KR20140084211A - 효과가 없는 적혈구생성 치료를 위한 방법 및 조성물 - Google Patents
효과가 없는 적혈구생성 치료를 위한 방법 및 조성물 Download PDFInfo
- Publication number
- KR20140084211A KR20140084211A KR1020147013325A KR20147013325A KR20140084211A KR 20140084211 A KR20140084211 A KR 20140084211A KR 1020147013325 A KR1020147013325 A KR 1020147013325A KR 20147013325 A KR20147013325 A KR 20147013325A KR 20140084211 A KR20140084211 A KR 20140084211A
- Authority
- KR
- South Korea
- Prior art keywords
- actriib
- anemia
- gdf trap
- seq
- amino acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 238000000034 method Methods 0.000 title claims abstract description 78
- 230000010437 erythropoiesis Effects 0.000 title claims description 61
- 239000000203 mixture Substances 0.000 title abstract description 31
- 210000003743 erythrocyte Anatomy 0.000 claims abstract description 230
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 321
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 312
- 229920001184 polypeptide Polymers 0.000 claims description 310
- 102000003951 Erythropoietin Human genes 0.000 claims description 159
- 108090000394 Erythropoietin Proteins 0.000 claims description 159
- 229940105423 erythropoietin Drugs 0.000 claims description 157
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 134
- 208000007502 anemia Diseases 0.000 claims description 118
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 claims description 114
- 150000001413 amino acids Chemical class 0.000 claims description 107
- 238000011282 treatment Methods 0.000 claims description 102
- 210000004369 blood Anatomy 0.000 claims description 72
- 239000008280 blood Substances 0.000 claims description 72
- 229910052742 iron Inorganic materials 0.000 claims description 67
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 59
- 208000005980 beta thalassemia Diseases 0.000 claims description 47
- 208000022806 beta-thalassemia major Diseases 0.000 claims description 46
- 210000000952 spleen Anatomy 0.000 claims description 46
- 210000001519 tissue Anatomy 0.000 claims description 37
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 35
- 206010065973 Iron Overload Diseases 0.000 claims description 31
- 210000000988 bone and bone Anatomy 0.000 claims description 27
- 208000035475 disorder Diseases 0.000 claims description 25
- 230000007170 pathology Effects 0.000 claims description 19
- 206010020880 Hypertrophy Diseases 0.000 claims description 18
- 238000001802 infusion Methods 0.000 claims description 17
- 208000011580 syndromic disease Diseases 0.000 claims description 17
- -1 DFO-B Chemical compound 0.000 claims description 16
- 239000000463 material Substances 0.000 claims description 14
- 230000000913 erythropoietic effect Effects 0.000 claims description 13
- 230000003393 splenic effect Effects 0.000 claims description 13
- 230000002378 acidificating effect Effects 0.000 claims description 12
- 239000000556 agonist Substances 0.000 claims description 9
- 230000002159 abnormal effect Effects 0.000 claims description 4
- 229940075525 iron chelating agent Drugs 0.000 claims description 4
- 239000000797 iron chelating agent Substances 0.000 claims description 4
- 210000002798 bone marrow cell Anatomy 0.000 claims description 3
- UBQYURCVBFRUQT-UHFFFAOYSA-N N-benzoyl-Ferrioxamine B Chemical compound CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN UBQYURCVBFRUQT-UHFFFAOYSA-N 0.000 claims 5
- TZXKOCQBRNJULO-UHFFFAOYSA-N Ferriprox Chemical compound CC1=C(O)C(=O)C=CN1C TZXKOCQBRNJULO-UHFFFAOYSA-N 0.000 claims 2
- 238000011360 adjunctive therapy Methods 0.000 claims 2
- BOFQWVMAQOTZIW-UHFFFAOYSA-N deferasirox Chemical compound C1=CC(C(=O)O)=CC=C1N1C(C=2C(=CC=CC=2)O)=NC(C=2C(=CC=CC=2)O)=N1 BOFQWVMAQOTZIW-UHFFFAOYSA-N 0.000 claims 2
- HRBYEPVOFCWVAZ-UHFFFAOYSA-N 3-(2H-triazol-4-yl)benzene-1,2-diol Chemical compound Oc1cccc(-c2c[nH]nn2)c1O HRBYEPVOFCWVAZ-UHFFFAOYSA-N 0.000 claims 1
- 229960003266 deferiprone Drugs 0.000 claims 1
- 229940099217 desferal Drugs 0.000 claims 1
- 229940024583 exjade Drugs 0.000 claims 1
- 229940025452 ferriprox Drugs 0.000 claims 1
- 230000002631 hypothermal effect Effects 0.000 claims 1
- 230000001965 increasing effect Effects 0.000 abstract description 68
- 108010054147 Hemoglobins Proteins 0.000 abstract description 57
- 102000001554 Hemoglobins Human genes 0.000 abstract description 57
- 241000251539 Vertebrata <Metazoa> Species 0.000 abstract description 12
- 241000283984 Rodentia Species 0.000 abstract description 3
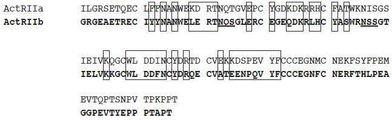
- 102220479051 CD59 glycoprotein_L79D_mutation Human genes 0.000 description 123
- 108090000623 proteins and genes Proteins 0.000 description 120
- 210000004027 cell Anatomy 0.000 description 115
- 235000001014 amino acid Nutrition 0.000 description 112
- 230000000694 effects Effects 0.000 description 111
- 229940024606 amino acid Drugs 0.000 description 103
- 102000004169 proteins and genes Human genes 0.000 description 98
- 241000699670 Mus sp. Species 0.000 description 97
- 235000018102 proteins Nutrition 0.000 description 95
- 230000027455 binding Effects 0.000 description 84
- 102000005962 receptors Human genes 0.000 description 83
- 108020003175 receptors Proteins 0.000 description 83
- 239000003446 ligand Substances 0.000 description 79
- 239000003981 vehicle Substances 0.000 description 61
- 102000037865 fusion proteins Human genes 0.000 description 58
- 108020001507 fusion proteins Proteins 0.000 description 58
- 150000001875 compounds Chemical class 0.000 description 53
- 230000014509 gene expression Effects 0.000 description 44
- 206010057190 Respiratory tract infections Diseases 0.000 description 39
- 230000035772 mutation Effects 0.000 description 39
- 238000004519 manufacturing process Methods 0.000 description 38
- 230000002829 reductive effect Effects 0.000 description 34
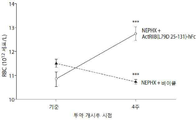
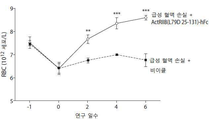
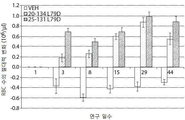
- 238000005534 hematocrit Methods 0.000 description 33
- 238000010172 mouse model Methods 0.000 description 33
- 150000007523 nucleic acids Chemical class 0.000 description 33
- 239000000126 substance Substances 0.000 description 33
- 210000001185 bone marrow Anatomy 0.000 description 31
- 230000000875 corresponding effect Effects 0.000 description 31
- 230000003394 haemopoietic effect Effects 0.000 description 28
- 239000002773 nucleotide Substances 0.000 description 27
- 125000003729 nucleotide group Chemical group 0.000 description 26
- 239000013598 vector Substances 0.000 description 26
- 102000039446 nucleic acids Human genes 0.000 description 25
- 108020004707 nucleic acids Proteins 0.000 description 25
- 230000004927 fusion Effects 0.000 description 23
- 238000001727 in vivo Methods 0.000 description 23
- 238000004458 analytical method Methods 0.000 description 22
- 108020001756 ligand binding domains Proteins 0.000 description 20
- 230000001225 therapeutic effect Effects 0.000 description 20
- 125000005647 linker group Chemical group 0.000 description 18
- 238000006467 substitution reaction Methods 0.000 description 18
- 206010028980 Neoplasm Diseases 0.000 description 17
- 102100030655 Platelet-activating factor acetylhydrolase IB subunit beta Human genes 0.000 description 17
- 230000015572 biosynthetic process Effects 0.000 description 17
- 230000036772 blood pressure Effects 0.000 description 17
- 230000009467 reduction Effects 0.000 description 17
- 102100022544 Bone morphogenetic protein 7 Human genes 0.000 description 16
- 241000699666 Mus <mouse, genus> Species 0.000 description 16
- 108091028043 Nucleic acid sequence Proteins 0.000 description 16
- 239000013543 active substance Substances 0.000 description 16
- 239000003814 drug Substances 0.000 description 16
- 210000002966 serum Anatomy 0.000 description 16
- 238000003556 assay Methods 0.000 description 15
- 230000004048 modification Effects 0.000 description 15
- 238000012986 modification Methods 0.000 description 15
- 210000001995 reticulocyte Anatomy 0.000 description 15
- 102000007469 Actins Human genes 0.000 description 14
- 108010085238 Actins Proteins 0.000 description 14
- 102100040898 Growth/differentiation factor 11 Human genes 0.000 description 14
- 101000937269 Homo sapiens Activin receptor type-2B Proteins 0.000 description 14
- 101000899361 Homo sapiens Bone morphogenetic protein 7 Proteins 0.000 description 14
- 230000004069 differentiation Effects 0.000 description 14
- 102000045412 human ACVR2B Human genes 0.000 description 14
- 210000003924 normoblast Anatomy 0.000 description 14
- 230000001105 regulatory effect Effects 0.000 description 14
- 101710194452 Growth/differentiation factor 11 Proteins 0.000 description 13
- 230000001154 acute effect Effects 0.000 description 13
- 125000000539 amino acid group Chemical group 0.000 description 13
- 239000013612 plasmid Substances 0.000 description 13
- 238000012360 testing method Methods 0.000 description 13
- 102100039939 Growth/differentiation factor 8 Human genes 0.000 description 12
- 108010056852 Myostatin Proteins 0.000 description 12
- 229930012538 Paclitaxel Natural products 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 229940009098 aspartate Drugs 0.000 description 12
- 201000011510 cancer Diseases 0.000 description 12
- 208000020832 chronic kidney disease Diseases 0.000 description 12
- 239000000306 component Substances 0.000 description 12
- 230000006870 function Effects 0.000 description 12
- 210000003205 muscle Anatomy 0.000 description 12
- 229960001592 paclitaxel Drugs 0.000 description 12
- 238000000746 purification Methods 0.000 description 12
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 12
- 241000282693 Cercopithecidae Species 0.000 description 11
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 11
- 239000012634 fragment Substances 0.000 description 11
- 239000003112 inhibitor Substances 0.000 description 11
- 210000003734 kidney Anatomy 0.000 description 11
- 239000002243 precursor Substances 0.000 description 11
- 210000000130 stem cell Anatomy 0.000 description 11
- 210000000689 upper leg Anatomy 0.000 description 11
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 10
- 101710196692 Actin A Proteins 0.000 description 10
- 238000007792 addition Methods 0.000 description 10
- 210000004899 c-terminal region Anatomy 0.000 description 10
- 230000008859 change Effects 0.000 description 10
- 230000001684 chronic effect Effects 0.000 description 10
- 230000006378 damage Effects 0.000 description 10
- 201000010099 disease Diseases 0.000 description 10
- 230000013595 glycosylation Effects 0.000 description 10
- 238000006206 glycosylation reaction Methods 0.000 description 10
- 230000002401 inhibitory effect Effects 0.000 description 10
- 102000040430 polynucleotide Human genes 0.000 description 10
- 108091033319 polynucleotide Proteins 0.000 description 10
- 239000002157 polynucleotide Substances 0.000 description 10
- 238000003860 storage Methods 0.000 description 10
- 238000002560 therapeutic procedure Methods 0.000 description 10
- XJOTXKZIRSHZQV-RXHOOSIZSA-N (3S)-3-amino-4-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[(2S)-2-[[(2S,3S)-1-[[(1R,6R,12R,17R,20S,23S,26R,31R,34R,39R,42S,45S,48S,51S,59S)-51-(4-aminobutyl)-31-[[(2S)-6-amino-1-[[(1S,2R)-1-carboxy-2-hydroxypropyl]amino]-1-oxohexan-2-yl]carbamoyl]-20-benzyl-23-[(2S)-butan-2-yl]-45-(3-carbamimidamidopropyl)-48-(hydroxymethyl)-42-(1H-imidazol-4-ylmethyl)-59-(2-methylsulfanylethyl)-7,10,19,22,25,33,40,43,46,49,52,54,57,60,63,64-hexadecaoxo-3,4,14,15,28,29,36,37-octathia-8,11,18,21,24,32,41,44,47,50,53,55,58,61,62,65-hexadecazatetracyclo[32.19.8.26,17.212,39]pentahexacontan-26-yl]amino]-3-methyl-1-oxopentan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-4-oxobutanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)[C@@H](C)O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@@H]4CSSC[C@H](NC(=O)[C@H](Cc5ccccc5)NC(=O)[C@@H](NC1=O)[C@@H](C)CC)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC3=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N2)C(=O)NCC(=O)N4)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XJOTXKZIRSHZQV-RXHOOSIZSA-N 0.000 description 9
- 208000013558 Developmental Bone disease Diseases 0.000 description 9
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 9
- 102220542870 Presenilins-associated rhomboid-like protein, mitochondrial_L79E_mutation Human genes 0.000 description 9
- 206010072610 Skeletal dysplasia Diseases 0.000 description 9
- 238000012217 deletion Methods 0.000 description 9
- 230000037430 deletion Effects 0.000 description 9
- 210000003527 eukaryotic cell Anatomy 0.000 description 9
- 239000013604 expression vector Substances 0.000 description 9
- 230000011132 hemopoiesis Effects 0.000 description 9
- 108060003558 hepcidin Proteins 0.000 description 9
- 102000018511 hepcidin Human genes 0.000 description 9
- 229940066919 hepcidin Drugs 0.000 description 9
- 239000002502 liposome Substances 0.000 description 9
- 210000004185 liver Anatomy 0.000 description 9
- 230000035800 maturation Effects 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 239000003642 reactive oxygen metabolite Substances 0.000 description 9
- 230000011664 signaling Effects 0.000 description 9
- 102220525561 Coiled-coil domain-containing protein 200_A24N_mutation Human genes 0.000 description 8
- 241000282412 Homo Species 0.000 description 8
- 108010076504 Protein Sorting Signals Proteins 0.000 description 8
- 241000700159 Rattus Species 0.000 description 8
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 8
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 8
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical group N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 description 8
- 230000009286 beneficial effect Effects 0.000 description 8
- 210000002960 bfu-e Anatomy 0.000 description 8
- 238000002512 chemotherapy Methods 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- 239000012091 fetal bovine serum Substances 0.000 description 8
- 238000000338 in vitro Methods 0.000 description 8
- 238000002347 injection Methods 0.000 description 8
- 239000007924 injection Substances 0.000 description 8
- 230000035755 proliferation Effects 0.000 description 8
- 230000004044 response Effects 0.000 description 8
- 230000001177 retroviral effect Effects 0.000 description 8
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 8
- 210000004906 toe nail Anatomy 0.000 description 8
- 108010059616 Activins Proteins 0.000 description 7
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 7
- 208000032843 Hemorrhage Diseases 0.000 description 7
- 101000835093 Homo sapiens Transferrin receptor protein 1 Proteins 0.000 description 7
- 206010020772 Hypertension Diseases 0.000 description 7
- 206010021143 Hypoxia Diseases 0.000 description 7
- 102100026818 Inhibin beta E chain Human genes 0.000 description 7
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 7
- 241001529936 Murinae Species 0.000 description 7
- 102100026144 Transferrin receptor protein 1 Human genes 0.000 description 7
- 241000700605 Viruses Species 0.000 description 7
- 239000000488 activin Substances 0.000 description 7
- 235000004279 alanine Nutrition 0.000 description 7
- 239000005557 antagonist Substances 0.000 description 7
- 238000004820 blood count Methods 0.000 description 7
- 238000000423 cell based assay Methods 0.000 description 7
- 238000004587 chromatography analysis Methods 0.000 description 7
- 230000000295 complement effect Effects 0.000 description 7
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 7
- 230000013632 homeostatic process Effects 0.000 description 7
- 230000001976 improved effect Effects 0.000 description 7
- 230000001939 inductive effect Effects 0.000 description 7
- 210000000265 leukocyte Anatomy 0.000 description 7
- 210000004962 mammalian cell Anatomy 0.000 description 7
- 230000001404 mediated effect Effects 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 239000000018 receptor agonist Substances 0.000 description 7
- 229940044601 receptor agonist Drugs 0.000 description 7
- 239000000523 sample Substances 0.000 description 7
- 238000012216 screening Methods 0.000 description 7
- 239000011780 sodium chloride Substances 0.000 description 7
- 230000000087 stabilizing effect Effects 0.000 description 7
- 230000004083 survival effect Effects 0.000 description 7
- 229940124597 therapeutic agent Drugs 0.000 description 7
- 238000012384 transportation and delivery Methods 0.000 description 7
- 239000003656 tris buffered saline Substances 0.000 description 7
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 6
- 102000014914 Carrier Proteins Human genes 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 239000007983 Tris buffer Substances 0.000 description 6
- 230000004913 activation Effects 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 108091008324 binding proteins Proteins 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- 150000001720 carbohydrates Chemical class 0.000 description 6
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 6
- 235000018417 cysteine Nutrition 0.000 description 6
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 230000002209 hydrophobic effect Effects 0.000 description 6
- 206010020718 hyperplasia Diseases 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 150000002632 lipids Chemical group 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 229920000609 methyl cellulose Polymers 0.000 description 6
- 239000001923 methylcellulose Substances 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- 238000010186 staining Methods 0.000 description 6
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- 206010018910 Haemolysis Diseases 0.000 description 5
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 5
- 230000004988 N-glycosylation Effects 0.000 description 5
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 5
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 5
- 239000004473 Threonine Substances 0.000 description 5
- 230000004075 alteration Effects 0.000 description 5
- 208000034158 bleeding Diseases 0.000 description 5
- 230000000740 bleeding effect Effects 0.000 description 5
- 238000003776 cleavage reaction Methods 0.000 description 5
- 230000001086 cytosolic effect Effects 0.000 description 5
- 230000002950 deficient Effects 0.000 description 5
- 238000001514 detection method Methods 0.000 description 5
- 235000019441 ethanol Nutrition 0.000 description 5
- 238000001415 gene therapy Methods 0.000 description 5
- 229930195712 glutamate Natural products 0.000 description 5
- 230000012010 growth Effects 0.000 description 5
- 239000003102 growth factor Substances 0.000 description 5
- 230000008588 hemolysis Effects 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 239000008194 pharmaceutical composition Substances 0.000 description 5
- 210000001236 prokaryotic cell Anatomy 0.000 description 5
- 230000001737 promoting effect Effects 0.000 description 5
- 230000007017 scission Effects 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 235000000346 sugar Nutrition 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 230000032258 transport Effects 0.000 description 5
- 239000004475 Arginine Substances 0.000 description 4
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 4
- 108091026890 Coding region Proteins 0.000 description 4
- 101150002621 EPO gene Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- 108091005902 Hemoglobin subunit alpha Proteins 0.000 description 4
- 241000238631 Hexapoda Species 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- 206010022971 Iron Deficiencies Diseases 0.000 description 4
- 208000015710 Iron-Deficiency Anemia Diseases 0.000 description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 4
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 4
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 102000002151 Microfilament Proteins Human genes 0.000 description 4
- 108010040897 Microfilament Proteins Proteins 0.000 description 4
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 4
- 208000001132 Osteoporosis Diseases 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 229920002684 Sepharose Polymers 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 4
- 230000002411 adverse Effects 0.000 description 4
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 4
- 235000009582 asparagine Nutrition 0.000 description 4
- 229960001230 asparagine Drugs 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 238000002655 chelation therapy Methods 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 238000002648 combination therapy Methods 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 235000005911 diet Nutrition 0.000 description 4
- 239000000539 dimer Substances 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 230000002708 enhancing effect Effects 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 230000007274 generation of a signal involved in cell-cell signaling Effects 0.000 description 4
- 230000036252 glycation Effects 0.000 description 4
- 235000011187 glycerol Nutrition 0.000 description 4
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 description 4
- 208000017169 kidney disease Diseases 0.000 description 4
- 238000002372 labelling Methods 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 208000019423 liver disease Diseases 0.000 description 4
- 239000011707 mineral Substances 0.000 description 4
- 230000037257 muscle growth Effects 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 108010004165 polystatin Proteins 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 238000002271 resection Methods 0.000 description 4
- 230000000717 retained effect Effects 0.000 description 4
- 238000012163 sequencing technique Methods 0.000 description 4
- 230000019491 signal transduction Effects 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- 150000008163 sugars Chemical class 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 102000035160 transmembrane proteins Human genes 0.000 description 4
- 108091005703 transmembrane proteins Proteins 0.000 description 4
- 241000701447 unidentified baculovirus Species 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- 230000003442 weekly effect Effects 0.000 description 4
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 3
- UZOVYGYOLBIAJR-UHFFFAOYSA-N 4-isocyanato-4'-methyldiphenylmethane Chemical compound C1=CC(C)=CC=C1CC1=CC=C(N=C=O)C=C1 UZOVYGYOLBIAJR-UHFFFAOYSA-N 0.000 description 3
- 108010088751 Albumins Proteins 0.000 description 3
- 102000009027 Albumins Human genes 0.000 description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 3
- 101100297347 Caenorhabditis elegans pgl-3 gene Proteins 0.000 description 3
- 208000031229 Cardiomyopathies Diseases 0.000 description 3
- 102220578642 Chorion-specific transcription factor GCMb_K74M_mutation Human genes 0.000 description 3
- 108020004705 Codon Proteins 0.000 description 3
- 206010010356 Congenital anomaly Diseases 0.000 description 3
- 241000699802 Cricetulus griseus Species 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 108091006020 Fc-tagged proteins Proteins 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 3
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 3
- 101000970954 Homo sapiens Activin receptor type-2A Proteins 0.000 description 3
- 101000987586 Homo sapiens Eosinophil peroxidase Proteins 0.000 description 3
- 101000869690 Homo sapiens Protein S100-A8 Proteins 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- 240000007472 Leucaena leucocephala Species 0.000 description 3
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 3
- 108060001084 Luciferase Proteins 0.000 description 3
- 239000005089 Luciferase Substances 0.000 description 3
- 102100025751 Mothers against decapentaplegic homolog 2 Human genes 0.000 description 3
- 101710143123 Mothers against decapentaplegic homolog 2 Proteins 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 102100032442 Protein S100-A8 Human genes 0.000 description 3
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 3
- 108091081021 Sense strand Proteins 0.000 description 3
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical class [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 3
- 208000007536 Thrombosis Diseases 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 239000011149 active material Substances 0.000 description 3
- 238000001042 affinity chromatography Methods 0.000 description 3
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 3
- 230000000692 anti-sense effect Effects 0.000 description 3
- 235000003704 aspartic acid Nutrition 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 3
- 210000000601 blood cell Anatomy 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 230000022159 cartilage development Effects 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 3
- 208000019425 cirrhosis of liver Diseases 0.000 description 3
- 238000001246 colloidal dispersion Methods 0.000 description 3
- 239000002131 composite material Substances 0.000 description 3
- 102000003675 cytokine receptors Human genes 0.000 description 3
- 108010057085 cytokine receptors Proteins 0.000 description 3
- 230000003111 delayed effect Effects 0.000 description 3
- 230000037213 diet Effects 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 210000000267 erythroid cell Anatomy 0.000 description 3
- 230000000925 erythroid effect Effects 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 235000013922 glutamic acid Nutrition 0.000 description 3
- 239000004220 glutamic acid Substances 0.000 description 3
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 3
- 239000005090 green fluorescent protein Substances 0.000 description 3
- 208000019622 heart disease Diseases 0.000 description 3
- 125000001165 hydrophobic group Chemical group 0.000 description 3
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 3
- 230000007954 hypoxia Effects 0.000 description 3
- 230000001771 impaired effect Effects 0.000 description 3
- 239000000411 inducer Substances 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 230000001926 lymphatic effect Effects 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 238000007726 management method Methods 0.000 description 3
- OVHQWOXKMOVDJP-UHFFFAOYSA-N melitin Chemical compound OC1C(O)C(O)C(C)OC1OC1C(O)C(OC=2C=C3C(C(C(OC4C(C(O)C(O)C(COC5C(C(O)C(O)C(CO)O5)O)O4)O)=C(C=4C=CC(O)=CC=4)O3)=O)=C(O)C=2)OC(C)C1O OVHQWOXKMOVDJP-UHFFFAOYSA-N 0.000 description 3
- 210000003716 mesoderm Anatomy 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 235000019198 oils Nutrition 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 3
- 239000000825 pharmaceutical preparation Substances 0.000 description 3
- 230000003285 pharmacodynamic effect Effects 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 229920002704 polyhistidine Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 230000017854 proteolysis Effects 0.000 description 3
- 239000012925 reference material Substances 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 238000007423 screening assay Methods 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- 210000004989 spleen cell Anatomy 0.000 description 3
- 238000010254 subcutaneous injection Methods 0.000 description 3
- 239000007929 subcutaneous injection Substances 0.000 description 3
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 238000011285 therapeutic regimen Methods 0.000 description 3
- 230000000451 tissue damage Effects 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical class [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 3
- 239000004474 valine Substances 0.000 description 3
- 229960004295 valine Drugs 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 2
- 208000004998 Abdominal Pain Diseases 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 102100034134 Activin receptor type-1B Human genes 0.000 description 2
- 208000030090 Acute Disease Diseases 0.000 description 2
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 2
- 102220556625 Acyl-coenzyme A thioesterase 11_K55A_mutation Human genes 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 208000030760 Anaemia of chronic disease Diseases 0.000 description 2
- 208000019901 Anxiety disease Diseases 0.000 description 2
- 102220638160 Arylamine N-acetyltransferase 1_R64K_mutation Human genes 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 108010049870 Bone Morphogenetic Protein 7 Proteins 0.000 description 2
- 108010078791 Carrier Proteins Proteins 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- 206010008479 Chest Pain Diseases 0.000 description 2
- 102220578645 Chorion-specific transcription factor GCMb_N65A_mutation Human genes 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 208000027205 Congenital disease Diseases 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 102220575187 Egl nine homolog 1_F82A_mutation Human genes 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 108010013369 Enteropeptidase Proteins 0.000 description 2
- 102100029727 Enteropeptidase Human genes 0.000 description 2
- 102000008857 Ferritin Human genes 0.000 description 2
- 108050000784 Ferritin Proteins 0.000 description 2
- 238000008416 Ferritin Methods 0.000 description 2
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 2
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 2
- 241000713858 Harvey murine sarcoma virus Species 0.000 description 2
- 101710154606 Hemagglutinin Proteins 0.000 description 2
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 2
- 101000799189 Homo sapiens Activin receptor type-1B Proteins 0.000 description 2
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 201000002980 Hyperparathyroidism Diseases 0.000 description 2
- 102000000646 Interleukin-3 Human genes 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- 102000004889 Interleukin-6 Human genes 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- 239000007760 Iscove's Modified Dulbecco's Medium Substances 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 208000029725 Metabolic bone disease Diseases 0.000 description 2
- 241000713869 Moloney murine leukemia virus Species 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- 102000007474 Multiprotein Complexes Human genes 0.000 description 2
- 108010085220 Multiprotein Complexes Proteins 0.000 description 2
- 101100238610 Mus musculus Msh3 gene Proteins 0.000 description 2
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 2
- 102000044547 Nodal Human genes 0.000 description 2
- 108700024442 Nodal Proteins 0.000 description 2
- 230000004989 O-glycosylation Effects 0.000 description 2
- 102220567352 Ornithine decarboxylase antizyme 1_K74F_mutation Human genes 0.000 description 2
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 2
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 2
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 102000013566 Plasminogen Human genes 0.000 description 2
- 108010051456 Plasminogen Proteins 0.000 description 2
- 102000004079 Prolyl Hydroxylases Human genes 0.000 description 2
- 108010043005 Prolyl Hydroxylases Proteins 0.000 description 2
- 101710176177 Protein A56 Proteins 0.000 description 2
- 102220495689 Putative uncharacterized protein FLJ43944_R40A_mutation Human genes 0.000 description 2
- 101100437153 Rattus norvegicus Acvr2b gene Proteins 0.000 description 2
- 208000009527 Refractory anemia Diseases 0.000 description 2
- 206010072684 Refractory cytopenia with unilineage dysplasia Diseases 0.000 description 2
- 108700008625 Reporter Genes Proteins 0.000 description 2
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 2
- 102000007374 Smad Proteins Human genes 0.000 description 2
- 108010007945 Smad Proteins Proteins 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 102000043168 TGF-beta family Human genes 0.000 description 2
- 108091085018 TGF-beta family Proteins 0.000 description 2
- 102000040945 Transcription factor Human genes 0.000 description 2
- 108091023040 Transcription factor Proteins 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 238000013019 agitation Methods 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 2
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 2
- 235000011130 ammonium sulphate Nutrition 0.000 description 2
- 208000022400 anemia due to chronic disease Diseases 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000036506 anxiety Effects 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 238000010322 bone marrow transplantation Methods 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 230000007248 cellular mechanism Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 210000000038 chest Anatomy 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 235000012000 cholesterol Nutrition 0.000 description 2
- 230000007882 cirrhosis Effects 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 230000001332 colony forming effect Effects 0.000 description 2
- 238000004040 coloring Methods 0.000 description 2
- 230000009918 complex formation Effects 0.000 description 2
- 239000003636 conditioned culture medium Substances 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 230000007850 degeneration Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 230000002526 effect on cardiovascular system Effects 0.000 description 2
- 230000013020 embryo development Effects 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 230000001804 emulsifying effect Effects 0.000 description 2
- 230000002124 endocrine Effects 0.000 description 2
- 230000003511 endothelial effect Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 230000001605 fetal effect Effects 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 239000000185 hemagglutinin Substances 0.000 description 2
- 230000002489 hematologic effect Effects 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 210000003494 hepatocyte Anatomy 0.000 description 2
- 238000013537 high throughput screening Methods 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 238000009396 hybridization Methods 0.000 description 2
- 208000000069 hyperpigmentation Diseases 0.000 description 2
- 230000003810 hyperpigmentation Effects 0.000 description 2
- 230000001146 hypoxic effect Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000005462 in vivo assay Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 239000003701 inert diluent Substances 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 229940076264 interleukin-3 Drugs 0.000 description 2
- 229940100601 interleukin-6 Drugs 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 230000004807 localization Effects 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 238000009115 maintenance therapy Methods 0.000 description 2
- 239000000693 micelle Substances 0.000 description 2
- 208000010555 moderate anemia Diseases 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- 230000001537 neural effect Effects 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 230000009437 off-target effect Effects 0.000 description 2
- 230000011164 ossification Effects 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 238000000059 patterning Methods 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 238000010647 peptide synthesis reaction Methods 0.000 description 2
- 239000000816 peptidomimetic Substances 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- 239000004626 polylactic acid Substances 0.000 description 2
- 102000054765 polymorphisms of proteins Human genes 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 230000001323 posttranslational effect Effects 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000002028 premature Effects 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- 229960004063 propylene glycol Drugs 0.000 description 2
- 230000006337 proteolytic cleavage Effects 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 238000011552 rat model Methods 0.000 description 2
- 230000008844 regulatory mechanism Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 102200089383 rs57977969 Human genes 0.000 description 2
- 208000007056 sickle cell anemia Diseases 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 210000004988 splenocyte Anatomy 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 239000000021 stimulant Substances 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 231100000827 tissue damage Toxicity 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000005030 transcription termination Effects 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- 238000011269 treatment regimen Methods 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 1
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 1
- PXEZTIWVRVSYOK-UHFFFAOYSA-N 2-(3,6-diacetyloxy-2,7-dichloro-9h-xanthen-9-yl)benzoic acid Chemical compound C1=2C=C(Cl)C(OC(=O)C)=CC=2OC2=CC(OC(C)=O)=C(Cl)C=C2C1C1=CC=CC=C1C(O)=O PXEZTIWVRVSYOK-UHFFFAOYSA-N 0.000 description 1
- ZBMRKNMTMPPMMK-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid;azane Chemical compound [NH4+].CP(O)(=O)CCC(N)C([O-])=O ZBMRKNMTMPPMMK-UHFFFAOYSA-N 0.000 description 1
- JNODDICFTDYODH-UHFFFAOYSA-N 2-hydroxytetrahydrofuran Chemical compound OC1CCCO1 JNODDICFTDYODH-UHFFFAOYSA-N 0.000 description 1
- OSJPPGNTCRNQQC-UWTATZPHSA-N 3-phospho-D-glyceric acid Chemical compound OC(=O)[C@H](O)COP(O)(O)=O OSJPPGNTCRNQQC-UWTATZPHSA-N 0.000 description 1
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 101710196690 Actin B Proteins 0.000 description 1
- 102100022900 Actin, cytoplasmic 1 Human genes 0.000 description 1
- 102220582491 Actin, cytoplasmic 1_D80A_mutation Human genes 0.000 description 1
- 102100034111 Activin receptor type-1 Human genes 0.000 description 1
- 102100034135 Activin receptor type-1C Human genes 0.000 description 1
- 101710173005 Activin receptor type-1C Proteins 0.000 description 1
- 206010000830 Acute leukaemia Diseases 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000269627 Amphiuma means Species 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000008873 Angiotensin II receptor Human genes 0.000 description 1
- 108050000824 Angiotensin II receptor Proteins 0.000 description 1
- 241000269350 Anura Species 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 244000186140 Asperula odorata Species 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 241000714235 Avian retrovirus Species 0.000 description 1
- 208000000412 Avitaminosis Diseases 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 108010007726 Bone Morphogenetic Proteins Proteins 0.000 description 1
- 102000007350 Bone Morphogenetic Proteins Human genes 0.000 description 1
- 206010006002 Bone pain Diseases 0.000 description 1
- 101000937267 Bos taurus Activin receptor type-2B Proteins 0.000 description 1
- 208000014644 Brain disease Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 208000020446 Cardiac disease Diseases 0.000 description 1
- 208000031404 Chromosome Aberrations Diseases 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 201000006306 Cor pulmonale Diseases 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 206010067477 Cytogenetic abnormality Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 241000388186 Deltapapillomavirus 4 Species 0.000 description 1
- 235000001602 Digitaria X umfolozi Nutrition 0.000 description 1
- 235000017898 Digitaria ciliaris Nutrition 0.000 description 1
- 235000005476 Digitaria cruciata Nutrition 0.000 description 1
- 235000006830 Digitaria didactyla Nutrition 0.000 description 1
- 235000005804 Digitaria eriantha ssp. eriantha Nutrition 0.000 description 1
- 235000010823 Digitaria sanguinalis Nutrition 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 244000025670 Eleusine indica Species 0.000 description 1
- 235000014716 Eleusine indica Nutrition 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 108010074604 Epoetin Alfa Proteins 0.000 description 1
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 1
- 108010075944 Erythropoietin Receptors Proteins 0.000 description 1
- 102100036509 Erythropoietin receptor Human genes 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 108010054218 Factor VIII Proteins 0.000 description 1
- 102000001690 Factor VIII Human genes 0.000 description 1
- 108010074864 Factor XI Proteins 0.000 description 1
- 108010074860 Factor Xa Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 101150050927 Fcgrt gene Proteins 0.000 description 1
- VTLYFUHAOXGGBS-UHFFFAOYSA-N Fe3+ Chemical compound [Fe+3] VTLYFUHAOXGGBS-UHFFFAOYSA-N 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 102100029379 Follistatin-related protein 3 Human genes 0.000 description 1
- 102000011852 GATA2 Transcription Factor Human genes 0.000 description 1
- 108010075641 GATA2 Transcription Factor Proteins 0.000 description 1
- 235000008526 Galium odoratum Nutrition 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- 208000033197 Genetic hemoglobinopathy Diseases 0.000 description 1
- KOSRFJWDECSPRO-WDSKDSINSA-N Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(O)=O KOSRFJWDECSPRO-WDSKDSINSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 102000005720 Glutathione transferase Human genes 0.000 description 1
- 108010070675 Glutathione transferase Proteins 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 102000028180 Glycophorins Human genes 0.000 description 1
- 108091005250 Glycophorins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 101150105462 HIS6 gene Proteins 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- 208000036581 Haemorrhagic anaemia Diseases 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 108010068323 Hemoglobin E Proteins 0.000 description 1
- 208000012925 Hemoglobin H disease Diseases 0.000 description 1
- 102100027685 Hemoglobin subunit alpha Human genes 0.000 description 1
- 206010019668 Hepatic fibrosis Diseases 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000756632 Homo sapiens Actin, cytoplasmic 1 Proteins 0.000 description 1
- 101000799140 Homo sapiens Activin receptor type-1 Proteins 0.000 description 1
- 101001062529 Homo sapiens Follistatin-related protein 3 Proteins 0.000 description 1
- 101000893545 Homo sapiens Growth/differentiation factor 11 Proteins 0.000 description 1
- 101001109620 Homo sapiens Nucleolar and coiled-body phosphoprotein 1 Proteins 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 208000004454 Hyperalgesia Diseases 0.000 description 1
- 208000035154 Hyperesthesia Diseases 0.000 description 1
- 206010062767 Hypophysitis Diseases 0.000 description 1
- 206010021135 Hypovitaminosis Diseases 0.000 description 1
- 206010021137 Hypovolaemia Diseases 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 206010022004 Influenza like illness Diseases 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000000704 Interleukin-7 Human genes 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 108010035210 Iron-Binding Proteins Proteins 0.000 description 1
- 102000008133 Iron-Binding Proteins Human genes 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 206010024305 Leukaemia monocytic Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 206010027439 Metal poisoning Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 102100025748 Mothers against decapentaplegic homolog 3 Human genes 0.000 description 1
- 101710143111 Mothers against decapentaplegic homolog 3 Proteins 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101000762244 Mus musculus Cadherin-17 Proteins 0.000 description 1
- 101100369076 Mus musculus Tdgf1 gene Proteins 0.000 description 1
- 206010028289 Muscle atrophy Diseases 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 description 1
- 101100395023 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) his-7 gene Proteins 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- RMIXHJPMNBXMBU-UHFFFAOYSA-N Nonactin Natural products CC1C(=O)OC(C)CC(O2)CCC2C(C)C(=O)OC(C)CC(O2)CCC2C(C)C(=O)OC(C)CC(O2)CCC2C(C)C(=O)OC(C)CC2CCC1O2 RMIXHJPMNBXMBU-UHFFFAOYSA-N 0.000 description 1
- 102100022726 Nucleolar and coiled-body phosphoprotein 1 Human genes 0.000 description 1
- 206010061876 Obstruction Diseases 0.000 description 1
- 241000207836 Olea <angiosperm> Species 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 239000004235 Orange GGN Substances 0.000 description 1
- 206010031252 Osteomyelitis Diseases 0.000 description 1
- 208000012868 Overgrowth Diseases 0.000 description 1
- 101150012394 PHO5 gene Proteins 0.000 description 1
- 101150079778 PREP gene Proteins 0.000 description 1
- 208000035467 Pancreatic insufficiency Diseases 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 108010022233 Plasminogen Activator Inhibitor 1 Proteins 0.000 description 1
- 102220509036 Platelet-activating factor acetylhydrolase IB subunit beta_L79A_mutation Human genes 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 102220577876 Poly(rC)-binding protein 1_W78A_mutation Human genes 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 208000008601 Polycythemia Diseases 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 208000018525 Postpartum Hemorrhage Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 208000012287 Prolapse Diseases 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102220469872 Protein argonaute-3_E37A_mutation Human genes 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 108700014121 Pyruvate Kinase Deficiency of Red Cells Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 208000000924 Right ventricular hypertrophy Diseases 0.000 description 1
- 108091006976 SLC40A1 Proteins 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 208000000223 Solitary Kidney Diseases 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- 206010041660 Splenomegaly Diseases 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 101710137500 T7 RNA polymerase Proteins 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- 108020005038 Terminator Codon Proteins 0.000 description 1
- 206010043390 Thalassaemia alpha Diseases 0.000 description 1
- 206010043391 Thalassaemia beta Diseases 0.000 description 1
- 102100036407 Thioredoxin Human genes 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 102000003978 Tissue Plasminogen Activator Human genes 0.000 description 1
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 1
- 238000008050 Total Bilirubin Reagent Methods 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 108700009124 Transcription Initiation Site Proteins 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 108700005077 Viral Genes Proteins 0.000 description 1
- 102220469729 Voltage-dependent L-type calcium channel subunit beta-2_D54K_mutation Human genes 0.000 description 1
- 102220479829 Voltage-dependent L-type calcium channel subunit beta-2_R56K_mutation Human genes 0.000 description 1
- 241000269370 Xenopus <genus> Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 108010023082 activin A Proteins 0.000 description 1
- 108010023079 activin B Proteins 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 230000001800 adrenalinergic effect Effects 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 238000004378 air conditioning Methods 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 230000002152 alkylating effect Effects 0.000 description 1
- 239000002160 alpha blocker Substances 0.000 description 1
- 201000006288 alpha thalassemia Diseases 0.000 description 1
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 1
- 229940124308 alpha-adrenoreceptor antagonist Drugs 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 150000004645 aluminates Chemical class 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 208000037868 anemia in chronic kidney disease Diseases 0.000 description 1
- 238000002399 angioplasty Methods 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003972 antineoplastic antibiotic Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 108010054176 apotransferrin Proteins 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 239000000823 artificial membrane Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 239000003462 bioceramic Substances 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 239000005312 bioglass Substances 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 210000001772 blood platelet Anatomy 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 210000000746 body region Anatomy 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 230000037182 bone density Effects 0.000 description 1
- 208000015322 bone marrow disease Diseases 0.000 description 1
- 230000005983 bone marrow dysfunction Effects 0.000 description 1
- 230000008993 bowel inflammation Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000000480 calcium channel blocker Substances 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical class [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 230000021523 carboxylation Effects 0.000 description 1
- 238000006473 carboxylation reaction Methods 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 238000005277 cation exchange chromatography Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000006369 cell cycle progression Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 210000004671 cell-free system Anatomy 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000009920 chelation Effects 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 210000000078 claw Anatomy 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000009643 clonogenic assay Methods 0.000 description 1
- 231100000096 clonogenic assay Toxicity 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 230000019771 cognition Effects 0.000 description 1
- 230000003920 cognitive function Effects 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000001447 compensatory effect Effects 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 108010084052 continuous erythropoietin receptor activator Proteins 0.000 description 1
- 230000002079 cooperative effect Effects 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000002498 deadly effect Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 229960003603 decitabine Drugs 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 238000005695 dehalogenation reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000003001 depressive effect Effects 0.000 description 1
- 230000000994 depressogenic effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 238000007877 drug screening Methods 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 210000002969 egg yolk Anatomy 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000003028 elevating effect Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 208000030172 endocrine system disease Diseases 0.000 description 1
- 210000001900 endoderm Anatomy 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000009786 epithelial differentiation Effects 0.000 description 1
- 229960003388 epoetin alfa Drugs 0.000 description 1
- 108010002601 epoetin beta Proteins 0.000 description 1
- 229960004579 epoetin beta Drugs 0.000 description 1
- 108010067416 epoetin delta Proteins 0.000 description 1
- 229950002109 epoetin delta Drugs 0.000 description 1
- 108010090921 epoetin omega Proteins 0.000 description 1
- 229950008767 epoetin omega Drugs 0.000 description 1
- 210000003013 erythroid precursor cell Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 229940093499 ethyl acetate Drugs 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 210000002436 femur neck Anatomy 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000002875 fluorescence polarization Methods 0.000 description 1
- 108091006047 fluorescent proteins Proteins 0.000 description 1
- 102000034287 fluorescent proteins Human genes 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 239000004052 folic acid antagonist Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 238000001476 gene delivery Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 102000018146 globin Human genes 0.000 description 1
- 108060003196 globin Proteins 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 150000004676 glycans Polymers 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 150000002333 glycines Chemical class 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 230000002414 glycolytic effect Effects 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 150000002338 glycosides Chemical class 0.000 description 1
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 1
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 1
- 230000002710 gonadal effect Effects 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 108010013846 hematide Proteins 0.000 description 1
- 210000000777 hematopoietic system Anatomy 0.000 description 1
- 208000034737 hemoglobinopathy Diseases 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- 238000012203 high throughput assay Methods 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 102000044890 human EPO Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 230000002390 hyperplastic effect Effects 0.000 description 1
- 230000001631 hypertensive effect Effects 0.000 description 1
- 230000001969 hypertrophic effect Effects 0.000 description 1
- 208000003532 hypothyroidism Diseases 0.000 description 1
- 230000002989 hypothyroidism Effects 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000010832 independent-sample T-test Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 208000018337 inherited hemoglobinopathy Diseases 0.000 description 1
- 239000000893 inhibin Substances 0.000 description 1
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 229940100994 interleukin-7 Drugs 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 230000010438 iron metabolism Effects 0.000 description 1
- DCYOBGZUOMKFPA-UHFFFAOYSA-N iron(2+);iron(3+);octadecacyanide Chemical compound [Fe+2].[Fe+2].[Fe+2].[Fe+3].[Fe+3].[Fe+3].[Fe+3].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-] DCYOBGZUOMKFPA-UHFFFAOYSA-N 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 210000004731 jugular vein Anatomy 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 210000001865 kupffer cell Anatomy 0.000 description 1
- 208000008127 lead poisoning Diseases 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 230000017687 left/right axis specification Effects 0.000 description 1
- 229960004942 lenalidomide Drugs 0.000 description 1
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 1
- 150000002614 leucines Chemical class 0.000 description 1
- 210000000982 limb bud Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000029226 lipidation Effects 0.000 description 1
- 230000004132 lipogenesis Effects 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 208000019420 lymphoid neoplasm Diseases 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 230000002934 lysing effect Effects 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 230000001071 malnutrition Effects 0.000 description 1
- 235000000824 malnutrition Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical class ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- 238000011177 media preparation Methods 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 210000004779 membrane envelope Anatomy 0.000 description 1
- 230000002175 menstrual effect Effects 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000037323 metabolic rate Effects 0.000 description 1
- 235000020938 metabolic status Nutrition 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 229960001046 methoxy polyethylene glycol-epoetin beta Drugs 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000029115 microtubule polymerization Effects 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 238000012900 molecular simulation Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 201000006894 monocytic leukemia Diseases 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 230000008383 multiple organ dysfunction Effects 0.000 description 1
- 238000002887 multiple sequence alignment Methods 0.000 description 1
- 201000000585 muscular atrophy Diseases 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 210000003643 myeloid progenitor cell Anatomy 0.000 description 1
- 210000003098 myoblast Anatomy 0.000 description 1
- 230000002107 myocardial effect Effects 0.000 description 1
- 229950006780 n-acetylglucosamine Drugs 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 238000013059 nephrectomy Methods 0.000 description 1
- 230000030363 nerve development Effects 0.000 description 1
- 230000004766 neurogenesis Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 208000015380 nutritional deficiency disease Diseases 0.000 description 1
- 210000001706 olfactory mucosa Anatomy 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000008816 organ damage Effects 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000002188 osteogenic effect Effects 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 125000001095 phosphatidyl group Chemical group 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000008288 physiological mechanism Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 210000003635 pituitary gland Anatomy 0.000 description 1
- 230000003169 placental effect Effects 0.000 description 1
- 238000013439 planning Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 150000004804 polysaccharides Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 208000037920 primary disease Diseases 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 235000021075 protein intake Nutrition 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 229960003351 prussian blue Drugs 0.000 description 1
- 239000013225 prussian blue Substances 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000010298 pulverizing process Methods 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000007261 regionalization Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 102220125997 rs141863326 Human genes 0.000 description 1
- 102220288227 rs141863326 Human genes 0.000 description 1
- 102220140258 rs146572379 Human genes 0.000 description 1
- 102200145357 rs1555341957 Human genes 0.000 description 1
- 102220036189 rs273585616 Human genes 0.000 description 1
- 102220004761 rs33990858 Human genes 0.000 description 1
- 102220005147 rs34173382 Human genes 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 230000007781 signaling event Effects 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- 210000003594 spinal ganglia Anatomy 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 238000009120 supportive therapy Methods 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229960003433 thalidomide Drugs 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 108060008226 thioredoxin Proteins 0.000 description 1
- 229940094937 thioredoxin Drugs 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 229960000187 tissue plasminogen activator Drugs 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-N triflic acid Chemical compound OS(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-N 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- 238000010396 two-hybrid screening Methods 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000005353 urine analysis Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229940124549 vasodilator Drugs 0.000 description 1
- 239000003071 vasodilator agent Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 238000011100 viral filtration Methods 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 230000007923 virulence factor Effects 0.000 description 1
- 239000000304 virulence factor Substances 0.000 description 1
- 208000002670 vitamin B12 deficiency Diseases 0.000 description 1
- 208000030401 vitamin deficiency disease Diseases 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/179—Receptors; Cell surface antigens; Cell surface determinants for growth factors; for growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/45—Transferases (2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/10—Transferases (2.)
- C12N9/12—Transferases (2.) transferring phosphorus containing groups, e.g. kinases (2.7)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y207/00—Transferases transferring phosphorus-containing groups (2.7)
- C12Y207/11—Protein-serine/threonine kinases (2.7.11)
- C12Y207/1103—Receptor protein serine/threonine kinase (2.7.11.30)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Endocrinology (AREA)
- Mycology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161547932P | 2011-10-17 | 2011-10-17 | |
| US61/547,932 | 2011-10-17 | ||
| PCT/US2012/060650 WO2013059347A1 (en) | 2011-10-17 | 2012-10-17 | Methods and compositions for treating ineffective erythropoiesis |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197025484A Division KR102079482B1 (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140084211A true KR20140084211A (ko) | 2014-07-04 |
Family
ID=48141315
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197025484A Active KR102079482B1 (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020227017193A Ceased KR20220075438A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020147013325A Ceased KR20140084211A (ko) | 2011-10-17 | 2012-10-17 | 효과가 없는 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020207004201A Ceased KR20200019261A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197025484A Active KR102079482B1 (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020227017193A Ceased KR20220075438A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207004201A Ceased KR20200019261A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Country Status (13)
| Country | Link |
|---|---|
| US (4) | US20130243743A1 (enExample) |
| EP (3) | EP2797620B1 (enExample) |
| JP (4) | JP6092883B2 (enExample) |
| KR (4) | KR102079482B1 (enExample) |
| CN (3) | CN107837390A (enExample) |
| AU (4) | AU2012326207B2 (enExample) |
| BR (3) | BR112014009528B1 (enExample) |
| CA (1) | CA2852683A1 (enExample) |
| DK (2) | DK3875104T3 (enExample) |
| EA (2) | EA034563B1 (enExample) |
| ES (3) | ES3010483T3 (enExample) |
| HK (2) | HK1250341A1 (enExample) |
| WO (1) | WO2013059347A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20180006437A (ko) * | 2015-05-13 | 2018-01-17 | 셀진 코포레이션 | Actrii 리간드 트랩을 사용한 베타-탈라세미아의 치료 |
Families Citing this family (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2551852T3 (es) | 2004-07-23 | 2015-11-24 | Acceleron Pharma Inc. | Polipéptidos del receptor ActRII |
| EA201692543A1 (ru) | 2005-11-23 | 2017-08-31 | Акселерон Фарма Инк. | Антагонисты активина-actriia и их применение для стимулирования роста кости |
| US8128933B2 (en) | 2005-11-23 | 2012-03-06 | Acceleron Pharma, Inc. | Method of promoting bone growth by an anti-activin B antibody |
| US8895016B2 (en) | 2006-12-18 | 2014-11-25 | Acceleron Pharma, Inc. | Antagonists of activin-actriia and uses for increasing red blood cell levels |
| CA2677007A1 (en) | 2007-02-01 | 2008-08-07 | Acceleron Pharma Inc. | Activin-actriia antagonists and uses for treating or preventing breast cancer |
| TWI782836B (zh) | 2007-02-02 | 2022-11-01 | 美商艾瑟勒朗法瑪公司 | 衍生自ActRIIB的變體與其用途 |
| CA3039330C (en) | 2007-02-09 | 2021-11-09 | Acceleron Pharma Inc. | Activin-actriia antagonists and uses for promoting bone growth in cancer patients |
| CN103877564A (zh) | 2007-09-18 | 2014-06-25 | 阿塞勒隆制药公司 | 活化素-actriia拮抗剂和减少或抑制fsh分泌的用途 |
| US8216997B2 (en) | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| JP5922928B2 (ja) | 2008-08-14 | 2016-05-24 | アクセルロン ファーマ, インコーポレイテッド | 赤血球レベルを高めるためのgdfトラップの使用 |
| CN102482339B (zh) | 2009-06-08 | 2015-06-17 | 阿塞勒隆制药公司 | 用于增加产热脂肪细胞的方法 |
| EP2440577A4 (en) | 2009-06-12 | 2013-01-23 | Acceleron Pharma Inc | SHORTEN ACTRIIB FC FUSION PROTEINS |
| CA2773494A1 (en) * | 2009-09-09 | 2011-03-17 | Acceleron Pharma Inc. | Actriib antagonists and dosing and uses thereof |
| ES2658292T3 (es) | 2009-11-17 | 2018-03-09 | Acceleron Pharma, Inc. | Proteínas ActRIIB y variantes y usos de las mismas con respecto a la inducción de la utrofina para el tratamiento de la distrofia muscular |
| CN103298832A (zh) | 2010-11-08 | 2013-09-11 | 阿塞勒隆制药公司 | Actriia结合剂及其用途 |
| WO2013055476A1 (en) | 2011-09-09 | 2013-04-18 | Anthrogenesis Corporation | Treatment of amyotrophic lateral sclerosis using placental stem cells |
| MX366336B (es) | 2012-11-02 | 2019-07-05 | Celgene Corp | Antagonistas de activina - actrii y usos para el tratar trastornos oseos y otros. |
| KR102520970B1 (ko) * | 2014-03-21 | 2023-04-12 | 악셀레론 파마 인코포레이티드 | 액티빈 b 및/또는 gdf11을 억제함으로써 적혈 혈액 세포 수준을 증가시키고 비효율적인 적혈구 생성을 치료하는 방법 |
| JP6649895B2 (ja) * | 2014-04-18 | 2020-02-19 | アクセルロン ファーマ, インコーポレイテッド | 赤血球レベルを増加させ、そして鎌状赤血球症を処置するための方法 |
| AU2015274277B2 (en) * | 2014-06-13 | 2021-03-18 | Acceleron Pharma, Inc. | Methods and compositions for treating ulcers |
| MA41052A (fr) | 2014-10-09 | 2017-08-15 | Celgene Corp | Traitement d'une maladie cardiovasculaire à l'aide de pièges de ligands d'actrii |
| SMT202300166T1 (it) * | 2014-12-03 | 2023-07-20 | Celgene Corp | Antagonisti di attivina- actrii e usi per il trattamento di sindrome mielodisplastica |
| MA41119A (fr) * | 2014-12-03 | 2017-10-10 | Acceleron Pharma Inc | Méthodes de traitement de syndromes myélodysplasiques et d'anémie sidéroblastique |
| RS61881B1 (sr) | 2015-04-22 | 2021-06-30 | Biogen Ma Inc | Novi hibridni actriib proteini zamke za ligand za lečenje bolesti gubljenja mišića |
| EP4190805A3 (en) | 2015-05-20 | 2023-08-16 | Celgene Corporation | In vitro cell culture methods for beta-thalassemia using activin type ii receptor ligand traps |
| US11123430B2 (en) | 2015-11-04 | 2021-09-21 | Acceleron Pharma Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis |
| CA3005975A1 (en) | 2015-11-23 | 2017-06-01 | Acceleron Pharma Inc. | Methods for treating eye disorders |
| MX2019001043A (es) | 2016-07-27 | 2019-09-26 | Acceleron Pharma Inc | Metodos y composiciones para el tratamiento de la mielofibrosis. |
| WO2018067874A1 (en) | 2016-10-05 | 2018-04-12 | Acceleron Pharma Inc. | Variant actriib proteins and uses thereof |
| JOP20190085A1 (ar) | 2016-10-20 | 2019-04-17 | Biogen Ma Inc | طرق علاج الضمور العضلي ومرض العظام باستخدام بروتينات احتجاز مركب ترابطي actriib هجين حديثة |
| EP3538123A4 (en) | 2016-11-10 | 2020-10-14 | Keros Therapeutics, Inc. | ACTIVIN RECEPTOR TYPE IIA VARIANTS AND THEIR METHODS OF USE |
| JP7496686B2 (ja) * | 2017-06-14 | 2024-06-07 | セルジーン コーポレイション | 骨髄増殖性腫瘍関連骨髄線維症及び貧血を治療するための方法 |
| CA3082146A1 (en) | 2017-11-09 | 2019-05-16 | Keros Therapeutics, Inc. | Activin receptor type iia variants and methods of use thereof |
| CN120399032A (zh) | 2018-01-12 | 2025-08-01 | 科乐斯疗法公司 | 激活素受体iib型变体及其使用方法 |
| EP3790572A4 (en) | 2018-05-09 | 2022-03-16 | Keros Therapeutics, Inc. | ACTIVIN RECEPTOR TYPE IIA VARIANTS AND METHODS OF USE THEREOF |
| KR20220088699A (ko) | 2019-09-27 | 2022-06-28 | 디스크 메디슨, 인크. | 골수섬유증 및 관련 상태의 치료 방법 |
| EP4121088A4 (en) | 2020-03-20 | 2024-07-03 | Keros Therapeutics, Inc. | Methods of using activin receptor type iib variants |
| KR20230012539A (ko) | 2020-05-13 | 2023-01-26 | 디스크 메디슨, 인크. | 골수섬유증을 치료하기 위한 항-헤모주벨린 (hjv) 항체 |
| US12186370B1 (en) | 2020-11-05 | 2025-01-07 | Celgene Corporation | ACTRIIB ligand trap compositions and uses thereof |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1265208A (en) | 1915-09-07 | 1918-05-07 | Edward C Kahn | Liquid-fuel burner. |
| EP0272253A4 (en) | 1986-03-07 | 1990-02-05 | Massachusetts Inst Technology | METHOD FOR IMPROVING GLYCOPROTE INSTABILITY. |
| US5080891A (en) | 1987-08-03 | 1992-01-14 | Ddi Pharmaceuticals, Inc. | Conjugates of superoxide dismutase coupled to high molecular weight polyalkylene glycols |
| WO1990008822A1 (en) | 1989-02-03 | 1990-08-09 | Genetics Institute, Inc. | Erythropoietin receptor |
| US5354934A (en) * | 1993-02-04 | 1994-10-11 | Amgen Inc. | Pulmonary administration of erythropoietin |
| US5677196A (en) | 1993-05-18 | 1997-10-14 | University Of Utah Research Foundation | Apparatus and methods for multi-analyte homogeneous fluoro-immunoassays |
| US5525490A (en) | 1994-03-29 | 1996-06-11 | Onyx Pharmaceuticals, Inc. | Reverse two-hybrid method |
| US5885574A (en) | 1994-07-26 | 1999-03-23 | Amgen Inc. | Antibodies which activate an erythropoietin receptor |
| US5814565A (en) | 1995-02-23 | 1998-09-29 | University Of Utah Research Foundation | Integrated optic waveguide immunosensor |
| CA2217545A1 (en) | 1995-04-11 | 1996-10-17 | Marc Vidal | Reverse two-hybrid systems |
| US5807839A (en) * | 1997-04-15 | 1998-09-15 | Advanced Viral Research Corp. | Method for stimulating red blood cell production |
| BR0008188A (pt) | 1999-01-21 | 2002-02-13 | Metamorphix Inc | Inibidores de fator de diferenciação de crescimento e usos para os mesmos |
| MXPA05003889A (es) * | 2002-10-15 | 2005-06-22 | Celgene Corp | Farmacos inhibidores de citosina selectiva para tratar sindrome mielodisplastico. |
| ES2551852T3 (es) | 2004-07-23 | 2015-11-24 | Acceleron Pharma Inc. | Polipéptidos del receptor ActRII |
| US8067562B2 (en) | 2005-11-01 | 2011-11-29 | Amgen Inc. | Isolated nucleic acid molecule comprising the amino acid sequence of SEQ ID NO:1 |
| US20100028332A1 (en) * | 2006-12-18 | 2010-02-04 | Acceleron Pharma Inc. | Antagonists of actriib and uses for increasing red blood cell levels |
| TWI782836B (zh) | 2007-02-02 | 2022-11-01 | 美商艾瑟勒朗法瑪公司 | 衍生自ActRIIB的變體與其用途 |
| JP5922928B2 (ja) * | 2008-08-14 | 2016-05-24 | アクセルロン ファーマ, インコーポレイテッド | 赤血球レベルを高めるためのgdfトラップの使用 |
| US8216997B2 (en) * | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| ES2796121T3 (es) * | 2009-08-13 | 2020-11-25 | Acceleron Pharma Inc | Uso combinado de trampas de GDF y activadores del receptor de la eritropoyetina para aumentar los niveles de glóbulos rojos |
-
2012
- 2012-10-17 CA CA2852683A patent/CA2852683A1/en active Pending
- 2012-10-17 CN CN201711069431.3A patent/CN107837390A/zh active Pending
- 2012-10-17 DK DK21153891.3T patent/DK3875104T3/da active
- 2012-10-17 ES ES21153891T patent/ES3010483T3/es active Active
- 2012-10-17 KR KR1020197025484A patent/KR102079482B1/ko active Active
- 2012-10-17 ES ES19161424T patent/ES2869168T3/es active Active
- 2012-10-17 KR KR1020227017193A patent/KR20220075438A/ko not_active Ceased
- 2012-10-17 KR KR1020147013325A patent/KR20140084211A/ko not_active Ceased
- 2012-10-17 DK DK19161424.7T patent/DK3520805T3/da active
- 2012-10-17 BR BR112014009528-0A patent/BR112014009528B1/pt active IP Right Grant
- 2012-10-17 WO PCT/US2012/060650 patent/WO2013059347A1/en not_active Ceased
- 2012-10-17 EA EA201490809A patent/EA034563B1/ru not_active IP Right Cessation
- 2012-10-17 EP EP12842601.2A patent/EP2797620B1/en active Active
- 2012-10-17 US US13/654,191 patent/US20130243743A1/en not_active Abandoned
- 2012-10-17 EA EA202090053A patent/EA202090053A3/ru unknown
- 2012-10-17 BR BR122019023166-4A patent/BR122019023166B1/pt active IP Right Grant
- 2012-10-17 CN CN201280061672.2A patent/CN103987403B/zh active Active
- 2012-10-17 BR BR122019023174-5A patent/BR122019023174B1/pt active IP Right Grant
- 2012-10-17 ES ES12842601T patent/ES2741477T3/es active Active
- 2012-10-17 EP EP19161424.7A patent/EP3520805B1/en active Active
- 2012-10-17 KR KR1020207004201A patent/KR20200019261A/ko not_active Ceased
- 2012-10-17 EP EP21153891.3A patent/EP3875104B1/en active Active
- 2012-10-17 CN CN201711069457.8A patent/CN107693776A/zh active Pending
- 2012-10-17 AU AU2012326207A patent/AU2012326207B2/en active Active
- 2012-10-17 JP JP2014537186A patent/JP6092883B2/ja active Active
-
2017
- 2017-01-23 US US15/413,184 patent/US20170137791A1/en not_active Abandoned
- 2017-02-09 JP JP2017022176A patent/JP6383821B2/ja active Active
- 2017-03-08 AU AU2017201580A patent/AU2017201580B2/en active Active
- 2017-07-28 US US15/663,361 patent/US20170327800A1/en not_active Abandoned
-
2018
- 2018-07-30 HK HK18109792.1A patent/HK1250341A1/zh unknown
- 2018-08-06 JP JP2018147507A patent/JP6796626B2/ja active Active
- 2018-09-26 HK HK18112295.7A patent/HK1252940A1/zh unknown
- 2018-10-11 AU AU2018247262A patent/AU2018247262B2/en active Active
-
2020
- 2020-08-07 US US16/987,756 patent/US20210207107A1/en active Pending
- 2020-08-24 JP JP2020140889A patent/JP2020186277A/ja active Pending
- 2020-12-09 AU AU2020286229A patent/AU2020286229A1/en not_active Abandoned
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20180006437A (ko) * | 2015-05-13 | 2018-01-17 | 셀진 코포레이션 | Actrii 리간드 트랩을 사용한 베타-탈라세미아의 치료 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7329573B2 (ja) | 赤血球レベルを高めるためのgdfトラップの使用 | |
| JP6796626B2 (ja) | 無効赤血球生成を処置するための方法および組成物 | |
| JP5909446B2 (ja) | 赤血球レベルを増大させるための、gdfトラップとエリスロポエチン受容体活性化因子とを組み合わせた使用 | |
| HK40011608A (en) | Compositions for treating myelofibrosis | |
| HK40011608B (en) | Compositions for treating myelofibrosis | |
| HK40041550A (en) | Gdf traps | |
| HK1203413B (en) | Compositions for treating iron overload in thalassemia | |
| HK1232466A1 (en) | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels | |
| HK40009493A (en) | Gdf traps | |
| HK40009493B (en) | Gdf traps |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20140519 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20171016 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20180917 Patent event code: PE09021S01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20190307 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20190731 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20190307 Comment text: Notification of reason for refusal Patent event code: PE06011S01I Patent event date: 20180917 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| J201 | Request for trial against refusal decision | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20190830 |
|
| PJ0201 | Trial against decision of rejection |
Patent event date: 20190830 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20190731 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20200922 Appeal identifier: 2019101002908 Request date: 20190830 |
|
| J301 | Trial decision |
Free format text: TRIAL NUMBER: 2019101002908; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20190830 Effective date: 20200922 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20200922 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20190830 Decision date: 20200922 Appeal identifier: 2019101002908 |