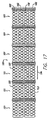
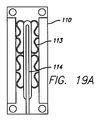
JP2008529593A - 電極および心外膜導線を有する心臓ハーネス - Google Patents
電極および心外膜導線を有する心臓ハーネス Download PDFInfo
- Publication number
- JP2008529593A JP2008529593A JP2007554133A JP2007554133A JP2008529593A JP 2008529593 A JP2008529593 A JP 2008529593A JP 2007554133 A JP2007554133 A JP 2007554133A JP 2007554133 A JP2007554133 A JP 2007554133A JP 2008529593 A JP2008529593 A JP 2008529593A
- Authority
- JP
- Japan
- Prior art keywords
- heart
- electrode
- cardiac harness
- pacing
- lead
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000000747 cardiac effect Effects 0.000 title claims abstract description 308
- 210000002216 heart Anatomy 0.000 claims abstract description 364
- 230000035939 shock Effects 0.000 claims abstract description 49
- 238000000034 method Methods 0.000 claims description 33
- 210000005241 right ventricle Anatomy 0.000 claims description 33
- 238000007920 subcutaneous administration Methods 0.000 claims description 25
- 210000001015 abdomen Anatomy 0.000 claims description 11
- 230000003187 abdominal effect Effects 0.000 claims description 9
- 210000005245 right atrium Anatomy 0.000 claims description 7
- 239000003989 dielectric material Substances 0.000 claims description 3
- 210000003205 muscle Anatomy 0.000 claims 2
- 230000033764 rhythmic process Effects 0.000 abstract description 21
- 239000004020 conductor Substances 0.000 abstract description 9
- 210000005242 cardiac chamber Anatomy 0.000 abstract description 7
- 230000002861 ventricular Effects 0.000 description 55
- 239000011810 insulating material Substances 0.000 description 40
- 230000006870 function Effects 0.000 description 38
- 210000005240 left ventricle Anatomy 0.000 description 30
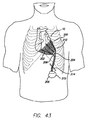
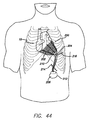
- 210000000038 chest Anatomy 0.000 description 27
- 239000000463 material Substances 0.000 description 27
- 229920002379 silicone rubber Polymers 0.000 description 25
- 229910052751 metal Inorganic materials 0.000 description 23
- 239000002184 metal Substances 0.000 description 23
- 210000004165 myocardium Anatomy 0.000 description 20
- 230000008602 contraction Effects 0.000 description 19
- 206010019280 Heart failures Diseases 0.000 description 16
- 239000013598 vector Substances 0.000 description 16
- 230000010339 dilation Effects 0.000 description 15
- 239000003814 drug Substances 0.000 description 15
- 238000002560 therapeutic procedure Methods 0.000 description 15
- 238000007726 management method Methods 0.000 description 14
- 206010007559 Cardiac failure congestive Diseases 0.000 description 13
- 229910001000 nickel titanium Inorganic materials 0.000 description 13
- 239000004945 silicone rubber Substances 0.000 description 13
- 238000005452 bending Methods 0.000 description 12
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 12
- 239000011295 pitch Substances 0.000 description 11
- 238000011282 treatment Methods 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 10
- 230000003601 intercostal effect Effects 0.000 description 10
- 238000013461 design Methods 0.000 description 9
- 210000003516 pericardium Anatomy 0.000 description 9
- 238000005086 pumping Methods 0.000 description 9
- 230000001360 synchronised effect Effects 0.000 description 9
- 239000011248 coating agent Substances 0.000 description 8
- 238000000576 coating method Methods 0.000 description 8
- 206010052428 Wound Diseases 0.000 description 7
- 208000027418 Wounds and injury Diseases 0.000 description 7
- 238000009125 cardiac resynchronization therapy Methods 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 230000004217 heart function Effects 0.000 description 7
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 7
- 238000003466 welding Methods 0.000 description 7
- 206010061592 cardiac fibrillation Diseases 0.000 description 6
- 230000002600 fibrillogenic effect Effects 0.000 description 6
- WABPQHHGFIMREM-OIOBTWANSA-N lead-204 Chemical compound [204Pb] WABPQHHGFIMREM-OIOBTWANSA-N 0.000 description 6
- 230000000638 stimulation Effects 0.000 description 6
- 229930012538 Paclitaxel Natural products 0.000 description 5
- 239000003146 anticoagulant agent Substances 0.000 description 5
- 230000036471 bradycardia Effects 0.000 description 5
- 208000006218 bradycardia Diseases 0.000 description 5
- 238000001514 detection method Methods 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 239000012811 non-conductive material Substances 0.000 description 5
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 5
- 229960001592 paclitaxel Drugs 0.000 description 5
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 5
- 229940124597 therapeutic agent Drugs 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- 208000001871 Tachycardia Diseases 0.000 description 4
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 4
- 230000001028 anti-proliverative effect Effects 0.000 description 4
- 229940127218 antiplatelet drug Drugs 0.000 description 4
- 239000004019 antithrombin Substances 0.000 description 4
- 230000001746 atrial effect Effects 0.000 description 4
- 230000004323 axial length Effects 0.000 description 4
- 239000002131 composite material Substances 0.000 description 4
- 239000007943 implant Substances 0.000 description 4
- 238000002324 minimally invasive surgery Methods 0.000 description 4
- 238000000465 moulding Methods 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- -1 polytetrafluoroethylene, tetrafluoroethylene Polymers 0.000 description 4
- 238000007634 remodeling Methods 0.000 description 4
- 150000003431 steroids Chemical class 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 239000005557 antagonist Substances 0.000 description 3
- 229940127219 anticoagulant drug Drugs 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 238000013194 cardioversion Methods 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000005684 electric field Effects 0.000 description 3
- 238000002594 fluoroscopy Methods 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 239000012212 insulator Substances 0.000 description 3
- 230000000149 penetrating effect Effects 0.000 description 3
- 239000000106 platelet aggregation inhibitor Substances 0.000 description 3
- 229910052697 platinum Inorganic materials 0.000 description 3
- 229920002635 polyurethane Polymers 0.000 description 3
- 239000004814 polyurethane Substances 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 230000006794 tachycardia Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 229940126585 therapeutic drug Drugs 0.000 description 3
- 210000000115 thoracic cavity Anatomy 0.000 description 3
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 2
- 206010003658 Atrial Fibrillation Diseases 0.000 description 2
- 108010092160 Dactinomycin Proteins 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- OHCQJHSOBUTRHG-KGGHGJDLSA-N FORSKOLIN Chemical compound O=C([C@@]12O)C[C@](C)(C=C)O[C@]1(C)[C@@H](OC(=O)C)[C@@H](O)[C@@H]1[C@]2(C)[C@@H](O)CCC1(C)C OHCQJHSOBUTRHG-KGGHGJDLSA-N 0.000 description 2
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 2
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 2
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 208000002847 Surgical Wound Diseases 0.000 description 2
- 241001433070 Xiphoides Species 0.000 description 2
- 210000003489 abdominal muscle Anatomy 0.000 description 2
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 230000003466 anti-cipated effect Effects 0.000 description 2
- 239000002260 anti-inflammatory agent Substances 0.000 description 2
- 229940121363 anti-inflammatory agent Drugs 0.000 description 2
- 239000003080 antimitotic agent Substances 0.000 description 2
- 229940034982 antineoplastic agent Drugs 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 206010003119 arrhythmia Diseases 0.000 description 2
- 238000010009 beating Methods 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000005422 blasting Methods 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 229960000640 dactinomycin Drugs 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- HTXDPTMKBJXEOW-UHFFFAOYSA-N dioxoiridium Chemical compound O=[Ir]=O HTXDPTMKBJXEOW-UHFFFAOYSA-N 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 229940126864 fibroblast growth factor Drugs 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- WQPDUTSPKFMPDP-OUMQNGNKSA-N hirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(OS(O)(=O)=O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]2CSSC[C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@H](C(NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N2)=O)CSSC1)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)CSSC1)C(C)C)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 WQPDUTSPKFMPDP-OUMQNGNKSA-N 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 229910000457 iridium oxide Inorganic materials 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- HWLDNSXPUQTBOD-UHFFFAOYSA-N platinum-iridium alloy Chemical compound [Ir].[Pt] HWLDNSXPUQTBOD-UHFFFAOYSA-N 0.000 description 2
- 229920000052 poly(p-xylylene) Polymers 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 231100000241 scar Toxicity 0.000 description 2
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 230000008467 tissue growth Effects 0.000 description 2
- 208000003663 ventricular fibrillation Diseases 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- 238000004804 winding Methods 0.000 description 2
- 210000002417 xiphoid bone Anatomy 0.000 description 2
- KWPACVJPAFGBEQ-IKGGRYGDSA-N (2s)-1-[(2r)-2-amino-3-phenylpropanoyl]-n-[(3s)-1-chloro-6-(diaminomethylideneamino)-2-oxohexan-3-yl]pyrrolidine-2-carboxamide Chemical compound C([C@@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl)C1=CC=CC=C1 KWPACVJPAFGBEQ-IKGGRYGDSA-N 0.000 description 1
- PUDHBTGHUJUUFI-SCTWWAJVSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-n-[(2s,3r)-1-amino-3-hydroxy-1-oxobutan-2-yl]-19-[[(2r)-2-amino-3-naphthalen-2-ylpropanoyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17-p Chemical compound C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1 PUDHBTGHUJUUFI-SCTWWAJVSA-N 0.000 description 1
- 102000015427 Angiotensins Human genes 0.000 description 1
- 108010064733 Angiotensins Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- SUZLHDUTVMZSEV-UHFFFAOYSA-N Deoxycoleonol Natural products C12C(=O)CC(C)(C=C)OC2(C)C(OC(=O)C)C(O)C2C1(C)C(O)CCC2(C)C SUZLHDUTVMZSEV-UHFFFAOYSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000018997 Growth Hormone Human genes 0.000 description 1
- 108010051696 Growth Hormone Proteins 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 102000007625 Hirudins Human genes 0.000 description 1
- 108010007267 Hirudins Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 239000004944 Liquid Silicone Rubber Substances 0.000 description 1
- 108010007859 Lisinopril Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 206010071436 Systolic dysfunction Diseases 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- 101000712605 Theromyzon tessulatum Theromin Proteins 0.000 description 1
- 229940122388 Thrombin inhibitor Drugs 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000702 anti-platelet effect Effects 0.000 description 1
- 230000002785 anti-thrombosis Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229960003856 argatroban Drugs 0.000 description 1
- KXNPVXPOPUZYGB-XYVMCAHJSA-N argatroban Chemical compound OC(=O)[C@H]1C[C@H](C)CCN1C(=O)[C@H](CCCN=C(N)N)NS(=O)(=O)C1=CC=CC2=C1NC[C@H](C)C2 KXNPVXPOPUZYGB-XYVMCAHJSA-N 0.000 description 1
- 230000006793 arrhythmia Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- YEESUBCSWGVPCE-UHFFFAOYSA-N azanylidyneoxidanium iron(2+) pentacyanide Chemical compound [Fe++].[C-]#N.[C-]#N.[C-]#N.[C-]#N.[C-]#N.N#[O+] YEESUBCSWGVPCE-UHFFFAOYSA-N 0.000 description 1
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 1
- 229960002170 azathioprine Drugs 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 239000000480 calcium channel blocker Substances 0.000 description 1
- 229960000830 captopril Drugs 0.000 description 1
- FAKRSMQSSFJEIM-RQJHMYQMSA-N captopril Chemical compound SC[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O FAKRSMQSSFJEIM-RQJHMYQMSA-N 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 229940082638 cardiac stimulant phosphodiesterase inhibitors Drugs 0.000 description 1
- 238000007675 cardiac surgery Methods 0.000 description 1
- 230000002612 cardiopulmonary effect Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- HHHKFGXWKKUNCY-FHWLQOOXSA-N cilazapril Chemical compound C([C@@H](C(=O)OCC)N[C@@H]1C(N2[C@@H](CCCN2CCC1)C(O)=O)=O)CC1=CC=CC=C1 HHHKFGXWKKUNCY-FHWLQOOXSA-N 0.000 description 1
- 229960005025 cilazapril Drugs 0.000 description 1
- 238000010073 coating (rubber) Methods 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- OHCQJHSOBUTRHG-UHFFFAOYSA-N colforsin Natural products OC12C(=O)CC(C)(C=C)OC1(C)C(OC(=O)C)C(O)C1C2(C)C(O)CCC1(C)C OHCQJHSOBUTRHG-UHFFFAOYSA-N 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 210000000555 contractile cell Anatomy 0.000 description 1
- 230000008828 contractile function Effects 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 210000004351 coronary vessel Anatomy 0.000 description 1
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- 229960002768 dipyridamole Drugs 0.000 description 1
- IZEKFCXSFNUWAM-UHFFFAOYSA-N dipyridamole Chemical compound C=12N=C(N(CCO)CCO)N=C(N3CCCCC3)C2=NC(N(CCO)CCO)=NC=1N1CCCCC1 IZEKFCXSFNUWAM-UHFFFAOYSA-N 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 238000002592 echocardiography Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 229960001123 epoprostenol Drugs 0.000 description 1
- KAQKFAOMNZTLHT-VVUHWYTRSA-N epoprostenol Chemical compound O1C(=CCCCC(O)=O)C[C@@H]2[C@@H](/C=C/[C@@H](O)CCCCC)[C@H](O)C[C@@H]21 KAQKFAOMNZTLHT-VVUHWYTRSA-N 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 229960005167 everolimus Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 229920000295 expanded polytetrafluoroethylene Polymers 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 239000000122 growth hormone Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002837 heart atrium Anatomy 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- ZFGMDIBRIDKWMY-PASTXAENSA-N heparin Chemical compound CC(O)=N[C@@H]1[C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O[C@@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O[C@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@@H](O[C@@H]3[C@@H](OC(O)[C@H](OS(O)(=O)=O)[C@H]3O)C(O)=O)O[C@@H]2O)CS(O)(=O)=O)[C@H](O)[C@H]1O ZFGMDIBRIDKWMY-PASTXAENSA-N 0.000 description 1
- 229940006607 hirudin Drugs 0.000 description 1
- 239000003668 hormone analog Substances 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 206010020871 hypertrophic cardiomyopathy Diseases 0.000 description 1
- 238000002847 impedance measurement Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000009413 insulation Methods 0.000 description 1
- 239000012774 insulation material Substances 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 108010021336 lanreotide Proteins 0.000 description 1
- 229960002437 lanreotide Drugs 0.000 description 1
- 238000003698 laser cutting Methods 0.000 description 1
- CNQCVBJFEGMYDW-UHFFFAOYSA-N lawrencium atom Chemical compound [Lr] CNQCVBJFEGMYDW-UHFFFAOYSA-N 0.000 description 1
- WABPQHHGFIMREM-OUBTZVSYSA-N lead-208 Chemical compound [208Pb] WABPQHHGFIMREM-OUBTZVSYSA-N 0.000 description 1
- 229960002394 lisinopril Drugs 0.000 description 1
- RLAWWYSOJDYHDC-BZSNNMDCSA-N lisinopril Chemical compound C([C@H](N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(O)=O)C(O)=O)CC1=CC=CC=C1 RLAWWYSOJDYHDC-BZSNNMDCSA-N 0.000 description 1
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 1
- 229960004844 lovastatin Drugs 0.000 description 1
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 1
- 239000003055 low molecular weight heparin Substances 0.000 description 1
- 229940127215 low-molecular weight heparin Drugs 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 102000006240 membrane receptors Human genes 0.000 description 1
- 108020004084 membrane receptors Proteins 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 229960001597 nifedipine Drugs 0.000 description 1
- HYIMSNHJOBLJNT-UHFFFAOYSA-N nifedipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1[N+]([O-])=O HYIMSNHJOBLJNT-UHFFFAOYSA-N 0.000 description 1
- 229960002460 nitroprusside Drugs 0.000 description 1
- 229940012843 omega-3 fatty acid Drugs 0.000 description 1
- 235000020660 omega-3 fatty acid Nutrition 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 1
- 238000001615 p wave Methods 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000002571 phosphodiesterase inhibitor Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 150000003815 prostacyclins Chemical class 0.000 description 1
- 239000002089 prostaglandin antagonist Substances 0.000 description 1
- 150000003180 prostaglandins Chemical class 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 229940076279 serotonin Drugs 0.000 description 1
- 239000012781 shape memory material Substances 0.000 description 1
- 150000003376 silicon Chemical class 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 210000001321 subclavian vein Anatomy 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 239000003868 thrombin inhibitor Substances 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 238000011277 treatment modality Methods 0.000 description 1
- 206010047302 ventricular tachycardia Diseases 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2478—Passive devices for improving the function of the heart muscle, i.e. devices for reshaping the external surface of the heart, e.g. bags, strips or bands
- A61F2/2481—Devices outside the heart wall, e.g. bags, strips or bands
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0587—Epicardial electrode systems; Endocardial electrodes piercing the pericardium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/3627—Heart stimulators for treating a mechanical deficiency of the heart, e.g. congestive heart failure or cardiomyopathy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2478—Passive devices for improving the function of the heart muscle, i.e. devices for reshaping the external surface of the heart, e.g. bags, strips or bands
- A61F2/2481—Devices outside the heart wall, e.g. bags, strips or bands
- A61F2002/2484—Delivery devices therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/056—Transvascular endocardial electrode systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3918—Heart defibrillators characterised by shock pathway, e.g. by electrode configuration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3956—Implantable devices for applying electric shocks to the heart, e.g. for cardioversion
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Radiology & Medical Imaging (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Hospice & Palliative Care (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Electrotherapy Devices (AREA)
- Prostheses (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/051,823 US20050137673A1 (en) | 2003-11-07 | 2005-02-04 | Cardiac harness having electrodes and epicardial leads |
| PCT/US2006/002434 WO2006083617A2 (en) | 2005-02-04 | 2006-01-19 | Cardiac harness having electrodes and epicardial leads |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008529593A true JP2008529593A (ja) | 2008-08-07 |
| JP2008529593A5 JP2008529593A5 (enExample) | 2008-12-04 |
Family
ID=36282726
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007554133A Pending JP2008529593A (ja) | 2005-02-04 | 2006-01-19 | 電極および心外膜導線を有する心臓ハーネス |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20050137673A1 (enExample) |
| EP (1) | EP1848494A2 (enExample) |
| JP (1) | JP2008529593A (enExample) |
| CA (1) | CA2588935A1 (enExample) |
| WO (1) | WO2006083617A2 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010124867A (ja) * | 2008-11-25 | 2010-06-10 | Tohoku Univ | 人工あるいは再生心筋の収縮力を増強する収縮支援装置 |
| JP2011056178A (ja) * | 2009-09-14 | 2011-03-24 | Olympus Corp | 電極体 |
| JP2013500071A (ja) * | 2009-07-22 | 2013-01-07 | ザ テキサス エー アンド エム ユニヴァーシティー システム | 心臓病態を治療するための補助及びリコイル機能を備える二相性及び動的調整可能サポートデバイス及び方法 |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7022063B2 (en) | 2002-01-07 | 2006-04-04 | Paracor Medical, Inc. | Cardiac harness |
| US7189203B2 (en) | 2002-11-15 | 2007-03-13 | Paracor Medical, Inc. | Cardiac harness delivery device and method |
| US7736299B2 (en) | 2002-11-15 | 2010-06-15 | Paracor Medical, Inc. | Introducer for a cardiac harness delivery |
| US7229405B2 (en) * | 2002-11-15 | 2007-06-12 | Paracor Medical, Inc. | Cardiac harness delivery device and method of use |
| US7781378B2 (en) * | 2003-09-12 | 2010-08-24 | Cornell Research Foundation, Inc. | Protective coating for array material deposition |
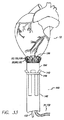
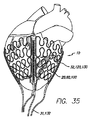
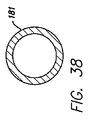
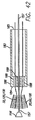
| US7155295B2 (en) * | 2003-11-07 | 2006-12-26 | Paracor Medical, Inc. | Cardiac harness for treating congestive heart failure and for defibrillating and/or pacing/sensing |
| US20060009831A1 (en) * | 2003-11-07 | 2006-01-12 | Lilip Lau | Cardiac harness having leadless electrodes for pacing and sensing therapy |
| US20050288715A1 (en) * | 2003-11-07 | 2005-12-29 | Lilip Lau | Cardiac harness for treating congestive heart failure and for defibrillating and/or pacing/sensing |
| US7587247B2 (en) | 2005-08-01 | 2009-09-08 | Paracor Medical, Inc. | Cardiac harness having an optimal impedance range |
| US7856260B1 (en) | 2006-09-06 | 2010-12-21 | Pacesetter, Inc. | Implantable cardiac patch for measuring physiologic information |
| US8192351B2 (en) | 2007-08-13 | 2012-06-05 | Paracor Medical, Inc. | Medical device delivery system having integrated introducer |
| FR2922460B1 (fr) * | 2007-10-22 | 2011-11-18 | Centre Nat Rech Scient | "dispositif de stimulation d'un tissu vivant par microelectrodes,ses modules amovibles et utilisation" |
| US8545387B2 (en) * | 2007-11-05 | 2013-10-01 | Corinnova Incorporated | Apparatus and method for minimally invasive implantation of heart assist device |
| WO2010040528A1 (en) * | 2008-10-10 | 2010-04-15 | Universitaetsklinikum Heidelberg | Arrangement for implanting and method for implanting |
| US10500394B1 (en) | 2011-10-11 | 2019-12-10 | A-Hamid Hakki | Pacemaker system equipped with a flexible intercostal generator |
| US9775991B1 (en) | 2011-10-11 | 2017-10-03 | A-Hamid Hakki | Endovascular electrode system for tissue stimulation with embedded generator |
| US9289593B1 (en) | 2011-10-11 | 2016-03-22 | A-Hamid Hakki | Endovascular electrode system for tissue stimulation |
| WO2013166292A1 (en) | 2012-05-02 | 2013-11-07 | The Charlotte-Mecklenburg Hospital Authority D/ B/ A Carolinas Healthcare System | Devices, systems, and methods for treating cardiac arrhythmias |
| US9242098B2 (en) | 2013-10-30 | 2016-01-26 | The Charlotte-Mecklenburg Hospital Authority | Devices, systems, and methods for treating cardiac arrhythmias |
| US11369784B2 (en) | 2018-06-06 | 2022-06-28 | The Regents Of The University Of California | Synchronizing a pulsatile cardiac assist device with a pacemaker |
| US11717674B2 (en) | 2018-07-31 | 2023-08-08 | Manicka Institute Llc | Subcutaneous device for use with remote device |
| US11179571B2 (en) | 2018-07-31 | 2021-11-23 | Manicka Institute Llc | Subcutaneous device for monitoring and/or providing therapies |
| US11433233B2 (en) | 2020-11-25 | 2022-09-06 | Calyan Technologies, Inc. | Electrode contact for a subcutaneous device |
| US10576291B2 (en) | 2018-07-31 | 2020-03-03 | Manicka Institute Llc | Subcutaneous device |
| US10716511B2 (en) | 2018-07-31 | 2020-07-21 | Manicka Institute Llc | Subcutaneous device for monitoring and/or providing therapies |
| US11660444B2 (en) | 2018-07-31 | 2023-05-30 | Manicka Institute Llc | Resilient body component contact for a subcutaneous device |
| US10987060B1 (en) | 2020-09-14 | 2021-04-27 | Calyan Technologies, Inc. | Clip design for a subcutaneous device |
| US12502531B2 (en) | 2021-04-26 | 2025-12-23 | Manicka Institute Llc | Subcutaneous device for preventing and treating atherosclerosis |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5824028A (en) * | 1996-09-20 | 1998-10-20 | The Uab Research Foundation | Line electrode oriented relative to fiber direction |
| US20020082647A1 (en) * | 2000-12-22 | 2002-06-27 | Acorn Cardiovascular, Inc. | Cardiac disease treatment and device |
| US6547716B1 (en) * | 2000-11-28 | 2003-04-15 | Abiomed, Inc. | Passive cardiac restraint systems having multiple layers of inflatable elements |
| JP2003526448A (ja) * | 2000-03-10 | 2003-09-09 | パラコー サージカル インコーポレイテッド | 鬱血性心不全を治療するための膨張可能な心臓ハーネス |
| US20030199955A1 (en) * | 2002-04-22 | 2003-10-23 | Chester Struble | Cardiac restraint with electrode attachment sites |
| WO2004021927A2 (en) * | 2002-09-05 | 2004-03-18 | Paracor Medical, Inc. | Cardiac harness |
Family Cites Families (88)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2826193A (en) * | 1956-08-01 | 1958-03-11 | Vineberg Heart Foundation | Cardiac resuscitation device |
| FR92798E (fr) * | 1966-09-05 | 1968-12-27 | Rhone Poulenc Sa | Nouvelle prothese d'assistance cardiaque et sa fabrication. |
| US3587567A (en) * | 1968-12-20 | 1971-06-28 | Peter Paul Schiff | Mechanical ventricular assistance assembly |
| US3966401A (en) * | 1974-07-01 | 1976-06-29 | Hancock Laboratories Incorporated | Preparing natural tissue for implantation so as to provide improved flexibility |
| US4011947A (en) * | 1975-05-22 | 1977-03-15 | Philip Nicholas Sawyer | Packaged prosthetic device |
| US4065816A (en) * | 1975-05-22 | 1978-01-03 | Philip Nicholas Sawyer | Surgical method of using a sterile packaged prosthesis |
| US4192293A (en) * | 1978-09-05 | 1980-03-11 | Manfred Asrican | Cardiac assist device |
| ES474582A1 (es) * | 1978-10-26 | 1979-11-01 | Aranguren Duo Iker | Procedimiento para la instalacion de valvulas mitrales en sulugar anatomico, mediante anclaje de cordajes en pilar arti-ficial |
| US4372293A (en) * | 1980-12-24 | 1983-02-08 | Vijil Rosales Cesar A | Apparatus and method for surgical correction of ptotic breasts |
| US4428375A (en) * | 1982-02-16 | 1984-01-31 | Ellman Barry R | Surgical bag for splenorrhaphy |
| US4665906A (en) * | 1983-10-14 | 1987-05-19 | Raychem Corporation | Medical devices incorporating sim alloy elements |
| US5190546A (en) * | 1983-10-14 | 1993-03-02 | Raychem Corporation | Medical devices incorporating SIM alloy elements |
| US4512471A (en) * | 1984-04-06 | 1985-04-23 | Angicor Limited | Storage unit |
| US4840626A (en) * | 1986-09-29 | 1989-06-20 | Johnson & Johnson Patient Care, Inc. | Heparin-containing adhesion prevention barrier and process |
| SU1604377A1 (ru) * | 1987-02-23 | 1990-11-07 | Благовещенский государственный медицинский институт | Искусственный перикард |
| US4821723A (en) * | 1987-02-27 | 1989-04-18 | Intermedics Inc. | Biphasic waveforms for defibrillation |
| US4827932A (en) * | 1987-02-27 | 1989-05-09 | Intermedics Inc. | Implantable defibrillation electrodes |
| US5098369A (en) * | 1987-02-27 | 1992-03-24 | Vascor, Inc. | Biocompatible ventricular assist and arrhythmia control device including cardiac compression pad and compression assembly |
| US4750619A (en) * | 1987-08-10 | 1988-06-14 | Osteonics Corp. | Package with tray for securing and presenting a sterile prosthetic implant element |
| US4834707A (en) * | 1987-09-16 | 1989-05-30 | Evans Phillip H | Venting apparatus and method for cardiovascular pumping application |
| US4838288A (en) * | 1988-03-14 | 1989-06-13 | Pioneering Technologies, Inc. | Heart valve and xenograft washing system |
| US5186711A (en) * | 1989-03-07 | 1993-02-16 | Albert Einstein College Of Medicine Of Yeshiva University | Hemostasis apparatus and method |
| US5904690A (en) * | 1989-08-16 | 1999-05-18 | Medtronic, Inc. | Device or apparatus for manipulating matter |
| US4997431A (en) * | 1989-08-30 | 1991-03-05 | Angeion Corporation | Catheter |
| US5087243A (en) * | 1990-06-18 | 1992-02-11 | Boaz Avitall | Myocardial iontophoresis |
| US5197978B1 (en) * | 1991-04-26 | 1996-05-28 | Advanced Coronary Tech | Removable heat-recoverable tissue supporting device |
| BR9206005A (pt) * | 1991-05-16 | 1994-08-02 | Mures Cardiovascular Research | Válvula cardíaca, processo para formar uma válvula cardiaca artificial, tecido flexível para a formação de uma válvula cardíaca, e, conjunto para formar membranas trapezoidais de pericárdio para a formação de uma válvula cardiaca |
| US5290217A (en) * | 1991-10-10 | 1994-03-01 | Earl K. Sipes | Method and apparatus for hernia repair |
| US5192314A (en) * | 1991-12-12 | 1993-03-09 | Daskalakis Michael K | Synthetic intraventricular implants and method of inserting |
| CA2089999A1 (en) * | 1992-02-24 | 1993-08-25 | H. Jonathan Tovey | Resilient arm mesh deployer |
| WO1993017635A1 (en) * | 1992-03-04 | 1993-09-16 | C.R. Bard, Inc. | Composite prosthesis and method for limiting the incidence of postoperative adhesions |
| US5383840A (en) * | 1992-07-28 | 1995-01-24 | Vascor, Inc. | Biocompatible ventricular assist and arrhythmia control device including cardiac compression band-stay-pad assembly |
| US5279539A (en) * | 1992-08-17 | 1994-01-18 | Ethicon, Inc. | Drawstring surgical pouch and method of use for preventing ovarian adhesions |
| EP0600140A1 (de) * | 1992-12-04 | 1994-06-08 | SULZER Medizinaltechnik AG | Behälter zum Verpacken einer hohlkörperartigen Endoprothese |
| US5385528A (en) * | 1993-06-17 | 1995-01-31 | Wilk; Peter J. | Intrapericardial assist device and associated method |
| AU686206B2 (en) * | 1993-07-12 | 1998-02-05 | Regents Of The University Of California, The | Soft tissue augmentation apparatus |
| US5385156A (en) * | 1993-08-27 | 1995-01-31 | Rose Health Care Systems | Diagnostic and treatment method for cardiac rupture and apparatus for performing the same |
| US6165210A (en) * | 1994-04-01 | 2000-12-26 | Gore Enterprise Holdings, Inc. | Self-expandable helical intravascular stent and stent-graft |
| US5507779A (en) * | 1994-04-12 | 1996-04-16 | Ventritex, Inc. | Cardiac insulation for defibrillation |
| US5509428A (en) * | 1994-05-31 | 1996-04-23 | Dunlop; Richard W. | Method and apparatus for the creation of tricuspid regurgitation |
| US5593424A (en) * | 1994-08-10 | 1997-01-14 | Segmed, Inc. | Apparatus and method for reducing and stabilizing the circumference of a vascular structure |
| US5749839A (en) * | 1994-08-18 | 1998-05-12 | Duke University | Direct mechanical bi-ventricular cardiac assist device |
| US6331188B1 (en) * | 1994-08-31 | 2001-12-18 | Gore Enterprise Holdings, Inc. | Exterior supported self-expanding stent-graft |
| US5603337A (en) * | 1994-12-05 | 1997-02-18 | Jarvik; Robert | Two-stage cardiomyoplasty |
| US5900245A (en) * | 1996-03-22 | 1999-05-04 | Focal, Inc. | Compliant tissue sealants |
| US6132438A (en) * | 1995-06-07 | 2000-10-17 | Ep Technologies, Inc. | Devices for installing stasis reducing means in body tissue |
| US5800528A (en) * | 1995-06-13 | 1998-09-01 | Abiomed R & D, Inc. | Passive girdle for heart ventricle for therapeutic aid to patients having ventricular dilatation |
| US5713954A (en) * | 1995-06-13 | 1998-02-03 | Abiomed R&D, Inc. | Extra cardiac ventricular assist device |
| US5836311A (en) * | 1995-09-20 | 1998-11-17 | Medtronic, Inc. | Method and apparatus for temporarily immobilizing a local area of tissue |
| DE19538796C2 (de) * | 1995-10-18 | 1999-09-23 | Fraunhofer Ges Forschung | Vorrichtung zur Unterstützung der Herzfunktion mit elastischen Füllkammern |
| US6592619B2 (en) * | 1996-01-02 | 2003-07-15 | University Of Cincinnati | Heart wall actuation device for the natural heart |
| US5957977A (en) * | 1996-01-02 | 1999-09-28 | University Of Cincinnati | Activation device for the natural heart including internal and external support structures |
| US5727569A (en) * | 1996-02-20 | 1998-03-17 | Cardiothoracic Systems, Inc. | Surgical devices for imposing a negative pressure to fix the position of cardiac tissue during surgery |
| US6059750A (en) * | 1996-08-01 | 2000-05-09 | Thomas J. Fogarty | Minimally invasive direct cardiac massage device and method |
| US6123662A (en) * | 1998-07-13 | 2000-09-26 | Acorn Cardiovascular, Inc. | Cardiac disease treatment and device |
| US5702343A (en) * | 1996-10-02 | 1997-12-30 | Acorn Medical, Inc. | Cardiac reinforcement device |
| US6183411B1 (en) * | 1998-09-21 | 2001-02-06 | Myocor, Inc. | External stress reduction device and method |
| US6050936A (en) * | 1997-01-02 | 2000-04-18 | Myocor, Inc. | Heart wall tension reduction apparatus |
| US6045497A (en) * | 1997-01-02 | 2000-04-04 | Myocor, Inc. | Heart wall tension reduction apparatus and method |
| US6390976B1 (en) * | 1997-09-17 | 2002-05-21 | Origin Medsystems, Inc. | System to permit offpump beating heart coronary bypass surgery |
| US6190408B1 (en) * | 1998-03-05 | 2001-02-20 | The University Of Cincinnati | Device and method for restructuring the heart chamber geometry |
| US6214047B1 (en) * | 1998-03-10 | 2001-04-10 | University Of Cincinnati | Article and method for coupling muscle to a prosthetic device |
| US6024096A (en) * | 1998-05-01 | 2000-02-15 | Correstore Inc | Anterior segment ventricular restoration apparatus and method |
| US6547821B1 (en) * | 1998-07-16 | 2003-04-15 | Cardiothoracic Systems, Inc. | Surgical procedures and devices for increasing cardiac output of the heart |
| US6685627B2 (en) * | 1998-10-09 | 2004-02-03 | Swaminathan Jayaraman | Modification of properties and geometry of heart tissue to influence heart function |
| US6360749B1 (en) * | 1998-10-09 | 2002-03-26 | Swaminathan Jayaraman | Modification of properties and geometry of heart tissue to influence heart function |
| US6230714B1 (en) * | 1998-11-18 | 2001-05-15 | Acorn Cardiovascular, Inc. | Cardiac constraint with prior venus occlusion methods |
| US6169922B1 (en) * | 1998-11-18 | 2001-01-02 | Acorn Cardiovascular, Inc. | Defibrillating cardiac jacket with interwoven electrode grids |
| US6701929B2 (en) * | 1999-03-03 | 2004-03-09 | Hany Hussein | Device and method for treatment of congestive heart failure |
| US6192280B1 (en) * | 1999-06-02 | 2001-02-20 | Medtronic, Inc. | Guidewire placed implantable lead with tip seal |
| US20040102804A1 (en) * | 1999-08-10 | 2004-05-27 | Chin Albert K. | Apparatus and methods for endoscopic surgical procedures |
| US6569082B1 (en) * | 1999-08-10 | 2003-05-27 | Origin Medsystems, Inc. | Apparatus and methods for cardiac restraint |
| US6179791B1 (en) * | 1999-09-21 | 2001-01-30 | Acorn Cardiovascular, Inc. | Device for heart measurement |
| US6193648B1 (en) * | 1999-09-21 | 2001-02-27 | Acorn Cardiovascular, Inc. | Cardiac constraint with draw string tensioning |
| US6174279B1 (en) * | 1999-09-21 | 2001-01-16 | Acorn Cardiovascular, Inc. | Cardiac constraint with tension indicator |
| US6702732B1 (en) * | 1999-12-22 | 2004-03-09 | Paracor Surgical, Inc. | Expandable cardiac harness for treating congestive heart failure |
| US6293906B1 (en) * | 2000-01-14 | 2001-09-25 | Acorn Cardiovascular, Inc. | Delivery of cardiac constraint jacket |
| US6564718B2 (en) * | 2000-05-20 | 2003-05-20 | Baker Hughes, Incorporated | Lead free liner composition for shaped charges |
| US6730016B1 (en) * | 2000-06-12 | 2004-05-04 | Acorn Cardiovascular, Inc. | Cardiac disease treatment and device |
| US6482146B1 (en) * | 2000-06-13 | 2002-11-19 | Acorn Cardiovascular, Inc. | Cardiac disease treatment and device |
| US6673009B1 (en) * | 2000-11-08 | 2004-01-06 | Acorn Cardiovascular, Inc. | Adjustment clamp |
| US6738674B2 (en) * | 2001-07-25 | 2004-05-18 | Oscor Inc. | Implantable coronary sinus lead with mapping capabilities |
| JP2005501652A (ja) * | 2001-09-10 | 2005-01-20 | パラコー メディカル インコーポレイテッド | 心不全治療用装置 |
| US6685620B2 (en) * | 2001-09-25 | 2004-02-03 | The Foundry Inc. | Ventricular infarct assist device and methods for using it |
| US6695769B2 (en) * | 2001-09-25 | 2004-02-24 | The Foundry, Inc. | Passive ventricular support devices and methods of using them |
| US7060023B2 (en) * | 2001-09-25 | 2006-06-13 | The Foundry Inc. | Pericardium reinforcing devices and methods of using them |
| US6682475B2 (en) * | 2002-06-11 | 2004-01-27 | Acorn Cardiovascular, Inc. | Tension indicator for cardiac support device and method therefore |
| US20050283042A1 (en) * | 2003-03-28 | 2005-12-22 | Steve Meyer | Cardiac harness having radiopaque coating and method of use |
-
2005
- 2005-02-04 US US11/051,823 patent/US20050137673A1/en not_active Abandoned
-
2006
- 2006-01-19 WO PCT/US2006/002434 patent/WO2006083617A2/en not_active Ceased
- 2006-01-19 CA CA002588935A patent/CA2588935A1/en not_active Abandoned
- 2006-01-19 JP JP2007554133A patent/JP2008529593A/ja active Pending
- 2006-01-19 EP EP06719334A patent/EP1848494A2/en not_active Withdrawn
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5824028A (en) * | 1996-09-20 | 1998-10-20 | The Uab Research Foundation | Line electrode oriented relative to fiber direction |
| JP2003526448A (ja) * | 2000-03-10 | 2003-09-09 | パラコー サージカル インコーポレイテッド | 鬱血性心不全を治療するための膨張可能な心臓ハーネス |
| US6547716B1 (en) * | 2000-11-28 | 2003-04-15 | Abiomed, Inc. | Passive cardiac restraint systems having multiple layers of inflatable elements |
| US20020082647A1 (en) * | 2000-12-22 | 2002-06-27 | Acorn Cardiovascular, Inc. | Cardiac disease treatment and device |
| US20030199955A1 (en) * | 2002-04-22 | 2003-10-23 | Chester Struble | Cardiac restraint with electrode attachment sites |
| WO2004021927A2 (en) * | 2002-09-05 | 2004-03-18 | Paracor Medical, Inc. | Cardiac harness |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010124867A (ja) * | 2008-11-25 | 2010-06-10 | Tohoku Univ | 人工あるいは再生心筋の収縮力を増強する収縮支援装置 |
| JP2013500071A (ja) * | 2009-07-22 | 2013-01-07 | ザ テキサス エー アンド エム ユニヴァーシティー システム | 心臓病態を治療するための補助及びリコイル機能を備える二相性及び動的調整可能サポートデバイス及び方法 |
| JP2015042259A (ja) * | 2009-07-22 | 2015-03-05 | ザ テキサス エー アンド エム ユニヴァーシティー システムThe Texas A&M University System | 心臓病態を治療するための補助及びリコイル機能を備える二相性及び動的調整可能サポートデバイス及び方法 |
| JP2011056178A (ja) * | 2009-09-14 | 2011-03-24 | Olympus Corp | 電極体 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20050137673A1 (en) | 2005-06-23 |
| WO2006083617A3 (en) | 2006-10-12 |
| WO2006083617A2 (en) | 2006-08-10 |
| EP1848494A2 (en) | 2007-10-31 |
| CA2588935A1 (en) | 2006-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7149588B2 (en) | Cardiac harness for treating congestive heart failure and for defibrillating and/or pacing/sensing | |
| US7158839B2 (en) | Cardiac harness for treating heart disease | |
| JP2008529593A (ja) | 電極および心外膜導線を有する心臓ハーネス | |
| US20090043152A1 (en) | Delivery device for cardiac harness | |
| US20050171589A1 (en) | Cardiac harness and method of delivery by minimally invasive access | |
| US20060009831A1 (en) | Cardiac harness having leadless electrodes for pacing and sensing therapy | |
| JP2010508083A (ja) | 鬱血性心不全の治療およびぺーシング/センシングのための心臓ハーネス組立体 | |
| US20050288715A1 (en) | Cardiac harness for treating congestive heart failure and for defibrillating and/or pacing/sensing | |
| US20070197859A1 (en) | Cardiac harness having diagnostic sensors and method of use | |
| US20100152531A1 (en) | Implantable medical device for drug delivery and method of use | |
| EP1198271A1 (en) | Devices and methods for vagus nerve stimulation | |
| US20050283042A1 (en) | Cardiac harness having radiopaque coating and method of use | |
| US20070106336A1 (en) | Cardiac harness assembly for treating congestive heart failure and for pacing/sensing |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081015 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20081015 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110719 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20111216 |