JP6976971B2 - ニコチン粒子送達消耗品 - Google Patents
ニコチン粒子送達消耗品 Download PDFInfo
- Publication number
- JP6976971B2 JP6976971B2 JP2018563904A JP2018563904A JP6976971B2 JP 6976971 B2 JP6976971 B2 JP 6976971B2 JP 2018563904 A JP2018563904 A JP 2018563904A JP 2018563904 A JP2018563904 A JP 2018563904A JP 6976971 B2 JP6976971 B2 JP 6976971B2
- Authority
- JP
- Japan
- Prior art keywords
- nicotine
- article
- container
- cavity
- particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000002245 particle Substances 0.000 title claims description 147
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 title claims description 122
- 229960002715 nicotine Drugs 0.000 title claims description 115
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 title claims description 115
- 239000000796 flavoring agent Substances 0.000 claims description 77
- 239000002775 capsule Substances 0.000 claims description 67
- 235000019634 flavors Nutrition 0.000 claims description 56
- 239000012528 membrane Substances 0.000 claims description 19
- 230000000149 penetrating effect Effects 0.000 claims description 14
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 12
- 210000004072 lung Anatomy 0.000 claims description 12
- 238000007789 sealing Methods 0.000 claims description 11
- 229960003136 leucine Drugs 0.000 claims description 7
- 235000019454 L-leucine Nutrition 0.000 claims description 5
- 239000004395 L-leucine Substances 0.000 claims description 5
- 229940024606 amino acid Drugs 0.000 claims description 4
- 150000001413 amino acids Chemical class 0.000 claims description 4
- 229910052751 metal Inorganic materials 0.000 claims description 4
- 239000002184 metal Substances 0.000 claims description 4
- FFYVQVZXWDGRAY-UHFFFAOYSA-N 3-(1-methylpyrrolidin-2-yl)pyridine;hydrate Chemical compound O.CN1CCCC1C1=CC=CN=C1 FFYVQVZXWDGRAY-UHFFFAOYSA-N 0.000 claims description 3
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 2
- 239000013013 elastic material Substances 0.000 claims description 2
- 239000000843 powder Substances 0.000 description 32
- 235000013355 food flavoring agent Nutrition 0.000 description 21
- 150000001875 compounds Chemical class 0.000 description 12
- 230000000391 smoking effect Effects 0.000 description 11
- 239000000203 mixture Substances 0.000 description 10
- 239000000779 smoke Substances 0.000 description 10
- 239000000463 material Substances 0.000 description 9
- 238000010521 absorption reaction Methods 0.000 description 8
- 239000000853 adhesive Substances 0.000 description 8
- 230000001070 adhesive effect Effects 0.000 description 8
- 210000000214 mouth Anatomy 0.000 description 8
- 238000000034 method Methods 0.000 description 6
- 230000002776 aggregation Effects 0.000 description 5
- 238000004220 aggregation Methods 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 4
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- 229930195725 Mannitol Natural products 0.000 description 4
- 150000001720 carbohydrates Chemical class 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 239000000594 mannitol Substances 0.000 description 4
- 235000010355 mannitol Nutrition 0.000 description 4
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 4
- 244000299461 Theobroma cacao Species 0.000 description 3
- 235000021474 generally recognized As safe (food) Nutrition 0.000 description 3
- 235000021473 generally recognized as safe (food ingredients) Nutrition 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 230000003434 inspiratory effect Effects 0.000 description 3
- CDOSHBSSFJOMGT-UHFFFAOYSA-N linalool Chemical compound CC(C)=CCCC(C)(O)C=C CDOSHBSSFJOMGT-UHFFFAOYSA-N 0.000 description 3
- 230000035515 penetration Effects 0.000 description 3
- -1 salt hydrates Chemical class 0.000 description 3
- 230000001953 sensory effect Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 description 2
- SKZDZXPBBYUFBY-WLHGVMLRSA-N (e)-but-2-enedioic acid;3-(1-methylpyrrolidin-2-yl)pyridine Chemical compound OC(=O)\C=C\C(O)=O.CN1CCCC1C1=CC=CN=C1 SKZDZXPBBYUFBY-WLHGVMLRSA-N 0.000 description 2
- JZBCTZLGKSYRSF-UHFFFAOYSA-N 2-Ethyl-3,5-dimethylpyrazine Chemical compound CCC1=NC=C(C)N=C1C JZBCTZLGKSYRSF-UHFFFAOYSA-N 0.000 description 2
- AIBWPBUAKCMKNS-PPHPATTJSA-N 2-hydroxybenzoic acid;3-[(2s)-1-methylpyrrolidin-2-yl]pyridine Chemical compound OC(=O)C1=CC=CC=C1O.CN1CCC[C@H]1C1=CC=CN=C1 AIBWPBUAKCMKNS-PPHPATTJSA-N 0.000 description 2
- MMOPGICOOYBFJU-UHFFFAOYSA-N 3-(1-methylpyrrolidin-2-yl)pyridine;2-oxopropanoic acid Chemical compound CC(=O)C(O)=O.CN1CCCC1C1=CC=CN=C1 MMOPGICOOYBFJU-UHFFFAOYSA-N 0.000 description 2
- INAXVXBDKKUCGI-UHFFFAOYSA-N 4-hydroxy-2,5-dimethylfuran-3-one Chemical compound CC1OC(C)=C(O)C1=O INAXVXBDKKUCGI-UHFFFAOYSA-N 0.000 description 2
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 description 2
- 244000024873 Mentha crispa Species 0.000 description 2
- 235000014749 Mentha crispa Nutrition 0.000 description 2
- 244000246386 Mentha pulegium Species 0.000 description 2
- 235000016257 Mentha pulegium Nutrition 0.000 description 2
- 235000004357 Mentha x piperita Nutrition 0.000 description 2
- 244000061176 Nicotiana tabacum Species 0.000 description 2
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- 235000019219 chocolate Nutrition 0.000 description 2
- 235000019504 cigarettes Nutrition 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- CBOQJANXLMLOSS-UHFFFAOYSA-N ethyl vanillin Chemical compound CCOC1=CC(C=O)=CC=C1O CBOQJANXLMLOSS-UHFFFAOYSA-N 0.000 description 2
- RRAFCDWBNXTKKO-UHFFFAOYSA-N eugenol Chemical compound COC1=CC(CC=C)=CC=C1O RRAFCDWBNXTKKO-UHFFFAOYSA-N 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 239000012458 free base Substances 0.000 description 2
- 239000008369 fruit flavor Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- LHGVFZTZFXWLCP-UHFFFAOYSA-N guaiacol Chemical compound COC1=CC=CC=C1O LHGVFZTZFXWLCP-UHFFFAOYSA-N 0.000 description 2
- CATSNJVOTSVZJV-UHFFFAOYSA-N heptan-2-one Chemical compound CCCCCC(C)=O CATSNJVOTSVZJV-UHFFFAOYSA-N 0.000 description 2
- 235000001050 hortel pimenta Nutrition 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- 229940041616 menthol Drugs 0.000 description 2
- DTUQWGWMVIHBKE-UHFFFAOYSA-N phenylacetaldehyde Chemical compound O=CCC1=CC=CC=C1 DTUQWGWMVIHBKE-UHFFFAOYSA-N 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 235000013599 spices Nutrition 0.000 description 2
- RUVINXPYWBROJD-ONEGZZNKSA-N trans-anethole Chemical compound COC1=CC=C(\C=C\C)C=C1 RUVINXPYWBROJD-ONEGZZNKSA-N 0.000 description 2
- 229940005605 valeric acid Drugs 0.000 description 2
- 238000009834 vaporization Methods 0.000 description 2
- 230000008016 vaporization Effects 0.000 description 2
- WTOYNNBCKUYIKC-JMSVASOKSA-N (+)-nootkatone Chemical compound C1C[C@@H](C(C)=C)C[C@@]2(C)[C@H](C)CC(=O)C=C21 WTOYNNBCKUYIKC-JMSVASOKSA-N 0.000 description 1
- GKTOAHZEMRRUKF-HVDRVSQOSA-N (2s)-2-aminopentanedioic acid;3-(1-methylpyrrolidin-2-yl)pyridine Chemical compound OC(=O)[C@@H](N)CCC(O)=O.CN1CCCC1C1=CC=CN=C1 GKTOAHZEMRRUKF-HVDRVSQOSA-N 0.000 description 1
- 239000001490 (3R)-3,7-dimethylocta-1,6-dien-3-ol Substances 0.000 description 1
- 229940098795 (3z)- 3-hexenyl acetate Drugs 0.000 description 1
- CDOSHBSSFJOMGT-JTQLQIEISA-N (R)-linalool Natural products CC(C)=CCC[C@@](C)(O)C=C CDOSHBSSFJOMGT-JTQLQIEISA-N 0.000 description 1
- 239000001363 2-ethyl-3,5-dimethylpyrazine Substances 0.000 description 1
- SDVKWBNZJFWIMO-UHFFFAOYSA-N 2-hydroxypropane-1,2,3-tricarboxylic acid;3-(1-methylpyrrolidin-2-yl)pyridine Chemical compound CN1CCCC1C1=CC=CN=C1.OC(=O)CC(O)(C(O)=O)CC(O)=O SDVKWBNZJFWIMO-UHFFFAOYSA-N 0.000 description 1
- VWTHFJXLFGINSW-PPHPATTJSA-N 2-hydroxypropanoic acid;3-[(2s)-1-methylpyrrolidin-2-yl]pyridine Chemical compound CC(O)C(O)=O.CN1CCC[C@H]1C1=CC=CN=C1 VWTHFJXLFGINSW-PPHPATTJSA-N 0.000 description 1
- WLJVXDMOQOGPHL-PPJXEINESA-N 2-phenylacetic acid Chemical compound O[14C](=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-PPJXEINESA-N 0.000 description 1
- NPBVQXIMTZKSBA-UHFFFAOYSA-N Chavibetol Natural products COC1=CC=C(CC=C)C=C1O NPBVQXIMTZKSBA-UHFFFAOYSA-N 0.000 description 1
- 244000223760 Cinnamomum zeylanicum Species 0.000 description 1
- 241000207199 Citrus Species 0.000 description 1
- 240000007154 Coffea arabica Species 0.000 description 1
- 244000004281 Eucalyptus maculata Species 0.000 description 1
- 239000005770 Eugenol Substances 0.000 description 1
- 241000208152 Geranium Species 0.000 description 1
- 240000004670 Glycyrrhiza echinata Species 0.000 description 1
- 235000001453 Glycyrrhiza echinata Nutrition 0.000 description 1
- 235000006200 Glycyrrhiza glabra Nutrition 0.000 description 1
- 235000017382 Glycyrrhiza lepidota Nutrition 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- 235000019501 Lemon oil Nutrition 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- YKVWPZJHENXDAJ-VOTSOKGWSA-N Megastigmatrienone Chemical compound CC1=CC(=O)CC(C)(C)C1\C=C\C=C YKVWPZJHENXDAJ-VOTSOKGWSA-N 0.000 description 1
- 235000006679 Mentha X verticillata Nutrition 0.000 description 1
- 235000002899 Mentha suaveolens Nutrition 0.000 description 1
- 235000001636 Mentha x rotundifolia Nutrition 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- UVMRYBDEERADNV-UHFFFAOYSA-N Pseudoeugenol Natural products COC1=CC(C(C)=C)=CC=C1O UVMRYBDEERADNV-UHFFFAOYSA-N 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- 244000223014 Syzygium aromaticum Species 0.000 description 1
- 235000016639 Syzygium aromaticum Nutrition 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- 235000009499 Vanilla fragrans Nutrition 0.000 description 1
- 244000263375 Vanilla tahitensis Species 0.000 description 1
- 235000012036 Vanilla tahitensis Nutrition 0.000 description 1
- 244000273928 Zingiber officinale Species 0.000 description 1
- 235000006886 Zingiber officinale Nutrition 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229940011037 anethole Drugs 0.000 description 1
- 230000001760 anti-analgesic effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- POIARNZEYGURDG-FNORWQNLSA-N beta-damascenone Chemical compound C\C=C\C(=O)C1=C(C)C=CCC1(C)C POIARNZEYGURDG-FNORWQNLSA-N 0.000 description 1
- POIARNZEYGURDG-UHFFFAOYSA-N beta-damascenone Natural products CC=CC(=O)C1=C(C)C=CCC1(C)C POIARNZEYGURDG-UHFFFAOYSA-N 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- QWJSAWXRUVVRLH-UHFFFAOYSA-M choline bitartrate Chemical compound C[N+](C)(C)CCO.OC(=O)C(O)C(O)C([O-])=O QWJSAWXRUVVRLH-UHFFFAOYSA-M 0.000 description 1
- 229960004874 choline bitartrate Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 235000017803 cinnamon Nutrition 0.000 description 1
- NPFVOOAXDOBMCE-PLNGDYQASA-N cis-3-Hexenyl acetate Natural products CC\C=C/CCOC(C)=O NPFVOOAXDOBMCE-PLNGDYQASA-N 0.000 description 1
- RRGOKSYVAZDNKR-ARJAWSKDSA-M cis-3-hexenylacetate Chemical compound CC\C=C/CCCC([O-])=O RRGOKSYVAZDNKR-ARJAWSKDSA-M 0.000 description 1
- 239000001926 citrus aurantium l. subsp. bergamia wright et arn. oil Substances 0.000 description 1
- 235000020971 citrus fruits Nutrition 0.000 description 1
- 235000016213 coffee Nutrition 0.000 description 1
- 235000013353 coffee beverage Nutrition 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 239000002781 deodorant agent Substances 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- WTOYNNBCKUYIKC-UHFFFAOYSA-N dl-nootkatone Natural products C1CC(C(C)=C)CC2(C)C(C)CC(=O)C=C21 WTOYNNBCKUYIKC-UHFFFAOYSA-N 0.000 description 1
- 229940112141 dry powder inhaler Drugs 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000003571 electronic cigarette Substances 0.000 description 1
- 229940073505 ethyl vanillin Drugs 0.000 description 1
- 229960002217 eugenol Drugs 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000005243 fluidization Methods 0.000 description 1
- 235000010855 food raising agent Nutrition 0.000 description 1
- 239000010648 geranium oil Substances 0.000 description 1
- 235000019717 geranium oil Nutrition 0.000 description 1
- 235000008397 ginger Nutrition 0.000 description 1
- 239000010649 ginger oil Substances 0.000 description 1
- 229960001867 guaiacol Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000002085 irritant Substances 0.000 description 1
- 231100000021 irritant Toxicity 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 239000010501 lemon oil Substances 0.000 description 1
- 229940010454 licorice Drugs 0.000 description 1
- 229930007744 linalool Natural products 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- LDMPZNTVIGIREC-ZGPNLCEMSA-N nicotine bitartrate Chemical compound O.O.OC(=O)[C@H](O)[C@@H](O)C(O)=O.OC(=O)[C@H](O)[C@@H](O)C(O)=O.CN1CCC[C@H]1C1=CC=CN=C1 LDMPZNTVIGIREC-ZGPNLCEMSA-N 0.000 description 1
- 229940069688 nicotine bitartrate Drugs 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- RUVINXPYWBROJD-UHFFFAOYSA-N para-methoxyphenyl Natural products COC1=CC=C(C=CC)C=C1 RUVINXPYWBROJD-UHFFFAOYSA-N 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 229940100595 phenylacetaldehyde Drugs 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 150000003216 pyrazines Chemical class 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229930004725 sesquiterpene Natural products 0.000 description 1
- 150000004354 sesquiterpene derivatives Chemical class 0.000 description 1
- PQDRXUSSKFWCFA-CFNZNRNTSA-N solanone Chemical compound CC(=O)CC[C@@H](C(C)C)\C=C\C(C)=C PQDRXUSSKFWCFA-CFNZNRNTSA-N 0.000 description 1
- PQDRXUSSKFWCFA-UHFFFAOYSA-N solanone Natural products CC(=O)CCC(C(C)C)C=CC(C)=C PQDRXUSSKFWCFA-UHFFFAOYSA-N 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 235000013616 tea Nutrition 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 235000007586 terpenes Nutrition 0.000 description 1
- NPFVOOAXDOBMCE-UHFFFAOYSA-N trans-3-hexenyl acetate Natural products CCC=CCCOC(C)=O NPFVOOAXDOBMCE-UHFFFAOYSA-N 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- MWOOGOJBHIARFG-UHFFFAOYSA-N vanillin Chemical compound COC1=CC(C=O)=CC=C1O MWOOGOJBHIARFG-UHFFFAOYSA-N 0.000 description 1
- FGQOOHJZONJGDT-UHFFFAOYSA-N vanillin Natural products COC1=CC(O)=CC(C=O)=C1 FGQOOHJZONJGDT-UHFFFAOYSA-N 0.000 description 1
- 235000012141 vanillin Nutrition 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 229930007850 β-damascenone Natural products 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/186—Treatment of tobacco products or tobacco substitutes by coating with a coating composition, encapsulation of tobacco particles
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/10—Chemical features of tobacco products or tobacco substitutes
- A24B15/16—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/24—Treatment of tobacco products or tobacco substitutes by extraction; Tobacco extracts
- A24B15/241—Extraction of specific substances
- A24B15/243—Nicotine
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F42/00—Simulated smoking devices other than electrically operated; Component parts thereof; Manufacture or testing thereof
- A24F42/20—Devices without heating means
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F42/00—Simulated smoking devices other than electrically operated; Component parts thereof; Manufacture or testing thereof
- A24F42/60—Constructional details
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F47/00—Smokers' requisites not otherwise provided for
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/02—Sprayers or atomisers specially adapted for therapeutic purposes operated by air or other gas pressure applied to the liquid or other product to be sprayed or atomised
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0035—Piercing means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0043—Non-destructive separation of the package, e.g. peeling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/06—Inhaling appliances shaped like cigars, cigarettes or pipes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Description
ニコチン粒子は、採用される特定の製剤に基づいてニコチンの任意の有用な濃度を有してもよい。ニコチン粒子は、少なくとも約5重量%〜最高約30重量%、または約5重量%〜約25重量%、または約5重量%〜約20重量%、または約5重量%〜約15重量%、または約7重量%〜約13重量%のニコチンを有してもよい。毎回の「吸煙」で、約50〜約150マイクログラムのニコチンがユーザーの肺に送達されることが好ましい。
Claims (15)
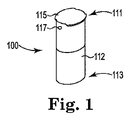
- ニコチン吸入器(200)で使用するための物品(100)であって、
容器(110)であって、
容器の第一の端(111)から反対側の容器の第二の端(113)に延び、かつ空洞(116)を画定する、側壁(112)を備える本体と、
前記容器の第一の端(111)を密封する貫通可能な膜(115)と、
前記容器の第二の端(113)を通して前記空洞(116)の中へと延びる空気出口(118)と、
前記本体側壁(112)を通し、かつ前記空洞(116)の中へと延びる空気吸込み口(117)であって、前記容器の第二の端(113)より前記容器の第一の端(111)に近い空気吸込み口(117)と、を備える容器と
前記空洞(116)の中に配置された貫通可能なカプセル(120)であって、ユーザーの肺の中への吸入送達用にサイズ設定されたニコチンを含む粒子を包含するカプセル(120)と、を備える物品。 - 前記貫通可能な膜(115)が、穿孔された後に再び封じるように構成された弾性材料である、請求項1に記載の物品(100)。
- 前記貫通可能な膜(115)が金属箔である、請求項1に記載の物品(100)。
- 前記空気吸込み口(117)が前記空洞(116)の質量中心長軸方向軸からずれており、かつ前記空気吸込み口(117)から前記空気出口(118)に流れる空気が前記カプセル(120)の長軸方向軸を中心として前記カプセルを回転させる、請求項1〜3のいずれか一項に記載の物品(100)。
- 前記空洞(116)が円形断面形状を有し、かつ前記空気吸込み口(117)の延びる方向が前記空洞(116)の前記円形断面形状に対して接線方向成分を備える、請求項1〜4のいずれか一項に記載の物品(100)。
- 前記容器(110)が二つの空気吸込み口(117)を備え、前記二つの空気吸込み口(117)が前記容器の第二の端(113)より前記容器の第一の端(111)に近い、請求項1〜5のいずれか一項に記載の物品(100)。
- 前記空洞(116)が円形断面形状を有し、かつ前記二つの空気吸込み口(117)の延びる方向が前記空洞(116)の前記円形断面形状に対して接線方向成分を備える、請求項6に記載の物品(100)。
- 前記空洞(116)が円形断面形状および第一の直径(D)を有し、また前記カプセル(120)が前記第一の直径(D)よりも小さい第二の直径を有し、前記第二の直径が前記第一の直径(D)の約80%〜約99%の範囲であるか、または前記第二の直径が前記第一の直径(D)の約90%〜約98%の範囲である、請求項1〜7のいずれか一項に記載の物品(100)。
- 前記ニコチンを含む粒子が、約10マイクロメートル以下、または約5マイクロメートル以下、または約1マイクロメートル〜約3マイクロメートルの範囲の空気動力学的中央粒子径を有する、請求項1〜8のいずれか一項に記載の物品(100)。
- 前記ニコチンがニコチン塩またはニコチン塩水和物を含む、請求項1〜9のいずれか一項に記載の物品(100)。
- 前記ニコチンが、ロイシンまたはL−ロイシンなどのアミノ酸を含む、請求項1〜10のいずれか一項に記載の物品(100)。
- 前記カプセル(120)が、風味を含む粒子を包含し、この粒子が約20マイクロメートル以上、または約50マイクロメートル〜約150マイクロメートルの範囲の空気動力学的中央粒子径を有する、請求項1〜11のいずれか一項に記載の物品(100)。
- 前記空気出口(118)の上に配置された空気出口密封層をさらに備え、前記密封層が前記空気出口(118)を露出するために穿孔されるように、または剥離可能なように構成されている、請求項1〜12のいずれか一項に記載の物品(100)。
- 前記空気吸込み口(117)の上に配置された空気吸込み口密封層をさらに備え、前記密封層が前記空気吸込み口(117)を露出するために穿孔されるように、または剥離可能なように構成されている、請求項1〜13のいずれか一項に記載の物品(100)。
- 請求項1〜14のいずれか一項に記載の物品(100)を備える吸入器(200)。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16178336.0 | 2016-07-07 | ||
| EP16178336 | 2016-07-07 | ||
| PCT/IB2017/053546 WO2018007887A1 (en) | 2016-07-07 | 2017-06-14 | Nicotine particle delivery consumable |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019524061A JP2019524061A (ja) | 2019-09-05 |
| JP2019524061A5 JP2019524061A5 (ja) | 2020-07-09 |
| JP6976971B2 true JP6976971B2 (ja) | 2021-12-08 |
Family
ID=56413481
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018563904A Active JP6976971B2 (ja) | 2016-07-07 | 2017-06-14 | ニコチン粒子送達消耗品 |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US10945460B2 (ja) |
| EP (1) | EP3481472B1 (ja) |
| JP (1) | JP6976971B2 (ja) |
| KR (1) | KR102446498B1 (ja) |
| CN (1) | CN109219462B (ja) |
| AU (1) | AU2017293424A1 (ja) |
| BR (1) | BR112018075142B1 (ja) |
| CA (1) | CA3022612A1 (ja) |
| ES (1) | ES2818542T3 (ja) |
| IL (1) | IL263326A (ja) |
| MX (1) | MX2018015620A (ja) |
| PH (1) | PH12018502716A1 (ja) |
| PL (1) | PL3481472T3 (ja) |
| RU (1) | RU2744863C2 (ja) |
| SG (1) | SG11201811746TA (ja) |
| UA (1) | UA124197C2 (ja) |
| WO (1) | WO2018007887A1 (ja) |
| ZA (1) | ZA201806848B (ja) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018158207A1 (en) | 2017-02-28 | 2018-09-07 | Philip Morris Products S.A. | Aerosol-generating device comprising a powder de-agglomerating actuator |
| JP7356430B2 (ja) * | 2018-02-19 | 2023-10-04 | フィリップ・モーリス・プロダクツ・ソシエテ・アノニム | 乾燥粉末吸入器 |
| WO2019186337A1 (en) * | 2018-03-26 | 2019-10-03 | Philip Morris Products S.A. | Inhaler with aperatured porous support element |
| KR102724157B1 (ko) | 2018-03-26 | 2024-11-01 | 필립모리스 프로덕츠 에스.에이. | 복합 다공성 지지 요소를 갖는 흡입기 물품 및 시스템 |
| CN114616010B (zh) * | 2019-10-25 | 2024-06-14 | 菲利普莫里斯生产公司 | 具有开放远端的吸入器制品以及吸入器系统 |
| US20230167115A1 (en) * | 2020-04-24 | 2023-06-01 | The University Of British Columbia | Antiviral Agents, Uses Thereof and Methods for Their Preparation |
| JP2022060276A (ja) | 2020-11-25 | 2022-04-14 | Future Technology株式会社 | 電子タバコカートリッジ |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3508558A (en) | 1969-03-19 | 1970-04-28 | Bernard M Seyburn | Cigarette filter |
| IT982765B (it) * | 1973-04-13 | 1974-10-21 | Farmaceutici Italia | Apparecchio inalatore |
| IT1116047B (it) | 1979-04-27 | 1986-02-10 | Sigma Tau Ind Farmaceuti | Dispositivo per la rapida inalazione di farmaci in polvere da parte di persone sofferenti di asma |
| CN1161866A (zh) * | 1996-03-21 | 1997-10-15 | 株式会社优尼希雅杰克斯 | 鼻腔给药器 |
| JPH09253208A (ja) * | 1996-03-21 | 1997-09-30 | Unisia Jecs Corp | 鼻腔用投薬器 |
| US20020134373A1 (en) | 2001-03-20 | 2002-09-26 | Igor Gonda | Aerosol generation using sterile multiple dose containers |
| ES2564165T3 (es) * | 2003-04-09 | 2016-03-18 | Novartis Ag | Aparato de aerosolización con guía de alineación para la perforación de la cápsula |
| CN100381083C (zh) * | 2003-04-29 | 2008-04-16 | 韩力 | 一种非可燃性电子喷雾香烟 |
| DE102006016904A1 (de) * | 2006-04-11 | 2007-10-25 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Inhalator |
| WO2009075794A1 (en) * | 2007-12-05 | 2009-06-18 | Nektar Therapeutics | Receptacle for an aerosolizable pharmaceutical formulation |
| ES2776003T3 (es) | 2011-05-27 | 2020-07-28 | Boehringer Ingelheim Int | Inhalador y cápsula para un inhalador |
| GB201110863D0 (en) * | 2011-06-27 | 2011-08-10 | British American Tobacco Co | Smoking article filter and insertable filter unit thereof |
| EP2925395B1 (en) * | 2012-11-28 | 2019-03-06 | Fontem Holdings 1 B.V. | Device for generating a condensation aerosol from a liquid formulation |
| WO2014089174A2 (en) * | 2012-12-06 | 2014-06-12 | Aerodesigns, Inc. | Aerosol dispenser with edible cartridge |
| WO2014150826A1 (en) * | 2013-03-15 | 2014-09-25 | Aerodesigns, Inc. | Aerosol dispenser with edible cartridge |
| MX2016016518A (es) | 2014-06-20 | 2017-05-01 | Philip Morris Products Sa | Sistema de siministro de polvo de nicotina con medios para el manejo del flujo de aire. |
| RU2688978C2 (ru) | 2014-12-15 | 2019-05-23 | Филип Моррис Продактс С.А. | Система, генерирующая аэрозоль, содержащая подвижный картридж |
| EP3419707B1 (de) * | 2016-02-24 | 2023-01-25 | Boehringer Ingelheim International GmbH | Inhalator |
-
2017
- 2017-06-14 PL PL17732585T patent/PL3481472T3/pl unknown
- 2017-06-14 EP EP17732585.9A patent/EP3481472B1/en active Active
- 2017-06-14 AU AU2017293424A patent/AU2017293424A1/en not_active Abandoned
- 2017-06-14 KR KR1020187034705A patent/KR102446498B1/ko active IP Right Grant
- 2017-06-14 MX MX2018015620A patent/MX2018015620A/es unknown
- 2017-06-14 JP JP2018563904A patent/JP6976971B2/ja active Active
- 2017-06-14 US US16/309,769 patent/US10945460B2/en active Active
- 2017-06-14 RU RU2018143531A patent/RU2744863C2/ru active
- 2017-06-14 CA CA3022612A patent/CA3022612A1/en not_active Abandoned
- 2017-06-14 SG SG11201811746TA patent/SG11201811746TA/en unknown
- 2017-06-14 CN CN201780034498.5A patent/CN109219462B/zh active Active
- 2017-06-14 ES ES17732585T patent/ES2818542T3/es active Active
- 2017-06-14 UA UAA201811266A patent/UA124197C2/uk unknown
- 2017-06-14 BR BR112018075142-1A patent/BR112018075142B1/pt active IP Right Grant
- 2017-06-14 WO PCT/IB2017/053546 patent/WO2018007887A1/en active Search and Examination
-
2018
- 2018-10-15 ZA ZA2018/06848A patent/ZA201806848B/en unknown
- 2018-11-27 IL IL263326A patent/IL263326A/en unknown
- 2018-12-21 PH PH12018502716A patent/PH12018502716A1/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| RU2018143531A3 (ja) | 2020-10-16 |
| AU2017293424A1 (en) | 2018-11-08 |
| EP3481472A1 (en) | 2019-05-15 |
| ES2818542T3 (es) | 2021-04-13 |
| CN109219462B (zh) | 2021-07-30 |
| US20200178611A1 (en) | 2020-06-11 |
| KR20190026656A (ko) | 2019-03-13 |
| CA3022612A1 (en) | 2018-01-11 |
| JP2019524061A (ja) | 2019-09-05 |
| BR112018075142B1 (pt) | 2023-05-02 |
| EP3481472B1 (en) | 2020-08-05 |
| PH12018502716A1 (en) | 2019-07-29 |
| PL3481472T3 (pl) | 2020-12-14 |
| RU2744863C2 (ru) | 2021-03-16 |
| US10945460B2 (en) | 2021-03-16 |
| WO2018007887A1 (en) | 2018-01-11 |
| CN109219462A (zh) | 2019-01-15 |
| ZA201806848B (en) | 2019-07-31 |
| IL263326A (en) | 2018-12-31 |
| RU2018143531A (ru) | 2020-08-07 |
| KR102446498B1 (ko) | 2022-09-23 |
| MX2018015620A (es) | 2019-04-11 |
| UA124197C2 (uk) | 2021-08-04 |
| BR112018075142A2 (pt) | 2019-03-26 |
| SG11201811746TA (en) | 2019-01-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6976970B2 (ja) | ニコチン吸入器システム | |
| JP7238043B2 (ja) | ニコチン粉末送達システム | |
| JP7061124B2 (ja) | 渦巻状端部プラグを備えた吸入器 | |
| JP6976971B2 (ja) | ニコチン粒子送達消耗品 | |
| EP3393565B1 (en) | Nicotine particle capsule | |
| JP7356430B2 (ja) | 乾燥粉末吸入器 | |
| JP7061123B2 (ja) | サイズ設定された空洞を備えた吸入器 | |
| JP2021500017A (ja) | 吸入器物品用の貫通付属品およびシステム | |
| JP2021500015A (ja) | 境界要素を備えた吸入器 | |
| US12233204B2 (en) | Nicotine powder delivery system | |
| RU2824230C2 (ru) | Система доставки никотинового порошка | |
| NZ747468A (en) | Nicotine particle delivery consumable |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190108 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200528 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200528 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210415 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210714 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20211011 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20211110 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6976971 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |