular shaped silica fine particles in the hard coat layer can be the same as the content of the reactive irregular shaped silica fine particles in the total solid content of the curable resin composition.
- Acrylic polymer The acrylic polymer contained in the hard coat layer in the present invention has a weight average molecular weight and an acrylic equivalent within predetermined ranges.
- description here is abbreviate
- the content of the acrylic polymer in the hard coat layer can be the same as the content of the acrylic polymer in the total solid content of the curable resin composition.
- the matrix resin contained in the hard coat layer in the present invention is a resin forming a composite with irregular-shaped silica fine particles and an acrylic polymer. It is considered that the hardness and scratch resistance of the hard coat layer are increased by forming a three-dimensional network structure with the matrix resin, the deformed silica fine particles, and the acrylic polymer.
- Such a matrix resin examples include acrylic resin, silicone resin, and polyether.
- acrylic resin is preferable. This is because the properties of the hard coat layer are improved because of its high affinity with the acrylic polymer.
- the matrix resin is preferably formed by crosslinking of monomers contained in the curable resin composition, or by crosslinking of the monomer with reactive deformed silica fine particles. That is, the hard coat layer preferably contains a matrix resin having a cross-linked bond or a matrix resin cross-linked with irregular shaped silica fine particles.
- the matrix resin is preferably a polymer having a structural unit derived from a monomer.
- matrix resins are polymers having structural units derived from hydroxy group-containing monomers such as pentaerythritol, dipentaerythritol, and trimethylolpropane.
- the structural unit is preferably a structural unit derived from a monomer of pentaerythritol.
- structures such as pentaerythritol trimethyl ether and pentaerythritol tetramethyl ether, dipentaerythritol hexamethyl ether, and dipentaerythritol pentamethyl ether can be given as the similar skeleton.
- the content of the matrix resin in the hard coat layer can be the same as the content of the monomer in the total solid content of the curable resin composition.
- the hard coat layer in the present invention may contain a polymerization initiator.
- a polymerization initiator since it is the same as that of the polymerization initiator contained in the said curable resin composition, description here is abbreviate
- the content of the polymerization initiator in the hard coat layer can be the same as the content of the polymerization initiator in the total solid content of the curable resin composition.
- the hard coat layer in the present invention may contain a surfactant.
- surfactant since it is the same as that of surfactant contained in the said curable resin composition, description here is abbreviate
- the content of the surfactant in the hard coat layer can be the same as the content of the surfactant in the total solid content of the curable resin composition.
- Blue color material The hard coat layer in the present invention may contain a blue color material.
- a blue color material since it is the same as that of the blue color material contained in the said curable resin composition, description here is abbreviate
- the content of the blue color material in the hard coat layer can be the same as the content of the blue color material in the total solid content of the curable resin composition.
- the hard coat layer in the invention may contain urethane acrylate.
- urethane acrylate since it is the same as that of the urethane acrylate contained in the said curable resin composition about urethane acrylate, description here is abbreviate
- the urethane acrylate content in the hard coat layer can be the same as the urethane acrylate content in the curable resin composition.
- the hardness of the hard coat layer can be evaluated by a pencil hardness test (4.9 N load) specified by JIS K5600-5-4 (1999).
- the pencil hardness of the hard coat layer is preferably 6H or more, more preferably 7H or more, and particularly preferably 9H or more.
- the hard coat layer is light transmissive.
- the transmittance of the hard coat layer in the visible light region is preferably 80% or more, and more preferably 90% or more. This is because when the transmittance is in the above range, a hard coat layer having excellent light transmittance can be formed.
- the transmittance of the hard coat layer is the total light transmittance measured by the method defined in JIS K 7105.
- the haze value of the hard coat layer is appropriately determined according to the type of reactive irregularly shaped silica fine particles and is not particularly limited. For example, it is 1.0 or less, particularly 0.8 or less, particularly 0. .5 or less is preferable. It is because it can be set as the hard-coat layer with favorable light transmittance because haze value is the said range.
- the haze value can be measured by a method according to JIS-K-7136. For example, it can be measured with a direct reading haze meter manufactured by Toyo Seiki Seisakusho using a semi-integrating sphere.
- the haze value can also be measured by a method according to JIS K-7105, for example, a haze meter HM150 manufactured by Murakami Color Research Laboratory.
- the hard coat layer preferably has antifouling properties.
- the antifouling property can be evaluated by wettability.
- the wettability of the hard coat layer surface is appropriately determined according to the components used in the curable resin composition, and is not particularly limited.
- the contact angle of water droplets on the hard coat layer surface is preferably 90 ° or more, more preferably 100 ° or more, and even more preferably 110 ° or more. This is because if the wettability is as described above, the hard coat layer can exhibit good antifouling properties.
- the contact angle of the water droplet is usually 120 ° or less. The contact angle of the water droplet is obtained by measuring the contact angle with water 30 seconds after dropping the water droplet from the microsyringe using a contact angle measuring device CA-Z type manufactured by Kyowa Interface Science Co., Ltd. be able to.
- a hard coat layer has slipperiness.
- the slipperiness can be evaluated by a dynamic friction coefficient. The smaller the dynamic friction coefficient, the better the slipperiness.
- the coefficient of dynamic friction on the surface of the hard coat layer is, for example, 0.300 or less, preferably 0.200 or less, and more preferably 0.100 or less. This is because if the dynamic friction coefficient is too large, it may be difficult to perform a good touch operation on the surface of the hard coat layer.
- the dynamic friction coefficient can be measured by a method in accordance with JIS K7125. For example, a dynamic friction tester HEIDON Type HHS2000 manufactured by Shinto Kagaku Co., Ltd. is used, a stainless hard ball having a diameter of 10 mm, a load of 200 g, and a speed. The dynamic friction coefficient can be measured at 5 mm / sec.
- the thickness of the hard coat layer is not particularly limited as long as the desired hardness and workability can be exhibited.
- the thickness can be about 5 ⁇ m to 40 ⁇ m, and particularly within the range of 10 ⁇ m to 30 ⁇ m. In particular, it is preferably in the range of 18 ⁇ m to 22 ⁇ m. This is because if the hard coat layer is thin, sufficient hardness cannot be exhibited, and if it is thick, cracks and warpage may occur.
- the method for forming the hard coat layer is not particularly limited as long as the hard coat layer can be formed using the curable resin composition, and the curable resin composition is applied onto the substrate, A method of curing the coating film can be used.
- the coating method of the curable resin composition is not particularly limited as long as the curable resin composition can be uniformly coated on the substrate, and is not limited to spin coating, dipping, spraying, slide coating.
- Various methods such as a method, a bar coat method, a roll coater method, a meniscus coater method, a flexographic printing method, a screen printing method, and a speed coater method can be used.
- substrate so that the hard-coat layer of a desired film thickness may be obtained.
- the method for drying the coating film include reduced-pressure drying, heat drying, and combinations thereof. When drying at normal pressure, it is preferable to dry in a temperature range in which the substrate does not deteriorate, for example, in the range of 30 ° C. to 110 ° C.
- At least one of light irradiation and heating can be used.
- light irradiation ultraviolet rays, visible light, electron beams, ionizing radiation, etc. are mainly used, and among these, ultraviolet rays are preferably used.
- ultraviolet curing ultraviolet rays emitted from light such as an ultra high pressure mercury lamp, a high pressure mercury lamp, a low pressure mercury lamp, a carbon arc, a xenon arc, a metal halide lamp are used.
- the amount of irradiation with the energy radiation source of accumulative exposure at an ultraviolet wavelength of 365 nm is preferably in the range of, for example, 50mJ / cm 2 ⁇ 5000mJ / cm 2.
- it is preferable to heat in a temperature range in which the substrate does not deteriorate for example, in the range of 40 ° C. to 120 ° C.
- substrate used in the present invention is not particularly limited as long as it has optical transparency and satisfies physical properties that can be used as a substrate for a hard coat film.
- the substrate used for the hard coat film may be transparent, translucent, colorless or colored, but is required to have light transmittance.
- Examples of the material for the substrate include acrylate polymers, polycarbonates, polyesters, cellulose acylates, cycloolefin polymers, and the like.
- Specific examples of the acrylate polymer include methyl poly (meth) acrylate, poly (meth) ethyl acrylate, methyl (meth) acrylate-butyl (meth) acrylate, and the like.
- Specific examples of the polycarbonate include aromatic polycarbonates based on bisphenols such as bisphenol A, and aliphatic polycarbonates such as diethylene glycol bisallyl carbonate.
- Specific examples of the polyester include polyethylene terephthalate and polyethylene naphthalate.
- cellulose acylate examples include cellulose triacetate, cellulose diacetate, and cellulose acetate butyrate.
- cycloolefin polymer examples include norbornene polymers, monocyclic olefin polymers, cyclic conjugated diene polymers, vinyl alicyclic hydrocarbon polymer resins, and the like.
- the substrate may be a single layer or a laminate of a plurality of layers.
- the substrate material is preferably an acrylate polymer, more preferably polymethyl methacrylate. This is because the transparency is high.
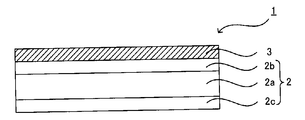
- the substrate in the case of a plurality of layers, has a plurality of resin layers.
- the number of resin layers may be two or more, preferably in the range of 3 to 5 layers, more preferably 3 layers.
- the outermost two layers are the outermost resin layers and the innermost resin layer is the innermost resin layer.
- the two outermost resin layers 2b and 2c are defined as the two outermost resin layers 2b and 2c
- the layer located inside 2c is referred to as an inner resin layer 2a.
- the inner resin layer 2a may be a single layer or a plurality of layers.
- the pencil hardness of the two outermost resin layers positioned on both sides of the substrate is higher than the pencil hardness of the inner resin layer.
- the difference in hardness between the outermost resin layer and the inner resin layer is preferably two or more steps, more preferably three or more steps, on the basis of pencil hardness.
- the pencil hardness of the outermost resin layer is preferably HB or higher, and more preferably H or higher and 5H or lower.
- the pencil hardness of the inner resin layer is preferably H or less, for example, and more preferably 3B or more and HB or less.
- the pencil hardness of the substrate is preferably 2H or more, and more preferably 3H or more. This is because the hardness of the hard coat film can be further improved.
- the pencil hardness of the substrate is preferably high, but is usually 4H or less.
- the pencil hardness of the substrate is preferably 2H or more.
- the inner resin layer 2a is polycarbonate
- the two outermost resin layers 2b and 2c are acrylate polymers. Is preferred. This is because the impact resistance is improved.
- the thickness of one outermost resin layer is preferably in the range of 60 ⁇ m to 110 ⁇ m.
- the substrate may contain a blue color material.
- a blue color material instead of adding a blue color material to the hard coat layer, a hard coat film capable of suppressing yellowishness and improving visibility and color reproducibility when used in an image display device is obtained by adding to a substrate. be able to.
- a blue color material it can be the same as that of what was described in the said hard-coat layer.
- the substrate transmits more light.
- the total light transmittance in the visible light region is preferably 80% or more, and more preferably 90% or more.
- the total light transmittance is a value measured by the method defined in JIS K 7105.
- the substrate may or may not have flexibility.
- the thickness of the substrate is not particularly limited, but in the case of a substrate that does not have flexibility, it is preferably 0.3 mm or more, more preferably in the range of 0.3 mm to 5 mm. . It is because sufficient impact resistance can be maintained within the above range.
- the thickness of the substrate varies depending on the material, configuration, etc., but can be set within a range of 10 ⁇ m to 500 ⁇ m, for example.
- the substrate may be subjected to surface treatment such as saponification treatment, glow discharge treatment, corona discharge treatment, ultraviolet treatment, flame treatment and the like.
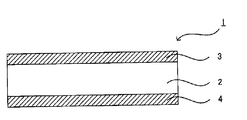
- the second hard coat layer 4 may be formed on the surface of the substrate 2 opposite to the surface on which the hard coat layer 3 is formed. . By forming the second hard coat layer, the hardness of the hard coat film can be further improved.
- the curable resin composition used for the second hard coat layer is the same as the curable resin composition used for the hard coat layer. Especially, it is preferable that the curable resin composition used for a 2nd hard-coat layer contains urethane acrylate. In this case, it is preferable that the curable resin composition used for the hard coat layer also contains urethane acrylate. As described above, by adding urethane acrylate, flexibility can be imparted to the hard coat layer and the second hard coat layer, occurrence of warpage can be suppressed, and impact resistance can be improved. Because.
- the curable resin composition used for the second hard coat layer may be the same as or different from the curable resin composition used for the hard coat layer. Especially, it is preferable that the curable resin composition used for a 2nd hard-coat layer is the same as the curable resin composition used for the said hard-coat layer. This is because warpage can be further suppressed.
- the thickness of the second hard coat layer is the same as the thickness of the hard coat layer.
- the thickness of the second hard coat layer may be the same as or different from the thickness of the hard coat layer.
- it is preferable that the thickness of a 2nd hard-coat layer is the same as the thickness of the said hard-coat layer. It is because generation
- the characteristics and formation method of the second hard coat layer are the same as those of the hard coat layer.
- the antiglare layer 5 may be formed on the hard coat layer 3 as illustrated in FIG.
- the antiglare layer reflection of light on the surface of the hard coat film of the present invention can be suppressed, and when the hard coat film of the present invention is used for an image display device, it reflects external light. Can suppress glare and glare.
- the hard coat layer in the present invention is excellent in hardness, high hardness can be achieved even when an antiglare layer is formed on the hard coat layer.
- the antiglare layer is not particularly limited as long as it has an antiglare property, but among them, a layer containing a resin and fine particles is preferable. Antiglare property can be exhibited by forming irregularities on the surface of the antiglare layer by the fine particles.
- the fine particles are not particularly limited as long as they exhibit antiglare properties, but are preferably translucent fine particles.
- the fine particles both inorganic and organic can be used.
- the inorganic fine particles include amorphous silica particles and inorganic silica particles.
- the organic fine particles include plastic beads, and specific examples include polystyrene beads, melamine resin beads, acrylic beads, acrylic-styrene beads, benzoguanamine beads, benzoguanamine formaldehyde condensation beads, polycarbonate beads, polyethylene beads, and the like.
- the plastic beads preferably have a hydrophobic group on the surface, and examples thereof include styrene beads.
- the fine particles may be used alone or in combination of two or more.
- the fine particles may be primary particles or secondary particles.
- inorganic fine particles are preferably used from the viewpoint of improving the film strength of the antiglare layer, and silica fine particles are particularly preferably used.
- silica fine particles are particularly preferably used.
- amorphous silica particles in order to obtain good dispersibility, it is preferable to use amorphous silica particles that have been subjected to organic treatment on the surface of the particles to make them hydrophobic.
- the organic matter treatment can be the same as the organic matter treatment described in JP2009-86361A.
- Examples of the shape of the fine particles include a true spherical shape, an elliptical shape, an indefinite shape, a rectangular parallelepiped shape, and a cubic shape.
- the average particle diameter of the fine particles is not particularly limited as long as the antiglare property can be imparted, but it is preferable that irregularities can be formed on the surface of the antiglare layer.
- the thickness is preferably in the range of 1 ⁇ m to 10 ⁇ m, more preferably in the range of 1 ⁇ m to 7 ⁇ m, and particularly preferably in the range of 2 ⁇ m to 5 ⁇ m. If the average particle size is small, it is difficult to provide the surface of the antiglare layer with irregularities large enough to exhibit antiglare properties, and even if irregularities can be provided, the amount of fine particles added is very large. Therefore, the film physical properties of the antiglare layer may be deteriorated.
- the average particle size is large, the surface shape of the antiglare layer becomes rough, and the surface quality may be deteriorated, or whiteness may increase due to an increase in surface haze.
- the fine particles may be agglomerated particles.
- the secondary particle size may be in the above range.
- the particle size of fine particles of 80% or more, especially 90% or more of the whole fine particles is in the range of average particle size ⁇ 300 nm. Thereby, the uniformity of the uneven shape on the surface of the antiglare layer can be improved.
- the average particle size of the fine particles means the average when each fine particle is a monodispersed fine particle, that is, a fine particle having a single shape, and is an irregular fine particle having a broad particle size distribution. Means the particle size of fine particles present most abundantly by particle size distribution measurement.
- the particle size of the fine particles can be measured mainly by the Coulter counter method. In addition to this method, measurement can also be performed by laser diffraction or SEM photography.
- the content of the fine particles in the antiglare layer may be adjusted as long as it is an amount capable of exhibiting antiglare properties.
- the content of the fine particles in the antiglare layer is, for example, preferably in the range of 2 to 35 parts by weight with respect to 100 parts by weight of the resin, and more preferably in the range of 5 to 25 parts by weight. preferable. If the content of the fine particles is small, sufficient antiglare properties may not be imparted. In addition, if the content of fine particles is large, the strength and hardness may decrease, or the internal scattering effect may be excessive and the contrast may decrease.
- the haze and gloss of the antiglare layer can be adjusted by adjusting the ratio of the resin and fine particle content in the antiglare layer.
- a curable resin is preferably used.
- a thermosetting resin or an ionizing radiation curable resin that is cured by an ionizing radiation such as an ultraviolet ray or an electron beam can be used.
- curable resin what is generally used for a glare-proof layer can be used. Examples thereof include those used for the antiglare layer described in JP-A-2009-86361.
- the antiglare layer may contain a surfactant.
- the surfactant those used for the hard coat layer can be used.
- the antiglare layer may contain a polymerization initiator, an antistatic agent, an antifouling agent, and the like as necessary.
- the antiglare layer may contain a blue color material.
- Hard coat film that can suppress yellowishness and improve visibility and color reproducibility when used in an image display device by adding a blue color material to the antiglare layer instead of adding it to the hard coat layer Can be obtained.
- a blue color material it can be the same as that of what was described in the said hard-coat layer.
- the hardness of the antiglare layer can be evaluated by a pencil hardness test (4.9 N load) defined in JIS K5600-5-4 (1999).
- the pencil hardness of the antiglare layer can be the same as the pencil hardness of the hard coat layer.
- the antiglare layer is light transmissive.
- the transmittance of the antiglare layer in the visible light region can be the same as the transmittance of the hard coat layer.
- the haze value of the antiglare layer is appropriately determined according to the use of the hard coat film of the present invention, and is not particularly limited, and is set within a range of 3% to 30%, for example. can do.
- the haze value can be measured according to JIS K-7105.
- As an apparatus used for the measurement for example, there is a haze meter HM150 manufactured by Murakami Color Research Laboratory.
- the antiglare layer preferably has antifouling properties.
- the antifouling property can be evaluated by wettability.
- the wettability of the antiglare layer surface can be the same as the wettability of the hard coat layer surface.
- the antiglare layer preferably has slipperiness.
- the slipperiness can be evaluated by a dynamic friction coefficient.
- the dynamic friction coefficient of the surface of the antiglare layer can be the same as the dynamic friction coefficient of the surface of the hard coat layer.
- the thickness of the antiglare layer is not particularly limited as long as the desired antiglare property can be exhibited, but it is preferably in the range of 1 ⁇ m to 5 ⁇ m, for example.
- the thickness of the antiglare layer is preferably in the range of 1 ⁇ m to 5 ⁇ m, for example.
- the amount of fine particles contained in the antiglare layer can be reduced.
- the excessive internal scattering effect in an anti-glare layer can be suppressed, and the fall of contrast can be suppressed, ensuring the desired internal scattering effect.
- the antiglare layer is an extremely thin film, high hardness can be obtained by the hard coat layer formed in contact with the antiglare layer.
- a method for forming the antiglare layer a method of applying a curable resin composition on the hard coat layer and curing the coating film can be used.
- a curable resin composition on the hard coat layer and curing the coating film.
- description here is abbreviate
- the hard coat film of the present invention may have a substrate and a hard coat layer, but may further have another layer depending on the use of the hard coat film.
- examples of other layers include a primer layer, an antistatic layer, an antiglare layer, and a low refractive index layer.
- a colored layer containing a blue color material may be formed on the surface of the substrate opposite to the surface on which the hard coat layer is formed.
- a blue color material to the hard coat layer
- a hard coat film can be obtained.
- the colored layer contains a blue color material and a binder resin.
- a blue color material it can be the same as that of what was described in the said hard-coat layer.
- the binder resin a general one can be used. For example, a resin used for a colored layer described in JP-A-2000-57976 can be used.
- the hard coat film of the present invention may or may not have flexibility.
- the use of the hard coat film of the present invention is not particularly limited.
- contact-type image display devices such as touch panels
- non-contact types such as liquid crystal displays, CRT displays, projection displays, plasma displays, and electroluminescence displays.
- image display device applications and solar cell applications such as dye-sensitized solar cells.
- the hard coat film of this invention is used as a front plate of a touch panel. This is because the front plate of the touch panel is a member in direct contact with a finger, and high hardness and scratch resistance are required.
- Curable resin composition for hard coat layer comprises reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, a monomer, It is characterized by containing.
- a hard coat layer having excellent hardness and scratch resistance can be obtained when the curable resin composition for a hard coat layer contains reactive deformed silica fine particles. Moreover, it is possible to achieve both high hardness and workability by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range to the curable resin composition for a hard coat layer.
- the curable resin composition for a hard coat layer of the present invention can be used as an ink.
- the viscosity and solid content concentration may be adjusted.
- the viscosity and solid content concentration can be adjusted by adding a solvent or heating.
- a solvent the solvent used for the curable resin composition described in the above-mentioned item of “A. Hard coat film 1. Hard coat layer” can be used.
- the method for producing a hard coat film of the present invention comprises a reactive irregularly shaped silica fine particle on a substrate, a weight average molecular weight in the range of 30,000 to 110,000, and an acrylic equivalent of 200 to A hard coat layer forming step of forming a hard coat layer by applying and curing a curable resin composition for a hard coat layer containing an acrylic polymer in the range of 1,200 and a monomer. And
- each process in the manufacturing method of the hard coat film of this invention is demonstrated.
- Hard coat layer forming step In the present invention, on the substrate, hardened layer hardenability containing reactive irregular silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. A hard coat layer forming step is performed in which the resin composition is applied and cured to form a hard coat layer.
- a hard coat layer forming step is performed in which the resin composition is applied and cured to form a hard coat layer.
- the coating film of the curable resin composition for hard-coat layers at a hard-coat layer formation process.
- the adhesion of the hard coat layer and the anti-glare layer can be improved by applying and curing the curable resin composition for the anti-glare layer on the semi-cured coating film of the curable resin composition for the hard coat layer. Because it can.
- the irradiation amount of the energy ray source may be reduced.
- an antiglare layer forming step of forming an antiglare layer by applying a curable resin composition for an antiglare layer on the hard coat layer and curing it may be performed.
- the hard coat layer resin composition was applied onto the substrate, the coating film of the hard coat layer curable resin composition was semi-cured, and then semi-cured.
- the curable resin composition for the antiglare layer is applied onto the coating film of the curable resin composition for the hard coat layer, and the curable resin composition for the hard coat layer and the curable resin composition for the antiglare layer are coated. It is preferable to cure the coating film to form a hard coat layer and an antiglare layer. This is because an antiglare layer having good adhesion to the hard coat layer can be obtained.
- the present invention is not limited to the above embodiment.
- the above-described embodiment is an exemplification, and the present invention has substantially the same configuration as the technical idea described in the claims of the present invention, and any device that exhibits the same function and effect is the present invention. It is included in the technical scope of the invention.
- Example 1 (Preparation) As a substrate, a laminate in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order was prepared.
- the laminate had an overall thickness of 1.0 mm, a PMMA thickness of 100 ⁇ m, and a pencil hardness of 3H.
- reactive irregularly shaped silica fine particles 3 to 10 silica fine particles having an average primary particle size of 55 nm are bonded by an inorganic chemical bond and have an average secondary particle size of 100 to 300 nm, and having a photocurable unsaturated group as a reactive functional group.
- a dispersion of propylene glycol-1-methyl ether acetate (PGMEA) solvent with a solid content concentration of 40.0% by mass was prepared using reactive irregular shaped silica fine particles.
- Pentaerythritol triacrylate (PETA) was prepared as a monomer.
- Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared.
- As the surfactant a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared.
- Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
- acrylic polymers and urethane acrylates having different weight average molecular weights and acrylic equivalents as shown in Table 1 below were prepared.
- the acrylic polymer BL-2002 and BL-2184 manufactured by Seiko PMC Co., Ltd. were used.
- the composition of BL-2002 manufactured by Seiko PMC Co., Ltd. is 30 to 40 parts by weight of acrylic resin, 60 to 70 parts by weight of methyl ethyl ketone, less than 1 part by weight of acetic acid, 2,6-di-tert-butyl-4-cresol. Less than 1 part by weight.
- the difference in physical properties refers to a material having a changed weight average molecular weight or acrylic equivalent.
- UV75550 manufactured by Nippon Synthetic Chemical Industry Co., Ltd.
- U15HA manufactured by Shin-Nakamura Chemical Industry Co., Ltd.
- UN904 manufactured by Negami Industrial Co., Ltd.
- a curable resin composition was prepared with the following composition.
- Reactive deformed silica fine particle dispersion 60 parts by weight (solid content: 40% by mass)
- Monomer 12.7 parts by weight
- Polymer 7.2 parts by weight
- Polymerization initiator 0.76 parts by weight
- Surfactant 0.905 parts by weight
- Solvent 19.2 parts by weight
- the content of reactive irregularly shaped silica fine particles is 60% by mass. Further, the content of the reactive irregularly shaped silica fine particles is 60% by mass with respect to the total solid content of the curable resin composition, and the ratio of the ratio of the components other than the polymer and reactive irregularly shaped silica fine particles is constant.
- the curable resin composition was prepared by changing the addition amount.
- a curable resin composition is applied to one side of the substrate by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, the solvent in the coating film is evaporated, and ultraviolet light having a central wavelength of 365 nm is applied to an integrated light amount of 3000 mJ.
- the hard coat layer with a film thickness of 20 ⁇ m was formed by curing the coating film by irradiating it to / cm 2 .
- the obtained hard coat layer contained a matrix resin, irregular-shaped silica fine particles, and an acrylic polymer or urethane acrylate having a predetermined weight average molecular weight and acrylic equivalent.
- the addition amount of a polymer shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
- Table 1 it was confirmed that the workability was improved while maintaining the hardness of the hard coat layer by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range.
- the addition amount of the acrylic polymer in which the weight average molecular weights and acrylic equivalents of No. 1-2 and 1-9 are within a predetermined range is 6.3% by mass, the polymer is added.
- the hardness of the hard coat layer was improved, and both the hardness and workability were excellent.
- Example 2 (Preparation of curable resin composition) A curable resin composition was prepared in the same manner as in Example 1 except that BL-2002 made by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used as the acrylic polymer. Moreover, curable resin compositions were prepared by changing the amount of the acrylic polymer added so that the ratio of the components other than the acrylic polymer was constant. Further, a curable resin composition was prepared by changing the addition amount of the reactive irregular shaped silica fine particles so that the ratio of the components other than the reactive irregular shaped silica fine particles was constant.
- a hard coat layer was formed in the same manner as in Example 1 except that the thickness of the hard coat layer was 17 ⁇ m or 20 ⁇ m.
- each component shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
- the workability was improved while maintaining the hardness of the hard coat layer by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range.
- the amount of acrylic polymer No.2-4 added is 6.3% by mass, the hardness of the hard coat layer is improved despite the addition of the polymer, and both hardness and workability are improved. It was.
- Example 3 (Preparation of curable resin composition) A curable resin composition was prepared in the same manner as in Example 1 except that the following reactive silica fine particles, acrylic polymer, and surfactant were used and the following composition was used.
- reactive silica fine particles a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. This dispersion of reactive irregularly shaped silica fine particles is DP1222 manufactured by JGC Catalysts & Chemicals.
- a spherical reactive silica fine particle having an average primary particle size of 15 nm, 45 nm or 55 nm and a photocurable unsaturated group as a reactive functional group is used, and the solid content concentration is 40.0 mass. %, A dispersion of methyl isobutyl ketone (MIBK) solvent was prepared.
- MIBK methyl isobutyl ketone
- These reactive silica fine particle dispersions are MIBK-SDL and MIBK-DL manufactured by Nissan Chemical Industries, Ltd., and V8803 manufactured by JGC Catalysts & Chemicals, Inc., respectively.
- As an acrylic polymer BL-2002 manufactured by Seiko PMC Co., Ltd.
- a curable resin composition was prepared by changing the addition amount of the reactive silica fine particles so that the ratio of the ratio of was constant.
- a hard coat layer was formed in the same manner as in Example 1 except that the thickness of the hard coat layer was changed to 10 ⁇ m to 20 ⁇ m.
- Abrasion resistance The surface of the hard coat layer of the hard coat film was subjected to 20 reciprocating frictions using # 0000 steel wool while applying a load, and then the presence or absence of scratches on the hard coat layer was observed and evaluated according to the following criteria.
- the content of the reactive silica fine particles indicates the amount with respect to the total solid content of the curable resin composition, that is, the amount in the hard coat layer. As shown in Table 3, it was confirmed that the hardness of the hard coat layer was improved by adding reactive irregular shaped silica fine particles.
- Example 4 (Preparation) As a substrate, Technoloy C101 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. Technoloy C101 had an overall thickness of 0.3 mm to 1 mm, a PMMA thickness of 70 ⁇ m, and a pencil hardness of 2H. As another substrate, FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared.
- FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order
- FX1190 had an overall thickness of 1 mm, a PMMA thickness of 100 ⁇ m, and a pencil hardness of 3H.
- reactive irregular shaped silica fine particles a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared.
- monomers pentaerythritol triacrylate (PETA), 3-4 functional PETA, SNP Clear 11 made by DNP Fine Chemical Co., Ltd., and acrylate monomers KARAYAD DPCA20 and DPCA120 made by Nippon Kayaku Co., Ltd. were prepared.
- As an acrylic polymer BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used.
- a polymerization initiator Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared.
- surfactants fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd., or fluorinated surfactant Megafac MCF350-5 manufactured by DIC Corporation was prepared.
- Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
- Curable resin compositions were prepared with the compositions shown in Table 4 below.
- a curable resin composition is applied to one side of the substrate by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, and the solvent in the coating film is evaporated.
- a hard coat layer having a film thickness of 10 ⁇ m to 22 ⁇ m was formed by irradiating ultraviolet rays having a wavelength of 365 nm so that the integrated light amount was 3000 mJ / cm 2 to cure the coating film.
- the obtained hard coat layer contained a matrix resin, irregular-shaped silica fine particles, and an acrylic polymer having a predetermined weight average molecular weight and acrylic equivalent.
- As the light source a high pressure mercury lamp, a low pressure mercury lamp, a metal halide lamp, a D bulb and an H bulb manufactured by Fusion UV Systems were used.
- the contact angle of water droplets in the hard coat layer of the hard coat film was measured by using a contact angle measuring device CA-Z type manufactured by Kyowa Interface Science Co., Ltd. Was evaluated by measuring.
- the hard coat layer of the hard coat film was evaluated by drawing a line on the thin side of the marker Mackie Extra Fine Black Model No .: MO-120-MC ⁇ BK manufactured by Zebra Corporation. A: Smoke within 2 seconds (Hajik) B: A line can be drawn (not relieved) within 2 seconds.
- content of each component shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
- the pencil hardness is It was confirmed that the processability was improved.
- the acrylic polymer content is 7.9% by mass, the hardness of the hard coat layer is the highest and the workability is improved. confirmed.
- From No. 4-2 and 4-10 to 4-13 it was confirmed that the pencil hardness was further improved when the thickness of the hard coat layer was 20 ⁇ m or more.
- Example 5 (Preparation of curable resin composition)
- the surfactant fluorine-based surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd., fluorine-based surfactant MegaFuck MCF350-5 manufactured by DIC Corporation, or silicon-based manufactured by Shin-Etsu Chemical Co., Ltd.
- a curable resin composition was prepared in the same manner as in Example 1 except that the surfactant X22-163A was used.
- a hard coat layer was formed in the same manner as in Example 1.
- This hard coat film is useful as a hard coat film used for a touch panel.
- Example 6 As a substrate, FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. FX1190 had an overall thickness of 0.65 mm, a PMMA thickness of 100 ⁇ m, and a pencil hardness of 3H. As reactive irregular shaped silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. As a blue color material, a blue pigment PB15: 6 was used. Pentaerythritol triacrylate (PETA) was prepared as a monomer.
- PMMA polymethyl methacrylate
- PC polycarbonate
- PMMA polymethyl methacrylate
- acrylic polymer BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used.
- a polymerization initiator Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared.
- surfactant a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared.
- PMEA Propylene glycol-1-methyl ether acetate
- Curable resin compositions were prepared with the compositions shown in Table 6 below.
- a hard coat layer was formed in the same manner as in Example 1.
- the obtained hard coat film was measured for spectrum with an ultraviolet-visible near-infrared spectrophotometer MPC-3100 manufactured by Shimadzu Corporation, and using a C light source, chromaticity coordinates x, y in the XYZ color system of CIE In addition, luminance Y, chromaticity b * and yellowness YI in CIE L * a * b * color system were measured.
- the chromaticity b * is an index in color coordinates defined in JIS-Z-8729.
- the chromaticity b * is an index of yellowness. When b * is large, the yellowness is strong, and when the value is negative, the yellowness is insufficient and the color becomes blue.
- Example 7 Formation of hard coat layer (Preparation of curable resin composition) A curable resin composition was prepared with the same composition as in Example 1 except that Megafac F568 manufactured by DIC Corporation was added as a surfactant. (Formation of hard coat layer) A hard coat layer was formed in the same manner as in Example 1 except that the exposure amount was 1/8.
- anti-glare layer TAC-D105 manufactured by Dainichi Chemical Industries as a clear material
- EXG40-77 (D-30M) manufactured by Dainichi Chemical Industries as a mat material containing amorphous silica
- Silicon 10 manufactured by Dainichi Chemical Industries as a surfactant
- a curable resin composition for an antiglare layer was prepared using -28, methyl isobutyl ketone (MIBK) as a solvent. At this time, 0.6 parts by weight of a surfactant was added to 100 parts by weight of the total amount of the clear material and the mat material, and the clear / mat ratio was changed to prepare a curable resin composition for an antiglare layer.
- MIBK methyl isobutyl ketone
- the anti-glare layer curable resin composition is applied by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, and the solvent in the coating film is evaporated.
- An anti-glare layer having a thickness of 5 ⁇ m or less was formed by irradiating ultraviolet rays having a central wavelength of 365 nm so that the integrated light amount was 3000 mJ / cm 2 to cure the coating film.
- haze and transmittance The haze and transmittance of the hard coat film were measured by a method based on JIS-K7105 using a haze meter HM150 manufactured by Murakami Color Research Laboratory.
- Example 8 (Preparation of curable resin composition) A curable resin composition was prepared with the same composition as in Example 1 except that urethane acrylate was further added. At this time, the ratio of the monomer, the acrylic polymer and the urethane acrylate was changed as shown in Table 8. As urethane acrylate, purple light UV-7000B made by Nippon Synthetic Chemical Industry was used. This urethane acrylate has an elongation of 10% to 40% when the elongation is measured with a tensile tester at room temperature of 25 ° C. and speed of 100 mm / min.
- a polyethylene terephthalate (PET) substrate having a thickness of 0.35 mm was used as a substrate, and a hard coat layer having a predetermined thickness was formed on one side of the PET substrate in the same manner as in Example 1.
- a second hard coat layer having a predetermined film thickness was similarly formed on the surface of the substrate opposite to the surface on which the hard coat layer was formed.
- warp The obtained hard coat film was set to 1/4 of the A4 size, the hard coat film was placed on a flat surface, and the average value of the height of lifting from the flat surfaces of the four corners and four sides was measured as warpage. Under the present circumstances, it measured about each after a hard coat film preparation (initial stage) and the following weathering test. The warpage was measured for each of the case where the substrate or the second hard coat layer was on the lower side and the case where the hard coat layer was on the lower side. In Table 8, the warp to the hard coat layer side when the substrate or the second hard coat layer is down is negative, and the substrate or second hard coat layer is when the hard coat layer is down The warp to the side is shown as a positive value.
- the weather resistance test was a test in which the hard coat film was allowed to stand for 24 hours at a temperature of 50 ° C. and a relative humidity of 95%, and a test for which the hard coat film was allowed to stand for 24 hours under a temperature of 85 ° C. and a relative humidity of 85%.
- warpage can be reduced by adding urethane acrylate.
- hard coat layers containing urethane acrylate were formed on both sides of the substrate, warpage after the weather resistance test was reduced.
Abstract
The main purpose of the present invention is to provide a hard coat film which has excellent hardness, scratch resistance and processability. The present invention achieves the above-mentioned purpose by providing a hard coat film which is obtained by forming a hard coat layer on a substrate, and which is characterized in that the hard coat layer contains irregularly shaped fine silica particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within specific ranges, and a matrix resin.
Description
本発明は、ディスプレイ等の表面を保護する目的等で使用されるハードコートフィルムに関するものである。
The present invention relates to a hard coat film used for the purpose of protecting the surface of a display or the like.
液晶ディスプレイ、CRTディスプレイ、プロジェクションディスプレイ、プラズマディスプレイ、エレクトロルミネッセンスディスプレイ等の画像表示装置における画像表示面は、取り扱い時に傷がつかないように、耐擦傷性を付与することが要求される。このような要求に対して、基板上にハードコート層を設けたハードコートフィルムを利用することにより、画像表示装置の画像表示面の耐擦傷性を向上させることが一般になされている。ハードコートフィルムに要求される性能は、近年益々高くなってきており、硬度および耐擦傷性にさらに優れたものが求められている。
Image display surfaces in image display devices such as liquid crystal displays, CRT displays, projection displays, plasma displays, and electroluminescence displays are required to be provided with scratch resistance so that they are not damaged during handling. In response to such demands, it has been common to improve the scratch resistance of the image display surface of an image display device by using a hard coat film having a hard coat layer provided on a substrate. The performance required for hard coat films has been increasing in recent years, and there is a demand for further improved hardness and scratch resistance.
ハードコートフィルムの硬度を向上させる手段として、例えば特許文献1には、特定の構成を有する基板を用い、ハードコートフィルムの総厚み、基板を構成するアクリル樹脂層の厚みおよびハードコート層の厚みを特定の範囲にする方法が開示されている。また特許文献2には、無機酸化物粒子が添加された硬化性組成物を用いてハードコート層を形成する方法が開示されている。さらに特許文献3~7には、反応性異形シリカ微粒子が添加された硬化性組成物を用いてハードコート層を形成する方法が開示されている。
As means for improving the hardness of the hard coat film, for example, in Patent Document 1, a substrate having a specific configuration is used, and the total thickness of the hard coat film, the thickness of the acrylic resin layer constituting the substrate, and the thickness of the hard coat layer are set. A method for making a specific range is disclosed. Patent Document 2 discloses a method of forming a hard coat layer using a curable composition to which inorganic oxide particles are added. Further, Patent Documents 3 to 7 disclose methods for forming a hard coat layer using a curable composition to which reactive irregularly shaped silica fine particles are added.
ハードコートフィルムは、画像表示装置等に使用される際に、打ち抜き加工、ルーター加工、切断加工、切削加工、曲げ加工等の加工が施されるため、加工性に優れることも要求される。
しかしながら、特許文献1のように基板を構成するアクリル樹脂層の厚みを厚くすると、ハードコートフィルムの硬度は向上するものの、加工時にクラックが生じやすいという問題がある。また、ハードコート層の厚みを単純に増加させても硬度を向上させることはできるが、この場合には加工時にハードコート層にクラックが発生しやすいという問題がある。また、特許文献2~7のように無機酸化物粒子や反応性異形シリカ微粒子を添加した場合でも、高硬度は達成できるが、加工性の要求に応えることはできない。 When a hard coat film is used in an image display device or the like, it is required to have excellent workability because it is subjected to punching, router processing, cutting processing, cutting processing, bending processing, and the like.
However, when the thickness of the acrylic resin layer constituting the substrate is increased as inPatent Document 1, the hardness of the hard coat film is improved, but there is a problem that cracks are likely to occur during processing. Further, the hardness can be improved by simply increasing the thickness of the hard coat layer, but in this case, there is a problem that cracks are likely to occur in the hard coat layer during processing. Moreover, even when inorganic oxide particles or reactive irregularly shaped silica fine particles are added as in Patent Documents 2 to 7, high hardness can be achieved, but the workability requirement cannot be met.
しかしながら、特許文献1のように基板を構成するアクリル樹脂層の厚みを厚くすると、ハードコートフィルムの硬度は向上するものの、加工時にクラックが生じやすいという問題がある。また、ハードコート層の厚みを単純に増加させても硬度を向上させることはできるが、この場合には加工時にハードコート層にクラックが発生しやすいという問題がある。また、特許文献2~7のように無機酸化物粒子や反応性異形シリカ微粒子を添加した場合でも、高硬度は達成できるが、加工性の要求に応えることはできない。 When a hard coat film is used in an image display device or the like, it is required to have excellent workability because it is subjected to punching, router processing, cutting processing, cutting processing, bending processing, and the like.
However, when the thickness of the acrylic resin layer constituting the substrate is increased as in
特許文献5には、ハードコート層用硬化性樹脂組成物に反応性異形シリカ微粒子を添加し、ポリマーをさらに添加することにより、良好な屈曲性が得られることが開示されている。しかしながら、ポリマーを単に添加するだけでは硬度が低くなり耐擦傷性が劣化することが懸念され、高硬度および加工性を両立することは困難である。
また、特許文献7には、硬度向上およびカール低減のために、特定の構造および重量平均分子量を有する反応性ポリマーをハードコート層用硬化性樹脂組成物に含有させることが提案されている。しかしながら、加工性については検討がなされていない。Patent Document 5 discloses that good flexibility can be obtained by adding reactive irregular-shaped silica fine particles to a curable resin composition for a hard coat layer and further adding a polymer. However, there is a concern that the hardness is lowered and the scratch resistance is deteriorated simply by adding a polymer, and it is difficult to achieve both high hardness and workability.
Patent Document 7 proposes that a reactive polymer having a specific structure and a weight average molecular weight is contained in a curable resin composition for a hard coat layer in order to improve hardness and reduce curl. However, the workability has not been studied.
また、特許文献7には、硬度向上およびカール低減のために、特定の構造および重量平均分子量を有する反応性ポリマーをハードコート層用硬化性樹脂組成物に含有させることが提案されている。しかしながら、加工性については検討がなされていない。
Patent Document 7 proposes that a reactive polymer having a specific structure and a weight average molecular weight is contained in a curable resin composition for a hard coat layer in order to improve hardness and reduce curl. However, the workability has not been studied.
本発明は、上記問題点に鑑みてなされたものであり、硬度、耐擦傷性および加工性に優れるハードコートフィルムを提供することを主目的とする。
The present invention has been made in view of the above problems, and has as its main object to provide a hard coat film that is excellent in hardness, scratch resistance and workability.
本発明者らは上記課題を解決するために鋭意検討を行った結果、反応性異形シリカ微粒子と、モノマーと、特定のポリマーとを含有する硬化性樹脂組成物を用いてハードコート層を形成することにより、高硬度および加工性を兼備するハードコートフィルムが得られることを知見し、本発明を完成させるに至った。
As a result of intensive studies to solve the above problems, the present inventors form a hard coat layer using a curable resin composition containing reactive irregularly shaped silica fine particles, a monomer, and a specific polymer. As a result, it was found that a hard coat film having both high hardness and workability was obtained, and the present invention was completed.
すなわち、本発明は、基板上にハードコート層が形成されたハードコートフィルムであって、上記ハードコート層は、異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有することを特徴とするハードコートフィルムを提供する。
That is, the present invention is a hard coat film in which a hard coat layer is formed on a substrate, and the hard coat layer has irregular-shaped silica fine particles and a weight average molecular weight in the range of 30,000 to 110,000. A hard coat film comprising an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200 and a matrix resin is provided.
本発明によれば、ハードコート層が異形シリカ微粒子を含むことにより、硬度および耐擦傷性を向上させることができる。また、ハードコート層に重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを用いることにより、高硬度を維持しつつ加工性を改善することが可能である。
According to the present invention, the hard coat layer contains irregular shaped silica fine particles, whereby the hardness and scratch resistance can be improved. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to improve workability while maintaining high hardness.
上記発明においては、上記ハードコート層は重合開始剤を含有していてもよい。
In the above invention, the hard coat layer may contain a polymerization initiator.
また本発明においては、上記ハードコート層は青色色材を含有していてもよい。本発明のハードコートフィルムを画像表示装置に用いた場合に、黄色味を抑え、視認性や色再現性を向上させることができるからである。
In the present invention, the hard coat layer may contain a blue color material. This is because when the hard coat film of the present invention is used in an image display device, yellowness can be suppressed and visibility and color reproducibility can be improved.
また本発明においては、上記ハードコート層上に防眩層が形成されていてもよい。ハードコート層上に防眩層が形成されている場合においても、ハードコート層が硬度に優れるため、高硬度を達成することができる。
In the present invention, an antiglare layer may be formed on the hard coat layer. Even in the case where the antiglare layer is formed on the hard coat layer, the hard coat layer is excellent in hardness, so that high hardness can be achieved.
また本発明は、基板上にハードコート層が形成されたハードコートフィルムであって、上記ハードコート層は、反応性異形シリカ微粒子と、上記アクリル系ポリマーと、モノマーとを含有する硬化性樹脂組成物の硬化物を含むことを特徴とするハードコートフィルムを提供する。
Further, the present invention is a hard coat film in which a hard coat layer is formed on a substrate, the hard coat layer is a curable resin composition containing reactive irregular silica fine particles, the acrylic polymer, and a monomer. A hard coat film comprising a cured product is provided.
本発明によれば、ハードコート層に反応性異形シリカ微粒子を用いることにより、硬度および耐擦傷性を向上させることができる。また、ハードコート層に重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを用いることにより、高硬度を維持しつつ加工性を改善することが可能である。
According to the present invention, hardness and scratch resistance can be improved by using reactive irregularly shaped silica fine particles in the hard coat layer. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to improve workability while maintaining high hardness.
また本発明は、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーとを含有することを特徴とするハードコート層用硬化性樹脂組成物を提供する。
The present invention also provides reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200, and a monomer. A curable resin composition for a hard coat layer is provided.
本発明によれば、ハードコート層用硬化性樹脂組成物が反応性異形シリカ微粒子を含むことにより、硬度および耐擦傷性に優れるハードコート層を得ることができる。また、ハードコート層用硬化性樹脂組成物に重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを添加することにより、高硬度および加工性の両立を図ることが可能である。
According to the present invention, when the curable resin composition for a hard coat layer contains reactive irregularly shaped silica fine particles, a hard coat layer having excellent hardness and scratch resistance can be obtained. Moreover, it is possible to achieve both high hardness and workability by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range to the curable resin composition for a hard coat layer.
また、本発明のハードコート層用硬化性樹脂組成物は重合開始剤をさらに含有していてもよい。
Moreover, the curable resin composition for a hard coat layer of the present invention may further contain a polymerization initiator.
また、本発明のハードコート層用硬化性樹脂組成物は青色色材をさらに含有していてもよい。画像表示装置に用いた場合に黄色味を抑え視認性や色再現性を向上させることが可能なハードコートフィルムを得ることができるからである。
Further, the curable resin composition for a hard coat layer of the present invention may further contain a blue color material. This is because when used in an image display device, a hard coat film capable of suppressing yellowness and improving visibility and color reproducibility can be obtained.
また本発明は、基板上に、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーとを含有するハードコート層用硬化性樹脂組成物を塗布し、硬化させてハードコート層を形成するハードコート層形成工程を有することを特徴とするハードコートフィルムの製造方法を提供する。
The present invention also provides a reactive irregularly shaped silica fine particle on a substrate, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200. There is provided a method for producing a hard coat film, comprising a hard coat layer forming step of applying a curable resin composition for a hard coat layer containing a monomer and curing to form a hard coat layer.
本発明によれば、上述のハードコート層用硬化性樹脂組成物を用いることにより、硬度、耐擦傷性および加工性に優れるハードコート層を得ることが可能である。
According to the present invention, it is possible to obtain a hard coat layer having excellent hardness, scratch resistance and workability by using the above-described curable resin composition for hard coat layer.
また本発明は、基板上にハードコート層が形成されたハードコートフィルムであって、上記ハードコート層は、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーと、重合開始剤とを含有する硬化性樹脂組成物の硬化物を含むことを特徴とするハードコートフィルムを提供する。
The present invention also relates to a hard coat film having a hard coat layer formed on a substrate, wherein the hard coat layer has reactive irregularly shaped silica fine particles and a weight average molecular weight in the range of 30,000 to 110,000. Provided is a hard coat film comprising a cured product of a curable resin composition containing an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200, a monomer, and a polymerization initiator To do.
また本発明は、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーと、重合開始剤とを含有することを特徴とするハードコート層用硬化性樹脂組成物を提供する。
The present invention also includes reactive irregularly shaped silica particles, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200, a monomer, A curable resin composition for a hard coat layer comprising a polymerization initiator is provided.
本発明によれば、ハードコート層が反応性異形シリカ微粒子を含むことにより、硬度および耐擦傷性を向上させることができる。また、ハードコート層に重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを用いることにより、高硬度を維持しつつ加工性を改善することが可能である。
According to the present invention, the hardness and scratch resistance can be improved by including the reactive deformed silica fine particles in the hard coat layer. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to improve workability while maintaining high hardness.
本発明は、硬度、耐擦傷性および加工性のいずれをも向上させることが可能であるという効果を奏する。
The present invention has an effect that it is possible to improve any of hardness, scratch resistance and workability.
以下、本発明のハードコートフィルム、ハードコート層用硬化性樹脂組成物およびハードコートフィルムの製造方法について詳細に説明する。
Hereinafter, the hard coat film, the curable resin composition for the hard coat layer, and the method for producing the hard coat film of the present invention will be described in detail.
A.ハードコートフィルム
本発明のハードコートフィルムは、基板上にハードコート層が形成されたハードコートフィルムであって、上記ハードコート層は、異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有することを特徴とするものである。 A. Hard coat film The hard coat film of the present invention is a hard coat film in which a hard coat layer is formed on a substrate, and the hard coat layer has irregular-shaped silica fine particles and a weight average molecular weight of 30,000 to 110,000. And an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200 and a matrix resin.
本発明のハードコートフィルムは、基板上にハードコート層が形成されたハードコートフィルムであって、上記ハードコート層は、異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有することを特徴とするものである。 A. Hard coat film The hard coat film of the present invention is a hard coat film in which a hard coat layer is formed on a substrate, and the hard coat layer has irregular-shaped silica fine particles and a weight average molecular weight of 30,000 to 110,000. And an acrylic polymer having an acrylic equivalent in the range of 200 to 1,200 and a matrix resin.
なお、本願明細書において、「フィルム」、「シート」、「板」等の用語は、呼称の違いのみに基づいて、互いから区別されるものではない。例えば、「ハードコートフィルム」はシートや板等とも呼ばれ得るような部材も含む概念である。
In the specification of the present application, terms such as “film”, “sheet”, and “plate” are not distinguished from each other based only on the difference in names. For example, the “hard coat film” is a concept including a member that can also be called a sheet or a plate.
本発明においては、ハードコート層が異形シリカ微粒子を含むことにより、硬度および耐擦傷性に優れるハードコート層を得ることができる。また、ハードコート層に重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを用いることにより、高硬度および加工性の両立を図ることが可能である。
In the present invention, when the hard coat layer contains irregular-shaped silica fine particles, a hard coat layer having excellent hardness and scratch resistance can be obtained. Further, by using an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range for the hard coat layer, it is possible to achieve both high hardness and workability.
本発明のハードコートフィルムについて図面を参照して説明する。
図1は、本発明のハードコートフィルムの一例を示す概略断面図である。図1に例示するように、ハードコートフィルム1は、基板2と、基板2上に形成されたハードコート層3とを有している。ハードコート層3は、異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有している。すなわち、ハードコート層3は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有する硬化性樹脂組成物の硬化物から構成されている。
以下、本発明のハードコートフィルムにおける各構成について説明する。 The hard coat film of the present invention will be described with reference to the drawings.
FIG. 1 is a schematic sectional view showing an example of the hard coat film of the present invention. As illustrated in FIG. 1, thehard coat film 1 includes a substrate 2 and a hard coat layer 3 formed on the substrate 2. The hard coat layer 3 contains irregular-shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within predetermined ranges, and a matrix resin. That is, the hard coat layer 3 is composed of a cured product of a curable resin composition containing reactive irregular shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Yes.
Hereafter, each structure in the hard coat film of this invention is demonstrated.
図1は、本発明のハードコートフィルムの一例を示す概略断面図である。図1に例示するように、ハードコートフィルム1は、基板2と、基板2上に形成されたハードコート層3とを有している。ハードコート層3は、異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有している。すなわち、ハードコート層3は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有する硬化性樹脂組成物の硬化物から構成されている。
以下、本発明のハードコートフィルムにおける各構成について説明する。 The hard coat film of the present invention will be described with reference to the drawings.
FIG. 1 is a schematic sectional view showing an example of the hard coat film of the present invention. As illustrated in FIG. 1, the
Hereafter, each structure in the hard coat film of this invention is demonstrated.
1.ハードコート層
本発明におけるハードコート層は、基板上に形成されるものであり、2つの態様を有する。第1態様のハードコート層は、異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有するものである。また、第2態様のハードコート層は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有する硬化性樹脂組成物の硬化物を含むものである。
以下、ハードコート層の構成について説明する。 1. Hard coat layer The hard coat layer in this invention is formed on a board | substrate, and has two aspects. The hard coat layer of the first embodiment contains irregular-shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within predetermined ranges, and a matrix resin. The hard coat layer of the second aspect is a cured product of a curable resin composition containing reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Is included.
Hereinafter, the configuration of the hard coat layer will be described.
本発明におけるハードコート層は、基板上に形成されるものであり、2つの態様を有する。第1態様のハードコート層は、異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、マトリクス樹脂とを含有するものである。また、第2態様のハードコート層は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有する硬化性樹脂組成物の硬化物を含むものである。
以下、ハードコート層の構成について説明する。 1. Hard coat layer The hard coat layer in this invention is formed on a board | substrate, and has two aspects. The hard coat layer of the first embodiment contains irregular-shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within predetermined ranges, and a matrix resin. The hard coat layer of the second aspect is a cured product of a curable resin composition containing reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Is included.
Hereinafter, the configuration of the hard coat layer will be described.
(1)硬化性樹脂組成物
本発明に用いられる硬化性樹脂組成物は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有するものである。
以下、硬化性樹脂組成物における各成分について説明する。 (1) Curable resin composition The curable resin composition used in the present invention contains reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Is.
Hereinafter, each component in the curable resin composition will be described.
本発明に用いられる硬化性樹脂組成物は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有するものである。
以下、硬化性樹脂組成物における各成分について説明する。 (1) Curable resin composition The curable resin composition used in the present invention contains reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. Is.
Hereinafter, each component in the curable resin composition will be described.
(a)反応性異形シリカ微粒子
本発明において、反応性異形シリカ微粒子は、ハードコート層の硬度向上に寄与する成分である。 (A) Reactive Modified Silica Fine Particle In the present invention, the reactive deformed silica fine particle is a component that contributes to improving the hardness of the hard coat layer.
本発明において、反応性異形シリカ微粒子は、ハードコート層の硬度向上に寄与する成分である。 (A) Reactive Modified Silica Fine Particle In the present invention, the reactive deformed silica fine particle is a component that contributes to improving the hardness of the hard coat layer.
反応性異形シリカ微粒子は、通常、反応性官能基を有する。反応性官能基としては、重合性不飽和基が好適に用いられ、好ましくは光硬化性不飽和基であり、特に好ましくは電離放射線硬化性不飽和基である。具体例としては、(メタ)アクリロイル基、ビニル基、アリル基等のエチレン性不飽和結合およびエポキシ基等が挙げられる。
The reactive irregular shaped silica fine particles usually have a reactive functional group. As the reactive functional group, a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group. Specific examples include ethylenically unsaturated bonds such as (meth) acryloyl groups, vinyl groups, allyl groups, and epoxy groups.
なお、本願明細書において、(メタ)アクリロイルはアクリロイルおよびメタクリロイルの少なくともいずれかを意味し、(メタ)アクリレートはアクリレートおよびメタクリレートの少なくともいずれかを意味し、(メタ)アクリルはアクリルおよびメタクリルの少なくともいずれかを意味する。
In the present specification, (meth) acryloyl means at least one of acryloyl and methacryloyl, (meth) acrylate means at least one of acrylate and methacrylate, and (meth) acryl is at least one of acrylic and methacrylic. Means.
反応性異形シリカ微粒子としては、複数のシリカ微粒子が無機の化学結合により結合した反応性異形シリカ微粒子が挙げられる。中でも、反応性異形シリカ微粒子は、平均1次粒径1nm~100nmのシリカ微粒子3個~20個が無機の化学結合により結合し、表面に反応性官能基を有する反応性異形シリカ微粒子であることが好ましい。反応性異形シリカ微粒子は、反応性官能基を有することにより、反応性異形シリカ微粒子同士、およびモノマーと架橋する硬化反応が可能であり、ハードコート層に耐擦傷性および硬度を付与することができる。
Examples of reactive irregularly shaped silica fine particles include reactive irregularly shaped silica fine particles in which a plurality of fine silica particles are bonded by an inorganic chemical bond. Among them, the reactive irregularly shaped silica fine particles are reactive irregularly shaped silica fine particles having 3 to 20 silica fine particles having an average primary particle diameter of 1 nm to 100 nm bonded by an inorganic chemical bond and having a reactive functional group on the surface. Is preferred. The reactive irregularly shaped silica fine particles have a reactive functional group, so that they can undergo a curing reaction that crosslinks with the reactive irregularly shaped silica fine particles and the monomer, and can impart scratch resistance and hardness to the hard coat layer. .
反応性異形シリカ微粒子を構成するシリカ微粒子の平均1次粒径は、1nm~100nmの範囲内であることが好ましく、5nm~80nmの範囲内であることがより好ましい。シリカ微粒子の平均1次粒径が小さいと、平均2次粒径が小さい反応性異形シリカ微粒子しか得られず、ハードコート層に十分な硬度を付与できない場合がある。また、シリカ微粒子の平均1次粒径が大きいと反応性異形シリカ微粒子の平均2次粒径が大きくなりやすく、平均2次粒径が大きいとハードコート層の透明性が低下し、透過率の悪化、ヘイズの上昇を招く場合がある。
反応性異形シリカ微粒子の平均2次粒径は、5nm~300nmの範囲内であることが好ましく、10nm~200nmの範囲内であることがより好ましい。反応性異形シリカ微粒子の平均2粒径が上記範囲内であれば、ハードコート層に硬度を付与しやすく、かつハードコート層の透明性を維持しやすい。 The average primary particle diameter of the silica fine particles constituting the reactive irregularly shaped silica fine particles is preferably in the range of 1 nm to 100 nm, and more preferably in the range of 5 nm to 80 nm. If the average primary particle size of the silica fine particles is small, only reactive irregularly shaped silica fine particles having a small average secondary particle size can be obtained, and sufficient hardness may not be imparted to the hard coat layer. Further, if the average primary particle size of the silica fine particles is large, the average secondary particle size of the reactive irregularly shaped silica fine particles tends to be large, and if the average secondary particle size is large, the transparency of the hard coat layer is lowered and the transmittance Deterioration and haze increase may occur.
The average secondary particle size of the reactive irregularly shaped silica fine particles is preferably in the range of 5 nm to 300 nm, and more preferably in the range of 10 nm to 200 nm. When the average two particle diameters of the reactive irregularly shaped silica fine particles are within the above range, it is easy to impart hardness to the hard coat layer and maintain the transparency of the hard coat layer.
反応性異形シリカ微粒子の平均2次粒径は、5nm~300nmの範囲内であることが好ましく、10nm~200nmの範囲内であることがより好ましい。反応性異形シリカ微粒子の平均2粒径が上記範囲内であれば、ハードコート層に硬度を付与しやすく、かつハードコート層の透明性を維持しやすい。 The average primary particle diameter of the silica fine particles constituting the reactive irregularly shaped silica fine particles is preferably in the range of 1 nm to 100 nm, and more preferably in the range of 5 nm to 80 nm. If the average primary particle size of the silica fine particles is small, only reactive irregularly shaped silica fine particles having a small average secondary particle size can be obtained, and sufficient hardness may not be imparted to the hard coat layer. Further, if the average primary particle size of the silica fine particles is large, the average secondary particle size of the reactive irregularly shaped silica fine particles tends to be large, and if the average secondary particle size is large, the transparency of the hard coat layer is lowered and the transmittance Deterioration and haze increase may occur.
The average secondary particle size of the reactive irregularly shaped silica fine particles is preferably in the range of 5 nm to 300 nm, and more preferably in the range of 10 nm to 200 nm. When the average two particle diameters of the reactive irregularly shaped silica fine particles are within the above range, it is easy to impart hardness to the hard coat layer and maintain the transparency of the hard coat layer.
ここで、シリカ微粒子の平均1次粒径は、硬化性樹脂組成物中のシリカ微粒子を動的光散乱方法で測定し、粒子径分布を累積分布で表したときの50%粒子径(d50 メジアン径)を意味する。平均1次粒径は、日機装(株)製のMicrotrac粒度分析計またはNanotrac粒度分析計を用いて測定することができる。また、反応性異形シリカ微粒子の平均2次粒径は、硬化性樹脂組成物においては、平均1次粒径と同様の方法により求めることができる。一方、反応性異形シリカ微粒子の平均2次粒径は、ハードコート層においては、ハードコート層の断面をSEM写真またはTEM写真を用いて観察し、観察された硬化した反応性異形シリカ微粒子を100個選び、その平均値として求めることができる。
Here, the average primary particle size of the silica fine particles is determined by measuring the silica fine particles in the curable resin composition by a dynamic light scattering method, and the 50% particle size (d50 median) when the particle size distribution is expressed as a cumulative distribution. Diameter). The average primary particle size can be measured using a Microtrac particle size analyzer or a Nanotrac particle size analyzer manufactured by Nikkiso Co., Ltd. In addition, the average secondary particle size of the reactive irregularly shaped silica fine particles can be determined by the same method as the average primary particle size in the curable resin composition. On the other hand, the average secondary particle size of the reactive irregularly shaped silica fine particles is such that, in the hard coat layer, the cross section of the hard coat layer is observed using an SEM photograph or a TEM photograph, and the observed cured reactive irregularly shaped silica fine particles are 100. It can be obtained as an average value of individual selection.
シリカ微粒子は、中空粒子のような粒子内部に空孔や多孔質組織を有する粒子の使用を排除するものではないが、粒子内部に空孔や多孔質組織を有しない中実粒子を用いることが硬度向上の点からより好ましい。
Silica fine particles do not exclude the use of particles having pores or porous structures inside the particles, such as hollow particles, but solid particles having no pores or porous structures inside the particles should be used. It is more preferable from the viewpoint of improving the hardness.
反応性異形シリカ微粒子は、上記シリカ微粒子が、好ましくは3個~20個、より好ましくは3個~10個、無機の化学結合によって結合してなる。無機の化学結合によって結合したシリカ微粒子数が少ないと、実質的に単分散粒子と変わらず、基板との密着性、耐擦傷性、鉛筆硬度に優れたハードコート層を得ることが困難である。また、無機の化学結合によって結合したシリカ微粒子数が多いと、ハードコート層の透明性が低下し、透過率の悪化、ヘイズの上昇を招く場合がある。
The reactive irregularly shaped silica fine particles are formed by bonding the above-mentioned silica fine particles, preferably 3 to 20, more preferably 3 to 10, by inorganic chemical bonds. When the number of silica fine particles bonded by inorganic chemical bonds is small, it is substantially the same as monodisperse particles, and it is difficult to obtain a hard coat layer excellent in adhesion to the substrate, scratch resistance, and pencil hardness. Moreover, when there are many silica fine particles couple | bonded by the inorganic chemical bond, the transparency of a hard-coat layer will fall, and the deterioration of the transmittance | permeability and the raise of a haze may be caused.
無機の化学結合としては、例えば、イオン結合、金属結合、配位結合、および共有結合が挙げられる。中でも、反応性異形シリカ微粒子を極性溶媒中に添加しても、結合したシリカ微粒子が分散しない結合、具体的には、金属結合、配位結合、および共有結合が好ましく、特に共有結合が好ましい。なお、極性溶媒としては、例えば、水、およびメタノール、エタノール、イソプロピルアルコール等の低級アルコール等が挙げられる。
Examples of inorganic chemical bonds include ionic bonds, metal bonds, coordination bonds, and covalent bonds. Among them, a bond in which the bonded silica fine particles are not dispersed even when the reactive deformed silica fine particles are added to the polar solvent, specifically, a metal bond, a coordination bond, and a covalent bond is preferable, and a covalent bond is particularly preferable. Examples of the polar solvent include water and lower alcohols such as methanol, ethanol and isopropyl alcohol.
反応性異形シリカ微粒子の粒子状態としては、3個~20個のシリカ微粒子が無機の化学結合により結合し、凝集した状態の粒子(凝集粒子)、および3個~20個のシリカ微粒子が無機の化学結合により結合し、鎖状に結合した鎖状粒子が挙げられる。中でも、ハードコート層の硬度を高める観点から、反応性異形シリカ微粒子の粒子状態としては、鎖状粒子が好ましい。また、反応性異形シリカ微粒子の少なくとも一部に、上記鎖状粒子が含まれていることが好ましい。
As the particle state of the reactive irregularly shaped silica fine particles, 3 to 20 silica fine particles are bonded by an inorganic chemical bond and aggregated particles (aggregated particles), and 3 to 20 silica fine particles are inorganic. Examples thereof include chain particles bonded by chemical bonds and bonded in a chain. Among these, from the viewpoint of increasing the hardness of the hard coat layer, chain particles are preferable as the particle state of the reactive irregularly shaped silica fine particles. Further, it is preferable that the chain particles are contained in at least a part of the reactive irregular shaped silica fine particles.
ここで、反応性異形シリカ微粒子が鎖状粒子の場合、シリカ微粒子の平均結合数は、ハードコート層の断面をSEM写真またはTEM写真を用いて観察し、観察された硬化した反応性異形シリカ微粒子を100個選び、各反応性異形シリカ微粒子中に含まれるシリカ微粒子を数え、その平均値として求めることができる。
Here, when the reactive irregularly shaped silica fine particles are chain particles, the average number of bonds of the silica fine particles is determined by observing the cross section of the hard coat layer using an SEM photograph or a TEM photograph and observing the cured reactive irregularly shaped silica fine particles. 100 are selected, the silica fine particles contained in each reactive deformed silica fine particle are counted, and the average value can be obtained.
反応性異形シリカ微粒子の製造方法は、上記シリカ微粒子が無機の結合により結合したものが得られれば特に限定されず、従来公知の方法を適宜選択して用いることができる。例えば、単分散のシリカ微粒子分散液の濃度、あるいはpHを調節し、100℃以上の高温で水熱処理することによって得ることができる。このとき、必要に応じてバインダー成分を添加してシリカ微粒子の結合を促進することもできる。また、使用されるシリカ微粒子分散液をイオン交換樹脂に通液することで、イオンを除去してもよい。このようなイオン交換処理によってシリカ微粒子の結合を促進することができる。水熱処理後、再度イオン交換処理を行ってもよい。
The method for producing reactive irregularly shaped silica fine particles is not particularly limited as long as the above-mentioned silica fine particles are bonded by an inorganic bond, and a conventionally known method can be appropriately selected and used. For example, it can be obtained by adjusting the concentration or pH of the monodispersed silica fine particle dispersion and performing hydrothermal treatment at a high temperature of 100 ° C. or higher. At this time, if necessary, a binder component can be added to promote the binding of the silica fine particles. Further, ions may be removed by passing the silica fine particle dispersion used through an ion exchange resin. Such ion exchange treatment can promote the binding of silica fine particles. After the hydrothermal treatment, the ion exchange treatment may be performed again.
反応性異形シリカ微粒子は、少なくとも表面の一部に有機成分が被覆され、この有機成分により導入された反応性官能基を表面に有する。ここで、有機成分とは、炭素を含有する成分である。また、少なくとも表面の一部に有機成分が被覆されている態様としては、例えば、シリカ微粒子の表面に存在する水酸基にシランカップリング剤等の有機成分を含む化合物が反応して、表面の一部に有機成分が結合した態様、または、シリカ微粒子の表面に存在する水酸基にイソシアネート基を有する有機成分を含む化合物が反応して、表面の一部に有機成分が結合した態様の他、例えば、シリカ微粒子の表面に存在する水酸基に水素結合等の相互作用により有機成分を付着させた態様や、ポリマー粒子中にシリカ微粒子を含有する態様等が含まれる。
The reactive irregular shaped silica fine particles have at least a part of the surface coated with an organic component, and have a reactive functional group introduced by the organic component on the surface. Here, the organic component is a component containing carbon. Moreover, as an aspect in which at least a part of the surface is coated with an organic component, for example, a compound containing an organic component such as a silane coupling agent reacts with a hydroxyl group present on the surface of the silica fine particles to cause a part of the surface In addition to the mode in which the organic component is bonded to the surface, or the mode in which the organic component having an isocyanate group is reacted with the hydroxyl group present on the surface of the silica fine particle and the organic component is bonded to a part of the surface, for example, silica Examples include a mode in which an organic component is attached to a hydroxyl group present on the surface of the fine particle by an interaction such as hydrogen bonding, a mode in which silica fine particles are contained in the polymer particle, and the like.
少なくとも表面の一部に有機成分が被覆され、有機成分により導入された反応性官能基を表面に有する反応性異形シリカ微粒子を調製する方法としては、反応性異形シリカ微粒子に導入したい反応性官能基により、従来公知の方法を適宜選択して用いることができる。中でも、反応性異形シリカ微粒子同士の凝集を抑制し、ハードコート層の硬度を向上させる点から、以下の(i)(ii)の反応性異形シリカ微粒子のいずれかを適宜選択して用いることが好ましい。
(i)飽和または不飽和カルボン酸、当該カルボン酸に対応する酸無水物、酸塩化物、エステルおよび酸アミド、アミノ酸、イミン、ニトリル、イソニトリル、エポキシ化合物、アミン、β-ジカルボニル化合物、シラン、および官能基を有する金属化合物よりなる群から選択される1種以上の分子量500以下の表面修飾化合物の存在下、分散媒としての水および有機溶媒の少なくともいずれの中に異形シリカ微粒子を分散させることにより得られる、表面に反応性官能基を有する反応性異形シリカ微粒子。
(ii)被覆前の異形シリカ微粒子に導入する反応性官能基、下記化学式(1)に示す基、およびシラノール基または加水分解によってシラノール基を生成する基を含む化合物と、金属酸化物微粒子とを結合することにより得られる、表面に反応性官能基を有する反応性異形シリカ微粒子。
-Q1-C(=Q2)-Q3- (1)
(式(1)中、Q1はNH、OまたはSを示し、Q2はOまたはSを示し、Q3はNHまたは2価以上の有機基を示す。)
以下、好適に用いられる反応性異形シリカ微粒子について説明する。 At least a part of the surface is coated with an organic component, and a method for preparing reactive deformed silica fine particles having a reactive functional group introduced by the organic component on the surface is a reactive functional group to be introduced into the reactive deformed silica fine particles. Thus, a conventionally known method can be appropriately selected and used. Among these, from the viewpoint of suppressing aggregation of the reactive deformed silica fine particles and improving the hardness of the hard coat layer, any one of the following reactive deformed silica fine particles (i) and (ii) may be appropriately selected and used. preferable.
(I) saturated or unsaturated carboxylic acid, acid anhydride, acid chloride, ester and acid amide corresponding to the carboxylic acid, amino acid, imine, nitrile, isonitrile, epoxy compound, amine, β-dicarbonyl compound, silane, And the modified silica fine particles are dispersed in at least one of water and an organic solvent as a dispersion medium in the presence of one or more surface modification compounds having a molecular weight of 500 or less selected from the group consisting of metal compounds having functional groups. Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by
(Ii) a compound containing a reactive functional group to be introduced into the deformed silica fine particle before coating, a group represented by the following chemical formula (1), and a silanol group or a group that generates a silanol group by hydrolysis, and metal oxide fine particles: Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by bonding.
-Q 1 -C (= Q 2 ) -Q 3- (1)
(In formula (1), Q 1 represents NH, O or S, Q 2 represents O or S, and Q 3 represents NH or a divalent or higher organic group.)
Hereinafter, the reactive irregular-shaped silica fine particle used suitably is demonstrated.
(i)飽和または不飽和カルボン酸、当該カルボン酸に対応する酸無水物、酸塩化物、エステルおよび酸アミド、アミノ酸、イミン、ニトリル、イソニトリル、エポキシ化合物、アミン、β-ジカルボニル化合物、シラン、および官能基を有する金属化合物よりなる群から選択される1種以上の分子量500以下の表面修飾化合物の存在下、分散媒としての水および有機溶媒の少なくともいずれの中に異形シリカ微粒子を分散させることにより得られる、表面に反応性官能基を有する反応性異形シリカ微粒子。
(ii)被覆前の異形シリカ微粒子に導入する反応性官能基、下記化学式(1)に示す基、およびシラノール基または加水分解によってシラノール基を生成する基を含む化合物と、金属酸化物微粒子とを結合することにより得られる、表面に反応性官能基を有する反応性異形シリカ微粒子。
-Q1-C(=Q2)-Q3- (1)
(式(1)中、Q1はNH、OまたはSを示し、Q2はOまたはSを示し、Q3はNHまたは2価以上の有機基を示す。)
以下、好適に用いられる反応性異形シリカ微粒子について説明する。 At least a part of the surface is coated with an organic component, and a method for preparing reactive deformed silica fine particles having a reactive functional group introduced by the organic component on the surface is a reactive functional group to be introduced into the reactive deformed silica fine particles. Thus, a conventionally known method can be appropriately selected and used. Among these, from the viewpoint of suppressing aggregation of the reactive deformed silica fine particles and improving the hardness of the hard coat layer, any one of the following reactive deformed silica fine particles (i) and (ii) may be appropriately selected and used. preferable.
(I) saturated or unsaturated carboxylic acid, acid anhydride, acid chloride, ester and acid amide corresponding to the carboxylic acid, amino acid, imine, nitrile, isonitrile, epoxy compound, amine, β-dicarbonyl compound, silane, And the modified silica fine particles are dispersed in at least one of water and an organic solvent as a dispersion medium in the presence of one or more surface modification compounds having a molecular weight of 500 or less selected from the group consisting of metal compounds having functional groups. Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by
(Ii) a compound containing a reactive functional group to be introduced into the deformed silica fine particle before coating, a group represented by the following chemical formula (1), and a silanol group or a group that generates a silanol group by hydrolysis, and metal oxide fine particles: Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by bonding.
-Q 1 -C (= Q 2 ) -Q 3- (1)
(In formula (1), Q 1 represents NH, O or S, Q 2 represents O or S, and Q 3 represents NH or a divalent or higher organic group.)
Hereinafter, the reactive irregular-shaped silica fine particle used suitably is demonstrated.
(i)飽和または不飽和カルボン酸、当該カルボン酸に対応する酸無水物、酸塩化物、エステル及び酸アミド、アミノ酸、イミン、ニトリル、イソニトリル、エポキシ化合物、アミン、β-ジカルボニル化合物、シラン、および官能基を有する金属化合物よりなる群から選択される1種以上の分子量500以下の表面修飾化合物の存在下、分散媒としての水および有機溶媒の少なくともいずれかの中に異形シリカ微粒子を分散させることにより得られる、表面に反応性官能基を有する反応性異形シリカ微粒子。
上記(i)の反応性異形シリカ微粒子を用いる場合には、有機成分含量が少なくてもハードコート層の強度を向上できるという利点がある。 (I) saturated or unsaturated carboxylic acid, acid anhydride, acid chloride, ester and acid amide corresponding to the carboxylic acid, amino acid, imine, nitrile, isonitrile, epoxy compound, amine, β-dicarbonyl compound, silane, And the modified silica fine particles are dispersed in at least one of water and an organic solvent as a dispersion medium in the presence of one or more surface modification compounds having a molecular weight of 500 or less selected from the group consisting of metal compounds having functional groups. Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by
When the reactive irregularly shaped silica fine particles (i) are used, there is an advantage that the strength of the hard coat layer can be improved even if the organic component content is small.
上記(i)の反応性異形シリカ微粒子を用いる場合には、有機成分含量が少なくてもハードコート層の強度を向上できるという利点がある。 (I) saturated or unsaturated carboxylic acid, acid anhydride, acid chloride, ester and acid amide corresponding to the carboxylic acid, amino acid, imine, nitrile, isonitrile, epoxy compound, amine, β-dicarbonyl compound, silane, And the modified silica fine particles are dispersed in at least one of water and an organic solvent as a dispersion medium in the presence of one or more surface modification compounds having a molecular weight of 500 or less selected from the group consisting of metal compounds having functional groups. Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by
When the reactive irregularly shaped silica fine particles (i) are used, there is an advantage that the strength of the hard coat layer can be improved even if the organic component content is small.
上記(i)の反応性異形シリカ微粒子に用いられる表面修飾化合物は、カルボキシル基、酸無水物基、酸塩化物基、酸アミド基、エステル基、イミノ基、ニトリル基、イソニトリル基、水酸基、チオール基、エポキシ基、第一級、第二級及び第三級アミノ基、Si-OH基、シランの加水分解性残基、またはβ-ジカルボニル化合物のようなC-H酸基等の、分散条件下において異形シリカ微粒子の表面に存在する基と化学結合可能な官能基を有する。ここでの化学結合は、好ましくは、共有結合、イオン結合または配位結合が含まれるが、水素結合も含まれる。配位結合は錯体形成であると考えられる。例えば、ブレンステッドまたはルイスに従う酸塩基反応、錯体形成またはエステル化が、上記表面修飾化合物の官能基と異形シリカ微粒子表面の基の間で生じる。上記(i)の反応性異形シリカ微粒子に用いられる表面修飾化合物は、1種または2種以上を混合して用いることができる。
The surface-modifying compound used for the reactive deformed silica fine particles (i) is a carboxyl group, acid anhydride group, acid chloride group, acid amide group, ester group, imino group, nitrile group, isonitrile group, hydroxyl group, thiol. Groups, epoxy groups, primary, secondary and tertiary amino groups, Si—OH groups, hydrolysable residues of silanes, or C—H acid groups such as β-dicarbonyl compounds It has a functional group capable of chemically bonding with a group present on the surface of the deformed silica fine particle under conditions. The chemical bond here preferably includes a covalent bond, an ionic bond or a coordination bond, but also includes a hydrogen bond. The coordination bond is considered to be complex formation. For example, an acid-base reaction, complex formation or esterification according to Bronsted or Lewis occurs between the functional group of the surface modifying compound and the group on the surface of the deformed silica fine particle. The surface modification compound used for the reactive irregular shaped silica fine particles (i) can be used alone or in combination of two or more.
上記表面修飾化合物は通常、異形シリカ微粒子の表面の基との化学結合に関与できる少なくとも1つの官能基に加えて、この官能基を介して上記表面修飾化合物に結びついた後に、異形シリカ微粒子に新たな特性を付与する分子残基を有する。なお、以下、異形シリカ微粒子の表面の基との化学結合に関与できる少なくとも1つの官能基を第1の官能基という。分子残基またはその一部は疎水性または親水性であり、例えば、異形シリカ微粒子を安定化、融和化、または活性化させる。例えば、疎水性分子残基としては、不活性化または反発作用をもたらす、アルキル、アリール、アルカリル、アラルキルまたはフッ素含有アルキル基等が挙げられる。親水性基としてはヒドロキシ基、アルコキシ基またはポリエステル基等が挙げられる。
The surface-modifying compound is usually added to at least one functional group capable of participating in chemical bonding with the surface group of the irregular-shaped silica fine particle, and after binding to the surface-modifying compound via this functional group, the irregular-shaped silica fine particle is newly added. It has molecular residues that give unique properties. Hereinafter, at least one functional group that can participate in chemical bonding with a group on the surface of the irregular shaped silica fine particle is referred to as a first functional group. The molecular residue or a part thereof is hydrophobic or hydrophilic and, for example, stabilizes, integrates, or activates irregular shaped silica fine particles. For example, the hydrophobic molecular residue includes an alkyl, aryl, alkaryl, aralkyl, or fluorine-containing alkyl group that causes inactivation or repulsion. Examples of the hydrophilic group include a hydroxy group, an alkoxy group, and a polyester group.
反応性異形シリカ微粒子が後述するモノマーと反応できるように表面に導入される反応性官能基は、モノマーに応じて適宜選択される。反応性官能基としては、重合性不飽和基が好適に用いられ、好ましくは光硬化性不飽和基であり、特に好ましくは電離放射線硬化性不飽和基である。その具体例としては、(メタ)アクリロイル基、ビニル基、アリル基等のエチレン性不飽和結合およびエポキシ基等が挙げられる。
The reactive functional group introduced to the surface so that the reactive irregular shaped silica fine particles can react with the monomer described later is appropriately selected according to the monomer. As the reactive functional group, a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group. Specific examples thereof include ethylenically unsaturated bonds such as (meth) acryloyl group, vinyl group and allyl group, and epoxy group.
上記表面修飾化合物の上記分子残基中に、後述するモノマーと反応できる反応性官能基が含まれる場合には、上記表面修飾化合物中に含まれる第1の官能基を異形シリカ微粒子表面に反応させることによって、上記(i)の反応性異形シリカ微粒子の表面にモノマーと反応できる反応性官能基を導入することが可能である。例えば、第1の官能基の他に、さらに重合性不飽和基を有する表面修飾化合物が、好適なものとして挙げられる。
When a reactive functional group capable of reacting with a monomer described later is contained in the molecular residue of the surface modifying compound, the first functional group contained in the surface modifying compound is reacted with the surface of the deformed silica fine particle. By this, it is possible to introduce a reactive functional group capable of reacting with the monomer on the surface of the reactive deformed silica fine particles (i). For example, in addition to the first functional group, a surface modification compound further having a polymerizable unsaturated group is preferable.
一方で、上記表面修飾化合物の上記分子残基中に、第2の反応性官能基を含有させ、当該第2の反応性官能基を足掛かりにして、上記(i)の反応性異形シリカ微粒子の表面にモノマーと反応できる反応性官能基が導入されてもよい。例えば、第2の反応性官能基として水酸基およびオキシ基のような水素結合が可能な水素結合形成基を導入し、この微粒子表面に導入された水素結合形成基に、さらに別の表面修飾化合物の水素結合形成基が反応することにより、モノマーと反応できる反応性官能基を導入することが好ましい。すなわち、表面修飾化合物として、水素結合形成基を有する化合物と、重合性不飽和基等のモノマーと反応できる反応性官能基と水素結合形成基を有する化合物とを併用して用いることが好適な例として挙げられる。水素結合形成基の具体例としては、水酸基、カルボキシル基、エポキシ基、グリシジル基、アミド基といった官能基、もしくはアミド結合を示すものである。ここで、アミド結合とは、-NHC(O)や>NC(O)-を結合単位に含むものを示す旨である。表面修飾化合物に用いられる水素結合形成基としては、中でもカルボキシル基、水酸基、アミド基が好ましい。
On the other hand, in the molecular residue of the surface modification compound, a second reactive functional group is contained, and the second reactive functional group is used as a foothold for the reactive deformed silica fine particles of (i). A reactive functional group capable of reacting with the monomer may be introduced on the surface. For example, a hydrogen bond-forming group capable of hydrogen bonding such as a hydroxyl group and an oxy group is introduced as the second reactive functional group, and another surface modification compound is added to the hydrogen bond-forming group introduced on the surface of the fine particles. It is preferable to introduce a reactive functional group capable of reacting with the monomer by the reaction of the hydrogen bond forming group. That is, as a surface modification compound, it is preferable to use a compound having a hydrogen bond forming group and a compound having a reactive functional group capable of reacting with a monomer such as a polymerizable unsaturated group and a compound having a hydrogen bond forming group in combination. As mentioned. Specific examples of the hydrogen bond-forming group indicate a functional group such as a hydroxyl group, a carboxyl group, an epoxy group, a glycidyl group, an amide group, or an amide bond. Here, the term “amide bond” means that the bond unit contains —NHC (O) or> NC (O) —. Among the hydrogen bond forming groups used in the surface modification compound, a carboxyl group, a hydroxyl group, and an amide group are particularly preferable.
上記(i)の反応性異形シリカ微粒子に用いられる上記表面修飾化合物は500以下の分子量、中でも400を超えない分子量、特に200を超えない分子量を有することが好ましい。このような低分子量を有するため、シリカ微粒子表面を急速に占有し、反応性異形シリカ微粒子同士の凝集を妨げることが可能であると推定される。
The surface-modifying compound used in the reactive irregularly shaped silica fine particles (i) above preferably has a molecular weight of 500 or less, particularly a molecular weight not exceeding 400, and particularly a molecular weight not exceeding 200. Since it has such a low molecular weight, it is presumed that the surface of the silica fine particles can be rapidly occupied and aggregation of the reactive irregular shaped silica fine particles can be prevented.
上記(i)の反応性異形シリカ微粒子に用いられる上記表面修飾化合物は、表面修飾のための反応条件下で好ましくは液体であり、分散媒中で溶解性または少なくとも乳化可能であるのが好ましい。中でも分散媒中で溶解し、分散媒中で離散した分子または分子イオンとして一様に分布して存在することが好ましい。
The surface-modifying compound used in the reactive deformed silica fine particles (i) is preferably a liquid under the reaction conditions for surface modification, and is preferably soluble or at least emulsifiable in a dispersion medium. Among these, it is preferable that the polymer is dissolved in the dispersion medium and uniformly distributed as discrete molecules or molecular ions in the dispersion medium.
飽和または不飽和カルボン酸としては、1~24の炭素原子を有しており、例えば、ギ酸、酢酸、プロピオン酸、酪酸、吉草酸、カプロン酸、アクリル酸、メタクリル酸、クロトン酸、クエン酸、アジピン酸、琥珀酸、グルタル酸、シュウ酸、マレイン酸、フマル酸、イタコン酸およびステアリン酸、ならびに対応する酸無水物、塩化物、エステルおよびアミド、例えば、カプロラクタム等が挙げられる。また、不飽和カルボン酸を用いると、重合性不飽和基を導入することができる。
Saturated or unsaturated carboxylic acids have 1 to 24 carbon atoms, such as formic acid, acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, acrylic acid, methacrylic acid, crotonic acid, citric acid, Examples include adipic acid, succinic acid, glutaric acid, oxalic acid, maleic acid, fumaric acid, itaconic acid and stearic acid, and the corresponding acid anhydrides, chlorides, esters and amides such as caprolactam. Further, when an unsaturated carboxylic acid is used, a polymerizable unsaturated group can be introduced.
好ましいアミンの例は、下記化学式を有するものである。
Q3-nNHn
上記式において、nは0,1または2である。残基Qは独立して、1~12、中でも1~6、特に好ましくは1~4の炭素原子を有するアルキル、例えば、メチル、エチル、n-プロピル、i-プロピルおよびブチル、ならびに6~24の炭素原子を有するアリール、アルカリルまたはアラルキル、例えば、フェニル、ナフチル、トリルおよびベンジルを表す。また、好ましいアミンの例としては、ポリアルキレンアミンが挙げられ、具体例は、メチルアミン、ジメチルアミン、トリメチルアミン、エチルアミン、アニリン、N-メチルアニリン、ジフェニルアミン、トリフェニルアミン、トルイジン、エチレンジアミン、ジエチレントリアミンである。 Examples of preferred amines are those having the following chemical formula:
Q 3-n NH n
In the above formula, n is 0, 1 or 2. Residue Q is independently alkyl having 1 to 12, especially 1 to 6, particularly preferably 1 to 4, carbon atoms such as methyl, ethyl, n-propyl, i-propyl and butyl, and 6 to 24 Represents aryl, alkaryl or aralkyl having 5 carbon atoms such as phenyl, naphthyl, tolyl and benzyl. Examples of preferred amines include polyalkyleneamines, and specific examples are methylamine, dimethylamine, trimethylamine, ethylamine, aniline, N-methylaniline, diphenylamine, triphenylamine, toluidine, ethylenediamine, and diethylenetriamine. .
Q3-nNHn
上記式において、nは0,1または2である。残基Qは独立して、1~12、中でも1~6、特に好ましくは1~4の炭素原子を有するアルキル、例えば、メチル、エチル、n-プロピル、i-プロピルおよびブチル、ならびに6~24の炭素原子を有するアリール、アルカリルまたはアラルキル、例えば、フェニル、ナフチル、トリルおよびベンジルを表す。また、好ましいアミンの例としては、ポリアルキレンアミンが挙げられ、具体例は、メチルアミン、ジメチルアミン、トリメチルアミン、エチルアミン、アニリン、N-メチルアニリン、ジフェニルアミン、トリフェニルアミン、トルイジン、エチレンジアミン、ジエチレントリアミンである。 Examples of preferred amines are those having the following chemical formula:
Q 3-n NH n
In the above formula, n is 0, 1 or 2. Residue Q is independently alkyl having 1 to 12, especially 1 to 6, particularly preferably 1 to 4, carbon atoms such as methyl, ethyl, n-propyl, i-propyl and butyl, and 6 to 24 Represents aryl, alkaryl or aralkyl having 5 carbon atoms such as phenyl, naphthyl, tolyl and benzyl. Examples of preferred amines include polyalkyleneamines, and specific examples are methylamine, dimethylamine, trimethylamine, ethylamine, aniline, N-methylaniline, diphenylamine, triphenylamine, toluidine, ethylenediamine, and diethylenetriamine. .
好ましいβ-ジカルボニル化合物は4~12、特に5~8の炭素原子を有するものであり、例えば、アセチルアセトン等のジケトン、2,3-ヘキサンジオン、3,5-ヘプタンジオン、アセト酢酸、アセト酢酸エチルエステル等のアセト酢酸-C1-C4-アルキルエステル、ジアセチルおよびアセトニルアセトンが挙げられる。
アミノ酸の例としては、β-アラニン、グリシン、バリン、アミノカプロン酸、ロイシンおよびイソロイシンが挙げられる。 Preferred β-dicarbonyl compounds are those having 4 to 12, particularly 5 to 8 carbon atoms, such as diketones such as acetylacetone, 2,3-hexanedione, 3,5-heptanedione, acetoacetate, acetoacetate Acetoacetic acid-C 1 -C 4 -alkyl esters such as ethyl esters, diacetyl and acetonyl acetone.
Examples of amino acids include β-alanine, glycine, valine, aminocaproic acid, leucine and isoleucine.
アミノ酸の例としては、β-アラニン、グリシン、バリン、アミノカプロン酸、ロイシンおよびイソロイシンが挙げられる。 Preferred β-dicarbonyl compounds are those having 4 to 12, particularly 5 to 8 carbon atoms, such as diketones such as acetylacetone, 2,3-hexanedione, 3,5-heptanedione, acetoacetate, acetoacetate Acetoacetic acid-C 1 -C 4 -alkyl esters such as ethyl esters, diacetyl and acetonyl acetone.
Examples of amino acids include β-alanine, glycine, valine, aminocaproic acid, leucine and isoleucine.
好ましいシランは、少なくとも1つの加水分解性基またはヒドロキシ基と、少なくとも1つの非加水分解性残基を有する加水分解性オルガノシランである。ここで加水分解性基としては、例えば、ハロゲン、アルコキシ基およびアシルオキシ基が挙げられる。非加水分解性残基としては、反応性官能基を有するまたは反応性官能基を有しない非加水分解性残基が用いられる。また、フッ素で置換されている有機残基を少なくとも部分的に有するシランを使用してもよい。
Preferred silanes are hydrolyzable organosilanes having at least one hydrolyzable group or hydroxy group and at least one non-hydrolyzable residue. Here, examples of the hydrolyzable group include a halogen, an alkoxy group, and an acyloxy group. As the non-hydrolyzable residue, a non-hydrolyzable residue having a reactive functional group or not having a reactive functional group is used. Silanes having at least partially organic residues substituted with fluorine may also be used.
用いられるシランとしては特に限定されないが、例えば、CH2=CHSi(OOCCH3)3、CH2=CHSiCl3、CH2=CHSi(OC2H5)3、CH2=CH-Si(OC2H4OCH3)3、CH2=CH-CH2-Si(OC2H5)3、CH2=CH-CH2-Si(OOCCH3)3、γ-グリシジルオキシプロピルトリメトキシシラン(GPTS)、γ-グリシジルオキシプロピルジメチルクロロシラン、3-アミノプロピルトリメトキシシラン(APTS)、3-アミノプロピルトリエトキシシラン(APTES)、N-(2-アミノエチル)-3-アミノプロピルトリメトキシシラン、N-[N′-(2′-アミノエチル)-2-アミノエチル]-3-アミノプロピルトリメトキシシラン、ヒドロキシメチルトリメトキシシラン、2-[メトキシ(ポリエチレンオキシ)プロピル]トリメトキシシラン、ビス-(ヒドロキシエチル)-3-アミノプロピルトリエトキシシラン、N-ヒドロキシエチル-N-メチルアミノプロピルトリエトキシシラン、3-(メタ)アクリルオキシプロピルトリエトキシシランおよび3-(メタ)アクリルオキシプロピルトリメトキシシラン等を挙げることができる。
As the silane used is not particularly limited, for example, CH 2 = CHSi (OOCCH 3 ) 3, CH 2 = CHSiCl 3, CH 2 = CHSi (OC 2 H 5) 3, CH 2 = CHSi (OC 2 H 4 OCH 3 ) 3 , CH 2 ═CH—CH 2 —Si (OC 2 H 5 ) 3 , CH 2 ═CH—CH 2 —Si (OOCCH 3 ) 3 , γ-glycidyloxypropyltrimethoxysilane (GPTS), γ-glycidyloxypropyldimethylchlorosilane, 3-aminopropyltrimethoxysilane (APTS), 3-aminopropyltriethoxysilane (APTES), N- (2-aminoethyl) -3-aminopropyltrimethoxysilane, N- [ N ′-(2′-Aminoethyl) -2-aminoethyl] -3-aminopropyl trimer Xysilane, hydroxymethyltrimethoxysilane, 2- [methoxy (polyethyleneoxy) propyl] trimethoxysilane, bis- (hydroxyethyl) -3-aminopropyltriethoxysilane, N-hydroxyethyl-N-methylaminopropyltriethoxysilane , 3- (meth) acryloxypropyltriethoxysilane, 3- (meth) acryloxypropyltrimethoxysilane, and the like.
上記シランカップリング剤としては、特に限定されず、公知のものを挙げることができ、例えば、信越化学工業(株)製のKBM-502、KBM-503、KBE-502、KBE-503等を挙げることができる。
The silane coupling agent is not particularly limited, and may include known ones such as KBM-502, KBM-503, KBE-502, KBE-503 manufactured by Shin-Etsu Chemical Co., Ltd. be able to.
官能基を有する金属化合物としては、元素周期表の第1群III~Vおよび第2群II~IVの少なくともいずれかからの金属Mの金属化合物が挙げられる。具体的には、下記化学式で表されるジルコニウムおよびチタニウムのアルコキシドが挙げられる。
M(OR)4
上記式において、MはTi、Zrである。OR基の一部はβ-ジカルボニル化合物またはモノカルボン酸等の錯生成剤により置換される。メタクリル酸等の重合性不飽和基を有する化合物が錯生成剤として使用される場合には、重合性不飽和基を導入することができる。 Examples of the metal compound having a functional group include a metal compound of metal M from at least one of the first group III to V and the second group II to IV of the periodic table. Specific examples include zirconium and titanium alkoxides represented by the following chemical formula.
M (OR) 4
In the above formula, M is Ti or Zr. A part of the OR group is substituted with a complexing agent such as a β-dicarbonyl compound or a monocarboxylic acid. When a compound having a polymerizable unsaturated group such as methacrylic acid is used as a complexing agent, a polymerizable unsaturated group can be introduced.
M(OR)4
上記式において、MはTi、Zrである。OR基の一部はβ-ジカルボニル化合物またはモノカルボン酸等の錯生成剤により置換される。メタクリル酸等の重合性不飽和基を有する化合物が錯生成剤として使用される場合には、重合性不飽和基を導入することができる。 Examples of the metal compound having a functional group include a metal compound of metal M from at least one of the first group III to V and the second group II to IV of the periodic table. Specific examples include zirconium and titanium alkoxides represented by the following chemical formula.
M (OR) 4
In the above formula, M is Ti or Zr. A part of the OR group is substituted with a complexing agent such as a β-dicarbonyl compound or a monocarboxylic acid. When a compound having a polymerizable unsaturated group such as methacrylic acid is used as a complexing agent, a polymerizable unsaturated group can be introduced.
分散媒として、水および有機溶媒の少なくともいずれかが好適に使用される。特に好ましい分散媒は、蒸留された純粋な水である。有機溶媒として、極性、非極性および非プロトン性溶媒が好ましい。それらの例として、メタノール、エタノール、n-およびi-プロパノールおよびブタノール等の炭素数1~6の脂肪族アルコール等のアルコール、メチルエチルケトン、ジエチルケトン、メチルイソブチルケトン、アセトンおよびブタノン等のケトン類、酢酸エチル等のエステル類;ジエチルエーテル、テトラヒドロフランおよびテトラヒドロピラン等のエーテル類;ジメチルアセトアミド、ジメチルホルムアミド等のアミド類;スルホランおよびジメチルスルホキシド等のスルホキシド類およびスルホン類;およびペンタン、ヘキサンおよびシクロヘキサン等の任意にハロゲン化されていてもよい脂肪族炭化水素類が挙げられる。これらの分散媒は混合物として使用することができる。
分散媒は、蒸留、任意には減圧下により容易に除去できる沸点を有することが好ましく、沸点が200℃以下、特に150℃以下の溶媒が好ましい。 As the dispersion medium, at least one of water and an organic solvent is preferably used. A particularly preferred dispersion medium is distilled pure water. As the organic solvent, polar, nonpolar and aprotic solvents are preferred. Examples thereof include alcohols such as C 1-6 aliphatic alcohols such as methanol, ethanol, n- and i-propanol and butanol, ketones such as methyl ethyl ketone, diethyl ketone, methyl isobutyl ketone, acetone and butanone, acetic acid Esters such as ethyl; ethers such as diethyl ether, tetrahydrofuran and tetrahydropyran; amides such as dimethylacetamide and dimethylformamide; sulfoxides and sulfones such as sulfolane and dimethyl sulfoxide; and optionally, such as pentane, hexane and cyclohexane Aliphatic hydrocarbons which may be halogenated are mentioned. These dispersion media can be used as a mixture.
The dispersion medium preferably has a boiling point that can be easily removed by distillation, optionally under reduced pressure, and a solvent having a boiling point of 200 ° C. or lower, particularly 150 ° C. or lower is preferable.
分散媒は、蒸留、任意には減圧下により容易に除去できる沸点を有することが好ましく、沸点が200℃以下、特に150℃以下の溶媒が好ましい。 As the dispersion medium, at least one of water and an organic solvent is preferably used. A particularly preferred dispersion medium is distilled pure water. As the organic solvent, polar, nonpolar and aprotic solvents are preferred. Examples thereof include alcohols such as C 1-6 aliphatic alcohols such as methanol, ethanol, n- and i-propanol and butanol, ketones such as methyl ethyl ketone, diethyl ketone, methyl isobutyl ketone, acetone and butanone, acetic acid Esters such as ethyl; ethers such as diethyl ether, tetrahydrofuran and tetrahydropyran; amides such as dimethylacetamide and dimethylformamide; sulfoxides and sulfones such as sulfolane and dimethyl sulfoxide; and optionally, such as pentane, hexane and cyclohexane Aliphatic hydrocarbons which may be halogenated are mentioned. These dispersion media can be used as a mixture.
The dispersion medium preferably has a boiling point that can be easily removed by distillation, optionally under reduced pressure, and a solvent having a boiling point of 200 ° C. or lower, particularly 150 ° C. or lower is preferable.
(i)の反応性異形シリカ微粒子の調製に際し、分散媒の濃度は、通常40質量%~90質量%の範囲内、好ましくは50質量%~80質量%の範囲内、特に55質量%~75質量%の範囲内である。分散液の残りは、未処理のシリカ微粒子および上記表面修飾化合物から構成される。ここで、シリカ微粒子:表面修飾化合物の重量比は、100:1~4:1とすることが好ましく、中でも50:1~8:1、特に25:1~10:1とすることが好ましい。
In preparing the reactive deformed silica fine particles (i), the concentration of the dispersion medium is usually within the range of 40% by mass to 90% by mass, preferably within the range of 50% by mass to 80% by mass, and particularly 55% by mass to 75%. It is in the range of mass%. The rest of the dispersion is composed of untreated silica fine particles and the surface modifying compound. Here, the weight ratio of silica fine particles: surface modification compound is preferably 100: 1 to 4: 1, more preferably 50: 1 to 8: 1, and particularly preferably 25: 1 to 10: 1.
(i)の反応性異形シリカ微粒子の調製は、好ましくは20℃程度の室温から分散媒の沸点までの温度範囲で行われる。特に好ましくは、分散温度は50℃~100℃である。分散時間は、特に使用される材料のタイプに依存するが、一般に数分から数時間、例えば、1時間~24時間である。
Preparation of the reactive deformed silica fine particles (i) is preferably carried out in a temperature range from room temperature of about 20 ° C. to the boiling point of the dispersion medium. Particularly preferably, the dispersion temperature is 50 ° C. to 100 ° C. The dispersion time depends in particular on the type of material used, but is generally from a few minutes to a few hours, for example 1 to 24 hours.
(ii)被覆前の異形シリカ微粒子に導入する反応性官能基、下記化学式(1)に示す基、およびシラノール基または加水分解によってシラノール基を生成する基を含む化合物と、コアとなるシリカ微粒子としての金属酸化物微粒子とを結合することにより得られる、表面に反応性官能基を有する反応性異形シリカ微粒子。
-Q1-C(=Q2)-Q3- (1)
(式(1)中、Q1はNH、OまたはSを示し、Q2はOまたはSを示し、Q3はNHまたは2価以上の有機基を示す。)
上記(ii)の反応性異形シリカ微粒子を用いる場合には、有機成分量が高まり、分散性、およびハードコート層の強度がより高まるという利点がある。 (Ii) a reactive functional group to be introduced into the irregular shaped silica fine particles before coating, a compound represented by the following chemical formula (1), and a silanol group or a group containing a group that generates a silanol group by hydrolysis, and silica fine particles as a core Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by bonding with metal oxide fine particles.
-Q 1 -C (= Q 2 ) -Q 3- (1)
(In formula (1), Q 1 represents NH, O or S, Q 2 represents O or S, and Q 3 represents NH or a divalent or higher organic group.)
When the reactive irregularly shaped silica fine particles (ii) are used, there are advantages that the amount of organic components is increased, and the dispersibility and the strength of the hard coat layer are further increased.
-Q1-C(=Q2)-Q3- (1)
(式(1)中、Q1はNH、OまたはSを示し、Q2はOまたはSを示し、Q3はNHまたは2価以上の有機基を示す。)
上記(ii)の反応性異形シリカ微粒子を用いる場合には、有機成分量が高まり、分散性、およびハードコート層の強度がより高まるという利点がある。 (Ii) a reactive functional group to be introduced into the irregular shaped silica fine particles before coating, a compound represented by the following chemical formula (1), and a silanol group or a group containing a group that generates a silanol group by hydrolysis, and silica fine particles as a core Reactive deformed silica fine particles having a reactive functional group on the surface, obtained by bonding with metal oxide fine particles.
-Q 1 -C (= Q 2 ) -Q 3- (1)
(In formula (1), Q 1 represents NH, O or S, Q 2 represents O or S, and Q 3 represents NH or a divalent or higher organic group.)
When the reactive irregularly shaped silica fine particles (ii) are used, there are advantages that the amount of organic components is increased, and the dispersibility and the strength of the hard coat layer are further increased.
まず、被覆前のシリカ微粒子に導入したい反応性官能基、上記化学式(1)に示す基、およびシラノール基または加水分解によってシラノール基を生成する基を含む化合物について説明する。なお、以下、上記化合物を反応性官能基修飾加水分解性シランという場合がある。
上記反応性官能基修飾加水分解性シランにおいて、シリカ微粒子に導入したい反応性官能基は、後述するモノマーと反応可能なように適宜選択すれば特に限定されない。上述したような重合性不飽和基を導入するのに適している。 First, a compound containing a reactive functional group to be introduced into the silica fine particles before coating, a group represented by the chemical formula (1), and a silanol group or a group that generates a silanol group by hydrolysis will be described. Hereinafter, the compound may be referred to as a reactive functional group-modified hydrolyzable silane.
In the reactive functional group-modified hydrolyzable silane, the reactive functional group to be introduced into the silica fine particles is not particularly limited as long as it is appropriately selected so as to be capable of reacting with a monomer described later. Suitable for introducing polymerizable unsaturated groups as described above.
上記反応性官能基修飾加水分解性シランにおいて、シリカ微粒子に導入したい反応性官能基は、後述するモノマーと反応可能なように適宜選択すれば特に限定されない。上述したような重合性不飽和基を導入するのに適している。 First, a compound containing a reactive functional group to be introduced into the silica fine particles before coating, a group represented by the chemical formula (1), and a silanol group or a group that generates a silanol group by hydrolysis will be described. Hereinafter, the compound may be referred to as a reactive functional group-modified hydrolyzable silane.
In the reactive functional group-modified hydrolyzable silane, the reactive functional group to be introduced into the silica fine particles is not particularly limited as long as it is appropriately selected so as to be capable of reacting with a monomer described later. Suitable for introducing polymerizable unsaturated groups as described above.
上記反応性官能基修飾加水分解性シランにおいて、上記化学式(1)に示す基の[-Q1-C(=Q2)-]部分は、具体的には、[-O-C(=O)-]、[-O-C(=S)-]、[-S-C(=O)-]、[-NH-C(=O)-]、[-NH-C(=S)-]、および[-S-C(=S)-]の6種である。これらの基は、1種単独でまたは2種以上を組み合わせて用いることができる。中でも、熱安定性の観点から、[-O-C(=O)-]基と、[-O-C(=S)-]基および[-S-C(=O)-]基の少なくとも1種を併用することが好ましい。上記式(1)に示す基[-Q1-C(=Q2)-Q3-]は、分子間において水素結合による適度の凝集力を発生させ、硬化物にした場合、優れた機械的強度、基板との密着性および耐熱性等の特性を付与することが可能になると考えられる。
In the reactive functional group-modified hydrolyzable silane, the [—Q 1 —C (═Q 2 ) —] moiety of the group represented by the chemical formula (1) is specifically [—O—C (═O )-], [—O—C (═S) —], [—S—C (═O) —], [—NH—C (═O) —], [—NH—C (═S) — ] And [-SC (= S)-]. These groups can be used alone or in combination of two or more. Among them, from the viewpoint of thermal stability, at least one of a [—O—C (═O) —] group, a [—O—C (═S) —] group, and a [—S—C (═O) —] group It is preferable to use one type in combination. The group [—Q 1 —C (= Q 2 ) —Q 3 —] represented by the above formula (1) generates an appropriate cohesive force due to hydrogen bonding between molecules, and has excellent mechanical properties when formed into a cured product. It is considered that properties such as strength, adhesion to the substrate, and heat resistance can be imparted.
また、加水分解によってシラノ-ル基を生成する基としては、ケイ素原子上にアルコキシ基、アリールオキシ基、アセトキシ基、アミノ基、ハロゲン原子等を有する基を挙げることができ、アルコキシシリル基またはアリールオキシシリル基が好ましい。シラノール基または、加水分解によってシラノ-ル基を生成する基は、縮合反応または加水分解に続いて生じる縮合反応によって、金属酸化物微粒子と結合することができる。
Examples of the group that generates a silanol group by hydrolysis include groups having an alkoxy group, an aryloxy group, an acetoxy group, an amino group, a halogen atom, etc. on the silicon atom. An oxysilyl group is preferred. A silanol group or a group that generates a silanol group by hydrolysis can be bonded to the metal oxide fine particles by a condensation reaction or a condensation reaction that occurs following hydrolysis.
上記反応性官能基修飾加水分解性シランの好ましい具体例としては、例えば、下記化学式(2)および(3)に示す化合物を挙げることができ、下記化学式(3)に示す化合物が硬度の点からより好ましく用いられる。
Preferable specific examples of the reactive functional group-modified hydrolyzable silane include, for example, compounds represented by the following chemical formulas (2) and (3), and the compound represented by the following chemical formula (3) is from the point of hardness. More preferably used.
上記式(2)および(3)中、Ra、Rbは同一でも異なっていてもよいが、水素原子またはC1からC8のアルキル基もしくはアリール基であり、例えば、メチル、エチル、プロピル、ブチル、オクチル、フェニル、キシリル基等を挙げることができる。ここでmは1、2または3である。
[(RaO)mRb 3-mSi-]で示される基としては、例えば、トリメトキシシリル基、トリエトキシシリル基、トリフェノキシシリル基、メチルジメトキシシリル基、ジメチルメトキシシリル基等を挙げることができる。このような基のうち、トリメトキシシリル基またはトリエトキシシリル基等が好ましい。 In the above formulas (2) and (3), R a and R b may be the same or different, but are a hydrogen atom or a C 1 to C 8 alkyl group or aryl group, for example, methyl, ethyl, propyl , Butyl, octyl, phenyl, xylyl group and the like. Here, m is 1, 2 or 3.
Examples of the group represented by [(R a O) m R b 3-m Si—] include a trimethoxysilyl group, a triethoxysilyl group, a triphenoxysilyl group, a methyldimethoxysilyl group, a dimethylmethoxysilyl group, and the like. Can be mentioned. Of these groups, a trimethoxysilyl group or a triethoxysilyl group is preferable.
[(RaO)mRb 3-mSi-]で示される基としては、例えば、トリメトキシシリル基、トリエトキシシリル基、トリフェノキシシリル基、メチルジメトキシシリル基、ジメチルメトキシシリル基等を挙げることができる。このような基のうち、トリメトキシシリル基またはトリエトキシシリル基等が好ましい。 In the above formulas (2) and (3), R a and R b may be the same or different, but are a hydrogen atom or a C 1 to C 8 alkyl group or aryl group, for example, methyl, ethyl, propyl , Butyl, octyl, phenyl, xylyl group and the like. Here, m is 1, 2 or 3.
Examples of the group represented by [(R a O) m R b 3-m Si—] include a trimethoxysilyl group, a triethoxysilyl group, a triphenoxysilyl group, a methyldimethoxysilyl group, a dimethylmethoxysilyl group, and the like. Can be mentioned. Of these groups, a trimethoxysilyl group or a triethoxysilyl group is preferable.
式(2)および(3)中、RcはC1からC12の脂肪族または芳香族構造を有する2価の有機基であり、鎖状、分岐状または環状の構造を含んでいてもよい。そのような有機基としては、例えば、メチレン、エチレン、プロピレン、ブチレン、ヘキサメチレン、シクロヘキシレン、フェニレン、キシリレン、ドデカメチレン等を挙げることができる。これらのうち好ましい例は、メチレン、プロピレン、シクロヘキシレン、フェニレン等である。
In formulas (2) and (3), R c is a divalent organic group having a C 1 to C 12 aliphatic or aromatic structure, and may contain a chain, branched or cyclic structure. . Examples of such an organic group include methylene, ethylene, propylene, butylene, hexamethylene, cyclohexylene, phenylene, xylylene, and dodecamethylene. Among these, preferred examples are methylene, propylene, cyclohexylene, phenylene and the like.
式(2)中、Rdは2価の有機基であり、通常、分子量14~10,000、好ましくは、分子量76~500の2価の有機基の中から選ばれる。例えば、ヘキサメチレン、オクタメチレン、ドデカメチレン等の鎖状ポリアルキレン基;シクロヘキシレン、ノルボルニレン等の脂環式または多環式の2価の有機基;フェニレン、ナフチレン、ビフェニレン、ポリフェニレン等の2価の芳香族基;およびこれらのアルキル基置換体、アリール基置換体を挙げることができる。また、これら2価の有機基は炭素および水素原子以外の元素を含む原子団を含んでいてもよく、ポリエーテル結合、ポリエステル結合、ポリアミド結合、ポリカーボネート結合、さらには上記式(1)に示す基を含むこともできる。
In the formula (2), R d is a divalent organic group and is usually selected from divalent organic groups having a molecular weight of 14 to 10,000, preferably a molecular weight of 76 to 500. For example, chain polyalkylene groups such as hexamethylene, octamethylene, dodecamethylene; alicyclic or polycyclic divalent organic groups such as cyclohexylene and norbornylene; divalent groups such as phenylene, naphthylene, biphenylene and polyphenylene An aromatic group; and these alkyl group-substituted and aryl group-substituted products. Further, these divalent organic groups may contain an atomic group containing an element other than carbon and hydrogen atoms, and include a polyether bond, a polyester bond, a polyamide bond, a polycarbonate bond, and a group represented by the above formula (1). Can also be included.
式(2)および(3)中、Reは(n+1)価の有機基であり、好ましくは鎖状、分岐状または環状の飽和炭化水素基、不飽和炭化水素基の中から選ばれる。
In the formulas (2) and (3), R e is an (n + 1) valent organic group, preferably selected from a chain, branched or cyclic saturated hydrocarbon group and unsaturated hydrocarbon group.
式(2)および(3)中、Y′は反応性官能基を有する1価の有機基を示す。上述のような反応性官能基そのものであってもよい。例えば、反応性官能基を重合性不飽和基から選択する場合、(メタ)アクリロイル(オキシ)基、ビニル(オキシ)基、プロペニル(オキシ)基、ブタジエニル(オキシ)基、スチリル(オキシ)基、エチニル(オキシ)基、シンナモイル(オキシ)基、マレエート基、(メタ)アクリルアミド基等を挙げることができる。また、nは好ましくは1~20の正の整数であり、さらに好ましくは1~10、特に好ましくは1~5である。
In formulas (2) and (3), Y ′ represents a monovalent organic group having a reactive functional group. The reactive functional group itself as described above may be used. For example, when the reactive functional group is selected from a polymerizable unsaturated group, (meth) acryloyl (oxy) group, vinyl (oxy) group, propenyl (oxy) group, butadienyl (oxy) group, styryl (oxy) group, Examples include ethynyl (oxy) group, cinnamoyl (oxy) group, maleate group, (meth) acrylamide group and the like. N is preferably a positive integer of 1 to 20, more preferably 1 to 10, and particularly preferably 1 to 5.
反応性官能基修飾加水分解性シランの合成は、例えば、特開平9-100111号公報に記載された方法を用いることができる。すなわち、例えば、重合性不飽和基を導入したい場合、(A)メルカプトアルコキシシランと、ポリイソシアネート化合物と、イソシアネート基と反応可能な活性水素基含有重合性不飽和化合物との付加反応により行うことができる。また、(B)分子中にアルコキシシリル基およびイソシアネート基を有する化合物と、活性水素基含有重合性不飽和化合物との直接的反応により行うことができる。さらに、(C)分子中に重合性不飽和基およびイソシアネート基を有する化合物と、メルカプトアルコキシシランまたはアミノシランとの付加反応により直接合成することもできる。
For the synthesis of the reactive functional group-modified hydrolyzable silane, for example, the method described in JP-A-9-100111 can be used. That is, for example, when it is desired to introduce a polymerizable unsaturated group, (A) an addition reaction between a mercaptoalkoxysilane, a polyisocyanate compound, and an active hydrogen group-containing polymerizable unsaturated compound capable of reacting with an isocyanate group is performed. it can. Moreover, it can carry out by (B) direct reaction with the compound which has an alkoxy silyl group and an isocyanate group in a molecule | numerator, and an active hydrogen group containing polymerizable unsaturated compound. Furthermore, it can also be directly synthesized by addition reaction of (C) a compound having a polymerizable unsaturated group and an isocyanate group in the molecule with mercaptoalkoxysilane or aminosilane.
(ii)の反応性異形シリカ微粒子の製造においては、反応性官能基修飾加水分解性シランを別途加水分解操作を行った後、これと異形シリカ微粒子を混合し、加熱、攪拌操作を行う方法、もしくは反応性官能基修飾加水分解性シランの加水分解を異形シリカ微粒子の存在下に行う方法、また、他の成分、例えば、多価不飽和有機化合物、単価不飽和有機化合物、放射線重合開始剤等の存在下、異形シリカ微粒子の表面処理を行う方法を選ぶことができるが、反応性官能基修飾加水分解性シランの加水分解を異形シリカ微粒子の存在下行う方法が好ましい。(ii)の反応性異形シリカ微粒子を製造する際、その温度は、通常20℃以上150℃以下であり、また処理時間は5分~24時間の範囲である。
In the production of the reactive deformed silica fine particles of (ii), after separately hydrolyzing the reactive functional group-modified hydrolyzable silane, this is mixed with the deformed silica fine particles, followed by heating and stirring operation, Alternatively, a method of hydrolyzing a reactive functional group-modified hydrolyzable silane in the presence of deformed silica fine particles, and other components such as polyunsaturated organic compounds, monounsaturated organic compounds, radiation polymerization initiators, etc. Although the method of performing the surface treatment of the deformed silica fine particles in the presence of can be selected, a method of hydrolyzing the reactive functional group-modified hydrolyzable silane in the presence of the deformed silica fine particles is preferable. When the reactive deformed silica fine particles (ii) are produced, the temperature is usually 20 ° C. or higher and 150 ° C. or lower, and the treatment time is in the range of 5 minutes to 24 hours.
加水分解反応を促進するため、触媒として酸、塩もしくは塩基を添加してもよい。酸としては有機酸および不飽和有機酸;塩基としては3級アミンまたは4級アンモニウムヒドロキシドが好適な物として挙げられる。これら酸もしくは塩基触媒の添加量は反応性官能基修飾加水分解性シランに対して0.001質量%~1.0質量%の範囲内、好ましくは0.01質量%~0.1質量%の範囲内である。
In order to accelerate the hydrolysis reaction, an acid, salt or base may be added as a catalyst. Preferable examples of the acid include organic acids and unsaturated organic acids; examples of the base include tertiary amines or quaternary ammonium hydroxides. The addition amount of these acid or base catalysts is within the range of 0.001% by mass to 1.0% by mass, preferably 0.01% by mass to 0.1% by mass with respect to the reactive functional group-modified hydrolyzable silane. Within range.
反応性異形シリカ微粒子としては、分散媒を含有しない粉末状の微粒子を用いてもよいが、分散工程を省略でき、生産性が高い点から微粒子を溶剤分散ゾルとしたものを用いることが好ましい。
As the reactive irregular shaped silica fine particles, powdery fine particles not containing a dispersion medium may be used. However, it is preferable to use a fine particle in a solvent-dispersed sol because the dispersion step can be omitted and the productivity is high.
なお、ハードコート層は、反応性異形シリカ微粒子が有する反応性官能基が反応したものだけでなく、反応性異形シリカ微粒子の反応性官能基が反応していないものを含んでいてもよい。
The hard coat layer may include not only those in which the reactive functional group of the reactive irregular shaped silica fine particles has reacted but also those in which the reactive functional group of the reactive irregular shaped silica fine particles has not reacted.
反応性異形シリカ微粒子の含有量は、硬化性樹脂組成物の全固形分に対して40質量%~70質量%の範囲内であることが好ましく、50質量%~60質量%の範囲内であることがより好ましい。含有量が少ないとハードコート層に十分な硬度を付与できないおそれがある。含有量が多いと、充填率が上がり過ぎ、反応性異形シリカ微粒子とモノマーとの密着性が悪化し、かえってハードコート層の硬度を低下させてしまうおそれがある。
ここで、固形分とは、硬化性樹脂組成物中に含まれる成分のうち溶剤以外のものを意味する。 The content of the reactive irregularly shaped silica fine particles is preferably in the range of 40% by mass to 70% by mass, and in the range of 50% by mass to 60% by mass with respect to the total solid content of the curable resin composition. It is more preferable. When the content is small, there is a possibility that sufficient hardness cannot be imparted to the hard coat layer. When the content is large, the filling rate is excessively increased, the adhesion between the reactive irregularly shaped silica fine particles and the monomer is deteriorated, and the hardness of the hard coat layer may be lowered.
Here, solid content means things other than a solvent among the components contained in curable resin composition.
ここで、固形分とは、硬化性樹脂組成物中に含まれる成分のうち溶剤以外のものを意味する。 The content of the reactive irregularly shaped silica fine particles is preferably in the range of 40% by mass to 70% by mass, and in the range of 50% by mass to 60% by mass with respect to the total solid content of the curable resin composition. It is more preferable. When the content is small, there is a possibility that sufficient hardness cannot be imparted to the hard coat layer. When the content is large, the filling rate is excessively increased, the adhesion between the reactive irregularly shaped silica fine particles and the monomer is deteriorated, and the hardness of the hard coat layer may be lowered.
Here, solid content means things other than a solvent among the components contained in curable resin composition.
(b)アクリル系ポリマー
本発明に用いられるアクリル系ポリマーは、重量平均分子量およびアクリル当量が所定の範囲内であり、ハードコートフィルムの加工性向上に寄与する成分である。 (B) Acrylic polymer The acrylic polymer used in the present invention has a weight average molecular weight and an acrylic equivalent within predetermined ranges, and is a component that contributes to improving the workability of the hard coat film.
本発明に用いられるアクリル系ポリマーは、重量平均分子量およびアクリル当量が所定の範囲内であり、ハードコートフィルムの加工性向上に寄与する成分である。 (B) Acrylic polymer The acrylic polymer used in the present invention has a weight average molecular weight and an acrylic equivalent within predetermined ranges, and is a component that contributes to improving the workability of the hard coat film.
アクリル系ポリマーの重量平均分子量は、ハードコート層に柔軟性を与え、加工時にクラックを防止する点から、30,000~110,000の範囲内であり、中でも50,000~110,000の範囲内であることが好ましく、特に60,000~80,000の範囲内であることが好ましい。
ここで、重量平均分子量とは、ゲル浸透クロマトグラフィーにより測定したポリスチレン換算値である重量平均分子量をいう。 The weight average molecular weight of the acrylic polymer is in the range of 30,000 to 110,000, particularly in the range of 50,000 to 110,000, from the viewpoint of imparting flexibility to the hard coat layer and preventing cracks during processing. It is preferably within the range of 60,000 to 80,000.
Here, the weight average molecular weight refers to a weight average molecular weight which is a polystyrene conversion value measured by gel permeation chromatography.
ここで、重量平均分子量とは、ゲル浸透クロマトグラフィーにより測定したポリスチレン換算値である重量平均分子量をいう。 The weight average molecular weight of the acrylic polymer is in the range of 30,000 to 110,000, particularly in the range of 50,000 to 110,000, from the viewpoint of imparting flexibility to the hard coat layer and preventing cracks during processing. It is preferably within the range of 60,000 to 80,000.
Here, the weight average molecular weight refers to a weight average molecular weight which is a polystyrene conversion value measured by gel permeation chromatography.
また、アクリル系ポリマーは、アクリル当量が200~1,200の範囲内であり、中でも200~1,000の範囲内であることが好ましい。
ここで、アクリル当量とは、アクリル系ポリマーの重量平均分子量を1分子中の(メタ)アクリル基の数で除した値を示す。 The acrylic polymer has an acrylic equivalent in the range of 200 to 1,200, and preferably in the range of 200 to 1,000.
Here, the acrylic equivalent indicates a value obtained by dividing the weight average molecular weight of the acrylic polymer by the number of (meth) acrylic groups in one molecule.
ここで、アクリル当量とは、アクリル系ポリマーの重量平均分子量を1分子中の(メタ)アクリル基の数で除した値を示す。 The acrylic polymer has an acrylic equivalent in the range of 200 to 1,200, and preferably in the range of 200 to 1,000.
Here, the acrylic equivalent indicates a value obtained by dividing the weight average molecular weight of the acrylic polymer by the number of (meth) acrylic groups in one molecule.
アクリル系ポリマーとしては、上記重量平均分子量およびアクリル当量を満たすものであれば特に限定されないが、グリセロール(メタ)アクリレートの重合体、またはメタクリル酸グリシジルに(メタ)アクリル酸を付加重合した化合物の重合体であることが好ましい。具体的には、下記化学式(4)または(5)で表されるアクリルモノマーの重合体が好ましく用いられる。
The acrylic polymer is not particularly limited as long as it satisfies the above weight average molecular weight and acrylic equivalent, but it is a polymer of glycerol (meth) acrylate or a compound of a compound obtained by addition polymerization of (meth) acrylic acid to glycidyl methacrylate. It is preferably a coalescence. Specifically, a polymer of an acrylic monomer represented by the following chemical formula (4) or (5) is preferably used.
上記式(4)において、R1~R3はそれぞれ独立してアクリレート基、メタクリレート基または水素原子であり、R1~R3のうち1つ以上はアクリレート基またはメタクリレート基である。すなわち、上記式(4)で表されるグリセロール(メタ)アクリレートは、単官能、2官能および3官能のいずれであってもよい。
また、上記式(5)において、Rはアクリル酸基またはメタクリル酸基である。 In the above formula (4), R 1 to R 3 are each independently an acrylate group, a methacrylate group or a hydrogen atom, and one or more of R 1 to R 3 are an acrylate group or a methacrylate group. That is, the glycerol (meth) acrylate represented by the above formula (4) may be monofunctional, bifunctional, or trifunctional.
In the above formula (5), R is an acrylic acid group or a methacrylic acid group.
また、上記式(5)において、Rはアクリル酸基またはメタクリル酸基である。 In the above formula (4), R 1 to R 3 are each independently an acrylate group, a methacrylate group or a hydrogen atom, and one or more of R 1 to R 3 are an acrylate group or a methacrylate group. That is, the glycerol (meth) acrylate represented by the above formula (4) may be monofunctional, bifunctional, or trifunctional.
In the above formula (5), R is an acrylic acid group or a methacrylic acid group.
このようなアクリル系ポリマーとしては、例えば、星光PMC(株)製のBL-2002が挙げられる。
また、アクリル系ポリマーとしては、1種単独で用いてもよく、2種以上を適宜混合して用いてもよい。 An example of such an acrylic polymer is BL-2002 manufactured by Seiko PMC Co., Ltd.
Moreover, as an acrylic polymer, you may use individually by 1 type, and may mix anduse 2 or more types suitably.
また、アクリル系ポリマーとしては、1種単独で用いてもよく、2種以上を適宜混合して用いてもよい。 An example of such an acrylic polymer is BL-2002 manufactured by Seiko PMC Co., Ltd.
Moreover, as an acrylic polymer, you may use individually by 1 type, and may mix and
アクリル系ポリマーの含有量は、硬化性樹脂組成物の全固形分に対して3質量%~20質量%の範囲内であることが好ましく、5質量%~10質量%であることがより好ましく、6質量%~8質量%の範囲内であることがさらに好ましい。アクリル系ポリマーの含有量が上記範囲内であれば、ハードコート層の硬さを維持しつつハードコートフィルムの加工性を向上させることができる。
また、アクリル系ポリマーの含有量は、後述のモノマー100重量部に対して5重量部~80重量部の範囲内で設定することができ、20重量部~40重量部の範囲内であることが好ましく、10重量部~30重量部の範囲内であることがさらに好ましい。 The content of the acrylic polymer is preferably in the range of 3% by mass to 20% by mass, more preferably in the range of 5% by mass to 10% by mass, based on the total solid content of the curable resin composition. More preferably, it is in the range of 6% by mass to 8% by mass. If the content of the acrylic polymer is within the above range, the workability of the hard coat film can be improved while maintaining the hardness of the hard coat layer.
The content of the acrylic polymer can be set in the range of 5 to 80 parts by weight with respect to 100 parts by weight of the monomer described later, and is in the range of 20 to 40 parts by weight. Preferably, it is in the range of 10 to 30 parts by weight.
また、アクリル系ポリマーの含有量は、後述のモノマー100重量部に対して5重量部~80重量部の範囲内で設定することができ、20重量部~40重量部の範囲内であることが好ましく、10重量部~30重量部の範囲内であることがさらに好ましい。 The content of the acrylic polymer is preferably in the range of 3% by mass to 20% by mass, more preferably in the range of 5% by mass to 10% by mass, based on the total solid content of the curable resin composition. More preferably, it is in the range of 6% by mass to 8% by mass. If the content of the acrylic polymer is within the above range, the workability of the hard coat film can be improved while maintaining the hardness of the hard coat layer.
The content of the acrylic polymer can be set in the range of 5 to 80 parts by weight with respect to 100 parts by weight of the monomer described later, and is in the range of 20 to 40 parts by weight. Preferably, it is in the range of 10 to 30 parts by weight.
(c)モノマー
本発明において、モノマーは、ハードコート層のマトリクス樹脂となる成分である。
モノマーは、通常、反応性官能基を有する。モノマーは、硬化した際にモノマー同士で架橋する。また、モノマーの反応性官能基は、反応性異形シリカ微粒子の反応性官能基と架橋反応性を有するため、モノマーは反応性異形シリカ微粒子と架橋し、網目構造が形成され、ハードコート層の硬度および耐擦傷性をさらに高める。反応性官能基としては、重合性不飽和基が好適に用いられ、好ましくは光硬化性不飽和基であり、特に好ましくは電離放射線硬化性不飽和基である。具体例としては、(メタ)アクリロイル基、ビニル基、アリル基等のエチレン性不飽和結合およびエポキシ基等が挙げられる。モノマーの反応性官能基は、反応性異形シリカ微粒子の反応性官能基と同じであっても異なっていてもよい。 (C) Monomer In this invention, a monomer is a component used as the matrix resin of a hard-coat layer.
The monomer usually has a reactive functional group. Monomers crosslink between monomers when cured. In addition, since the reactive functional group of the monomer has cross-linking reactivity with the reactive functional group of the reactive irregularly shaped silica fine particles, the monomer is crosslinked with the reactive irregularly shaped silica fine particles, forming a network structure, and the hardness of the hard coat layer And further improve the scratch resistance. As the reactive functional group, a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group. Specific examples include ethylenically unsaturated bonds such as (meth) acryloyl groups, vinyl groups, allyl groups, and epoxy groups. The reactive functional group of the monomer may be the same as or different from the reactive functional group of the reactive deformed silica fine particle.
本発明において、モノマーは、ハードコート層のマトリクス樹脂となる成分である。
モノマーは、通常、反応性官能基を有する。モノマーは、硬化した際にモノマー同士で架橋する。また、モノマーの反応性官能基は、反応性異形シリカ微粒子の反応性官能基と架橋反応性を有するため、モノマーは反応性異形シリカ微粒子と架橋し、網目構造が形成され、ハードコート層の硬度および耐擦傷性をさらに高める。反応性官能基としては、重合性不飽和基が好適に用いられ、好ましくは光硬化性不飽和基であり、特に好ましくは電離放射線硬化性不飽和基である。具体例としては、(メタ)アクリロイル基、ビニル基、アリル基等のエチレン性不飽和結合およびエポキシ基等が挙げられる。モノマーの反応性官能基は、反応性異形シリカ微粒子の反応性官能基と同じであっても異なっていてもよい。 (C) Monomer In this invention, a monomer is a component used as the matrix resin of a hard-coat layer.
The monomer usually has a reactive functional group. Monomers crosslink between monomers when cured. In addition, since the reactive functional group of the monomer has cross-linking reactivity with the reactive functional group of the reactive irregularly shaped silica fine particles, the monomer is crosslinked with the reactive irregularly shaped silica fine particles, forming a network structure, and the hardness of the hard coat layer And further improve the scratch resistance. As the reactive functional group, a polymerizable unsaturated group is suitably used, preferably a photocurable unsaturated group, and particularly preferably an ionizing radiation curable unsaturated group. Specific examples include ethylenically unsaturated bonds such as (meth) acryloyl groups, vinyl groups, allyl groups, and epoxy groups. The reactive functional group of the monomer may be the same as or different from the reactive functional group of the reactive deformed silica fine particle.
モノマーとしては、硬化性有機樹脂が好ましく、塗膜とした時に光が透過する透光性のものが好ましく、紫外線または電子線で代表される電離放射線により硬化する樹脂である電離放射線硬化性樹脂、その他公知の硬化性樹脂等を要求性能等に応じて適宜採用すればよい。電離放射線硬化性樹脂としては、アクリレート系、オキセタン系、シリコーン系等が挙げられる。モノマーとして、1種または2種以上のモノマーを用いることができる。
As the monomer, a curable organic resin is preferable, and a translucent material that transmits light when it is used as a coating film is preferable. An ionizing radiation curable resin that is a resin that is cured by ionizing radiation represented by ultraviolet rays or electron beams, Other known curable resins and the like may be appropriately employed according to required performance. Examples of the ionizing radiation curable resin include acrylate-based, oxetane-based, and silicone-based resins. As the monomer, one type or two or more types of monomers can be used.
モノマーは、反応性官能基を3つ以上有することが、架橋密度を高められる点から好ましい。反応性官能基を3つ以上有する多官能モノマーとしては、例えば、ペンタエリスリトールトリ(メタ)アクリレート、ペンタエリスリトールテトラ(メタ)アクリレート、ジペンタエリスリトールヘキサ(メタ)アクリレート、ジペンタエリスリトールペンタ(メタ)アクリレート、トリメチロールプロパントリ(メタ)アクリレート、トリメチロールプロパンヘキサ(メタ)アクリレート、およびこれらの変性体が挙げられる。なお、変性体としては、エチレンオキサイド変性体、プロピレンオキサイド変性体、カプロラクトン変性体、およびイソシアヌル酸変性体等が挙げられる。
中でも、多官能モノマーとしては、ペンタエリスリトールトリアクリレート、ペンタエリスリトールテトラ(メタ)アクリレート、ジペンタエリスリトールヘキサアクリレート、ペンタエリスリトールテトラアクリレート、およびジペンタエリスリトールペンタアクリレートが好ましく用いられ、ジペンタエリスリトールヘキサアクリレート、ペンタエリスリトールテトラアクリレート、およびジペンタエリスリトールペンタアクリレートが特に好ましく用いられる。 The monomer preferably has three or more reactive functional groups from the viewpoint of increasing the crosslinking density. Examples of the polyfunctional monomer having three or more reactive functional groups include pentaerythritol tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexa (meth) acrylate, and dipentaerythritol penta (meth) acrylate. , Trimethylolpropane tri (meth) acrylate, trimethylolpropane hexa (meth) acrylate, and modified products thereof. In addition, as a modified body, an ethylene oxide modified body, a propylene oxide modified body, a caprolactone modified body, an isocyanuric acid modified body, etc. are mentioned.
Among them, as the polyfunctional monomer, pentaerythritol triacrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexaacrylate, pentaerythritol tetraacrylate, and dipentaerythritol pentaacrylate are preferably used, and dipentaerythritol hexaacrylate, penta Erythritol tetraacrylate and dipentaerythritol pentaacrylate are particularly preferably used.
中でも、多官能モノマーとしては、ペンタエリスリトールトリアクリレート、ペンタエリスリトールテトラ(メタ)アクリレート、ジペンタエリスリトールヘキサアクリレート、ペンタエリスリトールテトラアクリレート、およびジペンタエリスリトールペンタアクリレートが好ましく用いられ、ジペンタエリスリトールヘキサアクリレート、ペンタエリスリトールテトラアクリレート、およびジペンタエリスリトールペンタアクリレートが特に好ましく用いられる。 The monomer preferably has three or more reactive functional groups from the viewpoint of increasing the crosslinking density. Examples of the polyfunctional monomer having three or more reactive functional groups include pentaerythritol tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexa (meth) acrylate, and dipentaerythritol penta (meth) acrylate. , Trimethylolpropane tri (meth) acrylate, trimethylolpropane hexa (meth) acrylate, and modified products thereof. In addition, as a modified body, an ethylene oxide modified body, a propylene oxide modified body, a caprolactone modified body, an isocyanuric acid modified body, etc. are mentioned.
Among them, as the polyfunctional monomer, pentaerythritol triacrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol hexaacrylate, pentaerythritol tetraacrylate, and dipentaerythritol pentaacrylate are preferably used, and dipentaerythritol hexaacrylate, penta Erythritol tetraacrylate and dipentaerythritol pentaacrylate are particularly preferably used.
なお、ハードコート層は、モノマーが架橋したものだけでなく、架橋していないモノマーを含んでいてもよい。
In addition, the hard coat layer may contain not only a monomer that is crosslinked but also a monomer that is not crosslinked.
モノマーの含有量は、硬化性樹脂組成物の全固形分に対して25質量%~44質量%の範囲内であることが好ましく、30質量%~40質量%の範囲内であることがより好ましい。含有量が少ないとハードコート層に十分な硬度を付与できないおそれがある。また、含有量が多いと、ハードコート層の硬度が上がり過ぎ、また上記アクリル系ポリマーの含有量が相対的に少なくなり、ハードコートフィルムの加工性を低下させてしまうおそれがある。
The monomer content is preferably in the range of 25% by mass to 44% by mass and more preferably in the range of 30% by mass to 40% by mass with respect to the total solid content of the curable resin composition. . When the content is small, there is a possibility that sufficient hardness cannot be imparted to the hard coat layer. On the other hand, when the content is large, the hardness of the hard coat layer is excessively increased, and the content of the acrylic polymer is relatively decreased, which may deteriorate the workability of the hard coat film.
(d)重合開始剤
本発明において、硬化性樹脂組成物は重合開始剤を含有していてもよい。硬化性樹脂組成物を紫外線照射または加熱等により硬化させる場合には重合開始剤が用いられるが、電子線照射により硬化させる場合には重合開始剤が不要である。 (D) Polymerization initiator In the present invention, the curable resin composition may contain a polymerization initiator. A polymerization initiator is used when the curable resin composition is cured by ultraviolet irradiation or heating, but when it is cured by electron beam irradiation, a polymerization initiator is unnecessary.
本発明において、硬化性樹脂組成物は重合開始剤を含有していてもよい。硬化性樹脂組成物を紫外線照射または加熱等により硬化させる場合には重合開始剤が用いられるが、電子線照射により硬化させる場合には重合開始剤が不要である。 (D) Polymerization initiator In the present invention, the curable resin composition may contain a polymerization initiator. A polymerization initiator is used when the curable resin composition is cured by ultraviolet irradiation or heating, but when it is cured by electron beam irradiation, a polymerization initiator is unnecessary.
重合開始剤は、光および熱の少なくともいずれかにより分解されて、ラジカルもしくはカチオンを発生してラジカル重合とカチオン重合を進行させるものである。
重合開始剤としては、ラジカル重合開始剤、カチオン重合開始剤、ラジカルおよびカチオン重合開始剤等を適宜選択して用いることができる。 The polymerization initiator is decomposed by at least one of light and heat to generate radicals or cations to advance radical polymerization and cationic polymerization.
As the polymerization initiator, radical polymerization initiators, cationic polymerization initiators, radicals and cationic polymerization initiators can be appropriately selected and used.
重合開始剤としては、ラジカル重合開始剤、カチオン重合開始剤、ラジカルおよびカチオン重合開始剤等を適宜選択して用いることができる。 The polymerization initiator is decomposed by at least one of light and heat to generate radicals or cations to advance radical polymerization and cationic polymerization.
As the polymerization initiator, radical polymerization initiators, cationic polymerization initiators, radicals and cationic polymerization initiators can be appropriately selected and used.
ラジカル重合開始剤は、光および熱の少なくともいずれかによりラジカル重合を開始させる物質を放出することが可能であればよい。例えば、光ラジカル重合開始剤としては、イミダゾール誘導体、ビスイミダゾール誘導体、N-アリールグリシン誘導体、有機アジド化合物、チタノセン類、アルミナート錯体、有機過酸化物、N-アルコキシピリジニウム塩、チオキサントン誘導体等が挙げられる。具体例としては、特開2010-102123号公報および特開2010-120182号公報に記載のものを挙げることができる。
The radical polymerization initiator only needs to be capable of releasing a substance that initiates radical polymerization by at least one of light and heat. For example, photo radical polymerization initiators include imidazole derivatives, bisimidazole derivatives, N-aryl glycine derivatives, organic azide compounds, titanocenes, aluminate complexes, organic peroxides, N-alkoxypyridinium salts, thioxanthone derivatives, and the like. It is done. Specific examples include those described in JP 2010-102123 A and JP 2010-120182 A.
また、カチオン重合開始剤は、光および熱の少なくともいずれかによりカチオン重合を開始させる物質を放出することが可能であればよい。カチオン重合開始剤としては、スルホン酸エステル、イミドスルホネート、ジアルキル-4-ヒドロキシスルホニウム塩、アリールスルホン酸-p-ニトロベンジルエステル、シラノール-アルミニウム錯体、(η6-ベンゼン)(η5-シクロペンタジエニル)鉄(II)等が例示される。具体例としては、特開2010-102123号公報および特開2010-120182号公報に記載のものを挙げることができる。
Moreover, the cationic polymerization initiator should just be able to discharge | release the substance which starts cationic polymerization by at least any one of light and a heat | fever. Examples of the cationic polymerization initiator include sulfonic acid ester, imide sulfonate, dialkyl-4-hydroxysulfonium salt, arylsulfonic acid-p-nitrobenzyl ester, silanol-aluminum complex, (η 6 -benzene) (η 5 -cyclopentadidiene). Enil) iron (II) and the like. Specific examples include those described in JP 2010-102123 A and JP 2010-120182 A.
ラジカル重合開始剤としてもカチオン重合開始剤としても用いられるものとしては、芳香族ヨードニウム塩、芳香族スルホニウム塩、芳香族ジアゾニウム塩、芳香族ホスホニウム塩、トリアジン化合物、鉄アレーン錯体等が例示される。具体例としては、特開2010-102123号公報および特開2010-120182号公報に記載のものを挙げることができる。
Examples of radical polymerization initiators and cationic polymerization initiators that can be used include aromatic iodonium salts, aromatic sulfonium salts, aromatic diazonium salts, aromatic phosphonium salts, triazine compounds, and iron arene complexes. Specific examples include those described in JP 2010-102123 A and JP 2010-120182 A.
中でも、重合開始剤は、可視光領域における吸収率が比較的低いことが好ましい。可視光領域における吸収率が高いと、ハードコートフィルムの光透過性が低下するおそれがあるからである。
Among them, the polymerization initiator preferably has a relatively low absorption rate in the visible light region. This is because if the absorption rate in the visible light region is high, the light transmittance of the hard coat film may be lowered.
重合開始剤の含有量は、硬化性樹脂組成物の全固形分に対して2質量%~5質量%の範囲内であることが好ましく、2質量%~2.5質量%の範囲内であることがより好ましい。含有量が少ないとモノマー等の重合反応が十分に進行せず、ハードコート層に十分な硬度を付与できないおそれがある。また、含有量が多いとモノマー等の重合反応が速く進行し、作業性が低下したり不均一な硬化物となったりするおそれがある。
The content of the polymerization initiator is preferably in the range of 2% by mass to 5% by mass with respect to the total solid content of the curable resin composition, and is preferably in the range of 2% by mass to 2.5% by mass. It is more preferable. If the content is small, the polymerization reaction of monomers or the like does not proceed sufficiently, and there is a possibility that sufficient hardness cannot be imparted to the hard coat layer. Moreover, when there is much content, polymerization reaction, such as a monomer, advances rapidly, and there exists a possibility that workability | operativity may fall or it may become a non-uniform cured | curing material.
(e)界面活性剤
本発明に用いられる硬化性樹脂組成物は、界面活性剤をさらに含有していてもよい。界面活性剤は、塗工安定性、滑り性、防汚性および耐擦傷性を付与する成分である。
界面活性剤としては、例えば、フッ素系界面活性剤、シリコン系界面活性剤、フッ素シリコン系界面活性剤等を挙げることができる。中でも、滑り性が良好であることから、フッ素シリコン系界面活性剤が好ましく用いられる。 (E) Surfactant The curable resin composition used in the present invention may further contain a surfactant. The surfactant is a component that imparts coating stability, slipperiness, antifouling properties, and scratch resistance.
Examples of the surfactant include a fluorine-based surfactant, a silicon-based surfactant, and a fluorine-silicon-based surfactant. Of these, a fluorosilicone surfactant is preferably used because of its good slipperiness.
本発明に用いられる硬化性樹脂組成物は、界面活性剤をさらに含有していてもよい。界面活性剤は、塗工安定性、滑り性、防汚性および耐擦傷性を付与する成分である。
界面活性剤としては、例えば、フッ素系界面活性剤、シリコン系界面活性剤、フッ素シリコン系界面活性剤等を挙げることができる。中でも、滑り性が良好であることから、フッ素シリコン系界面活性剤が好ましく用いられる。 (E) Surfactant The curable resin composition used in the present invention may further contain a surfactant. The surfactant is a component that imparts coating stability, slipperiness, antifouling properties, and scratch resistance.
Examples of the surfactant include a fluorine-based surfactant, a silicon-based surfactant, and a fluorine-silicon-based surfactant. Of these, a fluorosilicone surfactant is preferably used because of its good slipperiness.
界面活性剤は市販のものを使用することができ、例えば特開2010-102123号公報および特開2010-120182号公報に記載のレベリング剤を用いることができる。
As the surfactant, a commercially available one can be used, and for example, leveling agents described in JP 2010-102123 A and JP 2010-120182 A can be used.
また、フッ素シリコン系界面活性剤としては、例えばパーフルオロアルキル基およびシロキサン結合を有する化合物を挙げることができ、具体的にはパーフルオロアルキル基を有し、シロキサンとポリエーテルとが共重合した化合物が挙げられる。パーフルオロアルキル基の炭素数は例えば4~10である。パーフルオロアルキル基は、直鎖でも分岐鎖でもよい。ポリエーテル基としては、ポリエチレンオキシド鎖、ポリプロピレンオキシド鎖、それらの共重合体等が挙げられる。
Examples of the fluorosilicone surfactant include a compound having a perfluoroalkyl group and a siloxane bond. Specifically, a compound having a perfluoroalkyl group and a copolymer of siloxane and polyether. Is mentioned. The perfluoroalkyl group has, for example, 4 to 10 carbon atoms. The perfluoroalkyl group may be linear or branched. Examples of the polyether group include a polyethylene oxide chain, a polypropylene oxide chain, and a copolymer thereof.
このようなフッ素シリコン系界面活性剤としては、具体的には下記化学式(6)で表わされる化合物を挙げることができる。
Specific examples of such fluorine silicon surfactants include compounds represented by the following chemical formula (6).
上記式(6)中、Rは炭素数4~10のパーフルオロアルキル基、Qはポリエチレンオキシド鎖またはポリプロピレンオキシド鎖であり、kおよびmはそれぞれ0または1であり、nは1、2または3である。
In the above formula (6), R is a perfluoroalkyl group having 4 to 10 carbon atoms, Q is a polyethylene oxide chain or a polypropylene oxide chain, k and m are each 0 or 1, and n is 1, 2 or 3 It is.
また、フッ素シリコン系界面活性剤は反応性官能基を有していてもよい。反応性官能基としては、例えば重合性不飽和基が用いられ、具体的には光硬化性不飽和基であり、より具体的には電離放射線硬化性不飽和基である。反応性官能基は、具体的には、(メタ)アクリロイル基が挙げられる。
Further, the fluorosilicone surfactant may have a reactive functional group. As the reactive functional group, for example, a polymerizable unsaturated group is used, specifically a photocurable unsaturated group, and more specifically an ionizing radiation curable unsaturated group. Specific examples of the reactive functional group include a (meth) acryloyl group.
フッ素シリコン系界面活性剤の具体例としては、信越化学工業社製のX-71-1203M、X-70-090、X-70-091、X-70-092、X-70-093、DIC社製のメガファックR-08、XRB-4等を挙げることができる。
Specific examples of the fluorosilicone surfactant include X-71-1203M, X-70-090, X-70-091, X-70-092, X-70-093, manufactured by Shin-Etsu Chemical Co., Ltd., DIC Corporation. Examples thereof include Megafac R-08, XRB-4, and the like.
また、後述するようにハードコート層上に防眩層が形成されている場合には、ハードコート層上への防眩層の形成が可能になるように、表面の濡れ性が良いハードコート層が得られるような界面活性剤を用いることが好ましい。このような界面活性剤としては、上記の中から適宜選択して使用することができる。
In addition, when an antiglare layer is formed on the hard coat layer as will be described later, the hard coat layer has good surface wettability so that the antiglare layer can be formed on the hard coat layer. It is preferable to use a surfactant capable of obtaining Such a surfactant can be appropriately selected from the above and used.
界面活性剤の含有量は、硬化性樹脂組成物の全固形分に対して0.2質量%以下であることが好ましく、0.08質量%~0.1質量%の範囲内であることがより好ましい。
The content of the surfactant is preferably 0.2% by mass or less, and preferably in the range of 0.08% by mass to 0.1% by mass with respect to the total solid content of the curable resin composition. More preferred.
(f)青色色材
本発明に用いられる硬化性樹脂組成物は、青色色材をさらに含有していてもよい。本発明のハードコートフィルムを画像表示装置に用いた場合には、黄色味を抑え、視認性や色再現性を向上させることができるからである。
青色色材としては、一般的な顔料および染料を用いることができる。具体的には、フタロシアニン系顔料、インダンスレンブルー系顔料等が挙げられる。
青色色材の含有量は、後述するハードコート層の透過率が85%以上、好ましくは90%以上になるような量であればよく、適宜調整される。例えば、青色色材の含有量は、硬化性樹脂組成物の全固形分に対して0.05質量%以下であることが好ましい。 (F) Blue color material The curable resin composition used in the present invention may further contain a blue color material. This is because when the hard coat film of the present invention is used in an image display device, yellowness can be suppressed and visibility and color reproducibility can be improved.
As the blue color material, general pigments and dyes can be used. Specific examples include phthalocyanine pigments and indanthrene blue pigments.
The content of the blue color material may be an amount such that the transmittance of the hard coat layer described later is 85% or more, and preferably 90% or more, and is appropriately adjusted. For example, the content of the blue color material is preferably 0.05% by mass or less with respect to the total solid content of the curable resin composition.
本発明に用いられる硬化性樹脂組成物は、青色色材をさらに含有していてもよい。本発明のハードコートフィルムを画像表示装置に用いた場合には、黄色味を抑え、視認性や色再現性を向上させることができるからである。
青色色材としては、一般的な顔料および染料を用いることができる。具体的には、フタロシアニン系顔料、インダンスレンブルー系顔料等が挙げられる。
青色色材の含有量は、後述するハードコート層の透過率が85%以上、好ましくは90%以上になるような量であればよく、適宜調整される。例えば、青色色材の含有量は、硬化性樹脂組成物の全固形分に対して0.05質量%以下であることが好ましい。 (F) Blue color material The curable resin composition used in the present invention may further contain a blue color material. This is because when the hard coat film of the present invention is used in an image display device, yellowness can be suppressed and visibility and color reproducibility can be improved.
As the blue color material, general pigments and dyes can be used. Specific examples include phthalocyanine pigments and indanthrene blue pigments.
The content of the blue color material may be an amount such that the transmittance of the hard coat layer described later is 85% or more, and preferably 90% or more, and is appropriately adjusted. For example, the content of the blue color material is preferably 0.05% by mass or less with respect to the total solid content of the curable resin composition.
(g)ウレタンアクリレート
本発明に用いられる硬化性樹脂組成物は、ウレタンアクリレートをさらに含有していてもよい。ウレタンアクリレートを添加することにより、ハードコート層に柔軟性を付与することができ、反りの発生を抑制することができるからである。特に、図3に例示するように基板2の両面にそれぞれハードコート層3および第2のハードコート層4が形成されている場合には、ウレタンアクリレートが添加された硬化性樹脂組成物を用いてハードコート層3および第2のハードコート層4を形成することにより、反りの発生をさらに抑制するとともに、耐衝撃性を向上させることができる。 (G) Urethane acrylate The curable resin composition used in the present invention may further contain urethane acrylate. This is because by adding urethane acrylate, flexibility can be imparted to the hard coat layer and the occurrence of warpage can be suppressed. In particular, when thehard coat layer 3 and the second hard coat layer 4 are formed on both surfaces of the substrate 2 as illustrated in FIG. 3, a curable resin composition to which urethane acrylate is added is used. By forming the hard coat layer 3 and the second hard coat layer 4, it is possible to further suppress the occurrence of warping and improve the impact resistance.
本発明に用いられる硬化性樹脂組成物は、ウレタンアクリレートをさらに含有していてもよい。ウレタンアクリレートを添加することにより、ハードコート層に柔軟性を付与することができ、反りの発生を抑制することができるからである。特に、図3に例示するように基板2の両面にそれぞれハードコート層3および第2のハードコート層4が形成されている場合には、ウレタンアクリレートが添加された硬化性樹脂組成物を用いてハードコート層3および第2のハードコート層4を形成することにより、反りの発生をさらに抑制するとともに、耐衝撃性を向上させることができる。 (G) Urethane acrylate The curable resin composition used in the present invention may further contain urethane acrylate. This is because by adding urethane acrylate, flexibility can be imparted to the hard coat layer and the occurrence of warpage can be suppressed. In particular, when the
ウレタンアクリレートとしては、例えばハードコート層に用いられる一般的なウレタンアクリレートを使用することができる。具体的には、特開2011-31527号公報、特開2009-84328号公報、国際公開第2012/8444号パンフレットに記載されているものが挙げられる。
As the urethane acrylate, for example, a general urethane acrylate used for a hard coat layer can be used. Specific examples include those described in JP 2011-31527 A, JP 2009-84328 A, and International Publication No. 2012/8444.
ウレタンアクリレートの含有量は、後述するハードコート層の鉛筆硬度が所定の範囲になるような量であればよく、適宜調整される。例えば、ウレタンアクリレートの含有量は、上記モノマー100重量部に対して4重量部~100重量部の範囲内で設定することができる。
The content of urethane acrylate may be an amount such that the pencil hardness of the hard coat layer, which will be described later, falls within a predetermined range, and is appropriately adjusted. For example, the content of urethane acrylate can be set within the range of 4 to 100 parts by weight with respect to 100 parts by weight of the monomer.
(h)溶剤
本発明に用いられる硬化性樹脂組成物は、通常、溶剤を含有する。
溶剤は特に限定されないが、ハードコートフィルムの硬度を高める観点から非浸透性溶剤が好ましい。ここで、浸透とは、基板を溶解または膨潤させることをいう。
非浸透性溶剤の具体例としては、メチルイソブチルケトン、プロピレングリコールモノメチルエーテル、ノルマルプロパノール、イソプロパノール、ノルマルブタノール、sec-ブタノール、イソブタノール、およびtert-ブタノール等が挙げられる。 (H) Solvent The curable resin composition used in the present invention usually contains a solvent.
The solvent is not particularly limited, but a non-permeable solvent is preferable from the viewpoint of increasing the hardness of the hard coat film. Here, permeation refers to dissolving or swelling the substrate.
Specific examples of the non-permeable solvent include methyl isobutyl ketone, propylene glycol monomethyl ether, normal propanol, isopropanol, normal butanol, sec-butanol, isobutanol, and tert-butanol.
本発明に用いられる硬化性樹脂組成物は、通常、溶剤を含有する。
溶剤は特に限定されないが、ハードコートフィルムの硬度を高める観点から非浸透性溶剤が好ましい。ここで、浸透とは、基板を溶解または膨潤させることをいう。
非浸透性溶剤の具体例としては、メチルイソブチルケトン、プロピレングリコールモノメチルエーテル、ノルマルプロパノール、イソプロパノール、ノルマルブタノール、sec-ブタノール、イソブタノール、およびtert-ブタノール等が挙げられる。 (H) Solvent The curable resin composition used in the present invention usually contains a solvent.
The solvent is not particularly limited, but a non-permeable solvent is preferable from the viewpoint of increasing the hardness of the hard coat film. Here, permeation refers to dissolving or swelling the substrate.
Specific examples of the non-permeable solvent include methyl isobutyl ketone, propylene glycol monomethyl ether, normal propanol, isopropanol, normal butanol, sec-butanol, isobutanol, and tert-butanol.
(i)その他の成分
本発明に用いられる硬化性樹脂組成物は、必要に応じて、帯電防止剤、防眩剤、各種増感剤等を含有していてもよい。 (I) Other components The curable resin composition used for this invention may contain the antistatic agent, the glare-proof agent, various sensitizers, etc. as needed.
本発明に用いられる硬化性樹脂組成物は、必要に応じて、帯電防止剤、防眩剤、各種増感剤等を含有していてもよい。 (I) Other components The curable resin composition used for this invention may contain the antistatic agent, the glare-proof agent, various sensitizers, etc. as needed.
(j)硬化性樹脂組成物
硬化性樹脂組成物は、溶剤に反応性異形シリカ微粒子、アクリル系ポリマー、モノマー、重合開始剤等を一般的な調製方法に従って混合し分散処理することにより調製することができる。混合分散には、ペイントシェーカーまたはビーズミル等を用いることができる。 (J) Curable resin composition The curable resin composition is prepared by mixing a dispersion of reactive irregularly shaped silica fine particles, an acrylic polymer, a monomer, a polymerization initiator and the like in a solvent according to a general preparation method, and dispersing the mixture. Can do. For mixing and dispersing, a paint shaker or a bead mill can be used.
硬化性樹脂組成物は、溶剤に反応性異形シリカ微粒子、アクリル系ポリマー、モノマー、重合開始剤等を一般的な調製方法に従って混合し分散処理することにより調製することができる。混合分散には、ペイントシェーカーまたはビーズミル等を用いることができる。 (J) Curable resin composition The curable resin composition is prepared by mixing a dispersion of reactive irregularly shaped silica fine particles, an acrylic polymer, a monomer, a polymerization initiator and the like in a solvent according to a general preparation method, and dispersing the mixture. Can do. For mixing and dispersing, a paint shaker or a bead mill can be used.
(2)異形シリカ微粒子
本発明におけるハードコート層に含有される異形シリカ微粒子は、上記硬化性樹脂組成物に含有される反応性異形シリカ微粒子同士が架橋することにより、または反応性異形シリカ微粒子がモノマーと架橋することにより形成される。すなわち、ハードコート層には、異形シリカ微粒子同士が架橋されたものや、マトリクス樹脂と架橋された異形シリカ微粒子が含有される。
なお、ハードコート層には、異形シリカ微粒子として、反応性異形シリカ微粒子が有する反応性官能基が反応したものだけでなく、反応性異形シリカ微粒子の反応性官能基が反応していないものが含まれていてもよい。
ハードコート層中の異形シリカ微粒子の含有量としては、上記硬化性樹脂組成物の全固形分中の反応性異形シリカ微粒子の含有量と同様とすることができる。 (2) Deformed silica fine particles The deformed silica fine particles contained in the hard coat layer in the present invention are obtained by crosslinking the reactive deformed silica fine particles contained in the curable resin composition, or the reactive deformed silica fine particles It is formed by crosslinking with monomers. That is, the hard coat layer contains cross-linked irregular-shaped silica fine particles or irregular-shaped silica fine particles crosslinked with the matrix resin.
In addition, the hard coat layer includes not only the reactive functional group of the reactive irregular shaped silica fine particles reacted but also the reactive functional group of the reactive irregular shaped silica fine particles not reacting as the irregular shaped silica fine particles. It may be.
The content of the irregular shaped silica fine particles in the hard coat layer can be the same as the content of the reactive irregular shaped silica fine particles in the total solid content of the curable resin composition.
本発明におけるハードコート層に含有される異形シリカ微粒子は、上記硬化性樹脂組成物に含有される反応性異形シリカ微粒子同士が架橋することにより、または反応性異形シリカ微粒子がモノマーと架橋することにより形成される。すなわち、ハードコート層には、異形シリカ微粒子同士が架橋されたものや、マトリクス樹脂と架橋された異形シリカ微粒子が含有される。
なお、ハードコート層には、異形シリカ微粒子として、反応性異形シリカ微粒子が有する反応性官能基が反応したものだけでなく、反応性異形シリカ微粒子の反応性官能基が反応していないものが含まれていてもよい。
ハードコート層中の異形シリカ微粒子の含有量としては、上記硬化性樹脂組成物の全固形分中の反応性異形シリカ微粒子の含有量と同様とすることができる。 (2) Deformed silica fine particles The deformed silica fine particles contained in the hard coat layer in the present invention are obtained by crosslinking the reactive deformed silica fine particles contained in the curable resin composition, or the reactive deformed silica fine particles It is formed by crosslinking with monomers. That is, the hard coat layer contains cross-linked irregular-shaped silica fine particles or irregular-shaped silica fine particles crosslinked with the matrix resin.
In addition, the hard coat layer includes not only the reactive functional group of the reactive irregular shaped silica fine particles reacted but also the reactive functional group of the reactive irregular shaped silica fine particles not reacting as the irregular shaped silica fine particles. It may be.
The content of the irregular shaped silica fine particles in the hard coat layer can be the same as the content of the reactive irregular shaped silica fine particles in the total solid content of the curable resin composition.
(3)アクリル系ポリマー
本発明におけるハードコート層に含有されるアクリル系ポリマーは、重量平均分子量およびアクリル当量が所定の範囲内であるものである。
なお、アクリル系ポリマーについては、上記硬化性樹脂組成物に含有されるアクリル系ポリマーと同様であるので、ここでの説明は省略する。
ハードコート層中のアクリル系ポリマーの含有量としては、上記硬化性樹脂組成物の全固形分中のアクリル系ポリマーの含有量と同様とすることができる。 (3) Acrylic polymer The acrylic polymer contained in the hard coat layer in the present invention has a weight average molecular weight and an acrylic equivalent within predetermined ranges.
In addition, since it is the same as that of the acryl-type polymer contained in the said curable resin composition about an acryl-type polymer, description here is abbreviate | omitted.
The content of the acrylic polymer in the hard coat layer can be the same as the content of the acrylic polymer in the total solid content of the curable resin composition.
本発明におけるハードコート層に含有されるアクリル系ポリマーは、重量平均分子量およびアクリル当量が所定の範囲内であるものである。
なお、アクリル系ポリマーについては、上記硬化性樹脂組成物に含有されるアクリル系ポリマーと同様であるので、ここでの説明は省略する。
ハードコート層中のアクリル系ポリマーの含有量としては、上記硬化性樹脂組成物の全固形分中のアクリル系ポリマーの含有量と同様とすることができる。 (3) Acrylic polymer The acrylic polymer contained in the hard coat layer in the present invention has a weight average molecular weight and an acrylic equivalent within predetermined ranges.
In addition, since it is the same as that of the acryl-type polymer contained in the said curable resin composition about an acryl-type polymer, description here is abbreviate | omitted.
The content of the acrylic polymer in the hard coat layer can be the same as the content of the acrylic polymer in the total solid content of the curable resin composition.
(4)マトリクス樹脂
本発明におけるハードコート層に含有されるマトリクス樹脂は、異形シリカ微粒子およびアクリル系ポリマーと複合体を形成している樹脂である。マトリクス樹脂と異形シリカ微粒子とアクリル系ポリマーとにより三次元的な網目構造が形成されていることにより、ハードコート層の硬度や耐擦傷性が高くなると考えられる。 (4) Matrix resin The matrix resin contained in the hard coat layer in the present invention is a resin forming a composite with irregular-shaped silica fine particles and an acrylic polymer. It is considered that the hardness and scratch resistance of the hard coat layer are increased by forming a three-dimensional network structure with the matrix resin, the deformed silica fine particles, and the acrylic polymer.
本発明におけるハードコート層に含有されるマトリクス樹脂は、異形シリカ微粒子およびアクリル系ポリマーと複合体を形成している樹脂である。マトリクス樹脂と異形シリカ微粒子とアクリル系ポリマーとにより三次元的な網目構造が形成されていることにより、ハードコート層の硬度や耐擦傷性が高くなると考えられる。 (4) Matrix resin The matrix resin contained in the hard coat layer in the present invention is a resin forming a composite with irregular-shaped silica fine particles and an acrylic polymer. It is considered that the hardness and scratch resistance of the hard coat layer are increased by forming a three-dimensional network structure with the matrix resin, the deformed silica fine particles, and the acrylic polymer.
このようなマトリクス樹脂としては、例えばアクリル樹脂、シリコーン樹脂、ポリエーテル等を挙げることができる。中でも、アクリル樹脂が好ましい。アクリル系ポリマーと親和性が高いため、ハードコート層の特性が向上するためである。
Examples of such a matrix resin include acrylic resin, silicone resin, and polyether. Among these, an acrylic resin is preferable. This is because the properties of the hard coat layer are improved because of its high affinity with the acrylic polymer.
また、マトリクス樹脂は、上記硬化性樹脂組成物に含有されるモノマー同士が架橋することにより、またはモノマーが反応性異形シリカ微粒子と架橋することにより形成されるものであることが好ましい。すなわち、ハードコート層には、架橋結合を有するマトリクス樹脂や、異形シリカ微粒子と架橋されたマトリクス樹脂が含有されていることが好ましい。
Further, the matrix resin is preferably formed by crosslinking of monomers contained in the curable resin composition, or by crosslinking of the monomer with reactive deformed silica fine particles. That is, the hard coat layer preferably contains a matrix resin having a cross-linked bond or a matrix resin cross-linked with irregular shaped silica fine particles.
上記の場合、マトリクス樹脂は、モノマーに由来する構成単位を有する重合体であることが好ましい。特に、アクリル系ポリマーとの親和性や分子設計のしやすさのため、マトリクス樹脂は、ペンタエリスリトール、ジペンタエリスリトール、トリメチロールプロパン等のヒドロキシ基含有のモノマーに由来する構成単位を有する重合体であることが好ましい。この構成単位としては、中でも、ペンタエリスリトールのモノマーに由来する構成単位であることが好ましい。例えば、ペンタエリスリトールのモノマーがエーテル結合やエステル結合等の化学結合をすることで得られる構造が挙げられる。より具体的には、ペンタエリスリトールトリメチルエーテル、類似骨格としてペンタエリスリトールテトラメチルエーテル、ジペンタエリスリトールヘキサメチルエーテル、ジペンタエリスリトールペンタメチルエーテル等の構造を挙げることができる。
In the above case, the matrix resin is preferably a polymer having a structural unit derived from a monomer. In particular, because of its affinity with acrylic polymers and ease of molecular design, matrix resins are polymers having structural units derived from hydroxy group-containing monomers such as pentaerythritol, dipentaerythritol, and trimethylolpropane. Preferably there is. Among these, the structural unit is preferably a structural unit derived from a monomer of pentaerythritol. For example, the structure obtained when the monomer of pentaerythritol forms chemical bonds, such as an ether bond and an ester bond, is mentioned. More specifically, structures such as pentaerythritol trimethyl ether and pentaerythritol tetramethyl ether, dipentaerythritol hexamethyl ether, and dipentaerythritol pentamethyl ether can be given as the similar skeleton.
ハードコート層中のマトリクス樹脂の含有量としては、上記硬化性樹脂組成物の全固形分中のモノマーの含有量と同様とすることができる。
The content of the matrix resin in the hard coat layer can be the same as the content of the monomer in the total solid content of the curable resin composition.
(5)重合開始剤
本発明におけるハードコート層は重合開始剤を含有していてもよい。
なお、重合開始剤については、上記硬化性樹脂組成物に含有される重合開始剤と同様であるので、ここでの説明は省略する。
ハードコート層中の重合開始剤の含有量としては、上記硬化性樹脂組成物の全固形分中の重合開始剤の含有量と同様とすることができる。 (5) Polymerization initiator The hard coat layer in the present invention may contain a polymerization initiator.
In addition, about a polymerization initiator, since it is the same as that of the polymerization initiator contained in the said curable resin composition, description here is abbreviate | omitted.
The content of the polymerization initiator in the hard coat layer can be the same as the content of the polymerization initiator in the total solid content of the curable resin composition.
本発明におけるハードコート層は重合開始剤を含有していてもよい。
なお、重合開始剤については、上記硬化性樹脂組成物に含有される重合開始剤と同様であるので、ここでの説明は省略する。
ハードコート層中の重合開始剤の含有量としては、上記硬化性樹脂組成物の全固形分中の重合開始剤の含有量と同様とすることができる。 (5) Polymerization initiator The hard coat layer in the present invention may contain a polymerization initiator.
In addition, about a polymerization initiator, since it is the same as that of the polymerization initiator contained in the said curable resin composition, description here is abbreviate | omitted.
The content of the polymerization initiator in the hard coat layer can be the same as the content of the polymerization initiator in the total solid content of the curable resin composition.
(6)界面活性剤
本発明におけるハードコート層は界面活性剤を含有していてもよい。
なお、界面活性剤については、上記硬化性樹脂組成物に含有される界面活性剤と同様であるので、ここでの説明は省略する。
ハードコート層中の界面活性剤の含有量としては、上記硬化性樹脂組成物の全固形分中の界面活性剤の含有量と同様とすることができる。 (6) Surfactant The hard coat layer in the present invention may contain a surfactant.
In addition, about surfactant, since it is the same as that of surfactant contained in the said curable resin composition, description here is abbreviate | omitted.
The content of the surfactant in the hard coat layer can be the same as the content of the surfactant in the total solid content of the curable resin composition.
本発明におけるハードコート層は界面活性剤を含有していてもよい。
なお、界面活性剤については、上記硬化性樹脂組成物に含有される界面活性剤と同様であるので、ここでの説明は省略する。
ハードコート層中の界面活性剤の含有量としては、上記硬化性樹脂組成物の全固形分中の界面活性剤の含有量と同様とすることができる。 (6) Surfactant The hard coat layer in the present invention may contain a surfactant.
In addition, about surfactant, since it is the same as that of surfactant contained in the said curable resin composition, description here is abbreviate | omitted.
The content of the surfactant in the hard coat layer can be the same as the content of the surfactant in the total solid content of the curable resin composition.
(7)青色色材
本発明におけるハードコート層は青色色材を含有していてもよい。
なお、青色色材については、上記硬化性樹脂組成物に含有される青色色材と同様であるので、ここでの説明は省略する。
ハードコート層中の青色色材の含有量としては、上記硬化性樹脂組成物の全固形分中の青色色材の含有量と同様とすることができる。 (7) Blue color material The hard coat layer in the present invention may contain a blue color material.
In addition, about a blue color material, since it is the same as that of the blue color material contained in the said curable resin composition, description here is abbreviate | omitted.
The content of the blue color material in the hard coat layer can be the same as the content of the blue color material in the total solid content of the curable resin composition.
本発明におけるハードコート層は青色色材を含有していてもよい。
なお、青色色材については、上記硬化性樹脂組成物に含有される青色色材と同様であるので、ここでの説明は省略する。
ハードコート層中の青色色材の含有量としては、上記硬化性樹脂組成物の全固形分中の青色色材の含有量と同様とすることができる。 (7) Blue color material The hard coat layer in the present invention may contain a blue color material.
In addition, about a blue color material, since it is the same as that of the blue color material contained in the said curable resin composition, description here is abbreviate | omitted.
The content of the blue color material in the hard coat layer can be the same as the content of the blue color material in the total solid content of the curable resin composition.
(8)ウレタンアクリレート
本発明におけるハードコート層はウレタンアクリレートを含有していてもよい。
なお、ウレタンアクリレートについては、上記硬化性樹脂組成物に含有されるウレタンアクリレートと同様であるので、ここでの説明は省略する。
ハードコート層中のウレタンアクリレートの含有量としては、上記硬化性樹脂組成物中のウレタンアクリレートの含有量と同様とすることができる。 (8) Urethane acrylate The hard coat layer in the invention may contain urethane acrylate.
In addition, since it is the same as that of the urethane acrylate contained in the said curable resin composition about urethane acrylate, description here is abbreviate | omitted.
The urethane acrylate content in the hard coat layer can be the same as the urethane acrylate content in the curable resin composition.
本発明におけるハードコート層はウレタンアクリレートを含有していてもよい。
なお、ウレタンアクリレートについては、上記硬化性樹脂組成物に含有されるウレタンアクリレートと同様であるので、ここでの説明は省略する。
ハードコート層中のウレタンアクリレートの含有量としては、上記硬化性樹脂組成物中のウレタンアクリレートの含有量と同様とすることができる。 (8) Urethane acrylate The hard coat layer in the invention may contain urethane acrylate.
In addition, since it is the same as that of the urethane acrylate contained in the said curable resin composition about urethane acrylate, description here is abbreviate | omitted.
The urethane acrylate content in the hard coat layer can be the same as the urethane acrylate content in the curable resin composition.
(9)ハードコート層
ハードコート層の硬度は、JIS K5600-5-4(1999)で規定される鉛筆硬度試験(4.9N荷重)で評価できる。ハードコート層の鉛筆硬度は6H以上であることが好ましく、中でも7H以上、特に9H以上であることが好ましい。 (9) Hard Coat Layer The hardness of the hard coat layer can be evaluated by a pencil hardness test (4.9 N load) specified by JIS K5600-5-4 (1999). The pencil hardness of the hard coat layer is preferably 6H or more, more preferably 7H or more, and particularly preferably 9H or more.
ハードコート層の硬度は、JIS K5600-5-4(1999)で規定される鉛筆硬度試験(4.9N荷重)で評価できる。ハードコート層の鉛筆硬度は6H以上であることが好ましく、中でも7H以上、特に9H以上であることが好ましい。 (9) Hard Coat Layer The hardness of the hard coat layer can be evaluated by a pencil hardness test (4.9 N load) specified by JIS K5600-5-4 (1999). The pencil hardness of the hard coat layer is preferably 6H or more, more preferably 7H or more, and particularly preferably 9H or more.
ハードコート層は光透過性を有するものである。ハードコート層の可視光領域における透過率としては、具体的には、80%以上であることが好ましく、90%以上であることがより好ましい。上記透過率が上記範囲であることにより、光透過性に優れたハードコート層を形成することができるからである。
ここで、ハードコート層の透過率は、JIS K 7105で規定する方法により測定した全光線透過率とする。 The hard coat layer is light transmissive. Specifically, the transmittance of the hard coat layer in the visible light region is preferably 80% or more, and more preferably 90% or more. This is because when the transmittance is in the above range, a hard coat layer having excellent light transmittance can be formed.
Here, the transmittance of the hard coat layer is the total light transmittance measured by the method defined in JIS K 7105.
ここで、ハードコート層の透過率は、JIS K 7105で規定する方法により測定した全光線透過率とする。 The hard coat layer is light transmissive. Specifically, the transmittance of the hard coat layer in the visible light region is preferably 80% or more, and more preferably 90% or more. This is because when the transmittance is in the above range, a hard coat layer having excellent light transmittance can be formed.
Here, the transmittance of the hard coat layer is the total light transmittance measured by the method defined in JIS K 7105.
また、ハードコート層のヘイズ値としては、反応性異形シリカ微粒子の種類等に応じて適宜決定されるものであり、特に限定されないが、例えば、1.0以下、中でも0.8以下、特に0.5以下であることが好ましい。ヘイズ値が上記範囲であることにより、光透過性の良好なハードコート層とすることができるからである。
なお、ヘイズ値は、JIS-K-7136に準拠した方法で測定することができ、例えば準積分球を用いて、東洋精機製作所(株)製の直読ヘイズメーターにより測定することができる。また、ヘイズ値は、JIS K-7105に準拠した方法で測定することもでき、例えば村上色彩技術研究所製のヘイズメーターHM150により測定することができる。 Further, the haze value of the hard coat layer is appropriately determined according to the type of reactive irregularly shaped silica fine particles and is not particularly limited. For example, it is 1.0 or less, particularly 0.8 or less, particularly 0. .5 or less is preferable. It is because it can be set as the hard-coat layer with favorable light transmittance because haze value is the said range.
The haze value can be measured by a method according to JIS-K-7136. For example, it can be measured with a direct reading haze meter manufactured by Toyo Seiki Seisakusho using a semi-integrating sphere. The haze value can also be measured by a method according to JIS K-7105, for example, a haze meter HM150 manufactured by Murakami Color Research Laboratory.
なお、ヘイズ値は、JIS-K-7136に準拠した方法で測定することができ、例えば準積分球を用いて、東洋精機製作所(株)製の直読ヘイズメーターにより測定することができる。また、ヘイズ値は、JIS K-7105に準拠した方法で測定することもでき、例えば村上色彩技術研究所製のヘイズメーターHM150により測定することができる。 Further, the haze value of the hard coat layer is appropriately determined according to the type of reactive irregularly shaped silica fine particles and is not particularly limited. For example, it is 1.0 or less, particularly 0.8 or less, particularly 0. .5 or less is preferable. It is because it can be set as the hard-coat layer with favorable light transmittance because haze value is the said range.
The haze value can be measured by a method according to JIS-K-7136. For example, it can be measured with a direct reading haze meter manufactured by Toyo Seiki Seisakusho using a semi-integrating sphere. The haze value can also be measured by a method according to JIS K-7105, for example, a haze meter HM150 manufactured by Murakami Color Research Laboratory.
ハードコート層は防汚性を有することが好ましい。防汚性は濡れ性で評価することができ、具体的には、ハードコート層表面の濡れ性は、硬化性樹脂組成物に用いられる成分に応じて適宜決定されるものであり、特に限定されないが、ハードコート層表面での水滴の接触角が90°以上であることが好ましく、100°以上であることがより好ましく、110°以上であることがさらに好ましい。上記のような濡れ性であれば、ハードコート層が良好な防汚性を発揮できるからである。一方、上記水滴の接触角は、通常120°以下である。
なお、水滴の接触角は、協和界面科学(株)製の接触角測定器CA-Z型を用い、マイクロシリンジから水滴を滴下して30秒後の水との接触角を測定することで求めることができる。 The hard coat layer preferably has antifouling properties. The antifouling property can be evaluated by wettability. Specifically, the wettability of the hard coat layer surface is appropriately determined according to the components used in the curable resin composition, and is not particularly limited. However, the contact angle of water droplets on the hard coat layer surface is preferably 90 ° or more, more preferably 100 ° or more, and even more preferably 110 ° or more. This is because if the wettability is as described above, the hard coat layer can exhibit good antifouling properties. On the other hand, the contact angle of the water droplet is usually 120 ° or less.
The contact angle of the water droplet is obtained by measuring the contact angle with water 30 seconds after dropping the water droplet from the microsyringe using a contact angle measuring device CA-Z type manufactured by Kyowa Interface Science Co., Ltd. be able to.
なお、水滴の接触角は、協和界面科学(株)製の接触角測定器CA-Z型を用い、マイクロシリンジから水滴を滴下して30秒後の水との接触角を測定することで求めることができる。 The hard coat layer preferably has antifouling properties. The antifouling property can be evaluated by wettability. Specifically, the wettability of the hard coat layer surface is appropriately determined according to the components used in the curable resin composition, and is not particularly limited. However, the contact angle of water droplets on the hard coat layer surface is preferably 90 ° or more, more preferably 100 ° or more, and even more preferably 110 ° or more. This is because if the wettability is as described above, the hard coat layer can exhibit good antifouling properties. On the other hand, the contact angle of the water droplet is usually 120 ° or less.
The contact angle of the water droplet is obtained by measuring the contact angle with water 30 seconds after dropping the water droplet from the microsyringe using a contact angle measuring device CA-Z type manufactured by Kyowa Interface Science Co., Ltd. be able to.
また、本発明のハードコートフィルムがタッチパネル等に用いられる場合には、ハードコート層は易滑性を有することが好ましい。易滑性は動摩擦係数で評価することができ、動摩擦係数が小さいほど良好な易滑性を示す。ハードコート層の表面の動摩擦係数としては、例えば、0.300以下であり、0.200以下であることが好ましく、0.100以下であることがより好ましい。動摩擦係数が大きすぎると、ハードコート層の表面でのタッチ操作を良好に行うことが困難となる可能性があるからである。
なお、動摩擦係数は、JIS K7125に準拠した方法により測定することができ、例えば、新東科学(株)社製の動摩擦試験機HEIDON Type HHS2000で、直径10mmのステンレス剛球を用い、荷重200g、速度5mm/secにて動摩擦係数を測定することができる。 Moreover, when the hard coat film of this invention is used for a touch panel etc., it is preferable that a hard coat layer has slipperiness. The slipperiness can be evaluated by a dynamic friction coefficient. The smaller the dynamic friction coefficient, the better the slipperiness. The coefficient of dynamic friction on the surface of the hard coat layer is, for example, 0.300 or less, preferably 0.200 or less, and more preferably 0.100 or less. This is because if the dynamic friction coefficient is too large, it may be difficult to perform a good touch operation on the surface of the hard coat layer.
The dynamic friction coefficient can be measured by a method in accordance with JIS K7125. For example, a dynamic friction tester HEIDON Type HHS2000 manufactured by Shinto Kagaku Co., Ltd. is used, a stainless hard ball having a diameter of 10 mm, a load of 200 g, and a speed. The dynamic friction coefficient can be measured at 5 mm / sec.
なお、動摩擦係数は、JIS K7125に準拠した方法により測定することができ、例えば、新東科学(株)社製の動摩擦試験機HEIDON Type HHS2000で、直径10mmのステンレス剛球を用い、荷重200g、速度5mm/secにて動摩擦係数を測定することができる。 Moreover, when the hard coat film of this invention is used for a touch panel etc., it is preferable that a hard coat layer has slipperiness. The slipperiness can be evaluated by a dynamic friction coefficient. The smaller the dynamic friction coefficient, the better the slipperiness. The coefficient of dynamic friction on the surface of the hard coat layer is, for example, 0.300 or less, preferably 0.200 or less, and more preferably 0.100 or less. This is because if the dynamic friction coefficient is too large, it may be difficult to perform a good touch operation on the surface of the hard coat layer.
The dynamic friction coefficient can be measured by a method in accordance with JIS K7125. For example, a dynamic friction tester HEIDON Type HHS2000 manufactured by Shinto Kagaku Co., Ltd. is used, a stainless hard ball having a diameter of 10 mm, a load of 200 g, and a speed. The dynamic friction coefficient can be measured at 5 mm / sec.
ハードコート層の厚みとしては、所望の硬度および加工性を発揮することが可能であれば特に限定されるものではなく、例えば5μm~40μm程度にすることができ、中でも10μm~30μmの範囲内、特に18μm~22μmの範囲内であることが好ましい。ハードコート層が薄いと十分な硬度を発揮できず、厚いとクラックや反りが発生するおそれがあるからである。
The thickness of the hard coat layer is not particularly limited as long as the desired hardness and workability can be exhibited. For example, the thickness can be about 5 μm to 40 μm, and particularly within the range of 10 μm to 30 μm. In particular, it is preferably in the range of 18 μm to 22 μm. This is because if the hard coat layer is thin, sufficient hardness cannot be exhibited, and if it is thick, cracks and warpage may occur.
ハードコート層の形成方法は、上記硬化性樹脂組成物を用いてハードコート層を形成することができる方法であれば特に限定されるものではなく、基板上に硬化性樹脂組成物を塗布し、塗膜を硬化させる方法を用いることができる。
硬化性樹脂組成物の塗布方法は、基板上に硬化性樹脂組成物を均一に塗布することができる方法であれば特に限定されるものではなく、スピンコート法、ディップ法、スプレー法、スライドコート法、バーコート法、ロールコーター法、メニスカスコーター法、フレキソ印刷法、スクリーン印刷法、ピードコーター法等の各種方法を用いることができる。また、基板上への硬化性樹脂組成物の塗工量としては、所望の膜厚のハードコート層が得られるように調節することが好ましい。
塗膜の乾燥方法としては、例えば、減圧乾燥、加熱乾燥、およびこれらの組み合わせ等が挙げられる。常圧で乾燥させる場合、基板が劣化しない温度範囲で乾燥させることが好ましく、例えば30℃~110℃の範囲内で乾燥させることが好ましい。 The method for forming the hard coat layer is not particularly limited as long as the hard coat layer can be formed using the curable resin composition, and the curable resin composition is applied onto the substrate, A method of curing the coating film can be used.
The coating method of the curable resin composition is not particularly limited as long as the curable resin composition can be uniformly coated on the substrate, and is not limited to spin coating, dipping, spraying, slide coating. Various methods such as a method, a bar coat method, a roll coater method, a meniscus coater method, a flexographic printing method, a screen printing method, and a speed coater method can be used. Moreover, it is preferable to adjust as the coating amount of the curable resin composition on a board | substrate so that the hard-coat layer of a desired film thickness may be obtained.
Examples of the method for drying the coating film include reduced-pressure drying, heat drying, and combinations thereof. When drying at normal pressure, it is preferable to dry in a temperature range in which the substrate does not deteriorate, for example, in the range of 30 ° C. to 110 ° C.
硬化性樹脂組成物の塗布方法は、基板上に硬化性樹脂組成物を均一に塗布することができる方法であれば特に限定されるものではなく、スピンコート法、ディップ法、スプレー法、スライドコート法、バーコート法、ロールコーター法、メニスカスコーター法、フレキソ印刷法、スクリーン印刷法、ピードコーター法等の各種方法を用いることができる。また、基板上への硬化性樹脂組成物の塗工量としては、所望の膜厚のハードコート層が得られるように調節することが好ましい。
塗膜の乾燥方法としては、例えば、減圧乾燥、加熱乾燥、およびこれらの組み合わせ等が挙げられる。常圧で乾燥させる場合、基板が劣化しない温度範囲で乾燥させることが好ましく、例えば30℃~110℃の範囲内で乾燥させることが好ましい。 The method for forming the hard coat layer is not particularly limited as long as the hard coat layer can be formed using the curable resin composition, and the curable resin composition is applied onto the substrate, A method of curing the coating film can be used.
The coating method of the curable resin composition is not particularly limited as long as the curable resin composition can be uniformly coated on the substrate, and is not limited to spin coating, dipping, spraying, slide coating. Various methods such as a method, a bar coat method, a roll coater method, a meniscus coater method, a flexographic printing method, a screen printing method, and a speed coater method can be used. Moreover, it is preferable to adjust as the coating amount of the curable resin composition on a board | substrate so that the hard-coat layer of a desired film thickness may be obtained.
Examples of the method for drying the coating film include reduced-pressure drying, heat drying, and combinations thereof. When drying at normal pressure, it is preferable to dry in a temperature range in which the substrate does not deteriorate, for example, in the range of 30 ° C. to 110 ° C.
塗膜の硬化方法としては、光照射および加熱の少なくともいずれかを用いることができる。
光照射には、主に、紫外線、可視光、電子線、電離放射線等が使用され、中でも紫外線が好ましく用いられる。紫外線硬化の場合には、超高圧水銀灯、高圧水銀灯、低圧水銀灯、カーボンアーク、キセノンアーク、メタルハライドランプ等の光線から発する紫外線を使用する。エネルギー線源の照射量は、紫外線波長365nmでの積算露光量として、例えば50mJ/cm2~5000mJ/cm2の範囲内であることが好ましい。
また、加熱する場合、基板が劣化しない温度範囲で加熱することが好ましく、例えば40℃~120℃の範囲内で加熱することが好ましい。また、25℃程度の室温で24時間以上放置することにより反応を行ってもよい。 As a method for curing the coating film, at least one of light irradiation and heating can be used.
For light irradiation, ultraviolet rays, visible light, electron beams, ionizing radiation, etc. are mainly used, and among these, ultraviolet rays are preferably used. In the case of ultraviolet curing, ultraviolet rays emitted from light such as an ultra high pressure mercury lamp, a high pressure mercury lamp, a low pressure mercury lamp, a carbon arc, a xenon arc, a metal halide lamp are used. The amount of irradiation with the energy radiation source of accumulative exposure at an ultraviolet wavelength of 365 nm, is preferably in the range of, for example, 50mJ / cm 2 ~ 5000mJ / cm 2.
In addition, when heating, it is preferable to heat in a temperature range in which the substrate does not deteriorate, for example, in the range of 40 ° C. to 120 ° C. Moreover, you may react by leaving it to stand for 24 hours or more at room temperature of about 25 degreeC.
光照射には、主に、紫外線、可視光、電子線、電離放射線等が使用され、中でも紫外線が好ましく用いられる。紫外線硬化の場合には、超高圧水銀灯、高圧水銀灯、低圧水銀灯、カーボンアーク、キセノンアーク、メタルハライドランプ等の光線から発する紫外線を使用する。エネルギー線源の照射量は、紫外線波長365nmでの積算露光量として、例えば50mJ/cm2~5000mJ/cm2の範囲内であることが好ましい。
また、加熱する場合、基板が劣化しない温度範囲で加熱することが好ましく、例えば40℃~120℃の範囲内で加熱することが好ましい。また、25℃程度の室温で24時間以上放置することにより反応を行ってもよい。 As a method for curing the coating film, at least one of light irradiation and heating can be used.
For light irradiation, ultraviolet rays, visible light, electron beams, ionizing radiation, etc. are mainly used, and among these, ultraviolet rays are preferably used. In the case of ultraviolet curing, ultraviolet rays emitted from light such as an ultra high pressure mercury lamp, a high pressure mercury lamp, a low pressure mercury lamp, a carbon arc, a xenon arc, a metal halide lamp are used. The amount of irradiation with the energy radiation source of accumulative exposure at an ultraviolet wavelength of 365 nm, is preferably in the range of, for example, 50mJ / cm 2 ~ 5000mJ / cm 2.
In addition, when heating, it is preferable to heat in a temperature range in which the substrate does not deteriorate, for example, in the range of 40 ° C. to 120 ° C. Moreover, you may react by leaving it to stand for 24 hours or more at room temperature of about 25 degreeC.
2.基板
本発明に用いられる基板は、光透過性を有するものであり、ハードコートフィルムの基板として用い得る物性を満たすものであれば特に限定されない。通常、ハードコートフィルムに用いられる基板には、透明、半透明、無色または有色を問わないが、光透過性が要求される。 2. Substrate The substrate used in the present invention is not particularly limited as long as it has optical transparency and satisfies physical properties that can be used as a substrate for a hard coat film. Usually, the substrate used for the hard coat film may be transparent, translucent, colorless or colored, but is required to have light transmittance.
本発明に用いられる基板は、光透過性を有するものであり、ハードコートフィルムの基板として用い得る物性を満たすものであれば特に限定されない。通常、ハードコートフィルムに用いられる基板には、透明、半透明、無色または有色を問わないが、光透過性が要求される。 2. Substrate The substrate used in the present invention is not particularly limited as long as it has optical transparency and satisfies physical properties that can be used as a substrate for a hard coat film. Usually, the substrate used for the hard coat film may be transparent, translucent, colorless or colored, but is required to have light transmittance.
基板の材料としては、例えばアクリレート系ポリマー、ポリカーボネート、ポリエステル、セルロースアシレート、シクロオレフィンポリマー等が挙げられる。アクリレート系ポリマーの具体例としては、ポリ(メタ)アクリル酸メチル、ポリ(メタ)アクリル酸エチル、(メタ)アクリル酸メチル-(メタ)アクリル酸ブチル共重合体等が挙げられる。ポリカーボネートの具体例としては、ビスフェノールA等のビスフェノール類をベースとする芳香族ポリカーボネート、ジエチレングリコールビスアリルカーボネート等の脂肪族ポリカーボネート等が挙げられる。ポリエステルの具体例としては、ポリエチレンテレフタレート、ポリエチレンナフタレート等が挙げられる。セルロースアシレートの具体例としては、セルローストリアセテート、セルロースジアセテート、セルロースアセテートブチレート等が挙げられる。シクロオレフィンポリマーの具体例としては、ノルボルネン系重合体、単環の環状オレフィン系重合体、環状共役ジエン系重合体、ビニル脂環式炭化水素系重合体樹脂等が挙げられる。
Examples of the material for the substrate include acrylate polymers, polycarbonates, polyesters, cellulose acylates, cycloolefin polymers, and the like. Specific examples of the acrylate polymer include methyl poly (meth) acrylate, poly (meth) ethyl acrylate, methyl (meth) acrylate-butyl (meth) acrylate, and the like. Specific examples of the polycarbonate include aromatic polycarbonates based on bisphenols such as bisphenol A, and aliphatic polycarbonates such as diethylene glycol bisallyl carbonate. Specific examples of the polyester include polyethylene terephthalate and polyethylene naphthalate. Specific examples of cellulose acylate include cellulose triacetate, cellulose diacetate, and cellulose acetate butyrate. Specific examples of the cycloolefin polymer include norbornene polymers, monocyclic olefin polymers, cyclic conjugated diene polymers, vinyl alicyclic hydrocarbon polymer resins, and the like.
基板は、単層であってもよく、複数層が積層されたものであってもよい。単層の場合、基板の材料はアクリレート系ポリマーであることが好ましく、ポリメタクリル酸メチルがより好ましい。透明性が高いからである。一方、複数層の場合、基板は複数の樹脂層を有することになる。樹脂層の積層数は、2層以上であればよく、3層~5層の範囲内であることが好ましく、3層であることがより好ましい。
The substrate may be a single layer or a laminate of a plurality of layers. In the case of a single layer, the substrate material is preferably an acrylate polymer, more preferably polymethyl methacrylate. This is because the transparency is high. On the other hand, in the case of a plurality of layers, the substrate has a plurality of resin layers. The number of resin layers may be two or more, preferably in the range of 3 to 5 layers, more preferably 3 layers.
基板が3層以上の樹脂層を有する場合、最も外に位置する2つの層を最外樹脂層とし、2つの最外樹脂層の内側に位置する層を内側樹脂層とする。図2に示すように、例えば基板2が3層の樹脂層2a~2cを有する場合、最も外に位置する2つの樹脂層を最外樹脂層2b、2cとし、2つの最外樹脂層2b、2cの内側に位置する層を内側樹脂層2aとする。なお、内側樹脂層2aは単層であってもよく複数層であってもよい。
When the substrate has three or more resin layers, the outermost two layers are the outermost resin layers and the innermost resin layer is the innermost resin layer. As shown in FIG. 2, for example, when the substrate 2 has three resin layers 2a to 2c, the two outermost resin layers 2b and 2c are defined as the two outermost resin layers 2b and 2c, The layer located inside 2c is referred to as an inner resin layer 2a. The inner resin layer 2a may be a single layer or a plurality of layers.
基板が3層以上の樹脂層を有する場合、基板の両面にそれぞれ位置する2つの最外樹脂層の鉛筆硬度は、内側樹脂層の鉛筆硬度よりも高いことが好ましい。最外樹脂層の硬度を高くすることで、硬度の高いハードコートフィルムを形成しやすくなり、内側樹脂層の硬度を低くすることで、熱膨張率等の違いにより生じる応力を緩和でき、すなわち内側樹脂層がクッション層となり、例えば耐落球試験性のような耐衝撃性が向上するからである。最外樹脂層と内側樹脂層との硬度の差は、鉛筆硬度の基準において、2段階以上離れていることが好ましく、3段階以上離れていることがより好ましい。最外樹脂層の鉛筆硬度は、例えばHB以上であることが好ましく、H以上5H以下であることがより好ましい。内側樹脂層の鉛筆硬度は、例えばH以下であることが好ましく、3B以上HB以下であることがより好ましい。
When the substrate has three or more resin layers, it is preferable that the pencil hardness of the two outermost resin layers positioned on both sides of the substrate is higher than the pencil hardness of the inner resin layer. By increasing the hardness of the outermost resin layer, it becomes easier to form a hard coat film with high hardness, and by reducing the hardness of the inner resin layer, the stress caused by the difference in thermal expansion coefficient, etc. can be relieved, that is, the inner side This is because the resin layer becomes a cushion layer, and for example, impact resistance such as ball drop resistance is improved. The difference in hardness between the outermost resin layer and the inner resin layer is preferably two or more steps, more preferably three or more steps, on the basis of pencil hardness. For example, the pencil hardness of the outermost resin layer is preferably HB or higher, and more preferably H or higher and 5H or lower. The pencil hardness of the inner resin layer is preferably H or less, for example, and more preferably 3B or more and HB or less.
また、基板が複数層の樹脂層を有する場合、基板の鉛筆硬度は2H以上であることが好ましく、3H以上であることがより好ましい。ハードコートフィルムの硬度をさらに向上させることができるからである。なお、基板の鉛筆硬度は高いことが好ましいが、通常は4H以下である。
一方、基板が単層の場合でも、基板の鉛筆硬度は2H以上であることが好ましい。 When the substrate has a plurality of resin layers, the pencil hardness of the substrate is preferably 2H or more, and more preferably 3H or more. This is because the hardness of the hard coat film can be further improved. The pencil hardness of the substrate is preferably high, but is usually 4H or less.
On the other hand, even when the substrate is a single layer, the pencil hardness of the substrate is preferably 2H or more.
一方、基板が単層の場合でも、基板の鉛筆硬度は2H以上であることが好ましい。 When the substrate has a plurality of resin layers, the pencil hardness of the substrate is preferably 2H or more, and more preferably 3H or more. This is because the hardness of the hard coat film can be further improved. The pencil hardness of the substrate is preferably high, but is usually 4H or less.
On the other hand, even when the substrate is a single layer, the pencil hardness of the substrate is preferably 2H or more.
また、図2に例示するように、基板2が3層の樹脂層2a~2cを有する場合、内側樹脂層2aがポリカーボネートであり、2つの最外樹脂層2b、2cがアクリレート系ポリマーであることが好ましい。耐衝撃性が向上するからである。この場合、1つの最外樹脂層の厚さは、60μm~110μmの範囲内であることが好ましい。
Further, as illustrated in FIG. 2, when the substrate 2 has three resin layers 2a to 2c, the inner resin layer 2a is polycarbonate, and the two outermost resin layers 2b and 2c are acrylate polymers. Is preferred. This is because the impact resistance is improved. In this case, the thickness of one outermost resin layer is preferably in the range of 60 μm to 110 μm.
また、基板は青色色材を含有していてもよい。青色色材をハードコート層に添加する代わりに、基板に添加することにより、画像表示装置に用いた場合に黄色味を抑え視認性や色再現性を向上させることが可能なハードコートフィルムを得ることができる。なお、青色色材については、上記ハードコート層に記載したものと同様とすることができる。
In addition, the substrate may contain a blue color material. Instead of adding a blue color material to the hard coat layer, a hard coat film capable of suppressing yellowishness and improving visibility and color reproducibility when used in an image display device is obtained by adding to a substrate. be able to. In addition, about a blue color material, it can be the same as that of what was described in the said hard-coat layer.
基板は、より多くの光を透過することが好ましい。可視光領域における全光線透過率としては、80%以上であることが好ましく、90%以上であることがより好ましい。なお、全光線透過率は、JIS K 7105で規定する方法により測定した値とする。
It is preferable that the substrate transmits more light. The total light transmittance in the visible light region is preferably 80% or more, and more preferably 90% or more. The total light transmittance is a value measured by the method defined in JIS K 7105.
また、基板は可撓性を有していてもよく有さなくてもよい。
基板の厚さは、特に限定されるものではないが、可撓性を有さない基板の場合、0.3mm以上であることが好ましく、0.3mm~5mmの範囲内であることがより好ましい。上記範囲内であれば、十分な耐衝撃性を維持できるからである。一方、可撓性を有する基板の場合、基板の厚さは、材料や構成等により異なるが、例えば10μm~500μmの範囲内で設定することができる。
また、基板には、例えば、けん化処理、グロー放電処理、コロナ放電処理、紫外線処理、火炎処理等の表面処理が施されていてもよい。 Further, the substrate may or may not have flexibility.
The thickness of the substrate is not particularly limited, but in the case of a substrate that does not have flexibility, it is preferably 0.3 mm or more, more preferably in the range of 0.3 mm to 5 mm. . It is because sufficient impact resistance can be maintained within the above range. On the other hand, in the case of a flexible substrate, the thickness of the substrate varies depending on the material, configuration, etc., but can be set within a range of 10 μm to 500 μm, for example.
Further, the substrate may be subjected to surface treatment such as saponification treatment, glow discharge treatment, corona discharge treatment, ultraviolet treatment, flame treatment and the like.
基板の厚さは、特に限定されるものではないが、可撓性を有さない基板の場合、0.3mm以上であることが好ましく、0.3mm~5mmの範囲内であることがより好ましい。上記範囲内であれば、十分な耐衝撃性を維持できるからである。一方、可撓性を有する基板の場合、基板の厚さは、材料や構成等により異なるが、例えば10μm~500μmの範囲内で設定することができる。
また、基板には、例えば、けん化処理、グロー放電処理、コロナ放電処理、紫外線処理、火炎処理等の表面処理が施されていてもよい。 Further, the substrate may or may not have flexibility.
The thickness of the substrate is not particularly limited, but in the case of a substrate that does not have flexibility, it is preferably 0.3 mm or more, more preferably in the range of 0.3 mm to 5 mm. . It is because sufficient impact resistance can be maintained within the above range. On the other hand, in the case of a flexible substrate, the thickness of the substrate varies depending on the material, configuration, etc., but can be set within a range of 10 μm to 500 μm, for example.
Further, the substrate may be subjected to surface treatment such as saponification treatment, glow discharge treatment, corona discharge treatment, ultraviolet treatment, flame treatment and the like.
3.第2のハードコート層
本発明においては、図3に例示するように、基板2のハードコート層3の形成面とは反対側の面に第2のハードコート層4が形成されていてもよい。第2のハードコート層が形成されていることにより、ハードコートフィルムの硬度をさらに向上させることができる。 3. Second Hard Coat Layer In the present invention, as illustrated in FIG. 3, the secondhard coat layer 4 may be formed on the surface of the substrate 2 opposite to the surface on which the hard coat layer 3 is formed. . By forming the second hard coat layer, the hardness of the hard coat film can be further improved.
本発明においては、図3に例示するように、基板2のハードコート層3の形成面とは反対側の面に第2のハードコート層4が形成されていてもよい。第2のハードコート層が形成されていることにより、ハードコートフィルムの硬度をさらに向上させることができる。 3. Second Hard Coat Layer In the present invention, as illustrated in FIG. 3, the second
なお、第2のハードコート層に用いられる硬化性樹脂組成物については、上記ハードコート層に用いられる硬化性樹脂組成物と同様である。
中でも、第2のハードコート層に用いられる硬化性樹脂組成物はウレタンアクリレートを含有することが好ましい。この場合、上記ハードコート層に用いられる硬化性樹脂組成物もウレタンアクリレートを含有することが好ましい。上述したように、ウレタンアクリレートを添加することにより、ハードコート層および第2のハードコート層に柔軟性を付与することができ、反りの発生を抑制するとともに、耐衝撃性を向上させることができるからである。
第2のハードコート層に用いられる硬化性樹脂組成物は、上記ハードコート層に用いられる硬化性樹脂組成物と同じであってもよく異なっていてもよい。中でも、第2のハードコート層に用いられる硬化性樹脂組成物は、上記ハードコート層に用いられる硬化性樹脂組成物と同じであることが好ましい。反りの発生をさらに抑制できるからである。 The curable resin composition used for the second hard coat layer is the same as the curable resin composition used for the hard coat layer.
Especially, it is preferable that the curable resin composition used for a 2nd hard-coat layer contains urethane acrylate. In this case, it is preferable that the curable resin composition used for the hard coat layer also contains urethane acrylate. As described above, by adding urethane acrylate, flexibility can be imparted to the hard coat layer and the second hard coat layer, occurrence of warpage can be suppressed, and impact resistance can be improved. Because.
The curable resin composition used for the second hard coat layer may be the same as or different from the curable resin composition used for the hard coat layer. Especially, it is preferable that the curable resin composition used for a 2nd hard-coat layer is the same as the curable resin composition used for the said hard-coat layer. This is because warpage can be further suppressed.
中でも、第2のハードコート層に用いられる硬化性樹脂組成物はウレタンアクリレートを含有することが好ましい。この場合、上記ハードコート層に用いられる硬化性樹脂組成物もウレタンアクリレートを含有することが好ましい。上述したように、ウレタンアクリレートを添加することにより、ハードコート層および第2のハードコート層に柔軟性を付与することができ、反りの発生を抑制するとともに、耐衝撃性を向上させることができるからである。
第2のハードコート層に用いられる硬化性樹脂組成物は、上記ハードコート層に用いられる硬化性樹脂組成物と同じであってもよく異なっていてもよい。中でも、第2のハードコート層に用いられる硬化性樹脂組成物は、上記ハードコート層に用いられる硬化性樹脂組成物と同じであることが好ましい。反りの発生をさらに抑制できるからである。 The curable resin composition used for the second hard coat layer is the same as the curable resin composition used for the hard coat layer.
Especially, it is preferable that the curable resin composition used for a 2nd hard-coat layer contains urethane acrylate. In this case, it is preferable that the curable resin composition used for the hard coat layer also contains urethane acrylate. As described above, by adding urethane acrylate, flexibility can be imparted to the hard coat layer and the second hard coat layer, occurrence of warpage can be suppressed, and impact resistance can be improved. Because.
The curable resin composition used for the second hard coat layer may be the same as or different from the curable resin composition used for the hard coat layer. Especially, it is preferable that the curable resin composition used for a 2nd hard-coat layer is the same as the curable resin composition used for the said hard-coat layer. This is because warpage can be further suppressed.
また、第2のハードコート層の厚みについては、上記ハードコート層の厚みと同様である。第2のハードコート層の厚みは、上記ハードコート層の厚みと同一であってもよく異なっていてもよい。中でも、第2のハードコート層の厚みは、上記ハードコート層の厚みと同一であることが好ましい。上記の場合と同様に、反りの発生をさらに抑制できるからである。
Further, the thickness of the second hard coat layer is the same as the thickness of the hard coat layer. The thickness of the second hard coat layer may be the same as or different from the thickness of the hard coat layer. Especially, it is preferable that the thickness of a 2nd hard-coat layer is the same as the thickness of the said hard-coat layer. It is because generation | occurrence | production of curvature can further be suppressed similarly to said case.
第2のハードコート層の特性や形成方法等については、上記ハードコート層と同様である。
The characteristics and formation method of the second hard coat layer are the same as those of the hard coat layer.
4.防眩層
本発明においては、図4に例示するようにハードコート層3上に防眩層5が形成されていてもよい。防眩層が形成されていることで、本発明のハードコートフィルム表面での光の反射を抑制することができ、本発明のハードコートフィルムを画像表示装置に用いた場合には外光の映り込みを抑え、ぎらつきを抑制することができる。また、本発明におけるハードコート層は硬度に優れるため、ハードコート層上に防眩層を形成した場合においても高硬度を達成することができる。 4). Antiglare Layer In the present invention, theantiglare layer 5 may be formed on the hard coat layer 3 as illustrated in FIG. By forming the antiglare layer, reflection of light on the surface of the hard coat film of the present invention can be suppressed, and when the hard coat film of the present invention is used for an image display device, it reflects external light. Can suppress glare and glare. In addition, since the hard coat layer in the present invention is excellent in hardness, high hardness can be achieved even when an antiglare layer is formed on the hard coat layer.
本発明においては、図4に例示するようにハードコート層3上に防眩層5が形成されていてもよい。防眩層が形成されていることで、本発明のハードコートフィルム表面での光の反射を抑制することができ、本発明のハードコートフィルムを画像表示装置に用いた場合には外光の映り込みを抑え、ぎらつきを抑制することができる。また、本発明におけるハードコート層は硬度に優れるため、ハードコート層上に防眩層を形成した場合においても高硬度を達成することができる。 4). Antiglare Layer In the present invention, the
防眩層としては、防眩性を有するものであればよいが、中でも樹脂および微粒子を含有するものであることが好ましい。微粒子によって防眩層の表面に凹凸が形成されることで防眩性を発揮することができる。
The antiglare layer is not particularly limited as long as it has an antiglare property, but among them, a layer containing a resin and fine particles is preferable. Antiglare property can be exhibited by forming irregularities on the surface of the antiglare layer by the fine particles.
微粒子としては、防眩性を発揮するものであれば特に限定されるものではないが、透光性微粒子であることが好ましい。また、微粒子としては、無機系および有機系のいずれも用いることができる。
無機系微粒子としては、例えば不定形シリカ粒子、無機シリカ粒子等を挙げることができる。
有機系微粒子としては、例えばプラスチックビーズが挙げられ、具体例としてはポリスチレンビーズ、メラミン樹脂ビーズ、アクリルビーズ、アクリル-スチレンビーズ、ベンゾグアナミンビーズ、ベンゾグアナミンホルムアルデヒド縮合ビーズ、ポリカーボネートビーズ、ポリエチレンビーズ等が挙げられる。プラスチックビーズは、その表面に疎水性基を有することが好ましく、例えばスチレンビーズを挙げることができる。
微粒子は、1種単独で用いてもよく、2種以上を混合して用いてもよい。また、微粒子は、1次粒子であってもよく、2次粒子であってもよい。
中でも、防眩層の膜強度向上の点から無機系微粒子を使用することが好ましく、シリカ微粒子を用いることが特に好ましい。
また、不定形シリカ粒子の場合、良好な分散性を得るために、粒子表面に有機物処理を施して疎水化した不定形シリカ粒子を使用することが好ましい。有機物処理については、特開2009-86361号公報に記載されている有機物処理と同様とすることができる。 The fine particles are not particularly limited as long as they exhibit antiglare properties, but are preferably translucent fine particles. As the fine particles, both inorganic and organic can be used.
Examples of the inorganic fine particles include amorphous silica particles and inorganic silica particles.
Examples of the organic fine particles include plastic beads, and specific examples include polystyrene beads, melamine resin beads, acrylic beads, acrylic-styrene beads, benzoguanamine beads, benzoguanamine formaldehyde condensation beads, polycarbonate beads, polyethylene beads, and the like. The plastic beads preferably have a hydrophobic group on the surface, and examples thereof include styrene beads.
The fine particles may be used alone or in combination of two or more. The fine particles may be primary particles or secondary particles.
Among these, inorganic fine particles are preferably used from the viewpoint of improving the film strength of the antiglare layer, and silica fine particles are particularly preferably used.
In the case of amorphous silica particles, in order to obtain good dispersibility, it is preferable to use amorphous silica particles that have been subjected to organic treatment on the surface of the particles to make them hydrophobic. The organic matter treatment can be the same as the organic matter treatment described in JP2009-86361A.
無機系微粒子としては、例えば不定形シリカ粒子、無機シリカ粒子等を挙げることができる。
有機系微粒子としては、例えばプラスチックビーズが挙げられ、具体例としてはポリスチレンビーズ、メラミン樹脂ビーズ、アクリルビーズ、アクリル-スチレンビーズ、ベンゾグアナミンビーズ、ベンゾグアナミンホルムアルデヒド縮合ビーズ、ポリカーボネートビーズ、ポリエチレンビーズ等が挙げられる。プラスチックビーズは、その表面に疎水性基を有することが好ましく、例えばスチレンビーズを挙げることができる。
微粒子は、1種単独で用いてもよく、2種以上を混合して用いてもよい。また、微粒子は、1次粒子であってもよく、2次粒子であってもよい。
中でも、防眩層の膜強度向上の点から無機系微粒子を使用することが好ましく、シリカ微粒子を用いることが特に好ましい。
また、不定形シリカ粒子の場合、良好な分散性を得るために、粒子表面に有機物処理を施して疎水化した不定形シリカ粒子を使用することが好ましい。有機物処理については、特開2009-86361号公報に記載されている有機物処理と同様とすることができる。 The fine particles are not particularly limited as long as they exhibit antiglare properties, but are preferably translucent fine particles. As the fine particles, both inorganic and organic can be used.
Examples of the inorganic fine particles include amorphous silica particles and inorganic silica particles.
Examples of the organic fine particles include plastic beads, and specific examples include polystyrene beads, melamine resin beads, acrylic beads, acrylic-styrene beads, benzoguanamine beads, benzoguanamine formaldehyde condensation beads, polycarbonate beads, polyethylene beads, and the like. The plastic beads preferably have a hydrophobic group on the surface, and examples thereof include styrene beads.
The fine particles may be used alone or in combination of two or more. The fine particles may be primary particles or secondary particles.
Among these, inorganic fine particles are preferably used from the viewpoint of improving the film strength of the antiglare layer, and silica fine particles are particularly preferably used.
In the case of amorphous silica particles, in order to obtain good dispersibility, it is preferable to use amorphous silica particles that have been subjected to organic treatment on the surface of the particles to make them hydrophobic. The organic matter treatment can be the same as the organic matter treatment described in JP2009-86361A.
微粒子の形状としては、例えば真球状、楕円状、不定形、直方体状、立方体状等を挙げることができる。
Examples of the shape of the fine particles include a true spherical shape, an elliptical shape, an indefinite shape, a rectangular parallelepiped shape, and a cubic shape.
微粒子の平均粒径としては、防眩性を付与できる程度であれば特に限定されるものではないが、中でも防眩層の表面に凹凸を形成可能な程度であることが好ましく、具体的には1μm~10μmの範囲内であることが好ましく、中でも1μm~7μmの範囲内、特に2μm~5μmの範囲内であることが好ましい。平均粒径が小さいと、防眩層の表面に十分な防眩性を発揮できる大きさの凹凸を付与することが困難であり、仮に凹凸を付与できるとしても、微粒子の添加量を非常に多くしなければならないため、防眩層の膜物性が悪くなる場合がある。また、平均粒径が大きいと、防眩層の表面形状が粗くなり、面質を悪化させたり、表面へイズの上昇により白味が増したりするおそれがある。微粒子は凝集粒子であってもよく、凝集粒子である場合は2次粒径が上記範囲内であればよい。
The average particle diameter of the fine particles is not particularly limited as long as the antiglare property can be imparted, but it is preferable that irregularities can be formed on the surface of the antiglare layer. The thickness is preferably in the range of 1 μm to 10 μm, more preferably in the range of 1 μm to 7 μm, and particularly preferably in the range of 2 μm to 5 μm. If the average particle size is small, it is difficult to provide the surface of the antiglare layer with irregularities large enough to exhibit antiglare properties, and even if irregularities can be provided, the amount of fine particles added is very large. Therefore, the film physical properties of the antiglare layer may be deteriorated. On the other hand, if the average particle size is large, the surface shape of the antiglare layer becomes rough, and the surface quality may be deteriorated, or whiteness may increase due to an increase in surface haze. The fine particles may be agglomerated particles. In the case of agglomerated particles, the secondary particle size may be in the above range.
また、微粒子全体の80%以上、中でも90%以上の微粒子の粒径が、平均粒径±300nmの範囲内にあることが好ましい。これにより、防眩層表面の凹凸形状の均一性を良好なものとすることができる。
Further, it is preferable that the particle size of fine particles of 80% or more, especially 90% or more of the whole fine particles is in the range of average particle size ± 300 nm. Thereby, the uniformity of the uneven shape on the surface of the antiglare layer can be improved.
なお、微粒子の平均粒径は、各々の微粒子が、単分散型の微粒子、すなわち形状が単一な微粒子である場合はその平均を意味し、ブロードな粒度分布を持つ不定形の微粒子である場合は粒度分布測定により最も多く存在する微粒子の粒径を意味する。微粒子の粒径は、主にコールターカウンター法により計測できる。また、この方法以外に、レーザー回折法、SEM写真撮影による測定によっても計測できる。
The average particle size of the fine particles means the average when each fine particle is a monodispersed fine particle, that is, a fine particle having a single shape, and is an irregular fine particle having a broad particle size distribution. Means the particle size of fine particles present most abundantly by particle size distribution measurement. The particle size of the fine particles can be measured mainly by the Coulter counter method. In addition to this method, measurement can also be performed by laser diffraction or SEM photography.
防眩層中の微粒子の含有量としては、防眩性を発揮できる量であればよく適宜調整される。防眩層中の微粒子の含有量は、例えば樹脂100重量部に対し、2重量部~35重量部の範囲内であることが好ましく、5重量部~25重量部の範囲内であることがより好ましい。微粒子の含有量が少ないと十分な防眩性が付与できない場合がある。また、微粒子の含有量が多いと強度や硬度が低下したり、内部散乱効果が過剰となりコントラストが低下したりする場合がある。なお、防眩層中の樹脂および微粒子の含有量の比率を調整することにより、防眩層のヘイズおよび光沢を調整することができる。
The content of the fine particles in the antiglare layer may be adjusted as long as it is an amount capable of exhibiting antiglare properties. The content of the fine particles in the antiglare layer is, for example, preferably in the range of 2 to 35 parts by weight with respect to 100 parts by weight of the resin, and more preferably in the range of 5 to 25 parts by weight. preferable. If the content of the fine particles is small, sufficient antiglare properties may not be imparted. In addition, if the content of fine particles is large, the strength and hardness may decrease, or the internal scattering effect may be excessive and the contrast may decrease. The haze and gloss of the antiglare layer can be adjusted by adjusting the ratio of the resin and fine particle content in the antiglare layer.
樹脂としては、硬化性樹脂を用いることが好ましく、例えば熱硬化性樹脂、紫外線や電子線等の電離放射線により硬化する電離放射線硬化性樹脂を用いることができる。硬化性樹脂としては、防眩層に一般的に用いられるものを使用することができる。例えば、特開2009-86361号公報に記載されている防眩層に用いられるものを挙げることができる。
As the resin, a curable resin is preferably used. For example, a thermosetting resin or an ionizing radiation curable resin that is cured by an ionizing radiation such as an ultraviolet ray or an electron beam can be used. As curable resin, what is generally used for a glare-proof layer can be used. Examples thereof include those used for the antiglare layer described in JP-A-2009-86361.
防眩層は、界面活性剤を含有していてもよい。界面活性剤としては、上記ハードコート層に用いられるものを使用することができる。
また、防眩層は、必要に応じて、重合開始剤、帯電防止剤、防汚染剤等を含有していてもよい。 The antiglare layer may contain a surfactant. As the surfactant, those used for the hard coat layer can be used.
Further, the antiglare layer may contain a polymerization initiator, an antistatic agent, an antifouling agent, and the like as necessary.
また、防眩層は、必要に応じて、重合開始剤、帯電防止剤、防汚染剤等を含有していてもよい。 The antiglare layer may contain a surfactant. As the surfactant, those used for the hard coat layer can be used.
Further, the antiglare layer may contain a polymerization initiator, an antistatic agent, an antifouling agent, and the like as necessary.
また、防眩層は青色色材を含有していてもよい。青色色材をハードコート層に添加する代わりに、防眩層に添加することにより、画像表示装置に用いた場合に黄色味を抑え視認性や色再現性を向上させることが可能なハードコートフィルムを得ることができる。なお、青色色材については、上記ハードコート層に記載したものと同様とすることができる。
The antiglare layer may contain a blue color material. Hard coat film that can suppress yellowishness and improve visibility and color reproducibility when used in an image display device by adding a blue color material to the antiglare layer instead of adding it to the hard coat layer Can be obtained. In addition, about a blue color material, it can be the same as that of what was described in the said hard-coat layer.
防眩層の硬度は、JIS K5600-5-4(1999)で規定される鉛筆硬度試験(4.9N荷重)で評価できる。防眩層の鉛筆硬度としては、上記ハードコート層の鉛筆硬度と同様とすることができる。
The hardness of the antiglare layer can be evaluated by a pencil hardness test (4.9 N load) defined in JIS K5600-5-4 (1999). The pencil hardness of the antiglare layer can be the same as the pencil hardness of the hard coat layer.
防眩層は光透過性を有するものである。防眩層の可視光領域における透過率としては、上記ハードコート層の透過率と同様とすることができる。
The antiglare layer is light transmissive. The transmittance of the antiglare layer in the visible light region can be the same as the transmittance of the hard coat layer.
また、防眩層のヘイズ値としては、本発明のハードコートフィルムの用途等に応じて適宜決定されるものであり、特に限定されるものではなく、例えば3%~30%の範囲内で設定することができる。
なお、ヘイズ値は、JIS K-7105に従って測定することができる。測定に使用する機器としては、例えば村上色彩技術研究所製のヘイズメーターHM150が挙げられる。 Further, the haze value of the antiglare layer is appropriately determined according to the use of the hard coat film of the present invention, and is not particularly limited, and is set within a range of 3% to 30%, for example. can do.
The haze value can be measured according to JIS K-7105. As an apparatus used for the measurement, for example, there is a haze meter HM150 manufactured by Murakami Color Research Laboratory.
なお、ヘイズ値は、JIS K-7105に従って測定することができる。測定に使用する機器としては、例えば村上色彩技術研究所製のヘイズメーターHM150が挙げられる。 Further, the haze value of the antiglare layer is appropriately determined according to the use of the hard coat film of the present invention, and is not particularly limited, and is set within a range of 3% to 30%, for example. can do.
The haze value can be measured according to JIS K-7105. As an apparatus used for the measurement, for example, there is a haze meter HM150 manufactured by Murakami Color Research Laboratory.
防眩層は防汚性を有することが好ましい。防汚性は濡れ性で評価することができる。防眩層表面の濡れ性としては、上記ハードコート層表面の濡れ性と同様とすることができる。
The antiglare layer preferably has antifouling properties. The antifouling property can be evaluated by wettability. The wettability of the antiglare layer surface can be the same as the wettability of the hard coat layer surface.
また、本発明のハードコートフィルムがタッチパネル等に用いられる場合には、防眩層は易滑性を有することが好ましい。易滑性は動摩擦係数で評価することができる。防眩層の表面の動摩擦係数としては、上記ハードコート層の表面の動摩擦係数と同様とすることができる。
In addition, when the hard coat film of the present invention is used for a touch panel or the like, the antiglare layer preferably has slipperiness. The slipperiness can be evaluated by a dynamic friction coefficient. The dynamic friction coefficient of the surface of the antiglare layer can be the same as the dynamic friction coefficient of the surface of the hard coat layer.
防眩層の厚みとしては、所望の防眩性を発揮することが可能であれば特に限定されるものではないが、例えば1μm~5μmの範囲内であることが好ましい。防眩層の厚みを上記範囲内とすることにより、防眩層に含まれる微粒子の量を低減することができる。これにより、防眩層での過剰な内部散乱効果を抑制し、所望の内部散乱効果を確保しながら、コントラストの低下を抑制することができる。さらに、防眩層が極薄膜となることにより、防眩層に接して形成されているハードコート層によって高硬度を得ることができる。
The thickness of the antiglare layer is not particularly limited as long as the desired antiglare property can be exhibited, but it is preferably in the range of 1 μm to 5 μm, for example. By setting the thickness of the antiglare layer within the above range, the amount of fine particles contained in the antiglare layer can be reduced. Thereby, the excessive internal scattering effect in an anti-glare layer can be suppressed, and the fall of contrast can be suppressed, ensuring the desired internal scattering effect. Furthermore, when the antiglare layer is an extremely thin film, high hardness can be obtained by the hard coat layer formed in contact with the antiglare layer.
防眩層の形成方法は、ハードコート層上に硬化性樹脂組成物を塗布し、塗膜を硬化させる方法を用いることができる。
なお、防眩層の形成方法については、後述の「C.ハードコートフィルムの製造方法」に記載するので、ここでの説明は省略する。 As a method for forming the antiglare layer, a method of applying a curable resin composition on the hard coat layer and curing the coating film can be used.
In addition, since it forms in the below-mentioned "C. manufacturing method of a hard coat film" about the formation method of an anti-glare layer, description here is abbreviate | omitted.
なお、防眩層の形成方法については、後述の「C.ハードコートフィルムの製造方法」に記載するので、ここでの説明は省略する。 As a method for forming the antiglare layer, a method of applying a curable resin composition on the hard coat layer and curing the coating film can be used.
In addition, since it forms in the below-mentioned "C. manufacturing method of a hard coat film" about the formation method of an anti-glare layer, description here is abbreviate | omitted.
5.その他の構成
本発明のハードコートフィルムは、基板およびハードコート層を有していればよいが、ハードコートフィルムの用途等に応じて他の層をさらに有していてもよい。他の層としては、例えばプライマー層、帯電防止層、防眩層、低屈折率層等が挙げられる。 5. Other Configurations The hard coat film of the present invention may have a substrate and a hard coat layer, but may further have another layer depending on the use of the hard coat film. Examples of other layers include a primer layer, an antistatic layer, an antiglare layer, and a low refractive index layer.
本発明のハードコートフィルムは、基板およびハードコート層を有していればよいが、ハードコートフィルムの用途等に応じて他の層をさらに有していてもよい。他の層としては、例えばプライマー層、帯電防止層、防眩層、低屈折率層等が挙げられる。 5. Other Configurations The hard coat film of the present invention may have a substrate and a hard coat layer, but may further have another layer depending on the use of the hard coat film. Examples of other layers include a primer layer, an antistatic layer, an antiglare layer, and a low refractive index layer.
また本発明においては、基板のハードコート層の形成面とは反対側の面に青色色材を含有する着色層が形成されていてもよい。青色色材をハードコート層に添加する代わりに、青色色材を含有する着色層を形成することにより、画像表示装置に用いた場合に黄色味を抑え視認性や色再現性を向上させることができるハードコートフィルムを得ることができる。
着色層は青色色材およびバインダー樹脂を含有するものである。なお、青色色材については、上記ハードコート層に記載したものと同様とすることができる。バインダー樹脂としては、一般的なものを用いることができ、例えば特開2000-57976号公報に記載の着色層に用いられる樹脂を使用することができる。 In the present invention, a colored layer containing a blue color material may be formed on the surface of the substrate opposite to the surface on which the hard coat layer is formed. Instead of adding a blue color material to the hard coat layer, by forming a colored layer containing a blue color material, it is possible to suppress yellowness and improve visibility and color reproducibility when used in an image display device. A hard coat film can be obtained.
The colored layer contains a blue color material and a binder resin. In addition, about a blue color material, it can be the same as that of what was described in the said hard-coat layer. As the binder resin, a general one can be used. For example, a resin used for a colored layer described in JP-A-2000-57976 can be used.
着色層は青色色材およびバインダー樹脂を含有するものである。なお、青色色材については、上記ハードコート層に記載したものと同様とすることができる。バインダー樹脂としては、一般的なものを用いることができ、例えば特開2000-57976号公報に記載の着色層に用いられる樹脂を使用することができる。 In the present invention, a colored layer containing a blue color material may be formed on the surface of the substrate opposite to the surface on which the hard coat layer is formed. Instead of adding a blue color material to the hard coat layer, by forming a colored layer containing a blue color material, it is possible to suppress yellowness and improve visibility and color reproducibility when used in an image display device. A hard coat film can be obtained.
The colored layer contains a blue color material and a binder resin. In addition, about a blue color material, it can be the same as that of what was described in the said hard-coat layer. As the binder resin, a general one can be used. For example, a resin used for a colored layer described in JP-A-2000-57976 can be used.
また、本発明のハードコートフィルムは可撓性を有していてもよく有さなくてもよい。
Moreover, the hard coat film of the present invention may or may not have flexibility.
6.用途
本発明のハードコートフィルムの用途は特に限定されるものではなく、例えば、タッチパネル等の接触式画像表示装置用途、液晶ディスプレイ、CRTディスプレイ、プロジェクションディスプレイ、プラズマディスプレイ、エレクトロルミネッセンスディスプレイ等の非接触式画像表示装置用途、色素増感型太陽電池等の太陽電池用途等が挙げられる。中でも、本発明のハードコートフィルムは、タッチパネルの前面板として用いられることが好ましい。タッチパネルの前面板は、指が直接接触する部材であり、高い硬度および耐擦傷性が求められているからである。 6). Uses The use of the hard coat film of the present invention is not particularly limited. For example, contact-type image display devices such as touch panels, non-contact types such as liquid crystal displays, CRT displays, projection displays, plasma displays, and electroluminescence displays. Examples include image display device applications and solar cell applications such as dye-sensitized solar cells. Especially, it is preferable that the hard coat film of this invention is used as a front plate of a touch panel. This is because the front plate of the touch panel is a member in direct contact with a finger, and high hardness and scratch resistance are required.
本発明のハードコートフィルムの用途は特に限定されるものではなく、例えば、タッチパネル等の接触式画像表示装置用途、液晶ディスプレイ、CRTディスプレイ、プロジェクションディスプレイ、プラズマディスプレイ、エレクトロルミネッセンスディスプレイ等の非接触式画像表示装置用途、色素増感型太陽電池等の太陽電池用途等が挙げられる。中でも、本発明のハードコートフィルムは、タッチパネルの前面板として用いられることが好ましい。タッチパネルの前面板は、指が直接接触する部材であり、高い硬度および耐擦傷性が求められているからである。 6). Uses The use of the hard coat film of the present invention is not particularly limited. For example, contact-type image display devices such as touch panels, non-contact types such as liquid crystal displays, CRT displays, projection displays, plasma displays, and electroluminescence displays. Examples include image display device applications and solar cell applications such as dye-sensitized solar cells. Especially, it is preferable that the hard coat film of this invention is used as a front plate of a touch panel. This is because the front plate of the touch panel is a member in direct contact with a finger, and high hardness and scratch resistance are required.
B.ハードコート層用硬化性樹脂組成物
本発明のハードコート層用硬化性樹脂組成物は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有することを特徴とするものである。 B. Curable resin composition for hard coat layer The curable resin composition for hard coat layer of the present invention comprises reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, a monomer, It is characterized by containing.
本発明のハードコート層用硬化性樹脂組成物は、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有することを特徴とするものである。 B. Curable resin composition for hard coat layer The curable resin composition for hard coat layer of the present invention comprises reactive irregularly shaped silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, a monomer, It is characterized by containing.
本発明においては、ハードコート層用硬化性樹脂組成物が反応性異形シリカ微粒子を含むことにより、硬度および耐擦傷性に優れるハードコート層を得ることができる。また、ハードコート層用硬化性樹脂組成物に重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを添加することにより、高硬度および加工性の両立を図ることが可能である。
In the present invention, a hard coat layer having excellent hardness and scratch resistance can be obtained when the curable resin composition for a hard coat layer contains reactive deformed silica fine particles. Moreover, it is possible to achieve both high hardness and workability by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range to the curable resin composition for a hard coat layer.
なお、ハードコート層用硬化性樹脂組成物については、上記「A.ハードコートフィルム 1.ハードコート層」の項に詳しく記載したので、ここでの説明は省略する。
Since the curable resin composition for the hard coat layer was described in detail in the above section “A. Hard coat film 1. Hard coat layer”, description thereof is omitted here.
また、本発明のハードコート層用硬化性樹脂組成物は、インクとして用いることが可能である。インクを調製する際には、例えば粘度や固形分濃度を調整してもよい。具体的には溶剤の添加や加熱により粘度や固形分濃度を調整することができる。溶剤としては、上記「A.ハードコートフィルム 1.ハードコート層」の項に記載した硬化性樹脂組成物に用いられる溶剤を使用することができる。
Further, the curable resin composition for a hard coat layer of the present invention can be used as an ink. When preparing the ink, for example, the viscosity and solid content concentration may be adjusted. Specifically, the viscosity and solid content concentration can be adjusted by adding a solvent or heating. As a solvent, the solvent used for the curable resin composition described in the above-mentioned item of “A. Hard coat film 1. Hard coat layer” can be used.
C.ハードコートフィルムの製造方法
本発明のハードコートフィルムの製造方法は、基板上に、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーとを含有するハードコート層用硬化性樹脂組成物を塗布し、硬化させてハードコート層を形成するハードコート層形成工程を有することを特徴とする。
以下、本発明のハードコートフィルムの製造方法における各工程について説明する。 C. Method for producing hard coat film The method for producing a hard coat film of the present invention comprises a reactive irregularly shaped silica fine particle on a substrate, a weight average molecular weight in the range of 30,000 to 110,000, and an acrylic equivalent of 200 to A hard coat layer forming step of forming a hard coat layer by applying and curing a curable resin composition for a hard coat layer containing an acrylic polymer in the range of 1,200 and a monomer. And
Hereinafter, each process in the manufacturing method of the hard coat film of this invention is demonstrated.
本発明のハードコートフィルムの製造方法は、基板上に、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーとを含有するハードコート層用硬化性樹脂組成物を塗布し、硬化させてハードコート層を形成するハードコート層形成工程を有することを特徴とする。
以下、本発明のハードコートフィルムの製造方法における各工程について説明する。 C. Method for producing hard coat film The method for producing a hard coat film of the present invention comprises a reactive irregularly shaped silica fine particle on a substrate, a weight average molecular weight in the range of 30,000 to 110,000, and an acrylic equivalent of 200 to A hard coat layer forming step of forming a hard coat layer by applying and curing a curable resin composition for a hard coat layer containing an acrylic polymer in the range of 1,200 and a monomer. And
Hereinafter, each process in the manufacturing method of the hard coat film of this invention is demonstrated.
1.ハードコート層形成工程
本発明においては、基板上に、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有するハードコート層用硬化性樹脂組成物を塗布し、硬化させてハードコート層を形成するハードコート層形成工程を行う。
なお、ハードコート層用硬化性樹脂組成物およびハードコート層の形成方法については、上記「A.ハードコートフィルム 1.ハードコート層」に記載したので、ここでの説明は省略する。 1. Hard coat layer forming step In the present invention, on the substrate, hardened layer hardenability containing reactive irregular silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. A hard coat layer forming step is performed in which the resin composition is applied and cured to form a hard coat layer.
In addition, since it described in the said "A. hard-coat film 1. hard-coat layer" about the curable resin composition for hard-coat layers, and the formation method of a hard-coat layer, description here is abbreviate | omitted.
本発明においては、基板上に、反応性異形シリカ微粒子と、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーと、モノマーとを含有するハードコート層用硬化性樹脂組成物を塗布し、硬化させてハードコート層を形成するハードコート層形成工程を行う。
なお、ハードコート層用硬化性樹脂組成物およびハードコート層の形成方法については、上記「A.ハードコートフィルム 1.ハードコート層」に記載したので、ここでの説明は省略する。 1. Hard coat layer forming step In the present invention, on the substrate, hardened layer hardenability containing reactive irregular silica fine particles, an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range, and a monomer. A hard coat layer forming step is performed in which the resin composition is applied and cured to form a hard coat layer.
In addition, since it described in the said "A. hard-
また、後述するようにハードコート層上に防眩層を形成する場合には、ハードコート層形成工程にてハードコート層用硬化性樹脂組成物の塗膜を半硬化させることが好ましい。半硬化させたハードコート層用硬化性樹脂組成物の塗膜上に防眩層用硬化性樹脂組成物を塗布して硬化させることで、ハードコート層および防眩層の密着性を高めることができるからである。
ハードコート層用硬化性樹脂組成物の塗膜を半硬化させる場合には、例えばエネルギー線源の照射量を少なくすればよい。 Moreover, when forming a glare-proof layer on a hard-coat layer so that it may mention later, it is preferable to semi-harden the coating film of the curable resin composition for hard-coat layers at a hard-coat layer formation process. The adhesion of the hard coat layer and the anti-glare layer can be improved by applying and curing the curable resin composition for the anti-glare layer on the semi-cured coating film of the curable resin composition for the hard coat layer. Because it can.
In the case of semi-curing the coating film of the curable resin composition for the hard coat layer, for example, the irradiation amount of the energy ray source may be reduced.
ハードコート層用硬化性樹脂組成物の塗膜を半硬化させる場合には、例えばエネルギー線源の照射量を少なくすればよい。 Moreover, when forming a glare-proof layer on a hard-coat layer so that it may mention later, it is preferable to semi-harden the coating film of the curable resin composition for hard-coat layers at a hard-coat layer formation process. The adhesion of the hard coat layer and the anti-glare layer can be improved by applying and curing the curable resin composition for the anti-glare layer on the semi-cured coating film of the curable resin composition for the hard coat layer. Because it can.
In the case of semi-curing the coating film of the curable resin composition for the hard coat layer, for example, the irradiation amount of the energy ray source may be reduced.
2.防眩層形成工程
本発明においては、上記ハードコート層上に防眩層用硬化性樹脂組成物を塗布し、硬化させて防眩層を形成する防眩層形成工程を行ってもよい。
ハードコート層形成工程および防眩層形成工程では、基板上にハードコート層用樹脂組成物を塗布し、ハードコート層用硬化性樹脂組成物の塗膜を半硬化させた後、半硬化させたハードコート層用硬化性樹脂組成物の塗膜上に防眩層用硬化性樹脂組成物を塗布し、ハードコート層用硬化性樹脂組成物の塗膜および防眩層用硬化性樹脂組成物の塗膜を硬化させてハードコート層および防眩層を形成することが好ましい。ハードコート層との密着性が良好な防眩層を得ることができるからである。 2. Antiglare layer forming step In the present invention, an antiglare layer forming step of forming an antiglare layer by applying a curable resin composition for an antiglare layer on the hard coat layer and curing it may be performed.
In the hard coat layer forming step and the antiglare layer forming step, the hard coat layer resin composition was applied onto the substrate, the coating film of the hard coat layer curable resin composition was semi-cured, and then semi-cured. The curable resin composition for the antiglare layer is applied onto the coating film of the curable resin composition for the hard coat layer, and the curable resin composition for the hard coat layer and the curable resin composition for the antiglare layer are coated. It is preferable to cure the coating film to form a hard coat layer and an antiglare layer. This is because an antiglare layer having good adhesion to the hard coat layer can be obtained.
本発明においては、上記ハードコート層上に防眩層用硬化性樹脂組成物を塗布し、硬化させて防眩層を形成する防眩層形成工程を行ってもよい。
ハードコート層形成工程および防眩層形成工程では、基板上にハードコート層用樹脂組成物を塗布し、ハードコート層用硬化性樹脂組成物の塗膜を半硬化させた後、半硬化させたハードコート層用硬化性樹脂組成物の塗膜上に防眩層用硬化性樹脂組成物を塗布し、ハードコート層用硬化性樹脂組成物の塗膜および防眩層用硬化性樹脂組成物の塗膜を硬化させてハードコート層および防眩層を形成することが好ましい。ハードコート層との密着性が良好な防眩層を得ることができるからである。 2. Antiglare layer forming step In the present invention, an antiglare layer forming step of forming an antiglare layer by applying a curable resin composition for an antiglare layer on the hard coat layer and curing it may be performed.
In the hard coat layer forming step and the antiglare layer forming step, the hard coat layer resin composition was applied onto the substrate, the coating film of the hard coat layer curable resin composition was semi-cured, and then semi-cured. The curable resin composition for the antiglare layer is applied onto the coating film of the curable resin composition for the hard coat layer, and the curable resin composition for the hard coat layer and the curable resin composition for the antiglare layer are coated. It is preferable to cure the coating film to form a hard coat layer and an antiglare layer. This is because an antiglare layer having good adhesion to the hard coat layer can be obtained.
なお、防眩層の形成方法については、上記ハードコート層の形成方法と同様とすることができるので、ここでの説明は省略する。
In addition, about the formation method of a glare-proof layer, since it can be made to be the same as the formation method of the said hard-coat layer, description here is abbreviate | omitted.
本発明は、上記実施形態に限定されるものではない。上記実施形態は、例示であり、本発明の特許請求の範囲に記載された技術的思想と実質的に同一な構成を有し、同様な作用効果を奏するものは、いかなるものであっても本発明の技術的範囲に包含される。
The present invention is not limited to the above embodiment. The above-described embodiment is an exemplification, and the present invention has substantially the same configuration as the technical idea described in the claims of the present invention, and any device that exhibits the same function and effect is the present invention. It is included in the technical scope of the invention.
以下に実施例を示し、本発明をさらに詳細に説明する。
Hereinafter, the present invention will be described in more detail with reference to examples.
[実施例1]
(準備)
基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した積層体を用意した。積層体は全体の厚さ1.0mm、PMMAの厚さ100μm、鉛筆硬度3Hであった。
反応性異形シリカ微粒子として、平均1次粒径55nmのシリカ微粒子3~10個が無機の化学結合により結合した平均2次粒径100nm~300nm、反応性官能基として光硬化性不飽和基を有する反応性異形シリカ微粒子を用い、固形分濃度40.0質量%、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)溶剤の分散液を用意した。
モノマーとして、ペンタエリスリトールトリアクリレート(PETA)を用意した。重合開始剤として、チバ・ジャパン(株)製のイルガキュア184を用意した。界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203Mを用意した。溶剤として、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)を用意した。
ポリマーとして、下記表1に示すように重量平均分子量およびアクリル当量が異なるアクリル系ポリマーおよびウレタンアクリレートを用意した。アクリル系ポリマーとしては、星光PMC(株)製のBL-2002、BL-2184を用いた。なお、星光PMC(株)製のBL-2002の組成は、アクリル樹脂30~40重量部、メチルエチルケトン60~70重量部、酢酸1重量部未満、2,6-ジ-tert-ブチル-4-クレゾール1重量部未満である。また、表1において物性違いとは重量平均分子量やアクリル当量を変えたものをいう。また、ウレタンアクリレートとしては、日本合成化学工業(株)製のUV7550、新中村化学工業(株)製のU15HA、根上工業(株)製のUN904を用いた。 [Example 1]
(Preparation)
As a substrate, a laminate in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order was prepared. The laminate had an overall thickness of 1.0 mm, a PMMA thickness of 100 μm, and a pencil hardness of 3H.
As reactive irregularly shaped silica fine particles, 3 to 10 silica fine particles having an average primary particle size of 55 nm are bonded by an inorganic chemical bond and have an average secondary particle size of 100 to 300 nm, and having a photocurable unsaturated group as a reactive functional group. A dispersion of propylene glycol-1-methyl ether acetate (PGMEA) solvent with a solid content concentration of 40.0% by mass was prepared using reactive irregular shaped silica fine particles.
Pentaerythritol triacrylate (PETA) was prepared as a monomer. As a polymerization initiator, Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared. As the surfactant, a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared. Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
As polymers, acrylic polymers and urethane acrylates having different weight average molecular weights and acrylic equivalents as shown in Table 1 below were prepared. As the acrylic polymer, BL-2002 and BL-2184 manufactured by Seiko PMC Co., Ltd. were used. The composition of BL-2002 manufactured by Seiko PMC Co., Ltd. is 30 to 40 parts by weight of acrylic resin, 60 to 70 parts by weight of methyl ethyl ketone, less than 1 part by weight of acetic acid, 2,6-di-tert-butyl-4-cresol. Less than 1 part by weight. In Table 1, the difference in physical properties refers to a material having a changed weight average molecular weight or acrylic equivalent. Further, as urethane acrylate, UV75550 manufactured by Nippon Synthetic Chemical Industry Co., Ltd., U15HA manufactured by Shin-Nakamura Chemical Industry Co., Ltd., and UN904 manufactured by Negami Industrial Co., Ltd. were used.
(準備)
基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した積層体を用意した。積層体は全体の厚さ1.0mm、PMMAの厚さ100μm、鉛筆硬度3Hであった。
反応性異形シリカ微粒子として、平均1次粒径55nmのシリカ微粒子3~10個が無機の化学結合により結合した平均2次粒径100nm~300nm、反応性官能基として光硬化性不飽和基を有する反応性異形シリカ微粒子を用い、固形分濃度40.0質量%、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)溶剤の分散液を用意した。
モノマーとして、ペンタエリスリトールトリアクリレート(PETA)を用意した。重合開始剤として、チバ・ジャパン(株)製のイルガキュア184を用意した。界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203Mを用意した。溶剤として、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)を用意した。
ポリマーとして、下記表1に示すように重量平均分子量およびアクリル当量が異なるアクリル系ポリマーおよびウレタンアクリレートを用意した。アクリル系ポリマーとしては、星光PMC(株)製のBL-2002、BL-2184を用いた。なお、星光PMC(株)製のBL-2002の組成は、アクリル樹脂30~40重量部、メチルエチルケトン60~70重量部、酢酸1重量部未満、2,6-ジ-tert-ブチル-4-クレゾール1重量部未満である。また、表1において物性違いとは重量平均分子量やアクリル当量を変えたものをいう。また、ウレタンアクリレートとしては、日本合成化学工業(株)製のUV7550、新中村化学工業(株)製のU15HA、根上工業(株)製のUN904を用いた。 [Example 1]
(Preparation)
As a substrate, a laminate in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order was prepared. The laminate had an overall thickness of 1.0 mm, a PMMA thickness of 100 μm, and a pencil hardness of 3H.
As reactive irregularly shaped silica fine particles, 3 to 10 silica fine particles having an average primary particle size of 55 nm are bonded by an inorganic chemical bond and have an average secondary particle size of 100 to 300 nm, and having a photocurable unsaturated group as a reactive functional group. A dispersion of propylene glycol-1-methyl ether acetate (PGMEA) solvent with a solid content concentration of 40.0% by mass was prepared using reactive irregular shaped silica fine particles.
Pentaerythritol triacrylate (PETA) was prepared as a monomer. As a polymerization initiator, Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared. As the surfactant, a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared. Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
As polymers, acrylic polymers and urethane acrylates having different weight average molecular weights and acrylic equivalents as shown in Table 1 below were prepared. As the acrylic polymer, BL-2002 and BL-2184 manufactured by Seiko PMC Co., Ltd. were used. The composition of BL-2002 manufactured by Seiko PMC Co., Ltd. is 30 to 40 parts by weight of acrylic resin, 60 to 70 parts by weight of methyl ethyl ketone, less than 1 part by weight of acetic acid, 2,6-di-tert-butyl-4-cresol. Less than 1 part by weight. In Table 1, the difference in physical properties refers to a material having a changed weight average molecular weight or acrylic equivalent. Further, as urethane acrylate, UV75550 manufactured by Nippon Synthetic Chemical Industry Co., Ltd., U15HA manufactured by Shin-Nakamura Chemical Industry Co., Ltd., and UN904 manufactured by Negami Industrial Co., Ltd. were used.
(硬化性樹脂組成物の調製)
下記組成で、硬化性樹脂組成物を調製した。
反応性異形シリカ微粒子分散液:60重量部(固形分濃度40質量%)
モノマー:12.7重量部
ポリマー:7.2重量部
重合開始剤:0.76重量部
界面活性剤:0.905重量部
溶剤:19.2重量部
上記硬化性樹脂組成物の全固形分に対する反応性異形シリカ微粒子の含有量は60質量%である。また、反応性異形シリカ微粒子の硬化性樹脂組成物の全固形分に対する含有量が60質量%となり、ポリマーおよび反応性異形シリカ微粒子以外の成分の割合の比が一定になるようにして、ポリマーの添加量を変化させて、硬化性樹脂組成物を調製した。 (Preparation of curable resin composition)
A curable resin composition was prepared with the following composition.
Reactive deformed silica fine particle dispersion: 60 parts by weight (solid content: 40% by mass)
Monomer: 12.7 parts by weight Polymer: 7.2 parts by weight Polymerization initiator: 0.76 parts by weight Surfactant: 0.905 parts by weight Solvent: 19.2 parts by weight Based on the total solid content of the curable resin composition The content of reactive irregularly shaped silica fine particles is 60% by mass. Further, the content of the reactive irregularly shaped silica fine particles is 60% by mass with respect to the total solid content of the curable resin composition, and the ratio of the ratio of the components other than the polymer and reactive irregularly shaped silica fine particles is constant. The curable resin composition was prepared by changing the addition amount.
下記組成で、硬化性樹脂組成物を調製した。
反応性異形シリカ微粒子分散液:60重量部(固形分濃度40質量%)
モノマー:12.7重量部
ポリマー:7.2重量部
重合開始剤:0.76重量部
界面活性剤:0.905重量部
溶剤:19.2重量部
上記硬化性樹脂組成物の全固形分に対する反応性異形シリカ微粒子の含有量は60質量%である。また、反応性異形シリカ微粒子の硬化性樹脂組成物の全固形分に対する含有量が60質量%となり、ポリマーおよび反応性異形シリカ微粒子以外の成分の割合の比が一定になるようにして、ポリマーの添加量を変化させて、硬化性樹脂組成物を調製した。 (Preparation of curable resin composition)
A curable resin composition was prepared with the following composition.
Reactive deformed silica fine particle dispersion: 60 parts by weight (solid content: 40% by mass)
Monomer: 12.7 parts by weight Polymer: 7.2 parts by weight Polymerization initiator: 0.76 parts by weight Surfactant: 0.905 parts by weight Solvent: 19.2 parts by weight Based on the total solid content of the curable resin composition The content of reactive irregularly shaped silica fine particles is 60% by mass. Further, the content of the reactive irregularly shaped silica fine particles is 60% by mass with respect to the total solid content of the curable resin composition, and the ratio of the ratio of the components other than the polymer and reactive irregularly shaped silica fine particles is constant. The curable resin composition was prepared by changing the addition amount.
(ハードコート層の形成)
基板の片面に、硬化性樹脂組成物をスピンコート法にて塗布し、温度80℃のホットプレートで180秒間乾燥し、塗膜中の溶剤を蒸発させ、中心波長365nmの紫外線を積算光量が3000mJ/cm2になるように照射して塗膜を硬化させることにより、膜厚20μmのハードコート層を形成した。得られたハードコート層は、マトリクス樹脂と異形シリカ微粒子と所定の重量平均分子量およびアクリル当量を有するアクリル系ポリマーまたはウレタンアクリレートとを含有するものであった。 (Formation of hard coat layer)
A curable resin composition is applied to one side of the substrate by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, the solvent in the coating film is evaporated, and ultraviolet light having a central wavelength of 365 nm is applied to an integrated light amount of 3000 mJ. The hard coat layer with a film thickness of 20 μm was formed by curing the coating film by irradiating it to / cm 2 . The obtained hard coat layer contained a matrix resin, irregular-shaped silica fine particles, and an acrylic polymer or urethane acrylate having a predetermined weight average molecular weight and acrylic equivalent.
基板の片面に、硬化性樹脂組成物をスピンコート法にて塗布し、温度80℃のホットプレートで180秒間乾燥し、塗膜中の溶剤を蒸発させ、中心波長365nmの紫外線を積算光量が3000mJ/cm2になるように照射して塗膜を硬化させることにより、膜厚20μmのハードコート層を形成した。得られたハードコート層は、マトリクス樹脂と異形シリカ微粒子と所定の重量平均分子量およびアクリル当量を有するアクリル系ポリマーまたはウレタンアクリレートとを含有するものであった。 (Formation of hard coat layer)
A curable resin composition is applied to one side of the substrate by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, the solvent in the coating film is evaporated, and ultraviolet light having a central wavelength of 365 nm is applied to an integrated light amount of 3000 mJ. The hard coat layer with a film thickness of 20 μm was formed by curing the coating film by irradiating it to / cm 2 . The obtained hard coat layer contained a matrix resin, irregular-shaped silica fine particles, and an acrylic polymer or urethane acrylate having a predetermined weight average molecular weight and acrylic equivalent.
[評価1]
(鉛筆硬度試験)
得られたハードコートフィルムを温度25℃、相対湿度60%の条件で2時間調湿した後、JIS-S-6006が規定する試験用鉛筆を用いて、JIS K5600-5-4(1999)に規定する鉛筆硬度試験(4.9N荷重)を行い、傷がつかない最も高い鉛筆硬度を評価した。 [Evaluation 1]
(Pencil hardness test)
The obtained hard coat film was conditioned for 2 hours under the conditions of a temperature of 25 ° C. and a relative humidity of 60%. Then, using a test pencil specified by JIS-S-6006, JIS K5600-5-4 (1999) was used. The specified pencil hardness test (4.9 N load) was performed to evaluate the highest pencil hardness without scratches.
(鉛筆硬度試験)
得られたハードコートフィルムを温度25℃、相対湿度60%の条件で2時間調湿した後、JIS-S-6006が規定する試験用鉛筆を用いて、JIS K5600-5-4(1999)に規定する鉛筆硬度試験(4.9N荷重)を行い、傷がつかない最も高い鉛筆硬度を評価した。 [Evaluation 1]
(Pencil hardness test)
The obtained hard coat film was conditioned for 2 hours under the conditions of a temperature of 25 ° C. and a relative humidity of 60%. Then, using a test pencil specified by JIS-S-6006, JIS K5600-5-4 (1999) was used. The specified pencil hardness test (4.9 N load) was performed to evaluate the highest pencil hardness without scratches.
(マンドレル試験)
JIS-K5600-5-1に記載されているマンドレル試験(金属製円柱にサンプルを巻きつける試験)に準じ、円柱にハードコート層を外側にしてハードコートフィルムの長さ方向で巻きつけ、クラックが発生しなかった棒の最小直径を評価した。例えば、直径150mmの円柱でクラックが発生し、直径160mmの円柱でクラックが発生しなかった場合は、160mmとした。
ここで、本発明者らがハードコートフィルムの加工性について検討した結果、マンドレル試験と加工性には相関があり、マンドレル試験による屈曲性が良好である場合には打ち抜き加工、ルーター加工、切断加工等での加工性が良好であった。 (Mandrel test)
In accordance with the mandrel test described in JIS-K5600-5-1 (test in which a sample is wound around a metal cylinder), the hard coat layer is wound around the cylinder in the length direction of the hard coat film. The minimum diameter of the bar that did not occur was evaluated. For example, when a crack was generated in a cylinder having a diameter of 150 mm and no crack was generated in a cylinder having a diameter of 160 mm, the thickness was set to 160 mm.
Here, as a result of examining the processability of the hard coat film by the present inventors, there is a correlation between the mandrel test and the processability, and when the bendability by the mandrel test is good, the punching process, the router process, and the cutting process The processability with etc. was good.
JIS-K5600-5-1に記載されているマンドレル試験(金属製円柱にサンプルを巻きつける試験)に準じ、円柱にハードコート層を外側にしてハードコートフィルムの長さ方向で巻きつけ、クラックが発生しなかった棒の最小直径を評価した。例えば、直径150mmの円柱でクラックが発生し、直径160mmの円柱でクラックが発生しなかった場合は、160mmとした。
ここで、本発明者らがハードコートフィルムの加工性について検討した結果、マンドレル試験と加工性には相関があり、マンドレル試験による屈曲性が良好である場合には打ち抜き加工、ルーター加工、切断加工等での加工性が良好であった。 (Mandrel test)
In accordance with the mandrel test described in JIS-K5600-5-1 (test in which a sample is wound around a metal cylinder), the hard coat layer is wound around the cylinder in the length direction of the hard coat film. The minimum diameter of the bar that did not occur was evaluated. For example, when a crack was generated in a cylinder having a diameter of 150 mm and no crack was generated in a cylinder having a diameter of 160 mm, the thickness was set to 160 mm.
Here, as a result of examining the processability of the hard coat film by the present inventors, there is a correlation between the mandrel test and the processability, and when the bendability by the mandrel test is good, the punching process, the router process, and the cutting process The processability with etc. was good.
なお、表1中、ポリマーの添加量は硬化性樹脂組成物の全固形分に対する量、すなわちハードコート層中の量を示す。
表1に示すように、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを添加することで、ハードコート層の硬度を維持しつつ加工性が改善されることが確認された。特に、No.1-2、1-9の重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーの添加量が6.3質量%の場合には、ポリマーを添加したにもかかわらずハードコート層の硬度が向上し、硬度および加工性の両方に優れていた。 In addition, in Table 1, the addition amount of a polymer shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
As shown in Table 1, it was confirmed that the workability was improved while maintaining the hardness of the hard coat layer by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range. In particular, when the addition amount of the acrylic polymer in which the weight average molecular weights and acrylic equivalents of No. 1-2 and 1-9 are within a predetermined range is 6.3% by mass, the polymer is added. The hardness of the hard coat layer was improved, and both the hardness and workability were excellent.
表1に示すように、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを添加することで、ハードコート層の硬度を維持しつつ加工性が改善されることが確認された。特に、No.1-2、1-9の重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーの添加量が6.3質量%の場合には、ポリマーを添加したにもかかわらずハードコート層の硬度が向上し、硬度および加工性の両方に優れていた。 In addition, in Table 1, the addition amount of a polymer shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
As shown in Table 1, it was confirmed that the workability was improved while maintaining the hardness of the hard coat layer by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range. In particular, when the addition amount of the acrylic polymer in which the weight average molecular weights and acrylic equivalents of No. 1-2 and 1-9 are within a predetermined range is 6.3% by mass, the polymer is added. The hardness of the hard coat layer was improved, and both the hardness and workability were excellent.
[実施例2]
(硬化性樹脂組成物の調製)
アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いたこと以外は、実施例1と同様にして硬化性樹脂組成物を調製した。
また、アクリル系ポリマー以外の成分の割合の比が一定になるようにして、アクリル系ポリマーの添加量を変化させて、硬化性樹脂組成物を調製した。さらに、反応性異形シリカ微粒子以外の成分の割合の比が一定になるようにして、反応性異形シリカ微粒子の添加量を変化させて、硬化性樹脂組成物を調製した。 [Example 2]
(Preparation of curable resin composition)
A curable resin composition was prepared in the same manner as in Example 1 except that BL-2002 made by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used as the acrylic polymer.
Moreover, curable resin compositions were prepared by changing the amount of the acrylic polymer added so that the ratio of the components other than the acrylic polymer was constant. Further, a curable resin composition was prepared by changing the addition amount of the reactive irregular shaped silica fine particles so that the ratio of the components other than the reactive irregular shaped silica fine particles was constant.
(硬化性樹脂組成物の調製)
アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いたこと以外は、実施例1と同様にして硬化性樹脂組成物を調製した。
また、アクリル系ポリマー以外の成分の割合の比が一定になるようにして、アクリル系ポリマーの添加量を変化させて、硬化性樹脂組成物を調製した。さらに、反応性異形シリカ微粒子以外の成分の割合の比が一定になるようにして、反応性異形シリカ微粒子の添加量を変化させて、硬化性樹脂組成物を調製した。 [Example 2]
(Preparation of curable resin composition)
A curable resin composition was prepared in the same manner as in Example 1 except that BL-2002 made by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used as the acrylic polymer.
Moreover, curable resin compositions were prepared by changing the amount of the acrylic polymer added so that the ratio of the components other than the acrylic polymer was constant. Further, a curable resin composition was prepared by changing the addition amount of the reactive irregular shaped silica fine particles so that the ratio of the components other than the reactive irregular shaped silica fine particles was constant.
(ハードコート層の形成)
ハードコート層の膜厚を17μmまたは20μmとしたこと以外は、実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1 except that the thickness of the hard coat layer was 17 μm or 20 μm.
ハードコート層の膜厚を17μmまたは20μmとしたこと以外は、実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1 except that the thickness of the hard coat layer was 17 μm or 20 μm.
[評価2]
実施例1と同様にして、鉛筆硬度試験およびマンドレル試験を行い、評価した。 [Evaluation 2]
In the same manner as in Example 1, a pencil hardness test and a mandrel test were performed and evaluated.
実施例1と同様にして、鉛筆硬度試験およびマンドレル試験を行い、評価した。 [Evaluation 2]
In the same manner as in Example 1, a pencil hardness test and a mandrel test were performed and evaluated.
なお、表2中、各成分の含有量は硬化性樹脂組成物の全固形分に対する量、すなわちハードコート層中の量を示す。
表2に示すように、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを添加することで、ハードコート層の硬度を維持しつつ加工性が改善されることが確認された。特にNo.2-4のアクリル系ポリマーの添加量が6.3質量%の場合には、ポリマーを添加したにもかかわらずハードコート層の硬度が向上し、硬度および加工性の両方が改善された。 In addition, in Table 2, content of each component shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
As shown in Table 2, it was confirmed that the workability was improved while maintaining the hardness of the hard coat layer by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range. In particular, when the amount of acrylic polymer No.2-4 added is 6.3% by mass, the hardness of the hard coat layer is improved despite the addition of the polymer, and both hardness and workability are improved. It was.
表2に示すように、重量平均分子量およびアクリル当量が所定の範囲内であるアクリル系ポリマーを添加することで、ハードコート層の硬度を維持しつつ加工性が改善されることが確認された。特にNo.2-4のアクリル系ポリマーの添加量が6.3質量%の場合には、ポリマーを添加したにもかかわらずハードコート層の硬度が向上し、硬度および加工性の両方が改善された。 In addition, in Table 2, content of each component shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
As shown in Table 2, it was confirmed that the workability was improved while maintaining the hardness of the hard coat layer by adding an acrylic polymer having a weight average molecular weight and an acrylic equivalent within a predetermined range. In particular, when the amount of acrylic polymer No.2-4 added is 6.3% by mass, the hardness of the hard coat layer is improved despite the addition of the polymer, and both hardness and workability are improved. It was.
[実施例3]
(硬化性樹脂組成物の調製)
下記の反応性シリカ微粒子、アクリル系ポリマーおよび界面活性剤を用い、下記組成としたこと以外は、実施例1と同様にして硬化性樹脂組成物を調製した。
反応性シリカ微粒子として、実施例1で用いた反応性異形シリカ微粒子の分散液を用意した。この反応性異形シリカ微粒子の分散液は、日揮触媒化成(株)製のDP1222である。また、別の反応性シリカ微粒子として、平均1次粒径15nm、45nmまたは55nm、反応性官能基として光硬化性不飽和基を有する球形の反応性シリカ微粒子を用い、固形分濃度40.0質量%、メチルイソブチルケトン(MIBK)溶剤の分散液を用意した。これらの反応性シリカ微粒子の分散液はそれぞれ、日産化学工業(株)製のMIBK-SDL、MIBK-DL、日揮触媒化成(株)製のV8803である。
アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いた。
界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203Mを用意した。
反応性シリカ微粒子分散液:60重量部(固形分濃度40質量%)
モノマー:12.7重量部
アクリル系ポリマー:7.2重量部
重合開始剤:0.76重量部
界面活性剤:0.905重量部
溶剤:19.2重量部
また、反応性シリカ微粒子以外の成分の割合の比が一定になるようにして、反応性シリカ微粒子の添加量を変化させて、硬化性樹脂組成物を調製した。 [Example 3]
(Preparation of curable resin composition)
A curable resin composition was prepared in the same manner as in Example 1 except that the following reactive silica fine particles, acrylic polymer, and surfactant were used and the following composition was used.
As reactive silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. This dispersion of reactive irregularly shaped silica fine particles is DP1222 manufactured by JGC Catalysts & Chemicals. Further, as another reactive silica fine particle, a spherical reactive silica fine particle having an average primary particle size of 15 nm, 45 nm or 55 nm and a photocurable unsaturated group as a reactive functional group is used, and the solid content concentration is 40.0 mass. %, A dispersion of methyl isobutyl ketone (MIBK) solvent was prepared. These reactive silica fine particle dispersions are MIBK-SDL and MIBK-DL manufactured by Nissan Chemical Industries, Ltd., and V8803 manufactured by JGC Catalysts & Chemicals, Inc., respectively.
As an acrylic polymer, BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used.
As the surfactant, a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared.
Reactive silica fine particle dispersion: 60 parts by weight (solid content concentration 40% by mass)
Monomer: 12.7 parts by weight Acrylic polymer: 7.2 parts by weight Polymerization initiator: 0.76 parts by weight Surfactant: 0.905 parts by weight Solvent: 19.2 parts by weight Components other than reactive silica fine particles A curable resin composition was prepared by changing the addition amount of the reactive silica fine particles so that the ratio of the ratio of was constant.
(硬化性樹脂組成物の調製)
下記の反応性シリカ微粒子、アクリル系ポリマーおよび界面活性剤を用い、下記組成としたこと以外は、実施例1と同様にして硬化性樹脂組成物を調製した。
反応性シリカ微粒子として、実施例1で用いた反応性異形シリカ微粒子の分散液を用意した。この反応性異形シリカ微粒子の分散液は、日揮触媒化成(株)製のDP1222である。また、別の反応性シリカ微粒子として、平均1次粒径15nm、45nmまたは55nm、反応性官能基として光硬化性不飽和基を有する球形の反応性シリカ微粒子を用い、固形分濃度40.0質量%、メチルイソブチルケトン(MIBK)溶剤の分散液を用意した。これらの反応性シリカ微粒子の分散液はそれぞれ、日産化学工業(株)製のMIBK-SDL、MIBK-DL、日揮触媒化成(株)製のV8803である。
アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いた。
界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203Mを用意した。
反応性シリカ微粒子分散液:60重量部(固形分濃度40質量%)
モノマー:12.7重量部
アクリル系ポリマー:7.2重量部
重合開始剤:0.76重量部
界面活性剤:0.905重量部
溶剤:19.2重量部
また、反応性シリカ微粒子以外の成分の割合の比が一定になるようにして、反応性シリカ微粒子の添加量を変化させて、硬化性樹脂組成物を調製した。 [Example 3]
(Preparation of curable resin composition)
A curable resin composition was prepared in the same manner as in Example 1 except that the following reactive silica fine particles, acrylic polymer, and surfactant were used and the following composition was used.
As reactive silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. This dispersion of reactive irregularly shaped silica fine particles is DP1222 manufactured by JGC Catalysts & Chemicals. Further, as another reactive silica fine particle, a spherical reactive silica fine particle having an average primary particle size of 15 nm, 45 nm or 55 nm and a photocurable unsaturated group as a reactive functional group is used, and the solid content concentration is 40.0 mass. %, A dispersion of methyl isobutyl ketone (MIBK) solvent was prepared. These reactive silica fine particle dispersions are MIBK-SDL and MIBK-DL manufactured by Nissan Chemical Industries, Ltd., and V8803 manufactured by JGC Catalysts & Chemicals, Inc., respectively.
As an acrylic polymer, BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used.
As the surfactant, a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared.
Reactive silica fine particle dispersion: 60 parts by weight (solid content concentration 40% by mass)
Monomer: 12.7 parts by weight Acrylic polymer: 7.2 parts by weight Polymerization initiator: 0.76 parts by weight Surfactant: 0.905 parts by weight Solvent: 19.2 parts by weight Components other than reactive silica fine particles A curable resin composition was prepared by changing the addition amount of the reactive silica fine particles so that the ratio of the ratio of was constant.
(ハードコート層の形成)
ハードコート層の膜厚を10μm~20μmとしたこと以外は、実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1 except that the thickness of the hard coat layer was changed to 10 μm to 20 μm.
ハードコート層の膜厚を10μm~20μmとしたこと以外は、実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1 except that the thickness of the hard coat layer was changed to 10 μm to 20 μm.
[評価3]
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 3]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 3]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(マンドレル試験)
実施例1と同様にして、マンドレル試験を行い、評価した。 (Mandrel test)
A mandrel test was conducted and evaluated in the same manner as in Example 1.
実施例1と同様にして、マンドレル試験を行い、評価した。 (Mandrel test)
A mandrel test was conducted and evaluated in the same manner as in Example 1.
(耐擦傷性)
ハードコートフィルムのハードコート層表面を、#0000番のスチールウールを用いて、荷重をかけながら20往復摩擦し、その後のハードコート層の傷の有無を目視し下記の基準にて評価した。
A:1000g/cm2荷重で、20往復以上で傷なし
B:1000g/cm2荷重で、10往復以上20往復未満で傷なし
C:1000g/cm2荷重で、10往復未満で傷発生 (Abrasion resistance)
The surface of the hard coat layer of the hard coat film was subjected to 20 reciprocating frictions using # 0000 steel wool while applying a load, and then the presence or absence of scratches on the hard coat layer was observed and evaluated according to the following criteria.
A: No damage at 1000 g / cm 2 load at 20 reciprocations or more B: 1000 g / cm 2 load at 10 reciprocations or more but less than 20 reciprocations C: 1000 g / cm 2 load at 10 reciprocations or less flaws
ハードコートフィルムのハードコート層表面を、#0000番のスチールウールを用いて、荷重をかけながら20往復摩擦し、その後のハードコート層の傷の有無を目視し下記の基準にて評価した。
A:1000g/cm2荷重で、20往復以上で傷なし
B:1000g/cm2荷重で、10往復以上20往復未満で傷なし
C:1000g/cm2荷重で、10往復未満で傷発生 (Abrasion resistance)
The surface of the hard coat layer of the hard coat film was subjected to 20 reciprocating frictions using # 0000 steel wool while applying a load, and then the presence or absence of scratches on the hard coat layer was observed and evaluated according to the following criteria.
A: No damage at 1000 g / cm 2 load at 20 reciprocations or more B: 1000 g / cm 2 load at 10 reciprocations or more but less than 20 reciprocations C: 1000 g / cm 2 load at 10 reciprocations or less flaws
なお、表3中、反応性シリカ微粒子の含有量は硬化性樹脂組成物の全固形分に対する量、すなわちハードコート層中の量を示す。
表3に示すように、反応性異形シリカ微粒子を添加することで、ハードコート層の硬度が向上することが確認された。 In Table 3, the content of the reactive silica fine particles indicates the amount with respect to the total solid content of the curable resin composition, that is, the amount in the hard coat layer.
As shown in Table 3, it was confirmed that the hardness of the hard coat layer was improved by adding reactive irregular shaped silica fine particles.
表3に示すように、反応性異形シリカ微粒子を添加することで、ハードコート層の硬度が向上することが確認された。 In Table 3, the content of the reactive silica fine particles indicates the amount with respect to the total solid content of the curable resin composition, that is, the amount in the hard coat layer.
As shown in Table 3, it was confirmed that the hardness of the hard coat layer was improved by adding reactive irregular shaped silica fine particles.
[実施例4]
(準備)
基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した住友化学(株)製のテクノロイC101を用意した。テクノロイC101は全体の厚さ0.3mm~1mm、PMMAの厚さ70μm、鉛筆硬度2Hであった。また、別の基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した住友化学(株)製のFX1190を用意した。FX1190は全体の厚さ1mm、PMMAの厚さ100μm、鉛筆硬度3Hであった。
反応性異形シリカ微粒子として、実施例1で用いた反応性異形シリカ微粒子の分散液を用意した。モノマーとして、ペンタエリスリトールトリアクリレート(PETA)、3~4官能PETAである(株)DNPファインケミカル製のSFCクリア11、アクリレート系モノマーである日本化薬(株)製のKARAYAD DPCA20、DPCA120を用意した。アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いた。重合開始剤として、チバ・ジャパン(株)製のイルガキュア184を用意した。界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203M、あるいは、DIC(株)製のフッ素系界面活性剤メガファックMCF350-5を用意した。溶剤として、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)を用意した。 [Example 4]
(Preparation)
As a substrate, Technoloy C101 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. Technoloy C101 had an overall thickness of 0.3 mm to 1 mm, a PMMA thickness of 70 μm, and a pencil hardness of 2H. As another substrate, FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. FX1190 had an overall thickness of 1 mm, a PMMA thickness of 100 μm, and a pencil hardness of 3H.
As reactive irregular shaped silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. As monomers, pentaerythritol triacrylate (PETA), 3-4 functional PETA, SNP Clear 11 made by DNP Fine Chemical Co., Ltd., and acrylate monomers KARAYAD DPCA20 and DPCA120 made by Nippon Kayaku Co., Ltd. were prepared. As an acrylic polymer, BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used. As a polymerization initiator, Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared. As surfactants, fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd., or fluorinated surfactant Megafac MCF350-5 manufactured by DIC Corporation was prepared. Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
(準備)
基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した住友化学(株)製のテクノロイC101を用意した。テクノロイC101は全体の厚さ0.3mm~1mm、PMMAの厚さ70μm、鉛筆硬度2Hであった。また、別の基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した住友化学(株)製のFX1190を用意した。FX1190は全体の厚さ1mm、PMMAの厚さ100μm、鉛筆硬度3Hであった。
反応性異形シリカ微粒子として、実施例1で用いた反応性異形シリカ微粒子の分散液を用意した。モノマーとして、ペンタエリスリトールトリアクリレート(PETA)、3~4官能PETAである(株)DNPファインケミカル製のSFCクリア11、アクリレート系モノマーである日本化薬(株)製のKARAYAD DPCA20、DPCA120を用意した。アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いた。重合開始剤として、チバ・ジャパン(株)製のイルガキュア184を用意した。界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203M、あるいは、DIC(株)製のフッ素系界面活性剤メガファックMCF350-5を用意した。溶剤として、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)を用意した。 [Example 4]
(Preparation)
As a substrate, Technoloy C101 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. Technoloy C101 had an overall thickness of 0.3 mm to 1 mm, a PMMA thickness of 70 μm, and a pencil hardness of 2H. As another substrate, FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. FX1190 had an overall thickness of 1 mm, a PMMA thickness of 100 μm, and a pencil hardness of 3H.
As reactive irregular shaped silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. As monomers, pentaerythritol triacrylate (PETA), 3-4 functional PETA, SNP Clear 11 made by DNP Fine Chemical Co., Ltd., and acrylate monomers KARAYAD DPCA20 and DPCA120 made by Nippon Kayaku Co., Ltd. were prepared. As an acrylic polymer, BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used. As a polymerization initiator, Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared. As surfactants, fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd., or fluorinated surfactant Megafac MCF350-5 manufactured by DIC Corporation was prepared. Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
(硬化性樹脂組成物の調製)
下記表4に示す組成で、硬化性樹脂組成物を調製した。 (Preparation of curable resin composition)
Curable resin compositions were prepared with the compositions shown in Table 4 below.
下記表4に示す組成で、硬化性樹脂組成物を調製した。 (Preparation of curable resin composition)
Curable resin compositions were prepared with the compositions shown in Table 4 below.
(ハードコート層の形成)
基板の片面に、硬化性樹脂組成物をスピンコート法にて塗布し、温度80℃のホットプレートで180秒間乾燥し、塗膜中の溶剤を蒸発させ、下記表4に示す光源を用いて中心波長365nmの紫外線を積算光量が3000mJ/cm2になるように照射して塗膜を硬化させることにより、膜厚10μm~22μmのハードコート層を形成した。得られたハードコート層は、マトリクス樹脂と異形シリカ微粒子と所定の重量平均分子量およびアクリル当量を有するアクリル系ポリマーとを含有するものであった。
なお、光源としては、高圧水銀灯、低圧水銀灯、メタルハライドランプ、フュージョンUVシステムズ社製のDバルブ、Hバルブを用いた。 (Formation of hard coat layer)
A curable resin composition is applied to one side of the substrate by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, and the solvent in the coating film is evaporated. A hard coat layer having a film thickness of 10 μm to 22 μm was formed by irradiating ultraviolet rays having a wavelength of 365 nm so that the integrated light amount was 3000 mJ / cm 2 to cure the coating film. The obtained hard coat layer contained a matrix resin, irregular-shaped silica fine particles, and an acrylic polymer having a predetermined weight average molecular weight and acrylic equivalent.
As the light source, a high pressure mercury lamp, a low pressure mercury lamp, a metal halide lamp, a D bulb and an H bulb manufactured by Fusion UV Systems were used.
基板の片面に、硬化性樹脂組成物をスピンコート法にて塗布し、温度80℃のホットプレートで180秒間乾燥し、塗膜中の溶剤を蒸発させ、下記表4に示す光源を用いて中心波長365nmの紫外線を積算光量が3000mJ/cm2になるように照射して塗膜を硬化させることにより、膜厚10μm~22μmのハードコート層を形成した。得られたハードコート層は、マトリクス樹脂と異形シリカ微粒子と所定の重量平均分子量およびアクリル当量を有するアクリル系ポリマーとを含有するものであった。
なお、光源としては、高圧水銀灯、低圧水銀灯、メタルハライドランプ、フュージョンUVシステムズ社製のDバルブ、Hバルブを用いた。 (Formation of hard coat layer)
A curable resin composition is applied to one side of the substrate by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, and the solvent in the coating film is evaporated. A hard coat layer having a film thickness of 10 μm to 22 μm was formed by irradiating ultraviolet rays having a wavelength of 365 nm so that the integrated light amount was 3000 mJ / cm 2 to cure the coating film. The obtained hard coat layer contained a matrix resin, irregular-shaped silica fine particles, and an acrylic polymer having a predetermined weight average molecular weight and acrylic equivalent.
As the light source, a high pressure mercury lamp, a low pressure mercury lamp, a metal halide lamp, a D bulb and an H bulb manufactured by Fusion UV Systems were used.
[評価4]
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 4]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 4]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(マンドレル試験)
実施例1と同様にして、マンドレル試験を行い、評価した。 (Mandrel test)
A mandrel test was conducted and evaluated in the same manner as in Example 1.
実施例1と同様にして、マンドレル試験を行い、評価した。 (Mandrel test)
A mandrel test was conducted and evaluated in the same manner as in Example 1.
(耐擦傷性)
実施例1と同様にして評価した。 (Abrasion resistance)
Evaluation was performed in the same manner as in Example 1.
実施例1と同様にして評価した。 (Abrasion resistance)
Evaluation was performed in the same manner as in Example 1.
(耐熱クラック)
ハードコートフィルムを80℃のオーブンに30分間入れ、クラックの発生の有無を評価した。
A:80℃のオーブンに30分間入れてもクラックが発生しない。
B:80℃のオーブンに30分間入れた後にクラックが発生している。 (Heat-resistant crack)
The hard coat film was placed in an oven at 80 ° C. for 30 minutes, and the presence or absence of cracks was evaluated.
A: Cracks do not occur even when placed in an oven at 80 ° C. for 30 minutes.
B: Cracks occur after being placed in an oven at 80 ° C. for 30 minutes.
ハードコートフィルムを80℃のオーブンに30分間入れ、クラックの発生の有無を評価した。
A:80℃のオーブンに30分間入れてもクラックが発生しない。
B:80℃のオーブンに30分間入れた後にクラックが発生している。 (Heat-resistant crack)
The hard coat film was placed in an oven at 80 ° C. for 30 minutes, and the presence or absence of cracks was evaluated.
A: Cracks do not occur even when placed in an oven at 80 ° C. for 30 minutes.
B: Cracks occur after being placed in an oven at 80 ° C. for 30 minutes.
(接触角)
ハードコートフィルムのハードコート層における水滴の接触角を、協和界面科学(株)製の接触角測定器CA-Z型を用い、マイクロシリンジから水滴を滴下して30秒後の水との接触角を測定することにより評価した。 (Contact angle)
The contact angle of water droplets in the hard coat layer of the hard coat film was measured by using a contact angle measuring device CA-Z type manufactured by Kyowa Interface Science Co., Ltd. Was evaluated by measuring.
ハードコートフィルムのハードコート層における水滴の接触角を、協和界面科学(株)製の接触角測定器CA-Z型を用い、マイクロシリンジから水滴を滴下して30秒後の水との接触角を測定することにより評価した。 (Contact angle)
The contact angle of water droplets in the hard coat layer of the hard coat film was measured by using a contact angle measuring device CA-Z type manufactured by Kyowa Interface Science Co., Ltd. Was evaluated by measuring.
(防汚性)
ハードコートフィルムのハードコート層に、ゼブラ株式会社製のマーカー マッキー極細 黒色 型番:MO-120-MC・BKの細い側で線を引くことにより評価した。
A:2秒以内ににじむ(ハジク)
B:2秒以内ににじまず、線が引ける(ハジかない) (Anti-fouling property)
The hard coat layer of the hard coat film was evaluated by drawing a line on the thin side of the marker Mackie Extra Fine Black Model No .: MO-120-MC · BK manufactured by Zebra Corporation.
A: Smoke within 2 seconds (Hajik)
B: A line can be drawn (not relieved) within 2 seconds.
ハードコートフィルムのハードコート層に、ゼブラ株式会社製のマーカー マッキー極細 黒色 型番:MO-120-MC・BKの細い側で線を引くことにより評価した。
A:2秒以内ににじむ(ハジク)
B:2秒以内ににじまず、線が引ける(ハジかない) (Anti-fouling property)
The hard coat layer of the hard coat film was evaluated by drawing a line on the thin side of the marker Mackie Extra Fine Black Model No .: MO-120-MC · BK manufactured by Zebra Corporation.
A: Smoke within 2 seconds (Hajik)
B: A line can be drawn (not relieved) within 2 seconds.
なお、表4中、各成分の含有量は硬化性樹脂組成物の全固形分に対する量、すなわちハードコート層中の量を示す。
No.4-1~4-4から、反応性異形シリカ微粒子の含有量が硬化性樹脂組成物の全固形分に対して50質量%~60質量%の範囲内である場合に、鉛筆硬度が向上し、加工性が改善されることが確認された。No.4-2、4-5~4-9から、アクリル系ポリマーの含有量が7.9質量%である場合に、ハードコート層の硬度が最も高くなり、加工性が改善されることが確認された。No.4-2、4-10~4-13から、ハードコート層の膜厚が20μm以上である場合に、鉛筆硬度がさらに向上することが確認された。No.4-14~4-16から、基板の構成を変化させることで、鉛筆硬度がさらに向上することが確認された。No.4-2、4-17~4-19から、基板の厚さが0.5mm以上の場合に、鉛筆硬度がさらに向上することが確認された。No.4-20~4-22からモノマーとしてPETAを用いた場合に鉛筆硬度がさらに向上することが確認された。No.4-23~4-26から、硬化条件を変化させても、良好な鉛筆硬度が得られることが確認された。 In addition, in Table 4, content of each component shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
From No.4-1 to 4-4, when the content of the reactive irregular shaped silica fine particles is in the range of 50% by mass to 60% by mass with respect to the total solid content of the curable resin composition, the pencil hardness is It was confirmed that the processability was improved. From No. 4-2 and 4-5 to 4-9, when the acrylic polymer content is 7.9% by mass, the hardness of the hard coat layer is the highest and the workability is improved. confirmed. From No. 4-2 and 4-10 to 4-13, it was confirmed that the pencil hardness was further improved when the thickness of the hard coat layer was 20 μm or more. From No.4-14 to 4-16, it was confirmed that the pencil hardness was further improved by changing the configuration of the substrate. From No. 4-2 and 4-17 to 4-19, it was confirmed that the pencil hardness was further improved when the thickness of the substrate was 0.5 mm or more. From No. 4-20 to 4-22, it was confirmed that the pencil hardness was further improved when PETA was used as a monomer. From No.4-23 to 4-26, it was confirmed that good pencil hardness could be obtained even if the curing conditions were changed.
No.4-1~4-4から、反応性異形シリカ微粒子の含有量が硬化性樹脂組成物の全固形分に対して50質量%~60質量%の範囲内である場合に、鉛筆硬度が向上し、加工性が改善されることが確認された。No.4-2、4-5~4-9から、アクリル系ポリマーの含有量が7.9質量%である場合に、ハードコート層の硬度が最も高くなり、加工性が改善されることが確認された。No.4-2、4-10~4-13から、ハードコート層の膜厚が20μm以上である場合に、鉛筆硬度がさらに向上することが確認された。No.4-14~4-16から、基板の構成を変化させることで、鉛筆硬度がさらに向上することが確認された。No.4-2、4-17~4-19から、基板の厚さが0.5mm以上の場合に、鉛筆硬度がさらに向上することが確認された。No.4-20~4-22からモノマーとしてPETAを用いた場合に鉛筆硬度がさらに向上することが確認された。No.4-23~4-26から、硬化条件を変化させても、良好な鉛筆硬度が得られることが確認された。 In addition, in Table 4, content of each component shows the quantity with respect to the total solid of a curable resin composition, ie, the quantity in a hard-coat layer.
From No.4-1 to 4-4, when the content of the reactive irregular shaped silica fine particles is in the range of 50% by mass to 60% by mass with respect to the total solid content of the curable resin composition, the pencil hardness is It was confirmed that the processability was improved. From No. 4-2 and 4-5 to 4-9, when the acrylic polymer content is 7.9% by mass, the hardness of the hard coat layer is the highest and the workability is improved. confirmed. From No. 4-2 and 4-10 to 4-13, it was confirmed that the pencil hardness was further improved when the thickness of the hard coat layer was 20 μm or more. From No.4-14 to 4-16, it was confirmed that the pencil hardness was further improved by changing the configuration of the substrate. From No. 4-2 and 4-17 to 4-19, it was confirmed that the pencil hardness was further improved when the thickness of the substrate was 0.5 mm or more. From No. 4-20 to 4-22, it was confirmed that the pencil hardness was further improved when PETA was used as a monomer. From No.4-23 to 4-26, it was confirmed that good pencil hardness could be obtained even if the curing conditions were changed.
[実施例5]
(硬化性樹脂組成物の調製)
界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203M、DIC(株)製のフッ素系界面活性剤メガファックMCF350-5、あるいは、信越化学工業社製のシリコン系界面活性剤X22-163Aを用いたこと以外は、実施例1と同様にして硬化性樹脂組成物を調製した。 [Example 5]
(Preparation of curable resin composition)
As the surfactant, fluorine-based surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd., fluorine-based surfactant MegaFuck MCF350-5 manufactured by DIC Corporation, or silicon-based manufactured by Shin-Etsu Chemical Co., Ltd. A curable resin composition was prepared in the same manner as in Example 1 except that the surfactant X22-163A was used.
(硬化性樹脂組成物の調製)
界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203M、DIC(株)製のフッ素系界面活性剤メガファックMCF350-5、あるいは、信越化学工業社製のシリコン系界面活性剤X22-163Aを用いたこと以外は、実施例1と同様にして硬化性樹脂組成物を調製した。 [Example 5]
(Preparation of curable resin composition)
As the surfactant, fluorine-based surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd., fluorine-based surfactant MegaFuck MCF350-5 manufactured by DIC Corporation, or silicon-based manufactured by Shin-Etsu Chemical Co., Ltd. A curable resin composition was prepared in the same manner as in Example 1 except that the surfactant X22-163A was used.
(ハードコート層の形成)
実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1.
実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1.
[評価5]
(動摩擦係数)
ハードコートフィルムのハードコート層について、新東科学(株)社製の動摩擦試験機HEIDON Type HHS2000で、直径10mmのステンレス剛球を用い、荷重200g、速度5mm/secにて動摩擦係数を測定した。 [Evaluation 5]
(Dynamic friction coefficient)
With respect to the hard coat layer of the hard coat film, the dynamic friction coefficient was measured with a dynamic friction tester HEIDON Type HHS2000 manufactured by Shinto Kagaku Co., Ltd. using a stainless hard ball having a diameter of 10 mm at a load of 200 g and a speed of 5 mm / sec.
(動摩擦係数)
ハードコートフィルムのハードコート層について、新東科学(株)社製の動摩擦試験機HEIDON Type HHS2000で、直径10mmのステンレス剛球を用い、荷重200g、速度5mm/secにて動摩擦係数を測定した。 [Evaluation 5]
(Dynamic friction coefficient)
With respect to the hard coat layer of the hard coat film, the dynamic friction coefficient was measured with a dynamic friction tester HEIDON Type HHS2000 manufactured by Shinto Kagaku Co., Ltd. using a stainless hard ball having a diameter of 10 mm at a load of 200 g and a speed of 5 mm / sec.
表5に示すように、フッ素シリコン系界面活性剤を添加することで、易滑性が向上することが確認された。このハードコートフィルムは、タッチパネルに用いられるハードコートフィルムとして有用である。
As shown in Table 5, it was confirmed that the lubricity was improved by adding a fluorosilicone surfactant. This hard coat film is useful as a hard coat film used for a touch panel.
[実施例6]
(準備)
基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した住友化学(株)製のFX1190を用意した。FX1190は全体の厚さ0.65mm、PMMAの厚さ100μm、鉛筆硬度3Hであった。
反応性異形シリカ微粒子として、実施例1で用いた反応性異形シリカ微粒子の分散液を用意した。青色色材として、青色顔料PB15:6を用いた。モノマーとして、ペンタエリスリトールトリアクリレート(PETA)を用意した。アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いた。重合開始剤として、チバ・ジャパン(株)製のイルガキュア184を用意した。界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203Mを用意した。溶剤として、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)を用意した。 [Example 6]
(Preparation)
As a substrate, FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. FX1190 had an overall thickness of 0.65 mm, a PMMA thickness of 100 μm, and a pencil hardness of 3H.
As reactive irregular shaped silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. As a blue color material, a blue pigment PB15: 6 was used. Pentaerythritol triacrylate (PETA) was prepared as a monomer. As an acrylic polymer, BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used. As a polymerization initiator, Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared. As the surfactant, a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared. Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
(準備)
基板として、ポリメタクリル酸メチル(PMMA)、ポリカーボネート(PC)およびポリメタクリル酸メチル(PMMA)をこの順に積層した住友化学(株)製のFX1190を用意した。FX1190は全体の厚さ0.65mm、PMMAの厚さ100μm、鉛筆硬度3Hであった。
反応性異形シリカ微粒子として、実施例1で用いた反応性異形シリカ微粒子の分散液を用意した。青色色材として、青色顔料PB15:6を用いた。モノマーとして、ペンタエリスリトールトリアクリレート(PETA)を用意した。アクリル系ポリマーとして、重量平均分子量7万、アクリル当量265の星光PMC(株)製のBL-2002を用いた。重合開始剤として、チバ・ジャパン(株)製のイルガキュア184を用意した。界面活性剤として、信越化学工業社製のフッ素シリコン系界面活性剤X-71-1203Mを用意した。溶剤として、プロピレングリコール-1-メチルエーテルアセテート(PGMEA)を用意した。 [Example 6]
(Preparation)
As a substrate, FX1190 manufactured by Sumitomo Chemical Co., Ltd., in which polymethyl methacrylate (PMMA), polycarbonate (PC), and polymethyl methacrylate (PMMA) were laminated in this order, was prepared. FX1190 had an overall thickness of 0.65 mm, a PMMA thickness of 100 μm, and a pencil hardness of 3H.
As reactive irregular shaped silica fine particles, a dispersion of reactive irregular shaped silica fine particles used in Example 1 was prepared. As a blue color material, a blue pigment PB15: 6 was used. Pentaerythritol triacrylate (PETA) was prepared as a monomer. As an acrylic polymer, BL-2002 manufactured by Seiko PMC Co., Ltd. having a weight average molecular weight of 70,000 and an acrylic equivalent of 265 was used. As a polymerization initiator, Irgacure 184 manufactured by Ciba Japan Co., Ltd. was prepared. As the surfactant, a fluorosilicone surfactant X-71-1203M manufactured by Shin-Etsu Chemical Co., Ltd. was prepared. Propylene glycol-1-methyl ether acetate (PGMEA) was prepared as a solvent.
(硬化性樹脂組成物の調製)
下記表6に示す組成で、硬化性樹脂組成物を調製した。 (Preparation of curable resin composition)
Curable resin compositions were prepared with the compositions shown in Table 6 below.
下記表6に示す組成で、硬化性樹脂組成物を調製した。 (Preparation of curable resin composition)
Curable resin compositions were prepared with the compositions shown in Table 6 below.
(ハードコート層の形成)
実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1.
実施例1と同様にしてハードコート層を形成した。 (Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1.
[評価6]
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 6]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 6]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(光学特性)
得られたハードコートフィルムは、島津製作所社製の紫外可視近赤外分光光度計MPC-3100にて分光を測定し、C光源を用いて、CIEのXYZ表色系における色度座標x、yおよび輝度Yと、CIEのL*a*b*表色系における色度b*と、黄色度YIとを測定した。
なお、色度b*は、JIS-Z-8729にて規定されている色座標における指標である。色度b*は黄色味の指標であり、b*が大きい場合は黄色味が強いことを示し、負の値になると黄色味が不足して青くなることを示している。 (optical properties)
The obtained hard coat film was measured for spectrum with an ultraviolet-visible near-infrared spectrophotometer MPC-3100 manufactured by Shimadzu Corporation, and using a C light source, chromaticity coordinates x, y in the XYZ color system of CIE In addition, luminance Y, chromaticity b * and yellowness YI in CIE L * a * b * color system were measured.
The chromaticity b * is an index in color coordinates defined in JIS-Z-8729. The chromaticity b * is an index of yellowness. When b * is large, the yellowness is strong, and when the value is negative, the yellowness is insufficient and the color becomes blue.
得られたハードコートフィルムは、島津製作所社製の紫外可視近赤外分光光度計MPC-3100にて分光を測定し、C光源を用いて、CIEのXYZ表色系における色度座標x、yおよび輝度Yと、CIEのL*a*b*表色系における色度b*と、黄色度YIとを測定した。
なお、色度b*は、JIS-Z-8729にて規定されている色座標における指標である。色度b*は黄色味の指標であり、b*が大きい場合は黄色味が強いことを示し、負の値になると黄色味が不足して青くなることを示している。 (optical properties)
The obtained hard coat film was measured for spectrum with an ultraviolet-visible near-infrared spectrophotometer MPC-3100 manufactured by Shimadzu Corporation, and using a C light source, chromaticity coordinates x, y in the XYZ color system of CIE In addition, luminance Y, chromaticity b * and yellowness YI in CIE L * a * b * color system were measured.
The chromaticity b * is an index in color coordinates defined in JIS-Z-8729. The chromaticity b * is an index of yellowness. When b * is large, the yellowness is strong, and when the value is negative, the yellowness is insufficient and the color becomes blue.
[実施例7]
1.ハードコート層の形成
(硬化性樹脂組成物の調製)
界面活性剤としてDIC(株)製メガファックF568を添加したこと以外は、実施例1と同様の組成で、硬化性樹脂組成物を調製した。
(ハードコート層の形成)
露光量を1/8にしたこと以外は、実施例1と同様にしてハードコート層を形成した。 [Example 7]
1. Formation of hard coat layer (Preparation of curable resin composition)
A curable resin composition was prepared with the same composition as in Example 1 except that Megafac F568 manufactured by DIC Corporation was added as a surfactant.
(Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1 except that the exposure amount was 1/8.
1.ハードコート層の形成
(硬化性樹脂組成物の調製)
界面活性剤としてDIC(株)製メガファックF568を添加したこと以外は、実施例1と同様の組成で、硬化性樹脂組成物を調製した。
(ハードコート層の形成)
露光量を1/8にしたこと以外は、実施例1と同様にしてハードコート層を形成した。 [Example 7]
1. Formation of hard coat layer (Preparation of curable resin composition)
A curable resin composition was prepared with the same composition as in Example 1 except that Megafac F568 manufactured by DIC Corporation was added as a surfactant.
(Formation of hard coat layer)
A hard coat layer was formed in the same manner as in Example 1 except that the exposure amount was 1/8.
2.防眩層の形成
クリア材として大日精化工業製TAC-D105、不定形シリカを含むマット材として大日精化工業製EXG40-77(D-30M)、界面活性剤として大日精化工業製シリコン10-28、溶剤としてメチルイソブチルケトン(MIBK)を用い、防眩層用硬化性樹脂組成物を調製した。この際、クリア材およびマット材の総量100重量部に対して界面活性剤を0.6重量部添加し、クリア/マット比を変化させて、防眩層用硬化性樹脂組成物を調製した。
ハードコート層を形成した基板の片面に、防眩層用硬化性樹脂組成物をスピンコート法にて塗布し、温度80℃のホットプレートで180秒間乾燥し、塗膜中の溶剤を蒸発させ、中心波長365nmの紫外線を積算光量が3000mJ/cm2になるように照射して塗膜を硬化させることにより、膜厚5μm以下の防眩層を形成した。 2. Formation of anti-glare layer TAC-D105 manufactured by Dainichi Chemical Industries as a clear material, EXG40-77 (D-30M) manufactured by Dainichi Chemical Industries as a mat material containing amorphous silica, and Silicon 10 manufactured by Dainichi Chemical Industries as a surfactant A curable resin composition for an antiglare layer was prepared using -28, methyl isobutyl ketone (MIBK) as a solvent. At this time, 0.6 parts by weight of a surfactant was added to 100 parts by weight of the total amount of the clear material and the mat material, and the clear / mat ratio was changed to prepare a curable resin composition for an antiglare layer.
On one side of the substrate on which the hard coat layer is formed, the anti-glare layer curable resin composition is applied by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, and the solvent in the coating film is evaporated. An anti-glare layer having a thickness of 5 μm or less was formed by irradiating ultraviolet rays having a central wavelength of 365 nm so that the integrated light amount was 3000 mJ / cm 2 to cure the coating film.
クリア材として大日精化工業製TAC-D105、不定形シリカを含むマット材として大日精化工業製EXG40-77(D-30M)、界面活性剤として大日精化工業製シリコン10-28、溶剤としてメチルイソブチルケトン(MIBK)を用い、防眩層用硬化性樹脂組成物を調製した。この際、クリア材およびマット材の総量100重量部に対して界面活性剤を0.6重量部添加し、クリア/マット比を変化させて、防眩層用硬化性樹脂組成物を調製した。
ハードコート層を形成した基板の片面に、防眩層用硬化性樹脂組成物をスピンコート法にて塗布し、温度80℃のホットプレートで180秒間乾燥し、塗膜中の溶剤を蒸発させ、中心波長365nmの紫外線を積算光量が3000mJ/cm2になるように照射して塗膜を硬化させることにより、膜厚5μm以下の防眩層を形成した。 2. Formation of anti-glare layer TAC-D105 manufactured by Dainichi Chemical Industries as a clear material, EXG40-77 (D-30M) manufactured by Dainichi Chemical Industries as a mat material containing amorphous silica, and Silicon 10 manufactured by Dainichi Chemical Industries as a surfactant A curable resin composition for an antiglare layer was prepared using -28, methyl isobutyl ketone (MIBK) as a solvent. At this time, 0.6 parts by weight of a surfactant was added to 100 parts by weight of the total amount of the clear material and the mat material, and the clear / mat ratio was changed to prepare a curable resin composition for an antiglare layer.
On one side of the substrate on which the hard coat layer is formed, the anti-glare layer curable resin composition is applied by a spin coating method, dried on a hot plate at a temperature of 80 ° C. for 180 seconds, and the solvent in the coating film is evaporated. An anti-glare layer having a thickness of 5 μm or less was formed by irradiating ultraviolet rays having a central wavelength of 365 nm so that the integrated light amount was 3000 mJ / cm 2 to cure the coating film.
[評価7]
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 7]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 7]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(ヘイズおよび透過率)
ハードコートフィルムのヘイズおよび透過率は、村上色彩技術研究所製のヘイズメーターHM150を用いて、JIS-K7105に準拠する方法で測定した。 (Haze and transmittance)
The haze and transmittance of the hard coat film were measured by a method based on JIS-K7105 using a haze meter HM150 manufactured by Murakami Color Research Laboratory.
ハードコートフィルムのヘイズおよび透過率は、村上色彩技術研究所製のヘイズメーターHM150を用いて、JIS-K7105に準拠する方法で測定した。 (Haze and transmittance)
The haze and transmittance of the hard coat film were measured by a method based on JIS-K7105 using a haze meter HM150 manufactured by Murakami Color Research Laboratory.
(光沢)
村上色彩技術研究所製のグロスメーターGM-26PROを用い、入射角60度の条件で、ハードコートフィルムの表面のグロス値を測定した。数字が高いほど高光沢であることを示し、数字が低いほど低光沢であることを示す。 (Glossy)
Using a gloss meter GM-26PRO manufactured by Murakami Color Research Laboratory, the gloss value of the surface of the hard coat film was measured under the condition of an incident angle of 60 degrees. A higher number indicates higher gloss, and a lower number indicates lower gloss.
村上色彩技術研究所製のグロスメーターGM-26PROを用い、入射角60度の条件で、ハードコートフィルムの表面のグロス値を測定した。数字が高いほど高光沢であることを示し、数字が低いほど低光沢であることを示す。 (Glossy)
Using a gloss meter GM-26PRO manufactured by Murakami Color Research Laboratory, the gloss value of the surface of the hard coat film was measured under the condition of an incident angle of 60 degrees. A higher number indicates higher gloss, and a lower number indicates lower gloss.
(密着)
JIS K5600-5-6に準拠して、付着テープとしてニチバン(株)製の工業用24mmセロテープ(登録商標)を用いて、クロスカット法による付着性試験を行い、密着性を評価した。(剥がれなかったマス目の数)/(マス目の合計)として、下記の基準にて評価した。
A:100/100
B:50/100~99/100
C:50/100未満 (Close contact)
In accordance with JIS K5600-5-6, an adhesive test by cross-cut method was performed using an industrial 24 mm cello tape (registered trademark) manufactured by Nichiban Co., Ltd. as an adhesive tape, and the adhesion was evaluated. Evaluation was made according to the following criteria as (number of squares not peeled) / (total squares).
A: 100/100
B: 50/100 to 99/100
C: less than 50/100
JIS K5600-5-6に準拠して、付着テープとしてニチバン(株)製の工業用24mmセロテープ(登録商標)を用いて、クロスカット法による付着性試験を行い、密着性を評価した。(剥がれなかったマス目の数)/(マス目の合計)として、下記の基準にて評価した。
A:100/100
B:50/100~99/100
C:50/100未満 (Close contact)
In accordance with JIS K5600-5-6, an adhesive test by cross-cut method was performed using an industrial 24 mm cello tape (registered trademark) manufactured by Nichiban Co., Ltd. as an adhesive tape, and the adhesion was evaluated. Evaluation was made according to the following criteria as (number of squares not peeled) / (total squares).
A: 100/100
B: 50/100 to 99/100
C: less than 50/100
ハードコート層上に防眩層を形成した場合においても、高硬度が得られた。
Even when an antiglare layer was formed on the hard coat layer, high hardness was obtained.
[実施例8]
(硬化性樹脂組成物の調製)
ウレタンアクリレートをさらに添加したこと以外は、実施例1と同様の組成で、硬化性樹脂組成物を調製した。この際、モノマー、アクリル系ポリマーおよびウレタンアクリレートの比は表8に示すように変化させた。また、ウレタンアクリレートとしては、日本合成化学工業製の紫光UV-7000Bを使用した。このウレタンアクリレートは、引っ張り試験機で室温25℃、速度100mm/minの条件で伸び率を測定すると、伸び率が10%~40%となるものである。 [Example 8]
(Preparation of curable resin composition)
A curable resin composition was prepared with the same composition as in Example 1 except that urethane acrylate was further added. At this time, the ratio of the monomer, the acrylic polymer and the urethane acrylate was changed as shown in Table 8. As urethane acrylate, purple light UV-7000B made by Nippon Synthetic Chemical Industry was used. This urethane acrylate has an elongation of 10% to 40% when the elongation is measured with a tensile tester at room temperature of 25 ° C. and speed of 100 mm / min.
(硬化性樹脂組成物の調製)
ウレタンアクリレートをさらに添加したこと以外は、実施例1と同様の組成で、硬化性樹脂組成物を調製した。この際、モノマー、アクリル系ポリマーおよびウレタンアクリレートの比は表8に示すように変化させた。また、ウレタンアクリレートとしては、日本合成化学工業製の紫光UV-7000Bを使用した。このウレタンアクリレートは、引っ張り試験機で室温25℃、速度100mm/minの条件で伸び率を測定すると、伸び率が10%~40%となるものである。 [Example 8]
(Preparation of curable resin composition)
A curable resin composition was prepared with the same composition as in Example 1 except that urethane acrylate was further added. At this time, the ratio of the monomer, the acrylic polymer and the urethane acrylate was changed as shown in Table 8. As urethane acrylate, purple light UV-7000B made by Nippon Synthetic Chemical Industry was used. This urethane acrylate has an elongation of 10% to 40% when the elongation is measured with a tensile tester at room temperature of 25 ° C. and speed of 100 mm / min.
(ハードコート層の形成)
まず、基板として厚み0.35mmのポリエチレンテレフタレート(PET)基板を用い、PET基板の片面に実施例1と同様にして所定の膜厚のハードコート層を形成した。次に、一部の試料については、基板のハードコート層を形成した面とは反対側の面に同様に所定の膜厚の第2のハードコート層を形成した。 (Formation of hard coat layer)
First, a polyethylene terephthalate (PET) substrate having a thickness of 0.35 mm was used as a substrate, and a hard coat layer having a predetermined thickness was formed on one side of the PET substrate in the same manner as in Example 1. Next, for some of the samples, a second hard coat layer having a predetermined film thickness was similarly formed on the surface of the substrate opposite to the surface on which the hard coat layer was formed.
まず、基板として厚み0.35mmのポリエチレンテレフタレート(PET)基板を用い、PET基板の片面に実施例1と同様にして所定の膜厚のハードコート層を形成した。次に、一部の試料については、基板のハードコート層を形成した面とは反対側の面に同様に所定の膜厚の第2のハードコート層を形成した。 (Formation of hard coat layer)
First, a polyethylene terephthalate (PET) substrate having a thickness of 0.35 mm was used as a substrate, and a hard coat layer having a predetermined thickness was formed on one side of the PET substrate in the same manner as in Example 1. Next, for some of the samples, a second hard coat layer having a predetermined film thickness was similarly formed on the surface of the substrate opposite to the surface on which the hard coat layer was formed.
[評価8]
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 8]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(鉛筆硬度試験)
実施例1と同様にして、鉛筆硬度試験を行い、評価した。 [Evaluation 8]
(Pencil hardness test)
A pencil hardness test was performed and evaluated in the same manner as in Example 1.
(反り)
得られたハードコートフィルムをA4サイズの1/4の寸法とし、そのハードコートフィルムを平らな面に置き、四隅四辺の平面からの浮きあがりの高さの平均値を反りとして測定した。この際、ハードコートフィルム作製直後(初期)および下記の耐候試験後のそれぞれについて測定した。
なお、反りは基板または第2のハードコート層を下側にした場合、ハードコート層を下側にした場合のそれぞれについて測定を行った。表8では、基板または第2のハードコート層を下側にした場合のハードコート層側への反りを負の値に、ハードコート層を下側にした場合の基板または第2のハードコート層側への反りを正の値として示している。
また、耐候試験は、ハードコートフィルムを温度50℃、相対湿度95%の条件で24時間放置する試験、および、温度85℃、相対湿度85%の条件で24時間放置する試験を行った。 (warp)
The obtained hard coat film was set to 1/4 of the A4 size, the hard coat film was placed on a flat surface, and the average value of the height of lifting from the flat surfaces of the four corners and four sides was measured as warpage. Under the present circumstances, it measured about each after a hard coat film preparation (initial stage) and the following weathering test.
The warpage was measured for each of the case where the substrate or the second hard coat layer was on the lower side and the case where the hard coat layer was on the lower side. In Table 8, the warp to the hard coat layer side when the substrate or the second hard coat layer is down is negative, and the substrate or second hard coat layer is when the hard coat layer is down The warp to the side is shown as a positive value.
The weather resistance test was a test in which the hard coat film was allowed to stand for 24 hours at a temperature of 50 ° C. and a relative humidity of 95%, and a test for which the hard coat film was allowed to stand for 24 hours under a temperature of 85 ° C. and a relative humidity of 85%.
得られたハードコートフィルムをA4サイズの1/4の寸法とし、そのハードコートフィルムを平らな面に置き、四隅四辺の平面からの浮きあがりの高さの平均値を反りとして測定した。この際、ハードコートフィルム作製直後(初期)および下記の耐候試験後のそれぞれについて測定した。
なお、反りは基板または第2のハードコート層を下側にした場合、ハードコート層を下側にした場合のそれぞれについて測定を行った。表8では、基板または第2のハードコート層を下側にした場合のハードコート層側への反りを負の値に、ハードコート層を下側にした場合の基板または第2のハードコート層側への反りを正の値として示している。
また、耐候試験は、ハードコートフィルムを温度50℃、相対湿度95%の条件で24時間放置する試験、および、温度85℃、相対湿度85%の条件で24時間放置する試験を行った。 (warp)
The obtained hard coat film was set to 1/4 of the A4 size, the hard coat film was placed on a flat surface, and the average value of the height of lifting from the flat surfaces of the four corners and four sides was measured as warpage. Under the present circumstances, it measured about each after a hard coat film preparation (initial stage) and the following weathering test.
The warpage was measured for each of the case where the substrate or the second hard coat layer was on the lower side and the case where the hard coat layer was on the lower side. In Table 8, the warp to the hard coat layer side when the substrate or the second hard coat layer is down is negative, and the substrate or second hard coat layer is when the hard coat layer is down The warp to the side is shown as a positive value.
The weather resistance test was a test in which the hard coat film was allowed to stand for 24 hours at a temperature of 50 ° C. and a relative humidity of 95%, and a test for which the hard coat film was allowed to stand for 24 hours under a temperature of 85 ° C. and a relative humidity of 85%.
(落球試験)
所定の高さより36gの鋼球を落下させ、基板およびハードコートフィルムの割れやクラックの有無を確認する試験を行った。この際、高さは5cm刻みで試験を行った。表中の値は割れやクラックが見られず外観が良好であった高さを示している。 (Falling ball test)
A test was conducted in which a steel ball of 36 g was dropped from a predetermined height, and the substrate and the hard coat film were checked for cracks and cracks. At this time, the test was conducted at a height of 5 cm. The values in the table indicate the height at which the appearance was good with no cracks or cracks.
所定の高さより36gの鋼球を落下させ、基板およびハードコートフィルムの割れやクラックの有無を確認する試験を行った。この際、高さは5cm刻みで試験を行った。表中の値は割れやクラックが見られず外観が良好であった高さを示している。 (Falling ball test)
A test was conducted in which a steel ball of 36 g was dropped from a predetermined height, and the substrate and the hard coat film were checked for cracks and cracks. At this time, the test was conducted at a height of 5 cm. The values in the table indicate the height at which the appearance was good with no cracks or cracks.
ウレタンアクリレートを添加することにより、反りを低減できることが確認された。特に、ウレタンアクリレートを含有するハードコート層を基板の両面に形成した場合には、耐候試験後の反りが小さくなった。
It was confirmed that warpage can be reduced by adding urethane acrylate. In particular, when hard coat layers containing urethane acrylate were formed on both sides of the substrate, warpage after the weather resistance test was reduced.
1 … ハードコートフィルム
2 … 基板
3 … ハードコート層
4 … 第2のハードコート層
5 … 防眩層 DESCRIPTION OFSYMBOLS 1 ... Hard coat film 2 ... Board | substrate 3 ... Hard coat layer 4 ... 2nd hard coat layer 5 ... Anti-glare layer
2 … 基板
3 … ハードコート層
4 … 第2のハードコート層
5 … 防眩層 DESCRIPTION OF
Claims (9)
- 基板上にハードコート層が形成されたハードコートフィルムであって、
前記ハードコート層は、
異形シリカ微粒子と、
重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、
マトリクス樹脂と
を含有することを特徴とするハードコートフィルム。 A hard coat film having a hard coat layer formed on a substrate,
The hard coat layer is
Irregular shaped silica fine particles,
An acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200;
A hard coat film comprising a matrix resin. - 前記ハードコート層が重合開始剤を含有することを特徴とする請求項1に記載のハードコートフィルム。 The hard coat film according to claim 1, wherein the hard coat layer contains a polymerization initiator.
- 前記ハードコート層が青色色材を含有することを特徴とする請求項1または請求項2に記載のハードコートフィルム。 The hard coat film according to claim 1, wherein the hard coat layer contains a blue color material.
- 前記ハードコート層上に防眩層が形成されていることを特徴とする請求項1から請求項3までのいずれかに記載のハードコートフィルム。 The hard coat film according to any one of claims 1 to 3, wherein an antiglare layer is formed on the hard coat layer.
- 基板上にハードコート層が形成されたハードコートフィルムであって、
前記ハードコート層は、
反応性異形シリカ微粒子と、
前記アクリル系ポリマーと、
モノマーと
を含有する硬化性樹脂組成物の硬化物を含むことを特徴とするハードコートフィルム。 A hard coat film having a hard coat layer formed on a substrate,
The hard coat layer is
Reactive irregularly shaped silica fine particles;
The acrylic polymer;
A hard coat film comprising a cured product of a curable resin composition containing a monomer. - 反応性異形シリカ微粒子と、
重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、
モノマーと
を含有することを特徴とするハードコート層用硬化性樹脂組成物。 Reactive irregularly shaped silica fine particles;
An acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200;
A curable resin composition for a hard coat layer, comprising a monomer. - 重合開始剤をさらに含有することを特徴とする請求項6に記載のハードコート層用硬化性樹脂組成物。 The curable resin composition for a hard coat layer according to claim 6, further comprising a polymerization initiator.
- 青色色材をさらに含有することを特徴とする請求項6または請求項7に記載のハードコート層用硬化性樹脂組成物。 The curable resin composition for a hard coat layer according to claim 6 or 7, further comprising a blue color material.
- 基板上に、反応性異形シリカ微粒子と、重量平均分子量が30,000~110,000の範囲内であり、アクリル当量が200~1,200の範囲内であるアクリル系ポリマーと、モノマーとを含有するハードコート層用硬化性樹脂組成物を塗布し、硬化させてハードコート層を形成するハードコート層形成工程を有することを特徴とするハードコートフィルムの製造方法。 A reactive irregularly shaped silica particle, an acrylic polymer having a weight average molecular weight in the range of 30,000 to 110,000 and an acrylic equivalent in the range of 200 to 1,200, and a monomer are contained on the substrate. The manufacturing method of the hard coat film characterized by having the hard-coat layer formation process which apply | coats and hardens the curable resin composition for hard-coat layers to form a hard-coat layer.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013003902 | 2013-01-11 | ||
| JP2013-003902 | 2013-01-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014109407A1 true WO2014109407A1 (en) | 2014-07-17 |
Family
ID=51167051
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/050379 WO2014109407A1 (en) | 2013-01-11 | 2014-01-10 | Hard coat film, curable resin composition for hard coat layers, and method for producing hard coat film |
Country Status (2)
| Country | Link |
|---|---|
| JP (2) | JP2014149520A (en) |
| WO (1) | WO2014109407A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016109333A (en) * | 2014-12-04 | 2016-06-20 | 日本電気硝子株式会社 | Top plate for cooker and manufacturing method thereof |
| JP2016124293A (en) * | 2014-12-26 | 2016-07-11 | 三菱化学株式会社 | Laminate and display body cover |
| WO2020226600A3 (en) * | 2019-05-06 | 2020-12-24 | Alpay Aytekin | Composition used in coating inorganic compounds used in the production of laminate flooring |
| JPWO2021206066A1 (en) * | 2020-04-10 | 2021-10-14 | ||
| WO2022044870A1 (en) * | 2020-08-27 | 2022-03-03 | 日本ゼオン株式会社 | Light reducing film, method for manufacturing same, and laminate |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6276211B2 (en) | 2014-05-30 | 2018-02-07 | リケンテクノス株式会社 | Transparent resin laminate |
| JP6364297B2 (en) * | 2014-09-24 | 2018-07-25 | 帝人株式会社 | Conductive laminate and touch panel using the same |
| JP6153977B2 (en) | 2014-10-02 | 2017-06-28 | リケンテクノス株式会社 | Adhesive film |
| KR102479158B1 (en) | 2015-03-18 | 2022-12-19 | 리껭테크노스 가부시키가이샤 | Hard coat laminated film |
| US11065851B2 (en) | 2015-03-18 | 2021-07-20 | Riken Technos Corporation | Multilayer hard coating film |
| CN107405901B (en) | 2015-03-18 | 2020-04-17 | 理研科技株式会社 | Anti-glare hard-coat laminated film |
| US10596739B2 (en) | 2015-03-18 | 2020-03-24 | Riken Technos Corporation | Molded body |
| WO2016147716A1 (en) | 2015-03-18 | 2016-09-22 | リケンテクノス株式会社 | Adhesive film |
| US11433651B2 (en) | 2015-03-18 | 2022-09-06 | Riken Technos Corporation | Hard coat laminated film |
| WO2016147424A1 (en) * | 2015-03-18 | 2016-09-22 | リケンテクノス株式会社 | Hard coat laminate film and method for producing same |
| US10696016B2 (en) * | 2015-07-31 | 2020-06-30 | Samsung Sdi Co., Ltd. | Window film and flexible display including the same |
| JP6565573B2 (en) * | 2015-10-08 | 2019-08-28 | 三菱ケミカル株式会社 | Laminated body and manufacturing method thereof |
| US11774166B2 (en) | 2015-11-25 | 2023-10-03 | Riken Technos Corporation | Door body |
| TWI745316B (en) | 2015-11-25 | 2021-11-11 | 日商理研科技股份有限公司 | Door |
| JP2017100366A (en) * | 2015-12-02 | 2017-06-08 | 三菱ケミカル株式会社 | Resin laminate and method for producing the same |
| JP6644534B2 (en) | 2015-12-08 | 2020-02-12 | リケンテクノス株式会社 | Hard coat laminated film |
| JP6769676B2 (en) * | 2016-03-28 | 2020-10-14 | 日本製紙株式会社 | Hard coat paint composition and hard coat film for molding |
| JP2017206637A (en) * | 2016-05-20 | 2017-11-24 | 日立化成株式会社 | Resin composition, substrate with resin layer and method for producing the same, substrate with conductive layer and method for producing the same, and touch panel |
| KR102346427B1 (en) | 2016-05-20 | 2022-01-04 | 다이니폰 인사츠 가부시키가이샤 | Optical laminate and image display device |
| WO2018051653A1 (en) | 2016-09-14 | 2018-03-22 | リケンテクノス株式会社 | Hard coat laminated film |
| JP7064313B2 (en) | 2016-11-25 | 2022-05-10 | リケンテクノス株式会社 | Hardcourt laminated film |
| JP7013680B2 (en) * | 2017-06-01 | 2022-02-01 | 大日本印刷株式会社 | Hardcourt film, ionizing radiation curable resin composition, and display |
| WO2019171989A1 (en) * | 2018-03-09 | 2019-09-12 | 富士フイルム株式会社 | Hard coat film, and hard coat film having scratch-resistant layer attached thereto |
| JP2019191330A (en) * | 2018-04-24 | 2019-10-31 | 東洋紡株式会社 | Foldable display surface protective film |
| JP2023002293A (en) * | 2021-06-22 | 2023-01-10 | 株式会社ダイセル | Anti-glare film |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006168338A (en) * | 2004-11-19 | 2006-06-29 | Mitsubishi Plastics Ind Ltd | Sheet object for manufacturing name plate and its manufacturing method |
| JP2010120991A (en) * | 2008-11-17 | 2010-06-03 | Dainippon Printing Co Ltd | Curable resin composition for hard coat layer and hard coat film |
| JP2011075705A (en) * | 2009-09-29 | 2011-04-14 | Dainippon Printing Co Ltd | Method of manufacturing hard coat film, hard coat film, polarizing plate and display panel |
| JP2011201938A (en) * | 2010-03-24 | 2011-10-13 | Dic Corp | Inorganic particle dispersion, energy ray-curable resin composition, and film |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09159804A (en) * | 1995-12-13 | 1997-06-20 | Sumitomo Chem Co Ltd | Reflection preventing filter |
| JP4559097B2 (en) * | 2004-02-27 | 2010-10-06 | 株式会社リコー | Image forming apparatus |
| US20100028682A1 (en) * | 2006-09-29 | 2010-02-04 | Seiji Shinohara | Optical functional film |
| JP5181652B2 (en) * | 2007-12-17 | 2013-04-10 | 東レ株式会社 | Light transmissive electromagnetic wave shielding film, display filter using the same, and production method thereof |
| JP5379465B2 (en) * | 2008-12-17 | 2013-12-25 | パナソニック株式会社 | Light emitting device |
-
2014
- 2014-01-10 WO PCT/JP2014/050379 patent/WO2014109407A1/en active Application Filing
- 2014-01-10 JP JP2014003704A patent/JP2014149520A/en active Pending
- 2014-09-19 JP JP2014191199A patent/JP2014238614A/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006168338A (en) * | 2004-11-19 | 2006-06-29 | Mitsubishi Plastics Ind Ltd | Sheet object for manufacturing name plate and its manufacturing method |
| JP2010120991A (en) * | 2008-11-17 | 2010-06-03 | Dainippon Printing Co Ltd | Curable resin composition for hard coat layer and hard coat film |
| JP2011075705A (en) * | 2009-09-29 | 2011-04-14 | Dainippon Printing Co Ltd | Method of manufacturing hard coat film, hard coat film, polarizing plate and display panel |
| JP2011201938A (en) * | 2010-03-24 | 2011-10-13 | Dic Corp | Inorganic particle dispersion, energy ray-curable resin composition, and film |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016109333A (en) * | 2014-12-04 | 2016-06-20 | 日本電気硝子株式会社 | Top plate for cooker and manufacturing method thereof |
| JP2016124293A (en) * | 2014-12-26 | 2016-07-11 | 三菱化学株式会社 | Laminate and display body cover |
| WO2020226600A3 (en) * | 2019-05-06 | 2020-12-24 | Alpay Aytekin | Composition used in coating inorganic compounds used in the production of laminate flooring |
| JPWO2021206066A1 (en) * | 2020-04-10 | 2021-10-14 | ||
| JP7404511B2 (en) | 2020-04-10 | 2023-12-25 | 富士フイルム株式会社 | Anti-glare film and method for producing anti-glare film |
| WO2022044870A1 (en) * | 2020-08-27 | 2022-03-03 | 日本ゼオン株式会社 | Light reducing film, method for manufacturing same, and laminate |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2014238614A (en) | 2014-12-18 |
| JP2014149520A (en) | 2014-08-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2014109407A1 (en) | Hard coat film, curable resin composition for hard coat layers, and method for producing hard coat film | |
| JP5540495B2 (en) | Curable resin composition for hard coat layer and hard coat film | |
| JP5504605B2 (en) | Curable resin composition for hard coat layer and hard coat film | |
| KR102159140B1 (en) | Anti-smudge hard coat and anti-smudge hard coat precursor | |
| JP5526582B2 (en) | Hard coat film and method for producing hard coat film | |
| JP5103902B2 (en) | Hard coat film and method for producing the same | |
| JP5088297B2 (en) | Hard coat film | |
| JP5157819B2 (en) | Hard coat film | |
| JP5262610B2 (en) | Manufacturing method of optical sheet | |
| KR101194180B1 (en) | Antireflection laminate | |
| JP2015197487A (en) | hard coat film | |
| CN104379675A (en) | Nanostructured materials and methods of making the same | |
| JP5453777B2 (en) | Hard coat film | |
| CN104837908A (en) | Nanostructured materials and methods of making same | |
| JP2010122323A (en) | Optical sheet and method of manufacturing the same | |
| TW201411177A (en) | Anti-reflective hard coat and anti-reflective article | |
| JP2009086360A (en) | Antireflection film | |
| JP2008107762A (en) | Hard coat film and its manufacturing method | |
| JP2009084328A (en) | Curable resin composition and hard coat film | |
| JP2015222364A (en) | Hard coat film and manufacturing method thereof | |
| JP2010237572A (en) | Optical sheet | |
| JP2013210976A (en) | Protective body and touch panel module | |
| JP2008163252A (en) | Curable resin composition for anti-glaring layer, and anti-glaring film | |
| JP6089709B2 (en) | Front substrate for touch panel | |
| JP2010085931A (en) | Method for manufacturing hard coat film and method for manufacturing optical functional member |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14737793 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14737793 Country of ref document: EP Kind code of ref document: A1 |