WO2012066922A1 - Gas manufacturing apparatus, gas manufacturing method, and gas manufacturing apparatus array - Google Patents
Gas manufacturing apparatus, gas manufacturing method, and gas manufacturing apparatus array Download PDFInfo
- Publication number
- WO2012066922A1 WO2012066922A1 PCT/JP2011/074971 JP2011074971W WO2012066922A1 WO 2012066922 A1 WO2012066922 A1 WO 2012066922A1 JP 2011074971 W JP2011074971 W JP 2011074971W WO 2012066922 A1 WO2012066922 A1 WO 2012066922A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- photoelectric conversion
- gas
- electrolysis electrode
- electrode
- electrolysis
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/50—Processes
- C25B1/55—Photoelectrolysis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Definitions
- the present invention relates to a gas production apparatus, a gas production method, and a gas production apparatus array.
- renewable energy In recent years, the use of renewable energy is desired from the viewpoint of depletion of fossil fuel resources and the suppression of global warming gas emissions.
- renewable energy sources such as sunlight, hydropower, wind power, geothermal power, tidal power, and biomass.
- sunlight has a large amount of available energy, and there are geographical restrictions on other renewable energy sources. Because of the relatively small amount, early development and popularization of technology that can efficiently use energy from sunlight is desired.
- Possible forms of energy generated from sunlight include electrical energy produced using solar cells and solar thermal turbines, thermal energy by collecting solar energy in a heat medium, and other types of sunlight.
- Examples include storable fuel energy such as liquid fuel and hydrogen by substance reduction.
- Many solar cell technologies and solar heat utilization technologies have already been put into practical use, but the energy utilization efficiency is still low, and the cost of producing electricity and heat is still high. Technology development is underway.
- these forms of electricity and heat can be used to supplement short-term energy fluctuations, it is extremely difficult to supplement long-term fluctuations such as seasonal fluctuations, It is a problem that there is a possibility that the operating rate of the power generation equipment may be reduced due to the increase in power generation.
- storing energy as a substance, such as liquid fuel and hydrogen is extremely effective as a technology that efficiently supplements long-term fluctuations and increases the operating rate of power generation facilities. It is an indispensable technology to raise and reduce carbon dioxide emissions thoroughly.
- liquid fuels such as hydrocarbons
- gaseous fuels such as biogas and hydrogen
- solid pellets such as biomass-derived wood pellets and metals reduced by sunlight. It can.
- liquid fuel, gaseous fuel including hydrogen in terms of total utilization efficiency improvement with fuel cells, etc. solid fuel in terms of storability and energy density
- a hydrogen production technique by decomposing water with sunlight has attracted particular attention from the viewpoint that water that can be easily obtained as a raw material can be used.
- platinum is supported on a photocatalyst such as titanium oxide, and this substance is put in water to perform light separation in a semiconductor, and an electrolytic solution.
- the water is decomposed directly at high temperature using the photolysis method by reducing protons and oxidizing water, or by using thermal energy such as a high-temperature gas furnace, or indirectly by coupling with redox of metals, etc.
- Pyrolysis method that uses the metabolism of microorganisms that use light such as algae
- water electrolysis method that combines electricity generated by solar cells and water electrolysis hydrogen production equipment
- photoelectric conversion used in solar cells Examples of the method include a photovoltaic method in which electrons and holes obtained by photoelectric conversion are used in a reaction by a hydrogen generation catalyst and an oxygen generation catalyst by supporting a hydrogen generation catalyst and an oxygen generation catalyst on the material.
- the photolysis method the one that has the possibility of producing a small hydrogen production device by integrating the photoelectric conversion unit and the hydrogen generation unit is considered to be a photolysis method, a biological method, a photovoltaic method
- the photovoltaic method is considered to be one of the technologies closest to practical use.
- Patent Document 1 a titanium oxide photocatalyst electrode on which a ruthenium complex is adsorbed and a platinum electrode, an apparatus using oxidation reduction of iodine or iron is disclosed.
- Patent Documents 2 and 3 an integrated structure is adopted by connecting two layers of photocatalysts in tandem, connecting a platinum counter electrode, and sandwiching an ion exchange membrane therebetween.
- Non-Patent Document 1 a concept of a hydrogen production apparatus in which a photoelectric conversion unit, a hydrogen generation unit, and an oxygen generation unit are integrated has been announced (Non-Patent Document 1). According to this, charge separation is performed by using a photoelectric conversion unit, and hydrogen generation and oxygen generation are performed using corresponding catalysts.
- the photoelectric conversion part is made of a material used for solar cells. For example, in Non-Patent Document 2, after charge separation is performed with three silicon pin layers, a platinum catalyst is responsible for hydrogen generation and ruthenium oxide is responsible for oxygen generation.
- Non-Patent Document 3 a multi-junction photoelectric conversion material that absorbs light of different wavelengths is used by using Pt as a hydrogen generation catalyst and RuO 2 as an oxygen generation catalyst to achieve high efficiency.
- Patent Document 4 and Non-Patent Document 3 a hydrogen generation catalyst (NiFeO) and three layers of silicon pin are stacked in parallel on a substrate, and an oxygen generation catalyst (Co-Mo) is further formed on the silicon layer. ) To produce an integrated hydrogen production apparatus.
- the gas generated from the electrode for electrolysis is recovered as bubbles in the electrolytic solution, so that two gases generated from the two electrodes for electrolysis are used.
- the recovery ports are close to each other, and the piping is complicated.
- This invention is made
- the present invention includes a photoelectric conversion unit having a light receiving surface and a back surface thereof, and first and second electrodes for electrolysis that are provided side by side on the back surface and each have a surface that can contact an electrolytic solution, When the first and second electrolysis electrodes come into contact with the electrolytic solution, the first and second electrolysis electrodes use the electromotive force generated by the photoelectric conversion unit to receive light to electrolyze the electrolytic solution, respectively. When the light receiving surface of the photoelectric conversion unit is leveled, the first electrolysis electrode and the second electrolysis electrode are horizontal with the surface that can come into contact with the electrolytic solution.
- a gas production apparatus characterized in that an inclination angle with respect to a reference plane is different.
- the first and second electrolysis electrodes are configured to electrolyze the electrolytic solution using the electromotive force generated by the light received by the photoelectric conversion unit to generate the first gas and the second gas, respectively. Since it is provided, the first gas can be generated on the surface of the first electrolysis electrode, and the second gas can be generated on the surface of the second electrolysis electrode. According to the present invention, since the first electrolysis electrode and the second electrolysis electrode are provided on the back surface of the photoelectric conversion portion, light can be incident on the light receiving surface of the photoelectric conversion portion without passing through the electrolyte solution. It is possible to prevent absorption of incident light and scattering of incident light.
- the amount of incident light to the photoelectric conversion unit can be increased, and the light use efficiency can be increased.
- the first electrolysis electrode and the second electrolysis electrode are provided on the back surface of the photoelectric conversion unit, the light incident on the light receiving surface is generated from the first and second electrolysis electrodes, respectively. It is not absorbed or scattered by the first gas and the second gas. As a result, the amount of incident light to the photoelectric conversion unit can be increased, and the light use efficiency can be increased.
- the first electrolysis electrode and the second electrolysis electrode have different inclination angles between the surface that can come into contact with the electrolytic solution and the horizontal reference surface, so that the first gas rises as bubbles.
- the gas production apparatus when the gas production apparatus is provided with a plurality of at least one of the first electrolysis electrode and the second electrolysis electrode, or when a plurality of gas production apparatuses are installed side by side, a plurality of first gas recovery ports are provided.
- the piping for connecting and the piping for connecting the plurality of second gas recovery ports can be provided without overlapping, and the piping can be simplified. Thereby, the installation cost when providing piping for recovering the first gas and the second gas can be reduced, and piping connection errors and the like can be prevented.
- the first electrolysis electrode and the second electrolysis electrode have different inclination angles between the surface that can contact the electrolytic solution and the horizontal reference surface, so that the light receiving surface of the photoelectric conversion unit is substantially horizontal. It is also possible to install so that the installation cost can be reduced. Moreover, it becomes possible to install a gas production apparatus on the surface of a pond or the sea.
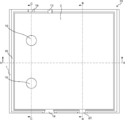
- FIG. 2 is a schematic cross-sectional view of the gas production apparatus taken along one-dot chain line AA in FIG.
- FIG. 2 is a schematic cross-sectional view of the gas production apparatus taken along a dotted line BB in FIG.
- FIG. 2 is a schematic cross-sectional view of a gas production apparatus taken along a dotted line CC in FIG.
- It is a schematic back view which shows the structure of the gas manufacturing apparatus of one Embodiment of this invention.
- It is a schematic sectional drawing which shows the structure of the gas manufacturing apparatus of one Embodiment of this invention.
- FIG. 18 is a schematic cross-sectional view of the gas production apparatus taken along dotted line AA in FIG. It is a schematic plan view which shows the structure of the gas manufacturing apparatus array of one Embodiment of this invention.
- the gas production apparatus of the present invention includes a photoelectric conversion unit having a light receiving surface and a back surface thereof, first and second electrodes for electrolysis that are provided side by side on the back surface and each have a surface that can contact an electrolyte solution
- first and second electrolysis electrodes electrolyze the electrolytic solution using electromotive force generated by the photoelectric conversion unit receiving light.
- the first electrolysis electrode and the second electrolysis electrode can contact the electrolyte when the first gas and the second gas are generated, respectively, and the light receiving surface of the photoelectric conversion unit is horizontal. The inclination angle between the surface and the horizontal reference surface is different.
- the inclination angle is an inclination angle with respect to a horizontal reference plane, and is an angle in a range of 0 degrees to 180 degrees.
- the first electrode and the second electrode are electrodes for outputting photovoltaic power of the photoelectric conversion unit, and the first electrolysis electrode and the second electrolysis electrode are for electrolyzing the electrolytic solution. Electrode.
- a first gas discharge port provided close to the upper end of the surface of the first electrolysis electrode that can contact the electrolytic solution
- the second electrolysis electrode may have a surface that can contact the electrolyte solution that is inclined so that the second gas can be recovered from the second gas discharge port. preferable. According to such a configuration, the first gas can be recovered from the first gas outlet, and the second gas can be recovered from the second gas outlet.
- the photoelectric conversion unit has a rectangular light receiving surface, and the first and second gas discharge ports are provided close to opposite sides of the light receiving surface of the photoelectric conversion unit, respectively.
- the first gas outlet and the second gas outlet can be provided on both sides of the photoelectric conversion unit, and the pipe for recovering the first gas and the pipe for recovering the second gas Can be simplified.
- At least one of the first and second electrolysis electrodes has a plurality of surfaces that can contact the strip-shaped electrolyte solution, and the long sides of the surfaces are adjacent to each other. It is preferable that they are provided alternately. According to such a configuration, the distance between the portion where the reaction generating the first gas occurs and the portion where the reaction generating the second gas occurs can be shortened, and the ion concentration generated in the electrolyte can be reduced. Imbalance can be reduced. Moreover, the 1st gas and 2nd gas can be collect
- the first mold part and the second electrolysis electrode provided between the first electrolysis electrode and the back surface of the photoelectric conversion unit and the back surface of the photoelectric conversion unit are provided.
- the first mold part and the second mold part have a horizontal reference with a surface that can contact the electrolyte of the first electrolysis electrode when the light receiving surface of the photoelectric conversion unit is horizontal. It is preferable that the tilt angle between the reference surface and the tilt angle between the surface and the reference surface is different from the tilt angle between the second electrolysis electrode and the electrolyte.
- the inclination angle between the surface of the first electrolysis electrode that can contact the electrolytic solution and the horizontal reference surface, the surface of the second electrolysis electrode that can contact the electrolytic solution, and the reference can be easily made different.
- the first mold part and the second mold part are preferably made of a solid resin. According to such a configuration, the first mold part and the second mold part can be easily molded.
- a first conductive part is further provided between the first mold part and the first electrolysis electrode or between the second mold part and the second electrolysis electrode. According to such a configuration, when the electromotive force generated by the photoelectric conversion unit receiving light is output to the first and second electrolysis electrodes, the internal resistance can be further reduced.
- each of the first and second electrolysis electrodes preferably has a groove-like depression extending in an inclined direction on a surface that can contact the electrolytic solution.
- the first gas and the second gas can be raised as bubbles along the groove-shaped depression, and the first gas and the second gas can be separated and recovered.
- the partition provided between the electrode for 1st electrolysis and the electrode for 2nd electrolysis is also omissible.
- the first electrolysis electrode when the light receiving surface of the photoelectric conversion unit is horizontal, the first electrolysis electrode can contact an electrolytic solution having an inclination angle of 1 degree to 60 degrees with the horizontal reference plane It is preferable that the second electrolysis electrode has a surface that can contact an electrolytic solution having an inclination angle of 120 degrees or more and 179 degrees or less with respect to the reference surface.
- a recovery port for recovering the first gas and a recovery port for recovering the second gas can be provided on both sides of the gas production device, respectively, in order to recover the first gas.
- a pipe for recovering the second gas can be provided on both sides of the gas production apparatus.
- the photoelectric conversion unit receives light to generate an electromotive force between the light receiving surface and the back surface, and the first electrolysis electrode is electrically connected to the back surface of the photoelectric conversion unit. And it is preferable that the 2nd electrode for electrolysis is electrically connected with the light-receiving surface of the said photoelectric conversion part. According to such a structure, the thing of a laminated structure can be utilized for a photoelectric conversion part.
- the gas production apparatus further includes a first electrode that contacts the light receiving surface of the photoelectric conversion unit. According to such a configuration, the internal resistance can be reduced.
- the second conductive portion is provided in a contact hole that penetrates the photoelectric conversion portion. According to such a configuration, the wiring distance between the light receiving surface of the photoelectric conversion unit and the second electrolysis electrode can be shortened, and the internal resistance can be reduced.
- the gas manufacturing apparatus of the present invention includes a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part, the second mold part having insulating properties, and the photoelectric conversion unit. It is preferable that the second conductive portion is provided on a portion of the second mold portion that covers the side surface of the photoelectric conversion portion. According to such a configuration, the second conductive portion can be provided with a small number of steps, and the manufacturing cost can be reduced.
- the gas manufacturing apparatus of the present invention includes a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part, the second mold part having insulating properties, and the photoelectric conversion unit.
- the second electrolysis electrode is preferably provided on a portion of the second mold part that covers the side surface of the photoelectric conversion unit, and is in contact with the first electrode. According to such a configuration, the first electrode and the second electrolysis electrode can be electrically connected without providing the second conductive portion.
- the first mold part provided between the first electrolysis electrode and the back surface of the photoelectric conversion unit, and the second electrolysis electrode and the back surface of the photoelectric conversion unit are provided.
- a second electrode provided between the back surface of the photoelectric conversion unit and the first mold unit and between the back surface of the photoelectric conversion unit and the second mold unit.
- the internal resistance between the back surface of the photoelectric conversion unit and the first electrolysis electrode Can be made smaller.
- the third conductive portion is provided so as to cover the side portion of the first mold portion and is in contact with the second electrode.
- the back surface of a photoelectric conversion part and the 1st electrode for electrolysis can be electrically connected easily.
- the photoelectric conversion unit generates a potential difference between the first and second areas on the back surface by receiving light, the first area is electrically connected to the first electrolysis electrode, The second area is preferably electrically connected to the second electrolysis electrode. According to such a configuration, an electromotive force can be generated between the first area and the second area in the photoelectric conversion unit.
- the photoelectric conversion part is made of at least one semiconductor material having an n-type semiconductor part and a p-type semiconductor part, and one of the first and second areas is the n-type semiconductor part. It is preferable that the other part is a part of the p-type semiconductor part. According to such a configuration, an electromotive force can be generated between the first and second areas on the back surface of the photoelectric conversion unit when the photoelectric conversion unit receives light.
- a translucent substrate is further provided, and the photoelectric conversion unit is provided on the translucent substrate. According to such a structure, a photoelectric conversion part can be formed on a translucent board
- the gas manufacturing apparatus of the present invention further includes a translucent substrate, the photoelectric conversion unit is provided on the translucent substrate, and the translucent substrate has a light receiving surface of the photoelectric conversion unit horizontally. Then, the inclination angle between the surface of the first electrolysis electrode that can contact the electrolytic solution and the horizontal reference surface, and the surface between the surface of the second electrolysis electrode that can contact the electrolytic solution and the reference surface. It is preferable that it is formed so as to have a different inclination angle. According to such a configuration, the first and second electrolysis electrodes are provided so that the inclination angles of the surfaces of the first and second electrolysis electrodes that can contact the electrolytic solution differ depending on the shape of the translucent substrate. Can do.
- the second electrolysis electrode is provided on the back surface of the photoelectric conversion unit via an insulating unit. According to such a configuration, it is possible to prevent a leak current from occurring.
- the photoelectric conversion unit has a photoelectric conversion layer including a p-type semiconductor layer, an i-type semiconductor layer, and an n-type semiconductor layer. According to such a configuration, an electromotive force can be generated by causing light to enter the photoelectric conversion unit.
- the photoelectric conversion unit includes a plurality of photoelectric conversion layers connected in series, and the plurality of photoelectric conversion layers generate electromotive force generated by receiving light in the first electrolysis electrode and the second electrolysis. It is preferable to supply it to the electrode for use. According to such a configuration, a high voltage electromotive force can be easily output to the first and second electrolysis electrodes.
- each photoelectric conversion layer is preferably connected in series by a fourth conductive portion. According to such a configuration, the photoelectric conversion layers can be provided side by side.
- one of the first electrolysis electrode and the second electrolysis electrode is a hydrogen generation unit that generates H 2 from the electrolytic solution, and the other is oxygen generation that generates O 2 from the electrolytic solution.
- the hydrogen generation unit and the oxygen generation unit include a catalyst for a reaction in which H 2 is generated from the electrolytic solution and a catalyst for a reaction in which O 2 is generated from the electrolytic solution, respectively. According to such a configuration, hydrogen serving as a fuel for the fuel cell can be produced.
- At least one of the hydrogen generation unit and the oxygen generation unit has a catalyst surface area larger than an area of the light receiving surface. According to such a configuration, hydrogen and oxygen can be produced more efficiently.
- at least one of the hydrogen generation part and the oxygen generation part is a porous conductor carrying a catalyst. According to such a configuration, the catalyst area of the reaction in which hydrogen or oxygen is generated can be increased.
- the hydrogen generation unit includes at least one of Pt, Ir, Ru, Pd, Rh, Au, Fe, Ni, and Se as a hydrogen generation catalyst. According to such a configuration, hydrogen can be efficiently generated from the electrolytic solution.
- the oxygen generation unit includes at least one of Mn, Ca, Zn, Co, and Ir as an oxygen generation catalyst. According to such a configuration, oxygen can be efficiently generated from the electrolytic solution.
- the gas manufacturing apparatus of the present invention further includes a light-transmitting substrate and an electrolyte chamber, and the photoelectric conversion unit is provided on the light-transmitting substrate, and includes a first electrolysis electrode and a second electrolysis electrode. It is preferable that a top plate is further provided, and the electrolytic solution chamber is provided between the first electrolysis electrode and the second electrolysis electrode and the top plate.
- the surface of the first electrolysis electrode that can contact the electrolyte solution and the surface of the second electrolysis electrode that can contact the electrolyte solution can be provided facing the electrolyte chamber, The first and second electrodes for electrolysis can be brought into contact with the electrolytic solution.
- the gas production apparatus further includes a partition partitioning the electrolyte chamber between the first electrolysis electrode and the top plate and the electrolyte chamber between the second electrolysis electrode and the top plate.
- the first gas and the second gas can be separated by the partition wall.
- the partition preferably includes an ion exchanger. According to such a structure, the imbalance of the ion concentration which arises in electrolyte solution can be eliminated easily.
- this invention installs the gas manufacturing apparatus of this invention so that the light-receiving surface of the said photoelectric conversion part may become substantially horizontal
- the said gas manufacturing apparatus has the 1st gas exhaust port which discharges
- the second electrolysis chamber is provided with a second gas exhaust port for discharging the second gas, and an electrolytic solution chamber.
- the electrolytic solution is introduced into the electrolytic solution chamber, and sunlight is incident on the light receiving surface of the photoelectric conversion unit.
- a gas production method is also provided in which the first gas and the second gas are generated from the electrode for electrode and the electrode for second electrolysis, respectively, and the first gas and the second gas are discharged from the first gas outlet and the second gas outlet, respectively.
- the first gas and the second gas can be produced by allowing light to enter the photoelectric conversion unit, and the first gas and the second gas can be easily recovered.
- the present invention further includes a plurality of the gas production apparatuses of the present invention, a first gas discharge path, and a second gas discharge path, and each gas production apparatus includes a first gas discharge port and a first gas discharge port for discharging the first gas.
- a second gas discharge port for discharging two gases the first gas discharge path is electrically connected to the first gas discharge port of each gas production apparatus, and the second gas discharge path is a second gas of each gas production apparatus.
- a gas production device array in communication with the outlet is also provided. According to the gas production device array of the present invention, the generation amounts of the first gas and the second gas can be increased, and the first gas and the second gas are recovered from the first gas discharge path and the second gas discharge path, respectively. can do. Moreover, the 1st gas exhaust path and the 2nd gas exhaust path can be simplified and provided.
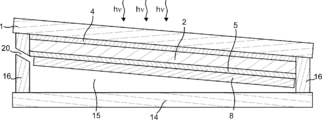
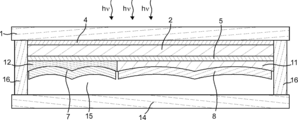
- Diagram 1 of a gas producing device is a schematic plan view showing the configuration of a gas producing device according to an embodiment of the present invention.
- 2 to 4 are schematic cross-sectional views of the gas production apparatus taken along one-dot chain line AA, dotted line BB, and dotted line CC in FIG. 1, respectively.
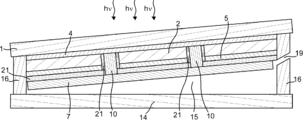
- FIG. 5 is a schematic back view showing the configuration of the gas production apparatus according to one embodiment of the present invention.
- 6 to 16 are schematic cross-sectional views showing the configuration of the gas production apparatus according to the embodiment of the present invention. 6, 13, and 15 correspond to the sectional view of the gas production apparatus taken along the dotted line BB in FIG. 1, and the sectional views of FIGS. 7, 8, 11, 12, 14, and 16 are 9 and 10 correspond to the cross-sectional view of the gas production apparatus taken along one-dot chain line AA in FIG.
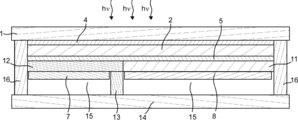
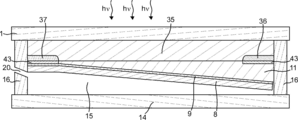
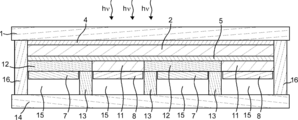
- the gas production apparatus 23 includes a photoelectric conversion unit 2 having a light receiving surface and a back surface thereof, and a surface that is provided side by side on the back surface of the photoelectric conversion unit 2 and that can contact an electrolytic solution. 1 and second electrolysis electrodes 8 and 7, and when the first and second electrolysis electrodes 8 and 7 are in contact with the electrolytic solution, the first and second electrolysis electrodes 8 and 7 are connected to the photoelectric conversion unit 2.
- the electrolysis solution is electrolyzed using the electromotive force generated by receiving the light to generate the first gas and the second gas, respectively, and when the light receiving surface of the photoelectric conversion unit 2 is leveled, the first electrolysis
- the electrode for electrode 8 and the electrode for second electrolysis 7 are characterized in that the inclination angles between the surface that can contact the electrolyte and the horizontal reference surface are different.
- the gas manufacturing apparatus 23 of the present embodiment may include the translucent substrate 1. Hereinafter, the gas manufacturing apparatus of this embodiment is demonstrated.
- the translucent substrate 1 may be provided in the gas manufacturing apparatus 23 of the present embodiment. Moreover, the photoelectric conversion part 2 may be provided on the translucent board
- substrate 1 is a member used as the foundation for comprising this gas manufacturing apparatus.
- a substrate material having a high light transmittance for example, a transparent rigid material such as soda glass, quartz glass, Pyrex (registered trademark), or a synthetic quartz plate, or a transparent resin plate or film material is preferably used. In view of chemical and physical stability, it is preferable to use a glass substrate.
- a fine uneven structure can be formed so that incident light is effectively irregularly reflected on the surface of the photoelectric conversion unit 2.
- This fine concavo-convex structure can be formed by a known method such as reactive ion etching (RIE) treatment or blast treatment.
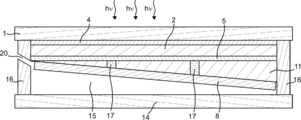
- the translucent substrate 1 has an inclination angle between the surface of the first electrolysis electrode 8 that can contact the electrolytic solution and a horizontal reference surface
- mold so that the inclination angle between the surface which can contact the electrolyte solution of the electrode 7 for 2 electrolysis and a horizontal reference plane differs.
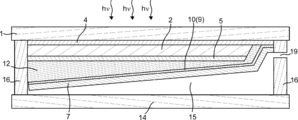
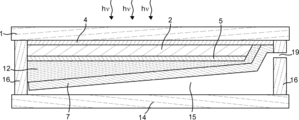
- the gas manufacturing apparatus of the present embodiment can have a cross section as shown in FIGS. 6 is a cross-sectional view corresponding to the cross-sectional view taken along the dotted line BB in FIG. 1, and FIG. 7 is a cross-sectional view corresponding to the cross-sectional view taken along the dotted line CC in FIG.
- the first gas recovery port 20 and the second gas recovery port 19 can be provided on both sides of the gas production apparatus. Thereby, the piping for recovering the first gas and the piping for recovering the second gas can be simplified.
- Such a molded translucent substrate 1 may be formed by pouring glass or the like into a mold, may be formed by deforming a plate-like substrate, or may be formed by combining plate-like substrates. May be.
- the first electrode 4 can be provided on the translucent substrate 1 and can be provided in contact with the light receiving surface of the photoelectric conversion part 2. Moreover, the 1st electrode 4 may have translucency. Moreover, the 1st electrode 4 may be directly provided in the light-receiving surface of the photoelectric conversion part 2, when the translucent board
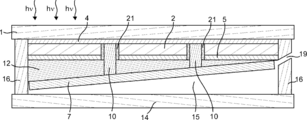
- the first electrode 4 can be electrically connected to the second electrolysis electrode 7. By providing the first electrode 4, the current flowing between the light receiving surface of the photoelectric conversion unit 2 and the second electrolysis electrode 7 can be increased. Further, when the photoelectric conversion unit 2 generates an electromotive force between the first area and the second area on the back surface of the photoelectric conversion unit 2 as shown in FIGS.
- the first electrode 4 is unnecessary.
- the first electrode 4 may be electrically connected to the second electrolysis electrode 7 through the second conductive portion 10 as shown in FIGS. 4, 7, 8, and 11, and as shown in FIG. It may be in contact with the electrode 7.
- the first electrode 4 may be made of a transparent conductive film such as ITO or SnO 2, or may be made of a metal finger electrode such as Ag or Au.
- the transparent conductive film is used to facilitate contact between the light receiving surface of the photoelectric conversion unit 2 and the second electrolysis electrode 7. What is generally used as a transparent electrode can be used. Specifically, In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, SnO 2 and the like can be given.
- the transparent conductive film preferably has a sunlight transmittance of 85% or more, particularly 90% or more, and particularly 92% or more. This is because the photoelectric conversion unit 2 can absorb light efficiently.
- a known method can be used, and examples thereof include sputtering, vacuum deposition, sol-gel method, cluster beam deposition method, and PLD (Pulse Laser Deposition) method.
- the second conductive portion 10 can be provided so as to contact the first electrode 4 and the second electrolysis electrode 7 respectively. By providing the second conductive portion 10, the first electrode 4 and the second electrolysis electrode 7 that are in contact with the light receiving surface of the photoelectric conversion portion 2 can be easily electrically connected.
- the 2nd electroconductive part 10 may be provided in the contact hole which penetrates the photoelectric conversion part 2 or the 2nd type
- the contact hole provided with the second conductive portion 10 may be one or more, and may have a circular cross section.
- the 2nd electroconductive part 10 may be provided so that the side surface of the photoelectric conversion part 2 may be covered like FIG.
- the material of the second conductive portion 10 is not particularly limited as long as it has conductivity.
- a paste containing conductive particles for example, a carbon paste, an Ag paste or the like applied by screen printing, an inkjet method, etc., dried or baked, a method of forming a film by a CVD method using a raw material gas, a PVD method, Examples thereof include a vapor deposition method, a sputtering method, a sol-gel method, and a method using an electrochemical redox reaction.
- the photoelectric conversion unit 2 has a light receiving surface and a back surface, and a first electrolysis electrode 8 and a second electrolysis electrode 7 are provided on the back surface of the photoelectric conversion unit 2.
- the light receiving surface is a surface that receives light for photoelectric conversion
- the back surface is the back surface of the light receiving surface.
- the photoelectric conversion part 2 can be provided on the translucent substrate 1 provided with the first electrode 4 with the light receiving surface facing down.
- the photoelectric conversion unit 2 may generate an electromotive force between the light receiving surface and the back surface as shown in FIGS. 2 to 4 and 6 to 13, and the first area on the back surface as shown in FIGS. And an electromotive force may be generated between the first area and the second area.
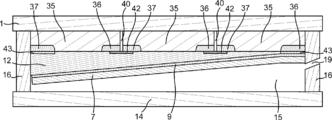
- the photoelectric conversion unit 2 as shown in FIGS. 14 to 16 can be formed by a semiconductor substrate on which the n-type semiconductor region 37 and the p-type semiconductor region 36 are formed. Moreover, when the translucent board
- the shape of the photoelectric conversion part 2 is not specifically limited, For example, it can be set as a square shape.
- the photoelectric conversion unit 2 is not particularly limited as long as it can separate charges by incident light and generates an electromotive force. For example, the photoelectric conversion unit using a silicon-based semiconductor or the photoelectric conversion unit using a compound semiconductor A photoelectric conversion part using a dye sensitizer, a photoelectric conversion part using an organic thin film, and the like.
- the photoelectric conversion unit 2 When the first gas and the second gas are hydrogen and oxygen, the photoelectric conversion unit 2 is necessary for generating hydrogen and oxygen in the first electrolysis electrode 8 and the second electrolysis electrode 7 by receiving light. It is necessary to use a material that generates an electromotive force.
- the potential difference between the first electrolysis electrode 8 and the second electrolysis electrode 7 needs to be larger than the theoretical voltage (1.23 V) for water decomposition, and for this purpose, a sufficiently large potential difference needs to be generated in the photoelectric conversion unit 2.
- the photoelectric conversion unit 2 connects two or more junctions in series such as a pn junction to generate an electromotive force.
- it can have a structure in which photoelectric conversion layers provided in parallel are connected by the fourth conductive portion 42.
- Examples of materials that perform photoelectric conversion include silicon-based semiconductors, compound semiconductors, and materials based on organic materials, and any photoelectric conversion material can be used.
- these photoelectric conversion materials can be stacked. In the case of stacking, it is possible to form a multi-junction structure with the same material, but stacking multiple photoelectric conversion layers with different optical band gaps and complementing the low sensitivity wavelength region of each photoelectric conversion layer mutually By doing so, incident light can be efficiently absorbed over a wide wavelength region.
- the plurality of photoelectric conversion layers preferably have different band gaps. According to such a configuration, the electromotive force generated in the photoelectric conversion unit 2 can be increased, and the electrolytic solution can be electrolyzed more efficiently.
- the photoelectric conversion unit 2 may be a combination of these.
- the example of the following photoelectric conversion parts 2 can also be made into a photoelectric converting layer.
- Photoelectric conversion part using a silicon-based semiconductor examples include a single crystal type, a polycrystalline type, an amorphous type, a spherical silicon type, and combinations thereof. Any of them can have a pn junction in which a p-type semiconductor and an n-type semiconductor are joined. Further, a pin junction in which an i-type semiconductor is provided between a p-type semiconductor and an n-type semiconductor may be provided. Further, it may have a plurality of pn junctions, a plurality of pin junctions, or a pn junction and a pin junction.
- the silicon-based semiconductor is a semiconductor containing silicon, such as silicon, silicon carbide, or silicon germanium.
- the photoelectric conversion unit 2 using a silicon-based semiconductor may be a thin film or a thick photoelectric conversion layer formed on the translucent substrate 1, or a pn junction or a wafer such as a silicon wafer.
- a pin junction may be formed, or a thin film photoelectric conversion layer may be formed on a wafer having a pn junction or a pin junction.
- a first conductivity type semiconductor layer is formed on the first electrode 4 laminated on the translucent substrate 1 by a method such as a plasma CVD method.
- a method such as a plasma CVD method.
- As the first conductive type semiconductor layer a p + type or n + type amorphous Si thin film doped with a conductivity type determining impurity atom concentration of about 1 ⁇ 10 18 to 5 ⁇ 10 21 / cm 3 , a polycrystalline or A microcrystalline Si thin film is used.
- the material of the first conductivity type semiconductor layer is not limited to Si, and it is also possible to use a compound such as SiC, SiGe, or Si x O 1-x .
- a polycrystalline or microcrystalline crystalline Si thin film is formed as a crystalline Si photoactive layer by a method such as plasma CVD.
- the conductivity type is the first conductivity type having a lower doping concentration than the first conductivity type semiconductor, or the i conductivity type.
- the material for the crystalline Si-based photoactive layer is not limited to Si, and it is also possible to use a compound such as SiC, SiGe, or Si x O 1-x .
- a second conductivity type semiconductor layer having a conductivity type opposite to the first conductivity type semiconductor layer is formed by a method such as plasma CVD.
- a method such as plasma CVD.
- an n + type or p + type amorphous Si thin film doped with about 1 ⁇ 10 18 to 5 ⁇ 10 21 / cm 3 of a conductivity type determining impurity atom, or a polycrystalline or microscopic A crystalline Si thin film is used.
- the material of the second conductivity type semiconductor layer is not limited to Si, and it is also possible to use a compound such as SiC, SiGe, or Si x O 1-x .
- the second photoelectric conversion layer includes a first conductive semiconductor layer, a crystalline Si-based photoactive layer, and a second conductive semiconductor layer, and each layer corresponds to the first photoelectric conversion layer.
- the first conductive type semiconductor layer, the crystalline Si-based photoactive layer, and the second conductive type semiconductor layer are formed.
- the volume crystallization fraction of the crystalline Si photoactive layer of the second photoelectric conversion layer is preferably higher than that of the first crystalline Si photoactive layer.
- the volume crystallization fraction is preferably increased as compared with the lower layer.
- the silicon substrate a single crystal silicon substrate, a polycrystalline silicon substrate, or the like can be used, and may be p-type, n-type, or i-type.
- An n-type semiconductor region 37 is formed by doping an n-type impurity such as P into a part of the silicon substrate by thermal diffusion or ion implantation, and a p-type impurity such as B is heated on the other part of the silicon substrate.
- the p-type semiconductor region 36 can be formed by doping by diffusion or ion implantation.
- pn junction in the silicon substrate, pin junction can be formed and npp + junction or pnn + junction, it is possible to form a photoelectric conversion unit 2.
- Each of the n-type semiconductor region 37 and the p-type semiconductor region 36 can be formed on the silicon substrate as shown in FIGS. 14 to 16, and one of the n-type semiconductor region 37 and the p-type semiconductor region 36 is formed. A plurality of can be formed.
- the photoelectric conversion unit 2 can be formed by arranging the silicon substrates on which the n-type semiconductor region 37 and the p-type semiconductor region 36 are arranged side by side and connecting them in series by the fourth conductive unit 42. Note that, although described with reference to a silicon substrate, pn junction, pin junction, may use other semiconductor substrate or the like can be formed npp + junction or pnn + junction.
- the semiconductor layer is not limited to the semiconductor substrate and may be a semiconductor layer formed on the substrate.
- Photoelectric conversion part using a compound semiconductor is, for example, GaP, GaAs, InP, InAs, or IId-VI elements composed of group III-V elements, CdTe / CdS, Examples thereof include those in which a pn junction is formed using CIGS (Copper Indium Gallium DiSelenide) composed of the I-III-VI group.
- CIGS Copper Indium Gallium DiSelenide
- a method for manufacturing a photoelectric conversion unit using a compound semiconductor is shown below.
- MOCVD metal organic chemical vapor deposition
- a group III element material for example, an organic metal such as trimethylgallium, trimethylaluminum, or trimethylindium is supplied to the growth apparatus using hydrogen as a carrier gas.
- a gas such as arsine (AsH 3 ), phosphine (PH 3 ), and stibine (SbH 3 ) is used as the material of the group V element.
- a dopant of p-type impurities or n-type impurities for example, diethyl zinc for p-type conversion, monosilane (SiH 4 ), disilane (Si 2 H 6 ), hydrogen selenide (H 2 Se) for n-type conversion, for example. Etc. are used.
- These source gases can be thermally decomposed by supplying them onto a substrate heated to, for example, 700 ° C., and a desired compound semiconductor material film can be epitaxially grown.
- the composition of these growth layers can be controlled by the gas composition to be introduced, and the film thickness can be controlled by the gas introduction time.
- a known window layer on the light receiving surface side or a known electric field layer on the non-light receiving surface side may be provided to improve carrier collection efficiency.
- a buffer layer for preventing diffusion of impurities may be provided.
- the photoelectric conversion part using a dye sensitizer is mainly composed of, for example, a porous semiconductor, a dye sensitizer, an electrolyte, a solvent, and the like.
- a material constituting the porous semiconductor for example, one or more kinds of known semiconductors such as titanium oxide, tungsten oxide, zinc oxide, barium titanate, strontium titanate, cadmium sulfide can be selected.
- a paste containing semiconductor particles is applied by a screen printing method, an ink jet method and the like, dried or baked, a method of forming a film by a CVD method using a raw material gas, etc. , PVD method, vapor deposition method, sputtering method, sol-gel method, method using electrochemical oxidation-reduction reaction, and the like.
- the dye sensitizer adsorbed on the porous semiconductor various dyes having absorption in the visible light region and the infrared light region can be used.
- the carboxylic acid group, carboxylic anhydride group, alkoxy group, sulfonic acid group, hydroxyl group, hydroxylalkyl group, ester group, mercapto group, phosphonyl in the dye molecule It is preferable that a group or the like is present.
- These functional groups provide an electrical bond that facilitates electron transfer between the excited state dye and the conduction band of the porous semiconductor.
- dyes containing these functional groups include ruthenium bipyridine dyes, quinone dyes, quinone imine dyes, azo dyes, quinacridone dyes, squarylium dyes, cyanine dyes, merocyanine dyes, and triphenylmethane dyes.
- ruthenium bipyridine dyes quinone dyes, quinone imine dyes, azo dyes, quinacridone dyes, squarylium dyes, cyanine dyes, merocyanine dyes, and triphenylmethane dyes.
- Xanthene dyes porphyrin dyes, phthalocyanine dyes, berylene dyes, indigo dyes, naphthalocyanine dyes, and the like.
- Examples of the method of adsorbing the dye to the porous semiconductor include a method of immersing the porous semiconductor in a solution in which the dye is dissolved (dye adsorption solution).
- the solvent used in the dye adsorption solution is not particularly limited as long as it dissolves the dye, and specifically, alcohols such as ethanol and methanol, ketones such as acetone, ethers such as diethyl ether and tetrahydrofuran.
- Nitrogen compounds such as acetonitrile, aliphatic hydrocarbons such as hexane, aromatic hydrocarbons such as benzene, esters such as ethyl acetate, water, and the like.
- the electrolyte is composed of a redox pair and a solid medium such as a liquid or polymer gel holding the redox pair.
- a redox pair iron- and cobalt-based metals and halogen substances such as chlorine, bromine, and iodine are preferably used as the redox pair, and metal iodides such as lithium iodide, sodium iodide, and potassium iodide and iodine are used.
- the combination of is preferably used.
- imidazole salts such as dimethylpropylimidazole iodide can also be mixed.
- the solvent examples include carbonate compounds such as propylene carbonate, nitrile compounds such as acetonitrile, alcohols such as ethanol and methanol, water, aprotic polar substances, and the like. Of these, carbonate compounds and nitrile compounds are preferred. Used.
- Photoelectric conversion part using organic thin film Photoelectric conversion part 2 using an organic thin film is an electron hole transport layer composed of an organic semiconductor material having electron donating properties and electron accepting properties, or an electron transport layer having electron accepting properties. And a hole transport layer having an electron donating property may be laminated.
- the electron-donating organic semiconductor material is not particularly limited as long as it has a function as an electron donor, but it is preferable that a film can be formed by a coating method, and among them, an electron-donating conductive polymer is preferably used.
- the conductive polymer refers to a ⁇ -conjugated polymer, which is composed of a ⁇ -conjugated system in which double bonds or triple bonds containing carbon-carbon or hetero atoms are alternately connected to single bonds, and exhibits semiconducting properties. Point.
- Examples of the electron-donating conductive polymer material include polyphenylene, polyphenylene vinylene, polythiophene, polycarbazole, polyvinyl carbazole, polysilane, polyacetylene, polypyrrole, polyaniline, polyfluorene, polyvinyl pyrene, polyvinyl anthracene, and derivatives, Examples thereof include a polymer, a phthalocyanine-containing polymer, a carbazole-containing polymer, and an organometallic polymer.
- thiophene-fluorene copolymer polyalkylthiophene, phenylene ethynylene-phenylene vinylene copolymer, fluorene-phenylene vinylene copolymer, thiophene-phenylene vinylene copolymer and the like are preferably used.
- the electron-accepting organic semiconductor material is not particularly limited as long as it has a function as an electron acceptor. However, it is preferable that a film can be formed by a coating method, and among them, an electron-donating conductive polymer is preferably used.
- the electron-accepting conductive polymer include polyphenylene vinylene, polyfluorene, and derivatives and copolymers thereof, or carbon nanotubes, fullerene and derivatives thereof, CN group or CF 3 group-containing polymers, and —CF Examples thereof include 3- substituted polymers.
- an electron-accepting organic semiconductor material doped with an electron-donating compound an electron-donating organic semiconductor material doped with an electron-accepting compound, or the like can be used.
- the electron-accepting conductive polymer material doped with the electron-donating compound include the above-described electron-accepting conductive polymer material.
- a Lewis base such as an alkali metal such as Li, K, Ca, or Cs or an alkaline earth metal can be used. The Lewis base acts as an electron donor.
- the electron-donating conductive polymer material doped with the electron-accepting compound include the above-described electron-donating conductive polymer material.
- a Lewis acid such as FeCl 3 , AlCl 3 , AlBr 3 , AsF 6 or a halogen compound can be used.
- Lewis acid acts as an electron acceptor.
- photoelectric conversion unit 2 In the photoelectric conversion unit 2 shown above, it is assumed that sunlight is received and photoelectric conversion is primarily performed. However, it is emitted from a fluorescent lamp, an incandescent lamp, an LED, or a specific heat source depending on the application. It is also possible to perform photoelectric conversion by irradiating artificial light such as light.
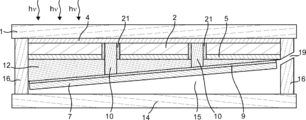
- the second electrode 5 can be provided between the back surface of the photoelectric conversion unit 2 and the first mold unit 11 and between the back surface of the photoelectric conversion unit 2 and the second mold unit 12. .
- the second electrode 5 can be electrically connected to the first electrolysis electrode 8.
- the second electrode 5 may be electrically connected to the first electrolysis electrode 8 through the third conductive portion 17 as shown in FIGS. 3 and 13, or may be in contact with the first electrolysis electrode 8.
- the third conductive part 17 may be provided in a contact hole provided in the first mold part 11 as shown in FIG.
- the second electrode 5 is provided between the back surface of the photoelectric conversion unit 2 and the insulating unit 21 and between the back surface of the photoelectric conversion unit 2 and the first electrolysis electrode 8. Also good. Moreover, it is preferable that the 2nd electrode 5 has the corrosion resistance with respect to electrolyte solution, and the liquid shielding property with respect to electrolyte solution. Thereby, corrosion of the photoelectric conversion part 2 by electrolyte solution can be prevented. Although it will not specifically limit if the 2nd electrode 5 has electroconductivity, For example, it is a metal thin film, for example, is thin films, such as Al, Ag, Au.
- IZO In—Zn—O
- ITO In—Sn—O
- ZnO—Al Zn—Sn—O
- SnO 2 SnO 2
- the material of the third conductive portion 17 is not particularly limited as long as it has conductivity.
- a paste containing conductive particles for example, a carbon paste, an Ag paste or the like applied by screen printing, an inkjet method, etc., dried or baked, a method of forming a film by a CVD method using a raw material gas, a PVD method, Examples thereof include a vapor deposition method, a sputtering method, a sol-gel method, and a method using an electrochemical redox reaction.
- mold part 11 can be provided between the back surface of the photoelectric conversion part 2, and the electrode 8 for 1st electrolysis
- mold part 12 is It can be provided between the back surface of the photoelectric conversion unit 2 and the second electrolysis electrode 7. Further, the first mold part 11 and the second mold part 12 are arranged between a surface that can contact the electrolytic solution of the first electrolysis electrode 8 and a horizontal reference surface when the light receiving surface of the photoelectric conversion unit 2 is leveled. And the inclination angle between the surface of the second electrolysis electrode 7 that can contact the electrolytic solution and the horizontal reference surface may be different. By providing the first mold part 11 and the second mold part 12 formed in this way, the inclination angle between the surface of the first electrolysis electrode in contact with the electrolytic solution and the surface of the second electrolysis electrode in contact with the electrolytic solution. Can be adjusted.
- mold part 12 may have a groove-shaped hollow like FIG.
- the first mold part and the second mold part may have electrical insulation.
- the second mold part 12 can be provided so as to cover the side surface of the photoelectric conversion part 2 as shown in FIGS. 11 and 12, and the second mold part 12 covering the side surface of the photoelectric conversion part 2 can be provided.
- the second conductive portion 10 or the second electrolysis electrode 7 can be provided thereon.
- leakage current can be prevented and the second electrolysis electrode 7 and the first electrode 4 can be electrically connected.
- the first mold part 11 and the second mold part 12 can be formed from, for example, a solid resin. By using a solid resin, the first mold part 11 and the second mold part 12 having a desired shape can be formed.
- the first mold part 11 may be made of a conductive material. Thereby, the back surface of the photoelectric conversion unit 2 and the first electrolysis electrode 8 can be electrically connected via the first mold unit.
- the first conductive part 9 can be provided between the first mold part 11 and the first electrolysis electrode 8 or between the second mold part 12 and the second electrolysis electrode 7.
- the first conductive portion 9 can be provided between the second mold portion 12 and the second electrolysis electrode 7 as shown in FIGS. 8, 11, 14, and 16, for example.
- the 1st electroconductive part 11 has electroconductivity
- it is a metal thin film, for example, is thin films, such as Al, Ag, Au.
- IZO In—Zn—O
- ITO In—Sn—O
- ZnO—Al Zn—Sn—O
- SnO 2 SnO 2
- Insulating part 21 can be provided in order to prevent the occurrence of leakage current.
- the insulating portion 21 can be provided on the side wall of the contact hole.
- the insulating part 21 can also be provided between the 2nd type
- the insulating part 11 can be used regardless of an organic material or an inorganic material.
- organic polymers and inorganic materials include metal oxides such as Al 2 O 3 , SiO 2 such as porous silica films, fluorine-added silicon oxide films (FSG), SiOC, HSQ (Hydrogen Silsesquioxane) films, SiN x , It is possible to use a method of forming a film by dissolving silanol (Si (OH) 4 ) in a solvent such as alcohol and applying and heating.
- a film containing a paste containing an insulating material is applied by a screen printing method, an ink jet method, a spin coating method, etc., dried or baked, or a CVD method using a source gas is used. And a method using a PVD method, a vapor deposition method, a sputtering method, a sol-gel method, and the like.
- the first electrolysis electrode 8 and the second electrolysis electrode 7 are respectively provided on the back surface of the photoelectric conversion unit 2, and the back surface side surface of the photoelectric conversion unit 2 Each has a surface which is the back surface and can contact the electrolyte. Thus, the first electrolysis electrode 8 does not block light incident on the photoelectric conversion unit 2.
- the electrolysis solution is electrolyzed by using the electromotive force generated by the photoelectric conversion unit 2 receiving light, and the first gas is obtained. And a second gas is generated.
- the first electrolysis electrode 8 is connected to the back surface of the photoelectric conversion unit 2 as shown in FIGS.
- the second electrolysis electrode 7 can be electrically connected to the light receiving surface of the photoelectric conversion unit 2 as shown in FIGS. 4, 7, 8, 11, and 12.
- the first electrolysis electrode 8 is connected to the first area and the second area as shown in FIGS.
- the second electrolysis electrode 7 can be electrically connected to one of the two areas and the other of the first and second areas.
- first electrolysis electrode 8 and the second electrolysis electrode 7 include a surface that can contact the electrolytic solution of the first electrolysis electrode 8 and a horizontal reference surface when the light receiving surface of the photoelectric conversion unit 2 is horizontal. And the inclination angle between the surface of the second electrolysis electrode 7 that can come into contact with the electrolytic solution and the horizontal reference surface are different.
- first gas rises in the electrolyte as bubbles
- the surface of the second electrolysis electrode 7 that becomes the guide surface can be shifted from the surface that can contact the electrolyte.
- the difference between the inclination angles of the first electrolysis electrode 8 and the second electrolysis electrode 7 is, for example, that the first gas outlet 20 and the second gas outlet 19 for recovering the first gas and the second gas are photoelectrically converted.
- the difference may be shifted up and down when the light receiving surface of the portion 2 is leveled.
- the first electrolysis electrode 8 is formed such that the first gas discharge port 20 is formed on one of the both sides of the gas production apparatus 23 and the second gas discharge port 19 is formed on the other side.
- the difference of the inclination angle of the electrode 7 for 2nd electrolysis may be given.
- the surface of the first electrolysis electrode 8 that can come into contact with the electrolytic solution may have, for example, an inclination angle of 1 to 60 degrees, preferably 5 degrees. It may be 30 degrees or less. Further, at this time, the surface of the second electrolysis electrode 7 that can come into contact with the electrolytic solution may have an inclination angle of 120 degrees or more and 179 degrees or less, preferably 150 degrees or more and 175 degrees or less. .
- the first gas can be discharged from one of the two sides of the gas production device 23, the second gas can be discharged from the other, and a pipe for collecting the first gas and the second gas can be provided. It can be simplified.
- a method for making a difference between the inclination angle of the surface of the first electrolysis electrode 8 that can contact the electrolytic solution and the inclination angle of the surface of the second electrolysis electrode 7 that can contact the electrolytic solution is not particularly limited.
- the first mold part 11 and the second mold part 12 may be provided to provide a difference in inclination angle.
- the surface of the first electrolysis electrode 8 and the second electrolysis electrode 7 that can contact the electrolytic solution is a guide surface when the first gas or the second gas rises as bubbles in the electrolytic solution.
- it may be a flat surface or a curved surface.
- the inclination angle of this curved surface means the average inclination angle.
- the first electrolysis electrode 8 and the second electrolysis electrode 7 can each have a groove-like recess extending in an inclined direction on a surface that can contact the electrolytic solution.
- the first gas or the second gas can be raised as bubbles in the electrolytic solution along the groove-like depression, and the first gas and the second gas can be easily separated.
- the partition wall 13 formed between the first electrolysis electrode 8 and the second electrolysis electrode 7 can be omitted by forming such a groove-like depression.
- one groove-like depression may be formed on each surface of the first electrolysis electrode 8 and the second electrolysis electrode 7 that can come into contact with the electrolytic solution.
- a plurality of electrodes may be formed on the surfaces of the electrode for electrolysis 8 and the electrode for second electrolysis 7 that can contact the electrolytic solution.
- At least one of the first electrolysis electrode 8 and the second electrolysis electrode 7 has a plurality of surfaces, each of which has a surface that can contact the strip-shaped electrolyte solution, and the long sides of the surfaces are adjacent to each other It may be provided alternately.
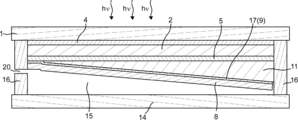
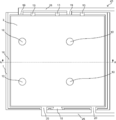
- FIG. 17 is a schematic plan view of the gas production apparatus of the present embodiment
- FIG. 18 is a schematic cross-sectional view of the gas production apparatus taken along the dotted line AA in FIG.
- the cross-sectional view of the gas production device 23 in the direction perpendicular to the dotted line AA has a cross section as shown in FIGS.
- the distance between the portion where the reaction generating the first gas occurs and the portion where the reaction generating the second gas occurs is increased. It can be shortened, and the ion concentration imbalance generated in the electrolyte can be reduced.
- the 1st gas and 2nd gas can be collect
- the some 1st gas exhaust port 20 can be formed in one among the both sides of the gas manufacturing apparatus 23, and the some 2nd gas exhaust port 19 can be formed in the other.
- the first gas discharge path 25 for recovering the first gas and the second gas discharge path 26 for recovering the second gas can be provided on both sides of the gas production device 23, thereby simplifying the piping. be able to.
- the gas discharged from the first gas discharge port 20 or the second gas discharge port 19 is replaced with water through a gas collecting unit (not shown). It is possible to collect gas by a method such as a method.
- At least one of the first electrolysis electrode 8 and the second electrolysis electrode 7 has a catalyst surface area larger than the area of the light receiving surface. According to such a configuration, the first gas or the second gas can be generated more efficiently by the electromotive force generated in the photoelectric conversion unit 2.
- at least one of the first electrolysis electrode 8 and the second electrolysis electrode 7 is preferably a porous conductor carrying a catalyst. According to such a configuration, the surface area of at least one of the first electrolysis electrode 8 and the second electrolysis electrode 7 can be increased, and the first gas or the second gas can be generated more efficiently. Can do.
- One of the first electrolysis electrode 8 and the second electrolysis electrode 7 may be a hydrogen generation unit, and the other may be an oxygen generation unit.
- one of the first gas and the second gas is hydrogen, and the other is oxygen.
- the hydrogen generating part is a part for generating H 2 from the electrolytic solution, and is one of the first electrolysis electrode 8 and the second electrolysis electrode 7. Further, the hydrogen generation unit may include a catalyst for a reaction in which H 2 is generated from the electrolytic solution. Thereby, the reaction rate of the reaction in which H 2 is generated from the electrolytic solution can be increased.
- the hydrogen generation part may consist only of a catalyst for the reaction in which H 2 is generated from the electrolytic solution, or this catalyst may be supported on a support. Further, the hydrogen generation unit may have a catalyst surface area larger than the area of the light receiving surface of the photoelectric conversion unit 2. Thereby, the reaction in which H 2 is generated from the electrolytic solution can be set to a faster reaction rate.
- the hydrogen generation part may be a porous conductor carrying a catalyst. This can increase the catalyst surface area. In addition, a change in potential due to a current flowing between the light receiving surface or the back surface of the photoelectric conversion unit 2 and the catalyst included in the hydrogen generation unit can be suppressed. Furthermore, the hydrogen generation unit may include at least one of Pt, Ir, Ru, Pd, Rh, Au, Fe, Ni, and Se as a hydrogen generation catalyst. According to such a configuration, hydrogen can be generated at a higher reaction rate by the electromotive force generated in the photoelectric conversion unit 2.
- the catalyst for the reaction of generating H 2 from the electrolyte is a catalyst that promotes the conversion of two protons and two electrons into one molecule of hydrogen, is chemically stable, and generates hydrogen overvoltage.
- platinum group metals such as Pt, Ir, Ru, Pd, Rh, and Au, which have catalytic activity for hydrogen, and alloys or compounds thereof, Fe, Ni, and Se that constitute the active center of hydrogenase that is a hydrogen-producing enzyme.
- An alloy or a compound, a combination thereof, or the like can be preferably used.
- a nanostructure containing Pt and Pt has a small hydrogen generation overvoltage and can be suitably used.
- Materials such as CdS, CdSe, ZnS, and ZrO 2 whose hydrogen generation reaction is confirmed by light irradiation can also be used.
- the hydrogen generating catalyst can be supported on the conductor.
- the conductor carrying the catalyst include metal materials, carbonaceous materials, and conductive inorganic materials.
- the metal material a material having electronic conductivity and resistance to corrosion in an acidic atmosphere is preferable.
- noble metals such as Au, Pt, Pd, metals such as Ti, Ta, W, Nb, Ni, Al, Cr, Ag, Cu, Zn, Su, Si, and nitrides and carbides of these metals
- Examples of the alloy include stainless steel, Cu—Cr, Ni—Cr, and Ti—Pt.
- the metal material contains at least one element selected from the group consisting of Pt, Ti, Au, Ag, Cu, Ni, and W from the viewpoint that there are few other chemical side reactions.
- These metal materials have a relatively small electric resistance, and can suppress a decrease in voltage even when a current is extracted in the surface direction.
- a metal material having poor corrosion resistance in an acidic atmosphere such as Cu, Ag, Zn, etc.
- noble metals and metals having corrosion resistance such as Au, Pt, Pd, carbon, graphite, glassy carbon
- a metal surface having poor corrosion resistance may be coated with a conductive polymer, a conductive nitride, a conductive carbide, a conductive oxide, or the like.
- the carbonaceous material a chemically stable and conductive material is preferable.
- examples thereof include carbon powders and carbon fibers such as acetylene black, vulcan, ketjen black, furnace black, VGCF, carbon nanotube, carbon nanohorn, and fullerene.
- Examples of the inorganic material having conductivity include In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, SnO 2 , and antimony oxide-doped tin oxide. .
- examples of the conductive polymer include polyacetylene, polythiophene, polyaniline, polypyrrole, polyparaphenylene, polyparaphenylene vinylene, and the like
- examples of the conductive nitride include carbon nitride, silicon nitride, gallium nitride, indium nitride, and nitride. Germanium, titanium nitride, zirconium nitride, thallium nitride, etc.
- conductive carbides include tantalum carbide, silicon carbide, zirconium carbide, titanium carbide, molybdenum carbide, niobium carbide, iron carbide, nickel carbide, hafnium carbide, tungsten carbide. , Vanadium carbide, chromium carbide, and the like.
- conductive oxide include tin oxide, indium tin oxide (ITO), and antimony oxide-doped tin oxide.
- the structure of the conductor supporting the hydrogen generation catalyst includes a plate shape, a foil shape, a rod shape, a mesh shape, a lath plate shape, a porous plate shape, a porous rod shape, a woven fabric shape, a nonwoven fabric shape, a fiber shape, and a felt shape. It can be used suitably. Further, a grooved conductor in which the surface of the felt-like electrode is pressure-bonded in a groove shape is preferable because the electric resistance and the flow resistance of the electrode liquid can be reduced.
- the oxygen generating portion is a portion that generates O 2 from the electrolytic solution, and is one of the first electrolysis electrode 8 and the second electrolysis electrode 7.
- the oxygen generation unit may include a catalyst for a reaction in which O 2 is generated from the electrolytic solution. Thereby, the reaction rate of the reaction in which O 2 is generated from the electrolytic solution can be increased.
- the oxygen generation part may consist only of a catalyst for the reaction that generates O 2 from the electrolytic solution, or the catalyst may be supported on a carrier.
- the oxygen generation unit may have a catalyst surface area larger than the area of the light receiving surface of the photoelectric conversion unit 2. Thereby, the reaction in which O 2 is generated from the electrolytic solution can be set to a faster reaction rate.
- the oxygen generation part may be a porous conductor carrying a catalyst. This can increase the catalyst surface area. In addition, a change in potential due to a current flowing between the light receiving surface or the back surface of the photoelectric conversion unit 2 and the catalyst included in the oxygen generation unit can be suppressed. Furthermore, the oxygen generation unit may include at least one of Mn, Ca, Zn, Co, and Ir as an oxygen generation catalyst. According to such a configuration, oxygen can be generated at a higher reaction rate by the electromotive force generated in the photoelectric conversion unit.
- the catalyst for the reaction of generating O 2 from the electrolyte is a catalyst that promotes the conversion of two water molecules into one molecule of oxygen, four protons, and four electrons, and is chemically stable.
- a material having a small oxygen generation overvoltage can be used.
- oxides or compounds containing Mn, Ca, Zn, Co, which are active centers of Photosystem II, which is an enzyme that catalyzes the reaction of generating oxygen from water using light and platinum such as Pt, RuO 2 , IrO 2
- compounds containing group metals, oxides or compounds containing transition metals such as Ti, Zr, Nb, Ta, W, Ce, Fe, Ni, and combinations of the above materials.
- iridium oxide, manganese oxide, cobalt oxide, and cobalt phosphate can be suitably used because they have low overvoltage and high oxygen generation efficiency.
- the oxygen generating catalyst can be supported on the conductor.
- the conductor carrying the catalyst include metal materials, carbonaceous materials, and conductive inorganic materials. These explanations apply as long as there is no contradiction in the explanation of the hydrogen generation catalyst described in “9.
- a promoter can be used. Examples thereof include oxides or compounds of Ni, Cr, Rh, Mo, Co, and Se.
- the method for supporting the hydrogen generating catalyst and the oxygen generating catalyst can be applied directly to a conductor or semiconductor, PVD methods such as vacuum deposition, sputtering, and ion plating, dry coating methods such as CVD,
- the method can be appropriately changed depending on the material such as an analysis method.
- a conductive material can be appropriately supported between the photoelectric conversion unit and the catalyst.
- the reaction surface area is increased by supporting it on porous materials such as metals and carbon, fibrous materials, nanoparticles, etc., and the hydrogen and oxygen generation rates are improved. It is possible to make it.
- the top plate 14 can be provided on the first electrolysis electrode 8 and the second electrolysis electrode 7 so as to face the translucent substrate 1.
- the top plate 14 can be provided such that a space is provided between the first electrolysis electrode 8 and the second electrolysis electrode 7 and the top plate 14. This space can be used as the electrolytic solution chamber 15, and the first electrolytic electrode 8 and the second electrolytic electrode 7 can be brought into contact with the electrolytic solution by introducing the electrolytic solution into the electrolytic solution chamber 15.
- the top plate 14 may be the bottom part of a box.
- the top plate 14 is a material that constitutes the electrolytic solution chamber 15 and confines the generated first gas and second gas, and a highly confidential substance is required. It is not particularly limited whether it is transparent or opaque, but it is preferably a transparent material in that it can be visually confirmed that the first gas and the second gas are generated. .
- the transparent top plate is not particularly limited, and examples thereof include a transparent rigid material such as quartz glass, Pyrex (registered trademark), and a synthetic quartz plate, a transparent resin plate, and a transparent resin film. Among them, it is preferable to use a glass material because it is a gas that is not chemically permeable and is chemically and physically stable.
- the partition wall 13 can be provided so as to partition the first electrolysis electrode 8 and the second electrolysis electrode 7.
- the partition wall 13 includes an electrolytic solution chamber 15 that is a space between the first electrolysis electrode 8 and the top plate 14 and an electrolytic solution chamber 15 that is a space between the second electrolysis electrode 7 and the top plate 14. It can provide so that it may partition. As a result, the first gas and the second gas generated by the first electrolysis electrode 8 and the second electrolysis electrode 7 can be prevented from mixing, and the first gas and the second gas can be separated. It can be recovered.
- the partition wall 13 may include an ion exchanger.
- the ion concentration that is unbalanced between the electrolytic solution in the space between the first electrolysis electrode 8 and the top plate 14 and the electrolytic solution in the space between the second electrolysis electrode 7 and the top plate 14 is obtained. Can be kept constant.
- an inorganic film such as porous glass, porous zirconia, or porous alumina or an ion exchanger
- an ion exchanger any ion exchanger known in the art can be used, and a proton conductive membrane, a cation exchange membrane, an anion exchange membrane, or the like can be used.
- the material of the proton conductive film is not particularly limited as long as it is a material having proton conductivity and electrical insulation, and a polymer film, an inorganic film, or a composite film can be used.
- polymer membrane examples include Nafion (registered trademark) manufactured by DuPont, Aciplex (registered trademark) manufactured by Asahi Kasei Co., and Flemion (registered trademark) manufactured by Asahi Glass Co., Ltd., which are perfluorosulfonic acid electrolyte membranes.
- membranes and hydrocarbon electrolyte membranes such as polystyrene sulfonic acid and sulfonated polyether ether ketone.
- Examples of the inorganic film include films made of phosphate glass, cesium hydrogen sulfate, polytungstophosphoric acid, ammonium polyphosphate, and the like.
- Examples of the composite membrane include a membrane made of a sulfonated polyimide polymer, a composite of an inorganic material such as tungstic acid and an organic material such as polyimide, and specifically, Gore Select membrane (registered trademark) or pores manufactured by Gore. Examples thereof include a filling electrolyte membrane.
- a high temperature environment for example, 100 ° C.
- sulfonated polyimide 2-acrylamido-2-methylpropanesulfonic acid (AMPS)
- APMS 2-acrylamido-2-methylpropanesulfonic acid
- sulfonated polybenzimidazole phosphonated polybenzimidazole
- sulfuric acid examples include cesium hydrogen and ammonium polyphosphate.
- the cation exchange membrane may be any solid polymer electrolyte that can move cations.
- fluorine ion exchange membranes such as perfluorocarbon sulfonic acid membranes and perfluorocarbon carboxylic acid membranes, polybenzimidazole membranes impregnated with phosphoric acid, polystyrene sulfonic acid membranes, sulfonated styrene / vinylbenzene copolymers Examples include membranes.
- an anion exchange membrane a solid polymer electrolyte capable of transferring anions can be used.
- a polyorthophenylenediamine film, a fluorine-based ion exchange film having an ammonium salt derivative group, a vinylbenzene polymer film having an ammonium salt derivative group, a film obtained by aminating a chloromethylstyrene / vinylbenzene copolymer, etc. can be mentioned.
- sealing material 16 adheres the translucent substrate 1 and the top plate 14, and seals the electrolyte flowing in the gas production device 23 and the first gas and the second gas generated in the gas production device 23. Material. When using a box-shaped thing for the top plate 14, the sealing material 16 is used in order to adhere
- an ultraviolet curable adhesive, a thermosetting adhesive, or the like is preferably used, but the type thereof is not limited.
- UV curable adhesives are resins that undergo polymerization when irradiated with light having a wavelength of 200 to 400 nm and undergo a curing reaction within a few seconds after light irradiation, and are classified into radical polymerization type and cationic polymerization type.
- the polymerization type resin include acrylates, unsaturated polyesters, and examples of the cationic polymerization type include epoxy, oxetane, and vinyl ether.
- thermosetting polymer adhesive include organic resins such as phenol resin, epoxy resin, melamine resin, urea resin, and thermosetting polyimide.
- thermosetting polymer adhesive is heated and polymerized in a state where pressure is applied at the time of thermocompression bonding, and then cooled to room temperature while being pressurized. I don't need it.
- a hybrid material having high adhesion to the glass substrate can be used. By using a hybrid material, mechanical properties such as elastic modulus and hardness are improved, and heat resistance and chemical resistance are dramatically improved.
- the hybrid material is composed of inorganic colloidal particles and an organic binder resin. For example, what is comprised from inorganic colloidal particles, such as a silica, and organic binder resin, such as an epoxy resin, a polyurethane acrylate resin, and a polyester acrylate resin, is mentioned.
- the sealing material 16 is described.
- the sealing material 16 is not limited as long as it has a function of bonding the translucent substrate 1 and the top plate 14, and a member such as a screw is externally used using a resin or metal gasket. It is also possible to appropriately use a method of applying pressure physically to increase confidentiality.
- the electrolytic solution chamber 15 can be a space between the first electrolysis electrode 8 and the top plate 14 and a space between the second electrolysis electrode 7 and the top plate 14. Further, the electrolyte chamber 15 can be partitioned by the partition wall 13. For example, a pump or a fan that circulates the electrolyte in the electrolyte chamber 15 so that the generated bubbles of the first gas and the second gas are efficiently separated from the first electrolysis electrode 8 or the second electrolysis electrode 7. It is also possible to provide a simple device such as a heat convection generator.
- the water supply port 18 is a part of the sealing material 16 included in the gas production device 23 or the top plate 14. It can be provided by making an opening in a part or the like.
- the water supply port 18 is arranged to replenish the electrolytic solution decomposed into the first gas and the second gas, and the arrangement location and shape of the water supply port 18 can be efficiently supplied to the gas production apparatus. For example, there is no particular limitation.
- the first gas outlet 20 is provided close to the upper end of the surface of the first electrolysis electrode 8 that can come into contact with the electrolytic solution when the light receiving surface of the photoelectric conversion unit 2 is leveled upward. it can.
- the second gas discharge port 19 is provided close to the upper end of the surface of the second electrolysis electrode 7 that can come into contact with the electrolytic solution when the light receiving surface of the photoelectric conversion unit 2 is leveled upward. it can.
- first gas discharge port 20 can be connected to the first gas discharge passage 25, and the second gas discharge port 19 can be connected to the second gas discharge passage 26.
- first gas exhaust path 25 can be electrically connected to the plurality of first gas exhaust ports 20, and the second gas exhaust path 26 can be electrically connected to the plurality of second gas exhaust ports 19.
- Electrolytic Solution is not particularly limited as long as it is a raw material for the first gas and the second gas.
- the electrolytic solution is an aqueous solution containing an electrolyte, for example, an electrolytic solution containing 0.1 M H 2 SO 4 , 0.1M potassium phosphate buffer.
- hydrogen and oxygen can be produced from the electrolytic solution as the first gas and the second gas.
- the gas manufacturing method of this embodiment installs the gas manufacturing apparatus 23 so that the said light-receiving surface becomes substantially horizontal, and the said gas manufacturing apparatus discharges
- the second electrolysis chamber is provided with a second gas exhaust port for discharging the second gas, and an electrolytic solution chamber.
- the electrolytic solution is introduced into the electrolytic solution chamber, and sunlight is incident on the light receiving surface of the photoelectric conversion unit.
- a first gas and a second gas are generated from the first electrode and the second electrolysis electrode, respectively, and the first gas and the second gas are discharged from the first gas outlet and the second gas outlet, respectively. Thereby, the first gas and the second gas can be produced.
- the gas manufacturing device array of the present embodiment includes a plurality of gas manufacturing devices 23 of the present embodiment, a first gas discharge path 25, and a second gas discharge path 26.
- the first gas discharge port 20 for discharging the first gas and the second gas discharge port 19 for discharging the second gas are provided, and the first gas discharge path 25 is the first gas discharge port 20 of each gas production device 23.
- the second gas discharge path 26 is connected to the second gas discharge port 19 of each gas production device 23.
- the gas production apparatus array can be formed by arranging the gas production apparatuses.
- the first gas discharge path 25 and the second gas discharge path 26 are connected to each gas production apparatus 23. Since it can provide in both sides, piping can be simplified.
- Second electrolysis electrode 8 First electrolysis electrode 9: First electroconductive part 10: Second electroconductive part 11: First type part 12: Second mold part 13: Bulkhead 14: Top plate 15: Electrolyte chamber 16: Sealing material 17: Third conductive part 18: Water supply port 19: Second gas outlet 20: First gas outlet 21: Insulating part 23: Gas production apparatus 25: First gas discharge path 26: Second gas discharge path 28: Photoelectric conversion layer 33: Third conductive part 35: Semiconductor region 36: p-type semiconductor region 37: n-type semiconductor region 40: Isolation 42: 4th conductive part 43: 5th conductive part 45: Gas production device array
Landscapes
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
Abstract
This gas manufacturing apparatus is characterized in being provided with a photoelectric conversion section, which has the light receiving surface and the rear surface thereof, and first and second electrodes for electrolysis, said electrodes being arranged on the rear surface and respectively having surfaces that can be in contact with the electrolyte solution. The gas manufacturing apparatus is also characterized in that the first and the second electrodes for electrolysis are provided such that, at the time when the first and the second electrodes for electrolysis are in contact with the electrolyte solution, the first and the second electrodes decompose the electrolyte solution by electrolysis using an electromotive force generated when the photoelectric conversion section receives light, and respectively generate a first gas and a second gas, and that the first electrode for electrolysis and the second electrode for electrolysis respectively have tilt angles different from each other, said tilt angles being formed between the surfaces that can be in contact with the electrolysis solution and the horizontal reference surface when the light receiving surface of the photoelectric conversion section is horizontally set.
Description
本発明は、気体製造装置、気体製造方法および気体製造装置アレイに関する。
The present invention relates to a gas production apparatus, a gas production method, and a gas production apparatus array.
近年、化石燃料資源の枯渇および地球温暖化ガス排出抑制などの観点から、再生可能エネルギーの利用が望まれている。再生可能エネルギー源としては太陽光、水力、風力、地熱、潮力、バイオマスなど多岐にわたるが、その中でも、太陽光は利用可能なエネルギー量が大きいこと、他の再生可能エネルギーに対し地理的制約が比較的少ないことから、太陽光から効率よく利用可能なエネルギーを生み出す技術の早期な開発と普及が望まれている。
In recent years, the use of renewable energy is desired from the viewpoint of depletion of fossil fuel resources and the suppression of global warming gas emissions. There are a wide variety of renewable energy sources such as sunlight, hydropower, wind power, geothermal power, tidal power, and biomass. Among them, sunlight has a large amount of available energy, and there are geographical restrictions on other renewable energy sources. Because of the relatively small amount, early development and popularization of technology that can efficiently use energy from sunlight is desired.
太陽光から生み出される利用可能なエネルギーの形態としては、太陽電池や太陽光熱タービンを用いて製造される電気エネルギー、太陽光エネルギーを熱媒体に集めることによる熱エネルギー、その他にも太陽光を用いた物質還元による液体燃料や水素などの貯蔵可能な燃料エネルギー等が挙げられる。太陽電池技術および太陽熱利用技術については、すでに実用化されている技術が多いものの、エネルギー利用効率が未だ低いことと、電気および熱を作り出す際のコストが依然高いことから、これらの改善に向けた技術開発が行われている。さらに、これら電気や熱というエネルギー形態は、短期のエネルギー変動を補完するような使用法は実現できるものの、例えば季節変動などの長期での変動を補完することは極めて困難であることや、エネルギー量の増加により発電設備の稼働率低下を招く可能性があることが課題である。これに対し、液体燃料や水素など、エネルギーを物質として蓄えておくことは、長期変動を効率よく補完するとともに発電設備の稼働率を高める技術として極めて有力であり、今後エネルギー利用効率を最大限に高め、二酸化炭素の排出量を徹底的に削減するためには必要不可欠な技術である。
Possible forms of energy generated from sunlight include electrical energy produced using solar cells and solar thermal turbines, thermal energy by collecting solar energy in a heat medium, and other types of sunlight. Examples include storable fuel energy such as liquid fuel and hydrogen by substance reduction. Many solar cell technologies and solar heat utilization technologies have already been put into practical use, but the energy utilization efficiency is still low, and the cost of producing electricity and heat is still high. Technology development is underway. Furthermore, while these forms of electricity and heat can be used to supplement short-term energy fluctuations, it is extremely difficult to supplement long-term fluctuations such as seasonal fluctuations, It is a problem that there is a possibility that the operating rate of the power generation equipment may be reduced due to the increase in power generation. On the other hand, storing energy as a substance, such as liquid fuel and hydrogen, is extremely effective as a technology that efficiently supplements long-term fluctuations and increases the operating rate of power generation facilities. It is an indispensable technology to raise and reduce carbon dioxide emissions thoroughly.
貯蔵可能な燃料の形態としては、炭化水素などの液体燃料や、バイオガス、水素などの気体燃料、バイオマス由来の木材ペレットや太陽光で還元された金属などの固体燃料などに大別することができる。インフラ整備の容易性、エネルギー密度の観点では液体燃料、燃料電池などとのトータルの利用効率向上の観点では水素をはじめとする気体燃料、貯蔵可能性とエネルギー密度の観点では固体燃料というように、各形態において長所短所を有するが、原料として容易に入手可能な水を利用できる観点から、太陽光により水を分解することによる水素製造技術が特に注目されている。
The types of fuel that can be stored are roughly divided into liquid fuels such as hydrocarbons, gaseous fuels such as biogas and hydrogen, solid pellets such as biomass-derived wood pellets and metals reduced by sunlight. it can. In terms of ease of infrastructure development and energy density, liquid fuel, gaseous fuel including hydrogen in terms of total utilization efficiency improvement with fuel cells, etc., solid fuel in terms of storability and energy density, Although each form has advantages and disadvantages, a hydrogen production technique by decomposing water with sunlight has attracted particular attention from the viewpoint that water that can be easily obtained as a raw material can be used.
水を原料として太陽光エネルギーを利用し水素を製造する方法としては、酸化チタン等の光触媒に白金を担持させ、この物質を水中に入れ光照射することにより半導体中で電荷分離を行い、電解液中のプロトンを還元、水を酸化することによる光分解法や、高温ガス炉などの熱エネルギーを利用して水を高温で直接分解する、あるいは金属等の酸化還元と共役させて間接的に分解する熱分解法、藻類など光を利用する微生物の代謝を利用した生物法、太陽電池で発電した電気と水の電気分解水素製造装置を組み合わせた水電気分解法、太陽電池に使用される光電変換材料に水素発生触媒、酸素発生触媒を担持することにより、光電変換で得られる電子と正孔を水素生成触媒、酸素発生触媒で反応に利用する光起電力法等が挙げられる。この中で、光電変換部と水素生成部を一体化することにより、小型の水素製造装置を作製することの可能性を有するものは光分解法、生物法、光起電力法と考えられるが、太陽光エネルギーの変換効率の観点から、光起電力法は実用化に最も近い技術の一つと考えられる。
As a method of producing hydrogen using solar energy using water as a raw material, platinum is supported on a photocatalyst such as titanium oxide, and this substance is put in water to perform light separation in a semiconductor, and an electrolytic solution. The water is decomposed directly at high temperature using the photolysis method by reducing protons and oxidizing water, or by using thermal energy such as a high-temperature gas furnace, or indirectly by coupling with redox of metals, etc. Pyrolysis method that uses the metabolism of microorganisms that use light such as algae, water electrolysis method that combines electricity generated by solar cells and water electrolysis hydrogen production equipment, photoelectric conversion used in solar cells Examples of the method include a photovoltaic method in which electrons and holes obtained by photoelectric conversion are used in a reaction by a hydrogen generation catalyst and an oxygen generation catalyst by supporting a hydrogen generation catalyst and an oxygen generation catalyst on the material. Among these, the one that has the possibility of producing a small hydrogen production device by integrating the photoelectric conversion unit and the hydrogen generation unit is considered to be a photolysis method, a biological method, a photovoltaic method, From the viewpoint of the conversion efficiency of solar energy, the photovoltaic method is considered to be one of the technologies closest to practical use.
これまでに、光分解法や光起電力法による光電変換と水素発生を一体化した水素製造装置の例が開示されている。光分解法では例えば、特許文献1によると、ルテニウム錯体を吸着させた酸化チタンの光触媒電極と、白金電極、ヨウ素もしくは鉄の酸化還元を利用した装置が開示されている。また、特許文献2、3によると、2層の光触媒をタンデム接続し、白金カウンター電極を接続、間にイオン交換膜を挟むことにより一体化構造を採用している。一方、光起電力法では、光電変換部と水素生成部、酸素生成部を一体化した水素製造装置のコンセプトが発表されている(非特許文献1)。これによると、電荷分離は光電変換部、水素生成と酸素生成はそれぞれに対応する触媒を用いることにより行われる。光電変換部は太陽電池に利用される材料が用いられている。例えば、非特許文献2の場合、3層のシリコンp-i-n層で電荷分離を行った上で、水素発生は白金触媒が担い、酸素発生は酸化ルテニウムが担っている。また、非特許文献3では、異なる波長の光を吸収する多接合光電変換材料を、水素発生触媒にPt、酸素発生触媒にRuO2を用い、高効率化を図っている。また特許文献4や非特許文献3では、基盤上に、水素発生触媒(NiFeO)と、3層のシリコンp-i-nを並列に積層、シリコン層の上にさらに酸素発生触媒(Co-Mo)を担持することにより、一体化水素製造装置を作製している。
Until now, the example of the hydrogen production apparatus which integrated photoelectric conversion and hydrogen generation by the photolysis method or the photovoltaic method has been disclosed. In the photolysis method, for example, according to Patent Document 1, a titanium oxide photocatalyst electrode on which a ruthenium complex is adsorbed and a platinum electrode, an apparatus using oxidation reduction of iodine or iron is disclosed. According to Patent Documents 2 and 3, an integrated structure is adopted by connecting two layers of photocatalysts in tandem, connecting a platinum counter electrode, and sandwiching an ion exchange membrane therebetween. On the other hand, in the photovoltaic method, a concept of a hydrogen production apparatus in which a photoelectric conversion unit, a hydrogen generation unit, and an oxygen generation unit are integrated has been announced (Non-Patent Document 1). According to this, charge separation is performed by using a photoelectric conversion unit, and hydrogen generation and oxygen generation are performed using corresponding catalysts. The photoelectric conversion part is made of a material used for solar cells. For example, in Non-Patent Document 2, after charge separation is performed with three silicon pin layers, a platinum catalyst is responsible for hydrogen generation and ruthenium oxide is responsible for oxygen generation. Further, in Non-Patent Document 3, a multi-junction photoelectric conversion material that absorbs light of different wavelengths is used by using Pt as a hydrogen generation catalyst and RuO 2 as an oxygen generation catalyst to achieve high efficiency. In Patent Document 4 and Non-Patent Document 3, a hydrogen generation catalyst (NiFeO) and three layers of silicon pin are stacked in parallel on a substrate, and an oxygen generation catalyst (Co-Mo) is further formed on the silicon layer. ) To produce an integrated hydrogen production apparatus.
しかし、従来の光電変換装置が受光することにより生じる起電力を利用した気体製造装置では、電解用電極から生じる気体を電解液中の気泡として回収するため、2つの電解用電極から生じる2つの気体の回収口が近接してしまい、配管が複雑になっている。
本発明は、このような事情に鑑みてなされたものであり、発生させた気体を簡素化された配管により回収できる気体製造装置を提供する。 However, in the gas manufacturing apparatus using the electromotive force generated by receiving light by the conventional photoelectric conversion device, the gas generated from the electrode for electrolysis is recovered as bubbles in the electrolytic solution, so that two gases generated from the two electrodes for electrolysis are used. The recovery ports are close to each other, and the piping is complicated.
This invention is made | formed in view of such a situation, and provides the gas manufacturing apparatus which can collect | recover the generated gas with the simplified piping.
本発明は、このような事情に鑑みてなされたものであり、発生させた気体を簡素化された配管により回収できる気体製造装置を提供する。 However, in the gas manufacturing apparatus using the electromotive force generated by receiving light by the conventional photoelectric conversion device, the gas generated from the electrode for electrolysis is recovered as bubbles in the electrolytic solution, so that two gases generated from the two electrodes for electrolysis are used. The recovery ports are close to each other, and the piping is complicated.
This invention is made | formed in view of such a situation, and provides the gas manufacturing apparatus which can collect | recover the generated gas with the simplified piping.
本発明は、受光面およびその裏面を有する光電変換部と、前記裏面の上に並べて設けられ、かつ、電解液に接触可能な面をそれぞれ有する第1および第2電解用電極とを備え、第1および第2電解用電極が電解液と接触するとき、第1および第2電解用電極は、前記光電変換部が受光することより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体が発生するように設けられ、前記光電変換部の受光面を水平にしたとき、第1電解用電極と第2電解用電極とは、電解液に接触可能な面と水平な基準面との間の傾斜角が異なることを特徴とする気体製造装置を提供する。
The present invention includes a photoelectric conversion unit having a light receiving surface and a back surface thereof, and first and second electrodes for electrolysis that are provided side by side on the back surface and each have a surface that can contact an electrolytic solution, When the first and second electrolysis electrodes come into contact with the electrolytic solution, the first and second electrolysis electrodes use the electromotive force generated by the photoelectric conversion unit to receive light to electrolyze the electrolytic solution, respectively. When the light receiving surface of the photoelectric conversion unit is leveled, the first electrolysis electrode and the second electrolysis electrode are horizontal with the surface that can come into contact with the electrolytic solution. Provided is a gas production apparatus characterized in that an inclination angle with respect to a reference plane is different.
本発明によれば、第1および第2電解用電極は、光電変換部が受光することより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体が発生するように設けられているため、第1電解用電極の表面で第1気体を発生させることができ、第2電解用電極の表面で第2気体を発生させることができる。
本発明によれば、光電変換部の裏面上に第1電解用電極および第2電解用電極を設けるため、光電変換部の受光面に電解液を介さず光を入射させることができ、電解液による入射光の吸収や入射光の散乱を防止することができる。このことにより、光電変換部へ入射光の量を多くすることができ、光利用効率を高くすることができる。
本発明によれば、光電変換部の裏面上に第1電解用電極および第2電解用電極を設けるため、受光面に入射する光が、第1および第2電解用電極、ならびにそこからそれぞれ発生する第1気体及び第2気体により吸収や散乱されることはない。このことにより、光電変換部へ入射光の量を多くすることができ、光利用効率を高くすることができる。 According to the present invention, the first and second electrolysis electrodes are configured to electrolyze the electrolytic solution using the electromotive force generated by the light received by the photoelectric conversion unit to generate the first gas and the second gas, respectively. Since it is provided, the first gas can be generated on the surface of the first electrolysis electrode, and the second gas can be generated on the surface of the second electrolysis electrode.
According to the present invention, since the first electrolysis electrode and the second electrolysis electrode are provided on the back surface of the photoelectric conversion portion, light can be incident on the light receiving surface of the photoelectric conversion portion without passing through the electrolyte solution. It is possible to prevent absorption of incident light and scattering of incident light. As a result, the amount of incident light to the photoelectric conversion unit can be increased, and the light use efficiency can be increased.
According to the present invention, since the first electrolysis electrode and the second electrolysis electrode are provided on the back surface of the photoelectric conversion unit, the light incident on the light receiving surface is generated from the first and second electrolysis electrodes, respectively. It is not absorbed or scattered by the first gas and the second gas. As a result, the amount of incident light to the photoelectric conversion unit can be increased, and the light use efficiency can be increased.
本発明によれば、光電変換部の裏面上に第1電解用電極および第2電解用電極を設けるため、光電変換部の受光面に電解液を介さず光を入射させることができ、電解液による入射光の吸収や入射光の散乱を防止することができる。このことにより、光電変換部へ入射光の量を多くすることができ、光利用効率を高くすることができる。
本発明によれば、光電変換部の裏面上に第1電解用電極および第2電解用電極を設けるため、受光面に入射する光が、第1および第2電解用電極、ならびにそこからそれぞれ発生する第1気体及び第2気体により吸収や散乱されることはない。このことにより、光電変換部へ入射光の量を多くすることができ、光利用効率を高くすることができる。 According to the present invention, the first and second electrolysis electrodes are configured to electrolyze the electrolytic solution using the electromotive force generated by the light received by the photoelectric conversion unit to generate the first gas and the second gas, respectively. Since it is provided, the first gas can be generated on the surface of the first electrolysis electrode, and the second gas can be generated on the surface of the second electrolysis electrode.
According to the present invention, since the first electrolysis electrode and the second electrolysis electrode are provided on the back surface of the photoelectric conversion portion, light can be incident on the light receiving surface of the photoelectric conversion portion without passing through the electrolyte solution. It is possible to prevent absorption of incident light and scattering of incident light. As a result, the amount of incident light to the photoelectric conversion unit can be increased, and the light use efficiency can be increased.
According to the present invention, since the first electrolysis electrode and the second electrolysis electrode are provided on the back surface of the photoelectric conversion unit, the light incident on the light receiving surface is generated from the first and second electrolysis electrodes, respectively. It is not absorbed or scattered by the first gas and the second gas. As a result, the amount of incident light to the photoelectric conversion unit can be increased, and the light use efficiency can be increased.
本発明によれば、第1電解用電極と第2電解用電極とは、電解液に接触可能な面と水平な基準面との間の傾斜角が異なるため、第1気体が気泡として上昇するときに案内面となる第1電解用電極の電解液に接触可能な面と、第2気体が気泡として上昇するときに案内面となる第2電解用電極の電解液に接触可能な面とをずらして設けることができる。このことにより、第1電解用電極の上端部に近接して設ける第1気体を回収するための配管と、第2電解用電極の上端部に近接して設ける第2気体を回収するための配管とを重なることなく設けることができ、配管を簡素化することができる。特に、気体製造装置が、第1電解用電極および第2電解用電極のうち少なくとも一方が複数設けられた場合や、複数の気体製造装置を並べて設置した場合、複数の第1気体の回収口を接続するための配管と、複数の第2気体の回収口を接続するための配管を重なることなく設けることができ、配管を簡素化することができる。このことにより、第1気体および第2気体を回収するための配管を設けるときの設置コストを低減することができ、配管の接続ミスなどを未然に防ぐことができる。また、第1電解用電極と第2電解用電極とは、電解液に接触可能な面と水平な基準面との間の傾斜角が異なるため、光電変換部の受光面が実質的に水平になるように設置することも可能になり、設置費用を低減することができる。また、気体製造装置を池や海の水面上に設置することも可能となる。
According to the present invention, the first electrolysis electrode and the second electrolysis electrode have different inclination angles between the surface that can come into contact with the electrolytic solution and the horizontal reference surface, so that the first gas rises as bubbles. A surface that can contact the electrolyte solution of the first electrolysis electrode that sometimes becomes the guide surface, and a surface that can contact the electrolyte solution of the second electrolysis electrode that becomes the guide surface when the second gas rises as bubbles. It can be shifted. Accordingly, a pipe for recovering the first gas provided close to the upper end of the first electrolysis electrode and a pipe for recovering the second gas provided close to the upper end of the second electrolysis electrode Can be provided without overlapping, and piping can be simplified. In particular, when the gas production apparatus is provided with a plurality of at least one of the first electrolysis electrode and the second electrolysis electrode, or when a plurality of gas production apparatuses are installed side by side, a plurality of first gas recovery ports are provided. The piping for connecting and the piping for connecting the plurality of second gas recovery ports can be provided without overlapping, and the piping can be simplified. Thereby, the installation cost when providing piping for recovering the first gas and the second gas can be reduced, and piping connection errors and the like can be prevented. In addition, the first electrolysis electrode and the second electrolysis electrode have different inclination angles between the surface that can contact the electrolytic solution and the horizontal reference surface, so that the light receiving surface of the photoelectric conversion unit is substantially horizontal. It is also possible to install so that the installation cost can be reduced. Moreover, it becomes possible to install a gas production apparatus on the surface of a pond or the sea.
本発明の気体製造装置は、受光面およびその裏面を有する光電変換部と、前記裏面の上に並べて設けられ、かつ、電解液に接触可能な面をそれぞれ有する第1および第2電解用電極とを備え、第1および第2電解用電極が電解液と接触するとき、第1および第2電解用電極は、前記光電変換部が受光することより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体が発生するように設けられ、前記光電変換部の受光面を水平にしたとき、第1電解用電極と第2電解用電極とは、電解液に接触可能な面と水平な基準面との間の傾斜角が異なることを特徴とする。
The gas production apparatus of the present invention includes a photoelectric conversion unit having a light receiving surface and a back surface thereof, first and second electrodes for electrolysis that are provided side by side on the back surface and each have a surface that can contact an electrolyte solution When the first and second electrolysis electrodes are in contact with the electrolytic solution, the first and second electrolysis electrodes electrolyze the electrolytic solution using electromotive force generated by the photoelectric conversion unit receiving light. The first electrolysis electrode and the second electrolysis electrode can contact the electrolyte when the first gas and the second gas are generated, respectively, and the light receiving surface of the photoelectric conversion unit is horizontal. The inclination angle between the surface and the horizontal reference surface is different.
本発明において、傾斜角とは、水平な基準面に対する傾斜角であり、0度以上180度以下の範囲の角度である。2つの面を傾斜角で比較する場合、一方の面が0度より大きく90度よりも小さいx度の傾斜角を有し、他方の面が(180―x)度の傾斜角を有する場合、この2つの面の傾斜角は異なる。
本発明において、第1電極および第2電極とは、光電変換部の光起電力の出力ための電極であり、第1電解用電極および第2電解用電極とは、電解液を電気分解するための電極である。
本発明の気体製造装置において、前記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面の上端に近接して設けられた第1気体排出口と、第2電解用電極の電解液に接触可能な面の上端に近接して設けられた第2気体排出口とをさらに備え、第1電解用電極は、第1気体を第1気体排出口から回収できるように傾斜した電解液に接触可能な面を有し、第2電解用電極は、第2気体を第2気体排出口から回収できるように傾斜した電解液に接触可能な面を有することが好ましい。
このような構成によれば、第1気体を第1気体排出口から回収することができ、第2気体を第2気体排出口から回収することができる。
本発明の気体製造装置において、前記光電変換部は、方形状の受光面を有し、第1および第2気体排出口は、それぞれ前記光電変換部の受光面の対向する辺に近接して設けられたことが好ましい。このような構成によれば、第1気体排出口および第2気体排出口を光電変換部の両側に設けることができ、第1気体を回収するための配管および第2気体を回収するための配管を簡素化することができる。 In the present invention, the inclination angle is an inclination angle with respect to a horizontal reference plane, and is an angle in a range of 0 degrees to 180 degrees. When comparing two surfaces with an inclination angle, if one surface has an inclination angle of x degrees greater than 0 degrees and less than 90 degrees and the other surface has an inclination angle of (180-x) degrees, The inclination angles of these two surfaces are different.
In the present invention, the first electrode and the second electrode are electrodes for outputting photovoltaic power of the photoelectric conversion unit, and the first electrolysis electrode and the second electrolysis electrode are for electrolyzing the electrolytic solution. Electrode.
In the gas production apparatus of the present invention, when the light receiving surface of the photoelectric conversion unit is leveled, a first gas discharge port provided close to the upper end of the surface of the first electrolysis electrode that can contact the electrolytic solution; A second gas discharge port provided close to the upper end of the surface of the second electrolysis electrode that can contact the electrolyte, and the first electrolysis electrode collects the first gas from the first gas discharge port. The second electrolysis electrode may have a surface that can contact the electrolyte solution that is inclined so that the second gas can be recovered from the second gas discharge port. preferable.
According to such a configuration, the first gas can be recovered from the first gas outlet, and the second gas can be recovered from the second gas outlet.
In the gas manufacturing apparatus of the present invention, the photoelectric conversion unit has a rectangular light receiving surface, and the first and second gas discharge ports are provided close to opposite sides of the light receiving surface of the photoelectric conversion unit, respectively. Preferably. According to such a configuration, the first gas outlet and the second gas outlet can be provided on both sides of the photoelectric conversion unit, and the pipe for recovering the first gas and the pipe for recovering the second gas Can be simplified.
本発明において、第1電極および第2電極とは、光電変換部の光起電力の出力ための電極であり、第1電解用電極および第2電解用電極とは、電解液を電気分解するための電極である。
本発明の気体製造装置において、前記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面の上端に近接して設けられた第1気体排出口と、第2電解用電極の電解液に接触可能な面の上端に近接して設けられた第2気体排出口とをさらに備え、第1電解用電極は、第1気体を第1気体排出口から回収できるように傾斜した電解液に接触可能な面を有し、第2電解用電極は、第2気体を第2気体排出口から回収できるように傾斜した電解液に接触可能な面を有することが好ましい。
このような構成によれば、第1気体を第1気体排出口から回収することができ、第2気体を第2気体排出口から回収することができる。
本発明の気体製造装置において、前記光電変換部は、方形状の受光面を有し、第1および第2気体排出口は、それぞれ前記光電変換部の受光面の対向する辺に近接して設けられたことが好ましい。このような構成によれば、第1気体排出口および第2気体排出口を光電変換部の両側に設けることができ、第1気体を回収するための配管および第2気体を回収するための配管を簡素化することができる。 In the present invention, the inclination angle is an inclination angle with respect to a horizontal reference plane, and is an angle in a range of 0 degrees to 180 degrees. When comparing two surfaces with an inclination angle, if one surface has an inclination angle of x degrees greater than 0 degrees and less than 90 degrees and the other surface has an inclination angle of (180-x) degrees, The inclination angles of these two surfaces are different.
In the present invention, the first electrode and the second electrode are electrodes for outputting photovoltaic power of the photoelectric conversion unit, and the first electrolysis electrode and the second electrolysis electrode are for electrolyzing the electrolytic solution. Electrode.
In the gas production apparatus of the present invention, when the light receiving surface of the photoelectric conversion unit is leveled, a first gas discharge port provided close to the upper end of the surface of the first electrolysis electrode that can contact the electrolytic solution; A second gas discharge port provided close to the upper end of the surface of the second electrolysis electrode that can contact the electrolyte, and the first electrolysis electrode collects the first gas from the first gas discharge port. The second electrolysis electrode may have a surface that can contact the electrolyte solution that is inclined so that the second gas can be recovered from the second gas discharge port. preferable.
According to such a configuration, the first gas can be recovered from the first gas outlet, and the second gas can be recovered from the second gas outlet.
In the gas manufacturing apparatus of the present invention, the photoelectric conversion unit has a rectangular light receiving surface, and the first and second gas discharge ports are provided close to opposite sides of the light receiving surface of the photoelectric conversion unit, respectively. Preferably. According to such a configuration, the first gas outlet and the second gas outlet can be provided on both sides of the photoelectric conversion unit, and the pipe for recovering the first gas and the pipe for recovering the second gas Can be simplified.
本発明の気体製造装置において、第1および第2電解用電極は、少なくとも一方が複数であり、かつ、それぞれ帯状の電解液に接触可能な面を有し、かつ、この面の長辺が隣接するように交互に設けられたことが好ましい。
このような構成によれば、第1気体が発生する反応が生じる部分と、第2気体が発生する反応が生じる部分との間の距離を短くすることができ、電解液中で生じるイオン濃度の不均衡をより少なくすることができる。また、電解液に接触可能な面を帯状とすることにより、第1気体および第2気体を容易に回収することができる。
本発明の気体製造装置において、第1電解用電極と前記光電変換部の裏面との間に設けられた第1型部および第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部をさらに備え、第1型部および第2型部は、前記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とが異なるように成形されたことが好ましい。
このような構成によれば、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とを容易に異なるようにすることができる。 In the gas production apparatus of the present invention, at least one of the first and second electrolysis electrodes has a plurality of surfaces that can contact the strip-shaped electrolyte solution, and the long sides of the surfaces are adjacent to each other. It is preferable that they are provided alternately.
According to such a configuration, the distance between the portion where the reaction generating the first gas occurs and the portion where the reaction generating the second gas occurs can be shortened, and the ion concentration generated in the electrolyte can be reduced. Imbalance can be reduced. Moreover, the 1st gas and 2nd gas can be collect | recovered easily by making the surface which can contact electrolyte solution into strip | belt shape.
In the gas manufacturing apparatus of the present invention, the first mold part and the second electrolysis electrode provided between the first electrolysis electrode and the back surface of the photoelectric conversion unit and the back surface of the photoelectric conversion unit are provided. The first mold part and the second mold part have a horizontal reference with a surface that can contact the electrolyte of the first electrolysis electrode when the light receiving surface of the photoelectric conversion unit is horizontal. It is preferable that the tilt angle between the reference surface and the tilt angle between the surface and the reference surface is different from the tilt angle between the second electrolysis electrode and the electrolyte.
According to such a configuration, the inclination angle between the surface of the first electrolysis electrode that can contact the electrolytic solution and the horizontal reference surface, the surface of the second electrolysis electrode that can contact the electrolytic solution, and the reference The inclination angle between the surfaces can be easily made different.
このような構成によれば、第1気体が発生する反応が生じる部分と、第2気体が発生する反応が生じる部分との間の距離を短くすることができ、電解液中で生じるイオン濃度の不均衡をより少なくすることができる。また、電解液に接触可能な面を帯状とすることにより、第1気体および第2気体を容易に回収することができる。
本発明の気体製造装置において、第1電解用電極と前記光電変換部の裏面との間に設けられた第1型部および第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部をさらに備え、第1型部および第2型部は、前記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とが異なるように成形されたことが好ましい。
このような構成によれば、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とを容易に異なるようにすることができる。 In the gas production apparatus of the present invention, at least one of the first and second electrolysis electrodes has a plurality of surfaces that can contact the strip-shaped electrolyte solution, and the long sides of the surfaces are adjacent to each other. It is preferable that they are provided alternately.
According to such a configuration, the distance between the portion where the reaction generating the first gas occurs and the portion where the reaction generating the second gas occurs can be shortened, and the ion concentration generated in the electrolyte can be reduced. Imbalance can be reduced. Moreover, the 1st gas and 2nd gas can be collect | recovered easily by making the surface which can contact electrolyte solution into strip | belt shape.
In the gas manufacturing apparatus of the present invention, the first mold part and the second electrolysis electrode provided between the first electrolysis electrode and the back surface of the photoelectric conversion unit and the back surface of the photoelectric conversion unit are provided. The first mold part and the second mold part have a horizontal reference with a surface that can contact the electrolyte of the first electrolysis electrode when the light receiving surface of the photoelectric conversion unit is horizontal. It is preferable that the tilt angle between the reference surface and the tilt angle between the surface and the reference surface is different from the tilt angle between the second electrolysis electrode and the electrolyte.
According to such a configuration, the inclination angle between the surface of the first electrolysis electrode that can contact the electrolytic solution and the horizontal reference surface, the surface of the second electrolysis electrode that can contact the electrolytic solution, and the reference The inclination angle between the surfaces can be easily made different.
本発明の気体製造装置において、第1型部および第2型部は、固体樹脂からなることが好ましい。
このような構成によれば、第1型部と第2型部を容易に成形することができる。
本発明の気体製造装置において、第1型部と第1電解用電極との間または第2型部と第2電解用電極との間に第1導電部をさらに備えることが好ましい。
このような構成によれば、光電変換部が受光することにより生じた起電力を第1および第2電解用電極に出力するとき、内部抵抗をより小さくすることができる。
本発明の気体製造装置において、第1および第2電解用電極は、それぞれ電解液に接触可能な面に傾斜方向に伸びる溝状のくぼみを有することが好ましい。
このような構成によれば、第1気体および第2気体をそれぞれ気泡として溝状のくぼみに沿って上昇させることができ、第1気体および第2気体を分離して回収することができる。また、この構成によれば、第1電解用電極と第2電解用電極との間に設ける隔壁も省略可能である。 In the gas production apparatus of the present invention, the first mold part and the second mold part are preferably made of a solid resin.
According to such a configuration, the first mold part and the second mold part can be easily molded.
In the gas production apparatus of the present invention, it is preferable that a first conductive part is further provided between the first mold part and the first electrolysis electrode or between the second mold part and the second electrolysis electrode.
According to such a configuration, when the electromotive force generated by the photoelectric conversion unit receiving light is output to the first and second electrolysis electrodes, the internal resistance can be further reduced.
In the gas production apparatus of the present invention, each of the first and second electrolysis electrodes preferably has a groove-like depression extending in an inclined direction on a surface that can contact the electrolytic solution.
According to such a configuration, the first gas and the second gas can be raised as bubbles along the groove-shaped depression, and the first gas and the second gas can be separated and recovered. Moreover, according to this structure, the partition provided between the electrode for 1st electrolysis and the electrode for 2nd electrolysis is also omissible.
このような構成によれば、第1型部と第2型部を容易に成形することができる。
本発明の気体製造装置において、第1型部と第1電解用電極との間または第2型部と第2電解用電極との間に第1導電部をさらに備えることが好ましい。
このような構成によれば、光電変換部が受光することにより生じた起電力を第1および第2電解用電極に出力するとき、内部抵抗をより小さくすることができる。
本発明の気体製造装置において、第1および第2電解用電極は、それぞれ電解液に接触可能な面に傾斜方向に伸びる溝状のくぼみを有することが好ましい。
このような構成によれば、第1気体および第2気体をそれぞれ気泡として溝状のくぼみに沿って上昇させることができ、第1気体および第2気体を分離して回収することができる。また、この構成によれば、第1電解用電極と第2電解用電極との間に設ける隔壁も省略可能である。 In the gas production apparatus of the present invention, the first mold part and the second mold part are preferably made of a solid resin.
According to such a configuration, the first mold part and the second mold part can be easily molded.
In the gas production apparatus of the present invention, it is preferable that a first conductive part is further provided between the first mold part and the first electrolysis electrode or between the second mold part and the second electrolysis electrode.
According to such a configuration, when the electromotive force generated by the photoelectric conversion unit receiving light is output to the first and second electrolysis electrodes, the internal resistance can be further reduced.
In the gas production apparatus of the present invention, each of the first and second electrolysis electrodes preferably has a groove-like depression extending in an inclined direction on a surface that can contact the electrolytic solution.
According to such a configuration, the first gas and the second gas can be raised as bubbles along the groove-shaped depression, and the first gas and the second gas can be separated and recovered. Moreover, according to this structure, the partition provided between the electrode for 1st electrolysis and the electrode for 2nd electrolysis is also omissible.
本発明の気体製造装置において、光電変換部の受光面を水平にしたとき、第1電解用電極は、水平な基準面との間の傾斜角が1度以上60度以下の電解液に接触可能な面を有し、第2電解用電極は、前記基準面との間の傾斜角が120度以上179度以下の電解液に接触可能な面を有することが好ましい。
このような構成によれば、第1気体を回収するための回収口と第2気体を回収するための回収口とをそれぞれ気体製造装置の両側に設けることができ、第1気体を回収するための配管と第2気体を回収するための配管をそれぞれ気体製造装置の両側に設けることができる。
本発明の気体製造装置において、前記光電変換部は、受光することによりその受光面と裏面との間に起電力が生じ、第1電解用電極は、前記光電変換部の裏面と電気的に接続し、第2電解用電極は、前記光電変換部の受光面と電気的に接続することが好ましい。
このような構成によれば、光電変換部に積層構造のものを利用することができる。 In the gas production apparatus of the present invention, when the light receiving surface of the photoelectric conversion unit is horizontal, the first electrolysis electrode can contact an electrolytic solution having an inclination angle of 1 degree to 60 degrees with the horizontal reference plane It is preferable that the second electrolysis electrode has a surface that can contact an electrolytic solution having an inclination angle of 120 degrees or more and 179 degrees or less with respect to the reference surface.
According to such a configuration, a recovery port for recovering the first gas and a recovery port for recovering the second gas can be provided on both sides of the gas production device, respectively, in order to recover the first gas. And a pipe for recovering the second gas can be provided on both sides of the gas production apparatus.
In the gas manufacturing apparatus of the present invention, the photoelectric conversion unit receives light to generate an electromotive force between the light receiving surface and the back surface, and the first electrolysis electrode is electrically connected to the back surface of the photoelectric conversion unit. And it is preferable that the 2nd electrode for electrolysis is electrically connected with the light-receiving surface of the said photoelectric conversion part.
According to such a structure, the thing of a laminated structure can be utilized for a photoelectric conversion part.
このような構成によれば、第1気体を回収するための回収口と第2気体を回収するための回収口とをそれぞれ気体製造装置の両側に設けることができ、第1気体を回収するための配管と第2気体を回収するための配管をそれぞれ気体製造装置の両側に設けることができる。
本発明の気体製造装置において、前記光電変換部は、受光することによりその受光面と裏面との間に起電力が生じ、第1電解用電極は、前記光電変換部の裏面と電気的に接続し、第2電解用電極は、前記光電変換部の受光面と電気的に接続することが好ましい。
このような構成によれば、光電変換部に積層構造のものを利用することができる。 In the gas production apparatus of the present invention, when the light receiving surface of the photoelectric conversion unit is horizontal, the first electrolysis electrode can contact an electrolytic solution having an inclination angle of 1 degree to 60 degrees with the horizontal reference plane It is preferable that the second electrolysis electrode has a surface that can contact an electrolytic solution having an inclination angle of 120 degrees or more and 179 degrees or less with respect to the reference surface.
According to such a configuration, a recovery port for recovering the first gas and a recovery port for recovering the second gas can be provided on both sides of the gas production device, respectively, in order to recover the first gas. And a pipe for recovering the second gas can be provided on both sides of the gas production apparatus.
In the gas manufacturing apparatus of the present invention, the photoelectric conversion unit receives light to generate an electromotive force between the light receiving surface and the back surface, and the first electrolysis electrode is electrically connected to the back surface of the photoelectric conversion unit. And it is preferable that the 2nd electrode for electrolysis is electrically connected with the light-receiving surface of the said photoelectric conversion part.
According to such a structure, the thing of a laminated structure can be utilized for a photoelectric conversion part.
本発明の気体製造装置において、前記光電変換部の受光面に接触する第1電極をさらに備えることが好ましい。
このような構成によれば、内部抵抗を小さくすることができる。
本発明の気体製造装置において、第1電極と第2電解用電極とを電気的に接続する第2導電部をさらに備えることが好ましい。
このような構成によれば、光電変換部の受光面と第2電解用電極とを電気的に接続することができる。
本発明の気体製造装置において、第2導電部は、前記光電変換部を貫通するコンタクトホールに設けられたことが好ましい。
このような構成によれば、光電変換部の受光面と第2電解用電極との間の配線距離を短くすることができ、内部抵抗を小さくすることができる。 In the gas production apparatus of the present invention, it is preferable that the gas production apparatus further includes a first electrode that contacts the light receiving surface of the photoelectric conversion unit.
According to such a configuration, the internal resistance can be reduced.
In the gas manufacturing apparatus of the present invention, it is preferable to further include a second conductive part that electrically connects the first electrode and the second electrolysis electrode.
According to such a structure, the light-receiving surface of a photoelectric conversion part and the 2nd electrode for electrolysis can be electrically connected.
In the gas manufacturing apparatus of the present invention, it is preferable that the second conductive portion is provided in a contact hole that penetrates the photoelectric conversion portion.
According to such a configuration, the wiring distance between the light receiving surface of the photoelectric conversion unit and the second electrolysis electrode can be shortened, and the internal resistance can be reduced.
このような構成によれば、内部抵抗を小さくすることができる。
本発明の気体製造装置において、第1電極と第2電解用電極とを電気的に接続する第2導電部をさらに備えることが好ましい。
このような構成によれば、光電変換部の受光面と第2電解用電極とを電気的に接続することができる。
本発明の気体製造装置において、第2導電部は、前記光電変換部を貫通するコンタクトホールに設けられたことが好ましい。
このような構成によれば、光電変換部の受光面と第2電解用電極との間の配線距離を短くすることができ、内部抵抗を小さくすることができる。 In the gas production apparatus of the present invention, it is preferable that the gas production apparatus further includes a first electrode that contacts the light receiving surface of the photoelectric conversion unit.
According to such a configuration, the internal resistance can be reduced.
In the gas manufacturing apparatus of the present invention, it is preferable to further include a second conductive part that electrically connects the first electrode and the second electrolysis electrode.
According to such a structure, the light-receiving surface of a photoelectric conversion part and the 2nd electrode for electrolysis can be electrically connected.
In the gas manufacturing apparatus of the present invention, it is preferable that the second conductive portion is provided in a contact hole that penetrates the photoelectric conversion portion.
According to such a configuration, the wiring distance between the light receiving surface of the photoelectric conversion unit and the second electrolysis electrode can be shortened, and the internal resistance can be reduced.
本発明の気体製造装置において、第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部を備え、第2型部は、絶縁性を有し、かつ、前記光電変換部の側面を覆うように設けられ、第2導電部は、第2型部の前記光電変換部の側面を覆う部分の上に設けられたことが好ましい。
このような構成によれば、第2導電部を少ない工程で設けることができ、製造コストを低減することができる。
本発明の気体製造装置において、第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部を備え、第2型部は、絶縁性を有し、かつ、前記光電変換部の側面を覆うように設けられ、第2電解用電極は、第2型部の前記光電変換部の側面を覆う部分の上に設けられ、かつ、第1電極と接触することが好ましい。
このような構成によれば、第2導電部を設けることなく、第1電極と第2電解用電極とを電気的に接続することができる。 The gas manufacturing apparatus of the present invention includes a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part, the second mold part having insulating properties, and the photoelectric conversion unit. It is preferable that the second conductive portion is provided on a portion of the second mold portion that covers the side surface of the photoelectric conversion portion.
According to such a configuration, the second conductive portion can be provided with a small number of steps, and the manufacturing cost can be reduced.
The gas manufacturing apparatus of the present invention includes a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part, the second mold part having insulating properties, and the photoelectric conversion unit. The second electrolysis electrode is preferably provided on a portion of the second mold part that covers the side surface of the photoelectric conversion unit, and is in contact with the first electrode.
According to such a configuration, the first electrode and the second electrolysis electrode can be electrically connected without providing the second conductive portion.
このような構成によれば、第2導電部を少ない工程で設けることができ、製造コストを低減することができる。
本発明の気体製造装置において、第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部を備え、第2型部は、絶縁性を有し、かつ、前記光電変換部の側面を覆うように設けられ、第2電解用電極は、第2型部の前記光電変換部の側面を覆う部分の上に設けられ、かつ、第1電極と接触することが好ましい。
このような構成によれば、第2導電部を設けることなく、第1電極と第2電解用電極とを電気的に接続することができる。 The gas manufacturing apparatus of the present invention includes a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part, the second mold part having insulating properties, and the photoelectric conversion unit. It is preferable that the second conductive portion is provided on a portion of the second mold portion that covers the side surface of the photoelectric conversion portion.
According to such a configuration, the second conductive portion can be provided with a small number of steps, and the manufacturing cost can be reduced.
The gas manufacturing apparatus of the present invention includes a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part, the second mold part having insulating properties, and the photoelectric conversion unit. The second electrolysis electrode is preferably provided on a portion of the second mold part that covers the side surface of the photoelectric conversion unit, and is in contact with the first electrode.
According to such a configuration, the first electrode and the second electrolysis electrode can be electrically connected without providing the second conductive portion.
本発明の気体製造装置において、第1電解用電極と前記光電変換部の裏面との間に設けられた第1型部と、第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部とを備え、前記光電変換部の裏面と第1型部との間および前記光電変換部の裏面と第2型部との間に設けられた第2電極をさらに備えることが好ましい。
このような構成によれば、光電変換部が受光することにより生じる起電力を第1および第2電解用電極に出力するとき、光電変換部の裏面と第1電解用電極との間の内部抵抗をより小さくすることができる。
本発明の気体製造装置において、第2電極と第1電解用電極とを電気的に接続する第3導電部をさらに備えることが好ましい。
このような構成によれば、光電変換部の裏面と第1電解用電極とを電気的に接続することができる。 In the gas manufacturing apparatus of the present invention, the first mold part provided between the first electrolysis electrode and the back surface of the photoelectric conversion unit, and the second electrolysis electrode and the back surface of the photoelectric conversion unit are provided. A second electrode provided between the back surface of the photoelectric conversion unit and the first mold unit and between the back surface of the photoelectric conversion unit and the second mold unit. Is preferred.
According to such a configuration, when the electromotive force generated when the photoelectric conversion unit receives light is output to the first and second electrolysis electrodes, the internal resistance between the back surface of the photoelectric conversion unit and the first electrolysis electrode Can be made smaller.
In the gas manufacturing apparatus of the present invention, it is preferable to further include a third conductive part that electrically connects the second electrode and the first electrolysis electrode.
According to such a structure, the back surface of a photoelectric conversion part and the 1st electrode for electrolysis can be electrically connected.
このような構成によれば、光電変換部が受光することにより生じる起電力を第1および第2電解用電極に出力するとき、光電変換部の裏面と第1電解用電極との間の内部抵抗をより小さくすることができる。
本発明の気体製造装置において、第2電極と第1電解用電極とを電気的に接続する第3導電部をさらに備えることが好ましい。
このような構成によれば、光電変換部の裏面と第1電解用電極とを電気的に接続することができる。 In the gas manufacturing apparatus of the present invention, the first mold part provided between the first electrolysis electrode and the back surface of the photoelectric conversion unit, and the second electrolysis electrode and the back surface of the photoelectric conversion unit are provided. A second electrode provided between the back surface of the photoelectric conversion unit and the first mold unit and between the back surface of the photoelectric conversion unit and the second mold unit. Is preferred.
According to such a configuration, when the electromotive force generated when the photoelectric conversion unit receives light is output to the first and second electrolysis electrodes, the internal resistance between the back surface of the photoelectric conversion unit and the first electrolysis electrode Can be made smaller.
In the gas manufacturing apparatus of the present invention, it is preferable to further include a third conductive part that electrically connects the second electrode and the first electrolysis electrode.
According to such a structure, the back surface of a photoelectric conversion part and the 1st electrode for electrolysis can be electrically connected.
本発明の気体製造装置において、第3導電部は、第1型部の側部を覆うように設けられ、かつ、第2電極と接触することが好ましい。
このような構成によれば、容易に光電変換部の裏面と第1電解用電極とを電気的に接続することができる。
本発明の気体製造装置において、前記光電変換部は、受光することにより前記裏面の第1および第2区域間に電位差が生じ、第1区域は、第1電解用電極と電気的に接続し、第2区域は、第2電解用電極と電気的に接続することが好ましい。
このような構成によれば、光電変換部を第1区域と第2区域との間に起電力が生じるものとすることができる。 In the gas manufacturing apparatus of the present invention, it is preferable that the third conductive portion is provided so as to cover the side portion of the first mold portion and is in contact with the second electrode.
According to such a structure, the back surface of a photoelectric conversion part and the 1st electrode for electrolysis can be electrically connected easily.
In the gas manufacturing apparatus of the present invention, the photoelectric conversion unit generates a potential difference between the first and second areas on the back surface by receiving light, the first area is electrically connected to the first electrolysis electrode, The second area is preferably electrically connected to the second electrolysis electrode.
According to such a configuration, an electromotive force can be generated between the first area and the second area in the photoelectric conversion unit.
このような構成によれば、容易に光電変換部の裏面と第1電解用電極とを電気的に接続することができる。
本発明の気体製造装置において、前記光電変換部は、受光することにより前記裏面の第1および第2区域間に電位差が生じ、第1区域は、第1電解用電極と電気的に接続し、第2区域は、第2電解用電極と電気的に接続することが好ましい。
このような構成によれば、光電変換部を第1区域と第2区域との間に起電力が生じるものとすることができる。 In the gas manufacturing apparatus of the present invention, it is preferable that the third conductive portion is provided so as to cover the side portion of the first mold portion and is in contact with the second electrode.
According to such a structure, the back surface of a photoelectric conversion part and the 1st electrode for electrolysis can be electrically connected easily.
In the gas manufacturing apparatus of the present invention, the photoelectric conversion unit generates a potential difference between the first and second areas on the back surface by receiving light, the first area is electrically connected to the first electrolysis electrode, The second area is preferably electrically connected to the second electrolysis electrode.
According to such a configuration, an electromotive force can be generated between the first area and the second area in the photoelectric conversion unit.
本発明の気体製造装置において、前記光電変換部は、n型半導体部およびp型半導体部を有する少なくとも1つの半導体材料からなり、第1および第2区域のうち、一方は前記n型半導体部の一部であり、他方は前記p型半導体部の一部であることが好ましい。
このような構成によれば、光電変換部が受光することにより、光電変換部の裏面の第1および第2区域間に起電力を生じさせることができる。
本発明の気体製造装置において、透光性基板をさらに備え、前記光電変換部は、前記透光性基板の上に設けられたことが好ましい。
このような構成によれば、光電変換部を透光性基板の上に形成することができる。 In the gas manufacturing apparatus of the present invention, the photoelectric conversion part is made of at least one semiconductor material having an n-type semiconductor part and a p-type semiconductor part, and one of the first and second areas is the n-type semiconductor part. It is preferable that the other part is a part of the p-type semiconductor part.
According to such a configuration, an electromotive force can be generated between the first and second areas on the back surface of the photoelectric conversion unit when the photoelectric conversion unit receives light.
In the gas manufacturing apparatus of the present invention, it is preferable that a translucent substrate is further provided, and the photoelectric conversion unit is provided on the translucent substrate.
According to such a structure, a photoelectric conversion part can be formed on a translucent board | substrate.
このような構成によれば、光電変換部が受光することにより、光電変換部の裏面の第1および第2区域間に起電力を生じさせることができる。
本発明の気体製造装置において、透光性基板をさらに備え、前記光電変換部は、前記透光性基板の上に設けられたことが好ましい。
このような構成によれば、光電変換部を透光性基板の上に形成することができる。 In the gas manufacturing apparatus of the present invention, the photoelectric conversion part is made of at least one semiconductor material having an n-type semiconductor part and a p-type semiconductor part, and one of the first and second areas is the n-type semiconductor part. It is preferable that the other part is a part of the p-type semiconductor part.
According to such a configuration, an electromotive force can be generated between the first and second areas on the back surface of the photoelectric conversion unit when the photoelectric conversion unit receives light.
In the gas manufacturing apparatus of the present invention, it is preferable that a translucent substrate is further provided, and the photoelectric conversion unit is provided on the translucent substrate.
According to such a structure, a photoelectric conversion part can be formed on a translucent board | substrate.
本発明の気体製造装置において、透光性基板をさらに備え、前記光電変換部は、前記透光性基板の上に設けられ、前記透光性基板は、前記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とが異なるように成形されたことが好ましい。
このような構成によれば、透光性基板の形により第1および第2電解用電極の電解液に接触可能な面の傾斜角が異なるように、第1および第2電解用電極を設けることができる。
本発明の気体製造装置において、第2電解用電極は、絶縁部を介して前記光電変換部の裏面上に設けられたことが好ましい。
このような構成によれば、リーク電流が生じることを防止することができる。
本発明の気体製造装置において、前記光電変換部は、p型半導体層、i型半導体層およびn型半導体層からなる光電変換層を有することが好ましい。
このような構成によれば、光電変換部に光を入射させることにより起電力を生じさせることができる。 The gas manufacturing apparatus of the present invention further includes a translucent substrate, the photoelectric conversion unit is provided on the translucent substrate, and the translucent substrate has a light receiving surface of the photoelectric conversion unit horizontally. Then, the inclination angle between the surface of the first electrolysis electrode that can contact the electrolytic solution and the horizontal reference surface, and the surface between the surface of the second electrolysis electrode that can contact the electrolytic solution and the reference surface. It is preferable that it is formed so as to have a different inclination angle.
According to such a configuration, the first and second electrolysis electrodes are provided so that the inclination angles of the surfaces of the first and second electrolysis electrodes that can contact the electrolytic solution differ depending on the shape of the translucent substrate. Can do.
In the gas production apparatus of the present invention, it is preferable that the second electrolysis electrode is provided on the back surface of the photoelectric conversion unit via an insulating unit.
According to such a configuration, it is possible to prevent a leak current from occurring.
In the gas production apparatus of the present invention, it is preferable that the photoelectric conversion unit has a photoelectric conversion layer including a p-type semiconductor layer, an i-type semiconductor layer, and an n-type semiconductor layer.
According to such a configuration, an electromotive force can be generated by causing light to enter the photoelectric conversion unit.
このような構成によれば、透光性基板の形により第1および第2電解用電極の電解液に接触可能な面の傾斜角が異なるように、第1および第2電解用電極を設けることができる。
本発明の気体製造装置において、第2電解用電極は、絶縁部を介して前記光電変換部の裏面上に設けられたことが好ましい。
このような構成によれば、リーク電流が生じることを防止することができる。
本発明の気体製造装置において、前記光電変換部は、p型半導体層、i型半導体層およびn型半導体層からなる光電変換層を有することが好ましい。
このような構成によれば、光電変換部に光を入射させることにより起電力を生じさせることができる。 The gas manufacturing apparatus of the present invention further includes a translucent substrate, the photoelectric conversion unit is provided on the translucent substrate, and the translucent substrate has a light receiving surface of the photoelectric conversion unit horizontally. Then, the inclination angle between the surface of the first electrolysis electrode that can contact the electrolytic solution and the horizontal reference surface, and the surface between the surface of the second electrolysis electrode that can contact the electrolytic solution and the reference surface. It is preferable that it is formed so as to have a different inclination angle.
According to such a configuration, the first and second electrolysis electrodes are provided so that the inclination angles of the surfaces of the first and second electrolysis electrodes that can contact the electrolytic solution differ depending on the shape of the translucent substrate. Can do.
In the gas production apparatus of the present invention, it is preferable that the second electrolysis electrode is provided on the back surface of the photoelectric conversion unit via an insulating unit.
According to such a configuration, it is possible to prevent a leak current from occurring.
In the gas production apparatus of the present invention, it is preferable that the photoelectric conversion unit has a photoelectric conversion layer including a p-type semiconductor layer, an i-type semiconductor layer, and an n-type semiconductor layer.
According to such a configuration, an electromotive force can be generated by causing light to enter the photoelectric conversion unit.
本発明の気体製造装置において、前記光電変換部は、直列接続した複数の光電変換層を含み、前記複数の光電変換層は、受光することにより生じる起電力を第1電解用電極および第2電解用電極に供給することが好ましい。
このような構成によれば、容易に高電圧の起電力を第1および第2電解用電極に出力することができる。
本発明の気体製造装置において、各光電変換層は、第4導電部により直列接続されたことが好ましい。
このような構成によれば、各光電変換層を並べて設けることができる。
本発明の気体製造装置において、第1電解用電極および第2電解用電極のうち、一方は電解液からH2を発生させる水素発生部であり、他方は電解液からO2を発生させる酸素発生部であり、前記水素発生部および前記酸素発生部は、それぞれ電解液からH2が発生する反応の触媒および電解液からO2が発生する反応の触媒を含むことが好ましい。
このような構成によれば、燃料電池の燃料となる水素を製造することができる。 In the gas production apparatus of the present invention, the photoelectric conversion unit includes a plurality of photoelectric conversion layers connected in series, and the plurality of photoelectric conversion layers generate electromotive force generated by receiving light in the first electrolysis electrode and the second electrolysis. It is preferable to supply it to the electrode for use.
According to such a configuration, a high voltage electromotive force can be easily output to the first and second electrolysis electrodes.
In the gas manufacturing apparatus of the present invention, each photoelectric conversion layer is preferably connected in series by a fourth conductive portion.
According to such a configuration, the photoelectric conversion layers can be provided side by side.
In the gas production apparatus of the present invention, one of the first electrolysis electrode and the second electrolysis electrode is a hydrogen generation unit that generates H 2 from the electrolytic solution, and the other is oxygen generation that generates O 2 from the electrolytic solution. It is preferable that the hydrogen generation unit and the oxygen generation unit include a catalyst for a reaction in which H 2 is generated from the electrolytic solution and a catalyst for a reaction in which O 2 is generated from the electrolytic solution, respectively.
According to such a configuration, hydrogen serving as a fuel for the fuel cell can be produced.
このような構成によれば、容易に高電圧の起電力を第1および第2電解用電極に出力することができる。
本発明の気体製造装置において、各光電変換層は、第4導電部により直列接続されたことが好ましい。
このような構成によれば、各光電変換層を並べて設けることができる。
本発明の気体製造装置において、第1電解用電極および第2電解用電極のうち、一方は電解液からH2を発生させる水素発生部であり、他方は電解液からO2を発生させる酸素発生部であり、前記水素発生部および前記酸素発生部は、それぞれ電解液からH2が発生する反応の触媒および電解液からO2が発生する反応の触媒を含むことが好ましい。
このような構成によれば、燃料電池の燃料となる水素を製造することができる。 In the gas production apparatus of the present invention, the photoelectric conversion unit includes a plurality of photoelectric conversion layers connected in series, and the plurality of photoelectric conversion layers generate electromotive force generated by receiving light in the first electrolysis electrode and the second electrolysis. It is preferable to supply it to the electrode for use.
According to such a configuration, a high voltage electromotive force can be easily output to the first and second electrolysis electrodes.
In the gas manufacturing apparatus of the present invention, each photoelectric conversion layer is preferably connected in series by a fourth conductive portion.
According to such a configuration, the photoelectric conversion layers can be provided side by side.
In the gas production apparatus of the present invention, one of the first electrolysis electrode and the second electrolysis electrode is a hydrogen generation unit that generates H 2 from the electrolytic solution, and the other is oxygen generation that generates O 2 from the electrolytic solution. It is preferable that the hydrogen generation unit and the oxygen generation unit include a catalyst for a reaction in which H 2 is generated from the electrolytic solution and a catalyst for a reaction in which O 2 is generated from the electrolytic solution, respectively.
According to such a configuration, hydrogen serving as a fuel for the fuel cell can be produced.
本発明の気体製造装置において、前記水素発生部および前記酸素発生部のうち少なくとも一方は、前記受光面の面積より大きい触媒表面積を有することが好ましい。
このような構成によれば、より効率的に水素および酸素を製造することができる。
本発明の気体製造装置において、前記水素発生部および前記酸素発生部のうち少なくとも一方は、触媒が担持された多孔質の導電体であることが好ましい。
このような構成によれば、水素または酸素が発生する反応の触媒面積を広くすることができる。
本発明の気体製造装置において、前記水素発生部は、水素発生触媒としてPt、Ir、Ru、Pd、Rh、Au、Fe、NiおよびSeのうち少なくとも1つを含むことが好ましい。
このような構成によれば、電解液から水素を効率よく発生させることができる。 In the gas production apparatus of the present invention, it is preferable that at least one of the hydrogen generation unit and the oxygen generation unit has a catalyst surface area larger than an area of the light receiving surface.
According to such a configuration, hydrogen and oxygen can be produced more efficiently.
In the gas production apparatus of the present invention, it is preferable that at least one of the hydrogen generation part and the oxygen generation part is a porous conductor carrying a catalyst.
According to such a configuration, the catalyst area of the reaction in which hydrogen or oxygen is generated can be increased.
In the gas production apparatus of the present invention, it is preferable that the hydrogen generation unit includes at least one of Pt, Ir, Ru, Pd, Rh, Au, Fe, Ni, and Se as a hydrogen generation catalyst.
According to such a configuration, hydrogen can be efficiently generated from the electrolytic solution.
このような構成によれば、より効率的に水素および酸素を製造することができる。
本発明の気体製造装置において、前記水素発生部および前記酸素発生部のうち少なくとも一方は、触媒が担持された多孔質の導電体であることが好ましい。
このような構成によれば、水素または酸素が発生する反応の触媒面積を広くすることができる。
本発明の気体製造装置において、前記水素発生部は、水素発生触媒としてPt、Ir、Ru、Pd、Rh、Au、Fe、NiおよびSeのうち少なくとも1つを含むことが好ましい。
このような構成によれば、電解液から水素を効率よく発生させることができる。 In the gas production apparatus of the present invention, it is preferable that at least one of the hydrogen generation unit and the oxygen generation unit has a catalyst surface area larger than an area of the light receiving surface.
According to such a configuration, hydrogen and oxygen can be produced more efficiently.
In the gas production apparatus of the present invention, it is preferable that at least one of the hydrogen generation part and the oxygen generation part is a porous conductor carrying a catalyst.
According to such a configuration, the catalyst area of the reaction in which hydrogen or oxygen is generated can be increased.
In the gas production apparatus of the present invention, it is preferable that the hydrogen generation unit includes at least one of Pt, Ir, Ru, Pd, Rh, Au, Fe, Ni, and Se as a hydrogen generation catalyst.
According to such a configuration, hydrogen can be efficiently generated from the electrolytic solution.
本発明の気体製造装置において、前記酸素発生部は、酸素発生触媒としてMn、Ca、Zn、CoおよびIrのうち少なくとも1つを含むことが好ましい。
このような構成によれば、電解液から酸素を効率よく発生させることができる。
本発明の気体製造装置において、透光性基板と電解液室とをさらに備え、前記光電変換部は、前記透光性基板の上に設けられ、第1電解用電極および第2電解用電極の上に天板をさらに備え、前記電解液室は、第1電解用電極および第2電解用電極と前記天板との間に設けられたことが好ましい。
このような構成によれば、第1電解用電極の電解液に接触可能な面と、第2電解用電極の電解液に接触可能な面とを電解液室に面して設けることができ、第1および第2電解用電極を電解液に接触させることができる。 In the gas production apparatus of the present invention, it is preferable that the oxygen generation unit includes at least one of Mn, Ca, Zn, Co, and Ir as an oxygen generation catalyst.
According to such a configuration, oxygen can be efficiently generated from the electrolytic solution.
The gas manufacturing apparatus of the present invention further includes a light-transmitting substrate and an electrolyte chamber, and the photoelectric conversion unit is provided on the light-transmitting substrate, and includes a first electrolysis electrode and a second electrolysis electrode. It is preferable that a top plate is further provided, and the electrolytic solution chamber is provided between the first electrolysis electrode and the second electrolysis electrode and the top plate.
According to such a configuration, the surface of the first electrolysis electrode that can contact the electrolyte solution and the surface of the second electrolysis electrode that can contact the electrolyte solution can be provided facing the electrolyte chamber, The first and second electrodes for electrolysis can be brought into contact with the electrolytic solution.
このような構成によれば、電解液から酸素を効率よく発生させることができる。
本発明の気体製造装置において、透光性基板と電解液室とをさらに備え、前記光電変換部は、前記透光性基板の上に設けられ、第1電解用電極および第2電解用電極の上に天板をさらに備え、前記電解液室は、第1電解用電極および第2電解用電極と前記天板との間に設けられたことが好ましい。
このような構成によれば、第1電解用電極の電解液に接触可能な面と、第2電解用電極の電解液に接触可能な面とを電解液室に面して設けることができ、第1および第2電解用電極を電解液に接触させることができる。 In the gas production apparatus of the present invention, it is preferable that the oxygen generation unit includes at least one of Mn, Ca, Zn, Co, and Ir as an oxygen generation catalyst.
According to such a configuration, oxygen can be efficiently generated from the electrolytic solution.
The gas manufacturing apparatus of the present invention further includes a light-transmitting substrate and an electrolyte chamber, and the photoelectric conversion unit is provided on the light-transmitting substrate, and includes a first electrolysis electrode and a second electrolysis electrode. It is preferable that a top plate is further provided, and the electrolytic solution chamber is provided between the first electrolysis electrode and the second electrolysis electrode and the top plate.
According to such a configuration, the surface of the first electrolysis electrode that can contact the electrolyte solution and the surface of the second electrolysis electrode that can contact the electrolyte solution can be provided facing the electrolyte chamber, The first and second electrodes for electrolysis can be brought into contact with the electrolytic solution.
本発明の気体製造装置において、第1電解用電極と前記天板との間の電解液室および第2電解用電極と天板との間の電解液室とを仕切る隔壁をさらに備えることが好ましい。
このような構成によれば、隔壁により第1気体と第2気体を分離することができる。
本発明の気体製造装置において、前記隔壁は、イオン交換体を含むことが好ましい。
このような構成によれば、電解液中で生じるイオン濃度の不均衡を容易に解消することができる。
また、本発明は、本発明の気体製造装置を前記光電変換部の受光面が実質的に水平となるように設置し、前記気体製造装置は、第1気体を排出する第1気体排出口と、第2気体を排出する第2気体排出口と、電解液室とを備え、前記電解液室に電解液を導入し、太陽光を前記光電変換部の受光面に入射させることにより第1電解用電極および第2電解用電極からそれぞれ第1気体および第2気体を発生させ、第1気体排出口および第2気体排出口からそれぞれ第1気体および第2気体を排出する気体製造方法も提供する。
本発明の気体製造方法によれば、光電変換部に光を入射させることにより、第1気体および第2気体を製造ことができ、容易に第1気体および第2気体を回収することができる。 In the gas production apparatus of the present invention, it is preferable that the gas production apparatus further includes a partition partitioning the electrolyte chamber between the first electrolysis electrode and the top plate and the electrolyte chamber between the second electrolysis electrode and the top plate. .
According to such a configuration, the first gas and the second gas can be separated by the partition wall.
In the gas production apparatus of the present invention, the partition preferably includes an ion exchanger.
According to such a structure, the imbalance of the ion concentration which arises in electrolyte solution can be eliminated easily.
Moreover, this invention installs the gas manufacturing apparatus of this invention so that the light-receiving surface of the said photoelectric conversion part may become substantially horizontal, The said gas manufacturing apparatus has the 1st gas exhaust port which discharges | emits 1st gas, The second electrolysis chamber is provided with a second gas exhaust port for discharging the second gas, and an electrolytic solution chamber. The electrolytic solution is introduced into the electrolytic solution chamber, and sunlight is incident on the light receiving surface of the photoelectric conversion unit. A gas production method is also provided in which the first gas and the second gas are generated from the electrode for electrode and the electrode for second electrolysis, respectively, and the first gas and the second gas are discharged from the first gas outlet and the second gas outlet, respectively. .
According to the gas production method of the present invention, the first gas and the second gas can be produced by allowing light to enter the photoelectric conversion unit, and the first gas and the second gas can be easily recovered.
このような構成によれば、隔壁により第1気体と第2気体を分離することができる。
本発明の気体製造装置において、前記隔壁は、イオン交換体を含むことが好ましい。
このような構成によれば、電解液中で生じるイオン濃度の不均衡を容易に解消することができる。
また、本発明は、本発明の気体製造装置を前記光電変換部の受光面が実質的に水平となるように設置し、前記気体製造装置は、第1気体を排出する第1気体排出口と、第2気体を排出する第2気体排出口と、電解液室とを備え、前記電解液室に電解液を導入し、太陽光を前記光電変換部の受光面に入射させることにより第1電解用電極および第2電解用電極からそれぞれ第1気体および第2気体を発生させ、第1気体排出口および第2気体排出口からそれぞれ第1気体および第2気体を排出する気体製造方法も提供する。
本発明の気体製造方法によれば、光電変換部に光を入射させることにより、第1気体および第2気体を製造ことができ、容易に第1気体および第2気体を回収することができる。 In the gas production apparatus of the present invention, it is preferable that the gas production apparatus further includes a partition partitioning the electrolyte chamber between the first electrolysis electrode and the top plate and the electrolyte chamber between the second electrolysis electrode and the top plate. .
According to such a configuration, the first gas and the second gas can be separated by the partition wall.
In the gas production apparatus of the present invention, the partition preferably includes an ion exchanger.
According to such a structure, the imbalance of the ion concentration which arises in electrolyte solution can be eliminated easily.
Moreover, this invention installs the gas manufacturing apparatus of this invention so that the light-receiving surface of the said photoelectric conversion part may become substantially horizontal, The said gas manufacturing apparatus has the 1st gas exhaust port which discharges | emits 1st gas, The second electrolysis chamber is provided with a second gas exhaust port for discharging the second gas, and an electrolytic solution chamber. The electrolytic solution is introduced into the electrolytic solution chamber, and sunlight is incident on the light receiving surface of the photoelectric conversion unit. A gas production method is also provided in which the first gas and the second gas are generated from the electrode for electrode and the electrode for second electrolysis, respectively, and the first gas and the second gas are discharged from the first gas outlet and the second gas outlet, respectively. .
According to the gas production method of the present invention, the first gas and the second gas can be produced by allowing light to enter the photoelectric conversion unit, and the first gas and the second gas can be easily recovered.
さらに本発明は、複数の本発明の気体製造装置と、第1気体排出路と、第2気体排出路とを備え、各気体製造装置は、第1気体を排出する第1気体排出口と第2気体を排出する第2気体排出口とを備え、第1気体排出路は、各気体製造装置の第1気体排出口と導通し、第2気体排出路は、各気体製造装置の第2気体排出口と導通する気体製造装置アレイも提供する。
本発明の気体製造装置アレイによれば、第1気体および第2気体の発生量を多くすることができ、第1気体および第2気体を第1気体排出路および第2気体排出路からそれぞれ回収することができる。また、第1気体排出路および第2気体排出路を簡素化して設けることができる。 The present invention further includes a plurality of the gas production apparatuses of the present invention, a first gas discharge path, and a second gas discharge path, and each gas production apparatus includes a first gas discharge port and a first gas discharge port for discharging the first gas. A second gas discharge port for discharging two gases, the first gas discharge path is electrically connected to the first gas discharge port of each gas production apparatus, and the second gas discharge path is a second gas of each gas production apparatus. A gas production device array in communication with the outlet is also provided.
According to the gas production device array of the present invention, the generation amounts of the first gas and the second gas can be increased, and the first gas and the second gas are recovered from the first gas discharge path and the second gas discharge path, respectively. can do. Moreover, the 1st gas exhaust path and the 2nd gas exhaust path can be simplified and provided.
本発明の気体製造装置アレイによれば、第1気体および第2気体の発生量を多くすることができ、第1気体および第2気体を第1気体排出路および第2気体排出路からそれぞれ回収することができる。また、第1気体排出路および第2気体排出路を簡素化して設けることができる。 The present invention further includes a plurality of the gas production apparatuses of the present invention, a first gas discharge path, and a second gas discharge path, and each gas production apparatus includes a first gas discharge port and a first gas discharge port for discharging the first gas. A second gas discharge port for discharging two gases, the first gas discharge path is electrically connected to the first gas discharge port of each gas production apparatus, and the second gas discharge path is a second gas of each gas production apparatus. A gas production device array in communication with the outlet is also provided.
According to the gas production device array of the present invention, the generation amounts of the first gas and the second gas can be increased, and the first gas and the second gas are recovered from the first gas discharge path and the second gas discharge path, respectively. can do. Moreover, the 1st gas exhaust path and the 2nd gas exhaust path can be simplified and provided.
以下、本発明の一実施形態を図面を用いて説明する。図面や以下の記述中で示す構成は、例示であって、本発明の範囲は、図面や以下の記述中で示すものに限定されない。
Hereinafter, an embodiment of the present invention will be described with reference to the drawings. The configurations shown in the drawings and the following description are merely examples, and the scope of the present invention is not limited to those shown in the drawings and the following description.
気体製造装置の構成
図1は本発明の一実施形態の気体製造装置の構成を示す概略平面図である。図2~4は、それぞれ図1の一点鎖線A-A、点線B-B、点線C-Cにおける気体製造装置の概略断面図である。図5は本発明の一実施形態の気体製造装置の構成を示す概略裏面図である。また図6~16はそれぞれ本発明の一実施形態の気体製造装置の構成を示す概略断面図である。なお、図6、13、15の断面図は、図1の点線B-Bにおける気体製造装置の断面図に対応し、図7、8、11、12、14、16の断面図は、図1の点線C-Cにおける気体製造装置の断面図に対応し、図9、10は、図1の一点鎖線A-Aにおける気体製造装置の断面図に対応する。 Diagram 1 of a gas producing device is a schematic plan view showing the configuration of a gas producing device according to an embodiment of the present invention. 2 to 4 are schematic cross-sectional views of the gas production apparatus taken along one-dot chain line AA, dotted line BB, and dotted line CC in FIG. 1, respectively. FIG. 5 is a schematic back view showing the configuration of the gas production apparatus according to one embodiment of the present invention. 6 to 16 are schematic cross-sectional views showing the configuration of the gas production apparatus according to the embodiment of the present invention. 6, 13, and 15 correspond to the sectional view of the gas production apparatus taken along the dotted line BB in FIG. 1, and the sectional views of FIGS. 7, 8, 11, 12, 14, and 16 are 9 and 10 correspond to the cross-sectional view of the gas production apparatus taken along one-dot chain line AA in FIG.
図1は本発明の一実施形態の気体製造装置の構成を示す概略平面図である。図2~4は、それぞれ図1の一点鎖線A-A、点線B-B、点線C-Cにおける気体製造装置の概略断面図である。図5は本発明の一実施形態の気体製造装置の構成を示す概略裏面図である。また図6~16はそれぞれ本発明の一実施形態の気体製造装置の構成を示す概略断面図である。なお、図6、13、15の断面図は、図1の点線B-Bにおける気体製造装置の断面図に対応し、図7、8、11、12、14、16の断面図は、図1の点線C-Cにおける気体製造装置の断面図に対応し、図9、10は、図1の一点鎖線A-Aにおける気体製造装置の断面図に対応する。 Diagram 1 of a gas producing device is a schematic plan view showing the configuration of a gas producing device according to an embodiment of the present invention. 2 to 4 are schematic cross-sectional views of the gas production apparatus taken along one-dot chain line AA, dotted line BB, and dotted line CC in FIG. 1, respectively. FIG. 5 is a schematic back view showing the configuration of the gas production apparatus according to one embodiment of the present invention. 6 to 16 are schematic cross-sectional views showing the configuration of the gas production apparatus according to the embodiment of the present invention. 6, 13, and 15 correspond to the sectional view of the gas production apparatus taken along the dotted line BB in FIG. 1, and the sectional views of FIGS. 7, 8, 11, 12, 14, and 16 are 9 and 10 correspond to the cross-sectional view of the gas production apparatus taken along one-dot chain line AA in FIG.
本実施形態の気体製造装置23は、受光面およびその裏面を有する光電変換部2と、光電変換部2の裏面の上に並べて設けられ、かつ、電解液に接触可能な面とをそれぞれ有する第1および第2電解用電極8、7とを備え、第1および第2電解用電極8、7が電解液と接触するとき、第1および第2電解用電極8、7は、光電変換部2が受光することより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体が発生するように設けられ、光電変換部2の受光面を水平にしたとき、第1電解用電極8と第2電解用電極7とは、電解液に接触可能な面と水平な基準面との間の傾斜角が異なることを特徴とする。
また、本実施形態の気体製造装置23は、透光性基板1を備えてもよい。
以下、本実施形態の気体製造装置について説明する。 Thegas production apparatus 23 according to the present embodiment includes a photoelectric conversion unit 2 having a light receiving surface and a back surface thereof, and a surface that is provided side by side on the back surface of the photoelectric conversion unit 2 and that can contact an electrolytic solution. 1 and second electrolysis electrodes 8 and 7, and when the first and second electrolysis electrodes 8 and 7 are in contact with the electrolytic solution, the first and second electrolysis electrodes 8 and 7 are connected to the photoelectric conversion unit 2. The electrolysis solution is electrolyzed using the electromotive force generated by receiving the light to generate the first gas and the second gas, respectively, and when the light receiving surface of the photoelectric conversion unit 2 is leveled, the first electrolysis The electrode for electrode 8 and the electrode for second electrolysis 7 are characterized in that the inclination angles between the surface that can contact the electrolyte and the horizontal reference surface are different.
Moreover, thegas manufacturing apparatus 23 of the present embodiment may include the translucent substrate 1.
Hereinafter, the gas manufacturing apparatus of this embodiment is demonstrated.
また、本実施形態の気体製造装置23は、透光性基板1を備えてもよい。
以下、本実施形態の気体製造装置について説明する。 The
Moreover, the
Hereinafter, the gas manufacturing apparatus of this embodiment is demonstrated.
1.透光性基板
透光性基板1は、本実施形態の気体製造装置23が備えてもよい。また、光電変換部2は、受光面が透光性基板1側となるように透光性基板1の上に設けられてもよい。なお、光電変換部2が、半導体基板などからなり一定の強度を有する場合、透光性基板1は省略することが可能である。また、光電変換部2が樹脂フィルムなど柔軟性を有する材料の上に形成可能な場合、透光性基板1は省略することができる。 1. Translucent substrate Thetranslucent substrate 1 may be provided in the gas manufacturing apparatus 23 of the present embodiment. Moreover, the photoelectric conversion part 2 may be provided on the translucent board | substrate 1 so that a light-receiving surface may become the translucent board | substrate 1 side. In addition, when the photoelectric conversion part 2 consists of semiconductor substrates etc. and has fixed intensity | strength, the translucent board | substrate 1 can be abbreviate | omitted. Moreover, when the photoelectric conversion part 2 can be formed on a flexible material such as a resin film, the translucent substrate 1 can be omitted.
透光性基板1は、本実施形態の気体製造装置23が備えてもよい。また、光電変換部2は、受光面が透光性基板1側となるように透光性基板1の上に設けられてもよい。なお、光電変換部2が、半導体基板などからなり一定の強度を有する場合、透光性基板1は省略することが可能である。また、光電変換部2が樹脂フィルムなど柔軟性を有する材料の上に形成可能な場合、透光性基板1は省略することができる。 1. Translucent substrate The
また、透光性基板1は、本気体製造装置を構成するための土台となる部材である。また、太陽光を光電変換部2の受光面で受光するためには、透明であり光透過率が高いことが好ましいが、光電変換部2へ効率的な光の入射が可能な構造であれば、光透過率に制限はない。
光透過率が高い基板材料として、例えば、ソーダガラス、石英ガラス、パイレックス(登録商標)、合成石英板等の透明なリジッド材、あるいは透明樹脂板やフィルム材等が好適に用いられる。化学的および物理的安定性を備える点より、ガラス基板を用いることが好ましい。
透光性基板1の光電変換部2側の表面には、入射した光が光電変換部2の表面で有効に乱反射されるように、微細な凹凸構造に形成することができる。この微細な凹凸構造は、例えば反応性イオンエッチング(RIE)処理もしくはブラスト処理等の公知の方法により形成することが可能である。 Moreover, the translucent board |substrate 1 is a member used as the foundation for comprising this gas manufacturing apparatus. Moreover, in order to receive sunlight with the light-receiving surface of the photoelectric conversion unit 2, it is preferable to be transparent and have a high light transmittance. However, as long as the light can be efficiently incident on the photoelectric conversion unit 2. There is no limit to the light transmittance.
As a substrate material having a high light transmittance, for example, a transparent rigid material such as soda glass, quartz glass, Pyrex (registered trademark), or a synthetic quartz plate, or a transparent resin plate or film material is preferably used. In view of chemical and physical stability, it is preferable to use a glass substrate.
On the surface of thetranslucent substrate 1 on the photoelectric conversion unit 2 side, a fine uneven structure can be formed so that incident light is effectively irregularly reflected on the surface of the photoelectric conversion unit 2. This fine concavo-convex structure can be formed by a known method such as reactive ion etching (RIE) treatment or blast treatment.
光透過率が高い基板材料として、例えば、ソーダガラス、石英ガラス、パイレックス(登録商標)、合成石英板等の透明なリジッド材、あるいは透明樹脂板やフィルム材等が好適に用いられる。化学的および物理的安定性を備える点より、ガラス基板を用いることが好ましい。
透光性基板1の光電変換部2側の表面には、入射した光が光電変換部2の表面で有効に乱反射されるように、微細な凹凸構造に形成することができる。この微細な凹凸構造は、例えば反応性イオンエッチング(RIE)処理もしくはブラスト処理等の公知の方法により形成することが可能である。 Moreover, the translucent board |
As a substrate material having a high light transmittance, for example, a transparent rigid material such as soda glass, quartz glass, Pyrex (registered trademark), or a synthetic quartz plate, or a transparent resin plate or film material is preferably used. In view of chemical and physical stability, it is preferable to use a glass substrate.
On the surface of the
また、透光性基板1は、光電変換部2の受光面を水平にしたとき、第1電解用電極8の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極7の電解液に接触可能な面と水平な基準面との間の傾斜角とが異なるように成形されてもよい。このように成形された透光性基板1の上に光電変換部2、第1および第2電解用電極8、7を設けることにより、第1電解用電極8の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極7の電解液に接触可能な面と水平な基準面との間の傾斜角とが異なるように設けることができる。たとえば、本実施形態の気体製造装置は、図6、7のような断面を有することができる。なお、図6は、図1の点線B-Bの断面図に対応した断面図であり、図7は、図1の点線C-Cの断面図に対応した断面図である。図6、7のような透光性基板1を用いることにより、第1および第2電解用電極8、7のそれぞれの電解液に接触可能な面の傾斜角を異なるように設けることができ、第1気体回収口20と第2気体回収口19とを気体製造装置の両側に設けることができる。このことにより第1気体を回収するための配管および第2気体を回収するための配管を簡素化することができる。
このような成形された透光性基板1は、ガラスなどを型に流し込むことにより形成してもよく、板状基板を変形させることにより形成してもよく、板状の基板を組み合わせることにより形成してもよい。 Further, when the light-receiving surface of thephotoelectric conversion unit 2 is leveled, the translucent substrate 1 has an inclination angle between the surface of the first electrolysis electrode 8 that can contact the electrolytic solution and a horizontal reference surface, You may shape | mold so that the inclination angle between the surface which can contact the electrolyte solution of the electrode 7 for 2 electrolysis and a horizontal reference plane differs. By providing the photoelectric conversion unit 2 and the first and second electrolysis electrodes 8 and 7 on the translucent substrate 1 formed in this manner, the surface of the first electrolysis electrode 8 that can contact the electrolytic solution and The tilt angle between the horizontal reference plane and the tilt angle between the horizontal reference plane and the plane that can contact the electrolytic solution of the second electrolysis electrode 7 can be different. For example, the gas manufacturing apparatus of the present embodiment can have a cross section as shown in FIGS. 6 is a cross-sectional view corresponding to the cross-sectional view taken along the dotted line BB in FIG. 1, and FIG. 7 is a cross-sectional view corresponding to the cross-sectional view taken along the dotted line CC in FIG. By using the translucent substrate 1 as shown in FIGS. 6 and 7, it is possible to provide different inclination angles of the surfaces of the first and second electrolysis electrodes 8 and 7 that can come into contact with the electrolytic solutions, The first gas recovery port 20 and the second gas recovery port 19 can be provided on both sides of the gas production apparatus. Thereby, the piping for recovering the first gas and the piping for recovering the second gas can be simplified.
Such a moldedtranslucent substrate 1 may be formed by pouring glass or the like into a mold, may be formed by deforming a plate-like substrate, or may be formed by combining plate-like substrates. May be.
このような成形された透光性基板1は、ガラスなどを型に流し込むことにより形成してもよく、板状基板を変形させることにより形成してもよく、板状の基板を組み合わせることにより形成してもよい。 Further, when the light-receiving surface of the
Such a molded
2.第1電極、第2導電部
第1電極4は、透光性基板1の上に設けることができ、光電変換部2の受光面と接触するように設けることができる。また、第1電極4は透光性を有してもよい。また、第1電極4は、透光性基板1を省略可能の場合、光電変換部2の受光面に直接設けられてもよい。第1電極4は、第2電解用電極7と電気的に接続することができる。第1電極4を設けることにより、光電変換部2の受光面と第2電解用電極7との間に流れる電流を大きくすることができる。また、光電変換部2が図14~16のように光電変換部2の裏面の第1区域と第2区域との間に起電力が生じるものである場合、第1電極4は不要である。
第1電極4は、図4、7、8、11のように第2導電部10を介して第2電解用電極7と電気的に接続してもよく、図12のように第2電解用電極7と接触してもよい。
第1電極4は、例えば、ITO、SnO2などの透明導電膜からなってもよく、Ag、Auなどの金属のフィンガー電極からなってもよい。 2. First Electrode, Second Conductive Part Thefirst electrode 4 can be provided on the translucent substrate 1 and can be provided in contact with the light receiving surface of the photoelectric conversion part 2. Moreover, the 1st electrode 4 may have translucency. Moreover, the 1st electrode 4 may be directly provided in the light-receiving surface of the photoelectric conversion part 2, when the translucent board | substrate 1 can be abbreviate | omitted. The first electrode 4 can be electrically connected to the second electrolysis electrode 7. By providing the first electrode 4, the current flowing between the light receiving surface of the photoelectric conversion unit 2 and the second electrolysis electrode 7 can be increased. Further, when the photoelectric conversion unit 2 generates an electromotive force between the first area and the second area on the back surface of the photoelectric conversion unit 2 as shown in FIGS. 14 to 16, the first electrode 4 is unnecessary.
Thefirst electrode 4 may be electrically connected to the second electrolysis electrode 7 through the second conductive portion 10 as shown in FIGS. 4, 7, 8, and 11, and as shown in FIG. It may be in contact with the electrode 7.
Thefirst electrode 4 may be made of a transparent conductive film such as ITO or SnO 2, or may be made of a metal finger electrode such as Ag or Au.
第1電極4は、透光性基板1の上に設けることができ、光電変換部2の受光面と接触するように設けることができる。また、第1電極4は透光性を有してもよい。また、第1電極4は、透光性基板1を省略可能の場合、光電変換部2の受光面に直接設けられてもよい。第1電極4は、第2電解用電極7と電気的に接続することができる。第1電極4を設けることにより、光電変換部2の受光面と第2電解用電極7との間に流れる電流を大きくすることができる。また、光電変換部2が図14~16のように光電変換部2の裏面の第1区域と第2区域との間に起電力が生じるものである場合、第1電極4は不要である。
第1電極4は、図4、7、8、11のように第2導電部10を介して第2電解用電極7と電気的に接続してもよく、図12のように第2電解用電極7と接触してもよい。
第1電極4は、例えば、ITO、SnO2などの透明導電膜からなってもよく、Ag、Auなどの金属のフィンガー電極からなってもよい。 2. First Electrode, Second Conductive Part The
The
The
以下に第1電極4を透明導電膜とした場合について説明する。
透明導電膜は、光電変換部2の受光面と第2電解用電極7とのコンタクトを取りやすくするために用いている。
一般に透明電極として使用されているものを用いることが可能である。具体的にはIn-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2等を挙げることができる。なお本透明導電膜は、太陽光の光線透過率が85%以上、中でも90%以上、特に92%以上であることが好ましい。このことにより光電変換部2が光を効率的に吸収することができるためである。
透明導電膜の作成方法としては公知の方法を用いることができ、スパッタリング、真空蒸着、ゾルゲル法、クラスタービーム蒸着法、PLD(Pulse Laser Deposition)法などが挙げられる。 A case where thefirst electrode 4 is a transparent conductive film will be described below.
The transparent conductive film is used to facilitate contact between the light receiving surface of thephotoelectric conversion unit 2 and the second electrolysis electrode 7.
What is generally used as a transparent electrode can be used. Specifically, In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, SnO 2 and the like can be given. The transparent conductive film preferably has a sunlight transmittance of 85% or more, particularly 90% or more, and particularly 92% or more. This is because thephotoelectric conversion unit 2 can absorb light efficiently.
As a method for producing the transparent conductive film, a known method can be used, and examples thereof include sputtering, vacuum deposition, sol-gel method, cluster beam deposition method, and PLD (Pulse Laser Deposition) method.
透明導電膜は、光電変換部2の受光面と第2電解用電極7とのコンタクトを取りやすくするために用いている。
一般に透明電極として使用されているものを用いることが可能である。具体的にはIn-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2等を挙げることができる。なお本透明導電膜は、太陽光の光線透過率が85%以上、中でも90%以上、特に92%以上であることが好ましい。このことにより光電変換部2が光を効率的に吸収することができるためである。
透明導電膜の作成方法としては公知の方法を用いることができ、スパッタリング、真空蒸着、ゾルゲル法、クラスタービーム蒸着法、PLD(Pulse Laser Deposition)法などが挙げられる。 A case where the
The transparent conductive film is used to facilitate contact between the light receiving surface of the
What is generally used as a transparent electrode can be used. Specifically, In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, SnO 2 and the like can be given. The transparent conductive film preferably has a sunlight transmittance of 85% or more, particularly 90% or more, and particularly 92% or more. This is because the
As a method for producing the transparent conductive film, a known method can be used, and examples thereof include sputtering, vacuum deposition, sol-gel method, cluster beam deposition method, and PLD (Pulse Laser Deposition) method.
第2導電部10は、第1電極4と第2電解用電極7とにそれぞれ接触するように設けることができる。第2の導電部10を設けることにより、容易に光電変換部2の受光面に接触した第1電極4と第2電解用電極7とを電気的に接続することができる。
The second conductive portion 10 can be provided so as to contact the first electrode 4 and the second electrolysis electrode 7 respectively. By providing the second conductive portion 10, the first electrode 4 and the second electrolysis electrode 7 that are in contact with the light receiving surface of the photoelectric conversion portion 2 can be easily electrically connected.
また、第2導電部10は、図4、7、8のように光電変換部2または第2型部12を貫通するコンタクトホールに設けられてもよい。このことにより、光電変換部2の受光面と第2電解用電極7との間の電流経路を短くすることができ、より効率的に第1気体および第2気体を発生させることができる。また、第2導電部10が設けられたコンタクトホールは、1つまたは複数でもよく、円形の断面を有してもよい。
また、第2導電部10は、図11のように光電変換部2の側面を覆うように設けられてもよい。 Moreover, the 2ndelectroconductive part 10 may be provided in the contact hole which penetrates the photoelectric conversion part 2 or the 2nd type | mold part 12 like FIG. Thus, the current path between the light receiving surface of the photoelectric conversion unit 2 and the second electrolysis electrode 7 can be shortened, and the first gas and the second gas can be generated more efficiently. In addition, the contact hole provided with the second conductive portion 10 may be one or more, and may have a circular cross section.
Moreover, the 2ndelectroconductive part 10 may be provided so that the side surface of the photoelectric conversion part 2 may be covered like FIG.
また、第2導電部10は、図11のように光電変換部2の側面を覆うように設けられてもよい。 Moreover, the 2nd
Moreover, the 2nd
第2導電部10の材料は、導電性を有しているものであれば特に制限されない。導電性粒子を含有するペースト、例えばカーボンペースト、Agペースト等をスクリーン印刷法、インクジェット法等で塗布し乾燥もしくは焼成する方法や、原料ガスを用いたCVD法等により製膜する方法、PVD法、蒸着法、スパッタ法、ゾルゲル法、電気化学的な酸化還元反応を利用した方法等が挙げられる。
The material of the second conductive portion 10 is not particularly limited as long as it has conductivity. A paste containing conductive particles, for example, a carbon paste, an Ag paste or the like applied by screen printing, an inkjet method, etc., dried or baked, a method of forming a film by a CVD method using a raw material gas, a PVD method, Examples thereof include a vapor deposition method, a sputtering method, a sol-gel method, and a method using an electrochemical redox reaction.
3.光電変換部
光電変換部2は、受光面および裏面を有し、光電変換部2の裏面の上に第1電解用電極8と第2電解用電極7が設けられる。なお、受光面とは、光電変換するための光を受光する面であり、裏面とは、受光面の裏の面である。また、光電変換部2は、第1電極4が設けられた透光性基板1の上に受光面を下にして設けることができる。光電変換部2は、例えば、図2~4、6~13のように受光面と裏面との間に起電力が生じるものであってもよく、図14~16のように裏面の第1区域と第2区域との間に起電力が生じるものであってもよい。図14~16のような光電変換部2は、n型半導体領域37とp型半導体領域36を形成した半導体基板などにより形成することができる。また、透光性基板1が図6、7のように傾斜角の異なる部分を有している場合、傾斜角の異なる部分の上にそれぞれ光電変換部2を形成し、これらの光電変換部を電気的に接続してもよい。
光電変換部2の形は、特に限定されないが、例えば、方形状とすることができる。
光電変換部2は、入射光により電荷分離することができ、起電力が生じるものであれば、特に限定されないが、例えば、シリコン系半導体を用いた光電変換部、化合物半導体を用いた光電変換部、色素増感剤を利用した光電変換部、有機薄膜を用いた光電変換部などである。 3. Photoelectric Conversion Unit Thephotoelectric conversion unit 2 has a light receiving surface and a back surface, and a first electrolysis electrode 8 and a second electrolysis electrode 7 are provided on the back surface of the photoelectric conversion unit 2. The light receiving surface is a surface that receives light for photoelectric conversion, and the back surface is the back surface of the light receiving surface. Moreover, the photoelectric conversion part 2 can be provided on the translucent substrate 1 provided with the first electrode 4 with the light receiving surface facing down. For example, the photoelectric conversion unit 2 may generate an electromotive force between the light receiving surface and the back surface as shown in FIGS. 2 to 4 and 6 to 13, and the first area on the back surface as shown in FIGS. And an electromotive force may be generated between the first area and the second area. The photoelectric conversion unit 2 as shown in FIGS. 14 to 16 can be formed by a semiconductor substrate on which the n-type semiconductor region 37 and the p-type semiconductor region 36 are formed. Moreover, when the translucent board | substrate 1 has a part from which an inclination angle differs like FIG.6, 7, the photoelectric conversion part 2 is formed on the part from which an inclination angle differs, respectively, and these photoelectric conversion parts are made into You may connect electrically.
Although the shape of thephotoelectric conversion part 2 is not specifically limited, For example, it can be set as a square shape.
Thephotoelectric conversion unit 2 is not particularly limited as long as it can separate charges by incident light and generates an electromotive force. For example, the photoelectric conversion unit using a silicon-based semiconductor or the photoelectric conversion unit using a compound semiconductor A photoelectric conversion part using a dye sensitizer, a photoelectric conversion part using an organic thin film, and the like.
光電変換部2は、受光面および裏面を有し、光電変換部2の裏面の上に第1電解用電極8と第2電解用電極7が設けられる。なお、受光面とは、光電変換するための光を受光する面であり、裏面とは、受光面の裏の面である。また、光電変換部2は、第1電極4が設けられた透光性基板1の上に受光面を下にして設けることができる。光電変換部2は、例えば、図2~4、6~13のように受光面と裏面との間に起電力が生じるものであってもよく、図14~16のように裏面の第1区域と第2区域との間に起電力が生じるものであってもよい。図14~16のような光電変換部2は、n型半導体領域37とp型半導体領域36を形成した半導体基板などにより形成することができる。また、透光性基板1が図6、7のように傾斜角の異なる部分を有している場合、傾斜角の異なる部分の上にそれぞれ光電変換部2を形成し、これらの光電変換部を電気的に接続してもよい。
光電変換部2の形は、特に限定されないが、例えば、方形状とすることができる。
光電変換部2は、入射光により電荷分離することができ、起電力が生じるものであれば、特に限定されないが、例えば、シリコン系半導体を用いた光電変換部、化合物半導体を用いた光電変換部、色素増感剤を利用した光電変換部、有機薄膜を用いた光電変換部などである。 3. Photoelectric Conversion Unit The
Although the shape of the
The
第1気体および第2気体が水素および酸素の場合、光電変換部2は、光を受光することにより、第1電解用電極8および第2電解用電極7において水素と酸素が発生するために必要な起電力が生じる材料を使用する必要がある。第1電解用電極8と第2電解用電極7の電位差は、水分解のための理論電圧(1.23V)より大きくする必要があり、そのためには光電変換部2で十分大きな電位差を生み出す必要がある。そのため光電変換部2は、pn接合など起電力を生じさせる部分を二接合以上直列に接続することが好ましい。例えば、図16のように並列に設けられた光電変換層を第4導電部42により接続した構造を有することができる。
When the first gas and the second gas are hydrogen and oxygen, the photoelectric conversion unit 2 is necessary for generating hydrogen and oxygen in the first electrolysis electrode 8 and the second electrolysis electrode 7 by receiving light. It is necessary to use a material that generates an electromotive force. The potential difference between the first electrolysis electrode 8 and the second electrolysis electrode 7 needs to be larger than the theoretical voltage (1.23 V) for water decomposition, and for this purpose, a sufficiently large potential difference needs to be generated in the photoelectric conversion unit 2. There is. Therefore, it is preferable that the photoelectric conversion unit 2 connects two or more junctions in series such as a pn junction to generate an electromotive force. For example, as shown in FIG. 16, it can have a structure in which photoelectric conversion layers provided in parallel are connected by the fourth conductive portion 42.
光電変換を行う材料は、シリコン系半導体、化合物半導体、有機材料をベースとしたものなどが挙げられるが、いずれの光電変換材料も使用することが可能である。また、起電力を大きくするために、これらの光電変換材料を積層することが可能である。積層する場合には同一材料で多接合構造を形成することが可能であるが、光学的バンドギャップの異なる複数の光電変換層を積層し、各々の光電変換層の低感度波長領域を相互に補完することにより、広い波長領域にわたり入射光を効率よく吸収することが可能となる。これらの複数の光電変換層は、それぞれ異なるバンドギャップを有することが好ましい。このような構成によれば、光電変換部2で生じる起電力をより大きくすることができ、電解液をより効率的に電気分解することができる。
Examples of materials that perform photoelectric conversion include silicon-based semiconductors, compound semiconductors, and materials based on organic materials, and any photoelectric conversion material can be used. In order to increase the electromotive force, these photoelectric conversion materials can be stacked. In the case of stacking, it is possible to form a multi-junction structure with the same material, but stacking multiple photoelectric conversion layers with different optical band gaps and complementing the low sensitivity wavelength region of each photoelectric conversion layer mutually By doing so, incident light can be efficiently absorbed over a wide wavelength region. The plurality of photoelectric conversion layers preferably have different band gaps. According to such a configuration, the electromotive force generated in the photoelectric conversion unit 2 can be increased, and the electrolytic solution can be electrolyzed more efficiently.
また、光電変換層間の直列接続特性の改善や、光電変換部2で発生する光電流の整合のために、層間に透明導電膜等の導電体を介在させることが可能である。これにより光電変換部2の劣化を抑制することが可能となる。
光電変換部2の例を以下に具体的に説明する。また、光電変換部2は、これらを組み合わせたものでもよい。また、以下の光電変換部2の例は、矛盾しない限り光電変換層とすることもできる。 Moreover, it is possible to interpose a conductor such as a transparent conductive film between the layers in order to improve the serial connection characteristics between the photoelectric conversion layers and to match the photocurrent generated in thephotoelectric conversion unit 2. Thereby, it becomes possible to suppress deterioration of the photoelectric conversion unit 2.
An example of thephotoelectric conversion unit 2 will be specifically described below. The photoelectric conversion unit 2 may be a combination of these. Moreover, as long as there is no contradiction, the example of the following photoelectric conversion parts 2 can also be made into a photoelectric converting layer.
光電変換部2の例を以下に具体的に説明する。また、光電変換部2は、これらを組み合わせたものでもよい。また、以下の光電変換部2の例は、矛盾しない限り光電変換層とすることもできる。 Moreover, it is possible to interpose a conductor such as a transparent conductive film between the layers in order to improve the serial connection characteristics between the photoelectric conversion layers and to match the photocurrent generated in the
An example of the
3-1.シリコン系半導体を用いた光電変換部
シリコン系半導体を用いた光電変換部2は、例えば、単結晶型、多結晶型、アモルファス型、球状シリコン型、及びこれらを組み合わせたもの等が挙げられる。いずれもp型半導体とn型半導体が接合したpn接合を有することができる。また、p型半導体とn型半導体との間にi型半導体を設けたpin接合を有するものとすることもできる。また、pn接合を複数有するもの、pin接合を複数有するもの、pn接合とpin接合を有するものとすることもできる。
シリコン系半導体とは、シリコンを含む半導体であり、例えば、シリコン、シリコンカーバイド、シリコンゲルマニウムなどである。また、シリコンなどにn型不純物またはp型不純物が添加されたものも含み、また、結晶質、非晶質、微結晶のものも含む。
また、シリコン系半導体を用いた光電変換部2は、透光性基板1の上に形成された薄膜または厚膜の光電変換層であってもよく、また、シリコンウェハなどのウェハにpn接合またはpin接合を形成したものでもよく、また、pn接合またはpin接合を形成したウェハの上に薄膜の光電変換層を形成したものでもよい。 3-1. Photoelectric conversion part using a silicon-based semiconductor Examples of thephotoelectric conversion part 2 using a silicon-based semiconductor include a single crystal type, a polycrystalline type, an amorphous type, a spherical silicon type, and combinations thereof. Any of them can have a pn junction in which a p-type semiconductor and an n-type semiconductor are joined. Further, a pin junction in which an i-type semiconductor is provided between a p-type semiconductor and an n-type semiconductor may be provided. Further, it may have a plurality of pn junctions, a plurality of pin junctions, or a pn junction and a pin junction.
The silicon-based semiconductor is a semiconductor containing silicon, such as silicon, silicon carbide, or silicon germanium. In addition, silicon or the like in which n-type impurities or p-type impurities are added is included, and crystalline, amorphous, or microcrystalline silicon is also included.
In addition, thephotoelectric conversion unit 2 using a silicon-based semiconductor may be a thin film or a thick photoelectric conversion layer formed on the translucent substrate 1, or a pn junction or a wafer such as a silicon wafer. A pin junction may be formed, or a thin film photoelectric conversion layer may be formed on a wafer having a pn junction or a pin junction.
シリコン系半導体を用いた光電変換部2は、例えば、単結晶型、多結晶型、アモルファス型、球状シリコン型、及びこれらを組み合わせたもの等が挙げられる。いずれもp型半導体とn型半導体が接合したpn接合を有することができる。また、p型半導体とn型半導体との間にi型半導体を設けたpin接合を有するものとすることもできる。また、pn接合を複数有するもの、pin接合を複数有するもの、pn接合とpin接合を有するものとすることもできる。
シリコン系半導体とは、シリコンを含む半導体であり、例えば、シリコン、シリコンカーバイド、シリコンゲルマニウムなどである。また、シリコンなどにn型不純物またはp型不純物が添加されたものも含み、また、結晶質、非晶質、微結晶のものも含む。
また、シリコン系半導体を用いた光電変換部2は、透光性基板1の上に形成された薄膜または厚膜の光電変換層であってもよく、また、シリコンウェハなどのウェハにpn接合またはpin接合を形成したものでもよく、また、pn接合またはpin接合を形成したウェハの上に薄膜の光電変換層を形成したものでもよい。 3-1. Photoelectric conversion part using a silicon-based semiconductor Examples of the
The silicon-based semiconductor is a semiconductor containing silicon, such as silicon, silicon carbide, or silicon germanium. In addition, silicon or the like in which n-type impurities or p-type impurities are added is included, and crystalline, amorphous, or microcrystalline silicon is also included.
In addition, the
シリコン系半導体を用いた光電変換部2の形成例を以下に示す。
透光性基板1上に積層した第1電極4上に、第1導電型半導体層をプラズマCVD法等の方法で形成する。この第1導電型半導体層としては、導電型決定不純物原子濃度が1×1018~5×1021/cm3程度ドープされた、p+型またはn+型の非晶質Si薄膜、または多結晶あるいは微結晶Si薄膜とする。第1導電型半導体層の材料としては、Siに限らず、SiCあるいはSiGe,SixO1-x等の化合物を用いることも可能である。 An example of forming thephotoelectric conversion unit 2 using a silicon-based semiconductor is shown below.
A first conductivity type semiconductor layer is formed on thefirst electrode 4 laminated on the translucent substrate 1 by a method such as a plasma CVD method. As the first conductive type semiconductor layer, a p + type or n + type amorphous Si thin film doped with a conductivity type determining impurity atom concentration of about 1 × 10 18 to 5 × 10 21 / cm 3 , a polycrystalline or A microcrystalline Si thin film is used. The material of the first conductivity type semiconductor layer is not limited to Si, and it is also possible to use a compound such as SiC, SiGe, or Si x O 1-x .
透光性基板1上に積層した第1電極4上に、第1導電型半導体層をプラズマCVD法等の方法で形成する。この第1導電型半導体層としては、導電型決定不純物原子濃度が1×1018~5×1021/cm3程度ドープされた、p+型またはn+型の非晶質Si薄膜、または多結晶あるいは微結晶Si薄膜とする。第1導電型半導体層の材料としては、Siに限らず、SiCあるいはSiGe,SixO1-x等の化合物を用いることも可能である。 An example of forming the
A first conductivity type semiconductor layer is formed on the
このように形成された第1導電型半導体層上に、結晶質Si系光活性層として多結晶あるいは微結晶の結晶質Si薄膜をプラズマCVD法等の方法で形成する。なお、導電型は第1導電型半導体よりドーピング濃度が低い第1導電型とするか、あるいはi型とする。結晶質Si系光活性層の材料としては、Siに限らず、SiCあるいはSiGe,SixO1-x等の化合物を用いることも可能である。
On the first conductivity type semiconductor layer thus formed, a polycrystalline or microcrystalline crystalline Si thin film is formed as a crystalline Si photoactive layer by a method such as plasma CVD. The conductivity type is the first conductivity type having a lower doping concentration than the first conductivity type semiconductor, or the i conductivity type. The material for the crystalline Si-based photoactive layer is not limited to Si, and it is also possible to use a compound such as SiC, SiGe, or Si x O 1-x .
次に、結晶質Si系光活性層上に半導体接合を形成するため、第1導電型半導体層とは反対導電型である第2導電型半導体層をプラズマCVD等の方法で形成する。この第2導電型半導体層としては、導電型決定不純物原子が1×1018~5×1021/cm3程度ドープされた、n+型またはp+型の非晶質Si薄膜、または多結晶あるいは微結晶Si薄膜とする。第2導電型半導体層の材料としては、Siに限らず、SiCあるいはSiGe,SixO1-x等の化合物を用いることも可能である。また接合特性をより改善するために、結晶質Si系光活性層と第2導電型半導体層との間に、実質的にi型の非単結晶Si系薄膜を挿入することも可能である。このようにして、受光面に最も近い光電変換層を一層積層することができる。
Next, in order to form a semiconductor junction on the crystalline Si-based photoactive layer, a second conductivity type semiconductor layer having a conductivity type opposite to the first conductivity type semiconductor layer is formed by a method such as plasma CVD. As the second conductive type semiconductor layer, an n + type or p + type amorphous Si thin film doped with about 1 × 10 18 to 5 × 10 21 / cm 3 of a conductivity type determining impurity atom, or a polycrystalline or microscopic A crystalline Si thin film is used. The material of the second conductivity type semiconductor layer is not limited to Si, and it is also possible to use a compound such as SiC, SiGe, or Si x O 1-x . In order to further improve the bonding characteristics, it is possible to insert a substantially i-type non-single-crystal Si-based thin film between the crystalline Si-based photoactive layer and the second conductive type semiconductor layer. In this manner, one photoelectric conversion layer closest to the light receiving surface can be stacked.
続けて第二層目の光電変換層を形成する。第二層目の光電変換層は、第1導電型半導体層、結晶質Si系光活性層、第2導電型半導体層からなり、それぞれの層は、第一層目の光電変換層中の対応する第1導電型半導体層、結晶質Si系光活性層、第2導電型半導体層と同様に形成する。二層のタンデムで水分解に十分な電位を得ることができない場合は、三層あるいはそれ以上の層状構造を取ることが好ましい。ただし第二層目の光電変換層の結晶質Si系光活性層の体積結晶化分率は、第一層目の結晶質Si系光活性層と比較すると高くすることが好ましい。三層以上積層する場合も同様に下層と比較すると体積結晶化分率を高くすることが好ましい。これは、長波長域での吸収が大きくなり、分光感度が長波長側にシフトし、同じSi材料を用いて光活性層を構成した場合においても、広い波長域で感度を向上させることが可能となるためである。すなわち、結晶化率の異なるSiでタンデム構造にすることにより、分光感度が広くなり、光の高効率利用が可能となる。このとき低結晶化率材料を受光面側にしないと高効率とならない。また結晶化率が40%以下に下がるとアモルファス成分が増え、劣化が生じてしまう。
Next, a second photoelectric conversion layer is formed. The second photoelectric conversion layer includes a first conductive semiconductor layer, a crystalline Si-based photoactive layer, and a second conductive semiconductor layer, and each layer corresponds to the first photoelectric conversion layer. The first conductive type semiconductor layer, the crystalline Si-based photoactive layer, and the second conductive type semiconductor layer are formed. When a potential sufficient for water splitting cannot be obtained with a two-layer tandem, it is preferable to take a three-layer structure or more. However, the volume crystallization fraction of the crystalline Si photoactive layer of the second photoelectric conversion layer is preferably higher than that of the first crystalline Si photoactive layer. Similarly, when three or more layers are laminated, the volume crystallization fraction is preferably increased as compared with the lower layer. This increases the absorption in the long wavelength region, shifts the spectral sensitivity to the longer wavelength side, and can improve the sensitivity in a wide wavelength region even when the photoactive layer is configured using the same Si material. This is because. That is, by using a tandem structure with Si having different crystallization rates, the spectral sensitivity is widened, and light can be used with high efficiency. At this time, if the low crystallization rate material is not on the light receiving surface side, high efficiency cannot be achieved. Further, when the crystallization rate is lowered to 40% or less, the amorphous component increases and deterioration occurs.
次に、シリコン基板を用いた光電変換部2の形成例を以下に示す。
シリコン基板としては、単結晶シリコン基板または多結晶シリコン基板などを用いることができ、p型であっても、n型であっても、i型であってもよい。このシリコン基板の一部にPなどのn型不純物を熱拡散またはイオン注入などによりドープすることによりn型半導体領域37を形成し、シリコン基板のほかの一部にBなどのp型不純物を熱拡散またはイオン注入などによりドープすることによりp型半導体領域36を形成することができる。このことにより、シリコン基板にpn接合、pin接合、npp+接合またはpnn+接合などを形成することができ、光電変換部2を形成することができる。 Next, the example of formation of thephotoelectric conversion part 2 using a silicon substrate is shown below.
As the silicon substrate, a single crystal silicon substrate, a polycrystalline silicon substrate, or the like can be used, and may be p-type, n-type, or i-type. An n-type semiconductor region 37 is formed by doping an n-type impurity such as P into a part of the silicon substrate by thermal diffusion or ion implantation, and a p-type impurity such as B is heated on the other part of the silicon substrate. The p-type semiconductor region 36 can be formed by doping by diffusion or ion implantation. Thus, pn junction in the silicon substrate, pin junction can be formed and npp + junction or pnn + junction, it is possible to form a photoelectric conversion unit 2.
シリコン基板としては、単結晶シリコン基板または多結晶シリコン基板などを用いることができ、p型であっても、n型であっても、i型であってもよい。このシリコン基板の一部にPなどのn型不純物を熱拡散またはイオン注入などによりドープすることによりn型半導体領域37を形成し、シリコン基板のほかの一部にBなどのp型不純物を熱拡散またはイオン注入などによりドープすることによりp型半導体領域36を形成することができる。このことにより、シリコン基板にpn接合、pin接合、npp+接合またはpnn+接合などを形成することができ、光電変換部2を形成することができる。 Next, the example of formation of the
As the silicon substrate, a single crystal silicon substrate, a polycrystalline silicon substrate, or the like can be used, and may be p-type, n-type, or i-type. An n-
n型半導体領域37およびp型半導体領域36は、図14~16のようにシリコン基板にそれぞれ1つの領域を形成することができ、n型半導体領域37およびp型半導体領域36のうちどちらか一方を複数形成することもできる。また、図16のようにn型半導体領域37およびp型半導体領域36を形成したシリコン基板を並べて設置し、第4導電部42により直列接続することにより光電変換部2を形成することもできる。
なお、ここではシリコン基板を用いて説明したが、pn接合、pin接合、npp+接合またはpnn+接合などを形成することができる他の半導体基板を用いてもよい。また、n型半導体領域37およびp型半導体領域36を形成することができれば、半導体基板に限定されず、基板上に形成された半導体層であってもよい。 Each of the n-type semiconductor region 37 and the p-type semiconductor region 36 can be formed on the silicon substrate as shown in FIGS. 14 to 16, and one of the n-type semiconductor region 37 and the p-type semiconductor region 36 is formed. A plurality of can be formed. In addition, as shown in FIG. 16, the photoelectric conversion unit 2 can be formed by arranging the silicon substrates on which the n-type semiconductor region 37 and the p-type semiconductor region 36 are arranged side by side and connecting them in series by the fourth conductive unit 42.
Note that, although described with reference to a silicon substrate, pn junction, pin junction, may use other semiconductor substrate or the like can be formed npp + junction or pnn + junction. In addition, as long as the n-type semiconductor region 37 and the p-type semiconductor region 36 can be formed, the semiconductor layer is not limited to the semiconductor substrate and may be a semiconductor layer formed on the substrate.
なお、ここではシリコン基板を用いて説明したが、pn接合、pin接合、npp+接合またはpnn+接合などを形成することができる他の半導体基板を用いてもよい。また、n型半導体領域37およびp型半導体領域36を形成することができれば、半導体基板に限定されず、基板上に形成された半導体層であってもよい。 Each of the n-
Note that, although described with reference to a silicon substrate, pn junction, pin junction, may use other semiconductor substrate or the like can be formed npp + junction or pnn + junction. In addition, as long as the n-
3-2.化合物半導体を用いた光電変換部
化合物半導体を用いた光電変換部は、例えば、III-V族元素で構成されるGaP、GaAsやInP、InAs、II-VI族元素で構成されるCdTe/CdS、I-III-VI族で構成されるCIGS(Copper Indium Gallium DiSelenide)などを用いpn接合を形成したものが挙げられる。 3-2. Photoelectric conversion part using a compound semiconductor The photoelectric conversion part using a compound semiconductor is, for example, GaP, GaAs, InP, InAs, or IId-VI elements composed of group III-V elements, CdTe / CdS, Examples thereof include those in which a pn junction is formed using CIGS (Copper Indium Gallium DiSelenide) composed of the I-III-VI group.
化合物半導体を用いた光電変換部は、例えば、III-V族元素で構成されるGaP、GaAsやInP、InAs、II-VI族元素で構成されるCdTe/CdS、I-III-VI族で構成されるCIGS(Copper Indium Gallium DiSelenide)などを用いpn接合を形成したものが挙げられる。 3-2. Photoelectric conversion part using a compound semiconductor The photoelectric conversion part using a compound semiconductor is, for example, GaP, GaAs, InP, InAs, or IId-VI elements composed of group III-V elements, CdTe / CdS, Examples thereof include those in which a pn junction is formed using CIGS (Copper Indium Gallium DiSelenide) composed of the I-III-VI group.
化合物半導体を用いた光電変換部の製造方法の一例を以下に示すが、本製造方法では、製膜処理等はすべて有機金属気相成長法(MOCVD;Metal Organic Chemical Vapor Deposition)装置を使って連続して行われる。III族元素の材料としては、例えばトリメチルガリウム、トリメチルアルミニウム、トリメチルインジウムなどの有機金属が水素をキャリアガスとして成長装置に供給される。V族元素の材料としては、例えばアルシン(AsH3)、ホスフィン(PH3)、スチビン(SbH3)等のガスが使われる。p型不純物またはn型不純物のドーパントとしては、例えばp型化にはジエチルジンク、またはn型化には、モノシラン(SiH4)やジシラン(Si2H6)、セレン化水素(H2Se)等が利用される。これらの原料ガスを、例えば700℃に加熱された基板上に供給することにより熱分解させ、所望の化合物半導体材料膜をエピタキシャル成長させることが可能である。これら成長層の組成は導入するガス組成により、また膜厚はガスの導入時間によって制御することが可能である。これらの光電変換部を多接合積層する場合は、層間での格子定数を可能な限り合わせることにより、結晶性に優れた成長層を形成することができ、光電変換効率を向上することが可能となる。
An example of a method for manufacturing a photoelectric conversion unit using a compound semiconductor is shown below. In this manufacturing method, all film-forming processes are continuously performed using a metal organic chemical vapor deposition (MOCVD) apparatus. Done. As a group III element material, for example, an organic metal such as trimethylgallium, trimethylaluminum, or trimethylindium is supplied to the growth apparatus using hydrogen as a carrier gas. For example, a gas such as arsine (AsH 3 ), phosphine (PH 3 ), and stibine (SbH 3 ) is used as the material of the group V element. As a dopant of p-type impurities or n-type impurities, for example, diethyl zinc for p-type conversion, monosilane (SiH 4 ), disilane (Si 2 H 6 ), hydrogen selenide (H 2 Se) for n-type conversion, for example. Etc. are used. These source gases can be thermally decomposed by supplying them onto a substrate heated to, for example, 700 ° C., and a desired compound semiconductor material film can be epitaxially grown. The composition of these growth layers can be controlled by the gas composition to be introduced, and the film thickness can be controlled by the gas introduction time. When multi-junction laminating these photoelectric conversion parts, it is possible to form a growth layer with excellent crystallinity by adjusting the lattice constant between layers as much as possible, and to improve the photoelectric conversion efficiency. Become.
pn接合を形成した部分以外にも、例えば受光面側に公知の窓層や、非受光面側に公知の電界層等を設けることによりキャリア収集効率を高める工夫を有してもよい。また不純物の拡散を防止するためのバッファ層を有していてもよい。
In addition to the portion where the pn junction is formed, for example, a known window layer on the light receiving surface side or a known electric field layer on the non-light receiving surface side may be provided to improve carrier collection efficiency. Further, a buffer layer for preventing diffusion of impurities may be provided.
3-3.色素増感剤を利用した光電変換部
色素増感剤を利用した光電変換部は、例えば、主に多孔質半導体、色素増感剤、電解質、溶媒などにより構成される。
多孔質半導体を構成する材料としては、例えば、酸化チタン、酸化タングステン、酸化亜鉛、チタン酸バリウム、チタン酸ストロンチウム、硫化カドミウム等公知の半導体から1種類以上を選択することが可能である。多孔質半導体を基板上に形成する方法としては、半導体粒子を含有するペーストをスクリーン印刷法、インクジェット法等で塗布し乾燥もしくは焼成する方法や、原料ガスを用いたCVD法等により製膜する方法、PVD法、蒸着法、スパッタ法、ゾルゲル法、電気化学的な酸化還元反応を利用した方法等が挙げられる。 3-3. Photoelectric conversion part using a dye sensitizer The photoelectric conversion part using a dye sensitizer is mainly composed of, for example, a porous semiconductor, a dye sensitizer, an electrolyte, a solvent, and the like.
As a material constituting the porous semiconductor, for example, one or more kinds of known semiconductors such as titanium oxide, tungsten oxide, zinc oxide, barium titanate, strontium titanate, cadmium sulfide can be selected. As a method for forming a porous semiconductor on a substrate, a paste containing semiconductor particles is applied by a screen printing method, an ink jet method and the like, dried or baked, a method of forming a film by a CVD method using a raw material gas, etc. , PVD method, vapor deposition method, sputtering method, sol-gel method, method using electrochemical oxidation-reduction reaction, and the like.
色素増感剤を利用した光電変換部は、例えば、主に多孔質半導体、色素増感剤、電解質、溶媒などにより構成される。
多孔質半導体を構成する材料としては、例えば、酸化チタン、酸化タングステン、酸化亜鉛、チタン酸バリウム、チタン酸ストロンチウム、硫化カドミウム等公知の半導体から1種類以上を選択することが可能である。多孔質半導体を基板上に形成する方法としては、半導体粒子を含有するペーストをスクリーン印刷法、インクジェット法等で塗布し乾燥もしくは焼成する方法や、原料ガスを用いたCVD法等により製膜する方法、PVD法、蒸着法、スパッタ法、ゾルゲル法、電気化学的な酸化還元反応を利用した方法等が挙げられる。 3-3. Photoelectric conversion part using a dye sensitizer The photoelectric conversion part using a dye sensitizer is mainly composed of, for example, a porous semiconductor, a dye sensitizer, an electrolyte, a solvent, and the like.
As a material constituting the porous semiconductor, for example, one or more kinds of known semiconductors such as titanium oxide, tungsten oxide, zinc oxide, barium titanate, strontium titanate, cadmium sulfide can be selected. As a method for forming a porous semiconductor on a substrate, a paste containing semiconductor particles is applied by a screen printing method, an ink jet method and the like, dried or baked, a method of forming a film by a CVD method using a raw material gas, etc. , PVD method, vapor deposition method, sputtering method, sol-gel method, method using electrochemical oxidation-reduction reaction, and the like.
多孔質半導体に吸着する色素増感剤としては、可視光領域および赤外光領域に吸収を持つ種々の色素を用いることが可能である。ここで、多孔質半導体に色素を強固に吸着させるには、色素分子中にカルボン酸基、カルボン酸無水基、アルコキシ基、スルホン酸基、ヒドロキシル基、ヒドロキシルアルキル基、エステル基、メルカプト基、ホスホニル基等が存在することが好ましい。これらの官能基は、励起状態の色素と多孔質半導体の伝導帯との間の電子移動を容易にする電気的結合を提供する。
As the dye sensitizer adsorbed on the porous semiconductor, various dyes having absorption in the visible light region and the infrared light region can be used. Here, in order to firmly adsorb the dye to the porous semiconductor, the carboxylic acid group, carboxylic anhydride group, alkoxy group, sulfonic acid group, hydroxyl group, hydroxylalkyl group, ester group, mercapto group, phosphonyl in the dye molecule It is preferable that a group or the like is present. These functional groups provide an electrical bond that facilitates electron transfer between the excited state dye and the conduction band of the porous semiconductor.
これらの官能基を含有する色素として、例えば、ルテニウムビピリジン系色素、キノン系色素、キノンイミン系色素、アゾ系色素、キナクリドン系色素、スクアリリウム系色素、シアニン系色素、メロシアニン系色素、トリフェニルメタン系色素、キサンテン系色素、ポルフィリン系色素、フタロシアニン系色素、ベリレン系色素、インジゴ系色素、ナフタロシアニン系色素等が挙げられる。
Examples of dyes containing these functional groups include ruthenium bipyridine dyes, quinone dyes, quinone imine dyes, azo dyes, quinacridone dyes, squarylium dyes, cyanine dyes, merocyanine dyes, and triphenylmethane dyes. Xanthene dyes, porphyrin dyes, phthalocyanine dyes, berylene dyes, indigo dyes, naphthalocyanine dyes, and the like.
多孔質半導体への色素の吸着方法としては、例えば多孔質半導体を、色素を溶解した溶液(色素吸着用溶液)に浸漬する方法が挙げられる。色素吸着用溶液に用いられる溶媒としては、色素を溶解するものであれば特に制限されず、具体的には、エタノール、メタノール等のアルコール類、アセトン等のケトン類、ジエチルエーテル、テトラヒドロフラン等のエーテル類、アセトニトリル等の窒素化合物類、ヘキサン等の脂肪族炭化水素、ベンゼン等の芳香族炭化水素、酢酸エチル等のエステル類、水等を挙げることができる。
Examples of the method of adsorbing the dye to the porous semiconductor include a method of immersing the porous semiconductor in a solution in which the dye is dissolved (dye adsorption solution). The solvent used in the dye adsorption solution is not particularly limited as long as it dissolves the dye, and specifically, alcohols such as ethanol and methanol, ketones such as acetone, ethers such as diethyl ether and tetrahydrofuran. Nitrogen compounds such as acetonitrile, aliphatic hydrocarbons such as hexane, aromatic hydrocarbons such as benzene, esters such as ethyl acetate, water, and the like.
電解質は、酸化還元対とこれを保持する液体または高分子ゲル等固体の媒体からなる。
酸化還元対としては一般に、鉄系、コバルト系等の金属類や塩素、臭素、ヨウ素等のハロゲン物質が好適に用いられ、ヨウ化リチウム、ヨウ化ナトリウム、ヨウ化カリウム等の金属ヨウ化物とヨウ素の組み合わせが好ましく用いられる。さらに、ジメチルプロピルイミダゾールアイオダイド等のイミダゾール塩等を混入することもできる。 The electrolyte is composed of a redox pair and a solid medium such as a liquid or polymer gel holding the redox pair.
In general, iron- and cobalt-based metals and halogen substances such as chlorine, bromine, and iodine are preferably used as the redox pair, and metal iodides such as lithium iodide, sodium iodide, and potassium iodide and iodine are used. The combination of is preferably used. Furthermore, imidazole salts such as dimethylpropylimidazole iodide can also be mixed.
酸化還元対としては一般に、鉄系、コバルト系等の金属類や塩素、臭素、ヨウ素等のハロゲン物質が好適に用いられ、ヨウ化リチウム、ヨウ化ナトリウム、ヨウ化カリウム等の金属ヨウ化物とヨウ素の組み合わせが好ましく用いられる。さらに、ジメチルプロピルイミダゾールアイオダイド等のイミダゾール塩等を混入することもできる。 The electrolyte is composed of a redox pair and a solid medium such as a liquid or polymer gel holding the redox pair.
In general, iron- and cobalt-based metals and halogen substances such as chlorine, bromine, and iodine are preferably used as the redox pair, and metal iodides such as lithium iodide, sodium iodide, and potassium iodide and iodine are used. The combination of is preferably used. Furthermore, imidazole salts such as dimethylpropylimidazole iodide can also be mixed.
また、溶媒としては、プロピレンカーボネート等のカーボネート化合物、アセトニトリル等のニトリル化合物、エタノール、メタノール等のアルコール、その他、水や非プロトン極性物質等が用いられるが、中でも、カーボネート化合物やニトリル化合物が好適に用いられる。
Examples of the solvent include carbonate compounds such as propylene carbonate, nitrile compounds such as acetonitrile, alcohols such as ethanol and methanol, water, aprotic polar substances, and the like. Of these, carbonate compounds and nitrile compounds are preferred. Used.
3-4.有機薄膜を用いた光電変換部
有機薄膜を用いた光電変換部2は、電子供与性および電子受容性を持つ有機半導体材料で構成される電子正孔輸送層、または電子受容性を有する電子輸送層と電子供与性を有する正孔輸送層とが積層されたものであってもよい。
電子供与性の有機半導体材料としては、電子供与体としての機能を有するものであれば特に限定されないが、塗布法により製膜できることが好ましく、中でも電子供与性の導電性高分子が好適に使用される。 3-4. Photoelectric conversion part using organic thin filmPhotoelectric conversion part 2 using an organic thin film is an electron hole transport layer composed of an organic semiconductor material having electron donating properties and electron accepting properties, or an electron transport layer having electron accepting properties. And a hole transport layer having an electron donating property may be laminated.
The electron-donating organic semiconductor material is not particularly limited as long as it has a function as an electron donor, but it is preferable that a film can be formed by a coating method, and among them, an electron-donating conductive polymer is preferably used. The
有機薄膜を用いた光電変換部2は、電子供与性および電子受容性を持つ有機半導体材料で構成される電子正孔輸送層、または電子受容性を有する電子輸送層と電子供与性を有する正孔輸送層とが積層されたものであってもよい。
電子供与性の有機半導体材料としては、電子供与体としての機能を有するものであれば特に限定されないが、塗布法により製膜できることが好ましく、中でも電子供与性の導電性高分子が好適に使用される。 3-4. Photoelectric conversion part using organic thin film
The electron-donating organic semiconductor material is not particularly limited as long as it has a function as an electron donor, but it is preferable that a film can be formed by a coating method, and among them, an electron-donating conductive polymer is preferably used. The
ここで導電性高分子とはπ共役高分子を示し、炭素-炭素またはヘテロ原子を含む二重結合または三重結合が、単結合と交互に連なったπ共役系からなり、半導体的性質を示すものをさす。
Here, the conductive polymer refers to a π-conjugated polymer, which is composed of a π-conjugated system in which double bonds or triple bonds containing carbon-carbon or hetero atoms are alternately connected to single bonds, and exhibits semiconducting properties. Point.
電子供与性の導電性高分子材料としては、例えばポリフェニレン、ポリフェニレンビニレン、ポリチオフェン、ポリカルバゾール、ポリビニルカルバゾール、ポリシラン、ポリアセチレン、ポリピロール、ポリアニリン、ポリフルオレン、ポリビニルピレン、ポリビニルアントラセン、およびこれらの誘導体、共重合体、あるいはフタロシアニン含有ポリマー、カルバゾール含有ポリマー、有機金属ポリマー等が挙げられる。中でも、チオフェン-フルオレン共重合体、ポリアルキルチオフェン、フェニレンエチニレン-フェニレンビニレン共重合体、フルオレン-フェニレンビニレン共重合体、チオフェン-フェニレンビニレン共重合体等が好適に利用される。
Examples of the electron-donating conductive polymer material include polyphenylene, polyphenylene vinylene, polythiophene, polycarbazole, polyvinyl carbazole, polysilane, polyacetylene, polypyrrole, polyaniline, polyfluorene, polyvinyl pyrene, polyvinyl anthracene, and derivatives, Examples thereof include a polymer, a phthalocyanine-containing polymer, a carbazole-containing polymer, and an organometallic polymer. Of these, thiophene-fluorene copolymer, polyalkylthiophene, phenylene ethynylene-phenylene vinylene copolymer, fluorene-phenylene vinylene copolymer, thiophene-phenylene vinylene copolymer and the like are preferably used.
電子受容性の有機半導体材料としては、電子受容体としての機能を有するものであれば特に限定されないが、塗布法により製膜できることが好ましく、中でも電子供与性の導電性高分子が好適に使用される。
電子受容性の導電性高分子としては、例えばポリフェニレンビニレン、ポリフルオレン、およびこれらの誘導体、共重合体、あるいはカーボンナノチューブ、フラーレンおよびこれらの誘導体、CN基またはCF3基含有ポリマーおよびそれらの-CF3置換ポリマー等が挙げられる。 The electron-accepting organic semiconductor material is not particularly limited as long as it has a function as an electron acceptor. However, it is preferable that a film can be formed by a coating method, and among them, an electron-donating conductive polymer is preferably used. The
Examples of the electron-accepting conductive polymer include polyphenylene vinylene, polyfluorene, and derivatives and copolymers thereof, or carbon nanotubes, fullerene and derivatives thereof, CN group or CF 3 group-containing polymers, and —CF Examples thereof include 3- substituted polymers.
電子受容性の導電性高分子としては、例えばポリフェニレンビニレン、ポリフルオレン、およびこれらの誘導体、共重合体、あるいはカーボンナノチューブ、フラーレンおよびこれらの誘導体、CN基またはCF3基含有ポリマーおよびそれらの-CF3置換ポリマー等が挙げられる。 The electron-accepting organic semiconductor material is not particularly limited as long as it has a function as an electron acceptor. However, it is preferable that a film can be formed by a coating method, and among them, an electron-donating conductive polymer is preferably used. The
Examples of the electron-accepting conductive polymer include polyphenylene vinylene, polyfluorene, and derivatives and copolymers thereof, or carbon nanotubes, fullerene and derivatives thereof, CN group or CF 3 group-containing polymers, and —CF Examples thereof include 3- substituted polymers.
また、電子供与性化合物がドープされた電子受容性の有機半導体材料や、電子受容性化合物がドープされた電子供与性の有機半導体材料等を用いることが可能である。電子供与性化合物がドープされる電子受容性の導電性高分子材料としては、上述の電子受容性の導電性高分子材料を挙げることができる。ドープされる電子供与性化合物としては、例えばLi、K、Ca、Cs等のアルカリ金属やアルカリ土類金属のようなルイス塩基を用いることができる。なお、ルイス塩基は電子供与体として作用する。また、電子受容性化合物がドープされる電子供与性の導電性高分子材料としては、上述した電子供与性の導電性高分子材料を挙げることができる。ドープされる電子受容性化合物としては、例えばFeCl3、AlCl3、AlBr3、AsF6やハロゲン化合物のようなルイス酸を用いることができる。なお、ルイス酸は電子受容体として作用する。
In addition, an electron-accepting organic semiconductor material doped with an electron-donating compound, an electron-donating organic semiconductor material doped with an electron-accepting compound, or the like can be used. Examples of the electron-accepting conductive polymer material doped with the electron-donating compound include the above-described electron-accepting conductive polymer material. As the electron-donating compound to be doped, for example, a Lewis base such as an alkali metal such as Li, K, Ca, or Cs or an alkaline earth metal can be used. The Lewis base acts as an electron donor. Examples of the electron-donating conductive polymer material doped with the electron-accepting compound include the above-described electron-donating conductive polymer material. As the electron-accepting compound to be doped, for example, a Lewis acid such as FeCl 3 , AlCl 3 , AlBr 3 , AsF 6 or a halogen compound can be used. In addition, Lewis acid acts as an electron acceptor.
上記にて示した光電変換部2においては、第一義的には太陽光を受光させ光電変換を行うことを想定しているが、用途により蛍光灯や白熱灯、LED、特定の熱源から発せられる光等の人工光を照射し光電変換を行うことも可能である。
In the photoelectric conversion unit 2 shown above, it is assumed that sunlight is received and photoelectric conversion is primarily performed. However, it is emitted from a fluorescent lamp, an incandescent lamp, an LED, or a specific heat source depending on the application. It is also possible to perform photoelectric conversion by irradiating artificial light such as light.
4.第2電極、第3導電部
第2電極5は、光電変換部2の裏面と第1型部11との間および光電変換部2の裏面と第2型部12との間に設けることができる。また、第2電極5は、第1電解用電極8と電気的に接続することができる。第2電極5を設けることにより、光電変換部2の裏面と第1電解用電極8との間のオーミックロスを低減することができる。また、第2電極5は、図3、13のように第3導電部17を介して第1電解用電極8と電気的に接続してもよく、第1電解用電極8と接触してもよい。また、第3導電部17は、図3のように第1型部11に設けられたコンタクトホール内に設けられてもよく、図13のように第1型部11の側部を覆うように設けられてもよい。
また、第2電極5は、図6、7のように、光電変換部2の裏面と絶縁部21との間および光電変換部2の裏面と第1電解用電極8との間に設けられてもよい。
また、第2電極5は、電解液に対する耐食性および電解液に対する遮液性を有することが好ましい。このことにより、電解液による光電変換部2の腐食を防止することができる。
第2電極5は、導電性を有すれば特に限定されないが、例えば、金属薄膜であり、また、例えば、Al、Ag、Auなどの薄膜である。これらは、例えば、スパッタリングなどにより形成することができる。また、例えば、In-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2等の透明導電膜である。 4). Second Electrode, Third Conductive Part Thesecond electrode 5 can be provided between the back surface of the photoelectric conversion unit 2 and the first mold unit 11 and between the back surface of the photoelectric conversion unit 2 and the second mold unit 12. . The second electrode 5 can be electrically connected to the first electrolysis electrode 8. By providing the second electrode 5, it is possible to reduce ohmic cross between the back surface of the photoelectric conversion unit 2 and the first electrolysis electrode 8. The second electrode 5 may be electrically connected to the first electrolysis electrode 8 through the third conductive portion 17 as shown in FIGS. 3 and 13, or may be in contact with the first electrolysis electrode 8. Good. The third conductive part 17 may be provided in a contact hole provided in the first mold part 11 as shown in FIG. 3 so as to cover the side part of the first mold part 11 as shown in FIG. It may be provided.
6 and 7, thesecond electrode 5 is provided between the back surface of the photoelectric conversion unit 2 and the insulating unit 21 and between the back surface of the photoelectric conversion unit 2 and the first electrolysis electrode 8. Also good.
Moreover, it is preferable that the2nd electrode 5 has the corrosion resistance with respect to electrolyte solution, and the liquid shielding property with respect to electrolyte solution. Thereby, corrosion of the photoelectric conversion part 2 by electrolyte solution can be prevented.
Although it will not specifically limit if the2nd electrode 5 has electroconductivity, For example, it is a metal thin film, for example, is thin films, such as Al, Ag, Au. These can be formed by, for example, sputtering. Further, for example, a transparent conductive film such as In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, and SnO 2 is used.
第2電極5は、光電変換部2の裏面と第1型部11との間および光電変換部2の裏面と第2型部12との間に設けることができる。また、第2電極5は、第1電解用電極8と電気的に接続することができる。第2電極5を設けることにより、光電変換部2の裏面と第1電解用電極8との間のオーミックロスを低減することができる。また、第2電極5は、図3、13のように第3導電部17を介して第1電解用電極8と電気的に接続してもよく、第1電解用電極8と接触してもよい。また、第3導電部17は、図3のように第1型部11に設けられたコンタクトホール内に設けられてもよく、図13のように第1型部11の側部を覆うように設けられてもよい。
また、第2電極5は、図6、7のように、光電変換部2の裏面と絶縁部21との間および光電変換部2の裏面と第1電解用電極8との間に設けられてもよい。
また、第2電極5は、電解液に対する耐食性および電解液に対する遮液性を有することが好ましい。このことにより、電解液による光電変換部2の腐食を防止することができる。
第2電極5は、導電性を有すれば特に限定されないが、例えば、金属薄膜であり、また、例えば、Al、Ag、Auなどの薄膜である。これらは、例えば、スパッタリングなどにより形成することができる。また、例えば、In-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2等の透明導電膜である。 4). Second Electrode, Third Conductive Part The
6 and 7, the
Moreover, it is preferable that the
Although it will not specifically limit if the
また、第3導電部17の材料は、導電性を有しているものであれば特に制限されない。導電性粒子を含有するペースト、例えばカーボンペースト、Agペースト等をスクリーン印刷法、インクジェット法等で塗布し乾燥もしくは焼成する方法や、原料ガスを用いたCVD法等により製膜する方法、PVD法、蒸着法、スパッタ法、ゾルゲル法、電気化学的な酸化還元反応を利用した方法等が挙げられる。
The material of the third conductive portion 17 is not particularly limited as long as it has conductivity. A paste containing conductive particles, for example, a carbon paste, an Ag paste or the like applied by screen printing, an inkjet method, etc., dried or baked, a method of forming a film by a CVD method using a raw material gas, a PVD method, Examples thereof include a vapor deposition method, a sputtering method, a sol-gel method, and a method using an electrochemical redox reaction.
5.第1型部、第2型部、第1導電部
第1型部11は、光電変換部2の裏面と第1電解用電極8との間に設けることができ、第2型部12は、光電変換部2の裏面と第2電解用電極7との間に設けることができる。また、第1型部11および第2型部12は、光電変換部2の受光面を水平にしたとき、第1電解用電極8の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極7の電解液に接触可能な面と水平な基準面との間の傾斜角とが異なるように成形されたものであってもよい。このように成形された第1型部11および第2型部12を設けることにより、第1電解用電極が電解液に接触する面と第2電解用電極の電解液に接触する面の傾斜角度を調節することができる。 5. 1st type | mold part, 2nd type | mold part, 1st electroconductive part The 1st type |mold part 11 can be provided between the back surface of the photoelectric conversion part 2, and the electrode 8 for 1st electrolysis, The 2nd type | mold part 12 is It can be provided between the back surface of the photoelectric conversion unit 2 and the second electrolysis electrode 7. Further, the first mold part 11 and the second mold part 12 are arranged between a surface that can contact the electrolytic solution of the first electrolysis electrode 8 and a horizontal reference surface when the light receiving surface of the photoelectric conversion unit 2 is leveled. And the inclination angle between the surface of the second electrolysis electrode 7 that can contact the electrolytic solution and the horizontal reference surface may be different. By providing the first mold part 11 and the second mold part 12 formed in this way, the inclination angle between the surface of the first electrolysis electrode in contact with the electrolytic solution and the surface of the second electrolysis electrode in contact with the electrolytic solution. Can be adjusted.
第1型部11は、光電変換部2の裏面と第1電解用電極8との間に設けることができ、第2型部12は、光電変換部2の裏面と第2電解用電極7との間に設けることができる。また、第1型部11および第2型部12は、光電変換部2の受光面を水平にしたとき、第1電解用電極8の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極7の電解液に接触可能な面と水平な基準面との間の傾斜角とが異なるように成形されたものであってもよい。このように成形された第1型部11および第2型部12を設けることにより、第1電解用電極が電解液に接触する面と第2電解用電極の電解液に接触する面の傾斜角度を調節することができる。 5. 1st type | mold part, 2nd type | mold part, 1st electroconductive part The 1st type |
また、第1型部11および第2型部12は、例えば、図9、10のように溝状のくぼみを有してもよい。このような第1型部11の上に第1電解用電極8を形成し、第2型部12の上に第2電解用電極7を形成することにより、第1電解用電極8の電解液に接触可能な面、および第2電解用電極7の電解液に接触可能な面に溝状のくぼみを形成することができる。
Moreover, the 1st type | mold part 11 and the 2nd type | mold part 12 may have a groove-shaped hollow like FIG. By forming the first electrolysis electrode 8 on the first mold part 11 and the second electrolysis electrode 7 on the second mold part 12, the electrolytic solution of the first electrolysis electrode 8 is formed. Groove-shaped depressions can be formed on the surface that can be contacted with each other and the surface that can be contacted with the electrolytic solution of the second electrolysis electrode 7.
また、第1型部および第2型部は、電気的絶縁性を有してもよい。このことにより、リーク電流を低減することができる。また、この場合、第2型部12は、図11、12のように光電変換部2の側面を覆うように設けることができ、この光電変換部2の側面を覆った第2型部12の上に第2導電部10または第2電解用電極7を設けることができる。このことにより、リーク電流を防止して第2電解用電極7と第1電極4とを電気的に接続することができる。
第1型部11および第2型部12は、例えば、固体樹脂から形成することができる。固体樹脂とすることにより、所望の形状を有する第1型部11および第2型部12を形成することができる。
また、第1型部11は導電性材料からなってもよい。このことにより、第1型部を介して光電変換部2の裏面と第1電解用電極8とを電気的に接続することができる。 Further, the first mold part and the second mold part may have electrical insulation. As a result, leakage current can be reduced. Further, in this case, thesecond mold part 12 can be provided so as to cover the side surface of the photoelectric conversion part 2 as shown in FIGS. 11 and 12, and the second mold part 12 covering the side surface of the photoelectric conversion part 2 can be provided. The second conductive portion 10 or the second electrolysis electrode 7 can be provided thereon. Thus, leakage current can be prevented and the second electrolysis electrode 7 and the first electrode 4 can be electrically connected.
Thefirst mold part 11 and the second mold part 12 can be formed from, for example, a solid resin. By using a solid resin, the first mold part 11 and the second mold part 12 having a desired shape can be formed.
Thefirst mold part 11 may be made of a conductive material. Thereby, the back surface of the photoelectric conversion unit 2 and the first electrolysis electrode 8 can be electrically connected via the first mold unit.
第1型部11および第2型部12は、例えば、固体樹脂から形成することができる。固体樹脂とすることにより、所望の形状を有する第1型部11および第2型部12を形成することができる。
また、第1型部11は導電性材料からなってもよい。このことにより、第1型部を介して光電変換部2の裏面と第1電解用電極8とを電気的に接続することができる。 Further, the first mold part and the second mold part may have electrical insulation. As a result, leakage current can be reduced. Further, in this case, the
The
The
また、第1型部11と第1電解用電極8との間、または第2型部12と第2電解用電極7との間に第1導電部9を設けることができる。第1導電部9を設けることにより、光電変換部2が受光することにより生じる起電力を第1電解用電極8と第2電解用電極7とに出力するときの内部抵抗を低減することができる。
第1導電部9は、例えば、図8、11、14、16のように第2型部12と第2電解用電極7との間に設けることができ、また、例えば、図13、15のように第1型部11と第1電解用電極8との間に設けることができる。
第1導電部11は、導電性を有すれば特に限定されないが、例えば、金属薄膜であり、また、例えば、Al、Ag、Auなどの薄膜である。これらは、例えば、スパッタリングなどにより形成することができる。また、例えば、In-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2等の透明導電膜である。 Further, the firstconductive part 9 can be provided between the first mold part 11 and the first electrolysis electrode 8 or between the second mold part 12 and the second electrolysis electrode 7. By providing the first conductive portion 9, it is possible to reduce internal resistance when the electromotive force generated by the photoelectric conversion portion 2 receiving light is output to the first electrolysis electrode 8 and the second electrolysis electrode 7. .
The firstconductive portion 9 can be provided between the second mold portion 12 and the second electrolysis electrode 7 as shown in FIGS. 8, 11, 14, and 16, for example. Thus, it can be provided between the first mold part 11 and the first electrolysis electrode 8.
Although it will not specifically limit if the 1stelectroconductive part 11 has electroconductivity, For example, it is a metal thin film, for example, is thin films, such as Al, Ag, Au. These can be formed by, for example, sputtering. Further, for example, a transparent conductive film such as In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, and SnO 2 is used.
第1導電部9は、例えば、図8、11、14、16のように第2型部12と第2電解用電極7との間に設けることができ、また、例えば、図13、15のように第1型部11と第1電解用電極8との間に設けることができる。
第1導電部11は、導電性を有すれば特に限定されないが、例えば、金属薄膜であり、また、例えば、Al、Ag、Auなどの薄膜である。これらは、例えば、スパッタリングなどにより形成することができる。また、例えば、In-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2等の透明導電膜である。 Further, the first
The first
Although it will not specifically limit if the 1st
6.絶縁部
絶縁部21は、リーク電流の発生を防止するために設けることができる。例えば、図4、7、8のように第2導電部10を光電変換部2を貫通するコンタクトホール内に設ける場合、コンタクトホールの側壁に絶縁部21を設けることができる。また、図7のように第2型部12を設けない場合、第2電解用電極7と光電変換部2の裏面との間に設けることができる。また、第2型部12が導電性を有する場合、第2型部12と光電変換部2の裏面との間に絶縁部21を設けることもできる。 6). Insulating part The insulatingpart 21 can be provided in order to prevent the occurrence of leakage current. For example, when the second conductive portion 10 is provided in a contact hole penetrating the photoelectric conversion portion 2 as shown in FIGS. 4, 7, and 8, the insulating portion 21 can be provided on the side wall of the contact hole. Moreover, when not providing the 2nd type | mold part 12 like FIG. 7, it can provide between the electrode 7 for 2nd electrolysis and the back surface of the photoelectric conversion part 2. FIG. Moreover, when the 2nd type | mold part 12 has electroconductivity, the insulating part 21 can also be provided between the 2nd type | mold part 12 and the back surface of the photoelectric conversion part 2. FIG.
絶縁部21は、リーク電流の発生を防止するために設けることができる。例えば、図4、7、8のように第2導電部10を光電変換部2を貫通するコンタクトホール内に設ける場合、コンタクトホールの側壁に絶縁部21を設けることができる。また、図7のように第2型部12を設けない場合、第2電解用電極7と光電変換部2の裏面との間に設けることができる。また、第2型部12が導電性を有する場合、第2型部12と光電変換部2の裏面との間に絶縁部21を設けることもできる。 6). Insulating part The insulating
絶縁部11としては、有機材料、無機材料を問わず用いることが可能であり、例えば、ポリアミド、ポリイミド、ポリアリーレン、芳香族ビニル化合物、フッ素系重合体、アクリル系重合体、ビニルアミド系重合体等の有機ポリマー、無機系材料としては、Al2O3等の金属酸化物、多孔質性シリカ膜等のSiO2や、フッ素添加シリコン酸化膜(FSG)、SiOC、HSQ(Hydrogen Silsesquioxane)膜、SiNx、シラノール(Si(OH)4)をアルコール等の溶媒に溶かし塗布・加熱することにより製膜する方法を用いることが可能である。
The insulating part 11 can be used regardless of an organic material or an inorganic material. For example, polyamide, polyimide, polyarylene, aromatic vinyl compound, fluorine polymer, acrylic polymer, vinylamide polymer, etc. Examples of organic polymers and inorganic materials include metal oxides such as Al 2 O 3 , SiO 2 such as porous silica films, fluorine-added silicon oxide films (FSG), SiOC, HSQ (Hydrogen Silsesquioxane) films, SiN x , It is possible to use a method of forming a film by dissolving silanol (Si (OH) 4 ) in a solvent such as alcohol and applying and heating.
絶縁部11を形成する方法としては、絶縁性材料を含有するペーストをスクリーン印刷法、インクジェット法、スピンコーティング法等で塗布し乾燥もしくは焼成する方法や、原料ガスを用いたCVD法等により製膜する方法、PVD法、蒸着法、スパッタ法、ゾルゲル法を利用した方法等が挙げられる。
As a method for forming the insulating portion 11, a film containing a paste containing an insulating material is applied by a screen printing method, an ink jet method, a spin coating method, etc., dried or baked, or a CVD method using a source gas is used. And a method using a PVD method, a vapor deposition method, a sputtering method, a sol-gel method, and the like.
7.第1電解用電極、第2電解用電極
第1電解用電極8および第2電解用電極7は、光電変換部2の裏面上にそれぞれ設けられ、かつ、光電変換部2の裏面側の面とその裏面であり電解液に接触可能な面をそれぞれ有する。このことにより、第1電解用電極8は光電変換部2に入射する光を遮ることはない。
また、第1電解用電極8および第2電解用電極7は、電解液と接触するとき、光電変換部2が受光することにより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体を発生するように設けられる。例えば、光電変換部2が受光することにより受光面とその裏面との間に起電力が生じる場合、第1電解用電極8は、図3、6、13のように光電変換部2の裏面と電気的に接続することができ、第2電解用電極7は、図4、7、8、11、12のように光電変換部2の受光面と電気的に接続することができる。また、光電変換部2が受光することによりその裏面の第1区域と第2区域との間に起電力が生じる場合、図14~16のように第1電解用電極8は第1区域と第2区域のうちどちらか一方と電気的に接続し、第2電解用電極7は第1区域と第2区域のうち他方と電気的に接続することとができる。 7. First Electrolysis Electrode, Second Electrolysis Electrode Thefirst electrolysis electrode 8 and the second electrolysis electrode 7 are respectively provided on the back surface of the photoelectric conversion unit 2, and the back surface side surface of the photoelectric conversion unit 2 Each has a surface which is the back surface and can contact the electrolyte. Thus, the first electrolysis electrode 8 does not block light incident on the photoelectric conversion unit 2.
In addition, when thefirst electrolysis electrode 8 and the second electrolysis electrode 7 are in contact with the electrolytic solution, the electrolysis solution is electrolyzed by using the electromotive force generated by the photoelectric conversion unit 2 receiving light, and the first gas is obtained. And a second gas is generated. For example, when an electromotive force is generated between the light receiving surface and the back surface when the photoelectric conversion unit 2 receives light, the first electrolysis electrode 8 is connected to the back surface of the photoelectric conversion unit 2 as shown in FIGS. The second electrolysis electrode 7 can be electrically connected to the light receiving surface of the photoelectric conversion unit 2 as shown in FIGS. 4, 7, 8, 11, and 12. In addition, when an electromotive force is generated between the first area and the second area on the back surface of the photoelectric conversion unit 2 by receiving light, the first electrolysis electrode 8 is connected to the first area and the second area as shown in FIGS. The second electrolysis electrode 7 can be electrically connected to one of the two areas and the other of the first and second areas.
第1電解用電極8および第2電解用電極7は、光電変換部2の裏面上にそれぞれ設けられ、かつ、光電変換部2の裏面側の面とその裏面であり電解液に接触可能な面をそれぞれ有する。このことにより、第1電解用電極8は光電変換部2に入射する光を遮ることはない。
また、第1電解用電極8および第2電解用電極7は、電解液と接触するとき、光電変換部2が受光することにより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体を発生するように設けられる。例えば、光電変換部2が受光することにより受光面とその裏面との間に起電力が生じる場合、第1電解用電極8は、図3、6、13のように光電変換部2の裏面と電気的に接続することができ、第2電解用電極7は、図4、7、8、11、12のように光電変換部2の受光面と電気的に接続することができる。また、光電変換部2が受光することによりその裏面の第1区域と第2区域との間に起電力が生じる場合、図14~16のように第1電解用電極8は第1区域と第2区域のうちどちらか一方と電気的に接続し、第2電解用電極7は第1区域と第2区域のうち他方と電気的に接続することとができる。 7. First Electrolysis Electrode, Second Electrolysis Electrode The
In addition, when the
また、第1電解用電極8および第2電解用電極7は、光電変換部2の受光面を水平にしたとき、第1電解用電極8の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極7の電解液に接触可能な面と水平な基準面との間の傾斜角とが異なるように設けられる。このことにより、第1気体が気泡として電解液中を上昇するときに案内面となる第1電解用電極8の電解液に接触可能な面と、第2気体が気泡として電解液中を上昇するときに案内面となる第2電解用電極7の電解液に接触可能な面とをずらして設けることができる。
第1電解用電極8および第2電解用電極7の傾斜角の差は、第1気体および第2気体を回収するための第1気体排出口20および第2気体排出口19が、例えば光電変換部2の受光面を水平にしたときに上下にずれるような差であってもよい。また、たとえば、図1のように気体製造装置23の両側のうち一方に第1気体排出口20が形成され、他方に第2気体排出口19が形成されるように、第1電解用電極8および第2電解用電極7の傾斜角の差がつけられてもよい。 In addition, thefirst electrolysis electrode 8 and the second electrolysis electrode 7 include a surface that can contact the electrolytic solution of the first electrolysis electrode 8 and a horizontal reference surface when the light receiving surface of the photoelectric conversion unit 2 is horizontal. And the inclination angle between the surface of the second electrolysis electrode 7 that can come into contact with the electrolytic solution and the horizontal reference surface are different. Thus, when the first gas rises in the electrolyte as bubbles, the surface of the first electrolysis electrode 8 that can be in contact with the electrolyte, and the second gas rises in the electrolyte as bubbles. Sometimes, the surface of the second electrolysis electrode 7 that becomes the guide surface can be shifted from the surface that can contact the electrolyte.
The difference between the inclination angles of thefirst electrolysis electrode 8 and the second electrolysis electrode 7 is, for example, that the first gas outlet 20 and the second gas outlet 19 for recovering the first gas and the second gas are photoelectrically converted. The difference may be shifted up and down when the light receiving surface of the portion 2 is leveled. Further, for example, as shown in FIG. 1, the first electrolysis electrode 8 is formed such that the first gas discharge port 20 is formed on one of the both sides of the gas production apparatus 23 and the second gas discharge port 19 is formed on the other side. And the difference of the inclination angle of the electrode 7 for 2nd electrolysis may be given.
第1電解用電極8および第2電解用電極7の傾斜角の差は、第1気体および第2気体を回収するための第1気体排出口20および第2気体排出口19が、例えば光電変換部2の受光面を水平にしたときに上下にずれるような差であってもよい。また、たとえば、図1のように気体製造装置23の両側のうち一方に第1気体排出口20が形成され、他方に第2気体排出口19が形成されるように、第1電解用電極8および第2電解用電極7の傾斜角の差がつけられてもよい。 In addition, the
The difference between the inclination angles of the
光電変換部2の受光面を水平にしたとき、第1電解用電極8の電解液に接触可能な面は、例えば、傾斜角が1度以上60度以下であってもよく、好ましくは5度以上30度以下であってもよい。また、このとき第2電解用電極7の電解液に接触可能な面は、例えば、傾斜角が120度以上179度以下であってもよく、好ましくは150度以上175度以下であってもよい。このことにより、気体製造装置23の両側のうち一方から第1気体を排出することができ、他方から第2気体を排出することができ、第1気体および第2気体を回収するための配管を簡素化することができる。
When the light receiving surface of the photoelectric conversion unit 2 is leveled, the surface of the first electrolysis electrode 8 that can come into contact with the electrolytic solution may have, for example, an inclination angle of 1 to 60 degrees, preferably 5 degrees. It may be 30 degrees or less. Further, at this time, the surface of the second electrolysis electrode 7 that can come into contact with the electrolytic solution may have an inclination angle of 120 degrees or more and 179 degrees or less, preferably 150 degrees or more and 175 degrees or less. . Thus, the first gas can be discharged from one of the two sides of the gas production device 23, the second gas can be discharged from the other, and a pipe for collecting the first gas and the second gas can be provided. It can be simplified.
第1電解用電極8の電解液に接触可能な面の傾斜角と、第2電解用電極7の電解液に接触可能な面の傾斜角に差をつける方法は、特に限定されないが、例えば、図2~4、8~16のように第1型部11および第2型部12を設けることにより傾斜角の差をつけてもよく、例えば図6、7のように透光性基板1を成形することにより傾斜角の差をつけてもよい。
なお、第1電解用電極8および第2電解用電極7の電解液に接触可能な面は、第1気体または第2気体が電解液中を気泡として上昇するときに案内面となるものであれば、平面であっても曲面であってもよい。また、電解液に接触可能な面が曲面であり、その傾斜角が場所により異なる場合、この曲面の傾斜角はその平均の傾斜角をいう。 A method for making a difference between the inclination angle of the surface of thefirst electrolysis electrode 8 that can contact the electrolytic solution and the inclination angle of the surface of the second electrolysis electrode 7 that can contact the electrolytic solution is not particularly limited. As shown in FIGS. 2 to 4 and 8 to 16, the first mold part 11 and the second mold part 12 may be provided to provide a difference in inclination angle. For example, as shown in FIGS. You may give the difference of an inclination angle by shape | molding.
It should be noted that the surface of thefirst electrolysis electrode 8 and the second electrolysis electrode 7 that can contact the electrolytic solution is a guide surface when the first gas or the second gas rises as bubbles in the electrolytic solution. For example, it may be a flat surface or a curved surface. Moreover, when the surface which can contact electrolyte solution is a curved surface and the inclination angle changes with places, the inclination angle of this curved surface means the average inclination angle.
なお、第1電解用電極8および第2電解用電極7の電解液に接触可能な面は、第1気体または第2気体が電解液中を気泡として上昇するときに案内面となるものであれば、平面であっても曲面であってもよい。また、電解液に接触可能な面が曲面であり、その傾斜角が場所により異なる場合、この曲面の傾斜角はその平均の傾斜角をいう。 A method for making a difference between the inclination angle of the surface of the
It should be noted that the surface of the
第1電解用電極8および第2電解用電極7は、それぞれ電解液に接触可能な面に傾斜方向に伸びる溝状のくぼみを有することができる。このことにより、第1気体または第2気体をこの溝状のくぼみに沿って電解液中を気泡として上昇させることができ、第1気体と第2気体とを容易に分離することができる。また、このような溝状のくぼみを形成することにより、第1電解用電極8と第2電解用電極7との間に形成する隔壁13を省略することもできる。溝状のくぼみは、例えば図9のように第1電解用電極8および第2電解用電極7の電解液に接触可能な面にそれぞれ1つ形成されてもよく、図10のように第1電解用電極8および第2電解用電極7の電解液に接触可能な面にそれぞれ複数形成されてもよい。
The first electrolysis electrode 8 and the second electrolysis electrode 7 can each have a groove-like recess extending in an inclined direction on a surface that can contact the electrolytic solution. Thus, the first gas or the second gas can be raised as bubbles in the electrolytic solution along the groove-like depression, and the first gas and the second gas can be easily separated. Moreover, the partition wall 13 formed between the first electrolysis electrode 8 and the second electrolysis electrode 7 can be omitted by forming such a groove-like depression. For example, as shown in FIG. 9, one groove-like depression may be formed on each surface of the first electrolysis electrode 8 and the second electrolysis electrode 7 that can come into contact with the electrolytic solution. A plurality of electrodes may be formed on the surfaces of the electrode for electrolysis 8 and the electrode for second electrolysis 7 that can contact the electrolytic solution.
第1電解用電極8および第2電解用電極7は、少なくとも一方が複数であり、かつ、それぞれ帯状の電解液に接触可能な面を有し、かつ、その面の長辺が隣接するように交互に設けられてもよい。図17は、本実施形態の気体製造装置の概略平面図であり、図18は、図17の点線A-Aにおける気体製造装置の概略断面図である。なお、この気体製造装置23の点線A-Aに垂直な方向の断面図は、図3、4のような断面を有している。このように、第1電解用電極8および第2電解用電極7を設けることにより、第1気体が発生する反応が生じる部分と、第2気体が発生する反応が生じる部分との間の距離を短くすることができ、電解液中で生じるイオン濃度の不均衡をより少なくすることができる。また、電解液に接触可能な面を帯状とすることにより、第1気体および第2気体を容易に回収することができる。また、気体製造装置23の両側のうち、一方に複数の第1気体排出口20を形成することができ、他方に複数の第2気体排出口19を形成することができる。このことにより第1気体を回収するための第1気体排出路25と第2気体を回収するための第2気体排出路26を気体製造装置23の両側に設けることができ、配管を簡素化することができる。もしくは、第1気体排出路25、第2気体排出路26を設けずとも、第1気体排出口20あるいは第2気体排出口19から排出される気体を、図示されない気体収集部を介して水上置換法等の方法により気体を収集することが可能である。
At least one of the first electrolysis electrode 8 and the second electrolysis electrode 7 has a plurality of surfaces, each of which has a surface that can contact the strip-shaped electrolyte solution, and the long sides of the surfaces are adjacent to each other It may be provided alternately. FIG. 17 is a schematic plan view of the gas production apparatus of the present embodiment, and FIG. 18 is a schematic cross-sectional view of the gas production apparatus taken along the dotted line AA in FIG. The cross-sectional view of the gas production device 23 in the direction perpendicular to the dotted line AA has a cross section as shown in FIGS. In this way, by providing the first electrolysis electrode 8 and the second electrolysis electrode 7, the distance between the portion where the reaction generating the first gas occurs and the portion where the reaction generating the second gas occurs is increased. It can be shortened, and the ion concentration imbalance generated in the electrolyte can be reduced. Moreover, the 1st gas and 2nd gas can be collect | recovered easily by making the surface which can contact electrolyte solution into strip | belt shape. Moreover, the some 1st gas exhaust port 20 can be formed in one among the both sides of the gas manufacturing apparatus 23, and the some 2nd gas exhaust port 19 can be formed in the other. As a result, the first gas discharge path 25 for recovering the first gas and the second gas discharge path 26 for recovering the second gas can be provided on both sides of the gas production device 23, thereby simplifying the piping. be able to. Alternatively, even if the first gas discharge path 25 and the second gas discharge path 26 are not provided, the gas discharged from the first gas discharge port 20 or the second gas discharge port 19 is replaced with water through a gas collecting unit (not shown). It is possible to collect gas by a method such as a method.
また、第1電解用電極8および第2電解用電極7のうち少なくとも一方は、前記受光面の面積より大きい触媒表面積を有することが好ましい。このような構成によれば、光電変換部2で生じる起電力により、より効率的に第1気体または第2気体を発生させることができる。
また、第1電解用電極8および第2電解用電極7のうち少なくとも一方は、触媒が担持された多孔質の導電体であることが好ましい。このような構成によれば、第1電解用電極8および第2電解用電極7のうち少なくとも一方の触媒表面積を大きくすることができ、より効率的に第1気体または第2気体を発生させることができる。また、多孔質の導電体を用いることにより、光電変換部2と触媒との間の電流が流れることによる電位の変化を抑制することができ、より効率的に第1気体または第2気体を発生させることができる。
第1電解用電極8および第2電解用電極7のうち、一方は水素発生部であってもよく、他方が酸素発生部であってもよい。この場合、第1気体および第2気体のうち一方は水素であり、他方は酸素である。 Moreover, it is preferable that at least one of thefirst electrolysis electrode 8 and the second electrolysis electrode 7 has a catalyst surface area larger than the area of the light receiving surface. According to such a configuration, the first gas or the second gas can be generated more efficiently by the electromotive force generated in the photoelectric conversion unit 2.
In addition, at least one of thefirst electrolysis electrode 8 and the second electrolysis electrode 7 is preferably a porous conductor carrying a catalyst. According to such a configuration, the surface area of at least one of the first electrolysis electrode 8 and the second electrolysis electrode 7 can be increased, and the first gas or the second gas can be generated more efficiently. Can do. Further, by using a porous conductor, it is possible to suppress a change in potential due to a current flowing between the photoelectric conversion unit 2 and the catalyst, and to generate the first gas or the second gas more efficiently. Can be made.
One of thefirst electrolysis electrode 8 and the second electrolysis electrode 7 may be a hydrogen generation unit, and the other may be an oxygen generation unit. In this case, one of the first gas and the second gas is hydrogen, and the other is oxygen.
また、第1電解用電極8および第2電解用電極7のうち少なくとも一方は、触媒が担持された多孔質の導電体であることが好ましい。このような構成によれば、第1電解用電極8および第2電解用電極7のうち少なくとも一方の触媒表面積を大きくすることができ、より効率的に第1気体または第2気体を発生させることができる。また、多孔質の導電体を用いることにより、光電変換部2と触媒との間の電流が流れることによる電位の変化を抑制することができ、より効率的に第1気体または第2気体を発生させることができる。
第1電解用電極8および第2電解用電極7のうち、一方は水素発生部であってもよく、他方が酸素発生部であってもよい。この場合、第1気体および第2気体のうち一方は水素であり、他方は酸素である。 Moreover, it is preferable that at least one of the
In addition, at least one of the
One of the
9.水素発生部
水素発生部は、電解液からH2を発生させる部分であり、第1電解用電極8および第2電解用電極7のうちどちらか一方である。
また、水素発生部は、電解液からH2が発生する反応の触媒を含んでもよい。このことにより、電解液からH2が発生する反応の反応速度を大きくすることができる。水素発生部は、電解液からH2が発生する反応の触媒のみからなってもよく、この触媒が担持体に担持されたものであってもよい。また、水素発生部は、光電変換部2の受光面の面積より大きい触媒表面積を有してもよい。このことにより、電解液からH2が発生する反応をより速い反応速度とすることができる。また、水素発生部は、触媒が担持された多孔質の導電体であってもよい。このことにより、触媒表面積を大きくすることができる。また、光電変換部2の受光面または裏面と水素発生部に含まれる触媒との間に電流が流れることによる電位の変化を抑制することができる。さらに、水素発生部は、水素発生触媒としてPt、Ir、Ru、Pd、Rh、Au、Fe、NiおよびSeのうち少なくとも1つを含んでもよい。このような構成によれば、光電変換部2で生じる起電力により、より速い反応速度で水素を発生させることができる。 9. Hydrogen generating part The hydrogen generating part is a part for generating H 2 from the electrolytic solution, and is one of thefirst electrolysis electrode 8 and the second electrolysis electrode 7.
Further, the hydrogen generation unit may include a catalyst for a reaction in which H 2 is generated from the electrolytic solution. Thereby, the reaction rate of the reaction in which H 2 is generated from the electrolytic solution can be increased. The hydrogen generation part may consist only of a catalyst for the reaction in which H 2 is generated from the electrolytic solution, or this catalyst may be supported on a support. Further, the hydrogen generation unit may have a catalyst surface area larger than the area of the light receiving surface of thephotoelectric conversion unit 2. Thereby, the reaction in which H 2 is generated from the electrolytic solution can be set to a faster reaction rate. The hydrogen generation part may be a porous conductor carrying a catalyst. This can increase the catalyst surface area. In addition, a change in potential due to a current flowing between the light receiving surface or the back surface of the photoelectric conversion unit 2 and the catalyst included in the hydrogen generation unit can be suppressed. Furthermore, the hydrogen generation unit may include at least one of Pt, Ir, Ru, Pd, Rh, Au, Fe, Ni, and Se as a hydrogen generation catalyst. According to such a configuration, hydrogen can be generated at a higher reaction rate by the electromotive force generated in the photoelectric conversion unit 2.
水素発生部は、電解液からH2を発生させる部分であり、第1電解用電極8および第2電解用電極7のうちどちらか一方である。
また、水素発生部は、電解液からH2が発生する反応の触媒を含んでもよい。このことにより、電解液からH2が発生する反応の反応速度を大きくすることができる。水素発生部は、電解液からH2が発生する反応の触媒のみからなってもよく、この触媒が担持体に担持されたものであってもよい。また、水素発生部は、光電変換部2の受光面の面積より大きい触媒表面積を有してもよい。このことにより、電解液からH2が発生する反応をより速い反応速度とすることができる。また、水素発生部は、触媒が担持された多孔質の導電体であってもよい。このことにより、触媒表面積を大きくすることができる。また、光電変換部2の受光面または裏面と水素発生部に含まれる触媒との間に電流が流れることによる電位の変化を抑制することができる。さらに、水素発生部は、水素発生触媒としてPt、Ir、Ru、Pd、Rh、Au、Fe、NiおよびSeのうち少なくとも1つを含んでもよい。このような構成によれば、光電変換部2で生じる起電力により、より速い反応速度で水素を発生させることができる。 9. Hydrogen generating part The hydrogen generating part is a part for generating H 2 from the electrolytic solution, and is one of the
Further, the hydrogen generation unit may include a catalyst for a reaction in which H 2 is generated from the electrolytic solution. Thereby, the reaction rate of the reaction in which H 2 is generated from the electrolytic solution can be increased. The hydrogen generation part may consist only of a catalyst for the reaction in which H 2 is generated from the electrolytic solution, or this catalyst may be supported on a support. Further, the hydrogen generation unit may have a catalyst surface area larger than the area of the light receiving surface of the
電解液からH2が発生する反応の触媒(水素発生触媒)は、2つのプロトンと2つの電子から1分子の水素への変換を促進する触媒であり、化学的に安定であり、水素生成過電圧が小さい材料を用いることができる。例えば、水素に対して触媒活性を有するPt,Ir,Ru,Pd,Rh,Au等の白金族金属およびその合金あるいは化合物、水素生成酵素であるヒドロゲナーゼの活性中心を構成するFe,Ni,Seの合金あるいは化合物、およびこれらの組み合わせ等を好適に用いることが可能である。中でもPtおよびPtを含有するナノ構造体は水素発生過電圧が小さく好適に用いることが可能である。光照射により水素発生反応が確認されるCdS,CdSe,ZnS,ZrO2などの材料を用いることもできる。
The catalyst for the reaction of generating H 2 from the electrolyte (hydrogen generation catalyst) is a catalyst that promotes the conversion of two protons and two electrons into one molecule of hydrogen, is chemically stable, and generates hydrogen overvoltage. Can be used. For example, platinum group metals such as Pt, Ir, Ru, Pd, Rh, and Au, which have catalytic activity for hydrogen, and alloys or compounds thereof, Fe, Ni, and Se that constitute the active center of hydrogenase that is a hydrogen-producing enzyme. An alloy or a compound, a combination thereof, or the like can be preferably used. Among them, a nanostructure containing Pt and Pt has a small hydrogen generation overvoltage and can be suitably used. Materials such as CdS, CdSe, ZnS, and ZrO 2 whose hydrogen generation reaction is confirmed by light irradiation can also be used.
水素発生触媒を導電体に担持することができる。触媒を担持する導電体としては、金属材料、炭素質材料、導電性を有する無機材料等が挙げられる。
金属材料としては、電子伝導性を有し、酸性雰囲気下で耐腐食性を有する材料が好ましい。具体的には、Au、Pt、Pd等の貴金属、Ti、Ta、W、Nb、Ni、Al、Cr、Ag、Cu、Zn、Su、Si等の金属並びにこれらの金属の窒化物および炭化物、ステンレス鋼、Cu-Cr、Ni-Cr、Ti-Pt等の合金が挙げられる。金属材料には、Pt、Ti、Au、Ag、Cu、Ni、Wからなる群より選ばれる少なくとも一つの元素を含むことが、他の化学的な副反応が少ないという観点から、より好ましい。これら金属材料は、比較的電気抵抗が小さく、面方向に電流を取り出しても電圧の低下を抑制することができる。また、Cu、Ag、Zn等の酸性雰囲気下での耐腐食性に乏しい金属材料を用いる場合には、Au、Pt、Pd等の耐腐食性を有する貴金属および金属、カーボン、グラファイト、グラッシーカーボン、導電性高分子、導電性窒化物、導電性炭化物、導電性酸化物等によって耐腐食性に乏しい金属の表面をコーティングしてもよい。 The hydrogen generating catalyst can be supported on the conductor. Examples of the conductor carrying the catalyst include metal materials, carbonaceous materials, and conductive inorganic materials.
As the metal material, a material having electronic conductivity and resistance to corrosion in an acidic atmosphere is preferable. Specifically, noble metals such as Au, Pt, Pd, metals such as Ti, Ta, W, Nb, Ni, Al, Cr, Ag, Cu, Zn, Su, Si, and nitrides and carbides of these metals, Examples of the alloy include stainless steel, Cu—Cr, Ni—Cr, and Ti—Pt. It is more preferable that the metal material contains at least one element selected from the group consisting of Pt, Ti, Au, Ag, Cu, Ni, and W from the viewpoint that there are few other chemical side reactions. These metal materials have a relatively small electric resistance, and can suppress a decrease in voltage even when a current is extracted in the surface direction. Further, when using a metal material having poor corrosion resistance in an acidic atmosphere such as Cu, Ag, Zn, etc., noble metals and metals having corrosion resistance such as Au, Pt, Pd, carbon, graphite, glassy carbon, A metal surface having poor corrosion resistance may be coated with a conductive polymer, a conductive nitride, a conductive carbide, a conductive oxide, or the like.
金属材料としては、電子伝導性を有し、酸性雰囲気下で耐腐食性を有する材料が好ましい。具体的には、Au、Pt、Pd等の貴金属、Ti、Ta、W、Nb、Ni、Al、Cr、Ag、Cu、Zn、Su、Si等の金属並びにこれらの金属の窒化物および炭化物、ステンレス鋼、Cu-Cr、Ni-Cr、Ti-Pt等の合金が挙げられる。金属材料には、Pt、Ti、Au、Ag、Cu、Ni、Wからなる群より選ばれる少なくとも一つの元素を含むことが、他の化学的な副反応が少ないという観点から、より好ましい。これら金属材料は、比較的電気抵抗が小さく、面方向に電流を取り出しても電圧の低下を抑制することができる。また、Cu、Ag、Zn等の酸性雰囲気下での耐腐食性に乏しい金属材料を用いる場合には、Au、Pt、Pd等の耐腐食性を有する貴金属および金属、カーボン、グラファイト、グラッシーカーボン、導電性高分子、導電性窒化物、導電性炭化物、導電性酸化物等によって耐腐食性に乏しい金属の表面をコーティングしてもよい。 The hydrogen generating catalyst can be supported on the conductor. Examples of the conductor carrying the catalyst include metal materials, carbonaceous materials, and conductive inorganic materials.
As the metal material, a material having electronic conductivity and resistance to corrosion in an acidic atmosphere is preferable. Specifically, noble metals such as Au, Pt, Pd, metals such as Ti, Ta, W, Nb, Ni, Al, Cr, Ag, Cu, Zn, Su, Si, and nitrides and carbides of these metals, Examples of the alloy include stainless steel, Cu—Cr, Ni—Cr, and Ti—Pt. It is more preferable that the metal material contains at least one element selected from the group consisting of Pt, Ti, Au, Ag, Cu, Ni, and W from the viewpoint that there are few other chemical side reactions. These metal materials have a relatively small electric resistance, and can suppress a decrease in voltage even when a current is extracted in the surface direction. Further, when using a metal material having poor corrosion resistance in an acidic atmosphere such as Cu, Ag, Zn, etc., noble metals and metals having corrosion resistance such as Au, Pt, Pd, carbon, graphite, glassy carbon, A metal surface having poor corrosion resistance may be coated with a conductive polymer, a conductive nitride, a conductive carbide, a conductive oxide, or the like.
炭素質材料としては、化学的に安定で導電性を有する材料が好ましい。例えば、アセチレンブラック、バルカン、ケッチェンブラック、ファーネスブラック、VGCF、カーボンナノチューブ、カーボンナノホーン、フラーレン等の炭素粉末や炭素繊維が挙げられる。
As the carbonaceous material, a chemically stable and conductive material is preferable. Examples thereof include carbon powders and carbon fibers such as acetylene black, vulcan, ketjen black, furnace black, VGCF, carbon nanotube, carbon nanohorn, and fullerene.
導電性を有する無機材料としては、例えば、In-Zn-O(IZO)、In-Sn-O(ITO)、ZnO-Al、Zn-Sn-O、SnO2、酸化アンチモンドープ酸化スズが挙げられる。
Examples of the inorganic material having conductivity include In—Zn—O (IZO), In—Sn—O (ITO), ZnO—Al, Zn—Sn—O, SnO 2 , and antimony oxide-doped tin oxide. .
なお、導電性高分子としては、ポリアセチレン、ポリチオフェン、ポリアニリン、ポリピロール、ポリパラフェニレン、ポリパラフェニレンビニレン等が挙げられ、導電性窒化物としては、窒化炭素、窒化ケイ素、窒化ガリウム、窒化インジウム、窒化ゲルマニウム、窒化チタニウム、窒化ジルコニウム、窒化タリウム等が挙げられ、導電性炭化物としては、炭化タンタル、炭化ケイ素、炭化ジルコニウム、炭化チタニウム、炭化モリブデン、炭化ニオブ、炭化鉄、炭化ニッケル、炭化ハフニウム、炭化タングステン、炭化バナジウム、炭化クロム等が挙げられ、導電性酸化物としては、酸化スズ、酸化インジウムスズ(ITO)、酸化アンチモンドープ酸化スズ等が挙げられる。
In addition, examples of the conductive polymer include polyacetylene, polythiophene, polyaniline, polypyrrole, polyparaphenylene, polyparaphenylene vinylene, and the like, and examples of the conductive nitride include carbon nitride, silicon nitride, gallium nitride, indium nitride, and nitride. Germanium, titanium nitride, zirconium nitride, thallium nitride, etc. are listed, and conductive carbides include tantalum carbide, silicon carbide, zirconium carbide, titanium carbide, molybdenum carbide, niobium carbide, iron carbide, nickel carbide, hafnium carbide, tungsten carbide. , Vanadium carbide, chromium carbide, and the like. Examples of the conductive oxide include tin oxide, indium tin oxide (ITO), and antimony oxide-doped tin oxide.
水素発生触媒を担持する導電体の構造としては、板状、箔状、棒状、メッシュ状、ラス板状、多孔質板状、多孔質棒状、織布状、不織布状、繊維状、フェルト状が好適に使用できる。また、フェルト状電極の表面を溝状に圧着した溝付き導電体は、電気抵抗と電極液の流動抵抗を低減できるので好適である。
The structure of the conductor supporting the hydrogen generation catalyst includes a plate shape, a foil shape, a rod shape, a mesh shape, a lath plate shape, a porous plate shape, a porous rod shape, a woven fabric shape, a nonwoven fabric shape, a fiber shape, and a felt shape. It can be used suitably. Further, a grooved conductor in which the surface of the felt-like electrode is pressure-bonded in a groove shape is preferable because the electric resistance and the flow resistance of the electrode liquid can be reduced.
10.酸素発生部
酸素発生部は、電解液からO2を発生させる部分であり、第1電解用電極8および第2電解用電極7のうちどちらか一方である。
また、酸素発生部は、電解液からO2が発生する反応の触媒を含んでもよい。このことにより、電解液からO2が発生する反応の反応速度を大きくすることができる。また、酸素発生部は、電解液からO2が発生する反応の触媒のみからなってもよく、この触媒が担持体に担持されたものであってもよい。また、酸素発生部は、光電変換部2の受光面の面積より大きい触媒表面積を有してもよい。このことにより、電解液からO2が発生する反応をより速い反応速度とすることができる。また、酸素発生部は、触媒が担持された多孔質の導電体であってもよい。このことにより、触媒表面積を大きくすることができる。また、光電変換部2の受光面または裏面と酸素発生部に含まれる触媒との間に電流が流れることによる電位の変化を抑制することができる。さらに、酸素発生部は、酸素発生触媒としてMn、Ca、Zn、CoおよびIrのうち少なくとも1つを含んでもよい。このような構成によれば、光電変換部で生じる起電力により、より速い反応速度で酸素を発生させることができる。 10. Oxygen generating portion The oxygen generating portion is a portion that generates O 2 from the electrolytic solution, and is one of thefirst electrolysis electrode 8 and the second electrolysis electrode 7.
Further, the oxygen generation unit may include a catalyst for a reaction in which O 2 is generated from the electrolytic solution. Thereby, the reaction rate of the reaction in which O 2 is generated from the electrolytic solution can be increased. Further, the oxygen generation part may consist only of a catalyst for the reaction that generates O 2 from the electrolytic solution, or the catalyst may be supported on a carrier. Further, the oxygen generation unit may have a catalyst surface area larger than the area of the light receiving surface of thephotoelectric conversion unit 2. Thereby, the reaction in which O 2 is generated from the electrolytic solution can be set to a faster reaction rate. The oxygen generation part may be a porous conductor carrying a catalyst. This can increase the catalyst surface area. In addition, a change in potential due to a current flowing between the light receiving surface or the back surface of the photoelectric conversion unit 2 and the catalyst included in the oxygen generation unit can be suppressed. Furthermore, the oxygen generation unit may include at least one of Mn, Ca, Zn, Co, and Ir as an oxygen generation catalyst. According to such a configuration, oxygen can be generated at a higher reaction rate by the electromotive force generated in the photoelectric conversion unit.
酸素発生部は、電解液からO2を発生させる部分であり、第1電解用電極8および第2電解用電極7のうちどちらか一方である。
また、酸素発生部は、電解液からO2が発生する反応の触媒を含んでもよい。このことにより、電解液からO2が発生する反応の反応速度を大きくすることができる。また、酸素発生部は、電解液からO2が発生する反応の触媒のみからなってもよく、この触媒が担持体に担持されたものであってもよい。また、酸素発生部は、光電変換部2の受光面の面積より大きい触媒表面積を有してもよい。このことにより、電解液からO2が発生する反応をより速い反応速度とすることができる。また、酸素発生部は、触媒が担持された多孔質の導電体であってもよい。このことにより、触媒表面積を大きくすることができる。また、光電変換部2の受光面または裏面と酸素発生部に含まれる触媒との間に電流が流れることによる電位の変化を抑制することができる。さらに、酸素発生部は、酸素発生触媒としてMn、Ca、Zn、CoおよびIrのうち少なくとも1つを含んでもよい。このような構成によれば、光電変換部で生じる起電力により、より速い反応速度で酸素を発生させることができる。 10. Oxygen generating portion The oxygen generating portion is a portion that generates O 2 from the electrolytic solution, and is one of the
Further, the oxygen generation unit may include a catalyst for a reaction in which O 2 is generated from the electrolytic solution. Thereby, the reaction rate of the reaction in which O 2 is generated from the electrolytic solution can be increased. Further, the oxygen generation part may consist only of a catalyst for the reaction that generates O 2 from the electrolytic solution, or the catalyst may be supported on a carrier. Further, the oxygen generation unit may have a catalyst surface area larger than the area of the light receiving surface of the
電解液からO2が発生する反応の触媒(酸素発生触媒)は、2つの水分子から1分子の酸素および4つのプロトンと4つの電子への変換を促進する触媒であり、化学的に安定であり、酸素発生過電圧が小さい材料を用いることができる。例えば、光を用い水から酸素発生を行う反応を触媒する酵素であるPhotosystem IIの活性中心を担うMn,Ca,Zn,Coを含む酸化物あるいは化合物や、Pt,RuO2,IrO2等の白金族金属を含む化合物や、Ti,Zr,Nb,Ta,W,Ce,Fe,Ni等の遷移金属を含む酸化物あるいは化合物、および上記材料の組み合わせ等を用いることが可能である。中でも酸化イリジウム、酸化マンガン、酸化コバルト、リン酸コバルトは、過電圧が小さく酸素発生効率が高いことから好適に用いることができる。
The catalyst for the reaction of generating O 2 from the electrolyte (oxygen generating catalyst) is a catalyst that promotes the conversion of two water molecules into one molecule of oxygen, four protons, and four electrons, and is chemically stable. In addition, a material having a small oxygen generation overvoltage can be used. For example, oxides or compounds containing Mn, Ca, Zn, Co, which are active centers of Photosystem II, which is an enzyme that catalyzes the reaction of generating oxygen from water using light, and platinum such as Pt, RuO 2 , IrO 2 It is possible to use compounds containing group metals, oxides or compounds containing transition metals such as Ti, Zr, Nb, Ta, W, Ce, Fe, Ni, and combinations of the above materials. Among these, iridium oxide, manganese oxide, cobalt oxide, and cobalt phosphate can be suitably used because they have low overvoltage and high oxygen generation efficiency.
酸素発生触媒を導電体に担持することができる。触媒を担持する導電体としては、金属材料、炭素質材料、導電性を有する無機材料等が挙げられる。これらの説明は、「9.水素発生部」に記載した水素発生触媒についての説明が矛盾がない限り当てはまる。
水素発生触媒および酸素発生触媒の単独の触媒活性が小さい場合、助触媒を用いることも可能である。例えば、Ni,Cr,Rh,Mo,Co,Seの酸化物あるいは化合物などが挙げられる。 The oxygen generating catalyst can be supported on the conductor. Examples of the conductor carrying the catalyst include metal materials, carbonaceous materials, and conductive inorganic materials. These explanations apply as long as there is no contradiction in the explanation of the hydrogen generation catalyst described in “9.
When the catalytic activity of the hydrogen generating catalyst and the oxygen generating catalyst alone is small, a promoter can be used. Examples thereof include oxides or compounds of Ni, Cr, Rh, Mo, Co, and Se.
水素発生触媒および酸素発生触媒の単独の触媒活性が小さい場合、助触媒を用いることも可能である。例えば、Ni,Cr,Rh,Mo,Co,Seの酸化物あるいは化合物などが挙げられる。 The oxygen generating catalyst can be supported on the conductor. Examples of the conductor carrying the catalyst include metal materials, carbonaceous materials, and conductive inorganic materials. These explanations apply as long as there is no contradiction in the explanation of the hydrogen generation catalyst described in “9.
When the catalytic activity of the hydrogen generating catalyst and the oxygen generating catalyst alone is small, a promoter can be used. Examples thereof include oxides or compounds of Ni, Cr, Rh, Mo, Co, and Se.
なお、水素発生触媒、酸素発生触媒の担持方法は、導電体もしくは半導体に直接塗布する方法や、真空蒸着法、スパッタ法、イオンプレーティング法等のPVD法、CVD法等の乾式塗工法、電析法など、材料により適宜その手法を変え作製ことが可能である。光電変換部と触媒の間に適宜導電物質を担持することが可能である。また水素発生および酸素発生のための触媒活性が十分でない場合、金属やカーボン等の多孔質体や繊維状物質、ナノ粒子等に担持することにより反応表面積を大きくし、水素及び酸素発生速度を向上させることが可能である。
The method for supporting the hydrogen generating catalyst and the oxygen generating catalyst can be applied directly to a conductor or semiconductor, PVD methods such as vacuum deposition, sputtering, and ion plating, dry coating methods such as CVD, The method can be appropriately changed depending on the material such as an analysis method. A conductive material can be appropriately supported between the photoelectric conversion unit and the catalyst. Also, when the catalytic activity for hydrogen generation and oxygen generation is not sufficient, the reaction surface area is increased by supporting it on porous materials such as metals and carbon, fibrous materials, nanoparticles, etc., and the hydrogen and oxygen generation rates are improved. It is possible to make it.
11.天板、電解液室
天板14は、第1電解用電極8および第2電解用電極7の上に透光性基板1と対向するように設けることができる。また、天板14は、第1電解用電極8および第2電解用電極7と天板14との間に空間が設けられるように設けることができる。この空間を電解液室15とすることができ、電解液室15に電解液を導入することにより、第1電解用電極8および第2電解用電極7を電解液に接触させることができる。また、天板に箱状のものを用いる場合、天板14は箱体の底の部分であってもよい。 11. Top Plate, Electrolyte Chamber Thetop plate 14 can be provided on the first electrolysis electrode 8 and the second electrolysis electrode 7 so as to face the translucent substrate 1. The top plate 14 can be provided such that a space is provided between the first electrolysis electrode 8 and the second electrolysis electrode 7 and the top plate 14. This space can be used as the electrolytic solution chamber 15, and the first electrolytic electrode 8 and the second electrolytic electrode 7 can be brought into contact with the electrolytic solution by introducing the electrolytic solution into the electrolytic solution chamber 15. Moreover, when using a box-shaped thing for a top plate, the top plate 14 may be the bottom part of a box.
天板14は、第1電解用電極8および第2電解用電極7の上に透光性基板1と対向するように設けることができる。また、天板14は、第1電解用電極8および第2電解用電極7と天板14との間に空間が設けられるように設けることができる。この空間を電解液室15とすることができ、電解液室15に電解液を導入することにより、第1電解用電極8および第2電解用電極7を電解液に接触させることができる。また、天板に箱状のものを用いる場合、天板14は箱体の底の部分であってもよい。 11. Top Plate, Electrolyte Chamber The
また、天板14は、電解液室15を構成し、生成した第1気体および第2気体を閉じ込めるために構成される材料であり、機密性が高い物質が求められる。透明なものであっても不透明なものであっても特に限定されるものではないが、第1気体および第2気体が発生していることを視認できる点においては透明な材料であることが好ましい。透明な天板としては特に限定されず、例えば石英ガラス、パイレックス(登録商標)、合成石英板等の透明なリジッド材、あるいは透明樹脂板、透明樹脂フィルムなどを挙げることができる。中でも、ガスの透過性がなく、化学的物理的に安定な物質である点でガラス材を用いることが好ましい。
Further, the top plate 14 is a material that constitutes the electrolytic solution chamber 15 and confines the generated first gas and second gas, and a highly confidential substance is required. It is not particularly limited whether it is transparent or opaque, but it is preferably a transparent material in that it can be visually confirmed that the first gas and the second gas are generated. . The transparent top plate is not particularly limited, and examples thereof include a transparent rigid material such as quartz glass, Pyrex (registered trademark), and a synthetic quartz plate, a transparent resin plate, and a transparent resin film. Among them, it is preferable to use a glass material because it is a gas that is not chemically permeable and is chemically and physically stable.
12.隔壁
隔壁13は、第1電解用電極8と第2電解用電極7とを仕切るように設けることができる。また、隔壁13は、第1電解用電極8と天板14との間の空間である電解液室15および第2電解用電極7と天板14との間の空間である電解液室15とを仕切るように設けることができる。このことにより、第1電解用電極8および第2電解用電極7で発生させた第1気体および第2気体が混合することを防止することができ、第1気体および第2気体を分離して回収することができる。
また、隔壁13は、イオン交換体を含んでもよい。このことにより、第1電解用電極8と天板14との間の空間の電解液と第2電解用電極7と天板14との間の空間の電解液でアンバランスとなったイオン濃度を一定に保つことができる。 12 Partition Wall Thepartition wall 13 can be provided so as to partition the first electrolysis electrode 8 and the second electrolysis electrode 7. The partition wall 13 includes an electrolytic solution chamber 15 that is a space between the first electrolysis electrode 8 and the top plate 14 and an electrolytic solution chamber 15 that is a space between the second electrolysis electrode 7 and the top plate 14. It can provide so that it may partition. As a result, the first gas and the second gas generated by the first electrolysis electrode 8 and the second electrolysis electrode 7 can be prevented from mixing, and the first gas and the second gas can be separated. It can be recovered.
Thepartition wall 13 may include an ion exchanger. As a result, the ion concentration that is unbalanced between the electrolytic solution in the space between the first electrolysis electrode 8 and the top plate 14 and the electrolytic solution in the space between the second electrolysis electrode 7 and the top plate 14 is obtained. Can be kept constant.
隔壁13は、第1電解用電極8と第2電解用電極7とを仕切るように設けることができる。また、隔壁13は、第1電解用電極8と天板14との間の空間である電解液室15および第2電解用電極7と天板14との間の空間である電解液室15とを仕切るように設けることができる。このことにより、第1電解用電極8および第2電解用電極7で発生させた第1気体および第2気体が混合することを防止することができ、第1気体および第2気体を分離して回収することができる。
また、隔壁13は、イオン交換体を含んでもよい。このことにより、第1電解用電極8と天板14との間の空間の電解液と第2電解用電極7と天板14との間の空間の電解液でアンバランスとなったイオン濃度を一定に保つことができる。 12 Partition Wall The
The
隔壁13は、例えば、多孔質ガラス、多孔質ジルコニア、多孔質アルミナ等の無機膜あるいはイオン交換体を用いることが可能である。
イオン交換体としては、当該分野で公知のイオン交換体をいずれも使用でき、プロトン伝導性膜、カチオン交換膜、アニオン交換膜等を使用できる。
プロトン伝導性膜の材質としては、プロトン伝導性を有しかつ電気的絶縁性を有する材質であれば特に限定されず、高分子膜、無機膜又はコンポジット膜を用いることができる。 For thepartition wall 13, for example, an inorganic film such as porous glass, porous zirconia, or porous alumina or an ion exchanger can be used.
As the ion exchanger, any ion exchanger known in the art can be used, and a proton conductive membrane, a cation exchange membrane, an anion exchange membrane, or the like can be used.
The material of the proton conductive film is not particularly limited as long as it is a material having proton conductivity and electrical insulation, and a polymer film, an inorganic film, or a composite film can be used.
イオン交換体としては、当該分野で公知のイオン交換体をいずれも使用でき、プロトン伝導性膜、カチオン交換膜、アニオン交換膜等を使用できる。
プロトン伝導性膜の材質としては、プロトン伝導性を有しかつ電気的絶縁性を有する材質であれば特に限定されず、高分子膜、無機膜又はコンポジット膜を用いることができる。 For the
As the ion exchanger, any ion exchanger known in the art can be used, and a proton conductive membrane, a cation exchange membrane, an anion exchange membrane, or the like can be used.
The material of the proton conductive film is not particularly limited as long as it is a material having proton conductivity and electrical insulation, and a polymer film, an inorganic film, or a composite film can be used.
高分子膜としては、例えば、パーフルオロスルホン酸系電解質膜である、デュポン社製のナフィオン(登録商標)、旭化成社製のアシプレックス(登録商標)、旭硝子社製のフレミオン(登録商標)等の膜や、ポリスチレンスルホン酸、スルホン化ポリエーテルエーテルケトン等の炭化水素系電解質膜等が挙げられる。
Examples of the polymer membrane include Nafion (registered trademark) manufactured by DuPont, Aciplex (registered trademark) manufactured by Asahi Kasei Co., and Flemion (registered trademark) manufactured by Asahi Glass Co., Ltd., which are perfluorosulfonic acid electrolyte membranes. Examples thereof include membranes and hydrocarbon electrolyte membranes such as polystyrene sulfonic acid and sulfonated polyether ether ketone.
無機膜としては、例えば、リン酸ガラス、硫酸水素セシウム、ポリタングストリン酸、ポリリン酸アンモニウム等からなる膜が挙げられる。コンポジット膜としては、スルホン化ポリイミド系ポリマー、タングステン酸等の無機物とポリイミド等の有機物とのコンポジット等からなる膜が挙げられ、具体的にはゴア社製のゴアセレクト膜(登録商標)や細孔フィリング電解質膜等が挙げられる。さらに、高温環境下(例えば、100℃以上)で使用する場合には、スルホン化ポリイミド、2-アクリルアミド-2-メチルプロパンスルホン酸(AMPS)、スルホン化ポリベンゾイミダゾール、ホスホン化ポリベンゾイミダゾール、硫酸水素セシウム、ポリリン酸アンモニウム等が挙げられる。
Examples of the inorganic film include films made of phosphate glass, cesium hydrogen sulfate, polytungstophosphoric acid, ammonium polyphosphate, and the like. Examples of the composite membrane include a membrane made of a sulfonated polyimide polymer, a composite of an inorganic material such as tungstic acid and an organic material such as polyimide, and specifically, Gore Select membrane (registered trademark) or pores manufactured by Gore. Examples thereof include a filling electrolyte membrane. Furthermore, when used in a high temperature environment (for example, 100 ° C. or higher), sulfonated polyimide, 2-acrylamido-2-methylpropanesulfonic acid (AMPS), sulfonated polybenzimidazole, phosphonated polybenzimidazole, sulfuric acid Examples include cesium hydrogen and ammonium polyphosphate.
カチオン交換膜としては、カチオンを移動させることができる固体高分子電解質であればよい。具体的には、パーフルオロカーボンスルフォン酸膜や、パーフルオロカーボンカルボン酸膜等のフッ素系イオン交換膜、リン酸を含浸させたポリベンズイミダゾール膜、ポリスチレンスルホン酸膜、スルホン酸化スチレン・ビニルベンゼン共重合体膜等が挙げられる。
支持電解質溶液のアニオン輸率が高い場合には、アニオン交換膜の使用が好ましい。アニオン交換膜としては、アニオンの移動可能な固体高分子電解質を使用できる。具体的には、ポリオルトフェニレンジアミン膜、アンモニウム塩誘導体基を有するフッ素系イオン交換膜、アンモニウム塩誘導体基を有するビニルベンゼンポリマー膜、クロロメチルスチレン・ビニルベンゼン共重合体をアミノ化した膜等が挙げられる。 The cation exchange membrane may be any solid polymer electrolyte that can move cations. Specifically, fluorine ion exchange membranes such as perfluorocarbon sulfonic acid membranes and perfluorocarbon carboxylic acid membranes, polybenzimidazole membranes impregnated with phosphoric acid, polystyrene sulfonic acid membranes, sulfonated styrene / vinylbenzene copolymers Examples include membranes.
When the anion transport number of the supporting electrolyte solution is high, it is preferable to use an anion exchange membrane. As the anion exchange membrane, a solid polymer electrolyte capable of transferring anions can be used. Specifically, a polyorthophenylenediamine film, a fluorine-based ion exchange film having an ammonium salt derivative group, a vinylbenzene polymer film having an ammonium salt derivative group, a film obtained by aminating a chloromethylstyrene / vinylbenzene copolymer, etc. Can be mentioned.
支持電解質溶液のアニオン輸率が高い場合には、アニオン交換膜の使用が好ましい。アニオン交換膜としては、アニオンの移動可能な固体高分子電解質を使用できる。具体的には、ポリオルトフェニレンジアミン膜、アンモニウム塩誘導体基を有するフッ素系イオン交換膜、アンモニウム塩誘導体基を有するビニルベンゼンポリマー膜、クロロメチルスチレン・ビニルベンゼン共重合体をアミノ化した膜等が挙げられる。 The cation exchange membrane may be any solid polymer electrolyte that can move cations. Specifically, fluorine ion exchange membranes such as perfluorocarbon sulfonic acid membranes and perfluorocarbon carboxylic acid membranes, polybenzimidazole membranes impregnated with phosphoric acid, polystyrene sulfonic acid membranes, sulfonated styrene / vinylbenzene copolymers Examples include membranes.
When the anion transport number of the supporting electrolyte solution is high, it is preferable to use an anion exchange membrane. As the anion exchange membrane, a solid polymer electrolyte capable of transferring anions can be used. Specifically, a polyorthophenylenediamine film, a fluorine-based ion exchange film having an ammonium salt derivative group, a vinylbenzene polymer film having an ammonium salt derivative group, a film obtained by aminating a chloromethylstyrene / vinylbenzene copolymer, etc. Can be mentioned.
13.シール材
シール材16は、透光性基板1と天板14を接着し、気体製造装置23内を流れる電解液および気体製造装置23内で生成した第1気体および第2気体を密閉するための材料である。天板14に箱状のものを用いる場合、この箱体と透光性基板1とを接着するためにシール材16が用いられる。シール材16は、例えば、紫外線硬化性接着剤、熱硬化性接着剤等が好適に使用されるが、その種類は限定されるものではない。紫外線硬化性の接着剤としては、200~400nmの波長を持つ光を照射することにより重合が起こり光照射後数秒で硬化反応が起こる樹脂であり、ラジカル重合型とカチオン重合型に分けられ、ラジカル重合型樹脂としてはアクリルレート、不飽和ポリエステル、カチオン重合型としては、エポキシ、オキセタン、ビニルエーテル等が挙げられる。また熱硬化性の高分子接着剤としては、フェノール樹脂、エポキシ樹脂、メラミン樹脂、尿素樹脂、熱硬化性ポリイミド等の有機樹脂が挙げられる。熱硬化性の高分子接着剤は、熱圧着時に圧力を掛けた状態で加熱重合し、その後、加圧したまま、室温まで冷却することにより、各部材を良好に接合させるため、締め付け部材等を要しない。また、有機樹脂に加えて、ガラス基板に対して密着性の高いハイブリッド材料を用いることが可能である。ハイブリッド材料を用いることによって、弾性率や硬度等の力学的特性が向上し、耐熱性や耐薬品性が飛躍的に向上する。ハイブリッド材料は、無機コロイド粒子と有機バインダ樹脂とから構成される。例えば、シリカなどの無機コロイド粒子と、エポキシ樹脂、ポリウレタンアクリレート樹脂やポリエステルアクリレート樹脂などの有機バインダ樹脂とから構成されるものが挙げられる。 13. Sealing material The sealingmaterial 16 adheres the translucent substrate 1 and the top plate 14, and seals the electrolyte flowing in the gas production device 23 and the first gas and the second gas generated in the gas production device 23. Material. When using a box-shaped thing for the top plate 14, the sealing material 16 is used in order to adhere | attach this box and the translucent board | substrate 1. FIG. As the sealing material 16, for example, an ultraviolet curable adhesive, a thermosetting adhesive, or the like is preferably used, but the type thereof is not limited. UV curable adhesives are resins that undergo polymerization when irradiated with light having a wavelength of 200 to 400 nm and undergo a curing reaction within a few seconds after light irradiation, and are classified into radical polymerization type and cationic polymerization type. Examples of the polymerization type resin include acrylates, unsaturated polyesters, and examples of the cationic polymerization type include epoxy, oxetane, and vinyl ether. Examples of the thermosetting polymer adhesive include organic resins such as phenol resin, epoxy resin, melamine resin, urea resin, and thermosetting polyimide. The thermosetting polymer adhesive is heated and polymerized in a state where pressure is applied at the time of thermocompression bonding, and then cooled to room temperature while being pressurized. I don't need it. In addition to the organic resin, a hybrid material having high adhesion to the glass substrate can be used. By using a hybrid material, mechanical properties such as elastic modulus and hardness are improved, and heat resistance and chemical resistance are dramatically improved. The hybrid material is composed of inorganic colloidal particles and an organic binder resin. For example, what is comprised from inorganic colloidal particles, such as a silica, and organic binder resin, such as an epoxy resin, a polyurethane acrylate resin, and a polyester acrylate resin, is mentioned.
シール材16は、透光性基板1と天板14を接着し、気体製造装置23内を流れる電解液および気体製造装置23内で生成した第1気体および第2気体を密閉するための材料である。天板14に箱状のものを用いる場合、この箱体と透光性基板1とを接着するためにシール材16が用いられる。シール材16は、例えば、紫外線硬化性接着剤、熱硬化性接着剤等が好適に使用されるが、その種類は限定されるものではない。紫外線硬化性の接着剤としては、200~400nmの波長を持つ光を照射することにより重合が起こり光照射後数秒で硬化反応が起こる樹脂であり、ラジカル重合型とカチオン重合型に分けられ、ラジカル重合型樹脂としてはアクリルレート、不飽和ポリエステル、カチオン重合型としては、エポキシ、オキセタン、ビニルエーテル等が挙げられる。また熱硬化性の高分子接着剤としては、フェノール樹脂、エポキシ樹脂、メラミン樹脂、尿素樹脂、熱硬化性ポリイミド等の有機樹脂が挙げられる。熱硬化性の高分子接着剤は、熱圧着時に圧力を掛けた状態で加熱重合し、その後、加圧したまま、室温まで冷却することにより、各部材を良好に接合させるため、締め付け部材等を要しない。また、有機樹脂に加えて、ガラス基板に対して密着性の高いハイブリッド材料を用いることが可能である。ハイブリッド材料を用いることによって、弾性率や硬度等の力学的特性が向上し、耐熱性や耐薬品性が飛躍的に向上する。ハイブリッド材料は、無機コロイド粒子と有機バインダ樹脂とから構成される。例えば、シリカなどの無機コロイド粒子と、エポキシ樹脂、ポリウレタンアクリレート樹脂やポリエステルアクリレート樹脂などの有機バインダ樹脂とから構成されるものが挙げられる。 13. Sealing material The sealing
ここではシール材16と記しているが、透光性基板1と天板14を接着させる機能を有するものであれば限定されず、樹脂製あるいは金属製のガスケットを用い外部からネジ等の部材を用いて物理的に圧力を加え機密性を高める方法等を適宜用いることも可能である。
Here, the sealing material 16 is described. However, the sealing material 16 is not limited as long as it has a function of bonding the translucent substrate 1 and the top plate 14, and a member such as a screw is externally used using a resin or metal gasket. It is also possible to appropriately use a method of applying pressure physically to increase confidentiality.
14.電解液室
電解液室15は、第1電解用電極8と天板14との間の空間および第2電解用電極7と天板14との間の空間とすることができる。また、電解液室15は、隔壁13により仕切ることができる。
生成した第1気体及び第2気体の気泡が効率よく第1電解用電極8または第2電解用電極7から離れるように、電解液室15の内部で電解液を循環させるような例えばポンプやファン、熱による対流発生装置などの簡易装置を備え付けることも可能である。 14 Electrolytic Solution Chamber Theelectrolytic solution chamber 15 can be a space between the first electrolysis electrode 8 and the top plate 14 and a space between the second electrolysis electrode 7 and the top plate 14. Further, the electrolyte chamber 15 can be partitioned by the partition wall 13.
For example, a pump or a fan that circulates the electrolyte in theelectrolyte chamber 15 so that the generated bubbles of the first gas and the second gas are efficiently separated from the first electrolysis electrode 8 or the second electrolysis electrode 7. It is also possible to provide a simple device such as a heat convection generator.
電解液室15は、第1電解用電極8と天板14との間の空間および第2電解用電極7と天板14との間の空間とすることができる。また、電解液室15は、隔壁13により仕切ることができる。
生成した第1気体及び第2気体の気泡が効率よく第1電解用電極8または第2電解用電極7から離れるように、電解液室15の内部で電解液を循環させるような例えばポンプやファン、熱による対流発生装置などの簡易装置を備え付けることも可能である。 14 Electrolytic Solution Chamber The
For example, a pump or a fan that circulates the electrolyte in the
15.給水口、第1気体排出口、第2気体排出口、第1気体排出路および第2気体排出路
給水口18は、気体製造装置23に含まれるシール材16の一部、もしくは天板14の一部などに開口を作ることにより設けることができる。給水口18は、第1気体及び第2気体へと分解された電解液を補充するために配置され、その配置箇所および形状は、原料となる電解液が効率よく気体製造装置へ供給されさえすれば、特に限定されるものではない。 15. Water supply port, first gas discharge port, second gas discharge port, first gas discharge channel and second gas discharge channel Thewater supply port 18 is a part of the sealing material 16 included in the gas production device 23 or the top plate 14. It can be provided by making an opening in a part or the like. The water supply port 18 is arranged to replenish the electrolytic solution decomposed into the first gas and the second gas, and the arrangement location and shape of the water supply port 18 can be efficiently supplied to the gas production apparatus. For example, there is no particular limitation.
給水口18は、気体製造装置23に含まれるシール材16の一部、もしくは天板14の一部などに開口を作ることにより設けることができる。給水口18は、第1気体及び第2気体へと分解された電解液を補充するために配置され、その配置箇所および形状は、原料となる電解液が効率よく気体製造装置へ供給されさえすれば、特に限定されるものではない。 15. Water supply port, first gas discharge port, second gas discharge port, first gas discharge channel and second gas discharge channel The
また、第1気体排出口20は、光電変換部2の受光面を上向きの状態で水平にしたとき、第1電解用電極8の電解液に接触可能な面の上端に近接して設けることができる。また、第2気体排出口19は、光電変換部2の受光面を上向きの状態で水平にしたとき、第2電解用電極7の電解液に接触可能な面の上端に近接して設けることができる。このことにより、気体製造装置23を光電変換部2の受光面が上向きの状態で水平になるように設置した場合に、第1電解用電極8で発生させた第1気体を気泡として電解液中を上昇させ第1気体排出口20から回収することができ、第2電解用電極7で発生させた第2気体を気泡として電解液中を上昇させ第2気体排出口19から回収することができる。
The first gas outlet 20 is provided close to the upper end of the surface of the first electrolysis electrode 8 that can come into contact with the electrolytic solution when the light receiving surface of the photoelectric conversion unit 2 is leveled upward. it can. Further, the second gas discharge port 19 is provided close to the upper end of the surface of the second electrolysis electrode 7 that can come into contact with the electrolytic solution when the light receiving surface of the photoelectric conversion unit 2 is leveled upward. it can. As a result, when the gas production device 23 is installed so that the light receiving surface of the photoelectric conversion unit 2 is horizontal, the first gas generated by the first electrolysis electrode 8 is used as bubbles in the electrolytic solution. Can be recovered from the first gas discharge port 20, and the second gas generated in the second electrolysis electrode 7 can be raised as bubbles to rise in the electrolytic solution and recovered from the second gas discharge port 19. .
また、第1気体排出口20は、第1気体排出路25と導通することができ、第2気体排出口19は第2気体排出路26と導通することができる。また、第1気体排出路25は、複数の第1気体排出口20と導通することができ、第2気体排出路26は、複数の第2気体排出口19と導通することができる。このことにより、気体製造装置23で発生させた第1気体および第2気体を回収することができる。
Further, the first gas discharge port 20 can be connected to the first gas discharge passage 25, and the second gas discharge port 19 can be connected to the second gas discharge passage 26. In addition, the first gas exhaust path 25 can be electrically connected to the plurality of first gas exhaust ports 20, and the second gas exhaust path 26 can be electrically connected to the plurality of second gas exhaust ports 19. As a result, the first gas and the second gas generated by the gas production device 23 can be recovered.
16.電解液
電解液は、第1気体および第2気体の原料となるものであれば特に限定されないが、例えば、電解質を含む水溶液であり、例えば、0.1MのH2SO4を含む電解液、0.1Mリン酸カリウム緩衝液などである。この場合、電解液から第1気体および第2気体として水素および酸素を製造することができる。 16. Electrolytic Solution The electrolytic solution is not particularly limited as long as it is a raw material for the first gas and the second gas. For example, the electrolytic solution is an aqueous solution containing an electrolyte, for example, an electrolytic solution containing 0.1 M H 2 SO 4 , 0.1M potassium phosphate buffer. In this case, hydrogen and oxygen can be produced from the electrolytic solution as the first gas and the second gas.
電解液は、第1気体および第2気体の原料となるものであれば特に限定されないが、例えば、電解質を含む水溶液であり、例えば、0.1MのH2SO4を含む電解液、0.1Mリン酸カリウム緩衝液などである。この場合、電解液から第1気体および第2気体として水素および酸素を製造することができる。 16. Electrolytic Solution The electrolytic solution is not particularly limited as long as it is a raw material for the first gas and the second gas. For example, the electrolytic solution is an aqueous solution containing an electrolyte, for example, an electrolytic solution containing 0.1 M H 2 SO 4 , 0.1M potassium phosphate buffer. In this case, hydrogen and oxygen can be produced from the electrolytic solution as the first gas and the second gas.
気体製造方法
本実施形態の気体製造方法は、気体製造装置23を前記受光面が実質的に水平となるように設置し、前記気体製造装置は、第1気体を排出する第1気体排出口と、第2気体を排出する第2気体排出口と、電解液室とを備え、前記電解液室に電解液を導入し、太陽光を前記光電変換部の受光面に入射させることにより第1電解用電極および第2電解用電極からそれぞれ第1気体および第2気体を発生させ、第1気体排出口および第2気体排出口からそれぞれ第1気体および第2気体を排出する。
このことにより第1気体および第2気体を製造することができる。 Gas manufacturing method The gas manufacturing method of this embodiment installs thegas manufacturing apparatus 23 so that the said light-receiving surface becomes substantially horizontal, and the said gas manufacturing apparatus discharges | emits 1st gas, 1st gas discharge port, The second electrolysis chamber is provided with a second gas exhaust port for discharging the second gas, and an electrolytic solution chamber. The electrolytic solution is introduced into the electrolytic solution chamber, and sunlight is incident on the light receiving surface of the photoelectric conversion unit. A first gas and a second gas are generated from the first electrode and the second electrolysis electrode, respectively, and the first gas and the second gas are discharged from the first gas outlet and the second gas outlet, respectively.
Thereby, the first gas and the second gas can be produced.
本実施形態の気体製造方法は、気体製造装置23を前記受光面が実質的に水平となるように設置し、前記気体製造装置は、第1気体を排出する第1気体排出口と、第2気体を排出する第2気体排出口と、電解液室とを備え、前記電解液室に電解液を導入し、太陽光を前記光電変換部の受光面に入射させることにより第1電解用電極および第2電解用電極からそれぞれ第1気体および第2気体を発生させ、第1気体排出口および第2気体排出口からそれぞれ第1気体および第2気体を排出する。
このことにより第1気体および第2気体を製造することができる。 Gas manufacturing method The gas manufacturing method of this embodiment installs the
Thereby, the first gas and the second gas can be produced.
気体製造装置アレイ
本実施形態の気体製造装置アレイは、複数の本実施形態の気体製造装置23と、第1気体排出路25と、第2気体排出路26とを備え、各気体製造装置23は、第1気体を排出する第1気体排出口20と第2気体を排出する第2気体排出口19とを備え、第1気体排出路25は、各気体製造装置23の第1気体排出口20と導通し、第2気体排出路26は、各気体製造装置23の第2気体排出口19と導通する。たとえば、図19のように気体製造装置を配置することにより気体製造装置アレイを形成することができ、この場合、第1気体排出路25と第2気体排出路26とを各気体製造装置23の両側に設けることができるため、配管を簡素化することができる。 Gas Manufacturing Device Array The gas manufacturing device array of the present embodiment includes a plurality ofgas manufacturing devices 23 of the present embodiment, a first gas discharge path 25, and a second gas discharge path 26. The first gas discharge port 20 for discharging the first gas and the second gas discharge port 19 for discharging the second gas are provided, and the first gas discharge path 25 is the first gas discharge port 20 of each gas production device 23. The second gas discharge path 26 is connected to the second gas discharge port 19 of each gas production device 23. For example, as shown in FIG. 19, the gas production apparatus array can be formed by arranging the gas production apparatuses. In this case, the first gas discharge path 25 and the second gas discharge path 26 are connected to each gas production apparatus 23. Since it can provide in both sides, piping can be simplified.
本実施形態の気体製造装置アレイは、複数の本実施形態の気体製造装置23と、第1気体排出路25と、第2気体排出路26とを備え、各気体製造装置23は、第1気体を排出する第1気体排出口20と第2気体を排出する第2気体排出口19とを備え、第1気体排出路25は、各気体製造装置23の第1気体排出口20と導通し、第2気体排出路26は、各気体製造装置23の第2気体排出口19と導通する。たとえば、図19のように気体製造装置を配置することにより気体製造装置アレイを形成することができ、この場合、第1気体排出路25と第2気体排出路26とを各気体製造装置23の両側に設けることができるため、配管を簡素化することができる。 Gas Manufacturing Device Array The gas manufacturing device array of the present embodiment includes a plurality of
1: 基板 2:光電変換部 4:第1電極 5:第2電極 7:第2電解用電極 8:第1電解用電極 9:第1導電部 10:第2導電部 11:第1型部 12:第2型部 13:隔壁 14:天板 15:電解液室 16:シール材 17:第3導電部 18:給水口 19:第2気体排出口 20:第1気体排出口 21:絶縁部 23:気体製造装置 25:第1気体排出路 26:第2気体排出路 28:光電変換層 33:第3導電部 35:半導体領域 36:p型半導体領域 37:n型半導体領域 40:アイソレーション 42:第4導電部 43:第5導電部 45:気体製造装置アレイ
1: Copper substrate 2: Photoelectric conversion part 4: First electrode 5: Second electrode 7: Second electrolysis electrode 8: First electrolysis electrode 9: First electroconductive part 10: Second electroconductive part 11: First type part 12: Second mold part 13: Bulkhead 14: Top plate 15: Electrolyte chamber 16: Sealing material 17: Third conductive part 18: Water supply port 19: Second gas outlet 20: First gas outlet 21: Insulating part 23: Gas production apparatus 25: First gas discharge path 26: Second gas discharge path 28: Photoelectric conversion layer 33: Third conductive part 35: Semiconductor region 36: p-type semiconductor region 37: n-type semiconductor region 40: Isolation 42: 4th conductive part 43: 5th conductive part 45: Gas production device array
Claims (36)
- 受光面およびその裏面を有する光電変換部と、前記裏面の上に並べて設けられ、かつ、電解液に接触可能な面をそれぞれ有する第1および第2電解用電極とを備え、
第1および第2電解用電極が電解液と接触するとき、第1および第2電解用電極は、前記光電変換部が受光することより生じる起電力を利用して電解液を電気分解しそれぞれ第1気体および第2気体が発生するように設けられ、
前記光電変換部の受光面を水平にしたとき、第1電解用電極と第2電解用電極とは、電解液に接触可能な面と水平な基準面との間の傾斜角が異なることを特徴とする気体製造装置。 A photoelectric conversion unit having a light receiving surface and a back surface thereof, and first and second electrodes for electrolysis that are provided side by side on the back surface and each have a surface that can come into contact with an electrolytic solution,
When the first and second electrolysis electrodes are in contact with the electrolytic solution, the first and second electrolysis electrodes use the electromotive force generated by the photoelectric conversion unit to receive light to electrolyze the electrolytic solution, respectively. 1 gas and a second gas are provided to be generated,
When the light receiving surface of the photoelectric conversion unit is horizontal, the first electrolysis electrode and the second electrolysis electrode have different inclination angles between a surface that can contact the electrolytic solution and a horizontal reference surface. Gas production equipment. - 前記電解液を収容する電解液室をさらに備え、
前記光電変換部の受光面を水平にしたとき、前記電解液室は、第1電解用電極の電解液に接触可能な面の上端に近接して設けられた第1気体排出口、または第2電解用電極の電解液に接触可能な面の上端に近接して設けられた第2気体排出口を備え、
第1電解用電極は、第1気体を第1気体排出口から回収できるように傾斜した電解液に接触可能な面を有し、
第2電解用電極は、第2気体を第2気体排出口から回収できるように傾斜した電解液に接触可能な面を有する請求項1に記載の装置。 An electrolyte chamber containing the electrolyte solution;
When the light receiving surface of the photoelectric conversion unit is leveled, the electrolyte chamber is a first gas exhaust port provided close to the upper end of the surface that can contact the electrolyte of the first electrolysis electrode, or second A second gas outlet provided close to the upper end of the surface of the electrode for electrolysis that can contact the electrolyte;
The electrode for first electrolysis has a surface that can contact the inclined electrolyte so that the first gas can be recovered from the first gas outlet,
2. The apparatus according to claim 1, wherein the second electrolysis electrode has a surface that can contact the inclined electrolyte so that the second gas can be recovered from the second gas discharge port. - 前記光電変換部は、方形状の受光面を有し、
第1および第2気体排出口は、それぞれ前記光電変換部の受光面の対向する辺に近接して設けられた請求項2に記載の装置。 The photoelectric conversion unit has a rectangular light receiving surface,
The device according to claim 2, wherein the first and second gas discharge ports are provided in proximity to opposing sides of the light receiving surface of the photoelectric conversion unit. - 第1および第2電解用電極は、少なくとも一方が複数であり、かつ、それぞれ帯状の電解液に接触可能な面を有し、かつ、この面の長辺が隣接するように交互に設けられた請求項1~3のいずれか1つに記載の装置。 At least one of the first and second electrolysis electrodes has a plurality of surfaces, each of which has a surface that can come into contact with the strip-shaped electrolyte solution, and the long sides of the surfaces are alternately provided so as to be adjacent to each other. The device according to any one of claims 1 to 3.
- 第1電解用電極と前記光電変換部の裏面との間に設けられた第1型部および第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部をさらに備え、
第1型部および第2型部は、前記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とが異なるように成形された請求項1~4のいずれか1つに記載の装置。 A first mold part provided between the first electrolysis electrode and the back surface of the photoelectric conversion part; and a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part. ,
When the light receiving surface of the photoelectric conversion unit is leveled, the first mold part and the second mold part have an inclination angle between a surface that can contact the electrolytic solution of the first electrolysis electrode and a horizontal reference surface, The apparatus according to any one of claims 1 to 4, wherein the second electrolysis electrode is formed so that an inclination angle between a surface that can come into contact with the electrolytic solution and the reference surface is different. - 第1型部および第2型部は、固体樹脂からなる請求項5に記載の装置。 The apparatus according to claim 5, wherein the first mold part and the second mold part are made of a solid resin.
- 第1型部と第1電解用電極との間または第2型部と第2電解用電極との間に第1導電部をさらに備える請求項5または6に記載の装置。 The apparatus according to claim 5 or 6, further comprising a first conductive part between the first mold part and the first electrolysis electrode or between the second mold part and the second electrolysis electrode.
- 第1および第2電解用電極は、それぞれ電解液に接触可能な面に傾斜方向に伸びる溝状のくぼみを有する請求項1~7のいずれか1つに記載の装置。 The device according to any one of claims 1 to 7, wherein each of the first and second electrolysis electrodes has a groove-like recess extending in an inclined direction on a surface that can contact the electrolytic solution.
- 光電変換部の受光面を水平にしたとき、
第1電解用電極は、水平な基準面との間の傾斜角が1度以上60度以下の電解液に接触可能な面を有し、第2電解用電極は、前記基準面との間の傾斜角が120度以上179度以下の電解液に接触可能な面を有する請求項1~8のいずれか1つに記載の装置。 When the light receiving surface of the photoelectric converter is leveled,
The first electrolysis electrode has a surface that can contact an electrolytic solution having an inclination angle of 1 degree to 60 degrees with respect to a horizontal reference surface, and the second electrolysis electrode is between the reference surface and the reference surface. The apparatus according to any one of claims 1 to 8, wherein the apparatus has a surface that can contact an electrolytic solution having an inclination angle of not less than 120 degrees and not more than 179 degrees. - 前記光電変換部は、受光することによりその受光面と裏面との間に起電力が生じ、
第1電解用電極は、前記光電変換部の裏面と電気的に接続し、
第2電解用電極は、前記光電変換部の受光面と電気的に接続する請求項1~9のいずれか1つに記載の装置。 The photoelectric conversion unit generates an electromotive force between the light receiving surface and the back surface by receiving light,
The first electrolysis electrode is electrically connected to the back surface of the photoelectric conversion unit,
The apparatus according to any one of claims 1 to 9, wherein the second electrolysis electrode is electrically connected to a light receiving surface of the photoelectric conversion unit. - 前記光電変換部の受光面に接触する第1電極をさらに備える請求項1~10のいずれか1つに記載の装置。 The apparatus according to any one of claims 1 to 10, further comprising a first electrode in contact with a light receiving surface of the photoelectric conversion unit.
- 第1電極と第2電解用電極とを電気的に接続する第2導電部をさらに備える請求項11に記載の装置。 The apparatus according to claim 11, further comprising a second conductive portion that electrically connects the first electrode and the second electrolysis electrode.
- 第2導電部は、前記光電変換部を貫通するコンタクトホールに設けられた請求項12に記載の装置。 The device according to claim 12, wherein the second conductive portion is provided in a contact hole penetrating the photoelectric conversion portion.
- 第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部を備え、
第2型部は、絶縁性を有し、かつ、前記光電変換部の側面を覆うように設けられ、
第2導電部は、第2型部の前記光電変換部の側面を覆う部分の上に設けられた請求項12に記載の装置。 A second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part;
The second mold part has insulating properties and is provided so as to cover the side surface of the photoelectric conversion part,
The device according to claim 12, wherein the second conductive portion is provided on a portion of the second mold portion that covers a side surface of the photoelectric conversion portion. - 第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部を備え、
第2型部は、絶縁性を有し、かつ、前記光電変換部の側面を覆うように設けられ、
第2電解用電極は、第2型部の前記光電変換部の側面を覆う部分の上に設けられ、かつ、第1電極と接触する請求項11に記載の装置。 A second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part;
The second mold part has insulating properties and is provided so as to cover the side surface of the photoelectric conversion part,
The device according to claim 11, wherein the second electrolysis electrode is provided on a portion of the second mold part that covers a side surface of the photoelectric conversion unit and is in contact with the first electrode. - 第1電解用電極と前記光電変換部の裏面との間に設けられた第1型部と、第2電解用電極と前記光電変換部の裏面との間に設けられた第2型部とを備え、
前記光電変換部の裏面と第1型部との間および前記光電変換部の裏面と第2型部との間に設けられた第2電極をさらに備える請求項1~15のいずれか1つに記載の装置。 A first mold part provided between the first electrolysis electrode and the back surface of the photoelectric conversion part; and a second mold part provided between the second electrolysis electrode and the back surface of the photoelectric conversion part. Prepared,
16. The method according to claim 1, further comprising a second electrode provided between the back surface of the photoelectric conversion unit and the first mold unit and between the back surface of the photoelectric conversion unit and the second mold unit. The device described. - 第2電極と第1電解用電極とを電気的に接続する第3導電部をさらに備える請求項16に記載の装置。 The apparatus according to claim 16, further comprising a third conductive portion that electrically connects the second electrode and the first electrolysis electrode.
- 第3導電部は、第1型部の側部を覆うように設けられ、かつ、第2電極と接触する請求項17に記載の装置。 The device according to claim 17, wherein the third conductive portion is provided so as to cover a side portion of the first mold portion and is in contact with the second electrode.
- 前記光電変換部は、受光することにより前記裏面の第1および第2区域間に電位差が生じ、
第1区域は、第1電解用電極と電気的に接続し、
第2区域は、第2電解用電極と電気的に接続する請求項1~9のいずれか1つに記載の装置。 The photoelectric conversion unit generates a potential difference between the first and second areas of the back surface by receiving light,
The first area is electrically connected to the first electrolysis electrode;
The apparatus according to any one of claims 1 to 9, wherein the second area is electrically connected to the second electrolysis electrode. - 前記光電変換部は、n型半導体部およびp型半導体部を有する少なくとも1つの半導体材料からなり、
第1および第2区域のうち、一方は前記n型半導体部の一部であり、他方は前記p型半導体部の一部である請求項19に記載の装置。 The photoelectric conversion part is made of at least one semiconductor material having an n-type semiconductor part and a p-type semiconductor part,
20. The device of claim 19, wherein one of the first and second areas is part of the n-type semiconductor part and the other is part of the p-type semiconductor part. - 透光性基板をさらに備え、
前記光電変換部は、前記透光性基板の上に設けられた請求項1~20のいずれか1つに記載の装置。 A translucent substrate;
The apparatus according to any one of claims 1 to 20, wherein the photoelectric conversion unit is provided on the translucent substrate. - 透光性基板をさらに備え、
前記光電変換部は、前記透光性基板の上に設けられ、
前記透光性基板は、記光電変換部の受光面を水平にしたとき、第1電解用電極の電解液に接触可能な面と水平な基準面との間の傾斜角と、第2電解用電極の電解液に接触可能な面と前記基準面との間の傾斜角とが異なるように成形された請求項1~6のいずれか1つに記載の装置。 A translucent substrate;
The photoelectric conversion unit is provided on the translucent substrate,
When the light-receiving surface of the photoelectric conversion unit is leveled, the translucent substrate has an inclination angle between a surface that can contact the electrolytic solution of the first electrolysis electrode and a horizontal reference surface, and a second electrolysis substrate. The apparatus according to any one of claims 1 to 6, wherein the device is shaped so that an inclination angle between a surface of the electrode that can contact the electrolyte and the reference surface is different. - 第2電解用電極は、絶縁部を介して前記光電変換部の裏面上に設けられた請求項22に記載の装置。 23. The apparatus according to claim 22, wherein the second electrolysis electrode is provided on the back surface of the photoelectric conversion unit via an insulating unit.
- 前記光電変換部は、p型半導体層、i型半導体層およびn型半導体層からなる光電変換層を有する請求項1~23のいずれか1つに記載の装置。 The apparatus according to any one of claims 1 to 23, wherein the photoelectric conversion unit includes a photoelectric conversion layer including a p-type semiconductor layer, an i-type semiconductor layer, and an n-type semiconductor layer.
- 前記光電変換部は、直列接続した複数の光電変換層を含み、
前記複数の光電変換層は、受光することにより生じる起電力を第1電解用電極および第2電解用電極に供給する請求項1~24のいずれか1つに記載の装置。 The photoelectric conversion unit includes a plurality of photoelectric conversion layers connected in series,
The apparatus according to any one of claims 1 to 24, wherein the plurality of photoelectric conversion layers supply an electromotive force generated by receiving light to the first electrolysis electrode and the second electrolysis electrode. - 各光電変換層は、第4導電部により直列接続された請求項25に記載の装置。 26. The apparatus according to claim 25, wherein each photoelectric conversion layer is connected in series by a fourth conductive portion.
- 第1電解用電極および第2電解用電極のうち、一方は電解液からH2を発生させる水素発生部であり、他方は電解液からO2を発生させる酸素発生部であり、
前記水素発生部および前記酸素発生部は、それぞれ電解液からH2が発生する反応の触媒および電解液からO2が発生する反応の触媒を含む請求項1~26のいずれか1つに記載の装置。 Of the first electrolysis electrode and the second electrolysis electrode, one is a hydrogen generation unit that generates H 2 from the electrolytic solution, and the other is an oxygen generation unit that generates O 2 from the electrolytic solution,
The hydrogen generation unit and the oxygen generation unit each include a catalyst for a reaction for generating H 2 from the electrolytic solution and a catalyst for a reaction for generating O 2 from the electrolytic solution, respectively. apparatus. - 前記水素発生部および前記酸素発生部のうち少なくとも一方は、前記受光面の面積より大きい触媒表面積を有する請求項27に記載の装置。 28. The apparatus according to claim 27, wherein at least one of the hydrogen generation part and the oxygen generation part has a catalyst surface area larger than an area of the light receiving surface.
- 前記水素発生部および前記酸素発生部のうち少なくとも一方は、触媒が担持された多孔質の導電体である請求項27または28に記載の装置。 29. The apparatus according to claim 27 or 28, wherein at least one of the hydrogen generator and the oxygen generator is a porous conductor carrying a catalyst.
- 前記水素発生部は、水素発生触媒としてPt、Ir、Ru、Pd、Rh、Au、Fe、NiおよびSeのうち少なくとも1つを含む請求項27~29のいずれか1つに記載の装置。 The apparatus according to any one of claims 27 to 29, wherein the hydrogen generation unit includes at least one of Pt, Ir, Ru, Pd, Rh, Au, Fe, Ni, and Se as a hydrogen generation catalyst.
- 前記酸素発生部は、酸素発生触媒としてMn、Ca、Zn、CoおよびIrのうち少なくとも1つを含む請求項27~30のいずれか1つに記載の装置。 The apparatus according to any one of claims 27 to 30, wherein the oxygen generation unit includes at least one of Mn, Ca, Zn, Co, and Ir as an oxygen generation catalyst.
- 透光性基板と電解液室とをさらに備え、
前記光電変換部は、前記透光性基板の上に設けられ、
第1電解用電極および第2電解用電極の上に天板をさらに備え、
前記電解液室は、第1電解用電極および第2電解用電極と前記天板との間に設けられた請求項1~31のいずれか1つに記載の装置。 A translucent substrate and an electrolyte chamber;
The photoelectric conversion unit is provided on the translucent substrate,
A top plate is further provided on the first electrolysis electrode and the second electrolysis electrode,
The apparatus according to any one of claims 1 to 31, wherein the electrolyte chamber is provided between the first electrolysis electrode and the second electrolysis electrode and the top plate. - 第1電解用電極と前記天板との間の電解液室および第2電解用電極と天板との間の電解液室とを仕切る隔壁をさらに備える請求項32に記載の装置。 The apparatus according to claim 32, further comprising a partition wall that partitions an electrolyte chamber between the first electrolysis electrode and the top plate and an electrolyte chamber between the second electrolysis electrode and the top plate.
- 前記隔壁は、イオン交換体を含む請求項33に記載の装置。 The apparatus according to claim 33, wherein the partition wall includes an ion exchanger.
- 請求項1~34のいずれか1つに記載の気体製造装置を前記光電変換部の受光面が実質的に水平となるように設置し、
前記気体製造装置は、第1気体を排出する第1気体排出口と、第2気体を排出する第2気体排出口と、電解液室とを備え、
前記電解液室に電解液を導入し、太陽光を前記光電変換部の受光面に入射させることにより第1電解用電極および第2電解用電極からそれぞれ第1気体および第2気体を発生させ、第1気体排出口および第2気体排出口からそれぞれ第1気体および第2気体を排出する気体製造方法。 The gas production apparatus according to any one of claims 1 to 34 is installed so that a light receiving surface of the photoelectric conversion unit is substantially horizontal,
The gas manufacturing apparatus includes a first gas outlet for discharging the first gas, a second gas outlet for discharging the second gas, and an electrolyte chamber.
An electrolyte is introduced into the electrolyte chamber, and sunlight is incident on the light receiving surface of the photoelectric conversion unit to generate a first gas and a second gas from the first electrolysis electrode and the second electrolysis electrode, respectively. A gas manufacturing method for discharging the first gas and the second gas from the first gas outlet and the second gas outlet, respectively. - 複数の請求項1~34のいずれか1つに記載の気体製造装置と、第1気体排出路と、第2気体排出路とを備え、
各気体製造装置は、第1気体を排出する第1気体排出口と第2気体を排出する第2気体排出口とを備え、
第1気体排出路は、各気体製造装置の第1気体排出口と導通し、
第2気体排出路は、各気体製造装置の第2気体排出口と導通する気体製造装置アレイ。 A plurality of the gas production apparatuses according to any one of claims 1 to 34, a first gas discharge path, and a second gas discharge path,
Each gas manufacturing apparatus includes a first gas outlet for discharging the first gas and a second gas outlet for discharging the second gas,
The first gas discharge path is electrically connected to the first gas discharge port of each gas production device,
The second gas discharge path is a gas production device array that is electrically connected to the second gas discharge port of each gas production device.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010255976A JP5676218B2 (en) | 2010-11-16 | 2010-11-16 | Gas production apparatus, gas production method, and gas production apparatus array |
| JP2010-255976 | 2010-11-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012066922A1 true WO2012066922A1 (en) | 2012-05-24 |
Family
ID=46083861
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/074971 WO2012066922A1 (en) | 2010-11-16 | 2011-10-28 | Gas manufacturing apparatus, gas manufacturing method, and gas manufacturing apparatus array |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5676218B2 (en) |
| WO (1) | WO2012066922A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013253269A (en) * | 2012-06-05 | 2013-12-19 | Sharp Corp | Carbon dioxide reduction device |
| JP2013253270A (en) * | 2012-06-05 | 2013-12-19 | Sharp Corp | Carbon dioxide reduction device |
| CN110093626A (en) * | 2019-05-22 | 2019-08-06 | 兰州大学 | A kind of Ni3Se4The preparation method and application of/NiO heterojunction composite |
| US10378116B2 (en) | 2014-03-24 | 2019-08-13 | Kabushiki Kaisha Toshiba | Photoelectrochemical reaction device |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6034151B2 (en) * | 2012-11-20 | 2016-11-30 | 株式会社東芝 | Photochemical reactor |
| JP5993768B2 (en) * | 2013-03-28 | 2016-09-14 | 富士フイルム株式会社 | Gas production equipment |
| JP6013251B2 (en) * | 2013-03-28 | 2016-10-25 | 富士フイルム株式会社 | Gas production equipment |
| KR101996479B1 (en) * | 2017-02-09 | 2019-07-03 | 정진호 | Electrolytic electrolysis device |
| WO2018147614A1 (en) * | 2017-02-09 | 2018-08-16 | 정진호 | Electrolytic electrolysis device |
| KR101992966B1 (en) * | 2017-11-30 | 2019-06-26 | 한국생산기술연구원 | Manufacturing method of high voltage photoelectricity for photoelectric chemical decantation using shingled array junction and high voltage photoelectricity thereof |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06316783A (en) * | 1993-04-30 | 1994-11-15 | Chlorine Eng Corp Ltd | Electrolyzer |
| JP2000192275A (en) * | 1998-12-25 | 2000-07-11 | Toshiba Corp | Apparatus for electrolysis of water |
| JP2004197167A (en) * | 2002-12-18 | 2004-07-15 | Honda Motor Co Ltd | Hydrogen producing apparatus |
| JP2006508253A (en) * | 2002-11-27 | 2006-03-09 | ザ・ユニバーシティ・オブ・トレド | Integrated photoelectrochemistry with liquid electrolyte and its system |
| JP2008507464A (en) * | 2004-05-18 | 2008-03-13 | ハイドロジェン ソーラー リミテッド | Photoelectrochemical system and method thereof |
| JP2009274891A (en) * | 2008-05-13 | 2009-11-26 | Sharp Corp | Semiconductor oxide film, production method thereof, and hydrogen generation apparatus using the semiconductor oxide film |
-
2010
- 2010-11-16 JP JP2010255976A patent/JP5676218B2/en active Active
-
2011
- 2011-10-28 WO PCT/JP2011/074971 patent/WO2012066922A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06316783A (en) * | 1993-04-30 | 1994-11-15 | Chlorine Eng Corp Ltd | Electrolyzer |
| JP2000192275A (en) * | 1998-12-25 | 2000-07-11 | Toshiba Corp | Apparatus for electrolysis of water |
| JP2006508253A (en) * | 2002-11-27 | 2006-03-09 | ザ・ユニバーシティ・オブ・トレド | Integrated photoelectrochemistry with liquid electrolyte and its system |
| JP2004197167A (en) * | 2002-12-18 | 2004-07-15 | Honda Motor Co Ltd | Hydrogen producing apparatus |
| JP2008507464A (en) * | 2004-05-18 | 2008-03-13 | ハイドロジェン ソーラー リミテッド | Photoelectrochemical system and method thereof |
| JP2009274891A (en) * | 2008-05-13 | 2009-11-26 | Sharp Corp | Semiconductor oxide film, production method thereof, and hydrogen generation apparatus using the semiconductor oxide film |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013253269A (en) * | 2012-06-05 | 2013-12-19 | Sharp Corp | Carbon dioxide reduction device |
| JP2013253270A (en) * | 2012-06-05 | 2013-12-19 | Sharp Corp | Carbon dioxide reduction device |
| US10378116B2 (en) | 2014-03-24 | 2019-08-13 | Kabushiki Kaisha Toshiba | Photoelectrochemical reaction device |
| CN110093626A (en) * | 2019-05-22 | 2019-08-06 | 兰州大学 | A kind of Ni3Se4The preparation method and application of/NiO heterojunction composite |
| CN110093626B (en) * | 2019-05-22 | 2021-01-19 | 兰州大学 | Ni3Se4Preparation method and application of/NiO heterojunction composite material |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5676218B2 (en) | 2015-02-25 |
| JP2012107280A (en) | 2012-06-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4594438B1 (en) | Hydrogen production apparatus and hydrogen production method | |
| JP5663254B2 (en) | Hydrogen production apparatus and hydrogen production method | |
| JP5802374B2 (en) | Solar cell integrated gas production system | |
| JP5792560B2 (en) | Power generation system | |
| JP5676218B2 (en) | Gas production apparatus, gas production method, and gas production apparatus array | |
| US9447508B2 (en) | Hydrogen production device and method for producing hydrogen | |
| JP5802403B2 (en) | Hydrogen production apparatus and hydrogen production method | |
| JP5860636B2 (en) | Anion exchange membrane fuel cell system | |
| JP5785736B2 (en) | Hydrogen production apparatus and hydrogen production method | |
| JP5427653B2 (en) | Gas production apparatus and gas production method | |
| JP5719576B2 (en) | Gas production apparatus and gas production method | |
| WO2013011843A1 (en) | Electrolytic cell, gas producing device, and gas producing method | |
| JP2012041623A (en) | Water electrolysis apparatus | |
| WO2012114787A1 (en) | Hydrogen production device and hydrogen production method | |
| JP2011116625A (en) | Hydrogen producing apparatus and hydrogen producing method | |
| JP2012021197A (en) | Device for producing gas | |
| JP2012107278A (en) | Gas producing device | |
| JP2011236466A (en) | Apparatus for manufacturing gas | |
| JP2011162428A (en) | Apparatus and method for producing hydrogen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11842377 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11842377 Country of ref document: EP Kind code of ref document: A1 |