WO2013134378A1 - Defined media for expansion and maintenance of pluripotent stem cells - Google Patents
Defined media for expansion and maintenance of pluripotent stem cells Download PDFInfo
- Publication number
- WO2013134378A1 WO2013134378A1 PCT/US2013/029360 US2013029360W WO2013134378A1 WO 2013134378 A1 WO2013134378 A1 WO 2013134378A1 US 2013029360 W US2013029360 W US 2013029360W WO 2013134378 A1 WO2013134378 A1 WO 2013134378A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cell culture
- cells
- culture formulation
- media
- defined cell
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0603—Embryonic cells ; Embryoid bodies
- C12N5/0606—Pluripotent embryonic cells, e.g. embryonic stem cells [ES]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/05—Inorganic components
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/05—Inorganic components
- C12N2500/10—Metals; Metal chelators
- C12N2500/20—Transition metals
- C12N2500/24—Iron; Fe chelators; Transferrin
- C12N2500/25—Insulin-transferrin; Insulin-transferrin-selenium
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/34—Sugars
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/36—Lipids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/38—Vitamins
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/105—Insulin-like growth factors [IGF]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/115—Basic fibroblast growth factor (bFGF, FGF-2)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/15—Transforming growth factor beta (TGF-β)
Definitions
- the present invention is in the field of proliferation and maintenance of
- pluripotent stem cells under defined media conditions.
- feeder cells which provide sufficient factors to support attachment, proliferation and maintenance of pluripotency markers.
- Early methods for the generation and culture of human embryonic stem cells required the use of mouse embryonic fibroblast (MEF) feeder cells. Subsequent techniques included use of "conditioned media” and an extracellular matrix coating to replace feeder cells.
- MEF mouse embryonic fibroblast
- Conditioned media is media that has been modified by feeder cells, such as MEFs.
- feeder cells such as MEFs.
- both methods suffer from inconsistencies in batches of conditioned media or feeder cells to continually support expansion of pluripotent stem cells.
- both systems provide undefined factors that may work differently on different pluripotent stem cells. Accordingly, establishing a defined, cheap, reproducible culture media that supports continual expansion of pluripotent stem cells is of great interest in the regenerative medicine field.
- hES cells human embryonic stem cells
- KSR knock-out serum replacer
- BSA bovine serum albumin
- mTeSR® 1 media to eight components (Nature Methods, 2011, 8:424-424) highlighting that even in defined media there exists unnecessary agent(s) that may actually slow the proliferation of ES cells or reduce their pluripotency state.
- the refined mTeSR®l media consists of DMEM/F12 basal media supplemented with insulin, selenium, transferrin, ascorbic acid, FGF2 (bFGF), and TGFP or nodal, having the pH adjusted with NaHCO 3 .
- the present invention provides a defined cell culture formulation for the culture, maintenance, and expansion of pluripotent stem cells, wherein the defined cell culture formulation comprises basal medium, insulin, transferrin, selenium, fatty- acid free albumin, a TGF- ⁇ ligand, bFGF, and ascorbic acid; and wherein culturing stem cells in the defined cell culture formulation maintains the pluripotency and karyotypic stability of the stem cells for at least 10 passages.
- the cell culture formulation further comprises insulin growth factor 1 (IGF-1).
- the cell culture formulation comprises DMEM-F12.
- the invention provides a defined cell culture formulation for the culture, maintenance, and expansion of pluripotent stem cells, wherein the defined cell culture formulation comprises basal medium, insulin, transferrin, selenium, fatty- acid free albumin, a TGF- ⁇ ligand, bFGF, ascorbic acid, Trace Elements C, 4-(2- hydroxyethyl)-l-piperazine-ethanesulfonic acid, lithium chloride, glucose, Defined Lipids, and L-alanyl-L-glutamine dipeptide; and wherein culturing stem cells in the defined cell culture formulation maintains the pluripotency and karyotypic stability of the stem cells for at least 10 passages.
- the cell culture formulation comprises MCDB-131.
- ITS-X provides the insulin, transferrin, and selenium for the defined cell culture formulation of the invention. In some embodiments of the invention, the ITS-X is present from about 0.5% to about 2%. In some embodiments of the invention, the ITS-X is present at about 1%. In some embodiments of the invention, the fatty acid free albumin is reagent grade. In some embodiments of the invention, the reagent grade fatty acid-free BSA is present from about 0.2% to about 2.5%. In some embodiments of the invention, the reagent grade fatty acid-free BSA is present at about 2%.
- the TGF- ⁇ ligand in the defined cell culture formulation of the invention is TGF- ⁇ .
- the TGF- ⁇ is present from about 0.5 ng/ml to about 10 ng/ml.
- the TGF-B1 is present at about 1 ng/ml.
- the bFGF is present in the cell culture formulation from about 50 ng/ml to about 100 ng/ml. In some embodiments of the invention, the bFGF is present in the defined cell culture formulation at about 50 ng/ml. In some embodiments, the bFGF is present in the defined cell culture formulation at about 100 ng/ml.
- the insulin growth factor 1 is present from about 10 ng/ml to about 50 ng/ml. In some embodiments of the invention, the IGF-1 is present in the defined cell culture formulation at about 20 ng/ml.
- ascorbic acid is present in the defined cell culture formulation from about 0.2 mM to about 0.3 mM. In some aspects of the invention, ascorbic acid is present in the defined cell culture formulation at about 0.25 mM.
- the invention concerns a defined cell culture formulation consisting essentially of DMEM-F12 basal medium, ITS-X (to provide insulin, transferrin, and selenium), fatty-acid free albumin, a TGF- ⁇ ligand, bFGF, insulin growth factor 1 (IGF-1), and ascorbic acid.
- the invention relates to a defined cell culture formulation consisting essentially of MCDB-131, ITS-X (as a source of insulin, transferrin, and selenium), fatty-acid free albumin, a TGF- ⁇ ligand, bFGF, ascorbic acid, Trace Elements C, 4-(2-hydroxyethyl)-l-piperazine-ethanesulfonic acid, lithium chloride, glucose, Defined Lipids, and L-alanyl-L-glutamine dipeptide.
- the invention concerns a method for the expansion of human pluripotent stem cells, where the method comprises culturing the human pluripotent stem cells on a feeder-free matrix in a defined cell culture formulation; where the defined cell culture formulation comprises basal medium, insulin, transferrin, selenium, fatty-acid free albumin, a TGF- ⁇ ligand, bFGF, and ascorbic acid; and where culturing the stem cells in the defined cell culture formulation maintains the pluripotency and karyotypic stability of the cells for at least 10 passages.
- the defined cell culture formulation further comprises insulin growth factor 1 (IGF-1).
- the cell culture formulation comprises DMEM-F12.
- the invention relates to a method for the expansion of human pluripotent stem cells, where the method comprises culturing the human pluripotent stem cells on a feeder-free matrix in a defined cell culture formulation; where the defined cell culture formulation comprises basal medium, insulin, transferrin, selenium, fatty-acid free albumin, a TGF- ⁇ ligand, bFGF, ascorbic acid, IGF-1, Trace Elements C, 4-(2-hydroxyethyl)-l-piperazineethanesulfonic acid, lithium chloride, glucose, Defined Lipids, and L-alanyl-L-glutamine dipeptide.
- the cell culture formulation used in the method for the expansion of human pluripotent stem cells comprises MCDB-131.
- An embodiment of the present invention is an in vitro cell population wherein greater than 50% of the cell population is positive for protein expression of OCT4, SOX2, NANOG, FOXA2 with negative or low protein expression of SSEA-4 and ZFP42.
- the population is obtained by culturing pluripotent stem cells in a defined cell culture formulation comprising basal media supplemented with IGF-1, insulin, bFGF, TGF-B ligand, and fatty-acid free albumin; and where the defined cell culture formulation does not comprise ascorbic acid.
- the defined cell culture formulation comprises DMEM/F12 basal media. In some embodiments of the invention the cell culture formulation comprises insulin as ITS-X. In some embodiments of the invention, the ITS-X is present from about 0.5% to about 2%. In some aspects of the invention, the ITS-X is present at about 1%. In some
- the fatty acid free albumin is reagent grade. In some aspects of the invention, the reagent grade fatty acid-free albumin is present from about 0.2% to about 2.5%. In some embodiments of the invention, the reagent grade fatty acid-free albumin is present at about 2%.
- the TGF-B ligand is TGF-B 1. In some embodiments of the invention, the TGF-B 1 is present from about 0.5 ng/ml to about 10 ng/ml. In some aspects of the invention, the TGF-B 1 is present at about 1 ng/ml.
- Figure 1A to Figure ID show phase-contrast images of HI cells cultured for 3 passages in IH-3 (FIG 1A), IH-1 (FIG IB), IH-6 (FIG 1C), and mTeSR®l (FIG ID).
- Figure 2 A to Figure 2C show phase-contrast images of HI cells cultured for 10 passages in IH-3 (FIG 2A), IH-1 (FIG 2B), and mTeSR®l (FIG 2C) media.
- Figure 3 A to Figure 3C show phase-contrast images of HI cells cultured for 18 passages in IH-3 (FIG 3A), IH-1 (FIG 3B), and mTeSR®l (FIG 3C) media.
- Figure 4A to Figure 4F show data from real-time PCR analyses of the expression of the following genes in cells of the human embryonic stem cell line HI cultured in media described in Example 1 and harvested at passages 1 to 5 (P1-P5); ZFP42 (FIG 4A), SOX2 (FIG 4B), POU5F1 (OCT4) (FIG 4C), Nanog (FIG 4D), FOXA2 (FIG 4E), and AFP (FIG 4F).
- Figure 5A to Figure 5B show data from real-time PCR analyses of the expression of Nanog, POU5F1 (OCT4), SOX2, and ZFP42 (FIG 5A), and of AFP and FOXA2 (FIG 5B) in cells of the human embryonic stem cell line HI cultured in media described in Example 1 and harvested at Passage 10.
- Figure 6A and Figure 6B show data from real-time PCR analyses of the expression of ZFP42, SOX2, POU5F1 (OCT4), and Nanog (FIG 6A), and of AFP and FOXA2 (FIG 6B) in cells of the human embryonic stem cell line HI cultured in media described in Example 1 and harvested at Passage 18.
- Figure 7 A to Figure 7F show FACS histogram expression profiles of the following markers in cells cultured for 18 passages in IH-3 media described in Example 1 : Isotype control (FIG 7A); KI-67 (FIG 7B); OCT4 (FIG 7C); SOX17 (FIG 7D); FOXA2 (FIG 7E); and SOX2 (FIG 7F). Percentage expression for each marker is shown on each histogram.
- Figure 8 A to Figure 8F show images of cells cultured for 18 passages in IH-3 media described in Example 1 and immunostained for OCT-4, FOXA2, SOX2, and fluorescent labeling of DNA using DAPI. Images obtained for OCT4 (Fig 8A), FOXA2 (FIG 8B), and DAPI-stained DNA (FIG 8C) were obtained from the same optical field but with different filters. Similarly, images for SOX2 (FIG 8D), FOXA2 (FIG 8E), and DAPI stained DNA (FIG 8F) were obtained from the same optical field but with different filters
- Figure 9 A to Figure 9F depict phase-contrast images of HI cells cultured for five passages in mTeSR®! media (FIG 9A) and in IH-3 (FIG 9B), IH-3-1 (FIG 9C), IH-3-2 (FIG 9D), IH-3-3 (FIG 9E), and IH-3-4 (FIG 9F) formulations described in Example 2.
- Figure 10A to Figure 10E show data from real-time PCR analyses of the expression of the following genes in cells of the human embryonic stem cell line HI cultured in media described in Example 2 and harvested at Passage 5: ZFP42 (FIG 10A), SOX2 (FIG 10B), FOXA2 (FIG IOC), Nanog (FIG 10 D), and POU5F1 (OCT4) (FIG 10E).
- Figure 11A to Figure 1 ID depict phase-contrast images of HI cells cultured for 20 passages in mTeSR® 1 media (Fig 1A), IH-3 (FIG 1 IB), IH-1 (FIG 11C), and IH-3RT (FIG 1 ID) media formulations described in Example 3.
- Figure 12A to Figure 12F show data from real-time PCR analyses of the expression of the following genes in cells of the human embryonic stem cell line HI cultured for 15 passages in media described in Example 3: AFP (FIG 12 A), FOXA2 (FIG 12B), SOX2 (FIG 12C), Nanog (FIG 12D), POU5F1 (OCT4) (FIG 12E), and ZFP42 (FIG 12F).
- Figure 13A to Figure 13F show data from real-time PCR analyses of the expression of the following genes in cells of the human embryonic stem cell line HI cultured for 20 passages in mTeSR®l media, and IH-1 and IH-3 media described in Example 3: AFP FIG 13 A), FOXA2 (FIG 13B), NANOG (FIG 13C), POU5F1 (OCT4) (FIG 13D), SOX2 (FIG 13E), and ZFP42 (FIG 13F).
- Figure 14A and Figure 14B depict phase-contrast images of HI cells cultured for 4 days in media formulations described in Example 5 containing Sigma BSA (FIG 14A) or containing fatty acid free BSA (FIG 14B).
- Figure 15A and Figure 15B depict phase-contrast images of HI cells cultured for three passages in media formulations described in Example 5 containing Sigma BSA (FIG 15 A) or containing fatty acid free BSA (FIG 15B).
- Figure 16A to Figure 16C show data from real-time PCR analyses of the expression of the following genes in cells of the human embryonic stem cell line HI cultured for three passages in media formulations described in Example 5 containing Sigma BSA or fatty acid free BSA: AFP (FIG 16A), MIXL1 (FIG 16B), and T (BRY) (FIG 16C).
- Figure 17A to Figure 17D show data from real-time PCR analyses of the expression of the following genes in cells of the human embryonic stem cell line HI cultured for ten passages in media formulations described in Example 6: SOX2 (FIG 17A), POU5F1 (FIG 17B), NANOG (FIG 17C), and FOXA2 (FIG 17C).
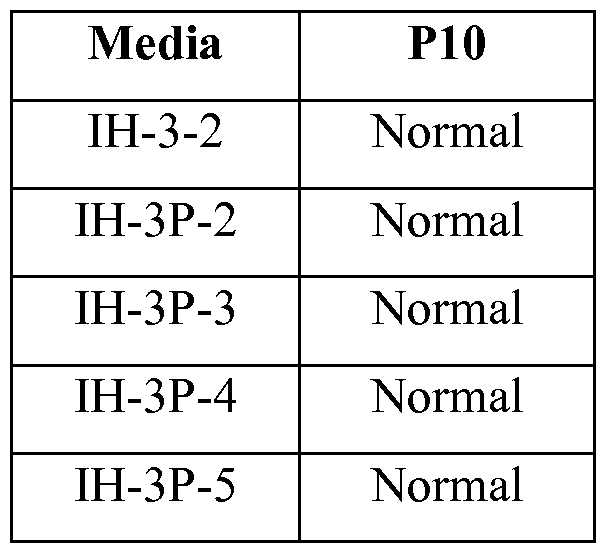
- Figure 18A to Figure 18E depict phase-contrast images of HI cells cultured for 10 passages in IH-3 (FIG 18 A), IH-3P-2 (FIG 18B), IH-3P-3 (FIG 18C), IH-3P-4 (FIG 18D), and IH-3P-5 (FIG 18E) media formulations described in Example 6.
- Stem cells are undifferentiated cells defined by their ability at the single cell level to both self-renew and differentiate to produce progeny cells, including self-renewing progenitors, non-renewing progenitors, and terminally differentiated cells. Stem cells are also characterized by their ability to differentiate in vitro into functional cells of various cell lineages from multiple germ layers
- endoderm endoderm, mesoderm and ectoderm
- endoderm mesoderm and ectoderm
- Stem cells are classified by their developmental potential as: (1) totipotent, meaning able to give rise to all embryonic and extra-embryonic cell types; (2) pluripotent, meaning able to give rise to all embryonic cell types; (3) multipotent, meaning able to give rise to a subset of cell lineages but all within a particular tissue, organ, or physiological system (for example, hematopoietic stem cells (HSC) can produce progeny that include HSC (self- renewal), blood cell restricted oligopotent progenitors, and all cell types and elements (e.g., platelets) that are normal
- HSC hematopoietic stem cells
- oligopotent meaning able to give rise to a more restricted subset of cell lineages than multipotent stem cells
- unipotent meaning able to give rise to a single cell lineage (e.g., spermatogenic stem cells).
- a differentiated or differentiation- induced cell is one that has taken on a more specialized ("committed") position within the lineage of a cell.
- the term "committed”, when applied to the process of differentiation, refers to a cell that has proceeded in the differentiation pathway to a point where, under normal circumstances, it will continue to differentiate into a specific cell type or subset of cell types, and cannot, under normal circumstances, differentiate into a different cell type or revert to a less differentiated cell type.
- De- differentiation refers to the process by which a cell reverts to a less specialized (or committed) position within the lineage of a cell.
- the lineage of a cell defines the heredity of the cell, i.e., which cells it came from and what cells it can give rise to.
- the lineage of a cell places the cell within a hereditary scheme of development and differentiation.
- a lineage-specific marker refers to a characteristic specifically associated with the phenotype of cells of a lineage of interest and can be used to assess the differentiation of an uncommitted cell to the lineage of interest.
- Markers are nucleic acid or polypeptide molecules that are differentially expressed in a cell of interest.
- differential expression means an increased level for a positive marker and a decreased level for a negative marker.
- the detectable level of the marker nucleic acid or polypeptide is sufficiently higher or lower in the cells of interest compared to other cells, such that the cell of interest can be identified and distinguished from other cells using any of a variety of methods known in the art.
- Basal Medium refers to a solution of salts, nutrients, and vitamins that can support the growth of pluripotent stem cells in culture.
- Basal media may be selected among others from Dulbecco's modified Eagle's media (DMEM), MCDB media, RPMI.
- DMEM may also be DMEM/F12 (also referred to as DM-F12), or DMEM -high glucose (also referred to as DMEM-hg).
- MCDB media may be selected from any of the MCDB media available, and specifically MCDB-131.
- basal media may be selected by mixing the basl media formulations listed above in the appropriate ratio to allow for proliferation and maintenance of pluripotency of embryonic stem cells/
- the basal media in the defined cell culture formulation of the invention is DMEM-F12.
- the basal media in the cell culture formulation of the invention is MCDB-131.
- Feeer Cells refers to non-pluripotent stem cells on which pluripotent stem cells are plated.
- the feeder cells provide sufficient soluble and insoluble factors to support for attachment, proliferation, and maintenance of pluripotency markers by pluripotent stem cells.
- Constant Medium refers to a medium that is further supplemented with soluble factors derived from feeder cells.
- Extracellular Matrix or “Defined Matrix” or “Synthetic Matrix” refers to one or more substances that can provide for attachment, proliferation, and maintenance of pluripotency markers by pluripotent stem cells. Used interchangeably herein are “IGF” and “IGF-1” which stand for Insulin-like growth factor 1. In humans this protein is made by the liver and is responsible for much of what is attributed to the human growth hormone.
- FGF2 and bFGF are used interchangeably to identify the human basic fibroblast growth factor.
- TGF beta used interchangeably herein are "TGF beta”, “TGF-B”, and "TGF- ⁇ ".
- a TGF- ⁇ ligand may be selected from bone morphogenetic proteins (BMPs), growth and differentiation factor (GDFs), activins (Activin A, Activin AB, Activin B, Activin C), nodal and TGF-Ps.
- BMPs bone morphogenetic proteins
- GDFs growth and differentiation factor
- Activins Activin A, Activin AB, Activin B, Activin C
- nodal and TGF-Ps used interchangeably herein are "TGF beta”, “TGF-B”, and "TGF- ⁇ ”.
- a TGF- ⁇ ligand may be selected from bone morphogenetic proteins (BMPs), growth and differentiation factor (GDFs), activins (Activin A, Activin AB, Activin B, Activin C), nodal and TGF-Ps.
- a TGF- ⁇ may be selected
- Pluripotent stem cells may express one or more of the stage-specific embryonic antigens (SSEA) 3 and 4, and markers detectable using antibodies designated Tra-1-60 and Tra-1-81 (Thomson et al., Science 282: 1145, 1998).
- SSEA stage-specific embryonic antigens
- Undifferentiated pluripotent stem cells typically have alkaline phosphatase activity, which can be detected by fixing the cells with 4% paraformaldehyde, followed by developing with Vector Red as a substrate, as described by the manufacturer (Vector Laboratories, Burlingame CA). Undifferentiated pluripotent stem cells also typically express OCT4 and TERT, as detected by RT-PCR.
- pluripotent stem cells Another desirable phenotype of propagated pluripotent stem cells is a potential to differentiate into cells of all three germinal layers: endoderm, mesoderm, and ectoderm tissues. Pluripotency of stem cells can be confirmed, for example, by injecting cells into severe combined immunodeficient (SCID) mice, fixing the teratomas that form using 4% paraformaldehyde, and then examining them histologically for evidence of cell types from the three germ layers. Alternatively, pluripotency may be determined by the creation of embryoid bodies and assessing the embryoid bodies for the presence of markers associated with the three germinal layers.
- SCID severe combined immunodeficient
- Propagated pluripotent stem cell lines may be karyotyped using a standard G-banding technique and compared to published karyotypes of the corresponding primate species. It is desirable to obtain cells that have a "normal karyotype," which means that the cells are euploid, wherein all human chromosomes are present and not noticeably altered. Pluripotent cells may be readily expanded in culture using various feeder layers or by using matrix protein coated vessels. Alternatively, chemically defined surfaces in combination with defined media such as mTeSR®l media (StemCell Technologies, Vancouver, Canada) may be used for routine expansion of the cells.
- defined media such as mTeSR®l media (StemCell Technologies, Vancouver, Canada) may be used for routine expansion of the cells.
- Pluripotent cells may be readily removed from culture plates using enzymatic, mechanical or use of various calcium chelators such as EDTA (Ethylenediaminetetraacetic acid). Alternatively, pluripotent cells may be expanded in suspension in the absence of any matrix proteins or a feeder layer.
- EDTA Ethylenediaminetetraacetic acid
- pluripotent stem cells include established lines of pluripotent cells derived from tissue formed after gestation, including pre-embryonic tissue (such as, for example, a blastocyst), embryonic tissue, or fetal tissue taken any time during gestation, typically but not necessarily before approximately 10 to 12 weeks gestation.
- pre-embryonic tissue such as, for example, a blastocyst
- embryonic tissue or fetal tissue taken any time during gestation, typically but not necessarily before approximately 10 to 12 weeks gestation.
- Non- limiting examples are established lines of human embryonic stem cells or human embryonic germ cells, such as, for example the human embryonic stem cell lines HI, H7, and H9 (WiCell Research Institute, Madison, WI).
- compositions of this disclosure during the initial establishment or stabilization of such cells, in which case the source cells would be primary pluripotent cells taken directly from the source tissues.
- cells taken from a pluripotent stem cell population already cultured in the absence of feeder cells are also suitable.
- inducible pluripotent cells (IPS) or reprogrammed pluripotent cells that can be derived from adult somatic cells using forced expression of a number of pluripotent related transcription factors, such as OCT4, Nanog, Sox2, KLF4,and ZFP42 (Annu Rev Genomics Hum Genet, 2011, 12: 165-185).
- Human embryonic stem cells may be prepared as described by
- pluripotent stem cell markers include, for example, the expression of one or more of the following: ABCG2, cripto, FOXD3, CONNEXIN43, CONNEXIN45, OCT4, SOX2, NANOG, hTERT, UTF1, ZFP42, SSEA-3, SSEA-4, Tra 1-60, Tra 1- 81.
- Differentiation markers typically present in cultures of embryonic stem cells include for example, AFP, FOXA2, SOX17, T(BRY), and MIXL1.
- human pluripotent stem cells are cultured in a defined media comprising ascorbic acid, IGF, insulin, bFGF, TGF-B ligand, and fatty-acid free albumin to sustain proliferation of the pluripotent stem cells while maintaining pluripotency and karyotypic stability of the expanded cells for at least 10 passages.
- An embodiment of the present invention is an in vitro cell population wherein greater than 50% of the cell population is positive for protein expression of OCT4, SOX2, NANOG, and FOXA2 positive but low protein expression of SSEA-4 and ZFP42.
- Another aspect of the present invention describes an in vitro defined cell culture formulation comprising IGF, insulin, bFGF, TGF-B, fatty-acid free albumin, and no ascorbic acid that results in a cell population wherein greater than 50% of the cell population is positive by protein staining for OCT4, SOX2, NANOG, FOXA2 and low protein expression of SSEA-4 and ZFP42.
- 11905031 contains 100.0 ml/L ethyl alcohol (200 proof) and 2 mg/L Arachidonic Acid, 220 mg/L Cholesterol, 70 mg/L DL-alpha-Tocopherol Acetate, 0 mg/L Ethyl Alcohol 100%, 10 mg/L Linoleic Acid, 10 mg/L Linolenic Acid, 10 mg/L Myristic Acid, 10 mg/L Oleic Acid, 10 mg/L Palmitic Acid, 10 mg/L Palmitoleic Acid, 90000 mg/L Pluronic F-68, 10 mg/L Stearic Acid, and 2200 mg/L Tween 80® (ICI Americas, Inc. Bridgewater, NJ).
- IH-1 and IH-3 were further compared to the cells cultured in mTeSR®l media.
- samples were collected from IH-1, IH-3, and mTeSR® 1 cultures and evaluated by FACS, PCR, karyotype analysis (G- banding or FISH), and immune fluorescence staining.
- the results from FISH analysis are shown in Table II. These results show that HI cells cultured in IH-1 media or IH- 3 media showed normal karyotype, whereas cells cultured in mTeSR® 1 media displayed abnormal trisomy 12 at passage 10 and 18.
- IH-1 media passaged continuously in IH-1 media maintained characteristic ES colony morphology with very few differentiated cells surrounding the colonies. However, cells grown in IH-3 media started to lose the characteristic ES colony morphology beyond passage 10 (See FIG 1A, FIG 2A, and FIG 3 A).
- HI cells cultured in IH-3 media maintained strong expression of OCT4 and SOX2 markers at passage 1 1 (Table IV). This was despite a very low expression level of SSEA-4 for HI cells cultured in IH-3 media.
- m NA expression of core pluripotency markers such as Nanog (FIG 4D), OCT4 (FIG 4C), SOX2 (FIG 4B), and ZPF42 (FIG 4A) were maintained through passage 5 for HI cells cultured in IH-1, and IH-3 media to the same level as HI cells cultured in mTeSR®l .
- core pluripotency markers such as Nanog (FIG 4D), OCT4 (FIG 4C), SOX2 (FIG 4B), and ZPF42 (FIG 4A) were maintained through passage 5 for HI cells cultured in IH-1, and IH-3 media to the same level as HI cells cultured in mTeSR®l .
- ZFP42 ZFP42
- HI cells cultured for 14 passages in IH-3 were subsequently cultured in the above media formulations and compared to cells cultured in IH-3 media.
- HI cells cultured using various media formulations were assayed for pluripotency markers.
- Table VI following five additional passages, HI cells cultured in IH-3-2 (IH-3 supplemented with ascorbic acid) media recovered a small percentage of their SSEA-4 expression as compared to cells cultured in the other tested media.
- HI cells cultured in IH-3-2 media retained typical embryonic stem cell morphology similar to cells cultured in mTeSR® 1 (FIG 9A) media.
- HI cells cultured in IH-3, IH-3-1, IH-3-3, and IH-3-4 showed loose colony morphology (See FIG 9B, FIG 9C, and FIG 9F).
- PCR analysis of cells cultured in the above media formulations further confirmed that HI cells cultured in IH-3 -2 media regained some of the expression of ZFP42 and down regulated expression of FOXA2 (see FIG 10A to FIG 10E).
- passage 40 cultured on MATRIGELTM (1 :30 dilution) coated dishes in mTeSR® 1 media and passaged using EDTA, as described in Example 1 , were used as the starting population to evaluate long-term cultures using IH-1, IH-3-2 and mTeSR®l media. Cells were passaged as small colonies using 5-10 minute EDTA treatment at room temperature. The components of the tested media are listed in Table VII.
- HI cells cultured for 20 passages in IH-1, IH-3-2, and IH-3RT retained typical ES morphology.
- the results of PCR analysis of HI cells cultured for 15 passages in IH-1, IH-3-2, and IH-3RT are shown in FIG 12A to FIG 12F.
- the results of PCR analysis of HI cells cultured for 20 passages in IH-1, IH-3-2, and IH-3RT are shown in FIG 13A to FIG13F.
- HI cells cultured continuously in IH-1, IH-3-2, and IH-3RT showed normal karyotype as measured by G-banding and FISH analysis. However, HI cells cultured for 10 to 20 passages in mTeSR®l showed abnormal chromosomal counts (See Table IX).
- HI cells cultured in IH-1, IH-3-2 and mTeSR®l media were released by using TrypLE (Invitrogen) and seeded at a density of 5 X 10 5 cells per 10 cm MATRIGELTM -coated dishes.
- released cells were pretreated with 10 ⁇ Rock inhibitor (Sigma). Media was changed daily until three days post-seeding. On day 3, cells were released as single cells and counted using a hemocytometer. As shown in Table X, cells cultured in all three media formulations showed equivalent doubling times Table X
- Cells of the human embryonic stem cells line HI (passage 35 to passage 40), cultured on MATRIGELTM (1 :30 dilution) coated dishes in mTeSR® 1 media and passaged using EDTA, were used as the starting population to evaluate short-term cultures using IH-3-2 media supplemented with either 2% Sigma BSA (catalog No. A2153; Lot: 061M1804V) or fatty-acid free BSA (Proliant, Catalog No. 7500804; Lot: 11G54001). Cells were passaged as small colonies using 5-10 minute EDTA treatment at room temperature.
- Figure 14A and Figure 14B depict phase- contrast images of HI cells cultured for 4 days in media formulations containing Sigma BSA (FIG 14A) or fatty acid free BSA (FIG 14B).
- Figure 15A and Figure 15B depict phase-contrast images of HI cells cultured for three passages in media formulations containing Sigma BSA (FIG 15 A) or fatty acid free BSA (FIG 15B).
- FIG 14 A As seen in FIG 14 A, as early as day 4 following seeding, there was morphological evidence of differentiated cells in cultures using Sigma BSA. However, there was no gross differentiated cell morphology evident in cultures treated with fatty acid-free BSA (see FIG 14B)). The same trend was noted at passage 3, there was
- FIG 16A 16A
- MIXL1 FIG. 16B
- T BRY
- FIG 16C 16C
- PCR data at passage 3 clearly showed significant upregulation of markers associated with a differentiated cell for cells cultured in media comprising Sigma BSA. This data clearly demonstrates that use of fatty-acid-free BSA is critical in the maintenance of pluripotency, colony morphology, and proliferation of cells.
- Pluripotent Stem Cells can be Propagated and Maintain Pluripotency in IH-3 Media Using a Wide Range of Fatty Acid Free BSA and bFGF Concentrations
- passage 40 cultured on MATRIGELTM (1 :30 dilution) coated dishes in mTesr® 1 media and passaged using EDTA, were used as the starting population to evaluate short and long-term cultures using IH-3 media supplemented as indicated in Table XI.
- FIG 17A to Figure 17D show data from real-time PCR analyses of the expression of SOX2 (FIG 17A), POU5F1 (FIG 17B), NANOG (FIG 17C), and FOXA2 (FIG 17C) in cells of the human embryonic stem cell line HI cultured for ten passages in media
- FIG. 18A to Figure 18E depict phase-contrast images of HI cells cultured for 10 passages in IH-3-2 (FIG
- IH-3P-2 (FIG 18B), IH-3P-3 (FIG 18C), IH-3P-4 (FIG 18D), and IH-3P-5 (FIG 18E) media formulations listed in Table XI. As indicated in these figures, all formulations tested in this example allowed for formation of ES colonies with minimal evidence of gross differentiated morphology.
- HI cells cultured for ten passages in media formulations listed in Table XI retained normal counts for chromosome 12 and 17 as measured by FISH analysis.
- defined media consisting of DMEM/F12 basal media supplemented with ITS-X, reagent-grade fatty acid- free BSA, TGF-B1, IGF-1, and ascorbic acid allows for expansion of pluripotent cells while maintaining pluripotency of the cells when using a wide range of concentrations of fatty acid -free BSA and bFGF.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Genetics & Genomics (AREA)
- Chemical & Material Sciences (AREA)
- Gynecology & Obstetrics (AREA)
- Biotechnology (AREA)
- Reproductive Health (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Developmental Biology & Embryology (AREA)
- Organic Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Description
Claims
Priority Applications (14)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| MX2014010782A MX354775B (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells. |
| EP13757652.6A EP2823037A4 (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells |
| KR1020147027965A KR20140131999A (en) | 2012-03-07 | 2013-03-06 | Defined Media for Expansion and Maintenance of Pluripotent Stem Cells |
| AU2013230020A AU2013230020B2 (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells |
| RU2014140371A RU2664467C2 (en) | 2012-03-07 | 2013-03-06 | Medium with defined composition for propagation and renewal of pluripotent stem cells |
| SG11201405052RA SG11201405052RA (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells |
| CA2866590A CA2866590A1 (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells |
| CN201380012670.9A CN104160018A (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells |
| JP2014561075A JP6383292B2 (en) | 2012-03-07 | 2013-03-06 | Clear media for proliferation and maintenance of pluripotent stem cells |
| IN7036DEN2014 IN2014DN07036A (en) | 2012-03-07 | 2014-08-21 | |
| PH12014501898A PH12014501898A1 (en) | 2012-03-07 | 2014-08-22 | Defined media for expansion and maintenance of pluripotent stem cells |
| ZA2014/07241A ZA201407241B (en) | 2012-03-07 | 2014-10-06 | Defined media for expansion and maintenance of pluripotent stem cells |
| HK15106542.3A HK1206058A1 (en) | 2012-03-07 | 2015-07-09 | Defined media for expansion and maintenance of pluripotent stem cells |
| AU2018260810A AU2018260810A1 (en) | 2012-03-07 | 2018-11-06 | Defined media for expansion and maintenance of pluripotent stem cells |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261607706P | 2012-03-07 | 2012-03-07 | |
| US61/607,706 | 2012-03-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013134378A1 true WO2013134378A1 (en) | 2013-09-12 |
Family
ID=49114468
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2013/029360 WO2013134378A1 (en) | 2012-03-07 | 2013-03-06 | Defined media for expansion and maintenance of pluripotent stem cells |
Country Status (16)
| Country | Link |
|---|---|
| US (3) | US9434920B2 (en) |
| EP (1) | EP2823037A4 (en) |
| JP (1) | JP6383292B2 (en) |
| KR (1) | KR20140131999A (en) |
| CN (1) | CN104160018A (en) |
| AR (1) | AR090276A1 (en) |
| AU (2) | AU2013230020B2 (en) |
| CA (1) | CA2866590A1 (en) |
| HK (1) | HK1206058A1 (en) |
| IN (1) | IN2014DN07036A (en) |
| MX (1) | MX354775B (en) |
| PH (1) | PH12014501898A1 (en) |
| RU (2) | RU2664467C2 (en) |
| SG (1) | SG11201405052RA (en) |
| WO (1) | WO2013134378A1 (en) |
| ZA (1) | ZA201407241B (en) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014192938A1 (en) | 2013-05-30 | 2014-12-04 | 味の素株式会社 | Medium for culturing stem cells |
| WO2017156580A1 (en) * | 2016-03-16 | 2017-09-21 | Cynata Therapeutics Limited | Colony forming medium and use thereof |
| CN111093680A (en) * | 2017-09-15 | 2020-05-01 | 洋蓟治疗有限公司 | Methods of treating Allergic Airway Disease (AAD)/asthma |
| WO2020243666A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | A biocompatible membrane composite |
| WO2020243663A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | A biocompatible membrane composite |
| WO2020243665A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | A biocompatible membrane composite |
| WO2020243668A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | Cell encapsulation devices with controlled oxygen diffusion distances |
| US11001810B1 (en) * | 2019-11-11 | 2021-05-11 | Lancell AB | Serum-free human pluripotent stem cell culture medium |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103088065B (en) * | 2013-01-25 | 2014-12-10 | 北京银杏德济生物技术有限公司 | Method capable of forming hematopoietic stem cells by quickly inducing reversal decision of mesenchymal stem cells in large scale with high purity |
| WO2015066631A2 (en) * | 2013-11-01 | 2015-05-07 | University Of Notre Dame Du Lac | Cell culture medium and bioprocess optimization |
| US11203739B2 (en) | 2014-04-07 | 2021-12-21 | Memorial Sloan-Kettering Cancer Center | Modulating cell proliferation and pluripotency |
| EP3177302A4 (en) * | 2014-08-07 | 2018-04-11 | Duke University | Compositions and methods for the reprogramming of cells into cardiomyocytes |
| WO2016107387A1 (en) * | 2014-12-31 | 2016-07-07 | 北京大学口腔医学院 | Device and application thereof in cell in-vitro experiment |
| KR102015815B1 (en) * | 2016-08-10 | 2019-08-29 | 가톨릭대학교 산학협력단 | Method for Culturing Cornea Epithealial Cell by Inducing Differentiation of Induced Pluripotent Stem Cell and System for the Same |
| AU2017381449B2 (en) * | 2016-12-21 | 2021-10-28 | Liminal Biosciences Limited | Methods and compositions for preventing or minimizing epithelial-mesenchymal transition |
| US10767164B2 (en) | 2017-03-30 | 2020-09-08 | The Research Foundation For The State University Of New York | Microenvironments for self-assembly of islet organoids from stem cells differentiation |
| DK3436568T3 (en) * | 2017-05-31 | 2023-09-18 | Promocell Gmbh | CULTURE MEDIUM FOR PLURIPOTENT STEM CELLS |
| KR20210021004A (en) * | 2018-06-15 | 2021-02-24 | 후소 야쿠힝 고교 가부시끼가이샤 | Reproductive aid medical media |
| CN112516291B (en) * | 2019-09-17 | 2023-07-14 | 通化安睿特生物制药股份有限公司 | Preparation containing human albumin and preparation method thereof |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6800480B1 (en) | 1997-10-23 | 2004-10-05 | Geron Corporation | Methods and materials for the growth of primate-derived primordial stem cells in feeder-free culture |
| US7005252B1 (en) | 2000-03-09 | 2006-02-28 | Wisconsin Alumni Research Foundation | Serum free cultivation of primate embryonic stem cells |
| US7297539B2 (en) | 2000-01-11 | 2007-11-20 | Geron Corporation | Medium for growing human embryonic stem cells |
| WO2008036447A2 (en) * | 2006-06-26 | 2008-03-27 | Lifescan, Inc. | Pluripotent stem cell culture |
| US7442548B2 (en) | 2004-09-08 | 2008-10-28 | Wisconsin Alumni Research Foundation | Culturing human embryonic stem cells in medium containing pipecholic acid and gamma amino butyric acid |
| US20080268534A1 (en) | 2006-02-23 | 2008-10-30 | Novocell, Inc. | Compositions and methods useful for culturing differentiable cells |
| US7449334B2 (en) | 2004-09-08 | 2008-11-11 | Wisconsin Alumni Research Foundation | Medium containing pipecholic acid and gamma amino butyric acid and culture of embryonic stem cells |
| US20090269845A1 (en) * | 2008-04-24 | 2009-10-29 | Alireza Rezania | Pluripotent cells |
| US20100255580A1 (en) * | 2007-07-18 | 2010-10-07 | Lifesccan, Inc. | Differentiation of Human Embryonic Stem Cells |
| US20110229441A1 (en) * | 2008-12-05 | 2011-09-22 | Association Francaise Contre Les Myopathies | Method and Medium for Neural Differentiation of Pluripotent Cells |
| WO2012019122A2 (en) * | 2010-08-05 | 2012-02-09 | Wisconsin Alumni Research Foundation | Simplified basic media for human pluripotent cell culture |
Family Cites Families (224)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3209652A (en) | 1961-03-30 | 1965-10-05 | Burgsmueller Karl | Thread whirling method |
| AT326803B (en) | 1968-08-26 | 1975-12-29 | Binder Fa G | MESHWARE AND METHOD OF MANUFACTURING THE SAME |
| US3935067A (en) | 1974-11-22 | 1976-01-27 | Wyo-Ben Products, Inc. | Inorganic support for culture media |
| CA1201400A (en) | 1982-04-16 | 1986-03-04 | Joel L. Williams | Chemically specific surfaces for influencing cell activity during culture |
| US4499802A (en) | 1982-09-29 | 1985-02-19 | Container Graphics Corporation | Rotary cutting die with scrap ejection |
| US4537773A (en) | 1983-12-05 | 1985-08-27 | E. I. Du Pont De Nemours And Company | α-Aminoboronic acid derivatives |
| US4557264A (en) | 1984-04-09 | 1985-12-10 | Ethicon Inc. | Surgical filament from polypropylene blended with polyethylene |
| US5215893A (en) | 1985-10-03 | 1993-06-01 | Genentech, Inc. | Nucleic acid encoding the ba chain prodomains of inhibin and method for synthesizing polypeptides using such nucleic acid |
| US5089396A (en) | 1985-10-03 | 1992-02-18 | Genentech, Inc. | Nucleic acid encoding β chain prodomains of inhibin and method for synthesizing polypeptides using such nucleic acid |
| US4737578A (en) | 1986-02-10 | 1988-04-12 | The Salk Institute For Biological Studies | Human inhibin |
| US5863531A (en) | 1986-04-18 | 1999-01-26 | Advanced Tissue Sciences, Inc. | In vitro preparation of tubular tissue structures by stromal cell culture on a three-dimensional framework |
| US5567612A (en) | 1986-11-20 | 1996-10-22 | Massachusetts Institute Of Technology | Genitourinary cell-matrix structure for implantation into a human and a method of making |
| US5759830A (en) | 1986-11-20 | 1998-06-02 | Massachusetts Institute Of Technology | Three-dimensional fibrous scaffold containing attached cells for producing vascularized tissue in vivo |
| CA1340581C (en) | 1986-11-20 | 1999-06-08 | Joseph P. Vacanti | Chimeric neomorphogenesis of organs by controlled cellular implantation using artificial matrices |
| NZ229354A (en) | 1988-07-01 | 1990-09-26 | Becton Dickinson Co | Treating polymer surfaces with a gas plasma and then applying a layer of endothelial cells to the surface |
| EP0363125A3 (en) | 1988-10-03 | 1990-08-16 | Hana Biologics Inc. | Proliferated pancreatic endocrine cell product and process |
| US5837539A (en) | 1990-11-16 | 1998-11-17 | Osiris Therapeutics, Inc. | Monoclonal antibodies for human mesenchymal stem cells |
| US5449383A (en) | 1992-03-18 | 1995-09-12 | Chatelier; Ronald C. | Cell growth substrates |
| GB9206861D0 (en) | 1992-03-28 | 1992-05-13 | Univ Manchester | Wound healing and treatment of fibrotic disorders |
| CA2114282A1 (en) | 1993-01-28 | 1994-07-29 | Lothar Schilder | Multi-layered implant |
| JP3525221B2 (en) | 1993-02-17 | 2004-05-10 | 味の素株式会社 | Immunosuppressants |
| AU687386B2 (en) | 1993-04-08 | 1998-02-26 | Human Cell Cultures, Inc. | Cell culturing method and medium |
| US5523226A (en) | 1993-05-14 | 1996-06-04 | Biotechnology Research And Development Corp. | Transgenic swine compositions and methods |
| GB9310557D0 (en) | 1993-05-21 | 1993-07-07 | Smithkline Beecham Plc | Novel process and apparatus |
| TW257671B (en) | 1993-11-19 | 1995-09-21 | Ciba Geigy | |
| US5834308A (en) | 1994-04-28 | 1998-11-10 | University Of Florida Research Foundation, Inc. | In vitro growth of functional islets of Langerhans |
| US6001647A (en) | 1994-04-28 | 1999-12-14 | Ixion Biotechnology, Inc. | In vitro growth of functional islets of Langerhans and in vivo uses thereof |
| US6703017B1 (en) | 1994-04-28 | 2004-03-09 | Ixion Biotechnology, Inc. | Reversal of insulin-dependent diabetes by islet-producing stem cells, islet progenitor cells and islet-like structures |
| US6083903A (en) | 1994-10-28 | 2000-07-04 | Leukosite, Inc. | Boronic ester and acid compounds, synthesis and uses |
| JP4079461B2 (en) | 1994-12-29 | 2008-04-23 | 中外製薬株式会社 | Action enhancer for antitumor agent comprising IL-6 antagonist |
| US5843780A (en) | 1995-01-20 | 1998-12-01 | Wisconsin Alumni Research Foundation | Primate embryonic stem cells |
| US5718922A (en) | 1995-05-31 | 1998-02-17 | Schepens Eye Research Institute, Inc. | Intravitreal microsphere drug delivery and method of preparation |
| US5908782A (en) | 1995-06-05 | 1999-06-01 | Osiris Therapeutics, Inc. | Chemically defined medium for human mesenchymal stem cells |
| AU7138298A (en) | 1997-04-24 | 1998-11-13 | Ortho-Mcneil Corporation, Inc. | Substituted imidazoles useful in the treatment of inflammatory diseases |
| ATE358493T1 (en) | 1997-07-03 | 2007-04-15 | Osiris Therapeutics Inc | HUMAN MESENCHYMAL STEM CELLS FROM PERIPHERAL BLOOD |
| WO1999014318A1 (en) | 1997-09-16 | 1999-03-25 | Board Of Regents, The University Of Texas System | Method for the complete chemical synthesis and assembly of genes and genomes |
| US6670127B2 (en) | 1997-09-16 | 2003-12-30 | Egea Biosciences, Inc. | Method for assembly of a polynucleotide encoding a target polypeptide |
| ZA9811898B (en) | 1997-12-29 | 2000-06-28 | Ortho Mcneil Pharm Inc | Anti-Inflammatory Compounds. |
| US6328960B1 (en) | 1998-03-18 | 2001-12-11 | Osiris Therapeutics, Inc. | Mesenchymal stem cells for prevention and treatment of immune responses in transplantation |
| MY132496A (en) | 1998-05-11 | 2007-10-31 | Vertex Pharma | Inhibitors of p38 |
| US6413773B1 (en) | 1998-06-01 | 2002-07-02 | The Regents Of The University Of California | Phosphatidylinositol 3-kinase inhibitors as stimulators of endocrine differentiation |
| US6667176B1 (en) | 2000-01-11 | 2003-12-23 | Geron Corporation | cDNA libraries reflecting gene expression during growth and differentiation of human pluripotent stem cells |
| US6610540B1 (en) | 1998-11-18 | 2003-08-26 | California Institute Of Technology | Low oxygen culturing of central nervous system progenitor cells |
| US6413556B1 (en) | 1999-01-08 | 2002-07-02 | Sky High, Llc | Aqueous anti-apoptotic compositions |
| NZ513598A (en) | 1999-01-21 | 2001-09-28 | Vitro Diagnostics Inc | Immortalized cell lines and methods of making the same |
| US6815203B1 (en) | 1999-06-23 | 2004-11-09 | Joslin Diabetes Center, Inc. | Methods of making pancreatic islet cells |
| US6306424B1 (en) | 1999-06-30 | 2001-10-23 | Ethicon, Inc. | Foam composite for the repair or regeneration of tissue |
| US6333029B1 (en) | 1999-06-30 | 2001-12-25 | Ethicon, Inc. | Porous tissue scaffoldings for the repair of regeneration of tissue |
| CA2385628A1 (en) | 1999-09-27 | 2001-04-05 | Ammon B. Peck | Reversal of insulin-dependent diabetes by islet-producing stem cells, islet progenitor cells and islet-like structures |
| US6685936B2 (en) | 1999-10-12 | 2004-02-03 | Osiris Therapeutics, Inc. | Suppressor cells induced by culture with mesenchymal stem cells for treatment of immune responses in transplantation |
| US20030082155A1 (en) | 1999-12-06 | 2003-05-01 | Habener Joel F. | Stem cells of the islets of langerhans and their use in treating diabetes mellitus |
| AU778155B2 (en) | 1999-12-13 | 2004-11-18 | Scripps Research Institute, The | Markers for identification and isolation of pancreatic islet alpha and beta cell progenitors |
| US7439064B2 (en) | 2000-03-09 | 2008-10-21 | Wicell Research Institute, Inc. | Cultivation of human embryonic stem cells in the absence of feeder cells or without conditioned medium |
| US6436704B1 (en) | 2000-04-10 | 2002-08-20 | Raven Biotechnologies, Inc. | Human pancreatic epithelial progenitor cells and methods of isolation and use thereof |
| US6458589B1 (en) | 2000-04-27 | 2002-10-01 | Geron Corporation | Hepatocyte lineage cells derived from pluripotent stem cells |
| EP1302534A4 (en) | 2000-06-26 | 2004-06-16 | Renomedix Inst Inc | Cell fraction containing cells capable of differentiating into neural cells |
| IL155367A0 (en) | 2000-10-23 | 2003-12-23 | Smithkline Beecham Corp | NOVEL 2,4,8-TRISUBSTITUTED-8h-PYRIDO[2,3,-d]PYRIMIDIN-7-ONE COMPOUNDS, PHARMACEUTICAL COMPOSITIONS COMPRISING THE SAME, PROCESSES FOR THE PREPARATION THEREOF, AND USE THEREOF IN THE PREPARATION OF MEDICAMENTS FOR TREATING CSBP/p38 KINASE MEDIATED DISEASES |
| CA2431166A1 (en) | 2000-12-08 | 2002-06-13 | Ortho-Mcneil Pharmaceutical, Inc. | Indazolyl-substituted pyrroline compounds as kinase inhibitors |
| ATE301661T1 (en) | 2000-12-08 | 2005-08-15 | Ortho Mcneil Pharm Inc | MACROHETEROCYCLIC COMPOUNDS AS KINASE INHIBITORS |
| US6599323B2 (en) | 2000-12-21 | 2003-07-29 | Ethicon, Inc. | Reinforced tissue implants and methods of manufacture and use |
| US20040121460A1 (en) | 2001-01-24 | 2004-06-24 | Lumelsky Nadya L | Differentiation of stem cells to pancreatic endocrine cells |
| EP3078667B1 (en) | 2001-01-25 | 2018-11-21 | The United States of America, represented by the Secretary, Department of Health and Human Services | Formulation of boronic acid compounds |
| US6656488B2 (en) | 2001-04-11 | 2003-12-02 | Ethicon Endo-Surgery, Inc. | Bioabsorbable bag containing bioabsorbable materials of different bioabsorption rates for tissue engineering |
| DE10290025T1 (en) | 2001-04-19 | 2003-10-09 | Develogen Ag | Procedure for differentiating stem cells into insulin-producing cells |
| DE60231035D1 (en) | 2001-04-24 | 2009-03-19 | Ajinomoto Kk | STEM CELLS AND METHOD FOR THEIR SEPARATION |
| JP2004531262A (en) | 2001-05-15 | 2004-10-14 | ラッパポート ファミリー インスチチュート フォア リサーチ イン ザ メディカル サイエンシズ | Human embryonic stem cell-derived insulin-producing cells |
| US6626950B2 (en) | 2001-06-28 | 2003-09-30 | Ethicon, Inc. | Composite scaffold with post anchor for the repair and regeneration of tissue |
| KR100418195B1 (en) | 2001-07-05 | 2004-02-11 | 주식회사 우리기술 | Apparatus and method for multi-testing insulation of power cables |
| GB0117583D0 (en) | 2001-07-19 | 2001-09-12 | Astrazeneca Ab | Novel compounds |
| CA2456981C (en) | 2001-08-06 | 2012-02-28 | Bresagen, Inc. | Alternative compositions and methods for the culture of stem cells |
| US6617152B2 (en) | 2001-09-04 | 2003-09-09 | Corning Inc | Method for creating a cell growth surface on a polymeric substrate |
| EP1298201A1 (en) | 2001-09-27 | 2003-04-02 | Cardion AG | Process for the production of cells exhibiting an islet-beta-cell-like state |
| JP2005506074A (en) | 2001-10-18 | 2005-03-03 | イクシオン・バイオテクノロジー・インコーポレーテッド | Conversion of hepatic stem and progenitor cells into functional pancreatic cells |
| CA2468171C (en) | 2001-11-15 | 2015-10-06 | Children's Medical Center Corporation | Methods of isolation, expansion and differentiation of fetal stem cells from chorionic villus, amniotic fluid, and placenta and therapeutic uses thereof |
| EP1463798A4 (en) | 2001-12-07 | 2005-01-19 | Geron Corp | Islet cells from human embryonic stem cells |
| EP2305276A3 (en) | 2001-12-07 | 2011-09-21 | Cytori Therapeutics, Inc. | Processed lipoaspirate cells for use in therapy |
| WO2003054169A1 (en) | 2001-12-21 | 2003-07-03 | Thromb-X Nv | Compositions for the in vitro derivation and culture of embryonic stem (es) cell lines with germline transmission capability |
| EP1461421A2 (en) | 2001-12-28 | 2004-09-29 | Cellartis AB | A method for the establishment of a pluripotent human blastocyst-derived stem cell line |
| US20030162290A1 (en) | 2002-01-25 | 2003-08-28 | Kazutomo Inoue | Method for inducing differentiation of embryonic stem cells into functioning cells |
| US20050208029A1 (en) | 2002-04-17 | 2005-09-22 | Akihiro Umezawa | Method of forming pancreatic beta cells from mesenchymal cells |
| US20040161419A1 (en) | 2002-04-19 | 2004-08-19 | Strom Stephen C. | Placental stem cells and uses thereof |
| AU2003225295A1 (en) | 2002-05-08 | 2003-11-11 | Janssen Pharmaceutica N.V. | Substituted pyrroline kinase inhibitors |
| GB0210539D0 (en) * | 2002-05-08 | 2002-06-19 | Univ Edinburgh | Control of es cell self renewal and lineage specification, and medium therefor |
| US20060003446A1 (en) | 2002-05-17 | 2006-01-05 | Gordon Keller | Mesoderm and definitive endoderm cell populations |
| WO2003102171A1 (en) | 2002-05-28 | 2003-12-11 | Becton, Dickinson And Company | Expansion and transdifferentiation of human acinar cells |
| AU2003238874A1 (en) | 2002-06-05 | 2003-12-22 | Janssen Pharmaceutica N.V. | Bisindolyl-maleimid derivatives as kinase inhibitors |
| GB0212976D0 (en) | 2002-06-06 | 2002-07-17 | Tonejet Corp Pty Ltd | Ejection method and apparatus |
| CN1171991C (en) | 2002-07-08 | 2004-10-20 | 徐如祥 | Culture process of human nerve stem cell |
| US6877147B2 (en) | 2002-07-22 | 2005-04-05 | Broadcom Corporation | Technique to assess timing delay by use of layout quality analyzer comparison |
| US7838290B2 (en) | 2002-07-25 | 2010-11-23 | The Scripps Research Institute | Hematopoietic stem cells and methods of treatment of neovascular eye diseases therewith |
| CA2494040A1 (en) | 2002-07-29 | 2004-02-05 | Es Cell International Pte Ltd. | Multi-step method for the differentiation of insulin positive, glucose |
| WO2004016747A2 (en) | 2002-08-14 | 2004-02-26 | University Of Florida | Bone marrow cell differentiation |
| AU2003268534A1 (en) | 2002-09-06 | 2004-03-29 | Amcyte Inc. | Cd56 positive human adult pancreatic endocrine progenitor cells |
| US9969977B2 (en) | 2002-09-20 | 2018-05-15 | Garnet Biotherapeutics | Cell populations which co-express CD49c and CD90 |
| US20040062753A1 (en) | 2002-09-27 | 2004-04-01 | Alireza Rezania | Composite scaffolds seeded with mammalian cells |
| AU2003285172A1 (en) | 2002-11-08 | 2004-06-03 | The Johns Hopkins University | Human embryonic stem cell cultures, and compositions and methods for growing same |
| US7144999B2 (en) | 2002-11-23 | 2006-12-05 | Isis Pharmaceuticals, Inc. | Modulation of hypoxia-inducible factor 1 alpha expression |
| WO2004050827A2 (en) | 2002-12-05 | 2004-06-17 | Technion Research & Development Foundation Ltd. | Cultured human pancreatic islets, and uses thereof |
| CN100549163C (en) | 2002-12-16 | 2009-10-14 | 技术研究及发展基金有限公司 | The stem cell culture for preparing the method for no feeder cell, no allogenic human embryo stem cell and use this method preparation |
| CA2514539C (en) | 2003-01-29 | 2012-03-06 | Takeda Pharmaceutical Company Limited | Process for producing coated preparation |
| RU2359671C2 (en) | 2003-01-29 | 2009-06-27 | Такеда Фармасьютикал Компани Лимитед | Method of obtaining of preparation with covering |
| US20070155661A1 (en) | 2003-02-14 | 2007-07-05 | The Board Of Trustees Of The Leland Standord Junior University | Methods and compositions for modulating the development of stem cells |
| US20070154981A1 (en) | 2003-02-14 | 2007-07-05 | The Board Of Trustees Of The Leland Stanford Junior University | Insulin-producing cells derived from stem cells |
| US20070020242A1 (en) | 2003-03-27 | 2007-01-25 | Ixion Biotechnology, Inc. | Method for transdifferentiation of non-pancreatic stem cells to the pancreatic pathway |
| US20060194315A1 (en) | 2003-03-31 | 2006-08-31 | Condie Brian G | Compositions and methods for the control, differentiaton and/or manipulation of pluripotent cells through a gamma-secretase signaling pathway |
| US20090203141A1 (en) | 2003-05-15 | 2009-08-13 | Shi-Lung Lin | Generation of tumor-free embryonic stem-like pluripotent cells using inducible recombinant RNA agents |
| ES2564044T3 (en) | 2003-06-27 | 2016-03-17 | DePuy Synthes Products, Inc. | Postpartum cells derived from umbilical cord tissue and methods of preparing and using them |
| IL161903A0 (en) | 2003-07-17 | 2005-11-20 | Gamida Cell Ltd | Ex vivo progenitor and stem cell expansion for usein the treatment of disease of endodermally- deri ved organs |
| ITRM20030395A1 (en) | 2003-08-12 | 2005-02-13 | Istituto Naz Per Le Malattie Infettive Lazz | CULTURE GROUND FOR MAINTENANCE, PROLIFERATION AND DIFFERENTIATION OF MAMMALIAN CELLS. |
| WO2005017117A2 (en) | 2003-08-14 | 2005-02-24 | Martin Haas | Multipotent amniotic fetal stem cells (mafsc) and banking of same |
| US7157275B2 (en) | 2003-08-15 | 2007-01-02 | Becton, Dickinson And Company | Peptides for enhanced cell attachment and growth |
| WO2005021728A2 (en) | 2003-08-27 | 2005-03-10 | Stemcells California, Inc. | Enriched pancreatic stem cell and progenitor cell populations, and methods for identifying, isolating and enriching for these populations |
| CA2550010A1 (en) | 2003-12-17 | 2005-06-30 | Allergan, Inc. | Methods for treating retinoid responsive disorders using selective inhibitors of cyp26a and cyp26b |
| US20060030042A1 (en) | 2003-12-19 | 2006-02-09 | Ali Brivanlou | Maintenance of embryonic stem cells by the GSK-3 inhibitor 6-bromoindirubin-3'-oxime |
| CN109628371B (en) | 2003-12-23 | 2021-02-19 | 维亚希特公司 | Definitive endoderm |
| CA2549605C (en) | 2003-12-23 | 2013-05-07 | Cythera, Inc. | Definitive endoderm |
| US20050266554A1 (en) | 2004-04-27 | 2005-12-01 | D Amour Kevin A | PDX1 expressing endoderm |
| US7625753B2 (en) | 2003-12-23 | 2009-12-01 | Cythera, Inc. | Expansion of definitive endoderm cells |
| TWI334443B (en) | 2003-12-31 | 2010-12-11 | Ind Tech Res Inst | Method of single cell culture of undifferentiated human embryonic stem cells |
| US20050233446A1 (en) | 2003-12-31 | 2005-10-20 | Parsons Xuejun H | Defined media for stem cell culture |
| WO2005071066A1 (en) | 2004-01-23 | 2005-08-04 | Board Of Regents, The University Of Texas System | Methods and compositions for preparing pancreatic insulin secreting cells |
| US7794704B2 (en) | 2004-01-23 | 2010-09-14 | Advanced Cell Technology, Inc. | Methods for producing enriched populations of human retinal pigment epithelium cells for treatment of retinal degeneration |
| GB2441530B (en) | 2004-02-12 | 2009-09-23 | Univ Newcastle | Stem Cells |
| JP4901471B2 (en) | 2004-02-19 | 2012-03-21 | 国立大学法人京都大学 | Screening method for somatic cell nuclear reprogramming substances |
| WO2005086860A2 (en) | 2004-03-09 | 2005-09-22 | Gang Xu | Methods for generating insulin-producing cells |
| CN1950498A (en) | 2004-03-10 | 2007-04-18 | 加利福尼亚大学董事会 | Compositions and methods for growth of embryonic stem cells |
| WO2005097980A2 (en) | 2004-03-26 | 2005-10-20 | Geron Corporation | New protocols for making hepatocytes from embryonic stem cells |
| EP1730268A2 (en) | 2004-04-01 | 2006-12-13 | Wisconsin Alumni Research Foundation | Differentiation of stem cells to endoderm and pancreatic lineage |
| KR101278421B1 (en) | 2004-04-27 | 2013-07-15 | 비아싸이트, 인크. | Pdx1 expressing endoderm |
| CA2573283C (en) | 2004-07-09 | 2023-03-14 | Cythera, Inc. | Methods for identifying factors for differentiating definitive endoderm |
| CA2576872C (en) | 2004-08-13 | 2013-11-12 | University Of Georgia Research Foundation, Inc. | Compositions and methods for self-renewal and differentiation in human embryonic stem cells |
| WO2006026473A2 (en) | 2004-08-25 | 2006-03-09 | University Of Georgia Research Foundation, Inc. | METHODS AND COMPOSITIONS UTILIZING MYC AND GSK3ß TO MANIPULATE THE PLURIPOTENCY OF EMBRYONIC STEM CELLS |
| DE102004043256B4 (en) | 2004-09-07 | 2013-09-19 | Rheinische Friedrich-Wilhelms-Universität Bonn | Scalable process for culturing undifferentiated stem cells in suspension |
| EP1859026A2 (en) | 2005-01-31 | 2007-11-28 | ES Cell International Pte Ltd. | Directed differentiation of embryonic stem cells and uses thereof |
| US20060182724A1 (en) * | 2005-02-15 | 2006-08-17 | Riordan Neil H | Method for expansion of stem cells |
| CN101188942B (en) | 2005-03-04 | 2011-11-30 | 生命扫描有限公司 | Adult pancreatic derived stromal cells |
| GB0505970D0 (en) | 2005-03-23 | 2005-04-27 | Univ Edinburgh | Culture medium containing kinase inhibitor, and uses thereof |
| CN100425694C (en) | 2005-04-15 | 2008-10-15 | 北京大学 | Method of inducing embryo stem cell to differentiate toward pancreatic cell |
| ATE553198T1 (en) | 2005-04-15 | 2012-04-15 | Geron Corp | TREATMENT OF CANCER THROUGH THE COMBINED INHIBITION OF PROTEASOME AND TELOMERASE ACTIVITIES |
| US20080208351A1 (en) | 2005-04-26 | 2008-08-28 | Aarhus Universitet | Biocompatible Material for Surgical Implants and Cell Guiding Tissue Culture Surfaces |
| EP1899344A1 (en) | 2005-06-10 | 2008-03-19 | Irm, Llc | Compounds that maintain pluripotency of embryonic stem cells |
| WO2006138433A2 (en) | 2005-06-14 | 2006-12-28 | The Regents Of The University Of California | Induction of cell differentiation by class i bhlh polypeptides |
| WO2006137787A1 (en) | 2005-06-21 | 2006-12-28 | Ge Healthcare Bio-Sciences Ab | Method for cell culture |
| KR20160116024A (en) | 2005-06-22 | 2016-10-06 | 아스테리아스 바이오세라퓨틱스, 인크. | Suspension culture of human embryonic stem cells |
| JP5345388B2 (en) | 2005-06-30 | 2013-11-20 | ジヤンセン・フアーマシユーチカ・ナームローゼ・フエンノートシヤツプ | Cyclic anilino-pyridinotriazine |
| US20080194021A1 (en) | 2005-07-29 | 2008-08-14 | Mays Robert W | Use of a Gsk-3 Inhibitor to Maintain Potency of Culture Cells |
| WO2007016366A2 (en) * | 2005-07-29 | 2007-02-08 | Yale University | Defined culture conditions of human embryonic stem cells |
| US20090087907A1 (en) | 2005-07-29 | 2009-04-02 | Alice Pebay | Compositions and Methods for Growth of Pluripotent Cells |
| WO2007025234A2 (en) | 2005-08-26 | 2007-03-01 | The Trustees Of Columbia University In The City Of New York | Generation of pancreatic endocrine cells from primary duct cell cultures and methods of use for treatment of diabetes |
| KR20080056182A (en) | 2005-09-02 | 2008-06-20 | 에이전시 포 사이언스, 테크놀로지 앤드 리서치 | Method of deriving mesenchymal stem cells |
| SG151259A1 (en) | 2005-09-12 | 2009-04-30 | Es Cell Int Pte Ltd | Cardiomyocyte production |
| SG169324A1 (en) | 2005-10-14 | 2011-03-30 | Univ Minnesota | Differentiation of non-embryonic stem cells to cells having a pancreatic phenotype |
| CA2627645C (en) | 2005-10-27 | 2015-07-07 | Cythera, Inc. | Pdx1-expressing dorsal and ventral foregut endoderm |
| CN103113463B (en) | 2005-12-13 | 2015-02-18 | 国立大学法人京都大学 | Nuclear reprogramming factor |
| WO2007082963A1 (en) | 2006-01-18 | 2007-07-26 | Fundación Instituto Valenciano De Infertilidad | Human embryo stem-cell lines and methods for using same |
| CA3147112A1 (en) | 2006-03-02 | 2007-09-13 | Viacyte, Inc. | Endocrine precursor cells, pancreatic hormone-expressing cells and methods of production |
| US7695965B2 (en) | 2006-03-02 | 2010-04-13 | Cythera, Inc. | Methods of producing pancreatic hormones |
| EP2021462B1 (en) | 2006-04-28 | 2019-01-09 | Lifescan, Inc. | Differentiation of human embryonic stem cells |
| US8741643B2 (en) | 2006-04-28 | 2014-06-03 | Lifescan, Inc. | Differentiation of pluripotent stem cells to definitive endoderm lineage |
| AU2007248609B2 (en) | 2006-05-02 | 2012-11-01 | Wisconsin Alumni Research Foundation | Method of differentiating stem cells into cells of the endoderm and pancreatic lineage |
| US8685730B2 (en) | 2006-05-02 | 2014-04-01 | Wisconsin Alumni Research Foundation | Methods and devices for differentiating pluripotent stem cells into cells of the pancreatic lineage |
| WO2007139929A2 (en) | 2006-05-25 | 2007-12-06 | The Burnham Institute For Medical Research | Methods for culture and production of single cell populations of human embryonic stem cells |
| CA2654196A1 (en) | 2006-06-02 | 2007-12-13 | University Of Georgia Research Foundation, Inc. | Pancreatic and liver endoderm cells and tissue by differentiation of definitive endoderm cells obtained from human embryonic stems |
| CN101541953A (en) | 2006-06-02 | 2009-09-23 | 佐治亚大学研究基金会 | Pancreatic and liver endoderm cells and tissue by differentiation of definitive endoderm cells obtained from human embryonic stems |
| US8415153B2 (en) | 2006-06-19 | 2013-04-09 | Geron Corporation | Differentiation and enrichment of islet-like cells from human pluripotent stem cells |
| CN100494359C (en) | 2006-06-23 | 2009-06-03 | 中日友好医院 | Method for in vitro amplifying and in 3D solid culturing for nerve stem cell |
| US20080003676A1 (en) | 2006-06-26 | 2008-01-03 | Millipore Corporation | Growth of embryonic stem cells |
| US8968994B2 (en) | 2006-07-06 | 2015-03-03 | Jeremy Micah Crook | Method for stem cell culture and cells derived therefrom |
| AU2007277364B2 (en) | 2006-07-26 | 2010-08-12 | Viacyte, Inc. | Methods of producing pancreatic hormones |
| KR101331510B1 (en) | 2006-08-30 | 2013-11-20 | 재단법인서울대학교산학협력재단 | Media compostions containing low concentrations of glucose useful for human embryonic stem cells, differentiation method of human embryonic stem cells into insulin-producing cells or cell clusters using thereof, and insulin-producing cells or cell clusters differentiated thereby |
| JP2008099662A (en) | 2006-09-22 | 2008-05-01 | Institute Of Physical & Chemical Research | Method for culturing stem cell |
| WO2008039521A2 (en) | 2006-09-26 | 2008-04-03 | Nmt Medical, Inc. | Method for modifying a medical implant surface for promoting tissue growth |
| WO2008048647A1 (en) | 2006-10-17 | 2008-04-24 | Cythera, Inc. | Modulation of the phosphatidylinositol-3-kinase pathway in the differentiation of human embryonic stem cells |
| CN101611016B (en) | 2006-10-17 | 2012-01-25 | 斯蒂菲尔实验室公司 | Talarazole metabolites |
| US8835163B2 (en) | 2006-10-18 | 2014-09-16 | The Board Of Trustees Of The University Of Illinois | Embryonic-like stem cells derived from adult human peripheral blood and methods of use |
| WO2008056779A1 (en) | 2006-11-09 | 2008-05-15 | Japan As Represented By The President Of International Medical Center Of Japan | Method for culture and passage of primate embryonic stem cell, and method for induction of differentiation of the embryonic stem cell |
| WO2008086005A1 (en) | 2007-01-09 | 2008-07-17 | University Of South Florida | Compositions including triciribine and bortezomib and derivatives thereof and methods of use thereof |
| CN101641436A (en) | 2007-01-30 | 2010-02-03 | 佐治亚大学研究基金会 | Be used to produce the promptly stable mesendoderm cell mass of early stage mesoblastema of entoderm and mesoblastema system and multipotency wandering cell (MMC) |
| GB0703188D0 (en) | 2007-02-19 | 2007-03-28 | Roger Land Building | Large scale production of stem cells |
| US20090053182A1 (en) | 2007-05-25 | 2009-02-26 | Medistem Laboratories, Inc. | Endometrial stem cells and methods of making and using same |
| EP2584034B8 (en) | 2007-07-31 | 2017-12-06 | Lifescan, Inc. | Pluripotent stem cell differentiation by using human feeder cells |
| KR101617243B1 (en) | 2007-07-31 | 2016-05-02 | 라이프스캔, 인코포레이티드 | Differentiation of human embryonic stem cells |
| MX2010002179A (en) | 2007-08-24 | 2010-04-27 | Stichting Het Nl Kanker I | Composition. |
| US20110151447A1 (en) | 2007-11-06 | 2011-06-23 | Children's Medical Center Corporation | Method to produce induced pluripotent stem (ips) cells from non-embryonic human cells |
| MX2010005805A (en) | 2007-11-27 | 2010-06-09 | Lifescan Inc | Differentiation of human embryonic stem cells. |
| SG154367A1 (en) | 2008-01-31 | 2009-08-28 | Es Cell Int Pte Ltd | Method of differentiating stem cells |
| WO2009096049A1 (en) | 2008-02-01 | 2009-08-06 | Kyoto University | Differentiated cells originating in artificial pluripotent stem cells |
| US20100330677A1 (en) | 2008-02-11 | 2010-12-30 | Cambridge Enterprise Limited | Improved Reprogramming of Mammalian Cells, and Cells Obtained |
| JP5733986B2 (en) | 2008-02-21 | 2015-06-10 | ヤンセン バイオテツク,インコーポレーテツド | Methods, surface modified plates, and compositions for cell attachment, culture and detachment |
| JPWO2009110215A1 (en) | 2008-03-03 | 2011-07-14 | 独立行政法人科学技術振興機構 | Ciliary cell differentiation induction method |
| WO2009116951A2 (en) | 2008-03-17 | 2009-09-24 | Agency For Science, Technology And Research | Microcarriers for stem cell culture |
| US8338170B2 (en) | 2008-04-21 | 2012-12-25 | Viacyte, Inc. | Methods for purifying endoderm and pancreatic endoderm cells derived from human embryonic stem cells |
| AU2008355123B2 (en) | 2008-04-21 | 2014-12-04 | Viacyte, Inc. | Methods for purifying endoderm and pancreatic endoderm cells derived from human embryonic stem cells |
| WO2009132083A2 (en) | 2008-04-22 | 2009-10-29 | President And Fellows Of Harvard College | Compositions and methods for promoting the generation of pdx1+ pancreatic cells |
| US8623648B2 (en) | 2008-04-24 | 2014-01-07 | Janssen Biotech, Inc. | Treatment of pluripotent cells |
| US20090298178A1 (en) | 2008-06-03 | 2009-12-03 | D Amour Kevin Allen | Growth factors for production of definitive endoderm |
| EP2297319B1 (en) | 2008-06-03 | 2015-10-07 | Viacyte, Inc. | Growth factors for production of definitive endoderm |
| KR101651661B1 (en) | 2008-06-30 | 2016-08-26 | 얀센 바이오테크 인코포레이티드 | Differentiation of pluripotent stem cells |
| DE102008032236A1 (en) | 2008-06-30 | 2010-04-01 | Eberhard-Karls-Universität Tübingen | Isolation and / or identification of stem cells with adipocytic, chondrocytic and pancreatic differentiation potential |
| US20100028307A1 (en) | 2008-07-31 | 2010-02-04 | O'neil John J | Pluripotent stem cell differentiation |
| PL2346988T3 (en) | 2008-10-31 | 2017-10-31 | Janssen Biotech Inc | Differentiation of human embryonic stem cells to the pancreatic endocrine lineage |
| US9012218B2 (en) | 2008-10-31 | 2015-04-21 | Janssen Biotech, Inc. | Differentiation of human embryonic stem cells |
| US8008075B2 (en) | 2008-11-04 | 2011-08-30 | Viacyte, Inc. | Stem cell aggregate suspension compositions and methods of differentiation thereof |
| WO2010053472A1 (en) | 2008-11-04 | 2010-05-14 | Novocell, Inc. | Stem cell aggregate suspension compositions and methods for differentiation thereof |
| EP4176888A1 (en) | 2008-11-14 | 2023-05-10 | ViaCyte, Inc. | Encapsulation of pancreatic cells derived from human pluripotent stem cells |
| KR101774546B1 (en) | 2008-11-20 | 2017-09-04 | 얀센 바이오테크 인코포레이티드 | Pluripotent stem cell culture on micro-carriers |
| CN102482643B (en) | 2009-07-20 | 2016-06-29 | 詹森生物科技公司 | The differentiation of human embryo stem cell |
| EP2456859A4 (en) | 2009-07-20 | 2015-03-18 | Janssen Biotech Inc | Differentiation of human embryonic stem cells |
| FI20096288A0 (en) * | 2009-12-04 | 2009-12-04 | Kristiina Rajala | Formulations and Methods for Culturing Stem Cells |
| CN102741395B (en) | 2009-12-23 | 2016-03-16 | 詹森生物科技公司 | The differentiation of human embryo stem cell |
| CN102858958A (en) * | 2010-02-03 | 2013-01-02 | 日本国立癌症研究中心 | Induced Hepatic Stem Cell And Process For Production Thereof, And Applications Of The Cell |
| SG183400A1 (en) | 2010-03-02 | 2012-09-27 | Univ Singapore | Culture additives to boost stem cell proliferation and differentiation response |
| JP2011177140A (en) * | 2010-03-03 | 2011-09-15 | Nippon Dental Univ | Serum-free medium for stem cell culture |
| CN102959076B (en) | 2010-03-31 | 2015-09-16 | 斯克里普斯研究所 | Reprogrammed cell |
| EP2563908B1 (en) | 2010-04-25 | 2019-01-09 | Icahn School of Medicine at Mount Sinai | Generation of anterior foregut endoderm from pluripotent cells |
| AU2011250912A1 (en) | 2010-05-12 | 2012-11-22 | Janssen Biotech, Inc. | Differentiation of human embryonic stem cells |
| EP2611907B1 (en) | 2010-08-31 | 2016-05-04 | Janssen Biotech, Inc. | Differentiation of pluripotent stem cells |
| WO2012117333A1 (en) | 2011-02-28 | 2012-09-07 | Stempeutics Research Malaysia Sdn Bhd | Isolation and expansion of adult stem cells, their therapeutic composition and uses thereof |
| US9133266B2 (en) * | 2011-05-06 | 2015-09-15 | Wisconsin Alumni Research Foundation | Vitronectin-derived cell culture substrate and uses thereof |
| WO2013055834A2 (en) | 2011-10-11 | 2013-04-18 | The New York Stem Cell Foundation | Er stress relievers in beta cell protection |
| SG10201608914WA (en) | 2011-12-22 | 2016-12-29 | Janssen Biotech Inc | Differentiation of human embryonic stem cells into single hormonal insulin positive cells |
| US10519422B2 (en) | 2012-02-29 | 2019-12-31 | Riken | Method of producing human retinal pigment epithelial cells |
| US10066210B2 (en) | 2012-06-08 | 2018-09-04 | Janssen Biotech, Inc. | Differentiation of human embryonic stem cells into pancreatic endocrine cells |
| TW201522637A (en) | 2013-03-15 | 2015-06-16 | Jackson Lab | Isolation of non-embryonic stem cells and uses thereof |
-
2013
- 2013-03-06 KR KR1020147027965A patent/KR20140131999A/en not_active Application Discontinuation
- 2013-03-06 RU RU2014140371A patent/RU2664467C2/en not_active IP Right Cessation
- 2013-03-06 WO PCT/US2013/029360 patent/WO2013134378A1/en active Application Filing
- 2013-03-06 SG SG11201405052RA patent/SG11201405052RA/en unknown
- 2013-03-06 AU AU2013230020A patent/AU2013230020B2/en not_active Ceased
- 2013-03-06 CA CA2866590A patent/CA2866590A1/en not_active Abandoned
- 2013-03-06 CN CN201380012670.9A patent/CN104160018A/en active Pending
- 2013-03-06 MX MX2014010782A patent/MX354775B/en active IP Right Grant
- 2013-03-06 JP JP2014561075A patent/JP6383292B2/en not_active Expired - Fee Related
- 2013-03-06 US US13/787,173 patent/US9434920B2/en not_active Expired - Fee Related
- 2013-03-06 RU RU2018128383A patent/RU2018128383A/en not_active Application Discontinuation
- 2013-03-06 EP EP13757652.6A patent/EP2823037A4/en not_active Withdrawn
- 2013-03-07 AR ARP130100747A patent/AR090276A1/en unknown
-
2014
- 2014-08-21 IN IN7036DEN2014 patent/IN2014DN07036A/en unknown
- 2014-08-22 PH PH12014501898A patent/PH12014501898A1/en unknown
- 2014-10-06 ZA ZA2014/07241A patent/ZA201407241B/en unknown
-
2015
- 2015-07-09 HK HK15106542.3A patent/HK1206058A1/en unknown
-
2016
- 2016-05-09 US US15/150,349 patent/US9593307B2/en active Active
-
2017
- 2017-03-13 US US15/457,952 patent/US20170183626A1/en not_active Abandoned
-
2018
- 2018-11-06 AU AU2018260810A patent/AU2018260810A1/en not_active Abandoned
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6800480B1 (en) | 1997-10-23 | 2004-10-05 | Geron Corporation | Methods and materials for the growth of primate-derived primordial stem cells in feeder-free culture |
| US7297539B2 (en) | 2000-01-11 | 2007-11-20 | Geron Corporation | Medium for growing human embryonic stem cells |
| US7005252B1 (en) | 2000-03-09 | 2006-02-28 | Wisconsin Alumni Research Foundation | Serum free cultivation of primate embryonic stem cells |
| US7410798B2 (en) | 2001-01-10 | 2008-08-12 | Geron Corporation | Culture system for rapid expansion of human embryonic stem cells |
| US7442548B2 (en) | 2004-09-08 | 2008-10-28 | Wisconsin Alumni Research Foundation | Culturing human embryonic stem cells in medium containing pipecholic acid and gamma amino butyric acid |
| US7449334B2 (en) | 2004-09-08 | 2008-11-11 | Wisconsin Alumni Research Foundation | Medium containing pipecholic acid and gamma amino butyric acid and culture of embryonic stem cells |
| US20080268534A1 (en) | 2006-02-23 | 2008-10-30 | Novocell, Inc. | Compositions and methods useful for culturing differentiable cells |
| WO2008036447A2 (en) * | 2006-06-26 | 2008-03-27 | Lifescan, Inc. | Pluripotent stem cell culture |
| US20100255580A1 (en) * | 2007-07-18 | 2010-10-07 | Lifesccan, Inc. | Differentiation of Human Embryonic Stem Cells |
| US20090269845A1 (en) * | 2008-04-24 | 2009-10-29 | Alireza Rezania | Pluripotent cells |
| US20110229441A1 (en) * | 2008-12-05 | 2011-09-22 | Association Francaise Contre Les Myopathies | Method and Medium for Neural Differentiation of Pluripotent Cells |
| WO2012019122A2 (en) * | 2010-08-05 | 2012-02-09 | Wisconsin Alumni Research Foundation | Simplified basic media for human pluripotent cell culture |
Non-Patent Citations (4)
| Title |
|---|
| CHEN ET AL., NATURE METHODS, vol. 8, 2011, pages 424 - 429 |
| NATURE METHODS, vol. 2, 2005, pages 185 - 189 |
| See also references of EP2823037A4 * |
| VALLIER ET AL., J CELL SCI, vol. 118, 2005, pages 4495 - 4509 |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20160012224A (en) | 2013-05-30 | 2016-02-02 | 아지노모토 가부시키가이샤 | Medium for culturing stem cells |
| US11859205B2 (en) | 2013-05-30 | 2024-01-02 | Ajinomoto Co., Inc. | Medium for culturing stem cells |
| WO2014192938A1 (en) | 2013-05-30 | 2014-12-04 | 味の素株式会社 | Medium for culturing stem cells |
| US11174461B2 (en) | 2016-03-16 | 2021-11-16 | Cynata Therapeutics Limited | Colony forming medium and use thereof |
| WO2017156580A1 (en) * | 2016-03-16 | 2017-09-21 | Cynata Therapeutics Limited | Colony forming medium and use thereof |
| JP2019509065A (en) * | 2016-03-16 | 2019-04-04 | サイナータ セラピューティクス リミテッド | Colony forming medium and use thereof |
| AU2017234378B2 (en) * | 2016-03-16 | 2022-10-13 | Cynata Therapeutics Limited | Colony forming medium and use thereof |
| JP7048977B2 (en) | 2016-03-16 | 2022-04-06 | サイナータ セラピューティクス リミテッド | Colonization medium and its use |
| CN111093680A (en) * | 2017-09-15 | 2020-05-01 | 洋蓟治疗有限公司 | Methods of treating Allergic Airway Disease (AAD)/asthma |
| WO2020243663A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | A biocompatible membrane composite |
| WO2020243668A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | Cell encapsulation devices with controlled oxygen diffusion distances |
| WO2020243665A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | A biocompatible membrane composite |
| WO2020243666A1 (en) | 2019-05-31 | 2020-12-03 | W. L. Gore & Associates, Inc. | A biocompatible membrane composite |
| US20210139858A1 (en) * | 2019-11-11 | 2021-05-13 | Lancell AB | Serum-free human pluripotent stem cell culture medium |
| US11001810B1 (en) * | 2019-11-11 | 2021-05-11 | Lancell AB | Serum-free human pluripotent stem cell culture medium |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2014010782A (en) | 2014-10-14 |
| HK1206058A1 (en) | 2015-12-31 |
| KR20140131999A (en) | 2014-11-14 |
| RU2018128383A (en) | 2019-03-14 |
| RU2664467C2 (en) | 2018-08-17 |
| AU2013230020A1 (en) | 2014-09-04 |
| EP2823037A1 (en) | 2015-01-14 |
| SG11201405052RA (en) | 2014-10-30 |
| ZA201407241B (en) | 2016-05-25 |
| US9434920B2 (en) | 2016-09-06 |
| RU2014140371A (en) | 2016-04-27 |
| IN2014DN07036A (en) | 2015-04-10 |
| US9593307B2 (en) | 2017-03-14 |
| EP2823037A4 (en) | 2015-09-16 |
| CA2866590A1 (en) | 2013-09-12 |
| AU2018260810A1 (en) | 2018-11-22 |
| RU2018128383A3 (en) | 2019-04-15 |
| AR090276A1 (en) | 2014-10-29 |
| US20160251615A1 (en) | 2016-09-01 |
| MX354775B (en) | 2018-03-20 |
| JP6383292B2 (en) | 2018-08-29 |
| AU2013230020B2 (en) | 2018-08-09 |
| US20130236973A1 (en) | 2013-09-12 |
| JP2015509381A (en) | 2015-03-30 |
| PH12014501898A1 (en) | 2014-11-24 |
| US20170183626A1 (en) | 2017-06-29 |
| CN104160018A (en) | 2014-11-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9593307B2 (en) | Defined media for expansion and maintenance of pluripotent stem cells | |
| US20200224157A1 (en) | Novel methods and culture media for culturing pluripotent stem cells | |
| ES2610812T3 (en) | Culture of pluripotent stem cells | |
| US20230151326A1 (en) | Simplified Compositions and Methods for Generating Neural Stem Cells from Human Pluripotent Stem Cells | |
| JP2018148921A (en) | Production of erythrocytes | |
| US20100081200A1 (en) | Formulations and methods for culturing stem cells | |
| KR20130009943A (en) | Formulations and methods for culturing stem cells | |
| JP2007228815A (en) | Method for maintaining embryonic stem cell | |
| Chaddah et al. | Clonal neural stem cells from human embryonic stem cell colonies | |
| KR101687344B1 (en) | Methods and compositions for cell attachment and cultivation on planar substrates | |
| WO2013054112A1 (en) | Culture media for pluripotent stem cells | |
| US20150037883A1 (en) | Method for derivation and long-term establishment of ground state pluripotent embryonic stem cells | |
| JP2019022509A (en) | Adhesive signature-based methods for isolating stem cells and cells derived therefrom | |
| US20090075374A1 (en) | Methods of generating epithelial lineage cells from embryoid bodies and pluripotent cells | |
| Burrell et al. | Stirred suspension bioreactor culture of porcine induced pluripotent stem cells | |
| Wu et al. | Derivation and characterization of human embryonic stem cell lines from the Chinese population | |
| US10542743B2 (en) | Isolation, expansion and characterization of wharton's jelly mesenchymal stem cells | |
| Balbasi et al. | Mouse embryonic stem cell culture in serum-containing or 2i conditions | |
| Coelho de Oliveira et al. | Hair follicle-derived mesenchymal cells support undifferentiated growth of embryonic stem cells | |
| Rostovskaya | Capacitation of human naïve pluripotent stem cells | |
| OJALA | Establishing and optimizing feeder cell-free culture methods for human embryonic stem cells | |
| WO2023191709A2 (en) | Method | |
| Young et al. | Feeder-free Culture |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13757652 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12014501898 Country of ref document: PH |
|
| ENP | Entry into the national phase |
Ref document number: 2013230020 Country of ref document: AU Date of ref document: 20130306 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IDP00201405259 Country of ref document: ID |
|
| ENP | Entry into the national phase |
Ref document number: 2014561075 Country of ref document: JP Kind code of ref document: A Ref document number: 2866590 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2014/010782 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013757652 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20147027965 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2014140371 Country of ref document: RU Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014022037 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014022037 Country of ref document: BR Kind code of ref document: A2 Effective date: 20140905 |