KR20140147835A - 나사 가공된 임플란트 - Google Patents
나사 가공된 임플란트 Download PDFInfo
- Publication number
- KR20140147835A KR20140147835A KR1020147028548A KR20147028548A KR20140147835A KR 20140147835 A KR20140147835 A KR 20140147835A KR 1020147028548 A KR1020147028548 A KR 1020147028548A KR 20147028548 A KR20147028548 A KR 20147028548A KR 20140147835 A KR20140147835 A KR 20140147835A
- Authority
- KR
- South Korea
- Prior art keywords
- bone
- threaded
- implant
- iliac
- sacrum
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
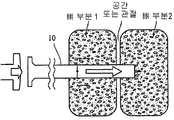
- 239000007943 implant Substances 0.000 title claims abstract description 233
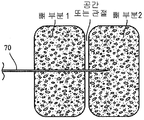
- 210000000988 bone and bone Anatomy 0.000 claims abstract description 134
- 239000011148 porous material Substances 0.000 claims abstract description 74
- 238000000034 method Methods 0.000 claims abstract description 50
- 230000012010 growth Effects 0.000 claims abstract description 48
- 230000035515 penetration Effects 0.000 claims abstract description 17
- 238000003780 insertion Methods 0.000 claims abstract description 11
- 230000037431 insertion Effects 0.000 claims abstract description 11
- 239000007787 solid Substances 0.000 claims abstract description 5
- 229910052751 metal Inorganic materials 0.000 claims description 20
- 239000002184 metal Substances 0.000 claims description 20
- 239000000463 material Substances 0.000 claims description 18
- 238000000576 coating method Methods 0.000 claims description 15
- 239000011248 coating agent Substances 0.000 claims description 14
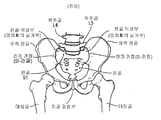
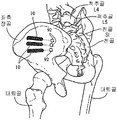
- 210000001621 ilium bone Anatomy 0.000 claims description 13
- 208000006820 Arthralgia Diseases 0.000 claims description 9
- 238000012546 transfer Methods 0.000 claims description 9
- 210000003692 ilium Anatomy 0.000 claims description 5
- 238000010079 rubber tapping Methods 0.000 claims description 5
- 238000013508 migration Methods 0.000 claims description 2
- 230000005012 migration Effects 0.000 claims description 2
- 238000007493 shaping process Methods 0.000 claims description 2
- 238000007500 overflow downdraw method Methods 0.000 claims 5
- 230000015572 biosynthetic process Effects 0.000 abstract 1
- 210000003131 sacroiliac joint Anatomy 0.000 description 20
- 239000002671 adjuvant Substances 0.000 description 14
- 238000013459 approach Methods 0.000 description 9
- 230000004927 fusion Effects 0.000 description 9
- 238000002513 implantation Methods 0.000 description 7
- 210000003049 pelvic bone Anatomy 0.000 description 6
- 210000004872 soft tissue Anatomy 0.000 description 6
- 208000002193 Pain Diseases 0.000 description 5
- 210000003484 anatomy Anatomy 0.000 description 5
- 239000003102 growth factor Substances 0.000 description 5
- 230000001012 protector Effects 0.000 description 5
- 238000005507 spraying Methods 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 4
- 230000005540 biological transmission Effects 0.000 description 4
- 229910052719 titanium Inorganic materials 0.000 description 4
- 239000010936 titanium Substances 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 3
- 239000012620 biological material Substances 0.000 description 3
- 239000000919 ceramic Substances 0.000 description 3
- 238000002224 dissection Methods 0.000 description 3
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000002591 computed tomography Methods 0.000 description 2
- 230000001054 cortical effect Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000003412 degenerative effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 238000006073 displacement reaction Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 230000009969 flowable effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 210000004705 lumbosacral region Anatomy 0.000 description 2
- 238000002595 magnetic resonance imaging Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 238000007794 visualization technique Methods 0.000 description 2
- 206010003267 Arthritis reactive Diseases 0.000 description 1
- 208000008035 Back Pain Diseases 0.000 description 1
- 206010017076 Fracture Diseases 0.000 description 1
- 101000911772 Homo sapiens Hsc70-interacting protein Proteins 0.000 description 1
- 208000008930 Low Back Pain Diseases 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- 206010049514 Traumatic fracture Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000003522 acrylic cement Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000002785 anti-thrombosis Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 208000037873 arthrodesis Diseases 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 239000005313 bioactive glass Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- CRQQGFGUEAVUIL-UHFFFAOYSA-N chlorothalonil Chemical compound ClC1=C(Cl)C(C#N)=C(Cl)C(C#N)=C1Cl CRQQGFGUEAVUIL-UHFFFAOYSA-N 0.000 description 1
- IUWCPXJTIPQGTE-UHFFFAOYSA-N chromium cobalt Chemical compound [Cr].[Co].[Co].[Co] IUWCPXJTIPQGTE-UHFFFAOYSA-N 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000000512 collagen gel Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- -1 for example Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000000642 iatrogenic effect Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 210000001696 pelvic girdle Anatomy 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 208000002574 reactive arthritis Diseases 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 229910000811 surgical stainless steel Inorganic materials 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/4455—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/70—Spinal positioners or stabilisers, e.g. stabilisers comprising fluid filler in an implant
- A61B17/7055—Spinal positioners or stabilisers, e.g. stabilisers comprising fluid filler in an implant connected to sacrum, pelvis or skull
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/84—Fasteners therefor or fasteners being internal fixation devices
- A61B17/86—Pins or screws or threaded wires; nuts therefor
- A61B17/864—Pins or screws or threaded wires; nuts therefor hollow, e.g. with socket or cannulated
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/1662—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans for particular parts of the body
- A61B17/1671—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans for particular parts of the body for the spine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/16—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans
- A61B17/1697—Instruments for performing osteoclasis; Drills or chisels for bones; Trepans specially adapted for wire insertion
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/84—Fasteners therefor or fasteners being internal fixation devices
- A61B17/86—Pins or screws or threaded wires; nuts therefor
- A61B17/8605—Heads, i.e. proximal ends projecting from bone
- A61B17/861—Heads, i.e. proximal ends projecting from bone specially shaped for gripping driver
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/84—Fasteners therefor or fasteners being internal fixation devices
- A61B17/86—Pins or screws or threaded wires; nuts therefor
- A61B17/8645—Headless screws, e.g. ligament interference screws
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B17/58—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor for osteosynthesis, e.g. bone plates, screws or setting implements
- A61B17/68—Internal fixation devices, including fasteners and spinal fixators, even if a part thereof projects from the skin
- A61B17/84—Fasteners therefor or fasteners being internal fixation devices
- A61B17/86—Pins or screws or threaded wires; nuts therefor
- A61B17/866—Material or manufacture
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/56—Surgical instruments or methods for treatment of bones or joints; Devices specially adapted therefor
- A61B2017/561—Implants with special means for releasing a drug
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30988—Other joints not covered by any of the groups A61F2/32 - A61F2/4425
- A61F2002/30995—Other joints not covered by any of the groups A61F2/32 - A61F2/4425 for sacro-iliac joints
Landscapes
- Health & Medical Sciences (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Neurology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Heart & Thoracic Surgery (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Neurosurgery (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Prostheses (AREA)
- Surgical Instruments (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261609211P | 2012-03-09 | 2012-03-09 | |
| US61/609,211 | 2012-03-09 | ||
| PCT/US2013/029945 WO2013134678A1 (en) | 2012-03-09 | 2013-03-08 | Threaded implant |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140147835A true KR20140147835A (ko) | 2014-12-30 |
Family
ID=49117393
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020147028548A Withdrawn KR20140147835A (ko) | 2012-03-09 | 2013-03-08 | 나사 가공된 임플란트 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20130245763A1 (enExample) |
| EP (1) | EP2827789A4 (enExample) |
| JP (1) | JP2015509435A (enExample) |
| KR (1) | KR20140147835A (enExample) |
| CN (1) | CN104302237A (enExample) |
| HK (1) | HK1206232A1 (enExample) |
| IN (1) | IN2014DN07038A (enExample) |
| WO (1) | WO2013134678A1 (enExample) |
Families Citing this family (62)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9662158B2 (en) | 2004-08-09 | 2017-05-30 | Si-Bone Inc. | Systems and methods for the fixation or fusion of bone at or near a sacroiliac joint |
| US9949843B2 (en) | 2004-08-09 | 2018-04-24 | Si-Bone Inc. | Apparatus, systems, and methods for the fixation or fusion of bone |
| US20070156241A1 (en) | 2004-08-09 | 2007-07-05 | Reiley Mark A | Systems and methods for the fixation or fusion of bone |
| US8986348B2 (en) | 2004-08-09 | 2015-03-24 | Si-Bone Inc. | Systems and methods for the fusion of the sacral-iliac joint |
| US20180228621A1 (en) | 2004-08-09 | 2018-08-16 | Mark A. Reiley | Apparatus, systems, and methods for the fixation or fusion of bone |
| US20060036251A1 (en) | 2004-08-09 | 2006-02-16 | Reiley Mark A | Systems and methods for the fixation or fusion of bone |
| CN105287056B (zh) * | 2010-01-13 | 2018-10-16 | Jcbd公司 | 骶髂关节固着融合系统 |
| US9333090B2 (en) | 2010-01-13 | 2016-05-10 | Jcbd, Llc | Systems for and methods of fusing a sacroiliac joint |
| US9381045B2 (en) * | 2010-01-13 | 2016-07-05 | Jcbd, Llc | Sacroiliac joint implant and sacroiliac joint instrument for fusing a sacroiliac joint |
| US8343224B2 (en) | 2010-03-16 | 2013-01-01 | Pinnacle Spine Group, Llc | Intervertebral implants and graft delivery systems and methods |
| EP2704647B1 (en) | 2011-05-05 | 2016-08-24 | Zyga Technology, Inc. | Sacroiliac fusion system |
| US9380932B1 (en) | 2011-11-02 | 2016-07-05 | Pinnacle Spine Group, Llc | Retractor devices for minimally invasive access to the spine |
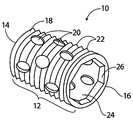
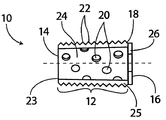
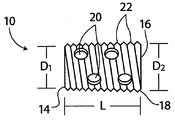
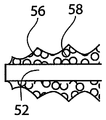
| US10363140B2 (en) | 2012-03-09 | 2019-07-30 | Si-Bone Inc. | Systems, device, and methods for joint fusion |
| KR20140147834A (ko) | 2012-03-09 | 2014-12-30 | 에스아이-본 인코포레이티드 | 일체형 임플란트 |
| WO2013166496A1 (en) | 2012-05-04 | 2013-11-07 | Si-Bone Inc. | Fenestrated implant |
| US20140012340A1 (en) * | 2012-07-05 | 2014-01-09 | Warsaw Orthopedic, Inc. | Sacro-iliac joint implant system and method |
| WO2014159739A1 (en) | 2013-03-14 | 2014-10-02 | Pinnacle Spine Group, Llc | Interbody implants and graft delivery systems |
| WO2014146018A1 (en) | 2013-03-15 | 2014-09-18 | Jcbd, Llc | Systems and methods for fusing a sacroiliac joint and anchoring an orthopedic appliance |
| US9826986B2 (en) | 2013-07-30 | 2017-11-28 | Jcbd, Llc | Systems for and methods of preparing a sacroiliac joint for fusion |
| US9936983B2 (en) | 2013-03-15 | 2018-04-10 | Si-Bone Inc. | Implants for spinal fixation or fusion |
| US10433880B2 (en) | 2013-03-15 | 2019-10-08 | Jcbd, Llc | Systems and methods for fusing a sacroiliac joint and anchoring an orthopedic appliance |
| US9717539B2 (en) | 2013-07-30 | 2017-08-01 | Jcbd, Llc | Implants, systems, and methods for fusing a sacroiliac joint |
| GB2512063B (en) * | 2013-03-18 | 2019-05-29 | Fitzbionics Ltd | Spinal implant assembly |
| US11147688B2 (en) | 2013-10-15 | 2021-10-19 | Si-Bone Inc. | Implant placement |
| WO2015057866A1 (en) * | 2013-10-15 | 2015-04-23 | Si-Bone Inc. | Implant placement |
| US9861375B2 (en) | 2014-01-09 | 2018-01-09 | Zyga Technology, Inc. | Undercutting system for use in conjunction with sacroiliac fusion |
| US9962265B2 (en) * | 2014-03-07 | 2018-05-08 | Arthrosurface Incorporated | System and method for repairing articular surfaces |
| JP2017513642A (ja) * | 2014-04-25 | 2017-06-01 | ネオ・メディカル・ソシエテ・アノニム | 脊椎ケージ |
| US9801546B2 (en) | 2014-05-27 | 2017-10-31 | Jcbd, Llc | Systems for and methods of diagnosing and treating a sacroiliac joint disorder |
| US10045803B2 (en) | 2014-07-03 | 2018-08-14 | Mayo Foundation For Medical Education And Research | Sacroiliac joint fusion screw and method |
| WO2016044731A1 (en) | 2014-09-18 | 2016-03-24 | Si-Bone Inc. | Implants for bone fixation or fusion |
| EP3782586B1 (en) * | 2014-09-18 | 2025-04-30 | SI-Bone, Inc. | Matrix implant |
| US10363075B2 (en) * | 2015-02-09 | 2019-07-30 | Yingze Zhang | Porous bionic internal fixation device for promoting healing of fractured bone |
| US10376206B2 (en) | 2015-04-01 | 2019-08-13 | Si-Bone Inc. | Neuromonitoring systems and methods for bone fixation or fusion procedures |
| AU2016246067B2 (en) * | 2015-04-09 | 2020-11-26 | Centinel Spine, Llc | Spinal implants configured for tissue sparing angle of insertion and related methods |
| CN105877828A (zh) * | 2016-04-05 | 2016-08-24 | 陈伟 | 一种治疗跟骨骨折的仿生固定装置 |
| WO2017180908A1 (en) | 2016-04-14 | 2017-10-19 | Cutting Edge Spine Llc | Implants and techniques for tissue fixation and fusion |
| CN105726114A (zh) * | 2016-04-15 | 2016-07-06 | 陈伟 | 一种治疗股骨近端骨折的空心螺钉 |
| US10413332B2 (en) | 2016-04-25 | 2019-09-17 | Imds Llc | Joint fusion implant and methods |
| US9833321B2 (en) | 2016-04-25 | 2017-12-05 | Imds Llc | Joint fusion instrumentation and methods |
| CN105919697B (zh) * | 2016-06-08 | 2018-01-02 | 王涛 | 一种胫骨中段假体 |
| WO2018209177A1 (en) | 2017-05-12 | 2018-11-15 | Cutting Edge Spine Llc | Implants for tissue fixation and fusion |
| US10603055B2 (en) | 2017-09-15 | 2020-03-31 | Jcbd, Llc | Systems for and methods of preparing and fusing a sacroiliac joint |
| WO2019067584A1 (en) | 2017-09-26 | 2019-04-04 | Si-Bone Inc. | SYSTEMS AND METHODS FOR DECORTICATING SACROILITIC JOINT |
| EP3773281B1 (en) * | 2018-03-28 | 2025-01-08 | SI-Bone, Inc. | Threaded implants for use across bone segments |
| CN108742899A (zh) * | 2018-06-12 | 2018-11-06 | 中国人民解放军总医院 | 一种多孔钽涂层的人工种植体及其制备方法 |
| US20220104856A1 (en) * | 2019-01-07 | 2022-04-07 | Anjali Investments Llc | Screw bone implant |
| USD929593S1 (en) | 2019-01-15 | 2021-08-31 | Neo Medical S.A. | Spine cage |
| USD883484S1 (en) | 2019-01-16 | 2020-05-05 | Neo Medical S.A. | Spine cage |
| US11369419B2 (en) | 2019-02-14 | 2022-06-28 | Si-Bone Inc. | Implants for spinal fixation and or fusion |
| WO2020168269A1 (en) | 2019-02-14 | 2020-08-20 | Si-Bone Inc. | Implants for spinal fixation and or fusion |
| CN112807065A (zh) * | 2019-11-15 | 2021-05-18 | 李锋涛 | 一种脊柱内镜下腰椎椎间融合系统及其使用方法 |
| EP4061262A1 (en) | 2019-11-21 | 2022-09-28 | SI-Bone, Inc. | Rod coupling assemblies for bone stabilization constructs |
| CN110916736A (zh) * | 2019-11-26 | 2020-03-27 | 杭州电子科技大学 | 一种多孔可降解螺钉及其制造方法 |
| US11672570B2 (en) * | 2019-11-27 | 2023-06-13 | Si-Bone Inc. | Bone stabilizing implants and methods of placement across SI Joints |
| US12083026B2 (en) | 2019-12-09 | 2024-09-10 | Si-Bone Inc. | Sacro-iliac joint stabilizing implants and methods of implantation |
| JP7411062B2 (ja) * | 2020-02-19 | 2024-01-10 | オリンパステルモバイオマテリアル株式会社 | 骨スクリュ |
| US11918257B2 (en) | 2020-03-04 | 2024-03-05 | Orthofix Us Llc | Implant system and method for joint fusion |
| CN215651798U (zh) * | 2020-12-04 | 2022-01-28 | 上海交通大学医学院附属第九人民医院 | 骨盆增强型髋关节假体 |
| WO2022125619A1 (en) | 2020-12-09 | 2022-06-16 | Si-Bone Inc. | Sacro-iliac joint stabilizing implants and methods of implantation |
| EP4440505A4 (en) | 2021-12-03 | 2025-11-19 | Si Bone Inc | INTERBODY CAGES AND SACROILIAC JOINT STABILIZATION METHODS |
| US12433733B2 (en) | 2023-08-15 | 2025-10-07 | Si-Bone Inc. | Pelvic stabilization implants, methods of use and manufacture |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7534254B1 (en) * | 1988-06-13 | 2009-05-19 | Warsaw Orthopedic, Inc. | Threaded frusto-conical interbody spinal fusion implants |
| US5334205A (en) * | 1993-06-30 | 1994-08-02 | The United States Of America As Represented By The Secretary Of The Air Force | Sacroiliac joint fixation guide |
| US5716358A (en) * | 1994-12-02 | 1998-02-10 | Johnson & Johnson Professional, Inc. | Directional bone fixation device |
| US6423095B1 (en) * | 1995-10-16 | 2002-07-23 | Sdgi Holdings, Inc. | Intervertebral spacers |
| US6053916A (en) * | 1999-02-17 | 2000-04-25 | Moore; Michael R. | Sacroiliac implant |
| US20050143837A1 (en) * | 2002-06-27 | 2005-06-30 | Ferree Bret A. | Arthroplasty devices configured to reduce shear stress |
| US8986348B2 (en) * | 2004-08-09 | 2015-03-24 | Si-Bone Inc. | Systems and methods for the fusion of the sacral-iliac joint |
| US20060036251A1 (en) * | 2004-08-09 | 2006-02-16 | Reiley Mark A | Systems and methods for the fixation or fusion of bone |
| US7951198B2 (en) * | 2005-05-10 | 2011-05-31 | Acumed Llc | Bone connector with pivotable joint |
| US20090024174A1 (en) * | 2007-07-17 | 2009-01-22 | Stark John G | Bone screws and particular applications to sacroiliac joint fusion |
| US20100237631A1 (en) * | 2009-03-20 | 2010-09-23 | Howard Yu | Oscillating Power Generator |
| US8579947B2 (en) | 2009-09-14 | 2013-11-12 | Yangguan Wu | Polyporous hollow bone screw |
| CN105287056B (zh) * | 2010-01-13 | 2018-10-16 | Jcbd公司 | 骶髂关节固着融合系统 |
-
2013
- 2013-03-08 US US13/791,801 patent/US20130245763A1/en not_active Abandoned
- 2013-03-08 HK HK15107028.4A patent/HK1206232A1/xx unknown
- 2013-03-08 WO PCT/US2013/029945 patent/WO2013134678A1/en not_active Ceased
- 2013-03-08 JP JP2014561153A patent/JP2015509435A/ja active Pending
- 2013-03-08 CN CN201380012916.2A patent/CN104302237A/zh active Pending
- 2013-03-08 KR KR1020147028548A patent/KR20140147835A/ko not_active Withdrawn
- 2013-03-08 EP EP13757448.9A patent/EP2827789A4/en not_active Withdrawn
-
2014
- 2014-08-21 IN IN7038DEN2014 patent/IN2014DN07038A/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| IN2014DN07038A (enExample) | 2015-04-10 |
| EP2827789A1 (en) | 2015-01-28 |
| US20130245763A1 (en) | 2013-09-19 |
| CN104302237A (zh) | 2015-01-21 |
| HK1206232A1 (en) | 2016-01-08 |
| JP2015509435A (ja) | 2015-03-30 |
| WO2013134678A1 (en) | 2013-09-12 |
| EP2827789A4 (en) | 2016-01-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20140147835A (ko) | 나사 가공된 임플란트 | |
| US11478287B2 (en) | Fenestrated implant | |
| US20220280303A1 (en) | Integrated implant | |
| US12427028B2 (en) | Threaded implants and methods of use across bone segments | |
| JP5771277B2 (ja) | 骨を使用する圧縮インプラントの定着または固定のためのシステムおよび方法 | |
| US20140276846A1 (en) | Systems and methods for implanting bone graft and implant |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20141010 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |