JP7689422B2 - 体外血液循環デバイス - Google Patents
体外血液循環デバイス Download PDFInfo
- Publication number
- JP7689422B2 JP7689422B2 JP2020204761A JP2020204761A JP7689422B2 JP 7689422 B2 JP7689422 B2 JP 7689422B2 JP 2020204761 A JP2020204761 A JP 2020204761A JP 2020204761 A JP2020204761 A JP 2020204761A JP 7689422 B2 JP7689422 B2 JP 7689422B2
- Authority
- JP
- Japan
- Prior art keywords
- blood
- circuit
- solution
- circulation circuit
- circulation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/36—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits
- A61M1/3672—Means preventing coagulation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L33/00—Antithrombogenic treatment of surgical articles, e.g. sutures, catheters, prostheses, or of articles for the manipulation or conditioning of blood; Materials for such treatment
- A61L33/04—Use of organic materials, e.g. acetylsalicylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L33/00—Antithrombogenic treatment of surgical articles, e.g. sutures, catheters, prostheses, or of articles for the manipulation or conditioning of blood; Materials for such treatment
- A61L33/06—Use of macromolecular materials
- A61L33/12—Polypeptides, proteins or derivatives thereof, e.g. degradation products thereof
- A61L33/128—Other specific proteins or polypeptides not covered by A61L33/122 - A61L33/126
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/04—Liquids
- A61M2202/0413—Blood
- A61M2202/0445—Proteins
- A61M2202/0447—Glycoproteins
- A61M2202/045—Fibrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/33—Controlling, regulating or measuring
- A61M2205/3331—Pressure; Flow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/33—Controlling, regulating or measuring
- A61M2205/3331—Pressure; Flow
- A61M2205/3334—Measuring or controlling the flow rate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/50—General characteristics of the apparatus with microprocessors or computers
- A61M2205/502—User interfaces, e.g. screens or keyboards
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/50—General characteristics of the apparatus with microprocessors or computers
- A61M2205/52—General characteristics of the apparatus with microprocessors or computers with memories providing a history of measured variating parameters of apparatus or patient
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Materials Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Surgery (AREA)
- Cardiology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- External Artificial Organs (AREA)
Description
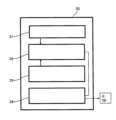
3 血液戻りライン
4’ 液状化溶液
5 血液修正手段
12 患者又はドナー
Claims (9)
- 体外血液循環デバイスであって、
少なくとも1つの血液抽出ライン(1)及び血液戻りライン(3)を含む血液循環回路と、
前記循環回路内で血液を循環させるように配置された手段(9)と、
前記循環回路内の血流に対する抵抗に影響されるパラメータのうちの少なくとも1つを測定するための手段(6、7、8)と、
を含み、
前記デバイスは、
血餅を液状化するための溶液(4’)のソースと、
前記液状化溶液(4’)を前記循環回路(1、3)の中に注入するための手段(4、4’’)と、 前記少なくとも1つのパラメータの少なくとも1つの閾値を決定し、それをメモリ(23)に記録し、かつ該少なくとも1つのパラメータの現在値を該記録された閾値と比較するように構成された計算手段(22)を含む制御ユニット(20)と、
を含み、
前記制御ユニット(20)は、前記液状化溶液(4’)を前記循環回路(1、3)の中に注入するために前記手段(4)を駆動するための駆動手段(24)を含み、
前記制御ユニット(20)は、前記液状化溶液(4’)が、前記回路内に形成された血餅を液状化するのに必要かつ十分であると予め決められた量でかつ時間にわたって該循環回路に存在するように、該血餅の存在を示す前記閾値が超えられた時に前記注入手段(4)を駆動するための前記駆動手段(24)を作動するように提供される、
ことを特徴とするデバイス。 - 前記制御ユニット(20)は、少なくとも1つの時間間隔を決定し又はそれをユーザインタフェース(21)から受け入れ、それをメモリ(23)に記録し、かつ前記時間の標準値を該間隔と比較して接続ライン(4’’)内で前記液状化溶液(4’)を循環させるように構成された前記注入手段(4)を駆動するための前記駆動手段(24)を作動するように構成されることを特徴とする請求項1に記載のデバイス。
- 前記計算手段(22)は、前記液状化溶液(4’)が前記循環回路内に十分と考えられる時間にわたって存在するように血液ポンプ(9)の流れを低減する又は停止することによって前記駆動手段(24)を制御することを特徴とする請求項1から請求項2のいずれか1項に記載のデバイス。
- 前記血液循環回路は、血液修正手段(5)及びライン(2)によって完了することを特徴とする請求項1から請求項3のいずれか1項に記載のデバイス。
- 前記循環回路内の血流に対する前記抵抗によって影響される前記パラメータのうちの少なくとも1つを測定するための前記手段(6、7、8)は、圧力を測定するように構成されることを特徴とする請求項1から請求項4のいずれか1項に記載のデバイス。
- 前記循環回路内の血流に対する前記抵抗によって影響される前記パラメータのうちの少なくとも1つを測定するための前記手段(6、7、8)は、流量を測定するように構成されることを特徴とする請求項1から請求項5のいずれか1項に記載のデバイス。
- 予防的抗凝固を行うための手段を更に含むことを特徴とする請求項1から請求項6のいずれか1項に記載のデバイス。
- 血餅を液状化するための溶液(4’)の前記ソース及び該液状化溶液(4’)を前記循環回路(1、3)の中に注入するための前記手段(4、4’’)は、予防的抗凝固を行うために使用されることを特徴とする請求項1から請求項7のいずれか1項に記載のデバイス。
- 前記液状化溶液(4’)は、クエン酸塩、ウロキナーゼ、ストレプトキナーゼ、及びその混合物を含む群から選択される成分を含むことを特徴とする請求項1から請求項8のいずれか1項に記載のデバイス。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP19215310.4 | 2019-12-11 | ||
| EP19215310.4A EP3834860B1 (fr) | 2019-12-11 | 2019-12-11 | Dispositif de circulation extracorporelle du sang |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2021090755A JP2021090755A (ja) | 2021-06-17 |
| JP7689422B2 true JP7689422B2 (ja) | 2025-06-06 |
Family
ID=68886879
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020204761A Active JP7689422B2 (ja) | 2019-12-11 | 2020-12-10 | 体外血液循環デバイス |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US11819599B2 (ja) |
| EP (1) | EP3834860B1 (ja) |
| JP (1) | JP7689422B2 (ja) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114404707B (zh) * | 2021-12-27 | 2023-08-18 | 健帆生物科技集团股份有限公司 | 血液氧合装置 |
| CN116400014B (zh) * | 2023-03-13 | 2025-06-27 | 宁波天益医疗器械股份有限公司 | 体外循环透析管路抗凝测试系统及测试方法 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994021124A1 (en) | 1993-03-16 | 1994-09-29 | Applied Immune Sciences, Inc. | Removal of selected factors from whole blood or its components |
| EP1095666A1 (fr) | 1999-10-29 | 2001-05-02 | Infomed S.A. | Dispositif d'épuration extracorporelle du sang |
| EP1430920A1 (fr) | 2002-12-20 | 2004-06-23 | Gambro Lundia AB | Dispositif et ligne disposable pour le traitement extracorporel du sang par anticoagulation au citrate |
| WO2007062197A1 (en) | 2005-11-22 | 2007-05-31 | Edwards Lifesciences Corporation | Citrate anticoagulation system for extracorporeal blood treatments |
| JP2011045450A (ja) | 2009-08-25 | 2011-03-10 | Hiroshima Univ | 血液粘度の推定方法、血液粘度比の推定方法、血液粘度モニタリング装置、及び、血液粘度比モニタリング装置 |
| US20120273415A1 (en) | 2011-04-29 | 2012-11-01 | Martin Gerber | Blood fluid removal system performance monitoring |
| JP2016007541A (ja) | 2014-06-26 | 2016-01-18 | アンフォムド ソシエテ アノニム | 体外循環による血液浄化のための装置 |
-
2019
- 2019-12-11 EP EP19215310.4A patent/EP3834860B1/fr active Active
-
2020
- 2020-12-10 JP JP2020204761A patent/JP7689422B2/ja active Active
- 2020-12-10 US US17/117,736 patent/US11819599B2/en active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994021124A1 (en) | 1993-03-16 | 1994-09-29 | Applied Immune Sciences, Inc. | Removal of selected factors from whole blood or its components |
| EP1095666A1 (fr) | 1999-10-29 | 2001-05-02 | Infomed S.A. | Dispositif d'épuration extracorporelle du sang |
| EP1430920A1 (fr) | 2002-12-20 | 2004-06-23 | Gambro Lundia AB | Dispositif et ligne disposable pour le traitement extracorporel du sang par anticoagulation au citrate |
| WO2007062197A1 (en) | 2005-11-22 | 2007-05-31 | Edwards Lifesciences Corporation | Citrate anticoagulation system for extracorporeal blood treatments |
| JP2011045450A (ja) | 2009-08-25 | 2011-03-10 | Hiroshima Univ | 血液粘度の推定方法、血液粘度比の推定方法、血液粘度モニタリング装置、及び、血液粘度比モニタリング装置 |
| US20120273415A1 (en) | 2011-04-29 | 2012-11-01 | Martin Gerber | Blood fluid removal system performance monitoring |
| JP2016007541A (ja) | 2014-06-26 | 2016-01-18 | アンフォムド ソシエテ アノニム | 体外循環による血液浄化のための装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| US11819599B2 (en) | 2023-11-21 |
| EP3834860C0 (fr) | 2024-08-14 |
| JP2021090755A (ja) | 2021-06-17 |
| US20210178049A1 (en) | 2021-06-17 |
| EP3834860A1 (fr) | 2021-06-16 |
| EP3834860B1 (fr) | 2024-08-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Jobes et al. | Increased accuracy and precision of heparin and protamine dosing reduces blood loss and transfusion in patients undergoing primary cardiac operations | |
| US8603021B2 (en) | Method and apparatus for ultrafiltration of blood | |
| US6533747B1 (en) | Extracorporeal circuit for peripheral vein fluid removal | |
| US20090192137A1 (en) | Dialysis method | |
| Hendrickx et al. | A comparative prospective study on the use of low concentrate citrate lock versus heparin lock in permanent dialysis catheters | |
| JP7689422B2 (ja) | 体外血液循環デバイス | |
| Romagnolib | Technical complications of continuous renal replacement therapy | |
| US20200188572A1 (en) | Device For An Extracorporeal Blood Treatment, And Method For Determining A Hemodynamic Parameter During An Extracorporeal Blood Treatment | |
| Stammers et al. | Hematological assessment of patients undergoing plasmapheresis during cardiac surgery | |
| Zaccaria et al. | Principles of extracorporeal circulation and transport phenomena | |
| Preuschof et al. | Heparin-free hemodialysis with prophylactic change of dialyser and blood lines | |
| Vanommeslaeghe et al. | Detection and scoring of extracorporeal circuit clotting during hemodialysis | |
| Liang et al. | Outcomes associated with a heparin-free hemodialysis protocol and review of the literature | |
| Hu et al. | Time course of activated coagulation time at various sites during continuous haemodiafiltration using nafamostat mesilate | |
| Clark et al. | Continuous Renal Replacement Therapy Machine Technology | |
| Kramer | Arteriovenous hemofiltration: A kidney replacement therapy for the intensive care unit | |
| Ashouri | Regional sodium citrate anticoagulation in patients with active bleeding undergoing hemodialysis | |
| De Vos | Anticoagulation therapy in acute renal failure extracorporeal treated patients | |
| CN112190779A (zh) | 一种crrt管路更换方法 | |
| Munshi et al. | Hemodialysis | |
| Majumdar et al. | Anticoagulation free hemodialysis | |
| Zope | Dialysis without anticoagulation (heparin free dialysis) | |
| Rees | 73 Hemodialysis | |
| JP2005046377A (ja) | 血液体外循環回路用抗凝固剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20231122 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20240823 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20240930 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20241216 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20250228 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20250428 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20250527 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7689422 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |