JP6976843B2 - Skin condition improving agent - Google Patents
Skin condition improving agent Download PDFInfo
- Publication number
- JP6976843B2 JP6976843B2 JP2017253491A JP2017253491A JP6976843B2 JP 6976843 B2 JP6976843 B2 JP 6976843B2 JP 2017253491 A JP2017253491 A JP 2017253491A JP 2017253491 A JP2017253491 A JP 2017253491A JP 6976843 B2 JP6976843 B2 JP 6976843B2
- Authority
- JP
- Japan
- Prior art keywords
- skin
- lactic acid
- skin condition
- test
- strain
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Description
本発明は、高いヒアルロン酸産生促進能力を有する乳児腸内由来のラクトバチルス属ガセリ種の乳酸菌及びその発酵物を有効成分として含有する皮膚状態改善剤、当該皮膚状態改善剤を有効成分として含む皮膚状態改善用医薬品、および皮膚状態改善用飲食品を提供する。 The present invention is a skin condition improving agent containing a lactic acid bacterium of the genus Lactobacillus gasseri derived from the infant intestine having a high ability to promote hyaluronic acid production and a fermented product thereof as an active ingredient, and a skin containing the skin condition improving agent as an active ingredient. Provide medicines for improving the condition and foods and drinks for improving the skin condition.
近年、皮膚の老化防止に関する研究が広く行われている。皮膚老化の原因は、加齢が重要な因子であるが、それに加えて乾燥、酸化、紫外線等による影響も皮膚老化に関わる直接的な因子として挙げられる。皮膚老化の具体的な現象としては、ヒアルロン酸をはじめとするムコ多糖類の減少、コラーゲンの架橋反応、紫外線による細胞の損傷などが知られている。 In recent years, research on skin aging prevention has been widely conducted. Aging is an important factor in the cause of skin aging, but in addition, the effects of dryness, oxidation, ultraviolet rays, etc. are also cited as direct factors related to skin aging. As specific phenomena of skin aging, reduction of mucopolysaccharides such as hyaluronic acid, collagen cross-linking reaction, and cell damage by ultraviolet rays are known.
その中でもヒアルロン酸は生体内では皮膚、関節、靭帯、硝子体、脳、肺、腎臓など種々の臓器・組織に広く存在しており、特に皮膚には、体全体の50%を占める量のヒアルロン酸が存在している(非特許文献1)。その保湿性の高さから皮膚の潤いを保つ重要な因子として知られている(非特許文献2)。 Among them, hyaluronic acid is widely present in various organs and tissues such as skin, joints, ligaments, vitreous body, brain, lungs, and kidneys in the living body, and especially in the skin, hyaluronic acid accounts for 50% of the whole body. Acid is present (Non-Patent Document 1). It is known as an important factor to keep the skin moisturized due to its high moisturizing property (Non-Patent Document 2).
本発明は、高いヒアルロン酸産生促進能力を有する乳児腸内由来のラクトバチルス属ガセリ種の乳酸菌及びその発酵物を有効成分として含有する皮膚状態改善剤、当該皮膚状態改善剤を有効成分として含む皮膚状態改善医薬品、および皮膚状態改善飲食品を提供することを目的とする。 The present invention is a skin condition improving agent containing a lactic acid bacterium of the genus Lactobacillus gasseri derived from the infant intestine having a high ability to promote hyaluronic acid production and a fermented product thereof as an active ingredient, and a skin containing the skin condition improving agent as an active ingredient. The purpose is to provide condition-improving drugs and skin condition-improving foods and drinks.
本発明者らは、肌の保湿力向上にはヒアルロン酸が必要不可欠である点に着目し、多数の乳酸菌についてスクリーニングを行った結果、乳児腸内由来のラクトバチルス属ガセリ種の乳酸菌、特にラクトバチルス・ガセリN320株(NITE BP-02287)には高いヒアルロン酸産生促進能力があり、例えば、乾燥による、皮膚の水分量の低下、頬や背中、頸の乾燥状態の悪化、洗顔後のつっぱり感、皮膚のつやの低下を含む、皮膚状態の改善に有効であることを見出し、本発明を完成するに至った。 The present inventors focused on the fact that hyaluronic acid is indispensable for improving the moisturizing power of the skin, and as a result of screening for a large number of lactic acid bacteria, the lactic acid bacteria of the genus Lactobacillus gasseri derived from the infant intestine, particularly lacto Bacillus gasseri N320 strain (NITE BP-02287) has a high ability to promote hyaluronic acid production. , It has been found that it is effective for improving the skin condition including the reduction of the gloss of the skin, and the present invention has been completed.
すなわち、本願第一の発明は、ラクトバチルス属ガセリ種の乳酸菌及び当該乳酸菌の発酵物を有効成分とする、経口投与可能な皮膚状態改善剤であり、本願第二の発明は、皮膚状態の改善は、皮膚の乾燥状態の改善、低下した皮膚のつやの改善、および洗顔後における皮膚のつっぱり感の改善を含む本願第一の発明に記載の皮膚状態改善剤であり、本願第三の発明は、乳酸菌が、ラクトバチルス・ガセリN320株(NITE BP-02287)である、本願第一の発明または本願第二の発明に記載の皮膚状態改善剤である。さらに、本出願人は、当該ラクトバチルス属ガセリ種の乳酸菌を含む皮膚状態を改善するための皮膚状態改善用医薬品、または皮膚状態改善用飲食品も意図している。 That is, the first invention of the present application is an orally administrable skin condition improving agent containing a lactic acid bacterium of the genus Lactobacillus gasseri and a fermented product of the lactic acid bacterium as an active ingredient, and the second invention of the present application is an improvement of the skin condition. Is a skin condition improving agent according to the first invention of the present application, which comprises improving the dry condition of the skin, improving the gloss of the lowered skin, and improving the feeling of firmness of the skin after washing the face. The lactic acid bacterium is Lactobacillus gasseri N320 strain (NITE BP-02287), which is the skin condition improving agent according to the first invention of the present application or the second invention of the present application. Furthermore, the applicant also intends a skin condition improving drug for improving the skin condition containing the lactic acid bacterium of the Lactobacillus gasseri species, or a food or drink for improving the skin condition.
本発明において皮膚状態の改善とは、乾燥による皮膚の水分量低下を改善し、客観的にも主観的にも皮膚状態の改善されたことをいう。 In the present invention, the improvement of the skin condition means that the decrease in the water content of the skin due to drying is improved, and the skin condition is improved both objectively and subjectively.
本発明によれば、日常的に経口摂取して皮膚の乾燥を改善することが可能な皮膚状態改善剤、医薬品、または飲食品が提供される。 According to the present invention, there is provided a skin condition improving agent, a drug, or a food or drink that can be orally ingested on a daily basis to improve the dryness of the skin.
以下、本発明を詳細に説明する。
1.ラクトバチルス・ガセリN320株(NITE BP-02287)
本発明の乳酸菌は、ラクトバチルス・ガセリ(lactobacillus gasseri)である。特にラクトバチルス・ガセリに属する乳酸菌のうち、ラクトバチルス・ガセリN320株(NITE BP-02287)である。本発明にいうN320の記号は日清食品ホールディングス株式会社で独自に菌株に付与した番号であり、本ラクトバチルス・ガセリN320株は本発明者によって初めて分離されたものである。
Hereinafter, the present invention will be described in detail.
1. 1. Lactobacillus gasseri N320 strain (NITE BP-02287)
The lactic acid bacterium of the present invention is lactobacillus gasseri. In particular, among the lactic acid bacteria belonging to Lactobacillus gasseri, Lactobacillus gasseri N320 strain (NITE BP-02287). The symbol of N320 referred to in the present invention is a number originally assigned to the strain by Nisshin Foods Holdings Co., Ltd., and the Lactobacillus gasseri N320 strain was isolated for the first time by the present inventor.
本発明のラクトバチルス・ガセリN320株は、下記条件で寄託されている。
(1)寄託機関名:独立行政法人製品技術基盤機構 特許微生物寄託センター
(2)連絡先:〒292−0818 千葉県木更津市かずさ鎌足2−5−8 122号室
(3)受託番号:NITE BP−02287
(4)識別のための表示:N320
(5)原寄託日:2016年6月14日
(6)ブダペスト条約に基づく寄託への移管日:2017年4月10日
本発明のラクトバチルス・ガセリN320株の菌学的性質は、以下の表1及び2に示す通りである。本菌学的性質は、Bergey’s manual of systematic bacteriology Vol.2(1986)に記載の方法による。表1は本菌株に関する形状等を、表2はアピ50CH及びアピCHL(ビオメリュー製)により、糖資化性を試験した結果を示す。表2において、「+」は発酵性あり、「−」は発酵性なしを示す。
The Lactobacillus gasseri N320 strain of the present invention has been deposited under the following conditions.
(1) Depositary organization name: National Institute of Technology and Technology Patented Microbial Deposit Center (2) Contact: 2-5-8 Kazusakamatari, Kisarazu City, Chiba Prefecture 292-0818 Room 122 (3) Deposit number: NITE BP -02287
(4) Display for identification: N320
(5) Original deposit date: June 14, 2016 (6) Transfer date to deposit based on the Budapest Treaty: April 10, 2017 The mycological properties of the Japanese invention Lactobacillus gasseri N320 strain are shown in the table below. It is as shown in 1 and 2. This bacteriological property is based on the method described in Bergey's manual of systematic bacteriology Vol.2 (1986). Table 1 shows the shape and the like related to this strain, and Table 2 shows the results of saccharification tests using Api 50CH and Api CHL (manufactured by BioMérieux). In Table 2, "+" indicates fermentable and "-" indicates non-fermentable.
2.ヒアルロン酸産生促進能試験
本発明のラクトバチルス・ガセリN320株は、後述する実験例に示すように、高いヒアルロン酸産生促進能を有する。ヒアルロン酸産生促進能の確認については以下の試験方法によって行った。
2. 2. Hyaluronic acid production promoting ability test The Lactobacillus gasseri N320 strain of the present invention has a high hyaluronic acid production promoting ability as shown in the experimental examples described later. The hyaluronic acid production promoting ability was confirmed by the following test method.
<ヒアルロン酸産生促進能評価に用いた被験体(培養液)の調製>
ヒアルロン酸産生促進能評価に用いた被験体(培養液)は、乳酸菌を表3に示すMRS培地(Difco Lactobacilli MRS Broth)で37℃ ・24時間培養し95℃20分加熱殺菌後、水酸化ナトリウム水溶液を用いてpH7.0にすることにより得た。
<Preparation of subject (culture solution) used for evaluation of hyaluronic acid production promoting ability>
The subject (culture solution) used for the evaluation of hyaluronic acid production promoting ability was lactic acid bacteria cultured in the MRS medium (Difco Lactobacilli MRS Broth) shown in Table 3 at 37 ° C for 24 hours, sterilized by heating at 95 ° C for 20 minutes, and then sodium hydroxide. It was obtained by adjusting the pH to 7.0 using an aqueous solution.
<ヒアルロン酸産生促進能評価>
上記で得た試料のヒアルロン酸産生促進作用を評価した。すなわち、24ウェルプレートに、5.0×104個/ウェルの濃度で正常ヒト成人表皮角化細胞を播種し、コンフルエント状態にしたところで、各試料群(最終濃度1%量)を含む500μLのHuMedia-KG2培養液を添加した。添加後、72時間培養した後、ELISA法にてヒアルロン酸産生量を測定した。ヒアルロン酸産生量の測定単位はng/mlとし、測定にはHyaluronan assay kit(生化学バイオビジネス社製)を使用した。なお、対照例(コントロール)として乳酸菌増殖培地(MRS)を使用した。
<Evaluation of hyaluronic acid production promoting ability>
The hyaluronic acid production promoting action of the sample obtained above was evaluated. That is, when normal human adult epidermal keratinized cells were seeded on a 24-well plate at a concentration of 5.0 × 10 4 cells / well and brought into a confluent state, 500 μL containing each sample group (final concentration 1% amount) was added. HuMedia-KG2 culture solution was added. After the addition, the cells were cultured for 72 hours, and then the amount of hyaluronic acid produced was measured by the ELISA method. The unit for measuring the amount of hyaluronic acid produced was ng / ml, and the Hyaluronan assay kit (manufactured by Biochemical Biobusiness) was used for the measurement. A lactic acid bacterium growth medium (MRS) was used as a control example.
3.スキムミルク培地での増殖性試験
<スキムミルク培地増殖性試験>
前培養液を10%SM(スキムミルク)培地に接種し、これを37℃ ・24時間培養した上で、乳酸菌数によってミルク培地での増殖性を評価した。
3. 3. Proliferative test in skim milk medium <Skim milk medium proliferative test>
The preculture solution was inoculated into a 10% SM (skimmed milk) medium, and after culturing this at 37 ° C for 24 hours, the proliferation in the milk medium was evaluated by the number of lactic acid bacteria.
4.飲食品
本発明の乳酸菌は飲食品に含有せしめて使用することができる。本発明の乳酸菌は特に乳製品に好適に用いることができるが、例えば、乳酸菌入り発酵乳及び乳酸菌入り乳酸菌飲料が考えられる。現行の乳及び乳製品の成分規格等に関する省令では、成分規格として乳酸菌数は特に規定はされていないが、発酵乳(無脂乳固形分8.0%以上のもの)や乳酸菌飲料(無脂乳固形分3.0%以上のもの)であれば1.0×107cfu/ml以上、乳酸菌飲料(無脂乳固形分3.0%未満のもの)であれば1.0×106cfu/ml以上が好ましく、乳などの発酵液中で増殖させたり、最終製品の形態で増殖させたりすることによって上記の菌数を実現することができる。また、乳酸菌入り発酵乳及び乳酸菌入り乳酸菌飲料以外にも、バター等の乳製品、マヨネーズ等の卵加工品、バターケーキ等の菓子パン類等にも利用することができる。また、即席麺やクッキー等の加工食品にも好適に利用することができる。上記の他、本発明の食品は、前記乳酸菌と共に、必要に応じて適当な担体及び添加剤を添加して製剤化された形態(例えば、粉末、顆粒、カプセル、錠剤等)であってもよい。
4. Food and Beverage The lactic acid bacterium of the present invention can be contained in food and drink and used. The lactic acid bacterium of the present invention can be particularly preferably used for dairy products, and for example, fermented milk containing lactic acid bacteria and a lactic acid bacteria beverage containing lactic acid bacteria can be considered. The current ministry ordinance on ingredient standards for milk and dairy products does not specifically specify the number of lactic acid bacteria as an ingredient standard, but fermented milk (non-fat milk solids content of 8.0% or more) and lactic acid bacteria beverages (non-fat milk solids). 1.0 x 10 7 cfu / ml or more is preferable for those with a minute of 3.0% or more, and 1.0 x 10 6 cfu / ml or more is preferable for lactic acid bacteria beverages (those with a non-fat milk solid content of less than 3.0%), such as milk. The above-mentioned number of bacteria can be realized by growing in a fermented liquid or in the form of a final product. In addition to fermented milk containing lactic acid bacteria and lactic acid bacteria beverages containing lactic acid bacteria, it can also be used for dairy products such as butter, processed egg products such as mayonnaise, and sweet breads such as butter cake. Further, it can be suitably used for processed foods such as instant noodles and cookies. In addition to the above, the food product of the present invention may be in a form (for example, powder, granules, capsules, tablets, etc.) formulated by adding appropriate carriers and additives together with the lactic acid bacteria as needed. ..
本発明の乳酸菌は、一般の飲料や食品以外にも特定保健用食品、栄養補助食品等に含有させることも有用である。 It is also useful to include the lactic acid bacterium of the present invention in foods for specified health use, dietary supplements, etc. in addition to general beverages and foods.
また、本発明の乳酸菌は、食品以外にも化粧水等の化粧品分野、整腸剤等の医薬品分野、歯磨き粉等の日用品分野、サイレージ、動物用餌、植物液体肥料等の動物飼料・植物肥料分野においても応用可能である。 In addition to foods, the lactic acid bacterium of the present invention is also used in the fields of cosmetics such as cosmetics, pharmaceuticals such as intestinal regulators, daily necessities such as toothpaste, silage, animal feeds, and animal feeds and plant fertilizers such as plant liquid fertilizers. It can be applied.
5.医薬品
本発明の乳酸菌は、発酵後に濃縮や分離等でエキス化及び粉末化した上で医薬品として使用することができる。例えば、エキス化したものを瓶等に詰めたものや、発酵液乾燥粉末を賦形剤なども用いて顆粒やカプセル化、打錠し錠剤の形態として提供が可能である。
5. Pharmaceuticals The lactic acid bacteria of the present invention can be used as pharmaceuticals after being extracted and powdered by concentration or separation after fermentation. For example, it is possible to provide an extract in a bottle or the like, or a dry fermented liquid powder in the form of granules, encapsulation, or tableting using an excipient or the like.
本発明の乳酸菌(ラクトバチルス・ガセリN320株)は高いヒアルロン酸産生促進能力を有するため、当該乳酸菌及びその発酵物を有効成分として含有する錠剤、医薬品、または飲食品を日常的に摂取することで、例えば、皮膚の乾燥状態(特に、頬、背中、頸など)、洗顔後のつっぱり感、皮膚のつやの低下を改善することができる。 Since the lactic acid bacterium of the present invention (Lactobacillus gasseri N320 strain) has a high ability to promote hyaluronic acid production, it is possible to routinely ingest tablets, pharmaceuticals, or foods and drinks containing the lactic acid bacterium and its fermented product as an active ingredient. For example, the dry state of the skin (particularly, cheeks, back, neck, etc.), the feeling of tension after washing the face, and the decrease in the gloss of the skin can be improved.
以下、本発明の実施例を示すが、本発明は以下の実施例に限定されるものではない。
<試験例1>ヒアルロン酸産生促進能力評価
本発明のラクトバチルス・ガセリN320株と、自社保有の3つのラクトバチルス・ガセリ比較菌株(N219、N220、N313)についてヒアルロン酸産生促進能評価を実施した。
Hereinafter, examples of the present invention will be shown, but the present invention is not limited to the following examples.
<Test Example 1> Evaluation of ability to promote hyaluronic acid production The ability to promote hyaluronic acid production was evaluated for the Lactobacillus gasseri N320 strain of the present invention and three Lactobacillus gasseri comparative strains (N219, N220, N313) owned by the company. ..
ヒアルロン酸産生促進能力評価は次の手順により行った。本発明の菌株と比較菌株のそれぞれについて、表3に示すMRS培地(Difco Lactobacilli MRS Broth)で37℃ ・24時間培養し95℃20分加熱殺菌後、水酸化ナトリウム水溶液を用いてpH7.0にすることにより被験体(培養液)を得た。 The hyaluronic acid production promoting ability was evaluated by the following procedure. Each of the strain of the present invention and the comparative strain was cultured in the MRS medium (Difco Lactobacilli MRS Broth) shown in Table 3 at 37 ° C for 24 hours, sterilized by heating at 95 ° C for 20 minutes, and then adjusted to pH 7.0 using an aqueous sodium hydroxide solution. By doing so, a subject (culture medium) was obtained.
別途、24ウェルプレートに、5.0×104個/ウェルの濃度で正常ヒト成人表皮角化細胞を播種し、コンフルエント状態にしたところで、各試料群(最終濃度1%量)を含む500μLのHuMedia -KG2培養液を添加した。添加後、72時間培養した後、ELISA法にてヒアルロン酸産生量を測定した。なお対照例(コントロール)としては、乳酸菌増殖培地(MRS)を用いた。測定にはHyaluronan assay kit(生化学バイオビジネス社製)を用いた。測定値の単位はヒアルロン酸産生量(ng/ml)を示す。 Separately, normal human adult epidermal keratinized cells were seeded on a 24-well plate at a concentration of 5.0 × 10 4 cells / well, and when the cells were brought into a confluent state, 500 μL containing each sample group (final concentration 1% amount) was added. HuMedia -KG2 culture solution was added. After the addition, the cells were cultured for 72 hours, and then the amount of hyaluronic acid produced was measured by the ELISA method. As a control example (control), a lactic acid bacterium growth medium (MRS) was used. A Hyaluronan assay kit (manufactured by Biochemical Biobusiness Co., Ltd.) was used for the measurement. The unit of the measured value is the amount of hyaluronic acid produced (ng / ml).
表4及び図1からも明らかなように、本発明の乳酸菌(ラクトバチルス・ガセリN320株)のヒアルロン酸産生促進能力は非常に強く、他の自社保有の乳酸菌と比べても高いヒアルロン酸産生促進能力を有していることが確認された。 As is clear from Table 4 and FIG. 1, the lactic acid bacterium of the present invention (Lactobacillus gasseri N320 strain) has a very strong ability to promote hyaluronic acid production, which is higher than that of other lactic acid bacteria owned by the company. It was confirmed that it had the ability.
<試験例2>スキムミルク培地増殖性試験
本発明のラクトバチルス・ガセリN320株と、自社保有の3つのラクトバチルス・ガセリ比較菌株(N219、N220、N313)について、スキムミルク培地増殖性試験を実施した。
<Test Example 2> Skim milk medium growth test A skim milk medium growth test was carried out on the Lactobacillus gasseri N320 strain of the present invention and three Lactobacillus gasseri comparative strains (N219, N220, N313) owned by the company.
10%SM(スキムミルク)培地に各菌株を植菌し、これを37℃ ・24時間培養した上で、乳酸菌数によってスキムミルク培地での増殖性を評価した。 Each strain was inoculated into a 10% SM (skimmed milk) medium, cultured at 37 ° C for 24 hours, and then the proliferative property in the skim milk medium was evaluated by the number of lactic acid bacteria.
結果を表5及び図2に示す。 The results are shown in Table 5 and FIG.
本発明の菌株(ラクトバチルス・ガセリN320株)の菌数は22×107cfu/mlであり、他の比較菌株と比べて増殖性が高く、乳酸菌入り発酵乳及び乳酸菌入り乳酸菌飲料を生産する上で問題のないレベルであった。 The strain of the present invention (Lactobacillus gasseri N320 strain) has a bacterial count of 22 × 10 7 cfu / ml, which is highly proliferative compared to other comparative strains, and produces fermented milk containing lactic acid bacteria and a lactic acid bacteria beverage containing lactic acid bacteria. It was a level that was not a problem above.
<試験例3>ヒト被験者に対する効果試験
被験者として年齢35歳以上59歳以下の男性22名、女性21名の合計43名を選択し効果試験を行った。被験者の割り付け方法は下記の通りである。
・スクリーニング時に測定した被験者右頬部の皮膚水分蒸散量の平均値が各群できるだけ同一になるように割り付けた。
・被験者の年齢に関しては、平均値が各群で、できるだけ同一になるように割り付けを行った。また、その際に各群の男女比が選抜時の男女比とできるだけ同一になるよう割り付けを行った。
<Test Example 3> Effect test on human subjects
A total of 43 subjects, 22 males and 21 females aged 35 to 59 years, were selected for the effect test. The method of allocating the subjects is as follows.
-Assigned so that the average value of the amount of skin water evaporation on the right cheek of the subject measured at the time of screening was the same for each group as much as possible.
-As for the ages of the subjects, the average values were assigned to each group so as to be as uniform as possible. At that time, the male-female ratio of each group was assigned to be as equal as possible to the male-female ratio at the time of selection.
<試験食品の作製>
試験食品として、表6に示すlactobacillus gasseri N320株の発酵食品である被験食品及びプラセボを作製した。被験食品が含有する本菌株は死菌体である。
<Preparation of test food>
As test foods, a test food and a placebo, which are fermented foods of the lactobacillus gasseri N320 strain shown in Table 6, were prepared. This strain contained in the test food is a dead cell.
<試験方法>
上記被験者を対象にプラセボ対照二重盲検試験を実施した。まず、被験者43名を、乱数を用いた割付表により被験食品摂取群22名、プラセボ摂取群21名に振り分け、各被験者には、1日に1回、各試験食品1本(100ml)を8週間(56日間)摂取させた。被験者の管理事項として、
(1)試験期間中は、日常使用している基礎化粧品の変更および追加は禁止した。
(2)観察前2週間以内の被験部位のむだ毛処理は禁止した。
(3)試験期間中の入浴剤等の使用は禁止した。
(4)観察日前日と観察日当日はボディーローションの使用は禁止した。
(5)試験期間中は、医薬品、新指定医薬部外品および漢方薬の使用は原則として禁止した。
(6)試験期間中は、新たに肌によい効果が明記された加工食品の摂取は原則として禁止した。
(7)試験期間中は、現在日常的に摂取している健康食品および保健機能食品(特定保健用食品及び食品機能性表示該当食品)以外の新規の健康食品、保健機能食品(特定保健用食品)の摂取は禁止した。
(8)試験期間中は、日常の摂取範囲を超えた過剰なアルコールの摂取は禁止した。また、観察日前日のアルコールの摂取は禁止した。
(9)試験期間中は、野外でのスポーツ、人工紫外線照射等、日焼けを起こすような行為は禁止した。日常生活でも屋内外で被験部位が紫外線に直接さらされないように注意した。具体的には、帽子、衣類、通常使用しているサンスクリーン等でケアした。
(10)観察日前3日間の温泉への入浴は禁止した。
(11)試験期間中は、ボディエステや岩盤浴等、肌状態を改善する目的の行為は禁止した。
(12)観察日当日の測定が終わるまでの激しい運動は禁止した。
(13)試験期間中は、海外旅行および海外出張は禁止した。
(14)観察日当日は辛いものなどの刺激性のある食品(カレー、唐辛子、タバスコ等)の摂取は禁止した。
(15)試験期間中は、遠赤外など特殊な効果により保湿効果を示す「暖かい下着等」の使用を禁止した。
(16)観察日前日は必ず就寝前に入浴し、観察日当日の測定前の入浴は禁止した。
(17)試験期間中は、測定部位(右頬)の剃毛・脱毛・除毛は禁止したが、髭処理についてはその限りではない。
<Test method>
A placebo-controlled, double-blind study was conducted on the above subjects. First, 43 subjects were divided into 22 test food intake groups and 21 placebo intake groups according to a random number allocation table, and each subject was given 8 test foods (100 ml) once a day. It was ingested for a week (56 days). As a subject's control item
(1) During the test period, it was prohibited to change or add basic cosmetics that are used daily.
(2) Treatment of waste hair at the test site within 2 weeks before observation was prohibited.
(3) The use of bath salts, etc. during the test period was prohibited.
(4) The use of body lotion was prohibited on the day before and on the day of observation.
(5) During the test period, the use of medicines, newly designated quasi-drugs and Chinese herbs was prohibited in principle.
(6) During the test period, in principle, the intake of processed foods with newly specified beneficial effects on the skin was prohibited.
(7) During the test period, new health foods and foods with health claims (foods for specified health use) other than the health foods and foods with health claims (foods for specified health use and foods with food functional claims) that are currently ingested on a daily basis. ) Was prohibited.
(8) During the test period, excessive alcohol intake beyond the daily intake range was prohibited. In addition, alcohol intake on the day before the observation day was prohibited.
(9) During the test period, activities that cause sunburn, such as outdoor sports and artificial ultraviolet irradiation, were prohibited. Care was taken not to expose the subject site to UV rays indoors or outdoors even in daily life. Specifically, care was taken with a hat, clothing, a sunscreen that is normally used, and the like.
(10) Bathing in hot springs for 3 days before the observation day was prohibited.
(11) During the test period, actions aimed at improving skin condition, such as body esthetics and bedrock baths, were prohibited.
(12) Vigorous exercise was prohibited until the measurement on the day of observation was completed.
(13) During the test period, overseas travel and overseas business trips were prohibited.
(14) On the day of observation, ingestion of irritating foods such as spicy foods (curry, chili pepper, tabasco, etc.) was prohibited.
(15) During the test period, the use of "warm underwear, etc." that show a moisturizing effect due to special effects such as far infrared rays was prohibited.
(16) The day before the observation day, the bath was always taken before going to bed, and the bathing before the measurement on the day of the observation was prohibited.
(17) During the test period, shaving, hair removal, and hair removal at the measurement site (right cheek) were prohibited, but this does not apply to beard treatment.
なお、上記被験者管理事項については遵守することを原則とするが、医療上の必要、その他生命身体の安全に危険を及ぼす場合はこの限りではないものとした。被験者管理事項に反する事項が生じた場合には、被験者は試験実施機関へ直ちに連絡をすることとした。被験者は、観察時期摂取前、摂取4週後、摂取8週後に、皮膚水分量測定、皮膚科医による医師所見、アンケート調査を実施した。 In principle, the above-mentioned subject management items shall be observed, but this shall not apply if there is a medical need or other danger to the safety of life and body. If any matter contrary to the subject management matters arises, the subject will immediately contact the testing organization. The subjects underwent skin water content measurement, doctor's findings by a dermatologist, and a questionnaire survey before, 4 weeks, and 8 weeks after ingestion.
皮膚水分量は、恒温恒湿ルームにおいて被験者の測定部位をCORNEOMETER CM 825((株)インテグラル)を用いて、5ミリずつずらして3箇所測定した。そして、摂取前の水分量測定値(μS)の平均値を100%として、摂取8週後の変化量を評価した。 The skin water content was measured at 3 points in a constant temperature and humidity room by shifting the measurement site of the subject by 5 mm using CORNEOMETER CM 825 (Integral Co., Ltd.). Then, the amount of change after 8 weeks of ingestion was evaluated with the average value of the measured water content (μS) before ingestion as 100%.
皮膚科医による医師所見は、被験者の頬部、上背部、頸部の計3部位を対象とし、乾燥状態を「なし (症状がみられない)」:0、「軽度 (少し症状が見られる)」:1、「中程度 (明らかな症状が見られる)」:2、「重度 (著しい症状が見られる)」:3の4段階で評価した。 The doctor's findings by the dermatologist targeted the subject's cheeks, upper back, and neck, and the dry state was "none (no symptoms)": 0, "mild (slight symptoms are seen). ) ”1,“ Moderate (with obvious symptoms) ”: 2,“ Severe (significant symptoms are seen) ”: 3
アンケート調査は、被験者の「いま現在、気になっているあなたのお肌状態(トラブル)についてお伺い致します」として、メイクのノリの悪さについて、顔のかさつきについて、顔の肌のつやについて、洗顔後のつっぱり感について、1.非常に気になる、2.少し気になる、3.どちらとも言えない、4.あまり気にならない、5.まったく気にならない、の5段階として評価した。 In the questionnaire survey, the subject said, "I would like to ask you about your skin condition (trouble) that you are currently worried about." About the feeling of tension after washing your face 1. Very worrisome 2. I'm a little worried 3. I can't say either 4. I don't really care 5. It was evaluated as 5 grades, which do not bother me at all.
<結果>
被験者の頬部の皮膚水分量を摂取前と摂取後8週間後に測定したところ、図3に示すように、被験食品を摂取した被験者の皮膚水分量(相対値)は、プラセボを摂取した被験者の値と比べて有意に増加した。なお、図3に示す被験食品とプラセボそれぞれの皮膚水分量(相対値)は、解析対象除外基準への抵触が疑われる被験者、検査前に長期間の有害事象が見られた被験者のデータを除外した、プラセボ摂取群の被験者21名、被験食品摂取群の被験者22名の平均値である。
<Result>
When the skin water content of the cheeks of the subjects was measured before and 8 weeks after ingestion, as shown in FIG. 3, the skin water content (relative value) of the subjects who ingested the test food was that of the subjects who ingested the placebo. Significantly increased compared to the value. The skin water content (relative value) of each of the test food and placebo shown in FIG. 3 excludes the data of subjects suspected of violating the analysis target exclusion criteria and subjects who had long-term adverse events before the test. It is the average value of 21 subjects in the placebo intake group and 22 subjects in the test food intake group.
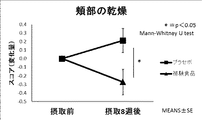
また、図4、図5に示すように、頬部の乾燥、上背部の乾燥に関する皮膚科医による医師の所見評価では、被験食品群のスコアは、摂取8週間でプラセボのスコアと比較して有意に改善した。頸部の乾燥については、図6に示すように被験食品群のスコアはプラセボと比較して摂取8週間で改善傾向が確認された。 In addition, as shown in FIGS. 4 and 5, in the evaluation of the doctor's findings regarding dry cheeks and dry upper back, the score of the test food group was compared with the placebo score at 8 weeks of ingestion. Significantly improved. As for the dryness of the neck, as shown in FIG. 6, the score of the test food group was confirmed to improve at 8 weeks after ingestion as compared with the placebo.
女性についてアンケート結果を解析したところ、図7〜図10に示すように、被験食品群のスコアは、洗顔後のつっぱり感と顔の肌のつやについて摂取8週間でプラセボと比べて有意に改善した。また、メイクのノリの悪さと顔のかさつきについても被験食品群のスコアはプラセボと比べて改善する傾向が認められた。 When the results of the questionnaire were analyzed for females, as shown in FIGS. 7 to 10, the scores of the test food groups were significantly improved compared to placebo in 8 weeks after ingestion of the feeling of tension after washing the face and the gloss of the skin on the face. .. In addition, the scores of the test food group tended to improve compared to placebo in terms of poor make-up and dry face.
乾燥肌の自覚のある者、肌のたるみを感じる者、肌荒れを自覚している者を対象としたプラセボ対照二重盲検の結果、ラクトバチルス属ガセリ種の乳酸菌及びその発酵物を主成分とする皮膚状態改善組成物には、乾燥による、皮膚の水分量の低下、頬や背中、頸の乾燥状態の悪化、洗顔後のつっぱり感、皮膚のつやの低下を含む、皮膚状態の改善に効果があることが確認された。 As a result of a placebo-controlled double-blind study for those who are aware of dry skin, those who feel sagging skin, and those who are aware of rough skin, the main component is lactic acid bacteria of Lactobacillus gasseri species and their fermented products. The skin condition improving composition is effective in improving the skin condition, including a decrease in the water content of the skin due to dryness, deterioration of the dry condition of the cheeks, back and neck, a feeling of tension after washing the face, and a decrease in the gloss of the skin. It was confirmed that there was.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017253491A JP6976843B2 (en) | 2017-12-28 | 2017-12-28 | Skin condition improving agent |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017253491A JP6976843B2 (en) | 2017-12-28 | 2017-12-28 | Skin condition improving agent |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019119689A JP2019119689A (en) | 2019-07-22 |
| JP2019119689A5 JP2019119689A5 (en) | 2019-09-05 |
| JP6976843B2 true JP6976843B2 (en) | 2021-12-08 |
Family
ID=67305977
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017253491A Active JP6976843B2 (en) | 2017-12-28 | 2017-12-28 | Skin condition improving agent |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP6976843B2 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7141245B2 (en) * | 2018-05-29 | 2022-09-22 | 日清食品ホールディングス株式会社 | Oral skin ultraviolet damage reducing agent or skin condition improving agent. |
| CN112618578B (en) * | 2019-09-24 | 2022-03-15 | 大江生医股份有限公司 | Use of leuconostoc mesenteroides TCI007 or metabolite thereof for improving allergic conditions |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2005257033B2 (en) * | 2004-06-23 | 2011-05-12 | L'oreal | Method and compositions useful for preventing and/or treating sensitive and/or dry skin |
| KR20140128675A (en) * | 2013-04-29 | 2014-11-06 | 주식회사한국야쿠르트 | Probiotics of Lactobacillus gasseri HY7025 for skin wrinkle inhibitory and moisturizing effects and use of thereof as skin anti-wrinkle or moisturizing products |
| JP5965368B2 (en) * | 2013-08-30 | 2016-08-03 | 森永乳業株式会社 | Method for producing D-amino acid |
| JP6495869B2 (en) * | 2016-07-15 | 2019-04-03 | 日清食品ホールディングス株式会社 | Lactic acid bacteria with the ability to promote hyaluronic acid production |
-
2017
- 2017-12-28 JP JP2017253491A patent/JP6976843B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019119689A (en) | 2019-07-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5791009B2 (en) | Lactic acid bacteria and food or drink using them | |
| US20100015110A1 (en) | Fermented milk for skin improvement and/or treatment and process for producing the same | |
| TWI387459B (en) | Wrinkle formation inhibitor | |
| JP5903380B2 (en) | Oral skin condition improver | |
| CN106727263B (en) | Skin Tiny ecosystem improves preparation and preparation method thereof | |
| JP6750903B2 (en) | Fermented honey and its manufacturing method | |
| KR102194315B1 (en) | Antioxidant or skin microbiome balance cosmetic composition comprising centella asiatica fermented extract fermented by inoculating lactobacillus rhamnosus strain | |
| KR102015180B1 (en) | Cosmetic composition comprising the fermented milk-cream broth of lactic acid bacteria | |
| US10251919B2 (en) | Method for improving skin condition or anti-microorganisms or anti-oxidation using Lactobacillus sakei MD honeysuckle | |
| TWI689585B (en) | Novel lactic acid strain and immune activating agent containing novel lactic acid strain | |
| WO2018155660A1 (en) | Composition containing dead cell bodies of lactic acid bacterium or treated product thereof, and cosmetic, household good, drug or food containing said composition | |
| KR102553057B1 (en) | Compositions comprising skin derived lactic acid bacteria having effects of anti-oxidation, anti-inflammation and whitening | |
| KR20160110829A (en) | The milk fermented product using Lactobacillus paracasei HY7301 for skin whitening and products containing thereof as effective component | |
| JP6976843B2 (en) | Skin condition improving agent | |
| JP6495869B2 (en) | Lactic acid bacteria with the ability to promote hyaluronic acid production | |
| KR102056916B1 (en) | Novel Lactobacillus bulgaricus UBC-U27 with anti-skin aging or anti-wrinkle activity, and compositions using the same | |
| KR102263454B1 (en) | Skin derived novel lactic acid bacteria having effects of enhancing skin barrier and anti-wrinkle and use thereof | |
| JP6261688B2 (en) | QOL improvement or persistence agent | |
| RU2453591C1 (en) | Strain lactobacillus rhamnosus used for making lactic acid bacillus containing products | |
| KR102485257B1 (en) | Composition comprising lactic acid bacteria derived from camellia japonica for caring damages of skin cells by microdust | |
| CN108938540A (en) | A kind of brilliant profit mildy wash | |
| JP7362081B2 (en) | Novel lactic acid bacteria strains and their uses | |
| JP7141245B2 (en) | Oral skin ultraviolet damage reducing agent or skin condition improving agent. | |
| CN110151672A (en) | A kind of lactobacillus plantarum GMNL-6 composition is used for the purposes of skin care | |
| KR102389753B1 (en) | Skin derived novel Lactobacillus plantarum subsp. shebah-202 strain having effects of enhancing skin barrier and anti-wrinkle and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190726 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200701 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210629 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210804 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20211109 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20211110 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6976843 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |