WO2006122806A2 - 1,3-dihydro-imidazo [4,5-c] quinolin-2-ones as lipid kinase inhibitors - Google Patents
1,3-dihydro-imidazo [4,5-c] quinolin-2-ones as lipid kinase inhibitors Download PDFInfo
- Publication number
- WO2006122806A2 WO2006122806A2 PCT/EP2006/004725 EP2006004725W WO2006122806A2 WO 2006122806 A2 WO2006122806 A2 WO 2006122806A2 EP 2006004725 W EP2006004725 W EP 2006004725W WO 2006122806 A2 WO2006122806 A2 WO 2006122806A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- quinolin
- methyl
- dihydro
- imidazo
- phenyl
- Prior art date
Links
- 0 C**1c2c(cc(c(*)c3)O)c3*(**)cc2*(*)C1=* Chemical compound C**1c2c(cc(c(*)c3)O)c3*(**)cc2*(*)C1=* 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4188—1,3-Diazoles condensed with other heterocyclic ring systems, e.g. biotin, sorbinil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/437—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a five-membered ring having nitrogen as a ring hetero atom, e.g. indolizine, beta-carboline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/16—Central respiratory analeptics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/14—Drugs for dermatological disorders for baldness or alopecia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
Definitions
- the invention relates to novel organic compounds, processes for the preparation thereof, the application thereof in a process for the treatment of the human or animal body, the use thereof - alone or in combination with one or more other pharmaceutically active compounds - for the treatment of an inflammatory or obstructive airway disease, such as asthma, disorders commonly occurring in connection with transplantation, or a proliferative disease, such as a tumor disease, which may be solid or liquid; a method for the treatment of such a disease in animals, especially in humans, and the use of such a compound - alone or in combination with one or more other pharmaceutically active compounds - for the manufacture of a pharmaceutical preparation for the treatment of said diseases.
- an inflammatory or obstructive airway disease such as asthma, disorders commonly occurring in connection with transplantation, or a proliferative disease, such as a tumor disease, which may be solid or liquid
- a method for the treatment of such a disease in animals, especially in humans and the use of such a compound - alone or in combination with one or more other pharmaceutically active compounds
- the present invention relates to compounds of formula (I)
- Ri is naphthyl or phenyl wherein said phenyl is substituted by one or two substituents independently selected from the group consisting of
- Halogen lower alkyl unsubstituted or substituted by halogen, cyano, imidazolyl or triazolyl; cycloalkyl; amino substituted by one or two substituents independently selected from the group consisting of lower alkyl, lower alkyl sulfonyl, lower alkoxy and lower alkoxy lower alkylamino; piperazinyl unsubstituted or substituted by one or two substituents independently selected from the group consisting of lower alkyl and lower alkyl sulfonyl;
- R 2 is O or S
- R 3 is lower alkyl
- R 4 is pyridyl unsubstituted or substituted by halogen, cyano, lower alkyl, lower alkoxy or piperazinyl unsubstituted or substituted by lower alkyl; pyrimidinyl unsubstituted or substituted by lower alkoxy; quinolinyl unsubstituted or substituted by halogen; quinoxalinyl; or phenyl substituted with alkoxy
- R 5 is hydrogen or halogen
- n 0 or 1 ;
- R 6 is oxido
- R 7 is hydrogen or amino
- alkyl has up to a maximum of 12 carbon atoms and is especially lower alkyl.
- Lower alkyl is preferably alkyl with from and including 1 up to and including 7, preferably from and including 1 to and including 4, and is linear or branched; preferably, lower alkyl is butyl, such as n-butyl, sec-butyl, isobutyl, tert-butyl, propyl, such as n-propyl or isopropyl, ethyl or preferably methyl.
- Cycloalkyl is preferably cycloalkyl with from and including 3 up to and including 6 carbon atoms in the ring; cycloalkyl is preferably cyclopropyl, cyclobutyl , cyclopently or cyclohexyl.
- Alkyl which is substituted by halogen is preferably perfluoro alkyl such as trifluoromethyl.
- Halogen is especially fluorine, chlorine, bromine, or iodine, especially fluorine, chlorine, or bromine.
- any reference to the free compounds hereinbefore and hereinafter is to be understood as referring also to the corresponding salts, as appropriate and expedient.
- Salts are formed, for example, as acid addition salts, preferably with organic or inorganic acids, from compounds of formula I with a basic nitrogen atom, especially the pharmaceutically acceptable salts.
- Suitable inorganic acids are, for example, halogen acids, such as hydrochloric acid, sulfuric acid, or phosphoric acid.
- Suitable organic acids are, for example, carboxylic, phosphonic, sulfonic or sulfamic acids, for example acetic acid, propionic acid, octanoic acid, decanoic acid, dodecanoic acid, glycolic acid, lactic acid, fumaric acid, succinic acid, malonic acid, adipic acid, pimelic acid, suberic acid, azelaic acid, malic acid, tartaric acid, citric acid, amino acids, such as glutamic acid or aspartic acid, maleic acid, hydroxymaleic acid, methylmaleic acid, cyclohexanecarboxylic acid, adamantanecarboxylic acid, benzoic acid, salicylic acid, 4-aminosalicylic acid, phthalic acid, phenylacetic acid, mandelic acid, cinnamic acid, methane- or ethane-sulfonic acid, 2-hydroxyethanesulfonic acid, ethane-1
- salts for isolation or purification purposes it is also possible to use pharmaceutically unacceptable salts, for example picrates or perchlorates.
- pharmaceutically acceptable salts or free compounds are employed (where applicable in the form of pharmaceutical preparations), and these are therefore preferred.
- R1 is preferably phenyl wherein said phenyl is substituted by one or two substituents independently selected from the group consisting of
- Halogen lower alkyl substituted by halogen, cyano, imidazolyl or triazolyl; amino substituted by one or two substituents independently selected from the group consisting of lower alkyl and lower alkyl sulfonyl; piperazinyl wherein said piperazinyl is unsubstituted or substituted by one or two lower alkyl substituents; imidazolyl; pyrazolyl; and triazolyl.
- R2 is preferably O.
- R3 is preferably Me.
- R4 is preferably pyrimidinyl or pyridyl unsubstituted or substituted by halogen, cyano, lower alkyl, lower alkoxy or piperazinyl unsubstituted or substituted by lower alkyl; quinolinyl unsubstituted or substituted by halogen; quinoxalinyl; or phenyl substituted with alkoxy.
- R5 is preferably hydrogen
- n is preferably 0.
- R 7 is preferably hydrogen.
- a preferred compound is a compound chosen from the group consisting of;
- R 1 is naphthyl or phenyl wherein said phenyl is substituted by one or two substituents independently selected from the group consisting of
- Halogen lower alkyl unsubstituted or substituted by halo or cyano; amino substituted by one or two substituents independently selected from the group consisting of lower alkyl, lower alkyl sulfonyl, lower alkoxy and lower alkoxy lower alkylamino; piperazinyl wherein said piperazinyl is unsubstituted or substituted by one or two substituents independently selected from the group consisting of lower alkyl and lower alkyl sulfonyl;
- R 2 is O or S
- R 3 is lower alkyl
- R 4 is pyridyl unsubstituted or substituted by lower alkoxy or piperazinyl unsubstituted or substituted by lower alkyl, or quinolinyl unsubstituted or substituted by halogen, or quinoxalinyl;
- R 5 is hydrogen or halogen
- n 0 or 1 ;
- R 6 is oxido
- the compounds of formula I have advantageous pharmacological properties and inhibit the activity of the lipid kinases, such as the PI3-kinase and/or members of the PI3-kinase-related protein kinase family (also called PIKK and include DNA-PK, ATM, ATR, hSMG-1 and mTOR), such as the DNA protein-kinase, and may be used to treat disease or disorders which depend on the activity of said kinases.
- the lipid kinases such as the PI3-kinase and/or members of the PI3-kinase-related protein kinase family (also called PIKK and include DNA-PK, ATM, ATR, hSMG-1 and mTOR), such as the DNA protein-kinase, and may be used to treat disease or disorders which depend on the activity of said kinases.
- compounds of formula (I) in free or pharmaceutically acceptable salt form are useful in the treatment of conditions which are mediated by the activation of the PI3 kinase enzymes, such as proliferative, inflammatory or allergic conditions, or disorders commonly occurring in connection with transplantation.
- Treatment in accordance with the invention may be symptomatic or prophylactic.
- Other diseases include Cowden syndrome, Lhermitte-Dudos disease and Bannayan-Zonana syndrome, or diseases in which the PI3K/PKB pathway is aberrantly activated.
- Inflammatory or obstructive airways diseases are useful in the treatment of inflammatory or obstructive airways diseases, resulting, for example, in reduction of tissue damage, airways inflammation, bronchial hyperreactivity, remodelling or disease progression.
- Inflammatory or obstructive airways diseases to which the present invention is applicable include asthma of whatever type or genesis including both intrinsic (non-allergic) asthma and extrinsic (allergic) asthma, mild asthma, moderate asthma, severe asthma, bronchitic asthma, exercise- induced asthma, occupational asthma and asthma induced following bacterial infection.
- Treatment of asthma is also to be understood as embracing treatment of subjects, e.g.
- Prophylactic efficacy in the treatment of asthma will be evidenced by reduced frequency or severity of symptomatic attack, e.g. of acute asthmatic or bronchoconstrictor attack, improvement in lung function or improved airways hyperreactivity. It may further be evidenced by reduced requirement for other, symptomatic therapy, i.e. therapy for or intended to restrict or abort symptomatic attack when it occurs, for example antiinflammatory (e.g. corticosteroid) or bronchodilatory.
- Prophylactic benefit in asthma may in particular be apparent in subjects prone to "morning dipping". "Morning dipping" is a recognised asthmatic syndrome, common to a substantial percentage of asthmatics and characterised by asthma attack, e.g. between the hours of about 4 to 6 am, i.e. at a time normally substantially distant form any previously administered symptomatic asthma therapy.
- Compounds according to formula I can be used for other inflammatory or obstructive airways diseases and conditions to which the present invention is applicable and include acute lung injury (ALI), adult/acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary, airways or lung disease (COPD, COAD or COLD), including chronic bronchitis or dyspnea associated therewith, emphysema, as well as exacerbation of airways hyperreactivity consequent to other drug therapy, in particular other inhaled drug therapy.
- the invention is also applicable to the treatment of bronchitis of whatever type or genesis including, e.g., acute, arachidic, catarrhal, croupus, chronic or phthinoid bronchitis.
- pneumoconiosis an inflammatory, commonly occupational, disease of the lungs, frequently accompanied by airways obstruction, whether chronic or acute, and occasioned by repeated inhalation of dusts
- pneumoconiosis an inflammatory, commonly occupational, disease of the lungs, frequently accompanied by airways obstruction, whether chronic or acute, and occasioned by repeated inhalation of dusts
- aluminosis an inflammatory, commonly occupational, disease of the lungs, frequently accompanied by airways obstruction, whether chronic or acute, and occasioned by repeated inhalation of dusts
- aluminosis anthracosis
- asbestosis chalicosis
- ptilosis ptilosis
- siderosis silicosis
- tabacosis tabacosis and byssinosis.
- compounds of the invention are also useful in the treatment of eosinophil related disorders, e.g. eosinophilia, in particular eosinophil related disorders of the airways (e.g.
- eosinophilic infiltration of pulmonary tissues including hypereosinophilia as it effects the airways and/or lungs as well as, for example, eosinophil- related disorders of the airways consequential or concomitant to Loffler's syndrome, eosinophilic pneumonia, parasitic (in particular metazoan) infestation (including tropical eosinophilia), bronchopulmonary aspergillosis, polyarteritis nodosa (including Churg-Strauss syndrome), eosinophilic granuloma and eosinophil-related disorders affecting the airways occasioned by drug-reaction.
- Compounds of the invention are also useful in the treatment of inflammatory or allergic conditions of the skin, for example psoriasis, contact dermatitis, atopic dermatitis, alopecia areata, erythema multiforma, dermatitis herpetiformis, scleroderma, vitiligo, hypersensitivity angiitis, urticaria, bullous pemphigoid, lupus erythematosus, pemphisus, epidermolysis bullosa acquisita, and other inflammatory or allergic conditions of the skin.
- Compounds of the invention may also be used for the treatment of other diseases or conditions, such as diseases or conditions having an inflammatory component, for example, treatment of diseases and conditions of the eye such as conjunctivitis, keratoconjunctivitis sicca, and vernal conjunctivitis, diseases affecting the nose including allergic rhinitis, and inflammatory disease in which autoimmune reactions are implicated or having an autoimmune component or aetiology, including autoimmune haematological disorders (e.g.
- haemolytic anaemia haemolytic anaemia, aplastic anaemia, pure red cell anaemia and idiopathic thrombocytopenia
- systemic lupus erythematosus polychondritis, sclerodoma, Wegener granulamatosis, dermatomyositis, chronic active hepatitis, myasthenia gravis, Steven- Johnson syndrome, idiopathic sprue, autoimmune inflammatory bowel disease (e.g.
- ulcerative colitis and Crohn's disease endocrine opthalmopathy
- Grave's disease sarcoidosis, alveolitis, chronic hypersensitivity pneumonitis, multiple sclerosis, primary billiary cirrhosis, uveitis (anterior and posterior), keratoconjunctivitis sicca and vernal keratoconjunctivitis, interstitial lung fibrosis, psoriatic arthritis and glomerulonephritis (with and without nephrotic syndrome, e.g. including idiopathic nephrotic syndrome or minal change nephropathy).
- the invention provides the use of a compound according to the definitions herein, or a pharmaceutically acceptable salt, or a hydrate or solvate thereof for the preparation of a medicament for the treatment of a proliferative disease, an inflammatory disease or an obstructive respiratory disease, or a disorder commonly occurring in connection with transplantation.
- the kinase reaction was performed in a final volume of 50 ⁇ L per well of a half area COSTAR, 96 well plate.
- the final concentrations of ATP and phosphatidyl inositol in the assay were 5 ⁇ M and 6 ⁇ g/mL respectively.
- the reaction was started by the addition of PI3 kinase p110 ⁇ .
- the components of the assay were added per well as follows:
- the assay plate was sealed using TopSeal-S and incubated at room temperature for at least 60 minutes. • The assay plate was then centrifuged at 1500 rpm for 2 minutes using the Jouan bench top centrifuge.
- the assay plate was counted using a Packard TopCount, each well being counted for 20 seconds.
- the volume of enzyme will be dependent on the enzymatic activity of the batch in use.
- Some of the compounds show a certain level of selectivity against the different paralogs PI3K alpha, gamma and delta.
- DNA-PK is a nuclear serine/threonine protein kinase that requires double-stranded DNA (dsDNA) for activity.
- dsDNA double-stranded DNA
- DNA-PK X5 reaction buffer 250 mM HEPES, 500 mM KCI, 50 mM MgCI 2 , 1 mM EGTA, 0.5 mM EDTA, 5 mM DTT, pH to 7.5 with KOH
- DNA-PK X5 reaction buffer 250 mM HEPES, 500 mM KCI, 50 mM MgCI 2 , 1 mM EGTA, 0.5 mM EDTA, 5 mM DTT, pH to 7.5 with KOH
- the activation buffer was made of 100 ⁇ g/ml of calf thymus DNA in control buffer (10 mM
- Tris-HCI pH 7.4
- 1 mM EDTA pH 8.0
- the DNA-PK enzyme (Promega V5811 , concentration ⁇ 00 U/ ⁇ L) was diluted 1/10 in X1 reaction buffer and kept on ice until imminent use. 10.8 ⁇ l of the diluted enzyme was incubated with 1.2 ⁇ l of 100 ⁇ M compounds (diluted 1/100 in water from 10 mM stock in neat
- U87MG cells (ATCC No. HTB-14) are trypsinized, counted in a Neubauer chamber, and diluted in fresh complete RPMI 1640 medium to a final concentration of 6 x 10 5 cells/mL
- Ten (10) cm tissue culture dishes are then loaded with 10 mL of the cell suspension, and incubated for 18 hours.
- the medium in plates is discarded and replaced by complete RPMI 1640 medium containing either DMSO or inhibitors [compounds of formula (I)].
- the medium is quickly removed by aspiration and the cells rinsed twice with pre- cooled PBS. Cells are then placed on ice and immediately lysed. Protein samples are then resolved by SDS-PAGE and transferred to Immbilon-P membrane for detection of levels of endogenous GSK3 ⁇ , PKB, PhosphoT308-PKB and PhosphoS9-GSK3 ⁇ by western-blotting.
- Membranes are then dried and covered with polyethylene film, and chemiluminescence measured in a MultilmageTM Light Cabinet (Alpha lnnotech Corp) driven with the FluorChemTM software (Alpha lnnotech Corp).
- mice with s.c. transplanted human glioblastoms U87MG tumors can be used to determine the anti-tumor activity of PI3 kinase inhibitors.
- a tumor fragment of approximately 25 mg is placed under the skin on the animals' left flank and the small incised wound is closed by means of suture clips.
- tumors reaches a volume of 100 mm 3 the mice are divided at random into groups of 6-8 animals and treatment commences.
- the treatment is carried out for a 2-3 weeks period with peroral, intravenous or intra-peritoneal administration once daily (or less frequently) of a compound of formula (I) in a suitable vehicle at defined doses.
- the tumors are measured twice a week with a slide gauge and the volume of the tumors is calculated.
- cell line U87MG As an alternative to cell line U87MG, other cell lines may also be used in the same manner, for example,
- PC-3 prostate carcinoma cell line PC-3 especially preferred; ATCC No. CRL 1435; see also Cancer Res. 40, 524-34 [1980]) and the PC-3M prostate carcinoma cell line;
- pancreatic cancer cell line SUIT-2 • the pancreatic cancer cell line SUIT-2 (see Tomioka et al., Cancer Res. 61., 7518- 24 [2001]).
- Compounds of the invention exhibit T cell inhibiting activity. More particular the compounds of the invention prevent T cell activation and/or proliferation in e.g. aqueous solution, e.g. as demonstrated in accordance with the following test method.
- the two-way MLR is performed according to standard procedures ( J. Immunol. Methods, 1973, 2, 279 and Meo T. et al., Immunological Methods, New York, Academic Press, 1979, 227-39).
- spleen cells from CBA and BALB/c mice (1.6 x 105 cells from each strain per well in flat bottom tissue culture microtiter plates, 3.2 x 105 in total) are incubated in RPMI medium containing 10% FCS, 100 U/ml penicillin, 100 ⁇ g/ml streptomycin (Gibco BRL, Basel, Switzerland), 50 ⁇ M 2- mercaptoethanol (Fluka, Buchs, Switzerland) and serially diluted compounds. Seven threefold dilution steps in duplicates per test compound are performed. After four days of incubation 1 ⁇ Ci 3H-thymidine is added.
- the compounds of the invention have IC50 values in the range of 1 nM to 10 ⁇ M, preferably from 1O nM tO 10O nM.
- a compound of the formula (I) may also be used to advantage in combination with other antiproliferative compounds.
- antiproliferative compounds include, but are not limited to aromatase inhibitors; antiestrogens; topoisomerase I inhibitors; topoisomerase Il inhibitors; microtubule active compounds; alkylating compounds; histone deacetylase inhibitors; compounds which induce cell differentiation processes; cyclooxygenase inhibitors; MMP inhibitors; mTOR inhibitors; antineoplastic antimetabolites; platin compounds; compounds targeting/decreasing a protein or lipid kinase activity and further anti-angiogenic compounds; compounds which target, decrease or inhibit the activity of a protein or lipid phosphatase; gonadorelin agonists; anti-androgens; methionine aminopeptidase inhibitors; bisphosphonates; biological response modifiers; antiproliferative antibodies; heparanase inhibitors; inhibitors of Ras oncogenic isoforms;
- aromatase inhibitor as used herein relates to a compound which inhibits the estrogen production, i.e. the conversion of the substrates androstenedione and testosterone to estrone and estradiol, respectively.
- the term includes, but is not limited to steroids, especially atamestane, exemestane and formestane and, in particular, non-steroids, especially aminoglutethimide, roglethimide, pyridoglutethimide, trilostane, testolactone, ketokonazole, vorozole, fadrozole, anastrozole and letrozole.
- Exemestane can be administered, e.g., in the form as it is marketed, e.g.
- AROMASIN Formestane can be administered, e.g., in the form as it is marketed, e.g. under the trademark LENTARON. Fadrozole can be administered, e.g., in the form as it is marketed, e.g. under the trademark AFEMA. Anastrozole can be administered, e.g., in the form as it is marketed, e.g. under the trademark ARIMIDEX. Letrozole can be administered, e.g., in the form as it is marketed, e.g. under the trademark FEMARA or FEMAR. Aminoglutethimide can be administered, e.g., in the form as it is marketed, e.g. under the trademark ORIMETEN.
- a combination of the invention comprising a chemotherapeutic agent which is an aromatase inhibitor is particularly useful for the treatment of hormone receptor positive tumors, e.g. breast tumors.
- antiestrogen as used herein relates to a compound which antagonizes the effect of estrogens at the estrogen receptor level.
- the term includes, but is not limited to tamoxifen, fulvestrant, raloxifene and raloxifene hydrochloride.
- Tamoxifen can be administered, e.g., in the form as it is marketed, e.g. under the trademark NOLVADEX.
- Raloxifene hydrochloride can be administered, e.g., in the form as it is marketed, e.g. under the trademark EVISTA.
- Fulvestrant can be formulated as disclosed in US 4,659,516 or it can be administered, e.g., in the form as it is marketed, e.g. under the trademark FASLODEX.
- a combination of the invention comprising a chemotherapeutic agent which is an antiestrogen is particularly useful for the treatment of estrogen receptor positive tumors, e.g. breast tumors.
- anti-androgen as used herein relates to any substance which is capable of inhibiting the biological effects of androgenic hormones and includes, but is not limited to, bicalutamide (CASODEX), which can be formulated, e.g. as disclosed in US 4,636,505.
- bicalutamide CASODEX
- gonadorelin agonist as used herein includes, but is not limited to abarelix, goserelin and goserelin acetate. Goserelin is disclosed in US 4,100,274 and can be administered, e.g., in the form as it is marketed, e.g. under the trademark ZOLADEX.
- Abarelix can be formulated, e.g. as disclosed in US 5,843,901.
- topoisomerase I inhibitor includes, but is not limited to topotecan, gimatecan, irinotecan, camptothecian and its analogues, 9-nitrocamptothecin and the macromolecular camptothecin conjugate PNU-166148 (compound A1 in WO99/ 17804).
- Irinotecan can be administered, e.g. in the form as it is marketed, e.g. under the trademark CAMPTOSAR.
- Topotecan can be administered, e.g., in the form as it is marketed, e.g. under the trademark HYCAMTIN.
- topoisomerase Il inhibitor includes, but is not limited to the an- thracyclines such as doxorubicin (including liposomal formulation, e.g. CAELYX), daunorubicin, epirubicin, idarubicin and nemorubicin, the anthraquinones mitoxantrone and losoxantrone, and the podophillotoxines etoposide and teniposide.
- Etoposide can be administered, e.g. in the form as it is marketed, e.g. under the trademark ETOPOPHOS.
- Teniposide can be administered, e.g. in the form as it is marketed, e.g.
- Doxorubicin can be administered, e.g. in the form as it is marketed, e.g. under the trademark ADRIBLASTIN or ADRIAMYCIN.
- Epirubicin can be administered, e.g. in the form as it is marketed, e.g. under the trademark FARMORUBICIN.
- Idarubicin can be administered, e.g. in the form as it is marketed, e.g. under the trademark ZAVEDOS.
- Mitoxantrone can be administered, e.g. in the form as it is marketed, e.g. under the trademark NOVANTRON.
- microtubule active agent relates to microtubule stabilizing, microtubule destabilizing compounds and microtublin polymerization inhibitors including, but not limited to taxanes, e.g. paclitaxel and docetaxel, vinca alkaloids, e.g., vinblastine, especially vinblastine sulfate, vincristine especially vincristine sulfate, and vinorelbine, discodermolides, cochicine and epothilones and derivatives thereof, e.g. epothilone B or D or derivatives thereof.
- Paclitaxel may be administered e.g. in the form as it is marketed, e.g. TAXOL.
- Docetaxel can be administered, e.g., in the form as it is marketed, e.g. under the trademark TAXOTERE.
- Vinblastine sulfate can be administered, e.g., in the form as it is marketed, e.g. under the trademark VINBLASTIN R.P..
- Vincristine sulfate can be administered, e.g., in the form as it is marketed, e.g. under the trademark FARMISTIN.
- Discodermolide can be obtained, e.g., as disclosed in US 5,010,099.
- Epothilone derivatives which are disclosed in WO 98/10121 , US 6,194,181 , WO 98/25929, WO 98/08849, WO 99/43653, WO 98/22461 and WO 00/31247. Especially preferred are Epothilone A and/or B.
- alkylating agent includes, but is not limited to, cyclophosphamide, ifosfamide, melphalan or nitrosourea (BCNU or Gliadel).
- Cyclophosphamide can be administered, e.g., in the form as it is marketed, e.g. under the trademark CYCLOSTIN.
- lfosfamide can be administered, e.g., in the form as it is marketed, e.g. under the trademark
- histone deacetylase inhibitors or "HDAC inhibitors” relates to compounds which inhibit the histone deacetylase and which possess antiproliferative activity. This includes compounds disclosed in WO 02/22577, especially N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-
- antimetabolite includes, but is not limited to, 5-Fluorouracil or 5-FU, capecitabine, gemcitabine, DNA demethylating compounds, such as 5-azacytidine and decitabine, methotrexate and edatrexate, and folic acid antagonists such as pemetrexed.
- Capecitabine can be administered, e.g., in the form as it is marketed, e.g. under the trademark
- Gemcitabine can be administered, e.g., in the form as it is marketed, e.g. under the trademark GEMZAR..
- platinum compound as used herein includes, but is not limited to, carboplatin, cis-platin, cisplatinum and oxaliplatin.
- Carboplatin can be administered, e.g., in the form as it is marketed, e.g. under the trademark CARBOPLAT.
- Oxaliplatin can be administered, e.g., in the form as it is marketed, e.g. under the trademark ELOXATIN.
- the term "compounds targeting/decreasing a protein or lipid kinase activity; or a protein or lipid phosphatase activity; or further anti-angiogenic compounds” as used herein includes, but is not limited to, protein tyrosine kinase and/or serine and/or threonine kinase inhibitors or lipid kinase inhibitors, e.g., a) compounds targeting, decreasing or inhibiting the activity of the platelet-derived growth factor-receptors (PDGFR), such as compounds which target, decrease or inhibit the activity of PDGFR, especially compounds which inhibit the PDGF receptor, e.g. a N-phenyl-2-pyrimidine-amine derivative, e.g.
- PDGFR platelet-derived growth factor-receptors
- imatinib, SU101 , SU6668 and GFB-111 ; b) compounds targeting, decreasing or inhibiting the activity of the fibroblast growth factor-receptors (FGFR); c) compounds targeting, decreasing or inhibiting the activity of the insulin-like growth factor receptor I (IGF-IR), such as compounds which target, decrease or inhibit the activity of IGF-IR, especially compounds which inhibit the kinase activity of IGF-I receptor, such as those compounds disclosed in WO 02/092599, or antibodies that target the extracellular domain of IGF-I receptor or its growth factors; d) compounds targeting, decreasing or inhibiting the activity of the Trk receptor tyrosine kinase family, or ephrin B4 inhibitors; e) compounds targeting, decreasing or inhibiting the activity of the AxI receptor tyrosine kinase family; f) compounds targeting, decreasing or inhibiting the activity of the Ret receptor tyrosine kinase; g) compounds targeting, decreasing or inhibiting
- imatinib compounds targeting, decreasing or inhibiting the activity of the C-kit receptor tyrosine kinases - (part of the PDGFR family), such as compounds which target, decrease or inhibit the activity of the c-Kit receptor tyrosine kinase family, especially compounds which inhibit the c-Kit receptor, e.g. imatinib; i) compounds targeting, decreasing or inhibiting the activity of members of the c-Abl family, their gene-fusion products (e.g. BCR-AbI kinase) and mutants, such as compounds which target decrease or inhibit the activity of c-Abl family members and their gene fusion products, e.g.
- N-phenyl-2-pyrimidine-amine derivative e.g. imatinib or nilotinib (AMN107); PD180970; AG957; NSC 680410; PD173955 from ParkeDavis; or dasatinib (BMS-354825) j) compounds targeting, decreasing or inhibiting the activity of members of the protein kinase C (PKC) and Raf family of serine/threonine kinases, members of the MEK, SRC, JAK, FAK, PDK1, PKB/Akt, and Ras/MAPK family members, and/or members of the cyclin-dependent kinase family (CDK) and are especially those staurosporine derivatives disclosed in US 5,093,330, e.g.
- PKC protein kinase C
- Raf family of serine/threonine kinases members of the MEK, SRC, JAK, FAK, PDK1, PKB/Akt, and Ras/
- examples of further compounds include e.g. UCN-01 , safingol, BAY 43-9006, Bryostatin 1 , Perifosine; llmofosine; RO 318220 and RO 320432; GO 6976; lsis 3521 ; LY333531/LY379196; isochinoline compounds such as those disclosed in WO 00/09495; FTIs; PD184352 or QAN697 (a P13K inhibitor) or AT7519 (CDK inhibitor); k) compounds targeting, decreasing or inhibiting the activity of protein-tyrosine kinase inhibitors, such as compounds which target, decrease or inhibit the activity of protein-tyrosine kinase inhibitors include imatinib mesylate (GLEEVEC) or tyrphostin.
- GLEEVEC imatinib mesylate
- tyrphostin include imatinib mesylate (GLEEVEC) or t
- a tyrphostin is preferably a low molecular weight (Mr ⁇ 1500) compound, or a pharmaceutically acceptable salt thereof, especially a compound selected from the benzylidenemalonitrile class or the S-arylbenzenemalonirile or bisubstrate quinoline class of compounds, more especially any compound selected from the group consisting of Tyrphostin A23/RG-50810; AG 99; Tyrphostin AG 213; Tyrphostin AG 1748; Tyrphostin AG 490; Tyrphostin B44; Tyrphostin B44 (+) enantiomer; Tyrphostin AG 555; AG 494; Tyrphostin AG 556, AG957 and adaphostin (4- ⁇ [(2,5- dihydroxyphenyl)methyl]amino ⁇ -benzoic acid adamantyl ester; NSC 680410, adaphostin);
- compounds targeting, decreasing or inhibiting the activity of the epidermal growth factor family of receptor tyrosine kinases are especially compounds, proteins or antibodies which inhibit members of the EGF receptor tyrosine kinase family, e.g. EGF receptor, ErbB2, ErbB3 and ErbB4 or bind to EGF or EGF related ligands, and are in particular those compounds, proteins or monoclonal antibodies generically and specifically disclosed in WO 97/02266, e.g. the compound of ex.
- trastuzumab HerceptinTM
- cetuximab ErbituxTM
- Iressa Tarceva
- OSI-774 Cl- 1033
- EKB-569 E1.1 , E2.4, E2.5, E6.2, E6.4, E2.11 , E6.3 or E7.6.3, and 7H-pyrrolo-[2,3-d]pyrimidine derivatives which are disclosed in WO 03/013541 ; and m) compounds targeting, decreasing or inhibiting the activity of the c-Met receptor, such as compounds which target, decrease or inhibit the activity of c-Met, especially compounds which inhibit the kinase activity of c-Met receptor, or antibodies that target the extracellular domain of c-Met or bind to HGF.
- compounds targeting, decreasing or inhibiting the activity of the c-Met receptor such as compounds which target, decrease or inhibit the activity of c-Met, especially compounds which inhibit the kinase activity of c-Met receptor, or antibodies that target the extracellular domain of c-Met or bind to HGF
- anti-angiogenic compounds include compounds having another mechanism for their activity, e.g. unrelated to protein or lipid kinase inhibition e.g. thalidomide (THALOMID) and
- Compounds which target, decrease or inhibit the activity of a protein or lipid phosphatase are e.g. inhibitors of phosphatase 1 , phosphatase 2A, or CDC25, e.g. okadaic acid or a derivative thereof.
- cyclooxygenase inhibitor as used herein includes, but is not limited to, e.g. Cox-2 inhibitors, 5-alkyl substituted 2-arylaminophenylacetic acid and derivatives, such as celecoxib (CELEBREX), rofecoxib (VIOXX), etoricoxib, valdecoxib or a 5-alkyl-2- arylaminophenylacetic acid, e.g. 5-methyl-2-(2'-chloro-6'-fluoroanilino)phenyl acetic acid, lumiracoxib.
- Cox-2 inhibitors 5-alkyl substituted 2-arylaminophenylacetic acid and derivatives, such as celecoxib (CELEBREX), rofecoxib (VIOXX), etoricoxib, valdecoxib or a 5-alkyl-2- arylaminophenylacetic acid, e.g. 5-methyl-2-(2'-chloro-6'-
- bisphosphonates as used herein includes, but is not limited to, etridonic, clodronic, tiludronic, pamidronic, alendronic, ibandronic, risedronic and zoledronic acid.
- Etridonic acid can be administered, e.g., in the form as it is marketed, e.g. under the trademark DIDRONEL.
- Clodronic acid can be administered, e.g., in the form as it is marketed, e.g. under the trademark BONEFOS.
- titaniumudronic acid can be administered, e.g., in the form as it is marketed, e.g. under the trademark SKELID.
- “Pamidronic acid” can be administered, e.g. in the form as it is marketed, e.g. under the trademark AREDIATM.
- “Alendronic acid” can be administered, e.g., in the form as it is marketed, e.g. under the trademark FOSAMAX.
- “Ibandronic acid” can be administered, e.g., in the form as it is marketed, e.g. under the trademark BONDRANAT.
- “Risedronic acid” can be administered, e.g., in the form as it is marketed, e.g. under the trademark ACTONEL.
- "Zoledronic acid” can be administered, e.g. in the form as it is marketed, e.g.
- mTOR inhibitors relates to compounds which inhibit the mammalian target of rapamycin (mTOR) and which possess antiproliferative activity such as sirolimus (Rapamune®), everolimus (CerticanTM), CCI-779 and ABT578.
- heparanase inhibitor refers to compounds which target, decrease or inhibit heparin sulfate degradation.
- the term includes, but is not limited to, PI-88.
- biological response modifier refers to a lymphokine or interferons, e.g. interferon ⁇ .
- inhibitor of Ras oncogenic isoforms e.g. H-Ras, K-Ras, or N-Ras
- a "farnesyl transferase inhibitor” e.g. L-744832, DK8G557 or R115777 (Zarnestra).
- telomerase inhibitor refers to compounds which target, decrease or inhibit the activity of telomerase. Compounds which target, decrease or inhibit the activity of telomerase are especially compounds which inhibit the telomerase receptor, e.g. telomestatin.
- methionine aminopeptidase inhibitor refers to compounds which target, decrease or inhibit the activity of methionine aminopeptidase.
- Compounds which target, decrease or inhibit the activity of methionine aminopeptidase are e.g. bengamide or a derivative thereof.
- proteasome inhibitor refers to compounds which target, decrease or inhibit the activity of the proteasome.
- Compounds which target, decrease or inhibit the activity of the proteasome include e.g. Bortezomid (VelcadeTM)and MLN 341.
- matrix metalloproteinase inhibitor or (“MMP” inhibitor) as used herein includes, but is not limited to, collagen peptidomimetic and nonpeptidomimetic inhibitors, tetracycline derivatives, e.g. hydroxamate peptidomimetic inhibitor batimastat and its orally bioavailable analogue marimastat (BB-2516), prinomastat (AG3340), metastat (NSC 683551) BMS-
- FMS-like tyrosine kinase inhibitors e.g. compounds targeting, decreasing or inhibiting the activity of FMS-like tyrosine kinase receptors (Flt-3R); interferon,
- ALK inhibitors e.g. compounds which target, decrease or inhibit anaplastic lymphoma kinase.
- FMS-like tyrosine kinase receptors are especially compounds, proteins or antibodies which inhibit members of the Flt-3R receptor kinase family, e.g. PKC412, midostaurin, a staurosporine derivative,
- HSP90 inhibitors includes, but is not limited to, compounds targeting, decreasing or inhibiting the intrinsic ATPase activity of HSP90; degrading, targeting, decreasing or inhibiting the HSP90 client proteins via the ubiquitin proteosome pathway. Compounds targeting, decreasing or inhibiting the intrinsic ATPase activity of HSP90; degrading, targeting, decreasing or inhibiting the HSP90 client proteins via the ubiquitin proteosome pathway. Compounds targeting, decreasing or inhibiting the intrinsic ATPase activity of
- HSP90 are especially compounds, proteins or antibodies which inhibit the ATPase activity of
- HSP90 e.g., 17-allylamino,17-demethoxygeldanamycin (17AAG), a geldanamycin derivative; other geldanamycin related compounds; radicicol and HDAC inhibitors.
- antiproliferative antibodies includes, but is not limited to, trastuzumab (HerceptinTM), Trastuzumab-DM1 ,erbitux, bevacizumab (AvastinTM), rituximab
- antibodies e.g. intact monoclonal antibodies, polyclonal antibodies, multispecific antibodies formed from at least 2 intact antibodies, and antibodies fragments so long as they exhibit the desired biological activity.
- compounds of formula (I) can be used in combination with standard leukemia therapies, especially in combination with therapies used for the treatment of AML.
- compounds of formula (I) can be administered in combination with, e.g., farnesyl transferase inhibitors and/or other drugs useful for the treatment of AML, such as Daunorubicin, Adriamycin, Ara-C, VP-16, Teniposide, Mitoxantrone, Idarubicin, Carboplatinum and PKC412.
- antigenemic compounds includes, for example, Ara-C, a pyrimidine analog, which is the 2 ' -alpha-hydroxy ribose (arabinoside) derivative of deoxycytidine. Also included is the purine analog of hypoxanthine, 6-mercaptopurine (6-MP) and fludarabine phosphate.
- HDAC histone deacetylase
- SAHA suberoylanilide hydroxamic acid
- HDAC inhibitors include MS275, SAHA, FK228 (formerly FR901228), Trichostatin A and compounds disclosed in US 6,552,065, in particular, ⁇ /-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)-ethyl]- amino]methyl]phenyl]-2£-2-propenamide, or a pharmaceutically acceptable salt thereof and ⁇ /-hydroxy-3-[4-[(2-hydroxyethyl) ⁇ 2-(1H-indol-3-yl)ethyl]-amino]methyl]phenyl]-2E-2- propenamide, or a pharmaceutically acceptable salt thereof, especially the lactate salt.
- Somatostatin receptor antagonists refers to compounds which target, treat or inhibit the somatostatin receptor such as octreotide, and SOM230.
- Tumor cell damaging approaches refer to approaches such as ionizing radiation.
- the term "ionizing radiation” referred to above and hereinafter means ionizing radiation that occurs as either electromagnetic rays (such as X-rays and gamma rays) or particles (such as alpha and beta particles). Ionizing radiation is provided in, but not limited to, radiation therapy and is known in the art. See Hellman, Principles of Radiation Therapy, Cancer, in Principles and Practice of Oncology, Devita et al., Eds., 4 th Edition, Vol. 1 , pp. 248-275 (1993).
- EDG binders refers a class of immunosuppressants that modulates lymphocyte recirculation, such as FTY720.
- ribonucleotide reductase inhibitors refers to pyrimidine or purine nucleoside analogs including, but not limited to, fludarabine and/or cytosine arabinoside (ara-C), 6-thioguanine, 5-fluorouracil, cladribine, 6-mercaptopurine (especially in combination with ara-C against ALL) and/or pentostatin.
- ara-C cytosine arabinoside
- 6-thioguanine 5-fluorouracil
- cladribine 6-mercaptopurine (especially in combination with ara-C against ALL) and/or pentostatin.
- Ribonucleotide reductase inhibitors are especially hydroxyurea or 2-hydroxy-1H-isoindole-1 ,3-dione derivatives, such as PL-1 , PL-2, PL-3, PL-4, PL-5, PL-6, PL-7 or PL-8 mentioned in Nandy et al., Acta Oncologica, Vol. 33, No. 8, pp. 953-961 (1994).
- S-adenosylmethionine decarboxylase inhibitors as used herein includes, but is not limited to the compounds disclosed in US 5,461 ,076.
- VEGF vascular endothelial growth factor
- WO 98/35958 e.g. 1-(4-chloroanilino)-4-(4-pyridylmethyl)phthalazine or a pharmaceutically acceptable salt thereof, e.g. the succinate, or in WO 00/09495,
- VEGF aptamer e.g. Macugon
- FLT-4 inhibitors FLT-3 inhibitors
- VEGFR-2 IgGI antibody Angiozyme (RPI 4610) and Bevacizumab (AvastinTM).
- Photodynamic therapy refers to therapy which uses certain chemicals known as photosensitizing compounds to treat or prevent cancers.
- Examples of photodynamic therapy includes treatment with compounds, such as e.g. VISUDYNE and porfimer sodium.
- Angiostatic steroids refers to compounds which block or inhibit angiogenesis, such as, e.g., anecortave, triamcinolone, hydrocortisone, 11- ⁇ -epihydrocotisol, cortexolone,
- Implants containing corticosteroids refers to compounds, such as e.g. fluocinolone, dexamethasone.
- chemotherapeutic compounds include, but are not limited to, plant alkaloids, hormonal compounds and antagonists; biological response modifiers, preferably lymphokines or interferons; antisense oligonucleotides or oligonucleotide derivatives; shRNA or siRNA; or miscellaneous compounds or compounds with other or unknown mechanism of action.
- the compounds of the invention are also useful as co-therapeutic compounds for use in combination with other drug substances such as anti-inflammatory, bronchodilatory or antihistamine drug substances, particularly in the treatment of obstructive or inflammatory airways diseases such as those mentioned hereinbefore, for example as potentiators of therapeutic activity of such drugs or as a means of reducing required dosaging or potential side effects of such drugs.
- a compound of the invention may be mixed with the other drug substance in a fixed pharmaceutical composition or it may be administered separately, before, simultaneously with or after the other drug substance.
- the invention includes a combination of a compound of the invention as hereinbefore described with an anti-inflammatory, bronchodilatory, antihistamine or anti-tussive drug substance, said compound of the invention and said drug substance being in the same or different pharmaceutical composition.
- Suitable anti-inflammatory drugs include steroids, in particular glucocorticosteroids such as budesonide, beclamethasone dipropionate, fluticasone propionate, ciclesonide or mometasone furoate, or steroids described in WO 02/88167, WO 02/12266, WO 02/100879, WO 02/00679 (especially those of Examples 3, 11 , 14, 17, 19, 26, 34, 37, 39, 51, 60, 67, 72, 73, 90, 99 and 101), WO 03/035668, WO 03/048181, WO 03/062259, WO 03/064445, WO 03/072592, non-steroidal glucocorticoid receptor agonists such as those described in WO 00/00531 , WO 02/10143, WO 03/082280, WO 03/082787, WO 03/104195, WO 04/005229; LTB4 antagonists such LY293111 , CGS02
- Suitable bronchodilatory drugs include anticholinergic or antimuscarinic compounds, in particular ipratropium bromide, oxitropium bromide, tiotropium salts and CHF 4226 (Chiesi), and glycopyrrolate, but also those described in WO 01/04118, WO 02/51841 , WO 02/53564, WO 03/00840, WO 03/87094, WO 04/05285, WO 02/00652, WO 03/53966, EP 424021 , US 5171744, US 3714357, WO 03/33495 and WO 04/018422.
- Suitable antihistamine drug substances include cetirizine hydrochloride, acetaminophen, clemastine fumarate, promethazine, loratidine, desloratidine, diphenhydramine and fexofenadine hydrochloride, activastine, astemizole, azelastine, ebastine, epinastine, mizolastine and tefenadine as well as those disclosed in WO 03/099807, WO 04/026841 and JP 2004107299.
- CCR-1 , CCR-2, CCR-3, CCR-4, CCR-5, CCR-6, CCR-7, CCR-8, CCR-9 and CCR10 CXCR1 , CXCR2, CXCR3, CXCR4, CXCR5, particularly CCR-5 antagonists such as Schering-Plough antagonists SC-351125, SCH- 55700 and SCH-D, Takeda antagonists such as N-[[4-[[[6,7-dihydro-2-(4-methylphenyl)-5H- benzo-cyclohepten- ⁇ -ylJcarbonylJaminoJphenylJ-methylJtetrahydro-N.N-dimethyl ⁇ H-pyran ⁇ - amin-ium chloride (TAK-770), and CCR-5 antagonists described in US 6166037 (particularly claims 18 and 19), WO 00/66558 (particularly claim 8),
- the structure of the active compounds identified by code nos., generic or trade names may be taken from the actual edition of the standard compendium "The Merck Index” or from databases, e.g. Patents International (e.g. IMS World Publications).
- the above-mentioned compounds, which can be used in combination with a compound of the formula (I), can be prepared and administered as described in the art, such as in the documents cited above.
- a compound of the formula (I) may also be used to advantage in combination with known therapeutic processes, for example, the administration of hormones or especially radiation.
- a compound of formula (I) may in particular be used as a radiosensitizer, especially for the treatment of tumors which exhibit poor sensitivity to radiotherapy.
- ком ⁇ онент there is meant either a fixed combination in one dosage unit form, or a kit of parts for the combined administration where a compound of the formula (I) and a combination partner may be administered independently at the same time or separately within time intervals that especially allow that the combination partners show a cooperative, e.g. synergistic effect.
- the invention also provides a pharmaceutical preparation, comprising a compound of formula I as defined herein, or an N-oxide or a tautomer thereof, or a pharmaceutically acceptable salt of such a compound, or a hydrate or solvate thereof, and at least one pharmaceutically acceptable carrier.
- a compound of formula I can be administered alone or in combination with one or more other therapeutic compounds, possible combination therapy taking the form of fixed combinations or the administration of a compound of the invention and one or more other therapeutic compounds being staggered or given independently of one another, or the combined administration of fixed combinations and one or more other therapeutic compounds.
- a compound of formula I can besides or in addition be administered especially for tumor therapy in combination with chemotherapy, radiotherapy, immunotherapy, phototherapy, surgical intervention, or a combination of these. Long-term therapy is equally possible as is adjuvant therapy in the context of other treatment strategies, as described above. Other possible treatments are therapy to maintain the patient's status after tumor regression, or even chemopreventive therapy, for example in patients at risk.
- the dosage of the active ingredient depends upon a variety of factors including type, species, age, weight, sex and medical condition of the patient; the severity of the condition to be treated; the route of administration; the renal and hepatic function of the patient; and the particular compound employed.
- a physician, clinician or veterinarian of ordinary skill can readily determine and prescribe the effective amount of the drug required to prevent, counter or arrest the progress of the condition.
- Optimal precision in achieving concentration of drug within the range that yields efficacy requires a regimen based on the kinetics of the drug's availability to target sites. This involves a consideration of the distribution, equilibrium, and elimination of a drug.
- the dose of a compound of the formula I or a pharmaceutically acceptable salt thereof to be administered to warm-blooded animals is preferably from approximately 3 mg to approximately 5 g, more preferably from approximately 10 mg to approximately 1.5 g, most preferably from about 100 mg to about 1000 mg per person per day, divided preferably into 1 to 3 single doses which may, for example, be of the same size. Usually, children receive half of the adult dose.
- the compounds of the invention may be administered by any conventional route, in particular parenterally, for example in the form of injectable solutions or suspensions, enterally, e.g. orally, for example in the form of tablets or capsules, topically, e.g. in the form of lotions, gels, ointments or creams, or in a nasal or a suppository form.
- Topical administration is e.g. to the skin.
- a further form of topical administration is to the eye.
- Pharmaceutical compositions comprising a compound of the invention in association with at least one pharmaceutical acceptable carrier or diluent may be manufactured in conventional manner by mixing with a pharmaceutically acceptable carrier or diluent.
- the invention relates also to pharmaceutical compositions comprising an effective amount, especially an amount effective in the treatment of one of the above-mentioned disorders, of a compound of formula I or an N-oxide or a tautomer thereof together with pharmaceutically acceptable carriers that are suitable for topical, enteral, for example oral or rectal, or parenteral administration and that may be inorganic or organic, solid or liquid.
- pharmaceutically acceptable carriers that are suitable for topical, enteral, for example oral or rectal, or parenteral administration and that may be inorganic or organic, solid or liquid.
- diluents for example lactose, dextrose, mannitol, and/or glycerol, and/or lubricants and/or polyethylene glycol.
- Tablets may also comprise binders, for example magnesium aluminum silicate, starches, such as corn, wheat or rice starch, gelatin, methylcellulose, sodium carboxymethylcellulose and/or polyvinylpyrrolidone, and, if desired, disintegrators, for example starches, agar, alginic acid or a salt thereof, such as sodium alginate, and/or effervescent mixtures, or adsorbents, dyes, flavorings and sweeteners. It is also possible to use the pharmacologically active compounds of the present invention in the form of parenterally administrable compositions or in the form of infusion solutions.
- binders for example magnesium aluminum silicate, starches, such as corn, wheat or rice starch, gelatin, methylcellulose, sodium carboxymethylcellulose and/or polyvinylpyrrolidone
- disintegrators for example starches, agar, alginic acid or a salt thereof, such as sodium alginate, and/or effervescent mixtures, or
- the pharmaceutical compositions may be sterilized and/or may comprise excipients, for example preservatives, stabilisers, wetting compounds and/or emulsifiers, solubilisers, salts for regulating the osmotic pressure and/or buffers.
- excipients for example preservatives, stabilisers, wetting compounds and/or emulsifiers, solubilisers, salts for regulating the osmotic pressure and/or buffers.
- the present pharmaceutical compositions which may, if desired, comprise other pharmacologically active substances are prepared in a manner known per se, for example by means of conventional mixing, granulating, confectioning, dissolving or lyophilising processes, and comprise approximately from 1% to 99%, especially from approximately 1% to approximately 20%, active ingredient(s).
- the present invention provides a compound of formula I or an N-oxide or a tautomer thereof, or a pharmaceutically acceptable salt of such a compound, for use in a method for the treatment of the human or animal body.
- the present invention also relates to the use of a compound of formula I or a tautomer thereof, or a pharmaceutically acceptable salt of such a compound, for the preparation of a medicament for the treatment of a proliferative disease, an inflammatory disease, or an obstructive airway disease, or disorders commonly occurring in connection with transplantation.
- the invention relates to a method for the treatment of a proliferative disease which responds to an inhibition of lipid kinases and/or PI3-kinase-related protein kinases, in particular the PI3 kinase, and/or DNA protein kinase activity, which comprises administering a compound of formula I or an N-oxide or a tautomer thereof, or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, wherein the radicals and symbols have the meanings as defined above, in a quantity effective against the said disease, to a warmblooded animal, in particular to humans, requiring such treatment.
- the invention relates to a pharmaceutical composition for treatment of solid or liquid tumours in warm-blooded animals, including humans, comprising an antitumourally effective dose of a compound of the formula I as described above or a pharmaceutically acceptable salt of such a compound together with a pharmaceutical carrier.
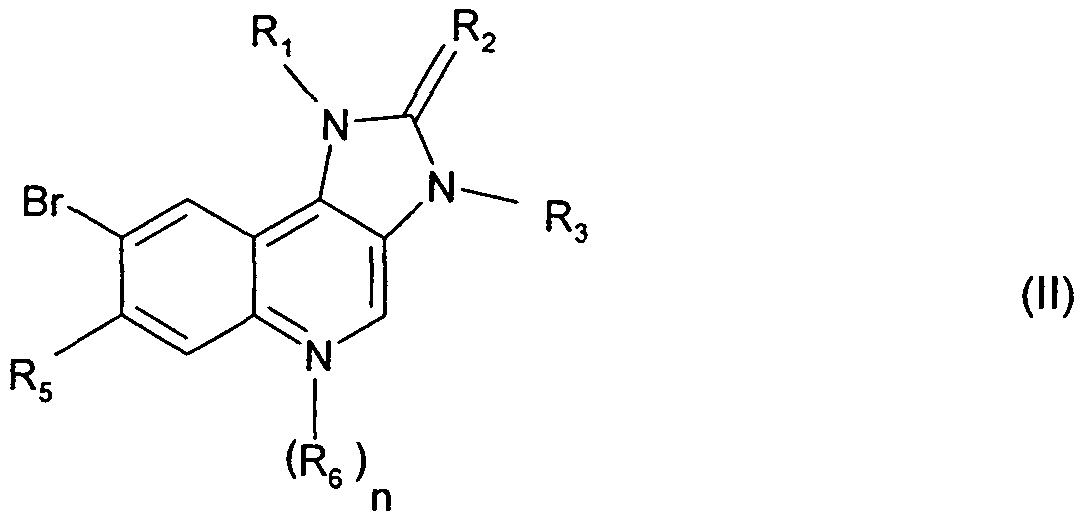
- the invention also provides a process for the preparation of a compound of formula I according to claim 1 , or a pharmaceutically acceptable salt thereof, characterized in that a imidazoquinoline derivative of the formula Il
- a compound of the invention may be prepared by processes that, though not applied hitherto for the new compounds of the present invention, are known per se, especially by a process characterized in that for the synthesis of a compound of the formula I wherein the symbols Ri ⁇ R 2 , R3, R4, R5, Re and n are as defined for a compound of the formula I, a compound of the formula Il
- R 1 , R 2 , R 3 , R 5 , R 6 and n are as defined for a compound of the formula I is reacted with a boronic acid of the formula III
- R 4 is as defined for a compound of the formula I in the presence of a base and a catalyst in a suitable solvent; where the above starting compounds Il and III may also be present with functional groups in protected form if necessary and/or in the form of salts, provided a salt-forming group is present and the reaction in salt form is possible;
- an obtainable compound of formula I is converted into another compound of formula I or a N-oxide thereof, a free compound of formula I is converted into a salt, an obtainable salt of a compound of formula I is converted into the free compound or another salt, and/or a mixture of isomeric compounds of formula I is separated into the individual isomers.
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and n are as defined for compounds of formula I, unless otherwise indicated.
- the reaction of compound of formula Il and III is preferably carried out under the conditions of a Suzuki-reaction, preferably in a mixture of a polar aprotic solvent such as DMF and water in the presence of a catalyst, especially a noble metal catalyst, such as palladium (II), preferable bis(triphenylphosphine)palladium (II) dichloride; in the presence of a base such as potassium carbonate.
- a catalyst especially a noble metal catalyst, such as palladium (II), preferable bis(triphenylphosphine)palladium (II) dichloride
- a base such as potassium carbonate.
- one or more other functional groups for example carboxy, hydroxy, amino, or mercapto, are or need to be protected in a compound of formulae Il or III, because they should not take part in the reaction, these are such groups as are usually used in the synthesis of peptide compounds, and also of cephalosporins and penicillins, as well as nucleic acid derivatives and sugars.
- the protecting groups may already be present in precursors and should protect the functional groups concerned against unwanted secondary reactions, such as acylations, etheri- fications, esterifications, oxidations, solvolysis, and similar reactions. It is a characteristic of protecting groups that they lend themselves readily, i.e. without undesired secondary reac- tions, to removal, typically by acetolysis, protonolysis, solvolysis, reduction, photolysis or also by enzyme activity, for example under conditions analogous to physiological conditions, and that they are not present in the end-products.
- the specialist knows, or can easily establish, which protecting groups are suitable with the reactions mentioned hereinabove and hereinafter.
- functional groups of the starting compounds which should not take part in the reaction may be present in unprotected form or may be protected for example by one or more of the protecting groups mentioned hereinabove under "protecting groups".
- the protecting groups are then wholly or partly removed according to one of the methods described there.
- Salts of a compound of formula I with a salt-forming group may be prepared in a manner known per se. Acid addition salts of compounds of formula I may thus be obtained by treatment with an acid or with a suitable anion exchange reagent.
- a salt with two acid molecules for example a dihalogenide of a compound of formula I
- Salts can usually be converted to free compounds, e.g. by treating with suitable basic compounds, for example with alkali metal carbonates, alkali metal hydrogencarbonates, or alkali metal hydroxides, typically potassium carbonate or sodium hydroxide.
- suitable basic compounds for example with alkali metal carbonates, alkali metal hydrogencarbonates, or alkali metal hydroxides, typically potassium carbonate or sodium hydroxide.
- Stereoisomeric mixtures e.g. mixtures of diastereomers
- Dia- stereomeric mixtures for example may be separated into their individual diastereomers by means of fractionated crystallization, chromatography, solvent distribution, and similar procedures. This separation may take place either at the level of a starting compound or in a compound of formula I itself.
- Enantiomers may be separated through the formation of dia- stereomeric salts, for example by salt formation with an enantiomer-pure chiral acid, or by means of chromatography, for example by HPLC, using chromatographic substrates with chiral ligands.
- a compound of the formula I, wherein R 2 is O, can be converted into the respective compound wherein R 2 is S, for example, by using an appropriate sulfur compound, e.g. using reaction with Lawesson's reagent (2,4-bis-(4-methoxyphenyl)2,4-dithioxo-1 , 2,3,4- dithiaphosphetan) in an appropriate solvent such as dioxane.
- an appropriate sulfur compound e.g. using reaction with Lawesson's reagent (2,4-bis-(4-methoxyphenyl)2,4-dithioxo-1 , 2,3,4- dithiaphosphetan) in an appropriate solvent such as dioxane.
- All process steps described here can be carried out under known reaction conditions, preferably under those specifically mentioned, in the absence of or usually in the presence of solvents or diluents, preferably such as are inert to the reagents used and able to dissolve these, in the absence or presence of catalysts, condensing agents or neutralising agents, for example ion exchangers, typically cation exchangers, for example in the H + form, depending on the type of reaction and/or reactants at reduced, normal, or elevated temperature, for example in the range from -100 0 C to about 190 0 C, preferably from about -80 0 C to about 150°C, for example at -80 to -60°C, at room temperature, at - 20 to 40°C or at the boiling point of the solvent used, under atmospheric pressure or in a closed vessel, where appropriate under pressure, and/or in an inert atmosphere, for example under argon or nitrogen.
- solvents or diluents preferably such as are inert to the rea
- Salts may be present in all starting compounds and transients, if these contain salt-forming groups. Salts may also be present during the reaction of such compounds, provided the reaction is not thereby disturbed.
- isomeric mixtures that occur can be separated into their individual isomers, e.g. diastereomers or enantiomers, or into any mixtures of isomers, e.g. racemates or diastereomeric mixtures, typically as described under "Additional process steps”.
- the solvents from which those can be selected which are suitable for the reaction in question include for example water, esters, typically lower alkyl-lower alkanoates, e.g ethyl acetate, ethers, typically aliphatic ethers, e.g. diethylether, or cyclic ethers, e.g.
- tetrahydro- furan liquid aromatic hydrocarbons, typically benzene or toluene, alcohols, typically methanol, ethanol or 1- or 2-propanol, 1-butanol, nitriles, typically acetonitrile, halogenated hydrocarbons, typically dichloromethane, acid amides, typically dimethylformamide, bases, typically heterocyclic nitrogen bases, e.g. pyridine, carboxylic acids, typically lower alkanecarboxylic acids, e.g. acetic acid, carboxylic acid anhydrides, typically lower alkane acid anhydrides, e.g.
- acetic anhydride cyclic, linear, or branched hydrocarbons, typically cyclohexane, hexane, or isopentane, or mixtures of these solvents, e.g. aqueous solutions, unless otherwise stated in the description of the process.
- solvent mixtures may also be used in processing, for example through chromatography or distribution.
- the compounds of formula I are also obtainable in the form of hydrates, or their crystals can include for example the solvent used for crystallization (present as solvates).
- a compound of formula I is prepared according to or in analogy to the processes and process steps defined in the Examples.
- Starting materials New starting materials and/or intermediates, as well as processes for the preparation thereof, are likewise the subject of this invention. In the preferred embodiment, such starting materials are used and reaction conditions so selected as to enable the preferred compounds to be obtained.
- a compound of the formula II, wherein n is 0, can be prepared by the alkylation of an amino compound of the formula IV,
- R 3 has the meaning as given under formula I and X is halogen or another suitable leaving group, in the presence of a base, e.g. sodium hydroxide, in a suitable solvent, e.g. a mixture of dichloromethane and water, preferably in the presence of a phase transfer catalyst, e.g. tetrabutylammonium bromide, at a temperature between 0 0 C and 50 0 C, preferably at room temperature.
- a base e.g. sodium hydroxide
- a suitable solvent e.g. a mixture of dichloromethane and water
- a phase transfer catalyst e.g. tetrabutylammonium bromide
- a compound of the formula II, wherein n is 0, can be converted into the respective compound wherein n is 1 , for example, by using an appropriate oxidant, e.g. using reaction with meta-chloroperbenzoic acid in an appropriate solvent such as dichloromethan at room temperature.
- an appropriate oxidant e.g. using reaction with meta-chloroperbenzoic acid in an appropriate solvent such as dichloromethan at room temperature.
- a compound of the formula IV, wherein R 2 is O, can be prepared by the cyclisation of a diamino compound of the formula Vl,
- Ri and R 5 have the meanings as given under formula I with trichloromethyl chloroformate in the presence of a base, such as triethylamine in an appropriate solvent, such as dichloromethane.
- a compound of the formula Vl can be prepared by the reduction of a nitro compound of the formula VII,
- the reduction preferably takes place in the presence of a suitable reducing agent, such as hydrogen in the presence of an appropriate catalyst, such as Raney nickel under pressure, e.g. between 1.1 and 2 bar, in an appropriate solvent, e.g. an alcohol or ether, such as methanol or tetrahydrofurane or a mixture thereof.
- a suitable reducing agent such as hydrogen
- an appropriate catalyst such as Raney nickel under pressure, e.g. between 1.1 and 2 bar
- an appropriate solvent e.g. an alcohol or ether, such as methanol or tetrahydrofurane or a mixture thereof.
- the reaction temperature is preferably between 0 and 80 °C, especially 15 to 30 0 C.
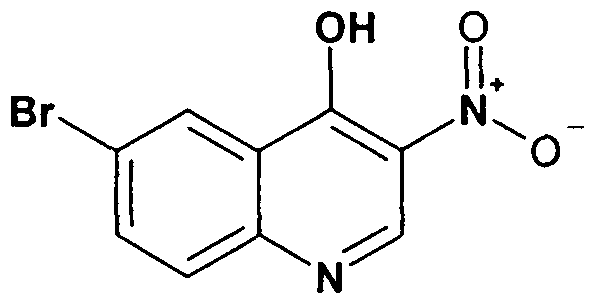
- a compound of the formula VII can be prepared by reaction of a compound VIII wherein R 5 is as defined for a compound of the formula I and Y is halogen or another suitable leaving group, is reacted with a compound of the formula IX,
- R 1 is as defined for a compound of the formula I, at a temperature between 0 0 C and 50 0 C, preferably at room temperature in a suitable solvent, i.e. acetic acid.
- Ratios of solvents are given in volume by volume (v/v).
- Example 1 The following compounds (Table 1) are prepared in a similar manner as described in example 1 by reacting 2-[4-(8-bromo-3-methyl-2-oxo-2,3-dihydro-imidazo[4,5-c]quinolin-1- yl)-phenyl]-2-methyl-propionitrile (Example 1i), with the appropriate boronic acid :
- Example 2 3-pyridineboronic acid (Aldrich, Buchs, Switzerland),
- Example 3 4-methoxy-3-pyridylboronic acid (Frontier Scientific, Logan, USA), Example 4: 3-methoxypyridine-5-boronic acid pinecol ester (Frontier Scientific,
- Example 5 4-[5-(4,4,5,5-Tetramethyl-[1 ,3,2]dioxaborolan-2-yl)-pyridin-2-yl]- piperazine-1-carboxylic acid tert-butyl ester (CB Research & Development, New Castle,
- Example 6 1 -Methyl-4-[4-(4,4,5,5-tetramethyl-[1 ,3,2]dioxaborolan-2-yl)-pyridin-2-yl]- piperazine (Oakwood Products, West Columbia, USA),
- Example 8 2-fluoroquinoline-3-boronic acid (Lancaster, Morecambe, UK), Example 9: 6-quinolineboronic acid (Asychem, Durham, USA), Example 10: 5-quinolineboronic acid (Asychem, Durham, USA), and
- Example 2 The following compounds (Table 2) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-(4-amino- phenyl)-2-ethyl-butyronitrile (Example 12a), and with the appropriate boronic acid:
- Example 1d The title compound is prepared in a similar manner as described in Example 1e using iodoethane (Fluka, Buchs, Switzerland) in Example 1d.
- Title compound: ES-MS: 189 (M + H) + , Br pattern; analytical HPLC: t ret 2.50 min (Grad 1).
- Example 3 The following compounds (Table 3) are prepared in a similar manner as described in Example 1 by reacting ⁇ -bromo ⁇ -chloro-S-nitro-quinoline (Example 1c) with 1-(4-amino-2- fluoro-phenyl)-pyrrolidin-2-one (Example 14a), and with the appropriate boronic acid:
- Example 14b 1-(2-Fluoro-4-nitro-phenyl)-pyrrolidin-2-one To 468 mg (5.5 mmol) of 2-pyrrolidone (Fluka, Buchs, Switzerland) in 10 ml of DMF at 0 0 C are added 240 mg (5.5 mmol) of 55% NaH in oil. The reaction mixture is stirred for 30 min at 0 0 C and for 30 min at it After this time, 795 mg (5 mmol) of 3,4-difluoronitrobenzene (Aldrich, Buchs, Switzerland) are added and the reaction mixture is stirred for 1 h at rt. The reaction mixture is quenched with 1 M aqueous HCI and extracted with EtOAc (2*).
- Example 4 The following compounds (Table 4) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 1-(4-amino- phenyl)-pyrrolidin-2-one (Example 16a), and with the appropriate boronic acid:
- Example 5 The following compounds (Table 5) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-fluoro-N1,N1- bis-(2-methoxy-ethyl)-benzene-1 ,4-diamine (Example 18a), and with the appropriate boronic acid:
- Example 18b The title compound is obtained in a similar manner as described in Example 14a starting with (2-fluoro-4-nitro-phenyl)-bis-(2-methoxy-ethyl)-amine (Example 18b).
- Title compound: ES- MS: 243 (M+H) + ; analytical HPLC: t ret 1.98 minutes (Grad 1).
- Example 6 The following compounds (Table 6) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with N,N-bis-(2- methoxy-ethyl)-benzene-1 ,4-diamine (Example 20a), and with the appropriate boronic acid:
- Example 7 The following compounds (Table 7) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2- naphthylamine (Aldrich, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 8 The following compounds (Table 8) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-chloroaniline (Fluka, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 9 The following compounds (Table 9) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-toluidine (Fluka, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 10 The following compounds (Table 10) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-ethylaniline (Aldrich, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 11 The following compounds (Table 11) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2- trifluoromethylaniline (Fluka, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 12 The following compounds (Table 12) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 4-fluoro-2- methylaniline (Aldrich, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 13 The following compounds (Table 13) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-chloro-4- fluoroaniline (Aldrich, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 14 The following compounds (Table 14) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 3-chloroaniline (Fluka, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 15 The following compounds (Table 15) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 3- trifluoromethylaniline (Fluka, Buchs, Switzerland), and with the appropriate boronic acid:
- Example 16 The following compounds (Table 16) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 4- methoxymethylaniline (Example 38a), and with the appropriate boronic acid: Table 16
- Example 17 The following compounds (Table 17) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 2-chloro-4-(2- methoxy-ethyl)-phenylamine (Example 42a), and with the appropriate boronic acid:
- Example 18 The following compounds (Table 18) are prepared in a similar manner as described in Example 1 by reacting 6-bromo-4-chloro-3-nitro-quinoline (Example 1c) with 4-(2-methoxy- ethyl)-phenylamine (Example 42b), and with the appropriate boronic acid:
- Example 50a 62 mg (0.128 mmol) of methyl-[4-(3-methyl-2-oxo-8-pyridin-3-yl-2,3-dihydro-imidazo[4,5- c]quinolin-1-yl)-phenyl]-carbamic acid tert-butyl ester (Example 50a) is treated with 2.5 ml of 1 M HCI in dioxane at rt for 1 h, and then the solution is evaporated to dryness. The residue is taken in 2 ml of CH 2 CI 2 together with 414 ⁇ l (5.13 mmol) of pyridine and 66 mg (0.579 mmol) of mesylchloride (Fluka, Buchs, Switzerland).
- Example 1c The title compound is prepared in a similar manner as described in Example 1 by reacting 6- bromo ⁇ 4-chloro-3-nitro-quinoline (Example 1c) with (4-amino-phenyl)-carbamic acid tert-butyl ester (Fluka, Buchs, Switzerland) and using 3-pyrineboronic acid (Aldrich, Buchs, Switzerland).
- Example 1h The title compound is prepared in a similar manner as described in Example 1 by reacting 2- [4-(8-bromo-2-oxo-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-2-methyl-propionitrile (Example 1h) with iodoethane (Fluka, Buchs, Switzerland) and using 3-pyridineboronic acid.
- Example 57a The title compound is prepared in a similar manner as described in Example 1 using 3- fluoro-4-(4-methanesulfonyl-piperazin-1-yl)-phenylamine (Example 57a) and 3- quinolineboronic acid.
- Title compound: ES-MS: 583.5 (M+H) + ; analytical HPLC: t ret 4.12 minutes (Grad 2).
- Example 59a The title compound is prepared in a similar manner described in Example 1 using 4-(4- amino-2-fluoro-phenyl)-piperazine-1-carboxylic acid tert-butyl ester (Example 59a) and 3- quinolineboronic acid.
- the removal of the tert-butoxycarbonyl protecting group is performed by using 4 N HCI in dioxane following protocols known in the art (The peptides, Vol. 3; ed. Edhard Gross and Johannes Meienhofer, Academic Press, New York).
- Title compound: ES- MS: 505.4 (M+H) + ; analytical HPLC: t ret 3.63 minutes (Grad 2).
- Example 14a The title compound is obtained as described in Example 14a using 4-(2-fluoro-4-nitro- phenyl)-piperazine-1-carboxylic acid tert-butyl ester (Example 59b).
- Title compound: ES-MS: 296.3 (M+H) + ; analytical HPLC: t ret 4.18 minutes (Grad 2).
- Example 63a The title compound is obtained as described in Example 1 using (6-bromo-3-nitro-quinolin-4- yl)-[2-chloro-4-(4-methyl-piperazin-1-yl)-phenyl]-amine (Example 63a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 535.4 (M+H) + ; analytical HPLC: t ret 3.93 minutes (Grad 2).
- Example 65a The title compound is obtained as described in Example 1 using 3-chloro-4-(4-methyl- piperazin-1-yl)-phenylamine (Example 65a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 536.4 (M+H) + ; analytical HPLC: W 3.78 minutes (Grad 2).
- Example 68a The title compound is obtained as described in Example 1 using 4-imidazol-1-yl-2-methyl- phenylamine (Example 68a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 483.4 (M+H) + ; analytical HPLC: U t 3.78 minutes (Grad 2).
- Example 69a The title compound is obtained as described in Example 1 using 4-pyrazol-1-yl-phenylamine (Example 69a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 469.4 (M+H) + ; analytical HPLC: t ret 4.18 minutes (Grad 2).
- Example 71a The title compound is obtained as described in Example 1 using 4-[1 ,2, 4]triazol-1 -yl- phenylamine (Example 71a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 470.3 (M+H) + ; analytical HPLC: W 3.99 minutes (Grad 2).
- Example 73a The title compound is obtained as described in Example 1 using 4-(4-methyl-piperazin-1-yl)- 3-trifluoromethyl-phenylamine (Example 73a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 569.5 (M+H) + ; analytical HPLC: W 4.08 minutes (Grad 2).
- Example 75 i-IS-Chloro ⁇ -piperazin-i-yl-phenyO-S-methyl- ⁇ -quinolin-S-yl-I.S-dihydro-imidazo ⁇ . ⁇ - c]quinolin-2-one
- the title compound is obtained as described in Example 1 using 4-(4-amino-2-chloro- phenyl)-piperazine-1-carboxylic acid tert-butyl ester (Example 75a) and 3-quinolineboronic acid and removal of the tert-butoxycarbonyl protecting group in a similar manner as described in Example 59.
- Title compound: ES-MS: 521.4 (M+H) + ; analytical HPLC: t ret 3.68 minutes (Grad 2).
- Example 75b The title compound is obtained as described in Example 1e using 2-chloro-4-(4-nitro-phenyl)- piperazine-1-carboxylic acid tert-butyl ester (Example 75b) as starting material.
- Title compound: ES-MS: 312.2, 314.3 (M+H) + ; analytical HPLC: t ret 4.58 minutes (Grad 2).
- Example 76 i-IS-Chloro ⁇ -piperazin-i-yl-phenyO-S-methyl- ⁇ -pyridin-S-yl-I.S-dihydro-imidazo ⁇ . ⁇ - c]quinolin-2-one
- the title compound is obtained as described in Example 75 using 3-pyridineboronic acid.
- Title compound: ES-MS: 471.3 (M+H) + ; analytical HPLC: t ret 3.42 minutes (Grad 2).
- Example 84a The title compound is obtained as described in Example 1 using 4-(4-amino-2- trifluoromethyl-phenyl)-piperazine-1-carboxylic acid tert-butyl ester (Example 84a).
- Title compound: Title compound: ES-MS: 555.0 (M+H) + ; analytical HPLC: t ret 3.86 minutes (Grad 2).
- Example 84a 4-(4-Amino-2-trifluoromethyl-phenyl)-piperazine-1-carboxylic acid tert-butyl ester
- the title compound is obtained as described in Example 1e using 4-(4-nitro-2-trifluoromethyl- phenyl)-piperazine-1-carboxylic acid tert-butyl ester (Example 84b) as starting material.
- Title compound: ES-MS: 346.2 (M+H) + ; analytical HPLC: W 4.95 minutes (Grad 2).
- Example 89a The title compound is obtained as described in Example 1 using 4-(4-amino-2-chloro- phenyl)-2,6-c/s-dimethyl-piperazine-1 -carboxylic acid tert-butyl ester (Example 89a) and 3- pyridineboronic acid and removal of the tert-butoxycarbonyl protecting group in a similar manner as described in Example 59.
- Title compound: ES-MS: 499 (M+H) + ; analytical HPLC: t r e t 2.24 minutes (Grad 1).
- Example 89b The title compound is obtained in a similar manner as described in Example 1e starting with 4-(2-chloro-4-nitro-phenyl)-2,6-c/s-dimethyl-piperazine-1 -carboxylic acid tert-butyl ester (Example 89b).
- Title compound: ES-MS: 340 (M+H) + ; analytical HPLC: t ret 3.35 minutes (Grad 1).
- Example 89c 1-(2-Chloro-4-nitro-phenyl)-3,5-c/s-dimethyl-piperazine 1.0 g (5.21 mmol) of 3,4-dichloronitrobenzene (Fluka, Buchs, Switzerland), 624 mg (5.47 mmol) cis-2,6-dimethylpiperazine (Aldrich, Buchs, Switzerland) and 580 mg (5.73 mmol) triethylamine in 20 ml of EtOH are heated in a microwave oven at 170 0 C for 6 h and 180 0 C for 2 h. The reaction mixture is evaporated to dryness and then taken in EtOAc.
- Example 93a The title compound is obtained in a similar manner as described in Example 1 using 3- chloro-4-(4-isopropyl-piperazin-1-yl)-phenylamine (Example 93a) and 3-pyridineboronic acid.
- Title compound: ES-MS: 513 (M+H) + ; analytical HPLC: t ret 2.32 minutes (Grad 1).
- Example 93b The title compound is obtained in a similar manner as described in Example 1e using 1-(2- Chloro-4-nitro-phenyl)-4-isopropyl-piperazine (Example 93b).
- Title compound: ES-MS: 254 (M+H) + ; analytical HPLC: t re , 1.80 minutes (Grad 1).
- Example 93a The title compound is obtained in a similar manner as described in Example 1 using 3- chloro-4-(4-isopropyl-piperazin-1-yl)-phenylamine (Example 93a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 563 (M+H) + ; analytical HPLC: t ret 2.68 minutes (Grad 1).
- Example 95a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- amino ⁇ -trifluoromethyl-phenyO ⁇ .e-c/s-dimethyl-piperazine-i-carboxylic acid tert-butyl ester (Example 95a) and 3-pyridineboronic acid and removal of the tert-butoxycarbonyl protecting group in a similar manner as described in Example 59.
- Title compound: ES-MS: 533 (M+H) + ; analytical HPLC: t ret 2.37 minutes (Grad 1).
- Example 95b The title compound is obtained in a similar manner as described in Example 1e starting with 4-(4-nitro-2-trifluoromethyl-phenyl)-2,6-c/s-dimethyl-piperazine-1 -carboxylic acid tert-butyl ester (Example 95b).
- Title compound: ES-MS: 374 (M+H) + ; analytical HPLC: t rel 3.79 minutes (Grad 1).
- Example 89c The title compound is obtained in a similar manner as described in Example 89b starting with c/s-3,5-Dimethyl-1-(4-nitro-2-trifluoromethyl-phenyl)-piperazine (Example 89c).
- Title compound: ES-MS: 404 (M+H) + ; analytical HPLC: t ret 4.76 minutes (Grad 1).
- Example 95a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- amino-2-trifluoromethyl-phenyl)-2,6-c/s-dimethyl-piperazine-1 -carboxylic acid tert-butyl ester (Example 95a) and 3-quinolineboronic acid and removal of the tert-butoxycarbonyl protecting group in a similar manner as described in Example 59.
- Title compound: ES-MS: 583 (M+H) + ; analytical HPLC: t ret 2.71 minutes (Grad 1).
- Example 97a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- ethyl-piperazin-1-yl)-3-trifluoromethyl-phenylamine (Example 97a) and 3-pyridineboronic acid.
- Title compound: ES-MS: 533 (M+H) + ; analytical HPLC: t ret 2.38 minutes (Grad 1).
- Example 97a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- ethyl-piperazin-1-yl)-3-trifluoromethyl-phenylamine (Example 97a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 583 (M+H) + ; analytical HPLC: t ret 2.73 minutes (Grad 1).
- Example 99a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- isopropyl-piperazin-1-yl)-3-trifluoromethyl-phenylamine (Example 99a) and 3-pyridineboronic acid.
- Title compound: ES-MS: 547 (M+H) + ; analytical HPLC: t ret 2.45 minutes (Grad 1).
- Example 99a 4-(4-lsopropyl-piperazin-1-yl)-3-trifluoromethyl-phenylamine
- the title compound is obtained in a similar manner as described in Example 95a/c using N- isopropylpiperazine (Aldrich, Buchs, Switzerland).
- Title compound: ES-MS: 288 (M+H) + ; analytical HPLC: t ret 2.17 minutes (Grad 1).
- Example 99a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- isopropyl-piperazin-1-yl)-3-trifluoromethyl-phenylamine (Example 99a) and 3- quinolineboronic acid.
- Title compound: ES-MS: 597 (M+H) + ; analytical HPLC: t rel 2.82 minutes (Grad 1).
- Example 104b The title compound is obtained in a similar manner as described in Example 1e starting with 1-(2-chloro-4-nitro-phenyl)-1H-imidazole (Example 104b).
- Title compound: ES-MS: 194 (M+H) + ; analytical HPLC: t ret 1.84 minutes (Grad 1).
- Example 105 i-JS-Chloro ⁇ -imidazol-i-yl-phenylJ-S-methyl- ⁇ -quinolin-S-yl-I.S-dihydro-imidazo ⁇ .S- c]quinolin-2-one
- the title compound is obtained in a similar manner as described in Example 1 using 3- chloro-4-imidazol-1-yl-phenylamine (Example 104a) and 3-quinolineboronic acid.
- Title compound: ES-MS: 503 (M+H) + ; analytical HPLC: t ret 2.44 minutes (Grad 1).
- Example 109d The title compound is obtained in a similar manner as described in Example 1 using 2-[4-(8- bromo-S-methyl ⁇ -oxo- ⁇ -oxy ⁇ .S-dihydro-imidazo ⁇ . ⁇ -cjquinolin-i-ylJ-phenylJ ⁇ -methyl- propionitrile (Example 109d) and 3-quinolineboronic acid.
- Title compound: ES-MS: 486 (M + H) + ; analytical HPLC: U t 3.14 minutes (Grad 1).
- Example 110a The title compound is obtained in a similar manner as described in Example 1 using 1-(4- amino-phenyl)-cyclopropanecarbonitrile (Example 110a) and 3-pyridineboronic acid.
- Title compound: ES-MS: 418 (M+H) + ; analytical HPLC: t re , 3.82 minutes (Grad 2).
- Example 110b 4-(1-Cyano-cyclopropyl)-benzoic acid
- To 2 g (12.4 mmol) of 4-(cyanomethyl)benzoic acid (Ubichem, Eastleigh, UK) and 10.9 ml (124 mmol) 1 ,2-dibromoethane (Fluka, Buchs, Switzerland) cooled at 0 G C with an ice-bath are added a solution of 14.4 g (62 mmol) benzyltriethylammonium chloride in 50 ml 8 M aqueous NaOH.
- the reaction mixture is stirred over night at rt and then is acidified at pH 1-2 with 6 M aqueous HCI and is extracted with EtOAc.
- Example 11Oa 1-(4- amino-phenyl)-cyclopropanecarbonitrile
- Example 11Oa 3-quinolineboronic acid.
- Title compound: ES-MS: 468 (M+H) + ; analytical HPLC: W 4.14 minutes (Grad 2).
- Example 11Oa The title compound is obtained in a similar manner as described in Example 1 using 1-(4- amino-phenyl)-cyclopropanecarbonitrile (Example 11Oa) and 2-methoxy-5-pyridineboronic acid.
- Title compound: ES-MS: 448.5 (M+H) + ; analytical HPLC: t ret 4.42 minutes (Grad 2).
- Example 95a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- amino ⁇ -trifluoromethyl-phenyl ⁇ . ⁇ -c/s-dimethyl-piperazine-i-carboxylic acid tert-butyl ester (Example 95a) and 2-methoxy-5-pyridineboronic acid and removal of the tert-butoxycarbonyl protecting group in a similar manner as described in Example 59.
- Title compound: ES-MS: 563 (M+H) + ; analytical HPLC: t re , 2.76 minutes (Grad 1).
- Example 95a The title compound is obtained in a similar manner as described in Example 1 using 4-(4- amino-2-trifluoromethyl-phenyl)-2,6-c/s-dimethyl-piperazine-1-carboxylic acid tert-butyl ester (Example 95a) and 3-methoxy-5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)pyridine and removal of the tert-butoxycarbonyl protecting group in a similar manner as described in Example 59.
- Title compound: ES-MS: 563 (M+H) + ; analytical HPLC: t ret 2.50 minutes (Grad 1).
- Example 128a The title compound is obtained as described in Example 1 using 4-[1 ,2,4]triazol-1-yl-3- trifluoromethylphenylamine (Example 128a).
- Title compound: ES-MS: 488 (M+H) + ; analytical HPLC: W 3.72 minutes (Grad 2).
- Example 128a 4-[1 ,2,4]Triazol-1 -yl-3-trif luoromethy l-phenylamine
- the title compound is obtained in a similar manner as Example 71a using 1-fluoro-4-nitro-2- trifluoromethyl-benzene (Aldrich, Buchs, Switzerland) and 1 ,2,4-triazole (Fluka, Buchs, Switzerland).
- Title compound: ES-MS: 229 (M+H) + ; analytical HPLC: t ret 4.14 minutes (Grad 2).
- Example 71a The title compound is obtained in a similar manner as Example 71a using 1-fluoro-4-nitro-2- trifluoromethyl-benzene (Aldrich, Buchs, Switzerland) and pyrazole (Fluka, Buchs, Switzerland).
- Title compound: ES-MS: 228 (M+H) + ; analytical HPLC: t ret 4.58 minutes (Grad 2).
- Example 142 i-tS-Chloro ⁇ -JI ⁇ Jtriazol-i-yl-phenyO-S-methyl-S-pyridin-S-yl-I.S-dihydro- imidazo[4,5-c]quinolin-2-one
- the title compound is obtained in a similar manner as described in Example 1 starting with 3-chloro-4-[1 ,2,4]triazol-1-yl-phenylamine (Example 142a).
- Title compound: ES-MS: 454 (M+H) + ; analytical HPLC: t ret 2.28 minutes (Grad 1).
- Example 144a The title compound is obtained as described in Example 1 using 4-pyrazol-1-yl-3- trifluoromethyl-phenylamine (Example 144a).
- Title compound: ES-MS: 487 (M+H) + ; analytical HPLC: t ret 3.54 minutes (Grad 2).
- Example 138a The title compound is obtained in a similar manner as Example 138a using imidazole.
- Title compound: ES-MS: 228 (M+H) + ; analytical HPLC: t ret 3.73 minutes (Grad 2).
- Example 146 1 -(4-lmidazol-1 -yl-3-trifluoromethyl-pheny l)-8-(6-methoxy-py ridin-3-yl)-3-methyl-1 ,3- dihydro-imidazo[4,5-c]quinoIin-2-one
- Example 148a The title compound is obtained in a similar manner as described in Example 1 starting with 4-[1 ,2,4]Triazol-1-ylmethyl-phenylamine (Example 148a).
- Title compound: ES-MS: 434 (M+H) + ; analytical HPLC: t ret 2.13 minutes (Grad 1).
- Example 148b The title compound is obtained in a similar manner as described in Example 1e starting with 1-(4-nitro-benzyl)-1 H-[1 ,2,4]triazole (Example 148b).
- Title compound: ES-MS: 175 (M+H) + ; analytical HPLC: t ret minutes (Grad 2).
- Example 150a The title compound is obtained in a similar manner as described in Example 1 starting with 4-imidazol-1-ylmethyl-phenylamine (Example 150a).
- Title compound: ES-MS: 433 (M+H) + ; analytical HPLC: t ret 1.96 minutes (Grad 1).
- maleic acid salts are prepared in a stoichiometric ratio of 1 :1 following standard reaction conditions in analogy to or according to methods that are known in the art.
- 153-1) Maleic acid 2-methyl-2- ⁇ 4-[3-methyl-2-oxo-8-(6-piperazin-1-yl-pyridin-3-yl)-2,3- dihydro-imidazo[4,5-c]quinolin-1-yl]-phenyl ⁇ -propionitrile salt;
- 153-3) Maleic acid 1-(3-fluoro-4-piperazin-1-yl-phenyl)-3-methyl-8-pyridin-3-yl-1 ,3- dihydro-imidazo[4,5-c]quinolin-2-one salt;
- Example 154 Soft capsules
- Preparation process The pulverized active ingredient is suspended in Lauroglykol® (propylene glycol laurate, Gattefosse S.A., Saint Priest, France) and ground in a wet pulverizer to produce a particle size of about 1 to 3 ⁇ m. 0.419 g portions of the mixture are then introduced into soft gelatin capsules using a capsule-filling machine.
- Lauroglykol® propylene glycol laurate, Gattefosse S.A., Saint Priest, France
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Rheumatology (AREA)
- Dermatology (AREA)
- Urology & Nephrology (AREA)
- Neurology (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Pain & Pain Management (AREA)
- Otolaryngology (AREA)
- Transplantation (AREA)
- Oncology (AREA)
- Ophthalmology & Optometry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Description
Claims
Priority Applications (27)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EA200702387A EA013434B1 (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| PL06753710T PL1888578T3 (en) | 2005-05-20 | 2006-05-18 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl)-phenyl]propionitrile as lipid kinase inhibitor |
| DK06753710.0T DK1888578T3 (en) | 2005-05-20 | 2006-05-18 | 2-Methyl-2- [4- (3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl) -phenyl] -propionitrile as a lipid kinase inhibitor |
| AT06753710T ATE535526T1 (en) | 2005-05-20 | 2006-05-18 | 2-METHYL-2-Ä4-(3-METHYL-2-OXO-8-QUINOLINE-3-YL-2 3-DIHYDRO-IMIDAZO Ä4,5-CÜ CHINOLINYL)-PHENYLUPROPIONITRIL AS A LIPID KINASE INHIBITOR |
| KR1020077026850A KR101237575B1 (en) | 2005-05-20 | 2006-05-18 | 1,3-dihydro-imidazo[4,5-c]quinolin-2-ones as lipid kinase inhibitors |
| GEAP200610380A GEP20105054B (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| SM200700051T SMAP200700051A (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| BRPI0610321A BRPI0610321B8 (en) | 2005-05-20 | 2006-05-18 | imidazoquinolines as lipid kinase inhibitors, their use and preparation process, and pharmaceutical preparation |
| NZ562890A NZ562890A (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| ES06753710T ES2378463T3 (en) | 2005-05-20 | 2006-05-18 | 2-methyl-2- [4- (3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl) -phenyl] propionitrile as a kinase inhibitor lipid |
| CA2608496A CA2608496C (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| JP2008511640A JP5220591B2 (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| SI200631230T SI1888578T1 (en) | 2005-05-20 | 2006-05-18 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl)-phenyl] propionitrile as lipid kinase inhibitor |
| EP06753710A EP1888578B8 (en) | 2005-05-20 | 2006-05-18 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl)-phenyl]propionitrile as lipid kinase inhibitor |
| AU2006249071A AU2006249071B2 (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors |
| US11/913,788 US7667039B2 (en) | 2005-05-20 | 2006-05-18 | 1,3-dihydro-imidazo [4,5-C] quinolin-2-ones as lipid kinase inhibitors |
| MX2007014380A MX2007014380A (en) | 2005-05-20 | 2006-05-18 | Imidazoquinolines as lipid kinase inhibitors. |
| ZA2007/09074A ZA200709074B (en) | 2005-05-20 | 2007-10-22 | Imidazoquinolines as lipid kinase inhibitors |
| IL187003A IL187003A (en) | 2005-05-20 | 2007-10-29 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile and use thereof for the preparation of a medicament for the treatment of a proliferative disease |
| TNP2007000431A TNSN07431A1 (en) | 2005-05-20 | 2007-11-19 | Imidazoquinolines as lipid kinase inhibitors |
| NO20076561A NO340584B1 (en) | 2005-05-20 | 2007-12-19 | New Imidazoquinolines, Methods for Preparing Such and Pharmaceutical Preparations Containing Such, as well as Use as a Pharmaceutical Preparation and Application for the Preparation of a Pharmaceutical Preparation. |
| HK08106290.6A HK1115871A1 (en) | 2005-05-20 | 2008-06-05 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl)-phenyl]propionitrile as lipid kinase inhibitor |
| US12/577,268 US7994170B2 (en) | 2005-05-20 | 2009-10-12 | 1,3-dihydro-imidazo[4,5-C]quinolin-2-ones as lipid kinase inhibitors |
| US13/165,144 US8431592B2 (en) | 2005-05-20 | 2011-06-21 | 1,3-dihydro-imidazo[4,5-c]quinolin-2-ones as lipid kinase inhibitors |
| US13/453,225 US20120207751A1 (en) | 2005-05-20 | 2012-04-23 | Imidazoquinolines as lipid kinase inhibitors |
| IL221573A IL221573A (en) | 2005-05-20 | 2012-08-22 | Imidazoquinolines and use thereof in the preparation of medicaments for treating proliferative, inflammatory and obstructive respiratory diseases |
| HRP20130794TT HRP20130794T1 (en) | 2005-05-20 | 2013-08-22 | 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo [4,5-c] quinolinyl)-phenyl]propionitrile as lipid kinase inhibitor |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB0510390.8A GB0510390D0 (en) | 2005-05-20 | 2005-05-20 | Organic compounds |
| GB0510390.8 | 2005-05-20 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/913,788 A-371-Of-International US7667039B2 (en) | 2005-05-20 | 2006-05-18 | 1,3-dihydro-imidazo [4,5-C] quinolin-2-ones as lipid kinase inhibitors |
| US12/577,268 Division US7994170B2 (en) | 2005-05-20 | 2009-10-12 | 1,3-dihydro-imidazo[4,5-C]quinolin-2-ones as lipid kinase inhibitors |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006122806A2 true WO2006122806A2 (en) | 2006-11-23 |
| WO2006122806A3 WO2006122806A3 (en) | 2009-03-12 |
Family
ID=34834420
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2006/004725 WO2006122806A2 (en) | 2005-05-20 | 2006-05-18 | 1,3-dihydro-imidazo [4,5-c] quinolin-2-ones as lipid kinase inhibitors |
Country Status (38)
| Country | Link |
|---|---|
| US (4) | US7667039B2 (en) |
| EP (3) | EP2292617B1 (en) |
| JP (2) | JP5220591B2 (en) |
| KR (1) | KR101237575B1 (en) |
| CN (2) | CN102796099A (en) |
| AR (2) | AR054127A1 (en) |
| AT (1) | ATE535526T1 (en) |
| AU (1) | AU2006249071B2 (en) |
| BR (1) | BRPI0610321B8 (en) |
| CA (1) | CA2608496C (en) |
| CR (1) | CR9504A (en) |
| CY (2) | CY1112369T1 (en) |
| DK (3) | DK1888578T3 (en) |
| EA (1) | EA013434B1 (en) |
| ES (3) | ES2397081T3 (en) |
| GB (1) | GB0510390D0 (en) |
| GE (1) | GEP20105054B (en) |
| GT (1) | GT200600193A (en) |
| HK (2) | HK1115871A1 (en) |
| HR (2) | HRP20130799T1 (en) |
| IL (2) | IL187003A (en) |
| JO (1) | JO2751B1 (en) |
| MA (1) | MA29462B1 (en) |
| MX (1) | MX2007014380A (en) |
| MY (1) | MY147442A (en) |
| NI (1) | NI200700296A (en) |
| NO (1) | NO340584B1 (en) |
| NZ (1) | NZ562890A (en) |
| PE (1) | PE20070004A1 (en) |
| PL (2) | PL2270008T3 (en) |
| PT (2) | PT2270008E (en) |
| SI (2) | SI2270008T1 (en) |
| SM (1) | SMAP200700051A (en) |
| TN (1) | TNSN07431A1 (en) |
| TW (1) | TWI383982B (en) |
| UA (1) | UA92490C2 (en) |
| WO (1) | WO2006122806A2 (en) |
| ZA (1) | ZA200709074B (en) |
Cited By (421)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007106854A2 (en) * | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| WO2008064093A2 (en) | 2006-11-20 | 2008-05-29 | Novartis Ag | Salts and crystal forms of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile |
| EP1956008A1 (en) * | 2005-11-30 | 2008-08-13 | Mitsubishi Chemical Corporation | Organic compound, charge-transporting material, composition for charge-transporting material and organic electroluminescent device |
| WO2008103636A1 (en) * | 2007-02-20 | 2008-08-28 | Novartis Ag | Imidazoquinolines as dual lipid kinase and mtor inhibitors |
| WO2008115974A2 (en) * | 2007-03-21 | 2008-09-25 | Wyeth | Pyrazolopyrimidine analogs and their use as mtor kinase and pi3 kinase inhibitors |
| WO2009013305A1 (en) * | 2007-07-24 | 2009-01-29 | Novartis Ag | Use of imidazoquinolines for the treatment of egfr dependent diseases or diseases that have acquired resistance to agents that target egfr family members |
| WO2009076170A2 (en) * | 2007-12-13 | 2009-06-18 | Novartis Ag | Combinations of therapeutic agents for treating cancer |
| WO2009008991A3 (en) * | 2007-07-06 | 2009-07-09 | Us Gov Nat Inst Health | Dna-pkcs modulates energy regulation and brain function |
| WO2009118324A1 (en) * | 2008-03-26 | 2009-10-01 | Novartis Ag | 5imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| WO2010006225A1 (en) * | 2008-07-11 | 2010-01-14 | Novartis Ag | Combination of (a) a phosphoinositide 3-kinase inhibitor and (b) a modulator of ras/raf/mek pathway |
| WO2010038165A1 (en) * | 2008-09-30 | 2010-04-08 | Pfizer Inc. | Imidazo[1,5]naphthyridine compounds, their pharmaceutical use and compositions |
| WO2010044401A1 (en) | 2008-10-14 | 2010-04-22 | 第一三共株式会社 | Morpholinopurine derivative |
| WO2010110686A1 (en) | 2009-03-27 | 2010-09-30 | Pathway Therapeutics Limited | Pyrimidinyl and 1,3,5 triazinyl benzimidazoles and their use in cancer therapy |
| WO2010110685A2 (en) | 2009-03-27 | 2010-09-30 | Pathway Therapeutics Limited | Pyrimddinyl and 1,3,5-triazinyl benzimtoazole sulfonamides and their use in cancer therapy |
| WO2010139747A1 (en) * | 2009-06-04 | 2010-12-09 | Novartis Ag | 1H-IMIDAZO[4, 5-c]QUINOLINONE COMPOUNDS, USEFUL FOR THE TREATMENT OF PROLIFERATIVE DISEASES |
| WO2010139731A1 (en) * | 2009-06-04 | 2010-12-09 | Novartis Ag | 1H-IMIDAZO[4,5-c]QUINOLINONE DERIVATIVES |
| WO2011001212A1 (en) * | 2009-06-30 | 2011-01-06 | Piramal Life Sciences Limited | Imidazo [4,5-c]quinoline derivatives and their use in the treatment of tumors and/or inflammation |
| WO2011005119A1 (en) | 2009-07-07 | 2011-01-13 | Pathway Therapeutics Limited | Pyrimidinyl and 1,3,5-triazinyl benzimidazoles and their use in cancer therapy |
| WO2011007819A1 (en) | 2009-07-17 | 2011-01-20 | 塩野義製薬株式会社 | Pharmaceutical product containing lactam or benzene sulfonamide compound |
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| WO2011104266A1 (en) | 2010-02-25 | 2011-09-01 | Novartis Ag | Dimeric iap inhibitors |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US8026366B2 (en) | 2004-06-18 | 2011-09-27 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| WO2011120911A1 (en) | 2010-03-30 | 2011-10-06 | Novartis Ag | Pkc inhibitors for the treatment of b-cell lymphoma having chronic active b-cell-receptor signalling |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| WO2012000632A1 (en) * | 2010-07-01 | 2012-01-05 | Merck Patent Gmbh | Pyrazolo-quinolines |
| WO2012004299A1 (en) | 2010-07-06 | 2012-01-12 | Novartis Ag | Tetrahydro-pyrido-pyrimidine derivatives |
| WO2012007926A1 (en) | 2010-07-16 | 2012-01-19 | Piramal Life Sciences Limited | Substituted imidazoquinoline derivatives as kinase inhibitors |
| JP2012504573A (en) * | 2008-10-01 | 2012-02-23 | ノバルティス アーゲー | Smoothened antagonism for the treatment of hedgehog pathway related disorders |
| WO2012022814A1 (en) | 2010-08-20 | 2012-02-23 | Novartis Ag | Antibodies for epidermal growth factor receptor 3 (her3) |
| WO2012028233A1 (en) * | 2010-08-28 | 2012-03-08 | Merck Patent Gmbh | Imidazo[4,5-c]quinolines as dna-pk inhibitors |
| CN102372712A (en) * | 2010-08-18 | 2012-03-14 | 山东轩竹医药科技有限公司 | Imidazodiazanaphthalene PI3K (phosphatidyl inositol 3 kinase) and mTOR (mammalian target of rapamycin) dual inhibitor |
| WO2012044641A1 (en) | 2010-09-29 | 2012-04-05 | Pathway Therapeutics Inc. | 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| WO2012047775A1 (en) * | 2010-10-04 | 2012-04-12 | Novartis Ag | Pharmaceutical combinations |
| WO2012068487A1 (en) | 2010-11-18 | 2012-05-24 | Synta Pharmaceuticals Corp. | Preselection of subjects for therapeutic treatment with oxygen sensitive agents based on hypoxic status |
| WO2012068483A1 (en) | 2010-11-18 | 2012-05-24 | Synta Pharmaceuticals Corp. | Preselection of subjects for therapeutic treatment based on hypoxic status |
| WO2012075253A2 (en) | 2010-12-03 | 2012-06-07 | Novartis Ag | Pharmaceutical compositions |
| WO2012077031A1 (en) | 2010-12-06 | 2012-06-14 | Piramal Life Sciences Limited | Substituted imidazoquinoline derivatives |
| WO2012080271A1 (en) | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| WO2012080260A1 (en) | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| AU2010247397B2 (en) * | 2009-05-15 | 2012-07-12 | Novartis Ag | Combination of a phosphoinositide 3-kinase inhibitor and an antidiabetic compound |
| WO2012110953A1 (en) | 2011-02-16 | 2012-08-23 | Novartis Ag | Combinations of therapeutic agents for use in the treatment of neurodegenerative diseases |
| US20120238562A1 (en) * | 2011-03-09 | 2012-09-20 | Cedars-Sinai Medical Center | Treatment of cancer by targeting molecules that influence mst1/stk4 signaling |
| WO2013006532A1 (en) | 2011-07-01 | 2013-01-10 | Novartis Ag | Combination therapy comprising a cdk4/6 inhibitor and a pi3k inhibitor for use in the treatment of cancer |
| WO2013010092A1 (en) | 2011-07-13 | 2013-01-17 | Novartis Ag | 4-oxo-3,5,7,8-tetrahydro-4h-pyrano {4,3-d} pyrminidinyl compounds for use as tankyrase inhibitors |
| WO2013008217A1 (en) | 2011-07-13 | 2013-01-17 | Novartis Ag | 4 - piperidinyl compounds for use as tankyrase inhibitors |
| WO2013012723A1 (en) | 2011-07-13 | 2013-01-24 | Novartis Ag | Novel 2-piperidin-1-yl-acetamide compounds for use as tankyrase inhibitors |
| WO2013023119A1 (en) | 2011-08-10 | 2013-02-14 | Novartis Pharma Ag | JAK P13K/mTOR COMBINATION THERAPY |
| US8394818B2 (en) | 2008-10-17 | 2013-03-12 | Dana-Farber Cancer Institute, Inc. | Soluble mTOR complexes and modulators thereof |
| WO2013049300A1 (en) | 2011-09-30 | 2013-04-04 | Dana-Farber Cancer Institute, Inc. | Method of treating mucoepidermoid carcinoma |
| WO2013053273A1 (en) * | 2011-10-10 | 2013-04-18 | 上海恒瑞医药有限公司 | Imidazo quinoline derivative and medicinal salt thereof, preparation method thereof and use in medicine thereof |
| WO2013057711A1 (en) | 2011-10-21 | 2013-04-25 | Novartis Ag | Quinazoline derivatives as pi3k modulators |
| WO2013063000A1 (en) | 2011-10-28 | 2013-05-02 | Novartis Ag | Method of treating gastrointestinal stromal tumors |
| US8440829B2 (en) | 2011-01-14 | 2013-05-14 | Eli Lilly And Company | PI3 kinase/mTOR dual inhibitor |
| WO2013071698A1 (en) | 2011-11-17 | 2013-05-23 | 山东轩竹医药科技有限公司 | Three-ring pi3k and/or mtor inhibitor |
| WO2013072392A1 (en) | 2011-11-15 | 2013-05-23 | Novartis Forschungsstiftung, Zweigniederlassung Friedrich Miescher Institute For Biomedical Research | Combination of a phosphoinositide 3-kinase inhibitor and a modulator of the janus kinase 2-signal transducer and activator of transcription 5 pathway |
| WO2013084138A1 (en) | 2011-12-05 | 2013-06-13 | Novartis Ag | Cyclic urea derivatives as androgen receptor antagonists |
| WO2013084148A2 (en) | 2011-12-05 | 2013-06-13 | Novartis Ag | Antibodies for epidermal growth factor receptor 3 (her3) directed to domain ii of her3 |
| WO2013084147A2 (en) | 2011-12-05 | 2013-06-13 | Novartis Ag | Antibodies for epidermal growth factor receptor 3 (her3) |
| WO2013088404A1 (en) | 2011-12-15 | 2013-06-20 | Novartis Ag | Use of inhibitors of the activity or function of PI3K |
| WO2013093849A1 (en) | 2011-12-22 | 2013-06-27 | Novartis Ag | Dihydro-benzo-oxazine and dihydro-pyrido-oxazine derivatives |
| WO2013093850A1 (en) | 2011-12-22 | 2013-06-27 | Novartis Ag | Quinoline derivatives |
| WO2013105022A2 (en) | 2012-01-09 | 2013-07-18 | Novartis Ag | Organic compositions to treat beta-catenin-related diseases |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| WO2013184621A1 (en) | 2012-06-06 | 2013-12-12 | Novartis Ag | Combination of a 17 -alpha -hydroxylase (c17, 20 - lyase) inhibitor and a specific pi-3k inhibitor for treating a tumor disease |
| WO2013192367A1 (en) | 2012-06-22 | 2013-12-27 | Novartis Ag | Neuroendocrine tumor treatment |
| WO2014006115A1 (en) | 2012-07-06 | 2014-01-09 | Novartis Ag | Combination of a phosphoinositide 3-kinase inhibitor and an inhibitor of the il-8/cxcr interaction |
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US8691807B2 (en) | 2011-06-20 | 2014-04-08 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US8697873B2 (en) | 2004-03-24 | 2014-04-15 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| EP2742940A1 (en) | 2012-12-13 | 2014-06-18 | IP Gesellschaft für Management mbH | Salts of aza-bicyclo-di-aryl ethers for adminstration once daily, twice daily or thrice daily |
| WO2014102630A1 (en) | 2012-11-26 | 2014-07-03 | Novartis Ag | Solid form of dihydro-pyrido-oxazine derivative |
| JP2014518887A (en) * | 2011-06-04 | 2014-08-07 | シュアンジュ・ファーマ・カンパニー・リミテッド | Pyridonaphthyridine-type PI3K and mTOR dual inhibitors and their preparation and use |
| US8802853B2 (en) | 2003-12-29 | 2014-08-12 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| WO2014130657A1 (en) | 2013-02-20 | 2014-08-28 | The Trustees Of The University Of Pennsylvania | Treatment of cancer using humanized anti-egfrviii chimeric antigen receptor |
| WO2014128612A1 (en) | 2013-02-20 | 2014-08-28 | Novartis Ag | Quinazolin-4-one derivatives |
| WO2014141118A1 (en) | 2013-03-14 | 2014-09-18 | Piramal Enterprises Limited | Imidazo[4,5-c]quinoline derivatives and uses thereof |
| WO2014147573A2 (en) | 2013-03-21 | 2014-09-25 | Novartis Ag | Combination therapy |
| WO2014160160A2 (en) | 2013-03-13 | 2014-10-02 | Novartis Ag | Antibody drug conjugates |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| WO2014177915A1 (en) | 2013-05-01 | 2014-11-06 | Piramal Enterprises Limited | Cancer combination therapy using imidazo[4,5-c]quinoline derivatives |
| WO2014203152A1 (en) | 2013-06-18 | 2014-12-24 | Novartis Ag | Pharmaceutical combinations |
| US8933086B2 (en) | 2005-12-13 | 2015-01-13 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B]pyridines and pyrrolo[2,3-B]pyrimidines as Janus kinase inhibitors |
| US8933085B2 (en) | 2010-11-19 | 2015-01-13 | Incyte Corporation | Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| WO2015010641A1 (en) | 2013-07-24 | 2015-01-29 | Novartis Ag | Substituted quinazolin-4-one derivatives |
| WO2015027667A1 (en) | 2013-08-30 | 2015-03-05 | 浙江医药股份有限公司新昌制药厂 | 2, 6-di-nitrogen-containing substituted purine derivative, and preparation method, pharmaceutical composition and use thereof |
| US8987443B2 (en) | 2013-03-06 | 2015-03-24 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9034884B2 (en) | 2010-11-19 | 2015-05-19 | Incyte Corporation | Heterocyclic-substituted pyrrolopyridines and pyrrolopyrimidines as JAK inhibitors |
| WO2015073644A1 (en) | 2013-11-13 | 2015-05-21 | Novartis Ag | Mtor inhibitors for enhancing the immune response |
| US9056852B2 (en) | 2011-03-28 | 2015-06-16 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| WO2015092634A1 (en) | 2013-12-16 | 2015-06-25 | Novartis Ag | 1,2,3,4-tetrahydroisoquinoline compounds and compositions as selective estrogen receptor antagonists and degraders |
| WO2015090229A1 (en) | 2013-12-20 | 2015-06-25 | Novartis Ag | Regulatable chimeric antigen receptor |
| WO2015090230A1 (en) | 2013-12-19 | 2015-06-25 | Novartis Ag | Human mesothelin chimeric antigen receptors and uses thereof |
| WO2015107495A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | N-azaspirocycloalkane substituted n-heteroaryl compounds and compositions for inhibiting the activity of shp2 |
| WO2015107494A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | 1 -(triazin-3-yi_/pyridazin-3-yl)-piper(-azine)idine derivatives and compositions thereof for inhibiting the activity of shp2 |
| WO2015107493A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | 1 -pyridazin-/triazin-3-yl-piper(-azine)/idine/pyrolidine derivatives and and compositions thereof for inhibiting the activity of shp2 |
| WO2015131080A1 (en) | 2014-02-28 | 2015-09-03 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| WO2015138920A1 (en) | 2014-03-14 | 2015-09-17 | Novartis Ag | Antibody molecules to lag-3 and uses thereof |
| WO2015142675A2 (en) | 2014-03-15 | 2015-09-24 | Novartis Ag | Treatment of cancer using chimeric antigen receptor |
| WO2015157252A1 (en) | 2014-04-07 | 2015-10-15 | BROGDON, Jennifer | Treatment of cancer using anti-cd19 chimeric antigen receptor |
| WO2015162584A1 (en) | 2014-04-24 | 2015-10-29 | Novartis Ag | Crystalline forms of the sulfate salt of n-[5-(3-imidazol-1-yl-4-methanesulfonyl-phenyl)-4-methyl-thiazol-2-yl]-acetamide |
| US9193733B2 (en) | 2012-05-18 | 2015-11-24 | Incyte Holdings Corporation | Piperidinylcyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| US9216984B2 (en) | 2009-05-22 | 2015-12-22 | Incyte Corporation | 3-[4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl]octane—or heptane-nitrile as JAK inhibitors |
| US9227969B2 (en) | 2013-08-14 | 2016-01-05 | Novartis Ag | Compounds and compositions as inhibitors of MEK |
| WO2016014553A1 (en) | 2014-07-21 | 2016-01-28 | Novartis Ag | Sortase synthesized chimeric antigen receptors |
| WO2016014530A1 (en) | 2014-07-21 | 2016-01-28 | Novartis Ag | Combinations of low, immune enhancing. doses of mtor inhibitors and cars |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US9249145B2 (en) | 2009-09-01 | 2016-02-02 | Incyte Holdings Corporation | Heterocyclic derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| WO2016020791A1 (en) | 2014-08-05 | 2016-02-11 | Novartis Ag | Ckit antibody drug conjugates |
| WO2016024195A1 (en) | 2014-08-12 | 2016-02-18 | Novartis Ag | Anti-cdh6 antibody drug conjugates |
| WO2016025880A1 (en) | 2014-08-14 | 2016-02-18 | Novartis Ag | Treatment of cancer using gfr alpha-4 chimeric antigen receptor |
| WO2016040892A1 (en) | 2014-09-13 | 2016-03-17 | Novartis Ag | Combination therapies |
| WO2016044605A1 (en) | 2014-09-17 | 2016-03-24 | Beatty, Gregory | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
| WO2016010869A3 (en) * | 2014-07-14 | 2016-04-07 | Advenchen Pharmaceuticals, LLC | FUSED QUINOLINE COMPUNDS AS PI3K, mTOR INHIBITORS |
| WO2016054555A2 (en) | 2014-10-03 | 2016-04-07 | Novartis Ag | Combination therapies |
| WO2016057705A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Biomarkers predictive of therapeutic responsiveness to chimeric antigen receptor therapy and uses thereof |
| WO2016057841A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Compositions and methods of use for augmented immune response and cancer therapy |
| WO2016061142A1 (en) | 2014-10-14 | 2016-04-21 | Novartis Ag | Antibody molecules to pd-l1 and uses thereof |
| US9334274B2 (en) | 2009-05-22 | 2016-05-10 | Incyte Holdings Corporation | N-(hetero)aryl-pyrrolidine derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines and pyrrol-3-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| WO2016075670A1 (en) | 2014-11-14 | 2016-05-19 | Novartis Ag | Antibody drug conjugates |
| WO2016079760A1 (en) | 2014-11-20 | 2016-05-26 | Council Of Scientific & Industrial Research | Novel 1,3,5 -triazine based pi3k inhibitors as anticancer agents and a process for the preparation thereof |
| US9359358B2 (en) | 2011-08-18 | 2016-06-07 | Incyte Holdings Corporation | Cyclohexyl azetidine derivatives as JAK inhibitors |
| WO2016092508A1 (en) | 2014-12-12 | 2016-06-16 | Massachusetts General Hospital | Treatment of breast cancer brain metastases |
| WO2016100882A1 (en) | 2014-12-19 | 2016-06-23 | Novartis Ag | Combination therapies |
| WO2016103155A1 (en) | 2014-12-23 | 2016-06-30 | Novartis Ag | Triazolopyrimidine compounds and uses thereof |
| US9428503B2 (en) | 2014-05-08 | 2016-08-30 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating Cancer |
| WO2016138352A1 (en) | 2015-02-27 | 2016-09-01 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| WO2016145102A1 (en) | 2015-03-10 | 2016-09-15 | Aduro Biotech, Inc. | Compositions and methods for activating "stimulator of interferon gene" -dependent signalling |
| WO2016142508A1 (en) | 2015-03-11 | 2016-09-15 | Centre Léon-Bérard | Composition for treating pancreatic neuroendocrine tumours |
| WO2016151499A1 (en) | 2015-03-25 | 2016-09-29 | Novartis Ag | Formylated n-heterocyclic derivatives as fgfr4 inhibitors |
| US9464088B2 (en) | 2010-03-10 | 2016-10-11 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| WO2016164580A1 (en) | 2015-04-07 | 2016-10-13 | Novartis Ag | Combination of chimeric antigen receptor therapy and amino pyrimidine derivatives |
| WO2016168595A1 (en) | 2015-04-17 | 2016-10-20 | Barrett David Maxwell | Methods for improving the efficacy and expansion of chimeric antigen receptor-expressing cells |
| WO2016172583A1 (en) | 2015-04-23 | 2016-10-27 | Novartis Ag | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| US9487521B2 (en) | 2011-09-07 | 2016-11-08 | Incyte Holdings Corporation | Processes and intermediates for making a JAK inhibitor |
| US9498467B2 (en) | 2014-05-30 | 2016-11-22 | Incyte Corporation | Treatment of chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML) by inhibitors of JAK1 |
| WO2016185443A1 (en) | 2015-05-20 | 2016-11-24 | Novartis Ag | Pharmaceutical combination of everolimus with dactolisib |
| WO2016203432A1 (en) | 2015-06-17 | 2016-12-22 | Novartis Ag | Antibody drug conjugates |
| WO2016203406A1 (en) | 2015-06-19 | 2016-12-22 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2016203404A1 (en) | 2015-06-19 | 2016-12-22 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2016203405A1 (en) | 2015-06-19 | 2016-12-22 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2017004134A1 (en) | 2015-06-29 | 2017-01-05 | Nimbus Iris, Inc. | Irak inhibitors and uses thereof |
| WO2017011363A1 (en) | 2015-07-11 | 2017-01-19 | Advenchen Pharmaceuticals, LLC | Fused quinoline compunds as pi3k/mtor inhibitors |
| WO2017019894A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to lag-3 |
| WO2017019897A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to tim-3 |
| WO2017040757A1 (en) | 2015-09-02 | 2017-03-09 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| WO2017037579A1 (en) | 2015-08-28 | 2017-03-09 | Novartis Ag | Mdm2 inhibitors and combinations thereof |
| WO2017070518A1 (en) | 2015-10-23 | 2017-04-27 | Navitor Pharmaceuticals, Inc. | Modulators of sestrin-gator2 interaction and uses thereof |
| WO2017072662A1 (en) | 2015-10-29 | 2017-05-04 | Novartis Ag | Antibody conjugates comprising toll-like receptor agonist |
| US9655854B2 (en) | 2013-08-07 | 2017-05-23 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| WO2017093905A1 (en) | 2015-12-03 | 2017-06-08 | Novartis Ag | Treatment of cancer with a pi3k inhibitor in a patient preselected for having a pik3ca mutation in the ctdna |
| US9675595B2 (en) | 2011-08-31 | 2017-06-13 | Novartis Ag | Synergistic combinations of PI3K- and MEK-inhibitors |
| WO2017106656A1 (en) | 2015-12-17 | 2017-06-22 | Novartis Ag | Antibody molecules to pd-1 and uses thereof |
| WO2017106352A1 (en) | 2015-12-14 | 2017-06-22 | Raze Therapeutics, Inc. | Caffeine inhibitors of mthfd2 and uses thereof |
| WO2017156179A1 (en) | 2016-03-09 | 2017-09-14 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| WO2017216706A1 (en) | 2016-06-14 | 2017-12-21 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2017221100A1 (en) | 2016-06-20 | 2017-12-28 | Novartis Ag | Imidazopyrimidine compounds useful for the treatment of cancer |
| WO2017223239A1 (en) | 2016-06-21 | 2017-12-28 | X4 Pharmaceuticals, Inc. | Cxcr4 inhibitors and uses thereof |
| WO2017221092A1 (en) | 2016-06-20 | 2017-12-28 | Novartis Ag | Triazolopyridine compounds and uses thereof |
| US9856255B2 (en) | 2015-09-17 | 2018-01-02 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| WO2018009638A1 (en) | 2016-07-06 | 2018-01-11 | The Regents Of The University Of Michigan | MULTIFUNCTIONAL INHIBITORS OF MEK/PI3K AND mTOR/MEK/PI3K BIOLOGICAL PATHWAYS AND THERAPEUTIC METHODS USING THE SAME |
| US9879003B2 (en) | 2012-04-11 | 2018-01-30 | Dana-Farber Cancer Institute, Inc. | Host targeted inhibitors of dengue virus and other viruses |
| WO2018064076A1 (en) | 2016-09-27 | 2018-04-05 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules |
| WO2018067992A1 (en) | 2016-10-07 | 2018-04-12 | Novartis Ag | Chimeric antigen receptors for the treatment of cancer |
| WO2018071794A1 (en) | 2016-10-14 | 2018-04-19 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| WO2018075937A1 (en) | 2016-10-21 | 2018-04-26 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US9968597B2 (en) | 2014-06-06 | 2018-05-15 | Advenchen Laboratories Nanjing Ltd | Methods and uses of quinoline derivatives in the treatment of soft tissue sarcomas and pharmaceutical compositions for treatment of same |
| WO2018092064A1 (en) | 2016-11-18 | 2018-05-24 | Novartis Ag | Combinations of mdm2 inhibitors and bcl-xl inhibitors |
| WO2018096402A1 (en) | 2016-11-23 | 2018-05-31 | Novartis Ag | Methods of enhancing immune response with everolimus, dactolisib or both |
| US9993480B2 (en) | 2011-02-18 | 2018-06-12 | Novartis Pharma Ag | mTOR/JAK inhibitor combination therapy |
| US10000483B2 (en) | 2012-10-19 | 2018-06-19 | Dana-Farber Cancer Institute, Inc. | Bone marrow on X chromosome kinase (BMX) inhibitors and uses thereof |
| WO2018119183A2 (en) | 2016-12-22 | 2018-06-28 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2018127699A1 (en) | 2017-01-06 | 2018-07-12 | Bicyclerd Limited | Compounds for treating cancer |
| WO2018142322A1 (en) | 2017-02-03 | 2018-08-09 | Novartis Ag | Anti-ccr7 antibody drug conjugates |
| EP3360870A1 (en) | 2013-02-19 | 2018-08-15 | Novartis AG | Benzothiophene derivatives and compositions thereof as selective estrogen receptor degraders |
| WO2018165240A1 (en) | 2017-03-08 | 2018-09-13 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors, uses, and methods for production thereof |
| WO2018163051A1 (en) | 2017-03-06 | 2018-09-13 | Novartis Ag | Methods of treatment of cancer with reduced ubb expression |
| WO2018185618A1 (en) | 2017-04-03 | 2018-10-11 | Novartis Ag | Anti-cdh6 antibody drug conjugates and anti-gitr antibody combinations and methods of treatment |
| US10100034B2 (en) | 2015-05-04 | 2018-10-16 | Advenchen Pharmaceuticals, LLC | Process for preparing an anti-cancer agent, 1-((4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropanamine, its crystalline form and its salts |
| WO2018197893A1 (en) | 2017-04-27 | 2018-11-01 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| WO2018201056A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Cells expressing a bcma-targeting chimeric antigen receptor, and combination therapy with a gamma secretase inhibitor |
| WO2018198091A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Antibody conjugates comprising toll-like receptor agonist and combination therapies |
| WO2018198076A1 (en) | 2017-04-28 | 2018-11-01 | Aduro Biotech, Inc. | Bis 2'-5'-rr-(3'f-a)(3'f-a) cyclic dinucleotide compound and uses thereof |
| WO2018215937A1 (en) | 2017-05-24 | 2018-11-29 | Novartis Ag | Interleukin-7 antibody cytokine engrafted proteins and methods of use in the treatment of cancer |
| WO2018217651A1 (en) | 2017-05-22 | 2018-11-29 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2018215938A1 (en) | 2017-05-24 | 2018-11-29 | Novartis Ag | Antibody-cytokine engrafted proteins and methods of use |
| WO2018215936A1 (en) | 2017-05-24 | 2018-11-29 | Novartis Ag | Antibody-cytokine engrafted proteins and methods of use in the treatment of cancer |
| US10166191B2 (en) | 2012-11-15 | 2019-01-01 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| WO2019002842A1 (en) | 2017-06-26 | 2019-01-03 | Bicyclerd Limited | Bicyclic peptide ligands with detectable moieties and uses thereof |
| WO2019032640A1 (en) | 2017-08-11 | 2019-02-14 | The Regents Of The University Of Michigan | Inhibitors of mek/pi3k, jak/mek, jak/pi3k/mtor and mek/pi3k/mtor biological pathways and methods for improving lymphatic uptake, bioavailability, and solubility of therapeutic compounds |
| WO2019034868A1 (en) | 2017-08-14 | 2019-02-21 | Bicyclerd Limited | Bicyclic peptide ligand prr-a conjugates and uses thereof |
| WO2019034866A1 (en) | 2017-08-14 | 2019-02-21 | Bicyclerd Limited | Bicyclic peptide ligand sting conjugates and uses thereof |
| WO2019046491A1 (en) | 2017-08-29 | 2019-03-07 | Ariya Therapeutics, Inc. | Lymphatic system-directing lipid prodrugs |
| WO2019051291A1 (en) | 2017-09-08 | 2019-03-14 | Amgen Inc. | Inhibitors of kras g12c and methods of using the same |
| WO2019051469A1 (en) | 2017-09-11 | 2019-03-14 | Krouzon Pharmaceuticals, Inc. | Octahydrocyclopenta[c]pyrrole allosteric inhibitors of shp2 |
| WO2019067328A1 (en) | 2017-09-26 | 2019-04-04 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules and methods of use |
| US10251876B2 (en) | 2014-06-06 | 2019-04-09 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Method for treating tumour, pharmaceutical composition and medicinal kit |
| US10259801B2 (en) | 2013-01-29 | 2019-04-16 | Avexxin As | Anti-inflammatory and antitumor 2-oxothiazoles ABD 2-oxothiophenes compounds |
| US10307412B2 (en) | 2014-12-09 | 2019-06-04 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Quinoline derivative against non-small cell lung cancer |
| WO2019126378A1 (en) | 2017-12-19 | 2019-06-27 | Ariya Therapeutics, Inc. | Lipid prodrugs of mycophenolic acid and uses thereof |
| EP3514178A1 (en) | 2013-03-15 | 2019-07-24 | Novartis AG | Antibody drug conjugates |
| EP3514179A1 (en) | 2014-01-24 | 2019-07-24 | Dana-Farber Cancer Institute, Inc. | Antibody molecules to pd-1 and uses thereof |
| WO2019148132A1 (en) | 2018-01-29 | 2019-08-01 | Merck Patent Gmbh | Gcn2 inhibitors and uses thereof |
| WO2019169001A1 (en) | 2018-02-27 | 2019-09-06 | Artax Biopharma Inc. | Chromene derivatives as inhibitors of tcr-nck interaction |
| US10407446B2 (en) | 2016-12-20 | 2019-09-10 | Astrazeneca Ab | Amino-triazolopyridine compounds and their use in treating cancer |
| US10414727B2 (en) | 2016-11-08 | 2019-09-17 | Navitor Pharmaceuticals, Inc. | Phenyl amino piperidine mTORC inhibitors and uses thereof |
| EP3539990A1 (en) | 2014-07-16 | 2019-09-18 | Dana-Farber Cancer Institute, Inc. | Her3 inhibition in low-grade serous cancers |
| WO2019191334A1 (en) | 2018-03-28 | 2019-10-03 | Cero Therapeutics, Inc. | Chimeric tim4 receptors and uses thereof |
| WO2019191339A1 (en) | 2018-03-28 | 2019-10-03 | Cero Therapeutics, Inc. | Expression vectors for chimeric engulfment receptors, genetically modified host cells, and uses thereof |
| WO2019191340A1 (en) | 2018-03-28 | 2019-10-03 | Cero Therapeutics, Inc. | Cellular immunotherapy compositions and uses thereof |
| US10457677B2 (en) | 2015-04-02 | 2019-10-29 | Merck Patent Gmbh | Imidazolonylquinolines and the use thereof as ATM kinase inhibitors |
| WO2019209759A1 (en) | 2018-04-24 | 2019-10-31 | Merck Patent Gmbh | Antiproliferation compounds and uses thereof |
| WO2019210153A1 (en) | 2018-04-27 | 2019-10-31 | Novartis Ag | Car t cell therapies with enhanced efficacy |
| WO2019209757A1 (en) | 2018-04-24 | 2019-10-31 | Vertex Pharmaceuticals Incorporated | Pteridinone compounds and uses thereof |
| WO2019213516A1 (en) | 2018-05-04 | 2019-11-07 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2019213526A1 (en) | 2018-05-04 | 2019-11-07 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2019213282A1 (en) | 2018-05-01 | 2019-11-07 | Novartis Ag | Biomarkers for evaluating car-t cells to predict clinical outcome |
| WO2019217691A1 (en) | 2018-05-10 | 2019-11-14 | Amgen Inc. | Kras g12c inhibitors for the treatment of cancer |
| WO2019232419A1 (en) | 2018-06-01 | 2019-12-05 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| US10508120B2 (en) | 2017-07-28 | 2019-12-17 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| WO2019241157A1 (en) | 2018-06-11 | 2019-12-19 | Amgen Inc. | Kras g12c inhibitors for treating cancer |
| WO2019241789A1 (en) | 2018-06-15 | 2019-12-19 | Navitor Pharmaceuticals, Inc. | Rapamycin analogs and uses thereof |
| WO2019243833A1 (en) | 2018-06-22 | 2019-12-26 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| WO2020010177A1 (en) | 2018-07-06 | 2020-01-09 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| US10577362B2 (en) | 2016-05-04 | 2020-03-03 | Genoscience Pharma | Substituted 2, 4-diamino-quinoline derivatives for use in the treatment of proliferative diseases |
| WO2020050890A2 (en) | 2018-06-12 | 2020-03-12 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2020051424A1 (en) | 2018-09-07 | 2020-03-12 | Pic Therapeutics | Eif4e inhibitors and uses thereof |
| US10596161B2 (en) | 2017-12-08 | 2020-03-24 | Incyte Corporation | Low dose combination therapy for treatment of myeloproliferative neoplasms |
| US10596165B2 (en) | 2018-02-12 | 2020-03-24 | resTORbio, Inc. | Combination therapies |
| WO2020061103A1 (en) | 2018-09-18 | 2020-03-26 | Nikang Therapeutics, Inc. | Fused tricyclic ring derivatives as src homology-2 phosphatase inhibitors |
| WO2020065453A1 (en) | 2018-09-29 | 2020-04-02 | Novartis Ag | Process of manufacture of a compound for inhibiting the activity of shp2 |
| WO2020068873A1 (en) | 2018-09-25 | 2020-04-02 | Black Diamond Therapeutics, Inc. | Tyrosine kinase inhibitor compositions, methods of making and methods of use |
| WO2020084305A1 (en) | 2018-10-23 | 2020-04-30 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| WO2020089811A1 (en) | 2018-10-31 | 2020-05-07 | Novartis Ag | Dc-sign antibody drug conjugates |
| WO2020102730A1 (en) | 2018-11-16 | 2020-05-22 | Amgen Inc. | Improved synthesis of key intermediate of kras g12c inhibitor compound |
| WO2020106647A2 (en) | 2018-11-19 | 2020-05-28 | Amgen Inc. | Combination therapy including a krasg12c inhibitor and one or more additional pharmaceutically active agents for the treatment of cancers |
| WO2020106640A1 (en) | 2018-11-19 | 2020-05-28 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| EP3660042A1 (en) | 2014-07-31 | 2020-06-03 | Novartis AG | Subset-optimized chimeric antigen receptor-containing t-cells |
| WO2020112937A1 (en) | 2018-11-30 | 2020-06-04 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US10683308B2 (en) | 2015-09-11 | 2020-06-16 | Navitor Pharmaceuticals, Inc. | Rapamycin analogs and uses thereof |
| EP3670659A1 (en) | 2018-12-20 | 2020-06-24 | Abivax | Biomarkers, and uses in treatment of viral infections, inflammations, or cancer |
| WO2020132653A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Heteroaryl amides useful as kif18a inhibitors |
| WO2020128612A2 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Antibodies to pmel17 and conjugates thereof |
| WO2020132651A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Kif18a inhibitors |
| WO2020132649A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Heteroaryl amides useful as kif18a inhibitors |
| WO2020132648A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Kif18a inhibitors |
| US10736887B2 (en) | 2016-04-15 | 2020-08-11 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Quinoline derivative for treating gastric cancer |
| WO2020165600A1 (en) | 2019-02-14 | 2020-08-20 | Bicycletx Limited | Bicyclic peptide ligand sting conjugates and uses thereof |
| US10758543B2 (en) | 2010-05-21 | 2020-09-01 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| WO2020180768A1 (en) | 2019-03-01 | 2020-09-10 | Revolution Medicines, Inc. | Bicyclic heteroaryl compounds and uses thereof |
| WO2020180770A1 (en) | 2019-03-01 | 2020-09-10 | Revolution Medicines, Inc. | Bicyclic heterocyclyl compounds and uses thereof |
| EP3712171A1 (en) | 2014-08-19 | 2020-09-23 | Novartis AG | Treatment of cancer using a cd123 chimeric antigen receptor |
| US10793563B2 (en) | 2018-01-29 | 2020-10-06 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| WO2020201753A1 (en) | 2019-04-02 | 2020-10-08 | Bicycletx Limited | Bicycle toxin conjugates and uses thereof |
| EP3722316A1 (en) | 2014-07-21 | 2020-10-14 | Novartis AG | Treatment of cancer using a cd33 chimeric antigen receptor |
| EP3738593A1 (en) | 2019-05-14 | 2020-11-18 | Amgen, Inc | Dosing of kras inhibitor for treatment of cancers |
| WO2020229685A1 (en) | 2019-05-15 | 2020-11-19 | Avexxin As | Combination therapy for proliferative conditions |
| WO2020229688A1 (en) | 2019-05-15 | 2020-11-19 | Avexxin As | Combination therapy for proliferative conditions |
| US10851114B2 (en) | 2014-08-01 | 2020-12-01 | Avexxin As | 2-oxothiatole compounds having activity as cPLA2 inhibitors for the treatment of inflammatory disorders and hyperproliferative disorders |
| WO2020243423A1 (en) | 2019-05-31 | 2020-12-03 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| US10874743B2 (en) | 2017-12-26 | 2020-12-29 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2021001743A1 (en) | 2019-07-02 | 2021-01-07 | Effector Therapeutics, Inc. | Translation inhibitors and uses thereof |
| US10894830B2 (en) | 2015-11-03 | 2021-01-19 | Janssen Biotech, Inc. | Antibodies specifically binding PD-1, TIM-3 or PD-1 and TIM-3 and their uses |
| US10899736B2 (en) | 2018-01-30 | 2021-01-26 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| WO2021014027A1 (en) | 2019-07-25 | 2021-01-28 | Coegin Pharma As | Phospholipase-a2 inhibitors for the prevention of cancer metastasis |
| WO2021026098A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Kif18a inhibitors |
| WO2021026099A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Kif18a inhibitors |
| WO2021026101A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Kif18a inhibitors |
| WO2021026100A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Pyridine derivatives as kif18a inhibitors |
| WO2021030711A1 (en) | 2019-08-15 | 2021-02-18 | Black Diamond Therapeutics, Inc. | Alkynyl quinazoline compounds |
| US10953004B2 (en) | 2016-03-14 | 2021-03-23 | Avexxin As | Combination therapy for proliferative diseases |
| US10954220B2 (en) | 2016-03-09 | 2021-03-23 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| WO2021055728A1 (en) | 2019-09-18 | 2021-03-25 | Merck Sharp & Dohme Corp. | Small molecule inhibitors of kras g12c mutant |
| WO2021057853A1 (en) | 2019-09-26 | 2021-04-01 | Novartis Ag | Aza-quinoline compounds and uses thereof |
| WO2021067875A1 (en) | 2019-10-03 | 2021-04-08 | Cero Therapeutics, Inc. | Chimeric tim4 receptors and uses thereof |
| WO2021076655A1 (en) | 2019-10-15 | 2021-04-22 | Amgen Inc. | Combination therapy of kras inhibitor and shp2 inhibitor for treatment of cancers |
| US10988465B2 (en) | 2016-06-21 | 2021-04-27 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US10988472B2 (en) | 2016-10-13 | 2021-04-27 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Compounds and method for blocking transmission of malarial parasite |
| WO2021081212A1 (en) | 2019-10-24 | 2021-04-29 | Amgen Inc. | Pyridopyrimidine derivatives useful as kras g12c and kras g12d inhibitors in the treatment of cancer |
| WO2021085653A1 (en) | 2019-10-31 | 2021-05-06 | Taiho Pharmaceutical Co., Ltd. | 4-aminobut-2-enamide derivatives and salts thereof |
| WO2021086833A1 (en) | 2019-10-28 | 2021-05-06 | Merck Sharp & Dohme Corp. | Small molecule inhibitors of kras g12c mutant |
| WO2021091967A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2021091982A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2021091956A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2021092115A1 (en) | 2019-11-08 | 2021-05-14 | Revolution Medicines, Inc. | Bicyclic heteroaryl compounds and uses thereof |
| WO2021097212A1 (en) | 2019-11-14 | 2021-05-20 | Amgen Inc. | Improved synthesis of kras g12c inhibitor compound |
| WO2021097207A1 (en) | 2019-11-14 | 2021-05-20 | Amgen Inc. | Improved synthesis of kras g12c inhibitor compound |
| US11021481B2 (en) | 2019-09-13 | 2021-06-01 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1h-pyrrolo[3,4-c]pyridin-1-ones as HPK1 antagonists |
| WO2021107160A1 (en) | 2019-11-29 | 2021-06-03 | Taiho Pharmaceutical Co., Ltd. | A compound having inhibitory activity against kras g12d mutation |
| WO2021108683A1 (en) | 2019-11-27 | 2021-06-03 | Revolution Medicines, Inc. | Covalent ras inhibitors and uses thereof |
| US11034672B1 (en) | 2018-09-25 | 2021-06-15 | Black Diamond Therapeutics, Inc. | Tyrosine kinase inhibitor compositions, methods of making and methods of use |
| WO2021126816A1 (en) | 2019-12-16 | 2021-06-24 | Amgen Inc. | Dosing regimen of a kras g12c inhibitor |
| US11045461B2 (en) | 2018-08-31 | 2021-06-29 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| WO2021142026A1 (en) | 2020-01-07 | 2021-07-15 | Revolution Medicines, Inc. | Shp2 inhibitor dosing and methods of treating cancer |
| US11091489B2 (en) | 2016-06-20 | 2021-08-17 | Novartis Ag | Crystalline forms of a triazolopyrimidine compound |
| US11091451B2 (en) | 2016-12-05 | 2021-08-17 | Raze Therapeutics, Inc. | SHMT inhibitors and uses thereof |
| US11098077B2 (en) | 2016-07-05 | 2021-08-24 | Chinook Therapeutics, Inc. | Locked nucleic acid cyclic dinucleotide compounds and uses thereof |
| WO2021178488A1 (en) | 2020-03-03 | 2021-09-10 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| US11117889B1 (en) | 2018-11-30 | 2021-09-14 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2021195206A1 (en) | 2020-03-24 | 2021-09-30 | Black Diamond Therapeutics, Inc. | Polymorphic forms and related uses |
| WO2021215544A1 (en) | 2020-04-24 | 2021-10-28 | Taiho Pharmaceutical Co., Ltd. | Kras g12d protein inhibitors |
| WO2021215545A1 (en) | 2020-04-24 | 2021-10-28 | Taiho Pharmaceutical Co., Ltd. | Anticancer combination therapy with n-(1-acryloyl-azetidin-3-yl)-2-((1h-indazol-3-yl)amino)methyl)-1h-imidazole-5-carboxamide inhibitor of kras-g12c |
| WO2021220199A1 (en) | 2020-04-30 | 2021-11-04 | Novartis Ag | Ccr7 antibody drug conjugates for treating cancer |
| US11174264B2 (en) | 2019-01-23 | 2021-11-16 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| WO2021257736A1 (en) | 2020-06-18 | 2021-12-23 | Revolution Medicines, Inc. | Methods for delaying, preventing, and treating acquired resistance to ras inhibitors |
| WO2021260580A1 (en) | 2020-06-24 | 2021-12-30 | Astrazeneca Uk Limited | Combination of antibody-drug conjugate and atm inhibitor |
| US11213528B2 (en) | 2007-06-13 | 2022-01-04 | Incyte Holdings Corporation | Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| WO2022014640A1 (en) | 2020-07-15 | 2022-01-20 | 大鵬薬品工業株式会社 | Pyrimidine compound-containing combination to be used in tumor treatment |
| US11236091B2 (en) | 2019-05-21 | 2022-02-01 | Amgen Inc. | Solid state forms |
| WO2022036285A1 (en) | 2020-08-14 | 2022-02-17 | Cero Therapeutics, Inc. | Compositions and methods for treating cancer with chimeric tim receptors in combination with inhibitors of poly (adp-ribose) polymerase |
| WO2022036265A1 (en) | 2020-08-14 | 2022-02-17 | Cero Therapeutics, Inc. | Chimeric tim receptors and uses thereof |
| WO2022033492A1 (en) | 2020-08-10 | 2022-02-17 | Novartis Ag | Quinoline compounds and compositions for inhibiting ezh2 |
| WO2022036287A1 (en) | 2020-08-14 | 2022-02-17 | Cero Therapeutics, Inc. | Anti-cd72 chimeric receptors and uses thereof |
| WO2022038158A1 (en) | 2020-08-17 | 2022-02-24 | Bicycletx Limited | Bicycle conjugates specific for nectin-4 and uses thereof |
| WO2022043557A1 (en) | 2020-08-31 | 2022-03-03 | Advanced Accelerator Applications International Sa | Method of treating psma-expressing cancers |
| WO2022043558A1 (en) | 2020-08-31 | 2022-03-03 | Advanced Accelerator Applications International Sa | Method of treating psma-expressing cancers |
| WO2022043556A1 (en) | 2020-08-31 | 2022-03-03 | Novartis Ag | Stable radiopharmaceutical composition |
| US11279676B2 (en) | 2018-03-02 | 2022-03-22 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Crystalline of compound as c-Met kinase inhibitor and preparation method therefor and use thereof |
| WO2022060836A1 (en) | 2020-09-15 | 2022-03-24 | Revolution Medicines, Inc. | Indole derivatives as ras inhibitors in the treatment of cancer |
| WO2022060583A1 (en) | 2020-09-03 | 2022-03-24 | Revolution Medicines, Inc. | Use of sos1 inhibitors to treat malignancies with shp2 mutations |
| US11304949B2 (en) | 2018-03-30 | 2022-04-19 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| US11304954B2 (en) | 2017-12-19 | 2022-04-19 | Puretech Lyt, Inc. | Lipid prodrugs of mycophenolic acid and uses thereof |
| US11311512B2 (en) | 2014-08-12 | 2022-04-26 | Monash University | Lymph directing prodrugs |
| EP3992191A1 (en) | 2020-11-03 | 2022-05-04 | Deutsches Krebsforschungszentrum | Imidazo[4,5-c]quinoline compounds and their use as atm kinase inhibitors |
| US11332470B2 (en) | 2016-06-21 | 2022-05-17 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11339144B2 (en) | 2017-04-10 | 2022-05-24 | Navitor Pharmaceuticals, Inc. | Heteroaryl Rheb inhibitors and uses thereof |
| US11337969B2 (en) | 2016-04-08 | 2022-05-24 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US11345654B2 (en) | 2018-10-24 | 2022-05-31 | Navitor Pharmaceuticals, Inc. | Polymorphic compounds and uses thereof |
| US11351176B2 (en) | 2017-08-14 | 2022-06-07 | Mei Pharma, Inc. | Combination therapy |
| US11351127B2 (en) | 2016-09-21 | 2022-06-07 | Avexxin As | Pharmaceutical composition |
| WO2022120354A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2022120353A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| US11358948B2 (en) | 2017-09-22 | 2022-06-14 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| WO2022123316A1 (en) | 2020-12-09 | 2022-06-16 | Takeda Pharmaceutical Company Limited | Compositions of guanylyl cyclase c (gcc) antigen binding agents and methods of use thereof |
| US11365252B2 (en) | 2016-07-20 | 2022-06-21 | University Of Utah Research Foundation | CD229 CAR T cells and methods of use thereof |
| WO2022140472A1 (en) | 2020-12-22 | 2022-06-30 | Nikang Therapeutics, Inc. | Compounds for degrading cyclin-dependent kinase 2 via ubiquitin proteosome pathway |
| EP4035659A1 (en) | 2016-11-29 | 2022-08-03 | PureTech LYT, Inc. | Exosomes for delivery of therapeutic agents |
| WO2022167457A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022167445A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022170052A1 (en) | 2021-02-05 | 2022-08-11 | Black Diamond Therapeutics, Inc. | Quinazoline derivatives, pyridopyrimidine derivatives, pyrimidopyrimidine derivatives, and uses thereof |
| US11414431B2 (en) | 2018-10-15 | 2022-08-16 | Nimbus Lakshmi, Inc. | Substituted pyrazolo[1,5-a]pyrimidines as TYK2 inhibitors |
| WO2022177267A1 (en) | 2021-02-16 | 2022-08-25 | 라이보텍(주) | Compound for suppressing nonsense-mediated mrna decay |
| WO2022183072A1 (en) | 2021-02-26 | 2022-09-01 | Kelonia Therapeutics, Inc. | Lymphocyte targeted lentiviral vectors |
| US11434239B2 (en) | 2017-03-14 | 2022-09-06 | Artax Biopharma Inc. | Aza-dihydro-acridone derivatives |
| US11439625B2 (en) | 2016-03-14 | 2022-09-13 | Avexxin As | Combination therapy for proliferative diseases |
| WO2022221866A1 (en) | 2021-04-16 | 2022-10-20 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2022221720A1 (en) | 2021-04-16 | 2022-10-20 | Novartis Ag | Antibody drug conjugates and methods for making thereof |
| US11485750B1 (en) | 2019-04-05 | 2022-11-01 | Kymera Therapeutics, Inc. | STAT degraders and uses thereof |
| US11485743B2 (en) | 2018-01-12 | 2022-11-01 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| WO2022235870A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Ras inhibitors for the treatment of cancer |
| WO2022235866A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Covalent ras inhibitors and uses thereof |
| WO2022235864A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Ras inhibitors |
| US11505526B2 (en) | 2017-03-14 | 2022-11-22 | Artax Biopharma Inc. | Aryl-piperidine derivatives |
| US11512080B2 (en) | 2018-01-12 | 2022-11-29 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| WO2022250170A1 (en) | 2021-05-28 | 2022-12-01 | Taiho Pharmaceutical Co., Ltd. | Small molecule inhibitors of kras mutated proteins |
| WO2023284730A1 (en) | 2021-07-14 | 2023-01-19 | Nikang Therapeutics, Inc. | Alkylidene derivatives as kras inhibitors |
| WO2023010097A1 (en) | 2021-07-28 | 2023-02-02 | Cero Therapeutics, Inc. | Chimeric tim4 receptors and uses thereof |
| US11591332B2 (en) | 2019-12-17 | 2023-02-28 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2023028235A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2023028238A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| US11608345B1 (en) | 2017-12-19 | 2023-03-21 | Puretech Lyt, Inc. | Lipid prodrugs of rapamycin and its analogs and uses thereof |
| US11623932B2 (en) | 2017-09-22 | 2023-04-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11623012B2 (en) | 2017-12-19 | 2023-04-11 | Bicyclerd Limited | Bicyclic peptide ligands specific for EphA2 |
| WO2023060253A1 (en) | 2021-10-08 | 2023-04-13 | Revolution Medicines, Inc. | Ras inhibitors |
| US11679090B2 (en) | 2017-04-26 | 2023-06-20 | Navitor Pharmaceuticals, Inc. | Modulators of Sestrin-GATOR2 interaction and uses thereof |
| US11679109B2 (en) | 2019-12-23 | 2023-06-20 | Kymera Therapeutics, Inc. | SMARCA degraders and uses thereof |
| WO2023114954A1 (en) | 2021-12-17 | 2023-06-22 | Genzyme Corporation | Pyrazolopyrazine compounds as shp2 inhibitors |
| WO2023114984A1 (en) | 2021-12-17 | 2023-06-22 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| US11685750B2 (en) | 2020-06-03 | 2023-06-27 | Kymera Therapeutics, Inc. | Crystalline forms of IRAK degraders |
| WO2023125877A1 (en) | 2021-12-30 | 2023-07-06 | 上海翰森生物医药科技有限公司 | Tricyclic derivative inhibitor, preparation method therefor, and application thereof |
| US11707457B2 (en) | 2019-12-17 | 2023-07-25 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11723890B2 (en) | 2019-11-01 | 2023-08-15 | Navitor Pharmaceuticals, Inc. | Methods of treatment using an mTORC1 modulator |
| EP4227307A1 (en) | 2022-02-11 | 2023-08-16 | Genzyme Corporation | Pyrazolopyrazine compounds as shp2 inhibitors |
| US11730819B2 (en) | 2016-12-23 | 2023-08-22 | Bicycletx Limited | Peptide derivatives having novel linkage structures |
| US11738087B2 (en) | 2015-09-08 | 2023-08-29 | Monash University | Lymph directing prodrugs |
| WO2023172940A1 (en) | 2022-03-08 | 2023-09-14 | Revolution Medicines, Inc. | Methods for treating immune refractory lung cancer |
| WO2023173057A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023173053A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| US11773103B2 (en) | 2021-02-15 | 2023-10-03 | Kymera Therapeutics, Inc. | IRAK4 degraders and uses thereof |
| WO2023192801A1 (en) | 2022-03-28 | 2023-10-05 | Nikang Therapeutics, Inc. | Sulfonamido derivatives as cyclin-dependent kinase 2 inhibitors |
| WO2023211889A1 (en) | 2022-04-25 | 2023-11-02 | Ikena Oncology, Inc. | Polymorphic compounds and uses thereof |
| US11814447B2 (en) | 2018-06-22 | 2023-11-14 | Bicyclerd Limited | Peptide ligands for binding to EphA2 |
| US11819476B2 (en) | 2019-12-05 | 2023-11-21 | Janssen Pharmaceutica Nv | Rapamycin analogs and uses thereof |
| WO2023230205A1 (en) | 2022-05-25 | 2023-11-30 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| US11833211B2 (en) | 2017-12-19 | 2023-12-05 | Bicycletx Limited | Methods of suppression and treatment of disease comprising administering bicycle peptide ligands specific for EphA2 |
| US11833155B2 (en) | 2020-06-03 | 2023-12-05 | Incyte Corporation | Combination therapy for treatment of myeloproliferative neoplasms |
| WO2023240263A1 (en) | 2022-06-10 | 2023-12-14 | Revolution Medicines, Inc. | Macrocyclic ras inhibitors |
| WO2023240024A1 (en) | 2022-06-08 | 2023-12-14 | Nikang Therapeutics, Inc. | Sulfamide derivatives as cyclin-dependent kinase 2 inhibitors |
| US11845724B2 (en) | 2019-09-11 | 2023-12-19 | Vincere Biosciences, Inc. | USP30 inhibitors and uses thereof |
| US11883497B2 (en) | 2017-08-29 | 2024-01-30 | Puretech Lyt, Inc. | Lymphatic system-directing lipid prodrugs |
| WO2024028364A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Aryl-triazolyl and related gpr84 antagonists and uses thereof |
| WO2024028363A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Heteroaryl carboxamide and related gpr84 antagonists and uses thereof |
| WO2024028365A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Substituted pyridone gpr84 antagonists and uses thereof |
| EP4324518A2 (en) | 2014-01-31 | 2024-02-21 | Novartis AG | Antibody molecules to tim-3 and uses thereof |
| US11926625B2 (en) | 2021-03-05 | 2024-03-12 | Nimbus Saturn, Inc. | HPK1 antagonists and uses thereof |
| US11932624B2 (en) | 2020-03-19 | 2024-03-19 | Kymera Therapeutics, Inc. | MDM2 degraders and uses thereof |
| WO2024081916A1 (en) | 2022-10-14 | 2024-04-18 | Black Diamond Therapeutics, Inc. | Methods of treating cancers using isoquinoline or 6-aza-quinoline derivatives |
| US11970553B2 (en) | 2019-07-30 | 2024-04-30 | Bicycletx Limited | Heterotandem bicyclic peptide complex |
| US11975073B2 (en) | 2020-02-05 | 2024-05-07 | Puretech Lyt, Inc. | Lipid prodrugs of neurosteroids |
| WO2024102849A1 (en) | 2022-11-11 | 2024-05-16 | Nikang Therapeutics, Inc. | Bifunctional compounds containing 2,5-substituted pyrimidine derivatives for degrading cyclin-dependent kinase 2 via ubiquitin proteasome pathway |
| WO2024112894A1 (en) | 2022-11-22 | 2024-05-30 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| EP4378957A2 (en) | 2015-07-29 | 2024-06-05 | Novartis AG | Combination therapies comprising antibody molecules to pd-1 |
| US12049520B2 (en) | 2017-08-04 | 2024-07-30 | Bicycletx Limited | Bicyclic peptide ligands specific for CD137 |
| US12071442B2 (en) | 2021-03-29 | 2024-08-27 | Nimbus Saturn, Inc. | Substituted pyrrolo[3,4-c]pyridines as HPK1 antagonists |
| US12091411B2 (en) | 2022-01-31 | 2024-09-17 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US12097261B2 (en) | 2021-05-07 | 2024-09-24 | Kymera Therapeutics, Inc. | CDK2 degraders and uses thereof |
| WO2024206858A1 (en) | 2023-03-30 | 2024-10-03 | Revolution Medicines, Inc. | Compositions for inducing ras gtp hydrolysis and uses thereof |
| WO2024211663A1 (en) | 2023-04-07 | 2024-10-10 | Revolution Medicines, Inc. | Condensed macrocyclic compounds as ras inhibitors |
| WO2024211712A1 (en) | 2023-04-07 | 2024-10-10 | Revolution Medicines, Inc. | Condensed macrocyclic compounds as ras inhibitors |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080269846A1 (en) * | 2003-03-14 | 2008-10-30 | Light Sciences Oncology, Inc. | Device for treatment of blood vessels using light |
| CN2885311Y (en) | 2006-01-18 | 2007-04-04 | 郑成福 | Via urethra prostate therapeutic equipment using photodynamic therapy |
| US10376711B2 (en) * | 2003-03-14 | 2019-08-13 | Light Sciences Oncology Inc. | Light generating guide wire for intravascular use |
| GB0510390D0 (en) * | 2005-05-20 | 2005-06-29 | Novartis Ag | Organic compounds |
| JP5250967B2 (en) * | 2005-11-30 | 2013-07-31 | 三菱化学株式会社 | Organic compound, charge transport material, composition for charge transport material, and organic electroluminescent device |
| US20090082387A1 (en) * | 2007-09-26 | 2009-03-26 | Protia, Llc | Deuterium-enriched nvp-bez234 |
| US9827212B2 (en) | 2009-03-18 | 2017-11-28 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treating asthma and other lung diseases |
| US20110008372A1 (en) * | 2009-07-08 | 2011-01-13 | Light Sciences Oncology, Inc. | Enhancement of light activated drug therapy through combination with other therapeutic agents |
| GB0919423D0 (en) * | 2009-11-05 | 2009-12-23 | Glaxosmithkline Llc | Novel compounds |
| CN102372711B (en) * | 2010-08-18 | 2014-09-17 | 山东轩竹医药科技有限公司 | Imidazo quinoline PI3K and mTOR (mammalian target of rapamycin) dual inhibitor |
| CN102382128A (en) * | 2010-08-27 | 2012-03-21 | 山东轩竹医药科技有限公司 | Three-ring fused PI3K and mTOR dual inhibitor |
| CN103012398B (en) * | 2011-09-19 | 2015-10-14 | 上海恒瑞医药有限公司 | Imidazoquinoline analog derivative and pharmacologically acceptable salt thereof, its preparation method and in application pharmaceutically |
| WO2013050921A1 (en) | 2011-10-03 | 2013-04-11 | Piramal Enterprises Limited | Hollow polymer microspheres as three-dimensional cell culture matrix |
| MX2014013725A (en) | 2012-05-23 | 2015-02-10 | Hoffmann La Roche | Compositions and methods of obtaining and using endoderm and hepatocyte cells. |
| KR20210049187A (en) * | 2012-08-16 | 2021-05-04 | 노파르티스 아게 | Combination of pi3k inhibitor and c-met inhibitor |
| EP3039126B1 (en) | 2013-08-26 | 2019-10-09 | The J. David Gladstone Institutes, A Testamentary Trust Established under The Will of J. David Gladstone | Small molecule cellular reprogramming to generate neuronal cells |
| US20160264570A1 (en) * | 2013-11-15 | 2016-09-15 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Serv | Method of blocking transmission of malarial parasite |
| CN105330699B (en) * | 2014-08-13 | 2018-12-04 | 山东汇睿迪生物技术有限公司 | A kind of phosphorous pyrido [2,3-d] pyrimidin-7-ones class compound or its pharmaceutically acceptable salt, pharmaceutical composition and its application |
| EP3265093A4 (en) | 2015-03-04 | 2018-11-07 | Dana-Farber Cancer Institute, Inc. | Tricyclic kinase inhibitors of melk and methods of use |
| CN110049783A (en) | 2016-12-14 | 2019-07-23 | 塔弗达治疗有限公司 | HSP90- targets conjugate and its preparation |
| WO2019180141A1 (en) | 2018-03-23 | 2019-09-26 | Bayer Aktiengesellschaft | Combinations of rogaratinib |
| CN108752336B (en) * | 2018-05-10 | 2021-06-04 | 常州润诺生物科技有限公司 | Imidazoquinoline derivatives and application thereof |
| TWI680973B (en) * | 2018-11-09 | 2020-01-01 | 長庚學校財團法人長庚科技大學 | Neutrophil inflammation inhibitor and uses thereof |
| CN115151253A (en) * | 2019-09-23 | 2022-10-04 | 南京征祥医药有限公司 | Phosphodiesterase inhibitors and uses thereof |
| US20230014730A1 (en) | 2019-09-23 | 2023-01-19 | Nanjing Zhengxiang Pharmaceuticals Co., Ltd. | Phosphodiesterase inhibitors and use |
| CN111187181B (en) * | 2019-11-22 | 2023-05-05 | 吉林大学 | Preparation method of 2- (4-aminophenyl) -2-methylpropanenitrile compound |
| EP4142710B1 (en) | 2020-09-21 | 2024-01-31 | Wei Zhong | Substituted 1-(3,3-difluoropiperidin-4-yl)-imidazo[4,5-c] quinolin-2-one compounds with blood-brain barrier penetrable capability |
| CN115232122B (en) * | 2021-04-23 | 2024-05-31 | 石药集团中奇制药技术(石家庄)有限公司 | Alkyne compound as well as preparation and application thereof |
Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3714357A (en) | 1968-07-15 | 1973-01-30 | Rech D Applic Scient Sogeras S | Pharmaceutical compositions comprising quinuclidinol derivatives |
| EP0424021A1 (en) | 1989-10-19 | 1991-04-24 | Pfizer Limited | Antimuscarinic bronchodilators |
| WO2000066558A1 (en) | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperazine derivatives useful as ccr5 antagonists |
| WO2000066559A1 (en) | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperidine derivatives useful as ccr5 antagonists |
| US6166037A (en) | 1997-08-28 | 2000-12-26 | Merck & Co., Inc. | Pyrrolidine and piperidine modulators of chemokine receptor activity |
| WO2001004118A2 (en) | 1999-07-14 | 2001-01-18 | Almirall Prodesfarma S.A. | Quinuclidine derivatives and their use as muscarinic m3 receptor ligands |
| WO2002000652A1 (en) | 2000-06-27 | 2002-01-03 | Laboratorios S.A.L.V.A.T., S.A. | Carbamates derived from arylalkylamines |
| WO2002051841A1 (en) | 2000-12-22 | 2002-07-04 | Almirall Prodesfarma Ag | Quinuclidine carbamate derivatives and their use as m3 antagonists |
| WO2002053564A2 (en) | 2000-12-28 | 2002-07-11 | Almirall Prodesfarma Ag | Quinuclidine derivatives and their use as m3 antagonists |
| WO2003000840A2 (en) | 2001-06-21 | 2003-01-03 | Diversa Corporation | Nitrilases |
| WO2003033495A1 (en) | 2001-10-17 | 2003-04-24 | Ucb, S.A. | Quinuclidine derivatives, processes for preparing them and their uses as m2 and/or m3 muscarinic receptor inhibitors |
| WO2003053966A2 (en) | 2001-12-20 | 2003-07-03 | Laboratorios S.A.L.V.A.T., S.A. | 1-alkyl-1-azoniabicyclo [2.2.2] octane carbamate derivatives and their use as muscarinic receptor antagonists |
| WO2003087094A2 (en) | 2002-04-16 | 2003-10-23 | Almirall Prodesfarma Ag | Pyrrolidinium derivatives as antagonists of m3 muscarinic receptors |
| WO2003099807A1 (en) | 2002-05-29 | 2003-12-04 | Almirall Prodesfarma S.A. | New indolylpiperidine derivatives as potent antihistaminic and antiallergic agents |
| WO2004005285A1 (en) | 2002-07-02 | 2004-01-15 | Almirall Prodesfarma Ag | New quinuclidine amide derivatives |
| WO2004016601A1 (en) | 2002-08-09 | 2004-02-26 | Novartis Ag | Benzothiazole derivatives having beta-2-adrenoreceptor agonist activity |
| WO2004018422A1 (en) | 2002-08-23 | 2004-03-04 | Ranbaxy Laboratories Limited | Fluoro and sulphonylamino containing 3,6-disubstituted azabicyclo (3.1.0) hexane derivatives as muscarinic receptor antagonists |
| WO2004018425A1 (en) | 2002-08-21 | 2004-03-04 | Astrazeneca Ab | N-4-piperidinyl compounds as ccr5 modulators |
| WO2004026841A1 (en) | 2002-09-18 | 2004-04-01 | Sumitomo Pharmaceuticals Co., Ltd. | Novel 6-substituted uracil derivative and therapeutic agent for allergic disease |
| WO2004026873A1 (en) | 2002-09-18 | 2004-04-01 | Ono Pharmaceutical Co., Ltd. | Triazaspiro[5.5]undecane derivatives and drugs comprising the same as the active ingredient |
| JP2004107299A (en) | 2002-09-20 | 2004-04-08 | Japan Energy Corp | New 1-substituted urasil derivative and therapeutic agent for allergic disease |
| WO2004033412A1 (en) | 2002-10-04 | 2004-04-22 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Novel beta mimetics with extended duration of action, method for production and use thereof as medicaments |
Family Cites Families (126)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1524747A (en) | 1976-05-11 | 1978-09-13 | Ici Ltd | Polypeptide |
| ATE28864T1 (en) | 1982-07-23 | 1987-08-15 | Ici Plc | AMIDE DERIVATIVES. |
| GB8327256D0 (en) | 1983-10-12 | 1983-11-16 | Ici Plc | Steroid derivatives |
| US5093330A (en) | 1987-06-15 | 1992-03-03 | Ciba-Geigy Corporation | Staurosporine derivatives substituted at methylamino nitrogen |
| US4929624A (en) | 1989-03-23 | 1990-05-29 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo(4,5-c)quinolin-4-amines |
| GB8916480D0 (en) | 1989-07-19 | 1989-09-06 | Glaxo Group Ltd | Chemical process |
| US5010099A (en) | 1989-08-11 | 1991-04-23 | Harbor Branch Oceanographic Institution, Inc. | Discodermolide compounds, compositions containing same and method of preparation and use |
| US5395855A (en) | 1990-05-07 | 1995-03-07 | Ciba-Geigy Corporation | Hydrazones |
| PT100441A (en) | 1991-05-02 | 1993-09-30 | Smithkline Beecham Corp | PIRROLIDINONES, ITS PREPARATION PROCESS, PHARMACEUTICAL COMPOSITIONS THAT CONTAIN THEM AND USE |
| US5451700A (en) | 1991-06-11 | 1995-09-19 | Ciba-Geigy Corporation | Amidino compounds, their manufacture and methods of treatment |
| NZ243082A (en) | 1991-06-28 | 1995-02-24 | Ici Plc | 4-anilino-quinazoline derivatives; pharmaceutical compositions, preparatory processes, and use thereof |
| US5268376A (en) | 1991-09-04 | 1993-12-07 | Minnesota Mining And Manufacturing Company | 1-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| GB9300059D0 (en) | 1992-01-20 | 1993-03-03 | Zeneca Ltd | Quinazoline derivatives |
| WO1993019750A1 (en) | 1992-04-02 | 1993-10-14 | Smithkline Beecham Corporation | Compounds useful for treating allergic or inflammatory diseases |
| EP0633776B1 (en) | 1992-04-02 | 2001-05-09 | Smithkline Beecham Corporation | Compounds useful for treating allergic and inflammatory diseases |
| WO1993019751A1 (en) | 1992-04-02 | 1993-10-14 | Smithkline Beecham Corporation | Compounds useful for treating inflammatory diseases and inhibiting production of tumor necrosis factor |
| TW225528B (en) | 1992-04-03 | 1994-06-21 | Ciba Geigy Ag | |
| DK0666868T4 (en) | 1992-10-28 | 2006-09-18 | Genentech Inc | Use of anti-VEGF antibodies to treat cancer |
| GB9301000D0 (en) | 1993-01-20 | 1993-03-10 | Glaxo Group Ltd | Chemical compounds |
| GB9314893D0 (en) | 1993-07-19 | 1993-09-01 | Zeneca Ltd | Quinazoline derivatives |
| GB9414193D0 (en) | 1994-07-14 | 1994-08-31 | Glaxo Group Ltd | Compounds |
| GB9414208D0 (en) | 1994-07-14 | 1994-08-31 | Glaxo Group Ltd | Compounds |
| EP1110953B1 (en) | 1995-03-30 | 2009-10-28 | Pfizer Products Inc. | Quinazoline derivatives |
| GB9508538D0 (en) | 1995-04-27 | 1995-06-14 | Zeneca Ltd | Quinazoline derivatives |
| US5747498A (en) | 1996-05-28 | 1998-05-05 | Pfizer Inc. | Alkynyl and azido-substituted 4-anilinoquinazolines |
| US5880141A (en) | 1995-06-07 | 1999-03-09 | Sugen, Inc. | Benzylidene-Z-indoline compounds for the treatment of disease |
| US5843901A (en) | 1995-06-07 | 1998-12-01 | Advanced Research & Technology Institute | LHRH antagonist peptides |
| DK0836605T3 (en) | 1995-07-06 | 2002-05-13 | Novartis Ag | Pyrrolopyrimidines and Methods for their Preparation |
| US5760041A (en) | 1996-02-05 | 1998-06-02 | American Cyanamid Company | 4-aminoquinazoline EGFR Inhibitors |
| GB9603095D0 (en) | 1996-02-14 | 1996-04-10 | Zeneca Ltd | Quinazoline derivatives |
| NZ332119A (en) | 1996-04-12 | 2001-08-31 | Warner Lambert Co | Quinazoline compounds which are irreversible inhibitors of tyrosine kinases |
| DE69734513T2 (en) | 1996-06-24 | 2006-07-27 | Pfizer Inc. | PHENYLAMINO-SUBSTITUTED TRICYCL DERIVATIVES FOR THE TREATMENT OF HYPERPROLIFERATIVE DISEASES |
| JP2001500851A (en) | 1996-08-30 | 2001-01-23 | ノバルティス アクチエンゲゼルシャフト | Process for producing epothilone and intermediate products obtained during the process |
| CA2264908C (en) | 1996-09-06 | 2006-04-25 | Obducat Ab | Method for anisotropic etching of structures in conducting materials |
| DE19638745C2 (en) | 1996-09-11 | 2001-05-10 | Schering Ag | Monoclonal antibodies against the extracellular domain of the human VEGF receptor protein (KDR) |
| AU4342997A (en) | 1996-09-13 | 1998-04-02 | Sugen, Inc. | Use of quinazoline derivatives for the manufacture of a medicament in the reatment of hyperproliferative skin disorders |
| EP0837063A1 (en) | 1996-10-17 | 1998-04-22 | Pfizer Inc. | 4-Aminoquinazoline derivatives |
| GB9622386D0 (en) | 1996-10-28 | 1997-01-08 | Sandoz Ltd | Organic compounds |
| HU229833B1 (en) | 1996-11-18 | 2014-09-29 | Biotechnolog Forschung Gmbh | Epothilone d production process, and its use as cytostatic as well as phytosanitary agents |
| US6441186B1 (en) | 1996-12-13 | 2002-08-27 | The Scripps Research Institute | Epothilone analogs |
| TW528755B (en) | 1996-12-24 | 2003-04-21 | Glaxo Group Ltd | 2-(purin-9-yl)-tetrahydrofuran-3,4-diol derivatives |
| CO4950519A1 (en) | 1997-02-13 | 2000-09-01 | Novartis Ag | PHTHALAZINES, PHARMACEUTICAL PREPARATIONS THAT UNDERSTAND THEM AND THE PROCESS FOR THEIR PREPARATION |
| CO4940418A1 (en) | 1997-07-18 | 2000-07-24 | Novartis Ag | MODIFICATION OF A CRYSTAL OF A DERIVATIVE OF N-PHENYL-2-PIRIMIDINAMINE, PROCESSES FOR ITS MANUFACTURE AND USE |
| WO1999016766A1 (en) | 1997-10-01 | 1999-04-08 | Kyowa Hakko Kogyo Co., Ltd. | Benzodioxole derivatives |
| GB9721069D0 (en) | 1997-10-03 | 1997-12-03 | Pharmacia & Upjohn Spa | Polymeric derivatives of camptothecin |
| GB9723589D0 (en) | 1997-11-08 | 1998-01-07 | Glaxo Group Ltd | Chemical compounds |
| GB9723590D0 (en) | 1997-11-08 | 1998-01-07 | Glaxo Group Ltd | Chemical compounds |
| GB9723566D0 (en) | 1997-11-08 | 1998-01-07 | Glaxo Group Ltd | Chemical compounds |
| YU44900A (en) | 1998-01-31 | 2003-01-31 | Glaxo Group Limited | 2-(purin-9-yl)-tetrahydrofuran-3,4-diol derivatives |
| CO4990969A1 (en) | 1998-02-14 | 2000-12-26 | Glaxo Group Ltd | DERIVATIVES OF 2- (PURIN-9-IL) -TETRAHYDROFURAN-3,4-DIOL |
| US6194181B1 (en) | 1998-02-19 | 2001-02-27 | Novartis Ag | Fermentative preparation process for and crystal forms of cytostatics |
| NZ506742A (en) | 1998-02-25 | 2003-09-26 | Sloan Kettering Inst Cancer | Synthesis of desoxyepothilones and hydroxy acid derivative intermediates |
| GB9813540D0 (en) | 1998-06-23 | 1998-08-19 | Glaxo Group Ltd | Chemical compounds |
| DE69910213T2 (en) | 1998-06-23 | 2004-07-01 | Glaxo Group Ltd., Greenford | 2- (PURIN-9-YL) -TETRAHYDROFURAN-3,4-DIOL DERIVATIVES |
| GB9813535D0 (en) | 1998-06-23 | 1998-08-19 | Glaxo Group Ltd | Chemical compounds |
| GB9813565D0 (en) | 1998-06-23 | 1998-08-19 | Glaxo Group Ltd | Chemical compounds |
| BR9815931A (en) | 1998-06-30 | 2001-02-20 | Dow Chemical Co | Polymeric polyols, a process for their production, and polyurethane foam obtained |
| ATE459616T1 (en) | 1998-08-11 | 2010-03-15 | Novartis Ag | ISOCHINOLINE DERIVATIVES WITH ANGIOGENESIS-INHIBITING EFFECT |
| JP2000119271A (en) | 1998-08-12 | 2000-04-25 | Hokuriku Seiyaku Co Ltd | 1h-imidazopyridine derivative |
| BR9914526A (en) | 1998-10-16 | 2001-07-03 | Pfizer | Adenine derivatives |
| GB9824579D0 (en) | 1998-11-10 | 1999-01-06 | Novartis Ag | Organic compounds |
| UA71587C2 (en) | 1998-11-10 | 2004-12-15 | Шерінг Акцієнгезелльшафт | Anthranilic acid amides and use thereof as medicaments |
| NZ511722A (en) | 1998-11-20 | 2004-05-28 | Kosan Biosciences Inc | Recombinant methods and materials for producing epothilone and epothilone derivatives |
| IL143596A0 (en) | 1998-12-22 | 2002-04-21 | Genentech Inc | Vascular endothelial cell growth factor antagonists and uses thereof |
| BR0009507A (en) | 1999-03-30 | 2002-01-15 | Novartis Ag | Phthalazine derivatives for the treatment of inflammatory diseases |
| GB9913083D0 (en) | 1999-06-04 | 1999-08-04 | Novartis Ag | Organic compounds |
| YU25500A (en) | 1999-05-11 | 2003-08-29 | Pfizer Products Inc. | Process for the synthesis of nucleosite analogues |
| GB9913932D0 (en) | 1999-06-15 | 1999-08-18 | Pfizer Ltd | Purine derivatives |
| US6322771B1 (en) | 1999-06-18 | 2001-11-27 | University Of Virginia Patent Foundation | Induction of pharmacological stress with adenosine receptor agonists |
| PT1212089E (en) | 1999-08-21 | 2006-08-31 | Altana Pharma Ag | ROFLUMILAST AND SALMETEROL SYNERGY COMBINATION |
| CO5180581A1 (en) | 1999-09-30 | 2002-07-30 | Pfizer Prod Inc | COMPOUNDS FOR THE TREATMENT OF THE ISCHEMIA PHARMACEUTICAL TIONS THAT CONTAIN THEM FOR THE TREATMENT OF THE ISCHEMIA |
| GB9924361D0 (en) | 1999-10-14 | 1999-12-15 | Pfizer Ltd | Purine derivatives |
| GB9924363D0 (en) | 1999-10-14 | 1999-12-15 | Pfizer Central Res | Purine derivatives |
| GB0003960D0 (en) | 2000-02-18 | 2000-04-12 | Pfizer Ltd | Purine derivatives |
| US6924410B2 (en) | 2000-03-17 | 2005-08-02 | Hisamitsu Pharmaceutical Co., Ltd. | Ultraviolet-screening patch |
| TWI227240B (en) | 2000-06-06 | 2005-02-01 | Pfizer | 2-aminocarbonyl-9H-purine derivatives |
| GB0015727D0 (en) | 2000-06-27 | 2000-08-16 | Pfizer Ltd | Purine derivatives |
| GB0015876D0 (en) | 2000-06-28 | 2000-08-23 | Novartis Ag | Organic compounds |
| DK1522590T3 (en) | 2000-06-28 | 2009-12-21 | Glycofi Inc | Process for Preparation of Modified Glycoproteins |
| DE10038639A1 (en) | 2000-07-28 | 2002-02-21 | Schering Ag | New and known N-aryl 2-hydroxy-omega-arylalkanamide derivatives, useful e.g. for treating inflammatory diseases such as rheumatism |
| BRPI0113042B8 (en) | 2000-08-05 | 2021-05-25 | Glaxo Group Ltd | compound of the formula or a physiologically acceptable solvate thereof, use thereof, pharmaceutical composition, pharmaceutical formulation, method of treating a human or animal subject with an inflammatory and/or allergic condition, and, process for preparing a compound or a solvate thereof |
| PE20020354A1 (en) | 2000-09-01 | 2002-06-12 | Novartis Ag | HYDROXAMATE COMPOUNDS AS HISTONE-DESACETILASE (HDA) INHIBITORS |
| GB0022695D0 (en) | 2000-09-15 | 2000-11-01 | Pfizer Ltd | Purine Derivatives |
| GB0028383D0 (en) | 2000-11-21 | 2001-01-03 | Novartis Ag | Organic compounds |
| EP1241176A1 (en) | 2001-03-16 | 2002-09-18 | Pfizer Products Inc. | Purine derivatives for the treatment of ischemia |
| BR0209271A (en) | 2001-04-30 | 2004-06-15 | Glaxo Group Ltd | Compound, use of a compound pharmaceutical composition, pharmaceutical aerosol formulation, method for treating a human or animal patient with an inflammatory and / or allergic condition, and process for preparing a compound |
| AR035885A1 (en) | 2001-05-14 | 2004-07-21 | Novartis Ag | DERIVATIVES OF 4-AMINO-5-FENIL-7-CYCLLOBUTILPIRROLO (2,3-D) PYRIMIDINE, A PROCESS FOR ITS PREPARATION, A PHARMACEUTICAL COMPOSITION AND THE USE OF SUCH DERIVATIVES FOR THE PREPARATION OF A PHARMACEUTICAL COMPOSITION |
| EP1395287A1 (en) | 2001-05-25 | 2004-03-10 | Pfizer Inc. | An adenosine a2a receptor agonist and an anticholinergic agent in combination for treating obstructive airways diseases |
| GB0119249D0 (en) | 2001-08-07 | 2001-10-03 | Novartis Ag | Organic compounds |
| GB0125259D0 (en) | 2001-10-20 | 2001-12-12 | Glaxo Group Ltd | Novel compounds |
| AR037517A1 (en) | 2001-11-05 | 2004-11-17 | Novartis Ag | DERIVATIVES OF NAFTIRIDINES, A PROCESS FOR THE PREPARATION, PHARMACEUTICAL COMPOSITION AND THE USE OF THEM FOR THE PREPARATION OF A MEDICINAL PRODUCT FOR THE TREATMENT OF AN INFLAMMATORY DISEASE |
| AU2002356759A1 (en) | 2001-12-01 | 2003-06-17 | Glaxo Group Limited | 17.alpha. -cyclic esters of 16-methylpregnan-3,20-dione as anti-inflammatory agents |
| AU2003202044A1 (en) | 2002-01-15 | 2003-09-09 | Glaxo Group Limited | 17.alpha-cycloalkyl/cycloylkenyl esters of alkyl-or haloalkyl-androst-4-en-3-on-11.beta.,17.alpha.-diol 17.beta.-carboxylates as anti-inflammatory agents |
| AU2003201693A1 (en) | 2002-01-21 | 2003-09-02 | Glaxo Group Limited | Non-aromatic 17.alpha.-esters of androstane-17.beta.-carboxylate esters as anti-inflammatory agents |
| GB0202216D0 (en) | 2002-01-31 | 2002-03-20 | Glaxo Group Ltd | Novel compounds |
| JP2005521717A (en) | 2002-03-26 | 2005-07-21 | ベーリンガー インゲルハイム ファーマシューティカルズ インコーポレイテッド | Glucocorticoid mimetics, process for producing the same, pharmaceutical composition thereof, and use thereof |
| CN1633296A (en) | 2002-03-26 | 2005-06-29 | 贝林格尔·英格海姆药物公司 | Glucocorticoid mimetics, methods of making them, pharmaceutical compositions, and uses thereof |
| ATE381336T1 (en) | 2002-04-10 | 2008-01-15 | Univ Virginia | USE OF A2A ADENOSINE RECEPTOR AGONIST AND ANTIPATHOGENE CONTAINING COMBINATIONS FOR THE TREATMENT OF INFLAMMATORY DISEASES |
| GB0211649D0 (en) * | 2002-05-21 | 2002-07-03 | Novartis Ag | Organic compounds |
| DE10224888A1 (en) | 2002-06-05 | 2003-12-24 | Merck Patent Gmbh | pyridazine |
| US7074806B2 (en) | 2002-06-06 | 2006-07-11 | Boehringer Ingelheim Pharmaceuticals, Inc. | Glucocorticoid mimetics, methods of making them, pharmaceutical compositions, and uses thereof |
| DE10225574A1 (en) | 2002-06-10 | 2003-12-18 | Merck Patent Gmbh | New 1-acyl-3-phenyl-5,6-dihydro-4H-pyridazine derivatives, are phosphodiesterase IV inhibitors useful e.g. for treating asthma, allergy, inflammation, autoimmune diseases or myocardial diseases |
| DE10227269A1 (en) | 2002-06-19 | 2004-01-08 | Merck Patent Gmbh | thiazole |
| AU2003243870B2 (en) | 2002-06-25 | 2008-11-20 | Merck Frosst Canada Ltd | 8-(biaryl) quinoline PDE4 inhibitors |
| EP1519922A1 (en) | 2002-07-02 | 2005-04-06 | Merck Frosst Canada & Co. | Di-aryl-substituted ethane pyridone pde4 inhibitors |
| EP1521733B1 (en) | 2002-07-08 | 2014-08-20 | Pfizer Products Inc. | Modulators of the glucocorticoid receptor |
| US20060167001A1 (en) | 2002-08-10 | 2006-07-27 | Sterk Jan G | Pyridazinone-derivatives as pde4 inhibitors |
| WO2004018457A1 (en) | 2002-08-10 | 2004-03-04 | Altana Pharma Ag | Pyrrolidinedione substituted piperidine-phthalazones as pde4 inhibitors |
| WO2004018450A1 (en) | 2002-08-10 | 2004-03-04 | Altana Pharma Ag | Piperidine-n-oxide-derivatives |
| WO2004018449A1 (en) | 2002-08-10 | 2004-03-04 | Altana Pharma Ag | Piperidine-derivatives as pde4 inhibitors |
| EP1537086A2 (en) | 2002-08-17 | 2005-06-08 | ALTANA Pharma AG | Novel phenanthridines |
| RS20050117A (en) | 2002-08-17 | 2007-06-04 | Altana Pharma Ag., | Novel benzonaphthyridines |
| JP4587294B2 (en) | 2002-08-29 | 2010-11-24 | ニコメッド ゲゼルシャフト ミット ベシュレンクテル ハフツング | 2-hydroxy-6-phenylphenanthridine as PDE4 inhibitor |
| ATE353217T1 (en) | 2002-08-29 | 2007-02-15 | Altana Pharma Ag | 3-HYDROXY-6-PHENYLPHENANTHRIDINE AS PDE-4 INHIBITORS |
| JP2006506379A (en) | 2002-10-23 | 2006-02-23 | グレンマーク・ファーマシューティカルズ・リミテッド | Novel tricyclic compounds useful for the treatment of inflammatory and allergic diseases: methods for their preparation and pharmaceutical compositions containing them |
| GB0225540D0 (en) | 2002-11-01 | 2002-12-11 | Glaxo Group Ltd | Medicinal compounds |
| GB0225535D0 (en) | 2002-11-01 | 2002-12-11 | Glaxo Group Ltd | Medicinal compounds |
| DE10253426B4 (en) | 2002-11-15 | 2005-09-22 | Elbion Ag | Novel hydroxyindoles, their use as inhibitors of phosphodiesterase 4 and methods for their preparation |
| DE10253220A1 (en) | 2002-11-15 | 2004-05-27 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | New 2-(N-phenylalkyl-amino)-1-phenyl-ethanol derivatives, are beta-adrenergic agents especially useful for treating inflammatory and obstructive respiratory diseases such as asthma or COPD |
| DE10253282A1 (en) | 2002-11-15 | 2004-05-27 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Treatment of chronic obstructive pulmonary disease, using new or known N-substituted 2-amino-1-(benz-(1,4)-oxazin-3-on-8-yl)-ethanol derivative beta-mimetic agents, suitable for once-daily administration |
| RS20050522A (en) * | 2003-01-09 | 2007-12-31 | Pfizer Inc., | Protein kinase inhibiting tricyclic compounds that increase the effects of anti-neoplastic agents and therapeutic radiation |
| EP1452173A1 (en) | 2003-02-25 | 2004-09-01 | Schering AG | UV-stable transdermal patch |
| MY150088A (en) * | 2003-05-19 | 2013-11-29 | Irm Llc | Immunosuppressant compounds and compositions |
| AR046845A1 (en) | 2003-11-21 | 2005-12-28 | Novartis Ag | DERIVATIVES OF 1H-IMIDAZO [4,5-C] QUINOLINE FOR THE TREATMENT OF PROTEIN-KINASE DEPENDENT DISEASES |
| ES2308272T3 (en) * | 2003-11-21 | 2008-12-01 | Novartis Ag | DERIVATIVES OF 1H-IMIDAZOQUINOLINE AS INHIBITORS OF PROTEIN QUINASA. |
| GB0510390D0 (en) * | 2005-05-20 | 2005-06-29 | Novartis Ag | Organic compounds |
-
2005
- 2005-05-20 GB GBGB0510390.8A patent/GB0510390D0/en not_active Ceased
-
2006
- 2006-05-09 GT GT200600193A patent/GT200600193A/en unknown
- 2006-05-16 MY MYPI20062243A patent/MY147442A/en unknown
- 2006-05-18 CN CN2012102906863A patent/CN102796099A/en active Pending
- 2006-05-18 ES ES10175375T patent/ES2397081T3/en active Active
- 2006-05-18 ES ES06753710T patent/ES2378463T3/en active Active
- 2006-05-18 GE GEAP200610380A patent/GEP20105054B/en unknown
- 2006-05-18 EP EP10175369A patent/EP2292617B1/en not_active Not-in-force
- 2006-05-18 EA EA200702387A patent/EA013434B1/en not_active IP Right Cessation
- 2006-05-18 EP EP10175375A patent/EP2270008B8/en active Active
- 2006-05-18 PL PL10175375T patent/PL2270008T3/en unknown
- 2006-05-18 EP EP06753710A patent/EP1888578B8/en active Active
- 2006-05-18 SM SM200700051T patent/SMAP200700051A/en unknown
- 2006-05-18 PE PE2006000525A patent/PE20070004A1/en not_active Application Discontinuation
- 2006-05-18 SI SI200631485T patent/SI2270008T1/en unknown
- 2006-05-18 AU AU2006249071A patent/AU2006249071B2/en not_active Ceased
- 2006-05-18 DK DK06753710.0T patent/DK1888578T3/en active
- 2006-05-18 UA UAA200712887A patent/UA92490C2/en unknown
- 2006-05-18 PL PL06753710T patent/PL1888578T3/en unknown
- 2006-05-18 CN CNA2006800175673A patent/CN101495477A/en active Pending
- 2006-05-18 CA CA2608496A patent/CA2608496C/en not_active Expired - Fee Related
- 2006-05-18 WO PCT/EP2006/004725 patent/WO2006122806A2/en active Application Filing
- 2006-05-18 BR BRPI0610321A patent/BRPI0610321B8/en not_active IP Right Cessation
- 2006-05-18 PT PT101753754T patent/PT2270008E/en unknown
- 2006-05-18 PT PT06753710T patent/PT1888578E/en unknown
- 2006-05-18 DK DK10175375.4T patent/DK2270008T3/en active
- 2006-05-18 ES ES10175369T patent/ES2391161T3/en active Active
- 2006-05-18 AT AT06753710T patent/ATE535526T1/en active
- 2006-05-18 NZ NZ562890A patent/NZ562890A/en not_active IP Right Cessation
- 2006-05-18 SI SI200631230T patent/SI1888578T1/en unknown
- 2006-05-18 KR KR1020077026850A patent/KR101237575B1/en active IP Right Grant
- 2006-05-18 JP JP2008511640A patent/JP5220591B2/en active Active
- 2006-05-18 JO JO2006141A patent/JO2751B1/en active
- 2006-05-18 MX MX2007014380A patent/MX2007014380A/en active IP Right Grant
- 2006-05-18 AR ARP060102006A patent/AR054127A1/en not_active Application Discontinuation
- 2006-05-18 US US11/913,788 patent/US7667039B2/en active Active
- 2006-05-18 DK DK10175369.7T patent/DK2292617T3/en active
- 2006-05-19 TW TW095117780A patent/TWI383982B/en not_active IP Right Cessation
-
2007
- 2007-10-22 ZA ZA2007/09074A patent/ZA200709074B/en unknown
- 2007-10-29 IL IL187003A patent/IL187003A/en active IP Right Grant
- 2007-11-07 CR CR9504A patent/CR9504A/en unknown
- 2007-11-16 NI NI200700296A patent/NI200700296A/en unknown
- 2007-11-19 TN TNP2007000431A patent/TNSN07431A1/en unknown
- 2007-11-20 MA MA30390A patent/MA29462B1/en unknown
- 2007-12-19 NO NO20076561A patent/NO340584B1/en not_active IP Right Cessation
-
2008
- 2008-06-05 HK HK08106290.6A patent/HK1115871A1/en not_active IP Right Cessation
-
2009
- 2009-10-12 US US12/577,268 patent/US7994170B2/en active Active
-
2011
- 2011-05-30 HK HK11105320.7A patent/HK1151286A1/en not_active IP Right Cessation
- 2011-06-21 US US13/165,144 patent/US8431592B2/en active Active
-
2012
- 2012-02-17 CY CY20121100169T patent/CY1112369T1/en unknown
- 2012-04-23 US US13/453,225 patent/US20120207751A1/en not_active Abandoned
- 2012-05-23 AR ARP120101818A patent/AR086528A2/en unknown
- 2012-08-08 JP JP2012176167A patent/JP5508485B2/en not_active Expired - Fee Related
- 2012-08-22 IL IL221573A patent/IL221573A/en active IP Right Grant
- 2012-12-21 CY CY20121101251T patent/CY1113494T1/en unknown
-
2013
- 2013-08-22 HR HRP20130799TT patent/HRP20130799T1/en unknown
- 2013-08-22 HR HRP20130794TT patent/HRP20130794T1/en unknown
Patent Citations (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3714357A (en) | 1968-07-15 | 1973-01-30 | Rech D Applic Scient Sogeras S | Pharmaceutical compositions comprising quinuclidinol derivatives |
| EP0424021A1 (en) | 1989-10-19 | 1991-04-24 | Pfizer Limited | Antimuscarinic bronchodilators |
| US5171744A (en) | 1989-10-19 | 1992-12-15 | Pfizer Inc. | Antimuscarinic bronchodilators |
| US6166037A (en) | 1997-08-28 | 2000-12-26 | Merck & Co., Inc. | Pyrrolidine and piperidine modulators of chemokine receptor activity |
| WO2000066558A1 (en) | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperazine derivatives useful as ccr5 antagonists |
| WO2000066559A1 (en) | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperidine derivatives useful as ccr5 antagonists |
| WO2001004118A2 (en) | 1999-07-14 | 2001-01-18 | Almirall Prodesfarma S.A. | Quinuclidine derivatives and their use as muscarinic m3 receptor ligands |
| WO2002000652A1 (en) | 2000-06-27 | 2002-01-03 | Laboratorios S.A.L.V.A.T., S.A. | Carbamates derived from arylalkylamines |
| WO2002051841A1 (en) | 2000-12-22 | 2002-07-04 | Almirall Prodesfarma Ag | Quinuclidine carbamate derivatives and their use as m3 antagonists |
| WO2002053564A2 (en) | 2000-12-28 | 2002-07-11 | Almirall Prodesfarma Ag | Quinuclidine derivatives and their use as m3 antagonists |
| WO2003000840A2 (en) | 2001-06-21 | 2003-01-03 | Diversa Corporation | Nitrilases |
| WO2003033495A1 (en) | 2001-10-17 | 2003-04-24 | Ucb, S.A. | Quinuclidine derivatives, processes for preparing them and their uses as m2 and/or m3 muscarinic receptor inhibitors |
| WO2003053966A2 (en) | 2001-12-20 | 2003-07-03 | Laboratorios S.A.L.V.A.T., S.A. | 1-alkyl-1-azoniabicyclo [2.2.2] octane carbamate derivatives and their use as muscarinic receptor antagonists |
| WO2003087094A2 (en) | 2002-04-16 | 2003-10-23 | Almirall Prodesfarma Ag | Pyrrolidinium derivatives as antagonists of m3 muscarinic receptors |
| WO2003099807A1 (en) | 2002-05-29 | 2003-12-04 | Almirall Prodesfarma S.A. | New indolylpiperidine derivatives as potent antihistaminic and antiallergic agents |
| WO2004005285A1 (en) | 2002-07-02 | 2004-01-15 | Almirall Prodesfarma Ag | New quinuclidine amide derivatives |
| WO2004016601A1 (en) | 2002-08-09 | 2004-02-26 | Novartis Ag | Benzothiazole derivatives having beta-2-adrenoreceptor agonist activity |
| WO2004018425A1 (en) | 2002-08-21 | 2004-03-04 | Astrazeneca Ab | N-4-piperidinyl compounds as ccr5 modulators |
| WO2004018422A1 (en) | 2002-08-23 | 2004-03-04 | Ranbaxy Laboratories Limited | Fluoro and sulphonylamino containing 3,6-disubstituted azabicyclo (3.1.0) hexane derivatives as muscarinic receptor antagonists |
| WO2004026841A1 (en) | 2002-09-18 | 2004-04-01 | Sumitomo Pharmaceuticals Co., Ltd. | Novel 6-substituted uracil derivative and therapeutic agent for allergic disease |
| WO2004026873A1 (en) | 2002-09-18 | 2004-04-01 | Ono Pharmaceutical Co., Ltd. | Triazaspiro[5.5]undecane derivatives and drugs comprising the same as the active ingredient |
| JP2004107299A (en) | 2002-09-20 | 2004-04-08 | Japan Energy Corp | New 1-substituted urasil derivative and therapeutic agent for allergic disease |
| WO2004033412A1 (en) | 2002-10-04 | 2004-04-22 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Novel beta mimetics with extended duration of action, method for production and use thereof as medicaments |
Cited By (722)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US8802853B2 (en) | 2003-12-29 | 2014-08-12 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| US8697873B2 (en) | 2004-03-24 | 2014-04-15 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US8026366B2 (en) | 2004-06-18 | 2011-09-27 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US10071156B2 (en) | 2005-02-04 | 2018-09-11 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| EP1956008A1 (en) * | 2005-11-30 | 2008-08-13 | Mitsubishi Chemical Corporation | Organic compound, charge-transporting material, composition for charge-transporting material and organic electroluminescent device |
| US8022617B2 (en) | 2005-11-30 | 2011-09-20 | Mitsubishi Chemical Corporation | Organic compound, charge-transporting material, composition for charge-transporting material and organic electroluminescent device |
| EP1956008A4 (en) * | 2005-11-30 | 2010-12-01 | Mitsubishi Chem Corp | Organic compound, charge-transporting material, composition for charge-transporting material and organic electroluminescent device |
| US11744832B2 (en) | 2005-12-13 | 2023-09-05 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US8933086B2 (en) | 2005-12-13 | 2015-01-13 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B]pyridines and pyrrolo[2,3-B]pyrimidines as Janus kinase inhibitors |
| US9206187B2 (en) | 2005-12-13 | 2015-12-08 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as Janus kinase |
| US9662335B2 (en) | 2005-12-13 | 2017-05-30 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors |
| US10639310B2 (en) | 2005-12-13 | 2020-05-05 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9814722B2 (en) | 2005-12-13 | 2017-11-14 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors |
| US11331320B2 (en) | 2005-12-13 | 2022-05-17 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9974790B2 (en) | 2005-12-13 | 2018-05-22 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors |
| US9079912B2 (en) | 2005-12-13 | 2015-07-14 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as Janus kinase inhibitors |
| US10398699B2 (en) | 2005-12-13 | 2019-09-03 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors |
| US8946245B2 (en) | 2005-12-13 | 2015-02-03 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| WO2007106854A3 (en) * | 2006-03-15 | 2008-10-16 | Coley Pharm Group Inc | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| WO2007106854A2 (en) * | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| EP2364981A1 (en) | 2006-11-20 | 2011-09-14 | Novartis AG | Salts and crystalline forms of 2-methyl-2-[4- (3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile |
| EA015677B1 (en) * | 2006-11-20 | 2011-10-31 | Новартис Аг | SALTS AND CRYSTAL FORMS OF 2-METHYL-2-[4-(3-METHYL-2-OXO-8-QUINOLIN-3-YL-2,3-DIHYDROIMIDAZO[4,5-c]QUINOLIN-1-YL)PHENYL]PROPIONITRILE |
| AU2007323820B2 (en) * | 2006-11-20 | 2012-02-23 | Novartis Ag | Salts and crystal forms of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl] -propionitrile |
| US8436177B2 (en) | 2006-11-20 | 2013-05-07 | Novartis Ag | Salts and crystall forms of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile |
| WO2008064093A3 (en) * | 2006-11-20 | 2008-08-14 | Novartis Ag | Salts and crystal forms of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile |
| WO2008064093A2 (en) | 2006-11-20 | 2008-05-29 | Novartis Ag | Salts and crystal forms of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2,3-dihydro-imidazo[4,5-c]quinolin-1-yl)-phenyl]-propionitrile |
| AU2008218806B2 (en) * | 2007-02-20 | 2011-12-01 | Novartis Ag | Imidazoquinolines as dual lipid kinase and mTOR inhibitors |
| JP2014028869A (en) * | 2007-02-20 | 2014-02-13 | Novartis Ag | IMIDAZOQUINOLINES AS DUAL LIPID KINASE AND mTOR INHIBITORS |
| KR101472607B1 (en) * | 2007-02-20 | 2014-12-15 | 노파르티스 아게 | Imidazoquinolines as dual lipid kinase and mtor inhibitors |
| JP2010519309A (en) * | 2007-02-20 | 2010-06-03 | ノバルティス アーゲー | Imidazoquinolines as dual inhibitors of lipid kinases and mTOR |
| CN104758288A (en) * | 2007-02-20 | 2015-07-08 | 诺华股份有限公司 | Imidazoquinolines as dual lipid kinase and mTOR inhibitors |
| WO2008103636A1 (en) * | 2007-02-20 | 2008-08-28 | Novartis Ag | Imidazoquinolines as dual lipid kinase and mtor inhibitors |
| JP2016041760A (en) * | 2007-02-20 | 2016-03-31 | ノバルティス アーゲー | IMIDAZOQUINOLINES AS DUAL LIPID KINASE AND mTOR INHIBITORS |
| US9370508B2 (en) | 2007-02-20 | 2016-06-21 | Novartis Ag | Imidazoquinolines as dual lipid kinase and mTOR inhibitors |
| WO2008115974A2 (en) * | 2007-03-21 | 2008-09-25 | Wyeth | Pyrazolopyrimidine analogs and their use as mtor kinase and pi3 kinase inhibitors |
| WO2008115974A3 (en) * | 2007-03-21 | 2008-12-18 | Wyeth Corp | Pyrazolopyrimidine analogs and their use as mtor kinase and pi3 kinase inhibitors |
| US11213528B2 (en) | 2007-06-13 | 2022-01-04 | Incyte Holdings Corporation | Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| WO2009008991A3 (en) * | 2007-07-06 | 2009-07-09 | Us Gov Nat Inst Health | Dna-pkcs modulates energy regulation and brain function |
| RU2481838C2 (en) * | 2007-07-24 | 2013-05-20 | Новартис Аг | Application of imidazoquinolines for treatment of egfr-dependent diseases or diseases with aquired resistance to agents binding members of egfr family |
| AU2008280105B2 (en) * | 2007-07-24 | 2011-10-20 | Novartis Ag | Use of imidazoquinolines for the treatment of EGFR dependent diseases or diseases that have acquired resistance to agents that target EGFR family members |
| CN103298471A (en) * | 2007-07-24 | 2013-09-11 | 诺瓦提斯公司 | Use of imidazoquinolines for the treatment of EGFR dependent diseases or diseases that have acquired resistance to agents that target EGFR family members |
| WO2009013305A1 (en) * | 2007-07-24 | 2009-01-29 | Novartis Ag | Use of imidazoquinolines for the treatment of egfr dependent diseases or diseases that have acquired resistance to agents that target egfr family members |
| JP2010534219A (en) * | 2007-07-24 | 2010-11-04 | ノバルティス アーゲー | Use of imidazoquinolines for the treatment of EGFR-dependent diseases or diseases that have acquired resistance to drugs targeting EGFR family members |
| WO2009076170A3 (en) * | 2007-12-13 | 2009-07-30 | Novartis Ag | Combinations of therapeutic agents for treating cancer |
| WO2009076170A2 (en) * | 2007-12-13 | 2009-06-18 | Novartis Ag | Combinations of therapeutic agents for treating cancer |
| CN101980708B (en) * | 2008-03-26 | 2013-03-13 | 诺瓦提斯公司 | Imidazoquinolines and pyrimidine derivatives as potent modulators of VEGF-driven angiogenic processes |
| WO2009118324A1 (en) * | 2008-03-26 | 2009-10-01 | Novartis Ag | 5imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| RU2509558C2 (en) * | 2008-03-26 | 2014-03-20 | Новартис Аг | Imidazoquinoline and pyrimidine derivatives as potential modulators of vbgf-stimulated angiogenic processes |
| EP2474323A2 (en) | 2008-03-26 | 2012-07-11 | Novartis AG | Imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| JP2011515440A (en) * | 2008-03-26 | 2011-05-19 | ノバルティス アーゲー | 5-Imidazoquinoline and pyrimidine derivatives as potent modulators of VEGF-promoted angiogenesis |
| JP2014141515A (en) * | 2008-03-26 | 2014-08-07 | Novartis Ag | 5imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| AU2009228797B2 (en) * | 2008-03-26 | 2012-07-19 | Novartis Ag | 5imidazoquinolines and pyrimidine derivatives as potent modulators of VEGF-driven angiogenic processes |
| EP2474323A3 (en) * | 2008-03-26 | 2012-10-10 | Novartis AG | Imidazoquinolines and pyrimidine derivatives as potent modulators of vegf-driven angiogenic processes |
| WO2010006225A1 (en) * | 2008-07-11 | 2010-01-14 | Novartis Ag | Combination of (a) a phosphoinositide 3-kinase inhibitor and (b) a modulator of ras/raf/mek pathway |
| US9241939B2 (en) | 2008-07-11 | 2016-01-26 | Novartis Ag | Combination of (a) a phosphoinositide 3-kinase inhibitor and (b) a modulator of RAS/RAF/MEK pathway |
| JP2011527703A (en) * | 2008-07-11 | 2011-11-04 | ノバルティス アーゲー | Formulation of (a) a phosphoinositide 3-kinase inhibitor and (b) a modulator of the Ras / Raf / Mek pathway |
| RU2508110C2 (en) * | 2008-07-11 | 2014-02-27 | Новартис Аг | COMBINATION OF (A) PHOSPHOINOSITIDE-3-KINASE INHIBITOR AND (B) Ras/Raf/Mek PATHAWAY MODULATOR |
| AU2009268469B2 (en) * | 2008-07-11 | 2013-02-28 | Novartis Ag | Combination of (a) a phosphoinositide 3-kinase inhibitor and (b) a modulator of Ras/Raf/Mek pathway |
| JP2015007110A (en) * | 2008-07-11 | 2015-01-15 | ノバルティス アーゲー | Combination of (a) phosphoinositide 3-kinase inhibitor and (b) modulator of ras/raf/mek pathway |
| WO2010038165A1 (en) * | 2008-09-30 | 2010-04-08 | Pfizer Inc. | Imidazo[1,5]naphthyridine compounds, their pharmaceutical use and compositions |
| US8791131B2 (en) | 2008-09-30 | 2014-07-29 | Pfizer Inc. | Imidazo[1,5]naphthyridine compounds, their pharmaceutical use and compositions |
| JP2012504573A (en) * | 2008-10-01 | 2012-02-23 | ノバルティス アーゲー | Smoothened antagonism for the treatment of hedgehog pathway related disorders |
| US8778927B2 (en) | 2008-10-01 | 2014-07-15 | Novartis Ag | Smoothened antagonism for the treatment of hedgehog pathway-related disorders |
| TWI478916B (en) * | 2008-10-01 | 2015-04-01 | Novartis Ag | Smoothened antagonism for the treatment of hedgehog pathway-related disorders |
| JP2015180654A (en) * | 2008-10-01 | 2015-10-15 | ノバルティス アーゲー | Smoothened antagonism for treatment of hedgehog pathway-related disorders |
| EP3023097A1 (en) | 2008-10-01 | 2016-05-25 | Novartis AG | Smoothened antagonism for the treatment of hedgehog pathway-related disorders |
| US8309546B2 (en) | 2008-10-14 | 2012-11-13 | Daiichi Sankyo Company, Limited | Morpholinopurine derivatives |
| WO2010044401A1 (en) | 2008-10-14 | 2010-04-22 | 第一三共株式会社 | Morpholinopurine derivative |
| US8097622B2 (en) | 2008-10-14 | 2012-01-17 | Daiichi Sankyo Company, Limited | Morpholinopurine derivatives |
| US8394818B2 (en) | 2008-10-17 | 2013-03-12 | Dana-Farber Cancer Institute, Inc. | Soluble mTOR complexes and modulators thereof |
| US8889706B2 (en) | 2008-10-17 | 2014-11-18 | Whitehead Institute For Biomedical Research | Soluble mTOR complexes and modulators thereof |
| WO2010110686A1 (en) | 2009-03-27 | 2010-09-30 | Pathway Therapeutics Limited | Pyrimidinyl and 1,3,5 triazinyl benzimidazoles and their use in cancer therapy |
| US8461158B2 (en) | 2009-03-27 | 2013-06-11 | Pathway Therapeutics Inc. | Pyrimidinyl and 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| US8772287B2 (en) | 2009-03-27 | 2014-07-08 | Vetdc, Inc. | Pyrimidinyl and 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| WO2010110685A2 (en) | 2009-03-27 | 2010-09-30 | Pathway Therapeutics Limited | Pyrimddinyl and 1,3,5-triazinyl benzimtoazole sulfonamides and their use in cancer therapy |
| US9108980B2 (en) | 2009-03-27 | 2015-08-18 | Vetdc, Inc. | Pyrimidinyl and 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| AU2010247397B2 (en) * | 2009-05-15 | 2012-07-12 | Novartis Ag | Combination of a phosphoinositide 3-kinase inhibitor and an antidiabetic compound |
| CN102958518A (en) * | 2009-05-15 | 2013-03-06 | 诺瓦提斯公司 | Combination of a phosphoinositide 3-kinase inhibitor and an antidiabetic compound for use in the treatment of proliferative diseases |
| WO2010130779A3 (en) * | 2009-05-15 | 2013-03-28 | Novartis Ag | Combination of (a) a phosphoinositide 3-kinase inhibitor and (b) an antidiabetic compound for use in the treatment of proliferative diseases |
| US9216984B2 (en) | 2009-05-22 | 2015-12-22 | Incyte Corporation | 3-[4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl]octane—or heptane-nitrile as JAK inhibitors |
| US9623029B2 (en) | 2009-05-22 | 2017-04-18 | Incyte Holdings Corporation | 3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]octane- or heptane-nitrile as JAK inhibitors |
| US9334274B2 (en) | 2009-05-22 | 2016-05-10 | Incyte Holdings Corporation | N-(hetero)aryl-pyrrolidine derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines and pyrrol-3-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| TWI464168B (en) * | 2009-06-04 | 2014-12-11 | Novartis Ag | 1h-imidazo[4,5-c]quinolinone derivatives |
| AU2010255727B2 (en) * | 2009-06-04 | 2013-02-28 | Novartis Ag | 1H-imidazo[4,5-c]quinolinone derivatives |
| US8476294B2 (en) | 2009-06-04 | 2013-07-02 | Novartis Ag | 1H-imidazo[4,5-c]quinolinone derivatives |
| EA020715B1 (en) * | 2009-06-04 | 2015-01-30 | Новартис Аг | 1H-IMIDAZO[4,5-c]QUINOLINONE DERIVATIVES |
| CN102574845B (en) * | 2009-06-04 | 2015-09-02 | 诺华股份有限公司 | 1H-imidazo [4,5-c] qualone derivative |
| WO2010139731A1 (en) * | 2009-06-04 | 2010-12-09 | Novartis Ag | 1H-IMIDAZO[4,5-c]QUINOLINONE DERIVATIVES |
| CN102574845A (en) * | 2009-06-04 | 2012-07-11 | 诺瓦提斯公司 | 1h-imidazo[4,5-c]quinolinone derivatives |
| WO2010139747A1 (en) * | 2009-06-04 | 2010-12-09 | Novartis Ag | 1H-IMIDAZO[4, 5-c]QUINOLINONE COMPOUNDS, USEFUL FOR THE TREATMENT OF PROLIFERATIVE DISEASES |
| US20120108627A1 (en) * | 2009-06-30 | 2012-05-03 | Sanjay Kumar | Imidazo [4,5-c]quinoline derivatives and their use in the treatment of tumors and/or inflammation |
| WO2011001212A1 (en) * | 2009-06-30 | 2011-01-06 | Piramal Life Sciences Limited | Imidazo [4,5-c]quinoline derivatives and their use in the treatment of tumors and/or inflammation |
| US8637670B2 (en) | 2009-06-30 | 2014-01-28 | Piramal Enterprises Limited | Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation |
| US8486939B2 (en) | 2009-07-07 | 2013-07-16 | Pathway Therapeutics Inc. | Pyrimidinyl and 1,3,5-triazinyl benzimidazoles and their use in cancer therapy |
| WO2011005119A1 (en) | 2009-07-07 | 2011-01-13 | Pathway Therapeutics Limited | Pyrimidinyl and 1,3,5-triazinyl benzimidazoles and their use in cancer therapy |
| WO2011007819A1 (en) | 2009-07-17 | 2011-01-20 | 塩野義製薬株式会社 | Pharmaceutical product containing lactam or benzene sulfonamide compound |
| US9249145B2 (en) | 2009-09-01 | 2016-02-02 | Incyte Holdings Corporation | Heterocyclic derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| WO2011104266A1 (en) | 2010-02-25 | 2011-09-01 | Novartis Ag | Dimeric iap inhibitors |
| US9464088B2 (en) | 2010-03-10 | 2016-10-11 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US9999619B2 (en) | 2010-03-10 | 2018-06-19 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US10695337B2 (en) | 2010-03-10 | 2020-06-30 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US11285140B2 (en) | 2010-03-10 | 2022-03-29 | Incyte Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| WO2011120911A1 (en) | 2010-03-30 | 2011-10-06 | Novartis Ag | Pkc inhibitors for the treatment of b-cell lymphoma having chronic active b-cell-receptor signalling |
| US10758543B2 (en) | 2010-05-21 | 2020-09-01 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US11590136B2 (en) | 2010-05-21 | 2023-02-28 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US11571425B2 (en) | 2010-05-21 | 2023-02-07 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US10869870B2 (en) | 2010-05-21 | 2020-12-22 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US11219624B2 (en) | 2010-05-21 | 2022-01-11 | Incyte Holdings Corporation | Topical formulation for a JAK inhibitor |
| WO2012000632A1 (en) * | 2010-07-01 | 2012-01-05 | Merck Patent Gmbh | Pyrazolo-quinolines |
| EA022430B1 (en) * | 2010-07-01 | 2015-12-30 | Мерк Патент Гмбх | Pyrazolo-quinolines as inhibitors of serine/threonine protein kinases |
| US8802712B2 (en) | 2010-07-01 | 2014-08-12 | Merck Patent Gmbh | Pyrazoloquinolines |
| AU2011273968B2 (en) * | 2010-07-01 | 2014-11-20 | Merck Patent Gmbh | Pyrazolo-quinolines |
| KR101921764B1 (en) | 2010-07-01 | 2018-11-23 | 메르크 파텐트 게엠베하 | Pyrazolo-quinolines |
| CN103080092A (en) * | 2010-07-01 | 2013-05-01 | 默克专利股份公司 | Pyrazolo-quinolines |
| WO2012004299A1 (en) | 2010-07-06 | 2012-01-12 | Novartis Ag | Tetrahydro-pyrido-pyrimidine derivatives |
| US9062046B2 (en) | 2010-07-16 | 2015-06-23 | Piramal Enterprises Limited | Substituted imidazoquinoline derivatives as kinase inhibitors |
| WO2012007926A1 (en) | 2010-07-16 | 2012-01-19 | Piramal Life Sciences Limited | Substituted imidazoquinoline derivatives as kinase inhibitors |
| CN102372712A (en) * | 2010-08-18 | 2012-03-14 | 山东轩竹医药科技有限公司 | Imidazodiazanaphthalene PI3K (phosphatidyl inositol 3 kinase) and mTOR (mammalian target of rapamycin) dual inhibitor |
| CN102372712B (en) * | 2010-08-18 | 2013-10-23 | 山东轩竹医药科技有限公司 | Imidazodiazanaphthalene PI3K (phosphatidyl inositol 3 kinase) and mTOR (mammalian target of rapamycin) dual inhibitor |
| WO2012022814A1 (en) | 2010-08-20 | 2012-02-23 | Novartis Ag | Antibodies for epidermal growth factor receptor 3 (her3) |
| US9598408B2 (en) | 2010-08-28 | 2017-03-21 | Merck Patent Gesellschaft Mit Beschrankter Haftung | Imidazo[4,5-C]quinolines as DNA-PK inhibitors |
| KR101912475B1 (en) | 2010-08-28 | 2018-10-26 | 메르크 파텐트 게엠베하 | Imidazo[4,5-c]quinolines as dna-pk inhibitors |
| KR20130106367A (en) * | 2010-08-28 | 2013-09-27 | 메르크 파텐트 게엠베하 | Imidazo[4,5-c]quinolines as dna-pk inhibitors |
| US9000153B2 (en) | 2010-08-28 | 2015-04-07 | Merck Patent Gmbh | Imidazo[4,5-c]quinolines as DNA-PK inhibitors |
| WO2012028233A1 (en) * | 2010-08-28 | 2012-03-08 | Merck Patent Gmbh | Imidazo[4,5-c]quinolines as dna-pk inhibitors |
| EA022095B1 (en) * | 2010-08-28 | 2015-10-30 | Мерк Патент Гмбх | IMIDAZO[4,5-c]QUINOLINES AS DNA-PK INHIBITORS |
| WO2012044641A1 (en) | 2010-09-29 | 2012-04-05 | Pathway Therapeutics Inc. | 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| WO2012047775A1 (en) * | 2010-10-04 | 2012-04-12 | Novartis Ag | Pharmaceutical combinations |
| WO2012068483A1 (en) | 2010-11-18 | 2012-05-24 | Synta Pharmaceuticals Corp. | Preselection of subjects for therapeutic treatment based on hypoxic status |
| WO2012068487A1 (en) | 2010-11-18 | 2012-05-24 | Synta Pharmaceuticals Corp. | Preselection of subjects for therapeutic treatment with oxygen sensitive agents based on hypoxic status |
| US9034884B2 (en) | 2010-11-19 | 2015-05-19 | Incyte Corporation | Heterocyclic-substituted pyrrolopyridines and pyrrolopyrimidines as JAK inhibitors |
| US10640506B2 (en) | 2010-11-19 | 2020-05-05 | Incyte Holdings Corporation | Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidines derivatives as JAK inhibitors |
| US8933085B2 (en) | 2010-11-19 | 2015-01-13 | Incyte Corporation | Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| WO2012075253A3 (en) * | 2010-12-03 | 2012-08-09 | Novartis Ag | Pharmaceutical compositions |
| WO2012075253A2 (en) | 2010-12-03 | 2012-06-07 | Novartis Ag | Pharmaceutical compositions |
| WO2012077031A1 (en) | 2010-12-06 | 2012-06-14 | Piramal Life Sciences Limited | Substituted imidazoquinoline derivatives |
| WO2012080260A1 (en) | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| WO2012080271A1 (en) | 2010-12-13 | 2012-06-21 | Novartis Ag | Dimeric iap inhibitors |
| JP2014502638A (en) * | 2011-01-14 | 2014-02-03 | イーライ リリー アンド カンパニー | Imidazo [4,5-c] quinolin-2-one compounds and their use as PI3 kinase / mTOR dual inhibitors |
| US8658668B2 (en) | 2011-01-14 | 2014-02-25 | Eli Lilly And Company | PI3 kinase/mTOR dual inhibitor |
| US8440829B2 (en) | 2011-01-14 | 2013-05-14 | Eli Lilly And Company | PI3 kinase/mTOR dual inhibitor |
| CN103391780A (en) * | 2011-02-16 | 2013-11-13 | 诺瓦提斯公司 | Combinations of therapeutic agents for use in the treatment of neurodegenerative diseases |
| WO2012110953A1 (en) | 2011-02-16 | 2012-08-23 | Novartis Ag | Combinations of therapeutic agents for use in the treatment of neurodegenerative diseases |
| US9358236B2 (en) | 2011-02-16 | 2016-06-07 | Novartis Ag | Combinations of therapeutic agents for use in the treatment of neurodegenerative diseases |
| US9993480B2 (en) | 2011-02-18 | 2018-06-12 | Novartis Pharma Ag | mTOR/JAK inhibitor combination therapy |
| US20120238562A1 (en) * | 2011-03-09 | 2012-09-20 | Cedars-Sinai Medical Center | Treatment of cancer by targeting molecules that influence mst1/stk4 signaling |
| US9056852B2 (en) | 2011-03-28 | 2015-06-16 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| US10603324B2 (en) | 2011-03-28 | 2020-03-31 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| US12059422B2 (en) | 2011-03-28 | 2024-08-13 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| US10335415B2 (en) | 2011-03-28 | 2019-07-02 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| US11400097B2 (en) | 2011-03-28 | 2022-08-02 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| US10064868B2 (en) | 2011-03-28 | 2018-09-04 | Mei Pharma, Inc. | (Alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| JP2014518887A (en) * | 2011-06-04 | 2014-08-07 | シュアンジュ・ファーマ・カンパニー・リミテッド | Pyridonaphthyridine-type PI3K and mTOR dual inhibitors and their preparation and use |
| US9475812B2 (en) | 2011-06-04 | 2016-10-25 | Xuanzhu Pharma Co., Ltd. | Pyridonaphthyridine type dual PI3K and mTOR inhibitor and its preparation and use |
| US11214573B2 (en) | 2011-06-20 | 2022-01-04 | Incyte Holdings Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US9611269B2 (en) | 2011-06-20 | 2017-04-04 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US9023840B2 (en) | 2011-06-20 | 2015-05-05 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US8691807B2 (en) | 2011-06-20 | 2014-04-08 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US10513522B2 (en) | 2011-06-20 | 2019-12-24 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| WO2013006532A1 (en) | 2011-07-01 | 2013-01-10 | Novartis Ag | Combination therapy comprising a cdk4/6 inhibitor and a pi3k inhibitor for use in the treatment of cancer |
| WO2013012723A1 (en) | 2011-07-13 | 2013-01-24 | Novartis Ag | Novel 2-piperidin-1-yl-acetamide compounds for use as tankyrase inhibitors |
| WO2013008217A1 (en) | 2011-07-13 | 2013-01-17 | Novartis Ag | 4 - piperidinyl compounds for use as tankyrase inhibitors |
| WO2013010092A1 (en) | 2011-07-13 | 2013-01-17 | Novartis Ag | 4-oxo-3,5,7,8-tetrahydro-4h-pyrano {4,3-d} pyrminidinyl compounds for use as tankyrase inhibitors |
| US9358229B2 (en) | 2011-08-10 | 2016-06-07 | Novartis Pharma Ag | JAK PI3K/mTOR combination therapy |
| WO2013023119A1 (en) | 2011-08-10 | 2013-02-14 | Novartis Pharma Ag | JAK P13K/mTOR COMBINATION THERAPY |
| US9359358B2 (en) | 2011-08-18 | 2016-06-07 | Incyte Holdings Corporation | Cyclohexyl azetidine derivatives as JAK inhibitors |
| US9675595B2 (en) | 2011-08-31 | 2017-06-13 | Novartis Ag | Synergistic combinations of PI3K- and MEK-inhibitors |
| US9718834B2 (en) | 2011-09-07 | 2017-08-01 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9487521B2 (en) | 2011-09-07 | 2016-11-08 | Incyte Holdings Corporation | Processes and intermediates for making a JAK inhibitor |
| WO2013049300A1 (en) | 2011-09-30 | 2013-04-04 | Dana-Farber Cancer Institute, Inc. | Method of treating mucoepidermoid carcinoma |
| CN103282363A (en) * | 2011-10-10 | 2013-09-04 | 上海恒瑞医药有限公司 | Imidazo quinoline derivative and medicinal salt thereof, preparation method thereof and use in medicine thereof |
| WO2013053273A1 (en) * | 2011-10-10 | 2013-04-18 | 上海恒瑞医药有限公司 | Imidazo quinoline derivative and medicinal salt thereof, preparation method thereof and use in medicine thereof |
| TWI601724B (en) * | 2011-10-10 | 2017-10-11 | 上海恆瑞醫藥有限公司 | Imidazo quinoline derivatives and salts thereof, preparation process and pharmaceutical use thereof |
| WO2013057711A1 (en) | 2011-10-21 | 2013-04-25 | Novartis Ag | Quinazoline derivatives as pi3k modulators |
| JP2014532647A (en) * | 2011-10-28 | 2014-12-08 | ノバルティス アーゲー | How to treat gastrointestinal stromal tumors |
| WO2013063000A1 (en) | 2011-10-28 | 2013-05-02 | Novartis Ag | Method of treating gastrointestinal stromal tumors |
| AU2012328979B2 (en) * | 2011-10-28 | 2016-04-21 | Novartis Ag | Method of treating gastrointestinal stromal tumors |
| WO2013072392A1 (en) | 2011-11-15 | 2013-05-23 | Novartis Forschungsstiftung, Zweigniederlassung Friedrich Miescher Institute For Biomedical Research | Combination of a phosphoinositide 3-kinase inhibitor and a modulator of the janus kinase 2-signal transducer and activator of transcription 5 pathway |
| EP2781520A4 (en) * | 2011-11-17 | 2015-07-08 | Xuanzhu Pharma Co Ltd | Three-ring pi3k and/or mtor inhibitor |
| US9284315B2 (en) | 2011-11-17 | 2016-03-15 | Zuanzhu Pharma Co., Ltd. | Three-ring PI3K and/or mTOR inhibitor |
| WO2013071698A1 (en) | 2011-11-17 | 2013-05-23 | 山东轩竹医药科技有限公司 | Three-ring pi3k and/or mtor inhibitor |
| EP3590538A1 (en) | 2011-12-05 | 2020-01-08 | Novartis AG | Antibodies for epidermal growth factor receptor 3 (her3) |
| WO2013084148A2 (en) | 2011-12-05 | 2013-06-13 | Novartis Ag | Antibodies for epidermal growth factor receptor 3 (her3) directed to domain ii of her3 |
| WO2013084147A2 (en) | 2011-12-05 | 2013-06-13 | Novartis Ag | Antibodies for epidermal growth factor receptor 3 (her3) |
| WO2013084138A1 (en) | 2011-12-05 | 2013-06-13 | Novartis Ag | Cyclic urea derivatives as androgen receptor antagonists |
| WO2013088404A1 (en) | 2011-12-15 | 2013-06-20 | Novartis Ag | Use of inhibitors of the activity or function of PI3K |
| WO2013093850A1 (en) | 2011-12-22 | 2013-06-27 | Novartis Ag | Quinoline derivatives |
| WO2013093849A1 (en) | 2011-12-22 | 2013-06-27 | Novartis Ag | Dihydro-benzo-oxazine and dihydro-pyrido-oxazine derivatives |
| US10023862B2 (en) | 2012-01-09 | 2018-07-17 | Arrowhead Pharmaceuticals, Inc. | Organic compositions to treat beta-catenin-related diseases |
| WO2013105022A2 (en) | 2012-01-09 | 2013-07-18 | Novartis Ag | Organic compositions to treat beta-catenin-related diseases |
| US9879003B2 (en) | 2012-04-11 | 2018-01-30 | Dana-Farber Cancer Institute, Inc. | Host targeted inhibitors of dengue virus and other viruses |
| US9193733B2 (en) | 2012-05-18 | 2015-11-24 | Incyte Holdings Corporation | Piperidinylcyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| WO2013184621A1 (en) | 2012-06-06 | 2013-12-12 | Novartis Ag | Combination of a 17 -alpha -hydroxylase (c17, 20 - lyase) inhibitor and a specific pi-3k inhibitor for treating a tumor disease |
| WO2013192367A1 (en) | 2012-06-22 | 2013-12-27 | Novartis Ag | Neuroendocrine tumor treatment |
| WO2014006115A1 (en) | 2012-07-06 | 2014-01-09 | Novartis Ag | Combination of a phosphoinositide 3-kinase inhibitor and an inhibitor of the il-8/cxcr interaction |
| US10000483B2 (en) | 2012-10-19 | 2018-06-19 | Dana-Farber Cancer Institute, Inc. | Bone marrow on X chromosome kinase (BMX) inhibitors and uses thereof |
| US11896717B2 (en) | 2012-11-15 | 2024-02-13 | Incyte Holdings Corporation | Sustained-release dosage forms of ruxolitinib |
| US10166191B2 (en) | 2012-11-15 | 2019-01-01 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US11576865B2 (en) | 2012-11-15 | 2023-02-14 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US11576864B2 (en) | 2012-11-15 | 2023-02-14 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US11337927B2 (en) | 2012-11-15 | 2022-05-24 | Incyte Holdings Corporation | Sustained-release dosage forms of ruxolitinib |
| US10874616B2 (en) | 2012-11-15 | 2020-12-29 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| WO2014102630A1 (en) | 2012-11-26 | 2014-07-03 | Novartis Ag | Solid form of dihydro-pyrido-oxazine derivative |
| EP2742940A1 (en) | 2012-12-13 | 2014-06-18 | IP Gesellschaft für Management mbH | Salts of aza-bicyclo-di-aryl ethers for adminstration once daily, twice daily or thrice daily |
| EP3251673A1 (en) | 2012-12-13 | 2017-12-06 | IP Gesellschaft für Management mbH | Combination therapy comprising a cdk4/6 inhibitor and a pi3k inhibitor for use in the treatment of cancer |
| US11034666B2 (en) | 2013-01-29 | 2021-06-15 | Avexxin As | Anti-inflammatory and antitumor 2-oxothiazoles and 2-oxothiophenes compounds |
| US10259801B2 (en) | 2013-01-29 | 2019-04-16 | Avexxin As | Anti-inflammatory and antitumor 2-oxothiazoles ABD 2-oxothiophenes compounds |
| US11691959B2 (en) | 2013-01-29 | 2023-07-04 | Avexxin As | Anti-inflammatory and antitumor 2-oxothiazoles and 2-oxothiophenes compounds |
| EP3360870A1 (en) | 2013-02-19 | 2018-08-15 | Novartis AG | Benzothiophene derivatives and compositions thereof as selective estrogen receptor degraders |
| EP3626741A1 (en) | 2013-02-20 | 2020-03-25 | The Trustees Of The University Of Pennsylvania | Treatment of cancer using humanized anti-egfrviii chimeric antigen receptor |
| WO2014130657A1 (en) | 2013-02-20 | 2014-08-28 | The Trustees Of The University Of Pennsylvania | Treatment of cancer using humanized anti-egfrviii chimeric antigen receptor |
| WO2014128612A1 (en) | 2013-02-20 | 2014-08-28 | Novartis Ag | Quinazolin-4-one derivatives |
| US8987443B2 (en) | 2013-03-06 | 2015-03-24 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9221845B2 (en) | 2013-03-06 | 2015-12-29 | Incyte Holdings Corporation | Processes and intermediates for making a JAK inhibitor |
| US9714233B2 (en) | 2013-03-06 | 2017-07-25 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| WO2014160160A2 (en) | 2013-03-13 | 2014-10-02 | Novartis Ag | Antibody drug conjugates |
| WO2014141118A1 (en) | 2013-03-14 | 2014-09-18 | Piramal Enterprises Limited | Imidazo[4,5-c]quinoline derivatives and uses thereof |
| EP3514178A1 (en) | 2013-03-15 | 2019-07-24 | Novartis AG | Antibody drug conjugates |
| WO2014147573A2 (en) | 2013-03-21 | 2014-09-25 | Novartis Ag | Combination therapy |
| WO2014177915A1 (en) | 2013-05-01 | 2014-11-06 | Piramal Enterprises Limited | Cancer combination therapy using imidazo[4,5-c]quinoline derivatives |
| WO2014203152A1 (en) | 2013-06-18 | 2014-12-24 | Novartis Ag | Pharmaceutical combinations |
| CN105338980A (en) * | 2013-06-18 | 2016-02-17 | 诺华股份有限公司 | Pharmaceutical combinations |
| WO2015010641A1 (en) | 2013-07-24 | 2015-01-29 | Novartis Ag | Substituted quinazolin-4-one derivatives |
| US9655854B2 (en) | 2013-08-07 | 2017-05-23 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US10561616B2 (en) | 2013-08-07 | 2020-02-18 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US11045421B2 (en) | 2013-08-07 | 2021-06-29 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US9629836B2 (en) | 2013-08-14 | 2017-04-25 | Novartis Ag | Compounds and compositions as inhibitors of MEK |
| US10011599B2 (en) | 2013-08-14 | 2018-07-03 | Novartis Ag | Compounds and compositions as inhibitors of MEK |
| US9227969B2 (en) | 2013-08-14 | 2016-01-05 | Novartis Ag | Compounds and compositions as inhibitors of MEK |
| WO2015027667A1 (en) | 2013-08-30 | 2015-03-05 | 浙江医药股份有限公司新昌制药厂 | 2, 6-di-nitrogen-containing substituted purine derivative, and preparation method, pharmaceutical composition and use thereof |
| WO2015073644A1 (en) | 2013-11-13 | 2015-05-21 | Novartis Ag | Mtor inhibitors for enhancing the immune response |
| WO2015092634A1 (en) | 2013-12-16 | 2015-06-25 | Novartis Ag | 1,2,3,4-tetrahydroisoquinoline compounds and compositions as selective estrogen receptor antagonists and degraders |
| WO2015090230A1 (en) | 2013-12-19 | 2015-06-25 | Novartis Ag | Human mesothelin chimeric antigen receptors and uses thereof |
| EP4026909A1 (en) | 2013-12-19 | 2022-07-13 | Novartis AG | Human mesothelin chimeric antigen receptors and uses thereof |
| WO2015090229A1 (en) | 2013-12-20 | 2015-06-25 | Novartis Ag | Regulatable chimeric antigen receptor |
| EP4420663A2 (en) | 2013-12-20 | 2024-08-28 | Novartis AG | Regulatable chimeric antigen receptor |
| WO2015107494A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | 1 -(triazin-3-yi_/pyridazin-3-yl)-piper(-azine)idine derivatives and compositions thereof for inhibiting the activity of shp2 |
| WO2015107493A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | 1 -pyridazin-/triazin-3-yl-piper(-azine)/idine/pyrolidine derivatives and and compositions thereof for inhibiting the activity of shp2 |
| WO2015107495A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | N-azaspirocycloalkane substituted n-heteroaryl compounds and compositions for inhibiting the activity of shp2 |
| EP3514179A1 (en) | 2014-01-24 | 2019-07-24 | Dana-Farber Cancer Institute, Inc. | Antibody molecules to pd-1 and uses thereof |
| EP4324518A2 (en) | 2014-01-31 | 2024-02-21 | Novartis AG | Antibody molecules to tim-3 and uses thereof |
| EP4019518A1 (en) | 2014-02-28 | 2022-06-29 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| WO2015131080A1 (en) | 2014-02-28 | 2015-09-03 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| EP3660050A1 (en) | 2014-03-14 | 2020-06-03 | Novartis AG | Antibody molecules to lag-3 and uses thereof |
| WO2015138920A1 (en) | 2014-03-14 | 2015-09-17 | Novartis Ag | Antibody molecules to lag-3 and uses thereof |
| WO2015142675A2 (en) | 2014-03-15 | 2015-09-24 | Novartis Ag | Treatment of cancer using chimeric antigen receptor |
| WO2015157252A1 (en) | 2014-04-07 | 2015-10-15 | BROGDON, Jennifer | Treatment of cancer using anti-cd19 chimeric antigen receptor |
| EP3888674A1 (en) | 2014-04-07 | 2021-10-06 | Novartis AG | Treatment of cancer using anti-cd19 chimeric antigen receptor |
| EP4406610A2 (en) | 2014-04-07 | 2024-07-31 | Novartis AG | Treatment of cancer using anti-cd19 chimeric antigen receptor |
| WO2015162584A1 (en) | 2014-04-24 | 2015-10-29 | Novartis Ag | Crystalline forms of the sulfate salt of n-[5-(3-imidazol-1-yl-4-methanesulfonyl-phenyl)-4-methyl-thiazol-2-yl]-acetamide |
| US10189834B2 (en) | 2014-05-08 | 2019-01-29 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| US9822111B2 (en) | 2014-05-08 | 2017-11-21 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| US9428503B2 (en) | 2014-05-08 | 2016-08-30 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating Cancer |
| US9498467B2 (en) | 2014-05-30 | 2016-11-22 | Incyte Corporation | Treatment of chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML) by inhibitors of JAK1 |
| US10183017B2 (en) | 2014-06-06 | 2019-01-22 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Methods and uses of quinoline derivatives in the treatment of soft tissue sarcomas and pharmaceutical compositions for treatment of same |
| US10251876B2 (en) | 2014-06-06 | 2019-04-09 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Method for treating tumour, pharmaceutical composition and medicinal kit |
| US9968597B2 (en) | 2014-06-06 | 2018-05-15 | Advenchen Laboratories Nanjing Ltd | Methods and uses of quinoline derivatives in the treatment of soft tissue sarcomas and pharmaceutical compositions for treatment of same |
| WO2016010869A3 (en) * | 2014-07-14 | 2016-04-07 | Advenchen Pharmaceuticals, LLC | FUSED QUINOLINE COMPUNDS AS PI3K, mTOR INHIBITORS |
| KR20170035944A (en) * | 2014-07-14 | 2017-03-31 | 어드벤첸 라보라토리스 난징 리미티드 | FUSED QUINOLINE COMPOUNDS AS PI3K, mTOR INHIBITORS |
| KR102426138B1 (en) | 2014-07-14 | 2022-07-28 | 어드벤첸 라보라토리스 난징 리미티드 | FUSED QUINOLINE COMPOUNDS AS PI3K, mTOR INHIBITORS |
| US10112945B2 (en) | 2014-07-14 | 2018-10-30 | Advenchen Pharmaceuticals, Nanjing Ltd. | Fused quinoline compunds as PI3K, mTOR inhibitors |
| EP3539990A1 (en) | 2014-07-16 | 2019-09-18 | Dana-Farber Cancer Institute, Inc. | Her3 inhibition in low-grade serous cancers |
| WO2016014530A1 (en) | 2014-07-21 | 2016-01-28 | Novartis Ag | Combinations of low, immune enhancing. doses of mtor inhibitors and cars |
| EP3722316A1 (en) | 2014-07-21 | 2020-10-14 | Novartis AG | Treatment of cancer using a cd33 chimeric antigen receptor |
| WO2016014553A1 (en) | 2014-07-21 | 2016-01-28 | Novartis Ag | Sortase synthesized chimeric antigen receptors |
| EP3660042A1 (en) | 2014-07-31 | 2020-06-03 | Novartis AG | Subset-optimized chimeric antigen receptor-containing t-cells |
| EP4205749A1 (en) | 2014-07-31 | 2023-07-05 | Novartis AG | Subset-optimized chimeric antigen receptor-containing cells |
| US10851114B2 (en) | 2014-08-01 | 2020-12-01 | Avexxin As | 2-oxothiatole compounds having activity as cPLA2 inhibitors for the treatment of inflammatory disorders and hyperproliferative disorders |
| WO2016020791A1 (en) | 2014-08-05 | 2016-02-11 | Novartis Ag | Ckit antibody drug conjugates |
| WO2016024195A1 (en) | 2014-08-12 | 2016-02-18 | Novartis Ag | Anti-cdh6 antibody drug conjugates |
| US11311512B2 (en) | 2014-08-12 | 2022-04-26 | Monash University | Lymph directing prodrugs |
| WO2016025880A1 (en) | 2014-08-14 | 2016-02-18 | Novartis Ag | Treatment of cancer using gfr alpha-4 chimeric antigen receptor |
| EP3712171A1 (en) | 2014-08-19 | 2020-09-23 | Novartis AG | Treatment of cancer using a cd123 chimeric antigen receptor |
| WO2016040880A1 (en) | 2014-09-13 | 2016-03-17 | Novartis Ag | Combination therapies of alk inhibitors |
| EP3659621A1 (en) | 2014-09-13 | 2020-06-03 | Novartis AG | Combination therapies for cancer |
| WO2016040892A1 (en) | 2014-09-13 | 2016-03-17 | Novartis Ag | Combination therapies |
| EP3925622A1 (en) | 2014-09-13 | 2021-12-22 | Novartis AG | Combination therapies |
| EP3967709A1 (en) | 2014-09-17 | 2022-03-16 | Novartis AG | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
| WO2016044605A1 (en) | 2014-09-17 | 2016-03-24 | Beatty, Gregory | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
| WO2016054555A2 (en) | 2014-10-03 | 2016-04-07 | Novartis Ag | Combination therapies |
| EP3662903A2 (en) | 2014-10-03 | 2020-06-10 | Novartis AG | Combination therapies |
| WO2016057841A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Compositions and methods of use for augmented immune response and cancer therapy |
| WO2016057705A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Biomarkers predictive of therapeutic responsiveness to chimeric antigen receptor therapy and uses thereof |
| WO2016061142A1 (en) | 2014-10-14 | 2016-04-21 | Novartis Ag | Antibody molecules to pd-l1 and uses thereof |
| EP4245376A2 (en) | 2014-10-14 | 2023-09-20 | Novartis AG | Antibody molecules to pd-l1 and uses thereof |
| WO2016075670A1 (en) | 2014-11-14 | 2016-05-19 | Novartis Ag | Antibody drug conjugates |
| WO2016079760A1 (en) | 2014-11-20 | 2016-05-26 | Council Of Scientific & Industrial Research | Novel 1,3,5 -triazine based pi3k inhibitors as anticancer agents and a process for the preparation thereof |
| US10307412B2 (en) | 2014-12-09 | 2019-06-04 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Quinoline derivative against non-small cell lung cancer |
| US10888559B2 (en) | 2014-12-09 | 2021-01-12 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Quinoline derivatives for non-small cell lung cancer |
| WO2016092508A1 (en) | 2014-12-12 | 2016-06-16 | Massachusetts General Hospital | Treatment of breast cancer brain metastases |
| WO2016100882A1 (en) | 2014-12-19 | 2016-06-23 | Novartis Ag | Combination therapies |
| WO2016103155A1 (en) | 2014-12-23 | 2016-06-30 | Novartis Ag | Triazolopyrimidine compounds and uses thereof |
| WO2016138352A1 (en) | 2015-02-27 | 2016-09-01 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US10968236B2 (en) | 2015-02-27 | 2021-04-06 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US10253046B2 (en) | 2015-02-27 | 2019-04-09 | Nimbus Lakshmi | TYK2 inhibitors and uses thereof |
| WO2016145102A1 (en) | 2015-03-10 | 2016-09-15 | Aduro Biotech, Inc. | Compositions and methods for activating "stimulator of interferon gene" -dependent signalling |
| US10449211B2 (en) | 2015-03-10 | 2019-10-22 | Aduro Biotech, Inc. | Compositions and methods for activating “stimulator of interferon gene”—dependent signalling |
| US11040053B2 (en) | 2015-03-10 | 2021-06-22 | Chinook Therapeutics, Inc. | Compositions and methods for activating “stimulator of interferon gene”13 dependent signalling |
| WO2016142508A1 (en) | 2015-03-11 | 2016-09-15 | Centre Léon-Bérard | Composition for treating pancreatic neuroendocrine tumours |
| WO2016151499A1 (en) | 2015-03-25 | 2016-09-29 | Novartis Ag | Formylated n-heterocyclic derivatives as fgfr4 inhibitors |
| US10745399B2 (en) | 2015-04-02 | 2020-08-18 | Merck Patent Gmbh | Imidazolonylquinolines and the use thereof as ATM kinase inhibitors |
| US10975075B2 (en) | 2015-04-02 | 2021-04-13 | Merck Patent Gmbh | Imidazolonylquinolines and the use thereof as ATM kinase inhibitors |
| US10457677B2 (en) | 2015-04-02 | 2019-10-29 | Merck Patent Gmbh | Imidazolonylquinolines and the use thereof as ATM kinase inhibitors |
| US11608338B2 (en) | 2015-04-02 | 2023-03-21 | Merck Patent Gmbh | Imidazolonylquinolines and the use thereof as ATM kinase inhibitors |
| WO2016164580A1 (en) | 2015-04-07 | 2016-10-13 | Novartis Ag | Combination of chimeric antigen receptor therapy and amino pyrimidine derivatives |
| WO2016168595A1 (en) | 2015-04-17 | 2016-10-20 | Barrett David Maxwell | Methods for improving the efficacy and expansion of chimeric antigen receptor-expressing cells |
| EP4234685A2 (en) | 2015-04-17 | 2023-08-30 | Novartis AG | Methods for improving the efficacy and expansion of chimeric antigen receptor-expressing cells |
| WO2016172583A1 (en) | 2015-04-23 | 2016-10-27 | Novartis Ag | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| US10544125B2 (en) | 2015-05-04 | 2020-01-28 | Advenchen Pharmaceuticals, LLC | Process for preparing an anti-cancer agent, 1-((4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropanamine, its crystalline form and its salts |
| US10100034B2 (en) | 2015-05-04 | 2018-10-16 | Advenchen Pharmaceuticals, LLC | Process for preparing an anti-cancer agent, 1-((4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropanamine, its crystalline form and its salts |
| US10576076B2 (en) | 2015-05-20 | 2020-03-03 | Novartis Ag | Pharmaceutical combination of everolimus with dactolisib |
| WO2016185443A1 (en) | 2015-05-20 | 2016-11-24 | Novartis Ag | Pharmaceutical combination of everolimus with dactolisib |
| WO2016203432A1 (en) | 2015-06-17 | 2016-12-22 | Novartis Ag | Antibody drug conjugates |
| WO2016203406A1 (en) | 2015-06-19 | 2016-12-22 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2016203405A1 (en) | 2015-06-19 | 2016-12-22 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2016203404A1 (en) | 2015-06-19 | 2016-12-22 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| WO2017004134A1 (en) | 2015-06-29 | 2017-01-05 | Nimbus Iris, Inc. | Irak inhibitors and uses thereof |
| US10730878B2 (en) | 2015-07-11 | 2020-08-04 | Advenchen Pharmaceuticals, LLC | Fused quinoline compounds as PI3K/mTOR inhibitors |
| WO2017011363A1 (en) | 2015-07-11 | 2017-01-19 | Advenchen Pharmaceuticals, LLC | Fused quinoline compunds as pi3k/mtor inhibitors |
| EP4378957A2 (en) | 2015-07-29 | 2024-06-05 | Novartis AG | Combination therapies comprising antibody molecules to pd-1 |
| WO2017019894A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to lag-3 |
| WO2017019897A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to tim-3 |
| EP3878465A1 (en) | 2015-07-29 | 2021-09-15 | Novartis AG | Combination therapies comprising antibody molecules to tim-3 |
| EP3964528A1 (en) | 2015-07-29 | 2022-03-09 | Novartis AG | Combination therapies comprising antibody molecules to lag-3 |
| EP4241850A2 (en) | 2015-08-28 | 2023-09-13 | Novartis AG | Mdm2 inhibitors and combinations thereof |
| WO2017037579A1 (en) | 2015-08-28 | 2017-03-09 | Novartis Ag | Mdm2 inhibitors and combinations thereof |
| EP4327809A2 (en) | 2015-09-02 | 2024-02-28 | Takeda Pharmaceutical Company Limited | Tyk2 inhibitors and uses thereof |
| US11434240B2 (en) | 2015-09-02 | 2022-09-06 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US10023571B2 (en) | 2015-09-02 | 2018-07-17 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| US10781204B2 (en) | 2015-09-02 | 2020-09-22 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| WO2017040757A1 (en) | 2015-09-02 | 2017-03-09 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US11738087B2 (en) | 2015-09-08 | 2023-08-29 | Monash University | Lymph directing prodrugs |
| US10683308B2 (en) | 2015-09-11 | 2020-06-16 | Navitor Pharmaceuticals, Inc. | Rapamycin analogs and uses thereof |
| US9856255B2 (en) | 2015-09-17 | 2018-01-02 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| US10457679B2 (en) | 2015-09-17 | 2019-10-29 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| US10882858B2 (en) | 2015-09-17 | 2021-01-05 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| US11613539B2 (en) | 2015-09-17 | 2023-03-28 | Astrazeneca Ab | Imidazo[4,5-c]quinolin-2-one compounds and their use in treating cancer |
| KR102469153B1 (en) | 2015-09-17 | 2022-11-22 | 아스트라제네카 아베 | 8-[6-[3-(amino)propoxy]-3-pyridyl]-1 -isopropyl-imidazo[4,5-c]quinolin-2-one derivatives as selective modulators of ataxia telangiectasia mutated (atm) kinase for the treatment of cancer |
| KR20190112852A (en) * | 2015-09-17 | 2019-10-07 | 아스트라제네카 아베 | 8-[6-[3-(amino)propoxy]-3-pyridyl]-1 -isopropyl-imidazo[4,5-c]quinolin-2-one derivatives as selective modulators of ataxia telangiectasia mutated (atm) kinase for the treatment of cancer |
| EP4112611A2 (en) | 2015-10-23 | 2023-01-04 | Navitor Pharmaceuticals, Inc. | Modulators of sestrin-gator2 interaction and uses thereof |
| US11325924B2 (en) | 2015-10-23 | 2022-05-10 | Navitor Pharmaceuticals, Inc. | Modulators of Sestrin-GATOR2 interaction and uses thereof |
| US10100066B2 (en) | 2015-10-23 | 2018-10-16 | Navitor Pharmaceuticals, Inc. | Modulators of sestrin-GATOR2 interaction and uses thereof |
| US10752644B2 (en) | 2015-10-23 | 2020-08-25 | Navitor Pharmaceuticals, Inc. | Modulators of Sestrin-GATOR2 interaction and uses thereof |
| WO2017070518A1 (en) | 2015-10-23 | 2017-04-27 | Navitor Pharmaceuticals, Inc. | Modulators of sestrin-gator2 interaction and uses thereof |
| US10414782B2 (en) | 2015-10-23 | 2019-09-17 | Navitor Pharmaceuticals, Inc. | Modulators of sestrin-GATOR2 interaction and uses thereof |
| EP3797797A1 (en) | 2015-10-29 | 2021-03-31 | Novartis AG | Antibody conjugates comprising toll-like receptor agonist |
| WO2017072662A1 (en) | 2015-10-29 | 2017-05-04 | Novartis Ag | Antibody conjugates comprising toll-like receptor agonist |
| US10894830B2 (en) | 2015-11-03 | 2021-01-19 | Janssen Biotech, Inc. | Antibodies specifically binding PD-1, TIM-3 or PD-1 and TIM-3 and their uses |
| WO2017093905A1 (en) | 2015-12-03 | 2017-06-08 | Novartis Ag | Treatment of cancer with a pi3k inhibitor in a patient preselected for having a pik3ca mutation in the ctdna |
| WO2017106352A1 (en) | 2015-12-14 | 2017-06-22 | Raze Therapeutics, Inc. | Caffeine inhibitors of mthfd2 and uses thereof |
| US11370792B2 (en) | 2015-12-14 | 2022-06-28 | Raze Therapeutics, Inc. | Caffeine inhibitors of MTHFD2 and uses thereof |
| WO2017106656A1 (en) | 2015-12-17 | 2017-06-22 | Novartis Ag | Antibody molecules to pd-1 and uses thereof |
| EP4424322A2 (en) | 2015-12-17 | 2024-09-04 | Novartis AG | Antibody molecules to pd-1 and uses thereof |
| US11634412B2 (en) | 2016-03-09 | 2023-04-25 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| EP3884939A1 (en) | 2016-03-09 | 2021-09-29 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| WO2017156179A1 (en) | 2016-03-09 | 2017-09-14 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| EP4234552A2 (en) | 2016-03-09 | 2023-08-30 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| US11014882B2 (en) | 2016-03-09 | 2021-05-25 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| US11535593B2 (en) | 2016-03-09 | 2022-12-27 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| US20190071400A1 (en) * | 2016-03-09 | 2019-03-07 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| US10954220B2 (en) | 2016-03-09 | 2021-03-23 | Raze Therapeutics, Inc. | 3-phosphoglycerate dehydrogenase inhibitors and uses thereof |
| US10953004B2 (en) | 2016-03-14 | 2021-03-23 | Avexxin As | Combination therapy for proliferative diseases |
| US11439625B2 (en) | 2016-03-14 | 2022-09-13 | Avexxin As | Combination therapy for proliferative diseases |
| US11337969B2 (en) | 2016-04-08 | 2022-05-24 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US10736887B2 (en) | 2016-04-15 | 2020-08-11 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Quinoline derivative for treating gastric cancer |
| US10577362B2 (en) | 2016-05-04 | 2020-03-03 | Genoscience Pharma | Substituted 2, 4-diamino-quinoline derivatives for use in the treatment of proliferative diseases |
| WO2017216706A1 (en) | 2016-06-14 | 2017-12-21 | Novartis Ag | Compounds and compositions for inhibiting the activity of shp2 |
| US10676479B2 (en) | 2016-06-20 | 2020-06-09 | Novartis Ag | Imidazolepyridine compounds and uses thereof |
| WO2017221092A1 (en) | 2016-06-20 | 2017-12-28 | Novartis Ag | Triazolopyridine compounds and uses thereof |
| WO2017221100A1 (en) | 2016-06-20 | 2017-12-28 | Novartis Ag | Imidazopyrimidine compounds useful for the treatment of cancer |
| US11091489B2 (en) | 2016-06-20 | 2021-08-17 | Novartis Ag | Crystalline forms of a triazolopyrimidine compound |
| US10689378B2 (en) | 2016-06-20 | 2020-06-23 | Novartis Ag | Triazolopyridine compounds and uses thereof |
| US11548897B2 (en) | 2016-06-20 | 2023-01-10 | Novartis Ag | Crystalline forms of a triazolopyrimidine compound |
| WO2017223239A1 (en) | 2016-06-21 | 2017-12-28 | X4 Pharmaceuticals, Inc. | Cxcr4 inhibitors and uses thereof |
| US11780837B2 (en) | 2016-06-21 | 2023-10-10 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11306088B2 (en) | 2016-06-21 | 2022-04-19 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US10988465B2 (en) | 2016-06-21 | 2021-04-27 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| EP3808748A1 (en) | 2016-06-21 | 2021-04-21 | X4 Pharmaceuticals, Inc. | Substituted piperidines as cxcr4-inhibitors |
| US11332470B2 (en) | 2016-06-21 | 2022-05-17 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11098077B2 (en) | 2016-07-05 | 2021-08-24 | Chinook Therapeutics, Inc. | Locked nucleic acid cyclic dinucleotide compounds and uses thereof |
| EP4086250A2 (en) | 2016-07-06 | 2022-11-09 | The Regents Of The University Of Michigan | Multifunctional inhibitors of mek/pi3k and mtor/mek/pi3k biological pathways and therapeutic methods using the same |
| WO2018009638A1 (en) | 2016-07-06 | 2018-01-11 | The Regents Of The University Of Michigan | MULTIFUNCTIONAL INHIBITORS OF MEK/PI3K AND mTOR/MEK/PI3K BIOLOGICAL PATHWAYS AND THERAPEUTIC METHODS USING THE SAME |
| US10919877B2 (en) | 2016-07-06 | 2021-02-16 | The Regents Of The University Of Michigan | Multifunctional inhibitors of MEK/PI3K and mTOR/MEK/PI3K biological pathways and therapeutic methods using the same |
| US11365252B2 (en) | 2016-07-20 | 2022-06-21 | University Of Utah Research Foundation | CD229 CAR T cells and methods of use thereof |
| US11351127B2 (en) | 2016-09-21 | 2022-06-07 | Avexxin As | Pharmaceutical composition |
| EP4089116A1 (en) | 2016-09-27 | 2022-11-16 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules |
| US11655282B2 (en) | 2016-09-27 | 2023-05-23 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules |
| WO2018064076A1 (en) | 2016-09-27 | 2018-04-05 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules |
| WO2018067992A1 (en) | 2016-10-07 | 2018-04-12 | Novartis Ag | Chimeric antigen receptors for the treatment of cancer |
| US11753408B2 (en) | 2016-10-13 | 2023-09-12 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Compounds and method for blocking transmission of malarial parasite |
| US10988472B2 (en) | 2016-10-13 | 2021-04-27 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Compounds and method for blocking transmission of malarial parasite |
| WO2018071794A1 (en) | 2016-10-14 | 2018-04-19 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US10793574B2 (en) | 2016-10-14 | 2020-10-06 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US10323036B2 (en) | 2016-10-14 | 2019-06-18 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US11220508B2 (en) | 2016-10-14 | 2022-01-11 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| EP3848370A2 (en) | 2016-10-14 | 2021-07-14 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| WO2018075937A1 (en) | 2016-10-21 | 2018-04-26 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US10647713B2 (en) | 2016-10-21 | 2020-05-12 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US11396508B2 (en) | 2016-10-21 | 2022-07-26 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US10844013B2 (en) | 2016-11-08 | 2020-11-24 | Navitor Pharmaceuticals, Inc. | Phenyl amino piperidine mTORC inhibitors and uses thereof |
| US10414727B2 (en) | 2016-11-08 | 2019-09-17 | Navitor Pharmaceuticals, Inc. | Phenyl amino piperidine mTORC inhibitors and uses thereof |
| WO2018092064A1 (en) | 2016-11-18 | 2018-05-24 | Novartis Ag | Combinations of mdm2 inhibitors and bcl-xl inhibitors |
| US10993940B2 (en) | 2016-11-23 | 2021-05-04 | Novartis Ag | Methods of enhancing immune response |
| US11045463B2 (en) | 2016-11-23 | 2021-06-29 | Novartis Ag | Methods of enhancing immune response |
| WO2018096402A1 (en) | 2016-11-23 | 2018-05-31 | Novartis Ag | Methods of enhancing immune response with everolimus, dactolisib or both |
| EP4035659A1 (en) | 2016-11-29 | 2022-08-03 | PureTech LYT, Inc. | Exosomes for delivery of therapeutic agents |
| US11091451B2 (en) | 2016-12-05 | 2021-08-17 | Raze Therapeutics, Inc. | SHMT inhibitors and uses thereof |
| US11136340B2 (en) | 2016-12-20 | 2021-10-05 | Astrazeneca Ab | Amino-triazolopyridine compounds and their use in treating cancer |
| US11746118B2 (en) | 2016-12-20 | 2023-09-05 | Astrazeneca Ab | Amino-triazolopyridine compounds and their use in treating cancer |
| US10407446B2 (en) | 2016-12-20 | 2019-09-10 | Astrazeneca Ab | Amino-triazolopyridine compounds and their use in treating cancer |
| US10532042B2 (en) | 2016-12-22 | 2020-01-14 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| EP4001269A1 (en) | 2016-12-22 | 2022-05-25 | Amgen Inc. | Benzoisothiazole, isothiazolo[3,4-b]pyridine, quinazoline, phthalazine, pyrido[2,3-d]pyridazine and pyrido[2,3-d]pyrimidine derivatives as kras g12c inhibitors for treating lung, pancreatic or colorectal cancer |
| WO2018119183A2 (en) | 2016-12-22 | 2018-06-28 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| US11285135B2 (en) | 2016-12-22 | 2022-03-29 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| US11730819B2 (en) | 2016-12-23 | 2023-08-22 | Bicycletx Limited | Peptide derivatives having novel linkage structures |
| US11433137B2 (en) | 2017-01-06 | 2022-09-06 | Bicyclerd Limited | Compounds for treating cancer |
| US10624968B2 (en) | 2017-01-06 | 2020-04-21 | Bicyclerd Limited | Compounds for treating cancer |
| WO2018127699A1 (en) | 2017-01-06 | 2018-07-12 | Bicyclerd Limited | Compounds for treating cancer |
| WO2018142322A1 (en) | 2017-02-03 | 2018-08-09 | Novartis Ag | Anti-ccr7 antibody drug conjugates |
| WO2018163051A1 (en) | 2017-03-06 | 2018-09-13 | Novartis Ag | Methods of treatment of cancer with reduced ubb expression |
| EP4338802A2 (en) | 2017-03-08 | 2024-03-20 | Takeda Pharmaceutical Company Limited | Tyk2 inhibitors, uses, and methods for production thereof |
| WO2018165240A1 (en) | 2017-03-08 | 2018-09-13 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors, uses, and methods for production thereof |
| US11040967B2 (en) | 2017-03-08 | 2021-06-22 | Nimbus Lakshmi, Inc. | TYK2 inhibitors, uses, and methods for production thereof |
| US10336752B2 (en) | 2017-03-08 | 2019-07-02 | Nimbus Lakshmi, Inc. | TYK2 inhibitors, uses, and methods for production thereof |
| US11434239B2 (en) | 2017-03-14 | 2022-09-06 | Artax Biopharma Inc. | Aza-dihydro-acridone derivatives |
| US11505526B2 (en) | 2017-03-14 | 2022-11-22 | Artax Biopharma Inc. | Aryl-piperidine derivatives |
| WO2018185618A1 (en) | 2017-04-03 | 2018-10-11 | Novartis Ag | Anti-cdh6 antibody drug conjugates and anti-gitr antibody combinations and methods of treatment |
| US11339144B2 (en) | 2017-04-10 | 2022-05-24 | Navitor Pharmaceuticals, Inc. | Heteroaryl Rheb inhibitors and uses thereof |
| US11679090B2 (en) | 2017-04-26 | 2023-06-20 | Navitor Pharmaceuticals, Inc. | Modulators of Sestrin-GATOR2 interaction and uses thereof |
| US11241473B2 (en) | 2017-04-27 | 2022-02-08 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| US10857196B2 (en) | 2017-04-27 | 2020-12-08 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| WO2018197893A1 (en) | 2017-04-27 | 2018-11-01 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| WO2018198091A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Antibody conjugates comprising toll-like receptor agonist and combination therapies |
| WO2018198076A1 (en) | 2017-04-28 | 2018-11-01 | Aduro Biotech, Inc. | Bis 2'-5'-rr-(3'f-a)(3'f-a) cyclic dinucleotide compound and uses thereof |
| WO2018201056A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Cells expressing a bcma-targeting chimeric antigen receptor, and combination therapy with a gamma secretase inhibitor |
| US10975114B2 (en) | 2017-04-28 | 2021-04-13 | Chinook Therapeutics, Inc. | Bis 2′-5′-RR-(3′F-A)(3′F-A) cyclic dinucleotide compound and uses thereof |
| WO2018217651A1 (en) | 2017-05-22 | 2018-11-29 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| US10519146B2 (en) | 2017-05-22 | 2019-12-31 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| EP3974429A1 (en) | 2017-05-22 | 2022-03-30 | Amgen Inc. | Precursors of kras g12c inhibitors |
| US11905281B2 (en) | 2017-05-22 | 2024-02-20 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| WO2018215937A1 (en) | 2017-05-24 | 2018-11-29 | Novartis Ag | Interleukin-7 antibody cytokine engrafted proteins and methods of use in the treatment of cancer |
| WO2018215936A1 (en) | 2017-05-24 | 2018-11-29 | Novartis Ag | Antibody-cytokine engrafted proteins and methods of use in the treatment of cancer |
| WO2018215938A1 (en) | 2017-05-24 | 2018-11-29 | Novartis Ag | Antibody-cytokine engrafted proteins and methods of use |
| WO2019002842A1 (en) | 2017-06-26 | 2019-01-03 | Bicyclerd Limited | Bicyclic peptide ligands with detectable moieties and uses thereof |
| US10899798B2 (en) | 2017-06-26 | 2021-01-26 | Bicyclerd Limited | Bicyclic peptide ligands with detectable moieties and uses thereof |
| US11746126B2 (en) | 2017-06-26 | 2023-09-05 | Bicyclerd Limited | Bicyclic peptide ligands with detectable moieties and uses thereof |
| US10508120B2 (en) | 2017-07-28 | 2019-12-17 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| US11046698B2 (en) | 2017-07-28 | 2021-06-29 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US10577373B2 (en) | 2017-07-28 | 2020-03-03 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| US10562906B2 (en) | 2017-07-28 | 2020-02-18 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| US10570145B2 (en) | 2017-07-28 | 2020-02-25 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| US10562907B2 (en) | 2017-07-28 | 2020-02-18 | Nimbus Lakshimi, Inc. | TYK2 inhibitors and uses thereof |
| US12049520B2 (en) | 2017-08-04 | 2024-07-30 | Bicycletx Limited | Bicyclic peptide ligands specific for CD137 |
| US11311624B2 (en) | 2017-08-11 | 2022-04-26 | The Regents Of The University Of Michigan | Inhibitors of MEK/PI3K, JAK/MEK, JAK/PI3K/mTOR and MEK/PI3K/mTOR biological pathways and methods for improving lymphatic uptake, bioavailability, and solubility of therapeutic compounds |
| WO2019032640A1 (en) | 2017-08-11 | 2019-02-14 | The Regents Of The University Of Michigan | Inhibitors of mek/pi3k, jak/mek, jak/pi3k/mtor and mek/pi3k/mtor biological pathways and methods for improving lymphatic uptake, bioavailability, and solubility of therapeutic compounds |
| WO2019034868A1 (en) | 2017-08-14 | 2019-02-21 | Bicyclerd Limited | Bicyclic peptide ligand prr-a conjugates and uses thereof |
| WO2019034866A1 (en) | 2017-08-14 | 2019-02-21 | Bicyclerd Limited | Bicyclic peptide ligand sting conjugates and uses thereof |
| US11351176B2 (en) | 2017-08-14 | 2022-06-07 | Mei Pharma, Inc. | Combination therapy |
| WO2019046491A1 (en) | 2017-08-29 | 2019-03-07 | Ariya Therapeutics, Inc. | Lymphatic system-directing lipid prodrugs |
| US11883497B2 (en) | 2017-08-29 | 2024-01-30 | Puretech Lyt, Inc. | Lymphatic system-directing lipid prodrugs |
| EP4403175A2 (en) | 2017-09-08 | 2024-07-24 | Amgen Inc. | Inhibitors of kras g12c and methods of using the same |
| EP4141005A1 (en) | 2017-09-08 | 2023-03-01 | Amgen Inc. | Inhibitors of kras g12c and methods of using the same |
| US11306087B2 (en) | 2017-09-08 | 2022-04-19 | Amgen Inc. | Inhibitors of KRAS G12C and methods of using the same |
| US11993597B2 (en) | 2017-09-08 | 2024-05-28 | Amgen Inc. | Inhibitors of KRAS G12C and methods of using the same |
| WO2019051291A1 (en) | 2017-09-08 | 2019-03-14 | Amgen Inc. | Inhibitors of kras g12c and methods of using the same |
| US10640504B2 (en) | 2017-09-08 | 2020-05-05 | Amgen Inc. | Inhibitors of KRAS G12C and methods of using the same |
| US10435389B2 (en) | 2017-09-11 | 2019-10-08 | Krouzon Pharmaccuticals, Inc. | Octahydrocyclopenta[c]pyrrole allosteric inhibitors of SHP2 |
| WO2019051469A1 (en) | 2017-09-11 | 2019-03-14 | Krouzon Pharmaceuticals, Inc. | Octahydrocyclopenta[c]pyrrole allosteric inhibitors of shp2 |
| US11358948B2 (en) | 2017-09-22 | 2022-06-14 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11623932B2 (en) | 2017-09-22 | 2023-04-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| WO2019067328A1 (en) | 2017-09-26 | 2019-04-04 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules and methods of use |
| US11708423B2 (en) | 2017-09-26 | 2023-07-25 | Cero Therapeutics, Inc. | Chimeric engulfment receptor molecules and methods of use |
| US10596161B2 (en) | 2017-12-08 | 2020-03-24 | Incyte Corporation | Low dose combination therapy for treatment of myeloproliferative neoplasms |
| US11278541B2 (en) | 2017-12-08 | 2022-03-22 | Incyte Corporation | Low dose combination therapy for treatment of myeloproliferative neoplasms |
| US11833211B2 (en) | 2017-12-19 | 2023-12-05 | Bicycletx Limited | Methods of suppression and treatment of disease comprising administering bicycle peptide ligands specific for EphA2 |
| US11938137B2 (en) | 2017-12-19 | 2024-03-26 | Puretech Lyt, Inc. | Lipid prodrugs of mycophenolic acid and uses thereof |
| US11608345B1 (en) | 2017-12-19 | 2023-03-21 | Puretech Lyt, Inc. | Lipid prodrugs of rapamycin and its analogs and uses thereof |
| WO2019126378A1 (en) | 2017-12-19 | 2019-06-27 | Ariya Therapeutics, Inc. | Lipid prodrugs of mycophenolic acid and uses thereof |
| US11304954B2 (en) | 2017-12-19 | 2022-04-19 | Puretech Lyt, Inc. | Lipid prodrugs of mycophenolic acid and uses thereof |
| US11623012B2 (en) | 2017-12-19 | 2023-04-11 | Bicyclerd Limited | Bicyclic peptide ligands specific for EphA2 |
| US10874743B2 (en) | 2017-12-26 | 2020-12-29 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11318205B1 (en) | 2017-12-26 | 2022-05-03 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11723980B2 (en) | 2017-12-26 | 2023-08-15 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US12006329B2 (en) | 2018-01-12 | 2024-06-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11932635B2 (en) | 2018-01-12 | 2024-03-19 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11485743B2 (en) | 2018-01-12 | 2022-11-01 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11512080B2 (en) | 2018-01-12 | 2022-11-29 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| WO2019148132A1 (en) | 2018-01-29 | 2019-08-01 | Merck Patent Gmbh | Gcn2 inhibitors and uses thereof |
| US10793563B2 (en) | 2018-01-29 | 2020-10-06 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| US12084438B2 (en) | 2018-01-29 | 2024-09-10 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| US10988477B2 (en) | 2018-01-29 | 2021-04-27 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| US10899736B2 (en) | 2018-01-30 | 2021-01-26 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US10596165B2 (en) | 2018-02-12 | 2020-03-24 | resTORbio, Inc. | Combination therapies |
| WO2019169001A1 (en) | 2018-02-27 | 2019-09-06 | Artax Biopharma Inc. | Chromene derivatives as inhibitors of tcr-nck interaction |
| US10696663B2 (en) | 2018-02-27 | 2020-06-30 | Artax Biopharma Inc. | Chromene derivatives as inhibitors of TCR-NCK interaction |
| US11008310B2 (en) | 2018-02-27 | 2021-05-18 | Artax Biopharma Inc. | Chromene derivatives as inhibitors of TCR-Nck interaction |
| US11807633B2 (en) | 2018-02-27 | 2023-11-07 | Artax Biopharma Inc. | Chromene derivatives as inhibitors of TCR-Nck interaction |
| US11279676B2 (en) | 2018-03-02 | 2022-03-22 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Crystalline of compound as c-Met kinase inhibitor and preparation method therefor and use thereof |
| WO2019191339A1 (en) | 2018-03-28 | 2019-10-03 | Cero Therapeutics, Inc. | Expression vectors for chimeric engulfment receptors, genetically modified host cells, and uses thereof |
| WO2019191334A1 (en) | 2018-03-28 | 2019-10-03 | Cero Therapeutics, Inc. | Chimeric tim4 receptors and uses thereof |
| WO2019191340A1 (en) | 2018-03-28 | 2019-10-03 | Cero Therapeutics, Inc. | Cellular immunotherapy compositions and uses thereof |
| US11304949B2 (en) | 2018-03-30 | 2022-04-19 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| WO2019209759A1 (en) | 2018-04-24 | 2019-10-31 | Merck Patent Gmbh | Antiproliferation compounds and uses thereof |
| EP4043460A1 (en) | 2018-04-24 | 2022-08-17 | Merck Patent GmbH | Antiproliferation compounds and uses thereof |
| WO2019209757A1 (en) | 2018-04-24 | 2019-10-31 | Vertex Pharmaceuticals Incorporated | Pteridinone compounds and uses thereof |
| WO2019210153A1 (en) | 2018-04-27 | 2019-10-31 | Novartis Ag | Car t cell therapies with enhanced efficacy |
| WO2019213282A1 (en) | 2018-05-01 | 2019-11-07 | Novartis Ag | Biomarkers for evaluating car-t cells to predict clinical outcome |
| US11766436B2 (en) | 2018-05-04 | 2023-09-26 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| US11090304B2 (en) | 2018-05-04 | 2021-08-17 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| US11045484B2 (en) | 2018-05-04 | 2021-06-29 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| WO2019213516A1 (en) | 2018-05-04 | 2019-11-07 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2019213526A1 (en) | 2018-05-04 | 2019-11-07 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2019217691A1 (en) | 2018-05-10 | 2019-11-14 | Amgen Inc. | Kras g12c inhibitors for the treatment of cancer |
| US10988485B2 (en) | 2018-05-10 | 2021-04-27 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| WO2019232419A1 (en) | 2018-06-01 | 2019-12-05 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| US11096939B2 (en) | 2018-06-01 | 2021-08-24 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| WO2019241157A1 (en) | 2018-06-11 | 2019-12-19 | Amgen Inc. | Kras g12c inhibitors for treating cancer |
| EP4268898A2 (en) | 2018-06-11 | 2023-11-01 | Amgen Inc. | Kras g12c inhibitors for treating cancer |
| US12083121B2 (en) | 2018-06-12 | 2024-09-10 | Amgen Inc. | Substituted piperazines as KRAS G12C inhibitors |
| US11285156B2 (en) | 2018-06-12 | 2022-03-29 | Amgen Inc. | Substituted piperazines as KRAS G12C inhibitors |
| WO2020050890A2 (en) | 2018-06-12 | 2020-03-12 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| US11944605B2 (en) | 2018-06-15 | 2024-04-02 | Janssen Pharmaceutica Nv | Rapamycin analogs and uses thereof |
| US10980784B2 (en) | 2018-06-15 | 2021-04-20 | Navitor Pharmaceuticals, Inc. | Rapamycin analogs and uses thereof |
| EP4302827A2 (en) | 2018-06-15 | 2024-01-10 | JANSSEN Pharmaceutica NV | Rapamycin analogs and uses thereof |
| WO2019241789A1 (en) | 2018-06-15 | 2019-12-19 | Navitor Pharmaceuticals, Inc. | Rapamycin analogs and uses thereof |
| US11814447B2 (en) | 2018-06-22 | 2023-11-14 | Bicyclerd Limited | Peptide ligands for binding to EphA2 |
| US11912792B2 (en) | 2018-06-22 | 2024-02-27 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| US11180531B2 (en) | 2018-06-22 | 2021-11-23 | Bicycletx Limited | Bicyclic peptide ligands specific for Nectin-4 |
| WO2019243833A1 (en) | 2018-06-22 | 2019-12-26 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| US11453702B2 (en) | 2018-06-22 | 2022-09-27 | Bicycletx Limited | Bicyclic peptide ligands specific for Nectin-4 |
| WO2019243832A1 (en) | 2018-06-22 | 2019-12-26 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| WO2020010177A1 (en) | 2018-07-06 | 2020-01-09 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| US11292792B2 (en) | 2018-07-06 | 2022-04-05 | Kymera Therapeutics, Inc. | Tricyclic CRBN ligands and uses thereof |
| US11897882B2 (en) | 2018-07-06 | 2024-02-13 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| US12115156B2 (en) | 2018-08-31 | 2024-10-15 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| US11045461B2 (en) | 2018-08-31 | 2021-06-29 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| WO2020051424A1 (en) | 2018-09-07 | 2020-03-12 | Pic Therapeutics | Eif4e inhibitors and uses thereof |
| US11034705B2 (en) | 2018-09-18 | 2021-06-15 | Nikang Therapeutics, Inc. | Fused tricyclic ring derivatives as Src homology-2 phosphate inhibitors |
| US10894797B2 (en) | 2018-09-18 | 2021-01-19 | Nikang Therapeutics, Inc. | Fused tricyclic ring derivatives as SRC homology-2 phosphatase inhibitors |
| WO2020061101A1 (en) | 2018-09-18 | 2020-03-26 | Nikang Therapeutics, Inc. | Tri-substituted heteroaryl derivatives as src homology-2 phosphatase inhibitors |
| WO2020061103A1 (en) | 2018-09-18 | 2020-03-26 | Nikang Therapeutics, Inc. | Fused tricyclic ring derivatives as src homology-2 phosphatase inhibitors |
| US11459340B2 (en) | 2018-09-18 | 2022-10-04 | Nikang Therapeutics, Inc. | Tri-substituted heteroaryl derivatives as Src homology-2 phosphatase inhibitors |
| US11518772B2 (en) | 2018-09-18 | 2022-12-06 | Nikang Therapeutics, Inc. | Fused tricyclic ring derivatives as Src homology-2 phosphate inhibitors |
| WO2020068873A1 (en) | 2018-09-25 | 2020-04-02 | Black Diamond Therapeutics, Inc. | Tyrosine kinase inhibitor compositions, methods of making and methods of use |
| US11034672B1 (en) | 2018-09-25 | 2021-06-15 | Black Diamond Therapeutics, Inc. | Tyrosine kinase inhibitor compositions, methods of making and methods of use |
| WO2020065453A1 (en) | 2018-09-29 | 2020-04-02 | Novartis Ag | Process of manufacture of a compound for inhibiting the activity of shp2 |
| EP4282416A2 (en) | 2018-09-29 | 2023-11-29 | Novartis AG | Process of manufacture of a compound for inhibiting the activity of shp2 |
| US11414431B2 (en) | 2018-10-15 | 2022-08-16 | Nimbus Lakshmi, Inc. | Substituted pyrazolo[1,5-a]pyrimidines as TYK2 inhibitors |
| US10919937B2 (en) | 2018-10-23 | 2021-02-16 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| WO2020084305A1 (en) | 2018-10-23 | 2020-04-30 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| US11396530B2 (en) | 2018-10-23 | 2022-07-26 | Bicycletx Limited | Bicyclic peptide ligands and uses thereof |
| US11697633B2 (en) | 2018-10-24 | 2023-07-11 | Navitor Pharmaceuticals, Inc. | Polymorphic compounds and uses thereof |
| US11345654B2 (en) | 2018-10-24 | 2022-05-31 | Navitor Pharmaceuticals, Inc. | Polymorphic compounds and uses thereof |
| WO2020089811A1 (en) | 2018-10-31 | 2020-05-07 | Novartis Ag | Dc-sign antibody drug conjugates |
| WO2020102730A1 (en) | 2018-11-16 | 2020-05-22 | Amgen Inc. | Improved synthesis of key intermediate of kras g12c inhibitor compound |
| US11299491B2 (en) | 2018-11-16 | 2022-04-12 | Amgen Inc. | Synthesis of key intermediate of KRAS G12C inhibitor compound |
| EP4234546A2 (en) | 2018-11-16 | 2023-08-30 | Amgen Inc. | Improved synthesis of key intermediate of kras g12c inhibitor compound |
| US11053226B2 (en) | 2018-11-19 | 2021-07-06 | Amgen Inc. | KRAS G12C inhibitors and methods of using the same |
| US11918584B2 (en) | 2018-11-19 | 2024-03-05 | Amgen Inc. | Combination therapy including a KRASG12C inhibitor and one or more additional pharmaceutically active agents for the treatment of cancers |
| WO2020106640A1 (en) | 2018-11-19 | 2020-05-28 | Amgen Inc. | Kras g12c inhibitors and methods of using the same |
| WO2020106647A2 (en) | 2018-11-19 | 2020-05-28 | Amgen Inc. | Combination therapy including a krasg12c inhibitor and one or more additional pharmaceutically active agents for the treatment of cancers |
| US11439645B2 (en) | 2018-11-19 | 2022-09-13 | Amgen Inc. | Combination therapy including a KRASG12C inhibitor and one or more additional pharmaceutically active agents for the treatment of cancers |
| US11834449B2 (en) | 2018-11-30 | 2023-12-05 | Takeda Pharmaceutical Company Limited | TYK2 inhibitors and uses thereof |
| US11807636B2 (en) | 2018-11-30 | 2023-11-07 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2020112937A1 (en) | 2018-11-30 | 2020-06-04 | Nimbus Lakshmi, Inc. | Tyk2 inhibitors and uses thereof |
| US11053241B2 (en) | 2018-11-30 | 2021-07-06 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| US11117889B1 (en) | 2018-11-30 | 2021-09-14 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11352350B2 (en) | 2018-11-30 | 2022-06-07 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2020132653A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Heteroaryl amides useful as kif18a inhibitors |
| WO2020127853A1 (en) | 2018-12-20 | 2020-06-25 | Abivax | Biomarkers, and uses in treatment of viral infections, inflammations, or cancer |
| WO2020132651A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Kif18a inhibitors |
| WO2020132649A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Heteroaryl amides useful as kif18a inhibitors |
| US11236069B2 (en) | 2018-12-20 | 2022-02-01 | Amgen Inc. | KIF18A inhibitors |
| EP3670659A1 (en) | 2018-12-20 | 2020-06-24 | Abivax | Biomarkers, and uses in treatment of viral infections, inflammations, or cancer |
| WO2020132648A1 (en) | 2018-12-20 | 2020-06-25 | Amgen Inc. | Kif18a inhibitors |
| US12054476B2 (en) | 2018-12-20 | 2024-08-06 | Amgen Inc. | KIF18A inhibitors |
| WO2020128612A2 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Antibodies to pmel17 and conjugates thereof |
| EP4406555A2 (en) | 2018-12-21 | 2024-07-31 | Novartis AG | Antibodies to pmel17 and conjugates thereof |
| US11174264B2 (en) | 2019-01-23 | 2021-11-16 | Nimbus Lakshmi, Inc. | TYK2 inhibitors and uses thereof |
| WO2020165600A1 (en) | 2019-02-14 | 2020-08-20 | Bicycletx Limited | Bicyclic peptide ligand sting conjugates and uses thereof |
| WO2020180768A1 (en) | 2019-03-01 | 2020-09-10 | Revolution Medicines, Inc. | Bicyclic heteroaryl compounds and uses thereof |
| WO2020180770A1 (en) | 2019-03-01 | 2020-09-10 | Revolution Medicines, Inc. | Bicyclic heterocyclyl compounds and uses thereof |
| WO2020201753A1 (en) | 2019-04-02 | 2020-10-08 | Bicycletx Limited | Bicycle toxin conjugates and uses thereof |
| US11746120B2 (en) | 2019-04-05 | 2023-09-05 | Kymera Therapeutics, Inc. | Stat degraders and uses thereof |
| US11485750B1 (en) | 2019-04-05 | 2022-11-01 | Kymera Therapeutics, Inc. | STAT degraders and uses thereof |
| US12077555B2 (en) | 2019-04-05 | 2024-09-03 | Kymera Therapeutics, Inc. | STAT degraders and uses thereof |
| US11426404B2 (en) | 2019-05-14 | 2022-08-30 | Amgen Inc. | Dosing of KRAS inhibitor for treatment of cancers |
| EP3738593A1 (en) | 2019-05-14 | 2020-11-18 | Amgen, Inc | Dosing of kras inhibitor for treatment of cancers |
| WO2020232130A1 (en) | 2019-05-14 | 2020-11-19 | Amgen Inc. | Dosing of kras inhibitor for treatment of cancers |
| WO2020229685A1 (en) | 2019-05-15 | 2020-11-19 | Avexxin As | Combination therapy for proliferative conditions |
| WO2020229688A1 (en) | 2019-05-15 | 2020-11-19 | Avexxin As | Combination therapy for proliferative conditions |
| US11827635B2 (en) | 2019-05-21 | 2023-11-28 | Amgen Inc. | Solid state forms |
| US11236091B2 (en) | 2019-05-21 | 2022-02-01 | Amgen Inc. | Solid state forms |
| WO2020243423A1 (en) | 2019-05-31 | 2020-12-03 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2021001743A1 (en) | 2019-07-02 | 2021-01-07 | Effector Therapeutics, Inc. | Translation inhibitors and uses thereof |
| WO2021014027A1 (en) | 2019-07-25 | 2021-01-28 | Coegin Pharma As | Phospholipase-a2 inhibitors for the prevention of cancer metastasis |
| US11970553B2 (en) | 2019-07-30 | 2024-04-30 | Bicycletx Limited | Heterotandem bicyclic peptide complex |
| WO2021026098A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Kif18a inhibitors |
| WO2021026100A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Pyridine derivatives as kif18a inhibitors |
| WO2021026099A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Kif18a inhibitors |
| WO2021026101A1 (en) | 2019-08-02 | 2021-02-11 | Amgen Inc. | Kif18a inhibitors |
| WO2021030711A1 (en) | 2019-08-15 | 2021-02-18 | Black Diamond Therapeutics, Inc. | Alkynyl quinazoline compounds |
| US11845724B2 (en) | 2019-09-11 | 2023-12-19 | Vincere Biosciences, Inc. | USP30 inhibitors and uses thereof |
| US11078201B2 (en) | 2019-09-13 | 2021-08-03 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1H-pyrrol[3,4-c]pyridin-1-ones as HPK1 antagonists |
| US11548890B1 (en) | 2019-09-13 | 2023-01-10 | Nimbus Saturn, Inc. | HPK1 antagonists and uses thereof |
| US11034694B2 (en) | 2019-09-13 | 2021-06-15 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1H-pyrrolo[3,4-c]pyridin-1-ones as HPK1 antagonists |
| US11021481B2 (en) | 2019-09-13 | 2021-06-01 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1h-pyrrolo[3,4-c]pyridin-1-ones as HPK1 antagonists |
| US11028085B2 (en) | 2019-09-13 | 2021-06-08 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1h-pyrrolo[3,4-c]pyridin-1-ones as hpk1 antagonists |
| WO2021055728A1 (en) | 2019-09-18 | 2021-03-25 | Merck Sharp & Dohme Corp. | Small molecule inhibitors of kras g12c mutant |
| WO2021057853A1 (en) | 2019-09-26 | 2021-04-01 | Novartis Ag | Aza-quinoline compounds and uses thereof |
| WO2021067875A1 (en) | 2019-10-03 | 2021-04-08 | Cero Therapeutics, Inc. | Chimeric tim4 receptors and uses thereof |
| WO2021076655A1 (en) | 2019-10-15 | 2021-04-22 | Amgen Inc. | Combination therapy of kras inhibitor and shp2 inhibitor for treatment of cancers |
| WO2021081212A1 (en) | 2019-10-24 | 2021-04-29 | Amgen Inc. | Pyridopyrimidine derivatives useful as kras g12c and kras g12d inhibitors in the treatment of cancer |
| WO2021086833A1 (en) | 2019-10-28 | 2021-05-06 | Merck Sharp & Dohme Corp. | Small molecule inhibitors of kras g12c mutant |
| WO2021085653A1 (en) | 2019-10-31 | 2021-05-06 | Taiho Pharmaceutical Co., Ltd. | 4-aminobut-2-enamide derivatives and salts thereof |
| US11723890B2 (en) | 2019-11-01 | 2023-08-15 | Navitor Pharmaceuticals, Inc. | Methods of treatment using an mTORC1 modulator |
| WO2021091982A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2021091967A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2021091956A1 (en) | 2019-11-04 | 2021-05-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2021092115A1 (en) | 2019-11-08 | 2021-05-14 | Revolution Medicines, Inc. | Bicyclic heteroaryl compounds and uses thereof |
| WO2021097212A1 (en) | 2019-11-14 | 2021-05-20 | Amgen Inc. | Improved synthesis of kras g12c inhibitor compound |
| WO2021097207A1 (en) | 2019-11-14 | 2021-05-20 | Amgen Inc. | Improved synthesis of kras g12c inhibitor compound |
| WO2021108683A1 (en) | 2019-11-27 | 2021-06-03 | Revolution Medicines, Inc. | Covalent ras inhibitors and uses thereof |
| WO2021107160A1 (en) | 2019-11-29 | 2021-06-03 | Taiho Pharmaceutical Co., Ltd. | A compound having inhibitory activity against kras g12d mutation |
| US11819476B2 (en) | 2019-12-05 | 2023-11-21 | Janssen Pharmaceutica Nv | Rapamycin analogs and uses thereof |
| WO2021126816A1 (en) | 2019-12-16 | 2021-06-24 | Amgen Inc. | Dosing regimen of a kras g12c inhibitor |
| US11779578B2 (en) | 2019-12-17 | 2023-10-10 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11591332B2 (en) | 2019-12-17 | 2023-02-28 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11707457B2 (en) | 2019-12-17 | 2023-07-25 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11679109B2 (en) | 2019-12-23 | 2023-06-20 | Kymera Therapeutics, Inc. | SMARCA degraders and uses thereof |
| WO2021142026A1 (en) | 2020-01-07 | 2021-07-15 | Revolution Medicines, Inc. | Shp2 inhibitor dosing and methods of treating cancer |
| US11975073B2 (en) | 2020-02-05 | 2024-05-07 | Puretech Lyt, Inc. | Lipid prodrugs of neurosteroids |
| WO2021178488A1 (en) | 2020-03-03 | 2021-09-10 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| US11932624B2 (en) | 2020-03-19 | 2024-03-19 | Kymera Therapeutics, Inc. | MDM2 degraders and uses thereof |
| WO2021195206A1 (en) | 2020-03-24 | 2021-09-30 | Black Diamond Therapeutics, Inc. | Polymorphic forms and related uses |
| WO2021215544A1 (en) | 2020-04-24 | 2021-10-28 | Taiho Pharmaceutical Co., Ltd. | Kras g12d protein inhibitors |
| WO2021215545A1 (en) | 2020-04-24 | 2021-10-28 | Taiho Pharmaceutical Co., Ltd. | Anticancer combination therapy with n-(1-acryloyl-azetidin-3-yl)-2-((1h-indazol-3-yl)amino)methyl)-1h-imidazole-5-carboxamide inhibitor of kras-g12c |
| WO2021220199A1 (en) | 2020-04-30 | 2021-11-04 | Novartis Ag | Ccr7 antibody drug conjugates for treating cancer |
| US11833155B2 (en) | 2020-06-03 | 2023-12-05 | Incyte Corporation | Combination therapy for treatment of myeloproliferative neoplasms |
| US11685750B2 (en) | 2020-06-03 | 2023-06-27 | Kymera Therapeutics, Inc. | Crystalline forms of IRAK degraders |
| WO2021257736A1 (en) | 2020-06-18 | 2021-12-23 | Revolution Medicines, Inc. | Methods for delaying, preventing, and treating acquired resistance to ras inhibitors |
| WO2021260580A1 (en) | 2020-06-24 | 2021-12-30 | Astrazeneca Uk Limited | Combination of antibody-drug conjugate and atm inhibitor |
| WO2022014640A1 (en) | 2020-07-15 | 2022-01-20 | 大鵬薬品工業株式会社 | Pyrimidine compound-containing combination to be used in tumor treatment |
| WO2022033492A1 (en) | 2020-08-10 | 2022-02-17 | Novartis Ag | Quinoline compounds and compositions for inhibiting ezh2 |
| WO2022036265A1 (en) | 2020-08-14 | 2022-02-17 | Cero Therapeutics, Inc. | Chimeric tim receptors and uses thereof |
| WO2022036285A1 (en) | 2020-08-14 | 2022-02-17 | Cero Therapeutics, Inc. | Compositions and methods for treating cancer with chimeric tim receptors in combination with inhibitors of poly (adp-ribose) polymerase |
| WO2022036287A1 (en) | 2020-08-14 | 2022-02-17 | Cero Therapeutics, Inc. | Anti-cd72 chimeric receptors and uses thereof |
| WO2022038158A1 (en) | 2020-08-17 | 2022-02-24 | Bicycletx Limited | Bicycle conjugates specific for nectin-4 and uses thereof |
| WO2022043556A1 (en) | 2020-08-31 | 2022-03-03 | Novartis Ag | Stable radiopharmaceutical composition |
| WO2022043558A1 (en) | 2020-08-31 | 2022-03-03 | Advanced Accelerator Applications International Sa | Method of treating psma-expressing cancers |
| WO2022043557A1 (en) | 2020-08-31 | 2022-03-03 | Advanced Accelerator Applications International Sa | Method of treating psma-expressing cancers |
| WO2022060583A1 (en) | 2020-09-03 | 2022-03-24 | Revolution Medicines, Inc. | Use of sos1 inhibitors to treat malignancies with shp2 mutations |
| WO2022060836A1 (en) | 2020-09-15 | 2022-03-24 | Revolution Medicines, Inc. | Indole derivatives as ras inhibitors in the treatment of cancer |
| WO2022096361A1 (en) | 2020-11-03 | 2022-05-12 | Deutsches Krebsforschungszentrum | Imidazo[4,5-c]quinoline compounds and their use as atm kinase inhibitors |
| EP3992191A1 (en) | 2020-11-03 | 2022-05-04 | Deutsches Krebsforschungszentrum | Imidazo[4,5-c]quinoline compounds and their use as atm kinase inhibitors |
| WO2022120353A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2022120354A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2022123316A1 (en) | 2020-12-09 | 2022-06-16 | Takeda Pharmaceutical Company Limited | Compositions of guanylyl cyclase c (gcc) antigen binding agents and methods of use thereof |
| WO2022140472A1 (en) | 2020-12-22 | 2022-06-30 | Nikang Therapeutics, Inc. | Compounds for degrading cyclin-dependent kinase 2 via ubiquitin proteosome pathway |
| WO2022167445A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022167457A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022170052A1 (en) | 2021-02-05 | 2022-08-11 | Black Diamond Therapeutics, Inc. | Quinazoline derivatives, pyridopyrimidine derivatives, pyrimidopyrimidine derivatives, and uses thereof |
| US11773103B2 (en) | 2021-02-15 | 2023-10-03 | Kymera Therapeutics, Inc. | IRAK4 degraders and uses thereof |
| WO2022177267A1 (en) | 2021-02-16 | 2022-08-25 | 라이보텍(주) | Compound for suppressing nonsense-mediated mrna decay |
| WO2022177269A1 (en) | 2021-02-16 | 2022-08-25 | 라이보텍(주) | Compound for inhibiting nonsense-mediated mrna decay |
| WO2022183072A1 (en) | 2021-02-26 | 2022-09-01 | Kelonia Therapeutics, Inc. | Lymphocyte targeted lentiviral vectors |
| US11926625B2 (en) | 2021-03-05 | 2024-03-12 | Nimbus Saturn, Inc. | HPK1 antagonists and uses thereof |
| US12071442B2 (en) | 2021-03-29 | 2024-08-27 | Nimbus Saturn, Inc. | Substituted pyrrolo[3,4-c]pyridines as HPK1 antagonists |
| EP4427590A2 (en) | 2021-04-16 | 2024-09-11 | Novartis AG | Antibody drug conjugates and methods for making thereof |
| WO2022221720A1 (en) | 2021-04-16 | 2022-10-20 | Novartis Ag | Antibody drug conjugates and methods for making thereof |
| WO2022221866A1 (en) | 2021-04-16 | 2022-10-20 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2022235864A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2022235866A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Covalent ras inhibitors and uses thereof |
| WO2022235870A1 (en) | 2021-05-05 | 2022-11-10 | Revolution Medicines, Inc. | Ras inhibitors for the treatment of cancer |
| US12097261B2 (en) | 2021-05-07 | 2024-09-24 | Kymera Therapeutics, Inc. | CDK2 degraders and uses thereof |
| WO2022250170A1 (en) | 2021-05-28 | 2022-12-01 | Taiho Pharmaceutical Co., Ltd. | Small molecule inhibitors of kras mutated proteins |
| WO2023284730A1 (en) | 2021-07-14 | 2023-01-19 | Nikang Therapeutics, Inc. | Alkylidene derivatives as kras inhibitors |
| WO2023010097A1 (en) | 2021-07-28 | 2023-02-02 | Cero Therapeutics, Inc. | Chimeric tim4 receptors and uses thereof |
| WO2023028235A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2023028238A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2023060253A1 (en) | 2021-10-08 | 2023-04-13 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2023114984A1 (en) | 2021-12-17 | 2023-06-22 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2023114954A1 (en) | 2021-12-17 | 2023-06-22 | Genzyme Corporation | Pyrazolopyrazine compounds as shp2 inhibitors |
| WO2023125877A1 (en) | 2021-12-30 | 2023-07-06 | 上海翰森生物医药科技有限公司 | Tricyclic derivative inhibitor, preparation method therefor, and application thereof |
| US12091411B2 (en) | 2022-01-31 | 2024-09-17 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| EP4227307A1 (en) | 2022-02-11 | 2023-08-16 | Genzyme Corporation | Pyrazolopyrazine compounds as shp2 inhibitors |
| WO2023172940A1 (en) | 2022-03-08 | 2023-09-14 | Revolution Medicines, Inc. | Methods for treating immune refractory lung cancer |
| WO2023173053A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023173057A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023192801A1 (en) | 2022-03-28 | 2023-10-05 | Nikang Therapeutics, Inc. | Sulfonamido derivatives as cyclin-dependent kinase 2 inhibitors |
| WO2023211889A1 (en) | 2022-04-25 | 2023-11-02 | Ikena Oncology, Inc. | Polymorphic compounds and uses thereof |
| WO2023230205A1 (en) | 2022-05-25 | 2023-11-30 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023240024A1 (en) | 2022-06-08 | 2023-12-14 | Nikang Therapeutics, Inc. | Sulfamide derivatives as cyclin-dependent kinase 2 inhibitors |
| WO2023240263A1 (en) | 2022-06-10 | 2023-12-14 | Revolution Medicines, Inc. | Macrocyclic ras inhibitors |
| WO2024028365A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Substituted pyridone gpr84 antagonists and uses thereof |
| WO2024028364A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Aryl-triazolyl and related gpr84 antagonists and uses thereof |
| WO2024028363A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Heteroaryl carboxamide and related gpr84 antagonists and uses thereof |
| WO2024081916A1 (en) | 2022-10-14 | 2024-04-18 | Black Diamond Therapeutics, Inc. | Methods of treating cancers using isoquinoline or 6-aza-quinoline derivatives |
| WO2024102849A1 (en) | 2022-11-11 | 2024-05-16 | Nikang Therapeutics, Inc. | Bifunctional compounds containing 2,5-substituted pyrimidine derivatives for degrading cyclin-dependent kinase 2 via ubiquitin proteasome pathway |
| WO2024112894A1 (en) | 2022-11-22 | 2024-05-30 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2024206858A1 (en) | 2023-03-30 | 2024-10-03 | Revolution Medicines, Inc. | Compositions for inducing ras gtp hydrolysis and uses thereof |
| WO2024211663A1 (en) | 2023-04-07 | 2024-10-10 | Revolution Medicines, Inc. | Condensed macrocyclic compounds as ras inhibitors |
| WO2024211712A1 (en) | 2023-04-07 | 2024-10-10 | Revolution Medicines, Inc. | Condensed macrocyclic compounds as ras inhibitors |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2270008B1 (en) | 8-heteroaryl-3-alkyl-1,3-dihydro-imidazo[4,5-c]quinolin-2-ones as PI-3 kinases inhibitors | |
| EP2081933B1 (en) | Pyrazolopyrimidines as pi3k lipid kinase inhibitors | |
| EP2049502B1 (en) | 2,4-substituted quinazolines as lipid kinase inhibitors | |
| AU2004295062B2 (en) | 1H-imidazo[4,5-C]quinoline derivatives in the treatment of protein kinase dependent diseases | |
| US20090318410A1 (en) | Imidazopyridazines as lipid kinase inhibitors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200680017567.3 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2006753710 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12007502354 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 562890 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 187003 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006249071 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 8464/DELNP/2007 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11913788 Country of ref document: US Ref document number: CR2007-009504 Country of ref document: CR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2608496 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/a/2007/014380 Country of ref document: MX Ref document number: 07121354 Country of ref document: CO |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008511640 Country of ref document: JP Ref document number: 1020077026850 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10380 Country of ref document: GE |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2006249071 Country of ref document: AU Date of ref document: 20060518 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200702387 Country of ref document: EA |
|
| WWP | Wipo information: published in national office |
Ref document number: 2006249071 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: DZP2007000798 Country of ref document: DZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1200702720 Country of ref document: VN |
|
| NENP | Non-entry into the national phase |
Ref country code: RU |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: RU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2006753710 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: PI0610321 Country of ref document: BR Kind code of ref document: A2 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: D2010214 Country of ref document: CU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 221573 Country of ref document: IL |