WO2012070639A1 - 神経細胞特異的な逆行性輸送ベクター - Google Patents
神経細胞特異的な逆行性輸送ベクター Download PDFInfo
- Publication number
- WO2012070639A1 WO2012070639A1 PCT/JP2011/077142 JP2011077142W WO2012070639A1 WO 2012070639 A1 WO2012070639 A1 WO 2012070639A1 JP 2011077142 W JP2011077142 W JP 2011077142W WO 2012070639 A1 WO2012070639 A1 WO 2012070639A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- gene
- vector
- vsv
- envelope
- kit
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
- A61K48/0058—Nucleic acids adapted for tissue specific expression, e.g. having tissue specific promoters as part of a contruct
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16041—Use of virus, viral particle or viral elements as a vector
- C12N2740/16045—Special targeting system for viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16071—Demonstrated in vivo effect
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/20011—Rhabdoviridae
- C12N2760/20111—Lyssavirus, e.g. rabies virus
- C12N2760/20122—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/20011—Rhabdoviridae
- C12N2760/20211—Vesiculovirus, e.g. vesicular stomatitis Indiana virus
- C12N2760/20222—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2810/00—Vectors comprising a targeting moiety
- C12N2810/50—Vectors comprising as targeting moiety peptide derived from defined protein
- C12N2810/60—Vectors comprising as targeting moiety peptide derived from defined protein from viruses
- C12N2810/6072—Vectors comprising as targeting moiety peptide derived from defined protein from viruses negative strand RNA viruses
- C12N2810/6081—Vectors comprising as targeting moiety peptide derived from defined protein from viruses negative strand RNA viruses rhabdoviridae, e.g. VSV
Definitions
- the present invention relates to a neuron-specific retrograde transport vector (NeuRet) system, that is, a highly retrograde transport ability, particularly in the brain, and high production efficiency.
- Vector system that enables selective gene transfer into the cell, more specifically, the N-terminal region of the extracellular domain of the rabies virus glycoprotein (RV-G) and the vesicular stomatitis virus glycoprotein (VSV- L) pseudotyped by a fusion polypeptide comprising a fusion extracellular domain comprising the C-terminal region of the extracellular domain of G), a transmembrane domain of RV-G or VSV-G, and an intracellular domain of VSV-G.
- the present invention relates to a virus vector system, a gene introduction method and a gene therapy method using the virus vector system, and the like.
- Non-proliferating (non-replicating) recombinant lentiviral vectors are used for gene therapy for various diseases such as a system in which a target gene is transported to non-dividing cells in the central nervous system (CNS) and maintained for a long period of time. It is used as a vector in many studies (Non-Patent Documents 1 to 4).
- primate lentiviral vectors derived from HIV-1 human immunodeficiency virus type 1
- Non-Patent Documents 5 to 8 are the most proven vectors for gene therapy.
- lentiviral vectors are integrated into the chromosome, it is known that there is a risk of carcinogenesis.
- leukemia develops in gene therapy for blood system diseases.
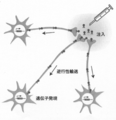
- the target is a cell body that is infected from a nerve terminal site, transported axons retrogradely, and located at a target site located away from the infected site.
- a viral vector into which a gene to be transferred can be introduced is useful (FIG. 1).
- Non-patent Document 9 Non-patent Document 9
- rabies virus is known to be infected from the synaptic terminal and transported axons retrogradely.
- RV rabies virus
- Non-patent Document 3 HIV-1 lentivirus pseudotyped with RV-G has been reported (Non-patent Document 3), no animal experiments (in vivo) using this viral vector have been actually conducted in this report.
- gene transfer in the CNS of an HIV-1 vector pseudotyped with glycoprotein of mocolala lyssavirus, which is a neurotropic virus causing rabies, or VSV-G has been studied.
- these vectors were comparable to each other in terms of retrograde transport to the olfactory nervous system (non- Patent Document 12).
- this document does not describe an example in which a viral vector is administered via the striatum.
- the present inventors have produced a pseudotyped HIV-1 lentivirus (RV-G / HIV-1 vector) with the rabies virus glycoprotein gene (RV-G) in the brain. It has been clarified that retrograde gene introduction can be carried out at a high frequency in various regions (Patent Document 2, Hum. Gene Ther., 2007). Furthermore, a fusion glycoprotein (FuG-B) in which the intracellular domain of RV-G was replaced with that of vesicular stomatitis virus glycoprotein (VSV-G) was prepared. Use this to construct a lentiviral vector system that retains highly efficient retrograde transport and has a higher titer (functional titer), and significantly increases the frequency of retrograde gene transfer. Successful (Hum. Gene Ther., 2010).
- NALDINI, L., BL Ö MER, U., GAGE, FH, TRONO, D., Oand VERMA, IM (1996). Efficient transfer, integration, integration, and sustained long-term expression of the transgene in adult rat brains injected with lentiviral vector. Proc. Natl. Acad. Sci. USA 93, 11382-11388. REISER, J., HARMISON, G., KLUEPFEL-STAHL, -S., BRADY, RO, KARLSSON, S., and SCHUBERT, M. (1996). Transduction of nondividing cells using pseudotyped defective high-titer HIparticles Proc. Natl. Acad. Sci.
- KORDOWER JH, EMBORG, ME, BLOCH, J., MA, SY, CHU, Y., LEVENTHAL, L., MCBRIDE, J., CHEN, E.-Y., PALFI, S., ROITBERG, BZ, BROWN , WD, HOLDEN, JE, PYZALSKI, R., TAYLOR, MD, CARVEY, P., LING, Z., TRONO, D., HANTRAYE, P., D É GLON, N., and AEBISCHER, P.
- Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery.
- RV-G and FuG-B vectors that show retrograde transport have the property of introducing genes into both neurons and glial cells at the injection site, and when these vector systems are used, neurons In addition, gene transfer also occurs in cell divisions.
- the object of the present invention is not only to enable gene transfer via high-frequency retrograde transport, but also by selective or specific gene transfer to nerve cells, thereby dividing cells such as glial cells and neural stem cells. To provide a safer lentiviral vector system with reduced risk of carcinogenesis and reduced safety.
- the present inventor has proposed a fusion extracellular domain comprising an N-terminal region of the extracellular domain of the rabies virus glycoprotein (RV-G) and a C-terminal region of the extracellular domain of the vesicular stomatitis virus glycoprotein (VSV-G).
- RV-G rabies virus glycoprotein
- VSV-G vesicular stomatitis virus glycoprotein
- the present invention relates to the following aspects.
- [Aspect 1] (1) a packaging plasmid containing HIV-1 gag and pol genes; (2) a packaging plasmid containing an accessory gene for HIV-1; (3) a transfer plasmid containing the gene of interest; and (4) as envelope genes, the N-terminal region of the extracellular domain of the rabies virus glycoprotein (RV-G) and the vesicular stomatitis virus glycoprotein (VSV-G).
- RV-G rabies virus glycoprotein
- VSV-G vesicular stomatitis virus glycoprotein
- An envelope plasmid comprising a gene encoding a fusion extracellular domain comprising the C-terminal region of the extracellular domain, a transmembrane domain of RV-G or VSV-G, and a fusion polypeptide comprising the intracellular domain of VSV-G,
- a kit for preparing a retrograde transportable virus vector comprising the kit for preparing a retrograde transportable virus vector.
- a kit for producing a producer cell comprising the kit for preparing the virus vector of aspect 1 and a host cell.
- a method for producing a producer cell which comprises co-transfecting an infected cell with a packaging plasmid, a transfer plasmid, and an envelope plasmid contained in the virus vector preparation kit of aspect 1.
- a method for producing a viral vector comprising culturing the producer cell of aspect 3 and collecting virus particles from the culture supernatant.
- a viral vector having a selective retrograde transport ability to nerve cells produced by the method of aspect 5.
- the virus vector of aspect 6 is infected to the nerve endings of an animal, and the virus vector is transported retrogradely through the nerve axons to selectively select the nerve cell body of the nerve in the target region in the brain.
- a gene introduction method comprising introducing and expressing a target gene in the nerve cell body.
- a gene therapy agent comprising the virus vector of aspect 6 as an active ingredient.
- a gene therapy method for brain disease which comprises incorporating and expressing a target gene introduced by the method of the aspect 7 method in a chromosome of a cell in a target region.
- a fusion extracellular domain comprising the N-terminal region of the extracellular domain of the rabies virus glycoprotein (RV-G) and the C-terminal region of the extracellular domain of the vesicular stomatitis virus glycoprotein (VSV-G), RV-G or An envelope for pseudotyping a lentiviral vector, comprising a fusion polypeptide comprising the transmembrane domain of VSV-G and the intracellular domain of VSV-G.
- An envelope plasmid comprising the gene of embodiment 11.
- a recombinant virus vector having a specific gene to be introduced is injected into a brain region where a nerve terminal (synaptic terminal) exists.
- the target gene is selectively transferred to a non-dividing cell in the central nervous system located far from the infection (injection) site of the viral vector. It was demonstrated in vivo that (transgene) can be efficiently introduced and expressed.
- a virus vector preparation kit using a specific packaging plasmid, transfer plasmid and envelope gene a virus vector having a virus titer higher than expected can be obtained. It is possible to produce a recombinant virus vector that exhibits a selective retrograde transport ability.
- the retrotransport virus vector of the present invention can suppress gene transfer into mitotic cells such as glial cells and neural stem cells, the risk of carcinogenesis is remarkably reduced, and gene transfer into cells other than nerve cells. And side effects caused by expression can be reduced.
- FIG. 1 An outline of an HIV-1 pseudotype vector showing high-frequency retrograde transport is schematically shown.
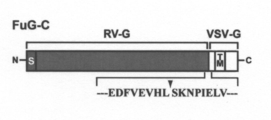
- the structure of FuG-C is shown.
- the extracellular domain C-terminal region derived from VSV-G is linked to the N-terminal region of the extracellular domain of RV-G, and further includes a transmembrane and intracellular domain derived from VSV-G.
- the connection site between RV-G and VSV-G is indicated by an arrow, and the amino acid sequence at the boundary is shown.
- S signal peptide
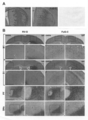
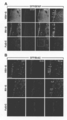
- TM transmembrane domain. It is a photograph which shows the expression pattern of a transgene.
- A Expression pattern in the striatum.
- M1 primary motor cortex
- S1 primary system sensory cortex
- PF parathalamic paranucleus
- SNc substantia nigra
- fr ream nucleo-leg
- SNr substantially nigra
- LV lateral ventricle
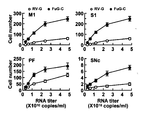
- Scale bar 500 ⁇ m It is a graph which shows the titer dependence of retrograde gene expression efficiency.
- the virus vector of the present invention is a neuron-specific retrograde transport vector (NeuRet) and is characterized by high titer.
- Such viral vectors can be prepared using the following preparation kit: (1) a packaging plasmid containing HIV-1 gag and pol genes; (2) a packaging plasmid containing an accessory gene for HIV-1; (3) a transfer plasmid containing the gene of interest; and (4) as envelope genes, the N-terminal region of the extracellular domain of the rabies virus glycoprotein (RV-G) and the vesicular stomatitis virus glycoprotein (VSV-G).
- RV-G rabies virus glycoprotein
- VSV-G vesicular stomatitis virus glycoprotein
- An envelope plasmid comprising a gene encoding a fusion extracellular domain comprising the C-terminal region of the extracellular domain, a transmembrane domain of RV-G or VSV-G, and a fusion polypeptide comprising the intracellular domain of VSV-G, A kit for preparing a retrograde transportable virus vector.
- “gag” is a gene encoding a retrovirus core protein
- “pol” is a gene encoding a reverse transcriptase or the like.
- the “envelope gene” is a gene encoding an envelope, which is a virus-specific protein present in the envelope, which is the outer membrane of a retrovirus composed of a lipid bilayer membrane.
- the envelope plays an important role for the virus to adsorb and enter cells.
- the “accessory gene” means, for example, a rev gene that regulates the expression of a structural gene.
- the transfer plasmid encodes the target gene “X” to be introduced downstream of the mouse stem cell virus promoter.
- Each of the plasmids included in the virus vector preparation kit is an HIV-1 vector system “SJ1” (HANAWA, H., et al., (2002) Mol, developed by Dr. Arthur Nienhuis of St. Jude Children's Research Hospital. Ther. 5, 242-251; (2004). Blood 103, 4062-4069 (provided by St. Jude Children's Research Hospital).
- This vector system is known to have a titer in HeLa cells of about 10 times that of other vector systems. Therefore, those skilled in the art can easily prepare each of these plasmids by referring to the present specification and the above-mentioned documents.
- the packaging plasmids (1) and (2) above may be constructed as a single plasmid.
- the envelope gene contained in the envelope plasmid of the kit for preparing the virus vector of the present invention includes the N-terminal region of the extracellular domain of the rabies virus glycoprotein (RV-G) and the cell of the glycoprotein ⁇ (VSV-G) of the vesicular stomatitis virus It encodes a fusion polypeptide comprising a fusion extracellular domain consisting of the C-terminal region of the outer domain, a transmembrane domain of RV-G or VSV-G, and an intracellular domain of VSV-G.
- RV-G rabies virus glycoprotein
- VSV-G glycoprotein ⁇
- a fusion polypeptide comprising a fusion extracellular domain consisting of the C-terminal region of the outer domain, a transmembrane domain of RV-G or VSV-G, and an intracellular domain of VSV-G.
- one or a plurality of amino acids can be appropriately changed by deletion, insertion, substitution, etc., and it is not
- the N-terminal region (including signal peptide) of the extracellular domain of the rabies virus glycoprotein (RV-G) constituting the fused extracellular domain is originally a rabies virus consisting of 458 amino acids.
- an envelope gene encoding a fusion polypeptide (FuG-C) consisting of the amino acid sequence shown in SEQ ID NO: 2 is preferred, preferably an N′-side 1 shown in SEQ ID NO: 1 (FIG. 7).
- ⁇ 1,365th base sequence in SEQ ID NO: 1, 5 ′ side 1st to 1,317th base (including start codon) is derived from RV-G
- 3 ′ side 1,318th to 1365th base is derived from VSV-G
- a nucleic acid molecule having In consideration of codon degeneracy the base sequence can be appropriately changed so as to be an optimal codon according to other elements in the envelope plasmid. Examples of the amino acid sequence of the rabies virus glycoprotein (RV-G) and the base sequence encoding it are shown in SEQ ID NO: 4 and SEQ ID NO: 3.
- the above fusion polypeptide is effective as an envelope for pseudotyping various lentiviral vectors, particularly HIV-1 lentiviral vectors.
- the present invention also relates to an envelope for pseudotyping a lentiviral vector comprising the fusion polypeptide, a gene encoding the envelope comprising the fusion polypeptide, and the envelope plasmid itself containing the gene.
- each gene is linked under the expression control of any expression regulatory sequence known to those skilled in the art.
- “Under expression control” means that a DNA encoding a predetermined amino acid sequence has the ability to express a protein having the amino acid sequence under predetermined conditions.
- expression control sequence means a nucleic acid sequence that regulates the expression of other nucleic acid sequences, and also controls and regulates the transcription and preferably translation of other nucleic acid sequences. Expression control sequences include appropriate promoters, enhancers, transcription terminators, initiation codons (ie, ATG) in the gene encoding the protein, splicing signals for introns, polyadenylation sites, and stop codons.
- Promoter means the minimum sequence necessary for transcription.
- the promoter also includes a promoter element that controls the expression of a gene in a promoter-dependent manner by a cell type-specific, tissue-specific, or external signal or regulator.
- the promoter element is linked to either the 5 ′ region or the 3 ′ region of the expressed DNA. Promoters include both constitutive and inducible promoters.
- a promoter known to those skilled in the art can be appropriately selected according to the type of target gene and viral vector to be used, the type of animal and brain disease to be treated, the pathology of the patient, and the like.
- the envelope gene is preferably ligated so that it is expressed under the control of the cytomegalovirus enhancer and the avian ⁇ -actin promoter.
- an envelope plasmid is the envelope domain “pCAGGS-VSV-G” contained in the vector system “SJ1”, and the extracellular domain and membrane of the vesicular stomatitis virus glycoprotein ⁇ (VSV-G) Nucleic acid encoding the rabies virus CVS glycoprotein (RV-G) (provided by Dr.
- the glycoprotein (RV-G) of the rabies virus CVS strain is not limited to the one having the base sequence shown in SEQ ID NO: 3 above, but is a glycoprotein (RV-G) derived from any other known rabies virus strain. ) Can be used.
- the target gene contained in the transfer plasmid can be appropriately selected from those known to those skilled in the art according to the intended use of the viral vector, the type of animal and brain disease to be treated, the patient's condition, etc. . Therefore, various genes of mammals such as mice, monkeys and humans, for example, genes necessary for the survival or protection of the nigrostriatal system used for the treatment of cranial nerve diseases or neurodegenerative diseases represented by Parkinson's disease (For example, tyrosine hydroxylase, glial cell line-derived neurotrophic factor), or interleukin 2 receptor ⁇ subunit (recombinant immunotoxin target molecule) and light-gated ion channel for cranial nervous system research You can list genes.
- Parkinson's disease For example, tyrosine hydroxylase, glial cell line-derived neurotrophic factor), or interleukin 2 receptor ⁇ subunit (recombinant immunotoxin target molecule) and light-gated ion channel for cranial nervous system research You can list genes
- Host cells included in the producer cell preparation kit of the present invention can be infected by the above-described virus vector preparation kit, and as a result, cells that produce retroviral particles called “producer cells” can be prepared.
- producer cells There is no particular limitation as long as it is a cell, and any cell known to those skilled in the art, for example, HEK293T cells (in which SV40 large T antigen has been introduced) or other suitable animal-derived cells, should be used. Can do.
- kits of the present invention include, for example, various reagents, buffers, various auxiliary agents, reaction plates (containers), etc., in addition to the plasmids and / or host cells, depending on the configuration and purpose of use.
- Other elements or components known to those skilled in the art can be included as appropriate.
- a producer cell can be prepared by co-transfecting the packaging plasmid, transfer plasmid, and envelope plasmid contained in the virus vector preparation kit into an infected cell. I can do it. This transfection is transient and can be performed by any method known to those skilled in the art, such as the calcium phosphate method.
- the producer cells thus obtained are cultured by any method / means known to those skilled in the art, and virus particles are collected from the culture supernatant, thereby providing a selective or specific retrograde transport ability to neurons in the brain.
- a virus vector having a high titer can be produced.
- the virus vector of the present invention is infected with the nerve endings of animals, and the virus vector is transported retrogradely through the nerve axons to selectively select the nerve cell body of the nerve in the target region in the brain.
- the target regions in the brain where the target gene can be expressed and expressed in the nerve cell body are, for example, primary motor cortex, primary somatosensory cortex, thalamic bundle paranucleus, substantia nigra that project to the striatum
- the central part of the brain such as the piriform cortex that projects into the ventral striatum (the nucleus accumbens), the sigmoid gyrus, the basolateral nucleus of the amygdala, the paraventricular nucleus, the dorsolateral nucleus of the thalamus, and the lateral hypothalamus It is.
- the viral vectors of the present invention are retrogradely transported through the axons of spinal motor neurons.
- the virus vector of the present invention is effective as an active ingredient of a gene therapy agent.
- the gene therapy agent can include other ingredients such as any pharmaceutical carrier or diluent known to those skilled in the art, in combination with the active ingredient, which are pharmaceutically acceptable.
- the effective amount of the active ingredient of the present invention is the type of transgene contained in the viral vector, the type / seriousness of brain disease or neurodegenerative disorder, treatment policy, patient age, weight, sex, general health condition Depending on the (genetic) racial background of the patient, those skilled in the art can select as appropriate.
- the dose of the active ingredient (virus vector) can be, for example, about a total amount of 10 8 to 10 9 TU (Transducing Unit) at several infection (injection) sites per administration.
- the virus vector or gene therapy agent can be infected (injected) into a predetermined site of a patient using any administration method / device known to those skilled in the art.
- the gene introduced into the nerve cell in the target region is integrated into the chromosome of the nerve cell, and the target gene is stably expressed. Therefore, gene therapy such as brain disease or neurodegenerative disease (for example, Parkinson's disease) in mammals including primates such as humans can be carried out using this gene transfer method.
- gene therapy such as brain disease or neurodegenerative disease (for example, Parkinson's disease) in mammals including primates such as humans can be carried out using this gene transfer method.
- Virus vector preparation The viral vectors of the present invention were prepared utilizing the HIV-1 vector system developed by Dr. Arthur Nienhuis of St. Jude Children's Research Hospital. That is, a packaging plasmid (pCAGkGP1.1R) containing gag and pol genes, a packaging plasmid (pCAG4-RTR2) containing an accessory gene, and a transfer plasmid (pCL20c-MSCV-) containing green fluorescent protein (GFP) as a target gene GFP) was used.
- pCAGkGP1.1R containing gag and pol genes
- pCAG4-RTR2 packaging plasmid
- pCL20c-MSCV- transfer plasmid containing green fluorescent protein (GFP) as a target gene GFP
- the envelope plasmid (pCAG-FuG-C: SEQ ID NO: 5) is an extracellular domain derived from VSV-G in the N-terminal region of the extracellular domain of the RV-G gene provided by Prof. Kinjiro Morimoto of National Institute of Infectious Diseases.
- a fused glycoprotein (FuG-C) was prepared by linking the C-terminal region and connecting the VSV-G transmembrane and intracellular domains (FIG. 2).
- the extracellular domain of FuG-C is composed of a 439-amino acid RV-G-derived extracellular domain N-terminal region and a 16-amino acid VSV-G-derived extracellular domain C-terminal region.
- comparative virus vectors were prepared using VSV-G and RV-G as envelope plasmids.
- Virus titer measurement Viral vector solutions containing these plasmids were transfected into HEK293T cells (10-cm dishes, 18 plates) using the calcium phosphate method. After culturing for 48 hours, virus particles were collected from the culture supernatant, centrifuged, and filtered through a 0.45 ⁇ m cellulose filter. The vector particles were then recovered by centrifugation (10,000 Xg, 16-18 hours) and suspended in 1 ml PBS. The suspension was subjected to Sepharose Q FF ion exchange column chromatography, washed with PBS, and eluted with a linear gradient of 0-1.5 M NaCl, and the fraction was monitored by absorbance at 260/280 nm. Fractions containing vector particles were collected, concentrated using an ultrafiltration filter, and stored at -80 ° C.
- cultured cells that can be easily obtained / purchased from public institutions, such as human kidney cells: HEK293T (available from RIKEN cell bank, deposit number: RCB2202), mouse nerve Blast cells: Neuro2A (available for purchase from ATCC, ID number: CCL-131TM), mouse neuroblasts: N1E-115 (available for purchase from ATCC, ID number: CRL-2263TM) for 6-well cell culture plate: MULTIWELL ( Registered trademark) FALCON), the cultured cells were infected with an appropriate concentration of virus solution, and the functional titer was measured using FACS Calibur (Nippon Becton Dickinson Co., Tokyo, Japan) 3 days after transformation. Next, the RNA level contained in the vector stock was measured using a quantitative RT-PCR method.
- mice Four weeks after injection, the mice were deeply anesthetized with sodium pentobarbital (50 mg / kg, ip) and perfusion-fixed with 4% formalin and 0.1 M phosphate buffer (PB: pH 7.4) via the heart. The brain was removed. Sections were prepared using a cryostat and analyzed using immunostaining.
- mice injected with the FuG-C vector an immunopositive signal was observed in a wide area of the striatum, but the intensity was significantly reduced as compared to the RV-G vector (FIG. 3A).
- the expression of transgenes in primary motor cortex (M1), primary somatosensory cortex (S1), parathalamic paraffin (PF), substantia nigra (SNc) Were analyzed by immunostaining.
- the cerebral cortex was observed on the ipsilateral and opposite brain regions of the injection site, and the PF and SNc were observed on the ipsilateral brain region (FIG.
- GFP + / NeuN + double positive cells per total NeuN + cells and GFP per total GFAP + cells
- the percentage of the number of + / GFAP + double positive cells was determined.
- the gene transfer efficiency of the FuG-C vector into striatal neurons was significantly reduced compared to other vectors (ANOVA, Tukey HSD, p ⁇ 0.001 vs VSV-G, p ⁇ 0.01 vs RV-G).
- VSV-G vectors are known to show high gene transfer efficiency into neural stem cells.
- the gene transfer characteristics of various vectors into neural stem cells localized in the periventricular region (SVZ) were analyzed.
- a viral vector solution (1.2 ⁇ 10 10 copies / ml) was injected into mouse SVZ, brain sections were prepared, and GFAP was used as a marker of neural stem cells, and double immunostaining of GFAP and GFP was performed (FIG. 6A). .
- VSV-G or RV-G vector was injected, transgene expression was observed in many GFAP-positive neural stem cells in SVZ.
- the FuG-C vector was used, gene transfer into these neural stem cells was hardly observed.
- a transverse section for mice: 30 ⁇ m thick was prepared using a cryostat, and a rabbit anti-GFP polyclonal antibody (Molecular Probes, Eugene, OR: 1: 2,000 dilution) and further with a biotinylated goat anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA: 1: 1,000 dilution). Immune response signals were visualized with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA).
- the sections were incubated with one of the above rabbit anti-GFP polyclonal antibody and anti-choline acetyltransferase mouse antibody (Chemicon, Temecula, CA: 1: 100 dilution). The sections were then incubated with FITC-conjugated goat anti-rabbit IgG and Cy3-conjugated donkey anti-mouse antibody (1: 500 dilution, Jackson, Immunoresearch Laboratories, West West Groove, PA). Fluorescence images were captured under a confocal laser scanning microscope (LSM510, Zeiss, Thornwood, NY) with appropriate filter cube specifications for FITC and Cy3 fluorescence channels. These fluorescence images were taken with a high-quality CCD camera system adjusted by the Zeiss Axiovision software package.
- LSM510 Zeiss, Thornwood, NY
- the retrograde transport virus vector of the present invention enables selective gene introduction into nerve cells mainly through retrograde transport, while the introduction into mitotic cells including glial cells and neural stem cells is remarkable. It is suppressed.
- This vector can significantly reduce the risk of carcinogenesis in the central nervous system, reduce side effects caused by non-specific gene expression outside of nerve cells, and is effective for gene therapy of cranial nerve diseases such as Parkinson's disease and Provide highly safe technology.
- the present invention provides an effective and highly safe experimental technique for gene therapy of cranial nerve disease and a technique for creating a disease state model.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- Virology (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Microbiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Plant Pathology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Physics & Mathematics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Epidemiology (AREA)
- Psychology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/988,973 US9006411B2 (en) | 2010-11-26 | 2011-11-25 | Neuron-specific retrograde transport vector |
| EP11843579.1A EP2644697B1 (en) | 2010-11-26 | 2011-11-25 | Neuron-specific retrograde transport vector |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010263148A JP5216072B2 (ja) | 2010-11-26 | 2010-11-26 | 神経細胞特異的な逆行性輸送ベクター |
| JP2010-263148 | 2010-11-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012070639A1 true WO2012070639A1 (ja) | 2012-05-31 |
Family
ID=46145976
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/077142 Ceased WO2012070639A1 (ja) | 2010-11-26 | 2011-11-25 | 神経細胞特異的な逆行性輸送ベクター |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US9006411B2 (enExample) |
| EP (1) | EP2644697B1 (enExample) |
| JP (1) | JP5216072B2 (enExample) |
| WO (1) | WO2012070639A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN119639818A (zh) * | 2025-02-18 | 2025-03-18 | 南京市计量监督检测院 | 一种假病毒颗粒的制备方法 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4587580A2 (en) * | 2022-09-12 | 2025-07-23 | The Board of Trustees of the Leland Stanford Junior University | Composition and method of universal pseudotyped retroviruses |
| CN117867029B (zh) * | 2023-12-29 | 2024-12-20 | 复百澳(苏州)生物医药科技有限公司 | 一种狂犬亚型假病毒的制备方法及应用 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004517057A (ja) * | 2000-11-03 | 2004-06-10 | オックスフォード バイオメディカ(ユーケイ)リミテッド | ベクターシステム |
| JP2009034029A (ja) * | 2007-08-01 | 2009-02-19 | Japan Science & Technology Agency | 逆行性輸送能を有するウィルスベクター系 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030124146A1 (en) | 2000-01-28 | 2003-07-03 | Schnell Matthias J. | Recombinant Rhabdoviruses as live-viral vaccines |
| JP5123922B2 (ja) * | 2009-12-02 | 2013-01-23 | 独立行政法人科学技術振興機構 | 融合糖タンパク質から成るエンベロープを有する逆行性輸送ウィルスベクター系 |
-
2010
- 2010-11-26 JP JP2010263148A patent/JP5216072B2/ja not_active Expired - Fee Related
-
2011
- 2011-11-25 US US13/988,973 patent/US9006411B2/en not_active Expired - Fee Related
- 2011-11-25 WO PCT/JP2011/077142 patent/WO2012070639A1/ja not_active Ceased
- 2011-11-25 EP EP11843579.1A patent/EP2644697B1/en not_active Not-in-force
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004517057A (ja) * | 2000-11-03 | 2004-06-10 | オックスフォード バイオメディカ(ユーケイ)リミテッド | ベクターシステム |
| JP2009034029A (ja) * | 2007-08-01 | 2009-02-19 | Japan Science & Technology Agency | 逆行性輸送能を有するウィルスベクター系 |
Non-Patent Citations (6)
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN119639818A (zh) * | 2025-02-18 | 2025-03-18 | 南京市计量监督检测院 | 一种假病毒颗粒的制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20130315872A1 (en) | 2013-11-28 |
| EP2644697A4 (en) | 2014-05-14 |
| US9006411B2 (en) | 2015-04-14 |
| EP2644697A1 (en) | 2013-10-02 |
| JP5216072B2 (ja) | 2013-06-19 |
| JP2012110290A (ja) | 2012-06-14 |
| EP2644697B1 (en) | 2018-12-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5123922B2 (ja) | 融合糖タンパク質から成るエンベロープを有する逆行性輸送ウィルスベクター系 | |
| Kato et al. | Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein | |
| Mazarakis et al. | Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery | |
| Kato et al. | A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein | |
| Desmaris et al. | Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins | |
| Cronin et al. | Altering the tropism of lentiviral vectors through pseudotyping | |
| Watson et al. | Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins | |
| Kato et al. | Neuron-specific gene transfer through retrograde transport of lentiviral vector pseudotyped with a novel type of fusion envelope glycoprotein | |
| ES2997865T3 (en) | Adapter-based retroviral vector system for the selective transduction of target cells | |
| Carpentier et al. | Enhanced pseudotyping efficiency of HIV-1 lentiviral vectors by a rabies/vesicular stomatitis virus chimeric envelope glycoprotein | |
| JP2008503230A (ja) | 非組み込み型且つ非複製型組換えレンチウイルス、その調製および使用 | |
| CA2839633C (en) | Peptides with viral infection enhancing properties and their use | |
| JP2008303215A (ja) | ベクターシステム | |
| Kobayashi et al. | Altering entry site preference of lentiviral vectors into neuronal cells by pseudotyping with envelope glycoproteins | |
| JP5216072B2 (ja) | 神経細胞特異的な逆行性輸送ベクター | |
| Loewen et al. | Lentiviral vectors | |
| JP2009034029A (ja) | 逆行性輸送能を有するウィルスベクター系 | |
| US7749973B2 (en) | Methods of gene transfer to astrocytes with mokola virus pseudotyped lentivirus vectors | |
| Trabalza et al. | Enhanced central nervous system transduction with lentiviral vectors pseudotyped with RVG/HIV-1gp41 chimeric envelope glycoproteins | |
| Liehl et al. | Simian immunodeficiency virus vector pseudotypes differ in transduction efficiency and target cell specificity in brain | |
| US20090233988A1 (en) | Therapeutic Agents for Diseases Associated With Apoptotic Degeneration in Ocular Tissue Cells That Use SIV-PEDF Vectors | |
| US20020168760A1 (en) | Retroviral vectors for gene transfer into neuronal cells | |
| Kato et al. | Highly efficient retrograde gene transfer for genetic treatment of neurological diseases | |
| Jakobsson et al. | Lentiviral vectors | |
| Eleftheriadou et al. | Lentiviral vectors for gene delivery to the nervous system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11843579 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011843579 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13988973 Country of ref document: US |