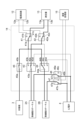
JP7263593B1 - 心腔内除細動システム - Google Patents
心腔内除細動システム Download PDFInfo
- Publication number
- JP7263593B1 JP7263593B1 JP2022076689A JP2022076689A JP7263593B1 JP 7263593 B1 JP7263593 B1 JP 7263593B1 JP 2022076689 A JP2022076689 A JP 2022076689A JP 2022076689 A JP2022076689 A JP 2022076689A JP 7263593 B1 JP7263593 B1 JP 7263593B1
- Authority
- JP
- Japan
- Prior art keywords
- defibrillation
- electrodes
- electrode group
- electrode
- catheter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000005242 cardiac chamber Anatomy 0.000 claims abstract description 13
- 230000002093 peripheral effect Effects 0.000 claims description 32
- 238000001356 surgical procedure Methods 0.000 claims description 2
- 238000013195 electrical cardioversion Methods 0.000 claims 1
- 238000012545 processing Methods 0.000 description 74
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical group [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 29
- 238000010586 diagram Methods 0.000 description 24
- 238000005259 measurement Methods 0.000 description 16
- 238000000034 method Methods 0.000 description 15
- 229920005989 resin Polymers 0.000 description 14
- 239000011347 resin Substances 0.000 description 14
- 239000000463 material Substances 0.000 description 12
- 239000003990 capacitor Substances 0.000 description 10
- 230000001976 improved effect Effects 0.000 description 10
- 238000003780 insertion Methods 0.000 description 10
- 230000037431 insertion Effects 0.000 description 10
- 230000008569 process Effects 0.000 description 10
- 210000004204 blood vessel Anatomy 0.000 description 9
- 239000012530 fluid Substances 0.000 description 8
- 230000004044 response Effects 0.000 description 8
- 210000001519 tissue Anatomy 0.000 description 8
- 230000001965 increasing effect Effects 0.000 description 7
- -1 polyethylene Polymers 0.000 description 7
- 239000004952 Polyamide Substances 0.000 description 6
- 208000005392 Spasm Diseases 0.000 description 6
- 230000001746 atrial effect Effects 0.000 description 6
- 239000011248 coating agent Substances 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- 235000019589 hardness Nutrition 0.000 description 6
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 6
- 229920002647 polyamide Polymers 0.000 description 6
- 230000002785 anti-thrombosis Effects 0.000 description 5
- 206010061592 cardiac fibrillation Diseases 0.000 description 5
- 229920001577 copolymer Polymers 0.000 description 5
- 229920001971 elastomer Polymers 0.000 description 5
- 239000000806 elastomer Substances 0.000 description 5
- 230000002600 fibrillogenic effect Effects 0.000 description 5
- 239000003292 glue Substances 0.000 description 5
- 210000005246 left atrium Anatomy 0.000 description 5
- 239000007769 metal material Substances 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 210000004165 myocardium Anatomy 0.000 description 5
- 208000003663 ventricular fibrillation Diseases 0.000 description 5
- 239000003146 anticoagulant agent Substances 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 230000006698 induction Effects 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 4
- 239000004810 polytetrafluoroethylene Substances 0.000 description 4
- 210000003492 pulmonary vein Anatomy 0.000 description 4
- 206010003658 Atrial Fibrillation Diseases 0.000 description 3
- 239000004677 Nylon Substances 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 230000001771 impaired effect Effects 0.000 description 3
- 229920001778 nylon Polymers 0.000 description 3
- 229910052697 platinum Inorganic materials 0.000 description 3
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 3
- 210000005245 right atrium Anatomy 0.000 description 3
- 230000000630 rising effect Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 229920001169 thermoplastic Polymers 0.000 description 3
- 239000004416 thermosoftening plastic Substances 0.000 description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 2
- 229910000566 Platinum-iridium alloy Inorganic materials 0.000 description 2
- 239000004962 Polyamide-imide Substances 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 102000003990 Urokinase-type plasminogen activator Human genes 0.000 description 2
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 2
- 150000001483 arginine derivatives Chemical class 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 229960002897 heparin Drugs 0.000 description 2
- 229920000669 heparin Polymers 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 239000011810 insulating material Substances 0.000 description 2
- 229920000126 latex Polymers 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 230000002107 myocardial effect Effects 0.000 description 2
- HWLDNSXPUQTBOD-UHFFFAOYSA-N platinum-iridium alloy Chemical class [Ir].[Pt] HWLDNSXPUQTBOD-UHFFFAOYSA-N 0.000 description 2
- 229920002492 poly(sulfone) Polymers 0.000 description 2
- 229920006122 polyamide resin Polymers 0.000 description 2
- 229920002312 polyamide-imide Polymers 0.000 description 2
- 229920001230 polyarylate Polymers 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 239000009719 polyimide resin Substances 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 239000004800 polyvinyl chloride Substances 0.000 description 2
- 229920000915 polyvinyl chloride Polymers 0.000 description 2
- 150000003180 prostaglandins Chemical class 0.000 description 2
- 229920002379 silicone rubber Polymers 0.000 description 2
- 229960005356 urokinase Drugs 0.000 description 2
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- 208000007101 Muscle Cramp Diseases 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229910001260 Pt alloy Inorganic materials 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229910001080 W alloy Inorganic materials 0.000 description 1
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000013153 catheter ablation Methods 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 210000003748 coronary sinus Anatomy 0.000 description 1
- 230000001862 defibrillatory effect Effects 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000004971 interatrial septum Anatomy 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229910001000 nickel titanium Inorganic materials 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000005476 soldering Methods 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 210000001631 vena cava inferior Anatomy 0.000 description 1
- 210000002620 vena cava superior Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/25—Bioelectric electrodes therefor
- A61B5/279—Bioelectric electrodes therefor specially adapted for particular uses
- A61B5/28—Bioelectric electrodes therefor specially adapted for particular uses for electrocardiography [ECG]
- A61B5/283—Invasive
- A61B5/287—Holders for multiple electrodes, e.g. electrode catheters for electrophysiological study [EPS]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/30—Input circuits therefor
- A61B5/304—Switching circuits
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/316—Modalities, i.e. specific diagnostic methods
- A61B5/318—Heart-related electrical modalities, e.g. electrocardiography [ECG]
- A61B5/33—Heart-related electrical modalities, e.g. electrocardiography [ECG] specially adapted for cooperation with other devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Public Health (AREA)
- Biophysics (AREA)
- Medical Informatics (AREA)
- Surgery (AREA)
- Cardiology (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- Pathology (AREA)
- Radiology & Medical Imaging (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Child & Adolescent Psychology (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Physiology (AREA)
- Electrotherapy Devices (AREA)
- Measurement And Recording Of Electrical Phenomena And Electrical Characteristics Of The Living Body (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022076689A JP7263593B1 (ja) | 2022-05-06 | 2022-05-06 | 心腔内除細動システム |
| JP2023061408A JP7482283B2 (ja) | 2022-05-06 | 2023-04-05 | 心腔内除細動システム |
| PCT/JP2023/016506 WO2023214529A1 (ja) | 2022-05-06 | 2023-04-26 | 心腔内除細動システム |
| JP2024018869A JP2024052698A (ja) | 2022-05-06 | 2024-02-09 | 心腔内除細動システム |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022076689A JP7263593B1 (ja) | 2022-05-06 | 2022-05-06 | 心腔内除細動システム |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2023061408A Division JP7482283B2 (ja) | 2022-05-06 | 2023-04-05 | 心腔内除細動システム |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP7263593B1 true JP7263593B1 (ja) | 2023-04-24 |
| JP2023165531A JP2023165531A (ja) | 2023-11-16 |
Family
ID=86054482
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2022076689A Active JP7263593B1 (ja) | 2022-05-06 | 2022-05-06 | 心腔内除細動システム |
| JP2023061408A Active JP7482283B2 (ja) | 2022-05-06 | 2023-04-05 | 心腔内除細動システム |
| JP2024018869A Pending JP2024052698A (ja) | 2022-05-06 | 2024-02-09 | 心腔内除細動システム |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2023061408A Active JP7482283B2 (ja) | 2022-05-06 | 2023-04-05 | 心腔内除細動システム |
| JP2024018869A Pending JP2024052698A (ja) | 2022-05-06 | 2024-02-09 | 心腔内除細動システム |
Country Status (2)
| Country | Link |
|---|---|
| JP (3) | JP7263593B1 (enExample) |
| WO (1) | WO2023214529A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023214529A1 (ja) * | 2022-05-06 | 2023-11-09 | 学 朝妻 | 心腔内除細動システム |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2025129734A (ja) * | 2024-02-26 | 2025-09-05 | 日本ライフライン株式会社 | 電源装置およびアブレーションシステム |
| WO2025205965A1 (ja) * | 2024-03-27 | 2025-10-02 | 株式会社カネカ | 心腔内除細動カテーテルシステムおよび心腔内除細動カテーテルシステムの制御方法 |
| WO2025205717A1 (ja) * | 2024-03-27 | 2025-10-02 | 株式会社カネカ | 心腔内除細動カテーテルシステムおよび心腔内除細動カテーテルシステムの制御方法 |
| WO2025205964A1 (ja) * | 2024-03-27 | 2025-10-02 | 株式会社カネカ | 心腔内除細動カテーテルシステムおよび心腔内除細動カテーテルシステムの制御方法 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001519216A (ja) | 1997-10-14 | 2001-10-23 | ユニヴァーシティ・オヴ・アラバマ・アト・バーミンガム・リサーチ・ファンデイション | 二重ショックによって心房細動を除去するシステム |
| US20070100382A1 (en) | 2005-10-31 | 2007-05-03 | Smits Karel F | Implantation of medical device with measurement of body surface potential |
| JP2010220778A (ja) | 2009-03-23 | 2010-10-07 | Japan Lifeline Co Ltd | 心腔内除細動カテーテルシステム |
| JP2017507699A (ja) | 2014-02-06 | 2017-03-23 | セント・ジュード・メディカル,カーディオロジー・ディヴィジョン,インコーポレイテッド | 面取りされたリング電極および可変シャフトを備えた細長医療機器 |
| WO2017149904A1 (ja) | 2016-02-29 | 2017-09-08 | 日本ライフライン株式会社 | 心腔内除細動カテーテル |
| WO2019156059A1 (ja) | 2018-02-06 | 2019-08-15 | 株式会社カネカ | カテーテルおよびその製造方法 |
| WO2020188642A1 (ja) | 2019-03-15 | 2020-09-24 | 日本ライフライン株式会社 | 心腔内除細動カテーテル |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6181967B1 (en) * | 1994-04-04 | 2001-01-30 | Eckhard Alt | Atrial defibrillator apparatus and method of use |
| JP7612195B2 (ja) * | 2021-01-29 | 2025-01-14 | 株式会社ニューロシューティカルズ | 心房細動除細動システム |
| JP7263593B1 (ja) * | 2022-05-06 | 2023-04-24 | 学 朝妻 | 心腔内除細動システム |
-
2022
- 2022-05-06 JP JP2022076689A patent/JP7263593B1/ja active Active
-
2023
- 2023-04-05 JP JP2023061408A patent/JP7482283B2/ja active Active
- 2023-04-26 WO PCT/JP2023/016506 patent/WO2023214529A1/ja not_active Ceased
-
2024
- 2024-02-09 JP JP2024018869A patent/JP2024052698A/ja active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001519216A (ja) | 1997-10-14 | 2001-10-23 | ユニヴァーシティ・オヴ・アラバマ・アト・バーミンガム・リサーチ・ファンデイション | 二重ショックによって心房細動を除去するシステム |
| US20070100382A1 (en) | 2005-10-31 | 2007-05-03 | Smits Karel F | Implantation of medical device with measurement of body surface potential |
| JP2010220778A (ja) | 2009-03-23 | 2010-10-07 | Japan Lifeline Co Ltd | 心腔内除細動カテーテルシステム |
| JP2017507699A (ja) | 2014-02-06 | 2017-03-23 | セント・ジュード・メディカル,カーディオロジー・ディヴィジョン,インコーポレイテッド | 面取りされたリング電極および可変シャフトを備えた細長医療機器 |
| WO2017149904A1 (ja) | 2016-02-29 | 2017-09-08 | 日本ライフライン株式会社 | 心腔内除細動カテーテル |
| WO2019156059A1 (ja) | 2018-02-06 | 2019-08-15 | 株式会社カネカ | カテーテルおよびその製造方法 |
| WO2020188642A1 (ja) | 2019-03-15 | 2020-09-24 | 日本ライフライン株式会社 | 心腔内除細動カテーテル |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023214529A1 (ja) * | 2022-05-06 | 2023-11-09 | 学 朝妻 | 心腔内除細動システム |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2023214529A1 (ja) | 2023-11-09 |
| JP2023165616A (ja) | 2023-11-16 |
| JP2023165531A (ja) | 2023-11-16 |
| JP7482283B2 (ja) | 2024-05-13 |
| JP2024052698A (ja) | 2024-04-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7263593B1 (ja) | 心腔内除細動システム | |
| JP7688153B2 (ja) | パルス場アブレーション装置および方法 | |
| JP4545216B1 (ja) | 心腔内除細動カテーテルシステム | |
| EP3139997A2 (en) | Methods and apparatus for selective tissue ablation | |
| JP4672802B1 (ja) | 心腔内除細動カテーテルシステム | |
| US11672973B2 (en) | Defibrillation catheter system, defibrillation power supply device and method for controlling defibrillation power supply device | |
| US11679269B2 (en) | Defibrillation catheter system, defibrillation power supply device and method for controlling defibrillation power supply device | |
| US20250269192A1 (en) | Control method for an intracardiac defibrillation catheter system | |
| US20230211154A1 (en) | Intracardiac defibrillation catheter system | |
| EP4212200A1 (en) | Medical device system and electrode contact detection method | |
| JP2012192124A (ja) | 心腔内除細動カテーテル | |
| JP2023175056A (ja) | 医療デバイスおよびシャント形成方法 | |
| US20220192742A1 (en) | Bipolar ablation graphical user interface | |
| WO2025014381A1 (en) | Spiral catheter for electrophysiological studies and irreversible cardiac electroporation | |
| CN120187369A (zh) | 消融导管导丝 | |
| HK40106124A (en) | Pulsed field ablation device | |
| JP2019122862A (ja) | 心腔内除細動カテーテル | |
| JP2019150526A (ja) | 心腔内除細動カテーテル |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20220606 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20220606 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20220906 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20221101 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20230124 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20230306 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20230314 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20230412 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7263593 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |