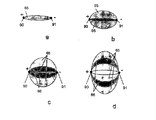
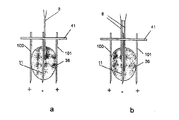
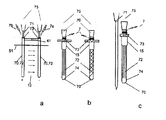
JP5197006B2 - 電気力を使用して分子を細胞に移動させるデバイス - Google Patents
電気力を使用して分子を細胞に移動させるデバイス Download PDFInfo
- Publication number
- JP5197006B2 JP5197006B2 JP2007517366A JP2007517366A JP5197006B2 JP 5197006 B2 JP5197006 B2 JP 5197006B2 JP 2007517366 A JP2007517366 A JP 2007517366A JP 2007517366 A JP2007517366 A JP 2007517366A JP 5197006 B2 JP5197006 B2 JP 5197006B2
- Authority
- JP
- Japan
- Prior art keywords
- electrode
- electrodes
- invasive
- tissue
- catheter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000004480 active ingredient Substances 0.000 claims description 118
- 230000005684 electric field Effects 0.000 claims description 70
- 238000000034 method Methods 0.000 claims description 55
- 238000002347 injection Methods 0.000 claims description 38
- 239000007924 injection Substances 0.000 claims description 38
- 239000012777 electrically insulating material Substances 0.000 claims description 9
- 241001465754 Metazoa Species 0.000 claims description 7
- 230000035515 penetration Effects 0.000 claims description 6
- 238000001727 in vivo Methods 0.000 claims description 5
- 238000001208 nuclear magnetic resonance pulse sequence Methods 0.000 claims description 5
- 230000001788 irregular Effects 0.000 claims description 3
- 230000009545 invasion Effects 0.000 claims description 2
- 210000001519 tissue Anatomy 0.000 description 132
- 238000010586 diagram Methods 0.000 description 18
- 230000004118 muscle contraction Effects 0.000 description 13
- 238000011282 treatment Methods 0.000 description 13
- 210000004027 cell Anatomy 0.000 description 11
- 230000036407 pain Effects 0.000 description 9
- 108090000623 proteins and genes Proteins 0.000 description 9
- 108020004414 DNA Proteins 0.000 description 8
- 102000004169 proteins and genes Human genes 0.000 description 8
- 210000003491 skin Anatomy 0.000 description 7
- 241000282412 Homo Species 0.000 description 6
- 230000006378 damage Effects 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 210000003205 muscle Anatomy 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 230000001988 toxicity Effects 0.000 description 5
- 231100000419 toxicity Toxicity 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 239000003204 tranquilizing agent Substances 0.000 description 4
- 230000002936 tranquilizing effect Effects 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 210000001503 joint Anatomy 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 108020004707 nucleic acids Proteins 0.000 description 3
- 102000039446 nucleic acids Human genes 0.000 description 3
- 150000007523 nucleic acids Chemical class 0.000 description 3
- 239000002773 nucleotide Substances 0.000 description 3
- 125000003729 nucleotide group Chemical group 0.000 description 3
- 230000008520 organization Effects 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 230000001681 protective effect Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 230000001960 triggered effect Effects 0.000 description 3
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 3
- 229960001600 xylazine Drugs 0.000 description 3
- 208000036487 Arthropathies Diseases 0.000 description 2
- 102000004506 Blood Proteins Human genes 0.000 description 2
- 108010017384 Blood Proteins Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 208000012659 Joint disease Diseases 0.000 description 2
- 208000021642 Muscular disease Diseases 0.000 description 2
- 201000009623 Myopathy Diseases 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 206010003246 arthritis Diseases 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000007769 metal material Substances 0.000 description 2
- 208000015122 neurodegenerative disease Diseases 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 238000000053 physical method Methods 0.000 description 2
- 239000002985 plastic film Substances 0.000 description 2
- 229920006255 plastic film Polymers 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 229940125725 tranquilizer Drugs 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000002255 vaccination Methods 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000008076 Angiogenic Proteins Human genes 0.000 description 1
- 108010074415 Angiogenic Proteins Proteins 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 206010068139 Device toxicity Diseases 0.000 description 1
- 102000003951 Erythropoietin Human genes 0.000 description 1
- 108090000394 Erythropoietin Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 208000031220 Hemophilia Diseases 0.000 description 1
- 208000009292 Hemophilia A Diseases 0.000 description 1
- 208000033868 Lysosomal disease Diseases 0.000 description 1
- 208000015439 Lysosomal storage disease Diseases 0.000 description 1
- 206010029113 Neovascularisation Diseases 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 102000007072 Nerve Growth Factors Human genes 0.000 description 1
- 206010036030 Polyarthritis Diseases 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229940035674 anesthetics Drugs 0.000 description 1
- 230000001772 anti-angiogenic effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 206010003230 arteritis Diseases 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 208000005980 beta thalassemia Diseases 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 210000004207 dermis Anatomy 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 210000005069 ears Anatomy 0.000 description 1
- 230000005672 electromagnetic field Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 229940105423 erythropoietin Drugs 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 210000001508 eye Anatomy 0.000 description 1
- 230000009969 flowable effect Effects 0.000 description 1
- 238000010230 functional analysis Methods 0.000 description 1
- 239000003193 general anesthetic agent Substances 0.000 description 1
- 208000037824 growth disorder Diseases 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 238000010999 medical injection Methods 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 239000006262 metallic foam Substances 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 208000018360 neuromuscular disease Diseases 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 210000003800 pharynx Anatomy 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 208000030428 polyarticular arthritis Diseases 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/327—Applying electric currents by contact electrodes alternating or intermittent currents for enhancing the absorption properties of tissue, e.g. by electroporation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/20—Applying electric currents by contact electrodes continuous direct currents
- A61N1/30—Apparatus for iontophoresis, i.e. transfer of media in ionic state by an electromotoric force into the body, or cataphoresis
- A61N1/303—Constructional details
- A61N1/306—Arrangements where at least part of the apparatus is introduced into the body
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Biomedical Technology (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Electrotherapy Devices (AREA)
- Immobilizing And Processing Of Enzymes And Microorganisms (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
Applications Claiming Priority (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0406943A FR2872055B1 (fr) | 2004-06-24 | 2004-06-24 | Dispositif pour le transfert de molecules aux cellules utilisant une force d'origine physique et combinaison permettant la mise en oeuvre du procede |
| FR0406943 | 2004-06-24 | ||
| FR0410172 | 2004-09-27 | ||
| FR0410172A FR2872056B1 (fr) | 2004-06-24 | 2004-09-27 | Dispositif pour l'administration de principe actif aux cellules et tissus utilisant une ou deux forces physiques |
| FR0500603 | 2005-01-20 | ||
| FR0500603A FR2880808A1 (fr) | 2005-01-20 | 2005-01-20 | Dispositif pour l'administration de principe actif aux cellules et tissus a l'aide d'electrodes de surface non invasives |
| FR0502215 | 2005-03-04 | ||
| FR0502215 | 2005-03-04 | ||
| PCT/FR2005/001611 WO2006010837A2 (fr) | 2004-06-24 | 2005-06-24 | Dispositif our le transfert de molecules aux cellules utilisant une force electrique |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012145616A Division JP2012250044A (ja) | 2004-06-24 | 2012-06-28 | 電気力を使用して分子を細胞に移動させるデバイス |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008503292A JP2008503292A (ja) | 2008-02-07 |
| JP5197006B2 true JP5197006B2 (ja) | 2013-05-15 |
Family
ID=35517221
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007517366A Expired - Fee Related JP5197006B2 (ja) | 2004-06-24 | 2005-06-24 | 電気力を使用して分子を細胞に移動させるデバイス |
| JP2012145616A Pending JP2012250044A (ja) | 2004-06-24 | 2012-06-28 | 電気力を使用して分子を細胞に移動させるデバイス |
| JP2015094612A Pending JP2015163230A (ja) | 2004-06-24 | 2015-05-07 | 電気力を使用して分子を細胞に移動させるデバイス |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012145616A Pending JP2012250044A (ja) | 2004-06-24 | 2012-06-28 | 電気力を使用して分子を細胞に移動させるデバイス |
| JP2015094612A Pending JP2015163230A (ja) | 2004-06-24 | 2015-05-07 | 電気力を使用して分子を細胞に移動させるデバイス |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20070156082A1 (enExample) |
| EP (1) | EP1786514B1 (enExample) |
| JP (3) | JP5197006B2 (enExample) |
| BR (1) | BRPI0512607A (enExample) |
| CA (1) | CA2572122A1 (enExample) |
| WO (1) | WO2006010837A2 (enExample) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2893256B1 (fr) * | 2005-11-14 | 2008-08-15 | Yves Scherman | Generateur d'impulsions electriques unipolaires. |
| EP2167188A1 (en) | 2007-07-10 | 2010-03-31 | Koninklijke Philips Electronics N.V. | Neurostimulation system |
| SG154343A1 (en) * | 2008-01-08 | 2009-08-28 | Ting Choon Meng | Method and apparatus for intra-articular injection or aspiration |
| FR2928536B1 (fr) * | 2008-03-14 | 2012-04-27 | Inst Nat Sante Rech Med | Dispositif d'injection dans l'oeil |
| AU2015201289B2 (en) * | 2008-03-14 | 2016-12-01 | Institut National De La Sante Et De La Recherche Medicale | Eye injection device |
| US10028782B2 (en) * | 2008-11-03 | 2018-07-24 | Magneto Thrombectomy Solutions Ltd. | Method and apparatus for thrombus dissolution/thrombectomy by an electrode catheter device |
| WO2011034939A1 (en) | 2009-09-15 | 2011-03-24 | Rush University Medical Center | Energy-releasing carbon nanotube transponder and method of using same |
| IT1395992B1 (it) * | 2009-10-27 | 2012-11-09 | Caccia | Testa per elettroporazione |
| WO2011109399A1 (en) * | 2010-03-01 | 2011-09-09 | Inovio Pharmaceuticals, Inc. | Multiple tissue layer electroporation applicator and device |
| ITTO20110411A1 (it) * | 2011-05-11 | 2012-11-12 | Consiglio Nazionale Ricerche | Dispositivo per elettroporazione |
| EP2720740A1 (en) | 2011-06-15 | 2014-04-23 | ChronTech Pharma AB | Injection needle and device |
| US11311332B2 (en) | 2011-08-23 | 2022-04-26 | Magneto Thrombectomy Solutions Ltd. | Thrombectomy devices |
| PL3277368T3 (pl) * | 2015-03-31 | 2021-01-25 | Oncosec Medical Incorporated | Układy do ulepszonej elektroporacji opartej na wykrywaniu tkanek |
| WO2017145689A1 (ja) * | 2016-02-22 | 2017-08-31 | 公益財団法人東京都医学総合研究所 | エレクトロポレーション用電極 |
| EP3436139B1 (en) * | 2016-03-28 | 2025-04-30 | PapiVax Biotech, Inc. | METHOD AND APPARATUS FOR ADMINISTERING THERAPEUTIC AGENTS |
| US12029475B2 (en) | 2017-03-22 | 2024-07-09 | Magneto Thrombectomy Solutions Ltd. | Thrombectomy using both electrostatic and suction forces |
| JP6884037B2 (ja) * | 2017-05-29 | 2021-06-09 | 株式会社ジェイメック | パルス電圧治療装置 |
| KR101889048B1 (ko) * | 2017-08-11 | 2018-08-16 | 문창수 | 전기적 자극을 가하는 약물주입용 기구 세트 |
| WO2019102307A1 (en) | 2017-11-23 | 2019-05-31 | Magneto Thrombectomy Solutions Ltd. | Tubular thrombectomy devices |
| US11071860B2 (en) | 2019-02-06 | 2021-07-27 | Oncosec Medical Incorporated | Systems and methods for detecting fault conditions in electroporation therapy |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5273525A (en) | 1992-08-13 | 1993-12-28 | Btx Inc. | Injection and electroporation apparatus for drug and gene delivery |
| US5318514A (en) | 1992-08-17 | 1994-06-07 | Btx, Inc. | Applicator for the electroporation of drugs and genes into surface cells |
| JPH06327777A (ja) * | 1993-03-26 | 1994-11-29 | Gijutsu Kenkyu Kumiai Iryo Fukushi Kiki Kenkyusho | 電気的薬剤導入装置 |
| US5993434A (en) * | 1993-04-01 | 1999-11-30 | Genetronics, Inc. | Method of treatment using electroporation mediated delivery of drugs and genes |
| JPH07299143A (ja) * | 1994-05-09 | 1995-11-14 | Kenji Tsubota | 医科用注射針 |
| AUPM982694A0 (en) * | 1994-12-02 | 1995-01-05 | University Of Queensland, The | Iontophoresis method and apparatus |
| AU8659598A (en) * | 1997-07-22 | 1999-02-16 | Emed Corporation | Iontophoretic delivery of an agent into cardiac tissue |
| JP2001506172A (ja) * | 1997-07-22 | 2001-05-15 | イーメッド コーポレイション | 作用物質のイオン導入法による送達のための針 |
| US6055453A (en) | 1997-08-01 | 2000-04-25 | Genetronics, Inc. | Apparatus for addressing needle array electrodes for electroporation therapy |
| US6241701B1 (en) * | 1997-08-01 | 2001-06-05 | Genetronics, Inc. | Apparatus for electroporation mediated delivery of drugs and genes |
| JP2003505114A (ja) * | 1998-07-13 | 2003-02-12 | ジェネトロニクス、インコーポレーテッド | パルス電場による皮膚および筋肉を標的とした遺伝子治療 |
| US6529776B1 (en) * | 1999-12-01 | 2003-03-04 | Vertis Neuroscience, Inc. | Method and apparatus for repositioning a percutaneous probe |
| EP1292359A4 (en) * | 2000-05-22 | 2005-03-30 | Merck & Co Inc | SYSTEM AND METHOD FOR EVALUATING THE EFFICACY OF A PHARMACEUTICAL AGENT DELIVERY DEVICE |
| US20040092860A1 (en) | 2000-07-26 | 2004-05-13 | Dev Nagendu B. | Skin and muscle-targeted gene therapy by pulsed electrical field |
| JP2002282371A (ja) * | 2001-03-28 | 2002-10-02 | Polytronics Ltd | 低電圧駆動型イオントフォレシス素子 |
| US20020198567A1 (en) | 2001-06-07 | 2002-12-26 | Yona Keisari | Electro-endocytotic therapy as a treatment modality of cancer |
| FR2830767B1 (fr) * | 2001-10-12 | 2004-03-12 | Optis France Sa | Dispositif de delivrance de medicaments par iontophorese ou electroporation introculaire |
| US20050154434A1 (en) * | 2002-03-07 | 2005-07-14 | Adam Simon | Clinical syringe with electrical stimulation aspects |
| US7245963B2 (en) * | 2002-03-07 | 2007-07-17 | Advisys, Inc. | Electrode assembly for constant-current electroporation and use |
| US6912417B1 (en) * | 2002-04-05 | 2005-06-28 | Ichor Medical Systmes, Inc. | Method and apparatus for delivery of therapeutic agents |
| CA2482183A1 (en) * | 2002-04-16 | 2003-10-30 | Cyto Pulse Sciences, Inc. | Method of treating biological materials with translating electrical fields and electrode polarity reversal |
| US20050070841A1 (en) | 2002-07-04 | 2005-03-31 | Inovio As | Electroporation device and injection apparatus |
| US7282033B2 (en) * | 2002-09-04 | 2007-10-16 | Urmey William F | Positioning system for a nerve stimulator needle |
| EE05601B2 (et) | 2010-12-31 | 2015-06-15 | As Myoton | Seade ja meetod pehme bioloogilise koe mehaanilise pingeseisundi, elastsuse, dünaamilise jäikuse, roomavuse ja mehaanilise pinge relaksatsiooniaega iseloomustavate parameetrite samaaegseks mõõtmiseks reaalajas ning arvutiprogrammi produkt |
-
2005
- 2005-06-24 CA CA002572122A patent/CA2572122A1/fr not_active Abandoned
- 2005-06-24 EP EP05779697.1A patent/EP1786514B1/fr not_active Expired - Lifetime
- 2005-06-24 BR BRPI0512607-0A patent/BRPI0512607A/pt not_active IP Right Cessation
- 2005-06-24 WO PCT/FR2005/001611 patent/WO2006010837A2/fr not_active Ceased
- 2005-06-24 JP JP2007517366A patent/JP5197006B2/ja not_active Expired - Fee Related
-
2006
- 2006-12-24 US US11/615,991 patent/US20070156082A1/en not_active Abandoned
-
2012
- 2012-06-28 JP JP2012145616A patent/JP2012250044A/ja active Pending
-
2015
- 2015-05-07 JP JP2015094612A patent/JP2015163230A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| EP1786514B1 (fr) | 2015-01-07 |
| BRPI0512607A (pt) | 2008-03-25 |
| JP2008503292A (ja) | 2008-02-07 |
| WO2006010837A2 (fr) | 2006-02-02 |
| US20070156082A1 (en) | 2007-07-05 |
| CA2572122A1 (fr) | 2006-02-02 |
| JP2012250044A (ja) | 2012-12-20 |
| EP1786514A2 (fr) | 2007-05-23 |
| JP2015163230A (ja) | 2015-09-10 |
| WO2006010837A3 (fr) | 2006-04-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2015163230A (ja) | 電気力を使用して分子を細胞に移動させるデバイス | |
| US20230001190A1 (en) | Device and method for single-needle in vivo electroporation | |
| US10252004B2 (en) | Method and apparatus for delivery of therapeutic agents | |
| US6738663B2 (en) | Implantable device and method for the electrical treatment of cancer | |
| US6366808B1 (en) | Implantable device and method for the electrical treatment of cancer | |
| US7742811B2 (en) | Implantable device and method for the electrical treatment of cancer | |
| US20110046540A1 (en) | Apparatus for Trans-Cerebral Electrophoresis and Methods of Use Thereof | |
| JP2002535100A (ja) | 細胞内への巨大分子の送達 | |
| US20250000569A1 (en) | Blood-brain barrier disruption using reversible or irreversible electroporation | |
| EP2211982A1 (en) | An electroporation device for improved electrical field control | |
| US20250228602A1 (en) | Methods of reducing adverse effects of non-thermal ablation | |
| CN101374569A (zh) | 单极电脉冲发生器 | |
| CA2686855C (en) | Device and method for single-needle in vivo electroporation | |
| MX2008008981A (es) | Dispositivo y metodo para electropermeacion in vivo de una sola aguja |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080526 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110512 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110808 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110815 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110912 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110920 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20111006 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20111014 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111114 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120228 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120628 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121010 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20121107 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130107 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130205 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20160215 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |