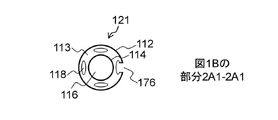
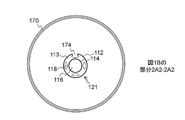
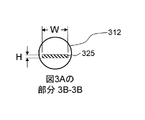
JP7061340B2 - 体管腔の偏向のためのシステムおよび方法 - Google Patents
体管腔の偏向のためのシステムおよび方法 Download PDFInfo
- Publication number
- JP7061340B2 JP7061340B2 JP2019526586A JP2019526586A JP7061340B2 JP 7061340 B2 JP7061340 B2 JP 7061340B2 JP 2019526586 A JP2019526586 A JP 2019526586A JP 2019526586 A JP2019526586 A JP 2019526586A JP 7061340 B2 JP7061340 B2 JP 7061340B2
- Authority
- JP
- Japan
- Prior art keywords
- catheter
- lumen
- deflection
- shaft
- balloon
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title description 17
- 230000007246 mechanism Effects 0.000 claims description 111
- 239000002184 metal Substances 0.000 claims description 32
- 229910052751 metal Inorganic materials 0.000 claims description 32
- 230000007935 neutral effect Effects 0.000 claims description 25
- 239000012530 fluid Substances 0.000 claims description 22
- 230000008859 change Effects 0.000 claims description 12
- 210000003238 esophagus Anatomy 0.000 description 41
- 239000000463 material Substances 0.000 description 28
- 230000010339 dilation Effects 0.000 description 15
- 238000006073 displacement reaction Methods 0.000 description 14
- 230000008901 benefit Effects 0.000 description 13
- 239000000853 adhesive Substances 0.000 description 11
- 230000001070 adhesive effect Effects 0.000 description 11
- 241001465754 Metazoa Species 0.000 description 10
- 230000006378 damage Effects 0.000 description 9
- 238000003780 insertion Methods 0.000 description 9
- 230000037431 insertion Effects 0.000 description 9
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 9
- 229910001000 nickel titanium Inorganic materials 0.000 description 9
- 229920003023 plastic Polymers 0.000 description 9
- 239000004033 plastic Substances 0.000 description 9
- 238000002679 ablation Methods 0.000 description 8
- 238000013461 design Methods 0.000 description 8
- 229910001220 stainless steel Inorganic materials 0.000 description 8
- 238000002594 fluoroscopy Methods 0.000 description 7
- 229920001778 nylon Polymers 0.000 description 7
- 239000010935 stainless steel Substances 0.000 description 7
- 238000001356 surgical procedure Methods 0.000 description 7
- 238000002627 tracheal intubation Methods 0.000 description 7
- 208000027418 Wounds and injury Diseases 0.000 description 6
- 210000005246 left atrium Anatomy 0.000 description 6
- 239000003550 marker Substances 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- 238000011282 treatment Methods 0.000 description 6
- 239000004677 Nylon Substances 0.000 description 5
- 210000004556 brain Anatomy 0.000 description 5
- -1 but not limited to Substances 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 206010033675 panniculitis Diseases 0.000 description 5
- 229920002635 polyurethane Polymers 0.000 description 5
- 239000004814 polyurethane Substances 0.000 description 5
- 210000004304 subcutaneous tissue Anatomy 0.000 description 5
- 206010003658 Atrial Fibrillation Diseases 0.000 description 4
- 208000006687 Esophageal Fistula Diseases 0.000 description 4
- 229910000760 Hardened steel Inorganic materials 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 206010065835 Oesophageal fistula Diseases 0.000 description 4
- 239000004696 Poly ether ether ketone Substances 0.000 description 4
- 238000005452 bending Methods 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 229920000728 polyester Polymers 0.000 description 4
- 229920002530 polyetherether ketone Polymers 0.000 description 4
- 229920000139 polyethylene terephthalate Polymers 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 229920000106 Liquid crystal polymer Polymers 0.000 description 3
- 239000004977 Liquid-crystal polymers (LCPs) Substances 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 230000000916 dilatatory effect Effects 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 210000000214 mouth Anatomy 0.000 description 3
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 238000007789 sealing Methods 0.000 description 3
- 230000007704 transition Effects 0.000 description 3
- 238000012800 visualization Methods 0.000 description 3
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 229920002292 Nylon 6 Polymers 0.000 description 2
- 239000004813 Perfluoroalkoxy alkane Substances 0.000 description 2
- 229920002614 Polyether block amide Polymers 0.000 description 2
- 239000004642 Polyimide Substances 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000000712 assembly Effects 0.000 description 2
- 238000000429 assembly Methods 0.000 description 2
- 230000001746 atrial effect Effects 0.000 description 2
- 238000013153 catheter ablation Methods 0.000 description 2
- 238000004891 communication Methods 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 229920000840 ethylene tetrafluoroethylene copolymer Polymers 0.000 description 2
- 229920002457 flexible plastic Polymers 0.000 description 2
- 229920001903 high density polyethylene Polymers 0.000 description 2
- 239000004700 high-density polyethylene Substances 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 229920001684 low density polyethylene Polymers 0.000 description 2
- 239000004702 low-density polyethylene Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 229920011301 perfluoro alkoxyl alkane Polymers 0.000 description 2
- 239000005020 polyethylene terephthalate Substances 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 210000001187 pylorus Anatomy 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 229920001651 Cyanoacrylate Polymers 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 108091084831 Teflon family Proteins 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- NLCKLZIHJQEMCU-UHFFFAOYSA-N cyano prop-2-enoate Chemical class C=CC(=O)OC#N NLCKLZIHJQEMCU-UHFFFAOYSA-N 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 238000011549 displacement method Methods 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 229940124645 emergency medicine Drugs 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- QHSJIZLJUFMIFP-UHFFFAOYSA-N ethene;1,1,2,2-tetrafluoroethene Chemical group C=C.FC(F)=C(F)F QHSJIZLJUFMIFP-UHFFFAOYSA-N 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 210000002837 heart atrium Anatomy 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000010297 mechanical methods and process Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 210000003105 phrenic nerve Anatomy 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920013745 polyesteretherketone Polymers 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000007674 radiofrequency ablation Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 229920002725 thermoplastic elastomer Polymers 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 210000003708 urethra Anatomy 0.000 description 1
- 230000001515 vagal effect Effects 0.000 description 1
- 210000001186 vagus nerve Anatomy 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/04—Protection of tissue around surgical sites against effects of non-mechanical surgery, e.g. laser surgery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M25/0147—Tip steering devices with movable mechanical means, e.g. pull wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0108—Steering means as part of the catheter or advancing means; Markers for positioning using radio-opaque or ultrasound markers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M25/0155—Tip steering devices with hydraulic or pneumatic means, e.g. balloons or inflatable compartments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1002—Balloon catheters characterised by balloon shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1011—Multiple balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B18/1492—Probes or electrodes therefor having a flexible, catheter-like structure, e.g. for heart ablation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00273—Anchoring means for temporary attachment of a device to tissue
- A61B2018/00279—Anchoring means for temporary attachment of a device to tissue deployable
- A61B2018/00285—Balloons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00345—Vascular system
- A61B2018/00351—Heart
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00571—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for achieving a particular surgical effect
- A61B2018/00577—Ablation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/04—Protection of tissue around surgical sites against effects of non-mechanical surgery, e.g. laser surgery
- A61B2090/0409—Specification of type of protection measures
- A61B2090/0427—Prevention of contact
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1084—Balloon catheters with special features or adapted for special applications having features for increasing the shape stability, the reproducibility or for limiting expansion, e.g. containments, wrapped around fibres, yarns or strands
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/02—Holding devices, e.g. on the body
- A61M25/04—Holding devices, e.g. on the body in the body, e.g. expansible
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/09—Guide wires
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Surgery (AREA)
- Child & Adolescent Psychology (AREA)
- Medical Informatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Molecular Biology (AREA)
- Otolaryngology (AREA)
- Pathology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Mechanical Engineering (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Surgical Instruments (AREA)
Description
本発明は「System and method for deflection of a body lumen」という表題で2016年11月23日にGregory G.Bruckerらによって出願された米国仮特許出願第62/426,223号の35U.S.C.§119(e)下での優先権の利益を主張するものであり、当該文献は全体として参照により本明細書に組み込まれるものとする。
(1)伸張ワイヤの動きに応じて偏向する、多数の長手方向の要素を収容する可撓性を有する部分(305);
(2)カラム(340)の内に長手方向に移動する伸張ワイヤ(322);
(3)伸張ワイヤによって生成された力に反応するカラム(340);および
(4)カラム(340)と接触するノブを収容するハンドル(350)。
この機器は、シャフト;機器の長さの少なくとも一部にわたって伸びるシャフト内の第1の管腔;シャフトに取り付けられ、体管腔内で拡張するように構成される、拡張可能な部材;および、第1の体管腔内に位置付けられ、拡張可能な部材に取り付けたシャフトの少なくとも一部に沿ったシャフトの対応する側方の偏向を引き起こすために、側方の偏向によって形状を変化するように構成される、偏向機構。
Claims (8)
- 体管腔の一部分を変位するための機器であって、該機器は、
シャフト、
第1の管腔であって、機器の長さの少なくとも一部にわたって伸びるシャフト内の第1の管腔、
シャフトに取り付けられ、体管腔内で拡張するように構成される、拡張可能な部材、及び
第1の管腔内の所望の深さまで第1の管腔内に挿入され、体管腔の対応する側方の偏向を引き起こすために、側方の偏向によって形状を変えるように構成された偏向機構であって、ここで、偏向機構がその偏向されていない中立の構成である場合、偏向カテーテルの遠位部は、偏向カテーテルの近位部と遠位部との間に定義される第1のラインに沿っており、および、偏向カテーテルの形状が偏向された構成に変えられる場合、前記遠位部と前記近位部との間の偏向カテーテルの偏向された部分は、前記遠位部および前記近位部が第1のラインに沿っている間、第1のラインから離れて湾曲し、第1のラインへと戻るように湾曲し、前記拡張可能な部材が前記シャフトを囲んでなり、前記拡張可能な部材の少なくとも一部が当該偏向機構の偏向された部分の少なくとも一部を囲む、偏向機構、
を含む、機器。 - 拡張可能な部材を体管腔内で拡張させるべく、拡張可能な部材に流体を注入するために、拡張可能な部材に動作可能に連結されたチューブをさらに含む、請求項1に記載の機器。
- 拡張可能な部材を体管腔内で拡張させるべく、拡張可能な部材に流体を注入するために、シャフト内にあり、および拡張可能な部材に動作可能に連結した第2の管腔をさらに含む、請求項1に記載の機器。
- シャフト内の第1の管腔は、拡張可能な部材を体管腔内で拡張させるべく、拡張可能な部材に流体を注入するために、拡張可能な部材に動作可能に連結される、請求項1に記載の機器。
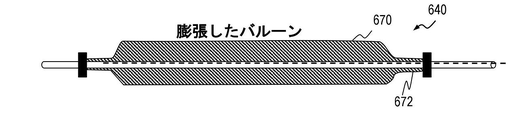
- 拡張可能な部材は、シャフトに沿って連続的に位置する複数の拡張可能なセグメントを含み、ここで、拡張可能な部材の複数の拡張可能なセグメントの各々が体管腔内で拡張する際に、シャフトが複数の拡張可能なセグメントの各々1つ内で実質的に中心になるように、複数の拡張可能なセグメントの各々はシャフトを囲む、請求項1に記載の機器。
- 偏向機構は、異なる長さの複数の平らな隣り合わせの金属セグメント、および張った状態で位置付けられる際に、複数の平らな隣り合わせの金属セグメントで弯曲を引き起こすように構成された短縮ケーブル、を含む、請求項1に記載の機器。
- 前記偏向機構は、シャフト内で円周方向に回転するようにさらに構成される、請求項1に記載の機器。
- 前記偏向機構は、向かい合って配置された、異なる長さの複数の平らな金属セグメント、および
張った状態で位置付けられる際に、当該複数の平らな金属セグメントで弯曲を引き起こすように構成された短縮ケーブルを含み、
ここで、前記複数の平らな金属セグメントの第1の金属セグメントは前記複数の平らな金属セグメントの最大の長さを有し、前記短縮ケーブルの遠位端は前記第1の金属セグメントに取り付けられ、
前記複数の平らな金属セグメントの第2の金属セグメントは、前記第1の金属セグメントと短縮ケーブルとの間に位置する、請求項1に記載の機器。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662426223P | 2016-11-23 | 2016-11-23 | |
| US62/426,223 | 2016-11-23 | ||
| PCT/US2017/063171 WO2018098388A1 (en) | 2016-11-23 | 2017-11-23 | System and method for deflection of a body lumen |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2020500065A JP2020500065A (ja) | 2020-01-09 |
| JP2020500065A5 JP2020500065A5 (ja) | 2021-01-07 |
| JP7061340B2 true JP7061340B2 (ja) | 2022-04-28 |
Family
ID=62195653
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019526586A Active JP7061340B2 (ja) | 2016-11-23 | 2017-11-23 | 体管腔の偏向のためのシステムおよび方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US11298203B2 (ja) |
| EP (1) | EP3544666A4 (ja) |
| JP (1) | JP7061340B2 (ja) |
| CA (1) | CA3044635A1 (ja) |
| WO (1) | WO2018098388A1 (ja) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020512169A (ja) * | 2017-03-24 | 2020-04-23 | ロバート ジェイ.コットーネRobert J. COTTONE | 組織変位のためのシステム及び方法 |
| US11090101B2 (en) * | 2018-05-02 | 2021-08-17 | Medtronic Cryocath Lp | Soft balloon device and system |
| WO2020068601A1 (en) * | 2018-09-24 | 2020-04-02 | Cottone Robert J | Systems and methods for tissue displacement |
| US20220126070A1 (en) * | 2019-02-20 | 2022-04-28 | Mohamed Abdalla Mahmoud Eltahlawi | Arterial balloon with variable pressures |
| CN110974323B (zh) * | 2020-01-04 | 2022-11-01 | 周龙安 | 一种普外科手术用肌肉牵开装置 |
| US12005216B2 (en) | 2020-03-03 | 2024-06-11 | St. Jude Medical, Cardiology Division, Inc. | Esophageal deviator |
| CN111419304A (zh) * | 2020-04-16 | 2020-07-17 | 上海科赐医疗技术有限公司 | 弯曲球囊导管牵开器 |
| WO2022024348A1 (ja) * | 2020-07-31 | 2022-02-03 | 朝日インテック株式会社 | バルーンカテーテル |
| US20240090901A1 (en) * | 2020-12-16 | 2024-03-21 | Sundaram Ravikumar | Catheter device and method for selective occlusion of arteries of the descending aorta or iliac vasculature |
| US11744993B2 (en) | 2021-07-19 | 2023-09-05 | Innovations In Medicine, Llc | System and method for deflection mechanism with expandable constraint |
| WO2024115418A1 (en) * | 2022-11-29 | 2024-06-06 | Fresenius Kabi Deutschland Gmbh | Port catheter system |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080033415A1 (en) | 2006-03-17 | 2008-02-07 | Rieker Gregory B | Method and apparatus to prevent esophageal damage |
| US20110034936A1 (en) | 2007-10-31 | 2011-02-10 | Maloney James D | Nasogastric tube for use during an ablation procedure |
| US20110082488A1 (en) | 2009-10-06 | 2011-04-07 | Niazi Imran K | Intra-esophageal balloon system |
| US20150245829A1 (en) | 2014-02-28 | 2015-09-03 | Manual Surgical Sciences, LLC | Expandable devices for positioning organs |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH667207A5 (fr) | 1985-11-21 | 1988-09-30 | Sarcem Sa | Catheter a commande a distance a micro-ballonet. |
| US4983167A (en) | 1988-11-23 | 1991-01-08 | Harvinder Sahota | Balloon catheters |
| WO1991011213A1 (en) | 1990-02-02 | 1991-08-08 | Ep Technologies, Inc. | Catheter steering mechanism |
| US5820591A (en) | 1990-02-02 | 1998-10-13 | E. P. Technologies, Inc. | Assemblies for creating compound curves in distal catheter regions |
| US5045061A (en) | 1990-02-02 | 1991-09-03 | C. R. Bard, Inc. | Balloon catheter and locking guidewire system |
| US5195968A (en) * | 1990-02-02 | 1993-03-23 | Ingemar Lundquist | Catheter steering mechanism |
| US5626618A (en) | 1993-09-24 | 1997-05-06 | The Ohio State University | Mechanical adjunct to cardiopulmonary resuscitation (CPR), and an electrical adjunct to defibrillation countershock, cardiac pacing, and cardiac monitoring |
| US5558665A (en) | 1994-06-24 | 1996-09-24 | Archimedes Surgical, Inc. | Surgical instrument and method for intraluminal retraction of an anatomic structure |
| US20030036070A1 (en) * | 1999-10-21 | 2003-02-20 | Shukti Chakravarti | Gene expression profiling of inflammatory bowel disease |
| US6733500B2 (en) * | 2000-03-31 | 2004-05-11 | Medtronic, Inc. | Method and system for delivering a medical electrical lead within a venous system |
| US7621908B2 (en) | 2005-11-18 | 2009-11-24 | Miller Steven W | Catheter for manipulation of the esophagus |
| US9931108B2 (en) * | 2005-11-18 | 2018-04-03 | Steven Miller | System and method for influencing an anatomical structure |
| US8273016B2 (en) | 2006-03-10 | 2012-09-25 | Biosense Webster, Inc. | Esophagus isolation device |
| US9119927B1 (en) | 2009-05-19 | 2015-09-01 | Jerry Blaine Ratterree | Apparatus and method for intubating humans and non-human animals |
| WO2014052368A1 (en) * | 2012-09-28 | 2014-04-03 | Ninepoint Medical, Inc. | Mechanical tensioning device |
| WO2015153595A1 (en) * | 2014-04-02 | 2015-10-08 | Gc Medtech Llc | Internal body cavity therapeutic applicators and methods for using them |
| DE102015103213A1 (de) * | 2015-03-05 | 2016-09-08 | Medfact Engineering Gmbh | Vorrichtung zur Verlagerung eines Hohlorgans eines Patienten |
| US9668720B2 (en) * | 2015-10-19 | 2017-06-06 | DNP Biomed, LLC | Systems, devices, components and methods for displacing and repositioning the esophagus away from the heart during atrial ablation surgical procedures |
| WO2017218836A2 (en) * | 2016-06-15 | 2017-12-21 | Miller Steven W | Gastric tube for ablation procedures |
| IL268174B2 (en) | 2017-01-19 | 2023-04-01 | Ohio State Innovation Foundation | Systems and methods for changing the mechanical position of an esophagus |
-
2017
- 2017-11-23 US US16/462,377 patent/US11298203B2/en active Active
- 2017-11-23 EP EP17873942.1A patent/EP3544666A4/en not_active Withdrawn
- 2017-11-23 JP JP2019526586A patent/JP7061340B2/ja active Active
- 2017-11-23 CA CA3044635A patent/CA3044635A1/en not_active Abandoned
- 2017-11-23 WO PCT/US2017/063171 patent/WO2018098388A1/en active Search and Examination
-
2022
- 2022-03-31 US US17/710,472 patent/US11504205B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080033415A1 (en) | 2006-03-17 | 2008-02-07 | Rieker Gregory B | Method and apparatus to prevent esophageal damage |
| US20110034936A1 (en) | 2007-10-31 | 2011-02-10 | Maloney James D | Nasogastric tube for use during an ablation procedure |
| US20110082488A1 (en) | 2009-10-06 | 2011-04-07 | Niazi Imran K | Intra-esophageal balloon system |
| US20150245829A1 (en) | 2014-02-28 | 2015-09-03 | Manual Surgical Sciences, LLC | Expandable devices for positioning organs |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3544666A1 (en) | 2019-10-02 |
| US20220218433A1 (en) | 2022-07-14 |
| JP2020500065A (ja) | 2020-01-09 |
| US11504205B2 (en) | 2022-11-22 |
| WO2018098388A1 (en) | 2018-05-31 |
| EP3544666A4 (en) | 2020-09-02 |
| US11298203B2 (en) | 2022-04-12 |
| US20190314109A1 (en) | 2019-10-17 |
| CA3044635A1 (en) | 2018-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7061340B2 (ja) | 体管腔の偏向のためのシステムおよび方法 | |
| US4822345A (en) | Controllable flexibility catheter | |
| US10272231B2 (en) | Expandable trans-septal sheath | |
| US8348892B2 (en) | Expandable transluminal sheath | |
| EP1942975B1 (en) | Steerable catheter devices and methods of articulating catheter devices | |
| US7993350B2 (en) | Shapeable or steerable guide sheaths and methods for making and using them | |
| EP3503956B1 (en) | Multilumen catheter | |
| US20060135963A1 (en) | Expandable gastrointestinal sheath | |
| EP1417984B1 (en) | Balloon catheter | |
| US20060259061A1 (en) | Expandable sheath for percutaneous upper gastrointestinal tract access | |
| JP6389267B2 (ja) | 臓器を位置調整するための拡張可能デバイス | |
| EP0256478B1 (en) | Controllable flexibility catheter | |
| US10994105B2 (en) | Apparatus and method for advancing catheters or other medical devices through a lumen | |
| US12048820B2 (en) | Apparatus and method for advancing catheters or other medical devices through a lumen | |
| US5984946A (en) | Diagnostic and guiding catheter | |
| WO2011127012A1 (en) | Endovascular catheter and method with hydraulic bladder system | |
| US11744993B2 (en) | System and method for deflection mechanism with expandable constraint | |
| US20220249804A1 (en) | Deflectable Sheath With Inflatable Balloon | |
| JP2022531345A (ja) | バルーン・カテーテル支持スリーブのためのシステム及び方法 | |
| JP2512790Y2 (ja) | 医療用バル―ンカテ―テル |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201118 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20201119 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20211125 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20211202 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220222 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220316 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220408 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7061340 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |