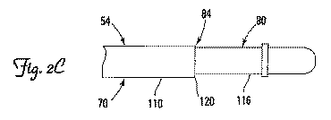
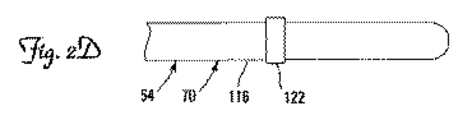
JP2009539425A - 展開可能な固定機構を有する医療用電気リード - Google Patents
展開可能な固定機構を有する医療用電気リード Download PDFInfo
- Publication number
- JP2009539425A JP2009539425A JP2009513360A JP2009513360A JP2009539425A JP 2009539425 A JP2009539425 A JP 2009539425A JP 2009513360 A JP2009513360 A JP 2009513360A JP 2009513360 A JP2009513360 A JP 2009513360A JP 2009539425 A JP2009539425 A JP 2009539425A
- Authority
- JP
- Japan
- Prior art keywords
- lead
- region
- tendon
- distal
- deflectable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000007246 mechanism Effects 0.000 title abstract description 49
- 210000002435 tendon Anatomy 0.000 claims abstract description 135
- 230000002526 effect on cardiovascular system Effects 0.000 claims abstract description 23
- 239000000463 material Substances 0.000 claims description 21
- 229920002635 polyurethane Polymers 0.000 claims description 4
- 239000004814 polyurethane Substances 0.000 claims description 4
- 230000007704 transition Effects 0.000 claims description 4
- 229920001296 polysiloxane Polymers 0.000 claims description 3
- 239000007779 soft material Substances 0.000 claims 2
- 230000008859 change Effects 0.000 abstract description 7
- 230000007774 longterm Effects 0.000 abstract description 7
- 230000001154 acute effect Effects 0.000 abstract description 5
- 238000003780 insertion Methods 0.000 description 17
- 230000037431 insertion Effects 0.000 description 17
- 210000003748 coronary sinus Anatomy 0.000 description 13
- 238000000034 method Methods 0.000 description 13
- 230000033001 locomotion Effects 0.000 description 11
- 230000036961 partial effect Effects 0.000 description 10
- 230000002792 vascular Effects 0.000 description 9
- 238000004873 anchoring Methods 0.000 description 8
- 230000000747 cardiac effect Effects 0.000 description 8
- 229920000642 polymer Polymers 0.000 description 8
- 238000000576 coating method Methods 0.000 description 7
- 239000004020 conductor Substances 0.000 description 7
- 210000002216 heart Anatomy 0.000 description 7
- 230000009471 action Effects 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 5
- 210000005166 vasculature Anatomy 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical group [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 4
- 229910001000 nickel titanium Inorganic materials 0.000 description 4
- 239000004810 polytetrafluoroethylene Substances 0.000 description 4
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 4
- 230000003014 reinforcing effect Effects 0.000 description 4
- 210000005245 right atrium Anatomy 0.000 description 4
- 210000003462 vein Anatomy 0.000 description 4
- 150000002739 metals Chemical class 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- 239000004696 Poly ether ether ketone Substances 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 210000004351 coronary vessel Anatomy 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 230000001965 increasing effect Effects 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- 210000005240 left ventricle Anatomy 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- 229920002530 polyetherether ketone Polymers 0.000 description 2
- 230000000452 restraining effect Effects 0.000 description 2
- 230000033764 rhythmic process Effects 0.000 description 2
- 210000005241 right ventricle Anatomy 0.000 description 2
- 238000007788 roughening Methods 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 210000001174 endocardium Anatomy 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 238000013152 interventional procedure Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 210000005246 left atrium Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 239000003356 suture material Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/056—Transvascular endocardial electrode systems
- A61N1/057—Anchoring means; Means for fixing the head inside the heart
- A61N1/0573—Anchoring means; Means for fixing the head inside the heart chacterised by means penetrating the heart tissue, e.g. helix needle or hook
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B18/1492—Probes or electrodes therefor having a flexible, catheter-like structure, e.g. for heart ablation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/056—Transvascular endocardial electrode systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/056—Transvascular endocardial electrode systems
- A61N2001/0585—Coronary sinus electrodes
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Vascular Medicine (AREA)
- Radiology & Medical Imaging (AREA)
- Surgery (AREA)
- Physics & Mathematics (AREA)
- Plasma & Fusion (AREA)
- Otolaryngology (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Electrotherapy Devices (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/421,990 US7890174B2 (en) | 2006-06-02 | 2006-06-02 | Medical electrical lead with deployable fixation features |
| PCT/US2007/067885 WO2007143304A2 (en) | 2006-06-02 | 2007-05-01 | Medical electrical lead with deployable fixation features |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009539425A true JP2009539425A (ja) | 2009-11-19 |
| JP2009539425A5 JP2009539425A5 (enExample) | 2010-06-17 |
Family
ID=38543930
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009513360A Pending JP2009539425A (ja) | 2006-06-02 | 2007-05-01 | 展開可能な固定機構を有する医療用電気リード |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US7890174B2 (enExample) |
| EP (1) | EP2051771B1 (enExample) |
| JP (1) | JP2009539425A (enExample) |
| AT (1) | ATE549059T1 (enExample) |
| WO (1) | WO2007143304A2 (enExample) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013526346A (ja) * | 2010-05-10 | 2013-06-24 | スパイナル・モデュレーション・インコーポレイテッド | 位置ずれを抑制するための方法、システムおよびデバイス |
| US8983624B2 (en) | 2006-12-06 | 2015-03-17 | Spinal Modulation, Inc. | Delivery devices, systems and methods for stimulating nerve tissue on multiple spinal levels |
| US9044592B2 (en) | 2007-01-29 | 2015-06-02 | Spinal Modulation, Inc. | Sutureless lead retention features |
| US9056197B2 (en) | 2008-10-27 | 2015-06-16 | Spinal Modulation, Inc. | Selective stimulation systems and signal parameters for medical conditions |
| US9205261B2 (en) | 2004-09-08 | 2015-12-08 | The Board Of Trustees Of The Leland Stanford Junior University | Neurostimulation methods and systems |
| US9259569B2 (en) | 2009-05-15 | 2016-02-16 | Daniel M. Brounstein | Methods, systems and devices for neuromodulating spinal anatomy |
| US9314618B2 (en) | 2006-12-06 | 2016-04-19 | Spinal Modulation, Inc. | Implantable flexible circuit leads and methods of use |
| US9327110B2 (en) | 2009-10-27 | 2016-05-03 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Devices, systems and methods for the targeted treatment of movement disorders |
| US9427570B2 (en) | 2006-12-06 | 2016-08-30 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Expandable stimulation leads and methods of use |
| US9468762B2 (en) | 2009-03-24 | 2016-10-18 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Pain management with stimulation subthreshold to paresthesia |
| US9486633B2 (en) | 2004-09-08 | 2016-11-08 | The Board Of Trustees Of The Leland Stanford Junior University | Selective stimulation to modulate the sympathetic nervous system |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030199961A1 (en) | 2002-04-03 | 2003-10-23 | Bjorklund Vicki L. | Method and apparatus for fixating a pacing lead of an implantable medical device |
| US7657482B1 (en) * | 2002-07-15 | 2010-02-02 | Paymentech, L.P. | System and apparatus for transaction fraud processing |
| EP2197539A1 (en) * | 2008-04-30 | 2010-06-23 | Medtronic, Inc. | Techniques for placing medical leads for electrical stimulation of nerve tissue |
| US8005539B2 (en) | 2008-10-31 | 2011-08-23 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US9775987B2 (en) | 2008-10-31 | 2017-10-03 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8260412B2 (en) | 2008-10-31 | 2012-09-04 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8611996B2 (en) | 2008-10-31 | 2013-12-17 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8532779B2 (en) | 2008-10-31 | 2013-09-10 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8249708B2 (en) | 2008-10-31 | 2012-08-21 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8688210B2 (en) | 2008-10-31 | 2014-04-01 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8774918B2 (en) | 2008-10-31 | 2014-07-08 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US9597505B2 (en) | 2008-10-31 | 2017-03-21 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US8452394B2 (en) | 2008-10-31 | 2013-05-28 | Medtronic, Inc. | Implantable medical device crosstalk evaluation and mitigation |
| US20110004288A1 (en) * | 2009-06-12 | 2011-01-06 | Terrance Ransbury | Intravascular implantable device having integrated anchor mechanism |
| US8521305B2 (en) | 2010-05-11 | 2013-08-27 | St. Jude Medical, Inc. | Percutaneous lead with distal fixation |
| US9744349B2 (en) | 2011-02-10 | 2017-08-29 | Respicardia, Inc. | Medical lead and implantation |
| EP2701795B1 (en) | 2011-04-28 | 2020-12-09 | Interventional Autonomics Corporation | Neuromodulation systems for treating acute heart failure syndromes |
| US9446240B2 (en) | 2011-07-11 | 2016-09-20 | Interventional Autonomics Corporation | System and method for neuromodulation |
| US20130072995A1 (en) | 2011-07-11 | 2013-03-21 | Terrance Ransbury | Catheter system for acute neuromodulation |
| EP2766086A4 (en) | 2011-07-11 | 2015-09-30 | Interventional Autonomics Corp | SYSTEM AND METHOD FOR NEUROMODULATION |
| US20130158640A1 (en) * | 2011-12-19 | 2013-06-20 | Brian D. Soltis | Lead anchoring system with limited movement of anchoring device along lead |
| WO2013096627A1 (en) * | 2011-12-21 | 2013-06-27 | Cardiac Pacemakers, Inc. | Directional features for implantable medical leads |
| US20130245739A1 (en) * | 2012-03-14 | 2013-09-19 | Anatoly Arber | Self-anchored stimulator lead and method of insertion |
| US8897892B2 (en) | 2012-10-29 | 2014-11-25 | Cardiac Pacemakers, Inc. | Suture sleeves having exterior surface tear resistance |
| US9486622B2 (en) | 2012-11-08 | 2016-11-08 | Cardiac Pacemakers, Inc. | Fixation and strain relief element for temporary therapy delivery device |
| US10315028B2 (en) | 2014-04-23 | 2019-06-11 | Medtronic, Inc. | Active fixation medical electrical lead |
| EP3294408B1 (en) * | 2015-05-13 | 2021-08-25 | Medtronic, Inc. | Securing an implantable medical device in position while reducing perforations |
| AU2016264610B2 (en) | 2015-05-20 | 2018-11-08 | Cardiac Pacemakers, Inc. | Fully integrated lead stabilizer for medical electrical leads and methods of attachment |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09506541A (ja) * | 1995-04-28 | 1997-06-30 | ターゲット セラピューティクス, インコーポレイテッド | 高性能ブレードカテーテル |
| JPH11239617A (ja) * | 1997-11-13 | 1999-09-07 | Impella Cardiotechnik Gmbh | 改良されたカニュ―ル装置 |
| JP2001029480A (ja) * | 1999-07-23 | 2001-02-06 | Terumo Corp | 生体植設用電極リード |
| JP2002501769A (ja) * | 1997-10-30 | 2002-01-22 | イー.ピー. テクノロジーズ, インコーポレイテッド | プルワイヤを備えるカテーテル遠位アセンブリ |
| WO2005076820A2 (en) * | 2004-02-09 | 2005-08-25 | Cryocor, Inc. | Catheter articulation segment with alternating cuts |
| JP2006507104A (ja) * | 2002-11-25 | 2006-03-02 | エドワーズ ライフサイエンシーズ アクチェンゲゼルシャフト | 血管外組織構造をリモデリングするための方法および装置 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4072154A (en) * | 1976-05-28 | 1978-02-07 | Cardiac Pacemakers, Inc. | Sealing arrangement for heart pacer electrode leads |
| US4401120A (en) * | 1978-11-06 | 1983-08-30 | Medtronic, Inc. | Digital cardiac pacemaker with program acceptance indicator |
| US5545206A (en) * | 1994-12-22 | 1996-08-13 | Ventritex, Inc. | Low profile lead with automatic tine activation |
| US6445958B1 (en) * | 1999-04-15 | 2002-09-03 | Intermedics, Inc. | Steerable coronary sinus defibrillation lead |
| US6697676B2 (en) * | 2000-12-21 | 2004-02-24 | Medtronic, Inc. | Medical electrical lead having an expandable electrode assembly |
| US6610058B2 (en) * | 2001-05-02 | 2003-08-26 | Cardiac Pacemakers, Inc. | Dual-profile steerable catheter |
| US6616628B2 (en) * | 2001-11-16 | 2003-09-09 | Cardiac Pacemakers, Inc. | Steerable catheter with a longitudinally adjustable curved core |
| ATE375127T1 (de) * | 2001-11-29 | 2007-10-15 | Medwaves Inc | Hochfrequenz-kathetersystem mit verbesserten ablenkungs- und steuermechanismen |
| US6755812B2 (en) * | 2001-12-11 | 2004-06-29 | Cardiac Pacemakers, Inc. | Deflectable telescoping guide catheter |
| US6869414B2 (en) * | 2002-03-22 | 2005-03-22 | Cardiac Pacemakers, Inc. | Pre-shaped catheter with proximal articulation and pre-formed distal end |
| US20030199961A1 (en) | 2002-04-03 | 2003-10-23 | Bjorklund Vicki L. | Method and apparatus for fixating a pacing lead of an implantable medical device |
-
2006
- 2006-06-02 US US11/421,990 patent/US7890174B2/en not_active Expired - Fee Related
-
2007
- 2007-05-01 WO PCT/US2007/067885 patent/WO2007143304A2/en not_active Ceased
- 2007-05-01 JP JP2009513360A patent/JP2009539425A/ja active Pending
- 2007-05-01 EP EP07761645A patent/EP2051771B1/en not_active Not-in-force
- 2007-05-01 AT AT07761645T patent/ATE549059T1/de active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09506541A (ja) * | 1995-04-28 | 1997-06-30 | ターゲット セラピューティクス, インコーポレイテッド | 高性能ブレードカテーテル |
| JP2002501769A (ja) * | 1997-10-30 | 2002-01-22 | イー.ピー. テクノロジーズ, インコーポレイテッド | プルワイヤを備えるカテーテル遠位アセンブリ |
| JPH11239617A (ja) * | 1997-11-13 | 1999-09-07 | Impella Cardiotechnik Gmbh | 改良されたカニュ―ル装置 |
| JP2001029480A (ja) * | 1999-07-23 | 2001-02-06 | Terumo Corp | 生体植設用電極リード |
| JP2006507104A (ja) * | 2002-11-25 | 2006-03-02 | エドワーズ ライフサイエンシーズ アクチェンゲゼルシャフト | 血管外組織構造をリモデリングするための方法および装置 |
| WO2005076820A2 (en) * | 2004-02-09 | 2005-08-25 | Cryocor, Inc. | Catheter articulation segment with alternating cuts |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9205261B2 (en) | 2004-09-08 | 2015-12-08 | The Board Of Trustees Of The Leland Stanford Junior University | Neurostimulation methods and systems |
| US10232180B2 (en) | 2004-09-08 | 2019-03-19 | The Board Of Trustees Of The Leland Stanford Junior University | Selective stimulation to modulate the sympathetic nervous system |
| US9486633B2 (en) | 2004-09-08 | 2016-11-08 | The Board Of Trustees Of The Leland Stanford Junior University | Selective stimulation to modulate the sympathetic nervous system |
| US9623233B2 (en) | 2006-12-06 | 2017-04-18 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Delivery devices, systems and methods for stimulating nerve tissue on multiple spinal levels |
| US9314618B2 (en) | 2006-12-06 | 2016-04-19 | Spinal Modulation, Inc. | Implantable flexible circuit leads and methods of use |
| US9427570B2 (en) | 2006-12-06 | 2016-08-30 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Expandable stimulation leads and methods of use |
| US8983624B2 (en) | 2006-12-06 | 2015-03-17 | Spinal Modulation, Inc. | Delivery devices, systems and methods for stimulating nerve tissue on multiple spinal levels |
| US9044592B2 (en) | 2007-01-29 | 2015-06-02 | Spinal Modulation, Inc. | Sutureless lead retention features |
| US9056197B2 (en) | 2008-10-27 | 2015-06-16 | Spinal Modulation, Inc. | Selective stimulation systems and signal parameters for medical conditions |
| US9409021B2 (en) | 2008-10-27 | 2016-08-09 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. | Selective stimulation systems and signal parameters for medical conditions |
| US11890472B2 (en) | 2008-10-27 | 2024-02-06 | Tc1 Llc | Selective stimulation systems and signal parameters for medical conditions |
| US9468762B2 (en) | 2009-03-24 | 2016-10-18 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Pain management with stimulation subthreshold to paresthesia |
| US9259569B2 (en) | 2009-05-15 | 2016-02-16 | Daniel M. Brounstein | Methods, systems and devices for neuromodulating spinal anatomy |
| US9327110B2 (en) | 2009-10-27 | 2016-05-03 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Devices, systems and methods for the targeted treatment of movement disorders |
| JP2013526346A (ja) * | 2010-05-10 | 2013-06-24 | スパイナル・モデュレーション・インコーポレイテッド | 位置ずれを抑制するための方法、システムおよびデバイス |
| US11413451B2 (en) | 2010-05-10 | 2022-08-16 | St. Jude Medical Luxembourg Holdings SMI S.A.R.L. (“SJM LUX SMI”) | Methods, systems and devices for reducing migration |
Also Published As
| Publication number | Publication date |
|---|---|
| US7890174B2 (en) | 2011-02-15 |
| EP2051771A2 (en) | 2009-04-29 |
| WO2007143304A2 (en) | 2007-12-13 |
| US20070282412A1 (en) | 2007-12-06 |
| WO2007143304A3 (en) | 2008-03-27 |
| EP2051771B1 (en) | 2012-03-14 |
| ATE549059T1 (de) | 2012-03-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5214595B2 (ja) | 展開可能な固定機構を有する医療用電気リード | |
| JP2009539425A (ja) | 展開可能な固定機構を有する医療用電気リード | |
| JP5108876B2 (ja) | 固定のための差し込み可能な剛化構造を有する心臓リードアセンブリ | |
| US7477946B2 (en) | Fixation device for coronary venous lead | |
| US7801627B2 (en) | Cardiac lead having self-expanding fixation features | |
| US8712553B2 (en) | Means to securely fixate pacing leads and/or sensors in vessels | |
| EP2827944B1 (en) | Systems and methods for stimulation of vagus nerve | |
| EP3420921B1 (en) | Devices for catheter advancement and delivery of anchors | |
| JP5048764B2 (ja) | 固定のための剛化構造を有する心臓リード | |
| JP5327875B2 (ja) | 静脈内リード固定のための拡張型部材 | |
| US8086324B1 (en) | Intrapericardial lead with distal region configured to optimize lead extraction | |
| HK40003127B (en) | Devices for catheter advancement and delivery of anchors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100422 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100422 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20120301 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120306 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120601 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120608 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120905 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20121010 |