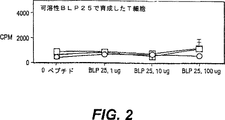
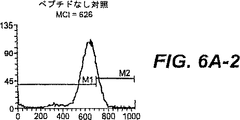
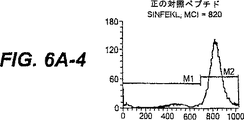
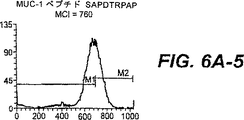
JP4480800B2 - 活性化t細胞および抗原パルス標識した抗原提示細胞を生成する方法 - Google Patents
活性化t細胞および抗原パルス標識した抗原提示細胞を生成する方法 Download PDFInfo
- Publication number
- JP4480800B2 JP4480800B2 JP54847798A JP54847798A JP4480800B2 JP 4480800 B2 JP4480800 B2 JP 4480800B2 JP 54847798 A JP54847798 A JP 54847798A JP 54847798 A JP54847798 A JP 54847798A JP 4480800 B2 JP4480800 B2 JP 4480800B2
- Authority
- JP
- Japan
- Prior art keywords
- cells
- antigen
- peptide
- cell
- muc
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 210000001744 T-lymphocyte Anatomy 0.000 title abstract description 200
- 108091007433 antigens Proteins 0.000 title abstract description 162
- 102000036639 antigens Human genes 0.000 title abstract description 162
- 239000000427 antigen Substances 0.000 title abstract description 161
- 238000000034 method Methods 0.000 title abstract description 88
- 210000000612 antigen-presenting cell Anatomy 0.000 title description 17
- 239000002502 liposome Substances 0.000 claims abstract description 88
- 238000000338 in vitro Methods 0.000 claims abstract description 26
- 239000000203 mixture Substances 0.000 claims abstract description 21
- 210000004027 cell Anatomy 0.000 claims description 110
- 108010008707 Mucin-1 Proteins 0.000 claims description 101
- 102100034256 Mucin-1 Human genes 0.000 claims description 92
- KXJTWOGIBOWZDJ-LELJLAJGSA-N l-blp25 Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1N(CCC1)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)[C@@H](C)O)C1=CNC=N1 KXJTWOGIBOWZDJ-LELJLAJGSA-N 0.000 claims description 47
- 108010028921 Lipopeptides Proteins 0.000 claims description 36
- 150000001413 amino acids Chemical class 0.000 claims description 29
- 108010017838 cyclo-(lysyl-prolyl-glycyl-prolyl-glycyl-glutamyl-prolyl-glycyl-prolyl-glycyl)cyclo(1epsilon-6gamma)glycyl-glycine Proteins 0.000 claims description 28
- 230000005867 T cell response Effects 0.000 claims description 26
- 150000002632 lipids Chemical class 0.000 claims description 21
- 230000027455 binding Effects 0.000 claims description 20
- 239000002671 adjuvant Substances 0.000 claims description 8
- 239000002691 unilamellar liposome Substances 0.000 claims description 8
- 125000001312 palmitoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 6
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 claims description 5
- -1 alum Chemical compound 0.000 claims description 5
- 210000004899 c-terminal region Anatomy 0.000 claims description 5
- 108010088652 Histocompatibility Antigens Class I Proteins 0.000 claims description 3
- 125000002252 acyl group Chemical group 0.000 claims description 3
- 125000001931 aliphatic group Chemical group 0.000 claims description 3
- 230000013595 glycosylation Effects 0.000 claims description 3
- 238000006206 glycosylation reaction Methods 0.000 claims description 3
- YVGGHNCTFXOJCH-UHFFFAOYSA-N DDT Chemical compound C1=CC(Cl)=CC=C1C(C(Cl)(Cl)Cl)C1=CC=C(Cl)C=C1 YVGGHNCTFXOJCH-UHFFFAOYSA-N 0.000 claims description 2
- 125000003074 decanoyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(*)=O 0.000 claims description 2
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 claims description 2
- 125000001419 myristoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 2
- 125000003696 stearoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 2
- 230000029226 lipidation Effects 0.000 claims 2
- 229940037003 alum Drugs 0.000 claims 1
- 125000001924 fatty-acyl group Chemical group 0.000 claims 1
- 125000003473 lipid group Chemical group 0.000 claims 1
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 abstract description 58
- 206010028980 Neoplasm Diseases 0.000 abstract description 30
- 201000011510 cancer Diseases 0.000 abstract description 12
- 230000004913 activation Effects 0.000 abstract description 9
- 210000003071 memory t lymphocyte Anatomy 0.000 abstract description 7
- 238000001727 in vivo Methods 0.000 abstract description 6
- 229940030156 cell vaccine Drugs 0.000 abstract description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 5
- 201000010099 disease Diseases 0.000 abstract description 4
- 210000000265 leukocyte Anatomy 0.000 abstract description 4
- 230000003612 virological effect Effects 0.000 abstract description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 209
- 102000004196 processed proteins & peptides Human genes 0.000 description 65
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 32
- 102000004127 Cytokines Human genes 0.000 description 25
- 108090000695 Cytokines Proteins 0.000 description 25
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 25
- 229940024606 amino acid Drugs 0.000 description 24
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 22
- 230000004044 response Effects 0.000 description 20
- 239000002609 medium Substances 0.000 description 19
- 230000009696 proliferative response Effects 0.000 description 19
- 238000002965 ELISA Methods 0.000 description 16
- 108090000978 Interleukin-4 Proteins 0.000 description 16
- 102000004388 Interleukin-4 Human genes 0.000 description 16
- 239000012634 fragment Substances 0.000 description 16
- 229940035032 monophosphoryl lipid a Drugs 0.000 description 16
- 108010002350 Interleukin-2 Proteins 0.000 description 15
- 102000000588 Interleukin-2 Human genes 0.000 description 15
- 238000003556 assay Methods 0.000 description 15
- 239000006228 supernatant Substances 0.000 description 15
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 14
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 14
- 230000000638 stimulation Effects 0.000 description 12
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 11
- 108091054437 MHC class I family Proteins 0.000 description 11
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 11
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 11
- 239000000463 material Substances 0.000 description 11
- 238000004007 reversed phase HPLC Methods 0.000 description 11
- 108090000174 Interleukin-10 Proteins 0.000 description 10
- 230000000890 antigenic effect Effects 0.000 description 10
- 238000004128 high performance liquid chromatography Methods 0.000 description 10
- 102000043129 MHC class I family Human genes 0.000 description 9
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 9
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 238000011534 incubation Methods 0.000 description 9
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 8
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 8
- 108010065805 Interleukin-12 Proteins 0.000 description 8
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- 125000003275 alpha amino acid group Chemical group 0.000 description 8
- 238000001516 cell proliferation assay Methods 0.000 description 8
- 230000001472 cytotoxic effect Effects 0.000 description 8
- 229960005160 dimyristoylphosphatidylglycerol Drugs 0.000 description 8
- BPHQZTVXXXJVHI-AJQTZOPKSA-N ditetradecanoyl phosphatidylglycerol Chemical compound CCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@@H](O)CO)OC(=O)CCCCCCCCCCCCC BPHQZTVXXXJVHI-AJQTZOPKSA-N 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 239000013642 negative control Substances 0.000 description 8
- 239000012071 phase Substances 0.000 description 8
- 230000009257 reactivity Effects 0.000 description 8
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 7
- 239000004698 Polyethylene Substances 0.000 description 7
- 208000009956 adenocarcinoma Diseases 0.000 description 7
- 238000010828 elution Methods 0.000 description 7
- 230000001965 increasing effect Effects 0.000 description 7
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 6
- JVJGCCBAOOWGEO-RUTPOYCXSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-4-amino-2-[[(2s,3s)-2-[[(2s,3s)-2-[[(2s)-2-azaniumyl-3-hydroxypropanoyl]amino]-3-methylpentanoyl]amino]-3-methylpentanoyl]amino]-4-oxobutanoyl]amino]-3-phenylpropanoyl]amino]-4-carboxylatobutanoyl]amino]-6-azaniumy Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 JVJGCCBAOOWGEO-RUTPOYCXSA-N 0.000 description 6
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 108010002586 Interleukin-7 Proteins 0.000 description 6
- 230000006052 T cell proliferation Effects 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 6
- 230000016396 cytokine production Effects 0.000 description 6
- 210000004443 dendritic cell Anatomy 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 238000011026 diafiltration Methods 0.000 description 6
- 230000028993 immune response Effects 0.000 description 6
- 230000036039 immunity Effects 0.000 description 6
- 238000009169 immunotherapy Methods 0.000 description 6
- 238000001819 mass spectrum Methods 0.000 description 6
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 6
- 230000003389 potentiating effect Effects 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 239000007790 solid phase Substances 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 238000005406 washing Methods 0.000 description 6
- 229920002684 Sepharose Polymers 0.000 description 5
- 230000006044 T cell activation Effects 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 238000004113 cell culture Methods 0.000 description 5
- 239000012228 culture supernatant Substances 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 229930195733 hydrocarbon Natural products 0.000 description 5
- 150000002430 hydrocarbons Chemical class 0.000 description 5
- 238000002955 isolation Methods 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 229960004857 mitomycin Drugs 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 230000035755 proliferation Effects 0.000 description 5
- 238000011160 research Methods 0.000 description 5
- 238000010186 staining Methods 0.000 description 5
- 230000004936 stimulating effect Effects 0.000 description 5
- 241000283707 Capra Species 0.000 description 4
- 238000012286 ELISA Assay Methods 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- 239000004677 Nylon Substances 0.000 description 4
- 108010058846 Ovalbumin Proteins 0.000 description 4
- 102000003992 Peroxidases Human genes 0.000 description 4
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- 238000001261 affinity purification Methods 0.000 description 4
- 239000012491 analyte Substances 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 231100000433 cytotoxic Toxicity 0.000 description 4
- 230000002950 deficient Effects 0.000 description 4
- 239000012636 effector Substances 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 239000010410 layer Substances 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- 229920001778 nylon Polymers 0.000 description 4
- 244000052769 pathogen Species 0.000 description 4
- 108040007629 peroxidase activity proteins Proteins 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 230000037452 priming Effects 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 230000004043 responsiveness Effects 0.000 description 4
- 238000012163 sequencing technique Methods 0.000 description 4
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 229960005486 vaccine Drugs 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 239000004215 Carbon black (E152) Substances 0.000 description 3
- 108010074328 Interferon-gamma Proteins 0.000 description 3
- 108010002352 Interleukin-1 Proteins 0.000 description 3
- 102000000589 Interleukin-1 Human genes 0.000 description 3
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 102000043131 MHC class II family Human genes 0.000 description 3
- 108091054438 MHC class II family Proteins 0.000 description 3
- 229930040373 Paraformaldehyde Natural products 0.000 description 3
- 239000012980 RPMI-1640 medium Substances 0.000 description 3
- 108010090804 Streptavidin Proteins 0.000 description 3
- 108091008874 T cell receptors Proteins 0.000 description 3
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 3
- 208000036142 Viral infection Diseases 0.000 description 3
- 102000015736 beta 2-Microglobulin Human genes 0.000 description 3
- 108010081355 beta 2-Microglobulin Proteins 0.000 description 3
- 229960002685 biotin Drugs 0.000 description 3
- 235000020958 biotin Nutrition 0.000 description 3
- 239000011616 biotin Substances 0.000 description 3
- 125000002091 cationic group Chemical group 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 235000012000 cholesterol Nutrition 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 230000000875 corresponding effect Effects 0.000 description 3
- 210000004748 cultured cell Anatomy 0.000 description 3
- 230000003013 cytotoxicity Effects 0.000 description 3
- 231100000135 cytotoxicity Toxicity 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 230000003511 endothelial effect Effects 0.000 description 3
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 3
- 210000002443 helper t lymphocyte Anatomy 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 238000010348 incorporation Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 230000002101 lytic effect Effects 0.000 description 3
- 210000002540 macrophage Anatomy 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 210000004379 membrane Anatomy 0.000 description 3
- 230000003278 mimic effect Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 229940092253 ovalbumin Drugs 0.000 description 3
- 230000020477 pH reduction Effects 0.000 description 3
- 229920002866 paraformaldehyde Polymers 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 230000000284 resting effect Effects 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 210000004881 tumor cell Anatomy 0.000 description 3
- 230000009385 viral infection Effects 0.000 description 3
- 239000011534 wash buffer Substances 0.000 description 3
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 description 2
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 2
- 108010015899 Glycopeptides Proteins 0.000 description 2
- 102000002068 Glycopeptides Human genes 0.000 description 2
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 description 2
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 2
- 102100037850 Interferon gamma Human genes 0.000 description 2
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 2
- 102000010789 Interleukin-2 Receptors Human genes 0.000 description 2
- 102000015696 Interleukins Human genes 0.000 description 2
- 108010063738 Interleukins Proteins 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- 229930182816 L-glutamine Natural products 0.000 description 2
- 102100033762 Proheparin-binding EGF-like growth factor Human genes 0.000 description 2
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 2
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 101710120037 Toxin CcdB Proteins 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 241000287433 Turdus Species 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- HMNZFMSWFCAGGW-XPWSMXQVSA-N [3-[hydroxy(2-hydroxyethoxy)phosphoryl]oxy-2-[(e)-octadec-9-enoyl]oxypropyl] (e)-octadec-9-enoate Chemical compound CCCCCCCC\C=C\CCCCCCCC(=O)OCC(COP(O)(=O)OCCO)OC(=O)CCCCCCC\C=C\CCCCCCCC HMNZFMSWFCAGGW-XPWSMXQVSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- OHDRQQURAXLVGJ-HLVWOLMTSA-N azane;(2e)-3-ethyl-2-[(e)-(3-ethyl-6-sulfo-1,3-benzothiazol-2-ylidene)hydrazinylidene]-1,3-benzothiazole-6-sulfonic acid Chemical compound [NH4+].[NH4+].S/1C2=CC(S([O-])(=O)=O)=CC=C2N(CC)C\1=N/N=C1/SC2=CC(S([O-])(=O)=O)=CC=C2N1CC OHDRQQURAXLVGJ-HLVWOLMTSA-N 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 230000022131 cell cycle Effects 0.000 description 2
- 238000009172 cell transfer therapy Methods 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000000139 costimulatory effect Effects 0.000 description 2
- 238000004163 cytometry Methods 0.000 description 2
- 230000007402 cytotoxic response Effects 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 239000003599 detergent Substances 0.000 description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000000132 electrospray ionisation Methods 0.000 description 2
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 2
- 239000012149 elution buffer Substances 0.000 description 2
- 239000008393 encapsulating agent Substances 0.000 description 2
- 238000005538 encapsulation Methods 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- VBZWSGALLODQNC-UHFFFAOYSA-N hexafluoroacetone Chemical compound FC(F)(F)C(=O)C(F)(F)F VBZWSGALLODQNC-UHFFFAOYSA-N 0.000 description 2
- 230000003308 immunostimulating effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- PGLTVOMIXTUURA-UHFFFAOYSA-N iodoacetamide Chemical compound NC(=O)CI PGLTVOMIXTUURA-UHFFFAOYSA-N 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 238000002794 lymphocyte assay Methods 0.000 description 2
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000011278 mitosis Effects 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N palmitic acid group Chemical group C(CCCCCCCCCCCCCCC)(=O)O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- 230000003071 parasitic effect Effects 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- 235000020030 perry Nutrition 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 125000006853 reporter group Chemical group 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000010532 solid phase synthesis reaction Methods 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 230000005068 transpiration Effects 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
- CJDRUOGAGYHKKD-XMTJACRCSA-N (+)-Ajmaline Natural products O[C@H]1[C@@H](CC)[C@@H]2[C@@H]3[C@H](O)[C@@]45[C@@H](N(C)c6c4cccc6)[C@@H](N1[C@H]3C5)C2 CJDRUOGAGYHKKD-XMTJACRCSA-N 0.000 description 1
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- PORPENFLTBBHSG-MGBGTMOVSA-N 1,2-dihexadecanoyl-sn-glycerol-3-phosphate Chemical group CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC PORPENFLTBBHSG-MGBGTMOVSA-N 0.000 description 1
- TWJNQYPJQDRXPH-UHFFFAOYSA-N 2-cyanobenzohydrazide Chemical group NNC(=O)C1=CC=CC=C1C#N TWJNQYPJQDRXPH-UHFFFAOYSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 101710145634 Antigen 1 Proteins 0.000 description 1
- 108010039627 Aprotinin Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- MJJIHRWNWSQTOI-VEVYYDQMSA-N Asp-Thr-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O MJJIHRWNWSQTOI-VEVYYDQMSA-N 0.000 description 1
- 102100035526 B melanoma antigen 1 Human genes 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 108010041397 CD4 Antigens Proteins 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 108010032795 CD8 receptor Proteins 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 238000011510 Elispot assay Methods 0.000 description 1
- 241000283074 Equus asinus Species 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 210000000712 G cell Anatomy 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 108010036972 HLA-A11 Antigen Proteins 0.000 description 1
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 1
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000874316 Homo sapiens B melanoma antigen 1 Proteins 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000908391 Homo sapiens Dipeptidyl peptidase 4 Proteins 0.000 description 1
- 101000935043 Homo sapiens Integrin beta-1 Proteins 0.000 description 1
- 101001063392 Homo sapiens Lymphocyte function-associated antigen 3 Proteins 0.000 description 1
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100025304 Integrin beta-1 Human genes 0.000 description 1
- 102100025390 Integrin beta-2 Human genes 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108090000176 Interleukin-13 Proteins 0.000 description 1
- 102000003812 Interleukin-15 Human genes 0.000 description 1
- 108090000172 Interleukin-15 Proteins 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- 241000222722 Leishmania <genus> Species 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 description 1
- 102100030984 Lymphocyte function-associated antigen 3 Human genes 0.000 description 1
- 102000008072 Lymphokines Human genes 0.000 description 1
- 108010074338 Lymphokines Proteins 0.000 description 1
- 108010037255 Member 7 Tumor Necrosis Factor Receptor Superfamily Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 1
- 108010063954 Mucins Proteins 0.000 description 1
- 102000015728 Mucins Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 101001133053 Mus musculus Mucin-1 Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 235000021360 Myristic acid Nutrition 0.000 description 1
- TUNFSRHWOTWDNC-UHFFFAOYSA-N Myristic acid Chemical group CCCCCCCCCCCCCC(O)=O TUNFSRHWOTWDNC-UHFFFAOYSA-N 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 244000046052 Phaseolus vulgaris Species 0.000 description 1
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 1
- 230000018199 S phase Effects 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- 241000145525 Spinach latent virus Species 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 238000011467 adoptive cell therapy Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 239000010441 alabaster Substances 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 230000003302 anti-idiotype Effects 0.000 description 1
- 230000007503 antigenic stimulation Effects 0.000 description 1
- 229960004405 aprotinin Drugs 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 230000000599 auto-anti-genic effect Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 210000004900 c-terminal fragment Anatomy 0.000 description 1
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 238000011072 cell harvest Methods 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- SURLGNKAQXKNSP-DBLYXWCISA-N chlorin Chemical compound C\1=C/2\N/C(=C\C3=N/C(=C\C=4NC(/C=C\5/C=CC/1=N/5)=CC=4)/C=C3)/CC\2 SURLGNKAQXKNSP-DBLYXWCISA-N 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 230000006957 competitive inhibition Effects 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- BFMYDTVEBKDAKJ-UHFFFAOYSA-L disodium;(2',7'-dibromo-3',6'-dioxido-3-oxospiro[2-benzofuran-1,9'-xanthene]-4'-yl)mercury;hydrate Chemical compound O.[Na+].[Na+].O1C(=O)C2=CC=CC=C2C21C1=CC(Br)=C([O-])C([Hg])=C1OC1=C2C=C(Br)C([O-])=C1 BFMYDTVEBKDAKJ-UHFFFAOYSA-L 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 238000012137 double-staining Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 235000015244 frankfurter Nutrition 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 102000054766 genetic haplotypes Human genes 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 230000007773 growth pattern Effects 0.000 description 1
- PJJJBBJSCAKJQF-UHFFFAOYSA-N guanidinium chloride Chemical compound [Cl-].NC(N)=[NH2+] PJJJBBJSCAKJQF-UHFFFAOYSA-N 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 239000013542 high molecular weight contaminant Substances 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 230000005745 host immune response Effects 0.000 description 1
- 210000005104 human peripheral blood lymphocyte Anatomy 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- 238000003125 immunofluorescent labeling Methods 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 230000001024 immunotherapeutic effect Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000004073 interleukin-2 production Effects 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 230000006674 lysosomal degradation Effects 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 238000012737 microarray-based gene expression Methods 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 229940051875 mucins Drugs 0.000 description 1
- 238000012243 multiplex automated genomic engineering Methods 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 108010028895 oncolipin Proteins 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000000242 pagocytic effect Effects 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 210000001539 phagocyte Anatomy 0.000 description 1
- 125000001095 phosphatidyl group Chemical group 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 210000004986 primary T-cell Anatomy 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 235000021251 pulses Nutrition 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 201000004409 schistosomiasis Diseases 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 238000010183 spectrum analysis Methods 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000012134 supernatant fraction Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 108010066082 tartrate-sensitive acid phosphatase Proteins 0.000 description 1
- WROMPOXWARCANT-UHFFFAOYSA-N tfa trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F WROMPOXWARCANT-UHFFFAOYSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000002992 thymic effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 201000002311 trypanosomiasis Diseases 0.000 description 1
- 210000004926 tubular epithelial cell Anatomy 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 229940125575 vaccine candidate Drugs 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4727—Mucins, e.g. human intestinal mucin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4256—Tumor associated carbohydrates
- A61K40/4257—Mucins, e.g. MUC-1
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55555—Liposomes; Vesicles, e.g. nanoparticles; Spheres, e.g. nanospheres; Polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Toxicology (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US4594997P | 1997-05-08 | 1997-05-08 | |
| US60/045,949 | 1997-05-08 | ||
| PCT/US1998/009288 WO1998050527A1 (en) | 1997-05-08 | 1998-05-07 | Method for generating activated t-cells and antigen-pulsed antigen-presenting cells |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2001525668A JP2001525668A (ja) | 2001-12-11 |
| JP2001525668A5 JP2001525668A5 (enExample) | 2005-12-02 |
| JP4480800B2 true JP4480800B2 (ja) | 2010-06-16 |
Family
ID=21940709
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP54847798A Expired - Fee Related JP4480800B2 (ja) | 1997-05-08 | 1998-05-07 | 活性化t細胞および抗原パルス標識した抗原提示細胞を生成する方法 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US6600012B1 (enExample) |
| EP (3) | EP1634949B1 (enExample) |
| JP (1) | JP4480800B2 (enExample) |
| AT (2) | ATE491465T1 (enExample) |
| AU (1) | AU727863B2 (enExample) |
| CA (1) | CA2289742C (enExample) |
| DE (2) | DE69839443D1 (enExample) |
| DK (1) | DK1634949T3 (enExample) |
| ES (1) | ES2357960T3 (enExample) |
| WO (1) | WO1998050527A1 (enExample) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19906744A1 (de) * | 1999-02-18 | 2000-08-24 | Martin Roecken | Verfahren zur Herstellung antitumoraler Th1-Zellen |
| CA2368967A1 (en) * | 1999-04-09 | 2000-10-19 | Biomira, Inc. | Telomerase-specific cancer vaccine |
| DE19917195B4 (de) * | 1999-04-16 | 2006-09-28 | Immatics Biotechnologies Gmbh | Peptid zur Auslösung einer Immunreaktion gegen Tumorzellen, diese enthaltende pharmzeutische Zusammensetzungen, dessen Verwendungen, dafür codierende Nukleinsäure und diese Nukleinsäure enthaltender Expressionsvektor |
| WO2001018035A2 (en) | 1999-09-08 | 2001-03-15 | Transgene S.A. | Muc-1 derived peptides |
| GB9921337D0 (en) * | 1999-09-09 | 1999-11-10 | Axis Genetics Plc | Modified plant viruses |
| GB0007150D0 (en) * | 2000-03-24 | 2000-05-17 | Lamellar Therapeutics Limited | Immunotherapeutic methods and compositions |
| EP1377275B1 (en) * | 2001-03-27 | 2006-10-25 | Biomira, Inc. | Vaccine for modulating between t1 and t2 immune responses |
| EP1499347A2 (en) | 2002-03-15 | 2005-01-26 | Department of Veterans Affairs, Rehabilitation R&D Service | Methods and compositions using cellular asialodeterminants and glycoconjugates for targeting cells to tissues and organs |
| CA2482477A1 (en) * | 2002-04-15 | 2003-10-30 | Biomira, Inc. | Synthetic glyco-lipo-peptides as vaccines |
| US20060142546A1 (en) * | 2002-09-05 | 2006-06-29 | Franz-Georg Hanisch | Immunogenic muc1 glycopeptides |
| WO2004033667A2 (en) * | 2002-10-10 | 2004-04-22 | Department Of Veterans Affairs | Detection, localization and staging of tumors using labeled activated lymphocytes directed to a tumor specific epitope |
| US20050045205A1 (en) * | 2003-08-29 | 2005-03-03 | Stach Steven R. | Apparatus and method for cleaning printed circuit boards |
| GB0322448D0 (en) | 2003-09-25 | 2003-10-29 | Lamellar Therapeutics Ltd | Using lamellar bodies to modify linear biological macro molecules |
| EP1697399B1 (en) | 2003-12-12 | 2016-11-23 | GOVERNMENT OF THE UNITED STATES OF AMERICA, as repr. by THE SECR. OF THE DEPT. OF HEALTH AND HUMAN SERVICES AND HIS SUCCESSORS | A human cytotoxic t-lymphocyte epitope and its agonist epitope from the non-variable number of tandem repeat sequence of muc-1 |
| TW201204410A (en) | 2004-04-01 | 2012-02-01 | Oncothyreon Inc | Mucinous glycoprotein (MUC-1) vaccine |
| EP1812052A1 (en) * | 2004-11-02 | 2007-08-01 | Biomedical Research Models, Inc. | Methods of cancer treatment/prevention using cancer cell-specific surface antigens |
| TW201615208A (zh) * | 2005-06-28 | 2016-05-01 | 安柯席爾伊恩股份有限公司 | 以黏液性糖蛋白(muc-1)疫苗治療病人之方法 |
| WO2010002478A2 (en) | 2008-07-03 | 2010-01-07 | University Of Georgia Research Foundation, Inc. | Glycopeptide and uses thereof |
| EP2025762A3 (en) | 2006-01-17 | 2009-09-30 | Health Research Inc. | Heteroduplex tracking assay |
| RU2377994C1 (ru) * | 2008-05-21 | 2010-01-10 | Леонид Валентинович Загребин | Способ иммунореабилитации онкозаболеваний |
| US20100059084A1 (en) * | 2008-09-10 | 2010-03-11 | Austin American Technology Corporation | Cleaning and testing ionic cleanliness of electronic assemblies |
| US9597392B2 (en) | 2010-05-10 | 2017-03-21 | Ascend Biopharmaceuticals Pty Ltd. | Use of high molecular weight mannan for inducing and/or enhancing an immune response |
| BR112013021779A2 (pt) | 2011-02-24 | 2017-09-19 | Oncothyreon Inc | vacina de glicolipopeptídeo baseada em muc1 com adjuvante. |
| EP2800762B1 (en) | 2012-01-03 | 2018-01-03 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Native and agonist ctl epitopes of the muc1 tumor antigen |
| BR112015028252A2 (pt) | 2013-05-14 | 2017-07-25 | Merck Patent Gmbh | método de tratamento de câncer de pulmão por vacinação com lipopeptídeo muc-1 |
| WO2015058831A1 (en) | 2013-10-25 | 2015-04-30 | Merck Patent Gmbh | Elevated biomarker expression in lung cancer patients responding to treatment with muc-1 lipopeptide vaccines |
| IL303806B2 (en) | 2016-12-22 | 2024-05-01 | Cue Biopharma Inc | Multimeric polypeptides modulate T cells and methods for their use |
| WO2018129474A1 (en) | 2017-01-09 | 2018-07-12 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| WO2018170168A1 (en) | 2017-03-15 | 2018-09-20 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| GB201812647D0 (en) | 2018-08-03 | 2018-09-19 | Chancellor Masters And Scholars Of The Univ Of Oxford | Viral vectors and methods for the prevention or treatment of cancer |
| WO2020257191A1 (en) * | 2019-06-19 | 2020-12-24 | Cue Biopharma, Inc. | Multimeric t-cell modulatory polypeptides and methods of use thereof |
| IL296209A (en) | 2020-05-12 | 2022-11-01 | Cue Biopharma Inc | Multimeric t-cell modulatory polypeptides and methods of using them |
| JP2023541366A (ja) | 2020-09-09 | 2023-10-02 | キュー バイオファーマ, インコーポレイテッド | 1型真性糖尿病(t1d)を治療するためのmhcクラスii t細胞調節多量体ポリペプチド及びその使用方法 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE69511410T2 (de) | 1994-06-13 | 1999-12-16 | Unilever N.V., Rotterdam | Bleichaktivierung |
| CA2192655A1 (en) * | 1994-06-14 | 1995-12-21 | Edgar G. Engleman | Methods for in vivo t cell activation by antigen-pulsed dendritic cells |
| AU5849796A (en) * | 1994-12-27 | 1996-08-07 | United Biomedical Inc. | Peptide ratchet libraries for ctl-inducing vaccines and therapeutics |
| AU6330896A (en) * | 1995-06-07 | 1996-12-30 | Governors Of The University Of Alberta, The | A method for eliciting a th1-specific immune response |
| WO1998037095A2 (en) | 1997-02-24 | 1998-08-27 | Therion Biologics Corporation | Recombinant pox virus for immunization against muc1 tumor-associated antigen |
-
1998
- 1998-05-07 JP JP54847798A patent/JP4480800B2/ja not_active Expired - Fee Related
- 1998-05-07 DK DK05076809.2T patent/DK1634949T3/da active
- 1998-05-07 EP EP05076809A patent/EP1634949B1/en not_active Expired - Lifetime
- 1998-05-07 ES ES05076809T patent/ES2357960T3/es not_active Expired - Lifetime
- 1998-05-07 CA CA2289742A patent/CA2289742C/en not_active Expired - Fee Related
- 1998-05-07 DE DE69839443T patent/DE69839443D1/de not_active Expired - Lifetime
- 1998-05-07 AU AU73712/98A patent/AU727863B2/en not_active Ceased
- 1998-05-07 AT AT05076809T patent/ATE491465T1/de active
- 1998-05-07 AT AT98921011T patent/ATE394474T1/de not_active IP Right Cessation
- 1998-05-07 WO PCT/US1998/009288 patent/WO1998050527A1/en not_active Ceased
- 1998-05-07 EP EP98921011A patent/EP0986636B1/en not_active Expired - Lifetime
- 1998-05-07 EP EP10180388.0A patent/EP2341072A3/en not_active Withdrawn
- 1998-05-07 DE DE69842060T patent/DE69842060D1/de not_active Expired - Lifetime
-
2000
- 2000-02-03 US US09/497,232 patent/US6600012B1/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| US6600012B1 (en) | 2003-07-29 |
| CA2289742C (en) | 2013-07-16 |
| EP0986636A1 (en) | 2000-03-22 |
| EP2341072A3 (en) | 2013-10-30 |
| EP1634949A1 (en) | 2006-03-15 |
| ATE394474T1 (de) | 2008-05-15 |
| DE69839443D1 (de) | 2008-06-19 |
| DK1634949T3 (da) | 2011-03-28 |
| AU727863B2 (en) | 2001-01-04 |
| ATE491465T1 (de) | 2011-01-15 |
| EP0986636B1 (en) | 2008-05-07 |
| JP2001525668A (ja) | 2001-12-11 |
| AU7371298A (en) | 1998-11-27 |
| CA2289742A1 (en) | 1998-11-12 |
| ES2357960T3 (es) | 2011-05-04 |
| EP2341072A2 (en) | 2011-07-06 |
| WO1998050527A1 (en) | 1998-11-12 |
| EP1634949B1 (en) | 2010-12-15 |
| DE69842060D1 (de) | 2011-01-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4480800B2 (ja) | 活性化t細胞および抗原パルス標識した抗原提示細胞を生成する方法 | |
| Reddish et al. | Anti‐MUC1 class I restricted CTLs in metastatic breast cancer patients immunized with a synthetic MUC1 peptide | |
| US20200157146A1 (en) | Controlled modulation of amino acid side chain length of peptide antigens | |
| US5662907A (en) | Induction of anti-tumor cytotoxic T lymphocytes in humans using synthetic peptide epitopes | |
| CN106220721B (zh) | Prame衍生的肽以及包括该肽的免疫原组合物 | |
| EP2089423B1 (en) | Antigen specific multi epitope vaccines | |
| Agrawal et al. | Rapid induction of primary human CD4+ and CD8+ T cell responses against cancer-associated MUC1 peptide epitopes. | |
| AU703120B2 (en) | Composition and methods for enhancing immune responses mediated by antigen-presenting cells | |
| US20060069238A1 (en) | Synthetic glyco-lipo-peptides as vaccines | |
| CA2441809C (en) | Liposomal vaccines comprising dilipopeptides for modulating t1 and t2 immune responses | |
| AU4139400A (en) | Telomerase-specific cancer vaccine | |
| Vivo | Interaction between Phosphatidylserine and | |
| AU4111999A (en) | Induction of anti-tumor cytotoxic T lymphocytes in humans using synthetic peptide epitopes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050427 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050427 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080507 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20080801 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20080908 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081107 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090317 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090617 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090727 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090917 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20091030 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20100223 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20100317 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130326 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130326 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140326 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140326 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |