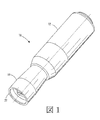
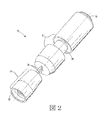
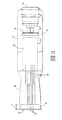
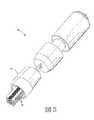
JP2012223560A - コリメートされたガス流及び放出活性化テープを用いる薬剤送達機器及び方法 - Google Patents
コリメートされたガス流及び放出活性化テープを用いる薬剤送達機器及び方法 Download PDFInfo
- Publication number
- JP2012223560A JP2012223560A JP2012061517A JP2012061517A JP2012223560A JP 2012223560 A JP2012223560 A JP 2012223560A JP 2012061517 A JP2012061517 A JP 2012061517A JP 2012061517 A JP2012061517 A JP 2012061517A JP 2012223560 A JP2012223560 A JP 2012223560A
- Authority
- JP
- Japan
- Prior art keywords
- drug
- collimator
- gas
- drug delivery
- delivery device
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000012377 drug delivery Methods 0.000 title claims abstract description 61
- 238000000034 method Methods 0.000 title abstract description 22
- 239000003814 drug Substances 0.000 claims abstract description 142
- 229940079593 drug Drugs 0.000 claims abstract description 138
- 239000002245 particle Substances 0.000 claims description 100
- 239000000463 material Substances 0.000 claims description 18
- 239000007787 solid Substances 0.000 claims description 13
- 238000013271 transdermal drug delivery Methods 0.000 claims description 7
- 239000003795 chemical substances by application Substances 0.000 claims description 6
- 239000000126 substance Substances 0.000 abstract description 20
- 241001465754 Metazoa Species 0.000 abstract description 5
- 239000007789 gas Substances 0.000 description 101
- 210000001519 tissue Anatomy 0.000 description 91
- 210000003491 skin Anatomy 0.000 description 24
- 239000007788 liquid Substances 0.000 description 15
- 210000004379 membrane Anatomy 0.000 description 14
- 239000012528 membrane Substances 0.000 description 14
- 239000003380 propellant Substances 0.000 description 10
- 239000010410 layer Substances 0.000 description 9
- 235000015097 nutrients Nutrition 0.000 description 9
- 239000011148 porous material Substances 0.000 description 9
- 210000000434 stratum corneum Anatomy 0.000 description 9
- 102000029749 Microtubule Human genes 0.000 description 5
- 108091022875 Microtubule Proteins 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 239000002537 cosmetic Substances 0.000 description 5
- 210000004688 microtubule Anatomy 0.000 description 5
- 239000000546 pharmaceutical excipient Substances 0.000 description 5
- 238000003825 pressing Methods 0.000 description 5
- 238000007790 scraping Methods 0.000 description 5
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 230000004888 barrier function Effects 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000009792 diffusion process Methods 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 239000007790 solid phase Substances 0.000 description 3
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 208000034656 Contusions Diseases 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical group [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 210000002510 keratinocyte Anatomy 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 210000004400 mucous membrane Anatomy 0.000 description 2
- 230000000149 penetrating effect Effects 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 239000012730 sustained-release form Substances 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 208000032912 Local swelling Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 230000003444 anaesthetic effect Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000011449 brick Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 239000012159 carrier gas Substances 0.000 description 1
- 238000002144 chemical decomposition reaction Methods 0.000 description 1
- 239000011247 coating layer Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 239000012792 core layer Substances 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 229910001882 dioxygen Inorganic materials 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 238000003487 electrochemical reaction Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000000976 ink Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000008776 intercellular pathway Effects 0.000 description 1
- 229940090046 jet injector Drugs 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 239000003589 local anesthetic agent Substances 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000003990 molecular pathway Effects 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000037368 penetrate the skin Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 210000003786 sclera Anatomy 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000001839 systemic circulation Effects 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 210000004127 vitreous body Anatomy 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0021—Intradermal administration, e.g. through microneedle arrays, needleless injectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0002—Galenical forms characterised by the drug release technique; Application systems commanded by energy
- A61K9/0009—Galenical forms characterised by the drug release technique; Application systems commanded by energy involving or responsive to electricity, magnetism or acoustic waves; Galenical aspects of sonophoresis, iontophoresis, electroporation or electroosmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/178—Syringes
- A61M5/30—Syringes for injection by jet action, without needle, e.g. for use with replaceable ampoules or carpules
- A61M5/3015—Syringes for injection by jet action, without needle, e.g. for use with replaceable ampoules or carpules for injecting a dose of particles in form of powdered drug, e.g. mounted on a rupturable membrane and accelerated by a gaseous shock wave or supersonic gas flow
Landscapes
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Dermatology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Vascular Medicine (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/089,777 US8486002B2 (en) | 2011-04-19 | 2011-04-19 | Drug delivery devices and methods with collimated gas stream and release-activatable tape |
| US13/089,777 | 2011-04-19 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012223560A true JP2012223560A (ja) | 2012-11-15 |
| JP2012223560A5 JP2012223560A5 (enExample) | 2015-05-07 |
Family
ID=46027656
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012061517A Pending JP2012223560A (ja) | 2011-04-19 | 2012-03-19 | コリメートされたガス流及び放出活性化テープを用いる薬剤送達機器及び方法 |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US8486002B2 (enExample) |
| EP (1) | EP2514408B1 (enExample) |
| JP (1) | JP2012223560A (enExample) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10130800B2 (en) | 2012-01-27 | 2018-11-20 | Invisiderm, Llc | Method of producing substances with supersaturated gas, transdermal delivery device thereof, and uses thereof |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63135179A (ja) * | 1986-11-26 | 1988-06-07 | 立花 俊郎 | 薬物の経皮投与具 |
| JPH04504671A (ja) * | 1989-04-28 | 1992-08-20 | アストラ アクティエボラーグ | 粉末吸入装置、粉末状薬剤の伸長型担体、及び伸長型担体を備えたカセット |
| JPH08509131A (ja) * | 1994-01-21 | 1996-10-01 | アグラシータス インコーポレイテッド | ガス駆動型遺伝子搬入装置 |
| CA2259194A1 (en) * | 1996-07-05 | 1998-01-15 | Orion Corporation | Transdermal compositions containing levosimendan |
| US20020065533A1 (en) * | 2000-06-08 | 2002-05-30 | Massachusetts Institute Of Technology | Localized molecular and ionic transport to and from tissues |
| JP2003529392A (ja) * | 1999-04-16 | 2003-10-07 | パウダージェクト リサーチ リミテッド | 無針注射器 |
| JP2004521677A (ja) * | 2001-01-11 | 2004-07-22 | パウダージェクト リサーチ リミテッド | 針無しシリンジ |
| EP1508379A1 (en) * | 2003-08-21 | 2005-02-23 | Delphi Technologies, Inc. | Gas collimator for a kinetic powder spray nozzle |
| US20100121262A1 (en) * | 2007-05-04 | 2010-05-13 | Lee's Pharmaceutical (Hk), Ltd. | Particle cassettes and processes therefor |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW404844B (en) | 1993-04-08 | 2000-09-11 | Oxford Biosciences Ltd | Needleless syringe |
| US5899880A (en) | 1994-04-08 | 1999-05-04 | Powderject Research Limited | Needleless syringe using supersonic gas flow for particle delivery |
| GB9416663D0 (en) | 1994-08-17 | 1994-10-12 | Oxford Bioscience Limited | Particle delivery |
| GB9426379D0 (en) | 1994-12-23 | 1995-03-01 | Oxford Biosciences Ltd | Particle delivery |
| US6893664B1 (en) | 1996-06-17 | 2005-05-17 | Powderject Research Limited | Particle delivery techniques |
| US5947928A (en) | 1997-06-19 | 1999-09-07 | Mile Creek Capital, Llc | Drug delivery system |
| EP0888790A1 (en) | 1997-07-04 | 1999-01-07 | PowderJect Research Limited | Drug particle delivery device |
| US6116718A (en) | 1998-09-30 | 2000-09-12 | Xerox Corporation | Print head for use in a ballistic aerosol marking apparatus |
| US7597692B2 (en) | 2000-06-08 | 2009-10-06 | Massachusetts Institute Of Technology | Microscission processes and procedures |
| US8061006B2 (en) | 2001-07-26 | 2011-11-22 | Powderject Research Limited | Particle cassette, method and kit therefor |
| GB2404865B (en) | 2001-09-11 | 2005-09-28 | Caretek Medical Ltd | Novel drug delivery technology |
-
2011
- 2011-04-19 US US13/089,777 patent/US8486002B2/en active Active
-
2012
- 2012-03-19 JP JP2012061517A patent/JP2012223560A/ja active Pending
- 2012-04-16 EP EP12164295.3A patent/EP2514408B1/en active Active
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63135179A (ja) * | 1986-11-26 | 1988-06-07 | 立花 俊郎 | 薬物の経皮投与具 |
| JPH04504671A (ja) * | 1989-04-28 | 1992-08-20 | アストラ アクティエボラーグ | 粉末吸入装置、粉末状薬剤の伸長型担体、及び伸長型担体を備えたカセット |
| JPH08509131A (ja) * | 1994-01-21 | 1996-10-01 | アグラシータス インコーポレイテッド | ガス駆動型遺伝子搬入装置 |
| CA2259194A1 (en) * | 1996-07-05 | 1998-01-15 | Orion Corporation | Transdermal compositions containing levosimendan |
| JP2003529392A (ja) * | 1999-04-16 | 2003-10-07 | パウダージェクト リサーチ リミテッド | 無針注射器 |
| US20020065533A1 (en) * | 2000-06-08 | 2002-05-30 | Massachusetts Institute Of Technology | Localized molecular and ionic transport to and from tissues |
| JP2004521677A (ja) * | 2001-01-11 | 2004-07-22 | パウダージェクト リサーチ リミテッド | 針無しシリンジ |
| EP1508379A1 (en) * | 2003-08-21 | 2005-02-23 | Delphi Technologies, Inc. | Gas collimator for a kinetic powder spray nozzle |
| US20100121262A1 (en) * | 2007-05-04 | 2010-05-13 | Lee's Pharmaceutical (Hk), Ltd. | Particle cassettes and processes therefor |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2514408A1 (en) | 2012-10-24 |
| EP2514408B1 (en) | 2018-01-10 |
| US8486002B2 (en) | 2013-07-16 |
| US20120271218A1 (en) | 2012-10-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5883330B2 (ja) | コリメートされたガス流及び粒子源を用いる送達機器及び方法 | |
| JP5883334B2 (ja) | コリメートされたガス流及び薬剤リザーバを用いる薬剤送達機器及び方法 | |
| ES2760973T3 (es) | Dispositivos de administración de fluido | |
| EP1471953B1 (en) | Gas pressure actuated microneedle arrays, and systems and methods relating to same | |
| US8251958B2 (en) | Medicament microdevice delivery system, cartridge and method of use | |
| EP1299147B1 (en) | Apparatus for transdermally sampling or administering a substance to a patient | |
| US20030208167A1 (en) | Microneedle drug delivery device | |
| JP4842807B2 (ja) | カートリッジ付きの医薬マイクロデバイス給送システム | |
| JP2010005411A (ja) | 針無しシリンジ | |
| WO2014025241A1 (ko) | 어븀야그 레이저를 이용한 마이크로젯 약물전달 시스템 | |
| US12115354B2 (en) | Alignment of elongated particles in a particle delivery device | |
| JP2012223560A (ja) | コリメートされたガス流及び放出活性化テープを用いる薬剤送達機器及び方法 | |
| US10039885B2 (en) | System and method for enhancing particle delivery to biological tissue | |
| Kumar et al. | Microneedle Drug Delivery System |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20131025 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150318 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150318 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20150318 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20150413 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150512 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150811 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150908 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20151124 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20160105 |