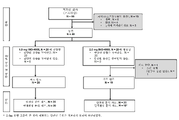
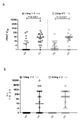
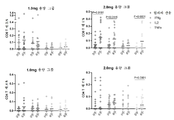
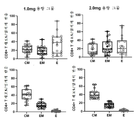
KR20220157969A - 코로나바이러스 백신 및 사용 방법 - Google Patents
코로나바이러스 백신 및 사용 방법 Download PDFInfo
- Publication number
- KR20220157969A KR20220157969A KR1020227032752A KR20227032752A KR20220157969A KR 20220157969 A KR20220157969 A KR 20220157969A KR 1020227032752 A KR1020227032752 A KR 1020227032752A KR 20227032752 A KR20227032752 A KR 20227032752A KR 20220157969 A KR20220157969 A KR 20220157969A
- Authority
- KR
- South Korea
- Prior art keywords
- cov
- sars
- nucleic acid
- seq
- acid sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/215—Coronaviridae, e.g. avian infectious bronchitis virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
- A61K2039/572—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2 cytotoxic response
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
- A61K2039/575—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2 humoral response
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/20011—Coronaviridae
- C12N2770/20021—Viruses as such, e.g. new isolates, mutants or their genomic sequences
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/20011—Coronaviridae
- C12N2770/20034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/20011—Coronaviridae
- C12N2770/20041—Use of virus, viral particle or viral elements as a vector
- C12N2770/20044—Chimeric viral vector comprising heterologous viral elements for production of another viral vector
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Virology (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Microbiology (AREA)
- Communicable Diseases (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Mycology (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Pulmonology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Oncology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (29)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202062981451P | 2020-02-25 | 2020-02-25 | |
| US202062981168P | 2020-02-25 | 2020-02-25 | |
| US62/981,168 | 2020-02-25 | ||
| US62/981,451 | 2020-02-25 | ||
| US202063004380P | 2020-04-02 | 2020-04-02 | |
| US63/004,380 | 2020-04-02 | ||
| US202063022032P | 2020-05-08 | 2020-05-08 | |
| US63/022,032 | 2020-05-08 | ||
| US202063028404P | 2020-05-21 | 2020-05-21 | |
| US63/028,404 | 2020-05-21 | ||
| US202063033349P | 2020-06-02 | 2020-06-02 | |
| US63/033,349 | 2020-06-02 | ||
| US202063040865P | 2020-06-18 | 2020-06-18 | |
| US63/040,865 | 2020-06-18 | ||
| US202063046415P | 2020-06-30 | 2020-06-30 | |
| US63/046,415 | 2020-06-30 | ||
| US202063056996P | 2020-07-27 | 2020-07-27 | |
| US63/056,996 | 2020-07-27 | ||
| US202063063157P | 2020-08-07 | 2020-08-07 | |
| US202063062762P | 2020-08-07 | 2020-08-07 | |
| US63/062,762 | 2020-08-07 | ||
| US63/063,157 | 2020-08-07 | ||
| US202063114858P | 2020-11-17 | 2020-11-17 | |
| US63/114,858 | 2020-11-17 | ||
| US202063130593P | 2020-12-24 | 2020-12-24 | |
| US63/130,593 | 2020-12-24 | ||
| US202163136973P | 2021-01-13 | 2021-01-13 | |
| US63/136,973 | 2021-01-13 | ||
| PCT/US2021/019662 WO2021173829A1 (en) | 2020-02-25 | 2021-02-25 | Vaccines against coronavirus and methods of use |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20220157969A true KR20220157969A (ko) | 2022-11-29 |
Family
ID=75108858
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227032752A Pending KR20220157969A (ko) | 2020-02-25 | 2021-02-25 | 코로나바이러스 백신 및 사용 방법 |
Country Status (15)
| Country | Link |
|---|---|
| US (3) | US11660335B2 (enExample) |
| EP (1) | EP4110468A1 (enExample) |
| JP (2) | JP2023511780A (enExample) |
| KR (1) | KR20220157969A (enExample) |
| CN (1) | CN115867349A (enExample) |
| AU (2) | AU2021226567B2 (enExample) |
| BR (1) | BR112022016507A2 (enExample) |
| CA (1) | CA3168353A1 (enExample) |
| CO (1) | CO2022012272A2 (enExample) |
| CU (1) | CU24743B1 (enExample) |
| IL (1) | IL295697A (enExample) |
| MX (1) | MX2022010460A (enExample) |
| PE (1) | PE20230171A1 (enExample) |
| PH (1) | PH12022552249A1 (enExample) |
| WO (1) | WO2021173829A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021252604A1 (en) * | 2020-06-09 | 2021-12-16 | The Wistar Institute Of Anatomy And Biology | Coronavirus disease 2019 (covid-19) combination vaccine |
| WO2021249116A1 (en) | 2020-06-10 | 2021-12-16 | Sichuan Clover Biopharmaceuticals, Inc. | Coronavirus vaccine compositions, methods, and uses thereof |
| EP4333887A4 (en) * | 2021-05-05 | 2025-05-21 | Inovio Pharmaceuticals, Inc. | Vaccines against coronavirus and methods of use |
| WO2023035016A1 (en) * | 2021-09-03 | 2023-03-09 | The Uab Research Foundation | Human neutralizing antibodies against sars-cov-2 spike s2 domain and uses thereof |
| WO2023073672A1 (en) * | 2021-10-29 | 2023-05-04 | Geneone Life Science, Inc. | Therapeutic and vaccine candidates against sars-cov-2 |
| WO2024231886A1 (en) * | 2023-05-10 | 2024-11-14 | Seqirus Inc. | Combination vaccine |
Family Cites Families (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US21579A (en) | 1858-09-21 | Rotary valve fob steam-engines | ||
| US3805783A (en) | 1971-02-12 | 1974-04-23 | A Ismach | Hand powered hypodermic jet injector gun |
| US4342310A (en) | 1980-07-08 | 1982-08-03 | Istvan Lindmayer | Hydro-pneumatic jet injector |
| US4447223A (en) | 1982-04-16 | 1984-05-08 | Cct Associates | Medicament implant applicator |
| US5703055A (en) | 1989-03-21 | 1997-12-30 | Wisconsin Alumni Research Foundation | Generation of antibodies through lipid mediated DNA delivery |
| US5843463A (en) | 1990-12-21 | 1998-12-01 | Antexbiologics, Inc. | Adhesin-oligosaccharide conjugate vaccine for Haemophilus influenzae |
| US5545130A (en) | 1992-04-08 | 1996-08-13 | Genetronics, Inc. | Flow through electroporation method |
| WO1993025673A1 (en) | 1992-06-04 | 1993-12-23 | The Regents Of The University Of California | In vivo gene therapy with intron-free sequence of interest |
| US5702359A (en) | 1995-06-06 | 1997-12-30 | Genetronics, Inc. | Needle electrodes for mediated delivery of drugs and genes |
| US5993434A (en) | 1993-04-01 | 1999-11-30 | Genetronics, Inc. | Method of treatment using electroporation mediated delivery of drugs and genes |
| US5679647A (en) | 1993-08-26 | 1997-10-21 | The Regents Of The University Of California | Methods and devices for immunizing a host against tumor-associated antigens through administration of naked polynucleotides which encode tumor-associated antigenic peptides |
| US5505697A (en) | 1994-01-14 | 1996-04-09 | Mckinnon, Jr.; Charles N. | Electrically powered jet injector |
| US5869326A (en) | 1996-09-09 | 1999-02-09 | Genetronics, Inc. | Electroporation employing user-configured pulsing scheme |
| US6055453A (en) | 1997-08-01 | 2000-04-25 | Genetronics, Inc. | Apparatus for addressing needle array electrodes for electroporation therapy |
| US6241701B1 (en) | 1997-08-01 | 2001-06-05 | Genetronics, Inc. | Apparatus for electroporation mediated delivery of drugs and genes |
| US6216034B1 (en) | 1997-08-01 | 2001-04-10 | Genetronics, Inc. | Method of programming an array of needle electrodes for electroporation therapy of tissue |
| US6009347A (en) | 1998-01-27 | 1999-12-28 | Genetronics, Inc. | Electroporation apparatus with connective electrode template |
| US6208893B1 (en) | 1998-01-27 | 2001-03-27 | Genetronics, Inc. | Electroporation apparatus with connective electrode template |
| US6120493A (en) | 1998-01-27 | 2000-09-19 | Genetronics, Inc. | Method for the introduction of therapeutic agents utilizing an electroporation apparatus |
| CA2337129A1 (en) | 1998-07-13 | 2000-01-20 | Genetronics, Inc. | Method and apparatus for electrically assisted topical delivery of agents for cosmetic applications |
| US6192270B1 (en) | 1998-08-14 | 2001-02-20 | Genetronics, Inc. | Apparatus and method for the delivery of drugs and genes into tissue |
| US6150148A (en) | 1998-10-21 | 2000-11-21 | Genetronics, Inc. | Electroporation apparatus for control of temperature during the process |
| US7171264B1 (en) | 1999-05-10 | 2007-01-30 | Genetronics, Inc. | Intradermal delivery of active agents by needle-free injection and electroporation |
| CA2369329A1 (en) | 1999-05-10 | 2000-11-16 | Gunter A. Hofmann | Method of electroporation-enhanced delivery of active agents |
| US7245963B2 (en) | 2002-03-07 | 2007-07-17 | Advisys, Inc. | Electrode assembly for constant-current electroporation and use |
| US7328064B2 (en) | 2002-07-04 | 2008-02-05 | Inovio As | Electroporation device and injection apparatus |
| US20050025788A1 (en) * | 2003-06-06 | 2005-02-03 | Chou George Chin-Sheng | Systemic delivery of non-viral vector expressing SARS viral genomic vaccine |
| CA2932055A1 (en) | 2013-11-29 | 2015-06-04 | The Trustees Of The University Of Pennsylvania | Mers-cov vaccine |
| US10953089B1 (en) | 2020-01-27 | 2021-03-23 | Novavax, Inc. | Coronavirus vaccine formulations |
| US20230075527A1 (en) | 2020-01-31 | 2023-03-09 | Janssen Pharmaceuticals, Inc | Compositions and Methods for Preventing and Treating Coronavirus Infection - Sars-Cov-2 Vaccines |
| US10906944B2 (en) | 2020-06-29 | 2021-02-02 | The Scripps Research Institute | Stabilized coronavirus spike (S) protein immunogens and related vaccines |
-
2021
- 2021-02-25 IL IL295697A patent/IL295697A/en unknown
- 2021-02-25 KR KR1020227032752A patent/KR20220157969A/ko active Pending
- 2021-02-25 US US17/185,458 patent/US11660335B2/en active Active
- 2021-02-25 PE PE2022001819A patent/PE20230171A1/es unknown
- 2021-02-25 BR BR112022016507A patent/BR112022016507A2/pt unknown
- 2021-02-25 CN CN202180030363.8A patent/CN115867349A/zh active Pending
- 2021-02-25 CA CA3168353A patent/CA3168353A1/en active Pending
- 2021-02-25 PH PH1/2022/552249A patent/PH12022552249A1/en unknown
- 2021-02-25 EP EP21713263.8A patent/EP4110468A1/en active Pending
- 2021-02-25 JP JP2022551031A patent/JP2023511780A/ja not_active Withdrawn
- 2021-02-25 AU AU2021226567A patent/AU2021226567B2/en active Active
- 2021-02-25 MX MX2022010460A patent/MX2022010460A/es unknown
- 2021-02-25 WO PCT/US2021/019662 patent/WO2021173829A1/en not_active Ceased
- 2021-02-25 CU CU2022000050A patent/CU24743B1/es unknown
-
2022
- 2022-08-29 CO CONC2022/0012272A patent/CO2022012272A2/es unknown
-
2023
- 2023-05-02 US US18/311,056 patent/US20230338515A1/en not_active Abandoned
-
2024
- 2024-10-18 JP JP2024184185A patent/JP2025010171A/ja active Pending
- 2024-12-20 US US18/990,242 patent/US20250121054A1/en active Pending
-
2025
- 2025-07-17 AU AU2025205555A patent/AU2025205555A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20210268102A1 (en) | 2021-09-02 |
| PH12022552249A1 (en) | 2023-11-13 |
| US20230338515A1 (en) | 2023-10-26 |
| US11660335B2 (en) | 2023-05-30 |
| AU2025205555A1 (en) | 2025-08-07 |
| EP4110468A1 (en) | 2023-01-04 |
| JP2023511780A (ja) | 2023-03-22 |
| CO2022012272A2 (es) | 2022-11-29 |
| AU2021226567A1 (en) | 2022-10-13 |
| WO2021173829A1 (en) | 2021-09-02 |
| CU20220050A7 (es) | 2023-04-10 |
| JP2025010171A (ja) | 2025-01-20 |
| PE20230171A1 (es) | 2023-02-01 |
| CU24743B1 (es) | 2025-03-10 |
| AU2021226567B2 (en) | 2025-04-17 |
| MX2022010460A (es) | 2022-09-19 |
| BR112022016507A2 (pt) | 2022-11-22 |
| CA3168353A1 (en) | 2021-09-02 |
| IL295697A (en) | 2022-10-01 |
| CN115867349A (zh) | 2023-03-28 |
| US20250121054A1 (en) | 2025-04-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11660335B2 (en) | Vaccines against coronavirus and methods of use | |
| US11382968B2 (en) | Coronavirus immunogenic compositions and uses thereof | |
| CN109152826B (zh) | 新抗寨卡病毒疫苗 | |
| AU2024205755B2 (en) | Treatment of covid-19 and methods therefor | |
| JP2019509350A (ja) | Dna抗体構築物及びその使用方法 | |
| US20210260181A1 (en) | Coronavirus immunogenic compositions and uses thereof | |
| US20220331421A1 (en) | Methods of Inducing Immune Response Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern | |
| US20220370598A1 (en) | Vaccines Against Coronavirus and Methods of Use | |
| US20220339280A1 (en) | Immunogenic Compositions Against SARS-COV-2 Variants and Their Methods of Use | |
| US20220370600A1 (en) | Multigenic mva-sars-cov-2 vaccine | |
| CN117479953A (zh) | 针对冠状病毒的疫苗及其使用方法 | |
| HK40091328A (zh) | 针对冠状病毒的疫苗和使用方法 | |
| EA046179B1 (ru) | Вакцины против коронавируса и способы их применения | |
| US20230210981A1 (en) | Coronavirus disease 2019 (covid-19) combination vaccine | |
| US12290558B2 (en) | SARS-CoV-2 vaccines comprising human adenovirus vectors encoding spike and nucleocapsid-ETSD immunogens | |
| CN117545502A (zh) | 诱导针对严重急性呼吸系统综合症冠状病毒2(sars-cov-2)有关变种的免疫应答的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| D21 | Rejection of application intended |
Free format text: ST27 STATUS EVENT CODE: A-1-2-D10-D21-EXM-PE0902 (AS PROVIDED BY THE NATIONAL OFFICE) |
|
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |