JP5545956B2 - 人工耳小骨 - Google Patents
人工耳小骨 Download PDFInfo
- Publication number
- JP5545956B2 JP5545956B2 JP2009552280A JP2009552280A JP5545956B2 JP 5545956 B2 JP5545956 B2 JP 5545956B2 JP 2009552280 A JP2009552280 A JP 2009552280A JP 2009552280 A JP2009552280 A JP 2009552280A JP 5545956 B2 JP5545956 B2 JP 5545956B2
- Authority
- JP
- Japan
- Prior art keywords
- fluid
- orp
- coupling
- container
- piston
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000012530 fluid Substances 0.000 claims description 104
- 230000008878 coupling Effects 0.000 claims description 59
- 238000010168 coupling process Methods 0.000 claims description 59
- 238000005859 coupling reaction Methods 0.000 claims description 59
- 230000033001 locomotion Effects 0.000 claims description 44
- 230000008859 change Effects 0.000 claims description 14
- 230000004044 response Effects 0.000 claims description 14
- 210000000959 ear middle Anatomy 0.000 claims description 12
- 230000009974 thixotropic effect Effects 0.000 claims description 4
- 230000007423 decrease Effects 0.000 claims description 3
- 230000003993 interaction Effects 0.000 claims description 3
- 238000007789 sealing Methods 0.000 claims description 3
- 230000001105 regulatory effect Effects 0.000 claims description 2
- 230000007613 environmental effect Effects 0.000 description 16
- 230000001965 increasing effect Effects 0.000 description 16
- 210000003454 tympanic membrane Anatomy 0.000 description 15
- 230000003068 static effect Effects 0.000 description 14
- 230000000694 effects Effects 0.000 description 12
- 210000000988 bone and bone Anatomy 0.000 description 9
- 238000013016 damping Methods 0.000 description 9
- 238000010586 diagram Methods 0.000 description 6
- 230000036316 preload Effects 0.000 description 6
- 210000003027 ear inner Anatomy 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 230000009471 action Effects 0.000 description 4
- 230000005540 biological transmission Effects 0.000 description 4
- 238000004891 communication Methods 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 230000001720 vestibular Effects 0.000 description 4
- 230000008901 benefit Effects 0.000 description 3
- 239000004568 cement Substances 0.000 description 3
- 210000003128 head Anatomy 0.000 description 3
- 238000013017 mechanical damping Methods 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 238000002788 crimping Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 239000013013 elastic material Substances 0.000 description 2
- 239000003292 glue Substances 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 210000001785 incus Anatomy 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000012080 ambient air Substances 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 230000009189 diving Effects 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 210000000613 ear canal Anatomy 0.000 description 1
- 210000003094 ear ossicle Anatomy 0.000 description 1
- 239000013536 elastomeric material Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 206010033103 otosclerosis Diseases 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 238000005549 size reduction Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/18—Internal ear or nose parts, e.g. ear-drums
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/18—Internal ear or nose parts, e.g. ear-drums
- A61F2002/183—Ear parts
Landscapes
- Health & Medical Sciences (AREA)
- Otolaryngology (AREA)
- Pulmonology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Description
Claims (20)
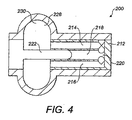
- 耳小骨連鎖の全部又は一部を置換し、患者の中耳における第1の点を第2の点にカップリングするための人工耳小骨(ORP)であって、
圧力差を構成する変化可能な構造を有するカップリングを備え、
前記カップリングは、前記カップリングの構造を変化させるための2つの相対移動可能部を有する流体充填チャンバーを有し、
前記カップリングは、リーク手段、及び前記チャンバー内部の流体移動を可能とする流路手段とを構成する非封止手段を備え、
前記相対移動可能部の相対移動は、前記チャンバー内の流体移動によって制御され、
前記移動可能部の相対移動が圧力の準静的変化に応じて可能とされ、かつ可聴周波数に対応した振動変化に応じて実質的に規制されるように、前記チャンバーは流体移動を制限するように構成されることを特徴とするORP。 - 前記カップリングが、前記非封止手段を備えた流体を収容するコンテナとピストンとを備え、前記コンテナの壁と前記ピストンとの間に設けられたクリアランスが、前記リーク手段および前記流路手段を構成することで前記流体が変位し、かつ
患者の聴覚系内部の要素間においてカップリングされるように構成された第1および第2の取付点を有し、
前記カップリングに対する音振動から導かれる力の付与に応じて、前記流路内部の前記流体の相互作用は、リーク手段を有する前記ピストンと前記コンテナによって、前記カップリングが実質的に非変形でかつ剛体であることを可能とし、さらに前記第1及び第2の取付点が実質的に互いに相対的に固定されることを可能とし、
前記カップリングに対する局地的な周囲圧力の変化から導かれる力の付与に応じて、前記流路内部の前記流体の相互作用は、リーク手段を有する前記ピストンと前記コンテナによって、前記第1及び第2の取付点間の相対移動が可能にされるように前記カップリングを変形させることを可能とすることを特徴とする請求項1に記載のORP。 - 前記流体は、非ニュートン流体であることを特徴とする請求項1または2に記載のORP。
- 前記非ニュートン流体はダイラタント流体であることを特徴とする請求項3に記載のORP。
- 前記非ニュートン流体は、揺変性の流体であることを特徴とする請求項3に記載のORP。
- 前記流体は、ニュートン流体を備えることを特徴とする請求項1又は2に記載のORP。
- 前記カップリングは、前記第1及び第2の取付点間において相対的に直線運動可能なように変形するように構成されることを特徴とする請求項2に記載のORP。
- 前記カップリングは、前記第1及び第2の取付点間において相対的に回転運動可能なように変形するように構成されることを特徴とする請求項2に記載のORP。
- 前記カップリングの剛性は、変化可能であることを特徴とする請求項1または2に記載のORP。
- 前記カップリングの剛性は、付与された荷重の周波数変化に応じて変化可能であることを特徴とする請求項1または2に記載のORP。
- 前記カップリングの剛性は、付与された荷重の周波数増加に応じて増加することを特徴とする請求項1または2に記載のORP。
- 前記カップリングの剛性は、付与された荷重の大きさの変化に応じて変化可能であることを特徴とする請求項1または2に記載のORP。
- 前記カップリングの剛性は、付与された荷重の大きさの増加に応じて低下することを特徴とする請求項1または2に記載のORP。
- 前記カップリングは、前記第1及び第2の取付点間に与圧が与えられるように構成されることを特徴とする請求項2に記載のORP。
- 前記カップリングの前記ピストンは、前記コンテナ内部に滑動自在に取り付けられ、前記ピストンは流体界面を定義することを特徴とする請求項2に記載のORP。
- 前記カップリングの前記ピストンは、前記コンテナ内部に回転自在に取り付けられる回転可能軸手段を構成することを特徴とする請求項2に記載のORP。
- 前記回転可能軸手段は、その上に取り付けられ、かつそこから径方向に延在する少なくとも1つの翼を備えることを特徴とする請求項16に記載のORP。
- 前記少なくとも1つの翼は、前記コンテナ内部に含まれる流体に係合するように採用されて、流体界面を定義することを特徴とする請求項17に記載のORP。
- 前記翼は、前記流体がそれを通って又はそれを横切って通ることが可能となるように構成されることを特徴とする請求項17又は18に記載のORP。
- 前記カップリングは、第1の摩擦要素が前記第1の取付点を支持し、第2の摩擦要素が前記第2の取付点を支持するように、摩擦カップリングによってともにカップリングされる第1及び第2の摩擦要素を備え、前記摩擦カップリングは、前記カップリングが患者の聴覚系内部の1つの要素から他の1つへ音誘起振動を伝達可能とするように構成されることを特徴とする請求項2に記載のORP。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0704125.4 | 2007-03-03 | ||
| GBGB0704125.4A GB0704125D0 (en) | 2007-03-03 | 2007-03-03 | Ossicular replacement prosthesis |
| PCT/GB2008/050147 WO2008107716A1 (en) | 2007-03-03 | 2008-03-03 | Ossicular replacement prosthesis |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010519999A JP2010519999A (ja) | 2010-06-10 |
| JP2010519999A5 JP2010519999A5 (ja) | 2014-03-06 |
| JP5545956B2 true JP5545956B2 (ja) | 2014-07-09 |
Family
ID=37965854
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009552280A Active JP5545956B2 (ja) | 2007-03-03 | 2008-03-03 | 人工耳小骨 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US8920496B2 (ja) |
| EP (1) | EP2134298B1 (ja) |
| JP (1) | JP5545956B2 (ja) |
| CN (1) | CN101677858B (ja) |
| AU (1) | AU2008222478B2 (ja) |
| CA (1) | CA2717361A1 (ja) |
| GB (1) | GB0704125D0 (ja) |
| WO (1) | WO2008107716A1 (ja) |
Families Citing this family (79)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9173661B2 (en) | 2006-02-27 | 2015-11-03 | Biomet Manufacturing, Llc | Patient specific alignment guide with cutting surface and laser indicator |
| US8377066B2 (en) * | 2006-02-27 | 2013-02-19 | Biomet Manufacturing Corp. | Patient-specific elbow guides and associated methods |
| US9113971B2 (en) | 2006-02-27 | 2015-08-25 | Biomet Manufacturing, Llc | Femoral acetabular impingement guide |
| US8473305B2 (en) | 2007-04-17 | 2013-06-25 | Biomet Manufacturing Corp. | Method and apparatus for manufacturing an implant |
| US20080257363A1 (en) * | 2007-04-17 | 2008-10-23 | Biomet Manufacturing Corp. | Method And Apparatus For Manufacturing An Implant |
| US8241293B2 (en) * | 2006-02-27 | 2012-08-14 | Biomet Manufacturing Corp. | Patient specific high tibia osteotomy |
| US8608749B2 (en) | 2006-02-27 | 2013-12-17 | Biomet Manufacturing, Llc | Patient-specific acetabular guides and associated instruments |
| US8858561B2 (en) | 2006-06-09 | 2014-10-14 | Blomet Manufacturing, LLC | Patient-specific alignment guide |
| US20150335438A1 (en) | 2006-02-27 | 2015-11-26 | Biomet Manufacturing, Llc. | Patient-specific augments |
| US9339278B2 (en) | 2006-02-27 | 2016-05-17 | Biomet Manufacturing, Llc | Patient-specific acetabular guides and associated instruments |
| US8568487B2 (en) | 2006-02-27 | 2013-10-29 | Biomet Manufacturing, Llc | Patient-specific hip joint devices |
| US9345548B2 (en) | 2006-02-27 | 2016-05-24 | Biomet Manufacturing, Llc | Patient-specific pre-operative planning |
| US9289253B2 (en) | 2006-02-27 | 2016-03-22 | Biomet Manufacturing, Llc | Patient-specific shoulder guide |
| US8864769B2 (en) | 2006-02-27 | 2014-10-21 | Biomet Manufacturing, Llc | Alignment guides with patient-specific anchoring elements |
| US8603180B2 (en) | 2006-02-27 | 2013-12-10 | Biomet Manufacturing, Llc | Patient-specific acetabular alignment guides |
| US8092465B2 (en) | 2006-06-09 | 2012-01-10 | Biomet Manufacturing Corp. | Patient specific knee alignment guide and associated method |
| US8133234B2 (en) * | 2006-02-27 | 2012-03-13 | Biomet Manufacturing Corp. | Patient specific acetabular guide and method |
| US8535387B2 (en) | 2006-02-27 | 2013-09-17 | Biomet Manufacturing, Llc | Patient-specific tools and implants |
| US8591516B2 (en) | 2006-02-27 | 2013-11-26 | Biomet Manufacturing, Llc | Patient-specific orthopedic instruments |
| US8407067B2 (en) | 2007-04-17 | 2013-03-26 | Biomet Manufacturing Corp. | Method and apparatus for manufacturing an implant |
| US8608748B2 (en) | 2006-02-27 | 2013-12-17 | Biomet Manufacturing, Llc | Patient specific guides |
| US9918740B2 (en) | 2006-02-27 | 2018-03-20 | Biomet Manufacturing, Llc | Backup surgical instrument system and method |
| US9907659B2 (en) | 2007-04-17 | 2018-03-06 | Biomet Manufacturing, Llc | Method and apparatus for manufacturing an implant |
| US10278711B2 (en) | 2006-02-27 | 2019-05-07 | Biomet Manufacturing, Llc | Patient-specific femoral guide |
| US7967868B2 (en) | 2007-04-17 | 2011-06-28 | Biomet Manufacturing Corp. | Patient-modified implant and associated method |
| US9795399B2 (en) | 2006-06-09 | 2017-10-24 | Biomet Manufacturing, Llc | Patient-specific knee alignment guide and associated method |
| DE102009028503B4 (de) | 2009-08-13 | 2013-11-14 | Biomet Manufacturing Corp. | Resektionsschablone zur Resektion von Knochen, Verfahren zur Herstellung einer solchen Resektionsschablone und Operationsset zur Durchführung von Kniegelenk-Operationen |
| US8632547B2 (en) | 2010-02-26 | 2014-01-21 | Biomet Sports Medicine, Llc | Patient-specific osteotomy devices and methods |
| US9271744B2 (en) | 2010-09-29 | 2016-03-01 | Biomet Manufacturing, Llc | Patient-specific guide for partial acetabular socket replacement |
| US9968376B2 (en) | 2010-11-29 | 2018-05-15 | Biomet Manufacturing, Llc | Patient-specific orthopedic instruments |
| US8147400B1 (en) | 2011-01-17 | 2012-04-03 | Coloplast A/S | Penile implant with dilatant liquid |
| US8419612B2 (en) | 2011-01-17 | 2013-04-16 | Coloplast A/S | Method treating erectile dysfunction via a penile implant with dilatant liquid |
| EP2665444B1 (en) * | 2011-01-17 | 2018-03-14 | Coloplast A/S | Penile implant with dilatant liquid |
| US9241745B2 (en) | 2011-03-07 | 2016-01-26 | Biomet Manufacturing, Llc | Patient-specific femoral version guide |
| US8715289B2 (en) | 2011-04-15 | 2014-05-06 | Biomet Manufacturing, Llc | Patient-specific numerically controlled instrument |
| US9675400B2 (en) | 2011-04-19 | 2017-06-13 | Biomet Manufacturing, Llc | Patient-specific fracture fixation instrumentation and method |
| US8956364B2 (en) | 2011-04-29 | 2015-02-17 | Biomet Manufacturing, Llc | Patient-specific partial knee guides and other instruments |
| US8668700B2 (en) | 2011-04-29 | 2014-03-11 | Biomet Manufacturing, Llc | Patient-specific convertible guides |
| US8532807B2 (en) | 2011-06-06 | 2013-09-10 | Biomet Manufacturing, Llc | Pre-operative planning and manufacturing method for orthopedic procedure |
| US9084618B2 (en) | 2011-06-13 | 2015-07-21 | Biomet Manufacturing, Llc | Drill guides for confirming alignment of patient-specific alignment guides |
| US20130001121A1 (en) | 2011-07-01 | 2013-01-03 | Biomet Manufacturing Corp. | Backup kit for a patient-specific arthroplasty kit assembly |
| US8764760B2 (en) | 2011-07-01 | 2014-07-01 | Biomet Manufacturing, Llc | Patient-specific bone-cutting guidance instruments and methods |
| US8597365B2 (en) | 2011-08-04 | 2013-12-03 | Biomet Manufacturing, Llc | Patient-specific pelvic implants for acetabular reconstruction |
| US9295497B2 (en) | 2011-08-31 | 2016-03-29 | Biomet Manufacturing, Llc | Patient-specific sacroiliac and pedicle guides |
| US9066734B2 (en) | 2011-08-31 | 2015-06-30 | Biomet Manufacturing, Llc | Patient-specific sacroiliac guides and associated methods |
| US9386993B2 (en) | 2011-09-29 | 2016-07-12 | Biomet Manufacturing, Llc | Patient-specific femoroacetabular impingement instruments and methods |
| US9451973B2 (en) | 2011-10-27 | 2016-09-27 | Biomet Manufacturing, Llc | Patient specific glenoid guide |
| US9301812B2 (en) | 2011-10-27 | 2016-04-05 | Biomet Manufacturing, Llc | Methods for patient-specific shoulder arthroplasty |
| US9554910B2 (en) | 2011-10-27 | 2017-01-31 | Biomet Manufacturing, Llc | Patient-specific glenoid guide and implants |
| KR20130046337A (ko) | 2011-10-27 | 2013-05-07 | 삼성전자주식회사 | 멀티뷰 디바이스 및 그 제어방법과, 디스플레이장치 및 그 제어방법과, 디스플레이 시스템 |
| WO2013062848A1 (en) | 2011-10-27 | 2013-05-02 | Biomet Manufacturing Corporation | Patient-specific glenoid guides |
| US9237950B2 (en) | 2012-02-02 | 2016-01-19 | Biomet Manufacturing, Llc | Implant with patient-specific porous structure |
| US9204977B2 (en) | 2012-12-11 | 2015-12-08 | Biomet Manufacturing, Llc | Patient-specific acetabular guide for anterior approach |
| US9060788B2 (en) | 2012-12-11 | 2015-06-23 | Biomet Manufacturing, Llc | Patient-specific acetabular guide for anterior approach |
| US11095994B2 (en) * | 2013-02-15 | 2021-08-17 | Cochlear Limited | Conformable pad bone conduction device |
| US9839438B2 (en) | 2013-03-11 | 2017-12-12 | Biomet Manufacturing, Llc | Patient-specific glenoid guide with a reusable guide holder |
| US9579107B2 (en) | 2013-03-12 | 2017-02-28 | Biomet Manufacturing, Llc | Multi-point fit for patient specific guide |
| US9498233B2 (en) | 2013-03-13 | 2016-11-22 | Biomet Manufacturing, Llc. | Universal acetabular guide and associated hardware |
| US9826981B2 (en) | 2013-03-13 | 2017-11-28 | Biomet Manufacturing, Llc | Tangential fit of patient-specific guides |
| US9517145B2 (en) | 2013-03-15 | 2016-12-13 | Biomet Manufacturing, Llc | Guide alignment system and method |
| US20150112349A1 (en) | 2013-10-21 | 2015-04-23 | Biomet Manufacturing, Llc | Ligament Guide Registration |
| US10282488B2 (en) | 2014-04-25 | 2019-05-07 | Biomet Manufacturing, Llc | HTO guide with optional guided ACL/PCL tunnels |
| US9408616B2 (en) | 2014-05-12 | 2016-08-09 | Biomet Manufacturing, Llc | Humeral cut guide |
| US9561040B2 (en) | 2014-06-03 | 2017-02-07 | Biomet Manufacturing, Llc | Patient-specific glenoid depth control |
| US9839436B2 (en) | 2014-06-03 | 2017-12-12 | Biomet Manufacturing, Llc | Patient-specific glenoid depth control |
| US10805744B2 (en) * | 2014-08-28 | 2020-10-13 | Cochlear Limited | Systems for accommodating separation of body parts in auditory prostheses |
| US9833245B2 (en) | 2014-09-29 | 2017-12-05 | Biomet Sports Medicine, Llc | Tibial tubercule osteotomy |
| US9826994B2 (en) | 2014-09-29 | 2017-11-28 | Biomet Manufacturing, Llc | Adjustable glenoid pin insertion guide |
| US9820868B2 (en) | 2015-03-30 | 2017-11-21 | Biomet Manufacturing, Llc | Method and apparatus for a pin apparatus |
| US10226262B2 (en) | 2015-06-25 | 2019-03-12 | Biomet Manufacturing, Llc | Patient-specific humeral guide designs |
| US10568647B2 (en) | 2015-06-25 | 2020-02-25 | Biomet Manufacturing, Llc | Patient-specific humeral guide designs |
| CN108886663B (zh) * | 2016-03-29 | 2021-09-03 | Med-El电气医疗器械有限公司 | 用于中耳植入物的s形联接弹簧 |
| US10595990B2 (en) | 2016-09-06 | 2020-03-24 | Gyrus Acmi, Inc. | Osseointegrative adjustable ossicular prosthesis |
| US10722310B2 (en) | 2017-03-13 | 2020-07-28 | Zimmer Biomet CMF and Thoracic, LLC | Virtual surgery planning system and method |
| EP3595475B1 (en) | 2017-04-17 | 2021-04-07 | Hewlett-Packard Development Company, L.P. | Vibrators in cells for footwear |
| CN108710775B (zh) * | 2018-07-31 | 2021-05-25 | 东北大学 | 一种基于听骨链传动机制的减震系统及设计方法 |
| KR102299713B1 (ko) | 2019-12-16 | 2021-09-07 | 인제대학교 산학협력단 | 이소골용 보철물 |
| US11819412B2 (en) | 2020-02-04 | 2023-11-21 | Coloplast A/S | Penile prostheses for treatment of erectile dysfunction |
| CN115836933B (zh) * | 2023-02-17 | 2023-09-01 | 北京理贝尔生物工程研究所有限公司 | 椎间融合器 |
Family Cites Families (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2394569A (en) * | 1941-07-14 | 1946-02-12 | Dictograph Products Co Inc | Fitting hearing aid device |
| US3277433A (en) * | 1963-10-17 | 1966-10-04 | William J Toulis | Flexural-extensional electromechanical transducer |
| US3594514A (en) * | 1970-01-02 | 1971-07-20 | Medtronic Inc | Hearing aid with piezoelectric ceramic element |
| US3710399A (en) * | 1970-06-23 | 1973-01-16 | H Hurst | Ossicle replacement prosthesis |
| US3764748A (en) * | 1972-05-19 | 1973-10-09 | J Branch | Implanted hearing aids |
| US4118599A (en) * | 1976-02-27 | 1978-10-03 | Victor Company Of Japan, Limited | Stereophonic sound reproduction system |
| JPS52125301A (en) * | 1976-04-13 | 1977-10-21 | Victor Co Of Japan Ltd | Signal processing circuit |
| JPS53114201U (ja) * | 1977-02-18 | 1978-09-11 | ||
| DE2844979C2 (de) | 1978-10-16 | 1989-08-31 | Juval Dr.-Ing. 8000 München Mantel | Hörgerät |
| JPS60154800A (ja) | 1984-01-24 | 1985-08-14 | Eastern Electric Kk | 補聴器 |
| US4624672A (en) | 1984-03-15 | 1986-11-25 | Edmundas Lenkauskas | Coiled wire prosthesis for complete or partial ossicular reconstruction |
| JPS6142284A (ja) | 1984-08-03 | 1986-02-28 | Nec Kansai Ltd | 変位拡大装置 |
| US4729366A (en) * | 1984-12-04 | 1988-03-08 | Medical Devices Group, Inc. | Implantable hearing aid and method of improving hearing |
| US4601723A (en) * | 1985-01-29 | 1986-07-22 | Mcgrew Robert N | Telescoping self-adjusting ossicular prostheses |
| DE3508830A1 (de) | 1985-03-13 | 1986-09-18 | Robert Bosch Gmbh, 7000 Stuttgart | Hoergeraet |
| US5015225A (en) * | 1985-05-22 | 1991-05-14 | Xomed, Inc. | Implantable electromagnetic middle-ear bone-conduction hearing aid device |
| US4774515A (en) * | 1985-09-27 | 1988-09-27 | Bo Gehring | Attitude indicator |
| US4759070A (en) * | 1986-05-27 | 1988-07-19 | Voroba Technologies Associates | Patient controlled master hearing aid |
| EP0349599B2 (en) * | 1987-05-11 | 1995-12-06 | Jay Management Trust | Paradoxical hearing aid |
| US4809708A (en) * | 1987-08-12 | 1989-03-07 | Nicolet Instrument Corporation | Method and apparatus for real bar measurements |
| US4957507A (en) | 1987-12-14 | 1990-09-18 | Edmundas Lenkauskas | Wire spring prosthesis for ossicular reconstruction |
| US4845688A (en) * | 1988-03-21 | 1989-07-04 | Image Acoustics, Inc. | Electro-mechanical transduction apparatus |
| US4901353A (en) * | 1988-05-10 | 1990-02-13 | Minnesota Mining And Manufacturing Company | Auditory prosthesis fitting using vectors |
| US4957478A (en) * | 1988-10-17 | 1990-09-18 | Maniglia Anthony J | Partially implantable hearing aid device |
| US5303306A (en) * | 1989-06-06 | 1994-04-12 | Audioscience, Inc. | Hearing aid with programmable remote and method of deriving settings for configuring the hearing aid |
| IT1248737B (it) | 1990-06-07 | 1995-01-26 | Franco Beoni | Protesi di orecchio medio |
| DE4104359A1 (de) * | 1991-02-13 | 1992-08-20 | Implex Gmbh | Ladesystem fuer implantierbare hoerhilfen und tinnitus-maskierer |
| FR2675372B1 (fr) * | 1991-04-19 | 1998-12-18 | Guy Charvin | Prothese ossiculaire auto-ajustable de l'oreille moyenne. |
| US5233665A (en) * | 1991-12-17 | 1993-08-03 | Gary L. Vaughn | Phonetic equalizer system |
| US5173944A (en) * | 1992-01-29 | 1992-12-22 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | Head related transfer function pseudo-stereophony |
| FR2691354A1 (fr) | 1992-05-22 | 1993-11-26 | France Chirurgie Instr | Prothèse ossiculaire pour l'oreille. |
| US5531787A (en) * | 1993-01-25 | 1996-07-02 | Lesinski; S. George | Implantable auditory system with micromachined microsensor and microactuator |
| JP2606069B2 (ja) | 1993-04-22 | 1997-04-30 | 日本電気株式会社 | 圧電素子変位増幅機構およびその駆動方法 |
| US5325436A (en) * | 1993-06-30 | 1994-06-28 | House Ear Institute | Method of signal processing for maintaining directional hearing with hearing aids |
| US5913815A (en) * | 1993-07-01 | 1999-06-22 | Symphonix Devices, Inc. | Bone conducting floating mass transducers |
| US5456654A (en) * | 1993-07-01 | 1995-10-10 | Ball; Geoffrey R. | Implantable magnetic hearing aid transducer |
| US5436975A (en) * | 1994-02-02 | 1995-07-25 | Qsound Ltd. | Apparatus for cross fading out of the head sound locations |
| US5825894A (en) * | 1994-08-17 | 1998-10-20 | Decibel Instruments, Inc. | Spatialization for hearing evaluation |
| RU2096027C1 (ru) | 1995-06-05 | 1997-11-20 | Юрий Петрович Ульянов | Способ улучшения слуха |
| US5729077A (en) * | 1995-12-15 | 1998-03-17 | The Penn State Research Foundation | Metal-electroactive ceramic composite transducer |
| JP2000504948A (ja) | 1995-12-20 | 2000-04-25 | デシベル インストルメンツ インコーポレイテッド | 補聴なし、シミュレーションされた補聴あり、補聴あり聴力評価のための仮想電気音響聴力測定 |
| US6001129A (en) * | 1996-08-07 | 1999-12-14 | St. Croix Medical, Inc. | Hearing aid transducer support |
| US5899847A (en) * | 1996-08-07 | 1999-05-04 | St. Croix Medical, Inc. | Implantable middle-ear hearing assist system using piezoelectric transducer film |
| US5879283A (en) * | 1996-08-07 | 1999-03-09 | St. Croix Medical, Inc. | Implantable hearing system having multiple transducers |
| US5707338A (en) * | 1996-08-07 | 1998-01-13 | St. Croix Medical, Inc. | Stapes vibrator |
| DE29701534U1 (de) | 1997-01-30 | 1997-03-27 | Voit, Sven, 21423 Winsen | Vorrichtung zur zangenartigen Halterung zweier Besteckteile |
| US6315710B1 (en) * | 1997-07-21 | 2001-11-13 | St. Croix Medical, Inc. | Hearing system with middle ear transducer mount |
| US6325755B1 (en) * | 1997-08-07 | 2001-12-04 | St. Croix Medical, Inc. | Mountable transducer assembly with removable sleeve |
| FR2769492B1 (fr) | 1997-10-10 | 1999-12-17 | Nogitek Sa | Prothese d'oreille moyenne |
| US6137889A (en) * | 1998-05-27 | 2000-10-24 | Insonus Medical, Inc. | Direct tympanic membrane excitation via vibrationally conductive assembly |
| GB2355287B (en) * | 1998-07-14 | 2002-07-03 | Csir | Generating displacement and thermoacoustic refrigerator |
| US6364825B1 (en) * | 1998-09-24 | 2002-04-02 | St. Croix Medical, Inc. | Method and apparatus for improving signal quality in implantable hearing systems |
| DE19923403C2 (de) * | 1999-05-21 | 2002-11-14 | Phonak Ag Staefa | Vorrichtung zum mechanischen Ankoppeln eines in einer Mastoidhöhle implantierbaren elektromechanischen Hörgerätewandlers |
| DE19935029C2 (de) * | 1999-07-26 | 2003-02-13 | Phonak Ag Staefa | Implantierbare Anordnung zum mechanischen Ankoppeln eines Treiberteils an eine Ankoppelstelle |
| DE19948375B4 (de) * | 1999-10-07 | 2004-04-01 | Phonak Ag | Anordnung zum mechanischen Ankoppeln eines Treibers an eine Ankoppelstelle der Ossikelkette |
| US6554761B1 (en) * | 1999-10-29 | 2003-04-29 | Soundport Corporation | Flextensional microphones for implantable hearing devices |
| US6629922B1 (en) * | 1999-10-29 | 2003-10-07 | Soundport Corporation | Flextensional output actuators for surgically implantable hearing aids |
| DE10015421C2 (de) * | 2000-03-28 | 2002-07-04 | Implex Ag Hearing Technology I | Teil- oder vollimplantierbares Hörsystem |
| DE10017332C2 (de) * | 2000-04-07 | 2002-04-18 | Daimler Chrysler Ag | Piezoelektrische Betätigungseinrichtung zur Klappensteuerung am Rotorblatt eines Hubschraubers |
| US6671559B2 (en) * | 2001-01-23 | 2003-12-30 | Microphonics, Inc. | Transcanal, transtympanic cochlear implant system for the rehabilitation of deafness and tinnitus |
| US6875166B2 (en) * | 2001-09-06 | 2005-04-05 | St. Croix Medical, Inc. | Method for creating a coupling between a device and an ear structure in an implantable hearing assistance device |
| US20030097178A1 (en) * | 2001-10-04 | 2003-05-22 | Joseph Roberson | Length-adjustable ossicular prosthesis |
| GB0201574D0 (en) | 2002-01-24 | 2002-03-13 | Univ Dundee | Hearing aid |
| CN1171567C (zh) * | 2002-02-28 | 2004-10-20 | 周星 | 记忆合金人工听骨 |
| DE10331644B3 (de) | 2003-07-08 | 2005-01-20 | Technische Universität Dresden | Gehörknöchelchenprothese |
| DE202004001008U1 (de) * | 2004-01-23 | 2004-04-01 | Heinz Kurz Gmbh Medizintechnik | Gehörknöchelchenprothese |
| AT7627U1 (de) * | 2004-02-26 | 2005-06-27 | Verdichter Oe Ges M B H | Kältemittelverdichter |
| DE202006002196U1 (de) * | 2005-07-21 | 2006-04-27 | Heinz Kurz Gmbh Medizintechnik | Gehörknöchelchenprothese |
| CA2620323A1 (en) * | 2005-08-22 | 2007-03-01 | 3Win N.V. | A combined set comprising a vibrator actuator and an implantable device |
| US20080065002A1 (en) * | 2006-09-07 | 2008-03-13 | Neurosystec Corporation | Catheter for Localized Drug Delivery and/or Electrical Stimulation |
| KR100859979B1 (ko) * | 2007-07-20 | 2008-09-25 | 경북대학교 산학협력단 | 튜브 진동 트랜스듀서에 의한 정원창 구동 방식의 인공중이 |
| KR100931209B1 (ko) * | 2007-11-20 | 2009-12-10 | 경북대학교 산학협력단 | 간편 설치가 가능한 정원창 구동 진동 트랜스듀서 및 이를이용한 이식형 보청기 |
| DE202007017910U1 (de) | 2007-12-21 | 2008-03-13 | Heinz Kurz Gmbh Medizintechnik | Modulare Mittelohr-Totalprothese |
-
2007
- 2007-03-03 GB GBGB0704125.4A patent/GB0704125D0/en not_active Ceased
-
2008
- 2008-03-03 JP JP2009552280A patent/JP5545956B2/ja active Active
- 2008-03-03 AU AU2008222478A patent/AU2008222478B2/en active Active
- 2008-03-03 CN CN200880014635.XA patent/CN101677858B/zh active Active
- 2008-03-03 US US12/529,461 patent/US8920496B2/en active Active
- 2008-03-03 WO PCT/GB2008/050147 patent/WO2008107716A1/en active Application Filing
- 2008-03-03 EP EP08709667.3A patent/EP2134298B1/en active Active
- 2008-03-03 CA CA2717361A patent/CA2717361A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008222478A1 (en) | 2008-09-12 |
| CN101677858A (zh) | 2010-03-24 |
| US8920496B2 (en) | 2014-12-30 |
| EP2134298A1 (en) | 2009-12-23 |
| JP2010519999A (ja) | 2010-06-10 |
| CN101677858B (zh) | 2014-07-02 |
| US20110106254A1 (en) | 2011-05-05 |
| CA2717361A1 (en) | 2008-09-12 |
| AU2008222478B2 (en) | 2013-08-29 |
| WO2008107716A1 (en) | 2008-09-12 |
| GB0704125D0 (en) | 2007-04-11 |
| EP2134298B1 (en) | 2018-01-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5545956B2 (ja) | 人工耳小骨 | |
| JP2010519999A5 (ja) | ||
| US9259311B2 (en) | Ossicle prosthesis comprising a built-up attaching element | |
| CA1282206C (en) | Middle-ear prosthesis | |
| US20070050027A1 (en) | Leak-proof breast implant | |
| CN104840279A (zh) | 膝关节假体 | |
| JPWO2019208315A1 (ja) | イヤーピース | |
| AU2010264285A1 (en) | Coupling apparatus | |
| US20240315878A1 (en) | Devices and methods for occluding a duct and attenuating sound | |
| US20160175093A1 (en) | Ossicular prosthesis having spikes | |
| Zenner et al. | Acoustomechanical properties of open TTP® titanium middle ear prostheses | |
| US20220015910A1 (en) | Method for limiting diffusion of wear debris of in vivo implant | |
| Hüttenbrink et al. | Biomechanical aspects in implantable microphones and hearing aids and development of a concept with a hydroacoustical transmission | |
| EP4134045B1 (en) | Ossicular replacement rosthesis with controllable stapedial conforming function, manufacture method thereof and applicator device therefor | |
| BORNITZ et al. | Design considerations for length variable prostheses finite element model simulations | |
| CN118742279A (zh) | 用于阻塞管道和衰减声音的装置和方法 | |
| HARDTKE | University Hospital Dresden, Dept. of Otorhinolaryngology, Fetscherstr. 74, D-01307 Dresden, Germany E-mail: hriolab (a) rcs. urz. tu-dresden. de | |
| BORNITZ et al. | FINITE ELEMENT MODEL SIMULATIONS | |
| Ladak et al. | Finite-element modelling of the normal and surgically repaired cat middle ear | |
| Prendergast et al. | A Finite Element Analysis of a Healthy Middle-Ear and a Middle-Ear Reconstructed With Prostheses | |
| JPH10216154A (ja) | 咬合圧均等分散型顎骨内埋入歯科用インプラント | |
| Navara et al. | A mathematical model of sound transmission through the middle ear | |
| Albrecht Eiber et al. | ON THE COUPLING OF PROSTHESES TO THE MIDDLE EAR STRUCTURE AND ITS INFLUENCE ON SOUND TRANSFER | |
| MILLS et al. | IN VITRO INVESTIGATION OF THE MOTION OF A MALLEUS HEAD TO STAPES HEAD ‘INCUS REPLICA’PROSTHESIS DURING CHANGES IN STATIC PRESSURE | |
| Ozkul et al. | Recent promising technological developments on hearing restoration |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110303 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120131 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20121115 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121127 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130226 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130322 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130415 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130425 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130716 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20131015 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20131024 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20140116 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140422 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140512 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5545956 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |