JP2007503286A - 心臓血管疾患を検知、診断および治療するためのシステムおよび方法 - Google Patents
心臓血管疾患を検知、診断および治療するためのシステムおよび方法 Download PDFInfo
- Publication number
- JP2007503286A JP2007503286A JP2006533330A JP2006533330A JP2007503286A JP 2007503286 A JP2007503286 A JP 2007503286A JP 2006533330 A JP2006533330 A JP 2006533330A JP 2006533330 A JP2006533330 A JP 2006533330A JP 2007503286 A JP2007503286 A JP 2007503286A
- Authority
- JP
- Japan
- Prior art keywords
- signal
- patient
- sensor
- pressure
- heart
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 208000024172 Cardiovascular disease Diseases 0.000 title claims abstract description 80
- 238000000034 method Methods 0.000 title claims description 132
- 238000011282 treatment Methods 0.000 claims abstract description 155
- 210000002216 heart Anatomy 0.000 claims abstract description 128
- 230000000747 cardiac effect Effects 0.000 claims abstract description 104
- 239000004020 conductor Substances 0.000 claims abstract description 100
- 210000005246 left atrium Anatomy 0.000 claims abstract description 98
- 238000002560 therapeutic procedure Methods 0.000 claims abstract description 76
- 230000008054 signal transmission Effects 0.000 claims abstract description 65
- 230000001225 therapeutic effect Effects 0.000 claims abstract description 60
- 239000012530 fluid Substances 0.000 claims abstract description 50
- 230000033764 rhythmic process Effects 0.000 claims abstract description 40
- 230000000638 stimulation Effects 0.000 claims abstract description 28
- 230000001746 atrial effect Effects 0.000 claims description 163
- 238000004891 communication Methods 0.000 claims description 111
- 206010019280 Heart failures Diseases 0.000 claims description 90
- 206010007559 Cardiac failure congestive Diseases 0.000 claims description 69
- 229940079593 drug Drugs 0.000 claims description 59
- 239000003814 drug Substances 0.000 claims description 59
- 238000012545 processing Methods 0.000 claims description 47
- 230000002861 ventricular Effects 0.000 claims description 45
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 18
- 239000001301 oxygen Substances 0.000 claims description 18
- 229910052760 oxygen Inorganic materials 0.000 claims description 18
- 230000005540 biological transmission Effects 0.000 claims description 14
- 230000000694 effects Effects 0.000 claims description 14
- 230000001133 acceleration Effects 0.000 claims description 12
- 230000000241 respiratory effect Effects 0.000 claims description 12
- 206010003658 Atrial Fibrillation Diseases 0.000 claims description 10
- 210000005240 left ventricle Anatomy 0.000 claims description 10
- 230000036961 partial effect Effects 0.000 claims description 10
- 210000005241 right ventricle Anatomy 0.000 claims description 10
- 230000029058 respiratory gaseous exchange Effects 0.000 claims description 6
- 238000012546 transfer Methods 0.000 claims description 6
- 230000004962 physiological condition Effects 0.000 claims description 5
- 210000000115 thoracic cavity Anatomy 0.000 claims description 4
- 230000037396 body weight Effects 0.000 claims description 3
- 230000000630 rising effect Effects 0.000 claims description 2
- 238000007726 management method Methods 0.000 description 53
- 238000005259 measurement Methods 0.000 description 51
- 210000003157 atrial septum Anatomy 0.000 description 41
- 230000008901 benefit Effects 0.000 description 25
- 210000003492 pulmonary vein Anatomy 0.000 description 25
- 230000004044 response Effects 0.000 description 25
- 210000005245 right atrium Anatomy 0.000 description 24
- 239000012528 membrane Substances 0.000 description 23
- 238000012544 monitoring process Methods 0.000 description 22
- 208000024891 symptom Diseases 0.000 description 20
- 230000006870 function Effects 0.000 description 19
- 230000036760 body temperature Effects 0.000 description 18
- 238000002513 implantation Methods 0.000 description 17
- 230000001965 increasing effect Effects 0.000 description 17
- 230000035487 diastolic blood pressure Effects 0.000 description 14
- 230000008859 change Effects 0.000 description 13
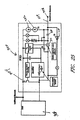
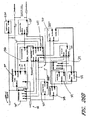
- 238000010586 diagram Methods 0.000 description 13
- 238000002604 ultrasonography Methods 0.000 description 13
- 230000001154 acute effect Effects 0.000 description 12
- 230000015654 memory Effects 0.000 description 12
- 210000001519 tissue Anatomy 0.000 description 12
- 238000001514 detection method Methods 0.000 description 11
- 239000002934 diuretic Substances 0.000 description 11
- 206010003119 arrhythmia Diseases 0.000 description 10
- 239000003990 capacitor Substances 0.000 description 10
- 238000000576 coating method Methods 0.000 description 10
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 10
- 239000007943 implant Substances 0.000 description 10
- 230000002685 pulmonary effect Effects 0.000 description 10
- 230000011664 signaling Effects 0.000 description 10
- 230000036772 blood pressure Effects 0.000 description 9
- 238000009530 blood pressure measurement Methods 0.000 description 9
- 210000005242 cardiac chamber Anatomy 0.000 description 9
- 239000011248 coating agent Substances 0.000 description 9
- 201000010099 disease Diseases 0.000 description 9
- 230000006793 arrhythmia Effects 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 206010061592 cardiac fibrillation Diseases 0.000 description 8
- 230000008602 contraction Effects 0.000 description 8
- 238000012937 correction Methods 0.000 description 8
- 238000003745 diagnosis Methods 0.000 description 8
- 238000005516 engineering process Methods 0.000 description 8
- 230000002600 fibrillogenic effect Effects 0.000 description 8
- 210000005248 left atrial appendage Anatomy 0.000 description 8
- 210000001147 pulmonary artery Anatomy 0.000 description 8
- 238000003860 storage Methods 0.000 description 8
- 230000002792 vascular Effects 0.000 description 8
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 7
- 230000004872 arterial blood pressure Effects 0.000 description 7
- 239000002876 beta blocker Substances 0.000 description 7
- 230000001684 chronic effect Effects 0.000 description 7
- 230000006866 deterioration Effects 0.000 description 7
- 210000004115 mitral valve Anatomy 0.000 description 7
- 230000000737 periodic effect Effects 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 210000003462 vein Anatomy 0.000 description 7
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 6
- 229940097320 beta blocking agent Drugs 0.000 description 6
- 210000004204 blood vessel Anatomy 0.000 description 6
- 208000006218 bradycardia Diseases 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 230000000875 corresponding effect Effects 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- 230000001882 diuretic effect Effects 0.000 description 6
- 230000002526 effect on cardiovascular system Effects 0.000 description 6
- 238000002594 fluoroscopy Methods 0.000 description 6
- 230000007774 longterm Effects 0.000 description 6
- 210000004072 lung Anatomy 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000036387 respiratory rate Effects 0.000 description 6
- 208000017667 Chronic Disease Diseases 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 230000002596 correlated effect Effects 0.000 description 5
- 238000006073 displacement reaction Methods 0.000 description 5
- 229940030606 diuretics Drugs 0.000 description 5
- 239000011888 foil Substances 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 208000028867 ischemia Diseases 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000002093 peripheral effect Effects 0.000 description 5
- 230000002265 prevention Effects 0.000 description 5
- 238000007920 subcutaneous administration Methods 0.000 description 5
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 4
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 4
- 206010037423 Pulmonary oedema Diseases 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000006399 behavior Effects 0.000 description 4
- 230000017531 blood circulation Effects 0.000 description 4
- 229960004195 carvedilol Drugs 0.000 description 4
- NPAKNKYSJIDKMW-UHFFFAOYSA-N carvedilol Chemical compound COC1=CC=CC=C1OCCNCC(O)COC1=CC=CC2=NC3=CC=C[CH]C3=C12 NPAKNKYSJIDKMW-UHFFFAOYSA-N 0.000 description 4
- 210000003109 clavicle Anatomy 0.000 description 4
- 230000001276 controlling effect Effects 0.000 description 4
- 230000002354 daily effect Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 206010012601 diabetes mellitus Diseases 0.000 description 4
- 210000002889 endothelial cell Anatomy 0.000 description 4
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 4
- 210000002837 heart atrium Anatomy 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 4
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 4
- 230000033001 locomotion Effects 0.000 description 4
- 238000002595 magnetic resonance imaging Methods 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 238000001615 p wave Methods 0.000 description 4
- 208000005333 pulmonary edema Diseases 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 238000005070 sampling Methods 0.000 description 4
- 238000004904 shortening Methods 0.000 description 4
- LTMHDMANZUZIPE-AMTYYWEZSA-N Digoxin Natural products O([C@H]1[C@H](C)O[C@H](O[C@@H]2C[C@@H]3[C@@](C)([C@@H]4[C@H]([C@]5(O)[C@](C)([C@H](O)C4)[C@H](C4=CC(=O)OC4)CC5)CC3)CC2)C[C@@H]1O)[C@H]1O[C@H](C)[C@@H](O[C@H]2O[C@@H](C)[C@H](O)[C@@H](O)C2)[C@@H](O)C1 LTMHDMANZUZIPE-AMTYYWEZSA-N 0.000 description 3
- 206010027727 Mitral valve incompetence Diseases 0.000 description 3
- 102100026827 Protein associated with UVRAG as autophagy enhancer Human genes 0.000 description 3
- 101710102978 Protein associated with UVRAG as autophagy enhancer Proteins 0.000 description 3
- 206010037368 Pulmonary congestion Diseases 0.000 description 3
- 208000037656 Respiratory Sounds Diseases 0.000 description 3
- 208000001871 Tachycardia Diseases 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 210000001367 artery Anatomy 0.000 description 3
- 210000001008 atrial appendage Anatomy 0.000 description 3
- 230000036471 bradycardia Effects 0.000 description 3
- 239000000496 cardiotonic agent Substances 0.000 description 3
- 230000003177 cardiotonic effect Effects 0.000 description 3
- 239000000919 ceramic Substances 0.000 description 3
- 239000002872 contrast media Substances 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 229960005156 digoxin Drugs 0.000 description 3
- LTMHDMANZUZIPE-PUGKRICDSA-N digoxin Chemical compound C1[C@H](O)[C@H](O)[C@@H](C)O[C@H]1O[C@@H]1[C@@H](C)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@@H]3C[C@@H]4[C@]([C@@H]5[C@H]([C@]6(CC[C@@H]([C@@]6(C)[C@H](O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)C[C@@H]2O)C)C[C@@H]1O LTMHDMANZUZIPE-PUGKRICDSA-N 0.000 description 3
- LTMHDMANZUZIPE-UHFFFAOYSA-N digoxine Natural products C1C(O)C(O)C(C)OC1OC1C(C)OC(OC2C(OC(OC3CC4C(C5C(C6(CCC(C6(C)C(O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)CC2O)C)CC1O LTMHDMANZUZIPE-UHFFFAOYSA-N 0.000 description 3
- 206010016256 fatigue Diseases 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 230000013632 homeostatic process Effects 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 229940063711 lasix Drugs 0.000 description 3
- 238000002483 medication Methods 0.000 description 3
- 230000028161 membrane depolarization Effects 0.000 description 3
- 238000012806 monitoring device Methods 0.000 description 3
- 230000003287 optical effect Effects 0.000 description 3
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 3
- 229920000052 poly(p-xylylene) Polymers 0.000 description 3
- 235000011164 potassium chloride Nutrition 0.000 description 3
- 239000001103 potassium chloride Substances 0.000 description 3
- 230000034225 regulation of ventricular cardiomyocyte membrane depolarization Effects 0.000 description 3
- 230000035939 shock Effects 0.000 description 3
- 210000001321 subclavian vein Anatomy 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 239000010936 titanium Substances 0.000 description 3
- 229910052719 titanium Inorganic materials 0.000 description 3
- 230000007704 transition Effects 0.000 description 3
- 229940124549 vasodilator Drugs 0.000 description 3
- 239000003071 vasodilator agent Substances 0.000 description 3
- 210000002620 vena cava superior Anatomy 0.000 description 3
- 230000000007 visual effect Effects 0.000 description 3
- 206010003662 Atrial flutter Diseases 0.000 description 2
- 229940127291 Calcium channel antagonist Drugs 0.000 description 2
- 208000002330 Congenital Heart Defects Diseases 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 206010016803 Fluid overload Diseases 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- 208000001953 Hypotension Diseases 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 108010007859 Lisinopril Proteins 0.000 description 2
- 208000020128 Mitral stenosis Diseases 0.000 description 2
- 208000009525 Myocarditis Diseases 0.000 description 2
- 108020001621 Natriuretic Peptide Proteins 0.000 description 2
- 102000004571 Natriuretic peptide Human genes 0.000 description 2
- 206010037660 Pyrexia Diseases 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 102000003978 Tissue Plasminogen Activator Human genes 0.000 description 2
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 2
- 206010057469 Vascular stenosis Diseases 0.000 description 2
- 206010052428 Wound Diseases 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 210000001015 abdomen Anatomy 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 238000002399 angioplasty Methods 0.000 description 2
- 210000000709 aorta Anatomy 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 230000000975 bioactive effect Effects 0.000 description 2
- 210000000748 cardiovascular system Anatomy 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 210000000038 chest Anatomy 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 208000028831 congenital heart disease Diseases 0.000 description 2
- 208000029078 coronary artery disease Diseases 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 230000003205 diastolic effect Effects 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 230000037213 diet Effects 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 2
- 238000001647 drug administration Methods 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 208000018578 heart valve disease Diseases 0.000 description 2
- 230000000004 hemodynamic effect Effects 0.000 description 2
- 230000023597 hemostasis Effects 0.000 description 2
- 230000036543 hypotension Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 239000011810 insulating material Substances 0.000 description 2
- 229940125396 insulin Drugs 0.000 description 2
- 210000004731 jugular vein Anatomy 0.000 description 2
- RLAWWYSOJDYHDC-BZSNNMDCSA-N lisinopril Chemical compound C([C@H](N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(O)=O)C(O)=O)CC1=CC=CC=C1 RLAWWYSOJDYHDC-BZSNNMDCSA-N 0.000 description 2
- 229960002394 lisinopril Drugs 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910052753 mercury Inorganic materials 0.000 description 2
- 208000006887 mitral valve stenosis Diseases 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- 239000000692 natriuretic peptide Substances 0.000 description 2
- 230000002644 neurohormonal effect Effects 0.000 description 2
- -1 obstructive and limited / invasive types Diseases 0.000 description 2
- 229940126701 oral medication Drugs 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 230000035479 physiological effects, processes and functions Effects 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 108091006082 receptor inhibitors Proteins 0.000 description 2
- 230000008929 regeneration Effects 0.000 description 2
- 238000011069 regeneration method Methods 0.000 description 2
- 230000004213 regulation of atrial cardiomyocyte membrane depolarization Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000008439 repair process Effects 0.000 description 2
- 230000000284 resting effect Effects 0.000 description 2
- 210000005247 right atrial appendage Anatomy 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 230000005236 sound signal Effects 0.000 description 2
- 238000010183 spectrum analysis Methods 0.000 description 2
- LXMSZDCAJNLERA-ZHYRCANASA-N spironolactone Chemical compound C([C@@H]1[C@]2(C)CC[C@@H]3[C@@]4(C)CCC(=O)C=C4C[C@H]([C@@H]13)SC(=O)C)C[C@@]21CCC(=O)O1 LXMSZDCAJNLERA-ZHYRCANASA-N 0.000 description 2
- 229960002256 spironolactone Drugs 0.000 description 2
- 230000003068 static effect Effects 0.000 description 2
- 230000008093 supporting effect Effects 0.000 description 2
- 229910052715 tantalum Inorganic materials 0.000 description 2
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 2
- 229960000187 tissue plasminogen activator Drugs 0.000 description 2
- 238000000108 ultra-filtration Methods 0.000 description 2
- 210000001631 vena cava inferior Anatomy 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 238000004804 winding Methods 0.000 description 2
- SFLSHLFXELFNJZ-QMMMGPOBSA-N (-)-norepinephrine Chemical compound NC[C@H](O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-QMMMGPOBSA-N 0.000 description 1
- UCTWMZQNUQWSLP-VIFPVBQESA-N (R)-adrenaline Chemical compound CNC[C@H](O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-VIFPVBQESA-N 0.000 description 1
- 229930182837 (R)-adrenaline Natural products 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- 208000010444 Acidosis Diseases 0.000 description 1
- 208000004476 Acute Coronary Syndrome Diseases 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 206010002329 Aneurysm Diseases 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- 208000031104 Arterial Occlusive disease Diseases 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 201000004569 Blindness Diseases 0.000 description 1
- 206010049765 Bradyarrhythmia Diseases 0.000 description 1
- 206010006580 Bundle branch block left Diseases 0.000 description 1
- 206010006578 Bundle-Branch Block Diseases 0.000 description 1
- 208000020446 Cardiac disease Diseases 0.000 description 1
- 206010007556 Cardiac failure acute Diseases 0.000 description 1
- 208000031229 Cardiomyopathies Diseases 0.000 description 1
- 206010062746 Carditis Diseases 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 229940123587 Cell cycle inhibitor Drugs 0.000 description 1
- 206010008479 Chest Pain Diseases 0.000 description 1
- 206010053567 Coagulopathies Diseases 0.000 description 1
- 108010031325 Cytidine deaminase Proteins 0.000 description 1
- 208000019505 Deglutition disease Diseases 0.000 description 1
- JRWZLRBJNMZMFE-UHFFFAOYSA-N Dobutamine Chemical compound C=1C=C(O)C(O)=CC=1CCNC(C)CCC1=CC=C(O)C=C1 JRWZLRBJNMZMFE-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 206010019315 Heart transplant rejection Diseases 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 108010007267 Hirudins Proteins 0.000 description 1
- 102000007625 Hirudins Human genes 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- 208000013016 Hypoglycemia Diseases 0.000 description 1
- 208000019025 Hypokalemia Diseases 0.000 description 1
- 206010021036 Hyponatraemia Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 208000010159 IgA glomerulonephritis Diseases 0.000 description 1
- 206010021263 IgA nephropathy Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000035478 Interatrial communication Diseases 0.000 description 1
- 208000009378 Low Cardiac Output Diseases 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- SNIOPGDIGTZGOP-UHFFFAOYSA-N Nitroglycerin Chemical compound [O-][N+](=O)OCC(O[N+]([O-])=O)CO[N+]([O-])=O SNIOPGDIGTZGOP-UHFFFAOYSA-N 0.000 description 1
- 239000000006 Nitroglycerin Substances 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 206010033557 Palpitations Diseases 0.000 description 1
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 206010038669 Respiratory arrest Diseases 0.000 description 1
- 206010057190 Respiratory tract infections Diseases 0.000 description 1
- 206010049418 Sudden Cardiac Death Diseases 0.000 description 1
- 206010049447 Tachyarrhythmia Diseases 0.000 description 1
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 206010047571 Visual impairment Diseases 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 230000007950 acidosis Effects 0.000 description 1
- 208000026545 acidosis disease Diseases 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 238000004873 anchoring Methods 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 230000002253 anti-ischaemic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 229940127218 antiplatelet drug Drugs 0.000 description 1
- 239000004019 antithrombin Substances 0.000 description 1
- 210000001765 aortic valve Anatomy 0.000 description 1
- 208000021328 arterial occlusion Diseases 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 230000003143 atherosclerotic effect Effects 0.000 description 1
- 208000013914 atrial heart septal defect Diseases 0.000 description 1
- 206010003664 atrial septal defect Diseases 0.000 description 1
- 210000001992 atrioventricular node Anatomy 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 229940125388 beta agonist Drugs 0.000 description 1
- 108010055460 bivalirudin Proteins 0.000 description 1
- 229960001500 bivalirudin Drugs 0.000 description 1
- OIRCOABEOLEUMC-GEJPAHFPSA-N bivalirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 OIRCOABEOLEUMC-GEJPAHFPSA-N 0.000 description 1
- 238000009529 body temperature measurement Methods 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 238000005219 brazing Methods 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 210000004375 bundle of his Anatomy 0.000 description 1
- 230000003683 cardiac damage Effects 0.000 description 1
- 239000002327 cardiovascular agent Substances 0.000 description 1
- 229940125692 cardiovascular agent Drugs 0.000 description 1
- 230000009084 cardiovascular function Effects 0.000 description 1
- 238000013131 cardiovascular procedure Methods 0.000 description 1
- 238000013153 catheter ablation Methods 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 230000002057 chronotropic effect Effects 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 230000002016 colloidosmotic effect Effects 0.000 description 1
- 239000003184 complementary RNA Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 238000013500 data storage Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000011863 diuretic therapy Methods 0.000 description 1
- 229960001089 dobutamine Drugs 0.000 description 1
- 229960003638 dopamine Drugs 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 238000010292 electrical insulation Methods 0.000 description 1
- 230000005670 electromagnetic radiation Effects 0.000 description 1
- 238000002001 electrophysiology Methods 0.000 description 1
- 230000007831 electrophysiology Effects 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 229960005139 epinephrine Drugs 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 229930182833 estradiol Natural products 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 210000003191 femoral vein Anatomy 0.000 description 1
- 239000003527 fibrinolytic agent Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229960003883 furosemide Drugs 0.000 description 1
- 230000007274 generation of a signal involved in cell-cell signaling Effects 0.000 description 1
- 210000004704 glottis Anatomy 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000002641 glycemic effect Effects 0.000 description 1
- 229960003711 glyceryl trinitrate Drugs 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 210000004013 groin Anatomy 0.000 description 1
- 230000009442 healing mechanism Effects 0.000 description 1
- 230000010247 heart contraction Effects 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 210000003709 heart valve Anatomy 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229940006607 hirudin Drugs 0.000 description 1
- WQPDUTSPKFMPDP-OUMQNGNKSA-N hirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(OS(O)(=O)=O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]2CSSC[C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@H](C(NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N2)=O)CSSC1)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)CSSC1)C(C)C)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 WQPDUTSPKFMPDP-OUMQNGNKSA-N 0.000 description 1
- 208000014471 histiocytoid cardiomyopathy Diseases 0.000 description 1
- 235000003642 hunger Nutrition 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 201000001421 hyperglycemia Diseases 0.000 description 1
- 230000002218 hypoglycaemic effect Effects 0.000 description 1
- 208000018875 hypoxemia Diseases 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000007373 indentation Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 230000001057 ionotropic effect Effects 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 230000003907 kidney function Effects 0.000 description 1
- 201000001715 left bundle branch hemiblock Diseases 0.000 description 1
- 231100000225 lethality Toxicity 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 230000005055 memory storage Effects 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910001092 metal group alloy Inorganic materials 0.000 description 1
- 230000027939 micturition Effects 0.000 description 1
- 229960003574 milrinone Drugs 0.000 description 1
- PZRHRDRVRGEVNW-UHFFFAOYSA-N milrinone Chemical compound N1C(=O)C(C#N)=CC(C=2C=CN=CC=2)=C1C PZRHRDRVRGEVNW-UHFFFAOYSA-N 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000006855 networking Effects 0.000 description 1
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 1
- 229910001000 nickel titanium Inorganic materials 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 229960002748 norepinephrine Drugs 0.000 description 1
- SFLSHLFXELFNJZ-UHFFFAOYSA-N norepinephrine Natural products NCC(O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-UHFFFAOYSA-N 0.000 description 1
- 230000000474 nursing effect Effects 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- 238000011369 optimal treatment Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000003538 oral antidiabetic agent Substances 0.000 description 1
- 229940127209 oral hypoglycaemic agent Drugs 0.000 description 1
- 230000003534 oscillatory effect Effects 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 210000003540 papillary muscle Anatomy 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 208000008494 pericarditis Diseases 0.000 description 1
- 210000003105 phrenic nerve Anatomy 0.000 description 1
- 230000037081 physical activity Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000000106 platelet aggregation inhibitor Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 231100000572 poisoning Toxicity 0.000 description 1
- 230000000607 poisoning effect Effects 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 208000024896 potassium deficiency disease Diseases 0.000 description 1
- 230000036316 preload Effects 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000036279 refractory period Effects 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 229940107685 reopro Drugs 0.000 description 1
- 230000008672 reprogramming Effects 0.000 description 1
- 201000003068 rheumatic fever Diseases 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 201000002859 sleep apnea Diseases 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 230000035900 sweating Effects 0.000 description 1
- 230000001360 synchronised effect Effects 0.000 description 1
- 230000035488 systolic blood pressure Effects 0.000 description 1
- 230000006794 tachycardia Effects 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 150000004579 taxol derivatives Chemical class 0.000 description 1
- IMCGHZIGRANKHV-AJNGGQMLSA-N tert-butyl (3s,5s)-2-oxo-5-[(2s,4s)-5-oxo-4-propan-2-yloxolan-2-yl]-3-propan-2-ylpyrrolidine-1-carboxylate Chemical compound O1C(=O)[C@H](C(C)C)C[C@H]1[C@H]1N(C(=O)OC(C)(C)C)C(=O)[C@H](C(C)C)C1 IMCGHZIGRANKHV-AJNGGQMLSA-N 0.000 description 1
- 238000011287 therapeutic dose Methods 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 230000035922 thirst Effects 0.000 description 1
- 229960000103 thrombolytic agent Drugs 0.000 description 1
- 201000005060 thrombophlebitis Diseases 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 238000010396 two-hybrid screening Methods 0.000 description 1
- 238000012285 ultrasound imaging Methods 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 208000003663 ventricular fibrillation Diseases 0.000 description 1
- 206010047302 ventricular tachycardia Diseases 0.000 description 1
- 208000029257 vision disease Diseases 0.000 description 1
- 230000004393 visual impairment Effects 0.000 description 1
- PJVWKTKQMONHTI-UHFFFAOYSA-N warfarin Chemical compound OC=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 PJVWKTKQMONHTI-UHFFFAOYSA-N 0.000 description 1
- 229960005080 warfarin Drugs 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Landscapes
- Measuring And Recording Apparatus For Diagnosis (AREA)
- Electrotherapy Devices (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US47046803P | 2003-05-13 | 2003-05-13 | |
| US10/697,960 US6970742B2 (en) | 2000-01-11 | 2003-10-29 | Method for detecting, diagnosing, and treating cardiovascular disease |
| US10/698,031 US7483743B2 (en) | 2000-01-11 | 2003-10-29 | System for detecting, diagnosing, and treating cardiovascular disease |
| PCT/US2004/016186 WO2005000206A2 (en) | 2003-05-13 | 2004-05-12 | System and method for detecting, diagnosing, and treating cardiovascular disease |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2007503286A true JP2007503286A (ja) | 2007-02-22 |
| JP2007503286A5 JP2007503286A5 (enExample) | 2007-07-05 |
Family
ID=37852361
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006533330A Pending JP2007503286A (ja) | 2003-05-13 | 2004-05-12 | 心臓血管疾患を検知、診断および治療するためのシステムおよび方法 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2007503286A (enExample) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008545471A (ja) * | 2005-05-24 | 2008-12-18 | カーディアック・ペースメーカーズ・インコーポレーテッド | 胸部体液蓄積の予測 |
| JP2009078148A (ja) * | 2007-09-25 | 2009-04-16 | Radi Medical Systems Ab | 圧力センサワイヤアセンブリ |
| JP2011509764A (ja) * | 2008-01-22 | 2011-03-31 | カーディアック ペースメイカーズ, インコーポレイテッド | 治療最適化のための引き金としての呼吸 |
| KR20110118632A (ko) * | 2008-12-19 | 2011-10-31 | 스카이 메디컬 테크놀러지 리미티드 | 치료 |
| US8414497B2 (en) | 2005-10-13 | 2013-04-09 | Cardiac Pacemakers, Inc. | Detection of hypovolemia using implantable medical device |
| US9138151B2 (en) | 2007-03-14 | 2015-09-22 | Cardiac Pacemakers, Inc. | Method and apparatus for management of heart failure hospitalization |
| WO2015194598A1 (ja) * | 2014-06-17 | 2015-12-23 | 日本電産コパル電子株式会社 | 感圧センサモジュール、圧力測定用ガイドワイヤ及び圧力測定装置 |
| KR20160075732A (ko) * | 2013-10-25 | 2016-06-29 | 신테프 또 에이에스 | 체내 압력 감시 시스템 |
| WO2019058792A1 (ja) * | 2017-09-20 | 2019-03-28 | マクセルホールディングス株式会社 | 電気的筋肉刺激装置 |
| JP2022553321A (ja) * | 2019-10-23 | 2022-12-22 | インパルス ダイナミクス エヌヴイ | 心臓電気刺激を計画し、送達する方法 |
| JP2023504565A (ja) * | 2019-12-06 | 2023-02-03 | ケー. ウルフ,ランドール | 低エネルギー心房カーディオバージョン、ペーシング及びセンシングのための埋め込み型血管内小型形状心臓内左心房拘束デバイス |
| JP2023018683A (ja) * | 2013-03-14 | 2023-02-08 | スティムウェイブ テクノロジーズ インコーポレイテッド | 無線植込み型電力受信機システム及び方法 |
| JP2023546057A (ja) * | 2020-10-13 | 2023-11-01 | ペーサーツール アーエス | 心臓の非同期に起因する非共同運動を検出するカテーテルおよび方法 |
| JP2023552162A (ja) * | 2020-11-30 | 2023-12-14 | ボストン サイエンティフィック サイムド,インコーポレイテッド | 植込型受動式平均圧センサ |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001051123A1 (en) * | 2000-01-11 | 2001-07-19 | Cedars-Sinai Medical Center | Permanently implantable system and method for detecting, diagnosing and treating congestive heart failure |
-
2004
- 2004-05-12 JP JP2006533330A patent/JP2007503286A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001051123A1 (en) * | 2000-01-11 | 2001-07-19 | Cedars-Sinai Medical Center | Permanently implantable system and method for detecting, diagnosing and treating congestive heart failure |
| JP2003519542A (ja) * | 2000-01-11 | 2003-06-24 | セダーズ−シナイ メディカル センター | うっ血性心不全を検知し、診断し、治療する、永久的に移植可能なシステムおよび方法 |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008545471A (ja) * | 2005-05-24 | 2008-12-18 | カーディアック・ペースメーカーズ・インコーポレーテッド | 胸部体液蓄積の予測 |
| US8414497B2 (en) | 2005-10-13 | 2013-04-09 | Cardiac Pacemakers, Inc. | Detection of hypovolemia using implantable medical device |
| US9730592B2 (en) | 2007-03-14 | 2017-08-15 | Cardiac Pacemakers, Inc. | Method and apparatus for management of heart failure hospitalization |
| US11723544B2 (en) | 2007-03-14 | 2023-08-15 | Cardiac Pacemakers, Inc. | Method and apparatus for management of heart failure hospitalization |
| US9138151B2 (en) | 2007-03-14 | 2015-09-22 | Cardiac Pacemakers, Inc. | Method and apparatus for management of heart failure hospitalization |
| US10441179B2 (en) | 2007-03-14 | 2019-10-15 | Cardiac Pacemakers, Inc. | Method and apparatus for management of heart failure hospitalization |
| JP2009078148A (ja) * | 2007-09-25 | 2009-04-16 | Radi Medical Systems Ab | 圧力センサワイヤアセンブリ |
| JP2011509764A (ja) * | 2008-01-22 | 2011-03-31 | カーディアック ペースメイカーズ, インコーポレイテッド | 治療最適化のための引き金としての呼吸 |
| KR101686829B1 (ko) * | 2008-12-19 | 2016-12-15 | 스카이 메디컬 테크놀러지 리미티드 | 신경의 전기자극을 통한 근육 치료 |
| KR20110118632A (ko) * | 2008-12-19 | 2011-10-31 | 스카이 메디컬 테크놀러지 리미티드 | 치료 |
| US11491328B2 (en) | 2008-12-19 | 2022-11-08 | Sky Medical Technology, Ltd. | Prevention and treatment of diastolic flow reversal |
| JP7498753B2 (ja) | 2013-03-14 | 2024-06-12 | クロニクス エルエルシー | 無線植込み型電力受信機システム及び方法 |
| JP2023018683A (ja) * | 2013-03-14 | 2023-02-08 | スティムウェイブ テクノロジーズ インコーポレイテッド | 無線植込み型電力受信機システム及び方法 |
| KR20160075732A (ko) * | 2013-10-25 | 2016-06-29 | 신테프 또 에이에스 | 체내 압력 감시 시스템 |
| JP2016533802A (ja) * | 2013-10-25 | 2016-11-04 | シンテフ ティーティーオー エーエス | 生体内圧力モニタリングシステム |
| KR102302422B1 (ko) * | 2013-10-25 | 2021-09-15 | 신테프 또 에이에스 | 체내 압력 감시 시스템 |
| WO2015194598A1 (ja) * | 2014-06-17 | 2015-12-23 | 日本電産コパル電子株式会社 | 感圧センサモジュール、圧力測定用ガイドワイヤ及び圧力測定装置 |
| JP2019054942A (ja) * | 2017-09-20 | 2019-04-11 | マクセルホールディングス株式会社 | 電気的筋肉刺激装置 |
| JP7066360B2 (ja) | 2017-09-20 | 2022-05-13 | マクセル株式会社 | 電気的筋肉刺激装置 |
| WO2019058792A1 (ja) * | 2017-09-20 | 2019-03-28 | マクセルホールディングス株式会社 | 電気的筋肉刺激装置 |
| JP2022553321A (ja) * | 2019-10-23 | 2022-12-22 | インパルス ダイナミクス エヌヴイ | 心臓電気刺激を計画し、送達する方法 |
| JP7707160B2 (ja) | 2019-10-23 | 2025-07-14 | インパルス ダイナミクス エヌヴイ | 心臓電気刺激を計画し、送達する方法 |
| JP2023504565A (ja) * | 2019-12-06 | 2023-02-03 | ケー. ウルフ,ランドール | 低エネルギー心房カーディオバージョン、ペーシング及びセンシングのための埋め込み型血管内小型形状心臓内左心房拘束デバイス |
| JP2023546057A (ja) * | 2020-10-13 | 2023-11-01 | ペーサーツール アーエス | 心臓の非同期に起因する非共同運動を検出するカテーテルおよび方法 |
| JP2023552162A (ja) * | 2020-11-30 | 2023-12-14 | ボストン サイエンティフィック サイムド,インコーポレイテッド | 植込型受動式平均圧センサ |
| JP7627761B2 (ja) | 2020-11-30 | 2025-02-06 | ボストン サイエンティフィック サイムド,インコーポレイテッド | 植込型受動式平均圧センサ |
| US12329500B2 (en) | 2020-11-30 | 2025-06-17 | Boston Scientific Scimed, Inc. | Implantable passive mean pressure sensor |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2525193C (en) | System and method for detecting, diagnosing, and treating cardiovascular disease | |
| US7590449B2 (en) | Patient signaling method for treating cardiovascular disease | |
| US7616991B2 (en) | Method for digital cardiac rhythm management | |
| US20060149330A1 (en) | Digitally controlled cardiac rhythm management | |
| US20060149324A1 (en) | Cardiac rhythm management with interchangeable components | |
| US20120046528A1 (en) | System and method for detecting and treating cardiovascular disease | |
| US8613705B2 (en) | Central venous pressure sensor and method to control a fluid or volume overload therapy | |
| US11969233B2 (en) | Measuring cardiovascular pressure based on patient state | |
| CN1819855A (zh) | 检测、诊断和治疗心血管疾病的系统和方法 | |
| JP2000350705A (ja) | 血管移植用の圧力/温度/流量モニタリング装置 | |
| CN110087534B (zh) | 针对测得的心血管压力值的静液压偏移调整 | |
| JP2007503286A (ja) | 心臓血管疾患を検知、診断および治療するためのシステムおよび方法 | |
| WO2024123547A1 (en) | Prediction or detection of major adverse cardiac events via disruption in sympathetic response | |
| WO2025219783A1 (en) | Medical device system configured to monitor pulmonary hypertension risk using optical and electrocardiogram signals | |
| WO2025125960A1 (en) | System to calibrate blood pressure values optically derived using an implantable medical device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070511 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20070511 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070511 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100106 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100406 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100921 |