JP2013523377A - 乾燥粉末吸入器のマウスピースボタン - Google Patents
乾燥粉末吸入器のマウスピースボタン Download PDFInfo
- Publication number
- JP2013523377A JP2013523377A JP2013504862A JP2013504862A JP2013523377A JP 2013523377 A JP2013523377 A JP 2013523377A JP 2013504862 A JP2013504862 A JP 2013504862A JP 2013504862 A JP2013504862 A JP 2013504862A JP 2013523377 A JP2013523377 A JP 2013523377A
- Authority
- JP
- Japan
- Prior art keywords
- dry powder
- inhaler
- drug
- component
- mouthpiece
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
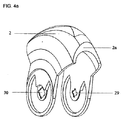
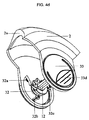
- A61M15/0021—Mouthpieces therefor
- A61M15/0025—Mouthpieces therefor with caps
- A61M15/0026—Hinged caps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0043—Non-destructive separation of the package, e.g. peeling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
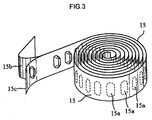
- A61M15/0051—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged on a tape, e.g. strips
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0053—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal
- A61M15/0055—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal the used dosages being coiled
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
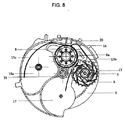
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/007—Mechanical counters
- A61M15/0071—Mechanical counters having a display or indicator
- A61M15/0075—Mechanical counters having a display or indicator on a disc
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/0081—Locking means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/062—Desiccants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0205—Materials having antiseptic or antimicrobial properties, e.g. silver compounds, rubber with sterilising agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0233—Conductive materials, e.g. antistatic coatings for spark prevention
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0238—General characteristics of the apparatus characterised by a particular materials the material being a coating or protective layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/27—General characteristics of the apparatus preventing use
- A61M2205/276—General characteristics of the apparatus preventing use preventing unwanted use
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Anesthesiology (AREA)
- Pulmonology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TR2010/02877A TR201002877A2 (tr) | 2010-04-13 | 2010-04-13 | Blister ambalaj içeren inhalasyon cihazı |
| TR2010/02877 | 2010-04-13 | ||
| TR201003091 | 2010-04-20 | ||
| TR2010/03091 | 2010-04-20 | ||
| TR2010/04310 | 2010-05-28 | ||
| TR201004310 | 2010-05-28 | ||
| PCT/TR2011/000087 WO2011129787A1 (en) | 2010-04-13 | 2011-04-13 | Dry powder inhaler mouthpiece button |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013523377A true JP2013523377A (ja) | 2013-06-17 |
| JP2013523377A5 JP2013523377A5 (enExample) | 2014-05-29 |
Family
ID=44315112
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013504862A Pending JP2013523377A (ja) | 2010-04-13 | 2011-04-13 | 乾燥粉末吸入器のマウスピースボタン |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP2558149A1 (enExample) |
| JP (1) | JP2013523377A (enExample) |
| EA (1) | EA201201393A1 (enExample) |
| IN (1) | IN2012DN04986A (enExample) |
| WO (1) | WO2011129787A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9345848B2 (en) | 2009-10-20 | 2016-05-24 | Sima Patent Ve Lisanslama Hizmetleri Ltd. Sti. | Dry powder inhaler |
| USD733287S1 (en) | 2011-04-13 | 2015-06-30 | Mahmut Bilgic | Dry powder inhaler |
| GB201115154D0 (en) * | 2011-09-01 | 2011-10-19 | Clement Clarke Int Ltd | Spacer |
| USD744087S1 (en) | 2013-10-01 | 2015-11-24 | Mahmut Bilgic | Dry powder inhaler |
| US20190076607A1 (en) | 2017-09-13 | 2019-03-14 | Lupin Atlantis Holdings Sa | Inhaler and mesh for an inhaler |
| CN116173358B (zh) * | 2021-11-29 | 2025-03-28 | 康希诺生物股份公司 | 一种雾化杯及其在雾化吸入给药中的应用 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5025092A (enExample) * | 1973-02-26 | 1975-03-17 | ||
| JPH05507637A (ja) * | 1990-06-14 | 1993-11-04 | ローン―プーラン・ロレ・リミテツド | 吸入器 |
| JP2003528644A (ja) * | 1998-12-23 | 2003-09-30 | バテル・メモリアル・インスティテュート | 肺疾患用エーロゾル送出装置およびエーロゾル化された液体治療薬を経口投与する方法並びに液体をエーロゾル化する装置。 |
| JP2006523486A (ja) * | 2003-04-16 | 2006-10-19 | トルーデル メディカル インターナショナル | 帯電防止型薬剤送出装置 |
| JP2009513203A (ja) * | 2005-10-28 | 2009-04-02 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | 微生物学的保護機能を有するマウスピースを備えた吸入器 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2771296B1 (fr) * | 1997-11-25 | 2000-03-10 | Sofab | Embout nasal avec fermeture d'extremite |
-
2011
- 2011-04-13 WO PCT/TR2011/000087 patent/WO2011129787A1/en not_active Ceased
- 2011-04-13 EA EA201201393A patent/EA201201393A1/ru unknown
- 2011-04-13 EP EP11726524A patent/EP2558149A1/en not_active Withdrawn
- 2011-04-13 JP JP2013504862A patent/JP2013523377A/ja active Pending
-
2012
- 2012-06-06 IN IN4986DEN2012 patent/IN2012DN04986A/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5025092A (enExample) * | 1973-02-26 | 1975-03-17 | ||
| JPH05507637A (ja) * | 1990-06-14 | 1993-11-04 | ローン―プーラン・ロレ・リミテツド | 吸入器 |
| JP2003528644A (ja) * | 1998-12-23 | 2003-09-30 | バテル・メモリアル・インスティテュート | 肺疾患用エーロゾル送出装置およびエーロゾル化された液体治療薬を経口投与する方法並びに液体をエーロゾル化する装置。 |
| JP2006523486A (ja) * | 2003-04-16 | 2006-10-19 | トルーデル メディカル インターナショナル | 帯電防止型薬剤送出装置 |
| JP2009513203A (ja) * | 2005-10-28 | 2009-04-02 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | 微生物学的保護機能を有するマウスピースを備えた吸入器 |
Also Published As
| Publication number | Publication date |
|---|---|
| IN2012DN04986A (enExample) | 2015-10-02 |
| EA201201393A1 (ru) | 2013-05-30 |
| WO2011129787A1 (en) | 2011-10-20 |
| EP2558149A1 (en) | 2013-02-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9345848B2 (en) | Dry powder inhaler | |
| JP6290969B2 (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| JP5855642B2 (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| JP2013523375A (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| DK2451514T3 (en) | Dosage unit, dosage unit pack and inhaler for inhalation of a combination of drugs | |
| JP2013523377A (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| JP5860869B2 (ja) | 吸入デバイス | |
| JP5857034B2 (ja) | ブリスター包装体を有する吸入器 | |
| JP2013523378A (ja) | 乱流を生成する吸入器 | |
| WO2011129788A1 (en) | User-friendly dry powder inhaler | |
| JP5873477B2 (ja) | 乾燥粉末形態の薬剤の送達に使用する吸入器 | |
| WO2011049538A2 (en) | Pharmaceutical composition in dry powder form |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20131009 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140410 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140410 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150127 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20150130 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150401 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150713 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20160120 |