KR20180002750A - 폐 전달용 졸미트립탄 분말 - Google Patents
폐 전달용 졸미트립탄 분말 Download PDFInfo
- Publication number
- KR20180002750A KR20180002750A KR1020177034671A KR20177034671A KR20180002750A KR 20180002750 A KR20180002750 A KR 20180002750A KR 1020177034671 A KR1020177034671 A KR 1020177034671A KR 20177034671 A KR20177034671 A KR 20177034671A KR 20180002750 A KR20180002750 A KR 20180002750A
- Authority
- KR
- South Korea
- Prior art keywords
- zolmitriptan
- powder
- powder formulation
- formulation
- particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000000843 powder Substances 0.000 title claims abstract description 256
- 229960001360 zolmitriptan Drugs 0.000 title claims abstract description 216
- 230000002685 pulmonary effect Effects 0.000 title claims abstract description 26
- UTAZCRNOSWWEFR-ZDUSSCGKSA-N zolmitriptan Chemical compound C=1[C]2C(CCN(C)C)=CN=C2C=CC=1C[C@H]1COC(=O)N1 UTAZCRNOSWWEFR-ZDUSSCGKSA-N 0.000 title claims abstract 27
- 239000000203 mixture Substances 0.000 claims abstract description 226
- 238000009472 formulation Methods 0.000 claims abstract description 198
- 150000003839 salts Chemical class 0.000 claims abstract description 30
- 238000011282 treatment Methods 0.000 claims abstract description 17
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 97
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical group [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 83
- 239000002245 particle Substances 0.000 claims description 79
- 235000002639 sodium chloride Nutrition 0.000 claims description 73
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 claims description 71
- 229960003136 leucine Drugs 0.000 claims description 61
- 239000011780 sodium chloride Substances 0.000 claims description 42
- 238000000034 method Methods 0.000 claims description 41
- 239000004395 L-leucine Substances 0.000 claims description 35
- 235000019454 L-leucine Nutrition 0.000 claims description 34
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 26
- 208000019695 Migraine disease Diseases 0.000 claims description 22
- 210000004072 lung Anatomy 0.000 claims description 22
- 206010027599 migraine Diseases 0.000 claims description 22
- 150000003904 phospholipids Chemical class 0.000 claims description 21
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 18
- 229940024606 amino acid Drugs 0.000 claims description 16
- 235000001014 amino acid Nutrition 0.000 claims description 16
- 150000001413 amino acids Chemical class 0.000 claims description 16
- 208000006561 Cluster Headache Diseases 0.000 claims description 13
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 13
- 208000018912 cluster headache syndrome Diseases 0.000 claims description 13
- 235000000346 sugar Nutrition 0.000 claims description 13
- 150000005846 sugar alcohols Chemical class 0.000 claims description 12
- 229920002774 Maltodextrin Polymers 0.000 claims description 11
- 239000005913 Maltodextrin Substances 0.000 claims description 11
- 229940035034 maltodextrin Drugs 0.000 claims description 11
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 claims description 8
- -1 polyglycitol Substances 0.000 claims description 7
- 239000001509 sodium citrate Substances 0.000 claims description 7
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 claims description 7
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 6
- 239000012458 free base Substances 0.000 claims description 5
- 239000001103 potassium chloride Substances 0.000 claims description 4
- 235000011164 potassium chloride Nutrition 0.000 claims description 4
- 150000008163 sugars Chemical class 0.000 claims description 4
- 239000004471 Glycine Substances 0.000 claims description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 3
- 239000008101 lactose Substances 0.000 claims description 3
- 210000002345 respiratory system Anatomy 0.000 claims description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 claims description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 2
- 229930195725 Mannitol Natural products 0.000 claims description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 claims description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 2
- 235000004279 alanine Nutrition 0.000 claims description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 claims description 2
- 230000002209 hydrophobic effect Effects 0.000 claims description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 2
- 229960000310 isoleucine Drugs 0.000 claims description 2
- 239000000594 mannitol Substances 0.000 claims description 2
- 235000010355 mannitol Nutrition 0.000 claims description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 2
- 230000029058 respiratory gaseous exchange Effects 0.000 claims description 2
- 235000011083 sodium citrates Nutrition 0.000 claims description 2
- 239000004474 valine Substances 0.000 claims description 2
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 claims 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 claims 1
- 239000001540 sodium lactate Substances 0.000 claims 1
- 229940005581 sodium lactate Drugs 0.000 claims 1
- 235000011088 sodium lactate Nutrition 0.000 claims 1
- 201000010099 disease Diseases 0.000 abstract 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract 1
- ULSDMUVEXKOYBU-ZDUSSCGKSA-N zolmitriptan Chemical compound C1=C2C(CCN(C)C)=CNC2=CC=C1C[C@H]1COC(=O)N1 ULSDMUVEXKOYBU-ZDUSSCGKSA-N 0.000 description 194
- 239000002775 capsule Substances 0.000 description 57
- 238000011156 evaluation Methods 0.000 description 47
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 35
- 239000007787 solid Substances 0.000 description 30
- 230000000694 effects Effects 0.000 description 24
- 238000000634 powder X-ray diffraction Methods 0.000 description 22
- 238000001694 spray drying Methods 0.000 description 21
- 239000000443 aerosol Substances 0.000 description 19
- 229910052757 nitrogen Inorganic materials 0.000 description 16
- 238000003860 storage Methods 0.000 description 16
- 230000008569 process Effects 0.000 description 15
- 239000007921 spray Substances 0.000 description 15
- 239000001301 oxygen Substances 0.000 description 14
- 229910052760 oxygen Inorganic materials 0.000 description 14
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 13
- 238000012512 characterization method Methods 0.000 description 13
- 239000000126 substance Substances 0.000 description 13
- 239000006096 absorbing agent Substances 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- 239000003814 drug Substances 0.000 description 12
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 12
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 12
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 12
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 12
- 239000000463 material Substances 0.000 description 12
- 238000005259 measurement Methods 0.000 description 12
- 230000002829 reductive effect Effects 0.000 description 12
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 10
- 230000008901 benefit Effects 0.000 description 10
- 230000008859 change Effects 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 10
- 239000007789 gas Substances 0.000 description 10
- 238000004806 packaging method and process Methods 0.000 description 10
- 239000012071 phase Substances 0.000 description 10
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 239000002904 solvent Substances 0.000 description 9
- 239000008346 aqueous phase Substances 0.000 description 8
- 230000001839 systemic circulation Effects 0.000 description 8
- 208000002193 Pain Diseases 0.000 description 7
- 238000010521 absorption reaction Methods 0.000 description 7
- 230000009477 glass transition Effects 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 238000011068 loading method Methods 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 208000016261 weight loss Diseases 0.000 description 7
- 230000004580 weight loss Effects 0.000 description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- 229910052782 aluminium Inorganic materials 0.000 description 6
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 6
- 238000011049 filling Methods 0.000 description 6
- 230000001965 increasing effect Effects 0.000 description 6
- 238000001953 recrystallisation Methods 0.000 description 6
- 230000007704 transition Effects 0.000 description 6
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 5
- 101100136727 Caenorhabditis elegans psd-1 gene Proteins 0.000 description 5
- 206010013911 Dysgeusia Diseases 0.000 description 5
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 5
- 206010028813 Nausea Diseases 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000001035 drying Methods 0.000 description 5
- 238000002844 melting Methods 0.000 description 5
- 230000008018 melting Effects 0.000 description 5
- 230000008693 nausea Effects 0.000 description 5
- 230000003647 oxidation Effects 0.000 description 5
- 238000007254 oxidation reaction Methods 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 238000012552 review Methods 0.000 description 5
- 239000004408 titanium dioxide Substances 0.000 description 5
- 238000012546 transfer Methods 0.000 description 5
- 239000002552 dosage form Substances 0.000 description 4
- 239000002207 metabolite Substances 0.000 description 4
- 238000010926 purge Methods 0.000 description 4
- 238000005245 sintering Methods 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- 230000010512 thermal transition Effects 0.000 description 4
- ZISSAWUMDACLOM-UHFFFAOYSA-N triptane Chemical compound CC(C)C(C)(C)C ZISSAWUMDACLOM-UHFFFAOYSA-N 0.000 description 4
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 4
- CITHEXJVPOWHKC-UUWRZZSWSA-N 1,2-di-O-myristoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCC CITHEXJVPOWHKC-UUWRZZSWSA-N 0.000 description 3
- 229920002245 Dextrose equivalent Polymers 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 206010034960 Photophobia Diseases 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000002250 absorbent Substances 0.000 description 3
- 230000002745 absorbent Effects 0.000 description 3
- 230000002411 adverse Effects 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 229960005070 ascorbic acid Drugs 0.000 description 3
- 238000002425 crystallisation Methods 0.000 description 3
- 230000008025 crystallization Effects 0.000 description 3
- 229960003724 dimyristoylphosphatidylcholine Drugs 0.000 description 3
- 229910001873 dinitrogen Inorganic materials 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 229940112141 dry powder inhaler Drugs 0.000 description 3
- 238000012854 evaluation process Methods 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 229940097496 nasal spray Drugs 0.000 description 3
- 239000007922 nasal spray Substances 0.000 description 3
- 239000012074 organic phase Substances 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 208000019899 phobic disease Diseases 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 238000013097 stability assessment Methods 0.000 description 3
- 230000003068 static effect Effects 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 206010019233 Headaches Diseases 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 229940087168 alpha tocopherol Drugs 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000003125 aqueous solvent Substances 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 235000019658 bitter taste Nutrition 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000000679 carrageenan Substances 0.000 description 2
- 235000010418 carrageenan Nutrition 0.000 description 2
- 229920001525 carrageenan Polymers 0.000 description 2
- 229940113118 carrageenan Drugs 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 238000002144 chemical decomposition reaction Methods 0.000 description 2
- 230000003750 conditioning effect Effects 0.000 description 2
- 238000000113 differential scanning calorimetry Methods 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 238000010579 first pass effect Methods 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 231100000869 headache Toxicity 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 210000003928 nasal cavity Anatomy 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 239000007935 oral tablet Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 231100000279 safety data Toxicity 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 239000007962 solid dispersion Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- KQKPFRSPSRPDEB-UHFFFAOYSA-N sumatriptan Chemical compound CNS(=O)(=O)CC1=CC=C2NC=C(CCN(C)C)C2=C1 KQKPFRSPSRPDEB-UHFFFAOYSA-N 0.000 description 2
- 229960003708 sumatriptan Drugs 0.000 description 2
- 235000019640 taste Nutrition 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 229960000984 tocofersolan Drugs 0.000 description 2
- 238000005303 weighing Methods 0.000 description 2
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 description 2
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 2
- 239000002076 α-tocopherol Substances 0.000 description 2
- 235000004835 α-tocopherol Nutrition 0.000 description 2
- 206010002383 Angina Pectoris Diseases 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 150000008575 L-amino acids Chemical class 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- 206010028391 Musculoskeletal Pain Diseases 0.000 description 1
- QGGCHSMZXKNGCK-GFCCVEGCSA-N N-Desmethylzolmitriptan Chemical compound C1=C2C(CCNC)=CNC2=CC=C1C[C@@H]1COC(=O)N1 QGGCHSMZXKNGCK-GFCCVEGCSA-N 0.000 description 1
- 206010028836 Neck pain Diseases 0.000 description 1
- 206010068319 Oropharyngeal pain Diseases 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002562 Polyethylene Glycol 3350 Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 206010040108 Serotonin syndrome Diseases 0.000 description 1
- 208000007613 Shoulder Pain Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 239000011358 absorbing material Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 230000006793 arrhythmia Effects 0.000 description 1
- 230000001174 ascending effect Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000013096 assay test Methods 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000001427 coherent effect Effects 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000002716 delivery method Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 229940126534 drug product Drugs 0.000 description 1
- 210000001198 duodenum Anatomy 0.000 description 1
- LMBMDLOSPKIWAP-UHFFFAOYSA-N embutramide Chemical class OCCCC(=O)NCC(CC)(CC)C1=CC=CC(OC)=C1 LMBMDLOSPKIWAP-UHFFFAOYSA-N 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 230000030136 gastric emptying Effects 0.000 description 1
- 230000030135 gastric motility Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 208000020442 loss of weight Diseases 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 235000019426 modified starch Nutrition 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 208000031225 myocardial ischemia Diseases 0.000 description 1
- GZYCQRZFJIZOKU-ZDUSSCGKSA-N n,n-dimethyl-2-[5-[[(4s)-2-oxo-1,3-oxazolidin-4-yl]methyl]-1h-indol-3-yl]ethanamine oxide Chemical compound C1=C2C(CC[N+](C)([O-])C)=CNC2=CC=C1C[C@H]1COC(=O)N1 GZYCQRZFJIZOKU-ZDUSSCGKSA-N 0.000 description 1
- 229940100656 nasal solution Drugs 0.000 description 1
- 229940126701 oral medication Drugs 0.000 description 1
- 229940096978 oral tablet Drugs 0.000 description 1
- 238000010525 oxidative degradation reaction Methods 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 108010094020 polyglycine Proteins 0.000 description 1
- 229920000232 polyglycine polymer Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- PHZLMBHDXVLRIX-UHFFFAOYSA-M potassium lactate Chemical compound [K+].CC(O)C([O-])=O PHZLMBHDXVLRIX-UHFFFAOYSA-M 0.000 description 1
- 239000001521 potassium lactate Substances 0.000 description 1
- 235000011085 potassium lactate Nutrition 0.000 description 1
- 229960001304 potassium lactate Drugs 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000009325 pulmonary function Effects 0.000 description 1
- 238000002106 pulse oximetry Methods 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 231100000628 reference dose Toxicity 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 239000013049 sediment Substances 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000013112 stability test Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000002076 thermal analysis method Methods 0.000 description 1
- 238000002411 thermogravimetry Methods 0.000 description 1
- 208000023409 throat pain Diseases 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- 230000002455 vasospastic effect Effects 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
- 239000011592 zinc chloride Substances 0.000 description 1
- 235000005074 zinc chloride Nutrition 0.000 description 1
- 229940003675 zomig Drugs 0.000 description 1
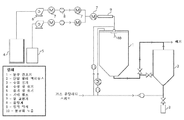
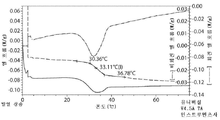
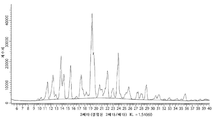
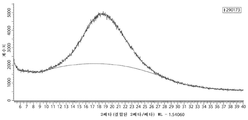
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
- A61K31/422—Oxazoles not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/02—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
- A61K47/183—Amino acids, e.g. glycine, EDTA or aspartame
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/24—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing atoms other than carbon, hydrogen, oxygen, halogen, nitrogen or sulfur, e.g. cyclomethicone or phospholipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
- A61K9/0075—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy for inhalation via a dry powder inhaler [DPI], e.g. comprising micronized drug mixed with lactose carrier particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1611—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Pulmonology (AREA)
- Otolaryngology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Inorganic Chemistry (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562155910P | 2015-05-01 | 2015-05-01 | |
| US62/155,910 | 2015-05-01 | ||
| PCT/US2016/030219 WO2016179026A1 (en) | 2015-05-01 | 2016-04-29 | Zolmitriptan powders for pulmonary delivery |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180002750A true KR20180002750A (ko) | 2018-01-08 |
Family
ID=56027187
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177034671A Withdrawn KR20180002750A (ko) | 2015-05-01 | 2016-04-29 | 폐 전달용 졸미트립탄 분말 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20160317503A1 (enExample) |
| EP (1) | EP3288591A1 (enExample) |
| JP (1) | JP2018514564A (enExample) |
| KR (1) | KR20180002750A (enExample) |
| AU (1) | AU2016257729A1 (enExample) |
| CA (1) | CA2984339A1 (enExample) |
| HK (1) | HK1251998A1 (enExample) |
| MX (1) | MX2017013950A (enExample) |
| WO (1) | WO2016179026A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017004501A1 (en) * | 2015-07-02 | 2017-01-05 | Civitas Therapeutics, Inc. | Triptan powders for pulmonary delivery |
| EP3727418A4 (en) * | 2017-12-21 | 2021-10-27 | Civitas Therapeutics, Inc. | SURFACTANT FORMULATIONS FOR INHALATION |
| MX2021005508A (es) * | 2018-11-13 | 2021-09-08 | Civitas Therapeutics Inc | Fórmulaciones y procesos de polinucleótido en polvo respirable para inhalación. |
| JP2023526098A (ja) | 2020-05-18 | 2023-06-20 | オレクソ・アクチエボラゲット | 薬物送達のための新しい医薬組成物 |
| GB202117016D0 (en) | 2021-11-25 | 2022-01-12 | Orexo Ab | New pharmaceutical device |
| CA3238850A1 (en) | 2021-11-25 | 2023-06-01 | Jonas Savmarker | Pharmaceutical composition comprising adrenaline |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6613308B2 (en) * | 2000-09-19 | 2003-09-02 | Advanced Inhalation Research, Inc. | Pulmonary delivery in treating disorders of the central nervous system |
| US6766799B2 (en) | 2001-04-16 | 2004-07-27 | Advanced Inhalation Research, Inc. | Inhalation device |
| US6848197B2 (en) | 2001-04-18 | 2005-02-01 | Advanced Inhalation Research, Inc. | Control of process humidity to produce large, porous particles |
| ES2718455T3 (es) * | 2002-03-20 | 2019-07-02 | Civitas Therapeutics Inc | Formulaciones terapéuticas sostenidas inhalables |
| US7763280B2 (en) | 2002-11-28 | 2010-07-27 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Tiotropium containing powder formulation for inhalation |
| WO2008156586A2 (en) | 2007-06-12 | 2008-12-24 | Alkermes, Inc. | Inhalation device for powdered substances |
| JP2011510964A (ja) * | 2008-02-01 | 2011-04-07 | ヴェクトゥラ リミテッド | トリプタン類の肺用製剤 |
| EP3536368B1 (en) * | 2010-11-09 | 2025-09-17 | MannKind Corporation | Dry powder inhaler comprising a serotonin receptor agonist and a diketopiperazine for treating migraines |
| ITMI20130572A1 (it) * | 2013-04-10 | 2014-10-11 | Eratech Srl | Composizione comprendente almeno due polveri secche ottenute per spray dry per aumentare la stabilita' della formulazione |
-
2016
- 2016-04-29 HK HK18111327.1A patent/HK1251998A1/zh unknown
- 2016-04-29 AU AU2016257729A patent/AU2016257729A1/en not_active Abandoned
- 2016-04-29 KR KR1020177034671A patent/KR20180002750A/ko not_active Withdrawn
- 2016-04-29 EP EP16724183.5A patent/EP3288591A1/en not_active Withdrawn
- 2016-04-29 WO PCT/US2016/030219 patent/WO2016179026A1/en not_active Ceased
- 2016-04-29 US US15/143,131 patent/US20160317503A1/en not_active Abandoned
- 2016-04-29 JP JP2017556853A patent/JP2018514564A/ja active Pending
- 2016-04-29 CA CA2984339A patent/CA2984339A1/en not_active Abandoned
- 2016-04-29 MX MX2017013950A patent/MX2017013950A/es unknown
Also Published As
| Publication number | Publication date |
|---|---|
| MX2017013950A (es) | 2018-08-28 |
| HK1251998A1 (zh) | 2019-05-10 |
| EP3288591A1 (en) | 2018-03-07 |
| US20160317503A1 (en) | 2016-11-03 |
| AU2016257729A1 (en) | 2017-11-09 |
| JP2018514564A (ja) | 2018-06-07 |
| CA2984339A1 (en) | 2016-11-10 |
| WO2016179026A1 (en) | 2016-11-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2018222983B2 (en) | Ultra Low Density Pulmonary Powders | |
| KR20180002750A (ko) | 폐 전달용 졸미트립탄 분말 | |
| JP2018087206A (ja) | エンザルタミドの製剤 | |
| PT2630954T (pt) | Administração pulmonar de levodopa | |
| JP2021054870A (ja) | 肺送達用ラパマイシン粉末 | |
| JP2018535984A (ja) | リバビリンの医薬組成物 | |
| CN116687887A (zh) | 用于吸入的干粉制剂 | |
| JP2021520397A (ja) | 甲状腺ホルモンを含む吸入用の薬学的乾燥粉末組成物 | |
| US10034857B2 (en) | Triptan powders for pulmonary delivery | |
| WO2022187222A1 (en) | Dihydroergotamine dry power formulations and methods of use | |
| HK40023697A (en) | Ultra low density pulmonary powders | |
| HK1213187B (en) | Ultra low density pulmonary powders |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20171130 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |