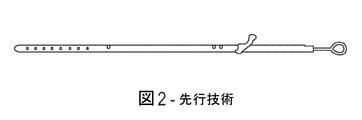
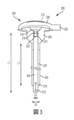
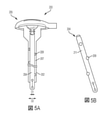
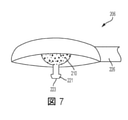
JP7002120B2 - カテーテル組立体 - Google Patents
カテーテル組立体 Download PDFInfo
- Publication number
- JP7002120B2 JP7002120B2 JP2017562264A JP2017562264A JP7002120B2 JP 7002120 B2 JP7002120 B2 JP 7002120B2 JP 2017562264 A JP2017562264 A JP 2017562264A JP 2017562264 A JP2017562264 A JP 2017562264A JP 7002120 B2 JP7002120 B2 JP 7002120B2
- Authority
- JP
- Japan
- Prior art keywords
- catheter
- sheath
- catheter assembly
- distal
- shunt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000004556 brain Anatomy 0.000 claims description 46
- 239000012530 fluid Substances 0.000 claims description 17
- 210000003625 skull Anatomy 0.000 claims description 6
- 239000003889 eye drop Substances 0.000 claims description 2
- 230000002861 ventricular Effects 0.000 description 35
- 238000000034 method Methods 0.000 description 27
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 21
- 238000001356 surgical procedure Methods 0.000 description 19
- 210000003128 head Anatomy 0.000 description 11
- 210000001015 abdomen Anatomy 0.000 description 9
- 208000003906 hydrocephalus Diseases 0.000 description 9
- 230000003187 abdominal effect Effects 0.000 description 7
- 210000000683 abdominal cavity Anatomy 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 230000000712 assembly Effects 0.000 description 5
- 238000000429 assembly Methods 0.000 description 5
- 210000000038 chest Anatomy 0.000 description 5
- 210000002987 choroid plexus Anatomy 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 230000001746 atrial effect Effects 0.000 description 4
- 230000006399 behavior Effects 0.000 description 4
- 230000006931 brain damage Effects 0.000 description 4
- 231100000874 brain damage Toxicity 0.000 description 4
- 208000029028 brain injury Diseases 0.000 description 4
- 210000005013 brain tissue Anatomy 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 238000009434 installation Methods 0.000 description 4
- 241001269524 Dura Species 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 210000003739 neck Anatomy 0.000 description 3
- 210000004761 scalp Anatomy 0.000 description 3
- 229920002379 silicone rubber Polymers 0.000 description 3
- 208000032843 Hemorrhage Diseases 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 239000003146 anticoagulant agent Substances 0.000 description 2
- 229940127219 anticoagulant drug Drugs 0.000 description 2
- 208000034158 bleeding Diseases 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 210000002837 heart atrium Anatomy 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 210000003281 pleural cavity Anatomy 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 208000028389 Nerve injury Diseases 0.000 description 1
- 208000031074 Reinjury Diseases 0.000 description 1
- 206010058028 Shunt infection Diseases 0.000 description 1
- 210000003815 abdominal wall Anatomy 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 210000003710 cerebral cortex Anatomy 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 210000004289 cerebral ventricle Anatomy 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 238000001839 endoscopy Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 210000004055 fourth ventricle Anatomy 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 210000003140 lateral ventricle Anatomy 0.000 description 1
- 210000005240 left ventricle Anatomy 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 206010033675 panniculitis Diseases 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 210000005241 right ventricle Anatomy 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 210000002330 subarachnoid space Anatomy 0.000 description 1
- 210000004304 subcutaneous tissue Anatomy 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 210000000779 thoracic wall Anatomy 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M27/00—Drainage appliance for wounds or the like, i.e. wound drains, implanted drains
- A61M27/002—Implant devices for drainage of body fluids from one part of the body to another
- A61M27/006—Cerebrospinal drainage; Accessories therefor, e.g. valves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0067—Catheters; Hollow probes characterised by the distal end, e.g. tips
- A61M25/0068—Static characteristics of the catheter tip, e.g. shape, atraumatic tip, curved tip or tip structure
- A61M25/007—Side holes, e.g. their profiles or arrangements; Provisions to keep side holes unblocked
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0067—Catheters; Hollow probes characterised by the distal end, e.g. tips
- A61M25/0068—Static characteristics of the catheter tip, e.g. shape, atraumatic tip, curved tip or tip structure
- A61M25/0071—Multiple separate lumens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0097—Catheters; Hollow probes characterised by the hub
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M27/00—Drainage appliance for wounds or the like, i.e. wound drains, implanted drains
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/02—Access sites
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/10—Tube connectors; Tube couplings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/02—Holding devices, e.g. on the body
- A61M2025/0293—Catheter, guide wire or the like with means for holding, centering, anchoring or frictionally engaging the device within an artificial lumen, e.g. tube
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/06—Body-piercing guide needles or the like
- A61M25/0662—Guide tubes
- A61M2025/0681—Systems with catheter and outer tubing, e.g. sheath, sleeve or guide tube
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/06—Head
- A61M2210/0687—Skull, cranium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/06—Head
- A61M2210/0693—Brain, cerebrum
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Public Health (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Pulmonology (AREA)
- Biophysics (AREA)
- Otolaryngology (AREA)
- Ophthalmology & Optometry (AREA)
- Neurology (AREA)
- External Artificial Organs (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562169186P | 2015-06-01 | 2015-06-01 | |
| US62/169,186 | 2015-06-01 | ||
| PCT/US2016/035211 WO2016196590A1 (en) | 2015-06-01 | 2016-06-01 | Catheter assemblies |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018516677A JP2018516677A (ja) | 2018-06-28 |
| JP2018516677A5 JP2018516677A5 (enExample) | 2019-07-04 |
| JP7002120B2 true JP7002120B2 (ja) | 2022-01-20 |
Family
ID=57441651
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017562264A Active JP7002120B2 (ja) | 2015-06-01 | 2016-06-01 | カテーテル組立体 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20180140810A1 (enExample) |
| EP (1) | EP3302677A4 (enExample) |
| JP (1) | JP7002120B2 (enExample) |
| AU (1) | AU2016270738B2 (enExample) |
| CA (1) | CA2987931A1 (enExample) |
| WO (1) | WO2016196590A1 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK3119797T3 (da) | 2014-03-18 | 2021-03-15 | Univ Massachusetts | Raav-baserede sammensætninger og fremgangsmåder til behandling af amyotrofisk lateralsklerose |
| US20220016338A1 (en) * | 2020-07-15 | 2022-01-20 | Cerebral Therapeutics, Inc. | Implantable cranial medical device |
| JP2019531787A (ja) | 2016-08-30 | 2019-11-07 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | 生物医学的ターゲティング及びデリバリーの方法並びにそれを実行するための装置及びシステム |
| JP7327803B2 (ja) | 2017-05-09 | 2023-08-16 | ユニバーシティ オブ マサチューセッツ | 筋萎縮性側索硬化症(als)を処置する方法 |
| CN111132626B (zh) | 2017-07-17 | 2024-01-30 | 沃雅戈治疗公司 | 轨迹阵列引导系统 |
| CN111448321A (zh) | 2017-09-22 | 2020-07-24 | 马萨诸塞大学 | Sod1双表达载体及其用途 |
| KR102223085B1 (ko) * | 2017-11-01 | 2021-03-04 | 사회복지법인 삼성생명공익재단 | 뇌내 약물 주입 장치 및 뇌내 약물 주입 방법 |
| US10493233B1 (en) * | 2018-06-05 | 2019-12-03 | Duke University | Bi-directional access to tumors |
| KR20220141869A (ko) * | 2020-02-18 | 2022-10-20 | 인큐브 랩스 엘엘씨 | 신체 내 치료 부위로의 약물 전달 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012071135A (ja) | 2010-09-29 | 2012-04-12 | Codman & Shurtleff Inc | 多ルーメン脳室排出カテーテル |
| US20130131576A1 (en) | 2011-11-18 | 2013-05-23 | Washington University | Catheter assembly for use with shunt systems and method of using same |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3583387A (en) * | 1968-12-20 | 1971-06-08 | John T Garner | Pressure absorbing appliance for treating hydrocephalus |
| US4382445A (en) * | 1980-12-04 | 1983-05-10 | Cosmos Research Associates | Physiological fluid shunt system and improvements therefor |
| US4583967A (en) * | 1984-02-13 | 1986-04-22 | Cordis Corporation | Telescoping catheter shunt system |
| US4578057A (en) * | 1984-08-31 | 1986-03-25 | Cordis Corporation | Ventricular right angle connector and system |
| US4632668A (en) * | 1984-12-31 | 1986-12-30 | University Of Virginia Alumni Patents Foundation | Ventricular catheter |
| US4936826A (en) * | 1988-10-24 | 1990-06-26 | Amarasinghe Disamodha C | Vena cava window |
| US5385541A (en) * | 1992-04-24 | 1995-01-31 | Loma Linda University Medical Center | Cerebrospinal fluid shunt capable of minimal invasive revision |
| US5713858A (en) * | 1995-04-28 | 1998-02-03 | Medtronic, Inc. | Permanently implantable guiding catheter |
| US6913589B2 (en) * | 2002-01-14 | 2005-07-05 | Codman & Shurtleff, Inc. | Multi-catheter insertion device and method |
| US7727225B2 (en) * | 2004-07-28 | 2010-06-01 | University Of Virginia Patent Foundation | Coaxial catheter systems for transference of medium |
| JP5820877B2 (ja) * | 2010-06-18 | 2015-11-24 | テクニオン リサーチ アンド ディベロップメント ファンデーション リミテッド | 自己洗浄シャント |
-
2016
- 2016-06-01 US US15/578,994 patent/US20180140810A1/en not_active Abandoned
- 2016-06-01 WO PCT/US2016/035211 patent/WO2016196590A1/en not_active Ceased
- 2016-06-01 AU AU2016270738A patent/AU2016270738B2/en active Active
- 2016-06-01 JP JP2017562264A patent/JP7002120B2/ja active Active
- 2016-06-01 EP EP16804305.7A patent/EP3302677A4/en not_active Withdrawn
- 2016-06-01 CA CA2987931A patent/CA2987931A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012071135A (ja) | 2010-09-29 | 2012-04-12 | Codman & Shurtleff Inc | 多ルーメン脳室排出カテーテル |
| US20130131576A1 (en) | 2011-11-18 | 2013-05-23 | Washington University | Catheter assembly for use with shunt systems and method of using same |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3302677A1 (en) | 2018-04-11 |
| US20180140810A1 (en) | 2018-05-24 |
| AU2016270738A1 (en) | 2017-12-14 |
| CA2987931A1 (en) | 2016-12-08 |
| WO2016196590A1 (en) | 2016-12-08 |
| JP2018516677A (ja) | 2018-06-28 |
| EP3302677A4 (en) | 2019-02-20 |
| AU2016270738B2 (en) | 2021-06-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7002120B2 (ja) | カテーテル組立体 | |
| EP1712252B1 (en) | Multi-catheter insertion device | |
| CN103842019B (zh) | 神经外科设备 | |
| US8672871B2 (en) | Endovascular cerebrospinal fluid shunt | |
| WO1996028200A1 (en) | Partially disposable surgical imaging assembly | |
| JP2006289086A (ja) | クモ膜下硬膜外シャント | |
| BR112013022857B1 (pt) | dispositivo intravascular compreendendo fatores de liberação de compressão | |
| BR102014005267A2 (pt) | Controlar o fluxo no cateter | |
| US12268830B2 (en) | Surgical method, device, system and kit for the treatment of hydrocephalus | |
| US11065421B2 (en) | Catheter curvature braces and methods of using same | |
| US8435226B2 (en) | Catheter | |
| WO2019171359A1 (en) | Fluid fitting for dilation instrument | |
| Sheth et al. | Ultrasound guidance for distal insertion of ventriculo-atrial shunt catheters | |
| US11439798B2 (en) | Cerebral spinal fluid shunt plug | |
| US12171966B2 (en) | Cerebral spinal fluid shunt plug | |
| RU2157120C1 (ru) | Устройство для пункции полостей | |
| WO2017021985A1 (en) | Guide for the intracranial positioning of cerebral catheters |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190531 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190531 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200629 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200929 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20201130 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210104 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20210611 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20211011 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20211011 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20211013 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20211109 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20211110 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20211129 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20211220 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7002120 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |