JP2009517381A - ユビキチンc末端ヒドロラーゼ−l1の新規な使用 - Google Patents
ユビキチンc末端ヒドロラーゼ−l1の新規な使用 Download PDFInfo
- Publication number
- JP2009517381A JP2009517381A JP2008542245A JP2008542245A JP2009517381A JP 2009517381 A JP2009517381 A JP 2009517381A JP 2008542245 A JP2008542245 A JP 2008542245A JP 2008542245 A JP2008542245 A JP 2008542245A JP 2009517381 A JP2009517381 A JP 2009517381A
- Authority
- JP
- Japan
- Prior art keywords
- uch
- cancer metastasis
- inhibitor
- screening
- composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 102100025038 Ubiquitin carboxyl-terminal hydrolase isozyme L1 Human genes 0.000 title claims abstract description 220
- 101000759926 Homo sapiens Ubiquitin carboxyl-terminal hydrolase isozyme L1 Proteins 0.000 title abstract description 209
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 137
- 201000011510 cancer Diseases 0.000 claims abstract description 132
- 206010027476 Metastases Diseases 0.000 claims abstract description 80
- 230000009401 metastasis Effects 0.000 claims abstract description 80
- 239000000203 mixture Substances 0.000 claims abstract description 60
- 238000000034 method Methods 0.000 claims abstract description 50
- 238000012216 screening Methods 0.000 claims abstract description 50
- 239000002257 antimetastatic agent Substances 0.000 claims abstract description 40
- 239000003112 inhibitor Substances 0.000 claims abstract description 38
- OPQRFPHLZZPCCH-LTGZKZEYSA-N [(e)-[5-chloro-1-[(2,5-dichlorophenyl)methyl]-2-oxoindol-3-ylidene]amino] acetate Chemical compound C12=CC=C(Cl)C=C2C(=N/OC(=O)C)\C(=O)N1CC1=CC(Cl)=CC=C1Cl OPQRFPHLZZPCCH-LTGZKZEYSA-N 0.000 claims abstract description 8
- 230000002401 inhibitory effect Effects 0.000 claims abstract description 8
- 101710186825 Ubiquitin carboxyl-terminal hydrolase isozyme L1 Proteins 0.000 claims description 30
- 102000005918 Ubiquitin Thiolesterase Human genes 0.000 claims description 24
- 108010005656 Ubiquitin Thiolesterase Proteins 0.000 claims description 24
- 238000012360 testing method Methods 0.000 claims description 19
- 150000001413 amino acids Chemical class 0.000 claims description 12
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 9
- 210000004897 n-terminal region Anatomy 0.000 claims description 6
- 239000002773 nucleotide Substances 0.000 claims description 4
- 125000003729 nucleotide group Chemical group 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 3
- OPQRFPHLZZPCCH-PGMHBOJBSA-N [(z)-[5-chloro-1-[(2,5-dichlorophenyl)methyl]-2-oxoindol-3-ylidene]amino] acetate Chemical compound C12=CC=C(Cl)C=C2C(=N/OC(=O)C)/C(=O)N1CC1=CC(Cl)=CC=C1Cl OPQRFPHLZZPCCH-PGMHBOJBSA-N 0.000 claims 25
- 108091034117 Oligonucleotide Proteins 0.000 claims 6
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 claims 6
- 125000003275 alpha amino acid group Chemical group 0.000 claims 3
- 239000013076 target substance Substances 0.000 claims 2
- 230000014509 gene expression Effects 0.000 abstract description 45
- 230000000694 effects Effects 0.000 abstract description 25
- 230000012292 cell migration Effects 0.000 abstract description 19
- 102000004190 Enzymes Human genes 0.000 abstract description 11
- 108090000790 Enzymes Proteins 0.000 abstract description 11
- 239000000758 substrate Substances 0.000 abstract description 11
- 238000003745 diagnosis Methods 0.000 abstract description 8
- 230000009400 cancer invasion Effects 0.000 abstract description 4
- 239000002671 adjuvant Substances 0.000 abstract description 2
- 238000011394 anticancer treatment Methods 0.000 abstract description 2
- 210000004027 cell Anatomy 0.000 description 124
- 108090000623 proteins and genes Proteins 0.000 description 57
- 102000004169 proteins and genes Human genes 0.000 description 49
- 235000018102 proteins Nutrition 0.000 description 41
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 21
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 21
- 230000009368 gene silencing by RNA Effects 0.000 description 21
- 239000000523 sample Substances 0.000 description 18
- 239000000463 material Substances 0.000 description 17
- 239000000499 gel Substances 0.000 description 14
- 210000001519 tissue Anatomy 0.000 description 14
- 210000004072 lung Anatomy 0.000 description 12
- 239000000126 substance Substances 0.000 description 12
- 241000713666 Lentivirus Species 0.000 description 11
- 102000007469 Actins Human genes 0.000 description 10
- 108010085238 Actins Proteins 0.000 description 10
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 10
- 229940088598 enzyme Drugs 0.000 description 10
- 238000013537 high throughput screening Methods 0.000 description 10
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 9
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 9
- 239000004480 active ingredient Substances 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 8
- 108010084455 Zeocin Proteins 0.000 description 8
- 201000005202 lung cancer Diseases 0.000 description 8
- 208000020816 lung neoplasm Diseases 0.000 description 8
- CWCMIVBLVUHDHK-ZSNHEYEWSA-N phleomycin D1 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC[C@@H](N=1)C=1SC=C(N=1)C(=O)NCCCCNC(N)=N)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C CWCMIVBLVUHDHK-ZSNHEYEWSA-N 0.000 description 8
- 101000990902 Homo sapiens Matrix metalloproteinase-9 Proteins 0.000 description 7
- 102100030412 Matrix metalloproteinase-9 Human genes 0.000 description 7
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 7
- 238000010586 diagram Methods 0.000 description 7
- 230000009545 invasion Effects 0.000 description 7
- 238000004445 quantitative analysis Methods 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 238000001262 western blot Methods 0.000 description 7
- 102100026802 72 kDa type IV collagenase Human genes 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 101000627872 Homo sapiens 72 kDa type IV collagenase Proteins 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 108090000848 Ubiquitin Proteins 0.000 description 6
- 102000044159 Ubiquitin Human genes 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 238000003556 assay Methods 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 239000013604 expression vector Substances 0.000 description 6
- 201000001441 melanoma Diseases 0.000 description 6
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- IDLAOWFFKWRNHB-UHFFFAOYSA-N 4,5,6,7-tetrachloroindene-1,3-dione Chemical compound ClC1=C(Cl)C(Cl)=C2C(=O)CC(=O)C2=C1Cl IDLAOWFFKWRNHB-UHFFFAOYSA-N 0.000 description 5
- 108010026132 Gelatinases Proteins 0.000 description 5
- 102000013382 Gelatinases Human genes 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 241000699670 Mus sp. Species 0.000 description 5
- 108010029485 Protein Isoforms Proteins 0.000 description 5
- 102000001708 Protein Isoforms Human genes 0.000 description 5
- 102100025040 Ubiquitin carboxyl-terminal hydrolase isozyme L3 Human genes 0.000 description 5
- 101710186831 Ubiquitin carboxyl-terminal hydrolase isozyme L3 Proteins 0.000 description 5
- 238000001114 immunoprecipitation Methods 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 238000000575 proteomic method Methods 0.000 description 5
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- 239000012980 RPMI-1640 medium Substances 0.000 description 4
- 102000004142 Trypsin Human genes 0.000 description 4
- 108090000631 Trypsin Proteins 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000001502 gel electrophoresis Methods 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- -1 isatin oxime compound Chemical class 0.000 description 4
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 238000003757 reverse transcription PCR Methods 0.000 description 4
- 239000012588 trypsin Substances 0.000 description 4
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 4
- 238000001419 two-dimensional polyacrylamide gel electrophoresis Methods 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 3
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 3
- 108010088751 Albumins Proteins 0.000 description 3
- 102000009027 Albumins Human genes 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 101710112780 Gene 1 protein Proteins 0.000 description 3
- 108020004459 Small interfering RNA Proteins 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 239000000427 antigen Substances 0.000 description 3
- 108091007433 antigens Proteins 0.000 description 3
- 102000036639 antigens Human genes 0.000 description 3
- 210000004899 c-terminal region Anatomy 0.000 description 3
- 239000004202 carbamide Substances 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 210000005265 lung cell Anatomy 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 230000001394 metastastic effect Effects 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 230000004853 protein function Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 108010042785 ubiquitin C-terminal 7-amido-4-methylcoumarin Proteins 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- UMCMPZBLKLEWAF-BCTGSCMUSA-N 3-[(3-cholamidopropyl)dimethylammonio]propane-1-sulfonate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 UMCMPZBLKLEWAF-BCTGSCMUSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 238000011729 BALB/c nude mouse Methods 0.000 description 2
- 101710163595 Chaperone protein DnaK Proteins 0.000 description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 108010007457 Extracellular Signal-Regulated MAP Kinases Proteins 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 101710178376 Heat shock 70 kDa protein Proteins 0.000 description 2
- 101710152018 Heat shock cognate 70 kDa protein Proteins 0.000 description 2
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- 238000000636 Northern blotting Methods 0.000 description 2
- KPKZJLCSROULON-QKGLWVMZSA-N Phalloidin Chemical compound N1C(=O)[C@@H]([C@@H](O)C)NC(=O)[C@H](C)NC(=O)[C@H](C[C@@](C)(O)CO)NC(=O)[C@H](C2)NC(=O)[C@H](C)NC(=O)[C@@H]3C[C@H](O)CN3C(=O)[C@@H]1CSC1=C2C2=CC=CC=C2N1 KPKZJLCSROULON-QKGLWVMZSA-N 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 206010041067 Small cell lung cancer Diseases 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 102000018390 Ubiquitin-Specific Proteases Human genes 0.000 description 2
- 108010066496 Ubiquitin-Specific Proteases Proteins 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- UDSAIICHUKSCKT-UHFFFAOYSA-N bromophenol blue Chemical compound C1=C(Br)C(O)=C(Br)C=C1C1(C=2C=C(Br)C(O)=C(Br)C=2)C2=CC=CC=C2S(=O)(=O)O1 UDSAIICHUKSCKT-UHFFFAOYSA-N 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 239000013592 cell lysate Substances 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 208000029742 colonic neoplasm Diseases 0.000 description 2
- GLNDAGDHSLMOKX-UHFFFAOYSA-N coumarin 120 Chemical compound C1=C(N)C=CC2=C1OC(=O)C=C2C GLNDAGDHSLMOKX-UHFFFAOYSA-N 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 239000012737 fresh medium Substances 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 230000004660 morphological change Effects 0.000 description 2
- 201000000050 myeloid neoplasm Diseases 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 2
- 230000001766 physiological effect Effects 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 208000000587 small cell lung carcinoma Diseases 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- UMGDCJDMYOKAJW-UHFFFAOYSA-N thiourea Chemical compound NC(N)=S UMGDCJDMYOKAJW-UHFFFAOYSA-N 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 239000000439 tumor marker Substances 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- MWWSFMDVAYGXBV-MYPASOLCSA-N (7r,9s)-7-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical compound Cl.O([C@@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 MWWSFMDVAYGXBV-MYPASOLCSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- YPLZVJKSYBUKBU-UHFFFAOYSA-N 3-amino-4-methylchromen-2-one Chemical compound C1=CC=CC2=C1OC(=O)C(N)=C2C YPLZVJKSYBUKBU-UHFFFAOYSA-N 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- 102100022142 Achaete-scute homolog 1 Human genes 0.000 description 1
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 1
- 229910000013 Ammonium bicarbonate Inorganic materials 0.000 description 1
- 108010039627 Aprotinin Proteins 0.000 description 1
- 101100489878 Arabidopsis thaliana ABCB9 gene Proteins 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 239000004604 Blowing Agent Substances 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 101000894568 Catharanthus roseus Catharanthine synthase Proteins 0.000 description 1
- 108090000712 Cathepsin B Proteins 0.000 description 1
- 102000004225 Cathepsin B Human genes 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 102000005927 Cysteine Proteases Human genes 0.000 description 1
- 108010005843 Cysteine Proteases Proteins 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102000001477 Deubiquitinating Enzymes Human genes 0.000 description 1
- 108010093668 Deubiquitinating Enzymes Proteins 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 240000001624 Espostoa lanata Species 0.000 description 1
- 235000009161 Espostoa lanata Nutrition 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 229920000209 Hexadimethrine bromide Polymers 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000901099 Homo sapiens Achaete-scute homolog 1 Proteins 0.000 description 1
- 102000004157 Hydrolases Human genes 0.000 description 1
- 108090000604 Hydrolases Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000005913 Maltodextrin Substances 0.000 description 1
- 229920002774 Maltodextrin Polymers 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 108010009711 Phalloidine Proteins 0.000 description 1
- 239000013614 RNA sample Substances 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 108091027967 Small hairpin RNA Proteins 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 235000012538 ammonium bicarbonate Nutrition 0.000 description 1
- 239000001099 ammonium carbonate Substances 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 229960004405 aprotinin Drugs 0.000 description 1
- 238000003491 array Methods 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000012131 assay buffer Substances 0.000 description 1
- 239000000022 bacteriostatic agent Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 102000023732 binding proteins Human genes 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000001045 blue dye Substances 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 238000002619 cancer immunotherapy Methods 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 190000008236 carboplatin Chemical compound 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000004709 cell invasion Effects 0.000 description 1
- 230000009087 cell motility Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 210000004720 cerebrum Anatomy 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000004624 confocal microscopy Methods 0.000 description 1
- 108091036078 conserved sequence Proteins 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 238000010799 enzyme reaction rate Methods 0.000 description 1
- 238000011067 equilibration Methods 0.000 description 1
- 239000006167 equilibration buffer Substances 0.000 description 1
- 230000005496 eutectics Effects 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 230000001815 facial effect Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 238000010185 immunofluorescence analysis Methods 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000001155 isoelectric focusing Methods 0.000 description 1
- 208000021601 lentivirus infection Diseases 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 201000005249 lung adenocarcinoma Diseases 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 229940035034 maltodextrin Drugs 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000001259 mesencephalon Anatomy 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 238000003358 metastasis assay Methods 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- UPSFMJHZUCSEHU-JYGUBCOQSA-N n-[(2s,3r,4r,5s,6r)-2-[(2r,3s,4r,5r,6s)-5-acetamido-4-hydroxy-2-(hydroxymethyl)-6-(4-methyl-2-oxochromen-7-yl)oxyoxan-3-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide Chemical compound CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@H]1[C@H](O)[C@@H](NC(C)=O)[C@H](OC=2C=C3OC(=O)C=C(C)C3=CC=2)O[C@@H]1CO UPSFMJHZUCSEHU-JYGUBCOQSA-N 0.000 description 1
- 239000005445 natural material Substances 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000004031 neuronal differentiation Effects 0.000 description 1
- 210000000607 neurosecretory system Anatomy 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- OSTGTTZJOCZWJG-UHFFFAOYSA-N nitrosourea Chemical compound NC(=O)N=NO OSTGTTZJOCZWJG-UHFFFAOYSA-N 0.000 description 1
- 229940127075 other antimetabolite Drugs 0.000 description 1
- 230000004792 oxidative damage Effects 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 230000036542 oxidative stress Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 108010091212 pepstatin Proteins 0.000 description 1
- FAXGPCHRFPCXOO-LXTPJMTPSA-N pepstatin A Chemical compound OC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)NC(=O)CC(C)C FAXGPCHRFPCXOO-LXTPJMTPSA-N 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 239000003507 refrigerant Substances 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000012723 sample buffer Substances 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 239000004055 small Interfering RNA Substances 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 235000014347 soups Nutrition 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000013077 target material Substances 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 230000006663 ubiquitin-proteasome pathway Effects 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- 238000001086 yeast two-hybrid system Methods 0.000 description 1
- 238000007805 zymography Methods 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4702—Regulators; Modulating activity
- C07K14/4703—Inhibitors; Suppressors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1137—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/50—Physical structure
- C12N2310/53—Physical structure partially self-complementary or closed
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/136—Screening for pharmacological compounds
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Wood Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Physics & Mathematics (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- General Engineering & Computer Science (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Urology & Nephrology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Hospice & Palliative Care (AREA)
- Veterinary Medicine (AREA)
- Cell Biology (AREA)
- Virology (AREA)
- Toxicology (AREA)
- General Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Plant Pathology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
Abstract
Description
UCH-L1はT1/T2 NSCLCと比べてT3/T4 NSCLCで優先的に発現する(Hidehumi Sasaki, et al., "expression of the protein gene product 9.5,PGP9.5 は非小細胞肺癌におけるT−ステータスと関係する。Jpn J Clin Oncol., 31(11) 532-535)。肺癌を有する患者において、循環するUCH-L1抗原及び/または抗体は血清中で検出される。(Frank
brichory, et al., 2Proteomics-based identifiction of protein Gene Product 9.5 as a Tumor Antigen That Induces A humoral imune Response in lung Cancer", CANCER RESEARCH, Vol. 61, p.7908-4912, 2001)。
(a)哺乳動物の組織または細胞から発現させたタンパク質試料を得;
(b)得られた試料をUCH-L1の基質と反応させ、蛍光を検出し、それによってUCH-L1の発現を調べ、定量的分析を行う。
シクロホスアミド、アジリジン、アルキルアルコンスルホネート、ニトロソウレア、ダカルバジン、カルボプラチン、シスプラチン等のアルキル化剤、マイトマイシンC、アントラサイクリン、ドキソルビシン(アドリアマイシン)等の抗生物質、メトトレキセート、5−フルオロウラシル、サイタラビン等の抗代謝剤、ビンカアルカロイドおよびホルモン等の植物由来薬剤等の当業者に周知の化学治療と共に用いうる。
UCH-L1遺伝子を含む癌転移阻害剤をスクリーニングするための組成物を、試験物質と接触させ、次にその反応を調べ、試験物質が遺伝子の発現を抑制するか否か調べる。
発明の態様
癌転移、癌転移の抑制、UCH-L1を用いる癌転移の阻害剤のスクリーニング
1−1.
癌転移に関連するタンパク質のスクリーニングと標的分子の同定
癌転移に関連するタンパク質を同定するために我々は侵襲ポテンシャル有するH157(扁平上皮細胞癌)、侵襲ポテンシャルを有さないH358(気管支肺胞癌)および正常な肺細胞ラインとしてW138(VA−13サブクローン2RA(線維芽細胞)のイントラまたはエクストラ細胞質画分で分化的に発現したタンパク質を分析した。H157,H358,およびW138細胞を10%(v/v)うし胎児血清(FBS),100単位/mLペニシリン、100μg/mLストレプトマイシン、3.75μg重炭酸ナトリウムおよび10mM HEPESを補ったRPMI−1640(2mMのL−グルタミンによるpH7.3)。
Martigel(登録商標)でコートしたトランスウエルを用いる侵襲アッセイ
細胞移動能力を調べるために、Martigel(登録商標)(Martigel, Becton Dickinson (BD) company, Cat no. 354234)(これはEHS(Englebreth-Holm-Swarm))から抽出した再建基部膜マトリックスである)コートしたトランスウエルを用いてインビトロで侵襲アッセイを行った。
チモグラフィーを用いるMMP2およびMMP9の活性試験
細胞移動とさまざまな癌細胞ライン中のMMPsの発現の間の関係を調べるためMMPsの活性をチモグラフィーを用いて調べた。
2Dゲル電気泳動およびMALDI−TOP質量分析
癌転移を制御する標的分子としてのUCH-L1の同定
1−2−1)
フラグタグで排他的に発現したWI38セルラインの侵襲試験
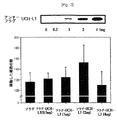
UCH-L1が癌細胞侵襲を制御する標的分子であるか否かを調べるために、我々はフラグタグとともに排他的に発現する正常な肺細胞セルラインW138の侵襲能力を調べた(UCH-L1の完全長cDNAの配列番号2を含むpFlag-CMV-2-UCH-L1, pFlag-CMV-2発現ベクター(sigma セイントルイス、USA))で、用量依存的(1,2,4,6μg)に、Martigel(登録商標)で被覆したトランスウエルを用いて排他的に発現させた。
フラグタッグで排他的に発現したHeLa細胞の侵襲試験
UCH-L1はHela中で発現しないが侵襲性である。Hela細胞は用量依存的に(0.5,1,2,および4μg)pFlag-CMV-2-UCH-L1で一時的にトランスフェクトした後に、Hela細胞移動能力が増加するか否か研究した。
UCH-L1のインターアクティングタンパク質の同定
A.
免疫沈澱を用いたUCH-L1インターアクティングタンパク質の同定
上の結果はUCH-L1は細胞移動を制御するキー物質であることを示唆する。UCH-L1細胞性相互作用タンパク質を同定するために免疫沈澱を行った。
HEK細胞をpFlag-CMV-2-UCH-L1で一時的にトランスフェクトした後にUCH-L1の細胞性相互作用タンパク質を同定するために免疫沈澱をおこなった
UCH-L1および細胞中のβアクチンの相互作用
細胞の形態学的変化は細胞の運動と深く関係している。ベータアクチンは細胞形態に関与している。少しのUCH-L1と共にH358細胞をpFlag-CMV-2-UCH-L1で一時的にトランスフェクトしUCH-L1を過発現させた場合、その形態は著しく変化し、この形態学的変化は細胞の移動能と密接に関連する。UCH-L1と細胞移動能の間の関係を確認するため、UCH-L1とベータアクチンの亜細胞分布を少しのUCH-L1有するH358細胞中で調べた。
UCH-L1特異的なRNAiを発現するB16F10マウスメラノーマ安定細胞中の細胞移動の減少
我々は動物モデルを用いて実験的転移によりUCH-L1が癌転移に関連するキーとなる標的タンパク質であるか否か解明することを試みた。正常なB16F10マウスメラノーマ細胞は非常に侵襲的であり、転移課程における最初の標的器官は肺である。レンチウイルスをベースにしたシステムを用いて我々はUCH-L1をノックダウンしたB16F10安定細胞を調製した。
UCH-L1特異的なRNAiを発現するB16F10マウスメラノーマの調製
B16F10マウスのメラノーマ細胞ラインにおけるUCH-L1の発現レベルを、UCH-L1を過発現するH157肺癌セルラインのそれと、抗UCH-L1抗体で、ウエスタンブロッティングを用いることによって分析した。この結果は図12に示す。UCH-L1はB16F10セルライン中でも発現されることが決定された
UCH-L1特異的なRNAiを発現するB16F10安定細胞を用いる癌転移の動物実験
上述のように、UCH-L1発現ノックダウンB16F10の安定な細胞をゼオシンで選択した後、これらの細胞(1x106)BALB/Cヌードマウスの尾の静脈に単一注入した。注入の14日後、肺に対する転移を調べた、(対照セットのために、BALB/Cマウスを無関係なRNAiを発現する同量のB16F10安定細胞を注入した(対照レンチウイルスベクター))。
UCH-L1を用いる癌転移に対する阻害剤のスクリーニング
UCH-L1の発現またはヒドロラーゼ阻害するための化学薬品(癌転移の阻害剤)をスクリーニングするために、ハイスループットスクリーニング(HTS)を用いることが出来る(図18および19)。
Claims (21)
- UCH−L1の阻害剤を含む癌転移を抑制するための組成物。
- UCH−L1の阻害剤がUCH−L1のshRNAiである請求項1に記載の組成物。
- UCH−L1のshRNAiが配列番号8,9および10のオリゴヌクレオチドから選択される1以上のオリゴヌクレオチドである請求項2に記載の組成物。
- UCH−L1の阻害剤がUCH−L1のモノクローナル抗体である請求項1に記載の組成物。
- UCH−L1の阻害剤がUCH−L1のN−末端領域に位置する11個のアミノ酸に対するポリモノクローナル抗体である請求項1に記載の組成物。
- 配列番号1のアミノ酸配列を有するUCH−L1を含む癌転移阻害剤をスクリーニングするための組成物。
- 配列番号2の塩基配列を有するUCH−L1を含む癌転移阻害剤をスクリーニングするための組成物。
- 癌転移を抑制するためのUCH−L1の阻害剤の使用。
- UCH−L1の阻害剤がUCH−L1のshRNAiである請求項8に記載の使用。
- UCH−L1のshRNAi配列番号が8,9および10のオリゴヌクレオチドから選択される1以上のオリゴヌクレオチドである請求項9に記載の使用。
- UCH−L1の阻害剤がUCH−L1のモノクローナル抗体である請求項8に記載の使用。
- UCH−L1の阻害剤がUCH−L1のN−末端領域に位置する11個のアミノ酸に対するポリクローナル抗体である請求項8に記載の使用。
- 癌転移阻害剤をスクリーニング配列番号1のアミノ酸配列を有するUCH−L1タンパク質の使用。
- 癌転移阻害剤をスクリーニングするための配列番号2の塩基配列を有するUCH−L1遺伝子の使用。
- 治療的に有効量のUCH−L1阻害剤および医薬的に許容し得る担体を患者に投与することを含む癌転移を抑制する方法。
- UCH−L1の阻害剤がUCH−L1のshRNAiである請求項15に記載の方法。
- UCH−L1のshRNAiが配列番号8,9および10のオリゴヌクレオチドから選択される1以上のオリゴヌクレオチドである請求項16に記載の方法。
- UCH−L1の阻害剤がUCH−L1に対するモノクローナル抗体である請求項15に記載の方法。
- UCH−L1の阻害剤がUCH−L1のN−末端領域に位置する11個のアミノ酸に対するポリクローナル抗体である請求項15に記載の使用。
- 試験試料を標的物質として配列番号1のアミノ酸配列を有するUCH−L1タンパク質を含む癌転移阻害剤スクリーニングするための組成物に対して接触することからなる癌転移阻害剤のスクリーニング方法。
- 試験試料を標的物質として配列番号2の塩基配列を有するUCH−L1遺伝子を含む癌転移阻害剤スクリーニングするための組成物に対して接触することからなる癌転移阻害剤のスクリーニング方法。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020050113101A KR100732298B1 (ko) | 2005-11-24 | 2005-11-24 | 유비키틴 c-말단 가수분해제-l1을 이용한 암전이 진단용조성물 |
| PCT/KR2006/004989 WO2007061256A1 (en) | 2005-11-24 | 2006-11-24 | Novel use of ubiquitin c-terminal hydrolase-l1 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009517381A true JP2009517381A (ja) | 2009-04-30 |
| JP2009517381A5 JP2009517381A5 (ja) | 2010-04-08 |
Family
ID=38067427
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008542245A Pending JP2009517381A (ja) | 2005-11-24 | 2006-11-24 | ユビキチンc末端ヒドロラーゼ−l1の新規な使用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20090208508A1 (ja) |
| EP (1) | EP1993581A4 (ja) |
| JP (1) | JP2009517381A (ja) |
| KR (1) | KR100732298B1 (ja) |
| CN (1) | CN101316605A (ja) |
| WO (1) | WO2007061256A1 (ja) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2195029A2 (en) * | 2007-08-24 | 2010-06-16 | Oryzon Genomics SA | Treatment and prevention of neurodegenerative diseases |
| WO2010123365A1 (en) * | 2009-04-24 | 2010-10-28 | Academisch Ziekenhuis Leiden H.O.D.N. Lumc | Increasing the immunogenicity of epithelial cells infected with human papilloma virus (hpv) |
| KR101384642B1 (ko) * | 2012-06-22 | 2014-04-22 | 이화여자대학교 산학협력단 | N―말단이 제거된 유비퀴틴 c―말단 가수분해효소―l1(nt―uch―l1)를 유효성분으로 포함하는 파킨슨병 예방 및 치료용 약학적 조성물 |
| WO2014185561A1 (ko) * | 2013-05-13 | 2014-11-20 | 이화여자대학교 산학협력단 | 신규한 화합물 또는 이의 약학적으로 허용 가능한 염 및 이를 유효성분으로 함유하는 uch-l1 관련 질환의 예방 또는 치료용 약학적 조성물 |
| US20230135030A1 (en) | 2018-11-26 | 2023-05-04 | Baseline Global, Inc. | Methods, Systems, and a Kit for Diagnosis, Detection, Monitoring & Treatment of Traumatic Brain Injury |
| CN110684740B (zh) * | 2019-08-09 | 2020-12-08 | 无锡傲锐东源生物科技有限公司 | 一种抗人泛素羧基末端水解酶-1(uch-l1)的单克隆抗体及其应用 |
| CN112891542B (zh) * | 2021-02-01 | 2021-11-16 | 暨南大学 | 一种包含UCHs抑制剂的药物组合物及其应用 |
| CN113970643B (zh) * | 2021-11-23 | 2023-06-30 | 北京大学人民医院 | 检测抗uch-l1表位的自身抗体的试剂在制备sle血清诊断相关产品中的应用 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005272350A (ja) * | 2004-03-24 | 2005-10-06 | Vitamin C60 Bioresearch Kk | 癌転移阻害剤 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998035985A1 (en) * | 1997-02-12 | 1998-08-20 | The Regents Of The University Of Michigan | Protein markers for lung cancer and use thereof |
| KR100545076B1 (ko) | 2003-01-27 | 2006-01-24 | 김현기 | 인간 원암유전자 hpp1 및 이에 의해 코드되는 단백질 |
| WO2004101762A2 (en) * | 2003-05-12 | 2004-11-25 | The Regents Of The University Of Michigan | Detection and treatment of cancers of the colon |
| KR100520800B1 (ko) | 2003-05-19 | 2005-10-12 | 김진우 | 인간 원암유전자 pig2 및 이에 의해 코드되는 단백질 |
-
2005
- 2005-11-24 KR KR1020050113101A patent/KR100732298B1/ko not_active IP Right Cessation
-
2006
- 2006-11-24 EP EP06843893A patent/EP1993581A4/en not_active Withdrawn
- 2006-11-24 CN CNA2006800437290A patent/CN101316605A/zh active Pending
- 2006-11-24 US US12/094,955 patent/US20090208508A1/en not_active Abandoned
- 2006-11-24 JP JP2008542245A patent/JP2009517381A/ja active Pending
- 2006-11-24 WO PCT/KR2006/004989 patent/WO2007061256A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005272350A (ja) * | 2004-03-24 | 2005-10-06 | Vitamin C60 Bioresearch Kk | 癌転移阻害剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR100732298B1 (ko) | 2007-06-25 |
| WO2007061256A1 (en) | 2007-05-31 |
| KR20070054952A (ko) | 2007-05-30 |
| US20090208508A1 (en) | 2009-08-20 |
| CN101316605A (zh) | 2008-12-03 |
| EP1993581A1 (en) | 2008-11-26 |
| EP1993581A4 (en) | 2009-08-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Ma et al. | USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis | |
| US9850543B2 (en) | Biomarkers associated with BRM inhibition | |
| US10139394B2 (en) | Method for controlling cancer metastasis or cancer cell migration by modulating the cellular level of lysyl tRNA synthetase | |
| Ridnour et al. | Nitric oxide synthase and breast cancer: role of TIMP-1 in NO-mediated Akt activation | |
| US7655402B2 (en) | Diagnoses and therapeutics for cancer | |
| JP2009517381A (ja) | ユビキチンc末端ヒドロラーゼ−l1の新規な使用 | |
| US20070141652A1 (en) | Isolation of the mitotic spindle matrix and its methods of use | |
| JP3771218B2 (ja) | 細胞老化関連核酸配列及びタンパク質 | |
| Wu et al. | Lopinavir enhances anoikis by remodeling autophagy in a circRNA-dependent manner | |
| Zhuang et al. | RNF144B-mediated p21 degradation regulated by HDAC3 contribute to enhancing ovarian cancer growth and metastasis | |
| US20080305102A1 (en) | Therapeutic Agent for Cancer Comprising Substance Capable of Inhibiting Expression or Function of Synoviolin as Active Ingredient and Screening Method for the Therapeutic Agent for Cancer | |
| JPWO2016148115A1 (ja) | ホスファチジルセリンシンターゼ1阻害剤への応答性を予測する方法 | |
| WO2006108225A1 (en) | Method of screening for compounds that modulate cell proliferation | |
| US20170108504A1 (en) | Treating bax(delta)2-positive cancer with chemotherapies targeting caspase 8 | |
| US10768179B2 (en) | Method for predicting responsiveness to cancer treatment using p300-inhibiting compound | |
| Ye et al. | Knockdown of casein kinase 1E inhibits cell proliferation and invasion of colorectal cancer cells via inhibition of the Wnt/beta-catenin signaling | |
| WO2021173712A1 (en) | Molecular biomarkers and targets for fuchs' endothelial corneal dystrophy and glaucoma | |
| KR101128297B1 (ko) | Tspyl5 유전자 발현을 억제하여 암세포의 화합물 또는 방사선에 대한 민감도를 증진하는 방법 | |
| US20080227734A1 (en) | Mutant Lrp5/6 Wnt-Signaling Receptors in Cancer Diagnosis, Prognosis, and Treatment | |
| EP4295844A2 (en) | Pharmaceutical composition for inhibiting cancer metastasis | |
| KR101796091B1 (ko) | 카복실 말단 조절 단백질을 포함하는 두경부암 진단용 바이오 마커 조성물 | |
| Lapi | Identification of novel and direct target genes of p73 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20090917 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091124 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091126 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100222 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120221 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120807 |