KR20160060133A - 다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 - Google Patents
다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 Download PDFInfo
- Publication number
- KR20160060133A KR20160060133A KR1020167010607A KR20167010607A KR20160060133A KR 20160060133 A KR20160060133 A KR 20160060133A KR 1020167010607 A KR1020167010607 A KR 1020167010607A KR 20167010607 A KR20167010607 A KR 20167010607A KR 20160060133 A KR20160060133 A KR 20160060133A
- Authority
- KR
- South Korea
- Prior art keywords
- arg arg
- leu
- ala
- val
- gln
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 230000004048 modification Effects 0.000 title description 6
- 238000012986 modification Methods 0.000 title description 6
- 238000001476 gene delivery Methods 0.000 title description 2
- 239000002105 nanoparticle Substances 0.000 claims abstract description 111
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims abstract description 106
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 54
- 239000000377 silicon dioxide Substances 0.000 claims abstract description 53
- 102000004196 processed proteins & peptides Human genes 0.000 claims abstract description 50
- 229920001184 polypeptide Polymers 0.000 claims abstract description 48
- 229920006317 cationic polymer Polymers 0.000 claims abstract description 46
- 102000040430 polynucleotide Human genes 0.000 claims abstract description 44
- 108091033319 polynucleotide Proteins 0.000 claims abstract description 44
- 239000002157 polynucleotide Substances 0.000 claims abstract description 44
- 238000000034 method Methods 0.000 claims abstract description 43
- 229920000642 polymer Polymers 0.000 claims abstract description 43
- 238000000576 coating method Methods 0.000 claims abstract description 42
- 125000002091 cationic group Chemical group 0.000 claims abstract description 40
- 239000011248 coating agent Substances 0.000 claims abstract description 40
- 229920006318 anionic polymer Polymers 0.000 claims abstract description 33
- 230000003834 intracellular effect Effects 0.000 claims abstract description 9
- 230000014509 gene expression Effects 0.000 claims description 37
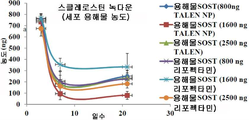
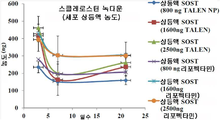
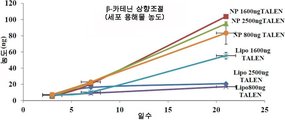
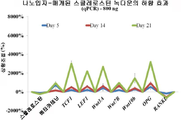
- 102000019307 Sclerostin Human genes 0.000 claims description 35
- 108050006698 Sclerostin Proteins 0.000 claims description 35
- 229920002643 polyglutamic acid Polymers 0.000 claims description 21
- 229920002873 Polyethylenimine Polymers 0.000 claims description 20
- 108010033040 Histones Proteins 0.000 claims description 17
- 108090000623 proteins and genes Proteins 0.000 claims description 15
- 241000282414 Homo sapiens Species 0.000 claims description 13
- 108010011110 polyarginine Proteins 0.000 claims description 13
- 101800000868 Tail peptide Proteins 0.000 claims description 12
- 102400001102 Tail peptide Human genes 0.000 claims description 12
- 101710163270 Nuclease Proteins 0.000 claims description 9
- 239000002773 nucleotide Substances 0.000 claims description 9
- 125000003729 nucleotide group Chemical group 0.000 claims description 9
- 235000018102 proteins Nutrition 0.000 claims description 9
- 102000004169 proteins and genes Human genes 0.000 claims description 9
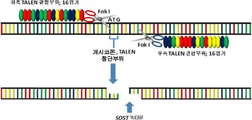
- 238000010459 TALEN Methods 0.000 claims description 5
- 108010043645 Transcription Activator-Like Effector Nucleases Proteins 0.000 claims description 5
- 108010041308 Endothelial Growth Factors Proteins 0.000 claims description 3
- 230000002227 vasoactive effect Effects 0.000 claims description 3
- 230000001939 inductive effect Effects 0.000 claims 2
- 238000001890 transfection Methods 0.000 abstract description 28
- 210000004027 cell Anatomy 0.000 description 61
- BUZMZDDKFCSKOT-CIUDSAMLSA-N Glu-Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O BUZMZDDKFCSKOT-CIUDSAMLSA-N 0.000 description 36
- QVVDVENEPNODSI-BTNSXGMBSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylidene Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O QVVDVENEPNODSI-BTNSXGMBSA-N 0.000 description 31
- 108020004414 DNA Proteins 0.000 description 30
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 26
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 25
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 25
- 230000000694 effects Effects 0.000 description 24
- LTFLDDDGWOVIHY-NAKRPEOUSA-N Val-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N LTFLDDDGWOVIHY-NAKRPEOUSA-N 0.000 description 20
- 239000013612 plasmid Substances 0.000 description 19
- XVZCXCTYGHPNEM-IHRRRGAJSA-N (2s)-1-[(2s)-2-[[(2s)-2-amino-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(O)=O XVZCXCTYGHPNEM-IHRRRGAJSA-N 0.000 description 18
- 108010068380 arginylarginine Proteins 0.000 description 16
- 239000010410 layer Substances 0.000 description 16
- UWZLBXOBVKRUFE-HGNGGELXSA-N Gln-Ala-His Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)N)N UWZLBXOBVKRUFE-HGNGGELXSA-N 0.000 description 15
- KVYVOGYEMPEXBT-GUBZILKMSA-N Gln-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(N)=O KVYVOGYEMPEXBT-GUBZILKMSA-N 0.000 description 15
- BMOFUVHDBROBSE-DCAQKATOSA-N Val-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N BMOFUVHDBROBSE-DCAQKATOSA-N 0.000 description 15
- 238000007792 addition Methods 0.000 description 15
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 13
- KLALXKYLOMZDQT-ZLUOBGJFSA-N Ala-Ser-Asn Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(N)=O KLALXKYLOMZDQT-ZLUOBGJFSA-N 0.000 description 12
- SOEXCCGNHQBFPV-DLOVCJGASA-N Gln-Val-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O SOEXCCGNHQBFPV-DLOVCJGASA-N 0.000 description 12
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 12
- KLSUAWUZBMAZCL-RHYQMDGZSA-N Leu-Thr-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(O)=O KLSUAWUZBMAZCL-RHYQMDGZSA-N 0.000 description 12
- QGVBFDIREUUSHX-IFFSRLJSSA-N Thr-Val-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O QGVBFDIREUUSHX-IFFSRLJSSA-N 0.000 description 12
- CCDFBRZVTDDJNM-GUBZILKMSA-N Ala-Leu-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O CCDFBRZVTDDJNM-GUBZILKMSA-N 0.000 description 11
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 11
- QITBQGJOXQYMOA-ZETCQYMHSA-N Gly-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)CN QITBQGJOXQYMOA-ZETCQYMHSA-N 0.000 description 11
- QKIBIXAQKAFZGL-GUBZILKMSA-N Leu-Cys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QKIBIXAQKAFZGL-GUBZILKMSA-N 0.000 description 11
- PRBLYKYHAJEABA-SRVKXCTJSA-N Gln-Arg-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O PRBLYKYHAJEABA-SRVKXCTJSA-N 0.000 description 10
- DLISPGXMKZTWQG-IFFSRLJSSA-N Glu-Thr-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O DLISPGXMKZTWQG-IFFSRLJSSA-N 0.000 description 10
- 210000000963 osteoblast Anatomy 0.000 description 10
- 239000000243 solution Substances 0.000 description 10
- PNALXAODQKTNLV-JBDRJPRFSA-N Ala-Ile-Ala Chemical compound C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O PNALXAODQKTNLV-JBDRJPRFSA-N 0.000 description 8
- FHQRLHFYVZAQHU-IUCAKERBSA-N Gly-Lys-Gln Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O FHQRLHFYVZAQHU-IUCAKERBSA-N 0.000 description 8
- RGPWUJOMKFYFSR-QWRGUYRKSA-N His-Gly-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O RGPWUJOMKFYFSR-QWRGUYRKSA-N 0.000 description 8
- ZFNLIDNJUWNIJL-WDCWCFNPSA-N Leu-Glu-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZFNLIDNJUWNIJL-WDCWCFNPSA-N 0.000 description 8
- JDBQSGMJBMPNFT-AVGNSLFASA-N Leu-Pro-Val Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O JDBQSGMJBMPNFT-AVGNSLFASA-N 0.000 description 8
- DFXQCCBKGUNYGG-GUBZILKMSA-N Lys-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN DFXQCCBKGUNYGG-GUBZILKMSA-N 0.000 description 8
- FISHYTLIMUYTQY-GUBZILKMSA-N Pro-Gln-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1 FISHYTLIMUYTQY-GUBZILKMSA-N 0.000 description 8
- 230000030648 nucleus localization Effects 0.000 description 8
- OKEWAFFWMHBGPT-XPUUQOCRSA-N Ala-His-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CN=CN1 OKEWAFFWMHBGPT-XPUUQOCRSA-N 0.000 description 7
- 229910019142 PO4 Inorganic materials 0.000 description 7
- 102000014128 RANK Ligand Human genes 0.000 description 7
- 108010025832 RANK Ligand Proteins 0.000 description 7
- XERQKTRGJIKTRB-CIUDSAMLSA-N Ser-His-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CO)N)CC1=CN=CN1 XERQKTRGJIKTRB-CIUDSAMLSA-N 0.000 description 7
- 108010047857 aspartylglycine Proteins 0.000 description 7
- 230000033558 biomineral tissue development Effects 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 230000015556 catabolic process Effects 0.000 description 7
- 230000009918 complex formation Effects 0.000 description 7
- 238000006731 degradation reaction Methods 0.000 description 7
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 7
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 7
- 239000012634 fragment Substances 0.000 description 7
- 238000003197 gene knockdown Methods 0.000 description 7
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 7
- 108010050848 glycylleucine Proteins 0.000 description 7
- 108010015792 glycyllysine Proteins 0.000 description 7
- 239000010452 phosphate Substances 0.000 description 7
- 108010070643 prolylglutamic acid Proteins 0.000 description 7
- JAMAWBXXKFGFGX-KZVJFYERSA-N Ala-Arg-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JAMAWBXXKFGFGX-KZVJFYERSA-N 0.000 description 6
- 125000000129 anionic group Chemical group 0.000 description 6
- 230000027455 binding Effects 0.000 description 6
- 230000001687 destabilization Effects 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 150000007523 nucleic acids Chemical class 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 5
- LWUWMHIOBPTZBA-DCAQKATOSA-N Ala-Arg-Lys Chemical compound NC(=N)NCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CCCCN)C(O)=O LWUWMHIOBPTZBA-DCAQKATOSA-N 0.000 description 5
- 102000015735 Beta-catenin Human genes 0.000 description 5
- 108060000903 Beta-catenin Proteins 0.000 description 5
- HTTSBEBKVNEDFE-AUTRQRHGSA-N Glu-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N HTTSBEBKVNEDFE-AUTRQRHGSA-N 0.000 description 5
- HEWWNLVEWBJBKA-WDCWCFNPSA-N Lys-Gln-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN HEWWNLVEWBJBKA-WDCWCFNPSA-N 0.000 description 5
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 5
- XTAUQCGQFJQGEJ-NHCYSSNCSA-N Val-Gln-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N XTAUQCGQFJQGEJ-NHCYSSNCSA-N 0.000 description 5
- 108010087924 alanylproline Proteins 0.000 description 5
- 108010093581 aspartyl-proline Proteins 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 108020004999 messenger RNA Proteins 0.000 description 5
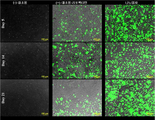
- 238000001000 micrograph Methods 0.000 description 5
- 102000039446 nucleic acids Human genes 0.000 description 5
- 108020004707 nucleic acids Proteins 0.000 description 5
- 229920000744 poly(arginines) Polymers 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- NHWYNIZWLJYZAG-XVYDVKMFSA-N Ala-Ser-His Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N NHWYNIZWLJYZAG-XVYDVKMFSA-N 0.000 description 4
- OMMIEVATLAGRCK-BYPYZUCNSA-N Asp-Gly-Gly Chemical compound OC(=O)C[C@H](N)C(=O)NCC(=O)NCC(O)=O OMMIEVATLAGRCK-BYPYZUCNSA-N 0.000 description 4
- BVFQOPGFOQVZTE-ACZMJKKPSA-N Cys-Gln-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O BVFQOPGFOQVZTE-ACZMJKKPSA-N 0.000 description 4
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 4
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 4
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 4
- HDOYNXLPTRQLAD-JBDRJPRFSA-N Ile-Ala-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)O)N HDOYNXLPTRQLAD-JBDRJPRFSA-N 0.000 description 4
- CDGLBYSAZFIIJO-RCOVLWMOSA-N Ile-Gly-Gly Chemical compound CC[C@H](C)[C@H]([NH3+])C(=O)NCC(=O)NCC([O-])=O CDGLBYSAZFIIJO-RCOVLWMOSA-N 0.000 description 4
- YJRSIJZUIUANHO-NAKRPEOUSA-N Ile-Val-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)O)N YJRSIJZUIUANHO-NAKRPEOUSA-N 0.000 description 4
- 102100022699 Lymphoid enhancer-binding factor 1 Human genes 0.000 description 4
- VDGTVWFMRXVQCT-GUBZILKMSA-N Pro-Glu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 VDGTVWFMRXVQCT-GUBZILKMSA-N 0.000 description 4
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 4
- OOKCGAYXSNJBGQ-ZLUOBGJFSA-N Ser-Asn-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O OOKCGAYXSNJBGQ-ZLUOBGJFSA-N 0.000 description 4
- PURRNJBBXDDWLX-ZDLURKLDSA-N Ser-Thr-Gly Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CO)N)O PURRNJBBXDDWLX-ZDLURKLDSA-N 0.000 description 4
- 230000009102 absorption Effects 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 108010070944 alanylhistidine Proteins 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 150000007942 carboxylates Chemical class 0.000 description 4
- -1 cationic amino acid Chemical class 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 239000012091 fetal bovine serum Substances 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- 108010038320 lysylphenylalanine Proteins 0.000 description 4
- 210000004940 nucleus Anatomy 0.000 description 4
- 108020003175 receptors Proteins 0.000 description 4
- 102000005962 receptors Human genes 0.000 description 4
- 230000011664 signaling Effects 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- RIIVUOJDDQXHRV-SRVKXCTJSA-N Arg-Lys-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O RIIVUOJDDQXHRV-SRVKXCTJSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- UFNSPPFJOHNXRE-AUTRQRHGSA-N Gln-Gln-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UFNSPPFJOHNXRE-AUTRQRHGSA-N 0.000 description 3
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 3
- GLYJPWIRLBAIJH-UHFFFAOYSA-N Ile-Lys-Pro Natural products CCC(C)C(N)C(=O)NC(CCCCN)C(=O)N1CCCC1C(O)=O GLYJPWIRLBAIJH-UHFFFAOYSA-N 0.000 description 3
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 3
- 108010043958 Peptoids Proteins 0.000 description 3
- IFMDQWDAJUMMJC-DCAQKATOSA-N Pro-Ala-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O IFMDQWDAJUMMJC-DCAQKATOSA-N 0.000 description 3
- DWGFLKQSGRUQTI-IHRRRGAJSA-N Pro-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1 DWGFLKQSGRUQTI-IHRRRGAJSA-N 0.000 description 3
- 238000011529 RT qPCR Methods 0.000 description 3
- 108091081024 Start codon Proteins 0.000 description 3
- LKJCABTUFGTPPY-HJGDQZAQSA-N Thr-Pro-Gln Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O LKJCABTUFGTPPY-HJGDQZAQSA-N 0.000 description 3
- 108050003627 Wnt Proteins 0.000 description 3
- 102000013814 Wnt Human genes 0.000 description 3
- 230000021736 acetylation Effects 0.000 description 3
- 238000006640 acetylation reaction Methods 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 108010077245 asparaginyl-proline Proteins 0.000 description 3
- 108010068265 aspartyltyrosine Proteins 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 239000013613 expression plasmid Substances 0.000 description 3
- 108010025306 histidylleucine Proteins 0.000 description 3
- 210000002997 osteoclast Anatomy 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 230000002035 prolonged effect Effects 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 108010073969 valyllysine Proteins 0.000 description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- DVWVZSJAYIJZFI-FXQIFTODSA-N Ala-Arg-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O DVWVZSJAYIJZFI-FXQIFTODSA-N 0.000 description 2
- WDIYWDJLXOCGRW-ACZMJKKPSA-N Ala-Asp-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WDIYWDJLXOCGRW-ACZMJKKPSA-N 0.000 description 2
- OQCPATDFWYYDDX-HGNGGELXSA-N Ala-Gln-His Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O OQCPATDFWYYDDX-HGNGGELXSA-N 0.000 description 2
- BLGHHPHXVJWCNK-GUBZILKMSA-N Ala-Gln-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O BLGHHPHXVJWCNK-GUBZILKMSA-N 0.000 description 2
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 2
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 2
- AETQNIIFKCMVHP-UVBJJODRSA-N Ala-Trp-Arg Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AETQNIIFKCMVHP-UVBJJODRSA-N 0.000 description 2
- 108091023037 Aptamer Proteins 0.000 description 2
- HJVGMOYJDDXLMI-AVGNSLFASA-N Arg-Arg-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N HJVGMOYJDDXLMI-AVGNSLFASA-N 0.000 description 2
- MAISCYVJLBBRNU-DCAQKATOSA-N Arg-Asn-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N MAISCYVJLBBRNU-DCAQKATOSA-N 0.000 description 2
- GRRXPUAICOGISM-RWMBFGLXSA-N Arg-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O GRRXPUAICOGISM-RWMBFGLXSA-N 0.000 description 2
- BSYKSCBTTQKOJG-GUBZILKMSA-N Arg-Pro-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O BSYKSCBTTQKOJG-GUBZILKMSA-N 0.000 description 2
- XYOVHPDDWCEUDY-CIUDSAMLSA-N Asn-Ala-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O XYOVHPDDWCEUDY-CIUDSAMLSA-N 0.000 description 2
- PCKRJVZAQZWNKM-WHFBIAKZSA-N Asn-Asn-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O PCKRJVZAQZWNKM-WHFBIAKZSA-N 0.000 description 2
- HUAOKVVEVHACHR-CIUDSAMLSA-N Asn-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)N)N HUAOKVVEVHACHR-CIUDSAMLSA-N 0.000 description 2
- CDGHMJJJHYKMPA-DLOVCJGASA-N Asn-Phe-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CC(=O)N)N CDGHMJJJHYKMPA-DLOVCJGASA-N 0.000 description 2
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 2
- RGKKALNPOYURGE-ZKWXMUAHSA-N Asp-Ala-Val Chemical compound N[C@@H](CC(=O)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O RGKKALNPOYURGE-ZKWXMUAHSA-N 0.000 description 2
- HMQDRBKQMLRCCG-GMOBBJLQSA-N Asp-Arg-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HMQDRBKQMLRCCG-GMOBBJLQSA-N 0.000 description 2
- DTNUIAJCPRMNBT-WHFBIAKZSA-N Asp-Gly-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](C)C(O)=O DTNUIAJCPRMNBT-WHFBIAKZSA-N 0.000 description 2
- KTTCQQNRRLCIBC-GHCJXIJMSA-N Asp-Ile-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O KTTCQQNRRLCIBC-GHCJXIJMSA-N 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 230000004568 DNA-binding Effects 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- XFKUFUJECJUQTQ-CIUDSAMLSA-N Gln-Gln-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O XFKUFUJECJUQTQ-CIUDSAMLSA-N 0.000 description 2
- YPMDZWPZFOZYFG-GUBZILKMSA-N Gln-Leu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YPMDZWPZFOZYFG-GUBZILKMSA-N 0.000 description 2
- CVRUVYDNRPSKBM-QEJZJMRPSA-N Gln-Trp-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N CVRUVYDNRPSKBM-QEJZJMRPSA-N 0.000 description 2
- FYBSCGZLICNOBA-XQXXSGGOSA-N Glu-Ala-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FYBSCGZLICNOBA-XQXXSGGOSA-N 0.000 description 2
- NCWOMXABNYEPLY-NRPADANISA-N Glu-Ala-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O NCWOMXABNYEPLY-NRPADANISA-N 0.000 description 2
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 2
- IRXNJYPKBVERCW-DCAQKATOSA-N Glu-Leu-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O IRXNJYPKBVERCW-DCAQKATOSA-N 0.000 description 2
- ZIYGTCDTJJCDDP-JYJNAYRXSA-N Glu-Phe-Lys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZIYGTCDTJJCDDP-JYJNAYRXSA-N 0.000 description 2
- JRDYDYXZKFNNRQ-XPUUQOCRSA-N Gly-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN JRDYDYXZKFNNRQ-XPUUQOCRSA-N 0.000 description 2
- STVHDEHTKFXBJQ-LAEOZQHASA-N Gly-Glu-Ile Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O STVHDEHTKFXBJQ-LAEOZQHASA-N 0.000 description 2
- GWNIGUKSRJBIHX-STQMWFEESA-N Gly-Tyr-Arg Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)CN)O GWNIGUKSRJBIHX-STQMWFEESA-N 0.000 description 2
- ZVXMEWXHFBYJPI-LSJOCFKGSA-N Gly-Val-Ile Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZVXMEWXHFBYJPI-LSJOCFKGSA-N 0.000 description 2
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 2
- VJJSDSNFXCWCEJ-DJFWLOJKSA-N His-Ile-Asn Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O VJJSDSNFXCWCEJ-DJFWLOJKSA-N 0.000 description 2
- UXSATKFPUVZVDK-KKUMJFAQSA-N His-Lys-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC1=CN=CN1)N UXSATKFPUVZVDK-KKUMJFAQSA-N 0.000 description 2
- ZVKDCQVQTGYBQT-LSJOCFKGSA-N His-Pro-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O ZVKDCQVQTGYBQT-LSJOCFKGSA-N 0.000 description 2
- 102000003893 Histone acetyltransferases Human genes 0.000 description 2
- 108090000246 Histone acetyltransferases Proteins 0.000 description 2
- 101001045751 Homo sapiens Hepatocyte nuclear factor 1-alpha Proteins 0.000 description 2
- 101000972291 Homo sapiens Lymphoid enhancer-binding factor 1 Proteins 0.000 description 2
- RWIKBYVJQAJYDP-BJDJZHNGSA-N Ile-Ala-Lys Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN RWIKBYVJQAJYDP-BJDJZHNGSA-N 0.000 description 2
- IGJWJGIHUFQANP-LAEOZQHASA-N Ile-Gly-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CCC(=O)N)C(=O)O)N IGJWJGIHUFQANP-LAEOZQHASA-N 0.000 description 2
- NGKPIPCGMLWHBX-WZLNRYEVSA-N Ile-Tyr-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N NGKPIPCGMLWHBX-WZLNRYEVSA-N 0.000 description 2
- KXUKTDGKLAOCQK-LSJOCFKGSA-N Ile-Val-Gly Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O KXUKTDGKLAOCQK-LSJOCFKGSA-N 0.000 description 2
- XBBKIIGCUMBKCO-JXUBOQSCSA-N Leu-Ala-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XBBKIIGCUMBKCO-JXUBOQSCSA-N 0.000 description 2
- STAVRDQLZOTNKJ-RHYQMDGZSA-N Leu-Arg-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O STAVRDQLZOTNKJ-RHYQMDGZSA-N 0.000 description 2
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 2
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 2
- VWHGTYCRDRBSFI-ZETCQYMHSA-N Leu-Gly-Gly Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(O)=O VWHGTYCRDRBSFI-ZETCQYMHSA-N 0.000 description 2
- UCDHVOALNXENLC-KBPBESRZSA-N Leu-Gly-Tyr Chemical compound CC(C)C[C@H]([NH3+])C(=O)NCC(=O)N[C@H](C([O-])=O)CC1=CC=C(O)C=C1 UCDHVOALNXENLC-KBPBESRZSA-N 0.000 description 2
- LXKNSJLSGPNHSK-KKUMJFAQSA-N Leu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N LXKNSJLSGPNHSK-KKUMJFAQSA-N 0.000 description 2
- AKVBOOKXVAMKSS-GUBZILKMSA-N Leu-Ser-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O AKVBOOKXVAMKSS-GUBZILKMSA-N 0.000 description 2
- SVBJIZVVYJYGLA-DCAQKATOSA-N Leu-Ser-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O SVBJIZVVYJYGLA-DCAQKATOSA-N 0.000 description 2
- AAKRWBIIGKPOKQ-ONGXEEELSA-N Leu-Val-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O AAKRWBIIGKPOKQ-ONGXEEELSA-N 0.000 description 2
- 108090001093 Lymphoid enhancer-binding factor 1 Proteins 0.000 description 2
- FZIJIFCXUCZHOL-CIUDSAMLSA-N Lys-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN FZIJIFCXUCZHOL-CIUDSAMLSA-N 0.000 description 2
- XFIHDSBIPWEYJJ-YUMQZZPRSA-N Lys-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN XFIHDSBIPWEYJJ-YUMQZZPRSA-N 0.000 description 2
- VHXMZJGOKIMETG-CQDKDKBSSA-N Lys-Ala-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CCCCN)N VHXMZJGOKIMETG-CQDKDKBSSA-N 0.000 description 2
- SJNZALDHDUYDBU-IHRRRGAJSA-N Lys-Arg-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(O)=O SJNZALDHDUYDBU-IHRRRGAJSA-N 0.000 description 2
- OWRUUFUVXFREBD-KKUMJFAQSA-N Lys-His-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O OWRUUFUVXFREBD-KKUMJFAQSA-N 0.000 description 2
- QOJDBRUCOXQSSK-AJNGGQMLSA-N Lys-Ile-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(O)=O QOJDBRUCOXQSSK-AJNGGQMLSA-N 0.000 description 2
- YUAXTFMFMOIMAM-QWRGUYRKSA-N Lys-Lys-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O YUAXTFMFMOIMAM-QWRGUYRKSA-N 0.000 description 2
- VVURYEVJJTXWNE-ULQDDVLXSA-N Lys-Tyr-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O VVURYEVJJTXWNE-ULQDDVLXSA-N 0.000 description 2
- HMZPYMSEAALNAE-ULQDDVLXSA-N Lys-Val-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O HMZPYMSEAALNAE-ULQDDVLXSA-N 0.000 description 2
- OXHSZBRPUGNMKW-DCAQKATOSA-N Met-Gln-Arg Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O OXHSZBRPUGNMKW-DCAQKATOSA-N 0.000 description 2
- DGNZGCQSVGGYJS-BQBZGAKWSA-N Met-Gly-Asp Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O DGNZGCQSVGGYJS-BQBZGAKWSA-N 0.000 description 2
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 108091093037 Peptide nucleic acid Proteins 0.000 description 2
- HTTYNOXBBOWZTB-SRVKXCTJSA-N Phe-Asn-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N HTTYNOXBBOWZTB-SRVKXCTJSA-N 0.000 description 2
- MSHZERMPZKCODG-ACRUOGEOSA-N Phe-Leu-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 MSHZERMPZKCODG-ACRUOGEOSA-N 0.000 description 2
- DEZCWWXTRAKZKJ-UFYCRDLUSA-N Phe-Phe-Met Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(O)=O DEZCWWXTRAKZKJ-UFYCRDLUSA-N 0.000 description 2
- 108010020346 Polyglutamic Acid Proteins 0.000 description 2
- OBVCYFIHIIYIQF-CIUDSAMLSA-N Pro-Asn-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O OBVCYFIHIIYIQF-CIUDSAMLSA-N 0.000 description 2
- AJCRQOHDLCBHFA-SRVKXCTJSA-N Pro-His-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O AJCRQOHDLCBHFA-SRVKXCTJSA-N 0.000 description 2
- RUDOLGWDSKQQFF-DCAQKATOSA-N Pro-Leu-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O RUDOLGWDSKQQFF-DCAQKATOSA-N 0.000 description 2
- IXUGADGDCQDLSA-FXQIFTODSA-N Ser-Gln-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N IXUGADGDCQDLSA-FXQIFTODSA-N 0.000 description 2
- OJPHFSOMBZKQKQ-GUBZILKMSA-N Ser-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CO OJPHFSOMBZKQKQ-GUBZILKMSA-N 0.000 description 2
- SZRNDHWMVSFPSP-XKBZYTNZSA-N Ser-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N)O SZRNDHWMVSFPSP-XKBZYTNZSA-N 0.000 description 2
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 2
- 108020004459 Small interfering RNA Proteins 0.000 description 2
- VFEHSAJCWWHDBH-RHYQMDGZSA-N Thr-Arg-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VFEHSAJCWWHDBH-RHYQMDGZSA-N 0.000 description 2
- SLUWOCTZVGMURC-BFHQHQDPSA-N Thr-Gly-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O SLUWOCTZVGMURC-BFHQHQDPSA-N 0.000 description 2
- VYEHBMMAJFVTOI-JHEQGTHGSA-N Thr-Gly-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O VYEHBMMAJFVTOI-JHEQGTHGSA-N 0.000 description 2
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 2
- 102100030627 Transcription factor 7 Human genes 0.000 description 2
- ASQFIHTXXMFENG-XPUUQOCRSA-N Val-Ala-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O ASQFIHTXXMFENG-XPUUQOCRSA-N 0.000 description 2
- OVLIFGQSBSNGHY-KKHAAJSZSA-N Val-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C(C)C)N)O OVLIFGQSBSNGHY-KKHAAJSZSA-N 0.000 description 2
- SYOMXKPPFZRELL-ONGXEEELSA-N Val-Gly-Lys Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)O)N SYOMXKPPFZRELL-ONGXEEELSA-N 0.000 description 2
- LKUDRJSNRWVGMS-QSFUFRPTSA-N Val-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N LKUDRJSNRWVGMS-QSFUFRPTSA-N 0.000 description 2
- JAKHAONCJJZVHT-DCAQKATOSA-N Val-Lys-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)O)N JAKHAONCJJZVHT-DCAQKATOSA-N 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 102000044880 Wnt3A Human genes 0.000 description 2
- 108700013515 Wnt3A Proteins 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 2
- 108010038633 aspartylglutamate Proteins 0.000 description 2
- 210000002798 bone marrow cell Anatomy 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 230000004700 cellular uptake Effects 0.000 description 2
- 230000000536 complexating effect Effects 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 230000005782 double-strand break Effects 0.000 description 2
- 238000013265 extended release Methods 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- HPAIKDPJURGQLN-UHFFFAOYSA-N glycyl-L-histidyl-L-phenylalanine Natural products C=1C=CC=CC=1CC(C(O)=O)NC(=O)C(NC(=O)CN)CC1=CN=CN1 HPAIKDPJURGQLN-UHFFFAOYSA-N 0.000 description 2
- 108010027668 glycyl-alanyl-valine Proteins 0.000 description 2
- 108010010096 glycyl-glycyl-tyrosine Proteins 0.000 description 2
- 108010078326 glycyl-glycyl-valine Proteins 0.000 description 2
- 108010037850 glycylvaline Proteins 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 108010028295 histidylhistidine Proteins 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- KXCLCNHUUKTANI-RBIYJLQWSA-N keratan Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@H](COS(O)(=O)=O)O[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@H](O[C@@H](O[C@H]3[C@H]([C@@H](COS(O)(=O)=O)O[C@@H](O)[C@@H]3O)O)[C@H](NC(C)=O)[C@H]2O)COS(O)(=O)=O)O[C@H](COS(O)(=O)=O)[C@@H]1O KXCLCNHUUKTANI-RBIYJLQWSA-N 0.000 description 2
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 2
- 108010047926 leucyl-lysyl-tyrosine Proteins 0.000 description 2
- 108010030617 leucyl-phenylalanyl-valine Proteins 0.000 description 2
- 108010025153 lysyl-alanyl-alanine Proteins 0.000 description 2
- 108010017391 lysylvaline Proteins 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000032965 negative regulation of cell volume Effects 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 239000013047 polymeric layer Substances 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 241000894007 species Species 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- DSLBDPPHINVUID-REOHCLBHSA-N (2s)-2-aminobutanediamide Chemical compound NC(=O)[C@@H](N)CC(N)=O DSLBDPPHINVUID-REOHCLBHSA-N 0.000 description 1
- LCTORNIWLGOBPB-GASJEMHNSA-N (3r,4s,5s,6r)-2-amino-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound NC1(O)O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O LCTORNIWLGOBPB-GASJEMHNSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- 102100036601 Aggrecan core protein Human genes 0.000 description 1
- 108010067219 Aggrecans Proteins 0.000 description 1
- FJVAQLJNTSUQPY-CIUDSAMLSA-N Ala-Ala-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN FJVAQLJNTSUQPY-CIUDSAMLSA-N 0.000 description 1
- QDRGPQWIVZNJQD-CIUDSAMLSA-N Ala-Arg-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O QDRGPQWIVZNJQD-CIUDSAMLSA-N 0.000 description 1
- CXZFXHGJJPVUJE-CIUDSAMLSA-N Ala-Cys-Leu Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)O)N CXZFXHGJJPVUJE-CIUDSAMLSA-N 0.000 description 1
- PAIHPOGPJVUFJY-WDSKDSINSA-N Ala-Glu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O PAIHPOGPJVUFJY-WDSKDSINSA-N 0.000 description 1
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 1
- QPBSRMDNJOTFAL-AICCOOGYSA-N Ala-Leu-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QPBSRMDNJOTFAL-AICCOOGYSA-N 0.000 description 1
- BHTBAVZSZCQZPT-GUBZILKMSA-N Ala-Pro-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N BHTBAVZSZCQZPT-GUBZILKMSA-N 0.000 description 1
- XCIGOVDXZULBBV-DCAQKATOSA-N Ala-Val-Lys Chemical compound CC(C)[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CCCCN)C(O)=O XCIGOVDXZULBBV-DCAQKATOSA-N 0.000 description 1
- SVHRPCMZTWZROG-DCAQKATOSA-N Arg-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)N SVHRPCMZTWZROG-DCAQKATOSA-N 0.000 description 1
- CYXCAHZVPFREJD-LURJTMIESA-N Arg-Gly-Gly Chemical compound NC(=N)NCCC[C@H](N)C(=O)NCC(=O)NCC(O)=O CYXCAHZVPFREJD-LURJTMIESA-N 0.000 description 1
- OVQJAKFLFTZDNC-GUBZILKMSA-N Arg-Pro-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O OVQJAKFLFTZDNC-GUBZILKMSA-N 0.000 description 1
- LRPZJPMQGKGHSG-XGEHTFHBSA-N Arg-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N)O LRPZJPMQGKGHSG-XGEHTFHBSA-N 0.000 description 1
- BECXEHHOZNFFFX-IHRRRGAJSA-N Arg-Ser-Tyr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BECXEHHOZNFFFX-IHRRRGAJSA-N 0.000 description 1
- UVTGNSWSRSCPLP-UHFFFAOYSA-N Arg-Tyr Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccc(O)cc1)C(=O)O UVTGNSWSRSCPLP-UHFFFAOYSA-N 0.000 description 1
- 102000004452 Arginase Human genes 0.000 description 1
- 108700024123 Arginases Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- GMRGSBAMMMVDGG-GUBZILKMSA-N Asn-Arg-Arg Chemical compound C(C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N GMRGSBAMMMVDGG-GUBZILKMSA-N 0.000 description 1
- HLTLEIXYIJDFOY-ZLUOBGJFSA-N Asn-Cys-Asn Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O HLTLEIXYIJDFOY-ZLUOBGJFSA-N 0.000 description 1
- OKZOABJQOMAYEC-NUMRIWBASA-N Asn-Gln-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OKZOABJQOMAYEC-NUMRIWBASA-N 0.000 description 1
- PPMTUXJSQDNUDE-CIUDSAMLSA-N Asn-Glu-Arg Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PPMTUXJSQDNUDE-CIUDSAMLSA-N 0.000 description 1
- KLKHFFMNGWULBN-VKHMYHEASA-N Asn-Gly Chemical compound NC(=O)C[C@H](N)C(=O)NCC(O)=O KLKHFFMNGWULBN-VKHMYHEASA-N 0.000 description 1
- ZTRJUKDEALVRMW-SRVKXCTJSA-N Asn-His-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CC(=O)N)N ZTRJUKDEALVRMW-SRVKXCTJSA-N 0.000 description 1
- YGHCVNQOZZMHRZ-DJFWLOJKSA-N Asn-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC(=O)N)N YGHCVNQOZZMHRZ-DJFWLOJKSA-N 0.000 description 1
- NVWJMQNYLYWVNQ-BYULHYEWSA-N Asn-Ile-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O NVWJMQNYLYWVNQ-BYULHYEWSA-N 0.000 description 1
- JLNFZLNDHONLND-GARJFASQSA-N Asn-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)N)N JLNFZLNDHONLND-GARJFASQSA-N 0.000 description 1
- FODVBOKTYKYRFJ-CIUDSAMLSA-N Asn-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)N)N FODVBOKTYKYRFJ-CIUDSAMLSA-N 0.000 description 1
- HPASIOLTWSNMFB-OLHMAJIHSA-N Asn-Thr-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O HPASIOLTWSNMFB-OLHMAJIHSA-N 0.000 description 1
- OERMIMJQPQUIPK-FXQIFTODSA-N Asp-Arg-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O OERMIMJQPQUIPK-FXQIFTODSA-N 0.000 description 1
- SOYOSFXLXYZNRG-CIUDSAMLSA-N Asp-Arg-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O SOYOSFXLXYZNRG-CIUDSAMLSA-N 0.000 description 1
- NZWDWXSWUQCNMG-GARJFASQSA-N Asp-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)C(=O)O NZWDWXSWUQCNMG-GARJFASQSA-N 0.000 description 1
- VWWAFGHMPWBKEP-GMOBBJLQSA-N Asp-Met-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(=O)O)N VWWAFGHMPWBKEP-GMOBBJLQSA-N 0.000 description 1
- DRCOAZZDQRCGGP-GHCJXIJMSA-N Asp-Ser-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DRCOAZZDQRCGGP-GHCJXIJMSA-N 0.000 description 1
- 102100021277 Beta-secretase 2 Human genes 0.000 description 1
- 101710150190 Beta-secretase 2 Proteins 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 108091033409 CRISPR Proteins 0.000 description 1
- 108091079001 CRISPR RNA Proteins 0.000 description 1
- 238000010354 CRISPR gene editing Methods 0.000 description 1
- 229920002567 Chondroitin Polymers 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- DEVDFMRWZASYOF-ZLUOBGJFSA-N Cys-Asn-Asp Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O DEVDFMRWZASYOF-ZLUOBGJFSA-N 0.000 description 1
- WTNLLMQAFPOCTJ-GARJFASQSA-N Cys-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CS)N)C(=O)O WTNLLMQAFPOCTJ-GARJFASQSA-N 0.000 description 1
- VDUPGIDTWNQAJD-CIUDSAMLSA-N Cys-Lys-Cys Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CS)C(O)=O VDUPGIDTWNQAJD-CIUDSAMLSA-N 0.000 description 1
- QQAYIVHVRFJICE-AEJSXWLSSA-N Cys-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CS)N QQAYIVHVRFJICE-AEJSXWLSSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-VANFPWTGSA-N D-mannopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@@H]1O AEMOLEFTQBMNLQ-VANFPWTGSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- LWDGZZGWDMHBOF-FXQIFTODSA-N Gln-Glu-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O LWDGZZGWDMHBOF-FXQIFTODSA-N 0.000 description 1
- CLPQUWHBWXFJOX-BQBZGAKWSA-N Gln-Gly-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O CLPQUWHBWXFJOX-BQBZGAKWSA-N 0.000 description 1
- GLAPJAHOPFSLKL-SRVKXCTJSA-N Gln-His-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)N)N GLAPJAHOPFSLKL-SRVKXCTJSA-N 0.000 description 1
- QBLMTCRYYTVUQY-GUBZILKMSA-N Gln-Leu-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O QBLMTCRYYTVUQY-GUBZILKMSA-N 0.000 description 1
- WOMUDRVDJMHTCV-DCAQKATOSA-N Glu-Arg-Arg Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WOMUDRVDJMHTCV-DCAQKATOSA-N 0.000 description 1
- MLCPTRRNICEKIS-FXQIFTODSA-N Glu-Asn-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MLCPTRRNICEKIS-FXQIFTODSA-N 0.000 description 1
- FLQAKQOBSPFGKG-CIUDSAMLSA-N Glu-Cys-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCN=C(N)N FLQAKQOBSPFGKG-CIUDSAMLSA-N 0.000 description 1
- VGUYMZGLJUJRBV-YVNDNENWSA-N Glu-Ile-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O VGUYMZGLJUJRBV-YVNDNENWSA-N 0.000 description 1
- HRBYTAIBKPNZKQ-AVGNSLFASA-N Glu-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCC(O)=O HRBYTAIBKPNZKQ-AVGNSLFASA-N 0.000 description 1
- LKOAAMXDJGEYMS-ZPFDUUQYSA-N Glu-Met-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LKOAAMXDJGEYMS-ZPFDUUQYSA-N 0.000 description 1
- SOEPMWQCTJITPZ-SRVKXCTJSA-N Glu-Met-Lys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N SOEPMWQCTJITPZ-SRVKXCTJSA-N 0.000 description 1
- HQOGXFLBAKJUMH-CIUDSAMLSA-N Glu-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)O)N HQOGXFLBAKJUMH-CIUDSAMLSA-N 0.000 description 1
- SOYWRINXUSUWEQ-DLOVCJGASA-N Glu-Val-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCC(O)=O SOYWRINXUSUWEQ-DLOVCJGASA-N 0.000 description 1
- PUUYVMYCMIWHFE-BQBZGAKWSA-N Gly-Ala-Arg Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PUUYVMYCMIWHFE-BQBZGAKWSA-N 0.000 description 1
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 1
- LXXLEUBUOMCAMR-NKWVEPMBSA-N Gly-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)CN)C(=O)O LXXLEUBUOMCAMR-NKWVEPMBSA-N 0.000 description 1
- HQRHFUYMGCHHJS-LURJTMIESA-N Gly-Gly-Arg Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N HQRHFUYMGCHHJS-LURJTMIESA-N 0.000 description 1
- IDOGEHIWMJMAHT-BYPYZUCNSA-N Gly-Gly-Cys Chemical compound NCC(=O)NCC(=O)N[C@@H](CS)C(O)=O IDOGEHIWMJMAHT-BYPYZUCNSA-N 0.000 description 1
- QPTNELDXWKRIFX-YFKPBYRVSA-N Gly-Gly-Gln Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O QPTNELDXWKRIFX-YFKPBYRVSA-N 0.000 description 1
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 1
- INLIXXRWNUKVCF-JTQLQIEISA-N Gly-Gly-Tyr Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 INLIXXRWNUKVCF-JTQLQIEISA-N 0.000 description 1
- OLPPXYMMIARYAL-QMMMGPOBSA-N Gly-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)CN OLPPXYMMIARYAL-QMMMGPOBSA-N 0.000 description 1
- ULZCYBYDTUMHNF-IUCAKERBSA-N Gly-Leu-Glu Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ULZCYBYDTUMHNF-IUCAKERBSA-N 0.000 description 1
- IUKIDFVOUHZRAK-QWRGUYRKSA-N Gly-Lys-His Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IUKIDFVOUHZRAK-QWRGUYRKSA-N 0.000 description 1
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 1
- OOCFXNOVSLSHAB-IUCAKERBSA-N Gly-Pro-Pro Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 OOCFXNOVSLSHAB-IUCAKERBSA-N 0.000 description 1
- ABPRMMYHROQBLY-NKWVEPMBSA-N Gly-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)CN)C(=O)O ABPRMMYHROQBLY-NKWVEPMBSA-N 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 229920002971 Heparan sulfate Polymers 0.000 description 1
- IPIVXQQRZXEUGW-UWJYBYFXSA-N His-Ala-His Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CN=CN1 IPIVXQQRZXEUGW-UWJYBYFXSA-N 0.000 description 1
- LSQHWKPPOFDHHZ-YUMQZZPRSA-N His-Asp-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N LSQHWKPPOFDHHZ-YUMQZZPRSA-N 0.000 description 1
- RAVLQPXCMRCLKT-KBPBESRZSA-N His-Gly-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RAVLQPXCMRCLKT-KBPBESRZSA-N 0.000 description 1
- NDKSHNQINMRKHT-PEXQALLHSA-N His-Ile-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC1=CN=CN1)N NDKSHNQINMRKHT-PEXQALLHSA-N 0.000 description 1
- ZRSJXIKQXUGKRB-TUBUOCAGSA-N His-Ile-Thr Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZRSJXIKQXUGKRB-TUBUOCAGSA-N 0.000 description 1
- ZFDKSLBEWYCOCS-BZSNNMDCSA-N His-Phe-Lys Chemical compound C([C@@H](C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@@H](N)CC=1NC=NC=1)C1=CC=CC=C1 ZFDKSLBEWYCOCS-BZSNNMDCSA-N 0.000 description 1
- 102100039869 Histone H2B type F-S Human genes 0.000 description 1
- 102000006947 Histones Human genes 0.000 description 1
- 101001035372 Homo sapiens Histone H2B type F-S Proteins 0.000 description 1
- 101001043594 Homo sapiens Low-density lipoprotein receptor-related protein 5 Proteins 0.000 description 1
- 101000808011 Homo sapiens Vascular endothelial growth factor A Proteins 0.000 description 1
- AQTWDZDISVGCAC-CFMVVWHZSA-N Ile-Asp-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N AQTWDZDISVGCAC-CFMVVWHZSA-N 0.000 description 1
- UBHUJPVCJHPSEU-GRLWGSQLSA-N Ile-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N UBHUJPVCJHPSEU-GRLWGSQLSA-N 0.000 description 1
- LPXHYGGZJOCAFR-MNXVOIDGSA-N Ile-Glu-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N LPXHYGGZJOCAFR-MNXVOIDGSA-N 0.000 description 1
- PNDMHTTXXPUQJH-RWRJDSDZSA-N Ile-Glu-Thr Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H]([C@H](O)C)C(=O)O PNDMHTTXXPUQJH-RWRJDSDZSA-N 0.000 description 1
- FFAUOCITXBMRBT-YTFOTSKYSA-N Ile-Lys-Ile Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FFAUOCITXBMRBT-YTFOTSKYSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- 101710134930 Import motor subunit, mitochondrial Proteins 0.000 description 1
- 229920000288 Keratan sulfate Polymers 0.000 description 1
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- KVRKAGGMEWNURO-CIUDSAMLSA-N Leu-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N KVRKAGGMEWNURO-CIUDSAMLSA-N 0.000 description 1
- BOFAFKVZQUMTID-AVGNSLFASA-N Leu-Gln-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N BOFAFKVZQUMTID-AVGNSLFASA-N 0.000 description 1
- LOLUPZNNADDTAA-AVGNSLFASA-N Leu-Gln-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LOLUPZNNADDTAA-AVGNSLFASA-N 0.000 description 1
- QVFGXCVIXXBFHO-AVGNSLFASA-N Leu-Glu-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O QVFGXCVIXXBFHO-AVGNSLFASA-N 0.000 description 1
- PRZVBIAOPFGAQF-SRVKXCTJSA-N Leu-Glu-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O PRZVBIAOPFGAQF-SRVKXCTJSA-N 0.000 description 1
- LAPSXOAUPNOINL-YUMQZZPRSA-N Leu-Gly-Asp Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O LAPSXOAUPNOINL-YUMQZZPRSA-N 0.000 description 1
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 1
- HGFGEMSVBMCFKK-MNXVOIDGSA-N Leu-Ile-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O HGFGEMSVBMCFKK-MNXVOIDGSA-N 0.000 description 1
- QJXHMYMRGDOHRU-NHCYSSNCSA-N Leu-Ile-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O QJXHMYMRGDOHRU-NHCYSSNCSA-N 0.000 description 1
- IEWBEPKLKUXQBU-VOAKCMCISA-N Leu-Leu-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IEWBEPKLKUXQBU-VOAKCMCISA-N 0.000 description 1
- 102100021926 Low-density lipoprotein receptor-related protein 5 Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- WGLAORUKDGRINI-WDCWCFNPSA-N Lys-Glu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGLAORUKDGRINI-WDCWCFNPSA-N 0.000 description 1
- YDDDRTIPNTWGIG-SRVKXCTJSA-N Lys-Lys-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O YDDDRTIPNTWGIG-SRVKXCTJSA-N 0.000 description 1
- MSSABBQOBUZFKZ-IHRRRGAJSA-N Lys-Pro-His Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCCCN)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O MSSABBQOBUZFKZ-IHRRRGAJSA-N 0.000 description 1
- XATKLFSXFINPSB-JYJNAYRXSA-N Lys-Tyr-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O XATKLFSXFINPSB-JYJNAYRXSA-N 0.000 description 1
- OHXUUQDOBQKSNB-AVGNSLFASA-N Lys-Val-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O OHXUUQDOBQKSNB-AVGNSLFASA-N 0.000 description 1
- VWJFOUBDZIUXGA-AVGNSLFASA-N Lys-Val-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCCCN)N VWJFOUBDZIUXGA-AVGNSLFASA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 208000003263 MASS syndrome Diseases 0.000 description 1
- QDMUMFDBUVOZOY-GUBZILKMSA-N Met-Arg-Cys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O)N QDMUMFDBUVOZOY-GUBZILKMSA-N 0.000 description 1
- ZEDVFJPQNNBMST-CYDGBPFRSA-N Met-Arg-Ile Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZEDVFJPQNNBMST-CYDGBPFRSA-N 0.000 description 1
- FZUNSVYYPYJYAP-NAKRPEOUSA-N Met-Ile-Ala Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O FZUNSVYYPYJYAP-NAKRPEOUSA-N 0.000 description 1
- DJJBHQHOZLUBCN-WDSOQIARSA-N Met-Lys-Trp Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DJJBHQHOZLUBCN-WDSOQIARSA-N 0.000 description 1
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 1
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 1
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- UNLYPPYNDXHGDG-IHRRRGAJSA-N Phe-Gln-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 UNLYPPYNDXHGDG-IHRRRGAJSA-N 0.000 description 1
- HOYQLNNGMHXZDW-KKUMJFAQSA-N Phe-Glu-Arg Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O HOYQLNNGMHXZDW-KKUMJFAQSA-N 0.000 description 1
- AUJWXNGCAQWLEI-KBPBESRZSA-N Phe-Lys-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O AUJWXNGCAQWLEI-KBPBESRZSA-N 0.000 description 1
- OAOLATANIHTNCZ-IHRRRGAJSA-N Phe-Met-Asp Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N OAOLATANIHTNCZ-IHRRRGAJSA-N 0.000 description 1
- 229920000805 Polyaspartic acid Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- YFNOUBWUIIJQHF-LPEHRKFASA-N Pro-Asp-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O YFNOUBWUIIJQHF-LPEHRKFASA-N 0.000 description 1
- SZZBUDVXWZZPDH-BQBZGAKWSA-N Pro-Cys-Gly Chemical compound OC(=O)CNC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1 SZZBUDVXWZZPDH-BQBZGAKWSA-N 0.000 description 1
- HQVPQXMCQKXARZ-FXQIFTODSA-N Pro-Cys-Ser Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O HQVPQXMCQKXARZ-FXQIFTODSA-N 0.000 description 1
- XZONQWUEBAFQPO-HJGDQZAQSA-N Pro-Gln-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XZONQWUEBAFQPO-HJGDQZAQSA-N 0.000 description 1
- AQGUSRZKDZYGGV-GMOBBJLQSA-N Pro-Ile-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O AQGUSRZKDZYGGV-GMOBBJLQSA-N 0.000 description 1
- UREQLMJCKFLLHM-NAKRPEOUSA-N Pro-Ile-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O UREQLMJCKFLLHM-NAKRPEOUSA-N 0.000 description 1
- WOIFYRZPIORBRY-AVGNSLFASA-N Pro-Lys-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O WOIFYRZPIORBRY-AVGNSLFASA-N 0.000 description 1
- CGSOWZUPLOKYOR-AVGNSLFASA-N Pro-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 CGSOWZUPLOKYOR-AVGNSLFASA-N 0.000 description 1
- CZCCVJUUWBMISW-FXQIFTODSA-N Pro-Ser-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O CZCCVJUUWBMISW-FXQIFTODSA-N 0.000 description 1
- 241000242739 Renilla Species 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 101150098533 SOST gene Proteins 0.000 description 1
- YMEXHZTVKDAKIY-GHCJXIJMSA-N Ser-Asn-Ile Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(O)=O YMEXHZTVKDAKIY-GHCJXIJMSA-N 0.000 description 1
- KMWFXJCGRXBQAC-CIUDSAMLSA-N Ser-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N KMWFXJCGRXBQAC-CIUDSAMLSA-N 0.000 description 1
- LALNXSXEYFUUDD-GUBZILKMSA-N Ser-Glu-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LALNXSXEYFUUDD-GUBZILKMSA-N 0.000 description 1
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 1
- IXCHOHLPHNGFTJ-YUMQZZPRSA-N Ser-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N IXCHOHLPHNGFTJ-YUMQZZPRSA-N 0.000 description 1
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 1
- 108091027967 Small hairpin RNA Proteins 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 1
- VASYSJHSMSBTDU-LKXGYXEUSA-N Thr-Asn-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N)O VASYSJHSMSBTDU-LKXGYXEUSA-N 0.000 description 1
- NRUPKQSXTJNQGD-XGEHTFHBSA-N Thr-Cys-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NRUPKQSXTJNQGD-XGEHTFHBSA-N 0.000 description 1
- UDQBCBUXAQIZAK-GLLZPBPUSA-N Thr-Glu-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UDQBCBUXAQIZAK-GLLZPBPUSA-N 0.000 description 1
- SIMKLINEDYOTKL-MBLNEYKQSA-N Thr-His-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](C)C(=O)O)N)O SIMKLINEDYOTKL-MBLNEYKQSA-N 0.000 description 1
- PUEWAXRPXOEQOW-HJGDQZAQSA-N Thr-Met-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(O)=O PUEWAXRPXOEQOW-HJGDQZAQSA-N 0.000 description 1
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 1
- ZHDQRPWESGUDST-JBACZVJFSA-N Trp-Phe-Gln Chemical compound C([C@H](NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)N)C(=O)N[C@@H](CCC(N)=O)C(O)=O)C1=CC=CC=C1 ZHDQRPWESGUDST-JBACZVJFSA-N 0.000 description 1
- XXJDYWYVZBHELV-TUSQITKMSA-N Trp-Trp-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CNC4=CC=CC=C43)C(=O)N[C@@H](CCCCN)C(=O)O)N XXJDYWYVZBHELV-TUSQITKMSA-N 0.000 description 1
- NSOMQRHZMJMZIE-GVARAGBVSA-N Tyr-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NSOMQRHZMJMZIE-GVARAGBVSA-N 0.000 description 1
- AYHSJESDFKREAR-KKUMJFAQSA-N Tyr-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 AYHSJESDFKREAR-KKUMJFAQSA-N 0.000 description 1
- NGALWFGCOMHUSN-AVGNSLFASA-N Tyr-Gln-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NGALWFGCOMHUSN-AVGNSLFASA-N 0.000 description 1
- ILTXFANLDMJWPR-SIUGBPQLSA-N Tyr-Ile-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N ILTXFANLDMJWPR-SIUGBPQLSA-N 0.000 description 1
- AZZLDIDWPZLCCW-ZEWNOJEFSA-N Tyr-Ile-Phe Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O AZZLDIDWPZLCCW-ZEWNOJEFSA-N 0.000 description 1
- XJPXTYLVMUZGNW-IHRRRGAJSA-N Tyr-Pro-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O XJPXTYLVMUZGNW-IHRRRGAJSA-N 0.000 description 1
- YYLHVUCSTXXKBS-IHRRRGAJSA-N Tyr-Pro-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YYLHVUCSTXXKBS-IHRRRGAJSA-N 0.000 description 1
- VPEFOFYNHBWFNQ-UFYCRDLUSA-N Tyr-Pro-Tyr Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 VPEFOFYNHBWFNQ-UFYCRDLUSA-N 0.000 description 1
- PQPWEALFTLKSEB-DZKIICNBSA-N Tyr-Val-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O PQPWEALFTLKSEB-DZKIICNBSA-N 0.000 description 1
- 108091008605 VEGF receptors Proteins 0.000 description 1
- XLDYBRXERHITNH-QSFUFRPTSA-N Val-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)C(C)C XLDYBRXERHITNH-QSFUFRPTSA-N 0.000 description 1
- CFSSLXZJEMERJY-NRPADANISA-N Val-Gln-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O CFSSLXZJEMERJY-NRPADANISA-N 0.000 description 1
- YCMXFKWYJFZFKS-LAEOZQHASA-N Val-Gln-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YCMXFKWYJFZFKS-LAEOZQHASA-N 0.000 description 1
- SZTTYWIUCGSURQ-AUTRQRHGSA-N Val-Glu-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SZTTYWIUCGSURQ-AUTRQRHGSA-N 0.000 description 1
- LAYSXAOGWHKNED-XPUUQOCRSA-N Val-Gly-Ser Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LAYSXAOGWHKNED-XPUUQOCRSA-N 0.000 description 1
- VENKIVFKIPGEJN-NHCYSSNCSA-N Val-Met-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N VENKIVFKIPGEJN-NHCYSSNCSA-N 0.000 description 1
- ZXYPHBKIZLAQTL-QXEWZRGKSA-N Val-Pro-Asp Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N ZXYPHBKIZLAQTL-QXEWZRGKSA-N 0.000 description 1
- NHXZRXLFOBFMDM-AVGNSLFASA-N Val-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C NHXZRXLFOBFMDM-AVGNSLFASA-N 0.000 description 1
- UGFMVXRXULGLNO-XPUUQOCRSA-N Val-Ser-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O UGFMVXRXULGLNO-XPUUQOCRSA-N 0.000 description 1
- CEKSLIVSNNGOKH-KZVJFYERSA-N Val-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](C(C)C)N)O CEKSLIVSNNGOKH-KZVJFYERSA-N 0.000 description 1
- DOBHJKVVACOQTN-DZKIICNBSA-N Val-Tyr-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC1=CC=C(O)C=C1 DOBHJKVVACOQTN-DZKIICNBSA-N 0.000 description 1
- PMKQKNBISAOSRI-XHSDSOJGSA-N Val-Tyr-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N PMKQKNBISAOSRI-XHSDSOJGSA-N 0.000 description 1
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 101150063416 add gene Proteins 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 235000009697 arginine Nutrition 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 108010008355 arginyl-glutamine Proteins 0.000 description 1
- 108010091092 arginyl-glycyl-proline Proteins 0.000 description 1
- 108010060035 arginylproline Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000001479 atomic absorption spectroscopy Methods 0.000 description 1
- 230000002902 bimodal effect Effects 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- DLGJWSVWTWEWBJ-HGGSSLSASA-N chondroitin Chemical compound CC(O)=N[C@@H]1[C@H](O)O[C@H](CO)[C@H](O)[C@@H]1OC1[C@H](O)[C@H](O)C=C(C(O)=O)O1 DLGJWSVWTWEWBJ-HGGSSLSASA-N 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 108010054813 diprotin B Proteins 0.000 description 1
- 230000002222 downregulating effect Effects 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 230000007783 downstream signaling Effects 0.000 description 1
- 238000002296 dynamic light scattering Methods 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 238000001917 fluorescence detection Methods 0.000 description 1
- 238000002073 fluorescence micrograph Methods 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 108010033719 glycyl-histidyl-glycine Proteins 0.000 description 1
- 108010020688 glycylhistidine Proteins 0.000 description 1
- 210000002288 golgi apparatus Anatomy 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 102000058223 human VEGFA Human genes 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- 108010057821 leucylproline Proteins 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 108010043322 lysyl-tryptophyl-alpha-lysine Proteins 0.000 description 1
- 108010064235 lysylglycine Proteins 0.000 description 1
- 230000034701 macropinocytosis Effects 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 108010056582 methionylglutamic acid Proteins 0.000 description 1
- 108091070501 miRNA Proteins 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 230000025608 mitochondrion localization Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 239000002539 nanocarrier Substances 0.000 description 1
- 239000002121 nanofiber Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000001937 non-anti-biotic effect Effects 0.000 description 1
- 210000000633 nuclear envelope Anatomy 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 108010064470 polyaspartate Proteins 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010090894 prolylleucine Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 108010048818 seryl-histidine Proteins 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000004055 small Interfering RNA Substances 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 1
- 229910052911 sodium silicate Inorganic materials 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 230000004960 subcellular localization Effects 0.000 description 1
- 239000012134 supernatant fraction Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- ORZHVTYKPFFVMG-UHFFFAOYSA-N xylenol orange Chemical compound OC(=O)CN(CC(O)=O)CC1=C(O)C(C)=CC(C2(C3=CC=CC=C3S(=O)(=O)O2)C=2C=C(CN(CC(O)=O)CC(O)=O)C(O)=C(C)C=2)=C1 ORZHVTYKPFFVMG-UHFFFAOYSA-N 0.000 description 1
- 125000002256 xylenyl group Chemical class C1(C(C=CC=C1)C)(C)* 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/645—Polycationic or polyanionic oligopeptides, polypeptides or polyamino acids, e.g. polylysine, polyarginine, polyglutamic acid or peptide TAT
- A61K47/6455—Polycationic oligopeptides, polypeptides or polyamino acids, e.g. for complexing nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6923—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being an inorganic particle, e.g. ceramic particles, silica particles, ferrite or synsorb
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6927—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores
- A61K47/6929—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L77/00—Compositions of polyamides obtained by reactions forming a carboxylic amide link in the main chain; Compositions of derivatives of such polymers
- C08L77/04—Polyamides derived from alpha-amino carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L79/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing nitrogen with or without oxygen or carbon only, not provided for in groups C08L61/00 - C08L77/00
- C08L79/04—Polycondensates having nitrogen-containing heterocyclic rings in the main chain; Polyhydrazides; Polyamide acids or similar polyimide precursors
- C08L79/08—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/90—Stable introduction of foreign DNA into chromosome
- C12N15/902—Stable introduction of foreign DNA into chromosome using homologous recombination
- C12N15/907—Stable introduction of foreign DNA into chromosome using homologous recombination in mammalian cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0008—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition
- A61K48/0025—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the non-active part clearly interacts with the delivered nucleic acid
- A61K48/0041—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the non-active part clearly interacts with the delivered nucleic acid the non-active part being polymeric
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/95—Protection of vectors from inactivation by agents such as antibodies or enzymes, e.g. using polymers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
- C12N9/22—Ribonucleases [RNase]; Deoxyribonucleases [DNase]
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Nanotechnology (AREA)
- Inorganic Chemistry (AREA)
- Immunology (AREA)
- Ceramic Engineering (AREA)
- Physical Education & Sports Medicine (AREA)
- Rheumatology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361881072P | 2013-09-23 | 2013-09-23 | |
| US61/881,072 | 2013-09-23 | ||
| PCT/US2014/057000 WO2015042585A1 (en) | 2013-09-23 | 2014-09-23 | Nanoparticle-mediated gene delivery, genomic editing and ligand-targeted modification in various cell populations |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160060133A true KR20160060133A (ko) | 2016-05-27 |
Family
ID=52689537
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167010607A Abandoned KR20160060133A (ko) | 2013-09-23 | 2014-09-23 | 다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US10526616B2 (enExample) |
| EP (2) | EP3520822A1 (enExample) |
| JP (1) | JP6595459B2 (enExample) |
| KR (1) | KR20160060133A (enExample) |
| CN (1) | CN105579068A (enExample) |
| AU (1) | AU2014321215B2 (enExample) |
| CA (2) | CA2924535C (enExample) |
| ES (1) | ES2719112T3 (enExample) |
| IL (1) | IL244585B (enExample) |
| PT (1) | PT3049116T (enExample) |
| RU (1) | RU2670512C2 (enExample) |
| WO (1) | WO2015042585A1 (enExample) |
Families Citing this family (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2734621B1 (en) | 2011-07-22 | 2019-09-04 | President and Fellows of Harvard College | Evaluation and improvement of nuclease cleavage specificity |
| US20150044192A1 (en) | 2013-08-09 | 2015-02-12 | President And Fellows Of Harvard College | Methods for identifying a target site of a cas9 nuclease |
| US9359599B2 (en) | 2013-08-22 | 2016-06-07 | President And Fellows Of Harvard College | Engineered transcription activator-like effector (TALE) domains and uses thereof |
| US9526784B2 (en) | 2013-09-06 | 2016-12-27 | President And Fellows Of Harvard College | Delivery system for functional nucleases |
| US9388430B2 (en) | 2013-09-06 | 2016-07-12 | President And Fellows Of Harvard College | Cas9-recombinase fusion proteins and uses thereof |
| US9340799B2 (en) | 2013-09-06 | 2016-05-17 | President And Fellows Of Harvard College | MRNA-sensing switchable gRNAs |
| US11053481B2 (en) | 2013-12-12 | 2021-07-06 | President And Fellows Of Harvard College | Fusions of Cas9 domains and nucleic acid-editing domains |
| WO2016022363A2 (en) | 2014-07-30 | 2016-02-11 | President And Fellows Of Harvard College | Cas9 proteins including ligand-dependent inteins |
| IL294014B2 (en) | 2015-10-23 | 2024-07-01 | Harvard College | Nucleobase editors and uses thereof |
| US10188749B2 (en) | 2016-04-14 | 2019-01-29 | Fred Hutchinson Cancer Research Center | Compositions and methods to program therapeutic cells using targeted nucleic acid nanocarriers |
| WO2018027078A1 (en) | 2016-08-03 | 2018-02-08 | President And Fellows Of Harard College | Adenosine nucleobase editors and uses thereof |
| CN109804066A (zh) | 2016-08-09 | 2019-05-24 | 哈佛大学的校长及成员们 | 可编程cas9-重组酶融合蛋白及其用途 |
| WO2018039438A1 (en) | 2016-08-24 | 2018-03-01 | President And Fellows Of Harvard College | Incorporation of unnatural amino acids into proteins using base editing |
| CN110214180A (zh) | 2016-10-14 | 2019-09-06 | 哈佛大学的校长及成员们 | 核碱基编辑器的aav递送 |
| CN110582301A (zh) * | 2016-12-14 | 2019-12-17 | 利甘达尔股份有限公司 | 用于核酸和蛋白质有效负载递送的方法和组合物 |
| US10745677B2 (en) | 2016-12-23 | 2020-08-18 | President And Fellows Of Harvard College | Editing of CCR5 receptor gene to protect against HIV infection |
| CA3049244A1 (en) | 2017-01-05 | 2018-07-12 | Fred Hutchinson Cancer Research Center | Systems and methods to improve vaccine efficacy |
| WO2018165504A1 (en) | 2017-03-09 | 2018-09-13 | President And Fellows Of Harvard College | Suppression of pain by gene editing |
| WO2018165631A1 (en) | 2017-03-09 | 2018-09-13 | President And Fellows Of Harvard College | Cancer vaccine |
| JP2020510439A (ja) | 2017-03-10 | 2020-04-09 | プレジデント アンド フェローズ オブ ハーバード カレッジ | シトシンからグアニンへの塩基編集因子 |
| KR20240116572A (ko) | 2017-03-23 | 2024-07-29 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | 핵산 프로그램가능한 dna 결합 단백질을 포함하는 핵염기 편집제 |
| US11560566B2 (en) | 2017-05-12 | 2023-01-24 | President And Fellows Of Harvard College | Aptazyme-embedded guide RNAs for use with CRISPR-Cas9 in genome editing and transcriptional activation |
| CN111801345A (zh) | 2017-07-28 | 2020-10-20 | 哈佛大学的校长及成员们 | 使用噬菌体辅助连续进化(pace)的进化碱基编辑器的方法和组合物 |
| US11319532B2 (en) | 2017-08-30 | 2022-05-03 | President And Fellows Of Harvard College | High efficiency base editors comprising Gam |
| CN111757937A (zh) | 2017-10-16 | 2020-10-09 | 布罗德研究所股份有限公司 | 腺苷碱基编辑器的用途 |
| US12406749B2 (en) | 2017-12-15 | 2025-09-02 | The Broad Institute, Inc. | Systems and methods for predicting repair outcomes in genetic engineering |
| WO2019204392A1 (en) * | 2018-04-17 | 2019-10-24 | Purdue Research Foundation | Modifying surface of a live cell and the uses thereof |
| CN112567032A (zh) * | 2018-04-18 | 2021-03-26 | 利甘达尔股份有限公司 | 基因组编辑的方法和组合物 |
| US20200149070A1 (en) * | 2018-04-24 | 2020-05-14 | Ligandal, Inc. | Methods and compositions for genome editing |
| IL314291A (en) * | 2018-04-27 | 2024-09-01 | Genedit Inc | Cationic polymer and use for biomolecule delivery |
| WO2019226953A1 (en) | 2018-05-23 | 2019-11-28 | The Broad Institute, Inc. | Base editors and uses thereof |
| EP3820495A4 (en) | 2018-07-09 | 2022-07-20 | The Broad Institute Inc. | RNA-PROGRAMMABLE EPIGENETIC RNA MODIFIERS AND THEIR USES |
| TWI764022B (zh) * | 2018-07-30 | 2022-05-11 | 中央研究院 | 凝聚複合物的治療性奈米粒子及其治療細菌的用途 |
| WO2020067142A1 (en) | 2018-09-25 | 2020-04-02 | The University Of Tokyo | Amphiphilic poly(amino acid), block copolymer using the amphiphilic poly(amino acid), and complex including the amphiphilic poly(amino acid) or the block copolymer and nucleic acid |
| US12281338B2 (en) | 2018-10-29 | 2025-04-22 | The Broad Institute, Inc. | Nucleobase editors comprising GeoCas9 and uses thereof |
| WO2020117706A1 (en) | 2018-12-03 | 2020-06-11 | Triplet Therapeutics, Inc. | Methods for the treatment of trinucleotide repeat expansion disorders associated with mlh3 activity |
| CN113631709A (zh) | 2018-12-20 | 2021-11-09 | 普拉克西斯精密药物股份有限公司 | 用于治疗kcnt1相关病症的组合物和方法 |
| US12351837B2 (en) | 2019-01-23 | 2025-07-08 | The Broad Institute, Inc. | Supernegatively charged proteins and uses thereof |
| CA3130488A1 (en) | 2019-03-19 | 2020-09-24 | David R. Liu | Methods and compositions for editing nucleotide sequences |
| US12473543B2 (en) | 2019-04-17 | 2025-11-18 | The Broad Institute, Inc. | Adenine base editors with reduced off-target effects |
| US20220296683A1 (en) * | 2019-09-20 | 2022-09-22 | University College Dublin | Oral delivery system |
| US12435330B2 (en) | 2019-10-10 | 2025-10-07 | The Broad Institute, Inc. | Methods and compositions for prime editing RNA |
| WO2021226558A1 (en) | 2020-05-08 | 2021-11-11 | The Broad Institute, Inc. | Methods and compositions for simultaneous editing of both strands of a target double-stranded nucleotide sequence |
| EP4150087A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of gap junction protein beta 2 (gjb2) |
| WO2021231692A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of otoferlin (otof) |
| EP4150086A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of leucine rich repeat kinase 2 (lrrk2) |
| WO2021231698A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of argininosuccinate lyase (asl) |
| EP4150076A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of methyl-cpg binding protein 2 (mecp2) |
| WO2021231691A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of retinoschisin 1 (rsi) |
| EP4150077A1 (en) | 2020-05-15 | 2023-03-22 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of transmembrane channel-like protein 1 (tmc1) |
| WO2021231675A1 (en) | 2020-05-15 | 2021-11-18 | Korro Bio, Inc. | Methods and compositions for the adar-mediated editing of argininosuccinate synthetase (ass1) |
| AR122534A1 (es) | 2020-06-03 | 2022-09-21 | Triplet Therapeutics Inc | Métodos para el tratamiento de los trastornos de expansión por repetición de nucleótidos asociados con la actividad de msh3 |
| EP4341405A1 (en) | 2021-05-20 | 2024-03-27 | Korro Bio, Inc. | Methods and compositions for adar-mediated editing |
| WO2022256283A2 (en) | 2021-06-01 | 2022-12-08 | Korro Bio, Inc. | Methods for restoring protein function using adar |
| EP4363574A1 (en) | 2021-06-29 | 2024-05-08 | Korro Bio, Inc. | Methods and compositions for adar-mediated editing |
| US20230194709A9 (en) | 2021-06-29 | 2023-06-22 | Seagate Technology Llc | Range information detection using coherent pulse sets with selected waveform characteristics |
| EP4419683A1 (en) | 2021-10-22 | 2024-08-28 | Korro Bio, Inc. | Methods and compositions for disrupting nrf2-keap1 protein interaction by adar mediated rna editing |
| WO2023122762A1 (en) | 2021-12-22 | 2023-06-29 | Camp4 Therapeutics Corporation | Modulation of gene transcription using antisense oligonucleotides targeting regulatory rnas |
| WO2023240277A2 (en) | 2022-06-10 | 2023-12-14 | Camp4 Therapeutics Corporation | Methods of modulating progranulin expression using antisense oligonucleotides targeting regulatory rnas |
| EP4627084A1 (en) | 2022-12-01 | 2025-10-08 | Camp4 Therapeutics Corporation | Modulation of syngap1 gene transcription using antisense oligonucleotides targeting regulatory rnas |
| AU2024287308A1 (en) | 2023-07-13 | 2025-12-18 | Korro Bio, Inc. | Rna-editing oligonucleotides and uses thereof |
| WO2025015338A1 (en) | 2023-07-13 | 2025-01-16 | Korro Bio, Inc. | Rna-editing oligonucleotides and uses thereof |
| WO2025128799A1 (en) | 2023-12-12 | 2025-06-19 | Korro Bio, Inc. | Double-stranded rna-editing oligonucleotides and uses thereof |
| WO2025255388A1 (en) | 2024-06-05 | 2025-12-11 | Camp4 Therapeutics Corporation | Modulation of syngap1 gene transcription using antisense oligonucleotides targeting regulatory rnas |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6051429A (en) | 1995-06-07 | 2000-04-18 | Life Technologies, Inc. | Peptide-enhanced cationic lipid transfections |
| US6379966B2 (en) | 1999-02-26 | 2002-04-30 | Mirus Corporation | Intravascular delivery of non-viral nucleic acid |
| US7098032B2 (en) * | 2001-01-02 | 2006-08-29 | Mirus Bio Corporation | Compositions and methods for drug delivery using pH sensitive molecules |
| RU2295954C2 (ru) * | 2000-09-28 | 2007-03-27 | Чирон Корпорейшн | Микрочастицы для доставки гетерологичных нуклеиновых кислот |
| US20040102606A1 (en) | 2001-04-24 | 2004-05-27 | Danuta Balicki | Histone H2A -derived peptides useful in gene delivery |
| US6805904B2 (en) | 2002-02-20 | 2004-10-19 | International Business Machines Corporation | Process of forming a multilayer nanoparticle-containing thin film self-assembly |
| RU2004133894A (ru) | 2002-04-22 | 2005-04-20 | Юниверсити Оф Флорида (Us) | Функционализированные наночастицы и способы их применения |
| WO2004085998A2 (en) | 2003-03-28 | 2004-10-07 | The Children's Hospital Of Philadelphia | Biomimetic hierarchies using functionalized nanoparticles as building blocks |
| EP1660053A2 (en) | 2003-08-21 | 2006-05-31 | Southwest Research Institute | Skeletally targeted nanoparticles |
| US20070190155A1 (en) | 2004-03-05 | 2007-08-16 | Leary James F | Molecular programming of nanoparticle systems for an ordered and controlled sequence of events for gene-drug delivery |
| WO2005123142A1 (en) | 2004-06-10 | 2005-12-29 | Abraxis Bioscience, Inc. | A method using inorganic nanoparticles as non-viral vectors for gene therapy |
| US20060088599A1 (en) | 2004-08-02 | 2006-04-27 | Prasad Paras N | Amino functionalized ORMOSIL nanoparticles as delivery vehicles |
| JP2010519203A (ja) | 2007-02-16 | 2010-06-03 | メルク・シャープ・エンド・ドーム・コーポレイション | 生物活性分子の活性を強化するための組成物及び方法 |
| US20100196492A1 (en) | 2007-03-08 | 2010-08-05 | Green Jordan J | Electrostatic coating of particles for drug delivery |
| JP2011517279A (ja) | 2007-10-29 | 2011-06-02 | ユニバーシティ オブ マサチューセッツ | 核酸(siRNA)送達用の酵母細胞壁粒子(YCWP)多層状ナノ粒子 |
| US8323618B2 (en) | 2007-11-07 | 2012-12-04 | University Of Houston System | Ultrasmall superparamagnetic iron oxide nanoparticles and uses thereof |
| BRPI0820302A2 (pt) | 2007-11-09 | 2015-05-19 | Univ Northeastern | Nanopartículas semelhantes a micelas de automontagem para liberação sistêmica de gene |
| US7999025B2 (en) | 2008-01-28 | 2011-08-16 | University Of Utah Research Foundation | Asymmetrically-functionalized nanoparticles organized on one-dimensional chains |
| US8324333B2 (en) | 2008-06-05 | 2012-12-04 | Wisconsin Alumni Research Foundation | Anionic charge-dynamic polymers for release of cationic agents |
| TWI576432B (zh) | 2009-04-07 | 2017-04-01 | 陶氏農業科學公司 | 序列特異性核酸酶之奈米粒子媒介式遞送技術 |
| KR102110725B1 (ko) | 2009-12-10 | 2020-05-13 | 리전츠 오브 더 유니버스티 오브 미네소타 | Tal 이펙터-매개된 dna 변형 |
| WO2011096408A1 (ja) | 2010-02-02 | 2011-08-11 | 国立大学法人 東京大学 | 複合体微粒子およびその製造方法、ならびに該複合体微粒子を用いた薬学組成物 |
| US9074187B2 (en) | 2011-03-21 | 2015-07-07 | Board Of Trustees Of The University Of Arkansas | Nanostructural materials that increase mineralization in bone cells and affect gene expression through miRNA regulation and applications of same |
| SG10201602651YA (en) * | 2011-04-05 | 2016-05-30 | Cellectis | Method for the generation of compact tale-nucleases and uses thereof |
| US8450107B1 (en) | 2011-11-30 | 2013-05-28 | The Broad Institute Inc. | Nucleotide-specific recognition sequences for designer TAL effectors |
| WO2013188979A1 (en) | 2012-06-20 | 2013-12-27 | Frank Gu | Mucoadhesive nanoparticle delivery system |
| WO2014093701A1 (en) | 2012-12-12 | 2014-06-19 | The Broad Institute, Inc. | Functional genomics using crispr-cas systems, compositions, methods, knock out libraries and applications thereof |
-
2014
- 2014-09-23 AU AU2014321215A patent/AU2014321215B2/en active Active
- 2014-09-23 US US15/024,264 patent/US10526616B2/en active Active
- 2014-09-23 ES ES14846422T patent/ES2719112T3/es active Active
- 2014-09-23 RU RU2016115721A patent/RU2670512C2/ru active
- 2014-09-23 EP EP18248103.6A patent/EP3520822A1/en active Pending
- 2014-09-23 CN CN201480052449.0A patent/CN105579068A/zh active Pending
- 2014-09-23 KR KR1020167010607A patent/KR20160060133A/ko not_active Abandoned
- 2014-09-23 PT PT14846422T patent/PT3049116T/pt unknown
- 2014-09-23 WO PCT/US2014/057000 patent/WO2015042585A1/en not_active Ceased
- 2014-09-23 CA CA2924535A patent/CA2924535C/en active Active
- 2014-09-23 JP JP2016516540A patent/JP6595459B2/ja not_active Expired - Fee Related
- 2014-09-23 CA CA3175320A patent/CA3175320A1/en active Pending
- 2014-09-23 EP EP14846422.5A patent/EP3049116B1/en active Active
-
2016
- 2016-03-14 IL IL24458516A patent/IL244585B/en active IP Right Grant
-
2019
- 2019-11-27 US US16/697,308 patent/US20200181642A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| AU2014321215B2 (en) | 2020-07-16 |
| PT3049116T (pt) | 2019-04-03 |
| EP3049116A4 (en) | 2017-05-17 |
| CN105579068A (zh) | 2016-05-11 |
| JP2016533322A (ja) | 2016-10-27 |
| WO2015042585A1 (en) | 2015-03-26 |
| US20200181642A1 (en) | 2020-06-11 |
| EP3520822A1 (en) | 2019-08-07 |
| EP3049116B1 (en) | 2019-01-02 |
| CA3175320A1 (en) | 2015-03-26 |
| US10526616B2 (en) | 2020-01-07 |
| RU2670512C2 (ru) | 2018-10-23 |
| EP3049116A1 (en) | 2016-08-03 |
| CA2924535C (en) | 2022-12-13 |
| JP6595459B2 (ja) | 2019-10-23 |
| CA2924535A1 (en) | 2015-03-26 |
| AU2014321215A1 (en) | 2016-04-21 |
| IL244585B (en) | 2019-11-28 |
| RU2016115721A (ru) | 2017-10-30 |
| RU2016115721A3 (enExample) | 2018-03-21 |
| US20160230189A1 (en) | 2016-08-11 |
| ES2719112T3 (es) | 2019-07-08 |
| IL244585A0 (en) | 2016-04-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20160060133A (ko) | 다양한 세포 집단에서 나노입자-매개된 유전자 전달, 게놈 편집 및 리간드-표적화된 변형 | |
| CN104981478B (zh) | 细胞穿透肽、包含该肽的缀合物、及包含该缀合物的组合物 | |
| Suh et al. | Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation | |
| Winton et al. | Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates | |
| Vaissière et al. | A retro-inverso cell-penetrating peptide for siRNA delivery | |
| EP4556567A2 (en) | Rationally-designed synthetic peptide shuttle agents for delivering polypeptide cargos from an extracellular space to the cytosol and/or nucleus of a target eukaryotic cell, uses thereof, methods and kits relating to same | |
| Mishra et al. | Functional role of the interaction between polysialic acid and extracellular histone H1 | |
| KR20210035022A (ko) | 핵산 및/또는 단백질 적재물 전달을 위한 조성물 및 방법 | |
| Sadeghian et al. | Design, engineering and preparation of a multi-domain fusion vector for gene delivery | |
| CN104768967A (zh) | 细胞穿透性肽、包含该肽的缀合物、及包含该缀合物的组合物 | |
| Park et al. | Receptor-mediated gene delivery into human mesenchymal stem cells using hyaluronic acid-shielded polyethylenimine/pDNA nanogels | |
| KR20220031545A (ko) | 펩타이드 기반 비단백질성 카고 전달 | |
| Lee et al. | Identification of cell‐penetrating osteogenic peptide from copine‐7 protein and its delivery system for enhanced bone formation | |
| Oh et al. | Multimeric Amphipathic α‐Helical Sequences for Rapid and Efficient Intracellular Protein Transport at Nanomolar Concentrations | |
| Du et al. | ATG101 single-stranded antisense RNA-loaded triangular DNA nanoparticles control human pulmonary endothelial growth via regulation of cell macroautophagy | |
| Ozbek et al. | The glycoprotein NOWA and minicollagens are part of a disulfidelinked polymer that forms the cnidarian nematocyst wall | |
| Nirasawa et al. | Development of A2G80 peptide-gene complex for targeted delivery to muscle cells | |
| HK1225274B (en) | Nanoparticle-mediated gene delivery, genomic editing and ligand-targeted modification in various cell populations | |
| JP7725041B2 (ja) | コラーゲン結合型膜透過性ペプチド、並びに、該ペプチド及びコラーゲン若しくはコラーゲン誘導体を含む運搬体 | |
| KR20220057542A (ko) | 펩타이드 내포 페리틴 | |
| KR20190108918A (ko) | Rna 간섭을 통한 줄기세포의 연골분화 유도를 위한 나노입자 전달체 및 이를 이용한 연골세포로의 분화방법 | |
| Goyal | Paralog specific role of COPI pathway in P19 neuronal differentiation | |
| KR20170002983A (ko) | 항암 및 항염증 활성을 갖는 약물 복합체 | |
| Shen | Understanding the Biological Barriers to Jurkat Transfection using Non-Viral Peptides | |
| Ping | Exploiting the Protein Corona Around Gold Nanoparticles in Modulation of Protein Expression |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160422 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20190724 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20200729 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20210125 |
|
| PC1904 | Unpaid initial registration fee |