JP4976384B2 - 質量分析法 - Google Patents
質量分析法 Download PDFInfo
- Publication number
- JP4976384B2 JP4976384B2 JP2008509289A JP2008509289A JP4976384B2 JP 4976384 B2 JP4976384 B2 JP 4976384B2 JP 2008509289 A JP2008509289 A JP 2008509289A JP 2008509289 A JP2008509289 A JP 2008509289A JP 4976384 B2 JP4976384 B2 JP 4976384B2
- Authority
- JP
- Japan
- Prior art keywords
- sample
- target
- ligand
- protein
- mass
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000004949 mass spectrometry Methods 0.000 title claims abstract description 50
- 238000000034 method Methods 0.000 claims abstract description 71
- 150000002500 ions Chemical class 0.000 claims abstract description 33
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 claims abstract description 30
- 239000003446 ligand Substances 0.000 claims description 64
- 102000004169 proteins and genes Human genes 0.000 claims description 36
- 108090000623 proteins and genes Proteins 0.000 claims description 36
- -1 imide esters Chemical class 0.000 claims description 28
- 239000000203 mixture Substances 0.000 claims description 28
- 239000011159 matrix material Substances 0.000 claims description 27
- 238000004132 cross linking Methods 0.000 claims description 26
- 238000006243 chemical reaction Methods 0.000 claims description 18
- 230000027455 binding Effects 0.000 claims description 17
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 13
- 239000003431 cross linking reagent Substances 0.000 claims description 11
- 238000001514 detection method Methods 0.000 claims description 11
- 239000007788 liquid Substances 0.000 claims description 9
- 239000002245 particle Substances 0.000 claims description 9
- 229920001184 polypeptide Polymers 0.000 claims description 8
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 8
- 150000001720 carbohydrates Chemical class 0.000 claims description 7
- 150000004676 glycans Chemical class 0.000 claims description 6
- 229920001282 polysaccharide Polymers 0.000 claims description 6
- 239000005017 polysaccharide Substances 0.000 claims description 6
- 239000000758 substrate Substances 0.000 claims description 6
- 241000700605 Viruses Species 0.000 claims description 5
- 230000005684 electric field Effects 0.000 claims description 5
- 238000005259 measurement Methods 0.000 claims description 5
- 239000000816 peptidomimetic Substances 0.000 claims description 5
- 108091033319 polynucleotide Proteins 0.000 claims description 5
- 239000002157 polynucleotide Substances 0.000 claims description 5
- 102000040430 polynucleotide Human genes 0.000 claims description 5
- 150000002632 lipids Chemical class 0.000 claims description 4
- 108020004707 nucleic acids Proteins 0.000 claims description 4
- 102000039446 nucleic acids Human genes 0.000 claims description 4
- 150000007523 nucleic acids Chemical class 0.000 claims description 4
- 238000004252 FT/ICR mass spectrometry Methods 0.000 claims description 3
- 229940042795 hydrazides for tuberculosis treatment Drugs 0.000 claims description 3
- 239000012948 isocyanate Substances 0.000 claims description 3
- 150000002513 isocyanates Chemical class 0.000 claims description 3
- 238000002156 mixing Methods 0.000 claims description 3
- 230000003287 optical effect Effects 0.000 claims description 3
- 238000000926 separation method Methods 0.000 claims description 3
- 239000007787 solid Substances 0.000 claims description 3
- 150000003923 2,5-pyrrolediones Chemical class 0.000 claims description 2
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 claims description 2
- 108091034117 Oligonucleotide Proteins 0.000 claims description 2
- 108010013639 Peptidoglycan Proteins 0.000 claims description 2
- 150000001718 carbodiimides Chemical class 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 claims description 2
- 238000000151 deposition Methods 0.000 claims description 2
- AFOSIXZFDONLBT-UHFFFAOYSA-N divinyl sulfone Chemical class C=CS(=O)(=O)C=C AFOSIXZFDONLBT-UHFFFAOYSA-N 0.000 claims description 2
- 125000005179 haloacetyl group Chemical group 0.000 claims description 2
- 125000005842 heteroatom Chemical group 0.000 claims description 2
- 238000005040 ion trap Methods 0.000 claims description 2
- 229920002521 macromolecule Polymers 0.000 claims description 2
- 229940126586 small molecule drug Drugs 0.000 claims description 2
- 230000003321 amplification Effects 0.000 claims 1
- 230000001678 irradiating effect Effects 0.000 claims 1
- 238000000691 measurement method Methods 0.000 claims 1
- 238000003199 nucleic acid amplification method Methods 0.000 claims 1
- 229920001542 oligosaccharide Polymers 0.000 claims 1
- 150000002482 oligosaccharides Chemical class 0.000 claims 1
- 230000005855 radiation Effects 0.000 claims 1
- 229920006395 saturated elastomer Polymers 0.000 claims 1
- 238000012512 characterization method Methods 0.000 abstract description 7
- 238000007876 drug discovery Methods 0.000 abstract description 6
- 239000000523 sample Substances 0.000 description 37
- 238000004458 analytical method Methods 0.000 description 34
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 21
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 14
- 230000003993 interaction Effects 0.000 description 13
- 239000000243 solution Substances 0.000 description 13
- 230000004850 protein–protein interaction Effects 0.000 description 12
- 230000006641 stabilisation Effects 0.000 description 10
- 238000011105 stabilization Methods 0.000 description 10
- PCMORTLOPMLEFB-ONEGZZNKSA-N sinapic acid Chemical compound COC1=CC(\C=C\C(O)=O)=CC(OC)=C1O PCMORTLOPMLEFB-ONEGZZNKSA-N 0.000 description 9
- 102000007474 Multiprotein Complexes Human genes 0.000 description 8
- 108010085220 Multiprotein Complexes Proteins 0.000 description 8
- 229940079593 drug Drugs 0.000 description 8
- 239000003814 drug Substances 0.000 description 8
- PCMORTLOPMLEFB-UHFFFAOYSA-N sinapinic acid Natural products COC1=CC(C=CC(O)=O)=CC(OC)=C1O PCMORTLOPMLEFB-UHFFFAOYSA-N 0.000 description 8
- 238000013467 fragmentation Methods 0.000 description 6
- 238000006062 fragmentation reaction Methods 0.000 description 6
- 238000013507 mapping Methods 0.000 description 6
- 238000001906 matrix-assisted laser desorption--ionisation mass spectrometry Methods 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 239000004971 Cross linker Substances 0.000 description 5
- 239000000427 antigen Substances 0.000 description 5
- 238000003556 assay Methods 0.000 description 5
- 235000014633 carbohydrates Nutrition 0.000 description 5
- 102000013515 cdc42 GTP-Binding Protein Human genes 0.000 description 5
- 108010051348 cdc42 GTP-Binding Protein Proteins 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 230000029087 digestion Effects 0.000 description 5
- KZNICNPSHKQLFF-UHFFFAOYSA-N dihydromaleimide Natural products O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 5
- 230000035945 sensitivity Effects 0.000 description 5
- 229960002317 succinimide Drugs 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 238000003795 desorption Methods 0.000 description 4
- 238000000132 electrospray ionisation Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 238000001228 spectrum Methods 0.000 description 4
- QLHLYJHNOCILIT-UHFFFAOYSA-N 4-o-(2,5-dioxopyrrolidin-1-yl) 1-o-[2-[4-(2,5-dioxopyrrolidin-1-yl)oxy-4-oxobutanoyl]oxyethyl] butanedioate Chemical compound O=C1CCC(=O)N1OC(=O)CCC(=O)OCCOC(=O)CCC(=O)ON1C(=O)CCC1=O QLHLYJHNOCILIT-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 102000008100 Human Serum Albumin Human genes 0.000 description 3
- 108091006905 Human Serum Albumin Proteins 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 230000000389 anti-prion effect Effects 0.000 description 3
- 230000009830 antibody antigen interaction Effects 0.000 description 3
- 230000009918 complex formation Effects 0.000 description 3
- 239000000356 contaminant Substances 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 238000010494 dissociation reaction Methods 0.000 description 3
- 230000005593 dissociations Effects 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 3
- 150000002894 organic compounds Chemical class 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- FXYPGCIGRDZWNR-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-[[3-(2,5-dioxopyrrolidin-1-yl)oxy-3-oxopropyl]disulfanyl]propanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCSSCCC(=O)ON1C(=O)CCC1=O FXYPGCIGRDZWNR-UHFFFAOYSA-N 0.000 description 2
- VILFTWLXLYIEMV-UHFFFAOYSA-N 1,5-difluoro-2,4-dinitrobenzene Chemical compound [O-][N+](=O)C1=CC([N+]([O-])=O)=C(F)C=C1F VILFTWLXLYIEMV-UHFFFAOYSA-N 0.000 description 2
- QQHITEBEBQNARV-UHFFFAOYSA-N 3-[[2-carboxy-2-(2,5-dioxopyrrolidin-1-yl)-2-sulfoethyl]disulfanyl]-2-(2,5-dioxopyrrolidin-1-yl)-2-sulfopropanoic acid Chemical compound O=C1CCC(=O)N1C(S(O)(=O)=O)(C(=O)O)CSSCC(S(O)(=O)=O)(C(O)=O)N1C(=O)CCC1=O QQHITEBEBQNARV-UHFFFAOYSA-N 0.000 description 2
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- 102000007079 Peptide Fragments Human genes 0.000 description 2
- 108010033276 Peptide Fragments Proteins 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 239000012472 biological sample Substances 0.000 description 2
- 239000000090 biomarker Substances 0.000 description 2
- NXVYSVARUKNFNF-UHFFFAOYSA-N bis(2,5-dioxopyrrolidin-1-yl) 2,3-dihydroxybutanedioate Chemical compound O=C1CCC(=O)N1OC(=O)C(O)C(O)C(=O)ON1C(=O)CCC1=O NXVYSVARUKNFNF-UHFFFAOYSA-N 0.000 description 2
- VYLDEYYOISNGST-UHFFFAOYSA-N bissulfosuccinimidyl suberate Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)CCCCCCC(=O)ON1C(=O)C(S(O)(=O)=O)CC1=O VYLDEYYOISNGST-UHFFFAOYSA-N 0.000 description 2
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 238000000451 chemical ionisation Methods 0.000 description 2
- 230000030609 dephosphorylation Effects 0.000 description 2
- 238000006209 dephosphorylation reaction Methods 0.000 description 2
- LRPQMNYCTSPGCX-UHFFFAOYSA-N dimethyl pimelimidate Chemical compound COC(=N)CCCCCC(=N)OC LRPQMNYCTSPGCX-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- WEHWNAOGRSTTBQ-UHFFFAOYSA-N dipropylamine Chemical compound CCCNCCC WEHWNAOGRSTTBQ-UHFFFAOYSA-N 0.000 description 2
- ZWIBGKZDAWNIFC-UHFFFAOYSA-N disuccinimidyl suberate Chemical compound O=C1CCC(=O)N1OC(=O)CCCCCCC(=O)ON1C(=O)CCC1=O ZWIBGKZDAWNIFC-UHFFFAOYSA-N 0.000 description 2
- 238000007877 drug screening Methods 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- LEQAOMBKQFMDFZ-UHFFFAOYSA-N glyoxal Chemical compound O=CC=O LEQAOMBKQFMDFZ-UHFFFAOYSA-N 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- DPBLXKKOBLCELK-UHFFFAOYSA-N pentan-1-amine Chemical compound CCCCCN DPBLXKKOBLCELK-UHFFFAOYSA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 2
- 230000017854 proteolysis Effects 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 239000012521 purified sample Substances 0.000 description 2
- 238000003653 radioligand binding assay Methods 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000006104 solid solution Substances 0.000 description 2
- JJAHTWIKCUJRDK-UHFFFAOYSA-N succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate Chemical compound C1CC(CN2C(C=CC2=O)=O)CCC1C(=O)ON1C(=O)CCC1=O JJAHTWIKCUJRDK-UHFFFAOYSA-N 0.000 description 2
- 150000003457 sulfones Chemical class 0.000 description 2
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 2
- 229920001059 synthetic polymer Polymers 0.000 description 2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 2
- DNIAPMSPPWPWGF-VKHMYHEASA-N (+)-propylene glycol Chemical compound C[C@H](O)CO DNIAPMSPPWPWGF-VKHMYHEASA-N 0.000 description 1
- FLCQLSRLQIPNLM-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 2-acetylsulfanylacetate Chemical compound CC(=O)SCC(=O)ON1C(=O)CCC1=O FLCQLSRLQIPNLM-UHFFFAOYSA-N 0.000 description 1
- VRDGQQTWSGDXCU-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 2-iodoacetate Chemical compound ICC(=O)ON1C(=O)CCC1=O VRDGQQTWSGDXCU-UHFFFAOYSA-N 0.000 description 1
- LLXVXPPXELIDGQ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-(2,5-dioxopyrrol-1-yl)benzoate Chemical compound C=1C=CC(N2C(C=CC2=O)=O)=CC=1C(=O)ON1C(=O)CCC1=O LLXVXPPXELIDGQ-UHFFFAOYSA-N 0.000 description 1
- WGMMKWFUXPMTRW-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-[(2-bromoacetyl)amino]propanoate Chemical compound BrCC(=O)NCCC(=O)ON1C(=O)CCC1=O WGMMKWFUXPMTRW-UHFFFAOYSA-N 0.000 description 1
- BQWBEDSJTMWJAE-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[(2-iodoacetyl)amino]benzoate Chemical compound C1=CC(NC(=O)CI)=CC=C1C(=O)ON1C(=O)CCC1=O BQWBEDSJTMWJAE-UHFFFAOYSA-N 0.000 description 1
- PMJWDPGOWBRILU-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[4-(2,5-dioxopyrrol-1-yl)phenyl]butanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCC(C=C1)=CC=C1N1C(=O)C=CC1=O PMJWDPGOWBRILU-UHFFFAOYSA-N 0.000 description 1
- RBAFCMJBDZWZIV-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-azido-2-hydroxybenzoate Chemical compound OC1=CC(N=[N+]=[N-])=CC=C1C(=O)ON1C(=O)CCC1=O RBAFCMJBDZWZIV-UHFFFAOYSA-N 0.000 description 1
- LWAVGNJLLQSNNN-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-azidobenzoate Chemical compound C1=CC(N=[N+]=[N-])=CC=C1C(=O)ON1C(=O)CCC1=O LWAVGNJLLQSNNN-UHFFFAOYSA-N 0.000 description 1
- FUOJEDZPVVDXHI-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 5-azido-2-nitrobenzoate Chemical compound [O-][N+](=O)C1=CC=C(N=[N+]=[N-])C=C1C(=O)ON1C(=O)CCC1=O FUOJEDZPVVDXHI-UHFFFAOYSA-N 0.000 description 1
- NGXDNMNOQDVTRL-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 6-(4-azido-2-nitroanilino)hexanoate Chemical compound [O-][N+](=O)C1=CC(N=[N+]=[N-])=CC=C1NCCCCCC(=O)ON1C(=O)CCC1=O NGXDNMNOQDVTRL-UHFFFAOYSA-N 0.000 description 1
- QYEAAMBIUQLHFQ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 6-[3-(pyridin-2-yldisulfanyl)propanoylamino]hexanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCCCNC(=O)CCSSC1=CC=CC=N1 QYEAAMBIUQLHFQ-UHFFFAOYSA-N 0.000 description 1
- IEUUDEWWMRQUDS-UHFFFAOYSA-N (6-azaniumylidene-1,6-dimethoxyhexylidene)azanium;dichloride Chemical compound Cl.Cl.COC(=N)CCCCC(=N)OC IEUUDEWWMRQUDS-UHFFFAOYSA-N 0.000 description 1
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- CDUQMGQIHYISOP-RMKNXTFCSA-N (e)-2-cyano-3-phenylprop-2-enoic acid Chemical compound OC(=O)C(\C#N)=C\C1=CC=CC=C1 CDUQMGQIHYISOP-RMKNXTFCSA-N 0.000 description 1
- YPFDHNVEDLHUCE-UHFFFAOYSA-N 1,3-propanediol Substances OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 1
- RVRLFABOQXZUJX-UHFFFAOYSA-N 1-[1-(2,5-dioxopyrrol-1-yl)ethyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1C(C)N1C(=O)C=CC1=O RVRLFABOQXZUJX-UHFFFAOYSA-N 0.000 description 1
- AASYSXRGODIQGY-UHFFFAOYSA-N 1-[1-(2,5-dioxopyrrol-1-yl)hexyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1C(CCCCC)N1C(=O)C=CC1=O AASYSXRGODIQGY-UHFFFAOYSA-N 0.000 description 1
- LVOYZSWOUDBRII-UHFFFAOYSA-N 1-[2-[(4-azido-2-hydroxybenzoyl)amino]hexanoyloxy]-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1CC(S(O)(=O)=O)C(=O)N1OC(=O)C(CCCC)NC(=O)C1=CC=C(N=[N+]=[N-])C=C1O LVOYZSWOUDBRII-UHFFFAOYSA-N 0.000 description 1
- SGVWDRVQIYUSRA-UHFFFAOYSA-N 1-[2-[2-(2,5-dioxopyrrol-1-yl)ethyldisulfanyl]ethyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1CCSSCCN1C(=O)C=CC1=O SGVWDRVQIYUSRA-UHFFFAOYSA-N 0.000 description 1
- FERLGYOHRKHQJP-UHFFFAOYSA-N 1-[2-[2-[2-(2,5-dioxopyrrol-1-yl)ethoxy]ethoxy]ethyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1CCOCCOCCN1C(=O)C=CC1=O FERLGYOHRKHQJP-UHFFFAOYSA-N 0.000 description 1
- VNJBTKQBKFMEHH-UHFFFAOYSA-N 1-[4-(2,5-dioxopyrrol-1-yl)-2,3-dihydroxybutyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1CC(O)C(O)CN1C(=O)C=CC1=O VNJBTKQBKFMEHH-UHFFFAOYSA-N 0.000 description 1
- WXXSHAKLDCERGU-UHFFFAOYSA-N 1-[4-(2,5-dioxopyrrol-1-yl)butyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1CCCCN1C(=O)C=CC1=O WXXSHAKLDCERGU-UHFFFAOYSA-N 0.000 description 1
- BMVXCPBXGZKUPN-UHFFFAOYSA-N 1-hexanamine Chemical compound CCCCCCN BMVXCPBXGZKUPN-UHFFFAOYSA-N 0.000 description 1
- SYEKJCKNTHYWOJ-UHFFFAOYSA-N 2-(2,5-dioxopyrrolidin-1-yl)-2-sulfobutanedioic acid;ethane-1,2-diol Chemical compound OCCO.OC(=O)CC(S(O)(=O)=O)(C(O)=O)N1C(=O)CCC1=O.OC(=O)CC(S(O)(=O)=O)(C(O)=O)N1C(=O)CCC1=O SYEKJCKNTHYWOJ-UHFFFAOYSA-N 0.000 description 1
- AWJWHBODZRSWCK-UHFFFAOYSA-O 2-carboxyethyl-tris(hydroxymethyl)phosphanium Chemical compound OC[P+](CO)(CO)CCC(O)=O AWJWHBODZRSWCK-UHFFFAOYSA-O 0.000 description 1
- XXUNIGZDNWWYED-UHFFFAOYSA-N 2-methylbenzamide Chemical compound CC1=CC=CC=C1C(N)=O XXUNIGZDNWWYED-UHFFFAOYSA-N 0.000 description 1
- HMMFDEBVQNRZLJ-UHFFFAOYSA-N 3-(2-azaniumylethyldisulfanyl)propanoate Chemical compound NCCSSCCC(O)=O HMMFDEBVQNRZLJ-UHFFFAOYSA-N 0.000 description 1
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 1
- NITXODYAMWZEJY-UHFFFAOYSA-N 3-(pyridin-2-yldisulfanyl)propanehydrazide Chemical compound NNC(=O)CCSSC1=CC=CC=N1 NITXODYAMWZEJY-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- ZMRMMAOBSFSXLN-UHFFFAOYSA-N 4-[4-(2,5-dioxopyrrol-1-yl)phenyl]butanehydrazide Chemical compound C1=CC(CCCC(=O)NN)=CC=C1N1C(=O)C=CC1=O ZMRMMAOBSFSXLN-UHFFFAOYSA-N 0.000 description 1
- QQZOUYFHWKTGEY-UHFFFAOYSA-N 4-azido-n-[2-[2-[(4-azido-2-hydroxybenzoyl)amino]ethyldisulfanyl]ethyl]-2-hydroxybenzamide Chemical compound OC1=CC(N=[N+]=[N-])=CC=C1C(=O)NCCSSCCNC(=O)C1=CC=C(N=[N+]=[N-])C=C1O QQZOUYFHWKTGEY-UHFFFAOYSA-N 0.000 description 1
- ZKCVIWAHVMYDIM-UHFFFAOYSA-N 4-azidobenzamide Chemical compound NC(=O)C1=CC=C(N=[N+]=[N-])C=C1 ZKCVIWAHVMYDIM-UHFFFAOYSA-N 0.000 description 1
- YRLKXQVDEQEYSN-UHFFFAOYSA-N 4-azidobenzohydrazide Chemical compound NNC(=O)C1=CC=C(N=[N+]=[N-])C=C1 YRLKXQVDEQEYSN-UHFFFAOYSA-N 0.000 description 1
- ILDXDBSWYJDHAL-UHFFFAOYSA-N 6-o-(2,5-dioxopyrrolidin-1-yl) 1-o-methyl hexanedioate Chemical compound COC(=O)CCCCC(=O)ON1C(=O)CCC1=O ILDXDBSWYJDHAL-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- NFSMUNQYUHPFAB-UHFFFAOYSA-N C(CCCCC)(=O)O.N1=C(C=CC=C1)SSCCC(=O)N Chemical compound C(CCCCC)(=O)O.N1=C(C=CC=C1)SSCCC(=O)N NFSMUNQYUHPFAB-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N Caprylic acid Natural products CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 241000819038 Chichester Species 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 101710204837 Envelope small membrane protein Proteins 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- WJYIASZWHGOTOU-UHFFFAOYSA-N Heptylamine Chemical compound CCCCCCCN WJYIASZWHGOTOU-UHFFFAOYSA-N 0.000 description 1
- 101710121996 Hexon protein p72 Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 241000102542 Kara Species 0.000 description 1
- 101710125418 Major capsid protein Proteins 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 101100298534 Mus musculus Prnp gene Proteins 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-O N,N,N-trimethylglycinium Chemical compound C[N+](C)(C)CC(O)=O KWIUHFFTVRNATP-UHFFFAOYSA-O 0.000 description 1
- LKJPYSCBVHEWIU-UHFFFAOYSA-N N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide Chemical compound C=1C=C(C#N)C(C(F)(F)F)=CC=1NC(=O)C(O)(C)CS(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-UHFFFAOYSA-N 0.000 description 1
- 108010061952 Orosomucoid Proteins 0.000 description 1
- 102000012404 Orosomucoid Human genes 0.000 description 1
- 108020002230 Pancreatic Ribonuclease Proteins 0.000 description 1
- 102000005891 Pancreatic ribonuclease Human genes 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000029797 Prion Human genes 0.000 description 1
- 108091000054 Prion Proteins 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- 101710088839 Replication initiation protein Proteins 0.000 description 1
- JQACFQMXPFVLQI-UHFFFAOYSA-N S(=O)(=O)(O)C1C(=O)N(C(C1)=O)NC(C1=C(C(=C(C(=C1F)F)F)F)N=[N+]=[N-])=O Chemical compound S(=O)(=O)(O)C1C(=O)N(C(C1)=O)NC(C1=C(C(=C(C(=C1F)F)F)F)N=[N+]=[N-])=O JQACFQMXPFVLQI-UHFFFAOYSA-N 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000044159 Ubiquitin Human genes 0.000 description 1
- 108090000848 Ubiquitin Proteins 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- CFVQWSRUQPIBLB-UHFFFAOYSA-N acetyllead Chemical compound CC([Pb])=O CFVQWSRUQPIBLB-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-L adipate(2-) Chemical compound [O-]C(=O)CCCCC([O-])=O WNLRTRBMVRJNCN-UHFFFAOYSA-L 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 229940125644 antibody drug Drugs 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 238000000065 atmospheric pressure chemical ionisation Methods 0.000 description 1
- GONOPSZTUGRENK-UHFFFAOYSA-N benzyl(trichloro)silane Chemical compound Cl[Si](Cl)(Cl)CC1=CC=CC=C1 GONOPSZTUGRENK-UHFFFAOYSA-N 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- XFLVBMBRLSCJAI-ZKWXMUAHSA-N biotin amide Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)N)SC[C@@H]21 XFLVBMBRLSCJAI-ZKWXMUAHSA-N 0.000 description 1
- XFLVBMBRLSCJAI-UHFFFAOYSA-N biotin amide Natural products N1C(=O)NC2C(CCCCC(=O)N)SCC21 XFLVBMBRLSCJAI-UHFFFAOYSA-N 0.000 description 1
- LNQHREYHFRFJAU-UHFFFAOYSA-N bis(2,5-dioxopyrrolidin-1-yl) pentanedioate Chemical compound O=C1CCC(=O)N1OC(=O)CCCC(=O)ON1C(=O)CCC1=O LNQHREYHFRFJAU-UHFFFAOYSA-N 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- DNSISZSEWVHGLH-UHFFFAOYSA-N butanamide Chemical compound CCCC(N)=O DNSISZSEWVHGLH-UHFFFAOYSA-N 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- BMRWNKZVCUKKSR-UHFFFAOYSA-N butane-1,2-diol Chemical compound CCC(O)CO BMRWNKZVCUKKSR-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 238000003926 complexometric titration Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- ZLFRJHOBQVVTOJ-UHFFFAOYSA-N dimethyl hexanediimidate Chemical compound COC(=N)CCCCC(=N)OC ZLFRJHOBQVVTOJ-UHFFFAOYSA-N 0.000 description 1
- ILKCDNKCNSNFMP-UHFFFAOYSA-N dimethyl octanediimidate;hydron;dichloride Chemical compound Cl.Cl.COC(=N)CCCCCCC(=N)OC ILKCDNKCNSNFMP-UHFFFAOYSA-N 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000000105 evaporative light scattering detection Methods 0.000 description 1
- 210000000416 exudates and transudate Anatomy 0.000 description 1
- 238000010265 fast atom bombardment Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 230000036252 glycation Effects 0.000 description 1
- 229940015043 glyoxal Drugs 0.000 description 1
- ALBYIUDWACNRRB-UHFFFAOYSA-N hexanamide Chemical compound CCCCCC(N)=O ALBYIUDWACNRRB-UHFFFAOYSA-N 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- MBAXWTVHCRPVFW-UHFFFAOYSA-N methyl 3-[(3-imino-3-methoxypropyl)disulfanyl]propanimidate Chemical compound COC(=N)CCSSCCC(=N)OC MBAXWTVHCRPVFW-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 1
- GSTZKFJRDDBPFD-UHFFFAOYSA-N n-(2,5-dioxopyrrolidin-1-yl)-3-(pyridin-2-yldisulfanyl)propanamide Chemical compound O=C1CCC(=O)N1NC(=O)CCSSC1=CC=CC=N1 GSTZKFJRDDBPFD-UHFFFAOYSA-N 0.000 description 1
- RQUGVTLRYOAFLV-UHFFFAOYSA-N n-(4-aminobutyl)-4-azido-2-hydroxybenzamide Chemical compound NCCCCNC(=O)C1=CC=C(N=[N+]=[N-])C=C1O RQUGVTLRYOAFLV-UHFFFAOYSA-N 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 230000004796 pathophysiological change Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- IPWFJLQDVFKJDU-UHFFFAOYSA-N pentanamide Chemical compound CCCCC(N)=O IPWFJLQDVFKJDU-UHFFFAOYSA-N 0.000 description 1
- 229940100684 pentylamine Drugs 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 150000003140 primary amides Chemical class 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- QLNJFJADRCOGBJ-UHFFFAOYSA-N propionamide Chemical compound CCC(N)=O QLNJFJADRCOGBJ-UHFFFAOYSA-N 0.000 description 1
- 229940080818 propionamide Drugs 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000006916 protein interaction Effects 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- 239000002287 radioligand Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000002133 sample digestion Methods 0.000 description 1
- 239000012488 sample solution Substances 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 238000002821 scintillation proximity assay Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 150000003334 secondary amides Chemical class 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 238000001004 secondary ion mass spectrometry Methods 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- WROMPOXWARCANT-UHFFFAOYSA-N tfa trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F WROMPOXWARCANT-UHFFFAOYSA-N 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000000101 transmission high energy electron diffraction Methods 0.000 description 1
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6803—General methods of protein analysis not limited to specific proteins or families of proteins
- G01N33/6848—Methods of protein analysis involving mass spectrometry
- G01N33/6851—Methods of protein analysis involving laser desorption ionisation mass spectrometry
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/14—Heterocyclic carbon compound [i.e., O, S, N, Se, Te, as only ring hetero atom]
- Y10T436/142222—Hetero-O [e.g., ascorbic acid, etc.]
- Y10T436/143333—Saccharide [e.g., DNA, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/24—Nuclear magnetic resonance, electron spin resonance or other spin effects or mass spectrometry
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Bioinformatics & Computational Biology (AREA)
- Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Microbiology (AREA)
- Medicinal Chemistry (AREA)
- Biotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Optics & Photonics (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
Description
標的分子とそのリガンドとの間の広い範囲の非共有結合的相互作用、たとえばタンパク質間の生体特異的相互作用又は薬物と特定の結合タンパク質との間の相互作用の同定及び特性決定は、生化学研究及び薬物発見において意義を増している。たとえば細胞レベルにおけるタンパク質−タンパク質相互作用は、シグナル伝達イベント、ひいては外部刺激に対する細胞応答及び罹患細胞の病態生理学的変化において大きな役割を演じる。そのような非共有結合的複合体の特異的直接検出のための従来技術としては、タンパク質抽出物をゲル電気泳動によって分離するウェスタンブロット法(Kemeny, D. M. and Challacombe, S.J. (eds.), "ELISA and other Solid Phase Immunoassays"; John Wiley & Sons, Chichester (1988))、ELISA及び放射性リガンド結合検定法(Berson et al., Clin. Chim. Acta, 22:51-60 (1968)、Chard, T. "An Introduction to Radioimmunoassay and Related Techniques," Elsevier Biomedical Press, Amsterdam, p95-110 (1982))、表面プラズモン共振(Karlsson et al., J. Immunol. Methods, 145:229-240 (1991)、Jonsson et al., Biotechniques, 11(5):620- 627 (1991))又はシンチレーション近接検定法(Udenfriend et al, Anal. Biochem., 161 :494-500 (1987))がある。放射性リガンド結合検定法は、一般に、結合部位における未知物質の競合的結合を放射性リガンドのそれに関して評価する場合のみ有用であり、放射能の使用を要する。表面プラズモン共振技術は、結合速度を計測するのに有用な技術であり、そのような計測から導出される解離及び会合定数は、標的−リガンド相互作用の性質を解明する際に役立つ。しかし、これらの検定法のすべては通常、労力と時間を要する実験を伴うか、特別に訓練を受けた個人によって実行されなければならない。また、これらの検定法はすべて、それぞれが標的又はリガンド分子の何らかの下準備(すなわち標識又は固定化)を要するため、ユーザが分析の前に標的分子又はリガンドを知っておくことを要する。
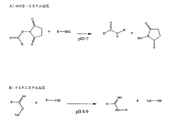
本発明の目的は、質量分析(MS)を使用して、試料の消化又は断片化なしに、精製された多成分試料又は不均一生物学的マトリックスからの共有結合的に安定化した超分子標的−リガンド複合体の存在を分析もしくは他の方法で検出する、又はその正体を決定する方法を提供することである。
a)非共有結合的に結合した超分子標的−リガンド複合体を架橋試薬と接触させて共有結合的に安定化した超分子標的−リガンド複合体を形成するステップ、及び
b)共有結合的に安定化した超分子標的−リガンド複合体からの無傷のイオンを質量分析によって分析するステップ
を含む方法を提供する。
本明細書で引用するすべての特許、特許出願及び刊行物を引用例として本明細書に取り込む。以下、本明細書で使用する特定の語句の意味を説明する。断りない限り、本明細書で使用するすべての科学技術用語は、対象が属する技術の当業者によって一般に理解される意味と同じ意味を有する。
a)非共有結合的に結合した超分子標的−リガンド複合体を架橋試薬と接触させて共有結合的に安定化した超分子標的−リガンド複合体を形成するステップ、及び
b)共有結合的に安定化した超分子標的−リガンド複合体からの無傷のイオンを質量分析によって分析するステップ
を含む方法を提供する。
質量分析
すべての質量計測は、マクロマイザ計器(Comet AG、Flamatt、スイス)で実行した。マクロマイザ計器は、本質的には、クリオ検出器アレイの小さな区域におけるイオン透過を最適化するように設計されたMALDI−TOF質量分析計である。すべての必要なイオン光学部品、レーザ及び電子部品を含む計器の各部品は、高質量イオンの検出を高めるように設計されていた。より詳細な計器の説明に関しては、Wenzel, et al. Anal. Chem. (2005)を参照すること。
この実施例は、非架橋条件(図3A)及び架橋安定化を使用する条件(図3B)下の抗体−抗原反応を実証する。反応は、650nMのヒト血清アルブミン(HSA)5μLを1μMの抗ヒト血清アルブミン(抗HSA)5μLと反応させることを含む。図3Bは、図3Aと同じ試料であったが、1mg/mLの架橋混合物1μLの使用を含み、架橋ピークの容易な特定を可能にした。標準的な作動条件下、マクロマイザ(Comet AG、Flamatt、スイス)MALDI質量分析計を使用して分析を実行した。シナピン酸1μL(70%アセトニトリル:30%水:0.1%トリフルオロ酢酸中10mg/mL)と混合した試料1μLを使用し、乾燥小滴技術を使用して1uLをスポッティングすることにより、試料を調製した。
この実施例は、非架橋条件(図4A)及び架橋安定化を使用する条件(図4B)下の細胞分裂周期42ホモログ(CDC42)とサルモネラ外タンパク質E(SopE)との間の(非抗体)タンパク質−タンパク質反応を実証する。反応は、1nMのSopE5μLを1μMのCDC42 5μLと反応させることを含む。図4Bは、図4Aと同じ試料であったが、1mg/mLの架橋混合物1μLの使用を含み、架橋ピークの容易な特定を可能にした。標準的な作動条件下、マクロマイザ(Comet AG、Flamatt、スイス)MALDI質量分析計を使用して分析を実行した。シナピン酸1μL(70%アセトニトリル:30%水:0.1%トリフルオロ酢酸中10mg/mL)と混合した試料1μLを使用し、乾燥小滴技術を使用して1uLをスポッティングすることにより、試料を調製した。
この実施例は、タンパク質の多成分混合物内の特異的タンパク質複合体を検出することが可能であることを実証する。反応は、6種の異なるタンパク質(インスリン、HetS、リボヌクレアーゼA、SopE、CDC42、HSA)それぞれ10μL、1μMを含む(図5A)。1mg/mLの架橋混合物1μLによる架橋安定化ののち、特異的複合体[CDC42・SopE]を検出した(図5B)。標準的な作動条件下、マクロマイザ(Comet AG、Flamatt、スイス)質量分析計を使用して分析を実行した。シナピン酸1μL(70%アセトニトリル:30%水:0.1%トリフルオロ酢酸中10mg/mL)と混合した試料1μLを使用し、乾燥小滴技術を使用して1uLをスポッティングすることにより、試料を調製した。
この実施例は、ヒト血清(1:200希釈)の試料内の10μM濃度のスパイクのとき架橋安定化を使用する抗体−抗原反応が特異的であることを実証する。反応は、650nMの抗プリオンタンパク質(6H4、Prionics、スイス)5μLを1pm/μLのマウスプリオンタンパク質(mPrP(121−230))の球状サブセクション5μL及び2mg/mLの架橋混合物1μLと反応させることを含み、架橋ピークの容易な特定を可能にした(図6)。標準的な作動条件下、マクロマイザ(Comet AG、Flamatt、スイス)MALDI質量分析計を使用して分析を実行した。シナピン酸1μL(70%アセトニトリル:30%水:0.1%トリフルオロ酢酸中10mg/mL)と混合した試料1μLを使用し、乾燥小滴技術を使用して1uLをスポッティングすることにより、試料を調製した。
この実施例は、多数の結合パートナーを一つの無傷の複合体として安定化できる方法を実証する。反応は、650nMの抗プリオンタンパク質(6H4、Prionics、スイス)5μLを1μMのウシプリオンタンパク質(bPrP)5μL及び1.3μMの抗プリオンタンパク質(3B8、Roboscreen、Leipzig、ドイツ)2.5μLと反応させることを含む。2mg/mLの架橋混合物1μLを使用して混合物を安定化して、架橋ピークの容易な特定を可能にした(図7)。標準的な作動条件下、マクロマイザ(Comet AG、Flamatt、スイス)MALDI質量分析計を使用して分析を実行した。シナピン酸1μL(70%アセトニトリル:30%水:0.1%トリフルオロ酢酸中10mg/mL)と混合した試料1μLを使用し、乾燥小滴技術を使用して1μLをスポッティングすることにより、試料を調製した。
bPrP(23−230)(6H4、600nM、300μl、H2O)に対するモノクロナール抗体の溶液をbPrP(23−230)(1μM、300μl、H2O)の溶液と時間0で混合した。インキュベート後、1〜30分の1分ごとに、次いで、30〜80分の5分ごとに10μlアリコートを抜き取り、10分間、架橋反応に付した。次いで、架橋した試料1μlをマトリックス(シナピン酸、7:3:1アセトニトリル:H2O:0.1%TFA中10mg/ml)1μlと混合することにより、反応を停止させた。インキュベート時間ごとに、反応生成物をマクロマイザ質量分析によって分析した。結果を図8及び9に図示する。図8は、t=0では、主ピークが非結合6H4ピークであり、5分後、主ピークが1個の6H4と1個のbPrPとの単一の結合であることを示す。60分後、反応は完了し、主ピークは、2個のbPrP分子と結合した1個の6H4である。図9は、異なるインキュベート時間で起こる非結合、結合及び多重結合タンパク質−タンパク質相互作用の強度を示す上記質量スペクトルのグラフ表示である。グラフは、各ピークに対応するイオン強度を計算し、バックグラウンドイオン強度を差し引き、各スペクトル内でピークの強度を正規化することによって得られた。そして、これらの修正され、正規化されたイオン強度が時間とともにグラフ化されている。
Claims (12)
- 質量分析を使用して、非消化で非断片化状態の超分子標的−リガンド複合体の無傷のイオンを分析する方法であって、
a)非共有結合的に結合した超分子標的−リガンド複合体を架橋試薬の溶液と接触させて共有結合的に安定化した超分子標的−リガンド複合体を形成し、架橋反応の完了の後、直接マトリックス溶液と混合して、試料/マトリックス混合物を得るステップ、及び
b)試料/マトリックス混合物中の共有結合的に安定化した非消化で非断片化状態の超分子標的−リガンド複合体からの無傷のイオンを高質量測定用のMALDI質量分析によって分析するステップ
を含む方法。 - MALDI質量分析を使用して、非消化で非断片化状態の超分子標的−リガンド複合体の無傷のイオンを検出する方法であって、(a)非共有結合的に結合した超分子標的−リガンド複合体を含む第一の試料を得ること、(b)前記第一の試料を架橋試薬の溶液と接触させて、共有結合的に安定化した超分子標的−リガンド複合体を含む液体の第二の試料を得ること、(c)工程(b)で得られる前記液体の第二の試料をマトリックス溶液と混合して試料/マトリックス混合物を得ること、(d)前記試料/マトリックス溶液を基材に堆積させ、それによって均一な薄層を形成すること、(e)基材にレーザからの放射線を照射して、試料/マトリックス混合物中の前記共有結合的に安定化した超分子標的−リガンド複合体を脱離させ、無傷のイオンを発生させること、及び(f)高質量測定用のMALDI質量分析計を使用して、非消化で非断片化状態の共有結合的に安定化した超分子標的−リガンド複合体の前記無傷のイオンを質量分離し、検出することを含む方法。
- 超分子標的−リガンド複合体が標的分子とその結合リガンドとの複合体を表し、前記標的分子が、タンパク質、ポリペプチド、糖ポリペプチド、リンポリペプチド、ペプチドグリカン、多糖、ペプチド様物質、脂質、炭水化物、ポリヌクレオチド及び他の天然又は合成高分子から選択され、前記結合リガンドが、小分子薬物、ペプチドもしくはポリペプチド、核酸もしくはオリゴヌクレオチド、炭水化物、たとえばオリゴ糖、ウイルス粒子、タンパク質又は他の有機誘導化合物から選択される、請求項1又は2記載の方法。
- 分析される超分子標的−リガンド複合体が、精製された多成分試料又は不均一生物学的マトリックスに存在する、請求項1〜3のいずれか1項記載の方法。
- イオン変換ダイノード(ICD)検出器、光学デカップリング、増幅又は特殊コートされた電子増倍管又はマイクロチャンネルプレート(MCP)検出器及び超伝導トンネル接合(STJ)検出器から選択される100kDa〜10MDaの範囲の高質量イオンに対して高感度であり、非飽和性である検出器が使用される、請求項1〜4のいずれか1項記載の方法。
- 2種以上の架橋試薬が使用される、請求項1〜5のいずれか1項記載の方法。
- 架橋試薬が、ホモ及びヘテロ多官能架橋剤から選択される、請求項1〜6のいずれか1項記載の方法。
- 架橋試薬が、イミドエステル、N−ヒドロキシスクシンイミド−エステル、マレイミド、ハロアセチル、ピリジルジスルフィド、ヒドラジド、カルボジイミド、アリールアジド、イソシアナート及びビニルスルホンから選択される、請求項1〜7のいずれか1項記載の方法。
- 工程(f)における質量分離及び検出が、線形及び非線形電場を有する線形型又はリフレクトロン飛行時間型;曲面電場リフレクトロン;単一又は複数の四重極;単一又は複数の磁気又は電気セクタ;フーリエ変換イオンサイクロトロン共鳴;イオントラップ及びこれらの組み合わせからなる群より選択される、請求項2〜8のいずれか1項記載の方法。
- 工程(f)で使用される検出器が、高質量イオンに対して高感度であり、非飽和性である検出器であって、超伝導トンネル接合(STJ)検出器、イオン変換ダイノード検出器、及び、光学デカップリング、増幅又は特殊コートされた電子増倍管又はマイクロチャンネルプレート検出器からなる群より選択される、請求項2〜9のいずれか1項記載の方法。
- 工程(f)で使用される検出器が、超伝導トンネル接合(STJ)検出器である、請求項2〜10のいずれか1項記載の方法。
- 工程(d)における試料/マトリックス混合物が固体支持体上に二次元配列で堆積され、工程(f)における質量分析の分析が高いスループット又は自動化されたやり方で実施される、請求項2〜11のいずれか1項記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05405338.4 | 2005-05-04 | ||
| EP05405338 | 2005-05-04 | ||
| PCT/CH2006/000245 WO2006116893A2 (en) | 2005-05-04 | 2006-05-04 | Mass spectrometric analysis method |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008541021A JP2008541021A (ja) | 2008-11-20 |
| JP2008541021A5 JP2008541021A5 (ja) | 2012-04-26 |
| JP4976384B2 true JP4976384B2 (ja) | 2012-07-18 |
Family
ID=36926813
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008509289A Active JP4976384B2 (ja) | 2005-05-04 | 2006-05-04 | 質量分析法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8323983B2 (ja) |
| EP (1) | EP1877783B1 (ja) |
| JP (1) | JP4976384B2 (ja) |
| AT (1) | ATE515697T1 (ja) |
| WO (1) | WO2006116893A2 (ja) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008074769A1 (en) * | 2006-12-18 | 2008-06-26 | Covalx Ag | Direct mass spectrometric analysis of aggregates of therapeutic proteins |
| EP2126584B1 (en) * | 2007-03-16 | 2012-12-19 | CovalX AG | Direct mass spectrometric analysis of drug candidates targeting protein complexes |
| US20110177617A1 (en) * | 2009-05-29 | 2011-07-21 | Claudia Bich | Cross-linking reagents for molecular interactions analysis |
| JP5947533B2 (ja) * | 2011-01-19 | 2016-07-06 | キヤノン株式会社 | 情報取得方法 |
| US11454628B2 (en) | 2018-08-06 | 2022-09-27 | Northeastern University | On-surface mass tagging |
| WO2021245184A1 (en) | 2020-06-02 | 2021-12-09 | Neurimmune Ag | HUMAN ANTIBODIES AGAINST SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 (SARS-CoV-2) |
| WO2022258437A1 (en) * | 2021-06-07 | 2022-12-15 | Paul Scherrer Institut | Quantification of protein-protein interaction of membrane proteins using high-mass mass spectrometry |
| CN113866257B (zh) * | 2021-09-03 | 2023-11-17 | 中国检验检疫科学研究院 | 评价鱼类环境污染程度的方法和试剂盒 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6428956B1 (en) * | 1998-03-02 | 2002-08-06 | Isis Pharmaceuticals, Inc. | Mass spectrometric methods for biomolecular screening |
| AU5298900A (en) * | 1999-05-26 | 2000-12-12 | Regents Of The University Of California, The | Method of determining the three-dimensional shape of a macromolecule |
| US20040014946A1 (en) | 2002-04-25 | 2004-01-22 | Heman Chao | Protein interaction method and composition |
| JP2004214293A (ja) * | 2002-12-27 | 2004-07-29 | National Institute Of Advanced Industrial & Technology | 粒子検出器 |
| JP2006512080A (ja) | 2002-12-31 | 2006-04-13 | ロディ ファーマ,インコーポレイティド | バインディングザイムアレイ及びハイスループットプロテオミクス方法 |
| US7368290B2 (en) | 2003-05-12 | 2008-05-06 | Sandia National Laboratories | Structural determination of intact proteins using mass spectrometry |
| US7691643B2 (en) * | 2003-06-23 | 2010-04-06 | Hitachi High-Technologies Corporation | Mass analysis method and mass analysis apparatus |
| WO2008074769A1 (en) * | 2006-12-18 | 2008-06-26 | Covalx Ag | Direct mass spectrometric analysis of aggregates of therapeutic proteins |
-
2006
- 2006-05-04 US US11/919,801 patent/US8323983B2/en active Active
- 2006-05-04 JP JP2008509289A patent/JP4976384B2/ja active Active
- 2006-05-04 EP EP06721947A patent/EP1877783B1/en active Active
- 2006-05-04 AT AT06721947T patent/ATE515697T1/de not_active IP Right Cessation
- 2006-05-04 WO PCT/CH2006/000245 patent/WO2006116893A2/en not_active Application Discontinuation
Also Published As
| Publication number | Publication date |
|---|---|
| US20090263786A1 (en) | 2009-10-22 |
| ATE515697T1 (de) | 2011-07-15 |
| EP1877783A2 (en) | 2008-01-16 |
| WO2006116893A3 (en) | 2007-01-18 |
| JP2008541021A (ja) | 2008-11-20 |
| US8323983B2 (en) | 2012-12-04 |
| EP1877783B1 (en) | 2011-07-06 |
| WO2006116893A2 (en) | 2006-11-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4976384B2 (ja) | 質量分析法 | |
| JP5331714B2 (ja) | タンパク質複合体をターゲティングする薬物候補の直接質量分析 | |
| Stults | Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) | |
| Mirgorodskaya et al. | Quantitation of peptides and proteins by matrix‐assisted laser desorption/ionization mass spectrometry using 18O‐labeled internal standards | |
| EP2286237B1 (en) | Mass spectrometric analysis | |
| Jiménez et al. | In‐gel digestion of proteins for MALDI‐MS fingerprint mapping | |
| CA2465297C (en) | Method of mass spectrometry | |
| Cristoni et al. | Development of new methodologies for the mass spectrometry study of bioorganic macromolecules | |
| Nelson et al. | Advances in surface plasmon resonance biomolecular interaction analysis mass spectrometry (BIA/MS) | |
| Gross et al. | Matrix-assisted laser desorption/ionisation–mass spectrometry applied to biological macromolecules | |
| Ashcroft | Recent developments in electrospray ionisation mass spectrometry: noncovalently bound protein complexes | |
| WO2007041520A2 (en) | Methods for direct protein identification by maldi | |
| Costello | Bioanalytic applications of mass spectrometry | |
| Mädler et al. | MALDI-ToF mass spectrometry for studying noncovalent complexes of biomolecules | |
| Nyman | The role of mass spectrometry in proteome studies | |
| Sinz | Investigation of protein–ligand interactions by mass spectrometry | |
| Cramer et al. | High-throughput proteomics using matrix-assisted laser desorption/ionization mass spectrometry | |
| Downard | Indirect study of non‐covalent protein complexes by MALDI mass spectrometry: Origins, advantages, and applications of the “intensity‐fading” approach | |
| Nakajima et al. | C-Terminal sequencing of protein by MALDI mass spectrometry through the specific derivatization of the α-carboxyl group with 3-aminopropyltris-(2, 4, 6-trimethoxyphenyl) phosphonium bromide | |
| JP5302212B2 (ja) | 治療タンパク質の凝集体の直接質量分光分析 | |
| Resing et al. | Applications of mass spectrometry to signal transduction | |
| EP1469314A1 (en) | Method of mass spectometry | |
| Sekera et al. | Sequencing proteins from bottom to top: Combining techniques for full sequence analysis of glucokinase | |
| Gibson et al. | Mass spectrometry of biomolecules | |
| Barry | Improving shotgun proteomics by: mass defect labeling of cysteines and aqueous-organic solvent system for trypsinolysis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20090212 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20110502 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110510 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110805 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110812 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110909 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110916 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111007 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111108 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120207 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120214 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120307 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20120307 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120403 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120412 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4976384 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150420 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |