WO2003023057A2 - Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells - Google Patents
Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells Download PDFInfo
- Publication number
- WO2003023057A2 WO2003023057A2 PCT/EP2002/005489 EP0205489W WO03023057A2 WO 2003023057 A2 WO2003023057 A2 WO 2003023057A2 EP 0205489 W EP0205489 W EP 0205489W WO 03023057 A2 WO03023057 A2 WO 03023057A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- sections
- antibodies
- mrna
- detection
- Prior art date
Links
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54313—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals the carrier being characterised by its particulate form
- G01N33/54326—Magnetic particles
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56966—Animal cells
Definitions
- the present invention relates to a method and a diagnostic kit for the selection and / or for the qualitative and / or quantitative detection of cells in a sample. Detection methods and diagnostic kits of this type are required in particular in the diagnosis or treatment control of tumor diseases. Because in the context of cancer screening or follow-up, it is of great importance to be able to detect malignant tumors or recurrent malignant tumors at an early stage based on the appearance of metastatic tumor cells in the blood. However, the present method and the present diagnostic kit are not only used here, but can in principle be used for the detection and recognition of rare cells in samples containing biological cells. For example, this can also be used to detect len cells in maternal blood or for the detection of stem cells.
- Testicular cancer is responsible for less than 2% of all malignant tumors in men.
- the tumor progression after the resection is primarily due to residual tumor cells. These cells are detached from the primary tumor preoperatively or intraoperatively and are given the possibility of being dispersed throughout the organism.
- tumor markers at the protein level are determined quantitatively in the blood or in other body fluids in cancer patients.
- these detection methods are only of limited suitability for tumor diagnosis or treatment control / aftercare for tumors, since increased tumor marker values in body fluids also due to non-tumor diseases, such as inflammation of the gastrointestinal tract, cirrhosis of the liver, viral infections or heavy smoking can be caused NEN.
- EP 0 520 794 B1 discloses such a method in which metastases in body tissues or
- nucleic acids are detected, for example by means of amplification by polymerase chain reaction.
- the method is now crucially based on the fact that the detected nucleic acid sequence is expressed in cells of the tissue of origin of a tumor, i.e. in tumor cells and, depending on the marker, also in the healthy cells of the original tissue.
- this sequence is normally not expressed in the cells whose tissue is being examined. If a corresponding sequence is found in the examined sample, it must be carried over from metastasizing cells from a non-resident tumor. This method is ultimately based on the detection of cells that should not be found in the blood sample of healthy people.
- the diagnostic methods currently used are too imprecise when it comes to assessing the malignant potency of residual tumors after chemotherapy in the metastatic stages. It is therefore still important to find evidence of an occult or residual metastasis that allows timely classification into the various primarily curative therapeutic options.
- the main problem here is that the cells to be detected, for example tumor cells in peripheral blood, occur only in extremely small numbers.
- the cells to be selected or detected are first marked using a combination of antibodies or antibody derivatives or a bispecific antibody or antibody derivative. This makes it possible, in particular, to mark, separate and thus enrich the cells sought. This means that a combined immunological detection or selection takes place in a first step.
- antibody derivative is understood to mean any type of modified antibody or antibody fragment that has a binding site, for example single-chain antibodies, antibody fragments such as FAB fragments or recombinant antibodies. In the following, when one speaks of “antibody *”, antibodies and / or antibody derivatives are always referred to.
- a second step at least one marker is then recorded on a molecular biological basis with a predefined combination of detection reagents, which marker is specific for the cells sought or can be found preferentially in these cells, so that the cells in question are specifically selected here.
- This is a combined molecular biological detection.
- the basic idea of the present invention is therefore to combine detection via a combination of immunological parameters with detection via a combination of molecular biological parameters. Surprisingly, this results in very excellent detection results, which are used to advance into detection areas which were previously not accessible to all known techniques from the prior art. In this way, concentrations of searched cells in blood samples down to two cells per 5 milliliter can still be detected. Such specificity and sensitivity have not been achieved in the prior art.
- Eukaryotic cells have a large number of different molecules on their cell surface.
- the combination of the expressed surface molecules differs according to the origin and the function of the individual cell, so that cell type-specific patterns arise.
- Antibodies are used to recognize these cell type-specific patterns.
- Antibodies bind to their antigen with high specificity, here to selected surface molecules. This property is used to recognize and differentiate cells by means of specific antibody binding based on their cell type-specific patterns. For example, the expression of special surface proteins of tumor cells differs from non-transformed cells of this cell type.
- the detection of the markers is preceded by a selection of the target cells by binding various antibodies to the cells sought.
- the expression of special surface proteins distinguishes cells of one type from cells of another type. For example, the expression of special surface pores differentiates a tumor cell from non-transformed cells of this cell type.
- tumor cells in the blood can be distinguished.
- antibodies that specifically recognize these special surface proteins are used as tools.
- the specific antibody binding is used for various analysis and separation methods.
- anti body coupled with fluorescent dyes For flow cytometric analysis, anti body coupled with fluorescent dyes. Scattered cells are individually guided past a light source (laser) in a constant liquid flow. When the cells are illuminated, the fluorescent dyes bound to the antibodies are excited and emit light of specific wavelengths. The emitted light is detected and the measured signal is stored digitally. The light signal can be assigned to individual cells. The antibody-labeled cell is recognized in this way and can now be separated from other cells. The cells are separated into tiny drops for separation. After detection of the antibody-labeled cell, the corresponding drop is directed into a collecting container. Such an enrichment can be done, for example, by FACS flow cytometry.
- enriched cells are incubated with fluorescence-labeled monoclonal antibodies against tumor-specific surface proteins.
- the labeled cells are washed twice with PBS and 10 7 cells are then resuspended in 1 ml PBS.
- a FACS Vanage SE flow cytometer (Becton Dickinson) is used to isolate the tumor cells.
- the CellQuest program is used for data acquisition, instrument control and data evaluation.
- the sorted cells are transferred to a 1.5 ml reaction vessel (filled with 1 ml PBS). The RNA can then be isolated as described later.
- Antibodies are coupled to pseudomagnetic particles for magnetic separation. After the pseudomagnetic particles have been introduced into a magnetic field, the particles migrate in the magnetic field. In the Movement in this magnetic field entrains cells to which these coupled antibodies are bound and separates them from other cells.
- the specificity of the separation is determined via the specificity of the antibodies.
- a blood sample containing target cells is mixed with antibody-coupled magnetic particles; Then the particles and blood are moved relative to each other, for example by "rotating overhead" samples in a closed container or by moving the particles due to changing magnetic fields.
- Those target cells that are recognized by one of the solid-phase-bound antibodies and thus firmly bound follow the movement of the particles. This makes it possible, when applying a magnetic field, to pull the particles with the cells attached to them from the blood (e.g. to the wall of the separation vessel).
- the target cell-depleted blood in this way can be exchanged for other solutions, the cells separated by magnetic particles until the shutdown / removal of the
- specific antibody mixtures are advantageously used for the detection of the tumor cells.
- a combination of the antibodies MOC-31 and Ber-EP4 is suitable for the detection of tumor cells in the blood.
- tumor cells are preferably detected, but with high specificity. This is due to the preferred expression of certain surface proteins that differentiate cancer cells from other cells.
- Antibody mixtures of this type show an increased sensitivity in comparison to the separately used antibodies in cell recognition and cell separation, regardless of the method used.
- the present invention is also based essentially on the fact that cell markers in the blood of patients are not detected at an immunological or enzymatic level, but that a molecular biological marker, for example the mRNA (messenger ribonucleic acid) of sought cells in a sample, for example a blood sample, is proven.
- a molecular biological marker for example the mRNA (messenger ribonucleic acid) of sought cells in a sample, for example a blood sample
- tumor cells can also be recognized if the expression of a particular marker in a patient or in a disease stage is relatively low, which could otherwise lead to a supposedly negative result.
- the use of markers mostly comes up against limits because mononuclear blood cells have a background expression ("illegitimate transcription *) that hinder an exact analysis.
- the expression of the genes mentioned in Table 2 is recorded as a marker, for example for tumors.
- the detection can be carried out for one or two markers or for any number of these tumor markers in combination with one another.
- the kit according to the invention can therefore contain two pairs of oligonucleotides for one, two or any selection or also all of the tumor markers.
- the tumor markers are also expressed due to other diseases and only enter the bloodstream as a protein. Consequently, based on the first immunological selection step, only cells are detected which, on the one hand, are in the blood sample and, on the other hand, express the tumor marker or markers. Consequently, these are tumor cells that originate from their original tumor tissue and have been carried over into the patient's blood. Since the mRNA of the markers examined does not normally exist in the blood of a tumor-infected person is expressed, there is a direct correlation between the occurrence of the associated mRNA and metastasis even at an early stage in the metastasis process.
- testicular tumors both seminous and non-seminal testicular cancer forms or mixed tumors with parts of a seminoma and thus 90-95% of all malignant tumors of the testicles, namely all germ cell tumors, are recorded.
- a combination of at least two of the following markers is therefore proposed according to the invention for the detection of testicular tumor cells:
- a combination of at least two tumor markers from the following marker groups is proposed according to the invention for the detection of breast cancer cells:
- CK20, EGFR, GA 733.2, CEA and Stanniocalcin a) CK20, EGFR, GA 733.2, CEA and Stanniocalcin b) CK20, EGFR, CEA and Stanniocalcin and c) EGFR, CEA and GA 733.2
- a peripheral blood sample was taken and a defined number of target cells added, for example 2, 10, 100 cells of a certain tumor type.
- Antibodies were also coupled to magnetic particles. pelt. The antibodies shown in Table 3 below were used as antibodies.
- the magnetic particles were used with a particle concentration of 4 x 10 8 beads / ml (CELLection TM Pan Mouse IgG Kit, Dynal). The relationships between the antibody concentration and the antibodies coupled to it are shown in Table 4.
- the magnetic particles prepared in this way were Test batch and detection system added to the blood.
- the corresponding addition of antibody-coupled magnetic particles per ml of blood at an initial concentration of 4 ⁇ 10 8 beads / ml of particles is shown in Table 5.
- the magnetic particles which were optionally in the form of cell-antibody-magnetic particle complexes, were washed three times with PBS (phosphate buffer saline) using a magnetic particle concentrator (MPC ® -S, Dynal) and the adherent cells were then treated according to the RNA isolation protocol described below.
- PBS phosphate buffer saline
- MPC ® -S, Dynal magnetic particle concentrator
- immunological separation using flow cytometry fluorescence-associated cell sorting, FACS is an option.
- a first relative enrichment of the tumor cells is achieved by depleting the erythrocytes.
- whole blood with EDTA
- a hypotonic erythrocyte lysis buffer and 30 minutes at room temperature incubated.
- the remaining nucleated cells are centrifuged and resuspended in PBS / BSA.
- the cells obtained in this way are then incubated with antibodies which are labeled with a fluorophore.
- the target cells which are fluorescently labeled by binding to an antibody, were then separated using FACS.
- enrichment by density gradient centrifugation can be used.
- Such a centrifugation with different density gradients separates cells of different average volume density from one another.
- Mononuclear blood cells are separated using a Ficoll-Hypaque gradient (company Pharmacia, Uppsala, Sweden) and then washed twice with PBS / 1% FCS. This is followed by a solid phase-coupled enrichment (e.g. via magnetic particles) or a liquid phase-based separation (FACS) of the target cells as described above.
- FACS liquid phase-based separation
- the first step is to isolate the total RNA of the cells separated as described above. This is done with the QIAamp RNA Blood Mini Kit (Qiaggen, Hilden) according to the manufacturer's instructions there, the lysis buffer being added directly to the cells bound to the magnetic particles. Additional DNA digestion on the column prevents contamination with genomic DNA. This DNA digestion is carried out with the RNase-Free DNase Set, from Qiagen, Hilden.
- an mRNA insulation e.g. B. using 01igo (dT) -coupled magnetic particles, Dynabeads ® mRNA Direct TM Micro Kit, (Dynal)). This isolation is also carried out in accordance with the manufacturer's instructions given in the kit.
- RNA isolation As a further alternative to RNA isolation, the isolated cells are lysed by adding trizole reagent (Gibco BRL, NY, USA) and homogenized using a pipette. After subsequent chloroform extraction, the RNA-containing aqueous phase is precipitated in isopropanol at -80 ° C. After washing twice and centrifuging in 80% ethanol, the pellet is air-dried and then resuspended in RNase-free water. This refurbishment step is also carried out according to conventional protocols.
- trizole reagent Gibco BRL, NY, USA
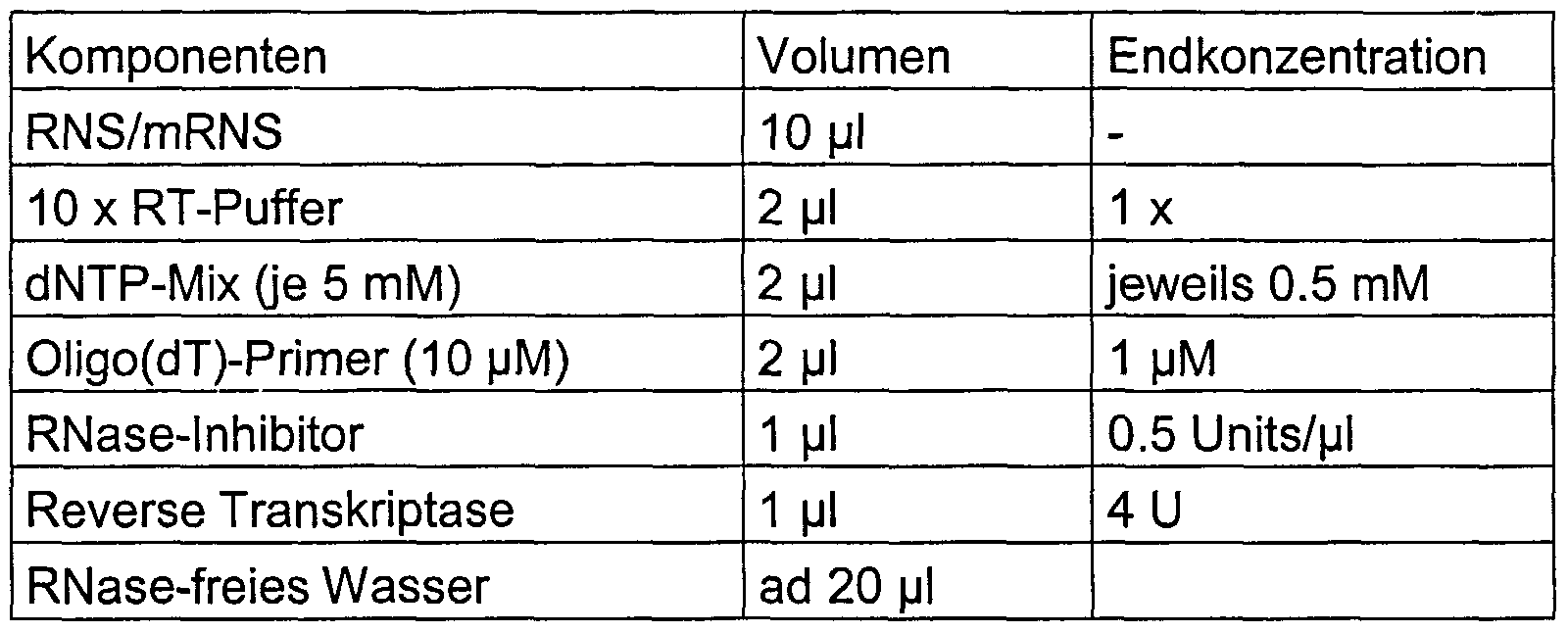
- RNA isolation of the RNA is followed by a reverse transcription in which the mRNA is transcribed into cDNA.
- RNA is denatured in a corresponding volume of water in accordance with the reaction mixture in Table 6 together with oligo (dT) 15 primers (from Promega, Mannheim) for 5 minutes at 65 ° C. and then incubated directly on ice.
- Table 6 Components of cDNA synthesis
- the cDNA synthesis takes place in a 20 ⁇ l reaction mixture
- the cDNA synthesis was carried out at 37 ° C for one hour with subsequent inactivation of the reverse transcriptase by heating for 5 minutes at 95 ° C and then cooling on ice.
- a Sensiscript Reverse Transcriptase Kit from Qiagen, Hilden was used according to the protocols specified there.
- oligo (dT) -coupled magnetic particles are used to isolate mRNA, the addition of oligo (dT) primers, i.e. the oligo (dT) linker also serves as a primer for reverse transcription, with the magnetic particles remaining in the approach.
- PCR polymerase chain reaction
- oligonucleotides listed in Table 7 were used as PCR primers for the amplification of cDNA corresponding to different marker genes, as indicated in the first column, are used.
- Table 7 contains the information in the first column Tu markers to be detected, two oligonucleotides (sense and antisense) each being specified as the primer pair.
- the length of the PCR product generated by the primers indicated in column two is indicated in column three. The PCR was carried out using the approach given in Table 8.
- the PCR synthesis was carried out in a 50 ⁇ l reaction mixture
- Table 9 gives a list of the specific combination of primers and primer concentrations as the final concentration in the PCR approach. In the following examples, a multiplex combination for these primers is shown for the different types of tumors, breast cancer, colon cancer and testicular cancer, as shown in Table 9. Table 9: List of the specific primer combinations and primer concentration (final concentration in the PCR approach)
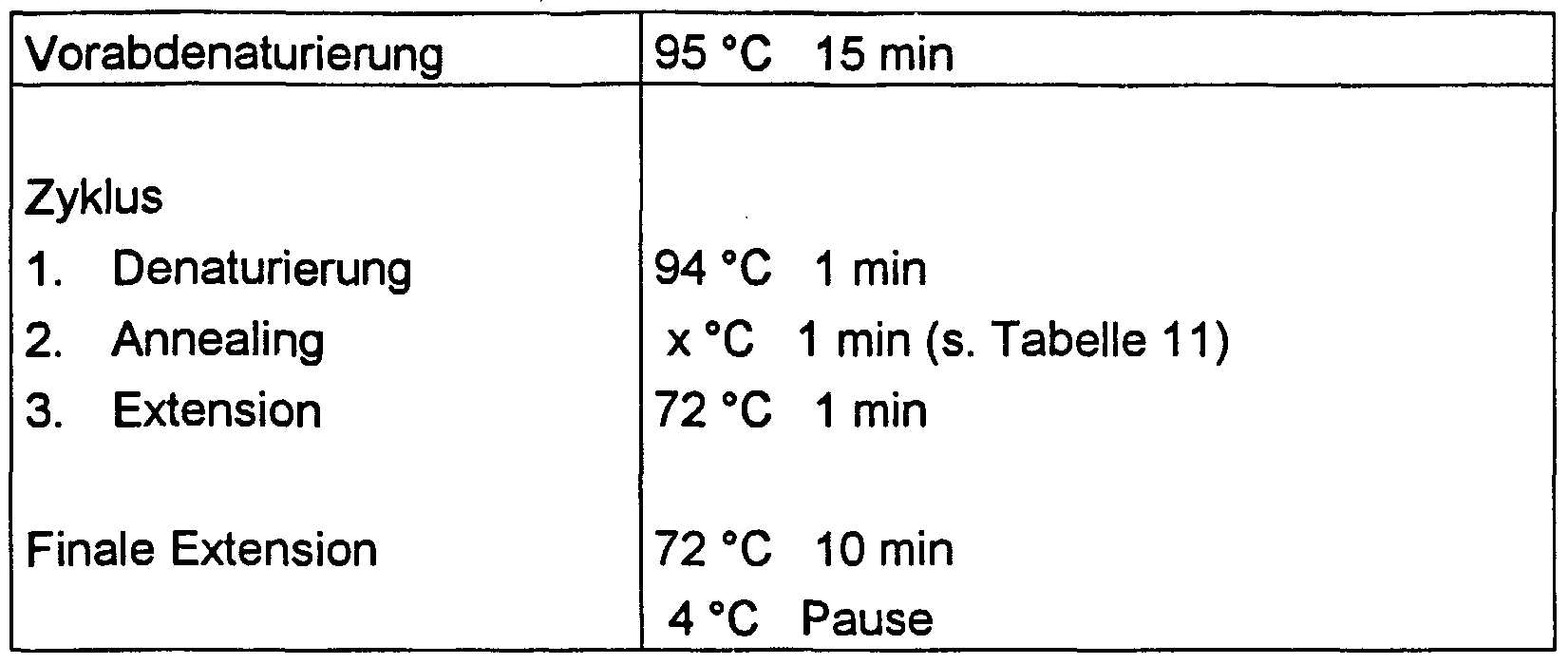
- PCR conditions (number of cycles, cycle management, etc.) are given in Tables 10 and 11.
- Table 10 PCR conditions
- a fragment analysis can also be carried out, for example, using an ABI Pris 310 genetic analyzer (PE Applied Biosystem, Rothstadt).
- ABI Pris 310 genetic analyzer PE Applied Biosystem, Rothstadt.
- 1 ⁇ l of the PCR product is used in a 1:50 dilution.
- fluorescence-labeled primers are used.
- FIGS. 1A and 1B now each show electrophoretic separations, the individual bands being labeled with the same terms here and below.
- the term “conductor” denotes calibrators with a length of 50-600 bp
- RT-Ko denotes a control that did not contain any mRNA
- PCR-Ko denotes a control measurement that did not contain any cDNA before the PCR.
- “Blood * is the blood sample without inoculated tumor cells
- 10 Z is the blood sample with 10 inoculated tumor cells per milliliter
- 100 Z is the blood sample with 100 inoculated tumor cells per milliliter or per 5 milliliter.
- Cell line * denotes a control measurement with a large number of cells from the tumor cell line in the sample.
- Results are shown in FIG. 1 when the selection in the first step with only one, two or all three of the following antibodies HMPV.2, GP1.4 and Ber-Ep 4 was performed. It can be seen immediately that the detection of the tumor marker CA 15.3 (Mucl) is only slight when using only one antibody. The best results are achieved when two of the antibodies, namely HMPV.2 and Ber-Ep 4 or GP1.4 and Ber-Ep 4 are used for detection. Already the combination of all three antibodies is more effective, as can be seen from the intensity of the bands for the tumor marker CA 15.3. This proves that with a suitable selection of a certain number of specific antibodies, a significantly improved result in the detection of tumor cells is possible is.
- FIG. 2 now shows results of detection methods in which no pre-selection by means of antibody marking was carried out in FIG. 2A and a pre-selection by means of antibody marking in FIG. 2B.
- combinations of the antibodies HMPV.2, Ber-Ep 4 and GP1.4 have been determined as a combination of two or all three antibodies.
- 10, 100 and 1000 tumor cells from a breast cancer cell line were inoculated in blood and subsequently detected. This shows that without antibody selection, background expression for some of the mRNA markers (GA 733.2, CA 15.3 and Her 2 / new) is recorded.
- FIG. 3 shows the result of a method according to the invention with tumor cells from a testicular cancer cell line inoculated in blood. Again, all tumor cells are selected from the blood sample that are marked by one of the antibodies shown. Subsequently, a total of four mRNA markers (GCAP, GA 733.2, GRPR and HGMI-C) are examined. It is shown here that when only one antibody such as Ber-Ep 4 is used, the HGMI-C marker only detects down to 10 cells per milliliter of blood and that when the MOC-31 antibody is used, no testicular cancer cells are detected at all. The same applies to the antibody 8B6, which has only a low sensitivity.
- GCAP mRNA markers
- the combination of the antibodies Ber-Ep 4 and 8B6 also leads to poor detection by means of the marker HGMI-C and also the combination of the antibodies Ber-Ep 4 and MOC-31.
- An optimal detection result for all four examined markers results testicular tumor cells using a total of three antibodies Ber-Ep 4, MOC-31 and 8B6, where down to 2 cells per milliliter of blood can be reliably detected via each of the markers. If two markers are detected according to the invention with the two detection reactions, then when two markers are selected from the markers used for FIG. 3, a reliable detection of a minimum number of cells while avoiding background detection can be carried out.
- FIG. 4 shows the detection of colon cancer cells that have been inoculated in the blood of a healthy person. This immediately shows that the use of a combination of the antibodies Ber-Ep 4 and MOC-31 leads to an improved sensitivity with regard to the mRNA marker EGF-R.
- a detection sensitivity of 100 cells per milliliter of blood is achieved, while when only one antibody is used, the sensitivity is around 1000 cells per milliliter of blood.
- Figure 5 shows an experiment in which by means of an antibody combination of Ber-Ep 4, HMPV.2 and GP1.4, which labeled with at least one of the antibodies
- Breast cancer cell lines (MCF-7 and SKBR-3) from a blood sample added.
- the antibodies Ber-Ep 4, HMPV.2 and GP1.4 were used in combination as antibodies.
- the use of the four mRNA markers GA 733.2, CA 15.3, Her 2 and Claudin 7 ensures that the breast cancer cells are recognized down to 10 cells of the individual cell lines per milliliter. An unspecific reaction in blood without breast cancer cells did not occur.
- the tumor markers GA 733.2 and CA 15.3 are more sensitive for cell line 2 (SKBR-3), while the tumor marker Her 2 is more sensitive for cell line 1 (MCF-7).
- the individual sub-types of breast cancer cells can then be distinguished from one another on the basis of the marker pattern that has occurred.
- breast cancer cells of different cell lines (MCF-7) in FIG. 7A and SKBR 3 in FIG. 7B have been inoculated in blood.
- the antibodies Ber-Ep 4, HMPV.2 and GP1.4 were used in combination as antibodies for the selection of the cells from the blood sample. It can be seen immediately that when a combination of the mRNA markers GA 733.2, CA 15.3 Her 2 and Claudin 7 is used, at least one of the markers reacts positively down to 2 cells per 5 milliliters in any case, without the blood being without tumor cells would provide expression background. Here too, a differential response of the two cell lines to the four different mRNA markers can be seen.
- the choice of marker combination would guarantee detection of both cell lines down to 2 cells per 5 milliliters of blood, ie the detection of breast cancer cells in the blood would be possible with high sensitivity regardless of the cell type of the breast cancer cell line.
- FIG. 8 shows the detection of colorectal cancer cells that have been inoculated in blood.
- the cells were selected with the two antibodies Ber-Ep 4 and MOC-31.
- the molecular biological detection step was carried out using the GA733.2, CEA and EGF-R mRNA markers. Two tumor cells were detectable in 5 milliliters of blood.
- FIG. 9 again shows a measurement with a combination of three antibodies Ber-Ep 4, MOC-31 and 8B6 and the mRNA markers GCAP / PLAP, GA 733.2, GRPR and HMGI-C on blood into which testicular cancer cells were inoculated.
- Figure 10 finally shows the separation of rare cells from the cell mixture of a biopsy material.
- biopsy material from breast tissue which contained tumor tissue with a suspected primary tumor, was mechanically separated and separated from cell debris, connective tissue etc. via gauze.
- the cell mixture obtained which contained both cells of the suspected tumor tissue and cells of the surrounding healthy tissue, was subjected to a cell selection with an antibody mixture coupled to a solid phase (magnetic particles with antibodies GP1.4, HMPV.2 and Ber-Ep 4) and after incubation magnetically separated to produce the antigen-antibody binding.
- mRNA was detected with regard to the markers GA 733.2 and Her 2.
- a cell line of a breast cancer was also determined in parallel. As can be seen, there is positive evidence that the biopsy actually contained a breast cancer tumor.
- the bands which are labeled “positive control” in FIGS. 8 and 9 show results of samples with the cell lines HT 29 for intestinal tumor (FIG. 8) or Tera / 1 for testicular tumor (FIG. 9).
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Cell Biology (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Urology & Nephrology (AREA)
- Biochemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Analytical Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Organic Chemistry (AREA)
- Oncology (AREA)
- Zoology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Hospice & Palliative Care (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
Abstract
Description
Claims
Priority Applications (16)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES02732726T ES2253533T3 (en) | 2001-09-06 | 2002-05-17 | PROCEDURE FOR QUALITATIVE AND / OR QUANTITATIVE DETECTION OF CELLS. |
| JP2003527120A JP4336198B2 (en) | 2001-09-06 | 2002-05-17 | Methods and diagnostic kits for cell selection and / or qualitative and / or quantitative detection |
| US10/488,729 US7507528B2 (en) | 2001-09-06 | 2002-05-17 | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| CA002466896A CA2466896A1 (en) | 2001-09-06 | 2002-05-17 | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| AU2002304640A AU2002304640A1 (en) | 2001-09-06 | 2002-05-17 | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| DE50204883T DE50204883D1 (en) | 2001-09-06 | 2002-05-17 | PROCESS FOR THE QUALITATIVE AND / OR QUANTITATIVE DETECTION OF CELLS |
| EP02732726A EP1409727B1 (en) | 2001-09-06 | 2002-05-17 | Method for qualitative and/or quantitative detection of cells |
| AT02732726T ATE309393T1 (en) | 2001-09-06 | 2002-05-17 | METHOD FOR THE QUALITATIVE AND/OR QUANTITATIVE DETECTION OF CELLS |
| EP02774579A EP1409746A2 (en) | 2001-09-06 | 2002-09-06 | Method and kit for diagnosing or controlling the treatment of breast cancer |
| DE50209932T DE50209932D1 (en) | 2001-09-06 | 2002-09-06 | METHOD AND KIT FOR THE DIAGNOSTIC OR TREATMENT CONTROL OF DARM CANCER |
| AT02772273T ATE359377T1 (en) | 2001-09-06 | 2002-09-06 | METHOD AND KIT FOR DIAGNOSTIC OR TREATMENT MONITORING OF COLON CANCER |
| PCT/EP2002/009999 WO2003023060A2 (en) | 2001-09-06 | 2002-09-06 | Method and kit for diagnosing or controlling the treatment of breast cancer |
| PCT/EP2002/009983 WO2003023059A2 (en) | 2001-09-06 | 2002-09-06 | Method and kit for the diagnosis or treatment control of intestinal carcinoma |
| EP02772273A EP1409745B1 (en) | 2001-09-06 | 2002-09-06 | Method and kit for the diagnosis or treatment control of intestinal carcinoma |
| US10/488,828 US20050014208A1 (en) | 2001-09-06 | 2002-09-06 | Method and kit for diagnosing or controlling the treatment of breast cancer |
| ES02772273T ES2284927T3 (en) | 2001-09-06 | 2002-09-06 | PROCEDURE AND KIT FOR DIAGNOSIS OR CONTROL OF CANCER TREATMENT. |
Applications Claiming Priority (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10143775.7 | 2001-09-06 | ||
| DE10143699.8 | 2001-09-06 | ||
| DE10143691.2 | 2001-09-06 | ||
| DE10143776.5 | 2001-09-06 | ||
| DE10143776A DE10143776A1 (en) | 2001-09-06 | 2001-09-06 | Selection and determination of specific cells, useful particularly for diagnosis and monitoring of tumors, by antibody-mediated selection then detecting specific mRNA |
| DE10143775A DE10143775A1 (en) | 2001-09-06 | 2001-09-06 | Selection and determination of specific cells, useful particularly for diagnosis and monitoring of tumors, by antibody-mediated selection then detecting specific mRNA |
| DE10143691 | 2001-09-06 | ||
| DE10143699 | 2001-09-06 | ||
| PCT/EP2001/013606 WO2003044224A1 (en) | 2001-11-22 | 2001-11-22 | Diagnosis kit, dna chip, and methods for diagnosing or supervising the treatment of testicular cancer |
| EPPCT/EP01/13606 | 2001-11-22 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2003023057A2 true WO2003023057A2 (en) | 2003-03-20 |
| WO2003023057A3 WO2003023057A3 (en) | 2003-12-18 |
Family
ID=27512423
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2002/005489 WO2003023057A2 (en) | 2001-09-06 | 2002-05-17 | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US7507528B2 (en) |
| EP (1) | EP1409727B1 (en) |
| JP (1) | JP4336198B2 (en) |
| AT (1) | ATE309393T1 (en) |
| AU (1) | AU2002304640A1 (en) |
| CA (1) | CA2466896A1 (en) |
| DE (1) | DE50204883D1 (en) |
| ES (1) | ES2253533T3 (en) |
| WO (1) | WO2003023057A2 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007515957A (en) * | 2003-12-12 | 2007-06-21 | バイエル・フアーマシユーチカルズ・コーポレーシヨン | Methods for cancer prognosis and prognosis and cancer therapy monitoring |
| EP1803822A1 (en) * | 2005-12-30 | 2007-07-04 | Adnagen AG | Method for the individual characterization of therapeutic target molecules and use thereof |
| US7507528B2 (en) | 2001-09-06 | 2009-03-24 | Adnagen Ag | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| US8137912B2 (en) | 2006-06-14 | 2012-03-20 | The General Hospital Corporation | Methods for the diagnosis of fetal abnormalities |
| US8168389B2 (en) | 2006-06-14 | 2012-05-01 | The General Hospital Corporation | Fetal cell analysis using sample splitting |
| US8195415B2 (en) | 2008-09-20 | 2012-06-05 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| EP2615180A1 (en) * | 2012-01-12 | 2013-07-17 | AdnaGen GmbH | Method for the quantification, qualitative genetic characterization and gene expression characterization of predetermined cells |
| US8585971B2 (en) | 2005-04-05 | 2013-11-19 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| US8895298B2 (en) | 2002-09-27 | 2014-11-25 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US8921102B2 (en) | 2005-07-29 | 2014-12-30 | Gpb Scientific, Llc | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US9850483B2 (en) | 2010-07-19 | 2017-12-26 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and systems for analysis of single cells |
| US10591391B2 (en) | 2006-06-14 | 2020-03-17 | Verinata Health, Inc. | Diagnosis of fetal abnormalities using polymorphisms including short tandem repeats |
| US10704090B2 (en) | 2006-06-14 | 2020-07-07 | Verinata Health, Inc. | Fetal aneuploidy detection by sequencing |
Families Citing this family (69)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6692952B1 (en) * | 1999-11-10 | 2004-02-17 | Massachusetts Institute Of Technology | Cell analysis and sorting apparatus for manipulation of cells |
| DE10143775A1 (en) * | 2001-09-06 | 2003-04-10 | Adnagen Ag | Selection and determination of specific cells, useful particularly for diagnosis and monitoring of tumors, by antibody-mediated selection then detecting specific mRNA |
| EP1604184A4 (en) * | 2003-02-27 | 2010-10-27 | Stephen A Lesko | Standardized evaluation of therapeutic efficacy based on cellular biomarkers |
| CA2529285A1 (en) * | 2003-06-13 | 2004-12-29 | The General Hospital Corporation | Microfluidic systems for size based removal of red blood cells and platelets from blood |
| JP2007533305A (en) * | 2004-03-03 | 2007-11-22 | ザ ジェネラル ホスピタル コーポレーション | Magnetic apparatus for isolating cells and biomolecules in a microfluidic environment |
| US20070026417A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| WO2006108087A2 (en) * | 2005-04-05 | 2006-10-12 | Cellpoint Diagnostics | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026414A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026413A1 (en) * | 2005-07-29 | 2007-02-01 | Mehmet Toner | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026415A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20090181421A1 (en) * | 2005-07-29 | 2009-07-16 | Ravi Kapur | Diagnosis of fetal abnormalities using nucleated red blood cells |
| US20070059680A1 (en) * | 2005-09-15 | 2007-03-15 | Ravi Kapur | System for cell enrichment |
| US20070026416A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US9957569B2 (en) | 2005-09-12 | 2018-05-01 | The Regents Of The University Of Michigan | Recurrent gene fusions in prostate cancer |
| CA2814598A1 (en) | 2005-09-12 | 2007-03-22 | The Regents Of The University Of Michigan | Recurrent gene fusions in prostate cancer |
| US20070059781A1 (en) * | 2005-09-15 | 2007-03-15 | Ravi Kapur | System for size based separation and analysis |
| US20070059716A1 (en) * | 2005-09-15 | 2007-03-15 | Ulysses Balis | Methods for detecting fetal abnormality |
| US20070059718A1 (en) * | 2005-09-15 | 2007-03-15 | Mehmet Toner | Systems and methods for enrichment of analytes |
| US20070059719A1 (en) * | 2005-09-15 | 2007-03-15 | Michael Grisham | Business methods for prenatal Diagnosis |
| US20070059774A1 (en) * | 2005-09-15 | 2007-03-15 | Michael Grisham | Kits for Prenatal Testing |
| US20070059683A1 (en) * | 2005-09-15 | 2007-03-15 | Tom Barber | Veterinary diagnostic system |
| WO2008111990A1 (en) * | 2006-06-14 | 2008-09-18 | Cellpoint Diagnostics, Inc. | Rare cell analysis using sample splitting and dna tags |
| CN101971033A (en) * | 2007-06-04 | 2011-02-09 | 戴诺普雷克斯公司 | Biomarker combinations for colorectal cancer |
| EP3018216B1 (en) | 2007-07-06 | 2018-09-12 | The Regents Of The University Of Michigan | Recurrent gene fusions in prostate cancer |
| ATE533861T1 (en) | 2007-07-06 | 2011-12-15 | Univ Michigan | MIPOL1-ETV1 GENE ARRANGEMENTS |
| EP2037265A1 (en) | 2007-09-17 | 2009-03-18 | Adnagen AG | Solid phase cell isolation and/or enrichment method |
| CA2725978A1 (en) | 2008-05-28 | 2009-12-03 | Genomedx Biosciences, Inc. | Systems and methods for expression-based discrimination of distinct clinical disease states in prostate cancer |
| WO2010081001A2 (en) | 2009-01-09 | 2010-07-15 | The Regents Of The University Of Michigan | Recurrent gene fusions in cancer |
| US10184937B2 (en) * | 2009-03-31 | 2019-01-22 | United Arab Emirates University | Minimally invasive assessment of IgE mediated allergy |
| CA2769219A1 (en) | 2009-08-04 | 2011-02-10 | E.I. Du Pont De Nemours And Company | Process and device for collecting nucleic acids of microorganisms from a particulate sample |
| JP5800817B2 (en) | 2009-09-17 | 2015-10-28 | ザ リージェンツ オブ ザ ユニバーシティ オブ ミシガン | Recurrent gene fusion in prostate cancer |
| JP5234666B2 (en) * | 2009-12-11 | 2013-07-10 | 国立大学法人大阪大学 | Measurement method for the determination of psoriasis |
| US8187979B2 (en) * | 2009-12-23 | 2012-05-29 | Varian Semiconductor Equipment Associates, Inc. | Workpiece patterning with plasma sheath modulation |
| WO2012068383A2 (en) | 2010-11-19 | 2012-05-24 | The Regents Of The University Of Michigan | ncRNA AND USES THEREOF |
| US8945556B2 (en) | 2010-11-19 | 2015-02-03 | The Regents Of The University Of Michigan | RAF gene fusions |
| CN102565404B (en) * | 2010-12-24 | 2014-04-16 | 牛刚 | Kit for detecting free colorectal cancer cell markers in blood |
| CN102109525B (en) * | 2011-01-26 | 2013-10-16 | 牛刚 | Kit for detecting free breast cancer cell marker in blood |
| CA2842359A1 (en) | 2011-08-01 | 2013-02-07 | Denovo Sciences | Cell capture system and method of use |
| US9404864B2 (en) | 2013-03-13 | 2016-08-02 | Denovo Sciences, Inc. | System for imaging captured cells |
| US10466160B2 (en) | 2011-08-01 | 2019-11-05 | Celsee Diagnostics, Inc. | System and method for retrieving and analyzing particles |
| US9174216B2 (en) | 2013-03-13 | 2015-11-03 | DeNovo Science, Inc. | System for capturing and analyzing cells |
| JP2014533100A (en) | 2011-11-04 | 2014-12-11 | オスロ ウニヴェルスィテーツスィーケフース ハーエフOslo Universitetssykehus Hf | Methods and biomarkers for the analysis of colorectal cancer |
| EP2773757B1 (en) | 2011-11-04 | 2019-01-09 | Gen-Probe Incorporated | Molecular assay reagents and methods |
| US20130189679A1 (en) | 2011-12-20 | 2013-07-25 | The Regents Of The University Of Michigan | Pseudogenes and uses thereof |
| EP2802673B1 (en) | 2012-01-09 | 2019-07-03 | Oslo Universitetssykehus HF | Methods and biomarkers for analysis of colorectal cancer |
| EP2817627A2 (en) | 2012-02-21 | 2014-12-31 | Oslo Universitetssykehus HF | Methods and biomarkers for detection and prognosis of cervical cancer |
| CA2881627A1 (en) | 2012-08-16 | 2014-02-20 | Genomedx Biosciences Inc. | Cancer diagnostics using biomarkers |
| RU2522923C1 (en) * | 2012-10-23 | 2014-07-20 | Федеральное Государственное Автономное Образовательное Учреждение Высшего Профессионального Образования "Московский Физико-Технический Институт (Государственный Университет)" | Integrated method for detecting circulating tumour cells in blood of patients suffering from breast cancer |
| US9606102B2 (en) | 2013-01-26 | 2017-03-28 | Denovo Sciences, Inc. | System and method for capturing and analyzing cells |
| JP6673698B2 (en) | 2013-02-15 | 2020-03-25 | エクソサム ダイアグノスティクス,インコーポレイティド | Novel EGFR variants |
| US9707562B2 (en) | 2013-03-13 | 2017-07-18 | Denovo Sciences, Inc. | System for capturing and analyzing cells |
| US10391490B2 (en) | 2013-05-31 | 2019-08-27 | Celsee Diagnostics, Inc. | System and method for isolating and analyzing cells |
| US9856535B2 (en) | 2013-05-31 | 2018-01-02 | Denovo Sciences, Inc. | System for isolating cells |
| WO2015107430A2 (en) | 2014-01-16 | 2015-07-23 | Oslo Universitetssykehus Hf | Methods and biomarkers for detection and prognosis of cervical cancer |
| EP3151733B1 (en) | 2014-06-06 | 2020-04-15 | The Regents Of The University Of Michigan | Compositions and methods for characterizing and diagnosing periodontal disease |
| US20170233826A1 (en) * | 2014-09-18 | 2017-08-17 | Adnagen Gmbh | Mrna and/or protein of ercc1 isoform 3 for use in diagnosing a resistance against a therapeutic agent and method for diagnosing a resistance against a therapeutic agent using said mrna and/or protein |
| CN110506127B (en) | 2016-08-24 | 2024-01-12 | 维拉科特Sd公司 | Use of genomic tags to predict responsiveness of prostate cancer patients to post-operative radiation therapy |
| WO2018127786A1 (en) | 2017-01-06 | 2018-07-12 | Oslo Universitetssykehus Hf | Compositions and methods for determining a treatment course of action |
| US11208697B2 (en) | 2017-01-20 | 2021-12-28 | Decipher Biosciences, Inc. | Molecular subtyping, prognosis, and treatment of bladder cancer |
| US11873532B2 (en) | 2017-03-09 | 2024-01-16 | Decipher Biosciences, Inc. | Subtyping prostate cancer to predict response to hormone therapy |
| AU2018266733A1 (en) | 2017-05-12 | 2020-01-16 | Veracyte, Inc. | Genetic signatures to predict prostate cancer metastasis and identify tumor aggressiveness |
| WO2019046307A1 (en) | 2017-08-29 | 2019-03-07 | Celsee Diagnostics, Inc. | System and method for isolating and analyzing cells |
| CN107843731B (en) * | 2017-09-14 | 2020-01-03 | 北京牛牛基因技术有限公司 | Kit for detecting pancreatic cancer cell markers in peripheral blood |
| US10633693B1 (en) | 2019-04-16 | 2020-04-28 | Celsee Diagnostics, Inc. | System and method for leakage control in a particle capture system |
| WO2020227309A1 (en) | 2019-05-07 | 2020-11-12 | Bio-Rad Laboratories, Inc. | System and method for automated single cell processing |
| US11273439B2 (en) | 2019-05-07 | 2022-03-15 | Bio-Rad Laboratories, Inc. | System and method for target material retrieval from microwells |
| JP7356519B2 (en) | 2019-06-14 | 2023-10-04 | バイオ-ラッド ラボラトリーズ インコーポレイテッド | Systems and methods for automated single cell processing and analysis |
| US11504719B2 (en) | 2020-03-12 | 2022-11-22 | Bio-Rad Laboratories, Inc. | System and method for receiving and delivering a fluid for sample processing |
| WO2023021330A1 (en) | 2021-08-16 | 2023-02-23 | University Of Oslo | Compositions and methods for determining a treatment course of action |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994007139A1 (en) * | 1992-09-14 | 1994-03-31 | Fodstad Oystein | Improved method for detection of specific target cells in specialized or mixed cell population and solutions containing mixed cell populations |
| WO1996029430A1 (en) * | 1995-03-17 | 1996-09-26 | John Wayne Cancer Institute | Detection of melanoma or breast metastases with a multiple marker assay |
| WO1997037226A1 (en) * | 1996-03-29 | 1997-10-09 | Mayo Foundation For Medical Education And Research | Methods of recovering colorectal epithelial cells or fragments thereof from stool |
| WO1998012227A1 (en) * | 1996-09-19 | 1998-03-26 | Diagnocure Inc. | Recombinant single chain antibodies directed against the gp54 cancer marker, composition comprising same and use thereof |
| DE19736691A1 (en) * | 1997-08-22 | 1999-02-25 | Michael Prof Dr Med Giesing | Characterising and identifying disseminated metastatic cancer cells |
| WO1999044064A1 (en) * | 1998-02-27 | 1999-09-02 | Cli Oncology, Inc. | Method and compositions for differential detection of primary tumor cells and metastatic cells |
| US6187546B1 (en) * | 1995-09-06 | 2001-02-13 | O'neill Ian Kenneth | Method of isolating cells |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU943280A1 (en) | 1978-05-29 | 1982-07-15 | Институт биоорганической химии им.М.М.Шемякина | Process for preserving immobilized enzymes |
| JPS61189454A (en) | 1985-02-18 | 1986-08-23 | Fujisawa Pharmaceut Co Ltd | Stabilized immobilizing antibody |
| DE3811659C1 (en) | 1988-04-07 | 1989-11-16 | Henning Berlin Gmbh Chemie- Und Pharmawerk, 1000 Berlin, De | |
| DD298055A5 (en) | 1989-09-14 | 1992-02-06 | ����������@�����������@���Kk�� | METHOD AND NUTRITIONAL APPARATUS FOR PREPARING PHARMACEUTICAL COMPOSITIONS |
| AU662906B2 (en) | 1991-06-26 | 1995-09-21 | F. Hoffmann-La Roche Ag | Methods for detection of carcinoma metastases by nucleic acid amplification |
| US6165467A (en) | 1991-07-20 | 2000-12-26 | Yoshihide Hagiwara | Stabilized human monoclonal antibody preparation |
| WO1996026011A1 (en) | 1995-02-21 | 1996-08-29 | Siddiqi Iqbal W | Apparatus and method for mixing and separation employing magnetic particles |
| WO1997007242A1 (en) | 1995-08-16 | 1997-02-27 | Steven Lehrer | Method for detecting circulating breast cancer cells |
| US6329179B1 (en) | 1996-03-26 | 2001-12-11 | Oncomedx, Inc. | Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer |
| AU2438497A (en) | 1996-04-05 | 1997-10-29 | Johns Hopkins University, The | A method of enriching rare cells |
| JP2000254472A (en) | 1999-03-15 | 2000-09-19 | Toshiba Corp | Device and method for agitating |
| US6558929B2 (en) * | 2000-09-15 | 2003-05-06 | Fraunhofer-Gesellschaft Zur Forderung Der Angewandten Forshung E.V. | PCR reaction mixture for fluorescence-based gene expression and gene mutation analyses |
| EP1409745B1 (en) | 2001-09-06 | 2007-04-11 | Adnagen AG | Method and kit for the diagnosis or treatment control of intestinal carcinoma |
| WO2003023057A2 (en) | 2001-09-06 | 2003-03-20 | Adnagen Ag | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| EP1409746A2 (en) | 2001-09-06 | 2004-04-21 | Adnagen AG | Method and kit for diagnosing or controlling the treatment of breast cancer |
| EP1448792A1 (en) | 2001-11-22 | 2004-08-25 | Adnagen AG | Diagnosis kit, dna chip, and methods for diagnosing or supervising the treatment of testicular cancer |
-
2002
- 2002-05-17 WO PCT/EP2002/005489 patent/WO2003023057A2/en active IP Right Grant
- 2002-05-17 CA CA002466896A patent/CA2466896A1/en not_active Abandoned
- 2002-05-17 DE DE50204883T patent/DE50204883D1/en not_active Expired - Lifetime
- 2002-05-17 EP EP02732726A patent/EP1409727B1/en not_active Expired - Lifetime
- 2002-05-17 JP JP2003527120A patent/JP4336198B2/en not_active Expired - Lifetime
- 2002-05-17 ES ES02732726T patent/ES2253533T3/en not_active Expired - Lifetime
- 2002-05-17 AT AT02732726T patent/ATE309393T1/en active
- 2002-05-17 AU AU2002304640A patent/AU2002304640A1/en not_active Abandoned
- 2002-05-17 US US10/488,729 patent/US7507528B2/en not_active Expired - Lifetime
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994007139A1 (en) * | 1992-09-14 | 1994-03-31 | Fodstad Oystein | Improved method for detection of specific target cells in specialized or mixed cell population and solutions containing mixed cell populations |
| WO1996029430A1 (en) * | 1995-03-17 | 1996-09-26 | John Wayne Cancer Institute | Detection of melanoma or breast metastases with a multiple marker assay |
| US6187546B1 (en) * | 1995-09-06 | 2001-02-13 | O'neill Ian Kenneth | Method of isolating cells |
| WO1997037226A1 (en) * | 1996-03-29 | 1997-10-09 | Mayo Foundation For Medical Education And Research | Methods of recovering colorectal epithelial cells or fragments thereof from stool |
| WO1998012227A1 (en) * | 1996-09-19 | 1998-03-26 | Diagnocure Inc. | Recombinant single chain antibodies directed against the gp54 cancer marker, composition comprising same and use thereof |
| DE19736691A1 (en) * | 1997-08-22 | 1999-02-25 | Michael Prof Dr Med Giesing | Characterising and identifying disseminated metastatic cancer cells |
| WO1999044064A1 (en) * | 1998-02-27 | 1999-09-02 | Cli Oncology, Inc. | Method and compositions for differential detection of primary tumor cells and metastatic cells |
Non-Patent Citations (7)
| Title |
|---|
| CHARPENTIER A H ET AL: "STC2 AND E2IG1, TWO NOVEL BREAST CANCER BIOMARKERS" PROCEEDINGS OF THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR CANCER RESEARCH, NEW YORK, NY, US, Bd. 42, Nr. 628, M{rz 2001 (2001-03), Seite 628 XP001119829 ISSN: 0197-016X * |
| CHRYSOGELOS S A ET AL: "EGF RECEPTOR EXPRESSION, REGULATION, AND FUNCTION IN BREAST CANCER" BREAST CANCER RESEARCH AND TREATMENT, NIJHOFF, BOSTON, US, Bd. 29, Nr. 1, 1994, Seiten 29-40, XP001118068 ISSN: 0167-6806 * |
| FUJIWARA Y ET AL: "ASSESSMENT OF STANNIOCALCIN-1 MRNA AS A MOLECULAR MARKER FOR MICROMETASTASES OF VARIOUS HUMAN CANCERS" INTERNATIONAL JOURNAL OF ONCOLOGY, EDITORIAL ACADEMY OF THE INTERNATIONAL JOURNAL OF ONCOLOGY,, GR, Bd. 16, April 2000 (2000-04), Seiten 799-804, XP002938551 ISSN: 1019-6439 * |
| HARDINGHAM J E ET AL: "IMMUNOBEAD-PCR: A TECHNIQUE FOR THE DETECTION OF CIRCULATING TUMOR CELLS USING IMMUNOMAGNETIC BEADS AND THE POLYMERASE CHAIN REACTION" CANCER RESEARCH, AMERICAN ASSOCIATION FOR CANCER RESEARCH, BALTIMORE, MD, US, Bd. 53, 1. August 1993 (1993-08-01), Seiten 3455-3458, XP000605489 ISSN: 0008-5472 * |
| NACHT MARIANA ET AL: "Combining serial analysis of gene expression and array technologies to identify genes differentially expressed in breast cancer" CANCER RESEARCH, AMERICAN ASSOCIATION FOR CANCER RESEARCH, BALTIMORE, MD, US, Bd. 59, Nr. 21, 1. November 1999 (1999-11-01), Seiten 5464-5470, XP002166291 ISSN: 0008-5472 * |
| REN-HEIDENREICH L ET AL: "SPECIFIC TARGETING OF EGP-2+ TUMOR CELLS BY PRIMARY LYMPHOCYTES MODIFIED WITH CHIMERIC T CELL RECEPTORS" HUMAN GENE THERAPY, XX, XX, Bd. 11, Nr. 1, 1. Januar 2000 (2000-01-01), Seiten 9-19, XP000944357 ISSN: 1043-0342 * |
| WATANABE S ET AL: "EXPRESSION OF THE GERM CELL ALKALINE PHOSPHATASE GENE IN HUMAN CHORIOCARCINOMA CELLS" JOURNAL OF BIOLOGICAL CHEMISTRY, AMERICAN SOCIETY OF BIOLOGICAL CHEMISTS, BALTIMORE, MD, US, Bd. 264, Nr. 21, 25. Juli 1989 (1989-07-25), Seiten 12611-12619, XP002225508 ISSN: 0021-9258 * |
Cited By (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7507528B2 (en) | 2001-09-06 | 2009-03-24 | Adnagen Ag | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| US11052392B2 (en) | 2002-09-27 | 2021-07-06 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US10081014B2 (en) | 2002-09-27 | 2018-09-25 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US8895298B2 (en) | 2002-09-27 | 2014-11-25 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| JP2007515957A (en) * | 2003-12-12 | 2007-06-21 | バイエル・フアーマシユーチカルズ・コーポレーシヨン | Methods for cancer prognosis and prognosis and cancer therapy monitoring |
| US8585971B2 (en) | 2005-04-05 | 2013-11-19 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| US10786817B2 (en) | 2005-04-05 | 2020-09-29 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| US9956562B2 (en) | 2005-04-05 | 2018-05-01 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| US8921102B2 (en) | 2005-07-29 | 2014-12-30 | Gpb Scientific, Llc | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| EP1803822A1 (en) * | 2005-12-30 | 2007-07-04 | Adnagen AG | Method for the individual characterization of therapeutic target molecules and use thereof |
| WO2007076989A1 (en) * | 2005-12-30 | 2007-07-12 | Adnagen Ag | Method for the individual characterization of therapeutic target molecules and use thereof |
| US8372584B2 (en) | 2006-06-14 | 2013-02-12 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US10591391B2 (en) | 2006-06-14 | 2020-03-17 | Verinata Health, Inc. | Diagnosis of fetal abnormalities using polymorphisms including short tandem repeats |
| US11781187B2 (en) | 2006-06-14 | 2023-10-10 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US11674176B2 (en) | 2006-06-14 | 2023-06-13 | Verinata Health, Inc | Fetal aneuploidy detection by sequencing |
| US9017942B2 (en) | 2006-06-14 | 2015-04-28 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US9273355B2 (en) | 2006-06-14 | 2016-03-01 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US9347100B2 (en) | 2006-06-14 | 2016-05-24 | Gpb Scientific, Llc | Rare cell analysis using sample splitting and DNA tags |
| US11261492B2 (en) | 2006-06-14 | 2022-03-01 | The General Hospital Corporation | Methods for the diagnosis of fetal abnormalities |
| US8137912B2 (en) | 2006-06-14 | 2012-03-20 | The General Hospital Corporation | Methods for the diagnosis of fetal abnormalities |
| US8168389B2 (en) | 2006-06-14 | 2012-05-01 | The General Hospital Corporation | Fetal cell analysis using sample splitting |
| US10704090B2 (en) | 2006-06-14 | 2020-07-07 | Verinata Health, Inc. | Fetal aneuploidy detection by sequencing |
| US10435751B2 (en) | 2006-06-14 | 2019-10-08 | Verinata Health, Inc. | Methods for the diagnosis of fetal abnormalities |
| US10041119B2 (en) | 2006-06-14 | 2018-08-07 | Verinata Health, Inc. | Methods for the diagnosis of fetal abnormalities |
| US10155984B2 (en) | 2006-06-14 | 2018-12-18 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US8195415B2 (en) | 2008-09-20 | 2012-06-05 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US8682594B2 (en) | 2008-09-20 | 2014-03-25 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US10669585B2 (en) | 2008-09-20 | 2020-06-02 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US8296076B2 (en) | 2008-09-20 | 2012-10-23 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuoploidy by sequencing |
| US9404157B2 (en) | 2008-09-20 | 2016-08-02 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US9353414B2 (en) | 2008-09-20 | 2016-05-31 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US9850483B2 (en) | 2010-07-19 | 2017-12-26 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and systems for analysis of single cells |
| US10006090B2 (en) | 2012-01-12 | 2018-06-26 | Adnagen Gmbh | Method for the quantification, qualitative genetic characterization and gene expression characterization of predetermined cells |
| EP2615180A1 (en) * | 2012-01-12 | 2013-07-17 | AdnaGen GmbH | Method for the quantification, qualitative genetic characterization and gene expression characterization of predetermined cells |
| WO2013104599A1 (en) * | 2012-01-12 | 2013-07-18 | Adnagen Gmbh | Method for the quantification, qualitative genetic characterization and gene expression characterization of predetermined cells |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2253533T3 (en) | 2006-06-01 |
| US7507528B2 (en) | 2009-03-24 |
| US20050042685A1 (en) | 2005-02-24 |
| EP1409727A2 (en) | 2004-04-21 |
| JP4336198B2 (en) | 2009-09-30 |
| JP2005502867A (en) | 2005-01-27 |
| ATE309393T1 (en) | 2005-11-15 |
| EP1409727B1 (en) | 2005-11-09 |
| WO2003023057A3 (en) | 2003-12-18 |
| DE50204883D1 (en) | 2005-12-15 |
| AU2002304640A1 (en) | 2003-03-24 |
| CA2466896A1 (en) | 2003-03-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1409727B1 (en) | Method for qualitative and/or quantitative detection of cells | |
| DE10143776A1 (en) | Selection and determination of specific cells, useful particularly for diagnosis and monitoring of tumors, by antibody-mediated selection then detecting specific mRNA | |
| DE60200248T2 (en) | Procedure for solution-based diagnosis | |
| EP1262776B1 (en) | Method for the quantitative detection of vital epithelial tumour cells in a body fluid | |
| DE69635377T3 (en) | EFFICIENT ENHANCEMENT AND DETECTION OF SOLID TUMOR CELLS | |
| CN101036055B (en) | Detection of elevated levels of Her-2/neu protein on circulating cancer cells and treatment | |
| EP0360822A1 (en) | Process for investigating an acellular biological fluid for cellular oncogenic transcripts or fragments thereof | |
| WO2003044224A1 (en) | Diagnosis kit, dna chip, and methods for diagnosing or supervising the treatment of testicular cancer | |
| EP1409745B1 (en) | Method and kit for the diagnosis or treatment control of intestinal carcinoma | |
| JP2024023284A (en) | Methods of using giant cell nucleic acid characterization in cancer screening, diagnostics, treatment and recurrence | |
| WO2003023060A2 (en) | Method and kit for diagnosing or controlling the treatment of breast cancer | |
| DE69822149T2 (en) | DETECTION OF TMORPECIFIC GENE PRODUCTS | |
| DE19859912C2 (en) | Test system for the detection of different markers, its production and use | |
| EP1529206A1 (en) | Method for the immunocytological or molecular detection of disseminated tumor cells in a body fluid and kit that is suitable therefor | |
| DE60031521T2 (en) | PRODUCTS AND METHODS FOR SINGLE-VALUE AND MULTI-VALUE PHENOTYPING OF CELLS | |
| US20060078949A1 (en) | Flow cytometry based micronucleus assays and kits | |
| EP0563635A1 (en) | Method for detecting micrometastases in mesenchymal tissue | |
| DE10217102B4 (en) | Method for the characterization of primary tumors | |
| DE10143775A1 (en) | Selection and determination of specific cells, useful particularly for diagnosis and monitoring of tumors, by antibody-mediated selection then detecting specific mRNA | |
| WO2023053574A1 (en) | Method for enriching cells or cell nuclei | |
| DE60216045T2 (en) | CANCER DIAGNOSTIC PROCEDURE | |
| WO2007028380A1 (en) | Functional in vitro immunoassay | |
| WO2002065889A1 (en) | Method for examining cell and tissue samples | |
| Heiba | Rapid Detection of Bcr-Abl Fusion Proteins by Immunobead Assay Flow Cytometry in Leukemia Patients | |
| DE102011053741A1 (en) | A method of verifying self-healing by the immune system of a human papillomavirus infected human |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BY BZ CA CH CN CO CR CU CZ DE DM DZ EC EE ES FI GB GD GE GH HR HU ID IL IN IS JP KE KG KP KR LC LK LR LS LT LU LV MA MD MG MN MW MX MZ NO NZ OM PH PL PT RU SD SE SG SI SK SL TJ TM TN TR TZ UA UG US UZ VN YU ZA ZM |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): GH GM KE LS MW MZ SD SL SZ UG ZM ZW AM AZ BY KG KZ RU TJ TM AT BE CH CY DE DK FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GQ ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2002732726 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2003527120 Country of ref document: JP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2002732726 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2466896 Country of ref document: CA |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10488729 Country of ref document: US |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2002732726 Country of ref document: EP |