JP6912852B2 - 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 - Google Patents
対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 Download PDFInfo
- Publication number
- JP6912852B2 JP6912852B2 JP2013540032A JP2013540032A JP6912852B2 JP 6912852 B2 JP6912852 B2 JP 6912852B2 JP 2013540032 A JP2013540032 A JP 2013540032A JP 2013540032 A JP2013540032 A JP 2013540032A JP 6912852 B2 JP6912852 B2 JP 6912852B2
- Authority
- JP
- Japan
- Prior art keywords
- pain
- testosterone
- androgen
- levels
- composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 208000002193 Pain Diseases 0.000 title claims description 326
- 230000036407 pain Effects 0.000 title claims description 227
- 230000001684 chronic effect Effects 0.000 title claims description 94
- 238000000034 method Methods 0.000 title description 84
- 239000008194 pharmaceutical composition Substances 0.000 title 1
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 claims description 628
- 229960003604 testosterone Drugs 0.000 claims description 317
- 210000002966 serum Anatomy 0.000 claims description 167
- 239000000203 mixture Substances 0.000 claims description 116
- 230000001965 increasing effect Effects 0.000 claims description 88
- 206010065390 Inflammatory pain Diseases 0.000 claims description 87
- 208000000114 Pain Threshold Diseases 0.000 claims description 87
- 230000037040 pain threshold Effects 0.000 claims description 87
- 206010002261 Androgen deficiency Diseases 0.000 claims description 81
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 60
- 108010093625 Opioid Peptides Proteins 0.000 claims description 59
- 102000001490 Opioid Peptides Human genes 0.000 claims description 59
- 239000003399 opiate peptide Substances 0.000 claims description 57
- 208000035475 disorder Diseases 0.000 claims description 50
- 206010049119 Emotional distress Diseases 0.000 claims description 45
- 230000009429 distress Effects 0.000 claims description 41
- 230000000694 effects Effects 0.000 claims description 33
- 239000000935 antidepressant agent Substances 0.000 claims description 31
- 229940005513 antidepressants Drugs 0.000 claims description 31
- 210000003743 erythrocyte Anatomy 0.000 claims description 30
- 238000004062 sedimentation Methods 0.000 claims description 30
- 239000003814 drug Substances 0.000 claims description 25
- 229940079593 drug Drugs 0.000 claims description 24
- 102100032752 C-reactive protein Human genes 0.000 claims description 23
- 108010074051 C-Reactive Protein Proteins 0.000 claims description 22
- 238000004519 manufacturing process Methods 0.000 claims description 21
- 108010092674 Enkephalins Proteins 0.000 claims description 16
- URLZCHNOLZSCCA-VABKMULXSA-N Leu-enkephalin Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=CC=C1 URLZCHNOLZSCCA-VABKMULXSA-N 0.000 claims description 16
- 208000014674 injury Diseases 0.000 claims description 15
- 241000282412 Homo Species 0.000 claims description 14
- 230000008733 trauma Effects 0.000 claims description 14
- 230000001225 therapeutic effect Effects 0.000 claims description 13
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 claims description 12
- 206010028980 Neoplasm Diseases 0.000 claims description 11
- 230000001154 acute effect Effects 0.000 claims description 11
- 239000003937 drug carrier Substances 0.000 claims description 11
- 201000011510 cancer Diseases 0.000 claims description 10
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 claims description 10
- 206010016256 fatigue Diseases 0.000 claims description 9
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 claims description 9
- 230000002950 deficient Effects 0.000 claims description 8
- 210000002149 gonad Anatomy 0.000 claims description 8
- 208000015181 infectious disease Diseases 0.000 claims description 8
- 230000002411 adverse Effects 0.000 claims description 7
- 239000000730 antalgic agent Substances 0.000 claims description 7
- 210000001175 cerebrospinal fluid Anatomy 0.000 claims description 7
- 208000028173 post-traumatic stress disease Diseases 0.000 claims description 7
- 208000004454 Hyperalgesia Diseases 0.000 claims description 6
- 229940035676 analgesics Drugs 0.000 claims description 6
- 229960005181 morphine Drugs 0.000 claims description 6
- 239000000014 opioid analgesic Substances 0.000 claims description 6
- 108010049140 Endorphins Proteins 0.000 claims description 5
- 102000009025 Endorphins Human genes 0.000 claims description 5
- 206010020751 Hypersensitivity Diseases 0.000 claims description 5
- 238000001356 surgical procedure Methods 0.000 claims description 5
- 208000023275 Autoimmune disease Diseases 0.000 claims description 4
- 208000026935 allergic disease Diseases 0.000 claims description 4
- 206010053552 allodynia Diseases 0.000 claims description 4
- 230000009610 hypersensitivity Effects 0.000 claims description 4
- 208000036142 Viral infection Diseases 0.000 claims description 3
- 210000005036 nerve Anatomy 0.000 claims description 3
- 230000007823 neuropathy Effects 0.000 claims description 3
- 201000001119 neuropathy Diseases 0.000 claims description 3
- 208000033808 peripheral neuropathy Diseases 0.000 claims description 3
- 208000012201 sexual and gender identity disease Diseases 0.000 claims description 3
- 208000015891 sexual disease Diseases 0.000 claims description 3
- 230000009385 viral infection Effects 0.000 claims description 3
- 108010065372 Dynorphins Proteins 0.000 claims description 2
- 102400000925 Opiorphin Human genes 0.000 claims description 2
- TWWFCOBVAKAKIT-SXYSDOLCSA-N Opiorphin Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)N)CC1=CC=CC=C1 TWWFCOBVAKAKIT-SXYSDOLCSA-N 0.000 claims description 2
- 102100024622 Proenkephalin-B Human genes 0.000 claims description 2
- 108700003635 adrenorphin Proteins 0.000 claims description 2
- XJOQRTJDYAHKPY-YVWIMRNGSA-N adrenorphin Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(N)=O)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=CC=C1 XJOQRTJDYAHKPY-YVWIMRNGSA-N 0.000 claims description 2
- 108010089466 amidorphin Proteins 0.000 claims description 2
- BRCPMMMERAGFCE-ZIFJQKEFSA-N amidorphin Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=CC=C1 BRCPMMMERAGFCE-ZIFJQKEFSA-N 0.000 claims description 2
- 108010074283 glutaminyl-arginyl-phenylalanyl-seryl-arginine Proteins 0.000 claims description 2
- 208000024732 dysthymic disease Diseases 0.000 claims 2
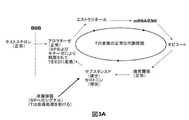
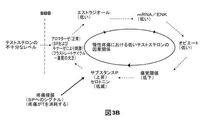
- 239000003098 androgen Substances 0.000 description 377
- 238000011282 treatment Methods 0.000 description 83
- 238000002560 therapeutic procedure Methods 0.000 description 81
- 229940030486 androgens Drugs 0.000 description 80
- 208000024891 symptom Diseases 0.000 description 78
- 208000000094 Chronic Pain Diseases 0.000 description 77
- 238000012360 testing method Methods 0.000 description 63
- 239000000499 gel Substances 0.000 description 53
- 230000035882 stress Effects 0.000 description 46
- 208000001640 Fibromyalgia Diseases 0.000 description 37
- 102100024304 Protachykinin-1 Human genes 0.000 description 33
- 238000009472 formulation Methods 0.000 description 33
- 239000003163 gonadal steroid hormone Substances 0.000 description 30
- 101800003906 Substance P Proteins 0.000 description 29
- 238000009093 first-line therapy Methods 0.000 description 29
- QDZOEBFLNHCSSF-PFFBOGFISA-N (2S)-2-[[(2R)-2-[[(2S)-1-[(2S)-6-amino-2-[[(2S)-1-[(2R)-2-amino-5-carbamimidamidopentanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-N-[(2R)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2S)-1-amino-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]pentanediamide Chemical compound C([C@@H](C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O)NC(=O)[C@@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](N)CCCNC(N)=N)C1=CC=CC=C1 QDZOEBFLNHCSSF-PFFBOGFISA-N 0.000 description 28
- 210000003169 central nervous system Anatomy 0.000 description 26
- 229940088597 hormone Drugs 0.000 description 25
- 239000005556 hormone Substances 0.000 description 25
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 24
- 229940011871 estrogen Drugs 0.000 description 23
- 239000000262 estrogen Substances 0.000 description 23
- 230000007246 mechanism Effects 0.000 description 23
- 230000003247 decreasing effect Effects 0.000 description 22
- -1 fluorimesterone Chemical compound 0.000 description 22
- 229940005483 opioid analgesics Drugs 0.000 description 22
- 230000036642 wellbeing Effects 0.000 description 22
- 108010078554 Aromatase Proteins 0.000 description 21
- 102000014654 Aromatase Human genes 0.000 description 21
- 230000003040 nociceptive effect Effects 0.000 description 20
- 210000000278 spinal cord Anatomy 0.000 description 20
- 230000008901 benefit Effects 0.000 description 19
- 230000036541 health Effects 0.000 description 19
- 230000004044 response Effects 0.000 description 19
- 210000002569 neuron Anatomy 0.000 description 18
- 230000000975 bioactive effect Effects 0.000 description 17
- 230000008499 blood brain barrier function Effects 0.000 description 16
- 210000001218 blood-brain barrier Anatomy 0.000 description 16
- 201000010066 hyperandrogenism Diseases 0.000 description 16
- 230000008058 pain sensation Effects 0.000 description 16
- 150000001875 compounds Chemical class 0.000 description 15
- 229960005309 estradiol Drugs 0.000 description 15
- 230000002829 reductive effect Effects 0.000 description 15
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 14
- 230000002757 inflammatory effect Effects 0.000 description 13
- 229940068196 placebo Drugs 0.000 description 13
- 239000000902 placebo Substances 0.000 description 13
- 241001465754 Metazoa Species 0.000 description 12
- 208000005298 acute pain Diseases 0.000 description 12
- 230000007812 deficiency Effects 0.000 description 12
- 239000003795 chemical substances by application Substances 0.000 description 11
- 230000037361 pathway Effects 0.000 description 11
- 230000002093 peripheral effect Effects 0.000 description 11
- 230000002992 thymic effect Effects 0.000 description 11
- GCKMFJBGXUYNAG-HLXURNFRSA-N Methyltestosterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)CC2 GCKMFJBGXUYNAG-HLXURNFRSA-N 0.000 description 10
- 230000007423 decrease Effects 0.000 description 10
- 201000010099 disease Diseases 0.000 description 10
- 210000004556 brain Anatomy 0.000 description 9
- 229940121991 Serotonin and norepinephrine reuptake inhibitor Drugs 0.000 description 8
- 108010089417 Sex Hormone-Binding Globulin Proteins 0.000 description 8
- 102100030758 Sex hormone-binding globulin Human genes 0.000 description 8
- 210000001744 T-lymphocyte Anatomy 0.000 description 8
- 238000003556 assay Methods 0.000 description 8
- 238000001514 detection method Methods 0.000 description 8
- 239000003623 enhancer Substances 0.000 description 8
- 210000000987 immune system Anatomy 0.000 description 8
- 238000012423 maintenance Methods 0.000 description 8
- 210000000653 nervous system Anatomy 0.000 description 8
- 229940127240 opiate Drugs 0.000 description 8
- 230000035935 pregnancy Effects 0.000 description 8
- 230000001105 regulatory effect Effects 0.000 description 8
- 229940124834 selective serotonin reuptake inhibitor Drugs 0.000 description 8
- 239000012896 selective serotonin reuptake inhibitor Substances 0.000 description 8
- 239000003775 serotonin noradrenalin reuptake inhibitor Substances 0.000 description 8
- 241000700159 Rattus Species 0.000 description 7
- 238000009098 adjuvant therapy Methods 0.000 description 7
- 108010080146 androgen receptors Proteins 0.000 description 7
- 238000010171 animal model Methods 0.000 description 7
- 230000008859 change Effects 0.000 description 7
- 238000011156 evaluation Methods 0.000 description 7
- 230000006870 function Effects 0.000 description 7
- 238000002657 hormone replacement therapy Methods 0.000 description 7
- 230000004054 inflammatory process Effects 0.000 description 7
- 230000007774 longterm Effects 0.000 description 7
- CWWARWOPSKGELM-SARDKLJWSA-N methyl (2s)-2-[[(2s)-2-[[2-[[(2s)-2-[[(2s)-2-[[(2s)-5-amino-2-[[(2s)-5-amino-2-[[(2s)-1-[(2s)-6-amino-2-[[(2s)-1-[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-5 Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)OC)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CCCN=C(N)N)C1=CC=CC=C1 CWWARWOPSKGELM-SARDKLJWSA-N 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 229940076279 serotonin Drugs 0.000 description 7
- 230000037317 transdermal delivery Effects 0.000 description 7
- NVKAWKQGWWIWPM-ABEVXSGRSA-N 17-β-hydroxy-5-α-Androstan-3-one Chemical compound C1C(=O)CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 NVKAWKQGWWIWPM-ABEVXSGRSA-N 0.000 description 6
- GCKMFJBGXUYNAG-UHFFFAOYSA-N 17alpha-methyltestosterone Natural products C1CC2=CC(=O)CCC2(C)C2C1C1CCC(C)(O)C1(C)CC2 GCKMFJBGXUYNAG-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 206010020112 Hirsutism Diseases 0.000 description 6
- 102000003840 Opioid Receptors Human genes 0.000 description 6
- 108090000137 Opioid Receptors Proteins 0.000 description 6
- 230000002159 abnormal effect Effects 0.000 description 6
- 239000002671 adjuvant Substances 0.000 description 6
- 238000009165 androgen replacement therapy Methods 0.000 description 6
- AEMFNILZOJDQLW-QAGGRKNESA-N androst-4-ene-3,17-dione Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1 AEMFNILZOJDQLW-QAGGRKNESA-N 0.000 description 6
- 229960003473 androstanolone Drugs 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 206010003246 arthritis Diseases 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 229930182833 estradiol Natural products 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 229960001566 methyltestosterone Drugs 0.000 description 6
- 210000003205 muscle Anatomy 0.000 description 6
- 239000002243 precursor Substances 0.000 description 6
- 239000000583 progesterone congener Substances 0.000 description 6
- 102000005962 receptors Human genes 0.000 description 6
- 108020003175 receptors Proteins 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 230000035807 sensation Effects 0.000 description 6
- 230000001629 suppression Effects 0.000 description 6
- 206010012289 Dementia Diseases 0.000 description 5
- 206010061218 Inflammation Diseases 0.000 description 5
- 208000001294 Nociceptive Pain Diseases 0.000 description 5
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical class C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 5
- 230000001919 adrenal effect Effects 0.000 description 5
- 229960005471 androstenedione Drugs 0.000 description 5
- AEMFNILZOJDQLW-UHFFFAOYSA-N androstenedione Natural products O=C1CCC2(C)C3CCC(C)(C(CC4)=O)C4C3CCC2=C1 AEMFNILZOJDQLW-UHFFFAOYSA-N 0.000 description 5
- 230000001430 anti-depressive effect Effects 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 230000036765 blood level Effects 0.000 description 5
- 239000002775 capsule Substances 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- FMGSKLZLMKYGDP-USOAJAOKSA-N dehydroepiandrosterone Chemical compound C1[C@@H](O)CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC=C21 FMGSKLZLMKYGDP-USOAJAOKSA-N 0.000 description 5
- 230000001976 improved effect Effects 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 230000037353 metabolic pathway Effects 0.000 description 5
- 230000004060 metabolic process Effects 0.000 description 5
- 239000002858 neurotransmitter agent Substances 0.000 description 5
- 230000036470 plasma concentration Effects 0.000 description 5
- 230000003389 potentiating effect Effects 0.000 description 5
- 230000003938 response to stress Effects 0.000 description 5
- 230000036299 sexual function Effects 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- 239000000829 suppository Substances 0.000 description 5
- 201000004384 Alopecia Diseases 0.000 description 4
- 102100032187 Androgen receptor Human genes 0.000 description 4
- 208000019901 Anxiety disease Diseases 0.000 description 4
- 206010006187 Breast cancer Diseases 0.000 description 4
- 208000026310 Breast neoplasm Diseases 0.000 description 4
- 208000024172 Cardiovascular disease Diseases 0.000 description 4
- FMGSKLZLMKYGDP-UHFFFAOYSA-N Dehydroepiandrosterone Natural products C1C(O)CCC2(C)C3CCC(C)(C(CC4)=O)C4C3CC=C21 FMGSKLZLMKYGDP-UHFFFAOYSA-N 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 102100038595 Estrogen receptor Human genes 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 4
- 101600111816 Homo sapiens Sex hormone-binding globulin (isoform 1) Proteins 0.000 description 4
- 102100027467 Pro-opiomelanocortin Human genes 0.000 description 4
- 102300044179 Sex hormone-binding globulin isoform 1 Human genes 0.000 description 4
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 description 4
- 210000004100 adrenal gland Anatomy 0.000 description 4
- 230000033228 biological regulation Effects 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 206010012601 diabetes mellitus Diseases 0.000 description 4
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 4
- 238000012377 drug delivery Methods 0.000 description 4
- 229960002464 fluoxetine Drugs 0.000 description 4
- 239000003862 glucocorticoid Substances 0.000 description 4
- 230000002710 gonadal effect Effects 0.000 description 4
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 description 4
- 229940035638 gonadotropin-releasing hormone Drugs 0.000 description 4
- 208000019423 liver disease Diseases 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 230000036651 mood Effects 0.000 description 4
- 210000001672 ovary Anatomy 0.000 description 4
- 230000008050 pain signaling Effects 0.000 description 4
- 239000008188 pellet Substances 0.000 description 4
- 230000000144 pharmacologic effect Effects 0.000 description 4
- 229960002847 prasterone Drugs 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 208000020016 psychiatric disease Diseases 0.000 description 4
- 230000001850 reproductive effect Effects 0.000 description 4
- 210000003491 skin Anatomy 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- 229940037128 systemic glucocorticoids Drugs 0.000 description 4
- HPFVBGJFAYZEBE-ZLQWOROUSA-N testosterone cypionate Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(CCC(=O)C=C4CC3)C)CC[C@@]21C)C(=O)CCC1CCCC1 HPFVBGJFAYZEBE-ZLQWOROUSA-N 0.000 description 4
- 229960000921 testosterone cypionate Drugs 0.000 description 4
- VOCBWIIFXDYGNZ-IXKNJLPQSA-N testosterone enanthate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](OC(=O)CCCCCC)[C@@]1(C)CC2 VOCBWIIFXDYGNZ-IXKNJLPQSA-N 0.000 description 4
- 229960003484 testosterone enanthate Drugs 0.000 description 4
- 150000003515 testosterones Chemical class 0.000 description 4
- 238000011200 topical administration Methods 0.000 description 4
- 238000012549 training Methods 0.000 description 4
- RTHCYVBBDHJXIQ-MRXNPFEDSA-N (R)-fluoxetine Chemical compound O([C@H](CCNC)C=1C=CC=CC=1)C1=CC=C(C(F)(F)F)C=C1 RTHCYVBBDHJXIQ-MRXNPFEDSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 208000002874 Acne Vulgaris Diseases 0.000 description 3
- 201000000736 Amenorrhea Diseases 0.000 description 3
- 206010001928 Amenorrhoea Diseases 0.000 description 3
- 108010087765 Antipain Proteins 0.000 description 3
- 208000017667 Chronic Disease Diseases 0.000 description 3
- 239000000055 Corticotropin-Releasing Hormone Substances 0.000 description 3
- 238000009007 Diagnostic Kit Methods 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 208000033830 Hot Flashes Diseases 0.000 description 3
- 206010060800 Hot flush Diseases 0.000 description 3
- 206010062767 Hypophysitis Diseases 0.000 description 3
- 208000000112 Myalgia Diseases 0.000 description 3
- JKWKMORAXJQQSR-MOPIKTETSA-N Nandrolone Decanoate Chemical compound C1CC2=CC(=O)CC[C@@H]2[C@@H]2[C@@H]1[C@@H]1CC[C@H](OC(=O)CCCCCCCCC)[C@@]1(C)CC2 JKWKMORAXJQQSR-MOPIKTETSA-N 0.000 description 3
- 206010030113 Oedema Diseases 0.000 description 3
- QSLJIVKCVHQPLV-PEMPUTJUSA-N Oxandrin Chemical compound C([C@@H]1CC2)C(=O)OC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@](C)(O)[C@@]2(C)CC1 QSLJIVKCVHQPLV-PEMPUTJUSA-N 0.000 description 3
- 102000045595 Phosphoprotein Phosphatases Human genes 0.000 description 3
- 108700019535 Phosphoprotein Phosphatases Proteins 0.000 description 3
- ORNBQBCIOKFOEO-YQUGOWONSA-N Pregnenolone Natural products O=C(C)[C@@H]1[C@@]2(C)[C@H]([C@H]3[C@@H]([C@]4(C)C(=CC3)C[C@@H](O)CC4)CC2)CC1 ORNBQBCIOKFOEO-YQUGOWONSA-N 0.000 description 3
- 102000001253 Protein Kinase Human genes 0.000 description 3
- 201000001880 Sexual dysfunction Diseases 0.000 description 3
- 208000013738 Sleep Initiation and Maintenance disease Diseases 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- LKAJKIOFIWVMDJ-IYRCEVNGSA-N Stanazolol Chemical compound C([C@@H]1CC[C@H]2[C@@H]3CC[C@@]([C@]3(CC[C@@H]2[C@@]1(C)C1)C)(O)C)C2=C1C=NN2 LKAJKIOFIWVMDJ-IYRCEVNGSA-N 0.000 description 3
- 229940123445 Tricyclic antidepressant Drugs 0.000 description 3
- 206010000496 acne Diseases 0.000 description 3
- 210000000577 adipose tissue Anatomy 0.000 description 3
- 238000011360 adjunctive therapy Methods 0.000 description 3
- 231100000540 amenorrhea Toxicity 0.000 description 3
- 230000036592 analgesia Effects 0.000 description 3
- 102000001307 androgen receptors Human genes 0.000 description 3
- SDNYTAYICBFYFH-TUFLPTIASA-N antipain Chemical compound NC(N)=NCCC[C@@H](C=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 SDNYTAYICBFYFH-TUFLPTIASA-N 0.000 description 3
- 230000036506 anxiety Effects 0.000 description 3
- 239000007900 aqueous suspension Substances 0.000 description 3
- 230000002146 bilateral effect Effects 0.000 description 3
- 210000000988 bone and bone Anatomy 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 230000010485 coping Effects 0.000 description 3
- POZRVZJJTULAOH-LHZXLZLDSA-N danazol Chemical compound C1[C@]2(C)[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=CC2=C1C=NO2 POZRVZJJTULAOH-LHZXLZLDSA-N 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000002405 diagnostic procedure Methods 0.000 description 3
- 230000002996 emotional effect Effects 0.000 description 3
- 230000013632 homeostatic process Effects 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 230000028709 inflammatory response Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 206010022437 insomnia Diseases 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 229960001935 nandrolone decanoate Drugs 0.000 description 3
- 229960000464 oxandrolone Drugs 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 230000007170 pathology Effects 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 230000035479 physiological effects, processes and functions Effects 0.000 description 3
- 210000003635 pituitary gland Anatomy 0.000 description 3
- 229960000249 pregnenolone Drugs 0.000 description 3
- ORNBQBCIOKFOEO-QGVNFLHTSA-N pregnenolone Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 ORNBQBCIOKFOEO-QGVNFLHTSA-N 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 239000000651 prodrug Substances 0.000 description 3
- 229940002612 prodrug Drugs 0.000 description 3
- 108060006633 protein kinase Proteins 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 230000008433 psychological processes and functions Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- VGKDLMBJGBXTGI-SJCJKPOMSA-N sertraline Chemical compound C1([C@@H]2CC[C@@H](C3=CC=CC=C32)NC)=CC=C(Cl)C(Cl)=C1 VGKDLMBJGBXTGI-SJCJKPOMSA-N 0.000 description 3
- 229960002073 sertraline Drugs 0.000 description 3
- 231100000872 sexual dysfunction Toxicity 0.000 description 3
- 230000001568 sexual effect Effects 0.000 description 3
- 230000007958 sleep Effects 0.000 description 3
- 229960000912 stanozolol Drugs 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 230000002459 sustained effect Effects 0.000 description 3
- 210000001541 thymus gland Anatomy 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- 239000003029 tricyclic antidepressant agent Substances 0.000 description 3
- 230000004584 weight gain Effects 0.000 description 3
- 235000019786 weight gain Nutrition 0.000 description 3
- ZEUITGRIYCTCEM-KRWDZBQOSA-N (S)-duloxetine Chemical compound C1([C@@H](OC=2C3=CC=CC=C3C=CC=2)CCNC)=CC=CS1 ZEUITGRIYCTCEM-KRWDZBQOSA-N 0.000 description 2
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 2
- 208000030507 AIDS Diseases 0.000 description 2
- 208000012639 Balance disease Diseases 0.000 description 2
- 101800005049 Beta-endorphin Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 108010066551 Cholestenone 5 alpha-Reductase Proteins 0.000 description 2
- 206010010144 Completed suicide Diseases 0.000 description 2
- 102400000739 Corticotropin Human genes 0.000 description 2
- 101800000414 Corticotropin Proteins 0.000 description 2
- 102000012289 Corticotropin-Releasing Hormone Human genes 0.000 description 2
- 108010022152 Corticotropin-Releasing Hormone Proteins 0.000 description 2
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 2
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 2
- 241001539473 Euphoria Species 0.000 description 2
- 206010015535 Euphoric mood Diseases 0.000 description 2
- 102000004300 GABA-A Receptors Human genes 0.000 description 2
- 108090000839 GABA-A Receptors Proteins 0.000 description 2
- 206010019233 Headaches Diseases 0.000 description 2
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 2
- 101000831616 Homo sapiens Protachykinin-1 Proteins 0.000 description 2
- 208000035154 Hyperesthesia Diseases 0.000 description 2
- 208000008454 Hyperhidrosis Diseases 0.000 description 2
- 206010058359 Hypogonadism Diseases 0.000 description 2
- 206010021067 Hypopituitarism Diseases 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 108090000862 Ion Channels Proteins 0.000 description 2
- 102000004310 Ion Channels Human genes 0.000 description 2
- 206010067125 Liver injury Diseases 0.000 description 2
- 208000008930 Low Back Pain Diseases 0.000 description 2
- 239000004909 Moisturizer Substances 0.000 description 2
- 208000019022 Mood disease Diseases 0.000 description 2
- 206010027951 Mood swings Diseases 0.000 description 2
- HOKKHZGPKSLGJE-GSVOUGTGSA-N N-Methyl-D-aspartic acid Chemical compound CN[C@@H](C(O)=O)CC(O)=O HOKKHZGPKSLGJE-GSVOUGTGSA-N 0.000 description 2
- 206010028813 Nausea Diseases 0.000 description 2
- 206010029216 Nervousness Diseases 0.000 description 2
- 101800000399 Neurokinin A Proteins 0.000 description 2
- 208000001132 Osteoporosis Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- 108010069820 Pro-Opiomelanocortin Proteins 0.000 description 2
- 239000000683 Pro-Opiomelanocortin Substances 0.000 description 2
- 208000025747 Rheumatic disease Diseases 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- 206010040880 Skin irritation Diseases 0.000 description 2
- 208000028911 Temporomandibular Joint disease Diseases 0.000 description 2
- 206010044565 Tremor Diseases 0.000 description 2
- 206010046788 Uterine haemorrhage Diseases 0.000 description 2
- 208000021017 Weight Gain Diseases 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000006978 adaptation Effects 0.000 description 2
- 230000032683 aging Effects 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 231100000360 alopecia Toxicity 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 239000003263 anabolic agent Substances 0.000 description 2
- 230000000202 analgesic effect Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 229940094957 androgens and estrogen Drugs 0.000 description 2
- 208000007502 anemia Diseases 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 230000002280 anti-androgenic effect Effects 0.000 description 2
- 239000000051 antiandrogen Substances 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 238000003339 best practice Methods 0.000 description 2
- WOPZMFQRCBYPJU-NTXHZHDSSA-N beta-endorphin Chemical compound C([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CCSC)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C1=CC=CC=C1 WOPZMFQRCBYPJU-NTXHZHDSSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000008236 biological pathway Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000037326 chronic stress Effects 0.000 description 2
- 230000007012 clinical effect Effects 0.000 description 2
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 2
- 238000009225 cognitive behavioral therapy Methods 0.000 description 2
- IDLFZVILOHSSID-OVLDLUHVSA-N corticotropin Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)NC(=O)[C@@H](N)CO)C1=CC=C(O)C=C1 IDLFZVILOHSSID-OVLDLUHVSA-N 0.000 description 2
- 229960000258 corticotropin Drugs 0.000 description 2
- 229940041967 corticotropin-releasing hormone Drugs 0.000 description 2
- KLVRDXBAMSPYKH-RKYZNNDCSA-N corticotropin-releasing hormone (human) Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(N)=O)[C@@H](C)CC)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1N(CCC1)C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CO)[C@@H](C)CC)C(C)C)C(C)C)C1=CNC=N1 KLVRDXBAMSPYKH-RKYZNNDCSA-N 0.000 description 2
- 229960004544 cortisone Drugs 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000000688 desorption electrospray ionisation Methods 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 208000002173 dizziness Diseases 0.000 description 2
- 229960003638 dopamine Drugs 0.000 description 2
- 230000003828 downregulation Effects 0.000 description 2
- 238000002651 drug therapy Methods 0.000 description 2
- 229960002866 duloxetine Drugs 0.000 description 2
- 230000000773 effect on pain Effects 0.000 description 2
- 239000012636 effector Substances 0.000 description 2
- 108010038795 estrogen receptors Proteins 0.000 description 2
- 230000003090 exacerbative effect Effects 0.000 description 2
- 230000002964 excitative effect Effects 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 2
- 108010047064 gamma-Endorphin Proteins 0.000 description 2
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 238000009499 grossing Methods 0.000 description 2
- 208000024963 hair loss Diseases 0.000 description 2
- 230000003676 hair loss Effects 0.000 description 2
- 231100000869 headache Toxicity 0.000 description 2
- 230000005802 health problem Effects 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 231100000234 hepatic damage Toxicity 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 210000003016 hypothalamus Anatomy 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 210000004969 inflammatory cell Anatomy 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 108091008042 inhibitory receptors Proteins 0.000 description 2
- 238000011221 initial treatment Methods 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 230000003907 kidney function Effects 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 230000008818 liver damage Effects 0.000 description 2
- 230000005976 liver dysfunction Effects 0.000 description 2
- 230000003908 liver function Effects 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000011004 medical lab test Methods 0.000 description 2
- 230000005906 menstruation Effects 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000001333 moisturizer Effects 0.000 description 2
- 229940035363 muscle relaxants Drugs 0.000 description 2
- 239000003158 myorelaxant agent Substances 0.000 description 2
- UBWXUGDQUBIEIZ-QNTYDACNSA-N nandrolone phenpropionate Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@H]4CCC(=O)C=C4CC3)CC[C@@]21C)C(=O)CCC1=CC=CC=C1 UBWXUGDQUBIEIZ-QNTYDACNSA-N 0.000 description 2
- 229960001133 nandrolone phenpropionate Drugs 0.000 description 2
- 230000008693 nausea Effects 0.000 description 2
- 208000004296 neuralgia Diseases 0.000 description 2
- 230000000955 neuroendocrine Effects 0.000 description 2
- 210000000929 nociceptor Anatomy 0.000 description 2
- 238000009806 oophorectomy Methods 0.000 description 2
- 230000037324 pain perception Effects 0.000 description 2
- 230000008533 pain sensitivity Effects 0.000 description 2
- 230000007310 pathophysiology Effects 0.000 description 2
- 239000008177 pharmaceutical agent Substances 0.000 description 2
- 230000003863 physical function Effects 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000004043 responsiveness Effects 0.000 description 2
- 230000000284 resting effect Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 229940125723 sedative agent Drugs 0.000 description 2
- 239000000932 sedative agent Substances 0.000 description 2
- 239000000849 selective androgen receptor modulator Substances 0.000 description 2
- 230000035946 sexual desire Effects 0.000 description 2
- 230000036556 skin irritation Effects 0.000 description 2
- 231100000475 skin irritation Toxicity 0.000 description 2
- 208000019116 sleep disease Diseases 0.000 description 2
- 208000020685 sleep-wake disease Diseases 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 239000003270 steroid hormone Substances 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 230000035900 sweating Effects 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 108060008037 tachykinin Proteins 0.000 description 2
- 210000001550 testis Anatomy 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- GASYAMBJHBRTOE-WHDBNHDESA-N γ-endorphin Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=CC=C1 GASYAMBJHBRTOE-WHDBNHDESA-N 0.000 description 2
- AHOUBRCZNHFOSL-YOEHRIQHSA-N (+)-Casbol Chemical compound C1=CC(F)=CC=C1[C@H]1[C@H](COC=2C=C3OCOC3=CC=2)CNCC1 AHOUBRCZNHFOSL-YOEHRIQHSA-N 0.000 description 1
- XMAYWYJOQHXEEK-OZXSUGGESA-N (2R,4S)-ketoconazole Chemical compound C1CN(C(=O)C)CCN1C(C=C1)=CC=C1OC[C@@H]1O[C@@](CN2C=NC=C2)(C=2C(=CC(Cl)=CC=2)Cl)OC1 XMAYWYJOQHXEEK-OZXSUGGESA-N 0.000 description 1
- YKFCISHFRZHKHY-NGQGLHOPSA-N (2s)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid;trihydrate Chemical compound O.O.O.OC(=O)[C@](N)(C)CC1=CC=C(O)C(O)=C1.OC(=O)[C@](N)(C)CC1=CC=C(O)C(O)=C1 YKFCISHFRZHKHY-NGQGLHOPSA-N 0.000 description 1
- PLSDWRYNZIVQKX-BGKYVOMCSA-N (3S)-3-amino-4-[[(2S)-1-[[2-[[(2S)-1-[[2-[[(2S)-5-amino-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S,3R)-1-[[(2S)-3-carboxy-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(1S)-1-carboxy-3-methylsulfanylpropyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-2-oxoethyl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-1-oxopropan-2-yl]amino]-4-oxobutanoic acid Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC2=CNC=N2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)CNC(=O)[C@H](CC4=CNC=N4)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(=O)O)N PLSDWRYNZIVQKX-BGKYVOMCSA-N 0.000 description 1
- HEAUFJZALFKPBA-JPQUDPSNSA-N (3s)-3-[[(2s,3r)-2-[[(2s)-6-amino-2-[[(2s)-2-amino-3-(1h-imidazol-5-yl)propanoyl]amino]hexanoyl]amino]-3-hydroxybutanoyl]amino]-4-[[(2s)-1-[[(2s)-1-[[(2s)-1-[[2-[[(2s)-1-[[(2s)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amin Chemical compound C([C@@H](C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)C(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)C1=CC=CC=C1 HEAUFJZALFKPBA-JPQUDPSNSA-N 0.000 description 1
- IBAFAEHLNXQMQA-OFLQVFCSSA-N (8r,9s,10s,13s,14s)-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15-dodecahydro-1h-cyclopenta[a]phenanthrene-3,17-diol Chemical compound C1C(O)CC[C@]2(C)[C@H]3CC[C@](C)(C(=CC4)O)[C@@H]4[C@@H]3CCC21 IBAFAEHLNXQMQA-OFLQVFCSSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- METKIMKYRPQLGS-GFCCVEGCSA-N (R)-atenolol Chemical compound CC(C)NC[C@@H](O)COC1=CC=C(CC(N)=O)C=C1 METKIMKYRPQLGS-GFCCVEGCSA-N 0.000 description 1
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- DBPWSSGDRRHUNT-UHFFFAOYSA-N 17alpha-hydroxy progesterone Natural products C1CC2=CC(=O)CCC2(C)C2C1C1CCC(C(=O)C)(O)C1(C)CC2 DBPWSSGDRRHUNT-UHFFFAOYSA-N 0.000 description 1
- DBPWSSGDRRHUNT-CEGNMAFCSA-N 17α-hydroxyprogesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 DBPWSSGDRRHUNT-CEGNMAFCSA-N 0.000 description 1
- SVUOLADPCWQTTE-UHFFFAOYSA-N 1h-1,2-benzodiazepine Chemical compound N1N=CC=CC2=CC=CC=C12 SVUOLADPCWQTTE-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- CBMYJHIOYJEBSB-KHOSGYARSA-N 5alpha-androstane-3alpha,17beta-diol Chemical compound C1[C@H](O)CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 CBMYJHIOYJEBSB-KHOSGYARSA-N 0.000 description 1
- USSIQXCVUWKGNF-UHFFFAOYSA-N 6-(dimethylamino)-4,4-diphenylheptan-3-one Chemical compound C=1C=CC=CC=1C(CC(C)N(C)C)(C(=O)CC)C1=CC=CC=C1 USSIQXCVUWKGNF-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 208000004611 Abdominal Obesity Diseases 0.000 description 1
- 206010000234 Abortion spontaneous Diseases 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- 208000020576 Adrenal disease Diseases 0.000 description 1
- 206010001367 Adrenal insufficiency Diseases 0.000 description 1
- 239000000275 Adrenocorticotropic Hormone Substances 0.000 description 1
- 108700022183 Ala(2)-MePhe(4)-Gly(5)- Enkephalin Proteins 0.000 description 1
- 208000007848 Alcoholism Diseases 0.000 description 1
- PQSUYGKTWSAVDQ-ZVIOFETBSA-N Aldosterone Chemical compound C([C@@]1([C@@H](C(=O)CO)CC[C@H]1[C@@H]1CC2)C=O)[C@H](O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 PQSUYGKTWSAVDQ-ZVIOFETBSA-N 0.000 description 1
- PQSUYGKTWSAVDQ-UHFFFAOYSA-N Aldosterone Natural products C1CC2C3CCC(C(=O)CO)C3(C=O)CC(O)C2C2(C)C1=CC(=O)CC2 PQSUYGKTWSAVDQ-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 208000000044 Amnesia Diseases 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 206010056292 Androgen-Insensitivity Syndrome Diseases 0.000 description 1
- QADHLRWLCPCEKT-UHFFFAOYSA-N Androstenediol Natural products C1C(O)CCC2(C)C3CCC(C)(C(CC4)O)C4C3CC=C21 QADHLRWLCPCEKT-UHFFFAOYSA-N 0.000 description 1
- 208000000103 Anorexia Nervosa Diseases 0.000 description 1
- 208000034048 Asymptomatic disease Diseases 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- 206010058019 Cancer Pain Diseases 0.000 description 1
- 101000841393 Candida albicans Probable NADPH dehydrogenase Proteins 0.000 description 1
- 206010065941 Central obesity Diseases 0.000 description 1
- GJSURZIOUXUGAL-UHFFFAOYSA-N Clonidine Chemical compound ClC1=CC=CC(Cl)=C1NC1=NCCN1 GJSURZIOUXUGAL-UHFFFAOYSA-N 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 206010010214 Compression fracture Diseases 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- OMFXVFTZEKFJBZ-UHFFFAOYSA-N Corticosterone Natural products O=C1CCC2(C)C3C(O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 OMFXVFTZEKFJBZ-UHFFFAOYSA-N 0.000 description 1
- 108700022182 D-Penicillamine (2,5)- Enkephalin Proteins 0.000 description 1
- MCMMCRYPQBNCPH-WMIMKTLMSA-N DPDPE Chemical compound C([C@H](N)C(=O)N[C@@H]1C(C)(C)SSC([C@@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)CNC1=O)C(O)=O)(C)C)C1=CC=C(O)C=C1 MCMMCRYPQBNCPH-WMIMKTLMSA-N 0.000 description 1
- 206010012335 Dependence Diseases 0.000 description 1
- 208000020401 Depressive disease Diseases 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 206010070840 Gastrointestinal tract irritation Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 206010056557 Gulf war syndrome Diseases 0.000 description 1
- 206010019196 Head injury Diseases 0.000 description 1
- 206010019851 Hepatotoxicity Diseases 0.000 description 1
- GVGLGOZIDCSQPN-PVHGPHFFSA-N Heroin Chemical compound O([C@H]1[C@H](C=C[C@H]23)OC(C)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4OC(C)=O GVGLGOZIDCSQPN-PVHGPHFFSA-N 0.000 description 1
- 206010020100 Hip fracture Diseases 0.000 description 1
- 101000655188 Homo sapiens Tachykinin-3 Proteins 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 206010020850 Hyperthyroidism Diseases 0.000 description 1
- 206010020864 Hypertrichosis Diseases 0.000 description 1
- 206010022998 Irritability Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 208000017924 Klinefelter Syndrome Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 108010022337 Leucine Enkephalin Proteins 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- 102000009151 Luteinizing Hormone Human genes 0.000 description 1
- 108010073521 Luteinizing Hormone Proteins 0.000 description 1
- 208000026139 Memory disease Diseases 0.000 description 1
- 206010027304 Menopausal symptoms Diseases 0.000 description 1
- YFGBQHOOROIVKG-FKBYEOEOSA-N Met-enkephalin Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(O)=O)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=CC=C1 YFGBQHOOROIVKG-FKBYEOEOSA-N 0.000 description 1
- 108010042237 Methionine Enkephalin Proteins 0.000 description 1
- 208000019695 Migraine disease Diseases 0.000 description 1
- 206010027603 Migraine headaches Diseases 0.000 description 1
- 206010027940 Mood altered Diseases 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010028289 Muscle atrophy Diseases 0.000 description 1
- RTHCYVBBDHJXIQ-UHFFFAOYSA-N N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine Chemical compound C=1C=CC=CC=1C(CCNC)OC1=CC=C(C(F)(F)F)C=C1 RTHCYVBBDHJXIQ-UHFFFAOYSA-N 0.000 description 1
- HOKKHZGPKSLGJE-UHFFFAOYSA-N N-methyl-D-aspartic acid Natural products CNC(C(O)=O)CC(O)=O HOKKHZGPKSLGJE-UHFFFAOYSA-N 0.000 description 1
- 108010021717 Nafarelin Proteins 0.000 description 1
- 102400000097 Neurokinin A Human genes 0.000 description 1
- HEAUFJZALFKPBA-YRVBCFNBSA-N Neurokinin A Chemical compound C([C@@H](C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)C(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC=1NC=NC=1)C(C)O)C1=CC=CC=C1 HEAUFJZALFKPBA-YRVBCFNBSA-N 0.000 description 1
- 102000046798 Neurokinin B Human genes 0.000 description 1
- NHXYSAFTNPANFK-HDMCBQFHSA-N Neurokinin B Chemical compound C([C@@H](C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)C(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C1=CC=CC=C1 NHXYSAFTNPANFK-HDMCBQFHSA-N 0.000 description 1
- 101800002813 Neurokinin-B Proteins 0.000 description 1
- 101800000923 Neuropeptide K Proteins 0.000 description 1
- 206010057852 Nicotine dependence Diseases 0.000 description 1
- 206010067482 No adverse event Diseases 0.000 description 1
- KYYIDSXMWOZKMP-UHFFFAOYSA-N O-desmethylvenlafaxine Chemical compound C1CCCCC1(O)C(CN(C)C)C1=CC=C(O)C=C1 KYYIDSXMWOZKMP-UHFFFAOYSA-N 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 229940121954 Opioid receptor agonist Drugs 0.000 description 1
- 208000005141 Otitis Diseases 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- 206010033372 Pain and discomfort Diseases 0.000 description 1
- 241000286209 Phasianidae Species 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 208000008601 Polycythemia Diseases 0.000 description 1
- 206010062519 Poor quality sleep Diseases 0.000 description 1
- 206010036297 Postpartum hypopituitarism Diseases 0.000 description 1
- 206010036595 Premature delivery Diseases 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 208000036992 Psychogenic pain disease Diseases 0.000 description 1
- 206010071368 Psychological trauma Diseases 0.000 description 1
- 208000010378 Pulmonary Embolism Diseases 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 102000005686 Serum Globulins Human genes 0.000 description 1
- 108010045362 Serum Globulins Proteins 0.000 description 1
- 201000009895 Sheehan syndrome Diseases 0.000 description 1
- 208000027520 Somatoform disease Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- 208000007271 Substance Withdrawal Syndrome Diseases 0.000 description 1
- 101150108167 TAC1 gene Proteins 0.000 description 1
- 101150100415 Tac3 gene Proteins 0.000 description 1
- 102100033009 Tachykinin-3 Human genes 0.000 description 1
- 208000025569 Tobacco Use disease Diseases 0.000 description 1
- 208000031674 Traumatic Acute Stress disease Diseases 0.000 description 1
- 201000004810 Vascular dementia Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 208000010399 Wasting Syndrome Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 210000002506 abnormal erythrocyte Anatomy 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 108010052004 acetyl-2-naphthylalanyl-3-chlorophenylalanyl-1-oxohexadecyl-seryl-4-aminophenylalanyl(hydroorotyl)-4-aminophenylalanyl(carbamoyl)-leucyl-ILys-prolyl-alaninamide Proteins 0.000 description 1
- 238000001467 acupuncture Methods 0.000 description 1
- 208000026345 acute stress disease Diseases 0.000 description 1
- 208000012826 adjustment disease Diseases 0.000 description 1
- 210000004404 adrenal cortex Anatomy 0.000 description 1
- 230000001780 adrenocortical effect Effects 0.000 description 1
- 208000017515 adrenocortical insufficiency Diseases 0.000 description 1
- 201000007930 alcohol dependence Diseases 0.000 description 1
- 208000028505 alcohol-related disease Diseases 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229960002478 aldosterone Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 108010041395 alpha-Endorphin Proteins 0.000 description 1
- 102000013529 alpha-Fetoproteins Human genes 0.000 description 1
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 229960000836 amitriptyline Drugs 0.000 description 1
- KRMDCWKBEZIMAB-UHFFFAOYSA-N amitriptyline Chemical compound C1CC2=CC=CC=C2C(=CCCN(C)C)C2=CC=CC=C21 KRMDCWKBEZIMAB-UHFFFAOYSA-N 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 210000004727 amygdala Anatomy 0.000 description 1
- 229940070021 anabolic steroids Drugs 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 230000003502 anti-nociceptive effect Effects 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 210000000617 arm Anatomy 0.000 description 1
- 230000037007 arousal Effects 0.000 description 1
- 230000001174 ascending effect Effects 0.000 description 1
- 238000013096 assay test Methods 0.000 description 1
- 229960002274 atenolol Drugs 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 230000005784 autoimmunity Effects 0.000 description 1
- 229940049706 benzodiazepine Drugs 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 210000000133 brain stem Anatomy 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 230000005189 cardiac health Effects 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 229960004195 carvedilol Drugs 0.000 description 1
- NPAKNKYSJIDKMW-UHFFFAOYSA-N carvedilol Chemical compound COC1=CC=CC=C1OCCNCC(O)COC1=CC=CC2=NC3=CC=C[CH]C3=C12 NPAKNKYSJIDKMW-UHFFFAOYSA-N 0.000 description 1
- 230000001925 catabolic effect Effects 0.000 description 1
- 230000001364 causal effect Effects 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000011712 cell development Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229940083181 centrally acting adntiadrenergic agent methyldopa Drugs 0.000 description 1
- 108700008462 cetrorelix Proteins 0.000 description 1
- SBNPWPIBESPSIF-MHWMIDJBSA-N cetrorelix Chemical compound C([C@@H](C(=O)N[C@H](CCCNC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(O)C=C1 SBNPWPIBESPSIF-MHWMIDJBSA-N 0.000 description 1
- 229960003230 cetrorelix Drugs 0.000 description 1
- 210000000038 chest Anatomy 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 208000037893 chronic inflammatory disorder Diseases 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- 229960002896 clonidine Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 229960003920 cocaine Drugs 0.000 description 1
- 208000010877 cognitive disease Diseases 0.000 description 1
- 230000001427 coherent effect Effects 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 230000008094 contradictory effect Effects 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- OMFXVFTZEKFJBZ-HJTSIMOOSA-N corticosterone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@H](CC4)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OMFXVFTZEKFJBZ-HJTSIMOOSA-N 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 229960000766 danazol Drugs 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 229960002272 degarelix Drugs 0.000 description 1
- MEUCPCLKGZSHTA-XYAYPHGZSA-N degarelix Chemical compound C([C@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CC=1C=CC(NC(=O)[C@H]2NC(=O)NC(=O)C2)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(NC(N)=O)C=C1 MEUCPCLKGZSHTA-XYAYPHGZSA-N 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- 239000007950 delayed release tablet Substances 0.000 description 1
- 229960001623 desvenlafaxine Drugs 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 229960002069 diamorphine Drugs 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 230000005059 dormancy Effects 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- ODQWQRRAPPTVAG-GZTJUZNOSA-N doxepin Chemical compound C1OC2=CC=CC=C2C(=C/CCN(C)C)/C2=CC=CC=C21 ODQWQRRAPPTVAG-GZTJUZNOSA-N 0.000 description 1
- 229960005426 doxepin Drugs 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 206010013663 drug dependence Diseases 0.000 description 1
- 229960004199 dutasteride Drugs 0.000 description 1
- JWJOTENAMICLJG-QWBYCMEYSA-N dutasteride Chemical compound O=C([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)N[C@@H]4CC3)C)CC[C@@]21C)NC1=CC(C(F)(F)F)=CC=C1C(F)(F)F JWJOTENAMICLJG-QWBYCMEYSA-N 0.000 description 1
- 208000019258 ear infection Diseases 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 230000003028 elevating effect Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000008451 emotion Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 210000000750 endocrine system Anatomy 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000002662 enteric coated tablet Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000009986 erectile function Effects 0.000 description 1
- 230000010437 erythropoiesis Effects 0.000 description 1
- 238000009164 estrogen replacement therapy Methods 0.000 description 1
- 235000010228 ethyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004403 ethyl p-hydroxybenzoate Substances 0.000 description 1
- 229940043351 ethyl-p-hydroxybenzoate Drugs 0.000 description 1
- NUVBSKCKDOMJSU-UHFFFAOYSA-N ethylparaben Chemical compound CCOC(=O)C1=CC=C(O)C=C1 NUVBSKCKDOMJSU-UHFFFAOYSA-N 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000005002 female reproductive tract Anatomy 0.000 description 1
- 229960004039 finasteride Drugs 0.000 description 1
- DBEPLOCGEIEOCV-WSBQPABSSA-N finasteride Chemical compound N([C@@H]1CC2)C(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)NC(C)(C)C)[C@@]2(C)CC1 DBEPLOCGEIEOCV-WSBQPABSSA-N 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- YLRFCQOZQXIBAB-RBZZARIASA-N fluoxymesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C[C@@H]2O YLRFCQOZQXIBAB-RBZZARIASA-N 0.000 description 1
- 229960001751 fluoxymesterone Drugs 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 229960003794 ganirelix Drugs 0.000 description 1
- 108700032141 ganirelix Proteins 0.000 description 1
- GJNXBNATEDXMAK-PFLSVRRQSA-N ganirelix Chemical compound C([C@@H](C(=O)N[C@H](CCCCN=C(NCC)NCC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN=C(NCC)NCC)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(O)C=C1 GJNXBNATEDXMAK-PFLSVRRQSA-N 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 230000011923 general adaptation syndrome Effects 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229940121381 gonadotrophin releasing hormone (gnrh) antagonists Drugs 0.000 description 1
- 230000036449 good health Effects 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 201000010235 heart cancer Diseases 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 208000024348 heart neoplasm Diseases 0.000 description 1
- 231100000304 hepatotoxicity Toxicity 0.000 description 1
- 230000007686 hepatotoxicity Effects 0.000 description 1
- 229960001340 histamine Drugs 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 230000003054 hormonal effect Effects 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- WVLOADHCBXTIJK-YNHQPCIGSA-N hydromorphone Chemical compound O([C@H]1C(CC[C@H]23)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O WVLOADHCBXTIJK-YNHQPCIGSA-N 0.000 description 1
- 229960001410 hydromorphone Drugs 0.000 description 1
- 229950000801 hydroxyprogesterone caproate Drugs 0.000 description 1
- 230000004179 hypothalamic–pituitary–adrenal axis Effects 0.000 description 1
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 1
- 230000008088 immune pathway Effects 0.000 description 1
- 208000026278 immune system disease Diseases 0.000 description 1
- 201000001881 impotence Diseases 0.000 description 1
- 208000022119 inability to concentrate Diseases 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 230000006749 inflammatory damage Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 229960004125 ketoconazole Drugs 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 210000004936 left thumb Anatomy 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- URLZCHNOLZSCCA-UHFFFAOYSA-N leu-enkephalin Chemical compound C=1C=C(O)C=CC=1CC(N)C(=O)NCC(=O)NCC(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=CC=C1 URLZCHNOLZSCCA-UHFFFAOYSA-N 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 210000003715 limbic system Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 235000004213 low-fat Nutrition 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 229940040129 luteinizing hormone Drugs 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000011418 maintenance treatment Methods 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 229960002985 medroxyprogesterone acetate Drugs 0.000 description 1
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 1
- 230000006984 memory degeneration Effects 0.000 description 1
- 208000023060 memory loss Diseases 0.000 description 1
- 230000009245 menopause Effects 0.000 description 1
- 208000007106 menorrhagia Diseases 0.000 description 1
- 230000007102 metabolic function Effects 0.000 description 1
- 229960001797 methadone Drugs 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 229960002237 metoprolol Drugs 0.000 description 1
- IUBSYMUCCVWXPE-UHFFFAOYSA-N metoprolol Chemical compound COCCC1=CC=C(OCC(O)CNC(C)C)C=C1 IUBSYMUCCVWXPE-UHFFFAOYSA-N 0.000 description 1
- 208000015994 miscarriage Diseases 0.000 description 1
- 230000007510 mood change Effects 0.000 description 1
- 230000008450 motivation Effects 0.000 description 1
- 230000007659 motor function Effects 0.000 description 1
- 208000015004 muscle tenderness Diseases 0.000 description 1
- 201000000585 muscular atrophy Diseases 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- RWHUEXWOYVBUCI-ITQXDASVSA-N nafarelin Chemical compound C([C@@H](C(=O)N[C@H](CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 RWHUEXWOYVBUCI-ITQXDASVSA-N 0.000 description 1
- 229960002333 nafarelin Drugs 0.000 description 1
- 229960004719 nandrolone Drugs 0.000 description 1
- NPAGDVCDWIYMMC-IZPLOLCNSA-N nandrolone Chemical compound O=C1CC[C@@H]2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 NPAGDVCDWIYMMC-IZPLOLCNSA-N 0.000 description 1
- 210000000944 nerve tissue Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 210000000118 neural pathway Anatomy 0.000 description 1
- 230000010004 neural pathway Effects 0.000 description 1
- 230000001722 neurochemical effect Effects 0.000 description 1
- 210000004412 neuroendocrine cell Anatomy 0.000 description 1
- 230000002314 neuroinflammatory effect Effects 0.000 description 1
- 239000003176 neuroleptic agent Substances 0.000 description 1
- 208000021722 neuropathic pain Diseases 0.000 description 1
- 210000000607 neurosecretory system Anatomy 0.000 description 1
- 230000008055 nociceptive signaling Effects 0.000 description 1
- 229940121367 non-opioid analgesics Drugs 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- UQDVHJGNIFVBLG-UHFFFAOYSA-N octadecanoic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.CCCCCCCCCCCCCCCCCC(O)=O.CCCCCCCCCCCCCCCCCC(O)=O UQDVHJGNIFVBLG-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 239000003402 opiate agonist Substances 0.000 description 1
- 239000006191 orally-disintegrating tablet Substances 0.000 description 1
- 238000011474 orchiectomy Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- ICMWWNHDUZJFDW-DHODBPELSA-N oxymetholone Chemical compound C([C@@H]1CC2)C(=O)\C(=C/O)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@](C)(O)[C@@]2(C)CC1 ICMWWNHDUZJFDW-DHODBPELSA-N 0.000 description 1
- 208000027753 pain disease Diseases 0.000 description 1
- 230000008052 pain pathway Effects 0.000 description 1
- 208000035824 paresthesia Diseases 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- 210000000578 peripheral nerve Anatomy 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 201000010076 persian gulf syndrome Diseases 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 208000022821 personality disease Diseases 0.000 description 1
- 239000002831 pharmacologic agent Substances 0.000 description 1
- 230000037081 physical activity Effects 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 230000001817 pituitary effect Effects 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 229940124272 protein stabilizer Drugs 0.000 description 1
- 229940124811 psychiatric drug Drugs 0.000 description 1
- 238000001671 psychotherapy Methods 0.000 description 1
- 239000003237 recreational drug Substances 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000026416 response to pain Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000011808 rodent model Methods 0.000 description 1
- 238000011300 routine therapy Methods 0.000 description 1
- 201000005404 rubella Diseases 0.000 description 1
- 238000009094 second-line therapy Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 210000000582 semen Anatomy 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000035911 sexual health Effects 0.000 description 1
- 230000006403 short-term memory Effects 0.000 description 1
- 210000002460 smooth muscle Anatomy 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L sodium carbonate Substances [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- LXMSZDCAJNLERA-ZHYRCANASA-N spironolactone Chemical compound C([C@@H]1[C@]2(C)CC[C@@H]3[C@@]4(C)CCC(=O)C=C4C[C@H]([C@@H]13)SC(=O)C)C[C@@]21CCC(=O)O1 LXMSZDCAJNLERA-ZHYRCANASA-N 0.000 description 1
- 229960002256 spironolactone Drugs 0.000 description 1
- 208000000995 spontaneous abortion Diseases 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000011301 standard therapy Methods 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 239000002438 stress hormone Substances 0.000 description 1
- 208000011117 substance-related disease Diseases 0.000 description 1
- 239000007940 sugar coated tablet Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 108010073085 tachykinin neuropeptide gamma Proteins 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 238000004885 tandem mass spectrometry Methods 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 229960004380 tramadol Drugs 0.000 description 1
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 description 1
- 229940100640 transdermal system Drugs 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 229960003991 trazodone Drugs 0.000 description 1
- PHLBKPHSAVXXEF-UHFFFAOYSA-N trazodone Chemical compound ClC1=CC=CC(N2CCN(CCCN3C(N4C=CC=CC4=N3)=O)CC2)=C1 PHLBKPHSAVXXEF-UHFFFAOYSA-N 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 210000001170 unmyelinated nerve fiber Anatomy 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 229940124549 vasodilator Drugs 0.000 description 1
- 239000003071 vasodilator agent Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229960004688 venlafaxine Drugs 0.000 description 1
- PNVNVHUZROJLTJ-UHFFFAOYSA-N venlafaxine Chemical compound C1=CC(OC)=CC=C1C(CN(C)C)C1(O)CCCCC1 PNVNVHUZROJLTJ-UHFFFAOYSA-N 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 230000005186 women's health Effects 0.000 description 1
- YYCPSEFQLGXPCO-UHFFFAOYSA-N xi-p-Menth-3-ene Chemical compound CC(C)C1=CCC(C)CC1 YYCPSEFQLGXPCO-UHFFFAOYSA-N 0.000 description 1
- 229960001475 zolpidem Drugs 0.000 description 1
- ZAFYATHCZYHLPB-UHFFFAOYSA-N zolpidem Chemical compound N1=C2C=CC(C)=CN2C(CC(=O)N(C)C)=C1C1=CC=C(C)C=C1 ZAFYATHCZYHLPB-UHFFFAOYSA-N 0.000 description 1
- NXSIJWJXMWBCBX-NWKQFZAZSA-N α-endorphin Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)NC(=O)CNC(=O)CNC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=CC=C1 NXSIJWJXMWBCBX-NWKQFZAZSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/565—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol
- A61K31/568—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol substituted in positions 10 and 13 by a chain having at least one carbon atom, e.g. androstanes, e.g. testosterone
- A61K31/5685—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol substituted in positions 10 and 13 by a chain having at least one carbon atom, e.g. androstanes, e.g. testosterone having an oxo group in position 17, e.g. androsterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/565—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol
- A61K31/568—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol substituted in positions 10 and 13 by a chain having at least one carbon atom, e.g. androstanes, e.g. testosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/26—Androgens
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5094—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for blood cell populations
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
- G01N33/6896—Neurological disorders, e.g. Alzheimer's disease
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/74—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving hormones or other non-cytokine intercellular protein regulatory factors such as growth factors, including receptors to hormones and growth factors
- G01N33/743—Steroid hormones
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/46—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans from vertebrates
- G01N2333/47—Assays involving proteins of known structure or function as defined in the subgroups
- G01N2333/4701—Details
- G01N2333/4737—C-reactive protein
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/28—Neurological disorders
- G01N2800/2842—Pain, e.g. neuropathic pain, psychogenic pain
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/70—Mechanisms involved in disease identification
- G01N2800/7095—Inflammation
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Biotechnology (AREA)
- Endocrinology (AREA)
- Pain & Pain Management (AREA)
- Dermatology (AREA)
- Communicable Diseases (AREA)
- Reproductive Health (AREA)
- Oncology (AREA)
- Diabetes (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41525810P | 2010-11-18 | 2010-11-18 | |
| US61/415,258 | 2010-11-18 | ||
| US201161534174P | 2011-09-13 | 2011-09-13 | |
| US61/534,174 | 2011-09-13 | ||
| PCT/US2011/061254 WO2012068410A1 (en) | 2010-11-18 | 2011-11-17 | Methods for treating chronic or unresolvable pain and/or increasing the pain threshold in a subject and pharmaceutical compositions for use therein |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018000146A Division JP2018087193A (ja) | 2010-11-18 | 2018-01-04 | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2013543004A JP2013543004A (ja) | 2013-11-28 |
| JP2013543004A5 JP2013543004A5 (cg-RX-API-DMAC7.html) | 2015-04-09 |
| JP6912852B2 true JP6912852B2 (ja) | 2021-08-04 |
Family
ID=46064977
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013540032A Expired - Fee Related JP6912852B2 (ja) | 2010-11-18 | 2011-11-17 | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 |
| JP2018000146A Pending JP2018087193A (ja) | 2010-11-18 | 2018-01-04 | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 |
| JP2019182786A Pending JP2020033354A (ja) | 2010-11-18 | 2019-10-03 | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018000146A Pending JP2018087193A (ja) | 2010-11-18 | 2018-01-04 | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 |
| JP2019182786A Pending JP2020033354A (ja) | 2010-11-18 | 2019-10-03 | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US9642863B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2640398A4 (cg-RX-API-DMAC7.html) |
| JP (3) | JP6912852B2 (cg-RX-API-DMAC7.html) |
| AU (3) | AU2011329782A1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2818491A1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2012068410A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040259852A1 (en) * | 2003-06-18 | 2004-12-23 | White Hillary D. | Trandsdermal compositions and methods for treatment of fibromyalgia and chronic fatigue syndrome |
| US20150248843A1 (en) * | 2012-10-12 | 2015-09-03 | Analgesic Solutions | Training methods for improved assaying of pain in clinical trial subjects |
| JP2022506944A (ja) * | 2018-11-08 | 2022-01-17 | ヴァーソナ セラピューティクス,インコーポレーテッド | 5-α-レダクターゼ阻害剤の局所製剤及びそれらの使用 |
| AU2020271839B2 (en) | 2019-04-09 | 2025-04-24 | Institut National de la Santé et de la Recherche Médicale | Prophylactic efficacy of serotonin 4 receptor agonists against stress |
| JP7493522B2 (ja) * | 2019-09-20 | 2024-05-31 | 株式会社あすか製薬メディカル | 動物体毛試料に含まれるステロイドホルモンを分析する方法、ストレス評価方法及び脱毛原因分析方法 |
| EP4143580A1 (en) * | 2020-04-30 | 2023-03-08 | Leibniz-Institut für Virologie | Method for predicting the course of a viral disease |
| US20250322463A1 (en) * | 2022-05-13 | 2025-10-16 | Imx, Inc. | Systems and methods for generating financial indexes for medical conditions |
| CN117982655B (zh) * | 2023-11-21 | 2024-10-15 | 北京大学人民医院 | 芳香化酶抑制剂治疗相关的疼痛动物模型构建方法及应用 |
Family Cites Families (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4542129A (en) | 1982-08-16 | 1985-09-17 | Norman Orentreich | DHEA Formulations and methods for treating dry skin |
| US5019395A (en) | 1988-03-08 | 1991-05-28 | Warner-Lambert Company | Compositions with enhanced penetration |
| US5461042A (en) | 1988-12-30 | 1995-10-24 | Loria; Roger M. | Regulation of the immune system |
| US5206008A (en) | 1991-04-15 | 1993-04-27 | Virginia Commonwealth University | Enhancement of immune response |
| US5676968A (en) | 1991-10-31 | 1997-10-14 | Schering Aktiengesellschaft | Transdermal therapeutic systems with crystallization inhibitors |
| US5656606A (en) | 1995-02-17 | 1997-08-12 | Merck & Co., Inc. | Camphor compounds promote release of growth hormone |
| US6197830B1 (en) * | 1995-09-22 | 2001-03-06 | Bruce M. Frome | Method for achieving relief from sympathetically mediated pain |
| US6923983B2 (en) | 1996-02-19 | 2005-08-02 | Acrux Dds Pty Ltd | Transdermal delivery of hormones |
| AUPN814496A0 (en) | 1996-02-19 | 1996-03-14 | Monash University | Dermal penetration enhancer |
| DE19619045C1 (de) * | 1996-05-02 | 1997-11-13 | Jenapharm Gmbh | Verwendung von Kombinationspräparaten zur Behandlung hypogonadaler Männer sowie Männern mit Hypophysenerkrankungen |
| US5709878A (en) | 1996-08-02 | 1998-01-20 | Rosenbaum; Jerry | Transdermal delivery of dehydroepiandrosterone |
| US5855920A (en) | 1996-12-13 | 1999-01-05 | Chein; Edmund Y. M. | Total hormone replacement therapy |
| DE19701949A1 (de) | 1997-01-13 | 1998-07-16 | Jenapharm Gmbh | Transdermales therapeutisches System |
| HUP0001748A3 (en) | 1997-02-28 | 2001-03-28 | Minnesota Mining And Mfg Co Sa | Transdermal device for the delivery of testosterone |
| US5968919A (en) | 1997-10-16 | 1999-10-19 | Macrochem Corporation | Hormone replacement therapy drug formulations for topical application to the skin |
| US20020013304A1 (en) | 1997-10-28 | 2002-01-31 | Wilson Leland F. | As-needed administration of an androgenic agent to enhance female sexual desire and responsiveness |
| DE69840426D1 (de) | 1997-11-10 | 2009-02-12 | Strakan Int Ltd | Penetrationsfördernde und irritationsvermindernde Systeme mit Testosteron |
| US5869090A (en) | 1998-01-20 | 1999-02-09 | Rosenbaum; Jerry | Transdermal delivery of dehydroepiandrosterone |
| US5935949A (en) | 1998-03-20 | 1999-08-10 | Trustees Of Dartmouth College | Use of androgen therapy in fibromyalgia and chronic fatigue syndrome |
| US6165504A (en) * | 1998-09-23 | 2000-12-26 | Barr Laboratories, Inc. | Methods for treating hot flashes and improving the quality of life of castrated prostatic cancer patients |
| FR2801507B1 (fr) | 1999-11-30 | 2003-06-27 | Pf Medicament | Dispositif transdermique auto-adhesif comprenant un reservoir et une matrice contenant le meme principe actif, son procede de preparation et ses utilisations |
| US20040198706A1 (en) | 2003-03-11 | 2004-10-07 | Carrara Dario Norberto R. | Methods and formulations for transdermal or transmucosal application of active agents |
| US8980290B2 (en) | 2000-08-03 | 2015-03-17 | Antares Pharma Ipl Ag | Transdermal compositions for anticholinergic agents |
| US7198801B2 (en) | 2000-08-03 | 2007-04-03 | Antares Pharma Ipl Ag | Formulations for transdermal or transmucosal application |
| ATE356636T1 (de) | 2000-08-03 | 2007-04-15 | Antares Pharma Ipl Ag | Zusammensetzung zur transdermalen und/oder transmukosalen verabreichung von wirkstoffen, die ausreichende therapeutische spiegel garantiert |
| JP2004524267A (ja) | 2000-08-30 | 2004-08-12 | ユニメッド・ファーマシューティカルズ・インコーポレーテッド | 男性の勃起障害の治療方法、及び性衝動の増強方法 |
| US6503894B1 (en) | 2000-08-30 | 2003-01-07 | Unimed Pharmaceuticals, Inc. | Pharmaceutical composition and method for treating hypogonadism |
| US20030139384A1 (en) | 2000-08-30 | 2003-07-24 | Dudley Robert E. | Method for treating erectile dysfunction and increasing libido in men |
| US20040002482A1 (en) | 2000-08-30 | 2004-01-01 | Dudley Robert E. | Androgen pharmaceutical composition and method for treating depression |
| US20040092494A9 (en) | 2000-08-30 | 2004-05-13 | Dudley Robert E. | Method of increasing testosterone and related steroid concentrations in women |
| NZ536633A (en) | 2000-08-30 | 2006-03-31 | Unimed Pharmaceuticals Inc | Percutaneous delivery of testosterone via a hydroalcoholic gel as a method of increasing testosterone and related steroid concentrations in women |
| US20040115175A1 (en) | 2000-11-10 | 2004-06-17 | The Board Of Trustees Of The Leland | Methods for treating disorders of neuronal deficiency with bone marrow-derived cells |
| US6743448B2 (en) | 2000-12-11 | 2004-06-01 | Abraham H. Kryger | Topical testosterone formulations and associated methods |
| CA2446060A1 (en) | 2001-05-07 | 2002-11-14 | Corium International | Compositions and delivery systems for administration of a local anesthetic agent |
| US20030027804A1 (en) | 2001-06-27 | 2003-02-06 | Van Der Hoop Roland Gerritsen | Therapeutic combinations for the treatment of hormone deficiencies |
| US20030175329A1 (en) | 2001-10-04 | 2003-09-18 | Cellegy Pharmaceuticals, Inc. | Semisolid topical hormonal compositions and methods for treatment |
| US7169107B2 (en) | 2002-01-25 | 2007-01-30 | Karen Jersey-Willuhn | Conductivity reconstruction based on inverse finite element measurements in a tissue monitoring system |
| OA12856A (en) | 2002-03-15 | 2006-09-15 | Unimed Pharmaceuticals Inc | Androgen pharmaceutical composition and method for treating depression. |
| MXPA04008988A (es) | 2002-03-15 | 2006-05-25 | Unimed Pharmaceuticals Inc | Composicion androgena farmaceutica y metodo para el tratamiento de la depresion. |
| MY139721A (en) | 2002-04-19 | 2009-10-30 | Cpex Pharmaceuticals Inc | Pharmaceutical composition |
| EP1558231A4 (en) * | 2002-10-03 | 2010-09-08 | Cypress Bioscience Inc | DOSAGE CLIMBING AND FRICTIONAL DAILY DOSE OF ANTIDEPRESSANTS TO TREAT NEUROLOGICAL DISORDERS |
| US20040259784A1 (en) | 2003-06-18 | 2004-12-23 | White Hillary D. | Compositions and methods for treatment of muscle pain and muscle wasting |
| US20040259852A1 (en) | 2003-06-18 | 2004-12-23 | White Hillary D. | Trandsdermal compositions and methods for treatment of fibromyalgia and chronic fatigue syndrome |
| US8883769B2 (en) | 2003-06-18 | 2014-11-11 | White Mountain Pharma, Inc. | Methods for the treatment of fibromyalgia and chronic fatigue syndrome |
| US20050042268A1 (en) | 2003-07-16 | 2005-02-24 | Chaim Aschkenasy | Pharmaceutical composition and method for transdermal drug delivery |
| US20050025833A1 (en) | 2003-07-16 | 2005-02-03 | Chaim Aschkenasy | Pharmaceutical composition and method for transdermal drug delivery |
| US20050020552A1 (en) | 2003-07-16 | 2005-01-27 | Chaim Aschkenasy | Pharmaceutical composition and method for transdermal drug delivery |
| DK1670433T3 (da) | 2003-10-10 | 2012-03-12 | Ferring Bv | Transdermal farmaceutisk formulering til mindskelse af hudrester |
| EP2374508A1 (en) * | 2005-03-21 | 2011-10-12 | Vicus Therapeutics SPE 1, LLC | Combination of angiotension receptor blockers (ARB) and NSAIDs for use in ameliorating cachexia |
| AU2006235453A1 (en) | 2005-04-12 | 2006-10-19 | Laboratories Besins International | Method of treating or preventing bone deterioration or osteoporosis |
| CA2604431A1 (en) | 2005-04-13 | 2006-10-26 | Unimed Pharmaceuticals, Inc. | Method of increasing testosterone and related steroid concentrations in women |
| AU2006236564B2 (en) | 2005-04-15 | 2011-02-17 | Besins Healthcare Luxembourg S.A.R.L. | Pharmaceutical delivery systems for hydrophobic drugs and compositions comprising same |
| US8492369B2 (en) | 2010-04-12 | 2013-07-23 | Clarus Therapeutics Inc | Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same |
| ES2607454T3 (es) | 2005-06-03 | 2017-03-31 | Acrux Dds Pty Ltd | Método y composición para el suministro transdérmico de testosterona |
| US20080261937A1 (en) | 2007-03-23 | 2008-10-23 | Dudley Robert E | Pharmaceutical compositions and method for treating pediatric hypogonadism |
| WO2008118423A1 (en) | 2007-03-26 | 2008-10-02 | Combinatorx, Incorporated | Compositions and methods for treating medical conditions |
-
2011
- 2011-11-17 CA CA2818491A patent/CA2818491A1/en not_active Abandoned
- 2011-11-17 US US13/988,245 patent/US9642863B2/en not_active Expired - Fee Related
- 2011-11-17 WO PCT/US2011/061254 patent/WO2012068410A1/en not_active Ceased
- 2011-11-17 JP JP2013540032A patent/JP6912852B2/ja not_active Expired - Fee Related
- 2011-11-17 US US13/299,232 patent/US9642862B2/en not_active Expired - Fee Related
- 2011-11-17 EP EP11841292.3A patent/EP2640398A4/en not_active Withdrawn
- 2011-11-17 AU AU2011329782A patent/AU2011329782A1/en not_active Abandoned
-
2017
- 2017-05-08 US US15/588,855 patent/US20170239270A1/en not_active Abandoned
- 2017-05-12 AU AU2017203161A patent/AU2017203161A1/en not_active Abandoned
-
2018
- 2018-01-04 JP JP2018000146A patent/JP2018087193A/ja active Pending
-
2019
- 2019-03-18 AU AU2019201863A patent/AU2019201863A1/en not_active Abandoned
- 2019-10-03 JP JP2019182786A patent/JP2020033354A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US20140018339A1 (en) | 2014-01-16 |
| CA2818491A1 (en) | 2012-05-24 |
| JP2020033354A (ja) | 2020-03-05 |
| JP2018087193A (ja) | 2018-06-07 |
| AU2019201863A1 (en) | 2019-04-11 |
| US20170239270A1 (en) | 2017-08-24 |
| US9642863B2 (en) | 2017-05-09 |
| WO2012068410A1 (en) | 2012-05-24 |
| JP2013543004A (ja) | 2013-11-28 |
| AU2017203161A1 (en) | 2017-06-01 |
| US20120130199A1 (en) | 2012-05-24 |
| US9642862B2 (en) | 2017-05-09 |
| EP2640398A1 (en) | 2013-09-25 |
| AU2011329782A1 (en) | 2013-06-20 |
| EP2640398A4 (en) | 2014-05-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6912852B2 (ja) | 対象における慢性または解決不能な疼痛を処置するための、および/または痛覚閾値を高めるための方法、およびこれに用いる医薬組成物 | |
| Davis et al. | Treating menopause—MHT and beyond | |
| Wang et al. | Androgen excess: a hallmark of polycystic ovary syndrome | |
| Bianchi | Impact of testosterone on Alzheimer’s disease | |
| Lunenfeld et al. | ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale | |
| Marques-Deak et al. | Brain-immune interactions and disease susceptibility | |
| Darby et al. | Male hypogonadism: an update on diagnosis and treatment | |
| Reddy | Pharmacology of endogenous neuroactive steroids | |
| Cagetti et al. | Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function | |
| Lund et al. | Testosterone and andropause: the feasibility of testosterone replacement therapy in elderly men | |
| Bhasin et al. | Age-related changes in the male reproductive system | |
| Marudhai et al. | Long-term opioids linked to hypogonadism and the role of testosterone supplementation therapy | |
| Clayton et al. | Pathophysiology and medical management of hypoactive sexual desire disorder | |
| JP2013543005A (ja) | 線維筋痛症候群および慢性疲労症候群を処置する方法 | |
| Misra et al. | Precocious puberty | |
| Ioannidou-Kadis et al. | Complete reversal of adult-onset isolated hypogonadotropic hypogonadism with clomiphene citrate | |
| Kaufman et al. | The effects of testosterone deficiency on male sexual function | |
| Mitsiades et al. | Cognitive effects of hormonal therapy in older adults | |
| US20090215738A1 (en) | Prevention and treatment of an increase in cardiovascular risk, adverse cardiovascular events, infertility, and adverse psychological effects after androgen or gnrh analogue intake | |
| Sokol et al. | Endocrine evaluation | |
| Burger et al. | The role of androgen therapy | |
| Bancroft | The behavioral correlates of testosterone | |
| Vetri et al. | Gender Identity Disorder Etiology and Medical Treatment: Between Scientific Evidence and Personal Experiences | |
| Čeponis et al. | Androgen replacement therapy in hypogonadal men | |
| Üstün | The Role of Androgens in Health and Disease in Females: A Narratıve Review |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20141117 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20141117 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20141225 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150216 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20151026 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160126 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160226 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160328 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160426 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20161003 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170104 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20170829 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180104 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20180104 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20180105 |
|
| C11 | Written invitation by the commissioner to file amendments |
Free format text: JAPANESE INTERMEDIATE CODE: C11 Effective date: 20180129 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20180302 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20180305 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20180420 |
|
| C211 | Notice of termination of reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C211 Effective date: 20180424 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20180802 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20190412 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20190805 |
|
| C13 | Notice of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: C13 Effective date: 20190829 |
|
| C28A | Non-patent document cited |
Free format text: JAPANESE INTERMEDIATE CODE: C2838 Effective date: 20190829 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20191129 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20191227 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200129 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200302 |
|
| C13 | Notice of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: C13 Effective date: 20200511 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200811 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200911 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20201009 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201111 |
|
| C13 | Notice of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: C13 Effective date: 20210104 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20210405 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210414 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210430 |
|
| C23 | Notice of termination of proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C23 Effective date: 20210519 |
|
| C302 | Record of communication |
Free format text: JAPANESE INTERMEDIATE CODE: C302 Effective date: 20210601 |
|
| C03 | Trial/appeal decision taken |
Free format text: JAPANESE INTERMEDIATE CODE: C03 Effective date: 20210616 |
|
| C30A | Notification sent |
Free format text: JAPANESE INTERMEDIATE CODE: C3012 Effective date: 20210616 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210709 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6912852 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |