WO1991011506A1 - Aqueous liquid cleaning agent - Google Patents
Aqueous liquid cleaning agent Download PDFInfo
- Publication number
- WO1991011506A1 WO1991011506A1 PCT/EP1991/000134 EP9100134W WO9111506A1 WO 1991011506 A1 WO1991011506 A1 WO 1991011506A1 EP 9100134 W EP9100134 W EP 9100134W WO 9111506 A1 WO9111506 A1 WO 9111506A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- content
- sulfates
- alkyl ether
- composition according
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/88—Ampholytes; Electroneutral compounds
- C11D1/94—Mixtures with anionic, cationic or non-ionic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/12—Sulfonic acids or sulfuric acid esters; Salts thereof
- C11D1/14—Sulfonic acids or sulfuric acid esters; Salts thereof derived from aliphatic hydrocarbons or mono-alcohols
- C11D1/146—Sulfuric acid esters
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/12—Sulfonic acids or sulfuric acid esters; Salts thereof
- C11D1/29—Sulfates of polyoxyalkylene ethers
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/662—Carbohydrates or derivatives
Definitions
- Liquid cleaning agents usually consist of aqueous solutions of synthetic anionic and / or nonionic surfactants and conventional additives. They are used in particular for cleaning hard surfaces, for example glass, ceramic materials, plastics, painted and polished surfaces.
- An important area of application for liquid cleaning agents is the manual washing of eating and cooking utensils.
- the cleaning of the dishes is usually carried out at slightly elevated temperatures of about 35 to 45 ° C. in very dilute liquors.
- the cleaning power of an agent is generally assessed by the consumer the better and the longer the cleaning liquor foams. Because of the contact of the hands with the cleaning liquid over a long period of time, the skin-friendliness of the product is of particular importance when washing dishes manually.
- agents are preferred which are free from the proportions of anionic surfactants which are generally customary for such agents petrochemical basis such as B. Alkylbenzenesulfonates and alkanesulfonates.
- alkyl sulfates and alkyl ether sulfates are not only very well finally degraded not only in the aerobic activation stage of the sewage treatment plants, but also under anaerobic conditions of sludge digestion (e.g. J. Steber, P. Gode, W Guhl: Fat. Sci. Technol. 90 (1), 32 (1988)).
- alkyl ether sulfates that is to say salts of sulfated adducts of about 2 to 5 moles of ethylene oxide with fatty alcohols having about 10 to 18, preferably 12 to 16, carbon atoms in the aliphatic radical have good foaming and cleaning power and skin-friendly properties own.
- the commercially available manual dishwashing agents also known as dishwashing detergents
- dishwashing detergents therefore generally represent aqueous solutions of such alkyl ether sulfates in conjunction with other surfactants, in particular alkylbenzenesulfonates and alkane sulfonates, and solubilizers, colorants and fragrances.
- Swiss patent 354 195 discloses liquid detergents for manual dishwashing, which are a combination of an alkyl ether sulfate containing alkyl sulfate and a nonionic surfactant of the fatty acid alkanolamide type from mono- or dialkanolamides with no more than 3 carbon atoms in each alkanol residue saturated fat containing 10 to 14 carbon atoms, together with water, solubilizers, colorants and fragrances and, inter alia, have a low solidification point.
- nonionic alkyl monoglucosides not only develop stable foam themselves, but also act as foam stabilizers for other anionic and nonionic surfactants.
- European patent application 70 074 discloses foaming liquid detergents containing anionic surfactants, in particular alkylbenzenesulfonates, alkylglucosides and amine oxides or fatty acid alkanolamides, the alkylglucosides being alkyloligoglucosides which the glucose unit (GE) about 1.5 to 10 times included, acts.
- This value is an average value and also takes into account the presence of alkyl monoglucosides in a corresponding proportion.
- Alkyl glucosides with a degree of oligomerization of higher than 2 are found to be particularly suitable. Mixtures of such alkyl glucosides and alkyl ether sulfates in a ratio of 3: 2 are described as only slightly foaming.
- liquid cleaning agents for the manual cleaning of dishes containing synthetic anionic surfactants of the sulfonate and / or or sulfate surfactants, fatty alkyl glucosides and optionally fatty acid alkanolamides which contain fatty alkyl glucosides with an average of less than 2 units per fatty alkyl radical, in particular 1 to 1.4 units.
- European patent application 341 071 describes liquid detergents for manual dishwashing with an active substance content of 5 to 60% by weight, 30 to 60% by weight of an alkyl glucoside with preferably 1 to 1.4 GE in the molecule 12 to 30 wt .-% of a betaine and up to 1.5 times the amount, based on the betaine, of alkyl sulfates and / or alkyl ether sulfates.
- These surfactant mixtures are said to be distinguished by good foam stability and good cleaning performance.
- the invention thus relates to aqueous, liquid cleaning agents, in particular for manual dishwashing, with an active substance content of 10 to 60, preferably 15 to 40 percent by weight, consisting of alkyl sulfates, alkyl ether sulfates, betaines and alkyl glucosides, which are characterized in that that they contain more than 45% of the active substance of alkyl sulfates and alkyl ether sulfates, their content being greater than 1.5 times the betaine content and being at least twice the alkyl glucoside content.
- the content of alkyl sulfates and alkyl ether sulfates can be a maximum of about 50 times the betaine content and / or the alkyl glucoside content.
- alkyl sulfates or alkyl ether sulfates are commercially available, they have a straight-chain, aliphatic C 1-4 g , preferably C. - alkyl radical.
- the associated cation is preferably an alkali metal or ammonium ion.
- Alkyl ether sulfates in the degree of ethoxylation is from 1 to 5, preferably preferably from 2 to 4.
- Alkylethersulfatgemisches consisting of 50 wt.-_ A C _-Alkylethersulfa.es and 50 wt -.% Of a C l ü - Alkyl ether sulfates with 1 to 5, preferably 2 to 4, ethylene oxide groups instead of or together with the alkyl ether sulfate mixture (es) used, which contains the same components in a weight ratio of 70:30. Small amounts of shorter or longer alkyl chains in alkyl sulfates and / or alkyl ether sulfates change the results only insignificantly.
- alkyl sulfate in industrially produced alkyl ether sulfates is included here.
- the claimed alkyl sulfate content is added separately.
- the quantitative ratio of alkyl sulfate to alkyl ether sulfate is preferably 4: 1 to 1: 5.
- betaines "simple betaines" of the type can:
- R 2 or R 3 CH 3 , C 2 H 5 , C 3 H ? ,
- the alkyl glucosides are of the fatty alcohol glucoside type. Compounds with an average of less than 2 GE per fatty alkyl radical are understood here, in particular those with 1.0 to 1.4 GE.
- the fatty alkyl radical has 10 to 18, in particular 12 to 14, carbon atoms.
- “Fatty alkyl” is understood to mean the residues of the fatty alcohols which are produced by hydrogenation of natural fatty acids and which are wholly or predominantly saturated.
- the agents according to the invention can preferably also contain solubilizers, solvents, colorants and fragrances.
- Alkanolamines, polyols, such as ethylene glycol, propylene glycol-1, 2 or glycerol, for example, can serve as solubilizers, for example for small amounts of dye and perfume oil.
- the amounts used are generally between 1 and 15 percent by weight, based on the total composition.
- solvents such as low molecular weight alkanols with 1 to 4 carbon atoms in the molecule, preferably ethanol and isopropyl alcohol.
- solvents such as low molecular weight alkanols with 1 to 4 carbon atoms in the molecule, preferably ethanol and isopropyl alcohol.
- the amounts used are up to 15 percent by weight, based on the total agent.
- Viscosity regulators such as urea, sodium chloride, ammonium chloride, magnesium chloride and salts of mono-, di- and tricarboxylic acids and mixtures thereof can also be used individually or in combination in the required amounts.
- Further customary optional additives are corrosion inhibitors and preservatives.
- a remainder, to be calculated for a total of 100 percent by weight, for the total composition consists of water.
- liquid cleaning agents according to the invention according to the following examples were obtained by stirring the individual constituents together and allowing the mixture to stand until there were no bubbles.
- FES-1 C 1 2 _ 1 4 alkyl ether sulfate, 2 EO, Na salt
- Betaine-1 C_, g-dimethylacylamidobetaine
- APG C 1 2 _ 1 4 alkyl glucoside 1, 4 GE
- the neutral combinations listed were adjusted with sodium chloride and / or ethanol to viscosities between 300 and 400 mPas (according to Höppler, 20 ° C).
- the samples were stored at -15 ° C for 24 hours.
- the temperature at which the products became optically clear was determined as a clear point.
- the saucers are each covered with 2 g of melted beef tallow (test soil A). Then 8 l of tap water (16 ° d) at 50 ° C. are placed in a bowl. To clean the plates soiled with (A), 4 g, i.e. 0.5 g / l, of the cleaning agent added and the plates washed. The plates listed in Table 3 could be rinsed clean until the foam of the initially foaming solution disappeared.
- the saucers are each covered with 2 g of mixed soiling (test soiling B). This soiling is obtained by mixing water, fat and carbohydrates (Mi No 1 from Henkel) with water. Then 8 l tap water (16 ° d) at 45 ° C are placed in a bowl. To clean the plates soiled with (B), 3, 2 g, i.e. 0.4 g / l, of the detergent produced are added and the plates are washed. The plates that were rinsed clean until the foam disappeared are listed in Table 3. Table 3
- FES-2 C 1-4 alkyl ether sulfate, 3.6 EO, Na salt
- Betain-2 C_- 1Q -dimethylalkylacetobetaine
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
Abstract
Aqueous liquid cleaning agent, especially for dishwashing by hand, with an active substance content of 10 to 60, preferably 15 to 40 % wt., consisting of alkyl sulphates, alkyl ether sulphates, betains and alkyl glucosides containing more than 45 % alkyl sulphates and alkyl ether sulphates in the active substance, whereby their content is more than 1.5 times the betain content and at least double the alkyl glucoside content.
Description
"Wäßriges flüssiges Reinigungsmittel" "Aqueous liquid detergent"
Flüssige Reinigungsmittel bestehen meist aus wäßrigen Lösungen von synthetischen anionischen und/oder nichtionischen Tensiden und üblichen Zusatzstoffen . Sie werden besonders zum Reinigen harter Oberflächen , zum Beispiel von Glas , keramischen Materia- lien , Kunststoffen , lackierten und polierten Oberflächen ver¬ wendet. Ein wichtiges Anwendungsgebiet für flüssige Reinigungs¬ mittel ist das manuelle Spülen von Eß- und Kochgeschirr. Die Ceschirreiπigung wird üblicherweise bei leicht erhöhten Tempe¬ raturen von etwa 35 bis 45 C in stark verdünnten Flotten durchgeführt. Dabei wird vom Verbraucher die Reinigungskraft eines Mittels im allgemeinen umso besser beurteilt je stärker und je länger die Reinigungsflotte schäumt. Wegen des Kontakts der Hände mit der Reinigungsfiotte über einen längeren Zeitraum ist bei manuellem Spülen von Geschirr auch die Hautfreundlichkeit des Mittels von besonderer Bedeutung . Schon aus diesen Gründen stellt der Fachmann bei der Auswahl der Komponenten und der Zusammensetzung eines Mittels für das manuelle Reinigen von Geschirr andere Überlegungen an , als bei flüssigen Reinigungs¬ mitteln für sonstige harte Oberflächen . Zunehmend sind aber auch ökologische Gesichtspunkte in daß Blickfeld des Interesses ge¬ rückt. So werden Mittel bevorzugt, die frei sind von für solche Mittel im allgemeinen üblichen Anteilen an Aniontensiden auf
petrochemischer Basis wie z . B . Alkylbenzolsulfonaten und Al- kansulfonaten .Liquid cleaning agents usually consist of aqueous solutions of synthetic anionic and / or nonionic surfactants and conventional additives. They are used in particular for cleaning hard surfaces, for example glass, ceramic materials, plastics, painted and polished surfaces. An important area of application for liquid cleaning agents is the manual washing of eating and cooking utensils. The cleaning of the dishes is usually carried out at slightly elevated temperatures of about 35 to 45 ° C. in very dilute liquors. The cleaning power of an agent is generally assessed by the consumer the better and the longer the cleaning liquor foams. Because of the contact of the hands with the cleaning liquid over a long period of time, the skin-friendliness of the product is of particular importance when washing dishes manually. For these reasons alone, the expert makes different considerations when selecting the components and the composition of an agent for the manual cleaning of dishes than with liquid detergents for other hard surfaces. However, ecological aspects are also increasingly becoming the focus of interest. Thus, agents are preferred which are free from the proportions of anionic surfactants which are generally customary for such agents petrochemical basis such as B. Alkylbenzenesulfonates and alkanesulfonates.
Aus der Literatur (z. B . Landesgewerbeanstalt, Bayern , Heft 5 (1987) , K. Goßler und R. Miess : Verhalten von linearen Alkyl¬ benzolsulfonaten aus Haushaltswaschmitteln bei der Abwasserbe¬ handlung - Literaturstudie) ist bekannt, daß die oben genannten Aniontenside unter aeroben Bedingungen - wie sie in der Belebt¬ stufe von Kläranlagen vorliegen - biologisch abbaubar sind , aber unter anaeroben Bedingungen der Schlammfaulung keinen oder nur partiellen Abbau aufweisen . Wählt man jedoch die hier bevor¬ zugten Alkylsulfate und Alkylethersulfate als Tenside, so werden diese nicht nur in der aeroben Belebtstufe der Kläranlagen , sondern auch unter anaeroben Bedingungen der Schlammfaulung sehr gut endabgebaut (z. B . J . Steber, P. Gode, W. Guhl : Fat. Sei. Technol . 90 (1 ) , 32 (1988) ) .From the literature (e.g. Landesgewerbeanstalt, Bayern, Issue 5 (1987), K. Goßler and R. Miess: Behavior of linear alkyl benzenesulfonates from household detergents during wastewater treatment - literature study) it is known that the anionic surfactants mentioned above are biodegradable under aerobic conditions - as they exist in the activated stage of sewage treatment plants - but show no or only partial degradation under anaerobic conditions of sludge digestion. However, if one chooses the preferred alkyl sulfates and alkyl ether sulfates as surfactants, these are not only very well finally degraded not only in the aerobic activation stage of the sewage treatment plants, but also under anaerobic conditions of sludge digestion (e.g. J. Steber, P. Gode, W Guhl: Fat. Sci. Technol. 90 (1), 32 (1988)).
Es ist auch allgemein bekannt, daß sogenannte Alkylethersulfate, das heißt Salze von sulfatierten Anlagerungsprodukten von etwa 2 bis 5 Mol Ethylenoxid an Fettalkohole mit etwa 10 bis 18 , vorzugs¬ weise 12 bis 16 Kohlenstoffatomen im aliphatischen Rest eine gute Schaum- und Reinigungskraft sowie hautfreundliche Eigenschaften besitzen . Die marktüblichen , manuell anwendbaren Geschirreini¬ gungsmittel (alias Geschirrspülmittel) stellen daher im allgemeinen wäßrige Lösungen solcher Alkylethersulfate in Verbindung mit anderen Tensideή , insbesondere Alkylbenzolsulfonaten und Alkan- sulfonaten , sowie Lösungsvermittlern, Färb- und Duftstoffen dar.It is also generally known that so-called alkyl ether sulfates, that is to say salts of sulfated adducts of about 2 to 5 moles of ethylene oxide with fatty alcohols having about 10 to 18, preferably 12 to 16, carbon atoms in the aliphatic radical have good foaming and cleaning power and skin-friendly properties own. The commercially available manual dishwashing agents (also known as dishwashing detergents) therefore generally represent aqueous solutions of such alkyl ether sulfates in conjunction with other surfactants, in particular alkylbenzenesulfonates and alkane sulfonates, and solubilizers, colorants and fragrances.
Aus der schweizerischen Patentschrift 354 195 sind flüssige Reinigungsmittel für das manuelle Geschirrspülen bekannt, die eine Kombination aus einem Alkylsulfat enthaltenden Alkylether- sulfat und einem nichtionischen Tensid vom Typ des Fettsäure- alkanolamids aus Mono- oder Dialkanolamiden mit nicht mehr als 3 Kohlenstoffatomen in jedem Alkanolrest von gesättigten Fettsäuren
mit 10 bis 14 Kohlenstoffatomen , zusammen mit Wasser, Lösungs¬ vermittlern , Färb- und Duftstoffen enthalten und unter anderem einen niedrigen Erstarrungspunkt aufweisen .Swiss patent 354 195 discloses liquid detergents for manual dishwashing, which are a combination of an alkyl ether sulfate containing alkyl sulfate and a nonionic surfactant of the fatty acid alkanolamide type from mono- or dialkanolamides with no more than 3 carbon atoms in each alkanol residue saturated fat containing 10 to 14 carbon atoms, together with water, solubilizers, colorants and fragrances and, inter alia, have a low solidification point.
Aus der US-Patentschrift 3 219 656 ist bereits bekannt, daß nichtionische Alkylmonoglucoside nicht nur selbst stabilen Schaum entwickeln , sondern als Schaumstabilisatoren für andere anioni¬ sche und nichtionische Tenside wirken .It is already known from US Pat. No. 3,219,656 that nonionic alkyl monoglucosides not only develop stable foam themselves, but also act as foam stabilizers for other anionic and nonionic surfactants.
Aus der europäischen Patentanmeldung 70 074 sind schäumende flüssige Reinigungsmittel mit einem Gehalt an Aniontensiden , insbesondere Alkylbenzolsulfonaten , Alkylglucosiden und Amin- oxiden beziehungsweise Fettsäurealkanolamiden bekannt, wobei es sich bei den Alkylglucosiden um Alkyloligoglucoside, welche die Glucoseeinheit (GE) etwa 1 ,5 bis 10 mal enthalten , handelt. Dieser Wert ist ein Mittelwert und berücksichtigt auch das Vorliegen von Alkylmonoglucosiden in einem entsprechenden Anteil . Als beson¬ ders geeignet werden Alkylglucoside mit einem Oligomerisierungs- grad von höher als 2 herausgestellt. Mischungen aus solchen Alkylglucosiden und Alkylethersulfaten im Verhältnis 3 : 2 werden als nur schwach schaumverbessernd beschrieben.European patent application 70 074 discloses foaming liquid detergents containing anionic surfactants, in particular alkylbenzenesulfonates, alkylglucosides and amine oxides or fatty acid alkanolamides, the alkylglucosides being alkyloligoglucosides which the glucose unit (GE) about 1.5 to 10 times included, acts. This value is an average value and also takes into account the presence of alkyl monoglucosides in a corresponding proportion. Alkyl glucosides with a degree of oligomerization of higher than 2 are found to be particularly suitable. Mixtures of such alkyl glucosides and alkyl ether sulfates in a ratio of 3: 2 are described as only slightly foaming.
In der deutschen Patentschrift DE 35 34 082 sowie in den inter¬ nationalen Patentanmeldungen WO 86/02943 und WO 89/02912 wer¬ den flüssige Reinigungsmittel für das manuelle Reinigen von Ge¬ schirr, enthaltend synthetische Aniontenside vom Typ der Sulfo- nat- und/oder Sulfattenside, Fettalkylglucoside und gegebenenfalls Fettsäurealkanolamide beschrieben , die Fettalkylglucoside mit durchschnittlich weniger als 2 GE pro Fettalkyl-Rest, insbeson¬ dere 1 bis 1 ,4 GE, enthalten.In the German patent specification DE 35 34 082 and in the international patent applications WO 86/02943 and WO 89/02912, liquid cleaning agents for the manual cleaning of dishes, containing synthetic anionic surfactants of the sulfonate and / or or sulfate surfactants, fatty alkyl glucosides and optionally fatty acid alkanolamides which contain fatty alkyl glucosides with an average of less than 2 units per fatty alkyl radical, in particular 1 to 1.4 units.
Aus der deutschen Offenlegungsschrift 37 06 015 ist bekannt , daß man durch Zusatz von Aniontensiden, vorzugsweise Alkylethersul¬ faten oder Alkylsulfaten und Betainen zu flüssigen Reinigungs-
mittein aus Sulfosuccinaten und Alkylglucosiden Leistungsstei¬ gerungen im Spül vermögen und Verbesserungen der Lagerstabili¬ tät erzielen kann .From German Offenlegungsschrift 37 06 015 it is known that by adding anionic surfactants, preferably alkyl ether sulfates or alkyl sulfates and betaines, to liquid cleaning agents with sulfosuccinates and alkyl glucosides can increase performance in rinsing and improve storage stability.
In der europäischen Patentanmeldung 341 071 werden flüssige Reinigungsmittel für das manuelle Geschirrspülen mit einem Aktivsubstanzgehalt von 5 bis 60 Gew.-% beschrieben , der zu 30 bis 60 Gew. -% aus einem Alkylglucosid mit vorzugsweise 1 bis 1 , 4 GE im Molekül , zu 12 bis 30 Gew.-% aus einem Betain und bis zur 1 ,5fachen Menge, bezogen auf das Betain , aus Alkylsulfaten und/oder Alkylethersulfaten bestehen kann. Diese Tensidmischun- gen sollen sich durch gute Schaumstabilität und gute Reini¬ gungsleistung auszeichnen .European patent application 341 071 describes liquid detergents for manual dishwashing with an active substance content of 5 to 60% by weight, 30 to 60% by weight of an alkyl glucoside with preferably 1 to 1.4 GE in the molecule 12 to 30 wt .-% of a betaine and up to 1.5 times the amount, based on the betaine, of alkyl sulfates and / or alkyl ether sulfates. These surfactant mixtures are said to be distinguished by good foam stability and good cleaning performance.
Von wäßrigen Reinigungsmitteln , die speziell für das manuelle Reinigen von Geschirr konzipiert sind , erwartet der Verbraucher aber nicht nur eine hohe Reinigungskraft und eine gute Schaum¬ bildung und Schaumstabilität bei Fettbelastung sowie ökologische Verträglichkeit, sondern auch ein optisch ansprechendes Erschei¬ nungsbild . Viele Produkte zur manuellen Reinigung von Geschirr werden häufig, in transparenten und/oder transluzenten Flaschen angeboten. Im Handel sollen die Produkte, auch wenn sie über längere Zeit in üblicherweise nicht temperierten Lagern gestanden haben , daher optisch einwandfrei , d. h. transparent vorliegen . Als besonders günstig werden solche Produkte betrachtet, die ei¬ nen Klarpunkt von unter ca. 15 °C aufweisen.However, the consumer expects not only a high cleaning power and good foam formation and foam stability when exposed to fat and ecological compatibility, but also an optically appealing appearance from aqueous cleaning agents which are specifically designed for the manual cleaning of dishes. Many products for manual cleaning of dishes are often offered in transparent and / or translucent bottles. In the trade, the products, even if they have been in warehouses that are usually not at a constant temperature, should be optically flawless. H. are transparent. Products which have a clear point below approximately 15 ° C. are considered to be particularly favorable.
Es wurde nun überraschenderweise gefunden , daß quaternäre Kombinationen aus Alkylsulfat, Alkylethersulfat, Betain und Alkylglucosid mit einer bestimmten quantitativen Zusammensetzung zu einem besseren Kälteverhalten , charakterisiert durch den Klar¬ punkt, führen als bekannte binäre , ternäre oder quaternäre Kombinationen aus diesen Tensiden. Je niedriger der Klarpunkt liegt, desto besser sind die Reinigungsmittel zu bewerten .
Die Erfindung betrifft somit wäßrige, flüssige Reinigungsmittel , insbesondere für die manuelle Geschirreinigung , mit einem Aktiv¬ substanzgehalt von 10 bis 60 , vorzugsweise 15 bis 40 Gewichts¬ prozent, bestehend aus Alkylsulfaten , Alkylethersulfaten , Beta¬ inen und Alkylglucosiden , die dadurch gekennzeichnet sind , daß sie mehr als 45 % der Aktivsubstanz an Alkylsulfaten und Al¬ kylethersulfaten enthalten , wobei deren Gehalt größer als das 1 ,5 fache des Betaingehalts ist und das wenigstens Doppelte des Alkylglucosidgehalts ausmacht.It has now surprisingly been found that quaternary combinations of alkyl sulfate, alkyl ether sulfate, betaine and alkyl glucoside with a certain quantitative composition lead to better cold behavior, characterized by the clear point, than known binary, ternary or quaternary combinations of these surfactants. The lower the clear point, the better the cleaning agents are to be rated. The invention thus relates to aqueous, liquid cleaning agents, in particular for manual dishwashing, with an active substance content of 10 to 60, preferably 15 to 40 percent by weight, consisting of alkyl sulfates, alkyl ether sulfates, betaines and alkyl glucosides, which are characterized in that that they contain more than 45% of the active substance of alkyl sulfates and alkyl ether sulfates, their content being greater than 1.5 times the betaine content and being at least twice the alkyl glucoside content.
Der Gehalt an Alkylsulfaten und Alkylethersulfaten kann maximal etwa das 50fache des Betaingehalts und/oder des Alkylglucosid¬ gehalts ausmachen .The content of alkyl sulfates and alkyl ether sulfates can be a maximum of about 50 times the betaine content and / or the alkyl glucoside content.
Die bevorzugt eingesetzten Alkylsulfate beziehungsweise Alkyl¬ ethersulfate sind handelsüblich , sie weisen einen geradkettigen , aliphatischen C- _ _ 1 g-, vorzugsweise C. - . - Alkylrest auf. Das zugehörige Kation ist vorzugsweise ein Alkalimetall- oder Ammoniumion . Bei Alkylethersulfaten liegt der Ethoxylierungsgrad bei 1 bis 5 , vorzugsweise bei 2 bis 4. Weiterhin bevorzugt ist der Einsatz eines Alkylethersulfatgemisches , bestehend aus 50 Gew.-_ eines C- _-Alkylethersulfa.es und 50 Gew. -% eines Cl ü-Alkylether- sulfates mit 1 bis 5 , vorzugsweise 2 bis 4 Ethylenoxidgruppen an¬ stelle des oder zusammen mit dem üblicherweise eingesetzten Al- kylethersulfatgemisch(es) , das die gleichen Komponenten im Ge¬ wichtsverhältnis 70 : 30 enthält. Geringe Mengen an kürzeren oder längeren Alkylketten in Alkylsulfaten und/oder Alkylether¬ sulfaten verändern die Ergebnisse nur unwesentlich . Der bekann¬ te Alkylsulfatanteil in technisch hergestellten Alkylethersulfaten wird hier diesen zugerechnet. Der beanspruchte Alkylsulfatgehalt wird gesondert zugesetzt. Das Mengenverhältnis von Alkylsulfat zu Alkylethersulfat beträgt dabei vorzugsweise 4 : 1 bis 1 : 5.
Als Betaine können "einfache Betaine" des Types:The preferred alkyl sulfates or alkyl ether sulfates are commercially available, they have a straight-chain, aliphatic C 1-4 g , preferably C. - alkyl radical. The associated cation is preferably an alkali metal or ammonium ion. Alkyl ether sulfates in the degree of ethoxylation is from 1 to 5, preferably preferably from 2 to 4. Furthermore, the use of a Alkylethersulfatgemisches, consisting of 50 wt.-_ A C _-Alkylethersulfa.es and 50 wt -.% Of a C l ü - Alkyl ether sulfates with 1 to 5, preferably 2 to 4, ethylene oxide groups instead of or together with the alkyl ether sulfate mixture (es) used, which contains the same components in a weight ratio of 70:30. Small amounts of shorter or longer alkyl chains in alkyl sulfates and / or alkyl ether sulfates change the results only insignificantly. The known proportion of alkyl sulfate in industrially produced alkyl ether sulfates is included here. The claimed alkyl sulfate content is added separately. The quantitative ratio of alkyl sulfate to alkyl ether sulfate is preferably 4: 1 to 1: 5. As betaines "simple betaines" of the type can:
R2 R 2
R . - N - CH-COO ©R. - N - CH-COO ©
R.R.
und/oder "Amidobetaine" des Types :and / or "amidobetaines" of the type:
CH.CH.
R4 ~ C C \ I © R 4 ~ C C \ I ©
N - CH2 - CH2 - CH2 - N - CH2C00 QN - CH 2 - CH 2 - CH 2 - N - CH 2 C00 Q
I II I
H CH3 H CH 3
eingesetzt werden; dabei gilt für:be used; the following applies to:
R. bzw. R„ = Cß _ l g linear oder verzweigt,R. or R „= C ß _ lg linear or branched,
R2 bzw. R3 = CH3 , C2H5 , C3H?.R 2 or R 3 = CH 3 , C 2 H 5 , C 3 H ? ,
Die Alkylglucoside sind vom Typ der Fettalkoholglucoside. Man versteht hier darunter Verbindungen mit durchschnittlich weniger als 2 GE pro Fettalkyl-Rest, insbesondere solche mit 1 ,0 bis 1 ,4 GE. Der Fettalkylrest weist 10 bis 18 , insbesondere 12 bis 14 Kohlenstoffatome auf. Unter "Fettalkyl" werden die Reste der durch Hydrierung von natürlichen Fettsäuren hergestellten Fett¬ alkohole, die ganz oder überwiegend gesättigt sind , verstanden.The alkyl glucosides are of the fatty alcohol glucoside type. Compounds with an average of less than 2 GE per fatty alkyl radical are understood here, in particular those with 1.0 to 1.4 GE. The fatty alkyl radical has 10 to 18, in particular 12 to 14, carbon atoms. "Fatty alkyl" is understood to mean the residues of the fatty alcohols which are produced by hydrogenation of natural fatty acids and which are wholly or predominantly saturated.
Weiterhin können die erfindungsgemäßen Mittel bevorzugt auch noch Lösungsvermittler, Lösungsmittel , Färb- und Duftstoffe ent¬ halten.
Als Lösungsvermittler, etwa für geringe Farbstoff- und Parfum- ölzusätze , können beispielsweise Alkanolamine, Polyole , wie Ethy- lenglykol , Propylenglykol-1 ,2 oder Glycerin dienen . Ihre Einsatz¬ mengen liegen im allgemeinen zwischen 1 und 15 Gewichtsprozent, bezogen auf das gesamte Mittel .Furthermore, the agents according to the invention can preferably also contain solubilizers, solvents, colorants and fragrances. Alkanolamines, polyols, such as ethylene glycol, propylene glycol-1, 2 or glycerol, for example, can serve as solubilizers, for example for small amounts of dye and perfume oil. The amounts used are generally between 1 and 15 percent by weight, based on the total composition.
Zusätzlich werden meist Lösungsmittel , wie niedermolekulare Alka- nole mit 1 bis 4 Kohlenstoffatomen im Molekül , vorzugsweise Etha- nol und Isopropylalkohol eingesetzt. Ihre Einsatzmengen betragen bis zu 15 Gewichtsprozent, bezogen auf das gesamte Mittel . Auch Viskositätsregulatoren wie Harnstoff, Natriumchlorid , Ammonium¬ chlorid , Magnesiumchlorid und Salze von Mono-, Di- und Tricar- bonsäuren und Gemischen daraus können einzeln oder kombiniert in erforderlichen Mengen eingesetzt werden . Weitere übliche fakultative Zusätze sind Korrosionsinhibitoren und Konservie¬ rungsmittel .In addition, mostly solvents, such as low molecular weight alkanols with 1 to 4 carbon atoms in the molecule, preferably ethanol and isopropyl alcohol, are used. The amounts used are up to 15 percent by weight, based on the total agent. Viscosity regulators such as urea, sodium chloride, ammonium chloride, magnesium chloride and salts of mono-, di- and tricarboxylic acids and mixtures thereof can also be used individually or in combination in the required amounts. Further customary optional additives are corrosion inhibitors and preservatives.
Ein etwa noch auf insgesamt 100 Gewichtsprozent zu berechnender Rest für das Gesamtmittel besteht jeweils aus Wasser.A remainder, to be calculated for a total of 100 percent by weight, for the total composition consists of water.
Die erfindungsgemäßen flüssigen Reinigungsmittel nach den fol¬ genden Beispielen wurden durch Zusammenrühren der einzelnen Bestandteile und Stehenlassen des Gemisches bis zur Blasenfrei¬ heit erhalten .
The liquid cleaning agents according to the invention according to the following examples were obtained by stirring the individual constituents together and allowing the mixture to stand until there were no bubbles.
BeispieleExamples
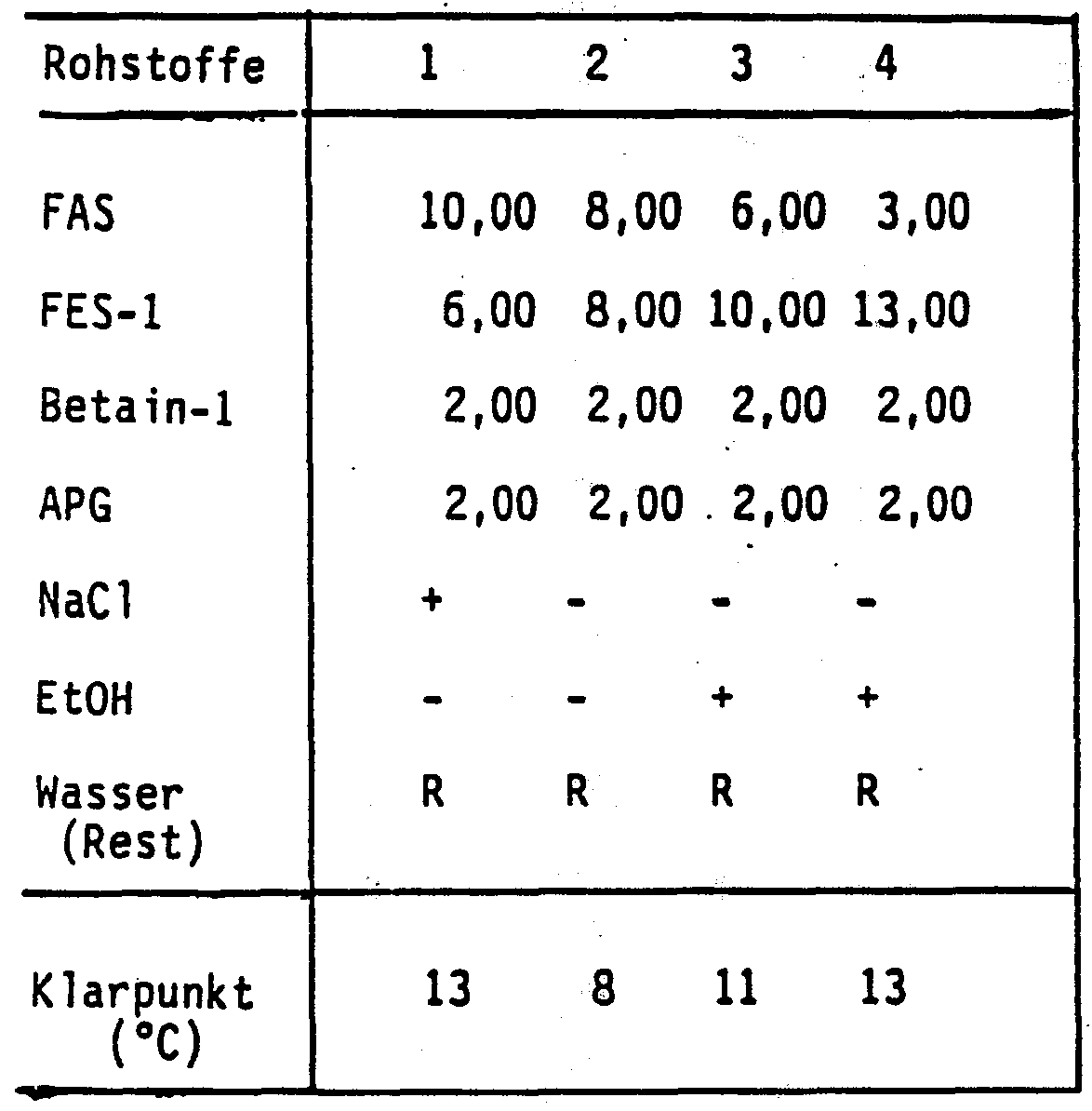
Beispiel 1 : .Example 1 : .
Folgende Substanzen wurden eingesetzt:The following substances were used:
FAS : C1 2_14-Alkylsulfat, Na-SalzFAS: C 1 2 _ 14 alkyl sulfate, Na salt
FES-1 : C1 2_1 4-Alkylethersulfat, 2 EO , Na-SalzFES-1: C 1 2 _ 1 4 alkyl ether sulfate, 2 EO, Na salt
Betain-1 : C_ , g-DimethylacylamidobetainBetaine-1: C_, g-dimethylacylamidobetaine
APG : C1 2_1 4-Alkylglucosid 1 ,4 GEAPG: C 1 2 _ 1 4 alkyl glucoside 1, 4 GE
EtOH : EthanolEtOH: ethanol
Die aufgeführten , neutralen Kombinationen wurden mit Kochsalz und/oder Ethanol auf Viskositäten zwischen 300 und 400 mPas (nach Höppler, 20 °C) eingestellt. Die Proben wurden 24 Stunden bei -15 °C gelagert. Als Klarpunkt wurde die Temperatur be¬ stimmt, bei welcher die Produkte wieder optisch klar wurden .The neutral combinations listed were adjusted with sodium chloride and / or ethanol to viscosities between 300 and 400 mPas (according to Höppler, 20 ° C). The samples were stored at -15 ° C for 24 hours. The temperature at which the products became optically clear was determined as a clear point.
Die folgenden Ergebnisse (Tabelle 1 ) zeigen , daß die quaternären Kombinationen aus Alkylsulfat, Alkylethersulfat, Betain und Alkylpolyglucosid über einen weiten Mischungsbereich ein hervorragendes Kälteverhalten aufweisen.
The following results (Table 1) show that the quaternary combinations of alkyl sulfate, alkyl ether sulfate, betaine and alkyl polyglucoside have excellent cold behavior over a wide range of mixtures.
Tabelle 1Table 1
Beispiel 2 :Example 2:
Die folgenden Ergebnisse (Tabelle 2) zeigen , daß die quaternären Kombinationen aus Alkylsulfat, Alkylethersulfat, Betain und Alkylpolyglucosid über einen weiten Mischungsbereich - auch in Gegenwart größerer Mengen an Alkohol - ein hervorragendes Kälteverhalten aufweisen . Die Viskositäten wurden wie in Beispiel 1 beschrieben eingestellt und der Klarpunkt entsprechend bestimmt.The following results (Table 2) show that the quaternary combinations of alkyl sulfate, alkyl ether sulfate, betaine and alkyl polyglucoside have an excellent low-temperature behavior over a wide mixing range - even in the presence of large amounts of alcohol. The viscosities were set as described in Example 1 and the clear point was determined accordingly.
Die folgenden Ergebnisse zeigen, daß die quaternären Kombinati¬ onen aus Alkylsulfat, Alkylethersulfat, Betain und Alkylpolygluco- sid nicht nur Vorteile bezüglich ihres Kälteverhaitens aufweisen , sondern auch zu ausgezeichneten spültechnischen Ergebnissen führen . Die Reinigungsleistung wurde mit Hilfe des Tellertestes bestimmt, der im folgenden beschrieben wird :The following results show that the quaternary combinations of alkyl sulfate, alkyl ether sulfate, betaine and alkyl polyglucoside not only have advantages in terms of their cold behavior, but also lead to excellent washing results. The cleaning performance was determined using the plate test, which is described below:
(A) : Rindertalganschmutzung(A): Beef tallow soiling
Die Untertassen werden mit jeweils 2 g geschmolzenem Rindertalg (Testanschmutzung A) überzogen. Dann werden 8 I Leitungswas¬ ser (16 °d) von 50 °C in eine Schüssel gegeben . Zum Reinigen der mit (A) angeschmutzten Teller wurden 4 g , d .h . 0 ,5 g/l , des Reinigungsmittels zugegeben und die Teller gewaschen . Bis zum Verschwinden des Schaumes der anfangs stark schäumenden Lösung konnten die in Tabelle 3 aufgeführten Teller sauber gespült werden .The saucers are each covered with 2 g of melted beef tallow (test soil A). Then 8 l of tap water (16 ° d) at 50 ° C. are placed in a bowl. To clean the plates soiled with (A), 4 g, i.e. 0.5 g / l, of the cleaning agent added and the plates washed. The plates listed in Table 3 could be rinsed clean until the foam of the initially foaming solution disappeared.
( B) : Mischanschmutzung(B): mixed soiling
Die Untertassen werden mit jeweils 2 g Mischanschmutzung (Test¬ anschmutzung B) überzogen. Diese Anschmutzung erhält man durch eine in Wasser verrührte Mischung aus Eiweiß, Fett und Kohlenhydraten (Mi No 1 von Henkel) . Dann werden 8 I Leitungs¬ wasser (16°d) von 45 °C in eine Schüssel gegeben. Zum Reinigen der mit (B) angeschmutzten Teller wurden 3 ,2 g , d .h . 0 ,4 g/l , des hergestellten Reinigungsmittels zugegeben und die Teller gewaschen . Die bis zum Verschwinden des Schaumes sauber gespülten Teller sind in Tabelle 3 aufgeführt.
Tabelle 3The saucers are each covered with 2 g of mixed soiling (test soiling B). This soiling is obtained by mixing water, fat and carbohydrates (Mi No 1 from Henkel) with water. Then 8 l tap water (16 ° d) at 45 ° C are placed in a bowl. To clean the plates soiled with (B), 3, 2 g, i.e. 0.4 g / l, of the detergent produced are added and the plates are washed. The plates that were rinsed clean until the foam disappeared are listed in Table 3. Table 3
(A): Anzahl sauber gespülter Teller: Fettanschπutzung (B): Anzahl sauber gespülter Teller: Hischanschπutzung
Beispiel 4:(A): Number of cleanly washed plates: use of grease (B): Number of cleanly washed plates: use of grease Example 4:
Die folgenden Ergebnisse (Tabelle 4) zeigen, daß nicht nur Amidobetaine (Betain-1), sondern auch einfache Betaine (Betain-2) eingesetzt werden können. Als Alkylethersulfate können nicht nur Produkte mit einem mittleren EO-Grad von ca. 2 und normaler Kokos-Ketten Verteilung (C :C_. =70:30) eingesetzt werden, sondern auch Produkte mit einem mittleren EO-Grad von ca. 3,6 und einer Kettenverteilung C_.-:C_. =50:50. Folgende Substanzen wurden eingesetzt:The following results (Table 4) show that not only amido betaines (betaine-1) but also simple betaines (betaine-2) can be used. Not only products with an average EO degree of approx. 2 and normal coconut chain distribution (C: C_. = 70: 30) can be used as alkyl ether sulfates, but also products with an average EO degree of approx. 3.6 and a chain distribution C _.-: C_. = 50: 50. The following substances were used:
FES-2 : C ^-Alkylethersulfat, 3,6 EO, Na-SalzFES-2: C 1-4 alkyl ether sulfate, 3.6 EO, Na salt
Betain-2: C_- 1Q-DimethylalkylacetobetainBetain-2: C_- 1Q -dimethylalkylacetobetaine
1 — 1 o1 - 1 o
Die folgenden Ergebnisse zeigen , daß nicht nur die binären Tensidkombinationen aus Alkylsulfat und Alkylethersulfat zu Produkten mit gutem Kälteverhalten führen , sondern daß auch die ternaren Tensidkombinationen aus Alkylsulfat und verschiedenen Alkylethersulfaten diesen Anforderungen genügen .The following results show that not only the binary surfactant combinations of alkyl sulfate and alkyl ether sulfate lead to products with good cold behavior, but also that the ternary surfactant combinations of alkyl sulfate and various alkyl ether sulfates meet these requirements.
Tabelle 5Table 5
Die folgenden Ergebnisse zeigen , daß der Aktivsubstanzgehalt der erfindungsgemäßen Formulierungen über einen weiten Bereich variieren kann und jedenfalls Vorteile gegenüber den entsprechenden Kombinationen nach der EP-Anmeldung 341 071 ( Formeln A und B) erhalten werden.The following results show that the active substance content of the formulations according to the invention can vary over a wide range and in any case advantages over the corresponding combinations according to EP application 341 071 (formulas A and B) are obtained.
Tabelle 6Table 6
Formel-Nr.Formula No.
Wasser ( Rest) R RWater (rest) R R
Klarpunkt (°C) 13 16 17Clear point (° C) 13 16 17
Teller (A) 12 18 17 16 18 18Plate (A) 12 18 17 16 18 18
Teller (B) 26 4,0 45 45 39 36
Plate (B) 26 4.0 45 45 39 36
Claims
1. Wäßrige flüssige- Reinigungsmittel, insbesondere für die manuelle Geschirreinigung, mit einem Aktivsubstanzgehalt von 10 bis 60, vorzugsweise 15 bis 40 Gewichtsprozent, bestehend aus Alkylsulfaten, Alkylethersulfaten, Betainen und Alkyl¬ glucosiden, dadurch gekennzeichnet, daß sie mehr als 45 % der Aktivsubstanz an Alkylsulfaten und Alkylethersulfaten ent¬ halten, wobei deren Gehalt größer als das 1 ,5 fache des Be¬ taingehaltes ist und wenigstens das Doppelte des Alkylglu- cosidgehalts ausmacht.1. Aqueous liquid cleaning agents, in particular for manual dishwashing, with an active substance content of 10 to 60, preferably 15 to 40 percent by weight, consisting of alkyl sulfates, alkyl ether sulfates, betaines and alkyl glucosides, characterized in that they contain more than 45% of the active ingredient contain alkyl sulfates and alkyl ether sulfates, the content of which is greater than 1.5 times the betaine content and is at least twice the alkyl glucoside content.
2. Mittel nach Anspruch 1, dadurch gekennzeichnet, daß der Gehalt an Alkylsulfaten und Alkylethersulfaten maximal etwa das 50fache des Betaingehaltes ausmacht.2. Composition according to claim 1, characterized in that the content of alkyl sulfates and alkyl ether sulfates makes up a maximum of about 50 times the betaine content.
3. Mittel nach den Ansprüchen 1 und 2, dadurch gekennzeichnet daß der Gehalt an Alkylsulfaten und Alkylethersulfaten maximal etwa das 50fache des Alkylglucosidgehaltes ausmacht.3. Composition according to claims 1 and 2, characterized in that the content of alkyl sulfates and alkyl ether sulfates makes up a maximum of about 50 times the alkyl glucoside content.
4. Mittel nach den Ansprüchen 1 bis 3, dadurch gekennzeichnet, daß die Alkylsulfate geradkettige, aliphatische C_._ _ _._-, vor¬ zugsweise C.« _ -„ - Alkylreste enthalten.4. Composition according to claims 1 to 3, characterized in that the alkyl sulfates contain straight-chain, aliphatic C _._ _ _._-, preferably C. «_ -" - alkyl radicals.
5. Mittel nach den Ansprüchen 1 bis 4, dadurch gekennzeichnet, daß die Alkylethersulfate geradkettige, aliphatische C.- _.g-, vorzugsweise C-- _ .„ - Alkylreste und 1 bis 5, vorzugsweise 2 bis 4 Ethylenoxidgruppen enthalten.5. Composition according to claims 1 to 4, characterized in that the alkyl ether sulfates straight-chain, aliphatic C.- _. g -, preferably C-- _. "- contain alkyl radicals and 1 to 5, preferably 2 to 4 ethylene oxide groups.
6. Mittel nach den Ansprüchen 1 bis 5, dadurch gekennzeichnet, daß das Gewichtsverhältnis von Alkylsulfat zu Alkylethersulfat 4 : 1 bis 1 : 5 beträgt. 6. Composition according to claims 1 to 5, characterized in that the weight ratio of alkyl sulfate to alkyl ether sulfate is 4: 1 to 1: 5.
7. Mittel nach den Ansprüchen 1 bis 6 , dadurch gekennzeichnet, daß sie zusätzlich Verbindungen aus der Gruppe der Lösungs¬ vermittler, Lösungsmittel , Färb-, Duftstoffe und Konservie¬ rungsmittel enthalten . 7. Composition according to claims 1 to 6, characterized in that they additionally contain compounds from the group of solvents, solvents, dyes, fragrances and preservatives.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE59102570T DE59102570D1 (en) | 1990-02-02 | 1991-01-24 | AQUEOUS LIQUID DETERGENT. |
| EP91903675A EP0513138B2 (en) | 1990-02-02 | 1991-01-24 | Aqueous liquid cleaning agent |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE4003098A DE4003098A1 (en) | 1990-02-02 | 1990-02-02 | WAESSFUL LIQUID CLEANING AGENT |
| DEP4003098.9 | 1990-02-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1991011506A1 true WO1991011506A1 (en) | 1991-08-08 |
Family
ID=6399292
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP1991/000134 WO1991011506A1 (en) | 1990-02-02 | 1991-01-24 | Aqueous liquid cleaning agent |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0513138B2 (en) |
| AT (1) | ATE110106T1 (en) |
| DE (2) | DE4003098A1 (en) |
| ES (1) | ES2059120T5 (en) |
| WO (1) | WO1991011506A1 (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994009102A1 (en) * | 1992-10-14 | 1994-04-28 | Henkel Kommanditgesellschaft Auf Aktien | Aqueous detergent mixtures |
| WO1995004592A1 (en) * | 1993-08-06 | 1995-02-16 | Societe D'exploitation De Produits Pour Les Industries Chimiques, S.E.P.P.I.C. | Concentrated aqueous compositions of alkylpolyglycosides, and applications thereof |
| WO1996035770A1 (en) * | 1995-05-10 | 1996-11-14 | Unilever Plc | Light duty cleaning composition |
| EP0763591A1 (en) * | 1995-09-15 | 1997-03-19 | Henkel Kommanditgesellschaft auf Aktien | Aqueous manual dishwashing composition |
| WO1997018189A1 (en) * | 1995-11-15 | 1997-05-22 | Henkel Kommanditgesellschaft Auf Aktien | Fatty alcohol (ether) sulphates with improved low-temperature behaviour |
| WO1997044423A1 (en) * | 1996-05-23 | 1997-11-27 | Henkel Kommanditgesellschaft Auf Aktien | Hand dishwashing agent acceptable to the skin |
| US5925747A (en) * | 1994-10-04 | 1999-07-20 | Henkel Kommanditgesellschaft Auf Aktien | Pumpable water-containing surfactant concentrates |
| US5932535A (en) * | 1995-12-21 | 1999-08-03 | Henkel Kommanditgesellschaft Auf Aktien | Process for the production of light-colored, low-viscosity surfactant concentrates |
| US6015780A (en) * | 1997-04-08 | 2000-01-18 | Henkel Kommanditgesellschaft Auf Aktien | Formulations for cleaning hard surfaces comprising a betaine surfactant having exactly 12 carbon atoms |
| FR2795659A1 (en) * | 1999-06-29 | 2001-01-05 | Cognis Deutschland Gmbh | MIXTURES OF HIGHLY CONCENTRATED FLUID ANIONIC SURFACTANTS |
| FR2826017A1 (en) * | 2001-06-15 | 2002-12-20 | Cognis France Sa | Surfactant mixtures useful in cosmetics, body care products, shower gels or shower baths and baby care products comprises oligoglycosides, betaines and alkyl ether sulfates |
| EP2133408A1 (en) * | 2000-06-29 | 2009-12-16 | Ecolab Inc. | Rinse agent composition and method for rinsing a substrate surface |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19506207A1 (en) * | 1995-02-23 | 1996-08-29 | Goldschmidt Ag Th | A storage stable, concentrated surfactant composition based on alkylglucosides |
| DE19548068C1 (en) | 1995-12-21 | 1997-06-19 | Henkel Kgaa | Process for the production of light colored, low viscosity surfactant concentrates |
| DE102023203495A1 (en) | 2023-04-18 | 2024-10-24 | Henkel Ag & Co. Kgaa | Grease-dissolving hand dishwashing detergent with non-ethoxylated alkyl sulfate |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0070074A2 (en) * | 1981-07-13 | 1983-01-19 | THE PROCTER & GAMBLE COMPANY | Foaming surfactant compositions |
| GB2114996A (en) * | 1982-02-19 | 1983-09-01 | Colgate Palmolive Co | Mild liquid detergent compositions |
| DE3534082C2 (en) * | 1985-09-25 | 1988-07-28 | Henkel Kgaa, 4000 Duesseldorf, De | |
| EP0341071A2 (en) * | 1988-05-06 | 1989-11-08 | Unilever Plc | Detergent compositions |
-
1990
- 1990-02-02 DE DE4003098A patent/DE4003098A1/en not_active Withdrawn
-
1991
- 1991-01-24 AT AT91903675T patent/ATE110106T1/en not_active IP Right Cessation
- 1991-01-24 ES ES91903675T patent/ES2059120T5/en not_active Expired - Lifetime
- 1991-01-24 EP EP91903675A patent/EP0513138B2/en not_active Expired - Lifetime
- 1991-01-24 DE DE59102570T patent/DE59102570D1/en not_active Expired - Fee Related
- 1991-01-24 WO PCT/EP1991/000134 patent/WO1991011506A1/en active IP Right Grant
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0070074A2 (en) * | 1981-07-13 | 1983-01-19 | THE PROCTER & GAMBLE COMPANY | Foaming surfactant compositions |
| GB2114996A (en) * | 1982-02-19 | 1983-09-01 | Colgate Palmolive Co | Mild liquid detergent compositions |
| DE3534082C2 (en) * | 1985-09-25 | 1988-07-28 | Henkel Kgaa, 4000 Duesseldorf, De | |
| EP0341071A2 (en) * | 1988-05-06 | 1989-11-08 | Unilever Plc | Detergent compositions |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994009102A1 (en) * | 1992-10-14 | 1994-04-28 | Henkel Kommanditgesellschaft Auf Aktien | Aqueous detergent mixtures |
| US5578560A (en) * | 1992-10-14 | 1996-11-26 | Henkel Corporation | Water-containing detergent mixtures comprising oligoglycoside surfactants |
| WO1995004592A1 (en) * | 1993-08-06 | 1995-02-16 | Societe D'exploitation De Produits Pour Les Industries Chimiques, S.E.P.P.I.C. | Concentrated aqueous compositions of alkylpolyglycosides, and applications thereof |
| FR2709679A1 (en) * | 1993-08-06 | 1995-03-17 | Seppic Sa | Concentrated aqueous compositions of alkylpolyglycosides and uses thereof |
| US5925747A (en) * | 1994-10-04 | 1999-07-20 | Henkel Kommanditgesellschaft Auf Aktien | Pumpable water-containing surfactant concentrates |
| AU695205B2 (en) * | 1995-05-10 | 1998-08-06 | Unilever Plc | Light duty cleaning composition |
| US5807816A (en) * | 1995-05-10 | 1998-09-15 | Lever Brothers Company, Division Of Conopco, Inc. | Light duty cleaning composition |
| WO1996035770A1 (en) * | 1995-05-10 | 1996-11-14 | Unilever Plc | Light duty cleaning composition |
| EP0763591A1 (en) * | 1995-09-15 | 1997-03-19 | Henkel Kommanditgesellschaft auf Aktien | Aqueous manual dishwashing composition |
| WO1997018189A1 (en) * | 1995-11-15 | 1997-05-22 | Henkel Kommanditgesellschaft Auf Aktien | Fatty alcohol (ether) sulphates with improved low-temperature behaviour |
| US5932535A (en) * | 1995-12-21 | 1999-08-03 | Henkel Kommanditgesellschaft Auf Aktien | Process for the production of light-colored, low-viscosity surfactant concentrates |
| WO1997044423A1 (en) * | 1996-05-23 | 1997-11-27 | Henkel Kommanditgesellschaft Auf Aktien | Hand dishwashing agent acceptable to the skin |
| US6015780A (en) * | 1997-04-08 | 2000-01-18 | Henkel Kommanditgesellschaft Auf Aktien | Formulations for cleaning hard surfaces comprising a betaine surfactant having exactly 12 carbon atoms |
| FR2795659A1 (en) * | 1999-06-29 | 2001-01-05 | Cognis Deutschland Gmbh | MIXTURES OF HIGHLY CONCENTRATED FLUID ANIONIC SURFACTANTS |
| EP2133408A1 (en) * | 2000-06-29 | 2009-12-16 | Ecolab Inc. | Rinse agent composition and method for rinsing a substrate surface |
| FR2826017A1 (en) * | 2001-06-15 | 2002-12-20 | Cognis France Sa | Surfactant mixtures useful in cosmetics, body care products, shower gels or shower baths and baby care products comprises oligoglycosides, betaines and alkyl ether sulfates |
| WO2002102949A1 (en) * | 2001-06-15 | 2002-12-27 | Cognis France S.A. | Surfactant mixtures |
| US7279456B2 (en) | 2001-06-15 | 2007-10-09 | Gognis France S.A. | Surfactant mixtures |
Also Published As
| Publication number | Publication date |
|---|---|
| DE4003098A1 (en) | 1991-08-08 |
| ATE110106T1 (en) | 1994-09-15 |
| ES2059120T5 (en) | 1997-08-01 |
| EP0513138B2 (en) | 1997-06-18 |
| EP0513138A1 (en) | 1992-11-19 |
| EP0513138B1 (en) | 1994-08-17 |
| ES2059120T3 (en) | 1994-11-01 |
| DE59102570D1 (en) | 1994-09-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0216301B1 (en) | Liquid cleaning agent | |
| EP0280143B1 (en) | Liquid cleaning agent | |
| DE68917195T2 (en) | Detergent compositions. | |
| DE69212045T2 (en) | Mild liquid detergent compositions | |
| AT396110B (en) | CLEAR, AQUEOUS DETERGENT | |
| DE2904945C2 (en) | Cleaning agents, in particular dishwashing detergents | |
| DE3533977A1 (en) | STRONG FOAMING LIQUID FINE CLEANER BASED ON NON-IONIC SURFACTANTS | |
| EP0513138B1 (en) | Aqueous liquid cleaning agent | |
| EP0664830B1 (en) | Aqueous detergent mixtures | |
| DE4311114A1 (en) | Detergent mixtures | |
| EP0574448A1 (en) | Liquid detergent containing di-salts of sulfo-oleic acid | |
| DE69311854T2 (en) | Liquid cleaning agent based on highly foaming, non-ionic, surface-active agents | |
| WO1994010279A1 (en) | Process for preparing aqueous solutions of anionic surfactants with improved low-temperature stability | |
| DE69202807T2 (en) | Liquid dishwashing liquid. | |
| EP1075502B1 (en) | Dishwashing detergent with an antibacterial effect | |
| EP0444262A2 (en) | Liquid foaming detergent | |
| DE4026809A1 (en) | Liq. aq. washing compsn. - contains anionic surfactant, nonionic surfactant and alkyl poly:glycoside as thickener, improving washing | |
| EP0863972A1 (en) | Aqueous surfactant mixture | |
| DE2359992C2 (en) | Liquid detergent | |
| EP0788537B1 (en) | Aqueous hand washing-up liquid | |
| DE19813042A1 (en) | Aqueous washing-up liquid mild to skin | |
| DE19519405A1 (en) | Aqueous detergent composition | |
| WO1994022995A1 (en) | Liquid detergent | |
| DE3220505A1 (en) | LIQUID CLEANING AGENTS | |
| WO1990008813A1 (en) | Liquid detergents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IT LU NL SE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1991903675 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1991903675 Country of ref document: EP |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1991903675 Country of ref document: EP |