Neue substituierte Phenyloxazolidon-DerivateNew substituted phenyloxazolidone derivatives
Die vorliegende Erfindung betrifft neue substituierte Phenyloxazolidon-Derivate, Verfahren zu ihrer Herstellung, sie umfassende pharmazeutische Zusammen- Setzungen sowie ihre Verwendung zur Herstellung von Arzneimitteln, insbesondere zur Herstellung von antibakteriellen Arzneimitteln zur Behandlung von Menschen und Tieren.The present invention relates to new substituted phenyloxazolidone derivatives, processes for their preparation, pharmaceutical compositions comprising them and their use for the production of medicaments, in particular for the production of antibacterial medicaments for the treatment of humans and animals.
Antibakteriell wirksame Oxazolidon-Derivate, die am Stickstoff des Oxazolidon- gerüstes eine substituierte Phenylgruppe aufweisen, sind im Stand der Technik bekannt (vgl. WO 96/23788, WO 93/09103, WO 93/23384, WO 95/14684, WO 94/13649, WO 95/07271, WO 97/09328, WO 97/21708, WO 97/30981, EP-A-0 127 902, EP-A-0 184 170, EP-A-0 316 594, EP-A-0 311 090,Antibacterial oxazolidone derivatives which have a substituted phenyl group on the nitrogen of the oxazolidone skeleton are known in the prior art (cf. WO 96/23788, WO 93/09103, WO 93/23384, WO 95/14684, WO 94 / 13649, WO 95/07271, WO 97/09328, WO 97/21708, WO 97/30981, EP-A-0 127 902, EP-A-0 184 170, EP-A-0 316 594, EP-A- 0 311 090,
EP-A-0 352 781 sowie WO 97/30995). Diese in 3-Stellung durch substituierte Phenylgruppen substituierte Oxazolidone weisen in der 5 -Stellung z. B. einenEP-A-0 352 781 and WO 97/30995). These substituted in the 3-position by substituted phenyl groups oxazolidones have in the 5-position z. B. one
Carbonylaminomethylsubstituenten auf, der am Carbonylkohlenstoffatom weitere Substituenten tragen kann. Die Erfinder der vorliegenden Erfindung stellten sich die Aufgabe, neue phenylsubstituierte Oxazolidon-Derivate mit antibakterieller Wirksamkeit zu finden, und es gelang ihnen, neue thiocarbonylaminomethyl-substituierte Oxazolidone mit substituierten Phenylresten in der 3 -Position des Oxazolidons zu finden, die über eine außerordentlich starke antibakterielle Wirksamkeit verfügen.Carbonylaminomethyl substituents, which can carry further substituents on the carbonyl carbon atom. The inventors of the present invention set themselves the task of finding new phenyl-substituted oxazolidone derivatives with antibacterial activity, and they succeeded in finding new thiocarbonylaminomethyl-substituted oxazolidones with substituted phenyl radicals in the 3-position of the oxazolidone, which have an extraordinarily strong antibacterial Effectiveness.
Gegenstand der vorliegenden Erfindung sind daher Verbindungen der allgemeinen Formel (I)
woπnThe present invention therefore relates to compounds of the general formula (I) woπn
R1 -OR4, worin R4 (C,-C8)AIkyl oder (C3-C8)Cycloalkyl ist, oderR 1 -OR 4 , wherein R 4 is (C, -C 8 ) alkyl or (C 3 -C 8 ) cycloalkyl, or
-NR5R6 ist, worin R5 und R6 gleich oder verschieden sind und Wasserstoff, Phenyl, Pyridyl oder (C,-C8)Alkyl, das gegebenenfalls über N-gebundenes Morpholin substituiert ist, bedeuten,-NR 5 R 6 , in which R 5 and R 6 are identical or different and are hydrogen, phenyl, pyridyl or (C, -C 8 ) alkyl, which is optionally substituted by N-linked morpholine,
R2 Wasserstoff, (C,-C4)Alkyl, Hydroxy oder Halogen ist,R 2 is hydrogen, (C, -C 4 ) alkyl, hydroxy or halogen,
R3 eine gesättigte, ungesättigte und/oder aromatische, gegebenfalls kondensierte und/oder substituierte, carbomono-, bi- oder tricyclische Gruppe oder eine gesättigte , ungesättigte und/oder aromatische, gegebenenfalls kondensierte und/oder substituierte, heteromono-, heterobi- oder heterotricyclische Gruppe ist, oderR 3 is a saturated, unsaturated and / or aromatic, optionally condensed and / or substituted, carbomono-, bi- or tricyclic group or a saturated, unsaturated and / or aromatic, optionally condensed and / or substituted, heteromono-, heterobi- or heterotricyclic Group is, or
OO
R3 — C— R7 , worin R7 Wasserstoff, (C,-C4)Alkyl oder (C3-C8)-Cycloalkyl,R 3 - C - R 7 , in which R 7 is hydrogen, (C, -C 4 ) alkyl or (C 3 -C 8 ) cycloalkyl,
oder — c — R7 ist > worin R7 wie oben definiert ist, und R8 -NR7 R9, worin R7' unabhängig wie R7 oben definiert und R9 Wasserstoff, (C,-C4)Alkyl oderor - c - R 7 i st > wherein R 7 is as defined above, and R 8 -NR 7 R 9 , wherein R 7 'is independently as R 7 defined above and R 9 is hydrogen, (C, -C 4 ) alkyl or
(C3-C8)-Cycloalkyl ist, oder -OR7' ist, worin R7 wie oben definiert ist, oder
Halogen,Is (C 3 -C 8 ) cycloalkyl, or is -OR 7 ' wherein R 7 is as defined above, or Halogen,
(C2-C5)Alkinyl,(C 2 -C 5 ) alkynyl,
5 worin R7 wie oben definiert ist, 5 wherein R 7 is as defined above,
, worin R7 und R wie oben definiert sind, und R" Wasserstoff
oder (C,-C4)Alkyl ist,wherein R 7 and R are as defined above and R "is hydrogen or (C, -C 4 ) alkyl,
, worin R7, R9 und Ru wie oben definiert sind,
, wherein R 7 , R 9 and R u are as defined above,
, worin R9 wie oben definiert ist und R12 und R13 unabhängig voneinander Wasserstoff oder (C1-C4)Alkyl sind oder gemeinsam eine (C2- C3)Alkandiylgruppe bilden, , wherein R 9 is as defined above and R 12 and R 13 are independently hydrogen or (C 1 -C 4 ) alkyl or together form a (C 2 - C 3 ) alkanediyl group,
, worin R7 und R9 wie oben definiert sind, und R15 und R16 unabhängig voneinander Wasserstoff, (C,-C4)Alkyl oder (C3-C8)Cyclo-alkyl sind, oder
R3
worin R" wie oben definiert ist, R'7 und R18 unabhängig voneinander Wasserstoff, (C,-C4)Alkyl, -NO2 oder -CN sind, oder , wherein R 7 and R 9 are as defined above, and R 15 and R 16 are independently hydrogen, (C, -C 4 ) alkyl or (C 3 -C 8 ) cycloalkyl, or R 3 wherein R "is as defined above, R ' 7 and R 18 are independently hydrogen, (C, -C 4 ) alkyl, -NO 2 or -CN, or
R2 und R3 gemeinsam eine Gruppe der FormelR 2 and R 3 together form a group of the formula
' bilden, worin m eine ganze Zahl von 1 bis 3 ist,
'where m is an integer from 1 to 3,
X -CH2-, -O-, -S- oder -NR" ist, worin R11 wie oben definiert ist, undX is -CH 2 -, -O-, -S- or -NR ", wherein R 11 is as defined above, and
R19 und R20 beide Wasserstoff, Wasserstoff und Hydroxy, Wasserstoff und -N(R,8)2 oder zusammen =O, =NOH, =NOR21, worin R21 (Cr R 19 and R 20 both hydrogen, hydrogen and hydroxy, hydrogen and -N (R , 8 ) 2 or together = O, = NOH, = NOR 21 , where R 21 (C r
OO
II 22 II 22
C4)Alkyl ist, =N— O— C— R j worin R22 Wasserstoff, (C,- C4)Alkyl, (C2-C4)-Alkenyl, (C3-C4)Cycloalkyl oder -OR21 ist, worinC 4) alkyl, = N- O- C- R j wor i n R 22 is hydrogen, (C, - C 4) alkyl, (C 2 -C 4) alkenyl, (C 3 -C 4) cycloalkyl, or -OR 21 is where
R21 wie oben definiert ist, oderR 21 is as defined above, or
— N— N N— CH3 sind,- N— NN— CH 3 ,
und pharmazeutisch verträgliche Salze davon.and pharmaceutically acceptable salts thereof.
Die erfindungsgemäßen Verbindungen können in stereoisomeren Formen, die sich entweder wie Bild und Spiegelbild (Enantiomere), oder die sich nicht wie Bild und Spiegelbild (Diastereomere) verhalten, existieren. Die Erfindung betrifft sowohl die Enantiomeren oder Diastereomeren oder deren jeweilige Mischungen. Die Racem- formen lassen sich ebenso wie die Diastereomeren in bekannter Weise in die stereoisomer einheitlichen Bestandteile trennen.
Folgendes Formelschema veranschaulicht die entsprechenden Schreibweisen für enantiomerenreine und racemische Formen des Oxazolidongerüstes:The compounds according to the invention can exist in stereoisomeric forms which either behave like image and mirror image (enantiomers) or do not behave like image and mirror image (diastereomers). The invention relates to both the enantiomers or diastereomers or their respective mixtures. Like the diastereomers, the racemic forms can be separated into the stereoisomerically uniform constituents in a known manner. The following formula shows the corresponding spellings for enantiomerically pure and racemic forms of the oxazolidone skeleton:
(A) (racemisch) <B) (enantiomer)(A) (racemic) < B ) (enantiomeric)
Physiologisch unbedenkliche Salze der erfindungsgemäßen Verbindungen können Salze der erfindungsgemäßen Stoffe mit Mineralsäuren, Carbonsäuren oder Sulfon- säuren sein. Besonders bevorzugt sind z.B. Salze mit Chlorwasserstoffsäure, Bromwasserstoffsäure, Schwefelsäure, Phosphorsäure, Methansulfonsäure, Ethansulfon- säure, Toluolsulfonsäure, Benzolsulfonsäure, Naphthalindisulfonsäure, Essigsäure,Physiologically acceptable salts of the compounds according to the invention can be salts of the substances according to the invention with mineral acids, carboxylic acids or sulfonic acids. For example, particular preference is given to Salts with hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, toluenesulfonic acid, benzenesulfonic acid, naphthalenedisulfonic acid, acetic acid,
Propionsäure, Milchsäure, Weinsäure, Zitronensäure, Fumarsäure, Maleinsäure oder Benzoesäure.Propionic acid, lactic acid, tartaric acid, citric acid, fumaric acid, maleic acid or benzoic acid.
Als Salze können weiterhin Salze mit üblichen Basen genannt werden, wie beispielsweise Alkalimetallsalze (z.B. Natrium- oder Kaliumsalze), Erdalkalisalze (z.B.Salts which can furthermore be mentioned are salts with customary bases, such as, for example, alkali metal salts (e.g. sodium or potassium salts), alkaline earth metal salts (e.g.
Calcium- oder Magnesiumsalze) oder Ammoniumsalze, abgeleitet von Ammoniak oder organischen A inen wie beispielsweise Diethylamin, Triethylamin, Ethyldiisopropyl- amin, Prokain, Dibenzylamin, N-Methylmorpholin, Dihydroabietylamin, 1-Ephenamin oder Methyl-piperidin.Calcium or magnesium salts) or ammonium salts derived from ammonia or organic amines such as diethylamine, triethylamine, ethyldiisopropylamine, procaine, dibenzylamine, N-methylmorpholine, dihydroabietylamine, 1-ephenamine or methyl-piperidine.
(CrC8)-Alkyl steht im Rahmen der Erfindung für geradkettiges oder verzweigtes Alkyl mit 1 bis 8 Kohlenstoffatomen wie z.B. Methyl, Ethyl, Propyl, Butyl, Pentyl, Hexyl, Heptyl und Octyl sowie deren verzweigtkettige isomere Formen. Aus dieser Definition leiten sich analog die entsprechenden Alkylgruppen mit weniger Koh- lenstoffatomen wie z.B. (CrC4)-Alkyl ab. Im allgemeinen gilt, daß (CrC4)-Alkyl bevorzugt ist.
Desweiteren leiten sich aus dieser Definition die entsprechenden Bedeutungen des Alkylanteils in funktionalisierten Gruppen ab, wie z.B. (C2-C5)-Alkinyl (z.B. Ethinyl, Propinyl etc.), (C2-C4)-Alkenyl (z.B. Vinyl, Propenyl etc.), (CrC4)-Acyl (z.B. Formyl, Acetyl, Propionyl, Butyryl etc.), (CrC4)-Alkoxy (wie z.B. Methoxy,In the context of the invention, (C r C 8 ) -alkyl stands for straight-chain or branched alkyl having 1 to 8 carbon atoms, such as, for example, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl and octyl and their branched-chain isomeric forms. The corresponding alkyl groups with fewer carbon atoms, such as (C r C 4 ) alkyl, are derived analogously from this definition. In general, (C r C 4 ) alkyl is preferred. Furthermore, the corresponding meanings of the alkyl moiety in functionalized groups are derived from this definition, such as (C 2 -C 5 ) alkynyl (eg ethynyl, propynyl etc.), (C 2 -C 4 ) alkenyl (eg vinyl, propenyl etc.), (C r C 4 ) acyl (e.g. formyl, acetyl, propionyl, butyryl etc.), (C r C 4 ) alkoxy (such as methoxy,
Ethoxy, Propoxy, Butoxy etc.), (Ci-C^-Alkoxycarbonyl (z.B. Methoxycarbonyl, Ethoxycarbonyl, Propoxycarbonyl, Isopropoxycarbonyl, tert.Butoxycarbonyl, n- Pentoxycarbonyl, n-Hexoxycarbonyl etc.) etc.Ethoxy, propoxy, butoxy etc.), (Ci-C ^ -alkoxycarbonyl (e.g. methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, tert.butoxycarbonyl, n-pentoxycarbonyl, n-hexoxycarbonyl etc.) etc.
(C3-C8)Cycloalkyl steht für einen cyclischen Kohlenwasserstoffrest mit 3 bis 8(C 3 -C 8 ) Cycloalkyl stands for a cyclic hydrocarbon residue with 3 to 8
Kohlenstoffatomen. Beispielsweise seien Cyclopropyl, Cyclobutyl, Cyclopentyl, Cyclohexyl, Cycloheptyl und Cyclooctyl genannt. Bevorzugt sind der Cyclopropyl-, Cyclopentan- und der Cyclohexanring.Carbon atoms. Examples include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl. The cyclopropyl, cyclopentane and cyclohexane rings are preferred.
Aryl steht im allgemeinen für einen aromatischen Rest mit 6 bis 10 Kohlenstoffatomen.Aryl generally represents an aromatic radical having 6 to 10 carbon atoms.
Bevorzugte Arylreste sind Phenyl und Naphthyl.Preferred aryl radicals are phenyl and naphthyl.
Halogen steht im Rahmen der Erfindung für Fluor, Chlor, Brom oder Iod, wobei Fluor und Chlor bevorzugt sind.Halogen in the context of the invention represents fluorine, chlorine, bromine or iodine, with fluorine and chlorine being preferred.
Eine gesättigte, ungesättigte und/oder aromatische, gegebenenfalls kondensierte und/oder substituierte, carbomono-, bi- oder tricyclische Gruppe für R3 schließt Carbomono-, bi- oder tricyclische Gruppen ein, wobei, wenn es sich um bi-oder tricyclische Reste handelt, die Zyklen aneinanderkondensiert vorliegen können, über eine Bindung miteinander verbunden sein können oder über zwei Bindungen miteinander verbunden sein können (spirocyclische Verbindungen), und diese Zyklen, sofern mehr als zwei im Rest vorliegen, unabhängig voneinander gesättigt, ungesättigt und/oder aromatisch sein können. Diese Gruppe kann ferner substituiert oder unsubstituiert sein. Derartige Gruppen sind an sich im Stand der Technik bekannt und sind beispielsweise in dem oben beschriebenen Stand der Technik beschrieben.
Analog schließen gesättigte, ungesättigte und/oder aromatische, gegebenenfalls kondensierte und/oder substituierte heteromono-, heterobi- oder heterotricyclische Gruppen für R3 heteromono-, heterobi- oder heterotricyclische Gruppen, die mindestens ein Heteroatom, bevorzugt 1 bis 3 Heteroatome ausgewählt aus S, N oderA saturated, unsaturated and / or aromatic, optionally fused and / or substituted, carbomono-, bi- or tricyclic group for R 3 includes carbomono-, bi- or tricyclic groups, if these are bi- or tricyclic radicals which can be fused to one another, linked via a bond or linked via two bonds (spirocyclic compounds), and these cycles, if there are more than two in the remainder, can be independently saturated, unsaturated and / or aromatic . This group can also be substituted or unsubstituted. Such groups are known per se in the prior art and are described, for example, in the prior art described above. Analogously, saturated, unsaturated and / or aromatic, optionally fused and / or substituted heteromono-, heterobi- or heterotricyclic groups for R 3 include heteromono-, heterobi- or heterotricyclic groups which have at least one heteroatom, preferably 1 to 3 heteroatoms selected from S, N or
O enthalten, ein, wobei die Zyklen kondensiert vorliegen können, durch eine Bindung miteinander verbunden sein können oder, wie im Falle der Spiroverbindungen über zwei Bindungen miteinander verbunden sein können. Diese Definition schließt auch Reste R3 ein, in denen gleichzeitig carbocyclische und heterocyclische Gruppen vorkommen. Die einzelnen Zyklen können, wenn mehr als zwei vorliegen, unabhängig voneinander gesättigt, ungesättigt und/oder aromatisch sein und substituiert sein. Derartige Gruppen sind an sich ebenfalls aus dem Stand der Technik bekannt und werden beispielsweise durch den in der Einleitung erwähnten Stand der Technik beschrieben. Konkrete Beispiele für R3 werden in der folgenden bevorzugten Ausfuhrungsform der Verbindungen der allgemeinen FormelContain O, a, where the cycles can be condensed, can be connected to one another by a bond or, as in the case of the spiro compounds, can be connected to one another by two bonds. This definition also includes radicals R 3 in which carbocyclic and heterocyclic groups occur simultaneously. If there are more than two, the individual cycles can be independently saturated, unsaturated and / or aromatic and substituted. Such groups are also known per se from the prior art and are described, for example, by the prior art mentioned in the introduction. Specific examples of R 3 are shown in the following preferred embodiment of the compounds of the general formula
(I) beschrieben, in der(I) described in the
R3 ausgewählt wird aus den Gruppen der Formeln:R 3 is selected from the groups of the formulas:
ist> worin R ausgewählt
wird, aus der Gruppe der Substituenten die besteht aus:i st > where R is selected from the group of substituents which consists of:
(a) Wasserstoff(a) hydrogen
(b) Halogen,(b) halogen,
(c) -OR24, (d) -SR24,(c) -OR 24 , (d) -SR 24 ,
(e) -S(O)nR24, worin n eine ganze Zahl von 1 oder 2 ist,(e) -S (O) n R 24 , where n is an integer of 1 or 2,
(f) Cyano,
O(f) cyano, O
(g) 24 , — O— C— R(g) 24, - O— C— R
H OH O
0000
H OH O
(i) 2 ' — N— C— O— R(i) 2 '- N - C - O - R
O — C-O-R24 O - COR 24
(k)(k)
(n) (C,-C8)Alkyl oder (C3-C8)Cycloalkyl ist, die wahlweise substituiert sein können mit einem oder mehreren Substi- tuenten, die aus der Gruppe der oben genannten Substituenten(n) is (C, -C 8 ) alkyl or (C 3 -C 8 ) cycloalkyl, which can optionally be substituted with one or more substituents, which come from the group of the abovementioned substituents
(a) bis (m) ausgewählt werden,(a) to (m) are selected,
(q) Phenyl ist, das wahlweise substituiert sein kann mit einem oder mehreren Substituenten, die aus der Gruppe der oben genannten Substituenten (a) bis (n) ausgewählt werden,(q) is phenyl which may be optionally substituted with one or more substituents selected from the group of the above-mentioned substituents (a) to (n),
worin R24 und R24 unabhängig ausgewählt werden aus:wherein R 24 and R 24 are independently selected from:
(a) Wasserstoff,(a) hydrogen,
(b) (C,-C6)Alkyl oder (C3-C8)Cycloalkyl, die wahlweise substi- tuiert sein können mit einem oder mehreren Substituenten, die aus der Gruppe ausgewählt werden, die aus Fluor, Chlor,
Hydroxy, (C,-C4)Alkoxy, (C,-C4)Acyl, (C,-C4)Acyloxy, oder(b) (C, -C 6 ) alkyl or (C 3 -C 8 ) cycloalkyl, which may optionally be substituted with one or more substituents selected from the group consisting of fluorine, chlorine, Hydroxy, (C, -C 4 ) alkoxy, (C, -C 4 ) acyl, (C, -C 4 ) acyloxy, or
(c) Phenyl, das wahlweise substituiert sein kann mit einem oder mehreren Substituenten, die aus der Gruppe ausgewählt wer- den, die aus Fluor, Chlor, (C,-C4)Alkyl, Hydroxy, (C,-C4)-(c) phenyl, which may be optionally substituted with one or more substituents selected from the group consisting of fluorine, chlorine, (C, -C 4 ) alkyl, hydroxy, (C, -C 4 ) -
Alkoxy, (C,-C4)Acyl, (C,-C4)Acyloxy, oderAlkoxy, (C, -C 4 ) acyl, (C, -C 4 ) acyloxy, or
besteht, oder Phenyl oder Pyridyl ist, die wahlweise substituiert sein können mit R25 und R26, worin R26 ausgewählt wird aus der Gruppe der Substituenten, die besteht aus: or is phenyl or pyridyl, which may be optionally substituted with R 25 and R 26 , wherein R 26 is selected from the group of substituents consisting of:
(a) Wasserstoff,(a) hydrogen,
(b) Halogen,(b) halogen,
(c) -R27 und -OR27, worin R27 Wasserstoff oder (C,-C4)Alkyl ist,(c) -R 27 and -OR 27 , wherein R 27 is hydrogen or (C, -C 4 ) alkyl,
(d) -NO2, und(d) -NO 2 , and
R25 ausgewählt wird aus der Gruppe der Substituenten, die besteht aus:R 25 is selected from the group of substituents, which consists of:
(a) Wasserstoff,(a) hydrogen,
(b) (C,-C8)Alkyl, das wahlweise substituiert sein kann mit einem oder mehreren Substituenten, die aus der Gruppe ausgewählt werden, die besteht aus: Halogen; -OH,(b) (C, -C 8 ) alkyl, which may be optionally substituted with one or more substituents selected from the group consisting of: halogen; -OH,
Oxo, daß nicht in α-Position vorliegt, - -S(O)0R28, worin o eine ganze Zahl von 0, 1 oder 2 ist und R28 (C,-C4)Alkyl oder (C3-C8)Cycloalkyl ist, -NR29R30 ist, worin R29 und R30 gleich oder verschieden sind, und Wasserstoff, (C,-C8)Alkyl, (C,-C8)Cycloalkyl,
-(CH2)— OR31 m , worin m eine ganze Zahl von 1, 2 oder 3 ist und R31 Wasserstoff oder (C,-C4)Alkyl ist, (Ob- (CH.) — NR32R33 Oxo that is not in the α-position, - -S (O) 0 R 28 , where o is an integer of 0, 1 or 2 and R 28 is (C, -C 4 ) alkyl or (C 3 -C 8 ) Is cycloalkyl, -NR 29 R 30 , in which R 29 and R 30 are identical or different, and hydrogen, (C, -C 8 ) alkyl, (C, -C 8 ) cycloalkyl, - (CH 2 ) - OR 31 m , where m is an integer of 1, 2 or 3 and R 31 is hydrogen or (C, -C 4 ) alkyl, (Ob- (CH.) - NR 32 R 33
P m , worin m wie oben definiert und p 0 oder 1 ist und R32 und R33 gleich oder 5 verschieden und Wasserstoff oder (C,-C4)Alkyl sind oder gemeinsam eine (C4-C6)Alkandiyl-Gruppe bilden, oder R29 und R30 gemeinsam -(CH2)2-O-(CH2)2- oder - (CH2)2-N(R31)-(CH2)2-, worin R3' wie oben definiert ist, bilden, 10 (c) (C2-C5)Alkenyl,P m , where m is as defined above and p is 0 or 1 and R 32 and R 33 are the same or 5 different and are hydrogen or (C, -C 4 ) alkyl or together form a (C 4 -C 6 ) alkanediyl group , or R 29 and R 30 together - (CH 2 ) 2 -O- (CH 2 ) 2 - or - (CH 2 ) 2 -N (R 31 ) - (CH 2 ) 2 -, wherein R 3 'as above is defined, form 10 (c) (C 2 -C 5 ) alkenyl,
(d) (C3-C8)Cycloalkyl,(d) (C 3 -C 8 ) cycloalkyl,
(e) -OR29, worin R29 wie oben definiert ist,(e) -OR 29 , wherein R 29 is as defined above,
(f) Cyano,(f) cyano,
(g) -S(O)0R34, worin o wie oben definiert ist und R34 (g) -S (O) 0 R 34 , wherein o is as defined above and R 34
15 - (C,-C4)Alkyl, das wahlweise mit einem oder mehreren Substituenten substituiert ist, die aus Gruppe ausgewählt werden, die aus Halogen, Hydroxy, Cyano, -NR29R30 ist, worin R29 und R30 wie oben definiert sind, und15 - (C, -C 4 ) alkyl optionally substituted with one or more substituents selected from the group consisting of halogen, hydroxy, cyano, -NR 29 R 30 , wherein R 29 and R 30 are as above are defined, and
20
worin R32 wie oben definiert ist, (C2-C4)Alkenyl,20 wherein R 32 is as defined above, (C 2 -C 4 ) alkenyl,
-NR35R36, worin R35 Wasserstoff, (C,-C4)Alkyl,-NR 35 R 36 , wherein R 35 is hydrogen, (C, -C 4 ) alkyl,
(C3-C8)-Cycloalkyl ist und R36 Wasserstoff, (C,-Is (C 3 -C 8 ) cycloalkyl and R 36 is hydrogen, (C, -
25 C4)Alkyl, Alkyl, (C2-C4)Alkenyl, (C3-C8)-25 C 4 ) alkyl, alkyl, (C 2 -C 4 ) alkenyl, (C 3 -C 8 ) -
Cycloalkyl, -OR37, worin R37 Wasserstoff oder (C,-C4)Alkyl ist, oder -NR32R33 ist, worin R32 und R33 wie oben definiert sind,
-N3,Cycloalkyl, -OR 37 , wherein R 37 is hydrogen or (C, -C 4 ) alkyl, or -NR 32 R 33 , wherein R 32 and R 33 are as defined above, -N 3 ,
H π , worin R38 (C,-C4)Alkyl ist, H π, where R 38 is (C, -C 4 ) alkyl,
— — C— R38 daß wahlweise mit einem oder mehreren Halogenatomen substituiert sein kann, 5- - C - R 38 that can be optionally substituted with one or more halogen atoms, 5
(h) 5 worin p ne ganze Zam
von 0 oder 1 ist und R39 und R40 unabhängig voneinander (CrC2)Alkyl oder gemeinsam eine (C3- C5)Alkandiyl Gruppe bilden,(h) 5 where p ne whole Zam is 0 or 1 and R 39 and R 40 independently of one another (C r C 2 ) alkyl or together form a (C 3 - C 5 ) alkanediyl group,
OO
10 (i) II ,R , worin R wie oben definiert ist,10 (i) II, R , where R is as defined above,
— S— C— R- S— C— R
(j) Tetrazolyl,(j) tetrazolyl,
(k) -NR29R30 ist, worin R29 und R30 wie oben definiert sind,(k) -NR 29 R 30 , wherein R 29 and R 30 are as defined above,
(1) -N(R29)COR38, worin R29 und R38 wie oben definiert sind,(1) -N (R 29 ) COR 38 , wherein R 29 and R 38 are as defined above,
15 (m) -NR29S(O)0R38 ist, worin R29, o und R38 wie oben definiert sind,15 (m) -NR 29 is S (O) 0 R 38 , wherein R 29 , o and R 38 are as defined above,
(n) -CONR29R30 ist, worin R29 und R30 wie oben definiert sind,(n) -CONR 29 R 30 , wherein R 29 and R 30 are as defined above,
(o) -COR41, worin R41 (o) -COR 41 , wherein R 41
20 - Wasserstoff20 - hydrogen
(C,-C8)Alkyl, daß wahlweise mit einem oder mehreren Halogenatomen substituiert sein kann,(C, -C 8 ) alkyl, which can optionally be substituted by one or more halogen atoms,
(C,-C4)Alkyl, daß wahlweise mit -OR37,(C, -C 4 ) alkyl that optionally with -OR 37 ,
25 -OC(O)R37, worin R37 jeweils wie oben definiert ist, -NR29R30, worin R29 und R30
wie oben definiert sind, -S(O)0R28, worin o und R28, wie oben definiert sind, (C3-C8)Cycloalkyl,25 -OC (O) R 37 , in which R 37 is in each case as defined above, -NR 29 R 30 , in which R 29 and R 30 are as defined above, -S (O) 0 R 28 , where o and R 28 are as defined above, (C 3 -C 8 ) cycloalkyl,
(C2-C5)Alkenyl, wahlweise substituiert mit -CHO, oder -CO2R37 ist,(C 2 -C 5 ) alkenyl, optionally substituted with -CHO, or -CO 2 R 37 ,
(p) -C(=NR42)R41, worin R41 wie oben definiert ist, und R42 (p) -C (= NR 42 ) R 41 , wherein R 41 is as defined above, and R 42
-NR29R30, worin R29 und R30 wie oben definiert sind,-NR 29 R 30 , wherein R 29 and R 30 are as defined above,
-OR29, worin R29 wie oben definiert ist, oder-OR 29 , wherein R 29 is as defined above, or
H O ist, worin P R29 wie oben definiert ist,H O is where P R29 is as defined above,
I. II ,29I. II, 29
— N— C— FT- N— C— FT
10 (q) -C(R41)(OR43)(OR43'), worin R4' wie oben definiert ist, und R43 und R43' gleich oder verschieden sein können und (C,-C4)Alkyl sind oder gemeinsam eine (C2-C3)- Alkandiylgruppe bilden,10 (q) -C (R 41 ) (OR 43 ) (OR 43 '), wherein R 4 ' is as defined above, and R 43 and R 43 'may be the same or different and (C, -C 4 ) alkyl are or together form a (C 2 -C 3 ) alkanediyl group,
(r) , worin R29, R35 und R4' wie oben defi-
(r), wherein R 29 , R 35 and R 4 'are as defined above
15 niert sind, und R44 R29 oder -NR29R31 ist, worin R29 und15 are renated, and R 44 is R 29 or -NR 29 R 31 , wherein R 29 and
R31 wie oben definiert sind,R 31 are as defined above,
(s) , worin R29, R31 und R41 wie oben
definiert sind,(s), wherein R 29 , R 31 and R 41 are as above are defined
(u) , worin m, R29, R30 und R31
wie oben definiert sind, oder (u), wherein m, R 29 , R 30 and R 31 are as defined above, or
R3 eine Gruppe der Formel:R 3 is a group of the formula:
, worin q eine ganze Zahl von 0 bis 4,
und R45 -COR46, worin R46 (C,-C6)Alkyl ist, daß mit Hydroxy substituiert sein kann, oder -C(O)-O-R47 ist, worin R47 (C,-C6)Alkyl ist, oderwhere q is an integer from 0 to 4, and R 45 -COR 46 , wherein R 46 is (C, -C 6 ) alkyl that may be substituted with hydroxy, or -C (O) -OR 47 , wherein R 47 is (C, -C 6 ) alkyl , or
R3 eine Gruppe der FormelR 3 is a group of the formula
ist, worin R47 wie oben definiert ist, oder
where R 47 is as defined above, or
R3 eine Gruppe der Formel:R 3 is a group of the formula:
ist, worin Y S oder O ist, oder is where Y is S or O, or
R3 eine Gruppe der Formel:R 3 is a group of the formula:
, worin R48 Wasserstoff, (C,-C6)Alkyl, daß
wahlweise einen oder mehrere Substituenten, ausgewählt aus OH, CN oder Halogen, aufweisen kann, -(CH2)r-(C6-C10)Aryl, worin r eine ganze Zahl von 1 bis 4 ist, oder -(CH2)r-OR49 ist, worin r wie oben definiert ist, und R49 Wasserstoff, (C,-C6)Alkyl, -(CH2)r-(C6-C10)Aryl,
worin r wie oben definiert ist, oder -C(O)R50 ist, worin R50 (C, C6)Alkyl ist, oder, wherein R 48 is hydrogen, (C, -C 6 ) alkyl, that can optionally have one or more substituents selected from OH, CN or halogen, - (CH 2 ) r - (C 6 -C 10 ) aryl, wherein r is an integer from 1 to 4, or - (CH 2 ) r is -OR 49 , wherein r is as defined above, and R 49 is hydrogen, (C, -C 6 ) alkyl, - (CH 2 ) r - (C 6 -C 10 ) aryl, wherein r is as defined above, or is -C (O) R 50 , wherein R 50 is (C, C 6 ) alkyl, or
R3 eine Gruppe der Formel:R 3 is a group of the formula:
R3 eine Gruppe der Formel:R 3 is a group of the formula:
voneinander ausgewählt werden aus Wasserstoff, -NO2 und Halogen, oder are selected from hydrogen, -NO 2 and halogen, or
R3 eine Gruppe der Formel:R 3 is a group of the formula:
5 worin R55 und R56 unabhängig von-
einander Wasserstoff, (C,-C4)Alkyl, (C3-C8)Cycloalkyl oder Phenyl sind oder gemeinsam eine (C,-C6)Alkandiylgruppe bilden, oder 5 wherein R 55 and R 56 are independent of are each other hydrogen, (C, -C 4 ) alkyl, (C 3 -C 8 ) cycloalkyl or phenyl or together form a (C, -C 6 ) alkanediyl group, or
R3 eine Gruppe der Formel:
R 3 is a group of the formula:
eine Gruppe der Formel:a group of the formula:
oder vorzugsweiseor preferably
R wie oben definiert ist,R is as defined above,
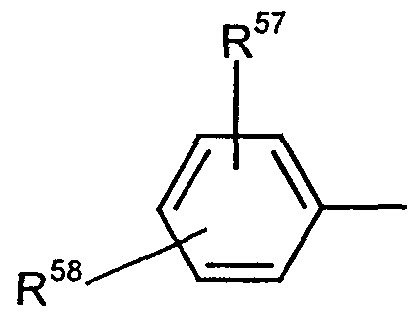
R 57 ausgewählt wird aus der Gruppe der Substituenten, die besteht aus:R 57 is selected from the group of substituents, which consists of:
(a) Wasserstoff,(a) hydrogen,
(b) -NO2,(b) -NO 2 ,
(c) -S(O)0R59 worin o wie oben definiert ist und R59 (C,- C4)Alkyl, daß wahlweise durch einen oder mehreren Substituenten substituiert sein kann, die ausgewählt werden aus der Gruppe die besteht aus, -OH, -CN, -NR29R30, worin R29 und R30 wie oben definiert sind, und -C(O)OR37, worin R37 wie oben definiert ist; (C2- C4)Alkenyl oder -NR7R60 ist, worin R7 wie oben definiert ist und R60 R22 oder -NR32R33 ist, worin R22, R32 und R33 wie oben definiert sind,
(d) Tetrazolyl,(c) -S (O) 0 R 59 where o is as defined above and R 59 (C, - C 4 ) alkyl, which may optionally be substituted by one or more substituents which are selected from the group consisting of -OH, -CN, -NR 29 R 30 , wherein R 29 and R 30 are as defined above, and -C (O) OR 37 , wherein R 37 is as defined above; (C 2 -C 4 ) is alkenyl or -NR 7 R 60 , wherein R 7 is as defined above and R 60 is R 22 or -NR 32 R 33 , wherein R 22 , R 32 and R 33 are as defined above, (d) tetrazolyl,
(e) worin p? R39 und R4O wie
oben definiert sind,(e) where p? R39 and R4 O as are defined above,
(f) -SH(f) -SH
O 5 (g) — S — C — R38 , worin R38 wie oben definiert ist,O 5 (g) - S - C - R 38 , wherein R 38 is as defined above,
(h) -COR41, worin R41 wie oben definiert ist,(h) -COR 41 , wherein R 41 is as defined above,
(i) -CONR29R30 ist, worin R29 und R30 wie oben definiert sind, (j) -C(=NR42)R41, worin R41 wie oben definiert ist, und R42 10 -NR29R30, worin R29 und R30 wie oben definiert sind,(i) -CONR 29 R 30 , wherein R 29 and R 30 are as defined above, (j) -C (= NR 42 ) R 41 , wherein R 41 is as defined above, and R 42 10 -NR 29 R 30 , wherein R 29 and R 30 are as defined above,
-OR29, worin R29 wie oben definiert ist, oder-OR 29 , wherein R 29 is as defined above, or
H OH O
I II ,Q ist, worin R wie oben definiert ist, — N— C— R29 I is II, Q , where R is as defined above, - N - C - R 29
(k) , worin R29, R3' und R41 wie oben definiert
sind,(k) wherein R 29 , R 3 'and R 41 are as defined above are,
15 (1) worin R29, R31 und R41 wie oben
definiert sind,15 (1) wherein R 29 , R 31 and R 41 are as above are defined
(m) , worin m, R29, R30 und R3'
wie oben definiert sind, (n) -CN
(o) -OR29, worin R29 wie oben definiert ist,(m), wherein m, R 29 , R 30 and R 3 ' are as defined above, (n) -CN (o) -OR 29 , wherein R 29 is as defined above,
(p) Halogen,(p) halogen,
(q) -NR29R30, worin R29 und R30 wie oben definiert sind,(q) -NR 29 R 30 , wherein R 29 and R 30 are as defined above,
R29 0 (r) N— C R38 ' worm R29 und R38 wie °ben definiertR 29 0 (r) N- CR 38 'worm R29 and R38 w i e ben defined °
5 sind,5 are
(s) , worin o, R29 und R38 wie oben
definiert sind, (t) -C(R 1)(OR43)(OR43'), worin R41 wie oben definiert ist, und R43 und R43' gleich oder verschieden sein können 10 und (C,-C4)Alkyl sind oder gemeinsam eine (C2-(s), wherein o, R 29 and R 38 are as above (t) -C (R 1 ) (OR 43 ) (OR 43 '), wherein R 41 is as defined above, and R 43 and R 43 ' may be the same or different 10 and (C, -C 4 ) Are alkyl or together form a (C 2 -
C3)Alkandiylgruppe bilden,C 3 ) form alkanediyl group,
(u) , worin R29, R35 und R41 wie oben definiert
sind, und R44 -R29 oder -NR29R31 ist, worin R29 und R31 wie oben definiert sind, 15 (v) (C,-C8)Alkyl, das wahlweise substituiert sein kann mit einem oder mehreren Substituenten, die aus der Gruppe ausgewählt werden, die besteht aus: Halogen; -OH, 20 - Oxo, daß nicht in α-Position vorliegt,(u), wherein R 29 , R 35 and R 41 are as defined above and R 44 is -R 29 or -NR 29 R 31 , wherein R 29 and R 31 are as defined above, 15 (v) (C, -C 8 ) alkyl, which may be optionally substituted with one or more substituents which are selected from the group consisting of: halogen; -OH, 20 - oxo that is not in the α position,
-S(O)0R28, worin o und R28 wie oben definiert sind, oder-S (O) 0 R 28 , wherein o and R 28 are as defined above, or
-NR29R30, worin R29 und R30 wie oben definiert ist, 25 (w) (C2-C5)Alkenyl, und-NR 29 R 30 , wherein R 29 and R 30 are as defined above, 25 (w) (C 2 -C 5 ) alkenyl, and
(x) (C3-C8)Cycloalkyl, und
R58 Wasserstoff, Halogen, OR37, worin R37 wie oben definiert ist, (C,-C3)Alkyl, oder NO2 ist, oder wenn R3 (x) (C 3 -C 8 ) cycloalkyl, and R 58 is hydrogen, halogen, OR 37 , wherein R 37 is as defined above, (C, -C 3 ) alkyl, or NO 2 , or when R 3
oder R57 und R58 gemeinsam
einen sechsgliedrigen carbocyclischen Ring bilden können.or R 57 and R 58 together can form a six-membered carbocyclic ring.
Besonders bevorzugt sind Verbindungen der allgemeinen Formel (I), worinCompounds of the general formula (I) in which
R1 -OR4, worin R4 (C,-C8)Alkyl ist, oder -NHR5 ist, worin R5 Wasserstoff oder (C,-C8)Alkyl ist,R 1 is -OR 4 , in which R 4 is (C, -C 8 ) alkyl, or -NHR 5 , in which R 5 is hydrogen or (C, -C 8 ) alkyl,
R2 Wasserstoff oder Halogen ist,R 2 is hydrogen or halogen,
OO
II 7II 7
R3 "C R , worin R7 (C,-C4)Alkyl ist,R 3 "CR , wherein R 7 is (C, -C 4 ) alkyl,
. worin R7 (CrC4)Alkyl ist und R8 -OR7' ist, worin R7' wie oben definiert ist, , wherein R 7 is (C r C 4 ) alkyl and R 8 is -OR 7 ' , where R 7' is as defined above,
, worin R11 wie oben definiert ist, R17 Wasserstoff und , wherein R 11 is as defined above, R 17 is hydrogen and
R18 -CN oder -NO2 ist oder R17 -CN oder -NO2 und R'8 Wasserstoff ist,R 18 is -CN or -NO 2 or R 17 -CN or -NO 2 and R ' 8 is hydrogen,
Pyridyl oder
ist, worin Y S oder O ist.Pyridyl or is where Y is S or O.
Ganz besonders bevorzugte Verbindungen der Erfindungen werden ausgewählt aus der Gruppe, die besteht aus:Very particularly preferred compounds of the inventions are selected from the group consisting of:
Die Erfindung betrifft weiterhin ein Verfahren zur Herstellung der Verbindungen der Formel (I) und deren pharmazeutisch verträgliche Salze, worinThe invention further relates to a process for the preparation of the compounds of formula (I) and their pharmaceutically acceptable salts, in which
a) Verbindungen der Formel (II)a) compounds of the formula (II)
worin R2 und R3 wie oben definiert sind, oder deren Salze mit Verbindungen der allgemeinen Formel (III) wherein R 2 and R 3 are as defined above, or their salts with compounds of the general formula (III)
worin R4 wie oben definiert ist, umgesetzt werden, um Verbindungen der Formel (Ia)
wherein R 4 is as defined above, are reacted to give compounds of the formula (Ia)
worin R4 wie oben definiert ist, oder ein Salz davon zu erhalten.wherein R 4 is as defined above, or to obtain a salt thereof.
Die Herstellung der Ausgangsverbindungen der allgemeinen Formel (II) sind an sich bekannt, und die Herstellung dieser Aminverbindungen wird z.B. in dem in der Einleitung erwähnten Stand der Technik beschrieben. Ebenso handelt es sich bei der Verbindung der allgemeinen Formel (III) um Verbindungen, die im Stand der Technik bekannt sind. Weiterhin werden Verbindungen der Erfindung durch die folgenden Verfahren erhalten:The preparation of the starting compounds of the general formula (II) are known per se and the preparation of these amine compounds is e.g. described in the prior art mentioned in the introduction. The compound of the general formula (III) is likewise a compound which is known in the prior art. Furthermore, compounds of the invention are obtained by the following methods:
(b) Verbindungen der allgemeinen Formel (II)(b) compounds of the general formula (II)
worin R2 und R3 wie oben definiert sind, werden zunächst in inerten Lösungsmitteln mit S=CC12 und anschließend mit Verbindungen der allgemeinen Formel (III) wherein R 2 and R 3 are as defined above, are first in inert solvents with S = CC1 2 and then with compounds of the general formula (III)
H-NR5R6 (III)
worin R5 und R6 wie oben definiert sind, umgesetzt, um Verbindungen der Formel (Ib) zu erhaltenH-NR 5 R 6 (III) wherein R 5 and R 6 are as defined above, reacted to obtain compounds of formula (Ib)
worin R2, R3, R5 oder R6 wie oben definiert sind, oderwherein R 2 , R 3 , R 5 or R 6 are as defined above, or
(c) Verbindungen der allgemeinen Formel (II)(c) compounds of the general formula (II)
worin R2 und R3 wie oben definiert sind, werden in inerten Lösungsmitteln mit Verbindungen der Formel (IV)wherein R 2 and R 3 are as defined above, in inert solvents with compounds of the formula (IV)
SS
A-C-R1 ACR 1
worin A für Halogen, bevorzugt für Chlor, steht, umgesetzt, um Verbindungen der Formel (I) zu erhalten, oderwherein A is halogen, preferably chlorine, reacted to obtain compounds of formula (I), or
(d) Verbindungen der allgemeinen Formel (II)
(d) compounds of the general formula (II)
worin R2 und R3 wie oben definiert sind, werden mit Ethyldithiocarboxylaten und Triethylamin und im Fall R1 = -NR5R6 mit Thioisocyanaten in inerten Lösungsmitteln umgesetzt, umwherein R 2 and R 3 are as defined above, are reacted with ethyl dithiocarboxylates and triethylamine and in the case R 1 = -NR 5 R 6 with thioisocyanates in inert solvents in order to
Verbindungen der Formel (I) zu erhalten.Obtaining compounds of formula (I).
Die Verbindungen der Erfindung finden Verwendung als Arzneimittel.The compounds of the invention find use as medicaments.
Gegenstand der Erfindung sind somit weiterhin pharmazeutischeThe invention therefore also relates to pharmaceuticals
Zusammensetzungen, die mindestens eine Verbindung der Erfindung in einer Mischung mit mindestens einem pharmazeutisch vertäglichen Trägerstoff umfassen, sowie die Verwendung der Verbindungen der Erfindung zur Herstellung eines Medikamentes zur Behandlung bakterieller Infektionen bei Menschen oder Tieren.Compositions comprising at least one compound of the invention in admixture with at least one pharmaceutically acceptable carrier, and the use of the compounds of the invention in the manufacture of a medicament for the treatment of bacterial infections in humans or animals.
Wie aus den folgenden Testergebnissen ersichtlich verfügen die Verbindungen der Erfindung über eine hohe antibakterielle Wirksamkeit, die der der analogen Verbindungen gemäß dem Stand der Technik, wie die Vergleichsbeispiele 1 und 2 zeigen, überlegen ist, was überaus überraschend ist.As can be seen from the following test results, the compounds of the invention have a high antibacterial activity, which is superior to that of the prior art analog compounds, as shown by comparative examples 1 and 2, which is extremely surprising.
Dazu wurden die MHK- Werte mit Hilfe der Mikrodilutionsmethode in BH-Medium bestimmt. Jede Prüfsubstanz wurde im Nährmedium gelöst. In der Mikrotiterplatte wurde durch serielle Verdünnung eine Konzentrationsreihe der Prüfsubstanzen angelegt. Zur Inokulation wurden Übernachtkulturen der Erreger verwandt, die zuvor im Nährmedium 1:250 verdünnt wurden. Zu 100 μl der verdünnten, wirkstoffhaltigenFor this purpose, the MIC values were determined using the microdilution method in BH medium. Each test substance was dissolved in the nutrient medium. A series of concentrations of the test substances was created in the microtiter plate by serial dilution. Overnight cultures of the pathogens were used for inoculation, which were previously diluted 1: 250 in the nutrient medium. To 100 μl of the diluted active ingredient
Nährlösungen wurden je 100 μl Inokulationslösung gegeben.
Die Mikrotiterplatten wurden bei 37°C bebrütet und nach ca. 20 Stunden oder nach 3 bis 5 Tagen abgelesen. Der MHK-Wert (μg/ml) gibt die niedrigste Wirkstoffkonzentration an, bei der kein Wachstum zu erkennen war.Nutrient solutions were given 100 μl inoculation solution. The microtiter plates were incubated at 37 ° C and read after about 20 hours or after 3 to 5 days. The MIC value (μg / ml) indicates the lowest active substance concentration at which no growth was discernible.
Es wurden die folgenden Ergebnisse gefunden:The following results were found:
MHK- Werte (μg/ml):MIC values (μg / ml):
Die erfindungsgemäßen Verbindungen weisen ein breites antibakterielles Spektrum, speziell gegen gram-positive Keime und einige gram-negative Bakterien sowie Mycobacterien, Corynebakterien, Haemophilus influenzae und anaerobe Keime auf.The compounds according to the invention have a broad antibacterial spectrum, especially against gram-positive germs and some gram-negative bacteria, as well as mycobacteria, corynebacteria, Haemophilus influenzae and anaerobic germs.
Diese Eigenschaften ermöglichen ihre Verwendung als chemotherapeutische Wirkstoffe in der Human- und Tiermedizin.These properties enable their use as chemotherapeutic agents in human and veterinary medicine.
Die erfindungsgemäßen Verbindungen sind gegen ein breites Spektrum von Mikroorganismen wirksam. Mit ihrer Hilfe können gram-positive Keime, gramnegative Bakterien und bakterienähnliche Mikroorganismen wie Mycoplasmen bekämpft sowie die durch diese Erreger hervorgerufenen Erkrankungen verhindert, gebessert und/oder geheilt werden.The compounds according to the invention are active against a broad spectrum of microorganisms. With their help, gram-positive germs, gram-negative bacteria and bacteria-like microorganisms such as mycoplasmas can be combated and the diseases caused by these pathogens can be prevented, improved and / or cured.
Besonders wirksam sind die erfindungsgemäßen Verbindungen gegen Bakterien und bakterienähnliche Mikroorganismen. Sie sind daher besonders gut zur Prophylaxe und Chemotherapie von lokalen und systemischen Infektionen in der Human- und Tiermedizin geeignet, die durch solche Erreger hervorgerufen werden.
Der oder die Wirkstoffe können gegebenenfalls in einem oder mehreren Trägerstoffe auch in mikroverkapselter Form vorliegen.The compounds according to the invention are particularly effective against bacteria and bacteria-like microorganisms. They are therefore particularly well suited for the prophylaxis and chemotherapy of local and systemic infections in human and veterinary medicine that are caused by such pathogens. The active ingredient (s) can optionally also be in microencapsulated form in one or more carriers.
Die therapeutisch wirksamen Verbindungen sollen in den oben aufgeführten pharmazeutischen Zusammensetzungen vorzugsweise in einer Konzentration von etwa 0,1 bis 99,5, vorzugsweise von etwa 0,5 bis 95 Gew.-% der Gesamtmischung, vorhanden sein.The therapeutically active compounds should preferably be present in the pharmaceutical compositions listed above in a concentration of approximately 0.1 to 99.5, preferably approximately 0.5 to 95% by weight of the total mixture.
Die oben aufgeführten pharmazeutischen Zubereitungen können außer den erfin- dungsgemäßen Verbindungen auch weitere pharmazeutische Wirkstoffe enthalten.In addition to the compounds according to the invention, the pharmaceutical preparations listed above can also contain further active pharmaceutical ingredients.
Im allgemeinen hat es sich sowohl in der Human- als auch in der Veterinärmedizin als vorteilhaft erwiesen, den oder die erfindungsgemäßen Wirkstoffe in Gesamtmengen von etwa 0,5 bis etwa 500, vorzugsweise 5 bis 100 mg kg Körpergewicht je 24 Stunden, gegebenenfalls in Form mehrerer Einzelgaben, zur Erzielung der gewünschten Ergebnisse zu verabreichen. Eine Einzelgabe enthält den oder die erfindungsgemäßen Wirkstoffe vorzugsweise in Mengen von etwa 1 bis etwa 80, insbesondere 3 bis 30 mg/kg, Körpergewicht.In general, both in human and in veterinary medicine, it has proven to be advantageous to use the active compound (s) according to the invention in total amounts of about 0.5 to about 500, preferably 5 to 100 mg, kg body weight per 24 hours, optionally in the form of several Single doses to be administered to achieve the desired results. A single dose contains the active ingredient (s) according to the invention preferably in amounts of about 1 to about 80, in particular 3 to 30 mg / kg, body weight.
Die erfindungsgemäßen Verbindungen können zum Zweck der Erweiterung desThe compounds of the invention can be used for the purpose of expanding the
Wirkungsspektrums und um eine Wirkungssteigerung zu erreichen, auch mit anderen Antibiotika kombiniert werden.
Range of effects and in order to achieve an increase in effectiveness, can also be combined with other antibiotics.
Beispiele Beispiel 1Examples Example 1
(5S)-3-(3-Fluoro-4-mo holinylphenyl)-5-(methoxy-thionocarbonylaminomethyl)- oxazolidin-2-on(5S) -3- (3-Fluoro-4-mo holinylphenyl) -5- (methoxothionocarbonylaminomethyl) oxazolidin-2-one
140 mg (0,46 mmol) (5S)-3-(3-Fluoro-4-morpholinylphenyl)-5-aminomethyl-oxa- zolidin-2-on, 110 mg (0,92 mmol) Thionocarbonylmonomethylmonothiomethylester und 0,2ml (1,15 mmol) Hünigbase werden in 5 ml Methanol über Nacht bei Raumtemperatur gerührt. Man engt ein und reinigt an Kieselgel (Dichlormethan/Methanol Gradient). Ausbeute: 60 mg, RF = 0,87 (Dichlormethan/Methanol 100:7)140 mg (0.46 mmol) (5S) -3- (3-fluoro-4-morpholinylphenyl) -5-aminomethyl-oxazolidin-2-one, 110 mg (0.92 mmol) thionocarbonylmonomethylmonothiomethyl ester and 0.2 ml ( 1.15 mmol) of Hunig base are stirred in 5 ml of methanol overnight at room temperature. It is concentrated and purified on silica gel (dichloromethane / methanol gradient). Yield: 60 mg, R F = 0.87 (dichloromethane / methanol 100: 7)
In Analogie zu Beispiel 1 werden aus den literaturbekannten Aminen die folgendenAnalogously to Example 1, the amines known from the literature become the following
Beispiele erhalten:Get examples:
Beispiel 10Example 10
(5S)-3-(3-Fluoro-4-moφholinylphenyl)-5-(aminothionocarbonylaminomethyl)- oxazolidin-2-on(5S) -3- (3-Fluoro-4-moφholinylphenyl) -5- (aminothionocarbonylaminomethyl) - oxazolidin-2-one
400 mg (1,35 mmol) (5S)-3-(3-Fluoro-4-moφholinylphenyl)-5-aminomethyl-oxa- zolidin-2-on in 12 ml Chloroform/Wasser 1 :1 werden bei 0°C mit 410 mg (4 mmol)400 mg (1.35 mmol) (5S) -3- (3-fluoro-4-moφholinylphenyl) -5-aminomethyl-oxazolidin-2-one in 12 ml chloroform / water 1: 1 are at 0 ° C with 410 mg (4 mmol)
Calciumcarbonat und 0,15 ml (2 mmol) Thiophosgen versetzt. Man rührt über Nacht bei Raumtemperatur nach, trennt die organische Phase ab und extrahiert die wäßrige Phase dreimal mit Chloroform. Die vereinigten organischen Phasen werden getrocknet und eingeengt. Das Zwischenprodukt wird in 38 ml Methanol aufgenommen und
mit 19 ml 2N Ammoniak in Methanol versetzt. Man rührt über Nacht bei Raumtemperatur, engt ein, filtriert ab und trocknet. Ausbeute: 142 mg, RF = 0,19 (Dichlormethan/Methanol 100:3)Calcium carbonate and 0.15 ml (2 mmol) thiophosgene added. The mixture is stirred overnight at room temperature, the organic phase is separated off and the aqueous phase is extracted three times with chloroform. The combined organic phases are dried and concentrated. The intermediate is taken up in 38 ml of methanol and mixed with 19 ml of 2N ammonia in methanol. The mixture is stirred overnight at room temperature, concentrated, filtered off and dried. Yield: 142 mg, R F = 0.19 (dichloromethane / methanol 100: 3)
In Analogie zu Beispiel 10 werden aus den literaturbekannten Aminen die folgenden Beispiele erhalten:In analogy to Example 10, the following examples are obtained from the amines known from the literature: