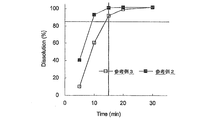
図1は、実施例1の小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の溶出挙動を示す図である。1 is a diagram showing the dissolution behavior of a miniaturized sarpogrelate hydrochloride oral administration preparation (100 mg preparation) of Example 1. FIG.
図2は、従来の製剤(100mg製剤)の溶出挙動を示す図である。FIG. 2 is a diagram showing the dissolution behavior of a conventional preparation (100 mg preparation).
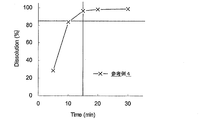
図3は、参考例1の小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の溶出挙動を示す図である。FIG. 3 is a view showing the dissolution behavior of the miniaturized sarpogrelate hydrochloride oral administration preparation (100 mg preparation) of Reference Example 1 .
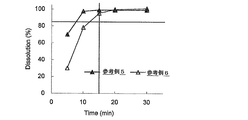
図4は、参考例2〜3の小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の溶出挙動を示す図である。FIG. 4 is a view showing the dissolution behavior of the miniaturized sarpogrelate hydrochloride oral administration preparation (100 mg preparation) of Reference Examples 2 to 3 .
図5は、参考例4の小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の溶出挙動を示す図である。FIG. 5 is a view showing the dissolution behavior of the miniaturized sarpogrelate hydrochloride oral administration preparation (100 mg preparation) of Reference Example 4 .
図6は、参考例5〜6の小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の溶出挙動を示す図である。FIG. 6 is a diagram showing the dissolution behavior of miniaturized sarpogrelate hydrochloride oral administration preparations (100 mg preparation) of Reference Examples 5 to 6 .
参考例1:カルメロースカルシウム含有小型化塩酸サルポグレラート経口投与製剤の製造
(1)塩酸サルポグレラート56重量%、結晶セルロース19重量%、カルメロースカルシウム(ニチリン化学製、ECG505)14重量%及び軽質無水ケイ酸1重量%を混合した。その後、高速撹拌造粒機(パウレック製、バーチカルグラニュレーター)を用いて、クエン酸1重量%及びヒドロキシプロピルセルロース2重量%を含む水溶液で造粒後、乾燥し、造粒顆粒を得た。この顆粒にステアリン酸マグネシウム2.4重量%を加え、ロータリー打錠機で圧縮成形(直径7.5mm)することにより、素錠を得た。
(2)ヒドロキシプロピルメチルセルロース(信越化学製、TC-5RG)3重量%、酸化チタン0.6重量%、マクロゴール6000 0.6重量%及びタルク0.4重量%を精製水に溶解又は分散し、コーティング液を得た。
(3)上記(1)で得られた素錠をコーティングパンに入れ、上記(2)で得られたコーティング液をスプレーすることにより、コーティング錠を得た。
Reference Example 1 : Production of a miniaturized sarpogrelate hydrochloride orally administered preparation containing carmellose calcium (1) 56% by weight of sarpogrelate hydrochloride, 19% by weight of crystalline cellulose, 14% by weight of carmellose calcium (manufactured by Nichirin Chemical Co., ECG505) and light anhydrous silicic acid 1 wt% was mixed. Thereafter, the mixture was granulated with an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose using a high-speed agitation granulator (manufactured by POWREC, vertical granulator), and dried to obtain granulated granules. An uncoated tablet was obtained by adding 2.4% by weight of magnesium stearate to this granule and compression-molding (diameter 7.5 mm) with a rotary tableting machine.
(2) 3% by weight of hydroxypropyl methylcellulose (manufactured by Shin-Etsu Chemical Co., Ltd., TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of Macrogol 6000, and 0.4% by weight of talc are dissolved or dispersed in purified water. A coating solution was obtained.
(3) The uncoated tablet obtained in the above (1) was put in a coating pan, and the coating liquid obtained in the above (2) was sprayed to obtain a coated tablet.
参考例2:カルボキシメチルスターチナトリウム含有小型化塩酸サルポグレラート経口投与製剤の製造(攪拌造粒)
(1)塩酸サルポグレラート56重量%、結晶セルロース23重量%、カルボキシメチルスターチナトリウム(DMV製、プリモジェル)10重量%及び軽質無水ケイ酸1重量%を混合した。その後、高速撹拌造粒機(パウレック製、バーチカルグラニュレーター)を用いて、クエン酸1重量%及びヒドロキシプロピルセルロース2重量%を含む水溶液で造粒後、乾燥し、造粒顆粒を得た。この顆粒にステアリン酸マグネシウム2.4重量%を加え、ロータリー打錠機で圧縮成形(直径7.5mm)することにより、素錠を得た。
(2)ヒドロキシプロピルメチルセルロース(信越化学製、TC-5RG)3重量%、酸化チタン0.6重量%、マクロゴール6000 0.6重量%及びタルク0.4重量%を精製水に溶解又は分散し、コーティング液を得た。
(3)上記(1)で得られた素錠をコーティングパンに入れ、上記(2)で得られたコーティング液をスプレーすることにより、コーティング錠を得た。
Reference Example 2 : Production of a miniaturized sarpogrelate hydrochloride-containing preparation containing sodium carboxymethyl starch (agitation granulation)
(1) 56% by weight of sarpogrelate hydrochloride, 23% by weight of crystalline cellulose, 10% by weight of sodium carboxymethyl starch (manufactured by DMV, Primogel) and 1% by weight of light anhydrous silicic acid were mixed. Thereafter, the mixture was granulated with an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose using a high-speed agitation granulator (manufactured by POWREC, vertical granulator), and dried to obtain granulated granules. An uncoated tablet was obtained by adding 2.4% by weight of magnesium stearate to this granule and compression-molding (diameter 7.5 mm) with a rotary tableting machine.
(2) 3% by weight of hydroxypropyl methylcellulose (manufactured by Shin-Etsu Chemical Co., Ltd., TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of Macrogol 6000, and 0.4% by weight of talc are dissolved or dispersed in purified water. A coating solution was obtained.
(3) The uncoated tablet obtained in the above (1) was put in a coating pan, and the coating liquid obtained in the above (2) was sprayed to obtain a coated tablet.
参考例3:カルボキシメチルスターチナトリウム含有小型化塩酸サルポグレラート経口投与製剤の製造(流動層造粒)
(1)塩酸サルポグレラート56重量%、乳糖17重量%、コーンスターチ7重量%を、流動層造粒機(パウレック製、マルチプレックス)を用いて、クエン酸1重量%及びヒドロキシプロピルセルロース2重量%を含む水溶液で造粒後、乾燥し、造粒顆粒を得た。この顆粒に、クロスカルメロースナトリウム10重量%(DMV製、アクチゾル)及びステアリン酸マグネシウム2.4重量%を加え、ロータリー打錠機で圧縮成形(直径7.5mm)することにより、素錠を得た。
(2)ヒドロキシプロピルメチルセルロース(信越化学製、TC-5RG)3重量%、酸化チタン0.6重量%、マクロゴール6000 0.6重量%及びタルク0.4重量%を精製水に溶解又は分散し、コーティング液を得た。
(3)上記(1)で得られた素錠をコーティングパンに入れ、上記(2)で得られたコーティング液をスプレーすることにより、コーティング錠を得た。
Reference Example 3 : Production of miniaturized sarpogrelate hydrochloride oral administration preparation containing sodium carboxymethyl starch (fluidized bed granulation)
(1) Sarpogrelate hydrochloride 56% by weight, lactose 17% by weight, corn starch 7% by weight, using a fluidized bed granulator (manufactured by POWREC, multiplex), containing 1% by weight citric acid and 2% by weight hydroxypropylcellulose After granulation with an aqueous solution, it was dried to obtain granulated granules. By adding 10% by weight of croscarmellose sodium (manufactured by DMV, Actisol) and 2.4% by weight of magnesium stearate to this granule, compression molding (diameter: 7.5 mm) with a rotary tableting machine is obtained. It was.
(2) 3% by weight of hydroxypropyl methylcellulose (manufactured by Shin-Etsu Chemical Co., Ltd., TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of Macrogol 6000, and 0.4% by weight of talc are dissolved or dispersed in purified water. A coating solution was obtained.
(3) The uncoated tablet obtained in the above (1) was put in a coating pan, and the coating liquid obtained in the above (2) was sprayed to obtain a coated tablet.
参考例4:ポビドン含有小型化塩酸サルポグレラート経口投与製剤の製造
(1)塩酸サルポグレラート56重量%、結晶セルロース19重量%、ポビドン(ISP製、プラスドンXL10)14重量%及び軽質無水ケイ酸1重量%を混合した。その後、高速撹拌造粒機(パウレック製、バーチカルグラニュレーター)を用いて、クエン酸1重量%及びヒドロキシプロピルセルロース2重量%を含む水溶液で造粒後、乾燥し、造粒顆粒を得た。この顆粒にステアリン酸マグネシウム2.4重量%を加え、ロータリー打錠機で圧縮成形(直径7.5mm)することにより、素錠を得た。
(2)ヒドロキシプロピルメチルセルロース(信越化学製、TC-5RG)3重量%、酸化チタン0.6重量%、マクロゴール6000 0.6重量%及びタルク0.4重量%を精製水に溶解又は分散し、コーティング液を得た。
(3)上記(1)で得られた素錠をコーティングパンに入れ、上記(2)で得られたコーティング液をスプレーすることにより、コーティング錠を得た。
Reference Example 4 : Production of povidone-containing miniaturized sarpogrelate hydrochloride oral administration preparation (1) 56% by weight of sarpogrelate hydrochloride, 19% by weight of crystalline cellulose, 14% by weight of povidone (manufactured by ISP, Plusdon XL10) and 1% by weight of light anhydrous silicic acid Mixed. Thereafter, the mixture was granulated with an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose using a high-speed agitation granulator (manufactured by POWREC, vertical granulator), and dried to obtain granulated granules. An uncoated tablet was obtained by adding 2.4% by weight of magnesium stearate to this granule and compression-molding (diameter 7.5 mm) with a rotary tableting machine.
(2) 3% by weight of hydroxypropyl methylcellulose (manufactured by Shin-Etsu Chemical Co., Ltd., TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of Macrogol 6000, and 0.4% by weight of talc are dissolved or dispersed in purified water. A coating solution was obtained.
(3) The uncoated tablet obtained in the above (1) was put in a coating pan, and the coating liquid obtained in the above (2) was sprayed to obtain a coated tablet.
参考例5:クロスカルメロースナトリウム含有小型化塩酸サルポグレラート経口投与製剤の製造(攪拌造粒)
(1)塩酸サルポグレラート56重量%、結晶セルロース23重量%、クロスカルメロースナトリウム(FMC製、アクチゾル)10重量%及び軽質無水ケイ酸1重量%を混合した。その後、高速撹拌造粒機(パウレック製、バーチカルグラニュレーター)を用いて、クエン酸1重量%及びヒドロキシプロピルセルロース2重量%を含む水溶液で造粒後、乾燥し、造粒顆粒を得た。この顆粒にステアリン酸マグネシウム2.4重量%を加え、ロータリー打錠機で圧縮成形(直径7.5mm)することにより、素錠を得た。
(2)ヒドロキシプロピルメチルセルロース(信越化学製、TC-5RG)3重量%、酸化チタン0.6重量%、マクロゴール6000 0.6重量%及びタルク0.4重量%を精製水に溶解又は分散し、コーティング液を得た。
(3)上記(1)で得られた素錠をコーティングパンに入れ、上記(2)で得られたコーティング液をスプレーすることにより、コーティング錠を得た。
Reference Example 5 : Production of miniaturized sarpogrelate hydrochloride oral administration preparation containing croscarmellose sodium (stir granulation)
(1) Sarpogrelate hydrochloride 56% by weight, crystalline cellulose 23% by weight, croscarmellose sodium (manufactured by FMC, Actisol) 10% by weight and light anhydrous silicic acid 1% by weight were mixed. Thereafter, the mixture was granulated with an aqueous solution containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose using a high-speed agitation granulator (manufactured by POWREC, vertical granulator), and dried to obtain granulated granules. An uncoated tablet was obtained by adding 2.4% by weight of magnesium stearate to this granule and compression-molding (diameter 7.5 mm) with a rotary tableting machine.
(2) 3% by weight of hydroxypropyl methylcellulose (manufactured by Shin-Etsu Chemical Co., Ltd., TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of Macrogol 6000, and 0.4% by weight of talc are dissolved or dispersed in purified water. A coating solution was obtained.
(3) The uncoated tablet obtained in the above (1) was put in a coating pan, and the coating liquid obtained in the above (2) was sprayed to obtain a coated tablet.
参考例6:クロスカルメロースナトリウム含有小型化塩酸サルポグレラート経口投与製剤の製造(流動層造粒)
(1)塩酸サルポグレラート59重量%、乳糖18重量%、コーンスターチ8重量%を、流動層造粒機(パウレック製、マルチプレックス)を用いて、クエン酸1重量%及びヒドロキシプロピルセルロース2重量%を含む水溶液で造粒後、乾燥し、造粒顆粒を得た。この顆粒に、クロスカルメロースナトリウム5重量%(DMV製、アクチゾル)及びステアリン酸マグネシウム2.4重量%を加え、ロータリー打錠機で圧縮成形(直径7.5mm)することにより、素錠を得た。
(2)ヒドロキシプロピルメチルセルロース(信越化学製、TC-5RG)3重量%、酸化チタン0.6重量%、マクロゴール6000 0.6重量%及びタルク0.4重量%を精製水に溶解又は分散し、コーティング液を得た。
(3)上記(1)で得られた素錠をコーティングパンに入れ、上記(2)で得られたコーティング液をスプレーすることにより、コーティング錠を得た。
Reference Example 6 : Production of croscarmellose sodium-containing miniaturized sarpogrelate hydrochloride oral administration formulation (fluidized bed granulation)
(1) 59% by weight of sarpogrelate hydrochloride, 18% by weight of lactose, and 8% by weight of corn starch, containing 1% by weight of citric acid and 2% by weight of hydroxypropylcellulose using a fluidized bed granulator (manufactured by POWREC, multiplex) After granulation with an aqueous solution, it was dried to obtain granulated granules. By adding 5% by weight of croscarmellose sodium (manufactured by DMV, Actisol) and 2.4% by weight of magnesium stearate to this granule, compression molding (diameter: 7.5 mm) with a rotary tableting machine is obtained. It was.
(2) 3% by weight of hydroxypropyl methylcellulose (manufactured by Shin-Etsu Chemical Co., Ltd., TC-5RG), 0.6% by weight of titanium oxide, 0.6% by weight of Macrogol 6000, and 0.4% by weight of talc are dissolved or dispersed in purified water. A coating solution was obtained.
(3) The uncoated tablet obtained in the above (1) was put in a coating pan, and the coating liquid obtained in the above (2) was sprayed to obtain a coated tablet.
試験例2:小型化塩酸サルポグレラート経口投与製剤の溶出試験
参考例1〜6で製造した小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の溶出試験を日本薬局方溶出試験法第2法(パドル法)に従って行った。具体的には、測定条件を、試験液量900mL、パドル回転数50min-1、温度37.5℃とした。
ここで、小型化塩酸サルポグレラート経口投与製剤(100mg製剤)の試験液としては、pH6.8(日本薬局方崩壊試験液第二液)を使用した。
この結果、参考例1〜6で製造した小型化塩酸サルポグレラート経口投与製剤において、有効成分である塩酸サルポグレラートの80重量%以上が、試験開始から15分以内に溶出することが明らかとなった。従って、本発明にかかる小型化塩酸サルポグレラート経口投与製剤は、従来の製剤に比べて、有効成分である塩酸サルポグレラートの含有率が高く、小型化されているにもかかわらず、従来の製剤と同様に速やかな溶出速度を示す薬剤であることが明らかとなった。
Test Example 2: Dissolution test of a miniaturized sarpogrelate hydrochloride oral dosage form
The dissolution test of the miniaturized sarpogrelate hydrochloride oral administration preparation (100 mg preparation) produced in Reference Examples 1 to 6 was performed according to the Japanese Pharmacopoeia Dissolution Test Method 2 (Paddle Method). Specifically, the measurement conditions were a test solution volume of 900 mL, a paddle rotation speed of 50 min −1 , and a temperature of 37.5 ° C.
Here, as a test solution of a miniaturized sarpogrelate hydrochloride oral administration formulation (100 mg formulation), pH 6.8 (Japanese Pharmacopoeia Disintegration Test Solution Second Solution) was used.
As a result, in the miniaturized sarpogrelate hydrochloride oral administration preparations produced in Reference Examples 1 to 6 , it was revealed that 80% by weight or more of sarpogrelate hydrochloride as an active ingredient was eluted within 15 minutes from the start of the test. Therefore, the miniaturized sarpogrelate hydrochloride oral administration preparation according to the present invention has a high content of sarpogrelate hydrochloride, which is an active ingredient, as compared with the conventional preparation, and is similar to the conventional preparation despite being miniaturized. It was revealed that the drug showed a rapid dissolution rate.