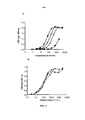
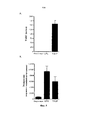
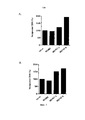
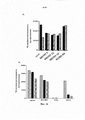
RU2734076C2 - Антитела, направленные на cd127 - Google Patents
Антитела, направленные на cd127 Download PDFInfo
- Publication number
- RU2734076C2 RU2734076C2 RU2016151265A RU2016151265A RU2734076C2 RU 2734076 C2 RU2734076 C2 RU 2734076C2 RU 2016151265 A RU2016151265 A RU 2016151265A RU 2016151265 A RU2016151265 A RU 2016151265A RU 2734076 C2 RU2734076 C2 RU 2734076C2
- Authority
- RU
- Russia
- Prior art keywords
- seq
- antibody
- disease
- antigen
- cells
- Prior art date
Links
- 101001043809 Homo sapiens Interleukin-7 receptor subunit alpha Proteins 0.000 claims abstract description 349
- 102100021593 Interleukin-7 receptor subunit alpha Human genes 0.000 claims abstract description 293
- 230000027455 binding Effects 0.000 claims abstract description 202
- 239000000427 antigen Substances 0.000 claims abstract description 198
- 102000036639 antigens Human genes 0.000 claims abstract description 198
- 108091007433 antigens Proteins 0.000 claims abstract description 198
- 239000012634 fragment Substances 0.000 claims abstract description 181
- 102000000704 Interleukin-7 Human genes 0.000 claims abstract description 158
- 108010002586 Interleukin-7 Proteins 0.000 claims abstract description 158
- 229920002521 macromolecule Polymers 0.000 claims abstract description 133
- 238000000034 method Methods 0.000 claims abstract description 92
- 238000004519 manufacturing process Methods 0.000 claims abstract description 50
- 125000003275 alpha amino acid group Chemical group 0.000 claims abstract description 49
- 150000007523 nucleic acids Chemical class 0.000 claims abstract description 35
- 102000039446 nucleic acids Human genes 0.000 claims abstract description 34
- 108020004707 nucleic acids Proteins 0.000 claims abstract description 34
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 28
- 230000011664 signaling Effects 0.000 claims abstract description 24
- 239000003814 drug Substances 0.000 claims abstract description 15
- 230000001976 improved effect Effects 0.000 claims abstract description 15
- 210000004027 cell Anatomy 0.000 claims description 261
- 241000282414 Homo sapiens Species 0.000 claims description 136
- 102100031294 Thymic stromal lymphopoietin Human genes 0.000 claims description 119
- 150000001413 amino acids Chemical class 0.000 claims description 119
- 230000014509 gene expression Effects 0.000 claims description 112
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 84
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 72
- 201000010099 disease Diseases 0.000 claims description 68
- 238000011282 treatment Methods 0.000 claims description 65
- 102000049284 human IL7R Human genes 0.000 claims description 53
- 230000005764 inhibitory process Effects 0.000 claims description 46
- 230000035800 maturation Effects 0.000 claims description 39
- 101000978362 Homo sapiens C-C motif chemokine 17 Proteins 0.000 claims description 26
- 102100023698 C-C motif chemokine 17 Human genes 0.000 claims description 25
- 230000003042 antagnostic effect Effects 0.000 claims description 23
- 201000006417 multiple sclerosis Diseases 0.000 claims description 23
- 208000023275 Autoimmune disease Diseases 0.000 claims description 22
- 108091033319 polynucleotide Proteins 0.000 claims description 21
- 102000040430 polynucleotide Human genes 0.000 claims description 21
- 239000002157 polynucleotide Substances 0.000 claims description 21
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 20
- 230000026731 phosphorylation Effects 0.000 claims description 20
- 238000006366 phosphorylation reaction Methods 0.000 claims description 20
- 230000003211 malignant effect Effects 0.000 claims description 19
- 230000019491 signal transduction Effects 0.000 claims description 19
- 208000027866 inflammatory disease Diseases 0.000 claims description 18
- 238000002054 transplantation Methods 0.000 claims description 18
- 208000026935 allergic disease Diseases 0.000 claims description 17
- 208000023504 respiratory system disease Diseases 0.000 claims description 16
- 238000006243 chemical reaction Methods 0.000 claims description 15
- 230000004041 dendritic cell maturation Effects 0.000 claims description 15
- 239000004480 active ingredient Substances 0.000 claims description 14
- 230000001225 therapeutic effect Effects 0.000 claims description 14
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 13
- 239000005557 antagonist Substances 0.000 claims description 12
- 230000003053 immunization Effects 0.000 claims description 12
- 206010025135 lupus erythematosus Diseases 0.000 claims description 11
- 208000030836 Hashimoto thyroiditis Diseases 0.000 claims description 10
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims description 10
- 201000002491 encephalomyelitis Diseases 0.000 claims description 10
- 206010025323 Lymphomas Diseases 0.000 claims description 8
- 125000003729 nucleotide group Chemical group 0.000 claims description 8
- 238000011360 adjunctive therapy Methods 0.000 claims description 7
- 230000033581 fucosylation Effects 0.000 claims description 7
- 208000032839 leukemia Diseases 0.000 claims description 7
- 239000002773 nucleotide Substances 0.000 claims description 7
- 230000001684 chronic effect Effects 0.000 claims description 6
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 5
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 claims description 5
- 101000845170 Homo sapiens Thymic stromal lymphopoietin Proteins 0.000 claims description 5
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 5
- 208000036142 Viral infection Diseases 0.000 claims description 5
- 230000001363 autoimmune Effects 0.000 claims description 5
- 102000003675 cytokine receptors Human genes 0.000 claims description 5
- 108010057085 cytokine receptors Proteins 0.000 claims description 5
- 230000009385 viral infection Effects 0.000 claims description 5
- 150000001875 compounds Chemical class 0.000 claims description 4
- 230000002519 immonomodulatory effect Effects 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 2
- 102000001712 STAT5 Transcription Factor Human genes 0.000 claims 1
- 108010029477 STAT5 Transcription Factor Proteins 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 47
- 239000000126 substance Substances 0.000 abstract description 10
- 229940100994 interleukin-7 Drugs 0.000 description 139
- 108010029307 thymic stromal lymphopoietin Proteins 0.000 description 115
- 235000001014 amino acid Nutrition 0.000 description 112
- 108090000765 processed proteins & peptides Proteins 0.000 description 77
- 210000004443 dendritic cell Anatomy 0.000 description 52
- 102000004196 processed proteins & peptides Human genes 0.000 description 50
- 102000005962 receptors Human genes 0.000 description 49
- 108020003175 receptors Proteins 0.000 description 49
- 102000010782 Interleukin-7 Receptors Human genes 0.000 description 47
- 108010038498 Interleukin-7 Receptors Proteins 0.000 description 47
- 241000700159 Rattus Species 0.000 description 47
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 description 46
- 102100038497 Cytokine receptor-like factor 2 Human genes 0.000 description 42
- 101710194733 Cytokine receptor-like factor 2 Proteins 0.000 description 42
- 241001465754 Metazoa Species 0.000 description 42
- 108090000623 proteins and genes Proteins 0.000 description 34
- 102000004169 proteins and genes Human genes 0.000 description 33
- 230000003993 interaction Effects 0.000 description 32
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 31
- 235000018102 proteins Nutrition 0.000 description 31
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 30
- 238000004458 analytical method Methods 0.000 description 29
- 238000002347 injection Methods 0.000 description 24
- 239000007924 injection Substances 0.000 description 24
- 239000000203 mixture Substances 0.000 description 23
- 241000699670 Mus sp. Species 0.000 description 22
- 101150013553 CD40 gene Proteins 0.000 description 21
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 21
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 21
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 21
- 241000880493 Leptailurus serval Species 0.000 description 20
- 238000003776 cleavage reaction Methods 0.000 description 19
- 230000007423 decrease Effects 0.000 description 19
- 210000003071 memory t lymphocyte Anatomy 0.000 description 19
- 230000007017 scission Effects 0.000 description 19
- 238000005516 engineering process Methods 0.000 description 18
- 238000001727 in vivo Methods 0.000 description 17
- 108010044426 integrins Proteins 0.000 description 17
- 102000006495 integrins Human genes 0.000 description 17
- 230000004913 activation Effects 0.000 description 16
- 125000000539 amino acid group Chemical group 0.000 description 16
- 230000000875 corresponding effect Effects 0.000 description 16
- 210000003289 regulatory T cell Anatomy 0.000 description 16
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 15
- 108020004414 DNA Proteins 0.000 description 15
- 238000002965 ELISA Methods 0.000 description 15
- 101001043807 Homo sapiens Interleukin-7 Proteins 0.000 description 15
- 102000052622 human IL7 Human genes 0.000 description 15
- 210000004408 hybridoma Anatomy 0.000 description 15
- 238000011534 incubation Methods 0.000 description 15
- 239000003446 ligand Substances 0.000 description 15
- 230000004083 survival effect Effects 0.000 description 15
- 210000001519 tissue Anatomy 0.000 description 15
- 230000001472 cytotoxic effect Effects 0.000 description 14
- 108010050848 glycylleucine Proteins 0.000 description 14
- 238000000338 in vitro Methods 0.000 description 14
- 108010031719 prolyl-serine Proteins 0.000 description 14
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 13
- 102000004190 Enzymes Human genes 0.000 description 13
- 108090000790 Enzymes Proteins 0.000 description 13
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 13
- 108010087924 alanylproline Proteins 0.000 description 13
- 238000003556 assay Methods 0.000 description 13
- 229940088598 enzyme Drugs 0.000 description 13
- 230000001404 mediated effect Effects 0.000 description 13
- 230000037361 pathway Effects 0.000 description 13
- 230000028993 immune response Effects 0.000 description 12
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 description 11
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 11
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 11
- 238000000749 co-immunoprecipitation Methods 0.000 description 11
- 238000001514 detection method Methods 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 239000000047 product Substances 0.000 description 11
- 108010077112 prolyl-proline Proteins 0.000 description 11
- 208000027930 type IV hypersensitivity disease Diseases 0.000 description 11
- 238000005406 washing Methods 0.000 description 11
- NHJVRSWLHSJWIN-UHFFFAOYSA-N 2,4,6-trinitrobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O NHJVRSWLHSJWIN-UHFFFAOYSA-N 0.000 description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 10
- SSYBNWFXCFNRFN-GUBZILKMSA-N Val-Pro-Ser Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SSYBNWFXCFNRFN-GUBZILKMSA-N 0.000 description 10
- 230000015572 biosynthetic process Effects 0.000 description 10
- 230000003247 decreasing effect Effects 0.000 description 10
- 230000004069 differentiation Effects 0.000 description 10
- 230000035755 proliferation Effects 0.000 description 10
- 230000002829 reductive effect Effects 0.000 description 10
- 108010073969 valyllysine Proteins 0.000 description 10
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 9
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 9
- 206010061218 Inflammation Diseases 0.000 description 9
- 241000699666 Mus <mouse, genus> Species 0.000 description 9
- 102000003992 Peroxidases Human genes 0.000 description 9
- 108010047857 aspartylglycine Proteins 0.000 description 9
- 210000004369 blood Anatomy 0.000 description 9
- 239000008280 blood Substances 0.000 description 9
- 239000000872 buffer Substances 0.000 description 9
- 230000001419 dependent effect Effects 0.000 description 9
- 238000000684 flow cytometry Methods 0.000 description 9
- 230000004927 fusion Effects 0.000 description 9
- 230000004054 inflammatory process Effects 0.000 description 9
- 239000003112 inhibitor Substances 0.000 description 9
- 210000004698 lymphocyte Anatomy 0.000 description 9
- 108040007629 peroxidase activity proteins Proteins 0.000 description 9
- 229920001184 polypeptide Polymers 0.000 description 9
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 8
- 108060003951 Immunoglobulin Proteins 0.000 description 8
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 8
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 8
- QARPMYDMYVLFMW-KKUMJFAQSA-N Phe-Pro-Glu Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(O)=O)C1=CC=CC=C1 QARPMYDMYVLFMW-KKUMJFAQSA-N 0.000 description 8
- FMLRRBDLBJLJIK-DCAQKATOSA-N Pro-Leu-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 FMLRRBDLBJLJIK-DCAQKATOSA-N 0.000 description 8
- YDTUEBLEAVANFH-RCWTZXSCSA-N Pro-Val-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 YDTUEBLEAVANFH-RCWTZXSCSA-N 0.000 description 8
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 8
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 8
- 108010092854 aspartyllysine Proteins 0.000 description 8
- 239000012636 effector Substances 0.000 description 8
- 108010049041 glutamylalanine Proteins 0.000 description 8
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 8
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 8
- 108010015792 glycyllysine Proteins 0.000 description 8
- 210000002865 immune cell Anatomy 0.000 description 8
- 238000002649 immunization Methods 0.000 description 8
- 102000018358 immunoglobulin Human genes 0.000 description 8
- 230000006698 induction Effects 0.000 description 8
- 210000000265 leukocyte Anatomy 0.000 description 8
- 230000007246 mechanism Effects 0.000 description 8
- 210000000822 natural killer cell Anatomy 0.000 description 8
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 8
- 210000003491 skin Anatomy 0.000 description 8
- 230000008685 targeting Effects 0.000 description 8
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 8
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 7
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 7
- 102100035361 Cerebellar degeneration-related protein 2 Human genes 0.000 description 7
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 7
- CQMFNTVQVLQRLT-JHEQGTHGSA-N Gly-Thr-Gln Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O CQMFNTVQVLQRLT-JHEQGTHGSA-N 0.000 description 7
- TTYKEFZRLKQTHH-MELADBBJSA-N His-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O TTYKEFZRLKQTHH-MELADBBJSA-N 0.000 description 7
- 101000737793 Homo sapiens Cerebellar degeneration-related antigen 1 Proteins 0.000 description 7
- 101000737796 Homo sapiens Cerebellar degeneration-related protein 2 Proteins 0.000 description 7
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 7
- NTBFKPBULZGXQL-KKUMJFAQSA-N Lys-Asp-Tyr Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NTBFKPBULZGXQL-KKUMJFAQSA-N 0.000 description 7
- QLFAPXUXEBAWEK-NHCYSSNCSA-N Lys-Val-Asp Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O QLFAPXUXEBAWEK-NHCYSSNCSA-N 0.000 description 7
- 241000282520 Papio Species 0.000 description 7
- 108010076504 Protein Sorting Signals Proteins 0.000 description 7
- UGJRQLURDVGULT-LKXGYXEUSA-N Ser-Asn-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UGJRQLURDVGULT-LKXGYXEUSA-N 0.000 description 7
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 7
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 7
- SDHZOOIGIUEPDY-JYJNAYRXSA-N Val-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 SDHZOOIGIUEPDY-JYJNAYRXSA-N 0.000 description 7
- JVGDAEKKZKKZFO-RCWTZXSCSA-N Val-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)N)O JVGDAEKKZKKZFO-RCWTZXSCSA-N 0.000 description 7
- 206010009887 colitis Diseases 0.000 description 7
- 238000011161 development Methods 0.000 description 7
- 230000018109 developmental process Effects 0.000 description 7
- 210000003162 effector t lymphocyte Anatomy 0.000 description 7
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 7
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 7
- 238000004949 mass spectrometry Methods 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 108010073101 phenylalanylleucine Proteins 0.000 description 7
- 108010070643 prolylglutamic acid Proteins 0.000 description 7
- 230000006798 recombination Effects 0.000 description 7
- 238000005215 recombination Methods 0.000 description 7
- 230000000638 stimulation Effects 0.000 description 7
- 239000006228 supernatant Substances 0.000 description 7
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 7
- 239000013598 vector Substances 0.000 description 7
- DPNZTBKGAUAZQU-DLOVCJGASA-N Ala-Leu-His Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N DPNZTBKGAUAZQU-DLOVCJGASA-N 0.000 description 6
- OINVDEKBKBCPLX-JXUBOQSCSA-N Ala-Lys-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OINVDEKBKBCPLX-JXUBOQSCSA-N 0.000 description 6
- ZJBUILVYSXQNSW-YTWAJWBKSA-N Arg-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ZJBUILVYSXQNSW-YTWAJWBKSA-N 0.000 description 6
- WQLJRNRLHWJIRW-KKUMJFAQSA-N Asn-His-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CC(=O)N)N)O WQLJRNRLHWJIRW-KKUMJFAQSA-N 0.000 description 6
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 6
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 6
- 108090000317 Chymotrypsin Proteins 0.000 description 6
- KSMSFCBQBQPFAD-GUBZILKMSA-N Cys-Pro-Pro Chemical compound SC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 KSMSFCBQBQPFAD-GUBZILKMSA-N 0.000 description 6
- HMIXCETWRYDVMO-GUBZILKMSA-N Gln-Pro-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O HMIXCETWRYDVMO-GUBZILKMSA-N 0.000 description 6
- AAJHGGDRKHYSDH-GUBZILKMSA-N Glu-Pro-Gln Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O AAJHGGDRKHYSDH-GUBZILKMSA-N 0.000 description 6
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 6
- FULZDMOZUZKGQU-ONGXEEELSA-N Gly-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN FULZDMOZUZKGQU-ONGXEEELSA-N 0.000 description 6
- UHNQRAFSEBGZFZ-YESZJQIVSA-N Leu-Phe-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N UHNQRAFSEBGZFZ-YESZJQIVSA-N 0.000 description 6
- DPURXCQCHSQPAN-AVGNSLFASA-N Leu-Pro-Pro Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DPURXCQCHSQPAN-AVGNSLFASA-N 0.000 description 6
- WSXTWLJHTLRFLW-SRVKXCTJSA-N Lys-Ala-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O WSXTWLJHTLRFLW-SRVKXCTJSA-N 0.000 description 6
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 6
- SJRQWEDYTKYHHL-SLFFLAALSA-N Phe-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O SJRQWEDYTKYHHL-SLFFLAALSA-N 0.000 description 6
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 6
- HZWAHWQZPSXNCB-BPUTZDHNSA-N Ser-Arg-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HZWAHWQZPSXNCB-BPUTZDHNSA-N 0.000 description 6
- VAUMZJHYZQXZBQ-WHFBIAKZSA-N Ser-Asn-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O VAUMZJHYZQXZBQ-WHFBIAKZSA-N 0.000 description 6
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 6
- BMKNXTJLHFIAAH-CIUDSAMLSA-N Ser-Ser-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O BMKNXTJLHFIAAH-CIUDSAMLSA-N 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- JMBRNXUOLJFURW-BEAPCOKYSA-N Thr-Phe-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N)O JMBRNXUOLJFURW-BEAPCOKYSA-N 0.000 description 6
- YOPQYBJJNSIQGZ-JNPHEJMOSA-N Thr-Tyr-Tyr Chemical compound C([C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 YOPQYBJJNSIQGZ-JNPHEJMOSA-N 0.000 description 6
- QYSBJAUCUKHSLU-JYJNAYRXSA-N Tyr-Arg-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O QYSBJAUCUKHSLU-JYJNAYRXSA-N 0.000 description 6
- GZUIDWDVMWZSMI-KKUMJFAQSA-N Tyr-Lys-Cys Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CS)C(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 GZUIDWDVMWZSMI-KKUMJFAQSA-N 0.000 description 6
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 6
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 6
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 6
- BGTDGENDNWGMDQ-KJEVXHAQSA-N Val-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N)O BGTDGENDNWGMDQ-KJEVXHAQSA-N 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 210000003719 b-lymphocyte Anatomy 0.000 description 6
- 229960000074 biopharmaceutical Drugs 0.000 description 6
- 229960002376 chymotrypsin Drugs 0.000 description 6
- 238000005520 cutting process Methods 0.000 description 6
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 6
- 108010010147 glycylglutamine Proteins 0.000 description 6
- 108010037850 glycylvaline Proteins 0.000 description 6
- 230000002163 immunogen Effects 0.000 description 6
- 210000002429 large intestine Anatomy 0.000 description 6
- 108010034529 leucyl-lysine Proteins 0.000 description 6
- 210000004072 lung Anatomy 0.000 description 6
- 108010064235 lysylglycine Proteins 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 230000007170 pathology Effects 0.000 description 6
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 6
- 230000000717 retained effect Effects 0.000 description 6
- 238000012216 screening Methods 0.000 description 6
- 238000006467 substitution reaction Methods 0.000 description 6
- 238000001262 western blot Methods 0.000 description 6
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 5
- SMEYEQDCCBHTEF-FXQIFTODSA-N Cys-Pro-Ala Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O SMEYEQDCCBHTEF-FXQIFTODSA-N 0.000 description 5
- 241000283074 Equus asinus Species 0.000 description 5
- RFDHKPSHTXZKLL-IHRRRGAJSA-N Glu-Gln-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N RFDHKPSHTXZKLL-IHRRRGAJSA-N 0.000 description 5
- BPCLDCNZBUYGOD-BPUTZDHNSA-N Glu-Trp-Glu Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 BPCLDCNZBUYGOD-BPUTZDHNSA-N 0.000 description 5
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 5
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 5
- 239000004471 Glycine Substances 0.000 description 5
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 5
- RFQATBGBLDAKGI-VHSXEESVSA-N Lys-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CCCCN)N)C(=O)O RFQATBGBLDAKGI-VHSXEESVSA-N 0.000 description 5
- RKIIYGUHIQJCBW-SRVKXCTJSA-N Met-His-Glu Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O RKIIYGUHIQJCBW-SRVKXCTJSA-N 0.000 description 5
- FWAHLGXNBLWIKB-NAKRPEOUSA-N Met-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCSC FWAHLGXNBLWIKB-NAKRPEOUSA-N 0.000 description 5
- 241000282516 Papio anubis Species 0.000 description 5
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 5
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 5
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 5
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 5
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 5
- OACSGBOREVRSME-NHCYSSNCSA-N Val-His-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(O)=O OACSGBOREVRSME-NHCYSSNCSA-N 0.000 description 5
- 108010068265 aspartyltyrosine Proteins 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 239000002299 complementary DNA Substances 0.000 description 5
- 238000007796 conventional method Methods 0.000 description 5
- 231100000433 cytotoxic Toxicity 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- 108010017391 lysylvaline Proteins 0.000 description 5
- 239000003550 marker Substances 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 238000002493 microarray Methods 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 210000000813 small intestine Anatomy 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 108010051110 tyrosyl-lysine Proteins 0.000 description 5
- 230000004580 weight loss Effects 0.000 description 5
- BVLIJXXSXBUGEC-SRVKXCTJSA-N Asn-Asn-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BVLIJXXSXBUGEC-SRVKXCTJSA-N 0.000 description 4
- SRUUBQBAVNQZGJ-LAEOZQHASA-N Asn-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC(=O)N)N SRUUBQBAVNQZGJ-LAEOZQHASA-N 0.000 description 4
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 4
- KBQOUDLMWYWXNP-YDHLFZDLSA-N Asn-Val-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CC(=O)N)N KBQOUDLMWYWXNP-YDHLFZDLSA-N 0.000 description 4
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 4
- 206010006187 Breast cancer Diseases 0.000 description 4
- NDNZRWUDUMTITL-FXQIFTODSA-N Cys-Ser-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NDNZRWUDUMTITL-FXQIFTODSA-N 0.000 description 4
- ALTQTAKGRFLRLR-GUBZILKMSA-N Cys-Val-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N ALTQTAKGRFLRLR-GUBZILKMSA-N 0.000 description 4
- 206010015150 Erythema Diseases 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- YHOJJFFTSMWVGR-HJGDQZAQSA-N Glu-Met-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YHOJJFFTSMWVGR-HJGDQZAQSA-N 0.000 description 4
- SYOJVRNQCXYEOV-XVKPBYJWSA-N Gly-Val-Glu Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SYOJVRNQCXYEOV-XVKPBYJWSA-N 0.000 description 4
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 4
- MKWSZEHGHSLNPF-NAKRPEOUSA-N Ile-Ala-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O)N MKWSZEHGHSLNPF-NAKRPEOUSA-N 0.000 description 4
- 102000007440 Interleukin-7 Receptor alpha Subunit Human genes 0.000 description 4
- 108010086134 Interleukin-7 Receptor alpha Subunit Proteins 0.000 description 4
- BKTXKJMNTSMJDQ-AVGNSLFASA-N Leu-His-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N BKTXKJMNTSMJDQ-AVGNSLFASA-N 0.000 description 4
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 4
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 4
- 201000003791 MALT lymphoma Diseases 0.000 description 4
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 4
- 206010035226 Plasma cell myeloma Diseases 0.000 description 4
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 4
- HQVPQXMCQKXARZ-FXQIFTODSA-N Pro-Cys-Ser Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O HQVPQXMCQKXARZ-FXQIFTODSA-N 0.000 description 4
- VPEVBAUSTBWQHN-NHCYSSNCSA-N Pro-Glu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O VPEVBAUSTBWQHN-NHCYSSNCSA-N 0.000 description 4
- ULWBBFKQBDNGOY-RWMBFGLXSA-N Pro-Lys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@@H]2C(=O)O ULWBBFKQBDNGOY-RWMBFGLXSA-N 0.000 description 4
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 4
- 102000001708 Protein Isoforms Human genes 0.000 description 4
- 108010029485 Protein Isoforms Proteins 0.000 description 4
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 4
- UEJYSALTSUZXFV-SRVKXCTJSA-N Rigin Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O UEJYSALTSUZXFV-SRVKXCTJSA-N 0.000 description 4
- 241000283984 Rodentia Species 0.000 description 4
- QEDMOZUJTGEIBF-FXQIFTODSA-N Ser-Arg-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O QEDMOZUJTGEIBF-FXQIFTODSA-N 0.000 description 4
- ZOHGLPQGEHSLPD-FXQIFTODSA-N Ser-Gln-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZOHGLPQGEHSLPD-FXQIFTODSA-N 0.000 description 4
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 4
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 4
- LAFLAXHTDVNVEL-WDCWCFNPSA-N Thr-Gln-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N)O LAFLAXHTDVNVEL-WDCWCFNPSA-N 0.000 description 4
- ZESGVALRVJIVLZ-VFCFLDTKSA-N Thr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O ZESGVALRVJIVLZ-VFCFLDTKSA-N 0.000 description 4
- XGFGVFMXDXALEV-XIRDDKMYSA-N Trp-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N XGFGVFMXDXALEV-XIRDDKMYSA-N 0.000 description 4
- SSSDKJMQMZTMJP-BVSLBCMMSA-N Trp-Tyr-Val Chemical compound C([C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C1=CC=C(O)C=C1 SSSDKJMQMZTMJP-BVSLBCMMSA-N 0.000 description 4
- SBLZVFCEOCWRLS-BPNCWPANSA-N Tyr-Met-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC1=CC=C(C=C1)O)N SBLZVFCEOCWRLS-BPNCWPANSA-N 0.000 description 4
- 238000001042 affinity chromatography Methods 0.000 description 4
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 4
- 108010082017 alpha chain interleukin-7 receptor Proteins 0.000 description 4
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 4
- 230000008485 antagonism Effects 0.000 description 4
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 4
- 229960000190 bacillus calmette–guérin vaccine Drugs 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 230000001276 controlling effect Effects 0.000 description 4
- 230000000139 costimulatory effect Effects 0.000 description 4
- 230000003013 cytotoxicity Effects 0.000 description 4
- 231100000135 cytotoxicity Toxicity 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 238000010494 dissociation reaction Methods 0.000 description 4
- 230000005593 dissociations Effects 0.000 description 4
- 231100000673 dose–response relationship Toxicity 0.000 description 4
- 230000003828 downregulation Effects 0.000 description 4
- 239000002158 endotoxin Substances 0.000 description 4
- 231100000321 erythema Toxicity 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 4
- 210000004602 germ cell Anatomy 0.000 description 4
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 4
- 208000024908 graft versus host disease Diseases 0.000 description 4
- 239000000833 heterodimer Substances 0.000 description 4
- 230000036039 immunity Effects 0.000 description 4
- 230000002871 immunocytoma Effects 0.000 description 4
- 229940072221 immunoglobulins Drugs 0.000 description 4
- 108010091871 leucylmethionine Proteins 0.000 description 4
- 108010057821 leucylproline Proteins 0.000 description 4
- 229920006008 lipopolysaccharide Polymers 0.000 description 4
- 210000004962 mammalian cell Anatomy 0.000 description 4
- 238000013507 mapping Methods 0.000 description 4
- 238000010172 mouse model Methods 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 201000000050 myeloid neoplasm Diseases 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 238000002823 phage display Methods 0.000 description 4
- 108010051242 phenylalanylserine Proteins 0.000 description 4
- 239000013612 plasmid Substances 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000033300 receptor internalization Effects 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000011160 research Methods 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 230000008054 signal transmission Effects 0.000 description 4
- 230000009870 specific binding Effects 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- 210000001541 thymus gland Anatomy 0.000 description 4
- 108010044292 tryptophyltyrosine Proteins 0.000 description 4
- 229960001005 tuberculin Drugs 0.000 description 4
- 230000005951 type IV hypersensitivity Effects 0.000 description 4
- LPXQRXLUHJKZIE-UHFFFAOYSA-N 8-azaguanine Chemical compound NC1=NC(O)=C2NN=NC2=N1 LPXQRXLUHJKZIE-UHFFFAOYSA-N 0.000 description 3
- UCIYCBSJBQGDGM-LPEHRKFASA-N Ala-Arg-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N UCIYCBSJBQGDGM-LPEHRKFASA-N 0.000 description 3
- DHBKYZYFEXXUAK-ONGXEEELSA-N Ala-Phe-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 DHBKYZYFEXXUAK-ONGXEEELSA-N 0.000 description 3
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 3
- SYIFFFHSXBNPMC-UWJYBYFXSA-N Ala-Ser-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N SYIFFFHSXBNPMC-UWJYBYFXSA-N 0.000 description 3
- VNFSAYFQLXPHPY-CIQUZCHMSA-N Ala-Thr-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O VNFSAYFQLXPHPY-CIQUZCHMSA-N 0.000 description 3
- ZCUFMRIQCPNOHZ-NRPADANISA-N Ala-Val-Gln Chemical compound C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N ZCUFMRIQCPNOHZ-NRPADANISA-N 0.000 description 3
- REWSWYIDQIELBE-FXQIFTODSA-N Ala-Val-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O REWSWYIDQIELBE-FXQIFTODSA-N 0.000 description 3
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 3
- KMFPQTITXUKJOV-DCAQKATOSA-N Arg-Ser-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O KMFPQTITXUKJOV-DCAQKATOSA-N 0.000 description 3
- NYGILGUOUOXGMJ-YUMQZZPRSA-N Asn-Lys-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O NYGILGUOUOXGMJ-YUMQZZPRSA-N 0.000 description 3
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 3
- JZLFYAAGGYMRIK-BYULHYEWSA-N Asn-Val-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O JZLFYAAGGYMRIK-BYULHYEWSA-N 0.000 description 3
- VPPXTHJNTYDNFJ-CIUDSAMLSA-N Asp-Ala-Lys Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)O)N VPPXTHJNTYDNFJ-CIUDSAMLSA-N 0.000 description 3
- HSWYMWGDMPLTTH-FXQIFTODSA-N Asp-Glu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HSWYMWGDMPLTTH-FXQIFTODSA-N 0.000 description 3
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 3
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 3
- VHUKCUHLFMRHOD-MELADBBJSA-N Asp-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC(=O)O)N)C(=O)O VHUKCUHLFMRHOD-MELADBBJSA-N 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- BYALSSDCQYHKMY-XGEHTFHBSA-N Cys-Arg-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CS)N)O BYALSSDCQYHKMY-XGEHTFHBSA-N 0.000 description 3
- YMBAVNPKBWHDAW-CIUDSAMLSA-N Cys-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CS)N YMBAVNPKBWHDAW-CIUDSAMLSA-N 0.000 description 3
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 3
- NVEASDQHBRZPSU-BQBZGAKWSA-N Gln-Gln-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O NVEASDQHBRZPSU-BQBZGAKWSA-N 0.000 description 3
- KCJJFESQRXGTGC-BQBZGAKWSA-N Gln-Glu-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O KCJJFESQRXGTGC-BQBZGAKWSA-N 0.000 description 3
- XFAUJGNLHIGXET-AVGNSLFASA-N Gln-Leu-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O XFAUJGNLHIGXET-AVGNSLFASA-N 0.000 description 3
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 3
- AQPZYBSRDRZBAG-AVGNSLFASA-N Gln-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N AQPZYBSRDRZBAG-AVGNSLFASA-N 0.000 description 3
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 3
- HPBKQFJXDUVNQV-FHWLQOOXSA-N Gln-Tyr-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O HPBKQFJXDUVNQV-FHWLQOOXSA-N 0.000 description 3
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 3
- WATXSTJXNBOHKD-LAEOZQHASA-N Glu-Asp-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O WATXSTJXNBOHKD-LAEOZQHASA-N 0.000 description 3
- HUFCEIHAFNVSNR-IHRRRGAJSA-N Glu-Gln-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUFCEIHAFNVSNR-IHRRRGAJSA-N 0.000 description 3
- JWNZHMSRZXXGTM-XKBZYTNZSA-N Glu-Ser-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWNZHMSRZXXGTM-XKBZYTNZSA-N 0.000 description 3
- DLISPGXMKZTWQG-IFFSRLJSSA-N Glu-Thr-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O DLISPGXMKZTWQG-IFFSRLJSSA-N 0.000 description 3
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 3
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 3
- NSTUFLGQJCOCDL-UWVGGRQHSA-N Gly-Leu-Arg Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N NSTUFLGQJCOCDL-UWVGGRQHSA-N 0.000 description 3
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 3
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 3
- OOCFXNOVSLSHAB-IUCAKERBSA-N Gly-Pro-Pro Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 OOCFXNOVSLSHAB-IUCAKERBSA-N 0.000 description 3
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 3
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 3
- FYVHHKMHFPMBBG-GUBZILKMSA-N His-Gln-Asp Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N FYVHHKMHFPMBBG-GUBZILKMSA-N 0.000 description 3
- NDKSHNQINMRKHT-PEXQALLHSA-N His-Ile-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC1=CN=CN1)N NDKSHNQINMRKHT-PEXQALLHSA-N 0.000 description 3
- TVMNTHXFRSXZGR-IHRRRGAJSA-N His-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O TVMNTHXFRSXZGR-IHRRRGAJSA-N 0.000 description 3
- PZUZIHRPOVVHOT-KBPBESRZSA-N His-Tyr-Gly Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)NCC(O)=O)C1=CN=CN1 PZUZIHRPOVVHOT-KBPBESRZSA-N 0.000 description 3
- DFJJAVZIHDFOGQ-MNXVOIDGSA-N Ile-Glu-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N DFJJAVZIHDFOGQ-MNXVOIDGSA-N 0.000 description 3
- NZGTYCMLUGYMCV-XUXIUFHCSA-N Ile-Lys-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N NZGTYCMLUGYMCV-XUXIUFHCSA-N 0.000 description 3
- FXJLRZFMKGHYJP-CFMVVWHZSA-N Ile-Tyr-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FXJLRZFMKGHYJP-CFMVVWHZSA-N 0.000 description 3
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 3
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 3
- 108010024121 Janus Kinases Proteins 0.000 description 3
- 102000015617 Janus Kinases Human genes 0.000 description 3
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 3
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical group SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 3
- HXWALXSAVBLTPK-NUTKFTJISA-N Leu-Ala-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(C)C)N HXWALXSAVBLTPK-NUTKFTJISA-N 0.000 description 3
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 3
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 3
- IZPVWNSAVUQBGP-CIUDSAMLSA-N Leu-Ser-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O IZPVWNSAVUQBGP-CIUDSAMLSA-N 0.000 description 3
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 3
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 3
- GQZMPWBZQALKJO-UWVGGRQHSA-N Lys-Gly-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O GQZMPWBZQALKJO-UWVGGRQHSA-N 0.000 description 3
- KYNNSEJZFVCDIV-ZPFDUUQYSA-N Lys-Ile-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O KYNNSEJZFVCDIV-ZPFDUUQYSA-N 0.000 description 3
- SKRGVGLIRUGANF-AVGNSLFASA-N Lys-Leu-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SKRGVGLIRUGANF-AVGNSLFASA-N 0.000 description 3
- YUAXTFMFMOIMAM-QWRGUYRKSA-N Lys-Lys-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O YUAXTFMFMOIMAM-QWRGUYRKSA-N 0.000 description 3
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 3
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 3
- JMNRXRPBHFGXQX-GUBZILKMSA-N Lys-Ser-Glu Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O JMNRXRPBHFGXQX-GUBZILKMSA-N 0.000 description 3
- WZVSHTFTCYOFPL-GARJFASQSA-N Lys-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCCCN)N)C(=O)O WZVSHTFTCYOFPL-GARJFASQSA-N 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- CIDICGYKRUTYLE-FXQIFTODSA-N Met-Ser-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O CIDICGYKRUTYLE-FXQIFTODSA-N 0.000 description 3
- 241001467552 Mycobacterium bovis BCG Species 0.000 description 3
- WUGMRIBZSVSJNP-UHFFFAOYSA-N N-L-alanyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C)C(O)=O)=CNC2=C1 WUGMRIBZSVSJNP-UHFFFAOYSA-N 0.000 description 3
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 108091007960 PI3Ks Proteins 0.000 description 3
- JOXIIFVCSATTDH-IHPCNDPISA-N Phe-Asn-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JOXIIFVCSATTDH-IHPCNDPISA-N 0.000 description 3
- LXVFHIBXOWJTKZ-BZSNNMDCSA-N Phe-Asn-Tyr Chemical compound N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O LXVFHIBXOWJTKZ-BZSNNMDCSA-N 0.000 description 3
- CUMXHKAOHNWRFQ-BZSNNMDCSA-N Phe-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CUMXHKAOHNWRFQ-BZSNNMDCSA-N 0.000 description 3
- FSPGBMWPNMRWDB-AVGNSLFASA-N Phe-Cys-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N FSPGBMWPNMRWDB-AVGNSLFASA-N 0.000 description 3
- FGWUALWGCZJQDJ-URLPEUOOSA-N Phe-Thr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FGWUALWGCZJQDJ-URLPEUOOSA-N 0.000 description 3
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 3
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 description 3
- ZCXQTRXYZOSGJR-FXQIFTODSA-N Pro-Asp-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZCXQTRXYZOSGJR-FXQIFTODSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 3
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 3
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 3
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 3
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 3
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 3
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 3
- UBRMZSHOOIVJPW-SRVKXCTJSA-N Ser-Leu-Lys Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O UBRMZSHOOIVJPW-SRVKXCTJSA-N 0.000 description 3
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 3
- 101000844753 Sulfolobus acidocaldarius (strain ATCC 33909 / DSM 639 / JCM 8929 / NBRC 15157 / NCIMB 11770) DNA-binding protein 7d Proteins 0.000 description 3
- SXAGUVRFGJSFKC-ZEILLAHLSA-N Thr-His-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SXAGUVRFGJSFKC-ZEILLAHLSA-N 0.000 description 3
- XTCNBOBTROGWMW-RWRJDSDZSA-N Thr-Ile-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N XTCNBOBTROGWMW-RWRJDSDZSA-N 0.000 description 3
- JLNMFGCJODTXDH-WEDXCCLWSA-N Thr-Lys-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O JLNMFGCJODTXDH-WEDXCCLWSA-N 0.000 description 3
- AHERARIZBPOMNU-KATARQTJSA-N Thr-Ser-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O AHERARIZBPOMNU-KATARQTJSA-N 0.000 description 3
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 3
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 3
- 206010052779 Transplant rejections Diseases 0.000 description 3
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 3
- 206010053613 Type IV hypersensitivity reaction Diseases 0.000 description 3
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 3
- VYQQQIRHIFALGE-UWJYBYFXSA-N Tyr-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 VYQQQIRHIFALGE-UWJYBYFXSA-N 0.000 description 3
- QFHRUCJIRVILCK-YJRXYDGGSA-N Tyr-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O QFHRUCJIRVILCK-YJRXYDGGSA-N 0.000 description 3
- SQUMHUZLJDUROQ-YDHLFZDLSA-N Tyr-Val-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O SQUMHUZLJDUROQ-YDHLFZDLSA-N 0.000 description 3
- MJFSRZZJQWZHFQ-SRVKXCTJSA-N Val-Met-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)O)N MJFSRZZJQWZHFQ-SRVKXCTJSA-N 0.000 description 3
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 3
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 3
- GVNLOVJNNDZUHS-RHYQMDGZSA-N Val-Thr-Lys Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O GVNLOVJNNDZUHS-RHYQMDGZSA-N 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 108010070944 alanylhistidine Proteins 0.000 description 3
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 3
- 108010008355 arginyl-glutamine Proteins 0.000 description 3
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 3
- 230000006472 autoimmune response Effects 0.000 description 3
- 150000001540 azides Chemical class 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 210000000601 blood cell Anatomy 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 239000002458 cell surface marker Substances 0.000 description 3
- 210000003169 central nervous system Anatomy 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 235000018417 cysteine Nutrition 0.000 description 3
- 230000009849 deactivation Effects 0.000 description 3
- 230000029087 digestion Effects 0.000 description 3
- 238000010828 elution Methods 0.000 description 3
- 239000012149 elution buffer Substances 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 3
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 3
- 108010081551 glycylphenylalanine Proteins 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 210000005260 human cell Anatomy 0.000 description 3
- 238000011577 humanized mouse model Methods 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 238000000126 in silico method Methods 0.000 description 3
- 230000008595 infiltration Effects 0.000 description 3
- 238000001764 infiltration Methods 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 238000011813 knockout mouse model Methods 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 210000001165 lymph node Anatomy 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 108010003700 lysyl aspartic acid Proteins 0.000 description 3
- 230000014759 maintenance of location Effects 0.000 description 3
- 238000000074 matrix-assisted laser desorption--ionisation tandem time-of-flight detection Methods 0.000 description 3
- 238000002625 monoclonal antibody therapy Methods 0.000 description 3
- 210000002501 natural regulatory T cell Anatomy 0.000 description 3
- 230000003472 neutralizing effect Effects 0.000 description 3
- 238000005457 optimization Methods 0.000 description 3
- 230000007310 pathophysiology Effects 0.000 description 3
- 230000006320 pegylation Effects 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 230000004481 post-translational protein modification Effects 0.000 description 3
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 3
- 108010090894 prolylleucine Proteins 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 230000000284 resting effect Effects 0.000 description 3
- 210000003705 ribosome Anatomy 0.000 description 3
- 238000007363 ring formation reaction Methods 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 3
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 210000000952 spleen Anatomy 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 2
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 2
- 229910000013 Ammonium bicarbonate Inorganic materials 0.000 description 2
- 101100152731 Arabidopsis thaliana TH2 gene Proteins 0.000 description 2
- JEOCWTUOMKEEMF-RHYQMDGZSA-N Arg-Leu-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JEOCWTUOMKEEMF-RHYQMDGZSA-N 0.000 description 2
- ULBHWNVWSCJLCO-NHCYSSNCSA-N Arg-Val-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N ULBHWNVWSCJLCO-NHCYSSNCSA-N 0.000 description 2
- WONGRTVAMHFGBE-WDSKDSINSA-N Asn-Gly-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N WONGRTVAMHFGBE-WDSKDSINSA-N 0.000 description 2
- RBOBTTLFPRSXKZ-BZSNNMDCSA-N Asn-Phe-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RBOBTTLFPRSXKZ-BZSNNMDCSA-N 0.000 description 2
- MJIJBEYEHBKTIM-BYULHYEWSA-N Asn-Val-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N MJIJBEYEHBKTIM-BYULHYEWSA-N 0.000 description 2
- YNQIDCRRTWGHJD-ZLUOBGJFSA-N Asp-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(O)=O YNQIDCRRTWGHJD-ZLUOBGJFSA-N 0.000 description 2
- WSGVTKZFVJSJOG-RCOVLWMOSA-N Asp-Gly-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O WSGVTKZFVJSJOG-RCOVLWMOSA-N 0.000 description 2
- VNXQRBXEQXLERQ-CIUDSAMLSA-N Asp-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N VNXQRBXEQXLERQ-CIUDSAMLSA-N 0.000 description 2
- YIDFBWRHIYOYAA-LKXGYXEUSA-N Asp-Ser-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YIDFBWRHIYOYAA-LKXGYXEUSA-N 0.000 description 2
- YUELDQUPTAYEGM-XIRDDKMYSA-N Asp-Trp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC(=O)O)N YUELDQUPTAYEGM-XIRDDKMYSA-N 0.000 description 2
- QOJJMJKTMKNFEF-ZKWXMUAHSA-N Asp-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC(O)=O QOJJMJKTMKNFEF-ZKWXMUAHSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000271566 Aves Species 0.000 description 2
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 2
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 2
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- 206010057248 Cell death Diseases 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- NDUSUIGBMZCOIL-ZKWXMUAHSA-N Cys-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CS)N NDUSUIGBMZCOIL-ZKWXMUAHSA-N 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- CGVWDTRDPLOMHZ-FXQIFTODSA-N Gln-Glu-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O CGVWDTRDPLOMHZ-FXQIFTODSA-N 0.000 description 2
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 2
- XUMFMAVDHQDATI-DCAQKATOSA-N Gln-Pro-Arg Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O XUMFMAVDHQDATI-DCAQKATOSA-N 0.000 description 2
- MWMJCGBSIORNCD-AVGNSLFASA-N Glu-Leu-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O MWMJCGBSIORNCD-AVGNSLFASA-N 0.000 description 2
- QDMVXRNLOPTPIE-WDCWCFNPSA-N Glu-Lys-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QDMVXRNLOPTPIE-WDCWCFNPSA-N 0.000 description 2
- QNJNPKSWAHPYGI-JYJNAYRXSA-N Glu-Phe-Leu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 QNJNPKSWAHPYGI-JYJNAYRXSA-N 0.000 description 2
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 2
- ZALGPUWUVHOGAE-GVXVVHGQSA-N Glu-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZALGPUWUVHOGAE-GVXVVHGQSA-N 0.000 description 2
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 2
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 2
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 2
- 208000031886 HIV Infections Diseases 0.000 description 2
- ALPXXNRQBMRCPZ-MEYUZBJRSA-N His-Thr-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ALPXXNRQBMRCPZ-MEYUZBJRSA-N 0.000 description 2
- 101000716124 Homo sapiens T-cell surface glycoprotein CD1c Proteins 0.000 description 2
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 2
- RWIKBYVJQAJYDP-BJDJZHNGSA-N Ile-Ala-Lys Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN RWIKBYVJQAJYDP-BJDJZHNGSA-N 0.000 description 2
- VGSPNSSCMOHRRR-BJDJZHNGSA-N Ile-Ser-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N VGSPNSSCMOHRRR-BJDJZHNGSA-N 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- 108010065920 Insulin Lispro Proteins 0.000 description 2
- 102000014150 Interferons Human genes 0.000 description 2
- 108010050904 Interferons Proteins 0.000 description 2
- 108010066719 Interleukin Receptor Common gamma Subunit Proteins 0.000 description 2
- 102000018682 Interleukin Receptor Common gamma Subunit Human genes 0.000 description 2
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 2
- 102000010789 Interleukin-2 Receptors Human genes 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- 229930182816 L-glutamine Natural products 0.000 description 2
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 2
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 2
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 2
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 2
- 206010025327 Lymphopenia Diseases 0.000 description 2
- MPGHETGWWWUHPY-CIUDSAMLSA-N Lys-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN MPGHETGWWWUHPY-CIUDSAMLSA-N 0.000 description 2
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 2
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 2
- KWUKZRFFKPLUPE-HJGDQZAQSA-N Lys-Asp-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KWUKZRFFKPLUPE-HJGDQZAQSA-N 0.000 description 2
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 2
- CAVRAQIDHUPECU-UVOCVTCTSA-N Lys-Thr-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAVRAQIDHUPECU-UVOCVTCTSA-N 0.000 description 2
- XABXVVSWUVCZST-GVXVVHGQSA-N Lys-Val-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN XABXVVSWUVCZST-GVXVVHGQSA-N 0.000 description 2
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 2
- IHRFZLQEQVHXFA-RHYQMDGZSA-N Met-Thr-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCCN IHRFZLQEQVHXFA-RHYQMDGZSA-N 0.000 description 2
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 2
- 108010038807 Oligopeptides Proteins 0.000 description 2
- 102000015636 Oligopeptides Human genes 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 108090000526 Papain Proteins 0.000 description 2
- 102000057297 Pepsin A Human genes 0.000 description 2
- 108090000284 Pepsin A Proteins 0.000 description 2
- HXSUFWQYLPKEHF-IHRRRGAJSA-N Phe-Asn-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HXSUFWQYLPKEHF-IHRRRGAJSA-N 0.000 description 2
- BWTKUQPNOMMKMA-FIRPJDEBSA-N Phe-Ile-Phe Chemical compound C([C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 BWTKUQPNOMMKMA-FIRPJDEBSA-N 0.000 description 2
- MHHQQZIFLWFZGR-DCAQKATOSA-N Pro-Lys-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O MHHQQZIFLWFZGR-DCAQKATOSA-N 0.000 description 2
- GMJDSFYVTAMIBF-FXQIFTODSA-N Pro-Ser-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GMJDSFYVTAMIBF-FXQIFTODSA-N 0.000 description 2
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- 206010070834 Sensitisation Diseases 0.000 description 2
- YUSRGTQIPCJNHQ-CIUDSAMLSA-N Ser-Arg-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O YUSRGTQIPCJNHQ-CIUDSAMLSA-N 0.000 description 2
- MOVJSUIKUNCVMG-ZLUOBGJFSA-N Ser-Cys-Ser Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N)O MOVJSUIKUNCVMG-ZLUOBGJFSA-N 0.000 description 2
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 2
- QJKPECIAWNNKIT-KKUMJFAQSA-N Ser-Lys-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O QJKPECIAWNNKIT-KKUMJFAQSA-N 0.000 description 2
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 2
- NADLKBTYNKUJEP-KATARQTJSA-N Ser-Thr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O NADLKBTYNKUJEP-KATARQTJSA-N 0.000 description 2
- DYEGLQRVMBWQLD-IXOXFDKPSA-N Ser-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CO)N)O DYEGLQRVMBWQLD-IXOXFDKPSA-N 0.000 description 2
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 2
- DWYAUVCQDTZIJI-VZFHVOOUSA-N Thr-Ala-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O DWYAUVCQDTZIJI-VZFHVOOUSA-N 0.000 description 2
- GKWNLDNXMMLRMC-GLLZPBPUSA-N Thr-Glu-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O GKWNLDNXMMLRMC-GLLZPBPUSA-N 0.000 description 2
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 2
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 2
- 101710120037 Toxin CcdB Proteins 0.000 description 2
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 2
- SLCSPPCQWUHPPO-JYJNAYRXSA-N Tyr-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SLCSPPCQWUHPPO-JYJNAYRXSA-N 0.000 description 2
- JJNXZIPLIXIGBX-HJPIBITLSA-N Tyr-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N JJNXZIPLIXIGBX-HJPIBITLSA-N 0.000 description 2
- PWKMJDQXKCENMF-MEYUZBJRSA-N Tyr-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O PWKMJDQXKCENMF-MEYUZBJRSA-N 0.000 description 2
- 101150117115 V gene Proteins 0.000 description 2
- SCBITHMBEJNRHC-LSJOCFKGSA-N Val-Asp-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](C(C)C)C(=O)O)N SCBITHMBEJNRHC-LSJOCFKGSA-N 0.000 description 2
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 2
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 2
- FMQGYTMERWBMSI-HJWJTTGWSA-N Val-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N FMQGYTMERWBMSI-HJWJTTGWSA-N 0.000 description 2
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 2
- TVGWMCTYUFBXAP-QTKMDUPCSA-N Val-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N)O TVGWMCTYUFBXAP-QTKMDUPCSA-N 0.000 description 2
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 2
- ZHWZDZFWBXWPDW-GUBZILKMSA-N Val-Val-Cys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(O)=O ZHWZDZFWBXWPDW-GUBZILKMSA-N 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 230000000172 allergic effect Effects 0.000 description 2
- 230000000961 alloantigen Effects 0.000 description 2
- 235000012538 ammonium bicarbonate Nutrition 0.000 description 2
- 239000001099 ammonium carbonate Substances 0.000 description 2
- 239000012491 analyte Substances 0.000 description 2
- 230000009830 antibody antigen interaction Effects 0.000 description 2
- 210000000628 antibody-producing cell Anatomy 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000007975 buffered saline Substances 0.000 description 2
- 210000004899 c-terminal region Anatomy 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000002771 cell marker Substances 0.000 description 2
- 210000001072 colon Anatomy 0.000 description 2
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 2
- 125000000151 cysteine group Chemical class N[C@@H](CS)C(=O)* 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 239000010432 diamond Substances 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 238000006471 dimerization reaction Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 230000006862 enzymatic digestion Effects 0.000 description 2
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 2
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 239000013613 expression plasmid Substances 0.000 description 2
- 210000001723 extracellular space Anatomy 0.000 description 2
- 239000012091 fetal bovine serum Substances 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 102000044739 human CD1C Human genes 0.000 description 2
- 230000028996 humoral immune response Effects 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 239000012133 immunoprecipitate Substances 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 210000004969 inflammatory cell Anatomy 0.000 description 2
- 108010021315 integrin beta7 Proteins 0.000 description 2
- 229940079322 interferon Drugs 0.000 description 2
- 230000000968 intestinal effect Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 238000011835 investigation Methods 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 210000003563 lymphoid tissue Anatomy 0.000 description 2
- 231100001023 lymphopenia Toxicity 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 210000005087 mononuclear cell Anatomy 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 230000002018 overexpression Effects 0.000 description 2
- 229940055729 papain Drugs 0.000 description 2
- 235000019834 papain Nutrition 0.000 description 2
- 230000008506 pathogenesis Effects 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- 229940111202 pepsin Drugs 0.000 description 2
- 230000003285 pharmacodynamic effect Effects 0.000 description 2
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 230000007115 recruitment Effects 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 238000003118 sandwich ELISA Methods 0.000 description 2
- 238000010187 selection method Methods 0.000 description 2
- 230000008313 sensitization Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000012679 serum free medium Substances 0.000 description 2
- 208000002491 severe combined immunodeficiency Diseases 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- -1 succinimide ester Chemical class 0.000 description 2
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000002992 thymic effect Effects 0.000 description 2
- 239000003053 toxin Substances 0.000 description 2
- 231100000765 toxin Toxicity 0.000 description 2
- 108700012359 toxins Proteins 0.000 description 2
- 102000035160 transmembrane proteins Human genes 0.000 description 2
- 108091005703 transmembrane proteins Proteins 0.000 description 2
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 2
- 239000011534 wash buffer Substances 0.000 description 2
- 230000003442 weekly effect Effects 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- 102220510016 52 kDa repressor of the inhibitor of the protein kinase_V54I_mutation Human genes 0.000 description 1
- XYTNPQNAZREREP-XQXXSGGOSA-N Ala-Glu-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XYTNPQNAZREREP-XQXXSGGOSA-N 0.000 description 1
- LBFXVAXPDOBRKU-LKTVYLICSA-N Ala-His-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O LBFXVAXPDOBRKU-LKTVYLICSA-N 0.000 description 1
- SUMYEVXWCAYLLJ-GUBZILKMSA-N Ala-Leu-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O SUMYEVXWCAYLLJ-GUBZILKMSA-N 0.000 description 1
- SOBIAADAMRHGKH-CIUDSAMLSA-N Ala-Leu-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O SOBIAADAMRHGKH-CIUDSAMLSA-N 0.000 description 1
- MDNAVFBZPROEHO-DCAQKATOSA-N Ala-Lys-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O MDNAVFBZPROEHO-DCAQKATOSA-N 0.000 description 1
- MDNAVFBZPROEHO-UHFFFAOYSA-N Ala-Lys-Val Natural products CC(C)C(C(O)=O)NC(=O)C(NC(=O)C(C)N)CCCCN MDNAVFBZPROEHO-UHFFFAOYSA-N 0.000 description 1
- OSRZOHXQCUFIQG-FPMFFAJLSA-N Ala-Phe-Pro Chemical compound C([C@H](NC(=O)[C@@H]([NH3+])C)C(=O)N1[C@H](CCC1)C([O-])=O)C1=CC=CC=C1 OSRZOHXQCUFIQG-FPMFFAJLSA-N 0.000 description 1
- WQLDNOCHHRISMS-NAKRPEOUSA-N Ala-Pro-Ile Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WQLDNOCHHRISMS-NAKRPEOUSA-N 0.000 description 1
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 1
- RTZCUEHYUQZIDE-WHFBIAKZSA-N Ala-Ser-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RTZCUEHYUQZIDE-WHFBIAKZSA-N 0.000 description 1
- YJHKTAMKPGFJCT-NRPADANISA-N Ala-Val-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O YJHKTAMKPGFJCT-NRPADANISA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 108010063104 Apoptosis Regulatory Proteins Proteins 0.000 description 1
- 102000010565 Apoptosis Regulatory Proteins Human genes 0.000 description 1
- 241000239290 Araneae Species 0.000 description 1
- RWWPBOUMKFBHAL-FXQIFTODSA-N Arg-Asn-Cys Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(O)=O RWWPBOUMKFBHAL-FXQIFTODSA-N 0.000 description 1
- SVHRPCMZTWZROG-DCAQKATOSA-N Arg-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)N SVHRPCMZTWZROG-DCAQKATOSA-N 0.000 description 1
- PNQWAUXQDBIJDY-GUBZILKMSA-N Arg-Glu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PNQWAUXQDBIJDY-GUBZILKMSA-N 0.000 description 1
- AUFHLLPVPSMEOG-YUMQZZPRSA-N Arg-Gly-Glu Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O AUFHLLPVPSMEOG-YUMQZZPRSA-N 0.000 description 1
- GIMTZGADWZTZGV-DCAQKATOSA-N Arg-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N GIMTZGADWZTZGV-DCAQKATOSA-N 0.000 description 1
- LRPZJPMQGKGHSG-XGEHTFHBSA-N Arg-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N)O LRPZJPMQGKGHSG-XGEHTFHBSA-N 0.000 description 1
- OQPAZKMGCWPERI-GUBZILKMSA-N Arg-Ser-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OQPAZKMGCWPERI-GUBZILKMSA-N 0.000 description 1
- LYJXHXGPWDTLKW-HJGDQZAQSA-N Arg-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O LYJXHXGPWDTLKW-HJGDQZAQSA-N 0.000 description 1
- MOGMYRUNTKYZFB-UNQGMJICSA-N Arg-Thr-Phe Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MOGMYRUNTKYZFB-UNQGMJICSA-N 0.000 description 1
- INOIAEUXVVNJKA-XGEHTFHBSA-N Arg-Thr-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O INOIAEUXVVNJKA-XGEHTFHBSA-N 0.000 description 1
- ZUVMUOOHJYNJPP-XIRDDKMYSA-N Arg-Trp-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZUVMUOOHJYNJPP-XIRDDKMYSA-N 0.000 description 1
- NVGWESORMHFISY-SRVKXCTJSA-N Asn-Asn-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O NVGWESORMHFISY-SRVKXCTJSA-N 0.000 description 1
- OLVIPTLKNSAYRJ-YUMQZZPRSA-N Asn-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N OLVIPTLKNSAYRJ-YUMQZZPRSA-N 0.000 description 1
- RCFGLXMZDYNRSC-CIUDSAMLSA-N Asn-Lys-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O RCFGLXMZDYNRSC-CIUDSAMLSA-N 0.000 description 1
- ZNYKKCADEQAZKA-FXQIFTODSA-N Asn-Ser-Met Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(O)=O ZNYKKCADEQAZKA-FXQIFTODSA-N 0.000 description 1
- LKIYSIYBKYLKPU-BIIVOSGPSA-N Asp-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)O)N)C(=O)O LKIYSIYBKYLKPU-BIIVOSGPSA-N 0.000 description 1
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 1
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 1
- KNDCWFXCFKSEBM-AVGNSLFASA-N Asp-Tyr-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O KNDCWFXCFKSEBM-AVGNSLFASA-N 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 208000004736 B-Cell Leukemia Diseases 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 108010035053 B7-1 Antigen Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 229940124292 CD20 monoclonal antibody Drugs 0.000 description 1
- 108010063916 CD40 Antigens Proteins 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 206010010099 Combined immunodeficiency Diseases 0.000 description 1
- XGHYKIDVGYYHDC-JBDRJPRFSA-N Cys-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CS)N XGHYKIDVGYYHDC-JBDRJPRFSA-N 0.000 description 1
- WVLZTXGTNGHPBO-SRVKXCTJSA-N Cys-Leu-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O WVLZTXGTNGHPBO-SRVKXCTJSA-N 0.000 description 1
- XMVZMBGFIOQONW-GARJFASQSA-N Cys-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N)C(=O)O XMVZMBGFIOQONW-GARJFASQSA-N 0.000 description 1
- SWJYSDXMTPMBHO-FXQIFTODSA-N Cys-Pro-Ser Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SWJYSDXMTPMBHO-FXQIFTODSA-N 0.000 description 1
- VIOQRFNAZDMVLO-NRPADANISA-N Cys-Val-Glu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O VIOQRFNAZDMVLO-NRPADANISA-N 0.000 description 1
- 108010008286 DNA nucleotidylexotransferase Proteins 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 101710096438 DNA-binding protein Proteins 0.000 description 1
- 102100029764 DNA-directed DNA/RNA polymerase mu Human genes 0.000 description 1
- 244000000626 Daucus carota Species 0.000 description 1
- 235000002767 Daucus carota Nutrition 0.000 description 1
- 208000006313 Delayed Hypersensitivity Diseases 0.000 description 1
- 208000016192 Demyelinating disease Diseases 0.000 description 1
- 206010012438 Dermatitis atopic Diseases 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 102100037362 Fibronectin Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- WLODHVXYKYHLJD-ACZMJKKPSA-N Gln-Asp-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N WLODHVXYKYHLJD-ACZMJKKPSA-N 0.000 description 1
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 1
- LOJYQMFIIJVETK-WDSKDSINSA-N Gln-Gln Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(O)=O LOJYQMFIIJVETK-WDSKDSINSA-N 0.000 description 1
- ZNZPKVQURDQFFS-FXQIFTODSA-N Gln-Glu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZNZPKVQURDQFFS-FXQIFTODSA-N 0.000 description 1
- XKBASPWPBXNVLQ-WDSKDSINSA-N Gln-Gly-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O XKBASPWPBXNVLQ-WDSKDSINSA-N 0.000 description 1
- DYVMTEWCGAVKSE-HJGDQZAQSA-N Gln-Thr-Arg Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O DYVMTEWCGAVKSE-HJGDQZAQSA-N 0.000 description 1
- BETSEXMYBWCDAE-SZMVWBNQSA-N Gln-Trp-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N BETSEXMYBWCDAE-SZMVWBNQSA-N 0.000 description 1
- FITIQFSXXBKFFM-NRPADANISA-N Gln-Val-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FITIQFSXXBKFFM-NRPADANISA-N 0.000 description 1
- IESFZVCAVACGPH-PEFMBERDSA-N Glu-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(O)=O IESFZVCAVACGPH-PEFMBERDSA-N 0.000 description 1
- BFEZQZKEPRKKHV-SRVKXCTJSA-N Glu-Pro-Lys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)O)N)C(=O)N[C@@H](CCCCN)C(=O)O BFEZQZKEPRKKHV-SRVKXCTJSA-N 0.000 description 1
- QOXDAWODGSIDDI-GUBZILKMSA-N Glu-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N QOXDAWODGSIDDI-GUBZILKMSA-N 0.000 description 1
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 1
- QXUPRMQJDWJDFR-NRPADANISA-N Glu-Val-Ser Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXUPRMQJDWJDFR-NRPADANISA-N 0.000 description 1
- IXKRSKPKSLXIHN-YUMQZZPRSA-N Gly-Cys-Leu Chemical compound [H]NCC(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O IXKRSKPKSLXIHN-YUMQZZPRSA-N 0.000 description 1
- UFPXDFOYHVEIPI-BYPYZUCNSA-N Gly-Gly-Asp Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O UFPXDFOYHVEIPI-BYPYZUCNSA-N 0.000 description 1
- MTBIKIMYHUWBRX-QWRGUYRKSA-N Gly-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)CN MTBIKIMYHUWBRX-QWRGUYRKSA-N 0.000 description 1
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 1
- FGPLUIQCSKGLTI-WDSKDSINSA-N Gly-Ser-Glu Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O FGPLUIQCSKGLTI-WDSKDSINSA-N 0.000 description 1
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 1
- 208000009329 Graft vs Host Disease Diseases 0.000 description 1
- 108060005986 Granzyme Proteins 0.000 description 1
- 102000001398 Granzyme Human genes 0.000 description 1
- 208000006968 Helminthiasis Diseases 0.000 description 1
- MAABHGXCIBEYQR-XVYDVKMFSA-N His-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MAABHGXCIBEYQR-XVYDVKMFSA-N 0.000 description 1
- HVCRQRQPIIRNLY-IUCAKERBSA-N His-Gln-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)NCC(=O)O)N HVCRQRQPIIRNLY-IUCAKERBSA-N 0.000 description 1
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 1
- SDTPKSOWFXBACN-GUBZILKMSA-N His-Glu-Asp Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O SDTPKSOWFXBACN-GUBZILKMSA-N 0.000 description 1
- BRQKGRLDDDQWQJ-MBLNEYKQSA-N His-Thr-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O BRQKGRLDDDQWQJ-MBLNEYKQSA-N 0.000 description 1
- CSTDQOOBZBAJKE-BWAGICSOSA-N His-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CN=CN2)N)O CSTDQOOBZBAJKE-BWAGICSOSA-N 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101001002508 Homo sapiens Immunoglobulin-binding protein 1 Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101001008922 Homo sapiens Kallikrein-11 Proteins 0.000 description 1
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 description 1
- 101100495232 Homo sapiens MS4A1 gene Proteins 0.000 description 1
- 101000997835 Homo sapiens Tyrosine-protein kinase JAK1 Proteins 0.000 description 1
- 101000997832 Homo sapiens Tyrosine-protein kinase JAK2 Proteins 0.000 description 1
- 101000934996 Homo sapiens Tyrosine-protein kinase JAK3 Proteins 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- XMYURPUVJSKTMC-KBIXCLLPSA-N Ile-Ser-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N XMYURPUVJSKTMC-KBIXCLLPSA-N 0.000 description 1
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 1
- KBDIBHQICWDGDL-PPCPHDFISA-N Ile-Thr-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N KBDIBHQICWDGDL-PPCPHDFISA-N 0.000 description 1
- RQZFWBLDTBDEOF-RNJOBUHISA-N Ile-Val-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N RQZFWBLDTBDEOF-RNJOBUHISA-N 0.000 description 1
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 1
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 1
- 102100021042 Immunoglobulin-binding protein 1 Human genes 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 108010041012 Integrin alpha4 Proteins 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 102100027612 Kallikrein-11 Human genes 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 125000000415 L-cysteinyl group Chemical group O=C([*])[C@@](N([H])[H])([H])C([H])([H])S[H] 0.000 description 1
- LEVWYRKDKASIDU-IMJSIDKUSA-N L-cystine Chemical compound [O-]C(=O)[C@@H]([NH3+])CSSC[C@H]([NH3+])C([O-])=O LEVWYRKDKASIDU-IMJSIDKUSA-N 0.000 description 1
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 1
- 102000017578 LAG3 Human genes 0.000 description 1
- 101150030213 Lag3 gene Proteins 0.000 description 1
- 235000019687 Lamb Nutrition 0.000 description 1
- STAVRDQLZOTNKJ-RHYQMDGZSA-N Leu-Arg-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O STAVRDQLZOTNKJ-RHYQMDGZSA-N 0.000 description 1
- TWQIYNGNYNJUFM-NHCYSSNCSA-N Leu-Asn-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O TWQIYNGNYNJUFM-NHCYSSNCSA-N 0.000 description 1
- VWHGTYCRDRBSFI-ZETCQYMHSA-N Leu-Gly-Gly Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(O)=O VWHGTYCRDRBSFI-ZETCQYMHSA-N 0.000 description 1
- BTNXKBVLWJBTNR-SRVKXCTJSA-N Leu-His-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O BTNXKBVLWJBTNR-SRVKXCTJSA-N 0.000 description 1
- AUNMOHYWTAPQLA-XUXIUFHCSA-N Leu-Met-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O AUNMOHYWTAPQLA-XUXIUFHCSA-N 0.000 description 1
- WMIOEVKKYIMVKI-DCAQKATOSA-N Leu-Pro-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O WMIOEVKKYIMVKI-DCAQKATOSA-N 0.000 description 1
- CHJKEDSZNSONPS-DCAQKATOSA-N Leu-Pro-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O CHJKEDSZNSONPS-DCAQKATOSA-N 0.000 description 1
- SBANPBVRHYIMRR-GARJFASQSA-N Leu-Ser-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N SBANPBVRHYIMRR-GARJFASQSA-N 0.000 description 1
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 1
- QWWPYKKLXWOITQ-VOAKCMCISA-N Leu-Thr-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(C)C QWWPYKKLXWOITQ-VOAKCMCISA-N 0.000 description 1
- DAYQSYGBCUKVKT-VOAKCMCISA-N Leu-Thr-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O DAYQSYGBCUKVKT-VOAKCMCISA-N 0.000 description 1
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 1
- 102000019298 Lipocalin Human genes 0.000 description 1
- 108050006654 Lipocalin Proteins 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 102100020862 Lymphocyte activation gene 3 protein Human genes 0.000 description 1
- NTSPQIONFJUMJV-AVGNSLFASA-N Lys-Arg-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O NTSPQIONFJUMJV-AVGNSLFASA-N 0.000 description 1
- NRQRKMYZONPCTM-CIUDSAMLSA-N Lys-Asp-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O NRQRKMYZONPCTM-CIUDSAMLSA-N 0.000 description 1
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 1
- PBIPLDMFHAICIP-DCAQKATOSA-N Lys-Glu-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PBIPLDMFHAICIP-DCAQKATOSA-N 0.000 description 1
- ODUQLUADRKMHOZ-JYJNAYRXSA-N Lys-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)N)O ODUQLUADRKMHOZ-JYJNAYRXSA-N 0.000 description 1
- GQFDWEDHOQRNLC-QWRGUYRKSA-N Lys-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN GQFDWEDHOQRNLC-QWRGUYRKSA-N 0.000 description 1
- QQPSCXKFDSORFT-IHRRRGAJSA-N Lys-Lys-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN QQPSCXKFDSORFT-IHRRRGAJSA-N 0.000 description 1
- ODTZHNZPINULEU-KKUMJFAQSA-N Lys-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N ODTZHNZPINULEU-KKUMJFAQSA-N 0.000 description 1
- MIFFFXHMAHFACR-KATARQTJSA-N Lys-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN MIFFFXHMAHFACR-KATARQTJSA-N 0.000 description 1
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 1
- UROWNMBTQGGTHB-DCAQKATOSA-N Met-Leu-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O UROWNMBTQGGTHB-DCAQKATOSA-N 0.000 description 1
- LPNWWHBFXPNHJG-AVGNSLFASA-N Met-Val-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN LPNWWHBFXPNHJG-AVGNSLFASA-N 0.000 description 1
- 102000007474 Multiprotein Complexes Human genes 0.000 description 1
- 108010085220 Multiprotein Complexes Proteins 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 108010047562 NGR peptide Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108010067902 Peptide Library Proteins 0.000 description 1
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 1
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 1
- MRWOVVNKSXXLRP-IHPCNDPISA-N Phe-Ser-Trp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O MRWOVVNKSXXLRP-IHPCNDPISA-N 0.000 description 1
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 1
- JSGWNFKWZNPDAV-YDHLFZDLSA-N Phe-Val-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 JSGWNFKWZNPDAV-YDHLFZDLSA-N 0.000 description 1
- 231100000742 Plant toxin Toxicity 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 208000009052 Precursor T-Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 208000017414 Precursor T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- XQLBWXHVZVBNJM-FXQIFTODSA-N Pro-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 XQLBWXHVZVBNJM-FXQIFTODSA-N 0.000 description 1
- GDXZRWYXJSGWIV-GMOBBJLQSA-N Pro-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@@H]1CCCN1 GDXZRWYXJSGWIV-GMOBBJLQSA-N 0.000 description 1
- UPJGUQPLYWTISV-GUBZILKMSA-N Pro-Gln-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UPJGUQPLYWTISV-GUBZILKMSA-N 0.000 description 1
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 1
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 1
- NMELOOXSGDRBRU-YUMQZZPRSA-N Pro-Glu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@@H]1CCCN1 NMELOOXSGDRBRU-YUMQZZPRSA-N 0.000 description 1
- NXEYSLRNNPWCRN-SRVKXCTJSA-N Pro-Glu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NXEYSLRNNPWCRN-SRVKXCTJSA-N 0.000 description 1
- WFHYFCWBLSKEMS-KKUMJFAQSA-N Pro-Glu-Phe Chemical compound N([C@@H](CCC(=O)O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C(=O)[C@@H]1CCCN1 WFHYFCWBLSKEMS-KKUMJFAQSA-N 0.000 description 1
- UIMCLYYSUCIUJM-UWVGGRQHSA-N Pro-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 UIMCLYYSUCIUJM-UWVGGRQHSA-N 0.000 description 1
- AFXCXDQNRXTSBD-FJXKBIBVSA-N Pro-Gly-Thr Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O AFXCXDQNRXTSBD-FJXKBIBVSA-N 0.000 description 1
- LPGSNRSLPHRNBW-AVGNSLFASA-N Pro-His-Val Chemical compound C([C@@H](C(=O)N[C@@H](C(C)C)C([O-])=O)NC(=O)[C@H]1[NH2+]CCC1)C1=CN=CN1 LPGSNRSLPHRNBW-AVGNSLFASA-N 0.000 description 1
- VZKBJNBZMZHKRC-XUXIUFHCSA-N Pro-Ile-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O VZKBJNBZMZHKRC-XUXIUFHCSA-N 0.000 description 1
- ZLXKLMHAMDENIO-DCAQKATOSA-N Pro-Lys-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLXKLMHAMDENIO-DCAQKATOSA-N 0.000 description 1
- WLJYLAQSUSIQNH-GUBZILKMSA-N Pro-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@@H]1CCCN1 WLJYLAQSUSIQNH-GUBZILKMSA-N 0.000 description 1
- RFWXYTJSVDUBBZ-DCAQKATOSA-N Pro-Pro-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 RFWXYTJSVDUBBZ-DCAQKATOSA-N 0.000 description 1
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 1
- KBUAPZAZPWNYSW-SRVKXCTJSA-N Pro-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KBUAPZAZPWNYSW-SRVKXCTJSA-N 0.000 description 1
- XDKKMRPRRCOELJ-GUBZILKMSA-N Pro-Val-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 XDKKMRPRRCOELJ-GUBZILKMSA-N 0.000 description 1
- 108010090931 Proto-Oncogene Proteins c-bcl-2 Proteins 0.000 description 1
- 102000013535 Proto-Oncogene Proteins c-bcl-2 Human genes 0.000 description 1
- 108700033844 Pseudomonas aeruginosa toxA Proteins 0.000 description 1
- LCTONWCANYUPML-UHFFFAOYSA-M Pyruvate Chemical compound CC(=O)C([O-])=O LCTONWCANYUPML-UHFFFAOYSA-M 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 101710146873 Receptor-binding protein Proteins 0.000 description 1
- 208000007400 Relapsing-Remitting Multiple Sclerosis Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 108010039491 Ricin Proteins 0.000 description 1
- 108010072819 STAT Transcription Factors Proteins 0.000 description 1
- 102000007078 STAT Transcription Factors Human genes 0.000 description 1
- 108010044012 STAT1 Transcription Factor Proteins 0.000 description 1
- 102000006381 STAT1 Transcription Factor Human genes 0.000 description 1
- 108010017324 STAT3 Transcription Factor Proteins 0.000 description 1
- 108010084592 Saporins Proteins 0.000 description 1
- MWMKFWJYRRGXOR-ZLUOBGJFSA-N Ser-Ala-Asn Chemical compound N[C@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)O)CC(N)=O)C)CO MWMKFWJYRRGXOR-ZLUOBGJFSA-N 0.000 description 1
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 1
- KAAPNMOKUUPKOE-SRVKXCTJSA-N Ser-Asn-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KAAPNMOKUUPKOE-SRVKXCTJSA-N 0.000 description 1
- BNFVPSRLHHPQKS-WHFBIAKZSA-N Ser-Asp-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O BNFVPSRLHHPQKS-WHFBIAKZSA-N 0.000 description 1
- HEQPKICPPDOSIN-SRVKXCTJSA-N Ser-Asp-Tyr Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HEQPKICPPDOSIN-SRVKXCTJSA-N 0.000 description 1
- KJMOINFQVCCSDX-XKBZYTNZSA-N Ser-Gln-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KJMOINFQVCCSDX-XKBZYTNZSA-N 0.000 description 1
- LALNXSXEYFUUDD-GUBZILKMSA-N Ser-Glu-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LALNXSXEYFUUDD-GUBZILKMSA-N 0.000 description 1
- VQBCMLMPEWPUTB-ACZMJKKPSA-N Ser-Glu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O VQBCMLMPEWPUTB-ACZMJKKPSA-N 0.000 description 1
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 1
- DJACUBDEDBZKLQ-KBIXCLLPSA-N Ser-Ile-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O DJACUBDEDBZKLQ-KBIXCLLPSA-N 0.000 description 1
- XNCUYZKGQOCOQH-YUMQZZPRSA-N Ser-Leu-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O XNCUYZKGQOCOQH-YUMQZZPRSA-N 0.000 description 1
- IUXGJEIKJBYKOO-SRVKXCTJSA-N Ser-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CO)N IUXGJEIKJBYKOO-SRVKXCTJSA-N 0.000 description 1
- GZSZPKSBVAOGIE-CIUDSAMLSA-N Ser-Lys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O GZSZPKSBVAOGIE-CIUDSAMLSA-N 0.000 description 1
- XUDRHBPSPAPDJP-SRVKXCTJSA-N Ser-Lys-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO XUDRHBPSPAPDJP-SRVKXCTJSA-N 0.000 description 1
- GDUZTEQRAOXYJS-SRVKXCTJSA-N Ser-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GDUZTEQRAOXYJS-SRVKXCTJSA-N 0.000 description 1
- RRVFEDGUXSYWOW-BZSNNMDCSA-N Ser-Phe-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RRVFEDGUXSYWOW-BZSNNMDCSA-N 0.000 description 1
- GZGFSPWOMUKKCV-NAKRPEOUSA-N Ser-Pro-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO GZGFSPWOMUKKCV-NAKRPEOUSA-N 0.000 description 1
- FLONGDPORFIVQW-XGEHTFHBSA-N Ser-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO FLONGDPORFIVQW-XGEHTFHBSA-N 0.000 description 1
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 1
- PCJLFYBAQZQOFE-KATARQTJSA-N Ser-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N)O PCJLFYBAQZQOFE-KATARQTJSA-N 0.000 description 1
- ZVBCMFDJIMUELU-BZSNNMDCSA-N Ser-Tyr-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CO)N ZVBCMFDJIMUELU-BZSNNMDCSA-N 0.000 description 1
- BEBVVQPDSHHWQL-NRPADANISA-N Ser-Val-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O BEBVVQPDSHHWQL-NRPADANISA-N 0.000 description 1
- RCOUFINCYASMDN-GUBZILKMSA-N Ser-Val-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O RCOUFINCYASMDN-GUBZILKMSA-N 0.000 description 1
- ODRUTDLAONAVDV-IHRRRGAJSA-N Ser-Val-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ODRUTDLAONAVDV-IHRRRGAJSA-N 0.000 description 1
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 1
- 208000009359 Sezary Syndrome Diseases 0.000 description 1
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 1
- 206010040914 Skin reaction Diseases 0.000 description 1
- 241000205098 Sulfolobus acidocaldarius Species 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 230000005867 T cell response Effects 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 1
- 208000029052 T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- LVHHEVGYAZGXDE-KDXUFGMBSA-N Thr-Ala-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(=O)O)N)O LVHHEVGYAZGXDE-KDXUFGMBSA-N 0.000 description 1
- JEDIEMIJYSRUBB-FOHZUACHSA-N Thr-Asp-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O JEDIEMIJYSRUBB-FOHZUACHSA-N 0.000 description 1
- ODSAPYVQSLDRSR-LKXGYXEUSA-N Thr-Cys-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O ODSAPYVQSLDRSR-LKXGYXEUSA-N 0.000 description 1
- FIFDDJFLNVAVMS-RHYQMDGZSA-N Thr-Leu-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O FIFDDJFLNVAVMS-RHYQMDGZSA-N 0.000 description 1
- YJVJPJPHHFOVMG-VEVYYDQMSA-N Thr-Met-Asp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)O YJVJPJPHHFOVMG-VEVYYDQMSA-N 0.000 description 1
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 1
- GFRIEEKFXOVPIR-RHYQMDGZSA-N Thr-Pro-Lys Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O GFRIEEKFXOVPIR-RHYQMDGZSA-N 0.000 description 1
- WPSKTVVMQCXPRO-BWBBJGPYSA-N Thr-Ser-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WPSKTVVMQCXPRO-BWBBJGPYSA-N 0.000 description 1
- VGNKUXWYFFDWDH-BEMMVCDISA-N Thr-Trp-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N3CCC[C@@H]3C(=O)O)N)O VGNKUXWYFFDWDH-BEMMVCDISA-N 0.000 description 1
- RPECVQBNONKZAT-WZLNRYEVSA-N Thr-Tyr-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H]([C@@H](C)O)N RPECVQBNONKZAT-WZLNRYEVSA-N 0.000 description 1
- LVRFMARKDGGZMX-IZPVPAKOSA-N Thr-Tyr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)O)C(O)=O)CC1=CC=C(O)C=C1 LVRFMARKDGGZMX-IZPVPAKOSA-N 0.000 description 1
- FYBFTPLPAXZBOY-KKHAAJSZSA-N Thr-Val-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O FYBFTPLPAXZBOY-KKHAAJSZSA-N 0.000 description 1
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 1
- 102220536881 Transcription factor JunD_Y77F_mutation Human genes 0.000 description 1
- BORCDLUWGBGTKL-XIRDDKMYSA-N Trp-Gln-Met Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O)=CNC2=C1 BORCDLUWGBGTKL-XIRDDKMYSA-N 0.000 description 1
- UDCHKDYNMRJYMI-QEJZJMRPSA-N Trp-Glu-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UDCHKDYNMRJYMI-QEJZJMRPSA-N 0.000 description 1
- QJBWZNTWJSZUOY-UWJYBYFXSA-N Tyr-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N QJBWZNTWJSZUOY-UWJYBYFXSA-N 0.000 description 1
- GFHYISDTIWZUSU-QWRGUYRKSA-N Tyr-Asn-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O GFHYISDTIWZUSU-QWRGUYRKSA-N 0.000 description 1
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 1
- BIWVVOHTKDLRMP-ULQDDVLXSA-N Tyr-Pro-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O BIWVVOHTKDLRMP-ULQDDVLXSA-N 0.000 description 1
- XFEMMSGONWQACR-KJEVXHAQSA-N Tyr-Thr-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O XFEMMSGONWQACR-KJEVXHAQSA-N 0.000 description 1
- OJCISMMNNUNNJA-BZSNNMDCSA-N Tyr-Tyr-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 OJCISMMNNUNNJA-BZSNNMDCSA-N 0.000 description 1
- KHPLUFDSWGDRHD-SLFFLAALSA-N Tyr-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N)C(=O)O KHPLUFDSWGDRHD-SLFFLAALSA-N 0.000 description 1
- PQPWEALFTLKSEB-DZKIICNBSA-N Tyr-Val-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O PQPWEALFTLKSEB-DZKIICNBSA-N 0.000 description 1
- 102100033438 Tyrosine-protein kinase JAK1 Human genes 0.000 description 1
- 102100033444 Tyrosine-protein kinase JAK2 Human genes 0.000 description 1
- 102100025387 Tyrosine-protein kinase JAK3 Human genes 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- REJBPZVUHYNMEN-LSJOCFKGSA-N Val-Ala-His Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N REJBPZVUHYNMEN-LSJOCFKGSA-N 0.000 description 1
- CGGVNFJRZJUVAE-BYULHYEWSA-N Val-Asp-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CGGVNFJRZJUVAE-BYULHYEWSA-N 0.000 description 1
- DIOSYUIWOQCXNR-ONGXEEELSA-N Val-Lys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O DIOSYUIWOQCXNR-ONGXEEELSA-N 0.000 description 1
- SBJCTAZFSZXWSR-AVGNSLFASA-N Val-Met-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N SBJCTAZFSZXWSR-AVGNSLFASA-N 0.000 description 1
- HJSLDXZAZGFPDK-ULQDDVLXSA-N Val-Phe-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N HJSLDXZAZGFPDK-ULQDDVLXSA-N 0.000 description 1
- KSFXWENSJABBFI-ZKWXMUAHSA-N Val-Ser-Asn Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KSFXWENSJABBFI-ZKWXMUAHSA-N 0.000 description 1
- VHIZXDZMTDVFGX-DCAQKATOSA-N Val-Ser-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N VHIZXDZMTDVFGX-DCAQKATOSA-N 0.000 description 1
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 1
- UVHFONIHVHLDDQ-IFFSRLJSSA-N Val-Thr-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O UVHFONIHVHLDDQ-IFFSRLJSSA-N 0.000 description 1
- LCHZBEUVGAVMKS-RHYQMDGZSA-N Val-Thr-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(O)=O LCHZBEUVGAVMKS-RHYQMDGZSA-N 0.000 description 1
- OFTXTCGQJXTNQS-XGEHTFHBSA-N Val-Thr-Ser Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](C(C)C)N)O OFTXTCGQJXTNQS-XGEHTFHBSA-N 0.000 description 1
- UEXPMFIAZZHEAD-HSHDSVGOSA-N Val-Thr-Trp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](C(C)C)N)O UEXPMFIAZZHEAD-HSHDSVGOSA-N 0.000 description 1
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 1
- SSKKGOWRPNIVDW-AVGNSLFASA-N Val-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N SSKKGOWRPNIVDW-AVGNSLFASA-N 0.000 description 1
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 1
- 235000010724 Wisteria floribunda Nutrition 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- 108010044940 alanylglutamine Proteins 0.000 description 1
- 108010047495 alanylglycine Proteins 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 108010029539 arginyl-prolyl-proline Proteins 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 239000012131 assay buffer Substances 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 201000008937 atopic dermatitis Diseases 0.000 description 1
- 239000012752 auxiliary agent Substances 0.000 description 1
- 210000003050 axon Anatomy 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 108700000707 bcl-2-Associated X Proteins 0.000 description 1
- 102000055102 bcl-2-Associated X Human genes 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229940046731 calcineurin inhibitors Drugs 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000007910 cell fusion Effects 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000010001 cellular homeostasis Effects 0.000 description 1
- 230000030570 cellular localization Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 230000035605 chemotaxis Effects 0.000 description 1
- 208000018805 childhood acute lymphoblastic leukemia Diseases 0.000 description 1
- 201000011633 childhood acute lymphocytic leukemia Diseases 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000004940 costimulation Effects 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 230000037029 cross reaction Effects 0.000 description 1
- 238000012926 crystallographic analysis Methods 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 238000004163 cytometry Methods 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- SUYVUBYJARFZHO-RRKCRQDMSA-N dATP Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-RRKCRQDMSA-N 0.000 description 1
- SUYVUBYJARFZHO-UHFFFAOYSA-N dATP Natural products C1=NC=2C(N)=NC=NC=2N1C1CC(O)C(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-UHFFFAOYSA-N 0.000 description 1
- 230000002354 daily effect Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000006240 deamidation Effects 0.000 description 1
- 230000005860 defense response to virus Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000003210 demyelinating effect Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- KZNICNPSHKQLFF-UHFFFAOYSA-N dihydromaleimide Natural products O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000012156 elution solvent Substances 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 238000002695 general anesthesia Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 108010079547 glutamylmethionine Proteins 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 1
- 108010051307 glycyl-glycyl-proline Proteins 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 108010087823 glycyltyrosine Proteins 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 108010092114 histidylphenylalanine Proteins 0.000 description 1
- 102000045535 human TSLP Human genes 0.000 description 1
- 230000008348 humoral response Effects 0.000 description 1
- 230000000521 hyperimmunizing effect Effects 0.000 description 1
- 210000003297 immature b lymphocyte Anatomy 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 238000002991 immunohistochemical analysis Methods 0.000 description 1
- 210000000428 immunological synapse Anatomy 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 229940124589 immunosuppressive drug Drugs 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 102000002467 interleukin receptors Human genes 0.000 description 1
- 108010093036 interleukin receptors Proteins 0.000 description 1
- 208000028774 intestinal disease Diseases 0.000 description 1
- 230000008944 intestinal immunity Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 230000035987 intoxication Effects 0.000 description 1
- 231100000566 intoxication Toxicity 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 1
- 108010027338 isoleucylcysteine Proteins 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000000207 lymphocyte subset Anatomy 0.000 description 1
- 230000000329 lymphopenic effect Effects 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 201000005962 mycosis fungoides Diseases 0.000 description 1
- 238000007857 nested PCR Methods 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 208000014837 parasitic helminthiasis infectious disease Diseases 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 229930192851 perforin Natural products 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 108010018625 phenylalanylarginine Proteins 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000003123 plant toxin Substances 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 230000003334 potential effect Effects 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 230000000861 pro-apoptotic effect Effects 0.000 description 1
- 210000001948 pro-b lymphocyte Anatomy 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 125000006239 protecting group Chemical group 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000006916 protein interaction Effects 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000007420 reactivation Effects 0.000 description 1
- 230000012121 regulation of immune response Effects 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 230000008521 reorganization Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 102220212795 rs1060501982 Human genes 0.000 description 1
- 102220065731 rs140562402 Human genes 0.000 description 1
- 102220011217 rs281865122 Human genes 0.000 description 1
- 102200026976 rs35086888 Human genes 0.000 description 1
- 102220058924 rs749212640 Human genes 0.000 description 1
- 102220340177 rs782563826 Human genes 0.000 description 1
- 102220097502 rs876660844 Human genes 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 238000007390 skin biopsy Methods 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 231100000430 skin reaction Toxicity 0.000 description 1
- 230000035483 skin reaction Effects 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000000547 structure data Methods 0.000 description 1
- 229960002317 succinimide Drugs 0.000 description 1
- 239000013595 supernatant sample Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 210000000225 synapse Anatomy 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000012085 test solution Substances 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 108010031491 threonyl-lysyl-glutamic acid Proteins 0.000 description 1
- 230000003614 tolerogenic effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 108091006106 transcriptional activators Proteins 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 108010079202 tyrosyl-alanyl-cysteine Proteins 0.000 description 1
- 108010020532 tyrosyl-proline Proteins 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 239000013585 weight reducing agent Substances 0.000 description 1
- 230000036642 wellbeing Effects 0.000 description 1
- 108010027345 wheylin-1 peptide Proteins 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2866—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for cytokines, lymphokines, interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/502—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects
- G01N33/5041—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects involving analysis of members of signalling pathways
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6854—Immunoglobulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/52—Assays involving cytokines
- G01N2333/54—Interleukins [IL]
- G01N2333/5418—IL-7
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/70546—Integrin superfamily, e.g. VLAs, leuCAM, GPIIb/GPIIIa, LPAM
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/715—Assays involving receptors, cell surface antigens or cell surface determinants for cytokines; for lymphokines; for interferons
- G01N2333/7155—Assays involving receptors, cell surface antigens or cell surface determinants for cytokines; for lymphokines; for interferons for interleukins [IL]
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2500/00—Screening for compounds of potential therapeutic value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Biochemistry (AREA)
- Diabetes (AREA)
- Urology & Nephrology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Microbiology (AREA)
- Endocrinology (AREA)
- Rheumatology (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Physics & Mathematics (AREA)
- Oncology (AREA)
- Pulmonology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Toxicology (AREA)
- Pain & Pain Management (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462010117P | 2014-06-10 | 2014-06-10 | |
| US62/010,117 | 2014-06-10 | ||
| EP15305078.6 | 2015-01-23 | ||
| EP15305078.6A EP2955196A1 (en) | 2014-06-10 | 2015-01-23 | Antibodies directed against CD127 |
| PCT/EP2015/062993 WO2015189302A1 (en) | 2014-06-10 | 2015-06-10 | Antibodies directed against cd127 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| RU2016151265A RU2016151265A (ru) | 2018-07-18 |
| RU2016151265A3 RU2016151265A3 (enExample) | 2019-02-08 |
| RU2734076C2 true RU2734076C2 (ru) | 2020-10-12 |
Family
ID=52434718
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2016151265A RU2734076C2 (ru) | 2014-06-10 | 2015-06-10 | Антитела, направленные на cd127 |
Country Status (26)
| Country | Link |
|---|---|
| US (3) | US10428152B2 (enExample) |
| EP (2) | EP2955196A1 (enExample) |
| JP (2) | JP7324565B2 (enExample) |
| KR (1) | KR102612930B1 (enExample) |
| CN (1) | CN106715471B (enExample) |
| AP (1) | AP2016009599A0 (enExample) |
| AU (1) | AU2015273532C1 (enExample) |
| BR (1) | BR112016028755B1 (enExample) |
| CA (1) | CA2950823A1 (enExample) |
| CL (1) | CL2016003172A1 (enExample) |
| CR (1) | CR20160576A (enExample) |
| EA (1) | EA039303B1 (enExample) |
| HK (1) | HK1231487A1 (enExample) |
| IL (1) | IL249449B (enExample) |
| MA (1) | MA40202A (enExample) |
| MX (1) | MX376066B (enExample) |
| MY (1) | MY190889A (enExample) |
| NZ (1) | NZ726932A (enExample) |
| PE (1) | PE20170324A1 (enExample) |
| PH (1) | PH12016502445A1 (enExample) |
| RU (1) | RU2734076C2 (enExample) |
| SA (1) | SA516380455B1 (enExample) |
| SG (1) | SG11201610036PA (enExample) |
| TN (1) | TN2016000528A1 (enExample) |
| UA (1) | UA125366C2 (enExample) |
| WO (1) | WO2015189302A1 (enExample) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SG10201606970UA (en) | 2012-02-23 | 2016-10-28 | Juno Therapeutics Gmbh | Chromatographic isolation of cells and other complex biological materials |
| EP2955196A1 (en) * | 2014-06-10 | 2015-12-16 | Effimune | Antibodies directed against CD127 |
| WO2016156466A1 (en) | 2015-03-31 | 2016-10-06 | Vhsquared Limited | Peptide construct having a protease-cleavable linker |
| CA2981103A1 (en) | 2015-03-31 | 2016-10-06 | Vhsquared Limited | Polypeptides |
| MA45488A (fr) | 2015-10-22 | 2018-08-29 | Juno Therapeutics Gmbh | Procédés, kits et appareil de culture de cellules |
| WO2017125578A1 (en) | 2016-01-21 | 2017-07-27 | Vhsquared Limited | Polypeptides |
| PL3423496T3 (pl) | 2016-02-29 | 2020-01-31 | Ose Immunotherapeutics | Nieantagonistyczne przeciwciała skierowane przeciw łańcuchowi alfa domeny zewnątrzkomórkowej receptora il7 i ich zastosowanie w leczeniu raka |
| PE20191152A1 (es) * | 2016-12-09 | 2019-09-05 | Ose Immunotherapeutics | Anticuerpos y polipeptidos dirigidos contra cd127 |
| WO2019043065A1 (en) * | 2017-08-29 | 2019-03-07 | Ose Immunotherapeutics | METHOD AND PREPARATION FOR SORTING T EFFECTOR LYMPHOCYTES USING ANTI-CD127 ANTIBODIES FOR CELL THERAPY APPLICATIONS |
| CN110117325B (zh) * | 2018-03-09 | 2023-06-20 | 重庆市畜牧科学院 | 一种猪cd127多肽及其编码基因和应用 |
| BR112021014106A2 (pt) | 2019-01-22 | 2021-10-13 | Bristol-Myers Squibb Company | Anticorpos contra subunidade alfa de il-7r e usos dos mesmos |
| JP7137696B2 (ja) | 2019-05-14 | 2022-09-14 | プロヴェンション・バイオ・インコーポレイテッド | 1型糖尿病を予防するための方法および組成物 |
| KR20220064953A (ko) | 2019-06-21 | 2022-05-19 | 소리소 파마슈티컬스 인크. | 폴리펩티드 |
| CN114555643B (zh) | 2019-06-21 | 2025-02-18 | 索瑞索制药公司 | 组合物 |
| EP3986571A1 (en) * | 2019-06-21 | 2022-04-27 | Sorriso Pharmaceuticals, Inc. | Polypeptides |
| CN110894237B (zh) * | 2019-12-05 | 2021-08-03 | 山东省分析测试中心 | 抗cd127的抗体、分泌该抗体的细胞株及其制备方法和应用 |
| CA3181394A1 (en) * | 2020-04-27 | 2021-11-04 | Memorial Sloan-Kettering Cancer Center | Chimeric antigen receptors targeting cd127 and use thereof |
| KR20230092863A (ko) | 2020-06-11 | 2023-06-26 | 프로벤션 바이오, 인코포레이티드 | 제1형 당뇨병을 예방하기 위한 방법 및 조성물 |
| US20220389104A1 (en) | 2021-05-28 | 2022-12-08 | Ose Immunotherapeutics | Method for Treating CD127-Positive Cancers by Administering an Anti-CD127 Agent |
| JP2025518164A (ja) | 2022-05-30 | 2025-06-12 | オセ イムノセラピューティクス | Il7rモジュレーター活性のバイオマーカー |
| CN115925955B (zh) * | 2022-12-30 | 2025-09-26 | 浙江正熙生物技术有限公司 | 一种抗cd127抗体及其制备方法和应用 |
| WO2024146955A1 (en) | 2023-01-06 | 2024-07-11 | Twain Therapeutics Pte. Ltd. | Antigen-binding molecules |
| EP4455308A1 (en) | 2023-04-24 | 2024-10-30 | Les Laboratoires Servier | Il-7r gene signatures |
| WO2024249954A1 (en) | 2023-05-31 | 2024-12-05 | Capstan Therapeutics, Inc. | Lipid nanoparticle formulations and compositions |
| WO2025076127A1 (en) | 2023-10-05 | 2025-04-10 | Capstan Therapeutics, Inc. | Constrained ionizable cationic lipids and lipid nanoparticles |
| WO2025076113A1 (en) | 2023-10-05 | 2025-04-10 | Capstan Therapeutics, Inc. | Ionizable cationic lipids with conserved spacing and lipid nanoparticles |
| WO2025089399A1 (ja) * | 2023-10-26 | 2025-05-01 | 国立研究開発法人国立がん研究センター | インターロイキン7受容体(il-7r)に対する抗体および当該抗体を含む抗体薬物コンジュゲート(adc) |
| US20250302763A1 (en) | 2024-02-22 | 2025-10-02 | Capstan Therapeutics, Inc. | Immune engineering amplification |
| WO2025202213A1 (en) | 2024-03-26 | 2025-10-02 | Institut National de la Santé et de la Recherche Médicale | Lipid nanoparticle loaded with antitumoral agent and functionnalized to target immosuppressive cells |
| WO2025217452A1 (en) | 2024-04-11 | 2025-10-16 | Capstan Therapeutics, Inc. | Constrained ionizable cationic lipids and lipid nanoparticles |
| WO2025217454A2 (en) | 2024-04-11 | 2025-10-16 | Capstan Therapeutics, Inc. | Ionizable cationic lipids and lipid nanoparticles |
| CN118638236B (zh) * | 2024-07-09 | 2025-03-14 | 武汉爱博泰克生物科技有限公司 | 抗人cd127蛋白的抗体、抗体偶联物及其应用 |
| WO2026022216A1 (en) | 2024-07-23 | 2026-01-29 | Ose Immunotherapeutics | Uses of anti-cd127 antibodies in the treatment of inflammatory bowel diseases |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011094259A2 (en) * | 2010-01-28 | 2011-08-04 | Glaxo Group Limited | Cd127 binding proteins |
| WO2011104687A1 (en) * | 2010-02-24 | 2011-09-01 | Rinat Neuroscience Corporation | Antagonist anti-il-7 receptor antibodies and methods |
| WO2013056984A1 (en) * | 2011-10-19 | 2013-04-25 | Effimune | Antibodies directed against the alpha chain of il7 receptor - their use for the preparation of drug candidates |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19742706B4 (de) | 1997-09-26 | 2013-07-25 | Pieris Proteolab Ag | Lipocalinmuteine |
| CA2655392A1 (en) | 2006-05-31 | 2007-12-06 | The Regents Of The University Of California | Cd127 expression inversely correlates with foxp3 and suppressive function of cd4+ tregs |
| ATE542830T1 (de) | 2006-12-04 | 2012-02-15 | Pasteur Institut | Als gerüst verwendeter ob-fold zur entwicklung neuer spezifischer bindemittel |
| UY32038A (es) | 2008-08-08 | 2010-03-26 | Glaxo Wellcome Mfg Pte Ltd | Inmunoblobulinas anti-cd127 y sus usos |
| WO2010085643A1 (en) | 2009-01-22 | 2010-07-29 | University Of Miami | Targeting il-7 signaling as a therapy for multiple sclerosis and other il-7 signaling dependent disorders |
| EP2955196A1 (en) * | 2014-06-10 | 2015-12-16 | Effimune | Antibodies directed against CD127 |
| PL3423496T3 (pl) | 2016-02-29 | 2020-01-31 | Ose Immunotherapeutics | Nieantagonistyczne przeciwciała skierowane przeciw łańcuchowi alfa domeny zewnątrzkomórkowej receptora il7 i ich zastosowanie w leczeniu raka |
-
2015
- 2015-01-23 EP EP15305078.6A patent/EP2955196A1/en not_active Withdrawn
- 2015-06-10 EP EP15727989.4A patent/EP3155014A1/en active Pending
- 2015-06-10 NZ NZ726932A patent/NZ726932A/en unknown
- 2015-06-10 TN TN2016000528A patent/TN2016000528A1/en unknown
- 2015-06-10 AP AP2016009599A patent/AP2016009599A0/en unknown
- 2015-06-10 AU AU2015273532A patent/AU2015273532C1/en active Active
- 2015-06-10 PE PE2016002699A patent/PE20170324A1/es unknown
- 2015-06-10 BR BR112016028755-0A patent/BR112016028755B1/pt active IP Right Grant
- 2015-06-10 RU RU2016151265A patent/RU2734076C2/ru active
- 2015-06-10 JP JP2017517406A patent/JP7324565B2/ja active Active
- 2015-06-10 MY MYPI2016002094A patent/MY190889A/en unknown
- 2015-06-10 MX MX2016016236A patent/MX376066B/es active IP Right Grant
- 2015-06-10 KR KR1020177000724A patent/KR102612930B1/ko active Active
- 2015-06-10 CN CN201580043066.1A patent/CN106715471B/zh active Active
- 2015-06-10 UA UAA201613258A patent/UA125366C2/uk unknown
- 2015-06-10 MA MA040202A patent/MA40202A/fr unknown
- 2015-06-10 CA CA2950823A patent/CA2950823A1/en active Pending
- 2015-06-10 WO PCT/EP2015/062993 patent/WO2015189302A1/en not_active Ceased
- 2015-06-10 CR CR20160576A patent/CR20160576A/es unknown
- 2015-06-10 US US15/317,355 patent/US10428152B2/en active Active
- 2015-06-10 SG SG11201610036PA patent/SG11201610036PA/en unknown
- 2015-06-10 EA EA201692460A patent/EA039303B1/ru unknown
- 2015-06-10 HK HK17104912.8A patent/HK1231487A1/zh unknown
-
2016
- 2016-12-06 PH PH12016502445A patent/PH12016502445A1/en unknown
- 2016-12-07 IL IL249449A patent/IL249449B/en active IP Right Grant
- 2016-12-08 SA SA516380455A patent/SA516380455B1/ar unknown
- 2016-12-09 CL CL2016003172A patent/CL2016003172A1/es unknown
-
2019
- 2019-08-05 US US16/532,000 patent/US11440964B2/en active Active
-
2021
- 2021-08-05 JP JP2021128846A patent/JP2021184731A/ja active Pending
-
2022
- 2022-09-09 US US17/941,885 patent/US12371502B2/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011094259A2 (en) * | 2010-01-28 | 2011-08-04 | Glaxo Group Limited | Cd127 binding proteins |
| WO2011104687A1 (en) * | 2010-02-24 | 2011-09-01 | Rinat Neuroscience Corporation | Antagonist anti-il-7 receptor antibodies and methods |
| WO2013056984A1 (en) * | 2011-10-19 | 2013-04-25 | Effimune | Antibodies directed against the alpha chain of il7 receptor - their use for the preparation of drug candidates |
Non-Patent Citations (4)
| Title |
|---|
| CHUNG B. et al. Prevention of graft-versus-host disease by anti IL-7R alpha, antibody. BLOOD. October 2007, vol. 110, n. 8, pp. 2803-2810. * |
| CHUNG B. et al. Prevention of graft-versus-host disease by anti IL-7R alpha, antibody. BLOOD. October 2007, vol. 110, n. 8, pp. 2803-2810. MCALLISTER F. et al. Role of IL-17A, IL-17F and the IL-17 receptor in regulating growth-related oncogene-a and granulocyte colony-stimulating factor in bronchial epithelium: Implications for airway inflammation in cystic fibrosis. J. Immunol., vol. 175, no. 1, 1 July 2005 (2005-07-01), pp. 404-412. АЛЬТШУЛЕР E.П. и др. Получение рекомбинантных антител и способы увеличения их аффинности. Успехи биологической химии, т. 50, 2010, с. 203-258. * |
| MCALLISTER F. et al. Role of IL-17A, IL-17F and the IL-17 receptor in regulating growth-related oncogene-a and granulocyte colony-stimulating factor in bronchial epithelium: Implications for airway inflammation in cystic fibrosis. J. Immunol., vol. 175, no. 1, 1 July 2005 (2005-07-01), pp. 404-412. * |
| АЛЬТШУЛЕР E.П. и др. Получение рекомбинантных антител и способы увеличения их аффинности. Успехи биологической химии, т. 50, 2010, с. 203-258. * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2734076C2 (ru) | Антитела, направленные на cd127 | |
| KR102444717B1 (ko) | 인간화 항-pacap 항체 및 그의 용도 | |
| KR102831970B1 (ko) | 항-sars-cov-2 스파이크 당단백질 항체 및 항원-결합 단편 | |
| KR101919170B1 (ko) | 중화 항-ccl20 항체 | |
| AU2015292406B2 (en) | Anti-CD3 antibodies, activatable anti-CD3 antibodies, multispecific anti-CD3 antibodies, multispecific activatable anti-CD3 antibodies, and methods of using the same | |
| KR101601387B1 (ko) | c-KIT 항체 및 이의 용도 | |
| KR102648281B1 (ko) | 항-pacap 항체 및 그의 용도 | |
| KR20240017912A (ko) | 항-ccr8 항체 및 이의 용도 | |
| KR20220002899A (ko) | 항-psma/cd3 항체로 전립선암을 치료하는 방법 | |
| KR20230017815A (ko) | 항-sars-cov-2 스파이크 당단백질 항체 및 항원-결합 단편 | |
| AU2011299443A1 (en) | Anti-VEGFR-3 antibody compositions | |
| KR102903323B1 (ko) | 고양이 맥도너 육종(fms)-유사 티로신 키나제 3 수용체 리간드(flt3l)에 대한 항체 및 자가면역 및 염증 질환을 치료하기 위한 이의 용도 | |
| KR102916165B1 (ko) | 중증근무력증의 치료 방법 | |
| KR20260014044A (ko) | 중증근무력증의 치료 방법 | |
| HK40004152A (en) | Humanized anti-pacap antibodies and uses thereof | |
| HK40004152B (en) | Humanized anti-pacap antibodies and uses thereof | |
| KR20210078517A (ko) | 중증근무력증의 치료 방법 | |
| KR20200018643A (ko) | 면역 혈소판감소증의 치료 방법 | |
| HK1256381B (en) | Anti-pacap antibodies and uses thereof |