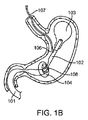
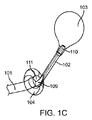
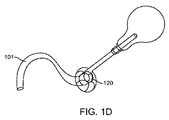
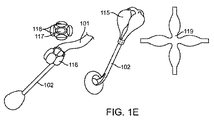
JP2014533981A - 胃内インプラントデバイス - Google Patents
胃内インプラントデバイス Download PDFInfo
- Publication number
- JP2014533981A JP2014533981A JP2014533674A JP2014533674A JP2014533981A JP 2014533981 A JP2014533981 A JP 2014533981A JP 2014533674 A JP2014533674 A JP 2014533674A JP 2014533674 A JP2014533674 A JP 2014533674A JP 2014533981 A JP2014533981 A JP 2014533981A
- Authority
- JP
- Japan
- Prior art keywords
- sleeve
- proximal
- anchor
- implant
- distal
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000007943 implant Substances 0.000 title claims abstract description 167
- 210000002784 stomach Anatomy 0.000 claims abstract description 148
- 210000001035 gastrointestinal tract Anatomy 0.000 claims abstract description 85
- 230000000968 intestinal effect Effects 0.000 claims abstract description 72
- 238000004873 anchoring Methods 0.000 claims abstract description 12
- 238000005452 bending Methods 0.000 claims abstract description 6
- 210000001198 duodenum Anatomy 0.000 claims description 62
- 238000000034 method Methods 0.000 claims description 53
- 230000002183 duodenal effect Effects 0.000 claims description 41
- 239000000463 material Substances 0.000 claims description 39
- 210000000936 intestine Anatomy 0.000 claims description 32
- 230000033001 locomotion Effects 0.000 claims description 31
- 210000001519 tissue Anatomy 0.000 claims description 22
- 230000002093 peripheral effect Effects 0.000 claims description 17
- 230000010339 dilation Effects 0.000 claims description 7
- 230000037406 food intake Effects 0.000 claims description 6
- 230000007797 corrosion Effects 0.000 claims description 4
- 238000005260 corrosion Methods 0.000 claims description 4
- 230000006835 compression Effects 0.000 claims description 3
- 238000007906 compression Methods 0.000 claims description 3
- 230000029087 digestion Effects 0.000 claims description 2
- 208000014674 injury Diseases 0.000 claims description 2
- 230000008733 trauma Effects 0.000 claims description 2
- 210000004877 mucosa Anatomy 0.000 claims 3
- 230000000916 dilatatory effect Effects 0.000 claims 1
- 239000002245 particle Substances 0.000 abstract description 28
- 230000002496 gastric effect Effects 0.000 abstract description 23
- 238000011282 treatment Methods 0.000 abstract description 22
- 230000001225 therapeutic effect Effects 0.000 abstract description 21
- 208000008589 Obesity Diseases 0.000 abstract description 19
- 235000020824 obesity Nutrition 0.000 abstract description 18
- 201000010099 disease Diseases 0.000 abstract description 15
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 15
- 206010012601 diabetes mellitus Diseases 0.000 abstract description 9
- 210000001187 pylorus Anatomy 0.000 description 35
- 210000000813 small intestine Anatomy 0.000 description 29
- 239000004743 Polypropylene Substances 0.000 description 17
- -1 polypropylene Polymers 0.000 description 17
- 229920001155 polypropylene Polymers 0.000 description 17
- 229920000642 polymer Polymers 0.000 description 15
- 230000008878 coupling Effects 0.000 description 14
- 238000010168 coupling process Methods 0.000 description 14
- 238000005859 coupling reaction Methods 0.000 description 14
- 239000004811 fluoropolymer Substances 0.000 description 14
- 229920002313 fluoropolymer Polymers 0.000 description 14
- 235000015097 nutrients Nutrition 0.000 description 14
- 229910001000 nickel titanium Inorganic materials 0.000 description 13
- 229920001971 elastomer Polymers 0.000 description 11
- 239000000806 elastomer Substances 0.000 description 11
- 229920001296 polysiloxane Polymers 0.000 description 11
- 229920003031 santoprene Polymers 0.000 description 11
- 229920002725 thermoplastic elastomer Polymers 0.000 description 11
- 230000002572 peristaltic effect Effects 0.000 description 10
- 230000003044 adaptive effect Effects 0.000 description 9
- 210000001630 jejunum Anatomy 0.000 description 9
- ORQBXQOJMQIAOY-UHFFFAOYSA-N nobelium Chemical compound [No] ORQBXQOJMQIAOY-UHFFFAOYSA-N 0.000 description 8
- 229910001220 stainless steel Inorganic materials 0.000 description 8
- 239000012530 fluid Substances 0.000 description 7
- 239000010935 stainless steel Substances 0.000 description 7
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 6
- 239000000853 adhesive Substances 0.000 description 6
- 230000002503 metabolic effect Effects 0.000 description 6
- 229920002635 polyurethane Polymers 0.000 description 6
- 239000004814 polyurethane Substances 0.000 description 6
- 230000001070 adhesive effect Effects 0.000 description 5
- 210000003484 anatomy Anatomy 0.000 description 5
- 210000002249 digestive system Anatomy 0.000 description 5
- 238000006073 displacement reaction Methods 0.000 description 5
- 239000000835 fiber Substances 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- 235000016709 nutrition Nutrition 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 230000028327 secretion Effects 0.000 description 5
- 206010020772 Hypertension Diseases 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 229910052788 barium Inorganic materials 0.000 description 4
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 239000002131 composite material Substances 0.000 description 4
- 210000003238 esophagus Anatomy 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 210000003041 ligament Anatomy 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 230000035764 nutrition Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 229920002379 silicone rubber Polymers 0.000 description 4
- 230000001154 acute effect Effects 0.000 description 3
- 210000004913 chyme Anatomy 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 230000001079 digestive effect Effects 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 229920001477 hydrophilic polymer Polymers 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 239000002923 metal particle Substances 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 229920000052 poly(p-xylylene) Polymers 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 210000005070 sphincter Anatomy 0.000 description 3
- 229910001256 stainless steel alloy Inorganic materials 0.000 description 3
- 229910052715 tantalum Inorganic materials 0.000 description 3
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 102000012004 Ghrelin Human genes 0.000 description 2
- 101800001586 Ghrelin Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 239000013536 elastomeric material Substances 0.000 description 2
- 229920005570 flexible polymer Polymers 0.000 description 2
- 238000002594 fluoroscopy Methods 0.000 description 2
- GNKDKYIHGQKHHM-RJKLHVOGSA-N ghrelin Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)CN)COC(=O)CCCCCCC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1N=CNC=1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=CC=CC=C1 GNKDKYIHGQKHHM-RJKLHVOGSA-N 0.000 description 2
- 235000003642 hunger Nutrition 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 230000037390 scarring Effects 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- 238000003466 welding Methods 0.000 description 2
- 206010061297 Mucosal erosion Diseases 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 206010053648 Vascular occlusion Diseases 0.000 description 1
- 239000004063 acid-resistant material Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- 238000009954 braiding Methods 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 208000012696 congenital leptin deficiency Diseases 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 102000038379 digestive enzymes Human genes 0.000 description 1
- 108091007734 digestive enzymes Proteins 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 210000005095 gastrointestinal system Anatomy 0.000 description 1
- 238000009998 heat setting Methods 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000002390 hyperplastic effect Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 238000012806 monitoring device Methods 0.000 description 1
- 208000001022 morbid obesity Diseases 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 230000000242 pagocytic effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000008855 peristalsis Effects 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 210000004203 pyloric antrum Anatomy 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000013464 silicone adhesive Substances 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 230000037339 smooth wrinkles Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 239000012815 thermoplastic material Substances 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 208000021331 vascular occlusion disease Diseases 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14503—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue invasive, e.g. introduced into the body by a catheter or needle or using implanted sensors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14507—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue specially adapted for measuring characteristics of body fluids other than blood
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/42—Detecting, measuring or recording for evaluating the gastrointestinal, the endocrine or the exocrine systems
- A61B5/4222—Evaluating particular parts, e.g. particular organs
- A61B5/4238—Evaluating particular parts, e.g. particular organs stomach
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6846—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be brought in contact with an internal body part, i.e. invasive
- A61B5/6879—Means for maintaining contact with the body
- A61B5/6884—Clamps or clips
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F5/00—Orthopaedic methods or devices for non-surgical treatment of bones or joints; Nursing devices ; Anti-rape devices
- A61F5/0003—Apparatus for the treatment of obesity; Anti-eating devices
- A61F5/0013—Implantable devices or invasive measures
- A61F5/0036—Intragastrical devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F5/00—Orthopaedic methods or devices for non-surgical treatment of bones or joints; Nursing devices ; Anti-rape devices
- A61F5/0003—Apparatus for the treatment of obesity; Anti-eating devices
- A61F5/0013—Implantable devices or invasive measures
- A61F5/0076—Implantable devices or invasive measures preventing normal digestion, e.g. Bariatric or gastric sleeves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F5/00—Orthopaedic methods or devices for non-surgical treatment of bones or joints; Nursing devices ; Anti-rape devices
- A61F5/0003—Apparatus for the treatment of obesity; Anti-eating devices
- A61F5/0013—Implantable devices or invasive measures
- A61F5/0076—Implantable devices or invasive measures preventing normal digestion, e.g. Bariatric or gastric sleeves
- A61F5/0079—Pyloric or esophageal obstructions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F5/00—Orthopaedic methods or devices for non-surgical treatment of bones or joints; Nursing devices ; Anti-rape devices
- A61F5/0003—Apparatus for the treatment of obesity; Anti-eating devices
- A61F5/0089—Instruments for placement or removal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M29/00—Dilators with or without means for introducing media, e.g. remedies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2560/00—Constructional details of operational features of apparatus; Accessories for medical measuring apparatus
- A61B2560/02—Operational features
- A61B2560/0204—Operational features of power management
- A61B2560/0214—Operational features of power management of power generation or supply
- A61B2560/0219—Operational features of power management of power generation or supply of externally powered implanted units
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2560/00—Constructional details of operational features of apparatus; Accessories for medical measuring apparatus
- A61B2560/02—Operational features
- A61B2560/0266—Operational features for monitoring or limiting apparatus function
- A61B2560/028—Arrangements to prevent overuse, e.g. by counting the number of uses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2560/00—Constructional details of operational features of apparatus; Accessories for medical measuring apparatus
- A61B2560/06—Accessories for medical measuring apparatus
- A61B2560/063—Devices specially adapted for delivering implantable medical measuring apparatus
- A61B2560/066—Devices specially adapted for delivering implantable medical measuring apparatus catheters therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14539—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue for measuring pH
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6846—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be brought in contact with an internal body part, i.e. invasive
- A61B5/6847—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be brought in contact with an internal body part, i.e. invasive mounted on an invasive device
- A61B5/686—Permanently implanted devices, e.g. pacemakers, other stimulators, biochips
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Physics & Mathematics (AREA)
- Vascular Medicine (AREA)
- Medical Informatics (AREA)
- Biophysics (AREA)
- Surgery (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- Nursing (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Child & Adolescent Psychology (AREA)
- Obesity (AREA)
- Gastroenterology & Hepatology (AREA)
- Optics & Photonics (AREA)
- Pulmonology (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Endocrinology (AREA)
- Physiology (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Surgical Instruments (AREA)
- Prostheses (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161539814P | 2011-09-27 | 2011-09-27 | |
| US61/539,814 | 2011-09-27 | ||
| PCT/US2012/057288 WO2013049167A1 (en) | 2011-09-27 | 2012-09-26 | Intragastric implant devices |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2014533981A true JP2014533981A (ja) | 2014-12-18 |
| JP2014533981A5 JP2014533981A5 (enExample) | 2015-11-19 |
Family
ID=47912004
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014533674A Pending JP2014533981A (ja) | 2011-09-27 | 2012-09-26 | 胃内インプラントデバイス |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9173734B2 (enExample) |
| EP (1) | EP2760523A1 (enExample) |
| JP (1) | JP2014533981A (enExample) |
| CN (1) | CN103974741A (enExample) |
| AU (1) | AU2012316159A1 (enExample) |
| CA (1) | CA2850162A1 (enExample) |
| WO (1) | WO2013049167A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021533897A (ja) * | 2018-08-14 | 2021-12-09 | ユナイテッド ステイツ エンドスコピー グループ,インコーポレイテッド | 体内の治療領域を他の領域から分離する装置および方法 |
| JP2023505759A (ja) * | 2019-11-29 | 2023-02-13 | アレヴェティクス メディカル リミテッド | 埋め込み可能なアンカーデバイス及び使用方法 |
| JP2024045363A (ja) * | 2016-04-27 | 2024-04-02 | サイナーズ メディカル,インコーポレイティド | 肥満治療用アンカーレス胃内デバイス |
Families Citing this family (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL144900A (en) | 2001-08-14 | 2013-12-31 | Neurim Pharma 1991 | Melatonin and drugs containing it for use in the treatment of primary insomnia, and the manufacture of those drugs |
| US7097665B2 (en) * | 2003-01-16 | 2006-08-29 | Synecor, Llc | Positioning tools and methods for implanting medical devices |
| US9060844B2 (en) | 2002-11-01 | 2015-06-23 | Valentx, Inc. | Apparatus and methods for treatment of morbid obesity |
| WO2005110280A2 (en) | 2004-05-07 | 2005-11-24 | Valentx, Inc. | Devices and methods for attaching an endolumenal gastrointestinal implant |
| EP2134303A1 (en) * | 2007-03-29 | 2009-12-23 | Jaime Vargas | Intragastric implant devices |
| US10420665B2 (en) | 2010-06-13 | 2019-09-24 | W. L. Gore & Associates, Inc. | Intragastric device for treating obesity |
| US10010439B2 (en) | 2010-06-13 | 2018-07-03 | Synerz Medical, Inc. | Intragastric device for treating obesity |
| US9526648B2 (en) | 2010-06-13 | 2016-12-27 | Synerz Medical, Inc. | Intragastric device for treating obesity |
| US8628554B2 (en) | 2010-06-13 | 2014-01-14 | Virender K. Sharma | Intragastric device for treating obesity |
| US9198791B2 (en) | 2010-07-22 | 2015-12-01 | Endobetix Ltd. | Pancreaticobiliary diversion device |
| FR2978345B1 (fr) | 2011-07-25 | 2013-08-30 | Charam Khosrovaninejad | Dispositif chirurgical d'ancrage controle dans l'intestin. |
| US9681975B2 (en) | 2012-05-31 | 2017-06-20 | Valentx, Inc. | Devices and methods for gastrointestinal bypass |
| US20130324906A1 (en) | 2012-05-31 | 2013-12-05 | Valen Tx, Inc. | Devices and methods for gastrointestinal bypass |
| US8956318B2 (en) | 2012-05-31 | 2015-02-17 | Valentx, Inc. | Devices and methods for gastrointestinal bypass |
| US10137023B2 (en) | 2012-09-14 | 2018-11-27 | Medibotics Llc | Colonnade (TM) expandable intragastric food flow lumen device |
| US9757264B2 (en) | 2013-03-13 | 2017-09-12 | Valentx, Inc. | Devices and methods for gastrointestinal bypass |
| CN105451685A (zh) * | 2013-06-07 | 2016-03-30 | 安朵贝提克斯有限公司 | 胰胆分流装置 |
| US9452072B2 (en) | 2013-06-25 | 2016-09-27 | J. Mark Provenza | Apparatus and methods for anchoring in the stomach and the duodenum |
| US9265640B2 (en) | 2013-08-28 | 2016-02-23 | Ethicon Endo-Surgery, Inc. | Conforming anchor for duodenal barrier |
| WO2015160662A1 (en) * | 2014-04-14 | 2015-10-22 | Mayo Foundation For Medical Education And Research | Gastrointestinal tract bypass devices |
| CN106659562B (zh) | 2014-04-30 | 2018-10-09 | 利恩医疗技术有限公司 | 胃肠装置 |
| US20160067466A1 (en) | 2014-09-05 | 2016-03-10 | Elwha LLC, a limited company of the State of Delaware | Systems, methods, and devices addressing the gastro-intestinal tract |
| US20160066855A1 (en) * | 2014-09-05 | 2016-03-10 | Elwha LLC, a limited liability company of the State of Delaware | Systems, methods, and devices addressing the gastro-intestinal tract |
| US10183154B2 (en) | 2014-09-05 | 2019-01-22 | Elwha Llc | Systems, methods, and devices addressing the gastro-intestinal tract |
| WO2017052694A1 (en) * | 2015-09-23 | 2017-03-30 | Synerz Medical, Inc. | Intragastric device for treating obesity |
| US10299795B2 (en) | 2016-04-28 | 2019-05-28 | Mayo Foundation For Medical Education And Research | Devices and methods for esophageal lengthening and anastomosis formation |
| IT201600097363A1 (it) * | 2016-09-28 | 2018-03-28 | Keyron Ltd | Dispositivo intragastrico espandibile |
| FR3072557B1 (fr) | 2017-10-19 | 2019-11-08 | Safeheal | Dispositif chirurgical complexe pour la realisation et protection d'une anastomose. |
| US11109890B2 (en) * | 2018-01-25 | 2021-09-07 | Kyphon Sarl | Vertebral body access cannula with enhanced bending stiffness |
| CN108261174B (zh) * | 2018-03-13 | 2024-06-07 | 南微医学科技股份有限公司 | 一种内窥镜端帽 |
| US10327938B1 (en) | 2018-05-23 | 2019-06-25 | Allium Medical Solutions Ltd. | Intestinal sleeve |
| EP3597156B1 (en) * | 2018-07-16 | 2021-03-17 | National Taiwan University of Science and Technology | Gastrointestinal implant and method for deploying the same |
| US11737745B2 (en) | 2018-10-24 | 2023-08-29 | Mayo Foundation For Medical Education And Research | Medical devices and methods for body conduit lengthening and anastomosis formation |
| CN109350217B (zh) * | 2018-12-21 | 2021-01-01 | 青岛智兴医疗器械有限公司 | 一种植骨材料用推入器 |
| US20220079791A1 (en) * | 2019-01-03 | 2022-03-17 | Alexander BUTZ | Gastrointestinal implant and positioning apparatus therefor |
| US12161574B2 (en) | 2019-06-14 | 2024-12-10 | Mayo Foundation For Medical Education And Research | Methods and devices for gastricintestinal tract bypass |
| FR3097421A1 (fr) | 2019-06-24 | 2020-12-25 | Safeheal | Dispositif pour le lavement d’une anastomose intestinale in situ |
| CN111544175A (zh) * | 2020-04-27 | 2020-08-18 | 王利 | 减肥水囊 |
| EP4281019A1 (en) * | 2021-04-28 | 2023-11-29 | Boston Scientific Scimed, Inc. | Devices, systems, and methods for duodenal exclusion and stomach capacity reduction |
| CN113057777B (zh) * | 2021-05-06 | 2022-03-25 | 哈尔滨医科大学 | 一种胃内支架减肥装置 |
| US12239321B2 (en) | 2023-03-17 | 2025-03-04 | SafeHeal SAS | Systems and methods for introducing and monitoring a negative pressure device for protecting an intestinal anastomosis |
| CN119075169A (zh) * | 2023-06-05 | 2024-12-06 | 丰凯利医疗器械(上海)有限公司 | 一种流体泵送导管 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050273060A1 (en) * | 2004-06-03 | 2005-12-08 | Mayo Foundation For Medical Education And Research | Obesity treatment and device |
| JP2008505690A (ja) * | 2004-07-09 | 2008-02-28 | ジーアイ・ダイナミックス・インコーポレーテッド | 胃腸内スリーブを配置するための方法および装置 |
| JP2008513129A (ja) * | 2004-09-17 | 2008-05-01 | ジーアイ・ダイナミックス・インコーポレーテッド | 内腔内アンカ装置 |
| JP2009538218A (ja) * | 2006-05-26 | 2009-11-05 | エンドスフィア・インコーポレイテッド | 食欲を抑制し及び/又は食物摂取量を低減する方法及び装置の改良 |
| JP2010537790A (ja) * | 2007-09-07 | 2010-12-09 | バロノバ, インコーポレイテッド | 胃開口部の断続的遮断の装置および使用方法 |
Family Cites Families (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4899747A (en) | 1981-12-10 | 1990-02-13 | Garren Lloyd R | Method and appartus for treating obesity |
| US4485805A (en) | 1982-08-24 | 1984-12-04 | Gunther Pacific Limited Of Hong Kong | Weight loss device and method |
| US4648383A (en) | 1985-01-11 | 1987-03-10 | Angelchik Jean P | Peroral apparatus for morbid obesity treatment |
| US4694827A (en) | 1986-01-14 | 1987-09-22 | Weiner Brian C | Inflatable gastric device for treating obesity and method of using the same |
| GB8603099D0 (en) | 1986-02-07 | 1986-03-12 | Blass K G | Gastrointestinal module |
| US4925446A (en) | 1988-07-06 | 1990-05-15 | Transpharm Group Inc. | Removable inflatable intragastrointestinal device for delivering beneficial agents |
| US5401241A (en) | 1992-05-07 | 1995-03-28 | Inamed Development Co. | Duodenal intubation catheter |
| US5306300A (en) | 1992-09-22 | 1994-04-26 | Berry H Lee | Tubular digestive screen |
| US5868141A (en) | 1997-05-14 | 1999-02-09 | Ellias; Yakub A. | Endoscopic stomach insert for treating obesity and method for use |
| US5820584A (en) | 1997-08-28 | 1998-10-13 | Crabb; Jerry A. | Duodenal insert and method of use |
| US6629987B1 (en) | 1999-07-30 | 2003-10-07 | C. R. Bard, Inc. | Catheter positioning systems |
| US6620122B2 (en) * | 2001-04-26 | 2003-09-16 | Scimed Life Systems, Inc. | Gastric pseudocyst drainage and stent delivery system for use therein |
| US6845776B2 (en) | 2001-08-27 | 2005-01-25 | Richard S. Stack | Satiation devices and methods |
| US6675809B2 (en) | 2001-08-27 | 2004-01-13 | Richard S. Stack | Satiation devices and methods |
| US6755869B2 (en) | 2001-11-09 | 2004-06-29 | Boston Scientific Corporation | Intragastric prosthesis for the treatment of morbid obesity |
| US7211114B2 (en) | 2002-08-26 | 2007-05-01 | The Trustees Of Columbia University In The City Of New York | Endoscopic gastric bypass |
| US7033384B2 (en) | 2002-08-30 | 2006-04-25 | Satiety, Inc. | Stented anchoring of gastric space-occupying devices |
| US7220237B2 (en) | 2002-10-23 | 2007-05-22 | Satiety, Inc. | Method and device for use in endoscopic organ procedures |
| US9060844B2 (en) | 2002-11-01 | 2015-06-23 | Valentx, Inc. | Apparatus and methods for treatment of morbid obesity |
| US7794447B2 (en) | 2002-11-01 | 2010-09-14 | Valentx, Inc. | Gastrointestinal sleeve device and methods for treatment of morbid obesity |
| US7025791B2 (en) * | 2002-12-02 | 2006-04-11 | Gi Dynamics, Inc. | Bariatric sleeve |
| JP4980569B2 (ja) | 2002-12-02 | 2012-07-18 | ジーアイ・ダイナミックス・インコーポレーテッド | 胃腸内埋込装置および該装置を体内に配置する送入システム |
| BR0302240B8 (pt) | 2003-06-24 | 2013-02-19 | balço semi-estacionÁrio no antro gÁstrico com haste de ancoragem para induÇço de emagrecimento em seres humanos. | |
| US7931693B2 (en) | 2004-02-26 | 2011-04-26 | Endosphere, Inc. | Method and apparatus for reducing obesity |
| US8147561B2 (en) | 2004-02-26 | 2012-04-03 | Endosphere, Inc. | Methods and devices to curb appetite and/or reduce food intake |
| US20050197714A1 (en) | 2004-03-02 | 2005-09-08 | Sayet Peter H. | System, system devices, and methods for regulating nutrient absorption and caloric intake |
| EP1750627B1 (en) | 2004-03-26 | 2018-10-10 | Ethicon Endo-Surgery, Inc. | System for reducing stomach volume |
| JP4934024B2 (ja) | 2004-05-03 | 2012-05-16 | フルフィリウム, インコーポレイテッド | 胃の容量を制御するための方法およびシステム |
| WO2005110280A2 (en) | 2004-05-07 | 2005-11-24 | Valentx, Inc. | Devices and methods for attaching an endolumenal gastrointestinal implant |
| US20060142731A1 (en) | 2004-12-27 | 2006-06-29 | Jeffrey Brooks | Floating gastro-intestinal anchor |
| US20070100369A1 (en) | 2005-10-31 | 2007-05-03 | Cragg Andrew H | Intragastric space filler |
| WO2007107990A2 (en) | 2006-03-20 | 2007-09-27 | Svip 2 Llc | Pyloric devices and methods |
| US8070768B2 (en) | 2006-04-19 | 2011-12-06 | Vibrynt, Inc. | Devices and methods for treatment of obesity |
| US9060835B2 (en) * | 2006-05-26 | 2015-06-23 | Endosphere, Inc. | Conformationally-stabilized intraluminal device for medical applications |
| EP2134303A1 (en) * | 2007-03-29 | 2009-12-23 | Jaime Vargas | Intragastric implant devices |
| AU2009239658A1 (en) | 2008-04-23 | 2009-10-29 | Duocure, Inc. | Duodenal liner device |
| US10350050B2 (en) | 2008-05-01 | 2019-07-16 | Ethicon Endo-Surgery, Inc. | Method for gastric volume reduction surgery |
| WO2010075569A1 (en) | 2008-12-24 | 2010-07-01 | Boston Scientific Scimed, Inc. | Methods of surgically modifying the duodenum |
| CA2746989A1 (en) | 2008-12-27 | 2010-07-01 | Chandra S. Duggirala | Devices for treating obesity and methods of using those devices |
| EP2801342A3 (en) | 2009-04-03 | 2015-03-25 | Metamodix, Inc. | Modular gastrointestinal prostheses |
| US20100286628A1 (en) | 2009-05-07 | 2010-11-11 | Rainbow Medical Ltd | Gastric anchor |
| US8574184B2 (en) | 2009-07-01 | 2013-11-05 | E2 Llc | Systems and methods for treatment of obesity and type 2 diabetes |
| US8496608B2 (en) | 2009-07-01 | 2013-07-30 | E2 Llc | Systems and methods for treating obesity and type 2 diabetes |
| WO2011006098A2 (en) | 2009-07-10 | 2011-01-13 | Metamodix, Inc. | External anchoring configurations for modular gastrointestinal prostheses |
| WO2011120047A1 (en) * | 2010-03-26 | 2011-09-29 | IBIS Medical, Inc. | Intragastric implant devices |
-
2012
- 2012-09-26 WO PCT/US2012/057288 patent/WO2013049167A1/en not_active Ceased
- 2012-09-26 AU AU2012316159A patent/AU2012316159A1/en not_active Abandoned
- 2012-09-26 CN CN201280057933.3A patent/CN103974741A/zh active Pending
- 2012-09-26 US US13/627,627 patent/US9173734B2/en not_active Expired - Fee Related
- 2012-09-26 EP EP12837376.8A patent/EP2760523A1/en not_active Withdrawn
- 2012-09-26 JP JP2014533674A patent/JP2014533981A/ja active Pending
- 2012-09-26 CA CA 2850162 patent/CA2850162A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050273060A1 (en) * | 2004-06-03 | 2005-12-08 | Mayo Foundation For Medical Education And Research | Obesity treatment and device |
| JP2008505690A (ja) * | 2004-07-09 | 2008-02-28 | ジーアイ・ダイナミックス・インコーポレーテッド | 胃腸内スリーブを配置するための方法および装置 |
| JP2008513129A (ja) * | 2004-09-17 | 2008-05-01 | ジーアイ・ダイナミックス・インコーポレーテッド | 内腔内アンカ装置 |
| JP2009538218A (ja) * | 2006-05-26 | 2009-11-05 | エンドスフィア・インコーポレイテッド | 食欲を抑制し及び/又は食物摂取量を低減する方法及び装置の改良 |
| JP2010537790A (ja) * | 2007-09-07 | 2010-12-09 | バロノバ, インコーポレイテッド | 胃開口部の断続的遮断の装置および使用方法 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2024045363A (ja) * | 2016-04-27 | 2024-04-02 | サイナーズ メディカル,インコーポレイティド | 肥満治療用アンカーレス胃内デバイス |
| JP2021533897A (ja) * | 2018-08-14 | 2021-12-09 | ユナイテッド ステイツ エンドスコピー グループ,インコーポレイテッド | 体内の治療領域を他の領域から分離する装置および方法 |
| JP7501826B2 (ja) | 2018-08-14 | 2024-06-18 | ユナイテッド ステイツ エンドスコピー グループ,インコーポレイテッド | 体内の治療領域を他の領域から分離する装置および方法 |
| JP2023505759A (ja) * | 2019-11-29 | 2023-02-13 | アレヴェティクス メディカル リミテッド | 埋め込み可能なアンカーデバイス及び使用方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103974741A (zh) | 2014-08-06 |
| WO2013049167A1 (en) | 2013-04-04 |
| US9173734B2 (en) | 2015-11-03 |
| US20130079603A1 (en) | 2013-03-28 |
| CA2850162A1 (en) | 2013-04-04 |
| EP2760523A1 (en) | 2014-08-06 |
| AU2012316159A1 (en) | 2014-04-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2014533981A (ja) | 胃内インプラントデバイス | |
| CN102917666B (zh) | 胃内植入装置 | |
| US8211186B2 (en) | Modular gastrointestinal prostheses | |
| US9744061B2 (en) | Intestinal sleeve | |
| EP1569582B1 (en) | Bariatric sleeve | |
| US7678068B2 (en) | Atraumatic delivery devices | |
| US20150088048A1 (en) | Intragastic implant devices | |
| US10201446B2 (en) | Conforming anchor for duodenal barrier | |
| US10292854B2 (en) | Gastrointestinal tract bypass devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150928 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150928 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20160819 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160823 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20161124 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170123 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20170516 |