JP6523323B2 - 尿管ステント - Google Patents
尿管ステント Download PDFInfo
- Publication number
- JP6523323B2 JP6523323B2 JP2016560994A JP2016560994A JP6523323B2 JP 6523323 B2 JP6523323 B2 JP 6523323B2 JP 2016560994 A JP2016560994 A JP 2016560994A JP 2016560994 A JP2016560994 A JP 2016560994A JP 6523323 B2 JP6523323 B2 JP 6523323B2
- Authority
- JP
- Japan
- Prior art keywords
- stent
- ureteral
- tail
- section
- proximal
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000003734 kidney Anatomy 0.000 claims description 23
- 238000003466 welding Methods 0.000 claims description 15
- 210000002105 tongue Anatomy 0.000 claims description 11
- 239000000853 adhesive Substances 0.000 claims description 2
- 230000001070 adhesive effect Effects 0.000 claims description 2
- 210000000626 ureter Anatomy 0.000 description 20
- 210000002700 urine Anatomy 0.000 description 18
- 238000004026 adhesive bonding Methods 0.000 description 13
- 239000000463 material Substances 0.000 description 10
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 201000010099 disease Diseases 0.000 description 5
- 239000004575 stone Substances 0.000 description 5
- 206010028980 Neoplasm Diseases 0.000 description 4
- 229920002614 Polyether block amide Polymers 0.000 description 4
- 229920003235 aromatic polyamide Polymers 0.000 description 4
- 239000004952 Polyamide Substances 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 229920002647 polyamide Polymers 0.000 description 3
- -1 polyethylene Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 210000003708 urethra Anatomy 0.000 description 3
- 239000004433 Thermoplastic polyurethane Substances 0.000 description 2
- 239000004760 aramid Substances 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 230000007794 irritation Effects 0.000 description 2
- 230000002572 peristaltic effect Effects 0.000 description 2
- VPRUMANMDWQMNF-UHFFFAOYSA-N phenylethane boronic acid Chemical compound OB(O)CCC1=CC=CC=C1 VPRUMANMDWQMNF-UHFFFAOYSA-N 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 239000004800 polyvinyl chloride Substances 0.000 description 2
- 229920000915 polyvinyl chloride Polymers 0.000 description 2
- 229920002725 thermoplastic elastomer Polymers 0.000 description 2
- 229920002803 thermoplastic polyurethane Polymers 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- 208000014001 urinary system disease Diseases 0.000 description 2
- 208000004998 Abdominal Pain Diseases 0.000 description 1
- 206010005052 Bladder irritation Diseases 0.000 description 1
- 208000002881 Colic Diseases 0.000 description 1
- 229920000271 Kevlar® Polymers 0.000 description 1
- 229920000784 Nomex Polymers 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 206010067171 Regurgitation Diseases 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 208000012931 Urologic disease Diseases 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 229920003232 aliphatic polyester Polymers 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000005489 elastic deformation Effects 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 239000004761 kevlar Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000027939 micturition Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000004763 nomex Substances 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002742 polystyrene-block-poly(ethylene/propylene) -block-polystyrene Polymers 0.000 description 1
- 229920002743 polystyrene-poly(ethylene-ethylene/propylene) block-polystyrene Polymers 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 229920000468 styrene butadiene styrene block copolymer Polymers 0.000 description 1
- 229920001935 styrene-ethylene-butadiene-styrene Polymers 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/94—Stents retaining their form, i.e. not being deformable, after placement in the predetermined place
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M27/00—Drainage appliance for wounds or the like, i.e. wound drains, implanted drains
- A61M27/002—Implant devices for drainage of body fluids from one part of the body to another
- A61M27/008—Implant devices for drainage of body fluids from one part of the body to another pre-shaped, for use in the urethral or ureteral tract
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2002/048—Ureters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0029—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in bending or flexure capacity
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0036—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in thickness
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/10—Trunk
- A61M2210/1078—Urinary tract
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/10—Trunk
- A61M2210/1078—Urinary tract
- A61M2210/1082—Kidney
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Otolaryngology (AREA)
- Ophthalmology & Optometry (AREA)
- Urology & Nephrology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Prostheses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Orthopedics, Nursing, And Contraception (AREA)
Description
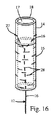
A. 患者の腎臓に配置されるように構成された腎領域、腎領域に接続されかつ患者の尿管に配置されるように構成された尿管領域、および尿管領域に接続されかつ本体部の近位端に配置された近位領域を有する、本体部と、
ステントに接続された糸を含む尾部と、
を含む、尿管ステントであって、
近位領域は第1の可撓性を備え、該第1の可撓性はステントの尿管領域の第2の可撓性を上回り、尾部は尿管領域に剛結合されかつ近位領域を越えて延在する、尿管ステント。
ステントに接続された糸を含む尾部と、
を含む、尿管ステントであって、
近位領域は第1の可撓性を備え、該第1の可撓性はステントの尿管領域の第2の可撓性を上回り、尾部は近位領域に剛結合されかつ近位領域を越えて延在する、尿管ステント。
Claims (3)
- 患者の腎臓に配置されるように構成された腎セクション、前記腎セクションに接続されかつ前記患者の尿管に配置されるように構成された尿管セクション、および前記尿管セクションに接続されかつ本体部の近位端に配置される近位セクションを有する、本体部と、
ステントに接続された糸を含む尾部と、
を含む、尿管ステントであって、
前記近位セクションは第1の可撓性を備え、該第1の可撓性は前記ステントの前記尿管セクションの第2の可撓性を上回り、
前記近位セクションに沿って縦溝が形成され、前記尾部が前記近位セクションに形成された前記縦溝内に挿入され、前記尾部が前記近位セクションを越えて延在し、
前記近位セクションが幾つかの舌部を含み、各舌部は1つ以上の開口部を有し前記1つ以上の開口部は前記ステントの前記尾部が通過するサイズにされた、尿管ステント。 - 前記近位セクションが1つ以上の貫通開口部を含み、前記尾部が前記貫通開口部の1つを介して固定される、請求項1に記載の尿管ステント。
- 前記尾部が結び目、接着接合、又は溶接によって前記本体部に固定される、請求項1又は2に記載の尿管ステント。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019043302A JP6898372B2 (ja) | 2014-04-11 | 2019-03-11 | 尿管ステント |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14290106.5 | 2014-04-11 | ||
| EP14290106 | 2014-04-11 | ||
| EP14178203.7 | 2014-07-23 | ||
| EP14178203.7A EP2977075B1 (en) | 2014-07-23 | 2014-07-23 | A Ureteral Stent |
| PCT/DK2015/050088 WO2015154782A1 (en) | 2014-04-11 | 2015-04-10 | A ureteral stent |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019043302A Division JP6898372B2 (ja) | 2014-04-11 | 2019-03-11 | 尿管ステント |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2017510371A JP2017510371A (ja) | 2017-04-13 |
| JP2017510371A5 JP2017510371A5 (ja) | 2018-05-17 |
| JP6523323B2 true JP6523323B2 (ja) | 2019-05-29 |
Family
ID=52991406
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016560994A Active JP6523323B2 (ja) | 2014-04-11 | 2015-04-10 | 尿管ステント |
| JP2019043302A Active JP6898372B2 (ja) | 2014-04-11 | 2019-03-11 | 尿管ステント |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019043302A Active JP6898372B2 (ja) | 2014-04-11 | 2019-03-11 | 尿管ステント |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20170119559A1 (ja) |
| JP (2) | JP6523323B2 (ja) |
| CN (1) | CN106456946B (ja) |
| WO (1) | WO2015154782A1 (ja) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10226606B2 (en) * | 2014-04-10 | 2019-03-12 | C.R. Bard, Inc. | Ureteral stents |
| US11957851B2 (en) * | 2017-04-10 | 2024-04-16 | Flat Medical Inc. | Catheter for guiding body fluid |
| US10933227B2 (en) | 2017-12-01 | 2021-03-02 | Gyrus Acmi, Inc. | Ureteral stent |
| CN117503421B (zh) * | 2023-11-22 | 2024-07-30 | 香港大学深圳医院 | 一种便于取放的输尿管支架管 |
Family Cites Families (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2156994C3 (de) * | 1971-11-17 | 1974-07-18 | Walter Prof. Dr. 6200 Wiesbaden Hartenbach | Gallengang-Endoprothese |
| US4307723A (en) * | 1978-04-07 | 1981-12-29 | Medical Engineering Corporation | Externally grooved ureteral stent |
| US4813925A (en) * | 1987-04-21 | 1989-03-21 | Medical Engineering Corporation | Spiral ureteral stent |
| US5738654A (en) * | 1995-03-20 | 1998-04-14 | Contimed, Inc. | Self cleansing bladder drainage device |
| DE19540731C2 (de) * | 1995-11-02 | 2001-03-01 | Wolf Gmbh Richard | Endoskopisches Instrument |
| BR9611541A (pt) * | 1995-11-07 | 1999-12-28 | Boston Scient Corp | Sonda uretral com pequena(s) cauda(s) de bexiga |
| US6676623B2 (en) * | 2001-05-04 | 2004-01-13 | Scimed Life Systems, Inc. | Drainage devices and methods |
| US6991614B2 (en) * | 1995-11-07 | 2006-01-31 | Boston Scientific Scimed, Inc. | Ureteral stent for improved patient comfort |
| DE69723137T2 (de) * | 1996-03-18 | 2004-05-27 | Ashiya, Hiroaki | Catheteranordnung |
| US6211904B1 (en) * | 1997-09-11 | 2001-04-03 | Edwin L. Adair | Surgical devices incorporating reduced area imaging devices |
| US6095990A (en) * | 1998-08-31 | 2000-08-01 | Parodi; Juan Carlos | Guiding device and method for inserting and advancing catheters and guidewires into a vessel of a patient in endovascular treatments |
| US6709465B2 (en) * | 1999-03-18 | 2004-03-23 | Fossa Medical, Inc. | Radially expanding ureteral device |
| GB0012764D0 (en) * | 2000-05-26 | 2000-07-19 | Tayside University Hospitals N | G-stent |
| AU2002350164A1 (en) * | 2001-11-08 | 2003-05-19 | William D. Hare | Rapid exchange catheter with stent deployment, therapeutic infusion, and lesion sampling features |
| US6908447B2 (en) * | 2002-04-18 | 2005-06-21 | Scimed Life Systems, Inc. | Anti-reflux ureteral stents and methods |
| US6929664B2 (en) * | 2003-12-05 | 2005-08-16 | Fossa Medical, Inc. | Open lumen stents |
| US7470247B2 (en) * | 2004-04-26 | 2008-12-30 | Gyrus Acmi, Inc. | Ureteral stent |
| US20080071343A1 (en) * | 2006-09-15 | 2008-03-20 | Kevin John Mayberry | Multi-segmented graft deployment system |
| US9125761B2 (en) * | 2007-01-25 | 2015-09-08 | Boston Scientific Scimed, Inc. | Endoscope with preloaded or preloadable stent |
| CN101249031B (zh) * | 2008-03-12 | 2010-06-02 | 北京望升伟业科技发展有限公司 | 一种输尿管支架 |
| US20100256731A1 (en) * | 2009-04-02 | 2010-10-07 | Mangiardi Eric K | Stent |
| WO2010141850A1 (en) * | 2009-06-05 | 2010-12-09 | Entrigue Surgical, Inc. | Systems and devices for providing therapy of an anatomical structure |
| US20120095566A1 (en) * | 2010-10-18 | 2012-04-19 | Boston Scientific Scimed, Inc. | Flexible ureteral stent |
| CA2817831C (en) * | 2010-11-19 | 2021-02-23 | Boston Scientific Scimed, Inc. | Rapid exchange stent delivery system |
| US8728169B2 (en) * | 2011-05-11 | 2014-05-20 | Boston Scientific Scimed, Inc. | Medical apparatuses for delivery of urologically beneficial agents |
| FR2999416A1 (fr) * | 2012-12-19 | 2014-06-20 | Benoit Vogt | Sonde endo-ureterale amelioree |
| CN109125893A (zh) * | 2013-02-28 | 2019-01-04 | 波士顿科学国际有限公司 | 沿胆道和/或胰管使用的医疗装置 |
| US10933227B2 (en) * | 2017-12-01 | 2021-03-02 | Gyrus Acmi, Inc. | Ureteral stent |
-
2015
- 2015-04-10 JP JP2016560994A patent/JP6523323B2/ja active Active
- 2015-04-10 CN CN201580018862.XA patent/CN106456946B/zh not_active Expired - Fee Related
- 2015-04-10 WO PCT/DK2015/050088 patent/WO2015154782A1/en active Application Filing
- 2015-04-10 US US15/302,021 patent/US20170119559A1/en not_active Abandoned
-
2019
- 2019-03-11 JP JP2019043302A patent/JP6898372B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN106456946A (zh) | 2017-02-22 |
| US20170119559A1 (en) | 2017-05-04 |
| JP2019141599A (ja) | 2019-08-29 |
| JP6898372B2 (ja) | 2021-07-07 |
| JP2017510371A (ja) | 2017-04-13 |
| WO2015154782A9 (en) | 2016-11-03 |
| CN106456946B (zh) | 2019-09-10 |
| WO2015154782A1 (en) | 2015-10-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240189089A1 (en) | Biliary Stents and Methods | |
| JP6898372B2 (ja) | 尿管ステント | |
| US11278436B2 (en) | Ureteral stent for placement in a kidney and bladder | |
| JP3226297U (ja) | 尿管ステント | |
| JP6357169B2 (ja) | 尿管ステントの改善および泌尿器系の問題を処理する方法 | |
| JP7212989B2 (ja) | 尿管ステント | |
| CN107847312B (zh) | 支架及其使用方法 | |
| KR19990067352A (ko) | 소형 방광 꼬리부를 갖는 요관 스텐트 | |
| EP2977075B1 (en) | A Ureteral Stent | |
| EP3517162B1 (en) | A ureteral stent | |
| WO2017193120A1 (en) | Ureteral stent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180328 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180328 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20181211 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190311 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20190326 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20190425 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6523323 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |