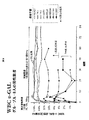
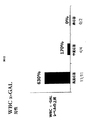
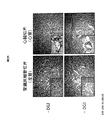
JP2010525084A - 薬理シャペロンを用いたリソソーム蓄積症治療のための投薬計画 - Google Patents
薬理シャペロンを用いたリソソーム蓄積症治療のための投薬計画 Download PDFInfo
- Publication number
- JP2010525084A JP2010525084A JP2010506557A JP2010506557A JP2010525084A JP 2010525084 A JP2010525084 A JP 2010525084A JP 2010506557 A JP2010506557 A JP 2010506557A JP 2010506557 A JP2010506557 A JP 2010506557A JP 2010525084 A JP2010525084 A JP 2010525084A
- Authority
- JP
- Japan
- Prior art keywords
- days
- administered
- dose
- day
- administering
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000011282 treatment Methods 0.000 title claims abstract description 153
- 108010006519 Molecular Chaperones Proteins 0.000 title claims abstract description 117
- 230000000144 pharmacologic effect Effects 0.000 title claims abstract description 94
- 208000015439 Lysosomal storage disease Diseases 0.000 title claims abstract description 15
- 229940079593 drug Drugs 0.000 title claims description 79
- 239000003814 drug Substances 0.000 title claims description 79
- 102000005431 Molecular Chaperones Human genes 0.000 title description 28
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 49
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 43
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 31
- LXBIFEVIBLOUGU-DPYQTVNSSA-N migalastat Chemical compound OC[C@H]1NC[C@H](O)[C@@H](O)[C@H]1O LXBIFEVIBLOUGU-DPYQTVNSSA-N 0.000 claims description 144
- 229950007469 migalastat Drugs 0.000 claims description 142
- 238000000034 method Methods 0.000 claims description 135
- QPYJXFZUIJOGNX-HSUXUTPPSA-N afegostat Chemical compound OC[C@H]1CNC[C@@H](O)[C@@H]1O QPYJXFZUIJOGNX-HSUXUTPPSA-N 0.000 claims description 130
- 102000004190 Enzymes Human genes 0.000 claims description 128
- 108090000790 Enzymes Proteins 0.000 claims description 128
- 238000012423 maintenance Methods 0.000 claims description 79
- 208000024720 Fabry Disease Diseases 0.000 claims description 66
- 230000035772 mutation Effects 0.000 claims description 66
- 238000009825 accumulation Methods 0.000 claims description 62
- 150000003839 salts Chemical class 0.000 claims description 56
- 208000015872 Gaucher disease Diseases 0.000 claims description 55
- 210000003712 lysosome Anatomy 0.000 claims description 32
- 230000001868 lysosomic effect Effects 0.000 claims description 32
- ULBPPCHRAVUQMC-MUMXBIPUSA-N (2r,3r)-2,3-dihydroxybutanedioic acid;(3r,4r,5r)-5-(hydroxymethyl)piperidine-3,4-diol Chemical compound OC[C@H]1CNC[C@@H](O)[C@@H]1O.OC(=O)[C@H](O)[C@@H](O)C(O)=O ULBPPCHRAVUQMC-MUMXBIPUSA-N 0.000 claims description 28
- 201000010099 disease Diseases 0.000 claims description 27
- 230000036470 plasma concentration Effects 0.000 claims description 27
- LXBIFEVIBLOUGU-JGWLITMVSA-N duvoglustat Chemical compound OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O LXBIFEVIBLOUGU-JGWLITMVSA-N 0.000 claims description 19
- 102100033448 Lysosomal alpha-glucosidase Human genes 0.000 claims description 11
- 208000032007 Glycogen storage disease due to acid maltase deficiency Diseases 0.000 claims description 10
- 206010053185 Glycogen storage disease type II Diseases 0.000 claims description 10
- 201000004502 glycogen storage disease II Diseases 0.000 claims description 10
- 239000003112 inhibitor Substances 0.000 claims description 10
- LXBIFEVIBLOUGU-UHFFFAOYSA-N Deoxymannojirimycin Natural products OCC1NCC(O)C(O)C1O LXBIFEVIBLOUGU-UHFFFAOYSA-N 0.000 claims description 9
- 230000002860 competitive effect Effects 0.000 claims description 6
- 235000013305 food Nutrition 0.000 claims description 6
- 238000010494 dissociation reaction Methods 0.000 claims description 4
- 230000005593 dissociations Effects 0.000 claims description 4
- 102200077295 rs104894828 Human genes 0.000 claims description 4
- 102200076442 rs28935197 Human genes 0.000 claims description 4
- 102220602509 Small integral membrane protein 1_M51K_mutation Human genes 0.000 claims description 3
- 102200076470 c.290C>T Human genes 0.000 claims description 3
- 102200077268 c.826A>G Human genes 0.000 claims description 3
- 102200076364 rs104894845 Human genes 0.000 claims description 3
- 102200076567 rs797044746 Human genes 0.000 claims description 2
- 102200077205 rs878853698 Human genes 0.000 claims description 2
- 102200018963 rs35470366 Human genes 0.000 claims 1
- 229940088598 enzyme Drugs 0.000 description 124
- 230000000694 effects Effects 0.000 description 98
- 230000002354 daily effect Effects 0.000 description 93
- 101000718529 Saccharolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) Alpha-galactosidase Proteins 0.000 description 65
- 230000035508 accumulation Effects 0.000 description 60
- NNISLDGFPWIBDF-MPRBLYSKSA-N alpha-D-Gal-(1->3)-beta-D-Gal-(1->4)-D-GlcNAc Chemical compound O[C@@H]1[C@@H](NC(=O)C)C(O)O[C@H](CO)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)[C@@H](O)[C@@H](CO)O1 NNISLDGFPWIBDF-MPRBLYSKSA-N 0.000 description 60
- 108010017544 Glucosylceramidase Proteins 0.000 description 52
- 102000004547 Glucosylceramidase Human genes 0.000 description 52
- 210000004027 cell Anatomy 0.000 description 50
- 230000007423 decrease Effects 0.000 description 30
- 210000002381 plasma Anatomy 0.000 description 30
- 102100026277 Alpha-galactosidase A Human genes 0.000 description 28
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 28
- 229940095064 tartrate Drugs 0.000 description 28
- 210000001519 tissue Anatomy 0.000 description 28
- 210000003734 kidney Anatomy 0.000 description 24
- 101000718525 Homo sapiens Alpha-galactosidase A Proteins 0.000 description 23
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 23
- 210000000265 leukocyte Anatomy 0.000 description 22
- 239000000758 substrate Substances 0.000 description 22
- 230000009467 reduction Effects 0.000 description 21
- 208000024891 symptom Diseases 0.000 description 21
- 238000000338 in vitro Methods 0.000 description 19
- 239000000902 placebo Substances 0.000 description 19
- 238000004458 analytical method Methods 0.000 description 18
- 229940068196 placebo Drugs 0.000 description 18
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 17
- 238000002641 enzyme replacement therapy Methods 0.000 description 17
- 230000002829 reductive effect Effects 0.000 description 17
- 230000008859 change Effects 0.000 description 16
- 210000002216 heart Anatomy 0.000 description 16
- 241000699670 Mus sp. Species 0.000 description 15
- 230000004044 response Effects 0.000 description 15
- 210000003491 skin Anatomy 0.000 description 15
- 230000003247 decreasing effect Effects 0.000 description 14
- 239000000203 mixture Substances 0.000 description 14
- 210000004369 blood Anatomy 0.000 description 12
- 239000008280 blood Substances 0.000 description 12
- 230000006872 improvement Effects 0.000 description 12
- 239000000243 solution Substances 0.000 description 12
- 238000012360 testing method Methods 0.000 description 10
- 230000002485 urinary effect Effects 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- 241000282414 Homo sapiens Species 0.000 description 9
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 9
- 210000000988 bone and bone Anatomy 0.000 description 9
- 239000002775 capsule Substances 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 230000002132 lysosomal effect Effects 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 238000002560 therapeutic procedure Methods 0.000 description 9
- 230000007306 turnover Effects 0.000 description 9
- 150000001413 amino acids Chemical class 0.000 description 8
- 210000002950 fibroblast Anatomy 0.000 description 8
- -1 small molecule derivatives of glucose Chemical class 0.000 description 8
- 210000002700 urine Anatomy 0.000 description 8
- 241000700159 Rattus Species 0.000 description 7
- 230000002411 adverse Effects 0.000 description 7
- 108010030291 alpha-Galactosidase Proteins 0.000 description 7
- 238000001574 biopsy Methods 0.000 description 7
- 230000001413 cellular effect Effects 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 238000011862 kidney biopsy Methods 0.000 description 7
- 230000003285 pharmacodynamic effect Effects 0.000 description 7
- 239000000843 powder Substances 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 230000002950 deficient Effects 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 150000002305 glucosylceramides Chemical class 0.000 description 6
- 235000011187 glycerol Nutrition 0.000 description 6
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 6
- 239000000546 pharmaceutical excipient Substances 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 102000004196 processed proteins & peptides Human genes 0.000 description 6
- 108090000765 processed proteins & peptides Proteins 0.000 description 6
- 230000035900 sweating Effects 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- 238000002562 urinalysis Methods 0.000 description 6
- 230000002861 ventricular Effects 0.000 description 6
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 208000006011 Stroke Diseases 0.000 description 5
- 230000005856 abnormality Effects 0.000 description 5
- 230000000747 cardiac effect Effects 0.000 description 5
- 239000013592 cell lysate Substances 0.000 description 5
- 238000005094 computer simulation Methods 0.000 description 5
- 210000003414 extremity Anatomy 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 150000002632 lipids Chemical class 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- 210000002540 macrophage Anatomy 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 239000003981 vehicle Substances 0.000 description 5
- 108060006698 EGF receptor Proteins 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 208000007177 Left Ventricular Hypertrophy Diseases 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 206010060860 Neurological symptom Diseases 0.000 description 4
- 208000002193 Pain Diseases 0.000 description 4
- 206010040021 Sensory abnormalities Diseases 0.000 description 4
- 108010056760 agalsidase beta Proteins 0.000 description 4
- 230000007812 deficiency Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 231100000673 dose–response relationship Toxicity 0.000 description 4
- 239000003651 drinking water Substances 0.000 description 4
- 235000020188 drinking water Nutrition 0.000 description 4
- 238000001647 drug administration Methods 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 230000004064 dysfunction Effects 0.000 description 4
- 235000019441 ethanol Nutrition 0.000 description 4
- 229940014516 fabrazyme Drugs 0.000 description 4
- 230000004217 heart function Effects 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 4
- 239000006166 lysate Substances 0.000 description 4
- 210000000557 podocyte Anatomy 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- 150000003384 small molecules Chemical class 0.000 description 4
- 210000000952 spleen Anatomy 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 230000002459 sustained effect Effects 0.000 description 4
- 206010043554 thrombocytopenia Diseases 0.000 description 4
- 210000000689 upper leg Anatomy 0.000 description 4
- ARQXEQLMMNGFDU-JHZZJYKESA-N 4-methylumbelliferone beta-D-glucuronide Chemical compound C1=CC=2C(C)=CC(=O)OC=2C=C1O[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O ARQXEQLMMNGFDU-JHZZJYKESA-N 0.000 description 3
- ARQXEQLMMNGFDU-UHFFFAOYSA-N 4MUG Natural products C1=CC=2C(C)=CC(=O)OC=2C=C1OC1OC(C(O)=O)C(O)C(O)C1O ARQXEQLMMNGFDU-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- YDNKGFDKKRUKPY-JHOUSYSJSA-N C16 ceramide Natural products CCCCCCCCCCCCCCCC(=O)N[C@@H](CO)[C@H](O)C=CCCCCCCCCCCCCC YDNKGFDKKRUKPY-JHOUSYSJSA-N 0.000 description 3
- 102000000013 Chemokine CCL3 Human genes 0.000 description 3
- 102000019034 Chemokines Human genes 0.000 description 3
- 108010012236 Chemokines Proteins 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- 208000020322 Gaucher disease type I Diseases 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- CRJGESKKUOMBCT-VQTJNVASSA-N N-acetylsphinganine Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@H](CO)NC(C)=O CRJGESKKUOMBCT-VQTJNVASSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 3
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 3
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 230000001668 ameliorated effect Effects 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 230000006793 arrhythmia Effects 0.000 description 3
- 206010003119 arrhythmia Diseases 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 230000027455 binding Effects 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 210000004413 cardiac myocyte Anatomy 0.000 description 3
- 229940106189 ceramide Drugs 0.000 description 3
- ZVEQCJWYRWKARO-UHFFFAOYSA-N ceramide Natural products CCCCCCCCCCCCCCC(O)C(=O)NC(CO)C(O)C=CCCC=C(C)CCCCCCCCC ZVEQCJWYRWKARO-UHFFFAOYSA-N 0.000 description 3
- 230000001876 chaperonelike Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 238000001493 electron microscopy Methods 0.000 description 3
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 210000003292 kidney cell Anatomy 0.000 description 3
- 201000006370 kidney failure Diseases 0.000 description 3
- 230000003907 kidney function Effects 0.000 description 3
- 235000019359 magnesium stearate Nutrition 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 208000004296 neuralgia Diseases 0.000 description 3
- 230000002981 neuropathic effect Effects 0.000 description 3
- 208000021722 neuropathic pain Diseases 0.000 description 3
- VVGIYYKRAMHVLU-UHFFFAOYSA-N newbouldiamide Natural products CCCCCCCCCCCCCCCCCCCC(O)C(O)C(O)C(CO)NC(=O)CCCCCCCCCCCCCCCCC VVGIYYKRAMHVLU-UHFFFAOYSA-N 0.000 description 3
- 238000005457 optimization Methods 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 230000004952 protein activity Effects 0.000 description 3
- 201000001474 proteinuria Diseases 0.000 description 3
- 238000003908 quality control method Methods 0.000 description 3
- 231100000279 safety data Toxicity 0.000 description 3
- 239000013049 sediment Substances 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 229940032147 starch Drugs 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 239000000375 suspending agent Substances 0.000 description 3
- 210000000106 sweat gland Anatomy 0.000 description 3
- 230000004797 therapeutic response Effects 0.000 description 3
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- YOQUZCNSUOJWQS-UHFFFAOYSA-N 3-chloro-4-methylaniline;hydron;chloride Chemical compound Cl.CC1=CC=C(N)C=C1Cl YOQUZCNSUOJWQS-UHFFFAOYSA-N 0.000 description 2
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 208000024827 Alzheimer disease Diseases 0.000 description 2
- 206010003591 Ataxia Diseases 0.000 description 2
- 206010049824 Bone infarction Diseases 0.000 description 2
- 206010006002 Bone pain Diseases 0.000 description 2
- 108700012434 CCL3 Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 206010007572 Cardiac hypertrophy Diseases 0.000 description 2
- 208000006029 Cardiomegaly Diseases 0.000 description 2
- 208000002177 Cataract Diseases 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 102100037328 Chitotriosidase-1 Human genes 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- 206010010904 Convulsion Diseases 0.000 description 2
- 208000006069 Corneal Opacity Diseases 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 206010012289 Dementia Diseases 0.000 description 2
- 101150028412 GBA gene Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-ZSJDYOACSA-N Heavy water Chemical compound [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 2
- 206010019842 Hepatomegaly Diseases 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- 102000013691 Interleukin-17 Human genes 0.000 description 2
- 108050003558 Interleukin-17 Proteins 0.000 description 2
- 102000004890 Interleukin-8 Human genes 0.000 description 2
- 108090001007 Interleukin-8 Proteins 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- 208000029725 Metabolic bone disease Diseases 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- 208000002678 Mucopolysaccharidoses Diseases 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 206010031264 Osteonecrosis Diseases 0.000 description 2
- 206010049088 Osteopenia Diseases 0.000 description 2
- 208000018737 Parkinson disease Diseases 0.000 description 2
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 2
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 208000001647 Renal Insufficiency Diseases 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 206010041660 Splenomegaly Diseases 0.000 description 2
- 238000000692 Student's t-test Methods 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 108010000134 Vascular Cell Adhesion Molecule-1 Proteins 0.000 description 2
- 102100023543 Vascular cell adhesion protein 1 Human genes 0.000 description 2
- 210000001766 X chromosome Anatomy 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000001464 adherent effect Effects 0.000 description 2
- 108010028144 alpha-Glucosidases Proteins 0.000 description 2
- 102000003802 alpha-Synuclein Human genes 0.000 description 2
- 108090000185 alpha-Synuclein Proteins 0.000 description 2
- 238000000540 analysis of variance Methods 0.000 description 2
- 208000007502 anemia Diseases 0.000 description 2
- 229960005070 ascorbic acid Drugs 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- WPIHMWBQRSAMDE-YCZTVTEBSA-N beta-D-galactosyl-(1->4)-beta-D-galactosyl-N-(pentacosanoyl)sphingosine Chemical compound CCCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@@H](CO[C@@H]1O[C@H](CO)[C@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O)[C@H](O)\C=C\CCCCCCCCCCCCC WPIHMWBQRSAMDE-YCZTVTEBSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 210000005013 brain tissue Anatomy 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 108010057052 chitotriosidase Proteins 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- CVSVTCORWBXHQV-UHFFFAOYSA-N creatine Chemical compound NC(=[NH2+])N(C)CC([O-])=O CVSVTCORWBXHQV-UHFFFAOYSA-N 0.000 description 2
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 239000003599 detergent Substances 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 239000002532 enzyme inhibitor Substances 0.000 description 2
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 230000009246 food effect Effects 0.000 description 2
- 235000021471 food effect Nutrition 0.000 description 2
- 239000012458 free base Substances 0.000 description 2
- 230000001434 glomerular Effects 0.000 description 2
- 230000024924 glomerular filtration Effects 0.000 description 2
- 150000002339 glycosphingolipids Chemical class 0.000 description 2
- 208000016354 hearing loss disease Diseases 0.000 description 2
- 230000002489 hematologic effect Effects 0.000 description 2
- 206010019847 hepatosplenomegaly Diseases 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- 229940027941 immunoglobulin g Drugs 0.000 description 2
- 238000011532 immunohistochemical staining Methods 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 239000007972 injectable composition Substances 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000007913 intrathecal administration Methods 0.000 description 2
- 230000000302 ischemic effect Effects 0.000 description 2
- 208000017169 kidney disease Diseases 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 244000005700 microbiome Species 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 206010028093 mucopolysaccharidosis Diseases 0.000 description 2
- 239000007922 nasal spray Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 208000033808 peripheral neuropathy Diseases 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 235000013772 propylene glycol Nutrition 0.000 description 2
- 208000002815 pulmonary hypertension Diseases 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 210000005084 renal tissue Anatomy 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 230000033764 rhythmic process Effects 0.000 description 2
- 102200065199 rs1057515579 Human genes 0.000 description 2
- 102200049940 rs1554402092 Human genes 0.000 description 2
- 102200077267 rs28935485 Human genes 0.000 description 2
- 102200019851 rs397515561 Human genes 0.000 description 2
- 102200060048 rs61754362 Human genes 0.000 description 2
- 102200077332 rs869312399 Human genes 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 210000004927 skin cell Anatomy 0.000 description 2
- 210000001626 skin fibroblast Anatomy 0.000 description 2
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 235000010199 sorbic acid Nutrition 0.000 description 2
- 239000004334 sorbic acid Substances 0.000 description 2
- 229940075582 sorbic acid Drugs 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 238000004611 spectroscopical analysis Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 238000012353 t test Methods 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- 210000005239 tubule Anatomy 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 230000009278 visceral effect Effects 0.000 description 2
- AAKDPDFZMNYDLR-JRTVQGFMSA-N (2R,3S,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine-3,4,5-triol Chemical compound CN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO AAKDPDFZMNYDLR-JRTVQGFMSA-N 0.000 description 1
- ZSNFEMNRWFDMNU-CVPUBMOQSA-N (2s,3s,4r,5s,6r)-2-butyl-6-(hydroxymethyl)piperidine-3,4,5-triol Chemical compound CCCC[C@@H]1N[C@H](CO)[C@H](O)[C@H](O)[C@H]1O ZSNFEMNRWFDMNU-CVPUBMOQSA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- RZRDQZQPTISYKY-MVIOUDGNSA-N (5r,6r,7s,8s)-5-(hydroxymethyl)-5,6,7,8-tetrahydroimidazo[1,2-a]pyridine-6,7,8-triol Chemical compound OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)C2=NC=CN12 RZRDQZQPTISYKY-MVIOUDGNSA-N 0.000 description 1
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N 1-behenoylglycerol Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)CO OKMWKBLSFKFYGZ-UHFFFAOYSA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- IDOQDZANRZQBTP-UHFFFAOYSA-N 2-[2-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol Chemical compound CC(C)(C)CC(C)(C)C1=CC=CC=C1OCCO IDOQDZANRZQBTP-UHFFFAOYSA-N 0.000 description 1
- 229940090248 4-hydroxybenzoic acid Drugs 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 235000019489 Almond oil Nutrition 0.000 description 1
- 102220486008 Alpha-galactosidase A_C94S_mutation Human genes 0.000 description 1
- 102220486515 Alpha-galactosidase A_D170V_mutation Human genes 0.000 description 1
- 102220486131 Alpha-galactosidase A_E59K_mutation Human genes 0.000 description 1
- 102220481462 Alpha-galactosidase A_V316E_mutation Human genes 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 102100022548 Beta-hexosaminidase subunit alpha Human genes 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 208000020084 Bone disease Diseases 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 201000006474 Brain Ischemia Diseases 0.000 description 1
- 102100023701 C-C motif chemokine 18 Human genes 0.000 description 1
- 101710155856 C-C motif chemokine 3 Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 206010008120 Cerebral ischaemia Diseases 0.000 description 1
- YBSQGNFRWZKFMJ-UHFFFAOYSA-N Cerebroside B Natural products CCCCCCCCCCCCCCC(O)C(=O)NC(C(O)C=CCCC=C(C)CCCCCCCCC)COC1OC(CO)C(O)C(O)C1O YBSQGNFRWZKFMJ-UHFFFAOYSA-N 0.000 description 1
- 102000012286 Chitinases Human genes 0.000 description 1
- 108010022172 Chitinases Proteins 0.000 description 1
- 208000028698 Cognitive impairment Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 208000032170 Congenital Abnormalities Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 206010011878 Deafness Diseases 0.000 description 1
- 206010011891 Deafness neurosensory Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 241000283070 Equus zebra Species 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- 102220571590 Fatty acid hydroxylase domain-containing protein 2_K79N_mutation Human genes 0.000 description 1
- 206010017076 Fracture Diseases 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 208000020916 Gaucher disease type II Diseases 0.000 description 1
- 208000028735 Gaucher disease type III Diseases 0.000 description 1
- 206010064571 Gene mutation Diseases 0.000 description 1
- 229940127551 Glucosyl Transferase Inhibitors Drugs 0.000 description 1
- 229920002527 Glycogen Polymers 0.000 description 1
- 208000011755 Hearing abnormality Diseases 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 208000016988 Hemorrhagic Stroke Diseases 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 101000978371 Homo sapiens C-C motif chemokine 18 Proteins 0.000 description 1
- 101001018026 Homo sapiens Lysosomal alpha-glucosidase Proteins 0.000 description 1
- 208000033830 Hot Flashes Diseases 0.000 description 1
- 206010060800 Hot flush Diseases 0.000 description 1
- 208000019758 Hypergammaglobulinemia Diseases 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 206010061216 Infarction Diseases 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 208000029523 Interstitial Lung disease Diseases 0.000 description 1
- 208000032382 Ischaemic stroke Diseases 0.000 description 1
- FFFHZYDWPBMWHY-VKHMYHEASA-N L-homocysteine Chemical compound OC(=O)[C@@H](N)CCS FFFHZYDWPBMWHY-VKHMYHEASA-N 0.000 description 1
- 206010024214 Lenticular opacities Diseases 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 101100236308 Mus musculus Gaa gene Proteins 0.000 description 1
- 208000007101 Muscle Cramp Diseases 0.000 description 1
- 208000008238 Muscle Spasticity Diseases 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 238000011887 Necropsy Methods 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 239000008118 PEG 6000 Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229920001030 Polyethylene Glycol 4000 Polymers 0.000 description 1
- 229920002584 Polyethylene Glycol 6000 Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 206010067171 Regurgitation Diseases 0.000 description 1
- 208000037656 Respiratory Sounds Diseases 0.000 description 1
- 208000009966 Sensorineural Hearing Loss Diseases 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- 208000005250 Spontaneous Fractures Diseases 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 208000007271 Substance Withdrawal Syndrome Diseases 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 206010061373 Sudden Hearing Loss Diseases 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 208000001871 Tachycardia Diseases 0.000 description 1
- 102000007591 Tartrate-Resistant Acid Phosphatase Human genes 0.000 description 1
- 108010032050 Tartrate-Resistant Acid Phosphatase Proteins 0.000 description 1
- 208000022292 Tay-Sachs disease Diseases 0.000 description 1
- 208000009205 Tinnitus Diseases 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 229920004929 Triton X-114 Polymers 0.000 description 1
- 102220468686 Tubulin polyglutamylase TTLL7_N264A_mutation Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 206010047924 Wheezing Diseases 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- 230000004598 abnormal eye movement Effects 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 108010049936 agalsidase alfa Proteins 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 229940022705 aldurazyme Drugs 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 239000008168 almond oil Substances 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- CLVUFWXGNIFGNC-UHFFFAOYSA-N alpha-homonojirimycin Natural products OCC1NC(CO)C(O)C(O)C1O CLVUFWXGNIFGNC-UHFFFAOYSA-N 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 210000002159 anterior chamber Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000003376 axonal effect Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000006399 behavior Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- ZSNFEMNRWFDMNU-UHFFFAOYSA-N beta-1-C-butyl-deoxygalactonojirimycin Natural products CCCCC1NC(CO)C(O)C(O)C1O ZSNFEMNRWFDMNU-UHFFFAOYSA-N 0.000 description 1
- 230000002715 bioenergetic effect Effects 0.000 description 1
- 230000008499 blood brain barrier function Effects 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 230000036471 bradycardia Effects 0.000 description 1
- 208000006218 bradycardia Diseases 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 102200076464 c.272T>C Human genes 0.000 description 1
- 102220364489 c.620A>C Human genes 0.000 description 1
- 102200076847 c.95T>C Human genes 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000006652 catabolic pathway Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 206010008118 cerebral infarction Diseases 0.000 description 1
- 229940049197 cerezyme Drugs 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 229940001468 citrate Drugs 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000010224 classification analysis Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 208000010877 cognitive disease Diseases 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 229940052810 complex b Drugs 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 231100000269 corneal opacity Toxicity 0.000 description 1
- 229960003624 creatine Drugs 0.000 description 1
- 239000006046 creatine Substances 0.000 description 1
- 229940109239 creatinine Drugs 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000002716 delivery method Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 210000005232 distal tubule cell Anatomy 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 239000008157 edible vegetable oil Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 210000003038 endothelium Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- 229940125532 enzyme inhibitor Drugs 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 230000007661 gastrointestinal function Effects 0.000 description 1
- 238000003304 gavage Methods 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 229940049654 glyceryl behenate Drugs 0.000 description 1
- 229940096919 glycogen Drugs 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 208000037824 growth disorder Diseases 0.000 description 1
- 230000010370 hearing loss Effects 0.000 description 1
- 231100000888 hearing loss Toxicity 0.000 description 1
- 238000005534 hematocrit Methods 0.000 description 1
- 238000010562 histological examination Methods 0.000 description 1
- 102000045921 human GAA Human genes 0.000 description 1
- 229910000042 hydrogen bromide Inorganic materials 0.000 description 1
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 1
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 108010039650 imiglucerase Proteins 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000000126 in silico method Methods 0.000 description 1
- 230000007574 infarction Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940096397 interleukin-8 Drugs 0.000 description 1
- XKTZWUACRZHVAN-VADRZIEHSA-N interleukin-8 Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](NC(C)=O)CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1[C@H](CCC1)C(=O)N1[C@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CO)C(=O)N1[C@H](CCC1)C(N)=O)C1=CC=CC=C1 XKTZWUACRZHVAN-VADRZIEHSA-N 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 208000020658 intracerebral hemorrhage Diseases 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 210000005240 left ventricle Anatomy 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- 201000002364 leukopenia Diseases 0.000 description 1
- 231100001022 leukopenia Toxicity 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 210000005229 liver cell Anatomy 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 210000003584 mesangial cell Anatomy 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- DWCZIOOZPIDHAB-UHFFFAOYSA-L methyl green Chemical compound [Cl-].[Cl-].C1=CC(N(C)C)=CC=C1C(C=1C=CC(=CC=1)[N+](C)(C)C)=C1C=CC(=[N+](C)C)C=C1 DWCZIOOZPIDHAB-UHFFFAOYSA-L 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- UQRORFVVSGFNRO-UTINFBMNSA-N miglustat Chemical compound CCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO UQRORFVVSGFNRO-UTINFBMNSA-N 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 229940051866 mouthwash Drugs 0.000 description 1
- 208000022018 mucopolysaccharidosis type 2 Diseases 0.000 description 1
- 230000004220 muscle function Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 230000002151 myoclonic effect Effects 0.000 description 1
- 229940103023 myozyme Drugs 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 210000004126 nerve fiber Anatomy 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 230000000626 neurodegenerative effect Effects 0.000 description 1
- 230000009251 neurologic dysfunction Effects 0.000 description 1
- 208000015015 neurological dysfunction Diseases 0.000 description 1
- 230000000926 neurological effect Effects 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 231100001079 no serious adverse effect Toxicity 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- 230000036963 noncompetitive effect Effects 0.000 description 1
- 230000037311 normal skin Effects 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 238000000399 optical microscopy Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 230000007331 pathological accumulation Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 210000002856 peripheral neuron Anatomy 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 206010035485 plasmacytosis Diseases 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 1
- 230000012846 protein folding Effects 0.000 description 1
- 230000029983 protein stabilization Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 230000011514 reflex Effects 0.000 description 1
- 230000008085 renal dysfunction Effects 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000004202 respiratory function Effects 0.000 description 1
- 102200030587 rs104893849 Human genes 0.000 description 1
- 102200077151 rs104894827 Human genes 0.000 description 1
- 102200077287 rs104894830 Human genes 0.000 description 1
- 102200076453 rs104894833 Human genes 0.000 description 1
- 102200076859 rs104894835 Human genes 0.000 description 1
- 102200076563 rs104894840 Human genes 0.000 description 1
- 102200077177 rs111422676 Human genes 0.000 description 1
- 102220087822 rs112341092 Human genes 0.000 description 1
- 102200050274 rs121913020 Human genes 0.000 description 1
- 102200158224 rs121918530 Human genes 0.000 description 1
- 102220126528 rs141810774 Human genes 0.000 description 1
- 102200076369 rs1555985829 Human genes 0.000 description 1
- 102200086549 rs17886395 Human genes 0.000 description 1
- 102220068566 rs201506921 Human genes 0.000 description 1
- 102200077148 rs28935493 Human genes 0.000 description 1
- 102200018556 rs587779420 Human genes 0.000 description 1
- 102220045034 rs587781775 Human genes 0.000 description 1
- 102200076593 rs727503948 Human genes 0.000 description 1
- 102200007201 rs750496798 Human genes 0.000 description 1
- 102220252093 rs751216929 Human genes 0.000 description 1
- 102220086768 rs760813493 Human genes 0.000 description 1
- 102220118498 rs777698606 Human genes 0.000 description 1
- 102200077160 rs797044774 Human genes 0.000 description 1
- 102220085241 rs879253808 Human genes 0.000 description 1
- 102200136953 rs879253837 Human genes 0.000 description 1
- 102220456815 rs886051894 Human genes 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 231100000879 sensorineural hearing loss Toxicity 0.000 description 1
- 208000023573 sensorineural hearing loss disease Diseases 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 201000002859 sleep apnea Diseases 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 229960003885 sodium benzoate Drugs 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 229940080313 sodium starch Drugs 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 208000018198 spasticity Diseases 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000009747 swallowing Effects 0.000 description 1
- 230000006794 tachycardia Effects 0.000 description 1
- 231100000886 tinnitus Toxicity 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- IMFACGCPASFAPR-UHFFFAOYSA-N tributylamine Chemical compound CCCCN(CCCC)CCCC IMFACGCPASFAPR-UHFFFAOYSA-N 0.000 description 1
- 210000004926 tubular epithelial cell Anatomy 0.000 description 1
- 210000005233 tubule cell Anatomy 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 208000037911 visceral disease Diseases 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940099072 zavesca Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/45—Non condensed piperidines, e.g. piperocaine having oxo groups directly attached to the heterocyclic ring, e.g. cycloheximide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Heart & Thoracic Surgery (AREA)
- Hospice & Palliative Care (AREA)
- Cardiology (AREA)
- Psychiatry (AREA)
- Obesity (AREA)
- Psychology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Hydrogenated Pyridines (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US91428807P | 2007-04-26 | 2007-04-26 | |
| US1474407P | 2007-12-18 | 2007-12-18 | |
| US2810508P | 2008-02-12 | 2008-02-12 | |
| PCT/US2008/061764 WO2008134628A2 (en) | 2007-04-26 | 2008-04-28 | Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010525084A true JP2010525084A (ja) | 2010-07-22 |
| JP2010525084A5 JP2010525084A5 (cg-RX-API-DMAC7.html) | 2011-05-19 |
Family
ID=39926311
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010506557A Pending JP2010525084A (ja) | 2007-04-26 | 2008-04-28 | 薬理シャペロンを用いたリソソーム蓄積症治療のための投薬計画 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US9056101B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2150254A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2010525084A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2008245578A1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2685332A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL201733A0 (cg-RX-API-DMAC7.html) |
| MX (1) | MX2009011473A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2008134628A2 (cg-RX-API-DMAC7.html) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015519329A (ja) * | 2012-05-03 | 2015-07-09 | アミカス セラピューティックス インコーポレイテッド | ポンペ病の処置のための投与計画 |
| JP2017511372A (ja) * | 2014-04-14 | 2017-04-20 | アミカス セラピューティックス インコーポレイテッド | 脳アミロイドーシスを処置及び/又は予防する投与計画 |
| JP2017161528A (ja) * | 2011-06-06 | 2017-09-14 | セントジーン アーゲー | ゴーシェ病の診断のための方法 |
| JP2018528953A (ja) * | 2015-08-31 | 2018-10-04 | アミカス セラピューティックス インコーポレイテッド | リソソーム障害及び中枢神経系の変性障害の治療及び予防のための(3r,4r,5s)−5−(ジフルオロメチル)ピペリジン−3,4−ジオールを含むレジメン |
| US10208299B2 (en) | 2014-09-30 | 2019-02-19 | Amicus Therapeutics, Inc. | Highly potent acid alpha-glucosidase with enhanced carbohydrates |
| US10227577B2 (en) | 2016-03-30 | 2019-03-12 | Amicus Therapeutics, Inc. | Method for selection of high M6P recombinant proteins |
| US10512676B2 (en) | 2016-03-30 | 2019-12-24 | Amicus Therapeutics, Inc. | Formulations comprising recombinant acid alpha-glucosidase |
| JP2020507562A (ja) * | 2017-05-30 | 2020-03-12 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
| US10857212B2 (en) | 2015-12-30 | 2020-12-08 | Amicus Therapeutics, Inc. | Augmented acid alpha-glucosidase for the treatment of Pompe disease |
| US12246062B2 (en) | 2017-05-15 | 2025-03-11 | Amicus Therapeutics, Inc. | Recombinant human acid alpha-glucosidase |
Families Citing this family (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2444102B1 (en) | 2003-01-31 | 2015-04-08 | The Mount Sinai School of Medicine of New York University | Combination therapy for treating protein deficiency disorders |
| PL2533050T6 (pl) | 2006-05-16 | 2016-05-31 | Amicus Therapeutics Inc | Możliwości leczenia choroby Fabry'ego |
| US9999618B2 (en) | 2007-04-26 | 2018-06-19 | Amicus Therapeutics, Inc. | Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones |
| EP2252313B1 (en) | 2008-02-12 | 2015-04-08 | Amicus Therapeutics, Inc. | Method to predict response to pharmacological chaperone treatment of diseases |
| WO2009155936A1 (en) | 2008-06-26 | 2009-12-30 | Orphazyme Aps | Use of hsp70 as a regulator of enzymatic activity |
| US8321148B2 (en) | 2008-10-24 | 2012-11-27 | Amicus Therapeutics, Inc. | Multiple compartment dosing model |
| WO2010056746A1 (en) * | 2008-11-11 | 2010-05-20 | Amicus Therapeutics, Inc. | Therapy regimens, dosing regimens and stable medicaments for the treatment of pompe disease |
| GB0906159D0 (en) * | 2009-04-09 | 2009-05-20 | Summit Corp Plc | Drug combination for the treatment of proteostatic diseases |
| ES2564093T3 (es) | 2009-04-09 | 2016-03-17 | Amicus Therapeutics, Inc. | Métodos para prevenir y/o tratar trastornos del almacenamiento lisosomal |
| ES2646099T3 (es) * | 2009-10-19 | 2017-12-12 | Amicus Therapeutics, Inc. | Método para el tratamiento de la enfermedad de Alzheimer utilizando chaperonas farmacológicas para aumentar la actividad de gangliosidasas |
| LT2995306T (lt) | 2009-10-19 | 2019-04-10 | Amicus Therapeutics, Inc. | Naujos kompozicijos lizosomų kaupimosi sutrikimų profilaktikai ir (arba) gydymui |
| DK2490532T3 (en) | 2009-10-19 | 2017-02-27 | Amicus Therapeutics Inc | Hitherto UNKNOWN COMPOSITIONS FOR PREVENTION AND / OR TREATMENT OF DEGENERATIVE DISORDERS IN THE CENTRAL Nervous System |
| CA2778669C (en) * | 2009-10-27 | 2019-04-16 | Erytech Pharma | Composition to induce specific immune tolerance |
| ES2768995T3 (es) * | 2009-11-17 | 2020-06-24 | Baylor Res Institute | 1-desoxigalactonojirimicina para el uso en el tratamiento de una enfermedad cardíaca |
| NZ625712A (en) * | 2009-11-27 | 2016-02-26 | Genzyme Corp | An amorphous and a crystalline form of genz 112638 hemitartrate as inhibitor of glucosylceramide synthase |
| CN105296447A (zh) | 2010-11-08 | 2016-02-03 | 阿米库斯治疗学公司 | 具有增强的稳定性和增强的保留催化活性的变体重组β-葡萄糖脑苷脂酶蛋白 |
| PT2646044T (pt) | 2010-11-30 | 2019-11-12 | Orphazyme As | Métodos para aumentar a atividade intracelular de hsp70 |
| SG10201604757RA (en) * | 2011-03-11 | 2016-08-30 | Amicus Therapeutics Inc | Dosing regimens for the treatment of fabry disease |
| EP2823043A4 (en) | 2012-03-07 | 2016-09-07 | Amicus Therapeutics Inc | HIGHLY CONCENTRATED ALPHA-GLUCOSIDASE COMPOSITIONS FOR THE TREATMENT OF MORBUS POMPE |
| ES2924829T3 (es) | 2012-03-27 | 2022-10-11 | Amicus Therapeutics Inc | Compuestos novedosos para prevenir y/o tratar tesaurismosis lisosómicas y/o trastornos degenerativos del sistema nervioso central |
| US10155027B2 (en) | 2012-07-17 | 2018-12-18 | Amicus Therapeutics, Inc. | Alpha-galactosidase A and 1-deoxygalactonojirimycin co-formulation for the treatment of fabry disease |
| EP2874648A4 (en) * | 2012-07-17 | 2015-12-30 | Amicus Therapeutics Inc | CO-FORMULATION OF ALPHA-GALACTOSIDASE A AND 1-DEOXYGALACTONOJIRIMYCIN |
| KR20250069686A (ko) | 2014-09-15 | 2025-05-19 | 제브라 덴마크 에이/에스 | 아리모클로몰 제제 |
| GB201508025D0 (en) | 2015-05-11 | 2015-06-24 | Ucl Business Plc | Fabry disease gene therapy |
| WO2017041051A1 (en) | 2015-09-04 | 2017-03-09 | Sqz Biotechnologies Company | Intracellular delivery of biomolecules to cells comprising a cell wall |
| JP6438421B2 (ja) * | 2016-02-17 | 2018-12-12 | ファナック株式会社 | 電動機のステータ |
| WO2017178029A1 (en) | 2016-04-13 | 2017-10-19 | Orphazyme Aps | Heat shock proteins and cholesterol homeostasis |
| CA3287846A1 (en) | 2016-04-29 | 2025-11-29 | Zevra Denmark A/S | Arimoclomol for treating glucocerebrosidase associated disorders |
| CA3023092A1 (en) | 2016-05-03 | 2017-11-09 | Sqz Biotechnologies Company | Intracellular delivery of biomolecules to induce tolerance |
| NL2017294B1 (en) | 2016-08-05 | 2018-02-14 | Univ Erasmus Med Ct Rotterdam | Natural cryptic exon removal by pairs of antisense oligonucleotides. |
| WO2018200618A1 (en) | 2017-04-25 | 2018-11-01 | Amicus Therapeutics, Inc. | Novel compositions for preventing and/or treating degenerative disorders of the central nervous system and/or lysosomal storage disorders |
| HUE065615T2 (hu) * | 2017-05-30 | 2024-06-28 | Amicus Therapeutics Inc | Migalasztát, vesekárosodással szövõdött Fabry-kórban szenvedõ betegeg kezelésére |
| EP3749307B1 (en) | 2018-02-06 | 2025-12-10 | Amicus Therapeutics, Inc. | Use of migalastat for treating fabry disease in pregnant patients |
| CN120044247A (zh) * | 2018-12-10 | 2025-05-27 | 戴纳立制药公司 | 溶酶体贮积症的生物标志物及其使用方法 |
| AU2020291002A1 (en) * | 2019-06-11 | 2022-01-06 | Amicus Therapeutics, Inc. | Methods of treating Fabry disease in patients having renal impairment |
| MX2022001623A (es) | 2019-08-07 | 2022-03-02 | Amicus Therapeutics Inc | Metodos para tratar la enfermedad de fabry en pacientes que tienen una mutacion en el gen gla. |
| AU2021380947C1 (en) | 2020-11-19 | 2025-02-20 | Zevra Denmark A/S | Processes for preparing arimoclomol citrate and intermediates thereof |
| US11623916B2 (en) | 2020-12-16 | 2023-04-11 | Amicus Therapeutics, Inc. | Highly purified batches of pharmaceutical grade migalastat and methods of producing the same |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003528799A (ja) * | 1998-06-01 | 2003-09-30 | マウント シナイ スクール オブ メディシン オブ ニューヨーク ユニバーシティー | リソソームα−ガラクトシダーゼAの増強方法 |
| JP2006516645A (ja) * | 2003-01-31 | 2006-07-06 | マウント シナイ スクール オブ メディシン オブ ニューヨーク ユニバーシティー | タンパク質欠損性障害の治療のための併用療法 |
| WO2006125141A2 (en) * | 2005-05-17 | 2006-11-23 | Amicus Therapeutics, Inc. | A method for the treatment of pompe disease using 1-deoxynojirimycin derivatives |
| WO2006133446A2 (en) * | 2005-06-08 | 2006-12-14 | Amicus Therapeutics, Inc. | Treatment of cns disorders associated with mutations in genes encoding lysosomal enzymes |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7446098B2 (en) * | 2003-02-18 | 2008-11-04 | Mount Sinai School Of Medicine Of New York University | Combination therapy for treating protein deficiencies |
| US7955262B2 (en) | 2005-07-26 | 2011-06-07 | Syneron Medical Ltd. | Method and apparatus for treatment of skin using RF and ultrasound energies |
| EP2374451A3 (en) * | 2005-07-27 | 2012-01-25 | University of Florida Research Foundation, Inc. | Histone deacetylase inhibitors (HDAC) that correct protein misfolding and uses thereof |
| PL2533050T6 (pl) * | 2006-05-16 | 2016-05-31 | Amicus Therapeutics Inc | Możliwości leczenia choroby Fabry'ego |
| EP2142197A4 (en) * | 2007-03-30 | 2010-11-10 | Amicus Therapeutics Inc | PROCESS FOR THE TREATMENT OF FABRY DISEASE USING PHARMACOLOGICAL CHAPERONS |
-
2008
- 2008-04-28 CA CA002685332A patent/CA2685332A1/en not_active Abandoned
- 2008-04-28 AU AU2008245578A patent/AU2008245578A1/en not_active Abandoned
- 2008-04-28 MX MX2009011473A patent/MX2009011473A/es not_active Application Discontinuation
- 2008-04-28 US US12/597,238 patent/US9056101B2/en active Active
- 2008-04-28 EP EP08747020A patent/EP2150254A4/en not_active Withdrawn
- 2008-04-28 WO PCT/US2008/061764 patent/WO2008134628A2/en not_active Ceased
- 2008-04-28 JP JP2010506557A patent/JP2010525084A/ja active Pending
-
2009
- 2009-10-25 IL IL201733A patent/IL201733A0/en unknown
-
2015
- 2015-05-15 US US14/713,821 patent/US20150352093A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003528799A (ja) * | 1998-06-01 | 2003-09-30 | マウント シナイ スクール オブ メディシン オブ ニューヨーク ユニバーシティー | リソソームα−ガラクトシダーゼAの増強方法 |
| JP2006516645A (ja) * | 2003-01-31 | 2006-07-06 | マウント シナイ スクール オブ メディシン オブ ニューヨーク ユニバーシティー | タンパク質欠損性障害の治療のための併用療法 |
| WO2006125141A2 (en) * | 2005-05-17 | 2006-11-23 | Amicus Therapeutics, Inc. | A method for the treatment of pompe disease using 1-deoxynojirimycin derivatives |
| WO2006133446A2 (en) * | 2005-06-08 | 2006-12-14 | Amicus Therapeutics, Inc. | Treatment of cns disorders associated with mutations in genes encoding lysosomal enzymes |
Non-Patent Citations (1)
| Title |
|---|
| 岡野定輔, 新.薬剤学総論, vol. 改訂第3版, JPN6013011899, 1987, pages 240 - 248, ISSN: 0002481224 * |
Cited By (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017161528A (ja) * | 2011-06-06 | 2017-09-14 | セントジーン アーゲー | ゴーシェ病の診断のための方法 |
| JP2020045346A (ja) * | 2012-05-03 | 2020-03-26 | アミカス セラピューティックス インコーポレイテッド | ポンペ病の処置のための投与計画 |
| JP2018109003A (ja) * | 2012-05-03 | 2018-07-12 | アミカス セラピューティックス インコーポレイテッド | ポンペ病の処置のための投与計画 |
| JP7046241B2 (ja) | 2012-05-03 | 2022-04-01 | アミカス セラピューティックス インコーポレイテッド | ポンペ病の処置のための投与計画 |
| JP2015519329A (ja) * | 2012-05-03 | 2015-07-09 | アミカス セラピューティックス インコーポレイテッド | ポンペ病の処置のための投与計画 |
| JP2021088561A (ja) * | 2012-05-03 | 2021-06-10 | アミカス セラピューティックス インコーポレイテッド | ポンペ病の処置のための投与計画 |
| JP2017511372A (ja) * | 2014-04-14 | 2017-04-20 | アミカス セラピューティックス インコーポレイテッド | 脳アミロイドーシスを処置及び/又は予防する投与計画 |
| US11591583B2 (en) | 2014-09-30 | 2023-02-28 | Amicus Therapeutics, Inc. | Highly potent acid alpha-glucosidase with enhanced carbohydrates |
| US11753632B2 (en) | 2014-09-30 | 2023-09-12 | Amicus Therapeutics, Inc. | Highly potent acid alpha-glucosidase with enhanced carbohydrates |
| US10208299B2 (en) | 2014-09-30 | 2019-02-19 | Amicus Therapeutics, Inc. | Highly potent acid alpha-glucosidase with enhanced carbohydrates |
| US10961522B2 (en) | 2014-09-30 | 2021-03-30 | Amicus Therapeutics, Inc. | Highly potent acid alpha-glucosidase with enhanced carbohydrates |
| JP2018528953A (ja) * | 2015-08-31 | 2018-10-04 | アミカス セラピューティックス インコーポレイテッド | リソソーム障害及び中枢神経系の変性障害の治療及び予防のための(3r,4r,5s)−5−(ジフルオロメチル)ピペリジン−3,4−ジオールを含むレジメン |
| US12414985B2 (en) | 2015-12-30 | 2025-09-16 | Amicus Therapeutics, Inc. | Augmented acid alpha-glucosidase for the treatment of Pompe disease |
| US10857212B2 (en) | 2015-12-30 | 2020-12-08 | Amicus Therapeutics, Inc. | Augmented acid alpha-glucosidase for the treatment of Pompe disease |
| US11278601B2 (en) | 2015-12-30 | 2022-03-22 | Amicus Therapeutics, Inc. | Augmented acid alpha-glucosidase for the treatment of Pompe disease |
| US11491211B2 (en) | 2016-03-30 | 2022-11-08 | Amicus Therapeutics, Inc. | Formulations comprising recombinant acid alpha-glucosidase |
| US11441138B2 (en) | 2016-03-30 | 2022-09-13 | Amicus Therapeutics, Inc. | Method for selection of high M6P recombinant proteins |
| US10512676B2 (en) | 2016-03-30 | 2019-12-24 | Amicus Therapeutics, Inc. | Formulations comprising recombinant acid alpha-glucosidase |
| US10227577B2 (en) | 2016-03-30 | 2019-03-12 | Amicus Therapeutics, Inc. | Method for selection of high M6P recombinant proteins |
| US12246062B2 (en) | 2017-05-15 | 2025-03-11 | Amicus Therapeutics, Inc. | Recombinant human acid alpha-glucosidase |
| KR20210066032A (ko) * | 2017-05-30 | 2021-06-04 | 아미쿠스 세라퓨틱스, 인코포레이티드 | 신장 손상을 갖는 파브리 환자를 치료하는 방법 |
| JP2020203886A (ja) * | 2017-05-30 | 2020-12-24 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
| JP2022071094A (ja) * | 2017-05-30 | 2022-05-13 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
| KR102427610B1 (ko) | 2017-05-30 | 2022-07-29 | 아미쿠스 세라퓨틱스, 인코포레이티드 | 신장 손상을 갖는 파브리 환자를 치료하는 방법 |
| JP2020073500A (ja) * | 2017-05-30 | 2020-05-14 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
| JP2020507562A (ja) * | 2017-05-30 | 2020-03-12 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
| JP2023052005A (ja) * | 2017-05-30 | 2023-04-11 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
| JP2023100658A (ja) * | 2017-05-30 | 2023-07-19 | アミカス セラピューティックス インコーポレイテッド | 腎機能障害を有するファブリー患者の治療方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008245578A1 (en) | 2008-11-06 |
| US20150352093A1 (en) | 2015-12-10 |
| US9056101B2 (en) | 2015-06-16 |
| MX2009011473A (es) | 2010-01-18 |
| WO2008134628A3 (en) | 2010-01-14 |
| WO2008134628A2 (en) | 2008-11-06 |
| EP2150254A2 (en) | 2010-02-10 |
| IL201733A0 (en) | 2010-06-16 |
| CA2685332A1 (en) | 2008-11-06 |
| EP2150254A4 (en) | 2010-11-10 |
| US20100266571A1 (en) | 2010-10-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230364071A1 (en) | Dosing Regimens for the Treatment of Lysosomal Storage Diseases Using Pharmacological Chaperones | |
| JP2010525084A (ja) | 薬理シャペロンを用いたリソソーム蓄積症治療のための投薬計画 | |
| AU2023206175B2 (en) | Treatment of fabry disease in ert-naïve and ert-experienced patients | |
| JP6788725B2 (ja) | 腎機能障害を有するファブリー患者の治療方法 | |
| US20100113517A1 (en) | Method for the treatment of fabry disease using pharmacological chaperones | |
| JP7677910B2 (ja) | 腎機能障害を有する患者のファブリー病を治療する方法 | |
| US20210315875A1 (en) | Methods of Treating Fabry Disease in Patients Having a Mutation in the GLA Gene | |
| JP2020531550A (ja) | ファブリー病患者における心臓機能の強化及び/または安定化方法 | |
| KR20220150902A (ko) | 파브리 질병을 치료하는 방법 | |
| JP7749572B2 (ja) | Gla遺伝子の突然変異を有する患者におけるファブリー病を治療する方法 | |
| EP4374918B1 (en) | Methods of treating fabry patients having renal impairment | |
| JP2022518733A (ja) | ファブリー病患者の脳血管事象のリスク低減におけるミガロスタットの使用 | |
| AU2018220047A1 (en) | A method for treatment of fabry disease |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110331 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110331 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20110927 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130319 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20131210 |