KR20150053769A - 프리바이오틱 틸라코이드 조성물 - Google Patents
프리바이오틱 틸라코이드 조성물 Download PDFInfo
- Publication number
- KR20150053769A KR20150053769A KR1020157008166A KR20157008166A KR20150053769A KR 20150053769 A KR20150053769 A KR 20150053769A KR 1020157008166 A KR1020157008166 A KR 1020157008166A KR 20157008166 A KR20157008166 A KR 20157008166A KR 20150053769 A KR20150053769 A KR 20150053769A
- Authority
- KR
- South Korea
- Prior art keywords
- composition
- thylakoid
- bifidobacterium
- lactobacillus
- use according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/21—Amaranthaceae (Amaranth family), e.g. pigweed, rockwort or globe amaranth
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/135—Bacteria or derivatives thereof, e.g. probiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/745—Bifidobacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/747—Lactobacilli, e.g. L. acidophilus or L. brevis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/30—Foods, ingredients or supplements having a functional effect on health
- A23V2200/32—Foods, ingredients or supplements having a functional effect on health having an effect on the health of the digestive tract
- A23V2200/3202—Prebiotics, ingredients fermented in the gastrointestinal tract by beneficial microflora
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/30—Foods, ingredients or supplements having a functional effect on health
- A23V2200/32—Foods, ingredients or supplements having a functional effect on health having an effect on the health of the digestive tract
- A23V2200/3204—Probiotics, living bacteria to be ingested for action in the digestive tract
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2250/00—Food ingredients
- A23V2250/20—Natural extracts
- A23V2250/206—Bacterial extracts
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2250/00—Food ingredients
- A23V2250/20—Natural extracts
- A23V2250/21—Plant extracts
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Mycology (AREA)
- Chemical & Material Sciences (AREA)
- Microbiology (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Botany (AREA)
- Polymers & Plastics (AREA)
- Nutrition Science (AREA)
- Food Science & Technology (AREA)
- Alternative & Traditional Medicine (AREA)
- Biotechnology (AREA)
- Medical Informatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- General Preparation And Processing Of Foods (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Plant Substances (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE1251019 | 2012-09-11 | ||
| SE1251019-4 | 2012-09-11 | ||
| PCT/EP2013/068656 WO2014040962A1 (en) | 2012-09-11 | 2013-09-10 | Prebiotic thylakoid composition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150053769A true KR20150053769A (ko) | 2015-05-18 |
Family
ID=49209335
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157008166A Withdrawn KR20150053769A (ko) | 2012-09-11 | 2013-09-10 | 프리바이오틱 틸라코이드 조성물 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20150209396A1 (enExample) |
| EP (1) | EP2895012B1 (enExample) |
| JP (1) | JP6475162B2 (enExample) |
| KR (1) | KR20150053769A (enExample) |
| CN (1) | CN104780783A (enExample) |
| AU (1) | AU2013314410B2 (enExample) |
| BR (1) | BR112015005355A2 (enExample) |
| CA (1) | CA2884281C (enExample) |
| DK (1) | DK2895012T3 (enExample) |
| ES (1) | ES2656619T3 (enExample) |
| NO (1) | NO2895012T3 (enExample) |
| WO (1) | WO2014040962A1 (enExample) |
| ZA (1) | ZA201502431B (enExample) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3082466B1 (en) * | 2013-12-18 | 2019-08-21 | Thylabisco AB | Use of thylakoids to reduce the urge for palatable food |
| DE112016002056T5 (de) * | 2015-05-06 | 2018-02-08 | Snipr Technologies Limited | Verändern mikrobieller Populationen und Modifizieren von Mikrobiomen |
| JP6551934B2 (ja) * | 2015-12-18 | 2019-07-31 | 森永乳業株式会社 | ビフィドバクテリウム属細菌および/または乳酸菌の増殖促進および/または減少抑制剤 |
| JP7244977B2 (ja) * | 2016-03-11 | 2023-03-23 | シオノギヘルスケア株式会社 | 腸内細菌叢調整用組成物 |
| GB202209518D0 (en) | 2022-06-29 | 2022-08-10 | Snipr Biome Aps | Treating & preventing E coli infections |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PT1056358E (pt) | 1998-02-24 | 2003-10-31 | Arla Foods Amba | Utilizacao de d-tagatose como componente alimentar prebiotico |
| US20030165557A1 (en) * | 2000-09-19 | 2003-09-04 | Sunstar, Inc. | Food, medicine and method relating to Bifidobacterium proliferation promoting action, allergy preventive action and human cholesterol lowering action |
| JP2006025669A (ja) * | 2004-07-14 | 2006-02-02 | Kumamotoken Kajitsu Nogyo Kyodo Kumiai Rengokai | 飲料の製造法 |
| US7700139B2 (en) * | 2004-12-30 | 2010-04-20 | Commonwealth Scientific And Industrial Research Organization | Method and means for improving bowel health |
| DK1893224T3 (en) | 2005-06-10 | 2018-08-06 | Thylabisco Ab | APPLICATION OF PLANT CELL MEMBRANE TO TREAT OBESITAS |
| JP4819767B2 (ja) * | 2006-12-12 | 2011-11-24 | 株式会社エス・エフ・シー | イグサ青汁 |
| US20080175899A1 (en) * | 2007-01-19 | 2008-07-24 | Ross Mairi R | Formulation for promoting sinus health in nasal cavities |
| CN101278752A (zh) * | 2008-01-29 | 2008-10-08 | 梁季鸿 | 即食卡尼汀天然果蔬饮料干粉系列配方及生产工艺 |
| CA2730753A1 (en) | 2008-07-14 | 2010-01-21 | Thylabisco Ab | Large-scale process for the preparation of thylakoids |
| WO2010146568A2 (en) * | 2009-06-19 | 2010-12-23 | Danisco A/S | Bifidobacteria for treating diabetes and related conditions |
| CN103491947A (zh) * | 2011-02-25 | 2014-01-01 | 西拉比索公司 | 一种用于延迟分子摄取的含有类囊体的组合物 |
-
2013
- 2013-09-10 AU AU2013314410A patent/AU2013314410B2/en active Active
- 2013-09-10 KR KR1020157008166A patent/KR20150053769A/ko not_active Withdrawn
- 2013-09-10 JP JP2015530443A patent/JP6475162B2/ja active Active
- 2013-09-10 CA CA2884281A patent/CA2884281C/en active Active
- 2013-09-10 EP EP13763020.8A patent/EP2895012B1/en active Active
- 2013-09-10 WO PCT/EP2013/068656 patent/WO2014040962A1/en not_active Ceased
- 2013-09-10 CN CN201380052341.7A patent/CN104780783A/zh active Pending
- 2013-09-10 NO NO13763020A patent/NO2895012T3/no unknown
- 2013-09-10 BR BR112015005355A patent/BR112015005355A2/pt not_active Application Discontinuation
- 2013-09-10 US US14/426,541 patent/US20150209396A1/en not_active Abandoned
- 2013-09-10 DK DK13763020.8T patent/DK2895012T3/en active
- 2013-09-10 ES ES13763020.8T patent/ES2656619T3/es active Active
-
2015
- 2015-04-10 ZA ZA2015/02431A patent/ZA201502431B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP2895012B1 (en) | 2017-10-18 |
| AU2013314410B2 (en) | 2017-02-23 |
| US20150209396A1 (en) | 2015-07-30 |
| AU2013314410A1 (en) | 2015-04-16 |
| ES2656619T3 (es) | 2018-02-27 |
| NO2895012T3 (enExample) | 2018-03-17 |
| JP2015533484A (ja) | 2015-11-26 |
| BR112015005355A2 (pt) | 2017-08-08 |
| WO2014040962A1 (en) | 2014-03-20 |
| CA2884281A1 (en) | 2014-03-20 |
| CA2884281C (en) | 2021-01-05 |
| ZA201502431B (en) | 2017-11-29 |
| DK2895012T3 (en) | 2018-01-22 |
| CN104780783A (zh) | 2015-07-15 |
| JP6475162B2 (ja) | 2019-02-27 |
| EP2895012A1 (en) | 2015-07-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Lim et al. | Lactobacillus sakei OK67 ameliorates high-fat diet–induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression | |
| Kerry et al. | Benefaction of probiotics for human health: A review | |
| Chiang et al. | Beneficial effects of Lactobacillus paracasei subsp. paracasei NTU 101 and its fermented products | |
| Boudry et al. | Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice | |
| Nakata et al. | Inhibitory effects of soybean oligosaccharides and water-soluble soybean fibre on formation of putrefactive compounds from soy protein by gut microbiota | |
| Önal Darilmaz et al. | The effects of inulin as a prebiotic supplement and the synbiotic interactions of probiotics to improve oxalate degrading activity | |
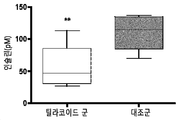
| Montelius et al. | Feeding spinach thylakoids to rats modulates the gut microbiota, decreases food intake and affects the insulin response | |
| EP2457575B1 (en) | New method for obtaining a Lactobacillus reuteri strain useful in medical and veterinary prophylaxis and treatment | |
| Pahumunto et al. | Anti-cancer properties of potential probiotics and their cell-free supernatants for the prevention of colorectal cancer: An in vitro study | |
| Mikelsaar et al. | Biodiversity of intestinal lactic acid bacteria in the healthy population | |
| EP2457576A1 (en) | New Lactobacillus reuteri strain useful in medical and veterinary prohylaxis and treatment | |
| AU2013314410B2 (en) | Prebiotic thylakoid composition | |
| Cheng et al. | Lactobacillus casei-fermented blueberry pomace augments sIgA production in high-fat diet mice by improving intestinal microbiota | |
| Li et al. | Apple polysaccharide could promote the growth of Bifidobacterium longum | |
| Guo et al. | Supplementation with yak (Bos grunniens) bone collagen hydrolysate altered the structure of gut microbiota and elevated short-chain fatty acid production in mice | |
| KR20200143944A (ko) | 글루텐 분해능을 가지는 락토바실러스 파라카제이 glu70 균주의 사균체를 유효성분으로 함유하는 글리아딘으로 야기된 염증성 장 질환의 예방, 개선 또는 치료용 조성물 | |
| Garcia-Gonzalez et al. | Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349 | |
| Miao et al. | Oral administration of fermented milk supplemented with synbiotics can influence the physiological condition of Wistar rats in a dose-sensitive and sex-specific manner | |
| Tijjani et al. | Probiotics and their attributes in human health therapy | |
| EP3793580A1 (en) | Probiotic strain lactobacillus kefiri sgl 13 and use thereof for the prevention and treatment of intestinal inflammation and in the prevention of intestinal neoplasms | |
| Shweta et al. | In vitro studies on anti-inflammatory, antioxidant and antihyperglycemic activities of potential probiotic Pediococcus acidilactici NCDC 252 | |
| Kuda et al. | Effect of food ingredients on susceptible gut indigenous bacteria | |
| Tian | The biotherapeutic potential of Lactobacillus reuteri DPC16 and bovine lactoferrin in controlling some pathogens, genotoxicity and inflammation in the gut: a thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Engineering and Advanced Technology at Massey University, Auckland, New Zealand | |
| Seshadri | Understanding the role of probiotics as a possible treatment strategy for alcoholism induced liver dysfunction | |
| Jangra et al. | Living medicines for health and disease management |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| P22-X000 | Classification modified |
St.27 status event code: A-2-2-P10-P22-nap-X000 |
|
| P22-X000 | Classification modified |
St.27 status event code: A-2-2-P10-P22-nap-X000 |
|
| PC1203 | Withdrawal of no request for examination |
St.27 status event code: N-1-6-B10-B12-nap-PC1203 |
|
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid | ||
| P22-X000 | Classification modified |
St.27 status event code: A-2-2-P10-P22-nap-X000 |
|
| P22-X000 | Classification modified |
St.27 status event code: A-2-2-P10-P22-nap-X000 |
|
| P22-X000 | Classification modified |
St.27 status event code: A-2-2-P10-P22-nap-X000 |