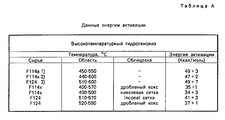
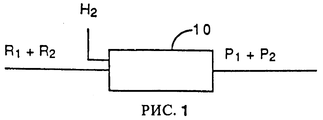
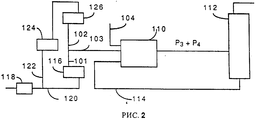
RU2067971C1 - Способ гидрогенолиза галогенуглеводородов - Google Patents
Способ гидрогенолиза галогенуглеводородов Download PDFInfo
- Publication number
- RU2067971C1 RU2067971C1 SU925011047A SU5011047A RU2067971C1 RU 2067971 C1 RU2067971 C1 RU 2067971C1 SU 925011047 A SU925011047 A SU 925011047A SU 5011047 A SU5011047 A SU 5011047A RU 2067971 C1 RU2067971 C1 RU 2067971C1
- Authority
- RU
- Russia
- Prior art keywords
- halogenated hydrocarbon
- ccl
- cclf
- starting material
- nickel
- Prior art date
Links
- 150000008282 halocarbons Chemical class 0.000 title claims abstract description 128
- 238000000034 method Methods 0.000 title claims abstract description 77
- 238000007327 hydrogenolysis reaction Methods 0.000 title claims description 93
- 239000000203 mixture Substances 0.000 claims abstract description 85
- 239000001257 hydrogen Substances 0.000 claims abstract description 52
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 52
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims abstract description 50
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims abstract description 24
- 229910045601 alloy Inorganic materials 0.000 claims abstract description 24
- 239000000956 alloy Substances 0.000 claims abstract description 24
- 125000004432 carbon atom Chemical group C* 0.000 claims abstract description 23
- 125000001153 fluoro group Chemical group F* 0.000 claims abstract description 23
- 238000009826 distribution Methods 0.000 claims abstract description 21
- 230000008569 process Effects 0.000 claims abstract description 20
- 229910052751 metal Inorganic materials 0.000 claims abstract description 14
- 239000002184 metal Substances 0.000 claims abstract description 14
- 229910052801 chlorine Inorganic materials 0.000 claims abstract description 13
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims abstract description 12
- 229910052731 fluorine Inorganic materials 0.000 claims abstract description 12
- 229910052742 iron Inorganic materials 0.000 claims abstract description 12
- 229910052750 molybdenum Inorganic materials 0.000 claims abstract description 12
- 239000011733 molybdenum Substances 0.000 claims abstract description 12
- 229910052794 bromium Inorganic materials 0.000 claims abstract description 10
- 239000011737 fluorine Substances 0.000 claims abstract description 10
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims abstract description 9
- 229910052799 carbon Inorganic materials 0.000 claims abstract description 9
- -1 halocarbon compounds Chemical class 0.000 claims abstract description 9
- 239000010936 titanium Substances 0.000 claims abstract description 9
- 229910052719 titanium Inorganic materials 0.000 claims abstract description 9
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims abstract description 8
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229910052782 aluminium Inorganic materials 0.000 claims abstract description 7
- 239000002245 particle Substances 0.000 claims abstract description 6
- KYKAJFCTULSVSH-UHFFFAOYSA-N chloro(fluoro)methane Chemical compound F[C]Cl KYKAJFCTULSVSH-UHFFFAOYSA-N 0.000 claims description 87
- 239000007858 starting material Substances 0.000 claims description 73
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 52
- 238000006243 chemical reaction Methods 0.000 claims description 45
- 229910052759 nickel Inorganic materials 0.000 claims description 26
- 125000004773 chlorofluoromethyl group Chemical group [H]C(F)(Cl)* 0.000 claims description 23
- 229910000990 Ni alloy Inorganic materials 0.000 claims description 21
- 150000001336 alkenes Chemical class 0.000 claims description 12
- 230000035484 reaction time Effects 0.000 claims description 10
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 9
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 claims description 8
- 229910052804 chromium Inorganic materials 0.000 claims description 8
- 239000011651 chromium Substances 0.000 claims description 8
- 229910017052 cobalt Inorganic materials 0.000 claims description 8
- 239000010941 cobalt Substances 0.000 claims description 8
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 8
- 229930195733 hydrocarbon Natural products 0.000 claims description 7
- 239000004215 Carbon black (E152) Substances 0.000 claims description 6
- 229910052736 halogen Inorganic materials 0.000 claims description 6
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 claims description 6
- 229910010271 silicon carbide Inorganic materials 0.000 claims description 6
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 4
- 229910052721 tungsten Inorganic materials 0.000 claims description 4
- 239000010937 tungsten Substances 0.000 claims description 4
- 238000006467 substitution reaction Methods 0.000 claims description 3
- 150000001721 carbon Chemical group 0.000 claims 1
- 239000000460 chlorine Substances 0.000 abstract description 14
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 abstract description 10
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 abstract description 7
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 abstract description 7
- 239000000126 substance Substances 0.000 abstract description 7
- 150000002739 metals Chemical class 0.000 abstract description 6
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 abstract description 2
- 229910052802 copper Inorganic materials 0.000 abstract description 2
- 239000010949 copper Substances 0.000 abstract description 2
- 239000002904 solvent Substances 0.000 abstract description 2
- 239000002826 coolant Substances 0.000 abstract 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 abstract 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 abstract 1
- 239000004411 aluminium Substances 0.000 abstract 1
- 239000000047 product Substances 0.000 description 61
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 38
- 230000015572 biosynthetic process Effects 0.000 description 20
- 238000003786 synthesis reaction Methods 0.000 description 16
- LVGUZGTVOIAKKC-UHFFFAOYSA-N 1,1,1,2-tetrafluoroethane Chemical compound FCC(F)(F)F LVGUZGTVOIAKKC-UHFFFAOYSA-N 0.000 description 14
- 239000003054 catalyst Substances 0.000 description 12
- 239000007788 liquid Substances 0.000 description 11
- 239000000463 material Substances 0.000 description 10
- RFCAUADVODFSLZ-UHFFFAOYSA-N 1-Chloro-1,1,2,2,2-pentafluoroethane Chemical compound FC(F)(F)C(F)(F)Cl RFCAUADVODFSLZ-UHFFFAOYSA-N 0.000 description 9
- BOUGCJDAQLKBQH-UHFFFAOYSA-N 1-chloro-1,2,2,2-tetrafluoroethane Chemical compound FC(Cl)C(F)(F)F BOUGCJDAQLKBQH-UHFFFAOYSA-N 0.000 description 9
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 9
- 235000019406 chloropentafluoroethane Nutrition 0.000 description 9
- BAMUEXIPKSRTBS-UHFFFAOYSA-N 1,1-dichloro-1,2,2,2-tetrafluoroethane Chemical compound FC(F)(F)C(F)(Cl)Cl BAMUEXIPKSRTBS-UHFFFAOYSA-N 0.000 description 8
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 8
- RWRIWBAIICGTTQ-UHFFFAOYSA-N difluoromethane Chemical compound FCF RWRIWBAIICGTTQ-UHFFFAOYSA-N 0.000 description 8
- VOPWNXZWBYDODV-UHFFFAOYSA-N Chlorodifluoromethane Chemical compound FC(F)Cl VOPWNXZWBYDODV-UHFFFAOYSA-N 0.000 description 7
- 239000004576 sand Substances 0.000 description 7
- WXGNWUVNYMJENI-UHFFFAOYSA-N 1,1,2,2-tetrafluoroethane Chemical compound FC(F)C(F)F WXGNWUVNYMJENI-UHFFFAOYSA-N 0.000 description 6
- YVOASHYXFVSAQN-UHFFFAOYSA-N 2,2-dichloro-1,1,1,3,3,3-hexafluoropropane Chemical compound FC(F)(F)C(Cl)(Cl)C(F)(F)F YVOASHYXFVSAQN-UHFFFAOYSA-N 0.000 description 6
- 239000007795 chemical reaction product Substances 0.000 description 6
- KVBKAPANDHPRDG-UHFFFAOYSA-N dibromotetrafluoroethane Chemical compound FC(F)(Br)C(F)(F)Br KVBKAPANDHPRDG-UHFFFAOYSA-N 0.000 description 6
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 6
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 6
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 5
- 239000006227 byproduct Substances 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 150000002430 hydrocarbons Chemical class 0.000 description 4
- 238000006317 isomerization reaction Methods 0.000 description 4
- 229910052763 palladium Inorganic materials 0.000 description 4
- 239000012084 conversion product Substances 0.000 description 3
- 150000002431 hydrogen Chemical class 0.000 description 3
- 238000005984 hydrogenation reaction Methods 0.000 description 3
- 229910001055 inconels 600 Inorganic materials 0.000 description 3
- 239000007791 liquid phase Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 238000012856 packing Methods 0.000 description 3
- GTLACDSXYULKMZ-UHFFFAOYSA-N pentafluoroethane Chemical compound FC(F)C(F)(F)F GTLACDSXYULKMZ-UHFFFAOYSA-N 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 239000012808 vapor phase Substances 0.000 description 3
- JQZFYIGAYWLRCC-UHFFFAOYSA-N 1-chloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)C(F)(F)Cl JQZFYIGAYWLRCC-UHFFFAOYSA-N 0.000 description 2
- 229910000838 Al alloy Inorganic materials 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 229910000792 Monel Inorganic materials 0.000 description 2
- WAIPAZQMEIHHTJ-UHFFFAOYSA-N [Cr].[Co] Chemical compound [Cr].[Co] WAIPAZQMEIHHTJ-UHFFFAOYSA-N 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 229910001063 inconels 617 Inorganic materials 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000004064 recycling Methods 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- YPFNIPKMNMDDDB-UHFFFAOYSA-K 2-[2-[bis(carboxylatomethyl)amino]ethyl-(2-hydroxyethyl)amino]acetate;iron(3+) Chemical compound [Fe+3].OCCN(CC([O-])=O)CCN(CC([O-])=O)CC([O-])=O YPFNIPKMNMDDDB-UHFFFAOYSA-K 0.000 description 1
- 229910016569 AlF 3 Inorganic materials 0.000 description 1
- 239000004340 Chloropentafluoroethane Substances 0.000 description 1
- 229910000531 Co alloy Inorganic materials 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- 229910000640 Fe alloy Inorganic materials 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- 229910001182 Mo alloy Inorganic materials 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000000571 coke Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000004070 electrodeposition Methods 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 229910000856 hastalloy Inorganic materials 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 229910001026 inconel Inorganic materials 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000011572 manganese Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 239000003507 refrigerant Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C17/00—Preparation of halogenated hydrocarbons
- C07C17/23—Preparation of halogenated hydrocarbons by dehalogenation
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/582—Recycling of unreacted starting or intermediate materials
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US07/682,764 US5208397A (en) | 1991-04-09 | 1991-04-09 | Hydrogenolysis of halocarbon mixtures |
| US682764 | 2003-10-09 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| RU2067971C1 true RU2067971C1 (ru) | 1996-10-20 |
Family
ID=24741032
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| SU925011047A RU2067971C1 (ru) | 1991-04-09 | 1992-01-31 | Способ гидрогенолиза галогенуглеводородов |
Country Status (11)
| Country | Link |
|---|---|
| US (3) | US5208397A (cg-RX-API-DMAC7.html) |
| EP (1) | EP0580718A1 (cg-RX-API-DMAC7.html) |
| JP (1) | JPH06506464A (cg-RX-API-DMAC7.html) |
| CN (1) | CN1065653A (cg-RX-API-DMAC7.html) |
| AU (1) | AU655265B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BR9205868A (cg-RX-API-DMAC7.html) |
| CA (1) | CA2107802A1 (cg-RX-API-DMAC7.html) |
| MX (1) | MX9201609A (cg-RX-API-DMAC7.html) |
| RU (1) | RU2067971C1 (cg-RX-API-DMAC7.html) |
| TW (1) | TW197961B (cg-RX-API-DMAC7.html) |
| WO (1) | WO1992018446A1 (cg-RX-API-DMAC7.html) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2125034C1 (ru) * | 1995-11-27 | 1999-01-20 | Научно-исследовательский физико-химический институт им.Л.Я.Карпова | Способ парофазного гидродехлорирования четыреххлористого углерода |
| RU2332396C2 (ru) * | 2003-08-13 | 2008-08-27 | Грейт Лейкс Кемикал Корпорейшн | Системы и способы получения с3 насыщенных и ненасыщенных фторуглеродов |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5208397A (en) * | 1991-04-09 | 1993-05-04 | E. I. Du Pont De Nemours And Company | Hydrogenolysis of halocarbon mixtures |
| US5817896A (en) * | 1993-03-26 | 1998-10-06 | The University Court Of The University Of Dundee | Catalytic method of replacing halogen in halocarbons |
| US5866730A (en) * | 1993-04-02 | 1999-02-02 | E. I. Du Pont De Nemours And Company | Process for enriching 1,2-dichloro-1,1,2,2-tetrafluoroethane from a mixture of dichlorotetrafluoroethane isomers |
| WO1995024369A1 (en) * | 1994-03-11 | 1995-09-14 | E.I. Du Pont De Nemours And Company | Process for converting chlorodifluoromethane and/or dichlorodifluoromethane |
| WO1996016009A2 (en) * | 1994-11-23 | 1996-05-30 | E.I. Du Pont De Nemours And Company | Hydrofluorocarbon production using heat carriers in high temperature hydrogenolysis |
| US5944876A (en) * | 1996-06-06 | 1999-08-31 | E. I. Du Pont De Nemours And Company | Cd-exchanged zeolite rho compositions and compositions of zeolite rho encapsulated with hydrogen made therefrom |
| US7329786B2 (en) * | 2001-09-28 | 2008-02-12 | Great Lakes Chemical Corporation | Processes for producing CF3CFHCF3 |
| US7223351B2 (en) * | 2003-04-17 | 2007-05-29 | Great Lakes Chemical Corporation | Fire extinguishing mixtures, methods and systems |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2005710A (en) * | 1931-05-20 | 1935-06-18 | Kinetic Chemicals Inc | Preparation of fluorine compounds |
| US2005708A (en) * | 1933-08-24 | 1935-06-18 | Kinetic Chemicals Inc | Production of halogenated ethane derivatives containing fluorine |
| US2062743A (en) * | 1935-06-12 | 1936-12-01 | Kinetic Chemicals Inc | Production of fluorine-containing carbon compounds |
| US2146725A (en) * | 1935-06-14 | 1939-02-14 | Kinetic Chemicals Inc | Process of preparing halogenated acyclic hydrocarbons containing fluorine |
| US2062748A (en) * | 1936-07-06 | 1936-12-01 | Magna Products Corp | Guard for lever openings |
| US2615926A (en) * | 1949-03-09 | 1952-10-28 | Du Pont | Process for preparing organic compounds containing fluorine |
| US2704775A (en) * | 1951-02-02 | 1955-03-22 | Union Carbide & Carbon Corp | Preparation of halogenated olefines |
| US2748177A (en) * | 1951-08-03 | 1956-05-29 | Allied Chem & Dye Corp | Fluorination |
| US3042727A (en) * | 1959-06-04 | 1962-07-03 | Dow Chemical Co | Preparation of fluoroform |
| US3138559A (en) * | 1960-03-30 | 1964-06-23 | Pennsalt Chemicals Corp | Method for the preparation of a catalyst composition by the reaction of a fluorocarbo with activated alumina |
| GB1578933A (en) * | 1977-05-24 | 1980-11-12 | Ici Ltd | Manufacture of halogenated hydrocarbons |
| US4155881A (en) * | 1978-03-08 | 1979-05-22 | E. I. Du Pont De Nemours And Company | Activation of chromic fluoride catalyst with hydrogen chloride and chlorine |
| US4319060A (en) * | 1980-12-08 | 1982-03-09 | Allied Chemical Corporation | Process for producing 1,2-dichloro-1,1,2,2-tetrafluoroethane substantially free of 1,1-dichloro-1,2,2,2-tetrafluoroethane |
| DE68912657T2 (de) * | 1988-06-21 | 1994-09-01 | Asahi Glass Co Ltd | Verfahren zur Herstellung von 1,1,1,2-Tetrafluorethan. |
| CA1328471C (en) * | 1988-06-23 | 1994-04-12 | Velliyur Nott Mallikarjuna Rao | Catalyzed hydrofluorination of halogenated alkanes |
| US5001283A (en) * | 1988-10-06 | 1991-03-19 | Mobil Oil Corp. | Process for preparing aromatic alkanols |
| AU641704B2 (en) * | 1989-10-10 | 1993-09-30 | E.I. Du Pont De Nemours And Company | Halocarbon hydrogenolysis |
| US5208397A (en) * | 1991-04-09 | 1993-05-04 | E. I. Du Pont De Nemours And Company | Hydrogenolysis of halocarbon mixtures |
-
1991
- 1991-04-09 US US07/682,764 patent/US5208397A/en not_active Expired - Lifetime
-
1992
- 1992-01-31 RU SU925011047A patent/RU2067971C1/ru active
- 1992-04-03 JP JP4509368A patent/JPH06506464A/ja active Pending
- 1992-04-03 CA CA002107802A patent/CA2107802A1/en not_active Abandoned
- 1992-04-03 WO PCT/US1992/002446 patent/WO1992018446A1/en not_active Ceased
- 1992-04-03 EP EP92909788A patent/EP0580718A1/en not_active Withdrawn
- 1992-04-03 AU AU16930/92A patent/AU655265B2/en not_active Ceased
- 1992-04-03 BR BR9205868A patent/BR9205868A/pt not_active Application Discontinuation
- 1992-04-07 TW TW081102648A patent/TW197961B/zh active
- 1992-04-08 MX MX9201609A patent/MX9201609A/es unknown
- 1992-04-09 CN CN92102624A patent/CN1065653A/zh active Pending
- 1992-12-29 US US07/998,005 patent/US5300713A/en not_active Expired - Lifetime
-
1994
- 1994-02-02 US US08/190,706 patent/US5446219A/en not_active Expired - Lifetime
Non-Patent Citations (1)
| Title |
|---|
| Патент Великобритании N 1578933, кл. С 07 С 19/08, 1980. * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2125034C1 (ru) * | 1995-11-27 | 1999-01-20 | Научно-исследовательский физико-химический институт им.Л.Я.Карпова | Способ парофазного гидродехлорирования четыреххлористого углерода |
| RU2332396C2 (ru) * | 2003-08-13 | 2008-08-27 | Грейт Лейкс Кемикал Корпорейшн | Системы и способы получения с3 насыщенных и ненасыщенных фторуглеродов |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0580718A1 (en) | 1994-02-02 |
| BR9205868A (pt) | 1994-12-27 |
| US5208397A (en) | 1993-05-04 |
| MX9201609A (es) | 1992-10-01 |
| AU1693092A (en) | 1992-11-17 |
| AU655265B2 (en) | 1994-12-08 |
| JPH06506464A (ja) | 1994-07-21 |
| US5446219A (en) | 1995-08-29 |
| US5300713A (en) | 1994-04-05 |
| TW197961B (cg-RX-API-DMAC7.html) | 1993-01-11 |
| CA2107802A1 (en) | 1992-10-10 |
| WO1992018446A1 (en) | 1992-10-29 |
| CN1065653A (zh) | 1992-10-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU641704B2 (en) | Halocarbon hydrogenolysis | |
| US6706935B2 (en) | Catalytic equilibration to increase the relative mole fraction of CF3CHCI, CF3CHCI2 or CF3CF2H in a composition | |
| RU2067971C1 (ru) | Способ гидрогенолиза галогенуглеводородов | |
| US5430204A (en) | Halocarbon hydrogenolysis | |
| US5446216A (en) | Process for manufacture of high purity 1,1-dichlorotetrafluoroethane | |
| JP3535516B2 (ja) | フッ素置換されたメタン類の製造 | |
| RU2010789C1 (ru) | Непрерывный способ получения 1,1-дихлортетрафторэтана | |
| US5395999A (en) | Catalytic chlorofluorination process for producing CHClFCF3 and CHF2 CF3 | |
| US6028026A (en) | Cubic chromium trifluoride and its use for halogenated hydrocarbon processing | |
| US5621151A (en) | Halocarbon hydrogenolysis | |
| US5516947A (en) | Process for converting chlorodifluoromethane and/or dichlorodifluoromethane | |
| US5406009A (en) | Catalytic equilibration to improve the relative yield of selected halocarbons | |
| US5463152A (en) | Halofluorocarbon hydrogenolysis | |
| AU618638B2 (en) | Improved process for the preparation of 1,1,1,2- tetrafluoroethane | |
| US5008476A (en) | Process for the preparation of 1,1,1,2-tetrafluoroethane | |
| US5841008A (en) | Hydrofluorocarbon production using heat carriers in high temperature hydrogenolysis | |
| WO2025019191A2 (en) | A process to produce 1252zc from 1230xa or 252dc and compositions thereof | |
| EP0724553A1 (en) | Process for manufacture of high purity 1,1-dichlorotetrafluoroethane |