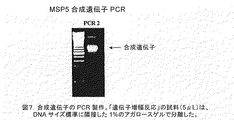
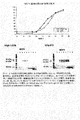
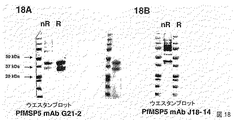
JP2009517016A - 組換え熱帯熱マラリア原虫(Plasmodiumfalciparum)メロゾイト表面タンパク質4および5およびこれらの使用 - Google Patents
組換え熱帯熱マラリア原虫(Plasmodiumfalciparum)メロゾイト表面タンパク質4および5およびこれらの使用 Download PDFInfo
- Publication number
- JP2009517016A JP2009517016A JP2008541848A JP2008541848A JP2009517016A JP 2009517016 A JP2009517016 A JP 2009517016A JP 2008541848 A JP2008541848 A JP 2008541848A JP 2008541848 A JP2008541848 A JP 2008541848A JP 2009517016 A JP2009517016 A JP 2009517016A
- Authority
- JP
- Japan
- Prior art keywords
- msp4
- recombinant
- polypeptide
- msp5
- antibody
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/44—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from protozoa
- C07K14/445—Plasmodium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/002—Protozoa antigens
- A61K39/015—Hemosporidia antigens, e.g. Plasmodium antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/06—Antimalarials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/20—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans from protozoa
- C07K16/205—Plasmodium
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2799/00—Uses of viruses
- C12N2799/02—Uses of viruses as vector
- C12N2799/021—Uses of viruses as vector for the expression of a heterologous nucleic acid
- C12N2799/026—Uses of viruses as vector for the expression of a heterologous nucleic acid where the vector is derived from a baculovirus
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Tropical Medicine & Parasitology (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Veterinary Medicine (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Mycology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Analytical Chemistry (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Biotechnology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US73997305P | 2005-11-23 | 2005-11-23 | |
| PCT/IB2006/003967 WO2007060550A2 (en) | 2005-11-23 | 2006-11-23 | Recombinant plasmodium falciparum merozoite surface proteins 4 and 5 and their use |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012158992A Division JP2012254082A (ja) | 2005-11-23 | 2012-07-17 | 組換え熱帯熱マラリア原虫(Plasmodiumfalciparum)メロゾイト表面タンパク質4および5およびこれらの使用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009517016A true JP2009517016A (ja) | 2009-04-30 |
| JP2009517016A5 JP2009517016A5 (enExample) | 2010-02-18 |
Family
ID=38067607
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008541848A Withdrawn JP2009517016A (ja) | 2005-11-23 | 2006-11-23 | 組換え熱帯熱マラリア原虫(Plasmodiumfalciparum)メロゾイト表面タンパク質4および5およびこれらの使用 |
| JP2012158992A Withdrawn JP2012254082A (ja) | 2005-11-23 | 2012-07-17 | 組換え熱帯熱マラリア原虫(Plasmodiumfalciparum)メロゾイト表面タンパク質4および5およびこれらの使用 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012158992A Withdrawn JP2012254082A (ja) | 2005-11-23 | 2012-07-17 | 組換え熱帯熱マラリア原虫(Plasmodiumfalciparum)メロゾイト表面タンパク質4および5およびこれらの使用 |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US8026354B2 (enExample) |
| EP (2) | EP1954305B1 (enExample) |
| JP (2) | JP2009517016A (enExample) |
| CN (1) | CN101415436A (enExample) |
| CA (1) | CA2630283A1 (enExample) |
| WO (1) | WO2007060550A2 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030161840A1 (en) * | 1992-10-19 | 2003-08-28 | Institut Pasteur | Plasmodium falciparum antigens inducing protective antibodies |
| US8026354B2 (en) * | 2005-11-23 | 2011-09-27 | Institut Pasteur | Recombinant plasmodium falciparum merozoite surface proteins 4 and 5 and their use |
| CN101671654B (zh) * | 2009-10-13 | 2012-11-07 | 黑龙江八一农垦大学 | 抗边缘无浆体msp5蛋白的单克隆抗体及其应用 |
| WO2011056216A1 (en) * | 2009-11-05 | 2011-05-12 | The United States Of America As Represented By The Secretary Of The Navy | Plasmodium falciparum sporozoite and liver stage antigens |
| CU20100083A7 (es) * | 2010-05-05 | 2012-06-21 | Inst Finlay | Tolerogenos adyuvados como vacuna de malaria |
| US20120244178A1 (en) * | 2011-03-25 | 2012-09-27 | Denise Doolan | Plasmodium falciparum antigens |
| CN102533678A (zh) * | 2012-01-10 | 2012-07-04 | 特菲(天津)生物医药科技有限公司 | 一种间日疟红内期疫苗及其制备方法 |
| WO2015055772A1 (en) | 2013-10-16 | 2015-04-23 | Shirley Longacre | Combination of plasmodium merozoite surface proteins msp4 and 1 and uses thereof |
| WO2015187652A1 (en) * | 2014-06-02 | 2015-12-10 | The Johns Hopkins University | Malarial antigens derived from subtilisin-like protease 2 and vaccines and methods of use |
| US10622025B2 (en) | 2018-06-05 | 2020-04-14 | Seagate Technology Llc | Carrierless storage chassis |
| US10354699B1 (en) | 2018-06-05 | 2019-07-16 | Seagate Technology Llc | Carrierless storage chassis drive locking units |
| US11304328B2 (en) | 2020-01-16 | 2022-04-12 | Seagate Technology Llc | Data storage retainer systems, methods, and devices |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003527435A (ja) * | 2000-03-23 | 2003-09-16 | アクゾ・ノベル・エヌ・ベー | 免疫療法におけるmiaの使用 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ZA811368B (en) | 1980-03-24 | 1982-04-28 | Genentech Inc | Bacterial polypedtide expression employing tryptophan promoter-operator |
| US4737462A (en) | 1982-10-19 | 1988-04-12 | Cetus Corporation | Structural genes, plasmids and transformed cells for producing cysteine depleted muteins of interferon-β |
| US4518584A (en) | 1983-04-15 | 1985-05-21 | Cetus Corporation | Human recombinant interleukin-2 muteins |
| DE69034168T3 (de) | 1989-03-21 | 2013-04-11 | Vical, Inc. | Expression von exogenen Polynukleotidsequenzen in Wirbeltieren |
| US5703055A (en) | 1989-03-21 | 1997-12-30 | Wisconsin Alumni Research Foundation | Generation of antibodies through lipid mediated DNA delivery |
| FR2681786A1 (fr) | 1991-09-27 | 1993-04-02 | Centre Nat Rech Scient | Vecteurs recombinants d'origine virale, leur procede d'obtention et leur utilisation pour l'expression de polypeptides dans des cellules musculaires. |
| FR2704145B1 (fr) | 1993-04-21 | 1995-07-21 | Pasteur Institut | Vecteur particulaire et composition pharmaceutique le contenant. |
| DE69939327D1 (de) | 1998-11-02 | 2008-09-25 | Curis Inc | Funktionelle antagonisten von hedgehog-aktivität |
| AU1818200A (en) * | 1998-11-05 | 2000-05-22 | Daniel Carucci | Chromosome 2 sequence of the human malaria parasite (plasmodium falciparum) and proteins of said chromosome useful in anti-malarial vaccines and diagnostic reagents |
| EP1529536A1 (en) * | 2003-11-05 | 2005-05-11 | Institut Pasteur | Immunogenic composition having improved immunostimulation capacity |
| US20080260763A1 (en) * | 2004-07-01 | 2008-10-23 | The Regents Of The University Of California | High Throughput Proteomics |
| US8026354B2 (en) * | 2005-11-23 | 2011-09-27 | Institut Pasteur | Recombinant plasmodium falciparum merozoite surface proteins 4 and 5 and their use |
-
2006
- 2006-11-22 US US11/603,310 patent/US8026354B2/en not_active Expired - Fee Related
- 2006-11-23 EP EP06842385A patent/EP1954305B1/en not_active Not-in-force
- 2006-11-23 EP EP10188189A patent/EP2329844A1/en not_active Withdrawn
- 2006-11-23 US US12/084,240 patent/US8350019B2/en not_active Expired - Fee Related
- 2006-11-23 CA CA002630283A patent/CA2630283A1/en not_active Abandoned
- 2006-11-23 WO PCT/IB2006/003967 patent/WO2007060550A2/en not_active Ceased
- 2006-11-23 JP JP2008541848A patent/JP2009517016A/ja not_active Withdrawn
- 2006-11-23 CN CNA200680044019XA patent/CN101415436A/zh active Pending
-
2011
- 2011-03-18 US US13/051,065 patent/US8378087B2/en not_active Expired - Fee Related
-
2012
- 2012-07-17 JP JP2012158992A patent/JP2012254082A/ja not_active Withdrawn
- 2012-11-01 US US13/666,601 patent/US20130129766A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003527435A (ja) * | 2000-03-23 | 2003-09-16 | アクゾ・ノベル・エヌ・ベー | 免疫療法におけるmiaの使用 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20120115156A1 (en) | 2012-05-10 |
| CA2630283A1 (en) | 2007-05-31 |
| EP2329844A1 (en) | 2011-06-08 |
| US20130129766A1 (en) | 2013-05-23 |
| JP2012254082A (ja) | 2012-12-27 |
| WO2007060550A2 (en) | 2007-05-31 |
| EP1954305B1 (en) | 2012-09-19 |
| US8026354B2 (en) | 2011-09-27 |
| EP1954305A2 (en) | 2008-08-13 |
| US20100092520A1 (en) | 2010-04-15 |
| CN101415436A (zh) | 2009-04-22 |
| WO2007060550A3 (en) | 2008-01-03 |
| US8350019B2 (en) | 2013-01-08 |
| US20080286805A1 (en) | 2008-11-20 |
| US8378087B2 (en) | 2013-02-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8378087B2 (en) | Recombinant Plasmodium falciparum merozoite surface proteins 4 and 5 and their use | |
| Baruch et al. | Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes | |
| Albano et al. | A homologue of Sar1p localises to a novel trafficking pathway in malaria-infected erythrocytes | |
| JP2584733B2 (ja) | 原虫類抗原用dnaのクロ−ニング | |
| US5231168A (en) | Malaria antigen | |
| Singh et al. | Antibodies raised against receptor-binding domain of Plasmodium knowlesi Duffy binding protein inhibit erythrocyte invasion | |
| Kaneko et al. | Disruption of the C-terminal region of EBA-175 in the Dd2/Nm clone of Plasmodium falciparum does not affect erythrocyte invasion | |
| EP0957937A1 (en) | VACCINES, ANTIBODIES, PROTEINS, GLYCOPROTEINS, DNAS AND RNAS FOR PROPHYLAXIS AND TREATMENT OF $i(CRYPTOSPORIDIUM PARVUM) INFECTIONS | |
| Ramasamy et al. | Antibodies to a merozoite surface protein promote multiple invasion of red blood cells by malaria parasites | |
| Black et al. | MSP8 is a non-essential merozoite surface protein in Plasmodium falciparum | |
| EP1526178B1 (en) | MSP-3-like family of genes | |
| CA2480786A1 (en) | Recombinant p. falciparum merozoite protein-142 vaccine | |
| EP1590368B1 (en) | Compounds useful in the diagnosis and treatment of pregnancy-associated malaria | |
| US6403103B1 (en) | Trypanosoma cruzi antigen, gene encoding therefore, and methods of detecting and treating chagas disease | |
| US5646247A (en) | Merozoite antigens localized at the apical end of the parasite | |
| US20090324628A1 (en) | Compounds useful in the diagnosis and treatment of malaria | |
| WO1990011772A1 (en) | Merozoite antigens localized at the apical end of the parasite | |
| HK1075066B (en) | Msp-3-like family of genes | |
| WO2004005489A2 (en) | Nucleic acids encoding sarcocystic neurona antigen and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091002 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091002 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091225 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120117 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120403 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120410 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120717 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120828 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121228 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20130128 |