JP6892079B2 - Regulatory T cell inducers, adjuvants and food compositions - Google Patents
Regulatory T cell inducers, adjuvants and food compositions Download PDFInfo
- Publication number
- JP6892079B2 JP6892079B2 JP2018122078A JP2018122078A JP6892079B2 JP 6892079 B2 JP6892079 B2 JP 6892079B2 JP 2018122078 A JP2018122078 A JP 2018122078A JP 2018122078 A JP2018122078 A JP 2018122078A JP 6892079 B2 JP6892079 B2 JP 6892079B2
- Authority
- JP
- Japan
- Prior art keywords
- lycopene
- regulatory
- food
- food composition
- allergen immunotherapy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 235000013305 food Nutrition 0.000 title claims description 106
- 210000003289 regulatory T cell Anatomy 0.000 title claims description 94
- 239000000203 mixture Substances 0.000 title claims description 93
- 239000002671 adjuvant Substances 0.000 title claims description 44
- 239000000411 inducer Substances 0.000 title claims description 28
- 239000013566 allergen Substances 0.000 claims description 104
- UPYKUZBSLRQECL-UKMVMLAPSA-N Lycopene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1C(=C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=C)CCCC2(C)C UPYKUZBSLRQECL-UKMVMLAPSA-N 0.000 claims description 97
- JEVVKJMRZMXFBT-XWDZUXABSA-N Lycophyll Natural products OC/C(=C/CC/C(=C\C=C\C(=C/C=C/C(=C\C=C\C=C(/C=C/C=C(\C=C\C=C(/CC/C=C(/CO)\C)\C)/C)\C)/C)\C)/C)/C JEVVKJMRZMXFBT-XWDZUXABSA-N 0.000 claims description 95
- 235000012661 lycopene Nutrition 0.000 claims description 95
- OAIJSZIZWZSQBC-GYZMGTAESA-N lycopene Chemical compound CC(C)=CCC\C(C)=C\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C=C(/C)CCC=C(C)C OAIJSZIZWZSQBC-GYZMGTAESA-N 0.000 claims description 95
- 239000001751 lycopene Substances 0.000 claims description 95
- 229960004999 lycopene Drugs 0.000 claims description 95
- ZCIHMQAPACOQHT-ZGMPDRQDSA-N trans-isorenieratene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/c1c(C)ccc(C)c1C)C=CC=C(/C)C=Cc2c(C)ccc(C)c2C ZCIHMQAPACOQHT-ZGMPDRQDSA-N 0.000 claims description 95
- 238000009169 immunotherapy Methods 0.000 claims description 79
- 208000026935 allergic disease Diseases 0.000 claims description 62
- 230000001939 inductive effect Effects 0.000 claims description 39
- 208000027866 inflammatory disease Diseases 0.000 claims description 34
- 239000003814 drug Substances 0.000 claims description 33
- 239000004480 active ingredient Substances 0.000 claims description 30
- 229940124597 therapeutic agent Drugs 0.000 claims description 27
- 208000004262 Food Hypersensitivity Diseases 0.000 claims description 24
- 235000020932 food allergy Nutrition 0.000 claims description 24
- 239000003795 chemical substances by application Substances 0.000 claims description 19
- 230000003449 preventive effect Effects 0.000 claims description 19
- 230000001225 therapeutic effect Effects 0.000 claims description 14
- 206010016946 Food allergy Diseases 0.000 claims description 13
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 claims description 13
- CBFCDTFDPHXCNY-UHFFFAOYSA-N octyldodecane Natural products CCCCCCCCCCCCCCCCCCCC CBFCDTFDPHXCNY-UHFFFAOYSA-N 0.000 claims description 13
- FMGSKLZLMKYGDP-UHFFFAOYSA-N Dehydroepiandrosterone Natural products C1C(O)CCC2(C)C3CCC(C)(C(CC4)=O)C4C3CC=C21 FMGSKLZLMKYGDP-UHFFFAOYSA-N 0.000 claims description 9
- FMGSKLZLMKYGDP-USOAJAOKSA-N dehydroepiandrosterone Chemical compound C1[C@@H](O)CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC=C21 FMGSKLZLMKYGDP-USOAJAOKSA-N 0.000 claims description 9
- 229960002847 prasterone Drugs 0.000 claims description 9
- 150000002664 lycopenes Chemical class 0.000 claims description 8
- 206010009887 colitis Diseases 0.000 claims description 7
- 230000000069 prophylactic effect Effects 0.000 claims description 6
- 230000006872 improvement Effects 0.000 claims description 5
- 239000013589 supplement Substances 0.000 claims description 4
- 235000015872 dietary supplement Nutrition 0.000 claims description 3
- 230000036541 health Effects 0.000 claims description 3
- 235000013402 health food Nutrition 0.000 claims description 3
- OCMSUPSDVXKDFY-UHFFFAOYSA-N (3E)-3,4-didehydro-psi,psi-carotene Chemical compound CC(C)=CCCC(C)=CC=CC(C)=CC=CC(C)=CC=CC=C(C)C=CC=C(C)C=CC=C(C)C=CC=C(C)C OCMSUPSDVXKDFY-UHFFFAOYSA-N 0.000 claims 4
- 210000004027 cell Anatomy 0.000 description 16
- 206010020751 Hypersensitivity Diseases 0.000 description 14
- 230000007815 allergy Effects 0.000 description 13
- 210000001744 T-lymphocyte Anatomy 0.000 description 12
- 239000000126 substance Substances 0.000 description 12
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 10
- 240000003768 Solanum lycopersicum Species 0.000 description 10
- 230000006698 induction Effects 0.000 description 10
- 239000000284 extract Substances 0.000 description 9
- 239000000047 product Substances 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- 241000282412 Homo Species 0.000 description 8
- 241000124008 Mammalia Species 0.000 description 8
- 201000010099 disease Diseases 0.000 description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 235000021466 carotenoid Nutrition 0.000 description 7
- 206010003645 Atopy Diseases 0.000 description 6
- 206010010744 Conjunctivitis allergic Diseases 0.000 description 6
- 206010039085 Rhinitis allergic Diseases 0.000 description 6
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 6
- 201000009961 allergic asthma Diseases 0.000 description 6
- 208000002205 allergic conjunctivitis Diseases 0.000 description 6
- 201000010105 allergic rhinitis Diseases 0.000 description 6
- 208000006673 asthma Diseases 0.000 description 6
- 208000024998 atopic conjunctivitis Diseases 0.000 description 6
- 150000001747 carotenoids Chemical class 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 231100000331 toxic Toxicity 0.000 description 6
- 230000002588 toxic effect Effects 0.000 description 6
- 206010012735 Diarrhoea Diseases 0.000 description 5
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 5
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 5
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 230000007774 longterm Effects 0.000 description 5
- 238000000034 method Methods 0.000 description 5
- 229930002330 retinoic acid Natural products 0.000 description 5
- 229960001727 tretinoin Drugs 0.000 description 5
- 241000283690 Bos taurus Species 0.000 description 4
- 241000282472 Canis lupus familiaris Species 0.000 description 4
- 102000004127 Cytokines Human genes 0.000 description 4
- 108090000695 Cytokines Proteins 0.000 description 4
- 241000283086 Equidae Species 0.000 description 4
- 241000282326 Felis catus Species 0.000 description 4
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 4
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 4
- 238000012258 culturing Methods 0.000 description 4
- 230000004069 differentiation Effects 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 210000002429 large intestine Anatomy 0.000 description 4
- 229960005375 lutein Drugs 0.000 description 4
- KBPHJBAIARWVSC-RGZFRNHPSA-N lutein Chemical compound C([C@H](O)CC=1C)C(C)(C)C=1\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\[C@H]1C(C)=C[C@H](O)CC1(C)C KBPHJBAIARWVSC-RGZFRNHPSA-N 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 229930014626 natural product Natural products 0.000 description 4
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 4
- KBPHJBAIARWVSC-XQIHNALSSA-N trans-lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C KBPHJBAIARWVSC-XQIHNALSSA-N 0.000 description 4
- FJHBOVDFOQMZRV-XQIHNALSSA-N xanthophyll Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C=C(C)C(O)CC2(C)C FJHBOVDFOQMZRV-XQIHNALSSA-N 0.000 description 4
- 241000699670 Mus sp. Species 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 235000013361 beverage Nutrition 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 238000002826 magnetic-activated cell sorting Methods 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 210000004400 mucous membrane Anatomy 0.000 description 3
- 210000000952 spleen Anatomy 0.000 description 3
- 210000004989 spleen cell Anatomy 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- JEBFVOLFMLUKLF-IFPLVEIFSA-N Astaxanthin Natural products CC(=C/C=C/C(=C/C=C/C1=C(C)C(=O)C(O)CC1(C)C)/C)C=CC=C(/C)C=CC=C(/C)C=CC2=C(C)C(=O)C(O)CC2(C)C JEBFVOLFMLUKLF-IFPLVEIFSA-N 0.000 description 2
- 238000011746 C57BL/6J (JAX™ mouse strain) Methods 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 208000001145 Metabolic Syndrome Diseases 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 206010035664 Pneumonia Diseases 0.000 description 2
- 210000004241 Th2 cell Anatomy 0.000 description 2
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 2
- OENHQHLEOONYIE-UKMVMLAPSA-N all-trans beta-carotene Natural products CC=1CCCC(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C OENHQHLEOONYIE-UKMVMLAPSA-N 0.000 description 2
- 230000000172 allergic effect Effects 0.000 description 2
- 235000013793 astaxanthin Nutrition 0.000 description 2
- 229940022405 astaxanthin Drugs 0.000 description 2
- 239000001168 astaxanthin Substances 0.000 description 2
- MQZIGYBFDRPAKN-ZWAPEEGVSA-N astaxanthin Chemical compound C([C@H](O)C(=O)C=1C)C(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1=C(C)C(=O)[C@@H](O)CC1(C)C MQZIGYBFDRPAKN-ZWAPEEGVSA-N 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 239000012752 auxiliary agent Substances 0.000 description 2
- 235000013734 beta-carotene Nutrition 0.000 description 2
- 239000011648 beta-carotene Substances 0.000 description 2
- TUPZEYHYWIEDIH-WAIFQNFQSA-N beta-carotene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCCC1(C)C)C=CC=C(/C)C=CC2=CCCCC2(C)C TUPZEYHYWIEDIH-WAIFQNFQSA-N 0.000 description 2
- 229960002747 betacarotene Drugs 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 150000001746 carotenes Chemical class 0.000 description 2
- 235000005473 carotenes Nutrition 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000013355 food flavoring agent Nutrition 0.000 description 2
- SJWWTRQNNRNTPU-ABBNZJFMSA-N fucoxanthin Chemical compound C[C@@]1(O)C[C@@H](OC(=O)C)CC(C)(C)C1=C=C\C(C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)C(=O)C[C@]1(C(C[C@H](O)C2)(C)C)[C@]2(C)O1 SJWWTRQNNRNTPU-ABBNZJFMSA-N 0.000 description 2
- AQLRNQCFQNNMJA-UHFFFAOYSA-N fucoxanthin Natural products CC(=O)OC1CC(C)(C)C(=C=CC(=CC=CC(=CC=CC=C(/C)C=CC=C(/C)C(=O)CC23OC2(C)CC(O)CC3(C)C)C)CO)C(C)(O)C1 AQLRNQCFQNNMJA-UHFFFAOYSA-N 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 230000006058 immune tolerance Effects 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- -1 kakkonto Chemical compound 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 235000012680 lutein Nutrition 0.000 description 2
- 239000001656 lutein Substances 0.000 description 2
- ORAKUVXRZWMARG-WZLJTJAWSA-N lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C ORAKUVXRZWMARG-WZLJTJAWSA-N 0.000 description 2
- 208000010125 myocardial infarction Diseases 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 239000001054 red pigment Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 235000015113 tomato pastes and purées Nutrition 0.000 description 2
- NCYCYZXNIZJOKI-UHFFFAOYSA-N vitamin A aldehyde Natural products O=CC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 235000008210 xanthophylls Nutrition 0.000 description 2
- OENHQHLEOONYIE-JLTXGRSLSA-N β-Carotene Chemical compound CC=1CCCC(C)(C)C=1\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C OENHQHLEOONYIE-JLTXGRSLSA-N 0.000 description 2
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- QCVGEOXPDFCNHA-UHFFFAOYSA-N 5,5-dimethyl-2,4-dioxo-1,3-oxazolidine-3-carboxamide Chemical compound CC1(C)OC(=O)N(C(N)=O)C1=O QCVGEOXPDFCNHA-UHFFFAOYSA-N 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 244000241235 Citrullus lanatus Species 0.000 description 1
- 235000012828 Citrullus lanatus var citroides Nutrition 0.000 description 1
- 240000000560 Citrus x paradisi Species 0.000 description 1
- 241000723267 Diospyros Species 0.000 description 1
- 235000011511 Diospyros Nutrition 0.000 description 1
- 108010000912 Egg Proteins Proteins 0.000 description 1
- 102000002322 Egg Proteins Human genes 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010058846 Ovalbumin Proteins 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 239000008122 artificial sweetener Substances 0.000 description 1
- 235000021311 artificial sweeteners Nutrition 0.000 description 1
- 235000015895 biscuits Nutrition 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 235000014121 butter Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 210000000991 chicken egg Anatomy 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 235000014510 cooky Nutrition 0.000 description 1
- 235000013365 dairy product Nutrition 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 235000014103 egg white Nutrition 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000015203 fruit juice Nutrition 0.000 description 1
- 201000005298 gastrointestinal allergy Diseases 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 238000002350 laparotomy Methods 0.000 description 1
- 239000011021 lapis lazuli Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 235000021056 liquid food Nutrition 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 235000021096 natural sweeteners Nutrition 0.000 description 1
- 239000008601 oleoresin Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229940092253 ovalbumin Drugs 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 208000026775 severe diarrhea Diseases 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 238000000194 supercritical-fluid extraction Methods 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 235000015193 tomato juice Nutrition 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 1
- 235000005282 vitamin D3 Nutrition 0.000 description 1
- 239000011647 vitamin D3 Substances 0.000 description 1
- 229940021056 vitamin d3 Drugs 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 235000013618 yogurt Nutrition 0.000 description 1
Images
Landscapes
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Description
本発明は、リコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導剤、アレルゲン免疫療法用アジュバント、及び、該制御性T細胞誘導剤を有効成分として含有する、炎症疾患の治療剤及び/又は予防剤、並びに、アレルゲン免疫療法によるアレルギー疾患の治療剤に関する。また、本発明は、リコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導用食品組成物、アレルゲン免疫療法のためのアジュバント用食品組成物、及び、該制御性T細胞誘導用食品組成物を含有する、炎症疾患の治療及び/又は予防用食品組成物、並びに、アレルゲン免疫療法によるアレルギー疾患治療用食品組成物に関する。 The present invention comprises a regulatory T cell inducer containing lycopene or a derivative thereof as an active ingredient, an adjuvant for allergen immunotherapy, and a therapeutic agent for inflammatory diseases containing the regulatory T cell inducer as an active ingredient. / Or related to prophylactic agents and therapeutic agents for allergic diseases by allergen immunotherapy. The present invention also comprises a regulatory T cell-inducing food composition containing lycopene or a derivative thereof as an active ingredient, an adjuvant food composition for allergen immunotherapy, and the regulatory T cell-inducing food composition. The present invention relates to a food composition for treating and / or preventing an inflammatory disease, and a food composition for treating an allergic disease by allergen immunotherapy.
制御性T細胞(以下、Tregともいう)は、リンパ球の中に存在する炎症抑制性の細胞であり、Tregの分化誘導を介した炎症抑制が炎症性疾患に対する新たな予防及び治療戦略として注目されている。なかでも食物アレルギーをはじめとするアレルギー疾患のアレルゲン免疫療法において、近年、Treg誘導作用を有する成分がアジュバントとして利用され始めている。例えば、ビタミンD3、葛根湯、フコキサンチン等が、Treg誘導作用を有することが報告されている(非特許文献1、2、特許文献1)。
カロテノイドの一つであるリコピン(Lycopene)は、トマト等に含まれている赤い色素であり、天然に存在するカロテノイド化合物の一種であって、様々な疾病に対して予防的又は治療的に働くことが知られている。
カロテノイドは、カロテンとキサントフィルに大別され、特許文献1に、このうちのキサントフィルであるフコキサンチンにTreg誘導作用があることが報告されているが、カロテンである、リコピンにはTreg誘導作用がないと報告されている。これまでにリコピンがTreg誘導作用を有することは報告されていない。
Regulatory T cells (hereinafter, also referred to as Treg) are anti-inflammatory cells existing in lymphocytes, and suppression of inflammation through induction of Treg differentiation is attracting attention as a new preventive and therapeutic strategy for inflammatory diseases. Has been done. In particular, in allergen immunotherapy for allergic diseases such as food allergies, components having a Treg-inducing action have begun to be used as adjuvants in recent years. For example, it has been reported that vitamin D3, kakkonto, fucoxanthin and the like have a Treg-inducing effect (Non-Patent Documents 1 and 2 and Patent Document 1).
Lycopene, one of the carotenoids, is a red pigment contained in tomatoes and the like, and is a kind of naturally occurring carotenoid compounds that act prophylactically or therapeutically against various diseases. It has been known.
Carotenoids are roughly classified into carotene and xanthophyll, and Patent Document 1 reports that fucoxanthin, which is a xanthophyll, has a Treg-inducing effect, but carotene, lycopene, has no Treg-inducing effect. Is reported. So far, it has not been reported that lycopene has a Treg-inducing effect.
本発明は上記事情に鑑みてなされたものであって、リコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導剤、アレルゲン免疫療法用アジュバント、及び、該制御性T細胞誘導剤を有効成分として含有する、炎症疾患の治療剤及び/又は予防剤、並びに、アレルゲン免疫療法によるアレルギー疾患の治療剤を提供すること、及び、制御性T細胞誘導用食品組成物、アレルゲン免疫療法のためのアジュバント用食品組成物、及び、該制御性T細胞誘導用食品組成物を含有する炎症疾患の治療及び/又は予防用食品、並びに、アレルゲン免疫療法によるアレルギー疾患治療用食品組成物を提供することを課題とする。 The present invention has been made in view of the above circumstances, and is effective for a regulatory T cell inducer, an allergen immunotherapy adjuvant, and the regulatory T cell inducer containing lycopene or a derivative thereof as an active ingredient. To provide therapeutic agents and / or preventive agents for inflammatory diseases and therapeutic agents for allergic diseases by allergen immunotherapy, which are contained as ingredients, and for regulatory T cell induction food compositions and allergen immunotherapy. To provide a food composition for adjuvant and a food for treating and / or preventing inflammatory diseases containing the regulatory T cell-inducing food composition, and a food composition for treating allergic diseases by allergen immunotherapy. Make it an issue.
本発明者らは、鋭意研究の結果、リコピンが単独で優れた制御性T細胞誘導作用を有し、アレルゲン免疫療法用アジュバントや炎症疾患の治療剤及び/又は予防剤、及び、アレルギー疾患の治療及び/又は予防用食品組成物として有効であることを見出し、本発明を完成するに至った。すなわち、本発明は、以下の[1]〜[27]に関する。 As a result of diligent research, the present inventors have found that lycopene alone has an excellent regulatory T cell-inducing effect, and is used as an adjuvant for allergen immunotherapy, a therapeutic agent and / or a preventive agent for inflammatory diseases, and a treatment for allergic diseases. And / or found to be effective as a preventive food composition, the present invention has been completed. That is, the present invention relates to the following [1] to [27].
[1]リコピン又は1−HO−3’,4’−ジデヒドロリコピン、1,1’−(HO) 2 −3,4,3’,4’−テトラデヒドロリコピン及び1,1’−(HO) 2 −3,4−ジデヒドロリコピンからなる群から選ばれるリコピン誘導体を有効成分として含有する、制御性T細胞誘導剤。
[2]炎症疾患を発症していない対象に対して投与する、[1]の制御性T細胞誘導剤。
[3]前記対象がヒトである[2]に記載の制御性T細胞誘導剤。
[4]前記制御性T細胞がFoxp3陽性である[1]〜[3]のいずれか一つの制御性T細胞誘導剤。
[5]アレルゲン免疫療法用アジュバントとして使用するための、[1]〜[4]のいずれか一つの制御性T細胞誘導剤。
[6]前記アレルゲン免疫療法が食物アレルギーに対するアレルゲン免疫療法である、[5]の制御性T細胞誘導剤。
[7][1]〜[4]のいずれか一つの制御性T細胞誘導剤を含有する、炎症疾患の治療剤及び/又は予防剤。
[8]前記炎症疾患がアレルギー疾患である、[7]の治療剤及び/又は予防剤。
[9]前記アレルギー疾患が食物アレルギーである、[8]の治療剤及び/又は予防剤。
[10]前記炎症疾患が大腸炎である、[7]の治療剤及び/又は予防剤。
[11]リコピン又は1−HO−3’,4’−ジデヒドロリコピン、1,1’−(HO) 2 −3,4,3’,4’−テトラデヒドロリコピン及び1,1’−(HO) 2 −3,4−ジデヒドロリコピンからなる群から選ばれるリコピン誘導体を有効成分として含有する、アレルゲン免疫療法用アジュバント。
[12]前記アレルゲン免疫療法が、食物アレルギーに対するアレルゲン免疫療法である、[11]のアジュバント。
[13][11]又は[12]のアジュバント及び、アレルゲンを有効成分として含有する、アレルゲン免疫療法によるアレルギー疾患の治療剤。
[14]前記アレルギー疾患が食物アレルギーである、[13]の治療剤。
[15]リコピン又は1−HO−3’,4’−ジデヒドロリコピン、1,1’−(HO) 2 −3,4,3’,4’−テトラデヒドロリコピン及び1,1’−(HO) 2 −3,4−ジデヒドロリコピンからなる群から選ばれるリコピン誘導体を有効成分として含有する、制御性T細胞誘導用食品組成物。
[16]炎症疾患を発症していない対象に摂取させる、[15]の食品組成物。
[17]前記対象がヒトである[16]の食品組成物。
[18]前記制御性T細胞がFoxp3陽性である[15]〜[17]のいずれか一つの食品組成物。
[19][15]〜[18]のいずれか一つの食品組成物を含有する、炎症疾患の治療及び/又は予防用食品組成物。
[20]前記炎症疾患がアレルギー疾患である、[19]の治療及び/又は予防用食品組成物。
[21]前記アレルギー疾患が食物アレルギーである、[20]の治療及び/又は予防用食品組成物。
[22]前記炎症疾患が大腸炎である、[19]の治療及び/又は予防用食品組成物。
[23]リコピン又は1−HO−3’,4’−ジデヒドロリコピン、1,1’−(HO) 2 −3,4,3’,4’−テトラデヒドロリコピン及び1,1’−(HO) 2 −3,4−ジデヒドロリコピンからなる群から選ばれるリコピン誘導体を有効成分として含有する、アレルギー疾患改善のためのアジュバント用食品組成物。
[24]前記アレルギー疾患改善が、アレルゲン免疫療法である、[23]のアジュバント用食品組成物。
[25]前記アレルゲン免疫療法が食物アレルギー疾患に対するアレルゲン免疫療法である、[24]の食品組成物。
[26][11]又は[12]のアジュバント及び、アレルゲンを含有する、アレルゲン免疫療法によるアレルギー疾患改善用食品組成物。
[27][23]〜[25]のいずれか一つのアジュバント用食品組成物及び、アレルゲンを含有する、アレルギー疾患改善用食品組成物。
[28]前記アレルギー疾患改善が、アレルゲン免疫療法によるものである、[26]又は[27]の食品組成物。
[29]前記食品組成物が、健康食品、機能性表示食品、特別用途食品、病者用食品、栄養補助食品、サプリメント又は特定保健用食品である、[15]〜[28]のいずれか一つの食品組成物。
[1] lycopene or 1-HO-3 ', 4'- didecyl hydro lycopene, 1,1' - (HO) 2 -3,4,3 ', 4'- tetra dehydroepiandrosterone lycopene and 1,1' - (HO ) lycopene derivative selected from the group consisting of 2-3,4 didecyl hydro lycopene as an active ingredient, regulatory T cell-inducing agent.
[2] The regulatory T cell inducer of [1] to be administered to a subject who has not developed an inflammatory disease.
[3] The regulatory T cell inducer according to [2], wherein the subject is a human.
[4] A regulatory T cell inducer according to any one of [1] to [3], wherein the regulatory T cells are Foxp3 positive.
[5] A regulatory T cell inducer according to any one of [1] to [4] for use as an adjuvant for allergen immunotherapy.
[6] The regulatory T cell inducer according to [5], wherein the allergen immunotherapy is an allergen immunotherapy for food allergies.
[7] A therapeutic and / or prophylactic agent for an inflammatory disease, which comprises a regulatory T cell inducer according to any one of [1] to [4].
[8] The therapeutic agent and / or preventive agent of [7], wherein the inflammatory disease is an allergic disease.
[9] The therapeutic agent and / or preventive agent according to [8], wherein the allergic disease is a food allergy.
[10] The therapeutic and / or prophylactic agent of [7], wherein the inflammatory disease is colitis.
[11] lycopene or 1-HO-3 ', 4'- didecyl hydro lycopene, 1,1' - (HO) 2 -3,4,3 ', 4'- tetra dehydroepiandrosterone lycopene and 1,1' - (HO ) lycopene derivative selected from the group consisting of 2-3,4 didecyl hydro lycopene as an active ingredient, allergen immunotherapy adjuvants.
[12] The adjuvant of [11], wherein the allergen immunotherapy is an allergen immunotherapy for food allergies.
[13] A therapeutic agent for allergic diseases by allergen immunotherapy, which contains the adjuvant of [11] or [12] and an allergen as an active ingredient.
[14] The therapeutic agent according to [13], wherein the allergic disease is a food allergy.
[15] lycopene or 1-HO-3 ', 4'- didecyl hydro lycopene, 1,1' - (HO) 2 -3,4,3 ', 4'- tetra dehydroepiandrosterone lycopene and 1,1' - (HO ) lycopene derivative selected from the group consisting of 2-3,4 didecyl hydro lycopene as an active ingredient, regulatory T cells induced food composition.
[16] The food composition of [15] to be ingested by a subject who has not developed an inflammatory disease.
[17] The food composition of [16], wherein the subject is a human.
[18] A food composition according to any one of [15] to [17], wherein the regulatory T cells are Foxp3 positive.
[19] A food composition for treating and / or preventing an inflammatory disease, which comprises the food composition according to any one of [15] to [18].
[20] The therapeutic and / or preventive food composition of [19], wherein the inflammatory disease is an allergic disease.
[21] The therapeutic and / or preventive food composition of [20], wherein the allergic disease is a food allergy.
[22] The therapeutic and / or preventive food composition of [19], wherein the inflammatory disease is colitis.
[23] lycopene or 1-HO-3 ', 4'- didecyl hydro lycopene, 1,1' - (HO) 2 -3,4,3 ', 4'- tetra dehydroepiandrosterone lycopene and 1,1' - (HO ) lycopene derivative selected from the group consisting of 2-3,4 didecyl hydro lycopene as an active ingredient, the adjuvant food composition for allergic diseases improvement.
[24] The food composition for an adjuvant according to [23], wherein the improvement of the allergic disease is allergen immunotherapy.
[ 25 ] The food composition of [24 ], wherein the allergen immunotherapy is an allergen immunotherapy for a food allergic disease.
[ 26 ] A food composition for improving allergic diseases by allergen immunotherapy, which contains the adjuvant of [11] or [12] and an allergen.
[27] [23] - one of A adjuvant food composition [25] and contains allergens, allergic diseases improved food composition.
[28] The food composition of [26] or [27], wherein the improvement of the allergic disease is due to allergen immunotherapy.
[ 29 ] Any one of [15] to [28 ], wherein the food composition is a health food, a food with a functional claim, a food for special use, a food for the sick, a nutritional supplement, a supplement or a food for specified health use. Two food compositions.
本発明に係るリコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導剤、アレルゲン免疫療法用アジュバントは、優れた制御性T細胞誘導作用を有し、炎症疾患、アレルギー疾患等の治療剤及び/又は予防剤として有用である。また、本発明に係るリコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導用食品組成物、アレルゲン免疫療法のためのアジュバント用食品組成物は、優れた制御性T細胞誘導作用を有し、炎症疾患の治療及び/又は予防用食品組成物として有用である。 The regulatory T cell inducer and the adjuvant for allergen immunotherapy containing lycopene or a derivative thereof as an active ingredient have an excellent regulatory T cell inducing action and are therapeutic agents for inflammatory diseases, allergic diseases and the like. And / or useful as a prophylactic agent. In addition, the regulatory T cell-inducing food composition and the adjuvant food composition for allergen immunotherapy containing lycopene or a derivative thereof as an active ingredient have an excellent regulatory T cell-inducing action. It is useful as a food composition for the treatment and / or prevention of inflammatory diseases.
≪リコピン≫
以下、本発明について詳しく説明する。
本発明に用いられるリコピンは、下記構造を有する分子量356.87のカロテノイドであり、水には殆ど溶けない脂溶性の赤色色素である。
≪Lycopene≫
Hereinafter, the present invention will be described in detail.
Lycopene used in the present invention is a carotenoid having the following structure and having a molecular weight of 356.87, and is a fat-soluble red pigment that is almost insoluble in water.
本発明のリコピンは、異性体が存在する。該異性体としては、オールトランス異性体と、11個の共役π結合のうち、少なくとも1個にシス型を含む、シス異性体等が挙げられる。該シス異性体としては、5−シス体、9−シス体、13−シス体等が挙げられる。本発明において用いられるリコピンは、これらのリコピンのいずれであってもよい。 The lycopene of the present invention has an isomer. Examples of the isomer include an all-trans isomer and a cis isomer in which at least one of the 11 conjugate π bonds contains a cis type. Examples of the cis isomer include 5-cis isomers, 9-cis isomers, 13-cis isomers and the like. The lycopene used in the present invention may be any of these lycopene.
リコピンは、天然においては、トマト、柿、スイカ、ピンクグレープフルーツなどの天然物に含まれており、本発明において用いられるリコピンは、これらの天然物から分離及び抽出されたのものであってもよい。 Lycopene is naturally contained in natural products such as tomatoes, persimmons, watermelons, and pink grapefruits, and the lycopene used in the present invention may be separated and extracted from these natural products.
また、本発明において用いられるリコピンは、天然物からの抽出物を必要に応じて適宜精製したものでもよいし、合成品であってもよい。 In addition, the lycopene used in the present invention may be an extract from a natural product that has been appropriately purified as necessary, or may be a synthetic product.
また、本発明において用いられるリコピンの誘導体としては、例えば、1−HO−3’,4’−ジデヒドロリコピン、1,1’−(HO)2−3,4,3’,4’−テトラデヒドロリコピン、及び1,1’−(HO)2−3,4−ジデヒドロリコピンが挙げられ、常法に従い、製造することができる。 As the derivatives of lapis Kopin used in the present invention, for example, 1 -HO-3 ', 4'- didecyl hydro lycopene, 1, 1' - (HO ) 2 -3,4,3 ', 4' - tetra dehydroepiandrosterone lycopene, and 1,1 '- (HO) 2 -3,4- Jidehidororikopi emissions and the like, may be a conventional method, to manufacture.
本発明において用いられるリコピンの形態の一つとしては、トマト加工品から抽出された脂溶性抽出物が挙げられる。トマト加工品としては、トマトペースト、トマトピューレ、トマトジュース、トマトパルプ等が挙げられる。該トマト加工品から抽出された脂溶性抽出物は、当該脂溶性抽出物を含む組成物中における安定性、品質、生産性の点から特に好ましい。
ここで、トマト加工品から抽出された脂溶性抽出物とは、トマト加工品から、有機溶剤や油を用いて抽出された抽出物や超臨界抽出法によって抽出された抽出物を意味する。 脂溶性抽出物であるリコピンとしては、リコピン含有オイル又はオレオレジンとして広く市販されているトマト抽出物を用いることができ、例えば、サンブライト(株)より販売されているLyc−O−Mato 15%、Lyc−O−Mato 6%、協和発酵バイオ(株)より販売されているリコピン18等が挙げられる。
One of the forms of lycopene used in the present invention is a fat-soluble extract extracted from a processed tomato product. Examples of processed tomato products include tomato paste, tomato puree, tomato juice, tomato pulp and the like. The fat-soluble extract extracted from the processed tomato product is particularly preferable from the viewpoint of stability, quality and productivity in the composition containing the fat-soluble extract.
Here, the fat-soluble extract extracted from the processed tomato product means an extract extracted from the processed tomato product using an organic solvent or oil or an extract extracted by a supercritical extraction method. As the lycopene, which is a fat-soluble extract, a lycopene-containing oil or a tomato extract widely marketed as oleoresin can be used. For example, Lyc-O-Mato 15% sold by Sunbright Co., Ltd. , Lyc-O-Mato 6%, lycopene 18 sold by Kyowa Hakko Bio Co., Ltd. and the like.
≪制御性T細胞誘導剤≫
リコピンは、後述の実施例に記載の通り、制御性T細胞誘導作用を有する。従って、リコピン又はその誘導体は、制御性T細胞誘導剤、制御性T細胞誘導作用を介するアレルゲン免疫療法用アジュバント、制御性T細胞誘導による炎症疾患の治療剤及び/又は予防剤の有効成分として用いることができる。
≪Regulatory T cell inducer≫
Lycopene has a regulatory T cell-inducing effect, as described in Examples below. Therefore, lycopene or a derivative thereof is used as an active ingredient of a regulatory T cell inducer, an adjuvant for allergen immunotherapy via a regulatory T cell inducing action, a therapeutic agent and / or a preventive agent for an inflammatory disease caused by regulatory T cell induction. be able to.
本発明において「制御性T細胞」とは、免疫反応を抑制する特定のT細胞サブポピュレーションを意味する。 In the present invention, "regulatory T cell" means a specific T cell subpopulation that suppresses an immune response.
制御性T細胞は、TGF−β存在下における抗原刺激により末梢血中のナイーブT細胞から分化誘導され、免疫寛容の機構に関与している。 Regulatory T cells are induced to differentiate from naive T cells in peripheral blood by antigen stimulation in the presence of TGF-β, and are involved in the mechanism of immune tolerance.
本発明における制御性T細胞は、Foxp3陽性細胞であり、好ましくは、Foxp3及びCD25陽性細胞であり、これらの表現マーカーを検出することにより、制御性T細胞の存在を確認することができる。 The regulatory T cells in the present invention are Foxp3 positive cells, preferably Foxp3 and CD25 positive cells, and the presence of regulatory T cells can be confirmed by detecting these expression markers.
本発明において、制御性T細胞誘導作用の測定は、ナイーブT細胞から、制御性T細胞への誘導を測定できる方法であれば、特に制限はされない。例えば、生体への被検物質の投与下、又は非投与下で、大腸粘膜固有層などの対象組織におけるFoxp3陽性CD25陽性細胞の割合を、フローサイトメーターを用いて測定することにより、被検物質の制御性T細胞誘導作用を測定することができる。被検物質投与下における制御性T細胞の割合が、被検物質非投与下での制御性T細胞の割合と比較して、高くなれば当該被検物質は制御性T細胞誘導作用を有すると評価することができる。 In the present invention, the measurement of the regulatory T cell inducing action is not particularly limited as long as it is a method capable of measuring the induction from naive T cells to the regulatory T cells. For example, by measuring the proportion of Foxp3-positive CD25-positive cells in a target tissue such as the lamina propria of the large intestine with or without administration of the test substance to a living body using a flow cytometer, the test substance The regulatory T cell-inducing effect of When the proportion of regulatory T cells under the administration of the test substance is higher than the proportion of regulatory T cells under the administration of the test substance, the test substance has a regulatory T cell-inducing effect. Can be evaluated.
また、制御性T細胞誘導作用は、脾臓より単離されたCD4陽性のナイーブT細胞を、制御性T細胞へ分化誘導することで直接評価することもできる。例えば、脾臓から単離した細胞を、さらにCD4の発現により選別することによって得られたナイーブT細胞をTGF−β等のサイトカインと被検物質とを添加して培養し、制御性T細胞の割合をフローサイトメーターにより測定することにより、制御性T細胞誘導作用を測定することができる。 The regulatory T cell-inducing effect can also be directly evaluated by inducing differentiation of CD4-positive naive T cells isolated from the spleen into regulatory T cells. For example, naive T cells obtained by further selecting cells isolated from the spleen by expressing CD4 are cultured by adding cytokines such as TGF-β and a test substance, and the proportion of regulatory T cells is increased. By measuring with a flow cytokine, the regulatory T cell-inducing action can be measured.
制御性T細胞は、免疫寛容機構に関与しており、本発明の制御性T細胞誘導剤は、制御性T細胞を誘導することにより、制御性T細胞が関与する疾患を治療及び/又は予防することができる。本発明において、制御性T細胞を誘導することにより治療及び/又は予防することができる制御性T細胞が関与する疾患としては、特に制限はないが、例えば、アレルギー疾患、大腸炎、肺炎等の炎症疾患、心筋梗塞、糖尿病、メタボリックシンドローム等が挙げられる。アレルギー疾患としては、アレルギー性喘息、アレルギー性鼻炎、食物アレルギー、毒物アレルギー、及びアレルギー性結膜炎等のI型即時型アトピー性アレルギー等を挙げることができる。 Regulatory T cells are involved in the immune tolerance mechanism, and the regulatory T cell inducers of the present invention treat and / or prevent diseases involving regulatory T cells by inducing regulatory T cells. can do. In the present invention, the diseases involving regulatory T cells that can be treated and / or prevented by inducing regulatory T cells are not particularly limited, but for example, allergic diseases, colitis, pneumonia and the like. Inflammatory diseases, myocardial infarction, diabetes, metabolic syndrome and the like can be mentioned. Examples of allergic diseases include allergic asthma, allergic rhinitis, food allergies, toxic allergies, and type I immediate atopic allergies such as allergic conjunctivitis.
本発明に係る制御性T細胞誘導剤の投与形態としては、例えば錠剤、被覆錠剤、カプセル剤、顆粒剤、散剤、溶液、シロップ剤、乳液等による経口投与を挙げることができる。これらの各種製剤は、常法に従って主薬であるリコピン又はその誘導体に加えて、賦形剤、結合剤、崩壊剤、滑沢剤、着色剤、矯味矯臭剤、溶解補助剤、懸濁剤、コーティング剤などの医薬等の製剤技術分野において通常使用しうる既知の補助剤を用いて製剤化することができる。 Examples of the administration form of the regulatory T cell inducer according to the present invention include oral administration with tablets, coated tablets, capsules, granules, powders, solutions, syrups, emulsions and the like. In addition to lycopene, which is the main drug, or derivatives thereof , these various formulations are prepared according to conventional methods, such as excipients, binders, disintegrants, lubricants, colorants, flavoring agents, solubilizing agents, suspending agents, and coatings. It can be formulated using a known auxiliary agent that can be usually used in the field of formulation technology such as pharmaceuticals such as a drug.
本発明の制御性T細胞誘導剤におけるリコピン又はその誘導体の含有量は、剤型によって異なるため特に限定されない。 The content of lycopene or a derivative thereof in the regulatory T cell inducer of the present invention is not particularly limited because it varies depending on the dosage form.
本発明に係る制御性T細胞誘導剤の投与量は、個々の薬剤の活性、患者の性別、症状、年齢、投与方法によって異なるが、例えば、リコピン又はその誘導体を、乾燥物換算で、通常、1回10mg〜100mg、好ましくは、15mg〜60mg投与することができる量である。 The dose of the regulatory T cell inducer according to the present invention varies depending on the activity of each drug, the sex, symptoms, age, and administration method of the patient. The amount is such that 10 mg to 100 mg, preferably 15 mg to 60 mg can be administered at a time.
本発明の制御性T細胞誘導剤を投与する対象は、動物、中でも哺乳類が挙げられる。哺乳類としては、特に制限はないが、例えば、ヒト、イヌ、ネコ、ウシ、ウマ等が挙げられ、ヒトであることが好ましい。また、本発明の制御性T細胞誘導剤は、炎症疾患に対する予防剤として、炎症疾患を発症していない上記対象に投与することもできる。 Targets to which the regulatory T cell inducer of the present invention is administered include animals, especially mammals. The mammal is not particularly limited, and examples thereof include humans, dogs, cats, cows, horses, and the like, and humans are preferable. In addition, the regulatory T cell inducer of the present invention can also be administered to the above-mentioned subject who has not developed an inflammatory disease as a preventive agent against an inflammatory disease.
≪アレルゲン免疫療法用アジュバント≫
本発明により、リコピン又はその誘導体を有効成分として含有する、アレルゲン免疫療法用アジュバントを提供することができる。アレルゲン免疫療法とは、アレルギー疾患の病因であるアレルゲンを投与していくことにより、アレルゲンに暴露された場合に引き起こされる関連症状を緩和する治療法である。
アレルゲン免疫療法の効果発現機序には種々の側面があるが、そのうちの一つとして、自然暴露より多量なアレルゲンを体内に取り込むことで、制御性T細胞の誘導やTh2細胞の増加抑制等が引き起こされ、これによりアレルギー反応の抑制効果を発現する機序が挙げられる。本発明におけるアレルゲン免疫療法用アジュバントは、該アレルゲン免疫療法において、アレルゲンと共に投与することで、制御性T細胞の誘導やTh2細胞の増加抑制等のアレルゲンの作用を増強させることができる。
≪Adjuliate for allergen immunotherapy≫
INDUSTRIAL APPLICABILITY According to the present invention, an adjuvant for allergen immunotherapy containing lycopene or a derivative thereof as an active ingredient can be provided. Allergen immunotherapy is a treatment method in which allergens, which are the etiology of allergic diseases, are administered to alleviate the related symptoms caused when exposed to allergens.
There are various aspects to the mechanism of manifestation of the effects of allergen immunotherapy, and one of them is the induction of regulatory T cells and the suppression of Th2 cell increase by taking in a larger amount of allergen than natural exposure. There is a mechanism by which it is triggered and thereby exerts an inhibitory effect on allergic reactions. The adjuvant for allergen immunotherapy in the present invention can enhance the action of allergens such as induction of regulatory T cells and suppression of Th2 cell increase by administration together with allergens in the allergen immunotherapy.
本発明に係るリコピン又はその誘導体は、制御性T細胞誘導作用を有するため、本発明のリコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導剤は、アレルゲン免疫療法用アジュバントとして使用することができる。本発明のアレルゲン免疫療法用アジュバントが有効なアレルゲン免疫療法としては、アレルギー疾患に対するアレルゲン免疫療法であれば特に制限はないが、例えば、食物アレルギーに対するアレルゲン免疫療法等が挙げられる。 Since the lycopene or its derivative according to the present invention has a regulatory T cell-inducing action , the regulatory T cell-inducing agent containing the lycopene or its derivative of the present invention as an active ingredient is used as an adjuvant for allergen immunotherapy. be able to. The allergen immunotherapy for which the adjuvant for allergen immunotherapy of the present invention is effective is not particularly limited as long as it is an allergen immunotherapy for allergic diseases, and examples thereof include allergen immunotherapy for food allergies.
本発明のリコピン又はその誘導体を有効成分として含有する、アレルゲン免疫療法用アジュバントは、アレルゲンと共に用いることで、アレルゲン免疫療法によるアレルギー疾患を治療することができる。
本発明のアレルゲン免疫療法用アジュバントにより治療することができるアレルギー疾患は、特に制限はないが、例えば、アレルギー性喘息、アレルギー性鼻炎、食物アレルギー、毒物アレルギー、及びアレルギー性結膜炎等のI型即時型アトピー性アレルギー等を挙げることができる。
The allergen immunotherapy adjuvant containing lycopene or a derivative thereof as an active ingredient can be used together with an allergen to treat allergic diseases caused by allergen immunotherapy.
The allergic diseases that can be treated by the adjuvant for allergen immunotherapy of the present invention are not particularly limited, but are, for example, type I immediate type such as allergic asthma, allergic rhinitis, food allergy, toxic allergy, and allergic conjunctivitis. Atopic allergies and the like can be mentioned.
本発明のアレルゲン免疫療法用アジュバントにおけるリコピン又はその誘導体の含有量は、剤型によって異なるため特に限定されない。 The content of lycopene or a derivative thereof in the adjuvant for allergen immunotherapy of the present invention is not particularly limited because it varies depending on the dosage form.
≪アレルギー疾患治療剤≫
本発明のリコピン又はその誘導体を有効成分として含有する、アレルゲン免疫療法用アジュバントは、アレルゲンと共に用いることで、アレルゲン免疫療法によるアレルギー疾患を治療することができるため、該アレルゲン免疫療法用アジュバント及び、アレルゲンは、アレルゲン免疫療法によるアレルギー疾患の治療剤の有効成分として用いることができる。
本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤により治療することができるアレルギー疾患は、特に制限はないが、例えば、アレルギー性喘息、アレルギー性鼻炎、食物アレルギー、毒物アレルギー、及びアレルギー性結膜炎等のI型即時型アトピー性アレルギー等を挙げることができる。
≪Therapeutic agent for allergic diseases≫
The allergen immunotherapy adjuvant containing lycopene or a derivative thereof as an active ingredient can be used together with an allergen to treat allergic diseases caused by allergen immunotherapy. Therefore, the allergen immunotherapy adjuvant and the allergen Can be used as an active ingredient of a therapeutic agent for allergic diseases by allergen immunotherapy.
The allergic disease that can be treated by the therapeutic agent for allergic disease by the allergen immunotherapy of the present invention is not particularly limited, but for example, allergic asthma, allergic rhinitis, food allergy, toxic allergy, allergic conjunctivitis and the like. Type I immediate atopic allergies and the like can be mentioned.
本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤の投与形態としては、例えば錠剤、被覆錠剤、カプセル剤、顆粒剤、散剤、溶液、シロップ剤、乳液等による経口投与を挙げることができる。これらの各種製剤は、常法に従って主薬であるリコピン又はその誘導体、及びアレルゲンに加えて、賦形剤、結合剤、崩壊剤、滑沢剤、着色剤、矯味矯臭剤、溶解補助剤、懸濁剤、コーティング剤などの医薬等の製剤技術分野において通常使用しうる既知の補助剤を用いて製剤化することができる。 Examples of the administration form of the therapeutic agent for allergic diseases by allergen immunotherapy of the present invention include oral administration with tablets, coated tablets, capsules, granules, powders, solutions, syrups, emulsions and the like. In addition to lycopene or its derivatives , which are the main agents, and allergens, these various preparations are prepared according to conventional methods, with excipients, binders, disintegrants, lubricants, colorants, flavoring agents, solubilizers, suspensions. It can be formulated using a known auxiliary agent that can be usually used in the field of formulation technology such as pharmaceuticals such as agents and coating agents.
本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤におけるリコピン又はその誘導体の含有量は、剤型によって異なるが、一般にアレルゲン免疫療法用によるアレルギー疾患の治療剤の全質量に対して、1質量%〜50質量%であることが好ましく、1〜30質量%であることがより好ましい。
また、本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤におけるアレルゲンの含有量は、剤型及び使用するアレルゲン、並びに治療期間等により異なるが、一般にアレルゲン免疫療法によるアレルギー疾患の治療剤の全質量に対して、1質量%〜50質量%であることが好ましく、1〜20質量%であることがより好ましい。
The content of lycopene or a derivative thereof in the therapeutic agent for allergic diseases by allergen immunotherapy of the present invention varies depending on the dosage form, but is generally 1% by mass to the total mass of the therapeutic agent for allergic diseases by allergen immunotherapy. It is preferably 50% by mass, more preferably 1 to 30% by mass.
The content of the allergen in the therapeutic agent for allergen disease by allergen immunotherapy of the present invention varies depending on the dosage type, the allergen used, the treatment period, etc., but is generally the total mass of the therapeutic agent for allergen disease by allergen immunotherapy. On the other hand, it is preferably 1% by mass to 50% by mass, and more preferably 1 to 20% by mass.
本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤の投与量は、個々の薬剤の活性、患者の性別、症状、年齢、投与方法によって異なるが、例えば、リコピン又はその誘導体を、乾燥物換算で、通常、1回10mg〜100mg、好ましくは、15mg〜60mg投与することができる量である。 The dose of the therapeutic agent for allergic diseases by the allergen immunotherapy of the present invention varies depending on the activity of each drug, the sex, symptom, age, and administration method of the patient. Usually, it is an amount that can be administered at a time of 10 mg to 100 mg, preferably 15 mg to 60 mg.
本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤を投与する対象は、動物、中でも哺乳類が挙げられる。哺乳類としては、特に制限はないが、例えば、ヒト、イヌ、ネコ、ウシ、ウマ等が挙げられ、ヒトであることが好ましい。また、本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤の有効成分の一つであるリコピンは、食経験のあるトマト等の天然物に含まれていることから、本発明のアレルゲン免疫療法によるアレルギー疾患の治療剤は、長期間にわたって行われるアレルゲン免疫療法において、副作用の可能性が低く、非常に安全性が高い。 The target for administering the therapeutic agent for allergic diseases by the allergen immunotherapy of the present invention includes animals, especially mammals. The mammal is not particularly limited, and examples thereof include humans, dogs, cats, cows, horses, and the like, and humans are preferable. In addition, since lycopene, which is one of the active ingredients of the therapeutic agent for allergen diseases by allergen immunotherapy of the present invention, is contained in natural products such as tomatoes that have eaten experience, allergies by allergen immunotherapy of the present invention Therapeutic agents for the disease are very safe with low potential for side effects in long-term allergen immunotherapy.
≪食品組成物≫
本発明により、リコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導用食品組成物を提供することができる。本発明に係るリコピン又はその誘導体は、制御性T細胞誘導作用を有するため、有効成分として食品組成物に含有させることにより、炎症疾患の治療用及び/又は予防用食品の有効成分として、各種食品組成物に含有させることができる。本発明において、食品組成物には、食品だけでなく飲料も含む。
≪Food composition≫
INDUSTRIAL APPLICABILITY According to the present invention, it is possible to provide a food composition for inducing regulatory T cells containing lycopene or a derivative thereof as an active ingredient. Since lycopene or a derivative thereof according to the present invention has a regulatory T cell-inducing action, various foods can be used as an active ingredient of a food for treating and / or preventing an inflammatory disease by being contained in a food composition as an active ingredient. It can be contained in the composition. In the present invention, the food composition includes not only foods but also beverages.
本発明の食品組成物において、制御性T細胞は、Foxp3陽性細胞であり、好ましくは、Foxp3及びCD25陽性細胞である。 In the food composition of the present invention, the regulatory T cells are Foxp3 positive cells, preferably Foxp3 and CD25 positive cells.
本発明の制御性T細胞誘導用食品組成物により治療及び/又は予防することができる疾患としては、特に制限はないが、例えば、例えば、大腸炎、肺炎等の炎症疾患、心筋梗塞、糖尿病、メタボリックシンドローム、アレルギー疾患等が挙げられる。アレルギー疾患としては、アレルギー性喘息、アレルギー性鼻炎、食物アレルギー、毒物アレルギー、及びアレルギー性結膜炎等のI型即時型アトピー性アレルギー等を挙げることができる。 The diseases that can be treated and / or prevented by the regulatory T cell-inducing food composition of the present invention are not particularly limited, but for example, inflammatory diseases such as colitis and pneumonia, myocardial infarction, diabetes, and the like. Examples include metabolic syndrome and allergic diseases. Examples of allergic diseases include allergic asthma, allergic rhinitis, food allergies, toxic allergies, and type I immediate atopic allergies such as allergic conjunctivitis.
本発明の制御性T細胞誘導用食品組成物を摂取させる対象は、動物、中でも哺乳類が挙げられる。哺乳類としては、特に制限はないが、例えば、ヒト、イヌ、ネコ、ウシ、ウマ等が挙げられ、ヒトであることが好ましい。また、本発明の制御性T細胞誘導用食品組成物の有効成分であるリコピン又はその誘導体は、アレルギー疾患に対する予防剤として、炎症疾患を発症していない対象に摂取させることもできる。 Targets for ingesting the regulatory T cell-inducing food composition of the present invention include animals, especially mammals. The mammal is not particularly limited, and examples thereof include humans, dogs, cats, cows, horses, and the like, and humans are preferable. In addition, lycopene or a derivative thereof, which is an active ingredient of the food composition for inducing regulatory T cells of the present invention, can be ingested as a preventive agent against allergic diseases by a subject who has not developed an inflammatory disease.
また、本発明により、リコピン又はその誘導体を有効成分として含有する、アレルゲン免疫療法のためのアジュバント用食品組成物を提供することができる。本発明に係るリコピンは、制御性T細胞誘導作用を有するため、アレルゲン免疫療法のためのアジュバントとして食品組成物に含有させることにより、アレルゲン免疫療法においてアレルゲンの作用を増強させることができる。本発明のアレルゲン免疫療法のためのアジュバント用食品組成物が有効なアレルゲン免疫療法としては、アレルギー疾患に対するアレルゲン免疫療法であれば特に制限はないが、例えば、食物アレルギーに対するアレルゲン免疫療法等が挙げられる。 Further, according to the present invention, it is possible to provide a food composition for an adjuvant for allergen immunotherapy, which contains lycopene or a derivative thereof as an active ingredient. Since the lycopene according to the present invention has a regulatory T cell-inducing action, the action of the allergen can be enhanced in the allergen immunotherapy by including it in the food composition as an adjuvant for the allergen immunotherapy. The allergen immunotherapy for which the food composition for adjuvant for the allergen immunotherapy of the present invention is effective is not particularly limited as long as it is an allergen immunotherapy for allergic diseases, and examples thereof include allergen immunotherapy for food allergies. ..
前記アレルゲン免疫療法用アジュバント及び、アレルゲンを含有する食品組成物、並びに、前記アレルゲン免疫療法のためのアジュバント用食品組成物及び、アレルゲンを含有する食品組成物は、アレルギー疾患治療用食品組成物として、アレルギー疾患を治療することができる。当該アレルギー疾患治療用食品組成物により治療することができるアレルギー疾患としては、特に制限はないが、例えば、アレルギー性喘息、アレルギー性鼻炎、食物アレルギー、毒物アレルギー、及びアレルギー性結膜炎等のI型即時型アトピー性アレルギー等を挙げることができる。 The adjuvant for allergen immunotherapy, the food composition containing the allergen, the food composition for the adjuvant for the allergen immunotherapy, and the food composition containing the allergen are used as the food composition for treating allergic diseases. Allergic diseases can be treated. The allergic disease that can be treated with the food composition for treating allergic disease is not particularly limited, but for example, type I immediate such as allergic asthma, allergic rhinitis, food allergy, toxic allergy, and allergic conjunctivitis. Type atopic allergies and the like can be mentioned.
本発明のアレルゲン免疫療法のためのアジュバント用食品組成物、及びアレルギー疾患治療用食品組成物を摂取させる対象は、動物、中でも哺乳類が挙げられる。哺乳類としては、特に制限はないが、例えば、ヒト、イヌ、ネコ、ウシ、ウマ等が挙げられ、ヒトであることが好ましい。 Targets for ingesting the food composition for adjuvant for allergen immunotherapy of the present invention and the food composition for treating allergic diseases include animals, especially mammals. The mammal is not particularly limited, and examples thereof include humans, dogs, cats, cows, horses, and the like, and humans are preferable.
本発明の制御性T細胞誘導用食品組成物、アレルゲン免疫療法のためのアジュバント用食品組成物、及びアレルギー疾患治療用食品組成物(以下、本発明の食品組成物と総称する)としては、特に限定されないが、例えば、各種飲料、ヨーグルト、チーズ、バター、乳酸菌発酵品等の各種乳製品、流動食、ゼリー、キャンディ、レトルト食品、錠菓、クッキー、カステラ、パン、ビスケット、などが挙げられる。また、健康食品、機能性表示食品、特別用途食品、病者用食品、栄養補助食品、サプリメント又は特定保健用食品等として使用することもできる。 The food composition for inducing controllable T cells of the present invention, the food composition for adjuvant for allergen immunotherapy, and the food composition for treating allergic diseases (hereinafter collectively referred to as the food composition of the present invention) are particularly selected. Examples include, but are not limited to, various beverages, yogurt, cheese, butter, various dairy products such as fermented lactic acid bacteria, liquid foods, jellies, candy, retort foods, tablets, cookies, castella, bread, biscuits, and the like. It can also be used as a health food, a food with a functional claim, a special purpose food, a food for the sick, a dietary supplement, a supplement, a food for specified health use, or the like.
本発明の食品組成物には、可食性の、炭水化物、蛋白質、脂質、ビタミン類、ミネラル類、糖質(ブドウ糖、等)、天然又は人工甘味剤、クエン酸、炭酸水、果汁、安定剤、保存剤、結合剤、増粘剤、乳化剤などを適宜配合することができる。 The food compositions of the present invention include edible carbohydrates, proteins, lipids, vitamins, minerals, sugars (glucose, etc.), natural or artificial sweeteners, citric acid, carbonated water, fruit juices, stabilizers, etc. Preservatives, binders, thickeners, emulsifiers and the like can be appropriately added.
本発明の食品組成物は、その形態によって異なるが、リコピン又はその誘導体を、1g当り、乾燥物換算で、0.1mg〜200mg程度配合することができる。例えば本発明の食品組成物を飲料とする場合、100mL当り、乾燥物換算で、好ましくは10mg〜200mg、より好ましくは10mg〜120mg配合することができる。例えば本発明の食品組成物をサプリメントとする場合、1g当り、乾燥物換算で、好ましくは0.1mg〜200mg程度配合することができる。 Although the food composition of the present invention varies depending on its form, lycopene or a derivative thereof can be blended in an amount of about 0.1 mg to 200 mg per 1 g in terms of dry matter. For example, when the food composition of the present invention is used as a beverage, preferably 10 mg to 200 mg, more preferably 10 mg to 120 mg can be blended per 100 mL in terms of dry matter. For example, when the food composition of the present invention is used as a supplement, it can be blended in an amount of preferably about 0.1 mg to 200 mg per 1 g in terms of dry matter.
また、本発明の食品組成物のうち、アレルギー疾患治療用食品組成物に含まれるアレルゲンの含有量は、食品組成物の形態や使用するアレルゲン、治療期間等により異なるが、一般にアレルギー疾患治療用食品組成物の全質量に対して、1質量%〜50質量%であることが好ましく、1〜20質量%であることがより好ましい。 Further, among the food compositions of the present invention, the content of allergens contained in the food composition for treating allergic diseases varies depending on the form of the food composition, the allergens used, the treatment period, etc., but is generally a food for treating allergic diseases. It is preferably 1% by mass to 50% by mass, more preferably 1 to 20% by mass, based on the total mass of the composition.
本発明の食品組成物において、リコピン又はその誘導体は、乾燥物換算で、通常、成人(体重60kg程度)1日当り10mg〜100mg、好ましくは、15mg〜60mg摂取することができる。摂取回数は、特に限定されないが、好ましくは1日1〜3回であり、必要に応じて摂取回数を増減してもよい。なお、「1日当り」とは、本発明の食品組成物の形態によって異なるが、表示される1日の摂取目安量や、通常1度で消費する飲みきりタイプの飲料であれば1本当りに含まれる量を指すものである。 In the food composition of the present invention, lycopene or a derivative thereof can be ingested from 10 mg to 100 mg, preferably 15 mg to 60 mg per day for an adult (body weight of about 60 kg) in terms of dry matter. The number of intakes is not particularly limited, but is preferably 1 to 3 times a day, and the number of intakes may be increased or decreased as needed. The term "per day" varies depending on the form of the food composition of the present invention, but it is per bottle if it is a displayed daily intake guideline amount or a drink that is normally consumed at one time. It refers to the amount contained.
上記の食品組成物は、大腸炎、アレルギー等の各種炎症疾患の治療及び/又は予防作用を有する旨の表示をした食品組成物であってもよい。該アレルギー治療作用を有する旨の表示をした用食品組成物としては、例えば、アレルギー性喘息、アレルギー性鼻炎、食物アレルギー、毒物アレルギー、及びアレルギー性結膜炎等のI型即時型アトピー性アレルギー治療作用等の作用を有する旨の表示を付した食品組成物等が挙げられる。 The above food composition may be a food composition labeled to have a therapeutic and / or preventive effect on various inflammatory diseases such as colitis and allergies. Examples of the food composition labeled as having the allergic therapeutic action include allergic asthma, allergic rhinitis, food allergy, toxic allergy, and type I immediate atopic allergic therapeutic action such as allergic conjunctivitis. Examples thereof include food compositions labeled as having the above-mentioned action.
以下、実施例により本発明をより具体的に説明するが、本発明は以下の実施例に限定されるものではない。 Hereinafter, the present invention will be described in more detail with reference to Examples, but the present invention is not limited to the following Examples.
[実施例1]
リコピン(和光純薬工業株式会社製)が0.01%配合されたCE−2飼料(日本クレア株式会社製)を6週齢のC57BL/6J系マウス(日本エスエルシー株式会社)6匹に4週間自由摂取させた。摂取28日目にマウスを安楽死させ、開腹下に大腸を摘出した。その後、摘出した大腸からLamina Propria Dissociation Kit mouse(ミルテニーバイオテク社製)を用いて大腸粘膜固有層(LPL)から免疫細胞を調製し、抗CD4抗体、抗Foxp3抗体及び抗CD25抗体で染色した。LPLにおける制御性T細胞の割合を、Foxp3陽性CD25陽性を指標として、フローサイトメーター(製品名CantoII;BD Biosciences社製)で観察し、CD4陽性細胞全体に対する制御性T細胞の割合を算出した。その結果を図1に示す。
[Example 1]
CE-2 feed (manufactured by Nippon Claire Co., Ltd.) containing 0.01% lycopene (manufactured by Wako Pure Chemical Industries, Ltd.) was added to 6 6-week-old C57BL / 6J mice (manufactured by Nippon SLC Co., Ltd.) 4 Free intake for a week. Mice were euthanized on the 28th day of ingestion, and the large intestine was removed under laparotomy. Then, immune cells were prepared from the large intestine lamina propria (LPL) using a Lamina Propria Dissociation Kit mouse (manufactured by Milteny Biotech) from the excised large intestine, and stained with anti-CD4 antibody, anti-Foxp3 antibody and anti-CD25 antibody. The ratio of regulatory T cells in LPL was observed with a flow cytometer (product name: CantoII; manufactured by BD Biosciences) using Foxp3-positive CD25-positive as an index, and the ratio of regulatory T cells to all CD4-positive cells was calculated. The result is shown in FIG.
[比較例1]
実施例1のリコピンの代わりに、リコピンと同じカロテノイド類である、β−カロテン(和光純薬工業株式会社製)、アスタキサンチン(和光純薬工業株式会社製)、ルテイン(ChromaDex社製)をそれぞれ0.01%配合した飼料を用いて、実施例1と同様にして制御性T細胞の割合を観察し、CD4陽性細胞全体に対する制御性T細胞の割合を算出した。その結果を図1に示す。なお、「コントロール」は、実施例1において、カロテノイドを含まない飼料を用いた結果を示す。
[Comparative Example 1]
Instead of lycopene in Example 1, β-carotene (manufactured by Wako Pure Chemical Industries, Ltd.), astaxanthin (manufactured by Wako Pure Chemical Industries, Ltd.), and lutein (manufactured by ChromaDex), which are the same carotenoids as lycopene, are 0 each. Using a diet containing 0.01%, the ratio of regulatory T cells was observed in the same manner as in Example 1, and the ratio of regulatory T cells to the total number of CD4 positive cells was calculated. The result is shown in FIG. In addition, "control" shows the result of using the feed containing no carotenoid in Example 1.
図1に示したように、リコピンには制御性T細胞誘導作用が認められたが、β−カロテン、アスタキサンチン、ルテインには、制御性T細胞誘導作用は認められなかった。このことから、リコピンには、制御性T細胞誘導作用があることが明らかとなった。 As shown in FIG. 1, lycopene had a regulatory T cell-inducing effect, but β-carotene, astaxanthin, and lutein had no regulatory T cell-inducing effect. From this, it was clarified that lycopene has a regulatory T cell-inducing effect.
[実施例2]
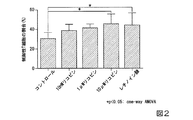
C57BL/6Jマウス(6週齢、雌、日本エスエルシー株式会社)より脾臓を摘出後、MACS Buffer(ミルテニーバイオテク社製)中ですり潰し、脾臓細胞を得た。その後、脾臓細胞を、ナイーブCD4単離キット(製品名;Naive CD4+ Tcell Isolation Kit mouse、ミルテニーバイオテク社製)を用いて、細胞へ磁気標識した。脾臓細胞をMACS Bufferで洗浄後、MACSカラム(ミルテニーバイオテク社製)によりナイーブCD4陽性細胞を分離・回収し、ナイーブCD4陽性T細胞として試験に用いた。ナイーブCD4陽性T細胞から制御性T細胞を誘導するために、ナイーブCD4陽性T細胞を96ウェルマイクロプレート(Thermo社製)において0.25μL / well 抗マウスCD3/28抗体(Gibco社製)、5ng/mL TGF−β(PEOROTECH社製)及び10%FBSを含有するRPMI1640培地中で0.5×105 cells/well(培養液量100μL)の密度で培養した。
[Example 2]
Spleens were removed from C57BL / 6J mice (6 weeks old, female, Nippon SLC Co., Ltd.) and then ground in MACS Buffer (Miltenyi Biotec) to obtain spleen cells. Then, the spleen cells were magnetically labeled with the naive CD4 isolation kit (product name; Naive CD4 + Tcell Isolation Kit mouse, manufactured by Milteny Biotech). After washing the spleen cells with MACS Buffer, naive CD4 positive cells were separated and recovered by a MACS column (manufactured by Miltenyi Biotec), and used as naive CD4 positive T cells in the test. To induce regulatory T cells from naive CD4-positive T cells, naive CD4-positive T cells were placed in 96-well microplates (Thermo) at 0.25 μL / well anti-mouse CD3 / 28 antibody (Gibco), 5 ng. The cells were cultured in RPMI1640 medium containing / mL TGF-β (manufactured by PEOROTECH) and 10% FBS at a density of 0.5 × 10 5 cells / well (culture solution volume 100 μL).
培養開始時に、レチノイン酸(ATRA;ポジティブコントロール、シグマ社製)又はリコピン(和光純薬工業株式会社製)を培地に添加した。レチノイン酸は終濃度10nMとなるように、リコピンは、終濃度10nM、1μM又は10μMとなるように添加した。培養96時間後、細胞を回収し、細胞を抗Foxp3抗体及び抗CD25抗体で染色した。制御性T細胞の割合を、Foxp3陽性CD25陽性を指標として、フローサイトメーター(製品名CantoII;BD Biosciences社製)で観察し、CD4陽性細胞全体に対する制御性T細胞の割合を算出した。 At the start of culturing, retinoic acid (ATRA; Positive Control, manufactured by Sigma) or lycopene (manufactured by Wako Pure Chemical Industries, Ltd.) was added to the medium. Retinoic acid was added to a final concentration of 10 nM, and lycopene was added to a final concentration of 10 nM, 1 μM or 10 μM. After 96 hours of culturing, cells were harvested and the cells were stained with anti-Foxp3 antibody and anti-CD25 antibody. The ratio of regulatory T cells was observed with a flow cytometer (product name: CantoII; manufactured by BD Biosciences) using Foxp3-positive CD25-positive as an index, and the ratio of regulatory T cells to all CD4-positive cells was calculated.
その結果を図2に示す。なお、図2中、「コントロール」は、リコピンを添加せずに同様の培地で培養した結果を示し、「レチノイン酸」は、ポジティブコントロールとしてレチノイン酸を添加して同様の培地で培養した結果を示す。レチノイン酸はナイーブT細胞からFoxp3陽性の制御性T細胞を誘導することが知られているが、リコピンにも濃度依存的に、ナイーブT細胞から、制御性T細胞への分化誘導作用があることが示された。また、TGF−βと抗CD3/28のみの刺激では、炎症性サイトカインによる刺激が存在しないため、炎症が発症していない状態を模しているが、当該条件においても、リコピンにより、制御性T細胞誘導が促進されたことから、炎症が発症していない対象に対しても、リコピンが制御性T細胞を誘導できることが示された。このことから、リコピンが、炎症疾患の予防効果があることが明らかとなった。 The result is shown in FIG. In FIG. 2, "control" shows the result of culturing in the same medium without adding lycopene, and "retinoic acid" shows the result of culturing in the same medium with retinoic acid added as a positive control. Shown. Retinoic acid is known to induce Foxp3-positive regulatory T cells from naive T cells, but lycopene also has a concentration-dependent effect of inducing differentiation of naive T cells into regulatory T cells. It has been shown. In addition, stimulation with only TGF-β and anti-CD3 / 28 imitates a state in which inflammation does not occur because stimulation by inflammatory cytokines does not exist, but even under these conditions, regulatory T by lycopene is used. Since cell induction was promoted, it was shown that cytokines can induce regulatory T cells even in non-inflamed subjects. From this, it was clarified that lycopene has a preventive effect on inflammatory diseases.
[実施例3]
6週齢のBALB/Cマウス (日本エスエルシー株式会社)9〜12匹に、20mgの鶏卵白アルブミン(OVA;ovalbumine)及び2mgの水酸化アルミニウムを2週間間隔で2回腹腔内投与した。最後の腹腔内投与の2週間後から、50mg OVA溶液を3日おきに1回の頻度で6回経口投与し、消化管アレルギーを誘発した。リコピン(和光純薬工業株式会社製)が0.1%配合されたCE−2飼料(日本クレア株式会社製)を、OVAの腹腔内投与開始時点(長期)、もしくは経口投与開始時点(短期)から試験終了時点まで自由摂取させた。最後のOVA経口投与後にマウスを個別のケージに移し、30〜60分後に観察した便性状の中で最も優位なものを、下記指標に基づき、その個体の便性状としてスコア化した。
0;正常便(Normal stool)
1;やや軟便(Soft stool)
2;軟便(Loose stool)
3;軽度の下痢(Mild diarrhea)
4;重度の下痢(Severe diarrhea)
5;水様弁(Fluid stool)]
その結果を図3に示す。図3中、「リコピン(短期)」は、リコピンを短期摂取させた結果を示し、「リコピン(長期)」は、リコピンを長期摂取させた結果を示す。
[Example 3]
9 to 12 6-week-old BALB / C mice (Nippon SLC Co., Ltd.) were intraperitoneally administered with 20 mg of chicken egg white albumin (OVA; ovalbumin) and 2 mg of aluminum hydroxide twice at 2-week intervals. From 2 weeks after the last intraperitoneal administration, a 50 mg OVA solution was orally administered once every 3 days for 6 times to induce gastrointestinal allergy. CE-2 feed (manufactured by Nippon Claire Co., Ltd.) containing 0.1% of lycopene (manufactured by Wako Pure Chemical Industries, Ltd.) at the start of intraperitoneal administration of OVA (long-term) or at the start of oral administration (short-term) From to the end of the test, they were allowed to take freely. After the last oral administration of OVA, the mice were transferred to individual cages, and the most predominant stool properties observed 30 to 60 minutes later were scored as the stool properties of the individual based on the following indicators.
0; Normal stool
1; Slightly loose stool (Soft school)
2; Loose stool
3; Mild diarrhea
4; Severe diarrhea
5; Water-like valve (Fluid store)]
The result is shown in FIG. In FIG. 3, "lycopene (short-term)" indicates the result of short-term intake of lycopene, and "lycopene (long-term)" indicates the result of long-term intake of lycopene.
[比較例2]
実施例3と同様にOVAを投与し、リコピンを含まないCE−2飼料を自由摂取させ、便性状を実施例3と同様にスコア化した。その結果を図3に示す。図3において、「食物アレルギー」が比較例2の結果を示す。なお、「コントロール」は、実施例3においてOVAを投与せず、リコピンを含まないCE−2飼料を用いた結果を示す。
[Comparative Example 2]
OVA was administered in the same manner as in Example 3, and a CE-2 feed containing no lycopene was freely ingested, and the stool properties were scored in the same manner as in Example 3. The result is shown in FIG. In FIG. 3, "food allergy" shows the result of Comparative Example 2. In addition, "control" shows the result of using the CE-2 feed containing no lycopene without administering OVA in Example 3.
図3に示したように、食物アレルギーの誘導にとって下痢スコアの有意な上昇が見られたが、リコピンの短期摂取、長期摂取ともに、リコピンの摂取によって下痢スコアの有意な低下が認められた。このことから、リコピンには食物アレルギーの抑制効果があることが明らかとなった。 As shown in FIG. 3, a significant increase in the diarrhea score was observed for the induction of food allergy, but a significant decrease in the diarrhea score was observed by the intake of lycopene in both the short-term intake and the long-term intake of lycopene. From this, it was clarified that lycopene has a suppressive effect on food allergies.
本発明の、リコピン又はその誘導体を有効成分として含有する、制御性T細胞誘導剤、アレルゲン免疫療法用アジュバント、制御性T細胞誘導用食品組成物、及び、アレルゲン免疫療法のためのアジュバント用食品組成物は、優れた制御性T細胞誘導作用を有し、炎症疾患等の治療及び/又は予防に利用可能である。
Regulatory T cell inducer, adjuvant for allergen immunotherapy, food composition for regulatory T cell induction, and food composition for adjuvant for allergen immunotherapy, which contain lycopene or a derivative thereof as an active ingredient of the present invention. The substance has an excellent regulatory T cell-inducing action and can be used for the treatment and / or prevention of inflammatory diseases and the like.
Claims (29)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017127846 | 2017-06-29 | ||
| JP2017127846 | 2017-06-29 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019011314A JP2019011314A (en) | 2019-01-24 |
| JP2019011314A5 JP2019011314A5 (en) | 2019-09-26 |
| JP6892079B2 true JP6892079B2 (en) | 2021-06-18 |
Family
ID=65227877
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018122078A Active JP6892079B2 (en) | 2017-06-29 | 2018-06-27 | Regulatory T cell inducers, adjuvants and food compositions |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP6892079B2 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021143142A (en) * | 2020-03-11 | 2021-09-24 | 花王株式会社 | Regulatory t-cell inducer |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4328514B2 (en) * | 2002-11-11 | 2009-09-09 | カゴメ株式会社 | Antiallergic agent |
| CN106102755B (en) * | 2014-03-14 | 2020-02-21 | 株式会社明治 | Agent for producing retinoic acid |
| WO2017005647A1 (en) * | 2015-07-03 | 2017-01-12 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for obtaining regulatory t cells and uses thereof |
| US10898568B2 (en) * | 2015-09-30 | 2021-01-26 | Boehringer Ingelheim Vetmedica Gmbh | Modular antigen transportation molecules and uses thereof in animals |
| JP2017081891A (en) * | 2015-10-27 | 2017-05-18 | 株式会社ホソダShc | Regulatory t cell activity compositions |
-
2018
- 2018-06-27 JP JP2018122078A patent/JP6892079B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019011314A (en) | 2019-01-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10555975B2 (en) | Prevotella histicola preparations and the treatment of autoimmune conditions | |
| Uranga et al. | Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease | |
| Von Geldern et al. | The influence of nutritional factors on the prognosis of multiple sclerosis | |
| TWI424840B (en) | Use of β-hydroxy-β-methylbutyrate | |
| US20160143941A1 (en) | Nutraceutical composition and methods for preventing or treating multiple sclerosis | |
| AU2012209285B2 (en) | Methods and compositions for treating, reducing or preventing deterioration of the visual system of animals | |
| US20050165105A1 (en) | Method of using punicic acid to enhance immune response and prevent metabolic disorders | |
| US20240058396A1 (en) | NOVEL STRAIN OF Bifidobacterium animalis subsp. lactis HEM20-01 AND COMPOSITION FOR treating depression, COMPRISING the same OR its culture fluid | |
| KR101778734B1 (en) | Extracellular Solute Binding Protein (ESBP) derived from Bifidobacterium longum KACC 91563 and Anti-allergy Composition using the Same | |
| US20140030807A1 (en) | Method for stimulating foxp3+ regulatory t cell expression of cd39 | |
| JP6892079B2 (en) | Regulatory T cell inducers, adjuvants and food compositions | |
| WO2005070412A2 (en) | Method of using punicic acid to enhance immune response and prevent metabolic disorders | |
| Larbi et al. | Nutrition as a Tool to Reverse Immunosenescence? | |
| WO2017191838A1 (en) | Safe and stable plasmalogen, formulation thereof, and method for assessing presymptomatic state of dementia | |
| US8580278B2 (en) | Nutraceutical composition and methods for preventing or treating multiple sclerosis | |
| US11638431B2 (en) | Fermented milk and polysaccharide with cancerous cachexia inhibitory effect | |
| JP2014166972A (en) | Intestinal inflammation inhibitor and food and drink | |
| JP5711616B2 (en) | IL-17 production inhibitor | |
| US20220249591A1 (en) | Composition for the treatment of emotional disorders | |
| JP6093100B2 (en) | Erythrocyte function improver | |
| JP7492222B2 (en) | Adiponectin receptor agonist and use thereof, and food composition for activating adiponectin receptor and use thereof | |
| JP2020531587A (en) | Compositions and nutritional supplements made from them | |
| JP7242219B2 (en) | Blood sugar elevation inhibitor, diabetes inhibitor, and food composition | |
| JP7206623B2 (en) | Composition for prevention and improvement of glucose metabolism disorder | |
| Kartikey et al. | Effects of oxidative stress on development of osteoporosis, carcinogenesis, atherosclerosis and brain degeneration |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AA64 | Notification of invalidation of claim of internal priority (with term) |
Free format text: JAPANESE INTERMEDIATE CODE: A241764 Effective date: 20180717 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180712 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190809 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190809 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200623 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20201117 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201222 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210420 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210518 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6892079 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |