JP4958905B2 - ペプチド鎖発現を増大させるための物質および方法 - Google Patents
ペプチド鎖発現を増大させるための物質および方法 Download PDFInfo
- Publication number
- JP4958905B2 JP4958905B2 JP2008526258A JP2008526258A JP4958905B2 JP 4958905 B2 JP4958905 B2 JP 4958905B2 JP 2008526258 A JP2008526258 A JP 2008526258A JP 2008526258 A JP2008526258 A JP 2008526258A JP 4958905 B2 JP4958905 B2 JP 4958905B2
- Authority
- JP
- Japan
- Prior art keywords
- dna
- seq
- cells
- expression
- peptide chain
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 62
- 230000014509 gene expression Effects 0.000 title abstract description 88
- 238000000034 method Methods 0.000 title abstract description 37
- 230000001965 increasing effect Effects 0.000 title description 17
- 239000000126 substance Substances 0.000 title 1
- 108020004414 DNA Proteins 0.000 claims description 185
- 150000007523 nucleic acids Chemical class 0.000 claims description 60
- 108020004707 nucleic acids Proteins 0.000 claims description 51
- 102000039446 nucleic acids Human genes 0.000 claims description 51
- 108020004511 Recombinant DNA Proteins 0.000 claims description 43
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 30
- 239000002299 complementary DNA Substances 0.000 claims description 14
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims description 10
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims description 10
- 108020002326 glutamine synthetase Proteins 0.000 claims description 8
- 102000005396 glutamine synthetase Human genes 0.000 claims description 7
- 108090000623 proteins and genes Proteins 0.000 abstract description 53
- 108091028043 Nucleic acid sequence Proteins 0.000 abstract description 21
- 210000004027 cell Anatomy 0.000 description 154
- 239000012634 fragment Substances 0.000 description 33
- 239000013598 vector Substances 0.000 description 27
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 26
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 26
- 108700008625 Reporter Genes Proteins 0.000 description 26
- 239000005090 green fluorescent protein Substances 0.000 description 26
- 241000699666 Mus <mouse, genus> Species 0.000 description 19
- 210000003527 eukaryotic cell Anatomy 0.000 description 19
- 239000013612 plasmid Substances 0.000 description 16
- 102000004169 proteins and genes Human genes 0.000 description 16
- 230000006798 recombination Effects 0.000 description 14
- 238000005215 recombination Methods 0.000 description 14
- 241000282414 Homo sapiens Species 0.000 description 13
- 230000010354 integration Effects 0.000 description 13
- 102000008297 Nuclear Matrix-Associated Proteins Human genes 0.000 description 12
- 108010035916 Nuclear Matrix-Associated Proteins Proteins 0.000 description 12
- 238000004458 analytical method Methods 0.000 description 12
- 239000003550 marker Substances 0.000 description 12
- 238000010561 standard procedure Methods 0.000 description 11
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 10
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 9
- 239000002609 medium Substances 0.000 description 9
- 210000000349 chromosome Anatomy 0.000 description 8
- 101150027568 LC gene Proteins 0.000 description 7
- 108091092195 Intron Proteins 0.000 description 6
- 241000053227 Themus Species 0.000 description 6
- 230000004888 barrier function Effects 0.000 description 6
- 230000027455 binding Effects 0.000 description 6
- 239000012212 insulator Substances 0.000 description 6
- 238000010367 cloning Methods 0.000 description 5
- 238000000684 flow cytometry Methods 0.000 description 5
- 210000000299 nuclear matrix Anatomy 0.000 description 5
- 102000004196 processed proteins & peptides Human genes 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- 102000053602 DNA Human genes 0.000 description 4
- 241000699660 Mus musculus Species 0.000 description 4
- 241000700605 Viruses Species 0.000 description 4
- 150000001413 amino acids Chemical class 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 238000001823 molecular biology technique Methods 0.000 description 4
- 229920001184 polypeptide Polymers 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 238000013518 transcription Methods 0.000 description 4
- 230000035897 transcription Effects 0.000 description 4
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 3
- 108010077544 Chromatin Proteins 0.000 description 3
- 241000699802 Cricetulus griseus Species 0.000 description 3
- 101150066002 GFP gene Proteins 0.000 description 3
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 description 3
- 210000003483 chromatin Anatomy 0.000 description 3
- 230000002759 chromosomal effect Effects 0.000 description 3
- 230000007547 defect Effects 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 238000000799 fluorescence microscopy Methods 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 201000000050 myeloid neoplasm Diseases 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 2
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 108700019146 Transgenes Proteins 0.000 description 2
- 208000036142 Viral infection Diseases 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 230000016784 immunoglobulin production Effects 0.000 description 2
- 229940072221 immunoglobulins Drugs 0.000 description 2
- 238000007901 in situ hybridization Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- SXTAYKAGBXMACB-UHFFFAOYSA-N methionine S-imide-S-oxide Natural products CS(=N)(=O)CCC(N)C(O)=O SXTAYKAGBXMACB-UHFFFAOYSA-N 0.000 description 2
- 210000003463 organelle Anatomy 0.000 description 2
- 238000000159 protein binding assay Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 230000005945 translocation Effects 0.000 description 2
- 230000009385 viral infection Effects 0.000 description 2
- BFFPVEVGHKMWLT-UHFFFAOYSA-N 2-amino-3,7-dihydropurin-6-one;3,7-dihydropurin-6-one Chemical compound O=C1NC=NC2=C1NC=N2.O=C1NC(N)=NC2=C1NC=N2 BFFPVEVGHKMWLT-UHFFFAOYSA-N 0.000 description 1
- 235000014653 Carica parviflora Nutrition 0.000 description 1
- 241000243321 Cnidaria Species 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 108010067770 Endopeptidase K Proteins 0.000 description 1
- 239000012739 FreeStyle 293 Expression medium Substances 0.000 description 1
- 208000034951 Genetic Translocation Diseases 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- 241000699684 Meriones unguiculatus Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241001125021 Mus booduga Species 0.000 description 1
- 241000699658 Mus musculus domesticus Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 238000010222 PCR analysis Methods 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 238000002105 Southern blotting Methods 0.000 description 1
- 102000002262 Thromboplastin Human genes 0.000 description 1
- 108010000499 Thromboplastin Proteins 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 241001512733 Zoanthus sp. Species 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000011543 agarose gel Substances 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- -1 antibody molecules Proteins 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 210000004507 artificial chromosome Anatomy 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 238000012832 cell culture technique Methods 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 238000001976 enzyme digestion Methods 0.000 description 1
- 230000001973 epigenetic effect Effects 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 102000034287 fluorescent proteins Human genes 0.000 description 1
- 108091006047 fluorescent proteins Proteins 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000000703 high-speed centrifugation Methods 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000003292 kidney cell Anatomy 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 238000007854 ligation-mediated PCR Methods 0.000 description 1
- 238000001638 lipofection Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- 210000000633 nuclear envelope Anatomy 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000000754 repressing effect Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000006152 selective media Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/244—Interleukins [IL]
- C07K16/248—IL-6
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2812—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/36—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against blood coagulation factors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/67—General methods for enhancing the expression
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Peptides Or Proteins (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Description
Furthら、Nucl.Acids.Res.、19、6205−6208(1991)
本発明の一局面は、配列番号1および配列番号2に示される配列を有する核酸を含んでなる単離されたDNAである。
本明細で引用される、限定されるものでないが特許および特許出願を挙げることができる全部の刊行物は、完全に示されるかのように引用することにより本明細書に組み込まれる。
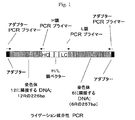
C128D細胞株は、ヒト/マウスキメラ抗体全体の発現を可能にするように2種の組換えDNA構築物で安定にトランスフェクトされているSp2/0ハツカネズミ(Mus musculus)系統BALB/c骨髄腫由来細胞株である。第一の構築物はヒトG1定常領域とともにマウス抗CD4 HC可変領域をコードした。第二の構築物はヒトκ定常領域とともにマウス抗CD4 LC可変領域をコードした。C128D細胞中で、HおよびL鎖ポリペプチドは極めて高レベル(消費済み(spent)振とうフラスコ中158mg/L;灌流バイオリアクター中1g/L/日)で発現された。加えて、最低1コピーのLC遺伝子および最低1コピーのHC遺伝子構築物が、C128D細胞株のゲノムDNA中に相互にすぐ隣接して組込んでいる。図1を参照されたい。
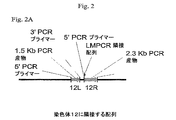
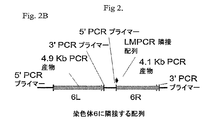
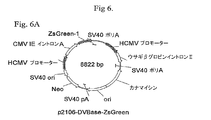
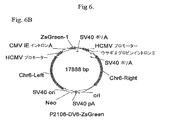
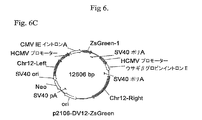
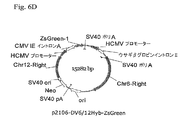
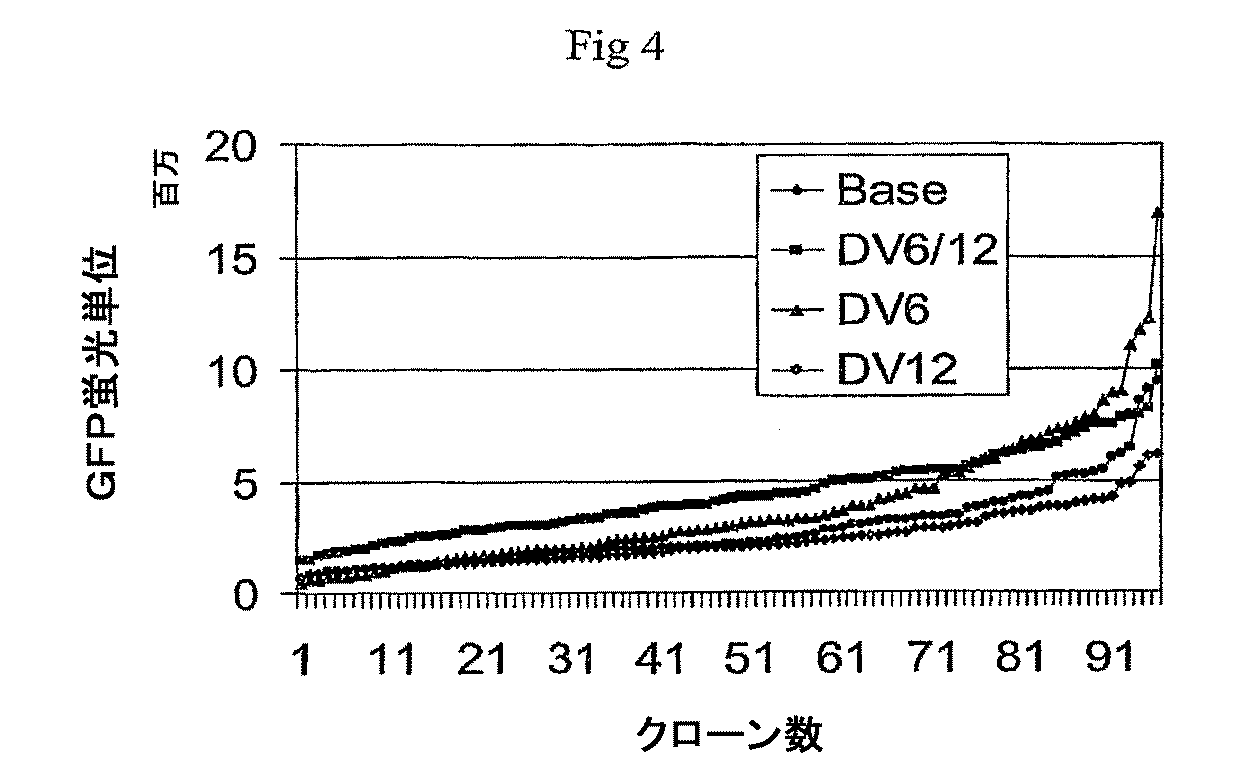
緑色蛍光タンパク質(GFP)レポーター遺伝子に連結された6R、6L、12Rおよび12L DNAを含有するベクター構築物を、組換え遺伝子発現を増大させる要素の同定を容易にするために作成した。全ベクターは標準的分子生物学的技術を使用して構築し、そしてプラスミド地図を全部図6に示す。
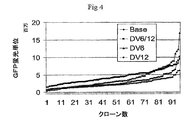
IL−6に対するヒトIgG1K抗体をコードするHCおよびLC遺伝子に連結した6Rおよび12R DNAを含有するベクター構築物を作成した。1構築物はp2106−DVBase−Antibody(DV−BASE−Ab)であった。DV−BASE−Abは、GFPレポーター遺伝子が抗体HC遺伝子で置換されかつLC遺伝子が該ベクターに存在するウサギβ−グロビンイントロンII要素に対し5’に挿入されたことを除き、p2106−DVBASE−ZsGreen(図6に示される)に本質的に同一である。第二の構築物はp2106−DV6/12Hyb−Antibody(DV6/12−Ab)であった。DV6/12−Abは、GFPレポーター遺伝子がHC遺伝子で置換されかつLC遺伝子が該ベクターに存在するウサギβグロビンイントロンII要素に対し5’に挿入されたことを除き、p2106−DV6/12Hyb−ZsGreen(図6に示される)に本質的に同一である。DV6/12−Ab中で、12R DNAはHC遺伝子の5’側に連結され、また、6R DNAはLC遺伝子の3’側に連結される。DV6/12は、C128D細胞中に見出される6Rおよび12R DNA要素の配置を表す。全ベクターは標準的分子生物学技術を使用して構築した。
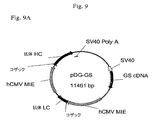
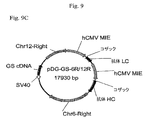
組織因子に対するヒト抗体をコードするHおよびL鎖遺伝子に連結された6Rおよび12R DNAを含有するベクター構築物を、示されるとおり作成した(プラスミド地図を図9に示す)。全ベクターは標準的分子生物学技術を使用して構築した。
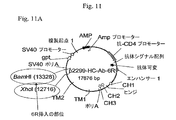
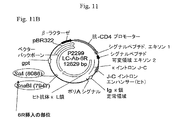
IL−6に対するマウス可変/ヒト定常キメラ抗体をコードするHCおよびLC遺伝子に連結した6R DNAを含有するベクター構築物を、示されるとおり作成した(プラスミド地図を図11に示す)。全ベクターは標準的分子生物学技術を使用して構築した。
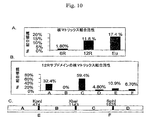
図10に示されるデータは、12R DNAおよびそのフラグメントが核マトリックス結合活性を有することを示す。核マトリックス結合活性は、マトリックス付着領域(MAR)を含んでなるDNAと一般に関連する。MARは、転写エンハンサーおよび障壁型インスレーターDNA要素としばしば緊密に関連しかつ頻繁にそれらに隣接する。「障壁型インスレーター」は、隣接する凝縮クロマチンがそれ以外は転写的に活性の遺伝子の遺伝子座に侵食しかつ抑制することを予防する障壁として作用することにより、遺伝子発現を増大させ得る。
組換え遺伝子発現を増大させかつ6Rおよび12R DNA中に存在する中核DNA要素は容易に同定し得る。最初に、6Rおよび12R DNAのフラグメントを生成しかつサブクローニングすることができる。6Rおよび12R DNAフラグメントは制限酵素消化若しくはPCRのような技術を使用して生成し得る。こうしたフラグメントは、いずれかの大きさの5’、3’若しくは内部に配置された欠失をもつ6Rおよび12R DNAを包含しうる。フラグメントは、5’、3’若しくは内的に配置された挿入若しくは置換をもつ6Rおよび12R DNAもまた包含しうる。必要な場合は、当業者に公知の技術を使用して、制限フラグメントのようなフラグメントから5’若しくは3’DNAオーバーハングを排除し得る。
Claims (8)
- 配列番号1および配列番号2に示される配列を有する核酸を含んでなる単離されたDNA。
- 配列番号1に示される配列を有する核酸が、ペプチド鎖をコードする組換えDNAの3’側に作動可能に連結される、請求項1に記載のDNA。
- グルタミン合成酵素をコードするDNAをさらに含んでなる、請求項2に記載のDNA。
- ペプチド鎖をコードする組換えDNAに作動可能に連結される、配列番号1および配列番号2に示される配列を有する核酸を含んでなる単離されたDNA。
- 組換えDNAがcDNAである、請求項4に記載のDNA。
- 配列番号1に示される配列を有する核酸が、組換えDNAの3’側に作動可能に連結され、かつ、配列番号2に示される配列を有する核酸が、組換えDNAの5’側に作動可能に連結される、請求項4に記載のDNA。
- グルタミン合成酵素をコードするDNAをさらに含んでなる、請求項6に記載のDNA。
- ペプチド鎖が抗体フラグメントである、請求項7に記載のDNA。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US70756405P | 2005-08-11 | 2005-08-11 | |
| US60/707,564 | 2005-08-11 | ||
| PCT/US2006/031506 WO2007022009A2 (en) | 2005-08-11 | 2006-08-11 | Materials and methods to increase peptide chain expression |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009504160A JP2009504160A (ja) | 2009-02-05 |
| JP4958905B2 true JP4958905B2 (ja) | 2012-06-20 |
Family
ID=37758241
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008526258A Expired - Fee Related JP4958905B2 (ja) | 2005-08-11 | 2006-08-11 | ペプチド鎖発現を増大させるための物質および方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US7498150B2 (ja) |
| EP (1) | EP1957660B1 (ja) |
| JP (1) | JP4958905B2 (ja) |
| AT (1) | ATE494386T1 (ja) |
| DE (1) | DE602006019486D1 (ja) |
| WO (1) | WO2007022009A2 (ja) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5528343B2 (ja) | 2007-08-20 | 2014-06-25 | マイ エフシー エイビー | フィードバックセンサーを有する燃料電池アッセンブリ |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1395669B1 (en) * | 2001-01-26 | 2009-07-22 | Selexis S.A. | Matrix attachment regions and methods for use thereof |
-
2006
- 2006-08-11 AT AT06789720T patent/ATE494386T1/de not_active IP Right Cessation
- 2006-08-11 EP EP06789720A patent/EP1957660B1/en not_active Not-in-force
- 2006-08-11 JP JP2008526258A patent/JP4958905B2/ja not_active Expired - Fee Related
- 2006-08-11 US US11/503,103 patent/US7498150B2/en active Active
- 2006-08-11 DE DE602006019486T patent/DE602006019486D1/de active Active
- 2006-08-11 WO PCT/US2006/031506 patent/WO2007022009A2/en not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| US20080213829A1 (en) | 2008-09-04 |
| JP2009504160A (ja) | 2009-02-05 |
| ATE494386T1 (de) | 2011-01-15 |
| EP1957660B1 (en) | 2011-01-05 |
| DE602006019486D1 (de) | 2011-02-17 |
| WO2007022009A3 (en) | 2007-11-22 |
| WO2007022009A2 (en) | 2007-02-22 |
| EP1957660A4 (en) | 2009-06-17 |
| EP1957660A2 (en) | 2008-08-20 |
| US7498150B2 (en) | 2009-03-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101183764B1 (ko) | 프로모터 | |
| PT939763E (pt) | Recombinação dirigida de adn medida por troca | |
| KR20110089846A (ko) | 키메라 항체의 제조를 위한 인간 이외의 포유동물 | |
| AU2017253240A1 (en) | Compositions and methods for making antibodies based on use of an expression-enhancing locus | |
| CN102165060A (zh) | 新的调控组件 | |
| EP2938728B1 (en) | Artificial introns | |
| US20230062964A1 (en) | Modified mice that produce heavy chain only antibodies | |
| US20220220509A1 (en) | Mammalian cell lines with sirt-1 gene knockout | |
| US20200087679A1 (en) | Expression cassette | |
| CN104870646A (zh) | 一种新颖的细胞系筛选方法 | |
| JP2013509188A (ja) | Sorf構築物および複数の遺伝子発現 | |
| JP4958905B2 (ja) | ペプチド鎖発現を増大させるための物質および方法 | |
| WO2014102101A1 (en) | Novel intron sequences | |
| EP2938726B1 (en) | Heterologous intron within a signal peptide | |
| CN114008081A (zh) | 通过以限定的组织形式靶向整合多个表达盒来产生二价双特异性抗体表达细胞的方法 | |
| CN114008212A (zh) | 通过以限定的组织形式靶向整合多个表达盒来产生三价抗体表达细胞的方法 | |
| US20240076405A1 (en) | Expression vectors for immunoglobulins and applications thereof | |
| HK40061338A (en) | Mammalian cell lines with sirt-1 gene knockout | |
| CN120322561A (zh) | 新型转座酶系统 | |
| HK40060850A (en) | Method for the generation of a bivalent, bispecific antibody expressing cell by targeted integration of multiple expression cassettes in a defined organization | |
| NZ626252B2 (en) | Expression cassette | |
| HK1232253B (zh) | 生产多肽的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20090710 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111018 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120118 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120130 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120220 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120313 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120319 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150330 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 4958905 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |