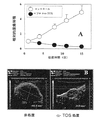
JP5396274B2 - プロオキシダント抗ガン化合物 - Google Patents
プロオキシダント抗ガン化合物 Download PDFInfo
- Publication number
- JP5396274B2 JP5396274B2 JP2009527656A JP2009527656A JP5396274B2 JP 5396274 B2 JP5396274 B2 JP 5396274B2 JP 2009527656 A JP2009527656 A JP 2009527656A JP 2009527656 A JP2009527656 A JP 2009527656A JP 5396274 B2 JP5396274 B2 JP 5396274B2
- Authority
- JP
- Japan
- Prior art keywords
- cells
- complex
- tos
- vitamin
- prooxidant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
- A61K31/353—3,4-Dihydrobenzopyrans, e.g. chroman, catechin
- A61K31/355—Tocopherols, e.g. vitamin E
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/662—Phosphorus acids or esters thereof having P—C bonds, e.g. foscarnet, trichlorfon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5076—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving cell organelles, e.g. Golgi complex, endoplasmic reticulum
- G01N33/5079—Mitochondria
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/82—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving vitamins or their receptors
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Toxicology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Tropical Medicine & Parasitology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Pyrane Compounds (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2006905109A AU2006905109A0 (en) | 2006-09-15 | Pro-oxidant Anti-cancer Compounds | |
| AU2006905109 | 2006-09-15 | ||
| PCT/AU2007/001371 WO2008031171A1 (en) | 2006-09-15 | 2007-09-17 | Pro-oxidant anti-cancer compounds |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010508242A JP2010508242A (ja) | 2010-03-18 |
| JP2010508242A5 JP2010508242A5 (enExample) | 2013-03-28 |
| JP5396274B2 true JP5396274B2 (ja) | 2014-01-22 |
Family
ID=39183283
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009527656A Expired - Fee Related JP5396274B2 (ja) | 2006-09-15 | 2007-09-17 | プロオキシダント抗ガン化合物 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US8410056B2 (enExample) |
| EP (1) | EP2063884B1 (enExample) |
| JP (1) | JP5396274B2 (enExample) |
| KR (1) | KR101482803B1 (enExample) |
| AU (1) | AU2007295877B2 (enExample) |
| CA (1) | CA2663474C (enExample) |
| NZ (1) | NZ575920A (enExample) |
| WO (1) | WO2008031171A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8598145B2 (en) | 2008-03-14 | 2013-12-03 | Stephen John Ralph | Mitochondrially delivered anti-cancer compounds |
| US8461362B2 (en) * | 2009-04-13 | 2013-06-11 | The Ohio State University Research Foundation | Protein phosphatase 2A-activating agents |
| KR20120100706A (ko) | 2009-05-20 | 2012-09-12 | 유니베르시떼 드 제네브 | 암 개시세포의 미토콘드리아 활성 저해제 및 이의 용도 |
| PT3122757T (pt) * | 2014-02-28 | 2023-11-03 | Hangzhou Dac Biotech Co Ltd | Ligantes carregados e as suas utilizações em conjugação |
| US11007182B2 (en) | 2015-04-09 | 2021-05-18 | Health Research, Inc. | Use of atpenin to activate innate immunity |
| KR102644587B1 (ko) * | 2015-12-24 | 2024-03-07 | (주)아모레퍼시픽 | 유사 세라마이드 화합물 및 그 제조방법 |
| WO2018148550A1 (en) * | 2017-02-10 | 2018-08-16 | Health Research, Inc. | Targeting mitochondrial complex ii to reduce effects of chronic hypoxia |
| WO2020051231A1 (en) * | 2018-09-04 | 2020-03-12 | H. Lee Moffitt Cancer Center & Research Institute Inc. | Use of delta-tocotrienol for treating cancer |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2220541A1 (en) * | 1997-11-07 | 1999-05-07 | Mcgill University | Analogs of vitamin e |
| RU2191903C2 (ru) | 1998-07-21 | 2002-10-27 | Карпенко Анатолий Григорьевич | Глушитель шума выхлопа двигателя внутреннего сгорания |
| CA2343893C (en) | 1998-09-15 | 2008-12-30 | Biophoretic Therapeutic Systems, Llc | Iontophoretic drug delivery electrodes |
| EP1169033A2 (en) * | 1999-04-02 | 2002-01-09 | Washington State University Research Foundation | Enhanced tissue and subcellular delivery of vitamin e compounds |
| JP4334472B2 (ja) * | 2002-06-10 | 2009-09-30 | 学校法人北里研究所 | 電子伝達系の複合体ii阻害剤 |
| US20040175415A1 (en) * | 2003-03-05 | 2004-09-09 | Chan Alvin C. | Formulations and methods of delivery of intact tocopheryl succinate to humans |
| US6716873B1 (en) * | 2003-03-20 | 2004-04-06 | Yasoo Health Inc. | Tocopherol ester compounds |
| RU2007107359A (ru) | 2004-07-28 | 2008-09-10 | ЭсДи ФАРМАСЬЮТИКАЛЗ, ИНК. (US) | Стабильная инъецируемая композиция альфа-токоферилсукцината, его аналогов и солей |
| AU2006211960A1 (en) * | 2005-02-08 | 2006-08-17 | Board Of Regents, The University Of Texas System | Compositions and methods involving MDA-7 for the treatment of cancer |
-
2007
- 2007-09-17 CA CA2663474A patent/CA2663474C/en active Active
- 2007-09-17 NZ NZ575920A patent/NZ575920A/xx unknown
- 2007-09-17 KR KR1020097007629A patent/KR101482803B1/ko not_active Expired - Fee Related
- 2007-09-17 EP EP07800325.8A patent/EP2063884B1/en active Active
- 2007-09-17 JP JP2009527656A patent/JP5396274B2/ja not_active Expired - Fee Related
- 2007-09-17 US US12/441,487 patent/US8410056B2/en not_active Expired - Fee Related
- 2007-09-17 AU AU2007295877A patent/AU2007295877B2/en active Active
- 2007-09-17 WO PCT/AU2007/001371 patent/WO2008031171A1/en not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| CA2663474C (en) | 2017-06-27 |
| EP2063884B1 (en) | 2014-08-13 |
| KR20090089845A (ko) | 2009-08-24 |
| EP2063884A1 (en) | 2009-06-03 |
| CA2663474A1 (en) | 2008-03-20 |
| US20110059898A1 (en) | 2011-03-10 |
| WO2008031171A1 (en) | 2008-03-20 |
| US8410056B2 (en) | 2013-04-02 |
| KR101482803B1 (ko) | 2015-01-15 |
| AU2007295877A1 (en) | 2008-03-20 |
| JP2010508242A (ja) | 2010-03-18 |
| AU2007295877B2 (en) | 2013-04-18 |
| NZ575920A (en) | 2012-08-31 |
| EP2063884A4 (en) | 2010-05-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5396274B2 (ja) | プロオキシダント抗ガン化合物 | |
| Fang et al. | The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease | |
| Nakamura et al. | Exploiting ferroptosis vulnerabilities in cancer | |
| Boutin et al. | Melatonin controversies, an update | |
| Fulda et al. | Targeting mitochondria for cancer therapy | |
| Dong et al. | α-Tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II | |
| KR101642157B1 (ko) | 미토콘드리아로 전달되는 항암 화합물 | |
| Ding et al. | Bioevaluation of human serum albumin–hesperidin bioconjugate: insight into protein vector function and conformation | |
| Mitani et al. | Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer | |
| Zhang et al. | Recent advances in the inhibition of membrane lipid peroxidation by food-borne plant polyphenols via the nrf2/gpx4 pathway | |
| WO2008150509A1 (en) | Methods and compositions for treating spinal muscular atrophy | |
| Xie et al. | In situ fluorescence imaging reveals contribution of cerebral hydroxyl radicals in hyperhomocysteinemia-induced Alzheimer-like dementia | |
| Fernandes et al. | Desrisking the cytotoxicity of a Mitochondriotropic antioxidant based on Caffeic acid by a PEGylated strategy | |
| Ates-Alagoz | Antioxidant activities of retinoidal benzimidazole or indole derivatives in In vitro model systems | |
| Viskupicova et al. | Inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA1) by rutin derivatives | |
| Nandha et al. | Anti-oxidants as therapeutic agents for oxidative stress associated pathologies: future challenges and opportunities | |
| Sun et al. | Targeting GDP-dissociation inhibitor beta (GDI2) with a benzo [a] quinolizidine library to induce paraptosis for cancer therapy | |
| Sirvins et al. | C-Nitrosation, C-Nitration, and Coupling of Flavonoids with N-Acetyltryptophan Limit This Amine N-Nitrosation in a Simulated Cured and Cooked Meat | |
| Mossalam et al. | Solid phase synthesis of mitochondrial triphenylphosphonium-vitamin E metabolite using a lysine linker for reversal of oxidative stress | |
| AU2009225261B2 (en) | Mitochondrially delivered anti-cancer compounds | |
| Ghanim | HAMLET and synthetic derivatives as pre-operative agents in the treatment of oral and oesophageal cancer | |
| Wilson et al. | Diet and Lifestyle in Prostate | |
| Dong et al. | α-Tocopheryl succinate induces apoptosis by targeting ubiquinone-binding | |
| Chung | Molecular Mechanisms of Vitamin E Secretion in Hepatocytes | |
| Gök | Investigation of the lipid metabolic changes induced by novel alpha tocopherol analog (TC6OH) in luminal A type breast cancer cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100917 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100917 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20100922 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120807 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121029 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20121105 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121206 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20121213 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121227 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130109 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20130205 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130206 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130402 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130701 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130708 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130731 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130924 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20131021 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5396274 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |