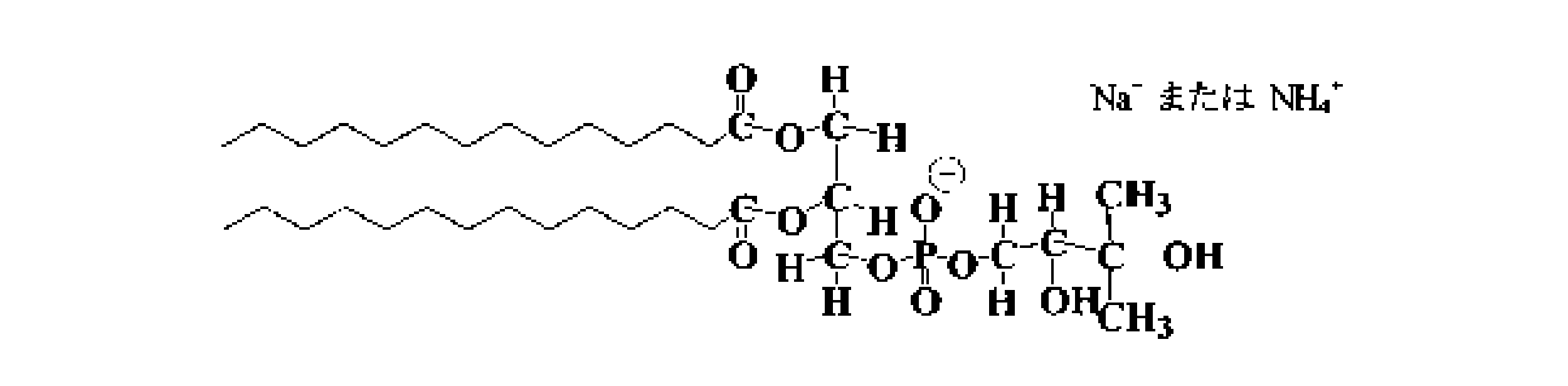WO2011162093A1 - Process for producing emulsion-producing hydrophilic nanoparticles - Google Patents
Process for producing emulsion-producing hydrophilic nanoparticles Download PDFInfo
- Publication number
- WO2011162093A1 WO2011162093A1 PCT/JP2011/063015 JP2011063015W WO2011162093A1 WO 2011162093 A1 WO2011162093 A1 WO 2011162093A1 JP 2011063015 W JP2011063015 W JP 2011063015W WO 2011162093 A1 WO2011162093 A1 WO 2011162093A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amphiphilic substance
- hydrophilic nanoparticles
- producing
- water
- hydrophilic
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K23/00—Use of substances as emulsifying, wetting, dispersing, or foam-producing agents
- C09K23/002—Inorganic compounds
Definitions
- the present invention relates to a method for producing hydrophilic nanoparticles used for producing an emulsion, a method for producing an emulsion using the hydrophilic nanoparticles, a method using the hydrophilic nanoparticles, a method for producing an emulsifier, and an emulsifier preparation solution.
- a method for producing hydrophilic nanoparticles used for producing an emulsion a method for producing an emulsion using the hydrophilic nanoparticles
- a method using the hydrophilic nanoparticles a method for producing an emulsifier, and an emulsifier preparation solution.
- a surfactant when emulsifying and dispersing a functional oil base or functional granule in water, a surfactant was selected according to the required HLB of the functional oil base and the properties of the granule surface, and emulsified and dispersed. .
- the required HLB value of the surfactant used as an emulsifier needs to be properly used depending on whether an O / W type emulsion is made or a W / O type emulsion, and further, thermal stability and aging Since the stability is not sufficient, a wide variety of surfactants were mixed and used (see Non-Patent Documents 1 to 4, etc.).
- physicochemical emulsification methods such as an HLB method, a phase inversion emulsification method, a phase inversion temperature emulsification method, and a gel emulsification method are generally performed. Since the basis of emulsion preparation is to reduce the interfacial energy at the oil / water interface and to stabilize the system thermodynamically, it is very cumbersome and labor intensive to select the most suitable emulsifier In addition, when many kinds of oils are mixed, stable emulsification is almost impossible.
- Patent Document 1 discloses an emulsifier containing hydrophilic nanoparticles formed by an amphiphilic substance that spontaneously forms closed vesicles and having a particle size distribution of 200 nm to 800 nm.
- Patent Document 1 since the emulsifier disclosed in Patent Document 1 has a high viscosity of the amphiphile and poor dispersibility in water, it is difficult to efficiently generate hydrophilic nanoparticles.
- gelation, solidification, and the like make it difficult to obtain the emulsifying ability expected by the prepared hydrophilic nanoparticle dispersion.
- the present invention has been made in view of the above circumstances, and provides a method for producing hydrophilic nanoparticles, a method for producing an emulsifier, and an emulsifier preparation solution capable of producing a wide range of amounts of hydrophilic nanoparticles.
- Another object of the present invention is to provide a novel use of hydrophilic nanoparticles.
- the present inventors have found that hydrophilic nanoparticles having an emulsifying action are precipitated by dissolving an amphiphilic substance in a good solvent and mixing the solution with water, thereby completing the present invention. .
- the present invention provides the following.
- a method for producing hydrophilic nanoparticles used in the production of an emulsion A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance; A method of producing hydrophilic nanoparticles comprising: a precipitation step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
- the amphiphilic substance is a derivative of polyoxyethylene hydrogenated castor oil represented by the following general formula, and the average added mole number (E) of ethylene oxide of the derivative is 3 to 100 (1 The manufacturing method of the hydrophilic nanoparticle as described in).
- the hydrophilic nanoparticle according to (2) in which a surfactant of 0.1 to 0.33 mole fraction is further dissolved in the polyoxyethylene hydrogenated castor oil derivative. Production method.
- the good solvent is methanol, ethanol, isopropanol, isobutanol, ter-butanol, sec-butanol, pentanol, methyl ethyl ketone, 1,3-propanediol, 2- (2-ethoxyethoxy) ethanol, 1,4
- (1) The manufacturing method of the hydrophilic nanoparticle in any one.
- a method for producing an emulsifier containing hydrophilic nanoparticles having an emulsifying action A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance; A method for producing an emulsifier, comprising: a step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
- hydrophilic nanoparticles having an emulsifying action are precipitated by mixing a solution in which an amphiphilic substance is dissolved in a good solvent with water. For this reason, by appropriately adjusting the amount of amphiphile dissolved in a good solvent, a desired amount of hydrophilic nanoparticles can be produced from a wide range, and hydrophilic nanoparticles can be produced.
- the hydrophilic nanoparticle of this invention is used for manufacture of an emulsion,
- the manufacturing method has a liquid preparation process and a precipitation process.
- a liquid preparation process and a precipitation process.
- the amphiphile is dissolved in a good solvent for the amphiphile.
- the amphiphilic substance used in the present invention is not particularly limited, but is a polyoxyethylene hydrogenated castor oil derivative represented by the following general formula 1, or a dialkyl ammonium derivative, a trialkyl ammonium derivative represented by the general formula 2, Examples thereof include tetraalkylammonium derivatives, dialkenylammonium derivatives, trialkenylammonium derivatives, and derivatives of halogenated salts of tetraalkenylammonium derivatives.
- E which is the average added mole number of ethylene oxide, is 3 to 100. If E is excessive, the type of good solvent that dissolves the amphiphilic substance is limited, and thus the degree of freedom in producing hydrophilic nanoparticles is narrowed.
- the upper limit of E is preferably 50, more preferably 40, and the lower limit of E is preferably 5.
- R 1 and R 2 are each independently an alkyl group or alkenyl group having 8 to 22 carbon atoms
- R 3 and R 4 are each independently hydrogen or an alkyl group having 1 to 4 carbon atoms
- X is F, Cl, Br or I.
- the hydrophilic nanoparticles may be ionized in order to increase the adhesion force of the hydrophilic nanoparticles to the emulsification target.
- alkyl or alkenyl trimethylammonium salt carbon chain length 12 to 22
- hexadecyltrimethylammonium bromide Hexadecyltrimethylammonium Bromide: hereinafter referred to as CTAB
- CTAB hexadecyltrimethylammonium bromide
- a mixed vesicle of a polyoxyethylene hydrogenated castor oil derivative such as HCO-10 (when E is 10 in the general formula 1) and CTAB the molar fraction (Xs) of CTAB is less than 0.1.
- Xs is preferably 0.1 or more and 0.33 or less.
- phospholipids As the amphiphilic substance forming the hydrophilic nanoparticles, phospholipids, phospholipid derivatives, and the like may be employed.
- phospholipid among the structures represented by the following general formula 3, DLPC (1,2-Dilauroyl-sn-glycero-3-phospho-rac-1-choline) having a carbon chain length of 12, DMPC (1,2-Dimyristol-sn-glycero-3-phospho-rac-1-choline), DPPC with a carbon chain length of 16 (1,2-Dipalmitoyyl-sn-glycero-3-phospho-rac-1-choline) Can be adopted.
- DLPG (1,2-Diilauroyl-sn-glycero-3-phospho-rac-1-glycerol) Na salt or NH4 salt
- DPPG 1,2-Dipalmitoyyl-sn-glycero-3 having a carbon chain length of 16 -Phospho-rac-1-glycerol
- examples of phospholipids include other glycerophospholipids (lecithin, ethanolamine, serine, inositol, etc.) and sphingophospholipids. Natural substances such as egg yolk lecithin or soybean lecithin may be employed.
- the mass ratio of the emulsified oil component and the emulsifier is preferably 4 to 200 and mixed.
- the good solvent used in the present invention has an ability to dissolve the amphiphilic substance to be used and is miscible with water.
- a good amount of amphiphile can be dissolved in the good solvent, and the solution can be mixed in an amount corresponding to the water miscibility of the good solvent, so that a desired amount of hydrophilic nanoparticles can be produced.
- the good solvent may be appropriately selected depending on the amphiphilic substance to be used.
- the good solvent is not limited to the above, and may be benzyl alcohol, n-butanol or the like. These solvents are not excellent in water miscibility, but are excellent in the ability to dissolve amphiphiles, so that a solution that dissolves many amphiphiles at a high concentration can be formed. Thereby, since the quantity of the solution mixed with water can be suppressed to a small amount, the low mixing property with water is not likely to be a problem. Thus, the good solvent in the present invention may be appropriately selected in consideration of the balance between the solubility of the amphiphile and the miscibility with water.
- the amphiphilic substance is precipitated as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
- the mixing of the solution and water may be performed through an external action such as stirring, or may be spontaneous by diffusion depending on the water mixing property of a good solvent.
- the former is preferable from the viewpoint of efficiency.
- Stirring may be performed simultaneously with the addition of the solution to water or with the solution of water, or after the addition.
- the water may be ion-exchanged water, pure water, ultrapure water, or tap water, and may be appropriately selected according to the required purity.
- the hydrophilic nanoparticles thus precipitated have an average particle diameter of about 20 nm to 800 nm when present at 0.1 to 20% by mass in the dispersion.
- the hydrophilic nanoparticles having such a particle size distribution exhibit an excellent and stable emulsion forming action with an average particle diameter of about 8 to 500 nm by making the hydrophilic nanoparticles finer in the emulsion forming step at the time of use.
- the average particle diameter in this specification refers to the average value based on the number frequency, and the measuring device is “FPAR (manufactured by Otsuka Electronics Co., Ltd.)”.
- the present invention also provides a method for producing an emulsifier containing hydrophilic nanoparticles. This method has the same steps as the method for producing the hydrophilic nanoparticles described above.
- the precipitated hydrophilic nanoparticles are dispersed in a mixed solvent of water and a good solvent. Such a dispersion may be appropriately treated according to the target of emulsification with hydrophilic nanoparticles, or may be used as an emulsifier without treatment.
- an emulsion for example, a product used in a living body, specifically, an ingested product (for example, a food or drink, a preparation for oral administration), an external preparation, a cosmetic, an agricultural chemical, or the like (for example, a protein) affected by a good solvent
- an ingested product for example, a food or drink, a preparation for oral administration
- an external preparation for example, a cosmetic, an agricultural chemical, or the like (for example, a protein) affected by a good solvent
- the content of the good solvent is low from the viewpoint of improving safety and reducing irritation. Then, you may perform the process (for example, fractional distillation, water dilution) which reduces content of a good solvent.
- the treatment for reducing the content of the good solvent for example, fractional distillation, water dilution
- the good solvent for example, fractional distillation, water dilution
- the treatment for reducing the content of the good solvent is performed for a long time before emulsification. Desirably not.
- an emulsion is used for the thing which is not used for a biological body, for example, a fuel, a coating material, etc., it is not necessary to perform the said process.
- the hydrophilic nanoparticles and the emulsifier produced by the method described above can be used for emulsification of various oily substances.
- an emulsion in which an oil phase containing an oily substance and an aqueous phase are dispersed is produced by mixing the oily substance with or without adding a substance that is distributed to the aqueous phase as appropriate to the emulsifier. it can.
- an oily substance means the substance which contains only oil or oil as a main component. A detailed use procedure is described in Japanese Patent No. 3855203.
- the hydrophilic nanoparticles and the emulsifier can form an emulsified dispersion having excellent thermal stability and stability over time with respect to the interface between the functional oil base and water or the functional granules and water.
- the emulsifying dispersant of the present invention can be used for stable emulsification in a wide temperature range over a long period of time.
- the hydrophilic nanoparticles and the emulsifier of the present invention include light oil, heavy oil A, heavy oil C, tar, biodiesel fuel, recycled heavy oil, waste cooking oil, cosmetic oil, cooking oil, industrial oil (for example, silicon oil, kerosene) and the like. Can be used to emulsify water in various oils.
- hydrophilic nanoparticles and the emulsifier of the present invention can be used to produce an emulsion under conditions that are difficult to emulsify with a surfactant.
- Such conditions include strongly acidic (for example, pH 4 or less) and strongly basic (for example, pH 12 or more), high salt concentration (for example, 0.1 mol / L or more) conditions, natural product oils (vegetable oil, mineral oil)
- Examples include emulsification and emulsification of an oil agent having a high melting point (for example, 50 ° C. or higher) (oil agent that is liquid at high temperature and solidifies after emulsification).
- HCO-100, water and ethanol or isopropyl alcohol were mixed at a ratio of 7: 1.5: 1.5 (mass ratio).
- the mixture was a uniform liquid regardless of whether ethanol or isopropyl alcohol was used. Accordingly, it was confirmed that a large amount of amphiphilic substance that gels when a solvent containing only water can be dissolved by using a good solvent.
- Example 1 Polyoxyethylene hydrogenated castor oil derivative 10 mL of a solution obtained by mixing HCO-10, water, and ethanol in a ratio of 7: 1.5: 1.5 (mass ratio) was added to 25 ° C. water. Added to 1 L and mixed by stirring at 500 rpm for 5 minutes.
- a particle size distribution analyzer FPAR manufactured by Otsuka Electronics Co., Ltd.
- the peak indicating the presence of hydrophilic nanoparticles was also around 400 nm and also at several ⁇ m. Was confirmed.
- Example 1 The dispersions of Example 1 and Comparative Example were emulsified with A-heavy oil at the respective mass ratios shown in Tables 3 and 4 by stirring at 8000 rpm for about 5 minutes using a homomixer at room temperature, and the emulsified state was evaluated. did.
- the evaluation criteria are as follows. ⁇ : No phase separation, ⁇ : Separation by specific gravity difference (coacervation), ⁇ : Separation 1: O / W emulsion, 2: W / O emulsion, 3: W / O emulsion and separated water phase
- the dispersion of Comparative Example could not emulsify 80% by mass or more of A heavy oil, and the emulsion stability of 60 to 70% by mass of A heavy oil was insufficient.
- the dispersion liquid of Example 1 A heavy oil of any amount, at least 95% by mass or less, was stably and satisfactorily emulsified.
- Example 2 Phospholipid A chloroform solution of hydrogenated lecithin shown in Table 2 was mixed with water in the same procedure as in Example 1. About the obtained mixture, when the particle size distribution was measured like Example 1, the peak which shows presence of a hydrophilic nanoparticle was confirmed by 100 nm vicinity.
- the obtained dispersion was stirred and emulsified in the same manner as in Example 1 with each oil within the range shown in Table 5, and the emulsified state was evaluated.
- the evaluation criteria are as described above.
- Example 5 As shown in Table 5, the dispersion liquid of Example 2 stably emulsified various oils over a wide range of mixing ratios.
Abstract
Provided are a process for producing hydrophilic nanoparticles that allows hydrophilic nanoparticles to be produced in a wide range of quantities, a process for producing an emulsifier, and a solution for preparing an emulsifier. The disclosed process for producing hydrophilic nanoparticles which are used for producing an emulsion involves: a solution preparation step of dissolving an amphiphilic substance into a good solvent of the amphiphilic substance; and a precipitation step of mixing the solution prepared in said solution preparation step with water, and thereby precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying effect.
Description
本発明は、乳化物製造用として用いる親水性ナノ粒子の製造方法、該親水性ナノ粒子を用いた乳化物の製造方法、該親水性ナノ粒子を使用する方法、乳化剤の製造方法、乳化剤調製液に関する。
The present invention relates to a method for producing hydrophilic nanoparticles used for producing an emulsion, a method for producing an emulsion using the hydrophilic nanoparticles, a method using the hydrophilic nanoparticles, a method for producing an emulsifier, and an emulsifier preparation solution. About.
従来、機能性油性基剤または機能性顆粒を水に乳化分散させる場合には、機能性油性基剤の所要HLBや顆粒表面の性質に応じて界面活性剤を選択し、乳化分散を行っていた。また、乳化剤として用いられる界面活性剤の所要HLB値は、O/W型エマルションを作る場合とW/O型エマルションを作る場合とのそれぞれに応じて使い分ける必要があり、しかも、熱安定性や経時安定性が十分でないため、多種多様な界面活性剤を混合して用いていた(非特許文献1~4等参照)。
Conventionally, when emulsifying and dispersing a functional oil base or functional granule in water, a surfactant was selected according to the required HLB of the functional oil base and the properties of the granule surface, and emulsified and dispersed. . In addition, the required HLB value of the surfactant used as an emulsifier needs to be properly used depending on whether an O / W type emulsion is made or a W / O type emulsion, and further, thermal stability and aging Since the stability is not sufficient, a wide variety of surfactants were mixed and used (see Non-Patent Documents 1 to 4, etc.).
しかしながら、界面活性剤は、生分解性が低く、泡立ちの原因となるので、環境汚染などの深刻な問題となっている。また、機能性油性基剤の乳化製剤の調製法として、HLB法、転相乳化法、転相温度乳化法、ゲル乳化法等の物理化学的な乳化方法が一般に行われているが、いずれも油/水界面の界面エネルギーを低下させ、熱力学的に系を安定化させる作用をエマルション調製の基本としているので、最適な乳化剤を選択するために非常に煩雑かつ多大な労力を有しており、まして、多種類の油が混在していると、安定に乳化させることは殆ど不可能であった。
However, surfactants have low biodegradability and cause foaming, which is a serious problem such as environmental pollution. In addition, as a method for preparing an emulsified preparation of a functional oil base, physicochemical emulsification methods such as an HLB method, a phase inversion emulsification method, a phase inversion temperature emulsification method, and a gel emulsification method are generally performed. Since the basis of emulsion preparation is to reduce the interfacial energy at the oil / water interface and to stabilize the system thermodynamically, it is very cumbersome and labor intensive to select the most suitable emulsifier In addition, when many kinds of oils are mixed, stable emulsification is almost impossible.
そこで、特許文献1には、自発的に閉鎖小胞を形成する両親媒性物質により形成され、200nm~800nmの粒度分布を有する親水性ナノ粒子を含有する乳化剤が開示されている。
Therefore, Patent Document 1 discloses an emulsifier containing hydrophilic nanoparticles formed by an amphiphilic substance that spontaneously forms closed vesicles and having a particle size distribution of 200 nm to 800 nm.
しかし、特許文献1に示される乳化剤は、両親媒性物質の粘性等が高く水への分散性が悪いため、親水性ナノ粒子を効率的に生成することが困難である。ここで、多量の両親媒性物質を水に添加すると、ゲル化や固化等するため、調製された親水性ナノ粒子分散液が期待する乳化能を得ることは難しくなる。
However, since the emulsifier disclosed in Patent Document 1 has a high viscosity of the amphiphile and poor dispersibility in water, it is difficult to efficiently generate hydrophilic nanoparticles. Here, when a large amount of an amphiphilic substance is added to water, gelation, solidification, and the like make it difficult to obtain the emulsifying ability expected by the prepared hydrophilic nanoparticle dispersion.
本発明は、以上の実情に鑑みてなされたものであり、幅広い範囲の量の親水性ナノ粒子を製造できる親水性ナノ粒子の製造方法、乳化剤の製造方法、及び乳化剤調製液を提供することを目的とする。また、本発明は、親水性ナノ粒子の新規用途を提供することを別の目的とする。
The present invention has been made in view of the above circumstances, and provides a method for producing hydrophilic nanoparticles, a method for producing an emulsifier, and an emulsifier preparation solution capable of producing a wide range of amounts of hydrophilic nanoparticles. Objective. Another object of the present invention is to provide a novel use of hydrophilic nanoparticles.
本発明者らは、両親媒性物質を良溶媒中に溶解させ、溶液を水と混合することで、乳化作用を有する親水性ナノ粒子が析出することを見出し、本発明を完成するに至った。具体的に、本発明は以下のようなものを提供する。
The present inventors have found that hydrophilic nanoparticles having an emulsifying action are precipitated by dissolving an amphiphilic substance in a good solvent and mixing the solution with water, thereby completing the present invention. . Specifically, the present invention provides the following.
(1) 乳化物の製造に用いられる親水性ナノ粒子の製造方法であって、
両親媒性物質を、前記両親媒性物質に対する良溶媒に溶解させる調液工程と、
前記調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として前記両親媒性物質を析出させる析出工程と、を有する親水性ナノ粒子の製造方法。 (1) A method for producing hydrophilic nanoparticles used in the production of an emulsion,
A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance;
A method of producing hydrophilic nanoparticles comprising: a precipitation step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
両親媒性物質を、前記両親媒性物質に対する良溶媒に溶解させる調液工程と、
前記調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として前記両親媒性物質を析出させる析出工程と、を有する親水性ナノ粒子の製造方法。 (1) A method for producing hydrophilic nanoparticles used in the production of an emulsion,
A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance;
A method of producing hydrophilic nanoparticles comprising: a precipitation step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
(2) 前記両親媒性物質が、下記の一般式で表されるポリオキシエチレン硬化ヒマシ油の誘導体であって、前記誘導体のエチレンオキシドの平均付加モル数(E)が3~100である(1)に記載の親水性ナノ粒子の製造方法。
(2) The amphiphilic substance is a derivative of polyoxyethylene hydrogenated castor oil represented by the following general formula, and the average added mole number (E) of ethylene oxide of the derivative is 3 to 100 (1 The manufacturing method of the hydrophilic nanoparticle as described in).
(3) 前記調液工程では、前記ポリオキシエチレン硬化ヒマシ油の誘導体に対して0.1~0.33モル分率の界面活性剤を更に溶解させる(2)に記載の親水性ナノ粒子の製造方法。
(3) In the preparation step, the hydrophilic nanoparticle according to (2), in which a surfactant of 0.1 to 0.33 mole fraction is further dissolved in the polyoxyethylene hydrogenated castor oil derivative. Production method.
(4) 前記良溶媒が、メタノール、エタノール、イソプロパノール、イソブタノール、ter-ブタノール、sec-ブタノール、ペンタノール、メチルエチルケトン、1,3-プロパンジオール、2-(2-エトキシエトキシ)エタノール、1,4-ジオキサン、ジエチレンオキシド、エチレングリコール、プロピレングリコール、ブチレングリコール、アセトン、及びテトラヒドロフランからなる群から選ばれる1種若しくは2種以上である溶媒、又は該溶媒と水との混合溶媒である(1)から(3)いずれかに記載の親水性ナノ粒子の製造方法。
(4) The good solvent is methanol, ethanol, isopropanol, isobutanol, ter-butanol, sec-butanol, pentanol, methyl ethyl ketone, 1,3-propanediol, 2- (2-ethoxyethoxy) ethanol, 1,4 A solvent that is one or more selected from the group consisting of dioxane, diethylene oxide, ethylene glycol, propylene glycol, butylene glycol, acetone, and tetrahydrofuran, or a mixed solvent of the solvent and water (1) (3) The manufacturing method of the hydrophilic nanoparticle in any one.
(5) 乳化作用を有する親水性ナノ粒子を含有する乳化剤の製造方法であって、
両親媒性物質を、前記両親媒性物質に対する良溶媒に溶解させる調液工程と、
前記調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として前記両親媒性物質を析出させる析出工程と、を有する乳化剤の製造方法。 (5) A method for producing an emulsifier containing hydrophilic nanoparticles having an emulsifying action,
A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance;
A method for producing an emulsifier, comprising: a step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
両親媒性物質を、前記両親媒性物質に対する良溶媒に溶解させる調液工程と、
前記調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として前記両親媒性物質を析出させる析出工程と、を有する乳化剤の製造方法。 (5) A method for producing an emulsifier containing hydrophilic nanoparticles having an emulsifying action,
A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance;
A method for producing an emulsifier, comprising: a step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
(6) 両親媒性物質を、前記両親媒性物質に対する良溶媒中に溶解状態で含有し、
水と混合することで、前記両親媒性物質が乳化作用を有する親水性ナノ粒子として析出する乳化剤調製液。 (6) containing an amphiphile in a dissolved state in a good solvent for the amphiphile,
An emulsifier preparation solution in which the amphiphilic substance is precipitated as hydrophilic nanoparticles having an emulsifying action by mixing with water.
水と混合することで、前記両親媒性物質が乳化作用を有する親水性ナノ粒子として析出する乳化剤調製液。 (6) containing an amphiphile in a dissolved state in a good solvent for the amphiphile,
An emulsifier preparation solution in which the amphiphilic substance is precipitated as hydrophilic nanoparticles having an emulsifying action by mixing with water.
(7) 界面活性剤では乳化させることが困難な条件下で乳化物を製造するために、両親媒性物質により形成された親水性ナノ粒子を使用する方法。
(7) A method of using hydrophilic nanoparticles formed from an amphiphilic substance to produce an emulsion under conditions that are difficult to emulsify with a surfactant.
本発明によれば、両親媒性物質が良溶媒中に溶解された溶液を水と混合することで、乳化作用を有する親水性ナノ粒子が析出する。このため、良溶媒への両親媒性物質の溶解量を適宜調節することで、幅広い範囲から所望の量の親水性ナノ粒子を製造でき、親水性ナノ粒子を製造できる。
According to the present invention, hydrophilic nanoparticles having an emulsifying action are precipitated by mixing a solution in which an amphiphilic substance is dissolved in a good solvent with water. For this reason, by appropriately adjusting the amount of amphiphile dissolved in a good solvent, a desired amount of hydrophilic nanoparticles can be produced from a wide range, and hydrophilic nanoparticles can be produced.
以下、本発明の実施形態を説明するが、これが本発明を限定するものではない。
Hereinafter, embodiments of the present invention will be described, but this does not limit the present invention.
[親水性ナノ粒子の製造方法]
本発明の親水性ナノ粒子は、乳化物の製造に用いるものであり、その製造方法は調液工程と析出工程とを有する。以下、各構成を詳細に説明する。 [Method for producing hydrophilic nanoparticles]
The hydrophilic nanoparticle of this invention is used for manufacture of an emulsion, The manufacturing method has a liquid preparation process and a precipitation process. Hereinafter, each configuration will be described in detail.
本発明の親水性ナノ粒子は、乳化物の製造に用いるものであり、その製造方法は調液工程と析出工程とを有する。以下、各構成を詳細に説明する。 [Method for producing hydrophilic nanoparticles]
The hydrophilic nanoparticle of this invention is used for manufacture of an emulsion, The manufacturing method has a liquid preparation process and a precipitation process. Hereinafter, each configuration will be described in detail.
<調液工程>
調液工程では、両親媒性物質を、この両親媒性物質に対する良溶媒に溶解させる。本発明で用いる両親媒性物質としては、特に限定されないが、下記の一般式1で表されるポリオキシエチレン硬化ひまし油の誘導体、もしくは一般式2で表されるジアルキルアンモニウム誘導体、トリアルキルアンモニウム誘導体、テトラアルキルアンモニウム誘導体、ジアルケニルアンモニウム誘導体、トリアルケニルアンモニウム誘導体、又はテトラアルケニルアンモニウム誘導体のハロゲン塩の誘導体が挙げられる。 <Preparation process>
In the preparation step, the amphiphile is dissolved in a good solvent for the amphiphile. The amphiphilic substance used in the present invention is not particularly limited, but is a polyoxyethylene hydrogenated castor oil derivative represented by the following general formula 1, or a dialkyl ammonium derivative, a trialkyl ammonium derivative represented by the general formula 2, Examples thereof include tetraalkylammonium derivatives, dialkenylammonium derivatives, trialkenylammonium derivatives, and derivatives of halogenated salts of tetraalkenylammonium derivatives.
調液工程では、両親媒性物質を、この両親媒性物質に対する良溶媒に溶解させる。本発明で用いる両親媒性物質としては、特に限定されないが、下記の一般式1で表されるポリオキシエチレン硬化ひまし油の誘導体、もしくは一般式2で表されるジアルキルアンモニウム誘導体、トリアルキルアンモニウム誘導体、テトラアルキルアンモニウム誘導体、ジアルケニルアンモニウム誘導体、トリアルケニルアンモニウム誘導体、又はテトラアルケニルアンモニウム誘導体のハロゲン塩の誘導体が挙げられる。 <Preparation process>
In the preparation step, the amphiphile is dissolved in a good solvent for the amphiphile. The amphiphilic substance used in the present invention is not particularly limited, but is a polyoxyethylene hydrogenated castor oil derivative represented by the following general formula 1, or a dialkyl ammonium derivative, a trialkyl ammonium derivative represented by the general formula 2, Examples thereof include tetraalkylammonium derivatives, dialkenylammonium derivatives, trialkenylammonium derivatives, and derivatives of halogenated salts of tetraalkenylammonium derivatives.
式中、エチレンオキシドの平均付加モル数であるEは、3~100である。Eが過大になると、両親媒性物質を溶解する良溶媒の種類が制限されるため、親水性ナノ粒子の製造の自由度が狭まる。Eの上限は好ましくは50であり、より好ましくは40であり、Eの下限は好ましくは5である。
In the formula, E, which is the average added mole number of ethylene oxide, is 3 to 100. If E is excessive, the type of good solvent that dissolves the amphiphilic substance is limited, and thus the degree of freedom in producing hydrophilic nanoparticles is narrowed. The upper limit of E is preferably 50, more preferably 40, and the lower limit of E is preferably 5.
式中、R1及びR2は、各々独立して炭素数8~22のアルキル基又はアルケニル基であり、R3及びR4は、各々独立して水素又は炭素数1~4のアルキル基であり、XはF、Cl、Br又はIである。
In the formula, R 1 and R 2 are each independently an alkyl group or alkenyl group having 8 to 22 carbon atoms, R 3 and R 4 are each independently hydrogen or an alkyl group having 1 to 4 carbon atoms, and X is F, Cl, Br or I.
調液工程では、親水性ナノ粒子の乳化対象への付着力を高めるために、親水性ナノ粒子をイオン化してもよい。カチオン化のためには、イオン性界面活性剤として、アルキルまたはアルケニルトリメチルアンモニウム塩(炭素鎖長12~22)、好ましくは、炭素鎖長16のヘキサデシルトリメチルアンモニウムブロミド(Hexadecyltrimethylammonium Bromide:以下、CTABという)、アニオン化のためには、アルキル硫酸エステル塩(CnSO4
-M+炭素鎖長n=8~22、M:アルカリ金属、アルカリ土類金属、アンモニウム塩など)、アルキルスルホン酸塩(CnSO3
- M+ 炭素鎖長8~22、M:アルカリ金属、アルカリ土類金属、アンモニウム塩など)などを用いることができる。
In the liquid preparation step, the hydrophilic nanoparticles may be ionized in order to increase the adhesion force of the hydrophilic nanoparticles to the emulsification target. For cationization, alkyl or alkenyl trimethylammonium salt (carbon chain length 12 to 22), preferably hexadecyltrimethylammonium bromide (Hexadecyltrimethylammonium Bromide: hereinafter referred to as CTAB) is used as an ionic surfactant. ), Anion sulfate alkyl salt (CnSO 4 − M + carbon chain length n = 8 to 22, M: alkali metal, alkaline earth metal, ammonium salt, etc.), alkyl sulfonate (CnSO 3 - M + carbon chain length 8 ~ 22, M: alkali metal, alkaline earth metal, ammonium salt), or the like can be used.
ポリオキシエチレン硬化ヒマシ油の誘導体、例えばHCO-10(一般式1においてEが10である場合)と、CTABとの混合ベシクルにおいて、CTABのモル分率(Xs)は、0.1未満であると、混合ベシクルのカチオン性が一定に保ちにくく、0.33超であると、安定した混合ベシクルを得られにくくなる。そこで、Xsは0.1以上0.33以下であることが好ましい。
In a mixed vesicle of a polyoxyethylene hydrogenated castor oil derivative such as HCO-10 (when E is 10 in the general formula 1) and CTAB, the molar fraction (Xs) of CTAB is less than 0.1. In addition, the cationicity of the mixed vesicle is difficult to keep constant, and if it exceeds 0.33, it becomes difficult to obtain a stable mixed vesicle. Therefore, Xs is preferably 0.1 or more and 0.33 or less.
また、親水性ナノ粒子を形成する両親媒性物質としては、リン脂質やリン脂質誘導体等を採用してもよい。リン脂質としては、下記の一般式3で示される構成のうち、炭素鎖長12のDLPC(1,2-Dilauroyl-sn-glycero-3-phospho-rac-1-choline)、炭素鎖長14のDMPC(1,2-Dimyristoyl-sn-glycero-3-phospho-rac-1-choline)、炭素鎖長16のDPPC(1,2-Dipalmitoyl-sn-glycero-3-phospho-rac-1-choline)が採用可能である。
Also, as the amphiphilic substance forming the hydrophilic nanoparticles, phospholipids, phospholipid derivatives, and the like may be employed. As the phospholipid, among the structures represented by the following general formula 3, DLPC (1,2-Dilauroyl-sn-glycero-3-phospho-rac-1-choline) having a carbon chain length of 12, DMPC (1,2-Dimyristol-sn-glycero-3-phospho-rac-1-choline), DPPC with a carbon chain length of 16 (1,2-Dipalmitoyyl-sn-glycero-3-phospho-rac-1-choline) Can be adopted.
また、下記の一般式4で示される構成のうち、炭素鎖長12のDLPG(1,2-Dilauroyl-sn-glycero-3-phospho-rac-1-glycerol)のNa塩又はNH4塩、炭素鎖長14のDMPG(1,2-Dimyristoyl-sn-glycero-3-phospho-rac-1-glycerol)のNa塩又はNH4塩、炭素鎖長16のDPPG(1,2-Dipalmitoyl-sn-glycero-3-phospho-rac-1-glycerol)のNa塩又はNH4塩を採用してもよい。
Further, among the structures represented by the following general formula 4, DLPG (1,2-Diilauroyl-sn-glycero-3-phospho-rac-1-glycerol) Na salt or NH4 salt, carbon chain of carbon chain length 12 Na or NH4 salt of long DMPG (1,2-Dimyristol-sn-glycero-3-phospho-rac-1-glycerol), DPPG (1,2-Dipalmitoyyl-sn-glycero-3) having a carbon chain length of 16 -Phospho-rac-1-glycerol) Na salt or NH4 salt may be employed.
さらに、リン脂質としては、他のグリセロリン脂質(レシチン、エタノールアミン,セリン,イノシトールなど)、スフィンゴリン脂質が挙げられる。卵黄レシチンまたは大豆レシチンなどの天然物質を採用してもよい。なお、被乳化油性成分を上記親水性ナノ粒子により形成される乳化剤を用いて乳化分散する場合には、被乳化油性成分と前記乳化剤との質量比を4~200として接触、混和させるとよい。
Furthermore, examples of phospholipids include other glycerophospholipids (lecithin, ethanolamine, serine, inositol, etc.) and sphingophospholipids. Natural substances such as egg yolk lecithin or soybean lecithin may be employed. When the emulsified oil component is emulsified and dispersed using an emulsifier formed of the above hydrophilic nanoparticles, the mass ratio of the emulsified oil component and the emulsifier is preferably 4 to 200 and mixed.
<良溶媒>
本発明で用いる良溶媒は、用いる両親媒性物質を溶解する能力を有し、かつ水への混合性を有するものである。良溶媒には多量の両親媒性物質を溶解でき、その溶液を良溶媒の水混合性に応じた量で混合できるので、所望量の親水性ナノ粒子の製造が可能である。 <Good solvent>
The good solvent used in the present invention has an ability to dissolve the amphiphilic substance to be used and is miscible with water. A good amount of amphiphile can be dissolved in the good solvent, and the solution can be mixed in an amount corresponding to the water miscibility of the good solvent, so that a desired amount of hydrophilic nanoparticles can be produced.
本発明で用いる良溶媒は、用いる両親媒性物質を溶解する能力を有し、かつ水への混合性を有するものである。良溶媒には多量の両親媒性物質を溶解でき、その溶液を良溶媒の水混合性に応じた量で混合できるので、所望量の親水性ナノ粒子の製造が可能である。 <Good solvent>
The good solvent used in the present invention has an ability to dissolve the amphiphilic substance to be used and is miscible with water. A good amount of amphiphile can be dissolved in the good solvent, and the solution can be mixed in an amount corresponding to the water miscibility of the good solvent, so that a desired amount of hydrophilic nanoparticles can be produced.
良溶媒は、前述のとおり、用いる両親媒性物質に応じて適宜選択されてよいが、例えば、水への混合性に優れる点で、メタノール、エタノール、イソプロパノール、イソブタノール、ter-ブタノール、sec-ブタノール、ペンタノール、メチルエチルケトン、1,3-プロパンジオール、2-(2-エトキシエトキシ)エタノール、1,4-ジオキサン、ジエチレンオキシド、エチレングリコール、プロピレングリコール、ブチレングリコール、アセトン、及びテトラヒドロフランからなる群から選ばれる1種若しくは2種以上である溶媒、又は該溶媒と水との混合溶媒であることが好ましい。水を含む混合溶媒の場合、水の含有率は、両親媒性物質の溶解度を所望の程度を下回らない範囲で、適宜設定されてよい。
As described above, the good solvent may be appropriately selected depending on the amphiphilic substance to be used. For example, methanol, ethanol, isopropanol, isobutanol, ter-butanol, sec- From the group consisting of butanol, pentanol, methyl ethyl ketone, 1,3-propanediol, 2- (2-ethoxyethoxy) ethanol, 1,4-dioxane, diethylene oxide, ethylene glycol, propylene glycol, butylene glycol, acetone, and tetrahydrofuran It is preferably a solvent that is one or more selected, or a mixed solvent of the solvent and water. In the case of a mixed solvent containing water, the water content may be appropriately set within a range that does not fall below the desired degree of solubility of the amphiphile.
なお、良溶媒は、上記のものに限られず、ベンジルアルコール、n-ブタノール等であってもよい。これらの溶媒は、水への混合性が優れてはいないが、両親媒性物質の溶解能に優れるため、多くの両親媒性物質を高濃度で溶解する溶液を形成できる。これにより、水と混合する溶液の量が少量に抑えられるため、水への混合性に低さが問題となりにくい。このように、本発明における良溶媒は、両親媒性物質の溶解度と水への混合性とのバランスを考慮して、適宜選択されてよい。
The good solvent is not limited to the above, and may be benzyl alcohol, n-butanol or the like. These solvents are not excellent in water miscibility, but are excellent in the ability to dissolve amphiphiles, so that a solution that dissolves many amphiphiles at a high concentration can be formed. Thereby, since the quantity of the solution mixed with water can be suppressed to a small amount, the low mixing property with water is not likely to be a problem. Thus, the good solvent in the present invention may be appropriately selected in consideration of the balance between the solubility of the amphiphile and the miscibility with water.
<析出工程>
析出工程では、調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として両親媒性物質を析出させる。 <Precipitation process>
In the precipitation step, the amphiphilic substance is precipitated as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
析出工程では、調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として両親媒性物質を析出させる。 <Precipitation process>
In the precipitation step, the amphiphilic substance is precipitated as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water.
溶液と水との混合は、撹拌等の外的作用を介して行ってもよく、良溶媒の水混合性に依存した拡散による自発的なものであってもよい。ただし、効率の観点からは前者が好ましい。なお、撹拌は、溶液の水への添加もしくは水の溶液への添加と同時に行ってもよく、添加後に行ってもよい。なお、水は、イオン交換水、純水、超純水、水道水のいずれでもよく、求められる純度に応じて適宜選択されてよい。
The mixing of the solution and water may be performed through an external action such as stirring, or may be spontaneous by diffusion depending on the water mixing property of a good solvent. However, the former is preferable from the viewpoint of efficiency. Stirring may be performed simultaneously with the addition of the solution to water or with the solution of water, or after the addition. The water may be ion-exchanged water, pure water, ultrapure water, or tap water, and may be appropriately selected according to the required purity.
このようにして析出する親水性ナノ粒子は、分散液中0.1~20質量%で存在する場合に、平均粒子径20nm~800nm程度である。かかる粒度分布を有する親水性ナノ粒子は、使用時にエマルション形成の工程で親水性ナノ粒子が細粒化されることで、平均粒子径8~500nm程度の優れた安定したエマルション形成作用を呈することになる。なお、本明細書における平均粒子径は個数頻度に基づく平均値を指し、測定装置は「FPAR(大塚電子(株)社製)」である。
The hydrophilic nanoparticles thus precipitated have an average particle diameter of about 20 nm to 800 nm when present at 0.1 to 20% by mass in the dispersion. The hydrophilic nanoparticles having such a particle size distribution exhibit an excellent and stable emulsion forming action with an average particle diameter of about 8 to 500 nm by making the hydrophilic nanoparticles finer in the emulsion forming step at the time of use. Become. In addition, the average particle diameter in this specification refers to the average value based on the number frequency, and the measuring device is “FPAR (manufactured by Otsuka Electronics Co., Ltd.)”.
[乳化剤の製造方法]
本発明は、親水性ナノ粒子を含有する乳化剤の製造方法も提供する。この方法は、前述した親水性ナノ粒子を製造する方法と同様の工程を有する。 [Method for producing emulsifier]
The present invention also provides a method for producing an emulsifier containing hydrophilic nanoparticles. This method has the same steps as the method for producing the hydrophilic nanoparticles described above.
本発明は、親水性ナノ粒子を含有する乳化剤の製造方法も提供する。この方法は、前述した親水性ナノ粒子を製造する方法と同様の工程を有する。 [Method for producing emulsifier]
The present invention also provides a method for producing an emulsifier containing hydrophilic nanoparticles. This method has the same steps as the method for producing the hydrophilic nanoparticles described above.
析出した親水性ナノ粒子は、水及び良溶媒の混合溶媒中に分散している。かかる分散液は、親水性ナノ粒子による乳化の対象に応じて適宜処理してもよく、無処理のまま乳化剤としてもよい。
The precipitated hydrophilic nanoparticles are dispersed in a mixed solvent of water and a good solvent. Such a dispersion may be appropriately treated according to the target of emulsification with hydrophilic nanoparticles, or may be used as an emulsifier without treatment.
乳化物が、例えば生体に使用される物、具体的には被摂取物(例えば飲食品、経口投与製剤)、外用剤、化粧品、農薬等、あるいは良溶媒に侵される物(例えば、タンパク質)として使用される場合には、安全性の向上や刺激性の低下等の観点から、良溶媒の含有量が低いことが好ましい。そこで、良溶媒の含有量を低減させる処理(例えば分留、水希釈)を行ってもよい。分散液における水含有率が過大であると、親水性ナノ粒子の保存性が低下するため、良溶媒の含有量を低減させる処理(例えば分留、水希釈)は、乳化の長時間前に行わないことが望ましい。なお、乳化物が、生体に使用されない物、例えば燃料、塗料等に使用される場合には、上記処理を行わなくてもよい。
As an emulsion, for example, a product used in a living body, specifically, an ingested product (for example, a food or drink, a preparation for oral administration), an external preparation, a cosmetic, an agricultural chemical, or the like (for example, a protein) affected by a good solvent When used, it is preferable that the content of the good solvent is low from the viewpoint of improving safety and reducing irritation. Then, you may perform the process (for example, fractional distillation, water dilution) which reduces content of a good solvent. If the water content in the dispersion is excessive, the preservability of the hydrophilic nanoparticles will decrease, so the treatment for reducing the content of the good solvent (for example, fractional distillation, water dilution) is performed for a long time before emulsification. Desirably not. In addition, when an emulsion is used for the thing which is not used for a biological body, for example, a fuel, a coating material, etc., it is not necessary to perform the said process.
[使用]
前述した方法で製造される親水性ナノ粒子及び乳化剤は、種々の油性物質の乳化に使用できる。具体的には、乳化剤に、水相に分配される物質を適宜添加し又は添加せずに、油性物質と混合することで、油性物質を含む油相と、水相とが分散したエマルションを製造できる。なお、油性物質とは、油のみ又は油を主成分として含む物質をいう。詳細な使用手順は、特許第3855203号公報に記載されている。 [use]
The hydrophilic nanoparticles and the emulsifier produced by the method described above can be used for emulsification of various oily substances. Specifically, an emulsion in which an oil phase containing an oily substance and an aqueous phase are dispersed is produced by mixing the oily substance with or without adding a substance that is distributed to the aqueous phase as appropriate to the emulsifier. it can. In addition, an oily substance means the substance which contains only oil or oil as a main component. A detailed use procedure is described in Japanese Patent No. 3855203.
前述した方法で製造される親水性ナノ粒子及び乳化剤は、種々の油性物質の乳化に使用できる。具体的には、乳化剤に、水相に分配される物質を適宜添加し又は添加せずに、油性物質と混合することで、油性物質を含む油相と、水相とが分散したエマルションを製造できる。なお、油性物質とは、油のみ又は油を主成分として含む物質をいう。詳細な使用手順は、特許第3855203号公報に記載されている。 [use]
The hydrophilic nanoparticles and the emulsifier produced by the method described above can be used for emulsification of various oily substances. Specifically, an emulsion in which an oil phase containing an oily substance and an aqueous phase are dispersed is produced by mixing the oily substance with or without adding a substance that is distributed to the aqueous phase as appropriate to the emulsifier. it can. In addition, an oily substance means the substance which contains only oil or oil as a main component. A detailed use procedure is described in Japanese Patent No. 3855203.
親水性ナノ粒子及び乳化剤は、機能性油性基剤と水、または機能性顆粒と水などの界面に対して、熱安定性や経時安定性に優れた乳化分散系を形成できる。このため、本発明の乳化分散剤は、長期間に亘る幅広い温度領域での安定な乳化に使用できる。また、一種類の乳化分散剤を用いて、被乳化油剤の所要HLB値又は機能性顆粒の表面状態に関係なく、油性物質を乳化分散できるので、炭化水素系油剤やシリコン系油剤の乳化にも使用できる。このため、多種類の混在している油を併せて乳化するためにも使用できる。従って、本発明の親水性ナノ粒子及び乳化剤は、軽油、A重油、C重油、タール、バイオディーゼル燃料、再生重油、廃食油、化粧油、食用油、工業用油剤(例えばシリコン油、灯油)等の種々の油への水の乳化に使用し得る。
The hydrophilic nanoparticles and the emulsifier can form an emulsified dispersion having excellent thermal stability and stability over time with respect to the interface between the functional oil base and water or the functional granules and water. For this reason, the emulsifying dispersant of the present invention can be used for stable emulsification in a wide temperature range over a long period of time. In addition, it is possible to emulsify and disperse oily substances using one type of emulsifying dispersant regardless of the required HLB value of the oil to be emulsified or the surface state of the functional granules. Can be used. For this reason, it can also be used to emulsify a mixture of various types of oil. Accordingly, the hydrophilic nanoparticles and the emulsifier of the present invention include light oil, heavy oil A, heavy oil C, tar, biodiesel fuel, recycled heavy oil, waste cooking oil, cosmetic oil, cooking oil, industrial oil (for example, silicon oil, kerosene) and the like. Can be used to emulsify water in various oils.
その他、本発明の親水性ナノ粒子及び乳化剤は、界面活性剤では乳化させることが困難な条件下で乳化物を製造するために使用し得る。かかる条件としては、強酸性(例えばpH4以下)及び強塩基性(例えばpH12以上)の条件、高塩濃度(例えば0.1モル/L以上)の条件、天然物油剤(植物油、鉱物油)の乳化、高融点(例えば50℃以上)の油剤(乳化時は高温で液状、乳化後に固化してしまう油剤)の乳化が挙げられる。
In addition, the hydrophilic nanoparticles and the emulsifier of the present invention can be used to produce an emulsion under conditions that are difficult to emulsify with a surfactant. Such conditions include strongly acidic (for example, pH 4 or less) and strongly basic (for example, pH 12 or more), high salt concentration (for example, 0.1 mol / L or more) conditions, natural product oils (vegetable oil, mineral oil) Examples include emulsification and emulsification of an oil agent having a high melting point (for example, 50 ° C. or higher) (oil agent that is liquid at high temperature and solidifies after emulsification).
(参考例)
前述した一般式1におけるエチレンオキシドの平均付加モル数(E)が100であるポリオキシエチレン硬化ヒマシ油の誘導体(HCO-100)を、種々の割合で60℃の水に滴下して撹拌した。その混合物の状態を表1に示す。 (Reference example)
The aforementioned polyoxyethylene hydrogenated castor oil derivative (HCO-100) having an average addition mole number (E) of ethylene oxide in the general formula 1 of 100 was added dropwise to water at 60 ° C. in various proportions and stirred. The state of the mixture is shown in Table 1.
前述した一般式1におけるエチレンオキシドの平均付加モル数(E)が100であるポリオキシエチレン硬化ヒマシ油の誘導体(HCO-100)を、種々の割合で60℃の水に滴下して撹拌した。その混合物の状態を表1に示す。 (Reference example)
The aforementioned polyoxyethylene hydrogenated castor oil derivative (HCO-100) having an average addition mole number (E) of ethylene oxide in the general formula 1 of 100 was added dropwise to water at 60 ° C. in various proportions and stirred. The state of the mixture is shown in Table 1.
次に、HCO-100、水及びエタノール又はイソプロピルアルコールを7:1.5:1.5(質量比)の比率で混合した。すると、エタノール及びイソプロピルアルコールのいずれを用いた場合も、混合物は均一な液体であった。従って、水のみの溶媒を用いた場合にはゲル化するような多量の両親媒性物質も、良溶媒を用いることで溶解できることが確認された。
Next, HCO-100, water and ethanol or isopropyl alcohol were mixed at a ratio of 7: 1.5: 1.5 (mass ratio). As a result, the mixture was a uniform liquid regardless of whether ethanol or isopropyl alcohol was used. Accordingly, it was confirmed that a large amount of amphiphilic substance that gels when a solvent containing only water can be dissolved by using a good solvent.
本発明者らが、各々の両親媒性物質に関し、溶解性に優れる良溶媒を調査した結果を次に示す。なお、表2において、○は溶液を形成したこと、×は溶液を形成できない又は沈殿を生じたことを示す。
The following shows the results of the investigation by the present inventors on a good solvent having excellent solubility for each amphiphile. In Table 2, o indicates that a solution was formed, and x indicates that the solution could not be formed or precipitation occurred.
<実施例1> ポリオキシエチレン硬化ヒマシ油の誘導体
HCO-10、水及びエタノールを7:1.5:1.5(質量比)の比率で混合して得られる溶液10mLを、25℃の水1Lに添加し、500rpmで5分間に亘って撹拌することによって混合した。得られた混合物について、粒度分布測定装置FPAR(大塚電子(株)社製)で粒度分布を測定したところ、親水性ナノ粒子の存在を示すピークが400nm付近の他、数μmの所にもピークが確認された。 <Example 1> Polyoxyethylene hydrogenated castor oil derivative 10 mL of a solution obtained by mixing HCO-10, water, and ethanol in a ratio of 7: 1.5: 1.5 (mass ratio) was added to 25 ° C. water. Added to 1 L and mixed by stirring at 500 rpm for 5 minutes. When the particle size distribution of the obtained mixture was measured with a particle size distribution analyzer FPAR (manufactured by Otsuka Electronics Co., Ltd.), the peak indicating the presence of hydrophilic nanoparticles was also around 400 nm and also at several μm. Was confirmed.
HCO-10、水及びエタノールを7:1.5:1.5(質量比)の比率で混合して得られる溶液10mLを、25℃の水1Lに添加し、500rpmで5分間に亘って撹拌することによって混合した。得られた混合物について、粒度分布測定装置FPAR(大塚電子(株)社製)で粒度分布を測定したところ、親水性ナノ粒子の存在を示すピークが400nm付近の他、数μmの所にもピークが確認された。 <Example 1> Polyoxyethylene hydrogenated castor oil derivative 10 mL of a solution obtained by mixing HCO-10, water, and ethanol in a ratio of 7: 1.5: 1.5 (mass ratio) was added to 25 ° C. water. Added to 1 L and mixed by stirring at 500 rpm for 5 minutes. When the particle size distribution of the obtained mixture was measured with a particle size distribution analyzer FPAR (manufactured by Otsuka Electronics Co., Ltd.), the peak indicating the presence of hydrophilic nanoparticles was also around 400 nm and also at several μm. Was confirmed.
(比較例)
前述した一般式1におけるエチレンオキシドの平均付加モル数(E)が10であるポリオキシエチレン硬化ヒマシ油の誘導体(HCO-10)を、60℃の水に滴下した。HCO-10の滴下量は、質量比1:9(HCO-10:水)とした。得られた混合物について、粒度分布測定装置FPAR(大塚電子(株)社製)で粒度分布を測定したところ、親水性ナノ粒子の存在を示すピークが400nm付近の他、数μmの所にもピークが確認された。 (Comparative example)
The polyoxyethylene hydrogenated castor oil derivative (HCO-10) having an average added mole number (E) of ethylene oxide in the general formula 1 described above of 10 was added dropwise to water at 60 ° C. The amount of HCO-10 added dropwise was 1: 9 (HCO-10: water). When the particle size distribution of the obtained mixture was measured with a particle size distribution analyzer FPAR (manufactured by Otsuka Electronics Co., Ltd.), the peak indicating the presence of hydrophilic nanoparticles was also around 400 nm and also at several μm. Was confirmed.
前述した一般式1におけるエチレンオキシドの平均付加モル数(E)が10であるポリオキシエチレン硬化ヒマシ油の誘導体(HCO-10)を、60℃の水に滴下した。HCO-10の滴下量は、質量比1:9(HCO-10:水)とした。得られた混合物について、粒度分布測定装置FPAR(大塚電子(株)社製)で粒度分布を測定したところ、親水性ナノ粒子の存在を示すピークが400nm付近の他、数μmの所にもピークが確認された。 (Comparative example)
The polyoxyethylene hydrogenated castor oil derivative (HCO-10) having an average added mole number (E) of ethylene oxide in the general formula 1 described above of 10 was added dropwise to water at 60 ° C. The amount of HCO-10 added dropwise was 1: 9 (HCO-10: water). When the particle size distribution of the obtained mixture was measured with a particle size distribution analyzer FPAR (manufactured by Otsuka Electronics Co., Ltd.), the peak indicating the presence of hydrophilic nanoparticles was also around 400 nm and also at several μm. Was confirmed.
(評価)
実施例1及び比較例の分散液を、表3及び4に示す各質量比でA-重油と、室温でホモミキサーを用いて、8000rpmで約5分間撹拌して乳化し、その乳化状態を評価した。なお、評価基準は、次の通りである。
○:相分離なし、△:比重差による分離(コアセルベーション)、×:分離
1:O/W型エマルション、2:W/O型エマルション、3:W/Oエマルションと分離水相 (Evaluation)
The dispersions of Example 1 and Comparative Example were emulsified with A-heavy oil at the respective mass ratios shown in Tables 3 and 4 by stirring at 8000 rpm for about 5 minutes using a homomixer at room temperature, and the emulsified state was evaluated. did. The evaluation criteria are as follows.
○: No phase separation, Δ: Separation by specific gravity difference (coacervation), ×: Separation 1: O / W emulsion, 2: W / O emulsion, 3: W / O emulsion and separated water phase
実施例1及び比較例の分散液を、表3及び4に示す各質量比でA-重油と、室温でホモミキサーを用いて、8000rpmで約5分間撹拌して乳化し、その乳化状態を評価した。なお、評価基準は、次の通りである。
○:相分離なし、△:比重差による分離(コアセルベーション)、×:分離
1:O/W型エマルション、2:W/O型エマルション、3:W/Oエマルションと分離水相 (Evaluation)
The dispersions of Example 1 and Comparative Example were emulsified with A-heavy oil at the respective mass ratios shown in Tables 3 and 4 by stirring at 8000 rpm for about 5 minutes using a homomixer at room temperature, and the emulsified state was evaluated. did. The evaluation criteria are as follows.
○: No phase separation, Δ: Separation by specific gravity difference (coacervation), ×: Separation 1: O / W emulsion, 2: W / O emulsion, 3: W / O emulsion and separated water phase
表3及び4に示されるように、比較例の分散液では、80質量%以上のA重油を乳化できず、また60~70質量%のA重油の乳化安定性が不充分であった。これに対し、実施例1の分散液では、あらゆる量、少なくとも95質量%以下の量のA重油が、安定的にかつ良好に乳化された。
As shown in Tables 3 and 4, the dispersion of Comparative Example could not emulsify 80% by mass or more of A heavy oil, and the emulsion stability of 60 to 70% by mass of A heavy oil was insufficient. On the other hand, in the dispersion liquid of Example 1, A heavy oil of any amount, at least 95% by mass or less, was stably and satisfactorily emulsified.
<実施例2> リン脂質
表2に示す、水素化レシチンのクロロホルム溶液を、実施例1と同様の手順で、水と混合した。得られた混合物について、実施例1と同様に粒度分布を測定したところ、親水性ナノ粒子の存在を示すピークが100nm付近に確認された。 <Example 2> Phospholipid A chloroform solution of hydrogenated lecithin shown in Table 2 was mixed with water in the same procedure as in Example 1. About the obtained mixture, when the particle size distribution was measured like Example 1, the peak which shows presence of a hydrophilic nanoparticle was confirmed by 100 nm vicinity.
表2に示す、水素化レシチンのクロロホルム溶液を、実施例1と同様の手順で、水と混合した。得られた混合物について、実施例1と同様に粒度分布を測定したところ、親水性ナノ粒子の存在を示すピークが100nm付近に確認された。 <Example 2> Phospholipid A chloroform solution of hydrogenated lecithin shown in Table 2 was mixed with water in the same procedure as in Example 1. About the obtained mixture, when the particle size distribution was measured like Example 1, the peak which shows presence of a hydrophilic nanoparticle was confirmed by 100 nm vicinity.
得られた分散液を、表5に示す条件の範囲で各油と実施例1と同様に撹拌して乳化し、その乳化状態を評価した。評価基準は前述のとおりである。
The obtained dispersion was stirred and emulsified in the same manner as in Example 1 with each oil within the range shown in Table 5, and the emulsified state was evaluated. The evaluation criteria are as described above.
表5に示されるように、実施例2の分散液は、種々の油を幅広い範囲の混合量比に亘って安定に乳化した。
As shown in Table 5, the dispersion liquid of Example 2 stably emulsified various oils over a wide range of mixing ratios.
Claims (7)
- 乳化物の製造に用いられる親水性ナノ粒子の製造方法であって、
両親媒性物質を、前記両親媒性物質に対する良溶媒に溶解させる調液工程と、
前記調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として前記両親媒性物質を析出させる析出工程と、を有する親水性ナノ粒子の製造方法。 A method for producing hydrophilic nanoparticles used in the production of an emulsion,
A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance;
A method of producing hydrophilic nanoparticles comprising: a precipitation step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water. - 前記両親媒性物質が、下記の一般式で表されるポリオキシエチレン硬化ヒマシ油の誘導体であって、前記誘導体のエチレンオキシドの平均付加モル数(E)が3~100である請求項1に記載の親水性ナノ粒子の製造方法。
- 前記調液工程では、前記ポリオキシエチレン硬化ヒマシ油の誘導体に対して0.1~0.33モル分率の界面活性剤を更に溶解させる請求項2に記載の親水性ナノ粒子の製造方法。 The method for producing hydrophilic nanoparticles according to claim 2, wherein, in the liquid preparation step, a surfactant having a 0.1 to 0.33 mole fraction is further dissolved in the polyoxyethylene hydrogenated castor oil derivative.
- 前記良溶媒が、メタノール、エタノール、イソプロパノール、イソブタノール、ter-ブタノール、sec-ブタノール、ペンタノール、メチルエチルケトン、1,3-プロパンジオール、2-(2-エトキシエトキシ)エタノール、1,4-ジオキサン、ジエチレンオキシド、エチレングリコール、プロピレングリコール、ブチレングリコール、アセトン、及びテトラヒドロフランからなる群から選ばれる1種若しくは2種以上である溶媒、又は該溶媒と水との混合溶媒である請求項1から3いずれかに記載の親水性ナノ粒子の製造方法。 The good solvent is methanol, ethanol, isopropanol, isobutanol, ter-butanol, sec-butanol, pentanol, methyl ethyl ketone, 1,3-propanediol, 2- (2-ethoxyethoxy) ethanol, 1,4-dioxane, 4. The solvent according to claim 1, which is one or more solvents selected from the group consisting of diethylene oxide, ethylene glycol, propylene glycol, butylene glycol, acetone, and tetrahydrofuran, or a mixed solvent of the solvent and water. The manufacturing method of the hydrophilic nanoparticle of description.
- 乳化作用を有する親水性ナノ粒子を含有する乳化剤の製造方法であって、
両親媒性物質を、前記両親媒性物質に対する良溶媒に溶解させる調液工程と、
前記調液工程において得られた溶液を水と混合することで、乳化作用を有する親水性ナノ粒子として前記両親媒性物質を析出させる析出工程と、を有する乳化剤の製造方法。 A method for producing an emulsifier containing hydrophilic nanoparticles having an emulsifying action,
A liquid preparation step of dissolving an amphiphilic substance in a good solvent for the amphiphilic substance;
A method for producing an emulsifier, comprising: a step of precipitating the amphiphilic substance as hydrophilic nanoparticles having an emulsifying action by mixing the solution obtained in the liquid preparation step with water. - 両親媒性物質を、前記両親媒性物質に対する良溶媒中に溶解状態で含有し、
水と混合することで、前記両親媒性物質が乳化作用を有する親水性ナノ粒子として析出する乳化剤調製液。 Containing the amphiphilic substance in a dissolved state in a good solvent for the amphiphilic substance,
An emulsifier preparation solution in which the amphiphilic substance is precipitated as hydrophilic nanoparticles having an emulsifying action by mixing with water. - 界面活性剤では乳化させることが困難な条件下で乳化物を製造するために、両親媒性物質により形成された親水性ナノ粒子を使用する方法。 A method of using hydrophilic nanoparticles formed of an amphiphilic substance to produce an emulsion under conditions that are difficult to emulsify with a surfactant.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011527914A JP5881042B2 (en) | 2010-06-23 | 2011-06-07 | Method for producing hydrophilic nanoparticles for producing emulsion |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-142968 | 2010-06-23 | ||
| JP2010142968 | 2010-06-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011162093A1 true WO2011162093A1 (en) | 2011-12-29 |
Family
ID=45371289
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/063015 WO2011162093A1 (en) | 2010-06-23 | 2011-06-07 | Process for producing emulsion-producing hydrophilic nanoparticles |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5881042B2 (en) |
| WO (1) | WO2011162093A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10186827B2 (en) | 2014-07-11 | 2019-01-22 | Trumpf Lasersystems For Semiconductor Manufacturing Gmbh | Amplifying pulsed laser radiation for EUV radiation production |
| US10638589B2 (en) | 2015-11-27 | 2020-04-28 | Trumpf Lasersystems For Semiconductor Manufacturing Gmbh | Amplifying laser pulses having different wavelengths for EUV radiation generation |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63116737A (en) * | 1986-07-15 | 1988-05-21 | シラグ・リミテツド | Manufacture of one membrane ribosome |
| JP2002536316A (en) * | 1999-02-08 | 2002-10-29 | アルザ・コーポレーション | How to control liposome size |
| JP2004130300A (en) * | 2002-08-12 | 2004-04-30 | Univ Kanagawa | Mixed vesicle, emulsion using same, and preparation process therefor |
| JP2006239666A (en) * | 2004-04-05 | 2006-09-14 | Univ Kanagawa | Emulsifying dispersant, method for emulsification and dispersion using it, and emulsion |
| WO2010064678A1 (en) * | 2008-12-03 | 2010-06-10 | 株式会社 資生堂 | Oil-in-water cosmetic |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1964778B (en) * | 2004-04-05 | 2011-10-19 | 学校法人神奈川大学 | Emulsifying dispersant, method for emulsification and dispersion using it, and emulsion and emulsion fuel |
| WO2006121017A1 (en) * | 2005-05-09 | 2006-11-16 | Fujifilm Corporation | Method for producing organic particle |
| JP2007061688A (en) * | 2005-08-29 | 2007-03-15 | Mitsubishi Chemicals Corp | Method of searching preparation condition of fine particle made of organic compound |
-
2011
- 2011-06-07 JP JP2011527914A patent/JP5881042B2/en active Active
- 2011-06-07 WO PCT/JP2011/063015 patent/WO2011162093A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63116737A (en) * | 1986-07-15 | 1988-05-21 | シラグ・リミテツド | Manufacture of one membrane ribosome |
| JP2002536316A (en) * | 1999-02-08 | 2002-10-29 | アルザ・コーポレーション | How to control liposome size |
| JP2004130300A (en) * | 2002-08-12 | 2004-04-30 | Univ Kanagawa | Mixed vesicle, emulsion using same, and preparation process therefor |
| JP2006239666A (en) * | 2004-04-05 | 2006-09-14 | Univ Kanagawa | Emulsifying dispersant, method for emulsification and dispersion using it, and emulsion |
| WO2010064678A1 (en) * | 2008-12-03 | 2010-06-10 | 株式会社 資生堂 | Oil-in-water cosmetic |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10186827B2 (en) | 2014-07-11 | 2019-01-22 | Trumpf Lasersystems For Semiconductor Manufacturing Gmbh | Amplifying pulsed laser radiation for EUV radiation production |
| US10638589B2 (en) | 2015-11-27 | 2020-04-28 | Trumpf Lasersystems For Semiconductor Manufacturing Gmbh | Amplifying laser pulses having different wavelengths for EUV radiation generation |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2011162093A1 (en) | 2013-08-19 |
| JP5881042B2 (en) | 2016-03-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3855203B2 (en) | Emulsifying dispersant, emulsifying dispersion method using the same, and emulsion | |
| US11708538B2 (en) | Emulsification dispersants, a method for emulsification and dispersion using the emulsification dispersants, emulsions, and emulsion fuels | |
| US9366387B2 (en) | Process of preparing improved heavy and extra heavy crude oil emulsions by use of biosurfactants in water and product thereof | |
| CN1324112C (en) | Functionalized cubic liquid crystalline phase materials and methods for their preparation and use | |
| JP6707727B2 (en) | Emulsion and method for producing emulsion | |
| Silva et al. | Size, Charge, and Stability of Fully Serine‐Based Catanionic Vesicles: Towards Versatile Biocompatible Nanocarriers | |
| CN103788402B (en) | A kind of carbon quantum dot/hectorite emulsion-stabilizing system and prepare the method for paraffin wax emulsions | |
| JPS5858392B2 (en) | Stabilized water-in-mineral oil emulsion | |
| KR102407260B1 (en) | Method of producing nanoparticle-in-oil dispersion | |
| JP2010104946A (en) | Water-in-oil (w/o) emulsion formed using reverse vesicle | |
| CN108159414B (en) | Water-in-oil adjuvant for animal vaccine and preparation method and application thereof | |
| US7001580B2 (en) | Emulsions including surface-modified organic molecules | |
| JP2007077178A (en) | Silicone type emulsion and its manufacturing method | |
| CN112108075A (en) | Pickering emulsifier and preparation method and application thereof | |
| JP5881042B2 (en) | Method for producing hydrophilic nanoparticles for producing emulsion | |
| JP2007074909A (en) | Edible emulsion and method for producing the same | |
| WO2023154747A1 (en) | Suspending systems constructed from hydrotropes | |
| TW201929835A (en) | Lipid particle dispersion | |
| JP3202984B2 (en) | O / W emulsion using lipophilic emulsifier | |
| CA2803770C (en) | Process for producing emulsifier-producing material, process for producing emulsifier, emulsifier for orally administered composition, and orally administered composition | |
| TWI510457B (en) | Aqueous dispersions of fatty amide | |
| RU2006139072A (en) | Emulsifying dispersants, a method of emulsifying and dispersing using emulsifying dispersants, emulsions and emulsive fuels | |
| JPH10298149A (en) | Dispersion of quaternary ammonium salt at high concentration and its preparation | |
| CN104667772B (en) | A kind of heat-resistant salt-resistant water-in-oil nano-emulsion and preparation method thereof | |
| JP2012130856A (en) | Method for producing emulsifier production material, emulsifier production material, and method for producing emulsifier |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011527914 Country of ref document: JP |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11797979 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11797979 Country of ref document: EP Kind code of ref document: A1 |