WO2010030038A1 - Method for producing pulverized waste plastic and solid fuel or mineral reduction material - Google Patents
Method for producing pulverized waste plastic and solid fuel or mineral reduction material Download PDFInfo
- Publication number
- WO2010030038A1 WO2010030038A1 PCT/JP2009/066205 JP2009066205W WO2010030038A1 WO 2010030038 A1 WO2010030038 A1 WO 2010030038A1 JP 2009066205 W JP2009066205 W JP 2009066205W WO 2010030038 A1 WO2010030038 A1 WO 2010030038A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- waste plastic
- pulverized
- plastic
- temperature
- melting
- Prior art date
Links
- 229920003023 plastic Polymers 0.000 title claims abstract description 188
- 239000004033 plastic Substances 0.000 title claims abstract description 188
- 239000002699 waste material Substances 0.000 title claims abstract description 142
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 21
- 239000000463 material Substances 0.000 title claims description 30
- 239000004449 solid propellant Substances 0.000 title claims description 16
- 229910052500 inorganic mineral Inorganic materials 0.000 title 1
- 239000011707 mineral Substances 0.000 title 1
- 238000002844 melting Methods 0.000 claims abstract description 46
- 230000008018 melting Effects 0.000 claims abstract description 46
- 238000004898 kneading Methods 0.000 claims abstract description 45
- 238000000354 decomposition reaction Methods 0.000 claims abstract description 21
- 239000002245 particle Substances 0.000 claims description 42
- 238000010298 pulverizing process Methods 0.000 claims description 34
- 239000007787 solid Substances 0.000 claims description 23
- 239000008187 granular material Substances 0.000 claims description 9
- 239000007789 gas Substances 0.000 abstract description 36
- 239000000047 product Substances 0.000 abstract 1
- 239000012265 solid product Substances 0.000 abstract 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 39
- 239000000460 chlorine Substances 0.000 description 39
- 229910052801 chlorine Inorganic materials 0.000 description 39
- 238000000034 method Methods 0.000 description 32
- 230000000052 comparative effect Effects 0.000 description 13
- 238000006298 dechlorination reaction Methods 0.000 description 13
- 238000012545 processing Methods 0.000 description 13
- 239000010903 husk Substances 0.000 description 12
- 238000010438 heat treatment Methods 0.000 description 11
- 241000209094 Oryza Species 0.000 description 10
- 235000007164 Oryza sativa Nutrition 0.000 description 10
- 238000001816 cooling Methods 0.000 description 10
- 238000005259 measurement Methods 0.000 description 10
- 235000009566 rice Nutrition 0.000 description 10
- 238000009826 distribution Methods 0.000 description 9
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- -1 etc. Substances 0.000 description 7
- 239000000446 fuel Substances 0.000 description 7
- 239000004800 polyvinyl chloride Substances 0.000 description 7
- 229920000915 polyvinyl chloride Polymers 0.000 description 7
- 239000000843 powder Substances 0.000 description 7
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 6
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 description 6
- 239000003245 coal Substances 0.000 description 6
- 238000002485 combustion reaction Methods 0.000 description 6
- 239000004698 Polyethylene Substances 0.000 description 5
- 238000004364 calculation method Methods 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 229920000573 polyethylene Polymers 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 4
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 4
- 229920001684 low density polyethylene Polymers 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 239000010816 packaging waste Substances 0.000 description 4
- 239000013618 particulate matter Substances 0.000 description 4
- 239000002994 raw material Substances 0.000 description 4
- 229920005989 resin Polymers 0.000 description 4
- 239000011347 resin Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000002023 wood Substances 0.000 description 4
- 239000004743 Polypropylene Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 229910052742 iron Inorganic materials 0.000 description 3
- 239000004702 low-density polyethylene Substances 0.000 description 3
- 239000000155 melt Substances 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 238000000465 moulding Methods 0.000 description 3
- 239000005020 polyethylene terephthalate Substances 0.000 description 3
- 229920000139 polyethylene terephthalate Polymers 0.000 description 3
- 229920001155 polypropylene Polymers 0.000 description 3
- 239000002028 Biomass Substances 0.000 description 2
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000000571 coke Substances 0.000 description 2
- 230000007797 corrosion Effects 0.000 description 2
- 238000005260 corrosion Methods 0.000 description 2
- 239000000428 dust Substances 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 239000002440 industrial waste Substances 0.000 description 2
- 229920005610 lignin Polymers 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000005416 organic matter Substances 0.000 description 2
- 239000005022 packaging material Substances 0.000 description 2
- 238000007711 solidification Methods 0.000 description 2
- 230000008023 solidification Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 235000017166 Bambusa arundinacea Nutrition 0.000 description 1
- 235000017491 Bambusa tulda Nutrition 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 239000004709 Chlorinated polyethylene Substances 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- HIZCTWCPHWUPFU-UHFFFAOYSA-N Glycerol tribenzoate Chemical compound C=1C=CC=CC=1C(=O)OCC(OC(=O)C=1C=CC=CC=1)COC(=O)C1=CC=CC=C1 HIZCTWCPHWUPFU-UHFFFAOYSA-N 0.000 description 1
- 229920002488 Hemicellulose Polymers 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 244000082204 Phyllostachys viridis Species 0.000 description 1
- 235000015334 Phyllostachys viridis Nutrition 0.000 description 1
- 229910000805 Pig iron Inorganic materials 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 229920001328 Polyvinylidene chloride Polymers 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 239000010426 asphalt Substances 0.000 description 1
- 239000011425 bamboo Substances 0.000 description 1
- 229910001570 bauxite Inorganic materials 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 239000003610 charcoal Substances 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000007033 dehydrochlorination reaction Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000002803 fossil fuel Substances 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000010309 melting process Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 238000010137 moulding (plastic) Methods 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 239000011236 particulate material Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000005033 polyvinylidene chloride Substances 0.000 description 1
- 238000010248 power generation Methods 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 238000000197 pyrolysis Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000012958 reprocessing Methods 0.000 description 1
- 238000010008 shearing Methods 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000002893 slag Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 238000000859 sublimation Methods 0.000 description 1
- 230000008022 sublimation Effects 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- 229920001187 thermosetting polymer Polymers 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B7/00—Working up raw materials other than ores, e.g. scrap, to produce non-ferrous metals and compounds thereof; Methods of a general interest or applied to the winning of more than two metals
- C22B7/001—Dry processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B09—DISPOSAL OF SOLID WASTE; RECLAMATION OF CONTAMINATED SOIL
- B09B—DISPOSAL OF SOLID WASTE NOT OTHERWISE PROVIDED FOR
- B09B3/00—Destroying solid waste or transforming solid waste into something useful or harmless
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B02—CRUSHING, PULVERISING, OR DISINTEGRATING; PREPARATORY TREATMENT OF GRAIN FOR MILLING
- B02C—CRUSHING, PULVERISING, OR DISINTEGRATING IN GENERAL; MILLING GRAIN
- B02C23/00—Auxiliary methods or auxiliary devices or accessories specially adapted for crushing or disintegrating not provided for in preceding groups or not specially adapted to apparatus covered by a single preceding group
- B02C23/08—Separating or sorting of material, associated with crushing or disintegrating
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L5/00—Solid fuels
- C10L5/40—Solid fuels essentially based on materials of non-mineral origin
- C10L5/46—Solid fuels essentially based on materials of non-mineral origin on sewage, house, or town refuse
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L5/00—Solid fuels
- C10L5/40—Solid fuels essentially based on materials of non-mineral origin
- C10L5/48—Solid fuels essentially based on materials of non-mineral origin on industrial residues and waste materials
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/10—Dry methods smelting of sulfides or formation of mattes by solid carbonaceous reducing agents
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/10—Biofuels, e.g. bio-diesel
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/30—Fuel from waste, e.g. synthetic alcohol or diesel
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Definitions
- the present invention relates to a method for producing a waste plastic pulverized product for producing ore reducing material, solid fuel and the like by reprocessing waste plastic.
- the conventional technology for converting plastic to solid fuel is, for example, directly pulverizing plastic with a pulverizer (see, for example, Non-Patent Document 1).
- a pulverizer see, for example, Non-Patent Document 1.
- hard plastic can only be pulverized to a particle size of 1 to 2 mm, and this pulverization requires a lot of time and cost, and fiber and film plastics are difficult to pulverize. For this reason, there is a problem that the process must be pulverized after being melted and solidified, resulting in a complicated process.
- waste plastic when waste plastic is finely pulverized, it can be made into a fuel that can be used as a combustion furnace such as a power generation boiler (see, for example, Patent Document 1). Is not described, and there is no technical disclosure regarding improvement of grindability of waste plastic itself.
- Plastic having a particle size of 2000 ⁇ m or less is excellent as a solid fuel, and is known to be produced by a dry pulverization method or a wet pulverization method (for example, see Patent Document 2).
- a jet mill or a vibration ball mill is suitable as a method for obtaining fine powder, but a technique for improving the pulverization property of waste plastic itself is not known.
- waste plastic is heated, dechlorinated, cooled and solidified, then pulverized and put into a furnace (for example, techniques such as Patent Document 4 and Patent Document 5) are known.
- plastics are excellent in impact resistance, so it is very difficult to pulverize them as they are.
- the pulverization time is lengthened or repeatedly put into the pulverizer several times. The method of doing is taken.
- the plastic is heated by shearing heat generation during pulverization and melts or the pulverized product becomes fibrous. In order to prevent this phenomenon, it is conceivable to cool the pulverizer or the pulverized product, but the equipment cost and the operating cost increase.
- So-called freeze pulverization is also an effective method for pulverizing plastic. That is, it is a method in which a plastic is cooled to a temperature range where a general plastic causes brittle fracture, for example, tens of degrees below zero, and then pulverized.
- a general plastic causes brittle fracture, for example, tens of degrees below zero
- pulverized since this method requires the plastic to be cooled in advance, the equipment cost and operation cost increase.
- the object of the present invention is to solve such problems of the prior art, and to pulverize waste plastic at a low cost to obtain a pulverized product, and to improve the productivity of the pulverized product. It is in providing the manufacturing method of a thing.
- Waste plastic is melted at a temperature higher than the softening melting temperature and at a temperature at which flammable decomposition gas is not generated, and further kneaded at a shear rate of 100 (1 / second) or more, and then cooled and solidified to obtain a solidified body.
- a method for producing a waste plastic pulverized product wherein the solidified product is pulverized.
- the waste plastic is melted at 160 ° C.
- a solid fuel or ore reducing material having excellent combustibility can be produced at low cost using waste plastic as a raw material without providing facilities for treating exhaust gas from waste plastic.
- Productivity of waste plastic crushed material is also improved.
- waste plastic processing method of the present invention by using the waste plastic processing method of the present invention, a large amount of waste plastic can be economically implemented.
- the present invention cools waste plastics and container packaging materials contained in municipal waste, industrial waste, general waste, etc., and waste plastics generated in the process of dismantling electrical appliances, automobiles, etc. after heating, melting and kneading.
- the present invention relates to a technology for producing a solidified body, pulverizing the solidified body, and producing a solid fuel, an ore reducing material, and the like.
- waste plastic is a mixture of a plurality of types of plastics, and it is effective to melt and knead various types of plastics as a method for improving the pulverization properties of waste plastics and easily pulverizing them. Since most waste plastics contain chlorine by mixing PVC or the like, conventionally, waste plastic is melted and kneaded at a high temperature that generates chlorine gas to generate chlorine-containing gas, Dechlorination treatment to remove the chlorine component was performed.
- PET polyethylene terephthalate
- paper / wood cellulose, hemicellulose, lignin
- flammable decomposition gas flammable organic matter
- a method for melting and kneading at a relatively low temperature was examined in order to prevent generation of flammable decomposition gas generated when melting and kneading waste plastic.
- the problem was that chlorine could not be removed sufficiently from waste plastic when melted at a relatively low temperature, but if waste plastic with low chlorine content is used, waste plastic with low chlorine concentration without dechlorination treatment. A solidified body and a pulverized product can be produced. Therefore, in the present invention, it is preferable to use a waste plastic having a low chlorine content (for example, a chlorine concentration of 2 mass% or less).
- the solid yield after the treatment is increased.
- the residual chlorine concentration in the solidified material after the treatment is somewhat higher.
- the calorific value of the solidified body is considered to increase by the amount of chlorine.
- the organic matter decomposes and separates as a side reaction, the calorific value of the solidified body after treatment is rather small. is there. Therefore, by performing the heat treatment of the waste plastic at a temperature equal to or higher than the softening melting temperature and not generating flammable decomposition gas, the calorific value of the solidified body does not decrease.
- Patent Document 4 for example, according to Patent Document 4, the heating temperature when waste plastic is heated, melted, cooled, and solidified is increased to a temperature for removing low-boiling compounds originally contained and / or generated by thermal decomposition.
- Heating is a major premise, specifically 150 to 450 ° C., more preferably 200 to 400 ° C., and even more preferably 250 to 380 ° C. In such a temperature range, combustible decomposition gas is generated together with hydrogen chloride by heating at a high temperature.
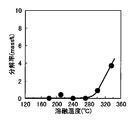
- the generation of waste plastic decomposition gas is very low up to 270 ° C. Therefore, the waste plastic is heated, melted and kneaded below the temperature at which such cracked gas is generated, and cooled and solidified, so that no cracked gas treatment equipment such as a combustion furnace is required, and the waste plastic is processed at low cost.
- a waste plastic solidified body can be produced.
- the waste plastic treated in the present invention uses a low chlorine concentration. It is preferable.
- a waste plastic having a chlorine concentration of 2 mass% or less treated by the method of the present invention can be sufficiently used for blowing blast furnace as an ore reducing material.
- the temperature at which the flammable decomposition gas is not generated varies slightly depending on the type of waste plastic, but in the normal case, it is preferably 270 ° C. or less from the result of FIG. Furthermore, if it is 200 degrees C or less, generation
- the temperature in this case is the highest temperature in the resin.
- the maximum temperature in the resin can be measured by a general method such as inserting a thermocouple into the resin.
- the temperature at which flammable decomposition gas is not generated is a temperature at which flammable organic substances are not substantially generated from waste plastic by heating, and is generated due to impurities in waste plastic (for example, liquefied gas in disposable lighters). Such gases are outside the scope of combustible cracked gas in this case.
- the lower limit of the melting temperature is set to a temperature at which the waste plastic is softened and melted so that the waste plastic can be kneaded well as described later.
- the softening and melting temperature varies depending on the type of waste plastic, but since the main components of ordinary waste plastic are polyethylene (PE) and polypropylene (PP), the lower limit of the softening and melting temperature is preferably 160 ° C. If the waste plastic is mainly composed of PE, the temperature can be set to 120 ° C.
- the waste plastic to be treated is a mixture of various plastics. That is, the only plastic that melts below 160 ° C is mainly PE, and if it is processed forcibly, the required power required for kneading becomes extremely large, and a blockage occurs in the processing apparatus, making it difficult to continue the processing. It may become.
- “Melting and further kneading” the waste plastic means that the waste plastic is kneaded simultaneously with the melting of the waste plastic or kneaded in a molten state after the melting.
- the kneading is performed at a shear rate of at least 100 (1 / second) or more.
- the molding shrinkage of different plastics differs depending on the plastic, and during the cooling process, the different plastics shrink according to their molding shrinkage and the bonding interface is separated.
- the inside must be evacuated (vacuum voids), and when void formation does not occur, a stress that "shall be separated but cannot be separated” remains at the bonding interface (residual stress).
- a dissimilar plastic highly dispersed by sufficient kneading has many adhesion interfaces, and the solidified body necessarily has a strong residual stress. When an impact is applied to the solidified body, stress concentration occurs at these different plastic bonding interfaces, and a breakage starting point is generated relatively easily.
- the entire structure becomes brittle and the grindability is improved.
- differences in dissolution parameters and the like also affect pulverizability improvement.
- Solid particulates other than waste plastics are also incompatible with plastics in most cases, so they are discarded before waste plastic melting and kneading, or during melting and kneading, or both before melting and kneading and during melting and kneading.
- solid particulates other than plastic By mixing solid particulates other than plastic with waste plastic and kneading with the waste plastic, after solidification by cooling, the solid particulate matter becomes a starting point of destruction, and the pulverization property of the solidified body can be further improved.
- a tumbler or the like may be used to mix waste plastic and solid particulate matter in advance.
- waste plastic and solid particulates may be separately supplied to the melting and kneading apparatus and mixed in the apparatus, or one of them is supplied first and then the other is supplied. May be.
- the melting point of the solid granular material is higher than the melting start temperature of the waste plastic, it is preferable to add the solid granular material after supplying the waste plastic first and achieving a certain molten state.
- Solid particulates include fossil fuels such as coal, coke and asphalt, synthetic polymers such as virgin plastic, natural polymers such as starch and cellulose, metals such as iron and aluminum, and those ores such as iron ore and bauxite, Examples include inorganic substances such as mica and talc.
- biomass solids such as waste derived from agricultural products, such as rice husk, tea husk, and coffee husk (rice husk), and lignin-containing plants such as wood, charcoal, and bamboo, as the solid particulate matter.
- wastes derived from agricultural crops and waste wood such as thinned wood and construction waste.
- the biomass solid contains ash and is likely to be a starting point of destruction.
- the rice husk tends to be elongated, and is further pulverized when kneading molten waste plastic, so that it can be effectively used as a starting point for destruction.
- the compounding quantity of the solid granular material in this case shall be less than 100 mass parts of solid granular materials with respect to 100 mass parts of waste plastics.
- the solid granular material is 100 parts by mass or more, the waste plastic is pulverized after the kneading process, and handling becomes difficult. Moreover, it is preferable to set it as 5 mass parts or more of solid granular materials with respect to 100 mass parts of waste plastics. This is because the effect of adding the solid particulate matter can be exhibited better. These solid particulate materials can be used if they can be introduced into the processing apparatus.
- the waste plastic can be heated and kneaded at a shear rate of 100 (1 / second) or more at the same time.
- the extruder can be further improved in kneadability by using a biaxial extruder.
- waste plastic pulverized product By crushing the produced waste plastic solidified body, waste plastic pulverized product can be produced, and ore reducing material and solid fuel can be produced.
- the solidified product cooled and solidified after heating and melting has improved pulverization properties, and a fine powder having a particle size of 2 mm or less can be easily produced using a normal pulverizer.
- the crushed solidified body is preferably passed through a sieve to adjust the particle size.
- the particle size after pulverization can be set by appropriately changing the sieve mesh. For example, fine powder which is a waste plastic pulverized product having a particle size of 0.5 mm or less can be obtained by passing through a sieve having an aperture of 0.5 mm using the production method of the present invention.
- Waste plastics subject to the present invention that is, raw material plastics in the present invention include waste plastics and container packaging materials contained in municipal waste, industrial waste, general waste, etc., and dismantling of electrical products, automobiles, etc. Examples include waste plastic generated in the process.
- polyolefins such as polyethylene and polypropylene, polyvinyl chloride, polyvinylidene chloride, chlorinated polyethylene, polystyrene, polyethylene terephthalate, polycarbonate, nylon, and other thermoplastic resins and thermosetting resins are all applicable.
- a plastic material in which any two or more of the plastics are mixed is used. Normally, it contains some chlorine-containing plastic due to the nature of waste plastic, which is waste, but the present invention can be used even with waste plastic that does not contain chlorine-containing plastic.
- the shape of the plastic to be heat-treated may be roughly pulverized, and a size of about 10 cm square is sufficient.
- For general waste plastic it is not necessary to pulverize again, and it is processed in the recovered state. Yes, film, sheet, and fiber plastics can be processed as they are. Of course, it may be finely pulverized, but the processing cost increases accordingly.
- the melting / kneading step examples include the following steps. That is, the waste plastic is melted at 160 ° C. or higher at a temperature at which combustible decomposition gas is not generated in a reactor or an extruder.
- the temperature at which the combustible decomposition gas is not generated varies depending on the composition of the waste plastic, but the upper limit is usually 270 ° C. In the temperature range where the combustible decomposition gas is not generated, it is preferable to melt at a higher temperature, preferably 200 ° C. or higher, more preferably 230 ° C. or higher.
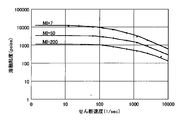
- MI Melt Index
- the molten plastic is a non-Newtonian fluid, and as shown in FIG. 2, the melt viscosity of low density polyethylene (LDPE) is substantially constant in the region where the shear rate is low.
- LDPE low density polyethylene
- kneading in a region where the shear rate is 100 (1 / second) or more is extremely effective for lowering kneading power and improving kneading efficiency.
- the melting and kneading treatment may be a batch type or a continuous type. An intermediate type such as batch switching may also be used.
- an extruder is preferable, and a twin screw extruder is more preferable from the viewpoint of kneadability.
- Processing time is suitably 0.5 minutes to 30 hours.
- the treatment time is less than 0.5 minutes, it is difficult to control the temperature in the reactor, and it is difficult to sufficiently knead the molten waste plastic product.
- processing time exceeds 30 hours, processing efficiency falls and it is not economical.
- a heat medium In the melting / kneading process, a heat medium can be allowed to coexist.
- the waste plastic after the melting treatment is cooled and solidified by supplying a fixed amount of the molten plastic to a belt cooler by a molten plastic conveying device.
- the amount of heat removal is calculated from the amount of enthalpy between the temperature after heat treatment and the temperature until fully solidified, and the treatment speed.
- the center temperature after cooling is about 110 ° C. It is sufficient to control so that
- a continuous heating and melting apparatus when using a continuous heating and melting apparatus, it can be cooled at the outlet of the apparatus, or it can be cooled without being cut, such as air cooling or charging into water.
- the solidified body that has undergone the cooling and solidification step is preferably pulverized so as to have a predetermined particle size.
- the pulverization of the plastic treated product which is a cooled solidified body obtained by the above-described method of the present invention, can be performed very easily as compared with the pulverization of untreated plastic. That is, the processed plastic product obtained by the method of the present invention can be pulverized by any type of pulverizer, and for example, a jaw crusher, a roll crusher, a ball mill, a centrifugal mill, or the like can be used.
- the particle size after pulverization may be determined according to the purpose of use of the processed plastic. If the particle size is adjusted to a predetermined particle size, for example, ore reducing material such as iron ore, that is, pig iron such as blast furnace is produced. It can be used as raw fuel such as reducing material for vertical furnaces, reducing material for converters, combustion fuel such as boilers and kilns, cupola fuel, and raw materials for coke ovens. Moreover, it can be used as a solid fuel other than the above-mentioned use.
- ore reducing material such as iron ore
- pig iron such as blast furnace
- the solidified body yield was calculated from the raw material charge amount and the solidified body recovered amount at this time. Further, the chlorine concentration in this solidified body (solidified body residual chlorine concentration) and the calorific value of the solidified body were measured. The results are shown in Table 1.
- This solidified body was coarsely pulverized by a small pulverizer (cutter mill) manufactured by Horai Co., Ltd., and passed through a 9 mm screen.
- the coarsely pulverized product was finely pulverized with an ACM pulverizer (hammer mill) manufactured by Hosokawa Micron Corporation, and then subjected to a classification test with a test sieve to measure the particle size distribution. The results are shown in Table 2.
- the average particle size was calculated from the particle size distribution.
- R (Dp) is the mass% on the integrated sieve of the sieve mesh Dp
- De is the particle size characteristic number [R (Dp) is the number corresponding to mass%]
- n is an equal number ( Index for evaluating the uniformity of the particle size distribution of the granular material)
- b is a constant and indicates an index for evaluating the fineness of the granular material.
- the average particle size of the waste plastic pulverized product of Example 1 of the present invention that was melt-treated at 180 ° C. was less than 500 ⁇ m and was sufficiently fine.
- Invention Example 2 The same operation as in Invention Example 1 was conducted except that 70 parts by mass of general waste container packaging waste plastic pulverized to about 1 cm and 30 parts by mass of rice husk were mixed and supplied to the twin screw extruder. The solidified product and the pulverized product thereof were manufactured. Tables 1 and 2 show the results of measurement of the solidified body yield, the residual chlorine concentration in the solidified body, the calorific value of the solidified body, the calculation result of the average particle size, and the measurement result of the particle size distribution after pulverization.
- Invention Example 3 Invention Example except that 70 parts by mass of general waste container packaging waste plastic pulverized to about 1 cm and 30 parts by mass of coal (brand: Xinglongzhuang) were mixed and supplied to a twin screw extruder. The same operation as in No. 1 was performed to produce a solidified product and a pulverized product thereof. Tables 1 and 2 show the results of measurement of the solidified body yield, the residual chlorine concentration in the solidified body, the calorific value of the solidified body, the calculation result of the average particle size, and the measurement result of the particle size distribution after pulverization.
- the average particle size after pulverization is smaller, but this is because the added coal itself has better pulverizability than rice husk.
- Comparative Example 1 the residual chlorine concentration in the solidified body was sufficiently reduced. Moreover, although the average particle diameter is small and pulverized, the main cause was a high ratio of ultrafine powder of 75 ⁇ m or less.
- the ultra fine powder of 75 ⁇ m or less has safety problems such as dust explosiveness, and costs for countermeasures such as nitrogen filling are required.
- the average particle size was small and pulverized, but the main cause was a high proportion of ultrafine powder of 75 ⁇ m or less.
- the ultra fine powder of 75 ⁇ m or less has safety problems such as dust explosiveness, and costs for countermeasures such as nitrogen filling are required.
- Example 4 The same processing as in Example 1 of the present invention was attempted except that the processing temperature at the time of kneading with a twin-screw extruder was set to 140 ° C. However, the screw motor stopped due to overload about 5 minutes after the start of the treatment, and the treatment could not be continued. When the screw was extracted after cooling the twin screw extruder, unmelted waste plastic solid was clogged inside.
- Example 1 of the present invention all the pulverized products could be finely pulverized to an average particle size of 500 ⁇ m or less, despite being melted and kneaded at a low temperature. By adding rice husk and coal, the grindability is further improved.
- Example 1 of the present invention and Comparative Example 1 were compared, in Example 1 of the present invention, the residual chlorine concentration in the solidified body did not decrease, but the solidified body yield increased and the calorific value of the solidified body increased. This clearly improves the productivity of the pulverized product. In Comparative Example 1, this is thought to be due to the fact that terephthalic acid, which is a combustion component, is also removed during the dechlorination process, but the combustion component remains in the present invention example.
- Comparative Example 3 when Comparative Example 3 is compared with Comparative Example 1, the average particle size is increased despite the addition of rice husk, and at first glance, it seems that the grindability is lowered.
- Comparative Example 3 has a higher particle size ratio of 150 to 500 ⁇ m and 75 to 150 ⁇ m than Comparative Example 1, and the width of the particle size distribution is It is narrower and the grindability is improved. Therefore, the effect of “better crushability is exhibited by forming the starting point of fracture” by adding rice husk was recognized.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Geology (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Food Science & Technology (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Separation, Recovery Or Treatment Of Waste Materials Containing Plastics (AREA)
- Solid Fuels And Fuel-Associated Substances (AREA)
- Processing Of Solid Wastes (AREA)
Abstract
Description
(1)廃プラスチックを軟化溶融温度以上、かつ可燃性分解ガスが生成しない温度で溶融し、更に100(1/秒)以上の剪断速度で混練した後、冷却・固化して固化体とし、該固化体を粉砕することを特徴とする、廃プラスチック粉砕物の製造方法。
(2)軟化溶融温度が160℃、可燃性分解ガスが生成しない温度が270℃以下であることを特徴とする、(1)に記載の廃プラスチック粉砕物の製造方法。
(3)廃プラスチックを160℃~270℃で溶融し、更に100(1/秒)以上の剪断速度で混練した後、冷却・固化して固化体とし、該固化体を粉砕することを特徴とする、廃プラスチック粉砕物の製造方法。
(4)溶融・混練を、押し出し機を用いて行なうことを特徴とする、(1)ないし(3)のいずれかに記載の廃プラスチック粉砕物の製造方法。
(5)押し出し機が、二軸押し出し機であることを特徴とする、(4)に記載の廃プラスチック粉砕物の製造方法。
(6)廃プラスチックの溶融・混練の前および/または溶融・混練時に、廃プラスチック以外の固体粒状物を混合し、廃プラスチックと共に混練することを特徴とする、(1)ないし(5)のいずれかに記載の廃プラスチック粉砕物の製造方法。
(7)篩を通過させて粉砕した固化体の粒度を調整することを特徴とする、(1)ないし(6)のいずれかに記載の廃プラスチック粉砕物の製造方法。
(8)(1)ないし(7)のいずれかで製造される粉砕物である鉱石還元材または固体燃料。
(9)目開き0.5mmのふるいを通過することを特徴とする、(8)に記載の鉱石還元材または固体燃料。 The features of the present invention for solving such problems are as follows.
(1) Waste plastic is melted at a temperature higher than the softening melting temperature and at a temperature at which flammable decomposition gas is not generated, and further kneaded at a shear rate of 100 (1 / second) or more, and then cooled and solidified to obtain a solidified body. A method for producing a waste plastic pulverized product, wherein the solidified product is pulverized.
(2) The method for producing a waste plastic pulverized product according to (1), wherein the softening and melting temperature is 160 ° C., and the temperature at which the combustible decomposition gas is not generated is 270 ° C. or less.
(3) The waste plastic is melted at 160 ° C. to 270 ° C., kneaded at a shear rate of 100 (1 / second) or more, then cooled and solidified to form a solidified body, and the solidified body is pulverized. The manufacturing method of waste plastic ground material.
(4) The method for producing a pulverized waste plastic according to any one of (1) to (3), wherein melting and kneading are performed using an extruder.
(5) The method for producing a waste plastic pulverized product according to (4), wherein the extruder is a twin screw extruder.
(6) Any one of (1) to (5), wherein solid particulates other than waste plastic are mixed and kneaded together with waste plastic before and / or during melting and kneading of waste plastic A method for producing a waste plastic pulverized product according to
(7) The method for producing a pulverized waste plastic according to any one of (1) to (6), wherein the particle size of the solidified body that has been pulverized by passing through a sieve is adjusted.
(8) An ore reducing material or a solid fuel which is a pulverized product produced in any one of (1) to (7).
(9) The ore reducing material or solid fuel according to (8), which passes through a sieve having an aperture of 0.5 mm.
R(Dp)=100・exp{−(Dp/De)n}・・・(1)
log{log[100/R(Dp)]}=n・logDp+log(b)・・・(2)
上記式(1)、(2)中、R(Dp)は篩い目Dpの積算篩い上質量%、Deは粒度特性数〔R(Dp)は質量%に対応する数〕、nは均等数(粉粒体の粒度分布の均一性を評価する指数)、bは定数であり、粉粒体の微細性を評価する指数を示す。
R (Dp) = 100 · exp {− (Dp / De) n} (1)
log {log [100 / R (Dp)]} = n · logDp + log (b) (2)
In the above formulas (1) and (2), R (Dp) is the mass% on the integrated sieve of the sieve mesh Dp, De is the particle size characteristic number [R (Dp) is the number corresponding to mass%], and n is an equal number ( Index for evaluating the uniformity of the particle size distribution of the granular material), b is a constant and indicates an index for evaluating the fineness of the granular material.
Claims (9)
- 廃プラスチックを軟化溶融温度以上、かつ可燃性分解ガスが生成しない温度で溶融し、更に100(1/秒)以上の剪断速度で混練した後、冷却・固化して固化体とし、該固化体を粉砕することを特徴とする、廃プラスチック粉砕物の製造方法。 The waste plastic is melted at a temperature not lower than the softening melting temperature and does not generate flammable decomposition gas, and further kneaded at a shear rate of 100 (1 / second) or more, and then cooled and solidified to obtain a solidified body. A method for producing a pulverized waste plastic, characterized by pulverizing.
- 軟化溶融温度が160℃、可燃性分解ガスが生成しない温度が270℃以下であることを特徴とする、請求項1に記載の廃プラスチック粉砕物の製造方法。 The method for producing a waste plastic pulverized product according to claim 1, wherein the softening and melting temperature is 160 ° C, and the temperature at which flammable decomposition gas is not generated is 270 ° C or less.
- 廃プラスチックを160℃~270℃で溶融し、更に100(1/秒)以上の剪断速度で混練した後、冷却・固化して固化体とし、該固化体を粉砕することを特徴とする、廃プラスチック粉砕物の製造方法。 Waste plastic characterized in that waste plastic is melted at 160 ° C. to 270 ° C., kneaded at a shear rate of 100 (1 / second) or more, then cooled and solidified to form a solidified body, and the solidified body is pulverized. Manufacturing method of plastic pulverized material.
- 溶融・混練を、押し出し機を用いて行なうことを特徴とする、請求項1ないし請求項3のいずれかに記載の廃プラスチック粉砕物の製造方法。 The method for producing a waste plastic pulverized product according to any one of claims 1 to 3, wherein melting and kneading are performed using an extruder.
- 押し出し機が、二軸押し出し機であることを特徴とする、請求項4に記載の廃プラスチック粉砕物の製造方法。 The method for producing a waste plastic pulverized product according to claim 4, wherein the extruder is a twin screw extruder.
- 廃プラスチックの溶融・混練の前および/または溶融・混練時に、廃プラスチック以外の固体粒状物を混合し、廃プラスチックと共に混練することを特徴とする、請求項1ないし請求項5のいずれかに記載の廃プラスチック粉砕物の製造方法。 The solid granular material other than the waste plastic is mixed and kneaded together with the waste plastic before and / or during the melting / kneading of the waste plastic. Of waste plastic crushed material.
- 篩を通過させて粉砕した固化体の粒度を調整することを特徴とする、請求項1ないし請求項6のいずれかに記載の廃プラスチック粉砕物の製造方法。 The method for producing a waste plastic pulverized product according to any one of claims 1 to 6, wherein the particle size of the solidified product pulverized by passing through a sieve is adjusted.
- 請求項1ないし請求項7のいずれかで製造される粉砕物である鉱石還元材または固体燃料。 An ore reducing material or a solid fuel which is a pulverized product produced according to any one of claims 1 to 7.
- 目開き0.5mmのふるいを通過することを特徴とする、請求項8に記載の鉱石還元材または固体燃料。 The ore reducing material or solid fuel according to claim 8, wherein the ore reducing material or the solid fuel passes through a sieve having an opening of 0.5 mm.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2009801351801A CN102149526B (en) | 2008-09-12 | 2009-09-10 | Method for producing pulverized waste plastic and solid fuel or mineral reduction material |
| KR1020117004950A KR101134809B1 (en) | 2008-09-12 | 2009-09-10 | Method for producing pulverized waste plastic |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008-234187 | 2008-09-12 | ||
| JP2008234187 | 2008-09-12 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010030038A1 true WO2010030038A1 (en) | 2010-03-18 |
Family
ID=42005285
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2009/066205 WO2010030038A1 (en) | 2008-09-12 | 2009-09-10 | Method for producing pulverized waste plastic and solid fuel or mineral reduction material |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP4775485B2 (en) |
| KR (1) | KR101134809B1 (en) |
| CN (1) | CN102149526B (en) |
| WO (1) | WO2010030038A1 (en) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102500602B (en) * | 2011-11-07 | 2014-07-16 | 英利集团有限公司 | Equipment and method for recycling photovoltaic module |
| CN102416401B (en) * | 2011-11-07 | 2015-05-27 | 英利集团有限公司 | Process and equipment for recovering photovoltaic component through thermal high-speed centrifugal decomposition |
| KR101308153B1 (en) | 2013-04-11 | 2013-09-12 | 이승환 | Method of recylcing waste plastics containing natural fiber filler |
| CN104073630B (en) * | 2014-07-21 | 2016-05-25 | 安徽工业大学 | A kind of waste plastics is iron-based carbonaceous pelletizing of carbon source and preparation method thereof |
| TWI570164B (en) * | 2014-08-29 | 2017-02-11 | Shiung Fire Cremator Co Ltd | Method of making environmentally friendly electronic board by recycling plastic waste |
| CN105647611A (en) * | 2014-11-14 | 2016-06-08 | 何湘贤 | Fuel extractive |
| JP7417852B2 (en) * | 2018-10-19 | 2024-01-19 | 三菱マテリアル株式会社 | How to treat covered wires |
| AU2019457152A1 (en) * | 2019-07-12 | 2021-11-25 | Technique Co., Ltd. | Method for grinding plastic waste and method for manufacturing synthetic resin molded product using plastic waste |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09118888A (en) * | 1995-10-24 | 1997-05-06 | Kawasaki Heavy Ind Ltd | Method of converting waste plastic into granular fuel |
| JPH11140474A (en) * | 1997-11-04 | 1999-05-25 | Kawasaki Steel Corp | Production of plastic solid fuel |
| JPH11147973A (en) * | 1997-11-14 | 1999-06-02 | Nkk Corp | Method of treating thermosetting resin powder waste material and method of supplying the waste material to a furnace |
| JPH11197630A (en) * | 1997-11-12 | 1999-07-27 | Kawasaki Steel Corp | Treating method for plastic, and solid fuel and reducing agent for ore each obtained by the method |
| JPH11209767A (en) * | 1998-01-30 | 1999-08-03 | Ube Ammonia Kogyo Kk | How to use waste plastic |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2968855B2 (en) * | 1991-05-08 | 1999-11-02 | 株式会社ネオス | Plastics powder containing fuel |
| JPH07119922A (en) * | 1993-09-01 | 1995-05-12 | Mitsui Eng & Shipbuild Co Ltd | Waste plastic combustion equipment and waste plastic powder fuel |
| JP2002067029A (en) * | 2000-08-24 | 2002-03-05 | Nkk Corp | Method for recycling used plastics and molded articles for recycling |
| CN1162263C (en) * | 2001-12-18 | 2004-08-18 | 上海交通大学 | Method for recovering cross-linked polyethylene with twin-screw extruder |
-
2009
- 2009-09-10 CN CN2009801351801A patent/CN102149526B/en active Active
- 2009-09-10 JP JP2009208864A patent/JP4775485B2/en active Active
- 2009-09-10 KR KR1020117004950A patent/KR101134809B1/en active Active
- 2009-09-10 WO PCT/JP2009/066205 patent/WO2010030038A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09118888A (en) * | 1995-10-24 | 1997-05-06 | Kawasaki Heavy Ind Ltd | Method of converting waste plastic into granular fuel |
| JPH11140474A (en) * | 1997-11-04 | 1999-05-25 | Kawasaki Steel Corp | Production of plastic solid fuel |
| JPH11197630A (en) * | 1997-11-12 | 1999-07-27 | Kawasaki Steel Corp | Treating method for plastic, and solid fuel and reducing agent for ore each obtained by the method |
| JPH11147973A (en) * | 1997-11-14 | 1999-06-02 | Nkk Corp | Method of treating thermosetting resin powder waste material and method of supplying the waste material to a furnace |
| JPH11209767A (en) * | 1998-01-30 | 1999-08-03 | Ube Ammonia Kogyo Kk | How to use waste plastic |
Also Published As
| Publication number | Publication date |
|---|---|
| KR101134809B1 (en) | 2012-04-13 |
| KR20110036771A (en) | 2011-04-08 |
| CN102149526A (en) | 2011-08-10 |
| JP2010089500A (en) | 2010-04-22 |
| CN102149526B (en) | 2013-11-20 |
| JP4775485B2 (en) | 2011-09-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4775485B2 (en) | Method for producing waste plastic crushed material and solid fuel or ore reducing material | |
| JP2014030824A (en) | Mixed plastic powder and manufacturing method of the same | |
| JP5446061B2 (en) | Method for producing fine powder of mixed plastic, method for operating blast furnace, and method for treating waste plastic | |
| JP5759097B2 (en) | Solid fuel | |
| JP2015189023A (en) | Method for producing waste plastic crushed material | |
| CN113891945A (en) | Method for producing polymer products | |
| JP5582685B2 (en) | Solid fuel and method for producing solid fuel | |
| JP5759099B2 (en) | Solid fuel | |
| JP2011056789A (en) | Method for manufacturing waste plastics pulverized powder, and ore reducing agent or solid fuel | |
| JP2006241442A (en) | Process for treatment of waste plastics | |
| JP5652441B2 (en) | Blowing synthetic resin into blast furnace | |
| JP5303855B2 (en) | Method for producing coke for blast furnace using waste plastic | |
| JP5135964B2 (en) | Blowing synthetic resin into blast furnace | |
| JP3359559B2 (en) | Plastic processing method, solid fuel obtained by the processing method, ore reducing agent | |
| JP4424054B2 (en) | How to use plastic | |
| JP4640014B2 (en) | Method for producing ore reducing agent | |
| JP4998657B2 (en) | How to blow plastic into the furnace | |
| JP4807112B2 (en) | Blowing synthetic resin into blast furnace | |
| JP6428137B2 (en) | Recycling method of textile waste | |
| JP5272362B2 (en) | Waste plastic grinding method | |
| JP3290630B2 (en) | Plastic processing method, solid fuel obtained by the processing method, ore reducing agent | |
| JP2953412B2 (en) | Regeneration method of inorganic filler | |
| JP4457753B2 (en) | Coke production method using waste plastic | |
| JP2001354982A (en) | Fuel for cement production | |
| JP2005314748A (en) | Method for utilizing plastics |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200980135180.1 Country of ref document: CN |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09813180 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20117004950 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 09813180 Country of ref document: EP Kind code of ref document: A1 |