WO2014054649A1 - Absorbent article - Google Patents
Absorbent article Download PDFInfo
- Publication number
- WO2014054649A1 WO2014054649A1 PCT/JP2013/076727 JP2013076727W WO2014054649A1 WO 2014054649 A1 WO2014054649 A1 WO 2014054649A1 JP 2013076727 W JP2013076727 W JP 2013076727W WO 2014054649 A1 WO2014054649 A1 WO 2014054649A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- chain hydrocarbon
- fiber
- hydrocarbon moiety
- top sheet
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/15577—Apparatus or processes for manufacturing
- A61F13/15804—Plant, e.g. involving several steps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/51—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers
- A61F13/511—Topsheet, i.e. the permeable cover or layer facing the skin
- A61F13/51104—Topsheet, i.e. the permeable cover or layer facing the skin the top sheet having a three-dimensional cross-section, e.g. corrugations, embossments, recesses or projections
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/51—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers
- A61F13/511—Topsheet, i.e. the permeable cover or layer facing the skin
- A61F13/51113—Topsheet, i.e. the permeable cover or layer facing the skin comprising an additive, e.g. lotion or odour control
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/84—Accessories, not otherwise provided for, for absorbent pads
- A61F13/8405—Additives, e.g. for odour, disinfectant or pH control
Definitions
- the present invention relates to an absorbent article.
- Patent Documents 1 to 4 As a top sheet of an absorbent article, a non-woven fabric having an uneven surface and a method for producing the same are known (Patent Documents 1 to 4).
- an object of this invention is to provide the absorbent article which has the improved menstrual blood transfer property from a top sheet to an absorber, and can reduce the menstrual blood remaining on a top sheet.
- the present invention provides a liquid-permeable top sheet having a skin contact surface, a liquid-impermeable back sheet having a non-skin contact surface, the top sheet, and the back sheet.
- An absorbent article comprising an absorbent body provided therebetween, wherein the top sheet intersects the first direction and the first direction at least in the excretory opening contact region of the skin contact surface.
- the absorbent article is provided with a blood slipperiness imparting agent having a mass% and a weight average molecular weight of less than 1,000.
- an absorbent article that has improved menstrual blood transfer from the top sheet to the absorbent body and can reduce menstrual blood remaining on the top sheet.
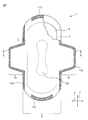
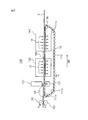
- FIG. 1 is a partially broken plan view of a sanitary napkin according to an embodiment of the absorbent article of the present invention.
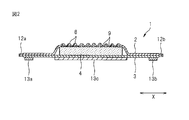
- 2 is a cross-sectional view taken along line AA in FIG.
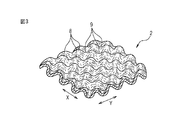
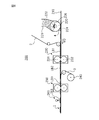
- FIG. 3 is a partial perspective view of a top sheet provided in the sanitary napkin shown in FIG. 1.
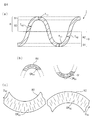
- 4A is a partially enlarged cross-sectional view of the top sheet shown in FIG. 3
- FIG. 4B is a diagram showing fiber orientations at the top of the convex portion and the bottom of the concave portion shown in FIG. 4A.
- FIG. 4C is a development view of the convex side wall and the concave side wall shown in FIG.
- FIG. 5 is a diagram illustrating a manufacturing process of the top sheet shown in FIG. FIG.
- FIG. 6 is a diagram for explaining a manufacturing process of the sanitary napkin shown in FIG.
- FIG. 7 is an electron micrograph of the skin contact surface of the top sheet in a sanitary napkin in which the top sheet contains tri-C2L oil fatty acid glycerides.
- FIG. 8 is a photomicrograph of menstrual blood with or without a blood slipping agent.
- FIG. 9 is a diagram for explaining a method of measuring the surface tension.
- An absorbent article according to the present invention is provided between a liquid-permeable top sheet having a skin contact surface, a liquid-impermeable back sheet having a non-skin contact surface, and the top sheet and the back sheet.
- An absorbent article comprising the absorbent body, wherein the top sheet has a first direction and a second direction intersecting the first direction at least in the excretory opening contact region of the skin contact surface.
- a plurality of concave portions each having a bottom portion and a concave portion side wall portion extending from the bottom portion, and the convex side wall portion extending from the top portion. Having a fiber orientation along the direction, and the recess side wall portion is a fiber along its extending direction. It has a tropism, wherein at least the convex portion of the excretory opening contact region, kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, water holding percentage of 0.01 to 4.0 mass%,
- the absorbent article is coated with a blood slipperiness-imparting agent having a weight average molecular weight of less than 1,000.
- the absorbent article according to the present invention when menstrual blood excreted from the wearer reaches the excretory opening contact region, it slides down together with the blood slipperiness-imparting agent present in the convex portion, passes through the top sheet, and becomes an absorbent body. Transition. Therefore, the absorbent article according to the present invention has improved menstrual blood transfer from the top sheet to the absorbent body, and can reduce menstrual blood remaining on the top sheet. For this reason, the sticky feeling of the skin contact surface of the top sheet is prevented, and the smooth feeling is maintained. The effect of such a blood slipperiness-imparting agent is exerted irrespective of changes in menstrual blood discharge (that is, whether menstrual blood discharged at a time is large or small). .
- the convex side wall portion extends from the top of the convex portion toward the bottom of the concave portion, and the concave side wall portion starts from the bottom of the concave portion at the top of the convex portion. It extends towards. Therefore, the convex side wall portion has a fiber orientation along the extending direction, that is, the direction from the top of the convex portion toward the bottom of the concave portion, and the concave side wall portion is in the extending direction, that is, the concave portion. It has fiber orientation along the direction from the bottom toward the top of the convex portion.
- menstrual blood excreted from the wearer easily slides along the extending direction of the convex side wall portion and the concave side wall portion together with the blood slipperiness imparting agent.
- menstrual blood excreted from the wearer does not spread in the surface direction of the top sheet, but easily moves in the thickness direction of the top sheet (that is, to the absorber).
- the convex side wall portion and the concave side wall portion have fiber orientation along each extending direction, and therefore the fiber orientation in the thickness direction of the top sheet is large, resulting from this.
- the top sheet has high compression deformation and compression recovery properties (cushioning properties). Therefore, even if the absorbent article is compressed, liquid return (rewetting back) from the absorbent body hardly occurs. Moreover, since the cushioning property of a top sheet is high, it gives a wearer good comfort.
- the skin contact surface of the top sheet has an uneven shape, the contact area between the wearer's skin and the top sheet is small, and the surface direction of the top sheet through the space in the recess is reduced. Good air permeability. Therefore, it is difficult to give the wearer stuffiness and itching.
- the fiber density at the top of the convex portion is lower than the fiber density at the bottom of the concave portion. Since the liquid is likely to move from the lower fiber density (the top of the convex portion in aspect 1) to the higher (the bottom of the concave part in aspect 1), the absorbent article according to aspect 1 has the effect of the blood slipperiness-imparting agent. In combination with the above, it has improved menstrual transfer from the top sheet to the absorbent body.
- the fiber density at the top of the convex portion is higher than the fiber density at the bottom of the concave portion.
- the liquid is less likely to move from the higher fiber density (the top of the convex part in aspect 2) to the lower (the bottom of the concave part in aspect 2), but the absorbent article according to aspect 2 has the effect of the blood slipperiness-imparting agent. Therefore, it has the improved menstrual blood transfer property from a top sheet to an absorber. Therefore, the effect of the blood slipperiness-imparting agent is significant in the absorbent article according to aspect 2.
- the convex side wall part and / or the concave side wall part have portions having different fiber orientations.
- Aspect 3 can be combined with Aspect 1 or Aspect 2.
- the fiber orientation in the thickness direction at the bottom of the recess is smaller than the fiber orientation in the thickness direction at the sidewall of the recess.
- menstrual blood is easy to transfer to a thickness direction (namely, to an absorber), without accumulating in a recessed part side wall part.
- the fiber orientation in a recessed part changes to the direction perpendicular
- Aspect 4 can be combined with one of Aspects 1 to 3, or two or more aspects that can be arranged side by side.
- the number of fiber fusion points at the top of the convex portion is smaller than the number of fiber fusion points at the bottom of the concave portion.
- the touch of a top sheet is favorable.
- Aspect 5 can be combined with one of aspects 1 to 4 or two or more aspects that can be arranged side by side.
- the constituent fibers of the top sheet are heat-sealed.
- corrugated shape of a top sheet is maintained by the heat sealing
- Aspect 6 can be combined with one of aspects 1 to 5 or two or more aspects that can be arranged side by side.
- the top sheet contains heat-extensible heat-extensible fibers. Due to the thermal elongation of the heat-extensible fibers, effective uneven shaping is possible. Moreover, the heat
- the IOB of the blood slipperiness imparting agent is an IOB of 0.00 to 0.60.
- Aspect 8 can be combined with one of Aspects 1 to 7, or two or more aspects that can be juxtaposed.
- the blood slipperiness imparting agent includes the following (i) to (iii): (I) hydrocarbons, (Ii) from (ii-1) a hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety.
- the hydrocarbon moiety A compound having one or a plurality of the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which replaces a hydrogen atom; Selected from the group consisting of any combination thereof (in the compound (ii) or (iii), when two or more oxy groups are inserted, each oxy group is not adjacent) ).
- Aspect 9 can be combined with one of aspects 1 to 8 or two or more aspects that can be arranged side by side.
- the blood slipperiness imparting agent includes the following (i ′) to (iii ′): (I ′) hydrocarbon, (Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—), an ester bond (—COO) inserted between the C—C single bonds of the hydrocarbon moiety.
- Aspect 10 can be combined with one of Aspects 1-9 or two or more aspects that can be juxtaposed.
- the blood slipperiness imparting agent includes the following (A) to (F): (A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain An ester with a compound having one carboxyl group for substituting a hydrogen atom in the hydrocarbon moiety, (B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety, (C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the chain
- Aspect 11 can be combined with one of Aspects 1 to 10 or two or more aspects that can be juxtaposed.
- the blood slipperiness imparting agent is (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) a chain carbonization.
- An ester of hydrogen triol and at least one fatty acid (a 3 ) an ester of a chain hydrocarbon diol and at least one fatty acid, and (b 1 ) a chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol.
- the blood slipperiness imparting agent has a vapor pressure of 0.00 to 0.01 Pa at 1 atm and 40 ° C.
- Aspect 13 can be combined with one of Aspects 1-12 or two or more aspects that can be juxtaposed.
- the kind and application of the absorbent article of the present invention are not particularly limited.
- the absorbent article include sanitary products and sanitary products such as sanitary napkins and panty liners, and these may be used for humans and non-human animals such as pets.
- the liquid to be absorbed by the absorbent article is not particularly limited, but is mainly liquid excrement such as menstrual blood.
- an embodiment of the absorbent article of the present invention will be described based on the drawings, taking a sanitary napkin as an example.
- a sanitary napkin 1 according to an embodiment of the absorbent article of the present invention includes a liquid-permeable top sheet 2, a liquid-impermeable back sheet 3, and a top sheet 2. And an absorbent body 4 provided between the back sheets 3.
- the X-axis direction corresponds to the width direction of the sanitary napkin 1
- the Y-axis direction corresponds to the longitudinal direction of the sanitary napkin 1
- the plane direction extending in the X-axis Y-axis direction corresponds to the plane direction of the sanitary napkin 1.
- the sanitary napkin 1 is worn for the purpose of absorbing liquid excretion such as menstrual blood.
- the top sheet 2 is worn on the skin side of the wearer, and the back sheet 3 is worn on the clothes (underwear) side of the wearer.
- Liquid excreta such as menstrual blood passes through the top sheet 2 to reach the absorber 4 and is absorbed and held by the absorber 4. Leakage of liquid excretion absorbed and held by the absorber 4 is prevented by the back sheet 3.
- the top sheet 2 and the back sheet 3 are joined at their longitudinal ends by seal portions 11 a and 11 b to form a main body portion 6, and end portions in the width direction are sealed portions 12 a. , 12b and substantially rectangular wings 7a, 7b extending in the width direction from the main body 6 are formed.
- the shape of the main body portion 6 can be adjusted as appropriate within a range suitable for the wearer's body, underwear, etc. Examples of the shape of the main body portion 6 include a substantially rectangular shape, a substantially oval shape, and a generally saddle shape. .
- the total length in the longitudinal direction of the main body 6 is usually 100 to 500 mm, preferably 150 to 350 mm.
- the total length in the width direction of the main body 6 is usually 30 to 200 mm, preferably 40 to 180 mm.
- Examples of the bonding mode by the seal portions 11a, 11b, 12a, and 12b include embossing, ultrasonic waves, hot-melt adhesives, and the like. In order to increase the bonding strength, two or more bonding modes may be combined (for example, embossing is performed after bonding with a hot-melt adhesive).
- embossing for example, a method of embossing by passing the top sheet 2 and the back sheet 3 together between an embossing roll having a patterned convex portion and a flat roll (a method called a so-called round seal), etc. Is mentioned.
- the emboss pattern include a lattice pattern, a staggered pattern, and a wavy pattern.
- the hot melt adhesive for example, styrene-ethylene-butadiene-styrene (SEBS), styrene-butadiene-styrene (SBS), styrene-isoprene-styrene (SIS), or the like, or linear Pressure-sensitive adhesives or heat-sensitive adhesives mainly composed of olefins such as low density polyethylene; water-soluble polymers (for example, polyvinyl alcohol, carboxymethyl cellulose, gelatin, etc.) or water-swellable polymers (for example, polyvinyl acetate, poly And water sensitive adhesives such as sodium acrylate).
- the method for applying the adhesive include spiral coating, coater coating, curtain coater coating, and summit gun coating.
- adhesive portions 13 a and 13 b are provided on the clothing side of the backsheet 3 that forms the wing portions 7 a and 7 b, and on the clothing side of the backsheet 3 that forms the main body portion 6, An adhesive portion 13c is provided.
- the sanitary napkin 1 is obtained by attaching the adhesive part 13c to the crotch part of the underwear, bending the wing parts 7a and 7b to the outer surface side of the underwear, and attaching the adhesive parts 13a and 13b to the crotch part of the underwear. Stablely fixed to underwear.
- Examples of the adhesive contained in the adhesive portions 13a, 13b, and 13c include styrene-ethylene-butylene-styrene block copolymers, styrene-butylene polymers, styrene-butylene-styrene block copolymers, and styrene-isobutylene- Styrene polymers such as styrene copolymers; tackifiers such as C5 petroleum resins, C9 petroleum resins, dicyclopentadiene petroleum resins, rosin petroleum resins, polyterpene resins, terpene phenol resins; trifresyl phosphate, phthalic acid Examples include monomer plasticizers such as dibutyl and dioctyl phthalate; polymer plasticizers such as vinyl polymer and polyester.
- the top sheet 2 is a sheet through which liquid excretion such as menstrual blood can pass.
- One surface (upper surface in FIG. 2) of the top sheet 2 is a skin contact surface with which the wearer's skin comes into contact, and the other surface (lower surface in FIG. 2) is an absorption on which the absorber 4 is disposed. It is a body placement surface.
- the top sheet 2 examples include a non-woven fabric, a synthetic resin film in which liquid permeation holes are formed, and a laminate of a synthetic resin film and a non-woven fabric, and a non-woven fabric is preferable.
- a nonwoven fabric an air through nonwoven fabric, a heat bond nonwoven fabric, a spun bond nonwoven fabric, a melt blown nonwoven fabric, a spunlace nonwoven fabric, a needle punch nonwoven fabric, etc. are mentioned, for example.
- the top sheet 2 contains a heat-fusible fiber as a constituent fiber.
- the constituent fibers of the top sheet 2 are heat-sealed by melting and solidifying the heat-fusible fibers, whereby the uneven shape of the top sheet 2 is maintained. Further, when the sanitary napkin 1 is used, it is difficult for fibers to fall out and fluff from the top sheet 2.
- the heat-fusible fiber examples include a heat-fusible fiber made of a thermoplastic resin such as polyolefin, polyester, or polyamide.
- a thermoplastic resin such as polyolefin, polyester, or polyamide.
- the polyolefin include, for example, linear low density polyethylene (LLDPE), low density polyethylene (LDPE), medium density polyethylene (MDPE), high density polyethylene (HDPE), polypropylene, polybutylene, and copolymers based on these (for example, Ethylene-vinyl acetate copolymer (EVA), ethylene-ethyl acrylate copolymer (EEA), ethylene-acrylic acid copolymer (EAA), ethylene-propylene random copolymer (EP)), and the like.
- LLDPE linear low density polyethylene
- LDPE low density polyethylene
- MDPE medium density polyethylene
- HDPE high density polyethylene
- EVA Ethylene-vinyl acetate copolymer
- Polyethylene particularly HDPE, is preferred because it has a relatively low softening point of around 100 ° C. and is excellent in heat workability, and has low rigidity and a supple feel.
- the polyester include linear or branched carbon atoms of up to 20 including polyethylene terephthalate (PET), polytrimethyl terephthalate (PTT), polybutylene terephthalate (PBT), polylactic acid, and polyglycolic acid. And polyesters such as polyhydroxyalkanoic acid, copolymers based on these, and copolymerized polyesters obtained by copolymerizing alkylene terephthalate as a main component with a small amount of other components. Since it has elastic resilience, it is possible to construct fibers and nonwoven fabrics having high cushioning properties, and PET is preferred from the economical point of being obtained industrially at a low cost.
- the polyamide include 6-nylon and 6,6-nylon.
- the fiber diameter of the heat-fusible fiber is usually 5 to 100 ⁇ m, preferably 10 to 40 ⁇ m, and the fineness of the heat-fusible fiber is usually 0.5 to 10 dtex, preferably 1 to 5 dtex.
- the content of the conductive fiber is usually 10 to 100% by mass, preferably 50 to 100% by mass, based on the entire constituent fibers of the top sheet 2.
- the top sheet 2 preferably contains a thermally stretchable fiber as a constituent fiber. Due to the thermal elongation of the heat-extensible fibers, effective uneven shaping is possible. Moreover, the heat
- the heat-extensible fiber may be a fiber whose actual fiber length is elongated by heat treatment (for example, a fiber whose actual fiber length is elongated due to a change in the crystalline state of the resin). Fibers that do not elongate but have an apparent fiber length (for example, crimped fibers in which the apparent fiber length is elongated by releasing the crimps such as zigzag, ⁇ , and spiral) It may be.
- the heat-extensible fibers include two types of resins having different melting points or softening points (resins having a relatively high melting point or softening point are called “high melting point resins”, and resins having a relatively low melting point are “low melting point resins”. And a two-component heat-extensible composite fiber in which a low melting point resin is continuously present in the length direction on at least a part of the fiber surface.
- the high-melting point resin is a component that develops heat extensibility
- the low melting point resin is a component that develops heat-fusibility
- the heat-extensible composite fiber is heated at a temperature lower than the melting point of the high-melting point resin component. It is extensible.
- the kind of the high melting point resin and the low melting point resin is not particularly limited as long as it has fiber forming ability.
- the melting point difference or softening point difference between the high melting point resin and the low melting point resin is usually 20 ° C. or higher, preferably 25 ° C. or higher.
- the melting point of the high melting point resin and the low melting point resin for example, the melting point measured by the following method is used. Using a differential scanning calorimeter (for example, DSC6200, manufactured by Seiko Instruments Inc.), a finely cut fiber sample (for example, 2 mg sample) was subjected to thermal analysis at a heating rate of 10 ° C./min to melt each resin. The peak temperature is measured and the measured melting peak temperature is defined as the melting point. When the melting point of the resin cannot be clearly measured by this method, the temperature at which the resin is fused to such an extent that the fiber fusing point strength can be measured as the temperature at which the molecular flow of the resin begins, is used as the softening point. Used for.
- a differential scanning calorimeter for example, DSC6200, manufactured by Seiko Instruments Inc.
- the orientation index of the high melting point resin and the low melting point resin can be appropriately adjusted depending on the type of the resin.
- the orientation index is usually 60% or less, preferably 40% or less, and more preferably 25% or less.
- polyester is used as the high melting point resin
- the orientation index is usually 25% or less, preferably 20% or less, more preferably 10% or less.
- the orientation index of the low melting point resin is usually 5% or more, preferably 15% or more, and more preferably 30% or more.
- the orientation index of the high melting point resin and the low melting point resin is an index of the degree of orientation of the polymer chain of the resin constituting the fiber, and is calculated by the following formula.
- Orientation index (%) X / Y ⁇ 100 [Wherein, X is the value of birefringence of the resin in the heat-extensible conjugate fiber, and Y is the value of intrinsic birefringence of the resin. ]
- the birefringence (X in the above formula) of the resin in the heat-extensible composite fiber is measured, for example, with a polarizing plate attached to an interference microscope and polarized light in a direction parallel to and perpendicular to the fiber axis.
- the immersion liquid for example, a standard refraction liquid manufactured by Cargille is used.
- the refractive index of the immersion liquid is measured by, for example, an Abbe refractometer. From the interference fringe image of the composite fiber obtained by the interference microscope, a known method (for example, Journal of the Fiber Society, “Fiber structure formation in high-speed spinning of core-sheath type composite fiber”, Vol. 51, No. 9, No. 408). Page, 1995), the refractive index in the direction parallel and perpendicular to the fiber axis is obtained, and the birefringence which is the difference between the two is calculated.
- the intrinsic birefringence of the resin (Y in the above formula) is the birefringence in a state where the polymer polymer chains are perfectly oriented, and the value is, for example, the first edition of “Plastic material in molding process”, and the attached surface molding process. It is described in typical plastic materials used (edited by the Japan Society for Plastic Processing), Sigma Publishing, published on February 10, 1998.
- the thermal elongation rate of the heat-stretchable composite fiber at a temperature 10 ° C. higher than the melting point or softening point of the low-melting resin is preferably 0.5 to 20%, more preferably 3 to 20%, still more preferably 7.5 to 20%.
- the thermal elongation rate for example, the thermal elongation rate measured by the following method is used.
- a thermomechanical analyzer TMA-50 manufactured by Shimadzu Corp.
- TMA-50 was mounted with parallel fibers arranged at a distance of 10 mm between chucks, and a constant load of 0.025 mN / tex was applied at a heating rate of 10 ° C./min. Raise the temperature.
- the change in the elongation rate of the fiber at that time was measured, and the elongation rate at the melting point or softening point of the low melting point resin and the elongation rate at a temperature 10 ° C. higher than the melting point or softening point of the low melting point resin were read.
- the thermal elongation rate for example, the thermal elongation rate measured by the following method is used.
- a thermomechanical analyzer TMA-50 manufactured by Shimadzu Corp.
- the reason why the thermal elongation rate is measured in the above temperature range is that, when the fiber intersection is heat-sealed, the temperature range is higher than the melting point or softening point of the low-melting resin and up to about 10 ° C. Is usually adopted.
- the heat-extensible conjugate fiber can take the form of a core-sheath type (concentric type, eccentric type), a side-by-side type, or the like.
- the sheath component and the core component can be composed of a low melting point resin and a high melting point resin, respectively.
- the core component include polypropylene (PP), polyethylene terephthalate (PET), polybutylene terephthalate (PBT), and the like.
- the sheath component when the core component is PP include polyethylene (PE) such as high density polyethylene (HDPE), low density polyethylene (LDPE), and linear low density polyethylene (LLDPE), and an ethylene propylene copolymer. , Polystyrene and the like.
- the sheath component when the core component is PET, PBT, and the like include PP and copolymer polyester.
- the ratio (weight ratio) of the low-melting point resin to the high-melting point resin in the heat-stretchable composite fiber can be appropriately adjusted in consideration of the heat-stretchability, heat-fusibility, mechanical properties, etc. of the fiber. 90 to 90:10, preferably 20:80 to 80:20, and more preferably 50:50 to 70:30.
- the thickness of the heat-extensible composite fiber can be adjusted to, for example, 1.0 to 10 dtex (particularly 1.7 to 8.0 dtex).
- the fiber length of the heat-extensible composite fiber is, for example, about 30 to 70 mm. Can be adjusted.
- a heat-extensible conjugate fiber having a desired thermal elongation rate can be produced, for example, by subjecting a spun conjugate fiber to a heat treatment or a crimping treatment without performing a drawing treatment.
- the conditions of the heat treatment applied to the composite fiber after spinning can be appropriately adjusted according to the types of the high melting point resin and the low melting point resin constituting the composite fiber.
- the heating temperature is usually 50 to 120 ° C., preferably 70 to 100 ° C.
- the heating time is usually 10 ° C. It is ⁇ 500 seconds, preferably 20 to 200 seconds.
- the heating medium include hot air and infrared rays.
- Examples of the crimping treatment performed on the spun composite fiber include mechanical crimping.
- the crimp may be in either a two-dimensional or three-dimensional manner, or a three-dimensional manifested crimp seen in an eccentric core-sheath composite fiber, side-by-side composite fiber, or the like. It may be.
- the mechanical crimping involves heat, the heating process and the crimping process are performed simultaneously.
- the fiber may be slightly stretched in the crimping process, but such stretching is not included in the stretching process.
- the drawing treatment usually means a drawing operation with a draw ratio of about 2 to 6 times performed on an undrawn yarn.
- the top sheet 2 may contain fibers other than heat-fusible fibers and heat-extensible fibers.
- fibers other than the heat-fusible fiber and the heat-extensible fiber include natural fibers (wool, cotton, etc.), regenerated fibers (rayon, acetate, etc.), inorganic fibers (glass fiber, carbon fiber, etc.), and the like.
- the content of the other fibers can be appropriately adjusted, but is usually 95% by weight or less, preferably 80% by weight or less of the top sheet 2.
- the skin contact surface of the top sheet 2 has a large number of convex portions 8 and concave portions 9, and the skin contact surface of the top sheet 2 has an uneven shape. . Since the skin contact surface of the top sheet 2 is uneven, the contact area between the wearer's skin and the top sheet 2 is small, and the air permeability in the surface direction of the top sheet 2 through the space in the recess 9 is good. is there. Therefore, it is difficult to give the wearer stuffiness and itching.
- the convex portion 8 and the concave portion 9 are formed on substantially the entire absorber arrangement region including the excretory opening abutting region 20 in the skin contact surface of the top sheet 2. 9 should just be formed in the excretion opening
- FIG. The absorber arrangement region is a region where the absorber 4 overlaps the top sheet 2 when the absorber 4 is projected onto the top sheet 2.
- the excretion opening contact area 20 is an area where the wearer's excretion opening (for example, small labia, large labia, etc.) abuts when the sanitary napkin 1 is worn.
- the excretory opening contact region 20 is a region surrounded by a dotted line in FIG. 1, and is set at substantially the center of the absorber arrangement region. The position, area, and the like of the excretion opening contact region 20 can be adjusted as appropriate.
- the excretory opening contact area 20 may be set as an area substantially the same as the area where the excretion opening actually contacts, or may be set as a larger area, but liquid excrement such as menstrual blood From the viewpoint of preventing leakage to the outside, it is preferably set as a region larger than the region where the excretion port actually contacts.
- the excretion opening contact region 20 has a length of usually 50 to 200 mm, preferably 70 to 150 mm, and a width of usually 10 to 80 mm, preferably 20 to 50 mm.
- the excretory opening contact area 20 is set as a virtual area, but may be set as a visually recognizable area.
- Visual recognition includes, for example, coloring of the excretory opening contact region 20 and formation of a recess (for example, a recess formed by a heat embossing process) extending continuously or intermittently along the periphery of the excretion opening contact region 20. Etc. are possible.
- substantially the entire excretory opening contact region 20 has a kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, a water retention of 0.01 to 4.0% by mass, and a weight average molecular weight.
- the blood slipperiness imparting agent which is less than 1,000 is applied. Refer to the separate item for details of the blood slipperiness-imparting agent.
- the blood slipperiness imparting agent is applied to substantially the entire excretory opening contact region 20, but the blood slipperiness imparting agent is at least the convex portion 8 (particularly convex in the excretion port contact region 20. It only has to be applied to the top 81) of the part 8.
- the blood slipperiness imparting agent is applied to at least the convex portion 8 in the excretory opening contact region 20, it is applied to a portion other than the convex portion 8 (for example, the concave portion 9).
- it may be applied to a region other than the excretion opening contact area 20 (for example, a peripheral area of the excretion opening contact area 20) on the skin contact surface.
- the blood slipperiness-imparting agent can be applied to substantially the entire skin contact surface or substantially the entire absorber arrangement region.
- the blood slipperiness imparting agent When the blood slipperiness imparting agent is applied to at least the convex portion 8 (particularly, the top portion 81 of the convex portion 8) in the excretory opening contact region 20, the following effects are exhibited.
- menstrual blood excreted from the wearer reaches the excretory opening contact region 20, it slides down into the concave portion 9 together with the blood slipperiness imparting agent present in the convex portion 8, passes through the top sheet 2, and shifts to the absorber 4. . Therefore, the sanitary napkin 1 has improved menstrual blood transfer from the top sheet 2 to the absorbent body 4, and can reduce menstrual blood remaining on the top sheet 2. For this reason, the sticky feeling of the skin contact surface of the top sheet 2 is prevented, and a smooth feeling is maintained. The effect of such a blood slipperiness-imparting agent is exerted irrespective of changes in menstrual blood discharge (that is, whether menstrual blood discharged at a time is large or small). .
- the convex side wall 82 extends from the top 81 of the convex 8 toward the bottom 91 of the concave portion 9, and the concave side wall 92 starts from the bottom 91 of the concave 9.
- the convex side wall 82 has a fiber orientation along the extending direction, that is, the direction from the top 81 of the convex 8 toward the bottom 91 of the concave 9, and the concave side 92 is extended. It has fiber orientation along the direction, that is, the direction from the bottom 91 of the concave portion 9 to the top 81 of the convex portion 8.
- menstrual blood excreted from the wearer easily slides along the extending direction of the convex side wall portion 82 and the concave side wall portion 92 together with the blood slipperiness imparting agent.
- menstrual blood excreted from the wearer does not spread in the surface direction of the top sheet 2 and easily moves in the thickness direction of the top sheet 2 (that is, to the absorber 4).
- lubricity imparting agent exhibits effectively. Is done.
- the effect of the blood slipperiness imparting agent can be enhanced by applying the blood slipperiness imparting agent to the concave portion 9 in addition to the convex portion 8.
- lubricity imparting agent acts also as a lubrication agent and reduces the friction between fibers, the suppleness of the whole top sheet 2 can be improved.
- the sanitary napkin 1 is different from known absorbent articles including skin care compositions, lotion compositions, etc., and does not require components such as emollients and immobilizing agents. It can be applied to the sheet 2.
- the basis weight of the blood slipperiness imparting agent is usually about 1 to 30 g / m 2 , preferably about 2 to 20 g / m 2 , more preferably about 3 to 10 g / m 2 .
- the basis weight of the blood slipperiness-imparting agent is less than about 1 g / m 2 , menstrual blood tends to remain in the top sheet 2, while when the basis weight of the blood slipperiness-imparting agent exceeds about 30 g / m 2 , The sticky feeling during wearing tends to increase.
- the basis weight of the blood slipperiness imparting agent can be measured, for example, by the following method.
- (1) The range to be measured of the top sheet is cut out with a sharp blade, for example, a cutter blade, so as not to change its thickness as much as possible, and a sample is obtained.
- (2) The area of the sample: SA (m 2 ) and the mass: SM 0 (g) are measured.
- (3) The sample is stirred for at least 3 minutes in a solvent capable of dissolving the blood lubricity-imparting agent, such as ethanol, acetone, etc., and the blood lubricity-imparting agent is dissolved in the solvent.
- a solvent capable of dissolving the blood lubricity-imparting agent such as ethanol, acetone, etc.
- the sample is filtered on the filter paper whose mass has been measured, and the sample is thoroughly washed with a solvent on the filter paper.
- the sample on the filter paper is dried in an oven at 60 ° C.
- the mass of the filter paper and the sample is measured, and the mass of the filter paper is subtracted therefrom to calculate the mass of the sample after drying: SM 1 (g).
- the basis weight BBS (g / m 2 ) of the blood slipperiness-imparting agent is calculated based on the following formula.
- BBS (g / m 2 ) [SM 0 (g) ⁇ SM 1 (g)] / SA (m 2 )
- a plurality of samples are collected from a plurality of absorbent articles so that the total area of the samples exceeds 100 cm 2 , the experiment is repeated a plurality of times, and an average value thereof is adopted.
- the blood slipperiness-imparting agent is preferably applied so as not to block the gaps between the fibers of the topsheet 2.
- the blood slipperiness imparting agent adheres to the surface of the fiber of the top sheet 2 in the form of droplets or particles, or covers the surface of the fiber.
- the blood slipperiness-imparting agent is preferably coated so that its surface area becomes large. Thereby, the contact area of a blood slipperiness
- the surface area can be increased by reducing the particle size.
- Examples of the method for applying the blood slipperiness-imparting agent include a method using a coating apparatus (for example, a non-contact type coater such as a spiral coater, curtain coater, spray coater, dip coater, contact type coater, etc.).
- a coating apparatus for example, a non-contact type coater such as a spiral coater, curtain coater, spray coater, dip coater, contact type coater, etc.
- a preferred coating apparatus is a non-contact type coater.
- the blood slipperiness imparting agent can be applied as a coating liquid containing a volatile solvent, for example, an alcohol solvent, an ester solvent, an aromatic solvent, or the like, if desired.
- a volatile solvent for example, an alcohol solvent, an ester solvent, an aromatic solvent, or the like.
- the blood slipperiness-imparting agent is, for example, a control seam HMA (Hot Melt Adhesive) as it is in the case of a liquid at room temperature or heated to lower the viscosity and heated to be liquefied in the case of a solid at room temperature. ) Can be applied by gun. By increasing the air pressure of the control seam HMA gun, it is possible to apply a particulate blood slipping agent. In addition, the application quantity of a blood slipperiness
- a control seam HMA Hot Melt Adhesive
- the blood slipperiness-imparting agent may be applied when the top sheet 2 is produced, or may be applied on the production line of the sanitary napkin 1. From the viewpoint of suppressing capital investment, it is preferable to apply a blood slipperiness-imparting agent in the production line of the sanitary napkin 1, and further suppress the blood slipperiness-imparting agent from dropping and contaminating the line. Therefore, it is preferable to apply the blood slipperiness imparting agent immediately downstream of the production line, specifically, immediately before the product is enclosed in the individual package.
- the convex part 8 has protruded to the skin contact surface side (upper side in FIG. 2) of the top sheet 2, and the recessed part 9 has the absorber arrangement
- the convex portions 8 and the concave portions 9 are alternately arranged in the first direction and the second direction intersecting with the first direction.
- the first direction is an arbitrary direction in the skin contact surface of the top sheet 2
- the second direction is a direction that intersects the first direction in the skin contact surface of the top sheet 2.
- the first direction is the X direction
- the second direction is the Y direction
- the angle at which the first direction and the second direction intersect is 90 °.
- the first direction, the second direction, and the angle at which the first direction and the second direction intersect are not limited to the present embodiment, and can be changed as appropriate.
- the angle at which the first direction and the second direction intersect is preferably 30 to 90 °.
- the convex portions 8 and the concave portions 9 are alternately arranged in the X direction and the Y direction to form a row, and adjacent convex portions in the rows in the X direction and the Y direction.
- the recesses 9 are positioned between the eight recesses 8 (that is, four recesses 9 are positioned around one protrusion 8).
- the convex portions 8 are arranged so as to be shifted by a half pitch, so that when the top sheet 2 is viewed in plan, the arrangement pattern of the convex portions 8 is a staggered pattern.
- the concave portions 9 are arranged so as to be shifted by a half pitch, so that when the top sheet 2 is viewed in plan, the arrangement pattern of the concave portions 9 is a staggered pattern.
- the arrangement pattern of the convex part 8 and the recessed part 9 is not limited to this embodiment, It can change suitably.
- the number of concave portions 9 positioned around one convex portion 8 and the number of convex portions 8 positioned around one concave portion 9 can be appropriately selected.
- the height of the convex portion 8 is usually 0.2 to 15 mm, preferably 0.5 to 5 mm, the width (X direction) is usually 1 to 20 mm, preferably 2 to 10 mm, and the length (Y direction). ) Is usually 1 to 20 mm, preferably 2 to 10 mm, and the interval between the convex portions 8 (width or length of the concave portion 9) is usually 1 to 20 mm, preferably 2 to 10 mm.
- the convex part 8 has a top part 81 and a convex part side wall part 82 extending from the top part 81, and the concave part 9 is a bottom part 91 and a concave part side wall part 92 extending from the bottom part 91. And have.
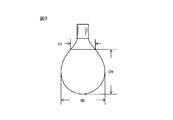
- the external shape of the convex portion 8 and the concave portion 9 is hemispherical, the cross-sectional view shape is arched, and the planar view shape is circular.
- the external shape of the convex part 8 is a shape when the convex part 8 is seen from the skin contact surface of the top sheet 2
- the external shape of the concave part 9 is the concave part 9 from the absorber arrangement surface of the top sheet 2. It is the shape when seen.
- the shape of the convex part 8 and the recessed part 9 is not limited to this embodiment, It can change into another shape. Examples of other external shapes include cone shapes such as a cone, a truncated cone, a pyramid, a truncated pyramid, and an oblique cone. Examples of other planar shapes include an elliptical shape, a rectangular shape, and a polygonal shape. Can be mentioned.
- the inside of the convex part 8 is hollow.
- a ridge-shaped connecting portion may be formed between the adjacent convex portions 8 and between the adjacent concave portions 9.
- the internal spaces of the adjacent convex portions 8 may or may not communicate with each other through this connecting portion.
- the convex portion side wall portion 82 When the skin contact surface side of the top sheet 2 is set to the upper side and the absorber arrangement surface side is set to the lower side, the convex portion side wall portion 82 has a tapered shape whose diameter gradually increases downward. Has a tapered shape with a diameter gradually increasing upward.
- the convex side wall portion 82 extends downward from the top portion 81, and the lower end portion thereof is continuous with the upper end portion of the concave side wall portion 92 extending upward from the bottom portion 91. That is, the convex side wall 82 extends in the direction from the top 81 to the bottom 91, and the concave side wall 92 extends in the direction from the bottom 91 to the top 81. Accordingly, the extending direction of the convex side wall 82 is a direction from the top 81 to the bottom 91, and the extending direction of the concave side wall 92 is a direction from the bottom 91 to the top 81.
- the shape of the convex side wall portion 82 and the concave side wall portion 92 is not limited to the shape of the present embodiment, and can be changed to other shapes. Examples of other shapes include a cylindrical shape and a rectangular tube shape.
- the convex side wall portion 82 has fiber orientation along the extending direction (that is, the direction from the top portion 81 to the bottom portion 91), and the concave side wall portion 92 is extended in the extending direction (that is, from the bottom portion 91 to the top portion). Fiber orientation along the direction toward 81).
- the convex side wall portion 82 and the concave side wall portion 92 are developed, the convex side wall portion 82 has a fiber orientation OR along its extending direction D 82.
- the recess side wall portion 92 has a fiber orientation OR 92 along the extending direction D 92 thereof.
- the convex side wall 82 has a radial fiber orientation that converges toward the top 81
- the concave side wall 92 is a radial fiber that converges toward the bottom 91.
- the convex side wall portion 82 and the concave side wall portion 92 have fiber orientation along the respective extending directions, whereby the fiber orientation in the thickness direction of the top sheet 2 is increased.
- the compression deformation property and compression recovery property (cushion property) of No. 2 are improved. Therefore, even if the sanitary napkin 1 is compressed, liquid return (rewetting back) from the absorbent body 4 hardly occurs. Moreover, since the cushioning property of the top sheet 2 is high, the wearer is given good comfort.
- the convex side wall portion 82 and the concave side wall portion 92 have substantially the same fiber orientation throughout the circumferential direction, but have portions with different fiber orientations. It may be.
- the convex part side wall part 82 and the concave part side wall part 92 may have a part in which the fiber orientation before uneven forming is maintained.
- the fiber orientation before the uneven shaping is maintained even after the uneven shaping.
- the portions having different fiber orientations have, for example, fiber orientations along a direction orthogonal to the extending direction of the convex side wall portions 82 or the concave side wall portions 92.
- the fibers are usually heat-sealed in a state where the fibers are oriented in the MD direction, the fibers are formed on the convex side wall portions 82 and the concave side wall portions 92 in the MD direction cross section. Although oriented in the extending direction of the side wall 92, the fibers are oriented in the direction perpendicular to the extending directions of the convex side wall 82 and the concave side wall 92 in the cross section in the CD direction.
- Fiber orientation is a concept consisting of the orientation angle and orientation strength of the fiber, and can be measured, for example, by the following method.
- the sample is allowed to stand so that the skin contact surface of the top sheet is positioned on the upper side.
- a microscope for example, a scanning electron microscope such as JCM-5100 manufactured by JEOL Ltd.
- an enlarged image is taken from a direction perpendicular to the measurement surface of the sample, printed, and transparent PET property Trace the fiber on the sheet.
- the enlarged image is an image enlarged to a magnification capable of measuring 10 or more fibers, and the enlargement magnification is, for example, 50 to 300 times.
- the image is taken into a personal computer, and the image is binarized by using a NexusNewQube (stand-alone version) image processing software manufactured by Nexus Corporation.
- the orientation angle is the angle at which the fibers are most oriented
- the orientation strength is the strength at that orientation angle. It shows that the fiber is orientating in the extending direction of a convex part side wall part or a recessed part side wall part, so that an orientation angle is near 90 degrees. Moreover, it shows that the direction of a fiber has gathered, so that orientation strength is large.
- the case where the orientation strength is 1.05 or more is assumed to be oriented. The measurement is repeated several times (for example, 3 to 5 times), and the average value is calculated.
- the fiber orientation at the top 81 of the convex portion 8 is different from the fiber orientation at the bottom 91 of the concave portion 9. That is, the fiber orientation OR 81 at the top 81 of the convex portion 8 is along the thickness direction, but the fiber orientation OR 91 at the bottom 91 of the concave portion 9 is not along the thickness direction. Along the direction perpendicular to the thickness direction.
- the orientation angle is preferably 50 ° to 130 °, and the orientation strength is preferably 1.05 or more.
- the orientation angle is preferably 0 ° to 50 ° or 130 ° to 180 °, and the orientation strength is preferably less than 1.05.
- the fiber orientation in the thickness direction at the bottom 91 of the recess 9 is preferably smaller than the fiber orientation in the thickness direction at the recess side wall 92. Thereby, menstrual blood easily moves in the thickness direction (that is, to the absorbent body 4) without accumulating in the recess side wall portion 92.
- the fiber orientation in the recessed part 9 changes in a direction perpendicular to the thickness direction as the absorber 4 is approached, whereby a gradient occurs in the fiber orientation, and menstrual blood is easily transmitted and diffused.
- the orientation angle of the recess side wall portion 92 is preferably 50 ° to 130 °, and the orientation strength is preferably 1.05 or more.
- the orientation angle of the bottom 91 of the recess 9 is preferably 0 ° to less than 50 ° or 130 ° to 180 °, and the orientation strength is preferably less than 1.05.
- the relationship between the fiber orientation OR 81 at the top 81 of the convex portion 8 and the fiber orientation OR 91 at the bottom 91 of the concave portion 9 is preferably OR 81 > OR 91 .
- the top part 81 of the convex part 8 has the compressive deformation property and compression recovery property (flexibility) superior to the bottom part 91 of the concave part 9.
- the fiber orientation in the skin contact surface side portion and the fiber orientation in the absorbent body placement surface side portion are substantially equal. That is, it is preferable that radial fiber orientation toward the bottom portion 91 of the recess 9 exists in both the skin contact surface side portion and the absorber arrangement surface side portion. Thereby, the compression deformability and the compression recovery property (flexibility) of the bottom 91 of the recess 9 are improved, and the bottom 91 of the recess 9 is not easily crushed.
- the fiber density of the top part 81 of the convex part 8 may be smaller or larger than the fiber density of the bottom part 91 of the concave part 9. Since the liquid easily moves from the lower fiber density to the higher fiber density according to the density gradient, when the fiber density at the top 81 of the convex portion 8 is smaller than the fiber density at the bottom 91 of the concave portion 9, the sanitary napkin 1 is Combined with the action and effect of the blood slipperiness-imparting agent, it has improved menstrual blood transfer from the top sheet 2 to the absorber 4.
- the liquid is less likely to move from the higher fiber density to the lower fiber density
- the fiber density at the top 81 of the convex portion 8 is smaller than the fiber density at the bottom 91 of the concave portion 9
- menstrual blood migration is less than the fiber density.
- the sanitary napkin 1 has an improved menstrual transfer property from the top sheet 2 to the absorbent body 4 due to the action effect of the blood slipperiness imparting agent. Therefore, the effect of the blood slipperiness imparting agent is remarkable when the fiber density of the top portion 81 of the convex portion 8 is larger than the fiber density of the bottom portion 91 of the concave portion 9.
- the fiber density of the top portion 81 of the convex portion 8 is larger than the fiber density of the bottom portion 91 of the concave portion 9, the followability of the uneven shape of the top sheet 2 to the wearer's skin becomes good, so the wearer Good comfort when rearranging and walking.
- the fiber density can be measured, for example, by the following method.
- the cut surface of the top sheet 2 is magnified and observed using a microscope (for example, a scanning electron microscope such as JCM-5100 manufactured by JEOL Ltd.).
- the magnification is adjusted to a magnification (for example, 150 to 500 times) at which 30 to 60 fiber cross sections can be measured.
- the fiber density (lines / mm 2 ) is calculated based on the number of fiber cross sections and the visual field area where the number of fiber cross sections is measured. Perform measurements at several locations (for example, 3 to 5 locations) and calculate the average value.
- the fiber density of the top portion 81 of the convex portion 8 measured in this way is preferably 30 to 150 pieces / mm 2 , more preferably 60 to 100 pieces / mm 2 , and the fiber density of the bottom portion 91 of the concave portion 9 is The number is preferably 150 to 600 / mm 2 , more preferably 300 to 550 / mm 2 .
- the fiber amount of the top part 81 of the convex part 8 and the fiber amount of the bottom part 91 of the recessed part 9 are substantially equal. Thereby, compression deformation property and compression recovery property improve. In addition, changes in feel and breakage due to fiber unevenness are less likely to occur.
- the fiber amount can be measured, for example, by the following method. Using a microscope (for example, Keyence digital microscope VHX-1000), the measurement site is enlarged. The enlargement magnification is, for example, 10 to 100 times. The thickness of the top of the convex part (T 81 in FIG. 4A) and the thickness of the bottom of the concave part (T 91 in FIG.
- the fiber density of the skin contact surface side portion of the top portion 81 of the convex portion 8 is smaller than the fiber density of the absorber arrangement surface side portion. Thereby, compression deformation property and compression recovery property improve.
- the fiber density of the skin contact surface side portion of the top portion 81 of the convex portion 8 is preferably 10 to 50 fibers / mm 2 , more preferably 15 to 30 fibers / mm 2 , and the fiber density in the absorber arrangement surface side portion. Is preferably 20 to 100 / mm 2 , more preferably 45 to 70 / mm 2 .
- the ratio of the fiber density of the absorber placement surface side portion to the fiber density of the skin contact surface side portion is preferably about 2 to 5 times. Thereby, compression deformation property and compression recovery property improve.
- the number of fiber fusion points at the top portion 81 of the convex portion 8 is preferably smaller than the number of fiber fusion points at the bottom portion 91 of the concave portion 9. Thereby, the touch of the top sheet 2 becomes favorable.
- the number of fiber fusion points is calculated by counting the number of fiber fusion points per unit area (1 mm 2 ).
- the number of fiber fusion points at the top 81 of the convex portion 8 is preferably 30 to 130 pieces / mm 2 , more preferably 50 to 100 pieces / mm 2 .
- the number of fiber fusion points at the bottom 91 of the recess 9 is preferably 250 to 500 / mm 2 , more preferably 300 to 450 / mm 2 .
- the thickness T 81 of the top portion 81 of the convex portion 8 is usually 0.1 to 5 mm, preferably 0.3 to 2.5 mm
- the thickness T 82 of the convex side wall portion 82 is usually 0.1 to 5 mm, preferably Is 0.3 to 2.5 mm
- the thickness T 91 of the bottom 91 of the recess 9 is usually 0.1 to 5 mm, preferably 0.3 to 2.5 mm
- the thickness T 92 of the recess side wall 92 is The thickness is usually 0.1 to 5 mm, preferably 0.3 to 2.5 mm. It is preferable that the relationship between the thicknesses of the respective parts is T 81 > T 82 and T 92 > T 91 .
- the thickness of the top sheet 2 under a load of 3 gf / cm 2 is usually 0.2 to 20 mm, preferably 0.5 to 10 mm.
- the basis weight (average value of the entire sheet) of the top sheet 2 is usually 10 to 100 g / m 2 , preferably 20 to 50 g / m 2 .
- the presence / absence and degree of the fiber density gradient of the top sheet 2 contains a colored liquid (for example, a pigment).
- a colored liquid for example, a pigment
- (Liquid) is dropped, and the color density after dropping can be evaluated. That is, the dark part can be evaluated as a part with a large liquid migration amount, that is, a part with a relatively high fiber density, and the light color part has a small liquid migration amount, that is, a fiber density. It can be evaluated as a relatively small portion.
- the top sheet 2 may be hydrophilized.
- the hydrophilic treatment include coating of the surface of the top sheet 2 with a hydrophilic agent, addition of a hydrophilic agent to a component, corona treatment, plasma treatment, and the like.
- the lipophilic region derived from the blood slipping agent and the hydrophilic region derived from the hydrophilic agent coexist sparsely in the top sheet 2, and menstrual blood is in the top sheet 2. It slips down from the convex part 8 of this, and it becomes easy to transfer to an absorber.
- the back sheet 3 is a sheet through which liquid excretion such as menstrual blood cannot permeate, and can prevent leakage of liquid excretion absorbed by the absorber 4.
- One surface (upper surface in FIG. 2) of the back sheet is an absorber arrangement surface on which the absorber 4 is arranged, and the other surface (lower surface in FIG. 2) is a non-skin contact surface (this embodiment). Then, the wearer's clothes (underwear) are in contact with each other).
- the backsheet 3 preferably has moisture permeability in addition to liquid impermeability in order to reduce stuffiness when worn.
- backsheet 3 examples include waterproof nonwoven fabric, synthetic resin (eg, polyethylene, polypropylene, polyethylene terephthalate, etc.) film, and composite sheet of nonwoven fabric and synthetic resin film (eg, nonwoven fabric such as spunbond and spunlace). And a composite film in which a breathable synthetic resin film is bonded), an SMS nonwoven fabric in which a melt-blown nonwoven fabric having high water resistance is sandwiched between strong spunbond nonwoven fabrics, and the like.
- synthetic resin eg, polyethylene, polypropylene, polyethylene terephthalate, etc.
- composite sheet of nonwoven fabric and synthetic resin film eg, nonwoven fabric such as spunbond and spunlace
- SMS nonwoven fabric in which a melt-blown nonwoven fabric having high water resistance is sandwiched between strong spunbond nonwoven fabrics, and the like.
- the absorber 4 contains an absorbent material that absorbs liquid excreta such as menstrual blood.
- the absorbent material contained in the absorber 4 is not particularly limited as long as it can absorb and retain liquid excretion such as menstrual blood.
- Examples of the absorbent material include a water-absorbing fiber and a highly water-absorbing material (for example, a highly water-absorbing resin and a highly water-absorbing fiber).
- the absorber 4 includes additives such as an antioxidant, a light stabilizer, an ultraviolet absorber, a neutralizer, a nucleating agent, an epoxy stabilizer, a lubricant, an antibacterial agent, a flame retardant, an antistatic agent, a pigment, and a plasticizer. You may contain as needed.
- water-absorbing fibers include wood pulp obtained from softwood or hardwood (for example, mechanical pulp such as groundwood pulp, refiner ground pulp, thermomechanical pulp, chemithermomechanical pulp; kraft pulp, sulfide pulp, alkaline pulp, etc. Chemical pulp; semi-chemical pulp, etc.]; mercerized pulp or crosslinked pulp obtained by chemically treating wood pulp; non-wood pulp such as bagasse, kenaf, bamboo, hemp, cotton (eg cotton linter); rayon, fibril Examples include regenerated cellulose such as rayon; semi-synthetic cellulose such as acetate and triacetate, but pulverized pulp is preferred because it is low in cost and easy to mold.
- wood pulp obtained from softwood or hardwood for example, mechanical pulp such as groundwood pulp, refiner ground pulp, thermomechanical pulp, chemithermomechanical pulp; kraft pulp, sulfide pulp, alkaline pulp, etc. Chemical pulp; semi-chemical pulp, etc.]; mercerized pulp or crosslinked pulp obtained by chemically treating wood pulp;
- the superabsorbent material examples include starch, cellulose, and synthetic polymer superabsorbent materials.
- starch-based or cellulose-based superabsorbent material examples include starch-acrylic acid (salt) graft copolymers, saponified starch-acrylonitrile copolymers, and crosslinked products of sodium carboxymethyl cellulose.
- Synthetic polymers examples include polyacrylates, polysulfonates, maleic anhydrides, polyacrylamides, polyvinyl alcohols, polyethylene oxides, polyaspartates, polyglutamates , Polyalginate-based, starch-based, and cellulose-based superabsorbent resins (Superabsorbent Polymer: SAP), and the like. Resins are preferred.
- shape of the superabsorbent material include particulates, fibers, and scales. In the case of particulates, the particle size is preferably 50 to 1000 ⁇ m, more preferably 100 to 600 ⁇ m. .
- the absorbent body 4 contains a highly water-absorbing material (for example, highly water-absorbing resin, highly water-absorbing fiber, etc.), the content of the highly water-absorbing material is usually 5 to 80% by mass, preferably 10%. It is ⁇ 60 mass%, more preferably 20 to 40 mass%.
- a highly water-absorbing material for example, highly water-absorbing resin, highly water-absorbing fiber, etc.
- the absorber 4 may contain silver, copper, zinc, silica, activated carbon, aluminosilicate compound, zeolite or the like. Thereby, functions, such as a deodorizing property, antibacterial property, and an endothermic effect, can be provided to an absorber.
- the thickness, basis weight, etc. of the absorbent body 4 can be appropriately adjusted according to the characteristics (for example, absorbency, strength, lightness, etc.) that the sanitary napkin 1 should have.
- the thickness of the absorber 4 is usually 0.1 to 15 mm, preferably 1 to 10 mm, more preferably 2 to 5 mm, and the basis weight is usually 20 to 1000 g / m 2 , preferably 50 to 800 g / m 2 , 100 to 500 g / m 2 is preferable.
- the thickness, basis weight, etc. of the absorber 4 may be constant throughout the absorber 4 or may be partially different.
- the absorbent body 4 is preferably in a form having a core containing an absorbent material and a core wrap covering the core.
- the core wrap is not particularly limited as long as it has liquid permeability and absorber retention.
- Examples of the core wrap include a nonwoven fabric, a woven fabric, a synthetic resin film in which liquid permeation holes are formed, and a net-like sheet having a mesh.
- a wet method using pulverized pulp as a main material A tissue molded with is preferred.
- the sanitary napkin 1 may include a second sheet disposed between the top sheet 2 and the absorbent body 4 in addition to the top sheet 2.
- the blood slipperiness imparting agent may be applied to the second sheet.
- the second sheet is not particularly limited as long as liquid excretion such as menstrual blood can permeate, and the thickness, basis weight, density, etc. of the second sheet are appropriately adjusted within a range where liquid excretion such as menstrual blood can permeate. can do.
- Examples of the second sheet include a nonwoven fabric, a woven fabric, a synthetic resin film in which liquid permeation holes are formed, and a net-like sheet having a mesh.
- Examples of the nonwoven fabric include air-through nonwoven fabric, spunbond nonwoven fabric, point bond nonwoven fabric, spunlace nonwoven fabric, needle punched nonwoven fabric, melt blown nonwoven fabric, and combinations thereof (for example, SMS).
- natural fibers wool, cotton, etc.
- regenerated fibers rayon, acetate, etc.
- inorganic fibers glass fibers, carbon fibers, etc.
- synthetic resin fibers polyethylene, polypropylene, polybutylene, ethylene-vinyl acetate copolymer, Polyolefins such as ethylene-ethyl acrylate copolymer, ethylene-acrylic acid copolymer, ionomer resin; polyesters such as polyethylene terephthalate, polybutylene terephthalate, polytrimethylene terephthalate, polylactic acid; De), and the like.
- Nonwoven fabrics include core / sheath fibers, side-by-side fibers, island / sea fibers, etc .; hollow fibers; flat fibers, Y-shaped fibers, C-shaped fibers, etc .; latent crimps or manifestations Crimped three-dimensional crimped fibers; split fibers that are split by a physical load such as water flow, heat, and embossing may be mixed.
- the manufacturing method which concerns on this embodiment cuts out the process (process 1) of laminating an absorber, the process of laminating a top sheet (process 2), the process of laminating a back sheet (process 3), and a sanitary napkin.
- a manufacturing apparatus 200 shown in FIG. 6 is used, including a step (step 4) and a step (step 5) of applying a blood slipperiness-imparting agent to a sanitary napkin.
- Step 1 On the peripheral surface of the suction drum 220 that rotates in the transport direction MD, recesses 224 are formed at a required pitch in the circumferential direction as a mold for filling the absorbent material 222.
- the suction unit 226 acts on the recess 224, and the absorber material 222 supplied from the material supply unit 221 is sucked into the recess 224 by vacuum.
- the material supply unit 221 is formed so as to cover the suction drum 220, and the material supply unit 221 supplies the absorber material 222 to the recess 224 by air conveyance, and the absorber 4 is formed in the recess 224.
- the absorber 4 formed in the recess 224 is transferred onto the carrier sheet 210 that proceeds in the transport direction MD.
- the top sheet 2 is laminated on the absorber 4 to form a laminate LB1.
- the manufacturing method of the top sheet 2 is mentioned later.
- a pressing groove is formed in the laminated body LB1 as necessary.
- the compressed groove is formed by the embossing device 230.
- the embossing device 230 includes an upper roll 231 having a convex portion (not shown) provided on the outer peripheral surface, and a lower roll 232 having a smooth outer peripheral surface.
- the convex portion of the upper roll 231 is formed so as to correspond to the shape of the compressed groove, the arrangement pattern, and the like.
- the laminated body LB1 passes between the upper roll 231 and the lower roll 232 of the embossing device 230, the laminated body LB1 is compressed in the thickness direction, and a compressed groove is formed in the laminated body LB1.
- the compressed groove is formed, for example, in the periphery of the excretory opening contact area 20, the peripheral area of the peripheral area of the excretion opening contact area 20, or the like in the top sheet 2.
- the top sheet 2 is integrated with the absorbent body 4 by forming the compressed grooves.
- the pressing groove forming step by the embossing device 230 is omitted when it is not necessary.
- Step 3 The back sheet 3 supplied from the back sheet roll 240 is laminated on the lower surface (opposite side of the top sheet 2) of the laminated body LB2 via an adhesive layer to form the laminated body LB3.
- the laminated body LB2 and the laminated body LB3 are the same.
- Step 4 The laminate LB3 is cut using the cutter 250, and a sanitary napkin is cut out.
- the blood slipperiness imparting agent 261 is applied to the top sheet 2 of the sanitary napkin using the spray 260 to form a blood slipperiness imparting agent layer on the surface of the top sheet 2.
- the blood slipperiness-imparting agent layer is formed at least in the excretion opening contact region 20 in the skin contact surface of the top sheet 2.
- the blood slipperiness-imparting agent is applied after cutting out the sanitary napkin, but it may be applied at any stage before cutting out, or it may be applied in the top sheet manufacturing process. Good. In order to prevent the blood slipperiness-imparting agent applied during production from flowing down, it is preferable to apply the blood slipperiness-imparting agent immediately downstream of the manufacturing process, for example, immediately before packaging a sanitary napkin.
- the manufacturing method of the sanitary napkin 1 can include, in addition to steps 1 to 5, a step of forming the seal portions 7a, 7b, 8a, 8b, a step of forming the adhesive portions 9a, 9b, 9c, and the like.
- the conveyor belt 110 rotates in the conveying direction MD while being supported by the upper rollers 111a and 111b and the lower rollers 111c and 111d.
- a plurality of protrusions 112 are formed on the web conveyance surface of the conveyor belt 110, and the web conveyance surface is uneven. Further, a plurality of vent holes (not shown) are formed on the web conveyance surface of the conveyor belt 110.
- the protrusion 112 has a tapered shape whose diameter gradually decreases toward the tip, and the tip has a rounded shape.
- the height of the protrusion 112 is, for example, 0.5 to 20 mm. If the height of the projection 112 is too low, the uneven shaping on the fiber web 50 may be insufficient, while if too high, the projection 112 may penetrate the fiber web 50 when hot air is blown. . From this viewpoint, the height of the protrusion 112 is preferably 1 to 10 mm.
- the pitch of the protrusions 112 in the MD direction is 1 to 20 mm, and the pitch in the direction perpendicular to the MD direction (CD direction) is 1 to 20 mm.
- the CD direction coincides with the width direction of the fiber web 50.
- the opening ratio (total area of the air holes / surface area of the conveyor belt 110) of the air holes (not shown) is preferably 20 to 45%, more preferably 25 to 40%. If the opening ratio is too low, the uneven shaping on the fiber web 50 may be insufficient, while if it is too high, the fiber web 50 will enter the vent hole when hot air is blown, and it will be difficult to peel off from the conveyor belt 110.
- the conveyor belt 110 which is supported by the rollers 111a to 111d and rotates, supports the fiber web 50 by the protrusions 112 formed on the conveying surface thereof and conveys it in the MD direction.
- a pair of drive rolls 151 and 152 are provided on the upstream side of the conveyor belt 110.
- the drive rolls 151 and 152 supply the fiber web 50 manufactured by the card machine to the conveyor belt 110.
- a tension detector (not shown) and the peripheral speed V1 of the driving rolls 151 and 152 are controlled based on the magnitude of the tension detected by the tension detector.
- a control unit (not shown). The controller adjusts the peripheral speed V1 of the drive rolls 151 and 152 relative to the peripheral speed V2 of the conveyor belt 110 based on the detection output of the tension detector. Thereby, the tension of the fiber web 50 supplied to the first nozzle 120 is adjusted to a desired tension.
- the control unit increases the circumferential speed V1 of the drive rolls 151 and 152 to the first nozzle 120 than the circumferential speed V2 of the conveyor belt.
- the tension of the fiber web 50 is reduced.
- the control unit makes the peripheral speed V1 of the drive rolls 151 and 152 smaller than the peripheral speed V2 of the conveyor belt 110, and causes the first nozzle 120 to The tension of the supplied fiber web 50 is increased.
- the peripheral speed V1 of the drive rolls 151 and 152 which is relatively adjusted, is the speed of the roll surface, and the peripheral speed V2 of the conveyor belt 110 is the speed of the web conveyance surface.
- the manufacturing apparatus 100 sprays the 1st nozzle 120 which performs the 1st air through process by spraying the 1st heating fluid H1 in order toward MD direction, and the 2nd heating fluid H2.
- a second nozzle 130 that performs the second air-through process and a third nozzle 140 that sprays the third heating fluid H3 and performs the third air-through process are provided.
- the manufacturing apparatus 100 includes a cooling unit 160 that is disposed between the second nozzle 130 and the third nozzle 140 and that cools the fiber web 50.
- the first nozzle 120 and the second nozzle 130 perform an uneven shape forming process for the fiber web 50, and the third nozzle 140 performs a fuzz reduction process. If it is not necessary to reduce fuzz, the processing by the third nozzle 140 may be omitted.
- the first air-through process is a process in which the first heating fluid H1 is sprayed by the first nozzle 120 onto the fiber web 50 that is supported and conveyed by the protrusion 112 of the conveyor belt 110.
- the fiber web 50 is shaped into a concavo-convex shape along the shape of the projection 112 of the conveyor belt 110, and the constituent fibers of the fiber web 50 are melted by heat-fusible fibers.
- the uneven shape is maintained by heat fusion by solidification.
- the first nozzle 120 ejects the first heated fluid H1 from its ejection hole (not shown) and sprays it almost perpendicularly to the fiber web 50 on the conveyor belt 110.
- the first heating fluid H1 air, water vapor or the like heated to a predetermined temperature by a first heater (not shown) provided in the first nozzle 120 is used.
- the first heating fluid H1 is heated by the first heater up to a temperature at which the uneven shape of the fiber web 50 applied by spraying the first heating fluid H1 is maintained by thermal fusion of the constituent fibers of the fiber web 50. Heated.
- the fiber web 50 contains a core-sheath type composite fiber in which the sheath component (low melting point component) is polyethylene (PE) and the core component (high melting point component) is polyethylene terephthalate (PET)
- the first The temperature of the heating fluid H1 is preferably 80 to 155 ° C., more preferably 130 to 135 ° C.
- the uneven shaping of the fiber web 50 becomes insufficient, while if it is too high than the melting point of the low melting point component, Since the constituent fibers of the fiber web 50 are rapidly fused and the degree of freedom of the fibers is reduced, the uneven shaping of the fiber web 50 becomes insufficient.
- the wind speed of the first heating fluid H1 is preferably 20 to 120 m / sec, more preferably 40 to 80 m / sec.
- the fiber web 50 will not fully follow the projection part 112, and the uneven
- the fiber web 50 constituent fibers are selected by the protrusion 112, and the uneven shaping of the fiber web 50 becomes insufficient.
- the spraying time of the first heating fluid H1 is preferably 0.01 to 0.5 seconds, more preferably 0.04 to 0.08 seconds.
- the spraying time is too short, the unevenness shaping of the fiber web 50 becomes insufficient.
- the spraying time is too long, the constituent fibers of the fiber web 50 are selected by the protrusions 112, and the unevenness shaping of the fiber web 50 is performed. It becomes insufficient.
- the desired speed is adjusted by adjusting the peripheral speed V1 of the drive rolls 151 and 152.
- the convex part side wall part and concave part side wall part which have the fiber density and fiber amount (basis weight) are formed.
- the fiber web 50 is processed by the first nozzle 120 by setting the peripheral speed V1 of the drive rolls 151 and 152 to be higher than the peripheral speed V2 of the conveyor belt 110, the fiber web 50 is over-fed and uneven. A decrease in fiber density and fiber amount (basis weight) of the convex side wall and the concave side wall that may occur during shaping is prevented.
- the circumferential speed V1 of the drive rolls 151, 152 is preferably 1 to 2 times, more preferably 1 to 1.5 times the circumferential speed V2 of the conveyor belt 110.
- the 1st heating fluid H1 which passed the fiber web 50 is discharged
- the fiber web 50 that has undergone the first air-through process is conveyed to the position of the second nozzle 130 while being placed on the conveyor belt 110.
- a 2nd air through process is a process of spraying the 2nd heating fluid H2 with the 2nd nozzle 130 with respect to the fiber web 50 which passed the 1st air through process.
- the constituent fibers of the fiber web 50 are heat-sealed by melting and solidifying the heat-fusible fibers, and the uneven shape of the fiber web 50 applied in the first air-through process is fixed. The In this way, the fibrous web 50 is formed with unevenness through the first and second air-through processes.
- the second nozzle 130 ejects the second heated fluid H2 from its ejection hole (not shown) and blows it almost perpendicularly to the fiber web 50 on the conveyor belt 110.
- the second heating fluid H2 air, water vapor or the like heated to a predetermined temperature by a second heater (not shown) provided in the second nozzle 130 is used.
- the second heating fluid H ⁇ b> 2 is heated by the second heater to a temperature at which the constituent fibers of the fiber web 50 are heat-sealed while the uneven shape of the fiber web 50 is maintained.
- the fiber web 50 is a composite fiber having a low-melting-point component and a high-melting-point component having a higher melting point than the low-melting-point component
- the second heating fluid H2 has a temperature from the melting point of the low-melting-point component to the melting point of the high-melting-point component. Be controlled.
- the temperature of the second heating fluid H2 is: The temperature is preferably 130 to 155 ° C, more preferably 135 to 150 ° C. If the temperature of the second heating fluid H2 is too low, the thermal fusion between the constituent fibers becomes insufficient, and the unevenness of the fiber web 50 is insufficiently fixed, while the temperature of the second heating fluid H2 is high. If it is too much, the texture of the nonwoven fabric becomes worse and it becomes difficult to produce bulk.
- the wind speed of the second heating fluid H2 is preferably 1 to 10 m / second, more preferably 2 to 8 m / second. If the wind speed of the second heating fluid H2 is too slow, the amount of heat will be insufficient, and the strength of the nonwoven fabric will be insufficient. The thickness is reduced due to heat fusion.
- the spraying time of the second heating fluid H2 is preferably 0.03 to 5 seconds, more preferably 0.1 to 1 second.
- the spraying time is too short, the constituent fibers of the fiber web 50 are not sufficiently fused to each other, and the unevenness of the fiber web 50 is insufficiently fixed.
- the spraying time is too long, the constituent fibers of the fiber web 50 are formed. The heat fusion between the two proceeds excessively.
- the cooling process is a process in which the cooling unit 160 cools the fiber web 50 that has been unevenly shaped through the first and second air-through processes. Cooling strengthens the thermal fusion point of the constituent fibers of the fiber web 50.
- the cooling unit 160 is a space arranged between the second nozzle 130 and the third nozzle 140. This space naturally cools the fiber web 50 after the second air-through process.
- the cooling unit 160 may be provided with a cooling device that forcibly cools the fiber web 50. Examples of the forced cooling method include spraying of a cooling fluid from a cooling nozzle and contact with a cooling roller.
- the cooling temperature is preferably a temperature lower than the melting point of the heat-fusible fiber contained in the fiber web 50. When the thermoplastic fiber is a core-sheath type composite fiber, it is preferably lower than the melting point of the low-melting point component. Is also a low temperature.
- the cooling temperature is, for example, 100 ° C. or less.
- a 3rd air through process is a process of spraying the 3rd heating fluid H3 with the 3rd nozzle 140 with respect to the fiber web 50 which passed through the cooling process.
- the fluffed fibers and the non-fluffed fibers are thermally fused at a new fusion point, and a shaped nonwoven fabric without fluff is produced.
- the third nozzle 140 ejects the third heating fluid H3 from its ejection hole (not shown) and sprays it almost perpendicularly to the fiber web 50 on the conveyor belt 110.
- the third heating fluid H3 air, water vapor or the like heated to a predetermined temperature by a third heater (not shown) provided in the third nozzle 140 is used.
- the third heating fluid H3 is heated by the third heater to a temperature at which the fuzzy fibers of the fiber web 50 and other constituent fibers are heat-sealed while the uneven shape of the fiber web 50 is maintained.
- the fiber web 50 is a composite fiber having a low melting point component and a high melting point component having a higher melting point than the low melting point component
- the third heating fluid H3 has a temperature ranging from the melting point of the low melting point component to the melting point of the high melting point component. Be controlled.
- the temperature of the third heating fluid H3 is preferably 130 to 155 ° C., more preferably 130-145 ° C. If the temperature of the third heating fluid H3 is too low, heat fusion between the fibers will be insufficient, and the reduction of fuzz will be insufficient. On the other hand, if the temperature of the third heating fluid H3 is too high, other than the fuzzy fibers These fibers are also fused, and the liquid permeability is lowered.
- the wind speed of the third heating fluid H3 is preferably 0.5 to 5 m / second, more preferably 2 to 8 m / second. If the wind speed of the third heating fluid H3 is too slow, the fluffy fibers cannot be laid down, and the reduction of the fuzz becomes insufficient. Since heat fusion occurs between the fibers, the thickness is reduced, and the feel and liquid permeability are insufficient.
- the spraying time of the third heating fluid H3 is preferably 0.3 to 10 seconds, more preferably 2 to 6 seconds. If the spraying time is too short, the fuzzy fiber and other fibers cannot be sufficiently heat-sealed, and it becomes difficult to reduce the fuzz. On the other hand, if the spraying time is too long, fibers other than the fluffy fibers of the fiber web 50 are too fused together, the thickness becomes small, and the texture and liquid permeability are difficult to obtain.
- the blood slipperiness imparting agent has a kinematic viscosity at 40 ° C. of about 0.01 to about 80 mm 2 / s, a water retention of about 0.05 to about 4.0% by mass, and a weight average molecular weight of about 1 Less than 1,000.
- the kinematic viscosity at 40 ° C. of the blood slipperiness-imparting agent can be appropriately adjusted in the range of about 0 to about 80 mm 2 / s, preferably about 1 to about 70 mm 2 / s, more preferably about 3 to about 60 mm 2 / s, even more preferably from about 5 to about 50 mm 2 / s, and even more preferably from about 7 to about 45 mm 2 / s.
- the kinematic viscosity at 40 ° C. may be simply referred to as “kinematic viscosity”.
- the kinematic viscosity is as follows: a) as the molecular weight of the blood slipping agent increases, b) polar groups such as carbonyl bond (—CO—), ether bond (—O—), carboxyl group (—COOH), hydroxyl group ( There is a tendency that the higher the ratio of —OH) and the like, and c) the higher the IOB, the higher the ratio.
- the melting point of the blood slipperiness imparting agent is preferably 45 ° C. or less. This is because when the blood slipperiness-imparting agent contains crystals at 40 ° C., the kinematic viscosity tends to increase.
- kinematic viscosity in the blood slipperiness-imparting agent will be described later.
- the kinematic viscosity exceeds about 80 mm 2 / s, the blood slipperiness-imparting agent is too viscous to reach the skin contact surface of the top sheet. At the same time, it tends to be difficult to slide from the convex portion to the concave portion and then to move into the absorber.
- the kinematic viscosity can be measured at a test temperature of 40 ° C. using a Cannon Fenceke reverse flow viscometer according to “5. Kinematic viscosity test method” of JIS K 2283: 2000.
- the water retention rate of the blood slipperiness-imparting agent can be appropriately adjusted within the range of about 0.01 to about 4.0% by mass, preferably about 0.02 to about 3.5% by mass, more preferably About 0.03 to about 3.0% by weight, even more preferably about 0.04 to about 2.5% by weight, even more preferably about 0.05 to about 2.0% by weight.
- the “water retention” means the ratio (mass) of water that a substance can hold, and can be measured as follows. (1) Place a 20 mL test tube, a rubber stopper, a substance to be measured, and deionized water in a constant temperature room at 40 ° C. overnight. (2) In a constant temperature room, 5.0 g of a substance to be measured and 5.0 g of deionized water are put into a test tube. (3) Put a rubber stopper on the mouth of the test tube in a thermostatic chamber, rotate the test tube once, and let it stand for 5 minutes.
- the affinity between the blood slipperiness-imparting agent and menstrual blood decreases and reaches the skin contact surface of the top sheet.
- the affinity with menstrual blood becomes very high like a surfactant, and the absorbed blood remains on the skin contact surface of the top sheet, and the skin contact of the top sheet The surface tends to be red and easily colored.
- the water retention rate is as follows: a) the smaller the molecular weight of the blood slipperiness agent, and b) polar groups such as carbonyl bond (—CO—), ether bond (—O—), carboxyl group (—COOH), hydroxyl
- polar groups such as carbonyl bond (—CO—), ether bond (—O—), carboxyl group (—COOH), hydroxyl
- the ratio of the group (—OH) or the like is higher, the value tends to increase. This is because the blood slipperiness imparting agent is more hydrophilic.
- the water retention rate tends to increase as the IOB increases, that is, as the inorganic value increases and as the organic value decreases. This is because the blood slipperiness-imparting agent is more hydrophilic.
- menstrual blood excreted from the wearer When menstrual blood excreted from the wearer reaches the excretory opening contact region, it contacts the blood slipperiness imparting agent present in the convex part, slides into the concave part together with this, passes through the top sheet, and moves to the absorber. .
- a blood slipperiness-imparting agent having a kinematic viscosity of about 0.01 to about 80 mm 2 / s at 40 ° C. has a very low viscosity around the body temperature of the wearer and has a certain affinity with menstrual blood Therefore, it is considered that menstrual blood can slide from the convex part to the concave part together with menstrual blood, and can pass through the top sheet and quickly migrate to the absorbent body using the momentum at the time of the sliding.
- the blood slipperiness-imparting agent present on the convex part has a water retention of about 0.01 to about 4.0% by mass, and therefore has an affinity for mainly hydrophilic components (such as plasma) in menstrual blood. Therefore, it is considered that menstrual blood hardly remains on the top sheet.
- menstrual blood If there is a large amount of menstrual blood discharged from the wearer, even if the kinetic energy of menstrual blood itself is large and the kinematic viscosity value of the blood slipperiness-imparting agent is relatively high, it is difficult to slip with menstrual blood. Even if the water retention value is relatively high and the affinity with the hydrophilic component of menstrual blood is high, the weight molecular weight value is relatively high and difficult to slide with menstrual blood, and the top sheet Even when there is no uneven structure on the skin contact surface, it is considered that menstrual blood easily migrates to the absorber.
- the blood slipperiness-imparting agent slides down from the convex part to the concave part together with menstrual blood, and draws menstrual blood into the top sheet, and then draws it into the absorber, thereby rapidly transferring menstrual blood to the absorber.
- the blood lubricity-imparting agent has a weight average molecular weight of less than about 1,000, and preferably has a weight average molecular weight of less than about 900. This is because if the weight average molecular weight is about 1,000 or more, the blood slipperiness-imparting agent itself has tackiness and tends to give the wearer discomfort. In addition, since the viscosity of the blood slipperiness-imparting agent tends to increase as the weight average molecular weight increases, it becomes difficult to lower the viscosity of the blood slipperiness-imparting agent to a viscosity suitable for coating by heating, As a result, the blood slipping agent may have to be diluted with a solvent.
- the blood slipperiness-imparting agent preferably has a weight average molecular weight of about 100 or more, and more preferably has a weight average molecular weight of about 200 or more. This is because when the weight average molecular weight is small, the vapor pressure of the blood slipperiness imparting agent becomes high and vaporizes during storage, causing problems such as a decrease in the amount and odor when worn.
- weight average molecular weight refers to a polydispersed compound (for example, a compound produced by sequential polymerization, a plurality of fatty acids, and an ester produced from a plurality of aliphatic monohydric alcohols).
- a weight average molecular weight means the value of polystyrene conversion calculated
- GPC measurement conditions include the following. Model: Hitachi High-Technologies Corporation High-Performance Liquid Chromatogram Lachrom Elite Column: SHODEX KF-801, KF-803 and KF-804 manufactured by Showa Denko K.K. Eluent: THF Flow rate: 1.0 mL / min Driving amount: 100 ⁇ L Detection: RI (differential refractometer)
- the weight average molecular weight described in the Example of this specification is measured on the said conditions.
- the blood slipperiness agent can have an IOB of about 0.00 to about 0.60.
- IOB Inorganic Organic Balance
- IOB is an index indicating a balance between hydrophilicity and lipophilicity.
- Oda et al. IOB value calculated by inorganic value / organic value.
- the IOB is preferably about 0.00 to about 0.60, more preferably about 0.00 to about 0.50, and about 0.00 to about 0.40. And more preferably from about 0.00 to about 0.30. This is because when the IOB is in the above range, the water holding power and kinematic viscosity easily satisfy the above requirements.
- the blood slipperiness-imparting agent preferably has a melting point of 45 ° C. or lower, more preferably 40 ° C. or lower. This is because when the blood slipperiness-imparting agent has a melting point of 45 ° C. or less, the blood slipperiness-imparting agent tends to have a kinematic viscosity in the above range.
- melting point means a peak top temperature of an endothermic peak when changing from a solid state to a liquid state when measured with a differential scanning calorimeter at a heating rate of 10 ° C./min.
- the melting point can be measured using, for example, a DSC-60 type DSC measuring apparatus manufactured by Shimadzu Corporation.
- the blood lubricity-imparting agent may be liquid at room temperature (about 25 ° C.) or solid as long as it has a melting point of about 45 ° C. or lower, ie, a melting point of about 25 ° C. or higher, or It may be less than about 25 ° C. and may have a melting point of, for example, about ⁇ 5 ° C., about ⁇ 20 ° C.
- the blood slipping agent does not have a lower limit for its melting point, but preferably has a low vapor pressure.
- the vapor pressure of the blood slipperiness imparting agent is preferably about 0 to about 200 Pa at 25 ° C. (1 atm), more preferably about 0 to about 100 Pa, and further preferably about 0 to about 10 Pa. Preferably, it is about 0 to about 1 Pa, and even more preferably about 0.0 to about 0.1 Pa.
- the vapor pressure is preferably about 0 to about 700 Pa at 40 ° C. (1 atm), and is about 0 to about 100 Pa. Is more preferably about 0 to about 10 Pa, still more preferably about 0 to about 1 Pa, and still more preferably about 0.0 to about 0.1 Pa. This is because if the vapor pressure of the blood slipperiness imparting agent is high, it may vaporize during storage, and problems such as reduction in the amount and odor during wearing may occur.
- the melting point of the blood slipperiness-imparting agent can be selected according to the climate, the length of wearing time, and the like. For example, in an area where the average temperature is about 10 ° C. or less, by adopting a blood slipperiness imparting agent having a melting point of about 10 ° C. or less, menstrual blood is excreted and then cooled by the ambient temperature. However, it is considered that the blood slipperiness-imparting agent is easy to function.
- the melting point of the blood slipperiness imparting agent is preferably higher in the range of about 45 ° C. or less. This is because the blood slipperiness-imparting agent is not easily biased even when worn for a long time, and is not easily affected by sweat, friction at the time of wearing.
- the skin contact surface of the top sheet is coated with a surfactant for the purpose of changing the surface tension of menstrual blood and the like and quickly absorbing menstrual blood.
- the top sheet coated with a surfactant has a high affinity with hydrophilic components (such as plasma) in menstrual blood, and tends to attract them and rather cause menstrual blood to remain on the top sheet.
- the blood slipperiness imparting agent has a low affinity for menstrual blood, and can pass the menstrual blood to the absorbent body quickly without remaining on the top sheet.
- the blood slipperiness imparting agent is preferably the following (i) to (iii): (I) hydrocarbons, (Ii) consisting of a (ii-1) hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety.
- One or more of the same or different groups selected from the group consisting of a carbonyl group (—CO—) and an oxy group (—O—), and (iii-3) a hydrogen atom of the hydrocarbon moiety A compound having one or a plurality of identical or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH) to be substituted; As well as any combination thereof.
- hydrocarbon means a compound composed of carbon and hydrogen, and a chain hydrocarbon, for example, a paraffinic hydrocarbon (also referred to as an alkane, which does not include a double bond and a triple bond).
- a paraffinic hydrocarbon also referred to as an alkane, which does not include a double bond and a triple bond.
- Olefinic hydrocarbons including one double bond, also referred to as alkene
- acetylenic hydrocarbons including one triple bond, also referred to as alkyne
- hydrocarbons containing two or more bonds selected from the above and cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
- the hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, paraffinic hydrocarbon, olefinic hydrocarbon, and two or more double bonds. More preferably, it is a hydrocarbon that does not contain a triple bond, and more preferably it is a paraffinic hydrocarbon.
- the chain hydrocarbon includes a straight chain hydrocarbon and a branched chain hydrocarbon.
- each oxy group (—O—) is not adjacent. Therefore, the compounds (ii) and (iii) do not include compounds having a continuous oxy group (so-called peroxides).
- At least one hydrogen atom in the hydrocarbon moiety is more hydroxyl group (—OH) than in the compound in which at least one hydrogen atom in the hydrocarbon moiety is substituted with a carboxyl group (—COOH).
- a carboxyl group —COOH
- IOB is preferred. This is because the carboxyl group binds to a metal or the like in menstrual blood, increases the water retention rate of the blood slipperiness imparting agent, and may exceed a predetermined range. This is the same from the viewpoint of IOB. As shown in Table 1, the carboxyl group binds to menstrual metals and the like, and the inorganic value increases significantly from 150 to 400 or more. Sometimes the value of IOB can be greater than about 0.60.
- the blood slipperiness imparting agent is more preferably the following (i ′) to (iii ′): (I ′) hydrocarbon, (Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—) or an ester bond (—COO— inserted between the C—C single bonds of the hydrocarbon moiety.
- the blood slipperiness imparting agent is more preferably about 1.8 or less carbonyl bonds (—CO—) and 2 or less ester bonds (—COO—) per 10 carbon atoms in the hydrocarbon moiety. About 1.5 or less bonds (—OCOO—), about 6 or less ether bonds (—O—), about 0.8 or less carboxyl groups (—COOH), and / or hydroxyl groups (—OH) About 1.2 or less.
- the blood slipperiness imparting agent is more preferably the following (A) to (F), (A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and a chain carbon An ester with a compound having one carboxyl group replacing a hydrogen atom of the hydrogen moiety, (B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and a chain hydrocarbon An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrogen moiety, (C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety, and (C2 ) An ester of a
- (A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, Esters with compounds having one carboxyl group replacing a hydrogen atom in the hydrocarbon moiety]
- (A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety;
- Esters (hereinafter sometimes referred to as “compound (A)”) with a compound having one carboxyl group that substitutes a hydrogen atom of the hydrogen moiety have the above kinematic viscosity, water retention and weight average molecular weight. As long as it has, all the hydroxyl groups do not need to be esterified.
- (A1) As a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (A1)”), for example, chain hydrocarbon tetraols such as alkane tetraols such as pentaerythritol, chain hydrocarbon triols such as alkane triols such as glycerin, and chain hydrocarbon diols such as alkane diols such as glycol Is mentioned.
- chain hydrocarbon tetraols such as alkane tetraols such as pentaerythritol
- chain hydrocarbon triols such as alkane triols such as glycerin
- chain hydrocarbon diols such as alkane diols such as glycol Is mentioned.
- Examples of the compound having a chain hydrocarbon moiety and one carboxyl group that replaces a hydrogen atom in the chain hydrocarbon moiety include, for example, one hydrogen atom on a hydrocarbon having one carboxyl group ( -COOH) substituted compounds, for example fatty acids.
- Examples of the compound (A) include (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) an ester of a chain hydrocarbon triol and at least one fatty acid, and (a 3 ) Esters of chain hydrocarbon diols with at least one fatty acid.
- ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid examples include the following formula (1): Tetraesters of pentaerythritol and fatty acids of the following formula (2): Triesters of pentaerythritol and fatty acids of the following formula (3): Diester of pentaerythritol and fatty acid of the following formula (4): And monoesters of pentaerythritol and fatty acids. (Wherein R 1 to R 4 are each a chain hydrocarbon)
- esters of pentaerythritol and fatty acids As fatty acids (R 1 COOH, R 2 COOH, R 3 COOH, and R 4 COOH) constituting esters of pentaerythritol and fatty acids, esters of pentaerythritol and fatty acids have kinematic viscosity, water retention and weight average.
- a saturated fatty acid for example, a C 2 to C 30 saturated fatty acid, for example, acetic acid (C 2 ) (C 2 represents the number of carbon atoms, R 1 C, R 2 C, R 3 C or R 4 C, the same applies hereinafter), propanoic acid (C 3 ), butanoic acid (C 4 ) and isomers thereof such as 2-methylpropanoic acid ( C 4 ), pentanoic acid (C 5 ) and isomers thereof such as 2-methylbutanoic acid (C 5 ), 2,2-dimethylpropanoic acid (C 5 ), hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8) Beauty isomers thereof, e.g., 2-ethylhexanoic acid (C 8), nonanoic acid (C 9), decanoic acid
- the fatty acid can also be an unsaturated fatty acid.
- unsaturated fatty acids include C 3 to C 20 unsaturated fatty acids such as monounsaturated fatty acids such as crotonic acid (C 4 ), myristoleic acid (C 14 ), palmitoleic acid (C 16 ), and olein.
- diunsaturated fatty acids such as linoleic acid (C 18
- Tetraunsaturated fatty acids such as stear Don acid (C 20), arachidonic acid (C 20), eicosatetraenoic acid (C 20), etc., penta unsaturated fatty acids, for example, bosseopentaenoic acid (C 18), such as eicosapentaenoic acid (C 20), as well as their Of the partial hydrogen adduct.
- the ester of pentaerythritol and a fatty acid is preferably an ester of pentaerythritol and a fatty acid derived from a saturated fatty acid, that is, an ester of pentaerythritol and a saturated fatty acid, considering the possibility of modification by oxidation or the like.
- the ester of pentaerythritol and fatty acid is preferably a diester, triester or tetraester, more preferably a triester or tetraester, and more preferably a tetraester from the viewpoint of reducing the water retention value. More preferably, it is an ester.
- the total number of carbon atoms of the fatty acid constituting the tetraester of pentaerythritol and fatty acid that is, the above formula In (1), the total number of carbon atoms of the R 1 C, R 2 C, R 3 C, and R 4 C moieties is preferably about 15 (when the total number of carbon atoms is 15, the IOB is 0.1. 60).
- Examples of tetraesters of pentaerythritol and fatty acids include pentaerythritol, hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8 ), such as 2-ethylhexanoic acid (C 8 ), nonane
- Examples include tetraesters with acid (C 9 ), decanoic acid (C 10 ) and / or dodecanoic acid (C 12 ).
- the triester of pentaerythritol and fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of pentaerythritol and fatty acid, that is, the above formula In (2), the total number of carbon atoms in the R 1 C, R 2 C and R 3 C moieties is preferably about 19 or more (when the total number of carbon atoms is 19, IOB is 0.58). ).
- the total number of carbon atoms of the fatty acid constituting the diester of pentaerythritol and fatty acid that is, the above formula (3 )
- the total number of carbon atoms in the R 1 C and R 2 C moieties is preferably about 22 or more (when the total number of carbon atoms is 22, IOB is 0.59).
- the carbon number of the fatty acid constituting the monoester of pentaerythritol and fatty acid that is, the above formula (4 )
- the number of carbon atoms in the R 1 C moiety is preferably about 25 or more (when the number of carbon atoms is 25, IOB is 0.60).
- the influence of double bond, triple bond, iso branch, and tert branch is not considered (the same applies hereinafter).
- esters of pentaerythritol and fatty acids examples include Unistar H-408BRS, H-2408BRS-22 (mixed product), etc. (above, manufactured by NOF Corporation).
- ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid examples include the following formula (5): Triester of glycerin and fatty acid of the following formula (6): Diesters of glycerin and fatty acids and the following formula (7): (Wherein R 5 to R 7 are each a chain hydrocarbon) And monoesters of glycerin and fatty acids.
- esters of glycerin and fatty acids satisfy the requirements of kinematic viscosity, water retention and weight average molecular weight. If there is, there is no particular limitation, for example, fatty acids listed in “esters of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”, that is, saturated fatty acids and unsaturated fatty acids, oxidation, etc. In view of the possibility of modification by the above, it is preferably an ester of glycerin and a fatty acid derived from a saturated fatty acid, that is, an ester of glycerin and a saturated fatty acid.
- ester of glycerin and fatty acid is preferably a diester or triester, more preferably a triester, from the viewpoint of reducing the water retention value.
- Triesters of glycerin and fatty acids are also called triglycerides, for example, triesters of glycerin and octanoic acid (C 8 ), triesters of glycerin and decanoic acid (C 10 ), glycerin and dodecanoic acid (C 12 ). And triesters of glycerin with 2 or 3 fatty acids, and mixtures thereof.
- Examples of triesters of glycerin and two or more fatty acids include triesters of glycerin and octanoic acid (C 8 ) and decanoic acid (C 10 ), glycerin, octanoic acid (C 8 ), and decanoic acid.
- the triester of glycerin and a fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of glycerin and a fatty acid, that is, in formula (5), R 5 C , R 6 C and R 7 C moieties preferably have a total carbon number of about 40 or less.
- the triester of glycerin and fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of glycerin and fatty acid, that is, the formula (5)
- the total number of carbon atoms of the R 5 C, R 6 C and R 7 C moieties is preferably about 12 or more (when the total number of carbon atoms is 12, IOB is 0.60).
- a triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute a human body, and thus is preferable from the viewpoint of safety.
- triesters of glycerin and fatty acid include tricoconut oil fatty acid glyceride, NA36, panacet 800, panacet 800B and panacet 810S, and tri-C2L oil fatty acid glyceride and tri-CL oil fatty acid glyceride (manufactured by NOF Corporation). Etc.
- Diesters of glycerin and fatty acids are also referred to as diglycerides.
- diesters of glycerin and decanoic acid C 10
- diesters of glycerin and dodecanoic acid C 12
- diesters of glycerin and hexadecanoic acid C 16
- diesters of glycerin and two fatty acids and mixtures thereof.
- the total number of carbon atoms of the fatty acid constituting the diester of glycerin and fatty acid that is, in the formula (6), the total number of carbon atoms in the R 5 C and R 6 C moieties is preferably about 16 or more (when the total number of carbon atoms is 16, IOB is 0.58).
- Monoesters of glycerin and fatty acids are also referred to as monoglycerides, and examples include glycerin octadecanoic acid (C 18 ) monoester, glycerin docosanoic acid (C 22 ) monoester, and the like.
- the number of carbon atoms of the fatty acid constituting the monoester of glycerin and fatty acid that is, in the formula (7), the number of carbon atoms in the R 5 C moiety is preferably about 19 or more (when the number of carbon atoms is 19, IOB is 0.59).
- esters of chain hydrocarbon diols with at least one fatty acid include C 2 to C 6 chain hydrocarbon diols such as C 2 to C 6 glycols such as ethylene glycol, propylene glycol, and butylene glycol. , Monoester or diester of pentylene glycol or hexylene glycol and a fatty acid.
- R 8 COOC k H 2k OCOR 9 (Wherein k is an integer from 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
- R 8 COOC k H 2k OH (9) (Wherein k is an integer from 2 to 6 and R 8 is a chain hydrocarbon)
- monoesters of C 2 -C 6 glycols and fatty acids for example, the following formula (8): R 8 COOC k H 2k OCOR 9 (8) (Wherein k is an integer from 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
- R 8 COOC k H 2k OH (Wherein k is an integer from 2 to 6 and R 8 is a chain hydrocarbon)
- monoesters of C 2 -C 6 glycols and fatty acids for example, the following formula (8): R 8 COOC k H 2k OCOR 9 (8) (Wherein k is an integer from 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
- the fatty acid to be esterified is C 2 -C 6 glycol and
- the ester with a fatty acid is not particularly limited as long as it satisfies the requirements of kinematic viscosity, water retention and weight average molecular weight.
- “(a 1 ) a chain hydrocarbon tetraol and at least one fatty acid
- the carbon number of the R 8 C and R 9 C moieties Is preferably about 6 or more (when the total number of carbon atoms is 6, IOB is 0.60).
- the carbon number of the R 8 C moiety is about It is preferably 12 or more (when the carbon number is 12, IOB is 0.57).
- esters of C 2 ⁇ C 6 glycols and fatty acid in view of the potential for degradation by oxidation and the like, derived from saturated fatty acids, esters of C 2 ⁇ C 6 glycols and fatty acid, Nachi Suwa, C 2 ⁇ An ester of C 6 glycol and a saturated fatty acid is preferred.
- an ester of C 2 -C 6 glycol and a fatty acid from the viewpoint of reducing the water retention value, an ester of glycol and a fatty acid derived from a glycol having a large number of carbon atoms, such as butylene glycol and pentylene.
- An ester of glycol and fatty acid derived from glycol or hexylene glycol is preferable.
- the ester of C 2 -C 6 glycol and a fatty acid is preferably a diester from the viewpoint of reducing the water retention value.
- Examples of commercially available esters of C 2 -C 6 glycol and fatty acid include Compol BL and Compol BS (manufactured by NOF Corporation).
- compound (B1) As a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (B1)”), What is enumerated as a compound (A1) in "Compound (A)", for example, a pentaerythritol, glycerol, and glycol are mentioned.
- Examples of the compound (B2) having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety include: A compound in which one hydrogen atom of a hydrocarbon is substituted with one hydroxyl group (—OH), for example, an aliphatic monohydric alcohol, for example, a saturated aliphatic monohydric alcohol and an unsaturated aliphatic monohydric alcohol Can be mentioned.
- —OH hydroxyl group
- saturated aliphatic monohydric alcohol examples include C 1 to C 20 saturated aliphatic monohydric alcohols such as methyl alcohol (C 1 ) (C 1 represents the number of carbon atoms, the same shall apply hereinafter), ethyl alcohol (C 2 ), propyl alcohol (C 3 ) and isomers thereof such as isopropyl alcohol (C 3 ), butyl alcohol (C 4 ) and isomers thereof such as sec-butyl alcohol (C 4 ) and tert-butyl alcohol ( C 4 ), pentyl alcohol (C 5 ), hexyl alcohol (C 6 ), heptyl alcohol (C 7 ), octyl alcohol (C 8 ) and isomers thereof such as 2-ethylhexyl alcohol (C 8 ), nonyl alcohol ( C 9), decyl alcohol (C 10), dodecyl alcohol (C 12), tetradecyl alcohol (C 14), hexyl Decyl alcohol (C 16),
- Examples of the unsaturated aliphatic monohydric alcohol include those obtained by substituting one of the C—C single bonds of the saturated aliphatic monohydric alcohol with a C ⁇ C double bond, such as oleyl alcohol. It is commercially available from Rika Co., Ltd. under the names of the Jamaica Coal series and the Angelo All series.
- Examples of the compound (B) include (b 1 ) ethers of chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, such as monoether, diether, triether and tetraether, preferably diether, triether.
- Ethers and tetraethers more preferably triethers and tetraethers, and even more preferably tetraethers, ethers of (b 2 ) chain hydrocarbon triols and at least one aliphatic monohydric alcohol, such as monoethers, diethers and Triethers, preferably diethers and triethers, and more preferably triethers, and (b 3 ) ethers of chain hydrocarbon diols with at least one aliphatic monohydric alcohol, such as monoethers and diethers, and preferably Diether It is below.
- ethers of chain hydrocarbon tetraols and at least one aliphatic monohydric alcohol include, for example, the following formulas (10) to (13): (In the formula, R 10 to R 13 are each a chain hydrocarbon.) And tetraethers, triethers, diethers and monoethers of pentaerythritol and aliphatic monohydric alcohols.
- ethers of chain hydrocarbon triols and at least one aliphatic monohydric alcohol include, for example, the following formulas (14) to (16): (Wherein R 14 to R 16 are each a chain hydrocarbon.) And triether, diether and monoether of glycerin and aliphatic monohydric alcohol.
- R 17 OC n H 2n OR 18 (Wherein n is an integer from 2 to 6 and R 17 and R 18 are each a chain hydrocarbon)
- R 17 OC n H 2n OH (18)
- n is an integer from 2 to 6 and R 17 is a chain hydrocarbon
- monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols
- the tetraether of pentaerythritol and aliphatic monohydric alcohol is aliphatic 1 constituting the tetraether of pentaerythritol and aliphatic monohydric alcohol.
- the total number of carbon atoms of the hydric alcohol that is, in the above formula (10), the total number of carbon atoms of the R 10 , R 11 , R 12 and R 13 moieties is preferably about 4 or more (the total number of carbon atoms is In the case of 4, the IOB is 0.44).
- the triether of pentaerythritol and aliphatic monohydric alcohol is aliphatic 1 constituting the triether of pentaerythritol and aliphatic monohydric alcohol.
- the total number of carbon atoms of the monohydric alcohol that is, in the above formula (11), the total number of carbon atoms of the R 10 , R 11 and R 12 portions is preferably about 9 or more (when the total number of carbon atoms is 9 IOB becomes 0.57).
- the diether of pentaerythritol and an aliphatic monohydric alcohol is an aliphatic monohydric alcohol constituting a diether of pentaerythritol and an aliphatic monohydric alcohol. That is, in the above formula (12), the total number of carbon atoms in the R 10 and R 11 moieties is preferably about 15 or more (when the total number of carbon atoms is 15, the IOB is 0). .60).
- the monoether of pentaerythritol and aliphatic monohydric alcohol is aliphatic 1 constituting the monoether of pentaerythritol and aliphatic monohydric alcohol.
- the number of carbon atoms of the polyhydric alcohol, i.e., in formula (13), the number of carbon atoms of R 10 moiety is preferably about 22 or more (when the number of carbon atoms is 22, IOB is 0.59).
- the triether of glycerin and aliphatic monohydric alcohol is aliphatic 1 constituting the triether of glycerin and aliphatic monohydric alcohol.
- the total number of carbon atoms of the R 14 , R 15 and R 16 moieties is preferably about 3 or more (when the total number of carbon atoms is 3). , IOB becomes 0.50).
- diether of glycerol and aliphatic monohydric alcohol is carbon of aliphatic monohydric alcohol constituting diether of glycerin and aliphatic monohydric alcohol.
- the total number of carbon atoms in the R 14 and R 15 moieties is preferably about 9 or more (when the total number of carbon atoms is 9, the IOB is 0.58). Clearly).
- the monoether of glycerin and an aliphatic monohydric alcohol is an aliphatic monohydric alcohol constituting a monoether of glycerin and an aliphatic monohydric alcohol.
- the carbon number of the R 14 moiety is preferably about 16 or more (when the carbon number is 16, IOB is 0.58).
- R 17 and R 18 moieties
- the total number of carbons is preferably about 2 or more (when the total number of carbons is 2, IOB is 0.33).
- the R 17 moieties The number of carbon atoms is preferably about 8 or more (when the number of carbon atoms is 8, IOB is 0.60).
- Compound (B) can be produced by dehydrating condensation of compound (B1) and compound (B2) in the presence of an acid catalyst.
- (C1) Carboxylic acid, hydroxy acid, alkoxy acid or oxo acid (hereinafter referred to as “compound (C1) containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety”
- a chain hydrocarbon carboxylic acid having 2 to 4 carboxyl groups such as a chain hydrocarbon dicarboxylic acid such as an alkanedicarboxylic acid such as ethanedioic acid, Propanedioic acid, butanedioic acid, pentanedioic acid, hexanedioic acid, heptanedioic acid, octanedioic acid, nonanedioic acid and decanedioic acid, chain hydrocarbon tricarboxylic acid such as alkanetricarboxylic acid such as propanetriacid , Butanetriacid, pentanetriacid, hexane
- the compound (C1) includes a chain hydrocarbon hydroxy acid having 2 to 4 carboxyl groups, for example, a chain chain having 2 to 4 carboxyl groups such as malic acid, tartaric acid, citric acid, isocitric acid and the like. Hydrocarbon alkoxy acids such as O-acetylcitric acid and chain hydrocarbon oxoacids having 2 to 4 carboxyl groups are included.
- C2 Examples of the compound having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety include those listed in the section of “Compound (B)”, for example, fat Group monohydric alcohols.
- compound (D) includes (d 1 ) aliphatic 1 And ethers of monohydric alcohols and aliphatic monohydric alcohols, (d 2 ) dialkyl ketones, (d 3 ) esters of fatty acids and aliphatic monohydric alcohols, and (d 4 ) dialkyl carbonates.
- the ether As the aliphatic monohydric alcohol constituting the ether (corresponding to R 19 OH and R 20 OH in the formula (19)), the ether satisfies the above-mentioned requirements for kinematic viscosity, water retention and weight average molecular weight. If it is, it will not restrict
- dialkylketone As the dialkyl ketone, the following formula (20): R 21 COR 22 (20) (Wherein R 21 and R 22 are each an alkyl group) The compound which has is mentioned.
- the dialkyl ketone can be obtained by a known method, for example, by oxidizing a secondary alcohol with chromic acid or the like.
- ester of a fatty acid and an aliphatic monohydric alcohol examples include the following formula (21): R 23 COOR 24 (21) (Wherein R 23 and R 24 are each a chain hydrocarbon) The compound which has is mentioned.
- Examples of the fatty acid constituting the ester include, for example, the fatty acids listed in “(a 1 ) ester of chain hydrocarbon tetraol and fatty acid”, that is, Saturated fatty acids or unsaturated fatty acids are exemplified, and saturated fatty acids are preferred in consideration of the possibility of modification by oxidation or the like.
- Examples of the aliphatic monohydric alcohol constituting the ester include the aliphatic monohydric alcohols listed in the section “Compound (B)”.
- esters of fatty acids and aliphatic monohydric alcohols include, for example, dodecanoic acid (C 12 ), dodecyl alcohol (C 12 ) ester, tetradecanoic acid (C 14 ), dodecyl alcohol (C 12 ) and Examples of commercially available esters of fatty acids and aliphatic monohydric alcohols include Electol WE20 and Electol WE40 (manufactured by NOF Corporation).
- the dialkyl carbonate is commercially available, and can be synthesized by a reaction of phosgene with an alcohol, a reaction of a formic chloride ester with an alcohol or an alcoholate, and a reaction of silver carbonate with an alkyl iodide.
- (d 1 ) an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, (d 2 ) a dialkyl ketone, (d 3 ) a fatty acid and an aliphatic monohydric alcohol And (d 4 ) dialkyl carbonates preferably have a weight average molecular weight of about 100 or more, and more preferably about 200 or more.
- dialkyl ketone when the total number of carbon atoms is about 8, for example, for 5-nonanone, the melting point is about ⁇ 50 ° C., and the vapor pressure is about 230 Pa at 20 ° C.
- (E) Polyoxy C 3 -C 6 alkylene glycol, or alkyl ester or alkyl ether thereof includes (e 1 ) polyoxy C 3 -C 6 alkylene glycol, (e 2 ) Esters of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid, (e 3 ) Ethers of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol. This will be described below.
- the polyoxy C 3 -C 6 alkylene glycol has the following formula (23): HO- (C m H 2m O) n -H (23) (Where m is an integer from 3 to 6) Is represented by
- Examples of commercially available poly C 3 -C 6 alkylene glycols include Uniol (trademark) PB-500 and PB-700 (manufactured by NOF Corporation).
- ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid examples include the OH terminal of the polyoxy C 3 -C 6 alkylene glycol described in the section “(e 1 ) Polyoxy C 3 -C 6 alkylene glycol”.
- One or both are esterified with fatty acids, ie monoesters and diesters.
- fatty acid to be esterified in the ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid are listed in “Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”.
- Fatty acid that is, saturated fatty acid or unsaturated fatty acid, and saturated fatty acid is preferable in consideration of possibility of modification by oxidation or the like.
- Examples of the aliphatic monohydric alcohol to be etherified in the ether of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, the aliphatic enumerated in the section of “Compound (B)”. A monohydric alcohol is mentioned.
- chain hydrocarbon examples include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes.
- the linear alkane has a carbon number of about 22 or less when the melting point is about 45 ° C. or less, and about 13 or less when the vapor pressure is about 0.01 Pa or less at 1 atm and 25 ° C. That's it.
- Branched-chain alkanes tend to have lower melting points at the same carbon number than straight-chain alkanes. Therefore, the branched chain alkane can include those having 22 or more carbon atoms even when the melting point is about 45 ° C. or lower.
- Pearl Ream 6 (NOF Corporation) can be mentioned.
- the blood slipperiness-imparting agent may be applied alone to at least the convex portion 8 in the excretory opening contact region 20, or the blood slipperiness-imparting agent and at least one other component are contained.
- a blood slipping agent-containing composition may be applied.
- the blood slipping agent-containing composition will be described.
- lubricity imparting agent since it is the same as that of the blood slipperiness
- the composition for containing a blood slipperiness agent contains the above-described blood slipperiness imparting agent and at least one other component.
- the other components are not particularly limited as long as they do not inhibit the effect of the blood slipperiness-imparting agent, and those conventionally used in absorbent articles, particularly top sheets, can be used in the industry. .
- Examples of other components include silicone oil, silicone, silicone resin, and the like.
- antioxidants such as BHT (2,6-di-t-butyl-p-cresol), BHA (butylated hydroxyanisole), propyl gallate and the like.
- vitamins such as natural vitamins and synthetic vitamins.
- vitamins include water-soluble vitamins such as vitamin B groups, such as vitamin B 1 , vitamin B 2 , vitamin B 3 , vitamin B 5 , vitamin B 6 , vitamin B 7 , vitamin B 9 , vitamin B 12, etc.
- vitamin C examples include water-soluble vitamins such as vitamin B groups, such as vitamin B 1 , vitamin B 2 , vitamin B 3 , vitamin B 5 , vitamin B 6 , vitamin B 7 , vitamin B 9 , vitamin B 12, etc.
- vitamin C such as water-soluble vitamins such as vitamin B groups, such as vitamin B 1 , vitamin B 2 , vitamin B 3 , vitamin B 5 , vitamin B 6 , vitamin B 7 , vitamin B 9 , vitamin B 12, etc.
- vitamins examples include fat-soluble vitamins such as vitamin A group, vitamin D group, vitamin E group, and vitamin K group. Vitamins also include their derivatives.
- Examples of other components include amino acids such as alanine, arginine, lysine, histidine, proline, hydroxyproline, and peptides.
- zeolites such as natural zeolites such as zeolitic, chabazite, pyroxenite, natrolite, zeolitic and somosonite, and synthetic zeolite.
- Examples of other components include cholesterol, hyaluronic acid, lecithin, ceramide and the like.
- Other components include, for example, drugs such as skin astringents, anti-acne agents, anti-wrinkle agents, anti-cellulite agents, whitening agents, antibacterial agents, anti-fungal agents and the like.
- Examples of the skin astringent include oil-soluble skin astringents such as zinc oxide, aluminum sulfate, and tannic acid, such as oil-soluble polyphenols.
- oil-soluble polyphenols include natural oil-soluble polyphenols, such as buckwheat extract, hypericum extract, nettle extract, chamomile extract, burdock extract, salvia extract, linden extract, scallop extract, birch extract, cedar extract, sage extract, salvia extract, Examples include teuchigurumi extract, hibiscus extract, loquat leaf extract, bodaiju extract, hop extract, marronnier extract, and yokuinin extract.
- anti-acne agent examples include salicylic acid, benzoyl peroxide, resorcinol, sulfur, erythromycin, and zinc.
- anti-wrinkle agent examples include lactic acid, salicylic acid, salicylic acid derivatives, glycolic acid, phytic acid, lipoic acid, and lysophosphatide acid.
- anti-cellulite agent examples include xanthine compounds such as aminophylline, caffeine, theophylline, theobromine and the like.
- whitening agents include niacinamide, kojic acid, arbutin, glucosamine and derivatives, phytosterol derivatives, ascorbic acid and derivatives thereof, and mulberry extract and placenta extract.
- Examples of other components include anti-inflammatory components, pH adjusters, antibacterial agents, moisturizers, fragrances, pigments, dyes, pigments, plant extracts and the like.
- Anti-inflammatory components include, for example, naturally-occurring anti-inflammatory agents such as buttons, ogon, hypericum, chamomile, licorice, momonoha, mugwort, perilla extract, synthetic anti-inflammatory agents such as allantoin, dipotassium glycyrrhizinate, etc. .
- Examples of the pH adjuster include those for keeping the skin weakly acidic, such as malic acid, succinic acid, citric acid, tartaric acid, and lactic acid.
- Examples of the pigment include titanium oxide.
- the blood slipping agent-containing composition preferably contains about 50 to about 99% by weight and about 1 to about 50% by weight, more preferably about 60%, of the blood slipping agent and at least one other component, respectively. To about 99% and about 1 to about 40%, more preferably about 70 to about 99% and about 1 to about 30%, even more preferably about 80 to about 99% and about 1 to about 20%. % By weight, even more preferably from about 90 to 99% by weight and from about 1 to about 10% by weight, even more preferably from about 95 to 99% by weight and from about 1 to about 5% by weight. This is from the viewpoint of the effect of the blood slipperiness-imparting agent and other components.
- the blood slipping agent-containing composition preferably contains a surfactant in an amount equal to or less than the amount derived from the hydrophilic treatment of the top sheet or the second sheet. More specifically, the composition containing a blood slipperiness agent preferably contains a surfactant, preferably about 0.0 to about 1.0 g / m 2 , more preferably about 0.0 to about 0.8 g / m 2. 2 , more preferably from about 0.1 to about 0.5 g / m 2 , and even more preferably from about 0.1 to about 0.3 g / m 2 in the basis weight range.
- a surfactant preferably about 0.0 to about 1.0 g / m 2 , more preferably about 0.0 to about 0.8 g / m 2. 2 , more preferably from about 0.1 to about 0.5 g / m 2 , and even more preferably from about 0.1 to about 0.3 g / m 2 in the basis weight range.
- the blood slipping agent-containing composition preferably contains water in an amount of about 0.0 to about 1.0 g / m 2 , more preferably about 0.0 to about 0.8 g / m 2 , and even more preferably about 0.0. It is included in the basis weight range of 1 to about 0.5 g / m 2 , even more preferably about 0.1 to about 0.3 g / m 2 . Since water reduces the absorption performance of an absorbent article, it is preferable that water is small.
- the blood slipping agent-containing composition like the blood slipping agent, preferably has a kinematic viscosity at 40 ° C. of about 0 to about 80 mm 2 / s, and about 1 to about 70 mm 2. More preferably, it has a kinematic viscosity of about 3 to about 60 mm 2 / s, even more preferably about 5 to about 50 mm 2 / s, and even more preferably about Even more preferably, it has a kinematic viscosity of from 7 to about 45 mm 2 / s.
- the kinematic viscosity of the composition containing a blood slipperiness agent exceeds about 80 mm 2 / s, the viscosity is high, and the blood slipperiness imparting composition is an absorbent article together with menstrual blood that has reached the skin contact surface of the top sheet. This is because it tends to be difficult to slide down inside.
- the other component preferably has a weight average molecular weight of less than about 1,000. More preferably having a weight average molecular weight of less than about 900. This is because when the weight average molecular weight is about 1,000 or more, the blood slipperiness-imparting agent-containing composition itself is tacky and tends to give the wearer discomfort. Moreover, since the viscosity of the composition containing a blood slipperiness-imparting agent tends to increase as the weight average molecular weight increases, the viscosity of the blood slipperiness-enhancing agent composition is lowered to a viscosity suitable for application by heating. As a result, the blood slipping agent may have to be diluted with a solvent.
- the composition containing a blood slipperiness imparting agent has a water content of about 0.01 to about 4.0% by mass and a water content of about 0.02 to about 3.5% by mass as the composition. Preferably about 0.03 to about 3.0% by weight, more preferably about 0.04 to about 2.5% by weight, and more preferably about 0.03 to about 3.0% by weight. More preferably, it has a water retention of 0.05 to about 2.0% by weight.
- the affinity between the blood slipperiness-imparting agent composition and menstrual blood is reduced, and menstrual blood that has reached the skin contact surface of the top sheet is less likely to slide into the absorbent article.
- lubricity imparting agent containing composition contains a solid substance, it is preferable to remove them by filtration in the measurement of kinematic viscosity and a water retention.
- Tri-C2L oil fatty acid glyceride manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid containing approximately 37: 7: 56 weight ratio, glycerol and fatty acid triester, Weight average molecular weight: about 570
- fatty acids manufactured by NOF Corporation C 8 fatty to C 10 are contained in approximately 85:15 weight ratio of triesters of glycerol with fatty acids, the weight average molecular weight: about 480 ⁇ Panasate 800, manufactured by NOF Corporation All fatty acids are octanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470
- Panaceate 800B manufactured by NOF Corporation All fatty acids are 2-ethylhexanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470 ⁇ NA36, manufactured by NOF Corporation C 16 fatty acid: fatty acid C 18: (including both saturated and unsaturated fatty acids) fatty acids to C 20 is approximately 5: contained in a weight ratio of 3: 92 Triester of glycerin and fatty acid, weight average molecular weight: about 880
- C 8 fatty C 10: fatty acid
- C 12 fatty acid
- C 14 (including both saturated and unsaturated fatty acids)
- C 16 is approximately 4 : Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670 -Caprylic acid diglyceride, manufactured by NOF Corporation
- Fatty acid is octanoic acid, diester of glycerin and fatty acid, weight average molecular weight: 340
- [Other materials] -NA50 manufactured by NOF Corporation Hydrogen added to NA36 to reduce the ratio of double bonds derived from the unsaturated fatty acid as a raw material Triester of glycerin and fatty acid, weight average molecular weight: about 880
- PEG 1500 manufactured by NOF Corporation, polyethylene glycol, weight average molecular weight: about 1,500 to about 1,600 -Wilbright cp9, manufactured by NOF Co., Ltd.
- Nonionic S-6 polyoxyethylene monostearate manufactured by NOF Corporation, about 7 repeating units, weight average molecular weight: about 880 ⁇ Unilube 5TP-300KB Polyoxyethylene polyoxypropylene pentaerythritol ether produced by adding 5 mol of ethylene oxide and 65 mol of propylene oxide to 1 mol of pentaerythritol, weight average molecular weight: 4,130
- Top sheet formed from air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 30 g) / M 2 ), a pulp (basis weight: 150 to 450 g / m 2 , more in the center), an acrylic superabsorbent polymer (basis weight: 15 g / m 2 ), and a tissue as a core wrap.
- An absorbent body, a side sheet treated with a water repellent, and a back sheet made of a polyethylene film were prepared.
- the top sheet is a top sheet having a ridge groove structure manufactured according to the method described in Japanese Patent Application Laid-Open No. 2008-2034.
- the ridge portion has a thickness of about 1.5 mm and the groove portion has a thickness of about 0.00 mm.
- the pitch of the ridge groove structure was about 4 mm, and an opening portion having an opening ratio of about 15% was formed in the groove portion.
- Unistar H-408BRS (manufactured by NOF Corporation, tetraester of pentaerythritol and fatty acid) is selected as a blood slipperiness-imparting agent, and the skin contact surface ( ⁇ ⁇ ⁇ ) of the top sheet from a control seam HMA gun at room temperature.
- the groove surface was coated with a basis weight of 5.0 g / m 2 .
- H-408BRS was in the form of fine particles and adhered to the fiber surface.
- the back sheet, the absorbent body, the second sheet, and the top sheet were sequentially laminated with the groove surface facing up, whereby a sanitary napkin no. 1-1 was formed.
- the blood slipperiness-imparting agent was changed from Unistar H-408BRS to that shown in Table 2, and sanitary napkin no. 1-2 to No. 1-49 was produced.
- the blood slipperiness-imparting agent is a liquid at room temperature
- the blood slipperiness-imparting agent is a solid at room temperature
- it is heated to the melting point + 20 ° C., and then a control seam HMA gun is used.
- the blood slipperiness imparting agent was atomized and applied to the skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
- lubricity imparting agent was applied to the substantially whole surface of the skin contact surface of a top sheet, and to both a collar part and a groove part.
- Mass of top sheet After measuring W 2 (g) (mass of top sheet before test), an acrylic plate having a hole in the center of the absorbent article in the longitudinal direction and width direction and on the top sheet ( Place 200mm x 100mm, 125g, 40mm x 10mm hole in the center, and from the hole, 37 ⁇ 1 ° C equine EDTA blood (ethylenediaminetetraacetic acid (hereinafter referred to as equine blood to prevent coagulation) 4.0 g) was added dropwise with a pipette.
- W 2 (g) mass of top sheet before test
- tackiness of the skin contact surface of the top sheet was measured at 35 ° C. according to the following criteria. ⁇ : No tackiness ⁇ : Some tackiness is present ⁇ : There is tackiness
- FIG. 7 shows an electron micrograph of the skin contact surface of the top sheet in a sanitary napkin where the top sheet contains tri-C2L oil fatty acid glycerides.
- Sanitary napkin No. which does not have a blood slipperiness imparting agent.
- the surface residual rate A was 7.5% by mass, but the kinematic viscosity and water retention rate were within the predetermined ranges. 1-1-No. In 1-21, the surface residual ratio A was 2.5 mass% or less.
- sanitary napkins no. 1-1-No. 1-21 suggests that when a large amount of menstrual blood reaches the top sheet at a time, menstrual blood can be rapidly transferred from the top sheet to the absorber.
- the top sheet is a top sheet having a ridge groove structure manufactured according to the method described in Japanese Patent Application Laid-Open No. 2008-2034.
- the ridge portion has a thickness of about 1.5 mm and the groove portion has a thickness of about 0.00 mm.
- the pitch of the ridge groove structure was about 4 mm, and an opening portion having an opening ratio of about 15% was formed in the groove portion.
- Unistar H-408BRS (manufactured by NOF Corporation, tetraester of pentaerythritol and fatty acid) is selected as a blood slipperiness-imparting agent, and the skin contact surface ( ⁇ ⁇ ⁇ ) of the top sheet from a control seam HMA gun at room temperature.
- the groove surface was coated with a basis weight of 5.0 g / m 2 .
- H-408BRS was in the form of fine particles and adhered to the fiber surface.
- the back sheet, the absorbent body, the second sheet, and the top sheet were sequentially laminated with the groove surface facing up, whereby a sanitary napkin no. 2-1 (i) was formed.
- a top sheet made of a flat, air-through nonwoven fabric composite fiber made of polyester and polyethylene terephthalate, basis weight: 35 g / m 2
- flat hydrophilic agent
- the blood slipperiness-imparting agent was changed from Unistar H-408BRS to that shown in Table 3, and sanitary napkin no. 2-2 (i) -No. 2-11 (i) and No. 2-11. 2-2 (ii) -No. 2-11 (ii) was prepared.
- the blood slipperiness-imparting agent is a liquid at room temperature
- the blood slipperiness-imparting agent is a solid at room temperature
- it is heated to the melting point + 20 ° C., and then a control seam HMA gun is used.
- the blood slipperiness imparting agent was atomized and applied to the skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
- lubricity imparting agent was apply
- Weight of top sheet W 4 (g) (mass of top sheet before test) was measured, and then the horse EDTA blood at 37 ⁇ 1 ° C. was placed on the center top sheet in the longitudinal direction and width direction of the absorbent article. About 0.25 g (2 drops) was dropped from the pipette. In the top sheet having the groin, equine EDTA blood was dropped on the top of the buttock.
- the viscosity of equine defibrinated blood containing 2% by mass of panacet 810s was 5.9 mPa ⁇ s, while the viscosity of equine defibrinated blood not containing a blood slipperiness agent was 50.4 mPa ⁇ s. Therefore, it can be seen that equine defibrinated blood containing 2% by mass of panacet 810s decreases the viscosity by about 90% compared to the case where no blood slipperiness-imparting agent is contained.
- blood contains components such as blood cells and has thixotropic properties, but the blood slipperiness imparting agent of the present disclosure has an action of lowering the viscosity of blood such as menstrual blood in a low viscosity region. It is thought to have. By reducing the viscosity of blood, it is considered that absorbed menstrual blood is easily transferred from the top sheet to the absorber.
- red blood cells form aggregates such as remuneration, but in menstrual blood that includes panacet 810s, red blood cells are each stably dispersed. I understand that. Therefore, it is suggested that the blood slipperiness imparting agent also has a function of stabilizing red blood cells in blood.
- the density ⁇ is defined in 5. of “Density Test Method and Density / Mass / Capacity Conversion Table” of JIS K 2249-1995. Based on the vibration density test method, the temperature was measured as shown in Table 4 below. For measurement, DA-505 of Kyoto Electronics Industry Co., Ltd. was used. The results are shown in Table 4 below.
- the blood slipperiness-imparting agent also has an action of lowering the surface tension of blood.
- the absorbed blood can be quickly transferred to the absorbent body without being held between the fibers of the top sheet.
Abstract
In order to solve the problem of providing an absorbent article that exhibits improved properties for migrating menses from a top sheet towards an absorbing body, and enables residual menses on the top sheet to be reduced, the present invention provides a sanitary napkin (1) including a liquid-permeable top sheet (2), a liquid-impermeable back sheet (3), and an absorbing body (4) disposed between the top sheet (2) and the back sheet (3), wherein a blood-lubricating agent that is coated on protrusions (8) formed in an excretory orifice contact region of the top sheet (2) has a kinetic viscosity of 0.01-80mm2/s at 40°C, a water retention rate of 0.01-4.0 mass%, and a weight-average molecular weight of less than 1,000.
Description
本発明は、吸収性物品に関する。
The present invention relates to an absorbent article.
吸収性物品のトップシートとして、表面が凹凸賦形された不織布及びその製造方法が知られている(特許文献1~4)。
As a top sheet of an absorbent article, a non-woven fabric having an uneven surface and a method for producing the same are known (Patent Documents 1 to 4).
しかしながら、経血は高粘度であるため、トップシートから吸収体に移行しにくく、トップシートに残存しやすい。
そこで、本発明は、トップシートから吸収体への向上した経血移行性を有し、トップシートに残存する経血を低減させることができる吸収性物品を提供することを目的とする。 However, menstrual blood has a high viscosity, so that it does not easily shift from the top sheet to the absorbent body and tends to remain on the top sheet.
Then, an object of this invention is to provide the absorbent article which has the improved menstrual blood transfer property from a top sheet to an absorber, and can reduce the menstrual blood remaining on a top sheet.
そこで、本発明は、トップシートから吸収体への向上した経血移行性を有し、トップシートに残存する経血を低減させることができる吸収性物品を提供することを目的とする。 However, menstrual blood has a high viscosity, so that it does not easily shift from the top sheet to the absorbent body and tends to remain on the top sheet.
Then, an object of this invention is to provide the absorbent article which has the improved menstrual blood transfer property from a top sheet to an absorber, and can reduce the menstrual blood remaining on a top sheet.
上記課題を解決するために、本発明は、肌当接面を有する液透過性のトップシートと、非肌当接面を有する液不透過性のバックシートと、前記トップシート及び前記バックシートの間に設けられた吸収体とを備えた吸収性物品であって、前記トップシートは、前記肌当接面のうち少なくとも排泄口当接領域に、第1の方向及び前記第1の方向と交差する第2の方向に交互に配置された、前記肌当接面側に隆起する複数の凸部と、前記吸収体側に陥没する複数の凹部とを有し、前記複数の凸部は、それぞれ、頂部と、前記頂部から延在する凸部側壁部とを有し、前記複数の凹部は、それぞれ、底部と、前記底部から延在する凹部側壁部とを有し、前記凸部側壁部は、その延在方向に沿った繊維配向性を有し、前記凹部側壁部は、その延在方向に沿った繊維配向性を有し、前記排泄口当接領域のうち少なくとも前記凸部には、40℃における動粘度が0.01~80mm2/s、抱水率が0.01~4.0質量%、重量平均分子量が1,000未満である血液滑性付与剤が塗工されている、前記吸収性物品を提供する。
In order to solve the above problems, the present invention provides a liquid-permeable top sheet having a skin contact surface, a liquid-impermeable back sheet having a non-skin contact surface, the top sheet, and the back sheet. An absorbent article comprising an absorbent body provided therebetween, wherein the top sheet intersects the first direction and the first direction at least in the excretory opening contact region of the skin contact surface. Alternately disposed in the second direction, and has a plurality of convex portions protruding on the skin contact surface side and a plurality of concave portions recessed on the absorber side, wherein the plurality of convex portions are respectively A plurality of concave portions each having a bottom portion and a concave portion side wall portion extending from the bottom portion; It has fiber orientation along the extending direction, and the concave side wall portion extends in the extending direction. Have fiber orientation that Tsu, at least on the convex portion of the excretory opening contact region, kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, water holding rate from 0.01 to 4.0 The absorbent article is provided with a blood slipperiness imparting agent having a mass% and a weight average molecular weight of less than 1,000.
本発明によれば、トップシートから吸収体への向上した経血移行性を有し、トップシートに残存する経血を低減させることができる吸収性物品が提供される。
According to the present invention, there is provided an absorbent article that has improved menstrual blood transfer from the top sheet to the absorbent body and can reduce menstrual blood remaining on the top sheet.
以下、本発明の吸収性物品について説明する。
本発明に係る吸収性物品は、肌当接面を有する液透過性のトップシートと、非肌当接面を有する液不透過性のバックシートと、前記トップシート及び前記バックシートの間に設けられた吸収体とを備えた吸収性物品であって、前記トップシートは、前記肌当接面のうち少なくとも排泄口当接領域に、第1の方向及び前記第1の方向と交差する第2の方向に交互に配置された、前記肌当接面側に隆起する複数の凸部と、前記吸収体側に陥没する複数の凹部とを有し、前記複数の凸部は、それぞれ、頂部と、前記頂部から延在する凸部側壁部とを有し、前記複数の凹部は、それぞれ、底部と、前記底部から延在する凹部側壁部とを有し、前記凸部側壁部は、その延在方向に沿った繊維配向性を有し、前記凹部側壁部は、その延在方向に沿った繊維配向性を有し、前記排泄口当接領域のうち少なくとも前記凸部には、40℃における動粘度が0.01~80mm2/s、抱水率が0.01~4.0質量%、重量平均分子量が1,000未満である血液滑性付与剤が塗工されている、前記吸収性物品である。 Hereinafter, the absorbent article of the present invention will be described.
An absorbent article according to the present invention is provided between a liquid-permeable top sheet having a skin contact surface, a liquid-impermeable back sheet having a non-skin contact surface, and the top sheet and the back sheet. An absorbent article comprising the absorbent body, wherein the top sheet has a first direction and a second direction intersecting the first direction at least in the excretory opening contact region of the skin contact surface. Alternately disposed in the direction of the skin, and has a plurality of protrusions protruding to the skin contact surface side, and a plurality of recesses recessed to the absorber side, the plurality of protrusions, respectively, A plurality of concave portions each having a bottom portion and a concave portion side wall portion extending from the bottom portion, and the convex side wall portion extending from the top portion. Having a fiber orientation along the direction, and the recess side wall portion is a fiber along its extending direction. It has a tropism, wherein at least the convex portion of the excretory opening contact region, kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, water holding percentage of 0.01 to 4.0 mass%, The absorbent article is coated with a blood slipperiness-imparting agent having a weight average molecular weight of less than 1,000.
本発明に係る吸収性物品は、肌当接面を有する液透過性のトップシートと、非肌当接面を有する液不透過性のバックシートと、前記トップシート及び前記バックシートの間に設けられた吸収体とを備えた吸収性物品であって、前記トップシートは、前記肌当接面のうち少なくとも排泄口当接領域に、第1の方向及び前記第1の方向と交差する第2の方向に交互に配置された、前記肌当接面側に隆起する複数の凸部と、前記吸収体側に陥没する複数の凹部とを有し、前記複数の凸部は、それぞれ、頂部と、前記頂部から延在する凸部側壁部とを有し、前記複数の凹部は、それぞれ、底部と、前記底部から延在する凹部側壁部とを有し、前記凸部側壁部は、その延在方向に沿った繊維配向性を有し、前記凹部側壁部は、その延在方向に沿った繊維配向性を有し、前記排泄口当接領域のうち少なくとも前記凸部には、40℃における動粘度が0.01~80mm2/s、抱水率が0.01~4.0質量%、重量平均分子量が1,000未満である血液滑性付与剤が塗工されている、前記吸収性物品である。 Hereinafter, the absorbent article of the present invention will be described.
An absorbent article according to the present invention is provided between a liquid-permeable top sheet having a skin contact surface, a liquid-impermeable back sheet having a non-skin contact surface, and the top sheet and the back sheet. An absorbent article comprising the absorbent body, wherein the top sheet has a first direction and a second direction intersecting the first direction at least in the excretory opening contact region of the skin contact surface. Alternately disposed in the direction of the skin, and has a plurality of protrusions protruding to the skin contact surface side, and a plurality of recesses recessed to the absorber side, the plurality of protrusions, respectively, A plurality of concave portions each having a bottom portion and a concave portion side wall portion extending from the bottom portion, and the convex side wall portion extending from the top portion. Having a fiber orientation along the direction, and the recess side wall portion is a fiber along its extending direction. It has a tropism, wherein at least the convex portion of the excretory opening contact region, kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, water holding percentage of 0.01 to 4.0 mass%, The absorbent article is coated with a blood slipperiness-imparting agent having a weight average molecular weight of less than 1,000.
本発明に係る吸収性物品において、着用者から排泄された経血が排泄口当接領域に到達すると、凸部に存在する血液滑性付与剤とともに滑落し、トップシートを通過して吸収体に移行する。したがって、本発明に係る吸収性物品は、トップシートから吸収体への向上した経血移行性を有し、トップシートに残存する経血を低減させることができる。このため、トップシートの肌当接面のべたつき感が防止され、サラサラ感が維持される。このような血液滑性付与剤の作用効果は、月経時の経血排出量の変化に関わらず(すなわち、一度に排出される経血が大量であっても少量であっても)発揮される。
In the absorbent article according to the present invention, when menstrual blood excreted from the wearer reaches the excretory opening contact region, it slides down together with the blood slipperiness-imparting agent present in the convex portion, passes through the top sheet, and becomes an absorbent body. Transition. Therefore, the absorbent article according to the present invention has improved menstrual blood transfer from the top sheet to the absorbent body, and can reduce menstrual blood remaining on the top sheet. For this reason, the sticky feeling of the skin contact surface of the top sheet is prevented, and the smooth feeling is maintained. The effect of such a blood slipperiness-imparting agent is exerted irrespective of changes in menstrual blood discharge (that is, whether menstrual blood discharged at a time is large or small). .
本発明に係る吸収性物品において、凸部側壁部は、凸部の頂部を起点として凹部の底部に向かって延在しており、凹部側壁部は、凹部の底部を起点として凸部の頂部に向かって延在している。したがって、凸部側壁部は、その延在方向、すなわち、凸部の頂部から凹部の底部に向かう方向に沿った繊維配向性を有し、凹部側壁部は、その延在方向、すなわち、凹部の底部から凸部の頂部に向かう方向に沿った繊維配向性を有する。このため、着用者から排泄された経血は、血液滑性付与剤とともに、凸部側壁部及び凹部側壁部の延在方向に沿って滑落しやすい。言い換えれば、着用者から排泄された経血は、トップシートの面方向に広がることなく、トップシートの厚み方向に(すなわち吸収体に)移行しやすい。
In the absorbent article according to the present invention, the convex side wall portion extends from the top of the convex portion toward the bottom of the concave portion, and the concave side wall portion starts from the bottom of the concave portion at the top of the convex portion. It extends towards. Therefore, the convex side wall portion has a fiber orientation along the extending direction, that is, the direction from the top of the convex portion toward the bottom of the concave portion, and the concave side wall portion is in the extending direction, that is, the concave portion. It has fiber orientation along the direction from the bottom toward the top of the convex portion. For this reason, menstrual blood excreted from the wearer easily slides along the extending direction of the convex side wall portion and the concave side wall portion together with the blood slipperiness imparting agent. In other words, menstrual blood excreted from the wearer does not spread in the surface direction of the top sheet, but easily moves in the thickness direction of the top sheet (that is, to the absorber).
本発明に係る吸収性物品において、凸部側壁部及び凹部側壁部は、それぞれの延在方向に沿った繊維配向性を有するので、トップシートの厚み方向の繊維配向性が大きく、これに起因してトップシートの圧縮変形性及び圧縮回復性(クッション性)が高い。したがって、吸収性物品が圧縮されても、吸収体からの液戻り(リウェットバック)が生じにくい。また、トップシートのクッション性が高いため、着用者に良好な着け心地を与える。
In the absorbent article according to the present invention, the convex side wall portion and the concave side wall portion have fiber orientation along each extending direction, and therefore the fiber orientation in the thickness direction of the top sheet is large, resulting from this. The top sheet has high compression deformation and compression recovery properties (cushioning properties). Therefore, even if the absorbent article is compressed, liquid return (rewetting back) from the absorbent body hardly occurs. Moreover, since the cushioning property of a top sheet is high, it gives a wearer good comfort.
本発明に係る吸収性物品において、トップシートの肌当接面が凹凸形状であるので、着用者の肌とトップシートとの接触面積が少ないとともに、凹部内の空間を通じたトップシートの面方向の通気性が良好である。したがって、着用者にムレ及びそれに起因する痒みを与えにくい。
In the absorbent article according to the present invention, since the skin contact surface of the top sheet has an uneven shape, the contact area between the wearer's skin and the top sheet is small, and the surface direction of the top sheet through the space in the recess is reduced. Good air permeability. Therefore, it is difficult to give the wearer stuffiness and itching.
本発明に係る吸収性物品の一態様(態様1)では、前記凸部の頂部の繊維密度が、前記凹部の底部の繊維密度よりも低い。液体は繊維密度が低い方(態様1における凸部の頂部)から高い方(態様1における凹部の底部)へ移行しやすいので、態様1に係る吸収性物品は、血液滑性付与剤の作用効果と相俟って、トップシートから吸収体への向上した経血移行性を有する。
In one aspect (Aspect 1) of the absorbent article according to the present invention, the fiber density at the top of the convex portion is lower than the fiber density at the bottom of the concave portion. Since the liquid is likely to move from the lower fiber density (the top of the convex portion in aspect 1) to the higher (the bottom of the concave part in aspect 1), the absorbent article according to aspect 1 has the effect of the blood slipperiness-imparting agent. In combination with the above, it has improved menstrual transfer from the top sheet to the absorbent body.
本発明に係る吸収性物品の一態様(態様2)では、前記凸部の頂部の繊維密度が、前記凹部の底部の繊維密度よりも高い。液体は繊維密度が高い方(態様2における凸部の頂部)から低い方(態様2における凹部の底部)へ移行しにくいが、態様2に係る吸収性物品は、血液滑性付与剤の作用効果によって、トップシートから吸収体への向上した経血移行性を有する。したがって、血液滑性付与剤の作用効果は、態様2に係る吸収性物品において顕著である。また、態様2に係る吸収性物品では、着用者の肌に対するトップシートの凹凸形状の追従性が良好であるので、着用者が足を組み替えたり歩いたりする際の着け心地が良好である。
In one aspect (Aspect 2) of the absorbent article according to the present invention, the fiber density at the top of the convex portion is higher than the fiber density at the bottom of the concave portion. The liquid is less likely to move from the higher fiber density (the top of the convex part in aspect 2) to the lower (the bottom of the concave part in aspect 2), but the absorbent article according to aspect 2 has the effect of the blood slipperiness-imparting agent. Therefore, it has the improved menstrual blood transfer property from a top sheet to an absorber. Therefore, the effect of the blood slipperiness-imparting agent is significant in the absorbent article according to aspect 2. Moreover, in the absorbent article which concerns on aspect 2, since the followable | trackability of the uneven | corrugated shape of a top sheet with respect to a wearer's skin is favorable, a wearer's comfort at the time of changing a leg or walking is favorable.
本発明に係る吸収性物品の一態様(態様3)では、前記凸部側壁部及び/又は前記凹部側壁部が、繊維配向性の異なる部分を有する。態様3は、態様1又は態様2と組み合わせることができる。
In one aspect (Aspect 3) of the absorbent article according to the present invention, the convex side wall part and / or the concave side wall part have portions having different fiber orientations. Aspect 3 can be combined with Aspect 1 or Aspect 2.
本発明に係る吸収性物品の一態様(態様4)では、前記凹部の底部における厚さ方向の繊維配向性が、前記凹部側壁部における厚さ方向の繊維配向性よりも小さい。態様4に係る吸収性物品では、経血が凹部側壁部に溜まることなく厚さ方向に(すなわち吸収体に)移行しやすい。なお、凹部における繊維配向性は、吸収体に近づくにつれて、厚さ方向と垂直な方向に変化することが好ましく、これにより、繊維配向性に勾配が生じ、経血が透過及び拡散しやすくなる。態様4は、態様1~態様3のうちの1つの態様又は並立し得る2以上の態様と組み合わせることができる。
In one aspect (Aspect 4) of the absorbent article according to the present invention, the fiber orientation in the thickness direction at the bottom of the recess is smaller than the fiber orientation in the thickness direction at the sidewall of the recess. In the absorbent article which concerns on aspect 4, menstrual blood is easy to transfer to a thickness direction (namely, to an absorber), without accumulating in a recessed part side wall part. In addition, it is preferable that the fiber orientation in a recessed part changes to the direction perpendicular | vertical to a thickness direction as it approaches an absorber, and thereby, a gradient arises in fiber orientation and menstrual blood becomes easy to permeate | transmit and diffuse. Aspect 4 can be combined with one of Aspects 1 to 3, or two or more aspects that can be arranged side by side.
本発明に係る吸収性物品の一態様(態様5)では、前記凸部の頂部における繊維融着点数が、前記凹部の底部における繊維融着点数よりも小さい。態様5に係る吸収性物品では、トップシートの肌触りが良好である。態様5は、態様1~態様4のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。
In one aspect (Aspect 5) of the absorbent article according to the present invention, the number of fiber fusion points at the top of the convex portion is smaller than the number of fiber fusion points at the bottom of the concave portion. In the absorbent article which concerns on aspect 5, the touch of a top sheet is favorable. Aspect 5 can be combined with one of aspects 1 to 4 or two or more aspects that can be arranged side by side.
本発明に係る吸収性物品の一態様(態様6)では、前記トップシートの構成繊維が熱融着している。態様6に係る吸収性物品では、トップシートの凹凸形状が、構成繊維の熱融着によって維持されている。また、吸収性物品の使用時に、トップシートからの繊維抜け及び毛羽立ちが生じにくい。態様6は、態様1~態様5のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。
In one aspect (Aspect 6) of the absorbent article according to the present invention, the constituent fibers of the top sheet are heat-sealed. In the absorbent article which concerns on aspect 6, the uneven | corrugated shape of a top sheet is maintained by the heat sealing | fusion of the constituent fiber. Further, when the absorbent article is used, it is difficult for fibers to fall out and fluff from the top sheet. Aspect 6 can be combined with one of aspects 1 to 5 or two or more aspects that can be arranged side by side.
本発明に係る吸収性物品の一態様(態様7)では、前記トップシートが、熱伸長した熱伸長性繊維を含有する。熱伸長性繊維の熱伸長により、効果的な凹凸賦形が可能となる。また、熱伸長性繊維の熱伸長により、凹凸賦形時に生じるおそれがる凸部側壁部及び凹部側壁部の繊維密度及び繊維量(坪量)の低下が防止される。態様7は、態様1~態様6のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。
In one aspect (Aspect 7) of the absorbent article according to the present invention, the top sheet contains heat-extensible heat-extensible fibers. Due to the thermal elongation of the heat-extensible fibers, effective uneven shaping is possible. Moreover, the heat | fever expansion | extension of a heat | fever extensible fiber prevents the fall of the fiber density and fiber amount (basis weight) of a convex part side wall part and a concave part side wall part which may arise at the time of uneven | corrugated shaping. Aspect 7 can be combined with one of aspects 1 to 6 or two or more aspects that can be arranged side by side.
本発明に係る吸収性物品の一態様(態様8)では、前記血液滑性付与剤のIOBが、0.00~0.60のIOBである。態様8は、態様1~態様7のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。
In one aspect (Aspect 8) of the absorbent article according to the present invention, the IOB of the blood slipperiness imparting agent is an IOB of 0.00 to 0.60. Aspect 8 can be combined with one of Aspects 1 to 7, or two or more aspects that can be juxtaposed.
本発明に係る吸収性物品の一態様(態様9)では、前記血液滑性付与剤が、次の(i)~(iii):
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、及び
(iii) (iii-1)炭化水素部分と、(iii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基と、(iii-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される(ここで、(ii)又は(iii)の化合物において、オキシ基が2つ以上挿入されている場合には、各オキシ基は隣接していない)。態様9は、態様1~態様8のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。 In one aspect (Aspect 9) of the absorbent article according to the present invention, the blood slipperiness imparting agent includes the following (i) to (iii):
(I) hydrocarbons,
(Ii) from (ii-1) a hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety. A compound having one or more identical or different groups selected from the group consisting of: (iii) (iii-1) a hydrocarbon moiety and (iii-2) a CC single bond of said hydrocarbon moiety One or more identical or different groups selected from the group consisting of a carbonyl group (—CO—) and an oxy group (—O—), and (iii-3) the hydrocarbon moiety A compound having one or a plurality of the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which replaces a hydrogen atom;
Selected from the group consisting of any combination thereof (in the compound (ii) or (iii), when two or more oxy groups are inserted, each oxy group is not adjacent) ).Aspect 9 can be combined with one of aspects 1 to 8 or two or more aspects that can be arranged side by side.
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、及び
(iii) (iii-1)炭化水素部分と、(iii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基と、(iii-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される(ここで、(ii)又は(iii)の化合物において、オキシ基が2つ以上挿入されている場合には、各オキシ基は隣接していない)。態様9は、態様1~態様8のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。 In one aspect (Aspect 9) of the absorbent article according to the present invention, the blood slipperiness imparting agent includes the following (i) to (iii):
(I) hydrocarbons,
(Ii) from (ii-1) a hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety. A compound having one or more identical or different groups selected from the group consisting of: (iii) (iii-1) a hydrocarbon moiety and (iii-2) a CC single bond of said hydrocarbon moiety One or more identical or different groups selected from the group consisting of a carbonyl group (—CO—) and an oxy group (—O—), and (iii-3) the hydrocarbon moiety A compound having one or a plurality of the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which replaces a hydrogen atom;
Selected from the group consisting of any combination thereof (in the compound (ii) or (iii), when two or more oxy groups are inserted, each oxy group is not adjacent) ).
本発明に係る吸収性物品の一態様(態様10)では、前記血液滑性付与剤が、次の(i’)~(iii’):
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合とを有する化合物、及び
(iii’) (iii’-1)炭化水素部分と、(iii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合と、(iii’-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される(ここで、(ii’)又は(iii’)の化合物において、2以上の同一又は異なる結合が挿入されている場合には、各結合は隣接していない)。態様10は、態様1~態様9のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。 In one aspect (Aspect 10) of the absorbent article according to the present invention, the blood slipperiness imparting agent includes the following (i ′) to (iii ′):
(I ′) hydrocarbon,
(Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—), an ester bond (—COO) inserted between the C—C single bonds of the hydrocarbon moiety. -), A carbonate bond (-OCOO-), and an ether bond (-O-) selected from the group consisting of one or more compounds having the same or different bonds, and (iii ') (iii'- 1) a hydrocarbon moiety and (iii′-2) a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (—OCOO) inserted between the CC single bonds of the hydrocarbon moiety. -), And one or a plurality of the same or different bonds selected from the group consisting of an ether bond (-O-) and (iii'-3) a carboxyl group that replaces the hydrogen atom of the hydrocarbon moiety ( -COOH) and hydroxy A compound having one or a plurality of the same or different groups selected from the group consisting of a sil group (—OH);
As well as any combination thereof (wherein (ii ′) or (iii ′) compounds wherein two or more identical or different bonds are inserted, each bond is adjacent) Not) Aspect 10 can be combined with one of Aspects 1-9 or two or more aspects that can be juxtaposed.
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合とを有する化合物、及び
(iii’) (iii’-1)炭化水素部分と、(iii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合と、(iii’-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される(ここで、(ii’)又は(iii’)の化合物において、2以上の同一又は異なる結合が挿入されている場合には、各結合は隣接していない)。態様10は、態様1~態様9のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。 In one aspect (Aspect 10) of the absorbent article according to the present invention, the blood slipperiness imparting agent includes the following (i ′) to (iii ′):
(I ′) hydrocarbon,
(Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—), an ester bond (—COO) inserted between the C—C single bonds of the hydrocarbon moiety. -), A carbonate bond (-OCOO-), and an ether bond (-O-) selected from the group consisting of one or more compounds having the same or different bonds, and (iii ') (iii'- 1) a hydrocarbon moiety and (iii′-2) a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (—OCOO) inserted between the CC single bonds of the hydrocarbon moiety. -), And one or a plurality of the same or different bonds selected from the group consisting of an ether bond (-O-) and (iii'-3) a carboxyl group that replaces the hydrogen atom of the hydrocarbon moiety ( -COOH) and hydroxy A compound having one or a plurality of the same or different groups selected from the group consisting of a sil group (—OH);
As well as any combination thereof (wherein (ii ′) or (iii ′) compounds wherein two or more identical or different bonds are inserted, each bond is adjacent) Not) Aspect 10 can be combined with one of Aspects 1-9 or two or more aspects that can be juxtaposed.
本発明に係る吸収性物品の一態様(態様11)では、前記血液滑性付与剤が、次の(A)~(F):
(A) (A1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、前記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル、及び
(F)鎖状炭化水素、
並びにそれらの任意の組み合わせから成る群から選択される。態様11は、態様1~態様10のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。 In one aspect (Aspect 11) of the absorbent article according to the present invention, the blood slipperiness imparting agent includes the following (A) to (F):
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain An ester with a compound having one carboxyl group for substituting a hydrogen atom in the hydrocarbon moiety,
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety,
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety; C2) an ester of a chain hydrocarbon moiety and a compound having one hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety,
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the CC single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—),
(E) polyoxy C 3 -C 6 alkylene glycol, or an alkyl ester or alkyl ether thereof, and (F) a chain hydrocarbon,
As well as any combination thereof. Aspect 11 can be combined with one ofAspects 1 to 10 or two or more aspects that can be juxtaposed.
(A) (A1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、前記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル、及び
(F)鎖状炭化水素、
並びにそれらの任意の組み合わせから成る群から選択される。態様11は、態様1~態様10のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。 In one aspect (Aspect 11) of the absorbent article according to the present invention, the blood slipperiness imparting agent includes the following (A) to (F):
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain An ester with a compound having one carboxyl group for substituting a hydrogen atom in the hydrocarbon moiety,
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety,
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety; C2) an ester of a chain hydrocarbon moiety and a compound having one hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety,
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the CC single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—),
(E) polyoxy C 3 -C 6 alkylene glycol, or an alkyl ester or alkyl ether thereof, and (F) a chain hydrocarbon,
As well as any combination thereof. Aspect 11 can be combined with one of
本発明に係る吸収性物品の一態様(態様12)では、前記血液滑性付与剤が、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル、(b1)鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテル、(b2)鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテル、(b3)鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテル、(c1)4個のカルボキシル基を有する鎖状炭化水素テトラカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、(d4)ジアルキルカーボネート、(e1)ポリオキシC3~C6アルキレングリコール、(e2)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル、及び(f1)鎖状アルカン、並びにそれらの任意の組み合わせから成る群から選択される。態様12は、態様1~態様11のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。
In one aspect (Aspect 12) of the absorbent article according to the present invention, the blood slipperiness imparting agent is (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) a chain carbonization. An ester of hydrogen triol and at least one fatty acid, (a 3 ) an ester of a chain hydrocarbon diol and at least one fatty acid, and (b 1 ) a chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol. An ether, (b 2 ) an ether of a chain hydrocarbon triol and at least one aliphatic monohydric alcohol, (b 3 ) an ether of a chain hydrocarbon diol and at least one aliphatic monohydric alcohol, (c 1 ) An ester of a chain hydrocarbon tetracarboxylic acid having 4 carboxyl groups, a hydroxy acid, an alkoxy acid or an oxo acid and at least one aliphatic monohydric alcohol; 2) a chain hydrocarbon tricarboxylic acids having 3 carboxyl groups, hydroxy acid, alkoxy acid or oxoacid, chains having at least one ester of an aliphatic monohydric alcohol, (c 3) 2 carboxyl groups Ester of a hydrocarbon dicarboxylic acid, hydroxy acid, alkoxy acid or oxo acid and at least one aliphatic monohydric alcohol, (d 1 ) an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, (d 2 ) Dialkyl ketone, (d 3 ) ester of fatty acid and aliphatic monohydric alcohol, (d 4 ) dialkyl carbonate, (e 1 ) polyoxy C 3 -C 6 alkylene glycol, (e 2 ) polyoxy C 3 -C 6 alkylene esters of glycol and at least one fatty acid, the least (e 3) polyoxy C 3 ~ C 6 alkylene glycol One aliphatic monohydric ethers of alcohols, and (f 1) a chain alkane, and is selected from the group consisting of any combination thereof. Aspect 12 can be combined with one of aspects 1 to 11 or two or more aspects that can be juxtaposed.
本発明に係る吸収性物品の一態様(態様13)では、前記血液滑性付与剤が、1気圧及び40℃において、0.00~0.01Paの蒸気圧を有する。態様13は、態様1~態様12のうちの1つの態様又は並立し得る2つ以上の態様と組み合わせることができる。
In one aspect (Aspect 13) of the absorbent article according to the present invention, the blood slipperiness imparting agent has a vapor pressure of 0.00 to 0.01 Pa at 1 atm and 40 ° C. Aspect 13 can be combined with one of Aspects 1-12 or two or more aspects that can be juxtaposed.
本発明の吸収性物品の種類及び用途は特に限定されない。吸収性物品としては、例えば、生理用ナプキン、パンティーライナー等の衛生用品・生理用品が挙げられ、これらはヒトを対象としてもよいし、ペット等のヒト以外の動物を対象としてもよい。吸収性物品が吸収対象とする液体は特に限定されないが、主として、経血等の液状排泄物である。
以下、生理用ナプキンを例とし、図面に基づいて、本発明の吸収性物品の実施形態を説明する。 The kind and application of the absorbent article of the present invention are not particularly limited. Examples of the absorbent article include sanitary products and sanitary products such as sanitary napkins and panty liners, and these may be used for humans and non-human animals such as pets. The liquid to be absorbed by the absorbent article is not particularly limited, but is mainly liquid excrement such as menstrual blood.
Hereinafter, an embodiment of the absorbent article of the present invention will be described based on the drawings, taking a sanitary napkin as an example.
以下、生理用ナプキンを例とし、図面に基づいて、本発明の吸収性物品の実施形態を説明する。 The kind and application of the absorbent article of the present invention are not particularly limited. Examples of the absorbent article include sanitary products and sanitary products such as sanitary napkins and panty liners, and these may be used for humans and non-human animals such as pets. The liquid to be absorbed by the absorbent article is not particularly limited, but is mainly liquid excrement such as menstrual blood.
Hereinafter, an embodiment of the absorbent article of the present invention will be described based on the drawings, taking a sanitary napkin as an example.
本発明の吸収性物品の一実施形態に係る生理用ナプキン1は、図1及び図2に示すように、液透過性のトップシート2と、液不透過性のバックシート3と、トップシート2及びバックシート3の間に設けられた吸収体4とを備える。
As shown in FIGS. 1 and 2, a sanitary napkin 1 according to an embodiment of the absorbent article of the present invention includes a liquid-permeable top sheet 2, a liquid-impermeable back sheet 3, and a top sheet 2. And an absorbent body 4 provided between the back sheets 3.
図1において、X軸方向は生理用ナプキン1の幅方向に、Y軸方向は生理用ナプキン1の長手方向に、X軸Y軸方向に広がる平面の方向は生理用ナプキン1の平面方向に相当する。他の図においても同様である。
In FIG. 1, the X-axis direction corresponds to the width direction of the sanitary napkin 1, the Y-axis direction corresponds to the longitudinal direction of the sanitary napkin 1, and the plane direction extending in the X-axis Y-axis direction corresponds to the plane direction of the sanitary napkin 1. To do. The same applies to the other drawings.
生理用ナプキン1は、経血等の液状排泄物を吸収する目的で着用される。この際、トップシート2が着用者の肌側に、バックシート3が着用者の着衣(下着)側に位置するように着用される。経血等の液状排泄物は、トップシート2を透過して吸収体4に至り、吸収体4で吸収・保持される。吸収体4で吸収・保持される液状排泄物の漏れは、バックシート3によって防止される。
The sanitary napkin 1 is worn for the purpose of absorbing liquid excretion such as menstrual blood. At this time, the top sheet 2 is worn on the skin side of the wearer, and the back sheet 3 is worn on the clothes (underwear) side of the wearer. Liquid excreta such as menstrual blood passes through the top sheet 2 to reach the absorber 4 and is absorbed and held by the absorber 4. Leakage of liquid excretion absorbed and held by the absorber 4 is prevented by the back sheet 3.
図1に示すように、トップシート2及びバックシート3は、長手方向の端部同士がシール部11a,11bによって接合され、本体部6を形成するとともに、幅方向の端部同士がシール部12a,12bによって接合され、本体部6から幅方向に延出する略矩形状のウイング部7a,7bを形成している。
As shown in FIG. 1, the top sheet 2 and the back sheet 3 are joined at their longitudinal ends by seal portions 11 a and 11 b to form a main body portion 6, and end portions in the width direction are sealed portions 12 a. , 12b and substantially rectangular wings 7a, 7b extending in the width direction from the main body 6 are formed.
本体部6の形状は、着用者の身体、下着等に適合する範囲で適宜調整することができ、本体部6の形状としては、例えば、略長方形、略楕円形、略瓢箪形等が挙げられる。本体部6の長手方向の延べ寸法は、通常100~500mm、好ましくは150~350mmであり、本体部6の幅方向の延べ寸法は、通常30~200mm、好ましくは40~180mmである。
The shape of the main body portion 6 can be adjusted as appropriate within a range suitable for the wearer's body, underwear, etc. Examples of the shape of the main body portion 6 include a substantially rectangular shape, a substantially oval shape, and a generally saddle shape. . The total length in the longitudinal direction of the main body 6 is usually 100 to 500 mm, preferably 150 to 350 mm. The total length in the width direction of the main body 6 is usually 30 to 200 mm, preferably 40 to 180 mm.
シール部11a,11b,12a,12bによる接合様式としては、例えば、エンボス加工、超音波、ホットメルト型接着剤等が挙げられる。接合強度を高めるために、2種以上の接合様式を組み合わせてもよい(例えば、ホットメルト型接着剤による接合後に、エンボス加工を施す等)。
Examples of the bonding mode by the seal portions 11a, 11b, 12a, and 12b include embossing, ultrasonic waves, hot-melt adhesives, and the like. In order to increase the bonding strength, two or more bonding modes may be combined (for example, embossing is performed after bonding with a hot-melt adhesive).
エンボス加工としては、例えば、パターニングされた凸部を有するエンボスロールとフラットロールとの間に、トップシート2及びバックシート3を合わせて通過させてエンボス加工する方法(いわゆるラウンドシールと呼ばれる方法)等が挙げられる。この方法では、エンボスロール及び/又はフラットロールの加熱により、各シートが軟化するため、シール部が明瞭になりやすい。エンボスパターンとしては、例えば、格子状パターン、千鳥状パターン、波状パターン等が挙げられる。
As the embossing, for example, a method of embossing by passing the top sheet 2 and the back sheet 3 together between an embossing roll having a patterned convex portion and a flat roll (a method called a so-called round seal), etc. Is mentioned. In this method, since each sheet is softened by heating the embossing roll and / or the flat roll, the seal portion tends to be clear. Examples of the emboss pattern include a lattice pattern, a staggered pattern, and a wavy pattern.
ホットメルト接着剤としては、例えば、スチレン-エチレン-ブタジエン-スチレン(SEBS)、スチレン-ブタジエン-スチレン(SBS)、スチレン-イソプレン-スチレン(SIS)等のゴム系を主体とした、又は直鎖状低密度ポリエチレン等のオレフィン系を主体とした感圧型接着剤又は感熱型接着剤;水溶性高分子(例えば、ポリビニルアルコール、カルボキシルメチルセルロース、ゼラチン等)又は水膨潤性高分子(例えば、ポリビニルアセテート、ポリアクリル酸ナトリウム等)からなる感水性接着剤等が挙げられる。接着剤の塗工方法としては、例えば、スパイラル塗工、コーター塗工、カーテンコーター塗工、サミットガン塗工等が挙げられる。
As the hot melt adhesive, for example, styrene-ethylene-butadiene-styrene (SEBS), styrene-butadiene-styrene (SBS), styrene-isoprene-styrene (SIS), or the like, or linear Pressure-sensitive adhesives or heat-sensitive adhesives mainly composed of olefins such as low density polyethylene; water-soluble polymers (for example, polyvinyl alcohol, carboxymethyl cellulose, gelatin, etc.) or water-swellable polymers (for example, polyvinyl acetate, poly And water sensitive adhesives such as sodium acrylate). Examples of the method for applying the adhesive include spiral coating, coater coating, curtain coater coating, and summit gun coating.
図2に示すように、ウイング部7a,7bを形成するバックシート3の着衣側には、粘着部13a,13bが設けられており、本体部6を形成するバックシート3の着衣側には、粘着部13cが設けられている。粘着部13cが下着のクロッチ部に貼付されるとともに、ウイング部7a,7bが下着の外面側に折り曲げられ、粘着部13a,13bが下着のクロッチ部に貼付されることにより、生理用ナプキン1は下着に安定して固定される。
As shown in FIG. 2, adhesive portions 13 a and 13 b are provided on the clothing side of the backsheet 3 that forms the wing portions 7 a and 7 b, and on the clothing side of the backsheet 3 that forms the main body portion 6, An adhesive portion 13c is provided. The sanitary napkin 1 is obtained by attaching the adhesive part 13c to the crotch part of the underwear, bending the wing parts 7a and 7b to the outer surface side of the underwear, and attaching the adhesive parts 13a and 13b to the crotch part of the underwear. Stablely fixed to underwear.
粘着部13a,13b,13cに含有される粘着剤としては、例えば、スチレン-エチレン-ブチレン-スチレンブロック共重合体、スチレン-ブチレン重合体、スチレン-ブチレン-スチレンブロック共重合体、スチレン-イソブチレン-スチレン共重合体等のスチレン系ポリマー;C5系石油樹脂、C9系石油樹脂、ジシクロペンタジエン系石油樹脂、ロジン系石油樹脂、ポリテルペン樹脂、テルペンフェノール樹脂等の粘着付与剤;リン酸トリフレシル、フタル酸ジブチル、フタル酸ジオクチル等のモノマー可塑剤;ビニル重合体、ポリエステル等のポリマー可塑剤等が挙げられる。
Examples of the adhesive contained in the adhesive portions 13a, 13b, and 13c include styrene-ethylene-butylene-styrene block copolymers, styrene-butylene polymers, styrene-butylene-styrene block copolymers, and styrene-isobutylene- Styrene polymers such as styrene copolymers; tackifiers such as C5 petroleum resins, C9 petroleum resins, dicyclopentadiene petroleum resins, rosin petroleum resins, polyterpene resins, terpene phenol resins; trifresyl phosphate, phthalic acid Examples include monomer plasticizers such as dibutyl and dioctyl phthalate; polymer plasticizers such as vinyl polymer and polyester.
トップシート2は、経血等の液状排泄物が透過できるシートである。トップシート2の一方の面(図2において上面)は、着用者の肌が当接する肌当接面となっており、他方の面(図2において下面)は、吸収体4が配置される吸収体配置面となっている。
The top sheet 2 is a sheet through which liquid excretion such as menstrual blood can pass. One surface (upper surface in FIG. 2) of the top sheet 2 is a skin contact surface with which the wearer's skin comes into contact, and the other surface (lower surface in FIG. 2) is an absorption on which the absorber 4 is disposed. It is a body placement surface.
トップシート2としては、例えば、不織布、液透過孔が形成され合成樹脂フィルム、合成樹脂フィルムと不織布との積層体等が挙げられるが、好ましくは、不織布である。不織布としては、例えば、エアースルー不織布、ヒートボンド不織布、スパンボンド不織布、メルトブローン不織布、スパンレース不織布、ニードルパンチ不織布等が挙げられる。
Examples of the top sheet 2 include a non-woven fabric, a synthetic resin film in which liquid permeation holes are formed, and a laminate of a synthetic resin film and a non-woven fabric, and a non-woven fabric is preferable. As a nonwoven fabric, an air through nonwoven fabric, a heat bond nonwoven fabric, a spun bond nonwoven fabric, a melt blown nonwoven fabric, a spunlace nonwoven fabric, a needle punch nonwoven fabric, etc. are mentioned, for example.
トップシート2は、構成繊維として熱融着性繊維を含有する。トップシート2の構成繊維は、熱融着性繊維の溶融固化によって熱融着しており、これにより、トップシート2の凹凸形状が保持されている。また、生理用ナプキン1の使用時に、トップシート2からの繊維抜け及び毛羽立ちが生じにくい。
The top sheet 2 contains a heat-fusible fiber as a constituent fiber. The constituent fibers of the top sheet 2 are heat-sealed by melting and solidifying the heat-fusible fibers, whereby the uneven shape of the top sheet 2 is maintained. Further, when the sanitary napkin 1 is used, it is difficult for fibers to fall out and fluff from the top sheet 2.
熱融着性繊維としては、例えば、ポリオレフィン、ポリエステル、ポリアミド等の熱可塑性樹脂で構成された熱融着性繊維が挙げられる。ポリオレフィンとしては、例えば、直鎖低密度ポリエチレン(LLDPE)、低密度ポリエチレン(LDPE)、中密度ポリエチレン(MDPE)、高密度ポリエチレン(HDPE)、ポリプロピレン、ポリブチレン、これらを主体とした共重合体(例えば、エチレン-酢酸ビニル共重合体(EVA)、エチレン-アクリル酸エチル共重合体(EEA)、エチレン-アクリル酸共重合体(EAA)、エチレン-プロピレンランダム共重合体(EP))等が挙げられる。軟化点が100℃前後と比較的低いので熱加工性に優れる点、並びに、剛性が低く、しなやかな触感である点から、ポリエチレン、特にHDPEが好ましい。ポリエステルとしては、例えば、ポリエチレンタレフタレート(PET)、ポリトリメチレテレフタレート(PTT)、ポリブチレンテレタレート(PBT)、ポリ乳酸、ポリグリコール酸をはじめとする直鎖状又は分岐状の炭素数20までのポリヒドロキシアルカン酸等のポリエステル、これらを主体とした共重合体、アルキレンテレフタレートを主成分として他の成分を少量共重合してなる共重合ポリエステル等が挙げられる。弾性反発性を有するのでクッション性が高い繊維及び不織布を構成することが可能である点、並びに工業的に安価に得られるという経済的な点から、PETが好ましい。ポリアミドとしては、例えば、6-ナイロン、6,6-ナイロン等が挙げられる。
Examples of the heat-fusible fiber include a heat-fusible fiber made of a thermoplastic resin such as polyolefin, polyester, or polyamide. Examples of the polyolefin include, for example, linear low density polyethylene (LLDPE), low density polyethylene (LDPE), medium density polyethylene (MDPE), high density polyethylene (HDPE), polypropylene, polybutylene, and copolymers based on these (for example, Ethylene-vinyl acetate copolymer (EVA), ethylene-ethyl acrylate copolymer (EEA), ethylene-acrylic acid copolymer (EAA), ethylene-propylene random copolymer (EP)), and the like. . Polyethylene, particularly HDPE, is preferred because it has a relatively low softening point of around 100 ° C. and is excellent in heat workability, and has low rigidity and a supple feel. Examples of the polyester include linear or branched carbon atoms of up to 20 including polyethylene terephthalate (PET), polytrimethyl terephthalate (PTT), polybutylene terephthalate (PBT), polylactic acid, and polyglycolic acid. And polyesters such as polyhydroxyalkanoic acid, copolymers based on these, and copolymerized polyesters obtained by copolymerizing alkylene terephthalate as a main component with a small amount of other components. Since it has elastic resilience, it is possible to construct fibers and nonwoven fabrics having high cushioning properties, and PET is preferred from the economical point of being obtained industrially at a low cost. Examples of the polyamide include 6-nylon and 6,6-nylon.
熱融着性繊維の繊維径は、通常5~100μm、好ましくは10~40μmであり、熱融着性繊維の繊度は、通常0.5~10dtex、好ましくは1~5dtexであり、熱融着性繊維の含有量は、トップシート2の構成繊維全体の通常10~100質量%、好ましくは50~100質量%である。
The fiber diameter of the heat-fusible fiber is usually 5 to 100 μm, preferably 10 to 40 μm, and the fineness of the heat-fusible fiber is usually 0.5 to 10 dtex, preferably 1 to 5 dtex. The content of the conductive fiber is usually 10 to 100% by mass, preferably 50 to 100% by mass, based on the entire constituent fibers of the top sheet 2.
トップシート2は、構成繊維として、熱伸長した熱伸長性繊維を含有することが好ましい。熱伸長性繊維の熱伸長により、効果的な凹凸賦形が可能となる。また、熱伸長性繊維の熱伸長により、凹凸賦形時に生じるおそれがる凸部側壁部82及び凹部側壁部92の繊維密度及び繊維量(坪量)の低下が防止される。熱伸長性繊維は、加熱処理により実際の繊維長が伸長する繊維(例えば、樹脂の結晶状態が変化して実際の繊維長が伸長する繊維)であってもよいし、加熱処理により実際の繊維長は伸長しないが、見かけ上の繊維長が伸長する繊維(例えば、発現していたジクザク状、Ω状、スパイラル状等の捲縮が解除されて見かけ上の繊維長が伸長する捲縮繊維)であってもよい。
The top sheet 2 preferably contains a thermally stretchable fiber as a constituent fiber. Due to the thermal elongation of the heat-extensible fibers, effective uneven shaping is possible. Moreover, the heat | fever elongation of a heat | fever extensible fiber prevents the fall of the fiber density and fiber amount (basis weight) of the convex part side wall part 82 and the concave part side wall part 92 which may arise at the time of uneven | corrugated shaping. The heat-extensible fiber may be a fiber whose actual fiber length is elongated by heat treatment (for example, a fiber whose actual fiber length is elongated due to a change in the crystalline state of the resin). Fibers that do not elongate but have an apparent fiber length (for example, crimped fibers in which the apparent fiber length is elongated by releasing the crimps such as zigzag, Ω, and spiral) It may be.
様々な熱伸長性繊維が公知であり(例えば、特開2004-218183号公報、特開2005-350836号公報、特開2007-303035号公報、特開2007-204899号公報、特開2007-204901号公報、特開2007-204902号公報、特開2008-101285号公報等)、適宜選択して使用することができる。
Various heat-extensible fibers are known (for example, JP-A No. 2004-218183, JP-A No. 2005-350836, JP-A No. 2007-303035, JP-A No. 2007-204899, JP-A No. 2007-204901). No. 2007, No. 2007-204902, No. 2008-101285, etc.) can be appropriately selected and used.
熱伸長性繊維としては、例えば、融点又は軟化点の異なる2種類の樹脂(融点又は軟化点が相対的に高い樹脂を「高融点樹脂」といい、相対的に低い樹脂を「低融点樹脂」という)とを含有し、低融点樹脂が、繊維表面の少なくとも一部に、長さ方向に連続して存在している2成分系の熱伸長性複合繊維が挙げられる。高融点樹脂は、熱伸長性を発現する成分であり、低融点樹脂は、熱融着性を発現する成分であり、熱伸長性複合繊維は、高融点樹脂成分の融点よりも低い温度において熱伸長可能である。
Examples of the heat-extensible fibers include two types of resins having different melting points or softening points (resins having a relatively high melting point or softening point are called “high melting point resins”, and resins having a relatively low melting point are “low melting point resins”. And a two-component heat-extensible composite fiber in which a low melting point resin is continuously present in the length direction on at least a part of the fiber surface. The high-melting point resin is a component that develops heat extensibility, the low melting point resin is a component that develops heat-fusibility, and the heat-extensible composite fiber is heated at a temperature lower than the melting point of the high-melting point resin component. It is extensible.
高融点樹脂及び低融点樹脂の種類は、繊維形成能をする限り特に限定されない。高融点樹脂及び低融点樹脂の融点差又は軟化点差は、通常20℃以上、好ましくは25℃以上である。
The kind of the high melting point resin and the low melting point resin is not particularly limited as long as it has fiber forming ability. The melting point difference or softening point difference between the high melting point resin and the low melting point resin is usually 20 ° C. or higher, preferably 25 ° C. or higher.
高融点樹脂及び低融点樹脂の融点としては、例えば、次の方法により測定された融点が使用される。示差走査型熱量計(例えば、セイコーインスツルメンツ株式会社製DSC6200)を使用して、細かく裁断した繊維試料(例えば、2mgのサンプル)の熱分析を昇温速度10℃/分で行い、各樹脂の融解ピーク温度を測定し、測定された融解ピーク温度を融点として定義する。この方法によって樹脂の融点を明確に測定できない場合には、樹脂の分子流動が開始する温度として繊維の融着点強度が計測できる程度に樹脂が融着する温度を軟化点とし、これが融点の代わりに使用される。
As the melting point of the high melting point resin and the low melting point resin, for example, the melting point measured by the following method is used. Using a differential scanning calorimeter (for example, DSC6200, manufactured by Seiko Instruments Inc.), a finely cut fiber sample (for example, 2 mg sample) was subjected to thermal analysis at a heating rate of 10 ° C./min to melt each resin. The peak temperature is measured and the measured melting peak temperature is defined as the melting point. When the melting point of the resin cannot be clearly measured by this method, the temperature at which the resin is fused to such an extent that the fiber fusing point strength can be measured as the temperature at which the molecular flow of the resin begins, is used as the softening point. Used for.
高融点樹脂及び低融点樹脂の配向指数は、樹脂の種類によって適宜調整することができる。例えば、高融点樹脂としてポリプロピレンを使用する場合、配向指数は、通常60%以下、好ましくは40%以下、さらに好ましくは25%以下である。また、高融点樹脂としてポリエステルを使用する場合、配向指数は、通常25%以下、好ましくは20%以下、さらに好ましくは10%以下である。これに対して、低融点樹脂の配向指数は、通常5%以上、好ましくは15%以上、さらに好ましくは30%以上である。高融点樹脂及び低融点樹脂の配向指数がそれぞれ上記範囲にある場合、熱伸長性複合繊維が加熱処理によって効率的に伸長可能となる。
The orientation index of the high melting point resin and the low melting point resin can be appropriately adjusted depending on the type of the resin. For example, when polypropylene is used as the high melting point resin, the orientation index is usually 60% or less, preferably 40% or less, and more preferably 25% or less. When polyester is used as the high melting point resin, the orientation index is usually 25% or less, preferably 20% or less, more preferably 10% or less. On the other hand, the orientation index of the low melting point resin is usually 5% or more, preferably 15% or more, and more preferably 30% or more. When the orientation indices of the high melting point resin and the low melting point resin are in the above ranges, the heat-extensible composite fiber can be efficiently stretched by heat treatment.
高融点樹脂及び低融点樹脂の配向指数は、繊維を構成する樹脂の高分子鎖の配向の程度の指標であり、下記式によって算出される。
配向指数(%)=X/Y×100
[式中、Xは、熱伸長性複合繊維における樹脂の複屈折の値であり、Yは、樹脂の固有複屈折の値である。] The orientation index of the high melting point resin and the low melting point resin is an index of the degree of orientation of the polymer chain of the resin constituting the fiber, and is calculated by the following formula.
Orientation index (%) = X / Y × 100
[Wherein, X is the value of birefringence of the resin in the heat-extensible conjugate fiber, and Y is the value of intrinsic birefringence of the resin. ]
配向指数(%)=X/Y×100
[式中、Xは、熱伸長性複合繊維における樹脂の複屈折の値であり、Yは、樹脂の固有複屈折の値である。] The orientation index of the high melting point resin and the low melting point resin is an index of the degree of orientation of the polymer chain of the resin constituting the fiber, and is calculated by the following formula.
Orientation index (%) = X / Y × 100
[Wherein, X is the value of birefringence of the resin in the heat-extensible conjugate fiber, and Y is the value of intrinsic birefringence of the resin. ]
熱伸長性複合繊維における樹脂の複屈折(上記式のX)は、例えば、干渉顕微鏡に偏光板を装着し、繊維軸に対して平行方向及び垂直方向の偏光下で測定される。浸漬液としては、例えば、Cargille社製の標準屈折液が使用される。浸漬液の屈折率は、例えば、アッベ屈折計によって測定される。そして、干渉顕微鏡により得られる複合繊維の干渉縞像から、公知の方法(例えば、繊維学会誌,「芯鞘型複合繊維の高速紡糸における繊維構造形成」,Vol.51,No.9,第408頁,1995年)に基づき、繊維軸に対して平行及び垂直方向の屈折率を求め、両者の差である複屈折が算出される。
The birefringence (X in the above formula) of the resin in the heat-extensible composite fiber is measured, for example, with a polarizing plate attached to an interference microscope and polarized light in a direction parallel to and perpendicular to the fiber axis. As the immersion liquid, for example, a standard refraction liquid manufactured by Cargille is used. The refractive index of the immersion liquid is measured by, for example, an Abbe refractometer. From the interference fringe image of the composite fiber obtained by the interference microscope, a known method (for example, Journal of the Fiber Society, “Fiber structure formation in high-speed spinning of core-sheath type composite fiber”, Vol. 51, No. 9, No. 408). Page, 1995), the refractive index in the direction parallel and perpendicular to the fiber axis is obtained, and the birefringence which is the difference between the two is calculated.
樹脂の固有複屈折(上記式のY)は、樹脂の高分子鎖が完全に配向した状態での複屈折であり、その値は、例えば、「成形加工におけるプラスチック材料」初版、付表 成形加工に用いられる代表的なプラスチック材料(プラスチック成形加工学会編、シグマ出版、1998年2月10日発行)に記載されている。
The intrinsic birefringence of the resin (Y in the above formula) is the birefringence in a state where the polymer polymer chains are perfectly oriented, and the value is, for example, the first edition of “Plastic material in molding process”, and the attached surface molding process. It is described in typical plastic materials used (edited by the Japan Society for Plastic Processing), Sigma Publishing, published on February 10, 1998.
低融点樹脂の融点又は軟化点より10℃高い温度における熱伸長性複合繊維の熱伸長率は、好ましくは0.5~20%、さらに好ましくは3~20%、さらに一層好ましくは7.5~20%である。これにより、熱伸長性複合繊維の伸長によって第1層21が嵩高くなり、第1層21の肌当接面の凹凸構造が顕著なものになる。
The thermal elongation rate of the heat-stretchable composite fiber at a temperature 10 ° C. higher than the melting point or softening point of the low-melting resin is preferably 0.5 to 20%, more preferably 3 to 20%, still more preferably 7.5 to 20%. Thereby, the 1st layer 21 becomes bulky by expansion | extension of a heat | fever extensible composite fiber, and the uneven structure of the skin contact surface of the 1st layer 21 becomes remarkable.
熱伸長率としては、例えば、次の方法により測定された熱伸長率が使用される。熱機械分析装置TMA-50(島津製作所製)に、平行に並べた繊維をチャック間距離10mmで装着し、0.025mN/texの一定荷重を負荷した状態で10℃/分の昇温速度で昇温させる。その際の繊維の伸長率変化を測定し、低融点樹脂の融点又は軟化点での伸長率、及び低融点樹脂の融点又は軟化点より10℃高い温度での伸長率をそれぞれ読み取って各温度の熱伸長率とする。なお、熱伸長率を上記温度範囲で測定する理由は、繊維の交点を熱融着させる場合、低融点樹脂の融点又は軟化点以上であって、かつ、それらより10℃程度高い温度までの範囲が通常採用するためである。
As the thermal elongation rate, for example, the thermal elongation rate measured by the following method is used. A thermomechanical analyzer TMA-50 (manufactured by Shimadzu Corp.) was mounted with parallel fibers arranged at a distance of 10 mm between chucks, and a constant load of 0.025 mN / tex was applied at a heating rate of 10 ° C./min. Raise the temperature. The change in the elongation rate of the fiber at that time was measured, and the elongation rate at the melting point or softening point of the low melting point resin and the elongation rate at a temperature 10 ° C. higher than the melting point or softening point of the low melting point resin were read. The thermal elongation rate. The reason why the thermal elongation rate is measured in the above temperature range is that, when the fiber intersection is heat-sealed, the temperature range is higher than the melting point or softening point of the low-melting resin and up to about 10 ° C. Is usually adopted.
熱伸長性複合繊維は、芯鞘型(同芯タイプ,偏芯タイプ)、サイド・バイ・サイド型等の形態をとることができる。芯鞘型複合繊維の場合、鞘成分及び芯成分をそれぞれ低融点樹脂及び高融点樹脂で構成することができる。芯成分としては、例えば、ポリプロピレン(PP)、ポリエチレンテレフタレート(PET)、ポリブチレンテレフタレート(PBT)等が挙げられる。芯成分がPPである場合の鞘成分としては、例えば、高密度ポリエチレン(HDPE)、低密度ポリエチレン(LDPE)、直鎖状低密度ポリエチレン(LLDPE)等のポリエチレン(PE)、エチレンプロピレン共重合体、ポリスチレン等が挙げられる。芯成分がPET、PBT等である場合の鞘成分としては、例えば、PP、共重合ポリエステル等が挙げられる。
The heat-extensible conjugate fiber can take the form of a core-sheath type (concentric type, eccentric type), a side-by-side type, or the like. In the case of the core-sheath type composite fiber, the sheath component and the core component can be composed of a low melting point resin and a high melting point resin, respectively. Examples of the core component include polypropylene (PP), polyethylene terephthalate (PET), polybutylene terephthalate (PBT), and the like. Examples of the sheath component when the core component is PP include polyethylene (PE) such as high density polyethylene (HDPE), low density polyethylene (LDPE), and linear low density polyethylene (LLDPE), and an ethylene propylene copolymer. , Polystyrene and the like. Examples of the sheath component when the core component is PET, PBT, and the like include PP and copolymer polyester.
熱伸長性複合繊維における高融点樹脂に対する低融点樹脂の比率(重量比)は、繊維の熱伸長性、熱融着性、力学特性等を考慮して適宜調整することができるが、通常10:90~90:10、好ましくは20:80~80:20、さらに好ましくは50:50~70:30である。
The ratio (weight ratio) of the low-melting point resin to the high-melting point resin in the heat-stretchable composite fiber can be appropriately adjusted in consideration of the heat-stretchability, heat-fusibility, mechanical properties, etc. of the fiber. 90 to 90:10, preferably 20:80 to 80:20, and more preferably 50:50 to 70:30.
熱伸長性複合繊維の太さは、例えば、1.0~10dtex(特に1.7~8.0dtex)に調整することができ、熱伸長性複合繊維の繊維長は、例えば、30~70mm程度に調整することができる。
The thickness of the heat-extensible composite fiber can be adjusted to, for example, 1.0 to 10 dtex (particularly 1.7 to 8.0 dtex). The fiber length of the heat-extensible composite fiber is, for example, about 30 to 70 mm. Can be adjusted.
所望の熱伸長率を有する熱伸長性複合繊維は、例えば、紡糸後の複合繊維に対して、延伸処理を施すことなく、加熱処理又は捲縮処理を施すことにより製造することができる。
A heat-extensible conjugate fiber having a desired thermal elongation rate can be produced, for example, by subjecting a spun conjugate fiber to a heat treatment or a crimping treatment without performing a drawing treatment.
紡糸後の複合繊維に対して施される加熱処理の条件は、複合繊維を構成する高融点樹脂及び低融点樹脂の種類に応じて適宜調整することができる。例えば、芯成分がポリプロピレンであり、鞘成分が高密度ポリエチレンである芯鞘型複合繊維の場合、加熱温度は、通常50~120℃、好ましくは70~100℃であり、加熱時間は、通常10~500秒、好ましくは20~200秒である。加熱媒体としては、例えば、熱風、赤外線等が挙げられる。
The conditions of the heat treatment applied to the composite fiber after spinning can be appropriately adjusted according to the types of the high melting point resin and the low melting point resin constituting the composite fiber. For example, in the case of a core-sheath type composite fiber in which the core component is polypropylene and the sheath component is high-density polyethylene, the heating temperature is usually 50 to 120 ° C., preferably 70 to 100 ° C., and the heating time is usually 10 ° C. It is ˜500 seconds, preferably 20 to 200 seconds. Examples of the heating medium include hot air and infrared rays.
紡糸後の複合繊維に対して施される捲縮処理としては、例えば、機械捲縮等が挙げられる。捲縮は、二次元状及び三次元状のいずれの態様であってもよいし、偏芯タイプの芯鞘型複合繊維、サイド・バイ・サイド型複合繊維等で見られる三次元の顕在捲縮であってもよい。機械捲縮が熱を伴う場合、加熱処理と捲縮処理とが同時に施されることになる。
Examples of the crimping treatment performed on the spun composite fiber include mechanical crimping. The crimp may be in either a two-dimensional or three-dimensional manner, or a three-dimensional manifested crimp seen in an eccentric core-sheath composite fiber, side-by-side composite fiber, or the like. It may be. When the mechanical crimping involves heat, the heating process and the crimping process are performed simultaneously.
なお、捲縮処理において繊維が若干引き伸ばされる場合があるが、そのような引き延ばしは、延伸処理には含まれない。延伸処理は、通常、未延伸糸に対して行われる延伸倍率2~6倍程度の延伸操作を意味する。
In addition, the fiber may be slightly stretched in the crimping process, but such stretching is not included in the stretching process. The drawing treatment usually means a drawing operation with a draw ratio of about 2 to 6 times performed on an undrawn yarn.
トップシート2は、熱融着性繊維及び熱伸長性繊維以外の繊維を含有してもよい。熱融着性繊維及び熱伸長性繊維以外の繊維としては、例えば、天然繊維(羊毛,コットン等)、再生繊維(レーヨン,アセテート等)、無機繊維(ガラス繊維,炭素繊維等)等が挙げられる。トップシート2がその他の繊維を含有する場合、その他の繊維の含有量は適宜調整することができるが、通常、トップシート2の95重量%以下、好ましくは80重量%以下である。
The top sheet 2 may contain fibers other than heat-fusible fibers and heat-extensible fibers. Examples of fibers other than the heat-fusible fiber and the heat-extensible fiber include natural fibers (wool, cotton, etc.), regenerated fibers (rayon, acetate, etc.), inorganic fibers (glass fiber, carbon fiber, etc.), and the like. . When the top sheet 2 contains other fibers, the content of the other fibers can be appropriately adjusted, but is usually 95% by weight or less, preferably 80% by weight or less of the top sheet 2.
図2及び図3に示すように、トップシート2の肌当接面には、多数の凸部8及び凹部9が形成されており、トップシート2の肌当接面は凹凸形状となっている。トップシート2の肌当接面が凹凸形状であるので、着用者の肌とトップシート2との接触面積が少ないとともに、凹部9内の空間を通じたトップシート2の面方向の通気性が良好である。したがって、着用者にムレ及びそれに起因する痒みを与えにくい。
As shown in FIGS. 2 and 3, the skin contact surface of the top sheet 2 has a large number of convex portions 8 and concave portions 9, and the skin contact surface of the top sheet 2 has an uneven shape. . Since the skin contact surface of the top sheet 2 is uneven, the contact area between the wearer's skin and the top sheet 2 is small, and the air permeability in the surface direction of the top sheet 2 through the space in the recess 9 is good. is there. Therefore, it is difficult to give the wearer stuffiness and itching.
本実施形態では、トップシート2の肌当接面のうち、排泄口当接領域20を含む吸収体配置領域の略全体に凸部8及び凹部9が形成されているが、凸部8及び凹部9は、トップシート2の肌当接面のうち少なくとも排泄口当接領域20に形成されていればよい。なお、吸収体配置領域は、吸収体4をトップシート2に投影したときに、吸収体4がトップシート2と重なる領域である。
In the present embodiment, the convex portion 8 and the concave portion 9 are formed on substantially the entire absorber arrangement region including the excretory opening abutting region 20 in the skin contact surface of the top sheet 2. 9 should just be formed in the excretion opening | mouth contact | abutting area | region 20 at least among the skin contact surfaces of the top sheet 2. FIG. The absorber arrangement region is a region where the absorber 4 overlaps the top sheet 2 when the absorber 4 is projected onto the top sheet 2.
排泄口当接領域20は、生理用ナプキン1の着用時に、着用者の排泄口(例えば、小陰唇、大陰唇等)が当接する領域である。排泄口当接領域20は、図1において点線で囲まれた領域であり、吸収体配置領域の略中央に設定されている。排泄口当接領域20の位置、面積等は、適宜調整することができる。排泄口当接領域20は、実際に排泄口が当接する領域と略同一の領域として設定されてもよいし、それよりも大きい領域として設定されてもよいが、経血等の液状排泄物の外部への漏れ出しを防止する点から、実際に排泄口が当接する領域よりも大きい領域として設定されることが好ましい。排泄口当接領域20の長さは通常50~200mm、好ましくは70~150mmであり、幅は通常10~80mm、好ましくは20~50mmである。
The excretion opening contact area 20 is an area where the wearer's excretion opening (for example, small labia, large labia, etc.) abuts when the sanitary napkin 1 is worn. The excretory opening contact region 20 is a region surrounded by a dotted line in FIG. 1, and is set at substantially the center of the absorber arrangement region. The position, area, and the like of the excretion opening contact region 20 can be adjusted as appropriate. The excretory opening contact area 20 may be set as an area substantially the same as the area where the excretion opening actually contacts, or may be set as a larger area, but liquid excrement such as menstrual blood From the viewpoint of preventing leakage to the outside, it is preferably set as a region larger than the region where the excretion port actually contacts. The excretion opening contact region 20 has a length of usually 50 to 200 mm, preferably 70 to 150 mm, and a width of usually 10 to 80 mm, preferably 20 to 50 mm.
本実施形態において、排泄口当接領域20は、仮想領域として設定されているが、視覚的に認識可能な領域として設定されていてもよい。視覚的な認識は、例えば、排泄口当接領域20の着色、排泄口当接領域20の周縁に沿って連続的又は断続的に延びる凹部(例えば、ヒートエンボス処理により形成される凹部)の形成等により可能である。
In the present embodiment, the excretory opening contact area 20 is set as a virtual area, but may be set as a visually recognizable area. Visual recognition includes, for example, coloring of the excretory opening contact region 20 and formation of a recess (for example, a recess formed by a heat embossing process) extending continuously or intermittently along the periphery of the excretion opening contact region 20. Etc. are possible.
図示されていないが、排泄口当接領域20の略全体に、40℃における動粘度が0.01~80mm2/s、抱水率が0.01~4.0質量%、重量平均分子量が1,000未満である血液滑性付与剤が塗工されている。なお、血液滑性付与剤の詳細については、別項目を参照のこと。
Although not shown, substantially the entire excretory opening contact region 20 has a kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, a water retention of 0.01 to 4.0% by mass, and a weight average molecular weight. The blood slipperiness imparting agent which is less than 1,000 is applied. Refer to the separate item for details of the blood slipperiness-imparting agent.
本実施形態では、排泄口当接領域20の略全体に血液滑性付与剤が塗工されているが、血液滑性付与剤は、排泄口当接領域20のうち少なくとも凸部8(特に凸部8の頂部81)に塗工されていればよい。血液滑性付与剤は、排泄口当接領域20のうち少なくとも凸部8に塗工されている限り、排泄口当接領域20のうち凸部8以外の部分(例えば、凹部9)に塗工されていてもよいし、肌当接面のうち排泄口当接領域20以外の領域(例えば、排泄口当接領域20の周辺領域)に塗工されていてもよい。例えば、血液滑性付与剤は、肌当接面の略全体又は吸収体配置領域の略全体に塗工することができる。
In the present embodiment, the blood slipperiness imparting agent is applied to substantially the entire excretory opening contact region 20, but the blood slipperiness imparting agent is at least the convex portion 8 (particularly convex in the excretion port contact region 20. It only has to be applied to the top 81) of the part 8. As long as the blood slipperiness imparting agent is applied to at least the convex portion 8 in the excretory opening contact region 20, it is applied to a portion other than the convex portion 8 (for example, the concave portion 9). Alternatively, it may be applied to a region other than the excretion opening contact area 20 (for example, a peripheral area of the excretion opening contact area 20) on the skin contact surface. For example, the blood slipperiness-imparting agent can be applied to substantially the entire skin contact surface or substantially the entire absorber arrangement region.
血液滑性付与剤が、排泄口当接領域20のうち少なくとも凸部8(特に凸部8の頂部81)に塗工されていることにより、次の作用効果が発揮される。着用者から排泄された経血が排泄口当接領域20に到達すると、凸部8に存在する血液滑性付与剤とともに凹部9に滑落し、トップシート2を通過して吸収体4に移行する。したがって、生理用ナプキン1は、トップシート2から吸収体4への向上した経血移行性を有し、トップシート2に残存する経血を低減させることができる。このため、トップシート2の肌当接面のべたつき感が防止され、サラサラ感が維持される。このような血液滑性付与剤の作用効果は、月経時の経血排出量の変化に関わらず(すなわち、一度に排出される経血が大量であっても少量であっても)発揮される。
When the blood slipperiness imparting agent is applied to at least the convex portion 8 (particularly, the top portion 81 of the convex portion 8) in the excretory opening contact region 20, the following effects are exhibited. When menstrual blood excreted from the wearer reaches the excretory opening contact region 20, it slides down into the concave portion 9 together with the blood slipperiness imparting agent present in the convex portion 8, passes through the top sheet 2, and shifts to the absorber 4. . Therefore, the sanitary napkin 1 has improved menstrual blood transfer from the top sheet 2 to the absorbent body 4, and can reduce menstrual blood remaining on the top sheet 2. For this reason, the sticky feeling of the skin contact surface of the top sheet 2 is prevented, and a smooth feeling is maintained. The effect of such a blood slipperiness-imparting agent is exerted irrespective of changes in menstrual blood discharge (that is, whether menstrual blood discharged at a time is large or small). .
特に、後述するように、凸部側壁部82は、凸部8の頂部81を起点として凹部9の底部91に向かって延在しており、凹部側壁部92は、凹部9の底部91を起点として凸部8の頂部81に向かって延在している。したがって、凸部側壁部82は、その延在方向、すなわち、凸部8の頂部81から凹部9の底部91に向かう方向に沿った繊維配向性を有し、凹部側壁部92は、その延在方向、すなわち、凹部9の底部91から凸部8の頂部81に向かう方向に沿った繊維配向性を有する。このため、着用者から排泄された経血は、血液滑性付与剤とともに、凸部側壁部82及び凹部側壁部92の延在方向に沿って滑落しやすい。言い換えれば、着用者から排泄された経血は、トップシート2の面方向に広がることなく、トップシート2の厚み方向に(すなわち吸収体4に)移行しやすい。
In particular, as will be described later, the convex side wall 82 extends from the top 81 of the convex 8 toward the bottom 91 of the concave portion 9, and the concave side wall 92 starts from the bottom 91 of the concave 9. As shown in FIG. Therefore, the convex side wall 82 has a fiber orientation along the extending direction, that is, the direction from the top 81 of the convex 8 toward the bottom 91 of the concave 9, and the concave side 92 is extended. It has fiber orientation along the direction, that is, the direction from the bottom 91 of the concave portion 9 to the top 81 of the convex portion 8. For this reason, menstrual blood excreted from the wearer easily slides along the extending direction of the convex side wall portion 82 and the concave side wall portion 92 together with the blood slipperiness imparting agent. In other words, menstrual blood excreted from the wearer does not spread in the surface direction of the top sheet 2 and easily moves in the thickness direction of the top sheet 2 (that is, to the absorber 4).
本実施形態では、排泄口当接領域20に凸部8及び凹部9が存在し、排泄口当接領域20が凹凸形状となっているので、血液滑性付与剤の作用効果が効果的に発揮される。血液滑性付与剤の作用効果は、凸部8に加えて凹部9にも血液滑性付与剤を塗工することにより、増強させることができる。
In this embodiment, since the convex part 8 and the recessed part 9 exist in the excretion opening | mouth contact | abutting area | region 20, and the excretion opening | mouth contact | abutting area | region 20 is uneven | corrugated shape, the effect of a blood slipperiness | lubricity imparting agent exhibits effectively. Is done. The effect of the blood slipperiness imparting agent can be enhanced by applying the blood slipperiness imparting agent to the concave portion 9 in addition to the convex portion 8.
なお、血液滑性付与剤は、潤滑剤としても作用し、繊維同士の摩擦を低減させるので、トップシート2全体のしなやかさを向上させることができる。
In addition, since the blood slipperiness | lubricity imparting agent acts also as a lubrication agent and reduces the friction between fibers, the suppleness of the whole top sheet 2 can be improved.
生理用ナプキン1は、スキンケア組成物、ローション組成物等を含む公知の吸収性物品とは異なり、エモリエント剤、固定化剤等の成分は不要であり、血液滑性付与剤は、単体で、トップシート2に適用することができる。
The sanitary napkin 1 is different from known absorbent articles including skin care compositions, lotion compositions, etc., and does not require components such as emollients and immobilizing agents. It can be applied to the sheet 2.
血液滑性付与剤の坪量は、通常約1~30g/m2、好ましくは約2~20g/m2、さらに好ましくは約3~10g/m2である。血液滑性付与剤の坪量が、約1g/m2を下回ると、経血がトップシート2に残存しやすくなる一方、血液滑性付与剤の坪量が約30g/m2を超えると、着用中のべたべた感が増加しやすい。
The basis weight of the blood slipperiness imparting agent is usually about 1 to 30 g / m 2 , preferably about 2 to 20 g / m 2 , more preferably about 3 to 10 g / m 2 . When the basis weight of the blood slipperiness-imparting agent is less than about 1 g / m 2 , menstrual blood tends to remain in the top sheet 2, while when the basis weight of the blood slipperiness-imparting agent exceeds about 30 g / m 2 , The sticky feeling during wearing tends to increase.
血液滑性付与剤の坪量は、例えば、次の方法により測定することができる。
(1)トップシートの測定すべき範囲を、鋭利な刃物、例えば、カッターの替え刃を用いて、できるだけその厚さを変化させないように切り出して、サンプルを得る。
(2)サンプルの面積:SA(m2)及び質量:SM0(g)を測定する。
(3)サンプルを、血液滑性付与剤を溶解させることができる溶媒、例えば、エタノール、アセトン等の中で、少なくとも3分間攪拌し、血液滑性付与剤を溶媒中に溶解させる。
(4)サンプルを、質量を測定したろ紙の上でろ過し、ろ紙上で、サンプルを溶媒で十分に洗浄する。ろ紙上のサンプルを、60℃のオーブン内で乾燥させる。
(5)ろ紙及びサンプルの質量を測定し、そこからろ紙の質量を減ずることにより、乾燥後のサンプルの質量:SM1(g)を算出する。
(6)血液滑性付与剤の坪量BBS(g/m2)を、次式に基づいて算出する。
BBS(g/m2)=[SM0(g)-SM1(g)]/SA(m2)
なお、誤差を少なくするために、サンプルの総面積が100cm2を超えるように、複数の吸収性物品から複数のサンプルを採取し、複数回実験を繰り返し、それらの平均値を採用する。 The basis weight of the blood slipperiness imparting agent can be measured, for example, by the following method.
(1) The range to be measured of the top sheet is cut out with a sharp blade, for example, a cutter blade, so as not to change its thickness as much as possible, and a sample is obtained.
(2) The area of the sample: SA (m 2 ) and the mass: SM 0 (g) are measured.
(3) The sample is stirred for at least 3 minutes in a solvent capable of dissolving the blood lubricity-imparting agent, such as ethanol, acetone, etc., and the blood lubricity-imparting agent is dissolved in the solvent.
(4) The sample is filtered on the filter paper whose mass has been measured, and the sample is thoroughly washed with a solvent on the filter paper. The sample on the filter paper is dried in an oven at 60 ° C.
(5) The mass of the filter paper and the sample is measured, and the mass of the filter paper is subtracted therefrom to calculate the mass of the sample after drying: SM 1 (g).
(6) The basis weight BBS (g / m 2 ) of the blood slipperiness-imparting agent is calculated based on the following formula.
BBS (g / m 2 ) = [SM 0 (g) −SM 1 (g)] / SA (m 2 )
In order to reduce the error, a plurality of samples are collected from a plurality of absorbent articles so that the total area of the samples exceeds 100 cm 2 , the experiment is repeated a plurality of times, and an average value thereof is adopted.
(1)トップシートの測定すべき範囲を、鋭利な刃物、例えば、カッターの替え刃を用いて、できるだけその厚さを変化させないように切り出して、サンプルを得る。
(2)サンプルの面積:SA(m2)及び質量:SM0(g)を測定する。
(3)サンプルを、血液滑性付与剤を溶解させることができる溶媒、例えば、エタノール、アセトン等の中で、少なくとも3分間攪拌し、血液滑性付与剤を溶媒中に溶解させる。
(4)サンプルを、質量を測定したろ紙の上でろ過し、ろ紙上で、サンプルを溶媒で十分に洗浄する。ろ紙上のサンプルを、60℃のオーブン内で乾燥させる。
(5)ろ紙及びサンプルの質量を測定し、そこからろ紙の質量を減ずることにより、乾燥後のサンプルの質量:SM1(g)を算出する。
(6)血液滑性付与剤の坪量BBS(g/m2)を、次式に基づいて算出する。
BBS(g/m2)=[SM0(g)-SM1(g)]/SA(m2)
なお、誤差を少なくするために、サンプルの総面積が100cm2を超えるように、複数の吸収性物品から複数のサンプルを採取し、複数回実験を繰り返し、それらの平均値を採用する。 The basis weight of the blood slipperiness imparting agent can be measured, for example, by the following method.
(1) The range to be measured of the top sheet is cut out with a sharp blade, for example, a cutter blade, so as not to change its thickness as much as possible, and a sample is obtained.
(2) The area of the sample: SA (m 2 ) and the mass: SM 0 (g) are measured.
(3) The sample is stirred for at least 3 minutes in a solvent capable of dissolving the blood lubricity-imparting agent, such as ethanol, acetone, etc., and the blood lubricity-imparting agent is dissolved in the solvent.
(4) The sample is filtered on the filter paper whose mass has been measured, and the sample is thoroughly washed with a solvent on the filter paper. The sample on the filter paper is dried in an oven at 60 ° C.
(5) The mass of the filter paper and the sample is measured, and the mass of the filter paper is subtracted therefrom to calculate the mass of the sample after drying: SM 1 (g).
(6) The basis weight BBS (g / m 2 ) of the blood slipperiness-imparting agent is calculated based on the following formula.
BBS (g / m 2 ) = [SM 0 (g) −SM 1 (g)] / SA (m 2 )
In order to reduce the error, a plurality of samples are collected from a plurality of absorbent articles so that the total area of the samples exceeds 100 cm 2 , the experiment is repeated a plurality of times, and an average value thereof is adopted.
血液滑性付与剤は、トップシート2の繊維間の空隙を閉塞しないように塗工されていることが好ましい。例えば、血液滑性付与剤は、トップシート2の繊維の表面に液滴状又は粒子状で付着しているか、又は繊維の表面を覆っている。
The blood slipperiness-imparting agent is preferably applied so as not to block the gaps between the fibers of the topsheet 2. For example, the blood slipperiness imparting agent adheres to the surface of the fiber of the top sheet 2 in the form of droplets or particles, or covers the surface of the fiber.
血液滑性付与剤は、その表面積が大きくなるように塗工されていることが好ましい。これにより、血液滑性付与剤と経血との接触面積が大きくなり、血液滑性付与剤が経血とともに滑落しやすくなる。血液滑性付与剤が液滴状又は粒子状で存在する場合には、粒径を小さくすることにより、表面積を大きくすることができる。
The blood slipperiness-imparting agent is preferably coated so that its surface area becomes large. Thereby, the contact area of a blood slipperiness | lubricity imparting agent and menstrual blood becomes large, and a blood slipperiness | lubricity imparting agent becomes easy to slide down with menstrual blood. When the blood slipperiness-imparting agent is present in the form of droplets or particles, the surface area can be increased by reducing the particle size.
血液滑性付与剤の塗工方法としては、例えば、塗布装置(例えば、スパイラルコーター、カーテンコーター、スプレーコーター、ディップコーター等の非接触式のコーター、接触式のコーター等)を用いる方法が挙げられる。好ましい塗布装置は、非接触式のコーターである。これにより、液滴状又は粒子状の血液滑性付与剤を全体に均一に分散させることができるとともに、トップシート2に与えるダメージを低減することができる。
Examples of the method for applying the blood slipperiness-imparting agent include a method using a coating apparatus (for example, a non-contact type coater such as a spiral coater, curtain coater, spray coater, dip coater, contact type coater, etc.). . A preferred coating apparatus is a non-contact type coater. Thereby, while being able to disperse | distribute the liquid slipperiness | lubricity imparting agent of a droplet form or a particle form to the whole uniformly, the damage given to the top sheet 2 can be reduced.
血液滑性付与剤は、所望により、揮発性溶媒、例えば、アルコール系溶媒、エステル系溶媒、芳香族系溶媒等を含む塗布液として塗装することができる。塗布液が揮発性溶媒を含むことにより、血液滑性付与剤を含む塗布液の粘度が下がるために、塗布が容易になる、塗装時の加温が不要になる等の塗布工程の簡易化が図れる。
The blood slipperiness imparting agent can be applied as a coating liquid containing a volatile solvent, for example, an alcohol solvent, an ester solvent, an aromatic solvent, or the like, if desired. When the coating solution contains a volatile solvent, the viscosity of the coating solution containing the blood slipperiness-imparting agent is lowered, so that the coating process can be simplified, such as easy application and no need for heating during coating. I can plan.
血液滑性付与剤は、例えば、室温で液体の場合にはそのまま、又は粘度を下げるために加熱して、室温で固体の場合には液化するように加熱して、コントロールシームHMA(Hot Melt Adhesive)ガンによって塗工することができる。コントロールシームHMAガンのエアー圧を高くすることにより、微粒子状の血液滑性付与剤を塗工することができる。なお、血液滑性付与剤の塗布量は、例えば、コントロールシームHMAガンからの塗出量を増減することにより調節することができる。
The blood slipperiness-imparting agent is, for example, a control seam HMA (Hot Melt Adhesive) as it is in the case of a liquid at room temperature or heated to lower the viscosity and heated to be liquefied in the case of a solid at room temperature. ) Can be applied by gun. By increasing the air pressure of the control seam HMA gun, it is possible to apply a particulate blood slipping agent. In addition, the application quantity of a blood slipperiness | lubricity imparting agent can be adjusted by increasing / decreasing the application quantity from a control seam HMA gun, for example.
血液滑性付与剤は、トップシート2を製造する際に塗工してもよいし、生理用ナプキン1の製造ラインにおいて塗工してもよい。設備投資を抑制する観点からは、生理用ナプキン1の製造ラインにおいて、血液滑性付与剤を塗工することが好ましく、さらに、血液滑性付与剤が脱落し、ラインを汚染することを抑制するためには、製造ラインの川下工程、具体的には、製品を個包装に封入する直前に、血液滑性付与剤を塗工することが好ましい。
The blood slipperiness-imparting agent may be applied when the top sheet 2 is produced, or may be applied on the production line of the sanitary napkin 1. From the viewpoint of suppressing capital investment, it is preferable to apply a blood slipperiness-imparting agent in the production line of the sanitary napkin 1, and further suppress the blood slipperiness-imparting agent from dropping and contaminating the line. Therefore, it is preferable to apply the blood slipperiness imparting agent immediately downstream of the production line, specifically, immediately before the product is enclosed in the individual package.
図2及び図3に示すように、凸部8は、トップシート2の肌当接面側(図2において上側)に隆起しており、凹部9は、トップシート2の吸収体配置面側(図2において下側)に陥没している。
As shown in FIG.2 and FIG.3, the convex part 8 has protruded to the skin contact surface side (upper side in FIG. 2) of the top sheet 2, and the recessed part 9 has the absorber arrangement | positioning surface side of the top sheet 2 ( It is depressed on the lower side in FIG.
凸部8及び凹部9は、第1の方向及び第1の方向と交差する第2の方向に交互に配置されている。第1の方向は、トップシート2の肌当接面内における任意の方向であり、第2の方向は、トップシート2の肌当接面内において第1の方向と交差する方向である。
The convex portions 8 and the concave portions 9 are alternately arranged in the first direction and the second direction intersecting with the first direction. The first direction is an arbitrary direction in the skin contact surface of the top sheet 2, and the second direction is a direction that intersects the first direction in the skin contact surface of the top sheet 2.
図3に示すように、本実施形態では、第1の方向はX方向であり、第2の方向はY方向であり、第1の方向及び第2の方向が交差する角度は90°である。但し、第1の方向、第2の方向並びに第1の方向及び第2の方向が交差する角度は、本実施形態に限定されるものではなく、適宜変更可能である。第1の方向及び第2の方向が交差する角度は、好ましくは30~90°である。
As shown in FIG. 3, in the present embodiment, the first direction is the X direction, the second direction is the Y direction, and the angle at which the first direction and the second direction intersect is 90 °. . However, the first direction, the second direction, and the angle at which the first direction and the second direction intersect are not limited to the present embodiment, and can be changed as appropriate. The angle at which the first direction and the second direction intersect is preferably 30 to 90 °.
図3に示すように、本実施形態では、凸部8及び凹部9は、X方向及びY方向に交互に配置されて列をなしており、X方向及びY方向の列において、隣り合う凸部8の間には凹部9が位置している(すなわち、1つの凸部8の周囲には4つの凹部9が位置している)。隣り合う2つの列において、凸部8は、半ピッチずつずれて配置されているので、トップシート2を平面視したとき、凸部8の配置パターンは千鳥格子状となっている。同様に、隣り合う2つの列において、凹部9は、半ピッチずつずれて配置されているので、トップシート2を平面視したとき、凹部9の配置パターンは千鳥格子状となっている。凸部8及び凹部9の配置パターンは本実施形態に限定されるものではなく、適宜変更可能である。例えば、1つの凸部8の周囲に位置する凹部9の数及び1つの凹部9の周囲に位置する凸部8の数は適宜選択することができる。
As shown in FIG. 3, in the present embodiment, the convex portions 8 and the concave portions 9 are alternately arranged in the X direction and the Y direction to form a row, and adjacent convex portions in the rows in the X direction and the Y direction. The recesses 9 are positioned between the eight recesses 8 (that is, four recesses 9 are positioned around one protrusion 8). In the two adjacent rows, the convex portions 8 are arranged so as to be shifted by a half pitch, so that when the top sheet 2 is viewed in plan, the arrangement pattern of the convex portions 8 is a staggered pattern. Similarly, in the two adjacent rows, the concave portions 9 are arranged so as to be shifted by a half pitch, so that when the top sheet 2 is viewed in plan, the arrangement pattern of the concave portions 9 is a staggered pattern. The arrangement pattern of the convex part 8 and the recessed part 9 is not limited to this embodiment, It can change suitably. For example, the number of concave portions 9 positioned around one convex portion 8 and the number of convex portions 8 positioned around one concave portion 9 can be appropriately selected.
凸部8の高さは、通常0.2~15mm、好ましくは0.5~5mmであり、幅(X方向)は、通常1~20mm、好ましくは2~10mmであり、長さ(Y方向)は、通常1~20mm、好ましくは2~10mmであり、凸部8の間隔(凹部9の幅又は長さ)は、通常1~20mm、好ましくは2~10mmである。
The height of the convex portion 8 is usually 0.2 to 15 mm, preferably 0.5 to 5 mm, the width (X direction) is usually 1 to 20 mm, preferably 2 to 10 mm, and the length (Y direction). ) Is usually 1 to 20 mm, preferably 2 to 10 mm, and the interval between the convex portions 8 (width or length of the concave portion 9) is usually 1 to 20 mm, preferably 2 to 10 mm.
図4に示すように、凸部8は、頂部81と、頂部81から延在する凸部側壁部82とを有し、凹部9は、底部91と、底部91から延在する凹部側壁部92とを有する。
As shown in FIG. 4, the convex part 8 has a top part 81 and a convex part side wall part 82 extending from the top part 81, and the concave part 9 is a bottom part 91 and a concave part side wall part 92 extending from the bottom part 91. And have.
凸部8及び凹部9の外観形状は半球状であり、断面視形状はアーチ状であり、平面視形状は円状である。なお、凸部8の外観形状は、トップシート2の肌当接面から凸部8を見たときの形状であり、凹部9の外観形状は、トップシート2の吸収体配置面から凹部9を見たときの形状である。凸部8及び凹部9の形状は、本実施形態に限定されものではなく、その他の形状に変更可能である。その他の外観形状としては、例えば、円錐、円錐台、角錐、角錐台、斜円錐等の錐体形状が挙げられ、その他の平面視形状としては、例えば、楕円状、矩形状、多角形状等が挙げられる。
The external shape of the convex portion 8 and the concave portion 9 is hemispherical, the cross-sectional view shape is arched, and the planar view shape is circular. In addition, the external shape of the convex part 8 is a shape when the convex part 8 is seen from the skin contact surface of the top sheet 2, and the external shape of the concave part 9 is the concave part 9 from the absorber arrangement surface of the top sheet 2. It is the shape when seen. The shape of the convex part 8 and the recessed part 9 is not limited to this embodiment, It can change into another shape. Examples of other external shapes include cone shapes such as a cone, a truncated cone, a pyramid, a truncated pyramid, and an oblique cone. Examples of other planar shapes include an elliptical shape, a rectangular shape, and a polygonal shape. Can be mentioned.
図2に示すように、凸部8の内部は空洞となっている。隣接する凸部8間及び隣接する凹部9間に、尾根状の連結部が形成されていてもよい。隣接する凸部8の内部空間同士は、この連結部によって連通していてもよいし、連通していなくてもよい。
As shown in FIG. 2, the inside of the convex part 8 is hollow. A ridge-shaped connecting portion may be formed between the adjacent convex portions 8 and between the adjacent concave portions 9. The internal spaces of the adjacent convex portions 8 may or may not communicate with each other through this connecting portion.
トップシート2の肌当接面側を上側、吸収体配置面側を下側としたとき、凸部側壁部82は、下方に向かって径が漸増するテーパ状となっており、凹部側壁部92は、上方に向かって径が漸増するテーパ状となっている。凸部側壁部82は、頂部81を起点として下方に延在し、その下端部は、底部91を起点として上方に延在する凹部側壁部92の上端部と連続している。すなわち、凸部側壁部82は、頂部81から底部91に向かう方向に延在しており、凹部側壁部92は、底部91から頂部81に向かう方向に延在している。したがって、凸部側壁部82の延在方向は、頂部81から底部91に向かう方向であり、凹部側壁部92の延在方向は、底部91から頂部81に向かう方向である。
When the skin contact surface side of the top sheet 2 is set to the upper side and the absorber arrangement surface side is set to the lower side, the convex portion side wall portion 82 has a tapered shape whose diameter gradually increases downward. Has a tapered shape with a diameter gradually increasing upward. The convex side wall portion 82 extends downward from the top portion 81, and the lower end portion thereof is continuous with the upper end portion of the concave side wall portion 92 extending upward from the bottom portion 91. That is, the convex side wall 82 extends in the direction from the top 81 to the bottom 91, and the concave side wall 92 extends in the direction from the bottom 91 to the top 81. Accordingly, the extending direction of the convex side wall 82 is a direction from the top 81 to the bottom 91, and the extending direction of the concave side wall 92 is a direction from the bottom 91 to the top 81.
凸部側壁部82及び凹部側壁部92の形状は、本実施形態の形状に限定されるものではなく、その他の形状に変更可能である。その他の形状としては、例えば、円筒状、角筒状等が挙げられる。
The shape of the convex side wall portion 82 and the concave side wall portion 92 is not limited to the shape of the present embodiment, and can be changed to other shapes. Examples of other shapes include a cylindrical shape and a rectangular tube shape.
凸部側壁部82は、その延在方向(すなわち、頂部81から底部91に向かう方向)に沿った繊維配向性を有し、凹部側壁部92は、その延在方向(すなわち、底部91から頂部81に向かう方向)に沿った繊維配向性を有する。具体的には、図4(c)に示すように、凸部側壁部82及び凹部側壁部92を展開したとき、凸部側壁部82は、その延在方向D82に沿った繊維配向性OR82を有し、凹部側壁部92は、その延在方向D92に沿った繊維配向性OR92を有する。図示しないが、平面視において、凸部側壁部82は、頂部81に向かって収束する放射状の繊維配向性を有しており、凹部部側壁部92は、底部91に向かって収束する放射状の繊維配向性を有している。凸部側壁部82及び凹部側壁部92がそれぞれの延在方向に沿った繊維配向性を有することにより、トップシート2の厚み方向の繊維配向性が大きくなっており、これに起因してトップシート2の圧縮変形性及び圧縮回復性(クッション性)が向上している。したがって、生理用ナプキン1が圧縮されても、吸収体4からの液戻り(リウェットバック)が生じにくい。また、トップシート2のクッション性が高いため、着用者に良好な着け心地を与える。
The convex side wall portion 82 has fiber orientation along the extending direction (that is, the direction from the top portion 81 to the bottom portion 91), and the concave side wall portion 92 is extended in the extending direction (that is, from the bottom portion 91 to the top portion). Fiber orientation along the direction toward 81). Specifically, as shown in FIG. 4C, when the convex side wall portion 82 and the concave side wall portion 92 are developed, the convex side wall portion 82 has a fiber orientation OR along its extending direction D 82. 82 , and the recess side wall portion 92 has a fiber orientation OR 92 along the extending direction D 92 thereof. Although not shown, in the plan view, the convex side wall 82 has a radial fiber orientation that converges toward the top 81, and the concave side wall 92 is a radial fiber that converges toward the bottom 91. Has orientation. The convex side wall portion 82 and the concave side wall portion 92 have fiber orientation along the respective extending directions, whereby the fiber orientation in the thickness direction of the top sheet 2 is increased. The compression deformation property and compression recovery property (cushion property) of No. 2 are improved. Therefore, even if the sanitary napkin 1 is compressed, liquid return (rewetting back) from the absorbent body 4 hardly occurs. Moreover, since the cushioning property of the top sheet 2 is high, the wearer is given good comfort.
図4(c)に示す具体例では、凸部側壁部82及び凹部側壁部92は、周方向全体にわたって、略同一の繊維配向性を有しているが、繊維配向性の異なる部分を有していてもよい。例えば、凸部側壁部82及び凹部側壁部92は、凹凸賦形前の繊維配向性が維持された部分を有していてもよい。例えば、凹凸賦形前に繊維同士が熱融着している場合、凹凸賦形後も凹凸賦形前の繊維配向性が維持される。繊維配向性の異なる部分は、例えば、凸部側壁部82又は凹部側壁部92の延在方向と直交する方向に沿った繊維配向性を有する。不織布が製造される際、通常、MD方向に繊維が配向した状態で熱融着されるため、凸部側壁部82及び凹部側壁部92のMD方向断面では、繊維が凸部側壁部82及び凹部側壁部92の延在方向に配向するものの、CD方向断面では、繊維が凸部側壁部82及び凹部側壁部92の延在方向と直交する方向に配向する。
In the specific example shown in FIG. 4C, the convex side wall portion 82 and the concave side wall portion 92 have substantially the same fiber orientation throughout the circumferential direction, but have portions with different fiber orientations. It may be. For example, the convex part side wall part 82 and the concave part side wall part 92 may have a part in which the fiber orientation before uneven forming is maintained. For example, when the fibers are heat-sealed before the uneven shaping, the fiber orientation before the uneven shaping is maintained even after the uneven shaping. The portions having different fiber orientations have, for example, fiber orientations along a direction orthogonal to the extending direction of the convex side wall portions 82 or the concave side wall portions 92. When the nonwoven fabric is manufactured, since the fibers are usually heat-sealed in a state where the fibers are oriented in the MD direction, the fibers are formed on the convex side wall portions 82 and the concave side wall portions 92 in the MD direction cross section. Although oriented in the extending direction of the side wall 92, the fibers are oriented in the direction perpendicular to the extending directions of the convex side wall 82 and the concave side wall 92 in the cross section in the CD direction.
繊維配向性は、繊維の配向角と配向強度とからなる概念であり、例えば、次の方法により測定することができる。トップシートの肌当接面が上側に位置するようにサンプルを静置する。顕微鏡(例えば、日本電子(株)社製JCM-5100等の走査型電子顕微鏡)を使用して、サンプルの測定面に対して垂直の方向からの拡大画像を撮影し、印刷し、透明PET性シート上に繊維をなぞる。拡大画像は、繊維が10本以上計測できる倍率に拡大された画像であり、拡大倍率は、例えば、50~300倍である。画像をパソコン内に取り込み、株式会社ネクサス社製のnexusNewQube(スタンドアロン版)画像処理ソフトウエアを使用し、画像を二値化する。繊維配向解析プログラムである、Fiber Orientation Analysis 8.13 Singleを使用して、二値化した画像から、配向角と配向強度を得る。配向角は、繊維が最も配向している角度であり、配向強度は、その配向角における強度である。配向角が90°に近いほど、凸部側壁部又は凹部側壁部の延在方向に繊維が配向していることを示す。また、配向強度が大きいほど、繊維の向きがそろっていることを示す。配向強度が1.05以上の場合を配向しているとする。測定を数回(例えば3~5回)繰り返し、平均値を算出する。
Fiber orientation is a concept consisting of the orientation angle and orientation strength of the fiber, and can be measured, for example, by the following method. The sample is allowed to stand so that the skin contact surface of the top sheet is positioned on the upper side. Using a microscope (for example, a scanning electron microscope such as JCM-5100 manufactured by JEOL Ltd.), an enlarged image is taken from a direction perpendicular to the measurement surface of the sample, printed, and transparent PET property Trace the fiber on the sheet. The enlarged image is an image enlarged to a magnification capable of measuring 10 or more fibers, and the enlargement magnification is, for example, 50 to 300 times. The image is taken into a personal computer, and the image is binarized by using a NexusNewQube (stand-alone version) image processing software manufactured by Nexus Corporation. Using Fiber Orientation Analysis 8.13 Single, which is a fiber orientation analysis program, the orientation angle and orientation strength are obtained from the binarized image. The orientation angle is the angle at which the fibers are most oriented, and the orientation strength is the strength at that orientation angle. It shows that the fiber is orientating in the extending direction of a convex part side wall part or a recessed part side wall part, so that an orientation angle is near 90 degrees. Moreover, it shows that the direction of a fiber has gathered, so that orientation strength is large. The case where the orientation strength is 1.05 or more is assumed to be oriented. The measurement is repeated several times (for example, 3 to 5 times), and the average value is calculated.
図4(b)に示すように、凸部8の頂部81の繊維配向性と、凹部9の底部91の繊維配向性とは相違する。すなわち、凸部8の頂部81における繊維配向性OR81は、厚さ方向に沿ったものであるが、凹部9の底部91における繊維配向性OR91は、厚さ方向に沿ったものではなく、厚さ方向と垂直な方向に沿ったものである。
As shown in FIG. 4B, the fiber orientation at the top 81 of the convex portion 8 is different from the fiber orientation at the bottom 91 of the concave portion 9. That is, the fiber orientation OR 81 at the top 81 of the convex portion 8 is along the thickness direction, but the fiber orientation OR 91 at the bottom 91 of the concave portion 9 is not along the thickness direction. Along the direction perpendicular to the thickness direction.
凸部8の頂部81の繊維配向性OR81に関し、配向角は好ましくは50°~130°であり、配向強度は好ましくは1.05以上である。これにより、経血等の液状排泄物が凸部8から凹部9へ移行しやすくなる。
Regarding the fiber orientation OR 81 at the top 81 of the convex portion 8, the orientation angle is preferably 50 ° to 130 °, and the orientation strength is preferably 1.05 or more. As a result, liquid excreta such as menstrual blood easily moves from the convex portion 8 to the concave portion 9.
凹部9の底部91の繊維配向性OR91に関し、配向角は好ましくは0°~50°又は130°~180°であり、配向強度は好ましくは1.05未満である。これにより、トップシート2のMD方向及びCD方向の引張強度のバランスが保たれ、生理用ナプキン1の使用時に生じ得るトップシート2の破断が防止される。
With respect to the fiber orientation OR 91 at the bottom 91 of the recess 9, the orientation angle is preferably 0 ° to 50 ° or 130 ° to 180 °, and the orientation strength is preferably less than 1.05. Thereby, the balance of the tensile strength of MD direction and CD direction of the top sheet 2 is maintained, and the fracture | rupture of the top sheet 2 which may arise at the time of use of the sanitary napkin 1 is prevented.
凹部9の底部91における厚さ方向の繊維配向性は、凹部側壁部92における厚さ方向の繊維配向性よりも小さいことが好ましい。これにより、経血が凹部側壁部92に溜まることなく厚さ方向に(すなわち吸収体4に)移行しやすい。なお、凹部9における繊維配向性は、吸収体4に近づくにつれて、厚さ方向と垂直な方向に変化することが好ましく、これにより、繊維配向性に勾配が生じ、経血が透過及び拡散しやすくなる。かかる観点から、凹部側壁部92の配向角は、好ましくは50°~130°であり、配向強度は、好ましくは1.05以上である。凹部9の底部91の配向角は、好ましくは0°~50°未満又は130°~180°であり、配向強度は、好ましくは1.05未満である。
The fiber orientation in the thickness direction at the bottom 91 of the recess 9 is preferably smaller than the fiber orientation in the thickness direction at the recess side wall 92. Thereby, menstrual blood easily moves in the thickness direction (that is, to the absorbent body 4) without accumulating in the recess side wall portion 92. In addition, it is preferable that the fiber orientation in the recessed part 9 changes in a direction perpendicular to the thickness direction as the absorber 4 is approached, whereby a gradient occurs in the fiber orientation, and menstrual blood is easily transmitted and diffused. Become. From such a viewpoint, the orientation angle of the recess side wall portion 92 is preferably 50 ° to 130 °, and the orientation strength is preferably 1.05 or more. The orientation angle of the bottom 91 of the recess 9 is preferably 0 ° to less than 50 ° or 130 ° to 180 °, and the orientation strength is preferably less than 1.05.
凸部8の頂部81の繊維配向性OR81と、凹部9の底部91の繊維配向性OR91との関係は、OR81>OR91であることが好ましい。これにより、凸部8の頂部81は、凹部9の底部91よりも優れた圧縮変形性及び圧縮回復性(柔軟性)を有する。
The relationship between the fiber orientation OR 81 at the top 81 of the convex portion 8 and the fiber orientation OR 91 at the bottom 91 of the concave portion 9 is preferably OR 81 > OR 91 . Thereby, the top part 81 of the convex part 8 has the compressive deformation property and compression recovery property (flexibility) superior to the bottom part 91 of the concave part 9.
凹部9の底部91の繊維配向性に関し、肌当接面側部分における繊維配向性と、吸収体配置面側部分における繊維配向性とは、実質的に等しいことが好ましい。すなわち、凹部9の底部91に向かって放射上の繊維配向性が、肌当接面側部分及び吸収体配置面側部分の両方に存在することが好ましい。これにより、凹部9の底部91の圧縮変形性及び圧縮回復性(柔軟性)が向上し、凹部9の底部91が潰れにくくなる。
Regarding the fiber orientation of the bottom 91 of the recess 9, it is preferable that the fiber orientation in the skin contact surface side portion and the fiber orientation in the absorbent body placement surface side portion are substantially equal. That is, it is preferable that radial fiber orientation toward the bottom portion 91 of the recess 9 exists in both the skin contact surface side portion and the absorber arrangement surface side portion. Thereby, the compression deformability and the compression recovery property (flexibility) of the bottom 91 of the recess 9 are improved, and the bottom 91 of the recess 9 is not easily crushed.
凸部8の頂部81の繊維密度は、凹部9の底部91の繊維密度よりも小さくてもよいし、大きくてもよい。液体は、疎密勾配に従って、繊維密度が低い方から高い方へ移行しやすいので、凸部8の頂部81の繊維密度が凹部9の底部91の繊維密度よりも小さい場合、生理用ナプキン1は、血液滑性付与剤の作用効果と相俟って、トップシート2から吸収体4への向上した経血移行性を有する。一方、液体は繊維密度が高い方から低い方へ移行しにくいので、凸部8の頂部81の繊維密度が凹部9の底部91の繊維密度よりも小さい場合、経血の移行性が繊維密度の疎密勾配によって阻害されるが、生理用ナプキン1は、血液滑性付与剤の作用効果によって、トップシート2から吸収体4への向上した経血移行性を有する。したがって、血液滑性付与剤の作用効果は、凸部8の頂部81の繊維密度が凹部9の底部91の繊維密度よりも大きい場合に顕著である。また、凸部8の頂部81の繊維密度が凹部9の底部91の繊維密度よりも大きい場合、着用者の肌に対するトップシート2の凹凸形状の追従性が良好となるので、着用者が足を組み替えたり歩いたりする際の着け心地が良好となる。
The fiber density of the top part 81 of the convex part 8 may be smaller or larger than the fiber density of the bottom part 91 of the concave part 9. Since the liquid easily moves from the lower fiber density to the higher fiber density according to the density gradient, when the fiber density at the top 81 of the convex portion 8 is smaller than the fiber density at the bottom 91 of the concave portion 9, the sanitary napkin 1 is Combined with the action and effect of the blood slipperiness-imparting agent, it has improved menstrual blood transfer from the top sheet 2 to the absorber 4. On the other hand, since the liquid is less likely to move from the higher fiber density to the lower fiber density, when the fiber density at the top 81 of the convex portion 8 is smaller than the fiber density at the bottom 91 of the concave portion 9, menstrual blood migration is less than the fiber density. Although it is inhibited by the density gradient, the sanitary napkin 1 has an improved menstrual transfer property from the top sheet 2 to the absorbent body 4 due to the action effect of the blood slipperiness imparting agent. Therefore, the effect of the blood slipperiness imparting agent is remarkable when the fiber density of the top portion 81 of the convex portion 8 is larger than the fiber density of the bottom portion 91 of the concave portion 9. In addition, when the fiber density of the top portion 81 of the convex portion 8 is larger than the fiber density of the bottom portion 91 of the concave portion 9, the followability of the uneven shape of the top sheet 2 to the wearer's skin becomes good, so the wearer Good comfort when rearranging and walking.
繊維密度は、例えば、次の方法により測定することができる。トップシート2の切断面を、顕微鏡(例えば、日本電子(株)社製JCM-5100等の走査型電子顕微鏡)を使用して拡大観察する。拡大倍率は、繊維断面数が30~60本計測できる倍率(例えば、150~500倍)に調整する。繊維断面数及び繊維断面数を測定した視野面積に基づいて、繊維密度(本/mm2)を算出する。測定は数ヶ所(例えば3~5ヶ所)で実施し、平均値を算出する。このようにして測定された凸部8の頂部81の繊維密度は、好ましくは30~150本/mm2、さらに好ましくは60~100本/mm2であり、凹部9の底部91の繊維密度は、好ましくは150~600本/mm2、さらに好ましくは300~550本/mm2である。
The fiber density can be measured, for example, by the following method. The cut surface of the top sheet 2 is magnified and observed using a microscope (for example, a scanning electron microscope such as JCM-5100 manufactured by JEOL Ltd.). The magnification is adjusted to a magnification (for example, 150 to 500 times) at which 30 to 60 fiber cross sections can be measured. The fiber density (lines / mm 2 ) is calculated based on the number of fiber cross sections and the visual field area where the number of fiber cross sections is measured. Perform measurements at several locations (for example, 3 to 5 locations) and calculate the average value. The fiber density of the top portion 81 of the convex portion 8 measured in this way is preferably 30 to 150 pieces / mm 2 , more preferably 60 to 100 pieces / mm 2 , and the fiber density of the bottom portion 91 of the concave portion 9 is The number is preferably 150 to 600 / mm 2 , more preferably 300 to 550 / mm 2 .
凸部8の頂部81の繊維量と、凹部9の底部91の繊維量とは、実質的に等しいことが好ましい。これにより、圧縮変形性及び圧縮回復性が向上する。また、繊維ムラによる感触の変化、破断が生じにくくなる。繊維量は、例えば、次の方法により測定することができる。顕微鏡(例えば、キーエンス製デジタルマイクロスコープVHX-1000)を使用して、測定部位を拡大する。拡大倍率は、例えば10~100倍である。凸部の頂部の厚み(図4(a)におけるT81)及び凹部の底部の厚み(図4(a)におけるT91)の測定を数回(例えば3~5回)実施し、その平均値(mm)を算出する。上記した繊維密度の測定方法により、繊維密度(本/mm2)を測定し、繊維量=厚み(mm)×繊維密度(本/mm2)に基づいて、繊維量(本/mm3)を算出する。
It is preferable that the fiber amount of the top part 81 of the convex part 8 and the fiber amount of the bottom part 91 of the recessed part 9 are substantially equal. Thereby, compression deformation property and compression recovery property improve. In addition, changes in feel and breakage due to fiber unevenness are less likely to occur. The fiber amount can be measured, for example, by the following method. Using a microscope (for example, Keyence digital microscope VHX-1000), the measurement site is enlarged. The enlargement magnification is, for example, 10 to 100 times. The thickness of the top of the convex part (T 81 in FIG. 4A) and the thickness of the bottom of the concave part (T 91 in FIG. 4A) were measured several times (for example, 3 to 5 times), and the average value (Mm) is calculated. The fiber density (lines / mm 2 ) is measured by the fiber density measurement method described above, and the fiber amount (lines / mm 3 ) is calculated based on the fiber amount = thickness (mm) × fiber density (lines / mm 2 ). calculate.
凸部8の頂部81のうち肌当接面側部分の繊維密度が、吸収体配置面側部分の繊維密度よりも小さいことが好ましい。これにより、圧縮変形性及び圧縮回復性が向上する。凸部8の頂部81の肌当接面側部分の繊維密度は、好ましくは10~50本/mm2、さらに好ましくは15~30本/mm2であり、吸収体配置面側部分における繊維密度は、好ましくは20~100本/mm2、さらに好ましくは45~70本/mm2である。肌当接面側部分の繊維密度に対する吸収体配置面側部分の繊維密度の比は、好ましくは2~5倍程度である。これにより、圧縮変形性及び圧縮回復性が向上する。
It is preferable that the fiber density of the skin contact surface side portion of the top portion 81 of the convex portion 8 is smaller than the fiber density of the absorber arrangement surface side portion. Thereby, compression deformation property and compression recovery property improve. The fiber density of the skin contact surface side portion of the top portion 81 of the convex portion 8 is preferably 10 to 50 fibers / mm 2 , more preferably 15 to 30 fibers / mm 2 , and the fiber density in the absorber arrangement surface side portion. Is preferably 20 to 100 / mm 2 , more preferably 45 to 70 / mm 2 . The ratio of the fiber density of the absorber placement surface side portion to the fiber density of the skin contact surface side portion is preferably about 2 to 5 times. Thereby, compression deformation property and compression recovery property improve.
凸部8の頂部81における繊維融着点数は、凹部9の底部91における繊維融着点数よりも少ないことが好ましい。これにより、トップシート2の肌触りが良好となる。繊維融着点数は、単位面積(1mm2)あたりの繊維融着点数をカウントすることにより算出される。凸部8の頂部81における繊維融着点数は、好ましくは30~130個/mm2、さらに好ましくは50~100個/mm2である。凹部9の底部91における繊維融着点数は、好ましくは250~500個/mm2、さらに好ましくは300~450個/mm2である。
The number of fiber fusion points at the top portion 81 of the convex portion 8 is preferably smaller than the number of fiber fusion points at the bottom portion 91 of the concave portion 9. Thereby, the touch of the top sheet 2 becomes favorable. The number of fiber fusion points is calculated by counting the number of fiber fusion points per unit area (1 mm 2 ). The number of fiber fusion points at the top 81 of the convex portion 8 is preferably 30 to 130 pieces / mm 2 , more preferably 50 to 100 pieces / mm 2 . The number of fiber fusion points at the bottom 91 of the recess 9 is preferably 250 to 500 / mm 2 , more preferably 300 to 450 / mm 2 .
凸部8の頂部81の厚みT81は、通常0.1~5mm、好ましくは0.3~2.5mmであり、凸部側壁部82の厚みT82は、通常0.1~5mm、好ましくは0.3~2.5mmであり、凹部9の底部91の厚みT91は、通常0.1~5mm、好ましくは0.3~2.5mmであり、凹部側壁部92の厚みT92は、通常0.1~5mm、好ましくは0.3~2.5mmである。各部分の厚みの関係は、T81>T82,T92>T91であることが好ましい。
The thickness T 81 of the top portion 81 of the convex portion 8 is usually 0.1 to 5 mm, preferably 0.3 to 2.5 mm, and the thickness T 82 of the convex side wall portion 82 is usually 0.1 to 5 mm, preferably Is 0.3 to 2.5 mm, and the thickness T 91 of the bottom 91 of the recess 9 is usually 0.1 to 5 mm, preferably 0.3 to 2.5 mm. The thickness T 92 of the recess side wall 92 is The thickness is usually 0.1 to 5 mm, preferably 0.3 to 2.5 mm. It is preferable that the relationship between the thicknesses of the respective parts is T 81 > T 82 and T 92 > T 91 .
トップシート2の3gf/cm2荷重下の厚みは、通常0.2~20mm、好ましくは0.5~10mmである。トップシート2の坪量(シート全体の平均値)は、通常10~100g/m2、好ましくは20~50g/m2である。
The thickness of the top sheet 2 under a load of 3 gf / cm 2 is usually 0.2 to 20 mm, preferably 0.5 to 10 mm. The basis weight (average value of the entire sheet) of the top sheet 2 is usually 10 to 100 g / m 2 , preferably 20 to 50 g / m 2 .
液体は繊維密度が低い部分から繊維密度が高い部分に移行する傾向があるので、トップシート2の繊維密度勾配の有無及び程度は、トップシート2に対して有色の液体(例えば、色素を含有する液体)を滴下し、滴下後の色の濃淡によって評価することができる。すなわち、色が濃い部分は、液体移行量の多い部分、すなわち、繊維密度が相対的に高い部分と評価することができ、色が薄い部分は、液体移行量が少ない部分、すなわち、繊維密度が相対的に小さい部分と評価することができる。
Since the liquid tends to shift from a portion having a low fiber density to a portion having a high fiber density, the presence / absence and degree of the fiber density gradient of the top sheet 2 contains a colored liquid (for example, a pigment). (Liquid) is dropped, and the color density after dropping can be evaluated. That is, the dark part can be evaluated as a part with a large liquid migration amount, that is, a part with a relatively high fiber density, and the light color part has a small liquid migration amount, that is, a fiber density. It can be evaluated as a relatively small portion.
トップシート2の坪量は、以下のように測定することができる。
(1)測定すべき範囲にマークを付け、その面積:SAα(m2)を測定する。なお、誤差を少なくするために、サンプルの総面積が5cm2を超えるように、マーキングする。
(2)マーキングされた範囲を、鋭利な刃物、例えば、カッターの替え刃で切り出し、その総質量:TM(g)を測定する。
(3)測定すべき範囲の坪量BSα(g/m2)を、次式に基づいて算出する。
BSα(g/m2)=TM(g)/SAα(m2) The basis weight of thetop sheet 2 can be measured as follows.
(1) Mark the range to be measured and measure its area: SA α (m 2 ). In order to reduce the error, marking is performed so that the total area of the sample exceeds 5 cm 2 .
(2) The marked range is cut out with a sharp blade, for example, a replacement blade of a cutter, and the total mass: TM (g) is measured.
(3) Basis weight BS α (g / m 2 ) of the range to be measured is calculated based on the following formula.
BS α (g / m 2 ) = TM (g) / SA α (m 2 )
(1)測定すべき範囲にマークを付け、その面積:SAα(m2)を測定する。なお、誤差を少なくするために、サンプルの総面積が5cm2を超えるように、マーキングする。
(2)マーキングされた範囲を、鋭利な刃物、例えば、カッターの替え刃で切り出し、その総質量:TM(g)を測定する。
(3)測定すべき範囲の坪量BSα(g/m2)を、次式に基づいて算出する。
BSα(g/m2)=TM(g)/SAα(m2) The basis weight of the
(1) Mark the range to be measured and measure its area: SA α (m 2 ). In order to reduce the error, marking is performed so that the total area of the sample exceeds 5 cm 2 .
(2) The marked range is cut out with a sharp blade, for example, a replacement blade of a cutter, and the total mass: TM (g) is measured.
(3) Basis weight BS α (g / m 2 ) of the range to be measured is calculated based on the following formula.
BS α (g / m 2 ) = TM (g) / SA α (m 2 )
トップシート2は、親水化処理してもよい。親水化処理としては、例えば、親水剤によるトップシート2の表面のコーティング、構成成分への親水剤の添加、コロナ処理、プラズマ処理等が挙げられる。トップシート2を親水化処理すると、血液滑性付与剤に由来する親油性領域と、親水剤に由来する親水性領域とがトップシート2にまばらに共存することになり、経血がトップシート2の凸部8から滑落し、吸収体に移行しやすくなる。
The top sheet 2 may be hydrophilized. Examples of the hydrophilic treatment include coating of the surface of the top sheet 2 with a hydrophilic agent, addition of a hydrophilic agent to a component, corona treatment, plasma treatment, and the like. When the top sheet 2 is hydrophilized, the lipophilic region derived from the blood slipping agent and the hydrophilic region derived from the hydrophilic agent coexist sparsely in the top sheet 2, and menstrual blood is in the top sheet 2. It slips down from the convex part 8 of this, and it becomes easy to transfer to an absorber.
バックシート3は、経血等の液状排泄物が透過できないシートであり、吸収体4に吸収された液状排泄物の漏れを防止することができる。バックシートの一方の面(図2において上面)は、吸収体4が配置される吸収体配置面となっており、他方の面(図2において下面)は、非肌当接面(本実施形態では、着用者の着衣(下着)が当接する面)となっている。バックシート3は、着用時のムレを低減させるために、液不透過性に加えて、透湿性を有することが好ましい。
The back sheet 3 is a sheet through which liquid excretion such as menstrual blood cannot permeate, and can prevent leakage of liquid excretion absorbed by the absorber 4. One surface (upper surface in FIG. 2) of the back sheet is an absorber arrangement surface on which the absorber 4 is arranged, and the other surface (lower surface in FIG. 2) is a non-skin contact surface (this embodiment). Then, the wearer's clothes (underwear) are in contact with each other). The backsheet 3 preferably has moisture permeability in addition to liquid impermeability in order to reduce stuffiness when worn.
バックシート3としては、例えば、防水処理を施した不織布、合成樹脂(例えば、ポリエチレン、ポリプロピレン、ポリエチレンテレフタレート等)フィルム、不織布と合成樹脂フィルムとの複合シート(例えば、スパンボンド、スパンレース等の不織布に通気性の合成樹脂フィルムが接合された複合フィルム)、耐水性の高いメルトブローン不織布を強度の強いスパンボンド不織布で挟んだSMS不織布等が挙げられる。
Examples of the backsheet 3 include waterproof nonwoven fabric, synthetic resin (eg, polyethylene, polypropylene, polyethylene terephthalate, etc.) film, and composite sheet of nonwoven fabric and synthetic resin film (eg, nonwoven fabric such as spunbond and spunlace). And a composite film in which a breathable synthetic resin film is bonded), an SMS nonwoven fabric in which a melt-blown nonwoven fabric having high water resistance is sandwiched between strong spunbond nonwoven fabrics, and the like.
吸収体4は、経血等の液状排泄物を吸収する吸収性材料を含有する。吸収体4に含有される吸収性材料は、経血等の液状排泄物を吸収・保持可能である限り特に限定されない。吸収性材料としては、例えば、吸水性繊維、高吸水性材料(例えば、高吸水性樹脂、高吸水性繊維等)が挙げられる。吸収体4は、酸化防止剤、光安定剤、紫外線吸収剤、中和剤、造核剤、エポキシ安定剤、滑剤、抗菌剤、難燃剤、帯電防止剤、顔料、可塑剤等の添加剤を必要に応じて含有してもよい。
The absorber 4 contains an absorbent material that absorbs liquid excreta such as menstrual blood. The absorbent material contained in the absorber 4 is not particularly limited as long as it can absorb and retain liquid excretion such as menstrual blood. Examples of the absorbent material include a water-absorbing fiber and a highly water-absorbing material (for example, a highly water-absorbing resin and a highly water-absorbing fiber). The absorber 4 includes additives such as an antioxidant, a light stabilizer, an ultraviolet absorber, a neutralizer, a nucleating agent, an epoxy stabilizer, a lubricant, an antibacterial agent, a flame retardant, an antistatic agent, a pigment, and a plasticizer. You may contain as needed.
吸水性繊維としては、例えば、針葉樹又は広葉樹を原料として得られる木材パルプ(例えば、砕木パルプ、リファイナーグランドパルプ、サーモメカニカルパルプ、ケミサーモメカニカルパルプ等の機械パルプ;クラフトパルプ、サルファイドパルプ、アルカリパルプ等の化学パルプ;半化学パルプ等);木材パルプに化学処理を施して得られるマーセル化パルプ又は架橋パルプ;バガス、ケナフ、竹、麻、綿(例えばコットンリンター)等の非木材パルプ;レーヨン、フィブリルレーヨン等の再生セルロース;アセテート、トリアセテート等の半合成セルロース等が挙げられるが、コストが低く、成形しやすいこと点から、粉砕パルプが好ましい。
Examples of water-absorbing fibers include wood pulp obtained from softwood or hardwood (for example, mechanical pulp such as groundwood pulp, refiner ground pulp, thermomechanical pulp, chemithermomechanical pulp; kraft pulp, sulfide pulp, alkaline pulp, etc. Chemical pulp; semi-chemical pulp, etc.]; mercerized pulp or crosslinked pulp obtained by chemically treating wood pulp; non-wood pulp such as bagasse, kenaf, bamboo, hemp, cotton (eg cotton linter); rayon, fibril Examples include regenerated cellulose such as rayon; semi-synthetic cellulose such as acetate and triacetate, but pulverized pulp is preferred because it is low in cost and easy to mold.
高吸水性材料としては、例えば、デンプン系、セルロース系、合成ポリマー系の高吸水性材料が挙げられる。デンプン系又はセルロース系の高吸水性材料としては、例えば、デンプン-アクリル酸(塩)グラフト共重合体、デンプン-アクリロニトリル共重合体のケン化物、ナトリウムカルボキシメチルセルロースの架橋物等が挙げられ、合成ポリマー系の高吸水性材料としては、例えば、ポリアクリル酸塩系、ポリスルホン酸塩系、無水マレイン酸塩系、ポリアクリルアミド系、ポリビニルアルコール系、ポリエチレンオキシド系、ポリアスパラギン酸塩系、ポリグルタミン酸塩系、ポリアルギン酸塩系、デンプン系、セルロース系等の高吸水性樹脂(Superabsorbent Polymer:SAP)等が挙げられるが、これらのうちポリアクリル酸塩系(特に、ポリアクリル酸ナトリウム系)の高吸水性樹脂が好ましい。高吸水性材料の形状としては、例えば、粒子状、繊維状、鱗片状等が挙げられ、粒子状である場合、粒径は、好ましくは50~1000μmであり、さらに好ましくは100~600μmである。
Examples of the superabsorbent material include starch, cellulose, and synthetic polymer superabsorbent materials. Examples of the starch-based or cellulose-based superabsorbent material include starch-acrylic acid (salt) graft copolymers, saponified starch-acrylonitrile copolymers, and crosslinked products of sodium carboxymethyl cellulose. Synthetic polymers Examples of high water-absorbing materials include polyacrylates, polysulfonates, maleic anhydrides, polyacrylamides, polyvinyl alcohols, polyethylene oxides, polyaspartates, polyglutamates , Polyalginate-based, starch-based, and cellulose-based superabsorbent resins (Superabsorbent Polymer: SAP), and the like. Resins are preferred. Examples of the shape of the superabsorbent material include particulates, fibers, and scales. In the case of particulates, the particle size is preferably 50 to 1000 μm, more preferably 100 to 600 μm. .
吸収体4が高吸水性材料(例えば、高吸水性樹脂、高吸水性繊維等)を含有する場合、高吸水性材料の含有量は、吸収体4の通常5~80質量%、好ましくは10~60質量%、さらに好ましくは20~40質量%である。
When the absorbent body 4 contains a highly water-absorbing material (for example, highly water-absorbing resin, highly water-absorbing fiber, etc.), the content of the highly water-absorbing material is usually 5 to 80% by mass, preferably 10%. It is ˜60 mass%, more preferably 20 to 40 mass%.
吸収体4は、銀、銅、亜鉛、シリカ、活性炭、アルミノケイ酸塩化合物、ゼオライト等を含有してもよい。これにより、消臭性、抗菌性、吸熱効果等の機能を吸収体に付与することができる。
The absorber 4 may contain silver, copper, zinc, silica, activated carbon, aluminosilicate compound, zeolite or the like. Thereby, functions, such as a deodorizing property, antibacterial property, and an endothermic effect, can be provided to an absorber.
吸収体4の厚み、目付等は、生理用ナプキン1が備えるべき特性(例えば吸収性、強度、軽量性等)に応じて適宜調整することができる。吸収体4の厚みは、通常0.1~15mm、好ましくは1~10mm、さらに好ましくは2~5mmであり、目付は、通常20~1000g/m2、好ましくは50~800g/m2、さらに好ましくは100~500g/m2である。なお、吸収体4の厚み、目付等は、吸収体4全体にわたって一定であってもよいし、部分的に異なっていてもよい。
The thickness, basis weight, etc. of the absorbent body 4 can be appropriately adjusted according to the characteristics (for example, absorbency, strength, lightness, etc.) that the sanitary napkin 1 should have. The thickness of the absorber 4 is usually 0.1 to 15 mm, preferably 1 to 10 mm, more preferably 2 to 5 mm, and the basis weight is usually 20 to 1000 g / m 2 , preferably 50 to 800 g / m 2 , 100 to 500 g / m 2 is preferable. In addition, the thickness, basis weight, etc. of the absorber 4 may be constant throughout the absorber 4 or may be partially different.
吸収体4は、吸収性材料を含有するコアと、コアを被覆するコアラップとを有する形態であることが好ましい。コアラップは、液透過性及び吸収体保持性を有する限り特に限定されない。コアラップとしては、例えば、不織布、織布、液体透過孔が形成された合成樹脂フィルム、網目を有するネット状シート等が挙げられるが、低コスト性等の点から、粉砕パルプを主材料として湿式法で成形されるティッシュが好ましい。
The absorbent body 4 is preferably in a form having a core containing an absorbent material and a core wrap covering the core. The core wrap is not particularly limited as long as it has liquid permeability and absorber retention. Examples of the core wrap include a nonwoven fabric, a woven fabric, a synthetic resin film in which liquid permeation holes are formed, and a net-like sheet having a mesh. However, from the viewpoint of low cost, a wet method using pulverized pulp as a main material A tissue molded with is preferred.
生理用ナプキン1は、トップシート2に加えて、トップシート2及び吸収体4の間に配置されたセカンドシートを備えていてもよい。この場合、血液滑性付与剤はセカンドシートに塗工されていてもよい。
The sanitary napkin 1 may include a second sheet disposed between the top sheet 2 and the absorbent body 4 in addition to the top sheet 2. In this case, the blood slipperiness imparting agent may be applied to the second sheet.
セカンドシートは、経血等の液状排泄物が透過可能である限り特に限定されず、セカンドシートの厚み、坪量、密度等は、経血等の液状排泄物が透過可能である範囲で適宜調整することができる。
The second sheet is not particularly limited as long as liquid excretion such as menstrual blood can permeate, and the thickness, basis weight, density, etc. of the second sheet are appropriately adjusted within a range where liquid excretion such as menstrual blood can permeate. can do.
セカンドシートとしては、例えば、不織布、織布、液体透過孔が形成された合成樹脂フィルム、網目を有するネット状シート等が挙げられる。不織布としては、例えば、エアスルー不織布、スパンボンド不織布、ポイントボンド不織布、スパンレース不織布、ニードルパンチ不織布、メルトブローン不織布、及びこれらの組み合わせ(例えば、SMS等)等が挙げられ、不織布を構成する繊維としては、例えば、天然繊維(羊毛,コットン等)、再生繊維(レーヨン,アセテート等)、無機繊維(ガラス繊維,炭素繊維等)、合成樹脂繊維(ポリエチレン、ポリプロピレン、ポリブチレン、エチレン-酢酸ビニル共重合体、エチレン-アクリル酸エチル共重合体、エチレン-アクリル酸共重合体、アイオノマー樹脂等のポリオレフィン;ポリエチレンテレフタレート、ポリブチレンテレフタラート、ポリトリメチレンテレフタラート、ポリ乳酸等のポリエステル;ナイロン等のポリアミド)等が挙げられる。不織布には、芯・鞘型繊維、サイド・バイ・サイド型繊維、島/海型繊維等の複合繊維;中空タイプの繊維;扁平、Y型、C型等の異型繊維;潜在捲縮又は顕在捲縮の立体捲縮繊維;水流、熱、エンボス加工等の物理的負荷により分割する分割繊維等が混合されていてもよい。
Examples of the second sheet include a nonwoven fabric, a woven fabric, a synthetic resin film in which liquid permeation holes are formed, and a net-like sheet having a mesh. Examples of the nonwoven fabric include air-through nonwoven fabric, spunbond nonwoven fabric, point bond nonwoven fabric, spunlace nonwoven fabric, needle punched nonwoven fabric, melt blown nonwoven fabric, and combinations thereof (for example, SMS). For example, natural fibers (wool, cotton, etc.), regenerated fibers (rayon, acetate, etc.), inorganic fibers (glass fibers, carbon fibers, etc.), synthetic resin fibers (polyethylene, polypropylene, polybutylene, ethylene-vinyl acetate copolymer, Polyolefins such as ethylene-ethyl acrylate copolymer, ethylene-acrylic acid copolymer, ionomer resin; polyesters such as polyethylene terephthalate, polybutylene terephthalate, polytrimethylene terephthalate, polylactic acid; De), and the like. Nonwoven fabrics include core / sheath fibers, side-by-side fibers, island / sea fibers, etc .; hollow fibers; flat fibers, Y-shaped fibers, C-shaped fibers, etc .; latent crimps or manifestations Crimped three-dimensional crimped fibers; split fibers that are split by a physical load such as water flow, heat, and embossing may be mixed.
以下、図面に基づいて、生理用ナプキン1の製造方法の一実施形態を説明する。
本実施形態に係る製造方法は、吸収体を形成する工程(工程1)と、トップシートを積層する工程(工程2)と、バックシートを積層する工程(工程3)と、生理用ナプキンを切り出す工程(工程4)と、生理用ナプキンに血液滑性付与剤を塗布する工程(工程5)とを含み、図6に示す製造装置200が使用される。 Hereinafter, an embodiment of a method for producing thesanitary napkin 1 will be described with reference to the drawings.
The manufacturing method which concerns on this embodiment cuts out the process (process 1) of laminating an absorber, the process of laminating a top sheet (process 2), the process of laminating a back sheet (process 3), and a sanitary napkin. Amanufacturing apparatus 200 shown in FIG. 6 is used, including a step (step 4) and a step (step 5) of applying a blood slipperiness-imparting agent to a sanitary napkin.
本実施形態に係る製造方法は、吸収体を形成する工程(工程1)と、トップシートを積層する工程(工程2)と、バックシートを積層する工程(工程3)と、生理用ナプキンを切り出す工程(工程4)と、生理用ナプキンに血液滑性付与剤を塗布する工程(工程5)とを含み、図6に示す製造装置200が使用される。 Hereinafter, an embodiment of a method for producing the
The manufacturing method which concerns on this embodiment cuts out the process (process 1) of laminating an absorber, the process of laminating a top sheet (process 2), the process of laminating a back sheet (process 3), and a sanitary napkin. A
[工程1]
搬送方向MDへ回転するサクションドラム220の周面には、吸収体材料222を詰める型として凹部224が周方向に所要のピッチで形成されている。サクションドラム220が回転して凹部224が材料供給部221へ進入すると、サクション部226が凹部224に作用し、材料供給部221から供給された吸収体材料222は凹部224に真空吸引される。材料供給部221は、サクションドラム220を覆うように形成されており、材料供給部221は、吸収体材料222を空気搬送により凹部224に対して供給し、凹部224には吸収体4が形成される。凹部224に形成された吸収体4は、搬送方向MDに向かって進むキャリアシート210上に転写される。 [Step 1]
On the peripheral surface of thesuction drum 220 that rotates in the transport direction MD, recesses 224 are formed at a required pitch in the circumferential direction as a mold for filling the absorbent material 222. When the suction drum 220 rotates and the recess 224 enters the material supply unit 221, the suction unit 226 acts on the recess 224, and the absorber material 222 supplied from the material supply unit 221 is sucked into the recess 224 by vacuum. The material supply unit 221 is formed so as to cover the suction drum 220, and the material supply unit 221 supplies the absorber material 222 to the recess 224 by air conveyance, and the absorber 4 is formed in the recess 224. The The absorber 4 formed in the recess 224 is transferred onto the carrier sheet 210 that proceeds in the transport direction MD.
搬送方向MDへ回転するサクションドラム220の周面には、吸収体材料222を詰める型として凹部224が周方向に所要のピッチで形成されている。サクションドラム220が回転して凹部224が材料供給部221へ進入すると、サクション部226が凹部224に作用し、材料供給部221から供給された吸収体材料222は凹部224に真空吸引される。材料供給部221は、サクションドラム220を覆うように形成されており、材料供給部221は、吸収体材料222を空気搬送により凹部224に対して供給し、凹部224には吸収体4が形成される。凹部224に形成された吸収体4は、搬送方向MDに向かって進むキャリアシート210上に転写される。 [Step 1]
On the peripheral surface of the
[工程2]
トップシート2が吸収体4に積層され、積層体LB1が形成される。なお、トップシート2の製造方法については後述する。
その後、積層体LB1には、必要に応じて圧搾溝が形成される。圧搾溝は、エンボス加工装置230により形成される。エンボス加工装置230は、凸部(不図示)が外周表面に設けられた上段ロール231と、外周の表面が平滑である下段ロール232とを有する。上段ロール231の凸部は、圧搾溝の形状、配置パターン等に対応するように形成されている。積層体LB1がエンボス加工装置230の上段ロール231と下段ロール232との間を通過すると、積層体LB1が厚さ方向に圧縮され、積層体LB1に圧搾溝が形成される。圧搾溝は、例えば、トップシート2のうち、排泄口当接領域20の周縁、排泄口当接領域20の周囲領域の周縁等に形成される。圧搾溝の形成により、トップシート2は吸収体4と一体化される。エンボス加工装置230による圧搾溝の形成工程は、必要がない場合には省略される。 [Step 2]
Thetop sheet 2 is laminated on the absorber 4 to form a laminate LB1. In addition, the manufacturing method of the top sheet 2 is mentioned later.
Then, a pressing groove is formed in the laminated body LB1 as necessary. The compressed groove is formed by theembossing device 230. The embossing device 230 includes an upper roll 231 having a convex portion (not shown) provided on the outer peripheral surface, and a lower roll 232 having a smooth outer peripheral surface. The convex portion of the upper roll 231 is formed so as to correspond to the shape of the compressed groove, the arrangement pattern, and the like. When the laminated body LB1 passes between the upper roll 231 and the lower roll 232 of the embossing device 230, the laminated body LB1 is compressed in the thickness direction, and a compressed groove is formed in the laminated body LB1. The compressed groove is formed, for example, in the periphery of the excretory opening contact area 20, the peripheral area of the peripheral area of the excretion opening contact area 20, or the like in the top sheet 2. The top sheet 2 is integrated with the absorbent body 4 by forming the compressed grooves. The pressing groove forming step by the embossing device 230 is omitted when it is not necessary.
トップシート2が吸収体4に積層され、積層体LB1が形成される。なお、トップシート2の製造方法については後述する。
その後、積層体LB1には、必要に応じて圧搾溝が形成される。圧搾溝は、エンボス加工装置230により形成される。エンボス加工装置230は、凸部(不図示)が外周表面に設けられた上段ロール231と、外周の表面が平滑である下段ロール232とを有する。上段ロール231の凸部は、圧搾溝の形状、配置パターン等に対応するように形成されている。積層体LB1がエンボス加工装置230の上段ロール231と下段ロール232との間を通過すると、積層体LB1が厚さ方向に圧縮され、積層体LB1に圧搾溝が形成される。圧搾溝は、例えば、トップシート2のうち、排泄口当接領域20の周縁、排泄口当接領域20の周囲領域の周縁等に形成される。圧搾溝の形成により、トップシート2は吸収体4と一体化される。エンボス加工装置230による圧搾溝の形成工程は、必要がない場合には省略される。 [Step 2]
The
Then, a pressing groove is formed in the laminated body LB1 as necessary. The compressed groove is formed by the
[工程3]
バックシートロール240から供給されたバックシート3を、積層体LB2の下側(トップシート2と反対側)の面に、接着剤層を介して積層し、積層体LB3を形成する。なお、エンボス加工装置230による圧搾溝の形成工程が省略される場合、積層体LB2及び積層体LB3は同一である。 [Step 3]
Theback sheet 3 supplied from the back sheet roll 240 is laminated on the lower surface (opposite side of the top sheet 2) of the laminated body LB2 via an adhesive layer to form the laminated body LB3. In addition, when the formation process of the pressing groove by the embossing apparatus 230 is abbreviate | omitted, the laminated body LB2 and the laminated body LB3 are the same.
バックシートロール240から供給されたバックシート3を、積層体LB2の下側(トップシート2と反対側)の面に、接着剤層を介して積層し、積層体LB3を形成する。なお、エンボス加工装置230による圧搾溝の形成工程が省略される場合、積層体LB2及び積層体LB3は同一である。 [Step 3]
The
[工程4]
カッター250を使用して積層体LB3を切断し、生理用ナプキンを切り出す。 [Step 4]
The laminate LB3 is cut using thecutter 250, and a sanitary napkin is cut out.
カッター250を使用して積層体LB3を切断し、生理用ナプキンを切り出す。 [Step 4]
The laminate LB3 is cut using the
[工程5]
スプレー260を使用して生理用ナプキンのトップシート2に血液滑性付与剤261を塗工して、トップシート2の表面に血液滑性付与剤層を形成する。血液滑性付与剤層は、トップシート2の肌当接面のうち、少なくとも排泄口当接領域20に形成される。 [Step 5]
The bloodslipperiness imparting agent 261 is applied to the top sheet 2 of the sanitary napkin using the spray 260 to form a blood slipperiness imparting agent layer on the surface of the top sheet 2. The blood slipperiness-imparting agent layer is formed at least in the excretion opening contact region 20 in the skin contact surface of the top sheet 2.
スプレー260を使用して生理用ナプキンのトップシート2に血液滑性付与剤261を塗工して、トップシート2の表面に血液滑性付与剤層を形成する。血液滑性付与剤層は、トップシート2の肌当接面のうち、少なくとも排泄口当接領域20に形成される。 [Step 5]
The blood
本実施形態では、生理用ナプキンを切り出した後に、血液滑性付与剤を塗工したが、切り出す前のいずれの段階で塗工してもよいし、トップシートの製造工程で塗工してもよい。製造途中で塗工した血液滑性付与剤が流れ落ちることを防止するために、製造工程の川下の段階、例えば、生理用ナプキンを包装する直前に血液滑性付与剤を塗工することが好ましい。
In this embodiment, the blood slipperiness-imparting agent is applied after cutting out the sanitary napkin, but it may be applied at any stage before cutting out, or it may be applied in the top sheet manufacturing process. Good. In order to prevent the blood slipperiness-imparting agent applied during production from flowing down, it is preferable to apply the blood slipperiness-imparting agent immediately downstream of the manufacturing process, for example, immediately before packaging a sanitary napkin.
生理用ナプキン1の製造方法は、工程1~5の他、シール部7a,7b,8a,8bを形成する工程、粘着部9a,9b,9cを形成する工程等を含むことができる。
The manufacturing method of the sanitary napkin 1 can include, in addition to steps 1 to 5, a step of forming the seal portions 7a, 7b, 8a, 8b, a step of forming the adhesive portions 9a, 9b, 9c, and the like.
<トップシートの製造方法>
以下、図面に基づいて、トップシート2の製造方法の一実施形態を説明する。
本実施形態に係る製造方法では、図5に示す製造装置100が使用される。 <Top sheet manufacturing method>
Hereinafter, an embodiment of a method for manufacturing thetop sheet 2 will be described with reference to the drawings.
In the manufacturing method according to the present embodiment, amanufacturing apparatus 100 shown in FIG. 5 is used.
以下、図面に基づいて、トップシート2の製造方法の一実施形態を説明する。
本実施形態に係る製造方法では、図5に示す製造装置100が使用される。 <Top sheet manufacturing method>
Hereinafter, an embodiment of a method for manufacturing the
In the manufacturing method according to the present embodiment, a
図5に示すように、コンベアベルト110は、上側ローラ111a,b及び下側ローラ111c,dに支持された状態で搬送方向MDに向かって回転する。コンベアベルト110のウェブ搬送面には、複数の突起部112が形成されており、ウェブ搬送面は凹凸状となっている。また、コンベアベルト110のウェブ搬送面には、複数の通気孔(不図示)が形成されている。
As shown in FIG. 5, the conveyor belt 110 rotates in the conveying direction MD while being supported by the upper rollers 111a and 111b and the lower rollers 111c and 111d. A plurality of protrusions 112 are formed on the web conveyance surface of the conveyor belt 110, and the web conveyance surface is uneven. Further, a plurality of vent holes (not shown) are formed on the web conveyance surface of the conveyor belt 110.
突起部112は、先端に向かって径が漸減する先細り形状となっており、その先端部は丸みを有している。突起部112の高さは、例えば、0.5~20mmである。突起部112の高さが低すぎると、繊維ウェブ50に対する凹凸賦形が不十分になるおそれがある一方、高すぎると、熱風を吹き付けたときに突起部112が繊維ウェブ50を突き抜けるおそれがある。かかる観点から、突起部112の高さは、好ましくは1~10mmである。突起部112のMD方向のピッチは1~20mmであり、MD方向と垂直な方向(CD方向)のピッチは1~20mmである。なお、CD方向は繊維ウェブ50の幅方向と一致する。
The protrusion 112 has a tapered shape whose diameter gradually decreases toward the tip, and the tip has a rounded shape. The height of the protrusion 112 is, for example, 0.5 to 20 mm. If the height of the projection 112 is too low, the uneven shaping on the fiber web 50 may be insufficient, while if too high, the projection 112 may penetrate the fiber web 50 when hot air is blown. . From this viewpoint, the height of the protrusion 112 is preferably 1 to 10 mm. The pitch of the protrusions 112 in the MD direction is 1 to 20 mm, and the pitch in the direction perpendicular to the MD direction (CD direction) is 1 to 20 mm. The CD direction coincides with the width direction of the fiber web 50.
通気孔(不図示)の開口率(通気孔の総面積/コンベアベルト110の表面積)は、好ましくは20~45%、さらに好ましくは25~40%である。開口率が低すぎると繊維ウェブ50に対する凹凸賦形が不十分になるおそれがある一方、高すぎると熱風を吹き付けたときに繊維ウェブ50が通気孔に入り込み、コンベアベルト110から剥離しにくくなる。
The opening ratio (total area of the air holes / surface area of the conveyor belt 110) of the air holes (not shown) is preferably 20 to 45%, more preferably 25 to 40%. If the opening ratio is too low, the uneven shaping on the fiber web 50 may be insufficient, while if it is too high, the fiber web 50 will enter the vent hole when hot air is blown, and it will be difficult to peel off from the conveyor belt 110.
ローラ111a~111dに支持されて回転するコンベアベルト110は、その搬送面に形成された突起部112によって繊維ウェブ50を支持してMD方向に搬送する。
The conveyor belt 110, which is supported by the rollers 111a to 111d and rotates, supports the fiber web 50 by the protrusions 112 formed on the conveying surface thereof and conveys it in the MD direction.
図5に示すように、コンベアベルト110の上流側には、一対の駆動ロール151,152が設けられている。駆動ロール151,152は、カード機で製造された繊維ウェブ50をコンベアベルト110に供給する。駆動ロール151,152と第1ノズル120との間には、張力検出器(不図示)と、張力検出器で検出された張力の大きさに基づいて駆動ロール151,152の周速度V1を制御する制御部(不図示)とが設けられている。制御部は、張力検出器の検出出力に基づいて、駆動ロール151,152の周速度V1を、コンベアベルト110の周速度V2に対して相対的に調整する。これにより、第1ノズル120に供給される繊維ウェブ50の張力は、所望の張力に調整される。張力検出器で検出された張力が所望の張力よりも大きい場合、制御部は、コンベアベルトの周速度V2よりも駆動ロール151,152の周速度V1を大きくし、第1ノズル120に供給される繊維ウェブ50の張力を低下させる。一方、張力検出器で検出された張力が所望の張力よりも小さい場合、制御部は、コンベアベルト110の周速度V2よりも駆動ロール151,152の周速度V1を小さくし、第1ノズル120に供給される繊維ウェブ50の張力を増加させる。なお、相対的に調整される、駆動ロール151,152の周速度V1はロール表面の速度であり、コンベアベルト110の周速度V2は、ウェブ搬送面の速度である。
As shown in FIG. 5, a pair of drive rolls 151 and 152 are provided on the upstream side of the conveyor belt 110. The drive rolls 151 and 152 supply the fiber web 50 manufactured by the card machine to the conveyor belt 110. Between the driving rolls 151 and 152 and the first nozzle 120, a tension detector (not shown) and the peripheral speed V1 of the driving rolls 151 and 152 are controlled based on the magnitude of the tension detected by the tension detector. And a control unit (not shown). The controller adjusts the peripheral speed V1 of the drive rolls 151 and 152 relative to the peripheral speed V2 of the conveyor belt 110 based on the detection output of the tension detector. Thereby, the tension of the fiber web 50 supplied to the first nozzle 120 is adjusted to a desired tension. When the tension detected by the tension detector is greater than the desired tension, the control unit increases the circumferential speed V1 of the drive rolls 151 and 152 to the first nozzle 120 than the circumferential speed V2 of the conveyor belt. The tension of the fiber web 50 is reduced. On the other hand, when the tension detected by the tension detector is smaller than the desired tension, the control unit makes the peripheral speed V1 of the drive rolls 151 and 152 smaller than the peripheral speed V2 of the conveyor belt 110, and causes the first nozzle 120 to The tension of the supplied fiber web 50 is increased. The peripheral speed V1 of the drive rolls 151 and 152, which is relatively adjusted, is the speed of the roll surface, and the peripheral speed V2 of the conveyor belt 110 is the speed of the web conveyance surface.
図5に示すように、製造装置100は、MD方向に向かって順に、第1の加熱流体H1を吹き付けて第1エアースルー工程を行う第1ノズル120と、第2の加熱流体H2を吹き付けて第2エアースルー工程を行う第2ノズル130と、第3の加熱流体H3を吹き付けて第3エアースルー工程を行う第3ノズル140とを備えている。また、製造装置100は、第2ノズル130と第3ノズル140との間に配置された、繊維ウェブ50を冷却する冷却部160を備えている。第1ノズル120及び第2ノズル130によって、繊維ウェブ50の凹凸賦形処理が実施され、第3ノズル140によって毛羽立ち低減処理が実施される。毛羽立ちを低減する必要がなければ、第3ノズル140による処理を省略してもよい。
As shown in FIG. 5, the manufacturing apparatus 100 sprays the 1st nozzle 120 which performs the 1st air through process by spraying the 1st heating fluid H1 in order toward MD direction, and the 2nd heating fluid H2. A second nozzle 130 that performs the second air-through process and a third nozzle 140 that sprays the third heating fluid H3 and performs the third air-through process are provided. In addition, the manufacturing apparatus 100 includes a cooling unit 160 that is disposed between the second nozzle 130 and the third nozzle 140 and that cools the fiber web 50. The first nozzle 120 and the second nozzle 130 perform an uneven shape forming process for the fiber web 50, and the third nozzle 140 performs a fuzz reduction process. If it is not necessary to reduce fuzz, the processing by the third nozzle 140 may be omitted.
[第1エアースルー工程]
第1エアースルー工程は、コンベアベルト110の突起部112に支持されて搬送された繊維ウェブ50に対して、第1ノズル120によって第1の加熱流体H1を吹き付ける工程である。第1の加熱流体H1の吹き付けによって、繊維ウェブ50は、コンベアベルト110の突起部112の形状に沿った凹凸形状に賦形されるとともに、繊維ウェブ50の構成繊維が熱融着性繊維の溶融固化により熱融着して凹凸形状が維持される。 [First air-through process]
The first air-through process is a process in which the first heating fluid H1 is sprayed by thefirst nozzle 120 onto the fiber web 50 that is supported and conveyed by the protrusion 112 of the conveyor belt 110. By spraying the first heating fluid H1, the fiber web 50 is shaped into a concavo-convex shape along the shape of the projection 112 of the conveyor belt 110, and the constituent fibers of the fiber web 50 are melted by heat-fusible fibers. The uneven shape is maintained by heat fusion by solidification.
第1エアースルー工程は、コンベアベルト110の突起部112に支持されて搬送された繊維ウェブ50に対して、第1ノズル120によって第1の加熱流体H1を吹き付ける工程である。第1の加熱流体H1の吹き付けによって、繊維ウェブ50は、コンベアベルト110の突起部112の形状に沿った凹凸形状に賦形されるとともに、繊維ウェブ50の構成繊維が熱融着性繊維の溶融固化により熱融着して凹凸形状が維持される。 [First air-through process]
The first air-through process is a process in which the first heating fluid H1 is sprayed by the
第1ノズル120は、その噴出孔(不図示)から第1の加熱流体H1を噴出し、コンベアベルト110上の繊維ウェブ50に対してほぼ垂直に吹き付ける。
The first nozzle 120 ejects the first heated fluid H1 from its ejection hole (not shown) and sprays it almost perpendicularly to the fiber web 50 on the conveyor belt 110.
第1の加熱流体H1としては、第1ノズル120が具備する第1ヒータ(不図示)によって所定温度に加熱された空気、水蒸気等が使用される。第1の加熱流体H1は、第1の加熱流体H1の吹き付けによって付与された繊維ウェブ50の凹凸形状が、繊維ウェブ50の構成繊維同士の熱融着によって維持される温度まで、第1ヒータによって加熱される。例えば、繊維ウェブ50が、鞘成分(低融点成分)がポリエチレン(PE)であり、芯成分(高融点成分)がポリエチレンテレフタレート(PET)である芯鞘型複合繊維を含有する場合、第1の加熱流体H1の温度は、好ましくは80~155℃、さらに好ましくは130~135℃である。第1の加熱流体H1の温度が、芯鞘型複合繊維の低融点成分の融点より低すぎると、繊維ウェブ50の凹凸賦形が不十分となる一方、低融点成分の融点より高すぎると、繊維ウェブ50の構成繊維同士が急速に融着し、繊維の自由度が低下するため、繊維ウェブ50の凹凸賦形が不十分となる。
As the first heating fluid H1, air, water vapor or the like heated to a predetermined temperature by a first heater (not shown) provided in the first nozzle 120 is used. The first heating fluid H1 is heated by the first heater up to a temperature at which the uneven shape of the fiber web 50 applied by spraying the first heating fluid H1 is maintained by thermal fusion of the constituent fibers of the fiber web 50. Heated. For example, when the fiber web 50 contains a core-sheath type composite fiber in which the sheath component (low melting point component) is polyethylene (PE) and the core component (high melting point component) is polyethylene terephthalate (PET), the first The temperature of the heating fluid H1 is preferably 80 to 155 ° C., more preferably 130 to 135 ° C. If the temperature of the first heating fluid H1 is too lower than the melting point of the low melting point component of the core-sheath composite fiber, the uneven shaping of the fiber web 50 becomes insufficient, while if it is too high than the melting point of the low melting point component, Since the constituent fibers of the fiber web 50 are rapidly fused and the degree of freedom of the fibers is reduced, the uneven shaping of the fiber web 50 becomes insufficient.
第1の加熱流体H1の風速は、好ましくは20~120m/秒、さらに好ましくは40~80m/秒である。なお、第1の加熱流体H1の風速が遅すぎると、繊維ウェブ50が突起部112に十分に沿わず、繊維ウェブ50の凹凸賦形が不十分となる一方、風速が速すぎると、繊維ウェブ50の構成繊維が突起部112により選り分けられ、繊維ウェブ50の凹凸賦形が不十分となる。
The wind speed of the first heating fluid H1 is preferably 20 to 120 m / sec, more preferably 40 to 80 m / sec. In addition, if the wind speed of the 1st heating fluid H1 is too slow, the fiber web 50 will not fully follow the projection part 112, and the uneven | corrugated shaping of the fiber web 50 will become inadequate, On the other hand, if the wind speed is too fast, the fiber web 50 constituent fibers are selected by the protrusion 112, and the uneven shaping of the fiber web 50 becomes insufficient.
第1の加熱流体H1の吹き付け時間は、好ましくは0.01~0.5秒、さらに好ましくは0.04~0.08秒である。吹き付け時間が短すぎると、繊維ウェブ50の凹凸賦形が不十分となる一方、吹き付け時間が長すぎると、繊維ウェブ50の構成繊維が突起部112により選り分けられ、繊維ウェブ50の凹凸賦形が不十分となる。
The spraying time of the first heating fluid H1 is preferably 0.01 to 0.5 seconds, more preferably 0.04 to 0.08 seconds. When the spraying time is too short, the unevenness shaping of the fiber web 50 becomes insufficient. On the other hand, when the spraying time is too long, the constituent fibers of the fiber web 50 are selected by the protrusions 112, and the unevenness shaping of the fiber web 50 is performed. It becomes insufficient.
コンベアベルト110の突起部112に支持されて搬送された繊維ウェブ50に対して、第1ノズル120によって第1の加熱流体H1を吹き付ける際、駆動ロール151,152の周速度V1の調整によって、所望の繊維密度及び繊維量(坪量)を有する凸部側壁部及び凹部側壁部が形成される。例えば、駆動ロール151,152の周速度V1を、コンベアベルト110の周速度V2より高速とすることにより、第1ノズル120によって繊維ウェブ50が処理される際、繊維ウェブ50がオーバーフィードされ、凹凸賦形時に生じるおそれがる凸部側壁部及び凹部側壁部の繊維密度及び繊維量(坪量)の低下が防止される。駆動ロール151,152の周速度V1は、コンベアベルト110の周速度V2の1~2倍であることが好ましく、1~1.5倍であることがさらに好ましい。
When the first heated fluid H1 is sprayed by the first nozzle 120 onto the fiber web 50 supported and conveyed by the protrusion 112 of the conveyor belt 110, the desired speed is adjusted by adjusting the peripheral speed V1 of the drive rolls 151 and 152. The convex part side wall part and concave part side wall part which have the fiber density and fiber amount (basis weight) are formed. For example, when the fiber web 50 is processed by the first nozzle 120 by setting the peripheral speed V1 of the drive rolls 151 and 152 to be higher than the peripheral speed V2 of the conveyor belt 110, the fiber web 50 is over-fed and uneven. A decrease in fiber density and fiber amount (basis weight) of the convex side wall and the concave side wall that may occur during shaping is prevented. The circumferential speed V1 of the drive rolls 151, 152 is preferably 1 to 2 times, more preferably 1 to 1.5 times the circumferential speed V2 of the conveyor belt 110.
繊維ウェブ50を通過した第1の加熱流体H1は、コンベアベルト110の通気孔を通ってダクト121から外部に排出される。
The 1st heating fluid H1 which passed the fiber web 50 is discharged | emitted from the duct 121 through the vent hole of the conveyor belt 110 outside.
第1エアースルー工程を経た繊維ウェブ50は、コンベアベルト110上に載置された状態のまま第2ノズル130の位置まで搬送される。
The fiber web 50 that has undergone the first air-through process is conveyed to the position of the second nozzle 130 while being placed on the conveyor belt 110.
[第2エアースルー工程]
第2エアースルー工程は、第1エアースルー工程を経た繊維ウェブ50に対して、第2ノズル130によって第2の加熱流体H2を吹き付ける工程である。第2の加熱流体H2の吹き付けによって、繊維ウェブ50の構成繊維が熱融着性繊維の溶融固化により熱融着して、第1エアースルー工程で付与された繊維ウェブ50の凹凸形状が固定される。こうして、第1及び第2エアースルー工程を通じて繊維ウェブ50は凹凸賦形される。 [Second air-through process]
A 2nd air through process is a process of spraying the 2nd heating fluid H2 with the2nd nozzle 130 with respect to the fiber web 50 which passed the 1st air through process. By spraying the second heating fluid H2, the constituent fibers of the fiber web 50 are heat-sealed by melting and solidifying the heat-fusible fibers, and the uneven shape of the fiber web 50 applied in the first air-through process is fixed. The In this way, the fibrous web 50 is formed with unevenness through the first and second air-through processes.
第2エアースルー工程は、第1エアースルー工程を経た繊維ウェブ50に対して、第2ノズル130によって第2の加熱流体H2を吹き付ける工程である。第2の加熱流体H2の吹き付けによって、繊維ウェブ50の構成繊維が熱融着性繊維の溶融固化により熱融着して、第1エアースルー工程で付与された繊維ウェブ50の凹凸形状が固定される。こうして、第1及び第2エアースルー工程を通じて繊維ウェブ50は凹凸賦形される。 [Second air-through process]
A 2nd air through process is a process of spraying the 2nd heating fluid H2 with the
第2ノズル130は、その噴出孔(不図示)から第2の加熱流体H2を噴出し、コンベアベルト110上の繊維ウェブ50に対してほぼ垂直に吹き付ける。
The second nozzle 130 ejects the second heated fluid H2 from its ejection hole (not shown) and blows it almost perpendicularly to the fiber web 50 on the conveyor belt 110.
第2の加熱流体H2としては、第2ノズル130が具備する第2ヒータ(不図示)によって所定温度に加熱された空気、水蒸気等が使用される。第2の加熱流体H2は、繊維ウェブ50の凹凸形状が維持された状態で繊維ウェブ50の構成繊維同士を熱融着させる温度まで、第2ヒータによって加熱される。繊維ウェブ50が、低融点成分と低融点成分よりも融点の高い高融点成分とを有する複合繊維の場合、第2の加熱流体H2は、低融点成分の融点~高融点成分の融点の温度に制御される。例えば、繊維ウェブ50が、鞘成分(低融点成分)がPEであり、芯成分(高融点成分)がPETである芯鞘型複合繊維を含有する場合、第2の加熱流体H2の温度は、好ましくは130~155℃、さらに好ましくは135~150℃である。第2の加熱流体H2の温度が低すぎると、構成繊維同士の熱融着が不十分となり、繊維ウェブ50の凹凸形状の固定が不十分となる一方、第2の加熱流体H2の温度が高すぎると、不織布の風合いが悪くなり、嵩が出にくくなる。
As the second heating fluid H2, air, water vapor or the like heated to a predetermined temperature by a second heater (not shown) provided in the second nozzle 130 is used. The second heating fluid H <b> 2 is heated by the second heater to a temperature at which the constituent fibers of the fiber web 50 are heat-sealed while the uneven shape of the fiber web 50 is maintained. When the fiber web 50 is a composite fiber having a low-melting-point component and a high-melting-point component having a higher melting point than the low-melting-point component, the second heating fluid H2 has a temperature from the melting point of the low-melting-point component to the melting point of the high-melting-point component. Be controlled. For example, when the fiber web 50 contains a core-sheath type composite fiber in which the sheath component (low melting point component) is PE and the core component (high melting point component) is PET, the temperature of the second heating fluid H2 is: The temperature is preferably 130 to 155 ° C, more preferably 135 to 150 ° C. If the temperature of the second heating fluid H2 is too low, the thermal fusion between the constituent fibers becomes insufficient, and the unevenness of the fiber web 50 is insufficiently fixed, while the temperature of the second heating fluid H2 is high. If it is too much, the texture of the nonwoven fabric becomes worse and it becomes difficult to produce bulk.
第2の加熱流体H2の風速は、好ましくは1~10m/秒、さらに好ましくは2~8m/秒である。第2の加熱流体H2の風速が遅すぎると、熱量が不足するため、不織布強度が不十分になる一方、風速が速すぎると、繊維ウェブ50の厚みが風圧で減少した状態で構成繊維同士が熱融着するため厚みが薄くなる。
The wind speed of the second heating fluid H2 is preferably 1 to 10 m / second, more preferably 2 to 8 m / second. If the wind speed of the second heating fluid H2 is too slow, the amount of heat will be insufficient, and the strength of the nonwoven fabric will be insufficient. The thickness is reduced due to heat fusion.
第2の加熱流体H2の吹き付け時間は、好ましくは0.03~5秒、さらに好ましくは0.1~1秒である。吹き付け時間が短すぎると、繊維ウェブ50の構成繊維同士の融着が不十分となり、繊維ウェブ50の凹凸形状の固定が不十分となる一方、吹き付け時間が長すぎると、繊維ウェブ50の構成繊維同士の熱融着が過剰に進む。
The spraying time of the second heating fluid H2 is preferably 0.03 to 5 seconds, more preferably 0.1 to 1 second. When the spraying time is too short, the constituent fibers of the fiber web 50 are not sufficiently fused to each other, and the unevenness of the fiber web 50 is insufficiently fixed. On the other hand, when the spraying time is too long, the constituent fibers of the fiber web 50 are formed. The heat fusion between the two proceeds excessively.
[冷却工程]
冷却工程は、第1及び第2エアースルー工程を経て凹凸賦形された繊維ウェブ50を、冷却部160によって冷却する工程である。冷却によって繊維ウェブ50の構成繊維の熱融着点が強固となる。
冷却部160は、第2ノズル130と第3ノズル140との間に配された空間である。この空間により、第2エアースルー工程後の繊維ウェブ50が自然冷却される。冷却部160には、繊維ウェブ50を強制冷却するさせる冷却装置を設けてもよい。強制冷却の方法としては、例えば、冷却ノズルからの冷却流体の吹き付け、冷却ローラとの接触等が挙げられる。冷却温度は、好ましくは、繊維ウェブ50に含有される熱融着性繊維の融点よりも低い温度であり、熱可塑性繊維が芯鞘型複合繊維である場合、好ましくは、低融点成分の融点よりも低い温度である。冷却温度は、例えば、100℃以下である。 [Cooling process]
The cooling process is a process in which thecooling unit 160 cools the fiber web 50 that has been unevenly shaped through the first and second air-through processes. Cooling strengthens the thermal fusion point of the constituent fibers of the fiber web 50.
Thecooling unit 160 is a space arranged between the second nozzle 130 and the third nozzle 140. This space naturally cools the fiber web 50 after the second air-through process. The cooling unit 160 may be provided with a cooling device that forcibly cools the fiber web 50. Examples of the forced cooling method include spraying of a cooling fluid from a cooling nozzle and contact with a cooling roller. The cooling temperature is preferably a temperature lower than the melting point of the heat-fusible fiber contained in the fiber web 50. When the thermoplastic fiber is a core-sheath type composite fiber, it is preferably lower than the melting point of the low-melting point component. Is also a low temperature. The cooling temperature is, for example, 100 ° C. or less.
冷却工程は、第1及び第2エアースルー工程を経て凹凸賦形された繊維ウェブ50を、冷却部160によって冷却する工程である。冷却によって繊維ウェブ50の構成繊維の熱融着点が強固となる。
冷却部160は、第2ノズル130と第3ノズル140との間に配された空間である。この空間により、第2エアースルー工程後の繊維ウェブ50が自然冷却される。冷却部160には、繊維ウェブ50を強制冷却するさせる冷却装置を設けてもよい。強制冷却の方法としては、例えば、冷却ノズルからの冷却流体の吹き付け、冷却ローラとの接触等が挙げられる。冷却温度は、好ましくは、繊維ウェブ50に含有される熱融着性繊維の融点よりも低い温度であり、熱可塑性繊維が芯鞘型複合繊維である場合、好ましくは、低融点成分の融点よりも低い温度である。冷却温度は、例えば、100℃以下である。 [Cooling process]
The cooling process is a process in which the
The
[第3エアースルー工程]
第3エアースルー工程は、冷却工程を経た繊維ウェブ50に対して、第3ノズル140によって第3の加熱流体H3を吹き付ける工程である。第3の加熱流体H3の吹き付けによって、毛羽立ちしている繊維と毛羽立ちしていない繊維とが新たな融着点で熱融着し、毛羽立ちがない賦形不織布が製造される。 [Third air-through process]
A 3rd air through process is a process of spraying the 3rd heating fluid H3 with the3rd nozzle 140 with respect to the fiber web 50 which passed through the cooling process. By spraying the third heating fluid H3, the fluffed fibers and the non-fluffed fibers are thermally fused at a new fusion point, and a shaped nonwoven fabric without fluff is produced.
第3エアースルー工程は、冷却工程を経た繊維ウェブ50に対して、第3ノズル140によって第3の加熱流体H3を吹き付ける工程である。第3の加熱流体H3の吹き付けによって、毛羽立ちしている繊維と毛羽立ちしていない繊維とが新たな融着点で熱融着し、毛羽立ちがない賦形不織布が製造される。 [Third air-through process]
A 3rd air through process is a process of spraying the 3rd heating fluid H3 with the
第3ノズル140は、その噴出孔(不図示)から第3の加熱流体H3を噴出し、コンベアベルト110上の繊維ウェブ50に対してほぼ垂直に吹き付ける。
The third nozzle 140 ejects the third heating fluid H3 from its ejection hole (not shown) and sprays it almost perpendicularly to the fiber web 50 on the conveyor belt 110.
第3の加熱流体H3としては、第3ノズル140が具備する第3ヒータ(不図示)によって所定温度に加熱された空気、水蒸気等が使用される。第3の加熱流体H3は、繊維ウェブ50の凹凸形状が維持された状態で繊維ウェブ50の毛羽立ち繊維と他の構成繊維とを熱融着させる温度まで、第3ヒータによって加熱される。繊維ウェブ50が、低融点成分と低融点成分よりも融点の高い高融点成分とを有する複合繊維の場合、第3の加熱流体H3は、低融点成分の融点~高融点成分の融点の温度に制御される。例えば、繊維ウェブ50が、鞘部がPEであり、芯成分がPETである芯鞘型複合繊維を含有する場合、第3の加熱流体H3の温度は、好ましくは130~155℃、さらに好ましくは130~145℃である。第3の加熱流体H3の温度が低すぎると、繊維同士の熱融着が不十分となり、毛羽立ちの低減が不十分となる一方、第3の加熱流体H3の温度が高すぎると、毛羽立ち繊維以外の繊維同士も融着されて、液透過性が低下する。
As the third heating fluid H3, air, water vapor or the like heated to a predetermined temperature by a third heater (not shown) provided in the third nozzle 140 is used. The third heating fluid H3 is heated by the third heater to a temperature at which the fuzzy fibers of the fiber web 50 and other constituent fibers are heat-sealed while the uneven shape of the fiber web 50 is maintained. When the fiber web 50 is a composite fiber having a low melting point component and a high melting point component having a higher melting point than the low melting point component, the third heating fluid H3 has a temperature ranging from the melting point of the low melting point component to the melting point of the high melting point component. Be controlled. For example, when the fiber web 50 contains core-sheath type composite fibers whose sheath is PE and whose core component is PET, the temperature of the third heating fluid H3 is preferably 130 to 155 ° C., more preferably 130-145 ° C. If the temperature of the third heating fluid H3 is too low, heat fusion between the fibers will be insufficient, and the reduction of fuzz will be insufficient. On the other hand, if the temperature of the third heating fluid H3 is too high, other than the fuzzy fibers These fibers are also fused, and the liquid permeability is lowered.
第3の加熱流体H3の風速は、好ましくは0.5~5m/秒、さらに好ましくは2~8m/秒である。第3の加熱流体H3の風速が遅すぎると、毛羽立ち繊維を寝せることができず、毛羽立ちの低減が不十分となる一方、風速が速すぎると、厚みが風圧で低下した状態で毛羽立ち繊維以外の繊維同士の熱融着が生じるため、厚みが小さくなり、感触と液浸透性が不十分になる。
The wind speed of the third heating fluid H3 is preferably 0.5 to 5 m / second, more preferably 2 to 8 m / second. If the wind speed of the third heating fluid H3 is too slow, the fluffy fibers cannot be laid down, and the reduction of the fuzz becomes insufficient. Since heat fusion occurs between the fibers, the thickness is reduced, and the feel and liquid permeability are insufficient.
第3の加熱流体H3の吹き付け時間は、好ましくは0.3~10秒、さらに好ましくは2~6秒である。吹き付け時間が短すぎると、毛羽立ち繊維と他の繊維同士の熱融着が十分にできず毛羽立ちを低減することが難しくなる。一方、吹き付け時間が長すぎると繊維ウェブ50の毛羽立ち繊維以外の繊維同士が融着され過ぎて、厚みが小さくなり、風合いと液透過性が得られ難くなる。
The spraying time of the third heating fluid H3 is preferably 0.3 to 10 seconds, more preferably 2 to 6 seconds. If the spraying time is too short, the fuzzy fiber and other fibers cannot be sufficiently heat-sealed, and it becomes difficult to reduce the fuzz. On the other hand, if the spraying time is too long, fibers other than the fluffy fibers of the fiber web 50 are too fused together, the thickness becomes small, and the texture and liquid permeability are difficult to obtain.
<血液滑性付与剤>
血液滑性付与剤は、40℃における動粘度が約0.01~約80mm2/sであり、抱水率が約0.05~約4.0質量%であり、重量平均分子量が約1,000未満である。 <Blood slipperiness agent>
The blood slipperiness imparting agent has a kinematic viscosity at 40 ° C. of about 0.01 to about 80 mm 2 / s, a water retention of about 0.05 to about 4.0% by mass, and a weight average molecular weight of about 1 Less than 1,000.
血液滑性付与剤は、40℃における動粘度が約0.01~約80mm2/sであり、抱水率が約0.05~約4.0質量%であり、重量平均分子量が約1,000未満である。 <Blood slipperiness agent>
The blood slipperiness imparting agent has a kinematic viscosity at 40 ° C. of about 0.01 to about 80 mm 2 / s, a water retention of about 0.05 to about 4.0% by mass, and a weight average molecular weight of about 1 Less than 1,000.
血液滑性付与剤の40℃における動粘度は、約0~約80mm2/sの範囲において適宜調整することができるが、好ましくは約1~約70mm2/s、さらに好ましくは約3~約60mm2/s、さらに一層好ましくは約5~約50mm2/s、さらに一層好ましくは約7~約45mm2/sである。なお、本明細書では、40℃における動粘度を、単に「動粘度」と称する場合がある。
The kinematic viscosity at 40 ° C. of the blood slipperiness-imparting agent can be appropriately adjusted in the range of about 0 to about 80 mm 2 / s, preferably about 1 to about 70 mm 2 / s, more preferably about 3 to about 60 mm 2 / s, even more preferably from about 5 to about 50 mm 2 / s, and even more preferably from about 7 to about 45 mm 2 / s. In this specification, the kinematic viscosity at 40 ° C. may be simply referred to as “kinematic viscosity”.
動粘度は、a)血液滑性付与剤の分子量が大きくなるほど、b)極性基、例えば、カルボニル結合(-CO-)、エーテル結合(-O-)、カルボキシル基(-COOH)、ヒドロキシル基(-OH)等の比率が高いほど、そしてc)IOBが大きくなるほど、高くなる傾向がある。
The kinematic viscosity is as follows: a) as the molecular weight of the blood slipping agent increases, b) polar groups such as carbonyl bond (—CO—), ether bond (—O—), carboxyl group (—COOH), hydroxyl group ( There is a tendency that the higher the ratio of —OH) and the like, and c) the higher the IOB, the higher the ratio.
40℃において、約0~約80mm2/sの動粘度を有するためには、血液滑性付与剤の融点が45℃以下であることが好ましい。血液滑性付与剤が40℃で結晶を含むと、その動粘度が高くなる傾向があるからである。
In order to have a kinematic viscosity of about 0 to about 80 mm 2 / s at 40 ° C., the melting point of the blood slipperiness imparting agent is preferably 45 ° C. or less. This is because when the blood slipperiness-imparting agent contains crystals at 40 ° C., the kinematic viscosity tends to increase.
血液滑性付与剤における動粘度の意義については後述するが、動粘度が約80mm2/sを超えると、血液滑性付与剤の粘性が高く、トップシートの肌当接面に到達した経血と共に、凸部から凹部に滑落し、次いで吸収体内部に移行することが難しくなる傾向がある。
The significance of kinematic viscosity in the blood slipperiness-imparting agent will be described later. When the kinematic viscosity exceeds about 80 mm 2 / s, the blood slipperiness-imparting agent is too viscous to reach the skin contact surface of the top sheet. At the same time, it tends to be difficult to slide from the convex portion to the concave portion and then to move into the absorber.
動粘度は、JIS K 2283:2000の「5.動粘度試験方法」に従って、キャノンフェンスケ逆流形粘度計を用いて、40℃の試験温度で測定されることができる。
The kinematic viscosity can be measured at a test temperature of 40 ° C. using a Cannon Fenceke reverse flow viscometer according to “5. Kinematic viscosity test method” of JIS K 2283: 2000.
血液滑性付与剤の抱水率は、約0.01~約4.0質量%の範囲で適宜調整することができるが、好ましくは約0.02~約3.5質量%、さらに好ましくは約0.03~約3.0質量%、さらに一層好ましくは約0.04~約2.5質量%、さらに一層好ましくは約0.05~約2.0質量%である。
The water retention rate of the blood slipperiness-imparting agent can be appropriately adjusted within the range of about 0.01 to about 4.0% by mass, preferably about 0.02 to about 3.5% by mass, more preferably About 0.03 to about 3.0% by weight, even more preferably about 0.04 to about 2.5% by weight, even more preferably about 0.05 to about 2.0% by weight.
本明細書において、「抱水率」は、物質が、保持することができる水の比率(質量)を意味し、以下の通りに測定することができる。
(1)40℃の恒温室に、20mLの試験管、ゴム栓、測定すべき物質及び脱イオン水を一昼夜静置する。
(2)恒温室で、試験管に、測定すべき物質5.0gと、脱イオン水5.0gを投入する。
(3)恒温室で、試験管の口をゴム栓をし、試験管を1回転させ、5分間静置する。
(4)恒温室で、測定すべき物質の層(通常は、上層)3.0gを、直径90mmの、質量:W0(g)のガラス製シャーレに採取する。
(5)シャーレを、オーブン内で、105℃で3時間加熱し、水分を蒸発させ、シャーレごと、質量:W1(g)を測定する。
(6)抱水率を、以下の式に従って算出する。
抱水率(質量%)=100×[W0(g)-W1(g)]/3.0(g)
測定は3回実施し、平均値を採用する。 In the present specification, the “water retention” means the ratio (mass) of water that a substance can hold, and can be measured as follows.
(1) Place a 20 mL test tube, a rubber stopper, a substance to be measured, and deionized water in a constant temperature room at 40 ° C. overnight.
(2) In a constant temperature room, 5.0 g of a substance to be measured and 5.0 g of deionized water are put into a test tube.
(3) Put a rubber stopper on the mouth of the test tube in a thermostatic chamber, rotate the test tube once, and let it stand for 5 minutes.
(4) In a thermostatic chamber, 3.0 g of the substance layer to be measured (usually the upper layer) is collected in a glass petri dish having a diameter of 90 mm and a mass of W 0 (g).
(5) The petri dish is heated in an oven at 105 ° C. for 3 hours to evaporate water, and the whole petri dish is measured for mass: W 1 (g).
(6) The water retention rate is calculated according to the following formula.
Water retention (mass%) = 100 × [W 0 (g) −W 1 (g)] / 3.0 (g)
The measurement is carried out three times and the average value is adopted.
(1)40℃の恒温室に、20mLの試験管、ゴム栓、測定すべき物質及び脱イオン水を一昼夜静置する。
(2)恒温室で、試験管に、測定すべき物質5.0gと、脱イオン水5.0gを投入する。
(3)恒温室で、試験管の口をゴム栓をし、試験管を1回転させ、5分間静置する。
(4)恒温室で、測定すべき物質の層(通常は、上層)3.0gを、直径90mmの、質量:W0(g)のガラス製シャーレに採取する。
(5)シャーレを、オーブン内で、105℃で3時間加熱し、水分を蒸発させ、シャーレごと、質量:W1(g)を測定する。
(6)抱水率を、以下の式に従って算出する。
抱水率(質量%)=100×[W0(g)-W1(g)]/3.0(g)
測定は3回実施し、平均値を採用する。 In the present specification, the “water retention” means the ratio (mass) of water that a substance can hold, and can be measured as follows.
(1) Place a 20 mL test tube, a rubber stopper, a substance to be measured, and deionized water in a constant temperature room at 40 ° C. overnight.
(2) In a constant temperature room, 5.0 g of a substance to be measured and 5.0 g of deionized water are put into a test tube.
(3) Put a rubber stopper on the mouth of the test tube in a thermostatic chamber, rotate the test tube once, and let it stand for 5 minutes.
(4) In a thermostatic chamber, 3.0 g of the substance layer to be measured (usually the upper layer) is collected in a glass petri dish having a diameter of 90 mm and a mass of W 0 (g).
(5) The petri dish is heated in an oven at 105 ° C. for 3 hours to evaporate water, and the whole petri dish is measured for mass: W 1 (g).
(6) The water retention rate is calculated according to the following formula.
Water retention (mass%) = 100 × [W 0 (g) −W 1 (g)] / 3.0 (g)
The measurement is carried out three times and the average value is adopted.
血液滑性付与剤における抱水率の意義については後述するが、抱水率が低くなると、血液滑性付与剤と、経血との親和性が低下し、トップシートの肌当接面に到達した経血と共に吸収体に移行しにくくなる傾向がある。一方、抱水率が高くなると、界面活性剤のように、経血との親和性が非常に高くなり、トップシートの肌当接面に、吸収した血液が残存し、トップシートの肌当接面が赤く着色しやすくなる傾向がある。
Although the significance of the water retention rate in the blood slipperiness-imparting agent will be described later, when the water retention rate decreases, the affinity between the blood slipperiness-imparting agent and menstrual blood decreases and reaches the skin contact surface of the top sheet. There is a tendency that it becomes difficult to transfer to the absorbent body with the menstrual blood. On the other hand, when the water retention rate becomes high, the affinity with menstrual blood becomes very high like a surfactant, and the absorbed blood remains on the skin contact surface of the top sheet, and the skin contact of the top sheet The surface tends to be red and easily colored.
抱水率は、a)血液滑性付与剤の分子量が小さくなるほど、そしてb)極性基、例えば、カルボニル結合(-CO-)、エーテル結合(-O-)、カルボキシル基(-COOH)、ヒドロキシル基(-OH)等の比率が高いほど、値が大きくなる傾向がある。血液滑性付与剤が、より親水性を有するからである。また、抱水率は、IOBが大きくなるほど、すなわち、無機性値が高いほど、そして有機性値が小さいほど、値が大きくなる傾向がある。血液滑性付与剤が、より親水性を有することになるからである。
The water retention rate is as follows: a) the smaller the molecular weight of the blood slipperiness agent, and b) polar groups such as carbonyl bond (—CO—), ether bond (—O—), carboxyl group (—COOH), hydroxyl As the ratio of the group (—OH) or the like is higher, the value tends to increase. This is because the blood slipperiness imparting agent is more hydrophilic. Further, the water retention rate tends to increase as the IOB increases, that is, as the inorganic value increases and as the organic value decreases. This is because the blood slipperiness-imparting agent is more hydrophilic.
血液滑性付与剤における動粘度と、抱水率との意義について説明する。
The significance of kinematic viscosity and water retention rate in the blood slipping agent will be described.
着用者から排泄された経血が排泄口当接領域に到達すると、凸部に存在する血液滑性付与剤と接触し、これとともに凹部に滑落し、トップシートを通過して吸収体に移行する。
When menstrual blood excreted from the wearer reaches the excretory opening contact region, it contacts the blood slipperiness imparting agent present in the convex part, slides into the concave part together with this, passes through the top sheet, and moves to the absorber. .
より詳細には、40℃において約0.01~約80mm2/sの動粘度を有する血液滑性付与剤は、着用者の体温付近で非常に低粘度であり且つ経血と一定の親和性を有するため、経血とともに、凸部から凹部に滑落し、その滑落の際の勢いを利用して、経血が、トップシートを通過し、吸収体に迅速に移行することができると考えられる。また、凸部に存在する血液滑性付与剤は、約0.01~約4.0質量%の抱水率を有するため、経血中の、主に親水性成分(血漿等)と親和性を有しないため、経血をトップシート上に残存させにくいと考えられる。
More specifically, a blood slipperiness-imparting agent having a kinematic viscosity of about 0.01 to about 80 mm 2 / s at 40 ° C. has a very low viscosity around the body temperature of the wearer and has a certain affinity with menstrual blood Therefore, it is considered that menstrual blood can slide from the convex part to the concave part together with menstrual blood, and can pass through the top sheet and quickly migrate to the absorbent body using the momentum at the time of the sliding. . In addition, the blood slipperiness-imparting agent present on the convex part has a water retention of about 0.01 to about 4.0% by mass, and therefore has an affinity for mainly hydrophilic components (such as plasma) in menstrual blood. Therefore, it is considered that menstrual blood hardly remains on the top sheet.
着用者から排出された経血が大量である場合には、経血そのものの運動エネルギーが大きく、血液滑性付与剤の動粘度の値が比較的高く経血と共に滑落しにくい場合であっても、抱水率の値が比較的高く経血の親水性成分と親和性が高い場合であっても、重量分子量の値が比較的高く経血と共に滑落しにくい場合であっても、そしてトップシートの肌当接面に凹凸構造がない場合であっても、経血は吸収体に移行しやすいと考えられる。
If there is a large amount of menstrual blood discharged from the wearer, even if the kinetic energy of menstrual blood itself is large and the kinematic viscosity value of the blood slipperiness-imparting agent is relatively high, it is difficult to slip with menstrual blood. Even if the water retention value is relatively high and the affinity with the hydrophilic component of menstrual blood is high, the weight molecular weight value is relatively high and difficult to slide with menstrual blood, and the top sheet Even when there is no uneven structure on the skin contact surface, it is considered that menstrual blood easily migrates to the absorber.
一方、着用者から排出された経血が少量である場合には、経血の運動エネルギーが小さく、トップシートの肌当接面に到達した経血が、その場に留まりやすい傾向がある。従って、血液滑性付与剤が、経血とともに、凸部から凹部に滑落し、そして経血をトップシートの内部に引き込み、次いで吸収体に引き込むことにより、経血を迅速に吸収体に移行させることができる。
On the other hand, when the menstrual blood discharged from the wearer is small, the kinetic energy of menstrual blood is small, and menstrual blood that has reached the skin contact surface of the top sheet tends to stay on the spot. Accordingly, the blood slipperiness-imparting agent slides down from the convex part to the concave part together with menstrual blood, and draws menstrual blood into the top sheet, and then draws it into the absorber, thereby rapidly transferring menstrual blood to the absorber. be able to.
血液滑性付与剤は、約1,000未満の重量平均分子量を有し、そして好ましくは約900未満の重量平均分子量を有する。重量平均分子量が約1,000以上であると、血液滑性付与剤そのものにタック性が生じ、着用者に不快感を与える傾向があるからである。また、重量平均分子量が高くなると、血液滑性付与剤の粘度が高くなる傾向があるため、加温により、血液滑性付与剤の粘度を、塗工に適した粘度に下げることが難しくなり、その結果、血液滑性付与剤を、溶媒で希釈しなければならない場合も生じうる。
The blood lubricity-imparting agent has a weight average molecular weight of less than about 1,000, and preferably has a weight average molecular weight of less than about 900. This is because if the weight average molecular weight is about 1,000 or more, the blood slipperiness-imparting agent itself has tackiness and tends to give the wearer discomfort. In addition, since the viscosity of the blood slipperiness-imparting agent tends to increase as the weight average molecular weight increases, it becomes difficult to lower the viscosity of the blood slipperiness-imparting agent to a viscosity suitable for coating by heating, As a result, the blood slipping agent may have to be diluted with a solvent.
血液滑性付与剤は、約100以上の重量平均分子量を有することが好ましく、そして約200以上の重量平均分子量を有することがより好ましい。重量平均分子量が小さくなると、血液滑性付与剤の蒸気圧が高くなり、保存中に気化し、量の減少、着用時の臭気等の問題が発生する場合があるからである。
The blood slipperiness-imparting agent preferably has a weight average molecular weight of about 100 or more, and more preferably has a weight average molecular weight of about 200 or more. This is because when the weight average molecular weight is small, the vapor pressure of the blood slipperiness imparting agent becomes high and vaporizes during storage, causing problems such as a decrease in the amount and odor when worn.
なお、本明細書において、「重量平均分子量」は、多分散系の化合物(例えば、逐次重合により製造された化合物、複数の脂肪酸と、複数の脂肪族1価アルコールとから生成されたエステル)と、単一化合物(例えば、1種の脂肪酸と、1種の脂肪族1価アルコールから生成されたエステル)とを含む概念であり、Ni個の分子量Miの分子(i=1、又はi=1,2・・・)からなる系において、次の式:
Mw=ΣNiMi 2/ΣNiMi
により求められるMwを意味する。 In the present specification, “weight average molecular weight” refers to a polydispersed compound (for example, a compound produced by sequential polymerization, a plurality of fatty acids, and an ester produced from a plurality of aliphatic monohydric alcohols). , A concept that includes a single compound (eg, an ester formed from one fatty acid and one aliphatic monohydric alcohol), and N i molecules of molecular weight M i (i = 1, or i = 1, 2, ...), the following formula:
M w = ΣN i M i 2 / ΣN i M i
Means M w obtained by
Mw=ΣNiMi 2/ΣNiMi
により求められるMwを意味する。 In the present specification, “weight average molecular weight” refers to a polydispersed compound (for example, a compound produced by sequential polymerization, a plurality of fatty acids, and an ester produced from a plurality of aliphatic monohydric alcohols). , A concept that includes a single compound (eg, an ester formed from one fatty acid and one aliphatic monohydric alcohol), and N i molecules of molecular weight M i (i = 1, or i = 1, 2, ...), the following formula:
M w = ΣN i M i 2 / ΣN i M i
Means M w obtained by
本明細書において、重量平均分子量は、ゲルパーミエーションクロマトグラフィー(GPC)により求められる、ポリスチレン換算の値を意味する。
GPCの測定条件としては、例えば、以下が挙げられる。
機種:(株)日立ハイテクノロジーズ製 高速液体クロマトグラム Lachrom Elite
カラム:昭和電工(株)製 SHODEX KF-801、KF-803及びKF-804
溶離液:THF
流量 :1.0mL/分
打込み量:100μL
検出:RI(示差屈折計)
なお、本明細書の実施例に記載される重量平均分子量は、上記条件により測定したものである。 In this specification, a weight average molecular weight means the value of polystyrene conversion calculated | required by gel permeation chromatography (GPC).
Examples of GPC measurement conditions include the following.
Model: Hitachi High-Technologies Corporation High-Performance Liquid Chromatogram Lachrom Elite
Column: SHODEX KF-801, KF-803 and KF-804 manufactured by Showa Denko K.K.
Eluent: THF
Flow rate: 1.0 mL / min Driving amount: 100 μL
Detection: RI (differential refractometer)
In addition, the weight average molecular weight described in the Example of this specification is measured on the said conditions.
GPCの測定条件としては、例えば、以下が挙げられる。
機種:(株)日立ハイテクノロジーズ製 高速液体クロマトグラム Lachrom Elite
カラム:昭和電工(株)製 SHODEX KF-801、KF-803及びKF-804
溶離液:THF
流量 :1.0mL/分
打込み量:100μL
検出:RI(示差屈折計)
なお、本明細書の実施例に記載される重量平均分子量は、上記条件により測定したものである。 In this specification, a weight average molecular weight means the value of polystyrene conversion calculated | required by gel permeation chromatography (GPC).
Examples of GPC measurement conditions include the following.
Model: Hitachi High-Technologies Corporation High-Performance Liquid Chromatogram Lachrom Elite
Column: SHODEX KF-801, KF-803 and KF-804 manufactured by Showa Denko K.K.
Eluent: THF
Flow rate: 1.0 mL / min Driving amount: 100 μL
Detection: RI (differential refractometer)
In addition, the weight average molecular weight described in the Example of this specification is measured on the said conditions.
血液滑性付与剤は、約0.00~約0.60のIOBを有することができる。
IOB(Inorganic Organic Balance)は、親水性及び親油性のバランスを示す指標であり、本明細書では、小田らによる次式:
IOB=無機性値/有機性値
により算出される値を意味する。 The blood slipperiness agent can have an IOB of about 0.00 to about 0.60.
IOB (Inorganic Organic Balance) is an index indicating a balance between hydrophilicity and lipophilicity. In the present specification, the following formula by Oda et al.
IOB = value calculated by inorganic value / organic value.
IOB(Inorganic Organic Balance)は、親水性及び親油性のバランスを示す指標であり、本明細書では、小田らによる次式:
IOB=無機性値/有機性値
により算出される値を意味する。 The blood slipperiness agent can have an IOB of about 0.00 to about 0.60.
IOB (Inorganic Organic Balance) is an index indicating a balance between hydrophilicity and lipophilicity. In the present specification, the following formula by Oda et al.
IOB = value calculated by inorganic value / organic value.
無機性値及び有機性値は、藤田穆「有機化合物の予測と有機概念図」化学の領域Vol.11,No.10(1957)p.719-725)に記載される有機概念図に基づく。
藤田氏による、主要な基の有機性値及び無機性値を、下記表1にまとめる。 Inorganic values and organic values are reported in the field of chemistry, Vol. 11, no. 10 (1957) p. 719-725).
Table 1 below summarizes the organic and inorganic values of the major groups by Mr. Fujita.
藤田氏による、主要な基の有機性値及び無機性値を、下記表1にまとめる。 Inorganic values and organic values are reported in the field of chemistry, Vol. 11, no. 10 (1957) p. 719-725).
Table 1 below summarizes the organic and inorganic values of the major groups by Mr. Fujita.
例えば、炭素数14のテトラデカン酸と、炭素数12のドデシルアルコールとのエステルの場合には、有機性値が520(CH2,20×26個)、無機性値が60(-COOR,60×1個)となるため、IOB=0.12となる。
For example, in the case of an ester of tetradecanoic acid having 14 carbon atoms and dodecyl alcohol having 12 carbon atoms, the organic value is 520 (CH 2 , 20 × 26), and the inorganic value is 60 (—COOR, 60 × 1), so that IOB = 0.12.
血液滑性付与剤において、IOBは、約0.00~約0.60であることが好ましく、約0.00~約0.50であることがより好ましく、約0.00~約0.40であることがさらに好ましく、そして約0.00~約0.30であることがさらに好ましい。IOBが上述の範囲にあると、抱水力及び動粘度が、上述の要件を満たしやすくなるからである。
In the blood slipping agent, the IOB is preferably about 0.00 to about 0.60, more preferably about 0.00 to about 0.50, and about 0.00 to about 0.40. And more preferably from about 0.00 to about 0.30. This is because when the IOB is in the above range, the water holding power and kinematic viscosity easily satisfy the above requirements.
血液滑性付与剤は、45℃以下の融点を有することが好ましく、40℃以下の融点を有することがさらに好ましい。血液滑性付与剤が45℃以下の融点を有することにより、血液滑性付与剤が、上述の範囲の動粘度を有しやすくなるからである。
The blood slipperiness-imparting agent preferably has a melting point of 45 ° C. or lower, more preferably 40 ° C. or lower. This is because when the blood slipperiness-imparting agent has a melting point of 45 ° C. or less, the blood slipperiness-imparting agent tends to have a kinematic viscosity in the above range.
本明細書において、「融点」は、示差走査熱量分析計において、昇温速度10℃/分で測定した場合の、固形状から液状に変化する際の吸熱ピークのピークトップ温度を意味する。融点は、例えば、島津製作所社製のDSC-60型DSC測定装置を用いて測定することができる。
In the present specification, “melting point” means a peak top temperature of an endothermic peak when changing from a solid state to a liquid state when measured with a differential scanning calorimeter at a heating rate of 10 ° C./min. The melting point can be measured using, for example, a DSC-60 type DSC measuring apparatus manufactured by Shimadzu Corporation.
血液滑性付与剤は、約45℃以下の融点を有すれば、室温(約25℃)で液体であっても、又は固体であってもよい、すなわち、融点が約25℃以上でも、又は約25℃未満でもよく、そして例えば、約-5℃、約-20℃等の融点を有することができる。
The blood lubricity-imparting agent may be liquid at room temperature (about 25 ° C.) or solid as long as it has a melting point of about 45 ° C. or lower, ie, a melting point of about 25 ° C. or higher, or It may be less than about 25 ° C. and may have a melting point of, for example, about −5 ° C., about −20 ° C.
血液滑性付与剤は、その融点に下限は存在しないが、その蒸気圧が低いことが好ましい。血液滑性付与剤の蒸気圧は、25℃(1気圧)で約0~約200Paであることが好ましく、約0~約100Paであることがより好ましく、約0~約10Paであることがさらに好ましく、約0~約1Paであることがさらに一層好ましく、約0.0~約0.1Paであることがさらに一層好ましい。
The blood slipping agent does not have a lower limit for its melting point, but preferably has a low vapor pressure. The vapor pressure of the blood slipperiness imparting agent is preferably about 0 to about 200 Pa at 25 ° C. (1 atm), more preferably about 0 to about 100 Pa, and further preferably about 0 to about 10 Pa. Preferably, it is about 0 to about 1 Pa, and even more preferably about 0.0 to about 0.1 Pa.
本開示の吸収性物品が、人体に接して用いられることを考慮すると、上記蒸気圧は、40℃(1気圧)で約0~約700Paであることが好ましく、約0~約100Paであることがより好ましく、約0~約10Paであることがさらに好ましく、約0~約1Paであることがさらに一層好ましく、約0.0~約0.1Paであることがさらに一層好ましい。血液滑性付与剤の蒸気圧が高いと、保存中に気化し、その量の減少、着用時の臭気等の問題が発生する場合があるからである。
Considering that the absorbent article of the present disclosure is used in contact with the human body, the vapor pressure is preferably about 0 to about 700 Pa at 40 ° C. (1 atm), and is about 0 to about 100 Pa. Is more preferably about 0 to about 10 Pa, still more preferably about 0 to about 1 Pa, and still more preferably about 0.0 to about 0.1 Pa. This is because if the vapor pressure of the blood slipperiness imparting agent is high, it may vaporize during storage, and problems such as reduction in the amount and odor during wearing may occur.
また、血液滑性付与剤の融点を、気候、着用時間の長さ等に応じて選択することができる。例えば、平均気温が約10℃以下の地域では、約10℃以下の融点を有する血液滑性付与剤を採用することにより、経血が排泄された後、周囲温度によって冷却された場合であっても、血液滑性付与剤が機能しやすいと考えられる。
Moreover, the melting point of the blood slipperiness-imparting agent can be selected according to the climate, the length of wearing time, and the like. For example, in an area where the average temperature is about 10 ° C. or less, by adopting a blood slipperiness imparting agent having a melting point of about 10 ° C. or less, menstrual blood is excreted and then cooled by the ambient temperature. However, it is considered that the blood slipperiness-imparting agent is easy to function.
また、吸収性物品が長時間にわたって使用される場合には、血液滑性付与剤の融点は、約45℃以下の範囲で高い方が好ましい。汗、着用時の摩擦等の影響を受けにくく、長時間着用した場合であっても、血液滑性付与剤が偏りにくいからである。
Further, when the absorbent article is used for a long time, the melting point of the blood slipperiness imparting agent is preferably higher in the range of about 45 ° C. or less. This is because the blood slipperiness-imparting agent is not easily biased even when worn for a long time, and is not easily affected by sweat, friction at the time of wearing.
当技術分野では、経血の表面張力等を変化させ、経血を迅速に吸収することを目的として、トップシートの肌当接面を、界面活性剤でコーティングすることが行われている。しかし、界面活性剤がコーティングされたトップシートは、経血中の親水性成分(血漿等)と親和性が高く、それらを引き寄せ、むしろ経血をトップシートに残存させるようにはたらく傾向がある。血液滑性付与剤は、従来公知の界面活性剤と異なり、経血と親和性が低く、経血をトップシートに残存させず、迅速に吸収体に移行させることができる。
In this technical field, the skin contact surface of the top sheet is coated with a surfactant for the purpose of changing the surface tension of menstrual blood and the like and quickly absorbing menstrual blood. However, the top sheet coated with a surfactant has a high affinity with hydrophilic components (such as plasma) in menstrual blood, and tends to attract them and rather cause menstrual blood to remain on the top sheet. Unlike conventionally known surfactants, the blood slipperiness imparting agent has a low affinity for menstrual blood, and can pass the menstrual blood to the absorbent body quickly without remaining on the top sheet.
血液滑性付与剤は、好ましくは、次の(i)~(iii)、
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、及び
(iii) (iii-1)炭化水素部分と、(iii-2)炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基と、(iii-3)炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される。 The blood slipperiness imparting agent is preferably the following (i) to (iii):
(I) hydrocarbons,
(Ii) consisting of a (ii-1) hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety. A compound having one or more, the same or different groups, selected from the group, and (iii) (iii-1) a hydrocarbon moiety and (iii-2) a CC single bond of the hydrocarbon moiety One or more of the same or different groups selected from the group consisting of a carbonyl group (—CO—) and an oxy group (—O—), and (iii-3) a hydrogen atom of the hydrocarbon moiety, A compound having one or a plurality of identical or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH) to be substituted;
As well as any combination thereof.
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、及び
(iii) (iii-1)炭化水素部分と、(iii-2)炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基と、(iii-3)炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される。 The blood slipperiness imparting agent is preferably the following (i) to (iii):
(I) hydrocarbons,
(Ii) consisting of a (ii-1) hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety. A compound having one or more, the same or different groups, selected from the group, and (iii) (iii-1) a hydrocarbon moiety and (iii-2) a CC single bond of the hydrocarbon moiety One or more of the same or different groups selected from the group consisting of a carbonyl group (—CO—) and an oxy group (—O—), and (iii-3) a hydrogen atom of the hydrocarbon moiety, A compound having one or a plurality of identical or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH) to be substituted;
As well as any combination thereof.
本明細書において、「炭化水素」は、炭素と水素とから成る化合物を意味し、鎖状炭化水素、例えば、パラフィン系炭化水素(二重結合及び三重結合を含まない、アルカンとも称される)、オレフィン系炭化水素(二重結合を1つ含む、アルケンとも称される)、アセチレン系炭化水素(三重結合を1つ含む、アルキンとも称される)、及び二重結合及び三重結合から成る群から選択される結合を2つ以上含む炭化水素、並びに環状炭化水素、例えば、芳香族炭化水素、脂環式炭化水素が挙げられる。
In the present specification, “hydrocarbon” means a compound composed of carbon and hydrogen, and a chain hydrocarbon, for example, a paraffinic hydrocarbon (also referred to as an alkane, which does not include a double bond and a triple bond). , Olefinic hydrocarbons (including one double bond, also referred to as alkene), acetylenic hydrocarbons (including one triple bond, also referred to as alkyne), and the group consisting of double and triple bonds And hydrocarbons containing two or more bonds selected from the above, and cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
炭化水素としては、鎖状炭化水素及び脂環式炭化水素であることが好ましく、鎖状炭化水素であることがより好ましく、パラフィン系炭化水素、オレフィン系炭化水素、及び二重結合を2つ以上含む炭化水素(三重結合を含まない)であることがさらに好ましく、そしてパラフィン系炭化水素であることがさらに好ましい。
鎖状炭化水素には、直鎖状炭化水素及び分岐鎖状炭化水素が含まれる。 The hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, paraffinic hydrocarbon, olefinic hydrocarbon, and two or more double bonds. More preferably, it is a hydrocarbon that does not contain a triple bond, and more preferably it is a paraffinic hydrocarbon.
The chain hydrocarbon includes a straight chain hydrocarbon and a branched chain hydrocarbon.
鎖状炭化水素には、直鎖状炭化水素及び分岐鎖状炭化水素が含まれる。 The hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, paraffinic hydrocarbon, olefinic hydrocarbon, and two or more double bonds. More preferably, it is a hydrocarbon that does not contain a triple bond, and more preferably it is a paraffinic hydrocarbon.
The chain hydrocarbon includes a straight chain hydrocarbon and a branched chain hydrocarbon.
上記(ii)及び(iii)の化合物において、オキシ基(-O-)が2つ以上挿入されている場合には、各オキシ基(-O-)は隣接していない。従って、上記(ii)及び(iii)の化合物には、オキシ基が連続する化合物(いわゆる、過酸化物)は含まれない。
In the compounds (ii) and (iii) above, when two or more oxy groups (—O—) are inserted, each oxy group (—O—) is not adjacent. Therefore, the compounds (ii) and (iii) do not include compounds having a continuous oxy group (so-called peroxides).
また、上記(iii)の化合物では、炭化水素部分の少なくとも1つの水素原子がカルボキシル基(-COOH)で置換された化合物よりも、炭化水素部分の少なくとも1つの水素原子が、ヒドロキシル基(-OH)で置換された化合物の方が好ましい。カルボキシル基は、経血中の金属等と結合し、血液滑性付与剤の抱水率が高くなり、所定の範囲を超える場合があるからである。これは、IOBの観点からも同様である。表1に示すように、カルボキシル基は、経血中の金属等と結合し、無機性値が150から、400以上へと大幅に上昇するため、カルボキシル基を有する血液滑性付与剤は、使用時にIOBの値が約0.60を上回る場合がありうる。
In the compound of (iii), at least one hydrogen atom in the hydrocarbon moiety is more hydroxyl group (—OH) than in the compound in which at least one hydrogen atom in the hydrocarbon moiety is substituted with a carboxyl group (—COOH). ) Is preferred. This is because the carboxyl group binds to a metal or the like in menstrual blood, increases the water retention rate of the blood slipperiness imparting agent, and may exceed a predetermined range. This is the same from the viewpoint of IOB. As shown in Table 1, the carboxyl group binds to menstrual metals and the like, and the inorganic value increases significantly from 150 to 400 or more. Sometimes the value of IOB can be greater than about 0.60.
血液滑性付与剤は、より好ましくは、次の(i’)~(iii’)、
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合とを有する化合物、及び
(iii’) (iii’-1)炭化水素部分と、(iii’-2)炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合と、(iii’-3)炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される。 The blood slipperiness imparting agent is more preferably the following (i ′) to (iii ′):
(I ′) hydrocarbon,
(Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—) or an ester bond (—COO— inserted between the C—C single bonds of the hydrocarbon moiety. ), A carbonate bond (—OCOO—), and an ether bond (—O—) selected from the group consisting of one or more, the same or different bonds, and (iii ′) (iii′-1 ) A hydrocarbon moiety and (iii′-2) a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (—OCOO—) inserted between the C—C single bonds of the hydrocarbon moiety. And a carboxyl group (—COOH) that replaces one or more of the same or different bonds selected from the group consisting of an ether bond (—O—) and a hydrogen atom of the hydrocarbon moiety (iii′-3) And a hydroxyl group (—O H) a compound having one or more identical or different groups selected from the group consisting of
As well as any combination thereof.
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合とを有する化合物、及び
(iii’) (iii’-1)炭化水素部分と、(iii’-2)炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合と、(iii’-3)炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択される。 The blood slipperiness imparting agent is more preferably the following (i ′) to (iii ′):
(I ′) hydrocarbon,
(Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—) or an ester bond (—COO— inserted between the C—C single bonds of the hydrocarbon moiety. ), A carbonate bond (—OCOO—), and an ether bond (—O—) selected from the group consisting of one or more, the same or different bonds, and (iii ′) (iii′-1 ) A hydrocarbon moiety and (iii′-2) a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (—OCOO—) inserted between the C—C single bonds of the hydrocarbon moiety. And a carboxyl group (—COOH) that replaces one or more of the same or different bonds selected from the group consisting of an ether bond (—O—) and a hydrogen atom of the hydrocarbon moiety (iii′-3) And a hydroxyl group (—O H) a compound having one or more identical or different groups selected from the group consisting of
As well as any combination thereof.
上記(ii’)及び(iii’)の化合物において、2以上の同一又は異なる結合が挿入されている場合、すなわち、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)及びエーテル結合(-O-)から選択される2以上の同一又は異なる結合が挿入されている場合には、各結合は隣接しておらず、各結合の間には、少なくとも、炭素原子が1つ介在する。
In the compounds (ii ′) and (iii ′) above, when two or more identical or different bonds are inserted, that is, a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (— When two or more identical or different bonds selected from OCOO-) and ether bonds (-O-) are inserted, the bonds are not adjacent, and at least carbon is interposed between the bonds. One atom intervenes.
血液滑性付与剤は、さらに好ましくは、炭化水素部分に、炭素原子10個当たり、カルボニル結合(-CO-)を約1.8個以下、エステル結合(-COO-)を2個以下、カーボネート結合(-OCOO-)を約1.5個以下、エーテル結合(-O-)を約6個以下、カルボキシル基(-COOH)を約0.8個以下、そして/又はヒドロキシル基(-OH)を約1.2個以下有することができる。
More preferably, the blood slipperiness imparting agent is more preferably about 1.8 or less carbonyl bonds (—CO—) and 2 or less ester bonds (—COO—) per 10 carbon atoms in the hydrocarbon moiety. About 1.5 or less bonds (—OCOO—), about 6 or less ether bonds (—O—), about 0.8 or less carboxyl groups (—COOH), and / or hydroxyl groups (—OH) About 1.2 or less.
血液滑性付与剤は、さらに好ましくは、次の(A)~(F)、
(A) (A1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル、及び
(F)鎖状炭化水素、
並びにそれらの任意の組み合わせから成る群から選択される。
以下、(A)~(F)に従う血液滑性付与剤について詳細に説明する。 The blood slipperiness imparting agent is more preferably the following (A) to (F),
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and a chain carbon An ester with a compound having one carboxyl group replacing a hydrogen atom of the hydrogen moiety,
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and a chain hydrocarbon An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrogen moiety,
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety, and (C2 ) An ester of a chain hydrocarbon moiety and a compound having a hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety;
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the C—C single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—),
(E) polyoxy C 3 -C 6 alkylene glycol, or an alkyl ester or alkyl ether thereof, and (F) a chain hydrocarbon,
As well as any combination thereof.
Hereinafter, the blood slipperiness imparting agent according to (A) to (F) will be described in detail.
(A) (A1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル、及び
(F)鎖状炭化水素、
並びにそれらの任意の組み合わせから成る群から選択される。
以下、(A)~(F)に従う血液滑性付与剤について詳細に説明する。 The blood slipperiness imparting agent is more preferably the following (A) to (F),
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and a chain carbon An ester with a compound having one carboxyl group replacing a hydrogen atom of the hydrogen moiety,
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and a chain hydrocarbon An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrogen moiety,
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety, and (C2 ) An ester of a chain hydrocarbon moiety and a compound having a hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety;
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the C—C single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—),
(E) polyoxy C 3 -C 6 alkylene glycol, or an alkyl ester or alkyl ether thereof, and (F) a chain hydrocarbon,
As well as any combination thereof.
Hereinafter, the blood slipperiness imparting agent according to (A) to (F) will be described in detail.
[(A) (A1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル]
(A)(A1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル(以下、「化合物(A)」と称する場合がある)は、上述の動粘度、抱水率及び重量平均分子量を有する限り、全てのヒドロキシル基がエステル化されていなくともよい。 [(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, Esters with compounds having one carboxyl group replacing a hydrogen atom in the hydrocarbon moiety]
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety; (A2) a chain hydrocarbon moiety; Esters (hereinafter sometimes referred to as “compound (A)”) with a compound having one carboxyl group that substitutes a hydrogen atom of the hydrogen moiety have the above kinematic viscosity, water retention and weight average molecular weight. As long as it has, all the hydroxyl groups do not need to be esterified.
(A)(A1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル(以下、「化合物(A)」と称する場合がある)は、上述の動粘度、抱水率及び重量平均分子量を有する限り、全てのヒドロキシル基がエステル化されていなくともよい。 [(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, Esters with compounds having one carboxyl group replacing a hydrogen atom in the hydrocarbon moiety]
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety; (A2) a chain hydrocarbon moiety; Esters (hereinafter sometimes referred to as “compound (A)”) with a compound having one carboxyl group that substitutes a hydrogen atom of the hydrogen moiety have the above kinematic viscosity, water retention and weight average molecular weight. As long as it has, all the hydroxyl groups do not need to be esterified.
(A1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物(以下、「化合物(A1)」と称する場合がある)としては、例えば、鎖状炭化水素テトラオール、例えば、アルカンテトラオール、例えば、ペンタエリトリトール、鎖状炭化水素トリオール、例えば、アルカントリオール、例えば、グリセリン、及び鎖状炭化水素ジオール、例えば、アルカンジオール、例えば、グリコールが挙げられる。
(A1) As a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (A1)”), For example, chain hydrocarbon tetraols such as alkane tetraols such as pentaerythritol, chain hydrocarbon triols such as alkane triols such as glycerin, and chain hydrocarbon diols such as alkane diols such as glycol Is mentioned.
(A2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物としては、例えば、炭化水素上の1つの水素原子が、1つのカルボキシル基(-COOH)で置換された化合物、例えば、脂肪酸が挙げられる。
化合物(A)としては、例えば、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、及び(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルが挙げられる。 (A2) Examples of the compound having a chain hydrocarbon moiety and one carboxyl group that replaces a hydrogen atom in the chain hydrocarbon moiety include, for example, one hydrogen atom on a hydrocarbon having one carboxyl group ( -COOH) substituted compounds, for example fatty acids.
Examples of the compound (A) include (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) an ester of a chain hydrocarbon triol and at least one fatty acid, and (a 3 ) Esters of chain hydrocarbon diols with at least one fatty acid.
化合物(A)としては、例えば、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、及び(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルが挙げられる。 (A2) Examples of the compound having a chain hydrocarbon moiety and one carboxyl group that replaces a hydrogen atom in the chain hydrocarbon moiety include, for example, one hydrogen atom on a hydrocarbon having one carboxyl group ( -COOH) substituted compounds, for example fatty acids.
Examples of the compound (A) include (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) an ester of a chain hydrocarbon triol and at least one fatty acid, and (a 3 ) Esters of chain hydrocarbon diols with at least one fatty acid.
[(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル]
鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステルとしては、例えば、次の式(1):
のペンタエリトリトールと脂肪酸とのテトラエステル、次の式(2):
のペンタエリトリトールと脂肪酸とのトリエステル、次の式(3):
のペンタエリトリトールと脂肪酸とのジエステル、次の式(4):
のペンタエリトリトールと脂肪酸とのモノエステルが挙げられる。
(式中、R1~R4は、それぞれ、鎖状炭化水素である) [Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
Examples of the ester of a chain hydrocarbon tetraol and at least one fatty acid include the following formula (1):
Tetraesters of pentaerythritol and fatty acids of the following formula (2):
Triesters of pentaerythritol and fatty acids of the following formula (3):
Diester of pentaerythritol and fatty acid of the following formula (4):
And monoesters of pentaerythritol and fatty acids.
(Wherein R 1 to R 4 are each a chain hydrocarbon)
鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステルとしては、例えば、次の式(1):
(式中、R1~R4は、それぞれ、鎖状炭化水素である) [Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
Examples of the ester of a chain hydrocarbon tetraol and at least one fatty acid include the following formula (1):
(Wherein R 1 to R 4 are each a chain hydrocarbon)
ペンタエリトリトールと脂肪酸とのエステルを構成する脂肪酸(R1COOH、R2COOH,R3COOH,及びR4COOH)としては、ペンタエリトリトールと脂肪酸とのエステルが、動粘度、抱水率及び重量平均分子量の要件を満たすものであれば、特に制限されないが、例えば、飽和脂肪酸、例えば、C2~C30の飽和脂肪酸、例えば、酢酸(C2)(C2は、炭素数を示し、R1C、R2C,R3C又はR4Cの炭素数に相当する、以下同じ)、プロパン酸(C3)、ブタン酸(C4)及びその異性体、例えば、2-メチルプロパン酸(C4)、ペンタン酸(C5)及びその異性体、例えば、2-メチルブタン酸(C5)、2,2-ジメチルプロパン酸(C5)、ヘキサン酸(C6)、ヘプタン酸(C7)、オクタン酸(C8)及びその異性体、例えば、2-エチルヘキサン酸(C8)、ノナン酸(C9)、デカン酸(C10)、ドデカン酸(C12)、テトラデカン酸(C14)、ヘキサデカン酸(C16)、ヘプタデカン酸(C17)、オクタデカン酸(C18)、エイコサン酸(C20)、ドコサン酸(C22)、テトラコサン酸(C24)、ヘキサコサン酸(C26)、オクタコサン酸(C28)、トリアコンタン酸(C30)等、並びに列挙されていないこれらの異性体が挙げられる。
As fatty acids (R 1 COOH, R 2 COOH, R 3 COOH, and R 4 COOH) constituting esters of pentaerythritol and fatty acids, esters of pentaerythritol and fatty acids have kinematic viscosity, water retention and weight average. Although it is not particularly limited as long as it satisfies the molecular weight requirement, for example, a saturated fatty acid, for example, a C 2 to C 30 saturated fatty acid, for example, acetic acid (C 2 ) (C 2 represents the number of carbon atoms, R 1 C, R 2 C, R 3 C or R 4 C, the same applies hereinafter), propanoic acid (C 3 ), butanoic acid (C 4 ) and isomers thereof such as 2-methylpropanoic acid ( C 4 ), pentanoic acid (C 5 ) and isomers thereof such as 2-methylbutanoic acid (C 5 ), 2,2-dimethylpropanoic acid (C 5 ), hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8) Beauty isomers thereof, e.g., 2-ethylhexanoic acid (C 8), nonanoic acid (C 9), decanoic acid (C 10), dodecanoic acid (C 12), tetradecanoic acid (C 14), hexadecanoic acid (C 16) Heptadecanoic acid (C 17 ), octadecanoic acid (C 18 ), eicosanoic acid (C 20 ), docosanoic acid (C 22 ), tetracosanoic acid (C 24 ), hexacosanoic acid (C 26 ), octacosanoic acid (C 28 ), Triacontanoic acid (C 30 ) and the like, as well as these isomers not listed.
脂肪酸はまた、不飽和脂肪酸であることができる。不飽和脂肪酸としては、例えば、C3~C20の不飽和脂肪酸、例えば、モノ不飽和脂肪酸、例えば、クロトン酸(C4)、ミリストレイン酸(C14)、パルミトレイン酸(C16)、オレイン酸(C18)、エライジン酸(C18)、バクセン酸(C18)、ガドレイン酸(C20)、エイコセン酸(C20)等、ジ不飽和脂肪酸、例えば、リノール酸(C18)、エイコサジエン酸(C20)等、トリ不飽和脂肪酸、例えば、リノレン酸、例えば、α-リノレン酸(C18)及びγ-リノレン酸(C18)、ピノレン酸(C18)、エレオステアリン酸、例えば、α-エレオステアリン酸(C18)及びβ-エレオステアリン酸(C18)、ミード酸(C20)、ジホモ-γ-リノレン酸(C20)、エイコサトリエン酸(C20)等、テトラ不飽和脂肪酸、例えば、ステアリドン酸(C20)、アラキドン酸(C20)、エイコサテトラエン酸(C20)等、ペンタ不飽和脂肪酸、例えば、ボセオペンタエン酸(C18)、エイコサペンタエン酸(C20)等、並びにこれらの部分水素付加物が挙げられる。
The fatty acid can also be an unsaturated fatty acid. Examples of unsaturated fatty acids include C 3 to C 20 unsaturated fatty acids such as monounsaturated fatty acids such as crotonic acid (C 4 ), myristoleic acid (C 14 ), palmitoleic acid (C 16 ), and olein. Acids (C 18 ), elaidic acid (C 18 ), vaccenic acid (C 18 ), gadoleic acid (C 20 ), eicosenoic acid (C 20 ), etc., diunsaturated fatty acids such as linoleic acid (C 18 ), eicosadiene Triunsaturated fatty acids such as acids (C 20 ) such as linolenic acid such as α-linolenic acid (C 18 ) and γ-linolenic acid (C 18 ), pinolenic acid (C 18 ), eleostearic acid such as , Α-eleostearic acid (C 18 ) and β-eleostearic acid (C 18 ), mead acid (C 20 ), dihomo-γ-linolenic acid (C 20 ), eicosatrienoic acid (C 20 ), etc. Tetraunsaturated fatty acids such as stear Don acid (C 20), arachidonic acid (C 20), eicosatetraenoic acid (C 20), etc., penta unsaturated fatty acids, for example, bosseopentaenoic acid (C 18), such as eicosapentaenoic acid (C 20), as well as their Of the partial hydrogen adduct.
ペンタエリトリトールと脂肪酸とのエステルとしては、酸化等により変性する可能性を考慮すると、飽和脂肪酸に由来する、ペンタエリトリトールと脂肪酸とのエステル、すなわち、ペンタエリトリトールと飽和脂肪酸とのエステルであることが好ましい。
また、ペンタエリトリトールと脂肪酸とのエステルとしては、抱水率の値を小さくする観点から、ジエステル、トリエステル又はテトラエステルであることが好ましく、トリエステル又はテトラエステルであることがより好ましく、そしてテトラエステルであることがさらに好ましい。 The ester of pentaerythritol and a fatty acid is preferably an ester of pentaerythritol and a fatty acid derived from a saturated fatty acid, that is, an ester of pentaerythritol and a saturated fatty acid, considering the possibility of modification by oxidation or the like. .
Further, the ester of pentaerythritol and fatty acid is preferably a diester, triester or tetraester, more preferably a triester or tetraester, and more preferably a tetraester from the viewpoint of reducing the water retention value. More preferably, it is an ester.
また、ペンタエリトリトールと脂肪酸とのエステルとしては、抱水率の値を小さくする観点から、ジエステル、トリエステル又はテトラエステルであることが好ましく、トリエステル又はテトラエステルであることがより好ましく、そしてテトラエステルであることがさらに好ましい。 The ester of pentaerythritol and a fatty acid is preferably an ester of pentaerythritol and a fatty acid derived from a saturated fatty acid, that is, an ester of pentaerythritol and a saturated fatty acid, considering the possibility of modification by oxidation or the like. .
Further, the ester of pentaerythritol and fatty acid is preferably a diester, triester or tetraester, more preferably a triester or tetraester, and more preferably a tetraester from the viewpoint of reducing the water retention value. More preferably, it is an ester.
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪酸とのテトラエステルでは、ペンタエリトリトールと脂肪酸とのテトラエステルを構成する脂肪酸の炭素数の合計、すなわち、上記式(1)において、R1C、R2C、R3C及びR4C部分の炭素数の合計が、約15であることが好ましい(炭素数の合計が15の場合に、IOBが0.60となる)。
Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the tetraester of pentaerythritol and fatty acid, the total number of carbon atoms of the fatty acid constituting the tetraester of pentaerythritol and fatty acid, that is, the above formula In (1), the total number of carbon atoms of the R 1 C, R 2 C, R 3 C, and R 4 C moieties is preferably about 15 (when the total number of carbon atoms is 15, the IOB is 0.1. 60).
ペンタエリトリトールと脂肪酸とのテトラエステルでは、例えば、ペンタエリトリトールと、ヘキサン酸(C6)、ヘプタン酸(C7)、オクタン酸(C8)、例えば、2-エチルヘキサン酸(C8)、ノナン酸(C9)、デカン酸(C10)及び/又はドデカン酸(C12)とのテトラエステルが挙げられる。
Examples of tetraesters of pentaerythritol and fatty acids include pentaerythritol, hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8 ), such as 2-ethylhexanoic acid (C 8 ), nonane Examples include tetraesters with acid (C 9 ), decanoic acid (C 10 ) and / or dodecanoic acid (C 12 ).
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪酸とのトリエステルでは、ペンタエリトリトールと脂肪酸とのトリエステルを構成する脂肪酸の炭素数の合計、すなわち、上記式(2)において、R1C、R2C及びR3C部分の炭素数の合計が、約19以上であることが好ましい(炭素数の合計が19の場合に、IOBが0.58となる)。
Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the triester of pentaerythritol and fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of pentaerythritol and fatty acid, that is, the above formula In (2), the total number of carbon atoms in the R 1 C, R 2 C and R 3 C moieties is preferably about 19 or more (when the total number of carbon atoms is 19, IOB is 0.58). ).
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪酸とのジエステルでは、ペンタエリトリトールと脂肪酸とのジエステルを構成する脂肪酸の炭素数の合計、すなわち、上記式(3)において、R1C及びR2C部分の炭素数の合計が、約22以上であることが好ましい(炭素数の合計が22の場合に、IOBが0.59となる)。
Considering from the viewpoint that IOB is about 0.00 to about 0.60, in the diester of pentaerythritol and fatty acid, the total number of carbon atoms of the fatty acid constituting the diester of pentaerythritol and fatty acid, that is, the above formula (3 ), The total number of carbon atoms in the R 1 C and R 2 C moieties is preferably about 22 or more (when the total number of carbon atoms is 22, IOB is 0.59).
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪酸とのモノエステルでは、ペンタエリトリトールと脂肪酸とのモノエステルを構成する脂肪酸の炭素数、すなわち、上記式(4)において、R1C部分の炭素数が、約25以上であることが好ましい(炭素数が25の場合に、IOBが0.60となる)。
なお、IOBの計算に当たっては、二重結合、三重結合、iso分岐、及びtert分岐の影響は、考慮していない(以下、同様である)。 Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the monoester of pentaerythritol and fatty acid, the carbon number of the fatty acid constituting the monoester of pentaerythritol and fatty acid, that is, the above formula (4 ), The number of carbon atoms in the R 1 C moiety is preferably about 25 or more (when the number of carbon atoms is 25, IOB is 0.60).
In the calculation of IOB, the influence of double bond, triple bond, iso branch, and tert branch is not considered (the same applies hereinafter).
なお、IOBの計算に当たっては、二重結合、三重結合、iso分岐、及びtert分岐の影響は、考慮していない(以下、同様である)。 Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the monoester of pentaerythritol and fatty acid, the carbon number of the fatty acid constituting the monoester of pentaerythritol and fatty acid, that is, the above formula (4 ), The number of carbon atoms in the R 1 C moiety is preferably about 25 or more (when the number of carbon atoms is 25, IOB is 0.60).
In the calculation of IOB, the influence of double bond, triple bond, iso branch, and tert branch is not considered (the same applies hereinafter).
ペンタエリトリトールと脂肪酸とのエステルの市販品としては、ユニスター H-408BRS、H-2408BRS-22(混合品)等(以上、日油株式会社製)が挙げられる。
Examples of commercially available esters of pentaerythritol and fatty acids include Unistar H-408BRS, H-2408BRS-22 (mixed product), etc. (above, manufactured by NOF Corporation).
[(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル]
鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステルとしては、例えば、次の式(5):
のグリセリンと脂肪酸とのトリエステル、次の式(6):
のグリセリンと脂肪酸とのジエステル、及び次の式(7):
(式中、R5~R7は、それぞれ、鎖状炭化水素である)
のグリセリンと脂肪酸とのモノエステルが挙げられる。 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
Examples of the ester of a chain hydrocarbon triol and at least one fatty acid include the following formula (5):
Triester of glycerin and fatty acid of the following formula (6):
Diesters of glycerin and fatty acids and the following formula (7):
(Wherein R 5 to R 7 are each a chain hydrocarbon)
And monoesters of glycerin and fatty acids.
鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステルとしては、例えば、次の式(5):
のグリセリンと脂肪酸とのモノエステルが挙げられる。 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
Examples of the ester of a chain hydrocarbon triol and at least one fatty acid include the following formula (5):
And monoesters of glycerin and fatty acids.
グリセリンと脂肪酸とのエステルを構成する脂肪酸(R5COOH、R6COOH及びR7COOH)としては、グリセリンと脂肪酸とのエステルが、動粘度、抱水率及び重量平均分子量の要件を満たすものであれば、特に制限されず、例えば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙される脂肪酸、すなわち、飽和脂肪酸及び不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸に由来する、グリセリンと脂肪酸とのエステル、すなわち、グリセリンと飽和脂肪酸とのエステルであることが好ましい。
As fatty acids (R 5 COOH, R 6 COOH and R 7 COOH) constituting esters of glycerin and fatty acids, the esters of glycerin and fatty acids satisfy the requirements of kinematic viscosity, water retention and weight average molecular weight. If there is, there is no particular limitation, for example, fatty acids listed in “esters of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”, that is, saturated fatty acids and unsaturated fatty acids, oxidation, etc. In view of the possibility of modification by the above, it is preferably an ester of glycerin and a fatty acid derived from a saturated fatty acid, that is, an ester of glycerin and a saturated fatty acid.
また、グリセリンと脂肪酸とのエステルとしては、抱水率の値を小さくする観点から、ジエステル又はトリエステルであることが好ましく、そしてトリエステルであることがより好ましい。
Also, the ester of glycerin and fatty acid is preferably a diester or triester, more preferably a triester, from the viewpoint of reducing the water retention value.
グリセリンと脂肪酸とのトリエステルは、トリグリセリドとも称され、例えば、グリセリンとオクタン酸(C8)とのトリエステル、グリセリンとデカン酸(C10)とのトリエステル、グリセリンとドデカン酸(C12)とのトリエステル、及びグリセリンと、2種又は3種の脂肪酸とのトリエステル、並びにこれらの混合物が挙げられる。
Triesters of glycerin and fatty acids are also called triglycerides, for example, triesters of glycerin and octanoic acid (C 8 ), triesters of glycerin and decanoic acid (C 10 ), glycerin and dodecanoic acid (C 12 ). And triesters of glycerin with 2 or 3 fatty acids, and mixtures thereof.
グリセリンと、2種以上の脂肪酸とのトリエステルとしては、例えば、グリセリンと、オクタン酸(C8)及びデカン酸(C10)とのトリエステル、グリセリンと、オクタン酸(C8)、デカン酸(C10)及びドデカン酸(C12)とのトリエステル、グリセリンと、オクタン酸(C8)、デカン酸(C10)、ドデカン酸(C12)、テトラデカン酸(C14)、ヘキサデカン酸(C16)及びオクタデカン酸(C18)とのトリエステル等が挙げられる。
Examples of triesters of glycerin and two or more fatty acids include triesters of glycerin and octanoic acid (C 8 ) and decanoic acid (C 10 ), glycerin, octanoic acid (C 8 ), and decanoic acid. (C 10 ) and triester with dodecanoic acid (C 12 ), glycerin and octanoic acid (C 8 ), decanoic acid (C 10 ), dodecanoic acid (C 12 ), tetradecanoic acid (C 14 ), hexadecanoic acid ( And a triester with C 16 ) and octadecanoic acid (C 18 ).
融点を約45℃以下とする観点から考察すると、グリセリンと脂肪酸とのトリエステルは、グリセリンと脂肪酸とのトリエステルを構成する脂肪酸の炭素数の合計、すなわち、式(5)において、R5C、R6C及びR7C部分の炭素数の合計が、約40以下であることが好ましい。
Considering from the viewpoint that the melting point is about 45 ° C. or less, the triester of glycerin and a fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of glycerin and a fatty acid, that is, in formula (5), R 5 C , R 6 C and R 7 C moieties preferably have a total carbon number of about 40 or less.
IOBを約0.00~約0.60とする観点から考察すると、グリセリンと脂肪酸とのトリエステルでは、グリセリンと脂肪酸とのトリエステルを構成する脂肪酸の炭素数の合計、すなわち、式(5)において、R5C、R6C及びR7C部分の炭素数の合計が、約12以上であることが好ましい(炭素数の合計が12の場合に、IOBが0.60となる)。
グリセリンと脂肪酸とのトリエステルは、いわゆる、脂肪であり、人体を構成しうる成分であるため、安全性の観点から好ましい。 Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the triester of glycerin and fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of glycerin and fatty acid, that is, the formula (5) The total number of carbon atoms of the R 5 C, R 6 C and R 7 C moieties is preferably about 12 or more (when the total number of carbon atoms is 12, IOB is 0.60).
A triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute a human body, and thus is preferable from the viewpoint of safety.
グリセリンと脂肪酸とのトリエステルは、いわゆる、脂肪であり、人体を構成しうる成分であるため、安全性の観点から好ましい。 Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the triester of glycerin and fatty acid is the total number of carbon atoms of the fatty acid constituting the triester of glycerin and fatty acid, that is, the formula (5) The total number of carbon atoms of the R 5 C, R 6 C and R 7 C moieties is preferably about 12 or more (when the total number of carbon atoms is 12, IOB is 0.60).
A triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute a human body, and thus is preferable from the viewpoint of safety.
グリセリンと脂肪酸とのトリエステルの市販品としては、トリヤシ油脂肪酸グリセリド、NA36、パナセート800、パナセート800B及びパナセート810S、並びにトリC2L油脂肪酸グリセリド及びトリCL油脂肪酸グリセリド(以上、日油株式会社製)等が挙げられる。
Commercial products of triesters of glycerin and fatty acid include tricoconut oil fatty acid glyceride, NA36, panacet 800, panacet 800B and panacet 810S, and tri-C2L oil fatty acid glyceride and tri-CL oil fatty acid glyceride (manufactured by NOF Corporation). Etc.
グリセリンと脂肪酸とのジエステルは、ジグリセリドとも称され、例えば、グリセリンとデカン酸(C10)とのジエステル、グリセリンとドデカン酸(C12)とのジエステル、グリセリンとヘキサデカン酸(C16)とのジエステル、及びグリセリンと、2種の脂肪酸とのジエステル、並びにこれらの混合物が挙げられる。
Diesters of glycerin and fatty acids are also referred to as diglycerides. For example, diesters of glycerin and decanoic acid (C 10 ), diesters of glycerin and dodecanoic acid (C 12 ), diesters of glycerin and hexadecanoic acid (C 16 ) And diesters of glycerin and two fatty acids, and mixtures thereof.
IOBを約0.00~約0.60とする観点から考察すると、グリセリンと脂肪酸とのジエステルでは、グリセリンと脂肪酸とのジエステルを構成する脂肪酸の炭素数の合計、すなわち、式(6)において、R5C及びR6C部分の炭素数の合計が、約16以上であることが好ましい(炭素数の合計が16の場合にIOBが0.58となる)。
Considering from the viewpoint that IOB is about 0.00 to about 0.60, in the diester of glycerin and fatty acid, the total number of carbon atoms of the fatty acid constituting the diester of glycerin and fatty acid, that is, in the formula (6), The total number of carbon atoms in the R 5 C and R 6 C moieties is preferably about 16 or more (when the total number of carbon atoms is 16, IOB is 0.58).
グリセリンと脂肪酸とのモノエステルは、モノグリセリドとも称され、例えば、グリセリンのオクタデカン酸(C18)モノエステル、グリセリンのドコサン酸(C22)モノエステル等が挙げられる。
Monoesters of glycerin and fatty acids are also referred to as monoglycerides, and examples include glycerin octadecanoic acid (C 18 ) monoester, glycerin docosanoic acid (C 22 ) monoester, and the like.
IOBを約0.00~約0.60とする観点から考察すると、グリセリンと脂肪酸とのモノエステルでは、グリセリンと脂肪酸とのモノエステルを構成する脂肪酸の炭素数、すなわち、式(7)において、R5C部分の炭素数が、約19以上であることが好ましい(炭素数が19の場合に、IOBが0.59となる)。
Considering from the viewpoint that the IOB is about 0.00 to about 0.60, in the monoester of glycerin and fatty acid, the number of carbon atoms of the fatty acid constituting the monoester of glycerin and fatty acid, that is, in the formula (7), The number of carbon atoms in the R 5 C moiety is preferably about 19 or more (when the number of carbon atoms is 19, IOB is 0.59).
[(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル]
鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルとしては、例えば、C2~C6の鎖状炭化水素ジオール、例えば、C2~C6のグリコール、例えば、エチレングリコール、プロピレングリコール、ブチレングリコール、ペンチレングリコール又はヘキシレングリコールと、脂肪酸とのモノエステル又はジエステルが挙げられる。 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
Examples of esters of chain hydrocarbon diols with at least one fatty acid include C 2 to C 6 chain hydrocarbon diols such as C 2 to C 6 glycols such as ethylene glycol, propylene glycol, and butylene glycol. , Monoester or diester of pentylene glycol or hexylene glycol and a fatty acid.
鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルとしては、例えば、C2~C6の鎖状炭化水素ジオール、例えば、C2~C6のグリコール、例えば、エチレングリコール、プロピレングリコール、ブチレングリコール、ペンチレングリコール又はヘキシレングリコールと、脂肪酸とのモノエステル又はジエステルが挙げられる。 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
Examples of esters of chain hydrocarbon diols with at least one fatty acid include C 2 to C 6 chain hydrocarbon diols such as C 2 to C 6 glycols such as ethylene glycol, propylene glycol, and butylene glycol. , Monoester or diester of pentylene glycol or hexylene glycol and a fatty acid.
具体的には、鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルとしては、例えば、次の式(8):
R8COOCkH2kOCOR9 (8)
(式中、kは、2~6の整数であり、そしてR8及びR9は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのジエステル、及び次の式(9):
R8COOCkH2kOH (9)
(式中、kは、2~6の整数であり、そしてR8は、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのモノエステルが挙げられる。 Specifically, as an ester of a chain hydrocarbon diol and at least one fatty acid, for example, the following formula (8):
R 8 COOC k H 2k OCOR 9 (8)
(Wherein k is an integer from 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
A diester of a C 2 -C 6 glycol with a fatty acid and the following formula (9):
R 8 COOC k H 2k OH (9)
(Wherein k is an integer from 2 to 6 and R 8 is a chain hydrocarbon)
And monoesters of C 2 -C 6 glycols and fatty acids.
R8COOCkH2kOCOR9 (8)
(式中、kは、2~6の整数であり、そしてR8及びR9は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのジエステル、及び次の式(9):
R8COOCkH2kOH (9)
(式中、kは、2~6の整数であり、そしてR8は、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのモノエステルが挙げられる。 Specifically, as an ester of a chain hydrocarbon diol and at least one fatty acid, for example, the following formula (8):
R 8 COOC k H 2k OCOR 9 (8)
(Wherein k is an integer from 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
A diester of a C 2 -C 6 glycol with a fatty acid and the following formula (9):
R 8 COOC k H 2k OH (9)
(Wherein k is an integer from 2 to 6 and R 8 is a chain hydrocarbon)
And monoesters of C 2 -C 6 glycols and fatty acids.
C2~C6グリコールと脂肪酸とのエステルにおいて、エステル化すべき脂肪酸(式(8)及び式(9)において、R8COOH及びR9COOHに相当する)としては、C2~C6グリコールと脂肪酸とのエステルが、動粘度、抱水率及び重量平均分子量の要件を満たすものであれば、特に制限されず、例えば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙されている脂肪酸、すなわち、飽和脂肪酸及び不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸が好ましい。
In the ester of C 2 -C 6 glycol and fatty acid, the fatty acid to be esterified (corresponding to R 8 COOH and R 9 COOH in formula (8) and formula (9)) is C 2 -C 6 glycol and The ester with a fatty acid is not particularly limited as long as it satisfies the requirements of kinematic viscosity, water retention and weight average molecular weight. For example, “(a 1 ) a chain hydrocarbon tetraol and at least one fatty acid The fatty acids listed in “Ester”, that is, saturated fatty acids and unsaturated fatty acids are mentioned, and saturated fatty acids are preferred in consideration of the possibility of modification by oxidation or the like.
IOBを約0.00~約0.60とする観点から考察すると、式(8)に示されるブチレングリコール(k=4)と脂肪酸とのジエステルでは、R8C及びR9C部分の炭素数の合計が、約6以上であることが好ましい(炭素数の合計が6の場合に、IOBが0.60となる)。
Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the diester of butylene glycol (k = 4) and a fatty acid represented by the formula (8), the carbon number of the R 8 C and R 9 C moieties Is preferably about 6 or more (when the total number of carbon atoms is 6, IOB is 0.60).
IOBを約0.00~約0.60とする観点から考察すると、式(9)に示されるエチレングリコール(k=2)と脂肪酸とのモノエステルでは、R8C部分の炭素数が、約12以上であることが好ましい(炭素数が12の場合に、IOBが0.57となる)。
Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the monoester of ethylene glycol (k = 2) and a fatty acid represented by the formula (9), the carbon number of the R 8 C moiety is about It is preferably 12 or more (when the carbon number is 12, IOB is 0.57).
C2~C6グリコールと脂肪酸とのエステルとしては、酸化等により変性する可能性を考慮すると、飽和脂肪酸に由来する、C2~C6グリコールと脂肪酸とのエステル、すわなち、C2~C6グリコールと飽和脂肪酸とのエステルであることが好ましい。
The esters of C 2 ~ C 6 glycols and fatty acid, in view of the potential for degradation by oxidation and the like, derived from saturated fatty acids, esters of C 2 ~ C 6 glycols and fatty acid, Nachi Suwa, C 2 ~ An ester of C 6 glycol and a saturated fatty acid is preferred.
また、C2~C6グリコールと脂肪酸とのエステルとしては、抱水率の値を小さくする観点から、炭素数の大きいグリコールに由来する、グリコールと脂肪酸とのエステル、例えば、ブチレングリコール、ペンチレングリコール又はヘキシレングリコールに由来するグリコールと脂肪酸とのエステルであることが好ましい。
さらに、C2~C6グリコールと脂肪酸とのエステルとしては、抱水率の値を小さくする観点から、ジエステルであることが好ましい。
C2~C6グリコールと脂肪酸とのエステルの市販品としては、例えば、コムポールBL、コムポールBS(以上、日油株式会社製)等が挙げられる。 Further, as an ester of C 2 -C 6 glycol and a fatty acid, from the viewpoint of reducing the water retention value, an ester of glycol and a fatty acid derived from a glycol having a large number of carbon atoms, such as butylene glycol and pentylene. An ester of glycol and fatty acid derived from glycol or hexylene glycol is preferable.
Further, the ester of C 2 -C 6 glycol and a fatty acid is preferably a diester from the viewpoint of reducing the water retention value.
Examples of commercially available esters of C 2 -C 6 glycol and fatty acid include Compol BL and Compol BS (manufactured by NOF Corporation).
さらに、C2~C6グリコールと脂肪酸とのエステルとしては、抱水率の値を小さくする観点から、ジエステルであることが好ましい。
C2~C6グリコールと脂肪酸とのエステルの市販品としては、例えば、コムポールBL、コムポールBS(以上、日油株式会社製)等が挙げられる。 Further, as an ester of C 2 -C 6 glycol and a fatty acid, from the viewpoint of reducing the water retention value, an ester of glycol and a fatty acid derived from a glycol having a large number of carbon atoms, such as butylene glycol and pentylene. An ester of glycol and fatty acid derived from glycol or hexylene glycol is preferable.
Further, the ester of C 2 -C 6 glycol and a fatty acid is preferably a diester from the viewpoint of reducing the water retention value.
Examples of commercially available esters of C 2 -C 6 glycol and fatty acid include Compol BL and Compol BS (manufactured by NOF Corporation).
[(B) (B1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル]
(B) (B1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル(以下、「化合物(B)」と称する場合がある)は、上述の動粘度、抱水率及び重量平均分子量を有する限り、全てのヒドロキシル基がエーテル化されていなくともよい。 [(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, Ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety]
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and a chain hydrocarbon The ether (hereinafter sometimes referred to as “compound (B)”) with a compound having one hydroxyl group substituting a hydrogen atom in the hydrogen moiety has the above kinematic viscosity, water retention and weight average molecular weight. As long as it has, all the hydroxyl groups do not need to be etherified.
(B) (B1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル(以下、「化合物(B)」と称する場合がある)は、上述の動粘度、抱水率及び重量平均分子量を有する限り、全てのヒドロキシル基がエーテル化されていなくともよい。 [(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, Ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety]
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and a chain hydrocarbon The ether (hereinafter sometimes referred to as “compound (B)”) with a compound having one hydroxyl group substituting a hydrogen atom in the hydrogen moiety has the above kinematic viscosity, water retention and weight average molecular weight. As long as it has, all the hydroxyl groups do not need to be etherified.
(B1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物(以下、「化合物(B1)」と称する場合がある)としては、「化合物(A)」において化合物(A1)として列挙されるもの、例えば、ペンタエリトリトール、グリセリン、及びグリコールが挙げられる。
(B1) As a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (B1)”), What is enumerated as a compound (A1) in "Compound (A)", for example, a pentaerythritol, glycerol, and glycol are mentioned.
(B2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物(以下、「化合物(B2)」と称する場合がある)としては、例えば、炭化水素の1個の水素原子が、1個のヒドロキシル基(-OH)で置換された化合物、例えば、脂肪族1価アルコール、例えば、飽和脂肪族1価アルコール及び不飽和脂肪族1価アルコールが挙げられる。
Examples of the compound (B2) having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (B2)”) include: A compound in which one hydrogen atom of a hydrocarbon is substituted with one hydroxyl group (—OH), for example, an aliphatic monohydric alcohol, for example, a saturated aliphatic monohydric alcohol and an unsaturated aliphatic monohydric alcohol Can be mentioned.
飽和脂肪族1価アルコールとしては、例えば、C1~C20の飽和脂肪族1価アルコール、例えば、メチルアルコール(C1)(C1は、炭素数を示す、以下同じ)、エチルアルコール(C2)、プロピルアルコール(C3)及びその異性体、例えば、イソプロピルアルコール(C3)、ブチルアルコール(C4)及びその異性体、例えば、sec-ブチルアルコール(C4)及びtert-ブチルアルコール(C4)、ペンチルアルコール(C5)、ヘキシルアルコール(C6)、ヘプチルアルコール(C7)、オクチルアルコール(C8)及びその異性体、例えば、2-エチルヘキシルアルコール(C8)、ノニルアルコール(C9)、デシルアルコール(C10)、ドデシルアルコール(C12)、テトラデシルアルコール(C14)、ヘキサデシルアルコール(C16)、へプラデシルアルコール(C17)、オクタデシルアルコール(C18)、及びエイコシルアルコール(C20)、並びに列挙されていないこれらの異性体が挙げられる。
Examples of the saturated aliphatic monohydric alcohol include C 1 to C 20 saturated aliphatic monohydric alcohols such as methyl alcohol (C 1 ) (C 1 represents the number of carbon atoms, the same shall apply hereinafter), ethyl alcohol (C 2 ), propyl alcohol (C 3 ) and isomers thereof such as isopropyl alcohol (C 3 ), butyl alcohol (C 4 ) and isomers thereof such as sec-butyl alcohol (C 4 ) and tert-butyl alcohol ( C 4 ), pentyl alcohol (C 5 ), hexyl alcohol (C 6 ), heptyl alcohol (C 7 ), octyl alcohol (C 8 ) and isomers thereof such as 2-ethylhexyl alcohol (C 8 ), nonyl alcohol ( C 9), decyl alcohol (C 10), dodecyl alcohol (C 12), tetradecyl alcohol (C 14), hexyl Decyl alcohol (C 16), heptadecyl alcohol (C 17) to, octadecyl alcohol (C 18), and eicosyl alcohol (C 20), and isomers thereof that are not listed.
不飽和脂肪族1価アルコールとしては、飽和脂肪族1価アルコールのC-C単結合の1つを、C=C二重結合で置換したもの、例えば、オレイルアルコールが挙げられ、例えば、新日本理化株式会社から、リカコールシリーズ及びアンジェコオールシリーズの名称で市販されている。
Examples of the unsaturated aliphatic monohydric alcohol include those obtained by substituting one of the C—C single bonds of the saturated aliphatic monohydric alcohol with a C═C double bond, such as oleyl alcohol. It is commercially available from Rika Co., Ltd. under the names of the Rica Coal series and the Angelo All series.
化合物(B)としては、例えば、(b1)鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテル、例えば、モノエーテル、ジエーテル、トリエーテル及びテトラエーテル、好ましくはジエーテル、トリエーテル及びテトラエーテル、より好ましくはトリエーテル及びテトラエーテル、そしてさらに好ましくはテトラエーテル、(b2)鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテル、例えば、モノエーテル、ジエーテル及びトリエーテル、好ましくはジエーテル及びトリエーテル、そしてより好ましくはトリエーテル、並びに(b3)鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテル、例えば、モノエーテル及びジエーテル、そして好ましくはジエーテルが挙げられる。
Examples of the compound (B) include (b 1 ) ethers of chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, such as monoether, diether, triether and tetraether, preferably diether, triether. Ethers and tetraethers, more preferably triethers and tetraethers, and even more preferably tetraethers, ethers of (b 2 ) chain hydrocarbon triols and at least one aliphatic monohydric alcohol, such as monoethers, diethers and Triethers, preferably diethers and triethers, and more preferably triethers, and (b 3 ) ethers of chain hydrocarbon diols with at least one aliphatic monohydric alcohol, such as monoethers and diethers, and preferably Diether It is below.
鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、例えば、次の式(10)~(13):
(式中、R10~R13は、それぞれ、鎖状炭化水素である。)
の、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテル、トリエーテル、ジエーテル及びモノエーテルが挙げられる。 Examples of ethers of chain hydrocarbon tetraols and at least one aliphatic monohydric alcohol include, for example, the following formulas (10) to (13):
(In the formula, R 10 to R 13 are each a chain hydrocarbon.)
And tetraethers, triethers, diethers and monoethers of pentaerythritol and aliphatic monohydric alcohols.
の、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテル、トリエーテル、ジエーテル及びモノエーテルが挙げられる。 Examples of ethers of chain hydrocarbon tetraols and at least one aliphatic monohydric alcohol include, for example, the following formulas (10) to (13):
And tetraethers, triethers, diethers and monoethers of pentaerythritol and aliphatic monohydric alcohols.
鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、例えば、次の式(14)~(16):
(式中、R14~R16は、それぞれ、鎖状炭化水素である。)
の、グリセリンと脂肪族1価アルコールとのトリエーテル、ジエーテル及びモノエーテルが挙げられる。 Examples of ethers of chain hydrocarbon triols and at least one aliphatic monohydric alcohol include, for example, the following formulas (14) to (16):
(Wherein R 14 to R 16 are each a chain hydrocarbon.)
And triether, diether and monoether of glycerin and aliphatic monohydric alcohol.
の、グリセリンと脂肪族1価アルコールとのトリエーテル、ジエーテル及びモノエーテルが挙げられる。 Examples of ethers of chain hydrocarbon triols and at least one aliphatic monohydric alcohol include, for example, the following formulas (14) to (16):
And triether, diether and monoether of glycerin and aliphatic monohydric alcohol.
鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、次の式(17):
R17OCnH2nOR18 (17)
(式中、nは、2~6の整数であり、そしてR17及びR18は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのジエーテル、及び次の式(18):
R17OCnH2nOH (18)
(式中、nは、2~6の整数であり、そしてR17は、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのモノエーテルが挙げられる。 As an ether of a chain hydrocarbon diol and at least one aliphatic monohydric alcohol, the following formula (17):
R 17 OC n H 2n OR 18 (17)
(Wherein n is an integer from 2 to 6 and R 17 and R 18 are each a chain hydrocarbon)
A diether of a C 2 -C 6 glycol and an aliphatic monohydric alcohol, and the following formula (18):
R 17 OC n H 2n OH (18)
(Wherein n is an integer from 2 to 6 and R 17 is a chain hydrocarbon)
And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
R17OCnH2nOR18 (17)
(式中、nは、2~6の整数であり、そしてR17及びR18は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのジエーテル、及び次の式(18):
R17OCnH2nOH (18)
(式中、nは、2~6の整数であり、そしてR17は、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのモノエーテルが挙げられる。 As an ether of a chain hydrocarbon diol and at least one aliphatic monohydric alcohol, the following formula (17):
R 17 OC n H 2n OR 18 (17)
(Wherein n is an integer from 2 to 6 and R 17 and R 18 are each a chain hydrocarbon)
A diether of a C 2 -C 6 glycol and an aliphatic monohydric alcohol, and the following formula (18):
R 17 OC n H 2n OH (18)
(Wherein n is an integer from 2 to 6 and R 17 is a chain hydrocarbon)
And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(10)において、R10、R11、R12及びR13部分の炭素数の合計が、約4以上であることが好ましい(炭素数の合計が4の場合に、IOBが0.44となる)。
Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the tetraether of pentaerythritol and aliphatic monohydric alcohol is aliphatic 1 constituting the tetraether of pentaerythritol and aliphatic monohydric alcohol. The total number of carbon atoms of the hydric alcohol, that is, in the above formula (10), the total number of carbon atoms of the R 10 , R 11 , R 12 and R 13 moieties is preferably about 4 or more (the total number of carbon atoms is In the case of 4, the IOB is 0.44).
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪族1価アルコールとのトリエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのトリエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(11)において、R10、R11及びR12部分の炭素数の合計が、約9以上であることが好ましい(炭素数の合計が9の場合に、IOBが0.57となる)。
Considering from the viewpoint that IOB is about 0.00 to about 0.60, the triether of pentaerythritol and aliphatic monohydric alcohol is aliphatic 1 constituting the triether of pentaerythritol and aliphatic monohydric alcohol. The total number of carbon atoms of the monohydric alcohol, that is, in the above formula (11), the total number of carbon atoms of the R 10 , R 11 and R 12 portions is preferably about 9 or more (when the total number of carbon atoms is 9 IOB becomes 0.57).
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪族1価アルコールとのジエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのジエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(12)において、R10及びR11部分の炭素数の合計が、約15以上であることが好ましい(炭素数の合計が15の場合に、IOBが0.60となる)。
Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the diether of pentaerythritol and an aliphatic monohydric alcohol is an aliphatic monohydric alcohol constituting a diether of pentaerythritol and an aliphatic monohydric alcohol. That is, in the above formula (12), the total number of carbon atoms in the R 10 and R 11 moieties is preferably about 15 or more (when the total number of carbon atoms is 15, the IOB is 0). .60).
IOBを約0.00~約0.60とする観点から考察すると、ペンタエリトリトールと脂肪族1価アルコールとのモノエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのモノエーテルを構成する脂肪族1価アルコールの炭素数、すなわち、上記式(13)において、R10部分の炭素数が、約22以上であることが好ましい(炭素数が22の場合に、IOBが0.59となる)。
Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the monoether of pentaerythritol and aliphatic monohydric alcohol is aliphatic 1 constituting the monoether of pentaerythritol and aliphatic monohydric alcohol. the number of carbon atoms of the polyhydric alcohol, i.e., in formula (13), the number of carbon atoms of R 10 moiety is preferably about 22 or more (when the number of carbon atoms is 22, IOB is 0.59).
また、IOBを約0.00~約0.60とする観点から考察すると、グリセリンと脂肪族1価アルコールとのトリエーテルでは、グリセリンと脂肪族1価アルコールとのトリエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、式(14)において、R14、R15及びR16部分の炭素数の合計が、約3以上であることが好ましい(炭素数の合計が3の場合に、IOBが0.50となる)。
Further, from the viewpoint of setting the IOB from about 0.00 to about 0.60, the triether of glycerin and aliphatic monohydric alcohol is aliphatic 1 constituting the triether of glycerin and aliphatic monohydric alcohol. In the formula (14), the total number of carbon atoms of the R 14 , R 15 and R 16 moieties is preferably about 3 or more (when the total number of carbon atoms is 3). , IOB becomes 0.50).
IOBを約0.00~約0.60とする観点から考察すると、グリセリンと脂肪族1価アルコールとのジエーテルでは、グリセリンと脂肪族1価アルコールとのジエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、式(15)において、R14及びR15部分の炭素数の合計が、約9以上であることが好ましい(炭素数の合計が9の場合に、IOBが0.58となる)。
Considering from the viewpoint that IOB is about 0.00 to about 0.60, diether of glycerol and aliphatic monohydric alcohol is carbon of aliphatic monohydric alcohol constituting diether of glycerin and aliphatic monohydric alcohol. In the formula (15), the total number of carbon atoms in the R 14 and R 15 moieties is preferably about 9 or more (when the total number of carbon atoms is 9, the IOB is 0.58). Become).
IOBを約0.00~約0.60とする観点から考察すると、グリセリンと脂肪族1価アルコールとのモノエーテルでは、グリセリンと脂肪族1価アルコールとのモノエーテルを構成する脂肪族1価アルコールの炭素数、すなわち、式(16)において、R14部分の炭素数が、約16以上であることが好ましい(炭素数が16の場合に、IOBが0.58となる)。
Considering from the viewpoint that the IOB is about 0.00 to about 0.60, the monoether of glycerin and an aliphatic monohydric alcohol is an aliphatic monohydric alcohol constituting a monoether of glycerin and an aliphatic monohydric alcohol. In the formula (16), the carbon number of the R 14 moiety is preferably about 16 or more (when the carbon number is 16, IOB is 0.58).
IOBを約0.00~約0.60とする観点から考察すると、式(17)に示されるブチレングリコール(n=4)と脂肪族1価アルコールとのジエーテルでは、R17及びR18部分の炭素数の合計が、約2以上であることが好ましい(炭素数の合計が2の場合に、IOBが0.33となる)。
また、IOBを約0.00~約0.60とする観点から考察すると、式(18)に示されるエチレングリコール(n=2)と脂肪族1価アルコールとのモノエーテルでは、R17部分の炭素数が、約8以上であることが好ましい(炭素数が8の場合に、IOBが0.60となる)。 Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the diether of butylene glycol (n = 4) and the aliphatic monohydric alcohol represented by the formula (17), R 17 and R 18 moieties The total number of carbons is preferably about 2 or more (when the total number of carbons is 2, IOB is 0.33).
Further, considering from the viewpoint of about 0.00 to about 0.60 the IOB, the monoether of ethylene glycol (n = 2) and the aliphatic monohydric alcohol represented by the formula (18), the R 17 moieties The number of carbon atoms is preferably about 8 or more (when the number of carbon atoms is 8, IOB is 0.60).
また、IOBを約0.00~約0.60とする観点から考察すると、式(18)に示されるエチレングリコール(n=2)と脂肪族1価アルコールとのモノエーテルでは、R17部分の炭素数が、約8以上であることが好ましい(炭素数が8の場合に、IOBが0.60となる)。 Considering from the viewpoint of setting the IOB to about 0.00 to about 0.60, in the diether of butylene glycol (n = 4) and the aliphatic monohydric alcohol represented by the formula (17), R 17 and R 18 moieties The total number of carbons is preferably about 2 or more (when the total number of carbons is 2, IOB is 0.33).
Further, considering from the viewpoint of about 0.00 to about 0.60 the IOB, the monoether of ethylene glycol (n = 2) and the aliphatic monohydric alcohol represented by the formula (18), the R 17 moieties The number of carbon atoms is preferably about 8 or more (when the number of carbon atoms is 8, IOB is 0.60).
化合物(B)としては、化合物(B1)と、化合物(B2)とを、酸触媒の存在下で、脱水縮合することにより生成することができる。
Compound (B) can be produced by dehydrating condensation of compound (B1) and compound (B2) in the presence of an acid catalyst.
[(C) (C1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル]
(C) (C1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル(以下、「化合物(C)」と称する場合がある)は、上述の動粘度、抱水率及び重量平均分子量を有する限り、全てのカルボキシル基がエステル化されていなくともよい。 [(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety; C2) Esters of a compound having a chain hydrocarbon moiety and one hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety]
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety, and (C2 ) An ester (hereinafter sometimes referred to as “compound (C)”) of a compound having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety is the above-mentioned All carboxyl groups may not be esterified as long as they have kinematic viscosity, water retention and weight average molecular weight.
(C) (C1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル(以下、「化合物(C)」と称する場合がある)は、上述の動粘度、抱水率及び重量平均分子量を有する限り、全てのカルボキシル基がエステル化されていなくともよい。 [(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety; C2) Esters of a compound having a chain hydrocarbon moiety and one hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety]
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety, and (C2 ) An ester (hereinafter sometimes referred to as “compound (C)”) of a compound having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety is the above-mentioned All carboxyl groups may not be esterified as long as they have kinematic viscosity, water retention and weight average molecular weight.
(C1)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸(以下、「化合物(C1)」と称する場合がある)としては、例えば、2~4個のカルボキシル基を有する鎖状炭化水素カルボン酸、例えば、鎖状炭化水素ジカルボン酸、例えば、アルカンジカルボン酸、例えば、エタン二酸、プロパン二酸、ブタン二酸、ペンタン二酸、ヘキサン二酸、ヘプタン二酸、オクタン二酸、ノナン二酸及びデカン二酸、鎖状炭化水素トリカルボン酸、例えば、アルカントリカルボン酸、例えば、プロパン三酸、ブタン三酸、ペンタン三酸、ヘキサン三酸、ヘプタン三酸、オクタン三酸、ノナン三酸及びデカン三酸、並びに鎖状炭化水素テトラカルボン酸、例えば、アルカンテトラカルボン酸、例えば、ブタン四酸、ペンタン四酸、ヘキサン四酸、ヘプタン四酸、オクタン四酸、ノナン四酸及びデカン四酸が挙げられる。
(C1) Carboxylic acid, hydroxy acid, alkoxy acid or oxo acid (hereinafter referred to as “compound (C1) containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atom of the chain hydrocarbon moiety” For example, a chain hydrocarbon carboxylic acid having 2 to 4 carboxyl groups, such as a chain hydrocarbon dicarboxylic acid such as an alkanedicarboxylic acid such as ethanedioic acid, Propanedioic acid, butanedioic acid, pentanedioic acid, hexanedioic acid, heptanedioic acid, octanedioic acid, nonanedioic acid and decanedioic acid, chain hydrocarbon tricarboxylic acid such as alkanetricarboxylic acid such as propanetriacid , Butanetriacid, pentanetriacid, hexanetriacid, heptanetriacid, octanetriacid, nonanetriacid and decanetriacid, and chain hydrocarbon tetracarbo Acids such as alkanetetracarboxylic acids such as butanetetraacid, pentanetetraacid, hexanetetraacid, heptanetetraacid, octanetetraacid, nonanetetraacid and decanetetraacid.
また、化合物(C1)には、2~4個のカルボキシル基を有する鎖状炭化水素ヒドロキシ酸、例えば、リンゴ酸、酒石酸、クエン酸、イソクエン酸等、2~4個のカルボキシル基を有する鎖状炭化水素アルコキシ酸、例えば、O-アセチルクエン酸、及び2~4個のカルボキシル基を有する鎖状炭化水素オキソ酸が含まれる。
(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物としては、「化合物(B)」の項で列挙されるもの、例えば、脂肪族1価アルコールが挙げられる。 Further, the compound (C1) includes a chain hydrocarbon hydroxy acid having 2 to 4 carboxyl groups, for example, a chain chain having 2 to 4 carboxyl groups such as malic acid, tartaric acid, citric acid, isocitric acid and the like. Hydrocarbon alkoxy acids such as O-acetylcitric acid and chain hydrocarbon oxoacids having 2 to 4 carboxyl groups are included.
(C2) Examples of the compound having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety include those listed in the section of “Compound (B)”, for example, fat Group monohydric alcohols.
(C2)鎖状炭化水素部分と、鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物としては、「化合物(B)」の項で列挙されるもの、例えば、脂肪族1価アルコールが挙げられる。 Further, the compound (C1) includes a chain hydrocarbon hydroxy acid having 2 to 4 carboxyl groups, for example, a chain chain having 2 to 4 carboxyl groups such as malic acid, tartaric acid, citric acid, isocitric acid and the like. Hydrocarbon alkoxy acids such as O-acetylcitric acid and chain hydrocarbon oxoacids having 2 to 4 carboxyl groups are included.
(C2) Examples of the compound having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety include those listed in the section of “Compound (B)”, for example, fat Group monohydric alcohols.
化合物(C)としては、(c1)4個のカルボキシル基を有する鎖状炭化水素テトラカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、例えば、モノエステル、ジエステル、トリエステル及びテトラエステル、好ましくはジエステル、トリエステル及びテトラエステル、より好ましくはトリエステル及びテトラエステル、そしてさらに好ましくはテトラエステル、(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、例えば、モノエステル、ジエステル及びトリエステル、好ましくはジエステル及びトリエステル、そしてより好ましくはトリエステル、並びに(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、例えば、モノエステル及びジエステル、好ましくはジエステルが挙げられる。
化合物(C)の例としては、アジピン酸ジオクチル、O-アセチルクエン酸トリブチル等が挙げられ、そして市販されている。 As the compound (C), (c 1 ) an ester of a linear hydrocarbon tetracarboxylic acid having 4 carboxyl groups, a hydroxy acid, an alkoxy acid or an oxo acid and at least one aliphatic monohydric alcohol, for example, Monoesters, diesters, triesters and tetraesters, preferably diesters, triesters and tetraesters, more preferably triesters and tetraesters, and even more preferably tetraesters, (c 2 ) a chain having three carboxyl groups Esters of hydrocarbon tricarboxylic acids, hydroxy acids, alkoxy acids or oxo acids with at least one aliphatic monohydric alcohol, such as monoesters, diesters and triesters, preferably diesters and triesters, and more preferably triesters And ( 3) chain hydrocarbon dicarboxylic acids having two carboxyl groups, hydroxy acids, esters of alkoxy acids or oxoacids, and at least one aliphatic monohydric alcohol, for example, mono- and diesters, preferably include diester It is done.
Examples of compound (C) include dioctyl adipate, tributyl O-acetylcitrate, and are commercially available.
化合物(C)の例としては、アジピン酸ジオクチル、O-アセチルクエン酸トリブチル等が挙げられ、そして市販されている。 As the compound (C), (c 1 ) an ester of a linear hydrocarbon tetracarboxylic acid having 4 carboxyl groups, a hydroxy acid, an alkoxy acid or an oxo acid and at least one aliphatic monohydric alcohol, for example, Monoesters, diesters, triesters and tetraesters, preferably diesters, triesters and tetraesters, more preferably triesters and tetraesters, and even more preferably tetraesters, (c 2 ) a chain having three carboxyl groups Esters of hydrocarbon tricarboxylic acids, hydroxy acids, alkoxy acids or oxo acids with at least one aliphatic monohydric alcohol, such as monoesters, diesters and triesters, preferably diesters and triesters, and more preferably triesters And ( 3) chain hydrocarbon dicarboxylic acids having two carboxyl groups, hydroxy acids, esters of alkoxy acids or oxoacids, and at least one aliphatic monohydric alcohol, for example, mono- and diesters, preferably include diester It is done.
Examples of compound (C) include dioctyl adipate, tributyl O-acetylcitrate, and are commercially available.
[(D)鎖状炭化水素部分と、鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物]
(D)鎖状炭化水素部分と、鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物(以下、「化合物(D)」と称する場合がある)としては、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、及び(d4)ジアルキルカーボネートが挙げられる。 [(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO— inserted between a chain hydrocarbon moiety and a CC single bond of the chain hydrocarbon moiety. , And any one bond selected from the group consisting of carbonate bonds (—OCOO—)]
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the C—C single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—) (hereinafter sometimes referred to as “compound (D)”) includes (d 1 ) aliphatic 1 And ethers of monohydric alcohols and aliphatic monohydric alcohols, (d 2 ) dialkyl ketones, (d 3 ) esters of fatty acids and aliphatic monohydric alcohols, and (d 4 ) dialkyl carbonates.
(D)鎖状炭化水素部分と、鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物(以下、「化合物(D)」と称する場合がある)としては、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、及び(d4)ジアルキルカーボネートが挙げられる。 [(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO— inserted between a chain hydrocarbon moiety and a CC single bond of the chain hydrocarbon moiety. , And any one bond selected from the group consisting of carbonate bonds (—OCOO—)]
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the C—C single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—) (hereinafter sometimes referred to as “compound (D)”) includes (d 1 ) aliphatic 1 And ethers of monohydric alcohols and aliphatic monohydric alcohols, (d 2 ) dialkyl ketones, (d 3 ) esters of fatty acids and aliphatic monohydric alcohols, and (d 4 ) dialkyl carbonates.
[(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル]
脂肪族1価アルコールと脂肪族1価アルコールとのエーテルとしては、次の式(19):
R19OR20 (19)
(式中、R19及びR20は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 1 ) Ether of aliphatic monohydric alcohol and aliphatic monohydric alcohol]
As an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, the following formula (19):
R 19 OR 20 (19)
(In the formula, R 19 and R 20 are each a chain hydrocarbon)
The compound which has is mentioned.
脂肪族1価アルコールと脂肪族1価アルコールとのエーテルとしては、次の式(19):
R19OR20 (19)
(式中、R19及びR20は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 1 ) Ether of aliphatic monohydric alcohol and aliphatic monohydric alcohol]
As an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, the following formula (19):
R 19 OR 20 (19)
(In the formula, R 19 and R 20 are each a chain hydrocarbon)
The compound which has is mentioned.
エーテルを構成する脂肪族1価アルコール(式(19)において、R19OH及びR20OHに相当する)としては、エーテルが、上述の動粘度、抱水率及び重量平均分子量の要件を満たすものであれば、特に制限されず、例えば、「化合物(B)」の項で列挙される脂肪族1価アルコールが挙げられる。
As the aliphatic monohydric alcohol constituting the ether (corresponding to R 19 OH and R 20 OH in the formula (19)), the ether satisfies the above-mentioned requirements for kinematic viscosity, water retention and weight average molecular weight. If it is, it will not restrict | limit in particular, For example, the aliphatic monohydric alcohol enumerated by the term of "compound (B)" is mentioned.
[(d2)ジアルキルケトン]
ジアルキルケトンとしては、次の式(20):
R21COR22 (20)
(式中、R21及びR22は、それぞれ、アルキル基である)
を有する化合物が挙げられる。
ジアルキルケトンは、市販されている他、公知の方法、例えば、第二級アルコールを、クロム酸等で酸化することにより得ることができる。 [(D 2 ) dialkylketone]
As the dialkyl ketone, the following formula (20):
R 21 COR 22 (20)
(Wherein R 21 and R 22 are each an alkyl group)
The compound which has is mentioned.
In addition to being commercially available, the dialkyl ketone can be obtained by a known method, for example, by oxidizing a secondary alcohol with chromic acid or the like.
ジアルキルケトンとしては、次の式(20):
R21COR22 (20)
(式中、R21及びR22は、それぞれ、アルキル基である)
を有する化合物が挙げられる。
ジアルキルケトンは、市販されている他、公知の方法、例えば、第二級アルコールを、クロム酸等で酸化することにより得ることができる。 [(D 2 ) dialkylketone]
As the dialkyl ketone, the following formula (20):
R 21 COR 22 (20)
(Wherein R 21 and R 22 are each an alkyl group)
The compound which has is mentioned.
In addition to being commercially available, the dialkyl ketone can be obtained by a known method, for example, by oxidizing a secondary alcohol with chromic acid or the like.
[(d3)脂肪酸と脂肪族1価アルコールとのエステル]
脂肪酸と脂肪族1価アルコールとのエステルとしては、例えば、次の式(21):
R23COOR24 (21)
(式中、R23及びR24は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
Examples of the ester of a fatty acid and an aliphatic monohydric alcohol include the following formula (21):
R 23 COOR 24 (21)
(Wherein R 23 and R 24 are each a chain hydrocarbon)
The compound which has is mentioned.
脂肪酸と脂肪族1価アルコールとのエステルとしては、例えば、次の式(21):
R23COOR24 (21)
(式中、R23及びR24は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
Examples of the ester of a fatty acid and an aliphatic monohydric alcohol include the following formula (21):
R 23 COOR 24 (21)
(Wherein R 23 and R 24 are each a chain hydrocarbon)
The compound which has is mentioned.
エステルを構成する脂肪酸(式(21)において、R23COOHに相当する)としては、例えば、「(a1)鎖状炭化水素テトラオールと脂肪酸とのエステル」において列挙されている脂肪酸、すなわち、飽和脂肪酸又は不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸が好ましい。エステルを構成する脂肪族1価アルコール(式(21)において、R24OHに相当する)としては、例えば、「化合物(B)」の項で列挙される脂肪族1価アルコールが挙げられる。
Examples of the fatty acid constituting the ester (corresponding to R 23 COOH in the formula (21)) include, for example, the fatty acids listed in “(a 1 ) ester of chain hydrocarbon tetraol and fatty acid”, that is, Saturated fatty acids or unsaturated fatty acids are exemplified, and saturated fatty acids are preferred in consideration of the possibility of modification by oxidation or the like. Examples of the aliphatic monohydric alcohol constituting the ester (corresponding to R 24 OH in formula (21)) include the aliphatic monohydric alcohols listed in the section “Compound (B)”.
脂肪酸と脂肪族1価アルコールとのエステルの例としては、例えば、ドデカン酸(C12)と、ドデシルアルコール(C12)とのエステル、テトラデカン酸(C14)と、ドデシルアルコール(C12)とのエステル等が挙げられ、脂肪酸と脂肪族1価アルコールとのエステルの市販品としては、例えば、エレクトールWE20、及びエレクトールWE40(以上、日油株式会社製)が挙げられる。
Examples of esters of fatty acids and aliphatic monohydric alcohols include, for example, dodecanoic acid (C 12 ), dodecyl alcohol (C 12 ) ester, tetradecanoic acid (C 14 ), dodecyl alcohol (C 12 ) and Examples of commercially available esters of fatty acids and aliphatic monohydric alcohols include Electol WE20 and Electol WE40 (manufactured by NOF Corporation).
[(d4)ジアルキルカーボネート]
ジアルキルカーボネートとしては、次の式(22):
R25OC(=O)OR26 (22)
(式中、R25及びR26は、それぞれ、アルキル基である)
を有する化合物が挙げられる。
ジアルキルカーボネートは、市販されている他、ホスゲンとアルコールとの反応、塩化ギ酸エステルとアルコール又はアルコラートとの反応、及び炭酸銀とヨウ化アルキルとの反応により合成することができる。 [(D 4 ) dialkyl carbonate]
As the dialkyl carbonate, the following formula (22):
R 25 OC (= O) OR 26 (22)
(Wherein R 25 and R 26 are each an alkyl group)
The compound which has is mentioned.
The dialkyl carbonate is commercially available, and can be synthesized by a reaction of phosgene with an alcohol, a reaction of a formic chloride ester with an alcohol or an alcoholate, and a reaction of silver carbonate with an alkyl iodide.
ジアルキルカーボネートとしては、次の式(22):
R25OC(=O)OR26 (22)
(式中、R25及びR26は、それぞれ、アルキル基である)
を有する化合物が挙げられる。
ジアルキルカーボネートは、市販されている他、ホスゲンとアルコールとの反応、塩化ギ酸エステルとアルコール又はアルコラートとの反応、及び炭酸銀とヨウ化アルキルとの反応により合成することができる。 [(D 4 ) dialkyl carbonate]
As the dialkyl carbonate, the following formula (22):
R 25 OC (= O) OR 26 (22)
(Wherein R 25 and R 26 are each an alkyl group)
The compound which has is mentioned.
The dialkyl carbonate is commercially available, and can be synthesized by a reaction of phosgene with an alcohol, a reaction of a formic chloride ester with an alcohol or an alcoholate, and a reaction of silver carbonate with an alkyl iodide.
抱水率、蒸気圧等の観点から考察すると、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、及び(d4)ジアルキルカーボネートでは、重量平均分子量が約100以上であることが好ましく、そして約200以上であることがより好ましい。
なお、(d2)ジアルキルケトンにおいて、炭素数の合計が約8の場合、例えば、5-ノナノンでは、融点は約-50℃であり、蒸気圧は20℃で約230Paである。 Considering from the viewpoint of water retention, vapor pressure, etc., (d 1 ) an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, (d 2 ) a dialkyl ketone, (d 3 ) a fatty acid and an aliphatic monohydric alcohol And (d 4 ) dialkyl carbonates preferably have a weight average molecular weight of about 100 or more, and more preferably about 200 or more.
In (d 2 ) dialkyl ketone, when the total number of carbon atoms is about 8, for example, for 5-nonanone, the melting point is about −50 ° C., and the vapor pressure is about 230 Pa at 20 ° C.
なお、(d2)ジアルキルケトンにおいて、炭素数の合計が約8の場合、例えば、5-ノナノンでは、融点は約-50℃であり、蒸気圧は20℃で約230Paである。 Considering from the viewpoint of water retention, vapor pressure, etc., (d 1 ) an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, (d 2 ) a dialkyl ketone, (d 3 ) a fatty acid and an aliphatic monohydric alcohol And (d 4 ) dialkyl carbonates preferably have a weight average molecular weight of about 100 or more, and more preferably about 200 or more.
In (d 2 ) dialkyl ketone, when the total number of carbon atoms is about 8, for example, for 5-nonanone, the melting point is about −50 ° C., and the vapor pressure is about 230 Pa at 20 ° C.
[(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル]
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル(以下、化合物(E)と称する場合がある)としては、(e1)ポリオキシC3~C6アルキレングリコール、(e2)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルが挙げられる。以下、説明する。 [(E) Polyoxy C 3 -C 6 alkylene glycol, or alkyl ester or alkyl ether thereof]
(E) Polyoxy C 3 -C 6 alkylene glycol, or alkyl ester or alkyl ether thereof (hereinafter sometimes referred to as compound (E)) includes (e 1 ) polyoxy C 3 -C 6 alkylene glycol, (e 2 ) Esters of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid, (e 3 ) Ethers of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol. This will be described below.
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル(以下、化合物(E)と称する場合がある)としては、(e1)ポリオキシC3~C6アルキレングリコール、(e2)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルが挙げられる。以下、説明する。 [(E) Polyoxy C 3 -C 6 alkylene glycol, or alkyl ester or alkyl ether thereof]
(E) Polyoxy C 3 -C 6 alkylene glycol, or alkyl ester or alkyl ether thereof (hereinafter sometimes referred to as compound (E)) includes (e 1 ) polyoxy C 3 -C 6 alkylene glycol, (e 2 ) Esters of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid, (e 3 ) Ethers of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol. This will be described below.
[(e1)ポリオキシC3~C6アルキレングリコール]
ポリオキシC3~C6アルキレングリコールは、i)オキシC3~C6アルキレン骨格、すなわち、オキシプロピレン骨格、オキシブチレン骨格、オキシペンチレン骨格、及びオキシヘキシレン骨格から成る群から選択されるいずれか1種の骨格を有し且つ両末端にヒドロキシ基を有するホモポリマー、ii)上記群から選択される2種以上の骨格を有し且つ両末端にヒドロキシ基を有するブロックコポリマー、又はiii)上記群から選択される2種以上の骨格を有し且つ両末端にヒドロキシ基を有するランダムコポリマーを意味する。 [(E 1 ) polyoxy C 3 -C 6 alkylene glycol]
Polyoxy C 3 ~ C 6 alkylene glycol, i) oxy C 3 ~ C 6 alkylene backbone, i.e., oxypropylene backbone, oxybutylene backbone, any one selected from the group consisting of oxypentylene skeleton, and oxy hexylene backbone A homopolymer having one skeleton and having hydroxy groups at both ends, ii) a block copolymer having two or more skeletons selected from the above group and having hydroxy groups at both ends, or iii) the above group Means a random copolymer having two or more skeletons selected from the above and having hydroxy groups at both ends.
ポリオキシC3~C6アルキレングリコールは、i)オキシC3~C6アルキレン骨格、すなわち、オキシプロピレン骨格、オキシブチレン骨格、オキシペンチレン骨格、及びオキシヘキシレン骨格から成る群から選択されるいずれか1種の骨格を有し且つ両末端にヒドロキシ基を有するホモポリマー、ii)上記群から選択される2種以上の骨格を有し且つ両末端にヒドロキシ基を有するブロックコポリマー、又はiii)上記群から選択される2種以上の骨格を有し且つ両末端にヒドロキシ基を有するランダムコポリマーを意味する。 [(E 1 ) polyoxy C 3 -C 6 alkylene glycol]
Polyoxy C 3 ~ C 6 alkylene glycol, i) oxy C 3 ~ C 6 alkylene backbone, i.e., oxypropylene backbone, oxybutylene backbone, any one selected from the group consisting of oxypentylene skeleton, and oxy hexylene backbone A homopolymer having one skeleton and having hydroxy groups at both ends, ii) a block copolymer having two or more skeletons selected from the above group and having hydroxy groups at both ends, or iii) the above group Means a random copolymer having two or more skeletons selected from the above and having hydroxy groups at both ends.
ポリオキシC3~C6アルキレングリコールは、次の式(23):
HO-(CmH2mO)n-H (23)
(式中、mは3~6の整数である)
により表わされる。 The polyoxy C 3 -C 6 alkylene glycol has the following formula (23):
HO- (C m H 2m O) n -H (23)
(Where m is an integer from 3 to 6)
Is represented by
HO-(CmH2mO)n-H (23)
(式中、mは3~6の整数である)
により表わされる。 The polyoxy C 3 -C 6 alkylene glycol has the following formula (23):
HO- (C m H 2m O) n -H (23)
(Where m is an integer from 3 to 6)
Is represented by
本発明者が確認したところ、ポリプロピレングリコール(式(23)において、m=3のホモポリマーに相当する)では、重量平均分子量が約1,000未満の場合には、抱水率の要件を満たさないことが見いだされた。従って、血液滑性付与剤の範囲に、ポリプロピレングリコールのホモポリマーは含まれず、プロピレングリコールは、他のグリコールとのコポリマー又はランダムポリマーとして、(e1)ポリオキシC3~C6アルキレングリコールに含まれるべきである。
As a result of confirmation by the present inventor, polypropylene glycol (corresponding to a homopolymer of m = 3 in the formula (23)) satisfies the water retention requirement when the weight average molecular weight is less than about 1,000. I found nothing. Therefore, the range of blood lubricity-imparting agents does not include homopolymers of polypropylene glycol, and propylene glycol is included in (e 1 ) polyoxy C 3 -C 6 alkylene glycol as a copolymer or random polymer with other glycols. Should.
なお、本発明者が確認したところ、ポリエチレングリコール(式(23)において、m=2のホモポリマーに相当する)では、重量平均分子量が1,000未満では、動粘度及び抱水率の要件を満たし得ないことが示唆された。
As a result of confirmation by the present inventor, polyethylene glycol (corresponding to a homopolymer of m = 2 in the formula (23)) has a weight average molecular weight of less than 1,000 and satisfies the requirements for kinematic viscosity and water retention. It was suggested that it could not be satisfied.
IOBを約0.00~約0.60とする観点から考察すると、例えば、式(23)がポリブチレングリコール(m=4のホモポリマー)である場合には、n≧約7であることが好ましい(n=7の場合に、IOBが0.57となる)。
Considering from the viewpoint of setting the IOB from about 0.00 to about 0.60, for example, when the formula (23) is polybutylene glycol (homopolymer of m = 4), n ≧ about 7. Preferred (when n = 7, the IOB is 0.57).
ポリC3~C6アルキレングリコールの市販品としては、例えば、ユニオール(商標)PB-500及びPB-700(以上、日油株式会社製)が挙げられる。
Examples of commercially available poly C 3 -C 6 alkylene glycols include Uniol (trademark) PB-500 and PB-700 (manufactured by NOF Corporation).
[(e2)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル]
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステルとしては、「(e1)ポリオキシC3~C6アルキレングリコール」の項で説明したポリオキシC3~C6アルキレングリコールのOH末端の一方又は両方が、脂肪酸によりエステル化されているもの、すなわち、モノエステル及びジエステルが挙げられる。 [(E 2 ) ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid]
Examples of the ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid include the OH terminal of the polyoxy C 3 -C 6 alkylene glycol described in the section “(e 1 ) Polyoxy C 3 -C 6 alkylene glycol”. One or both are esterified with fatty acids, ie monoesters and diesters.
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステルとしては、「(e1)ポリオキシC3~C6アルキレングリコール」の項で説明したポリオキシC3~C6アルキレングリコールのOH末端の一方又は両方が、脂肪酸によりエステル化されているもの、すなわち、モノエステル及びジエステルが挙げられる。 [(E 2 ) ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid]
Examples of the ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid include the OH terminal of the polyoxy C 3 -C 6 alkylene glycol described in the section “(e 1 ) Polyoxy C 3 -C 6 alkylene glycol”. One or both are esterified with fatty acids, ie monoesters and diesters.
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステルにおいて、エステル化すべき脂肪酸としては、例えば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙されている脂肪酸、すなわち、飽和脂肪酸又は不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸が好ましい。
Examples of the fatty acid to be esterified in the ester of polyoxy C 3 -C 6 alkylene glycol and at least one fatty acid are listed in “Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”. Fatty acid, that is, saturated fatty acid or unsaturated fatty acid, and saturated fatty acid is preferable in consideration of possibility of modification by oxidation or the like.
[(e3)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル]
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、「(e1)ポリオキシC3~C6アルキレングリコール」の項で説明したポリオキシC3~C6アルキレングリコールのOH末端の一方又は両方が、脂肪族1価アルコールによりエーテル化されているもの、すなわち、モノエーテル及びジエーテルが挙げられる。
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルにおいて、エーテル化すべき脂肪族1価アルコールとしては、例えば、「化合物(B)」の項で列挙されている脂肪族1価アルコールが挙げられる。 [(E 3 ) ether of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol]
The ether of polyoxy C 3 ~ C 6 alkylene glycol and at least one aliphatic monohydric alcohol, "(e 1) polyoxy C 3 ~ C 6 alkylene glycol" polyoxy C 3 ~ C 6 alkylene glycol is described in the section of And those in which one or both of the OH ends are etherified with an aliphatic monohydric alcohol, that is, monoethers and diethers.
Examples of the aliphatic monohydric alcohol to be etherified in the ether of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, the aliphatic enumerated in the section of “Compound (B)”. A monohydric alcohol is mentioned.
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、「(e1)ポリオキシC3~C6アルキレングリコール」の項で説明したポリオキシC3~C6アルキレングリコールのOH末端の一方又は両方が、脂肪族1価アルコールによりエーテル化されているもの、すなわち、モノエーテル及びジエーテルが挙げられる。
ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルにおいて、エーテル化すべき脂肪族1価アルコールとしては、例えば、「化合物(B)」の項で列挙されている脂肪族1価アルコールが挙げられる。 [(E 3 ) ether of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol]
The ether of polyoxy C 3 ~ C 6 alkylene glycol and at least one aliphatic monohydric alcohol, "(e 1) polyoxy C 3 ~ C 6 alkylene glycol" polyoxy C 3 ~ C 6 alkylene glycol is described in the section of And those in which one or both of the OH ends are etherified with an aliphatic monohydric alcohol, that is, monoethers and diethers.
Examples of the aliphatic monohydric alcohol to be etherified in the ether of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, the aliphatic enumerated in the section of “Compound (B)”. A monohydric alcohol is mentioned.
[(F)鎖状炭化水素]
鎖状炭化水素としては、例えば、(f1)鎖状アルカン、例えば、直鎖アルカン及び分岐鎖アルカンが挙げられる。直鎖アルカンは、融点が約45℃以下の場合には、炭素数が約22以下となり、そして蒸気圧が1気圧及び25℃で約0.01Pa以下である場合には、炭素数が約13以上となる。分岐鎖アルカンは、直鎖アルカンよりも、同一炭素数において融点が低い傾向がある。従って、分岐鎖アルカンは、融点が約45℃以下の場合でも、炭素数が22以上のものも含むことができる。
炭化水素の市販品としては、例えば、パールリーム6(日油株式会社)が挙げられる。 [(F) chain hydrocarbon]
Examples of the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes. The linear alkane has a carbon number of about 22 or less when the melting point is about 45 ° C. or less, and about 13 or less when the vapor pressure is about 0.01 Pa or less at 1 atm and 25 ° C. That's it. Branched-chain alkanes tend to have lower melting points at the same carbon number than straight-chain alkanes. Therefore, the branched chain alkane can include those having 22 or more carbon atoms even when the melting point is about 45 ° C. or lower.
As a commercial item of hydrocarbons, for example, Pearl Ream 6 (NOF Corporation) can be mentioned.
鎖状炭化水素としては、例えば、(f1)鎖状アルカン、例えば、直鎖アルカン及び分岐鎖アルカンが挙げられる。直鎖アルカンは、融点が約45℃以下の場合には、炭素数が約22以下となり、そして蒸気圧が1気圧及び25℃で約0.01Pa以下である場合には、炭素数が約13以上となる。分岐鎖アルカンは、直鎖アルカンよりも、同一炭素数において融点が低い傾向がある。従って、分岐鎖アルカンは、融点が約45℃以下の場合でも、炭素数が22以上のものも含むことができる。
炭化水素の市販品としては、例えば、パールリーム6(日油株式会社)が挙げられる。 [(F) chain hydrocarbon]
Examples of the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes. The linear alkane has a carbon number of about 22 or less when the melting point is about 45 ° C. or less, and about 13 or less when the vapor pressure is about 0.01 Pa or less at 1 atm and 25 ° C. That's it. Branched-chain alkanes tend to have lower melting points at the same carbon number than straight-chain alkanes. Therefore, the branched chain alkane can include those having 22 or more carbon atoms even when the melting point is about 45 ° C. or lower.
As a commercial item of hydrocarbons, for example, Pearl Ream 6 (NOF Corporation) can be mentioned.
排泄口当接領域20のうち少なくとも凸部8には、血液滑性付与剤が単独で塗工されていてもよいし、血液滑性付与剤と、少なくとも1種の他の成分とを含有する血液滑性付与剤含有組成物が塗工されていてもよい。
以下、血液滑性付与剤含有組成物について説明する。なお、血液滑性付与剤含有組成物の塗工に関しては、血液滑性付与剤の塗工と同様であるので、説明を省略する。 The blood slipperiness-imparting agent may be applied alone to at least theconvex portion 8 in the excretory opening contact region 20, or the blood slipperiness-imparting agent and at least one other component are contained. A blood slipping agent-containing composition may be applied.
Hereinafter, the blood slipping agent-containing composition will be described. In addition, since it is the same as that of the blood slipperiness | lubricity imparting agent about application of a blood slipperiness | lubricity imparting agent containing composition, description is abbreviate | omitted.
以下、血液滑性付与剤含有組成物について説明する。なお、血液滑性付与剤含有組成物の塗工に関しては、血液滑性付与剤の塗工と同様であるので、説明を省略する。 The blood slipperiness-imparting agent may be applied alone to at least the
Hereinafter, the blood slipping agent-containing composition will be described. In addition, since it is the same as that of the blood slipperiness | lubricity imparting agent about application of a blood slipperiness | lubricity imparting agent containing composition, description is abbreviate | omitted.
[血液滑性付与剤含有組成物]
血液滑性付与剤含有組成物は、上述の血液滑性付与剤と、少なくとも1種の他の成分とを含有する。他の成分としては、血液滑性付与剤の作用効果を阻害しないものであれば特に制限されず、当業界で吸収性物品、特にトップシートに慣用的に適用されるものを使用することができる。 [Blood lubricity-containing composition]
The composition for containing a blood slipperiness agent contains the above-described blood slipperiness imparting agent and at least one other component. The other components are not particularly limited as long as they do not inhibit the effect of the blood slipperiness-imparting agent, and those conventionally used in absorbent articles, particularly top sheets, can be used in the industry. .
血液滑性付与剤含有組成物は、上述の血液滑性付与剤と、少なくとも1種の他の成分とを含有する。他の成分としては、血液滑性付与剤の作用効果を阻害しないものであれば特に制限されず、当業界で吸収性物品、特にトップシートに慣用的に適用されるものを使用することができる。 [Blood lubricity-containing composition]
The composition for containing a blood slipperiness agent contains the above-described blood slipperiness imparting agent and at least one other component. The other components are not particularly limited as long as they do not inhibit the effect of the blood slipperiness-imparting agent, and those conventionally used in absorbent articles, particularly top sheets, can be used in the industry. .
他の成分としては、例えば、シリコーンオイル、シリコーン、シリコーン系レジン等が挙げられる。
Examples of other components include silicone oil, silicone, silicone resin, and the like.
他の成分としては、例えば、酸化防止剤、例えば、BHT(2,6-ジ-t-ブチル-p-クレゾール)、BHA(ブチル化ヒドロキシアニソール)、没食子酸プロピル等が挙げられる。
Examples of other components include antioxidants such as BHT (2,6-di-t-butyl-p-cresol), BHA (butylated hydroxyanisole), propyl gallate and the like.
他の成分としては、例えば、ビタミン、例えば、天然ビタミン又は合成ビタミンが挙げられる。ビタミンとしては、例えば、水溶性ビタミン、例えば、ビタミンB群、例えば、ビタミンB1,ビタミンB2,ビタミンB3,ビタミンB5,ビタミンB6,ビタミンB7,ビタミンB9,ビタミンB12等、ビタミンCが挙げられる。
Examples of other components include vitamins such as natural vitamins and synthetic vitamins. Examples of vitamins include water-soluble vitamins such as vitamin B groups, such as vitamin B 1 , vitamin B 2 , vitamin B 3 , vitamin B 5 , vitamin B 6 , vitamin B 7 , vitamin B 9 , vitamin B 12, etc. And vitamin C.
ビタミンとしては、例えば、脂溶性ビタミン、例えば、ビタミンA群、ビタミンD群、ビタミンE群、およびビタミンK群等が挙げられる。ビタミンにはまた、それらの誘導体も含まれる。
Examples of vitamins include fat-soluble vitamins such as vitamin A group, vitamin D group, vitamin E group, and vitamin K group. Vitamins also include their derivatives.
他の成分としては、例えば、アミノ酸、例えば、アラニン、アルギニン、リジン、ヒスチジン、プロリン、ヒドロキシプロリン等、並びにペプチドが挙げられる。
Examples of other components include amino acids such as alanine, arginine, lysine, histidine, proline, hydroxyproline, and peptides.
他の成分としては、例えば、ゼオライト、例えば、天然ゼオライト、例えば、方沸石、菱沸石、輝沸石、ナトロライト、束沸石、及びソモソナイト、並びに、合成ゼオライトが挙げられる。
Other components include, for example, zeolites such as natural zeolites such as zeolitic, chabazite, pyroxenite, natrolite, zeolitic and somosonite, and synthetic zeolite.
他の成分としては、例えば、コレステロール、ヒアルロン酸、レシチン、セラミド等が挙げられる。
Examples of other components include cholesterol, hyaluronic acid, lecithin, ceramide and the like.
他の成分としては、例えば、薬剤、例えば、皮膚収斂剤、抗ニキビ剤、抗シワ剤、抗セルライト剤、美白剤、抗菌剤、抗カビ剤等が挙げられる。
Other components include, for example, drugs such as skin astringents, anti-acne agents, anti-wrinkle agents, anti-cellulite agents, whitening agents, antibacterial agents, anti-fungal agents and the like.
皮膚収斂剤としては、例えば、酸化亜鉛、硫酸アルミニウム、タンニン酸等、油溶性皮膚収斂剤、例えば、油溶性ポリフェノールが挙げられる。油溶性ポリフェノールとしては、天然の油溶性ポリフェノール、例えば、オオバクエキス、オトギリソウエキス、オドリコソウエキス、カモミラエキス、ゴボウエキス、サルビアエキス、シナノキエキス、セイヨウボダイジュエキス、シラカバエキス、スギナエキス、セージエキス、サルビアエキス、テウチグルミエキス、ハイビスカスエキス、ビワ葉エキス、ボダイジュエキス、ホップエキス、マロニエエキス、ヨクイニンエキス等が挙げられる。
Examples of the skin astringent include oil-soluble skin astringents such as zinc oxide, aluminum sulfate, and tannic acid, such as oil-soluble polyphenols. Examples of oil-soluble polyphenols include natural oil-soluble polyphenols, such as buckwheat extract, hypericum extract, nettle extract, chamomile extract, burdock extract, salvia extract, linden extract, scallop extract, birch extract, cedar extract, sage extract, salvia extract, Examples include teuchigurumi extract, hibiscus extract, loquat leaf extract, bodaiju extract, hop extract, marronnier extract, and yokuinin extract.
抗ニキビ剤としては、例えば、サリチル酸、過酸化ベンゾイル、レゾルシノール、イオウ、エリスロマイシン、亜鉛等が挙げられる。
Examples of the anti-acne agent include salicylic acid, benzoyl peroxide, resorcinol, sulfur, erythromycin, and zinc.
抗シワ剤としては、例えば、乳酸、サリチル酸、サリチル酸誘導体、グリコール酸、フィチン酸、リポ酸、リソフォスファチド酸が挙げられる。
Examples of the anti-wrinkle agent include lactic acid, salicylic acid, salicylic acid derivatives, glycolic acid, phytic acid, lipoic acid, and lysophosphatide acid.
抗セルライト剤としては、例えば、キサンチン化合物、例えば、アミノフィリン、カフェイン、テオフィリン、テオブロミン等が挙げられる。
Examples of the anti-cellulite agent include xanthine compounds such as aminophylline, caffeine, theophylline, theobromine and the like.
美白剤としては、例えば、ナイアシンアミド、コウジ酸、アルブチン、グルコサミン及び誘導体、フィトステロール誘導体、アスコルビン酸及びその誘導体、並びにクワ抽出物及び胎盤抽出物が挙げられる。
Examples of whitening agents include niacinamide, kojic acid, arbutin, glucosamine and derivatives, phytosterol derivatives, ascorbic acid and derivatives thereof, and mulberry extract and placenta extract.
他の成分としては、例えば、抗炎症成分、pH調整剤、抗菌剤、保湿剤、香料、色素、染料、顔料、植物抽出エキス等が挙げられる。
Examples of other components include anti-inflammatory components, pH adjusters, antibacterial agents, moisturizers, fragrances, pigments, dyes, pigments, plant extracts and the like.
抗炎症成分としては、例えば、天然由来の抗炎症剤、例えば、ボタン、オオゴン、オトギリソウ、カモミール、甘草、モモノハ、ヨモギ、シソエキス等、合成抗炎症剤、例えば、アラントイン、グリチルリチン酸ジカリウム等が挙げられる。
Anti-inflammatory components include, for example, naturally-occurring anti-inflammatory agents such as buttons, ogon, hypericum, chamomile, licorice, momonoha, mugwort, perilla extract, synthetic anti-inflammatory agents such as allantoin, dipotassium glycyrrhizinate, etc. .
pH調整剤としては、皮膚を弱酸性に保つためのもの、例えば、リンゴ酸、コハク酸、クエン酸、酒石酸、乳酸等が挙げられる。
Examples of the pH adjuster include those for keeping the skin weakly acidic, such as malic acid, succinic acid, citric acid, tartaric acid, and lactic acid.
顔料としては、例えば、酸化チタンが挙げられる。
Examples of the pigment include titanium oxide.
血液滑性付与剤含有組成物は、血液滑性付与剤及び少なくとも1種の他の成分を、それぞれ、好ましくは約50~約99質量%及び約1~約50質量%、より好ましくは約60~約99質量%及び約1~約40質量%、さらに好ましくは約70~約99質量%及び約1~約30質量%、さらに一層好ましくは約80~約99質量%及び約1~約20質量%、さらに一層好ましくは約90~99質量%及び約1~約10質量%、さらに一層好ましくは約95~99質量%及び約1~約5質量%含む。血液滑性付与剤及び他の成分の作用効果の観点からである。
The blood slipping agent-containing composition preferably contains about 50 to about 99% by weight and about 1 to about 50% by weight, more preferably about 60%, of the blood slipping agent and at least one other component, respectively. To about 99% and about 1 to about 40%, more preferably about 70 to about 99% and about 1 to about 30%, even more preferably about 80 to about 99% and about 1 to about 20%. % By weight, even more preferably from about 90 to 99% by weight and from about 1 to about 10% by weight, even more preferably from about 95 to 99% by weight and from about 1 to about 5% by weight. This is from the viewpoint of the effect of the blood slipperiness-imparting agent and other components.
血液滑性付与剤含有組成物は、界面活性剤を、トップシート又はセカンドシートの親水化処理に由来する量以下で含むことが好ましい。より具体的には、血液滑性付与剤含有組成物は、界面活性剤を、好ましくは約0.0~約1.0g/m2、より好ましくは約0.0~約0.8g/m2、さらに好ましくは約0.1~約0.5g/m2、さらに一層好ましくは約0.1~約0.3g/m2の坪量の範囲で含む。
The blood slipping agent-containing composition preferably contains a surfactant in an amount equal to or less than the amount derived from the hydrophilic treatment of the top sheet or the second sheet. More specifically, the composition containing a blood slipperiness agent preferably contains a surfactant, preferably about 0.0 to about 1.0 g / m 2 , more preferably about 0.0 to about 0.8 g / m 2. 2 , more preferably from about 0.1 to about 0.5 g / m 2 , and even more preferably from about 0.1 to about 0.3 g / m 2 in the basis weight range.
界面活性剤の量が増えると、経血がトップシートに残存しやすい傾向があるからである。なお、界面活性剤は、抱水率の値を有しない。水と混和するため、測定すべき物質の層が存在しないからである。
This is because menstrual blood tends to remain on the top sheet when the amount of the surfactant increases. The surfactant does not have a water retention value. This is because there is no layer of material to be measured because it is miscible with water.
血液滑性付与剤含有組成物は、水を、好ましくは約0.0~約1.0g/m2、より好ましくは約0.0~約0.8g/m2、さらに好ましくは約0.1~約0.5g/m2、さらに一層好ましくは約0.1~約0.3g/m2の坪量の範囲で含む。水は、吸収性物品の吸収性能を低下させるため、少ないことが好ましい。
The blood slipping agent-containing composition preferably contains water in an amount of about 0.0 to about 1.0 g / m 2 , more preferably about 0.0 to about 0.8 g / m 2 , and even more preferably about 0.0. It is included in the basis weight range of 1 to about 0.5 g / m 2 , even more preferably about 0.1 to about 0.3 g / m 2 . Since water reduces the absorption performance of an absorbent article, it is preferable that water is small.
血液滑性付与剤含有組成物は、血液滑性付与剤と同様に、組成物として、40℃において、約0~約80mm2/sの動粘度を有することが好ましく、約1~約70mm2/sの動粘度を有することがより好ましく、約3~約60mm2/sの動粘度を有することがさらに好ましく、約5~約50mm2/sの動粘度を有することがさらに一層好ましく、約7~約45mm2/sの動粘度を有することがさらに一層好ましい。
The blood slipping agent-containing composition, like the blood slipping agent, preferably has a kinematic viscosity at 40 ° C. of about 0 to about 80 mm 2 / s, and about 1 to about 70 mm 2. More preferably, it has a kinematic viscosity of about 3 to about 60 mm 2 / s, even more preferably about 5 to about 50 mm 2 / s, and even more preferably about Even more preferably, it has a kinematic viscosity of from 7 to about 45 mm 2 / s.
血液滑性付与剤含有組成物の動粘度が約80mm2/sを超えると、粘性が高く、トップシートの肌当接面に到達した経血と共に、血液滑性付与剤組成物が吸収性物品の内部に滑落することが難しくなる傾向があるからである。
When the kinematic viscosity of the composition containing a blood slipperiness agent exceeds about 80 mm 2 / s, the viscosity is high, and the blood slipperiness imparting composition is an absorbent article together with menstrual blood that has reached the skin contact surface of the top sheet. This is because it tends to be difficult to slide down inside.
血液滑性付与剤含有組成物が、少なくとも1種の他の成分として血液滑性付与剤と混和する成分を含む場合には、その他の成分は、好ましくは約1,000未満の重量平均分子量を有し、より好ましくは約900未満の重量平均分子量を有する。重量平均分子量が約1,000以上であると、血液滑性付与剤含有組成物そのものにタック性が生じ、着用者に不快感を与える傾向があるからである。また、重量平均分子量が高くなると、血液滑性付与剤含有組成物の粘度が高くなる傾向があるため、加温により、血液滑性付与剤組成物の粘度を、塗布に適した粘度に下げることが難しくなり、その結果、血液滑性付与剤を、溶媒で希釈しなければならない場合も生じうる。
If the composition containing a blood slipperiness agent contains at least one other component that is miscible with the blood slipperiness agent, the other component preferably has a weight average molecular weight of less than about 1,000. More preferably having a weight average molecular weight of less than about 900. This is because when the weight average molecular weight is about 1,000 or more, the blood slipperiness-imparting agent-containing composition itself is tacky and tends to give the wearer discomfort. Moreover, since the viscosity of the composition containing a blood slipperiness-imparting agent tends to increase as the weight average molecular weight increases, the viscosity of the blood slipperiness-enhancing agent composition is lowered to a viscosity suitable for application by heating. As a result, the blood slipping agent may have to be diluted with a solvent.
血液滑性付与剤含有組成物は、組成物として、約0.01~約4.0質量%の抱水率を有し、約0.02~約3.5質量%の抱水率を有することが好ましく、約0.03~約3.0質量%の抱水率を有することがより好ましく、約0.04~約2.5質量%の抱水率を有することがさらに好ましく、そして約0.05~約2.0質量%の抱水率を有することがさらに好ましい。
The composition containing a blood slipperiness imparting agent has a water content of about 0.01 to about 4.0% by mass and a water content of about 0.02 to about 3.5% by mass as the composition. Preferably about 0.03 to about 3.0% by weight, more preferably about 0.04 to about 2.5% by weight, and more preferably about 0.03 to about 3.0% by weight. More preferably, it has a water retention of 0.05 to about 2.0% by weight.
抱水率が低くなると、血液滑性付与剤組成物と、経血との親和性が低下し、トップシートの肌当接面に到達した経血が吸収性物品の内部に滑落しにくくなる傾向がある。
なお、血液滑性付与剤含有組成物が固形物を含む場合には、動粘度及び抱水率の測定において、それらを濾過により取り除くことが好ましい。 When the water retention rate is low, the affinity between the blood slipperiness-imparting agent composition and menstrual blood is reduced, and menstrual blood that has reached the skin contact surface of the top sheet is less likely to slide into the absorbent article. There is.
In addition, when a blood slipperiness | lubricity imparting agent containing composition contains a solid substance, it is preferable to remove them by filtration in the measurement of kinematic viscosity and a water retention.
なお、血液滑性付与剤含有組成物が固形物を含む場合には、動粘度及び抱水率の測定において、それらを濾過により取り除くことが好ましい。 When the water retention rate is low, the affinity between the blood slipperiness-imparting agent composition and menstrual blood is reduced, and menstrual blood that has reached the skin contact surface of the top sheet is less likely to slide into the absorbent article. There is.
In addition, when a blood slipperiness | lubricity imparting agent containing composition contains a solid substance, it is preferable to remove them by filtration in the measurement of kinematic viscosity and a water retention.
<試験例1>
以下に、本試験例で用いられた血液滑性付与剤を列挙する。
[(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル]
・ユニスター H-408BRS,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトール,重量平均分子量:約640
・ユニスター H-2408BRS-22,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトールと、ジ2-エチルヘキサン酸ネオペンチルグリコールとの混合物(58:42、重量比),重量平均分子量:約520 <Test Example 1>
The blood slipperiness imparting agents used in this test example are listed below.
[Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
Unistar H-408BRS, manufactured by NOF Corporation Tetra-2-ethylhexanoate pentaerythritol, weight average molecular weight: about 640
Unistar H-2408BRS-22, manufactured by NOF Corporation Mixture of tetraerythritol tetra-2-ethylhexanoate and neopentyl glycol di-2-ethylhexanoate (58:42, weight ratio), weight average molecular weight: about 520
以下に、本試験例で用いられた血液滑性付与剤を列挙する。
[(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル]
・ユニスター H-408BRS,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトール,重量平均分子量:約640
・ユニスター H-2408BRS-22,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトールと、ジ2-エチルヘキサン酸ネオペンチルグリコールとの混合物(58:42、重量比),重量平均分子量:約520 <Test Example 1>
The blood slipperiness imparting agents used in this test example are listed below.
[Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
Unistar H-408BRS, manufactured by NOF Corporation Tetra-2-ethylhexanoate pentaerythritol, weight average molecular weight: about 640
Unistar H-2408BRS-22, manufactured by NOF Corporation Mixture of tetraerythritol tetra-2-ethylhexanoate and neopentyl glycol di-2-ethylhexanoate (58:42, weight ratio), weight average molecular weight: about 520
[(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル]
・Cetiol SB45DEO,コグニスジャパン株式会社製
脂肪酸が、オレイン酸又はステアリル酸である、グリセリンと脂肪酸とのトリエステル
・SOY42,日油株式会社製
C14の脂肪酸:C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸及び不飽和脂肪酸の両方を含む)がおおよそ0.2:11:88:0.8の質量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:880 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
· Cetiol SB45DEO, the Cognis Corp. fatty acids, oleic acid or stearic acid, triesters · SOY42 of glycerin and fatty acids, fatty acid manufactured by NOF Corporation C 14: fatty acid C 16: fatty acid C 18: Triester of glycerin and fatty acid, weight average molecular weight, containing C 20 fatty acids (including both saturated and unsaturated fatty acids) in a mass ratio of approximately 0.2: 11: 88: 0.8 880
・Cetiol SB45DEO,コグニスジャパン株式会社製
脂肪酸が、オレイン酸又はステアリル酸である、グリセリンと脂肪酸とのトリエステル
・SOY42,日油株式会社製
C14の脂肪酸:C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸及び不飽和脂肪酸の両方を含む)がおおよそ0.2:11:88:0.8の質量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:880 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
· Cetiol SB45DEO, the Cognis Corp. fatty acids, oleic acid or stearic acid, triesters · SOY42 of glycerin and fatty acids, fatty acid manufactured by NOF Corporation C 14: fatty acid C 16: fatty acid C 18: Triester of glycerin and fatty acid, weight average molecular weight, containing C 20 fatty acids (including both saturated and unsaturated fatty acids) in a mass ratio of approximately 0.2: 11: 88: 0.8 880
・トリC2L油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸がおおよそ37:7:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570
・トリCL油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C12の脂肪酸がおおよそ44:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570 Tri-C2L oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid containing approximately 37: 7: 56 weight ratio, glycerol and fatty acid triester, Weight average molecular weight: about 570
Tri-CL oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: Triester of glycerin and fatty acid containing fatty acid of C 12 in a weight ratio of about 44:56, weight average molecular weight: about 570
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸がおおよそ37:7:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570
・トリCL油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C12の脂肪酸がおおよそ44:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570 Tri-C2L oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid containing approximately 37: 7: 56 weight ratio, glycerol and fatty acid triester, Weight average molecular weight: about 570
Tri-CL oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: Triester of glycerin and fatty acid containing fatty acid of C 12 in a weight ratio of about 44:56, weight average molecular weight: about 570
・パナセート810s,日油株式会社製
C8の脂肪酸:C10の脂肪酸がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約480
・パナセート800,日油株式会社製
脂肪酸が全てオクタン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470 · PANACET 810s, fatty acids manufactured by NOF Corporation C 8: fatty to C 10 are contained in approximately 85:15 weight ratio of triesters of glycerol with fatty acids, the weight average molecular weight: about 480
・ Panasate 800, manufactured by NOF Corporation All fatty acids are octanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470
C8の脂肪酸:C10の脂肪酸がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約480
・パナセート800,日油株式会社製
脂肪酸が全てオクタン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470 · PANACET 810s, fatty acids manufactured by NOF Corporation C 8: fatty to C 10 are contained in approximately 85:15 weight ratio of triesters of glycerol with fatty acids, the weight average molecular weight: about 480
・ Panasate 800, manufactured by NOF Corporation All fatty acids are octanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470
・パナセート800B,日油株式会社製
脂肪酸が全て2-エチルヘキサン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470
・NA36,日油株式会社製
C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸及び不飽和脂肪酸の両方を含む)がおおよそ5:92:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約880 Panaceate 800B, manufactured by NOF Corporation All fatty acids are 2-ethylhexanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470
· NA36, manufactured by NOF Corporation C 16 fatty acid: fatty acid C 18: (including both saturated and unsaturated fatty acids) fatty acids to C 20 is approximately 5: contained in a weight ratio of 3: 92 Triester of glycerin and fatty acid, weight average molecular weight: about 880
脂肪酸が全て2-エチルヘキサン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470
・NA36,日油株式会社製
C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸及び不飽和脂肪酸の両方を含む)がおおよそ5:92:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約880 Panaceate 800B, manufactured by NOF Corporation All fatty acids are 2-ethylhexanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470
· NA36, manufactured by NOF Corporation C 16 fatty acid: fatty acid C 18: (including both saturated and unsaturated fatty acids) fatty acids to C 20 is approximately 5: contained in a weight ratio of 3: 92 Triester of glycerin and fatty acid, weight average molecular weight: about 880
・トリヤシ油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸:C14の脂肪酸:C16の脂肪酸(飽和脂肪酸及び不飽和脂肪酸の両方を含む)がおおよそ4:8:60:25:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:670
・カプリル酸ジグリセリド,日油株式会社製
脂肪酸がオクタン酸である、グリセリンと脂肪酸とのジエステル,重量平均分子量:340 · Tri-coconut oil fatty acid glyceride, NOF fatty Ltd. C 8: fatty C 10: fatty acid C 12: fatty acid C 14: (including both saturated and unsaturated fatty acids) fatty acids C 16 is approximately 4 : Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670
-Caprylic acid diglyceride, manufactured by NOF Corporation Fatty acid is octanoic acid, diester of glycerin and fatty acid, weight average molecular weight: 340
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸:C14の脂肪酸:C16の脂肪酸(飽和脂肪酸及び不飽和脂肪酸の両方を含む)がおおよそ4:8:60:25:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:670
・カプリル酸ジグリセリド,日油株式会社製
脂肪酸がオクタン酸である、グリセリンと脂肪酸とのジエステル,重量平均分子量:340 · Tri-coconut oil fatty acid glyceride, NOF fatty Ltd. C 8: fatty C 10: fatty acid C 12: fatty acid C 14: (including both saturated and unsaturated fatty acids) fatty acids C 16 is approximately 4 : Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670
-Caprylic acid diglyceride, manufactured by NOF Corporation Fatty acid is octanoic acid, diester of glycerin and fatty acid, weight average molecular weight: 340
[(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル]
・ユニスター H-208BRS,日油株式会社製
ジ2-エチルヘキサン酸ネオペンチルグリコール,重量平均分子量:約360
・コムポールBL,日油株式会社製
ブチレングリコールのドデカン酸(C12)モノエステル,重量平均分子量:約270
・コムポールBS,日油株式会社製
ブチレングリコールのオクタデカン酸(C18)モノエステル,重量平均分子量:約350 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
・ Unistar H-208BRS, manufactured by NOF Corporation di-2-ethylhexanoic acid neopentyl glycol, weight average molecular weight: about 360
-Compol BL, manufactured by NOF Corporation Butylene glycol dodecanoic acid (C 12 ) monoester, weight average molecular weight: about 270
Compole BS, manufactured by NOF Corporation Butylene glycol octadecanoic acid (C 18 ) monoester, weight average molecular weight: about 350
・ユニスター H-208BRS,日油株式会社製
ジ2-エチルヘキサン酸ネオペンチルグリコール,重量平均分子量:約360
・コムポールBL,日油株式会社製
ブチレングリコールのドデカン酸(C12)モノエステル,重量平均分子量:約270
・コムポールBS,日油株式会社製
ブチレングリコールのオクタデカン酸(C18)モノエステル,重量平均分子量:約350 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
・ Unistar H-208BRS, manufactured by NOF Corporation di-2-ethylhexanoic acid neopentyl glycol, weight average molecular weight: about 360
-Compol BL, manufactured by NOF Corporation Butylene glycol dodecanoic acid (C 12 ) monoester, weight average molecular weight: about 270
Compole BS, manufactured by NOF Corporation Butylene glycol octadecanoic acid (C 18 ) monoester, weight average molecular weight: about 350
[(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル]
・O-アセチルクエン酸トリブチル,東京化成工業株式会社製
重量平均分子量:約400
・クエン酸トリブチル,東京化成工業株式会社製
重量平均分子量:約360 [(C 2 ) Ester of linear hydrocarbon tricarboxylic acid having 3 carboxyl groups, hydroxy acid, alkoxy acid or oxo acid and at least one aliphatic monohydric alcohol]
・ O-acetyl citrate tributyl, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 400
Tributyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 360
・O-アセチルクエン酸トリブチル,東京化成工業株式会社製
重量平均分子量:約400
・クエン酸トリブチル,東京化成工業株式会社製
重量平均分子量:約360 [(C 2 ) Ester of linear hydrocarbon tricarboxylic acid having 3 carboxyl groups, hydroxy acid, alkoxy acid or oxo acid and at least one aliphatic monohydric alcohol]
・ O-acetyl citrate tributyl, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 400
Tributyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 360
[(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル]
・アジピン酸ジオクチル,和光純薬工業製
重量平均分子量:約380 [(C 3 ) ester of a chain hydrocarbon dicarboxylic acid having two carboxyl groups, a hydroxy acid, an alkoxy acid or an oxo acid and at least one aliphatic monohydric alcohol]
・ Dioctyl adipate, manufactured by Wako Pure Chemical Industries, Ltd. Weight average molecular weight: about 380
・アジピン酸ジオクチル,和光純薬工業製
重量平均分子量:約380 [(C 3 ) ester of a chain hydrocarbon dicarboxylic acid having two carboxyl groups, a hydroxy acid, an alkoxy acid or an oxo acid and at least one aliphatic monohydric alcohol]
・ Dioctyl adipate, manufactured by Wako Pure Chemical Industries, Ltd. Weight average molecular weight: about 380
[(d3)脂肪酸と脂肪族1価アルコールとのエステル]
・エレクトールWE20,日油株式会社製
ドデカン酸(C12)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約360
・エレクトールWE40,日油株式会社製
テトラデカン酸(C14)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約390 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
Electol WE20, an ester of dodecanoic acid (C 12 ) and dodecyl alcohol (C 12 ) manufactured by NOF Corporation, weight average molecular weight: about 360
Electol WE40, an ester of tetradecanoic acid (C 14 ) and dodecyl alcohol (C 12 ) manufactured by NOF Corporation, weight average molecular weight: about 390
・エレクトールWE20,日油株式会社製
ドデカン酸(C12)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約360
・エレクトールWE40,日油株式会社製
テトラデカン酸(C14)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約390 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
Electol WE20, an ester of dodecanoic acid (C 12 ) and dodecyl alcohol (C 12 ) manufactured by NOF Corporation, weight average molecular weight: about 360
Electol WE40, an ester of tetradecanoic acid (C 14 ) and dodecyl alcohol (C 12 ) manufactured by NOF Corporation, weight average molecular weight: about 390
[(e1)ポリオキシC3~C6アルキレングリコール]
・ユニオールPB500,日油株式会社製
ポリブチレングリコール,重量平均分子量:約500
・ユニオールPB700,日油株式会社製
ポリオキシブチレンポリオキシプロピレングリコール,重量平均分子量:約700 [(E 1 ) polyoxy C 3 -C 6 alkylene glycol]
・ Uniol PB500, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 500
-Uniol PB700, manufactured by NOF Corporation polyoxybutylene polyoxypropylene glycol, weight average molecular weight: about 700
・ユニオールPB500,日油株式会社製
ポリブチレングリコール,重量平均分子量:約500
・ユニオールPB700,日油株式会社製
ポリオキシブチレンポリオキシプロピレングリコール,重量平均分子量:約700 [(E 1 ) polyoxy C 3 -C 6 alkylene glycol]
・ Uniol PB500, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 500
-Uniol PB700, manufactured by NOF Corporation polyoxybutylene polyoxypropylene glycol, weight average molecular weight: about 700
[(f1)鎖状アルカン]
・パールリーム6,日油株式会社製
流動イソパラフィン、イソブテン及びn-ブテンを共重合し、次いで水素を付加することにより生成された分岐鎖炭化水素、重合度:約5~約10,重量平均分子量:約330 [(F 1 ) chain alkane]
-Pearl Ream 6, manufactured by NOF Corporation Branched hydrocarbons produced by copolymerizing liquid isoparaffin, isobutene and n-butene and then adding hydrogen, degree of polymerization: about 5 to about 10, weight average molecular weight : About 330
・パールリーム6,日油株式会社製
流動イソパラフィン、イソブテン及びn-ブテンを共重合し、次いで水素を付加することにより生成された分岐鎖炭化水素、重合度:約5~約10,重量平均分子量:約330 [(F 1 ) chain alkane]
-
[その他の材料]
・NA50,日油株式会社製
NA36に水素を付加し、原料である不飽和脂肪酸に由来する二重結合の比率を下げたグリセリンと脂肪酸とのトリエステル,重量平均分子量:約880
・(カプリル酸/カプリン酸)モノグリセリド,日油株式会社製
オクタン酸(C8)及びデカン酸(C10)がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのモノエステル,重量平均分子量:約220
・Monomuls 90-L2ラウリン酸モノグリセリド,コグニスジャパン株式会社製 [Other materials]
-NA50, manufactured by NOF Corporation Hydrogen added to NA36 to reduce the ratio of double bonds derived from the unsaturated fatty acid as a raw material Triester of glycerin and fatty acid, weight average molecular weight: about 880
A monoester of glycerin and a fatty acid, containing (caprylic acid / capric acid) monoglyceride, NOF Corporation octanoic acid (C 8 ) and decanoic acid (C 10 ) in a weight ratio of approximately 85:15, Weight average molecular weight: about 220
-Monomuls 90-L2 lauric acid monoglyceride, manufactured by Cognis Japan
・NA50,日油株式会社製
NA36に水素を付加し、原料である不飽和脂肪酸に由来する二重結合の比率を下げたグリセリンと脂肪酸とのトリエステル,重量平均分子量:約880
・(カプリル酸/カプリン酸)モノグリセリド,日油株式会社製
オクタン酸(C8)及びデカン酸(C10)がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのモノエステル,重量平均分子量:約220
・Monomuls 90-L2ラウリン酸モノグリセリド,コグニスジャパン株式会社製 [Other materials]
-NA50, manufactured by NOF Corporation Hydrogen added to NA36 to reduce the ratio of double bonds derived from the unsaturated fatty acid as a raw material Triester of glycerin and fatty acid, weight average molecular weight: about 880
A monoester of glycerin and a fatty acid, containing (caprylic acid / capric acid) monoglyceride, NOF Corporation octanoic acid (C 8 ) and decanoic acid (C 10 ) in a weight ratio of approximately 85:15, Weight average molecular weight: about 220
-Monomuls 90-L2 lauric acid monoglyceride, manufactured by Cognis Japan
・クエン酸イソプロピル,東京化成工業株式会社製
重量平均分子量:約230
・リンゴ酸ジイソステアリル
重量平均分子量:約640
・ユニオールPB1000R,日油株式会社製
ポリブチレングリコール,重量平均分子量:約1,000
・ユニオールD-250,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約250 ・ Isopropyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 230
・ Diisostearyl malate Weight average molecular weight: about 640
・ Uniol PB1000R, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,000
・ Uniol D-250, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 250
重量平均分子量:約230
・リンゴ酸ジイソステアリル
重量平均分子量:約640
・ユニオールPB1000R,日油株式会社製
ポリブチレングリコール,重量平均分子量:約1,000
・ユニオールD-250,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約250 ・ Isopropyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 230
・ Diisostearyl malate Weight average molecular weight: about 640
・ Uniol PB1000R, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,000
・ Uniol D-250, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 250
・ユニオールD-400,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約400
・ユニオールD-700,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約700
・ユニオールD-1000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,000
・ユニオールD-1200,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,160 ・ Uniol D-400, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 400
-Uniol D-700, NOF Corporation polypropylene glycol, weight average molecular weight: about 700
・ Uniol D-1000, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 1,000
・ Uniol D-1200, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 1,160
ポリプロピレングリコール,重量平均分子量:約400
・ユニオールD-700,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約700
・ユニオールD-1000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,000
・ユニオールD-1200,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,160 ・ Uniol D-400, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 400
-Uniol D-700, NOF Corporation polypropylene glycol, weight average molecular weight: about 700
・ Uniol D-1000, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 1,000
・ Uniol D-1200, manufactured by NOF Corporation, polypropylene glycol, weight average molecular weight: about 1,160
・ユニオールD-2000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約2,030
・ユニオールD-3000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約3,000
・ユニオールD-4000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約4,000 ・ Uniol D-2000, NOF Corporation Polypropylene glycol, weight average molecular weight: about 2,030
・ Uniol D-3000, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 3,000
・ Uniol D-4000, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 4,000
ポリプロピレングリコール,重量平均分子量:約2,030
・ユニオールD-3000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約3,000
・ユニオールD-4000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約4,000 ・ Uniol D-2000, NOF Corporation Polypropylene glycol, weight average molecular weight: about 2,030
・ Uniol D-3000, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 3,000
・ Uniol D-4000, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 4,000
・PEG1500,日油株式会社製
ポリエチレングリコール,重量平均分子量:約1,500~約1,600
・ウィルブライトcp9,日油株式会社製
ポリブチレングリコールの両末端のOH基が、ヘキサデカン酸(C16)によりエステル化された化合物,重量平均分子量:約1,150
・ユニルーブMS-70K,日油株式会社製
ポリプロピレングリコールのステアリルエーテル,約15の繰返し単位,重量平均分子量:約1,140 PEG 1500, manufactured by NOF Corporation, polyethylene glycol, weight average molecular weight: about 1,500 to about 1,600
-Wilbright cp9, manufactured by NOF Co., Ltd. A compound in which the OH groups at both ends of polybutylene glycol are esterified with hexadecanoic acid (C 16 ), weight average molecular weight: about 1,150
Unilube MS-70K, NOF Corporation Polypropylene glycol stearyl ether, about 15 repeating units, weight average molecular weight: about 1,140
ポリエチレングリコール,重量平均分子量:約1,500~約1,600
・ウィルブライトcp9,日油株式会社製
ポリブチレングリコールの両末端のOH基が、ヘキサデカン酸(C16)によりエステル化された化合物,重量平均分子量:約1,150
・ユニルーブMS-70K,日油株式会社製
ポリプロピレングリコールのステアリルエーテル,約15の繰返し単位,重量平均分子量:約1,140 PEG 1500, manufactured by NOF Corporation, polyethylene glycol, weight average molecular weight: about 1,500 to about 1,600
-Wilbright cp9, manufactured by NOF Co., Ltd. A compound in which the OH groups at both ends of polybutylene glycol are esterified with hexadecanoic acid (C 16 ), weight average molecular weight: about 1,150
Unilube MS-70K, NOF Corporation Polypropylene glycol stearyl ether, about 15 repeating units, weight average molecular weight: about 1,140
・ノニオンS-6,日油株式会社製
ポリオキシエチレンモノステアレート、約7の繰返し単位、重量平均分子量:約880
・ユニルーブ5TP-300KB
ペンタエリトリトール1モルに、エチレンオキシド5モルと、プロピレンオキシド65モルとを付加させることにより生成した、ポリオキシエチレンポリオキシプロピレンペンタエリスリトールエーテル,重量平均分子量:4,130 Nonionic S-6, polyoxyethylene monostearate manufactured by NOF Corporation, about 7 repeating units, weight average molecular weight: about 880
・ Unilube 5TP-300KB
Polyoxyethylene polyoxypropylene pentaerythritol ether produced by adding 5 mol of ethylene oxide and 65 mol of propylene oxide to 1 mol of pentaerythritol, weight average molecular weight: 4,130
ポリオキシエチレンモノステアレート、約7の繰返し単位、重量平均分子量:約880
・ユニルーブ5TP-300KB
ペンタエリトリトール1モルに、エチレンオキシド5モルと、プロピレンオキシド65モルとを付加させることにより生成した、ポリオキシエチレンポリオキシプロピレンペンタエリスリトールエーテル,重量平均分子量:4,130 Nonionic S-6, polyoxyethylene monostearate manufactured by NOF Corporation, about 7 repeating units, weight average molecular weight: about 880
・ Unilube 5TP-300KB
Polyoxyethylene polyoxypropylene pentaerythritol ether produced by adding 5 mol of ethylene oxide and 65 mol of propylene oxide to 1 mol of pentaerythritol, weight average molecular weight: 4,130
・ウィルブライトs753,日油株式会社製
ポリオキシエチレンポリオキシプロピレンポリオキシブチレングリセリン,重量平均分子量:約960
・ユニオール TG-330,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約6の繰返し単位,重量平均分子量:約330 -Wilbright s753, NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
・ Uniol TG-330, manufactured by NOF Corporation Polypropylene glycol glyceryl ether, about 6 repeating units, weight average molecular weight: about 330
ポリオキシエチレンポリオキシプロピレンポリオキシブチレングリセリン,重量平均分子量:約960
・ユニオール TG-330,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約6の繰返し単位,重量平均分子量:約330 -Wilbright s753, NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
・ Uniol TG-330, manufactured by NOF Corporation Polypropylene glycol glyceryl ether, about 6 repeating units, weight average molecular weight: about 330
・ユニオール TG-1000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約1,000
・ユニオール TG-3000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約3,000
・ユニオール TG-4000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約4,000 -Uniol TG-1000, manufactured by NOF Corporation Polyglycol glycol glyceryl ether, about 16 repeating units, weight average molecular weight: about 1,000
・ Uniol TG-3000, manufactured by NOF Corporation Polyglycol glycol glyceryl ether, about 16 repeating units, weight average molecular weight: about 3,000
・ Uniol TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約1,000
・ユニオール TG-3000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約3,000
・ユニオール TG-4000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約4,000 -Uniol TG-1000, manufactured by NOF Corporation Polyglycol glycol glyceryl ether, about 16 repeating units, weight average molecular weight: about 1,000
・ Uniol TG-3000, manufactured by NOF Corporation Polyglycol glycol glyceryl ether, about 16 repeating units, weight average molecular weight: about 3,000
・ Uniol TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
・ユニルーブ DGP-700,日油株式会社製
ポリプロピレングリコールのジグリセリルエーテル,約9の繰返し単位,重量平均分子量:約700
・ユニオックスHC60,日油株式会社製
ポリオキシエチレン硬化ヒマシ油,重量平均分子量:約3,570
・ワセリン,コグニスジャパン株式会社製
石油に由来する炭化水素、半固形 ・ Unilube DGP-700, manufactured by NOF Corporation Polyglycol diglyceryl ether, about 9 repeating units, weight average molecular weight: about 700
・ Uniox HC60, polyoxyethylene hydrogenated castor oil manufactured by NOF Corporation, weight average molecular weight: about 3,570
・ Vaseline, manufactured by Cognis Japan Co., Ltd. Petroleum-derived hydrocarbon, semi-solid
ポリプロピレングリコールのジグリセリルエーテル,約9の繰返し単位,重量平均分子量:約700
・ユニオックスHC60,日油株式会社製
ポリオキシエチレン硬化ヒマシ油,重量平均分子量:約3,570
・ワセリン,コグニスジャパン株式会社製
石油に由来する炭化水素、半固形 ・ Unilube DGP-700, manufactured by NOF Corporation Polyglycol diglyceryl ether, about 9 repeating units, weight average molecular weight: about 700
・ Uniox HC60, polyoxyethylene hydrogenated castor oil manufactured by NOF Corporation, weight average molecular weight: about 3,570
・ Vaseline, manufactured by Cognis Japan Co., Ltd. Petroleum-derived hydrocarbon, semi-solid
<試験例2>
[大量の血液を吸収した際の経血の表面残存率A]
生理用ナプキンが一度に大量の血液を吸収した場合の吸収性を評価する実験を行った。
親水剤で処理されたエアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシートと、エアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:30g/m2)から形成されたセカンドシートと、パルプ(坪量:150~450g/m2、中央部ほど多い)、アクリル系高吸収ポリマー(坪量:15g/m2)及びコアラップとしてのティッシュを含む吸収体と、撥水剤処理されたサイドシートと、ポリエチレンフィルムから成るバックシートとを準備した。 <Test Example 2>
[A surface residual rate A of menstrual blood when a large amount of blood is absorbed]
An experiment was conducted to evaluate the absorbability when a sanitary napkin absorbed a large amount of blood at one time.
Top sheet formed from air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 30 g) / M 2 ), a pulp (basis weight: 150 to 450 g / m 2 , more in the center), an acrylic superabsorbent polymer (basis weight: 15 g / m 2 ), and a tissue as a core wrap. An absorbent body, a side sheet treated with a water repellent, and a back sheet made of a polyethylene film were prepared.
[大量の血液を吸収した際の経血の表面残存率A]
生理用ナプキンが一度に大量の血液を吸収した場合の吸収性を評価する実験を行った。
親水剤で処理されたエアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシートと、エアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:30g/m2)から形成されたセカンドシートと、パルプ(坪量:150~450g/m2、中央部ほど多い)、アクリル系高吸収ポリマー(坪量:15g/m2)及びコアラップとしてのティッシュを含む吸収体と、撥水剤処理されたサイドシートと、ポリエチレンフィルムから成るバックシートとを準備した。 <Test Example 2>
[A surface residual rate A of menstrual blood when a large amount of blood is absorbed]
An experiment was conducted to evaluate the absorbability when a sanitary napkin absorbed a large amount of blood at one time.
Top sheet formed from air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 30 g) / M 2 ), a pulp (basis weight: 150 to 450 g / m 2 , more in the center), an acrylic superabsorbent polymer (basis weight: 15 g / m 2 ), and a tissue as a core wrap. An absorbent body, a side sheet treated with a water repellent, and a back sheet made of a polyethylene film were prepared.
上記トップシートは、特開2008-2034号公報に記載の方法に従って製造された、畝溝構造を有するトップシートであり、畝部の厚みが約1.5mmであり、溝部の厚みが約0.4mmであり、畝溝構造のピッチ(畝部の幅+溝部の幅)が約4mmであり、そして溝部には、開孔率約15%の開孔部が形成されていた。
The top sheet is a top sheet having a ridge groove structure manufactured according to the method described in Japanese Patent Application Laid-Open No. 2008-2034. The ridge portion has a thickness of about 1.5 mm and the groove portion has a thickness of about 0.00 mm. The pitch of the ridge groove structure (width of the ridge portion + width of the groove portion) was about 4 mm, and an opening portion having an opening ratio of about 15% was formed in the groove portion.
血液滑性付与剤として、ユニスター H-408BRS(日油株式会社製、ペンタエリトリトールと脂肪酸とのテトラエステル)を選択し、室温において、コントロールシームHMAガンから、上記トップシートの肌当接面(畝溝面)に、5.0g/m2の坪量で塗工した。電子顕微鏡で確認したところ、H-408BRSは、微粒子状で、繊維の表面に付着していた。
次いで、バックシート、吸収体、セカンドシート、そして畝溝面を上にしてトップシートを順に重ね合わせることにより、生理用ナプキンNo.1-1を形成した。 Unistar H-408BRS (manufactured by NOF Corporation, tetraester of pentaerythritol and fatty acid) is selected as a blood slipperiness-imparting agent, and the skin contact surface (畝 上 記) of the top sheet from a control seam HMA gun at room temperature. The groove surface was coated with a basis weight of 5.0 g / m 2 . When confirmed with an electron microscope, H-408BRS was in the form of fine particles and adhered to the fiber surface.
Next, the back sheet, the absorbent body, the second sheet, and the top sheet were sequentially laminated with the groove surface facing up, whereby a sanitary napkin no. 1-1 was formed.
次いで、バックシート、吸収体、セカンドシート、そして畝溝面を上にしてトップシートを順に重ね合わせることにより、生理用ナプキンNo.1-1を形成した。 Unistar H-408BRS (manufactured by NOF Corporation, tetraester of pentaerythritol and fatty acid) is selected as a blood slipperiness-imparting agent, and the skin contact surface (畝 上 記) of the top sheet from a control seam HMA gun at room temperature. The groove surface was coated with a basis weight of 5.0 g / m 2 . When confirmed with an electron microscope, H-408BRS was in the form of fine particles and adhered to the fiber surface.
Next, the back sheet, the absorbent body, the second sheet, and the top sheet were sequentially laminated with the groove surface facing up, whereby a sanitary napkin no. 1-1 was formed.
血液滑性付与剤を、ユニスター H-408BRSから、表2に示されるものに変更して、生理用ナプキンNo.1-2~No.1-49を製造した。なお、血液滑性付与剤が室温で液体である場合には、そのまま、そして血液滑性付与剤が室温で固体である場合には、融点+20℃まで加熱し、次いで、コントロールシームHMAガンを用いて、血液滑性付与剤を微粒化し、トップシートの肌当接面に、坪量がおおよそ5g/m2となるように塗工した。
また、血液滑性付与剤は、トップシートの肌当接面のほぼ全面に、そして畝部及び溝部の両方に塗工された。 The blood slipperiness-imparting agent was changed from Unistar H-408BRS to that shown in Table 2, and sanitary napkin no. 1-2 to No. 1-49 was produced. When the blood slipperiness-imparting agent is a liquid at room temperature, and when the blood slipperiness-imparting agent is a solid at room temperature, it is heated to the melting point + 20 ° C., and then a control seam HMA gun is used. Then, the blood slipperiness imparting agent was atomized and applied to the skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
Moreover, the blood slipperiness | lubricity imparting agent was applied to the substantially whole surface of the skin contact surface of a top sheet, and to both a collar part and a groove part.
また、血液滑性付与剤は、トップシートの肌当接面のほぼ全面に、そして畝部及び溝部の両方に塗工された。 The blood slipperiness-imparting agent was changed from Unistar H-408BRS to that shown in Table 2, and sanitary napkin no. 1-2 to No. 1-49 was produced. When the blood slipperiness-imparting agent is a liquid at room temperature, and when the blood slipperiness-imparting agent is a solid at room temperature, it is heated to the melting point + 20 ° C., and then a control seam HMA gun is used. Then, the blood slipperiness imparting agent was atomized and applied to the skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
Moreover, the blood slipperiness | lubricity imparting agent was applied to the substantially whole surface of the skin contact surface of a top sheet, and to both a collar part and a groove part.
[試験方法]
トップシートの質量:W2(g)(試験前のトップシートの質量)を測定した後、吸収性物品の長手方向及び幅方向の中央部且つトップシートの上に、穴の開いたアクリル板(200mm×100mm,125g,中央に、40mm×10mmの穴が開いている)を置き、上記穴から、37±1℃のウマEDTA血(ウマの血液に、凝結防止のため、エチレンジアミン四酢酸(以下、「EDTA」と称する)が添加されたもの)4.0gを、ピペットを用いて滴下した。 [Test method]
Mass of top sheet: After measuring W 2 (g) (mass of top sheet before test), an acrylic plate having a hole in the center of the absorbent article in the longitudinal direction and width direction and on the top sheet ( Place 200mm x 100mm, 125g, 40mm x 10mm hole in the center, and from the hole, 37 ± 1 ° C equine EDTA blood (ethylenediaminetetraacetic acid (hereinafter referred to as equine blood to prevent coagulation) 4.0 g) was added dropwise with a pipette.
トップシートの質量:W2(g)(試験前のトップシートの質量)を測定した後、吸収性物品の長手方向及び幅方向の中央部且つトップシートの上に、穴の開いたアクリル板(200mm×100mm,125g,中央に、40mm×10mmの穴が開いている)を置き、上記穴から、37±1℃のウマEDTA血(ウマの血液に、凝結防止のため、エチレンジアミン四酢酸(以下、「EDTA」と称する)が添加されたもの)4.0gを、ピペットを用いて滴下した。 [Test method]
Mass of top sheet: After measuring W 2 (g) (mass of top sheet before test), an acrylic plate having a hole in the center of the absorbent article in the longitudinal direction and width direction and on the top sheet ( Place 200mm x 100mm, 125g, 40mm x 10mm hole in the center, and from the hole, 37 ± 1 ° C equine EDTA blood (ethylenediaminetetraacetic acid (hereinafter referred to as equine blood to prevent coagulation) 4.0 g) was added dropwise with a pipette.
ウマEDTA血の滴下後、直ちに上記アクリル板を外し、トップシートを取出し、その質量:W3(g)(試験後のトップシートの質量)を測定し、以下の式に従って、「表面残存率A(質量%)」を算出した。
表面残存率A(質量%)
=100×[W3(g)-W2(g)]/4.0(g) Immediately after dropping the equine EDTA blood, the above acrylic plate is removed, the top sheet is taken out, and its mass: W 3 (g) (the mass of the top sheet after the test) is measured. (Mass%) "was calculated.
Surface residual rate A (mass%)
= 100 × [W 3 (g) −W 2 (g)] / 4.0 (g)
表面残存率A(質量%)
=100×[W3(g)-W2(g)]/4.0(g) Immediately after dropping the equine EDTA blood, the above acrylic plate is removed, the top sheet is taken out, and its mass: W 3 (g) (the mass of the top sheet after the test) is measured. (Mass%) "was calculated.
Surface residual rate A (mass%)
= 100 × [W 3 (g) −W 2 (g)] / 4.0 (g)
また、トップシートの肌当接面のタック性を、以下の基準に従って35℃で測定した。
○:タック性なし
△:若干のタック性有り
×:タック性有り Further, the tackiness of the skin contact surface of the top sheet was measured at 35 ° C. according to the following criteria.
○: No tackiness △: Some tackiness is present ×: There is tackiness
○:タック性なし
△:若干のタック性有り
×:タック性有り Further, the tackiness of the skin contact surface of the top sheet was measured at 35 ° C. according to the following criteria.
○: No tackiness △: Some tackiness is present ×: There is tackiness
各吸収性物品の表面残存率A、及びタック性、並びに各血液滑性付与剤の特性を、以下の表2に示す。また、図7に、トップシートがトリC2L油脂肪酸グリセリドを含む生理用ナプキンにおける、トップシートの肌当接面の電子顕微鏡写真を示す。
Table 2 below shows the surface residual ratio A, tackiness, and characteristics of each blood slipperiness imparting agent of each absorbent article. FIG. 7 shows an electron micrograph of the skin contact surface of the top sheet in a sanitary napkin where the top sheet contains tri-C2L oil fatty acid glycerides.
血液滑性付与剤を有しない生理用ナプキンNo.1-49では、表面残存率Aが7.5質量%であったが、動粘度及び抱水率が所定の範囲内にある生理用ナプキンNo.1-1~No.1-21では、表面残存率Aが2.5質量%以下であった。
Sanitary napkin No. which does not have a blood slipperiness imparting agent. In No. 1-49, the surface residual rate A was 7.5% by mass, but the kinematic viscosity and water retention rate were within the predetermined ranges. 1-1-No. In 1-21, the surface residual ratio A was 2.5 mass% or less.
生理用ナプキンNo.1-1~No.1-21では、トップシートの畝部に滴下されたウマEDTA血が、畝部から溝部へと滑落し、溝部から吸収体内部に迅速に吸収される様子が観察された。一方、血液滑性付与剤を有しない生理用ナプキンNo.1-49では、滴下したウマEDTA血は、溝部に滑落するのではなく、溝部にゆっくりと垂れ落ち、その多くがトップシートの畝部に残存した。また、抱水率が高い吸収性物品、例えば、No.1-30では、トップシートの畝部に滴下されたウマEDTA血は、溝部に滑落するのではなく、トップシートに一部残存しながらゆっくりと垂れ落ち、そして一部が畝部に残存した。
Sanitary napkin No. 1-1-No. In 1-21, it was observed that equine EDTA blood dripped onto the buttocks of the top sheet slid down from the buttocks into the groove and was quickly absorbed into the absorber from the groove. On the other hand, in the sanitary napkin No. 1-49 having no blood slipperiness-imparting agent, the dropped equine EDTA blood does not slide down into the groove part, but slowly drops down into the groove part, and most of it is the ridge part of the top sheet. Remained. Further, in an absorbent article having a high water retention rate, for example, No. 1-30, the equine EDTA blood dripped onto the buttocks of the top sheet does not slide down into the groove part, but partially remains in the top sheet. Slowly dropped and some remained in the buttocks.
以上より、生理用ナプキンNo.1-1~No.1-21は、一度に大量の経血がトップシートに到達した際に、経血をトップシートから吸収体に迅速に移行させることができることが示唆される。
From the above, sanitary napkins no. 1-1-No. 1-21 suggests that when a large amount of menstrual blood reaches the top sheet at a time, menstrual blood can be rapidly transferred from the top sheet to the absorber.
次に、No.1-1~1-49の生理用ナプキンを、複数のボランティアの被験者に着用してもらったところ、No.1-1~1-21の血液滑性付与剤を含む生理用ナプキンでは、経血を吸収した後であってもトップシートにべたつき感がなく、トップシートがサラサラしているとの回答が多かった。
Next, No. When the sanitary napkins 1-1 to 1-49 were worn by a plurality of volunteer subjects, In the sanitary napkin containing the blood slipping agent of 1-1 to 1-21, the top sheet is not sticky even after menstrual blood is absorbed, and many respondents say that the top sheet is smooth. It was.
<試験例3>
[少量の血液を吸収した際の経血の表面残存率B]
生理用ナプキンが少量の血液を吸収した場合の吸収性を評価する実験を行った。
親水剤で処理されたエアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシート(以下、「畝溝を有するトップシート」と称する場合がある)と、エアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:30g/m2)から形成されたセカンドシートと、パルプ(坪量:150~450g/m2、中央部ほど多い)、アクリル系高吸収ポリマー(坪量:15g/m2)及びコアラップとしてのティッシュを含む吸収体と、撥水剤処理されたサイドシートと、ポリエチレンフィルムから成るバックシートとを準備した。 <Test Example 3>
[Surface remaining rate B of menstrual blood when a small amount of blood is absorbed]
An experiment was conducted to evaluate the absorbability when a sanitary napkin absorbed a small amount of blood.
An air-through nonwoven fabric treated with a hydrophilic agent (a composite fiber composed of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) and a top sheet (hereinafter sometimes referred to as “top sheet having a ridge”) , Second sheet formed from air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the center), acrylic high An absorbent body including an absorbent polymer (basis weight: 15 g / m 2 ) and a tissue as a core wrap, a water repellent-treated side sheet, and a back sheet made of a polyethylene film were prepared.
[少量の血液を吸収した際の経血の表面残存率B]
生理用ナプキンが少量の血液を吸収した場合の吸収性を評価する実験を行った。
親水剤で処理されたエアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシート(以下、「畝溝を有するトップシート」と称する場合がある)と、エアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:30g/m2)から形成されたセカンドシートと、パルプ(坪量:150~450g/m2、中央部ほど多い)、アクリル系高吸収ポリマー(坪量:15g/m2)及びコアラップとしてのティッシュを含む吸収体と、撥水剤処理されたサイドシートと、ポリエチレンフィルムから成るバックシートとを準備した。 <Test Example 3>
[Surface remaining rate B of menstrual blood when a small amount of blood is absorbed]
An experiment was conducted to evaluate the absorbability when a sanitary napkin absorbed a small amount of blood.
An air-through nonwoven fabric treated with a hydrophilic agent (a composite fiber composed of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) and a top sheet (hereinafter sometimes referred to as “top sheet having a ridge”) , Second sheet formed from air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the center), acrylic high An absorbent body including an absorbent polymer (basis weight: 15 g / m 2 ) and a tissue as a core wrap, a water repellent-treated side sheet, and a back sheet made of a polyethylene film were prepared.
上記トップシートは、特開2008-2034号公報に記載の方法に従って製造された、畝溝構造を有するトップシートであり、畝部の厚みが約1.5mmであり、溝部の厚みが約0.4mmであり、畝溝構造のピッチ(畝部の幅+溝部の幅)が約4mmであり、そして溝部には、開孔率約15%の開孔部が形成されていた。
The top sheet is a top sheet having a ridge groove structure manufactured according to the method described in Japanese Patent Application Laid-Open No. 2008-2034. The ridge portion has a thickness of about 1.5 mm and the groove portion has a thickness of about 0.00 mm. The pitch of the ridge groove structure (width of the ridge portion + width of the groove portion) was about 4 mm, and an opening portion having an opening ratio of about 15% was formed in the groove portion.
血液滑性付与剤として、ユニスター H-408BRS(日油株式会社製、ペンタエリトリトールと脂肪酸とのテトラエステル)を選択し、室温において、コントロールシームHMAガンから、上記トップシートの肌当接面(畝溝面)に、5.0g/m2の坪量で塗工した。電子顕微鏡で確認したところ、H-408BRSは、微粒子状で、繊維の表面に付着していた。
次いで、バックシート、吸収体、セカンドシート、そして畝溝面を上にしてトップシートを順に重ね合わせることにより、生理用ナプキンNo.2-1(i)を形成した。 Unistar H-408BRS (manufactured by NOF Corporation, tetraester of pentaerythritol and fatty acid) is selected as a blood slipperiness-imparting agent, and the skin contact surface (畝 上 記) of the top sheet from a control seam HMA gun at room temperature. The groove surface was coated with a basis weight of 5.0 g / m 2 . When confirmed with an electron microscope, H-408BRS was in the form of fine particles and adhered to the fiber surface.
Next, the back sheet, the absorbent body, the second sheet, and the top sheet were sequentially laminated with the groove surface facing up, whereby a sanitary napkin no. 2-1 (i) was formed.
次いで、バックシート、吸収体、セカンドシート、そして畝溝面を上にしてトップシートを順に重ね合わせることにより、生理用ナプキンNo.2-1(i)を形成した。 Unistar H-408BRS (manufactured by NOF Corporation, tetraester of pentaerythritol and fatty acid) is selected as a blood slipperiness-imparting agent, and the skin contact surface (畝 上 記) of the top sheet from a control seam HMA gun at room temperature. The groove surface was coated with a basis weight of 5.0 g / m 2 . When confirmed with an electron microscope, H-408BRS was in the form of fine particles and adhered to the fiber surface.
Next, the back sheet, the absorbent body, the second sheet, and the top sheet were sequentially laminated with the groove surface facing up, whereby a sanitary napkin no. 2-1 (i) was formed.
トップシートを、畝溝構造を有しないフラットな、親水剤で処理されたエアスルー不織布(ポリエステル及びポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシート(以下、「フラットなトップシート」と称する場合がある)に変更した以外は、生理用ナプキンNo.2-1(i)と同様にして、生理用ナプキンNo.2-1(ii)を形成した。
A top sheet made of a flat, air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) that has no groove structure and is treated with a hydrophilic agent (hereinafter referred to as “flat”) The sanitary napkin No. 4 was changed to “No. In the same manner as in 2-1 (i), the sanitary napkin no. 2-1 (ii) was formed.
血液滑性付与剤を、ユニスター H-408BRSから、表3に示されるものに変更して、生理用ナプキンNo.2-2(i)~No.2-11(i)及びNo.2-2(ii)~No.2-11(ii)を製造した。なお、血液滑性付与剤が室温で液体である場合には、そのまま、そして血液滑性付与剤が室温で固体である場合には、融点+20℃まで加熱し、次いで、コントロールシームHMAガンを用いて、血液滑性付与剤を微粒化し、トップシートの肌当接面に、坪量がおおよそ5g/m2となるように塗工した。
また、血液滑性付与剤は、トップシートの肌当接面のほぼ全面に、そして畝溝構造を有するトップシートでは、畝部及び溝部の両方に塗工された。 The blood slipperiness-imparting agent was changed from Unistar H-408BRS to that shown in Table 3, and sanitary napkin no. 2-2 (i) -No. 2-11 (i) and No. 2-11. 2-2 (ii) -No. 2-11 (ii) was prepared. When the blood slipperiness-imparting agent is a liquid at room temperature, and when the blood slipperiness-imparting agent is a solid at room temperature, it is heated to the melting point + 20 ° C., and then a control seam HMA gun is used. Then, the blood slipperiness imparting agent was atomized and applied to the skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
Moreover, the blood slipperiness | lubricity imparting agent was apply | coated to both the ridge part and a groove part in the substantially whole surface of the skin contact surface of a top sheet, and the top sheet which has a ridge groove structure.
また、血液滑性付与剤は、トップシートの肌当接面のほぼ全面に、そして畝溝構造を有するトップシートでは、畝部及び溝部の両方に塗工された。 The blood slipperiness-imparting agent was changed from Unistar H-408BRS to that shown in Table 3, and sanitary napkin no. 2-2 (i) -No. 2-11 (i) and No. 2-11. 2-2 (ii) -No. 2-11 (ii) was prepared. When the blood slipperiness-imparting agent is a liquid at room temperature, and when the blood slipperiness-imparting agent is a solid at room temperature, it is heated to the melting point + 20 ° C., and then a control seam HMA gun is used. Then, the blood slipperiness imparting agent was atomized and applied to the skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
Moreover, the blood slipperiness | lubricity imparting agent was apply | coated to both the ridge part and a groove part in the substantially whole surface of the skin contact surface of a top sheet, and the top sheet which has a ridge groove structure.
[試験方法]
トップシートの質量:W4(g)(試験前のトップシートの質量)を測定した後、吸収性物品の長手方向及び幅方向の中央のトップシートの上に、37±1℃のウマEDTA血約0.25g(2滴)をピペットから滴下した。なお、畝溝を有するトップシートでは、畝部の頂部にウマEDTA血を滴下した。 [Test method]
Weight of top sheet: W 4 (g) (mass of top sheet before test) was measured, and then the horse EDTA blood at 37 ± 1 ° C. was placed on the center top sheet in the longitudinal direction and width direction of the absorbent article. About 0.25 g (2 drops) was dropped from the pipette. In the top sheet having the groin, equine EDTA blood was dropped on the top of the buttock.
トップシートの質量:W4(g)(試験前のトップシートの質量)を測定した後、吸収性物品の長手方向及び幅方向の中央のトップシートの上に、37±1℃のウマEDTA血約0.25g(2滴)をピペットから滴下した。なお、畝溝を有するトップシートでは、畝部の頂部にウマEDTA血を滴下した。 [Test method]
Weight of top sheet: W 4 (g) (mass of top sheet before test) was measured, and then the horse EDTA blood at 37 ± 1 ° C. was placed on the center top sheet in the longitudinal direction and width direction of the absorbent article. About 0.25 g (2 drops) was dropped from the pipette. In the top sheet having the groin, equine EDTA blood was dropped on the top of the buttock.
滴下から30秒後、トップシートを取出し、その質量:W5(g)(試験後のトップシートの質量)を測定し、以下の式に従って、「表面残存率B(質量%)」を算出した。
表面残存率B(質量%)
=100×(W5(g)-W4(g))/W6(g)
なお、W6(g)は、滴下前後のピペットの質量から算出した、滴下されたウマEDTA血の質量である。
結果を、下記表3に示す。 30 seconds after dropping, the top sheet was taken out, its mass: W 5 (g) (the mass of the top sheet after the test) was measured, and “surface residual ratio B (mass%)” was calculated according to the following formula. .
Surface residual rate B (mass%)
= 100 × (W 5 (g) −W 4 (g)) / W 6 (g)
W 6 (g) is the mass of the equine EDTA blood that was dripped, calculated from the mass of the pipette before and after the dripping.
The results are shown in Table 3 below.
表面残存率B(質量%)
=100×(W5(g)-W4(g))/W6(g)
なお、W6(g)は、滴下前後のピペットの質量から算出した、滴下されたウマEDTA血の質量である。
結果を、下記表3に示す。 30 seconds after dropping, the top sheet was taken out, its mass: W 5 (g) (the mass of the top sheet after the test) was measured, and “surface residual ratio B (mass%)” was calculated according to the following formula. .
Surface residual rate B (mass%)
= 100 × (W 5 (g) −W 4 (g)) / W 6 (g)
W 6 (g) is the mass of the equine EDTA blood that was dripped, calculated from the mass of the pipette before and after the dripping.
The results are shown in Table 3 below.
表3から、血液滑性付与剤がH-408BRS、パナセート810S、カプリン酸ジグリセリド、コムポールBL、O-アセチルクエン酸トリブチル、アジピン酸ジオクチル、エレクトールWE40、ユニオールPB500、及びパールリーム6である場合には、畝溝を有するトップシートにおいて、表面残存率Bが低いことがわかる。これは、所定の特性を有する血液滑性付与剤が、少量の血液を畝部から、溝部、及び吸収体に迅速に移行させたことを示唆していると思われる。
From Table 3, when the blood slipperiness-imparting agent is H-408BRS, Panacet 810S, Capric acid diglyceride, Compol BL, O-acetyl tributyl citrate, Dioctyl adipate, Electol WE40, Uniol PB500, and Pearl Ream 6 Shows that the surface residual ratio B is low in the top sheet having the ridges. This seems to suggest that the blood slipperiness-imparting agent having the predetermined characteristics quickly transferred a small amount of blood from the groin to the groove and the absorber.
<試験例4>
[血液滑性付与剤を含む血液の粘性]
血液滑性付与剤を含む血液の粘性を、Rheometric Expansion System ARES(Rheometric Scientific,Inc)を用いて測定した。ウマ脱繊維血に、パナセート810sを2質量%添加し、軽く撹拌して試料を形成し、直径50mmのパラレルプレートに試料を載せ、ギャップを100μmとし、37±0.5℃で粘度を測定した。パラレルプレートゆえ、試料に均一なせん断速度はかかっていないが、機器に表示された平均せん断速度は、10s-1であった。 <Test Example 4>
[Viscosity of blood containing blood slipping agent]
The viscosity of blood containing a blood slipping agent was measured using Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by mass of panacet 810s was added to equine defibrinated blood, lightly stirred to form a sample, the sample was placed on a parallel plate with a diameter of 50 mm, the gap was 100 μm, and the viscosity was measured at 37 ± 0.5 ° C. . Because of the parallel plate, the sample did not have a uniform shear rate, but the average shear rate displayed on the instrument was 10 s −1 .
[血液滑性付与剤を含む血液の粘性]
血液滑性付与剤を含む血液の粘性を、Rheometric Expansion System ARES(Rheometric Scientific,Inc)を用いて測定した。ウマ脱繊維血に、パナセート810sを2質量%添加し、軽く撹拌して試料を形成し、直径50mmのパラレルプレートに試料を載せ、ギャップを100μmとし、37±0.5℃で粘度を測定した。パラレルプレートゆえ、試料に均一なせん断速度はかかっていないが、機器に表示された平均せん断速度は、10s-1であった。 <Test Example 4>
[Viscosity of blood containing blood slipping agent]
The viscosity of blood containing a blood slipping agent was measured using Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by mass of panacet 810s was added to equine defibrinated blood, lightly stirred to form a sample, the sample was placed on a parallel plate with a diameter of 50 mm, the gap was 100 μm, and the viscosity was measured at 37 ± 0.5 ° C. . Because of the parallel plate, the sample did not have a uniform shear rate, but the average shear rate displayed on the instrument was 10 s −1 .
パナセート810sを2質量%含むウマ脱繊維血の粘度は、5.9mPa・sであり、一方、血液滑性付与剤を含まないウマ脱繊維血の粘度は、50.4mPa・sであった。従って、パナセート810sを2質量%含むウマ脱繊維血は、血液滑性付与剤を含まない場合と比較して、約90%粘度を下げることが分かる。
The viscosity of equine defibrinated blood containing 2% by mass of panacet 810s was 5.9 mPa · s, while the viscosity of equine defibrinated blood not containing a blood slipperiness agent was 50.4 mPa · s. Therefore, it can be seen that equine defibrinated blood containing 2% by mass of panacet 810s decreases the viscosity by about 90% compared to the case where no blood slipperiness-imparting agent is contained.
血液は、血球等の成分を含み、チキソトロピーの性質を有することが知られているが、本開示の血液滑性付与剤は、低粘度域で、経血等の血液の粘度を下げる作用をも有すると考えられる。血液の粘度を下げることにより、吸収した経血を、トップシートから吸収体に速やかに移行しやすくなると考えられる。
It is known that blood contains components such as blood cells and has thixotropic properties, but the blood slipperiness imparting agent of the present disclosure has an action of lowering the viscosity of blood such as menstrual blood in a low viscosity region. It is thought to have. By reducing the viscosity of blood, it is considered that absorbed menstrual blood is easily transferred from the top sheet to the absorber.
<試験例5>
[血液滑性付与剤を含む血液の顕微鏡写真]
健常ボランティアの経血を、食品保護用ラップフィルム上に採取し、その一部に、10倍の質量のリン酸緩衝生理食塩水中に分散されたパナセート810sを、パナセート810sの濃度が1質量%となるように添加した。経血を、スライドグラスに適下し、カバーグラスをかけ、光学顕微鏡にて、赤血球の状態を観察した。血液滑性付与剤を含まない経血の顕微鏡写真を図8(a)に、そしてパナセート810sを含む経血の顕微鏡写真を図8(b)に示す。 <Test Example 5>
[Micrograph of blood containing blood slipping agent]
A healthy volunteer's menstrual blood was collected on a food protective wrap film, and a portion of the panacet 810s dispersed in 10 times the mass of phosphate buffered saline was used, and the concentration of panacet 810s was 1% by mass. It added so that it might become. Menstrual blood was appropriately applied to a slide glass, covered with a cover glass, and the state of red blood cells was observed with an optical microscope. A photomicrograph of menstrual blood containing no blood slipping agent is shown in FIG. 8 (a), and a photomicrograph of menstrual blood containing panacet 810s is shown in FIG. 8 (b).
[血液滑性付与剤を含む血液の顕微鏡写真]
健常ボランティアの経血を、食品保護用ラップフィルム上に採取し、その一部に、10倍の質量のリン酸緩衝生理食塩水中に分散されたパナセート810sを、パナセート810sの濃度が1質量%となるように添加した。経血を、スライドグラスに適下し、カバーグラスをかけ、光学顕微鏡にて、赤血球の状態を観察した。血液滑性付与剤を含まない経血の顕微鏡写真を図8(a)に、そしてパナセート810sを含む経血の顕微鏡写真を図8(b)に示す。 <Test Example 5>
[Micrograph of blood containing blood slipping agent]
A healthy volunteer's menstrual blood was collected on a food protective wrap film, and a portion of the panacet 810s dispersed in 10 times the mass of phosphate buffered saline was used, and the concentration of panacet 810s was 1% by mass. It added so that it might become. Menstrual blood was appropriately applied to a slide glass, covered with a cover glass, and the state of red blood cells was observed with an optical microscope. A photomicrograph of menstrual blood containing no blood slipping agent is shown in FIG. 8 (a), and a photomicrograph of menstrual blood containing panacet 810s is shown in FIG. 8 (b).
図8から、血液滑性付与剤を含まない経血では、赤血球が連銭等の集合塊を形成しているが、パナセート810sを含む経血では、赤血球が、それぞれ、安定に分散していることが分かる。従って、血液滑性付与剤は、血液の中で、赤血球を安定化させる働きをも有することが示唆される。
From FIG. 8, in menstrual blood that does not contain a blood slipperiness-imparting agent, red blood cells form aggregates such as remuneration, but in menstrual blood that includes panacet 810s, red blood cells are each stably dispersed. I understand that. Therefore, it is suggested that the blood slipperiness imparting agent also has a function of stabilizing red blood cells in blood.
<試験例6>
[血液滑性付与剤を含む血液の表面張力]
血液滑性付与剤を含む血液の表面張力を、協和界面科学社製接触角計 Drop Master500を用い、ペンダントドロップ法にて測定した。表面張力は、ヒツジ脱繊維血に、所定の量の血液滑性付与剤を添加し、十分振とうした後に測定した。
測定は、機器が自動で行うが、表面張力γは、以下の式により求められる(図9を参照)。 <Test Example 6>
[Surface tension of blood containing blood slipperiness agent]
The surface tension of blood containing a blood slipperiness imparting agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd. The surface tension was measured after adding a predetermined amount of blood slipperiness-imparting agent to sheep defibrinated blood and shaking sufficiently.
Although the measurement is automatically performed by the instrument, the surface tension γ is obtained by the following equation (see FIG. 9).
[血液滑性付与剤を含む血液の表面張力]
血液滑性付与剤を含む血液の表面張力を、協和界面科学社製接触角計 Drop Master500を用い、ペンダントドロップ法にて測定した。表面張力は、ヒツジ脱繊維血に、所定の量の血液滑性付与剤を添加し、十分振とうした後に測定した。
測定は、機器が自動で行うが、表面張力γは、以下の式により求められる(図9を参照)。 <Test Example 6>
[Surface tension of blood containing blood slipperiness agent]
The surface tension of blood containing a blood slipperiness imparting agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd. The surface tension was measured after adding a predetermined amount of blood slipperiness-imparting agent to sheep defibrinated blood and shaking sufficiently.
Although the measurement is automatically performed by the instrument, the surface tension γ is obtained by the following equation (see FIG. 9).
γ=g×ρ×(de)2×1/H
g:重力定数
1/H:ds/deから求められる補正項
ρ:密度
de:最大直径
ds:滴下端よりdeだけ上がった位置での径 γ = g × ρ × (de) 2 × 1 / H
g: Gravity constant 1 / H: Correction term obtained from ds / de ρ: Density de: Maximum diameter ds: Diameter at a position that is de higher than the dropping end
g:重力定数
1/H:ds/deから求められる補正項
ρ:密度
de:最大直径
ds:滴下端よりdeだけ上がった位置での径 γ = g × ρ × (de) 2 × 1 / H
g: Gravity constant 1 / H: Correction term obtained from ds / de ρ: Density de: Maximum diameter ds: Diameter at a position that is de higher than the dropping end
密度ρは、JIS K 2249-1995の「密度試験方法及び密度・質量・容量換算表」の5.振動式密度試験方法に準拠し、下記表4に示される温度で測定した。
測定には、京都電子工業株式会社のDA-505を用いた。
結果を、下記表4に示す。 The density ρ is defined in 5. of “Density Test Method and Density / Mass / Capacity Conversion Table” of JIS K 2249-1995. Based on the vibration density test method, the temperature was measured as shown in Table 4 below.
For measurement, DA-505 of Kyoto Electronics Industry Co., Ltd. was used.
The results are shown in Table 4 below.
測定には、京都電子工業株式会社のDA-505を用いた。
結果を、下記表4に示す。 The density ρ is defined in 5. of “Density Test Method and Density / Mass / Capacity Conversion Table” of JIS K 2249-1995. Based on the vibration density test method, the temperature was measured as shown in Table 4 below.
For measurement, DA-505 of Kyoto Electronics Industry Co., Ltd. was used.
The results are shown in Table 4 below.
表4から、血液滑性付与剤は、血液の表面張力を下げる作用をも有することが分かる。
血液の表面張力を下げることにより、吸収した血液をトップシートの繊維間に保持せず、速やかに吸収体に移行させることができると考えられる。 From Table 4, it can be seen that the blood slipperiness-imparting agent also has an action of lowering the surface tension of blood.
By reducing the surface tension of the blood, it is considered that the absorbed blood can be quickly transferred to the absorbent body without being held between the fibers of the top sheet.
血液の表面張力を下げることにより、吸収した血液をトップシートの繊維間に保持せず、速やかに吸収体に移行させることができると考えられる。 From Table 4, it can be seen that the blood slipperiness-imparting agent also has an action of lowering the surface tension of blood.
By reducing the surface tension of the blood, it is considered that the absorbed blood can be quickly transferred to the absorbent body without being held between the fibers of the top sheet.
1 生理用ナプキン(吸収性物品)
2 トップシート
3 バックシート
4 吸収体
8 凸部
9 凹部 1 Sanitary napkin (absorbent article)
2Top sheet 3 Back sheet 4 Absorber 8 Convex part 9 Concave part
2 トップシート
3 バックシート
4 吸収体
8 凸部
9 凹部 1 Sanitary napkin (absorbent article)
2
Claims (14)
- 肌当接面を有する液透過性のトップシートと、非肌当接面を有する液不透過性のバックシートと、前記トップシート及び前記バックシートの間に設けられた吸収体とを備えた吸収性物品であって、
前記トップシートは、前記肌当接面のうち少なくとも排泄口当接領域に、第1の方向及び前記第1の方向と交差する第2の方向に交互に配置された、前記肌当接面側に隆起する複数の凸部と、前記吸収体側に陥没する複数の凹部とを有し、
前記複数の凸部は、それぞれ、頂部と、前記頂部から延在する凸部側壁部とを有し、
前記複数の凹部は、それぞれ、底部と、前記底部から延在する凹部側壁部とを有し、
前記凸部側壁部は、その延在方向に沿った繊維配向性を有し、
前記凹部側壁部は、その延在方向に沿った繊維配向性を有し、
前記排泄口当接領域のうち少なくとも前記凸部には、40℃における動粘度が0.01~80mm2/s、抱水率が0.01~4.0質量%、重量平均分子量が1,000未満である血液滑性付与剤が塗工されている、前記吸収性物品。 Absorption comprising a liquid-permeable top sheet having a skin contact surface, a liquid-impermeable back sheet having a non-skin contact surface, and an absorber provided between the top sheet and the back sheet A sex product,
The skin contact surface side where the top sheet is alternately arranged in the first direction and the second direction intersecting the first direction at least in the excretory opening contact region of the skin contact surface. A plurality of protrusions protruding to the absorber, and a plurality of recesses recessed to the absorber side,
Each of the plurality of convex portions has a top portion and a convex portion side wall portion extending from the top portion,
Each of the plurality of recesses has a bottom part and a recess side wall part extending from the bottom part,
The convex side wall portion has a fiber orientation along the extending direction,
The concave side wall has fiber orientation along the extending direction,
At least the convex portion of the excretory opening contact region has a kinematic viscosity at 40 ° C. of 0.01 to 80 mm 2 / s, a water retention of 0.01 to 4.0% by mass, a weight average molecular weight of 1, The said absorbent article with which the blood slipperiness | lubricity imparting agent which is less than 000 is coated. - 前記凸部の頂部の繊維密度が、前記凹部の底部の繊維密度よりも低い、請求項1に記載の吸収性物品。 The absorbent article according to claim 1, wherein the fiber density at the top of the convex part is lower than the fiber density at the bottom of the concave part.
- 前記凸部の頂部の繊維密度が、前記凹部の底部の繊維密度よりも高い、請求項1に記載の吸収性物品。 The absorbent article according to claim 1, wherein the fiber density at the top of the convex part is higher than the fiber density at the bottom of the concave part.
- 前記凸部側壁部及び/又は前記凹部側壁部が、繊維配向性の異なる部分を有する、請求項1~3のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 3, wherein the convex side wall portion and / or the concave side wall portion has portions having different fiber orientations.
- 前記凹部の底部における厚さ方向の繊維配向性が、前記凹部側壁部における厚さ方向の繊維配向性よりも小さい、請求項1~4のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 4, wherein the fiber orientation in the thickness direction at the bottom of the recess is smaller than the fiber orientation in the thickness direction at the sidewall of the recess.
- 前記凸部の頂部における繊維融着点数が、前記凹部の底部における繊維融着点数よりも小さい、請求項1~5のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 5, wherein the number of fiber fusion points at the top of the convex portion is smaller than the number of fiber fusion points at the bottom of the concave portion.
- 前記トップシートの構成繊維が熱融着している、請求項1~6のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 6, wherein the constituent fibers of the top sheet are heat-sealed.
- 前記トップシートが、熱伸長した熱伸長性繊維を含有する、請求項1~7のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 7, wherein the top sheet contains thermally stretchable fibers.
- 前記血液滑性付与剤のIOBが、0.00~0.60のIOBである、請求項1~8のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 8, wherein the blood lubricity-imparting agent has an IOB of 0.00 to 0.60.
- 前記血液滑性付与剤が、次の(i)~(iii):
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、及び
(iii) (iii-1)炭化水素部分と、(iii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)及びオキシ基(-O-)から成る群から選択される、一又は複数の、同一又は異なる基と、(iii-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択され、
ここで、(ii)又は(iii)の化合物において、オキシ基が2つ以上挿入されている場合には、各オキシ基は隣接していない、
請求項1~9のいずれか1項に記載の吸収性物品。 The blood slipperiness-imparting agent comprises the following (i) to (iii):
(I) hydrocarbons,
(Ii) from (ii-1) a hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety. A compound having one or more identical or different groups selected from the group consisting of: (iii) (iii-1) a hydrocarbon moiety and (iii-2) a CC single bond of said hydrocarbon moiety One or more identical or different groups selected from the group consisting of a carbonyl group (—CO—) and an oxy group (—O—), and (iii-3) the hydrocarbon moiety A compound having one or a plurality of the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which replaces a hydrogen atom;
As well as selected from the group consisting of any combination thereof,
Here, in the compound of (ii) or (iii), when two or more oxy groups are inserted, each oxy group is not adjacent,
The absorbent article according to any one of claims 1 to 9. - 前記血液滑性付与剤が、次の(i’)~(iii’):
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合とを有する化合物、及び
(iii’) (iii’-1)炭化水素部分と、(iii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、及びエーテル結合(-O-)から成る群から選択される、一又は複数の、同一又は異なる結合と、(iii’-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)及びヒドロキシル基(-OH)から成る群から選択される、一又は複数の、同一又は異なる基とを有する化合物、
並びにそれらの任意の組み合わせから成る群から選択され、
ここで、(ii’)又は(iii’)の化合物において、2以上の同一又は異なる結合が挿入されている場合には、各結合は隣接していない、
請求項1~10のいずれか1項に記載の吸収性物品。 The blood slipperiness-imparting agent is any of the following (i ′) to (iii ′):
(I ′) hydrocarbon,
(Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—), an ester bond (—COO) inserted between the C—C single bonds of the hydrocarbon moiety. -), A carbonate bond (-OCOO-), and an ether bond (-O-) selected from the group consisting of one or more compounds having the same or different bonds, and (iii ') (iii'- 1) a hydrocarbon moiety and (iii′-2) a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (—OCOO) inserted between the CC single bonds of the hydrocarbon moiety. -), And one or a plurality of the same or different bonds selected from the group consisting of an ether bond (-O-) and (iii'-3) a carboxyl group that replaces the hydrogen atom of the hydrocarbon moiety ( -COOH) and hydroxy A compound having one or a plurality of the same or different groups selected from the group consisting of a sil group (—OH);
As well as selected from the group consisting of any combination thereof,
Here, in the compound of (ii ′) or (iii ′), when two or more identical or different bonds are inserted, each bond is not adjacent,
The absorbent article according to any one of claims 1 to 10. - 前記血液滑性付与剤が、次の(A)~(F):
(A) (A1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、(C2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、前記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、及びカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC3~C6アルキレングリコール、又はそのアルキルエステル若しくはアルキルエーテル、及び
(F)鎖状炭化水素、
並びにそれらの任意の組み合わせから成る群から選択される、請求項1~11のいずれか1項に記載の吸収性物品。 The blood slipperiness-imparting agent includes the following (A) to (F):
(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain An ester with a compound having one carboxyl group for substituting a hydrogen atom in the hydrocarbon moiety,
(B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups substituting for hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety,
(C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety; C2) an ester of a chain hydrocarbon moiety and a compound having one hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety,
(D) an ether bond (—O—), a carbonyl bond (—CO—), an ester bond (—COO—) inserted between the chain hydrocarbon moiety and the CC single bond of the chain hydrocarbon moiety. And a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—),
(E) polyoxy C 3 -C 6 alkylene glycol, or an alkyl ester or alkyl ether thereof, and (F) a chain hydrocarbon,
The absorbent article according to any one of claims 1 to 11, which is selected from the group consisting of any combination thereof. - 前記血液滑性付与剤が、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル、(b1)鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテル、(b2)鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテル、(b3)鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテル、(c1)4個のカルボキシル基を有する鎖状炭化水素テトラカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸又はオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、(d4)ジアルキルカーボネート、(e1)ポリオキシC3~C6アルキレングリコール、(e2)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC3~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル、及び(f1)鎖状アルカン、並びにそれらの任意の組み合わせから成る群から選択される、請求項1~12のいずれか1項に記載の吸収性物品。 The blood lubricity-imparting agent is (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) an ester of a chain hydrocarbon triol and at least one fatty acid, (a 3 ) chain Ester of linear hydrocarbon diol and at least one fatty acid, (b 1 ) ether of chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, (b 2 ) chain hydrocarbon triol and at least one fat Ethers with aliphatic monohydric alcohols, (b 3 ) ethers of chain hydrocarbon diols with at least one aliphatic monohydric alcohol, (c 1 ) chain hydrocarbon tetracarboxylic acids having four carboxyl groups, hydroxy acid, alkoxy acid or an oxo acid, at least one ester of an aliphatic monohydric alcohol, (c 2) a chain hydrocarbon bets with 3 carboxyl groups Carboxylic acid, hydroxy acid, alkoxy acid or an oxo acid, at least one ester of an aliphatic monohydric alcohol, (c 3) a chain hydrocarbon dicarboxylic acids having two carboxyl groups, hydroxy acid, alkoxy acid or an oxo An ester of an acid and at least one aliphatic monohydric alcohol, (d 1 ) an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, (d 2 ) a dialkyl ketone, (d 3 ) a fatty acid and an aliphatic 1 Esters with dihydric alcohols, (d 4 ) dialkyl carbonates, (e 1 ) polyoxy C 3 -C 6 alkylene glycols, (e 2 ) esters of polyoxy C 3 -C 6 alkylene glycols with at least one fatty acid, (e 3 ) An ether of polyoxy C 3 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol, and (f 1 The absorbent article according to any one of claims 1 to 12, which is selected from the group consisting of :) chain alkanes, and any combination thereof.
- 前記血液滑性付与剤が、1気圧及び40℃において、0.00~0.01Paの蒸気圧を有する、請求項1~13のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 13, wherein the blood slipperiness-imparting agent has a vapor pressure of 0.00 to 0.01 Pa at 1 atm and 40 ° C.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201380062904.0A CN104822349B (en) | 2012-10-03 | 2013-10-01 | Absorbent commodity |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-221737 | 2012-10-03 | ||
| JP2012221737A JP6004878B2 (en) | 2012-10-03 | 2012-10-03 | Absorbent articles |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014054649A1 true WO2014054649A1 (en) | 2014-04-10 |
Family
ID=50434974
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/076727 WO2014054649A1 (en) | 2012-10-03 | 2013-10-01 | Absorbent article |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP6004878B2 (en) |
| CN (1) | CN104822349B (en) |
| WO (1) | WO2014054649A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016060212A1 (en) * | 2014-10-17 | 2016-04-21 | 花王株式会社 | Absorbent product |
| JP2016077888A (en) * | 2014-10-17 | 2016-05-16 | 花王株式会社 | Absorbent article |
| CN107072831A (en) * | 2014-10-17 | 2017-08-18 | 花王株式会社 | Absorbent commodity |
| US10667961B2 (en) | 2014-06-30 | 2020-06-02 | Unicharm Corporation | Absorbent article and wearable article including absorbent article |
| US11154431B1 (en) | 2020-11-06 | 2021-10-26 | Mast Industries (Far East) Limited | Absorbent garment and method of manufacture thereof |
| TWI763882B (en) * | 2017-10-11 | 2022-05-11 | 日商優你 嬌美股份有限公司 | Non-woven fabrics and non-woven fabric rolls for liquid-permeable sheets of absorbent articles |
| US11957552B2 (en) | 2021-09-15 | 2024-04-16 | Mast Industries (Far East) Limited | Absorbent garment and method of manufacture thereof |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6467242B2 (en) * | 2015-02-18 | 2019-02-06 | 花王株式会社 | Absorbent articles |
| JP6629542B2 (en) * | 2015-08-04 | 2020-01-15 | 花王株式会社 | Absorbent articles |
| JP6351648B2 (en) | 2016-03-23 | 2018-07-04 | ユニ・チャーム株式会社 | Absorbent articles |
| JP6087462B1 (en) * | 2016-05-13 | 2017-03-01 | ユニ・チャーム株式会社 | Absorbent articles |
| JP7212985B2 (en) * | 2018-09-13 | 2023-01-26 | 大王製紙株式会社 | Articulated disposable wearing article |
| CN111150559B (en) * | 2018-11-08 | 2022-08-19 | 尤妮佳股份有限公司 | Absorbent article |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010518918A (en) * | 2007-02-16 | 2010-06-03 | ザ プロクター アンド ギャンブル カンパニー | Absorbent article comprising a lotion comprising a polypropylene glycol material |
| JP2011510801A (en) * | 2008-02-15 | 2011-04-07 | ザ プロクター アンド ギャンブル カンパニー | Absorbent article comprising a lotion comprising a polypropylene glycol material |
| JP2012136791A (en) * | 2010-12-24 | 2012-07-19 | Kao Corp | Nonwoven fabric and absorbent article including the same |
| JP2012236001A (en) * | 2011-04-28 | 2012-12-06 | Unicharm Corp | Absorbent article |
| WO2013129236A1 (en) * | 2012-02-29 | 2013-09-06 | ユニ・チャーム株式会社 | Absorbent article |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5643588A (en) * | 1994-11-28 | 1997-07-01 | The Procter & Gamble Company | Diaper having a lotioned topsheet |
| JP2003192563A (en) * | 2001-12-26 | 2003-07-09 | Lion Corp | Composition for impregnating use, and impregnated material for skin protection |
| JP4514630B2 (en) * | 2005-03-16 | 2010-07-28 | 花王株式会社 | Absorbent article surface sheet |
| EP2258408A1 (en) * | 2006-04-05 | 2010-12-08 | The Procter & Gamble Company | Absorbent articles including odour control system |
| WO2012086730A1 (en) * | 2010-12-24 | 2012-06-28 | 花王株式会社 | Non-woven fabric, and absorbent article using same |
-
2012
- 2012-10-03 JP JP2012221737A patent/JP6004878B2/en not_active Expired - Fee Related
-
2013
- 2013-10-01 CN CN201380062904.0A patent/CN104822349B/en not_active Expired - Fee Related
- 2013-10-01 WO PCT/JP2013/076727 patent/WO2014054649A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010518918A (en) * | 2007-02-16 | 2010-06-03 | ザ プロクター アンド ギャンブル カンパニー | Absorbent article comprising a lotion comprising a polypropylene glycol material |
| JP2011510801A (en) * | 2008-02-15 | 2011-04-07 | ザ プロクター アンド ギャンブル カンパニー | Absorbent article comprising a lotion comprising a polypropylene glycol material |
| JP2012136791A (en) * | 2010-12-24 | 2012-07-19 | Kao Corp | Nonwoven fabric and absorbent article including the same |
| JP2012236001A (en) * | 2011-04-28 | 2012-12-06 | Unicharm Corp | Absorbent article |
| WO2013129236A1 (en) * | 2012-02-29 | 2013-09-06 | ユニ・チャーム株式会社 | Absorbent article |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10667961B2 (en) | 2014-06-30 | 2020-06-02 | Unicharm Corporation | Absorbent article and wearable article including absorbent article |
| WO2016060212A1 (en) * | 2014-10-17 | 2016-04-21 | 花王株式会社 | Absorbent product |
| JP2016077888A (en) * | 2014-10-17 | 2016-05-16 | 花王株式会社 | Absorbent article |
| CN107072831A (en) * | 2014-10-17 | 2017-08-18 | 花王株式会社 | Absorbent commodity |
| TWI683655B (en) * | 2014-10-17 | 2020-02-01 | 日商花王股份有限公司 | Absorbent article |
| TWI763882B (en) * | 2017-10-11 | 2022-05-11 | 日商優你 嬌美股份有限公司 | Non-woven fabrics and non-woven fabric rolls for liquid-permeable sheets of absorbent articles |
| US11154431B1 (en) | 2020-11-06 | 2021-10-26 | Mast Industries (Far East) Limited | Absorbent garment and method of manufacture thereof |
| US11957552B2 (en) | 2021-09-15 | 2024-04-16 | Mast Industries (Far East) Limited | Absorbent garment and method of manufacture thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN104822349A (en) | 2015-08-05 |
| CN104822349B (en) | 2019-08-23 |
| JP6004878B2 (en) | 2016-10-12 |
| JP2014073213A (en) | 2014-04-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6062199B2 (en) | Absorbent articles | |
| JP6004878B2 (en) | Absorbent articles | |
| JP6184721B2 (en) | Absorbent articles | |
| JP6112816B2 (en) | Absorbent articles | |
| JP6021566B2 (en) | Absorbent articles | |
| JP6021565B2 (en) | Absorbent articles | |
| JP6195770B2 (en) | Absorbent articles | |
| WO2014050767A1 (en) | Absorbent article | |
| JP5745487B2 (en) | Absorbent articles | |
| WO2013154072A1 (en) | Absorbent article | |
| JP2014068934A (en) | Absorbent article | |
| JP6202967B2 (en) | Absorbent articles | |
| JPWO2013146711A1 (en) | Absorber and absorbent article provided with the same | |
| JP6041473B2 (en) | Absorbent articles | |
| JP6073101B2 (en) | Absorbent articles | |
| JP5988811B2 (en) | Absorbent articles | |
| JP3211401U (en) | Absorber and absorbent article provided with the same | |
| JP5885633B2 (en) | Absorbent articles | |
| JP3211431U (en) | Absorbent articles |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13843260 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IDP00201502499 Country of ref document: ID |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13843260 Country of ref document: EP Kind code of ref document: A1 |