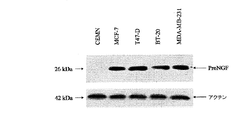
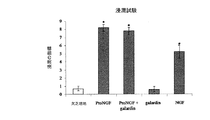
JP5091876B2 - 癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 - Google Patents
癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 Download PDFInfo
- Publication number
- JP5091876B2 JP5091876B2 JP2008552858A JP2008552858A JP5091876B2 JP 5091876 B2 JP5091876 B2 JP 5091876B2 JP 2008552858 A JP2008552858 A JP 2008552858A JP 2008552858 A JP2008552858 A JP 2008552858A JP 5091876 B2 JP5091876 B2 JP 5091876B2
- Authority
- JP
- Japan
- Prior art keywords
- prongf
- cancer
- cells
- breast
- thyroid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 206010028980 Neoplasm Diseases 0.000 title claims description 124
- 201000011510 cancer Diseases 0.000 title claims description 80
- 238000000034 method Methods 0.000 title claims description 61
- 208000026310 Breast neoplasm Diseases 0.000 title claims description 53
- 206010006187 Breast cancer Diseases 0.000 title claims description 52
- 208000024770 Thyroid neoplasm Diseases 0.000 title claims description 33
- 208000020816 lung neoplasm Diseases 0.000 title claims description 32
- 206010058467 Lung neoplasm malignant Diseases 0.000 title claims description 31
- 201000005202 lung cancer Diseases 0.000 title claims description 31
- 201000002510 thyroid cancer Diseases 0.000 title claims description 30
- 230000001225 therapeutic effect Effects 0.000 title claims description 19
- 238000003745 diagnosis Methods 0.000 title claims description 15
- 238000000338 in vitro Methods 0.000 title claims description 7
- 238000003556 assay Methods 0.000 title description 2
- 210000004027 cell Anatomy 0.000 claims description 143
- 210000001519 tissue Anatomy 0.000 claims description 40
- 238000001514 detection method Methods 0.000 claims description 35
- 206010060862 Prostate cancer Diseases 0.000 claims description 32
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 32
- 238000001574 biopsy Methods 0.000 claims description 27
- 239000012472 biological sample Substances 0.000 claims description 23
- 210000001124 body fluid Anatomy 0.000 claims description 17
- 238000012216 screening Methods 0.000 claims description 17
- 208000005443 Circulating Neoplastic Cells Diseases 0.000 claims description 16
- 239000010839 body fluid Substances 0.000 claims description 16
- 238000004393 prognosis Methods 0.000 claims description 12
- 206010027476 Metastases Diseases 0.000 claims description 11
- 238000004949 mass spectrometry Methods 0.000 claims description 11
- 239000000523 sample Substances 0.000 claims description 10
- 230000009401 metastasis Effects 0.000 claims description 9
- 230000000903 blocking effect Effects 0.000 claims description 6
- 238000013399 early diagnosis Methods 0.000 claims description 6
- 238000003018 immunoassay Methods 0.000 claims description 6
- 238000012258 culturing Methods 0.000 claims description 5
- 239000002609 medium Substances 0.000 description 46
- 108010025020 Nerve Growth Factor Proteins 0.000 description 38
- 102000015336 Nerve Growth Factor Human genes 0.000 description 37
- 108090000623 proteins and genes Proteins 0.000 description 29
- 230000002950 deficient Effects 0.000 description 28
- 239000003112 inhibitor Substances 0.000 description 28
- 102000004169 proteins and genes Human genes 0.000 description 24
- 102000005962 receptors Human genes 0.000 description 24
- 108020003175 receptors Proteins 0.000 description 24
- 238000011282 treatment Methods 0.000 description 23
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 21
- 230000014509 gene expression Effects 0.000 description 19
- 210000000069 breast epithelial cell Anatomy 0.000 description 18
- 102100032889 Sortilin Human genes 0.000 description 17
- 230000002452 interceptive effect Effects 0.000 description 17
- 108010014657 sortilin Proteins 0.000 description 17
- 238000001262 western blot Methods 0.000 description 17
- 239000000427 antigen Substances 0.000 description 16
- 108091007433 antigens Proteins 0.000 description 16
- 102000036639 antigens Human genes 0.000 description 16
- 230000000694 effects Effects 0.000 description 16
- 210000000481 breast Anatomy 0.000 description 15
- 239000003636 conditioned culture medium Substances 0.000 description 14
- 239000012528 membrane Substances 0.000 description 13
- 210000002966 serum Anatomy 0.000 description 13
- 102000007469 Actins Human genes 0.000 description 11
- 108010085238 Actins Proteins 0.000 description 11
- 108010030545 N-(2(R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl)-L-tryptophan methylamide Proteins 0.000 description 11
- NITYDPDXAAFEIT-DYVFJYSZSA-N ilomastat Chemical compound C1=CC=C2C(C[C@@H](C(=O)NC)NC(=O)[C@H](CC(C)C)CC(=O)NO)=CNC2=C1 NITYDPDXAAFEIT-DYVFJYSZSA-N 0.000 description 11
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 11
- 210000002307 prostate Anatomy 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 10
- 210000000056 organ Anatomy 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- 108020004459 Small interfering RNA Proteins 0.000 description 9
- 230000012292 cell migration Effects 0.000 description 9
- 201000009030 Carcinoma Diseases 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 230000034994 death Effects 0.000 description 8
- 231100000517 death Toxicity 0.000 description 8
- 239000000499 gel Substances 0.000 description 8
- 230000008595 infiltration Effects 0.000 description 8
- 238000001764 infiltration Methods 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 238000013508 migration Methods 0.000 description 8
- 239000000700 radioactive tracer Substances 0.000 description 8
- 238000001890 transfection Methods 0.000 description 8
- 238000012546 transfer Methods 0.000 description 8
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 7
- 241000699670 Mus sp. Species 0.000 description 7
- 229920001213 Polysorbate 20 Polymers 0.000 description 7
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 7
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 7
- 230000004071 biological effect Effects 0.000 description 7
- 230000012010 growth Effects 0.000 description 7
- 239000001963 growth medium Substances 0.000 description 7
- 238000001727 in vivo Methods 0.000 description 7
- 230000005012 migration Effects 0.000 description 7
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 7
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 7
- 210000001685 thyroid gland Anatomy 0.000 description 7
- 239000003656 tris buffered saline Substances 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 6
- 239000004480 active ingredient Substances 0.000 description 6
- 230000004709 cell invasion Effects 0.000 description 6
- 238000002512 chemotherapy Methods 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- 230000018109 developmental process Effects 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 229940088597 hormone Drugs 0.000 description 6
- 239000005556 hormone Substances 0.000 description 6
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 6
- 230000009545 invasion Effects 0.000 description 6
- 108020004999 messenger RNA Proteins 0.000 description 6
- 239000013641 positive control Substances 0.000 description 6
- 238000001959 radiotherapy Methods 0.000 description 6
- 230000028327 secretion Effects 0.000 description 6
- 238000002098 selective ion monitoring Methods 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 6
- 102000012422 Collagen Type I Human genes 0.000 description 5
- 108010022452 Collagen Type I Proteins 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- 239000000020 Nitrocellulose Substances 0.000 description 5
- 239000000512 collagen gel Substances 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 150000002500 ions Chemical class 0.000 description 5
- 206010061289 metastatic neoplasm Diseases 0.000 description 5
- 239000002105 nanoparticle Substances 0.000 description 5
- 229920001220 nitrocellulos Polymers 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 239000012047 saturated solution Substances 0.000 description 5
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 4
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- 102000003781 Inhibitor of growth protein 1 Human genes 0.000 description 4
- 108090000191 Inhibitor of growth protein 1 Proteins 0.000 description 4
- 108091034117 Oligonucleotide Proteins 0.000 description 4
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 4
- 238000011033 desalting Methods 0.000 description 4
- 208000010932 epithelial neoplasm Diseases 0.000 description 4
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 4
- 230000002055 immunohistochemical effect Effects 0.000 description 4
- 238000003364 immunohistochemistry Methods 0.000 description 4
- 238000002372 labelling Methods 0.000 description 4
- 210000004072 lung Anatomy 0.000 description 4
- 238000001819 mass spectrum Methods 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 230000019491 signal transduction Effects 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 239000000439 tumor marker Substances 0.000 description 4
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- 229930182566 Gentamicin Natural products 0.000 description 3
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 3
- 102000004877 Insulin Human genes 0.000 description 3
- 108090001061 Insulin Proteins 0.000 description 3
- 229930182555 Penicillin Natural products 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- 206010041067 Small cell lung cancer Diseases 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 3
- 102000011923 Thyrotropin Human genes 0.000 description 3
- 108010061174 Thyrotropin Proteins 0.000 description 3
- 239000007983 Tris buffer Substances 0.000 description 3
- 208000009956 adenocarcinoma Diseases 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000006907 apoptotic process Effects 0.000 description 3
- 229940098773 bovine serum albumin Drugs 0.000 description 3
- 230000005907 cancer growth Effects 0.000 description 3
- 231100000504 carcinogenesis Toxicity 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 210000000038 chest Anatomy 0.000 description 3
- 210000003040 circulating cell Anatomy 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 230000001143 conditioned effect Effects 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 229960002518 gentamicin Drugs 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 210000004408 hybridoma Anatomy 0.000 description 3
- 238000009169 immunotherapy Methods 0.000 description 3
- 229940125396 insulin Drugs 0.000 description 3
- 210000001165 lymph node Anatomy 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 230000003211 malignant effect Effects 0.000 description 3
- 238000009607 mammography Methods 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 230000001394 metastastic effect Effects 0.000 description 3
- 230000027939 micturition Effects 0.000 description 3
- 230000001575 pathological effect Effects 0.000 description 3
- 229940049954 penicillin Drugs 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- 239000012857 radioactive material Substances 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 208000000587 small cell lung carcinoma Diseases 0.000 description 3
- 238000001228 spectrum Methods 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 229940113082 thymine Drugs 0.000 description 3
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 3
- 210000002700 urine Anatomy 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- PNDPGZBMCMUPRI-HVTJNCQCSA-N 10043-66-0 Chemical compound [131I][131I] PNDPGZBMCMUPRI-HVTJNCQCSA-N 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 2
- 102100023003 Ankyrin repeat domain-containing protein 30A Human genes 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 210000003771 C cell Anatomy 0.000 description 2
- 102000055006 Calcitonin Human genes 0.000 description 2
- 108060001064 Calcitonin Proteins 0.000 description 2
- 102000009016 Cholera Toxin Human genes 0.000 description 2
- 108010049048 Cholera Toxin Proteins 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 2
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 2
- 102000016359 Fibronectins Human genes 0.000 description 2
- 108010067306 Fibronectins Proteins 0.000 description 2
- 229920001917 Ficoll Polymers 0.000 description 2
- 206010018498 Goitre Diseases 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- 101000757191 Homo sapiens Ankyrin repeat domain-containing protein 30A Proteins 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- BLTCBVOJNNKFKC-QUDYQQOWSA-N N-acetylsphingosine Chemical class CCCCCCCCCCCCC\C=C\[C@@H](O)[C@H](CO)NC(C)=O BLTCBVOJNNKFKC-QUDYQQOWSA-N 0.000 description 2
- 108700020796 Oncogene Proteins 0.000 description 2
- 102000043276 Oncogene Human genes 0.000 description 2
- 206010036790 Productive cough Diseases 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- 208000009453 Thyroid Nodule Diseases 0.000 description 2
- 102000004338 Transferrin Human genes 0.000 description 2
- 108090000901 Transferrin Proteins 0.000 description 2
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 2
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 2
- 102000044209 Tumor Suppressor Genes Human genes 0.000 description 2
- 108700025716 Tumor Suppressor Genes Proteins 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 239000003098 androgen Substances 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 229960004015 calcitonin Drugs 0.000 description 2
- BBBFJLBPOGFECG-VJVYQDLKSA-N calcitonin Chemical compound N([C@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(N)=O)C(C)C)C(=O)[C@@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 BBBFJLBPOGFECG-VJVYQDLKSA-N 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 239000002254 cytotoxic agent Substances 0.000 description 2
- 229940127089 cytotoxic agent Drugs 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 238000004520 electroporation Methods 0.000 description 2
- 239000003797 essential amino acid Substances 0.000 description 2
- 102000015694 estrogen receptors Human genes 0.000 description 2
- 108010038795 estrogen receptors Proteins 0.000 description 2
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 2
- 229940126864 fibroblast growth factor Drugs 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 230000003325 follicular Effects 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 230000003054 hormonal effect Effects 0.000 description 2
- 238000001794 hormone therapy Methods 0.000 description 2
- 229960000890 hydrocortisone Drugs 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 230000002601 intratumoral effect Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 239000011630 iodine Substances 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- UAEPNZWRGJTJPN-UHFFFAOYSA-N methylcyclohexane Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 description 2
- 239000007758 minimum essential medium Substances 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 238000010844 nanoflow liquid chromatography Methods 0.000 description 2
- 230000001613 neoplastic effect Effects 0.000 description 2
- 210000000653 nervous system Anatomy 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 210000002381 plasma Anatomy 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 230000035935 pregnancy Effects 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 238000003672 processing method Methods 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 238000000751 protein extraction Methods 0.000 description 2
- 230000005180 public health Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 230000000405 serological effect Effects 0.000 description 2
- 208000002491 severe combined immunodeficiency Diseases 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 208000000649 small cell carcinoma Diseases 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 210000003802 sputum Anatomy 0.000 description 2
- 208000024794 sputum Diseases 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 229960002175 thyroglobulin Drugs 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 239000012581 transferrin Substances 0.000 description 2
- 230000004565 tumor cell growth Effects 0.000 description 2
- NMWKYTGJWUAZPZ-WWHBDHEGSA-N (4S)-4-[[(4R,7S,10S,16S,19S,25S,28S,31R)-31-[[(2S)-2-[[(1R,6R,9S,12S,18S,21S,24S,27S,30S,33S,36S,39S,42R,47R,53S,56S,59S,62S,65S,68S,71S,76S,79S,85S)-47-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-methylbutanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-3-phenylpropanoyl]amino]-4-oxobutanoyl]amino]-3-carboxypropanoyl]amino]-18-(4-aminobutyl)-27,68-bis(3-amino-3-oxopropyl)-36,71,76-tribenzyl-39-(3-carbamimidamidopropyl)-24-(2-carboxyethyl)-21,56-bis(carboxymethyl)-65,85-bis[(1R)-1-hydroxyethyl]-59-(hydroxymethyl)-62,79-bis(1H-imidazol-4-ylmethyl)-9-methyl-33-(2-methylpropyl)-8,11,17,20,23,26,29,32,35,38,41,48,54,57,60,63,66,69,72,74,77,80,83,86-tetracosaoxo-30-propan-2-yl-3,4,44,45-tetrathia-7,10,16,19,22,25,28,31,34,37,40,49,55,58,61,64,67,70,73,75,78,81,84,87-tetracosazatetracyclo[40.31.14.012,16.049,53]heptaoctacontane-6-carbonyl]amino]-3-methylbutanoyl]amino]-7-(3-carbamimidamidopropyl)-25-(hydroxymethyl)-19-[(4-hydroxyphenyl)methyl]-28-(1H-imidazol-4-ylmethyl)-10-methyl-6,9,12,15,18,21,24,27,30-nonaoxo-16-propan-2-yl-1,2-dithia-5,8,11,14,17,20,23,26,29-nonazacyclodotriacontane-4-carbonyl]amino]-5-[[(2S)-1-[[(2S)-1-[[(2S)-3-carboxy-1-[[(2S)-1-[[(2S)-1-[[(1S)-1-carboxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid Chemical compound CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]4CCCN4C(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N3CCC[C@H]3C(=O)N[C@@H](C)C(=O)N2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)C(=O)N[C@@H](C)C(O)=O NMWKYTGJWUAZPZ-WWHBDHEGSA-N 0.000 description 1
- HNSDLXPSAYFUHK-UHFFFAOYSA-N 1,4-bis(2-ethylhexyl) sulfosuccinate Chemical compound CCCCC(CC)COC(=O)CC(S(O)(=O)=O)C(=O)OCC(CC)CCCC HNSDLXPSAYFUHK-UHFFFAOYSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- PRDFBSVERLRRMY-UHFFFAOYSA-N 2'-(4-ethoxyphenyl)-5-(4-methylpiperazin-1-yl)-2,5'-bibenzimidazole Chemical compound C1=CC(OCC)=CC=C1C1=NC2=CC=C(C=3NC4=CC(=CC=C4N=3)N3CCN(C)CC3)C=C2N1 PRDFBSVERLRRMY-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- YYYARFHFWYKNLF-UHFFFAOYSA-N 4-[(2,4-dimethylphenyl)diazenyl]-3-hydroxynaphthalene-2,7-disulfonic acid Chemical compound CC1=CC(C)=CC=C1N=NC1=C(O)C(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=C12 YYYARFHFWYKNLF-UHFFFAOYSA-N 0.000 description 1
- 102100031126 6-phosphogluconolactonase Human genes 0.000 description 1
- 108010029731 6-phosphogluconolactonase Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 108010039627 Aprotinin Proteins 0.000 description 1
- 241000501754 Astronotus ocellatus Species 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 206010006784 Burning sensation Diseases 0.000 description 1
- 206010007269 Carcinogenicity Diseases 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 244000258136 Costus speciosus Species 0.000 description 1
- 235000000385 Costus speciosus Nutrition 0.000 description 1
- XUIIKFGFIJCVMT-GFCCVEGCSA-N D-thyroxine Chemical compound IC1=CC(C[C@@H](N)C(O)=O)=CC(I)=C1OC1=CC(I)=C(O)C(I)=C1 XUIIKFGFIJCVMT-GFCCVEGCSA-N 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102100037738 Fatty acid-binding protein, heart Human genes 0.000 description 1
- 101710136552 Fatty acid-binding protein, heart Proteins 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- 206010060891 General symptom Diseases 0.000 description 1
- 108010018962 Glucosephosphate Dehydrogenase Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 101001008951 Homo sapiens Kinesin-like protein KIF15 Proteins 0.000 description 1
- 101000574648 Homo sapiens Retinoid-inducible serine carboxypeptidase Proteins 0.000 description 1
- 101000632529 Homo sapiens Shugoshin 1 Proteins 0.000 description 1
- 101000610980 Homo sapiens Tumor protein D52 Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100027630 Kinesin-like protein KIF15 Human genes 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- GDBQQVLCIARPGH-UHFFFAOYSA-N Leupeptin Natural products CC(C)CC(NC(C)=O)C(=O)NC(CC(C)C)C(=O)NC(C=O)CCCN=C(N)N GDBQQVLCIARPGH-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 208000009018 Medullary thyroid cancer Diseases 0.000 description 1
- 208000002406 Mucoepidermoid Tumor Diseases 0.000 description 1
- 101150064037 NGF gene Proteins 0.000 description 1
- 102000007072 Nerve Growth Factors Human genes 0.000 description 1
- 102000003797 Neuropeptides Human genes 0.000 description 1
- 108090000189 Neuropeptides Proteins 0.000 description 1
- KUIFHYPNNRVEKZ-VIJRYAKMSA-N O-(N-acetyl-alpha-D-galactosaminyl)-L-threonine Chemical compound OC(=O)[C@@H](N)[C@@H](C)O[C@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1NC(C)=O KUIFHYPNNRVEKZ-VIJRYAKMSA-N 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- 108010053210 Phycocyanin Proteins 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 208000002151 Pleural effusion Diseases 0.000 description 1
- 101710100968 Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- 102100028402 Shugoshin 1 Human genes 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- GKLVYJBZJHMRIY-OUBTZVSYSA-N Technetium-99 Chemical compound [99Tc] GKLVYJBZJHMRIY-OUBTZVSYSA-N 0.000 description 1
- 102000009843 Thyroglobulin Human genes 0.000 description 1
- 108010034949 Thyroglobulin Proteins 0.000 description 1
- 208000033781 Thyroid carcinoma Diseases 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000009618 Transforming Growth Factors Human genes 0.000 description 1
- 108010009583 Transforming Growth Factors Proteins 0.000 description 1
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 1
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102100040418 Tumor protein D52 Human genes 0.000 description 1
- 206010046543 Urinary incontinence Diseases 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- KRHYYFGTRYWZRS-BJUDXGSMSA-N ac1l2y5h Chemical compound [18FH] KRHYYFGTRYWZRS-BJUDXGSMSA-N 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 238000011226 adjuvant chemotherapy Methods 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 102000005840 alpha-Galactosidase Human genes 0.000 description 1
- 108010030291 alpha-Galactosidase Proteins 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 230000001621 anti-mitogenic effect Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 229960004405 aprotinin Drugs 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 230000003305 autocrine Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- AFYNADDZULBEJA-UHFFFAOYSA-N bicinchoninic acid Chemical compound C1=CC=CC2=NC(C=3C=C(C4=CC=CC=C4N=3)C(=O)O)=CC(C(O)=O)=C21 AFYNADDZULBEJA-UHFFFAOYSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 239000003914 blood derivative Substances 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- UDSAIICHUKSCKT-UHFFFAOYSA-N bromophenol blue Chemical compound C1=C(Br)C(O)=C(Br)C=C1C1(C=2C=C(Br)C(O)=C(Br)C=2)C2=CC=CC=C2S(=O)(=O)O1 UDSAIICHUKSCKT-UHFFFAOYSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 229940022399 cancer vaccine Drugs 0.000 description 1
- 238000009566 cancer vaccine Methods 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 231100000260 carcinogenicity Toxicity 0.000 description 1
- 230000007670 carcinogenicity Effects 0.000 description 1
- 208000002458 carcinoid tumor Diseases 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 229940096422 collagen type i Drugs 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000007819 coupling partner Substances 0.000 description 1
- 239000006059 cover glass Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000010217 densitometric analysis Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000002224 dissection Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 230000010393 epithelial cell migration Effects 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 229930182833 estradiol Natural products 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 230000001076 estrogenic effect Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000001400 expression cloning Methods 0.000 description 1
- 210000000416 exudates and transudate Anatomy 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 201000003872 goiter Diseases 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- -1 hapten / antibody Proteins 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 230000003118 histopathologic effect Effects 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 230000004727 humoral immunity Effects 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 238000003312 immunocapture Methods 0.000 description 1
- 238000003365 immunocytochemistry Methods 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 201000001881 impotence Diseases 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 238000005040 ion trap Methods 0.000 description 1
- 208000003849 large cell carcinoma Diseases 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- GDBQQVLCIARPGH-ULQDDVLXSA-N leupeptin Chemical compound CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C=O)CCCN=C(N)N GDBQQVLCIARPGH-ULQDDVLXSA-N 0.000 description 1
- 108010052968 leupeptin Proteins 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 238000011454 long-term hormonal therapy Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 208000037841 lung tumor Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 239000002122 magnetic nanoparticle Substances 0.000 description 1
- 201000010893 malignant breast melanoma Diseases 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 208000023356 medullary thyroid gland carcinoma Diseases 0.000 description 1
- 230000009245 menopause Effects 0.000 description 1
- GYNNXHKOJHMOHS-UHFFFAOYSA-N methyl-cycloheptane Natural products CC1CCCCCC1 GYNNXHKOJHMOHS-UHFFFAOYSA-N 0.000 description 1
- 210000004088 microvessel Anatomy 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 238000007479 molecular analysis Methods 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 210000003061 neural cell Anatomy 0.000 description 1
- 210000000933 neural crest Anatomy 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000013421 nuclear magnetic resonance imaging Methods 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 230000003076 paracrine Effects 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 102000013415 peroxidase activity proteins Human genes 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- INAAIJLSXJJHOZ-UHFFFAOYSA-N pibenzimol Chemical compound C1CN(C)CCN1C1=CC=C(N=C(N2)C=3C=C4NC(=NC4=CC=3)C=3C=CC(O)=CC=3)C2=C1 INAAIJLSXJJHOZ-UHFFFAOYSA-N 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000000861 pro-apoptotic effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000011471 prostatectomy Methods 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000012797 qualification Methods 0.000 description 1
- 238000011472 radical prostatectomy Methods 0.000 description 1
- 239000013643 reference control Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 210000001625 seminal vesicle Anatomy 0.000 description 1
- 239000004055 small Interfering RNA Substances 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 210000001913 submandibular gland Anatomy 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 208000013077 thyroid gland carcinoma Diseases 0.000 description 1
- 229940034208 thyroxine Drugs 0.000 description 1
- XUIIKFGFIJCVMT-UHFFFAOYSA-N thyroxine-binding globulin Natural products IC1=CC(CC([NH3+])C([O-])=O)=CC(I)=C1OC1=CC(I)=C(O)C(I)=C1 XUIIKFGFIJCVMT-UHFFFAOYSA-N 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000005747 tumor angiogenesis Effects 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 210000002229 urogenital system Anatomy 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- WQEVDHBJGNOKKO-UHFFFAOYSA-K vanadic acid Chemical compound O[V](O)(O)=O WQEVDHBJGNOKKO-UHFFFAOYSA-K 0.000 description 1
- 201000010653 vesiculitis Diseases 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57415—Specifically defined cancers of breast
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/475—Assays involving growth factors
- G01N2333/48—Nerve growth factor [NGF]
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Biochemistry (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Microbiology (AREA)
- Oncology (AREA)
- Cell Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Hospice & Palliative Care (AREA)
- Food Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Mycology (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0600851 | 2006-01-31 | ||
| FR0600851A FR2896881B1 (fr) | 2006-01-31 | 2006-01-31 | Procede de dosage du prongf pour le diagnostic in vitro du cancer du sein et utilisation du prongf en therapie |
| PCT/FR2007/050708 WO2007088305A1 (fr) | 2006-01-31 | 2007-01-30 | Procede de dosage du prongf pour le diagnostic in vitro du cancer en particulier du cancer du sein, de la thyroide ou du poumon et utilisation du prongf en therapie |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012124525A Division JP5792121B2 (ja) | 2006-01-31 | 2012-05-31 | 癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009525478A JP2009525478A (ja) | 2009-07-09 |
| JP2009525478A5 JP2009525478A5 (enExample) | 2010-03-04 |
| JP5091876B2 true JP5091876B2 (ja) | 2012-12-05 |
Family
ID=36741378
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008552858A Active JP5091876B2 (ja) | 2006-01-31 | 2007-01-30 | 癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 |
| JP2012124525A Active JP5792121B2 (ja) | 2006-01-31 | 2012-05-31 | 癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012124525A Active JP5792121B2 (ja) | 2006-01-31 | 2012-05-31 | 癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 |
Country Status (7)
| Country | Link |
|---|---|
| US (3) | US8008009B2 (enExample) |
| EP (1) | EP1982192B1 (enExample) |
| JP (2) | JP5091876B2 (enExample) |
| CN (2) | CN101395478B (enExample) |
| ES (1) | ES2471126T3 (enExample) |
| FR (1) | FR2896881B1 (enExample) |
| WO (1) | WO2007088305A1 (enExample) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2896881B1 (fr) * | 2006-01-31 | 2008-04-18 | Biomerieux Sa | Procede de dosage du prongf pour le diagnostic in vitro du cancer du sein et utilisation du prongf en therapie |
| WO2008058018A2 (en) | 2006-11-02 | 2008-05-15 | Mayo Foundation For Medical Education And Research | Predicting cancer outcome |
| EP2806054A1 (en) | 2008-05-28 | 2014-11-26 | Genomedx Biosciences Inc. | Systems and methods for expression-based discrimination of distinct clinical disease states in prostate cancer |
| US10407731B2 (en) | 2008-05-30 | 2019-09-10 | Mayo Foundation For Medical Education And Research | Biomarker panels for predicting prostate cancer outcomes |
| US9495515B1 (en) | 2009-12-09 | 2016-11-15 | Veracyte, Inc. | Algorithms for disease diagnostics |
| US10236078B2 (en) | 2008-11-17 | 2019-03-19 | Veracyte, Inc. | Methods for processing or analyzing a sample of thyroid tissue |
| US9074258B2 (en) | 2009-03-04 | 2015-07-07 | Genomedx Biosciences Inc. | Compositions and methods for classifying thyroid nodule disease |
| FR2944019B1 (fr) * | 2009-04-03 | 2011-04-22 | Biomerieux Sa | Procede de dosage de la prodefensine-a6 pour le diagnostic in vitro du cancer colorectal. |
| JP6078339B2 (ja) | 2009-05-07 | 2017-02-08 | ベラサイト インコーポレイテッド | 甲状腺状態の診断のための方法および組成物 |
| US10446272B2 (en) | 2009-12-09 | 2019-10-15 | Veracyte, Inc. | Methods and compositions for classification of samples |
| CN101782585A (zh) * | 2010-02-10 | 2010-07-21 | 广州医学院 | 肺癌组织蛋白印迹膜片及其制备方法 |
| WO2011159762A1 (en) * | 2010-06-15 | 2011-12-22 | Cornell University | Methods of limiting microvascular damage following acute myocardial ischemia |
| US20130267443A1 (en) | 2010-11-19 | 2013-10-10 | The Regents Of The University Of Michigan | ncRNA AND USES THEREOF |
| JP2014521958A (ja) * | 2011-07-28 | 2014-08-28 | ザ・トラステイーズ・オブ・ザ・ユニバーシテイ・オブ・ペンシルベニア | 胸腔内液若しくは漿液と関連する腫瘍細胞の特徴づけによる癌の診断方法 |
| WO2013090620A1 (en) | 2011-12-13 | 2013-06-20 | Genomedx Biosciences, Inc. | Cancer diagnostics using non-coding transcripts |
| ES2945036T3 (es) | 2012-08-16 | 2023-06-28 | Veracyte Sd Inc | Pronóstico del cáncer de próstata mediante biomarcadores |
| CN103122390B (zh) * | 2013-03-07 | 2015-04-08 | 上海市疾病预防控制中心 | Frat1基因作为检测甲状腺癌的血清标志物及其应用 |
| US10526655B2 (en) | 2013-03-14 | 2020-01-07 | Veracyte, Inc. | Methods for evaluating COPD status |
| US11976329B2 (en) | 2013-03-15 | 2024-05-07 | Veracyte, Inc. | Methods and systems for detecting usual interstitial pneumonia |
| CN103866039B (zh) * | 2014-04-04 | 2015-05-27 | 厦门大学 | 人类甲状腺癌组织基因特异位点甲基化水平检测引物及其应用 |
| US12297505B2 (en) | 2014-07-14 | 2025-05-13 | Veracyte, Inc. | Algorithms for disease diagnostics |
| EP2977759B1 (en) * | 2014-07-25 | 2017-07-12 | Serum Institute of India Private Limited | Highly sensitive immunoassay for rapid quantification of meningococcal capsular polysaccharide antigens |
| EP3770274A1 (en) | 2014-11-05 | 2021-01-27 | Veracyte, Inc. | Systems and methods of diagnosing idiopathic pulmonary fibrosis on transbronchial biopsies using machine learning and high dimensional transcriptional data |
| LT3280441T (lt) | 2015-04-07 | 2021-11-25 | Alector Llc | Anti-sortilino antikūnai ir jų naudojimo būdai |
| US10849992B1 (en) | 2015-04-07 | 2020-12-01 | Alector Llc | Methods of screening for sortilin binding antagonists |
| US11414708B2 (en) | 2016-08-24 | 2022-08-16 | Decipher Biosciences, Inc. | Use of genomic signatures to predict responsiveness of patients with prostate cancer to post-operative radiation therapy |
| AU2018210695B2 (en) | 2017-01-20 | 2024-07-18 | The University Of British Columbia | Molecular subtyping, prognosis, and treatment of bladder cancer |
| CA3051488A1 (en) * | 2017-01-24 | 2018-08-02 | Genetic Technologies Limited | Improved methods for assessing risk of developing breast cancer |
| US11873532B2 (en) | 2017-03-09 | 2024-01-16 | Decipher Biosciences, Inc. | Subtyping prostate cancer to predict response to hormone therapy |
| WO2018205035A1 (en) | 2017-05-12 | 2018-11-15 | Genomedx Biosciences, Inc | Genetic signatures to predict prostate cancer metastasis and identify tumor agressiveness |
| EP3635399B1 (fr) * | 2017-06-08 | 2025-10-29 | Gnaho, Sylvain | Méthode d'isolement et de détection de cellules souches cancéreuses |
| US11217329B1 (en) | 2017-06-23 | 2022-01-04 | Veracyte, Inc. | Methods and systems for determining biological sample integrity |
| PE20210186A1 (es) | 2018-07-13 | 2021-02-02 | Alector Llc | Anticuerpos anti-sortilina y metodos para su uso |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6548062B2 (en) * | 2000-02-29 | 2003-04-15 | Cephalon, Inc. | Method of treating cancer with anti-neurotrophin agents |
| WO2003076942A2 (fr) | 2002-03-13 | 2003-09-18 | Biomerieux | Quantification de cellules tumorales circulantes issues de cancers solides |
| FR2846426B1 (fr) * | 2002-10-28 | 2004-12-10 | Bio Merieux | Procede de dosage du ngf pour le diagnostic in vitro du cancer du sein et utilisation en therapie |
| ATE545429T1 (de) * | 2002-12-20 | 2012-03-15 | Lundbeck & Co As H | Modulation der neurotrophinaktivität;screeningsverfahren |
| WO2005076695A2 (en) * | 2004-02-11 | 2005-08-25 | Painceptor Pharma Corporation | Methods of modulating neurotrophin-mediated activity |
| FR2896881B1 (fr) * | 2006-01-31 | 2008-04-18 | Biomerieux Sa | Procede de dosage du prongf pour le diagnostic in vitro du cancer du sein et utilisation du prongf en therapie |
-
2006
- 2006-01-31 FR FR0600851A patent/FR2896881B1/fr not_active Expired - Fee Related
-
2007
- 2007-01-30 ES ES07731536.4T patent/ES2471126T3/es active Active
- 2007-01-30 EP EP07731536.4A patent/EP1982192B1/fr active Active
- 2007-01-30 CN CN2007800040238A patent/CN101395478B/zh active Active
- 2007-01-30 JP JP2008552858A patent/JP5091876B2/ja active Active
- 2007-01-30 WO PCT/FR2007/050708 patent/WO2007088305A1/fr not_active Ceased
- 2007-01-30 CN CN2013103889291A patent/CN103446585A/zh active Pending
- 2007-01-30 US US12/087,606 patent/US8008009B2/en active Active
-
2011
- 2011-07-20 US US13/137,101 patent/US20110293636A1/en not_active Abandoned
-
2012
- 2012-05-31 JP JP2012124525A patent/JP5792121B2/ja active Active
- 2012-06-26 US US13/533,908 patent/US9061045B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN101395478B (zh) | 2013-08-28 |
| JP5792121B2 (ja) | 2015-10-07 |
| FR2896881B1 (fr) | 2008-04-18 |
| JP2009525478A (ja) | 2009-07-09 |
| US9061045B2 (en) | 2015-06-23 |
| WO2007088305A1 (fr) | 2007-08-09 |
| CN101395478A (zh) | 2009-03-25 |
| US20090068200A1 (en) | 2009-03-12 |
| EP1982192B1 (fr) | 2014-03-26 |
| FR2896881A1 (fr) | 2007-08-03 |
| US20110293636A1 (en) | 2011-12-01 |
| EP1982192A1 (fr) | 2008-10-22 |
| US20130171173A1 (en) | 2013-07-04 |
| ES2471126T3 (es) | 2014-06-25 |
| JP2012211148A (ja) | 2012-11-01 |
| CN103446585A (zh) | 2013-12-18 |
| US8008009B2 (en) | 2011-08-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5091876B2 (ja) | 癌、特に乳癌、甲状腺癌及び肺癌のインビトロ診断のためのProNGFアッセイのための方法とProNGFの治療的使用 | |
| US8101713B2 (en) | Prostate cancer diagnosis and treatment | |
| Nitori et al. | Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma | |
| Rajamanickam et al. | Selective targeting of the TLR4 co-receptor, MD2, prevents colon cancer growth and lung metastasis | |
| US20110111431A1 (en) | Method for identifying pre-neoplastic and/or neoplastic states in mammals | |
| US20160208008A1 (en) | Monoclonal antibodies to transferrin and transferrin receptor antigens, and uses thereof | |
| EP3287143A1 (en) | Ckap4-molecular-targeted antitumor agent | |
| AU2003285472B2 (en) | Method for NGF assay for in vitro diagnosis of breast cancer and therapeutic use | |
| Gu et al. | Expression of thymosin β10 and its role in non–small cell lung cancer | |
| US20240174762A1 (en) | Methods, compositions and uses for targeting sema7a in the diagnosis and treatment of health conditions | |
| US7026454B1 (en) | Neuroendocrine marker of prostate cancer and method for producing same | |
| He et al. | Local blockage of self-sustainable erythropoietin signaling suppresses tumor progression in non-small cell lung cancer | |
| Wu et al. | Chronic stress-induced ANPEP drives liver cancer progression by increasing glutathione synthesis and inhibiting ferroptosis | |
| Wei et al. | Activation of vitamin D/VDR signaling reverses gemcitabine resistance of pancreatic cancer cells through inhibition of MUC1 expression | |
| CN118252922A (zh) | Sirt6激活剂mdl-800在乳腺癌逆转治疗抵抗与化疗耐药中的应用 | |
| KR20110122468A (ko) | 재발암, 내성암 또는 암전이 치료용 조성물 | |
| AU2002344775A1 (en) | Neuroendocrine marker for cancer and method for producing same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100108 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100108 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20111214 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120131 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120427 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120509 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120531 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20120604 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120821 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120914 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150921 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5091876 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |