JP2008206875A - 皮膚コンダクタンス測定装置、成分濃度分析装置、皮膚コンダクタンス測定方法および成分濃度分析方法 - Google Patents
皮膚コンダクタンス測定装置、成分濃度分析装置、皮膚コンダクタンス測定方法および成分濃度分析方法 Download PDFInfo
- Publication number
- JP2008206875A JP2008206875A JP2007048262A JP2007048262A JP2008206875A JP 2008206875 A JP2008206875 A JP 2008206875A JP 2007048262 A JP2007048262 A JP 2007048262A JP 2007048262 A JP2007048262 A JP 2007048262A JP 2008206875 A JP2008206875 A JP 2008206875A
- Authority
- JP
- Japan
- Prior art keywords
- skin
- conductance
- subject
- extraction
- tissue fluid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000004458 analytical method Methods 0.000 title claims abstract description 39
- 238000000034 method Methods 0.000 title claims description 30
- 238000000605 extraction Methods 0.000 claims abstract description 178
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims abstract description 157
- 239000008103 glucose Substances 0.000 claims abstract description 157
- 239000012530 fluid Substances 0.000 claims abstract description 84
- 238000005259 measurement Methods 0.000 claims description 61
- 230000008569 process Effects 0.000 claims description 19
- 230000001737 promoting effect Effects 0.000 claims description 17
- 238000004364 calculation method Methods 0.000 claims description 10
- 239000000126 substance Substances 0.000 claims description 6
- 231100000223 dermal penetration Toxicity 0.000 abstract 1
- 210000003491 skin Anatomy 0.000 description 133
- 210000001519 tissue Anatomy 0.000 description 68
- 239000008280 blood Substances 0.000 description 20
- 210000004369 blood Anatomy 0.000 description 20
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- 230000000875 corresponding effect Effects 0.000 description 7
- 238000010586 diagram Methods 0.000 description 7
- 238000004040 coloring Methods 0.000 description 6
- 239000012491 analyte Substances 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 238000000691 measurement method Methods 0.000 description 5
- 239000003054 catalyst Substances 0.000 description 4
- 210000004207 dermis Anatomy 0.000 description 4
- 239000000284 extract Substances 0.000 description 4
- 239000000049 pigment Substances 0.000 description 4
- 238000003825 pressing Methods 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108010015776 Glucose oxidase Proteins 0.000 description 3
- 239000004366 Glucose oxidase Substances 0.000 description 3
- 102000003992 Peroxidases Human genes 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 150000002303 glucose derivatives Chemical class 0.000 description 3
- 229940116332 glucose oxidase Drugs 0.000 description 3
- 235000019420 glucose oxidase Nutrition 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 238000004088 simulation Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- -1 2-hydroxy-3-sulfopropyl Chemical group 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 229910021607 Silver chloride Inorganic materials 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- CVSVTCORWBXHQV-UHFFFAOYSA-N creatine Chemical compound NC(=[NH2+])N(C)CC([O-])=O CVSVTCORWBXHQV-UHFFFAOYSA-N 0.000 description 2
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 230000003204 osmotic effect Effects 0.000 description 2
- 230000001151 other effect Effects 0.000 description 2
- 206010033675 panniculitis Diseases 0.000 description 2
- 108040007629 peroxidase activity proteins Proteins 0.000 description 2
- 239000004065 semiconductor Substances 0.000 description 2
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 2
- 210000004304 subcutaneous tissue Anatomy 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 238000002834 transmittance Methods 0.000 description 2
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 241000208011 Digitalis Species 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- 229920002527 Glycogen Polymers 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 1
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 239000001961 anticonvulsive agent Substances 0.000 description 1
- 229960004676 antithrombotic agent Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000002763 arrhythmic effect Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229960003624 creatine Drugs 0.000 description 1
- 239000006046 creatine Substances 0.000 description 1
- 229940109239 creatinine Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- KCIDZIIHRGYJAE-YGFYJFDDSA-L dipotassium;[(2r,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] phosphate Chemical class [K+].[K+].OC[C@H]1O[C@H](OP([O-])([O-])=O)[C@H](O)[C@@H](O)[C@H]1O KCIDZIIHRGYJAE-YGFYJFDDSA-L 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 229960002737 fructose Drugs 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 229960003082 galactose Drugs 0.000 description 1
- 108010046301 glucose peroxidase Proteins 0.000 description 1
- 229940096919 glycogen Drugs 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 238000002847 impedance measurement Methods 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 229960000448 lactic acid Drugs 0.000 description 1
- IUBSYMUCCVWXPE-UHFFFAOYSA-N metoprolol Chemical compound COCCC1=CC=C(OCC(O)CNC(C)C)C=C1 IUBSYMUCCVWXPE-UHFFFAOYSA-N 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 150000002972 pentoses Chemical class 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 239000003910 polypeptide antibiotic agent Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 210000000434 stratum corneum Anatomy 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- 229940041492 toprol Drugs 0.000 description 1
- 229940116269 uric acid Drugs 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
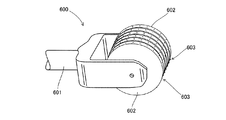
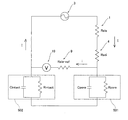
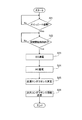
Images
Landscapes
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measurement And Recording Of Electrical Phenomena And Electrical Characteristics Of The Living Body (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007048262A JP2008206875A (ja) | 2007-02-28 | 2007-02-28 | 皮膚コンダクタンス測定装置、成分濃度分析装置、皮膚コンダクタンス測定方法および成分濃度分析方法 |
| CN2008100816194A CN101254093B (zh) | 2007-02-28 | 2008-02-27 | 透皮率测定方法及皮肤电导率检测仪 |
| EP08003552A EP1964512A3 (en) | 2007-02-28 | 2008-02-27 | Method of measuring skin conductance, method of analyzing component concentration, skin conductive measuring apparatus, and component concentration analyzer |
| US12/074,198 US20080208024A1 (en) | 2007-02-28 | 2008-02-28 | Method of measuring skin conductance, method of analyzing component concentration, skin conductance measuring apparatus, and component concentration analyzer |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007048262A JP2008206875A (ja) | 2007-02-28 | 2007-02-28 | 皮膚コンダクタンス測定装置、成分濃度分析装置、皮膚コンダクタンス測定方法および成分濃度分析方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008206875A true JP2008206875A (ja) | 2008-09-11 |
| JP2008206875A5 JP2008206875A5 (enExample) | 2010-04-02 |
Family
ID=39783686
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007048262A Withdrawn JP2008206875A (ja) | 2007-02-28 | 2007-02-28 | 皮膚コンダクタンス測定装置、成分濃度分析装置、皮膚コンダクタンス測定方法および成分濃度分析方法 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2008206875A (enExample) |
| CN (1) | CN101254093B (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010169662A (ja) * | 2008-12-22 | 2010-08-05 | Sysmex Corp | 生体内成分測定方法および生体内成分測定装置 |
| JP2017029487A (ja) * | 2015-08-03 | 2017-02-09 | 国立大学法人 千葉大学 | リンパ浮腫モニタ装置 |
| JP2024510045A (ja) * | 2021-02-22 | 2024-03-05 | ゼレミック リミテッド | インピーダンスの経皮測定を行う方法及び装置 |
| CN119880888A (zh) * | 2025-03-25 | 2025-04-25 | 军事科学院系统工程研究院军需工程技术研究所 | 一种组织液葡萄糖比色检测装置及方法 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105877694A (zh) * | 2014-08-30 | 2016-08-24 | 孙宗正 | 组织液组分传感器 |
| CN104224120B (zh) * | 2014-09-15 | 2017-02-01 | 北京智谷技术服务有限公司 | 肢体内外侧识别方法和设备 |
| CN105158213B (zh) * | 2015-09-11 | 2018-08-17 | 暨南大学 | 基于光纤表面等离子体共振的葡萄糖检测装置及方法 |
| CN107361768A (zh) * | 2017-06-27 | 2017-11-21 | 深圳市宏电技术股份有限公司 | 一种检测皮肤衰老程度的方法和装置 |
| CN112354080B (zh) * | 2020-09-30 | 2024-04-19 | 未来穿戴技术有限公司 | 离子导入进度的监控方法、装置、离子导入仪及存储介质 |
| CN114931378B (zh) * | 2022-04-28 | 2025-11-21 | 中国科学院大学 | 一种无创血糖检测方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006068492A (ja) * | 2004-08-04 | 2006-03-16 | Sysmex Corp | 分析装置および分析方法 |
-
2007
- 2007-02-28 JP JP2007048262A patent/JP2008206875A/ja not_active Withdrawn
-
2008
- 2008-02-27 CN CN2008100816194A patent/CN101254093B/zh not_active Expired - Fee Related
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006068492A (ja) * | 2004-08-04 | 2006-03-16 | Sysmex Corp | 分析装置および分析方法 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010169662A (ja) * | 2008-12-22 | 2010-08-05 | Sysmex Corp | 生体内成分測定方法および生体内成分測定装置 |
| JP2017029487A (ja) * | 2015-08-03 | 2017-02-09 | 国立大学法人 千葉大学 | リンパ浮腫モニタ装置 |
| JP2024510045A (ja) * | 2021-02-22 | 2024-03-05 | ゼレミック リミテッド | インピーダンスの経皮測定を行う方法及び装置 |
| CN119880888A (zh) * | 2025-03-25 | 2025-04-25 | 军事科学院系统工程研究院军需工程技术研究所 | 一种组织液葡萄糖比色检测装置及方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101254093B (zh) | 2011-11-23 |
| CN101254093A (zh) | 2008-09-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2008206875A (ja) | 皮膚コンダクタンス測定装置、成分濃度分析装置、皮膚コンダクタンス測定方法および成分濃度分析方法 | |
| US7066884B2 (en) | System, method, and device for non-invasive body fluid sampling and analysis | |
| JP4817692B2 (ja) | 分析装置 | |
| EP2152358B1 (en) | Skin permeation device for analyte sensing or transdermal drug delivery | |
| JP3328290B2 (ja) | イオン導入サンプリング装置および方法 | |
| US7174199B2 (en) | Monitoring a physiological analytes | |
| US20060094946A1 (en) | System and method for analyte sampling and analysis with hydrogel | |
| JP2005137416A (ja) | 経皮的分析物抽出システム及び経皮的分析物分析システム | |
| JP2006167428A (ja) | 分析物抽出装置、分析装置、分析物抽出方法および分析方法 | |
| JP2012068263A (ja) | 体液の非侵襲性のサンプリングおよび分析のためのシステム、方法およびデバイス | |
| EP1964512A2 (en) | Method of measuring skin conductance, method of analyzing component concentration, skin conductive measuring apparatus, and component concentration analyzer | |
| JP4381705B2 (ja) | 経皮的分析物抽出システムと分析システムおよび経皮的分析物抽出方法と分析方法 | |
| JP4987527B2 (ja) | 皮膚透過率測定方法、成分濃度分析方法、皮膚透過率測定装置および成分濃度分析装置 | |
| JP2005218855A (ja) | 経皮的分析物抽出キット、経皮的分析物抽出装置、血糖値測定装置、経皮的分析物抽出方法及び血糖値測定方法 | |
| JP4248283B2 (ja) | 経皮的分析物測定システムおよび経皮的分析物測定方法 | |
| Nair et al. | Therapeutic drug monitoring by reverse iontophoresis | |
| JP4340463B2 (ja) | 経皮的分析物測定システムおよび経皮的分析物測定装置 | |
| WO2006077750A1 (ja) | 分析装置および分析物抽出カートリッジ | |
| Liu et al. | 26‐1: A Wearable Glucose Sensor Integrated with Hollow Microneedles and Reverse Iontophoresis Extraction | |
| HK1140709B (en) | Skin permeation device for analyte sensing or transdermal drug delivery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100208 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100208 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120223 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120228 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20120302 |