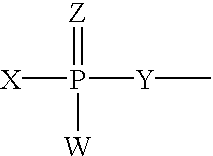US20030143732A1 - RNA interference mediated inhibition of adenosine A1 receptor (ADORA1) gene expression using short interfering RNA - Google Patents
RNA interference mediated inhibition of adenosine A1 receptor (ADORA1) gene expression using short interfering RNA Download PDFInfo
- Publication number
- US20030143732A1 US20030143732A1 US10/224,005 US22400502A US2003143732A1 US 20030143732 A1 US20030143732 A1 US 20030143732A1 US 22400502 A US22400502 A US 22400502A US 2003143732 A1 US2003143732 A1 US 2003143732A1
- Authority
- US
- United States
- Prior art keywords
- sirna
- sequence
- sirna molecule
- rna
- alkyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [1*]*P(=C)([W])[Y][2*] Chemical compound [1*]*P(=C)([W])[Y][2*] 0.000 description 11
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1137—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1138—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against receptors or cell surface proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/12—Type of nucleic acid catalytic nucleic acids, e.g. ribozymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/12—Type of nucleic acid catalytic nucleic acids, e.g. ribozymes
- C12N2310/121—Hammerhead
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/13—Decoys
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/18—Type of nucleic acid acting by a non-sequence specific mechanism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/317—Chemical structure of the backbone with an inverted bond, e.g. a cap structure
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
- C12N2310/332—Abasic residue
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/346—Spatial arrangement of the modifications having a combination of backbone and sugar modifications
Definitions
- the present invention concerns methods and reagents useful in modulating gene expression associated with asthma, inflammation and allergic response in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications.
- the invention relates to short interfering nucleic acid molecules (siRNA) capable of mediating RNA interference (RNAi) against adenosine A1 receptor gene expression.
- siRNA short interfering nucleic acid molecules
- RNA interference refers to the process of sequence-specific post transcriptional gene silencing in animals mediated by short interfering RNAs (siRNA) (Fire et al., 1998, Nature, 391, 806).
- siRNA short interfering RNAs
- the corresponding process in plants is commonly referred to as post transcriptional gene silencing or RNA silencing and is also referred to as quelling in fungi.
- the process of post transcriptional gene silencing is thought to be an evolutionarily conserved cellular defense mechanism used to prevent the expression of foreign genes which is commonly shared by diverse flora and phyla (Fire et al., 1999, Trends Genet., 15, 358).
- Such protection from foreign gene expression may have evolved in response to the production of double stranded RNAs (dsRNA) derived from viral infection or the random integration of transposon elements into a host genome via a cellular response that specifically destroys homologous single stranded RNA or viral genomic RNA.
- dsRNA double stranded RNAs
- the presence of dsRNA in cells triggers the RNAi response though a mechanism that has yet to be fully characterized. This mechanism appears to be different from the interferon response that results from dsRNA mediated activation of protein kinase PKR and 2′, 5′-oligoadenylate synthetase resulting in non-specific cleavage of mRNA by ribonuclease L.
- Dicer a ribonuclease III enzyme referred to as Dicer.
- Dicer is involved in the processing of the dsRNA into short pieces of dsRNA known as short interfering RNAs (siRNA) (Berstein et al., 2001, Nature, 409, 363).
- Short interfering RNAs derived from Dicer activity are typically about 21-23 nucleotides in length and comprise about 19 base pair duplexes.
- Dicer has also been implicated in the excision of 21 and 22 nucleotide small temporal RNAs (stRNA) from precursor RNA of conserved structure that are implicated in translational control (Hutvagner et al., 2001, Science, 293, 834).
- the RNAi response also features an endonuclease complex containing a siRNA, commonly referred to as an RNA-induced silencing complex (RISC), which mediates cleavage of single stranded RNA having sequence complementary to the antisense strand of the siRNA duplex. Cleavage of the target RNA takes place in the middle of the region complementary to the antisense strand of the siRNA duplex (Elbashir et al., 2001, Genes Dev., 15, 188).
- RISC RNA-induced silencing complex
- RNAi has been studied in a variety of systems. Fire et al., 1998, Nature, 391, 806, were the first to observe RNAi in C. elegans. Wianny and Goetz, 1999, Nature Cell Biol., 2, 70, describe RNAi mediated by dsRNA in mouse embryos. Hammond et al., 2000, Nature, 404, 293, describe RNAi in Drosophila cells transfected with dsRNA. Elbashir et al., 2001, Nature, 411, 494, describe RNAi induced by introduction of duplexes of synthetic 21-nucleotide RNAs in cultured mammalian cells including human embryonic kidney and HeLa cells.
- RNAi activity Single mismatch sequences in the center of the siRNA duplex were also shown to abolish RNAi activity.
- these studies also indicate that the position of the cleavage site in the target RNA is defined by the 5′-end of the siRNA guide sequence rather than the 3′-end (Elbashir et al., 2001, EMBO J., 20, 6877).
- Other studies have indicated that a 5′-phosphate on the target-complementary strand of a siRNA duplex is required for siRNA activity and that ATP is utilized to maintain the 5′-phosphate moiety on the siRNA (Nykanen et al., 2001, Cell, 107, 309).
- siRNA may include modifications to either the phosphate-sugar back bone or the nucleoside to include at least one of a nitrogen or sulfur heteroatom”, however neither application teaches to what extent these modifications are tolerated in siRNA molecules nor provide any examples of such modified siRNA. Kreutzer and Limmer, Canadian Patent Application No.
- 2,359,180 also describe certain chemical modifications for use in dsRNA constructs in order to counteract activation of double stranded-RNA-dependent protein kinase PKR, specifically 2′-amino or 2′-O-methyl nucleotides, and nucleotides containing a 2′-O or 4′-C methylene bridge.
- PKR double stranded-RNA-dependent protein kinase
- 2′-amino or 2′-O-methyl nucleotides specifically 2′-amino or 2′-O-methyl nucleotides, and nucleotides containing a 2′-O or 4′-C methylene bridge.
- Kreutzer and Limmer similarly fail to show to what extent these modifications are tolerated in siRNA molecules nor do they provide any examples of such modified siRNA.
- Zernicka-Goetz et al. International PCT Publication No. WO 01/36646, describes certain methods for inhibiting the expression of particular genes in mammalian cells using certain dsRNA molecules.
- Fire et al. International PCT Publication No. WO 99/32619, describes particular methods for introducing certain dsRNA molecules into cells for use in inhibiting gene expression.
- Plaetinck et al. International PCT Publication No. WO 00/01846, describes certain methods for identifying specific genes responsible for conferring a particular phenotype in a cell using specific dsRNA molecules.
- Mello et al. International PCT Publication No. WO 01/29058, describes the identification of specific genes involved in dsRNA mediated RNAi.
- WO 01/38551 describes certain methods for regulating polycomb gene expression in plants.
- Churikov et al., International PCT Publication No. WO 01/42443 describes certain methods for modifying genetic characteristics of an organism.
- Cogoni et al., International PCT Publication No. WO 01/53475 describes certain methods for isolating a Neurospora silending gene and uses thereof.
- Reed et al., International PCT Publication No. WO 01/68836 describes certain methods for gene silencing in plants.
- Honer et al, International PCT Publication No. WO 01/70944 describes certain methods of drug screening using transgenic nematodes as Parkinson's disease models. Deak et al., International PCT Publication No.
- WO 01/72774 describes certain Drosophila derived gene products.
- Arndt et al., International PCT Publication No. WO 01/92513 describes certain methods for mediating gene suppression by using factors that enhance RNAi. Tuschl et al., International PCT Publication No. WO 02/44321, describe certain synthetic siRNA constructs.
- Pachuk et al., International PCT Publication No. WO 00/63364, and Satishchandran et al., International PCT Publication No. WO 01/04313 describes certain methods and compositions for inhibiting the function of certain polynucleotide sequences.
- Echeverri et al., International PCT Publication No. WO 02/38805 describes certain C. elegans genes identified via RNAi. Kreutzer et al., International PCT Publication No. WO 02/055692 and WO 02/055693, describes certain methods for inhibiting gene expression using RNAi.
- Asthma is a chronic inflammatory disorder of the lungs characterized by airflow obstruction, bronchial hyper-responsiveness, and airway inflammation. T-lymphocytes that produce TH2 cytokines and eosinophilic leukocytes infiltrate the airways. In the airway and in bronchial alveolar lavage (BAL) fluid of individuals with asthma, high concentrations of TH2 cytokines, interleukin-4 (IL-4), IL-5, and IL-13, are present along with increased levels of adenosine. In contrast to normal individuals, asthmatics respond to adenosine challenge with marked airway obstruction. Upon allergen challenge, mast cells are activated by cross-linked IgE-allergen complexes.
- BAL bronchial alveolar lavage
- PGD2 prostaglandin D2
- PGD2 the major cyclooxygenase product of arachidonic acid are released.
- PGD2 is generated from PGH2 via the activity of prostaglandin D2 synthetase (PTGDS).
- PGD2 receptors and adenosine A1 receptors are present in the lungs and airway along with various other tissues in response to allergic stimuli (Howarth, 1997, Allergy, 52, 12).
- DP PGD2 receptor
- PGD2 receptor a heterotrimeric GTP-binding protein-coupled, rhodopsin-type receptor specific for PGD2 (Hirata et al., 1994, PNAS USA., 91, 11192). These mice fail to develop airway hyperreactivity and have greatly reduced eosinophil infiltration and cytokine accumulation in response to allergens.
- PGD2 prostaglandin D2 receptor
- siRNA short interfering RNA
- ADORA1 adenosine A1 receptor
- the siRNA molecule can be adapted for use to treat, for example allergic/inflammatory diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and any other indications that can respond to the level of ADORA1.
- the siRNA molecule can comprise a sense region and an antisense region.
- the antisense region can comprise sequence complementary to an RNA sequence encoding ADORA1 and the sense region can comprise sequence complementary to the antisense region.
- An siRNA molecule of the invention can be adapted for use to treat asthma.
- An siRNA molecule can comprise a sense region and an antisense region and wherein said antisense region comprises sequence complementary to an RNA sequence encoding ADORA1 and the sense region comprises sequence complementary to the antisense region.
- the siRNA molecule can be assembled from two nucleic acid fragments wherein one fragment comprises the sense region and the second fragment comprises the antisense region of said siRNA molecule.
- the sense region and antisense region can be covalently connected via a linker molecule.
- the linker molecule can be a polynucleotide linker or a non-nucleotide linker.
- the antisense region of ADORA1 siRNA constructs can comprise a sequence complementary to sequence having any of SEQ ID NOs. 1-161.
- the antisense region can also comprise sequence having any of SEQ ID NOs. 162-322, 336, 338, 340, 342, 344, or 346.
- the sequences shown in SEQ ID NO: 1-346 are not limiting.
- a siRNA molecule of the invention can comprise any contiguous ADORA1 sequences (e.g., about 19 contiguous ADORA1 nucleotides.
- the sense region of ADORA1 siRNA constructs can comprise sequence having any of SEQ ID NOs. 1-161, 335, 337, 339, 341, 343, or 345.
- the sense region can comprise a sequence of SEQ ID NO. 323 and the antisense region can comprise a sequence of SEQ ID NO. 324.
- the sense region can comprise a sequence of SEQ ID NO. 325 and the antisense region can comprise a sequence of SEQ ID NO. 326.
- the sense region can comprise a sequence of SEQ ID NO. 327 and the antisense region can comprise a sequence of SEQ ID NO. 328.
- the sense region can comprise a sequence of SEQ ID NO. 329 and the antisense region can comprise a sequence of SEQ ID NO. 330.
- the sense region can comprise a sequence of SEQ ID NO. 331 and the antisense region can comprise a sequence of SEQ ID NO. 332.
- the sense region can comprise a sequence of SEQ ID NO. 333 and the antisense region can comprise a sequence of SEQ ID NO. 334.
- the sense region of a siRNA molecule of the invention can comprise a 3′-terminal overhang and the antisense region can comprise a 3′-terminal overhang.
- the 3′-terminal overhangs each can comprise about 2 nucleotides.
- the antisense region of the 3′-terminal nucleotide overhang can be complementary to RNA encoding ADORA1.
- the sense region of a siRNA molecule can comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-O-methyl modified pyrimidine nucleotides.
- the sense region can comprise a terminal cap moiety at the 5′-end, 3′-end, or both 5′ and 3′ ends of said sense region.
- the antisense region of a siRNA molecule can comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy-2′-fluoro modified pyrimidine nucleotides.
- the antisense region can also comprise a phosphorothioate internucleotide linkage at the 3′ end of said antisense region.
- the antisense region can comprise between about one and about five phosphorothioate internucleotide linkages at the 5′ end of said antisense region.
- the 3′-terminal nucleotide overhangs of a siRNA molecule can comprise ribonucleotides or deoxyribonucleotides that are chemically modified at a nucleic acid sugar, base, or backbone.
- the 3′-terminal nucleotide overhangs can also comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base ribonucleotides. Additionally, the 3′-terminal nucleotide overhangs can comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) acyclic nucleotides.
- the 3′-terminal nucleotide overhangs can comprise nucleotides comprising internucleotide linkages having Formula I:
- each R1 and R2 is independently any nucleotide, non-nucleotide, or polynucleotide which can be naturally occurring or chemically modified
- each X and Y is independently O, S, N, alkyl, or substituted alkyl
- each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, or aralkyl, and wherein W, X, Y and Z are not all O.
- the 3′-terminal nucleotide overhangs can comprise nucleotides or non-nucleotides having Formula II:
- each R3, R4, R5, R6, R7, R8, R10, R11 and R12 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino,
- Another embodiment of the invention provides an expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of the invention in a manner that allows expression of the nucleic acid molecule.
- the expression vector can be in a mammalian cell, such as a human cell.
- the siRNA molecule can comprise a sense region and an antisense region.
- the antisense region can comprise sequence complementary to an RNA sequence encoding ADORA1 and the sense region comprises sequence complementary to the antisense region.
- the siRNA molecule can comprise two distinct strands having complementarity sense and antisense regions or can comprise a single strand having complementary sense and antisense regions.
- this invention relates to compounds, compositions, and methods useful for modulating gene expression, for example, genes associated with asthma, inflammation and allergic response by RNA interference (RNAi) using short interfering RNA (siRNA).
- the siRNA of the invention can be unmodified or chemically modified.
- the siRNA of the instant invention can be chemically synthesized, expressed from a vector or enzymatically synthesized.
- the instant invention also features various chemically modified synthetic short interfering RNA (siRNA) molecules capable of modulating ADORA1 gene expression/activity in cells by RNA inference (RNAi).
- siRNA molecules of the instant invention provide useful reagents and methods for a variety of therapeutic, diagnostic, agricultural, target validation, genomic discovery, genetic engineering and pharmacogenomic applications.
- the invention features one or more siRNA molecules and methods that independently or in combination modulate the expression of gene(s) encoding proteins associated with asthma, inflammation, and the allergic response.
- the present invention features siRNA molecules that modulate the expression of ADORA1 genes such as GenBank accession No. NM — 000674.
- ADORA1 exemplary gene
- A2A, A2B, and/or A3 genes which express other adenosine receptors
- Those additional genes can be analyzed for target sites using the methods described for ADORA1.
- the inhibition and the effects of such inhibition of the other genes can be performed as described herein.
- the inhibition and the effects of such inhibition of the other genes can be performed as described herein.
- the invention features a siRNA molecule that down regulates expression of an ADORA1 gene, for example, wherein the ADORA1 gene comprises ADORA1 sequence.
- the invention features a siRNA molecule having RNAi activity against ADORA1 RNA, wherein the siRNA molecule comprises a sequence complimentary to any RNA having ADORA1 encoding sequence, such as GenBank accession No. NM — 000674.
- the invention features a siRNA molecule comprising sequences selected from the group consisting of SEQ ID NOs: 1-322.
- the invention features an ADORA1 siRNA molecule having an antisense region complementary to any sequence having SEQ ID NOs: 1-161.
- the invention features an ADORA1 siRNA molecule having an antisense region having any of SEQ ID NOs: 162-322, 336, 338, 340, 342, 344, 346, 348, 350, 352 or 354.
- the invention features an ADORA1 siRNA molecule having a sense region having any of SEQ ID NOs.
- the sense region can comprise a sequence of SEQ ID NO. 323 and the antisense region can comprise a sequence of SEQ ID NO. 324.
- the sense region can comprise a sequence of SEQ ID NO. 325 and the antisense region can comprise a sequence of SEQ ID NO. 326.
- the sense region can comprise a sequence of SEQ ID NO. 327 and the antisense region can comprise a sequence of SEQ ID NO. 328.
- the sense region can comprise a sequence of SEQ ID NO. 329 and the antisense region can comprise a sequence of SEQ ID NO. 330.
- the sense region can comprise a sequence of SEQ ID NO.
- the antisense region can comprise a sequence of SEQ ID NO. 332.
- the sense region can comprise a sequence of SEQ ID NO. 333 and the antisense region can comprise a sequence of SEQ ID NO. 334.
- the invention features a siRNA molecule comprising a sequence, for example the antisense sequence of the siRNA construct, complementary to a sequence or portion of sequence comprising GenBank accession No. NM — 000674.
- a siRNA molecule of the invention has RNAi activity that modulates expression of RNA encoded by an ADORA1 gene.
- nucleic acid molecules of the invention that act as mediators of the RNA interference gene silencing response are double stranded RNA molecules.
- the siRNA molecules of the invention consist of duplexes containing about 19 base pairs between oligonucleotides comprising about 19 to about 25 nucleotides (e.g., about 19, 20, 21, 22, 23, 24, or 25).
- siRNA molecules of the invention comprise duplexes with overhanging ends of 1-3 (e.g., 1, 2, or 3) nucleotides, for example 21 nucleotide duplexes with 19 base pairs and 2 nucleotide 3′-overhangs. These nucleotide overhangs in the antisense strand are optionally complementary to the target sequence.
- the invention features chemically modified siRNA constructs having specificity for ADORA1 expressing nucleic acid molecules.
- chemical modifications include without limitation phosphorothioate internucleotide linkages, 2′-O-methyl ribonucleotides, 2′-deoxy-2′-fluoro ribonucleotides, “universal base” nucleotides, 5-C-methyl nucleotides, and inverted deoxyabasic residue incorporation.
- siRNA constructs can also be used to improve the stability of the interaction with the target RNA sequence and to improve nuclease resistance.
- nucleic acid molecules will provide a powerful tool in overcoming potential limitations of in vivo stability and bioavailability inherent to native RNA molecules that are delivered exogenously.
- the use of chemically modified nucleic acid molecules can enable a lower dose of a particular nucleic acid molecule for a given therapeutic effect since chemically modified nucleic acid molecules tend to have a longer half-life in serum.
- certain chemical modifications can improve the bioavailability of nucleic acid molecules by targeting particular cells or tissues and/or improving cellular uptake of the nucleic acid molecule.
- the overall activity of the modified nucleic acid molecule can be greater than the native molecule due to improved stability and/or delivery of the molecule.
- chemically modified siRNA can also minimize the possibility of activating interferon activity in humans.
- the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more nucleotides comprising a backbone modified internucleotide linkage having Formula I:
- each R1 and R2 is independently any nucleotide, non-nucleotide, or polynucleotide which can be naturally occurring or chemically modified
- each X and Y is independently O, S, N, alkyl, or substituted alkyl
- each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, or aralkyl, and wherein W, X, Y and Z are not all O.
- the chemically modified internucleotide linkages having Formula I can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands.
- the siRNA molecules of the invention can comprise one or more chemically modified internucleotide linkages having Formula I at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified internucleotide linkages having Formula I at the 5′-end of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) pyrimidine nucleotides with chemically modified internucleotide linkages having Formula I in the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) purine nucleotides with chemically modified internucleotide linkages having Formula I in the sense strand, antisense strand, or both strands.
- a siRNA molecule of the invention having internucleotide linkage(s) of Formula I also comprises a chemically modified nucleotide or non-nucleotide having any of Formulae II, III, V, or VI.
- the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more nucleotides or non-nucleotides having Formula II:
- each R3, R4, R5, R6, R7, R8, R10, R 11 and R12 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino,
- the chemically modified nucleotide or non-nucleotide of Formula II can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands.
- the siRNA molecules of the invention can comprise one or more chemically modified nucleotide or non-nucleotide of Formula II at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula II at the 5′-end of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula II at the 3′-end of the sense strand, antisense strand, or both strands.
- the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more nucleotides or non-nucleotides having Formula III:
- each R3, R4, R5, R6, R7, R8, R10, R11 and R12 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino,
- the chemically modified nucleotide or non-nucleotide of Formula III can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands.
- the siRNA molecules of the invention can comprise one or more chemically modified nucleotide or non-nucleotide of Formula III at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula III at the 5′-end of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula III at the 3′-end of the sense strand, antisense strand, or both strands.
- a siRNA molecule of the invention comprises a nucleotide having Formula II or III, wherein the nucleotide having Formula II or III is in an inverted configuration.
- the nucleotide having Formula II or III is connected to the siRNA construct in a 3′,3′, 3′-2′, 2′-3′, or 5′,5′configuration, such as at the 3′-end, 5′-end, or both 3′ and 5′ ends of one or both siRNA strands.
- the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises a 5′-terminal phosphate group having Formula IV:
- each X and Y is independently O, S, N, alkyl, substituted alkyl, or alkylhalo; each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, aralkyl, or alkylhalo; and wherein W, X, Y and Z are not all O.
- the invention features a siRNA molecule having a 5′-terminal phosphate group having Formula IV on the target-complementary strand, for example a strand complementary to ADORA1 RNA, wherein the siRNA molecule comprises an all RNA siRNA molecule.
- the invention features a siRNA molecule having a 5′-terminal phosphate group having Formula IV on the target-complementary strand wherein the siRNA molecule also comprises 1-3 (e.g., 1, 2, or 3) nucleotide 3′-overhangs having between about 1 and about 4 (e.g., about 1, 2, 3, or 4) deoxyribonucleotides on the 3′-end of one or both strands.
- a 5′-terminal phosphate group having Formula IV is present on the target-complementary strand of a siRNA molecule of the invention, for example a siRNA molecule having chemical modifications having Formula I, Formula II and/or Formula III.
- the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more phosphorothioate internucleotide linkages.
- siRNA short interfering RNA
- the invention features a chemically modified short interfering RNA (siRNA) having about 1, 2, 3, 4, 5, 6, 7, 8 or more phosphorothioate internucleotide linkages in one siRNA strand.
- the invention features a chemically modified short interfering RNA (siRNA) individually having about 1, 2, 3, 4, 5, 6, 7, 8 or more phosphorothioate internucleotide linkages in both siRNA strands.
- the phosphorothioate internucleotide linkages can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands.
- the siRNA molecules of the invention can comprise one or more phosphorothioate internucleotide linkages at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) consecutive phosphorothioate internucleotide linkages at the 5′-end of the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) pyrimidine phosphorothioate internucleotide linkages in the sense strand, antisense strand, or both strands.
- an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) purine phosphorothioate internucleotide linkages in the sense strand, antisense strand, or both strands.
- the invention features a siRNA molecule, wherein the sense strand comprises one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8 , 9 , 10 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between 1 and 10 or more, specifically about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl
- one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- the invention features a siRNA molecule, wherein the sense strand comprises between about 1 and about 5, specifically about 1, 2, 3, 4, or 5 phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between about 1 and about 5 or more, specifically about 1, 2, 3, 4, 5, or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro
- one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without between about 1 and about 5 or more, for example about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- the invention features a siRNA molecule, wherein the antisense strand comprises one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8 , 9 , 10 or more phosphorothioate internucleotide linkages, and/or between one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between about 1 and about 10, specifically about 1, 2, 3, 4, 5, 6, 7, 8 , 9, 10 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′
- one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- the invention features a siRNA molecule, wherein the antisense strand comprises between about 1 and about 5 or more, specifically about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between about 1 and about 5 or more, specifically about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy
- one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without between about 1 and about 5, for example about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- the invention features a chemically modified short interfering RNA (siRNA) molecule having between about 1 and about 5, specifically about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages in each strand of the siRNA molecule.
- siRNA short interfering RNA
- the invention features a siRNA molecule comprising 2′-5′ internucleotide linkages.
- the 2′-5′ internucleotide linkage(s) can be at the 5′-end, 3′-end, or both 5′ and 3′ ends of one or both siRNA sequence strands.
- the 2′-5′ internucleotide linkage(s) can be present at various other positions within one or both siRNA sequence strands, for example, about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more including every internucleotide linkage of a pyrimidine nucleotide in one or both strands of the siRNA molecule can comprise a 2′-5′ internucleotide linkage, or about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more including every internucleotide linkage of a purine nucleotide in one or both strands of the siRNA molecule can comprise a 2′-5′ internucleotide linkage.
- a chemically modified siRNA molecule of the invention comprises a duplex having two strands, one or both of which can be chemically modified, wherein each strand is between about 18 and about 27 (e.g., about 18, 19, 20, 21, 22, 23, 24, 25, 26, or 27) nucleotides in length, wherein the duplex has between about 18 and about 23 (e.g., about 18, 19, 20, 21, 22, or 23) base pairs, and wherein the chemical modification comprises a structure having Formula I, Formula II, Formula III and/or Formula IV.
- an exemplary chemically modified siRNA molecule of the invention comprises a duplex having two strands, one or both of which can be chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein each strand consists of 21 nucleotides, each having 2 nucleotide 3′-overhangs, and wherein the duplex has 19 base pairs.
- a siRNA molecule of the invention comprises a single stranded hairpin structure, wherein the siRNA is between about 36 and about 70 (e.g., about 36, 40, 45, 50, 55, 60, 65, or 70) nucleotides in length having between about 18 and about 23 (e.g., about 18, 19, 20, 21, 22, or 23) base pairs, and wherein the siRNA can include a chemical modification comprising a structure having Formula I, Formula II, Formula III and/or Formula IV.
- an exemplary chemically modified siRNA molecule of the invention comprises a linear oligonucleotide having between about 42 and about 50 (e.g., about 42, 43, 44, 45, 46, 47, 48, 49, or 50) nucleotides that is chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein the linear oligonucleotide forms a hairpin structure having 19 base pairs and a 2 nucleotide 3′-overhang.
- a linear oligonucleotide having between about 42 and about 50 (e.g., about 42, 43, 44, 45, 46, 47, 48, 49, or 50) nucleotides that is chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein the linear oligonucleotide forms a hairpin structure having 19 base pairs and a 2 nucleotide 3′-overhang.
- a linear hairpin siRNA molecule of the invention contains a stem loop motif, wherein the loop portion of the siRNA molecule is biodegradable.
- a linear hairpin siRNA molecule of the invention is designed such that degradation of the loop portion of the siRNA molecule in vivo can generate a double stranded siRNA molecule with 3′-overhangs, such as 3′-overhangs comprising about 2 nucleotides.
- a siRNA molecule of the invention comprises a circular nucleic acid molecule, wherein the siRNA is between about 38 and about 70 (e.g., about 38, 40, 45, 50, 55, 60, 65, or 70) nucleotides in length having between about 18 and about 23 (e.g., about 18, 19, 20, 21, 22, or 23) base pairs, and wherein the siRNA can include a chemical modification, which comprises a structure having Formula I, Formula II, Formula III and/or Formula IV.
- an exemplary chemically modified siRNA molecule of the invention comprises a circular oligonucleotide having between about 42 and about 50 (e.g., about 42, 43, 44, 45, 46, 47, 48, 49, or 50) nucleotides that is chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein the circular oligonucleotide forms a dumbbell shaped structure having 19 base pairs and 2 loops.
- a circular siRNA molecule of the invention contains two loop motifs, wherein one or both loop portions of the siRNA molecule is biodegradable.
- a circular siRNA molecule of the invention is designed such that degradation of the loop portions of the siRNA molecule in vivo can generate a double stranded siRNA molecule with 3′-overhangs, such as 3′-overhangs comprising about 2 nucleotides.
- a siRNA molecule of the invention comprises at least one abasic residue, for example a compound having Formula V:
- each R3, R4, R5, R6, R7, R8, R10, R11, R12, and R13 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkyl, aminoa
- a siRNA molecule of the invention comprises at least one inverted abasic residue, for example a compound having Formula VI:
- each R3, R4, R5, R6, R7, R8, R10, R11, R12, and R13 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkyl, aminoa
- a siRNA molecule of the invention comprises an abasic residue having Formula II or III, wherein the abasic residue having Formula II or III is connected to the siRNA construct in a 3′,3′, 3′-2′, 2′-3′, or 5′,5′ configuration, such as at the 3′-end, 5′-end, or both 3′ and 5′ ends of one or both siRNA strands.
- a siRNA molecule of the invention comprises one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) locked nucleic acid (LNA) nucleotides, for example at the 5′-end, 3′-end, 5′ and 3′-end, or any combination thereof, of the siRNA molecule.
- LNA locked nucleic acid
- a siRNA molecule of the invention comprises one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) acyclic nucleotides, for example at the 5′-end, 3′-end, 5′ and 3′-end, or any combination thereof, of the siRNA molecule.
- the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises a conjugate covalently attached to the siRNA molecule.
- the conjugate is covalently attached to the siRNA molecule via a biodegradable linker.
- the conjugate molecule is attached at the 3′-end of either the sense strand, antisense strand, or both strands of the siRNA.
- the conjugate molecule is attached at the 5′-end of either the sense strand, antisense strand, or both strands of the siRNA.
- the conjugate molecule is attached both the 3′-end and 5′-end of either the sense strand, antisense strand, or both strands of the siRNA, or any combination thereof.
- a conjugate molecule of the invention comprises a molecule that facilitates delivery of a siRNA molecule into a biological system such as a cell.
- the conjugate molecule attached to the siRNA is a poly ethylene glycol, human serum albumin, or a ligand for a cellular receptor that can mediate cellular uptake. Examples of specific conjugate molecules contemplated by the instant invention that can be attached to siRNA molecules are described in Vargeese et al., U.S. Ser. No. 60/311,865, incorporated by reference herein.
- the invention features a siRNA molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein one or both strands of the siRNA comprise ribonucleotides at positions withing the siRNA that are critical for siRNA mediated RNAi in a cell. All other positions within the siRNA can include chemically modified nucleotides and/or non-nucleotides such as nucleotides and or non-nucleotides having Formula I, II, III, IV, V, or VI, or any combination thereof to the extent that the ability of the siRNA molecule to support RNAi activity in a cell is maintained.
- RNA interference RNA interference
- the invention features a method for modulating the expression of an ADORA1 gene within a cell, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene; and (b) introducing the siRNA molecule into a cell under conditions suitable to modulate the expression of the ADORA1 gene in the cell.
- the invention features a method for modulating the expression of an ADORA1 gene within a cell, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene and wherein the sense strand sequence of the siRNA is identical to the complementary sequence of the ADORA1 RNA; and (b) introducing the siRNA molecule into a cell under conditions suitable to modulate the expression of the ADORA1 gene in the cell.
- the invention features a method for modulating the expression of more than one ADORA1 gene within a cell, comprising: (a) synthesizing siRNA molecules of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 genes; and (b) introducing the siRNA molecules into a cell under conditions suitable to modulate the expression of the ADORA1 genes in the cell.
- the invention features a method for modulating the expression of more than one ADORA1 gene within a cell, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene and wherein the sense strand sequence of the siRNA is identical to the complementary sequence of the ADORA1 RNA; and (b) introducing the siRNA molecules into a cell under conditions suitable to modulate the expression of the ADORA1 genes in the cell.
- the invention features a method of modulating the expression of an ADORA1 gene in a tissue explant, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene; (b) introducing the siRNA molecule into a cell of the tissue explant derived from a particular organism under conditions suitable to modulate the expression of the ADORA1 gene in the tissue explant, and (c) optionally introducing the tissue explant back into the organism the tissue was derived from or into another organism under conditions suitable to modulate the expression of the ADORA1 gene in that organism.
- the invention features a method of modulating the expression of an ADORA1 gene in a tissue explant, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene and wherein the sense strand sequence of the siRNA is identical to the complementary sequence of the ADORA1 RNA; (b) introducing the siRNA molecule into a cell of the tissue explant derived from a particular organism under conditions suitable to modulate the expression of the ADORA1 gene in the tissue explant, and (c) optionally introducing the tissue explant back into the organism the tissue was derived from or into another organism under conditions suitable to modulate the expression of the ADORA1 gene in that organism.
- the invention features a method of modulating the expression of more than one ADORA1 gene in a tissue explant, comprising: (a) synthesizing siRNA molecules of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 genes; (b) introducing the siRNA molecules into a cell of the tissue explant derived from a particular organism under conditions suitable to modulate the expression of the ADORA1 genes in the tissue explant, and (c) optionally introducing the tissue explant back into the organism the tissue was derived from or into another organism under conditions suitable to modulate the expression of the ADORA1 genes in that organism.
- the invention features a method of modulating the expression of an ADORA1 gene in an organism, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene; and (b) introducing the siRNA molecule into the organism under conditions suitable to modulate the expression of the ADORA1 gene in the organism.
- the invention features a method of modulating the expression of more than one ADORA1 gene in an organism, comprising: (a) synthesizing siRNA molecules of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 genes; and (b) introducing the siRNA molecules into the organism under conditions suitable to modulate the expression of the ADORA1 genes in the organism.
- the siRNA molecules of the invention can be designed to inhibit ADORA1 gene expression through RNAi targeting of a variety of RNA molecules.
- the siRNA molecules of the invention are used to target various RNAs corresponding to a target gene.
- Non-limiting examples of such RNAs include messenger RNA (mRNA), alternate RNA splice variants of target gene(s), post-transcriptionally modified RNA of target gene(s), pre-mRNA of target gene(s), and/or RNA templates used for ADORA1 activity. If alternate splicing produces a family of transcipts that are distinguished by usage of appropriate exons, the instant invention can be used to inhibit gene expression through the appropriate exons to specifically inhibit or to distinguish among the functions of gene family members.
- mRNA messenger RNA
- alternate RNA splice variants of target gene(s) post-transcriptionally modified RNA of target gene(s)
- pre-mRNA of target gene(s) pre-mRNA of target gene(s)
- a protein that contains an alternatively spliced transmembrane domain can be expressed in both membrane bound and secreted forms.
- Use of the invention to target the exon containing the transmembrane domain can be used to determine the functional consequences of pharmaceutical targeting of membrane bound as opposed to the secreted form of the protein.
- Non-limiting examples of applications of the invention relating to targeting these RNA molecules include therapeutic pharmaceutical applications, pharmaceutical discovery applications, molecular diagnostic and gene function applications, and gene mapping, for example using single nucleotide polymorphism mapping with siRNA molecules of the invention.
- Such applications can be implemented using known gene sequences or from partial sequences available from an expressed sequence tag (EST).
- the siRNA molecules of the invention are used to target conserved sequences corresponding to a gene family or gene families such as checkpoint kinase genes. As such, siRNA molecules targeting multiple checkpoint kinase targets can provide increased therapeutic effect.
- siRNA can be used to characterize pathways of gene function in a variety of applications.
- the present invention can be used to inhibit the activity of target gene(s) in a pathway to determine the function of uncharacterized gene(s) in gene function analysis, mRNA function analysis, or translational analysis.
- the invention can be used to determine potential target gene pathways involved in various diseases and conditions toward pharmaceutical development.
- the invention can be used to understand pathways of gene expression involved in development, such as prenatal development, postnatal development and/or aging.
- siRNA molecule(s) and/or methods of the invention are used to inhibit the expression of gene(s) that encode RNA referred to by Genbank Accession number, for example genes such as Genbank Accession No. NM — 000674. Such sequences are readily obtained using this Genbank Accession number.
- the invention features a method comprising: (a) generating a randomized library of siRNA constructs having a predetermined complexity, such as of 4 N , where N represents the number of base paired nucleotides in each of the siRNA construct strands (eg. for a siRNA construct having 21 nucleotide sense and antisense strands with 19 base pairs, the complexity would be 4 19 ); and (b) assaying the siRNA constructs of (a) above, under conditions suitable to determine RNAi target sites within the target ADORA1 RNA sequence.
- the siRNA molecules of (a) have strands of a fixed length, for example about 23 nucleotides in length.
- the siRNA molecules of (a) are of differing length, for example having strands of about 19 to about 25 (e.g., about 19, 20, 21, 22, 23, 24, or 25) nucleotides in length.
- the assay can comprise a reconstituted in vitro siRNA assay as described in Example 6 herein.
- the assay can comprise a cell culture system in which target RNA is expressed.
- fragments of ADORA1 RNA are analyzed for detectable levels of cleavage, for example by gel electrophoresis, northern blot analysis, or RNAse protection assays, to determine the most suitable target site(s) within the target ADORA1 RNA sequence.
- the target ADORA1 RNA sequence can be obtained as is known in the art, for example, by cloning and/or transcription for in vitro systems, and by cellular expression in in vivo systems.
- the invention features a method comprising: (a) analyzing the sequence of a RNA target encoded by an ADORA1 gene; (b) synthesizing one or more sets of siRNA molecules having sequence complementary to one or more regions of the RNA of (a); and (c) assaying the siRNA molecules of (b) under conditions suitable to determine RNAi targets within the target RNA sequence.
- the siRNA molecules of (b) have strands of a fixed length, for example about 23 nucleotides in length.
- the siRNA molecules of (b) are of differing length, for example having strands of about 19 to about 25 (e.g., about 19, 20, 21, 22, 23, 24, or 25) nucleotides in length.
- the assay can comprise a reconstituted in vitro siRNA assay as described in Example 6 herein.
- the assay can comprise a cell culture system in which target RNA is expressed. Fragments of ADORA1 RNA are analyzed for detectable levels of cleavage, for example by gel electrophoresis, northern blot analysis, or RNAse protection assays, to determine the most suitable target site(s) within the target ADORA1 RNA sequence.
- the target ADORA1 RNA sequence can be obtained as is known in the art, for example, by cloning and/or transcription for in vitro systems, and by expression in in vivo systems.
- target site is meant a sequence within a target RNA that is “targeted” for cleavage mediated by a siRNA construct which contains sequences within its antisense region that are complementary to the target sequence.
- detecttable level of cleavage is meant cleavage of target RNA (and formation of cleaved product RNAs) to an extent sufficient to discern cleavage products above the background of RNAs produced by random degradation of the target RNA. Production of cleavage products from 1-5% of the target RNA is sufficient to detect above the background for most methods of detection.
- the invention features a composition comprising a siRNA molecule of the invention, which can be chemically modified, in a pharmaceutically acceptable carrier or diluent.
- the invention features a pharmaceutical composition comprising siRNA molecules of the invention, which can be chemically modified, targeting one or more genes in a pharmaceutically acceptable carrier or diluent.
- the invention features a method for treating or preventing a disease or condition in a subject, comprising administering to the subject a composition of the invention under conditions suitable for the treatment or prevention of the disease or condition in the subject, alone or in conjunction with one or more other therapeutic compounds.
- the invention features a method for validating an ADORA1 gene target, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of an ADORA1 target gene; (b) introducing the siRNA molecule into a cell, tissue, or organism under conditions suitable for modulating expression of the ADORA1 target gene in the cell, tissue, or organism; and (c) determining the function of the gene by assaying for any phenotypic change in the cell, tissue, or organism.
- the invention features a kit containing a siRNA molecule of the invention, which can be chemically modified, that can be used to modulate the expression of an ADORA1 target gene in a cell, tissue, or organism.
- the invention features a kit containing more than one siRNA molecule of the invention, which can be chemically modified, that can be used to modulate the expression of more than one ADORA1 target gene in a cell, tissue, or organism.
- the invention features a cell containing one or more siRNA molecules of the invention, which can be chemically modified.
- the cell containing a siRNA molecule of the invention is a mammalian cell.
- the cell containing a siRNA molecule of the invention is a human cell.
- the synthesis of a siRNA molecule of the invention comprises: (a) synthesis of two complementary strands of the siRNA molecule; (b) annealing the two complementary strands together under conditions suitable to obtain a double stranded siRNA molecule.
- synthesis of the two complementary strands of the siRNA molecule is by solid phase oligonucleotide synthesis.
- synthesis of the two complementary strands of the siRNA molecule is by solid phase tandem oligonucleotide synthesis.
- the invention features a method for synthesizing a siRNA duplex molecule comprising: (a) synthesizing a first oligonucleotide sequence strand of the siRNA molecule, wherein the first oligonucleotide sequence strand comprises a cleavable linker molecule that can be used as a scaffold for the synthesis of the second oligonucleotide sequence strand of the siRNA; (b) synthesizing the second oligonucleotide sequence strand of siRNA on the scaffold of the first oligonucleotide sequence strand, wherein the second oligonucleotide sequence strand further comprises a chemical moiety than can be used to purify the siRNA duplex; (c) cleaving the linker molecule of (a) under conditions suitable for the two siRNA oligonucleotide strands to hybridize and form a stable duplex; and (d) purifying the siRNA duplex utilizing the chemical moiety
- cleavage of the linker molecule in (c) above takes place during deprotection of the oligonucleotide, for example under hydrolysis conditions using an alkylamine base such as methylamine.
- the method of synthesis comprises solid phase synthesis on a solid support such as controlled pore glass (CPG) or polystyrene, wherein the first sequence of (a) is synthesized on a cleavable linker, such as a succinyl linker, using the solid support as a scaffold.
- CPG controlled pore glass
- a cleavable linker such as a succinyl linker
- the cleavable linker in (a) used as a scaffold for synthesizing the second strand can comprise similar reactivity as the solid support derivatized linker, such that cleavage of the solid support derivatized linker and the cleavable linker of (a) takes place concomitantly.
- the chemical moiety of (b) that can used to isolate the attached oligonucleotide sequence comprises a trityl group, for example a dimethoxytrityl group, which can be employed in a trityl-on synthesis strategy as described herein.
- the chemical moiety, such as a dimethoxytrityl group is removed during purification, for example using acidic conditions.
- the method for siRNA synthesis is a solution phase synthesis or hybrid phase synthesis wherein both strands of the siRNA duplex are synthesized in tandem using a cleavable linker attached to the first sequence which acts a scaffold for synthesis of the second sequence. Cleavage of the linker under conditions suitable for hybridization of the separate siRNA sequence strands results in formation of the double stranded siRNA molecule.
- the invention features a method for synthesizing a siRNA duplex molecule comprising: (a) synthesizing one oligonucleotide sequence strand of the siRNA molecule, wherein the sequence comprises a cleavable linker molecule that can be used as a scaffold for the synthesis of another oligonucleotide sequence; (b) synthesizing a second oligonucleotide sequence having complementarity to the first sequence strand on the scaffold of (a), wherein the second sequence comprises the other strand of the double stranded siRNA molecule and wherein the second sequence further comprises a chemical moiety than can be used to isolate the attached oligonucleotide sequence; (c) purifying the product of (b) utilizing the chemical moiety of the second oligonucleotide sequence strand under conditions suitable for isolating the full length sequence comprising both siRNA oligonucleotide strands connected by the cleavable linker; and
- cleavage of the linker molecule in (c) above takes place during deprotection of the oligonucleotide, for example under hydrolysis conditions. In another embodiment, cleavage of the linker molecule in (c) above takes place after deprotection of the oligonucleotide.
- the method of synthesis comprises solid phase synthesis on a solid support such as controlled pore glass (CPG) or polystyrene, wherein the first sequence of (a) is synthesized on a cleavable linker, such as a succinyl linker, using the solid support as a scaffold.
- the cleavable linker in (a) used as a scaffold for synthesizing the second strand can comprise similar reactivity or differing reactivity as the solid support derivatized linker, such that cleavage of the solid support derivatized linker and the cleavable linker of (a) takes place either concomitantly or sequentially.
- the chemical moiety of (b) that can used to isolate the attached oligonucleotide sequence comprises a trityl group, for example a dimethoxytrityl group.
- the invention features a method for making a double stranded siRNA molecule in a single synthetic process, comprising: (a) synthesizing an oligonucleotide having a first and a second sequence, wherein the first sequence is complementary to the second sequence, and the first oligonucleotide sequence is linked to the second sequence via a cleavable linker, and wherein a terminal 5′-protecting group, for example a 5′-O-dimethoxytrityl group (5′-O-DMT) remains on the oligonucleotide having the second sequence; (b) deprotecting the oligonucleotide whereby the deprotection results in the cleavage of the linker joining the two oligonucleotide sequences; and (c) purifying the product of (b) under conditions suitable for isolating the double stranded siRNA molecule, for example using a trityl-on synthesis strategy as described here
- the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications, for example one or more chemical modifications having Formula I, II, III, IV, or V, that increases the nuclease resistance of the siRNA construct.
- the invention features a method for generating siRNA molecules with increased nuclease resistance comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having increased nuclease resistance.
- the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulates the binding affinity between the sense and antisense strands of the siRNA construct.
- the invention features a method for generating siRNA molecules with increased binding affinity between the sense and antisense strands of the siRNA molecule comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having increased binding affinity between the sense and antisense strands of the siRNA molecule.
- the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulates the binding affinity between the antisense strand of the siRNA construct and a complementary target RNA sequence within a cell.
- the invention features a method for generating siRNA molecules with increased binding affinity between the antisense strand of the siRNA molecule and a complementary target RNA sequence, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having increased binding affinity between the antisense strand of the siRNA molecule and a complementary target RNA sequence.
- the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulate the polymerase activity of a cellular polymerase capable of generating additional endogenous siRNA molecules having sequence homology to the chemically modified siRNA construct.
- the invention features a method for generating siRNA molecules capable of mediating increased polymerase activity of a cellular polymerase capable of generating additional endogenous siRNA molecules having sequence homology to the chemically modified siRNA molecule comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules capable of mediating increased polymerase activity of a cellular polymerase capable of generating additional endogenous siRNA molecules having sequence homology to the chemically modified siRNA molecule.
- the invention features chemically modified siRNA constructs that mediate RNAi against ADORA1 in a cell, wherein the chemical modifications do not significantly effect the interaction of siRNA with a target RNA molecule and/or proteins or other factors that are essential for RNAi in a manner that would decrease the efficacy of RNAi mediated by such siRNA constructs.
- the invention features a method for generating siRNA molecules with improved RNAi activity against ADORA1, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved RNAi activity.
- the invention features a method for generating siRNA molecules with improved RNAi activity against an ADORA1 target RNA, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved RNAi activity against the target RNA.
- the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulates the cellular uptake of the siRNA construct.
- the invention features a method for generating siRNA molecules against ADORA1 with improved cellular uptake, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved cellular uptake.
- the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that increases the bioavailability of the siRNA construct, for example by attaching polymeric conjugates such as polyethyleneglycol or equivalent conjugates that improve the pharmacokinetics of the siRNA construct, or by attaching conjugates that target specific tissue types or cell types in vivo.
- polymeric conjugates such as polyethyleneglycol or equivalent conjugates that improve the pharmacokinetics of the siRNA construct
- conjugates that target specific tissue types or cell types in vivo.
- Non-limiting examples of such conjugates are described in Vargeese et al., U.S. Serial No. 60/311,865 incorporated by reference herein.
- the invention features a method for generating siRNA molecules of the invention with improved bioavailability, comprising (a) introducing a conjugate into the structure of a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved bioavailability.
- Such conjugates can include ligands for cellular receptors such as peptides derived from naturally occurring protein ligands, protein localization sequences including cellular ZIP code sequences, antibodies, nucleic acid aptamers, vitamins and other co-factors such as folate and N-acetylgalactosamine, polymers such as polyethyleneglycol (PEG), phospholipids, polyamines such as spermine or spermidine, and others.
- ligands for cellular receptors such as peptides derived from naturally occurring protein ligands, protein localization sequences including cellular ZIP code sequences, antibodies, nucleic acid aptamers, vitamins and other co-factors such as folate and N-acetylgalactosamine, polymers such as polyethyleneglycol (PEG), phospholipids, polyamines such as spermine or spermidine, and others.
- PEG polyethyleneglycol
- phospholipids such as spermine or spermidine
- the invention features a method for generating siRNA molecules of the invention with improved bioavailability, comprising (a) introducing an excipient formulation to a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved bioavailability.
- excipients include polymers such as cyclodextrins, lipids, cationic lipids, polyamines, phospholipids, and others.
- the invention features a method for generating siRNA molecules of the invention with improved bioavailability, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved bioavailability.
- polyethylene glycol can be covalently attached to siRNA compounds of the present invention.
- the attached PEG can be any molecular weight, preferably from about 2,000 to about 50,000 daltons (Da).
- the present invention can be used alone or as a component of a kit having at least one of the reagents necessary to carry out the in vitro or in vivo introduction of RNA to test samples and/or subjects.
- preferred components of the kit include the siRNA and a vehicle that promotes introduction of the siRNA.
- Such a kit can also include instructions to allow a user of the kit to practice the invention.
- RNA interference refers to any nucleic acid molecule capable of mediating RNA interference “RNAi” or gene silencing; see for example Bass, 2001, Nature, 411, 428-429; Elbashir et al., 2001, Nature, 411, 494-498; and Kreutzer et al., International PCT Publication No. WO 00/44895; Zernicka-Goetz et al., International PCT Publication No. WO 01/36646; Fire, International PCT Publication No. WO 99/32619; Plaetinck et al, International PCT Publication No.
- siRNA molecules of the invention are shown in FIG. 2.
- the siRNA can be a double stranded polynucleotide molecule comprising self complementary sense and antisense regions, wherein the antisense region comprises complementarity to a target nucleic acid molecule.
- the siRNA can be a single stranded hairpin polynucleotide having self complementary sense and antisense regions, wherein the antisense region comprises complementarity to a target nucleic acid molecule.
- the siRNA can be a circular single stranded polynucleotide having two or more loop structures and a stem comprising self complementary sense and antisense regions, wherein the antisense region comprises complementarity to a target nucleic acid molecule, and wherein the circular polynucleotide can be processed either in vivo or in vitro to generate an active siRNA capable of mediating RNAi.
- siRNA molecules need not be limited to those molecules containing only RNA, but further encompasses chemically modified nucleotides and non-nucleotides.
- module is meant that the expression of the gene, or level of RNA molecule or equivalent RNA molecules encoding one or more proteins or protein subunits, or activity of one or more proteins or protein subunits is up regulated or down regulated, such that expression, level, or activity is greater than or less than that observed in the absence of the modulator.
- modulate can mean “inhibit,” but the use of the word “modulate” is not limited to this definition.
- inhibitor it is meant that the activity of a gene expression product or level of RNAs or equivalent RNAs encoding one or more gene products is reduced below that observed in the absence of the nucleic acid molecule of the invention.
- inhibition with a siRNA molecule preferably is below that level observed in the presence of an inactive or attenuated molecule that is unable to mediate an RNAi response.
- inhibition of gene expression with the siRNA molecule of the instant invention is greater in the presence of the siRNA molecule than in its absence.
- RNA nucleic acid that encodes an RNA
- the target gene can be a gene derived from a cell, an endogenous gene, a transgene, or exogenous genes such as genes of a pathogen, for example a virus, which is present in the cell after infection thereof.
- the cell containing the target gene can be derived from or contained in any organism, for example a plant, animal, protozoan, virus, bacterium, or fungus.
- Non-limiting examples of plants include monocots, dicots, or gymnosperms.
- animals include vertebrates or invertebrates.
- fungi include molds or yeasts.
- ADORA1 is meant, a polypeptide comprising an adenosine A1 receptor or polynucleotide encoding an Ets adenosine A1 receptor, for example a polynucleotide having Genbank Accession No. NM — 000674.
- highly conserved sequence region is meant, a nucleotide sequence of one or more regions in a target gene does not vary significantly from one generation to the other or from one biological system to the other.
- complementarity or “complementary” is meant that a nucleic acid can form hydrogen bond(s) with another nucleic acid sequence by either traditional Watson-Crick or other non-traditional types of interaction.
- the binding free energy for a nucleic acid molecule with its complementary sequence is sufficient to allow the relevant function of the nucleic acid to proceed, e.g., RNAi activity.
- the degree of complementarity between the sense and antisense strand of the siRNA construct can be the same or different from the degree of complementarity between the antisense strand of the siRNA and the target RNA sequence.
- a percent complementarity indicates the percentage of contiguous residues in a nucleic acid molecule that can form hydrogen bonds (e.g., Watson-Crick base pairing) with a second nucleic acid sequence (e.g., 5, 6, 7, 8, 9, 10 out of 10 being 50%, 60%, 70%, 80%, 90%, and 100% complementary). “Perfectly complementary” means that all the contiguous residues of a nucleic acid sequence will hydrogen bond with the same number of contiguous residues in a second nucleic acid sequence.
- siRNA molecules of the invention represent a novel therapeutic approach to treat a variety of allergic/inflammatory diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and other indications that can respond to the level of ADORA1.
- each sequence of a siRNA molecule of the invention is independently about 18 to about 24 nucleotides in length, in specific embodiments about 18, 19, 20, 21, 22, 23, or 24 nucleotides in length.
- the siRNA duplexes of the invention independently comprise between about 17 and about 23 (e.g., about 17, 18, 19, 20, 21, 22, or 23) base pairs.
- siRNA molecules of the invention comprising hairpin or circular structures are about 35 to about 55 (e.g., about 35, 40, 45, 50, or 55) nucleotides in length, or about 38 to about 44 (e.g., about 38, 39, 40, 41, 42, 43, or 44) nucleotides in length and comprising about 16 to about 22 (e.g., about 16, 17, 18, 19, 20, 21, or 22) base pairs.
- Exemplary siRNA molecules of the invention are shown in Table I and III (all sequences are shown 5′-3′) and/or FIGS. 4 and 5.
- cell is used in its usual biological sense, and does not refer to an entire multicellular organism, e.g., specifically does not refer to a human.
- the cell can be present in an organism, e.g., mammals such as humans, cows, sheep, apes, monkeys, swine, dogs, and cats.
- the cell can be eukaryotic (e.g., a mammalian cell, such as a human cell).
- the cell can be of somatic or germ line origin, totipotent or pluripotent, dividing or non-dividing.
- the cell can also be derived from or can comprise a gamete or embryo, a stem cell, or a fully differentiated cell.
- the siRNA molecules of the invention are added directly, or can be complexed with cationic lipids, packaged within liposomes, or otherwise delivered to target cells or tissues.
- the nucleic acid or nucleic acid complexes can be locally administered to relevant tissues ex vivo, or in vivo through injection, infusion pump or stent, with or without their incorporation in biopolymers.
- the nucleic acid molecules of the invention comprise sequences shown in Table I, III and/or FIGS. 4 and 5. Examples of such nucleic acid molecules consist essentially of sequences defined in these tables/figures.
- the invention provides mammalian cells containing one or more siRNA molecules of this invention.
- the one or more siRNA molecules can independently be targeted to the same or different sites.
- RNA is meant a molecule comprising at least one ribonucleotide residue.
- ribonucleotide is meant a nucleotide with a hydroxyl group at the 2′ position of a ⁇ -D-ribo-furanose moiety.
- the terms include double stranded RNA, single stranded RNA, isolated RNA such as partially purified RNA, essentially pure RNA, synthetic RNA, recombinantly produced RNA, as well as altered RNA that differs from naturally occurring RNA by the addition, deletion, substitution and/or alteration of one or more nucleotides.
- Such alterations can include addition of non-nucleotide material, such as to the end(s) of the siRNA or internally, for example at one or more nucleotides of the RNA.
- Nucleotides in the RNA molecules of the instant invention can also comprise non-standard nucleotides, such as non-naturally occurring nucleotides or chemically synthesized nucleotides or deoxynucleotides. These altered RNAs can be referred to as analogs or analogs of naturally-occurring RNA.
- subject is meant an organism, which is a donor or recipient of explanted cells or the cells themselves. “Subject” also refers to an organism to which the nucleic acid molecules of the invention can be administered. In one embodiment, a subject is a mammal or mammalian cells. In another embodiment, a subject is a human or human cells.
- phosphorothioate refers to an internucleotide linkage having Formula I, wherein Z and/or W comprise a sulfur atom.
- phosphorothioate refers to both phosphorothioate and phosphorodithioate internucleotide linkages.
- universal base refers to nucleotide base analogs that form base pairs with each of the natural DNA/RNA bases with little discrimination between them.
- Non-limiting examples of universal bases include C-phenyl, C-naphthyl and other aromatic derivatives, inosine, azole carboxamides, and nitroazole derivatives such as 3-nitropyrrole, 4-nitroindole, 5-nitroindole, and 6-nitroindole as known in the art (see for example Loakes, 2001, Nucleic Acids Research, 29, 2437-2447).
- acyclic nucleotide refers to any nucleotide having an acyclic ribose sugar, for example where any of the ribose carbons (C1, C2, C3, C4, or C5), are independently or in combination absent from the nucleotide.
- nucleic acid molecules of the instant invention can be used to treat diseases or conditions discussed herein.
- the siRNA molecules can be administered to a subject or can be administered to other appropriate cells evident to those skilled in the art, individually or in combination with one or more drugs under conditions suitable for the treatment.
- the siRNA molecules can be used in combination with other known treatments to treat conditions or diseases discussed above.
- the described molecules could be used in combination with one or more known therapeutic agents to treat a disease or condition.
- Non-limiting examples of other therapeutic agents that can be readily combined with a siRNA molecule of the invention are enzymatic nucleic acid molecules, allosteric nucleic acid molecules, antisense, decoy, or aptamer nucleic acid molecules, antibodies such as monoclonal antibodies, small molecules, and other organic and/or inorganic compounds including metals, salts and ions.
- the invention features an expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of the invention, in a manner which allows expression of the siRNA molecule.
- the vector can contain sequence(s) encoding both strands of a siRNA molecule comprising a duplex.
- the vector can also contain sequence(s) encoding a single nucleic acid molecule that is self complementary and thus forms a siRNA molecule.
- Non-limiting examples of such expression vectors are described in Paul et al, 2002, Nature Biotechnology, 19, 505; Miyagishi and Taira, 2002, Nature Biotechnology, 19, 497; Lee et al., 2002, Nature Biotechnology, 19, 500; and Novina et al, 2002, Nature Medicine, advance online publication doi: 10.1038/nm725.
- the invention features a mammalian cell, for example, a human cell, including an expression vector of the invention.
- the expression vector of the invention comprises a sequence for a siRNA molecule having complementarity to a RNA molecule referred to by a Genbank Accession numbers, for example genes such as Genbank Accession No. No. NM — 000674.
- an expression vector of the invention comprises a nucleic acid sequence encoding two or more siRNA molecules, which can be the same or different.
- siRNA molecules that interact with target RNA molecules and down-regulate gene encoding target RNA molecules are expressed from transcription units inserted into DNA or RNA vectors.
- the recombinant vectors can be DNA plasmids or viral vectors.
- siRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus, retrovirus, adenovirus, or alphavirus.
- the recombinant vectors capable of expressing the siRNA molecules can be delivered as described herein, and persist in target cells.
- viral vectors can be used that provide for transient expression of siRNA molecules. Such vectors can be repeatedly administered as necessary.
- siRNA molecules bind and down-regulate gene function or expression via RNA interference (RNAi).
- Delivery of siRNA expressing vectors can be systemic, such as by intravenous or intramuscular administration, by administration to target cells ex-planted from a subject followed by reintroduction into the subject, or by any other means that would allow for introduction into the desired target cell.
- vectors any nucleic acid- and/or viral-based technique used to deliver a desired nucleic acid.
- FIG. 1 shows a non-limiting example of a scheme for the synthesis of siRNA molecules.
- the complementary siRNA sequence strands, strand 1 and strand 2 are synthesized in tandem and are connected by a cleavable linkage, such as a nucleotide succinate or abasic succinate, which can be the same or different from the cleavable linker used for solid phase synthesis on a solid support.
- the synthesis can be either solid phase or solution phase, in the example shown, the synthesis is a solid phase synthesis.
- the synthesis is performed such that a protecting group, such as a dimethoxytrityl group, remains intact on the terminal nucleotide of the tandem oligonucleotide.
- the two siRNA strands spontaneously hybridize to form a siRNA duplex, which allows the purification of the duplex by utilizing the properties of the terminal protecting group, for example by applying a trityl on purification method wherein only duplexes/oligonucleotides with the terminal protecting group are isolated.
- FIG. 2 shows a MALDI-TOV mass spectrum of a purified siRNA duplex synthesized by a method of the invention.
- the two peaks shown correspond to the predicted mass of the separate siRNA sequence strands. This result demonstrates that the siRNA duplex generated from tandem synthesis can be purified as a single entity using a simple trityl-on purification methodology.
- FIG. 3 shows a non-limiting proposed mechanistic representation of target RNA degradation involved in RNAi.
- Double stranded RNA (dsRNA), which is generated by RNA dependent RNA polymerase (RdRP) from foreign single stranded RNA, for example viral, transposon, or other exogenous RNA, activates the DICER enzyme which in turn generates siRNA duplexes having terminal phosphate groups (P).
- RdRP RNA dependent RNA polymerase
- An active siRNA complex forms which recognizes a target RNA, resulting in degradation of the target RNA by the RISC endonuclease complex or in the synthesis of additional RNA by RNA dependent RNA polymerase (RdRP), which can activate DICER and result in additional siRNA molecules, thereby amplifying the RNAi response.
- RdRP RNA dependent RNA polymerase
- FIG. 4 shows non-limiting examples of chemically modified siRNA constructs of the present invention.
- N stands for any nucleotide (adenosine, guanosine, cytosine, uridine, or optionally thymidine, for example thymidine can be substituted in the overhanging regions designated by parenthesis (N N).
- Various modifications are shown for the sense and antisense strands of the siRNA constructs.
- the sense strand comprises 21 nucleotides having four phosphorothioate 5′ and 3′-terminal internucleotide linkages, wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and having one 3′-terminal phosphorothioate internucleotide linkage and four 5′-terminal phosphorothioate internucleotide linkages and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the sense strand comprises 21 nucleotides wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the sense strand comprises 21 nucleotides having 5′- and 3′-terminal cap moieties wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the sense strand comprises 21 nucleotides having five phosphorothioate 5′ and 3′-terminal internucleotide linkages, wherein the two terminal 3′-nucleotides are optionally base paired and wherein all nucleotides are ribonucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and having one 3′-terminal phosphorothioate internucleotide linkage and five 5′-terminal phosphorothioate internucleotide linkages and wherein all nucleotides are ribonucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the sense strand comprises 21 nucleotides wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand comprises 21 nucleotides all having phosphorothioate internucleotide linkages, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and wherein all nucleotides are ribonucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the sense strand comprises 21 nucleotides having 5′- and 3′-terminal cap moieties, wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and having one 3′-termninal phosphorothioate internucleotide linkage and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein.
- the antisense strand of constructs A-F comprise sequence complimentary to target RNA sequence of the invention.
- FIG. 5 shows non-limiting examples of specific chemically modified siRNA sequences of the invention.
- A-F applies the chemical modifications described in FIG. 4A-F to an ADORA1 siRNA sequence.
- FIG. 6 shows non-limiting examples of different siRNA constructs of the invention.
- the examples shown (constructs 1, 2, and 3) have 19 representative base pairs, however, different embodiments of the invention include any number of base pairs described herein.
- Bracketed regions represent nucleotide overhangs, for example comprising between about 1, 2, 3, or 4 nucleotides in length, preferably about 2 nucleotides.
- Constructs 1 and 2 can be used independently for RNAi activity.
- Construct 2 can comprise a polynucleotide or non-nucleotide linker, which can optionally be designed as a biodegradable linker.
- the loop structure shown in construct 2 can comprise a biodegradable linker that results in the formation of construct 1 in vivo and/or in vitro.
- construct 3 can be used to generate construct 2 under the same principle wherein a linker is used to generate the active siRNA construct 2 in vivo and/or in vitro, which can optionally utilize another biodegradable linker to generate the active siRNA construct 1 in vivo and/or in vitro.
- the stability and/or activity of the siRNA constructs can be modulated based on the design of the siRNA construct for use in vivo or in vitro and/or in vitro.
- FIG. 7 is a diagrammatic representation of a scheme utilized in generating an expression cassette to generate siRNA hairpin constructs.
- a DNA oligomer is synthesized with a 5′-restriction site (R1) sequence followed by a region having sequence identical (sense region of siRNA) to a predetermined ADORA1 target seqeunce, wherein the sense region comprises, for example, about 19, 20, 21, or 22 nucleotides (N) in length, which is followed by a loop sequence of defined sequence (X), comprising, for example, between about 3 and 10 nucleotides.
- R1 5′-restriction site
- X loop sequence of defined sequence
- the synthetic construct is then extended by DNA polymerase to generate a hairpin structure having self complementary sequence that will result in a siRNA transcript having specificity for an ADORA1 target sequence and having self complementary sense and antisense regions.
- C The construct is heated (for example to about 95° C.) to linearize the sequence, thus allowing extension of a complementary second DNA strand using a primer to the 3′-restriction sequence of the first strand.
- the double stranded DNA is then inserted into an appropriate vector for expression in cells.
- the construct can be designed such that a 3′-overhang results from the transcription, for example by engineering restriction sites and/or utilizing a poly-U termination region as described in Paul et al., 2002, Nature Biotechnology, 29, 505-508.
- FIG. 8 is a diagrammatic representation of a scheme utilized in generating an expression cassette to generate double stranded siRNA constructs.
- a DNA oligomer is synthesized with a 5′-restriction (R1) site sequence followed by a region having sequence identical (sense region of siRNA) to a predetermined ADORA1 target seqeunce, wherein the sense region comprises, for example, about 19, 20, 21, or 22 nucleotides (N) in length, and which is followed by a 3′-restriction site (R2) which is adjacent to a loop sequence of defined sequence (X).
- R1 5′-restriction
- R2 3′-restriction site
- the construct is processed by restriction enzymes specific to R1 and R2 to generate a double stranded DNA which is then inserted into an appropriate vector for expression in cells.
- the transcription cassette is designed such that a U6 promoter region flanks each side of the dsDNA which generates the separate sense and antisense strands of the siRNA.
- Poly T termination sequences can be added to the constructs to generate U overhangs in the resulting transcript.
- FIG. 9 is a diagrammatic representation of a method used to determine target sites for siRNA mediated RNAi within a particular target nucleic acid sequence, such as messenger RNA.
- a pool of siRNA oligonucleotides are synthesized wherein the antisense region of the siRNA constructs has complementarity to target sites across the target nucleic acid sequence, and wherein the sense region comprises sequence complementary to the antisense region of the siRNA.
- the sequences are pooled and are inserted into vectors such that (C) transfection of a vector into cells results in the expression of the siRNA.
- D Cells are sorted based on phenotypic change that is associated with modulation of the target nucleic acid sequence.
- E The siRNA is isolated from the sorted cells and is sequenced to identify efficacious target sites within the target nucleic acid sequence.
- RNA interference refers to the process of sequence specific post transcriptional gene silencing in animals mediated by short interfering RNAs (siRNA) (Fire et al., 1998, Nature, 391, 806). The corresponding process in plants is commonly referred to as post transcriptional gene silencing or RNA silencing and is also referred to as quelling in fungi. The process of post transcriptional gene silencing is thought to be an evolutionarily conserved cellular defense mechanism used to prevent the expression of foreign genes which is commonly shared by diverse flora and phyla (Fire et al., 1999, Trends Genet., 15, 358).
- Such protection from foreign gene expression may have evolved in response to the production of double stranded RNAs (dsRNA) derived from viral infection or the random integration of transposon elements into a host genome via a cellular response that specifically destroys homologous single stranded RNA or viral genomic RNA.
- dsRNA double stranded RNAs
- the presence of dsRNA in cells triggers the RNAi response though a mechanism that has yet to be fully characterized. This mechanism appears to be different from the interferon response that results from dsRNA mediated activation of protein kinase PKR and 2′,5′-oligoadenylate synthetase resulting in non-specific cleavage of mRNA by ribonuclease L.
- Dicer a ribonuclease III enzyme referred to as Dicer.
- Dicer is involved in the processing of the dsRNA into short pieces of dsRNA known as short interfering RNAs (siRNA) (Berstein et al., 2001, Nature, 409, 363).
- Short interfering RNAs derived from Dicer activity are typically about 21-23 nucleotides in length and comprise about 19 base pair duplexes.
- Dicer has also been implicated in the excision of 21 and 22 nucleotide small temporal RNAs (stRNA) from precursor RNA of conserved structure that are implicated in translational control (Hutvagner et al., 2001, Science, 293, 834).
- the RNAi response also features an endonuclease complex containing a siRNA, commonly referred to as an RNA-induced silencing complex (RISC), which mediates cleavage of single stranded RNA having sequence homologous to the siRNA. Cleavage of the target RNA takes place in the middle of the region complementary to the guide sequence of the siRNA duplex (Elbashir et al., 2001, Genes Dev., 15, 188).
- RISC RNA-induced silencing complex
- RNAi has been studied in a variety of systems. Fire et al., 1998, Nature, 391, 806, were the first to observe RNAi in C. elegans. Wianny and Goetz, 1999, Nature Cell Biol., 2, 70, describes RNAi mediated by dsRNA in mouse embryos. Hammond et al., 2000, Nature, 404, 293, describe RNAi in Drosophila cells transfected with dsRNA. Elbashir et al., 2001, Nature, 411, 494, describe RNAi induced by introduction of duplexes of synthetic 21-nucleotide RNAs in cultured mammalian cells including human embryonic kidney and HeLa cells.
- nucleic acids greater than 100 nucleotides in length is difficult using automated methods, and the therapeutic cost of such molecules is prohibitive.
- small nucleic acid motifs (“small” refers to nucleic acid motifs no more than 100 nucleotides in length, preferably no more than 80 nucleotides in length, and most preferably no more than 50 nucleotides in length; e.g., individual siRNA oligonucleotide sequences or siRNA sequences synthesized in tandem) are preferably used for exogenous delivery.
- the simple structure of these molecules increases the ability of the nucleic acid to invade targeted regions of protein and/or RNA structure.
- Exemplary molecules of the instant invention are chemically synthesized, and others can similarly be synthesized.
- Oligonucleotides are synthesized using protocols known in the art, for example as described in Caruthers et al., 1992, Methods in Enzymology 211, 3-19, Thompson et al., International PCT Publication No. WO 99/54459, Wincott et al., 1995, Nucleic Acids Res. 23, 2677-2684, Wincott et al., 1997, Methods Mol. Bio., 74, 59, Brennan et al., 1998, Biotechnol Bioeng., 61, 33-45, and Brennan, U.S.
- oligonucleotides makes use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5′-end, and phosphoramidites at the 3′-end.
- small scale syntheses are conducted on a 394 Applied Biosystems, Inc. synthesizer using a 0.2 ⁇ mol scale protocol with a 2.5 min coupling step for 2′-O-methylated nucleotides and a 45 sec coupling step for 2′-deoxy nucleotides or 2′-deoxy-2′-fluoro nucleotides.
- Table II outlines the amounts and the contact times of the reagents used in the synthesis cycle.
- syntheses at the 0.2 ⁇ mol scale can be performed on a 96-well plate synthesizer, such as the instrument produced by Protogene (Palo Alto, Calif.) with minimal modification to the cycle.
- Average coupling yields on the 394 Applied Biosystems, Inc. synthesizer, determined by calorimetric quantitation of the trityl fractions, are typically 97.5-99%.
- synthesizer include the following: detritylation solution is 3% TCA in methylene chloride (ABI); capping is performed with 16% N-methyl imidazole in THF (ABI) and 10% acetic anhydride/10% 2,6-lutidine in THF (ABI); and oxidation solution is 16.9 mM I 2 , 49 mM pyridine, 9% water in THF (PERSEPTIVETM). Burdick & Jackson Synthesis Grade acetonitrile is used directly from the reagent bottle. S-Ethyltetrazole solution (0.25 M in acetonitrile) is made up from the solid obtained from American International Chemical, Inc. Alternately, for the introduction of phosphorothioate linkages, Beaucage reagent (3H-1,2-Benzodithiol-3-one 1,1-dioxide, 0.05 M in acetonitrile) is used.
- Deprotection of the DNA-based oligonucleotides is performed as follows: the polymer-bound trityl-on oligoribonucleotide is transferred to a 4 mL glass screw top vial and suspended in a solution of 40% aq. methylamine (1 mL) at 65° C. for 10 min. After cooling to ⁇ 20° C., the supernatant is removed from the polymer support. The support is washed three times with 1.0 mL of EtOH:MeCN:H2O/3:1:1, vortexed and the supernatant is then added to the first supernatant. The combined supernatants, containing the oligoribonucleotide, are dried to a white powder.
- RNA including certain siRNA molecules of the invention follows the procedure as described in Usman et al., 1987, J. Am. Chem. Soc., 109, 7845; Scaringe et al., 1990, Nucleic Acids Res., 18, 5433; and Wincott et al., 1995, Nucleic Acids Res. 23, 2677-2684 Wincott et al., 1997, Methods Mol. Bio., 74, 59, and makes use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5′-end, and phosphoramidites at the 3′-end.
- common nucleic acid protecting and coupling groups such as dimethoxytrityl at the 5′-end, and phosphoramidites at the 3′-end.
- small scale syntheses are conducted on a 394 Applied Biosystems, Inc. synthesizer using a 0.2 ⁇ mol scale protocol with a 7.5 min coupling step for alkylsilyl protected nucleotides and a 2.5 min coupling step for 2′-O-methylated nucleotides.
- Table II outlines the amounts and the contact times of the reagents used in the synthesis cycle.
- syntheses at the 0.2 ⁇ mol scale can be done on a 96-well plate synthesizer, such as the instrument produced by Protogene (Palo Alto, Calif.) with minimal modification to the cycle.
- Average coupling yields on the 394 Applied Biosystems, Inc. synthesizer, determined by colorimetric quantitation of the trityl fractions, are typically 97.5-99%.
- synthesizer include the following: detritylation solution is 3% TCA in methylene chloride (ABI); capping is performed with 16% N-methyl imidazole in THF (ABI) and 10% acetic anhydride/10% 2,6-lutidine in THF (ABI); oxidation solution is 16.9 mM I 2 , 49 mM pyridine, 9% water in THF (PERSEPTIVETM). Burdick & Jackson Synthesis Grade acetonitrile is used directly from the reagent bottle. S-Ethyltetrazole solution (0.25 M in acetonitrile) is made up from the solid obtained from American International Chemical, Inc. Alternately, for the introduction of phosphorothioate linkages, Beaucage reagent (3H-1,2-Benzodithiol-3-one 1,1-dioxide0.05 M in acetonitrile) is used.
- RNA deprotection of the RNA is performed using either a two-pot or one-pot protocol.
- the polymer-bound trityl-on oligoribonucleotide is transferred to a 4 mL glass screw top vial and suspended in a solution of 40% aq. methylamine (1 mL) at 65° C. for 10 min. After cooling to ⁇ 20° C., the supernatant is removed from the polymer support. The support is washed three times with 1.0 mL of EtOH:MeCN:H2O/3:1:1, vortexed and the supernatant is then added to the first supernatant.
- the combined supernatants, containing the oligoribonucleotide, are dried to a white powder.
- the base deprotected oligoribonucleotide is resuspended in anhydrous TEA/HF/NMP solution (300 ⁇ L of a solution of 1.5 mL N-methylpyrrolidinone, 750 ⁇ L TEA and 1 mL TEA ⁇ 3HF to provide a 1.4 M HF concentration) and heated to 65° C. After 1.5 h, the oligomer is quenched with 1.5 M NH 4 HCO 3 .
- the polymer-bound trityl-on oligoribonucleotide is transferred to a 4 mL glass screw top vial and suspended in a solution of 33% ethanolic methylamine/DMSO: 1/1 (0.8 mL) at 65° C. for 15 min.
- the vial is brought to r.t. TEA ⁇ 3HF (0.1 mL) is added and the vial is heated at 65° C. for 15 min.
- the sample is cooled at ⁇ 20° C. and then quenched with 1.5 M NH 4 HCO 3 .
- the quenched NH 4 HCO 3 solution is loaded onto a C-18 containing cartridge that had been prewashed with acetonitrile followed by 50 mM TEAA. After washing the loaded cartridge with water, the RNA is detritylated with 0.5% TFA for 13 min. The cartridge is then washed again with water, salt exchanged with 1 M NaCl and washed with water again. The oligonucleotide is then eluted with 30% acetonitrile.
- the average stepwise coupling yields are typically >98% (Wincott et al., 1995 Nucleic Acids Res. 23, 2677-2684).
- the scale of synthesis can be adapted to be larger or smaller than the example described above including but not limited to 96-well format, all that is important is the ratio of chemicals used in the reaction.
- nucleic acid molecules of the present invention can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., 1992, Science 256, 9923; Draper et al., International PCT publication No. WO 93/23569; Shabarova et al., 1991, Nucleic Acids Research 19, 4247; Bellon et al., 1997, Nucleosides & Nucleotides, 16, 951; Bellon et al., 1997, Bioconjugate Chem. 8, 204), or by hybridization following synthesis and/or deprotection.
- siRNA molecules of the invention can also be synthesized via a tandem synthesis methodology as described in Example 1 herein, wherein both siRNA strands are synthesized as a single contiguous oligonucleotide fragment or strand separated by a cleavable linker which is subsequently cleaved to provide separate siRNA fragments or strands that hybridize and permit purification of the siRNA duplex.
- the linker can be a polynucleotide linker or a non-nucleotide linker.
- the tandem synthesis of siRNA as described herein can be readily adapted to both multiwell/multiplate synthesis platforms such as 96 well or similarly larger multi-well platforms.
- the tandem synthesis of siRNA as described herein can also be readily adapted to large scale synthesis platforms employing batch reactors, synthesis columns and the like.
- a siRNA molecule can also be assembled from two distinct nucleic acid strands or fragments wherein one fragment includes the sense region and the second fragment includes the antisense region of the RNA molecule.
- nucleic acid molecules of the present invention can be modified extensively to enhance stability by modification with nuclease resistant groups, for example, 2′-amino, 2′-C-allyl, 2′-flouro, 2′-O-methyl, 2′-H (for a review see Usman and Cedergren, 1992, TIBS 17, 34; Usman et al., 1994, Nucleic Acids Symp. Ser. 31, 163).
- siRNA constructs can be purified by gel electrophoresis using general methods or can be purified by high pressure liquid chromatography (HPLC; see Wincott et al., supra, the totality of which is hereby incorporated herein by reference) and re-suspended in water.
- siRNA molecules of the invention are expressed from transcription units inserted into DNA or RNA vectors.
- the recombinant vectors can be DNA plasmids or viral vectors.
- siRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus, retrovirus, adenovirus, or alphavirus.
- the recombinant vectors capable of expressing the siRNA molecules can be delivered as described herein, and persist in target cells.
- viral vectors can be used that provide for transient expression of siRNA molecules.
- nucleic acid molecules with modifications can prevent their degradation by serum ribonucleases, which can increase their potency (see e.g., Eckstein et al., International Publication No. WO 92/07065; Perrault et al., 1990 Nature 344, 565; Pieken et al., 1991, Science 253, 314; Usman and Cedergren, 1992, Trends in Biochem. Sci. 17, 334; Usman et al., International Publication No. WO 93/15187; and Rossi et al., International Publication No. WO 91/03162; Sproat, U.S. Pat. No.
- oligonucleotides are modified to enhance stability and/or enhance biological activity by modification with nuclease resistant groups, for example, 2′-amino, 2′-C-allyl, 2′-flouro, 2′-O-methyl, 2′-O-allyl, 2′-H, nucleotide base modifications (for a review see Usman and Cedergren, 1992, TIBS. 17, 34; Usman et al., 1994, Nucleic Acids Symp. Ser.
- RNA molecules having chemical modifications that maintain or enhance activity are provided.
- Such a nucleic acid is also generally more resistant to nucleases than an unmodified nucleic acid. Accordingly, the in vitro and/or in vivo activity should not be significantly lowered.
- therapeutic nucleic acid molecules delivered exogenously should optimally be stable within cells until translation of the target RNA has been modulated long enough to reduce the levels of the undesirable protein. This period of time varies between hours to days depending upon the disease state. Improvements in the chemical synthesis of RNA and DNA (Wincott et al., 1995 Nucleic Acids Res.
- nucleic acid molecules of the invention include one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) G-clamp nucleotides.
- a G-clamp nucleotide is a modified cytosine analog wherein the modifications confer the ability to hydrogen bond both Watson-Crick and Hoogsteen faces of a complementary guanine within a duplex, see for example Lin and Matteucci, 1998, J. Am. Chem. Soc., 120, 8531-8532.
- a single G-clamp analog substitution within an oligonucleotide can result in substantially enhanced helical thermal stability and mismatch discrimination when hybridized to complementary oligonucleotides.
- nucleic acid molecules of the invention include one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) LNA “locked nucleic acid” nucleotides such as a 2′, 4′-C mythylene bicyclo nucleotide (see for example Wengel et al., International PCT Publication No. WO 00/66604 and WO 99/14226).
- the invention features conjugates and/or complexes of siRNA molecules of the invention.
- Such conjugates and/or complexes can be used to facilitate delivery of siRNA molecules into a biological system, such as a cell.
- the conjugates and complexes provided by the instant invention can impart therapeutic activity by transferring therapeutic compounds across cellular membranes, altering the pharmacokinetics, and/or modulating the localization of nucleic acid molecules of the invention.
- the present invention encompasses the design and synthesis of novel conjugates and complexes for the delivery of molecules, including, but not limited to, small molecules, lipids, phospholipids, nucleosides, nucleotides, nucleic acids, antibodies, toxins, negatively charged polymers and other polymers, for example proteins, peptides, hormones, carbohydrates, polyethylene glycols, or polyamines, across cellular membranes.
- molecules including, but not limited to, small molecules, lipids, phospholipids, nucleosides, nucleotides, nucleic acids, antibodies, toxins, negatively charged polymers and other polymers, for example proteins, peptides, hormones, carbohydrates, polyethylene glycols, or polyamines, across cellular membranes.
- the transporters described are designed to be used either individually or as part of a multi-component system, with or without degradable linkers.
- Conjugates of the molecules described herein can be attached to biologically active molecules via linkers that are biodegradable, such as biodegradable nucleic acid linker molecules.
- biodegradable linker refers to a nucleic acid or non-nucleic acid linker molecule that is designed as a biodegradable linker to connect one molecule to another molecule, for example, a biologically active molecule to a siRNA molecule of the invention or the sense and antisense strands of a siRNA molecule of the invention.
- the biodegradable linker is designed such that its stability can be modulated for a particular purpose, such as delivery to a particular tissue or cell type.
- the stability of a nucleic acid-based biodegradable linker molecule can be modulated by using various chemistries, for example combinations of ribonucleotides, deoxyribonucleotides, and chemically modified nucleotides, such as 2′-O-methyl, 2′-fluoro, 2′-amino, 2′-O-amino, 2′-C-allyl, 2′-O-allyl, and other 2′-modified or base modified nucleotides.
- the biodegradable nucleic acid linker molecule can be a dimer, trimer, tetramer or longer nucleic acid molecule, for example, an oligonucleotide of about 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20 nucleotides in length, or can comprise a single nucleotide with a phosphorus-based linkage, for example, a phosphoramidate or phosphodiester linkage.
- the biodegradable nucleic acid linker molecule can also comprise nucleic acid backbone, nucleic acid sugar, or nucleic acid base modifications.
- biodegradable refers to degradation in a biological system, for example enzymatic degradation or chemical degradation.
- biologically active molecule refers to compounds or molecules that are capable of eliciting or modifying a biological response in a system.
- biologically active siRNA molecules either alone or in combination with othe molecules contemplated by the instant invention include therapeutically active molecules such as antibodies, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, siRNA, dsRNA, allozymes, aptamers, decoys and analogs thereof.
- Biologically active molecules of the invention also include molecules capable of modulating the pharmacokinetics and/or pharmacodynamics of other biologically active molecules, for example, lipids and polymers such as polyamines, polyamides, polyethylene glycol and other polyethers.
- phospholipid refers to a hydrophobic molecule comprising at least one phosphorus group.
- a phospholipid can comprise a phosphorus-containing group and saturated or unsaturated alkyl group, optionally substituted with OH, COOH, oxo, amine, or substituted or unsubstituted aryl groups.
- nucleic acid molecules e.g., siRNA molecules
- delivered exogenously optimally are stable within cells until reverse trascription of the RNA has been modulated long enough to reduce the levels of the RNA transcript.
- the nucleic acid molecules are resistant to nucleases in order to function as effective intracellular therapeutic agents. Improvements in the chemical synthesis of nucleic acid molecules described in the instant invention and in the art have expanded the ability to modify nucleic acid molecules by introducing nucleotide modifications to enhance their nuclease stability as described above.
- siRNA molecules having chemical modifications that maintain or enhance enzymatic activity of proteins involved in RNAi are provided.
- Such nucleic acids are also generally more resistant to nucleases than unmodified nucleic acids. Thus, in vitro and/or in vivo the activity should not be significantly lowered.
- nucleic acid-based molecules of the invention will lead to better treatment of the disease progression by affording the possibility of combination therapies (e.g., multiple siRNA molecules targeted to different genes; nucleic acid molecules coupled with known small molecule modulators; or intermittent treatment with combinations of molecules, including different motifs and/or other chemical or biological molecules).
- combination therapies e.g., multiple siRNA molecules targeted to different genes; nucleic acid molecules coupled with known small molecule modulators; or intermittent treatment with combinations of molecules, including different motifs and/or other chemical or biological molecules.
- the treatment of subjects with siRNA molecules can also include combinations of different types of nucleic acid molecules, such as enzymatic nucleic acid molecules (ribozymes), allozymes, antisense, 2,5-A oligoadenylate, decoys, aptamers etc.
- a siRNA molecule of the invention comprises one or more 5′ and/or a 3′-cap structure, for example on only the sense siRNA strand, antisense siRNA strand, or both siRNA strands.
- cap structure is meant chemical modifications, which have been incorporated at either terminus of the oligonucleotide (see, for example, Adamic et al., U.S. Pat. No. 5,998,203, incorporated by reference herein). These terminal modifications protect the nucleic acid molecule from exonuclease degradation, and may help in delivery and/or localization within a cell.
- the cap may be present at the 5′-terminus (5′-cap) or at the 3′-terminal (3′-cap) or may be present on both termini.
- the 5′-cap is selected from the group comprising inverted abasic residue (moiety); 4′,5′-methylene nucleotide; 1-(beta-D-erythrofuranosyl) nucleotide, 4′-thio nucleotide; carbocyclic nucleotide; 1,5-anhydrohexitol nucleotide; L-nucleotides; alpha-nucleotides; modified base nucleotide; phosphorodithioate linkage; threo-pentofuranosyl nucleotide; acyclic 3′,4′-seco nucleotide; acyclic 3,4-dihydroxybutyl nucleotide; acyclic 3,5-dihydroxypentyl nucleotide, 3′-3′-inverted nucleotide moiety; 3′-3′-inverted abasic moiety; 3′-2′-inverted nucleotide
- the 3′-cap is selected from a group comprising, 4′,5′-methylene nucleotide; 1-(beta-D-erythrofuranosyl) nucleotide; 4′-thio nucleotide, carbocyclic nucleotide; 5′-amino-alkyl phosphate; 1,3-diamino-2-propyl phosphate; 3-aminopropyl phosphate; 6-aminohexyl phosphate; 1,2-aminododecyl phosphate; hydroxypropyl phosphate; 1,5-anhydrohexitol nucleotide; L-nucleotide; alpha-nucleotide; modified base nucleotide; phosphorodithioate; threo-pentofuranosyl nucleotide; acyclic 3′,4′-seco nucleotide; 3,4-dihydroxybutyl
- non-nucleotide any group or compound which can be incorporated into a nucleic acid chain in the place of one or more nucleotide units, including either sugar and/or phosphate substitutions, and allows the remaining bases to exhibit their enzymatic activity.
- the group or compound is abasic in that it does not contain a commonly recognized nucleotide base, such as adenosine, guanine, cytosine, uracil or thymine and therefore lacks a base at the 1′-position.
- alkyl refers to a saturated aliphatic hydrocarbon, including straight-chain, branched-chain, and cyclic alkyl groups.
- the alkyl group has 1 to 12 carbons. More preferably, it is a lower alkyl of from 1 to 7 carbons, more preferably 1 to 4 carbons.
- the alkyl group can be substituted or unsubstituted. When substituted the substituted group(s) is preferably, hydroxyl, cyano, alkoxy, ⁇ O, ⁇ S, NO 2 or N(CH 3 ) 2 , amino, or SH.
- alkenyl groups that are unsaturated hydrocarbon groups containing at least one carbon-carbon double bond, including straight-chain, branched-chain, and cyclic groups.
- the alkenyl group has 1 to 12 carbons. More preferably, it is a lower alkenyl of from 1 to 7 carbons, more preferably 1 to 4 carbons.
- the alkenyl group may be substituted or unsubstituted. When substituted the substituted group(s) is preferably, hydroxyl, cyano, alkoxy, ⁇ O, ⁇ S, NO 2, halogen, N(CH 3 ) 2 , amino, or SH.
- alkyl also includes alkynyl groups that have an unsaturated hydrocarbon group containing at least one carbon-carbon triple bond, including straight-chain, branched-chain, and cyclic groups.
- the alkynyl group has 1 to 12 carbons. More preferably, it is a lower alkynyl of from 1 to 7 carbons, more preferably 1 to 4 carbons.
- the alkynyl group may be substituted or unsubstituted. When substituted the substituted group(s) is preferably, hydroxyl, cyano, alkoxy, ⁇ O, ⁇ S, NO 2 or N(CH 3 ) 2 , amino or SH.
- Such alkyl groups can also include aryl, alkylaryl, carbocyclic aryl, heterocyclic aryl, amide and ester groups.
- An “aryl” group refers to an aromatic group that has at least one ring having a conjugated pi electron system and includes carbocyclic aryl, heterocyclic aryl and biaryl groups, all of which may be optionally substituted.
- the preferred substituent(s) of aryl groups are halogen, trihalomethyl, hydroxyl, SH, OH, cyano, alkoxy, alkyl, alkenyl, alkynyl, and amino groups.
- alkylaryl refers to an alkyl group (as described above) covalently joined to an aryl group (as described above).
- Carbocyclic aryl groups are groups wherein the ring atoms on the aromatic ring are all carbon atoms. The carbon atoms are optionally substituted.
- Heterocyclic aryl groups are groups having from 1 to 3 heteroatoms as ring atoms in the aromatic ring and the remainder of the ring atoms are carbon atoms.
- Suitable heteroatoms include oxygen, sulfur, and nitrogen, and include furanyl, thienyl, pyridyl, pyrrolyl, N-lower alkyl pyrrolo, pyrimidyl, pyrazinyl, imidazolyl and the like, all optionally substituted.
- An “amide” refers to an —C(O)—NH—R, where R is either alkyl, aryl, alkylaryl or hydrogen.
- An “ester” refers to an —C(O)—OR′, where R is either alkyl, aryl, alkylaryl or hydrogen.
- nucleotide as used herein is as recognized in the art to include natural bases (standard), and modified bases well known in the art. Such bases are generally located at the 1′ position of a nucleotide sugar moiety. Nucleotides generally comprise a base, sugar and a phosphate group. The nucleotides can be unmodified or modified at the sugar, phosphate and/or base moiety, (also referred to interchangeably as nucleotide analogs, modified nucleotides, non-natural nucleotides, non-standard nucleotides and other; see, for example, Usman and McSwiggen, supra; Eckstein et al., International PCT Publication No.
- base modifications that can be introduced into nucleic acid molecules include, inosine, purine, pyridin-4-one, pyridin-2-one, phenyl, pseudouracil, 2, 4, 6-trimethoxy benzene, 3-methyl uracil, dihydrouridine, naphthyl, aminophenyl, 5-alkylcytidines (e.g., 5-methylcytidine), 5-alkyluridines (e.g., ribothymidine), 5-halouridine (e.g., 5-bromouridine) or 6-azapyrimidines or 6-alkylpyrimidines (e.g.
- modified bases in this aspect is meant nucleotide bases other than adenine, guanine, cytosine and uracil at 1′ position or their equivalents.
- the invention features modified siRNA molecules, with phosphate backbone modifications comprising one or more phosphorothioate, phosphorodithioate, methylphosphonate, phosphotriester, morpholino, amidate carbamate, carboxymethyl, acetamidate, polyamide, sulfonate, sulfonamide, sulfamate, formacetal, thioformacetal, and/or alkylsilyl, substitutions.
- phosphate backbone modifications comprising one or more phosphorothioate, phosphorodithioate, methylphosphonate, phosphotriester, morpholino, amidate carbamate, carboxymethyl, acetamidate, polyamide, sulfonate, sulfonamide, sulfamate, formacetal, thioformacetal, and/or alkylsilyl, substitutions.
- abasic sugar moieties lacking a base or having other chemical groups in place of a base at the 1′ position, see for example Adamic et al., U.S. Pat. No. 5,998,203.
- unmodified nucleoside is meant one of the bases adenine, cytosine, guanine, thymine, uracil joined to the 1′ carbon of a ⁇ -D-ribo-furanose.
- modified nucleoside is meant any nucleotide base which contains a modification in the chemical structure of an unmodified nucleotide base, sugar and/or phosphate.
- amino is meant 2′-NH 2 or 2′-O—NH 2 , which may be modified or unmodified.
- modified groups are described, for example, in Eckstein et al., U.S. Pat. No. 5,672,695 and Matulic-Adamic et al., U.S. Pat. No. 6,248,878, which are both incorporated by reference in their entireties.
- nucleic acid siRNA structure can be made to enhance the utility of these molecules. Such modifications will enhance shelf-life, half-life in vitro, stability, and ease of introduction of such oligonucleotides to the target site, e.g., to enhance penetration of cellular membranes, and confer the ability to recognize and bind to targeted cells.
- a siRNA molecule of the invention can be adapted for use to treat, for example allergic/inflammatory diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and any other indications that can respond to the level of ADORA1 in a cell or tissue, alone or in combination with other therapies.
- a siRNA molecule can comprise a delivery vehicle, including liposomes, for administration to a subject, carriers and diluents and their salts, and/or can be present in pharmaceutically acceptable formulations.
- Nucleic acid molecules can be administered to cells by a variety of methods known to those of skill in the art, including, but not restricted to, encapsulation in liposomes, by iontophoresis, or by incorporation into other delivery vehicles, such as hydrogels, cyclodextrins, biodegradable nanocapsules, and bioadhesive microspheres, or by proteinaceous vectors (O'Hare and Normand, International PCT Publication No. WO 00/53722).
- the nucleic acid/vehicle combination is locally delivered by direct injection or by use of an infusion pump.
- Direct injection of the nucleic acid molecules of the invention can take place using standard needle and syringe methodologies, or by needle-free technologies such as those described in Conry et al., 1999, Clin. Cancer Res., 5, 2330-2337 and Barry et al., International PCT Publication No. WO 99/31262.
- CNS delivery methods of oligonucleotides by osmotic pump see Chun et al., 1998, Neuroscience Letters, 257, 135-138, D'Aldin et al., 1998, Mol.
- nucleic acid delivery and administration are provided in Sullivan et al., supra, Draper et al., PCT W093/23569, Beigelman et al., PCT W099/05094, and Klimuk et al., PCT W099/04819 all of which have been incorporated by reference herein.
- the invention features the use of methods to deliver the nucleic acid molecules of the instant invention to hematopoietic cells, including monocytes and lymphocytes. These methods are described in detail by Hartmann et al., 1998, J. Phamacol. Exp. Ther., 285(2), 920-928; Kronenwett et al., 1998, Blood, 91(3), 852-862; Filion and Phillips, 1997, Biochim. Biophys. Acta., 1329(2), 345-356; Ma and Wei, 1996, Leuk. Res., 20(11/12), 925-930; and Bongartz et al., 1994, Nucleic Acids Research, 22(22), 4681-8.
- Such methods include the use of free oligonucleitide, cationic lipid formulations, liposome formulations including pH sensitive liposomes and immunoliposomes, and bioconjugates including oligonucleotides conjugated to fusogenic peptides, for the transfection of hematopoietic cells with oligonucleotides.
- the invention features a pharmaceutical composition
- a pharmaceutical composition comprising one or more nucleic acid(s) of the invention in an acceptable carrier, such as a stabilizer, buffer, and the like.
- the polynucleotides of the invention can be administered (e.g., RNA, DNA or protein) and introduced into a subject by any standard means, with or without stabilizers, buffers, and the like, to form a pharmaceutical composition.
- a liposome delivery mechanism standard protocols for formation of liposomes can be followed.
- the compositions of the present invention may also be formulated and used as tablets, capsules or elixirs for oral administration, suppositories for rectal administration, sterile solutions, suspensions for injectable administration, and the other compositions known in the art.
- the present invention also includes pharmaceutically acceptable formulations of the compounds described.
- formulations include salts of the above compounds, e.g., acid addition salts, for example, salts of hydrochloric, hydrobromic, acetic acid, and benzene sulfonic acid.
- a pharmacological composition or formulation refers to a composition or formulation in a form suitable for administration, e.g., systemic administration, into a cell or subject, including for example a human. Suitable forms, in part, depend upon the use or the route of entry, for example oral, transdermal, or by injection. Such forms should not prevent the composition or formulation from reaching a target cell (i.e., a cell to which the negatively charged nucleic acid is desirable for delivery). For example, pharmacological compositions injected into the blood stream should be soluble. Other factors are known in the art, and include considerations such as toxicity and forms that prevent the composition or formulation from exerting its effect.
- systemic administration in vivo systemic absorption or accumulation of drugs in the blood stream followed by distribution throughout the entire body.
- Administration routes which lead to systemic absorption include, without limitation: intravenous, subcutaneous, intraperitoneal, inhalation, oral, intrapulmonary and intramuscular.
- Each of these administration routes expose the siRNA molecules of the invention to an accessible diseased tissue.
- the rate of entry of a drug into the circulation has been shown to be a function of molecular weight or size.
- the use of a liposome or other drug carrier comprising the compounds of the instant invention can potentially localize the drug, for example, in certain tissue types, such as the tissues of the reticular endothelial system (RES).
- RES reticular endothelial system
- a liposome formulation that can facilitate the association of drug with the surface of cells, such as, lymphocytes and macrophages is also useful. This approach may provide enhanced delivery of the drug to target cells by taking advantage of the specificity of macrophage and lymphocyte immune recognition of abnormal cells, such as cancer cells.
- compositions or formulations that allows for the effective distribution of the nucleic acid molecules of the instant invention in the physical location most suitable for their desired activity.
- agents suitable for formulation with the nucleic acid molecules of the instant invention include the forulations and conjugates described herein, as well as other target area specific formulations including CNS formulations including P-glycoprotein inhibitors (such as Pluronic P85), which can enhance entry of drugs into the CNS (Jolliet-Riant and Tillement, 1999, Fundam. Clin.
- biodegradable polymers such as poly (DL-lactide-coglycolide) microspheres for sustained release delivery after intracerebral implantation (Emerich, D F et al, 1999, Cell Transplant, 8, 47-58) (Alkermes, Inc. Cambridge, Mass.); and loaded nanoparticles, such as those made of polybutylcyanoacrylate, which can deliver drugs across the blood brain barrier and can alter neuronal uptake mechanisms ( Prog Neuropsychopharmacol Biol Psychiatry, 23, 941-949, 1999).
- Other non-limiting examples of delivery strategies for the nucleic acid molecules of the instant invention include material described in Boado et al., 1998, J. Pharm.
- the invention also features the use of the composition comprising surface-modified liposomes containing poly (ethylene glycol) lipids (PEG-modified, or long-circulating liposomes or stealth liposomes).
- PEG-modified, or long-circulating liposomes or stealth liposomes offer a method for increasing the accumulation of drugs in target tissues.
- This class of drug carriers resists opsonization and elimination by the mononuclear phagocytic system (MPS or RES), thereby enabling longer blood circulation times and enhanced tissue exposure for the encapsulated drug (Lasic et al. Chem. Rev. 1995, 95, 2601-2627; Ishiwata et al., Chem. Pharm. Bull. 1995, 43, 1005-1011).
- liposomes have been shown to accumulate selectively in tumors, presumably by extravasation and capture in the neovascularized target tissues (Lasic et al., Science 1995, 267, 1275-1276; Oku et al., 1995, Biochim. Biophys. Acta, 1238, 86-90).
- the long-circulating liposomes enhance the pharmacokinetics and pharmacodynamics of DNA and RNA, particularly compared to conventional cationic liposomes which are known to accumulate in tissues of the MPS (Liu et al., J. Biol. Chem. 1995, 42, 24864-24870; Choi et al., International PCT Publication No.
- WO 96/10391 Ansell et al., International PCT Publication No. WO 96/10390; Holland et al., International PCT Publication No. WO 96/10392).
- Long-circulating liposomes are also likely to protect drugs from nuclease degradation to a greater extent compared to cationic liposomes, based on their ability to avoid accumulation in metabolically aggressive MPS tissues such as the liver and spleen.
- compositions prepared for storage or administration which include a pharmaceutically effective amount of the desired compounds in a pharmaceutically acceptable carrier or diluent.
- Acceptable carriers or diluents for therapeutic use are well known in the pharmaceutical art, and are described, for example, in Remington's Pharmaceutical Sciences, Mack Publishing Co. (A. R. Gennaro edit. 1985) hereby incorporated by reference herein.
- preservatives, stabilizers, dyes and flavoring agents may be provided. These include sodium benzoate, sorbic acid and esters of p-hydroxybenzoic acid.
- antioxidants and suspending agents can be used.
- a pharmaceutically effective dose is that dose required to prevent, inhibit the occurrence, or treat (alleviate a symptom to some extent, preferably all of the symptoms) of a disease state.
- the pharmaceutically effective dose depends on the type of disease, the composition used, the route of administration, the type of mammal being treated, the physical characteristics of the specific mammal under consideration, concurrent medication, and other factors that those skilled in the medical arts will recognize. Generally, an amount between 0.1 mg/kg and 100 mg/kg body weight/day of active ingredients is administered dependent upon potency of the negatively charged polymer.
- nucleic acid molecules of the invention and formulations thereof can be administered orally, topically, parenterally, by inhalation or spray, or rectally in dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and/or vehicles.
- parenteral as used herein includes percutaneous, subcutaneous, intravascular (e.g., intravenous), intramuscular, or intrathecal injection or infusion techniques and the like.
- a pharmaceutical formulation comprising a nucleic acid molecule of the invention and a pharmaceutically acceptable carrier.
- nucleic acid molecules of the invention can be present in association with one or more non-toxic pharmaceutically acceptable carriers and/or diluents and/or adjuvants, and if desired other active ingredients.
- the pharmaceutical compositions containing nucleic acid molecules of the invention can be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion, hard or soft capsules, or syrups or elixirs.
- compositions intended for oral use can be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions can contain one or more such sweetening agents, flavoring agents, coloring agents or preservative agents in order to provide pharmaceutically elegant and palatable preparations.
- Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients that are suitable for the manufacture of tablets.
- excipients can be, for example, inert diluents; such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia; and lubricating agents, for example magnesium stearate, stearic acid or talc.
- the tablets can be uncoated or they can be coated by known techniques. In some cases such coatings can be prepared by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monosterate or glyceryl distearate can be employed.
- Formulations for oral use can also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin or olive oil.
- an inert solid diluent for example, calcium carbonate, calcium phosphate or kaolin
- water or an oil medium for example peanut oil, liquid paraffin or olive oil.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions.
- excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydropropyl-methylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents can be a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan mono
- the aqueous suspensions can also contain one or more preservatives, for example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- preservatives for example ethyl, or n-propyl p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl p-hydroxybenzoate
- flavoring agents for example ethyl, or n-propyl p-hydroxybenzoate
- sweetening agents such as sucrose or saccharin.
- Oily suspensions can be formulated by suspending the active ingredients in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions can contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol.
- Sweetening agents and flavoring agents can be added to provide palatable oral preparations. These compositions can be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives.
- a dispersing or wetting agent for example sweetening, flavoring and coloring agents, can also be present.
- compositions of the invention can also be in the form of oil-in-water emulsions.
- the oily phase can be a vegetable oil or a mineral oil or mixtures of these.
- Suitable emulsifying agents can be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol, anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate.
- the emulsions can also contain sweetening and flavoring agents.
- Syrups and elixirs can be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol, glucose or sucrose. Such formulations can also contain a demulcent, a preservative and flavoring and coloring agents.
- the pharmaceutical compositions can be in the form of a sterile injectable aqueous or oleaginous suspension. This suspension can be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents that have been mentioned above.
- the sterile injectable preparation can also be a sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example as a solution in 1,3-butanediol.
- Suitable vehicles and solvents that can be employed are water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil can be employed including synthetic mono-or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- the nucleic acid molecules of the invention can also be administered in the form of suppositories, e.g., for rectal administration of the drug.
- suppositories e.g., for rectal administration of the drug.
- These compositions can be prepared by mixing the drug with a suitable non-irritating excipient that is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- suitable non-irritating excipient that is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- Such materials include cocoa butter and polyethylene glycols.
- Nucleic acid molecules of the invention can be administered parenterally in a sterile medium.
- the drug depending on the vehicle and concentration used, can either be suspended or dissolved in the vehicle.
- adjuvants such as local anesthetics, preservatives and buffering agents can be dissolved in the vehicle.
- Dosage levels of the order of from about 0.1 mg to about 140 mg per kilogram of body weight per day are useful in the treatment of the above-indicated conditions (about 0.5 mg to about 7 g per subject per day).
- the amount of active ingredient that can be combined with the carrier materials to produce a single dosage form varies depending upon the host treated and the particular mode of administration.
- Dosage unit forms generally contain between from about 1 mg to about 500 mg of an active ingredient.
- the specific dose level for any particular subject depends upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diet, time of administration, route of administration, and rate of excretion, drug combination and the severity of the particular disease undergoing therapy.
- the composition can also be added to the animal feed or drinking water. It can be convenient to formulate the animal feed and drinking water compositions so that the animal takes in a therapeutically appropriate quantity of the composition along with its diet. It can also be convenient to present the composition as a premix for addition to the feed or drinking water.
- nucleic acid molecules of the present invention may also be administered to a subject in combination with other therapeutic compounds to increase the overall therapeutic effect.
- the use of multiple compounds to treat an indication may increase the beneficial effects while reducing the presence of side effects.
- the invention compositions suitable for administering nucleic acid molecules of the invention to specific cell types are suitable for administering nucleic acid molecules of the invention to specific cell types.
- ASGPr asialoglycoprotein receptor
- ASOR asialoorosomucoid
- Binding of such glycoproteins or synthetic glycoconjugates to the receptor takes place with an affinity that strongly depends on the degree of branching of the oligosaccharide chain, for example, triatennary structures are bound with greater affinity than biatenarry or monoatennary chains (Baenziger and Fiete, 1980, Cell, 22, 611-620; Connolly et al., 1982, J. Biol. Chem., 257, 939-945).
- Lee and Lee, 1987, Glycoconjugate J., 4, 317-328 obtained this high specificity through the use of N-acetyl-D-galactosamine as the carbohydrate moiety, which has higher affinity for the receptor, compared to galactose.
- bioconjugates are described in Vargeese et al., U.S. Ser. No. 60/311,865, filed Aug. 13, 2001; and Matulic-Adamic et al., U.S. Ser. No. 60/362,016, filed Mar. 6, 2002.
- siRNA molecules of the instant invention can be expressed within cells from eukaryotic promoters (e.g., Izant and Weintraub, 1985, Science, 229, 345; McGarry and Lindquist, 1986, Proc. Natl. Acad. Sci., USA 83, 399; Scanlon et al., 1991, Proc. Natl. Acad. Sci. USA, 88, 10591-5; Kashani-Sabet et al., 1992, Antisense Res. Dev., 2, 3-15; Dropulic et al., 1992, J. Virol., 66, 1432-41; Weerasinghe et al., 1991, J.
- eukaryotic promoters e.g., Izant and Weintraub, 1985, Science, 229, 345; McGarry and Lindquist, 1986, Proc. Natl. Acad. Sci., USA 83, 399; Scanlon et al
- nucleic acids can be augmented by their release from the primary transcript by a enzymatic nucleic acid (Draper et al., PCT WO 93/23569, and Sullivan et al., PCT WO 94/02595; Ohkawa et al., 1992, Nucleic Acids Symp. Ser., 27, 15-6; Taira et al., 1991, Nucleic Acids Res., 19, 5125-30; Ventura et al., 1993, Nucleic Acids Res., 21, 3249-55; Chowrira et al., 1994, J. Biol Chem., 269, 25856.
- RNA molecules of the present invention can be expressed from transcription units (see for example Couture et al., 1996, TIG., 12, 510) inserted into DNA or RNA vectors.
- the recombinant vectors can be DNA plasmids or viral vectors.
- siRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus, retrovirus, adenovirus, or alphavirus.
- pol III based constructs are used to express nucleic acid molecules of the invention (see for example Thompson, U.S. Pat. Nos. 5,902,880 and 6,146,886).
- the recombinant vectors capable of expressing the siRNA molecules can be delivered as described above, and persist in target cells.
- viral vectors can be used that provide for transient expression of nucleic acid molecules.
- Such vectors can be repeatedly administered as necessary.
- the siRNA molecule interacts with the target mRNA and generates an RNAi response.
- Delivery of siRNA molecule expressing vectors can be systemic, such as by intravenous or intra-muscular administration, by administration to target cells ex-planted from a subject followed by reintroduction into the subject, or by any other means that would allow for introduction into the desired target cell (for a review see Couture et al., 1996, TIG., 12, 510).
- the invention features an expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of the instant invention.
- the expression vector can encode one or both strands of a siRNA duplex, or a single self complementary strand that self hybridizes into a siRNA duplex.
- the nucleic acid sequences encoding the siRNA molecules of the instant invention can be operably linked in a manner that allows expression of the siRNA molecule (see for example Paul et al., 2002, Nature Biotechnology, 19, 505; Miyagishi and Taira, 2002, Nature Biotechnology, 19, 497; Lee et al., 2002, Nature Biotechnology, 19, 500; and Novina et al., 2002, Nature Medicine, advance online publication doi: 10.1038/nm725).
- the invention features an expression vector comprising: a) a transcription initiation region (e.g., eukaryotic pol I, II or III initiation region); b) a transcription termination region (e.g., eukaryotic pol I, II or III termination region); and c) a nucleic acid sequence encoding at least one of the siRNA molecules of the instant invention; wherein said sequence is operably linked to said initiation region and said termination region, in a manner that allows expression and/or delivery of the siRNA molecule.
- the vector can optionally include an open reading frame (ORF) for a protein operably linked on the 5′ side or the 3′-side of the sequence encoding the siRNA of the invention; and/or an intron (intervening sequences).
- ORF open reading frame
- RNA polymerase I eukaryotic RNA polymerase I
- polymerase II RNA polymerase II
- poly III RNA polymerase III
- Transcripts from pol II or pol III promoters are expressed at high levels in all cells; the levels of a given pol II promoter in a given cell type depends on the nature of the gene regulatory sequences (enhancers, silencers, etc.) present nearby.
- Prokaryotic RNA polymerase promoters are also used, providing that the prokaryotic RNA polymerase enzyme is expressed in the appropriate cells (Elroy-Stein and Moss, 1990, Proc. Natl. Acad. Sci.
- nucleic acid molecules expressed from such promoters can function in mammalian cells (e.g. Kashani-Sabet et al., 1992, Antisense Res. Dev., 2, 3-15; Ojwang et al., 1992, Proc. Natl. Acad. Sci.
- transcription units such as the ones derived from genes encoding U6 small nuclear (snRNA), transfer RNA (tRNA) and adenovirus VA RNA are useful in generating high concentrations of desired RNA molecules such as siRNA in cells (Thompson et al., supra; Couture and Stinchcomb, 1996, supra; Noonberg et al., 1994, Nucleic Acid Res., 22, 2830; Noonberg et al., U.S. Pat. No. 5,624,803; Good et al, 1997, Gene Ther., 4, 45; Beigelman et al., International PCT Publication No. WO 96/18736.
- siRNA transcription units can be incorporated into a variety of vectors for introduction into mammalian cells, including but not restricted to, plasmid DNA vectors, viral DNA vectors (such as adenovirus or adeno-associated virus vectors), or viral RNA vectors (such as retroviral or alphavirus vectors) (for a review see Couture and Stinchcomb, 1996, supra).
- plasmid DNA vectors such as adenovirus or adeno-associated virus vectors
- viral RNA vectors such as retroviral or alphavirus vectors
- the invention features an expression vector comprising a nucleic acid sequence encoding at least one of the siRNA molecules of the invention, in a manner that allows expression of that siRNA molecule.
- the expression vector comprises in one embodiment; a) a transcription initiation region; b) a transcription termination region; and c) a nucleic acid sequence encoding at least one strand of the siRNA molecule; wherein the sequence is operably linked to the initiation region and the termination region, in a manner that allows expression and/or delivery of the siRNA molecule.
- the expression vector comprises: a) a transcription initiation region; b) a transcription termination region; c) an open reading frame; and d) a nucleic acid sequence encoding at least one strand of a siRNA molecule, wherein the sequence is operably linked to the 3′-end of the open reading frame; and wherein the sequence is operably linked to the initiation region, the open reading frame and the termination region, in a manner that allows expression and/or delivery of the siRNA molecule.
- the expression vector comprises: a) a transcription initiation region; b) a transcription termination region; c) an intron; and d) a nucleic acid sequence encoding at least one siRNA molecule; wherein the sequence is operably linked to the initiation region, the intron and the termination region, in a manner which allows expression and/or delivery of the nucleic acid molecule.
- the expression vector comprises: a) a transcription initiation region; b) a transcription termination region; c) an intron; d) an open reading frame; and e) a nucleic acid sequence encoding at least one strand of a siRNA molecule, wherein the sequence is operably linked to the 3′-end of the open reading frame; and wherein the sequence is operably linked to the initiation region, the intron, the open reading frame and the termination region, in a manner which allows expression and/or delivery of the siRNA molecule.
- siRNA molecules of the invention are synthesized in tandem using a cleavable linker, for example a succinyl-based linker. Tandem synthesis as described herein is followed by a one step purification process that provides RNAi molecules in high yield. This approach is highly amenable to siRNA synthesis in support of high throughput RNAi screening, and can be readily adapted to multi-column or multi-well synthesis platforms.
- a cleavable linker for example a succinyl-based linker.
- the oligonucleotides are deprotected as described above. Following deprotection, the siRNA sequence strands are allowed to spontaneously hybridize. This hybridization yields a duplex in which one strand has retained the 5′-O-DMT group while the complementary strand comprises a terminal 5′-hydroxyl. The newly formed duplex to behaves as a single molecule during routine solid-phase extraction purification (Trityl-On purification) even though only one molecule has a dimethoxytrityl group.
- this dimethoxytrityl group (or an equivalent group, such as other trityl groups or other hydrophobic moieties) is all that is required to purify the pair of oligos, for example by using a C18 cartridge.
- Standard phosphoramidite synthesis chemistry is used up to point of introducing a tandem linker, such as an inverted deoxyabasic succinate linker (see FIG. 1) or an equivalent cleavable linker.
- linker coupling conditions that can be used includes a hindered base such as diisopropylethylamine (DIPA) and/or DMAP in the presence of an activator reagent such as Bromotripyrrolidinophosphoniumhexaflurorophosphate (PyBrOP).
- DIPA diisopropylethylamine
- PyBrOP Bromotripyrrolidinophosphoniumhexaflurorophosphate
- standard synthesis chemistry is utilized to complete synthesis of the second sequence leaving the terminal the 5′-O-DMT intact.
- the resulting oligonucleotide is deprotected according to the procedures described herein and quenched with a suitable buffer, for example with 50 mM NaOAc or 1.5M
- siRNA duplex can be readily accomplished using solid phase extraction, for example using a Waters C18 SepPak 1 g cartridge conditioned with 1 column volume (CV) of acetonitrile, 2 CV H2O, and 2 CV 50 mM NaOAc. The sample is loaded and then washed with 1 CV H2O or 50 mM NaOAc. Failure sequences are eluted with 1 CV 14% ACN (Aqueous with 50 mM NaOAc and 50mM NaCl).
- CV column volume
- the column is then washed, for example with 1 CV H2O followed by on-column detritylation, for example by passing 1 CV of 1% aqueous trifluoroacetic acid (TFA) over the column, then adding a second CV of 1% aqueous TFA to the column and allowing to stand for approx. 10 minutes.
- TFA trifluoroacetic acid
- the remaining TFA solution is removed and the column washed with H2O followed by 1 CV 1M NaCl and additional H2O.
- the siRNA duplex product is then eluted, for example using 1 CV 20% aqueous CAN.
- FIG. 2 provides an example of MALDI-TOV mass spectrometry analysis of a purified siRNA construct in which each peak corresponds to the calculated mass of an individual siRNA strand of the siRNA duplex.
- the same purified siRNA provides three peaks when analyzed by capillary gel electrophoresis (CGE), one peak presumably corresponding to the duplex siRNA, and two peaks presumably corresponding to the separate siRNA sequence strands. Ion exchange HPLC analysis of the same siRNA contract only shows a single peak.
- CGE capillary gel electrophoresis
- RNA target of interest such as a human mRNA transcript
- sequence of a gene or RNA gene transcript derived from a database is used to generate siRNA targets having complimentarity to the target.
- Such sequences can be obtained from a database, or can be determined experimentally as known in the art.
- Target sites that are known, for example, those target sites determined to be effective target sites based on studies with other nucleic acid molecules, for example ribozymes or antisense, or those targets known to be associated with a disease or condition such as those sites containing mutations or deletions, can be used to design siRNA molecules targeting those sites as well.
- RNA transcripts can be chosen to screen siRNA molecules for efficacy, for example by using in vitro RNA cleavage assays, cell culture, or animal models. In a non-limiting example, anywhere from 1 to 1000 target sites are chosen within the transcript based on the size of the siRNA contruct to be used. High throughput screening assays can be developed for screening siRNA molecules using methods known in the art, such as with multi-well or multi-plate assays to determine efficient reduction in target gene expression.
- the target sequence is parsed in silico into a list of all fragments or subsequences of a particular length, for example 23 nucleotide fragments, contained within the target sequence. This step is typically carried out using a custom Perl script, but commercial sequence analysis programs such as Oligo, MacVector, or the GCG Wisconsin Package can be employed as well.
- the siRNAs correspond to more than one target sequence; such would be the case for example in targeting different transcipts of the same gene, targeting different transcipts of more than one gene, or for targeting both the human gene and an animal homolog.
- a subsequence list of a particular length is generated for each of the targets, and then the lists are compared to find matching sequences in each list.
- the subsequences are then ranked according to the number of target sequences that contain the given subsequence; the goal is to find subsequences that are present in most or all of the target sequences.
- the ranking can indentify subsequences that are unique to a target sequence, such as a mutant target sequence. Such an approach would enable the use of siRNA to target specifically the mutant sequence and not effect the expression of the normal sequence.
- siRNA subsequences are absent in one or more sequences while present in the desired target sequence; such would be the case if the siRNA targets a gene with a paralogous family member that is to remain untargeted.
- a subsequence list of a particular length is generated for each of the targets, and then the lists are compared to find sequences that are present in the target gene but are absent in the untargeted paralog.
- the ranked siRNA subsequences can be further analyzed and ranked according to GC content. A preference can be given to sites containing 30-70% GC, with a further preference to sites containing 40-60% GC.
- the ranked siRNA subsequences can be further analyzed and ranked according to self-folding and internal hairpins. Weaker internal folds are preferred; strong hairpin structures are to be avoided.
- the ranked siRNA subsequences can be further analyzed and ranked according to whether they have runs of GGG or CCC in the sequence.
- GGG or even more Gs in either strand can make oligonucleotide synthesis problematic, so it is avoided whenever better sequences are available.
- CCC is searched in the target strand because that will place GGG in the antisense strand.
- the ranked siRNA subsequences can be further analyzed and ranked according to whether they have the dinucleotide UU (uridine dinucleotide) on the 3′ end of the sequence, and/or AA on the 5′ end of the sequence (to yield 3′ UU on the antisense sequence). These sequences allow one to design siRNA molecules with terminal TT thymidine dinucleotides.
- UU uridine dinucleotide
- target sites are chosen from the ranked list of subsequences as described above. For example, in subsequences having 23 nucleotides, the right 21 nucleotides of each chosen 23-mer subsequence are then designed and synthesized for the upper (sense) strand of the siRNA duplex, while the reverse complement of the left 21 nucleotides of each chosen 23-mer subsequence are then designed and synthesized for the lower (antisense) strand of the siRNA duplex. If terminal TT residues are desired for the sequence (as described in paragraph 7), then the two 3′ terminal nucleotides of both the sense and antisense strands are replaced by TT prior to synthesizing the oligos.
- siRNA molecules are screened in an in vitro, cell culture or animal model system to identify the most active siRNA molecule or the most preferred target site within the target RNA sequence.
- a pool of siRNA constructs specific to an ADORA1 target sequence is used to screen for target sites in cells expressing ADORA1 RNA, such as human lung mast cells.
- ADORA1 RNA such as human lung mast cells.
- the general strategy used in this approach is shown in FIG. 9.
- a non-limiting example of such as pool is a pool comprising sequences having sense sequences comprising SEQ ID NOs. 1-161 and antisense sequences comprising SEQ ID NOs. 162-322 respectively.
- Human lung mast cells expressing ADORA1 are transfected with the pool of siRNA constructs and cells that demonstrate a phenotype associated with ADORA1 inhibition are sorted.
- the pool of siRNA constructs can be expressed from transciption cassettes inserted into appropriate vectors (see for example FIG. 7 and FIG.
- siRNA from cells demonstrating a positive phenotypic change are sequenced to determine the most suitable target site(s) within the target ADORA1 RNA sequence.
- siRNA target sites were chosen by analyzing sequences of the ADORA1 RNA target and optionally prioritizing the target sites on the basis of folding (structure of any given sequence analyzed to determine siRNA accessibility to the target), using a library of siRNA molecules as described in Example 3, or alternately by using an in vitro siRNA system as described in Example 6 herein.
- siRNA molecules were designed that could bind each target and are optionally individually analyzed by computer folding to assess whether the siRNA molecule can interact with the target sequence. Varying the length of the siRNA molecules can be chosen to optimize activity.
- siRNA molecules can be designed to target sites within any known RNA sequence, for example those RNA sequences corresponding to the any gene transcript.
- siRNA molecules can be designed to interact with various sites in the RNA message, for example target sequences within the RNA sequences described herein.
- the sequence of one strand of the siRNA molecule(s) are complementary to the target site sequences described above.
- the siRNA molecules can be chemically synthesized using methods described herein.
- Inactive siRNA molecules that are used as control sequences can be synthesized by scrambling the sequence of the siRNA molecules such that it is not complimentary to the target sequence.
- An in vitro assay that recapitulates RNAi in a cell free system is used to evaluate siRNA constructs targeting ADORA1 RNA targets.
- the assay comprises the system described by Tuschl et al., 1999, Genes and Development, 13, 3191-3197 and Zamore et al., 2000, Cell, 101, 25-33 adapted for use with ADORA1 target RNA.
- a Drosophila extract derived from syncytial blastoderm is used to reconstitute RNAi activity in vitro.
- Target RNA is generated via in vitro transcription from an appropriate ADORA1 expressing plasmid using T7 RNA polymerase or via chemical synthesis as described herein.
- Sense and antisense siRNA strands are annealed by incubation in buffer (such as 100 mM potassium acetate, 30 mM HEPES-KOH, pH 7.4, 2 mM magnesium acetate) for 1 min. at 90° C. followed by 1 hour at 37° C., then diluted in lysis buffer (for example 100 mM potassium acetate, 30 mM HEPES-KOH at pH 7.4, 2 mM magnesium acetate). Annealing can be monitored by gel electrophoresis on an agarose gel in TBE buffer and stained with ethidium bromide.
- buffer such as 100 mM potassium acetate, 30 mM HEPES-KOH, pH 7.4, 2 mM magnesium acetate
- the Drosophila lysate is prepared using zero to two hour old embryos from Oregon R flies collected on yeasted molasses agar that are dechorionated and lysed. The lysate is centrifuged and the supernatant isolated.
- the assay comprises a reaction mixture containing 50% lysate [vol/vol], RNA (10-50 pM final concentration), and 10% [vol/vol] lysis buffer containing siRNA (10 nM final concentration).
- the reaction mixture also contains 10 mM creatine phosphate, 10 ug.ml creatine phosphokinase, 100 um GTP, 100 uM UTP, 100 uM CTP, 500 uM ATP, 5 mM DTT, 0.1 U/uL RNasin (Promega), and 100 uM of each amino acid.
- the final concentration of potassium acetate is adjusted to 100 mM.
- the reactions are pre-assembled on ice and preincubated at 25° C. for 10 minutes before adding RNA, then incubated at 25° C. for an additional 60 minutes. Reactions are quenched with 4 volumes of 1.25 ⁇ Passive Lysis Buffer (Promega).
- Target RNA cleavage is assayed by RT-PCR analysis or other methods known in the art and are compared to control reactions in which siRNA is omitted from the reaction.
- target RNA for the assay is prepared by in vitro transcription in the presence of [a- 32 P] CTP, passed over a G 50 Sephadex column by spin chromatography and used as target RNA without further purification.
- target RNA is 5′- 32 P-end labeled using T4 polynucleotide kinase enzyme. Assays are performed as described above and target RNA and the specific RNA cleavage products generated by RNAi are visualized on an autoradiograph of a gel. The percentage of cleavage is determined by Phosphor Imager® quantitation of bands representing intact control RNA or RNA from control reactions without siRNA and the cleavage products generated by the assay.
- this assay is used to determine target sites the ADORA1 RNA target for siRNA mediated RNAi cleavage, wherein a plurality of siRNA constructs are screened for RNAi mediated cleavage of the ADORA1 RNA target, for example by analysing the assay reaction by electrophoresis of labelled target RNA, or by northern blotting, as well as by other methodology well known in the art.
- siRNA molecules targeted to the human ADORA1 RNA are designed and synthesized as described above. These nucleic acid molecules can be tested for cleavage activity in vivo, for example, using the following procedure.
- the target sequences and the nucleotide location within the ADORA1 RNA are given in Table I and III.
- RNA inhibition is measured after delivery of these reagents by a suitable transfection agent to human lung epithelial cells. Relative amounts of target RNA are measured versus actin using real-time PCR monitoring of amplification (eg. ABI 7700 Taqman®).
- a comparison is made to a mixture of oligonucleotide sequences made to unrelated targets or to a randomized siRNA control with the same overall length and chemistry, but randomly substituted at each position.
- Primary and secondary lead reagents are chosen for the target and optimization performed. After an optimal transfection agent concentration is chosen, a RNA time-course of inhibition is performed with the lead siRNA molecule.
- a cell-plating format can be used to determine RNA inhibition.
- Human lung epithelial cells e.g., A549 are seeded, for example, at 1 ⁇ 10 5 cells per well of a six well dish in EGM-2 (BioWhittaker) the day before transfection.
- siRNA final concentration, for example 20 nM
- cationic lipid e.g., final concentration 2 ⁇ g/ml
- EGM basal media Biowhittaker
- the complexed siRNA is added to each well and incubated for the times indicated.
- cells are seeded, for example, at 1 ⁇ 10 3 in 96 well plates and siRNA complex added as described.
- Efficiency of delivery of siRNA to A549 is determined using a fluorescent siRNA complexed with lipid.
- A549 in 6 well dishes are incubated with siRNA for 24 hours, rinsed with PBS and fixed in 2% paraformaldehyde for 15 minutes at room temperature. Uptake of siRNA is visualised using a fluorescent microscope.
- Total RNA is prepared from cells following siRNA delivery, for example using Qiagen RNA purification kits for 6 well or Rneasy extraction kits for 96 well assays.
- dual-labeled probes are synthesized with the reporter dye, FAM or JOE, covalently linked at the 5′ end and the quencher dye TAMRA conjugated to the 3′ end.
- RT-PCR amplifications are performed on, for example, an ABI PRISM 7700 Sequence Detector using 50 ⁇ l reactions consisting of 10 ⁇ l total RNA, 100 nM forward primer, 900 nM reverse primer, 100 nM probe, 1 ⁇ TaqMan PCR reaction buffer (PE-Applied Biosystems), 5.5 mM MgCl 2 , 300 ⁇ M each dATP, dCTP, dGTP, and dTTP, 10U RNase Inhibitor (Promega), 1.25U AmpliTaq Gold (PE-Applied Biosystems) and 10U M-MLV Reverse Transcriptase (Promega).
- ABI PRISM 7700 Sequence Detector using 50 ⁇ l reactions consisting of 10 ⁇ l total RNA, 100 nM forward primer, 900 nM reverse primer, 100 nM probe, 1 ⁇ TaqMan PCR reaction buffer (PE-Applied Biosystems), 5.5 mM MgCl 2 , 300 ⁇ M
- the thermal cycling conditions can consist of 30 min at 48° C., 10 min at 95° C., followed by 40 cycles of 15 sec at 95° C. and 1 min at 60° C.
- Quantitation of mRNA levels are determined relative to standards generated from serially diluted total cellular RNA (300, 100, 33, 11 ng/rxn) and normalizing to ⁇ -actin or GAPDH mRNA in parallel TaqMan reactions.
- an upper and lower primer and a flourescently labelled probe are designed.
- Real time incorporation of SYBR Green I dye into a specific PCR product can be measured in glass capillary tubes using a lightcyler.
- a standard curve is generated for each primer pair using control c RNA allularnd values are represented as relative expression to GAPDH in each sample.
- Nuclear extracts can be prepared using a standard micropreparation technique (see for example Andrews and Faller, 1991, Nucleic Acids Research, 19, 2499). Protein extracts from supernatants are prepared, for example using TCA precipitation. An equal volume of 20% TCA is added to the cell supernatant, incubated on ice for 1 hour and pelleted by centrifugation for 5 minutes. Pellets are washed in acetone, dried and resuspended in water. Cellular protein extracts are run on a 10% Bis-Tris NuPage (nuclear extracts) or 4-12% Tris-Glycine (supernatant extracts) polyacrylamide gel and transferred onto nitro-cellulose membranes.
- Non-specific binding can be blocked by incubation, for example, with 5% non-fat milk for 1 hour followed by primary antibody for 16 hour at 4° C. Following washes, the secondary antibody is applied, for example (1:10,000 dilution) for 1 hour at room temperature and the signal detected with SuperSignal reagent (Pierce).
- anti-ADORA-1 agents e.g., siRNA
- Nyce and Metzger, 1997, Nature, 385, 721-725 describe a useful dust mite conditioned allergic rabbit model of human asthma.
- Allergic rabbits treated with aerosolized siRNA are compared to untreated controls or animals treated with a non-specific siRNA constrol with regard to adenosine challenge.
- the concentration of aerolsolized adenosine required to reduce the dynamic compliance of the bronchial airway 50% from a baseline values is determined in both groups of animals. Additionally, dose response studies using this same endpoint are performed.
- Airway smooth muscle is surgically dissected from the animals and is processed for quantitative assessment of adenosine A1 receptors.
- adenosine A2 receptors and/or bradykinin receptors are quantitated as well.
- Adenosine A1 receptor density can be assayed by specific binding of a [ 3 H]DPCPX.
- a dose dependent reduction in adenosine A1 receptor densitiy is indicative of a therapeutic response
- This model can be used to evaluate animals that are treated with nucleic acid molecules of the invention and can furthermore be used as a positive control in determining the response of animals treated with nucleic acid molecules of the invention by using such factors as airway obstruction, lung capacity, and bronchiolar alveolar lavage (BAL) fluid in the evaluation.
- Human epithelial lung cell lines such as NPE cells and NCB-20 cells, can be used to express ADORA1. Cloned human ADORA1 is therefore expressed in CHO and COS7 cells and used in various studies. These ADORA1 expressing lung cell lines can be used in cell culture assays to evaluate nucleic acid molecules of the invention. A primary endpoint in these experiments would be the RT-PCR analysis of ADORA1 ntRNA expression in ADORA1 expressing cells. In addition, ligand binding assays can be developed where binding of [ 3 H]DPCPX can be evaluated in response to treatment with nucleic acid molecules of the invention.
- nucleic acid molecules of the present invention can be used in assays to diagnose disease state related of ADORA1 levels.
- nucleic acid molecules can be used to treat disease state related to ADORA1 levels.
- Particular degenerative and disease states that can be associated with ADORA1 levels include, but are not limited to allergic diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and any other diseases or conditions that are related to or will respond to the levels of ADORA1 in a cell or tissue, alone or in combination with other therapies.
- siRNA molecules of the invention can be used in a variety of diagnostic applications, such as in identifying molecular targets such as RNA in a variety of applications, for example, in clinical, industrial, environmental, agricultural and/or research settings.
- diagnostic use of siRNA molecules involves utilizing reconstituted RNAi systems, for example using cellular lysates or partially purified cellular lysates.
- siRNA molecules of this invention may be used as diagnostic tools to examine genetic drift and mutations within diseased cells or to detect the presence of endogenous or exogenous, for example viral, RNA in a cell.
- siRNA activity allows the detection of mutations in any region of the molecule, which alters the base-pairing and three-dimensional structure of the target RNA.
- siRNA molecules described in this invention one may map nucleotide changes, which are important to RNA structure and function in vitro, as well as in cells and tissues. Cleavage of target RNAs with siRNA molecules can be used to inhibit gene expression and define the role (essentially) of specified gene products in the progression of disease or infection. In this manner, other genetic targets may be defined as important mediators of the disease.
- siRNA molecules of this invention include detection of the presence of mRNAs associated with a disease, infection, or related condition. Such RNA is detected by determining the presence of a cleavage product after treatment with a siRNA using standard methodologies, for example fluorescence resonance emission transfer (FRET).
- FRET fluorescence resonance emission transfer
- siRNA molecules that can cleave only wild-type or mutant forms of the target RNA are used for the assay.
- the first siRNA molecules is used to identify wild-type RNA present in the sample and the second siRNA molecules will be used to identify mutant RNA in the sample.
- synthetic substrates of both wild-type and mutant RNA will be cleaved by both siRNA molecules to demonstrate the relative siRNA efficiencies in the reactions and the absence of cleavage of the “non-targeted” RNA species.
- the cleavage products from the synthetic substrates will also serve to generate size markers for the analysis of wild-type and mutant RNAs in the sample population.
- each analysis will require two siRNA molecules, two substrates and one unknown sample which will be combined into six reactions.
- the presence of cleavage products will be determined using an RNase protection assay so that full-length and cleavage fragments of each RNA can be analyzed in one lane of a polyacrylamide gel. It is not absolutely required to quantify the results to gain insight into the expression of mutant RNAs and putative risk of the desired phenotypic changes in target cells.
- the expression of mRNA whose protein product is implicated in the development of the phenotype is adequate to establish risk. If probes of comparable specific activity are used for both transcripts, then a qualitative comparison of RNA levels will be adequate and will decrease the cost of the initial diagnosis. Higher mutant form to wild-type ratios will be correlated with higher risk whether RNA levels are compared qualitatively or quantitatively.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Virology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The present invention concerns methods and reagents useful in modulating adenosine A1 receptor (ADORA1) gene expression in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to small interfering RNA (siRNA) molecules capable of mediating RNA interference (RNAi) against ADORA1 and related receptors.
Description
- This application claims the benefit of U.S. Patent Application 60/315,315 filed on Aug. 28, 2001.
- The present invention concerns methods and reagents useful in modulating gene expression associated with asthma, inflammation and allergic response in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to short interfering nucleic acid molecules (siRNA) capable of mediating RNA interference (RNAi) against adenosine A1 receptor gene expression.
- The following is a discussion of relevant art pertaining to RNAi. The discussion is provided only for understanding of the invention that follows. The summary is not an admission that any of the work described below is prior art to the claimed invention.
- RNA interference refers to the process of sequence-specific post transcriptional gene silencing in animals mediated by short interfering RNAs (siRNA) (Fire et al., 1998, Nature, 391, 806). The corresponding process in plants is commonly referred to as post transcriptional gene silencing or RNA silencing and is also referred to as quelling in fungi. The process of post transcriptional gene silencing is thought to be an evolutionarily conserved cellular defense mechanism used to prevent the expression of foreign genes which is commonly shared by diverse flora and phyla (Fire et al., 1999, Trends Genet., 15, 358). Such protection from foreign gene expression may have evolved in response to the production of double stranded RNAs (dsRNA) derived from viral infection or the random integration of transposon elements into a host genome via a cellular response that specifically destroys homologous single stranded RNA or viral genomic RNA. The presence of dsRNA in cells triggers the RNAi response though a mechanism that has yet to be fully characterized. This mechanism appears to be different from the interferon response that results from dsRNA mediated activation of protein kinase PKR and 2′, 5′-oligoadenylate synthetase resulting in non-specific cleavage of mRNA by ribonuclease L.
- The presence of long dsRNAs in cells stimulates the activity of a ribonuclease III enzyme referred to as Dicer. Dicer is involved in the processing of the dsRNA into short pieces of dsRNA known as short interfering RNAs (siRNA) (Berstein et al., 2001, Nature, 409, 363). Short interfering RNAs derived from Dicer activity are typically about 21-23 nucleotides in length and comprise about 19 base pair duplexes. Dicer has also been implicated in the excision of 21 and 22 nucleotide small temporal RNAs (stRNA) from precursor RNA of conserved structure that are implicated in translational control (Hutvagner et al., 2001, Science, 293, 834). The RNAi response also features an endonuclease complex containing a siRNA, commonly referred to as an RNA-induced silencing complex (RISC), which mediates cleavage of single stranded RNA having sequence complementary to the antisense strand of the siRNA duplex. Cleavage of the target RNA takes place in the middle of the region complementary to the antisense strand of the siRNA duplex (Elbashir et al., 2001, Genes Dev., 15, 188).
- RNAi has been studied in a variety of systems. Fire et al., 1998, Nature, 391, 806, were the first to observe RNAi in C. elegans. Wianny and Goetz, 1999, Nature Cell Biol., 2, 70, describe RNAi mediated by dsRNA in mouse embryos. Hammond et al., 2000, Nature, 404, 293, describe RNAi in Drosophila cells transfected with dsRNA. Elbashir et al., 2001, Nature, 411, 494, describe RNAi induced by introduction of duplexes of synthetic 21-nucleotide RNAs in cultured mammalian cells including human embryonic kidney and HeLa cells. Recent work in Drosophila embryonic lysates (Elbashir et al., 2001, EMBO J., 20, 6877) has revealed certain requirements for siRNA length, structure, chemical composition, and sequence that are essential to mediate efficient RNAi activity. These studies have shown that 21 nucleotide siRNA duplexes are most active when containing two
nucleotide 3′-overhangs. Furthermore, complete substitution of one or both siRNA strands with 2′-deoxy (2′-H) or 2′-O-methyl nucleotides abolishes RNAi activity, whereas substitution of the 3′-terminal siRNA overhang nucleotides with deoxy nucleotides (2′-H) was shown to be tolerated. Single mismatch sequences in the center of the siRNA duplex were also shown to abolish RNAi activity. In addition, these studies also indicate that the position of the cleavage site in the target RNA is defined by the 5′-end of the siRNA guide sequence rather than the 3′-end (Elbashir et al., 2001, EMBO J., 20, 6877). Other studies have indicated that a 5′-phosphate on the target-complementary strand of a siRNA duplex is required for siRNA activity and that ATP is utilized to maintain the 5′-phosphate moiety on the siRNA (Nykanen et al., 2001, Cell, 107, 309). - Studies have shown that replacing the 3′-overhanging segments of a 21-mer siRNA duplex having 2
nucleotide 3′ overhangs with deoxyribonucleotides does not have an adverse effect on RNAi activity. Replacing up to 4 nucleotides on each end of the siRNA with deoxyribonucleotides has been reported to be well tolerated whereas complete substitution with deoxyribonucleotides results in no RNAi activity (Elbashir et al., 2001, EMBO J., 20, 6877). In addition, Elbashir et al., supra, also report that substitution of siRNA with 2′-O-methyl nucleotides completely abolishes RNAi activity. Li et al., International PCT Publication No. WO 00/44914, and Beach et al., International PCT Publication No. WO 01/68836 both suggest that siRNA “may include modifications to either the phosphate-sugar back bone or the nucleoside to include at least one of a nitrogen or sulfur heteroatom”, however neither application teaches to what extent these modifications are tolerated in siRNA molecules nor provide any examples of such modified siRNA. Kreutzer and Limmer, Canadian Patent Application No. 2,359,180, also describe certain chemical modifications for use in dsRNA constructs in order to counteract activation of double stranded-RNA-dependent protein kinase PKR, specifically 2′-amino or 2′-O-methyl nucleotides, and nucleotides containing a 2′-O or 4′-C methylene bridge. However, Kreutzer and Limmer similarly fail to show to what extent these modifications are tolerated in siRNA molecules nor do they provide any examples of such modified siRNA. - Parrish et al., 2000, Molecular Cell, 6, 1977-1087, tested certain chemical modifications targeting the unc-22 gene in C. elegans using long (>25 nt) siRNA transcripts. The authors describe the introduction of thiophosphate residues into these siRNA transcripts by incorporating thiophosphate nucleotide analogs with T7 and T3 RNA polymerase and observed that “RNAs with two [phosphorothioate] modified bases also had substantial decreases in effectiveness as RNAi triggers (data not shown); [phosphorothioate] modification of more than two residues greatly destabilized the RNAs in vitro and we were not able to assay interference activities.” Id. at 1081. The authors also tested certain modifications at the 2′-position of the nucleotide sugar in the long siRNA transcripts and observed that substituting deoxynucleotides for ribonucleotides “produced a substantial decrease in interference activity”, especially in the case of Uridine to Thymidine and/or Cytidine to deoxy-Cytidine substitutions. Id. In addition, the authors tested certain base modifications, including substituting 4-thiouracil, 5-bromouracil, 5-iodouracil, 3-(aminoallyl)uracil for uracil, and inosine for guanosine in sense and antisense strands of the siRNA, and found that whereas 4-thiouracil and 5-bromouracil were all well tolerated, inosine “produced a substantial decrease in interference activity” when incorporated in either strand. Incorporation of 5-iodouracil and 3-(aminoallyl)uracil in the antisense strand resulted in substantial decrease in RNAi activity as well.
- Beach et al., International PCT Publication No. WO 01/68836, describes specific methods for attenuating gene expression using endogenously derived dsRNA. Tuschl et al., International PCT Publication No. WO 01/75164, describes a Drosophila in vitro RNAi system and the use of specific siRNA molecules for certain functional genomic and certain therapeutic applications; although Tuschl, 2001, Chem. Biochem., 2, 239-245, doubts that RNAi can be used to cure genetic diseases or viral infection due “to the danger of activating interferon response”. Li et al., International PCT Publication No. WO 00/44914, describes the use of specific dsRNAs for use in attenuating the expression of certain target genes. Zernicka-Goetz et al., International PCT Publication No. WO 01/36646, describes certain methods for inhibiting the expression of particular genes in mammalian cells using certain dsRNA molecules. Fire et al., International PCT Publication No. WO 99/32619, describes particular methods for introducing certain dsRNA molecules into cells for use in inhibiting gene expression. Plaetinck et al., International PCT Publication No. WO 00/01846, describes certain methods for identifying specific genes responsible for conferring a particular phenotype in a cell using specific dsRNA molecules. Mello et al., International PCT Publication No. WO 01/29058, describes the identification of specific genes involved in dsRNA mediated RNAi. Deschamps Depaillette et al., International PCT Publication No. WO 99/07409, describes specific compositions consisting of particular dsRNA molecules combined with certain anti-viral agents. Waterhouse et al., International PCT Publication No. 99/53050, describes certain methods for decreasing the phenotypic expression of a nucleic acid in plant cells. Driscoll et al., International PCT Publication No. WO 01/49844, describes specific DNA constructs for use in facilitating gene silencing in targeted organisms. Parrish et al., 2000, Molecular Cell, 6, 1977-1087, describes specific chemically modified siRNA constructs targeting the unc-22 gene of C. elegans. Grossniklaus, International PCT Publication No. WO 01/38551, describes certain methods for regulating polycomb gene expression in plants. Churikov et al., International PCT Publication No. WO 01/42443, describes certain methods for modifying genetic characteristics of an organism. Cogoni et al., International PCT Publication No. WO 01/53475, describes certain methods for isolating a Neurospora silending gene and uses thereof. Reed et al., International PCT Publication No. WO 01/68836, describes certain methods for gene silencing in plants. Honer et al, International PCT Publication No. WO 01/70944, describes certain methods of drug screening using transgenic nematodes as Parkinson's disease models. Deak et al., International PCT Publication No. WO 01/72774, describes certain Drosophila derived gene products. Arndt et al., International PCT Publication No. WO 01/92513 describes certain methods for mediating gene suppression by using factors that enhance RNAi. Tuschl et al., International PCT Publication No. WO 02/44321, describe certain synthetic siRNA constructs. Pachuk et al., International PCT Publication No. WO 00/63364, and Satishchandran et al., International PCT Publication No. WO 01/04313 describes certain methods and compositions for inhibiting the function of certain polynucleotide sequences. Echeverri et al., International PCT Publication No. WO 02/38805, describes certain C. elegans genes identified via RNAi. Kreutzer et al., International PCT Publication No. WO 02/055692 and WO 02/055693, describes certain methods for inhibiting gene expression using RNAi.
- Asthma is a chronic inflammatory disorder of the lungs characterized by airflow obstruction, bronchial hyper-responsiveness, and airway inflammation. T-lymphocytes that produce TH2 cytokines and eosinophilic leukocytes infiltrate the airways. In the airway and in bronchial alveolar lavage (BAL) fluid of individuals with asthma, high concentrations of TH2 cytokines, interleukin-4 (IL-4), IL-5, and IL-13, are present along with increased levels of adenosine. In contrast to normal individuals, asthmatics respond to adenosine challenge with marked airway obstruction. Upon allergen challenge, mast cells are activated by cross-linked IgE-allergen complexes. Large amounts of prostaglandin D2 (PGD2), the major cyclooxygenase product of arachidonic acid are released. PGD2 is generated from PGH2 via the activity of prostaglandin D2 synthetase (PTGDS). PGD2 receptors and adenosine A1 receptors are present in the lungs and airway along with various other tissues in response to allergic stimuli (Howarth, 1997, Allergy, 52, 12).
- The significance of PGD2 as a mediator of allergic asthma has been established with the development of mice deficient in the PGD2 receptor (DP). DP is a heterotrimeric GTP-binding protein-coupled, rhodopsin-type receptor specific for PGD2 (Hirata et al., 1994, PNAS USA., 91, 11192). These mice fail to develop airway hyperreactivity and have greatly reduced eosinophil infiltration and cytokine accumulation in response to allergens. Upon allergen challenge mice deficient in the prostaglandin D2 (PGD2) receptor (DP) did not develop airway hyperactivity. Cytokine, lymphocyte and eosinophil accumulation in the lungs were greatly reduced (Matsuoka et al., 2000, Science, 287, 2013). The DP -/- mice exhibited no behavioral, anatomic, or histological abnormalities. Primary immune response is not affected by DP disruption.
- Asthma affects more than 100 million people worldwide and more than 17 million Americans (5% of the population). Since 1980 the incidence has more than doubled and deaths have tripled (5,000 deaths in 1995). Annual asthma-related healthcare costs in the US alone were estimated to exceed $14.5 billion in 2000. Current therapies such as inhalant anti-inflammatories and bronchodilators can be used to treat symptoms, however, these therapies do not prevent or cure asthma.
- One embodiment of the invention provides a short interfering RNA (siRNA) molecule that down regulates expression of adenosine A1 receptor (ADORA1) by RNA interference. The siRNA molecule can be adapted for use to treat, for example allergic/inflammatory diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and any other indications that can respond to the level of ADORA1. The siRNA molecule can comprise a sense region and an antisense region. The antisense region can comprise sequence complementary to an RNA sequence encoding ADORA1 and the sense region can comprise sequence complementary to the antisense region. An siRNA molecule of the invention can be adapted for use to treat asthma.
- An siRNA molecule can comprise a sense region and an antisense region and wherein said antisense region comprises sequence complementary to an RNA sequence encoding ADORA1 and the sense region comprises sequence complementary to the antisense region.
- The siRNA molecule can be assembled from two nucleic acid fragments wherein one fragment comprises the sense region and the second fragment comprises the antisense region of said siRNA molecule. The sense region and antisense region can be covalently connected via a linker molecule. The linker molecule can be a polynucleotide linker or a non-nucleotide linker.
- The antisense region of ADORA1 siRNA constructs can comprise a sequence complementary to sequence having any of SEQ ID NOs. 1-161. The antisense region can also comprise sequence having any of SEQ ID NOs. 162-322, 336, 338, 340, 342, 344, or 346. The sequences shown in SEQ ID NO: 1-346 are not limiting. A siRNA molecule of the invention can comprise any contiguous ADORA1 sequences (e.g., about 19 contiguous ADORA1 nucleotides. The sense region of ADORA1 siRNA constructs can comprise sequence having any of SEQ ID NOs. 1-161, 335, 337, 339, 341, 343, or 345. The sense region can comprise a sequence of SEQ ID NO. 323 and the antisense region can comprise a sequence of SEQ ID NO. 324. The sense region can comprise a sequence of SEQ ID NO. 325 and the antisense region can comprise a sequence of SEQ ID NO. 326. The sense region can comprise a sequence of SEQ ID NO. 327 and the antisense region can comprise a sequence of SEQ ID NO. 328. The sense region can comprise a sequence of SEQ ID NO. 329 and the antisense region can comprise a sequence of SEQ ID NO. 330. The sense region can comprise a sequence of SEQ ID NO. 331 and the antisense region can comprise a sequence of SEQ ID NO. 332. The sense region can comprise a sequence of SEQ ID NO. 333 and the antisense region can comprise a sequence of SEQ ID NO. 334.
- The sense region of a siRNA molecule of the invention can comprise a 3′-terminal overhang and the antisense region can comprise a 3′-terminal overhang. The 3′-terminal overhangs each can comprise about 2 nucleotides. The antisense region of the 3′-terminal nucleotide overhang can be complementary to RNA encoding ADORA1.
- The sense region of a siRNA molecule can comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-O-methyl modified pyrimidine nucleotides. The sense region can comprise a terminal cap moiety at the 5′-end, 3′-end, or both 5′ and 3′ ends of said sense region.
- The antisense region of a siRNA molecule can comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy-2′-fluoro modified pyrimidine nucleotides. The antisense region can also comprise a phosphorothioate internucleotide linkage at the 3′ end of said antisense region. The antisense region can comprise between about one and about five phosphorothioate internucleotide linkages at the 5′ end of said antisense region.
- The 3′-terminal nucleotide overhangs of a siRNA molecule can comprise ribonucleotides or deoxyribonucleotides that are chemically modified at a nucleic acid sugar, base, or backbone. The 3′-terminal nucleotide overhangs can also comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base ribonucleotides. Additionally, the 3′-terminal nucleotide overhangs can comprise one or more (e.g., about 1, 2, 3, 4, 5, or more) acyclic nucleotides.
-
- wherein each R1 and R2 is independently any nucleotide, non-nucleotide, or polynucleotide which can be naturally occurring or chemically modified, each X and Y is independently O, S, N, alkyl, or substituted alkyl, each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, or aralkyl, and wherein W, X, Y and Z are not all O.
-
- wherein each R3, R4, R5, R6, R7, R8, R10, R11 and R12 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalklylamino, substituted silyl, or group having Formula I; R9 is O, S, CH2, S═O, CHF, or CF2, and B is a nucleosidic base or any other non-naturally occurring base that can be complementary or non-complementary to ADORA1 RNA or a non-nucleosidic base or any other non-naturally occurring universal base that can be complementary or non-complementary to ADORA1 RNA.
- Another embodiment of the invention provides an expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of the invention in a manner that allows expression of the nucleic acid molecule. The expression vector can be in a mammalian cell, such as a human cell. The siRNA molecule can comprise a sense region and an antisense region. The antisense region can comprise sequence complementary to an RNA sequence encoding ADORA1 and the sense region comprises sequence complementary to the antisense region. The siRNA molecule can comprise two distinct strands having complementarity sense and antisense regions or can comprise a single strand having complementary sense and antisense regions.
- Therefore, this invention relates to compounds, compositions, and methods useful for modulating gene expression, for example, genes associated with asthma, inflammation and allergic response by RNA interference (RNAi) using short interfering RNA (siRNA). In particular, the instant invention features siRNA molecules and methods to modulate the expression of ADORA1. The siRNA of the invention can be unmodified or chemically modified. The siRNA of the instant invention can be chemically synthesized, expressed from a vector or enzymatically synthesized. The instant invention also features various chemically modified synthetic short interfering RNA (siRNA) molecules capable of modulating ADORA1 gene expression/activity in cells by RNA inference (RNAi). The use of chemically modified siRNA is expected to improve various properties of native siRNA molecules through increased resistance to nuclease degradation in vivo and/or improved cellular uptake. The siRNA molecules of the instant invention provide useful reagents and methods for a variety of therapeutic, diagnostic, agricultural, target validation, genomic discovery, genetic engineering and pharmacogenomic applications.
- In one embodiment, the invention features one or more siRNA molecules and methods that independently or in combination modulate the expression of gene(s) encoding proteins associated with asthma, inflammation, and the allergic response. Specifically, the present invention features siRNA molecules that modulate the expression of ADORA1 genes such as GenBank accession No. NM —000674.
- The description below of the various aspects and embodiments is provided with reference to the exemplary gene ADORA1. However, the various aspects and embodiments are also directed to other genes which express other adenosine receptors (A2A, A2B, and/or A3). Those additional genes can be analyzed for target sites using the methods described for ADORA1. Thus, the inhibition and the effects of such inhibition of the other genes can be performed as described herein. Thus, the inhibition and the effects of such inhibition of the other genes can be performed as described herein.
- In one embodiment, the invention features a siRNA molecule that down regulates expression of an ADORA1 gene, for example, wherein the ADORA1 gene comprises ADORA1 sequence.
- In one embodiment, the invention features a siRNA molecule having RNAi activity against ADORA1 RNA, wherein the siRNA molecule comprises a sequence complimentary to any RNA having ADORA1 encoding sequence, such as GenBank accession No. NM —000674.
- In another embodiment, the invention features a siRNA molecule comprising sequences selected from the group consisting of SEQ ID NOs: 1-322. In another embodiment, the invention features an ADORA1 siRNA molecule having an antisense region complementary to any sequence having SEQ ID NOs: 1-161. In another embodiment, the invention features an ADORA1 siRNA molecule having an antisense region having any of SEQ ID NOs: 162-322, 336, 338, 340, 342, 344, 346, 348, 350, 352 or 354. In another embodiment, the invention features an ADORA1 siRNA molecule having a sense region having any of SEQ ID NOs. 1-161, 335, 337, 339, 341, 343, or 345, 347, 349, 351 or 353. The sense region can comprise a sequence of SEQ ID NO. 323 and the antisense region can comprise a sequence of SEQ ID NO. 324. The sense region can comprise a sequence of SEQ ID NO. 325 and the antisense region can comprise a sequence of SEQ ID NO. 326. The sense region can comprise a sequence of SEQ ID NO. 327 and the antisense region can comprise a sequence of SEQ ID NO. 328. The sense region can comprise a sequence of SEQ ID NO. 329 and the antisense region can comprise a sequence of SEQ ID NO. 330. The sense region can comprise a sequence of SEQ ID NO. 331 and the antisense region can comprise a sequence of SEQ ID NO. 332. The sense region can comprise a sequence of SEQ ID NO. 333 and the antisense region can comprise a sequence of SEQ ID NO. 334. In yet another embodiment, the invention features a siRNA molecule comprising a sequence, for example the antisense sequence of the siRNA construct, complementary to a sequence or portion of sequence comprising GenBank accession No. NM —000674.
- In one embodiment, a siRNA molecule of the invention has RNAi activity that modulates expression of RNA encoded by an ADORA1 gene.
- In one embodiment, nucleic acid molecules of the invention that act as mediators of the RNA interference gene silencing response are double stranded RNA molecules. In another embodiment, the siRNA molecules of the invention consist of duplexes containing about 19 base pairs between oligonucleotides comprising about 19 to about 25 nucleotides (e.g., about 19, 20, 21, 22, 23, 24, or 25). In yet another embodiment, siRNA molecules of the invention comprise duplexes with overhanging ends of 1-3 (e.g., 1, 2, or 3) nucleotides, for example 21 nucleotide duplexes with 19 base pairs and 2
nucleotide 3′-overhangs. These nucleotide overhangs in the antisense strand are optionally complementary to the target sequence. - In one embodiment, the invention features chemically modified siRNA constructs having specificity for ADORA1 expressing nucleic acid molecules. Non-limiting examples of such chemical modifications include without limitation phosphorothioate internucleotide linkages, 2′-O-methyl ribonucleotides, 2′-deoxy-2′-fluoro ribonucleotides, “universal base” nucleotides, 5-C-methyl nucleotides, and inverted deoxyabasic residue incorporation. These chemical modifications, when used in various siRNA constructs, are shown to preserve RNAi activity in cells while at the same time, dramatically increasing the serum stability of these compounds. Furthermore, contrary to the data published by Parrish et al., supra, applicant demonstrates that multiple (greater than one) phosphorothioate substitutions are well tolerated and confer substantial increases in serum stability for modified siRNA constructs. Chemical modifications of the siRNA constructs can also be used to improve the stability of the interaction with the target RNA sequence and to improve nuclease resistance.
- In a non-limiting example, the introduction of chemically modified nucleotides into nucleic acid molecules will provide a powerful tool in overcoming potential limitations of in vivo stability and bioavailability inherent to native RNA molecules that are delivered exogenously. For example, the use of chemically modified nucleic acid molecules can enable a lower dose of a particular nucleic acid molecule for a given therapeutic effect since chemically modified nucleic acid molecules tend to have a longer half-life in serum. Furthermore, certain chemical modifications can improve the bioavailability of nucleic acid molecules by targeting particular cells or tissues and/or improving cellular uptake of the nucleic acid molecule. Therefore, even if the activity of a chemically modified nucleic acid molecule is reduced as compared to a native nucleic acid molecule, for example when compared to an all RNA nucleic acid molecule, the overall activity of the modified nucleic acid molecule can be greater than the native molecule due to improved stability and/or delivery of the molecule. Unlike native unmodified siRNA, chemically modified siRNA can also minimize the possibility of activating interferon activity in humans.
- In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more nucleotides comprising a backbone modified internucleotide linkage having Formula I:
- wherein each R1 and R2 is independently any nucleotide, non-nucleotide, or polynucleotide which can be naturally occurring or chemically modified, each X and Y is independently O, S, N, alkyl, or substituted alkyl, each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, or aralkyl, and wherein W, X, Y and Z are not all O.
- The chemically modified internucleotide linkages having Formula I, for example wherein any Z, W, X, and/or Y independently comprises a sulphur atom, can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands. The siRNA molecules of the invention can comprise one or more chemically modified internucleotide linkages having Formula I at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands. For example, an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified internucleotide linkages having Formula I at the 5′-end of the sense strand, antisense strand, or both strands. In another non-limiting example, an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) pyrimidine nucleotides with chemically modified internucleotide linkages having Formula I in the sense strand, antisense strand, or both strands. In yet another non-limiting example, an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) purine nucleotides with chemically modified internucleotide linkages having Formula I in the sense strand, antisense strand, or both strands. In another embodiment, a siRNA molecule of the invention having internucleotide linkage(s) of Formula I also comprises a chemically modified nucleotide or non-nucleotide having any of Formulae II, III, V, or VI.
- In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more nucleotides or non-nucleotides having Formula II:
- wherein each R3, R4, R5, R6, R7, R8, R10, R 11 and R12 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalklylamino, substituted silyl, or group having Formula I; R9 is O, S, CH2, S═O, CHF, or CF2, and B is a nucleosidic base such as adenine, guanine, uracil, cytosine, thymine, 2-aminoadenosine, 5-methylcytosine, 2,6-diaminopurine, or any other non-naturally occurring base that can be complementary or non-complementary to ADORA1 RNA or a non-nucleosidic base such as phenyl, naphthyl, 3-nitropyrrole, 5-nitroindole, nebularine, pyridone, pyridinone, or any other non-naturally occurring universal base that can be complementary or non-complementary to ADORA1 RNA.
- The chemically modified nucleotide or non-nucleotide of Formula II can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands. The siRNA molecules of the invention can comprise one or more chemically modified nucleotide or non-nucleotide of Formula II at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands. For example, an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula II at the 5′-end of the sense strand, antisense strand, or both strands. In anther non-limiting example, an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula II at the 3′-end of the sense strand, antisense strand, or both strands.
- In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more nucleotides or non-nucleotides having Formula III:
- wherein each R3, R4, R5, R6, R7, R8, R10, R11 and R12 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalklylamino, substituted silyl, or group having Formula I; R9 is O, S, CH2, S═O, CHF, or CF2, and B is a nucleosidic base such as adenine, guanine, uracil, cytosine, thymine, 2-aminoadenosine, 5-methylcytosine, 2,6-diaminopurine, or any other non-naturally occurring base that can be employed to be complementary or non-complementary to ADORA1 RNA or a non-nucleosidic base such as phenyl, naphthyl, 3-nitropyrrole, 5-nitroindole, nebularine, pyridone, pyridinone, or any other non-naturally occurring universal base that can be complementary or non-complementary to ADORA1 RNA.
- The chemically modified nucleotide or non-nucleotide of Formula III can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands. The siRNA molecules of the invention can comprise one or more chemically modified nucleotide or non-nucleotide of Formula III at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands. For example, an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula III at the 5′-end of the sense strand, antisense strand, or both strands. In anther non-limiting example, an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) chemically modified nucleotide or non-nucleotide of Formula III at the 3′-end of the sense strand, antisense strand, or both strands.
- In another embodiment, a siRNA molecule of the invention comprises a nucleotide having Formula II or III, wherein the nucleotide having Formula II or III is in an inverted configuration. For example, the nucleotide having Formula II or III is connected to the siRNA construct in a 3′,3′, 3′-2′, 2′-3′, or 5′,5′configuration, such as at the 3′-end, 5′-end, or both 3′ and 5′ ends of one or both siRNA strands.
- In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises a 5′-terminal phosphate group having Formula IV:
- wherein each X and Y is independently O, S, N, alkyl, substituted alkyl, or alkylhalo; each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, aralkyl, or alkylhalo; and wherein W, X, Y and Z are not all O.
- In one embodiment, the invention features a siRNA molecule having a 5′-terminal phosphate group having Formula IV on the target-complementary strand, for example a strand complementary to ADORA1 RNA, wherein the siRNA molecule comprises an all RNA siRNA molecule. In another embodiment, the invention features a siRNA molecule having a 5′-terminal phosphate group having Formula IV on the target-complementary strand wherein the siRNA molecule also comprises 1-3 (e.g., 1, 2, or 3)
nucleotide 3′-overhangs having between about 1 and about 4 (e.g., about 1, 2, 3, or 4) deoxyribonucleotides on the 3′-end of one or both strands. In another embodiment, a 5′-terminal phosphate group having Formula IV is present on the target-complementary strand of a siRNA molecule of the invention, for example a siRNA molecule having chemical modifications having Formula I, Formula II and/or Formula III. - In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises one or more phosphorothioate internucleotide linkages. For example, in a non-limiting example, the invention features a chemically modified short interfering RNA (siRNA) having about 1, 2, 3, 4, 5, 6, 7, 8 or more phosphorothioate internucleotide linkages in one siRNA strand. In yet another embodiment, the invention features a chemically modified short interfering RNA (siRNA) individually having about 1, 2, 3, 4, 5, 6, 7, 8 or more phosphorothioate internucleotide linkages in both siRNA strands. The phosphorothioate internucleotide linkages can be present in one or both oligonucleotide strands of the siRNA duplex, for example in the sense strand, antisense strand, or both strands. The siRNA molecules of the invention can comprise one or more phosphorothioate internucleotide linkages at the 3′-end, 5′-end, or both 3′ and 5′-ends of the sense strand, antisense strand, or both strands. For example, an exemplary siRNA molecule of the invention can comprise between about 1 and about 5 or more (e.g., about 1, 2, 3, 4, 5, or more) consecutive phosphorothioate internucleotide linkages at the 5′-end of the sense strand, antisense strand, or both strands. In another non-limiting example, an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) pyrimidine phosphorothioate internucleotide linkages in the sense strand, antisense strand, or both strands. In yet another non-limiting example, an exemplary siRNA molecule of the invention can comprise one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) purine phosphorothioate internucleotide linkages in the sense strand, antisense strand, or both strands.
- In one embodiment, the invention features a siRNA molecule, wherein the sense strand comprises one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8 , 9 , 10 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between 1 and 10 or more, specifically about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the antisense strand. In another embodiment, one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- In another embodiment, the invention features a siRNA molecule, wherein the sense strand comprises between about 1 and about 5, specifically about 1, 2, 3, 4, or 5 phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between about 1 and about 5 or more, specifically about 1, 2, 3, 4, 5, or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the antisense strand. In another embodiment, one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without between about 1 and about 5 or more, for example about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- In one embodiment, the invention features a siRNA molecule, wherein the antisense strand comprises one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8 , 9 , 10 or more phosphorothioate internucleotide linkages, and/or between one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between about 1 and about 10, specifically about 1, 2, 3, 4, 5, 6, 7, 8 , 9, 10 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the antisense strand. In another embodiment, one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- In another embodiment, the invention features a siRNA molecule, wherein the antisense strand comprises between about 1 and about 5 or more, specifically about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5 or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the sense strand; and wherein the antisense strand comprises any of between about 1 and about 5 or more, specifically about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) 2′-deoxy, 2′-O-methyl, 2′-deoxy-2′-fluoro, and/or one or more (e.g., about 1, 2, 3, 4, 5, or more) universal base modified nucleotides, and optionally a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends of the antisense strand. In another embodiment, one or more, for example about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more pyrimidine nucleotides of the sense and/or antisense siRNA stand are chemically modified with 2′-deoxy, 2′-O-methyl and/or 2′-deoxy-2′-fluoro nucleotides, with or without between about 1 and about 5, for example about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages and/or a terminal cap molecule at the 3′, 5′, or both 3′ and 5′-ends, being present in the same or different strand.
- In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule having between about 1 and about 5, specifically about 1, 2, 3, 4, 5 or more phosphorothioate internucleotide linkages in each strand of the siRNA molecule.
- In another embodiment, the invention features a siRNA molecule comprising 2′-5′ internucleotide linkages. The 2′-5′ internucleotide linkage(s) can be at the 5′-end, 3′-end, or both 5′ and 3′ ends of one or both siRNA sequence strands. In addition, the 2′-5′ internucleotide linkage(s) can be present at various other positions within one or both siRNA sequence strands, for example, about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more including every internucleotide linkage of a pyrimidine nucleotide in one or both strands of the siRNA molecule can comprise a 2′-5′ internucleotide linkage, or about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more including every internucleotide linkage of a purine nucleotide in one or both strands of the siRNA molecule can comprise a 2′-5′ internucleotide linkage.
- In another embodiment, a chemically modified siRNA molecule of the invention comprises a duplex having two strands, one or both of which can be chemically modified, wherein each strand is between about 18 and about 27 (e.g., about 18, 19, 20, 21, 22, 23, 24, 25, 26, or 27) nucleotides in length, wherein the duplex has between about 18 and about 23 (e.g., about 18, 19, 20, 21, 22, or 23) base pairs, and wherein the chemical modification comprises a structure having Formula I, Formula II, Formula III and/or Formula IV. For example, an exemplary chemically modified siRNA molecule of the invention comprises a duplex having two strands, one or both of which can be chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein each strand consists of 21 nucleotides, each having 2
nucleotide 3′-overhangs, and wherein the duplex has 19 base pairs. - In another embodiment, a siRNA molecule of the invention comprises a single stranded hairpin structure, wherein the siRNA is between about 36 and about 70 (e.g., about 36, 40, 45, 50, 55, 60, 65, or 70) nucleotides in length having between about 18 and about 23 (e.g., about 18, 19, 20, 21, 22, or 23) base pairs, and wherein the siRNA can include a chemical modification comprising a structure having Formula I, Formula II, Formula III and/or Formula IV. For example, an exemplary chemically modified siRNA molecule of the invention comprises a linear oligonucleotide having between about 42 and about 50 (e.g., about 42, 43, 44, 45, 46, 47, 48, 49, or 50) nucleotides that is chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein the linear oligonucleotide forms a hairpin structure having 19 base pairs and a 2
nucleotide 3′-overhang. - In another embodiment, a linear hairpin siRNA molecule of the invention contains a stem loop motif, wherein the loop portion of the siRNA molecule is biodegradable. For example, a linear hairpin siRNA molecule of the invention is designed such that degradation of the loop portion of the siRNA molecule in vivo can generate a double stranded siRNA molecule with 3′-overhangs, such as 3′-overhangs comprising about 2 nucleotides.
- In another embodiment, a siRNA molecule of the invention comprises a circular nucleic acid molecule, wherein the siRNA is between about 38 and about 70 (e.g., about 38, 40, 45, 50, 55, 60, 65, or 70) nucleotides in length having between about 18 and about 23 (e.g., about 18, 19, 20, 21, 22, or 23) base pairs, and wherein the siRNA can include a chemical modification, which comprises a structure having Formula I, Formula II, Formula III and/or Formula IV. For example, an exemplary chemically modified siRNA molecule of the invention comprises a circular oligonucleotide having between about 42 and about 50 (e.g., about 42, 43, 44, 45, 46, 47, 48, 49, or 50) nucleotides that is chemically modified with a chemical modification having Formula I, Formula II, Formula III, and/or Formula IV, wherein the circular oligonucleotide forms a dumbbell shaped structure having 19 base pairs and 2 loops.
- In another embodiment, a circular siRNA molecule of the invention contains two loop motifs, wherein one or both loop portions of the siRNA molecule is biodegradable. For example, a circular siRNA molecule of the invention is designed such that degradation of the loop portions of the siRNA molecule in vivo can generate a double stranded siRNA molecule with 3′-overhangs, such as 3′-overhangs comprising about 2 nucleotides.
-
- wherein each R3, R4, R5, R6, R7, R8, R10, R11, R12, and R13 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalklylamino, substituted silyl, or group having Formula I; R9 is O, S, CH2, S═O, CHF, or CF2.
-
- wherein each R3, R4, R5, R6, R7, R8, R10, R11, R12, and R13 is independently H, OH, alkyl, substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalklylamino, substituted silyl, or group having Formula I; R9 is O, S, CH2, S═O, CHF, or CF2, and either R2, R3, R8 or R13 serve as points of attachment to the siRNA molecule of the invention.
- In another embodiment, a siRNA molecule of the invention comprises an abasic residue having Formula II or III, wherein the abasic residue having Formula II or III is connected to the siRNA construct in a 3′,3′, 3′-2′, 2′-3′, or 5′,5′ configuration, such as at the 3′-end, 5′-end, or both 3′ and 5′ ends of one or both siRNA strands.
- In one embodiment, a siRNA molecule of the invention comprises one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) locked nucleic acid (LNA) nucleotides, for example at the 5′-end, 3′-end, 5′ and 3′-end, or any combination thereof, of the siRNA molecule.
- In another embodiment, a siRNA molecule of the invention comprises one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) acyclic nucleotides, for example at the 5′-end, 3′-end, 5′ and 3′-end, or any combination thereof, of the siRNA molecule.
- In one embodiment, the invention features a chemically modified short interfering RNA (siRNA) molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein the chemical modification comprises a conjugate covalently attached to the siRNA molecule. In another embodiment, the conjugate is covalently attached to the siRNA molecule via a biodegradable linker. In one embodiment, the conjugate molecule is attached at the 3′-end of either the sense strand, antisense strand, or both strands of the siRNA. In another embodiment, the conjugate molecule is attached at the 5′-end of either the sense strand, antisense strand, or both strands of the siRNA. In yet another embodiment, the conjugate molecule is attached both the 3′-end and 5′-end of either the sense strand, antisense strand, or both strands of the siRNA, or any combination thereof. In one embodiment, a conjugate molecule of the invention comprises a molecule that facilitates delivery of a siRNA molecule into a biological system such as a cell. In another embodiment, the conjugate molecule attached to the siRNA is a poly ethylene glycol, human serum albumin, or a ligand for a cellular receptor that can mediate cellular uptake. Examples of specific conjugate molecules contemplated by the instant invention that can be attached to siRNA molecules are described in Vargeese et al., U.S. Ser. No. 60/311,865, incorporated by reference herein.
- In one embodiment, the invention features a siRNA molecule capable of mediating RNA interference (RNAi) against ADORA1 inside a cell or reconstituted in vitro system, wherein one or both strands of the siRNA comprise ribonucleotides at positions withing the siRNA that are critical for siRNA mediated RNAi in a cell. All other positions within the siRNA can include chemically modified nucleotides and/or non-nucleotides such as nucleotides and or non-nucleotides having Formula I, II, III, IV, V, or VI, or any combination thereof to the extent that the ability of the siRNA molecule to support RNAi activity in a cell is maintained.
- In one embodiment, the invention features a method for modulating the expression of an ADORA1 gene within a cell, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene; and (b) introducing the siRNA molecule into a cell under conditions suitable to modulate the expression of the ADORA1 gene in the cell.
- In one embodiment, the invention features a method for modulating the expression of an ADORA1 gene within a cell, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene and wherein the sense strand sequence of the siRNA is identical to the complementary sequence of the ADORA1 RNA; and (b) introducing the siRNA molecule into a cell under conditions suitable to modulate the expression of the ADORA1 gene in the cell.
- In another embodiment, the invention features a method for modulating the expression of more than one ADORA1 gene within a cell, comprising: (a) synthesizing siRNA molecules of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 genes; and (b) introducing the siRNA molecules into a cell under conditions suitable to modulate the expression of the ADORA1 genes in the cell.
- In another embodiment, the invention features a method for modulating the expression of more than one ADORA1 gene within a cell, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene and wherein the sense strand sequence of the siRNA is identical to the complementary sequence of the ADORA1 RNA; and (b) introducing the siRNA molecules into a cell under conditions suitable to modulate the expression of the ADORA1 genes in the cell.
- In one embodiment, the invention features a method of modulating the expression of an ADORA1 gene in a tissue explant, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene; (b) introducing the siRNA molecule into a cell of the tissue explant derived from a particular organism under conditions suitable to modulate the expression of the ADORA1 gene in the tissue explant, and (c) optionally introducing the tissue explant back into the organism the tissue was derived from or into another organism under conditions suitable to modulate the expression of the ADORA1 gene in that organism.
- In one embodiment, the invention features a method of modulating the expression of an ADORA1 gene in a tissue explant, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene and wherein the sense strand sequence of the siRNA is identical to the complementary sequence of the ADORA1 RNA; (b) introducing the siRNA molecule into a cell of the tissue explant derived from a particular organism under conditions suitable to modulate the expression of the ADORA1 gene in the tissue explant, and (c) optionally introducing the tissue explant back into the organism the tissue was derived from or into another organism under conditions suitable to modulate the expression of the ADORA1 gene in that organism.
- In another embodiment, the invention features a method of modulating the expression of more than one ADORA1 gene in a tissue explant, comprising: (a) synthesizing siRNA molecules of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 genes; (b) introducing the siRNA molecules into a cell of the tissue explant derived from a particular organism under conditions suitable to modulate the expression of the ADORA1 genes in the tissue explant, and (c) optionally introducing the tissue explant back into the organism the tissue was derived from or into another organism under conditions suitable to modulate the expression of the ADORA1 genes in that organism.
- In one embodiment, the invention features a method of modulating the expression of an ADORA1 gene in an organism, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 gene; and (b) introducing the siRNA molecule into the organism under conditions suitable to modulate the expression of the ADORA1 gene in the organism.
- In another embodiment, the invention features a method of modulating the expression of more than one ADORA1 gene in an organism, comprising: (a) synthesizing siRNA molecules of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of the ADORA1 genes; and (b) introducing the siRNA molecules into the organism under conditions suitable to modulate the expression of the ADORA1 genes in the organism.
- The siRNA molecules of the invention can be designed to inhibit ADORA1 gene expression through RNAi targeting of a variety of RNA molecules. In one embodiment, the siRNA molecules of the invention are used to target various RNAs corresponding to a target gene. Non-limiting examples of such RNAs include messenger RNA (mRNA), alternate RNA splice variants of target gene(s), post-transcriptionally modified RNA of target gene(s), pre-mRNA of target gene(s), and/or RNA templates used for ADORA1 activity. If alternate splicing produces a family of transcipts that are distinguished by usage of appropriate exons, the instant invention can be used to inhibit gene expression through the appropriate exons to specifically inhibit or to distinguish among the functions of gene family members. For example, a protein that contains an alternatively spliced transmembrane domain can be expressed in both membrane bound and secreted forms. Use of the invention to target the exon containing the transmembrane domain can be used to determine the functional consequences of pharmaceutical targeting of membrane bound as opposed to the secreted form of the protein. Non-limiting examples of applications of the invention relating to targeting these RNA molecules include therapeutic pharmaceutical applications, pharmaceutical discovery applications, molecular diagnostic and gene function applications, and gene mapping, for example using single nucleotide polymorphism mapping with siRNA molecules of the invention. Such applications can be implemented using known gene sequences or from partial sequences available from an expressed sequence tag (EST).
- In another embodiment, the siRNA molecules of the invention are used to target conserved sequences corresponding to a gene family or gene families such as checkpoint kinase genes. As such, siRNA molecules targeting multiple checkpoint kinase targets can provide increased therapeutic effect. In addition, siRNA can be used to characterize pathways of gene function in a variety of applications. For example, the present invention can be used to inhibit the activity of target gene(s) in a pathway to determine the function of uncharacterized gene(s) in gene function analysis, mRNA function analysis, or translational analysis. The invention can be used to determine potential target gene pathways involved in various diseases and conditions toward pharmaceutical development. The invention can be used to understand pathways of gene expression involved in development, such as prenatal development, postnatal development and/or aging.
- In one embodiment, siRNA molecule(s) and/or methods of the invention are used to inhibit the expression of gene(s) that encode RNA referred to by Genbank Accession number, for example genes such as Genbank Accession No. NM —000674. Such sequences are readily obtained using this Genbank Accession number.
- In one embodiment, the invention features a method comprising: (a) generating a randomized library of siRNA constructs having a predetermined complexity, such as of 4 N, where N represents the number of base paired nucleotides in each of the siRNA construct strands (eg. for a siRNA construct having 21 nucleotide sense and antisense strands with 19 base pairs, the complexity would be 419); and (b) assaying the siRNA constructs of (a) above, under conditions suitable to determine RNAi target sites within the target ADORA1 RNA sequence. In another embodiment, the siRNA molecules of (a) have strands of a fixed length, for example about 23 nucleotides in length. In yet another embodiment, the siRNA molecules of (a) are of differing length, for example having strands of about 19 to about 25 (e.g., about 19, 20, 21, 22, 23, 24, or 25) nucleotides in length. In yet another embodiment, the assay can comprise a reconstituted in vitro siRNA assay as described in Example 6 herein. In another embodiment, the assay can comprise a cell culture system in which target RNA is expressed. In another embodiment, fragments of ADORA1 RNA are analyzed for detectable levels of cleavage, for example by gel electrophoresis, northern blot analysis, or RNAse protection assays, to determine the most suitable target site(s) within the target ADORA1 RNA sequence. In another embodiment, the target ADORA1 RNA sequence can be obtained as is known in the art, for example, by cloning and/or transcription for in vitro systems, and by cellular expression in in vivo systems.
- In another embodiment, the invention features a method comprising: (a) analyzing the sequence of a RNA target encoded by an ADORA1 gene; (b) synthesizing one or more sets of siRNA molecules having sequence complementary to one or more regions of the RNA of (a); and (c) assaying the siRNA molecules of (b) under conditions suitable to determine RNAi targets within the target RNA sequence. In another embodiment, the siRNA molecules of (b) have strands of a fixed length, for example about 23 nucleotides in length. In yet another embodiment, the siRNA molecules of (b) are of differing length, for example having strands of about 19 to about 25 (e.g., about 19, 20, 21, 22, 23, 24, or 25) nucleotides in length. In yet another embodiment, the assay can comprise a reconstituted in vitro siRNA assay as described in Example 6 herein. In another embodiment, the assay can comprise a cell culture system in which target RNA is expressed. Fragments of ADORA1 RNA are analyzed for detectable levels of cleavage, for example by gel electrophoresis, northern blot analysis, or RNAse protection assays, to determine the most suitable target site(s) within the target ADORA1 RNA sequence. The target ADORA1 RNA sequence can be obtained as is known in the art, for example, by cloning and/or transcription for in vitro systems, and by expression in in vivo systems.
- By “target site” is meant a sequence within a target RNA that is “targeted” for cleavage mediated by a siRNA construct which contains sequences within its antisense region that are complementary to the target sequence.
- By “detectable level of cleavage” is meant cleavage of target RNA (and formation of cleaved product RNAs) to an extent sufficient to discern cleavage products above the background of RNAs produced by random degradation of the target RNA. Production of cleavage products from 1-5% of the target RNA is sufficient to detect above the background for most methods of detection.
- In one embodiment, the invention features a composition comprising a siRNA molecule of the invention, which can be chemically modified, in a pharmaceutically acceptable carrier or diluent. In another embodiment, the invention features a pharmaceutical composition comprising siRNA molecules of the invention, which can be chemically modified, targeting one or more genes in a pharmaceutically acceptable carrier or diluent. In another embodiment, the invention features a method for treating or preventing a disease or condition in a subject, comprising administering to the subject a composition of the invention under conditions suitable for the treatment or prevention of the disease or condition in the subject, alone or in conjunction with one or more other therapeutic compounds.
- In another embodiment, the invention features a method for validating an ADORA1 gene target, comprising: (a) synthesizing a siRNA molecule of the invention, which can be chemically modified, wherein one of the siRNA strands includes a sequence complementary to RNA of an ADORA1 target gene; (b) introducing the siRNA molecule into a cell, tissue, or organism under conditions suitable for modulating expression of the ADORA1 target gene in the cell, tissue, or organism; and (c) determining the function of the gene by assaying for any phenotypic change in the cell, tissue, or organism.
- In one embodiment, the invention features a kit containing a siRNA molecule of the invention, which can be chemically modified, that can be used to modulate the expression of an ADORA1 target gene in a cell, tissue, or organism. In another embodiment, the invention features a kit containing more than one siRNA molecule of the invention, which can be chemically modified, that can be used to modulate the expression of more than one ADORA1 target gene in a cell, tissue, or organism.
- In one embodiment, the invention features a cell containing one or more siRNA molecules of the invention, which can be chemically modified. In another embodiment, the cell containing a siRNA molecule of the invention is a mammalian cell. In yet another embodiment, the cell containing a siRNA molecule of the invention is a human cell.
- In one embodiment, the synthesis of a siRNA molecule of the invention, which can be chemically modified, comprises: (a) synthesis of two complementary strands of the siRNA molecule; (b) annealing the two complementary strands together under conditions suitable to obtain a double stranded siRNA molecule. In another embodiment, synthesis of the two complementary strands of the siRNA molecule is by solid phase oligonucleotide synthesis. In yet another embodiment, synthesis of the two complementary strands of the siRNA molecule is by solid phase tandem oligonucleotide synthesis.
- In one embodiment, the invention features a method for synthesizing a siRNA duplex molecule comprising: (a) synthesizing a first oligonucleotide sequence strand of the siRNA molecule, wherein the first oligonucleotide sequence strand comprises a cleavable linker molecule that can be used as a scaffold for the synthesis of the second oligonucleotide sequence strand of the siRNA; (b) synthesizing the second oligonucleotide sequence strand of siRNA on the scaffold of the first oligonucleotide sequence strand, wherein the second oligonucleotide sequence strand further comprises a chemical moiety than can be used to purify the siRNA duplex; (c) cleaving the linker molecule of (a) under conditions suitable for the two siRNA oligonucleotide strands to hybridize and form a stable duplex; and (d) purifying the siRNA duplex utilizing the chemical moiety of the second oligonucleotide sequence strand. In another embodiment, cleavage of the linker molecule in (c) above takes place during deprotection of the oligonucleotide, for example under hydrolysis conditions using an alkylamine base such as methylamine. In another embodiment, the method of synthesis comprises solid phase synthesis on a solid support such as controlled pore glass (CPG) or polystyrene, wherein the first sequence of (a) is synthesized on a cleavable linker, such as a succinyl linker, using the solid support as a scaffold. The cleavable linker in (a) used as a scaffold for synthesizing the second strand can comprise similar reactivity as the solid support derivatized linker, such that cleavage of the solid support derivatized linker and the cleavable linker of (a) takes place concomitantly. In another embodiment, the chemical moiety of (b) that can used to isolate the attached oligonucleotide sequence comprises a trityl group, for example a dimethoxytrityl group, which can be employed in a trityl-on synthesis strategy as described herein. In yet another embodiment, the chemical moiety, such as a dimethoxytrityl group, is removed during purification, for example using acidic conditions.
- In a further embodiment, the method for siRNA synthesis is a solution phase synthesis or hybrid phase synthesis wherein both strands of the siRNA duplex are synthesized in tandem using a cleavable linker attached to the first sequence which acts a scaffold for synthesis of the second sequence. Cleavage of the linker under conditions suitable for hybridization of the separate siRNA sequence strands results in formation of the double stranded siRNA molecule.
- In another embodiment, the invention features a method for synthesizing a siRNA duplex molecule comprising: (a) synthesizing one oligonucleotide sequence strand of the siRNA molecule, wherein the sequence comprises a cleavable linker molecule that can be used as a scaffold for the synthesis of another oligonucleotide sequence; (b) synthesizing a second oligonucleotide sequence having complementarity to the first sequence strand on the scaffold of (a), wherein the second sequence comprises the other strand of the double stranded siRNA molecule and wherein the second sequence further comprises a chemical moiety than can be used to isolate the attached oligonucleotide sequence; (c) purifying the product of (b) utilizing the chemical moiety of the second oligonucleotide sequence strand under conditions suitable for isolating the full length sequence comprising both siRNA oligonucleotide strands connected by the cleavable linker; and (d) under conditions suitable for the two siRNA oligonucleotide strands to hybridize and form a stable duplex. In another embodiment, cleavage of the linker molecule in (c) above takes place during deprotection of the oligonucleotide, for example under hydrolysis conditions. In another embodiment, cleavage of the linker molecule in (c) above takes place after deprotection of the oligonucleotide. In another embodiment, the method of synthesis comprises solid phase synthesis on a solid support such as controlled pore glass (CPG) or polystyrene, wherein the first sequence of (a) is synthesized on a cleavable linker, such as a succinyl linker, using the solid support as a scaffold. The cleavable linker in (a) used as a scaffold for synthesizing the second strand can comprise similar reactivity or differing reactivity as the solid support derivatized linker, such that cleavage of the solid support derivatized linker and the cleavable linker of (a) takes place either concomitantly or sequentially. In another embodiment, the chemical moiety of (b) that can used to isolate the attached oligonucleotide sequence comprises a trityl group, for example a dimethoxytrityl group.
- In another embodiment, the invention features a method for making a double stranded siRNA molecule in a single synthetic process, comprising: (a) synthesizing an oligonucleotide having a first and a second sequence, wherein the first sequence is complementary to the second sequence, and the first oligonucleotide sequence is linked to the second sequence via a cleavable linker, and wherein a
terminal 5′-protecting group, for example a 5′-O-dimethoxytrityl group (5′-O-DMT) remains on the oligonucleotide having the second sequence; (b) deprotecting the oligonucleotide whereby the deprotection results in the cleavage of the linker joining the two oligonucleotide sequences; and (c) purifying the product of (b) under conditions suitable for isolating the double stranded siRNA molecule, for example using a trityl-on synthesis strategy as described herein. - In one embodiment, the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications, for example one or more chemical modifications having Formula I, II, III, IV, or V, that increases the nuclease resistance of the siRNA construct.
- In another embodiment, the invention features a method for generating siRNA molecules with increased nuclease resistance comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having increased nuclease resistance.
- In one embodiment, the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulates the binding affinity between the sense and antisense strands of the siRNA construct.
- In another embodiment, the invention features a method for generating siRNA molecules with increased binding affinity between the sense and antisense strands of the siRNA molecule comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having increased binding affinity between the sense and antisense strands of the siRNA molecule.
- In one embodiment, the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulates the binding affinity between the antisense strand of the siRNA construct and a complementary target RNA sequence within a cell.
- In another embodiment, the invention features a method for generating siRNA molecules with increased binding affinity between the antisense strand of the siRNA molecule and a complementary target RNA sequence, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having increased binding affinity between the antisense strand of the siRNA molecule and a complementary target RNA sequence.
- In one embodiment, the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulate the polymerase activity of a cellular polymerase capable of generating additional endogenous siRNA molecules having sequence homology to the chemically modified siRNA construct.
- In another embodiment, the invention features a method for generating siRNA molecules capable of mediating increased polymerase activity of a cellular polymerase capable of generating additional endogenous siRNA molecules having sequence homology to the chemically modified siRNA molecule comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules capable of mediating increased polymerase activity of a cellular polymerase capable of generating additional endogenous siRNA molecules having sequence homology to the chemically modified siRNA molecule.
- In one embodiment, the invention features chemically modified siRNA constructs that mediate RNAi against ADORA1 in a cell, wherein the chemical modifications do not significantly effect the interaction of siRNA with a target RNA molecule and/or proteins or other factors that are essential for RNAi in a manner that would decrease the efficacy of RNAi mediated by such siRNA constructs.
- In another embodiment, the invention features a method for generating siRNA molecules with improved RNAi activity against ADORA1, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved RNAi activity.
- In yet another embodiment, the invention features a method for generating siRNA molecules with improved RNAi activity against an ADORA1 target RNA, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved RNAi activity against the target RNA.
- In one embodiment, the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that modulates the cellular uptake of the siRNA construct.
- In another embodiment, the invention features a method for generating siRNA molecules against ADORA1 with improved cellular uptake, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved cellular uptake.
- In one embodiment, the invention features siRNA constructs that mediate RNAi against ADORA1, wherein the siRNA construct comprises one or more chemical modifications described herein that increases the bioavailability of the siRNA construct, for example by attaching polymeric conjugates such as polyethyleneglycol or equivalent conjugates that improve the pharmacokinetics of the siRNA construct, or by attaching conjugates that target specific tissue types or cell types in vivo. Non-limiting examples of such conjugates are described in Vargeese et al., U.S. Serial No. 60/311,865 incorporated by reference herein.
- In one embodiment, the invention features a method for generating siRNA molecules of the invention with improved bioavailability, comprising (a) introducing a conjugate into the structure of a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved bioavailability. Such conjugates can include ligands for cellular receptors such as peptides derived from naturally occurring protein ligands, protein localization sequences including cellular ZIP code sequences, antibodies, nucleic acid aptamers, vitamins and other co-factors such as folate and N-acetylgalactosamine, polymers such as polyethyleneglycol (PEG), phospholipids, polyamines such as spermine or spermidine, and others.
- In another embodiment, the invention features a method for generating siRNA molecules of the invention with improved bioavailability, comprising (a) introducing an excipient formulation to a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved bioavailability. Such excipients include polymers such as cyclodextrins, lipids, cationic lipids, polyamines, phospholipids, and others.
- In another embodiment, the invention features a method for generating siRNA molecules of the invention with improved bioavailability, comprising (a) introducing nucleotides having any of Formula I-VI into a siRNA molecule, and (b) assaying the siRNA molecule of step (a) under conditions suitable for isolating siRNA molecules having improved bioavailability.
- In another embodiment, polyethylene glycol (PEG) can be covalently attached to siRNA compounds of the present invention. The attached PEG can be any molecular weight, preferably from about 2,000 to about 50,000 daltons (Da).
- The present invention can be used alone or as a component of a kit having at least one of the reagents necessary to carry out the in vitro or in vivo introduction of RNA to test samples and/or subjects. For example, preferred components of the kit include the siRNA and a vehicle that promotes introduction of the siRNA. Such a kit can also include instructions to allow a user of the kit to practice the invention.
- The term “short interfering RNA” or “siRNA” as used herein refers to any nucleic acid molecule capable of mediating RNA interference “RNAi” or gene silencing; see for example Bass, 2001, Nature, 411, 428-429; Elbashir et al., 2001, Nature, 411, 494-498; and Kreutzer et al., International PCT Publication No. WO 00/44895; Zernicka-Goetz et al., International PCT Publication No. WO 01/36646; Fire, International PCT Publication No. WO 99/32619; Plaetinck et al, International PCT Publication No. WO 00/01846; Mello and Fire, International PCT Publication No. WO 01/29058; Deschamps-Depaillette, International PCT Publication No. WO 99/07409; and Li et al., International PCT Publication No. WO 00/44914. Non limiting examples of siRNA molecules of the invention are shown in FIG. 2. For example the siRNA can be a double stranded polynucleotide molecule comprising self complementary sense and antisense regions, wherein the antisense region comprises complementarity to a target nucleic acid molecule. The siRNA can be a single stranded hairpin polynucleotide having self complementary sense and antisense regions, wherein the antisense region comprises complementarity to a target nucleic acid molecule. The siRNA can be a circular single stranded polynucleotide having two or more loop structures and a stem comprising self complementary sense and antisense regions, wherein the antisense region comprises complementarity to a target nucleic acid molecule, and wherein the circular polynucleotide can be processed either in vivo or in vitro to generate an active siRNA capable of mediating RNAi. As used herein, siRNA molecules need not be limited to those molecules containing only RNA, but further encompasses chemically modified nucleotides and non-nucleotides.
- By “modulate” is meant that the expression of the gene, or level of RNA molecule or equivalent RNA molecules encoding one or more proteins or protein subunits, or activity of one or more proteins or protein subunits is up regulated or down regulated, such that expression, level, or activity is greater than or less than that observed in the absence of the modulator. For example, the term “modulate” can mean “inhibit,” but the use of the word “modulate” is not limited to this definition.
- By “inhibit” it is meant that the activity of a gene expression product or level of RNAs or equivalent RNAs encoding one or more gene products is reduced below that observed in the absence of the nucleic acid molecule of the invention. In one embodiment, inhibition with a siRNA molecule preferably is below that level observed in the presence of an inactive or attenuated molecule that is unable to mediate an RNAi response. In another embodiment, inhibition of gene expression with the siRNA molecule of the instant invention is greater in the presence of the siRNA molecule than in its absence.
- By “gene” or “target gene” is meant, a nucleic acid that encodes an RNA, for example, nucleic acid sequences including, but not limited to, structural genes encoding a polypeptide. The target gene can be a gene derived from a cell, an endogenous gene, a transgene, or exogenous genes such as genes of a pathogen, for example a virus, which is present in the cell after infection thereof. The cell containing the target gene can be derived from or contained in any organism, for example a plant, animal, protozoan, virus, bacterium, or fungus. Non-limiting examples of plants include monocots, dicots, or gymnosperms. Non-limiting examples of animals include vertebrates or invertebrates. Non-limiting examples of fungi include molds or yeasts.
- By “ADORA1” is meant, a polypeptide comprising an adenosine A1 receptor or polynucleotide encoding an Ets adenosine A1 receptor, for example a polynucleotide having Genbank Accession No. NM —000674.
- By “highly conserved sequence region” is meant, a nucleotide sequence of one or more regions in a target gene does not vary significantly from one generation to the other or from one biological system to the other.
- By “complementarity” or “complementary” is meant that a nucleic acid can form hydrogen bond(s) with another nucleic acid sequence by either traditional Watson-Crick or other non-traditional types of interaction. In reference to the nucleic molecules of the present invention, the binding free energy for a nucleic acid molecule with its complementary sequence is sufficient to allow the relevant function of the nucleic acid to proceed, e.g., RNAi activity. For example, the degree of complementarity between the sense and antisense strand of the siRNA construct can be the same or different from the degree of complementarity between the antisense strand of the siRNA and the target RNA sequence. Complementarity to the target sequence of less than 100% in the antisense strand of the siRNA duplex, including point mutations, is reported not to be tolerated when these changes are located between the 3′-end and the middle of the antisense siRNA (completely abolishes siRNA activity), whereas mutations near the 5′-end of the antisense siRNA strand can exhibit a small degree of RNAi activity (Elbashir et al., 2001, The EMBO Journal, 20, 6877-6888). Determination of binding free energies for nucleic acid molecules is well known in the art (see, e.g., Turner et al., 1987, CSH Symp. Quant. Biol. LII pp.123-133; Frier et al., 1986, Proc. Nat. Acad. Sci. USA 83:9373-9377; Turner et al., 1987, J. Am. Chem. Soc. 109:3783-3785). A percent complementarity indicates the percentage of contiguous residues in a nucleic acid molecule that can form hydrogen bonds (e.g., Watson-Crick base pairing) with a second nucleic acid sequence (e.g., 5, 6, 7, 8, 9, 10 out of 10 being 50%, 60%, 70%, 80%, 90%, and 100% complementary). “Perfectly complementary” means that all the contiguous residues of a nucleic acid sequence will hydrogen bond with the same number of contiguous residues in a second nucleic acid sequence.
- The siRNA molecules of the invention represent a novel therapeutic approach to treat a variety of allergic/inflammatory diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and other indications that can respond to the level of ADORA1.
- In one embodiment of the present invention, each sequence of a siRNA molecule of the invention is independently about 18 to about 24 nucleotides in length, in specific embodiments about 18, 19, 20, 21, 22, 23, or 24 nucleotides in length. In another embodiment, the siRNA duplexes of the invention independently comprise between about 17 and about 23 (e.g., about 17, 18, 19, 20, 21, 22, or 23) base pairs. In yet another embodiment, siRNA molecules of the invention comprising hairpin or circular structures are about 35 to about 55 (e.g., about 35, 40, 45, 50, or 55) nucleotides in length, or about 38 to about 44 (e.g., about 38, 39, 40, 41, 42, 43, or 44) nucleotides in length and comprising about 16 to about 22 (e.g., about 16, 17, 18, 19, 20, 21, or 22) base pairs. Exemplary siRNA molecules of the invention are shown in Table I and III (all sequences are shown 5′-3′) and/or FIGS. 4 and 5.
- As used herein “cell” is used in its usual biological sense, and does not refer to an entire multicellular organism, e.g., specifically does not refer to a human. The cell can be present in an organism, e.g., mammals such as humans, cows, sheep, apes, monkeys, swine, dogs, and cats. The cell can be eukaryotic (e.g., a mammalian cell, such as a human cell). The cell can be of somatic or germ line origin, totipotent or pluripotent, dividing or non-dividing. The cell can also be derived from or can comprise a gamete or embryo, a stem cell, or a fully differentiated cell.
- The siRNA molecules of the invention are added directly, or can be complexed with cationic lipids, packaged within liposomes, or otherwise delivered to target cells or tissues. The nucleic acid or nucleic acid complexes can be locally administered to relevant tissues ex vivo, or in vivo through injection, infusion pump or stent, with or without their incorporation in biopolymers. In particular embodiments, the nucleic acid molecules of the invention comprise sequences shown in Table I, III and/or FIGS. 4 and 5. Examples of such nucleic acid molecules consist essentially of sequences defined in these tables/figures.
- In another aspect, the invention provides mammalian cells containing one or more siRNA molecules of this invention. The one or more siRNA molecules can independently be targeted to the same or different sites.
- By “RNA” is meant a molecule comprising at least one ribonucleotide residue. By “ribonucleotide” is meant a nucleotide with a hydroxyl group at the 2′ position of a β-D-ribo-furanose moiety. The terms include double stranded RNA, single stranded RNA, isolated RNA such as partially purified RNA, essentially pure RNA, synthetic RNA, recombinantly produced RNA, as well as altered RNA that differs from naturally occurring RNA by the addition, deletion, substitution and/or alteration of one or more nucleotides. Such alterations can include addition of non-nucleotide material, such as to the end(s) of the siRNA or internally, for example at one or more nucleotides of the RNA. Nucleotides in the RNA molecules of the instant invention can also comprise non-standard nucleotides, such as non-naturally occurring nucleotides or chemically synthesized nucleotides or deoxynucleotides. These altered RNAs can be referred to as analogs or analogs of naturally-occurring RNA.
- By “subject” is meant an organism, which is a donor or recipient of explanted cells or the cells themselves. “Subject” also refers to an organism to which the nucleic acid molecules of the invention can be administered. In one embodiment, a subject is a mammal or mammalian cells. In another embodiment, a subject is a human or human cells.
- The term “phosphorothioate” as used herein refers to an internucleotide linkage having Formula I, wherein Z and/or W comprise a sulfur atom. Hence, the term phosphorothioate refers to both phosphorothioate and phosphorodithioate internucleotide linkages.
- The term “universal base” as used herein refers to nucleotide base analogs that form base pairs with each of the natural DNA/RNA bases with little discrimination between them. Non-limiting examples of universal bases include C-phenyl, C-naphthyl and other aromatic derivatives, inosine, azole carboxamides, and nitroazole derivatives such as 3-nitropyrrole, 4-nitroindole, 5-nitroindole, and 6-nitroindole as known in the art (see for example Loakes, 2001, Nucleic Acids Research, 29, 2437-2447).
- The term “acyclic nucleotide” as used herein refers to any nucleotide having an acyclic ribose sugar, for example where any of the ribose carbons (C1, C2, C3, C4, or C5), are independently or in combination absent from the nucleotide.
- The nucleic acid molecules of the instant invention, individually, or in combination or in conjunction with other drugs, can be used to treat diseases or conditions discussed herein. For example, to treat a particular disease or condition, the siRNA molecules can be administered to a subject or can be administered to other appropriate cells evident to those skilled in the art, individually or in combination with one or more drugs under conditions suitable for the treatment.
- In a further embodiment, the siRNA molecules can be used in combination with other known treatments to treat conditions or diseases discussed above. For example, the described molecules could be used in combination with one or more known therapeutic agents to treat a disease or condition. Non-limiting examples of other therapeutic agents that can be readily combined with a siRNA molecule of the invention are enzymatic nucleic acid molecules, allosteric nucleic acid molecules, antisense, decoy, or aptamer nucleic acid molecules, antibodies such as monoclonal antibodies, small molecules, and other organic and/or inorganic compounds including metals, salts and ions.
- In one embodiment, the invention features an expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of the invention, in a manner which allows expression of the siRNA molecule. For example, the vector can contain sequence(s) encoding both strands of a siRNA molecule comprising a duplex. The vector can also contain sequence(s) encoding a single nucleic acid molecule that is self complementary and thus forms a siRNA molecule. Non-limiting examples of such expression vectors are described in Paul et al, 2002, Nature Biotechnology, 19, 505; Miyagishi and Taira, 2002, Nature Biotechnology, 19, 497; Lee et al., 2002, Nature Biotechnology, 19, 500; and Novina et al, 2002, Nature Medicine, advance online publication doi: 10.1038/nm725.
- In another embodiment, the invention features a mammalian cell, for example, a human cell, including an expression vector of the invention.
- In yet another embodiment, the expression vector of the invention comprises a sequence for a siRNA molecule having complementarity to a RNA molecule referred to by a Genbank Accession numbers, for example genes such as Genbank Accession No. No. NM —000674.
- In one embodiment, an expression vector of the invention comprises a nucleic acid sequence encoding two or more siRNA molecules, which can be the same or different.
- In another aspect of the invention, siRNA molecules that interact with target RNA molecules and down-regulate gene encoding target RNA molecules (for example target RNA molecules referred to by Genbank Accession numbers herein) are expressed from transcription units inserted into DNA or RNA vectors. The recombinant vectors can be DNA plasmids or viral vectors. siRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus, retrovirus, adenovirus, or alphavirus. The recombinant vectors capable of expressing the siRNA molecules can be delivered as described herein, and persist in target cells. Alternatively, viral vectors can be used that provide for transient expression of siRNA molecules. Such vectors can be repeatedly administered as necessary. Once expressed, the siRNA molecules bind and down-regulate gene function or expression via RNA interference (RNAi). Delivery of siRNA expressing vectors can be systemic, such as by intravenous or intramuscular administration, by administration to target cells ex-planted from a subject followed by reintroduction into the subject, or by any other means that would allow for introduction into the desired target cell.
- By “vectors” is meant any nucleic acid- and/or viral-based technique used to deliver a desired nucleic acid.
- By “comprising” is meant including, but not limited to, whatever follows the word “comprising”. Thus, use of the term “comprising” indicates that the listed elements are required or mandatory, but that other elements are optional and may or may not be present. By “consisting of” is meant including, and limited to, whatever follows the phrase “consisting of”. Thus, the phrase “consisting of” indicates that the listed elements are required or mandatory, and that no other elements may be present. By “consisting essentially of” is meant including any elements listed after the phrase, and limited to other elements that do not interfere with or contribute to the activity or action specified in the disclosure for the listed elements. Thus, the phrase “consisting essentially of” indicates that the listed elements are required or mandatory, but that other elements are optional and may or may not be present depending upon whether or not they affect the activity or action of the listed elements.
- Other features and advantages of the invention will be apparent from the following description of the preferred embodiments thereof, and from the claims.
- First the drawings will be described briefly.
- FIG. 1 shows a non-limiting example of a scheme for the synthesis of siRNA molecules. The complementary siRNA sequence strands,
strand 1 andstrand 2, are synthesized in tandem and are connected by a cleavable linkage, such as a nucleotide succinate or abasic succinate, which can be the same or different from the cleavable linker used for solid phase synthesis on a solid support. The synthesis can be either solid phase or solution phase, in the example shown, the synthesis is a solid phase synthesis. The synthesis is performed such that a protecting group, such as a dimethoxytrityl group, remains intact on the terminal nucleotide of the tandem oligonucleotide. Upon cleavage and deprotection of the oligonucleotide, the two siRNA strands spontaneously hybridize to form a siRNA duplex, which allows the purification of the duplex by utilizing the properties of the terminal protecting group, for example by applying a trityl on purification method wherein only duplexes/oligonucleotides with the terminal protecting group are isolated. - FIG. 2 shows a MALDI-TOV mass spectrum of a purified siRNA duplex synthesized by a method of the invention. The two peaks shown correspond to the predicted mass of the separate siRNA sequence strands. This result demonstrates that the siRNA duplex generated from tandem synthesis can be purified as a single entity using a simple trityl-on purification methodology.
- FIG. 3 shows a non-limiting proposed mechanistic representation of target RNA degradation involved in RNAi. Double stranded RNA (dsRNA), which is generated by RNA dependent RNA polymerase (RdRP) from foreign single stranded RNA, for example viral, transposon, or other exogenous RNA, activates the DICER enzyme which in turn generates siRNA duplexes having terminal phosphate groups (P). An active siRNA complex forms which recognizes a target RNA, resulting in degradation of the target RNA by the RISC endonuclease complex or in the synthesis of additional RNA by RNA dependent RNA polymerase (RdRP), which can activate DICER and result in additional siRNA molecules, thereby amplifying the RNAi response.
- FIG. 4 shows non-limiting examples of chemically modified siRNA constructs of the present invention. In the figure, N stands for any nucleotide (adenosine, guanosine, cytosine, uridine, or optionally thymidine, for example thymidine can be substituted in the overhanging regions designated by parenthesis (N N). Various modifications are shown for the sense and antisense strands of the siRNA constructs. A The sense strand comprises 21 nucleotides having four
phosphorothioate 5′ and 3′-terminal internucleotide linkages, wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and having one 3′-terminal phosphorothioate internucleotide linkage and four 5′-terminal phosphorothioate internucleotide linkages and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. B The sense strand comprises 21 nucleotides wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. C The sense strand comprises 21 nucleotides having 5′- and 3′-terminal cap moieties wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro modified nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. D The sense strand comprises 21 nucleotides having fivephosphorothioate 5′ and 3′-terminal internucleotide linkages, wherein the two terminal 3′-nucleotides are optionally base paired and wherein all nucleotides are ribonucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and having one 3′-terminal phosphorothioate internucleotide linkage and five 5′-terminal phosphorothioate internucleotide linkages and wherein all nucleotides are ribonucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. E The sense strand comprises 21 nucleotides wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand comprises 21 nucleotides all having phosphorothioate internucleotide linkages, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and wherein all nucleotides are ribonucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. F The sense strand comprises 21 nucleotides having 5′- and 3′-terminal cap moieties, wherein the two terminal 3′-nucleotides are optionally base paired and wherein all pyrimidine nucleotides that may be present are 2′-O-methyl nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand comprises 21 nucleotides, wherein the two terminal 3′-nucleotides are optionally complimentary to the target RNA sequence, and having one 3′-termninal phosphorothioate internucleotide linkage and wherein all pyrimidine nucleotides that may be present are 2′-deoxy-2′-fluoro nucleotides except for (N N) nucleotides, which can comprise naturally occurring ribonucleotides, deoxynucleotides, universal bases, or other chemical modifications described herein. The antisense strand of constructs A-F comprise sequence complimentary to target RNA sequence of the invention. - FIG. 5 shows non-limiting examples of specific chemically modified siRNA sequences of the invention. A-F applies the chemical modifications described in FIG. 4A-F to an ADORA1 siRNA sequence.
- FIG. 6 shows non-limiting examples of different siRNA constructs of the invention. The examples shown (constructs 1, 2, and 3) have 19 representative base pairs, however, different embodiments of the invention include any number of base pairs described herein. Bracketed regions represent nucleotide overhangs, for example comprising between about 1, 2, 3, or 4 nucleotides in length, preferably about 2 nucleotides.
Constructs Construct 2 can comprise a polynucleotide or non-nucleotide linker, which can optionally be designed as a biodegradable linker. In one embodiment, the loop structure shown inconstruct 2 can comprise a biodegradable linker that results in the formation ofconstruct 1 in vivo and/or in vitro. In another example, construct 3 can be used to generateconstruct 2 under the same principle wherein a linker is used to generate the active siRNA construct 2 in vivo and/or in vitro, which can optionally utilize another biodegradable linker to generate the active siRNA construct 1 in vivo and/or in vitro. As such, the stability and/or activity of the siRNA constructs can be modulated based on the design of the siRNA construct for use in vivo or in vitro and/or in vitro. - FIG. 7 is a diagrammatic representation of a scheme utilized in generating an expression cassette to generate siRNA hairpin constructs. (A) A DNA oligomer is synthesized with a 5′-restriction site (R1) sequence followed by a region having sequence identical (sense region of siRNA) to a predetermined ADORA1 target seqeunce, wherein the sense region comprises, for example, about 19, 20, 21, or 22 nucleotides (N) in length, which is followed by a loop sequence of defined sequence (X), comprising, for example, between about 3 and 10 nucleotides. (B) The synthetic construct is then extended by DNA polymerase to generate a hairpin structure having self complementary sequence that will result in a siRNA transcript having specificity for an ADORA1 target sequence and having self complementary sense and antisense regions. (C) The construct is heated (for example to about 95° C.) to linearize the sequence, thus allowing extension of a complementary second DNA strand using a primer to the 3′-restriction sequence of the first strand. The double stranded DNA is then inserted into an appropriate vector for expression in cells. The construct can be designed such that a 3′-overhang results from the transcription, for example by engineering restriction sites and/or utilizing a poly-U termination region as described in Paul et al., 2002, Nature Biotechnology, 29, 505-508.
- FIG. 8 is a diagrammatic representation of a scheme utilized in generating an expression cassette to generate double stranded siRNA constructs. (A) A DNA oligomer is synthesized with a 5′-restriction (R1) site sequence followed by a region having sequence identical (sense region of siRNA) to a predetermined ADORA1 target seqeunce, wherein the sense region comprises, for example, about 19, 20, 21, or 22 nucleotides (N) in length, and which is followed by a 3′-restriction site (R2) which is adjacent to a loop sequence of defined sequence (X). (B) The synthetic construct is then extended by DNA polymerase to generate a hairpin structure having self complementary sequence. (C) The construct is processed by restriction enzymes specific to R1 and R2 to generate a double stranded DNA which is then inserted into an appropriate vector for expression in cells. The transcription cassette is designed such that a U6 promoter region flanks each side of the dsDNA which generates the separate sense and antisense strands of the siRNA. Poly T termination sequences can be added to the constructs to generate U overhangs in the resulting transcript.
- FIG. 9 is a diagrammatic representation of a method used to determine target sites for siRNA mediated RNAi within a particular target nucleic acid sequence, such as messenger RNA. (A) A pool of siRNA oligonucleotides are synthesized wherein the antisense region of the siRNA constructs has complementarity to target sites across the target nucleic acid sequence, and wherein the sense region comprises sequence complementary to the antisense region of the siRNA. (B) The sequences are pooled and are inserted into vectors such that (C) transfection of a vector into cells results in the expression of the siRNA. (D) Cells are sorted based on phenotypic change that is associated with modulation of the target nucleic acid sequence. (E) The siRNA is isolated from the sorted cells and is sequenced to identify efficacious target sites within the target nucleic acid sequence.
- RNA interference refers to the process of sequence specific post transcriptional gene silencing in animals mediated by short interfering RNAs (siRNA) (Fire et al., 1998, Nature, 391, 806). The corresponding process in plants is commonly referred to as post transcriptional gene silencing or RNA silencing and is also referred to as quelling in fungi. The process of post transcriptional gene silencing is thought to be an evolutionarily conserved cellular defense mechanism used to prevent the expression of foreign genes which is commonly shared by diverse flora and phyla (Fire et al., 1999, Trends Genet., 15, 358). Such protection from foreign gene expression may have evolved in response to the production of double stranded RNAs (dsRNA) derived from viral infection or the random integration of transposon elements into a host genome via a cellular response that specifically destroys homologous single stranded RNA or viral genomic RNA. The presence of dsRNA in cells triggers the RNAi response though a mechanism that has yet to be fully characterized. This mechanism appears to be different from the interferon response that results from dsRNA mediated activation of protein kinase PKR and 2′,5′-oligoadenylate synthetase resulting in non-specific cleavage of mRNA by ribonuclease L.
- The presence of long dsRNAs in cells stimulates the activity of a ribonuclease III enzyme referred to as Dicer. Dicer is involved in the processing of the dsRNA into short pieces of dsRNA known as short interfering RNAs (siRNA) (Berstein et al., 2001, Nature, 409, 363). Short interfering RNAs derived from Dicer activity are typically about 21-23 nucleotides in length and comprise about 19 base pair duplexes. Dicer has also been implicated in the excision of 21 and 22 nucleotide small temporal RNAs (stRNA) from precursor RNA of conserved structure that are implicated in translational control (Hutvagner et al., 2001, Science, 293, 834). The RNAi response also features an endonuclease complex containing a siRNA, commonly referred to as an RNA-induced silencing complex (RISC), which mediates cleavage of single stranded RNA having sequence homologous to the siRNA. Cleavage of the target RNA takes place in the middle of the region complementary to the guide sequence of the siRNA duplex (Elbashir et al., 2001, Genes Dev., 15, 188).
- RNAi has been studied in a variety of systems. Fire et al., 1998, Nature, 391, 806, were the first to observe RNAi in C. elegans. Wianny and Goetz, 1999, Nature Cell Biol., 2, 70, describes RNAi mediated by dsRNA in mouse embryos. Hammond et al., 2000, Nature, 404, 293, describe RNAi in Drosophila cells transfected with dsRNA. Elbashir et al., 2001, Nature, 411, 494, describe RNAi induced by introduction of duplexes of synthetic 21-nucleotide RNAs in cultured mammalian cells including human embryonic kidney and HeLa cells. Recent work in Drosophila embryonic lysates has revealed certain requirements for siRNA length, structure, chemical composition, and sequence that are essential to mediate efficient RNAi activity. These studies have shown that 21 nucleotide siRNA duplexes are most active when containing two
nucleotide 3′-overhangs. Furthermore, substitution of one or both siRNA strands with 2′-deoxy or 2′-O-methyl nucleotides abolishes RNAi activity, whereas substitution of 3′-terminal siRNA nucleotides with deoxy nucleotides was shown to be tolerated. Mismatch sequences in the center of the siRNA duplex were also shown to abolish RNAi activity. In addition, these studies also indicate that the position of the cleavage site in the target RNA is defined by the 5′-end of the siRNA guide sequence rather than the 3′-end (Elbashir et al., 2001, EMBO J., 20, 6877). Other studies have indicated that a 5′-phosphate on the target-complementary strand of a siRNA duplex is required for siRNA activity and that ATP is utilized to maintain the 5′-phosphate moiety on the siRNA (Nykanen et al., 2001, Cell, 107, 309), however siRNA molecules lacking a 5′-phosphate are active when introduced exogenously, suggesting that 5′-phosphorylation of siRNA constructs may occur in vivo. - Synthesis of nucleic acids greater than 100 nucleotides in length is difficult using automated methods, and the therapeutic cost of such molecules is prohibitive. In this invention, small nucleic acid motifs (“small” refers to nucleic acid motifs no more than 100 nucleotides in length, preferably no more than 80 nucleotides in length, and most preferably no more than 50 nucleotides in length; e.g., individual siRNA oligonucleotide sequences or siRNA sequences synthesized in tandem) are preferably used for exogenous delivery. The simple structure of these molecules increases the ability of the nucleic acid to invade targeted regions of protein and/or RNA structure. Exemplary molecules of the instant invention are chemically synthesized, and others can similarly be synthesized.
- Oligonucleotides (e.g., certain modified oligonucleotides or portions of oligonucleotides lacking ribonucleotides) are synthesized using protocols known in the art, for example as described in Caruthers et al., 1992, Methods in Enzymology 211, 3-19, Thompson et al., International PCT Publication No. WO 99/54459, Wincott et al., 1995, Nucleic Acids Res. 23, 2677-2684, Wincott et al., 1997, Methods Mol. Bio., 74, 59, Brennan et al., 1998, Biotechnol Bioeng., 61, 33-45, and Brennan, U.S. Pat. No. 6,001,311. All of these references are incorporated herein by reference. The synthesis of oligonucleotides makes use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5′-end, and phosphoramidites at the 3′-end. In a non-limiting example, small scale syntheses are conducted on a 394 Applied Biosystems, Inc. synthesizer using a 0.2 μmol scale protocol with a 2.5 min coupling step for 2′-O-methylated nucleotides and a 45 sec coupling step for 2′-deoxy nucleotides or 2′-deoxy-2′-fluoro nucleotides. Table II outlines the amounts and the contact times of the reagents used in the synthesis cycle. Alternatively, syntheses at the 0.2 μmol scale can be performed on a 96-well plate synthesizer, such as the instrument produced by Protogene (Palo Alto, Calif.) with minimal modification to the cycle. A 33-fold excess (60 μL of 0.11 M=6.6 μmol) of 2′-O-methyl phosphoramidite and a 105-fold excess of S-ethyl tetrazole (60 μL of 0.25 M=15 μmol) can be used in each coupling cycle of 2′-O-methyl residues relative to polymer-bound 5′-hydroxyl. A 22-fold excess (40 μL of 0.11 M=4.4 μmol) of deoxy phosphoramidite and a 70-fold excess of S-ethyl tetrazole (40 μL of 0.25 M=10 μmol) can be used in each coupling cycle of deoxy residues relative to polymer-bound 5′-hydroxyl. Average coupling yields on the 394 Applied Biosystems, Inc. synthesizer, determined by calorimetric quantitation of the trityl fractions, are typically 97.5-99%. Other oligonucleotide synthesis reagents for the 394 Applied Biosystems, Inc. synthesizer include the following: detritylation solution is 3% TCA in methylene chloride (ABI); capping is performed with 16% N-methyl imidazole in THF (ABI) and 10% acetic anhydride/10% 2,6-lutidine in THF (ABI); and oxidation solution is 16.9 mM I2, 49 mM pyridine, 9% water in THF (PERSEPTIVE™). Burdick & Jackson Synthesis Grade acetonitrile is used directly from the reagent bottle. S-Ethyltetrazole solution (0.25 M in acetonitrile) is made up from the solid obtained from American International Chemical, Inc. Alternately, for the introduction of phosphorothioate linkages, Beaucage reagent (3H-1,2-Benzodithiol-3-
one 1,1-dioxide, 0.05 M in acetonitrile) is used. - Deprotection of the DNA-based oligonucleotides is performed as follows: the polymer-bound trityl-on oligoribonucleotide is transferred to a 4 mL glass screw top vial and suspended in a solution of 40% aq. methylamine (1 mL) at 65° C. for 10 min. After cooling to −20° C., the supernatant is removed from the polymer support. The support is washed three times with 1.0 mL of EtOH:MeCN:H2O/3:1:1, vortexed and the supernatant is then added to the first supernatant. The combined supernatants, containing the oligoribonucleotide, are dried to a white powder.
- The method of synthesis used for RNA including certain siRNA molecules of the invention follows the procedure as described in Usman et al., 1987, J. Am. Chem. Soc., 109, 7845; Scaringe et al., 1990, Nucleic Acids Res., 18, 5433; and Wincott et al., 1995, Nucleic Acids Res. 23, 2677-2684 Wincott et al., 1997, Methods Mol. Bio., 74, 59, and makes use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5′-end, and phosphoramidites at the 3′-end. In a non-limiting example, small scale syntheses are conducted on a 394 Applied Biosystems, Inc. synthesizer using a 0.2 μmol scale protocol with a 7.5 min coupling step for alkylsilyl protected nucleotides and a 2.5 min coupling step for 2′-O-methylated nucleotides. Table II outlines the amounts and the contact times of the reagents used in the synthesis cycle. Alternatively, syntheses at the 0.2 μmol scale can be done on a 96-well plate synthesizer, such as the instrument produced by Protogene (Palo Alto, Calif.) with minimal modification to the cycle. A 33-fold excess (60 μL of 0.11 M=6.6 μmol) of 2′-O-methyl phosphoramidite and a 75-fold excess of S-ethyl tetrazole (60 μL of 0.25 M=15 μmol) can be used in each coupling cycle of 2′-O-methyl residues relative to polymer-bound 5′-hydroxyl. A 66-fold excess (120 μL of 0.11 M=13.2 μmol) of alkylsilyl (ribo) protected phosphoramidite and a 150-fold excess of S-ethyl tetrazole (120 μL of 0.25 M=30 μmol) can be used in each coupling cycle of ribo residues relative to polymer-bound 5′-hydroxyl. Average coupling yields on the 394 Applied Biosystems, Inc. synthesizer, determined by colorimetric quantitation of the trityl fractions, are typically 97.5-99%. Other oligonucleotide synthesis reagents for the 394 Applied Biosystems, Inc. synthesizer include the following: detritylation solution is 3% TCA in methylene chloride (ABI); capping is performed with 16% N-methyl imidazole in THF (ABI) and 10% acetic anhydride/10% 2,6-lutidine in THF (ABI); oxidation solution is 16.9 mM I2, 49 mM pyridine, 9% water in THF (PERSEPTIVE™). Burdick & Jackson Synthesis Grade acetonitrile is used directly from the reagent bottle. S-Ethyltetrazole solution (0.25 M in acetonitrile) is made up from the solid obtained from American International Chemical, Inc. Alternately, for the introduction of phosphorothioate linkages, Beaucage reagent (3H-1,2-Benzodithiol-3-
one 1,1-dioxide0.05 M in acetonitrile) is used. - Deprotection of the RNA is performed using either a two-pot or one-pot protocol. For the two-pot protocol, the polymer-bound trityl-on oligoribonucleotide is transferred to a 4 mL glass screw top vial and suspended in a solution of 40% aq. methylamine (1 mL) at 65° C. for 10 min. After cooling to −20° C., the supernatant is removed from the polymer support. The support is washed three times with 1.0 mL of EtOH:MeCN:H2O/3:1:1, vortexed and the supernatant is then added to the first supernatant. The combined supernatants, containing the oligoribonucleotide, are dried to a white powder. The base deprotected oligoribonucleotide is resuspended in anhydrous TEA/HF/NMP solution (300 μL of a solution of 1.5 mL N-methylpyrrolidinone, 750 μL TEA and 1 mL TEA·3HF to provide a 1.4 M HF concentration) and heated to 65° C. After 1.5 h, the oligomer is quenched with 1.5 M NH 4HCO3.
- Alternatively, for the one-pot protocol, the polymer-bound trityl-on oligoribonucleotide is transferred to a 4 mL glass screw top vial and suspended in a solution of 33% ethanolic methylamine/DMSO: 1/1 (0.8 mL) at 65° C. for 15 min. The vial is brought to r.t. TEA·3HF (0.1 mL) is added and the vial is heated at 65° C. for 15 min. The sample is cooled at −20° C. and then quenched with 1.5 M NH 4HCO3.
- For purification of the trityl-on oligomers, the quenched NH 4HCO3 solution is loaded onto a C-18 containing cartridge that had been prewashed with acetonitrile followed by 50 mM TEAA. After washing the loaded cartridge with water, the RNA is detritylated with 0.5% TFA for 13 min. The cartridge is then washed again with water, salt exchanged with 1 M NaCl and washed with water again. The oligonucleotide is then eluted with 30% acetonitrile.
- The average stepwise coupling yields are typically >98% (Wincott et al., 1995 Nucleic Acids Res. 23, 2677-2684). Those of ordinary skill in the art will recognize that the scale of synthesis can be adapted to be larger or smaller than the example described above including but not limited to 96-well format, all that is important is the ratio of chemicals used in the reaction.
- Alternatively, the nucleic acid molecules of the present invention can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., 1992, Science 256, 9923; Draper et al., International PCT publication No. WO 93/23569; Shabarova et al., 1991, Nucleic Acids Research 19, 4247; Bellon et al., 1997, Nucleosides & Nucleotides, 16, 951; Bellon et al., 1997, Bioconjugate Chem. 8, 204), or by hybridization following synthesis and/or deprotection.
- The siRNA molecules of the invention can also be synthesized via a tandem synthesis methodology as described in Example 1 herein, wherein both siRNA strands are synthesized as a single contiguous oligonucleotide fragment or strand separated by a cleavable linker which is subsequently cleaved to provide separate siRNA fragments or strands that hybridize and permit purification of the siRNA duplex. The linker can be a polynucleotide linker or a non-nucleotide linker. The tandem synthesis of siRNA as described herein can be readily adapted to both multiwell/multiplate synthesis platforms such as 96 well or similarly larger multi-well platforms. The tandem synthesis of siRNA as described herein can also be readily adapted to large scale synthesis platforms employing batch reactors, synthesis columns and the like.
- A siRNA molecule can also be assembled from two distinct nucleic acid strands or fragments wherein one fragment includes the sense region and the second fragment includes the antisense region of the RNA molecule.
- The nucleic acid molecules of the present invention can be modified extensively to enhance stability by modification with nuclease resistant groups, for example, 2′-amino, 2′-C-allyl, 2′-flouro, 2′-O-methyl, 2′-H (for a review see Usman and Cedergren, 1992, TIBS 17, 34; Usman et al., 1994, Nucleic Acids Symp. Ser. 31, 163). siRNA constructs can be purified by gel electrophoresis using general methods or can be purified by high pressure liquid chromatography (HPLC; see Wincott et al., supra, the totality of which is hereby incorporated herein by reference) and re-suspended in water.
- In another aspect of the invention, siRNA molecules of the invention are expressed from transcription units inserted into DNA or RNA vectors. The recombinant vectors can be DNA plasmids or viral vectors. siRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus, retrovirus, adenovirus, or alphavirus. The recombinant vectors capable of expressing the siRNA molecules can be delivered as described herein, and persist in target cells. Alternatively, viral vectors can be used that provide for transient expression of siRNA molecules.
- Chemically synthesizing nucleic acid molecules with modifications (base, sugar and/or phosphate) can prevent their degradation by serum ribonucleases, which can increase their potency (see e.g., Eckstein et al., International Publication No. WO 92/07065; Perrault et al., 1990
Nature 344, 565; Pieken et al., 1991, Science 253, 314; Usman and Cedergren, 1992, Trends in Biochem. Sci. 17, 334; Usman et al., International Publication No. WO 93/15187; and Rossi et al., International Publication No. WO 91/03162; Sproat, U.S. Pat. No. 5,334,711; Gold et al., U.S. Pat. No. 6,300,074; and Burgin et al., supra; all of which are incorporated by reference herein). All of the above references describe various chemical modifications that can be made to the base, phosphate and/or sugar moieties of the nucleic acid molecules described herein. Modifications that enhance their efficacy in cells, and removal of bases from nucleic acid molecules to shorten oligonucleotide synthesis times and reduce chemical requirements are desired. - There are several examples in the art describing sugar, base and phosphate modifications that can be introduced into nucleic acid molecules with significant enhancement in their nuclease stability and efficacy. For example, oligonucleotides are modified to enhance stability and/or enhance biological activity by modification with nuclease resistant groups, for example, 2′-amino, 2′-C-allyl, 2′-flouro, 2′-O-methyl, 2′-O-allyl, 2′-H, nucleotide base modifications (for a review see Usman and Cedergren, 1992, TIBS. 17, 34; Usman et al., 1994, Nucleic Acids Symp. Ser. 31, 163; Burgin et al., 1996, Biochemistry, 35, 14090). Sugar modification of nucleic acid molecules have been extensively described in the art (see Eckstein et al., International Publication PCT No. WO 92/07065; Perrault et al. Nature, 1990, 344, 565-568; Pieken et al. Science, 1991, 253, 314-317; Usman and Cedergren, Trends in Biochem. Sci. , 1992, 17, 334-339; Usman et al. International Publication PCT No. WO 93/15187; Sproat, U.S. Pat. No. 5,334,711 and Beigelman et al., 1995, J. Biol. Chem., 270, 25702; Beigelman et al., International PCT publication No. WO 97/26270; Beigelman et al., U.S. Pat. No. 5,716,824; Usman et al., U.S. Pat. No. 5,627,053; Woolf et al., International PCT Publication No. WO 98/13526; Thompson et al., U.S. Ser. No. 60/082,404 which was filed on Apr. 20, 1998; Karpeisky et al., 1998, Tetrahedron Lett., 39, 1131; Earnshaw and Gait, 1998, Biopolymers (Nucleic Acid Sciences), 48, 39-55; Verma and Eckstein, 1998, Annu. Rev. Biochem., 67, 99-134; and Burlina et al., 1997, Bioorg. Med. Chem., 5, 1999-2010; all of the references are hereby incorporated in their totality by reference herein). Such publications describe general methods and strategies to determine the location of incorporation of sugar, base and/or phosphate modifications and the like into nucleic acid molecules without modulating catalysis, and are incorporated by reference herein. In view of such teachings, similar modifications can be used as described herein to modify the siRNA nucleic acid molecules of the instant invention so long as the ability of siRNA to promote RNAi is cells is not significantly inhibited.
- While chemical modification of oligonucleotide internucleotide linkages with phosphorothioate, phosphorothioate, and/or 5′-methylphosphonate linkages improves stability, excessive modifications can cause some toxicity or decreased activity. Therefore, when designing nucleic acid molecules, the amount of these internucleotide linkages should be minimized. The reduction in the concentration of these linkages should lower toxicity, resulting in increased efficacy and higher specificity of these molecules.
- Small interfering RNA (siRNA) molecules having chemical modifications that maintain or enhance activity are provided. Such a nucleic acid is also generally more resistant to nucleases than an unmodified nucleic acid. Accordingly, the in vitro and/or in vivo activity should not be significantly lowered. In cases in which modulation is the goal, therapeutic nucleic acid molecules delivered exogenously should optimally be stable within cells until translation of the target RNA has been modulated long enough to reduce the levels of the undesirable protein. This period of time varies between hours to days depending upon the disease state. Improvements in the chemical synthesis of RNA and DNA (Wincott et al., 1995 Nucleic Acids Res. 23, 2677; Caruthers et al., 1992, Methods in Enzymology 211,3-19 (incorporated by reference herein)) have expanded the ability to modify nucleic acid molecules by introducing nucleotide modifications to enhance their nuclease stability, as described above.
- In one embodiment, nucleic acid molecules of the invention include one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) G-clamp nucleotides. A G-clamp nucleotide is a modified cytosine analog wherein the modifications confer the ability to hydrogen bond both Watson-Crick and Hoogsteen faces of a complementary guanine within a duplex, see for example Lin and Matteucci, 1998, J. Am. Chem. Soc., 120, 8531-8532. A single G-clamp analog substitution within an oligonucleotide can result in substantially enhanced helical thermal stability and mismatch discrimination when hybridized to complementary oligonucleotides. The inclusion of such nucleotides in nucleic acid molecules of the invention results in both enhanced affinity and specificity to nucleic acid targets, complementary sequences, or template strands. In another embodiment, nucleic acid molecules of the invention include one or more (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more) LNA “locked nucleic acid” nucleotides such as a 2′, 4′-C mythylene bicyclo nucleotide (see for example Wengel et al., International PCT Publication No. WO 00/66604 and WO 99/14226).
- In another embodiment, the invention features conjugates and/or complexes of siRNA molecules of the invention. Such conjugates and/or complexes can be used to facilitate delivery of siRNA molecules into a biological system, such as a cell. The conjugates and complexes provided by the instant invention can impart therapeutic activity by transferring therapeutic compounds across cellular membranes, altering the pharmacokinetics, and/or modulating the localization of nucleic acid molecules of the invention. The present invention encompasses the design and synthesis of novel conjugates and complexes for the delivery of molecules, including, but not limited to, small molecules, lipids, phospholipids, nucleosides, nucleotides, nucleic acids, antibodies, toxins, negatively charged polymers and other polymers, for example proteins, peptides, hormones, carbohydrates, polyethylene glycols, or polyamines, across cellular membranes. In general, the transporters described are designed to be used either individually or as part of a multi-component system, with or without degradable linkers. These compounds are expected to improve delivery and/or localization of nucleic acid molecules of the invention into a number of cell types originating from different tissues, in the presence or absence of serum (see Sullenger and Cech, U.S. Pat. No. 5,854,038). Conjugates of the molecules described herein can be attached to biologically active molecules via linkers that are biodegradable, such as biodegradable nucleic acid linker molecules.
- The term “biodegradable linker” as used herein, refers to a nucleic acid or non-nucleic acid linker molecule that is designed as a biodegradable linker to connect one molecule to another molecule, for example, a biologically active molecule to a siRNA molecule of the invention or the sense and antisense strands of a siRNA molecule of the invention. The biodegradable linker is designed such that its stability can be modulated for a particular purpose, such as delivery to a particular tissue or cell type. The stability of a nucleic acid-based biodegradable linker molecule can be modulated by using various chemistries, for example combinations of ribonucleotides, deoxyribonucleotides, and chemically modified nucleotides, such as 2′-O-methyl, 2′-fluoro, 2′-amino, 2′-O-amino, 2′-C-allyl, 2′-O-allyl, and other 2′-modified or base modified nucleotides. The biodegradable nucleic acid linker molecule can be a dimer, trimer, tetramer or longer nucleic acid molecule, for example, an oligonucleotide of about 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20 nucleotides in length, or can comprise a single nucleotide with a phosphorus-based linkage, for example, a phosphoramidate or phosphodiester linkage. The biodegradable nucleic acid linker molecule can also comprise nucleic acid backbone, nucleic acid sugar, or nucleic acid base modifications.
- The term “biodegradable” as used herein, refers to degradation in a biological system, for example enzymatic degradation or chemical degradation.
- The term “biologically active molecule” as used herein, refers to compounds or molecules that are capable of eliciting or modifying a biological response in a system. Non-limiting examples of biologically active siRNA molecules either alone or in combination with othe molecules contemplated by the instant invention include therapeutically active molecules such as antibodies, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, siRNA, dsRNA, allozymes, aptamers, decoys and analogs thereof. Biologically active molecules of the invention also include molecules capable of modulating the pharmacokinetics and/or pharmacodynamics of other biologically active molecules, for example, lipids and polymers such as polyamines, polyamides, polyethylene glycol and other polyethers.
- The term “phospholipid” as used herein, refers to a hydrophobic molecule comprising at least one phosphorus group. For example, a phospholipid can comprise a phosphorus-containing group and saturated or unsaturated alkyl group, optionally substituted with OH, COOH, oxo, amine, or substituted or unsubstituted aryl groups.
- Therapeutic nucleic acid molecules (e.g., siRNA molecules) delivered exogenously optimally are stable within cells until reverse trascription of the RNA has been modulated long enough to reduce the levels of the RNA transcript. The nucleic acid molecules are resistant to nucleases in order to function as effective intracellular therapeutic agents. Improvements in the chemical synthesis of nucleic acid molecules described in the instant invention and in the art have expanded the ability to modify nucleic acid molecules by introducing nucleotide modifications to enhance their nuclease stability as described above.
- In yet another embodiment, siRNA molecules having chemical modifications that maintain or enhance enzymatic activity of proteins involved in RNAi are provided. Such nucleic acids are also generally more resistant to nucleases than unmodified nucleic acids. Thus, in vitro and/or in vivo the activity should not be significantly lowered.
- Use of the nucleic acid-based molecules of the invention will lead to better treatment of the disease progression by affording the possibility of combination therapies (e.g., multiple siRNA molecules targeted to different genes; nucleic acid molecules coupled with known small molecule modulators; or intermittent treatment with combinations of molecules, including different motifs and/or other chemical or biological molecules). The treatment of subjects with siRNA molecules can also include combinations of different types of nucleic acid molecules, such as enzymatic nucleic acid molecules (ribozymes), allozymes, antisense, 2,5-A oligoadenylate, decoys, aptamers etc.
- In another aspect a siRNA molecule of the invention comprises one or more 5′ and/or a 3′-cap structure, for example on only the sense siRNA strand, antisense siRNA strand, or both siRNA strands.
- By “cap structure” is meant chemical modifications, which have been incorporated at either terminus of the oligonucleotide (see, for example, Adamic et al., U.S. Pat. No. 5,998,203, incorporated by reference herein). These terminal modifications protect the nucleic acid molecule from exonuclease degradation, and may help in delivery and/or localization within a cell. The cap may be present at the 5′-terminus (5′-cap) or at the 3′-terminal (3′-cap) or may be present on both termini. In non-limiting examples: the 5′-cap is selected from the group comprising inverted abasic residue (moiety); 4′,5′-methylene nucleotide; 1-(beta-D-erythrofuranosyl) nucleotide, 4′-thio nucleotide; carbocyclic nucleotide; 1,5-anhydrohexitol nucleotide; L-nucleotides; alpha-nucleotides; modified base nucleotide; phosphorodithioate linkage; threo-pentofuranosyl nucleotide; acyclic 3′,4′-seco nucleotide; acyclic 3,4-dihydroxybutyl nucleotide; acyclic 3,5-dihydroxypentyl nucleotide, 3′-3′-inverted nucleotide moiety; 3′-3′-inverted abasic moiety; 3′-2′-inverted nucleotide moiety; 3′-2′-inverted abasic moiety; 1,4-butanediol phosphate; 3′-phosphoramidate; hexylphosphate; aminohexyl phosphate; 3′-phosphate; 3′-phosphorothioate; phosphorodithioate; or bridging or non-bridging methylphosphonate moiety.
- In yet another preferred embodiment, the 3′-cap is selected from a group comprising, 4′,5′-methylene nucleotide; 1-(beta-D-erythrofuranosyl) nucleotide; 4′-thio nucleotide, carbocyclic nucleotide; 5′-amino-alkyl phosphate; 1,3-diamino-2-propyl phosphate; 3-aminopropyl phosphate; 6-aminohexyl phosphate; 1,2-aminododecyl phosphate; hydroxypropyl phosphate; 1,5-anhydrohexitol nucleotide; L-nucleotide; alpha-nucleotide; modified base nucleotide; phosphorodithioate; threo-pentofuranosyl nucleotide; acyclic 3′,4′-seco nucleotide; 3,4-dihydroxybutyl nucleotide; 3,5-dihydroxypentyl nucleotide, 5′-5′-inverted nucleotide moiety; 5′-5′-inverted abasic moiety; 5′-phosphoramidate; 5′-phosphorothioate; 1,4-butanediol phosphate; 5′-amino; bridging and/or
non-bridging 5′-phosphoramidate, phosphorothioate and/or phosphorodithioate, bridging or non bridging methylphosphonate and 5′-mercapto moieties (for more details see Beaucage and Jyer, 1993, Tetrahedron 49, 1925; incorporated by reference herein). - By the term “non-nucleotide” is meant any group or compound which can be incorporated into a nucleic acid chain in the place of one or more nucleotide units, including either sugar and/or phosphate substitutions, and allows the remaining bases to exhibit their enzymatic activity. The group or compound is abasic in that it does not contain a commonly recognized nucleotide base, such as adenosine, guanine, cytosine, uracil or thymine and therefore lacks a base at the 1′-position.
- An “alkyl” group refers to a saturated aliphatic hydrocarbon, including straight-chain, branched-chain, and cyclic alkyl groups. Preferably, the alkyl group has 1 to 12 carbons. More preferably, it is a lower alkyl of from 1 to 7 carbons, more preferably 1 to 4 carbons. The alkyl group can be substituted or unsubstituted. When substituted the substituted group(s) is preferably, hydroxyl, cyano, alkoxy, ═O, ═S, NO 2 or N(CH3)2, amino, or SH. The term also includes alkenyl groups that are unsaturated hydrocarbon groups containing at least one carbon-carbon double bond, including straight-chain, branched-chain, and cyclic groups. Preferably, the alkenyl group has 1 to 12 carbons. More preferably, it is a lower alkenyl of from 1 to 7 carbons, more preferably 1 to 4 carbons. The alkenyl group may be substituted or unsubstituted. When substituted the substituted group(s) is preferably, hydroxyl, cyano, alkoxy, ═O, ═S, NO2, halogen, N(CH3)2, amino, or SH. The term “alkyl” also includes alkynyl groups that have an unsaturated hydrocarbon group containing at least one carbon-carbon triple bond, including straight-chain, branched-chain, and cyclic groups. Preferably, the alkynyl group has 1 to 12 carbons. More preferably, it is a lower alkynyl of from 1 to 7 carbons, more preferably 1 to 4 carbons. The alkynyl group may be substituted or unsubstituted. When substituted the substituted group(s) is preferably, hydroxyl, cyano, alkoxy, ═O, ═S, NO2 or N(CH3)2, amino or SH.
- Such alkyl groups can also include aryl, alkylaryl, carbocyclic aryl, heterocyclic aryl, amide and ester groups. An “aryl” group refers to an aromatic group that has at least one ring having a conjugated pi electron system and includes carbocyclic aryl, heterocyclic aryl and biaryl groups, all of which may be optionally substituted. The preferred substituent(s) of aryl groups are halogen, trihalomethyl, hydroxyl, SH, OH, cyano, alkoxy, alkyl, alkenyl, alkynyl, and amino groups. An “alkylaryl” group refers to an alkyl group (as described above) covalently joined to an aryl group (as described above). Carbocyclic aryl groups are groups wherein the ring atoms on the aromatic ring are all carbon atoms. The carbon atoms are optionally substituted. Heterocyclic aryl groups are groups having from 1 to 3 heteroatoms as ring atoms in the aromatic ring and the remainder of the ring atoms are carbon atoms. Suitable heteroatoms include oxygen, sulfur, and nitrogen, and include furanyl, thienyl, pyridyl, pyrrolyl, N-lower alkyl pyrrolo, pyrimidyl, pyrazinyl, imidazolyl and the like, all optionally substituted. An “amide” refers to an —C(O)—NH—R, where R is either alkyl, aryl, alkylaryl or hydrogen. An “ester” refers to an —C(O)—OR′, where R is either alkyl, aryl, alkylaryl or hydrogen.
- By “nucleotide” as used herein is as recognized in the art to include natural bases (standard), and modified bases well known in the art. Such bases are generally located at the 1′ position of a nucleotide sugar moiety. Nucleotides generally comprise a base, sugar and a phosphate group. The nucleotides can be unmodified or modified at the sugar, phosphate and/or base moiety, (also referred to interchangeably as nucleotide analogs, modified nucleotides, non-natural nucleotides, non-standard nucleotides and other; see, for example, Usman and McSwiggen, supra; Eckstein et al., International PCT Publication No. WO 92/07065; Usman et al., International PCT Publication No. WO 93/15187; Uhlman & Peyman, supra, all are hereby incorporated by reference herein). There are several examples of modified nucleic acid bases known in the art as summarized by Limbach et al, 1994, Nucleic Acids Res. 22, 2183. Some of the non-limiting examples of base modifications that can be introduced into nucleic acid molecules include, inosine, purine, pyridin-4-one, pyridin-2-one, phenyl, pseudouracil, 2, 4, 6-trimethoxy benzene, 3-methyl uracil, dihydrouridine, naphthyl, aminophenyl, 5-alkylcytidines (e.g., 5-methylcytidine), 5-alkyluridines (e.g., ribothymidine), 5-halouridine (e.g., 5-bromouridine) or 6-azapyrimidines or 6-alkylpyrimidines (e.g. 6-methyluridine), propyne, and others (Burgin et al., 1996, Biochemistry, 35, 14090; Uhlman & Peyman, supra). By “modified bases” in this aspect is meant nucleotide bases other than adenine, guanine, cytosine and uracil at 1′ position or their equivalents.
- In one embodiment, the invention features modified siRNA molecules, with phosphate backbone modifications comprising one or more phosphorothioate, phosphorodithioate, methylphosphonate, phosphotriester, morpholino, amidate carbamate, carboxymethyl, acetamidate, polyamide, sulfonate, sulfonamide, sulfamate, formacetal, thioformacetal, and/or alkylsilyl, substitutions. For a review of oligonucleotide backbone modifications, see Hunziker and Leumann, 1995, Nucleic Acid Analogues: Synthesis and Properties, in Modern Synthetic Methods, VCH, 331-417, and Mesmaeker et al., 1994, Novel Backbone Replacements for Oligonucleotides, in Carbohydrate Modifications in Antisense Research, ACS, 24-39.
- By “abasic” is meant sugar moieties lacking a base or having other chemical groups in place of a base at the 1′ position, see for example Adamic et al., U.S. Pat. No. 5,998,203.
- By “unmodified nucleoside” is meant one of the bases adenine, cytosine, guanine, thymine, uracil joined to the 1′ carbon of a β-D-ribo-furanose.
- By “modified nucleoside” is meant any nucleotide base which contains a modification in the chemical structure of an unmodified nucleotide base, sugar and/or phosphate.
- In connection with 2′-modified nucleotides as described for the present invention, by “amino” is meant 2′-NH 2 or 2′-O—NH2, which may be modified or unmodified. Such modified groups are described, for example, in Eckstein et al., U.S. Pat. No. 5,672,695 and Matulic-Adamic et al., U.S. Pat. No. 6,248,878, which are both incorporated by reference in their entireties.
- Various modifications to nucleic acid siRNA structure can be made to enhance the utility of these molecules. Such modifications will enhance shelf-life, half-life in vitro, stability, and ease of introduction of such oligonucleotides to the target site, e.g., to enhance penetration of cellular membranes, and confer the ability to recognize and bind to targeted cells.
- A siRNA molecule of the invention can be adapted for use to treat, for example allergic/inflammatory diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and any other indications that can respond to the level of ADORA1 in a cell or tissue, alone or in combination with other therapies. For example, a siRNA molecule can comprise a delivery vehicle, including liposomes, for administration to a subject, carriers and diluents and their salts, and/or can be present in pharmaceutically acceptable formulations. Methods for the delivery of nucleic acid molecules are described in Akhtar et al., 1992, Trends Cell Bio., 2, 139; Delivery Strategies for Antisense Oligonucleotide Therapeutics, ed. Akhtar, 1995, Maurer et al., 1999, Mol. Membr. Biol., 16, 129-140; Hofland and Huang, 1999, Handb. Exp. Pharmacol., 137, 165-192; and Lee et al., 2000, ACS Symp. Ser., 752, 184-192, all of which are incorporated herein by reference. Beigelman et al., U.S. Pat. No. 6,395,713 and Sullivan et al., PCT WO 94/02595, further describes the general methods for delivery of nucleic acid molecules. These protocols can be utilized for the delivery of virtually any nucleic acid molecule. Nucleic acid molecules can be administered to cells by a variety of methods known to those of skill in the art, including, but not restricted to, encapsulation in liposomes, by iontophoresis, or by incorporation into other delivery vehicles, such as hydrogels, cyclodextrins, biodegradable nanocapsules, and bioadhesive microspheres, or by proteinaceous vectors (O'Hare and Normand, International PCT Publication No. WO 00/53722). Alternatively, the nucleic acid/vehicle combination is locally delivered by direct injection or by use of an infusion pump. Direct injection of the nucleic acid molecules of the invention, whether subcutaneous, intramuscular, or intradermal, can take place using standard needle and syringe methodologies, or by needle-free technologies such as those described in Conry et al., 1999, Clin. Cancer Res., 5, 2330-2337 and Barry et al., International PCT Publication No. WO 99/31262. Many examples in the art describe CNS delivery methods of oligonucleotides by osmotic pump, (see Chun et al., 1998, Neuroscience Letters, 257, 135-138, D'Aldin et al., 1998, Mol. Brain Research, 55, 151-164, Dryden et al., 1998, J. Endocrinol., 157, 169-175, Ghimikar et al, 1998, Neuroscience Letters, 247, 21-24) or direct infusion (Broaddus et al., 1997, Neurosurg. Focus, 3, article 4). Other routes of delivery include, but are not limited to oral (tablet or pill form) and/or intrathecal delivery (Gold, 1997, Neuroscience, 76, 1153-1158). More detailed descriptions of nucleic acid delivery and administration are provided in Sullivan et al., supra, Draper et al., PCT W093/23569, Beigelman et al., PCT W099/05094, and Klimuk et al., PCT W099/04819 all of which have been incorporated by reference herein.
- In addition, the invention features the use of methods to deliver the nucleic acid molecules of the instant invention to hematopoietic cells, including monocytes and lymphocytes. These methods are described in detail by Hartmann et al., 1998, J. Phamacol. Exp. Ther., 285(2), 920-928; Kronenwett et al., 1998, Blood, 91(3), 852-862; Filion and Phillips, 1997, Biochim. Biophys. Acta., 1329(2), 345-356; Ma and Wei, 1996, Leuk. Res., 20(11/12), 925-930; and Bongartz et al., 1994, Nucleic Acids Research, 22(22), 4681-8. Such methods, as described above, include the use of free oligonucleitide, cationic lipid formulations, liposome formulations including pH sensitive liposomes and immunoliposomes, and bioconjugates including oligonucleotides conjugated to fusogenic peptides, for the transfection of hematopoietic cells with oligonucleotides.
- Thus, the invention features a pharmaceutical composition comprising one or more nucleic acid(s) of the invention in an acceptable carrier, such as a stabilizer, buffer, and the like. The polynucleotides of the invention can be administered (e.g., RNA, DNA or protein) and introduced into a subject by any standard means, with or without stabilizers, buffers, and the like, to form a pharmaceutical composition. When it is desired to use a liposome delivery mechanism, standard protocols for formation of liposomes can be followed. The compositions of the present invention may also be formulated and used as tablets, capsules or elixirs for oral administration, suppositories for rectal administration, sterile solutions, suspensions for injectable administration, and the other compositions known in the art.
- The present invention also includes pharmaceutically acceptable formulations of the compounds described. These formulations include salts of the above compounds, e.g., acid addition salts, for example, salts of hydrochloric, hydrobromic, acetic acid, and benzene sulfonic acid.
- A pharmacological composition or formulation refers to a composition or formulation in a form suitable for administration, e.g., systemic administration, into a cell or subject, including for example a human. Suitable forms, in part, depend upon the use or the route of entry, for example oral, transdermal, or by injection. Such forms should not prevent the composition or formulation from reaching a target cell (i.e., a cell to which the negatively charged nucleic acid is desirable for delivery). For example, pharmacological compositions injected into the blood stream should be soluble. Other factors are known in the art, and include considerations such as toxicity and forms that prevent the composition or formulation from exerting its effect.
- By “systemic administration” is meant in vivo systemic absorption or accumulation of drugs in the blood stream followed by distribution throughout the entire body. Administration routes which lead to systemic absorption include, without limitation: intravenous, subcutaneous, intraperitoneal, inhalation, oral, intrapulmonary and intramuscular. Each of these administration routes expose the siRNA molecules of the invention to an accessible diseased tissue. The rate of entry of a drug into the circulation has been shown to be a function of molecular weight or size. The use of a liposome or other drug carrier comprising the compounds of the instant invention can potentially localize the drug, for example, in certain tissue types, such as the tissues of the reticular endothelial system (RES). A liposome formulation that can facilitate the association of drug with the surface of cells, such as, lymphocytes and macrophages is also useful. This approach may provide enhanced delivery of the drug to target cells by taking advantage of the specificity of macrophage and lymphocyte immune recognition of abnormal cells, such as cancer cells.
- By “pharmaceutically acceptable formulation” is meant, a composition or formulation that allows for the effective distribution of the nucleic acid molecules of the instant invention in the physical location most suitable for their desired activity. Non-limiting examples of agents suitable for formulation with the nucleic acid molecules of the instant invention include the forulations and conjugates described herein, as well as other target area specific formulations including CNS formulations including P-glycoprotein inhibitors (such as Pluronic P85), which can enhance entry of drugs into the CNS (Jolliet-Riant and Tillement, 1999, Fundam. Clin. Pharmacol., 13, 16-26); biodegradable polymers, such as poly (DL-lactide-coglycolide) microspheres for sustained release delivery after intracerebral implantation (Emerich, D F et al, 1999, Cell Transplant, 8, 47-58) (Alkermes, Inc. Cambridge, Mass.); and loaded nanoparticles, such as those made of polybutylcyanoacrylate, which can deliver drugs across the blood brain barrier and can alter neuronal uptake mechanisms (Prog Neuropsychopharmacol Biol Psychiatry, 23, 941-949, 1999). Other non-limiting examples of delivery strategies for the nucleic acid molecules of the instant invention include material described in Boado et al., 1998, J. Pharm. Sci., 87, 1308-1315; Tyler et al., 1999, FEBS Lett., 421, 280-284; Pardridge et al., 1995, PNAS USA., 92, 5592-5596; Boado, 1995, Adv. Drug Delivery Rev., 15, 73-107; Aldrian-Herrada et al., 1998, Nucleic Acids Res., 26, 4910-4916; and Tyler et al., 1999, PNAS USA., 96, 7053-7058.
- The invention also features the use of the composition comprising surface-modified liposomes containing poly (ethylene glycol) lipids (PEG-modified, or long-circulating liposomes or stealth liposomes). These formulations offer a method for increasing the accumulation of drugs in target tissues. This class of drug carriers resists opsonization and elimination by the mononuclear phagocytic system (MPS or RES), thereby enabling longer blood circulation times and enhanced tissue exposure for the encapsulated drug (Lasic et al. Chem. Rev. 1995, 95, 2601-2627; Ishiwata et al., Chem. Pharm. Bull. 1995, 43, 1005-1011). Such liposomes have been shown to accumulate selectively in tumors, presumably by extravasation and capture in the neovascularized target tissues (Lasic et al., Science 1995, 267, 1275-1276; Oku et al., 1995, Biochim. Biophys. Acta, 1238, 86-90). The long-circulating liposomes enhance the pharmacokinetics and pharmacodynamics of DNA and RNA, particularly compared to conventional cationic liposomes which are known to accumulate in tissues of the MPS (Liu et al., J. Biol. Chem. 1995, 42, 24864-24870; Choi et al., International PCT Publication No. WO 96/10391; Ansell et al., International PCT Publication No. WO 96/10390; Holland et al., International PCT Publication No. WO 96/10392). Long-circulating liposomes are also likely to protect drugs from nuclease degradation to a greater extent compared to cationic liposomes, based on their ability to avoid accumulation in metabolically aggressive MPS tissues such as the liver and spleen.
- The present invention also includes compositions prepared for storage or administration, which include a pharmaceutically effective amount of the desired compounds in a pharmaceutically acceptable carrier or diluent. Acceptable carriers or diluents for therapeutic use are well known in the pharmaceutical art, and are described, for example, in Remington's Pharmaceutical Sciences, Mack Publishing Co. (A. R. Gennaro edit. 1985) hereby incorporated by reference herein. For example, preservatives, stabilizers, dyes and flavoring agents may be provided. These include sodium benzoate, sorbic acid and esters of p-hydroxybenzoic acid. In addition, antioxidants and suspending agents can be used.
- A pharmaceutically effective dose is that dose required to prevent, inhibit the occurrence, or treat (alleviate a symptom to some extent, preferably all of the symptoms) of a disease state. The pharmaceutically effective dose depends on the type of disease, the composition used, the route of administration, the type of mammal being treated, the physical characteristics of the specific mammal under consideration, concurrent medication, and other factors that those skilled in the medical arts will recognize. Generally, an amount between 0.1 mg/kg and 100 mg/kg body weight/day of active ingredients is administered dependent upon potency of the negatively charged polymer.
- The nucleic acid molecules of the invention and formulations thereof can be administered orally, topically, parenterally, by inhalation or spray, or rectally in dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and/or vehicles. The term parenteral as used herein includes percutaneous, subcutaneous, intravascular (e.g., intravenous), intramuscular, or intrathecal injection or infusion techniques and the like. In addition, there is provided a pharmaceutical formulation comprising a nucleic acid molecule of the invention and a pharmaceutically acceptable carrier. One or more nucleic acid molecules of the invention can be present in association with one or more non-toxic pharmaceutically acceptable carriers and/or diluents and/or adjuvants, and if desired other active ingredients. The pharmaceutical compositions containing nucleic acid molecules of the invention can be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion, hard or soft capsules, or syrups or elixirs.
- Compositions intended for oral use can be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions can contain one or more such sweetening agents, flavoring agents, coloring agents or preservative agents in order to provide pharmaceutically elegant and palatable preparations. Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients that are suitable for the manufacture of tablets. These excipients can be, for example, inert diluents; such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia; and lubricating agents, for example magnesium stearate, stearic acid or talc. The tablets can be uncoated or they can be coated by known techniques. In some cases such coatings can be prepared by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period. For example, a time delay material such as glyceryl monosterate or glyceryl distearate can be employed.
- Formulations for oral use can also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin or olive oil.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions. Such excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydropropyl-methylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents can be a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan monooleate. The aqueous suspensions can also contain one or more preservatives, for example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- Oily suspensions can be formulated by suspending the active ingredients in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin. The oily suspensions can contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents and flavoring agents can be added to provide palatable oral preparations. These compositions can be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives. Suitable dispersing or wetting agents or suspending agents are exemplified by those already mentioned above. Additional excipients, for example sweetening, flavoring and coloring agents, can also be present.
- Pharmaceutical compositions of the invention can also be in the form of oil-in-water emulsions. The oily phase can be a vegetable oil or a mineral oil or mixtures of these. Suitable emulsifying agents can be naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol, anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate. The emulsions can also contain sweetening and flavoring agents.
- Syrups and elixirs can be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol, glucose or sucrose. Such formulations can also contain a demulcent, a preservative and flavoring and coloring agents. The pharmaceutical compositions can be in the form of a sterile injectable aqueous or oleaginous suspension. This suspension can be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents that have been mentioned above. The sterile injectable preparation can also be a sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that can be employed are water, Ringer's solution and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose, any bland fixed oil can be employed including synthetic mono-or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of injectables.
- The nucleic acid molecules of the invention can also be administered in the form of suppositories, e.g., for rectal administration of the drug. These compositions can be prepared by mixing the drug with a suitable non-irritating excipient that is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug. Such materials include cocoa butter and polyethylene glycols.
- Nucleic acid molecules of the invention can be administered parenterally in a sterile medium. The drug, depending on the vehicle and concentration used, can either be suspended or dissolved in the vehicle. Advantageously, adjuvants such as local anesthetics, preservatives and buffering agents can be dissolved in the vehicle.
- Dosage levels of the order of from about 0.1 mg to about 140 mg per kilogram of body weight per day are useful in the treatment of the above-indicated conditions (about 0.5 mg to about 7 g per subject per day). The amount of active ingredient that can be combined with the carrier materials to produce a single dosage form varies depending upon the host treated and the particular mode of administration. Dosage unit forms generally contain between from about 1 mg to about 500 mg of an active ingredient.
- It is understood that the specific dose level for any particular subject depends upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diet, time of administration, route of administration, and rate of excretion, drug combination and the severity of the particular disease undergoing therapy.
- For administration to non-human animals, the composition can also be added to the animal feed or drinking water. It can be convenient to formulate the animal feed and drinking water compositions so that the animal takes in a therapeutically appropriate quantity of the composition along with its diet. It can also be convenient to present the composition as a premix for addition to the feed or drinking water.
- The nucleic acid molecules of the present invention may also be administered to a subject in combination with other therapeutic compounds to increase the overall therapeutic effect. The use of multiple compounds to treat an indication may increase the beneficial effects while reducing the presence of side effects.
- In one embodiment, the invention compositions suitable for administering nucleic acid molecules of the invention to specific cell types. For example, the asialoglycoprotein receptor (ASGPr) (Wu and Wu, 1987, J. Biol. Chem. 262, 4429-4432) is unique to hepatocytes and binds branched galactose-terminal glycoproteins, such as asialoorosomucoid (ASOR). Binding of such glycoproteins or synthetic glycoconjugates to the receptor takes place with an affinity that strongly depends on the degree of branching of the oligosaccharide chain, for example, triatennary structures are bound with greater affinity than biatenarry or monoatennary chains (Baenziger and Fiete, 1980, Cell, 22, 611-620; Connolly et al., 1982, J. Biol. Chem., 257, 939-945). Lee and Lee, 1987, Glycoconjugate J., 4, 317-328, obtained this high specificity through the use of N-acetyl-D-galactosamine as the carbohydrate moiety, which has higher affinity for the receptor, compared to galactose. This “clustering effect” has also been described for the binding and uptake of mannosyl-terminating glycoproteins or glycoconjugates (Ponpipom et al., 1981, J. Med. Chem., 24, 1388-1395). The use of galactose and galactosamine based conjugates to transport exogenous compounds across cell membranes can provide a targeted delivery approach to the treatment of liver disease or hepatocellular carcinoma. The use of bioconjugates can also provide a reduction in the required dose of therapeutic compounds required for treatment. Furthermore, therapeutic bioavialability, pharmacodynamics, and pharmacokinetic parameters can be modulated through the use of nucleic acid bioconjugates of the invention. Non-limiting examples of such bioconjugates are described in Vargeese et al., U.S. Ser. No. 60/311,865, filed Aug. 13, 2001; and Matulic-Adamic et al., U.S. Ser. No. 60/362,016, filed Mar. 6, 2002.
- Alternatively, certain siRNA molecules of the instant invention can be expressed within cells from eukaryotic promoters (e.g., Izant and Weintraub, 1985, Science, 229, 345; McGarry and Lindquist, 1986, Proc. Natl. Acad. Sci., USA 83, 399; Scanlon et al., 1991, Proc. Natl. Acad. Sci. USA, 88, 10591-5; Kashani-Sabet et al., 1992, Antisense Res. Dev., 2, 3-15; Dropulic et al., 1992, J. Virol., 66, 1432-41; Weerasinghe et al., 1991, J. Virol., 65, 5531-4; Ojwang et al., 1992, Proc. Natl. Acad. Sci. USA, 89, 10802-6; Chen et al., 1992, Nucleic Acids Res., 20, 4581-9; Sarver et al., 1990 Science, 247, 1222-1225; Thompson et al., 1995, Nucleic Acids Res., 23, 2259; Good et al., 1997, Gene Therapy, 4, 45. Those skilled in the art realize that any nucleic acid can be expressed in eukaryotic cells from the appropriate DNA/RNA vector. The activity of such nucleic acids can be augmented by their release from the primary transcript by a enzymatic nucleic acid (Draper et al., PCT WO 93/23569, and Sullivan et al., PCT WO 94/02595; Ohkawa et al., 1992, Nucleic Acids Symp. Ser., 27, 15-6; Taira et al., 1991, Nucleic Acids Res., 19, 5125-30; Ventura et al., 1993, Nucleic Acids Res., 21, 3249-55; Chowrira et al., 1994, J. Biol Chem., 269, 25856.
- In another aspect of the invention, RNA molecules of the present invention can be expressed from transcription units (see for example Couture et al., 1996, TIG., 12, 510) inserted into DNA or RNA vectors. The recombinant vectors can be DNA plasmids or viral vectors. siRNA expressing viral vectors can be constructed based on, but not limited to, adeno-associated virus, retrovirus, adenovirus, or alphavirus. In another embodiment, pol III based constructs are used to express nucleic acid molecules of the invention (see for example Thompson, U.S. Pat. Nos. 5,902,880 and 6,146,886). The recombinant vectors capable of expressing the siRNA molecules can be delivered as described above, and persist in target cells. Alternatively, viral vectors can be used that provide for transient expression of nucleic acid molecules. Such vectors can be repeatedly administered as necessary. Once expressed, the siRNA molecule interacts with the target mRNA and generates an RNAi response. Delivery of siRNA molecule expressing vectors can be systemic, such as by intravenous or intra-muscular administration, by administration to target cells ex-planted from a subject followed by reintroduction into the subject, or by any other means that would allow for introduction into the desired target cell (for a review see Couture et al., 1996, TIG., 12, 510).
- In one aspect the invention features an expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of the instant invention. The expression vector can encode one or both strands of a siRNA duplex, or a single self complementary strand that self hybridizes into a siRNA duplex. The nucleic acid sequences encoding the siRNA molecules of the instant invention can be operably linked in a manner that allows expression of the siRNA molecule (see for example Paul et al., 2002, Nature Biotechnology, 19, 505; Miyagishi and Taira, 2002, Nature Biotechnology, 19, 497; Lee et al., 2002, Nature Biotechnology, 19, 500; and Novina et al., 2002, Nature Medicine, advance online publication doi: 10.1038/nm725).
- In another aspect, the invention features an expression vector comprising: a) a transcription initiation region (e.g., eukaryotic pol I, II or III initiation region); b) a transcription termination region (e.g., eukaryotic pol I, II or III termination region); and c) a nucleic acid sequence encoding at least one of the siRNA molecules of the instant invention; wherein said sequence is operably linked to said initiation region and said termination region, in a manner that allows expression and/or delivery of the siRNA molecule. The vector can optionally include an open reading frame (ORF) for a protein operably linked on the 5′ side or the 3′-side of the sequence encoding the siRNA of the invention; and/or an intron (intervening sequences).
- Transcription of the siRNA molecule sequences can be driven from a promoter for eukaryotic RNA polymerase I (pol I), RNA polymerase II (pol II), or RNA polymerase III (pol III). Transcripts from pol II or pol III promoters are expressed at high levels in all cells; the levels of a given pol II promoter in a given cell type depends on the nature of the gene regulatory sequences (enhancers, silencers, etc.) present nearby. Prokaryotic RNA polymerase promoters are also used, providing that the prokaryotic RNA polymerase enzyme is expressed in the appropriate cells (Elroy-Stein and Moss, 1990, Proc. Natl. Acad. Sci. US A, 87, 6743-7; Gao and Huang 1993, Nucleic Acids Res., 21, 2867-72; Lieber et al., 1993, Methods Enzymol., 217, 47-66; Zhou et al., 1990, Mol. Cell. Biol., 10, 4529-37). Several investigators have demonstrated that nucleic acid molecules expressed from such promoters can function in mammalian cells (e.g. Kashani-Sabet et al., 1992, Antisense Res. Dev., 2, 3-15; Ojwang et al., 1992, Proc. Natl. Acad. Sci. U S A, 89, 10802-6; Chen et al., 1992, Nucleic Acids Res., 20, 4581-9; Yu et al., 1993, Proc. Natl. Acad. Sci. U S A, 90, 6340-4; L'Huillier et al., 1992, EMBO J., 11, 4411-8; Lisziewicz et al., 1993, Proc. Natl. Acad. Sci. U. S. A, 90, 8000-4; Thompson et al., 1995, Nucleic Acids Res., 23, 2259; Sullenger & Cech, 1993, Science, 262, 1566). More specifically, transcription units such as the ones derived from genes encoding U6 small nuclear (snRNA), transfer RNA (tRNA) and adenovirus VA RNA are useful in generating high concentrations of desired RNA molecules such as siRNA in cells (Thompson et al., supra; Couture and Stinchcomb, 1996, supra; Noonberg et al., 1994, Nucleic Acid Res., 22, 2830; Noonberg et al., U.S. Pat. No. 5,624,803; Good et al, 1997, Gene Ther., 4, 45; Beigelman et al., International PCT Publication No. WO 96/18736. The above siRNA transcription units can be incorporated into a variety of vectors for introduction into mammalian cells, including but not restricted to, plasmid DNA vectors, viral DNA vectors (such as adenovirus or adeno-associated virus vectors), or viral RNA vectors (such as retroviral or alphavirus vectors) (for a review see Couture and Stinchcomb, 1996, supra).
- In another aspect the invention features an expression vector comprising a nucleic acid sequence encoding at least one of the siRNA molecules of the invention, in a manner that allows expression of that siRNA molecule. The expression vector comprises in one embodiment; a) a transcription initiation region; b) a transcription termination region; and c) a nucleic acid sequence encoding at least one strand of the siRNA molecule; wherein the sequence is operably linked to the initiation region and the termination region, in a manner that allows expression and/or delivery of the siRNA molecule.
- In another embodiment the expression vector comprises: a) a transcription initiation region; b) a transcription termination region; c) an open reading frame; and d) a nucleic acid sequence encoding at least one strand of a siRNA molecule, wherein the sequence is operably linked to the 3′-end of the open reading frame; and wherein the sequence is operably linked to the initiation region, the open reading frame and the termination region, in a manner that allows expression and/or delivery of the siRNA molecule. In yet another embodiment the expression vector comprises: a) a transcription initiation region; b) a transcription termination region; c) an intron; and d) a nucleic acid sequence encoding at least one siRNA molecule; wherein the sequence is operably linked to the initiation region, the intron and the termination region, in a manner which allows expression and/or delivery of the nucleic acid molecule.
- In another embodiment, the expression vector comprises: a) a transcription initiation region; b) a transcription termination region; c) an intron; d) an open reading frame; and e) a nucleic acid sequence encoding at least one strand of a siRNA molecule, wherein the sequence is operably linked to the 3′-end of the open reading frame; and wherein the sequence is operably linked to the initiation region, the intron, the open reading frame and the termination region, in a manner which allows expression and/or delivery of the siRNA molecule.
- The following are non-limiting examples showing the selection, isolation, synthesis and activity of nucleic acids of the instant invention.
- Exemplary siRNA molecules of the invention are synthesized in tandem using a cleavable linker, for example a succinyl-based linker. Tandem synthesis as described herein is followed by a one step purification process that provides RNAi molecules in high yield. This approach is highly amenable to siRNA synthesis in support of high throughput RNAi screening, and can be readily adapted to multi-column or multi-well synthesis platforms.
- After completing a tandem synthesis of an siRNA oligo and its compliment in which the 5′-terminal dimethoxytrityl (5′-O-DMT) group remains intact (trityl on synthesis), the oligonucleotides are deprotected as described above. Following deprotection, the siRNA sequence strands are allowed to spontaneously hybridize. This hybridization yields a duplex in which one strand has retained the 5′-O-DMT group while the complementary strand comprises a
terminal 5′-hydroxyl. The newly formed duplex to behaves as a single molecule during routine solid-phase extraction purification (Trityl-On purification) even though only one molecule has a dimethoxytrityl group. Because the strands form a stable duplex, this dimethoxytrityl group (or an equivalent group, such as other trityl groups or other hydrophobic moieties) is all that is required to purify the pair of oligos, for example by using a C18 cartridge. - Standard phosphoramidite synthesis chemistry is used up to point of introducing a tandem linker, such as an inverted deoxyabasic succinate linker (see FIG. 1) or an equivalent cleavable linker. A non-limiting example of linker coupling conditions that can be used includes a hindered base such as diisopropylethylamine (DIPA) and/or DMAP in the presence of an activator reagent such as Bromotripyrrolidinophosphoniumhexaflurorophosphate (PyBrOP). After the linker is coupled, standard synthesis chemistry is utilized to complete synthesis of the second sequence leaving the terminal the 5′-O-DMT intact. Following synthesis, the resulting oligonucleotide is deprotected according to the procedures described herein and quenched with a suitable buffer, for example with 50 mM NaOAc or 1.5M NH 4H2CO3.
- Purification of the siRNA duplex can be readily accomplished using solid phase extraction, for example using a Waters C18 SepPak 1 g cartridge conditioned with 1 column volume (CV) of acetonitrile, 2 CV H2O, and 2
CV 50 mM NaOAc. The sample is loaded and then washed with 1 CV H2O or 50 mM NaOAc. Failure sequences are eluted with 1 CV 14% ACN (Aqueous with 50 mM NaOAc and 50mM NaCl). The column is then washed, for example with 1 CV H2O followed by on-column detritylation, for example by passing 1 CV of 1% aqueous trifluoroacetic acid (TFA) over the column, then adding a second CV of 1% aqueous TFA to the column and allowing to stand for approx. 10 minutes. The remaining TFA solution is removed and the column washed with H2O followed by 1 CV 1M NaCl and additional H2O. The siRNA duplex product is then eluted, for example using 1CV 20% aqueous CAN. - FIG. 2 provides an example of MALDI-TOV mass spectrometry analysis of a purified siRNA construct in which each peak corresponds to the calculated mass of an individual siRNA strand of the siRNA duplex. The same purified siRNA provides three peaks when analyzed by capillary gel electrophoresis (CGE), one peak presumably corresponding to the duplex siRNA, and two peaks presumably corresponding to the separate siRNA sequence strands. Ion exchange HPLC analysis of the same siRNA contract only shows a single peak.
- The sequence of an RNA target of interest, such as a human mRNA transcript, is screened for target sites, for example by using a computer folding algorithm. In a non-limiting example, the sequence of a gene or RNA gene transcript derived from a database, such as Genbank, is used to generate siRNA targets having complimentarity to the target. Such sequences can be obtained from a database, or can be determined experimentally as known in the art. Target sites that are known, for example, those target sites determined to be effective target sites based on studies with other nucleic acid molecules, for example ribozymes or antisense, or those targets known to be associated with a disease or condition such as those sites containing mutations or deletions, can be used to design siRNA molecules targeting those sites as well. Various parameters can be used to determine which sites are the most suitable target sites within the target RNA sequence. These parameters include but are not limited to secondary or tertiary RNA structure, the nucleotide base composition of the target sequence, the degree of homology between various regions of the target sequence, or the relative position of the target sequence within the RNA transcript. Based on these determinations, any number of target sites within the RNA transcript can be chosen to screen siRNA molecules for efficacy, for example by using in vitro RNA cleavage assays, cell culture, or animal models. In a non-limiting example, anywhere from 1 to 1000 target sites are chosen within the transcript based on the size of the siRNA contruct to be used. High throughput screening assays can be developed for screening siRNA molecules using methods known in the art, such as with multi-well or multi-plate assays to determine efficient reduction in target gene expression.
- The following non-limiting steps can be used to carry out the selection of siRNAs targeting a given gene sequence or transcipt.
- 1. The target sequence is parsed in silico into a list of all fragments or subsequences of a particular length, for example 23 nucleotide fragments, contained within the target sequence. This step is typically carried out using a custom Perl script, but commercial sequence analysis programs such as Oligo, MacVector, or the GCG Wisconsin Package can be employed as well.
- 2. In some instances the siRNAs correspond to more than one target sequence; such would be the case for example in targeting different transcipts of the same gene, targeting different transcipts of more than one gene, or for targeting both the human gene and an animal homolog. In this case, a subsequence list of a particular length is generated for each of the targets, and then the lists are compared to find matching sequences in each list. The subsequences are then ranked according to the number of target sequences that contain the given subsequence; the goal is to find subsequences that are present in most or all of the target sequences. Alternately, the ranking can indentify subsequences that are unique to a target sequence, such as a mutant target sequence. Such an approach would enable the use of siRNA to target specifically the mutant sequence and not effect the expression of the normal sequence.
- 3. In some instances the siRNA subsequences are absent in one or more sequences while present in the desired target sequence; such would be the case if the siRNA targets a gene with a paralogous family member that is to remain untargeted. As in
case 2 above, a subsequence list of a particular length is generated for each of the targets, and then the lists are compared to find sequences that are present in the target gene but are absent in the untargeted paralog. - 4. The ranked siRNA subsequences can be further analyzed and ranked according to GC content. A preference can be given to sites containing 30-70% GC, with a further preference to sites containing 40-60% GC.
- 5. The ranked siRNA subsequences can be further analyzed and ranked according to self-folding and internal hairpins. Weaker internal folds are preferred; strong hairpin structures are to be avoided.
- 6. The ranked siRNA subsequences can be further analyzed and ranked according to whether they have runs of GGG or CCC in the sequence. GGG (or even more Gs) in either strand can make oligonucleotide synthesis problematic, so it is avoided whenever better sequences are available. CCC is searched in the target strand because that will place GGG in the antisense strand.
- 7. The ranked siRNA subsequences can be further analyzed and ranked according to whether they have the dinucleotide UU (uridine dinucleotide) on the 3′ end of the sequence, and/or AA on the 5′ end of the sequence (to yield 3′ UU on the antisense sequence). These sequences allow one to design siRNA molecules with terminal TT thymidine dinucleotides.
- 8. Four or five target sites are chosen from the ranked list of subsequences as described above. For example, in subsequences having 23 nucleotides, the right 21 nucleotides of each chosen 23-mer subsequence are then designed and synthesized for the upper (sense) strand of the siRNA duplex, while the reverse complement of the left 21 nucleotides of each chosen 23-mer subsequence are then designed and synthesized for the lower (antisense) strand of the siRNA duplex. If terminal TT residues are desired for the sequence (as described in paragraph 7), then the two 3′ terminal nucleotides of both the sense and antisense strands are replaced by TT prior to synthesizing the oligos.
- 9. The siRNA molecules are screened in an in vitro, cell culture or animal model system to identify the most active siRNA molecule or the most preferred target site within the target RNA sequence.
- In an alternate approach, a pool of siRNA constructs specific to an ADORA1 target sequence is used to screen for target sites in cells expressing ADORA1 RNA, such as human lung mast cells. The general strategy used in this approach is shown in FIG. 9. A non-limiting example of such as pool is a pool comprising sequences having sense sequences comprising SEQ ID NOs. 1-161 and antisense sequences comprising SEQ ID NOs. 162-322 respectively. Human lung mast cells expressing ADORA1 are transfected with the pool of siRNA constructs and cells that demonstrate a phenotype associated with ADORA1 inhibition are sorted. The pool of siRNA constructs can be expressed from transciption cassettes inserted into appropriate vectors (see for example FIG. 7 and FIG. 8). The siRNA from cells demonstrating a positive phenotypic change (e.g., decreased adenosine receptor expression, for example as determined by a [ 3H]DPCPX binding assay as described in Nyce and Metzger, 1997, Nature, 385, 721-725), are sequenced to determine the most suitable target site(s) within the target ADORA1 RNA sequence.
- siRNA target sites were chosen by analyzing sequences of the ADORA1 RNA target and optionally prioritizing the target sites on the basis of folding (structure of any given sequence analyzed to determine siRNA accessibility to the target), using a library of siRNA molecules as described in Example 3, or alternately by using an in vitro siRNA system as described in Example 6 herein. siRNA molecules were designed that could bind each target and are optionally individually analyzed by computer folding to assess whether the siRNA molecule can interact with the target sequence. Varying the length of the siRNA molecules can be chosen to optimize activity. Generally, a sufficient number of complimentary nucleotide bases are chosen to bind to, or otherwise interact with, the target RNA, but the degree of complementarity can be modulated to accommodate siRNA duplexes or varying length or base composition. By using such methodologies, siRNA molecules can be designed to target sites within any known RNA sequence, for example those RNA sequences corresponding to the any gene transcript.
- siRNA molecules can be designed to interact with various sites in the RNA message, for example target sequences within the RNA sequences described herein. The sequence of one strand of the siRNA molecule(s) are complementary to the target site sequences described above. The siRNA molecules can be chemically synthesized using methods described herein. Inactive siRNA molecules that are used as control sequences can be synthesized by scrambling the sequence of the siRNA molecules such that it is not complimentary to the target sequence.
- An in vitro assay that recapitulates RNAi in a cell free system is used to evaluate siRNA constructs targeting ADORA1 RNA targets. The assay comprises the system described by Tuschl et al., 1999, Genes and Development, 13, 3191-3197 and Zamore et al., 2000, Cell, 101, 25-33 adapted for use with ADORA1 target RNA. A Drosophila extract derived from syncytial blastoderm is used to reconstitute RNAi activity in vitro. Target RNA is generated via in vitro transcription from an appropriate ADORA1 expressing plasmid using T7 RNA polymerase or via chemical synthesis as described herein. Sense and antisense siRNA strands (for example 20 uM each) are annealed by incubation in buffer (such as 100 mM potassium acetate, 30 mM HEPES-KOH, pH 7.4, 2 mM magnesium acetate) for 1 min. at 90° C. followed by 1 hour at 37° C., then diluted in lysis buffer (for example 100 mM potassium acetate, 30 mM HEPES-KOH at pH 7.4, 2 mM magnesium acetate). Annealing can be monitored by gel electrophoresis on an agarose gel in TBE buffer and stained with ethidium bromide. The Drosophila lysate is prepared using zero to two hour old embryos from Oregon R flies collected on yeasted molasses agar that are dechorionated and lysed. The lysate is centrifuged and the supernatant isolated. The assay comprises a reaction mixture containing 50% lysate [vol/vol], RNA (10-50 pM final concentration), and 10% [vol/vol] lysis buffer containing siRNA (10 nM final concentration). The reaction mixture also contains 10 mM creatine phosphate, 10 ug.ml creatine phosphokinase, 100 um GTP, 100 uM UTP, 100 uM CTP, 500 uM ATP, 5 mM DTT, 0.1 U/uL RNasin (Promega), and 100 uM of each amino acid. The final concentration of potassium acetate is adjusted to 100 mM. The reactions are pre-assembled on ice and preincubated at 25° C. for 10 minutes before adding RNA, then incubated at 25° C. for an additional 60 minutes. Reactions are quenched with 4 volumes of 1.25× Passive Lysis Buffer (Promega). Target RNA cleavage is assayed by RT-PCR analysis or other methods known in the art and are compared to control reactions in which siRNA is omitted from the reaction.
- Alternately, internally-labeled target RNA for the assay is prepared by in vitro transcription in the presence of [a- 32P] CTP, passed over a
G 50 Sephadex column by spin chromatography and used as target RNA without further purification. Optionally, target RNA is 5′-32P-end labeled using T4 polynucleotide kinase enzyme. Assays are performed as described above and target RNA and the specific RNA cleavage products generated by RNAi are visualized on an autoradiograph of a gel. The percentage of cleavage is determined by Phosphor Imager® quantitation of bands representing intact control RNA or RNA from control reactions without siRNA and the cleavage products generated by the assay. - In one embodiment, this assay is used to determine target sites the ADORA1 RNA target for siRNA mediated RNAi cleavage, wherein a plurality of siRNA constructs are screened for RNAi mediated cleavage of the ADORA1 RNA target, for example by analysing the assay reaction by electrophoresis of labelled target RNA, or by northern blotting, as well as by other methodology well known in the art.
- siRNA molecules targeted to the human ADORA1 RNA are designed and synthesized as described above. These nucleic acid molecules can be tested for cleavage activity in vivo, for example, using the following procedure. The target sequences and the nucleotide location within the ADORA1 RNA are given in Table I and III.
- Two formats are used to test the efficacy of siRNAs targeting ADORA1. First, the reagents are tested on human lung epithelial cells (e.g., A549), to determine the extent of RNA and protein inhibition. siRNA reagents (e.g.; see Table I, and III) are selected against the ADORA1 target. RNA inhibition is measured after delivery of these reagents by a suitable transfection agent to human lung epithelial cells. Relative amounts of target RNA are measured versus actin using real-time PCR monitoring of amplification (eg. ABI 7700 Taqman®). A comparison is made to a mixture of oligonucleotide sequences made to unrelated targets or to a randomized siRNA control with the same overall length and chemistry, but randomly substituted at each position. Primary and secondary lead reagents are chosen for the target and optimization performed. After an optimal transfection agent concentration is chosen, a RNA time-course of inhibition is performed with the lead siRNA molecule. In addition, a cell-plating format can be used to determine RNA inhibition.
- Human lung epithelial cells (e.g., A549) are seeded, for example, at 1×10 5 cells per well of a six well dish in EGM-2 (BioWhittaker) the day before transfection. siRNA (final concentration, for example 20 nM) and cationic lipid (e.g.,
final concentration 2 μg/ml) are complexed in EGM basal media (Biowhittaker) at 37° C. for 30 mins in polystyrene tubes. Following vortexing, the complexed siRNA is added to each well and incubated for the times indicated. For initial optimization experiments, cells are seeded, for example, at 1×103 in 96 well plates and siRNA complex added as described. Efficiency of delivery of siRNA to A549 is determined using a fluorescent siRNA complexed with lipid. A549 in 6 well dishes are incubated with siRNA for 24 hours, rinsed with PBS and fixed in 2% paraformaldehyde for 15 minutes at room temperature. Uptake of siRNA is visualised using a fluorescent microscope. - Total RNA is prepared from cells following siRNA delivery, for example using Qiagen RNA purification kits for 6 well or Rneasy extraction kits for 96 well assays. For Taqman analysis, dual-labeled probes are synthesized with the reporter dye, FAM or JOE, covalently linked at the 5′ end and the quencher dye TAMRA conjugated to the 3′ end. One-step RT-PCR amplifications are performed on, for example, an ABI PRISM 7700 Sequence Detector using 50 μl reactions consisting of 10 μl total RNA, 100 nM forward primer, 900 nM reverse primer, 100 nM probe, 1× TaqMan PCR reaction buffer (PE-Applied Biosystems), 5.5 mM MgCl 2, 300 μM each dATP, dCTP, dGTP, and dTTP, 10U RNase Inhibitor (Promega), 1.25U AmpliTaq Gold (PE-Applied Biosystems) and 10U M-MLV Reverse Transcriptase (Promega). The thermal cycling conditions can consist of 30 min at 48° C., 10 min at 95° C., followed by 40 cycles of 15 sec at 95° C. and 1 min at 60° C. Quantitation of mRNA levels are determined relative to standards generated from serially diluted total cellular RNA (300, 100, 33, 11 ng/rxn) and normalizing to β-actin or GAPDH mRNA in parallel TaqMan reactions. For each gene of interest an upper and lower primer and a flourescently labelled probe are designed. Real time incorporation of SYBR Green I dye into a specific PCR product can be measured in glass capillary tubes using a lightcyler. A standard curve is generated for each primer pair using control c RNA allularnd values are represented as relative expression to GAPDH in each sample.
- Nuclear extracts can be prepared using a standard micropreparation technique (see for example Andrews and Faller, 1991, Nucleic Acids Research, 19, 2499). Protein extracts from supernatants are prepared, for example using TCA precipitation. An equal volume of 20% TCA is added to the cell supernatant, incubated on ice for 1 hour and pelleted by centrifugation for 5 minutes. Pellets are washed in acetone, dried and resuspended in water. Cellular protein extracts are run on a 10% Bis-Tris NuPage (nuclear extracts) or 4-12% Tris-Glycine (supernatant extracts) polyacrylamide gel and transferred onto nitro-cellulose membranes. Non-specific binding can be blocked by incubation, for example, with 5% non-fat milk for 1 hour followed by primary antibody for 16 hour at 4° C. Following washes, the secondary antibody is applied, for example (1:10,000 dilution) for 1 hour at room temperature and the signal detected with SuperSignal reagent (Pierce).
- Evaluating the efficacy of anti-ADORA-1 agents (e.g., siRNA) in animal models is an important prerequisite to human clinical trials. Nyce and Metzger, 1997, Nature, 385, 721-725, describe a useful dust mite conditioned allergic rabbit model of human asthma. Allergic rabbits treated with aerosolized siRNA are compared to untreated controls or animals treated with a non-specific siRNA constrol with regard to adenosine challenge. The concentration of aerolsolized adenosine required to reduce the dynamic compliance of the
bronchial airway 50% from a baseline values is determined in both groups of animals. Additionally, dose response studies using this same endpoint are performed. Airway smooth muscle is surgically dissected from the animals and is processed for quantitative assessment of adenosine A1 receptors. As a control for specificity, adenosine A2 receptors and/or bradykinin receptors are quantitated as well. Adenosine A1 receptor density can be assayed by specific binding of a [3H]DPCPX. A dose dependent reduction in adenosine A1 receptor densitiy is indicative of a therapeutic response This model can be used to evaluate animals that are treated with nucleic acid molecules of the invention and can furthermore be used as a positive control in determining the response of animals treated with nucleic acid molecules of the invention by using such factors as airway obstruction, lung capacity, and bronchiolar alveolar lavage (BAL) fluid in the evaluation. - Human epithelial lung cell lines, such as NPE cells and NCB-20 cells, can be used to express ADORA1. Cloned human ADORA1 is therefore expressed in CHO and COS7 cells and used in various studies. These ADORA1 expressing lung cell lines can be used in cell culture assays to evaluate nucleic acid molecules of the invention. A primary endpoint in these experiments would be the RT-PCR analysis of ADORA1 ntRNA expression in ADORA1 expressing cells. In addition, ligand binding assays can be developed where binding of [ 3H]DPCPX can be evaluated in response to treatment with nucleic acid molecules of the invention.
- The present body of knowledge in ADORA1 research indicates the need for methods to assay ADORA1 activity and for compounds that can regulate ADORA1 expression for research, diagnostic, and therapeutic use. As described herein, the nucleic acid molecules of the present invention can be used in assays to diagnose disease state related of ADORA1 levels. In addition, the nucleic acid molecules can be used to treat disease state related to ADORA1 levels.
- Particular degenerative and disease states that can be associated with ADORA1 levels include, but are not limited to allergic diseases and conditions, including but not limited to asthma, allergic rhinitis, atopic dermatitis, and any other diseases or conditions that are related to or will respond to the levels of ADORA1 in a cell or tissue, alone or in combination with other therapies.
- The use of anti-inflammatories, bronchodilators, adenosine inhibitors and adenosine A1 receptor inhibitors are examples of other treatments or therapies can be combined with the nucleic acid molecules of the invention. Those skilled in the art will recognize that other drug compounds and therapies can be similarly be readily combined with the nucleic acid molecules of the instant invention (e.g., siRNA molecules) are hence within the scope of the instant invention.
- The siRNA molecules of the invention can be used in a variety of diagnostic applications, such as in identifying molecular targets such as RNA in a variety of applications, for example, in clinical, industrial, environmental, agricultural and/or research settings. Such diagnostic use of siRNA molecules involves utilizing reconstituted RNAi systems, for example using cellular lysates or partially purified cellular lysates. siRNA molecules of this invention may be used as diagnostic tools to examine genetic drift and mutations within diseased cells or to detect the presence of endogenous or exogenous, for example viral, RNA in a cell. The close relationship between siRNA activity and the structure of the target RNA allows the detection of mutations in any region of the molecule, which alters the base-pairing and three-dimensional structure of the target RNA. By using multiple siRNA molecules described in this invention, one may map nucleotide changes, which are important to RNA structure and function in vitro, as well as in cells and tissues. Cleavage of target RNAs with siRNA molecules can be used to inhibit gene expression and define the role (essentially) of specified gene products in the progression of disease or infection. In this manner, other genetic targets may be defined as important mediators of the disease. These experiments will lead to better treatment of the disease progression by affording the possibility of combination therapies (e.g., multiple siRNA molecules targeted to different genes, siRNA molecules coupled with known small molecule inhibitors, or intermittent treatment with combinations siRNA molecules and/or other chemical or biological molecules). Other in vitro uses of siRNA molecules of this invention are well known in the art, and include detection of the presence of mRNAs associated with a disease, infection, or related condition. Such RNA is detected by determining the presence of a cleavage product after treatment with a siRNA using standard methodologies, for example fluorescence resonance emission transfer (FRET).
- In a specific example, siRNA molecules that can cleave only wild-type or mutant forms of the target RNA are used for the assay. The first siRNA molecules is used to identify wild-type RNA present in the sample and the second siRNA molecules will be used to identify mutant RNA in the sample. As reaction controls, synthetic substrates of both wild-type and mutant RNA will be cleaved by both siRNA molecules to demonstrate the relative siRNA efficiencies in the reactions and the absence of cleavage of the “non-targeted” RNA species. The cleavage products from the synthetic substrates will also serve to generate size markers for the analysis of wild-type and mutant RNAs in the sample population. Thus each analysis will require two siRNA molecules, two substrates and one unknown sample which will be combined into six reactions. The presence of cleavage products will be determined using an RNase protection assay so that full-length and cleavage fragments of each RNA can be analyzed in one lane of a polyacrylamide gel. It is not absolutely required to quantify the results to gain insight into the expression of mutant RNAs and putative risk of the desired phenotypic changes in target cells. The expression of mRNA whose protein product is implicated in the development of the phenotype (i.e., disease related or infection related) is adequate to establish risk. If probes of comparable specific activity are used for both transcripts, then a qualitative comparison of RNA levels will be adequate and will decrease the cost of the initial diagnosis. Higher mutant form to wild-type ratios will be correlated with higher risk whether RNA levels are compared qualitatively or quantitatively.
- All patents and publications mentioned in the specification are indicative of the levels of skill of those skilled in the art to which the invention pertains. All references cited in this disclosure are incorporated by reference to the same extent as if each reference had been incorporated by reference in its entirety individually.
- One skilled in the art would readily appreciate that the present invention is well adapted to carry out the objects and obtain the ends and advantages mentioned, as well as those inherent therein. The methods and compositions described herein as presently representative of preferred embodiments are exemplary and are not intended as limitations on the scope of the invention. Changes therein and other uses will occur to those skilled in the art, which are encompassed within the spirit of the invention, are defined by the scope of the claims.
- It will be readily apparent to one skilled in the art that varying substitutions and modifications may be made to the invention disclosed herein without departing from the scope and spirit of the invention. Thus, such additional embodiments are within the scope of the present invention and the following claims.
- The invention illustratively described herein suitably may be practiced in the absence of any element or elements, limitation or limitations that are not specifically disclosed herein. Thus, for example, in each instance herein any of the terms “comprising”, “consisting essentially of” and “consisting of” may be replaced with either of the other two terms. The terms and expressions which have been employed are used as terms of description and not of limitation, and there is no intention that in the use of such terms and expressions of excluding any equivalents of the features shown and described or portions thereof, but it is recognized that various modifications are possible within the scope of the invention claimed. Thus, it should be understood that although the present invention has been specifically disclosed by preferred embodiments, optional features, modification and variation of the concepts herein disclosed may be resorted to by those skilled in the art, and that such modifications and variations are considered to be within the scope of this invention as defined by the description and the appended claims.
- In addition, where features or aspects of the invention are described in terms of Markush groups or other grouping of alternatives, those skilled in the art will recognize that the invention is also thereby described in terms of any individual member or subgroup of members of the Markush group or other group.
TABLE I ADORA1 target and siRNA sequences (5′-3′) Seq Seq Seq Pos Target Sequence ID UPos Upper seq ID LPos Lower seq ID 3 GAGUGUCAGAAGUGUGAAG 1 3 GAGUGUCAGAAGUGUGAAG 1 21 CUUCACACUUCUGACACUC 162 21 GGGUGCCUGUUCUGAAUCC 2 21 GGGUGCCUGUUCUGAAUCC 2 39 GGAUUCAGAACAGGCACCC 163 39 CCAGAGCCUCCUCUCCCUC 3 39 CCAGAGCCUCCUCUCCCUC 3 57 GAGGGAGAGGAGGCUCUGG 164 57 CUGUGAGGCUGGCAGGUGA 4 57 CUGUGAGGCUGGCAGGUGA 4 75 UCACCUGCCAGCCUCACAG 165 75 AGGAAGGGUUUAACCUCAC 5 75 AGGAAGGGUUUAACCUCAC 5 93 GUGAGGUUAAACCCUUCCU 166 93 CUGGAAGGAAUCCCUGGAG 6 93 CUGGAAGGAAUCCCUGGAG 6 111 CUCCAGGGAUUCCUUCCAG 167 111 GCUAGCGGCUGCUGAAGGC 7 111 GCUAGCGGCUGCUGAAGGC 7 129 GCCUUCAGCAGCCGCUAGC 168 129 CGUCGAGGUGUGGGGGCAC 8 129 CGUCGAGGUGUGGGGGCAC 8 147 GUGCCCCCACACCUCGACG 169 147 CUUGGACAGAACAGUCAGG 9 147 CUUGGACAGAACAGUCAGG 9 165 CCUGACUGUUCUGUCCAAG 170 165 GCAGCCGGGAGCUCUGCCA 10 165 GCAGCCGGGAGCUCUGCCA 10 183 UGGCAGAGCUCCCGGCUGC 171 183 AGCUUUGGUGACCUUGGGC 11 183 AGCUUUGGUGACCUUGGGC 11 201 GCCCAAGGUCACCAAAGCU 172 201 CCGGGCUGGGAGCGCUGCG 12 201 CCGGGCUGGGAGCGCUGCG 12 219 CGCAGCGCUCCCAGCCCGG 173 219 GGCGGGAGCCGGAGGACUA 13 219 GGCGGGAGCCGGAGGACUA 13 237 UAGUCCUCCGGCUCCCGCC 174 237 AUGAGCUGCCGCGCGUUGU 14 237 AUGAGCUGCCGCGCGUUGU 14 255 ACAACGCGCGGCAGCUCAU 175 255 UCCAGAGCCCAGCCCAGCC 15 255 UCCAGAGCCCAGCCCAGCC 15 273 GGCUGGGCUGGGCUCUGGA 176 273 CCUACGCGCGCGGCCCGGA 16 273 CCUACGCGCGCGGCCCGGA 16 291 UCCGGGCCGCGCGCGUAGG 177 291 AGCUCUGUUCCCUGGAACU 17 291 AGCUCUGUUCCCUGGAACU 17 309 AGUUCCAGGGAACAGAGCU 178 309 UUUGGGCACUGCCUCUGGG 18 309 UUUGGGCACUGCCUCUGGG 18 327 CCCAGAGGCAGUGCCCAAA 179 327 GACCCCUGCCGGCCAGCAG 19 327 GACCCCUGCCGGCCAGCAG 19 345 CUGCUGGCCGGCAGGGGUC 180 345 GGCAGGAUGGUGCUUGCCU 20 345 GGCAGGAUGGUGCUUGCCU 20 363 AGGCAAGCACCAUCCUGCC 181 363 UCGUGCCCCUUGGUGCCCG 21 363 UCGUGCCCCUUGGUGCCCG 21 381 CGGGCACCAAGGGGCACGA 182 381 GUCUGCUGAUGUGCCCAGC 22 381 GUCUGCUGAUGUGCCCAGC 22 399 GCUGGGCACAUCAGCAGAC 183 399 CCUGUGCCCGCCAUGCCGC 23 399 CCUGUGCCCGCCAUGCCGC 23 417 GCGGCAUGGCGGGCACAGG 184 417 CCCUCCAUCUCAGCUUUCC 24 417 CCCUCCAUCUCAGCUUUCC 24 435 GGAAAGCUGAGAUGGAGGG 185 435 CAGGCCGCCUACAUCGGCA 25 435 CAGGCCGCCUACAUCGGCA 25 453 UGCCGAUGUAGGCGGCCUG 186 453 AUCGAGGUGCUCAUCGCCC 26 453 AUCGAGGUGCUCAUCGCCC 26 471 GGGCGAUGAGCACCUCGAU 187 471 CUGGUCUCUGUGCCCGGGA 27 471 CUGGUCUCUGUGCCCGGGA 27 489 UCCCGGGCACAGAGACCAG 188 489 AACGUGCUGGUGAUCUGGG 28 489 AACGUGCUGGUGAUCUGGG 28 507 CCCAGAUCACCAGCACGUU 189 507 GCGGUGAAGGUGAACCAGG 29 507 GCGGUGAAGGUGAACCAGG 29 525 CCUGGUUCACCUUCACCGC 190 525 GCGCUGCGGGAUGCCACCU 30 525 GCGCUGCGGGAUGCCACCU 30 543 AGGUGGCAUCCCGCAGCGC 191 543 UUCUGCUUCAUCGUGUCGC 31 543 UUCUGCUUCAUCGUGUCGC 31 561 GCGACACGAUGAAGCAGAA 192 561 CUGGCGGUGGCUGAUGUGG 32 561 CUGGCGGUGGCUGAUGUGG 32 579 CCACAUCAGCCACCGCCAG 193 579 GCCGUGGGUGCCCUGGUCA 33 579 GCCGUGGGUGCCCUGGUCA 33 597 UGACCAGGGCACCCACGGC 194 597 AUCCCCCUCGCCAUCCUCA 34 597 AUCCCCCUCGCCAUCCUCA 34 615 UGAGGAUGGCGAGGGGGAU 195 615 AUCAACAUUGGGCCACAGA 35 615 AUCAACAUUGGGCCACAGA 35 633 UCUGUGGCCCAAUGUUGAU 196 633 ACCUACUUCCACACCUGCC 36 633 ACCUACUUCCACACCUGCC 36 651 GGCAGGUGUGGAAGUAGGU 197 651 CUCAUGGUUGCCUGUCCGG 37 651 CUCAUGGUUGCCUGUCCGG 37 669 CCGGACAGGCAACCAUGAG 198 669 GUCCUCAUCCUCACCCAGA 38 669 GUCCUCAUCCUCACCCAGA 38 687 UCUGGGUGAGGAUGAGGAC 199 687 AGCUCCAUCCUGGCCCUGC 39 687 AGCUCCAUCCUGGCCCUGC 39 705 GCAGGGCCAGGAUGGAGCU 200 705 CUGGCAAUUGCUGUGGACC 40 705 CUGGCAAUUGCUGUGGACC 40 723 GGUCCACAGCAAUUGCCAG 201 723 CGCUACCUCCGGGUCAAGA 41 723 CGCUACCUCCGGGUCAAGA 41 741 UCUUGACCCGGAGGUAGCG 202 741 AUCCCUCUCCGGUACAAGA 42 741 AUCCCUCUCCGGUACAAGA 42 759 UCUUGUACCGGAGAGGGAU 203 759 AUGGUGGUGACCCCCCGGA 43 759 AUGGUGGUGACCCCCCGGA 43 777 UCCGGGGGGUCACCACCAU 204 777 AGGGCGGCGGUGGCCAUAG 44 777 AGGGCGGCGGUGGCCAUAG 44 795 CUAUGGCCACCGCCGCCCU 205 795 GCCGGCUGCUGGAUCCUCU 45 795 GCCGGCUGCUGGAUCCUCU 45 813 AGAGGAUCCAGCAGCCGGC 206 813 UCCUUCGUGGUGGGACUGA 46 813 UCCUUCGUGGUGGGACUGA 46 831 UCAGUCCCACCACGAAGGA 207 831 ACCCCUAUGUUUGGCUGGA 47 831 ACCCCUAUGUUUGGCUGGA 47 849 UCCAGCCAAACAUAGGGGU 208 849 AACAAUCUGAGUGCGGUGG 48 849 AACAAUCUGAGUGCGGUGG 48 867 CCACCGCACUCAGAUUGUU 209 867 GAGCGGGCCUGGGCAGCCA 49 867 GAGCGGGCCUGGGCAGCCA 49 885 UGGCUGCCCAGGCCCGCUC 210 885 AACGGCAGCAUGGGGGAGC 50 885 AACGGCAGCAUGGGGGAGC 50 903 GCUCCCCCAUGCUGCCGUU 211 903 CCCGUGAUCAAGUGCGAGU 51 903 CCCGUGAUCAAGUGCGAGU 51 921 ACUCGCACUUGAUCACGGG 212 921 UUCGAGAAGGUCAUCAGCA 52 921 UUCGAGAAGGUCAUCAGCA 52 939 UGCUGAUGACCUUCUCGAA 213 939 AUGGAGUACAUGGUCUACU 53 939 AUGGAGUACAUGGUCUACU 53 957 AGUAGACCAUGUACUCCAU 214 957 UUCAACUUCUUUGUGUGGG 54 957 UUCAACUUCUUUGUGUGGG 54 975 CCCACACAAAGAAGUUGAA 215 975 GUGCUGCCCCCGCUUCUCC 55 975 GUGCUGCCCCCGCUUCUCC 55 993 GGAGAAGCGGGGGCAGCAC 216 993 CUCAUGGUCCUCAUCUACC 56 993 CUCAUGGUCCUCAUCUACC 56 1011 GGUAGAUGAGGACCAUGAG 217 1011 CUGGAGGUCUUCUACCUAA 57 1011 CUGGAGGUCUUCUACCUAA 57 1029 UUAGGUAGAAGACCUCCAG 218 1029 AUCCGCAAGCAGCUCAACA 58 1029 AUCCGCAAGCAGCUCAACA 58 1047 UGUUGAGCUGCUUGCGGAU 219 1047 AAGAAGGUGUCGGCCUCCU 59 1047 AAGAAGGUGUCGGCCUCCU 59 1065 AGGAGGCCGACACCUUCUU 220 1065 UCCGGCGACCCGCAGAAGU 60 1065 UCCGGCGACCCGCAGAAGU 60 1083 ACUUCUGCGGGUCGCCGGA 221 1083 UACUAUGGGAAGGAGCUGA 61 1083 UACUAUGGGAAGGAGCUGA 61 1101 UCAGCUCCUUCCCAUAGUA 222 1101 AAGAUCGCCAAGUCGCUGG 62 1101 AAGAUCGCCAAGUCGCUGG 62 1119 CCAGCGACUUGGCGAUCUU 223 1119 GCCCUCAUCCUCUUCCUCU 63 1119 GCCCUCAUCCUCUUCCUCU 63 1137 AGAGGAAGAGGAUGAGGGC 224 1137 UUUGCCCUCAGCUGGCUGC 64 1137 UUUGCCCUCAGCUGGCUGC 64 1155 GCAGCCAGCUGAGGGCAAA 225 1155 CCUUUGCACAUCCUCAACU 65 1155 CCUUUGCACAUCCUCAACU 65 1173 AGUUGAGGAUGUGCAAAGG 226 1173 UGCAUCACCCUCUUCUGCC 66 1173 UGCAUCACCCUCUUCUGCC 66 1191 GGCAGAAGAGGGUGAUGCA 227 1191 CCGUCCUGCCACAAGCCCA 67 1191 CCGUCCUGCCACAAGCCCA 67 1209 UGGGCUUGUGGCAGGACGG 228 1209 AGCAUCCUUACCUACAUUG 68 1209 AGCAUCCUUACCUACAUUG 68 1227 CAAUGUAGGUAAGGAUGCU 229 1227 GCCAUCUUCCUCACGCACG 69 1227 GCCAUCUUCCUCACGCACG 69 1245 CGUGCGUGAGGAAGAUGGC 230 1245 GGCAACUCGGCCAUGAACC 70 1245 GGCAACUCGGCCAUGAACC 70 1263 GGUUCAUGGCCGAGUUGCC 231 1263 CCCAUUGUCUAUGCCUUCC 71 1263 CCCAUUGUCUAUGCCUUCC 71 1281 GGAAGGCAUAGACAAUGGG 232 1281 CGCAUCCAGAAGUUCCGCG 72 1281 CGCAUCCAGAAGUUCCGCG 72 1299 CGCGGAACUUCUGGAUGCG 233 1299 GUCACCUUCCUUAAGAUUU 73 1299 GUCACCUUCCUUAAGAUUU 73 1317 AAAUCUUAAGGAAGGUGAC 234 1317 UGGAAUGACCAUUUCCGCU 74 1317 UGGAAUGACCAUUUCCGCU 74 1335 AGCGGAAAUGGUCAUUCCA 235 1335 UGCCAGCCUGCACCUCCCA 75 1335 UGCCAGCCUGCACCUCCCA 75 1353 UGGGAGGUGCAGGCUGGCA 236 1353 AUUGACGAGGAUCUCCCAG 76 1353 AUUGACGAGGAUCUCCCAG 76 1371 CUGGGAGAUCCUCGUCAAU 237 1371 GAAGAGAGGCCUGAUGACU 77 1371 GAAGAGAGGCCUGAUGACU 77 1389 AGUCAUCAGGCCUCUCUUC 238 1389 UAGACCCCGCCUUCCGCUC 78 1389 UAGACCCCGCCUUCCGCUC 78 1407 GAGCGGAAGGCGGGGUCUA 239 1407 CCCACCAGCCCACAUCCAG 79 1407 CCCACCAGCCCACAUCCAG 79 1425 CUGGAUGUGGGCUGGUGGG 240 1425 GUGGGGUCUCAGUCCAGUC 80 1425 GUGGGGUCUCAGUCCAGUC 80 1443 GACUGGACUGAGACCCCAC 241 1443 CCUCACAUGCCCGCUGUCC 81 1443 CCUCACAUGCCCGCUGUCC 81 1461 GGACAGCGGGCAUGUGAGG 242 1461 CCAGGGGUCUCCCUGAGCC 82 1461 CCAGGGGUCUCCCUGAGCC 82 1479 GGCUCAGGGAGACCCCUGG 243 1479 CUGCCCCAGCUGGGCUGUU 83 1479 CUGCCCCAGCUGGGCUGUU 83 1497 AACAGCCCAGCUGGGGCAG 244 1497 UGGCUGGGGGCAUGGGGGA 84 1497 UGGCUGGGGGCAUGGGGGA 84 1515 UCCCCCAUGCCCCCAGCCA 245 1515 AGGCUCUGAAGAGAUACCC 85 1515 AGGCUCUGAAGAGAUACCC 85 1533 GGGUAUCUCUUCAGAGCCU 246 1533 CACAGAGUGUGGUCCCUCC 86 1533 CACAGAGUGUGGUCCCUCC 86 1551 GGAGGGACCACACUCUGUG 247 1551 CACUAGGAGUUAACUACCC 87 1551 CACUAGGAGUUAACUACCC 87 1569 GGGUAGUUAACUCCUAGUG 248 1569 CUACACCUCUGGGCCCUGC 88 1569 CUACACCUCUGGGCCCUGC 88 1587 GCAGGGCCCAGAGGUGUAG 249 1587 CAGGAGGCCUGGGAGGGCA 89 1587 CAGGAGGCCUGGGAGGGCA 89 1605 UGCCCUCCCAGGCCUCCUG 250 1605 AAGGGUCCUACGGAGGGAC 90 1605 AAGGGUCCUACGGAGGGAC 90 1623 GUCCCUCCGUAGGACCCUU 251 1623 CCAGGUGUCUAGAGGCAAC 91 1623 CCAGGUGUCUAGAGGCAAC 91 1641 GUUGCCUCUAGACACCUGG 252 1641 CAGUGUUCUGAGCCCCCAC 92 1641 CAGUGUUCUGAGCCCCCAC 92 1659 GUGGGGGCUCAGAACACUG 253 1659 CCUGCCUGACCAUCCCAUG 93 1659 CCUGCCUGACCAUCCCAUG 93 1677 CAUGGGAUGGUCAGGCAGG 254 1677 GAGCAGUCCAGCGCUUCAG 94 1677 GAGCAGUCCAGCGCUUCAG 94 1695 CUGAAGCGCUGGACUGCUC 255 1695 GGGCUGGGCAGGUCCUGGG 95 1695 GGGCUGGGCAGGUCCUGGG 95 1713 CCCAGGACCUGCCCAGCCC 256 1713 GGAGGCUGAGACUGCAGAG 96 1713 GGAGGCUGAGACUGCAGAG 96 1731 CUCUGCAGUCUCAGCCUCC 257 1731 GGAGCCACCUGGGCUGGGA 97 1731 GGAGCCACCUGGGCUGGGA 97 1749 UCCCAGCCCAGGUGGCUCC 258 1749 AGAAGGUGCUUGGGCUUCU 98 1749 AGAAGGUGCUUGGGCUUCU 98 1767 AGAAGCCCAAGCACCUUCU 259 1767 UGCGGUGAGGCAGGGGAGU 99 1767 UGCGGUGAGGCAGGGGAGU 99 1785 ACUCCCCUGCCUCACCGCA 260 1785 UCUGCUUGUCUUAGAUGUU 100 1785 UCUGCUUGUCUUAGAUGUU 100 1803 AACAUCUAAGACAAGCAGA 261 1803 UGGUGGUGCAGCCCCAGGA 101 1803 UGGUGGUGCAGCCCCAGGA 101 1821 UCCUGGGGCUGCACCACCA 262 1821 ACCAAGCUUAAGGAGAGGA 102 1821 ACCAAGCUUAAGGAGAGGA 102 1839 UCCUCUCCUUAAGCUUGGU 263 1839 AGAGCAUCUGCUCUGAGAC 103 1839 AGAGCAUCUGCUCUGAGAC 103 1857 GUCUCAGAGCAGAUGCUCU 264 1857 CGGAUGGAAGGAGAGAGGU 104 1857 CGGAUGGAAGGAGAGAGGU 104 1875 ACCUCUCUCCUUCCAUCCG 265 1875 UUGAGGAUGCACUGGCCUG 105 1875 UUGAGGAUGCACUGGCCUG 105 1893 CAGGCCAGUGCAUCCUCAA 266 1893 GUUCUGUAGGAGAGACUGG 106 1893 GUUCUGUAGGAGAGACUGG 106 1911 CCAGUCUCUCCUACAGAAC 267 1911 GCCAGAGGCAGCUAAGGGG 107 1911 GCCAGAGGCAGCUAAGGGG 107 1929 CCCCUUAGCUGCCUCUGGC 268 1929 GCAGGAAUCAAGGAGCCUC 108 1929 GCAGGAAUCAAGGAGCCUC 108 1947 GAGGCUCCUUGAUUCCUGC 269 1947 CCGUUCCCACCUCUGAGGA 109 1947 CCGUUCCCACCUCUGAGGA 109 1965 UCCUCAGAGGUGGGAACGG 270 1965 ACUCUGGACCCCAGGCCAU 110 1965 ACUCUGGACCCCAGGCCAU 110 1983 AUGGCCUGGGGUCCAGAGU 271 1983 UACCAGGUGCUAGGGUGCC 111 1983 UACCAGGUGCUAGGGUGCC 111 2001 GGCACCCUAGCACCUGGUA 272 2001 CUGCUCUCCUUGCCCUGGG 112 2001 CUGCUCUCCUUGCCCUGGG 112 2019 CCCAGGGCAAGGAGAGCAG 273 2019 GCCAGCCCAGGAUUGUACG 113 2019 GCCAGCCCAGGAUUGUACG 113 2037 CGUACAAUCCUGGGCUGGC 274 2037 GUGGGAGAGGCAGAAAGGG 114 2037 GUGGGAGAGGCAGAAAGGG 114 2055 CCCUUUCUGCCUCUCCCAC 275 2055 GUAGGUUCAGUAAUCAUUU 115 2055 GUAGGUUCAGUAAUCAUUU 115 2073 AAAUGAUUACUGAACCUAC 276 2073 UCUGAUGAUUUGCUGGAGU 116 2073 UCUGAUGAUUUGCUGGAGU 116 2091 ACUCCAGCAAAUCAUCAGA 277 2091 UGCUGGCUCCACGCCCUGG 117 2091 UGCUGGCUCCACGCCCUGG 117 2109 CCAGGGCGUGGAGCCAGCA 278 2109 GGGAGUGAGCUUGGUGCGG 118 2109 GGGAGUGAGCUUGGUGCGG 118 2127 CCGCACCAAGCUCACUCCC 279 2127 GUAGGUGCUGGCCUCAAAC 119 2127 GUAGGUGCUGGCCUCAAAC 119 2145 GUUUGAGGCCAGCACCUAC 280 2145 CAGCCACGAGGUGGUAGCU 120 2145 CAGCCACGAGGUGGUAGCU 120 2163 AGCUACCACCUCGUGGCUG 281 2163 UCUGAGCCCUCCUUCUUGC 121 2163 UCUGAGCCCUCCUUCUUGC 121 2181 GCAAGAAGGAGGGCUCAGA 282 2181 CCCUGAGCUUUCCGGGGAG 122 2181 CCCUGAGCUUUCCGGGGAG 122 2199 CUCCCCGGAAAGCUCAGGG 283 2199 GGAGCCUGGAGUGUAAUUA 123 2199 GGAGCCUGGAGUGUAAUUA 123 2217 UAAUUACACUCCAGGCUCC 284 2217 ACCUGUCAUCUGGGCCACC 124 2217 ACCUGUCAUCUGGGCCACC 124 2235 GGUGGCCCAGAUGACAGGU 285 2235 CAGCUCCACUGGCCCCCGU 125 2235 CAGCUCCACUGGCCCCCGU 125 2253 ACGGGGGCCAGUGGAGCUG 286 2253 UUGCCGGGCCUGGACUGUC 126 2253 UUGCCGGGCCUGGACUGUC 126 2271 GACAGUCCAGGCCCGGCAA 287 2271 CCUAGGUGACCCCAUCUCU 127 2271 CCUAGGUGACCCCAUCUCU 127 2289 AGAGAUGGGGUCACCUAGG 288 2289 UGCUGCUUCUGGGCCUGAU 128 2289 UGCUGCUUCUGGGCCUGAU 128 2307 AUCAGGCCCAGAAGCAGCA 289 2307 UGGAGAGGAGAACACUAGA 129 2307 UGGAGAGGAGAACACUAGA 129 2325 UCUAGUGUUCUCCUCUCCA 290 2325 ACAUGCCAACUCGGGAGCA 130 2325 ACAUGCCAACUCGGGAGCA 130 2343 UGCUCCCGAGUUGGCAUGU 291 2343 AUUCUGCCUGCCUGGGAAC 131 2343 AUUCUGCCUGCCUGGGAAC 131 2361 GUUCCCAGGCAGGCAGAAU 292 2361 CGGGGUGGACGAGGGAGUG 132 2361 CGGGGUGGACGAGGGAGUG 132 2379 CACUCCCUCGUCCACCCCG 293 2379 GUCUGUAAGGACUCAGUGU 133 2379 GUCUGUAAGGACUCAGUGU 133 2397 ACACUGAGUCCUUACAGAC 294 2397 UUGACUGUAGGCGCCCCUG 134 2397 UUGACUGUAGGCGCCCCUG 134 2415 CAGGGGCGCCUACAGUCAA 295 2415 GGGGUGGGUUUAGCAGGCU 135 2415 GGGGUGGGUUUAGCAGGCU 135 2433 AGCCUGCUAAACCCACCCC 296 2433 UGCAGCAGGCAGAGGAGGA 136 2433 UGCAGCAGGCAGAGGAGGA 136 2451 UCCUCCUCUGCCUGCUGCA 297 2451 AGUACCCCCCUGAGAGCAU 137 2451 AGUACCCCCCUGAGAGCAU 137 2469 AUGCUCUCAGGGGGGUACU 298 2469 UGUGGGGGAAGGCCUUGCU 138 2469 UGUGGGGGAAGGCCUUGCU 138 2487 AGCAAGGCCUUCCCCCACA 299 2487 UGUCAUGUGAAUCCCUCAA 139 2487 UGUCAUGUGAAUCCCUCAA 139 2505 UUGAGGGAUUCACAUGACA 300 2505 AUACCCCUAGUAUCUGGCU 140 2505 AUACCCCUAGUAUCUGGCU 140 2523 AGCCAGAUACUAGGGGUAU 301 2523 UGGGUUUUCAGGGGCUUUG 141 2523 UGGGUUUUCAGGGGCUUUG 141 2541 CAAAGCCCCUGAAAACCCA 302 2541 GGAAGCUCUGUUGCAGGUG 142 2541 GGAAGCUCUGUUGCAGGUG 142 2559 CACCUGCAACAGAGCUUCC 303 2559 GUCCGGGGGUCUAGGACUU 143 2559 GUCCGGGGGUCUAGGACUU 143 2577 AAGUCCUAGACCCCCGGAC 304 2577 UUAGGGAUCUGGGAUCUGG 144 2577 UUAGGGAUCUGGGAUCUGG 144 2595 CCAGAUCCCAGAUCCCUAA 305 2595 GGGAAGGACCAACCCAUGC 145 2595 GGGAAGGACCAACCCAUGC 145 2613 GCAUGGGUUGGUCCUUCCC 306 2613 CCCUGCCAAGCCUGGAGCC 146 2613 CCCUGCCAAGCCUGGAGCC 146 2631 GGCUCCAGGCUUGGCAGGG 307 2631 CCCUGUGUUGGGGGGCAAG 147 2631 CCCUGUGUUGGGGGGCAAG 147 2649 CUUGCCCCCCAACACAGGG 308 2649 GGUGGGGGAGCCUGGAGCC 148 2649 GGUGGGGGAGCCUGGAGCC 148 2667 GGCUCCAGGCUCCCCCACC 309 2667 CCCUGUGUGGGAGGGCGAG 149 2667 CCCUGUGUGGGAGGGCGAG 149 2685 CUCGCCCUCCCACACAGGG 310 2685 GGCGGGGGAGCCUGGAGCC 150 2685 GGCGGGGGAGCCUGGAGCC 150 2703 GGCUCCAGGCUCCCCCGCC 311 2703 CCCUGUGUGGGAGGGCGAG 151 2703 CCCUGUGUGGGAGGGCGAG 151 2721 CUCGCCCUCCCACACAGGG 312 2721 GGCGGGGGAUCCUGGAGCC 152 2721 GGCGGGGGAUCCUGGAGCC 152 2739 GGCUCCAGGAUCCCCCGCC 313 2739 CCCUGUGUCGGGGGGCGAG 153 2739 CCCUGUGUCGGGGGGCGAG 153 2757 CUCGCCCCCCGACACAGGG 314 2757 GGGAGGGGAGGUGGCCGUC 154 2757 GGGAGGGGAGGUGGCCGUC 154 2775 GACGGCCACCUCCCCUCCC 315 2775 CGGUUGACCUUCUGAACAU 155 2775 CGGUUGACCUUCUGAACAU 155 2793 AUGUUCAGAAGGUCAACCG 316 2793 UGAGUGUCAACUCCAGGAC 156 2793 UGAGUGUCAACUCCAGGAC 156 2811 GUCCUGGAGUUGACACUCA 317 2811 CUUGCUUCCAAGCCCUUCC 157 2811 CUUGCUUCCAAGCCCUUCC 157 2829 GGAAGGGCUUGGAAGCAAG 318 2829 CCUCUGUUGGAAAUUGGGU 158 2829 CCUCUGUUGGAAAUUGGGU 158 2847 ACCCAAUUUCCAACAGAGG 319 2847 UGUGCCCUGGCUCCCAAGG 159 2847 UGUGCCCUGGCUCCCAAGG 159 2865 CCUUGGGAGCCAGGGCACA 320 2865 GGAGGCCCAUGUGACUAAU 160 2865 GGAGGCCCAUGUGACUAAU 160 2883 AUUAGUCACAUGGGCCUCC 321 2880 UAAUAAAAAACUGUGAACC 161 2880 UAAUAAAAAACUGUGAACC 161 2898 GGUUCACAGUUUUUUAUUA 322 # the lower sequence is also referred to as the antisense strand. -
TABLE II Wait Time* 2′-O- Reagent Equivalents Amount Wait Time* DNA methyl Wait Time* RNA A. 2.5 μmol Synthesis Cycle ABI 394 Instrument Phosphoramidites 6.5 163 μL 45 sec 2.5 min 7.5 min S-Ethyl Tetrazole 23.8 238 μL 45 sec 2.5 min 7.5 min Acetic Anhydride 100 233 μL 5 sec 5 sec 5 sec N-Methyl 186 233 μL 5 sec 5 sec 5 sec Imidazole TCA 176 2.3 mL 21 sec 21 sec 21 sec Iodine 11.2 1.7 mL 45 sec 45 sec 45 sec Beaucage 12.9 645 μL 100 sec 300 sec 300 sec Acetonitrile NA 6.67 mL NA NA NA B. 0.2 μmol Synthesis Cycle ABI 394 Instrument Phosphoramidites 15 31 μL 45 sec 233 sec 465 sec S-Ethyl Tetrazole 38.7 31 μL 45 sec 233 min 465 sec Acetic Anhydride 655 124 μL 5 sec 5 sec 5 sec N-Methyl 1245 124 μL 5 sec 5 sec 5 sec Imidazole TCA 700 732 μL 10 sec 10 sec 10 sec Iodine 20.6 244 μL 15 sec 15 sec 15 sec Beaucage 7.7 232 μL 100 sec 300 sec 300 sec Acetonitrile NA 2.64 mL NA NA NA C. 0.2 μmol Synthesis Cycle 96 well Instrument Equivalents: DNA/ Amount: DNA/2′-O- Wait Time* 2′-O- Reagent 2′-O-methyl/Ribo methyl/Ribo Wait Time* DNA methyl Wait Time* Ribo Phosphoramidites 22/33/66 40/60/120 μL 60 sec 180 sec 360 sec S-Ethyl Tetrazole 70/105/210 40/60/120 μL 60 sec 180 min 360 sec Acetic Anhydride 265/265/265 50/50/50 μL 10 sec 10 sec 10 sec N-Methyl 502/502/502 50/50/50 μL 10 sec 10 sec 10 sec Imidazole TCA 238/475/475 250/500/500 μL 15 sec 15 sec 15 sec Iodine 6.8/6.8/6.8 80/80/80 μL 30 sec 30 sec 30 sec Beaucage 34/51/51 80/120/120 100 sec 200 sec 200 sec Acetonitrile NA 1150/1150/1150 μL NA NA NA -
TABLE III Chemically Modified siRNAs Target Pos Aliases Sequence (5′-3′) Seq ID Strand 1819 ADORA1:1821U21 siRNA stab4 B AccAAGcuuAAGGAGAGGAGA B 347 Upper 919 ADORA1:921U21 siRNA stab4 B uucGAGAAGGucAucAGcAuG B 349 Upper 1621 ADORA1:1623U21 siRNA stab4 B ccAGGuGucuAGAGGcAAcAG B 351 Upper 2773 ADORA1:2775U21 siRNA stab4 B cGGuuGAccuucuGAAcAuGA B 353 Upper 1819 ADORA1:1839L21 siRNA (1821C) stab5 uccucuccuuAAGcuuGGuTsT 348 Lower 919 ADORA1:939L21 siRNA (921C) stab5 uGcuGAuGAccuucucGAATsT 350 Lower 1621 ADORA1:1641L21 siRNA(1623C) stab5 GuuGccucuAGAcAceuGGTsT 352 Lower 2773 ADORA1:2793L21 siRNA (2775C) stab5 AuGuucAGAAGGucAAccGTsT 354 Lower
Claims (36)
1. A short interfering RNA (siRNA) molecule that down regulates expression of an ADORA1 gene by RNA interference.
2. The siRNA molecule of claim 1 , wherein said siRNA molecule is adapted for use to treat asthma.
3. The siRNA molecule of claim 1 , wherein said siRNA molecule comprises a sense region and an antisense region and wherein said antisense region comprises sequence complementary to an RNA sequence encoding ADORA1 and the sense region comprises sequence complementary to the antisense region.
4. The siRNA molecule of claim 3 , wherein said siRNA molecule is assembled from two nucleic acid fragments wherein one fragment comprises the sense region and the second fragment comprises the antisense region of said siRNA molecule.
5. The siRNA molecule of claim 4 , wherein said sense region and antisense region are covalently connected via a linker molecule.
6. The siRNA molecule of claim 5 , wherein said linker molecule is a polynucleotide linker.
7. The siRNA molecule of claim 5 , wherein said linker molecule is a non-nucleotide linker.
8. The siRNA molecule of claim 3 , wherein said antisense region comprises sequence complementary to sequence having any of SEQ ID NOs. 1-161.
9. The siRNA molecule of claim 3 , wherein said antisense region comprises sequence having any of SEQ ID NOs. 162-322, 336, 338, 340, 342, 344, or 346.
10. The siRNA molecule of claim 3 , wherein said sense region comprises sequence having any of SEQ ID NOs. 1-161, 335, 337, 339, 341, 343, or 345.
11. The siRNA molecule of claim 3 , wherein said sense region comprises a sequence of SEQ ID NO. 323 and said antisense region comprises a sequence of SEQ ID NO. 324.
12. The siRNA molecule of claim 3 , wherein said sense region comprises a sequence of SEQ ID NO. 325 and said antisense region comprises a sequence of SEQ ID NO. 326.
13. The siRNA molecule of claim 3 , wherein said sense region comprises a sequence of SEQ ID NO. 327 and said antisense region comprises a sequence of SEQ ID NO. 328.
14. The siRNA molecule of claim 3 , wherein said sense region comprises a sequence of SEQ ID NO. 329 and said antisense region comprises a sequence of SEQ ID NO. 330.
15. The siRNA molecule of claim 3 , wherein said sense region comprises a sequence of SEQ ID NO. 331 and said antisense region comprises a sequence of SEQ ID NO. 332.
16. The siRNA molecule of claim 3 , wherein said sense region comprises a sequence of SEQ ID NO. 333 and said antisense region comprises a sequence of SEQ ID NO. 334.
17. The siRNA molecule of claim 3 , wherein said sense region comprises a 3′-terminal overhang and said antisense region comprises a 3′-terminal overhang.
18. The siRNA molecule of claim 17 , wherein said 3′-terminal overhangs each comprise about 2 nucleotides.
19. The siRNA molecule of claim 17 , wherein said antisense region 3′-terminal nucleotide overhang is complementary to RNA encoding ADORA1.
20. The siRNA molecule of claim 3 , wherein said sense region comprises one or more 2′-O-methyl modified pyrimidine nucleotides.
21. The siRNA molecule of claim 3 , wherein said sense region comprises a terminal cap moiety at the 5′-end, 3′-end, or both 5′ and 3′ ends of said sense region.
22. The siRNA molecule of claim 3 , wherein said antisense region comprises one or more 2′-deoxy-2′-fluoro modified pyrimidine nucleotides.
23. The siRNA molecule of claim 3 , wherein said antisense region comprises a phosphorothioate internucleotide linkage at the 3′ end of said antisense region.
24. The siRNA molecule of claim 3 , wherein said antisense region comprises between about one and about five phosphorothioate internucleotide linkages at the 5′ end of said antisense region.
25. The siRNA molecule of claim 17 , wherein said 3′-terminal nucleotide overhangs comprise ribonucleotides that are chemically modified at a nucleic acid sugar, base, or backbone.
26. The siRNA molecule of claim 17 , wherein said 3′-terminal nucleotide overhangs comprise deoxyribonucleotides that are chemically modified at a nucleic acid sugar, base, or backbone.
27. The siRNA molecule of claim 17 , wherein said 3′-terminal nucleotide overhangs comprise one or more universal base ribonucleotides.
28. The siRNA molecule of claim 17 , wherein said 3′-terminal nucleotide overhangs comprise one or more acyclic nucleotides.
29. The siRNA molecule of claim 17 , wherein said 3′-terminal nucleotide overhangs comprise nucleotides comprising internucleotide linkages having Formula I:
wherein each R1 and R2 is independently any nucleotide, non-nucleotide, or polynucleotide which can be naturally occurring or chemically modified, each X and Y is independently O, S, N, alkyl, or substituted alkyl, each Z and W is independently O, S, N, alkyl, substituted alkyl, O-alkyl, S-alkyl, alkaryl, or aralkyl, and wherein W, X, Y and Z are not all O.
30. The siRNA molecule of claim 17 , wherein said 3′-terminal nucleotide overhangs comprise nucleotides or non-nucleotides having Formula II:
wherein each R3, R4, R5, R6, R7, R8, R10, R11 and R12 is independently H, OH, alkyl substituted alkyl, alkaryl or aralkyl, F, Cl, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, N-alkyl, O-alkenyl, S-alkenyl, N-alkenyl, SO-alkyl, alkyl-OSH, alkyl-OH, O-alkyl-OH, O-alkyl-SH, S-alkyl-OH, S-alkyl-SH, alkyl-S-alkyl, alkyl-O-alkyl, ONO2, NO2, N3, NH2, aminoalkyl, aminoacid, aminoacyl, ONH2, O-aminoalkyl, O-aminoacid, O-aminoacyl, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalklylamino, substituted silyl, or group having Formula I; R9 is O, S, CH2, S═O, CHF, or CF2, and B is a nucleosidic base or any other non-naturally occurring base that can be complementary or non-complementary to ADORA1 RNA or a non-nucleosidic base or any other non-naturally occurring universal base that can be complementary or non-complementary to ADORA1 RNA.
31. An expression vector comprising a nucleic acid sequence encoding at least one siRNA molecule of claim 1 in a manner that allows expression of the nucleic acid molecule.
32. A mammalian cell comprising an expression vector of claim 31 .
33. The mammalian cell of claim 32 , wherein said mammalian cell is a human cell.
34. The expression vector of claim 31 , wherein said siRNA molecule comprises a sense region and an antisense region and wherein said antisense region comprises sequence complementary to an RNA sequence encoding ADORA1 and the sense region comprises sequence complementary to the antisense region.
35. The expression vector of claim 34 , wherein said siRNA molecule comprises two distinct strands having complementarity sense and antisense regions.
36. The expression vector of claim 34 , wherein said siRNA molecule comprises a single strand having complementary sense and antisense regions.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/224,005 US20030143732A1 (en) | 2001-04-05 | 2002-08-20 | RNA interference mediated inhibition of adenosine A1 receptor (ADORA1) gene expression using short interfering RNA |
| US12/200,693 US20090247606A1 (en) | 2001-08-28 | 2008-08-28 | RNA Interference Mediated Inhibition of Adenosine A1 Receptor (ADORA1) Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| US12/754,111 US20100305191A1 (en) | 2001-08-28 | 2010-04-05 | Rna interference mediated inhibition of adenosine a1 receptor (adora1) gene expression using short interfering rna |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/827,395 US20030113891A1 (en) | 2000-02-11 | 2001-04-05 | Method and reagent for the inhibition of NOGO and NOGO receptor genes |
| US29441201P | 2001-05-29 | 2001-05-29 | |
| US31531501P | 2001-08-28 | 2001-08-28 | |
| US10/224,005 US20030143732A1 (en) | 2001-04-05 | 2002-08-20 | RNA interference mediated inhibition of adenosine A1 receptor (ADORA1) gene expression using short interfering RNA |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/200,693 Continuation US20090247606A1 (en) | 2001-08-28 | 2008-08-28 | RNA Interference Mediated Inhibition of Adenosine A1 Receptor (ADORA1) Gene Expression Using Short Interfering Nucleic Acid (siNA) |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030143732A1 true US20030143732A1 (en) | 2003-07-31 |
Family
ID=40293860
Family Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/471,271 Abandoned US20070026394A1 (en) | 2000-02-11 | 2002-04-03 | Modulation of gene expression associated with inflammation proliferation and neurite outgrowth using nucleic acid based technologies |
| US10/156,306 Expired - Fee Related US7022828B2 (en) | 2001-04-05 | 2002-05-28 | siRNA treatment of diseases or conditions related to levels of IKK-gamma |
| US10/206,693 Abandoned US20050261212A1 (en) | 2000-02-11 | 2002-07-26 | RNA interference mediated inhibition of NOGO and NOGO receptor gene expression using short interfering RNA |
| US10/224,005 Abandoned US20030143732A1 (en) | 2001-04-05 | 2002-08-20 | RNA interference mediated inhibition of adenosine A1 receptor (ADORA1) gene expression using short interfering RNA |
| US10/226,992 Abandoned US20030148507A1 (en) | 2001-04-05 | 2002-08-23 | RNA interference mediated inhibition of prostaglandin D2 receptor (PTGDR) and prostaglandin D2 synthetase (PTGDS) gene expression using short interfering RNA |
| US10/230,006 Abandoned US20030191077A1 (en) | 2001-04-05 | 2002-08-28 | Method and reagent for the treatment of asthma and allergic conditions |
| US11/255,139 Abandoned US20060154271A1 (en) | 2001-04-05 | 2005-10-20 | Enzymatic nucleic acid treatment of diseases or conditions related to levels of IKK-gamma and PKR |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/471,271 Abandoned US20070026394A1 (en) | 2000-02-11 | 2002-04-03 | Modulation of gene expression associated with inflammation proliferation and neurite outgrowth using nucleic acid based technologies |
| US10/156,306 Expired - Fee Related US7022828B2 (en) | 2001-04-05 | 2002-05-28 | siRNA treatment of diseases or conditions related to levels of IKK-gamma |
| US10/206,693 Abandoned US20050261212A1 (en) | 2000-02-11 | 2002-07-26 | RNA interference mediated inhibition of NOGO and NOGO receptor gene expression using short interfering RNA |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/226,992 Abandoned US20030148507A1 (en) | 2001-04-05 | 2002-08-23 | RNA interference mediated inhibition of prostaglandin D2 receptor (PTGDR) and prostaglandin D2 synthetase (PTGDS) gene expression using short interfering RNA |
| US10/230,006 Abandoned US20030191077A1 (en) | 2001-04-05 | 2002-08-28 | Method and reagent for the treatment of asthma and allergic conditions |
| US11/255,139 Abandoned US20060154271A1 (en) | 2001-04-05 | 2005-10-20 | Enzymatic nucleic acid treatment of diseases or conditions related to levels of IKK-gamma and PKR |
Country Status (3)
| Country | Link |
|---|---|
| US (7) | US20070026394A1 (en) |
| EP (1) | EP1386004A4 (en) |
| WO (1) | WO2002081628A2 (en) |
Cited By (491)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030096287A1 (en) * | 1996-06-06 | 2003-05-22 | Crooke Stanley T. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US20030105051A1 (en) * | 2001-05-29 | 2003-06-05 | Mcswiggen James | Nucleic acid treatment of diseases or conditions related to levels of HER2 |
| US20030157030A1 (en) * | 2001-11-02 | 2003-08-21 | Insert Therapeutics, Inc. | Methods and compositions for therapeutic use of rna interference |
| US20030191077A1 (en) * | 2001-04-05 | 2003-10-09 | Kathy Fosnaugh | Method and reagent for the treatment of asthma and allergic conditions |
| US20030190654A1 (en) * | 2002-01-22 | 2003-10-09 | Ribopharma | Double-stranded RNA (dsRNA) and method of use for inhibiting expression of a fusion gene |
| US20030206887A1 (en) * | 1992-05-14 | 2003-11-06 | David Morrissey | RNA interference mediated inhibition of hepatitis B virus (HBV) using short interfering nucleic acid (siNA) |
| US20040001811A1 (en) * | 2001-01-09 | 2004-01-01 | Ribopharma Ag | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20040005593A1 (en) * | 2002-03-06 | 2004-01-08 | Rigel Pharmaceuticals, Inc. | Novel method for delivery and intracellular synthesis of siRNA molecules |
| US20040019001A1 (en) * | 2002-02-20 | 2004-01-29 | Mcswiggen James A. | RNA interference mediated inhibition of protein typrosine phosphatase-1B (PTP-1B) gene expression using short interfering RNA |
| US20040023390A1 (en) * | 2002-08-05 | 2004-02-05 | Davidson Beverly L. | SiRNA-mediated gene silencing with viral vectors |
| US20040038921A1 (en) * | 2001-10-26 | 2004-02-26 | Ribopharma Ag | Composition and method for inhibiting expression of a target gene |
| US20040053875A1 (en) * | 1999-01-30 | 2004-03-18 | Ribopharma Ag | Method and medicament for inhibiting the expression of a given gene |
| US20040053289A1 (en) * | 2002-09-09 | 2004-03-18 | The Regents Of The University Of California | Short interfering nucleic acid hybrids and methods thereof |
| US20040054155A1 (en) * | 2002-02-01 | 2004-03-18 | Sequitur, Inc. | Oligonucleotide compositions with enhanced efficiency |
| US20040063654A1 (en) * | 2001-11-02 | 2004-04-01 | Davis Mark E. | Methods and compositions for therapeutic use of RNA interference |
| US20040096882A1 (en) * | 2002-08-21 | 2004-05-20 | Martin Gleave | RNAi probes targeting cancer-related proteins |
| US20040121348A1 (en) * | 2001-10-26 | 2004-06-24 | Ribopharma Ag | Compositions and methods for treating pancreatic cancer |
| US20040137471A1 (en) * | 2002-09-18 | 2004-07-15 | Timothy Vickers | Efficient reduction of target RNA's by single-and double-stranded oligomeric compounds |
| US20040138163A1 (en) * | 2002-05-29 | 2004-07-15 | Mcswiggen James | RNA interference mediated inhibition of vascular edothelial growth factor and vascular edothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20040146902A1 (en) * | 1996-06-06 | 2004-07-29 | Ecker David J. | Structural motifs and oligomeric compounds and their use in gene modulation |
| US20040147023A1 (en) * | 1996-06-06 | 2004-07-29 | Baker Brenda F. | Chimeric oligomeric compounds and their use in gene modulation |
| US20040161777A1 (en) * | 1996-06-06 | 2004-08-19 | Baker Brenda F. | Modified oligonucleotides for use in RNA interference |
| US20040161844A1 (en) * | 1996-06-06 | 2004-08-19 | Baker Brenda F. | Sugar and backbone-surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US20040167090A1 (en) * | 2003-02-21 | 2004-08-26 | Monahan Sean D. | Covalent modification of RNA for in vitro and in vivo delivery |
| US20040171032A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | Non-phosphorous-linked oligomeric compounds and their use in gene modulation |
| US20040171570A1 (en) * | 2002-11-05 | 2004-09-02 | Charles Allerson | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US20040171030A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | Oligomeric compounds having modified bases for binding to cytosine and uracil or thymine and their use in gene modulation |
| US20040171028A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | Phosphorous-linked oligomeric compounds and their use in gene modulation |
| US20040175703A1 (en) * | 1999-11-24 | 2004-09-09 | Ribopharma Ag | Compositions and methods for inhibiting expression of a target gene |
| US20040192626A1 (en) * | 2002-02-20 | 2004-09-30 | Mcswiggen James | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| WO2004044132A3 (en) * | 2002-11-05 | 2004-10-07 | Isis Pharmaceuticals Inc | Modified oligonucleotides for use in rna interference |
| US20040198682A1 (en) * | 2001-11-30 | 2004-10-07 | Mcswiggen James | RNA interference mediated inhibition of placental growth factor gene expression using short interfering nucleic acid (siNA) |
| US20040203145A1 (en) * | 2002-08-07 | 2004-10-14 | University Of Massachusetts | Compositions for RNA interference and methods of use thereof |
| US20040220132A1 (en) * | 2002-11-26 | 2004-11-04 | Medtronic, Inc. | Treatment of neurodegenerative disease through intracranial delivery of siRNA |
| US20040224405A1 (en) * | 2003-05-06 | 2004-11-11 | Dharmacon Inc. | siRNA induced systemic gene silencing in mammalian systems |
| US20040242521A1 (en) * | 1999-10-25 | 2004-12-02 | Board Of Regents, The University Of Texas System | Thio-siRNA aptamers |
| US20040241854A1 (en) * | 2002-08-05 | 2004-12-02 | Davidson Beverly L. | siRNA-mediated gene silencing |
| US20040248174A1 (en) * | 2003-04-18 | 2004-12-09 | Thetrustees Of The University Of Pennsylvania | Compositions and methods for siRNA inhibition of angiopoietin 1and 2 and their receptor Tie2 |
| US20040254358A1 (en) * | 2003-06-12 | 2004-12-16 | Muthiah Manoharan | Phosphorous-linked oligomeric compounds and their use in gene modulation |
| US20040265912A1 (en) * | 2003-05-23 | 2004-12-30 | Board Of Regents, The University Of Texas System | Structure based and combinatorially selected oligonucleoside phosphorothioate and phosphorodithioate aptamer targeting AP-1 transcription factors |
| US20050004064A1 (en) * | 2001-11-21 | 2005-01-06 | Mitsubishi Chemical Corporation | Method of inhibiting gene expression |
| WO2004043977A3 (en) * | 2002-11-05 | 2005-01-20 | Isis Pharmaceuticals Inc | 2’-fluoro substituted oligomeric compounds and compositions for use in gene modulations |
| WO2004086047A3 (en) * | 2003-03-28 | 2005-01-20 | Bayer Healthcare Ag | Diagnostics and therapeutics for diseases associated with g-protein-coupled receptor adenosine a1 (adora1) |
| US20050020521A1 (en) * | 2002-09-25 | 2005-01-27 | University Of Massachusetts | In vivo gene silencing by chemically modified and stable siRNA |
| US20050020525A1 (en) * | 2002-02-20 | 2005-01-27 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20050032733A1 (en) * | 2001-05-18 | 2005-02-10 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (SiNA) |
| US20050037370A1 (en) * | 1996-06-06 | 2005-02-17 | Baker Brenda F. | Oligomeric compounds having modified bases for binding to adenine and guanine and their use in gene modulation |
| US20050042646A1 (en) * | 2002-08-05 | 2005-02-24 | Davidson Beverly L. | RNA interference suppresion of neurodegenerative diseases and methods of use thereof |
| US20050048529A1 (en) * | 2002-02-20 | 2005-03-03 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of intercellular adhesion molecule (ICAM) gene expression using short interfering nucleic acid (siNA) |
| US20050053922A1 (en) * | 2003-06-30 | 2005-03-10 | Schaffer David V. | Mutant adeno-associated virus virions and methods of use thereof |
| US20050054836A1 (en) * | 2000-11-09 | 2005-03-10 | Cold Spring Harbor Laboratory | Chimeric molecules to modulate gene expression |
| US20050058982A1 (en) * | 2002-07-26 | 2005-03-17 | Chiron Corporation | Modified small interfering RNA molecules and methods of use |
| WO2004044138A3 (en) * | 2002-11-05 | 2005-03-24 | Isis Pharmaceuticals Inc | Chimeric oligomeric compounds and their use in gene modulation |
| US20050074757A1 (en) * | 2001-10-12 | 2005-04-07 | Ribopharma Ag | Compositions and methods for inhibiting expression of a mutant gene |
| US20050075304A1 (en) * | 2001-11-30 | 2005-04-07 | Mcswiggen James | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050079610A1 (en) * | 2001-05-18 | 2005-04-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of Fos gene expression using short interfering nucleic acid (siNA) |
| US20050080246A1 (en) * | 2002-11-05 | 2005-04-14 | Charles Allerson | Compositions comprising alternating 2'-modified nucleosides for use in gene modulation |
| US20050096284A1 (en) * | 2002-02-20 | 2005-05-05 | Sirna Therapeutics, Inc. | RNA interference mediated treatment of polyglutamine (polyQ) repeat expansion diseases using short interfering nucleic acid (siNA) |
| WO2005012483A3 (en) * | 2003-08-01 | 2005-06-02 | Internat Therapeutics Inc | Vpr selective rnai agents and methods for using the same |
| US20050118611A1 (en) * | 2003-07-24 | 2005-06-02 | Board Of Regents, The University Of Texas System | Thioaptamers enable discovery of physiological pathways and new therapeutic strategies |
| US20050119212A1 (en) * | 2001-05-18 | 2005-06-02 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of FAS and FASL gene expression using short interfering nucleic acid (siNA) |
| WO2005001110A3 (en) * | 2003-05-29 | 2005-06-02 | Salk Inst For Biological Studi | Transcriptional regulation of gene expression by small double-stranded modulatory rna |
| US20050124569A1 (en) * | 2001-05-18 | 2005-06-09 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of CXCR4 gene expression using short interfering nucleic acid (siNA) |
| US20050124566A1 (en) * | 2001-05-18 | 2005-06-09 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of myostatin gene expression using short interfering nucleic acid (siNA) |
| US20050136437A1 (en) * | 2003-08-25 | 2005-06-23 | Nastech Pharmaceutical Company Inc. | Nanoparticles for delivery of nucleic acids and stable double-stranded RNA |
| US20050137155A1 (en) * | 2001-05-18 | 2005-06-23 | Sirna Therapeutics, Inc. | RNA interference mediated treatment of Parkinson disease using short interfering nucleic acid (siNA) |
| US20050136436A1 (en) * | 2001-05-18 | 2005-06-23 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of G72 and D-amino acid oxidase (DAAO) gene expression using short interfering nucleic acid (siNA) |
| US20050136430A1 (en) * | 2003-07-15 | 2005-06-23 | California Institute Of Technology | Inhibitor nucleic acids |
| US20050143333A1 (en) * | 2001-05-18 | 2005-06-30 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of interleukin and interleukin receptor gene expression using short interfering nucleic acid (SINA) |
| US20050153914A1 (en) * | 2001-05-18 | 2005-07-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of MDR P-glycoprotein gene expression using short interfering nucleic acid (siNA) |
| US20050153337A1 (en) * | 2003-04-03 | 2005-07-14 | Muthiah Manoharan | iRNA conjugates |
| US20050158735A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of proliferating cell nuclear antigen (PCNA) gene expression using short interfering nucleic acid (siNA) |
| US20050159380A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of angiopoietin gene expression using short interfering nucleic acid (siNA) |
| US20050159378A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of Myc and/or Myb gene expression using short interfering nucleic acid (siNA) |
| US20050159379A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc | RNA interference mediated inhibition of gastric inhibitory polypeptide (GIP) and gastric inhibitory polypeptide receptor (GIPR) gene expression using short interfering nucleic acid (siNA) |
| US20050164224A1 (en) * | 2001-05-18 | 2005-07-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of cyclin D1 gene expression using short interfering nucleic acid (siNA) |
| US20050164967A1 (en) * | 2001-05-18 | 2005-07-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of platelet-derived endothelial cell growth factor (ECGF1) gene expression using short interfering nucleic acid (siNA) |
| US20050164968A1 (en) * | 2001-05-18 | 2005-07-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of ADAM33 gene expression using short interfering nucleic acid (siNA) |
| US20050171040A1 (en) * | 2001-05-18 | 2005-08-04 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of cholesteryl ester transfer protein (CEPT) gene expression using short interfering nucleic acid (siNA) |
| US20050176666A1 (en) * | 2001-05-18 | 2005-08-11 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of GPRA and AAA1 gene expression using short interfering nucleic acid (siNA) |
| US20050176018A1 (en) * | 1998-04-20 | 2005-08-11 | Sirna Therapeutics, Inc. | Chemically modified double stranded nucleic acid molecules |
| US20050176663A1 (en) * | 2001-05-18 | 2005-08-11 | Sima Therapeutics, Inc. | RNA interference mediated inhibition of protein tyrosine phosphatase type IVA (PRL3) gene expression using short interfering nucleic acid (siNA) |
| US20050176667A1 (en) * | 2001-01-09 | 2005-08-11 | Alnylam Europe Ag | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20050176025A1 (en) * | 2001-05-18 | 2005-08-11 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of B-cell CLL/Lymphoma-2 (BCL-2) gene expression using short interfering nucleic acid (siNA) |
| US20050181382A1 (en) * | 2003-06-02 | 2005-08-18 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNAi |
| US20050182007A1 (en) * | 2001-05-18 | 2005-08-18 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of interleukin and interleukin receptor gene expression using short interfering nucleic acid (SINA) |
| US20050187178A1 (en) * | 2002-11-05 | 2005-08-25 | Charles Allerson | Compositions comprising alternating 2'-modified nucleosides for use in gene modulation |
| US20050186591A1 (en) * | 2003-06-09 | 2005-08-25 | Alnylam Pharmaceuticals | Method of treating neurodegenerative disease |
| US20050187174A1 (en) * | 2001-05-18 | 2005-08-25 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of intercellular adhesion molecule (ICAM) gene expression using short interfering nucleic acid (siNA) |
| US20050191618A1 (en) * | 2001-05-18 | 2005-09-01 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA) |
| US20050197310A1 (en) * | 2004-01-30 | 2005-09-08 | Orna Mor | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| US20050196767A1 (en) * | 2001-05-18 | 2005-09-08 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of GRB2 associated binding protein (GAB2) gene expression using short interfering nucleic acis (siNA) |
| US20050196781A1 (en) * | 2001-05-18 | 2005-09-08 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of STAT3 gene expression using short interfering nucleic acid (siNA) |
| US20050209182A1 (en) * | 2002-02-20 | 2005-09-22 | Sirna Therapeutics, Inc. | Nucleic acid mediated inhibition of enterococcus infection and cytolysin toxin activity |
| US20050208658A1 (en) * | 2003-11-21 | 2005-09-22 | The University Of Maryland | RNA interference mediated inhibition of 11beta hydroxysteriod dehydrogenase-1 (11beta HSD-1) gene expression |
| US20050214772A1 (en) * | 1998-10-26 | 2005-09-29 | Board Of Regents, The University Of Texas System | Thio modified aptamer synthetic methods and compositions |
| US20050222066A1 (en) * | 2001-05-18 | 2005-10-06 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050233344A1 (en) * | 2001-05-18 | 2005-10-20 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of platelet derived growth factor (PDGF) and platelet derived growth factor receptor (PDGFR) gene expression using short interfering nucleic acid (siNA) |
| US20050233998A1 (en) * | 2001-05-18 | 2005-10-20 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050233997A1 (en) * | 2001-05-18 | 2005-10-20 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of matrix metalloproteinase 13 (MMP13) gene expression using short interfering nucleic acid (siNA) |
| US20050234003A1 (en) * | 1998-12-29 | 2005-10-20 | Bahramian Mohammad B | Method of using nucleic acid compositions for muting expression of a gene in animals |
| US20050239134A1 (en) * | 2004-04-21 | 2005-10-27 | Board Of Regents, The University Of Texas System | Combinatorial selection of phosphorothioate single-stranded DNA aptamers for TGF-beta-1 protein |
| US20050239739A1 (en) * | 2001-05-18 | 2005-10-27 | Sirna Therapeutics, Inc. | Conjugates and compositions for cellular delivery |
| US20050239731A1 (en) * | 2001-05-18 | 2005-10-27 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of MAP kinase gene expression using short interfering nucleic acid (siNA) |
| US20050245475A1 (en) * | 2002-11-14 | 2005-11-03 | Dharmacon, Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20050256071A1 (en) * | 2003-07-15 | 2005-11-17 | California Institute Of Technology | Inhibitor nucleic acids |
| US20050255086A1 (en) * | 2002-08-05 | 2005-11-17 | Davidson Beverly L | Nucleic acid silencing of Huntington's Disease gene |
| US20050261219A1 (en) * | 2001-05-18 | 2005-11-24 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of interleukin and interleukin receptor gene expression using short interfering nucleic acid (siNA) |
| US20050267058A1 (en) * | 2001-05-18 | 2005-12-01 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of placental growth factor gene expression using short interfering nucleic acid (sINA) |
| US20050267300A1 (en) * | 2004-04-05 | 2005-12-01 | Muthiah Manoharan | Processes and reagents for oligonucleotide synthesis and purification |
| US20050266561A1 (en) * | 2003-11-21 | 2005-12-01 | Revivicor, Inc. | Use of interfering RNA in the production of transgenic animals |
| US20050282188A1 (en) * | 2001-05-18 | 2005-12-22 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20050288242A1 (en) * | 2001-05-18 | 2005-12-29 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of RAS gene expression using short interfering nucleic acid (siNA) |
| US20050287128A1 (en) * | 2001-05-18 | 2005-12-29 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of TGF-beta and TGF-beta receptor gene expression using short interfering nucleic acid (siNA) |
| US20050288244A1 (en) * | 2004-04-30 | 2005-12-29 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a C5-modified pyrimidine |
| US20060008910A1 (en) * | 2004-06-07 | 2006-01-12 | Protiva Biotherapeuties, Inc. | Lipid encapsulated interfering RNA |
| US20060009409A1 (en) * | 2002-02-01 | 2006-01-12 | Woolf Tod M | Double-stranded oligonucleotides |
| US20060014289A1 (en) * | 2004-04-20 | 2006-01-19 | Nastech Pharmaceutical Company Inc. | Methods and compositions for enhancing delivery of double-stranded RNA or a double-stranded hybrid nucleic acid to regulate gene expression in mammalian cells |
| US20060025366A1 (en) * | 2004-07-02 | 2006-02-02 | Protiva Biotherapeutics, Inc. | Immunostimulatory siRNA molecules and uses therefor |
| US20060040882A1 (en) * | 2004-05-04 | 2006-02-23 | Lishan Chen | Compostions and methods for enhancing delivery of nucleic acids into cells and for modifying expression of target genes in cells |
| WO2006023491A2 (en) | 2004-08-16 | 2006-03-02 | The Cbr Institute For Biomedical Research, Inc. | Method of delivering rna interference and uses thereof |
| US20060069050A1 (en) * | 2004-02-17 | 2006-03-30 | University Of Massachusetts | Methods and compositions for mediating gene silencing |
| US20060073127A1 (en) * | 2004-07-09 | 2006-04-06 | Umass Medical School | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US20060084621A1 (en) * | 2001-01-09 | 2006-04-20 | Hans-Peter Vornlocher | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20060083780A1 (en) * | 2004-06-07 | 2006-04-20 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods of use |
| US20060094678A1 (en) * | 2004-05-27 | 2006-05-04 | Hans-Peter Vornlocher | Nuclease resistant double-stranded ribonucleic acid |
| US20060121489A1 (en) * | 2003-05-23 | 2006-06-08 | Board Of Regents, The University Of Texas System | High throughput screening of aptamer libraries for specific binding to proteins on viruses and other pathogens |
| US20060134189A1 (en) * | 2004-11-17 | 2006-06-22 | Protiva Biotherapeutics, Inc | siRNA silencing of apolipoprotein B |
| US20060142225A1 (en) * | 2001-05-18 | 2006-06-29 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of cyclin dependent kinase-2 (CDK2) gene expression using short interfering nucleic acid (siNA) |
| US20060142557A1 (en) * | 1994-03-29 | 2006-06-29 | Sirna Therapeutics, Inc. | 2'-deoxy-2'alkylnucleotide containing nucleic acid |
| US20060166921A1 (en) * | 2005-01-07 | 2006-07-27 | Rachel Meyers | RNAi modulation of RSV and therapeutic uses thereof |
| US20060178324A1 (en) * | 2003-01-21 | 2006-08-10 | Philipp Hadwiger | Lipophilic derivatives of double-stranded ribonucleic acid |
| US20060178297A1 (en) * | 2003-01-28 | 2006-08-10 | Troy Carol M | Systems and methods for silencing expression of a gene in a cell and uses thereof |
| US20060178328A1 (en) * | 2002-11-26 | 2006-08-10 | Medtronic Inc. | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of siRNA |
| US20060205635A1 (en) * | 2005-03-14 | 2006-09-14 | Board Of Regents, The University Of Texas System | Antigene oligomers inhibit transcription |
| US20060211642A1 (en) * | 2001-05-18 | 2006-09-21 | Sirna Therapeutics, Inc. | RNA inteference mediated inhibition of hepatitis C virus (HVC) gene expression using short interfering nucleic acid (siNA) |
| US20060216747A1 (en) * | 2001-05-18 | 2006-09-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of checkpoint kinase-1 (CHK-1) gene expression using short interfering nucleic acid (siNA) |
| US20060217324A1 (en) * | 2005-01-24 | 2006-09-28 | Juergen Soutschek | RNAi modulation of the Nogo-L or Nogo-R gene and uses thereof |
| US20060217331A1 (en) * | 2001-05-18 | 2006-09-28 | Sirna Therapeutics, Inc. | Chemically modified double stranded nucleic acid molecules that mediate RNA interference |
| US20060223990A1 (en) * | 1992-05-11 | 2006-10-05 | Sirna Therapeutics, Inc. | Synthesis, deprotection, analysis & purification of RNA & ribozymes |
| US20060223773A1 (en) * | 2005-03-11 | 2006-10-05 | Alcon, Inc. | RNAi-mediated inhibition of Frizzled Related Protein-1 for treatment of glaucoma |
| US20060223749A1 (en) * | 2001-09-19 | 2006-10-05 | University Of South Florida | Inhibition of SHIP to enhance stem cell harvest and transplantation |
| US20060240093A1 (en) * | 2003-07-16 | 2006-10-26 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering rna |
| US20060240425A1 (en) * | 2002-09-30 | 2006-10-26 | Oncotherapy Science, Inc | Genes and polypeptides relating to myeloid leukemia |
| US20060241075A1 (en) * | 2001-05-18 | 2006-10-26 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of desmoglein gene expression using short interfering nucleic acid (siNA) |
| US20060239971A1 (en) * | 2003-02-21 | 2006-10-26 | Mohapatra Shyam S | Vectors for regulating gene expression |
| US20060253068A1 (en) * | 2005-04-20 | 2006-11-09 | Van Bilsen Paul | Use of biocompatible in-situ matrices for delivery of therapeutic cells to the heart |
| US20060270623A1 (en) * | 2001-05-18 | 2006-11-30 | Sirna Therapeutics, Inc. | RNA interference mediated treatment of polyglutamine (polyQ) repeat expansion diseases using short interfering nucleic acid (siNA) |
| US20060276635A1 (en) * | 2002-09-05 | 2006-12-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060287269A1 (en) * | 2002-09-09 | 2006-12-21 | The Regents Of The University Of California | Short interfering nucleic acid hybrids and methods thereof |
| US20060287260A1 (en) * | 2004-06-30 | 2006-12-21 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a non-phosphate backbone linkage |
| US20070015722A1 (en) * | 2003-06-20 | 2007-01-18 | Kraynack Brian A | Double stranded compositions comprising a 3'-endo modified strand for use in gene modulation |
| US20070026002A1 (en) * | 2004-07-20 | 2007-02-01 | Genentech, Inc. | Inhibitors of angiopoietin-like 4 protein, combinations, and their use |
| US20070042984A1 (en) * | 2005-07-21 | 2007-02-22 | Juergen Soutschek | RNAi modulation of the Rho-A gene and uses thereof |
| US20070054873A1 (en) * | 2005-08-26 | 2007-03-08 | Protiva Biotherapeutics, Inc. | Glucocorticoid modulation of nucleic acid-mediated immune stimulation |
| US20070078085A1 (en) * | 2004-10-13 | 2007-04-05 | Chung Leland W | Methods and compositions for the utilization and targeting of osteomimicry |
| US20070093437A1 (en) * | 2001-05-18 | 2007-04-26 | Sirna Therapeutics, Inc. | Rna interference mediated inhibition of xiap gene expression using short interfering nucleic acid (sina) |
| US20070093440A1 (en) * | 2004-01-28 | 2007-04-26 | Champion Brian R | Medical treatment |
| US20070099858A1 (en) * | 2005-10-03 | 2007-05-03 | Sirna Therapeutics, Inc. | RNA interference mediated of inhibition of influenza virus gene expression using short interfering nucleic acid (siNA) |
| US20070105806A1 (en) * | 2005-11-04 | 2007-05-10 | Dinah Sah | Compositions and methods for inhibiting expression of Nav1.8 gene |
| US20070111963A1 (en) * | 2005-11-17 | 2007-05-17 | Board Of Regents, The University Of Texas System | Modulation of gene expression by oligomers targeted to chromosomal DNA |
| US20070128640A1 (en) * | 2002-11-14 | 2007-06-07 | Dharmacon, Inc. | siRNA targeting ras-related nuclear protein |
| US20070135370A1 (en) * | 2005-10-20 | 2007-06-14 | Protiva Biotherapeutics, Inc. | siRNA silencing of filovirus gene expression |
| US20070135372A1 (en) * | 2005-11-02 | 2007-06-14 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US20070141601A1 (en) * | 2004-05-12 | 2007-06-21 | Dharmacon, Inc. | siRNA targeting cAMP-specific phosphodiesterase 4D |
| US20070149470A1 (en) * | 2004-09-10 | 2007-06-28 | Kaspar Roger L | Inhibition of viral gene expression using small interfering RNA |
| US20070161595A1 (en) * | 2003-06-09 | 2007-07-12 | Mayo Foundation For Medical Education And Research | Method of treating neurodegenerative disease |
| US20070160980A1 (en) * | 2001-05-18 | 2007-07-12 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20070161586A1 (en) * | 2004-01-16 | 2007-07-12 | Takeda Pharmaceutical Company Limited | Drug for preventing and treating atherosclerosis |
| US20070167389A1 (en) * | 2003-11-25 | 2007-07-19 | Kaemmerer William F | Compositions, devices and methods for treatment of huntington's disease through intracranial delivery of sirna |
| US20070172948A1 (en) * | 2004-06-03 | 2007-07-26 | Balkrishen Bhat | Double strand compositions comprising differentially modified strands for use in gene modulation |
| US20070173473A1 (en) * | 2001-05-18 | 2007-07-26 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of proprotein convertase subtilisin Kexin 9 (PCSK9) gene expression using short interfering nucleic acid (siNA) |
| US20070180242A1 (en) * | 2006-01-30 | 2007-08-02 | Nagaraj Thadi M | GSM authentication in a CDMA network |
| US20070203084A1 (en) * | 2003-08-28 | 2007-08-30 | Jan Weiler | Interfering Rna Duplex Having Blunt-Ends And 3'-Modifications |
| US20070203333A1 (en) * | 2001-11-30 | 2007-08-30 | Mcswiggen James | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20070219148A1 (en) * | 2003-07-02 | 2007-09-20 | Commissariat A L'energie Atomique | Small Interfering RNA Specific to Sub-Units $g(a),$g(a)' and $g(b) of the Kinase Protein ck2,and the Applications of the Same |
| US20070218122A1 (en) * | 2005-11-18 | 2007-09-20 | Protiva Biotherapeutics, Inc. | siRNA silencing of influenza virus gene expression |
| US20070218551A1 (en) * | 2003-10-02 | 2007-09-20 | Chuan-Yuan Li | Novel Sirna-Based Approach to Target the Hif-Alpha Factor for Gene Therapy |
| US20070238691A1 (en) * | 2006-03-29 | 2007-10-11 | Senesco Technologies, Inc. | Inhibition of HIV replication and expression of p24 with eIF-5A |
| US20070244311A1 (en) * | 2002-11-14 | 2007-10-18 | Dharmacon, Inc. | siRNA targeting coatomer protein complex, subunit beta 2 (CPOB2) |
| US20070249550A1 (en) * | 2004-09-01 | 2007-10-25 | Sitkovsky Michail V | Modulation of immune response and inflammation by targeting hypoxia inducible factors |
| US20070249549A1 (en) * | 2003-12-17 | 2007-10-25 | Index Pharmaceuticals Ab | Compounds and Methods for Rna Interference of the P65 Subunit of Nf-Kappa-B |
| US20070254362A1 (en) * | 2005-09-02 | 2007-11-01 | Nastech Pharmaceutical Company Inc. | COMPOSITIONS AND METHODS EMPLOYING UNIVERSAL-BINDING NUCLEOTIDES FOR TARGETING MULTIPLE GENE VARIANTS WITH A SINGLE siRNA DUPLEX |
| US20070261126A1 (en) * | 2005-05-06 | 2007-11-08 | Kaemmerer William F | Methods and sequences to suppress primate huntington gene expression in vivo |
| US20070270579A1 (en) * | 2001-05-18 | 2007-11-22 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20070269892A1 (en) * | 2006-05-18 | 2007-11-22 | Nastech Pharmaceutical Company Inc. | FORMULATIONS FOR INTRACELLULAR DELIVERY dsRNA |
| US20070270366A1 (en) * | 2005-12-20 | 2007-11-22 | Karras James G | Double stranded nucleic acid molecules targeted to il-4 receptor alpha |
| US20070270360A1 (en) * | 2003-04-15 | 2007-11-22 | Sirna Therapeutics, Inc. | Rna Interference Mediated Inhibition of Severe Acute Respiratory Syndrome (Sars) Gene Expression Using Short Interfering Nucleic Acid |
| US20070275921A1 (en) * | 1996-06-06 | 2007-11-29 | Isis Pharmaceuticals, Inc. | Oligomeric Compounds That Facilitate Risc Loading |
| US20070275923A1 (en) * | 2006-05-25 | 2007-11-29 | Nastech Pharmaceutical Company Inc. | CATIONIC PEPTIDES FOR siRNA INTRACELLULAR DELIVERY |
| US20070275914A1 (en) * | 2003-03-07 | 2007-11-29 | Muthiah Manoharan | Therapeutic Compositions |
| US20080039415A1 (en) * | 2006-08-11 | 2008-02-14 | Gregory Robert Stewart | Retrograde transport of sirna and therapeutic uses to treat neurologic disorders |
| US20080039414A1 (en) * | 2002-02-20 | 2008-02-14 | Sima Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20080057062A1 (en) * | 2006-07-18 | 2008-03-06 | National Institute Of Advanced Industrial Science And Technology | Agent for Inducing senescence and apoptosis of cancer cell |
| US20080085999A1 (en) * | 2003-03-06 | 2008-04-10 | Oligoengine, Inc. | Modulation of gene expression using dna-rna hybrids |
| US20080119787A1 (en) * | 2006-11-21 | 2008-05-22 | Kaemmerer William F | Microsyringe for pre-packaged delivery of pharmaceuticals |
| US20080124379A1 (en) * | 2006-11-03 | 2008-05-29 | Kaemmerer William F | Compositions and methods for making therapies delivered by viral vectors reversible for safety and allele-specificity |
| US20080125386A1 (en) * | 2006-01-26 | 2008-05-29 | Universtiy Of Massachusetts | RNA interference agents for therapeutic use |
| EP1660631A4 (en) * | 2003-08-01 | 2008-06-25 | Invitrogen Corp | Compositions and methods for preparing short rna molecules and other nucleic acids |
| WO2005120230A3 (en) * | 2004-06-03 | 2008-06-26 | Isis Pharmaceuticals Inc | POSITIONALLY MODIFIED siRNA CONSTRUCTS |
| US20080153772A1 (en) * | 2005-06-01 | 2008-06-26 | Jean-Paul Behr | Oligonucleotides For Rna Interference and Biological Applications Thereof |
| US20080161256A1 (en) * | 2001-05-18 | 2008-07-03 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20080171906A1 (en) * | 2007-01-16 | 2008-07-17 | Everaerts Frank J L | Tissue performance via hydrolysis and cross-linking |
| US20080171719A1 (en) * | 2006-11-28 | 2008-07-17 | Alcon Manufacturing, Ltd. | RNAi-MEDIATED INHIBITION OF AQUAPORIN 1 FOR TREATMENT OF IOP-RELATED CONDITIONS |
| US20080177051A1 (en) * | 2002-11-14 | 2008-07-24 | Dharmacon, Inc. | siRNA targeting cyclin-dependent kinase inhibitor 1B (p27, Kip1) (CDKN1B) |
| US20080188430A1 (en) * | 2001-05-18 | 2008-08-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of hypoxia inducible factor 1 (HIF1) gene expression using short interfering nucleic acid (siNA) |
| US20080188429A1 (en) * | 2002-12-27 | 2008-08-07 | Iyer Radhakrishnan P | Synthetic siRNA compounds and methods for the downregulation of gene expression |
| US20080200340A1 (en) * | 2001-11-15 | 2008-08-21 | Board Of Regents, The University Of Texas System | Bead Bound Combinatorial Oligonucleoside Phosphorothioate And Phosphorodithioate Aptamer Libraries |
| US20080214486A1 (en) * | 2006-11-28 | 2008-09-04 | Alcon Manufacturing, Ltd. | RNAi-MEDIATED INHIBITION OF AQUAPORIN 4 FOR TREATMENT OF IOP-RELATED CONDITIONS |
| US20080227967A1 (en) * | 2002-11-14 | 2008-09-18 | Dharmacon, Inc. | siRNA targeting ribonucleotide reductase M2 polypeptide (RRM2 or RNR-R2) |
| US20080249046A1 (en) * | 2006-06-09 | 2008-10-09 | Protiva Biotherapeutics, Inc. | MODIFIED siRNA MOLECULES AND USES THEREOF |
| US20080249038A1 (en) * | 2003-10-07 | 2008-10-09 | Quark Biotech, Inc. | Bone Morphogenetic Protein (Bmp) 2A and Uses Thereof |
| US20080253989A1 (en) * | 2004-10-22 | 2008-10-16 | Neuregenix Limited | Neuron Regeneration |
| US20080255345A1 (en) * | 2006-11-21 | 2008-10-16 | Alnylam Pharmaceuticals, Inc. | IRNA Agents Targeting CCR5 Expressing Cells And Uses Thereof |
| WO2008036933A3 (en) * | 2006-09-21 | 2008-10-23 | Alnylam Pharmaceuticals Inc | Compositions and methods for inhibiting expression of the hamp gene |
| US20080269148A1 (en) * | 2004-10-01 | 2008-10-30 | Jang Han | Modified Small Interfering Rna Molecules and Methods of Use |
| US20080268457A1 (en) * | 2002-11-14 | 2008-10-30 | Dharmacon, Inc. | siRNA targeting forkhead box P3 (FOXP3) |
| US20080280843A1 (en) * | 2006-05-24 | 2008-11-13 | Van Bilsen Paul | Methods and kits for linking polymorphic sequences to expanded repeat mutations |
| WO2008121963A3 (en) * | 2007-03-30 | 2008-11-20 | Univ Rutgers | Compositions and methods for gene silencing |
| US20080300145A1 (en) * | 2003-05-27 | 2008-12-04 | Cold Spring Harbor Laboratory | In vivo high throughput selection of RNAi probes |
| US20080318894A1 (en) * | 2007-05-11 | 2008-12-25 | Santaris Pharma A/S | Rna antagonist compounds for the modulation of her3 |
| US20080319180A1 (en) * | 2002-11-14 | 2008-12-25 | Dharmacon, Inc. | siRNA targeting protein kinase N-3 (PKN-3) |
| US20090005548A1 (en) * | 2002-11-14 | 2009-01-01 | Dharmacon, Inc. | siRNA targeting nuclear receptor interacting protein 1 (NRIP1) |
| US20090005330A1 (en) * | 2004-08-31 | 2009-01-01 | Sylentis S.A. | Methods and Compositions to Inhibit P2x7 Receptor Expression |
| US20090018321A1 (en) * | 2004-03-15 | 2009-01-15 | Integrated Dna Technologies, Inc. | Methods and compositions for the specific inhibition of gene expression by double-stranded rna |
| US20090023675A1 (en) * | 2002-02-20 | 2009-01-22 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition of Gene Expression Using Chemically Modified Short Interfering Nucleic Acid (siNA) |
| US20090029466A1 (en) * | 2007-05-01 | 2009-01-29 | City Of Hope | Methods and compositions for the specific inhibition of gene expression by double-stranded rna |
| WO2007057897A3 (en) * | 2005-11-17 | 2009-02-12 | Tel Hashomer Medical Res Infrastructure & Services Ltd | Pharmaceutical composition and method for regulating abnormal cellular proliferation |
| US20090048192A1 (en) * | 2004-06-03 | 2009-02-19 | Isis Pharmaceuticals, Inc. | Double Strand Compositions Comprising Differentially Modified Strands for Use in Gene Modulation |
| US20090054365A1 (en) * | 2007-01-26 | 2009-02-26 | Alcon Research, Ltd. | RNAi-MEDIATED INHIBITION OF AQUAPORIN 1 FOR TREATMENT OF OCULAR NEOVASCULARIZATION |
| WO2009040319A1 (en) | 2007-09-28 | 2009-04-02 | Imba - Institut Für Molekulare Biotechnologie Gmbh | Methods for modulating the proliferation and differentiation potential of stem cells and progenitor cells |
| US20090093439A1 (en) * | 2002-02-20 | 2009-04-09 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF CHROMOSOME TRANSLOCATION GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20090093425A1 (en) * | 2006-07-12 | 2009-04-09 | The Regents Of The University Of California | Transducible delivery of nucleic acids by reversible phosphotriester charge neutralization protecting groups |
| US20090092988A1 (en) * | 2007-10-04 | 2009-04-09 | Schwartz Jacob C | Modulating Gene Expression with agRNA and Gapmers Targeting Antisense Transcripts |
| US7517864B2 (en) | 2001-05-18 | 2009-04-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| WO2008036638A3 (en) * | 2006-09-18 | 2009-04-16 | Alnylam Pharmaceuticals Inc | Rnai modulation of scap and therapeutic uses thereof |
| US20090099116A1 (en) * | 2002-02-20 | 2009-04-16 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF FOS GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20090118489A1 (en) * | 2002-11-14 | 2009-05-07 | Dharmacon, Inc. | siRNA targeting nucleoporin 62kDa (Nup62) |
| US20090137500A1 (en) * | 2002-02-20 | 2009-05-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20090137512A1 (en) * | 2002-02-20 | 2009-05-28 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition of Cyclin D1 Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| US20090143321A1 (en) * | 2005-07-07 | 2009-06-04 | Avraham Hochberg | Nucleic acid agents for downregulating h19 and methods of using same |
| US20090149403A1 (en) * | 2006-05-26 | 2009-06-11 | Protiva Biotherapeutics, Inc. | siRNA silencing of genes expressed in cancer |
| US20090176725A1 (en) * | 2005-08-17 | 2009-07-09 | Sirna Therapeutics Inc. | Chemically modified short interfering nucleic acid molecules that mediate rna interference |
| US20090197332A1 (en) * | 2005-03-08 | 2009-08-06 | Ioanna Andreou | Modified Short Interfering RNA |
| US7579451B2 (en) | 2004-07-21 | 2009-08-25 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a modified or non-natural nucleobase |
| US20090215863A1 (en) * | 2005-08-18 | 2009-08-27 | Rachel Bar-Shavit | Gene Silencing of Protease Activated Receptor 1(Par1) |
| US20090226446A1 (en) * | 2006-04-06 | 2009-09-10 | Deutsches Krebsforschungszentrum Stiftung Des Offentilchen Rechts | Method to Inhibit the Propagation of an Undesired Cell Population |
| US20090227780A1 (en) * | 2002-11-14 | 2009-09-10 | Dharmacon, Inc. | siRNA targeting connexin 43 |
| US20090234102A1 (en) * | 2008-03-14 | 2009-09-17 | Juridical Foundation The Chemo-Sero-Therapeutic Research Institute | Hepatitis c virus inhibitors |
| US20090238772A1 (en) * | 2007-12-13 | 2009-09-24 | Alnylam Pharmaceuticals, Inc. | Methods and compositions for prevention or treatment of rsv infection |
| US20090247607A1 (en) * | 2006-03-24 | 2009-10-01 | John Benson | dsRNA COMPOSITIONS AND METHODS FOR TREATING HPV INFECTION |
| US20090258931A1 (en) * | 2003-09-09 | 2009-10-15 | Isis Pharmaceuticals, Inc. | Chimeric oligomeric compounds comprising alternating regions of northern and southern conformational geometry |
| US7605249B2 (en) | 2002-11-26 | 2009-10-20 | Medtronic, Inc. | Treatment of neurodegenerative disease through intracranial delivery of siRNA |
| US20090275638A1 (en) * | 2008-04-17 | 2009-11-05 | Kevin Fitzgerald | Compositions and Methods for Inhibiting Expression of XBP-1 Gene |
| US20090286969A1 (en) * | 2003-07-31 | 2009-11-19 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US20090291131A1 (en) * | 2007-12-27 | 2009-11-26 | Protiva Biotherapeutics, Inc. | Silencing of polo-like kinase expression using interfering rna |
| US7626014B2 (en) | 2004-04-27 | 2009-12-01 | Alnylam Pharmaceuticals | Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety |
| US20090299045A1 (en) * | 2001-05-18 | 2009-12-03 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition Of Interleukin and Interleukin Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| US20090298910A1 (en) * | 2004-12-10 | 2009-12-03 | Griffey Richard H | Regulation of epigenetic control of gene expression |
| US20090306182A1 (en) * | 2002-02-20 | 2009-12-10 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF MAP KINASE GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20090306356A1 (en) * | 2002-11-14 | 2009-12-10 | Dharmacon,Inc. | siRNA Targeting TNFalpha |
| US7632932B2 (en) | 2004-08-04 | 2009-12-15 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a ligand tethered to a modified or non-natural nucleobase |
| US20100003317A1 (en) * | 2008-03-27 | 2010-01-07 | Akin Akinc | Compositions and methods for mediating rnai in vivo |
| US20100008981A1 (en) * | 2005-05-06 | 2010-01-14 | Medtronic, Inc. | Methods and sequences to suppress primate huntington gene expression |
| US20100009451A1 (en) * | 2008-05-30 | 2010-01-14 | Sigma Aldrich Company | Compositions and methods for specifically silencing a target nucleic acid |
| US20100010066A1 (en) * | 2008-01-31 | 2010-01-14 | Kevin Fitzgerald | Optimized Methods For Delivery Of DSRNA Targeting The PCSK9 Gene |
| US20100015707A1 (en) * | 2006-05-04 | 2010-01-21 | Francois Jean-Charles Natt | SHORT INTERFERING RIBONUCLEIC ACID (siRNA) FOR ORAL ADMINISTRATION |
| US20100016405A1 (en) * | 2006-07-10 | 2010-01-21 | Alnylam Pharmaceuticals, Inc | Compositions and Methods for Inhibiting Expression of the MYC Gene |
| US20100056606A1 (en) * | 2005-10-03 | 2010-03-04 | Isis Pharmaceuticals, Inc. | Combination therapy using budesonide and antisense oligonucleotide targeted to IL4-receptor alpha |
| US20100069461A1 (en) * | 2005-11-09 | 2010-03-18 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor v leiden mutant gene |
| US20100076054A1 (en) * | 2006-07-31 | 2010-03-25 | Universite Joseph Fourier | Sensizitation of cancer cells to therapy using sina targeting genes from the 1p and 19q chromosomal regions |
| US20100087508A1 (en) * | 2008-03-05 | 2010-04-08 | David Bumcrot | Compositions and Methods for Inhibiting Expression of Eg5 and VEGF Genes |
| US20100086526A1 (en) * | 2007-01-16 | 2010-04-08 | Abraham Hochberg | Nucleic acid constructs and methods for specific silencing of h19 |
| US20100093836A1 (en) * | 2007-01-29 | 2010-04-15 | Isis Pharmaceuticals, Inc | Compounds and methods for modulating protein expression |
| US20100093830A1 (en) * | 2006-08-30 | 2010-04-15 | Dolly Mehta | Modulation of MLCK-L Expression and Uses Thereof |
| US20100093831A1 (en) * | 2003-03-12 | 2010-04-15 | Vasgene Therapeutics, Inc. | Nucleic acid compounds for inhibiting angiogenesis and tumor growth |
| US20100099739A1 (en) * | 2007-03-30 | 2010-04-22 | Samuel Ian Gunderson | Compositions and Methods for Gene Silencing |
| US20100098664A1 (en) * | 2007-11-28 | 2010-04-22 | Mathieu Jean-Francois Desclaux | Lentiviral vectors allowing RNAi mediated inhibition of GFAP and vimentin expression |
| US20100113307A1 (en) * | 2002-11-14 | 2010-05-06 | Dharmacon, Inc. | siRNA targeting vascular endothelial growth factor (VEGF) |
| US20100112686A1 (en) * | 2008-10-15 | 2010-05-06 | Qing Ge | Short hairpin rnas for inhibition of gene expression |
| US7713945B2 (en) | 2000-09-19 | 2010-05-11 | University Of South Florida | Control of NK cell function and survival by modulation of SHIP activity |
| US20100120891A1 (en) * | 2005-04-12 | 2010-05-13 | Universite Libre De Bruxelles | Use of a galectin-1-targeted rnai-based approach for the treatment of cancer |
| US20100120893A1 (en) * | 2008-10-20 | 2010-05-13 | Dinah Wen-Yee Sah | Compositions and Methods for Inhibiting Expression of Transthyretin |
| US20100120892A1 (en) * | 2003-04-23 | 2010-05-13 | Georgetown University | Methods and compositions for the inhibition of stat5 in prostate cancer cells |
| US20100130588A1 (en) * | 2008-04-15 | 2010-05-27 | Protiva Biotherapeutics, Inc. | Novel lipid formulations for nucleic acid delivery |
| US20100136026A1 (en) * | 2007-09-26 | 2010-06-03 | Kerr William G | Ship Inhibition to Direct Hematopoietic Stem Cells and Induce Extramedullary Hematopoiesis |
| US7745418B2 (en) | 2001-10-12 | 2010-06-29 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting viral replication |
| US20100168206A1 (en) * | 2008-12-10 | 2010-07-01 | Jared Gollob | GNAQ Targeted dsRNA Compositions And Methods For Inhibiting Expression |
| US20100168205A1 (en) * | 2008-10-23 | 2010-07-01 | Alnylam Pharmaceuticals, Inc. | Methods and Compositions for Prevention or Treatment of RSV Infection Using Modified Duplex RNA Molecules |
| US20100173974A1 (en) * | 2008-12-18 | 2010-07-08 | Dicerna Pharmaceuticals, Inc. | Extended dicer substrate agents and methods for the specific inhibition of gene expression |
| US20100184828A1 (en) * | 2003-06-02 | 2010-07-22 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of rna silencing |
| US20100183696A1 (en) * | 2007-01-30 | 2010-07-22 | Allergan, Inc | Treating Ocular Diseases Using Peroxisome Proliferator-Activated Receptor Delta Antagonists |
| US7763592B1 (en) | 2003-11-20 | 2010-07-27 | University Of South Florida | SHIP-deficiency to increase megakaryocyte progenitor production |
| US20100197773A1 (en) * | 2009-02-03 | 2010-08-05 | Birgit Bramlage | Compositions and methods for inhibiting expression of ptp1b genes |
| US20100196403A1 (en) * | 2007-01-29 | 2010-08-05 | Jacob Hochman | Antibody conjugates for circumventing multi-drug resistance |
| US20100196450A1 (en) * | 2005-10-14 | 2010-08-05 | Donald Carlton D | Targeting pax2 for the induction of defbi-mediated tumor immunity and cancer therapy |
| US20100204306A1 (en) * | 2007-12-14 | 2010-08-12 | Alnylam Pharmaceuticals, Inc. | Method of Treating Neurodegenerative Disease |
| US7781575B2 (en) | 2002-11-14 | 2010-08-24 | Dharmacon, Inc. | siRNA targeting tumor protein 53 (p53) |
| US20100216866A1 (en) * | 2004-09-24 | 2010-08-26 | Alnylam Pharmaceuticals, Inc. | RNAi Modulation of APOB and Uses Thereof |
| US20100222564A1 (en) * | 2003-07-25 | 2010-09-02 | Life Technologies Corporation | Methods and compositions for isolating small rna molecules |
| US20100227912A1 (en) * | 2002-02-20 | 2010-09-09 | Mcswiggen James | RNA INTERFERENCE MEDIATED INHIBITION OF MYOSTATIN GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20100227915A1 (en) * | 2006-04-28 | 2010-09-09 | Pamela Tan | Compositions and methods for inhibiting expression of a gene from the jc virus |
| US20100234446A1 (en) * | 2004-11-24 | 2010-09-16 | Philipp Hadwiger | RNAi Modulation of the BCR-ABL Fusion Gene and Uses Thereof |
| EP2229946A2 (en) | 2006-03-16 | 2010-09-22 | Jukka Westermarck | Use of the growth-stimulating protein KIAA1524 |
| US20100240730A1 (en) * | 2002-02-20 | 2010-09-23 | Merck Sharp And Dohme Corp. | RNA Interference Mediated Inhibition of Gene Expression Using Chemically Modified Short Interfering Nucleic Acid (siNA) |
| US20100239228A1 (en) * | 2005-03-30 | 2010-09-23 | Sony Corporation | Information processing system, information processing method, and information processing program |
| US20100249052A1 (en) * | 2007-03-26 | 2010-09-30 | Alnylam Pharmaceuticals, Inc. | Dsrna compositions and methods for treating hpv infections |
| US7807646B1 (en) * | 2003-11-20 | 2010-10-05 | University Of South Florida | SHIP-deficiency to increase megakaryocyte progenitor production |
| US20100256218A1 (en) * | 2004-12-14 | 2010-10-07 | Olaf Heidenreich | RNAi MODULATION OF MLL-AF4 AND USES THEREOF |
| US7812149B2 (en) | 1996-06-06 | 2010-10-12 | Isis Pharmaceuticals, Inc. | 2′-Fluoro substituted oligomeric compounds and compositions for use in gene modulations |
| US7819842B2 (en) | 2006-11-21 | 2010-10-26 | Medtronic, Inc. | Chronically implantable guide tube for repeated intermittent delivery of materials or fluids to targeted tissue sites |
| WO2010122217A1 (en) | 2009-04-22 | 2010-10-28 | Faron Pharmaceuticals Oy | A novel cell and therapeutical and diagnostical methods based thereon |
| US20100273863A1 (en) * | 2009-04-24 | 2010-10-28 | Board Of Regents, The University Of Texas System | Modulation of Gene Expression Using Oligomers That Target Gene Regions Downstream of 3' Untranslated Regions |
| US20100292304A1 (en) * | 2007-10-04 | 2010-11-18 | Isis Pharmaceuticals, Inc. | Compounds and methods for improving cellular uptake of oligomeric compounds |
| US20100292305A1 (en) * | 2005-06-27 | 2010-11-18 | Akin Akinc | RNAi MODULATION OF HIF-1 AND THERAPUTIC USES THEREOF |
| US20100298405A1 (en) * | 2005-10-28 | 2010-11-25 | Dinah Wen-Yee Sah | Compositions And Methods For Inhibiting Expression Of Huntingtin Gene |
| US7858769B2 (en) | 2004-02-10 | 2010-12-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using multifunctional short interfering nucleic acid (multifunctional siNA) |
| US20110003882A1 (en) * | 2006-05-19 | 2011-01-06 | Alnylam Pharmaceuticals, Inc. | RNAi Modulation of AHA and Therapeutic Uses Thereof |
| EP2272982A1 (en) | 2006-08-23 | 2011-01-12 | Valtion Teknillinen Tutkimuskeskus | Method for treatment of prostate cancer and diagnosing of patients benefiting from the same |
| US20110015252A1 (en) * | 2009-06-15 | 2011-01-20 | Kevin Fitzgerald | Lipid formulated dsrna targeting the pcsk9 gene |
| US20110021606A1 (en) * | 2004-10-22 | 2011-01-27 | South Alabama Medical Science Foundation | RNAi Modulation of RSV, PIV and Other Respiratory Viruses and Uses Thereof |
| US7884086B2 (en) * | 2004-09-08 | 2011-02-08 | Isis Pharmaceuticals, Inc. | Conjugates for use in hepatocyte free uptake assays |
| US20110034537A1 (en) * | 2008-02-12 | 2011-02-10 | De Fougerolles Antonin | Compositions and methods for inhibiting expression of cd45 gene |
| US20110038922A1 (en) * | 2005-06-16 | 2011-02-17 | Faron Pharmaceuticals Oy (A Finnish Company) | Compounds for treating or preventing amine oxidase related diseases or disorders |
| US20110039914A1 (en) * | 2008-02-11 | 2011-02-17 | Rxi Pharmaceuticals Corporation | Modified rnai polynucleotides and uses thereof |
| US20110055965A1 (en) * | 2008-02-15 | 2011-03-03 | Hiroshi Abe | Cycle single-stranded nucleic acid complex and method for producing the same |
| US20110052546A1 (en) * | 2000-09-19 | 2011-03-03 | University Of South Florida | Inhibition of SHIP to Enhance Stem Cell Harvest and Transplantation |
| US20110060031A1 (en) * | 2007-03-29 | 2011-03-10 | Alnylam Pharmaceuticals, Inc. | Compositions And Methods For Inhibiting Expression Of A Gene From The Ebola Virus |
| US20110077286A1 (en) * | 2008-06-05 | 2011-03-31 | Damha Masad J | Oligonucleotide duplexes comprising dna-like and rna-like nucleotides and uses thereof |
| US20110082185A1 (en) * | 2007-09-17 | 2011-04-07 | Ludwig Institute For Cancer Research Ltd. | Cancer-testis gene silencing agents and uses thereof |
| US20110091525A1 (en) * | 2003-09-15 | 2011-04-21 | Protiva Biotherapeutics, Inc. | Polyethyleneglycol-modified lipid compounds and uses thereof |
| US20110110483A1 (en) * | 2009-11-06 | 2011-05-12 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Methods and systems for migrating fuel assemblies in a nuclear fission reactor |
| US20110112178A1 (en) * | 2006-05-22 | 2011-05-12 | Alnylam Pharmaceuticals, Inc. | Compositions And Methods For Inhibiting Expression Of IKK-B Gene |
| US20110117088A1 (en) * | 2004-05-12 | 2011-05-19 | Simon Michael R | Composition and method for introduction of rna interference sequences into targeted cells and tissues |
| US20110124706A1 (en) * | 2009-11-25 | 2011-05-26 | Zhigang He | SOCS3 Inhibition Promotes CNS Neuron Regeneration |
| US7951935B2 (en) | 2002-11-14 | 2011-05-31 | Dharmacon, Inc. | siRNA targeting v-myc myelocytomatosis viral oncogene homolog (MYC) |
| US20110129461A1 (en) * | 2008-04-07 | 2011-06-02 | University Of Cincinnati | Mat II Beta Subunit RNAi and Therapeutic Methods Using Same |
| WO2011067420A1 (en) | 2009-12-04 | 2011-06-09 | Vib Vzw | Arf6 as a new target for treating alzheimer's disease |
| US20110142848A1 (en) * | 2008-08-07 | 2011-06-16 | Chung Leland W K | Anti-beta-2-microglobulin agents and the use thereof |
| US20110172286A1 (en) * | 2007-07-10 | 2011-07-14 | Neurim Pharmaceuticals (1991) Ltd. | Cd44 splice variants in neurodegenerative diseases |
| US20110184046A1 (en) * | 2008-07-11 | 2011-07-28 | Dinah Wen-Yee Sah | Compositions And Methods For Inhibiting Expression Of GSK-3 Genes |
| US20110201667A1 (en) * | 2009-07-20 | 2011-08-18 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing ebola virus gene expression |
| US20110213328A1 (en) * | 2004-03-18 | 2011-09-01 | Medtronic, Inc. | Methods and Systems for Treatment of Neurological Diseases of the Central Nervous System |
| US20110212520A1 (en) * | 2002-08-05 | 2011-09-01 | University Of Iowa Research Foundation | Rna interference suppression of neurodegenerative diseases and methods of use thereof |
| US20110217365A1 (en) * | 2007-03-30 | 2011-09-08 | Samuel Ian Gunderson | Compositions and Methods for Gene Silencing |
| US20110223665A1 (en) * | 2008-07-25 | 2011-09-15 | Alnylam Pharmaceuticals, Inc. | ENHANCEMENT OF siRNA SILENCING ACTIVITY USING UNIVERSAL BASES OR MISMATCHES IN THE SENSE STRAND |
| US20110230542A1 (en) * | 2006-05-11 | 2011-09-22 | Pamela Tan | Compositions and Methods for Inhibiting Expression of the PCSK9 Gene |
| US20110245325A1 (en) * | 2008-12-12 | 2011-10-06 | Kureha Corporation | Pharmaceutical composition for treatment of cancer and asthma |
| US20110311524A1 (en) * | 2004-07-20 | 2011-12-22 | Genentech, Inc. | Inhibitors of Angiopoietin-Like 4 Protein, Combinations, and Their Use |
| US20110313024A1 (en) * | 2004-08-20 | 2011-12-22 | Leonid Beigelman | RNA INTERFERENCE MEDIATED INHIBITION OF PROPROTEIN CONVERTASE SUBTILISIN KEXIN 9 (PCSK9) GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20110313020A1 (en) * | 2008-12-03 | 2011-12-22 | Marina Biotech, Inc. | UsiRNA Complexes |
| US20110318361A1 (en) * | 2006-05-30 | 2011-12-29 | Young Woo Park | Anticancer Drug Comprising Inhibitor of Tmprss4 |
| US20120016011A1 (en) * | 2009-03-19 | 2012-01-19 | Merck Sharp & Dohme Corp. | RNA Interference Mediated Inhibition of Connective Tissue Growth Factor (CTGF) Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| US8101743B2 (en) * | 2004-04-05 | 2012-01-24 | Isis Pharmaceuticals, Inc. | Modulation of transthyretin expression |
| US8153603B2 (en) | 2005-02-25 | 2012-04-10 | Isis Pharmaceuticals, Inc. | Compositions and their uses directed to IL-4R alpha |
| US20120142758A1 (en) * | 2009-06-26 | 2012-06-07 | OPKO CuRNA,, LLC | Treatment of down syndrome gene related diseases by inhibition of natural antisense transcript to a down syndrome gene |
| WO2012075337A2 (en) | 2010-12-01 | 2012-06-07 | Spinal Modulation, Inc. | Directed delivery of agents to neural anatomy |
| US8198427B1 (en) | 2002-11-14 | 2012-06-12 | Dharmacon, Inc. | SiRNA targeting catenin, beta-1 (CTNNB1) |
| US20120157511A1 (en) * | 2009-07-07 | 2012-06-21 | Alnylam Pharmaceuticals, Inc. | 5' phosphate mimics |
| US20120165397A1 (en) * | 2009-07-13 | 2012-06-28 | Qing Ge | Chemical modification of short small hairpin rnas for inhibition of gene expression |
| US8222221B2 (en) | 2008-06-04 | 2012-07-17 | The Board Of Regents Of The University Of Texas System | Modulation of gene expression through endogenous small RNA targeting of gene promoters |
| US20120202215A1 (en) * | 2005-11-24 | 2012-08-09 | Jichi Medical University | Mitochondrial function of prohibitin 2 (phb2) |
| WO2012110500A1 (en) | 2011-02-15 | 2012-08-23 | Vib Vzw | Means and methods for improvement of synaptic dysfunction disorders |
| WO2012119949A1 (en) | 2011-03-04 | 2012-09-13 | Vib Vzw | Means and methods for the treatment of neurodegenerative disorders |
| US8283333B2 (en) | 2009-07-01 | 2012-10-09 | Protiva Biotherapeutics, Inc. | Lipid formulations for nucleic acid delivery |
| US8293719B2 (en) | 2004-03-12 | 2012-10-23 | Alnylam Pharmaceuticals, Inc. | iRNA agents targeting VEGF |
| US20120283309A1 (en) * | 2009-11-26 | 2012-11-08 | Sharon Avkin-Nachum | Sirna compounds comprising terminal substitutions |
| US8324366B2 (en) | 2008-04-29 | 2012-12-04 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for delivering RNAI using lipoproteins |
| US8324367B2 (en) | 2006-11-03 | 2012-12-04 | Medtronic, Inc. | Compositions and methods for making therapies delivered by viral vectors reversible for safety and allele-specificity |
| US8334273B2 (en) | 2007-12-10 | 2012-12-18 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor VII gene |
| WO2012175735A1 (en) | 2011-06-23 | 2012-12-27 | Vib Vzw | A20 inhibitors for the treatment of respiratory viral infections |
| WO2012175798A2 (en) | 2011-06-22 | 2012-12-27 | Turun Yliopisto | Combination therapy |
| WO2013007766A1 (en) | 2011-07-13 | 2013-01-17 | Vib Vzw | Means and methods for the treatment of pathological angiogenesis |
| WO2013034806A1 (en) | 2011-09-06 | 2013-03-14 | Turun Yliopisto | Pharmaceutical combination comprising a cip2a silencing agent for use in the treatment of a hyperproliferative disorder, preferably one with impaired p53 function |
| US8455455B1 (en) | 2010-03-31 | 2013-06-04 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing genes involved in hemorrhagic fever |
| US20130164846A1 (en) * | 2010-06-23 | 2013-06-27 | Mina Therapeutics Limited | Rna molecules and uses thereof |
| US8524680B2 (en) | 2002-02-01 | 2013-09-03 | Applied Biosystems, Llc | High potency siRNAS for reducing the expression of target genes |
| US8546554B2 (en) | 2008-09-25 | 2013-10-01 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of Serum Amyloid A gene |
| US8569256B2 (en) | 2009-07-01 | 2013-10-29 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods for the delivery of therapeutic agents |
| US8569474B2 (en) | 2004-03-09 | 2013-10-29 | Isis Pharmaceuticals, Inc. | Double stranded constructs comprising one or more short strands hybridized to a longer strand |
| US8580493B2 (en) | 2009-06-08 | 2013-11-12 | Vib Vzw | Screening for compounds that modulate GPR3-mediated beta-arrestin signaling and amyloid beta peptide generation |
| US8592570B2 (en) | 2008-10-06 | 2013-11-26 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of an RNA from West Nile virus |
| WO2014009609A1 (en) | 2012-07-13 | 2014-01-16 | Turun Yliopisto | Combination therapy iii |
| US8648185B2 (en) | 2002-02-20 | 2014-02-11 | Merck Sharp & Dohme Corp. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20140088300A1 (en) * | 2012-09-24 | 2014-03-27 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Modified siRNA Molecules Incorporating 5-Fluoro-2'-Deoxyuridine Residues to Enhance Cytotoxicity |
| US8691971B2 (en) | 2008-09-23 | 2014-04-08 | Scott G. Petersen | Self delivering bio-labile phosphate protected pro-oligos for oligonucleotide based therapeutics and mediating RNA interference |
| US8697860B1 (en) | 2010-04-29 | 2014-04-15 | Isis Pharmaceuticals, Inc. | Diagnosis and treatment of disease |
| US20140135379A1 (en) * | 2007-06-29 | 2014-05-15 | Niigata University | Method of fixing and expressing physiologically active substance |
| US8765704B1 (en) | 2008-02-28 | 2014-07-01 | Novartis Ag | Modified small interfering RNA molecules and methods of use |
| US8765709B2 (en) | 2004-11-12 | 2014-07-01 | Asuragen, Inc. | Methods and compositions involving miRNA and miRNA inhibitor molecules |
| US8859516B2 (en) | 2009-09-15 | 2014-10-14 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of Eg5 and VEGF genes |
| US8957198B2 (en) | 2003-02-03 | 2015-02-17 | Medtronic, Inc. | Compositions, devices and methods for treatment of Huntington's disease through intracranial delivery of sirna |
| WO2015051366A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Novel formats for organic compounds for use in rna interference |
| WO2015051045A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | 3'END CAPS FOR RNAi AGENTS FOR USE IN RNA INTERFERENCE |
| WO2015050871A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Organic compounds to treat hepatitis b virus |
| WO2015051044A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Novel formats for organic compounds for use in rna interference |
| US9006417B2 (en) | 2010-06-30 | 2015-04-14 | Protiva Biotherapeutics, Inc. | Non-liposomal systems for nucleic acid delivery |
| US9018187B2 (en) | 2009-07-01 | 2015-04-28 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods for the delivery of therapeutic agents |
| US9023820B2 (en) | 2009-01-26 | 2015-05-05 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing apolipoprotein C-III expression |
| US9029338B2 (en) | 2009-08-14 | 2015-05-12 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of a gene from the ebola virus |
| US9051570B2 (en) | 2007-05-22 | 2015-06-09 | Arcturus Therapeutics, Inc. | UNA oligomers for therapeutics |
| US9051567B2 (en) | 2009-06-15 | 2015-06-09 | Tekmira Pharmaceuticals Corporation | Methods for increasing efficacy of lipid formulated siRNA |
| US9057069B2 (en) | 2006-03-31 | 2015-06-16 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of Eg5 gene |
| US9068184B2 (en) | 2011-06-21 | 2015-06-30 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of protein C (PROC) genes |
| US9074213B2 (en) | 2001-01-09 | 2015-07-07 | Alnylam Pharmacuticals, Inc. | Compositions and methods for inhibiting expression of a target gene |
| US9089590B2 (en) | 2003-12-04 | 2015-07-28 | University Of South Florida | Polynucleotides for reducing respiratory syncytial virus gene expression |
| US9101643B2 (en) | 2009-11-03 | 2015-08-11 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of transthyretin (TTR) |
| US9133517B2 (en) | 2005-06-28 | 2015-09-15 | Medtronics, Inc. | Methods and sequences to preferentially suppress expression of mutated huntingtin |
| US9139554B2 (en) | 2008-10-09 | 2015-09-22 | Tekmira Pharmaceuticals Corporation | Amino lipids and methods for the delivery of nucleic acids |
| US9145556B2 (en) | 2010-04-13 | 2015-09-29 | Life Technologies Corporation | Compositions and methods for inhibition of nucleic acids function |
| US9181551B2 (en) | 2002-02-20 | 2015-11-10 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US9187746B2 (en) | 2009-09-22 | 2015-11-17 | Alnylam Pharmaceuticals, Inc. | Dual targeting siRNA agents |
| US9193956B2 (en) | 2011-04-22 | 2015-11-24 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US9228188B2 (en) | 2011-06-21 | 2016-01-05 | Alnylam Pharmaceuticals, Inc. | Compositions and method for inhibiting hepcidin antimicrobial peptide (HAMP) or HAMP-related gene expression |
| US9228186B2 (en) | 2002-11-14 | 2016-01-05 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US9233131B2 (en) | 2003-06-30 | 2016-01-12 | The Regents Of The University Of California | Mutant adeno-associated virus virions and methods of use thereof |
| US9260471B2 (en) | 2010-10-29 | 2016-02-16 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acids (siNA) |
| US9260715B2 (en) | 2007-01-16 | 2016-02-16 | The University Of Queensland | Method of inducing an immune response |
| US9273356B2 (en) | 2006-05-24 | 2016-03-01 | Medtronic, Inc. | Methods and kits for linking polymorphic sequences to expanded repeat mutations |
| US9315813B2 (en) | 2011-06-21 | 2016-04-19 | Alnylam Pharmaceuticals, Inc | Compositions and methods for inhibition of expression of apolipoprotein C-III (APOC3) genes |
| US9399775B2 (en) | 2011-11-18 | 2016-07-26 | Alnylam Pharmaceuticals, Inc. | RNAi agents, compositions and methods of use thereof for treating transthyretin (TTR) associated diseases |
| US9441221B2 (en) | 2007-03-30 | 2016-09-13 | Rutgers, The State University Of New Jersey | Compositions and methods for gene silencing |
| US9457103B2 (en) | 2010-10-06 | 2016-10-04 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| WO2016161388A1 (en) | 2015-04-03 | 2016-10-06 | University Of Massachusetts | Fully stabilized asymmetric sirna |
| US9492386B2 (en) | 2002-06-28 | 2016-11-15 | Protiva Biotherapeutics, Inc. | Liposomal apparatus and manufacturing methods |
| US9506033B2 (en) * | 2012-05-22 | 2016-11-29 | University Of Massachusetts | Compositions and methods for inducing myoblast differentiation and myotube formation |
| US9605259B2 (en) | 2007-11-13 | 2017-03-28 | Ionis Pharmaceuticals, Inc. | Compounds and methods for modulating protein expression |
| US9657294B2 (en) | 2002-02-20 | 2017-05-23 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| CN106701758A (en) * | 2009-12-09 | 2017-05-24 | 日东电工株式会社 | Modulation of hsp47 expression |
| US9719092B2 (en) | 2002-11-14 | 2017-08-01 | Thermo Fisher Scientific Inc. | RNAi targeting CNTD2 |
| US9719094B2 (en) | 2002-11-14 | 2017-08-01 | Thermo Fisher Scientific Inc. | RNAi targeting SEC61G |
| US9771586B2 (en) | 2002-11-14 | 2017-09-26 | Thermo Fisher Scientific Inc. | RNAi targeting ZNF205 |
| US20170283795A1 (en) * | 2016-04-01 | 2017-10-05 | Avidity Biosciences Llc | Phosphatidylinositol-3-kinase nucleic acids and uses thereof |
| US20170283806A1 (en) * | 2016-04-01 | 2017-10-05 | Avidity Biosciences Llc | Kras nucleic acids and uses thereof |
| US20170283476A1 (en) * | 2016-04-01 | 2017-10-05 | Avidity Biosciences Llc | Myc nucleic acids and uses thereof |
| US9809817B2 (en) | 2015-04-03 | 2017-11-07 | University Of Massachusetts | Oligonucleotide compounds for targeting huntingtin mRNA |
| US9827263B2 (en) * | 2002-11-05 | 2017-11-28 | Ionis Pharmaceuticals, Inc. | 2′-methoxy substituted oligomeric compounds and compositions for use in gene modulations |
| US9839649B2 (en) | 2002-11-14 | 2017-12-12 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US9856475B2 (en) | 2014-03-25 | 2018-01-02 | Arcturus Therapeutics, Inc. | Formulations for treating amyloidosis |
| US9862952B2 (en) | 2015-04-03 | 2018-01-09 | University Of Massachusetts | Oligonucleotide compounds for treatment of preeclampsia and other angiogenic disorders |
| US9879253B2 (en) | 2003-12-22 | 2018-01-30 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of single and double blunt-ended siRNA |
| US9879266B2 (en) | 2002-11-14 | 2018-01-30 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US9950001B2 (en) | 2012-08-20 | 2018-04-24 | The Regents Of The University Of California | Polynucleotides having bioreversible groups |
| US9982259B2 (en) | 2014-03-25 | 2018-05-29 | Arcturus Therapeutics, Inc. | Transthyretin allele selective UNA oligomers for gene silencing |
| US9994853B2 (en) | 2001-05-18 | 2018-06-12 | Sirna Therapeutics, Inc. | Chemically modified multifunctional short interfering nucleic acid molecules that mediate RNA interference |
| US10011836B2 (en) | 2002-11-14 | 2018-07-03 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US10022454B2 (en) | 2008-09-23 | 2018-07-17 | Liposciences, Llc | Functionalized phosphorodiamites for therapeutic oligonucleotide synthesis |
| US20180216112A1 (en) * | 2015-07-21 | 2018-08-02 | Università Degli Studi Di Torino | Process for inducing resistance to diphtheria toxin in human cells, products and uses thereof |
| US10060921B2 (en) | 2014-08-29 | 2018-08-28 | Alnylam Pharmaceuticals, Inc. | Methods of treating transthyretin (TTR) mediated amyloidosis |
| US10208307B2 (en) | 2015-07-31 | 2019-02-19 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof for treating or preventing TTR-associated diseases |
| US10246709B2 (en) | 2016-03-07 | 2019-04-02 | Arrowhead Pharmaceuticals, Inc. | Targeting ligands for therapeutic compounds |
| US10294474B2 (en) | 2016-09-02 | 2019-05-21 | Arrowhead Pharmaceuticals, Inc. | Targeting ligands |
| US10421964B2 (en) | 2015-07-23 | 2019-09-24 | Arcturus Therapeutics, Inc. | UNA oligomers and compositions for treating amyloidosis |
| US10478503B2 (en) | 2016-01-31 | 2019-11-19 | University Of Massachusetts | Branched oligonucleotides |
| US10478500B2 (en) | 2014-10-10 | 2019-11-19 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of HAO1 (Hydroxyacid Oxidase 1 (Glycolate Oxidase)) gene expression |
| US10508277B2 (en) | 2004-05-24 | 2019-12-17 | Sirna Therapeutics, Inc. | Chemically modified multifunctional short interfering nucleic acid molecules that mediate RNA interference |
| US10513703B2 (en) | 2014-11-10 | 2019-12-24 | Alnylam Pharmaceuticals, Inc. | Hepatitis B virus (HBV) iRNA compositions and methods of use thereof |
| US10519447B2 (en) | 2015-04-01 | 2019-12-31 | Arcturus Therapeutics, Inc. | Therapeutic UNA oligomers and uses thereof |
| US10633653B2 (en) | 2015-08-14 | 2020-04-28 | University Of Massachusetts | Bioactive conjugates for oligonucleotide delivery |
| US10683500B2 (en) | 2014-03-25 | 2020-06-16 | Arcturus Therapeutics, Inc. | UNA oligomers having reduced off-target effects in gene silencing |
| US10844377B2 (en) | 2017-06-23 | 2020-11-24 | University Of Massachusetts | Two-tailed self-delivering siRNA |
| US10883117B2 (en) | 2015-03-24 | 2021-01-05 | The Regents Of The University Of California | Adeno-associated virus variants and methods of use thereof |
| US10982212B2 (en) | 2003-06-12 | 2021-04-20 | Alnylam Pharmaceuticals, Inc. | Conserved HBV and HCV sequences useful for gene silencing |
| US11021519B2 (en) | 2015-03-02 | 2021-06-01 | Adverum Biotechnologies, Inc. | Compositions and methods for intravitreal delivery of polynucleotides to retinal cones |
| WO2021160937A1 (en) | 2020-02-11 | 2021-08-19 | Turun Yliopisto | Therapy of ras-dependent cancers |
| US11136557B2 (en) | 2013-05-31 | 2021-10-05 | The Regents Of The University Of California | Adeno-associated virus variants and methods of use thereof |
| US11142766B2 (en) | 2014-11-17 | 2021-10-12 | Alnylam Pharmaceuticals, Inc. | Apolipoprotein C3 (APOC3) iRNA compositions and methods of use thereof |
| US11192925B2 (en) | 2016-10-19 | 2021-12-07 | Adverum Biotechnologies, Inc. | Modified AAV capsids and uses thereof |
| US11202795B2 (en) | 2014-11-20 | 2021-12-21 | Vib Vzw | Means and methods for treatment of early-onset Parkinson's disease |
| US11248214B2 (en) | 2014-03-17 | 2022-02-15 | Adverum Biotechnologies, Inc. | Compositions and methods for enhanced gene expression in cone cells |
| US11261447B2 (en) | 2017-07-13 | 2022-03-01 | Alnylam Pharmaceuticals, Inc. | Methods for inhibition of HAO1 (hydroxyacid oxidase 1 (glycolate oxidase)) gene expression |
| US11279930B2 (en) | 2018-08-23 | 2022-03-22 | University Of Massachusetts | O-methyl rich fully stabilized oligonucleotides |
| US11324820B2 (en) | 2017-04-18 | 2022-05-10 | Alnylam Pharmaceuticals, Inc. | Methods for the treatment of subjects having a hepatitis b virus (HBV) infection |
| US11492623B2 (en) | 2018-08-13 | 2022-11-08 | Alnylam Pharmaceuticals, Inc. | Hepatitis B virus (HBV) dsRNA agent compositions and methods of use thereof |
| US11554180B2 (en) | 2016-07-29 | 2023-01-17 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US11591544B2 (en) | 2020-11-25 | 2023-02-28 | Akagera Medicines, Inc. | Ionizable cationic lipids |
| US11597929B2 (en) | 2008-12-18 | 2023-03-07 | Dicerna Pharmaceuticals, Inc. | Extended dicer substrate agents and methods for the specific inhibition of gene expression |
| US11597744B2 (en) | 2017-06-30 | 2023-03-07 | Sirius Therapeutics, Inc. | Chiral phosphoramidite auxiliaries and methods of their use |
| US11680249B2 (en) | 2017-08-28 | 2023-06-20 | The Regents Of The University Of California | Adeno-associated virus capsid variants and methods of use thereof |
| US11702659B2 (en) | 2021-06-23 | 2023-07-18 | University Of Massachusetts | Optimized anti-FLT1 oligonucleotide compounds for treatment of preeclampsia and other angiogenic disorders |
| US11753638B2 (en) | 2016-08-12 | 2023-09-12 | University Of Massachusetts | Conjugated oligonucleotides |
| US11806360B2 (en) | 2017-09-19 | 2023-11-07 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for treating transthyretin (TTR) mediated amyloidosis |
| US11827882B2 (en) | 2018-08-10 | 2023-11-28 | University Of Massachusetts | Modified oligonucleotides targeting SNPs |
| US11959081B2 (en) | 2021-08-03 | 2024-04-16 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof |
| US11981703B2 (en) | 2016-08-17 | 2024-05-14 | Sirius Therapeutics, Inc. | Polynucleotide constructs |
| US12024706B2 (en) | 2019-08-09 | 2024-07-02 | University Of Massachusetts | Modified oligonucleotides targeting SNPs |
| US12064479B2 (en) | 2022-05-25 | 2024-08-20 | Akagera Medicines, Inc. | Lipid nanoparticles for delivery of nucleic acids and methods of use thereof |
| US12180477B2 (en) | 2019-01-18 | 2024-12-31 | University Of Massachusetts | Dynamic pharmacokinetic-modifying anchors |
| US12310997B2 (en) | 2017-06-30 | 2025-05-27 | The Regents Of The University Of California | Compositions and methods of treating ocular diseases |
| US12331297B2 (en) | 2020-11-13 | 2025-06-17 | Alnylam Pharmaceuticals, Inc. | Coagulation factor V (F5) iRNA compositions and methods of use thereof |
| US12365894B2 (en) | 2019-09-16 | 2025-07-22 | University Of Massachusetts | Branched lipid conjugates of siRNA for specific tissue delivery |
Families Citing this family (82)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050124568A1 (en) * | 2001-05-18 | 2005-06-09 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of acetyl-CoA-carboxylase gene expression using short interfering nucleic acid (siNA) |
| US20050054596A1 (en) * | 2001-11-30 | 2005-03-10 | Mcswiggen James | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050203040A1 (en) * | 2001-05-18 | 2005-09-15 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular cell adhesion molecule (VCAM) gene expression using short interfering nucleic acid (siNA) |
| US20050159382A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of polycomb group protein EZH2 gene expression using short interfering nucleic acid (siNA) |
| US7795422B2 (en) * | 2002-02-20 | 2010-09-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of hypoxia inducible factor 1 (HIF1) gene expression using short interfering nucleic acid (siNA) |
| US20090137513A1 (en) * | 2002-02-20 | 2009-05-28 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition of Acetyl-CoA-Carboxylase Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| EP1504126B1 (en) | 2002-05-03 | 2014-02-26 | Duke University | A method of regulating gene expression |
| EP1506020A4 (en) * | 2002-05-23 | 2007-08-29 | Mirus Bio Corp | Processes for inhibiting gene expression using polynucleotides |
| US20040248094A1 (en) * | 2002-06-12 | 2004-12-09 | Ford Lance P. | Methods and compositions relating to labeled RNA molecules that reduce gene expression |
| US20100075423A1 (en) * | 2002-06-12 | 2010-03-25 | Life Technologies Corporation | Methods and compositions relating to polypeptides with rnase iii domains that mediate rna interference |
| GB2406169B (en) * | 2002-06-12 | 2006-11-01 | Ambion Inc | Methods and compositions relating to labeled rna molecules that reduce gene expression |
| US8318922B2 (en) * | 2002-08-29 | 2012-11-27 | The Hong Kong Polytechnic University | Treatment and prevention of hyperproliferative conditions in humans and antisense oligonucleotide inhibition of human replication-initiation proteins |
| US20080214437A1 (en) * | 2002-09-06 | 2008-09-04 | Mohapatra Shyam S | Methods and compositions for reducing activity of the atrial natriuretic peptide receptor and for treatment of diseases |
| WO2004022003A2 (en) | 2002-09-06 | 2004-03-18 | University Of South Florida | Materials and methods for treatment of allergic diseases |
| EP1555874A4 (en) * | 2002-10-10 | 2006-10-04 | Oxford Biomedica Ltd | Gene regulation with aptamer and modulator complexes for gene therapy |
| AU2003297062A1 (en) * | 2002-12-11 | 2004-06-30 | University Of Massachusetts | METHOD OF INTRODUCING siRNA INTO ADIPOCYTES |
| US7521534B1 (en) | 2003-03-03 | 2009-04-21 | The University Board Of Regents Of Texas System | IKK gamma gene products and methods for making and using same |
| DK1606406T4 (en) | 2003-03-21 | 2013-12-16 | Santaris Pharma As | Short Interfering RNA (siRNA) Analogues |
| US20050020526A1 (en) * | 2003-06-03 | 2005-01-27 | Cytogenix, Inc. | Oligodeoxynucleotide intervention for prevention and treatment of sepsis |
| US20070202505A1 (en) * | 2003-09-08 | 2007-08-30 | Alex Chenchik | Methods for gene function analysis |
| US20050059019A1 (en) * | 2003-09-11 | 2005-03-17 | Sven Bulow | Gene-related RNAi transfection method |
| JP2007505634A (en) * | 2003-09-22 | 2007-03-15 | ロゼッタ インファーマティクス エルエルシー | Synthetic lethal screening using RNA interference |
| DE10346721A1 (en) * | 2003-10-08 | 2005-05-04 | Holger Kalthoff | New oligonucleotides, useful for treating cancer, especially of the pancreas, are not species specific but induce apoptosis or inhibit proliferation |
| WO2005037868A2 (en) * | 2003-10-16 | 2005-04-28 | Case Western Reserve University | Methods of treating nfat-related disorders |
| JP2007522793A (en) * | 2003-10-23 | 2007-08-16 | サーナ・セラピューティクス・インコーポレイテッド | Inhibition of NOGO and / or NOGO receptor gene expression via RNA interference using short interfering nucleic acids (siNA) |
| US20050164970A1 (en) * | 2003-12-22 | 2005-07-28 | University Of Kansas Medical Center | Method for treating prostate cancer using siRNA duplex for androgen receptor |
| EP1713912B1 (en) * | 2004-01-30 | 2013-09-18 | Santaris Pharma A/S | Modified short interfering rna (modified sirna) |
| WO2005094420A2 (en) * | 2004-02-17 | 2005-10-13 | University Of South Florida | Materials and methods for treatment of inflammatory and cell proliferation disorders |
| WO2005085443A2 (en) * | 2004-03-01 | 2005-09-15 | Massachusetts Institute Of Technology | Rnai-based therapeutics for allergic rhinitis and asthma |
| DE102004010547A1 (en) * | 2004-03-03 | 2005-11-17 | Beiersdorf Ag | Oligoribonucleotides for the treatment of irritative and / or inflammatory skin conditions by RNA interference |
| US20050272682A1 (en) * | 2004-03-22 | 2005-12-08 | Evers Bernard M | SiRNA targeting PI3K signal transduction pathway and siRNA-based therapy |
| US7563885B1 (en) * | 2004-05-24 | 2009-07-21 | Isis Pharmaceuticals, Inc. | Modulation of Tudor-SN expression |
| US20060253100A1 (en) | 2004-10-22 | 2006-11-09 | Medtronic, Inc. | Systems and Methods to Treat Pain Locally |
| US20090111786A1 (en) * | 2004-12-03 | 2009-04-30 | Glass Christopher K | Compounds that Prevent Macrophage Apoptosis and Uses Thereof |
| AU2005315143B2 (en) * | 2004-12-14 | 2011-05-26 | National Institute Of Immunology | Dnazymes for inhibition of Japanese Encephalitis Virus replication |
| US20060142228A1 (en) * | 2004-12-23 | 2006-06-29 | Ambion, Inc. | Methods and compositions concerning siRNA's as mediators of RNA interference |
| TW200639252A (en) * | 2005-02-01 | 2006-11-16 | Alcon Inc | RNAi-mediated inhibition of ocular hypertension targets |
| US20100129288A1 (en) * | 2005-06-28 | 2010-05-27 | Elior Peles | Gliomedin, Fragments Thereof and Methods of Using Same |
| US7919583B2 (en) * | 2005-08-08 | 2011-04-05 | Discovery Genomics, Inc. | Integration-site directed vector systems |
| TWI333959B (en) * | 2005-08-31 | 2010-12-01 | Academia Sinica | Methods and reagents for the analysis and purification of polysaccharides |
| US7943134B2 (en) | 2005-08-31 | 2011-05-17 | Academia Sinica | Compositions and methods for identifying response targets and treating flavivirus infection responses |
| CA2638837A1 (en) * | 2006-01-27 | 2007-08-02 | Santaris Pharma A/S | Lna modified phosphorothiolated oligonucleotides |
| EP2002004B1 (en) * | 2006-03-23 | 2015-10-14 | Roche Innovation Center Copenhagen A/S | Small internally segmented interfering rna |
| EP2051585A4 (en) * | 2006-04-28 | 2010-06-02 | Univ South Florida | MATERIALS AND METHODS FOR REDUCING INFLAMMATION BY INHIBITION OF THE ATRIAL NATRIURETIC PEPTIDE RECEPTOR |
| EP2471815B1 (en) | 2006-07-11 | 2016-03-30 | University Of Medicine And Dentistry Of New Jersey | Proteins, nucleic acids encoding the same and associated methods of use |
| US7951789B2 (en) | 2006-12-28 | 2011-05-31 | Idenix Pharmaceuticals, Inc. | Compounds and pharmaceutical compositions for the treatment of viral infections |
| EP2769731A1 (en) | 2007-02-23 | 2014-08-27 | The Trustees of Columbia University in the City of New York | Methods to activate or block the HLA-E/QA-1 restricted CD8+ T cell regulatory pathway to treat immunological disease |
| DK2494993T3 (en) | 2007-05-04 | 2018-11-12 | Marina Biotech Inc | Amino acid lipids and uses thereof |
| DK2548962T3 (en) | 2007-09-19 | 2016-04-11 | Applied Biosystems Llc | Sirna sequence-independent modification formats to reduce off-target phenotype effects in RNAI and stabilized forms thereof |
| RU2542973C2 (en) * | 2007-11-30 | 2015-02-27 | Ноксон Фарма Аг | Mcp-1 binding nucleic acid and using it |
| USRE48948E1 (en) | 2008-04-18 | 2022-03-01 | Warsaw Orthopedic, Inc. | Clonidine compounds in a biodegradable polymer |
| US20090291073A1 (en) * | 2008-05-20 | 2009-11-26 | Ward Keith W | Compositions Comprising PKC-theta and Methods for Treating or Controlling Ophthalmic Disorders Using Same |
| US20100015708A1 (en) * | 2008-06-18 | 2010-01-21 | Mdrna, Inc. | Ribonucleic acids with non-standard bases and uses thereof |
| EP2307434B1 (en) * | 2008-07-02 | 2014-02-12 | IDENIX Pharmaceuticals, Inc. | Compounds and pharmaceutical compositions for the treatment of viral infections |
| US8309791B2 (en) * | 2008-07-16 | 2012-11-13 | Recombinectics, Inc. | Method for producing a transgenic pig using a hyper-methylated transposon |
| CA2738625C (en) * | 2008-09-22 | 2017-12-12 | Dicerna Pharmaceuticals, Inc. | Compositions and methods for the specific inhibition of gene expression by dsrna possessing modifications |
| CN104382853A (en) | 2008-10-16 | 2015-03-04 | 玛瑞纳生物技术有限公司 | Processes and Compositions for Liposomal and Efficient Delivery of Gene Silencing Therapeutics |
| EP2381934A2 (en) | 2008-12-23 | 2011-11-02 | Carmel - Haifa University Economic Corp Ltd. | Improving cognitive function |
| US20100239632A1 (en) | 2009-03-23 | 2010-09-23 | Warsaw Orthopedic, Inc. | Drug depots for treatment of pain and inflammation in sinus and nasal cavities or cardiac tissue |
| US8598327B2 (en) * | 2009-08-18 | 2013-12-03 | Baxter International Inc. | Aptamers to tissue factor pathway inhibitor and their use as bleeding disorder therapeutics |
| WO2011123586A1 (en) | 2010-04-01 | 2011-10-06 | Idenix Pharmaceuticals, Inc. | Compounds and pharmaceutical compositions for the treatment of viral infections |
| US9243246B2 (en) | 2010-08-24 | 2016-01-26 | Sirna Therapeutics, Inc. | Single-stranded RNAi agents containing an internal, non-nucleic acid spacer |
| US10184942B2 (en) | 2011-03-17 | 2019-01-22 | University Of South Florida | Natriuretic peptide receptor as a biomarker for diagnosis and prognosis of cancer |
| WO2012123591A1 (en) * | 2011-03-17 | 2012-09-20 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Method for targeting nucleic acids to the nucleus |
| EP2691409B1 (en) | 2011-03-31 | 2018-02-21 | Idenix Pharmaceuticals LLC. | Compounds and pharmaceutical compositions for the treatment of viral infections |
| AU2012250924B2 (en) | 2011-05-02 | 2017-05-25 | Immunomedics, Inc. | Ultrafiltration concentration of allotype selected antibodies for small-volume administration |
| US9403863B2 (en) | 2011-09-12 | 2016-08-02 | Idenix Pharmaceuticals Llc | Substituted carbonyloxymethylphosphoramidate compounds and pharmaceutical compositions for the treatment of viral infections |
| CN109513003A (en) | 2012-08-14 | 2019-03-26 | Ibc药品公司 | T- cell for treating disease redirects bispecific antibody |
| CN105143203A (en) | 2013-04-17 | 2015-12-09 | 辉瑞大药厂 | N-piperidin-3-ylbenzamide derivatives for treating cardiovascular diseases |
| CN106132436B (en) | 2014-02-21 | 2021-06-15 | Ibc药品公司 | Disease therapy by inducing an immune response to TROP-2 expressing cells |
| US9139649B2 (en) | 2014-02-25 | 2015-09-22 | Immunomedics, Inc. | Humanized anti-CD22 antibody |
| KR101668074B1 (en) * | 2015-02-12 | 2016-10-21 | 전북대학교산학협력단 | Composition comprising PKR inhibitor for preventing or treating severe bronchial asthma |
| JP6770522B2 (en) | 2015-02-13 | 2020-10-14 | アンスティチュ ナショナル ドゥ ラ サンテ エ ドゥ ラ ルシェルシュ メディカル | PTGDR-1 and / or PTGDR-2 antagonists for preventing and / or treating systemic lupus erythematosus |
| US10988765B2 (en) | 2015-08-27 | 2021-04-27 | The General Hospital Corporation | Methods and compositions for inhibiting detoxification response |
| WO2017161043A1 (en) | 2016-03-16 | 2017-09-21 | The J. David Gladstone Institutes | Methods and compositions for treating obesity and/or diabetes and for identifying candidate treatment agents |
| ES2674128B1 (en) * | 2016-12-27 | 2019-04-10 | Univ Salamanca | Method for diagnosing allergic sensitization in a subject |
| WO2018224162A1 (en) * | 2017-06-09 | 2018-12-13 | Biontech Rna Pharmaceuticals Gmbh | Methods for characterizing loss of antigen presentation |
| CA3103756A1 (en) * | 2018-06-22 | 2019-12-26 | F. Hoffman-La Roche Ag | Oligonucleotides for modulating scn9a expression |
| EP3856908A1 (en) | 2018-09-26 | 2021-08-04 | Greenlight Biosciences, Inc. | Control of coleopteran insects |
| GB201817990D0 (en) | 2018-11-02 | 2018-12-19 | Univ Of Essex Enterprise Limited | Enzymatic nucleic acid molecules |
| CA3118148A1 (en) | 2018-11-08 | 2020-05-14 | Greenlight Biosciences, Inc. | Control of insect infestation |
| JP2023528434A (en) * | 2020-06-03 | 2023-07-04 | パイオニア ハイ-ブレッド インターナショナル, インコーポレイテッド | Corn event DP-915635-4 and its detection method |
Citations (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5320962A (en) * | 1992-07-22 | 1994-06-14 | Duke University | DNA encoding the human A1 adenosine receptor |
| US5334711A (en) * | 1991-06-20 | 1994-08-02 | Europaisches Laboratorium Fur Molekularbiologie (Embl) | Synthetic catalytic oligonucleotide structures |
| US5624803A (en) * | 1993-10-14 | 1997-04-29 | The Regents Of The University Of California | In vivo oligonucleotide generator, and methods of testing the binding affinity of triplex forming oligonucleotides derived therefrom |
| US5627053A (en) * | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| US5670633A (en) * | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US5672695A (en) * | 1990-10-12 | 1997-09-30 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften E.V. | Modified ribozymes |
| US5716824A (en) * | 1995-04-20 | 1998-02-10 | Ribozyme Pharmaceuticals, Inc. | 2'-O-alkylthioalkyl and 2-C-alkylthioalkyl-containing enzymatic nucleic acids (ribozymes) |
| US5792847A (en) * | 1989-10-24 | 1998-08-11 | Gilead Sciences, Inc. | 2' Modified Oligonucleotides |
| US5801154A (en) * | 1993-10-18 | 1998-09-01 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of multidrug resistance-associated protein |
| US5814620A (en) * | 1993-07-27 | 1998-09-29 | Hybridon, Inc. | Inhibition of neovascularization using vegf-specific oligonucleotides |
| US5854038A (en) * | 1993-01-22 | 1998-12-29 | University Research Corporation | Localization of a therapeutic agent in a cell in vitro |
| US5898031A (en) * | 1996-06-06 | 1999-04-27 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides for cleaving RNA |
| US5902880A (en) * | 1994-08-19 | 1999-05-11 | Ribozyme Pharmaceuticals, Inc. | RNA polymerase III-based expression of therapeutic RNAs |
| US5994315A (en) * | 1995-06-07 | 1999-11-30 | East Carolina University | Low adenosine agent, composition, kit and method for treatment of airway disease |
| US5998203A (en) * | 1996-04-16 | 1999-12-07 | Ribozyme Pharmaceuticals, Inc. | Enzymatic nucleic acids containing 5'-and/or 3'-cap structures |
| US6001311A (en) * | 1997-02-05 | 1999-12-14 | Protogene Laboratories, Inc. | Apparatus for diverse chemical synthesis using two-dimensional array |
| US6005087A (en) * | 1995-06-06 | 1999-12-21 | Isis Pharmaceuticals, Inc. | 2'-modified oligonucleotides |
| US6248878B1 (en) * | 1996-12-24 | 2001-06-19 | Ribozyme Pharmaceuticals, Inc. | Nucleoside analogs |
| US6258601B1 (en) * | 2000-09-07 | 2001-07-10 | Isis Pharmaceuticals, Inc. | Antisense modulation of ubiquitin protein ligase expression |
| US6300074B1 (en) * | 1990-06-11 | 2001-10-09 | Gilead Sciences, Inc. | Systematic evolution of ligands by exponential enrichment: Chemi-SELEX |
| US6395713B1 (en) * | 1997-07-23 | 2002-05-28 | Ribozyme Pharmaceuticals, Inc. | Compositions for the delivery of negatively charged molecules |
| US6506559B1 (en) * | 1997-12-23 | 2003-01-14 | Carnegie Institute Of Washington | Genetic inhibition by double-stranded RNA |
| US6573099B2 (en) * | 1998-03-20 | 2003-06-03 | Benitec Australia, Ltd. | Genetic constructs for delaying or repressing the expression of a target gene |
| US6617438B1 (en) * | 1997-11-05 | 2003-09-09 | Sirna Therapeutics, Inc. | Oligoribonucleotides with enzymatic activity |
Family Cites Families (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2359130A (en) * | 1942-02-13 | 1944-09-26 | Gen Electric | Electric valve circuits |
| US2359180A (en) * | 1942-08-11 | 1944-09-26 | Gen Motors Corp | Dynamic balancer |
| US4987071A (en) | 1986-12-03 | 1991-01-22 | University Patents, Inc. | RNA ribozyme polymerases, dephosphorylases, restriction endoribonucleases and methods |
| PT88550A (en) | 1987-09-21 | 1989-07-31 | Ml Tecnology Ventures Lp | PROCESS FOR THE PREPARATION OF NON-NUCLEOTIDIC LIGACATION REAGENTS FOR NUCLEOTIDIAL PROBES |
| US5719197A (en) * | 1988-03-04 | 1998-02-17 | Noven Pharmaceuticals, Inc. | Compositions and methods for topical administration of pharmaceutically active agents |
| CA1340323C (en) | 1988-09-20 | 1999-01-19 | Arnold E. Hampel | Rna catalyst for cleaving specific rna sequences |
| JPH04507083A (en) | 1989-05-19 | 1992-12-10 | ヘム・リサーチ・インコーポレーテッド | Short therapeutic dsRNA of defined structure |
| WO1991003162A1 (en) | 1989-08-31 | 1991-03-21 | City Of Hope | Chimeric dna-rna catalytic sequences |
| US5567588A (en) * | 1990-06-11 | 1996-10-22 | University Research Corporation | Systematic evolution of ligands by exponential enrichment: Solution SELEX |
| US5652094A (en) | 1992-01-31 | 1997-07-29 | University Of Montreal | Nucleozymes |
| US5294433A (en) * | 1992-04-15 | 1994-03-15 | The Procter & Gamble Company | Use of H-2 antagonists for treatment of gingivitis |
| WO1993023569A1 (en) | 1992-05-11 | 1993-11-25 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for inhibiting viral replication |
| EP0641212A4 (en) | 1992-05-14 | 1997-05-21 | Ribozyme Pharm Inc | Method and reagent for inhibiting cancer development. |
| US5525468A (en) * | 1992-05-14 | 1996-06-11 | Ribozyme Pharmaceuticals, Inc. | Assay for Ribozyme target site |
| US20030206887A1 (en) * | 1992-05-14 | 2003-11-06 | David Morrissey | RNA interference mediated inhibition of hepatitis B virus (HBV) using short interfering nucleic acid (siNA) |
| RU94046425A (en) | 1992-07-02 | 1997-03-20 | Хайбрайдон | Self-stabilized oligonucleotide and method of genetic expression inhibition |
| EP0654077A4 (en) | 1992-07-17 | 1996-03-13 | Ribozyme Pharm Inc | Method and reagent for treatment of animal diseases. |
| EP1195442A3 (en) * | 1992-12-04 | 2002-07-31 | Innovir Laboratories, Inc. | Ribozyme amplified diagnostics |
| WO1994013791A1 (en) * | 1992-12-04 | 1994-06-23 | Innovir Laboratories, Inc. | Regulatable nucleic acid therapeutic and methods of use thereof |
| US5616488A (en) * | 1992-12-07 | 1997-04-01 | Ribozyme Pharmaceuticals, Inc. | IL-5 targeted ribozymes |
| US5871914A (en) * | 1993-06-03 | 1999-02-16 | Intelligene Ltd. | Method for detecting a nucleic acid involving the production of a triggering RNA and transcription amplification |
| US6410322B1 (en) | 1993-07-27 | 2002-06-25 | Hybridon Inc | Antisense oligonucleotide inhibition of vascular endothelial growth factor expression |
| WO1995004818A1 (en) | 1993-08-06 | 1995-02-16 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for inhibiting human immunodeficiency virus replication |
| PT748382E (en) | 1993-09-02 | 2003-03-31 | Ribozyme Pharm Inc | NUCLEIC ENZYMAL ACIDS CONTAINING NON-NUCLEOTIDES |
| US5861288A (en) | 1993-10-18 | 1999-01-19 | Ribozyme Pharmaceuticals, Inc. | Catalytic DNA |
| WO1995011910A1 (en) | 1993-10-27 | 1995-05-04 | Ribozyme Pharmaceuticals, Inc. | 2'-amido and 2'-peptido modified oligonucleotides |
| AU704687B2 (en) | 1993-11-12 | 1999-04-29 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for treatment of arthritic conditions |
| US6060456A (en) * | 1993-11-16 | 2000-05-09 | Genta Incorporated | Chimeric oligonucleoside compounds |
| US5587471A (en) * | 1994-01-11 | 1996-12-24 | Isis Pharmaceuticals, Inc. | Method of making oligonucleotide libraries |
| US5631359A (en) * | 1994-10-11 | 1997-05-20 | Ribozyme Pharmaceuticals, Inc. | Hairpin ribozymes |
| CA2183992A1 (en) | 1994-02-23 | 1995-08-31 | Dan T. Stinchcomb | Method and reagent for inhibiting the expression of disease related genes |
| JPH10501686A (en) * | 1994-04-13 | 1998-02-17 | ザ ロックフェラー ユニヴァーシティ | AAV-mediated delivery of DNA to cells of the nervous system |
| US5633133A (en) * | 1994-07-14 | 1997-05-27 | Long; David M. | Ligation with hammerhead ribozymes |
| US5519059A (en) * | 1994-08-17 | 1996-05-21 | Sawaya; Assad S. | Antifungal formulation |
| US6146886A (en) * | 1994-08-19 | 2000-11-14 | Ribozyme Pharmaceuticals, Inc. | RNA polymerase III-based expression of therapeutic RNAs |
| US5753613A (en) | 1994-09-30 | 1998-05-19 | Inex Pharmaceuticals Corporation | Compositions for the introduction of polyanionic materials into cells |
| US5885613A (en) | 1994-09-30 | 1999-03-23 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| US5820873A (en) | 1994-09-30 | 1998-10-13 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| DE4445700A1 (en) | 1994-12-21 | 1996-06-27 | Forschungszentrum Juelich Gmbh | Gradiometer |
| US6025339A (en) * | 1995-06-07 | 2000-02-15 | East Carolina University | Composition, kit and method for treatment of disorders associated with bronchoconstriction and lung inflammation |
| US6346398B1 (en) * | 1995-10-26 | 2002-02-12 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for the treatment of diseases or conditions related to levels of vascular endothelial growth factor receptor |
| EP1108724B1 (en) | 1996-01-16 | 2007-09-19 | Sirna Therpeutics, Inc. | Synthesis of methoxy nucleosides and enzymatic nucleic acid molecules |
| US6214805B1 (en) * | 1996-02-15 | 2001-04-10 | The United States Of America As Represented By The Department Of Health And Human Services | RNase L activators and antisense oligonucleotides effective to treat RSV infections |
| US20040161844A1 (en) * | 1996-06-06 | 2004-08-19 | Baker Brenda F. | Sugar and backbone-surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US5849902A (en) * | 1996-09-26 | 1998-12-15 | Oligos Etc. Inc. | Three component chimeric antisense oligonucleotides |
| US5989912A (en) * | 1996-11-21 | 1999-11-23 | Oligos Etc. Inc. | Three component chimeric antisense oligonucleotides |
| EP0958303A4 (en) | 1996-12-19 | 2004-03-31 | Univ Yale | BIOREACTIVE ALLOSTER POLYNUCLEOTIDES |
| JP3903392B2 (en) | 1996-12-24 | 2007-04-11 | サーナ・セラピューティクス・インコーポレイテッド | Chemical synthesis of nucleoside analogues and their introduction into polynucleotides. |
| US20030064945A1 (en) * | 1997-01-31 | 2003-04-03 | Saghir Akhtar | Enzymatic nucleic acid treatment of diseases or conditions related to levels of epidermal growth factor receptors |
| WO1998043993A2 (en) | 1997-03-31 | 1998-10-08 | Yale University | Nucleic acid catalysts |
| WO1998058058A1 (en) | 1997-06-19 | 1998-12-23 | Ribozyme Pharmaceuticals, Inc. | Hammerhead ribozymes with extended cleavage rule |
| US20030035829A1 (en) | 1997-07-24 | 2003-02-20 | Townsend And Townsend And Crew | Liposomal compositions for the delivery of nucleic acid catalysts |
| WO1999016871A2 (en) | 1997-09-22 | 1999-04-08 | Max-Planck-Gesellschaft Zur Forderung Der Wissensc | Nucleic acid catalysts with endonuclease activity |
| JP2002506612A (en) | 1997-12-05 | 2002-03-05 | デューク・ユニバーシティー | Nucleic acid-mediated RNA tagging and RNA repair |
| ES2374534T3 (en) | 1998-03-20 | 2012-02-17 | Commonwealth Scientific And Industrial Research Organisation | GENES EXPRESSION CONTROL. |
| NZ507093A (en) | 1998-04-08 | 2003-08-29 | Commw Scient Ind Res Org | Methods and means for reducing the phenotypic expression of a nucleic acid of interest in a plant |
| CA2326823A1 (en) | 1998-04-20 | 1999-10-28 | Ribozyme Pharmaceuticals, Inc. | Nucleic acid molecules with novel chemical compositions capable of modulating gene expression |
| AU751480B2 (en) | 1998-04-29 | 2002-08-15 | Ribozyme Pharmaceuticals, Inc. | Nucleoside triphosphates and their incorporation into ribozymes |
| AR020078A1 (en) | 1998-05-26 | 2002-04-10 | Syngenta Participations Ag | METHOD FOR CHANGING THE EXPRESSION OF AN OBJECTIVE GENE IN A PLANT CELL |
| IL126731A0 (en) | 1998-10-23 | 1999-08-17 | Intelligene Ltd | A method of detection |
| EP1133513A4 (en) | 1998-11-03 | 2002-07-03 | Univ Yale | MOLECULAR SENSORS CONSISTING OF SEVERAL DOMAINS OF POLYNUCLEOTIDES |
| EP2314700A1 (en) | 1999-01-28 | 2011-04-27 | Medical College of Georgia Research Institute, Inc | Composition and method for in vivo and in vitro attenuation of gene expression using double stranded RNA |
| DE19956568A1 (en) | 1999-01-30 | 2000-08-17 | Roland Kreutzer | Method and medicament for inhibiting the expression of a given gene |
| AU3369900A (en) | 1999-02-19 | 2000-09-04 | General Hospital Corporation, The | Gene silencing |
| US5998206A (en) * | 1999-02-23 | 1999-12-07 | Isis Pharmaceuticals Inc. | Antisense inhibiton of human G-alpha-12 expression |
| US6197061B1 (en) * | 1999-03-01 | 2001-03-06 | Koichi Masuda | In vitro production of transplantable cartilage tissue cohesive cartilage produced thereby, and method for the surgical repair of cartilage damage |
| JP2000253884A (en) | 1999-03-10 | 2000-09-19 | Toagosei Co Ltd | Antisense nucleic acid compound |
| US5998148A (en) * | 1999-04-08 | 1999-12-07 | Isis Pharmaceuticals Inc. | Antisense modulation of microtubule-associated protein 4 expression |
| CA2370628A1 (en) | 1999-04-21 | 2000-10-26 | American Home Products Corporation | Methods and compositions for inhibiting the function of polynucleotide sequences |
| GB9927444D0 (en) | 1999-11-19 | 2000-01-19 | Cancer Res Campaign Tech | Inhibiting gene expression |
| EP1248803B1 (en) * | 2000-01-12 | 2010-05-05 | Yale University | Nogo receptor-mediated blockade of axonal growth |
| US6602857B1 (en) | 2000-01-18 | 2003-08-05 | Isis Pharmaceuticals, Inc. | Antisense modulation of PTP1B expression |
| WO2001057206A2 (en) * | 2000-02-03 | 2001-08-09 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for the inhibition of checkpoint kinase-1 (chk 1) enzyme |
| US6831171B2 (en) * | 2000-02-08 | 2004-12-14 | Yale University | Nucleic acid catalysts with endonuclease activity |
| WO2002081628A2 (en) * | 2001-04-05 | 2002-10-17 | Ribozyme Pharmaceuticals, Incorporated | Modulation of gene expression associated with inflammation proliferation and neurite outgrowth, using nucleic acid based technologies |
| PT1309726E (en) * | 2000-03-30 | 2010-03-08 | Whitehead Biomedical Inst | RNA INTERFERENCE-SPECIFIC RNA INTERFERENCE MEDIATORS |
| US6824972B2 (en) * | 2000-05-22 | 2004-11-30 | Baylor College Of Medicine | Diagnosis and treatment of medical conditions associated with defective NFkappa B(NF-κB) activation |
| AU2001275474A1 (en) | 2000-06-12 | 2001-12-24 | Akkadix Corporation | Materials and methods for the control of nematodes |
| JP2003535910A (en) | 2000-06-23 | 2003-12-02 | シエーリング アクチエンゲゼルシャフト | Combinations and compositions that interfere with VEGF / VEGF and angiopoietin / Tie receptor function and their use (II) |
| AU2001276934A1 (en) | 2000-07-18 | 2002-02-05 | Joslin Diabetes Center Inc. | Methods of modulating fibrosis |
| US20030190635A1 (en) * | 2002-02-20 | 2003-10-09 | Mcswiggen James A. | RNA interference mediated treatment of Alzheimer's disease using short interfering RNA |
| US6613567B1 (en) | 2000-09-15 | 2003-09-02 | Isis Pharmaceuticals, Inc. | Antisense inhibition of Her-2 expression |
| CA2429814C (en) * | 2000-12-01 | 2014-02-18 | Thomas Tuschl | Rna interference mediating small rna molecules |
| US20020096927A1 (en) * | 2001-01-24 | 2002-07-25 | Tsang-Ying Chen | Foldable backrest of electric cart |
| WO2003070910A2 (en) | 2002-02-20 | 2003-08-28 | Ribozyme Pharmaceuticals, Incorporated | INHIBITION OF VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) AND VEGF RECEPTOR GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20040019001A1 (en) * | 2002-02-20 | 2004-01-29 | Mcswiggen James A. | RNA interference mediated inhibition of protein typrosine phosphatase-1B (PTP-1B) gene expression using short interfering RNA |
| CA2448320A1 (en) | 2001-05-29 | 2002-12-05 | Sirna Therapeutics, Inc. | Ribozyme based treatment of female reproductive diseases |
| US6580879B2 (en) * | 2001-08-27 | 2003-06-17 | Xerox Corporation | Method and system for managing replenishment of toners |
| US7919309B2 (en) * | 2001-09-13 | 2011-04-05 | California Institute Of Technology | Method for expression of small antiviral RNA molecules within a cell |
| US6540559B1 (en) * | 2001-09-28 | 2003-04-01 | Tyco Electronics Corporation | Connector with staggered contact pattern |
| JP2003109708A (en) * | 2001-09-28 | 2003-04-11 | D D K Ltd | Multicore high speed signal transmission connector |
| EP1325955A1 (en) | 2002-01-04 | 2003-07-09 | atugen AG | Compounds and methods for the identification and/or validation of a target |
| AU2003209128B2 (en) | 2002-02-14 | 2008-05-15 | City Of Hope | Methods for producing interfering RNA molecules in mammalian cells and therapeutic uses for such molecules |
| CA2480308C (en) | 2002-03-27 | 2011-10-04 | Aegera Therapeutics Inc. | Antisense iap nucleobase oligomers and uses thereof |
| WO2004029212A2 (en) * | 2002-09-25 | 2004-04-08 | University Of Massachusetts | In vivo gene silencing by chemically modified and stable sirna |
| AU2003295388A1 (en) | 2002-11-05 | 2004-06-03 | Isis Pharmaceuticals, Inc. | 2'-substituted oligomeric compounds and compositions for use in gene modulations |
| AU2004210972A1 (en) | 2003-02-11 | 2004-08-26 | Immusol Incorporated | siRNA libraries optimized for predetermined protein families |
| GB2424887B (en) * | 2003-11-26 | 2008-05-21 | Univ Massachusetts | Sequence-specific inhibition of small RNA function |
| US20050182005A1 (en) * | 2004-02-13 | 2005-08-18 | Tuschl Thomas H. | Anti-microRNA oligonucleotide molecules |
-
2002
- 2002-04-03 WO PCT/US2002/010512 patent/WO2002081628A2/en not_active Application Discontinuation
- 2002-04-03 US US10/471,271 patent/US20070026394A1/en not_active Abandoned
- 2002-04-03 EP EP02763926A patent/EP1386004A4/en not_active Withdrawn
- 2002-05-28 US US10/156,306 patent/US7022828B2/en not_active Expired - Fee Related
- 2002-07-26 US US10/206,693 patent/US20050261212A1/en not_active Abandoned
- 2002-08-20 US US10/224,005 patent/US20030143732A1/en not_active Abandoned
- 2002-08-23 US US10/226,992 patent/US20030148507A1/en not_active Abandoned
- 2002-08-28 US US10/230,006 patent/US20030191077A1/en not_active Abandoned
-
2005
- 2005-10-20 US US11/255,139 patent/US20060154271A1/en not_active Abandoned
Patent Citations (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6476205B1 (en) * | 1989-10-24 | 2002-11-05 | Isis Pharmaceuticals, Inc. | 2′ Modified oligonucleotides |
| US5792847A (en) * | 1989-10-24 | 1998-08-11 | Gilead Sciences, Inc. | 2' Modified Oligonucleotides |
| US5670633A (en) * | 1990-01-11 | 1997-09-23 | Isis Pharmaceuticals, Inc. | Sugar modified oligonucleotides that detect and modulate gene expression |
| US6300074B1 (en) * | 1990-06-11 | 2001-10-09 | Gilead Sciences, Inc. | Systematic evolution of ligands by exponential enrichment: Chemi-SELEX |
| US5672695A (en) * | 1990-10-12 | 1997-09-30 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften E.V. | Modified ribozymes |
| US5334711A (en) * | 1991-06-20 | 1994-08-02 | Europaisches Laboratorium Fur Molekularbiologie (Embl) | Synthetic catalytic oligonucleotide structures |
| US5320962A (en) * | 1992-07-22 | 1994-06-14 | Duke University | DNA encoding the human A1 adenosine receptor |
| US5854038A (en) * | 1993-01-22 | 1998-12-29 | University Research Corporation | Localization of a therapeutic agent in a cell in vitro |
| US5814620A (en) * | 1993-07-27 | 1998-09-29 | Hybridon, Inc. | Inhibition of neovascularization using vegf-specific oligonucleotides |
| US5624803A (en) * | 1993-10-14 | 1997-04-29 | The Regents Of The University Of California | In vivo oligonucleotide generator, and methods of testing the binding affinity of triplex forming oligonucleotides derived therefrom |
| US5801154A (en) * | 1993-10-18 | 1998-09-01 | Isis Pharmaceuticals, Inc. | Antisense oligonucleotide modulation of multidrug resistance-associated protein |
| US5627053A (en) * | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| US5902880A (en) * | 1994-08-19 | 1999-05-11 | Ribozyme Pharmaceuticals, Inc. | RNA polymerase III-based expression of therapeutic RNAs |
| US5716824A (en) * | 1995-04-20 | 1998-02-10 | Ribozyme Pharmaceuticals, Inc. | 2'-O-alkylthioalkyl and 2-C-alkylthioalkyl-containing enzymatic nucleic acids (ribozymes) |
| US6005087A (en) * | 1995-06-06 | 1999-12-21 | Isis Pharmaceuticals, Inc. | 2'-modified oligonucleotides |
| US5994315A (en) * | 1995-06-07 | 1999-11-30 | East Carolina University | Low adenosine agent, composition, kit and method for treatment of airway disease |
| US5998203A (en) * | 1996-04-16 | 1999-12-07 | Ribozyme Pharmaceuticals, Inc. | Enzymatic nucleic acids containing 5'-and/or 3'-cap structures |
| US6107094A (en) * | 1996-06-06 | 2000-08-22 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US5898031A (en) * | 1996-06-06 | 1999-04-27 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides for cleaving RNA |
| US6248878B1 (en) * | 1996-12-24 | 2001-06-19 | Ribozyme Pharmaceuticals, Inc. | Nucleoside analogs |
| US6001311A (en) * | 1997-02-05 | 1999-12-14 | Protogene Laboratories, Inc. | Apparatus for diverse chemical synthesis using two-dimensional array |
| US6395713B1 (en) * | 1997-07-23 | 2002-05-28 | Ribozyme Pharmaceuticals, Inc. | Compositions for the delivery of negatively charged molecules |
| US6617438B1 (en) * | 1997-11-05 | 2003-09-09 | Sirna Therapeutics, Inc. | Oligoribonucleotides with enzymatic activity |
| US6506559B1 (en) * | 1997-12-23 | 2003-01-14 | Carnegie Institute Of Washington | Genetic inhibition by double-stranded RNA |
| US6573099B2 (en) * | 1998-03-20 | 2003-06-03 | Benitec Australia, Ltd. | Genetic constructs for delaying or repressing the expression of a target gene |
| US6258601B1 (en) * | 2000-09-07 | 2001-07-10 | Isis Pharmaceuticals, Inc. | Antisense modulation of ubiquitin protein ligase expression |
Cited By (1186)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060223990A1 (en) * | 1992-05-11 | 2006-10-05 | Sirna Therapeutics, Inc. | Synthesis, deprotection, analysis & purification of RNA & ribozymes |
| US20030206887A1 (en) * | 1992-05-14 | 2003-11-06 | David Morrissey | RNA interference mediated inhibition of hepatitis B virus (HBV) using short interfering nucleic acid (siNA) |
| US20060142557A1 (en) * | 1994-03-29 | 2006-06-29 | Sirna Therapeutics, Inc. | 2'-deoxy-2'alkylnucleotide containing nucleic acid |
| US20070275921A1 (en) * | 1996-06-06 | 2007-11-29 | Isis Pharmaceuticals, Inc. | Oligomeric Compounds That Facilitate Risc Loading |
| US7695902B2 (en) | 1996-06-06 | 2010-04-13 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US9096636B2 (en) | 1996-06-06 | 2015-08-04 | Isis Pharmaceuticals, Inc. | Chimeric oligomeric compounds and their use in gene modulation |
| US7812149B2 (en) | 1996-06-06 | 2010-10-12 | Isis Pharmaceuticals, Inc. | 2′-Fluoro substituted oligomeric compounds and compositions for use in gene modulations |
| US20050118605A9 (en) * | 1996-06-06 | 2005-06-02 | Baker Brenda F. | Oligomeric compounds having modified bases for binding to adenine and guanine and their use in gene modulation |
| US20030119777A1 (en) * | 1996-06-06 | 2003-06-26 | Crooke Stanley T. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US20030096287A1 (en) * | 1996-06-06 | 2003-05-22 | Crooke Stanley T. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US7919612B2 (en) | 1996-06-06 | 2011-04-05 | Isis Pharmaceuticals, Inc. | 2′-substituted oligomeric compounds and compositions for use in gene modulations |
| US20050037370A1 (en) * | 1996-06-06 | 2005-02-17 | Baker Brenda F. | Oligomeric compounds having modified bases for binding to adenine and guanine and their use in gene modulation |
| US7432250B2 (en) | 1996-06-06 | 2008-10-07 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US20040171028A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | Phosphorous-linked oligomeric compounds and their use in gene modulation |
| US20040171030A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | Oligomeric compounds having modified bases for binding to cytosine and uracil or thymine and their use in gene modulation |
| US20040171033A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | 2'-substituted oligomeric compounds and compositions for use in gene modulations |
| US7432249B2 (en) | 1996-06-06 | 2008-10-07 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US20040171032A1 (en) * | 1996-06-06 | 2004-09-02 | Baker Brenda F. | Non-phosphorous-linked oligomeric compounds and their use in gene modulation |
| US20040161844A1 (en) * | 1996-06-06 | 2004-08-19 | Baker Brenda F. | Sugar and backbone-surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US20040161777A1 (en) * | 1996-06-06 | 2004-08-19 | Baker Brenda F. | Modified oligonucleotides for use in RNA interference |
| US7629321B2 (en) | 1996-06-06 | 2009-12-08 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides and ribonucleases for cleaving RNA |
| US20040147023A1 (en) * | 1996-06-06 | 2004-07-29 | Baker Brenda F. | Chimeric oligomeric compounds and their use in gene modulation |
| US20040146902A1 (en) * | 1996-06-06 | 2004-07-29 | Ecker David J. | Structural motifs and oligomeric compounds and their use in gene modulation |
| US20050176018A1 (en) * | 1998-04-20 | 2005-08-11 | Sirna Therapeutics, Inc. | Chemically modified double stranded nucleic acid molecules |
| US20050214772A1 (en) * | 1998-10-26 | 2005-09-29 | Board Of Regents, The University Of Texas System | Thio modified aptamer synthetic methods and compositions |
| US20050282764A1 (en) * | 1998-12-29 | 2005-12-22 | Bahramian Mohammad B | Method of identifying nucleic acid compositions for muting expression of a gene |
| US20050234003A1 (en) * | 1998-12-29 | 2005-10-20 | Bahramian Mohammad B | Method of using nucleic acid compositions for muting expression of a gene in animals |
| US20040072779A1 (en) * | 1999-01-30 | 2004-04-15 | Ribopharma Ag | Method and medicament for inhibiting the expression of a given gene |
| US8119608B2 (en) | 1999-01-30 | 2012-02-21 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US8101584B2 (en) | 1999-01-30 | 2012-01-24 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US20080166800A1 (en) * | 1999-01-30 | 2008-07-10 | Roland Kreutzer | Method and medicament for inhibiting the expression of a given gene |
| US20050100907A1 (en) * | 1999-01-30 | 2005-05-12 | Ribopharma, Ag | Method and medicament for inhibiting the expression of a given gene |
| US20080182981A1 (en) * | 1999-01-30 | 2008-07-31 | Roland Kreutzer | Method and medicament for inhibiting the expression of a given gene |
| US20080261303A1 (en) * | 1999-01-30 | 2008-10-23 | Roland Kreutzer | Method and medicament for inhibiting the expression of a given gene |
| US20040053875A1 (en) * | 1999-01-30 | 2004-03-18 | Ribopharma Ag | Method and medicament for inhibiting the expression of a given gene |
| US20040102408A1 (en) * | 1999-01-30 | 2004-05-27 | Ribopharma Ag | Method and medicament for inhibiting the expression of a given gene |
| US8202980B2 (en) * | 1999-01-30 | 2012-06-19 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US8729037B2 (en) | 1999-01-30 | 2014-05-20 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US8101742B2 (en) | 1999-01-30 | 2012-01-24 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US8114851B2 (en) | 1999-01-30 | 2012-02-14 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US9133454B2 (en) | 1999-01-30 | 2015-09-15 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US20080171861A1 (en) * | 1999-01-30 | 2008-07-17 | Roland Kreutzer | Method and medicament for inhibiting the expression of a given gene |
| US8114981B2 (en) | 1999-01-30 | 2012-02-14 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US8183362B2 (en) | 1999-01-30 | 2012-05-22 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US9902955B2 (en) | 1999-01-30 | 2018-02-27 | Alnylam Pharmaceuticals, Inc. | Method and medicament for inhibiting the expression of a given gene |
| US8168776B2 (en) | 1999-01-30 | 2012-05-01 | Alnylam Pharmaceuticals, Inc. | Method for making a 21 nucleotide double stranded RNA chemically linked at one end |
| US20040242521A1 (en) * | 1999-10-25 | 2004-12-02 | Board Of Regents, The University Of Texas System | Thio-siRNA aptamers |
| US20040175703A1 (en) * | 1999-11-24 | 2004-09-09 | Ribopharma Ag | Compositions and methods for inhibiting expression of a target gene |
| US7829693B2 (en) | 1999-11-24 | 2010-11-09 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a target gene |
| US20050261212A1 (en) * | 2000-02-11 | 2005-11-24 | Mcswiggen James A | RNA interference mediated inhibition of NOGO and NOGO receptor gene expression using short interfering RNA |
| US20070026394A1 (en) * | 2000-02-11 | 2007-02-01 | Lawrence Blatt | Modulation of gene expression associated with inflammation proliferation and neurite outgrowth using nucleic acid based technologies |
| US7713945B2 (en) | 2000-09-19 | 2010-05-11 | University Of South Florida | Control of NK cell function and survival by modulation of SHIP activity |
| US20100144841A1 (en) * | 2000-09-19 | 2010-06-10 | University Of South Florida | Control of nk cell function and survival by modulation of ship activity |
| US20110052546A1 (en) * | 2000-09-19 | 2011-03-03 | University Of South Florida | Inhibition of SHIP to Enhance Stem Cell Harvest and Transplantation |
| US8163710B2 (en) | 2000-09-19 | 2012-04-24 | University Of South Florida | Reduction of graft-versus-host disease by modulation of SHIP activity |
| US20110159587A1 (en) * | 2000-11-09 | 2011-06-30 | Krainer Adrian R | Chimeric Molecules to Modulate Gene Expression |
| US20050054836A1 (en) * | 2000-11-09 | 2005-03-10 | Cold Spring Harbor Laboratory | Chimeric molecules to modulate gene expression |
| US9074213B2 (en) | 2001-01-09 | 2015-07-07 | Alnylam Pharmacuticals, Inc. | Compositions and methods for inhibiting expression of a target gene |
| US7423142B2 (en) | 2001-01-09 | 2008-09-09 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20090053808A1 (en) * | 2001-01-09 | 2009-02-26 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting the expression of anti-apoptopic genes |
| US7473525B2 (en) | 2001-01-09 | 2009-01-06 | Alnylam Europe Ag | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20050176667A1 (en) * | 2001-01-09 | 2005-08-11 | Alnylam Europe Ag | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US7868160B2 (en) | 2001-01-09 | 2011-01-11 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US9587240B2 (en) | 2001-01-09 | 2017-03-07 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a target gene |
| US20060084621A1 (en) * | 2001-01-09 | 2006-04-20 | Hans-Peter Vornlocher | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US7767802B2 (en) | 2001-01-09 | 2010-08-03 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20040001811A1 (en) * | 2001-01-09 | 2004-01-01 | Ribopharma Ag | Compositions and methods for inhibiting expression of anti-apoptotic genes |
| US20060154271A1 (en) * | 2001-04-05 | 2006-07-13 | Sirna Therapeutics, Inc. | Enzymatic nucleic acid treatment of diseases or conditions related to levels of IKK-gamma and PKR |
| US20030191077A1 (en) * | 2001-04-05 | 2003-10-09 | Kathy Fosnaugh | Method and reagent for the treatment of asthma and allergic conditions |
| US20050136436A1 (en) * | 2001-05-18 | 2005-06-23 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of G72 and D-amino acid oxidase (DAAO) gene expression using short interfering nucleic acid (siNA) |
| US20050233997A1 (en) * | 2001-05-18 | 2005-10-20 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of matrix metalloproteinase 13 (MMP13) gene expression using short interfering nucleic acid (siNA) |
| US20050119212A1 (en) * | 2001-05-18 | 2005-06-02 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of FAS and FASL gene expression using short interfering nucleic acid (siNA) |
| US20060216747A1 (en) * | 2001-05-18 | 2006-09-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of checkpoint kinase-1 (CHK-1) gene expression using short interfering nucleic acid (siNA) |
| US20050124569A1 (en) * | 2001-05-18 | 2005-06-09 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of CXCR4 gene expression using short interfering nucleic acid (siNA) |
| US20050124566A1 (en) * | 2001-05-18 | 2005-06-09 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of myostatin gene expression using short interfering nucleic acid (siNA) |
| US20060217331A1 (en) * | 2001-05-18 | 2006-09-28 | Sirna Therapeutics, Inc. | Chemically modified double stranded nucleic acid molecules that mediate RNA interference |
| US20050137155A1 (en) * | 2001-05-18 | 2005-06-23 | Sirna Therapeutics, Inc. | RNA interference mediated treatment of Parkinson disease using short interfering nucleic acid (siNA) |
| US20060211642A1 (en) * | 2001-05-18 | 2006-09-21 | Sirna Therapeutics, Inc. | RNA inteference mediated inhibition of hepatitis C virus (HVC) gene expression using short interfering nucleic acid (siNA) |
| US20080161256A1 (en) * | 2001-05-18 | 2008-07-03 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20050143333A1 (en) * | 2001-05-18 | 2005-06-30 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of interleukin and interleukin receptor gene expression using short interfering nucleic acid (SINA) |
| US20050153914A1 (en) * | 2001-05-18 | 2005-07-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of MDR P-glycoprotein gene expression using short interfering nucleic acid (siNA) |
| US20070173473A1 (en) * | 2001-05-18 | 2007-07-26 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of proprotein convertase subtilisin Kexin 9 (PCSK9) gene expression using short interfering nucleic acid (siNA) |
| US20050158735A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of proliferating cell nuclear antigen (PCNA) gene expression using short interfering nucleic acid (siNA) |
| US20050159380A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of angiopoietin gene expression using short interfering nucleic acid (siNA) |
| US20050159378A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of Myc and/or Myb gene expression using short interfering nucleic acid (siNA) |
| US20050159379A1 (en) * | 2001-05-18 | 2005-07-21 | Sirna Therapeutics, Inc | RNA interference mediated inhibition of gastric inhibitory polypeptide (GIP) and gastric inhibitory polypeptide receptor (GIPR) gene expression using short interfering nucleic acid (siNA) |
| US20050164224A1 (en) * | 2001-05-18 | 2005-07-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of cyclin D1 gene expression using short interfering nucleic acid (siNA) |
| US20050164967A1 (en) * | 2001-05-18 | 2005-07-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of platelet-derived endothelial cell growth factor (ECGF1) gene expression using short interfering nucleic acid (siNA) |
| US20050164968A1 (en) * | 2001-05-18 | 2005-07-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of ADAM33 gene expression using short interfering nucleic acid (siNA) |
| US20050171040A1 (en) * | 2001-05-18 | 2005-08-04 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of cholesteryl ester transfer protein (CEPT) gene expression using short interfering nucleic acid (siNA) |
| US20050176666A1 (en) * | 2001-05-18 | 2005-08-11 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of GPRA and AAA1 gene expression using short interfering nucleic acid (siNA) |
| US20060142225A1 (en) * | 2001-05-18 | 2006-06-29 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of cyclin dependent kinase-2 (CDK2) gene expression using short interfering nucleic acid (siNA) |
| US20050176663A1 (en) * | 2001-05-18 | 2005-08-11 | Sima Therapeutics, Inc. | RNA interference mediated inhibition of protein tyrosine phosphatase type IVA (PRL3) gene expression using short interfering nucleic acid (siNA) |
| US20050079610A1 (en) * | 2001-05-18 | 2005-04-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of Fos gene expression using short interfering nucleic acid (siNA) |
| US20050176025A1 (en) * | 2001-05-18 | 2005-08-11 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of B-cell CLL/Lymphoma-2 (BCL-2) gene expression using short interfering nucleic acid (siNA) |
| US20090299045A1 (en) * | 2001-05-18 | 2009-12-03 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition Of Interleukin and Interleukin Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| US20050182007A1 (en) * | 2001-05-18 | 2005-08-18 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of interleukin and interleukin receptor gene expression using short interfering nucleic acid (SINA) |
| US20060241075A1 (en) * | 2001-05-18 | 2006-10-26 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of desmoglein gene expression using short interfering nucleic acid (siNA) |
| US20080188430A1 (en) * | 2001-05-18 | 2008-08-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of hypoxia inducible factor 1 (HIF1) gene expression using short interfering nucleic acid (siNA) |
| US20050187174A1 (en) * | 2001-05-18 | 2005-08-25 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of intercellular adhesion molecule (ICAM) gene expression using short interfering nucleic acid (siNA) |
| US20050191618A1 (en) * | 2001-05-18 | 2005-09-01 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA) |
| US20060270623A1 (en) * | 2001-05-18 | 2006-11-30 | Sirna Therapeutics, Inc. | RNA interference mediated treatment of polyglutamine (polyQ) repeat expansion diseases using short interfering nucleic acid (siNA) |
| US20070160980A1 (en) * | 2001-05-18 | 2007-07-12 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20050196767A1 (en) * | 2001-05-18 | 2005-09-08 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of GRB2 associated binding protein (GAB2) gene expression using short interfering nucleic acis (siNA) |
| US20050196781A1 (en) * | 2001-05-18 | 2005-09-08 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of STAT3 gene expression using short interfering nucleic acid (siNA) |
| US7858625B2 (en) | 2001-05-18 | 2010-12-28 | Sirna Therapeutics, Inc. | Conjugates and compositions for cellular delivery |
| US20050287128A1 (en) * | 2001-05-18 | 2005-12-29 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of TGF-beta and TGF-beta receptor gene expression using short interfering nucleic acid (siNA) |
| US7517864B2 (en) | 2001-05-18 | 2009-04-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050222066A1 (en) * | 2001-05-18 | 2005-10-06 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050288242A1 (en) * | 2001-05-18 | 2005-12-29 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of RAS gene expression using short interfering nucleic acid (siNA) |
| US20050233344A1 (en) * | 2001-05-18 | 2005-10-20 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of platelet derived growth factor (PDGF) and platelet derived growth factor receptor (PDGFR) gene expression using short interfering nucleic acid (siNA) |
| US20050233998A1 (en) * | 2001-05-18 | 2005-10-20 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050282188A1 (en) * | 2001-05-18 | 2005-12-22 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20050267058A1 (en) * | 2001-05-18 | 2005-12-01 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of placental growth factor gene expression using short interfering nucleic acid (sINA) |
| US20070270579A1 (en) * | 2001-05-18 | 2007-11-22 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) |
| US20050239739A1 (en) * | 2001-05-18 | 2005-10-27 | Sirna Therapeutics, Inc. | Conjugates and compositions for cellular delivery |
| US20050239731A1 (en) * | 2001-05-18 | 2005-10-27 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of MAP kinase gene expression using short interfering nucleic acid (siNA) |
| US20070093437A1 (en) * | 2001-05-18 | 2007-04-26 | Sirna Therapeutics, Inc. | Rna interference mediated inhibition of xiap gene expression using short interfering nucleic acid (sina) |
| US20050032733A1 (en) * | 2001-05-18 | 2005-02-10 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (SiNA) |
| US9994853B2 (en) | 2001-05-18 | 2018-06-12 | Sirna Therapeutics, Inc. | Chemically modified multifunctional short interfering nucleic acid molecules that mediate RNA interference |
| US20050261219A1 (en) * | 2001-05-18 | 2005-11-24 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of interleukin and interleukin receptor gene expression using short interfering nucleic acid (siNA) |
| US20030105051A1 (en) * | 2001-05-29 | 2003-06-05 | Mcswiggen James | Nucleic acid treatment of diseases or conditions related to levels of HER2 |
| US7691821B2 (en) | 2001-09-19 | 2010-04-06 | University Of South Florida | Inhibition of SHIP to enhance stem cell harvest and transplantation |
| US20060223749A1 (en) * | 2001-09-19 | 2006-10-05 | University Of South Florida | Inhibition of SHIP to enhance stem cell harvest and transplantation |
| US20050074757A1 (en) * | 2001-10-12 | 2005-04-07 | Ribopharma Ag | Compositions and methods for inhibiting expression of a mutant gene |
| US7348314B2 (en) | 2001-10-12 | 2008-03-25 | Alnylam Europe Ag | Compositions and methods for inhibiting viral replication |
| US7745418B2 (en) | 2001-10-12 | 2010-06-29 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting viral replication |
| US7763590B2 (en) | 2001-10-12 | 2010-07-27 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a mutant gene |
| US20040126791A1 (en) * | 2001-10-26 | 2004-07-01 | Ribopharma Ag | Compositions and methods for treating trail-resistant cancer cells |
| US20040038921A1 (en) * | 2001-10-26 | 2004-02-26 | Ribopharma Ag | Composition and method for inhibiting expression of a target gene |
| US20040121348A1 (en) * | 2001-10-26 | 2004-06-24 | Ribopharma Ag | Compositions and methods for treating pancreatic cancer |
| US20090304798A1 (en) * | 2001-11-02 | 2009-12-10 | Insert Therapeutics, Inc. | Methods and compositions for therapeutic use of RNA interference |
| US20030157030A1 (en) * | 2001-11-02 | 2003-08-21 | Insert Therapeutics, Inc. | Methods and compositions for therapeutic use of rna interference |
| US20040063654A1 (en) * | 2001-11-02 | 2004-04-01 | Davis Mark E. | Methods and compositions for therapeutic use of RNA interference |
| US20080200340A1 (en) * | 2001-11-15 | 2008-08-21 | Board Of Regents, The University Of Texas System | Bead Bound Combinatorial Oligonucleoside Phosphorothioate And Phosphorodithioate Aptamer Libraries |
| US20080255005A1 (en) * | 2001-11-15 | 2008-10-16 | Board Of Regents, The University Of Texas System | Bead Bound Combinatorial Oligonucleoside Phosphorothioate And Phosphorodithioate Aptamer Libraries |
| US20110224099A1 (en) * | 2001-11-15 | 2011-09-15 | Board Of Regents, The University Of Texas System | Bead bound combinatorial oligonucleoside phosphorothioate and phosphorodithioate aptamer libraries |
| US20050004064A1 (en) * | 2001-11-21 | 2005-01-06 | Mitsubishi Chemical Corporation | Method of inhibiting gene expression |
| US20040198682A1 (en) * | 2001-11-30 | 2004-10-07 | Mcswiggen James | RNA interference mediated inhibition of placental growth factor gene expression using short interfering nucleic acid (siNA) |
| US20070203333A1 (en) * | 2001-11-30 | 2007-08-30 | Mcswiggen James | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US20050075304A1 (en) * | 2001-11-30 | 2005-04-07 | Mcswiggen James | RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US7846907B2 (en) | 2002-01-22 | 2010-12-07 | Alnylam Pharmaceuticals, Inc. | Double-stranded RNA (dsRNA) and method of use for inhibiting expression of a fusion gene |
| US20030190654A1 (en) * | 2002-01-22 | 2003-10-09 | Ribopharma | Double-stranded RNA (dsRNA) and method of use for inhibiting expression of a fusion gene |
| US7196184B2 (en) | 2002-01-22 | 2007-03-27 | Alnylam Europe Ag | Double-stranded RNA (DSRNA) and method of use for inhibiting expression of the AML-1/MTG8 fusion gene |
| US10626398B2 (en) | 2002-02-01 | 2020-04-21 | Life Technologies Corporation | Oligonucleotide compositions with enhanced efficiency |
| US20040054155A1 (en) * | 2002-02-01 | 2004-03-18 | Sequitur, Inc. | Oligonucleotide compositions with enhanced efficiency |
| US9777275B2 (en) | 2002-02-01 | 2017-10-03 | Life Technologies Corporation | Oligonucleotide compositions with enhanced efficiency |
| US8524680B2 (en) | 2002-02-01 | 2013-09-03 | Applied Biosystems, Llc | High potency siRNAS for reducing the expression of target genes |
| US9592250B2 (en) | 2002-02-01 | 2017-03-14 | Life Technologies Corporation | Double-stranded oligonucleotides |
| US20060009409A1 (en) * | 2002-02-01 | 2006-01-12 | Woolf Tod M | Double-stranded oligonucleotides |
| US10036025B2 (en) | 2002-02-01 | 2018-07-31 | Life Technologies Corporation | Oligonucleotide compositions with enhanced efficiency |
| US20120107897A1 (en) * | 2002-02-01 | 2012-05-03 | Life Technologies Corporation | Double-stranded oligonucleotides |
| US9796978B1 (en) | 2002-02-01 | 2017-10-24 | Life Technologies Corporation | Oligonucleotide compositions with enhanced efficiency |
| US10106793B2 (en) | 2002-02-01 | 2018-10-23 | Life Technologies Corporation | Double-stranded oligonucleotides |
| US8815821B2 (en) * | 2002-02-01 | 2014-08-26 | Life Technologies Corporation | Double-stranded oligonucleotides |
| US10196640B1 (en) | 2002-02-01 | 2019-02-05 | Life Technologies Corporation | Oligonucleotide compositions with enhanced efficiency |
| US20050096284A1 (en) * | 2002-02-20 | 2005-05-05 | Sirna Therapeutics, Inc. | RNA interference mediated treatment of polyglutamine (polyQ) repeat expansion diseases using short interfering nucleic acid (siNA) |
| US7910724B2 (en) * | 2002-02-20 | 2011-03-22 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of Fos gene expression using short interfering nucleic acid (siNA) |
| US8846894B2 (en) | 2002-02-20 | 2014-09-30 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20100227911A1 (en) * | 2002-02-20 | 2010-09-09 | Mcswiggen James | RNA INTERFERENCE MEDIATED INHIBITION OF CHROMOSOME TRANSLOCATION GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20100240730A1 (en) * | 2002-02-20 | 2010-09-23 | Merck Sharp And Dohme Corp. | RNA Interference Mediated Inhibition of Gene Expression Using Chemically Modified Short Interfering Nucleic Acid (siNA) |
| US20100227912A1 (en) * | 2002-02-20 | 2010-09-09 | Mcswiggen James | RNA INTERFERENCE MEDIATED INHIBITION OF MYOSTATIN GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20040019001A1 (en) * | 2002-02-20 | 2004-01-29 | Mcswiggen James A. | RNA interference mediated inhibition of protein typrosine phosphatase-1B (PTP-1B) gene expression using short interfering RNA |
| US8648185B2 (en) | 2002-02-20 | 2014-02-11 | Merck Sharp & Dohme Corp. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US8618277B2 (en) | 2002-02-20 | 2013-12-31 | Merck Sharp & Dohme Corp. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20040192626A1 (en) * | 2002-02-20 | 2004-09-30 | Mcswiggen James | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US10000754B2 (en) | 2002-02-20 | 2018-06-19 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060275903A1 (en) * | 2002-02-20 | 2006-12-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20080039414A1 (en) * | 2002-02-20 | 2008-02-14 | Sima Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060281175A1 (en) * | 2002-02-20 | 2006-12-14 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US8067575B2 (en) * | 2002-02-20 | 2011-11-29 | Merck, Sharp & Dohme Corp. | RNA interference mediated inhibition of cyclin D1 gene expression using short interfering nucleic acid (siNA) |
| US9957517B2 (en) | 2002-02-20 | 2018-05-01 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060293272A1 (en) * | 2002-02-20 | 2006-12-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060292691A1 (en) * | 2002-02-20 | 2006-12-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060293271A1 (en) * | 2002-02-20 | 2006-12-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20070004663A1 (en) * | 2002-02-20 | 2007-01-04 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20070004665A1 (en) * | 2002-02-20 | 2007-01-04 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20070004667A1 (en) * | 2002-02-20 | 2007-01-04 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20050020525A1 (en) * | 2002-02-20 | 2005-01-27 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20090023675A1 (en) * | 2002-02-20 | 2009-01-22 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition of Gene Expression Using Chemically Modified Short Interfering Nucleic Acid (siNA) |
| US20050048529A1 (en) * | 2002-02-20 | 2005-03-03 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of intercellular adhesion molecule (ICAM) gene expression using short interfering nucleic acid (siNA) |
| US20090306182A1 (en) * | 2002-02-20 | 2009-12-10 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF MAP KINASE GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US7977472B2 (en) * | 2002-02-20 | 2011-07-12 | Leonid Beigelman | RNA interference mediated inhibition of myostatin gene expression using short interfering nucleic acid (siNA) |
| US8232383B2 (en) | 2002-02-20 | 2012-07-31 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20070167393A1 (en) * | 2002-02-20 | 2007-07-19 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF GENE EXPRESSION USING CHEMICALLY MODIFIED SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20090093439A1 (en) * | 2002-02-20 | 2009-04-09 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF CHROMOSOME TRANSLOCATION GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US10889815B2 (en) | 2002-02-20 | 2021-01-12 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US10351852B2 (en) | 2002-02-20 | 2019-07-16 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20090099116A1 (en) * | 2002-02-20 | 2009-04-16 | Sirna Therapeutics, Inc. | RNA INTERFERENCE MEDIATED INHIBITION OF FOS GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20090137500A1 (en) * | 2002-02-20 | 2009-05-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20090137512A1 (en) * | 2002-02-20 | 2009-05-28 | Sirna Therapeutics, Inc. | RNA Interference Mediated Inhibition of Cyclin D1 Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| US9771588B2 (en) | 2002-02-20 | 2017-09-26 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US9738899B2 (en) | 2002-02-20 | 2017-08-22 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US9732344B2 (en) | 2002-02-20 | 2017-08-15 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US9657294B2 (en) | 2002-02-20 | 2017-05-23 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US9181551B2 (en) | 2002-02-20 | 2015-11-10 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US10662428B2 (en) | 2002-02-20 | 2020-05-26 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US8273866B2 (en) | 2002-02-20 | 2012-09-25 | Merck Sharp & Dohme Corp. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (SINA) |
| US8202979B2 (en) | 2002-02-20 | 2012-06-19 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid |
| US7989612B2 (en) | 2002-02-20 | 2011-08-02 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20050209182A1 (en) * | 2002-02-20 | 2005-09-22 | Sirna Therapeutics, Inc. | Nucleic acid mediated inhibition of enterococcus infection and cytolysin toxin activity |
| US20040005593A1 (en) * | 2002-03-06 | 2004-01-08 | Rigel Pharmaceuticals, Inc. | Novel method for delivery and intracellular synthesis of siRNA molecules |
| US20040138163A1 (en) * | 2002-05-29 | 2004-07-15 | Mcswiggen James | RNA interference mediated inhibition of vascular edothelial growth factor and vascular edothelial growth factor receptor gene expression using short interfering nucleic acid (siNA) |
| US11298320B2 (en) | 2002-06-28 | 2022-04-12 | Arbutus Biopharma Corporation | Liposomal apparatus and manufacturing methods |
| US9504651B2 (en) | 2002-06-28 | 2016-11-29 | Protiva Biotherapeutics, Inc. | Lipid compositions for nucleic acid delivery |
| US11318098B2 (en) | 2002-06-28 | 2022-05-03 | Arbutus Biopharma Corporation | Liposomal apparatus and manufacturing methods |
| US9492386B2 (en) | 2002-06-28 | 2016-11-15 | Protiva Biotherapeutics, Inc. | Liposomal apparatus and manufacturing methods |
| US20050058982A1 (en) * | 2002-07-26 | 2005-03-17 | Chiron Corporation | Modified small interfering RNA molecules and methods of use |
| US9139833B2 (en) | 2002-07-26 | 2015-09-22 | Arrowhead Research Corporation | Modified small interfering RNA molecules and methods of use |
| US20040241854A1 (en) * | 2002-08-05 | 2004-12-02 | Davidson Beverly L. | siRNA-mediated gene silencing |
| US8524879B2 (en) | 2002-08-05 | 2013-09-03 | University Of Iowa Research Foundation | RNA interference suppresion of neurodegenerative diseases and methods of use thereof |
| US10072264B2 (en) | 2002-08-05 | 2018-09-11 | University Of Iowa Research Foundation | RNA interference suppression of neurodegenerative diseases and methods of use |
| US9260716B2 (en) | 2002-08-05 | 2016-02-16 | University Of Iowa Research Foundation | RNA interference suppression of neurodegenerative diseases and methods of use thereof |
| US20040023390A1 (en) * | 2002-08-05 | 2004-02-05 | Davidson Beverly L. | SiRNA-mediated gene silencing with viral vectors |
| US8329890B2 (en) | 2002-08-05 | 2012-12-11 | University Of Iowa Research Foundation | SiRNA-mediated gene silencing |
| US20110212520A1 (en) * | 2002-08-05 | 2011-09-01 | University Of Iowa Research Foundation | Rna interference suppression of neurodegenerative diseases and methods of use thereof |
| US8779116B2 (en) | 2002-08-05 | 2014-07-15 | University Of Iowa Research Foundation | SiRNA-mediated gene silencing |
| US20050042646A1 (en) * | 2002-08-05 | 2005-02-24 | Davidson Beverly L. | RNA interference suppresion of neurodegenerative diseases and methods of use thereof |
| US20060009408A1 (en) * | 2002-08-05 | 2006-01-12 | University Of Iowa Research Foundation, A Iowa Corporation | siRNA-Mediated gene silencing with viral vectors |
| US8481710B2 (en) | 2002-08-05 | 2013-07-09 | University Of Iowa Research Foundation | RNA interference suppression of neurodegenerative diseases and methods of use thereof |
| US20050255086A1 (en) * | 2002-08-05 | 2005-11-17 | Davidson Beverly L | Nucleic acid silencing of Huntington's Disease gene |
| US20110111491A1 (en) * | 2002-08-05 | 2011-05-12 | University Of Iowa Research Foundation | Rna interference suppresion of neurodegenerative diseases and methods of use thereof |
| US9487779B2 (en) | 2002-08-05 | 2016-11-08 | University Of Iowa Research Foundation | siRNA-mediated gene silencing |
| US20040203145A1 (en) * | 2002-08-07 | 2004-10-14 | University Of Massachusetts | Compositions for RNA interference and methods of use thereof |
| US9611472B2 (en) | 2002-08-07 | 2017-04-04 | University Of Massachusetts | Compositions for RNA interference and methods of use thereof |
| US8729036B2 (en) * | 2002-08-07 | 2014-05-20 | University Of Massachusetts | Compositions for RNA interference and methods of use thereof |
| US8252918B2 (en) * | 2002-08-21 | 2012-08-28 | The University Of British Columbia | RNAi probes targeting cancer-related proteins |
| US9487777B2 (en) * | 2002-08-21 | 2016-11-08 | The University Of British Columbia | RNAi probes targeting cancer-related proteins |
| US20040096882A1 (en) * | 2002-08-21 | 2004-05-20 | Martin Gleave | RNAi probes targeting cancer-related proteins |
| US7956176B2 (en) | 2002-09-05 | 2011-06-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US7923547B2 (en) | 2002-09-05 | 2011-04-12 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20060276635A1 (en) * | 2002-09-05 | 2006-12-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20070004664A1 (en) * | 2002-09-05 | 2007-01-04 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| WO2004022771A3 (en) * | 2002-09-09 | 2009-07-16 | Univ California | Short interfering nucleic acid hybrids and methods thereof |
| US20060287269A1 (en) * | 2002-09-09 | 2006-12-21 | The Regents Of The University Of California | Short interfering nucleic acid hybrids and methods thereof |
| US20040053289A1 (en) * | 2002-09-09 | 2004-03-18 | The Regents Of The University Of California | Short interfering nucleic acid hybrids and methods thereof |
| US20040137471A1 (en) * | 2002-09-18 | 2004-07-15 | Timothy Vickers | Efficient reduction of target RNA's by single-and double-stranded oligomeric compounds |
| US20100041047A1 (en) * | 2002-09-18 | 2010-02-18 | Timothy Vickers | Efficient reduction of target rna's by single- and double-stranded oligomeric compounds |
| US20190085328A1 (en) * | 2002-09-25 | 2019-03-21 | University Of Massachusetts | IN VIVO SILENCING BY CHEMICALLY MODIFIED AND STABLE siRNA |
| US11136578B2 (en) | 2002-09-25 | 2021-10-05 | University Of Massachusetts | In vivo gene silencing by chemically modified and stable siRNA |
| US20150299703A1 (en) * | 2002-09-25 | 2015-10-22 | University Of Massachusetts | IN VIVO GENE SILENCING BY CHEMICALLY MODIFIED AND STABLE siRNA |
| US10087441B2 (en) * | 2002-09-25 | 2018-10-02 | University Of Massachusetts | In vivo gene silencing by chemically modified and stable siRNA |
| US9012623B2 (en) | 2002-09-25 | 2015-04-21 | University Of Massachusetts | In vivo gene silencing by chemically modified and stable siRNA |
| US20050020521A1 (en) * | 2002-09-25 | 2005-01-27 | University Of Massachusetts | In vivo gene silencing by chemically modified and stable siRNA |
| US20060240425A1 (en) * | 2002-09-30 | 2006-10-26 | Oncotherapy Science, Inc | Genes and polypeptides relating to myeloid leukemia |
| US20090123922A1 (en) * | 2002-10-16 | 2009-05-14 | Board Of Regents, The University Of Texas System | Bead Bound Combinatorial Oligonucleoside Phosphorothioate And Phosphorodithioate Aptamer Libraries |
| US9150606B2 (en) * | 2002-11-05 | 2015-10-06 | Isis Pharmaceuticals, Inc. | Compositions comprising alternating 2'-modified nucleosides for use in gene modulation |
| WO2004043977A3 (en) * | 2002-11-05 | 2005-01-20 | Isis Pharmaceuticals Inc | 2’-fluoro substituted oligomeric compounds and compositions for use in gene modulations |
| US8124745B2 (en) | 2002-11-05 | 2012-02-28 | Isis Pharmaceuticals, Inc | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| US9943539B1 (en) * | 2002-11-05 | 2018-04-17 | Ionis Pharmaceuticals, Inc. | 2′-methoxy substituted oligomeric compounds and compositions for use in gene modulations |
| US8604183B2 (en) * | 2002-11-05 | 2013-12-10 | Isis Pharmaceuticals, Inc. | Compositions comprising alternating 2′-modified nucleosides for use in gene modulation |
| US9943538B1 (en) * | 2002-11-05 | 2018-04-17 | Ionis Pharmaceuticals, Inc. | 2′-methoxy substituted oligomeric compounds and compositions for use in gene modulations |
| WO2004044132A3 (en) * | 2002-11-05 | 2004-10-07 | Isis Pharmaceuticals Inc | Modified oligonucleotides for use in rna interference |
| US20050080246A1 (en) * | 2002-11-05 | 2005-04-14 | Charles Allerson | Compositions comprising alternating 2'-modified nucleosides for use in gene modulation |
| US20040171570A1 (en) * | 2002-11-05 | 2004-09-02 | Charles Allerson | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| WO2004044136A3 (en) * | 2002-11-05 | 2005-02-24 | Isis Pharmaceuticals Inc | Compositions comprising alternating 2’-modified nucleosides for use in gene modulation |
| US9150605B2 (en) * | 2002-11-05 | 2015-10-06 | Isis Pharmaceuticals, Inc. | Compositions comprising alternating 2′-modified nucleosides for use in gene modulation |
| WO2004044140A3 (en) * | 2002-11-05 | 2005-04-14 | Isis Pharmaceticals Inc | 2’-substituted oligomeric compounds and compositions for use in gene modulations |
| US9827263B2 (en) * | 2002-11-05 | 2017-11-28 | Ionis Pharmaceuticals, Inc. | 2′-methoxy substituted oligomeric compounds and compositions for use in gene modulations |
| US7696345B2 (en) | 2002-11-05 | 2010-04-13 | Isis Pharmaceuticals, Inc. | Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| WO2004043979A3 (en) * | 2002-11-05 | 2005-03-24 | Isis Pharmaceuticals Inc | Sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation |
| WO2004044138A3 (en) * | 2002-11-05 | 2005-03-24 | Isis Pharmaceuticals Inc | Chimeric oligomeric compounds and their use in gene modulation |
| US20050187178A1 (en) * | 2002-11-05 | 2005-08-25 | Charles Allerson | Compositions comprising alternating 2'-modified nucleosides for use in gene modulation |
| WO2004044133A3 (en) * | 2002-11-05 | 2005-04-07 | Isis Pharmaceuticals Inc | Modified oligonucleotides for use in rna interference |
| US20090163702A1 (en) * | 2002-11-14 | 2009-06-25 | Dharmacon Inc. | siRNA targeting Myeloid cell leukemia sequence 1 |
| US7576196B2 (en) | 2002-11-14 | 2009-08-18 | Dharmacon, Inc. | siRNA targeting transducin (beta)-like 3 (TBL3) |
| US20080113378A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting interleukin-1 receptor-associated kinase 4 (IRAK4) |
| US20080113372A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting glucagon receptor (GCGR) |
| US20100267587A1 (en) * | 2002-11-14 | 2010-10-21 | Dharmacon, Inc. | siRNA targeting cyclin dependent kinase 11 (CDK11) |
| US20080113374A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting fructose-1,6-bisphosphatase 1 (FBP1) |
| US20100267586A1 (en) * | 2002-11-14 | 2010-10-21 | Dharmacon Inc. | siRNA targeting KRAS |
| US20080132691A1 (en) * | 2002-11-14 | 2008-06-05 | Dharmacon, Inc. | siRNA targeting kinase insert domain receptor (KDR) |
| US7807819B2 (en) | 2002-11-14 | 2010-10-05 | Dharmacon, Inc. | siRNA targeting survivin |
| US20120010106A1 (en) * | 2002-11-14 | 2012-01-12 | Dharmacon, Inc. | siRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US20080113375A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting superoxide dismutase 1 (SOD1) |
| US20080114162A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20080113369A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting diacylglycerol O-acyltransferase homolog 2 (DGAT2) |
| US20080113370A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting apolipoprotein B (APOB) |
| US20080113371A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting beta secretase (BACE) |
| US7820809B2 (en) | 2002-11-14 | 2010-10-26 | Dharmacon, Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20080177051A1 (en) * | 2002-11-14 | 2008-07-24 | Dharmacon, Inc. | siRNA targeting cyclin-dependent kinase inhibitor 1B (p27, Kip1) (CDKN1B) |
| US20080113376A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US20080113373A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA targeting amyloid beta (A4) precursor protein (APP) |
| US20100248990A1 (en) * | 2002-11-14 | 2010-09-30 | Dharmacon, Inc. | siRNA targeting ribonucleotide reductase M2 polypeptide (RRM2 or RNR-R2) |
| US20080108803A1 (en) * | 2002-11-14 | 2008-05-08 | Dharmacon Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US7803933B2 (en) | 2002-11-14 | 2010-09-28 | Dharmacon, Inc. | siRNA targeting TATA box binding protein (TBP)-associated factor (TAF1) |
| US20080097090A1 (en) * | 2002-11-14 | 2008-04-24 | Dharmacon Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20080227967A1 (en) * | 2002-11-14 | 2008-09-18 | Dharmacon, Inc. | siRNA targeting ribonucleotide reductase M2 polypeptide (RRM2 or RNR-R2) |
| US20080090997A1 (en) * | 2002-11-14 | 2008-04-17 | Dharmacon, Inc. | siRNA targeting complement component 3 (C3) |
| US20080091003A1 (en) * | 2002-11-14 | 2008-04-17 | Dharmacon Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20080091001A1 (en) * | 2002-11-14 | 2008-04-17 | Dharmacon Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US8093370B2 (en) | 2002-11-14 | 2012-01-10 | Dharmacon, Inc. | siRNA targeting spleen tyrosine kinase |
| US20080085998A1 (en) * | 2002-11-14 | 2008-04-10 | Dharmacon, Inc. | siRNA targeting transient receptor potential cation channel, subfamily V, member 1 (TRPV1) |
| US10011836B2 (en) | 2002-11-14 | 2018-07-03 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US20100240554A1 (en) * | 2002-11-14 | 2010-09-23 | Dharmacon, Inc. | siRNA Targeting Glucagon Receptor (GCGR) |
| US20080064865A1 (en) * | 2002-11-14 | 2008-03-13 | Dharmacon, Inc. | siRNA targeting cyclin dependent kinase 11 (CDK11) |
| US7829696B2 (en) | 2002-11-14 | 2010-11-09 | Dharmacon, Inc. | siRNA targeting amyloid beta (A4) precursor protein (APP) |
| US8090542B2 (en) | 2002-11-14 | 2012-01-03 | Dharmacon Inc. | Functional and hyperfunctional siRNA |
| US20080268457A1 (en) * | 2002-11-14 | 2008-10-30 | Dharmacon, Inc. | siRNA targeting forkhead box P3 (FOXP3) |
| US20070276135A1 (en) * | 2002-11-14 | 2007-11-29 | Dharmacon, Inc. | siRNA targeting dual specificity phosphate 5 (DUSP5) |
| US7795421B2 (en) | 2002-11-14 | 2010-09-14 | Dharmacon, Inc. | siRNA targeting apolipoprotein B (APOB) |
| US7795420B2 (en) | 2002-11-14 | 2010-09-14 | Dharmacon, Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20080293595A1 (en) * | 2002-11-14 | 2008-11-27 | Dharmacon, Inc. | siRNA targeting protein tyrosine phosphatase-1B (PTP1B) |
| US20080293593A1 (en) * | 2002-11-14 | 2008-11-27 | Dharmacon, Inc. | siRNA targeting casitas B cell lymphoma-B (CBL-B) |
| US11198870B2 (en) | 2002-11-14 | 2021-12-14 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US20080306015A1 (en) * | 2002-11-14 | 2008-12-11 | Dharmacon, Inc. | siRNA targeting proprotein convertase subtilisin/kexin type 9 (PCSK9) |
| US10233449B2 (en) | 2002-11-14 | 2019-03-19 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US20080319180A1 (en) * | 2002-11-14 | 2008-12-25 | Dharmacon, Inc. | siRNA targeting protein kinase N-3 (PKN-3) |
| US20090005548A1 (en) * | 2002-11-14 | 2009-01-01 | Dharmacon, Inc. | siRNA targeting nuclear receptor interacting protein 1 (NRIP1) |
| US7834170B2 (en) * | 2002-11-14 | 2010-11-16 | Dharmacon, Inc. | Functional and hyperfunctional siRNA |
| US20070276136A1 (en) * | 2002-11-14 | 2007-11-29 | Dharmacon, Inc. | siRNA targeting serine/threonine kinase 12 (STK12 or aurora B kinase) |
| US7833989B2 (en) | 2002-11-14 | 2010-11-16 | Dharmacon, Inc. | siRNA targeting connective tissue growth factor (CTGF) |
| US7781575B2 (en) | 2002-11-14 | 2010-08-24 | Dharmacon, Inc. | siRNA targeting tumor protein 53 (p53) |
| US8633306B2 (en) | 2002-11-14 | 2014-01-21 | Thermo Fisher Scientific Biosciences Inc. | SiRNA targeting histamine receptor H1 |
| US8883998B2 (en) * | 2002-11-14 | 2014-11-11 | Thermo Fisher Scientific Inc. | siRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US8071754B2 (en) | 2002-11-14 | 2011-12-06 | Dharmacon, Inc. | siRNA targeting apolipoprotein B (APOB) |
| US8067576B2 (en) | 2002-11-14 | 2011-11-29 | Dharmacon, Inc. | siRNA targeting serine/threonine kinase 12 (STK12 or aurora B kinase) |
| US9879266B2 (en) | 2002-11-14 | 2018-01-30 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US7855186B2 (en) | 2002-11-14 | 2010-12-21 | Dharmacon, Inc. | siRNA targeting TIE-2 |
| US20100323922A1 (en) * | 2002-11-14 | 2010-12-23 | Dharmacon, Inc. | siRNA targeting TATA box binding protein (TBP)-associated factor (TAF1) |
| US20100331214A1 (en) * | 2002-11-14 | 2010-12-30 | Dharmacon Inc. | siRNA Targeting Survivin |
| US20090082556A1 (en) * | 2002-11-14 | 2009-03-26 | Dharmacon, Inc. | siRNA targeting TATA box binding protein (TBP)-associated factor (TAF1) |
| US20090088563A1 (en) * | 2002-11-14 | 2009-04-02 | Dharmacon, Inc. | siRNA targeting Transducin (beta)-like 3 (TBL3) |
| US9839649B2 (en) | 2002-11-14 | 2017-12-12 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US20070265438A1 (en) * | 2002-11-14 | 2007-11-15 | Dharmacon, Inc. | siRNA targeting polo-like kinase-1 (PLK-1) |
| US20110003714A1 (en) * | 2002-11-14 | 2011-01-06 | Dharmacon, Inc. | siRNA Targeting Beta Secretase (BACE) |
| US20110003713A1 (en) * | 2002-11-14 | 2011-01-06 | Dharmacon, Inc. | siRNA targeting apolipoprotein B (APOB) |
| US7816512B2 (en) | 2002-11-14 | 2010-10-19 | Dharmacon, Inc. | siRNA targeting proto-oncogene MET |
| US20070244311A1 (en) * | 2002-11-14 | 2007-10-18 | Dharmacon, Inc. | siRNA targeting coatomer protein complex, subunit beta 2 (CPOB2) |
| US8039610B2 (en) | 2002-11-14 | 2011-10-18 | Dharmacon, Inc. | siRNA targeting superoxide dismutase 1 (SOD1) |
| US20090118489A1 (en) * | 2002-11-14 | 2009-05-07 | Dharmacon, Inc. | siRNA targeting nucleoporin 62kDa (Nup62) |
| US8138329B2 (en) | 2002-11-14 | 2012-03-20 | Dharmacon, Inc. | siRNA targeting connective tissue growth factor (CTGF) |
| US20100190971A1 (en) * | 2002-11-14 | 2010-07-29 | Dharmacon, Inc. | siRNA Targeting Diacylglycerol O-Acyltransferase Homolog 2 (DGAT2) |
| US20110034349A1 (en) * | 2002-11-14 | 2011-02-10 | Dharmacon, Inc. | siRNA targeting proto-oncogene MET |
| US8030476B2 (en) | 2002-11-14 | 2011-10-04 | Dharmacon, Inc. | siRNA targeting gremlin |
| US9777270B2 (en) | 2002-11-14 | 2017-10-03 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US9771586B2 (en) | 2002-11-14 | 2017-09-26 | Thermo Fisher Scientific Inc. | RNAi targeting ZNF205 |
| US20090149644A1 (en) * | 2002-11-14 | 2009-06-11 | Dharmacon Inc. | siRNA Targeting KRAS |
| US20090156797A1 (en) * | 2002-11-14 | 2009-06-18 | Dharmacon, Inc. | siRNA Targeting Hypoxia-inducible Factor 1 |
| US7550572B2 (en) * | 2002-11-14 | 2009-06-23 | Dharmacon, Inc. | siRNA targeting cell division cycle 20 homolog (CDC20) |
| US20130210676A1 (en) * | 2002-11-14 | 2013-08-15 | Dharmacon, Inc. | siRNA Targeting Myeloid Differentiation Primary Response Gene (88) (MYD88) |
| US20090163701A1 (en) * | 2002-11-14 | 2009-06-25 | Dharmacon Inc. | siRNA targeting tumor necrosis factor receptor superfamily member 1A |
| US8030474B2 (en) | 2002-11-14 | 2011-10-04 | Dharmacon, Inc. | siRNA targeting cyclin-dependent kinase 4 (CDK4) |
| US20070219362A1 (en) * | 2002-11-14 | 2007-09-20 | Dharmacon, Inc. | siRNA targeting azurocidin 1 (Cartionic Antimicrobial protein 37) |
| US20070207974A1 (en) * | 2002-11-14 | 2007-09-06 | Dharmacon Inc. | Functional and hyperfunctional siRNA |
| US8022199B2 (en) | 2002-11-14 | 2011-09-20 | Dharmacon, Inc. | SiRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US20090191625A1 (en) * | 2002-11-14 | 2009-07-30 | Dharmacon, Inc. | siRNA targeting connective tissue growth factor (CTGF) |
| US8022198B2 (en) | 2002-11-14 | 2011-09-20 | Dharmacon, Inc. | siRNA targeting histamine receptor H1 |
| US20090203895A1 (en) * | 2002-11-14 | 2009-08-13 | Dharmacon, Inc. | siRNA targeting cyclin-dependent kinase 4 (CDK4) |
| US7893247B2 (en) | 2002-11-14 | 2011-02-22 | Dharmacon, Inc. | siRNA targeting spleen tyrosine kinase |
| US7576197B2 (en) | 2002-11-14 | 2009-08-18 | Dharmacon, Inc. | SiRNA targeting KRAS |
| US20050246794A1 (en) * | 2002-11-14 | 2005-11-03 | Dharmacon Inc. | Functional and hyperfunctional siRNA |
| US8013145B2 (en) | 2002-11-14 | 2011-09-06 | Dharmacon, Inc. | SiRNA targeting cyclin-dependent kinase inhibitor 1B (p27, Kip1) (CDKN1B) |
| US7897754B2 (en) | 2002-11-14 | 2011-03-01 | Dharmacon, Inc. | SiRNA targeting ras-related nuclear protein RAN |
| US9719094B2 (en) | 2002-11-14 | 2017-08-01 | Thermo Fisher Scientific Inc. | RNAi targeting SEC61G |
| US9719092B2 (en) | 2002-11-14 | 2017-08-01 | Thermo Fisher Scientific Inc. | RNAi targeting CNTD2 |
| US7582746B2 (en) | 2002-11-14 | 2009-09-01 | Dharmacon, Inc. | siRNA targeting complement component 3 (C3) |
| US8461326B2 (en) | 2002-11-14 | 2013-06-11 | Dharmacon, Inc. | SiRNA targeting connective tissue growth factor (CTGF) |
| US20090227780A1 (en) * | 2002-11-14 | 2009-09-10 | Dharmacon, Inc. | siRNA targeting connexin 43 |
| US7589191B2 (en) | 2002-11-14 | 2009-09-15 | Dharmacon, Inc. | siRNA targeting hypoxia-inducible factor 1 |
| US8008474B2 (en) | 2002-11-14 | 2011-08-30 | Dharmacon, Inc. | siRNA targeting KRAS |
| US20070185320A1 (en) * | 2002-11-14 | 2007-08-09 | Dharmacon, Inc. | siRNA targeting cell division cycle 20 homolog (CDC20) |
| US7592442B2 (en) | 2002-11-14 | 2009-09-22 | Dharmacon, Inc. | siRNA targeting ribonucleotide reductase M2 polypeptide (RRM2 or RNR-R2) |
| US7592444B2 (en) | 2002-11-14 | 2009-09-22 | Dharmacon, Inc. | siRNA targeting myeloid cell leukemia sequence 1 |
| US7592443B2 (en) | 2002-11-14 | 2009-09-22 | Dharmacon, Inc. | siRNA targeting interleukin-1 receptor-associated kinase 4 (IRAK4) |
| US7745610B2 (en) | 2002-11-14 | 2010-06-29 | Dharmacon, Inc. | siRNA targeting cyclin dependent kinase 11 (CDK11) |
| US7745612B2 (en) | 2002-11-14 | 2010-06-29 | Dharmacon, Inc. | siRNA targeting interleukin-1 receptor-associated kinase 4 (IRAK4) |
| US7595389B2 (en) | 2002-11-14 | 2009-09-29 | Dharmacon, Inc. | siRNA targeting casitas B cell lymphoma-B (CBL-B) |
| US20070031844A1 (en) * | 2002-11-14 | 2007-02-08 | Anastasia Khvorova | Functional and hyperfunctional siRNA |
| US7598370B2 (en) | 2002-11-14 | 2009-10-06 | Dharmacon, Inc. | siRNA targeting polo-like kinase-1 (PLK-1) |
| US7598369B2 (en) | 2002-11-14 | 2009-10-06 | Dharmacon, Inc. | siRNA targeting histamine receptor H1 |
| US20090253776A1 (en) * | 2002-11-14 | 2009-10-08 | Dharmacon, Inc. | siRNA targeting gremlin |
| US8198427B1 (en) | 2002-11-14 | 2012-06-12 | Dharmacon, Inc. | SiRNA targeting catenin, beta-1 (CTNNB1) |
| US8000902B2 (en) | 2002-11-14 | 2011-08-16 | Dharmacon, Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US7745611B2 (en) | 2002-11-14 | 2010-06-29 | Dharmacon, Inc. | siRNA targeting KRAS |
| US7605252B2 (en) | 2002-11-14 | 2009-10-20 | Dharmacon, Inc. | siRNA targeting kinase insert domain receptor (KDR) |
| US7999097B2 (en) | 2002-11-14 | 2011-08-16 | Dharmacon, Inc. | siRNA targeting beta secretase (BACE) |
| US7741470B2 (en) | 2002-11-14 | 2010-06-22 | Dharmacon, Inc. | siRNA targeting gremlin |
| US7608707B2 (en) * | 2002-11-14 | 2009-10-27 | Dharmacon, Inc. | siRNA targeting survivin |
| US7608706B2 (en) * | 2002-11-14 | 2009-10-27 | Dharmacon, Inc. | siRNA targeting ras-related nuclear protein |
| US7612196B2 (en) | 2002-11-14 | 2009-11-03 | Dharmacon, Inc. | siRNA targeting cyclin-dependent kinase inhibitor 1B (p27, Kip1) (CDKN1B) |
| US7737267B2 (en) | 2002-11-14 | 2010-06-15 | Dharmacon, Inc. | siRNA targeting hypoxia-inducible factor 1 |
| US7615541B2 (en) | 2002-11-14 | 2009-11-10 | Dharmacon, Inc. | siRNA targeting TIE-2 |
| US20100144552A1 (en) * | 2002-11-14 | 2010-06-10 | Dharmacon, Inc. | siRNA targeting serine/threonine kinase 12 (STK12 or aurora B kinase) |
| US8426579B2 (en) * | 2002-11-14 | 2013-04-23 | Dharmacon, Inc. | SiRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US7619081B2 (en) | 2002-11-14 | 2009-11-17 | Dharmacon, Inc. | siRNA targeting coatomer protein complex, subunit beta 2 (COPB2) |
| US20070072823A1 (en) * | 2002-11-14 | 2007-03-29 | Dharmacon Inc. | siRNA targeting survivin |
| US20080113377A1 (en) * | 2002-11-14 | 2008-05-15 | Dharmacon, Inc. | siRNA Targeting proto-oncogene MET |
| US10765695B2 (en) | 2002-11-14 | 2020-09-08 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US8217162B2 (en) | 2002-11-14 | 2012-07-10 | Dharmacon, Inc. | siRNA targeting interleukin-1 receptor-associated kinase 4(IRAK4) |
| US8222395B2 (en) | 2002-11-14 | 2012-07-17 | Dharmacon, Inc. | siRNA targeting kinase insert domain receptor (KDR) |
| US7985854B2 (en) | 2002-11-14 | 2011-07-26 | Dharmacon, Inc. | siRNA targeting TATA box binding protein (TBP)-associated factor (TAF1) |
| US8222396B2 (en) | 2002-11-14 | 2012-07-17 | Dharmacon, Inc. | SiRNA targeting proto-oncogene MET |
| US8232386B2 (en) | 2002-11-14 | 2012-07-31 | Dharmacon, Inc. | SiRNA targeting apolipoprotein B (APOB) |
| US7935813B2 (en) | 2002-11-14 | 2011-05-03 | Dharmacon, Inc. | siRNA target hypoxia-inducible factor 1 |
| US7977471B2 (en) | 2002-11-14 | 2011-07-12 | Dharmacon, Inc. | siRNA targeting TNFα |
| US8232385B2 (en) | 2002-11-14 | 2012-07-31 | Dharmacon, Inc. | siRNA targeting cyclin-dependent kinase inhibitor 1B (p27, Kip1) (CDKN1B) |
| US20070088154A1 (en) * | 2002-11-14 | 2007-04-19 | Dharmacon Inc. | siRNA targeting complement factor B |
| US20090306356A1 (en) * | 2002-11-14 | 2009-12-10 | Dharmacon,Inc. | siRNA Targeting TNFalpha |
| US7632938B2 (en) | 2002-11-14 | 2009-12-15 | Dharmacon, Inc. | siRNA targeting superoxide dismutase 1 (SOD1) |
| US7632939B2 (en) | 2002-11-14 | 2009-12-15 | Dharmacon, Inc. | siRNA targeting proto-oncogene MET |
| US8236942B2 (en) | 2002-11-14 | 2012-08-07 | Dharmacon, Inc. | SiRNA targeting glucagon receptor (GCGR) |
| US7635770B2 (en) | 2002-11-14 | 2009-12-22 | Dharmacon, Inc. | siRNA targeting protein kinase N-3 (PKN-3) |
| US7635771B2 (en) | 2002-11-14 | 2009-12-22 | Dharmacon, Inc. | siRNA targeting amyloid beta (A4) precursor protein (APP) |
| US8247169B2 (en) | 2002-11-14 | 2012-08-21 | Dharmacon, Inc. | SiRNA targeting diacylglycerol O-acyltransferase homolog 2 (DGAT2) |
| US20100113306A1 (en) * | 2002-11-14 | 2010-05-06 | Dharmacon, Inc. | siRNA Targeting connective tissue growth factor (CTGF) |
| US20090325818A1 (en) * | 2002-11-14 | 2009-12-31 | Dharmacon, Inc. | siRNA targeting interleukin-1 receptor-associated kinase 4 (IRAK4) |
| US7642349B2 (en) | 2002-11-14 | 2010-01-05 | Dharmacon, Inc. | siRNA targeting TATA box binding protein (TBP)-associated factor (TAF1) |
| US8293887B2 (en) | 2002-11-14 | 2012-10-23 | Dharmacon, Inc. | SiRNA targeting beta secretase (BACE) |
| US20050245475A1 (en) * | 2002-11-14 | 2005-11-03 | Dharmacon, Inc. | Functional and hyperfunctional siRNA directed against Bcl-2 |
| US20070141602A1 (en) * | 2002-11-14 | 2007-06-21 | Dharmacon, Inc. | siRNA targeting aquaporin 4 |
| US8304528B2 (en) | 2002-11-14 | 2012-11-06 | Dharmacon, Inc. | SiRNA targeting fructose-1, 6-bisphosphatase 1 (FBP1) |
| US7951935B2 (en) | 2002-11-14 | 2011-05-31 | Dharmacon, Inc. | siRNA targeting v-myc myelocytomatosis viral oncogene homolog (MYC) |
| US20100113760A1 (en) * | 2002-11-14 | 2010-05-06 | Dharmacon, Inc. | siRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US20100016176A1 (en) * | 2002-11-14 | 2010-01-21 | Dharmacon. Inc. | siRNA targeting histamine receptor H1 |
| US20100022763A1 (en) * | 2002-11-14 | 2010-01-28 | Dharmacon, Inc. | siRNA targeting kinase insert domain receptor (KDR) |
| US20100022413A1 (en) * | 2002-11-14 | 2010-01-28 | Dharmacon, Inc. | siRNA targeting Ras-related nuclear protein RAN |
| US7655789B2 (en) | 2002-11-14 | 2010-02-02 | Dharmacon, Inc. | siRNA targeting transient receptor potential cation channel, subfamily V, member 1 (TRPV1) |
| US7662950B2 (en) * | 2002-11-14 | 2010-02-16 | Dharmacon, Inc. | siRNA targeting myeloid differentiation primary response gene (88) (MYD88) |
| US20100113307A1 (en) * | 2002-11-14 | 2010-05-06 | Dharmacon, Inc. | siRNA targeting vascular endothelial growth factor (VEGF) |
| US7666853B2 (en) | 2002-11-14 | 2010-02-23 | Dharmacon, Inc. | siRNA targeting connective tissue growth factor (CTGF) |
| US7709629B2 (en) | 2002-11-14 | 2010-05-04 | Dharmacon, Inc. | siRNA targeting diacylglycerol O-acyltransferase homolog 2 (DGAT2) |
| US20110105363A1 (en) * | 2002-11-14 | 2011-05-05 | Dharmacon, Inc. | siRNA targeting TNFa |
| US20100062951A1 (en) * | 2002-11-14 | 2010-03-11 | Dharmacon, Inc. | siRNA targeting TIE-2 |
| US7678896B2 (en) | 2002-11-14 | 2010-03-16 | Dharmacon, Inc. | siRNA targeting serine/threonine kinase 12 (STK12 or aurora B kinase) |
| US20070134697A1 (en) * | 2002-11-14 | 2007-06-14 | Dharmacon, Inc. | siRNA targeting TIE-2 |
| US10696968B2 (en) | 2002-11-14 | 2020-06-30 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US20100075869A1 (en) * | 2002-11-14 | 2010-03-25 | Dharmacon, Inc. | siRNA targeting TATA box binding protein (TBP)-associated factor (TAF1) |
| US7691998B2 (en) | 2002-11-14 | 2010-04-06 | Dharmacon, Inc. | siRNA targeting nucleoporin 62kDa (Nup62) |
| US7691997B2 (en) | 2002-11-14 | 2010-04-06 | Dharmacon, Inc. | Functional and hyperfunctional siRNA |
| US20070134698A1 (en) * | 2002-11-14 | 2007-06-14 | Dharmacon, Inc. | siRNA targeting histamine receptor H1 |
| US9228186B2 (en) | 2002-11-14 | 2016-01-05 | Thermo Fisher Scientific Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US20100099578A1 (en) * | 2002-11-14 | 2010-04-22 | Dharmacon, Inc. | siRNA Targeting Fructose-1, 6-bisphosphatase 1 (FBP1) |
| US7696344B2 (en) | 2002-11-14 | 2010-04-13 | Dharmacon, Inc. | siRNA targeting complement factor B |
| US8314229B2 (en) | 2002-11-14 | 2012-11-20 | Dharmacon, Inc. | siRNA targeting tie-2 |
| US20070128640A1 (en) * | 2002-11-14 | 2007-06-07 | Dharmacon, Inc. | siRNA targeting ras-related nuclear protein |
| US20050255487A1 (en) * | 2002-11-14 | 2005-11-17 | Dharmacon, Inc. | Methods and compositions for selecting siRNA of improved functionality |
| US8058251B2 (en) | 2002-11-26 | 2011-11-15 | Kaemmerer William F | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of siRNA |
| US8618069B2 (en) | 2002-11-26 | 2013-12-31 | Medtronic, Inc. | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of siRNA |
| US7829694B2 (en) * | 2002-11-26 | 2010-11-09 | Medtronic, Inc. | Treatment of neurodegenerative disease through intracranial delivery of siRNA |
| US7605249B2 (en) | 2002-11-26 | 2009-10-20 | Medtronic, Inc. | Treatment of neurodegenerative disease through intracranial delivery of siRNA |
| US20090060987A1 (en) * | 2002-11-26 | 2009-03-05 | Kaemmerer William F | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of sirna |
| US7618948B2 (en) * | 2002-11-26 | 2009-11-17 | Medtronic, Inc. | Devices, systems and methods for improving and/or cognitive function through brain delivery of siRNA |
| US20040220132A1 (en) * | 2002-11-26 | 2004-11-04 | Medtronic, Inc. | Treatment of neurodegenerative disease through intracranial delivery of siRNA |
| US8415319B2 (en) | 2002-11-26 | 2013-04-09 | Medtronic, Inc. | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of siRNA |
| US8119611B2 (en) | 2002-11-26 | 2012-02-21 | Medtronic, Inc. | Treatment of neurodegenerative disease through intracranial delivery of SIRNA |
| US20060178328A1 (en) * | 2002-11-26 | 2006-08-10 | Medtronic Inc. | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of siRNA |
| US20080188429A1 (en) * | 2002-12-27 | 2008-08-07 | Iyer Radhakrishnan P | Synthetic siRNA compounds and methods for the downregulation of gene expression |
| US20060178324A1 (en) * | 2003-01-21 | 2006-08-10 | Philipp Hadwiger | Lipophilic derivatives of double-stranded ribonucleic acid |
| US20060178297A1 (en) * | 2003-01-28 | 2006-08-10 | Troy Carol M | Systems and methods for silencing expression of a gene in a cell and uses thereof |
| US8957198B2 (en) | 2003-02-03 | 2015-02-17 | Medtronic, Inc. | Compositions, devices and methods for treatment of Huntington's disease through intracranial delivery of sirna |
| US20040167090A1 (en) * | 2003-02-21 | 2004-08-26 | Monahan Sean D. | Covalent modification of RNA for in vitro and in vivo delivery |
| US8796235B2 (en) | 2003-02-21 | 2014-08-05 | University Of South Florida | Methods for attenuating dengue virus infection |
| US20060239971A1 (en) * | 2003-02-21 | 2006-10-26 | Mohapatra Shyam S | Vectors for regulating gene expression |
| US20080085999A1 (en) * | 2003-03-06 | 2008-04-10 | Oligoengine, Inc. | Modulation of gene expression using dna-rna hybrids |
| US11530408B2 (en) | 2003-03-07 | 2022-12-20 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US10669544B2 (en) | 2003-03-07 | 2020-06-02 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| AU2009201569B2 (en) * | 2003-03-07 | 2011-11-17 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US20070275914A1 (en) * | 2003-03-07 | 2007-11-29 | Muthiah Manoharan | Therapeutic Compositions |
| US8809516B2 (en) | 2003-03-07 | 2014-08-19 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US20100240881A1 (en) * | 2003-03-07 | 2010-09-23 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US8754201B2 (en) | 2003-03-07 | 2014-06-17 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US8420799B2 (en) | 2003-03-07 | 2013-04-16 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US9708615B2 (en) | 2003-03-07 | 2017-07-18 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US9222091B2 (en) | 2003-03-07 | 2015-12-29 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US10273477B2 (en) | 2003-03-07 | 2019-04-30 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US8110674B2 (en) * | 2003-03-07 | 2012-02-07 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US20110201798A1 (en) * | 2003-03-07 | 2011-08-18 | Alnylam Pharmaceuticals | Therapeutic compositions |
| US8445665B2 (en) | 2003-03-07 | 2013-05-21 | Alnylam Pharmaceuticals, Inc. | Therapeutic compositions |
| US20100093831A1 (en) * | 2003-03-12 | 2010-04-15 | Vasgene Therapeutics, Inc. | Nucleic acid compounds for inhibiting angiogenesis and tumor growth |
| WO2004086047A3 (en) * | 2003-03-28 | 2005-01-20 | Bayer Healthcare Ag | Diagnostics and therapeutics for diseases associated with g-protein-coupled receptor adenosine a1 (adora1) |
| US20050153337A1 (en) * | 2003-04-03 | 2005-07-14 | Muthiah Manoharan | iRNA conjugates |
| US20070270360A1 (en) * | 2003-04-15 | 2007-11-22 | Sirna Therapeutics, Inc. | Rna Interference Mediated Inhibition of Severe Acute Respiratory Syndrome (Sars) Gene Expression Using Short Interfering Nucleic Acid |
| US7994305B2 (en) * | 2003-04-18 | 2011-08-09 | The Trustees Of The University Of Pennsylvania | Compositions and methods for siRNA inhibition of angiopoietin 1 and 2 and their receptor Tie2 |
| US20040248174A1 (en) * | 2003-04-18 | 2004-12-09 | Thetrustees Of The University Of Pennsylvania | Compositions and methods for siRNA inhibition of angiopoietin 1and 2 and their receptor Tie2 |
| US8202849B2 (en) * | 2003-04-23 | 2012-06-19 | Georgetown University | Methods and compositions for the inhibition of Stat5 in prostate cancer cells |
| US20100120892A1 (en) * | 2003-04-23 | 2010-05-13 | Georgetown University | Methods and compositions for the inhibition of stat5 in prostate cancer cells |
| US20040224405A1 (en) * | 2003-05-06 | 2004-11-11 | Dharmacon Inc. | siRNA induced systemic gene silencing in mammalian systems |
| WO2004099387A3 (en) * | 2003-05-06 | 2005-09-01 | Dharmacon Inc | siRNA INDUCED SYSTEMIC GENE SILENCING IN MAMMALIAN SYSTEMS |
| US20040265912A1 (en) * | 2003-05-23 | 2004-12-30 | Board Of Regents, The University Of Texas System | Structure based and combinatorially selected oligonucleoside phosphorothioate and phosphorodithioate aptamer targeting AP-1 transcription factors |
| US9567579B2 (en) | 2003-05-23 | 2017-02-14 | Board Of Regents, The University Of Texas System | Structure based and combinatorially selected oligonucleoside phosphorothioate and phosphorodithioate aptamer targeting AP-1 transcription factors |
| US7910523B2 (en) | 2003-05-23 | 2011-03-22 | Board Of Regents, The University Of Texas System | Structure based and combinatorially selected oligonucleoside phosphorothioate and phosphorodithioate aptamer targeting AP-1 transcription factors |
| US20060121489A1 (en) * | 2003-05-23 | 2006-06-08 | Board Of Regents, The University Of Texas System | High throughput screening of aptamer libraries for specific binding to proteins on viruses and other pathogens |
| US20110212843A1 (en) * | 2003-05-23 | 2011-09-01 | Board Of Regents, The University Of Texas System | Structure based and combinatorially selected oligonucleoside phosphorothioate and phosphorodithioate aptamer targeting ap-1 transcription factors |
| US20080300145A1 (en) * | 2003-05-27 | 2008-12-04 | Cold Spring Harbor Laboratory | In vivo high throughput selection of RNAi probes |
| WO2005001110A3 (en) * | 2003-05-29 | 2005-06-02 | Salk Inst For Biological Studi | Transcriptional regulation of gene expression by small double-stranded modulatory rna |
| US20050226848A1 (en) * | 2003-05-29 | 2005-10-13 | Tomoko Kuwabara | Transcriptional regulation of gene expression by small double-stranded modulatory RNA |
| US8092992B2 (en) | 2003-05-29 | 2012-01-10 | Salk Institute For Biological Studies | Transcriptional regulation of gene expression by small double-stranded modulatory RNA |
| US9121018B2 (en) | 2003-06-02 | 2015-09-01 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNA silencing |
| US8309705B2 (en) * | 2003-06-02 | 2012-11-13 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNA silencing |
| US20100184827A1 (en) * | 2003-06-02 | 2010-07-22 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of rna silencing |
| US8329892B2 (en) * | 2003-06-02 | 2012-12-11 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNA silencing |
| US20100184826A1 (en) * | 2003-06-02 | 2010-07-22 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of rna silencing |
| US8309704B2 (en) | 2003-06-02 | 2012-11-13 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNAi |
| US10604754B2 (en) | 2003-06-02 | 2020-03-31 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNA silencing |
| US8304530B2 (en) | 2003-06-02 | 2012-11-06 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNA silencing |
| US20100184828A1 (en) * | 2003-06-02 | 2010-07-22 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of rna silencing |
| US20050181382A1 (en) * | 2003-06-02 | 2005-08-18 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of RNAi |
| US20050186591A1 (en) * | 2003-06-09 | 2005-08-25 | Alnylam Pharmaceuticals | Method of treating neurodegenerative disease |
| US20070161595A1 (en) * | 2003-06-09 | 2007-07-12 | Mayo Foundation For Medical Education And Research | Method of treating neurodegenerative disease |
| US20110092565A1 (en) * | 2003-06-09 | 2011-04-21 | Alnylam Pharmaceuticals, Inc. | Method of treating neurodegenerative disease |
| US7595306B2 (en) | 2003-06-09 | 2009-09-29 | Alnylam Pharmaceuticals Inc | Method of treating neurodegenerative disease |
| US10982212B2 (en) | 2003-06-12 | 2021-04-20 | Alnylam Pharmaceuticals, Inc. | Conserved HBV and HCV sequences useful for gene silencing |
| US20040254358A1 (en) * | 2003-06-12 | 2004-12-16 | Muthiah Manoharan | Phosphorous-linked oligomeric compounds and their use in gene modulation |
| US7790691B2 (en) * | 2003-06-20 | 2010-09-07 | Isis Pharmaceuticals, Inc. | Double stranded compositions comprising a 3′-endo modified strand for use in gene modulation |
| WO2004113496A3 (en) * | 2003-06-20 | 2007-11-29 | Isis Pharmaceuticals Inc | Double stranded compositions comprising a 3’-endo modified strand for use in gene modulation |
| US20070015722A1 (en) * | 2003-06-20 | 2007-01-18 | Kraynack Brian A | Double stranded compositions comprising a 3'-endo modified strand for use in gene modulation |
| US10046016B2 (en) | 2003-06-30 | 2018-08-14 | The Regents Of The University Of California | Mutant adeno-associated virus virions and methods of use thereof |
| US9441244B2 (en) * | 2003-06-30 | 2016-09-13 | The Regents Of The University Of California | Mutant adeno-associated virus virions and methods of use thereof |
| US10214566B2 (en) | 2003-06-30 | 2019-02-26 | The Regents Of The University Of California | Mutant adeno-associated virus virions and methods of use thereof |
| US20050053922A1 (en) * | 2003-06-30 | 2005-03-10 | Schaffer David V. | Mutant adeno-associated virus virions and methods of use thereof |
| US9233131B2 (en) | 2003-06-30 | 2016-01-12 | The Regents Of The University Of California | Mutant adeno-associated virus virions and methods of use thereof |
| US20070219148A1 (en) * | 2003-07-02 | 2007-09-20 | Commissariat A L'energie Atomique | Small Interfering RNA Specific to Sub-Units $g(a),$g(a)' and $g(b) of the Kinase Protein ck2,and the Applications of the Same |
| US8106179B2 (en) * | 2003-07-02 | 2012-01-31 | Commissariat A L'energie Atomique | Small interfering RNA specific to sub-units α, α′and β of the Kinase Protein ck2, and the applications of the same |
| US20050256071A1 (en) * | 2003-07-15 | 2005-11-17 | California Institute Of Technology | Inhibitor nucleic acids |
| US20050136430A1 (en) * | 2003-07-15 | 2005-06-23 | California Institute Of Technology | Inhibitor nucleic acids |
| US7982027B2 (en) * | 2003-07-16 | 2011-07-19 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering RNA |
| US20060240093A1 (en) * | 2003-07-16 | 2006-10-26 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering rna |
| US20120058188A1 (en) * | 2003-07-16 | 2012-03-08 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering rna |
| US20050118611A1 (en) * | 2003-07-24 | 2005-06-02 | Board Of Regents, The University Of Texas System | Thioaptamers enable discovery of physiological pathways and new therapeutic strategies |
| US20100222564A1 (en) * | 2003-07-25 | 2010-09-02 | Life Technologies Corporation | Methods and compositions for isolating small rna molecules |
| US8404439B2 (en) | 2003-07-25 | 2013-03-26 | Applied Biosystems, Llc | Methods and compositions for isolating small RNA molecules |
| US9193748B2 (en) | 2003-07-25 | 2015-11-24 | Applied Biosystems, Llc | Methods and compositions for isolating small RNA molecules |
| US10619150B2 (en) | 2003-07-25 | 2020-04-14 | Applied Biosystems, Llc | Methods and compositions for isolating small RNA |
| US10138475B2 (en) | 2003-07-25 | 2018-11-27 | Applied Biosystems, Llc | Methods and compositions for isolating small RNA molecules |
| US8178506B2 (en) | 2003-07-31 | 2012-05-15 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8765701B2 (en) * | 2003-07-31 | 2014-07-01 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US10072265B2 (en) | 2003-07-31 | 2018-09-11 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US9139832B2 (en) | 2003-07-31 | 2015-09-22 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8809294B2 (en) | 2003-07-31 | 2014-08-19 | Regulus Therapeutics Inc. | Method of inhibiting miR-33 using a modified oligonucleotide |
| US20100249215A1 (en) * | 2003-07-31 | 2010-09-30 | Regulus Therapeutics, Inc. | Oligomeric Compounds And Compositions For Use In Modulation Of Pri-miRNAs |
| US8697663B2 (en) | 2003-07-31 | 2014-04-15 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8133876B2 (en) | 2003-07-31 | 2012-03-13 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8106025B2 (en) | 2003-07-31 | 2012-01-31 | Regulus Therapeutics Inc. | Method for inhibiting the activity of mir-155 |
| US8859521B2 (en) | 2003-07-31 | 2014-10-14 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8110558B2 (en) | 2003-07-31 | 2012-02-07 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAS |
| US9752146B2 (en) | 2003-07-31 | 2017-09-05 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8466120B2 (en) | 2003-07-31 | 2013-06-18 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of pri-miRNAs |
| US20110224277A1 (en) * | 2003-07-31 | 2011-09-15 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US20100267813A1 (en) * | 2003-07-31 | 2010-10-21 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US9447413B2 (en) | 2003-07-31 | 2016-09-20 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US9663787B2 (en) | 2003-07-31 | 2017-05-30 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US10584336B2 (en) | 2003-07-31 | 2020-03-10 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8946179B2 (en) | 2003-07-31 | 2015-02-03 | Regulus Therapeutics, Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US20090286969A1 (en) * | 2003-07-31 | 2009-11-19 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US9267138B2 (en) | 2003-07-31 | 2016-02-23 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US20090291907A1 (en) * | 2003-07-31 | 2009-11-26 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US9528108B2 (en) | 2003-07-31 | 2016-12-27 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAS |
| US20090291906A1 (en) * | 2003-07-31 | 2009-11-26 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US10093926B2 (en) | 2003-07-31 | 2018-10-09 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US8546350B2 (en) | 2003-07-31 | 2013-10-01 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAs |
| US20090298174A1 (en) * | 2003-07-31 | 2009-12-03 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US20090317907A1 (en) * | 2003-07-31 | 2009-12-24 | Regulus Therapeutics, Llc. | Oligomeric Compounds And Compositions For Use In Modulation Of Small Non-Coding RNAs |
| US9447412B2 (en) | 2003-07-31 | 2016-09-20 | Regulus Therapeutics Inc. | Oligomeric compounds and compositions for use in modulation of small non-coding RNAS |
| EP1660631A4 (en) * | 2003-08-01 | 2008-06-25 | Invitrogen Corp | Compositions and methods for preparing short rna molecules and other nucleic acids |
| EP2311994A1 (en) * | 2003-08-01 | 2011-04-20 | Life Technologies Corporation | Compositions and methods for preparing short RNA molecules and other nucleic acids |
| WO2005012483A3 (en) * | 2003-08-01 | 2005-06-02 | Internat Therapeutics Inc | Vpr selective rnai agents and methods for using the same |
| US20070155658A1 (en) * | 2003-08-25 | 2007-07-05 | Nastech Pharmaceutical Company Inc. | Nanoparticles for delivery of nucleic acids and stable double-stranded rna |
| US20060142230A1 (en) * | 2003-08-25 | 2006-06-29 | Nastech Pharmaceutical Company Inc. | Double-stranded ribonucleic acid molecules having ribothymidine |
| US20060160123A1 (en) * | 2003-08-25 | 2006-07-20 | Nastech Pharmaceutical Company Inc. | Method of minimizing off-target effects of siRNA molecules |
| US20050136437A1 (en) * | 2003-08-25 | 2005-06-23 | Nastech Pharmaceutical Company Inc. | Nanoparticles for delivery of nucleic acids and stable double-stranded RNA |
| US20060122137A1 (en) * | 2003-08-25 | 2006-06-08 | Nastech Pharmaceutical Company Inc. | 5'-methylpyrimidine and 2'-O-methyl ribonucleotide modified double-stranded ribonucleic acid molecules |
| US20070203084A1 (en) * | 2003-08-28 | 2007-08-30 | Jan Weiler | Interfering Rna Duplex Having Blunt-Ends And 3'-Modifications |
| AU2004269150B2 (en) * | 2003-08-28 | 2009-06-04 | Novartis Ag | Interfering RNA duplex having blunt-ends and 3'-modifications |
| AU2004269150C1 (en) * | 2003-08-28 | 2010-11-18 | Novartis Ag | Interfering RNA duplex having blunt-ends and 3'-modifications |
| US20090192113A1 (en) * | 2003-08-28 | 2009-07-30 | Jan Weiler | Interfering RNA Duplex Having Blunt-Ends and 3`-Modifications |
| US8097716B2 (en) | 2003-08-28 | 2012-01-17 | Novartis Ag | Interfering RNA duplex having blunt-ends and 3′-modifications |
| US8791083B2 (en) | 2003-09-09 | 2014-07-29 | Isis Pharmaceuticals, Inc. | Chimeric oligomeric compounds comprising alternating regions of northern and southern conformational geometry |
| US20090258931A1 (en) * | 2003-09-09 | 2009-10-15 | Isis Pharmaceuticals, Inc. | Chimeric oligomeric compounds comprising alternating regions of northern and southern conformational geometry |
| US20110091525A1 (en) * | 2003-09-15 | 2011-04-21 | Protiva Biotherapeutics, Inc. | Polyethyleneglycol-modified lipid compounds and uses thereof |
| US8936942B2 (en) * | 2003-09-15 | 2015-01-20 | Protiva Biotherapeutics, Inc. | Polyethyleneglycol-modified lipid compounds and uses thereof |
| US20070218551A1 (en) * | 2003-10-02 | 2007-09-20 | Chuan-Yuan Li | Novel Sirna-Based Approach to Target the Hif-Alpha Factor for Gene Therapy |
| US20080249038A1 (en) * | 2003-10-07 | 2008-10-09 | Quark Biotech, Inc. | Bone Morphogenetic Protein (Bmp) 2A and Uses Thereof |
| US7807646B1 (en) * | 2003-11-20 | 2010-10-05 | University Of South Florida | SHIP-deficiency to increase megakaryocyte progenitor production |
| US8008273B2 (en) | 2003-11-20 | 2011-08-30 | University Of South Florida | SHIP-deficiency to increase megakaryocyte progenitor production |
| US20100260730A1 (en) * | 2003-11-20 | 2010-10-14 | University Of South Florida | SHIP-Deficiency to Increase Megakaryocyte Progenitor Production |
| US20110064704A1 (en) * | 2003-11-20 | 2011-03-17 | University Of South Florida | Ship-deficiency to increase megakaryocyte and platelet production |
| US7763592B1 (en) | 2003-11-20 | 2010-07-27 | University Of South Florida | SHIP-deficiency to increase megakaryocyte progenitor production |
| US10793873B2 (en) | 2003-11-21 | 2020-10-06 | Revivicor, Inc. | Use of interfering RNA in the production of transgenic animals |
| US20050208658A1 (en) * | 2003-11-21 | 2005-09-22 | The University Of Maryland | RNA interference mediated inhibition of 11beta hydroxysteriod dehydrogenase-1 (11beta HSD-1) gene expression |
| US20050266561A1 (en) * | 2003-11-21 | 2005-12-01 | Revivicor, Inc. | Use of interfering RNA in the production of transgenic animals |
| US20070167389A1 (en) * | 2003-11-25 | 2007-07-19 | Kaemmerer William F | Compositions, devices and methods for treatment of huntington's disease through intracranial delivery of sirna |
| US7732591B2 (en) | 2003-11-25 | 2010-06-08 | Medtronic, Inc. | Compositions, devices and methods for treatment of huntington's disease through intracranial delivery of sirna |
| US9089590B2 (en) | 2003-12-04 | 2015-07-28 | University Of South Florida | Polynucleotides for reducing respiratory syncytial virus gene expression |
| US20070249549A1 (en) * | 2003-12-17 | 2007-10-25 | Index Pharmaceuticals Ab | Compounds and Methods for Rna Interference of the P65 Subunit of Nf-Kappa-B |
| US9879253B2 (en) | 2003-12-22 | 2018-01-30 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of single and double blunt-ended siRNA |
| US10385339B2 (en) | 2003-12-22 | 2019-08-20 | University Of Massachusetts | Methods and compositions for enhancing the efficacy and specificity of single and double blunt-ended siRNA |
| US20070161586A1 (en) * | 2004-01-16 | 2007-07-12 | Takeda Pharmaceutical Company Limited | Drug for preventing and treating atherosclerosis |
| US20090082303A1 (en) * | 2004-01-16 | 2009-03-26 | Takeda Pharmaceutical Company Limited | Drug for preventing and treating atherosclerosis |
| US20070093440A1 (en) * | 2004-01-28 | 2007-04-26 | Champion Brian R | Medical treatment |
| US20050197310A1 (en) * | 2004-01-30 | 2005-09-08 | Orna Mor | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| US20080287386A1 (en) * | 2004-01-30 | 2008-11-20 | Quark Biotech, Inc. | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| US20110201670A1 (en) * | 2004-01-30 | 2011-08-18 | Quark Pharmaceuticals, Inc. | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| WO2005072057A3 (en) * | 2004-01-30 | 2007-07-19 | Quark Biotech Inc | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| US7939652B2 (en) | 2004-01-30 | 2011-05-10 | Quark Pharmaceuticals Inc. | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| US8198258B2 (en) | 2004-01-30 | 2012-06-12 | Quark Pharmaceuticals Inc. | Oligoribonucleotides and methods of use thereof for treatment of fibrotic conditions and other diseases |
| US20110118335A1 (en) * | 2004-02-10 | 2011-05-19 | Vasant Jadhav | RNA Interference Mediated Inhibition Of Gene Expression Using Multifunctional Short Interfering Nucleic Acid (Multifunctional siNA) |
| US7858769B2 (en) | 2004-02-10 | 2010-12-28 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using multifunctional short interfering nucleic acid (multifunctional siNA) |
| US20060069050A1 (en) * | 2004-02-17 | 2006-03-30 | University Of Massachusetts | Methods and compositions for mediating gene silencing |
| US8569474B2 (en) | 2004-03-09 | 2013-10-29 | Isis Pharmaceuticals, Inc. | Double stranded constructs comprising one or more short strands hybridized to a longer strand |
| US8293719B2 (en) | 2004-03-12 | 2012-10-23 | Alnylam Pharmaceuticals, Inc. | iRNA agents targeting VEGF |
| US20090018321A1 (en) * | 2004-03-15 | 2009-01-15 | Integrated Dna Technologies, Inc. | Methods and compositions for the specific inhibition of gene expression by double-stranded rna |
| US20110213328A1 (en) * | 2004-03-18 | 2011-09-01 | Medtronic, Inc. | Methods and Systems for Treatment of Neurological Diseases of the Central Nervous System |
| US20110196145A1 (en) * | 2004-04-05 | 2011-08-11 | Alnylam Pharmaceuticals, Inc. | Process for desilylation of oligonucleotides |
| US8101743B2 (en) * | 2004-04-05 | 2012-01-24 | Isis Pharmaceuticals, Inc. | Modulation of transthyretin expression |
| US8058448B2 (en) | 2004-04-05 | 2011-11-15 | Alnylam Pharmaceuticals, Inc. | Processes and reagents for sulfurization of oligonucleotides |
| US8063198B2 (en) | 2004-04-05 | 2011-11-22 | Alnylam Pharmaceuticals, Inc. | Processes and reagents for desilylation of oligonucleotides |
| US8431693B2 (en) | 2004-04-05 | 2013-04-30 | Alnylam Pharmaceuticals, Inc. | Process for desilylation of oligonucleotides |
| US20050267300A1 (en) * | 2004-04-05 | 2005-12-01 | Muthiah Manoharan | Processes and reagents for oligonucleotide synthesis and purification |
| US20060014289A1 (en) * | 2004-04-20 | 2006-01-19 | Nastech Pharmaceutical Company Inc. | Methods and compositions for enhancing delivery of double-stranded RNA or a double-stranded hybrid nucleic acid to regulate gene expression in mammalian cells |
| US20050239134A1 (en) * | 2004-04-21 | 2005-10-27 | Board Of Regents, The University Of Texas System | Combinatorial selection of phosphorothioate single-stranded DNA aptamers for TGF-beta-1 protein |
| US8470988B2 (en) | 2004-04-27 | 2013-06-25 | Alnylam Pharmaceuticals, Inc. | Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety |
| US7626014B2 (en) | 2004-04-27 | 2009-12-01 | Alnylam Pharmaceuticals | Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety |
| US20100197899A1 (en) * | 2004-04-27 | 2010-08-05 | Alnylam Pharmaceuticals, Inc. | Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety |
| US20050288244A1 (en) * | 2004-04-30 | 2005-12-29 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a C5-modified pyrimidine |
| US7674778B2 (en) | 2004-04-30 | 2010-03-09 | Alnylam Pharmaceuticals | Oligonucleotides comprising a conjugate group linked through a C5-modified pyrimidine |
| US20060040882A1 (en) * | 2004-05-04 | 2006-02-23 | Lishan Chen | Compostions and methods for enhancing delivery of nucleic acids into cells and for modifying expression of target genes in cells |
| US20110117088A1 (en) * | 2004-05-12 | 2011-05-19 | Simon Michael R | Composition and method for introduction of rna interference sequences into targeted cells and tissues |
| US20070207491A1 (en) * | 2004-05-12 | 2007-09-06 | Dharmacon, Inc. | siRNA targeting minichromosome maintenance deficient 4 (MCM4) |
| US20070141601A1 (en) * | 2004-05-12 | 2007-06-21 | Dharmacon, Inc. | siRNA targeting cAMP-specific phosphodiesterase 4D |
| US7605250B2 (en) | 2004-05-12 | 2009-10-20 | Dharmacon, Inc. | siRNA targeting cAMP-specific phosphodiesterase 4D |
| US10508277B2 (en) | 2004-05-24 | 2019-12-17 | Sirna Therapeutics, Inc. | Chemically modified multifunctional short interfering nucleic acid molecules that mediate RNA interference |
| AU2005247509B2 (en) * | 2004-05-27 | 2012-02-23 | Alnylam Pharmaceuticals, Inc. | Nuclease resistant double-stranded ribonucleic acid |
| EP1765847A4 (en) * | 2004-05-27 | 2010-10-20 | Alnylam Pharmaceuticals Inc | Nuclease resistant double-stranded ribonucleic acid |
| US20060094678A1 (en) * | 2004-05-27 | 2006-05-04 | Hans-Peter Vornlocher | Nuclease resistant double-stranded ribonucleic acid |
| US8993746B2 (en) | 2004-05-27 | 2015-03-31 | Alnylam Pharmaceuticals, Inc. | Nuclease resistant double-stranded ribonucleic acid |
| US8334373B2 (en) | 2004-05-27 | 2012-12-18 | Alnylam Pharmaceuticals, Inc. | Nuclease resistant double-stranded ribonucleic acid |
| EP2399924A3 (en) * | 2004-05-27 | 2012-02-29 | Alnylam Pharmaceuticals, Inc. | Nuclease resistant double-stranded ribonucleic acid |
| US7928217B2 (en) | 2004-05-27 | 2011-04-19 | Alnylam Pharmaceuticals, Inc. | Nuclease resistant double-stranded ribonucleic acid |
| AU2005247509C1 (en) * | 2004-05-27 | 2012-09-20 | Alnylam Pharmaceuticals, Inc. | Nuclease resistant double-stranded ribonucleic acid |
| WO2005120230A3 (en) * | 2004-06-03 | 2008-06-26 | Isis Pharmaceuticals Inc | POSITIONALLY MODIFIED siRNA CONSTRUCTS |
| US20090048192A1 (en) * | 2004-06-03 | 2009-02-19 | Isis Pharmaceuticals, Inc. | Double Strand Compositions Comprising Differentially Modified Strands for Use in Gene Modulation |
| US8394947B2 (en) | 2004-06-03 | 2013-03-12 | Isis Pharmaceuticals, Inc. | Positionally modified siRNA constructs |
| WO2005121371A3 (en) * | 2004-06-03 | 2008-01-24 | Isis Pharmaceuticals Inc | Double strand compositions comprising differentially modified strands for use in gene modulation |
| US20070179108A1 (en) * | 2004-06-03 | 2007-08-02 | Balkrishen Bhat | Double strand compositions comprising differentially modified strands for use in gene modulation |
| US20070172948A1 (en) * | 2004-06-03 | 2007-07-26 | Balkrishen Bhat | Double strand compositions comprising differentially modified strands for use in gene modulation |
| US9926560B2 (en) | 2004-06-07 | 2018-03-27 | Protiva Biotherapeutics, Inc. | Lipid encapsulating interfering RNA |
| US9181545B2 (en) | 2004-06-07 | 2015-11-10 | Protiva Biotherapeutics, Inc. | Lipid encapsulating interfering RNA |
| US20060083780A1 (en) * | 2004-06-07 | 2006-04-20 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods of use |
| US7799565B2 (en) | 2004-06-07 | 2010-09-21 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering RNA |
| US20060008910A1 (en) * | 2004-06-07 | 2006-01-12 | Protiva Biotherapeuties, Inc. | Lipid encapsulated interfering RNA |
| US20110060032A1 (en) * | 2004-06-07 | 2011-03-10 | Protiva Biotherapeutics, Inc. | Lipid encapsulating interfering rna |
| US7745651B2 (en) | 2004-06-07 | 2010-06-29 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods of use |
| US20060287260A1 (en) * | 2004-06-30 | 2006-12-21 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a non-phosphate backbone linkage |
| US7615618B2 (en) | 2004-06-30 | 2009-11-10 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a non-phosphate backbone linkage |
| US20090318676A1 (en) * | 2004-06-30 | 2009-12-24 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a non-phosphate backbone linkage |
| US7723512B2 (en) | 2004-06-30 | 2010-05-25 | Alnylam Pharmaceuticals | Oligonucleotides comprising a non-phosphate backbone linkage |
| US8013136B2 (en) | 2004-06-30 | 2011-09-06 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a non-phosphate backbone linkage |
| US20090281299A1 (en) * | 2004-06-30 | 2009-11-12 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a non-phosphate backbone linkage |
| US20060025366A1 (en) * | 2004-07-02 | 2006-02-02 | Protiva Biotherapeutics, Inc. | Immunostimulatory siRNA molecules and uses therefor |
| US7807815B2 (en) | 2004-07-02 | 2010-10-05 | Protiva Biotherapeutics, Inc. | Compositions comprising immunostimulatory siRNA molecules and DLinDMA or DLenDMA |
| US10260066B2 (en) | 2004-07-09 | 2019-04-16 | University Of Massachusetts | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US11220686B2 (en) | 2004-07-09 | 2022-01-11 | University Of Massachusetts | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US8940709B2 (en) | 2004-07-09 | 2015-01-27 | University Of Massachusetts | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US8361976B2 (en) * | 2004-07-09 | 2013-01-29 | University Of Massachusetts | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US20060073127A1 (en) * | 2004-07-09 | 2006-04-06 | Umass Medical School | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US9150861B2 (en) | 2004-07-09 | 2015-10-06 | University Of Massachusetts | Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules |
| US8604185B2 (en) * | 2004-07-20 | 2013-12-10 | Genentech, Inc. | Inhibitors of angiopoietin-like 4 protein, combinations, and their use |
| US20070026002A1 (en) * | 2004-07-20 | 2007-02-01 | Genentech, Inc. | Inhibitors of angiopoietin-like 4 protein, combinations, and their use |
| US20110311524A1 (en) * | 2004-07-20 | 2011-12-22 | Genentech, Inc. | Inhibitors of Angiopoietin-Like 4 Protein, Combinations, and Their Use |
| US7579451B2 (en) | 2004-07-21 | 2009-08-25 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a modified or non-natural nucleobase |
| US7772387B2 (en) | 2004-07-21 | 2010-08-10 | Alnylam Pharmaceuticals | Oligonucleotides comprising a modified or non-natural nucleobase |
| US7893224B2 (en) | 2004-08-04 | 2011-02-22 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a ligand tethered to a modified or non-natural nucleobase |
| US7632932B2 (en) | 2004-08-04 | 2009-12-15 | Alnylam Pharmaceuticals, Inc. | Oligonucleotides comprising a ligand tethered to a modified or non-natural nucleobase |
| WO2006023491A2 (en) | 2004-08-16 | 2006-03-02 | The Cbr Institute For Biomedical Research, Inc. | Method of delivering rna interference and uses thereof |
| US20110313024A1 (en) * | 2004-08-20 | 2011-12-22 | Leonid Beigelman | RNA INTERFERENCE MEDIATED INHIBITION OF PROPROTEIN CONVERTASE SUBTILISIN KEXIN 9 (PCSK9) GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) |
| US20090005330A1 (en) * | 2004-08-31 | 2009-01-01 | Sylentis S.A. | Methods and Compositions to Inhibit P2x7 Receptor Expression |
| US20090264501A9 (en) * | 2004-08-31 | 2009-10-22 | Sylentis S.A. | Methods and Compositions to Inhibit P2x7 Receptor Expression |
| US7718624B2 (en) * | 2004-09-01 | 2010-05-18 | Sitkovsky Michail V | Modulation of immune response and inflammation by targeting hypoxia inducible factors |
| US20070249550A1 (en) * | 2004-09-01 | 2007-10-25 | Sitkovsky Michail V | Modulation of immune response and inflammation by targeting hypoxia inducible factors |
| US7884086B2 (en) * | 2004-09-08 | 2011-02-08 | Isis Pharmaceuticals, Inc. | Conjugates for use in hepatocyte free uptake assays |
| US20070149470A1 (en) * | 2004-09-10 | 2007-06-28 | Kaspar Roger L | Inhibition of viral gene expression using small interfering RNA |
| US20090170794A1 (en) * | 2004-09-10 | 2009-07-02 | Somagenics Inc. | Small interfering rnas that efficiently inhibit viral expression and methods of use thereof |
| US7902351B2 (en) * | 2004-09-10 | 2011-03-08 | Somagenics Inc. | Inhibition of viral gene expression using small interfering RNA |
| US8426380B2 (en) | 2004-09-10 | 2013-04-23 | Somagenics, Inc. | Inhibition of viral gene expression using small interfering RNA |
| US9187747B2 (en) | 2004-09-24 | 2015-11-17 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of ApoB and uses thereof |
| US20100216866A1 (en) * | 2004-09-24 | 2010-08-26 | Alnylam Pharmaceuticals, Inc. | RNAi Modulation of APOB and Uses Thereof |
| US8188061B2 (en) | 2004-09-24 | 2012-05-29 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of APOB and uses thereof |
| US8592571B2 (en) | 2004-09-24 | 2013-11-26 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of APOB and uses thereof |
| US20100113332A1 (en) * | 2004-09-27 | 2010-05-06 | Nastech Pharmaceutical Company Inc. | Method of treating an inflammatory disease by double stranded ribonucleic acid |
| US20080269148A1 (en) * | 2004-10-01 | 2008-10-30 | Jang Han | Modified Small Interfering Rna Molecules and Methods of Use |
| US9084808B2 (en) | 2004-10-01 | 2015-07-21 | Arrowhead Research Corporation | Modified small interfering RNA molecules and methods of use |
| US8138161B2 (en) | 2004-10-01 | 2012-03-20 | Novartis Vaccines And Diagnostics, Inc. | Modified small interfering RNA molecules and methods of use |
| US20070078085A1 (en) * | 2004-10-13 | 2007-04-05 | Chung Leland W | Methods and compositions for the utilization and targeting of osteomimicry |
| US20110021606A1 (en) * | 2004-10-22 | 2011-01-27 | South Alabama Medical Science Foundation | RNAi Modulation of RSV, PIV and Other Respiratory Viruses and Uses Thereof |
| US8598134B2 (en) | 2004-10-22 | 2013-12-03 | South Alabama Medical Science Foundation | RNAi modulation of RSV, PIV and other respiratory viruses and uses thereof |
| US7943755B2 (en) * | 2004-10-22 | 2011-05-17 | Neuregenix Limited | Neuron regeneration |
| US20080253989A1 (en) * | 2004-10-22 | 2008-10-16 | Neuregenix Limited | Neuron Regeneration |
| US9506061B2 (en) | 2004-11-12 | 2016-11-29 | Asuragen, Inc. | Methods and compositions involving miRNA and miRNA inhibitor molecules |
| US9447414B2 (en) | 2004-11-12 | 2016-09-20 | Asuragen, Inc. | Methods and compositions involving miRNA and miRNA inhibitor molecules |
| US8765709B2 (en) | 2004-11-12 | 2014-07-01 | Asuragen, Inc. | Methods and compositions involving miRNA and miRNA inhibitor molecules |
| US20060134189A1 (en) * | 2004-11-17 | 2006-06-22 | Protiva Biotherapeutics, Inc | siRNA silencing of apolipoprotein B |
| US20110189300A1 (en) * | 2004-11-17 | 2011-08-04 | Protiva Biotherapeutics, Inc. | siRNA SILENCING OF APOLIPOPROTEIN B |
| US7994307B2 (en) | 2004-11-24 | 2011-08-09 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of the BCR-ABL fusion gene and uses thereof |
| US20100234446A1 (en) * | 2004-11-24 | 2010-09-16 | Philipp Hadwiger | RNAi Modulation of the BCR-ABL Fusion Gene and Uses Thereof |
| US20090298910A1 (en) * | 2004-12-10 | 2009-12-03 | Griffey Richard H | Regulation of epigenetic control of gene expression |
| US9550990B2 (en) | 2004-12-10 | 2017-01-24 | Ionis Pharmaceuticals, Inc. | Regulation of epigenetic control of gene expression |
| US8034793B2 (en) | 2004-12-14 | 2011-10-11 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of MLL-AF4 and uses thereof |
| US20100256218A1 (en) * | 2004-12-14 | 2010-10-07 | Olaf Heidenreich | RNAi MODULATION OF MLL-AF4 AND USES THEREOF |
| US8263572B2 (en) | 2005-01-07 | 2012-09-11 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of RSV and therapeutic uses thereof |
| US20060166921A1 (en) * | 2005-01-07 | 2006-07-27 | Rachel Meyers | RNAi modulation of RSV and therapeutic uses thereof |
| US7981869B2 (en) | 2005-01-07 | 2011-07-19 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of RSV and therapeutic uses thereof |
| US8859750B2 (en) | 2005-01-07 | 2014-10-14 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of RSV and therapeutic uses thereof |
| US7507809B2 (en) * | 2005-01-07 | 2009-03-24 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of RSV and therapeutic uses thereof |
| US8158773B2 (en) | 2005-01-07 | 2012-04-17 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of RSV and therapeutic uses thereof |
| US20090233984A1 (en) * | 2005-01-07 | 2009-09-17 | Alnylam Pharmaceuticals, Inc. | RNAi Modulation of RSV and Therapeutic Uses Thereof |
| US20060217324A1 (en) * | 2005-01-24 | 2006-09-28 | Juergen Soutschek | RNAi modulation of the Nogo-L or Nogo-R gene and uses thereof |
| US8153603B2 (en) | 2005-02-25 | 2012-04-10 | Isis Pharmaceuticals, Inc. | Compositions and their uses directed to IL-4R alpha |
| US8859749B2 (en) * | 2005-03-08 | 2014-10-14 | Qiagen Gmbh | Modified short interfering RNA |
| US20090197332A1 (en) * | 2005-03-08 | 2009-08-06 | Ioanna Andreou | Modified Short Interfering RNA |
| US20110190381A1 (en) * | 2005-03-11 | 2011-08-04 | Alcon Inc. | Rnai-mediated inhibition of frizzled related protein-1 for treatment of glaucoma |
| US9040494B2 (en) | 2005-03-11 | 2015-05-26 | Novartis Ag | RNAi-mediated inhibition of frizzled related protein-1 for treatment of glaucoma |
| US7947660B2 (en) * | 2005-03-11 | 2011-05-24 | Alcon, Inc. | RNAi-mediated inhibition of frizzled related protein-1 for treatment of glaucoma |
| US8173617B2 (en) | 2005-03-11 | 2012-05-08 | Novartis Ag | RNAi-mediated inhibition of frizzled related protein-1 for treatment of glaucoma |
| US9550994B2 (en) | 2005-03-11 | 2017-01-24 | Arrowhead Pharmaceuticals, Inc. | RNAI-mediated inhibition of frizzled related protein-1 for treatment of glaucoma |
| US20060223773A1 (en) * | 2005-03-11 | 2006-10-05 | Alcon, Inc. | RNAi-mediated inhibition of Frizzled Related Protein-1 for treatment of glaucoma |
| US8999943B2 (en) | 2005-03-14 | 2015-04-07 | Board Of Regents, The University Of Texas System | Antigene oligomers inhibit transcription |
| US20060205635A1 (en) * | 2005-03-14 | 2006-09-14 | Board Of Regents, The University Of Texas System | Antigene oligomers inhibit transcription |
| US20100239228A1 (en) * | 2005-03-30 | 2010-09-23 | Sony Corporation | Information processing system, information processing method, and information processing program |
| US7964575B2 (en) * | 2005-04-12 | 2011-06-21 | Universite Libre De Bruxelles | Use of a galectin-1-targeted RNAi-based approach for the treatment of cancer |
| US20100120891A1 (en) * | 2005-04-12 | 2010-05-13 | Universite Libre De Bruxelles | Use of a galectin-1-targeted rnai-based approach for the treatment of cancer |
| US20060253068A1 (en) * | 2005-04-20 | 2006-11-09 | Van Bilsen Paul | Use of biocompatible in-situ matrices for delivery of therapeutic cells to the heart |
| US7902352B2 (en) | 2005-05-06 | 2011-03-08 | Medtronic, Inc. | Isolated nucleic acid duplex for reducing huntington gene expression |
| US8258112B2 (en) | 2005-05-06 | 2012-09-04 | Medtronic, Inc | Methods and sequences to suppress primate huntington gene Expression |
| US20100008981A1 (en) * | 2005-05-06 | 2010-01-14 | Medtronic, Inc. | Methods and sequences to suppress primate huntington gene expression |
| US20070261126A1 (en) * | 2005-05-06 | 2007-11-08 | Kaemmerer William F | Methods and sequences to suppress primate huntington gene expression in vivo |
| US20100325746A9 (en) * | 2005-05-06 | 2010-12-23 | Kaemmerer William F | Methods and sequences to suppress primate huntington gene expression in vivo |
| US9243248B2 (en) | 2005-06-01 | 2016-01-26 | Polyplus-Transfection Sa | Oligonucleotides for RNA interference and biological applications thereof |
| US8802640B2 (en) * | 2005-06-01 | 2014-08-12 | Polyplus-Transfection Sa | Oligonucleotides for RNA interference and biological applications thereof |
| US20080153772A1 (en) * | 2005-06-01 | 2008-06-26 | Jean-Paul Behr | Oligonucleotides For Rna Interference and Biological Applications Thereof |
| US20110038922A1 (en) * | 2005-06-16 | 2011-02-17 | Faron Pharmaceuticals Oy (A Finnish Company) | Compounds for treating or preventing amine oxidase related diseases or disorders |
| US20100292305A1 (en) * | 2005-06-27 | 2010-11-18 | Akin Akinc | RNAi MODULATION OF HIF-1 AND THERAPUTIC USES THEREOF |
| US9133517B2 (en) | 2005-06-28 | 2015-09-15 | Medtronics, Inc. | Methods and sequences to preferentially suppress expression of mutated huntingtin |
| US20090143321A1 (en) * | 2005-07-07 | 2009-06-04 | Avraham Hochberg | Nucleic acid agents for downregulating h19 and methods of using same |
| US8067574B2 (en) * | 2005-07-07 | 2011-11-29 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Nucleic acid agents for downregulating H19, and methods of using same |
| US20090203121A1 (en) * | 2005-07-07 | 2009-08-13 | Avraham Hochberg | Nucleic acid agents for downregulating h19, and methods of using same |
| US8067573B2 (en) * | 2005-07-07 | 2011-11-29 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Nucleic acid agents for downregulating H19 and methods of using same |
| US20070042984A1 (en) * | 2005-07-21 | 2007-02-22 | Juergen Soutschek | RNAi modulation of the Rho-A gene and uses thereof |
| US7772200B2 (en) * | 2005-07-21 | 2010-08-10 | Alnylam Pharmaceuticals, Inc. | iRNA agents targeted to the Rho-A gene |
| US20070044161A1 (en) * | 2005-07-21 | 2007-02-22 | Juergen Soutschek | RNAi modulation of the Rho-A gene in research models |
| US20090176725A1 (en) * | 2005-08-17 | 2009-07-09 | Sirna Therapeutics Inc. | Chemically modified short interfering nucleic acid molecules that mediate rna interference |
| US20090215863A1 (en) * | 2005-08-18 | 2009-08-27 | Rachel Bar-Shavit | Gene Silencing of Protease Activated Receptor 1(Par1) |
| US20070054873A1 (en) * | 2005-08-26 | 2007-03-08 | Protiva Biotherapeutics, Inc. | Glucocorticoid modulation of nucleic acid-mediated immune stimulation |
| US20070254362A1 (en) * | 2005-09-02 | 2007-11-01 | Nastech Pharmaceutical Company Inc. | COMPOSITIONS AND METHODS EMPLOYING UNIVERSAL-BINDING NUCLEOTIDES FOR TARGETING MULTIPLE GENE VARIANTS WITH A SINGLE siRNA DUPLEX |
| US20100056606A1 (en) * | 2005-10-03 | 2010-03-04 | Isis Pharmaceuticals, Inc. | Combination therapy using budesonide and antisense oligonucleotide targeted to IL4-receptor alpha |
| US20070099858A1 (en) * | 2005-10-03 | 2007-05-03 | Sirna Therapeutics, Inc. | RNA interference mediated of inhibition of influenza virus gene expression using short interfering nucleic acid (siNA) |
| US20100196450A1 (en) * | 2005-10-14 | 2010-08-05 | Donald Carlton D | Targeting pax2 for the induction of defbi-mediated tumor immunity and cancer therapy |
| US7964577B2 (en) * | 2005-10-14 | 2011-06-21 | Donald Carlton D | Targeting PAX2 for the induction of DEFB1-mediated tumor immunity and cancer therapy |
| US8735365B2 (en) | 2005-10-14 | 2014-05-27 | Phigenix, Inc. | Targeting PAX2 for the induction of DEFB1-mediated tumor immunity and cancer therapy |
| US8431546B2 (en) | 2005-10-14 | 2013-04-30 | Phigenix, Inc. | Targeting PAX2 for the induction of DEFB1-mediated tumor immunity and cancer therapy |
| US8318692B2 (en) | 2005-10-14 | 2012-11-27 | Donald Carlton D | Targeting PAX2 for the induction of DEFB1-mediated tumor immunity and cancer therapy |
| WO2007047692A3 (en) * | 2005-10-19 | 2007-08-02 | Medtronic Inc | Devices, systems and methods for improving memory and/or cognitive function through brain delivery of sirna |
| US20070135370A1 (en) * | 2005-10-20 | 2007-06-14 | Protiva Biotherapeutics, Inc. | siRNA silencing of filovirus gene expression |
| US20110177131A1 (en) * | 2005-10-20 | 2011-07-21 | Protiva Biotherapeutics, Inc. | siRNA SILENCING OF FILOVIRUS GENE EXPRESSION |
| US7838658B2 (en) * | 2005-10-20 | 2010-11-23 | Ian Maclachlan | siRNA silencing of filovirus gene expression |
| US8314075B2 (en) | 2005-10-28 | 2012-11-20 | Alynylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of huntingtin gene |
| US8080532B2 (en) | 2005-10-28 | 2011-12-20 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of Huntingtin gene |
| US20100298405A1 (en) * | 2005-10-28 | 2010-11-25 | Dinah Wen-Yee Sah | Compositions And Methods For Inhibiting Expression Of Huntingtin Gene |
| US9074208B2 (en) | 2005-11-02 | 2015-07-07 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US20070135372A1 (en) * | 2005-11-02 | 2007-06-14 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US8513403B2 (en) | 2005-11-02 | 2013-08-20 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US8101741B2 (en) * | 2005-11-02 | 2012-01-24 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US8188263B2 (en) | 2005-11-02 | 2012-05-29 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US20110124711A1 (en) * | 2005-11-04 | 2011-05-26 | Alnylam Pharmaceuticals, Inc. | COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF Nav1.8 GENE |
| US7902168B2 (en) | 2005-11-04 | 2011-03-08 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of Nav1.8 gene |
| US20090258934A1 (en) * | 2005-11-04 | 2009-10-15 | Alnylam Pharmaceuticals, Inc. | COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF Nav1.8 GENE |
| US20070105806A1 (en) * | 2005-11-04 | 2007-05-10 | Dinah Sah | Compositions and methods for inhibiting expression of Nav1.8 gene |
| US7582745B2 (en) * | 2005-11-04 | 2009-09-01 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of Nav1.8 gene |
| US10501740B2 (en) | 2005-11-09 | 2019-12-10 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor V |
| US20100069461A1 (en) * | 2005-11-09 | 2010-03-18 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor v leiden mutant gene |
| US20110130443A1 (en) * | 2005-11-09 | 2011-06-02 | Hans-Peter Vornlocher | Compositions And Methods For Inhibiting Expression Of Factor V Leiden Mutant Gene |
| US9441225B2 (en) | 2005-11-09 | 2016-09-13 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor V |
| US8658782B2 (en) * | 2005-11-09 | 2014-02-25 | Alynylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor V |
| US20100278797A1 (en) * | 2005-11-17 | 2010-11-04 | Tel HaShomer Medical Research Infrastracture and Services Ltd. | Pharmaceutical Composition and Method for Regulating Abnormal Cellular Proliferation |
| US7709456B2 (en) * | 2005-11-17 | 2010-05-04 | Board Of Regents, The University Of Texas System | Modulation of gene expression by oligomers targeted to chromosomal DNA |
| US8841266B2 (en) | 2005-11-17 | 2014-09-23 | Tel Hashomer Medical Research Infrastructure And Services Ltd. | Pharmaceutical composition and method for regulating abnormal cellular proliferation |
| US8324181B2 (en) | 2005-11-17 | 2012-12-04 | Board Of Regents, The University Of Texas System | Modulation of gene expression by oligomers targeted to chromosomal DNA |
| WO2007057897A3 (en) * | 2005-11-17 | 2009-02-12 | Tel Hashomer Medical Res Infrastructure & Services Ltd | Pharmaceutical composition and method for regulating abnormal cellular proliferation |
| US20070111963A1 (en) * | 2005-11-17 | 2007-05-17 | Board Of Regents, The University Of Texas System | Modulation of gene expression by oligomers targeted to chromosomal DNA |
| US20070218122A1 (en) * | 2005-11-18 | 2007-09-20 | Protiva Biotherapeutics, Inc. | siRNA silencing of influenza virus gene expression |
| US20120202215A1 (en) * | 2005-11-24 | 2012-08-09 | Jichi Medical University | Mitochondrial function of prohibitin 2 (phb2) |
| US20070270366A1 (en) * | 2005-12-20 | 2007-11-22 | Karras James G | Double stranded nucleic acid molecules targeted to il-4 receptor alpha |
| US20080125386A1 (en) * | 2006-01-26 | 2008-05-29 | Universtiy Of Massachusetts | RNA interference agents for therapeutic use |
| US7951784B2 (en) * | 2006-01-26 | 2011-05-31 | University Of Massachusetts | RNA interference agents for therapeutic use |
| US20070180242A1 (en) * | 2006-01-30 | 2007-08-02 | Nagaraj Thadi M | GSM authentication in a CDMA network |
| EP2229946A2 (en) | 2006-03-16 | 2010-09-22 | Jukka Westermarck | Use of the growth-stimulating protein KIAA1524 |
| US7956177B2 (en) * | 2006-03-24 | 2011-06-07 | Alnylam Pharmaceuticals, Inc. | dsRNA compositions and methods for treating HPV infection |
| US20090247607A1 (en) * | 2006-03-24 | 2009-10-01 | John Benson | dsRNA COMPOSITIONS AND METHODS FOR TREATING HPV INFECTION |
| US20110230546A1 (en) * | 2006-03-24 | 2011-09-22 | John Benson | dsRNA COMPOSITIONS AND METHODS FOR TREATING HPV INFECTION |
| US20070238691A1 (en) * | 2006-03-29 | 2007-10-11 | Senesco Technologies, Inc. | Inhibition of HIV replication and expression of p24 with eIF-5A |
| US9057069B2 (en) | 2006-03-31 | 2015-06-16 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of Eg5 gene |
| US20090226446A1 (en) * | 2006-04-06 | 2009-09-10 | Deutsches Krebsforschungszentrum Stiftung Des Offentilchen Rechts | Method to Inhibit the Propagation of an Undesired Cell Population |
| US8058257B2 (en) | 2006-04-28 | 2011-11-15 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the JC virus |
| US8410261B2 (en) | 2006-04-28 | 2013-04-02 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the JC virus |
| US9012624B2 (en) | 2006-04-28 | 2015-04-21 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the JC virus |
| US20100227915A1 (en) * | 2006-04-28 | 2010-09-09 | Pamela Tan | Compositions and methods for inhibiting expression of a gene from the jc virus |
| US8404831B2 (en) | 2006-05-04 | 2013-03-26 | Novartis Ag | Short interfering ribonucleic acid (siRNA) for oral administration |
| US20100015707A1 (en) * | 2006-05-04 | 2010-01-21 | Francois Jean-Charles Natt | SHORT INTERFERING RIBONUCLEIC ACID (siRNA) FOR ORAL ADMINISTRATION |
| US9493771B2 (en) | 2006-05-04 | 2016-11-15 | Novartis Ag | Short interfering ribonucleic acid (siRNA) for oral administration |
| US8404832B2 (en) | 2006-05-04 | 2013-03-26 | Novartis Ag | Short interfering ribonucleic acid (siRNA) for oral administration |
| US8957041B2 (en) | 2006-05-04 | 2015-02-17 | Novartis Ag | Short interfering ribonucleic acid (siRNA) for oral administration |
| US8344128B2 (en) | 2006-05-04 | 2013-01-01 | Novartis Ag | Short interfering ribonucleic acid (siRNA) for oral administration |
| US8084600B2 (en) * | 2006-05-04 | 2011-12-27 | Novartis Ag | Short interfering ribonucleic acid (siRNA) with improved pharmacological properties |
| US10501742B2 (en) | 2006-05-11 | 2019-12-10 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the PCSK9 gene |
| US8222222B2 (en) | 2006-05-11 | 2012-07-17 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the PCSK9 gene |
| US20110230542A1 (en) * | 2006-05-11 | 2011-09-22 | Pamela Tan | Compositions and Methods for Inhibiting Expression of the PCSK9 Gene |
| US9822365B2 (en) | 2006-05-11 | 2017-11-21 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the PCSK9 gene |
| US8809292B2 (en) | 2006-05-11 | 2014-08-19 | Alnylam Pharmaceuticals, Inc | Compositions and methods for inhibiting expression of the PCSK9 gene |
| US9260718B2 (en) | 2006-05-11 | 2016-02-16 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the PCSK9 gene |
| US20070269892A1 (en) * | 2006-05-18 | 2007-11-22 | Nastech Pharmaceutical Company Inc. | FORMULATIONS FOR INTRACELLULAR DELIVERY dsRNA |
| US20110003882A1 (en) * | 2006-05-19 | 2011-01-06 | Alnylam Pharmaceuticals, Inc. | RNAi Modulation of AHA and Therapeutic Uses Thereof |
| US8114984B2 (en) | 2006-05-19 | 2012-02-14 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of Aha and therapeutic uses thereof |
| US20110112178A1 (en) * | 2006-05-22 | 2011-05-12 | Alnylam Pharmaceuticals, Inc. | Compositions And Methods For Inhibiting Expression Of IKK-B Gene |
| US9000143B2 (en) | 2006-05-22 | 2015-04-07 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of IKK-B gene |
| US20080280843A1 (en) * | 2006-05-24 | 2008-11-13 | Van Bilsen Paul | Methods and kits for linking polymorphic sequences to expanded repeat mutations |
| US9273356B2 (en) | 2006-05-24 | 2016-03-01 | Medtronic, Inc. | Methods and kits for linking polymorphic sequences to expanded repeat mutations |
| US20070275923A1 (en) * | 2006-05-25 | 2007-11-29 | Nastech Pharmaceutical Company Inc. | CATIONIC PEPTIDES FOR siRNA INTRACELLULAR DELIVERY |
| US8598333B2 (en) | 2006-05-26 | 2013-12-03 | Alnylam Pharmaceuticals, Inc. | SiRNA silencing of genes expressed in cancer |
| US20090149403A1 (en) * | 2006-05-26 | 2009-06-11 | Protiva Biotherapeutics, Inc. | siRNA silencing of genes expressed in cancer |
| US20110318361A1 (en) * | 2006-05-30 | 2011-12-29 | Young Woo Park | Anticancer Drug Comprising Inhibitor of Tmprss4 |
| US8778901B2 (en) * | 2006-05-30 | 2014-07-15 | Korea Research Institute Of Bioscience And Biotechnology | Anticancer drug comprising inhibitor of TMPRSS4 |
| US7915399B2 (en) | 2006-06-09 | 2011-03-29 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| US20080249046A1 (en) * | 2006-06-09 | 2008-10-09 | Protiva Biotherapeutics, Inc. | MODIFIED siRNA MOLECULES AND USES THEREOF |
| US8124752B2 (en) | 2006-07-10 | 2012-02-28 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the MYC gene |
| US20100016405A1 (en) * | 2006-07-10 | 2010-01-21 | Alnylam Pharmaceuticals, Inc | Compositions and Methods for Inhibiting Expression of the MYC Gene |
| US20090093425A1 (en) * | 2006-07-12 | 2009-04-09 | The Regents Of The University Of California | Transducible delivery of nucleic acids by reversible phosphotriester charge neutralization protecting groups |
| US20080057062A1 (en) * | 2006-07-18 | 2008-03-06 | National Institute Of Advanced Industrial Science And Technology | Agent for Inducing senescence and apoptosis of cancer cell |
| US8293886B2 (en) | 2006-07-31 | 2012-10-23 | Universite Joseph Fourier | Sensizitation of cancer cells to therapy using sina targeting genes from the 1P and 19Q chromosomal regions |
| US20100076054A1 (en) * | 2006-07-31 | 2010-03-25 | Universite Joseph Fourier | Sensizitation of cancer cells to therapy using sina targeting genes from the 1p and 19q chromosomal regions |
| US7939653B2 (en) * | 2006-07-31 | 2011-05-10 | Universite Joseph Fourier | Sensizitation of cancer cells to therapy using siNA targeting genes from the 1p and 19q chromosomal regions |
| US20080039415A1 (en) * | 2006-08-11 | 2008-02-14 | Gregory Robert Stewart | Retrograde transport of sirna and therapeutic uses to treat neurologic disorders |
| EP2272982A1 (en) | 2006-08-23 | 2011-01-12 | Valtion Teknillinen Tutkimuskeskus | Method for treatment of prostate cancer and diagnosing of patients benefiting from the same |
| US20100093830A1 (en) * | 2006-08-30 | 2010-04-15 | Dolly Mehta | Modulation of MLCK-L Expression and Uses Thereof |
| US7825101B2 (en) * | 2006-08-30 | 2010-11-02 | The Board Of Trustees Of The University Of Illinois | Modulation of MLCK-L expression and uses thereof |
| US20110092568A1 (en) * | 2006-08-30 | 2011-04-21 | The Board Of Trustees Of The University Of Illinois | Modulation of mlck-l expression and uses thereof |
| US9102940B2 (en) | 2006-09-18 | 2015-08-11 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of SCAP and therapeutic uses thereof |
| US20110184047A1 (en) * | 2006-09-18 | 2011-07-28 | Juergen Soutschek | Rnai modulation of scap and therapeutic uses thereof |
| WO2008036638A3 (en) * | 2006-09-18 | 2009-04-16 | Alnylam Pharmaceuticals Inc | Rnai modulation of scap and therapeutic uses thereof |
| US20100184829A1 (en) * | 2006-09-18 | 2010-07-22 | Juergen Soutschek | Rnai modulation of scap and therapeutic uses thereof |
| US7919613B2 (en) | 2006-09-18 | 2011-04-05 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of SCAP and therapeutic uses thereof |
| US7737266B2 (en) | 2006-09-18 | 2010-06-15 | Board Of Regents, The University Of Texas System | RNAi modulation of SCAP and therapeutics uses thereof |
| US8383805B2 (en) | 2006-09-18 | 2013-02-26 | Alnylam Pharmaceuticals, Inc. | RNAi modulation of SCAP and therapeutic uses thereof |
| WO2008036933A3 (en) * | 2006-09-21 | 2008-10-23 | Alnylam Pharmaceuticals Inc | Compositions and methods for inhibiting expression of the hamp gene |
| US8163711B2 (en) | 2006-09-21 | 2012-04-24 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the HAMP gene |
| US8268799B2 (en) | 2006-09-21 | 2012-09-18 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the HAMP gene |
| US8791250B2 (en) | 2006-09-21 | 2014-07-29 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the HAMP gene |
| US20090209478A1 (en) * | 2006-09-21 | 2009-08-20 | Tomoko Nakayama | Compositions and methods for inhibiting expression of the hamp gene |
| US20100204307A1 (en) * | 2006-09-21 | 2010-08-12 | Tomoko Nakayama | Compositions And Methods For Inhibiting Expression Of The HAMP Gene |
| US9506067B2 (en) | 2006-09-21 | 2016-11-29 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the HAMP gene |
| US9090895B2 (en) | 2006-09-21 | 2015-07-28 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the HAMP gene |
| US8470799B2 (en) | 2006-09-21 | 2013-06-25 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of the HAMP gene |
| US8324367B2 (en) | 2006-11-03 | 2012-12-04 | Medtronic, Inc. | Compositions and methods for making therapies delivered by viral vectors reversible for safety and allele-specificity |
| US20080124379A1 (en) * | 2006-11-03 | 2008-05-29 | Kaemmerer William F | Compositions and methods for making therapies delivered by viral vectors reversible for safety and allele-specificity |
| US9375440B2 (en) | 2006-11-03 | 2016-06-28 | Medtronic, Inc. | Compositions and methods for making therapies delivered by viral vectors reversible for safety and allele-specificity |
| US8034921B2 (en) | 2006-11-21 | 2011-10-11 | Alnylam Pharmaceuticals, Inc. | IRNA agents targeting CCR5 expressing cells and uses thereof |
| US7988668B2 (en) | 2006-11-21 | 2011-08-02 | Medtronic, Inc. | Microsyringe for pre-packaged delivery of pharmaceuticals |
| US7819842B2 (en) | 2006-11-21 | 2010-10-26 | Medtronic, Inc. | Chronically implantable guide tube for repeated intermittent delivery of materials or fluids to targeted tissue sites |
| US20080119787A1 (en) * | 2006-11-21 | 2008-05-22 | Kaemmerer William F | Microsyringe for pre-packaged delivery of pharmaceuticals |
| US20080255345A1 (en) * | 2006-11-21 | 2008-10-16 | Alnylam Pharmaceuticals, Inc. | IRNA Agents Targeting CCR5 Expressing Cells And Uses Thereof |
| US20080214486A1 (en) * | 2006-11-28 | 2008-09-04 | Alcon Manufacturing, Ltd. | RNAi-MEDIATED INHIBITION OF AQUAPORIN 4 FOR TREATMENT OF IOP-RELATED CONDITIONS |
| US20080171719A1 (en) * | 2006-11-28 | 2008-07-17 | Alcon Manufacturing, Ltd. | RNAi-MEDIATED INHIBITION OF AQUAPORIN 1 FOR TREATMENT OF IOP-RELATED CONDITIONS |
| US20100086526A1 (en) * | 2007-01-16 | 2010-04-08 | Abraham Hochberg | Nucleic acid constructs and methods for specific silencing of h19 |
| US20080171906A1 (en) * | 2007-01-16 | 2008-07-17 | Everaerts Frank J L | Tissue performance via hydrolysis and cross-linking |
| US20100105759A1 (en) * | 2007-01-16 | 2010-04-29 | Abraham Hochberg | H19 silencing nucleic acid agents for treating rheumatoid arthritis |
| US9260715B2 (en) | 2007-01-16 | 2016-02-16 | The University Of Queensland | Method of inducing an immune response |
| US7928083B2 (en) | 2007-01-16 | 2011-04-19 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | H19 silencing nucleic acid agents for treating rheumatoid arthritis |
| US20090054365A1 (en) * | 2007-01-26 | 2009-02-26 | Alcon Research, Ltd. | RNAi-MEDIATED INHIBITION OF AQUAPORIN 1 FOR TREATMENT OF OCULAR NEOVASCULARIZATION |
| US20100196403A1 (en) * | 2007-01-29 | 2010-08-05 | Jacob Hochman | Antibody conjugates for circumventing multi-drug resistance |
| US20100093836A1 (en) * | 2007-01-29 | 2010-04-15 | Isis Pharmaceuticals, Inc | Compounds and methods for modulating protein expression |
| US20100183696A1 (en) * | 2007-01-30 | 2010-07-22 | Allergan, Inc | Treating Ocular Diseases Using Peroxisome Proliferator-Activated Receptor Delta Antagonists |
| US20120136041A1 (en) * | 2007-01-30 | 2012-05-31 | Allergan, Inc. | Treating Ocular Diseases Using Peroxisome Proliferator-Activated Receptor Delta Antagonists |
| US8729042B2 (en) * | 2007-01-30 | 2014-05-20 | Allergan, Inc. | Treating ocular diseases using peroxisome proliferator—activated receptor delta antagonists |
| US20100249052A1 (en) * | 2007-03-26 | 2010-09-30 | Alnylam Pharmaceuticals, Inc. | Dsrna compositions and methods for treating hpv infections |
| US20110201671A1 (en) * | 2007-03-29 | 2011-08-18 | Alnylam Pharmaceuticals, Inc. | Compositions And Methods For Inhibiting Expression Of A Gene From The Ebola Virus |
| US20110060031A1 (en) * | 2007-03-29 | 2011-03-10 | Alnylam Pharmaceuticals, Inc. | Compositions And Methods For Inhibiting Expression Of A Gene From The Ebola Virus |
| US8735369B2 (en) | 2007-03-29 | 2014-05-27 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the Ebola virus |
| US9187516B2 (en) | 2007-03-29 | 2015-11-17 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the Ebola virus |
| US8354390B2 (en) | 2007-03-29 | 2013-01-15 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the ebola virus |
| US7973020B2 (en) | 2007-03-29 | 2011-07-05 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of a gene from the ebola virus |
| US9078823B2 (en) | 2007-03-30 | 2015-07-14 | Rutgers, The State University Of New Jersey | Compositions and methods for gene silencing |
| US9441221B2 (en) | 2007-03-30 | 2016-09-13 | Rutgers, The State University Of New Jersey | Compositions and methods for gene silencing |
| WO2008121963A3 (en) * | 2007-03-30 | 2008-11-20 | Univ Rutgers | Compositions and methods for gene silencing |
| US8343941B2 (en) | 2007-03-30 | 2013-01-01 | Rutgers, The State University Of New Jersey | Compositions and methods for gene silencing |
| US20100099739A1 (en) * | 2007-03-30 | 2010-04-22 | Samuel Ian Gunderson | Compositions and Methods for Gene Silencing |
| US20110217365A1 (en) * | 2007-03-30 | 2011-09-08 | Samuel Ian Gunderson | Compositions and Methods for Gene Silencing |
| US8907075B2 (en) | 2007-03-30 | 2014-12-09 | Samuel Ian Gunderson | Compositions and methods for gene silencing |
| US20090029466A1 (en) * | 2007-05-01 | 2009-01-29 | City Of Hope | Methods and compositions for the specific inhibition of gene expression by double-stranded rna |
| US20080318894A1 (en) * | 2007-05-11 | 2008-12-25 | Santaris Pharma A/S | Rna antagonist compounds for the modulation of her3 |
| US8268793B2 (en) * | 2007-05-11 | 2012-09-18 | Santaris Pharma A/S | RNA antagonist compounds for the modulation of HER3 |
| US9051570B2 (en) | 2007-05-22 | 2015-06-09 | Arcturus Therapeutics, Inc. | UNA oligomers for therapeutics |
| US10457945B2 (en) | 2007-05-22 | 2019-10-29 | Arcturus Therapeutics, Inc. | UNA oligomers for therapeutics with prolonged stability |
| US9297009B2 (en) | 2007-05-22 | 2016-03-29 | Arcturus Therapeutics, Inc. | UNA oligomers targeting micro-RNA for therapeutics |
| US9944929B2 (en) | 2007-05-22 | 2018-04-17 | Arcturus Therapeutics, Inc. | UNA single stranded oligomers for therapeutics |
| US9303260B2 (en) | 2007-05-22 | 2016-04-05 | Arcturus Therapeutics, Inc. | UNA duplex oligomers for therapeutics |
| US10689650B2 (en) | 2007-06-29 | 2020-06-23 | Stelic Institute & Co. | Method of fixing and expressing physiologically active substance |
| US20140135379A1 (en) * | 2007-06-29 | 2014-05-15 | Niigata University | Method of fixing and expressing physiologically active substance |
| US11485974B2 (en) | 2007-06-29 | 2022-11-01 | Stelic Institute & Co. | Method of fixing and expressing physiologically active substance |
| US9018180B2 (en) * | 2007-07-10 | 2015-04-28 | Neurim Pharmaceuticals (1991) Ltd. | CD44 splice variants in neurodegenerative diseases |
| US9416166B2 (en) | 2007-07-10 | 2016-08-16 | Neurim Pharmaceuticals (1991) Ltd. | CD44 splice variants in neurodegenerative diseases |
| US20110172286A1 (en) * | 2007-07-10 | 2011-07-14 | Neurim Pharmaceuticals (1991) Ltd. | Cd44 splice variants in neurodegenerative diseases |
| US20110082185A1 (en) * | 2007-09-17 | 2011-04-07 | Ludwig Institute For Cancer Research Ltd. | Cancer-testis gene silencing agents and uses thereof |
| US20100136026A1 (en) * | 2007-09-26 | 2010-06-03 | Kerr William G | Ship Inhibition to Direct Hematopoietic Stem Cells and Induce Extramedullary Hematopoiesis |
| WO2009040319A1 (en) | 2007-09-28 | 2009-04-02 | Imba - Institut Für Molekulare Biotechnologie Gmbh | Methods for modulating the proliferation and differentiation potential of stem cells and progenitor cells |
| US20100317563A1 (en) * | 2007-09-28 | 2010-12-16 | Imba-Institut Fur Molekulare Biotechnologie Gmbh | Methods for modulating the proliferation and differentiation potential of stem cells and progenitor cells |
| US8431398B2 (en) | 2007-09-28 | 2013-04-30 | Imba-Institut Fur Molekulare Biotechnologie Gmbh | Methods for modulating the proliferation and differentiation potential of stem cells and progenitor cells |
| US20090092988A1 (en) * | 2007-10-04 | 2009-04-09 | Schwartz Jacob C | Modulating Gene Expression with agRNA and Gapmers Targeting Antisense Transcripts |
| US8318496B2 (en) * | 2007-10-04 | 2012-11-27 | Isis Pharmaceuticals, Inc. | Compounds and methods for improving cellular uptake of oligomeric compounds |
| US20100292304A1 (en) * | 2007-10-04 | 2010-11-18 | Isis Pharmaceuticals, Inc. | Compounds and methods for improving cellular uptake of oligomeric compounds |
| US9605259B2 (en) | 2007-11-13 | 2017-03-28 | Ionis Pharmaceuticals, Inc. | Compounds and methods for modulating protein expression |
| US20100098664A1 (en) * | 2007-11-28 | 2010-04-22 | Mathieu Jean-Francois Desclaux | Lentiviral vectors allowing RNAi mediated inhibition of GFAP and vimentin expression |
| US8664193B2 (en) | 2007-12-10 | 2014-03-04 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor VII gene |
| US8334273B2 (en) | 2007-12-10 | 2012-12-18 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor VII gene |
| US9062310B2 (en) | 2007-12-10 | 2015-06-23 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of factor VII gene |
| US9127277B2 (en) | 2007-12-13 | 2015-09-08 | Alnylam Pharmaceuticals, Inc. | Methods and compositions for prevention or treatment of RSV infection |
| US20090238772A1 (en) * | 2007-12-13 | 2009-09-24 | Alnylam Pharmaceuticals, Inc. | Methods and compositions for prevention or treatment of rsv infection |
| US8410073B2 (en) | 2007-12-13 | 2013-04-02 | Alnylam Pharmaceuticals, Inc. | Methods and compositions for prevention or treatment of RSV infection |
| US20100204306A1 (en) * | 2007-12-14 | 2010-08-12 | Alnylam Pharmaceuticals, Inc. | Method of Treating Neurodegenerative Disease |
| US20090291131A1 (en) * | 2007-12-27 | 2009-11-26 | Protiva Biotherapeutics, Inc. | Silencing of polo-like kinase expression using interfering rna |
| US9006191B2 (en) | 2007-12-27 | 2015-04-14 | Protiva Biotherapeutics, Inc. | Silencing of polo-like kinase expression using interfering RNA |
| US20100010066A1 (en) * | 2008-01-31 | 2010-01-14 | Kevin Fitzgerald | Optimized Methods For Delivery Of DSRNA Targeting The PCSK9 Gene |
| US20110039914A1 (en) * | 2008-02-11 | 2011-02-17 | Rxi Pharmaceuticals Corporation | Modified rnai polynucleotides and uses thereof |
| US10131904B2 (en) * | 2008-02-11 | 2018-11-20 | Rxi Pharmaceuticals Corporation | Modified RNAi polynucleotides and uses thereof |
| CN104975020A (en) * | 2008-02-11 | 2015-10-14 | 阿克赛医药公司 | Modified Rnai Polynucleotides And Uses Thereof |
| US10633654B2 (en) | 2008-02-11 | 2020-04-28 | Phio Pharmaceuticals Corp. | Modified RNAi polynucleotides and uses thereof |
| JP2011511636A (en) * | 2008-02-11 | 2011-04-14 | アールエックスアイ ファーマシューティカルズ コーポレーション | Modified RNAi polynucleotides and uses thereof |
| US8288525B2 (en) | 2008-02-12 | 2012-10-16 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of CD45 gene |
| US8912316B2 (en) | 2008-02-12 | 2014-12-16 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of CD45 gene |
| US20110034537A1 (en) * | 2008-02-12 | 2011-02-10 | De Fougerolles Antonin | Compositions and methods for inhibiting expression of cd45 gene |
| US20110055965A1 (en) * | 2008-02-15 | 2011-03-03 | Hiroshi Abe | Cycle single-stranded nucleic acid complex and method for producing the same |
| US8765704B1 (en) | 2008-02-28 | 2014-07-01 | Novartis Ag | Modified small interfering RNA molecules and methods of use |
| US20100087508A1 (en) * | 2008-03-05 | 2010-04-08 | David Bumcrot | Compositions and Methods for Inhibiting Expression of Eg5 and VEGF Genes |
| US9006197B2 (en) | 2008-03-05 | 2015-04-14 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of Eg5 and VEGF genes |
| US20090234102A1 (en) * | 2008-03-14 | 2009-09-17 | Juridical Foundation The Chemo-Sero-Therapeutic Research Institute | Hepatitis c virus inhibitors |
| US8466273B2 (en) * | 2008-03-14 | 2013-06-18 | Juridical Foundation The Chemo-Sero-Therapeutic Research Institute | Hepatitis C virus inhibitors |
| US8148344B2 (en) | 2008-03-27 | 2012-04-03 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for mediating RNAi in vivo |
| US20100003317A1 (en) * | 2008-03-27 | 2010-01-07 | Akin Akinc | Compositions and methods for mediating rnai in vivo |
| US8420616B2 (en) * | 2008-04-07 | 2013-04-16 | University Of Cincinnati | MAT II beta subunit RNAi and therapeutic methods using same |
| US20110129461A1 (en) * | 2008-04-07 | 2011-06-02 | University Of Cincinnati | Mat II Beta Subunit RNAi and Therapeutic Methods Using Same |
| US8058069B2 (en) | 2008-04-15 | 2011-11-15 | Protiva Biotherapeutics, Inc. | Lipid formulations for nucleic acid delivery |
| US11141378B2 (en) | 2008-04-15 | 2021-10-12 | Arbutus Biopharma Corporation | Lipid formulations for nucleic acid delivery |
| US8822668B2 (en) | 2008-04-15 | 2014-09-02 | Protiva Biotherapeutics, Inc. | Lipid formulations for nucleic acid delivery |
| US20100130588A1 (en) * | 2008-04-15 | 2010-05-27 | Protiva Biotherapeutics, Inc. | Novel lipid formulations for nucleic acid delivery |
| US8492359B2 (en) | 2008-04-15 | 2013-07-23 | Protiva Biotherapeutics, Inc. | Lipid formulations for nucleic acid delivery |
| US9364435B2 (en) | 2008-04-15 | 2016-06-14 | Protiva Biotherapeutics, Inc. | Lipid formulations for nucleic acid delivery |
| US20090275638A1 (en) * | 2008-04-17 | 2009-11-05 | Kevin Fitzgerald | Compositions and Methods for Inhibiting Expression of XBP-1 Gene |
| US8344126B2 (en) | 2008-04-17 | 2013-01-01 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of XBP-1 gene |
| US20110152350A1 (en) * | 2008-04-17 | 2011-06-23 | Kevin Fitzgerald | Compositions and Methods for Inhibiting Expression of XBP-1 Gene |
| US8765932B2 (en) | 2008-04-17 | 2014-07-01 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of XBP-1 gene |
| US7875711B2 (en) | 2008-04-17 | 2011-01-25 | Alnylam Pharamaceuticals, Inc. | Compositions and methods for inhibiting expression of XBP-1 gene |
| US8324366B2 (en) | 2008-04-29 | 2012-12-04 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for delivering RNAI using lipoproteins |
| US20100009451A1 (en) * | 2008-05-30 | 2010-01-14 | Sigma Aldrich Company | Compositions and methods for specifically silencing a target nucleic acid |
| US8222221B2 (en) | 2008-06-04 | 2012-07-17 | The Board Of Regents Of The University Of Texas System | Modulation of gene expression through endogenous small RNA targeting of gene promoters |
| US20110077286A1 (en) * | 2008-06-05 | 2011-03-31 | Damha Masad J | Oligonucleotide duplexes comprising dna-like and rna-like nucleotides and uses thereof |
| US9090649B2 (en) * | 2008-06-05 | 2015-07-28 | Paladin Labs, Inc. | Oligonucleotide duplexes comprising DNA-like and RNA-like nucleotides and uses thereof |
| US9719091B2 (en) | 2008-06-05 | 2017-08-01 | Paladin Labs, Inc. | Oligonucleotide duplexes comprising DNA-like and RNA-like nucleotides and uses thereof |
| US9029525B2 (en) | 2008-07-11 | 2015-05-12 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of GSK-3 genes |
| US20110184046A1 (en) * | 2008-07-11 | 2011-07-28 | Dinah Wen-Yee Sah | Compositions And Methods For Inhibiting Expression Of GSK-3 Genes |
| US20110223665A1 (en) * | 2008-07-25 | 2011-09-15 | Alnylam Pharmaceuticals, Inc. | ENHANCEMENT OF siRNA SILENCING ACTIVITY USING UNIVERSAL BASES OR MISMATCHES IN THE SENSE STRAND |
| US20110142848A1 (en) * | 2008-08-07 | 2011-06-16 | Chung Leland W K | Anti-beta-2-microglobulin agents and the use thereof |
| US10022454B2 (en) | 2008-09-23 | 2018-07-17 | Liposciences, Llc | Functionalized phosphorodiamites for therapeutic oligonucleotide synthesis |
| US8691971B2 (en) | 2008-09-23 | 2014-04-08 | Scott G. Petersen | Self delivering bio-labile phosphate protected pro-oligos for oligonucleotide based therapeutics and mediating RNA interference |
| US11884919B2 (en) | 2008-09-25 | 2024-01-30 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of serum amyloid a gene |
| US9868950B2 (en) | 2008-09-25 | 2018-01-16 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of serum amyloid A gene |
| US9206421B2 (en) | 2008-09-25 | 2015-12-08 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of serum amyloid A gene |
| US10472628B2 (en) | 2008-09-25 | 2019-11-12 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of Serum Amyloid A gene |
| US8546554B2 (en) | 2008-09-25 | 2013-10-01 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of Serum Amyloid A gene |
| US11149273B2 (en) | 2008-09-25 | 2021-10-19 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of serum amyloid A gene |
| US9200282B2 (en) | 2008-10-06 | 2015-12-01 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of an RNA from west nile virus |
| US8592570B2 (en) | 2008-10-06 | 2013-11-26 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of an RNA from West Nile virus |
| US9139554B2 (en) | 2008-10-09 | 2015-09-22 | Tekmira Pharmaceuticals Corporation | Amino lipids and methods for the delivery of nucleic acids |
| US10653780B2 (en) | 2008-10-09 | 2020-05-19 | The University Of British Columbia | Amino lipids and methods for the delivery of nucleic acids |
| US20100112686A1 (en) * | 2008-10-15 | 2010-05-06 | Qing Ge | Short hairpin rnas for inhibition of gene expression |
| US8779115B2 (en) | 2008-10-15 | 2014-07-15 | Somagenics Inc. | Short hairpin RNAs for inhibition of gene expression |
| US8283460B2 (en) | 2008-10-15 | 2012-10-09 | Somagenics, Inc. | Short hairpin RNAs for inhibition of gene expression |
| US12227744B2 (en) | 2008-10-20 | 2025-02-18 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of transthyretin |
| US8741866B2 (en) | 2008-10-20 | 2014-06-03 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of transthyretin |
| US20100120893A1 (en) * | 2008-10-20 | 2010-05-13 | Dinah Wen-Yee Sah | Compositions and Methods for Inhibiting Expression of Transthyretin |
| US10240152B2 (en) | 2008-10-20 | 2019-03-26 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of transthyretin |
| US8168775B2 (en) | 2008-10-20 | 2012-05-01 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of transthyretin |
| US9234196B2 (en) | 2008-10-20 | 2016-01-12 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of transthyretin |
| US20100168205A1 (en) * | 2008-10-23 | 2010-07-01 | Alnylam Pharmaceuticals, Inc. | Methods and Compositions for Prevention or Treatment of RSV Infection Using Modified Duplex RNA Molecules |
| US9340789B2 (en) * | 2008-12-03 | 2016-05-17 | Arcturus Therapeutics, Inc. | UNA oligomer structures for therapeutic agents |
| JP2012510297A (en) * | 2008-12-03 | 2012-05-10 | マリーナ バイオテック,インコーポレイテッド | UsiRNA complex |
| US20110313020A1 (en) * | 2008-12-03 | 2011-12-22 | Marina Biotech, Inc. | UsiRNA Complexes |
| US9963700B2 (en) | 2008-12-10 | 2018-05-08 | Alnylam Pharmaceuticals, Inc. | GNAQ targeted dsRNA compositions and methods for inhibiting expression |
| US8889644B2 (en) | 2008-12-10 | 2014-11-18 | Alnylam Pharmaceuticals, Inc. | GNAQ targeted dsRNA compositions and methods for inhibiting expression |
| US10954516B2 (en) | 2008-12-10 | 2021-03-23 | Alnylam Pharmaceuticals, Inc. | GNAQ targeted dsRNA compositions and methods for inhibiting expression |
| US12031133B2 (en) | 2008-12-10 | 2024-07-09 | Alnylam Pharmaceuticals, Inc. | GNAQ targeted dsRNA compositions and methods for inhibiting expression |
| US20100168206A1 (en) * | 2008-12-10 | 2010-07-01 | Jared Gollob | GNAQ Targeted dsRNA Compositions And Methods For Inhibiting Expression |
| US8324368B2 (en) | 2008-12-10 | 2012-12-04 | Alnylam Pharmaceuticals, Inc. | GNAQ targeted dsRNA compositions and methods for inhibiting expression |
| US9566295B2 (en) | 2008-12-10 | 2017-02-14 | Alnylam Pharmaceuticals, Inc. | GNAQ targeted dsRNA compositions and methods for inhibiting expression |
| US20110245325A1 (en) * | 2008-12-12 | 2011-10-06 | Kureha Corporation | Pharmaceutical composition for treatment of cancer and asthma |
| US8389711B2 (en) * | 2008-12-12 | 2013-03-05 | Kureha Corporation | Pharmaceutical composition for treatment of cancer and asthma |
| US11597929B2 (en) | 2008-12-18 | 2023-03-07 | Dicerna Pharmaceuticals, Inc. | Extended dicer substrate agents and methods for the specific inhibition of gene expression |
| US20100173974A1 (en) * | 2008-12-18 | 2010-07-08 | Dicerna Pharmaceuticals, Inc. | Extended dicer substrate agents and methods for the specific inhibition of gene expression |
| US8513207B2 (en) * | 2008-12-18 | 2013-08-20 | Dicerna Pharmaceuticals, Inc. | Extended dicer substrate agents and methods for the specific inhibition of gene expression |
| US10131912B2 (en) | 2008-12-18 | 2018-11-20 | Dicerna Pharmaceuticals, Inc. | Extended dicer substrate agents and methods for the specific inhibition of gene expression |
| US9428751B2 (en) | 2009-01-26 | 2016-08-30 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing apolipoprotein C-III expression |
| US9023820B2 (en) | 2009-01-26 | 2015-05-05 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing apolipoprotein C-III expression |
| US20100197773A1 (en) * | 2009-02-03 | 2010-08-05 | Birgit Bramlage | Compositions and methods for inhibiting expression of ptp1b genes |
| US20120016011A1 (en) * | 2009-03-19 | 2012-01-19 | Merck Sharp & Dohme Corp. | RNA Interference Mediated Inhibition of Connective Tissue Growth Factor (CTGF) Gene Expression Using Short Interfering Nucleic Acid (siNA) |
| EP3330281A1 (en) | 2009-04-22 | 2018-06-06 | Faron Pharmaceuticals OY | Clever-1 levels as marker for increased anti-tumor response |
| WO2010122217A1 (en) | 2009-04-22 | 2010-10-28 | Faron Pharmaceuticals Oy | A novel cell and therapeutical and diagnostical methods based thereon |
| US8815586B2 (en) | 2009-04-24 | 2014-08-26 | The Board Of Regents Of The University Of Texas System | Modulation of gene expression using oligomers that target gene regions downstream of 3′ untranslated regions |
| US20100273863A1 (en) * | 2009-04-24 | 2010-10-28 | Board Of Regents, The University Of Texas System | Modulation of Gene Expression Using Oligomers That Target Gene Regions Downstream of 3' Untranslated Regions |
| US8580493B2 (en) | 2009-06-08 | 2013-11-12 | Vib Vzw | Screening for compounds that modulate GPR3-mediated beta-arrestin signaling and amyloid beta peptide generation |
| US9051567B2 (en) | 2009-06-15 | 2015-06-09 | Tekmira Pharmaceuticals Corporation | Methods for increasing efficacy of lipid formulated siRNA |
| US8598139B2 (en) | 2009-06-15 | 2013-12-03 | Alnylam Pharmaceuticals, Inc. | Lipid formulated dsRNA targeting the PCSK9 gene |
| US20110015252A1 (en) * | 2009-06-15 | 2011-01-20 | Kevin Fitzgerald | Lipid formulated dsrna targeting the pcsk9 gene |
| US8273869B2 (en) | 2009-06-15 | 2012-09-25 | Alnylam Pharmaceuticals, Inc. | Lipid formulated dsRNA targeting the PCSK9 gene |
| US10053689B2 (en) | 2009-06-15 | 2018-08-21 | Arbutus Biopharma Corporation | Methods for increasing efficacy of lipid formulated siRNA |
| US20120142758A1 (en) * | 2009-06-26 | 2012-06-07 | OPKO CuRNA,, LLC | Treatment of down syndrome gene related diseases by inhibition of natural antisense transcript to a down syndrome gene |
| US8921330B2 (en) * | 2009-06-26 | 2014-12-30 | Curna, Inc. | Treatment of down syndrome gene related diseases by inhibition of natural antisense transcript to a down syndrome gene |
| US9018187B2 (en) | 2009-07-01 | 2015-04-28 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods for the delivery of therapeutic agents |
| US11786598B2 (en) | 2009-07-01 | 2023-10-17 | Arbutus Biopharma Corporation | Lipid formulations for delivery of therapeutic agents |
| US12016929B2 (en) | 2009-07-01 | 2024-06-25 | Arbutus Biopharma Corporation | Lipid formulations for delivery of therapeutic agents |
| US9878042B2 (en) | 2009-07-01 | 2018-01-30 | Protiva Biotherapeutics, Inc. | Lipid formulations for delivery of therapeutic agents to solid tumors |
| US8569256B2 (en) | 2009-07-01 | 2013-10-29 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods for the delivery of therapeutic agents |
| US8283333B2 (en) | 2009-07-01 | 2012-10-09 | Protiva Biotherapeutics, Inc. | Lipid formulations for nucleic acid delivery |
| US11446383B2 (en) | 2009-07-01 | 2022-09-20 | Arbutus Biopharma Corporation | Lipid formulations for delivery of therapeutic agents |
| US8927513B2 (en) * | 2009-07-07 | 2015-01-06 | Alnylam Pharmaceuticals, Inc. | 5′ phosphate mimics |
| US20120157511A1 (en) * | 2009-07-07 | 2012-06-21 | Alnylam Pharmaceuticals, Inc. | 5' phosphate mimics |
| US10131908B2 (en) | 2009-07-07 | 2018-11-20 | Alnylam Pharmaceuticals, Inc. | 5′ phosphate mimics |
| US20150152414A1 (en) * | 2009-07-13 | 2015-06-04 | Somagenics, Inc. | Chemical modification of short small hairpin rnas for inhibition of gene expression |
| US10870850B2 (en) | 2009-07-13 | 2020-12-22 | Somagenics, Inc. | Chemical modification of short small hairpin RNAs for inhibition of gene expression |
| US9816091B2 (en) * | 2009-07-13 | 2017-11-14 | Somagenics, Inc. | Chemical modification of short small hairpin RNAs for inhibition of gene expression |
| US8871730B2 (en) * | 2009-07-13 | 2014-10-28 | Somagenics Inc. | Chemical modification of short small hairpin RNAs for inhibition of gene expression |
| US20120165397A1 (en) * | 2009-07-13 | 2012-06-28 | Qing Ge | Chemical modification of short small hairpin rnas for inhibition of gene expression |
| US8716464B2 (en) | 2009-07-20 | 2014-05-06 | Thomas W. Geisbert | Compositions and methods for silencing Ebola virus gene expression |
| US20110201667A1 (en) * | 2009-07-20 | 2011-08-18 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing ebola virus gene expression |
| US9187748B2 (en) | 2009-07-20 | 2015-11-17 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing ebola virus gene expression |
| US9029338B2 (en) | 2009-08-14 | 2015-05-12 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of a gene from the ebola virus |
| US8859516B2 (en) | 2009-09-15 | 2014-10-14 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of Eg5 and VEGF genes |
| US9187746B2 (en) | 2009-09-22 | 2015-11-17 | Alnylam Pharmaceuticals, Inc. | Dual targeting siRNA agents |
| US9101643B2 (en) | 2009-11-03 | 2015-08-11 | Alnylam Pharmaceuticals, Inc. | Lipid formulated compositions and methods for inhibiting expression of transthyretin (TTR) |
| US20110110483A1 (en) * | 2009-11-06 | 2011-05-12 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Methods and systems for migrating fuel assemblies in a nuclear fission reactor |
| US20110124706A1 (en) * | 2009-11-25 | 2011-05-26 | Zhigang He | SOCS3 Inhibition Promotes CNS Neuron Regeneration |
| US8796239B2 (en) * | 2009-11-26 | 2014-08-05 | Quark Pharmaceuticals, Inc. | Sirna compounds comprising terminal substitutions |
| US20120283309A1 (en) * | 2009-11-26 | 2012-11-08 | Sharon Avkin-Nachum | Sirna compounds comprising terminal substitutions |
| US10494631B2 (en) | 2009-11-26 | 2019-12-03 | Quark Pharmaceuticals, Inc. | siRNA compounds comprising terminal substitutions |
| US8790887B2 (en) | 2009-12-04 | 2014-07-29 | Vib Vzw | Screening methods for compounds that modulate ARF-6 mediated endosomal redistribution |
| WO2011067420A1 (en) | 2009-12-04 | 2011-06-09 | Vib Vzw | Arf6 as a new target for treating alzheimer's disease |
| CN106701758A (en) * | 2009-12-09 | 2017-05-24 | 日东电工株式会社 | Modulation of hsp47 expression |
| US8455455B1 (en) | 2010-03-31 | 2013-06-04 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing genes involved in hemorrhagic fever |
| US9783804B2 (en) | 2010-04-13 | 2017-10-10 | Life Technologies Corporation | Compositions and methods for inhibition of nucleic acids function |
| US9410153B2 (en) | 2010-04-13 | 2016-08-09 | Life Technologies Corporation | Compositions and methods for inhibition of nucleic acids function |
| US9145556B2 (en) | 2010-04-13 | 2015-09-29 | Life Technologies Corporation | Compositions and methods for inhibition of nucleic acids function |
| US10023866B2 (en) | 2010-04-13 | 2018-07-17 | Life Technologies Corporation | Compositions and methods for inhibition of nucleic acids function |
| US11535849B2 (en) | 2010-04-29 | 2022-12-27 | Ionis Pharmaceuticals, Inc. | Modulation of transthyretin expression |
| US9061044B2 (en) | 2010-04-29 | 2015-06-23 | Isis Pharmaceuticals, Inc. | Modulation of transthyretin expression |
| US9399774B2 (en) | 2010-04-29 | 2016-07-26 | Ionis Pharmaceuticals, Inc. | Modulation of transthyretin expression |
| US8697860B1 (en) | 2010-04-29 | 2014-04-15 | Isis Pharmaceuticals, Inc. | Diagnosis and treatment of disease |
| US20130164846A1 (en) * | 2010-06-23 | 2013-06-27 | Mina Therapeutics Limited | Rna molecules and uses thereof |
| US9404127B2 (en) | 2010-06-30 | 2016-08-02 | Protiva Biotherapeutics, Inc. | Non-liposomal systems for nucleic acid delivery |
| US9006417B2 (en) | 2010-06-30 | 2015-04-14 | Protiva Biotherapeutics, Inc. | Non-liposomal systems for nucleic acid delivery |
| US9518272B2 (en) | 2010-06-30 | 2016-12-13 | Protiva Biotherapeutics, Inc. | Non-liposomal systems for nucleic acid delivery |
| US12129467B2 (en) | 2010-06-30 | 2024-10-29 | Arbutus Biopharma Corporation | Non-liposomal systems for nucleic acid delivery |
| US11718852B2 (en) | 2010-06-30 | 2023-08-08 | Arbutus Biopharma Corporation | Non-liposomal systems for nucleic acid delivery |
| US9457103B2 (en) | 2010-10-06 | 2016-10-04 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US10494612B2 (en) | 2010-10-06 | 2019-12-03 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US11932854B2 (en) | 2010-10-29 | 2024-03-19 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acids (siNA) |
| US9970005B2 (en) | 2010-10-29 | 2018-05-15 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acids (siNA) |
| US11193126B2 (en) | 2010-10-29 | 2021-12-07 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acids (siNA) |
| US9260471B2 (en) | 2010-10-29 | 2016-02-16 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using short interfering nucleic acids (siNA) |
| WO2012075337A2 (en) | 2010-12-01 | 2012-06-07 | Spinal Modulation, Inc. | Directed delivery of agents to neural anatomy |
| WO2012110500A1 (en) | 2011-02-15 | 2012-08-23 | Vib Vzw | Means and methods for improvement of synaptic dysfunction disorders |
| WO2012119949A1 (en) | 2011-03-04 | 2012-09-13 | Vib Vzw | Means and methods for the treatment of neurodegenerative disorders |
| US10202657B2 (en) | 2011-04-22 | 2019-02-12 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US10214785B2 (en) | 2011-04-22 | 2019-02-26 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US9458517B2 (en) | 2011-04-22 | 2016-10-04 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US9193956B2 (en) | 2011-04-22 | 2015-11-24 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US11236402B2 (en) | 2011-04-22 | 2022-02-01 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid |
| US9856539B2 (en) | 2011-04-22 | 2018-01-02 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US9587282B2 (en) | 2011-04-22 | 2017-03-07 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US9315813B2 (en) | 2011-06-21 | 2016-04-19 | Alnylam Pharmaceuticals, Inc | Compositions and methods for inhibition of expression of apolipoprotein C-III (APOC3) genes |
| US9228188B2 (en) | 2011-06-21 | 2016-01-05 | Alnylam Pharmaceuticals, Inc. | Compositions and method for inhibiting hepcidin antimicrobial peptide (HAMP) or HAMP-related gene expression |
| US11118181B2 (en) | 2011-06-21 | 2021-09-14 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of protein C (PROC) genes |
| US10273478B2 (en) | 2011-06-21 | 2019-04-30 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of protein C (PROC) genes |
| US9970006B2 (en) | 2011-06-21 | 2018-05-15 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of apolipoprotein C-III (APOC3) genes |
| US9068184B2 (en) | 2011-06-21 | 2015-06-30 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of protein C (PROC) genes |
| US9725718B2 (en) | 2011-06-21 | 2017-08-08 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of expression of protein C (PROC) genes |
| WO2012175798A2 (en) | 2011-06-22 | 2012-12-27 | Turun Yliopisto | Combination therapy |
| US9476050B2 (en) | 2011-06-22 | 2016-10-25 | Turun Yliopisto | Combination therapy |
| WO2012175735A1 (en) | 2011-06-23 | 2012-12-27 | Vib Vzw | A20 inhibitors for the treatment of respiratory viral infections |
| WO2013007766A1 (en) | 2011-07-13 | 2013-01-17 | Vib Vzw | Means and methods for the treatment of pathological angiogenesis |
| US9968630B2 (en) | 2011-09-06 | 2018-05-15 | Turun Yliopisto | Pharmaceutical combination comprising a CIP2A silencing agent for use in the treatment of a hyperproliferative disorder, preferably one with impaired P53 function |
| US9457042B2 (en) | 2011-09-06 | 2016-10-04 | Turun Yliopisto | Pharmaceutical combination comprising a CIP2A silencing agent for use in the treatment of a hyperproliferative disorder, preferably one with impaired p53 function |
| WO2013034806A1 (en) | 2011-09-06 | 2013-03-14 | Turun Yliopisto | Pharmaceutical combination comprising a cip2a silencing agent for use in the treatment of a hyperproliferative disorder, preferably one with impaired p53 function |
| US10570391B2 (en) | 2011-11-18 | 2020-02-25 | Alnylam Pharmaceuticals, Inc. | RNAi agents, compositions and methods of use thereof for treating transthyretin (TTR) associated diseases |
| US9399775B2 (en) | 2011-11-18 | 2016-07-26 | Alnylam Pharmaceuticals, Inc. | RNAi agents, compositions and methods of use thereof for treating transthyretin (TTR) associated diseases |
| US9506033B2 (en) * | 2012-05-22 | 2016-11-29 | University Of Massachusetts | Compositions and methods for inducing myoblast differentiation and myotube formation |
| US10166241B2 (en) | 2012-07-13 | 2019-01-01 | Turun Yliopisto | Combination Therapy III |
| WO2014009609A1 (en) | 2012-07-13 | 2014-01-16 | Turun Yliopisto | Combination therapy iii |
| US9950001B2 (en) | 2012-08-20 | 2018-04-24 | The Regents Of The University Of California | Polynucleotides having bioreversible groups |
| US20140088300A1 (en) * | 2012-09-24 | 2014-03-27 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Modified siRNA Molecules Incorporating 5-Fluoro-2'-Deoxyuridine Residues to Enhance Cytotoxicity |
| US9096853B2 (en) * | 2012-09-24 | 2015-08-04 | U.S. Department Of Veterans Affairs | Modified siRNA molecules incorporating 5-fluoro-2′-deoxyuridine residues to enhance cytotoxicity |
| US11634691B2 (en) | 2013-05-31 | 2023-04-25 | The Regents Of The University Of California | Compositions and methods of treatment |
| US11136557B2 (en) | 2013-05-31 | 2021-10-05 | The Regents Of The University Of California | Adeno-associated virus variants and methods of use thereof |
| WO2015051045A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | 3'END CAPS FOR RNAi AGENTS FOR USE IN RNA INTERFERENCE |
| US10519446B2 (en) | 2013-10-04 | 2019-12-31 | Novartis Ag | Organic compounds to treat hepatitis B virus |
| US9988627B2 (en) | 2013-10-04 | 2018-06-05 | Novartis Ag | Formats for organic compounds for use in RNA interference |
| EP3722277A2 (en) | 2013-10-04 | 2020-10-14 | Novartis AG | 3'end caps for rna-interferring agents for use in rna |
| WO2015051366A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Novel formats for organic compounds for use in rna interference |
| US10227588B2 (en) | 2013-10-04 | 2019-03-12 | Novartis Ag | 3′end caps for RNAi agents for use in RNA interference |
| WO2015050871A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Organic compounds to treat hepatitis b virus |
| US11008570B2 (en) | 2013-10-04 | 2021-05-18 | Novartis Ag | 3′ end caps for RNAi agents for use in RNA interference |
| WO2015051044A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Novel formats for organic compounds for use in rna interference |
| US11248214B2 (en) | 2014-03-17 | 2022-02-15 | Adverum Biotechnologies, Inc. | Compositions and methods for enhanced gene expression in cone cells |
| US12275959B2 (en) | 2014-03-17 | 2025-04-15 | Adverum Biotechnologies, Inc. | Compositions and methods for enhanced gene expression in cone cells |
| US10683500B2 (en) | 2014-03-25 | 2020-06-16 | Arcturus Therapeutics, Inc. | UNA oligomers having reduced off-target effects in gene silencing |
| US9856475B2 (en) | 2014-03-25 | 2018-01-02 | Arcturus Therapeutics, Inc. | Formulations for treating amyloidosis |
| US9982259B2 (en) | 2014-03-25 | 2018-05-29 | Arcturus Therapeutics, Inc. | Transthyretin allele selective UNA oligomers for gene silencing |
| US10604758B2 (en) | 2014-03-25 | 2020-03-31 | Arcturus Therapeutics, Inc. | Therapeutic oligomers for treating amyloidosis |
| US11079379B2 (en) | 2014-08-29 | 2021-08-03 | Alnylam Pharmaceuticals, Inc. | Methods of treating transthyretin (TTR) mediated amyloidosis |
| US10060921B2 (en) | 2014-08-29 | 2018-08-28 | Alnylam Pharmaceuticals, Inc. | Methods of treating transthyretin (TTR) mediated amyloidosis |
| US11446380B2 (en) | 2014-10-10 | 2022-09-20 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of HAO1 (hydroxyacid oxidase 1 (glycolate oxidase)) gene expression |
| US10478500B2 (en) | 2014-10-10 | 2019-11-19 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibition of HAO1 (Hydroxyacid Oxidase 1 (Glycolate Oxidase)) gene expression |
| US10513703B2 (en) | 2014-11-10 | 2019-12-24 | Alnylam Pharmaceuticals, Inc. | Hepatitis B virus (HBV) iRNA compositions and methods of use thereof |
| US11060091B2 (en) | 2014-11-10 | 2021-07-13 | Alnylam Pharmaceuticals, Inc. | Hepatitis B virus (HBV) iRNA compositions and methods of use thereof |
| US11408001B1 (en) | 2014-11-17 | 2022-08-09 | Alnylam Pharmaceuticals, Inc. | Apolipoprotein C3 (APOC3) iRNA compositions and methods of use thereof |
| US11142766B2 (en) | 2014-11-17 | 2021-10-12 | Alnylam Pharmaceuticals, Inc. | Apolipoprotein C3 (APOC3) iRNA compositions and methods of use thereof |
| US11202795B2 (en) | 2014-11-20 | 2021-12-21 | Vib Vzw | Means and methods for treatment of early-onset Parkinson's disease |
| US11021519B2 (en) | 2015-03-02 | 2021-06-01 | Adverum Biotechnologies, Inc. | Compositions and methods for intravitreal delivery of polynucleotides to retinal cones |
| US10883117B2 (en) | 2015-03-24 | 2021-01-05 | The Regents Of The University Of California | Adeno-associated virus variants and methods of use thereof |
| US10519447B2 (en) | 2015-04-01 | 2019-12-31 | Arcturus Therapeutics, Inc. | Therapeutic UNA oligomers and uses thereof |
| WO2016161388A1 (en) | 2015-04-03 | 2016-10-06 | University Of Massachusetts | Fully stabilized asymmetric sirna |
| US9809817B2 (en) | 2015-04-03 | 2017-11-07 | University Of Massachusetts | Oligonucleotide compounds for targeting huntingtin mRNA |
| US12173286B2 (en) | 2015-04-03 | 2024-12-24 | University Of Massachusetts | Fully stabilized asymmetric siRNA |
| US11230713B2 (en) | 2015-04-03 | 2022-01-25 | University Of Massachusetts | Oligonucleotide compounds for targeting huntingtin mRNA |
| US10519451B2 (en) | 2015-04-03 | 2019-12-31 | University Of Massachusetts | Oligonucleotide compounds for treatment of preeclampsia and other angiogenic disorders |
| US9862952B2 (en) | 2015-04-03 | 2018-01-09 | University Of Massachusetts | Oligonucleotide compounds for treatment of preeclampsia and other angiogenic disorders |
| US10774327B2 (en) | 2015-04-03 | 2020-09-15 | University Of Massachusetts | Oligonucleotide compounds for targeting huntingtin mRNA |
| EP3929293A2 (en) | 2015-04-03 | 2021-12-29 | University Of Massachusetts | Fully stabilized asymmetric sirna |
| US11345917B2 (en) | 2015-04-03 | 2022-05-31 | University Of Massachusetts | Oligonucleotide compounds for treatment of preeclampsia and other angiogenic disorders |
| US10435688B2 (en) | 2015-04-03 | 2019-10-08 | University Of Massachusetts | Oligonucleotide compounds for targeting huntingtin mRNA |
| US20180216112A1 (en) * | 2015-07-21 | 2018-08-02 | Università Degli Studi Di Torino | Process for inducing resistance to diphtheria toxin in human cells, products and uses thereof |
| US10421964B2 (en) | 2015-07-23 | 2019-09-24 | Arcturus Therapeutics, Inc. | UNA oligomers and compositions for treating amyloidosis |
| US12049628B2 (en) | 2015-07-31 | 2024-07-30 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof for treating or preventing TTR-associated diseases |
| US11286486B2 (en) | 2015-07-31 | 2022-03-29 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof for treating or preventing TTR-associated diseases |
| US10683501B2 (en) | 2015-07-31 | 2020-06-16 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof for treating or preventing TTR-associated diseases |
| US10208307B2 (en) | 2015-07-31 | 2019-02-19 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof for treating or preventing TTR-associated diseases |
| US10633653B2 (en) | 2015-08-14 | 2020-04-28 | University Of Massachusetts | Bioactive conjugates for oligonucleotide delivery |
| US12077755B2 (en) | 2015-08-14 | 2024-09-03 | University Of Massachusetts | Bioactive conjugates for oligonucleotide delivery |
| US10478503B2 (en) | 2016-01-31 | 2019-11-19 | University Of Massachusetts | Branched oligonucleotides |
| US10799591B2 (en) | 2016-01-31 | 2020-10-13 | University Of Massachusetts | Branched oligonucleotides |
| US11896669B2 (en) | 2016-01-31 | 2024-02-13 | University Of Massachusetts | Branched oligonucleotides |
| US12116573B2 (en) | 2016-03-07 | 2024-10-15 | Arrowhead Pharmaceuticals, Inc. | Targeting ligands for therapeutic compounds |
| US10246709B2 (en) | 2016-03-07 | 2019-04-02 | Arrowhead Pharmaceuticals, Inc. | Targeting ligands for therapeutic compounds |
| US20170283806A1 (en) * | 2016-04-01 | 2017-10-05 | Avidity Biosciences Llc | Kras nucleic acids and uses thereof |
| US20170283795A1 (en) * | 2016-04-01 | 2017-10-05 | Avidity Biosciences Llc | Phosphatidylinositol-3-kinase nucleic acids and uses thereof |
| US10858656B2 (en) * | 2016-04-01 | 2020-12-08 | Avidity Biosciences, Inc. | KRAS nucleic acids and uses thereof |
| US20170283476A1 (en) * | 2016-04-01 | 2017-10-05 | Avidity Biosciences Llc | Myc nucleic acids and uses thereof |
| US11565001B2 (en) | 2016-07-29 | 2023-01-31 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US11554180B2 (en) | 2016-07-29 | 2023-01-17 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US11565000B2 (en) | 2016-07-29 | 2023-01-31 | The Regents Of The University Of California | Adeno-associated virus virions with variant capsid and methods of use thereof |
| US11753638B2 (en) | 2016-08-12 | 2023-09-12 | University Of Massachusetts | Conjugated oligonucleotides |
| US11981703B2 (en) | 2016-08-17 | 2024-05-14 | Sirius Therapeutics, Inc. | Polynucleotide constructs |
| US11174481B2 (en) | 2016-09-02 | 2021-11-16 | Arrowhead Pharmaceuticals, Inc. | Targeting ligands |
| US10294474B2 (en) | 2016-09-02 | 2019-05-21 | Arrowhead Pharmaceuticals, Inc. | Targeting ligands |
| US11192925B2 (en) | 2016-10-19 | 2021-12-07 | Adverum Biotechnologies, Inc. | Modified AAV capsids and uses thereof |
| US12030914B2 (en) | 2016-10-19 | 2024-07-09 | Adverum Biotechnologies, Inc. | Modified AAV capsids and uses thereof |
| US11324820B2 (en) | 2017-04-18 | 2022-05-10 | Alnylam Pharmaceuticals, Inc. | Methods for the treatment of subjects having a hepatitis b virus (HBV) infection |
| US12049627B2 (en) | 2017-06-23 | 2024-07-30 | University Of Massachusetts | Two-tailed self-delivering siRNA |
| US10844377B2 (en) | 2017-06-23 | 2020-11-24 | University Of Massachusetts | Two-tailed self-delivering siRNA |
| US11597744B2 (en) | 2017-06-30 | 2023-03-07 | Sirius Therapeutics, Inc. | Chiral phosphoramidite auxiliaries and methods of their use |
| US12310997B2 (en) | 2017-06-30 | 2025-05-27 | The Regents Of The University Of California | Compositions and methods of treating ocular diseases |
| US12269839B2 (en) | 2017-06-30 | 2025-04-08 | Sirius Therapeutics, Inc. | Chiral phosphoramidite auxiliaries and methods of their use |
| US11261447B2 (en) | 2017-07-13 | 2022-03-01 | Alnylam Pharmaceuticals, Inc. | Methods for inhibition of HAO1 (hydroxyacid oxidase 1 (glycolate oxidase)) gene expression |
| US11680249B2 (en) | 2017-08-28 | 2023-06-20 | The Regents Of The University Of California | Adeno-associated virus capsid variants and methods of use thereof |
| US11806360B2 (en) | 2017-09-19 | 2023-11-07 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for treating transthyretin (TTR) mediated amyloidosis |
| US11827882B2 (en) | 2018-08-10 | 2023-11-28 | University Of Massachusetts | Modified oligonucleotides targeting SNPs |
| US12173289B2 (en) | 2018-08-13 | 2024-12-24 | Alnylam Pharmaceuticals, Inc. | Hepatitis B virus (HBV) dsRNA agent compositions and methods of use thereof |
| US11492623B2 (en) | 2018-08-13 | 2022-11-08 | Alnylam Pharmaceuticals, Inc. | Hepatitis B virus (HBV) dsRNA agent compositions and methods of use thereof |
| US11279930B2 (en) | 2018-08-23 | 2022-03-22 | University Of Massachusetts | O-methyl rich fully stabilized oligonucleotides |
| US12297430B2 (en) | 2018-08-23 | 2025-05-13 | University Of Massachusetts | O-methyl rich fully stabilized oligonucleotides |
| US12180477B2 (en) | 2019-01-18 | 2024-12-31 | University Of Massachusetts | Dynamic pharmacokinetic-modifying anchors |
| US12024706B2 (en) | 2019-08-09 | 2024-07-02 | University Of Massachusetts | Modified oligonucleotides targeting SNPs |
| US12365894B2 (en) | 2019-09-16 | 2025-07-22 | University Of Massachusetts | Branched lipid conjugates of siRNA for specific tissue delivery |
| WO2021160937A1 (en) | 2020-02-11 | 2021-08-19 | Turun Yliopisto | Therapy of ras-dependent cancers |
| US12331297B2 (en) | 2020-11-13 | 2025-06-17 | Alnylam Pharmaceuticals, Inc. | Coagulation factor V (F5) iRNA compositions and methods of use thereof |
| US11591544B2 (en) | 2020-11-25 | 2023-02-28 | Akagera Medicines, Inc. | Ionizable cationic lipids |
| US12077725B2 (en) | 2020-11-25 | 2024-09-03 | Akagera Medicines, Inc. | Ionizable cationic lipids |
| US12331264B2 (en) | 2020-11-25 | 2025-06-17 | Akagera Medicines, Inc. | Lipid nanoparticles for delivery of nucleic acids and methods of use thereof |
| US11702659B2 (en) | 2021-06-23 | 2023-07-18 | University Of Massachusetts | Optimized anti-FLT1 oligonucleotide compounds for treatment of preeclampsia and other angiogenic disorders |
| US11959081B2 (en) | 2021-08-03 | 2024-04-16 | Alnylam Pharmaceuticals, Inc. | Transthyretin (TTR) iRNA compositions and methods of use thereof |
| US12064479B2 (en) | 2022-05-25 | 2024-08-20 | Akagera Medicines, Inc. | Lipid nanoparticles for delivery of nucleic acids and methods of use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| US20030119017A1 (en) | 2003-06-26 |
| EP1386004A4 (en) | 2005-02-16 |
| WO2002081628A3 (en) | 2003-02-20 |
| US20060154271A1 (en) | 2006-07-13 |
| EP1386004A2 (en) | 2004-02-04 |
| US20050261212A1 (en) | 2005-11-24 |
| US7022828B2 (en) | 2006-04-04 |
| US20030148507A1 (en) | 2003-08-07 |
| WO2002081628A2 (en) | 2002-10-17 |
| US20030191077A1 (en) | 2003-10-09 |
| WO2002081628A8 (en) | 2003-08-28 |
| US20070026394A1 (en) | 2007-02-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20030143732A1 (en) | RNA interference mediated inhibition of adenosine A1 receptor (ADORA1) gene expression using short interfering RNA | |
| AU2003213090B2 (en) | RNA interference mediated treatment of alzheimer's disease using short interfering nucleic acid ( siNA) | |
| EP2287306B2 (en) | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) | |
| US20040019001A1 (en) | RNA interference mediated inhibition of protein typrosine phosphatase-1B (PTP-1B) gene expression using short interfering RNA | |
| US7858771B2 (en) | RNA interference mediated inhibition of muscarinic colinergic receptor gene expression using short interfering nucleic acid (siNA) | |
| US20030170891A1 (en) | RNA interference mediated inhibition of epidermal growth factor receptor gene expression using short interfering nucleic acid (siNA) | |
| US20030206887A1 (en) | RNA interference mediated inhibition of hepatitis B virus (HBV) using short interfering nucleic acid (siNA) | |
| US20050096284A1 (en) | RNA interference mediated treatment of polyglutamine (polyQ) repeat expansion diseases using short interfering nucleic acid (siNA) | |
| AU2003217550A1 (en) | RNA INTERFERENCE MEDIATED INHIBITION OF TNF AND TNF RECEPTOR SUPERFAMILY GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) | |
| WO2003070887A2 (en) | RNA INTERFERENCE MEDIATED INHIBITION OF POLYCOMB GROUP PROTEIN EZH2 GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) | |
| US20050191638A1 (en) | RNA interference mediated treatment of polyglutamine (polyQ) repeat expansion diseases using short interfering nucleic acid (siNA) | |
| US20100305191A1 (en) | Rna interference mediated inhibition of adenosine a1 receptor (adora1) gene expression using short interfering rna | |
| EP1432725A1 (en) | RNA INTERFERENCE MEDIATED INHIBITION OF TELOMERASE GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) | |
| WO2003070888A2 (en) | Rna interference mediated inhibition of checkpoint kinase-1 (chk-1) gene expression using short interfering nucleic acid | |
| US8017765B2 (en) | RNA interference mediated treatment of alzheimer's disease using short interfering nucleic acid (siNA) | |
| WO2003070885A2 (en) | SHORT INTERFERING NUCLEIC ACID INHIBITION OF STEAROYL-CoA DESATURASE (SCD) GENE | |
| EP1710307A2 (en) | RNA interference mediated inhibition of gene expression using short interfering nucleic acid (siNA) | |
| EP1495041A1 (en) | RNA INTERFERENCE MEDIATED INHIBITION OF G72 AND D-AMINO ACID OXIDASE (DAAO) GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA) | |
| EP1741781A2 (en) | RNA interference mediated treatment of Alzheimer's disease using short interfering nucleic acid (siNA) | |
| US20090233983A1 (en) | RNA Interference Mediated Inhibition of Protein Tyrosine Phosphatase-1B (PTP-1B) Gene Expression Using Short Interfering RNA |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: RIBOZYME PHARMACEUTICALS, INC., COLORADO Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:FOSNAUGH, KATHY;MCSWIGGEN, JAMES A.;REEL/FRAME:013453/0361;SIGNING DATES FROM 20021002 TO 20021007 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |