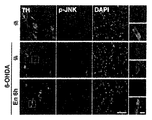
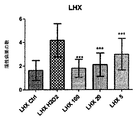
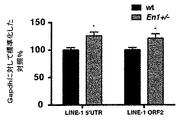
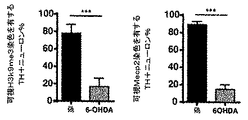
JP6860575B2 - 神経変性障害の処置に用いられるホメオタンパク質 - Google Patents
神経変性障害の処置に用いられるホメオタンパク質 Download PDFInfo
- Publication number
- JP6860575B2 JP6860575B2 JP2018534025A JP2018534025A JP6860575B2 JP 6860575 B2 JP6860575 B2 JP 6860575B2 JP 2018534025 A JP2018534025 A JP 2018534025A JP 2018534025 A JP2018534025 A JP 2018534025A JP 6860575 B2 JP6860575 B2 JP 6860575B2
- Authority
- JP
- Japan
- Prior art keywords
- neurons
- mice
- ohda
- disease
- snpc
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Epidemiology (AREA)
- Psychology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15306482 | 2015-09-23 | ||
| EP15306482.9 | 2015-09-23 | ||
| PCT/EP2016/072675 WO2017071889A1 (en) | 2015-09-23 | 2016-09-23 | Homeoproteins for use in the treatment of neurodegenerative disorders |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018536697A JP2018536697A (ja) | 2018-12-13 |
| JP2018536697A5 JP2018536697A5 (cg-RX-API-DMAC7.html) | 2019-09-05 |
| JP6860575B2 true JP6860575B2 (ja) | 2021-04-14 |
Family
ID=54207439
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018534025A Active JP6860575B2 (ja) | 2015-09-23 | 2016-09-23 | 神経変性障害の処置に用いられるホメオタンパク質 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US20190247461A1 (cg-RX-API-DMAC7.html) |
| EP (2) | EP3960195B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP6860575B2 (cg-RX-API-DMAC7.html) |
| CN (1) | CN108289929A (cg-RX-API-DMAC7.html) |
| CA (1) | CA2999209A1 (cg-RX-API-DMAC7.html) |
| ES (2) | ES2895154T3 (cg-RX-API-DMAC7.html) |
| PL (2) | PL3960195T3 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2017071889A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024149784A1 (en) | 2023-01-13 | 2024-07-18 | Brainever | Stabilized engrailed protein aqueous compositions |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2662698A1 (fr) * | 1990-06-05 | 1991-12-06 | Centre Nat Rech Scient | Nouveaux facteurs de croissance neurotropes comprenant un peptide homeoboite. |
| FR2855178B1 (fr) * | 2003-05-20 | 2005-08-05 | Centre Nat Rech Scient | Peptides modulateurs de l'activite du facteur de transcription engrailed |
| FR2897780B1 (fr) | 2006-02-28 | 2008-05-23 | Centre Nat Rech Scient | Utilisation de la proteine a homeodomaine engrailed comme anxiolytique |
| GB0625321D0 (en) * | 2006-12-19 | 2007-01-24 | Univ Surrey | Cancer biomarker |
| FR2926023B1 (fr) | 2008-01-09 | 2010-02-26 | Centre Nat Rech Scient | Utilisation d'une homoproteine de la famille bicoid pour le traitement du glaucome. |
| EP2454597A1 (en) * | 2009-07-13 | 2012-05-23 | The University of Surrey | Therapeutic peptides, polypeptides ans nucleic acid sequences |
| WO2013128239A1 (en) | 2012-02-29 | 2013-09-06 | Centre National De La Recherche Scientifique | Use of engrailed for increasing dopamine synthesis by dopaminergic neurons |
-
2016
- 2016-09-23 US US15/761,702 patent/US20190247461A1/en not_active Abandoned
- 2016-09-23 WO PCT/EP2016/072675 patent/WO2017071889A1/en not_active Ceased
- 2016-09-23 CN CN201680059070.1A patent/CN108289929A/zh active Pending
- 2016-09-23 PL PL21189549.5T patent/PL3960195T3/pl unknown
- 2016-09-23 ES ES16777596T patent/ES2895154T3/es active Active
- 2016-09-23 ES ES21189549T patent/ES3010130T3/es active Active
- 2016-09-23 CA CA2999209A patent/CA2999209A1/en active Pending
- 2016-09-23 EP EP21189549.5A patent/EP3960195B1/en active Active
- 2016-09-23 JP JP2018534025A patent/JP6860575B2/ja active Active
- 2016-09-23 PL PL16777596T patent/PL3352777T3/pl unknown
- 2016-09-23 EP EP16777596.4A patent/EP3352777B1/en active Active
-
2021
- 2021-06-16 US US17/349,746 patent/US20210379144A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US20210379144A1 (en) | 2021-12-09 |
| ES3010130T3 (en) | 2025-04-01 |
| EP3352777B1 (en) | 2021-09-15 |
| JP2018536697A (ja) | 2018-12-13 |
| PL3352777T3 (pl) | 2022-02-07 |
| CA2999209A1 (en) | 2017-05-04 |
| EP3960195B1 (en) | 2025-01-08 |
| PL3960195T3 (pl) | 2025-06-09 |
| WO2017071889A1 (en) | 2017-05-04 |
| EP3352777A1 (en) | 2018-08-01 |
| US20190247461A1 (en) | 2019-08-15 |
| EP3960195A1 (en) | 2022-03-02 |
| CN108289929A (zh) | 2018-07-17 |
| ES2895154T3 (es) | 2022-02-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| He et al. | Targets of neuroprotection in glaucoma | |
| Bertrand et al. | Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats | |
| Carty et al. | SARM: From immune regulator to cell executioner | |
| Shindler et al. | SIRT1 activation confers neuroprotection in experimental optic neuritis | |
| Shah et al. | Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration | |
| Conforti et al. | Wallerian degeneration: an emerging axon death pathway linking injury and disease | |
| Wu et al. | Recombinant adiponectin peptide promotes neuronal survival after intracerebral haemorrhage by suppressing mitochondrial and ATF4‐CHOP apoptosis pathways in diabetic mice via Smad3 signalling inhibition | |
| CN102449140A (zh) | 抑制光感受器凋亡的方法 | |
| Sun et al. | Nrf2 loss of function exacerbates endoplasmic reticulum stress-induced apoptosis in TBI mice | |
| Gu et al. | Piperlongumine attenuates experimental autoimmune encephalomyelitis through inhibition of NF-kappaB activity | |
| Akaiwa et al. | Topical ripasudil suppresses retinal ganglion cell death in a mouse model of normal tension glaucoma | |
| KR20170104457A (ko) | 신경변성 장애 | |
| KR20190120197A (ko) | 치료 및 신경보호 펩티드 | |
| US20130197069A1 (en) | Methods for treating stress induced emotional disorders | |
| Kojima et al. | Axonal protection by modulation of p62 expression in TNF-induced optic nerve degeneration | |
| JP6860575B2 (ja) | 神経変性障害の処置に用いられるホメオタンパク質 | |
| Song et al. | Fasudil, a Rho-associated protein kinase inhibitor, attenuates retinal ischemia and reperfusion injury in rats | |
| US7749496B2 (en) | Neuronal regeneration | |
| WO2019134574A1 (zh) | 防治端粒功能异常相关疾病的多肽及医药用途 | |
| JP7630540B2 (ja) | 神経保護ペプチド | |
| CA3204205A1 (en) | Lectin protein for treatment and prevention of neurodegenerative diseases | |
| Fritzinger et al. | Complement depletion with humanized cobra venom factor in a mouse model of age-related macular degeneration | |
| CN115475247A (zh) | β2-微球蛋白或其抑制剂的制药用途 | |
| US20240209038A1 (en) | Targeting piezo1 to treat inherited and age-related macular degenerations | |
| US20130130993A1 (en) | Pimap39 modulates inflammatory response |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180322 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180731 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190729 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190729 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200807 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201029 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210106 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210107 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210303 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210326 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6860575 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |