JP2022166313A - 個別化冠状動脈ステント - Google Patents
個別化冠状動脈ステント Download PDFInfo
- Publication number
- JP2022166313A JP2022166313A JP2022134599A JP2022134599A JP2022166313A JP 2022166313 A JP2022166313 A JP 2022166313A JP 2022134599 A JP2022134599 A JP 2022134599A JP 2022134599 A JP2022134599 A JP 2022134599A JP 2022166313 A JP2022166313 A JP 2022166313A
- Authority
- JP
- Japan
- Prior art keywords
- stent
- mandrel
- shape
- sleeve
- establishing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B34/00—Computer-aided surgery; Manipulators or robots specially adapted for use in surgery
- A61B34/10—Computer-aided planning, simulation or modelling of surgical operations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/844—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents folded prior to deployment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B34/00—Computer-aided surgery; Manipulators or robots specially adapted for use in surgery
- A61B34/10—Computer-aided planning, simulation or modelling of surgical operations
- A61B2034/101—Computer-aided simulation of surgical operations
- A61B2034/102—Modelling of surgical devices, implants or prosthesis
- A61B2034/104—Modelling the effect of the tool, e.g. the effect of an implanted prosthesis or for predicting the effect of ablation or burring
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B34/00—Computer-aided surgery; Manipulators or robots specially adapted for use in surgery
- A61B34/10—Computer-aided planning, simulation or modelling of surgical operations
- A61B2034/101—Computer-aided simulation of surgical operations
- A61B2034/105—Modelling of the patient, e.g. for ligaments or bones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B34/00—Computer-aided surgery; Manipulators or robots specially adapted for use in surgery
- A61B34/10—Computer-aided planning, simulation or modelling of surgical operations
- A61B2034/108—Computer aided selection or customisation of medical implants or cutting guides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B34/00—Computer-aided surgery; Manipulators or robots specially adapted for use in surgery
- A61B34/20—Surgical navigation systems; Devices for tracking or guiding surgical instruments, e.g. for frameless stereotaxis
- A61B2034/2046—Tracking techniques
- A61B2034/2048—Tracking techniques using an accelerometer or inertia sensor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/36—Image-producing devices or illumination devices not otherwise provided for
- A61B90/37—Surgical systems with images on a monitor during operation
- A61B2090/373—Surgical systems with images on a monitor during operation using light, e.g. by using optical scanners
- A61B2090/3735—Optical coherence tomography [OCT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/39—Markers, e.g. radio-opaque or breast lesions markers
- A61B2090/3966—Radiopaque markers visible in an X-ray image
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
- A61F2002/91575—Adjacent bands being connected to each other connected peak to trough
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
- A61F2002/9583—Means for holding the stent on the balloon, e.g. using protrusions, adhesives or an outer sleeve
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2240/00—Manufacturing or designing of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2240/001—Designing or manufacturing processes
- A61F2240/002—Designing or making customized prostheses
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Heart & Thoracic Surgery (AREA)
- General Health & Medical Sciences (AREA)
- Surgery (AREA)
- Vascular Medicine (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Robotics (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Prostheses (AREA)
Abstract
【解決手段】血管の実際の形状の3Dモデルに応答して、血管の非狭窄形状の3Dモデルを生成することと、しぼんだ構成から、非狭窄形状に並置される最終構成に拡張されるステントのパラメータ記述を確立することと、しぼんだ構成と最終構成の間での塑性変形中にステント支柱が破損するリスクを含んだ発見的設計に応答して、パラメータ記述のパラメータを変えることによってステント用の設計を作り出すことと、ステント用の設計に従ってステントを具体化することと、ステントをそのしぼんだ構成で血管に挿入することと、血管を通って狭窄部に至るようにステントを操作することと、ステントをその最終構成に拡張することとを含む方法。
【選択図】図9
Description
本発明は、システム、方法、またはコンピュータ・プログラム製品、あるいはそれらの組合せであってもよい。コンピュータ・プログラム製品は、本発明の態様をプロセッサに実施させるためのコンピュータ読取り可能プログラム命令を有するコンピュータ読取り可能な1つ(または複数)の記憶媒体を含んでもよい。
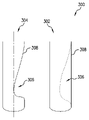
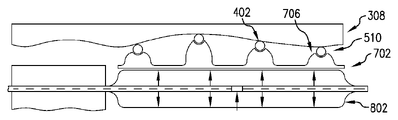
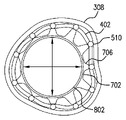
302 非狭窄形状
304 狭窄形状
306 狭窄部
308 血管壁
402 個別化冠状動脈ステント
403 汎用ステント・テンプレート
406 候補構成
416 しぼんだ構成
412 慣性力
418 修正されたステント・テンプレート
420 新しい候補構成
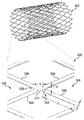
500 細部
502,504 アーチ
506 支柱
508 アーチの頂点
510 ブリッジ
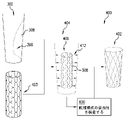
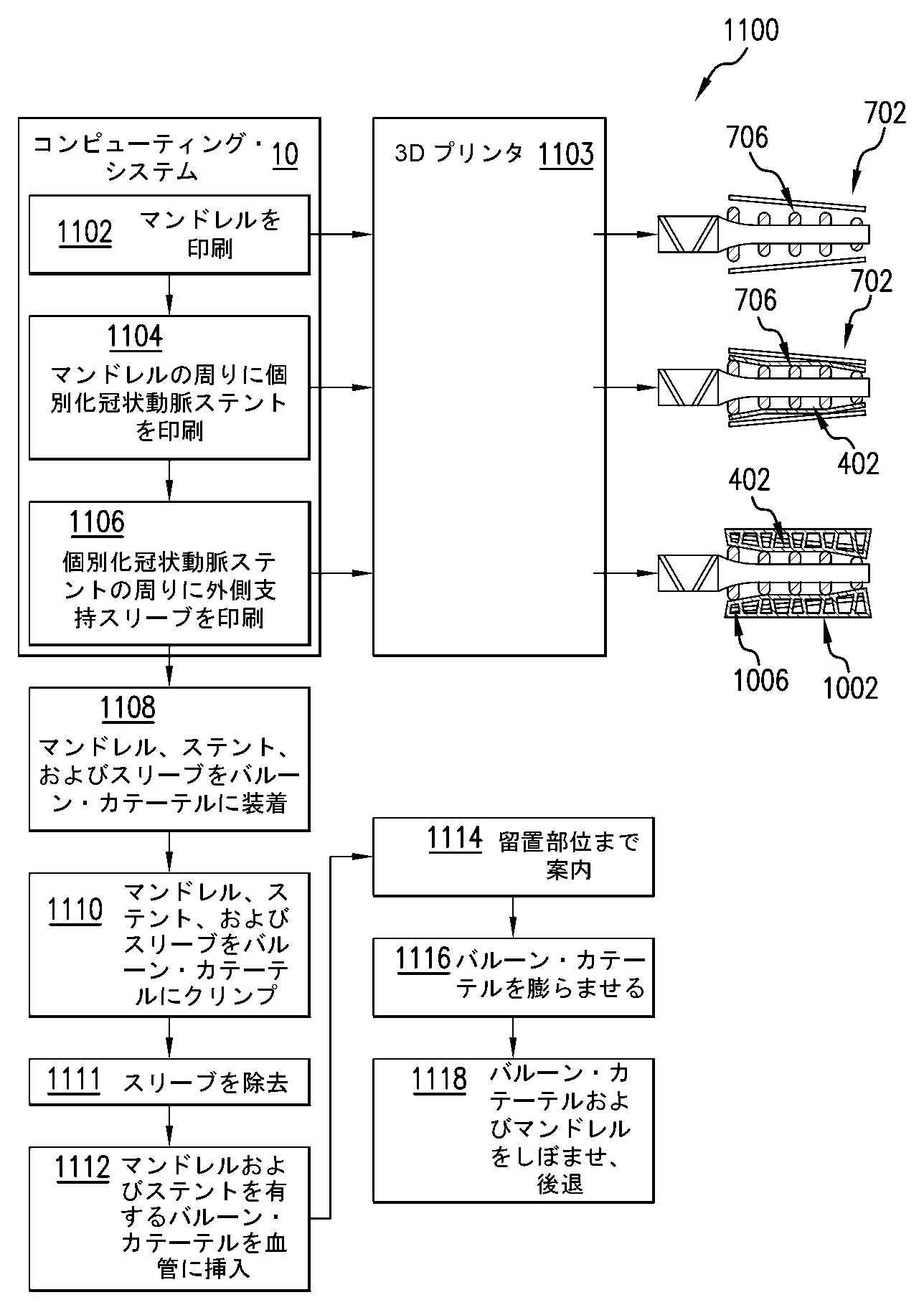
702 マンドレル
704 マンドレル・テンプレート
705 膜
706 柱
707 案内区分
708 マーカ
802 バルーン・カテーテル
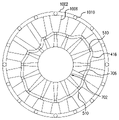
1002 スリーブ
1006 指部
1010 ブリッジ
Claims (12)
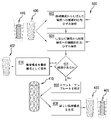
- ステントを提供する方法であって、
血管の実際の形状の三次元(3D)モデルに応答して、前記血管の非狭窄形状の3Dモデルを生成することと、
ステントのパラメータ記述を確立することであって、前記ステントは、複数の支柱を含み、前記ステントは、血管に挿入できる所定の構成から、前記複数の支柱の間の間隙が広がることにより前記非狭窄形状に並置される最終構成に拡張することができるものであり、前記パラメータ記述が、前記ステントの支柱の寸法を特徴付けるパラメータを含む、前記確立することと、
機械的応力/ひずみ解析により、前記所定の構成と前記最終構成の間での前記ステントの塑性変形中に前記支柱が破損するリスクを有するか否かの判断をすることと、前記リスクを有すると判断されたことに従って前記パラメータ記述のパラメータを変えて前記判断を反復することとを含む発見的設計によって、前記ステント用の設計を作り出すことと、
前記ステント用の前記設計に従って前記ステントを具体化することと
を含む方法。 - 前記所定の構成の前記ステントを支持するマンドレルの形状を確立することと、
前記マンドレルの前記形状に従って前記マンドレルを具体化することと
をさらに含む、請求項1に記載の方法。 - 前記マンドレルの前記形状を確立することが、前記マンドレルの膜から突出する複数の柱を確立することであって、前記柱のうちの少なくとも1つが、前記柱のうちの少なくとも1つの他の柱との比較において、前記マンドレルの中心軸から異なる距離まで延在する、前記確立することを含む、請求項2に記載の方法。
- 前記マンドレルの前記形状を確立することが、前記所定の構成の前記ステントのブリッジを支持するように、前記マンドレルの前記柱を構成することを含む、請求項3に記載の方法。
- 前記マンドレルの前記形状を確立することが、前記マンドレルが伸長形状に拡張されたときに前記最終構成の前記ステントのブリッジも支持するように、前記マンドレルの前記柱を構成することを含む、請求項4に記載の方法。
- 前記マンドレルを具体化することが、前記マンドレルを3D印刷することを含み、前記ステントを具体化することが、前記ステントのブリッジが前記マンドレルの前記柱に位置合わせされるように前記マンドレルの周りに前記ステントを3D印刷することを含む、請求項3ないし5のいずれかに記載の方法。
- 前記ステントを具体化することが、前記最終構成の前記ステントを3D印刷することを含み、前記方法は、
前記最終構成から前記所定の構成に前記ステントをクリンプするのを容易にするようにスリーブの形状を確立することと、
前記スリーブの前記形状に従って前記スリーブを具体化することと
をさらに含む、請求項1ないし6のいずれかに記載の方法。 - 前記スリーブの前記形状が、概ね円筒形の本体、および前記本体から内向きに突出する複数の指部を含む、請求項7に記載の方法。
- 前記スリーブの前記形状を確立することが、前記指部のそれぞれに対応する前記ステントのブリッジを前記スリーブの中心軸に向く方向に均一な長さで変位させることによって前記ステントをその最終構成からその所定の構成に圧縮するように前記スリーブの前記指部を構成することを含む、請求項8に記載の方法。
- 前記スリーブを具体化することが、前記ステントの周りに前記スリーブを3D印刷することを含む、請求項7ないし9のいずれかに記載の方法。
- 前記所定の構成の前記ステントを支持するマンドレルの形状を確立することと、
前記マンドレルの前記形状に従って前記マンドレルを具体化することと、
前記ステントをその最終構成で前記マンドレルの周りに配置することと、
前記最終構成から前記所定の構成に前記ステントをクリンプするのを容易にするようにスリーブの形状を確立することと、
前記スリーブの前記形状に従って前記スリーブを具体化することと、
前記ステントの周りに前記スリーブを配置することと、
前記スリーブを使用して前記ステントの非対称的な複数のブリッジに前記スリーブの中心軸を向く均一な大きさの力を分散させることによって、前記マンドレルに前記ステントをクリンプすることと
をさらに含む、請求項1ないし10のいずれかに記載の方法。 - コンピュータ読取り可能媒体に記憶され、デジタル・コンピュータの内部メモリにロード可能なコンピュータ・プログラムであって、前記プログラムがコンピュータ上で走らされたときに、請求項1ないし11のいずれかに記載の方法を実施するためのソフトウェア・コード部分を含む、コンピュータ・プログラム。
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/651,197 | 2017-07-17 | ||
| US15/651,197 US10568696B2 (en) | 2017-07-17 | 2017-07-17 | Apparatus for supporting personalized coronary stents |
| US15/859,558 | 2017-12-31 | ||
| US15/859,558 US10568697B2 (en) | 2017-07-17 | 2017-12-31 | Personalized coronary stent methods |
| JP2020501177A JP7164928B2 (ja) | 2017-07-17 | 2018-07-16 | 個別化冠状動脈ステント |
| PCT/IB2018/055253 WO2019016676A1 (en) | 2017-07-17 | 2018-07-16 | PERSONALIZED CORONARY ENDOPROSTHESES |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020501177A Division JP7164928B2 (ja) | 2017-07-17 | 2018-07-16 | 個別化冠状動脈ステント |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2022166313A true JP2022166313A (ja) | 2022-11-01 |
Family
ID=65000269
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020501177A Active JP7164928B2 (ja) | 2017-07-17 | 2018-07-16 | 個別化冠状動脈ステント |
| JP2022134599A Pending JP2022166313A (ja) | 2017-07-17 | 2022-08-26 | 個別化冠状動脈ステント |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020501177A Active JP7164928B2 (ja) | 2017-07-17 | 2018-07-16 | 個別化冠状動脈ステント |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US10568696B2 (ja) |
| JP (2) | JP7164928B2 (ja) |
| CN (1) | CN110891529B (ja) |
| DE (1) | DE112018003648B4 (ja) |
| GB (1) | GB2579290B (ja) |
| WO (1) | WO2019016676A1 (ja) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10568696B2 (en) | 2017-07-17 | 2020-02-25 | International Business Machines Corporation | Apparatus for supporting personalized coronary stents |
| WO2020185851A1 (en) * | 2019-03-11 | 2020-09-17 | The Board Of Supervisors Of Louisiana State University And Agricultural And Mechanical College | Anastomosing stent and methods of use |
| CN110403706B (zh) * | 2019-06-28 | 2021-06-01 | 吉林大学 | 一种结直肠术后肿瘤标本经肛取出充气式通道装置 |
| AU2020309595A1 (en) * | 2019-07-11 | 2022-02-24 | New Cos Inc. | System and method for model-based stent design and placement |
| US12115089B2 (en) | 2020-03-26 | 2024-10-15 | Medtronic, Inc. | Clamshell iris-style crimper for medical devices |
| US11844713B2 (en) * | 2020-08-06 | 2023-12-19 | Medtronic, Inc. | Iris-style crimpers for medical devices |
| CN112741690B (zh) * | 2020-12-31 | 2022-04-19 | 杭州脉流科技有限公司 | 用于血管支架释放的模拟方法、装置、计算机设备和存储介质 |
| CN113283023B (zh) * | 2021-04-30 | 2024-05-14 | 中国空间技术研究院 | 一种通用的航天器设备安装支架参数化构型设计方法 |
| WO2023086385A1 (en) * | 2021-11-09 | 2023-05-19 | Infinite Composites, Inc. | Additive manufacturing process for high performance composite pressure vessels and structures |
| CN116211463B (zh) * | 2023-05-05 | 2023-08-01 | 杭州脉流科技有限公司 | 一种编织支架虚拟套叠装置 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004528858A (ja) * | 2000-10-04 | 2004-09-24 | トライバスキュラー・インコーポレイテッド | 医療デバイスの開発のための仮想的原型作製および試験 |
Family Cites Families (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5554181A (en) * | 1994-05-04 | 1996-09-10 | Regents Of The University Of Minnesota | Stent |
| US5776141A (en) * | 1995-08-28 | 1998-07-07 | Localmed, Inc. | Method and apparatus for intraluminal prosthesis delivery |
| US5935135A (en) * | 1995-09-29 | 1999-08-10 | United States Surgical Corporation | Balloon delivery system for deploying stents |
| US5658311A (en) * | 1996-07-05 | 1997-08-19 | Schneider (Usa) Inc. | High pressure expander bundle for large diameter stent deployment |
| US6027510A (en) * | 1997-12-08 | 2000-02-22 | Inflow Dynamics Inc. | Stent delivery system |
| US5938697A (en) | 1998-03-04 | 1999-08-17 | Scimed Life Systems, Inc. | Stent having variable properties |
| US6558415B2 (en) | 1998-03-27 | 2003-05-06 | Intratherapeutics, Inc. | Stent |
| US6547814B2 (en) * | 1998-09-30 | 2003-04-15 | Impra, Inc. | Selective adherence of stent-graft coverings |
| US6273910B1 (en) | 1999-03-11 | 2001-08-14 | Advanced Cardiovascular Systems, Inc. | Stent with varying strut geometry |
| US6258099B1 (en) * | 1999-03-31 | 2001-07-10 | Scimed Life Systems, Inc. | Stent security balloon/balloon catheter |
| US6048350A (en) * | 1999-06-14 | 2000-04-11 | Scimed Life Systems, Inc. | Segmented balloon delivery system |
| ATE514392T1 (de) * | 2000-07-24 | 2011-07-15 | Jeffrey Grayzel | Versteifter ballonkatheter zur ausdehnung und anbringung von stents |
| US20020068968A1 (en) * | 2000-08-16 | 2002-06-06 | Thomas Hupp | Virtual stent making process based upon novel enhanced plate tectonics derived from endoluminal mapping |
| US7327862B2 (en) | 2001-04-30 | 2008-02-05 | Chase Medical, L.P. | System and method for facilitating cardiac intervention |
| US6695920B1 (en) * | 2001-06-27 | 2004-02-24 | Advanced Cardiovascular Systems, Inc. | Mandrel for supporting a stent and a method of using the mandrel to coat a stent |
| US6679836B2 (en) | 2002-06-21 | 2004-01-20 | Scimed Life Systems, Inc. | Universal programmable guide catheter |
| WO2004068406A2 (en) | 2003-01-30 | 2004-08-12 | Chase Medical, L.P. | A method and system for image processing and contour assessment |
| US7776078B2 (en) * | 2003-05-22 | 2010-08-17 | Boston Scientfic Scimed, Inc. | Catheter balloon with improved retention |
| US8042485B1 (en) * | 2003-12-30 | 2011-10-25 | Advanced Cardiovascular Systems, Inc. | Stent mandrel fixture and method for coating stents |
| US20110066222A1 (en) * | 2009-09-11 | 2011-03-17 | Yunbing Wang | Polymeric Stent and Method of Making Same |
| US20070118243A1 (en) | 2005-10-14 | 2007-05-24 | Vantus Technology Corporation | Personal fit medical implants and orthopedic surgical instruments and methods for making |
| US20070100420A1 (en) | 2005-11-02 | 2007-05-03 | Kavanagh Joseph T | Guided stent delivery systems of minimal diameter |
| US20070293936A1 (en) * | 2006-04-28 | 2007-12-20 | Dobak John D Iii | Systems and methods for creating customized endovascular stents and stent grafts |
| US8551155B2 (en) * | 2006-06-16 | 2013-10-08 | The Invention Science Fund I, Llc | Stent customization system and method |
| US7818084B2 (en) * | 2006-06-16 | 2010-10-19 | The Invention Science Fund, I, LLC | Methods and systems for making a blood vessel sleeve |
| DE07841109T1 (de) * | 2006-08-22 | 2010-02-11 | Abbott Cardiovascular Systems Inc., Santa Clara | Intravaskulärer stent |
| US8165360B2 (en) * | 2006-12-06 | 2012-04-24 | Siemens Medical Solutions Usa, Inc. | X-ray identification of interventional tools |
| US8200466B2 (en) | 2008-07-21 | 2012-06-12 | The Board Of Trustees Of The Leland Stanford Junior University | Method for tuning patient-specific cardiovascular simulations |
| US9597214B2 (en) * | 2008-10-10 | 2017-03-21 | Kevin Heraty | Medical device |
| US8311312B1 (en) * | 2009-05-14 | 2012-11-13 | Abbott Cardiovascular Systems Inc. | Apparatus, systems and methods for accepting or rejecting a manufactured medical device |
| US9566177B2 (en) * | 2009-10-06 | 2017-02-14 | Artertial Remodeling Technologies, S.A. | Bioresorbable vascular implant having homogenously distributed stresses under a radial load |
| US20140072610A1 (en) | 2011-03-21 | 2014-03-13 | National University Of Singapore | Bioabsorbable tracheal stent, and method of manufacturing thereof |
| US8852257B2 (en) | 2011-06-21 | 2014-10-07 | Abbott Cardiovascular Systems Inc. | Sheaths used with polymer scaffold |
| EP2763623B1 (en) | 2011-10-07 | 2016-07-06 | Materialise N.V. | Methods for the manufacture of intraluminal endoprosthesis |
| US9782282B2 (en) * | 2011-11-14 | 2017-10-10 | W. L. Gore & Associates, Inc. | External steerable fiber for use in endoluminal deployment of expandable devices |
| JP6271555B2 (ja) | 2012-09-21 | 2018-01-31 | マテリアライズ・ナムローゼ・フエンノートシャップMaterialise Nv | 患者固有の腔内インプラント |
| MX2015004101A (es) * | 2012-10-01 | 2015-07-06 | Bard Inc C R | Cateter con globo que tiene multiples lumenes inflables y metodos relacionados. |
| EP2956823B2 (en) | 2013-02-12 | 2019-07-03 | CARBON3D, Inc. | Continuous liquid interphase printing |
| EP2991587A4 (en) * | 2013-05-01 | 2016-05-18 | Aneumed Inc | PERSONALIZED AORTIC FLUTE PROSTHESIS |
| US9043191B2 (en) | 2013-08-16 | 2015-05-26 | Heartflow, Inc. | Systems and methods for identifying personalized vascular implants from patient-specific anatomic data |
| US10098771B2 (en) | 2013-09-25 | 2018-10-16 | Abbott Cardiovascular Systems Inc. | Clip sheath for a polymer scaffold |
| US20150105852A1 (en) * | 2013-10-16 | 2015-04-16 | Covidien Lp | Vascular Stent |
| EP3171763B1 (en) | 2014-07-24 | 2019-07-17 | Lightlab Imaging, Inc. | Stent and vessel visualization and diagnostic methods |
| CN104224412B (zh) * | 2014-08-20 | 2016-08-17 | 湖南瀚德微创医疗科技有限公司 | 一种基于3d打印技术制备血管内支架的方法 |
| CN107847330B (zh) * | 2015-03-03 | 2020-04-03 | 埃夫莫拉尔医疗有限责任公司 | 多元生物可吸收血管内支架 |
| US9943627B2 (en) | 2015-03-03 | 2018-04-17 | Yujie Zhou | Method of producing personalized biomimetic drug-eluting coronary stents by 3D-printing |
| CN105877881A (zh) | 2015-03-03 | 2016-08-24 | 周玉杰 | 利用3d打印技术制备个性化仿生药物洗脱冠状动脉支架的方法 |
| WO2016172629A1 (en) | 2015-04-22 | 2016-10-27 | Aneumed, Inc. | Personalized prosthesis and methods of deployment |
| DE102015207596A1 (de) * | 2015-04-24 | 2016-10-27 | Siemens Healthcare Gmbh | Verfahren sowie Rechen- und Druckeinheit zum Erstellen einer Gefäßstütze |
| US20180117219A1 (en) * | 2015-04-29 | 2018-05-03 | Northwestern University | 3d printing of biomedical implants |
| CN105853036B (zh) | 2016-05-18 | 2017-12-26 | 周玉杰 | 一种可降解个性化非柱形仿生药物洗脱冠状动脉支架 |
| CN106491241A (zh) * | 2016-11-21 | 2017-03-15 | 清华大学 | 一种主动脉覆膜支架的成型方法 |
| US10251708B2 (en) | 2017-04-26 | 2019-04-09 | International Business Machines Corporation | Intravascular catheter for modeling blood vessels |
| US11026583B2 (en) | 2017-04-26 | 2021-06-08 | International Business Machines Corporation | Intravascular catheter including markers |
| US10568696B2 (en) | 2017-07-17 | 2020-02-25 | International Business Machines Corporation | Apparatus for supporting personalized coronary stents |
-
2017
- 2017-07-17 US US15/651,197 patent/US10568696B2/en active Active
- 2017-12-31 US US15/859,558 patent/US10568697B2/en active Active
-
2018
- 2018-07-16 DE DE112018003648.0T patent/DE112018003648B4/de active Active
- 2018-07-16 WO PCT/IB2018/055253 patent/WO2019016676A1/en not_active Ceased
- 2018-07-16 GB GB2001224.1A patent/GB2579290B/en active Active
- 2018-07-16 CN CN201880047323.2A patent/CN110891529B/zh active Active
- 2018-07-16 JP JP2020501177A patent/JP7164928B2/ja active Active
-
2020
- 2020-01-20 US US16/747,060 patent/US11660141B2/en active Active
-
2022
- 2022-08-26 JP JP2022134599A patent/JP2022166313A/ja active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004528858A (ja) * | 2000-10-04 | 2004-09-24 | トライバスキュラー・インコーポレイテッド | 医療デバイスの開発のための仮想的原型作製および試験 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190015159A1 (en) | 2019-01-17 |
| CN110891529B (zh) | 2021-12-03 |
| GB2579290A (en) | 2020-06-17 |
| GB202001224D0 (en) | 2020-03-11 |
| CN110891529A (zh) | 2020-03-17 |
| US11660141B2 (en) | 2023-05-30 |
| JP7164928B2 (ja) | 2022-11-02 |
| US10568697B2 (en) | 2020-02-25 |
| DE112018003648T5 (de) | 2020-04-23 |
| JP2020528301A (ja) | 2020-09-24 |
| US20200163720A1 (en) | 2020-05-28 |
| US20190015158A1 (en) | 2019-01-17 |
| US10568696B2 (en) | 2020-02-25 |
| WO2019016676A1 (en) | 2019-01-24 |
| DE112018003648B4 (de) | 2024-09-12 |
| GB2579290B (en) | 2022-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7164928B2 (ja) | 個別化冠状動脈ステント | |
| US11026749B1 (en) | Computational simulation platform for planning of interventional procedures | |
| US20250235264A1 (en) | Computational simulation platform for planning of interventional procedures | |
| US11026583B2 (en) | Intravascular catheter including markers | |
| US10390888B2 (en) | Intravascular catheter for modeling blood vessels | |
| CN100452095C (zh) | 从投影照片进行对象的三维重构 | |
| Cai et al. | VR simulated training for less invasive vascular intervention | |
| Li et al. | A Novel FEM‐Based Numerical Solver for Interactive Catheter Simulation in Virtual Catheterization | |
| Wu et al. | A Preliminary Real‐Time and Realistic Simulation Environment for Percutaneous Coronary Intervention | |
| KR102806459B1 (ko) | 가이드와이어 팁의 형상 결정 방법 및 장치 | |
| WO2023152237A1 (en) | Method for simulating a device deployment | |
| Valencia et al. | 3D models for vascular lumen segmentation in MRA images and for artery-stenting simulation | |
| Qiu et al. | An improved real-time endovascular guidewire position simulation using shortest path algorithm | |
| EP4623833A1 (en) | Providing feedback during balloon angioplasty | |
| HK40075241B (zh) | 用於模拟可植入医疗装置植入後的变形的方法 | |
| HK40077780A (en) | Computational simulation platform for planning of interventional procedures | |
| Cai et al. | Using VR technology for training in minimally invasive vascular surgery | |
| Khaleel et al. | Voice activation visualisation of cardiovascular angiography and 3D coronary arteries in surgery | |
| Iannaccone | Computer simulations in stroke prevention: design tools and strategies towards virtual procedure planning | |
| Selvarasu | Investigation of the Hemodynamics of Coronary Arteries-Effect of Stenting | |
| Vayalappil | An Automated Instrumentation System for Expansion and Deployment Characterization of Tubular Stent Devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20220830 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20230825 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20230919 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20240319 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240702 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20240724 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20240913 |