JP2010519274A - TNFおよびα−ガラクトシルセラミドを使用する併用療法 - Google Patents
TNFおよびα−ガラクトシルセラミドを使用する併用療法 Download PDFInfo
- Publication number
- JP2010519274A JP2010519274A JP2009550701A JP2009550701A JP2010519274A JP 2010519274 A JP2010519274 A JP 2010519274A JP 2009550701 A JP2009550701 A JP 2009550701A JP 2009550701 A JP2009550701 A JP 2009550701A JP 2010519274 A JP2010519274 A JP 2010519274A
- Authority
- JP
- Japan
- Prior art keywords
- tnf
- galcer
- treatment
- galactosylceramide
- administration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/191—Tumor necrosis factors [TNF], e.g. lymphotoxin [LT], i.e. TNF-beta
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP07102787 | 2007-02-21 | ||
| PCT/EP2008/052055 WO2008101951A1 (en) | 2007-02-21 | 2008-02-20 | Combination therapy using tnf and alfa-galactosyl ceramide |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010519274A true JP2010519274A (ja) | 2010-06-03 |
| JP2010519274A5 JP2010519274A5 (enExample) | 2011-04-07 |
Family
ID=39495301
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009550701A Pending JP2010519274A (ja) | 2007-02-21 | 2008-02-20 | TNFおよびα−ガラクトシルセラミドを使用する併用療法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US7959907B2 (enExample) |
| EP (1) | EP2117583B1 (enExample) |
| JP (1) | JP2010519274A (enExample) |
| AU (1) | AU2008219269B2 (enExample) |
| CA (1) | CA2678088A1 (enExample) |
| WO (1) | WO2008101951A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7959907B2 (en) | 2007-02-21 | 2011-06-14 | Vib Vzw | Method of treating cancer by combination therapy using TNF and alpha-galactosylceramide |
| WO2016065353A1 (en) * | 2014-10-24 | 2016-04-28 | University Of Miami | Combination therapy with fenofibrate and 2-deoxyglucose or 2-deoxymannose |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4677063A (en) * | 1985-05-02 | 1987-06-30 | Cetus Corporation | Human tumor necrosis factor |
| US4677064A (en) * | 1984-11-09 | 1987-06-30 | Cetus Corporation | Human tumor necrosis factor |
| JPH0559081A (ja) | 1991-08-29 | 1993-03-09 | Kirin Brewery Co Ltd | 新規スフインゴ糖脂質、その製造法および使用 |
| US6001619A (en) | 1995-10-04 | 1999-12-14 | Cold Spring Harbor Laboratory | Ubiquitin ligases, and uses related thereto |
| EP1192174A4 (en) | 1999-06-11 | 2002-08-28 | Univ New York State Res Found | ANTAGONISTS OF BMP AND TGF BETA SIGNAL PATHS |
| US7273852B2 (en) * | 2002-06-13 | 2007-09-25 | The Research Foundation Of The City University Of New York | Synthetic C-glycolipid and its use for treating cancer, infectious diseases and autoimmune diseases |
| EP1639008A1 (en) | 2003-07-01 | 2006-03-29 | VIB vzw | Ubiquitinated tnf receptor 2 and its uses |
| US7772380B2 (en) | 2004-08-27 | 2010-08-10 | Albert Einstein College Of Medicine Of Yeshiva University | Ceramide derivatives as modulators of immunity and autoimmunity |
| US7959907B2 (en) | 2007-02-21 | 2011-06-14 | Vib Vzw | Method of treating cancer by combination therapy using TNF and alpha-galactosylceramide |
-
2008
- 2008-02-20 US US12/449,652 patent/US7959907B2/en not_active Expired - Fee Related
- 2008-02-20 EP EP08716968.6A patent/EP2117583B1/en not_active Not-in-force
- 2008-02-20 CA CA002678088A patent/CA2678088A1/en not_active Abandoned
- 2008-02-20 WO PCT/EP2008/052055 patent/WO2008101951A1/en not_active Ceased
- 2008-02-20 AU AU2008219269A patent/AU2008219269B2/en not_active Expired - Fee Related
- 2008-02-20 JP JP2009550701A patent/JP2010519274A/ja active Pending
Non-Patent Citations (3)
| Title |
|---|
| JPN6012054914; Eur.J.Biochem., 1985, Vol. 152, No. 3, P. 515-522 * |
| JPN6012054918; Clin.Exp.Metastasis, 2000, Vol. 18, No. 2, P. 147-153 * |
| JPN6012054920; Cancer Sci., 2008.Jan., Vol. 99, No. 1, P. 113-120 * |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008219269B2 (en) | 2012-10-25 |
| US20100166697A1 (en) | 2010-07-01 |
| US7959907B2 (en) | 2011-06-14 |
| CA2678088A1 (en) | 2008-08-28 |
| EP2117583B1 (en) | 2013-05-22 |
| WO2008101951A1 (en) | 2008-08-28 |
| AU2008219269A1 (en) | 2008-08-28 |
| EP2117583A1 (en) | 2009-11-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Colombo et al. | Interleukin-12 in anti-tumor immunity and immunotherapy | |
| Younes et al. | Interferon‐γ therapy: Evaluation of routes of administration and delivery systems | |
| KR101192136B1 (ko) | 혈액 줄기 세포의 동원에 유효한 g-csf와 plgf의조합 약제 | |
| Sarkar et al. | Protective effect of neoglycoprotein-conjugated muramyl dipeptide against Leishmania donovani infection: the role of cytokines | |
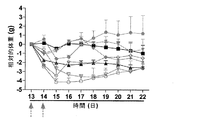
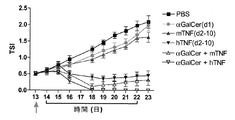
| EP2117583B1 (en) | Combination therapy using TNF and alpha-galactosyl ceramide | |
| Negrier et al. | Phase I trial of recombinant interleukin-2 followed by recombinant tumor necrosis factor in patients with metastatic cancer | |
| Takahashi et al. | Induction of tolerance allows separation of lethal and antitumor activities of tumor necrosis factor in mice | |
| EP0248516B1 (en) | Compositions and the use of interleukin-2 and/or interferon-beta and tumour necrosis factor for combination therapy or in providing medicaments or formulations | |
| US5096707A (en) | Flavone-8-acetic acid and interleukin-2 in a method of treating certain cancers | |
| ES2448837T3 (es) | Agente que comprende G-CSF para la prevención y el tratamiento de neuropatía periférica diabética | |
| Ohno | Granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor in the treatment of acute myeloid leukemia and acute lymphoblastic leukemia | |
| AU2012340446B2 (en) | Apoaequorin for reducing neuronal injury due to ischemia | |
| CN101068564B (zh) | 含有vegf抑制剂与5fu或其中一种衍生物的抗肿瘤组合物 | |
| Sone et al. | Local lnterleukin-2 Therapy for Cancer, and Its Effector Induction Mechanisms | |
| JP2010529984A (ja) | 癌を治療するためのインターフェロンアルファ逐次レジメン | |
| Balemans et al. | PEG‐IL‐2 therapy of advanced cancer in the guinea pig. Impact of the primary tumor and beneficial effect of cyclopphosphamide | |
| Teicher et al. | Optimal scheduling of interleukin‐12 and fractionated radiation therapy in the murine lewis lung carcinoma | |
| van Moorselaar et al. | Synergistic antitumor effects of rat γ-interferon and human tumor necrosis factor α against androgen-dependent and-independent rat prostatic tumors | |
| IJzermans et al. | Injection of recombinant tumor necrosis factor directly into liver metastases: an experimental and clinical approach | |
| Frankfurt et al. | Growth factors in leukemia | |
| Gottlieb | Cytokine manipulation of the immune response in the treatment of human acute leukaemia | |
| JPH09510737A (ja) | 化学療法剤を増強するためのil−4の使用 | |
| TW202019440A (zh) | 用於治療癌症之組合療法 | |
| Krosnick et al. | Studies of the mechanisms of toxicity of the administration of recombinant tumor necrosis factor α in normal and tumor-bearing mice | |
| Ravandi et al. | CYTOKINES IN THE TREATMENT OF ACUTE |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110218 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110218 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121023 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20130423 |